Abstract
Superovulation procedures are routinely and widely used in mouse reproductive technology. Previous studies have shown that a large number of oocytes can be obtained from adult mice (> 10 weeks old) using a combined treatment with progesterone (P4) and anti-inhibin serum (AIS). However, these effects have not been fully investigated in young (4 weeks) C57BL/6J mice. Here, we found that a modified superovulation protocol (combined treatment with P4, AIS, eCG (equine chorionic gonadotropin), and hCG (human chorionic gonadotropin); P4D2-Ae-h) improved the number of oocytes compared to the control (eCG and hCG) (39.7 vs. 21.3 oocytes/mouse). After in vitro fertilization, pronuclear formation rates were 69.3% (P4D2-Ae-h group) and 66.2% (control group). After embryo transfer, 46.4% (116/250) of the embryos in the P4D2-Ae-h group successfully developed to term, which was comparable to the control group (42.9%; 123/287 embryos). In conclusion, our protocol (P4D2-Ae-h) was effective for superovulation in young C57BL/6J mice.
Keywords: Anti-inhibin serum, eCG, Progesterone, Reproductive engineering, Superovulation
Laboratory animals are widely used in various fields of research, including medicine and physiology. Mice are among the most commonly used laboratory animals and many genetically modified mice have been produced. These mice are stored in mouse banks and available for lots of researchers [1,2,3]. Various breeding techniques have been developed to efficiently store, transport, and produce genetically modified mice [4]. However, research using animals must be conducted based on the 3R principle (replacement, reduction, and refinement), which addresses the issues of breeding space and “the application of humane methods in animal research” [5]. Therefore, it is necessary to improve and upgrade reproductive engineering techniques for efficient mouse production.
It is important to consider reducing the number of animals used, based on the 3R principle [6]. Researchers have attempted to reduce the number of mice used based on the 3R principle. In particular, reproductive technologies, such as oocyte/embryo cryopreservation, somatic cell nuclear transfer, and genome editing, require large numbers of oocytes. Superovulation has been used to collect large numbers of oocytes. Superovulation using a combination of eCG and hCG has been widely used in many rodents. An increase in the number of oocytes collected via superovulation led to a decrease in the number of donors. Recently, it has been reported that the administration of anti-inhibin antibodies (AIS) dramatically increases the number of oocytes retrieved from several mammalian species, including golden hamsters [7], rats [8], guinea pigs [9], cows [10], and horses [11]. In mice, a protocol using AIS and progesterone (P4) reportedly increases the number of retrieved oocytes [12], and a new method for hyperovulation treatment using the mixed administration of AIS and eCG (AISe) [13] has been developed. Another important objective was to obtain more oocytes from younger mice. If many oocytes can be obtained from a young mouse, the breeding period can be shortened, saving breeding space and costs. Previous studies demonstrated an improvement in the superovulation protocol using P4 and AIS in adult mice [12, 14]. There has been a demand for a more efficient superovulation protocol for young mice. However, whether the superovulation protocol using P4 and AISe is applicable to young mice has not been fully investigated. The C57BL/6J (B6) strain is an inbred strain and widely used as the donor strain to generate genetically engineered mice [15, 16]. In this study, we compared different superovulation protocols for young B6 mice using AIS, AISe, and P4, and attempted to improve the number of oocytes obtained from young B6 mice. We also evaluated the fertility and developmental ability of the oocytes after in vitro fertilization.
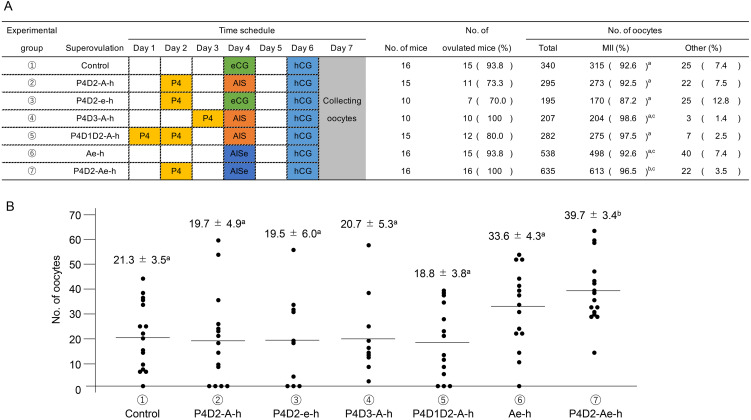
As shown in Fig. 1A, various superovulation protocols were compared. More than 70% of the treated mice ovulated, regardless of the protocol. The rates of MII oocytes were 92.6 ± 3.5% (control), 92.5 ± 4.9% (P4D2-A-h), 87.2 ± 6.0% (P4D2-e-h), 98.6 ± 5.3% (P4D3-A-h), 97.5 ± 3.8% (P4D1D2-A-h), 92.6 ± 4.3% (Ae-h) and 96.5 ± 3.4% oocytes (P4D2-Ae-h). In the P4D2-Ae-h group, the number of MII oocytes was significantly higher than that in the control group. However, the values in the other groups were not significantly different from those in the control group. These results showed that the P4D2-Ae-h protocol collected the largest number of mature oocytes among all protocols examined in this study.
Fig. 1.
The time schedule of hormone injection and result of superovulation in each experimental group of young C57BL/6J strain. (A) Schematic representation of treatments to synchronize the estrus cycle and induce superovulation. Progesterone (P4) was injected subcutaneously in the neck, and the other hormones were injected intraperitoneally at 1700–1800 h on each day. In addition, results of the number of ovulated mice and oocytes are shown. The number of oocytes were counted with classifications based on their morphology. The oocytes with fragmentation, degeneration, regression, and small ooplasm were defined as “other”. These experiments were repeated at least thrice. Statistical differences were calculated according to one-way ANOVA and Tukey–Kramer’s test, as appropriate. Letters represent significant differences (P < 0.05). (B) The results of average number of oocytes in each superovulation procedure. Data represents means ± SEM. These experiments were repeated at least thrice. Statistical differences were calculated according to one-way ANOVA and Tukey-Kramer’s test, as appropriate. Letters represent significant differences (P < 0.05).
Next, we examined the effect of our modified superovulation protocol on the number of oocytes collected from individual mice. As shown in Fig. 1B, different superovulation protocols were compared. The average numbers of oocytes collected from one female mouse were 21.3 ± 3.5 (control), 19.7 ± 4.9 (P4D2-A-h), 19.5 ± 6.0 (P4D2-e-h), 20.7 ± 5.3 (P4D3-A-h), 18.8 ± 3.8 (P4D1D2-A-h), 33.6 ± 4.3 (Ae-h) and 39.7 ± 3.4 oocytes (P4D2-Ae-h). In the P4D2-Ae-h group, the number of oocytes retrieved was significantly higher than those in the other groups (P < 0.05). Oocytes were collected from all the female mice in the P4D2-Ae-h group.
The embryonic development of oocytes collected using the P4D2-Ae-h protocol after in vitro fertilization was examined. The results are summarized in Table 1. The percentages of 2PN (69.3%), 2-cell (67.4%), and blastocysts (53.9%) were comparable to those of the control (66.2%, 62.6%, and 53.6%, respectively). To test their ability to develop to term, fresh and vitrified-warmed 2-cell embryos were transferred into the oviducts of the recipients. Table 2 presents the results of the study. The progeny rates were 42.9 ± 2.4% (123 pups/287 embryos) in the control group and 46.4 ± 2.8% (116 pups/250 embryos) in the P4D2-Ae-h group. The offspring rate in the P4D2-Ae-h group was comparable to that in the control group (P > 0.05). These results indicate that our modified superovulation protocol is effective for improving both the number of oocytes and embryonic development in young B6 mice.
Table 1. The results of in vitro fertilization and embryo development in young C57BL/6J strain.
| Superovulation procedure | No. of oocytes | No. of embryo development |
||
|---|---|---|---|---|
| 2PN (%) | 2 cell (%) | Blastocyst (%) | ||
| Control | 222 | 147 (66.2) | 139 (62.6) | 119 (53.6) |
| P4D2-Ae-h | 267 | 185 (69.3) | 180 (67.4) | 144 (53.9) |
Control and P4D2-Ae-h: Details are shown in Fig. 1. Data are presented as percentages. These experiments were repeated at least thrice. Fertilization and embryo development rates were analyzed using the chi-square test (P > 0.05; no significant difference).
Table 2. Embryonic development after embryo transfer in modified superovulation protocol.
| Treatment | No. of recipients | No. of transferred embryos | No. of delivery |
No. of pups (%) | Average No. of offspring (Average ± SE) |
|---|---|---|---|---|---|
| delivery / recipient (%) | |||||
| Control | 13 | 287 | 13/13 (100) | 123 (42.9) | 9.5 ± 0.9 |
| P4D2-Ae-h | 11 | 250 | 11/11 (100) | 116 (46.4) | 10.5 ± 1.3 |
Control and P4D2-Ae-h: Details are shown in Fig. 1. The number of mice that were pregnant and bore offspring are represented with percentages. The average number of offspring is represented with the mean ± SEM. These experiments were repeated at least thrice. Offspring rates were analyzed using the chi-square test (P > 0.05; no significant difference).
Studies have been conducted on the estrous cycle and number of oocytes collected. The relationship between estrous cycle stage and superovulation efficiency has been extensively studied in rats [17, 18]. Estrous cycle stage affects the number, quality, and morphology of oocytes in mice [19, 20]. Two days of sequential progesterone injections synchronize the estrous cycle in mice [12]. In addition, progesterone treatment inhibits ovulation during the estrous cycle in guinea pigs [9]. Furthermore, a previous study showed that the first ovulation in the estrus cycle occurs at approximately 4.5 weeks of age [21]. We also preliminarily examined vaginal smears from mice at 4 weeks of age and confirmed that the estrous cycle was already rotating in 2 out of 7 mice, as in a previous study. In addition, administering progesterone to mice can synchronize a rotating estrous cycle [12, 22], and in our preliminary study, a single administration of progesterone could also induce metestrus in young mice (data not shown). Therefore, synchronization of the estrous cycle by progesterone administration appears to be effective even in young mice. The increase in oocyte retrieval was not due to an increase in the number of abnormal oocytes but due to an increase in the number of normal MII oocytes (data not shown). An increase in FSH concentration reportedly recruits growing follicles and rescues them from apoptotic degeneration [23]. Honda et al. reported that the estrous cycle can be synchronized using LHRH in immature rats, and that the number of eggs retrieved can be increased [24]. This result indicates an effect similar to that of progesterone in mice. In other words, we speculate that synchronization of the estrous cycle increased the number of follicles responding to FSH and that AIS plus eCG (resulting in a high concentration of FSH) suppressed follicular degeneration, thus increasing the number of ovulations.
Regarding the relationship between age and the number of oocytes collected in mice, the number of oocytes collected decreases at 5 weeks of age compared with that at 4 weeks of age [25, 26]. However, in this study, there was no significant difference in the number of oocytes collected from 4- and 5-week-old mice in any of the experimental groups (data not shown). The reasons for these differences in the results of previous studies are unclear; however, possible reasons include differences in the breeders from which the mice were purchased and the environment in which the mice were kept.
In conclusion, we have developed an improved superovulation protocol that enables the stable collection of many normal oocytes from the young B6 strain. This protocol will also lead to a reduction in the number of animals and breeding space used.
Method
All chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA), unless otherwise noted. Crlj: C57BL/6J female mice (4–5 weeks old), male mice (8–12 weeks old), Crli: ICR female mice (8–14 weeks old), and male mice (12–50 weeks old) The mice were obtained from Charles River Laboratories (Yokohama, Japan). The mice were housed under controlled lighting conditions (daily light period, 0600–1800 h). This study was approved by the Animal Experimentation Committee of Azabu University and conducted in accordance with the guidelines of the committees (200318-13). All animal experiments were performed according to the ARRIVE guidelines.
The schedule of hormone injections for each condition is summarized in Fig. 1. The day of oocyte collection was defined as Day 7. Equine chorionic gonadotropin (eCG) (PMS; Nippon Zenyaku Kogyo, Fukushima, Japan) was injected intraperitoneally at 5 IU. Human chorionic gonadotropin (hCG) (Gonatropin; ASKA Pharmaceutical, Tokyo, Japan) was injected intraperitoneally at a concentration of 5 IU. Two milligrams of progesterone (P4) dissolved in sesame oil (100 μl per female mouse) was injected subcutaneously into the neck. AIS (Central Research, Tokyo, Japan) was administered 100 μL intraperitoneally. A combination of 100 μl AIS and 3.75 IU of eCG (AISe) was administered intraperitoneally. In experimental group 1 (eCG-hCG, control), female mice were injected with eCG (Day 4), followed by the injection of hCG 48 h later (Day 6). In experimental group 2 (P4D2-A-h), female mice were injected with P4 on Day 2. On Day 4, P4-treated mice were administered AIS, followed by an injection of hCG 48 h later (Day 6). In experimental group 3 (P4D2-e-h), female mice were injected with P4 on Day 2. On Day 4, P4-treated mice were administered eCG, followed by an injection of hCG 48 h later (Day 6). In experimental group 4 (P4D3-A-h), female mice were injected with P4 on Day 3. On Day 4, P4-treated mice received AIS, followed by an hCG injection 48 h later (Day 6). In experimental group 5 (P4D1D2-A-h), female mice were injected with P4 on Days 1 and 2. On Day 4, P4-treated mice were injected with AIS, followed by an injection of hCG 48 h later (Day 6). In experimental group 6 (Ae-h), female mice were intraperitoneally injected with AISe on Day 4. At 48 h after AISe administration, the mice were injected with hCG (Day 6). In experimental group 7 (P4D2-Ae-h), female mice were injected with P4 on Day 2. On Day 4, P4-treated mice were administered AISe, followed by an injection of hCG 48 h later (Day 6).
Cumulus-oocyte complexes were collected from the oviductal ampulla 14–16 h after the hCG injection. The number of ovulated oocytes was counted using a classification system based on morphology. Oocytes with fragmentation, degeneration, regression, and small ooplasms were classified as ‘other’. In vitro fertilization and sperm collection and retrieval were performed according to a method described in our previous study [27] with some modifications. Briefly, ovulated oocytes were collected from the oviductal ampulla as described above and preincubated for 1 h in 80 µl human tubal fluid (HTF) droplets supplemented with 1.25 mM reduced glutathione (GSH). Frozen-thawed sperm suspensions were suspended in 200 µl preincubation medium (HTF containing 0.4 mM methyl-β-cyclodextrin) and 0.1 mg/ml polyvinyl alcohol, but without bovine serum albumin, and were incubated at 37°C under 5% CO2 in humidified air for 1 h. At the time of insemination, preincubated spermatozoa were transferred into droplets containing oocytes at a final concentration of 2.0 × 106 spermatozoa/ml. After 6 h, oocytes were separated from spermatozoa and cumulus cells with a fine glass pipette and transferred into 50 µl KSOMaa. Cells were cultured at 37°C under 5% CO2 in humidified air for approximately 24–96 h.
Fresh 2-cell embryos were cultured until embryo transfer. If embryos could not be transferred, embryo vitrification was performed as previously described [28]. At 24 h after IVF, 2-cell embryos were placed in a drop of 1 M DMSO and then transferred to another drop of 1 M DMSO. Five microliters of DMSO containing 2-cell embryos were placed in a cryotube and equilibrated at 0°C for 5 min. Then, 45 µl of DAP213 was added to the cryotube and equilibrated for 5 min at 0°C. Cryotubes were stored in liquid nitrogen until further use. The vitrified embryos in the cryotube were warmed to 37°C with 0.25 M sucrose solution. The embryos were removed from the 0.25 M sucrose solution and transferred to a drop of KSOMaa medium. After 10 min, morphologically normal embryos were transferred to KSOMaa drops and cultured until embryo transfer. Fresh and vitrified-warmed 2-cell embryos produced by IVF were transferred into the oviducts (7–15 embryos/oviduct) of pseudopregnant mice that had been mated with a vasectomized male the night before transfer. Nineteen days after embryo transfer, offspring were obtained by natural delivery. If the offspring were not delivered by natural vaginal delivery on day 20, cesarean section was performed.
Statistical analyses were performed using Statcel 3 software (OMS Ltd., Saitama, Japan). Each experiment was repeated at least thrice. The number of MII oocytes and the average number of oocytes were analyzed by one-way analysis of variance (ANOVA) for significant differences and multiple comparison tests using the Tukey–Kramer’s test. The average number of oocytes is shown as mean ± SEM. Fertilization, embryo development, and offspring rates were analyzed by Chi-squared test. Differences were considered significant at P values < 0.05.
Conflict of interests
The authors declare no conflicts of interest.
Acknowledgments
This work was partially supported by Grants-in-Aid for Scientific Research from the JSPS (KAKENHI, 21H02384 to J.I., and 21K05977 to N.K.). This study was supported by the MEXT*-Supported Program for the Private University Research Branding Project (2016–2019) (*Ministry of Education, Culture, Sports, Science and Technology). This research was supported in part by the Center for Human and Animal Symbiosis Science, Azabu University and a research project grant from the Research Services Division of Azabu University. This work was supported by the NIBB Collaborative Research Program (18–908 and 19–903) to J.I.
References
- 1.Eppig JT, Strivens M. Finding a mouse: the International Mouse Strain Resource (IMSR). Trends Genet 1999; 15: 81–82. [DOI] [PubMed] [Google Scholar]
- 2.Yoshiki A, Ike F, Mekada K, Kitaura Y, Nakata H, Hiraiwa N, Mochida K, Ijuin M, Kadota M, Murakami A, Ogura A, Abe K, Moriwaki K, Obata Y. The mouse resources at the RIKEN BioResource center. Exp Anim 2009; 58: 85–96. [DOI] [PubMed] [Google Scholar]
- 3.Nakagata N, Takeo T. Basic mouse reproductive techniques developed and modified at the Center for Animal Resources and Development (CARD), Kumamoto University. Exp Anim 2019; 68: 391–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeo T, Nakao S, Nakagawa Y, Sztein JM, Nakagata N. Cryopreservation of mouse resources. Lab Anim Res 2020; 36: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Díaz L, Zambrano E, Flores ME, Contreras M, Crispín JC, Alemán G, Bravo C, Armenta A, Valdés VJ, Tovar A, Gamba G, Barrios-Payán J, Bobadilla NA. Ethical Considerations in Animal Research: The Principle of 3R’s. Rev Invest Clin 2020; 73: 199–209. [DOI] [PubMed] [Google Scholar]
- 6.Richmond J. Refinement, reduction, and replacement of animal use for regulatory testing: future improvements and implementation within the regulatory framework. ILAR J 2002; 43(Suppl): S63–S68. [DOI] [PubMed] [Google Scholar]
- 7.Kishi H, Okada T, Otsuka M, Watanabe G, Taya K, Sasamoto S. Induction of superovulation by immunoneutralization of endogenous inhibin through the increase in the secretion of follicle-stimulating hormone in the cyclic golden hamster. J Endocrinol 1996; 151: 65–75. [DOI] [PubMed] [Google Scholar]
- 8.Rivier C, Vale W. Immunoneutralization of endogenous inhibin modifies hormone secretion and ovulation rate in the rat. Endocrinology 1989; 125: 152–157. [DOI] [PubMed] [Google Scholar]
- 9.Shi F, Ozawa M, Komura H, Watanabe G, Tsonis CG, Suzuki AK, Taya K. Induction of superovulation by inhibin vaccine in cyclic guinea-pigs. J Reprod Fertil 2000; 118: 1–7. [DOI] [PubMed] [Google Scholar]
- 10.Akagi S, Kaneko H, Nakanishi Y, Takedomi T, Watanabe G, Taya K. Ovarian response and FSH profile in cows following injection of various doses of inhibin antiserum. J Vet Med Sci 1997; 59: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 11.Nambo Y, Kaneko H, Nagata S, Oikawa M, Yoshihara T, Nagamine N, Watanabe G, Taya K. Effect of passive immunization against inhibin on FSH secretion, folliculogenesis and ovulation rate during the follicular phase of the estrous cycle in mares. Theriogenology 1998; 50: 545–557. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa A, Mochida K, Inoue H, Noda Y, Endo T, Watanabe G, Ogura A. High-yield superovulation in adult mice by anti-inhibin serum treatment combined with estrous cycle synchronization. Biol Reprod 2016; 94: 21. [DOI] [PubMed] [Google Scholar]
- 13.Takeo T, Nakagata N. Superovulation using the combined administration of inhibin antiserum and equine chorionic gonadotropin increases the number of ovulated oocytes in C57BL/6 female mice. PLoS One 2015; 10: e0128330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mochida K. Development of assisted reproductive technologies in small animal species for their efficient preservation and production. J Reprod Dev 2020; 66: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontaine DA, Davis DB. Attention to background strain is essential for metabolic research: C57BL/6 and the international knockout mouse consortium. Diabetes 2016; 65: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kobayashi Y, Aoshima T, Ito R, Shinmura R, Ohtsuka M, Akasaka E, Sato M, Takabayashi S. Modification of i-GONAD suitable for production of genome-edited C57BL/6 inbred mouse strain. Cells 2020; 9: 957. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishigame H, Medan MS, Watanabe G, Shi Z, Kishi H, Arai KY, Taya K. A new alternative method for superovulation using passive immunization against inhibin in adult rats. Biol Reprod 2004; 71: 236–243. [DOI] [PubMed] [Google Scholar]
- 18.Kon H, Hokao R, Shinoda M. Fertilizability of superovulated eggs by estrous stage-independent PMSG/hCG treatment in adult Wistar-Imamichi rats. Exp Anim 2014; 63: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redina OE, Amstislavsky SYa, Maksimovsky LF. Induction of superovulation in DD mice at different stages of the oestrous cycle. J Reprod Fertil 1994; 102: 263–267. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Herath CB, Xia G, Watanabe G, Taya K. Superovulation, fertilization and in vitro embryo development in mice after administration of an inhibin-neutralizing antiserum. Reproduction 2001; 122: 809–816. [DOI] [PubMed] [Google Scholar]
- 21.Lenert ME, Chaparro MM, Burton MD. Homeostatic regulation of estrus cycle of young female mice on Western Diet. J Endocr Soc 2021; 5: bvab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hasegawa A, Mochida K, Ogonuki N, Hirose M, Tomishima T, Inoue K, Ogura A. Efficient and scheduled production of pseudopregnant female mice for embryo transfer by estrous cycle synchronization. J Reprod Dev 2017; 63: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markström E, Svensson EC, Shao R, Svanberg B, Billig H. Survival factors regulating ovarian apoptosis -- dependence on follicle differentiation. Reproduction 2002; 123: 23–30. [DOI] [PubMed] [Google Scholar]
- 24.Honda A, Tachibana R, Hamada K, Morita K, Mizuno N, Morita K, Asano M. Efficient derivation of knock-out and knock-in rats using embryos obtained by in vitro fertilization. Sci Rep 2019; 9: 11571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zarrow MX, Wilson ED. The influence of age on superovulation in the immature rat and mouse. Endocrinology 1961; 69: 851–855. [DOI] [PubMed] [Google Scholar]
- 26.Takeo T, Mukunoki A, Nakagata N. Ovulation of juvenile, mature, and aged female C57BL/6 mice following coadministration of inhibin antiserum and equine chorionic gonadotropin. Theriogenology 2019; 135: 1–6. [DOI] [PubMed] [Google Scholar]
- 27.Kageyama A, Suyama A, Kinoshita R, Ito J, Kashiwazaki N. Dynamic changes of intracellular zinc ion level during maturation, fertilization, activation, and development in mouse oocytes. Anim Sci J 2022; 93: e13759. [DOI] [PubMed] [Google Scholar]
- 28.Nakao K, Nakagata N, Katsuki M. Simple and efficient vitrification procedure for cryopreservation of mouse embryos. Exp Anim 1997; 46: 231–234. [DOI] [PubMed] [Google Scholar]