Abstract
Berberine (BBR) is an isoquinoline alkaloid that is widely distributed in the plant kingdom and is commonly found in Coptis chinensis Franch. It has low bioavailability, but it can interact with gut microbiota and affect a variety of diseases. The effects of BBR in diabetes, hyperlipidemia, atherosclerosis, liver diseases, intestinal diseases, mental disorders, autoimmune diseases, and other diseases are all thought to be related to gut microbiota. This review systematically and comprehensively summarize these interactions and their effects, and describes the changes of gut microbiota after the intervention of different doses of berberine and its potential clinical consequences, in order to provide a basis for the rational application of BBR in the future clinical treatment.
Keywords: berberine, gut microbiota, metabolic diseases, liver disease, intestinal diseases, autoimmune diseases
1. Background
Berberine (BBR) is able to be extracted from the roots and rhizomes of a variety of medicinal plants, such as Berberis kansuensis C.K. Schneid. (Berberidaceae), Coptis chinensis Franch. (Ranunculaceae), Coscinium fenestratum (Goetgh.) Colebr. (Menispermaceae), Argemone mexicana L. (Papaveraceae), and Phellodendron amurense Rupr. (Rutaceae),whose chemical composition is an isoquinoline alkaloid (1). It is used to treat a wide range of diseases, including tumor, endocrine diseases, cardiovascular diseases, neurological diseases, and digestive diseases (2). Animal and clinical studies have demonstrated that BBR promotes insulin secretion, increases insulin sensitivity, inhibits gluconeogenesis, reduces lipid accumulation, inhibits steatosis and fibrosis, has properties that reduce inflammatory responses and oxidative stress, and modulates the immune system [(1, 3, 4)]. Recent advancements in microbial sequencing technology, metabolomics technology, and sterile animal models have focused attention on the study of intestinal microecology (5). The gut microbiota plays a crucial role in the digestion and absorption of nutrients, metabolism, immune function, and disease development in the host. Maintaining the stability of the intestinal microecological environment is essential for regulating the health of the host. So it is important to keep the intestinal micro-ecological environment stable for controlling the health of the host. In recent years, there is growing evidence that BBR can reverse the composition and value of gut microbiota in non-healthy state (Tables 1, 2). Hyperlipidemia, diabetes, cancer, and inflammatory diseases suggest an key correlation between gut microbiota and BBR (6). But the target of BBR needs to be further investigated. With low oral bioavailability, it may influence gut microbiota. It is not thoroughly understood what the results of BBR for gut microbiota is and how altered flora relate to the metabolic benefits of BBR. This paper aims to review the role of gut microbiota under pathological conditions after BBR treatment in the background of fundamental studies and application of BBR in clinical, described the modulatory effects of BBR on the composition of the gut microbiota and its metabolites, and discussed the interaction between gut microbiota and BBR to provide more support for basic research and clinical trials.
TABLE 1.
Berberine regulates gut microbiota in animal models.
| Bioassay model | Dosage | Key finding | References |
| diabetic db/db mice | 136.5 mg/kg, i.g. for 11 weeks | ↑SCFAs-producing bacteria | (11) |
| diabetic rats | 200 mg/kg, i.g. 22 for weeks | ↑Butyrate-producing bacteria | (13) |
| diabetic rats | 200 mg/kg, i.g. for 6 weeks | ↑Bacteroides,Lactobacillaceae;↓ Proteobacteria, Verrucomicrobia | (14) |
| diabetic db/db mice |
BBR (210 mg/kg), oryzanol (33.6 mg/kg),vitamin B6 (7 mg/kg), for 4 weeks | ↑BSH-producing bacteria | (17) |
| diabetic rats | 100 mg/kg, p.o. for 30 days + 150 mg/kg, p.o.for 18 days | ↑Akkermansiaceae, Erysipelotrichaceae, Desulfovibrionaceae; ↓ Enterobacteriaceae, Christensenellaceae, Bifidobacteriace | (18) |
| Hyperlipidemic rats | 150 mg/kg,p.o. for 16 weeks | ↓Firmicutes | (22) |
| NAFLD rats | 200 mg/kg. i.g. for 8 weeks | ↑Bifidobacteria | (25) |
| NAFLD rats | 150 mg/kg. i.p. for 4 weeks | ↑Bacteroides;↓Faecalibacterium prausnitzii | (26) |
| NASH mice | 100 mg/kg. i.g. for 4 weeks | ↑Clostridiales, Lactobacillaceae, Bacteroidale | (27) |
| ALD mice | 10, 50, 100 mg/kg. i.g. for 33 days | ↑Akkermansia muciniphila↓Pseudoflavonifractor, Mucisirillum, Alistipes, Ruminiclostridium, Lachnoclostridium | (28) |
| AS mice | 100, 200 mg/kg, p.o. for 4 months | ↑Lachnospiraceae NK4A136group, Bacteroidales S24-7 group (unclassified), Eubacterium | (33) |
| AS mice | 50 mg/kg, i.g. for 12 weeks | ↑Verrucomicrobia;↓Firmicutes | (35) |
| AS mice | 0.5 g/L, p.o. for 14 weeks | ↑Akkermansia | (36) |
| Colitis rats | 40 mg/kg, i.g. for 7 days | ↑Bacteroides,Akkermansia | (41) |
| Colitis mice | 40 mg/kg, i.g. for 7 days | ↑Lactococcus;↓Mouse intestinal Bacteroides, Segmented filamentous bacteria,Enterobacteriaceae | (42) |
| UC mice | 40 mg/kg, i.g. for 7 days | ↑Lactic acid-producing bacteria, carbohydrate hydrolysis bacteria;↓conditional pathogenic bacteria | (43) |
| UC mice | 100 mg/kg, p.o. for 8 days | ↑Bacteroides fragilis | (44) |
| IBS rats | 200 mg/kg, p.o. for 14 days | ↑SCFAs-producing bacteria; | (47) |
| IBS rats | BA-BBR 1.715 mg, i.g. for 10 days | ↓ Bacteroidia, Deferribacteres, Verrucomicrobia, Candidatus_Saccharibacteria, Cyanobacteria | (48) |
| CRC mice | 100 mg/kg, p.o. for 10 weeks | ↑SCFAs-producing bacteria;↓f_Erysipelotrichaceae, Alistipes | (52) |
| CRC Apcmin/+ mice | 500 ppm, p.o. for 12 weeks | ↑Lachnospiraceae;↓Akkermansia | (53) |
| CRC mice | 7.5, 15 mg/kg, i.g. for 4 weeks | ↑Lactobacillus,Dubosiella;↓Bacteroides, Escherichia-Shigella,Akkermansia |
(54) |
| Anxiety rats | 100 mg/kg, i.g. for 4 weeks | ↑Bacteroides, Bifidobacterium, Lactobacillus, Akkermansia | (62) |
| EAU mice | 100 mg/kg, i.g. for 2 weeks | ↑Lactobacillus,Akkermansia,Oscillibacter, Ruminocococaceae |
(67) |
| GVHD mice | 50 mg/kg, i.g. for 25 days | ↑Actinobacteria, Bacteroidetes, Adlercreutzia, Lactobacillus, Dorea, Sutterella,Plesiomonas | (68) |
| Allograft mice | 200 mg/kg, i.g. for 3 weeks | ↓Bacillus cereus | (69) |
| CIA mice | 200 mg/kg, i.g. for 2 weeks | ↑SCFAs-producing bacteria | (70) |
| PD mice | 100, 200 mg/kg, i.g. for 24 h | Enterococcus might be an interesting genus for dopa/dopamine biosynthesis in the intestine, and BBR might promote the body dopa/dopamine levels through the bacteria in intestine. | (71) |
| Periodontitis rats | 120 mg/kg, i.g. for 7 weeks | ↑Butyrate-producing bacteria | (72) |
TABLE 2.
Changes of gut microbiota in clinical subjects regulated by berberine.
| Bioassay model | Dosage | Key finding | References |
| Hyperglycemia patients | 0.5 g, bid, p.o. for 16 weeks | ↑Blautia;↓Roseburia | (15) |
| T2DM patients | 3.6 g, bid, p.o. for 12 weeks | BBR is mediated by the inhibition of DCA biotransformation by Ruminococcus bromii | (16) |
| Hyperlipidemic patients | 0.5 g, bid, p.o. for 12 weeks | the baseline abundance of Alistipes and Blautia could effectively predict the cholesterol-decreasing efficacy of BBR | (21) |
| postprandial lipidemia patients | BBR (0.6 g per 6 pills, bid) + probiotics (4 g per 2 strips of powder, qd), p.o. for 12 weeks | Bifidobacterium breve and BBR could exert a synergistic hypolipidemic effect on postprandial lipidemia | (24) |
| Schizophrenia or bipolar disorder patients | 100–300 mg/tid, p.o. for 12 weeks | ↑Bacteroides;↓Firmicutes | (63) |
| Graves patients | 300 mg/tid, p.o. for 24 weeks | ↑Lactococcus lactis;↓Enterobacter hormaechei, Chryseobacterium indologenes | (66) |
2. Methods
Two authors searched the literature published in 2022 06 through MEDLINE (PubMed), EMBASE. Using “berberine” and “gut microbiota” as keywords, they included the literature meeting the following criteria: patients and animal models of gut microbiota were studied; studies were clinical trials or animal experiments of berberine intervention; and the primary endpoints were changes in organ function, metabolic status, and inflammatory response. Authors conducted this study in three stages: analyzing the title followed by the abstract and, finally, reading the full text in detail. They were able to retrieve 170 articles from PubMed and 286 (with 3 duplicates) from EMBASE, for a total of 124 duplicates in both databases 260 irrelevant articles were excluded by title, and 3 systematic reviews were excluded after reading the abstracts. 16 non-disease studies, and 15 low-quality literature pieces were also excluded. Finally, 35 studies were included after reading the full text. The literature had to meet the following criteria: all clinical and basic studies on diseases connected with intervention of gut microbiota through the BBR active ingredient pathway, and the language of the literature was limited to English. There were no BBR-related compounding agents involved in the study.
3. Effect of BBR on gut microbiota in different diseases
3.1. Diseases related to glycolipid metabolism
Glycolipid metabolic diseases are common chronic diseases in the clinic that have been attracting increasing attention. Approximately 1.5 billion people worldwide have metabolism-related diseases, making it a global public health issue (7).
It is important for BBR in treating these diseases by affecting the gut microbiota according to the latest research. This promotes insulin secretion, improves insulin resistance, and inhibits lipogenesis which is associated with changes in the composition of the gut microbiota and its metabolites [(1, 8, 9)].
3.1.1. Diabetes mellitus
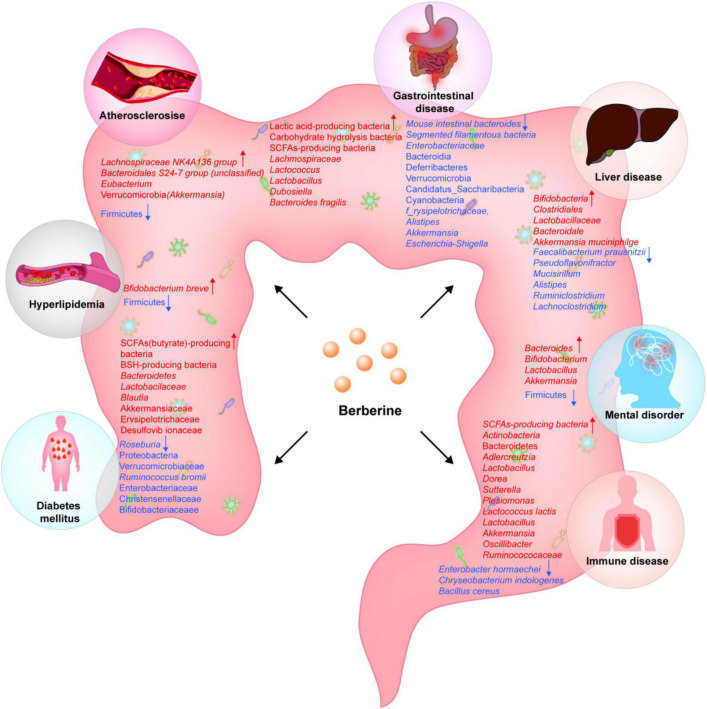
Modern pharmacological studies show the importance of gut microbiota in the developmental phases of type 2 diabetes mellitus (T2DM) similar to that of genetic-, environmental-, and dietary factors (9, 10). Berberine is intragastric in db/db mice with the dosage of 136.5 mg/kg, and the proportion of Butyricimonas, Coprococcus, and Ruminococcus bacteria producing short-chain fatty acids (SCFAs) increases (11) (Figure 1). Short chain fatty acids cause an increase in glucagon-like peptide-1 (GLP-1) secretion, enhance insulin secretion and suppress glucagon secretion to improve blood glucose levels (12). Gegen Qinlian decoction (containing BBR as the key component) and BBR (200 mg/kg, 22 weeks) alone enrich butyrate-producing bacteria, such as Faecalibacterium and Roseburia, and increase the level of SCFAs in the feces (13).
FIGURE 1.
Berberine affects the changes of gut flora in different diseases.
In a rat model of diabetes, intragastric administration of berberine at a dosage of 200 mg/kg suppressed blood glucose levels, improved glucose tolerance, and serum lipid parameters after 6 weeks. The relative abundance of Bacteroides increases in the BBR group, while the relative abundance of Proteobacteria and Verrucomicrobia phyla decreases. Probiotic Lactobacillaceae are significantly up-regulated in the BBR group and have a negative correlation with the risk of T2DM (14).
The combined application of BBR and probiotics shows that BBR regulates the structure and action of gut microbiota, and Bifidobacterium potentially enhances the hypoglycemic effect of BBR (15) (There is a significant reduction in blood glucose in the group of BBR and the group of BBR- Bifidobacterium combination after 16 weeks of treatment with oral BBR (0.5 g twice daily) at 2 h postprandial compared with that in the control. Both groups show reduced abundance of intestinal bacteria Roseburia, including Ruminococcus gnavus and Ruminococcus, while the abundance of Blautia increases. In addition, the abundance of Proteobacteria dramatically increases in the BBR group, but not in the combination group, which may be related to the use of Bifidobacterium (15). A randomized double-blind controlled trial enrolling 409 patients with T2DM receiving probiotics, BBR (0.6 g/6 capsules twice daily), probiotics + BBR, or placebo for 12 weeks after 1 week of gentamicin pretreatment shows that glycated hemoglobin is more significantly altered in the probiotics + BBR group and BBR alone group compared with that in the placebo and probiotics alone group (16). Berberine alters gut microbiota, microbiota-associated bile acid metabolism, and blood bile acid composition; it may exert hypoglycemic effects by inhibiting secondary bile acid production by Ruminococcus bromii (16).
The combination of BBR with other drugs is important in T2DM. The use of BBR (210 mg/kg), oryzanol (33.6 mg/kg), and vitamin B6 (7 mg/kg) for 4 weeks restores the relative abundance of Bacteroidaceae, Clostridiaceae in db/db mice. Bacteroidaceae and Clostridiaceae are considered to be bacteria that produce bile acid hydrolase, and the combination improves hyperglycemia. This effect may be connected to increased gut microbiota-mediated deoxycholic acid (DCA) production resulting in the upregulation of colonic TGR5 expression and glucagon-like peptide secretion, and improved glucose, lipid, and energy metabolism in db/db mice (17).
In addition, BBR (100 mg/kg, 150 mg/kg) and combined treatment with stachyose for 48 days can significantly improve glucose metabolism and reshape gut microbiota in Zucker diabetic fatty rats. Both BBR and BBR + stachyose have increased the abundance of Akkermansiaceae, Erysipelotrichaceae and Desulfovibrionaceae, and decreased the abundance of Enterobacteriaceae, Christensenellaceae, and Bifidobacteriaceae (18).
3.1.2. Hyperlipidemia
Hyperlipidemia is a condition in which the level of fat in the blood [mainly total cholesterol (TC), triglycerides (TG) and low-density lipoprotein cholesterol (LDL-C)] is abnormally high (19). The prevalence of hyperlipidemia is rapidly increasing due to improvements in lifestyle and the popularity of high-calorie diets. It can suffer from an increased risk of causing various cardiovascular diseases (20). Various studies show that BBR has a good lipid-lowering activity; it significantly reduces TC, TG, and LDL-C concentrations and enhances serum high-density lipoprotein cholesterol (HDL-C)concentrations (1).
Berberine reduces blood lipids after 12 weeks of oral treatment (0.5 g, twice daily) in patients with hyperlipidemia. However, there were significant individual differences. BBR lowers cholesterol by regulating the gut microbiota. Baseline levels of Alistipes and Blautia accurately predict the anti-cholesterolemia effectiveness of BBR in subsequent treatment; the cholesterol-lowering effect of BBR is diminished in Blautia-deficient mice (21). Berberine also alters the intestinal microbial structure of rats on a high-fat diet., Species diversity and flora richness were markedly reduced after 4 months of intervention with BBR (150 mg/kg, orally). The abundance of Christensenellaceae, Dehalobacteriaceae, Erysipelotrichaceae, and Peptococcaceae (all Firmicutes) was significantly reduced (22). In addition, clinical studies have shown that nitroreductase (NR) from intestinal bacteria plays an important role in promoting intestinal absorption of BBR. Fecal NR activity is higher in patients with hyperlipidemia than in healthy individuals; blood BBR and fecal NR activity are positively correlated (23).
Berberine combined with probiotics improves postprandial hyperlipidemia in patients with T2DM. The effect of combined probiotic (with nine strains) and BBR treatment on postprandial lipids was assessed in 365 T2DM subjects (24). The combination of probiotics + BBR improves postprandial lipids (reduced TC, LDL-C, and multiple lipid metabolites) in patients compared with the treatment alone; this effect is associated with fecal Bifidobacterium breve enrichment; the presence of four fadD genes encoding long-chain acyl-CoA synthetase in Bifidobacterium breve strains. Berberine up-regulates fadD gene expression in vitro and further reduces the free fatty acid level in the culture medium. This may be the basis for probiotics + BBR to reduce the intestinal lipid uptake and blood cholesterol level of the host (24).
3.1.3. Viscera injury related to glucose and lipid metabolism
The improvement of glycolipid metabolism also has a certain therapeutic effect on organ function impairment secondary to metabolic diseases. BBR can obviously promote the above metabolic processes, so it can ameliorate the organ function damage caused by metabolic related diseases to a certain extent. Ecological dysbiosis of gut microbiota is thought to underlie non-alcoholic steatohepatitis (NASH). The relative levels of Bifidobacteria and the proportion of Bacteroidetes: Firmicutes are restored in HFD-fed treated mice receiving BBR (200 mg/kg/d) by gavage for 8 weeks (25). Four weeks of intraperitoneal administration of BBR (150 mg/kg/d) alleviates HFD-induced hepatic steatosis and histopathological changes in the intestinal mucosa. The abundance of gut bacteria Faecalibacterium prausnitzii decreases and the abundance of Bacteroides increases (26). Also, BBR treatment of mice (100 mg/kg/d by gavage) for 4 weeks increases the relative abundance of Clostridiales, Lactobacillaceae, and Bacteroidales, which mediates the activation of intestinal Farnesoid X receptor to alleviate NASH (27).
Berberine alters the gut microbiota environment in mice with alcoholic liver disease treated with BBR (10, 50, 100 mg/kg) by gavage, decreases the abundance of Pseudoflavonifractor, Mucisirillum, Alistipes, Ruminiclostridium, and Lachnoclostridium and increases the abundance of Akkermansia muciniphila. Among them, Akkermansia muciniphila is essential in maintenance of the gut barrier integrity and may induce the activation of specific cell subpopulations with immunosuppressive functions, thereby alleviating alcoholic liver injury (28).
Diabetes and hypercholesterolemia are high risk factors for atherosclerosis. Atherosclerosis (AS) is often the leading cause of cardiovascular disease due to the growth of connective tissue, deposition of intracellular and intracellular cholesterol, fatty acids, and calcium carbonate, accumulation of collagen and proteoglycan, hardening and thickening of artery walls, thinning of arteries, and loss of elasticity of the entire artery (29). In recent years, the incidence has been on the rise and is difficult to treat [(30, 31)]. BBR improves glycolipid metabolism through the regulation of gut microbiota, and at the same time improves the progression of atherosclerosis. Current studies have shown that gut microbiota is an influential factor in the development and deterioration of AS (32). BBR can change the intestinal microbial composition of mice (Lachnospiraceae NK4A136 group, Bacteroidales S24-7 group) (unclassified), increased abundance of Eubacterium), and cutC/cntA gene abundance associated with trimethylamine (TMA) production; Under anaerobic conditions in vitro, BBR inhibits the formation of d9-TMA in a dose-dependent manner, and in mice, BBR can significantly reduce the elevated level of TMA-producing bacteria (33). Trimethylamine oxide (TMAO) is an independent risk factor and initiator of Atherosclerosis (34). Similarly, other studies show that BBR administration (50 mg/kg, twice weekly, by gavage) reduces the expression of TMAO and inflammatory cytokines with an increased abundance of Verrucomicrobia and decreased abundance of Firmicutes in BBR-treated mice (35). Akkermansia is an important bacterium in the Verrucomicrobia phylum; its abundance increases after 14 weeks of BBR (0.5 g/L) administration in the drinking water of ApoE–/– mice. Meanwhile, BBR attenuates high-fat diet (HFD)-induced metabolic endotoxemia and reduces arterial and intestinal expression levels of inflammatory cytokines and chemokines. BBR attenuates metabolic endotoxemia caused by a high-fat diet (HFD) and reduces the expression of inflammatory and chemokines in the arteries and intestines, Anti-AS and metabolic management effects of BBR may be associated with increased abundance of Akkermansia (36). In conclusion, regulation of gut microbiota by BBR contributes to anti-AS, and BBR can be considered as one of the effective drugs for the treatment of AS.
3.2. Gastrointestinal disease
3.2.1. Intestinal inflammatory disease
Inflammatory bowel disease (IBD) is a chronic inflammatory disease of the intestine of unknown origin, and its pathogenesis includes host genetics and immune response, gut microbiota, and environmental stimulation (37). Dysbiosis of gut microbiota is associated with IBD (38–40). Berberine (40 mg/kg for 7 days) alleviates dysbiosis in rats with dextran sodium sulfate (DSS)-induced colitis and significantly upregulates Bacteroides and Akkermansia; both animal and Caco-2 cell models show that BBR regulates gut microbiota by tryptophan metabolism and activation of the tryptophan receptor (AhR) pathway to improve the damaged intestinal barrier (41). Berberine may also prevent and treat ulcerative colitis by regulating intestinal microecology and protecting the intestinal mucosal barrier (UC) Berberine restores DSS-induced colonic inflammation by modulating intestinal microbes (42). It protects the colon by reconstructing the disrupted epithelial barrier, regulating the expression of immune factors, and enhancing the expression of the Wnt/β -catenin pathway. For DSS-induced colitis, the biological barrier was repaired after 7 days of treatment with BBR (40 mg/kg/d). Berberine increases the relative level of bacteria. Also, the expression of probiotic bacteria Lactococcus is upregulated compared to the model group, but the expression of conditionally pathogenic bacteria such asmouse intestinal Bacteroides, segmented filamentous bacteria, and Enterobacteriaceae decreases. Similar studies confirm that BBR increases the expression of lactic acid producing bacteria (F. rodentium and Lactobacillus) and carbohydrate hydrolyzing bacteria (R. flavefaciens and B. pseudolongum) and decreases the expression of conditionally pathogenic bacteria (Mucispirillum, Oscillospira, B. uniformis, and Allobaculum) to regulate the gut microbiota (43). In addition, BBR is significantly related to immune homeostasis in the gut. Berberine (100 mg/kg oral) regulates the differentiation of intestinal immune cells by affecting the growth of Bacteroides fragilis to relieve DSS induced colitis after 8 days of treatment (44).
3.2.2. Irritable bowel syndrome
Irritable bowel syndrome (IBS) is a functional disorder of the intestine characterized by abdominal pain and abnormal bowel movements (45). IBS treatment focuses on a variety of causes, including changes to the gut microbiota, visceral hypersensitivity, intestinal permeability, and other factors that contribute to the disease’s pathophysiology (46). Patients with IBS have visceral hypersensitivity; this is thought to be related to the activity of spinal microglia. Berberine significantly alleviates chronic water avoidance stress-induced visceral hypersensitivity and reduces the activation of colonic mast cells and spinal microglia in rats (47). Berberine (200 mg/kg,14 days) does not directly inhibit LPS-induced microglia activation, but may inhibit it through the enrichment of SCFA-producing bacteria (Anaerostipes, Eubacterium, Lachnoclostridium, and Eisenbergiella).
Berberine has promising therapeutic effects on IBS in combination with other drugs. Berberine and baicalin (BA) form natural self-assemblies such as BA-BBR nanoparticles (BA-BBR NPs) and show synergistic effects on IBS-D. The 1:1 ratio of BA:BBR was mixed to form BBR-BA NPs and BA-BBR NPs (1.715 mg/d) followed by administration by gavage for 10 days. The relative abundance of Bacteroidia, Deferribacteres, Verrucomicrobia, Candidatus, Saccharibacteria, and Cyanobacteria was significantly remarkably higher in the IBS-D mouse model group than that in the normal group. However, BA-BBR NP treatment reduces the relative abundance of these phyla. BA-BBR NPs are most effective in treating visceral hypersensitivity and diarrhea in IBS-D model mice (48).
3.2.3. Gastrointestinal tumor
Colorectal cancer (CRC) is one of the leading causes of cancer deaths worldwide (49). The ratio of intestinal flora plays a crucial role in the development of CRC (50). Berberine improves the tumor microenvironment by regulating the disturbed gut microbiota (51).
Oral administration of BBR (100 mg/kg for 10 weeks) to CRC mice significantly alters gut microbiota composition. Berberine inhibits pathogenic species such as f_Erysipelotrichaceae and Alistipes and increases the abundance of SCFA-producing bacteria including Alloprevotella and Flavonifractor. Also, metabolic data suggest that BBR can alter fecal metabolism by regulating the metabolism of sugars, amino acids and SCFA. These fecal metabolites are the product of the combined action of the host and the intestinal flora (52). The important role of SCFA bacteria was confirmed in another study. BBR significantly attenuates CRC progression and alters the gut microbiota structure in HFD-fed Apc min/+ mice after 12 weeks of oral treatment with BBR (500 ppm). Berberine significantly inhibits the increase of Verrucomicrobia at the phylum level, inhibits Akkermansia at the genus level, and elevates the levels of SCFA-producing bacteria (Lachnospiraceae) (53). Recent studies show that BBR prevents p-azomethane (AOM)/DSS-induced CRC in mice by reducing inflammatory activation and improving intestinal flora dysbiosis. The release of inflammatory factors and cell proliferation markers is suppressed under BBR intervention (7.5 and 15 mg/kg), and key pathway proteins involved in the inflammatory process (p-STAT3 and p-JNK) and cell cycle regulatory molecules (β-catenin, c-Myc, and CylinD1) have lower expression levels, AOM/DSS stimulation results in a sharp decrease in abundance of beneficial bacteria, Lactobacillus and Dubosiella, and an increase in abundance of undesirable bacteria Bacteroides, Escherichia, Shigella, and Akkermansia. Meanwhile, the use of BBR restored the ratio of these bacteria to a relatively normal state (54). In conclusion, the anticancer effect of BBR is achieved through its regulation of the intestinal microbiota.
3.3. Liver disease
Liver disease is a life-threatening condition that includes liver fibrosis, cirrhosis, and drug-induced hepatotoxicity is one of the main reasons for mortality and morbidity all over the world. Hepatic fibrosis is a pathological marker and precursor of cirrhosis, and fibrosis occurs in relation to liver metabolism and gut microbiota homeostasis (55). It is suggested that gut microbiota can be an independent regulator of liver metabolism, affecting the fibrosis progression as well as the regression (56). Compared to normal mice, Germ-free mice show more severe signs of liver fibrosis (57). These studies suggest that dysbiosis of gut microbiota is the important driver of liver fibrosis. Berberine strengthens the endocrine capabilities of the gut microbiota, which further regulates the liver microenvironment and ameliorates fibrosis. The abundance of SCFA secreting bacteria increased due to berberine treatment (58). Short chain fatty acids are essentialin liver diseases. For example, butyrate alleviates inflammation and liver fibrosis by promoting anti-inflammatory cytokines including IL-4 and IL-10 and inhibits inflammatory genes such as TGF-β1 and IL-1α(59). In addition, BBR reduces hepatotoxicity caused by pathological or pharmacological interventions by improving the dysbiosis of gut microbiota (60).
Berberine has potential value in the treatment of liver diseases by reshaping the structure of the gut microbiota, especially by modulating the abundance of SCFA-producing bacteria (for example, Clostridium and Bacillus) and Akkermansia muciniphila.
3.4. Mental disorder
Berberine protects the central nervous system and has been shown to be effective in anti-depressant, anti-anxiety, and anti-inflammatory conditions. It reduces depressive and anxious behavior by suppressing neuroinflammation in mice under stress (61). The anxiety model of ovariectomized rats treated with BBR (100 mg/kg) for 4 weeks show significant improvement in anxious behavior and increased levels of the bacterial community metabolite equol (which has potential estrogen-like effects). These changes may be caused by an increase in beneficial bacteria such as Bacteroides, Bifidobacterium, Lactobacillus, and Akkermansia (62).
Berberine modulates gut microbiota and metabolic disturbances of patients with schizophrenia or bipolar disorder, as well as mild olanzapine-induced metabolic disturbances (63). For the patients with schizophrenia or bipolar disorder treated with olanzapine for at least 9 months, followed by 12 weeks of treatment with BBR (100–300 mg/tid), there is a remarkable decrease in the abundance of Firmicutes while a remarkable increase in the abundance of Bacteroides. Antipsychotic treatment can cause changes in the gut microbiota that induce chronic low-grade inflammation, suppress resting metabolic rates, and activate multiple signal transduction pathways, leading to metabolic dysfunction (64). Gut microbiota is promising for research of antipsychotic-induced metabolic disturbances, and BBR is a candidate for treatment.
3.5. Immune disease
Recent studies have shown that BBR has increasing importance in immune diseases. Graves’ disease is a multisystemic syndrome of autoimmune diseases (65). Berberine significantly upregulates the enterobactin synthesis and restores the thyroid function by increasing iron uptake. Methimazole alone did not affect the gut microbiota structure of patients alone, while combined treatment with BBR (0.3 g/three times a day) for 6 months significantly changes the flora structure of patients, increases the abundance of beneficial bacterium (such as Lactococcus lactis, and decreases the abundance of disease-causing bacteria (such as Enterobacter hormaechei,Chryseobacterium indologenes) (66). The gut microbiota of autoimmune uveitis mice is modified after 14 days of intragastric administration of BBR (100 mg/kg/d). Bacteria with immunoregulatory ability (such as Lactobacillus, Akkermansia, Oscillibacter, and Ruminococcosaceae) are enriched and play an important for immune homeostasis during autoimmune uveitis (67). Berberine (50 mg/kg,25 days) was used to treat acute graft-versus-host disease mice through gut microbiota remodeling (the abundance of Actinobacteria and Bacteroidetes and genus Adlercreutzia, Lactobacillus, Dorea, Sutterella and Plesiomonas were increased) and intestinal mucosal barrier protection, inhibition of TLR4 signaling pathway activation, and suppression of NLRP3 inflammatory vesicles and their cytokine release (68). In addition, another study demonstrates that BBR (200 mg/kg/d) inhibits CD8 + TCM cells by reducing the abundance of Bacillus cereus to inhibit mouse islet allograft rejection (69). Berberine (200 mg/kg/d for 14 days) reduces collagen-induced arthritis (CIA) in rats by upregulating the relative abundance of intestinal SCFA-producing bacteria (Blautia, Buttericicoccus, and Parabacteroides) and significantly increases the content and proportion of butyric acid (70).
3.6. Other diseases
Oral BBR (100, 200 mg/kg) increases the amount of dopamine secretion in the brain to improve Parkinson’s disease (PD) symptoms by enhancing tyrosine hydroxylase activity in Enterococcus and promoting levodopa production in the intestine of a PD mouse model (71). Similar clinical findings show that oral administration of BBR (0.5 g, bid) for 8 weeks in 28 patients with hyperlipidemia increases the relative abundance of blood/fecal levodopa. Meanwhile, the relative abundance of Enterococcus increases by 11%, wherein E. faecalis and E. faecium are dominant. Enterococcus may synthesize dopa/dopamine in the gut, and BBR may promote dopa/dopamine levels in vivo through intestinal bacteria. On the other hand, berberine may have the same effect (71). Berberine treatment of osteoporosis in a ovariectomy-periodontitis rat model for 7 weeks (120 mg/kg) results in a significant increase in butyric acid-producing bacteria (Blautia, norank_f_Bacteroidales_S24-7_group, and Roseburia) compared to the control group, and the intestinal barrier integrity improves. Berberine treatment attenuates IL-17A-related immune responses in rats and reduces serum levels of pro-inflammatory factors; this suggests that BBR may treat periodontal bone loss caused by estrogen deficiency by regulating the gut microbiota (72).
4. Outlook
Gut microbiota can regulate the efficiency of BBR absorption and utilization in vivo; meanwhile, the structure and function of the gut microbiota will be changed due to the intervention of BBR (9). The effect of BBR on the gut microbiota varies depending on its dose (73). Good therapeutic effects on a variety of diseases can be achieved by the BBR-gut microbiota axis multi-target drug in future studies. However, BBR can cause therapeutic diarrhea, and the treatment-emergent mild diarrhea of BBR in normal rats is likely to be caused by ecological dysbiosis of gut microbiota (74). The pharmacological action and clinical research of BBR requires further investigation. Many preclinical experiments proved the role of BBR, and some clinical experiments also show good results. However, BBR has poor bioavailability. Therefore, attempts were made to use different dosage forms, drug delivery systems, and technologies such as microcapsules, nanoparticles, and other new drug carriers to improve its bioavailability and therapeutic effect. Future work should involve more clinical experiments to explore the mechanism of BBR-mediated gut microbiota regulating various diseases and accumulate more evidence to support its early intervention as a routine treatment.
5. Conclusion
This paper mainly describes the effects of different BBR doses on various diseases by regulating gut microbiota. The multi-pharmacological effect of BBR can be explained at least in part by its regulatory role in the gut microbiota. The evidences presented in this paper shows that the different roles of BBR in diseases are related to the diversity of gut microbiota. In diabetes, BBR plays a hypoglycemic role mainly through SCFA producing bacteria and bacteria related to bile acid metabolism. In patients with hyperlipidemia, BBR alters host lipid and cholesterol levels by regulating lipid synthesis related microbiota. In AS, BBR reduces the production of TMA, TMAO, inflammatory factors and chemokines by remodeling gut microbiota, thus playing an anti-AS role. In intestinal diseases, BBR mainly maintains the intestinal mucosal barrier, regulates immune homeostasis, reduces visceral hypersensitivity, and anti-inflammatory effects by altering lactic acid producing bacteria, carbohydrate producing bacteria, and SCFA producing bacteria. In the liver, BBR mainly alleviates liver damage by regulating SCFA producing bacteria and FXR related bacteria. In mental disorders, BBR plays an anti-inflammatory and anxiety relieving role by regulating microbiota related metabolites. In immune diseases, changes in the immune regulatory microbiota and SCFA producing bacteria in the body after BBR intervention play a role in regulating immunity and anti-inflammatory effects. But the effect of BBR on gut microbiota under pathological conditions seems to be quite different due to the large individual differences in the composition of gut microbiota. Moreover, there is a genetic gap between rodents and humans. Therefore, more advanced and large-scale clinical research is required in order to investigate the impact of BBR on the regulation of the gut microbiota under pathological conditions.
Author contributions
DX and RL conceived the study and took responsibility for the integrity of the study. FY, RG, and XL joined in study design, data analysis, data interpretation, manuscript preparation, and revised the manuscript. All authors contributed to the intellectual content of the manuscript and approved the final version submitted for publication.
Acknowledgments
We thank all the staff members in participating centers to help in data collection.
Funding Statement
This work was supported by the Key Project of Sichuan Provincial Department of Science and Technology (No. 2020YFS0375).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Xu X, Yi H, Wu J, Kuang T, Zhang J, Li Q, et al. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed Pharmacother. (2021) 133:110984. [DOI] [PubMed] [Google Scholar]
- 2.Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. (2020) 14:564–82. 10.1007/s11684-019-0724-6 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Liu Y, Du X, Ma H, Yao J. The anti-cancer mechanisms of berberine: a review. Cancer Manag Res. (2020) 12:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang M, Yuan D, Liao P. Berberine improves intestinal barrier function and reduces inflammation, immunosuppression, and oxidative stress by regulating the NF-κB/MAPK signaling pathway in deoxynivalenol-challenged piglets. Environ Pollut. (2021) 289:117865. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Wei H. Establishment of an efficient germ-free animal system to support functional microbiome research. Sci China Life Sci. (2019) 62:1400–3. [DOI] [PubMed] [Google Scholar]
- 6.Habtemariam S. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res. (2020) 155:104722. 10.1016/j.phrs.2020.104722 [DOI] [PubMed] [Google Scholar]
- 7.Maifeld A, Bartolomaeus H, Löber U, Avery EG, Steckhan N, Markó L, et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat Commun. (2021) 12:1970. 10.1038/s41467-021-22097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang S, Li D, Yu Z, Li Y, Wu M. Multi-pharmacology of berberine in atherosclerosis and metabolic diseases: potential contribution of gut microbiota. Front Pharmacol. (2021) 12:709629. 10.3389/fphar.2021.709629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Zhang H, Gao Z, Zhang Q, Gu C. The mechanism of berberine alleviating metabolic disorder based on gut microbiome. Front Cell Infect Microbiol. (2022) 12:854885. 10.3389/fcimb.2022.854885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeon J, Jang J, Park K. Effects of consuming calcium-rich foods on the incidence of type 2 diabetes mellitus. Nutrients. (2018) 11:31. 10.3390/nu11010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang W, Xu JH, Yu T, Chen QK. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed Pharmacother. (2019) 118:109131. 10.1016/j.biopha.2019.109131 [DOI] [PubMed] [Google Scholar]
- 12.Ducastel S, Touche V, Trabelsi MS, Boulinguiez A, Butruille L, Nawrot M, et al. The nuclear receptor FXR inhibits Glucagon-Like Peptide-1 secretion in response to microbiota-derived Short-Chain Fatty Acids. Sci Rep. (2020) 10:174. 10.1038/s41598-019-56743-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Gao Z, Yang F, Yang Y, Chen L, Han L, et al. Antidiabetic effects of gegen qinlian decoction via the gut microbiota are attributable to its key ingredient berberine. Genomics Proteomics Bioinformatics. (2020) 18:721–36. 10.1016/j.gpb.2019.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Chen H, Yan L, Wang W, Wang D. Berberine alleviates type 2 diabetic symptoms by altering gut microbiota and reducing aromatic amino acids. Biomed Pharmacother. (2020) 131:110669. 10.1016/j.biopha.2020.110669 [DOI] [PubMed] [Google Scholar]
- 15.Ming J, Yu X, Xu X, Wang L, Ding C, Wang Z, et al. Effectiveness and safety of Bifidobacterium and berberine in human hyperglycemia and their regulatory effect on the gut microbiota: a multi-center, double-blind, randomized, parallel-controlled study. Genome Med. (2021) 13:125. 10.1186/s13073-021-00942-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Gu Y, Ren H, Wang S, Zhong H, Zhao X, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. (2020) 11:5015. 10.1038/s41467-020-18414-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Zhou W, Dang Y, Li C, Ji G, Zhang L. Berberine compounds improves hyperglycemia via microbiome mediated colonic TGR5-GLP pathway in db/db mice. Biomed Pharmacother. (2020) 132:110953. 10.1016/j.biopha.2020.110953 [DOI] [PubMed] [Google Scholar]
- 18.Li C, Cao H, Huan Y, Ji W, Liu S, Sun S, et al. Berberine combined with stachyose improves glycometabolism and gut microbiota through regulating colonic microRNA and gene expression in diabetic rats. Life Sci. (2021) 284:119928. 10.1016/j.lfs.2021.119928 [DOI] [PubMed] [Google Scholar]
- 19.Guo WL, Pan YY, Li L, Li TT, Liu B, Lv XC. Ethanol extract of Ganoderma lucidum ameliorates lipid metabolic disorders and modulates the gut microbiota composition in high-fat diet fed rats. Food Funct. (2018) 9:3419–31. 10.1039/c8fo00836a [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Li K, Zhao H, Hao Z, Yang Y, Gao M, et al. Integrated lipidomics and network pharmacology analysis to reveal the mechanisms of berberine in the treatment of hyperlipidemia. J Transl Med. (2022) 20:412. 10.1186/s12967-022-03623-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu C, Zhao Y, Zhang Y, Yang Y, Su W, Yang Y, et al. Gut microbiota specifically mediates the anti-hypercholesterolemic effect of berberine (BBR) and facilitates to predict BBR’s cholesterol-decreasing efficacy in patients. J Adv Res. (2022) 37:197–208. 10.1016/j.jare.2021.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun H, Wang N, Cang Z, Zhu C, Zhao L, Nie X, et al. Modulation of microbiota-gut-brain axis by berberine resulting in improved metabolic status in high-fat diet-fed rats. Obes Facts. (2016) 9:365–78. 10.1159/000449507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Tong Q, Shou JW, Zhao ZX, Li XY, Zhang XF, et al. Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics. (2017) 7:2443–51. 10.7150/thno.18290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang S, Ren H, Zhong H, Zhao X, Li C, Ma J, et al. Combined berberine and probiotic treatment as an effective regimen for improving postprandial hyperlipidemia in type 2 diabetes patients: a double blinded placebo controlled randomized study. Gut Microbes. (2022) 14:2003176. 10.1080/19490976.2021.2003176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao Y, Pan Q, Cai W, Shen F, Chen GY, Xu LM, et al. Modulation of gut microbiota by berberine improves steatohepatitis in high-fat diet-fed BALB/C mice. Arch Iran Med. (2016) 19:197–203. [PubMed] [Google Scholar]
- 26.Li D, Zheng J, Hu Y, Hou H, Hao S, Liu N, et al. Amelioration of intestinal barrier dysfunction by berberine in the treatment of nonalcoholic fatty liver disease in rats. Pharmacogn Mag. (2017) 13:677–82. 10.4103/pm.pm_584_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu X, Li M, Cao Y, Li C, Zhou W, Ji G, et al. Berberine alleviates non-alcoholic steatohepatitis through modulating gut microbiota mediated intestinal FXR activation. Front Pharmacol. (2021) 12:750826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Wang N, Tan HY, Chueng F, Zhang ZJ, Yuen MF, et al. Modulation of gut microbiota mediates berberine-induced expansion of immuno-suppressive cells to against alcoholic liver disease. Clin Transl Med. (2020) 10:e112. 10.1002/ctm2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. (2010) 30:1282–92. 10.1161/ATVBAHA.108.179739 [DOI] [PubMed] [Google Scholar]
- 30.Basaria S, Harman SM, Travison TG, Hodis H, Tsitouras P, Budoff M, et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: a randomized clinical trial. JAMA. (2015) 314:570–81. [DOI] [PubMed] [Google Scholar]
- 31.Libby P. The changing landscape of atherosclerosis. Nature. (2021) 592:524–33. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadmehrabi S, Tang WHW. Gut microbiome and its role in cardiovascular diseases. Curr Opin Cardiol. (2017) 32:761–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Su C, Jiang Z, Yang Y, Zhang Y, Yang M, et al. Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-N-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes. (2021) 7:36. 10.1038/s41522-021-00205-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. (2013) 368:1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y, Hu J, Geng J, Hu T, Wang B, Yan W, et al. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/- mice. Biomed Pharmacother. (2018) 107:1556–63. [DOI] [PubMed] [Google Scholar]
- 36.Zhu L, Zhang D, Zhu H, Zhu J, Weng S, Dong L, et al. Berberine treatment increases Akkermansia in the gut and improves high-fat diet-induced atherosclerosis in Apoe(-/-) mice. Atherosclerosis. (2018) 268:117–26. 10.1016/j.atherosclerosis.2017.11.023 [DOI] [PubMed] [Google Scholar]
- 37.Zuo T, Ng SC. The gut microbiota in the pathogenesis and therapeutics of inflammatory bowel disease. Front Microbiol (2018) 9:2247. 10.3389/fmicb.2018.02247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casén C, Vebø HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E, et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. (2015) 42:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putignani L, Del Chierico F, Vernocchi P, Cicala M, Cucchiara S, Dallapiccola B. Gut microbiota dysbiosis as risk and premorbid factors of IBD and IBS along the childhood-adulthood transition. Inflamm Bowel Dis. (2016) 22:487–504. 10.1097/MIB.0000000000000602 [DOI] [PubMed] [Google Scholar]
- 40.Halfvarson J, Brislawn CJ, Lamendella R, Vázquez-Baeza Y, Walters WA, Bramer LM, et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat Microbiol. (2017) 2:17004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing W, Dong S, Luo X, Liu J, Wei B, Du W, et al. Berberine improves colitis by triggering AhR activation by microbial tryptophan catabolites. Pharmacol Res. (2021) 164:105358. 10.1016/j.phrs.2020.105358 [DOI] [PubMed] [Google Scholar]
- 42.Dong Y, Fan H, Zhang Z, Jiang F, Li M, Zhou H, et al. Berberine ameliorates DSS-induced intestinal mucosal barrier dysfunction through microbiota-dependence and Wnt/β-catenin pathway. Int J Biol Sci. (2022) 18:1381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao Z, Xie Y, Zhou B, Zou B, Xiao D, Liu W, et al. Berberine ameliorates colonic damage accompanied with the modulation of dysfunctional bacteria and functions in ulcerative colitis rats. Appl Microbiol Biotechnol. (2020) 104:1737–49. 10.1007/s00253-019-10307-1 [DOI] [PubMed] [Google Scholar]
- 44.Zheng C, Wang Y, Xu Y, Zhou L, Hassan S, Xu G, et al. Berberine inhibits dendritic cells differentiation in DSS-induced colitis by promoting Bacteroides fragilis. Int Immunopharmacol. (2021) 101:108329. 10.1016/j.intimp.2021.108329 [DOI] [PubMed] [Google Scholar]
- 45.Altomare A, Di Rosa C, Imperia E, Emerenziani S, Cicala M, Guarino MPL. Diarrhea predominant-irritable bowel syndrome (IBS-D): effects of different nutritional patterns on intestinal dysbiosis and symptoms. Nutrients. (2021) 13:1506. 10.3390/nu13051506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nee J, Lembo A. Review article: current and future treatment approaches for IBS with diarrhoea (IBS-D) and IBS mixed pattern (IBS-M). Aliment Pharmacol Ther. (2021) 54:S63–74. 10.1111/apt.16625 [DOI] [PubMed] [Google Scholar]
- 47.Zhang JD, Liu J, Zhu SW, Fang Y, Wang B, Jia Q, et al. Berberine alleviates visceral hypersensitivity in rats by altering gut microbiome and suppressing spinal microglial activation. Acta Pharmacol Sin. (2021) 42:1821–33. 10.1038/s41401-020-00601-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Cui H, Li T, Qi J, Chen H, Gao F, et al. Synergistic effect of berberine-based chinese medicine assembled nanostructures on diarrhea-predominant irritable bowel syndrome in vivo. Front Pharmacol. (2020) 11:1210. 10.3389/fphar.2020.01210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. [DOI] [PubMed] [Google Scholar]
- 50.Gagnière J, Raisch J, Veziant J, Barnich N, Bonnet R, Buc E, et al. Gut microbiota imbalance and colorectal cancer. World J Gastroenterol. (2016) 22:501–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu YN, Yu TC, Zhao HJ, Sun TT, Chen HM, Chen HY, et al. Berberine may rescue Fusobacterium nucleatum-induced colorectal tumorigenesis by modulating the tumor microenvironment. Oncotarget. (2015) 6:32013–26. 10.18632/oncotarget.5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen H, Zhang F, Zhang J, Zhang X, Guo Y, Yao Q. A holistic view of berberine inhibiting intestinal carcinogenesis in conventional mice based on microbiome-metabolomics analysis. Front Immunol. (2020) 11:588079. 10.3389/fimmu.2020.588079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Guan L, Li J, Lai M, Wen X. The effects of berberine on the gut microbiota in Apc (min/+) mice fed with a high fat diet. Molecules. (2018) 23:2298. 10.3390/molecules23092298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng J, Zhao L, Yuan X, Li Y, Shi J, Zhang H, et al. Pre-Administration of berberine exerts chemopreventive effects in AOM/DSS-Induced colitis-associated carcinogenesis mice via modulating inflammation and intestinal microbiota. Nutrients. (2022) 14:726. 10.3390/nu14040726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, Wang L, Tan S, Chen Z, Wu B, Wu X. Therapeutic effects of berberine on liver fibrosis are associated with lipid metabolism and intestinal flora. Front Pharmacol. (2022) 13:814871. 10.3389/fphar.2022.814871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei X, Yan X, Zou D, Yang Z, Wang X, Liu W, et al. Abnormal fecal microbiota community and functions in patients with hepatitis B liver cirrhosis as revealed by a metagenomic approach. BMC Gastroenterol. (2013) 13:175. 10.1186/1471-230X-13-175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature. (2020) 587:555–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui HX, Hu YN, Li JW, Yuan K. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid Med Cell Longev. (2018) 2018:8930374. 10.1155/2018/8930374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ye J, Lv L, Wu W, Li Y, Shi D, Fang D, et al. Butyrate protects mice against methionine-choline-deficient diet-induced non-alcoholic steatohepatitis by improving gut barrier function, attenuating inflammation and reducing endotoxin levels. Front Microbiol. (2018) 9:1967. 10.3389/fmicb.2018.01967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qin C, Zhang H, Zhao L, Zeng M, Huang W, Fu G, et al. Microbiota transplantation reveals beneficial impact of berberine on hepatotoxicity by improving gut homeostasis. Sci China Life Sci. (2018) 61:1537–44. 10.1007/s11427-017-9202-0 [DOI] [PubMed] [Google Scholar]
- 61.Liu YM, Niu L, Wang LL, Bai L, Fang XY, Li YC, et al. Berberine attenuates depressive-like behaviors by suppressing neuro-inflammation in stressed mice. Brain Res Bull. (2017) 134:220–7. 10.1016/j.brainresbull.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 62.Fang Y, Zhang J, Zhu S, He M, Ma S, Jia Q, et al. Berberine ameliorates ovariectomy-induced anxiety-like behaviors by enrichment in equol generating gut microbiota. Pharmacol Res. (2021) 165:105439. 10.1016/j.phrs.2021.105439 [DOI] [PubMed] [Google Scholar]
- 63.Pu Z, Sun Y, Jiang H, Hou Q, Yan H, Wen H, et al. Effects of berberine on gut microbiota in patients with mild metabolic disorders induced by olanzapine. Am J Chin Med. (2021) 49:1949–63. 10.1142/S0192415X21500920 [DOI] [PubMed] [Google Scholar]
- 64.Skonieczna-Żydecka K, Łoniewski I, Misera A, Stachowska E, Maciejewska D, Marlicz W, et al. Second-generation antipsychotics and metabolism alterations: a systematic review of the role of the gut microbiome. Psychopharmacology. (2019) 236:1491–512. 10.1007/s00213-018-5102-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Q, Hou Q, Huang S, Ou Q, Huo D, Vázquez-Baeza Y, et al. Compositional and genetic alterations in Graves’ disease gut microbiome reveal specific diagnostic biomarkers. ISME J. (2021) 15:3399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han Z, Cen C, Ou Q, Pan Y, Zhang J, Huo D, et al. The potential prebiotic berberine combined with methimazole improved the therapeutic effect of graves’ disease patients through regulating the intestinal microbiome. Front Immunol. (2021) 12:826067. 10.3389/fimmu.2021.826067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Du Z, Wang Q, Huang X, Yi S, Mei S, Yuan G, et al. Effect of berberine on spleen transcriptome and gut microbiota composition in experimental autoimmune uveitis. Int Immunopharmacol. (2020) 81:106270. 10.1016/j.intimp.2020.106270 [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y, Huang J, Li T, Zhang S, Wen C, Wang L. Berberine ameliorates aGVHD by gut microbiota remodelling, TLR4 signalling suppression and colonic barrier repairment for NLRP3 inflammasome inhibition. J Cell Mol Med. (2022) 26:1060–70. 10.1111/jcmm.17158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu F, Lu W, Ye S, Liu H, Zeng Q, Huang H, et al. Berberine promotes induction of immunological tolerance to an allograft via downregulating memory CD8(+) T-Cells through altering the gut microbiota. Front Immunol. (2021) 12:646831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yue M, Tao Y, Fang Y, Lian X, Zhang Q, Xia Y, et al. The gut microbiota modulator berberine ameliorates collagen-induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply. FASEB J. (2019) 33:12311–23. 10.1096/fj.201900425RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y, Tong Q, Ma SR, Zhao ZX, Pan LB, Cong L, et al. Oral berberine improves brain dopa/dopamine levels to ameliorate Parkinson’s disease by regulating gut microbiota. Signal Transduct Target Ther. (2021) 6:77. 10.1038/s41392-020-00456-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jia X, Jia L, Mo L, Yuan S, Zheng X, He J, et al. Berberine ameliorates periodontal bone loss by regulating gut microbiota. J Dent Res. (2019) 98:107–16. 10.1177/0022034518797275 [DOI] [PubMed] [Google Scholar]
- 73.Guo Y, Zhang Y, Huang W, Selwyn FP, Klaassen CD. Dose-response effect of berberine on bile acid profile and gut microbiota in mice. BMC Complement Altern Med. (2016) 16:394. 10.1186/s12906-016-1367-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yue SJ, Liu J, Wang WX, Wang AT, Yang XY, Guan HS, et al. Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed Pharmacother. (2019) 116:109002. 10.1016/j.biopha.2019.109002 [DOI] [PubMed] [Google Scholar]