Abstract
Gonadal function is often suppressed during lactation in mammals including rodents, ruminants, and primates. This suppression is thought to be mostly due to the inhibition of the tonic (pulsatile) release of gonadotropin-releasing hormone (GnRH) and consequent gonadotropin. Accumulating evidence suggests that kisspeptin neurons in the arcuate nucleus (ARC) play a critical role in the regulation of pulsatile GnRH/gonadotropin release, and kisspeptin mRNA (Kiss1) and/or kisspeptin expression in the ARC are strongly suppressed by the suckling stimuli in lactating rats. This study aimed to examine whether the central enkephalin-δ-opioid receptor (DOR) signaling mediates the suckling-induced suppression of luteinizing hormone (LH) release in lactating rats. Central administration of a selective DOR antagonist increased the mean plasma LH levels and baseline of LH pulses in ovariectomized lactating mother rats compared to vehicle-injected control dams on day 8 of lactation without affecting the number of Kiss1-expressing cells and the intensity of Kiss1 mRNA signals in the ARC. Furthermore, the suckling stimuli significantly increased the number of enkephalin mRNA (Penk)-expressing cells and the intensity of Penk mRNA signals in the ARC compared to non-lactating control rats. Collectively, these results suggest that central DOR signaling, at least in part, mediates the suppression of LH release induced by suckling stimuli in lactating rats via indirect and/or direct inhibition of ARC kisspeptin neurons.
Keywords: Arcuate nucleus, GnRH neuron, Kisspeptin neuron, Suckling stimulus
Gonadal function is often suppressed in lactating animals including rodents, ruminants, primates, and humans [1,2,3]. Lactational anestrus is thought to be a strategic adaptation to avoid pregnancy during lactation. Stimulation of the mother’s nipple by suckling pups (suckling stimulus) and a negative energy balance for milk production are thought to be the main causes of gonadal dysfunction during the lactation period. Lactational suppression of gonadal function is suggested to be mainly due to the suppression of tonic (pulsatile) release of gonadotropin-releasing hormone (GnRH) and consequent gonadotropin in cows [3,4,5,6], ewes [7], sows [8], rhesus monkeys [9], mice [10], and rats [11, 12]. Luteinizing hormone (LH) pulses are strongly suppressed in lactating rats [11] and recover within a few hours after pups are removed from maternal rats [13].
Accumulating evidence suggests that kisspeptin neurons in the arcuate nucleus (ARC) play a critical role in regulating pulsatile GnRH/gonadotropin release, because LH pulses, as an indicator of GnRH pulses, are suppressed by conditional knockout (KO) of the kisspeptin gene (Kiss1) in the ARC of rats [14] and mice [15, 16]. Notably, Kiss1 and/or kisspeptin expression in the ARC is strongly inhibited by suckling stimuli during lactation in rats [17,18,19], suggesting that the lactational suppression of GnRH/LH pulses in lactating rats is mainly due to the inhibition of ARC Kiss1 expression.
Our previous studies have suggested that central opioid peptides, such as dynorphin A (Dyn), β-endorphin, and enkephalin, and their receptor signaling mediate the malnutritional suppression of GnRH/LH pulses in female rats [20,21,22]. Particularly, administration of nor-binaltorphimine (nor-BNI), a selective antagonist for Dyn receptor (κ-opioid receptor, KOR); D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP), a selective antagonist for β-endorphin receptor (μ-opioid receptor, MOR); or naltrindole hydrochloride (NTI), a selective antagonist for enkephalin receptor (δ-opioid receptor, DOR) into the third ventricle (3V) blocked the suppression of LH pulses induced by a central or peripheral injection of 2-deoxy-D-glucose (2DG), an inhibitor of glucose utilization, in female rats [20,21,22]. Furthermore, our recent study revealed that Dyn-KOR signaling mediates suckling-induced LH pulse suppression in rats during late lactation [23]. This is because central administration of a KOR selective antagonist (nor-BNI) blocked the suppression of LH release in rats during late lactation, and re-suckling increased fos (a marker for neural activation)-expressing Dyn neurons in the paraventricular nucleus (PVN) of mother rats from which pups were removed for 24 h [23]. On the other hand, unlike 2DG-induced suppression of LH release, β-endorphin-MOR signaling may not mediate suckling-induced LH suppression because the central administration of a MOR selective antagonist (CTOP) failed to block LH suppression in lactating rats during late lactation [23]. A previous study showed that enkephalin mRNA (Penk) expression and immunoreactivity were significantly increased in the ARC of lactating mice between days 7 and 10 postpartum compared to those in diestrous mice [24]. This implies that enkephalin-DOR signaling may be involved in the suppression of LH release during lactation [24]. However, to the best of our knowledge, no study has demonstrated the role of enkephalin-DOR signaling in the suppression of LH release during lactation.
Therefore, this study aimed to examine whether enkephalin-DOR signaling is involved in the suppression of LH release in rats during lactation. To address this issue, we examined whether the 3V administration of a selective DOR antagonist (NTI) blocked the suppression of LH release in ovariectomized (OVX) lactating rats on day 8 of lactation. Furthermore, we investigated whether 3V NTI administration affected ARC Kiss1 expression. Moreover, we examined the effect of the suckling stimuli on Penk expression in the hypothalamus of female rats by investigating the difference in Penk mRNA expression between OVX lactating and non-lactating rats from which pups were deprived after parturition.
Materials and Methods
Animals
Adult Wistar-Imamichi strain female rats (8–13 weeks old, 200–250 g; Institute for Animal Reproduction, Kasumigaura, Japan) were housed in a controlled environment (14-h light, 10-h darkness with lights on at 0500 h, 22 ± 3°C) and had free access to food (CE-2; CLEA Japan, Tokyo, Japan) and water. Female rats that showed at least two consecutive estrous cycles were mated with male Wistar Imamichi rats. Pregnant female rats were housed individually until the day of parturition, and the day on which the newborn litter was found at noon was designated as Day 0 of lactation. The litter size of the lactating group was adjusted to eight on Day 1, whereas the rats in the non-lactating group were deprived of their litters since Day 1. All lactating and non-lactating rats underwent OVX on Day 2 to avoid the influence of ovarian steroids [13, 18], and blood or brain sampling was performed on Day 8. These surgical procedures, if not otherwise specified, were performed under ketamine (27 mg/kg; Fujita Pharmaceutical, Tokyo, Japan) and xylazine (5.3 mg/kg; Bayer, Leverkusen, Germany) mixtures and inhalant 1%–2% isoflurane (Pfizer Japan, Tokyo, Japan) anesthesia. This study was approved by the Committee on Animal Experiments of the Graduate School of Bioagricultural Sciences, Nagoya University.
Blood and brain sampling to determine the effects of central DOR antagonism on the suppression of LH release and ARC Kiss1 expression during early lactation
OVX lactating rats (n = 9 and 6 for blood and brain sampling, respectively) were stereotaxically implanted with a stainless-steel guide cannula (22G; Plastics ONE, Roanoke, VA, USA) into the 3V according to the rat brain atlas [25] as follows: at 0.8 mm posterior and 7.5 mm ventral to the bregma at the midline. Brain surgery was performed on Day 2 of lactation under anesthesia using an intraperitoneal injection of a mixture of medetomidine (0.375 mg/kg), midazolam (2 mg/kg), and butorphanol (2.5 mg/kg). Anesthesia was immediately reversed with an intraperitoneal injection of atipamezole (0.75 mg/kg) after brain surgery. Some lactating rats (n = 9) were inserted with a silicon cannula (inner diameter = 0.5 mm, outer diameter = 1.0 mm; Shin-Etsu Polymer, Tokyo, Japan) into the right atrium through the jugular vein on the day before blood sampling. The free-moving conscious rats were administered NTI (a selective DOR antagonist; Sigma-Aldrich, St. Louis, MO, USA), dissolved in ultrapure water at 22.175 nmol/µl, into the 3V at a flow rate of 1 µl/min for 2 min using a microsyringe pump (EICOM, Kyoto, Japan) through an internal cannula (28G; Plastics ONE, Boerne, TX, USA) at 1300 h on Day 8 of lactation. The dose of NTI was selected according to our previous study [22], showing that this dose of NTI blocked the suppression of LH pulses by peripheral 2DG administration in OVX female rats treated with diestrous levels of estradiol-17β (E2). Ultrapure water was used for vehicle-treated control rats.
Blood samples (100 µl) were collected every 6 min for 3 h (1300–1600 h, the first sample was collected immediately before the NTI administration), and red blood cells taken from donor rats and washed with heparinized saline were replaced after each blood collection to keep the hematocrit level constant. Plasma samples (50 µl) were obtained using immediate centrifugation and stored at –20 °C until assayed for LH. At the end of blood sampling, the placement of the 3V cannula was verified via visual inspection after injection of the same amount of 3% brilliant blue solution using a microsyringe pump. Brain samples were collected 1 h after the NTI or vehicle administration. Briefly, OVX lactating rats (n = 6) were deeply anesthetized with sodium pentobarbital (40 mg/kg; Tokyo Chemical Industry, Tokyo, Japan) and perfused with 4% paraformaldehyde (Sigma-Aldrich) in 0.05 M phosphate buffer (PB). The brain was immersed in 30% sucrose in 0.05 M PB at 4°C. Frozen sections (50-µm thick) of the hypothalamus, including the ARC, were obtained using a cryostat (CM1860; Leica, Wetzlar, Germany). Brain sections were subjected to a single in situ hybridization (ISH) for Kiss1.
Radioimmunoassay for plasma LH concentrations and LH pulse analysis
Plasma LH concentrations were measured by a double-antibody radioimmunoassay (RIA), as previously described [26], using a rat LH RIA kit provided by the National Hormone and Peptide Program (Harbor-UCLA Medical Center, Torrance, CA, USA) and were expressed in terms of NIDDK rat LH-RP-3. The least detectable level in 50-µl plasma samples was 0.078 ng/ml, and the intra- and inter-assay coefficients of variation were 9.0% and 13.6% at 0.29 ng/ml, respectively. LH pulses were identified using the PULSAR computer program [27], as previously described [20]. The mean LH concentrations and the baseline, frequency, and amplitude of LH pulses for a 3-h sampling period were calculated for each individual and then for the groups.
Brain sampling to determine the effect of suckling stimuli on Penk expression
OVX lactating and non-lactating rats (n = 3 in each group) were subjected to brain sampling on Day 8 postpartum. The animals were perfused and their brains were collected as described above. Frozen sections (50-µm thick) of the hypothalamus, including the ARC, PVN, and ventromedial hypothalamus (VMH), were obtained using a cryostat. Brain sections obtained from lactating and non-lactating rats were subjected to a single ISH for Penk.
Single ISH for Kiss1 or Penk in rat hypothalamus
A free-floating single ISH for Kiss1 was performed using every fourth hypothalamic section, including the ARC, as previously described [28]. ISH for Penk was performed using every fourth hypothalamic section, including the ARC, PVN, and VMH, as previously described [22]. Kiss1- (position 33–348; GenBank accession number AY196983) and Penk- (position 447–1256; GenBank accession number NM_017139) specific digoxigenin (DIG)-labeled complementary RNA (cRNA) probes were designed and synthesized from whole rat hypothalamic cDNA using a DIG-labeling kit (Roche Diagnostics, Basel, Switzerland). The hybridized DIG-labeled probe was detected overnight with an alkaline phosphatase-conjugated anti-DIG antibody (RRID:AB_2734716, 1:1000; Roche Diagnostics) at 4°C. The sections were treated with a chromogen solution (338 µg/ml 4-nitroblue tetrazolium chloride, 175 µg/ml 5-bromo-4-chloro-3-indoyl-phosphate; Roche Diagnostics) for 1 h. The brain sections were then mounted, and Penk signals were examined using an optical microscope (BX53; Olympus, Tokyo, Japan). The number of Kiss1- or Penk-expressing cells in the ARC (every 200 µm from 1.72 to 4.36 mm posterior to the bregma) and the number of Penk-expressing cells in the PVN (every 200 µm from 0.96 to 1.92 mm posterior to the bregma) were counted unilaterally in duplicates and averaged. Note that the number of Penk-expressing cells in the VMH (every 200 µm from 2.04 to 3.36 mm posterior to the bregma) could not be counted because Penk-positive cells overlapped each other in the VMH, thus, each Penk-positive cell was not distinguishable. The intensity of Kiss1 mRNA signals in a digital photomicrograph of the ARC brain sections and the intensity of Penk mRNA signals in a digital photomicrograph of the ARC, PVN, and VMH brain sections of each rat were also quantitatively analyzed using Fiji software (version:2.3.0/1.53p; ImageJ2). Each nucleus was outlined in a grayscale image and processed for intensity measurements. Non-specific background intensity was eliminated using the same threshold for each rat. The intensity was calculated as the integrated density, that is, the sum of the 8-bit grayscale values of all pixels in the threshold area. No positive signals for Kiss1 or Penk mRNA were detected in the brain sections hybridized with the corresponding sense probe as a negative control (data not shown).
Statistical analysis
Statistical differences in LH parameters (mean LH concentrations and the baseline, frequency, and amplitude of LH pulses), the number of ARC Kiss1-expressing cells, and intensity of Kiss1 mRNA signals between the NTI- and vehicle-injected lactating rats; and the number of Penk-expressing cells and intensity of Penk mRNA signals between lactating and non-lactating rats were determined using Student’s t-test. All statistical analyses were performed using SAS OnDemand for Academics (https://welcome.oda.sas.com/).
Results
Central DOR antagonism increased LH release in OVX lactating rats
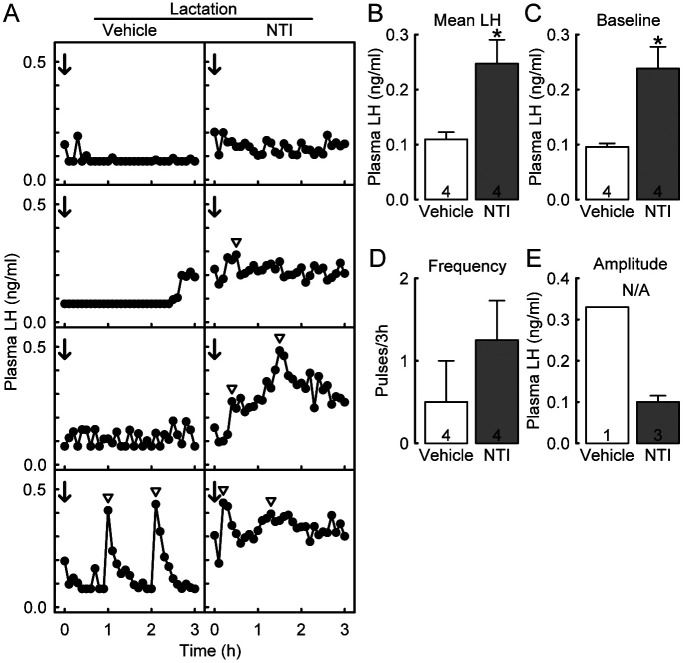
Figure 1A shows the LH profiles of individual OVX lactating rats administered with NTI or vehicle at the 3V on Day 8 of lactation. Pulsatile LH release was inhibited in 3 out of 4 vehicle-injected lactating dams, whereas plasma LH levels increased in 3 out of 4 NTI-injected lactating dams. The mean LH concentration in the NTI-injected lactating dams was significantly higher than that in the vehicle-injected dams (P = 0.0230, Student’s t-test; Fig. 1B). The baseline of LH pulses was also significantly higher than that of the vehicle-injected lactating dams (P = 0.0121, Student’s t-test; Fig. 1C). LH pulse frequency tended to be higher in NTI-injected lactating rats than in vehicle-treated controls; however, no significant difference was observed between the groups (Fig. 1D). Statistical analysis was not applicable to the amplitude of LH pulses because of the small sample size (only one vehicle-treated and three NTI-treated rats showed LH pulses; Fig. 1E).
Fig. 1.
Effects of central administration of naltrindole hydrochloride (NTI), a selective antagonist of δ-opioid receptors (DOR), on the suppression of luteinizing hormone (LH) pulses induced by suckling stimuli in ovariectomized (OVX) lactating rats during early lactation. Plasma LH profiles of individual OVX lactating rats, which were treated with the third ventricle (3V) injection of NTI (or vehicle) during early lactation (A). Blood samples were collected every 6 min for 3 h. Immediately after the first blood sampling, NTI or vehicle (timing indicated by arrows) was injected into the 3V. Arrowheads indicate the peaks of LH pulses identified by the PULSAR computer program. The mean plasma LH concentrations (B) and the baseline (C), frequency (D), and amplitude (E) of LH pulses in each group. Values are means ± SEM. The number in each column indicates the number of animals used. * Significant difference between NTI- and vehicle-treated rats (P < 0.05, Student’s t-test). N/A, not applicable.
Central DOR antagonism did not affect Kiss1 mRNA expression in the ARC of OVX lactating rats
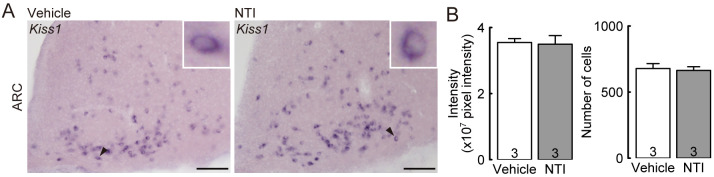
Figure 2A shows the expression of Kiss1 mRNA in the ARC of representative OVX lactating rats administered with NTI or vehicle at the 3V on Day 8 of lactation. The intensity of ARC Kiss1 mRNA signals and the number of Kiss1-expressing cells were comparable between the NTI- and vehicle-injected lactating rats (Fig. 2B).
Fig. 2.
Effects of central administration of NTI on the Kiss1 (kisspeptin gene) expression in the arcuate nucleus (ARC) of OVX lactating rats during early lactation. Kiss1-expressing cells in the ARC in representative OVX lactating rats treated with vehicle or NTI in the 3V 1 h before brain sampling (A). The insets indicate Kiss1-expressing cells (black arrowheads) at higher magnification. NTI administration did not affect the intensity of Kiss1 mRNA signals and the number of Kiss1-expressing cells in the ARC of OVX lactating rats (B). Scale bars = 100 µm. Values are means ± SEM. The number in each column indicates the number of animals used.
Suckling stimuli increased Penk mRNA expression in the ARC of OVX lactating rats
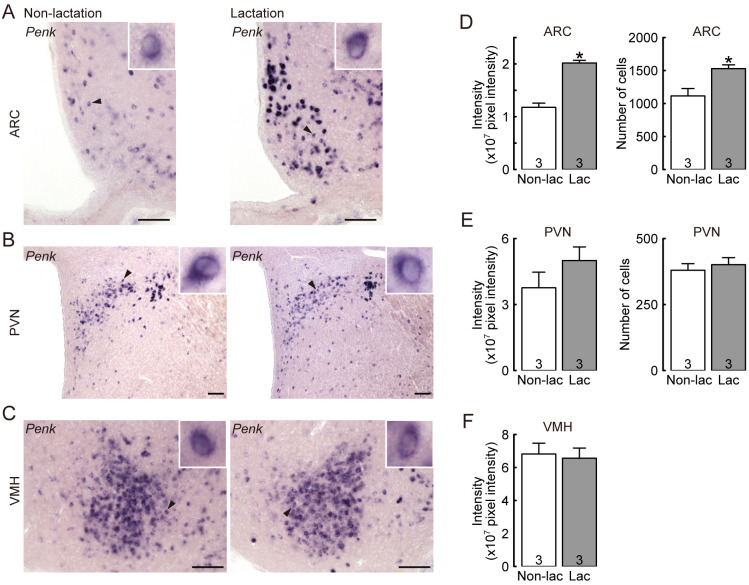
Figures 3A, B, and C show the expression of Penk mRNA in the ARC, PVN, and VMH of representative OVX lactating and non-lactating rats. The intensity of Penk mRNA signals in the ARC of OVX lactating dams was significantly higher than in the non-lactating controls (P = 0.0009, Student’s t-test; Fig. 3D). The number of Penk-expressing cells in the ARC of OVX lactating dams was also significantly higher than in the non-lactating controls (P = 0.0303, Student’s t-test; Fig. 3D). In contrast, the number of Penk-expressing cells in the PVN (Fig. 3E) and the intensity of Penk mRNA signals in the PVN (Fig. 3E) and VMH (Fig. 3F) were comparable between OVX lactating and non-lactating control rats.
Fig. 3.
Effect of suckling stimuli on hypothalamic Penk (enkephalin gene) expression in OVX lactating rats during early lactation. Penk-expressing cells in the ARC (A), paraventricular nucleus (PVN) (B), and ventromedial hypothalamus (VMH) (C) in representative OVX lactating and non-lactating rats. The insets indicate Penk-expressing cells (black arrowheads) at higher magnification. The suckling stimuli increased the number of Penk-expressing cells and the intensity of Penk mRNA signals in the ARC (D), but not in the PVN (E) and VMH (F), of OVX lactating rats. Note that only Penk signal intensity is provided for the VMH because Penk-positive cells overlapped each other, thus, each Penk-positive cell was not distinguishable. Scale bars = 100 µm. Values are means ± SEM. The number in each column indicates the number of animals used. * Significant difference between lactating and non-lactating OVX rats (P < 0.05, Student’s t-test).
Discussion
The present study demonstrated that enkephalin-DOR signaling mediates, at least in part, the suppression of LH release in lactating rats during early lactation. This is because administration of NTI, a selective DOR antagonist, increased the mean LH levels and baseline of LH pulses in OVX lactating rats. Furthermore, this study showed that the suckling stimuli enhanced Penk mRNA expression in the ARC, suggesting that ARC enkephalin neurons are involved in the suppression of LH release in response to the suckling stimuli in lactating rats. Taken together, these findings suggest that ARC enkephalin neurons are, at least in part, involved in the suppression of LH release in response to suckling stimuli in lactating rats. To the best of our knowledge, this is the first report demonstrating the physiological role of enkephalin-DOR signaling in lactational anestrus.
In this study, the number of Penk-expressing cells and the intensity of Penk mRNA expression were significantly increased only in the ARC of lactating rats compared to those in non-lactating control rats. This result is largely consistent with a previous study demonstrating that Penk mRNA levels and enkephalin immunoreactivity in the ARC were higher in lactating mice than in diestrus mice [24]. These findings suggest that the suckling stimuli promote the transcription and translation of enkephalin to mediate the suckling-induced suppression of LH release in rodents. Remarkably, our previous study showed that Penk mRNA was expressed in the ARC of OVX virgin rats treated with diestrous levels of E2 (low E2) but not in ARC kisspeptin neurons [22]. ARC kisspeptin neurons (also known as KNDy neurons because of the co-expression of neurokinin B and Dyn) are now well accepted as the GnRH pulse generator in rats because rescuing KNDy neurons restores GnRH/LH pulses in global Kiss1 KO rats showing no LH pulse [14]. In addition, excitatory neurotransmitters such as glutamate and noradrenaline failed to stimulate LH release in global Kiss1 KO rats [29]. Therefore, we speculate that ARC enkephalin neurons may exert an inhibitory action on neighboring kisspeptin neurons of lactating rats to suppress GnRH/LH release. Notably, our previous study showed that the same central DOR antagonism completely blocked 2DG-induced LH pulse suppression in female rats, because the 3V NTI administration restored LH pulse frequency and mean LH levels in 2DG-administared OVX virgin rats treated with low E2 [22]. Similarly, KOR antagonism completely blocked 2DG-induced LH pulse suppression in OVX virgin rats treated with low E2 [21]. In contrast, DOR antagonism significantly increased only the mean plasma LH levels and baseline of LH pulses in OVX rats during early lactation, and our previous study showed that KOR antagonism also increased only the mean plasma LH levels and baseline of LH pulses in low E2-treated OVX lactating rats during late lactation [23]. These findings suggest that, unlike the glucoprivic suppression of LH pulses, other inhibitory signaling pathways may also be involved in the lactational suppression of ARC kisspeptin neurons and LH pulses in rats.
DOR antagonism did not affect the intensity of Kiss1 mRNA levels or the number of ARC Kiss1-expressing cells in OVX lactating rats 1 h after NTI treatment. Notably, central NTI administration immediately increased plasma LH levels in lactating rats. Thus, we speculate that the current NTI treatment may rapidly stimulate ARC kisspeptin neurons without affecting ARC Kiss1 expression in OVX lactating rats. In addition, the current enkephalin antagonism may have partly restored GnRH release at the median eminence because Pimpinelli et al. [30] reported that DOR immunoreactivity was found in some GnRH neuronal fibers in the median eminence in male rats.
A previous study has shown that approximately 80% of tuberoinfundibular dopaminergic (TIDA) neurons in the ARC co-express enkephalin in mice [24]. Furthermore, subcutaneous administration of bromocriptine, a dopamine agonist, decreases enkephalin-immunoreactive cells in lactating mice, whereas intraperitoneal injection of prolactin in bromocriptine-treated mice increases ARC enkephalin-immunoreactive cells [24]. Therefore, we inferred that prolactin may promote enkephalin synthesis in TIDA/enkephalin neurons in lactating rats. To date, it has not been clearly demonstrated whether TIDA/enkephalin neurons project their fibers to ARC kisspeptin neurons in female rats. In addition, it is unclear which neurons express DOR in the hypothalamus of female rats. In contrast to the current findings, a previous study reported that DOR was rarely expressed in the ARC of female rats [31]. Further studies are required to address the projection of TIDA/enkephalin neurons and whether ARC kisspeptin neurons or other hypothalamic interneurons express DOR to mediate the inhibitory role of enkephalin in the lactational suppression of GnRH/LH release.
Previous studies have shown that enkephalin neurons are also located in the A1, A2, and C1 regions of the hindbrain [32, 33]. Furthermore, re-suckling for 90 min increased c-Fos expression in several hindbrain nuclei, including A1 and A2, of lactating rats from which pups were removed for 48 h [34]. Thus, it is also possible that A1 and A2 enkephalin neurons activated by suckling stimuli are involved in the suppression of ARC kisspeptin neurons and LH release in lactating rats. Further studies are required to clarify whether suckling stimuli activate A1 and A2 enkephalin neurons in lactating rats.
In conclusion, the present study demonstrated that enkephalin-DOR signaling partly mediates the suppression of LH release in lactating rats and that the suckling stimuli increase ARC Penk mRNA expression. Thus, ARC enkephalin neurons may be involved in the suppression of LH pulses induced by suckling stimuli in lactating rats. To the best of our knowledge, this is the first report to demonstrate the role of enkephalin-DOR signaling in mediating ovarian dysfunction during lactation.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgments
We thank the National Hormone and Peptide Program for providing the rat LH RIA kit. RIA was performed at the Radioisotope Research Center of Nagoya University, Nagoya, Japan. This work was supported in part by the Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Numbers: 21H05031 and 21K19186 to H. Tsukamura, 19H03103 to N. I., and 20H03127 and 22K19245 to Y. U.); the Graduate Program of Transformative Chem‐Bio Research in Nagoya University, supported by MEXT (WISE Program); and the Sasakawa Scientific Research Grant from the Japan Science Society (2021-4093).
References
- 1.Tsukamura H. Kobayashi Award 2019: The neuroendocrine regulation of the mammalian reproduction. Gen Comp Endocrinol 2022; 315: 113755. [DOI] [PubMed] [Google Scholar]
- 2.Van Ginneken JK. Prolonged breastfeeding as a birth spacing method. Stud Fam Plann 1974; 5: 201–206. [PubMed] [Google Scholar]
- 3.Walters DL, Short RE, Convey EM, Staigmiller RB, Dunn TG, Kaltenbach CC. Pituitary and ovarian function in postpartum beef cows. III. Induction of estrus, ovulation and luteal function with intermittent small-dose injections of GnRH. Biol Reprod 1982; 26: 655–662. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Wakabayashi Y, Yamamura T, Ohkura S, Matsuyama S. A neurokinin 3 receptor-selective agonist accelerates pulsatile luteinizing hormone secretion in lactating cattle. Biol Reprod 2017; 97: 81–90. [DOI] [PubMed] [Google Scholar]
- 5.Beam SW, Butler WR. Effects of energy balance on follicular development and first ovulation in postpartum dairy cows. J Reprod Fertil Suppl 1999; 54: 411–424. [PubMed] [Google Scholar]
- 6.Williams GL, Talavera F, Petersen BJ, Kirsch JD, Tilton JE. Coincident secretion of follicle-stimulating hormone and luteinizing hormone in early postpartum beef cows: effects of suckling and low-level increases of systemic progesterone. Biol Reprod 1983; 29: 362–373. [DOI] [PubMed] [Google Scholar]
- 7.Hernández-Hernández JM, Martin GB, Becerril-Pérez CM, Pro-Martínez A, Cortez-Romero C, Gallegos-Sánchez J. Kisspeptin stimulates the pulsatile secretion of luteinizing hormone (LH) during postpartum anestrus in ewes undergoing continuous and restricted suckling. Animals (Basel) 2021; 11: 2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunavongkrit A. Clinical and endocrinological studies in primiparous post partum sows. Effects of lactation length and litter size. Acta Vet Scand 1984; 25: 260–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ordög T, Chen MD, O’Byrne KT, Goldsmith JR, Connaughton MA, Hotchkiss J, Knobil E. On the mechanism of lactational anovulation in the rhesus monkey. Am J Physiol 1998; 274: E665–E676. [DOI] [PubMed] [Google Scholar]
- 10.Naik SI, Young LS, Charlton HM, Clayton RN. Pituitary gonadotropin-releasing hormone receptor regulation in mice. II: Females. Endocrinology 1984; 115: 114–120. [DOI] [PubMed] [Google Scholar]
- 11.Fox SR, Smith MS. The suppression of pulsatile luteinizing hormone secretion during lactation in the rat. Endocrinology 1984; 115: 2045–2051. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, Kirigiti MA, Grove KL, Smith MS. Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology 2009; 150: 4231–4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda K-I, Tsukamura H, Uchida E, Ohkura N, Ohkura S, Yokoyama A. Changes in the pulsatile secretion of LH after the removal of and subsequent resuckling by pups in ovariectomized lactating rats. J Endocrinol 1989; 121: 277–283. [DOI] [PubMed] [Google Scholar]
- 14.Nagae M, Uenoyama Y, Okamoto S, Tsuchida H, Ikegami K, Goto T, Majarune S, Nakamura S, Sanbo M, Hirabayashi M, Kobayashi K, Inoue N, Tsukamura H. Direct evidence that KNDy neurons maintain gonadotropin pulses and folliculogenesis as the GnRH pulse generator. Proc Natl Acad Sci USA 2021; 118: e2009156118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minabe S, Nakamura S, Fukushima E, Sato M, Ikegami K, Goto T, Sanbo M, Hirabayashi M, Tomikawa J, Imamura T, Inoue N, Uenoyama Y, Tsukamura H, Maeda K-I, Matsuda F. Inducible Kiss1 knockdown in the hypothalamic arcuate nucleus suppressed pulsatile secretion of luteinizing hormone in male mice. J Reprod Dev 2020; 66: 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikegami K, Goto T, Nakamura S, Watanabe Y, Sugimoto A, Majarune S, Horihata K, Nagae M, Tomikawa J, Imamura T, Sanbo M, Hirabayashi M, Inoue N, Maeda K-I, Tsukamura H, Uenoyama Y. Conditional kisspeptin neuron-specific Kiss1 knockout with newly generated Kiss1-floxed and Kiss1-Cre mice replicates a hypogonadal phenotype of global Kiss1 knockout mice. J Reprod Dev 2020; 66: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higo S, Aikawa S, Iijima N, Ozawa H. Rapid modulation of hypothalamic Kiss1 levels by the suckling stimulus in the lactating rat. J Endocrinol 2015; 227: 105–115. [DOI] [PubMed] [Google Scholar]
- 18.Yamada S, Uenoyama Y, Kinoshita M, Iwata K, Takase K, Matsui H, Adachi S, Inoue K, Maeda K-I, Tsukamura H. Inhibition of metastin (kisspeptin-54)-GPR54 signaling in the arcuate nucleus-median eminence region during lactation in rats. Endocrinology 2007; 148: 2226–2232. [DOI] [PubMed] [Google Scholar]
- 19.Yamada S, Uenoyama Y, Deura C, Minabe S, Naniwa Y, Iwata K, Kawata M, Maeda K-I, Tsukamura H. Oestrogen-dependent suppression of pulsatile luteinising hormone secretion and kiss1 mRNA expression in the arcuate nucleus during late lactation in rats. J Neuroendocrinol 2012; 24: 1234–1242. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchida H, Kawai N, Yamada K, Takizawa M, Inoue N, Uenoyama Y, Tsukamura H. Central μ-opioid receptor antagonism blocks glucoprivic LH pulse suppression and gluconeogenesis/feeding in female rats. Endocrinology 2021; 162: bqab140. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchida H, Mostari P, Yamada K, Miyazaki S, Enomoto Y, Inoue N, Uenoyama Y, Tsukamura H. Paraventricular dynorphin a neurons mediate LH pulse suppression induced by hindbrain glucoprivation in female rats. Endocrinology 2020; 161: bqaa161. [DOI] [PubMed] [Google Scholar]
- 22.Tsuchida H, Nonogaki M, Takizawa M, Inoue N, Uenoyama Y, Tsukamura H. Enkephalin-δ opioid receptor signaling mediates glucoprivic suppression of LH Pulse and gluconeogenesis in female rats. Endocrinology 2023; 164: bqac216. [DOI] [PubMed] [Google Scholar]
- 23.Tsuchida H, Nonogaki M, Inoue N, Uenoyama Y, Tsukamura H. Dynorphin-κ-opioid receptor signaling, but not µ-opioid receptor signaling, partly mediates the suppression of luteinizing hormone release during late lactation in rats. Neurosci Lett 2022; 791: 136920. [DOI] [PubMed] [Google Scholar]
- 24.Yip SH, Romanò N, Gustafson P, Hodson DJ, Williams EJ, Kokay IC, Martin AO, Mollard P, Grattan DR, Bunn SJ. Elevated prolactin during pregnancy drives a phenotypic switch in mouse hypothalamic dopaminergic neurons. Cell Rep 2019; 26: 1787–1799.e5. [DOI] [PubMed] [Google Scholar]
- 25.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates, 6th Edition. San Diego: Academic Press; 2008. [Google Scholar]
- 26.Sugimoto A, Tsuchida H, Ieda N, Ikegami K, Inoue N, Uenoyama Y, Tsukamura H. Somatostatin-somatostatin receptor 2 signaling mediates LH pulse suppression in lactating rats. Endocrinology 2019; 160: 473–483. [DOI] [PubMed] [Google Scholar]
- 27.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol 1982; 243: E310–E318. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto A, Tsuchida H, Nagae M, Inoue N, Uenoyama Y, Tsukamura H. Central somatostatin-somatostatin receptor 2 signaling mediates lactational suppression of luteinizing hormone release via the inhibition of glutamatergic interneurons during late lactation in rats. J Reprod Dev 2022; 68: 190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Uenoyama Y, Nakamura S, Hayakawa Y, Ikegami K, Watanabe Y, Deura C, Minabe S, Tomikawa J, Goto T, Ieda N, Inoue N, Sanbo M, Tamura C, Hirabayashi M, Maeda K-I, Tsukamura H. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rat. J Neuroendocrinol 2015; 27: 187–197. [DOI] [PubMed] [Google Scholar]
- 30.Pimpinelli F, Parenti M, Guzzi F, Piva F, Hokfelt T, Maggi R. Presence of delta opioid receptors on a subset of hypothalamic gonadotropin releasing hormone (GnRH) neurons. Brain Res 2006; 1070: 15–23. [DOI] [PubMed] [Google Scholar]
- 31.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol 1994; 350: 412–438. [DOI] [PubMed] [Google Scholar]
- 32.Parker LM, Kumar NN, Lonergan T, McMullan S, Goodchild AK. Distribution and neurochemical characterization of neurons in the rat ventrolateral medulla activated by glucoprivation. Brain Struct Funct 2015; 220: 117–134. [DOI] [PubMed] [Google Scholar]
- 33.Ceccatelli S, Millhorn DE, Hökfelt T, Goldstein M. Evidence for the occurrence of an enkephalin-like peptide in adrenaline and noradrenaline neurons of the rat medulla oblongata. Exp Brain Res 1989; 74: 631–640. [DOI] [PubMed] [Google Scholar]
- 34.Li C, Chen P, Smith MS. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience 1999; 94: 117–129. [DOI] [PubMed] [Google Scholar]