Abstract
Molecular docking is a computational technique that predicts the binding affinity of ligands to receptor proteins. Although it has potential uses in nutraceutical research, it has developed into a formidable tool for drug development. Bioactive substances called nutraceuticals are present in food sources and can be used in the management of diseases. Finding their molecular targets can help in the creation of disease-specific new therapies. The purpose of this review was to explore molecular docking's application to the study of dietary supplements and disease management. First, an overview of the fundamentals of molecular docking and the various software tools available for docking was presented. The limitations and difficulties of using molecular docking in nutraceutical research are also covered, including the reliability of scoring functions and the requirement for experimental validation. Additionally, there was a focus on the identification of molecular targets for nutraceuticals in numerous disease models, including those for sickle cell disease, cancer, cardiovascular, gut, reproductive, and neurodegenerative disorders. We further highlighted biochemistry pathways and models from recent studies that have revealed molecular mechanisms to pinpoint new nutraceuticals' effects on disease pathogenesis. It is convincingly true that molecular docking is a useful tool for identifying the molecular targets of nutraceuticals in the management of diseases. It may offer information about how nutraceuticals work and support the creation of new therapeutics. Therefore, molecular docking has a bright future in nutraceutical research and has a lot of potentials to lead to the creation of brand-new medicines for the treatment of disease.
Subject terms: Pharmaceutics, Target identification, Target validation
Introduction
Molecular Docking has become an essential aspect of in-silico drug development in recent years. This technique involves predicting the interaction between a small molecule and a protein at the atomic level1. This enables researchers to study the behavior of small molecules, such as nutraceuticals, within the binding site of a target protein and understand the fundamental biochemical process underlying this interaction2. The technique is structure-based and requires a high-resolution 3D representation of the target protein obtained through techniques like X-ray crystallography, Nuclear Magnetic Resonance Spectroscopy, or Cryo-Electron Microscopy3–5.
There are several computational tools and algorithms available for molecular docking techniques, both commercial and free-of-charge. These programs and tools have been developed and are currently being used in drug research and academic fields6–9. According to Sahoo et al.1 some of the most commonly used docking programs include AutoDock Vina, Discovery Studio, Surflex, AutoDock GOLD, Glide, MCDock, MOE-Dock, FlexX, DOCK, LeDock, rDock, ICM, Cdcker, LigandFit, FRED, and UCSF Dock. Among these programs, AutoDock Vina, Glide, and AutoDock GOLD have been identified as top-ranking choices with the best scores10–12. Additionally, some of these programs have been effective in predicting Root Mean Square Deviations (RMSDs) ranging from 1.5 to 2 Å, depending on the experimental poses1. However, flexible receptor docking, specifically receptor backbone flexibility, remains a challenge for contemporary docking programs13.
The ligand-receptor complex's computational electrostatics can be assessed, screened, and predicted through the docking study, as stated by Sahoo et al.1. This study typically follows two distinct steps, according to Mohapatra et al.14. First, ligand conformations are sampled according to the protein's active site. Second, the conformations are ranked according to a scoring function. Sampling algorithms should theoretically reproduce experimental binding modes, and the confirmations obtained should be ranked according to a scoring function, as per Dash et al.15. The dry lab approach offers a significant advantage over in vivo lab studies in terms of resource and time investment, as noted by Sahoo et al.16 and Pramanik et al.17. Nanda et al.18 explained that this approach predicts the ligand orientation in a complex formed by the ligand itself with proteins or enzymes. Also, the docked complex's shape and electrostatic interaction quantify the interaction.
The utility of Molecular Docking in drug discovery and design has been well-established19. However, Tao et al.20 have recently reported a surge of interest in the application of this method in food science. Specifically, molecular docking is being utilized to authenticate the molecular targets of nutraceuticals in disease management21.
Nutraceuticals are natural substances with therapeutic benefits on human health that are often sourced from dietary sources22. Because of their potential to prevent and control chronic diseases including cancer, diabetes, cardiovascular, and neurodegenerative disorders, these have grown in popularity over the past several years23–25. Before in vitro investigations, molecular docking studies are used in the field of nutraceutical research to provide crucial information26. The goal of this review is to examine the most pertinent molecular docking applications for assessing the possible health-promoting effects of nutraceuticals.
Molecular docking
Molecular docking rudiments
Molecular docking aims to predict the ligand-receptor complex through computer-based methods27. The process of docking involves two main steps which include sampling the ligand and utilizing a scoring function28. Sampling algorithms help to identify the most energetically favorable conformations of the ligand within the protein's active site, taking into account their binding mode. These confirmations are then ranked using a scoring function7,28.
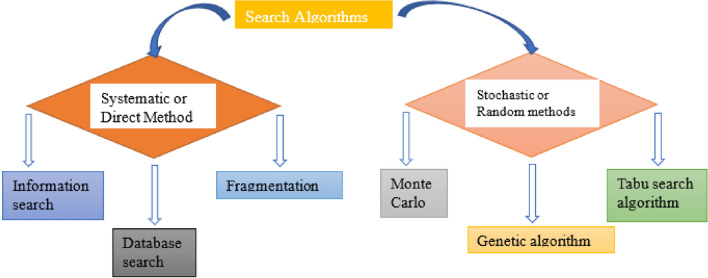
Search algorithms
The principal target of the search algorithm is to locate every single imaginable direction and conformation of the protein combined with the ligand28. The search algorithms are classified as shown in Fig. 1.
- Systematic or direct method There are three subtypes of systematic methods as follows:
- i.
-
ii.Fragmentation Here, multiple fragments may be docked during the molecular docking process to form bonds between them, or the fragments may be anchored separately, with the first fragment being docked first and subsequent fragments being built outward in steps from that initial bound position. It uses the tools like Flex XTM, DOCK, LUDI, etc.
-
iii.Database Search By using this technique, it is possible to create many reasonable conformations of every tiny molecule that is already recorded in the database and then dock them as hard bodies. FLOG is an example of the tools it uses.
- Stochastic methods or Random methods There are three subtypes of stochastic methods.
-
i.Monte Carlo This approach involves arbitrarily placing ligands in the receptor binding site, scoring it, and then generating a new configuration. It employs instruments such as MCDOCK, ICM, etc.
-
ii.Genetic algorithm It starts with a population of postures, where the configuration and location concerning the receptor are described by the “gene” and the score is the "fitness." Perform transformations, hybrids, etc., of the fittest to produce the next generation and repeat the agreement31,32. It uses programs like GOLD, AutoDock, and others.
-
i.
Tabu search It operates by striking limitations that facilitate the research of a fresh configuration by preventing the previously exposed areas of the ligands’ conformational space from being examined again. The tools it uses are PRO LEADS, Molegro Virtual Docker (MVD)TM, etc.
Figure 1.
Classes of search algorithm mechanisms.
Scoring functions
By applying virtual screening, ligands are evaluated according to their binding affinity, which aids in the evaluation of which ligand structure and rotation is most advantageous concerning the receptor (protein)28,31. According to Fig. 2, four main groupings make up the scoring function.
Force field-based In an ace capacity, it adds the contribution of non-bonded interactions including van der Waal forces, hydrogen bonding, and Columbic electrostatics as well as bond-like angle bonding and torsional deviation to determine the binding affinity33. Tools like AutoDock, DOCK, GoldScore, etc. are employed.
Empirical function It relies on repeated linear relapse analysis of a prepared set of complex structures using protein–ligand complexes with known binding affinities, comprising functional groups and some type of interaction. Examples include the N–O hydrogen link, the O–O hydrogen bond, the salt scaffold, the stacking of aromatic rings, etc.34. It makes use of technologies like LUDI score, ChemScore, AutoDock scoring, etc.
Knowledge-based By statistically assessing a collection of complex structures, it provides elements, atoms, and functional groupings with the possibility for separation into ward pairs35. Instruments like PMF and DrugScore are employed.
Consensus Fundamentally, it fuses the evaluations or orders acquired through multiple evaluation methods in various arrangements28.
Figure 2.
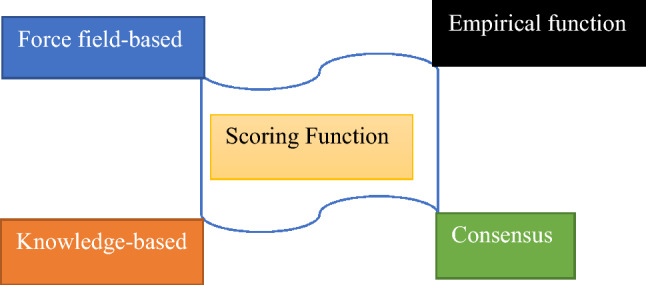
Classes of scoring function mechanisms.
Molecular docking software
Molecular docking program design
In many drug discovery initiatives, molecular docking has become crucial, especially for the virtual screening of phytochemicals or nutraceuticals as possible therapeutic compounds7. Irwin Kuntz of the University of California created the first docking program in the middle of the 1980s, and efforts are constantly being made to enhance docking computations. Current developments in docking techniques identify an enzyme’s natural substrates to forecast its capacity36. By determining that the protein of interest belongs to a certain superfamily, protein complexes can be successfully predicted by limiting the search for probable substrates and reaction types to that region37.
Methodologies of ranking docked molecules
The docked molecules are carefully ranked using a variety of approaches and systems. This section highlights the often used.
DOCK 3.5.x
The idea behind this program is that enzymes catalyze processes by restricting the transition state that is preferable to the substrate. Furthermore, amidohydrolase superfamily hydrolysis processes the protein to maintain its stiffness, therefore docking molecules that match transition states should yield a stronger signal than docking substrates38.
Glide
The program distinguishes the enzymes having a place with a specific subgroup of the enolase superfamily allowing tapering the arrangement of potential substrates, and precision in the positioning was improved by fine-tuning and rescoring the docked complex with an increasingly perplexed material science-based scoring capacity and allowing receptor side chains to move39.
Highlights of molecular docking software
Many programs are available for docking, and some of the most popular ones are discussed in this section.
Dock
Dock is a molecular docking software developed by the UCSF Chimera team. It is a user-friendly tool that can be used to dock small molecules into a receptor-binding site. Dock uses a grid-based method to evaluate the binding affinity of ligands to the receptor. It also includes scoring functions to rank the poses generated during the docking process. The dock supports several input file formats, including PDB, MOL2, and SDF. The dock is available from http://dock.compbio.ucsf.edu/.
Autodock
Autodock is a widely used molecular docking software developed by the Scripps Research Institute. It is a free, open-source software that can perform both rigid and flexible docking. Autodock uses a Lamarckian genetic algorithm to optimize the placement of ligands within a receptor binding site. It also includes several scoring functions to evaluate the binding affinity of ligands to the receptor. Autodock supports a variety of input file formats, including PDB, MOL2, and SDF. AutoDock is available from http://autodock.scripps.edu
Argus lab 4.0.1
Argus lab, a molecular modeling software created by Mark Thomson of the Department of Energy at Pacific Northwest National Laboratory in the USA, utilizes a combination of quantum mechanics and classical mechanics algorithms to model solvent effects. This software is capable of performing tasks such as drug design, producing graphics, and molecular modeling. Argus lab is available at http://www.arguslab.com.
Genetic optimization for ligand docking (GOLDTM)
GOLD is a protein–ligand docking software that offers several key features. It allows for the inclusion of spine and side chain adaptability in computations and uses user-defined scoring functions that can adapt accordingly. The energy functions are based on both conformational and non-reinforced contact information. There are also various options available for docking, including the ability to remove crystallographic water molecules in the ligand binding site. Additionally, GOLD can handle metal atoms automatically if they are correctly set up in the protein data file. Finally, virtual screening high-throughput screening results can be analyzed and post-processed efficiently using the companion programs SILVERTM or GoldMineTM. The latest version of GOLD Suite 5.2 includes three components: Gold 5.2 for protein–ligand docking, Hermes 1.6 for comprehensive protein visualization, and Gold Mine 1.5 for analysis of docking grades. This software is available at http://www.ccdc.cam.ac.uk/ products/lifesciences/gold.
MolDock
MolDock is a molecular docking software developed by MolSoft LLC40. It is a fast and efficient docking program that can be used to dock small molecules into a receptor-binding site. MolDock uses a fast Fourier transform (FFT) algorithm to evaluate the binding affinity of ligands to the receptor. It also includes a scoring function that takes into account the shape complementarity, electrostatic interactions, and van der Waals forces between the ligand and the receptor. MolDock supports several input file formats, including PDB, MOL2, and SDF. It is available at https://www.molsoft.com/about.html.
Discovery studio
Discovery Studio is a software package for molecular modeling and simulation, developed by Dassault Systèmes BIOVIA. It includes a range of tools for molecular docking, virtual screening, protein modeling, and analysis of molecular dynamics simulations. The molecular docking component is used to predict the binding mode of a ligand (small molecule) to a target protein and to estimate the strength of the interaction between them. Discovery Studio employs a variety of docking algorithms, such as CDOCKER, GOLD, and LibDock, to generate a set of possible binding poses for the ligand, and to rank them based on their predicted binding energy. The software also provides tools for visualizing and analyzing the docking results, and for comparing the binding modes of different ligands to the same protein target. It is available at https://discover.3ds.com/discovery-studio-visualizer-download.
Chimera
Chimera is a software package for visualizing, analyzing, and modeling molecular structures, developed by the University of California, San Francisco. It provides a range of tools for displaying 3D structures of proteins, nucleic acids, and small molecules, and for performing molecular docking simulations. The molecular docking component in Chimera is called “Dock Prep” and is used to prepare the ligand and target protein for docking simulations. It includes tools for adding hydrogens, assigning charges, and generating molecular surfaces, which can be used to guide the placement of the ligand in the binding site of the protein. Chimera also provides tools for analyzing the docking results, such as visualizing the binding poses, calculating binding energies, and generating interaction maps between the ligand and protein residues. Additionally, Chimera can interface with other molecular docking software packages, such as AutoDock, to perform more advanced docking simulations. Chimera is available from https://www.cgl.ucsf.edu/chimera/.2.4
Representation of molecular docking
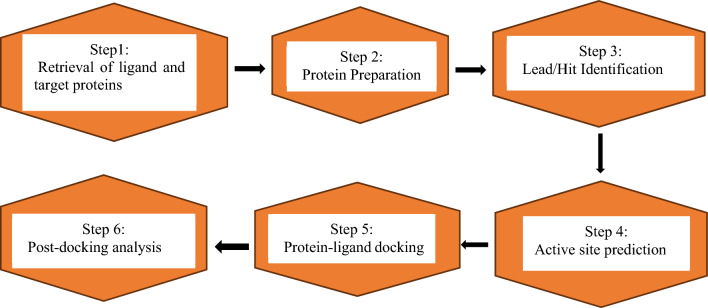
Generally, the Docking process can be represented in a flowchart as shown in Fig. 3.
Figure 3.
A prototype flow chart of a molecular docking study.
Retrieval of protein and ligand structure
Finding practical target proteins and ligands is crucial for carrying out docking. Thus, one must check to see if the target protein has been stored in the Swiss UniProt knowledge base (http://expasy.org/sprot) or the Protein Data Bank (PDB) database (http://pdb.org). Homology modeling may be done utilizing the Swiss model repository (http://swissmodel.expasy.org/repository/), modeller programs like I-TASSER, etc. if the target protein is not in the database but comparable sequences are. The next step is to locate the ligand using the PubChem database (http://pubchem.org), Zinc (http://blaster.docking.org/zinc/), ChmBl, or if none of these databases include the ligand, ChemDraw or ChemSketch can be used to synthesize the ligand from scratch41.
Protein preparation
The process of optimizing the protein structure and getting it ready for precise docking simulations is known as protein preparation, and it is an essential stage in the molecular docking process. The protein structure is first obtained from a database like Protein Databank (PDB) or created using molecular modeling tools like SWISS MODELLER. The structure is then completed by adding any extra atoms or residues. The protein is subsequently put through energy minimization to loosen up the structure and get rid of any steric interference. The protonation states of ionizable residues are then established to provide proper electrostatic interactions during docking. To further simplify the system, water molecules, and extraneous ligands are eliminated from the protein structure. To appropriately reflect the protein's behavior during docking simulations, the proper force field parameters are lastly supplied to it. Through these preparation steps, the protein structure is optimized and refined, providing a suitable starting point for successful molecular docking studies42.
Lead or hit identification
The ligands to be docked are selected based on various criteria, such as their chemical diversity, their known biological activity, or their potential for drug development. The ligands are then prepared for docking by assigning charges, generating conformers, and optimizing their geometry40,42.
Active site prediction
During molecular docking, the binding pockets can be initially specified or identified after docking. Consequently, to validate the binding pocket of interest during molecular docking three different approaches can be conceived28 as highlighted below.
-
i.
Site-directed docking Here, first, identify the protein–ligand binding site and then dock the ligand.
-
ii.
Blind docking Here, the docked ligand is directly onto the complete receptor structure without prior knowledge of the binding site43.
-
iii.
Docking with a standard Here, you dock the protein with the test ligands and/or standard small molecule(s)41. The standard ligand facilitates the prediction of the relevant binding pocket.
Additionally, calculating the inhibition constant for docked ligands and proteins is an important step in evaluating the binding affinity and potential inhibitory activity of the ligands. However, it is not always necessary or applicable in all cases. The decision to calculate the inhibition constant depends on the specific research question, experimental design, and the objectives of the study.
Protein–ligand docking
Ligand is docked against the protein and the interactions are analyzed. The scoring function gives a score based on the best-docked ligand complex picked out44.
Post-docking analysis
After the ligands have been docked to the protein, the results are analyzed to identify the most promising candidates for further study. The binding affinity of each ligand is calculated based on the predicted interaction energy, and the ligands are ranked based on their affinity scores. The docked structures are also analyzed to identify key interactions between the ligands and the protein, such as hydrogen bonds, hydrophobic interactions, and electrostatic interactions. These interactions can provide insights into the mechanism of action of the ligands and guide further optimization of their structure19.
General application of molecular docking
Hit identification/virtual screening
Molecular docking is widely used in hit identification in drug discovery. It helps in identifying potential drug candidates by predicting the binding affinity of small molecules to a protein or receptor of interest. Docking can be used to screen a large database of small molecules to identify those that can bind to a protein of interest with high affinity45.
Lead optimization
Once a hit compound is identified, molecular docking can be used to optimize the lead compound's structure to improve its binding affinity and selectivity. Docking can also be used to design new analogs by predicting the binding modes of modified structures46.
Bioremediation
Molecular docking is used in bioremediation to predict the binding affinity of small molecules to enzymes involved in the degradation of environmental pollutants. Docking can help in designing inhibitors or activators of these enzymes to enhance bioremediation efficiency47.
ADMET prediction
Docking can also be used to predict the Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) properties of small molecules. The predicted ADMET properties can be used to screen out compounds with unfavorable properties early in the drug discovery process28. Some notable examples include AutoDock Vina, GOLD (Genetic Optimization for Ligand Docking), Glide, and Schrödinger Suite. These software packages provide advanced algorithms and computational techniques for efficient ligand-receptor docking simulations, allowing for the prediction of binding affinities and identifying potential drug candidates. Furthermore, they incorporate ADMET prediction modules, enabling the assessment of the drug's behavior in terms of its absorption, distribution within the body, metabolism, excretion, and potential toxicity.
Molecular dynamics simulation
Molecular docking can be combined with molecular dynamic simulations to study the dynamic behavior of protein–ligand complexes. The simulations can help in understanding the conformational changes that occur upon ligand binding and the stability of the complex48. Several software tools combine molecular docking and dynamics simulation. These include frequently used software like AutoDock, Vina, Glide, and GOLD. In addition to molecular docking, they provide capabilities for conducting molecular dynamics simulations, allowing for the exploration of protein–ligand interactions over time and the analysis of their dynamic behavior.
Structure elucidation
Molecular docking can also be used to elucidate the structure of proteins with unknown structures. Docking can be used to predict the binding modes of small molecules to the protein and generate a homology model of the protein based on the binding mode prediction. The generated model can then be refined using experimental data to obtain an accurate structure of the protein49.
Nutraceuticals
Concept of nutraceuticals
In 1989, a term called “nutraceutical” was coined by DeFelice and the Foundation for Innovation in Medicine, combining the words “nutrition” and “pharmaceutical”50. A press release in 1994 defined nutraceuticals as substances that provide medical or health benefits and can be considered food or a component of food, including disease prevention and treatment51,52. As further explained by Raj et al.53, nutraceuticals can include a wide range of substances, such as isolated nutrients, dietary supplements, diets, herbal products, genetically engineered designer foods, and processed foods like cereals, soups, and beverages.
Despite their distinctions, nutraceuticals and functional foods are occasionally used interchangeably. Nutraceuticals as well as functional foods are two phrases used to refer to food items that offer extra health advantages over and beyond basic nutrition52,54. Nutraceuticals are isolated chemicals that have been taken from food sources and are sold in supplement form50,55, whereas functional foods are entire foods that have been fortified or augmented with nutrients or bioactive components that give a specific health benefit24,56. Nutraceuticals are frequently categorized as dietary supplements and are subject to different laws than functional foods in many nations50,56.
The idea of nutraceuticals is fundamentally influenced by personal interests and degree of expertise. Cardiologists could, for instance, prioritize dietary supplements that have been linked to decreasing heart diseases risk factors, such as those that have a positive effect on hypertension, hypercholesterolemia, and the decrease of free radical or platelet-dependent thrombotic activity57. Phytosterols, N-3 fatty acids, quercetin, and grape flavonoids are of particular significance to cardiologists24. On the other hand, oncologists may focus on nutraceuticals that have anticarcinogenic effects, such as those that enhance antioxidant and microsomal detoxification systems or decrease the growth of cancer that has already manifested56.
Classifications of nutraceuticals
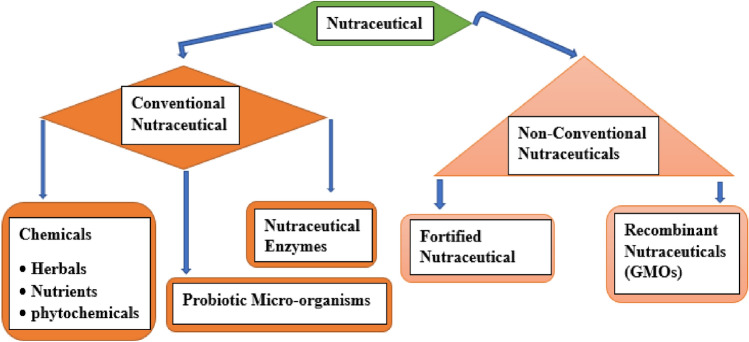
Several authors used different methods to classify nutraceuticals. However, this seminar considers the food availability framework according to Bairagi and Patel22. Therefore, nutraceuticals are classified as shown in Fig. 4.
Figure 4.
Classification of nutraceuticals22.
Conventional nutraceuticals
Nutraceuticals that have been extensively researched for their health benefits and are widely recognized as traditional include vitamins, minerals, herbal extracts, and plant-based supplements58. These conventional nutraceuticals have been in use for centuries for their medicinal properties and are readily available in the market59. Conventional nutraceuticals such as vitamin C, vitamin D, omega-3 fatty acids, and probiotics have been widely accepted22.
Chemicals (Nutrients, Herbals, and Phytocompounds) Nutraceuticals encompass a variety of compounds, with chemicals being one of the most commonly studied22. These chemicals can be further classified into three distinct groups: nutrients, herbals, and phytocompounds. Nutrients, such as vitamins and minerals, play a crucial role in maintaining normal bodily functions60. Herbals are derived from plant sources and are thought to provide various health benefits. Examples of herbals include ginger, turmeric, and ginseng. Phytocompounds, on the other hand, refer to bioactive compounds found in plants that are believed to have therapeutic properties. Prominent examples of phytocompounds include polyphenols, flavonoids, and carotenoids61.
Probiotic micro-organisms Live bacteria known as probiotics are said to have health advantages when ingested. Typically, they may be discovered in fermented foods like yogurt, kefir, and sauerkraut. Probiotics are supposed to promote gut health by balancing out the beneficial bacteria present in the gut microbiome. Moreover, they could give the immune system the vitality required to reduce inflammation22.
Nutraceutical enzymes Proteins called enzymes speed up chemical processes in the body. Enzymes that are considered to offer therapeutic advantages when taken as supplements are known as nutraceutical enzymes. For instance, proteolytic enzymes may assist lower inflammation in the body while digestive enzymes may aid enhance food digestion56.
Non-conventional nutraceuticals
Unconventional sources such as algae, fungi, and animal by-products have given rise to a new generation of less familiar nutraceuticals22,56. Among these non-conventional nutraceuticals are exotic fruits, novel proteins, and unique bioactive compounds. Despite the growing interest in these nutraceuticals, their potential health benefits, safety, and efficacy are still under investigation61. Spirulina, chlorella, mushroom extracts, and insect-based proteins are examples of non-conventional nutraceuticals that have emerged in recent years22,24,59.
- Fortified Nutraceuticals To enhance the nutritional value of products, fortified nutraceuticals are enriched with extra vitamins, minerals, and nutrients. This process is aimed at providing more health benefits beyond their natural form. As stated by Rajasekaran and Kalaivani62, “fortified nutraceuticals are defined as food or food products that are fortified with additional nutrients to provide a health benefit beyond their normal nutritional content”. Some examples of fortified nutraceuticals include:
-
i.Fortified fruit juices These drinks include added vitamins and minerals to improve their nutritional value. For instance, calcium and vitamin D may be added to orange juice to promote the health of your bones.
-
ii.Fortified breakfast cereals These are fortified cereals containing added vitamins and minerals. For instance, some cereals could be iron-fortified to guard against iron deficiency anemia.
-
iii.Fortified milk Milk may be fortified with vitamin D to support bone health and calcium absorption.
-
iv.Fortified energy drinks To boost energy metabolism, energy drinks may be supplemented with vitamins and minerals.
-
i.
- Recombinant Nutraceuticals Innovative nutraceuticals are novel products obtained from genetically engineered organisms (GMOs) that are programmed to synthesize targeted nutrients or bioactive molecules. These novel products are intended to confer extra health benefits beyond the native versions63. A few instances of innovative nutraceuticals comprise:
-
i.Recombinant antibodies These are antibodies that are produced using recombinant DNA technology. For example, recombinant monoclonal antibodies are used to treat cancer and autoimmune diseases.
-
ii.Recombinant vitamins These vitamins are created by the use of recombinant DNA technology. Recombinant vitamin B12, for instance, is used to treat vitamin B12 insufficiency.
-
iii.Recombinant proteins Recombinant DNA technology is employed to produce proteins such as human insulin, which is utilized in the management of diabetes22.
-
iv.Recombinant enzymes Recombinant DNA technology is employed to create enzymes like lactase, which are used to break down lactose in individuals with lactose intolerance64.
-
i.
Industrial dynamics of nutraceuticals
Cannabis blueprint
The popularity of cannabis together with its byproducts in nutraceuticals is on the rise, as they are believed to offer a wide range of health benefits65. In a surprising move, the Cannabis Act of 2018 was passed, allowing for the legal cultivation of hemp and marijuana and their derivative products in the United States22. Nutraceuticals containing cannabidiol, the active ingredient derived from cannabis or hemp, are becoming increasingly popular66. As cannabis becomes legal in more countries, further research is being conducted to determine the potential benefits and risks of cannabis-based products. It is expected that the trend toward cannabis-based nutraceuticals will continue to grow as more consumers become aware of their potential health benefits64,66.
Nutricosmetics
According to Bairagi and Patel22, nutricosmetics are supplements aimed at enhancing the health and appearance of the hair, skin, and nails. As consumers increasingly prioritize natural and holistic approaches to augment their physical appearance, the demand for nutricosmetics is expected to surge. Taeymans et al.67 suggest that this trend will continue to gain momentum as consumers become more health-conscious and seek multifaceted benefits from the products they purchase.
Problem of plastic packaging
The nutraceutical industry is currently grappling with the challenges arising from plastic packaging, which has emerged as a significant worry for both producers and consumers22,53. With increasing awareness about the environment, more consumers are seeking out eco-friendly alternatives for packaging68. Consequently, manufacturers are devising innovative, sustainable packaging options to cater to this demand69.
Sports nutrition space
As interest in fitness and sports-related activities increases, so too does the demand for products that can improve athletic performance and overall health. Accordingly, Bairagi and Patel22 noted that this trend has led to rapid growth in the sports nutrition market, with no signs of slowing down.
Domestic anima food development
Pet owners are increasingly looking for high-quality, nutrient-dense food options for their pets. The trend toward pet food optimization is expected to continue as more consumers become aware of the importance of proper nutrition for their pets70.
Online marketing of nutraceuticals
The rise of e-commerce and online marketing has made it easier for nutraceutical companies to reach consumers directly. Online marketing allows companies to target specific demographics and provide more personalized marketing messages. This trend is expected to continue as more consumers turn to online shopping for their nutraceutical needs22.
Seed oil as nutraceutical deposit
Seed oils, such as flaxseed oil as well as chia seed oil are becoming popular as a source of essential fatty acids and other nutrients71,72. These oils are being incorporated into a wide range of nutraceutical products, including supplements and functional foods. The trend toward using seed oils as a nutraceutical deposit is expected to continue as consumers seek out more plant-based sources of nutrients73.
Nutraceuticals against bisphenol A and other endocrine disruptors
Nutraceuticals are gaining popularity as a means of combating the effects of endocrine disruptors such as Bisphenol A (BPA)74. BPA is commonly found in plastics, and exposure to it has been linked to a variety of negative health effects, including disruption of the endocrine system75. Nutraceuticals such as resveratrol, curcumin, and green tea extract have been shown to have protective effects against BPA-induced damage, with research indicating that they can mitigate the effects of BPA on the body76,77. As consumers become more aware of the potential dangers of endocrine disruptors, nutraceuticals are likely to become an increasingly popular choice for those looking to protect their health77,78.
Generally, the nutraceuticals industry is experiencing significant growth as consumers become more health-conscious and seek out natural alternatives to traditional pharmaceuticals. Key trends in this industry include the use of innovative delivery methods, the development of personalized nutrition, and the incorporation of technology such as artificial intelligence and blockchain to ensure product quality and traceability. In addition to a rise in interest in the use of nutraceuticals for mental health and wellbeing, there is a rising demand for components that are derived from plants and are sustainably harvested. As the industry continues to evolve, companies that prioritize innovation and sustainability are likely to see the greatest success.
Molecular docking validations of nutraceuticals targets in diseases
Theory of molecular docking validation of nutraceuticals
Molecular docking validation is a computational approach that is increasingly being used in the field of nutraceutical research to identify potential targets for the management of various diseases. Nutraceuticals are naturally occurring compounds that have potential health benefits and are found in food sources such as fruits, vegetables, and herbs73. With the rise of chronic diseases such as diabetes, cardiovascular disease, and cancer, there is a growing interest in the use of nutraceuticals as a complementary approach to conventional medical treatments15,22. Molecular docking validation can help researchers identify potential nutraceutical targets for disease management, providing a more efficient and cost-effective way to screen potential treatments before proceeding with costly clinical trials79. This method makes drug discovery more ethical since it lessens the need for animal testing throughout the development of novel medicines80,81.
Molecular docking discovery of nutraceuticals targets
Molecular docking is a computational technique used to predict the interactions between small molecules, such as nutraceuticals, and larger biomolecules, such as enzymes, receptors, RNA, DNA, and other proteins82. The technique involves the simulation of the molecular interactions between the small molecule and the target biomolecule, which can provide insights into the binding affinity, binding site, and possible mechanism of action83.
Enzymes
Enzymes are proteins that catalyze chemical reactions in the body84. Nutraceuticals such as curcumin, resveratrol, quercetin, hesperidin, etc., have been shown to interact with enzymes and modulate their activity21,85. For example, curcumin has been shown to inhibit the activity of the enzyme COX-2, which is involved in the inflammatory response86. Molecular docking can predict the binding site and the strength of the interaction between the nutraceutical and the enzyme, which can help in understanding the mechanism of action and the potential therapeutic benefits82.
Receptors
Receptors are proteins that bind to specific molecules, such as hormones, neurotransmitters, and drugs, and initiate a cellular response. Nutraceuticals can also interact with receptors and modulate their activity73. For example, resveratrol was able to activate the sirtuin family of proteins, which are involved in cellular metabolism and aging85. Molecular docking can predict the binding site and the strength of the interaction between the nutraceutical and the receptor, which can help in understanding the mechanism of action and the potential therapeutic benefits80.
RNA and DNA
Nucleic acids such as RNA and DNA are essential for the storage and transfer of genetic information. By attaching to their structures or changing their expression, nutraceuticals can also interact with RNA or DNA and affect their function87–89. For instance, it has been demonstrated that the enzyme topoisomerase, which is involved in DNA replication and repair, is inhibited by quercetin90. According to Singh et al.86, molecular docking can forecast how nutraceuticals may interact with DNA and RNA sequences like telomerase reverse transcriptase (TERT) and microRNAs (miRNAs). Furthermore, molecular docking can forecast the binding location and intensity of the interaction between the nutraceutical and RNA or DNA, which aids in understanding the mechanism of action91.
Epigenetic markers
According to recent studies82,92, epigenetic changes, which can change gene expression without affecting the underlying DNA sequence, are significant targets for nutraceuticals. Molecular docking can predict how nutraceuticals may interact with proteins involved in epigenetic regulation, such as histone deacetylases (HDACs), DNA methyltransferases (DNMTs), and transcription factors/nuclear receptors like estrogen receptors, androgen receptors, fibroblast growth factors21,93. These epigenetic modifications can lead to transgenerational effects.
Other proteins
Nutraceuticals may potentially target other proteins in the body, including transporters, ion channels, and structural proteins94. Hesperidin, for instance, has been demonstrated to block the activity of alpha-glucosidase, an enzyme involved in the breakdown of carbohydrates21,73. To understand the mechanism of action and possible therapeutic effects, molecular docking can predict the binding site and the degree of interaction between the nutraceutical and the protein target91.
Applications of nutraceuticals in disease management
Nutraceutical in cancer
Numerous research95–98 have demonstrated that some nutraceuticals may have a positive impact on cancer prevention and therapy by regulating important signaling pathways involved in tumor development and metastasis (Fig. 5).
Figure 5.
The mechanism of nutraceuticals in cancer management96.
Phytocompounds such as phenolics, flavonoids, and others possess anticancer potentials41. One of the most well-known nutraceuticals in cancer prevention is curcumin, a compound found in turmeric96,98. As shown in Fig. 5, Curcumin has been shown to inhibit the activation of NF-κB, a transcription factor that plays a critical role in inflammation and cancer development. NF-κB activation can result in the expression of genes that promote tumor growth, invasion, and metastasis97,99. By inhibiting NF-κB, curcumin can suppress the development and progression of cancer95,100.
Another nutraceutical that has been studied extensively in cancer prevention is resveratrol, a polyphenol found in grapes, berries, and peanuts. The PI3K/Akt/mTOR pathway, which controls cell proliferation and survival, is one of the signaling pathways that resveratrol modulates and is associated with the development of cancer101. Resveratrol inhibits the activation of Akt, a kinase that is frequently overexpressed in cancer and can induce cell cycle arrest and apoptosis in cancer cells97.
According to research by Melzer et al.102, green tea polyphenol epigallocatechin-3-gallate (EGCG) may have anticancer properties. The Wnt/-catenin pathway, which is crucial for cell division and proliferation, is one of the signaling pathways that can be inhibited by EGCG103. EGCG can also prevent the activation of other signaling pathways that are implicated in the growth of cancer102. In addition, EGCG has been shown in animal models to stop tumor development and spread by causing cell cycle arrest and death in cancer cells100,103.
Numerous additional substances contained in foods or supplements, in addition to these nutraceuticals, have also been proven to have potential anticancer benefits104. These include sulforaphane, a substance found in cruciferous vegetables, which can activate Nrf2, a transcription factor that controls antioxidant and detoxification pathways and can induce apoptosis in cancer cells96.
Overall, the use of nutraceuticals in cancer prevention and treatment is an exciting area of research that holds promise for developing novel therapies for this devastating disease. By targeting key signaling pathways involved in tumor growth and metastasis, nutraceuticals have the potential to complement conventional cancer treatments and improve patient outcomes100.
Nutraceuticals in cardiovascular health
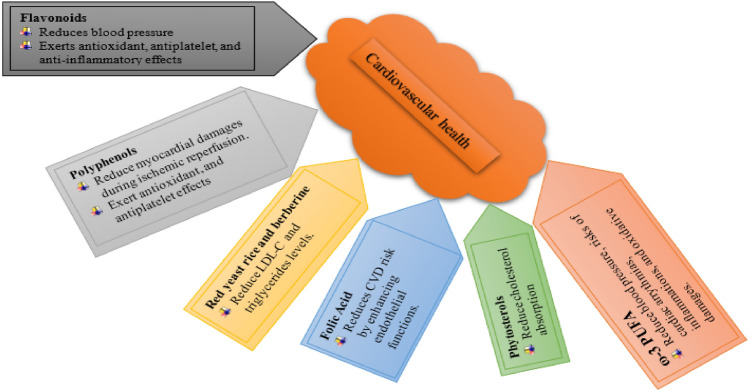
It has been demonstrated that several nutraceuticals help to promote cardiovascular health by lowering blood pressure, cholesterol levels, and inflammation105–108. The probable processes through which dietary supplements support cardiovascular health are shown in Fig. 6.
Figure 6.
Nutraceutical’s effects on the cardiovascular system.
Omega-3 fatty acids are a type of polyunsaturated fatty acid that is found in fatty fish, nuts, and seeds109. They have been shown to lower blood pressure and reduce the risk of heart disease110. The molecular mechanism by which omega-3 fatty acids regulate blood pressure is not fully understood, but it is thought to involve the inhibition of the production of inflammatory cytokines, which can cause the constriction of blood vessels and increase blood pressure106. Omega-3 fatty acids have also been shown to reduce levels of triglycerides and LDL cholesterol and increase levels of HDL cholesterol110 which can help to prevent the buildup of plaque in the arteries and reduce the risk of heart disease111.
Fruits, vegetables, and tea all include a group of substances known as flavonoids112. Research by Ohishi et al.113 has demonstrated that they contain anti-inflammatory and antioxidant characteristics as well as the ability to lower the risk of cardiovascular disease. The activation of the endothelial nitric oxide synthase enzyme, which can relax blood arteries and lower blood pressure, is assumed to be the molecular mechanism by which flavonoids modulate blood pressure114. Furthermore, flavonoids have been shown to increase HDL cholesterol levels and decrease triglyceride and LDL cholesterol levels107.
In fruits, vegetables, and tea, a group of substances known as polyphenols can be discovered115. They have been shown to have antioxidant and anti-inflammatory properties, and to reduce the risk of cardiovascular disease116. The molecular mechanism by which polyphenols regulate blood pressure is thought to involve the inhibition of the renin–angiotensin–aldosterone system, which can cause the constriction of blood vessels and increase blood pressure114. Similar to flavonoids, polyphenols have also been shown to reduce levels of LDL cholesterol and triglycerides and to increase levels of HDL cholesterol107.
Therefore, nutraceuticals such as omega-3 fatty acids, flavonoids, and polyphenols have been found to play a role in promoting cardiovascular health by regulating blood pressure, cholesterol levels, and inflammation. The molecular mechanisms by which these compounds exert their beneficial effects are not fully understood, but they are thought to involve the inhibition of inflammatory cytokines, the activation of endothelial nitric oxide synthase, and the inhibition of the renin–angiotensin–aldosterone system.
Nutraceuticals in neurodegenerative diseases
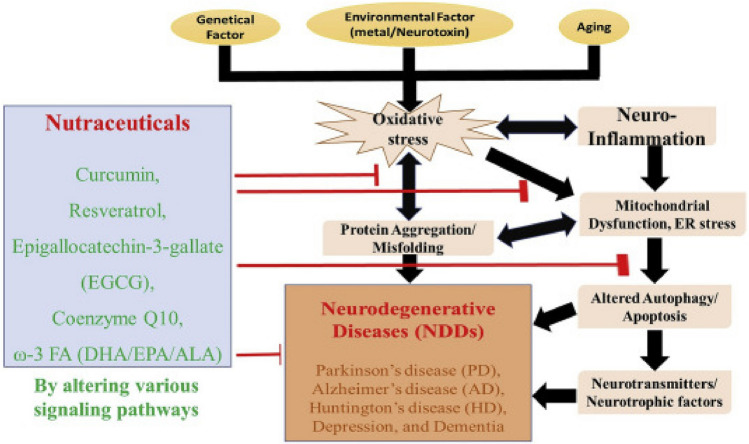
Nutraceuticals have been studied for their potential role in preventing and treating neurodegenerative diseases such as Alzheimer's, Parkinson's, etc.117–119 (Fig. 7).
Figure 7.
Molecular mechanisms of nutraceuticals in neurodegenerative diseases117.
According to Chiu et al.117, oxidative stress is one of the molecular targets for nutraceuticals in neurodegenerative disorders. This happens with the emergence of an imbalance between the body’s ability to use antioxidants to neutralize reactive oxygen species (ROS) and their synthesis119,120. According to Teter et al.121, ROS has been connected to the emergence of neurodegenerative illnesses and can harm cellular components including proteins, lipids, and DNA.
Fish oil and flaxseed oil contain omega-3 fatty acids, which have been demonstrated to have anti-inflammatory and antioxidant characteristics, which can both aids prevent neurodegeneration71. According to studies117,121, omega-3 fatty acids may also enhance cognitive performance in persons with moderate cognitive impairment and lower the chance of acquiring Alzheimer’s disease.
Specifically, against Parkinson’s disease, flavonoids have been investigated for their possible neuroprotective properties120. Flavonoids have been demonstrated to affect signaling pathways involved in cell survival and death, as well as to have antioxidant and anti-inflammatory activities117. In animal models of Parkinson's disease, the flavonoid quercetin in particular has been demonstrated to shield neurons from harm and degeneration118,120.
Typically, oxidative stress, inflammation, and cell survival pathways are the molecular targets of nutraceuticals in neurodegenerative disorders. Nutraceuticals like flavonoids and omega-3 fatty acids have both been investigated for their possible neuroprotective properties71,107. They could influence these targets by lowering inflammation and oxidative stress while enhancing cell survival pathways117.
Nutraceuticals and gut health
Numerous areas of health, including gut health, can be supported by nutritional supplements122. The collection of microbes that live in the human gastrointestinal system, or the gut microbiome, is essential to gut health123,124. Nutraceuticals including probiotics, prebiotics, and fiber can all help control the gut microbiota and immune system106.
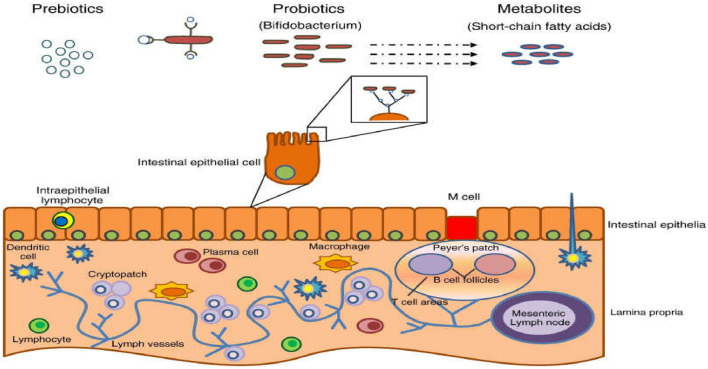
Figure 8 illustrates how probiotics, which are living microbes, can help the host's health when given in sufficient doses. According to Abdulhussein et al.125, probiotics can alter the makeup and operation of the gut microbiome and enhance gut health. For instance, it has been demonstrated that certain probiotic strains can boost immunological function, increase gut barrier function, and reduce gut inflammation126.
Figure 8.
Mechanism of prebiotic actions125.
Probiotics have many different and intricate molecular processes that contribute to their positive benefits106. Through some signaling pathways, including toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors, and pattern recognition receptors (PRRs), probiotics can interact with immune cells and gut epithelial cells126. Additionally, probiotics can generate many metabolites that influence the immune system and gut flora (Fig. 8).
Prebiotics are non-digestible dietary components that support the development and activity of healthy bacteria in the gut. By boosting the number of advantageous bacteria in the gut microbiome, such as Bifidobacteria and Lactobacilli, prebiotics can enhance gut health126. Short-chain fatty acids (SCFAs), which have anti-inflammatory characteristics and can enhance gut barrier function, can also be produced by prebiotics106. Prebiotics’ interaction with the gut microbiota is one of the molecular processes behind the positive benefits of prebiotics127. Prebiotics can specifically encourage the development of good bacteria that can create SCFAs, which can alter the gut microbiota and immune response122.
According to Das et al.128, fiber is a kind of complex carbohydrate that is not broken down by human enzymes and can serve as a prebiotic. For instance, fiber can enhance gut health by boosting the generation of SCFAs, accelerating gut motility, and encouraging the proliferation of advantageous bacteria in the gut microbiome106,126. Additionally, poisons and other hazardous chemicals in the gut can be bound by fiber and eliminated from the body. The chemical processes that underlie fiber's beneficial effects are equally intricate and varied. Prebiotics that resemble fiber can specifically encourage the growth of good bacteria that can create SCFAs, which can alter the gut microbiota and immune response. The release of many gut hormones, including peptide YY (PYY) and glucagon-like peptide 1 (GLP-1), which control gut motility and hunger, can also be stimulated by fiber128,129. Furthermore, according to Das et al.128, fiber can bind to bile acids and other toxic chemicals in the gut and remove them from the body.
Probiotics and prebiotics, like fiber, are nutraceuticals that can promote gut health and help control gut flora. The intricate molecular processes behind their beneficial effects include many signaling channels and metabolites. People can boost their gut health and general wellbeing by including these nutraceuticals in a balanced diet.
Nutraceuticals and sickle cell disorder
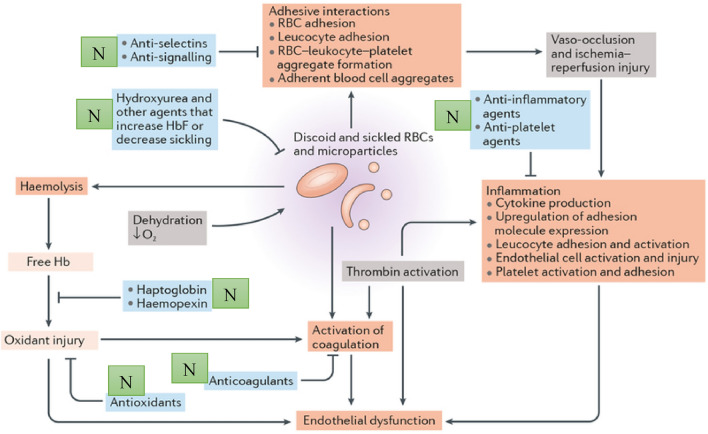
Red blood cells with an irregular shape are thought to contribute to sickle cell disease (SCD), which can result in discomfort and organ damage due to blockages in tiny blood arteries130,131. Due to their alleged anti-inflammatory, antioxidant, and antiplatelet effects, nutraceuticals have been researched as a possible anti-sickling chemotherapeutic drug in SCD132–134. The molecular targets of nutritional supplements in sickle cell disorders are depicted in Fig. 9.
Figure 9.
Mechanisms of nutraceuticals in sickle cell diseases135 with modifications (N = Nutraceuticals: Curcumin, Resveratrol, Quercetin, N-Acetyl cysteine, etc.).
Resveratrol, a phytonutrient that is present in grapes and other fruits, is one nutraceutical that has been investigated for SCD134. Resveratrol has been demonstrated to lessen oxidative stress, inflammation, and platelet activation, all of which are implicated in the pathophysiology of SCD135. Resveratrol oral administration decreased inflammatory and oxidative stress markers in a study of SCD patients, pointing to a potential preventive benefit against SCD consequences136,137.
A flavonoid present in many fruits and vegetables called quercetin is another nutraceutical that has demonstrated potential as an anti-sickling agent138. Quercetin has been proven to increase red blood cell deformability and decrease sickling in red blood cells when tested in vitro139,140. These effects may assist avoid blockages in tiny blood arteries. Al Balushi et al.132 observed that quercetin also has anti-inflammatory and antioxidant properties that may aid to lessen the consequences of SCD problems.
A component of turmeric called curcumin has also been investigated for its possible anti-sickling properties137. The pathophysiology of SCD is influenced by oxidative stress, inflammation, and platelet activation, all of which can be reduced by curcumin (Vona et al.136). Curcumin administration reduced inflammatory and oxidative stress indicators in a study of SCD patients and improved blood flow, suggesting a possible preventive benefit against SCD consequences137,141.
Nutraceuticals have therefore demonstrated potential as anti-sickling chemotherapeutic agents in SCD because of their alleged anti-inflammatory, antioxidant, and antiplatelet characteristics142. Several dietary supplements have been investigated for their potential to offer protection against the side effects of SCD, including resveratrol, quercetin, and curcumin. The best dosages, formulations, and supplementation duration for these substances, as well as their effectiveness and safety in bigger clinical studies, need to be determined via more studies.
Nutraceuticals in reproductive health
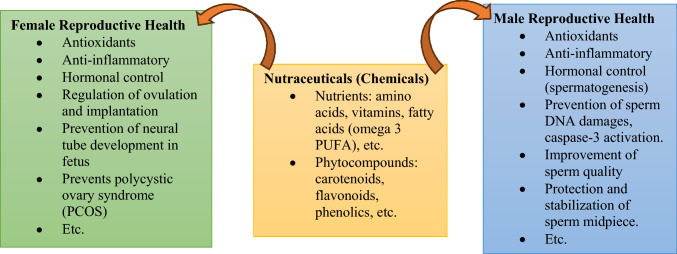
The use of nutraceuticals as possible chemotherapeutic agents to enhance both male and female reproductive health has grown in prominence in recent years143 (see Fig. 10). According to research by Ma et al.144, these items include natural substances that have been demonstrated to enhance sexual function, hormonal balance, and fertility. Examples of these substances include vitamins, minerals, amino acids, and phytochemicals.
Figure 10.
Actions of the Nutraceuticals on reproductive health.
Nutraceuticals contained in natural goods like seed oil formulations, such as L-carnitine, CoQ10, zinc, and phytocompounds, have been demonstrated to increase sperm count, motility, and morphology for male reproductive health78,144. These substances have antioxidant properties that shield sperm from oxidative stress and enhance mitochondrial performance145. Men’s sexual drive and performance have also been demonstrated to be enhanced by nutraceuticals containing maca root extract146.
Nutraceuticals including folic acid, vitamin D, and omega-3 fatty acids have been demonstrated to enhance female fertility and hormonal balance. Vitamin D is important for ovulation and implantation whereas folic acid helps to prevent neural tube abnormalities in growing babies147. Omega-3 fatty acids may also boost fertility by regulating hormone levels and lowering inflammation in the reproductive system148.
In general, the use of nutraceuticals as possible chemotherapeutic agents has demonstrated positive outcomes for enhancing both male and female reproductive health78,147. A starting point for the identification of innovative medication formulations is the invalidation of these chemotherapeutic actions of nutraceuticals through the use of molecular docking. While research continues to fully understand the usefulness and safety of nutraceuticals, using these natural substances in a balanced diet may aid those who struggle with infertility or hormone imbalances148. However, it's crucial to speak with a doctor before including nutraceuticals in your diet, especially if you take any drugs or have underlying medical issues.
Conclusion
Molecular docking is a useful approach for identifying the molecular targets of nutraceuticals in the treatment of illness. To identify possible treatment targets, it enables the prediction of the binding affinity and conformation of nutraceuticals with target proteins. The availability of databases and the improvement of computational tools have made molecular docking a crucial tool in the drug discovery process. The usage of this technology has improved drug discovery's efficiency and efficacy by cutting the time and expense needed for conventional experimental procedures. Therefore, the use of molecular docking in research on dietary supplements has considerable potential for the identification of novel therapeutic targets and the creation of secure and efficient dietary supplements for the treatment of disease.
Future perspectives
A potent approach for identifying the molecular targets of nutraceuticals in the treatment of illness is molecular docking. Future developments in molecular docking tools and algorithms will improve the precision and effectiveness of discovering prospective nutraceutical targets. Additionally, molecular docking will be combined with other computational techniques like network analysis and machine learning to provide a more thorough knowledge of the complicated interactions between nutraceuticals and their molecular targets. Additionally, the use of molecular docking in personalized medicine will enable the development of customized treatment plans based on a person's genetic make-up and illness condition.
Author contributions
A.P.C., O.U.O., O.I.H., A.P.M. and A.C.A wrote the main manuscript text and A.P.C., U.E.I., and A.P.M prepared the figures. All authors reviewed the manuscript.
Data availability
Data relating to this review are available on request. Kindly contact the corresponding author to request the data from this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
P. C. Agu, Email: sirpfoundation@gmail.com
P. M. Aja, Email: Patrick.aja@ebsu.edu.ng
References
- 1.Sahoo RN, Pattanaik S, Pattnaik G, Mallick S, Mohapatra R. Review on the use of molecular docking as the first line tool in drug discovery and development. Indian J. Pharm. Sci. 2022;84(5):1334–1337. [Google Scholar]
- 2.Meng XY, Zhang HX, Mezei M, Cui M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011;7:146–157. doi: 10.2174/157340911795677602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyth MS, Martin JH. X-ray crystallography. Mol. Pathol. 2000;53:8–14. doi: 10.1136/mp.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugiki T, Kobayashi N, Fujiwara T. Modern technologies of solution nuclear magnetic resonance spectroscopy for three-dimensional structure determination of proteins open avenues for life scientists. Comput. Struct. Biotechnol. J. 2017;15:328–339. doi: 10.1016/j.csbj.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakane T, Kotecha A, Sente A, McMullan G, Masiulis S, Brown PMGE, Grigoras IT, Malinauskaite L, Malinauskas T, Miehling J. Single-particle cryo-EM at atomic resolution. Nature. 2020;587:152–156. doi: 10.1038/s41586-020-2829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jorgensen WL. The many roles of computation in drug discovery. Science. 2004;303(5665):1813–1818. doi: 10.1126/science.1096361. [DOI] [PubMed] [Google Scholar]
- 7.Kitchen DB, Decornez H, Furr JR, Bajorath J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Natl. Rev. Drug Discov. 2004;3(11):935–949. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 8.Bajorath J. Integration of virtual and high-throughput screening. Natl. Rev. Drug Discov. 2002;1(11):882–894. doi: 10.1038/nrd941. [DOI] [PubMed] [Google Scholar]
- 9.Langer T, Hoffmann RD. Virtual screening an effective tool for lead structure discovery. Curr. Pharm. Discov. 2001;7(7):509–527. doi: 10.2174/1381612013397861. [DOI] [PubMed] [Google Scholar]
- 10.Potluri H, Prasanth DS, Atmakuri LR. In vivo antinociceptive effect of methanolic extract of Ipomoea marginata Desr. in rodents as well as in silico molecular docking of some phytoconstituents from the plant. Indian J. Pharm. Sci. 2021;83(4):732–741. [Google Scholar]
- 11.Ghode P, Jain SK. Structural requirements for some 3-amino-N-substituted-4-(substituted phenyl) butanamides as dipeptidyl peptidase-IV inhibitors using 3D-QSAR and molecular docking approaches. Indian J. Pharm. Sci. 2018;79(6):974–986. [Google Scholar]
- 12.Tomar NR, Singh V, Marla SS, Chandra R, Kumar R, Kumar A. Molecular docking studies with rabies virus glycoprotein to design viral therapeutics. Indian J. Pharm. Sci. 2018;72(4):486. doi: 10.4103/0250-474X.73905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bissantz C, Folkers G, Rognan D. Protein-based virtual screening of chemical databases. 1. Evaluation of different docking/scoring combinations. J. Med. Chem. 2000;43(25):4759–4767. doi: 10.1021/jm001044l. [DOI] [PubMed] [Google Scholar]
- 14.Mohapatra R, Mallick S, Nanda A, Sahoo RN, Pramanik A, Bose A. Analysis of steady state and non-steady state corneal permeation of diclofenac. RSC Adv. 2016;6(38):31976–31987. [Google Scholar]
- 15.Dash R, Sahoo RN, Nandi S, Swain R, Mallick S. Sustained release bioadhesive suppository formulation for systemic delivery of ornidazole: In silico docking study. Indian J. Pharm. Educ. Res. 2019;53(4):S580–S586. [Google Scholar]
- 16.Sahoo RN, Nanda A, Pramanik A, Nandi S, Swain R, Pradhan SK, Mallick S. Interactions between Ibuprofen and Silicified MCC: Characterization, drug release, and modeling approaches. Acta Chima Slovica. 2019;66(4):923–933. [PubMed] [Google Scholar]
- 17.Pramanik A, Sahoo RN, Pradhan SK, Mallick S. Characterization and molecular docking of kaolin-based cellulosic film for extending ophthalmic drug delivery. Indian J. Pharm. Sci. 2021;83(4):794–807. [Google Scholar]
- 18.Nanda A, Sahoo RN, Pramanik A, Mohapatra R, Pradhan SK, Thirumurugan A. Drug-in-mucoadhesive type film for the ocular anti-inflammatory potential of amlodipine: Effect of sulphobutyl-ether-beta-cyclodextrin on permeation and molecular docking characterization. Colloids Surf. B Biointerfaces. 2018;172:555–564. doi: 10.1016/j.colsurfb.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Pinzi L, Rastelli G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 2019;20:4331. doi: 10.3390/ijms20184331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao X, Huang Y, Wang C, Chen F, Yang L, Ling L, Che Z, Chen X. Recent developments in molecular docking technology applied in food science: A review. Int. J. Food Sci. Technol. 2020;55:33–45. [Google Scholar]
- 21.Aja PM, Awoke JN, Agu PC, Adegboyega AE, Ezeh EM, Igwenyi IO, Orji OU, Ani OG, Ale BA, Ibiam UA. Hesperidin abrogates Bisphenol A endocrine disruption through binding with fibroblast growth factor 21 (FGF-21), α-amylase and α-glucosidase: An in silico molecular study. J. Genet. Eng. Biotechnol. 2022;20(1):84. doi: 10.1186/s43141-022-00370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bairagi GR, Patel VP. Nutraceutical a review on basic need, classification, recent trends in industry and delivery systems. J. Emerg. Technol. Innov. Res. (JETIR) 2021;8(5):c183–c199. [Google Scholar]
- 23.Heyland DK. In search of the magic nutraceutical: Problems with current approaches. J. Nutr. 2001;131(9):2591S–2595S. doi: 10.1093/jn/131.9.2591S. [DOI] [PubMed] [Google Scholar]
- 24.Miller EG, Gonzales SAP, Couvillon AM, Binnie WH, Hasegawa S, Lam LKT. Emerging trends in dietary components for preventing and combating disease. Food Technol. 2012;5:114. [Google Scholar]
- 25.Madley WR. Functional foods. New Prod. 2003;66:125. [Google Scholar]
- 26.Carpio LE, Sanz Y, Gozalbes R, Barigye SJ. Computational strategies for the discovery of biological functions of health foods, nutraceuticals, and cosmeceuticals: A review. Mol. Divers. 2021;25:1425–1438. doi: 10.1007/s11030-021-10277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain AN. Virtual screening in lead discovery and optimization. Curr. Opin. Drug Discov. Devel. 2004;7(4):396–403. [PubMed] [Google Scholar]
- 28.Das DR, Kumar D, Kumar P, Dash BP. Molecular docking and its application in search of antisickling agent from Carica papaya. J. Appl. Biol. Biotechnol. 2020;8(01):105–116. [Google Scholar]
- 29.Williams PA, Cosme J, Ward A, Angove HC, Matak Vinković D, Jhoti H. Crystal structure of human cytochrome P450 2C9 with bound warfarin. Nature. 2003;424(6947):464–468. doi: 10.1038/nature01862. [DOI] [PubMed] [Google Scholar]
- 30.Sousa SF, Fernandes PA, Ramos MJ. Protein-ligand docking: Current status and future challenges. Proteins Struct. Funct. Bioinform. 2006;65(1):15–26. doi: 10.1002/prot.21082. [DOI] [PubMed] [Google Scholar]
- 31.Brooijmans N, Kuntz ID. Molecular recognition and docking algorithms. Annu. Rev. Biophys. Biomol. Struct. 2003;32:335–373. doi: 10.1146/annurev.biophys.32.110601.142532. [DOI] [PubMed] [Google Scholar]
- 32.Halperin I, Ma B, Wolfson H, Nussinov R. Principles of docking: An overview of search algorithms and a guide to scoring functions. Proteins. 2002;47:409–443. doi: 10.1002/prot.10115. [DOI] [PubMed] [Google Scholar]
- 33.Burnett RM, Taylor JS. DARWIN: A program for docking flexible molecules. Proteins. 2000;41:173–191. [PubMed] [Google Scholar]
- 34.Li Y, Zhang X, Cao D. The role of shape complementarity in the protein-protein interactions. Sci. Replication. 2013;3:3271. doi: 10.1038/srep03271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hari KV, Bhaskar D. A novel volumetric criterion for optimal shape matching of surfaces for protein-protein docking. J. Comput. Des. Eng. 2018;5(2):180–190. [Google Scholar]
- 36.Gohlke H, Klebe G. Approaches to the description and prediction of the binding affinity of small-molecule ligands to macromolecular receptors. Angew. Chem. Int. Ed. 2002;41:2644–2676. doi: 10.1002/1521-3773(20020802)41:15<2644::AID-ANIE2644>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Noureldeen AFH, Aziz SW, Shouman SA, Mohamed MM, Attia YM, Ramadan RM, Elhady MM. Molecular design, spectroscopic, DFT, pharmacological, and molecular docking studies of novel ruthenium(III)–Schiff base complex: An inhibitor of progression in HepG2 cells. Int. J. Environ. Res. Public Health. 2022;19:13624. doi: 10.3390/ijerph192013624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing D, Yi L, Yuan-Ling X, Shi-Meng Ai, Jing L, Peng S, Xing-Lai J, Shu-Qun L. Insights into protein-ligand interactions: Mechanisms, models, and methods. Int. J. Mol. Sci. 2016;17(2):144. doi: 10.3390/ijms17020144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venhorst J, ter Laak AM, Commandeur JN, Funae Y, Hiroi T, Vermeulen NP. Homology modeling of rat and human cytochrome P450 2D (CYP2D) isoforms and computational rationalization of experimental ligand-binding specificities. J. Med. Chem. 2003;46(1):74–86. doi: 10.1021/jm0209578. [DOI] [PubMed] [Google Scholar]
- 40.De Azevedo J, Filgueira W. MolDock applied to structure-based virtual screening. Curr. Drug Targets. 2010;11(3):327–334. doi: 10.2174/138945010790711941. [DOI] [PubMed] [Google Scholar]
- 41.Aja PM, Agu PC, Ezeh EM, Awoke JN, Ogwoni HA, Deusdedit T, Ekpono EU, Igwenyi IO, Alum EU, Ugwuja EI, Ibiam AU, Afiukwa CA, Adegboyega AE. Prospect into therapeutic potentials of Moringa oleifera phytocompounds against cancer upsurge: De novo synthesis of test compounds, molecular docking, and ADMET studies. Bull. Natl. Res. Cent. 2021;45:99. [Google Scholar]
- 42.Chaudhary KK, Mishra N. A review on molecular docking: Novel tool for drug discovery. JSM Chem. 2016;4(3):1029. [Google Scholar]
- 43.Pujadas G, Vaque M, Ardevol A, Blade C, Salvado MJ, Blay M. Protein-ligand docking: A review of recent advances and future perspectives. Curr. Pharm. Anal. 2008;4(1):1–9. [Google Scholar]
- 44.Torres PHM, Sodero ACR, Jofily P, Silva-Jr FP. Key topics in molecular docking for drug design. Int. J. Mol. Sci. 2019;20(18):4574. doi: 10.3390/ijms20184574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaba M, Gaba P, Singh S, Gupta GD. An overview of molecular docking. Int. J. Drug Dev. Res. 2010;2(2):219–231. [Google Scholar]
- 46.Eweas AF, Maghrabi IA, Namarneh AI. Advances in molecular modeling and docking as a tool for modern drug discovery. Deriv. Pharm. Chem. 2014;6:211–228. [Google Scholar]
- 47.Suresh PS, Kumar A, Kumar R, Singh VP. An in silico [correction of insilico] approach to bioremediation: Laccase as a case study. J. Mol. Graph Model. 2008;26(5):845.9. doi: 10.1016/j.jmgm.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Dhanik A, McMurray JS, Kavraki LE. DINC: A new AutoDock-based protocol for docking large ligands. BMC Struct. Biol. 2013;13(1):S11. doi: 10.1186/1472-6807-13-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferreira L, dos Santos R, Oliva G, Andricopulo A. Molecular docking and structure-based drug design strategies. Molecules. 2015;20(7):13384–133421. doi: 10.3390/molecules200713384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalra EK. Nutraceutical–definition and introduction. AAPS Pharm. Sci. 2003;5(3):E25. doi: 10.1208/ps050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andlauer W, Fürst P. Nutraceuticals: A piece of history, present status, and outlook. Food Res. Int. 2002;35(2–3):171–176. [Google Scholar]
- 52.Wildman R, Kelley M. Handbook of nutraceuticals and functional foods. In: Wildman R, editor. Nutraceuticals and Functional Foods. Taylor & Francis; 2007. pp. 1–9. [Google Scholar]
- 53.Raj KK, Rajesh KK, Narendra V, Sarang J, Ramsaneh R, Anil KS. Nutraceutical and functional food as future food: A review. Pharm. Lett. 2010;2(1):106–116. [Google Scholar]
- 54.Whitman M. Understanding the perceived need for complementary and alternative nutraceuticals: Lifestyle issues. Clin. J. Oncol. Nurs. 2001;5(5):190–194. [PubMed] [Google Scholar]
- 55.Egbuna C, Tupas GD, editors. Functional Foods and Nutraceuticals—Bioactive Components, Formulations, and Innovations. 1. Springer Nature; 2020. [Google Scholar]
- 56.Aronson JK. Defining ‘nutraceuticals’: Neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017;83(1):8–19. doi: 10.1111/bcp.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parikh PD, Kumar V. Nutraceuticals: The link between foods and pharmaceuticals. Int. J. Pharm. Technol. Biotechnol. 2021;8(2):08–16. [Google Scholar]
- 58.Gupta S, Chauhan D, Meheka K, Sood P, Nair A. An overview of nutraceutical current scenario. J. Basic Clin. Pharm. 2010;2(3):55–62. [PMC free article] [PubMed] [Google Scholar]
- 59.Kumari M, Jain S, Singh J. Nutraceutical-medicine of future. J. Glob. Biosci. 2015;4(7):2790–2794. [Google Scholar]
- 60.da Costa J. A current look at nutraceuticals—Key concepts and future prospects. Trends Food Sci. Technol. 2017;62:68–78. [Google Scholar]
- 61.Verma G, Mishra M. A review on nutraceutical: Classification and its role in various diseases. Int. J. Pharm. Ther. 2016;7(4):152–160. [Google Scholar]
- 62.Rajasekaran A, Kalaivani M. Designer foods and their benefits: A review. J. Food Sci. Technol. 2013;50:1–16. doi: 10.1007/s13197-012-0726-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaman T, Adetunji H, Salih E. Nutraceutical: A slow transition from preventive to curative healthcare and pretition about the physicians and patient—A study of South Delhi India. Int. J. Pharm. Sci. Res. 2017;8(7):3113–3117. [Google Scholar]
- 64.Facioni MS, Raspini B, Pivari F, Dogliotti E, Cena H. Nutritional management of lactose intolerance: The importance of diet and food labeling. J. Transl. Med. 2020;18(1):260. doi: 10.1186/s12967-020-02429-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pollastro F, Minassi A, Fresu LG. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018;25(10):1160–1185. doi: 10.2174/0929867324666170810164636. [DOI] [PubMed] [Google Scholar]
- 66.Das, R. Nutraceutical from hemp (Cannabis sativa L.) and functional evaluation (2016).
- 67.Taeymans J, Clary P, Barel A. Use of food supplements as nutricosmetics in health and fitness: A review. Handb. Cosmet. Sci. Technol. 2014;14:587–596. [Google Scholar]
- 68.Pareek VD, Khuntera A. Pharmaceutical packaging current trends and future. Int. J. Pharm. Pharm. Sci. 2014;6(6):480–485. [Google Scholar]
- 69.Ojha A, Sharma A, Sihag M, Ojha S. Food packaging—materials and sustainability: A review. Agric. Rev. 2015;36(3):241–245. [Google Scholar]
- 70.Schleicher M, Cash SB, Freeman LM. Determinants of pet food purchasing decisions. Cand. Vet. J. 2019;60(6):644–650. [PMC free article] [PubMed] [Google Scholar]
- 71.Gunstone FD. Oilseeds, vegetable oils, and seed meal: An overview by commodity. Lipid Technol. 2008;20:96. [Google Scholar]
- 72.Parker J, Schellenberger AN, Roe AL, Oketch-Rabah H, Calderón AI. Therapeutic perspectives on chia seed and its oil: A review. Planta Med. 2018;84(9–10):606–612. doi: 10.1055/a-0586-4711. [DOI] [PubMed] [Google Scholar]
- 73.Vergallo C. Nutraceutical vegetable oil nanoformulations for prevention and management of diseases. Nanomaterials (Basel) 2020;10(6):1232. doi: 10.3390/nano10061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hart RJ, Doherty DA, Keelan JA, Minaee NS, Thorstensen EB, Dickinson JE, Pennell CE, Newnham JP, McLachlan R, Norman RJ, Handelsman DJ. The impact of antenatal Bisphenol A exposure on male reproductive function at 20–22 years of age. Reprod. Biomed. Online. 2018;36(3):340–347. doi: 10.1016/j.rbmo.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 75.D'Angelo S, Scafuro M, Meccariello R. BPA and nutraceuticals, simultaneous effects on endocrine functions. Endocr. Metab. Immune Disord. Drug Targets. 2019;19(5):594–604. doi: 10.2174/1871530319666190101120119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar P, Kumar N, Omer T. A review on nutraceutical “Critical supplement for building a healthy world”. World J. Pharm. Sci. 2016;5(3):579–594. [Google Scholar]
- 77.Aja PM, Agu PC, Ogwoni HA, Ekpono UE, Ezeh EM, Ani OG, Asuk AA, Awoke JN, Ale BA, Nwite FE, Ukachi OU, Orji OU, Nweke PC, Ekpono UE, Ewa GO, Igwenyi IO, Alum EU, Chukwu DC, Maduagwuna E, Nwiziogo FC. Cucumeropsis mannii (African white melon) seed oil mitigates dysregulation of redox homeostasis, inflammatory response, and apoptosis in testis of Bisphenol A exposed male rats. Niger. J. Biochem. Mol. Biol. 2022;37(4):272–281. [Google Scholar]
- 78.Agu PC, Aja PM, Ugbala EE, Ogwoni HA, Ezeh EM, Oscar-Amobi PC, Asuk Atamgba A, Ani OG, Awoke JN, Nwite FE, Kachi OU, Orji OU, Nweke PC, Ugbala EE, Ewa GO, Igwenyi IO, Egwu CO, Alum EU, Chukwu DC, Famurewa AC. Cucumeropsis mannii seed oil (CMSO) attenuates alterations in testicular biochemistry and histology against Bisphenol A-induced toxicity in male Wister albino rats. Heliyon. 2022;8(3):e09162. doi: 10.1016/j.heliyon.2022.e09162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kumar V, Kancharla S, Jena MK. In silico virtual screening-based study of nutraceuticals predicts the therapeutic potentials of folic acid and its derivatives against COVID-19. Virus Dis. 2021;32(1):29–37. doi: 10.1007/s13337-020-00643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chandra A, Gurjar V, Qamar I, Singh N. Identification of potential inhibitors of SARS-COV-2 endoribonuclease (EndoU) from FDA approved drugs: A drug repurposing approach to finding therapeutics for COVID-19. J. Biomol. Struct. Dyn. 2021;39(12):4201–4211. doi: 10.1080/07391102.2020.1775127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kirschner KM. Reduce, replace, refine-Animal experiments. Acta Physiol. 2021;223(3):e13726. doi: 10.1111/apha.13726. [DOI] [PubMed] [Google Scholar]
- 82.Teimouri M, Junaid M, Saleem S, Khan A, Ali A. In-vitro analysis of selective nutraceuticals binding to human transcription factors through computer-aided molecular docking predictions. Bioinformation. 2016;12(7):354–358. doi: 10.6026/97320630012354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sliwoski G, Kothiwale S, Meiler J, Lowe EW., Jr Computational methods in drug discovery. Pharmacol. Rev. 2013;66(1):334–395. doi: 10.1124/pr.112.007336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ohmatsu K, Ooi T. Cationic organic catalysts or ligands in concert with metal catalysts. Top. Curr. Chem. (Cham) 2019;377(6):31. doi: 10.1007/s41061-019-0256-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhao J. Nutraceuticals, nutritional therapy, phytonutrients, and phytotherapy for improvement of human health: A perspective on plant biotechnology application. Recent Path Biotechnol. 2008;1:75–97. doi: 10.2174/187220807779813893. [DOI] [PubMed] [Google Scholar]
- 86.Singh S, Malik BK, Sharma DK. Molecular drug targets and structure-based drug design: A holistic approach. Bioinformation. 2006;1(8):314–320. doi: 10.6026/97320630001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ecker DJ, Griffey RH. RNA as a small-molecule drug target: Doubling the value of genomics. Drug Discov. Today. 1999;4(9):420–429. doi: 10.1016/s1359-6446(99)01389-6. [DOI] [PubMed] [Google Scholar]
- 88.Fu WJ, Stromberg AJ, Viele K, Carroll RJ, Wu G. Statistics and bioinformatics in nutritional sciences: Analysis of complex data in the era of systems biology. J. Nutr. Biochem. 2010;21:561–572. doi: 10.1016/j.jnutbio.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pandita D, Pandita A. Omics technology for the promotion of nutraceuticals and functional foods. Front. Physiol. 2022;13:817247. doi: 10.3389/fphys.2022.817247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gagna CE, Lambert WC. Novel drug discovery and molecular biological methods, via DNA, RNA, and protein changes using structure-function transitions: Transitional structural chemogenomics, transitional structural chemo-proteomics, and novel multi-stranded nucleic acid microarray. Med. Hypotheses. 2006;67(5):1099–10114. doi: 10.1016/j.mehy.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 91.Majumder K, Wu J. A new approach for identification of novel antihypertensive peptides from egg proteins by QSAR and bioinformatics. Food Res. Int. 2010;43:1371–1378. [Google Scholar]
- 92.Fernandez A, O'Leary C, O'Byrne KJ, Burgess J, Richard DJ, Suraweera A. Epigenetic mechanisms in DNA double strand break repair: A clinical review. Front. Mol. Biosci. 2021;8:685440. doi: 10.3389/fmolb.2021.685440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Boran AD, Iyengar R. Systems approach polypharmacology and drug discovery. Curr. Opin. Drug Discov. Devel. 2010;13(3):297–309. [PMC free article] [PubMed] [Google Scholar]
- 94.Fernández-Ballester G, Fernández-Carvajal A, González-Ros JM, Ferrer-Montiel A. Ionic channels as targets for drug design: a review on computational methods. Pharmaceutics. 2011;3(4):932–953. doi: 10.3390/pharmaceutics3040932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Calvani M, Pasha A, Favre C. Nutraceutical boom in cancer: Inside the labyrinth of reactive oxygen species. Int. J. Mol. Sci. 2010;21(6):1936. doi: 10.3390/ijms21061936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arora D, Jaglan S. Nanocarriers based delivery of nutraceuticals for cancer prevention and treatment: A review of recent research developments. Trends Food Sci. Technol. 2016;54:114–126. [Google Scholar]
- 97.Tripathi YB, Tripathi P, Arjmandi BH. Nutraceuticals and cancer management. Front. Biosci. 2005;10:1607–1618. doi: 10.2741/1644. [DOI] [PubMed] [Google Scholar]
- 98.Awadelkareem AM, Al-Shammari E, Elkhalifa AEO, Adnan M, Siddiqui AJ, Snoussi M, Khan MI, Azad ZRAA, Patel M, Ashraf SA. Phytochemical and in silico ADME/Tox analysis of Eruca sativa extract with antioxidant, antibacterial and anticancer potential against Caco-2 and HCT-116 colorectal carcinoma cell lines. Molecules. 2022;27:1409. doi: 10.3390/molecules27041409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jimi E, Fei H, Nakatomi C. NF-κB signaling regulates physiological and pathological chondrogenesis. Int. J. Mol. Sci. 2019;20(24):6275. doi: 10.3390/ijms20246275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Das L, Vinayak M. Long-term effect of curcumin in restoration of tumor suppressor p53 and phase-II antioxidant enzymes via activation of Nrf2 signaling and modulation of inflammation in the prevention of cancer. PLoS ONE. 2015;10(4):e0124000. doi: 10.1371/journal.pone.0124000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: A review of clinical trials. npj Precis. Oncol. 2017;1:35. doi: 10.1038/s41698-017-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Melzer J, Brignoli R, Saller R. Komplementärmedizin, phytotherapie und sojaisoflavone als phytoöstrogene [complementary medicine: Phytotherapy and soyaisoflavones as phytoestrogens] Zentralbl. Gynakol. 2004;126(3):138–147. doi: 10.1055/s-2004-822694. [DOI] [PubMed] [Google Scholar]
- 103.Zhou J, Farah BL, Sinha RA, Wu Y, Singh BK, Bay BH, Yang CS, Yen PM. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE. 2014;9(1):e87161. doi: 10.1371/journal.pone.0087161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nitzan-Kaluski D, Stern F, Kachel J, Leventhal A. Soy and phytoestrogens consumption and health policy hesitation or certitude. Harefuah. 2002;141(1):61–66. [PubMed] [Google Scholar]
- 105.Dwyer J. Overview: dietary approaches for reducing cardiovascular disease risks. J. Nutrition. 1995;125:656S–665S. doi: 10.1093/jn/125.suppl_3.656S. [DOI] [PubMed] [Google Scholar]
- 106.Cencic A, Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients. 2010;2(6):611–625. doi: 10.3390/nu2060611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martino A, Goanta E, Magnano R, Bencivenga S, Pezzi L, Acitelli A, Piccarozzi A, Penco M, Calo L. Diets and heart disease. Myths and reality. J. Nutr. Health Food Sci. 2016;4(2):1–10. [Google Scholar]
- 108.Ashraf SA, Elkhalifa AEO, Siddiqui AJ, Patel M, Awadelkareem AM, Snoussi M, Ashraf MS, Adnan M, Hadi S. Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic medicinal fungus Cordyceps with its nutraceutical and therapeutic potential. Molecules. 2020;25:2735. doi: 10.3390/molecules25122735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.American Heart Association News. Consuming about 3 grams of omega-3 fatty acids a day may lower blood pressure. Available from https://www.heart.org/en/news/2022/06/01/consuming-about-3-grams-of-omega-3-fatty-acids-a-day-may-lower-blood-pressure. (2022).
- 110.Jain AP, Aggarwal KK, Zhang PY. Omega-3 fatty acids and cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2015;19(3):441–445. [PubMed] [Google Scholar]
- 111.Wang CZ, Mehendale SR, Yuan CS. Commonly used antioxidant botanicals: Active constituents and their potential role in cardiovascular illness. Am. J. Chin. Med. 2007;35:543–558. doi: 10.1142/S0192415X07005053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lampe JW. Health effects of vegetables and fruit: Assessing mechanisms of action in human experimental studies. Am. J. Clin. Nutr. 1999;70:475S–490S. doi: 10.1093/ajcn/70.3.475s. [DOI] [PubMed] [Google Scholar]
- 113.Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y. Anti-inflammatory action of green tea. Antiinflamm. Antiallergy Agents Med. Chem. 2016;15(2):74–90. doi: 10.2174/1871523015666160915154443. [DOI] [PubMed] [Google Scholar]
- 114.National Cholesterol Education Program (NCEP) Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 115.Ahmad A, Ahmad V, Zamzami MA, Chaudhary H, Baothman OA, Hosawi S, Kashif M, Akhtar MS, Khan MJ. Introduction and classification of natural polyphenols. In: Tabrez S, Imran Khan M, editors. Polyphenols-Based Nanotherapeutics for Cancer Management. Singapore: Springer; 2021. [Google Scholar]
- 116.Riccioni G, Mancini B, Di Ilio E, Bucciarelli T, D'Orazio N. Protective effect of lycopene in cardiovascular disease. Eur. Rev. Med. Pharmacol. Sci. 2008;12:183–190. [PubMed] [Google Scholar]
- 117.Chiu H-F, Venkatakrishnan F, Wang C-K. The role of nutraceuticals as a complementary therapy against various neurodegenerative diseases: A mini-review. J. Tradit. Complement. Med. 2020;10(5):434–439. doi: 10.1016/j.jtcme.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ashraf SA, Elkhalifa AEO, Mehmood K, Adnan M, Khan MA, Eltoum NE, Krishnan A, Baig MS. Multi-targeted molecular docking, pharmacokinetics, and drug-likeness evaluation of okra-derived ligand abscisic acid targeting signaling proteins involved in the development of diabetes. Molecules. 2021;26:5957. doi: 10.3390/molecules26195957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Agu PC, Aja PM, Ezeh EM, Ekpono UE. Neuroprotective potentials of Ageratum conyzoides phyto-constituents via inhibition of monoamine oxidase: An in-silico study. Niger. J. Biochem. Mol. Biol. 2023;38(1):43–55. [Google Scholar]
- 120.Limanaqi F, Biagioni F, Busceti CL. Phytochemicals bridging autophagy induction and alpha-synuclein degradation in parkinsonism. Int. J. Mol. Sci. 2019;20:3274. doi: 10.3390/ijms20133274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Teter B, Morihara T, Lim GP. Curcumin restores innate immune Alzheimer’s disease risk gene expression to ameliorate Alzheimer’s pathogenesis. Neurobiol. Disord. 2019;127:432–448. doi: 10.1016/j.nbd.2019.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Morowitz MJ, Carlisle EM, Alverdy JC. Contributions of intestinal bacteria to nutrition and metabolism in the critically ill. Surg. Clin. N. Am. 2011;91(4):771–785. doi: 10.1016/j.suc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nissen L, Chingwaru W, Sgorbati B, Biavati B, Cencic A. Gut health-promoting activity of new putative probiotic/protective Lactobacillus spp. strains: A functional study in the small intestinal cell model. Int. J. Foodst. Microbiol. 2009;135:288–294. doi: 10.1016/j.ijfoodmicro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 124.Siddeeg A, Afzaal M, Saeed F, Ali R, Shah YA, Shehzadi U, Ateeq H, Waris N, Hussain M, Raza MA, Al-Farga A. Recent updates and perspectives of fermented healthy superfood sauerkraut: A review. Int. J. Food Prop. 2022;25(1):2320–2331. [Google Scholar]
- 125.Abdulhussein AJ, Mtasher AS, Mutleg S. Probiotics and prebiotics. Int. J. Curr. Res. 2018;10(11):75341–75352. [Google Scholar]
- 126.Maragkoudakis PA, Chingwaru W, Gradisnik L, Tsakalidou E, Cencic A. Lactic acid bacteria efficiently protect human and animal intestinal epithelial and immune cells from enteric virus infection. Int. J. Food Microbiol. 2010;141(1):S91–S97. doi: 10.1016/j.ijfoodmicro.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rijkers GT, Bengmark S, Enck P, Haller D, Herz U, Kalliomaki M, Kudo S, Lenoir-Wijnkoop I, Mercenier A, Myllyluoma E, Rabot S, Rafter J, Szajewska H, Watzl B, Wells J, Wolvers D, Antoine JM. Guidance for substantiating the evidence for beneficial effects of probiotics: Current status and recommendations for future research. J. Nutr. 2010;140:671S–676S. doi: 10.3945/jn.109.113779. [DOI] [PubMed] [Google Scholar]
- 128.Das L, Bhaumik E, Raychaudhuri U, Chakraborty R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012;49(2):173–183. doi: 10.1007/s13197-011-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Watzl B, Girrbach S, Roller M. Inulin, oligofructose and immunomodulation. Br. J. Nutr. 2005;93(1):S49–S55. doi: 10.1079/bjn20041357. [DOI] [PubMed] [Google Scholar]
- 130.Bunn HF. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997;337(11):762–769. doi: 10.1056/NEJM199709113371107. [DOI] [PubMed] [Google Scholar]
- 131.Migotsky M, Beestrum M, Badawy SM. Recent advances in sickle-cell disease therapies: A review of voxelotor, crizanlizumab, and L-glutamine. Pharmacy (Basel) 2022;10(5):123. doi: 10.3390/pharmacy10050123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Al Balushi H, Hannemann A, Rees D, Brewin J, Gibson JS. The effect of Antio Morowitz xidants on the properties of red blood cells from patients with sickle cell anemia. Front. Physiol. 2019;10:976. doi: 10.3389/fphys.2019.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Imaga NA. Phytomedicines and nutraceuticals: Alternative therapeutics for sickle cell anemia. Sci. World J. 2013;13:269659. doi: 10.1155/2013/269659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kotue TC. Functional foods and nutraceuticals in the primary prevention of sickle cell disease crises. Indian J. Nutr. 2018;5(1):186. [Google Scholar]
- 135.Telen MJ, Malik P, Vercellotti GM. Therapeutic strategies for sickle cell disease: Towards a multi-agent approach. Natl. Rev. Drug Discov. 2019;18(2):139–158. doi: 10.1038/s41573-018-0003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vona R, Sposi NM, Mattia L, Gambardella L, Straface E, Pietraforte D. Sickle cell disease: Role of oxidative stress and antioxidant therapy. Antioxidants (Basel) 2021;10(2):296. doi: 10.3390/antiox10020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kaddam L, Fadl-Elmula I, Eisawi OA, Abdelrazig HA, Salih MA, Lang F, Saeed AM. Gum Arabic as a novel anti-oxidant agent in sickle cell anemia, phase II trial. BMC Hematol. 2017;17:4. doi: 10.1186/s12878-017-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salehi B, Machin L, Monzote L, Sharifi-Rad J, Ezzat MS, Salem MA, Merghany RM, El Mahdy NM, Kılıç CS, Sytar O, Sharifi-Rad M, Sharopov F, Martins N, Martorell M, Cho WC. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega. 2020;5(20):11849–11872. doi: 10.1021/acsomega.0c01818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Afanas’ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem. Pharmacol. 1989;38:1763–1769. doi: 10.1016/0006-2952(89)90410-3. [DOI] [PubMed] [Google Scholar]
- 140.Brandow AM, Liem RI. Advances in the diagnosis and treatment of sickle cell disease. J. Hematol. Oncol. 2022;15:20. doi: 10.1186/s13045-022-01237-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ali M, Thomson M, Afzal M. Garlic and onions: Their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot. Essent. Fat. Acids. 2000;6:55–73. doi: 10.1054/plef.1999.0124. [DOI] [PubMed] [Google Scholar]
- 142.Nur E, Brandjes DP, Teerlink T, Otten HM, Oude Elferink RP, Muskiet F, Evers LM, ten Cate H, Biemond BJ, Duits AJ, Schnog JJ. CURAMA study group. N-acetylcysteine reduces oxidative stress in sickle cell patients. Ann. Hematol. 2012;91(7):1097–1105. doi: 10.1007/s00277-011-1404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Akbaribazm M, Goodarzi N, Rahimi M. Female infertility and herbal medicine: An overview of the new findings. Food Sci. Nutr. 2021;9(10):5869–5882. doi: 10.1002/fsn3.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ma X, Wu L, Wang Y, Han S, El-Dalatony MM, Feng F, Tao Z, Yu L, Wang Y. Diet and human reproductive system: Insight of omics approaches. Food Sci. Nutr. 2022;10:1368–1384. doi: 10.1002/fsn3.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ahmadi S, Bashiri R, Ghadiri-Anari A, Nadjarzadeh A. Antioxidant supplements and semen parameters: An evidence-based review. Int. J. Reprod. Biomed. 2016;14(12):729–736. [PMC free article] [PubMed] [Google Scholar]
- 146.Hosen MB, Islam MR, Begum F, Kabir Y, Howlader MZH. Oxidative stress-induced sperm DNA damage, a possible reason for male infertility. Iran. J. Reprod. Med. 2015;13:525–532. [PMC free article] [PubMed] [Google Scholar]
- 147.Silvestris E, Lovero D, Palmirotta R. Nutrition and female fertility: An interdependent correlation. Front. Endocrinol. (Lausanne) 2019;10:346. doi: 10.3389/fendo.2019.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS. Polycystic ovary syndrome. Natl. Rev. Dis. Prim. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data relating to this review are available on request. Kindly contact the corresponding author to request the data from this study.