Abstract
An increasing number of studies have suggested that oxidative stress and inflammation play momentous roles in acute pulmonary embolism (APE). Honokiol, a bioactive biphenolic phytochemical substance, is known for its strong anti-oxidative and anti-inflammatory effects, and it served as an activator of sirtuin3 (SIRT3) in the present study. The purposes of the study were to explore the effects of honokiol on APE rats and investigate whether the function of honokiol is mediated by SIRT3 activation. In the study, the rats received a right femoral vein injection of dextran gel G-50 particles (12 mg/kg) to establish the APE model and were subsequently administered honokiol and/or a selective SIRT3 inhibitor 3-(1H-1,2,3-triazol-4-yl)pyridine (3-TYP; 5 mg/kg) intraperitoneally. The results showed that SIRT3 activation by honokiol attenuated the loss in lung function, ameliorated the inflammatory response and oxidative damage, and inhibited apoptosis in lung tissues of the rats with APE but that this was reversed by 3-TYP. In addition, we found that the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway might be activated by honokiol but restrained by 3-TYP. These results indicated that honokiol was capable of suppressing the adverse effects of APE and that this was diminished by SIRT3 suppression, implying that activation of SIRT3 might serve as a therapeutic method for APE.
Keywords: acute pulmonary embolism (APE), apoptosis, inflammation, oxidative stress, sirtuin3 (SIRT3)
Introduction
Acute pulmonary embolism (APE) is the third most common acute cardiovascular disease [1]. In the clinical setting, deep vein thrombosis (DVT) is the most common risk factor for APE [2]. APE normally causes chest pain, breathlessness, and cough [3, 4]. Inflammation and oxidative stress play significant roles in APE progression. On the one hand, APE is capable of inciting inflammation induced by multiple stimuli [5, 6], including pulmonary artery hypertension [7,8,9], hypoxia [10], and ischemia [11]. On the other hand, APE causes perturbations in the redox balance, thereby inducing oxidative stress [12,13,14]. Here, we attempted to explore a molecule associated with inflammation and oxidative stress, so as to further study the mechanism related to APE, which has an important guiding significance for treatment of this disease.
Mammalian sirtuins (SIRTs), NAD+-dependent protein deacetylases, comprise seven isoforms (SIRT1 to SIRT7) based on distinct differences in subcellular localization and substrate specificity [15,16,17,18]. Numerous studies have shown that SIRTs possess protective effects against aging, apoptosis, inflammation, and oxidative stress [19, 20]. SIRTs, especially SIRT3, are linked to several pathologies, including cancer, heart disease, fibrosis, acute kidney injury, and neurodegeneration diseases [21,22,23,24,25].
SIRT3 mostly controls mitochondrial acetylation [26, 27], which plays important roles in regulating systemic inflammation, cellular energy metabolism, oxidative stress, and apoptosis [28,29,30]. Previous studies have indicated that mitochondrial SIRT3 is highly expressed in a variety of metabolic tissues, such as the heart, kidney, and liver [26, 31,32,33]. SIRT3 is associated with cardiovascular disease and aging [34,35,36]. Global SIRT3 depletion (Sirt3−/−) induces oxidative stress, increases vascular inflammation, and accelerates vascular senescence and age-dependent hypertension in mice. Nevertheless, genetic SIRT3 overexpression prevents these deleterious effects [37]. Similarly, SIRT3 serves as a novel therapeutic molecule in endotoxin-induced acute lung injury (ALI) by repressing macrophage metabolic reprogramming and its pro-inflammatory phenotype [38]. Besides, a study has pointed out that melatonin treatment protects the heart from myocardial ischemia/reperfusion injury by reducing oxidative stress and apoptosis via activation of the SIRT3 signaling pathway [39]. SIRT3 deficiency also promotes pulmonary fibrosis by augmenting mitochondrial DNA damage and apoptosis in alveolar epithelial cells (AEC) [40]. These studies provide hints about the anti-inflammatory, anti-oxidative, and anti-apoptotic properties of SIRT3 in diseases. However, there is a lack of evidence demonstrating the effects of SIRT3 on APE.
Therefore, we used the SIRT3 activator honokiol and inhibitor 3-(1H-1,2,3-triazol-4-yl)pyridine (3-TYP) to appraise the effects of SIRT3 on inflammation, oxidative stress, and apoptosis induced by APE in lung tissues of rats and explore the underlying molecular mechanisms.
Materials and Methods
Animals
This study used male Sprague-Dawley (SD) rats, which were housed under a 12-h light/dark cycle at 22 ± 1°C with 45–55% humidity and free access to food and water. SD rats were treated with a dextran gel G-50 particles (diameter 300 µm; Macklin, Shanghai, China) suspension (12 mg/kg) via the right femoral vein to establish the APE model. Some of the APE rats were also administered an intraperitoneal injection of 5 mg/kg honokiol (MedChemExpress, Shanghai, China) as described in the previous studies [41, 42]. Furthermore, some of the APE rats administered honokiol were also injected with 5 mg/kg 3-TYP (MedChemExpress) intraperitoneally [39, 43, 44]. Subsequently, arterial blood was drawn from the common carotid artery of the rats under oxygen inhalation. The blood samples were immediately analyzed using a blood gas analyzer. The levels of arterial partial pressure of oxygen (PaO2), fraction of inspiration O2 (FiO2), oxygenation index (PaO2/FiO2), and arterial partial pressure of carbon dioxide (PaCO2) were recorded. The lung tissues were obtained from the euthanized rats for subsequent experiments. All animal experiments were carried out in strict adherence with the Guidelines of Laboratory Animals Center of Soochow University.
Real-time PCR
Total RNA was extracted by TRIpure Reagent (BioTeke Corp., Beijing, China) according to manufacturer’s instructions. The corresponding cDNA was obtained by reverse transcription using BeyoRT II M-MLV Reverse Transcriptase (Beyotime Biotechnology, Shanghai, China). Real-time quantitative PCR was performed using SYBR Green (Solarbio Science & Technology, Beijing, China) to measure the Sirt3 levels of the control and APE groups with specific primer (SIRT3 forward primer, 5′-GCCCAATGTCGCTCACTA-3′; SIRT3 reverse primer, 5′-CGTCAGCCCGTATGTCTT-3′). β-actin was used as the endogenous control (β-actin forward primer, 5′-GGAGATTACTGCCCTGGCTCCTAGC-3′; β-actin reverse primer, 5′-GGCCGGACTCATCGTACTCCTGCTT-3′). The relative expression levels of Sirt3 were calculated using the 2-ΔΔCt method.
Immunohistochemistry (IHC)
Pulmonary tissues from the rats were embedded in paraffin, and the paraffin-embedded blocks of pulmonary tissues from the control and APE rats were processed to obtain 5-µm-thick histologic sections. The samples were incubated with anti-SIRT3 (1:100; Abclonal Technology, Hubei, China) overnight at 4°C, followed by incubation with HRP-linked goat anti-rabbit IgG (1:500; Thermo Fisher Scientific, Waltham, MA, USA) at 37°C for 1 h. Eventually, 3,3-diaminobenzidine (DAB; Maixin Biotech, Fujian, China) and hematoxylin (Solarbio Science & Technology) were used for staining and counterstaining, respectively. The results were observed under a microscope (×400 magnification; Olympus, Tokyo, Japan).
Immunofluorescence (IF) staining
Lung tissues obtained from the rats were processed into 5-µm-thick sections and incubated with anti-SIRT3 overnight at 4°C, as in the case of the IHC. Next, the tissues were kept in Cy3-labeled goat anti-rabbit IgG (1:200; Invitrogen, Thermo Fisher Scientific) for 1 h. Finally, all samples were stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI; Aladdin, Shanghai, China) and visualized with a fluorescence microscope (×400 magnification; Olympus).
Hematoxylin-eosin (HE) staining
Briefly, 5-µm-thick sections of lung tissues from the rats were stained with hematoxylin (Solarbio Science & Technology) and eosin (Sangon Biotech, Shanghai, China) and then photographed (40× and 200× magnification). Severity of lung injury was evaluated by referring to the criteria of previous reports [45,46,47].
Inflammatory cell count
Bronchoalveolar lavage was performed, and the collected bronchoalveolar lavage fluid was stained with Giemsa stain (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). Then, inflammatory cells, including eosinophils, neutrophils, lymphocytes, and monocyte macrophages, were counted under a microscope (400× magnification; Olympus).
Reactive oxygen species (ROS) determination
Lung tissues were embedded using optimal cutting temperature (OCT) compound. The embedded lung tissues were cut into 10-µm-thick slices. ROS levels were detected with an ROS Assay Kit (BestBio, Shanghai, China). Images were captured with a microscope (400× magnification; Olympus).
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) staining
Apoptotic cells in lung tissues were analyzed by TUNEL staining. In short, 5-µm-thick sections of lung tissues were permeabilized with 0.1% Triton X-100 (Beyotime Biotechnology) for 8 min. The sections were then placed in a TUNEL solution mixture for 1 h. An In Situ Cell Death Detection Kit (Roche, Basel, Switzerland) was used following the manufacturer’s instructions. The results were observed under a fluorescence microscope (400× magnification; Olympus).
Assessment of pulmonary injury markers
The dry and wet weights of lung tissues were determined, and then the wet/dry weight ratios (W/D) were calculated. Moreover, a Rat TNF-α ELISA Kit (Multisciences Lianke Biotech, Zhejiang, China) and IL-6 ELISA Kit (Multisciences Lianke Biotech, Zhejiang, China) were utilized to measure the levels of tumor necrosis factor-α (TNF-α) and IL-6. Myeloperoxidase (MPO) was quantified in accordance with the instructions of an MPO Assay Kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). The above biomarkers were used to evaluate the effect of SIRT3 on lung injury in the rats.
Assessment of pulmonary oxidative stress markers
The influence of SIRT3 on oxidative stress in the APE rats was assessed by measuring the content of malondialdehyde (MDA) in lung tissues as well as the activity of serum lactic dehydrogenase (LDH) and superoxide dismutase (SOD) with assay kits from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China).
Western blot
Total proteins were collected from lung tissues and separated on sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) gels (Beyotime Biotechnology). After being blocked by 5% skim milk, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (MilliporeSigma, St. Louis, USA). Then the membranes were incubated overnight at 4°C with anti-cleaved caspase-3 (1:1,000), B-cell lymphoma 2 (Bcl-2; 1:1,000), Bcl-2-associated X (Bax; 1:500), p-AMPK (1:1,000), AMPK (1:1,000), p-mTOR (1:500), and mTOR (1:500) from Affinity Biosciences (Jiangsu, China) as well as β-actin (1:50,000) from Abclonal Technology (Hubei, China). Subsequently, the membranes were exposed to secondary antibodies, horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:10,000, Abclonal Technology, Hubei, China), at 37°C for 40 min. Protein signals were observed with an enhanced chemiluminescence (ECL; Beyotime Biotechnology) system.
Statistical analysis
All experiments were repeated at least six times. The data are presented as the mean ± SD. Statistical significance was determined by Student’s t-test for comparisons between two groups and by one-way ANOVA followed by Tukey’s test for comparisons among multiple groups. The data were analyzed using the GraphPad Prism 8.0 software. A P-value <0.05 indicated statistical significance.
Results
SIRT3 was decreased in the pulmonary tissues
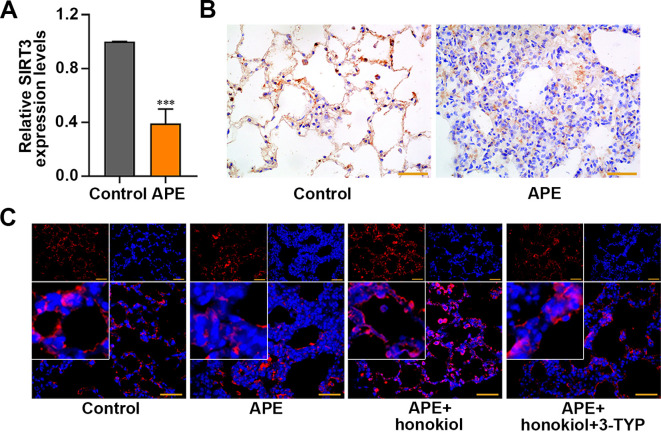
As shown in Figs. 1A and B, the levels of SIRT3 expression were downregulated in the pulmonary tissues of APE rats (P<0.001). Furthermore, IF staining assays demonstrated that honokiol elevated the expression of SIRT3 (Fig. 1C).
Fig. 1.
Sirtuin3 (SIRT3) expression in the lung tissues of rats with acute pulmonary embolism (APE). (A) The levels of SIRT3 were assessed by real-time quantitative PCR and (B) immunohistochemistry. (C) Immunofluorescence (IF) staining showed the expression of SIRT3 (red) in the lung tissues of APE rats after honokiol and/or 3-TYP treatment. Scale bar=50 µm. Data are presented as the mean ± SD (n=6 in each group). ***P<0.001 versus the control group.
SIRT3 alleviated pulmonary function loss and lung injury
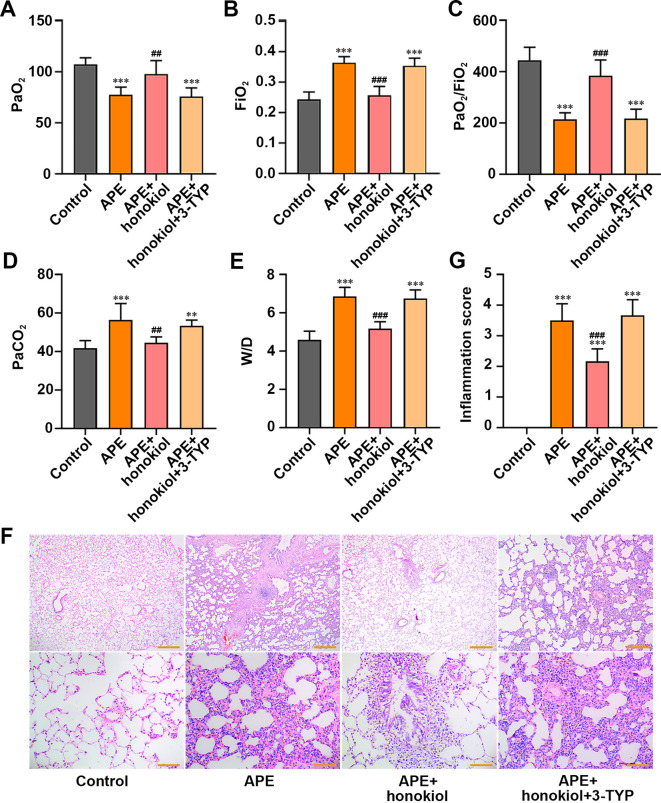
Honokiol enhanced the PaO2/FiO2 ratio and reduced the PaCO2 of the APE rats. These results were reversed by 3-TYP (Figs. 2A–D, P<0.01). Measurement of pulmonary injury revealed that the W/D ratio was reduced by honokiol but was restored by 3-TYP (Fig. 2E, P<0.001). We performed HE staining to estimate the degrees of inflammation, and the results were consistent with the above (Figs. 2F and G, P<0.001). These results suggested that SIRT3 preserved pulmonary function and reduced the lung injury induced by APE.
Fig. 2.
Effect of honokiol or 3-TYP on pulmonary function and injury in the rats with acute pulmonary embolism (APE). (A) Arterial partial pressure of oxygen (PaO2). (B) Fraction of inspiration O2 (FiO2). (C) Oxygenation index (PaO2/FiO2). (D) Arterial partial pressure of carbon dioxide (PaCO2). (E) Wet/dry weight ratio (W/D). (F, G) Hematoxylin-eosin (HE) staining was used to observe the pathological changes at 40× (Scale bar=500 µm) and 200× magnification (Scale bar=100 µm) and determine the inflammation grade. Data are presented as the mean ± SD (n=6 in each group). ***P<0.001 versus the control group. **P<0.01 versus the control group. ###P<0.001 versus the APE group. ##P<0.01 versus the APE group.
SIRT3 reduced inflammation
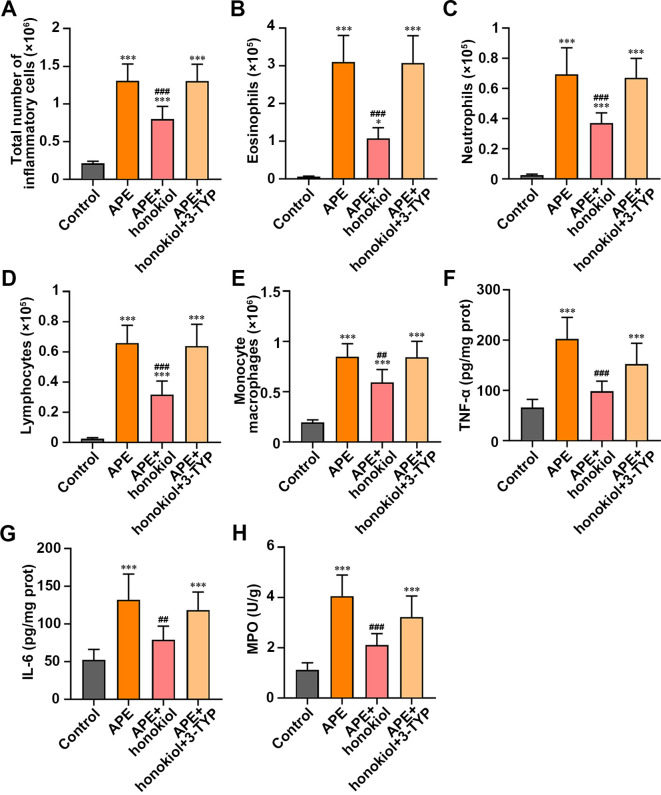
The increases in inflammatory cells, including eosinophils, neutrophils, lymphocytes, and monocyte macrophages, caused by APE were suppressed by honokiol in the APE rats (Figs. 3A–E, P<0.05). TNF-α, IL-6, and MPO, known as pro-inflammatory cytokines, were augmented in the rats with APE compared with the controls. The levels of these markers were reduced after honokiol treatment in the rats with G-50 particle-induced APE (Figs. 3F–H, P<0.01). On the other hand, 3-TYP reversed the changes (Fig. 3). Our results proved that SIRT3 might play an anti-inflammatory role in the rats suffering from APE.
Fig. 3.
Effect of honokiol or 3-TYP treatment on inflammation in the acute pulmonary embolism (APE) rats. (A) Total number of inflammatory cells. (B) Eosinophils. (C) Neutrophils. (D) Lymphocytes. (E) Monocyte macrophages. (F) Measurement of the inflammation-linked indices tumor necrosis factor-α (TNF-α), (G) IL-6, and (H) myeloperoxidase (MPO). Scale bar=50 µm. Data are presented as the mean ± SD (n=6 in each group). ***P<0.001 versus the control group. *P<0.05 versus the control group. ###P<0.001 versus the APE group. ##P<0.01 versus the APE group.
SIRT3 decreased oxidative stress and apoptosis
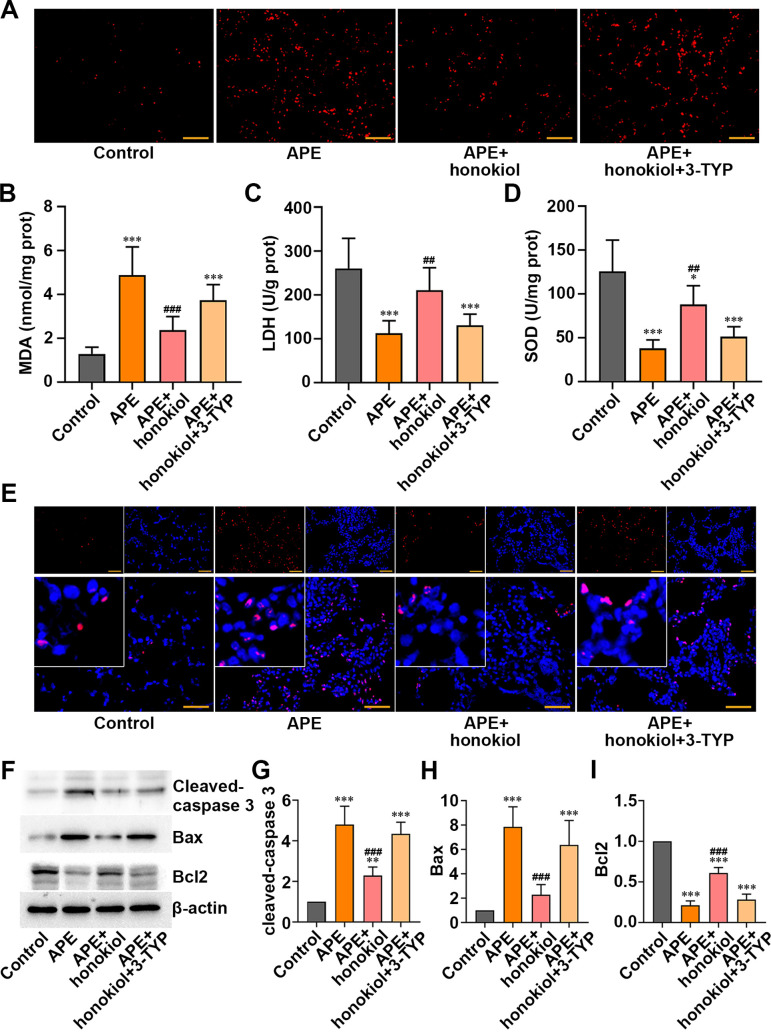
The results shown in Fig. 4A revealed that honokiol prevented elevation of ROS. Similarly, the growth of MDA was inhibited by honokiol (Fig. 4B, P<0.001). In addition, the activities of LDH and SOD were lower in the APE rats than in the controls. Honokiol was able to recover their activities (Figs. 4C and D, P<0.05). However, 3-TYP reversed these effects (Figs. 4A–D). Besides, honokiol restrained cell apoptosis, and 3-TYP mitigated this effect (Fig. 4E). Moreover, honokiol-induced decreases of pro-apoptosis genes, including cleaved caspase-3 and Bax, and elevation of the anti-apoptotic gene Bcl-2 were reversed by 3-TYP treatment (Figs. 4F–I, P<0.001). Taking into consideration all the above results, SIRT3 might protect the rats from oxidative stress and apoptosis induced by APE.
Fig. 4.
Effect of honokiol or 3-TYP on oxidative stress and apoptosis in the acute pulmonary embolism (APE) rats. (A) Reactive oxygen species (ROS). (B) Measurement of the oxidative stress-linked indices malondialdehyde (MDA), (C) lactic dehydrogenase (LDH), and (D) superoxide dismutase (SOD). (E) Apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) (red), and nuclei were detected by 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) (blue). (F-I) Apoptosis-linked indices (cleaved caspase-3, B-cell lymphoma 2 (Bcl-2)-associated X (Bax), Bcl2) were assessed by western blot. Scale bar=50 µm. Data are presented as the mean ± SD (n=6 in each group). ***P<0.001 versus the control group. **P<0.01 versus the control group. *P<0.05 versus the control group. ###P<0.001 versus the APE group. ##P<0.01 versus the APE group.
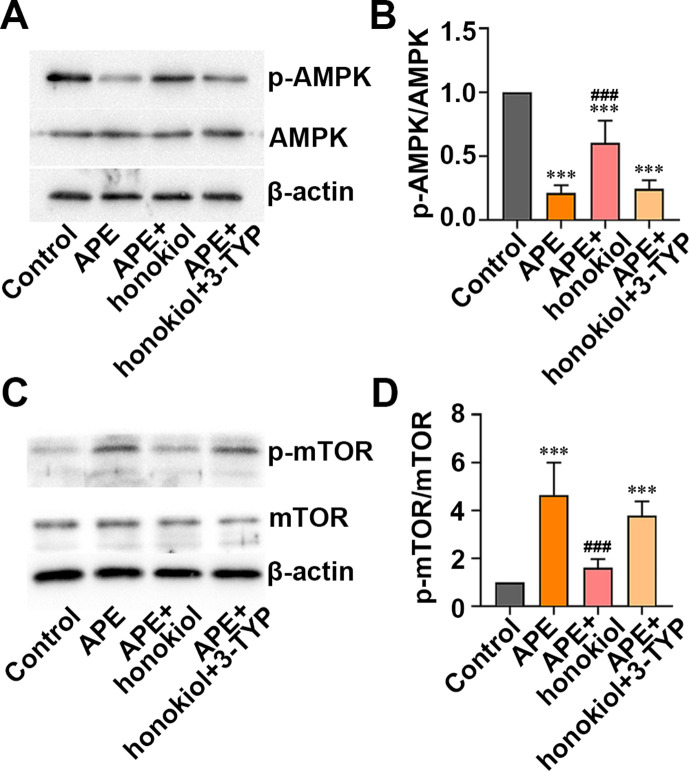
SIRT3 enhanced the AMPK/mTOR signaling pathway
The results of the western blot assay revealed that the p-AMPK/AMPK ratios, which were reduced by APE, were augmented by honokiol, whereas 3-TYP removed this effect in the APE rats (Figs. 5A and B, P<0.001). Similarly, Fig. 5C shows that the p-mTOR/mTOR ratios, which were increased by APE, were restored by honokiol in the rats with APE, and this was reversed by 3-TYP (Figs. 5C and D, P<0.001). Based on the above results, honokiol might be capable of activating the AMPK/mTOR signaling pathway, whereas 3-TYP might inhibit this. In other words, SIRT3 might protect rats from APE-induced the deleterious effects by activating the AMPK/mTOR signaling pathway.
Fig. 5.
Effect of honokiol or 3-TYP on AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway in the rats with acute pulmonary embolism (APE). (A, B) The levels of p-AMPK/AMPK were determined by western blot. (C, D) The levels of p-mTOR/mTOR were analyzed by western blot. Data are presented as the mean ± SD (n=6 in each group). ***P<0.001 versus the control group. ###P<0.001 versus the APE group.
Discussion
APE is typically caused by a thrombus that travels from a vein in a lower limb [2], and most deaths from APE occur within the first several hours to days. Therefore, early diagnosis and intervention are of vital importance [48,49,50]. In the present study, we investigated the effects of SIRT3 on rats with G-50 particle-induced APE via its modulators and explored the possible mechanisms. Previous studies have found that the abnormal expression of SIRT3 is closely pertinent to cardiovascular diseases [28, 37, 51]. We provided the evidence that SIRT3 was downregulated in the rats suffering from APE. Our data showed that SIRT3 activation induced by honokiol alleviated the abnormality of lung function in the APE rats, while SIRT3 suppression induced by 3-TYP exacerbated it. Furthermore, the increased anti-inflammatory, anti-oxidative, and anti-apoptotic capacities induced by honokiol were inhibited by 3-TYP, indicating that SIRT3 exhibited a protective effect in the APE rats. Notably, we suggested that this phenomenon might involve the AMPK/mTOR signaling pathway.
In our investigation of the anti-inflammatory effect of SIRT3 in APE rats, HE staining revealed that the lung tissues of APE rats suffered serious damage, including inflammatory cells infiltration and alveolar structure destruction. Significantly, SIRT3 activation diminished, but SIRT3 suppression aggravated these symptoms of APE. Besides, honokiol administration effectively decreased the levels of eosinophils, neutrophils, lymphocytes, and monocyte macrophages. This was sufficient evidence to suggest that SIRT3 had anti-inflammatory effects.
Oxidative stress, defined as a disturbance in the balance between oxidation and anti-oxidant defense, is able to cause excessive accumulation of ROS [52, 53]. SIRT3 is reported to rapidly balance ROS levels via deacetylation and activation of superoxide dismutase 2 (SOD2) [31, 54, 55]. In addition to oxidative stress, SIRT3 plays a regulatory role in controlling the inflammatory response to diseases [37, 38]. Honokiol (C18H18O2), a SIRT3 activator [56], possesses anti-oxidative, anti-inflammatory, and anti-apoptotic properties [57,58,59]. Zheng et al. [60] found that honokiol attenuates oxidative stress injury by deacetylating SOD2 and then scavenging excess ROS after intracerebral hemorrhage in diabetic rats. As shown in a study by Chiang et al. [42], honokiol effectively protects skeletal muscle from eccentric exercise-induced damage by repressing oxidative stress and inflammation in rats. Additionally, honokiol ameliorates the cognitive decline induced by laparotomy under sevoflurane anesthesia in mice through the inhibition of neuronal apoptosis, oxidative stress, and inflammation in the hippocampus [44]. In contrast to honokiol, 3-TYP (C7H6N4) is a selective SIRT3 inhibitor that removes honokiol’s therapeutic effects. A previous study reported that 3-TYP increases hepatic damage caused by oxidative stress and endoplasmic reticulum stress and facilitates the hepatocyte apoptosis in mice with acute liver failure [61]. Interestingly, it has been reported that 3-TYP administration abolishes the neuroprotective effects of honokiol [44]. In the present study, we found that the anti-oxidative, anti-inflammatory, and anti-apoptotic capacities provided by honokiol were eliminated by 3-TYP in the lung tissues of the APE rats, which was consistent with the previous studies.
AMPK, a regulator of cellular energy homeostasis [62], plays a crucial role in cellular physiology and the pathological development of chronic diseases [63]. It is noteworthy that high expression of SIRT3 ameliorates sepsis-related cardiomyocyte injury by facilitating AMPK activity [64]. mTOR is one of the downstream targets of AMPK. It functions as an intracellular nutrient sensor to control protein synthesis, cell growth, and metabolism [65,66,67]. A stress-inducing protein, Sestrin2, serves as a guardian by activating AMPK, reducing mTOR, and maintaining the redox balance under the conditions of various stress environments [68]. A previous study clarified that dapagliflozin activates AMPK and suppresses the phosphorylation of mTOR in Zucker diabetic fatty rats, thereby mitigating hepatic steatosis [69]. Given these studies, we conjectured that SIRT3 might exert a protective effect on the lung tissues of APE rats by inducing anti-inflammation, anti-oxidative stress, and anti-apoptosis effects through the AMPK/mTOR pathway.
Conclusions
Collectively, we demonstrated that SIRT3 was downregulated in the lung tissues of APE rats. We investigated the effects of SIRT3 on the APE rats using the SIRT3 activator honokiol and inhibitor 3-TYP and showed that honokiol diminished APE’s negative influence by alleviating oxidative stress, inflammation, and apoptosis. However, 3-TYP treatment eradicated the rescuing effects (Fig. 6). Our study indicated that SIRT3 might serve as a promising molecular target in APE therapy.
Fig. 6.
Graphical abstract.
Funding Information
This research was supported by the Major Scientific Projects of the Wuxi Municipal Health Commission (grant no. Z202105), the Science and Technology Development Fund Project of Wuxi (grant no. Y20212057), and the Health Committee General Project of Wuxi, Jiangsu, China (grant no. M202045).
References
- 1.Demelo-Rodriguez P, Galeano-Valle F, Salzano A, Biskup E, Vriz O, Cittadini A, et al. Pulmonary embolism: a practical guide for the busy clinician. Heart Fail Clin. 2020; 16: 317–330. doi: 10.1016/j.hfc.2020.03.004 [DOI] [PubMed] [Google Scholar]
- 2.Yavuz S, Toktas F, Goncu T, Eris C, Gucu A, Ay D, et al. Surgical embolectomy for acute massive pulmonary embolism. Int J Clin Exp Med. 2014; 7: 5362–5375. [PMC free article] [PubMed] [Google Scholar]
- 3.Riedel M. Acute pulmonary embolism 1: pathophysiology, clinical presentation, and diagnosis. Heart. 2001; 85: 229–240. doi: 10.1136/heart.85.2.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez Licha CR, McCurdy CM, Maldonado SM, Lee LS. Current management of acute pulmonary embolism. Ann Thorac Cardiovasc Surg. 2020; 26: 65–71. doi: 10.5761/atcs.ra.19-00158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eagleton MJ, Henke PK, Luke CE, Hawley AE, Bedi A, Knipp BS, et al. Southern Association for Vascular Surgery William J. von Leibig Award. Inflammation and intimal hyperplasia associated with experimental pulmonary embolism. J Vasc Surg. 2002; 36: 581–588. doi: 10.1067/mva.2002.126556 [DOI] [PubMed] [Google Scholar]
- 6.Zagorski J, Debelak J, Gellar M, Watts JA, Kline JA. Chemokines accumulate in the lungs of rats with severe pulmonary embolism induced by polystyrene microspheres. J Immunol. 2003; 171: 5529–5536. doi: 10.4049/jimmunol.171.10.5529 [DOI] [PubMed] [Google Scholar]
- 7.Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997; 59: 89–144. doi: 10.1146/annurev.physiol.59.1.89 [DOI] [PubMed] [Google Scholar]
- 8.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther. 2001; 92: 1–20. doi: 10.1016/S0163-7258(01)00157-7 [DOI] [PubMed] [Google Scholar]
- 9.Moser KM, Auger WR, Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulation. 1990; 81: 1735–1743. doi: 10.1161/01.CIR.81.6.1735 [DOI] [PubMed] [Google Scholar]
- 10.Rose F, Grimminger F, Appel J, Heller M, Pies V, Weissmann N, et al. Hypoxic pulmonary artery fibroblasts trigger proliferation of vascular smooth muscle cells: role of hypoxia-inducible transcription factors. FASEB J. 2002; 16: 1660–1661. doi: 10.1096/fj.02-0420fje [DOI] [PubMed] [Google Scholar]
- 11.Eppinger MJ, Deeb GM, Bolling SF, Ward PA. Mediators of ischemia-reperfusion injury of rat lung. Am J Pathol. 1997; 150: 1773–1784. [PMC free article] [PubMed] [Google Scholar]
- 12.Mühl D, Füredi R, Cristofari J, Ghosh S, Bogár L, Borsiczki B, et al. Evaluation of oxidative stress in the thrombolysis of pulmonary embolism. J Thromb Thrombolysis. 2006; 22: 221–228. doi: 10.1007/s11239-006-9035-2 [DOI] [PubMed] [Google Scholar]
- 13.Re G, Lanzarini C, Vaona I, Pazzaglia M, Palareti G, Bassein L, et al. Systemically circulating oxidative species in human deep venous thrombosis. Eur J Emerg Med. 1998; 5: 9–12. doi: 10.1097/00063110-199803000-00004 [DOI] [PubMed] [Google Scholar]
- 14.Fisher AB. Reactive oxygen species and cell signaling with lung ischemia. Undersea Hyperb Med. 2004; 31: 97–103. [PubMed] [Google Scholar]
- 15.Mesquita I, Varela P, Belinha A, Gaifem J, Laforge M, Vergnes B, et al. Exploring NAD+ metabolism in host-pathogen interactions. Cell Mol Life Sci. 2016; 73: 1225–1236. doi: 10.1007/s00018-015-2119-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilopoulos A, Fritz KS, Petersen DR, Gius D. The human sirtuin family: evolutionary divergences and functions. Hum Genomics. 2011; 5: 485–496. doi: 10.1186/1479-7364-5-5-485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebastián C, Satterstrom FK, Haigis MC, Mostoslavsky R. From sirtuin biology to human diseases: an update. J Biol Chem. 2012; 287: 42444–42452. doi: 10.1074/jbc.R112.402768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA. 2002; 99: 13653–13658. doi: 10.1073/pnas.222538099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guarente L. Franklin H. Epstein Lecture: Sirtuins, aging, and medicine. N Engl J Med. 2011; 364: 2235–2244. doi: 10.1056/NEJMra1100831 [DOI] [PubMed] [Google Scholar]
- 20.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012; 13: 225–238. doi: 10.1038/nrm3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol Metab. 2015; 26: 486–492. doi: 10.1016/j.tem.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Kim Y, Liu T, Hwang YJ, Hyeon SJ, Im H, et al. SIRT3 deregulation is linked to mitochondrial dysfunction in Alzheimer’s disease. Aging Cell. 2018; 17: e1267. doi: 10.1111/acel.12679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akamata K, Wei J, Bhattacharyya M, Cheresh P, Bonner MY, Arbiser JL, et al. SIRT3 is attenuated in systemic sclerosis skin and lungs, and its pharmacologic activation mitigates organ fibrosis. Oncotarget. 2016; 7: 69321–69336. doi: 10.18632/oncotarget.12504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morigi M, Perico L, Rota C, Longaretti L, Conti S, Rottoli D, et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015; 125: 715–726. doi: 10.1172/JCI77632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, et al. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell. 2011; 19: 416–428. doi: 10.1016/j.ccr.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dittenhafer-Reed KE, Richards AL, Fan J, Smallegan MJ, Fotuhi Siahpirani A, Kemmerer ZA, et al. SIRT3 mediates multi-tissue coupling for metabolic fuel switching. Cell Metab. 2015; 21: 637–646. doi: 10.1016/j.cmet.2015.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, et al. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 2013; 49: 186–199. doi: 10.1016/j.molcel.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W, Liu C, Chen Q, Liu N, Yan Y, Liu B. SIRT3: A new regulator of cardiovascular diseases. Oxid Med Cell Longev. 2018; 2018: 7293861. doi: 10.1155/2018/7293861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010; 464: 121–125. doi: 10.1038/nature08778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA. 2008; 105: 14447–14452. doi: 10.1073/pnas.0803790105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Ven RAH, Santos D, Haigis MC. Mitochondrial sirtuins and molecular mechanisms of aging. Trends Mol Med. 2017; 23: 320–331. doi: 10.1016/j.molmed.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi T, Wang F, Stieren E, Tong Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 2005; 280: 13560–13567. doi: 10.1074/jbc.M414670200 [DOI] [PubMed] [Google Scholar]
- 33.Jiang DQ, Wang Y, Li MX, Ma YJ, Wang Y. SIRT3 in neural stem cells attenuates microglia activation-induced oxidative stress injury through mitochondrial pathway. Front Cell Neurosci. 2017; 11: 7. doi: 10.3389/fncel.2017.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Fu LL, Wen X, Wang XY, Liu J, Cheng Y, et al. Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer. Cell Death Dis. 2014; 5: e1047. doi: 10.1038/cddis.2014.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005; 85: 258–263. doi: 10.1016/j.ygeno.2004.11.003 [DOI] [PubMed] [Google Scholar]
- 36.Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013; 5: 48. doi: 10.3389/fnagi.2013.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dikalova AE, Pandey A, Xiao L, Arslanbaeva L, Sidorova T, Lopez MG, et al. Mitochondrial deacetylase Sirt3 reduces vascular dysfunction and hypertension while sirt3 depletion in essential hypertension is linked to vascular inflammation and oxidative stress. Circ Res. 2020; 126: 439–452. doi: 10.1161/CIRCRESAHA.119.315767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kurundkar D, Kurundkar AR, Bone NB, Becker EJ, Jr, Liu W, Chacko B, et al. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight. 2019; 4: e120722. doi: 10.1172/jci.insight.120722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017; 63: jpi.12419. doi: 10.1111/jpi.12419 [DOI] [PubMed] [Google Scholar]
- 40.Jablonski RP, Kim SJ, Cheresh P, Williams DB, Morales-Nebreda L, Cheng Y, et al. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J. 2017; 31: 2520–2532. doi: 10.1096/fj.201601077R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weng TI, Wu HY, Kuo CW, Liu SH. Honokiol rescues sepsis-associated acute lung injury and lethality via the inhibition of oxidative stress and inflammation. Intensive Care Med. 2011; 37: 533–541. doi: 10.1007/s00134-010-2104-1 [DOI] [PubMed] [Google Scholar]
- 42.Chiang J, Shen YC, Wang YH, Hou YC, Chen CC, Liao JF, et al. Honokiol protects rats against eccentric exercise-induced skeletal muscle damage by inhibiting NF-kappaB induced oxidative stress and inflammation. Eur J Pharmacol. 2009; 610: 119–127. doi: 10.1016/j.ejphar.2009.03.035 [DOI] [PubMed] [Google Scholar]
- 43.Shumin C, Wei X, Yunfeng L, Jiangshui L, Youguang G, Zhongqing C, et al. Genipin alleviates vascular hyperpermeability following hemorrhagic shock by up-regulation of SIRT3/autophagy. Cell Death Discov. 2018; 4: 52. doi: 10.1038/s41420-018-0057-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye JS, Chen L, Lu YY, Lei SQ, Peng M, Xia ZY. SIRT3 activator honokiol ameliorates surgery/anesthesia-induced cognitive decline in mice through anti-oxidative stress and anti-inflammatory in hippocampus. CNS Neurosci Ther. 2019; 25: 355–366. doi: 10.1111/cns.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niu F, Li H, Xu X, Sun L, Gan N, Wang A. Ursodeoxycholic acid protects against lung injury induced by fat embolism syndrome. J Cell Mol Med. 2020; 24: 14626–14632. doi: 10.1111/jcmm.15985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Zheng X, Cheng Y, Zhang YL, Wen HX, Tao Z, et al. Resolvin D1 stimulates alveolar fluid clearance through alveolar epithelial sodium channel, Na,K-ATPase via ALX/cAMP/PI3K pathway in lipopolysaccharide-induced acute lung injury. J Immunol. 2014; 192: 3765–3777. doi: 10.4049/jimmunol.1302421 [DOI] [PubMed] [Google Scholar]
- 47.Takada M, Chiba S, Nagai T, Takeshita H, Kanno S, Ikawa T, et al. Inflammatory responses to neutral fat and fatty acids in multiple organs in a rat model of fat embolism syndrome. Forensic Sci Int. 2015; 254: 126–132. doi: 10.1016/j.forsciint.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 48.Fukuda I, Daitoku K. Surgical embolectomy for acute pulmonary thromboembolism. Ann Vasc Dis. 2017; 10: 107–114. doi: 10.3400/avd.ra.17-00038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes G, Tamura A, Guerrero JL, Hernando LM. [Surgical treatment of a massive pulmonary embolism after a double cardiac arrest]. Rev Esp Cardiol. 2007; 60: 887–889. doi: 10.1157/13109007 [DOI] [PubMed] [Google Scholar]
- 50.Jiménez D, Bikdeli B, Quezada A, Muriel A, Lobo JL, de Miguel-Diez J, et al. RIETE investigators. Hospital volume and outcomes for acute pulmonary embolism: multinational population based cohort study. BMJ. 2019; 366: l4416. doi: 10.1136/bmj.l4416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang C, Wang Y, Shen L. Mitochondrial proteins in heart failure: The role of deacetylation by SIRT3. Pharmacol Res. 2021; 172: 105802. doi: 10.1016/j.phrs.2021.105802 [DOI] [PubMed] [Google Scholar]
- 52.Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016; 2016: 7432797. doi: 10.1155/2016/7432797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betteridge DJ. What is oxidative stress? Metabolism. 2000; 49:(Suppl 1): 3–8. doi: 10.1016/S0026-0495(00)80077-3 [DOI] [PubMed] [Google Scholar]
- 54.Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010; 12: 662–667. doi: 10.1016/j.cmet.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 55.Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011; 12: 534–541. doi: 10.1038/embor.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pillai VB, Kanwal A, Fang YH, Sharp WW, Samant S, Arbiser J, et al. Honokiol, an activator of Sirtuin-3 (SIRT3) preserves mitochondria and protects the heart from doxorubicin-induced cardiomyopathy in mice. Oncotarget. 2017; 8: 34082–34098. doi: 10.18632/oncotarget.16133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chang KH, Yan MD, Yao CJ, Lin PC, Lai GM. Honokiol-induced apoptosis and autophagy in glioblastoma multiforme cells. Oncol Lett. 2013; 6: 1435–1438. doi: 10.3892/ol.2013.1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee TY, Chang CC, Lu WJ, Yen TL, Lin KH, Geraldine P, et al. Honokiol as a specific collagen receptor glycoprotein VI antagonist on human platelets: Functional ex vivo and in vivo studies. Sci Rep. 2017; 7: 40002. doi: 10.1038/srep40002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhai H, Nakade K, Oda M, Mitsumoto Y, Akagi M, Sakurai J, et al. Honokiol-induced neurite outgrowth promotion depends on activation of extracellular signal-regulated kinases (ERK1/2). Eur J Pharmacol. 2005; 516: 112–117. doi: 10.1016/j.ejphar.2005.04.035 [DOI] [PubMed] [Google Scholar]
- 60.Zheng J, Shi L, Liang F, Xu W, Li T, Gao L, et al. Sirt3 ameliorates oxidative stress and mitochondrial dysfunction after intracerebral hemorrhage in diabetic rats. Front Neurosci. 2018; 12: 414. doi: 10.3389/fnins.2018.00414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shi C, Jiao F, Wang Y, Chen Q, Wang L, Gong Z. SIRT3 inhibitor 3-TYP exacerbates thioacetamide-induced hepatic injury in mice. Front Physiol. 2022; 13: 915193. doi: 10.3389/fphys.2022.915193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ha J, Guan KL, Kim J. AMPK and autophagy in glucose/glycogen metabolism. Mol Aspects Med. 2015; 46: 46–62. doi: 10.1016/j.mam.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 63.Jiang X, Tan HY, Teng S, Chan YT, Wang D, Wang N. The Role of AMP-activated protein kinase as a potential target of treatment of hepatocellular carcinoma. Cancers (Basel). 2019; 11: 647. doi: 10.3390/cancers11050647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xin T, Lu C. SirT3 activates AMPK-related mitochondrial biogenesis and ameliorates sepsis-induced myocardial injury. Aging (Albany NY). 2020; 12: 16224–16237. doi: 10.18632/aging.103644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reiter AK, Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. Repression of protein synthesis and mTOR signaling in rat liver mediated by the AMPK activator aminoimidazole carboxamide ribonucleoside. Am J Physiol Endocrinol Metab. 2005; 288: E980–E988. doi: 10.1152/ajpendo.00333.2004 [DOI] [PubMed] [Google Scholar]
- 66.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004; 279: 15719–15722. doi: 10.1074/jbc.C300534200 [DOI] [PubMed] [Google Scholar]
- 67.Xu J, Ji J, Yan XH. Cross-talk between AMPK and mTOR in regulating energy balance. Crit Rev Food Sci Nutr. 2012; 52: 373–381. doi: 10.1080/10408398.2010.500245 [DOI] [PubMed] [Google Scholar]
- 68.Tian Z, Yan BJ, Luo W, Gui DD, Zhou K, Tian KJ, et al. Sestrin2 in atherosclerosis. Clin Chim Acta. 2021; 523: 325–329. doi: 10.1016/j.cca.2021.10.019 [DOI] [PubMed] [Google Scholar]
- 69.Li L, Li Q, Huang W, Han Y, Tan H, An M, et al. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front Pharmacol. 2021; 12: 589273. doi: 10.3389/fphar.2021.589273 [DOI] [PMC free article] [PubMed] [Google Scholar]