Abstract
One of the most significant medical advancements in human history is the development of vaccines. Progress in vaccine development has always been greatly influenced by scientific human innovation. The main objective of vaccine development would be to acquire sufficient evidence of vaccine effectiveness, immunogenicity, safety, and/or quality to support requests for marketing approval. Vaccines are biological products that enhance the body’s defenses against infectious diseases. From the first smallpox vaccine to the latest notable coronavirus disease 2019 nasal vaccine, India has come a long way. The development of numerous vaccines, driven by scientific innovation and advancement, combined with researcher’s knowledge, has helped to reduce the global burden of disease and mortality rates. The Drugs and Cosmetics Rules of 1945 and the New Drugs and Clinical Trials Rules of 2019 specify the requirements and guidelines for CMC (chemistry, manufacturing, and controls) for all manufactured and imported vaccines, including those against coronavirus infections. This article provides an overview of the regulation pertaining to the development process, registration, and approval procedures for vaccines, particularly in India, along with their brief history.
Keywords: Vaccine, Ethics committee, Registration, Intranasal route, Health
Introduction
Progress in vaccine development has always been greatly influenced by scientific human innovation. A biological product that has the potential to prevent the spread of certain diseases is known as a vaccine [1]. Thus, vaccination becomes the most practical and cost-effective way to combat infectious diseases and safeguard the public’s health. To produce protection against a pathogen, vaccinations aim to simulate a pathogen’s normal interaction with the human body’s immune system [2]. It has been identified as one of the best tools for preventing morbidity and mortality from endemic as well as emerging threats [3]. Many live attenuated viruses, inactive bacteria, or parasites are used in the creation of vaccines today to fight various diseases [3]. Also, vaccines have lately served as a boon for increasing child survival and lowering morbidity [4], as many childhood diseases are either eliminated or reduced in numbers with their use. According to World Health Organization (WHO), 2–3 million deaths are prevented annually owing to vaccinations, i.e., vaccinations are reported to save five lives every minute [5]. Beginning with the first smallpox vaccine created in 1796 to the latest notable coronavirus disease 2019 (COVID-19) vaccine, numerous vaccines have helped to lessen the burden of disease in addition to a decrease in death rates all across the globe [6]. Numerous new advancements and improvements in techniques such as cell culture and genome sequencing have greatly influenced the growth of vaccines including viral vector vaccine, nucleic acid vaccine, and RNA vaccine [6].
History: The Sojourn
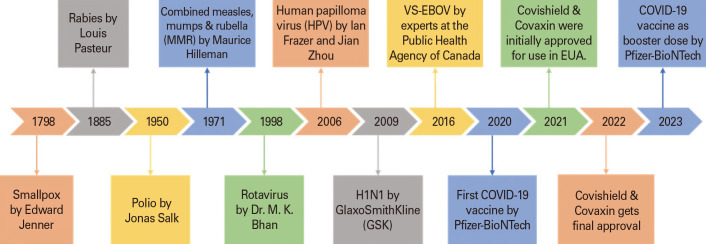
The termination of the smallpox epidemic in 1980 is considered as one of the greatest achievements in medicine. For at least 3,000 years, smallpox had plagued mankind, leading to death of 300 million people. Edward Jenner an English physician coined the word “vaccine” for the first time in the 18th century. The term “vaccine,” originated from the Latin word “Vacca,” meaning “cow,” relates to Jenner’s work, which is universally viewed as the basic principle of disease prevention by vaccination [7]. In 1796, when Jenner employed cowpox-infected human pustule material to immunize against smallpox, it marked the beginning of the genuine vaccination procedure [8]. After Edward, a Frenchman named Louis Pasteur developed the first live vaccine against rabies disease which protected several humans in 1880’s. In the 19th century when the vaccination program had taken a rise, diseases such as diphtheria, tetanus, whooping cough, tuberculosis treatment was also a big task which leads to the development of toxoid vaccines. Since 1924 in the United States, 103 million cases of childhood diseases have been prevented. As per the studies reported in the United States, toxoid vaccines have successfully helped in curing 35 million cases of measles, 40 million cases of diphtheria [5]. Patients with hepatitis B were treated with the first genetically engineered vaccine in 1986, which came out to be successful. Later on, after 15 years polio vaccine was developed as a consequence of advancements in cell culture, which lead to the entry in the golden age of vaccines. A number of significant vaccines, such as those against measles, rubella, mumps, were developed during this time. Through international vaccination campaigns, polio has almost entirely disappeared, and smallpox has been completely eliminated. VSV-EBOV was the first approved vaccine in 2019 for Ebola virus, which turned out to be a boon for saving human lives with good success rate [6,7,9]. Amongst all diseases, the recent occurrence of COVID-19 pandemic has been recognized as one of the largest in recent human history. Almost all the nations in the world were deeply affected by COVID-19 [10,11]. The outbreak of this pandemic’s unexpected worldwide crisis in 2020 served as an alert for the need of immunizations [1]. Fig. 1 shows the vital milestones in the journey of vaccine development. It became vital to develop vaccinations at an incredible speed and in abundance following the appearance of the severe acute respiratory syndrome coronavirus 2 [12] virus at the end of 2019 and realizing its potential for worldwide spread, causing the COVID-19 disease [13]. In this devastating endemic to treat people with vaccines it was made clear that those in phase III clinical trials that were found to be safe and effective may hit the market by giving them an emergency use status [14]. After which several vaccines have been approved in market worldwide, such as COVAXIN (BBV152), Sputnik V, COVISHIELD, Nuvaxovid, Spikevax, and many more. As per the updated report of emergency use listing by WHO on 16th of September 2022 there are 11 vaccines granted with Emergency Use Authorization (EUA) [13]. Amongst which the Covaxin developed by Bharat Biotech International Ltd. of Hyderabad and Covishield by Oxford-AstraZeneca have received regular full market authorization from EUA in Indian market, which will now reduce hesitancy in people related to the vaccination effects [15]. Both vaccinations have an excellent immunogenic response and, are secure and efficient [16]. Table 1 tabulates the information on various vaccines developed and manufactured in India, along with their indications in different diseases [17,18,19,20,21,22,23,24,25,26]. Beyond the person who has received the vaccination, these have also substantially helped in improving the public health. Increase in vaccinated population guarantees increase in population immunity [25,26,27]. Several social, scientific, and geopolitical events that took place at the start of the 20th century had an ongoing impact on the nation’s vaccination programs such as increase in cholera, plague in India from 1896 to 1907. Beginning in 1914 and lasting through 1918, the First World War was accompanied by the influenza pandemic, which the government prioritized due to its alleged death count of 17 million Indians [28]. This time period offers insight into how sociopolitical circumstances can negatively impact people’s health. One such milestone in the history of vaccines in India was “The 1919 Act” which was passed with the best of intentions, but due to the local government’s limited financial resources, vaccination efforts and progress were occasionally inconsistent [28]. Vaccination efforts persisted through 1939, when World War II officially began, with varying degrees of success. Even though they were still a priority for local government, vaccination programs suffered from the conflict. The vaccination rate decreased, and the highest numbers of smallpox cases in the previous 20 years were recorded in India in 1944–1945. As soon as World War II ended, attention was once again directed toward smallpox vaccination, and cases abruptly decreased [29]. India has a long history of producing vaccines, and the Haffkine Institute is one example of a facility that was prequalified to produce vaccines by the WHO before the country gained independence from the United Kingdom in 1947. By the way, Waldemar Haffkine created the first plague vaccine in the world in 1897 [30]. Biological E. Ltd., India’s first privately-funded vaccine organization, was founded in 1953 [31]; since then, a number of companies have begun to advocate for public-private partnership initiatives [32].
Fig. 1. Vital milestones in the journey of vaccines development. EUA, Emergency Use Authorization; COVID-19, coronavirus disease 2019.
Table 1. Various vaccines developed and manufactured in India, along with their indications in different diseases.
| No. | Vaccines developed & manufactured in India | Route of administration | Indication/disease | Reference |
|---|---|---|---|---|
| 1. | iNCOVACC | Intra nasal (drops) | Corona virus | [17] |
| 2. | Covaxin | Intramuscular injection in the deltoid muscles of upper arm | Corona virus | [18] |
| 3. | ZyCoV-D | Corona virus | [19] | |
| 4. | CadiFlu-S | Intramuscular injection (sterile liquid) | Seasonal influenza | [20] |
| 5. | Tetanus Toxoid (Adsorbed) I.P | Injectable suspension for intramuscular use | Tetanus | [21] |
| 6. | Rabies Vaccine (SURE RAB) | Intramuscular or intradermal route | Rabies | [22] |
| 7. | Freeze Dried Measles Vaccine (live attenuated) | Subcutaneous route | Measles | [23] |
| 8. | Boostrix | Intramuscular injection | Diphtheria, pertussis & tetanus | [24] |
| 9. | Cervarix | Intramuscular | Human papilloma virus | [24] |
| 10. | Rotavac | Oral | Rotavirus | [25] |
Merits, Challenges, and Current Perspective of Vaccination
Vaccination has good effects on productivity, well-being, and cognitive growth of individuals as well. Vaccination not only protects a person from diseases, but also provides several other benefits such as the cost cutting in medical expenses that includes the doctor consultation fees, the cost of drugs, the hospitalization charges and travel costs. These provide benefits to the population in developing countries like India where 51 million individuals are into the hands of poverty and cannot afford for treatment in private sectors due to high cost [33,34,35]. Thus, socioeconomic as well as health equity of populations is also improved by routine vaccination. The cycle of poverty, low income, and illness could be lessened if the burden of infectious disease in early infants is reduced by maintaining hygiene, providing proper nutrition, drinking sanitized clean water, and nurturing the kids with care and routine vaccination provided [36]. The medications are designed to cure or treat a disease or virus, whereas vaccines are believed to prevent from a disease. Patient driven with vaccine can last with memory for years or forever by training the immune system to identify a particular pathogen, such as a virus or bacteria stopping people from getting infected from that disease in future [37].
A vaccine is given to the entire healthy population which brings the whole community at a lower risk factor whereas, on the other hand, medicines are consumed only by people with illness or disorder and therefore do not involve the entire population at risk. The efficacy of vaccines cannot be assessed immediately after entering into market, it may take few years. Besides all these advantages, vaccines cannot protect all individuals from a disease entirely with 100% successful outcomes as its overall efficacy depends on type of vaccine being injected and health of the person receiving it. Vaccines do have several adverse effects such as redness at the site of injection, headache, and swelling might occur, children have more chances to get fever after getting vaccinated, and few people might develop with some allergic reactions. Though severe adverse complications through vaccination including, seizures, Guillian–Barre, a neurological status and fatalities harming the society are rare [38]. The recent outbreak of COVID-19 has highlighted the significance of vaccines in protecting the world population and they are generally considered to be more effective than prescription medications in preventing the spread of infectious diseases. Further, taking manufacturing into consideration, all in-process for drugs are tested and validated so that there is no batch-to-batch variation, but in case of vaccines all batches are similar though not identical. The validation and testing procedures for vaccines being short and less complicated, these have a different monitoring system for safety from medicines [39]. The global scenario shows that vaccine manufacturers are relatively less as compared to drug manufacturers owing to major challenge of high costs involved in producing these biological products. Moreover, a specialized infrastructure, including laboratories, equipment, and skilled manpower is required for developing vaccines. However, most of the Indian research institutions and companies lack these necessary infrastructures, resulting in impeding vaccine development growth. It may be however noteworthy that of late, during COVID-19, India has invested heavily in developing infrastructure for vaccine development. This enabled India to position itself as one of the global leaders in COVID-19 vaccine production [40]. There are currently initiatives underway to expand the physical infrastructure required for vaccine production in areas where it is not yet present [41]. Another greater challenge faced in India for vaccine development is limited funding and it is a time-consuming process. It requires significant financial investment for research, development, and clinical trials. During COVID-19, the quick adoption of effective vaccines was made possible owing to governments and non-profit organizations fundings, which facilitated construction and expansion of production facilities, and set up contract manufacturing and distribution networks [42]. Furthermore, the stability of vaccines is also critical as these have different stability requirements with varying temperature, and therefore require more care and attention while storing them to maintain their efficacy. They are generally stored in refrigerators or coolers with a specific temperature range. Nowadays, efforts are put in to develop intranasal vaccines just to avoid these instabilities [43]. Further, it is quite well known that pharmaceutical regulation plays an essential role for product approval and eventual product marketing. As a result, it has several intricate laws and directives framed which are dependent upon the regulatory organizations. Although vaccines are typically considered pharmaceutical products and are therefore subject to drug regulatory affairs, there are some additional considerations that must be made when regulating and controlling these products. Thus, the current review mainly focuses regulations for approval of vaccines in India.
Routes of Administration of Vaccines
The route of administration of vaccines is a vital aspect to be considered. This would ultimately govern the mechanism by which it will get absorbed into the body, while impacting its effectiveness and safety. The route of delivery must be such that it maximizes their benefits while minimizing any potential risks or adverse effects. The most common routes of vaccine administration are injection (viz., intramuscular, subcutaneous, or intradermal), oral and intranasal. Each route has its advantages and disadvantages depending on the type of vaccine and the targeted population.
Injection
It is the most common route of vaccine administration, as it allows rapid absorption and a quick immune response. However, some individuals may have a fear of needles, making this route of administration less desirable. It can be given intramuscularly, subcutaneously, or intradermally.
Intramuscular injections
Intramuscular injections are given into the muscle tissue, typically in the upper arms, thigh, or buttock. Intramuscularly injected medications enhanced patients’ ability to integrate into society and take part in social activities while enabling them healthy lives [44]. Since almost 50 years, intramuscular vaccines have played a significant role in the administration of medications in nursing practices [45]. Large muscle mass can be reached with drug delivery via intramuscular injection. In comparison to subcutaneous tissues, muscles receive more venous blood. As a result, drug absorption after intramuscular injection is quicker than in subcutaneous tissues [46]. Healthcare professional frequently administer intramuscular injections. One way to administer parental medications is by way of this injection. In general, intramuscular injections are used to administer medications that need to be absorbed by the body relatively quickly but have a reasonably long duration of action. To decrease the discomfort among the individuals while administration of intramuscular vaccine, it is suggested to administer under the muscle fascia part that is found below the fatty layer of subcutaneous tissue. According to this, there are five different locations where intramuscular injections can be given which include rectus femoris, dorsogluteal site, deltoid site, ventrogluteal site, and the vastus lateralis site. According to the researchers and the hospital staff the study conducted shows that giving intramuscular injection through the ventrogluteal site does not cause any severe injuries to the patients [47]. The intramuscular route besides being a boon to mankind has created hesitancy in the community by causing local irritation at the site, hematomas, infection at the site of administration and at times causing major gangrene at the site. Influenza vaccine, hepatitis A and B vaccine, measles–mumps–rubella vaccine, and the latest Pfizer-BioNTech COVID-19 vaccine and Moderna COVID-19 vaccine are a few vaccines administered via intramuscular route.
Subcutaneous injections
Subcutaneous injections are given under the skin, typically upper arms, and abdomen. This route of administration is typically shallower than intramuscular injections. It delivers the vaccine into fatty tissue just below the skin. Few diseases cured through this vaccine are rabies vaccine, pneumococcal vaccine, and yellow fever vaccine [48].
Intradermal injections
Intradermal injections are given into the dermis, the layer of skin just below the epidermis. This route of administration is less commonly used for vaccines, but it may be used for certain types of vaccines, such as the tuberculosis vaccine, hepatitis B vaccine, and rabies vaccine (for prophylaxis) [49].
Oral vaccines
These are convenient to administer and can be given without the need for a trained healthcare professional. However, they may require multiple doses to achieve adequate immunity. Over 60% of the marketed novel molecular drug products use the oral route of administration, making it the most preferred and patient-accepted approach [50]. An oral route of administration is said to be ideal when it fulfills three basic criteria which include: (1) Despite the temporary restrictions of the gastrointestinal tract, the dose should be maintained inside it; (2) till the protein molecule reaches the site of absorption, its integrity should be preserved; and (3) helping the dose to reach the site of action through specific interaction done by the delivery system. The oral route often leads to reduced bioavailability by 1% to 2% or less than that due to the obstruction caused by the gastrointestinal tract chemically or physically during the absorption process for the proteins [51]. In comparison to conventional injection-based formulations, the oral route is preferable due to the ease of administration and simpler manufacturing. This route develops a long-term defense against diseases by activating the humoral as well as the cellular immune activity near the mucosal and systemic sites. While the development of vaccines through the oral route, the formulation must be made in such a way that it is protected from the harsh environment of the gastrointestinal tract. Some examples include (1) the oral polio vaccine, a live attenuated vaccine, that protects against polio; (2) the rotavirus vaccine, protects against rotavirus infection; and (3) the typhoid vaccine taken to protect against typhoid fever.
Nasal vaccines
The nasal vaccines more accurately called intranasal vaccines are preferred over the parental way of administration because of ease of administration. These intranasal vaccines can be administered by self, which serves as to be the major advantage of this route. It is most effective at defending against pathogenic microbes which enter through the nasal passages, such as in the case of influenza and the most recently found COVID-19 [52]. The mucosal membrane of the respiratory tract is the most common site for the spread of transmittable viral as well as bacterial diseases [53]. A great way to administer vaccines is through the nose because it allows for lower doses and has no risk of intestinal problems or high pH. The immunization process through the nose provides both systemic and mucosal protection, unlike other routes for vaccines. Today intranasal vaccines are being preferred over intramuscular vaccines because a nasal cavity is easily accessible. Unlike other mode of administration, these intranasal vaccines are easy to store and transport. Formulations that are liquid or dry powder can be administered intranasally. Nasal devices such as a spray or a dropper are used for the administration of vaccines, these are not invasive but cause little discomfort to patients [54]. In addition to increasing protection and enforcement, saving costs, and lessening the discomfort associated with immunizations, this needle-free vaccine administration will aid in mass vaccination by making it simpler and faster to administer the shots [55]. The fear of injections related to pain or disease transmission such as human immunodeficiency virus or hepatitis B is also reduced after the birth of nasal vaccines. Intranasal vaccines are a good alternative for immunizing kids who normally dislike injections. Additionally, nasal vaccines can be administered in a single- or bi-dose, which greatly reduces the need for a sterile setting during administration and is especially advantageous for immunization in nations like the third world [56]. Recently two countries though with the highest population in the world, i.e., China and India, got approval for the use of nasal vaccines for fighting against COVID-19. Convidecia, a recombinant vaccine given via inhalation and administered as a nasal spray just gained approval from the National Medical Products Administration of China [57]. The iNCOVACC recombinant vaccine, which is administered intranasally as a nasal drop, has recently received approval under Restricted Use in Emergency Situation in India from Bharat Biotech International [58]. The development and approval of these nasal vaccines against COVID-19 have turned out to be a global game changer [59].
Vaccine Approval Process in India
About the regulatory body
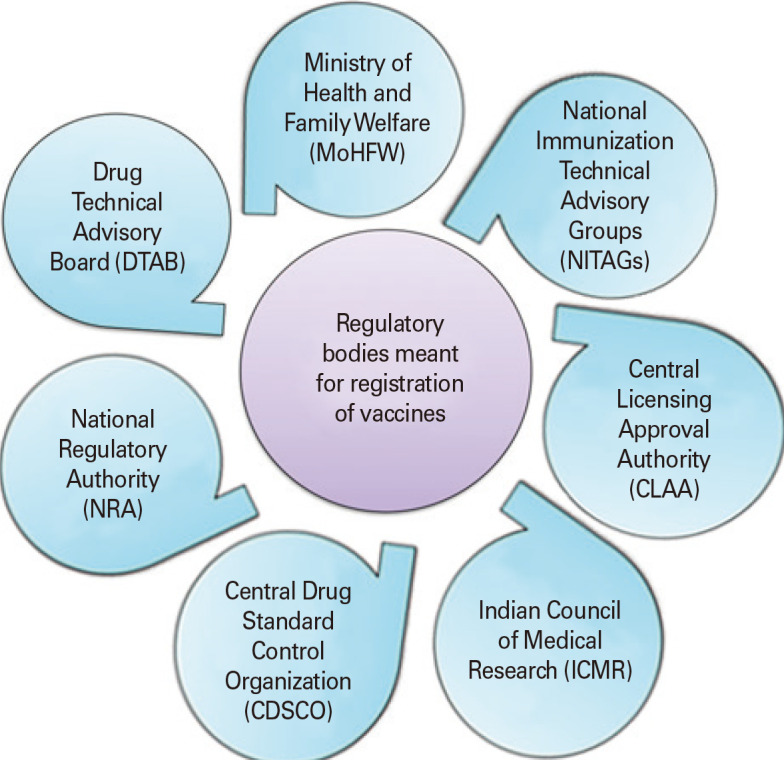
The Directorate General of Health Services, the Ministry of Health and Family Welfare (MoHFW), and the Government of India oversee the Central Drug Standard Control Organization (CDSCO), a national regulatory agency that is led by the Drug Controller General of India (DCGI). New Delhi serves as the location of its corporate headquarters. It has six zonal offices, four sub-zonal offices, 13 port offices, and seven laboratories spread out over the country. CDSCO enforces regulatory power over the nation’s notified medical devices, cosmetics, and drug quality. It develops standards and procedures and regulates the guidelines amending the Drug and Cosmetic Act 1940 and Rules 1945. Harmonizing clinical research and introducing safe and sustainable medications to the Indian market are its main objectives. Clinical trial approval, oversight, and execution in India are all handled by the DCGI. It controls the import and sale of pharmaceuticals used in clinical trials. It oversees carrying out the responsibilities given to the federal government by the Drug and Cosmetics Act. The DCGI is advised by the Drug Technical Advisory Board (DTAB) and the Drug Consultative Committee. The DTAB approves the inclusion of vaccines in the immunization programs. By guaranteeing the efficacy, quality, and safety of pharmaceuticals, cosmetics, and medical devices, CDSCO seeks to safeguard and enhance public health. Vaccines in India with regards to its licensing, approval, and follow-up by Good Manufacturing Practices is also regulated by DCGI, along with CDSCO and Drug Regulatory Authorities which are regarded as the central and state drug control departments [60]. The MoHFW is an important part of it, accountable for the national implementation of multiple initiatives in the sectors of health and family welfare, combating and preventing of major contagious infections. It also provides technical assistance to the states in controlling and preventing the outbreaks of seasonal disease and epidemics [61]. Since vaccination is a universal health priority, low- and middle-income countries established the National Immunization Technical Advisory Groups (NITAGs) to enhance decision-making and the quality of the available evidence. NITAGs offer independent technical advice to national policymakers and program managers to support decisions regarding immunization policy and programs that are grounded in data and relevant to local conditions [62]. The Indian Drugs Controller, as mentioned in Rule 3, is referred to as the “Central Licensing Approval Authority” (New Drugs and Clinical Trials Rules, 2019). In order to achieve uniformity in the application of the Drugs and Cosmetics Act, the central authorities are in charge of approving new drugs, conducting clinical trials in the nation, establishing drug standards, monitoring the quality of imported drugs, coordinating the activities of state drug control organizations, and offering expert advice [63]. The top organization in charge of formulating, coordinating, and promoting biomedical research is the Indian Council of Medical Research (ICMR). Approvals must adhere to ICMR’s good clinical practice (GCP) and ethical standards. The Department of Health Research and the MoHFW of the Government of India both provide funding for it [64]. National regulatory agencies/authority oversee ensuring that products made available for public consumption (typically pharmaceuticals and biological products, such as vaccines and medical devices, including test kits), have undergone adequate evaluation, and comply with all applicable quality, safety, and efficacy standards internationally [65]. Fig. 2 portrays the regulatory authorities responsible for laying down guidelines for registration process of vaccines in India. However, there could be some overlap in the areas of vaccine research and clinical trials, as both the CDSCO and ICMR are involved in this process. Additionally, there could be gaps in the regulation of certain aspects of vaccine manufacturing or distribution too. Thus, to address these issues, the regulatory bodies in India often work together to ensure that all aspects of the vaccine approval process are properly regulated and monitored. They also regularly review their processes and procedures to identify and address any potential gaps or overlaps in their jurisdiction [66].
Fig. 2. Regulatory authorities responsible for laying down guidelines for registration process of vaccines in India.
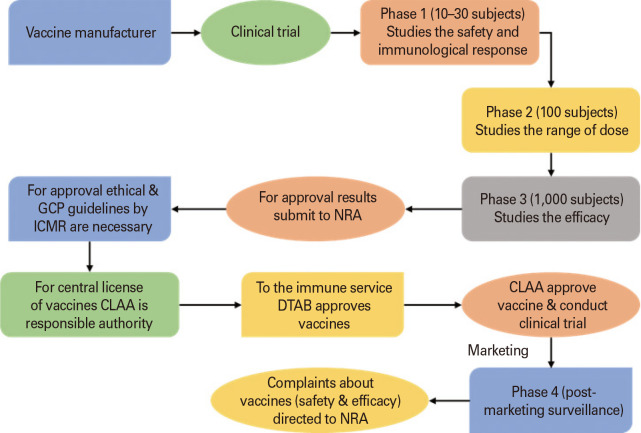
Development process for vaccine
Due to the stringent regulatory guidelines, vaccine registration is a complex and time-consuming process. To ensure vaccine safety, quality, and efficacy, the conduct of clinical trials is of utmost importance. Vaccines undergo rigorous testing phases to ensure and protect public health so that countries can conduct vaccination programs on a large scale for people. A vaccine before getting approved for the Indian market undergoes a pre-clinical investigation followed by three phases of clinical trials. There needs to be a specific plan to ensure the effectiveness and progress of the vaccine prior to clinical evaluation and fulfillment of the demands of the market manufacturers. This plan should include taking socioeconomic factors, target disease susceptibility, vaccine safety, the occurrence of the target infection, and environmental exposure, into consideration. The amount of drug, infusion process, as well as the awareness training program for the community should be evaluated [67]. The fact that there have not been many severe injuries brought on by vaccines in the decades since their evolution and use is evidence of their general safety. Though unfavorable events have been reported, but they are extremely rare [68]. Current Schedule Y regulations as outlined in the Drug and Cosmetic Act of 1940 and its Rules of 1945 should be followed when conducting clinical trials today. The prerequisites and guidelines for authorization to import new drugs for sale or to carry out clinical trials in the nation are set forth in Schedule Y [60]. All phases of clinical trials in India are required to adhere to the International Conference of Harmonization-Good Clinical Practice standards. For the purpose of conducting clinical trials in India for biologics, the clinical trial sponsor should submit a clinical trial application, i.e., form 44 and a CMC (chemistry, manufacturing, and controls) report to the DCGI. Data generated, documented, collected, and reported should be in compliance to the protocol issued by CDSCO and should be monitored and implemented by Quality Assurance [69]. Periodically the clinical trial sponsors need to submit a status report to the licensing authority and other investigators about the clinical trial data gathered along with any expected serious adverse event (SAE). Any SAE found during the ongoing clinical trial must be communicated within 14 calendar days. The sponsor needs to submit a summary report within 3 months to the licensing authority in case of premature discontinuation of trials for any reason. A summary report includes all the information starting from the purpose of the study, the number of patients exposed to the trial drug, its dosage to the duration exposed. Before COVID-19 vaccine development and its approval took at least 10–15 years to enter the market which was a long period. But now this time frame got shortened to 12–18 months, this is possible only due to modern technologies, expedited approvals, and medical experts [70].
Exploratory stage
This is the first basic step carried out by scientists and medical experts to plan and identify the specific antigen to be used for treating a particular disease. The antigen found can be natural or scientific. This basic lab research may take about 2–4 years. This stage is a slow and extensive process as it requires many approvals, funding, and a minute study of every result. Research is conducted to identify whether the antigen could be either a virus-like particle, a weakened bacteria or virus, or any other substance obtained from a pathogen [71].
Pre-clinical stage
Once the vaccine is developed then to evaluate its safety, efficacy, and capacity to elicit an immune response is tested on animals such as mice or monkeys in order to check whether these have any positive cellular response. The pre-clinical studies can also be conducted by the use of cell-culturing or tissue–culturing techniques. The outcomes of this stage aid in selecting a safe dose for the upcoming phase. The researchers can also decide the safest dose and route of administration of the vaccine to humans. Mostly, they may even conduct challenge studies, where subjected animals are vaccinated and then infected with the targeted pathogen to assess the results. Due to their inability to elicit the desired immune response, many vaccines, however, may fail at this stage. The pre-clinical stage typically lasts between one to 2 years and involves researchers from private industry [72]. Once completed, the sponsor submits an application that includes a summary of the laboratory reports, production and testing procedures, and the intended study. The clinical protocol then needs to be approved by an institutional review board on behalf of the institution hosting the clinical research. After approval of the application, the vaccination is tested in three stages. For obtaining permission to market or import or to conduct clinical trials for vaccines, Form 44/45/46 are employed [73].
Clinical trial phases
Phase I trials
It is the first phase that involves human beings. Phase I trials are designed to determine the highest safe dosage that a human may tolerate without experiencing any negative side effects. These studies are carried out on small human groups consisting of less than 100 individuals. Vaccine safety and its tolerability, along with local and systemic side effects are closely monitored during the study. After the results are obtained, it is assessed whether the study induces the desired immune response in individuals or not. By this point in the trial, both the researchers and the participants being tested know whether they are receiving a real vaccine or a dummy one. This open-label condition is known as a non-blinded state. The vaccinated study participants are carefully monitored and conditions are kept under control. This phase may take several months to gather data. The researchers progress to the next phase II trial only if this trial (phase I) comes out with 70% or more successful outcomes.
Phase II trials
These trials involve several hundred individuals from the targeted population for the vaccine. Some participants who are most vulnerable to contracting the disease are also taking part in the trial. The most effective and safest dose, together with the immunological response, can be further refined in phase II trials. During this phase, common short-term vaccine side effects are also observed. The study conducted is randomized and well-controlled along with a placebo group [74]. This phase is continued for at least 2 years for gathering the data. The vaccine may progress to the next phase only if vaccines under phase II trials come out with a 33% success rate. During this phase, numerous trials are carried out to assess the effectiveness of the vaccine in various age groups and formulations. In this phase along with the proposed doses, vaccination schedules and method of delivery are also determined.
Phase III trials
Phase III clinical trials are conducted on a large population of around 1,000–3,000 human population involved. This study may require a trial period of 1–4 years. These tests are being conducted in a randomized, double-blind fashion. Vaccines under this phase find difficult to pass as their success rate is only 25%–30%. Phase III trials make a comparison of the unvaccinated population with the vaccinated participants in terms of its safety, efficacy, and dosage. This stage determines the benefit versus risks of the vaccine, which ultimately grants the approval for vaccines. As the phase III trial is the last clinical trial stage so more care is taken on the consistency of vaccines while manufacturing under different lots. Even the effect plausibly owing to the co-administration of any other vaccine is also considered [75]. Phase III studies are often carried out in a variety of countries and places within a single country to ensure that the results are generalizable to a wide range of populations and the effectiveness of the vaccines.
Phase IV trials
After a vaccine is licensed and approved, it often goes through phase IV trials, which are ongoing studies. During this phase, the manufacturer of the vaccine continues to test the vaccine for its effectiveness and safety in the market. The term “post-marketing surveillance studies” also applies to phase IV trials that evaluates optimal use along with safety and efficacy. The pharmacovigilance programs tend to assess the adverse events of the vaccine and records are maintained during this ongoing trial.
Role of ethics committee in India
An ethics committee is a committee comprising seven members, where according to the Central Licensing Authority, each member undergoes regular training and development programs. These members come from various backgrounds, including medical and non-medical, scientific, and non-scientific areas. To make sure that the proposed clinical trial adheres to ethical standards that are recognized globally and locally, the ethics committee examines as well as monitors the studies carefully. Once the research or the trial is completed the body even takes part in the follow-up and surveillance actions of the studies. During the ongoing trial process, the ethics committee has the power to reject or end the studies if not satisfied or even demand changes in the protocols of the trial process. They may also carry out other duties, like making decisions or expressing their viewpoints on persistent ethical dilemmas in research [76]. Its primary duty is to safeguard the rights, safety, and well-being of the human participants involved in the study. It also considers potential risks as well as benefits associated with the community where the research will be conducted. The ultimate objective is to support and encourage high ethical standards in medical research for reduced adverse events. All members of the ethics committee are highly qualified and experienced individuals amongst which some remain to be silent and good observers during all discussions and meetings to conclude or put forth any questions [77]. From assessing the SAE reports to reviewing the annual status records of the trials, makes the ethics committees to perform their duties carefully and responsibly. The ethical committee plays a major role in the conduction of clinical trials by reviewing and documenting the informed consent of trial participants, safeguarding the confidentiality of the trials, approving protocols, and ensuring the suitability of the investigators involved. The registration of the clinical trials approval granted by the ethics committee is acceptable for a period of 3 years. For re-registration purposes as well as post-approval changes there is a need for the involvement of the ethics committees in India. Minimum 3 months prior to expiry, the re-registration application needs to be filled.
According to Rule 7, an ethics committee must be established and registered under Rule 8 to conduct a clinical trial, and its approval is required prior to the beginning of the trials [62]. The ethics committee will submit an application as per the form clinical trial-04 to the central licensing authority. If the central licensing authority is satisfied after receiving the application, it registers the ethics committee using form clinical trial-06. If not satisfied then the application will be rejected and the reason will be recorded in writing. The application must be reviewed by the central licensing authority within 90 working days of the date of receipt. Unless suspended, extended, or revoked by the central licensing authority, the registration of ethics committees granted in the form clinical trial-06 under Rule 22 or automatic approval under Rule 23 in the form clinical trial-4A remains valid for 2 years from the date of its issuance. The ethics committee also oversees the safety and welfare of trial participants in addition to reviewing and approving clinical trials in accordance with GCP guidelines. When any SAE occurs in the ongoing clinical trial then the ethics committee shall analyze the reports and forward them to the central licensing authority for further discussions.
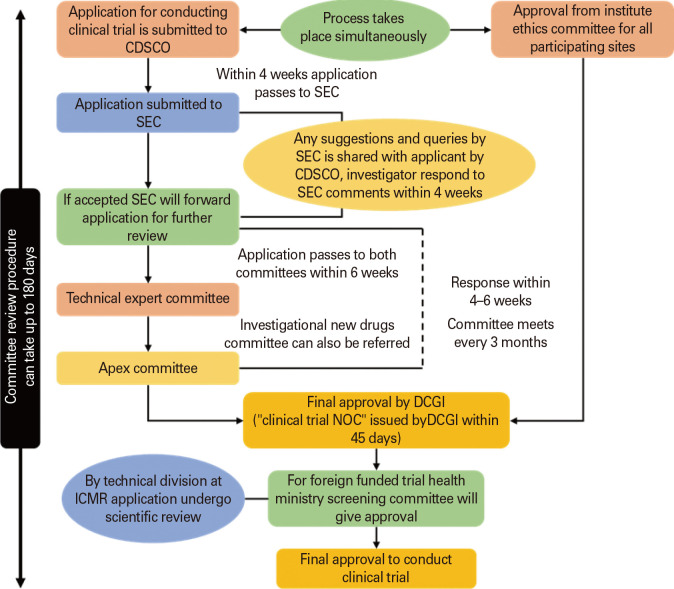
The committee may order the discontinuation or suspension of the clinical trial if it is determined that continuing the trial will hinder the safety, rights, and general welfare of the trial subject [75]. Any officer of the central licensing authority who is accompanied by a member of the state licensing authority may visit the sites of ongoing clinical trials, as well as their facilities, records, and documents, either with or without prior notice, to ensure that they are in accordance with the standards for GCPs. As per the new drug clinical trial 2019, when any institution or organization fails to comply with any provisions of the act then the central licensing authority may issue a warning letter and give an opportunity to show cause, if satisfied with the justifications then no further actions will be taken otherwise even the authority may debar the investigator along with its representatives to conduct any clinical trial in future for such period. Fig. 3 portrays the pathway for approval of clinical trial applications.
Fig. 3. Pathway for approval of clinical trial applications. SEC, subject expert committee; CDSCO, Central Drug Standard Control Organization; DCGI, Drug Controller General of India; ICMR, Indian Council of Medical Research; NOC, No Objection Certificate.
Approval process of vaccines
Vaccine approval is crucial in ensuring public health for several reasons. First and foremost, vaccines are designed to prevent the spread of infectious diseases by stimulating the immune system to recognize and fight off specific pathogens. When vaccines are approved, it means that these have undergone rigorous testing and have been found to be safe and effective in protecting individuals from specific diseases [74]. The approval of vaccines also plays an important role in promoting public trust in the healthcare system. By following strict guidelines and regulations, vaccine manufacturers are held accountable for producing, distributing, and using safe and effective vaccines. Therefore, the approval of vaccines is critical to ensure public health. The documents designed for vaccine approval are as per the common technical document (CTD) format which consists of five modules that are administrative information, quality overall summary, quality information, non-clinical data, and last clinical data [78]. A hard copy and a digital copy of each document submitted to the CDSCO by the manufacturer must be kept for possible future use as a reference. As per the requirements documents are submitted in paper format as well as in electronic CTD format.
Legislation in India that controls the authorization to use vaccines in emergencies
In India, the DCGI is the regulatory body that makes decisions regarding a drug’s efficacy and safety before dispensing it to the general public in the event of a proclaimed emergency. The regulator has the power to provide an EUA to a vaccination, medication, or other medical product in order to make it widely available for use upon having evidence that suggests that a drug or vaccine may benefit people. It should be emphasized that before being used, a regulatory authority must approve the vaccines, medications, diagnostic tests, and even medical equipment. The application for EUA is only taken into consideration when the vaccine producers submit adequate efficacy data from phase 3 studies [79]. It should be highlighted that phase 1 or phase 2 trial data are insufficient to meet the requirements for getting EUA. However, phase 1 and phase 2 tests are necessary to demonstrate that the product is secure enough to proceed to phase 3 tests. This means that the vaccination or drug should include all necessary safety trials when filing for a EUA. Before approving a product, the Regulatory Authority investigates it and determines for them if it complies with reasonable safety threshold limitations and is usable. Fig. 4 delineates the flowchart pathway for the registration process of vaccines.
Fig. 4. Flowchart pathway for registration of vaccines in India. GCP, good clinical practice; ICMR, Indian Council of Medical Research; NRA, national regulatory agencies/authority; CLAA, Central Licensing Approval Authority; DTAB, Drug Technical Advisory Board.
Challenges in Vaccine Development
Today’s worldwide vaccine market has grown in response to the spread of infectious illnesses. Despite the positive results, the entire process—from vaccine development to market entry-remains extremely difficult. After administration of a vaccine, its efficacy in the population varies from person to person, age being the major factor. Due to physiological variations in cell-mediated immune function that take place over the course of a person’s lifetime, age influences vaccine design [77]. The young infants and the aged individuals (above 60 years old) are the major groups with varied immune activity. The difference in the vaccine immune status of them gets a major concern while developing vaccines. Most vaccinations advised for older adults are administered to strengthen preexisting immune memory from earlier vaccinations or infections. Although the disease burden is somewhat reduced by these booster shots, infections like influenza, those brought on by Streptococcus pneumoniae, or herpes zoster reactivation are still very common in the elderly population, indicating ineffective recall responses. It can be challenging to interpret vaccination studies in older people, in part because of the high heterogeneity of immune responses owing to aging and the abundance of underlying comorbidities [80]. The immune response to the influenza vaccine can also vary depending on age. Children and the elderly may have a weaker immune response to the vaccine, which means that they may not be as well protected from the flu as healthy adults. This has led to the development of high-dose influenza vaccines specifically for the elderly. Additionally, research has shown that women produce a strong immune response to the influenza vaccine than men. Genetics of the person is another major barrier to vaccine development. The vaccine responses are influenced by genetic factors such as hereditary diseases like diabetes and cancer. A healthy person getting vaccinated versus a person with already existing medical conditions or compromised immunity getting vaccinated also gives different outcomes, thus acting as a challenge to the researchers. The human papillomavirus (HPV) vaccine has been shown to be less effective in individuals with certain genetic variations. Some individuals may have genetic variations that reduce their immune response to the vaccine, which can impact their protection against HPV-related cancers. Depending on the specific vaccine, the degree of heritability varies ranging from 36%–90% for humoral responses and 39%–90% for cellular responses [81]. The lifestyle of individuals and the gender populations too play a challenging role in vaccine development. The ratio of antibody development in males to females is largely consistent but may vary depending on the vaccines for particular diseases. Vaccine manufacturers are also few in number because of the critical steps involved in order to assure its quality. The developing nations earlier could not afford high-cost vaccines, but lately, the situation is changing, and the cost-effectiveness is benefiting a lot of nations [82]. The world is growing increasingly worried about vaccination skepticism, especially in populous and uneducated countries, including India. As a result, the WHO has named it as one of the top 10 health concerns in 2019 [83]. The success of mass vaccination campaigns is also influenced by how well people accept the vaccine. It would be difficult to achieve herd immunity if significant numbers of society are hesitant [84]. An international survey with 742 respondents from India was conducted in June 2020. According to the findings, respectively, 44% and 30% of the participants were completely and somewhat willing to receive the vaccine. In response to receiving the vaccine if it were available, 14% of respondents were neutral, 5.66% disagreed somewhat, and 5.66% disagreed completely [85]. The challenge of vaccination depends critically on ensuring that there are adequate doses available everywhere. A lack of COVID-19 vaccine in 2021 could have a significant global impact on billions of people, prolonging the pandemic and raising the risk of new virus mutations that could reduce the efficacy of current vaccines. During the 2009 H1N1 influenza pandemic, wealthy nations purchased the majority of the pandemic influenza vaccines that were available globally, leaving resource-poor nations—many of which were among the worst affected in the world-with insufficient supplies. To avoid a repeat of the H1N1 situation, the WHO announced in April 2020 that the COVID-19 Vaccine Global Access (COVAX) Facility would be created in collaboration with CEPI and Gavi. This facility has not only made vaccines more accessible to all citizens, regardless of wealth but has also assisted in making vaccines more affordable [42]. India has a vast and diverse population, and delivering vaccines to remote areas can be challenging. Apart from the lack of laboratory facilities and the high costs involved, the other challenges in vaccine development in India are the lack of adequate cold chain infrastructure for storage and stability of vaccines and transport facilities.
Conclusion
By avoiding the spread of numerous diseases around the globe, vaccines play a crucial role in promoting global health. Additionally, vaccines have a substantial economic impact by saving over $500 billion in medical expenses and hospital stays. Combating outbreaks requires stringent regulation for vaccine clinical testing and trials to be performed for a specified time period in a clean and hygienic space. Through the consolidation of interdisciplinary approaches and the large commitment of resources, vaccines have been able to fight against various infectious diseases as well as eradicate few of them completely. From the eradication of smallpox to helping in preventing the spread of coronavirus infection today vaccines have become a global game changer. Just like chemical drugs, these biological products in India are also clinically evaluated through three phases, i.e., phase I, II, and III, and their approval is regulated by CDSCO. There are certain provisions for emergency use as well. As per the Drug and Cosmetic Rules of 1945, the ethics committee is the committee constituted under Rule 7 and registered under Rule 8, meant for the purpose of registration, safeguarding the rights and well-being of the trial subjects, reviewing protocols, documenting, and maintaining the informed consents of the healthy volunteers. This article focuses on the stages of clinical trials and the involvement of the ethics committee in the licensing and approval of the trials for vaccines. The CDSCO being the national regulatory body is in charge of maintaining transparency, accountability, and uniformity in its service from conducting clinical trials to its approval in the market. The licensing of biological products such as vaccines is regulated and approved by CDSCO. Besides being in the most successful period of vaccine development, the innovation of science and the knowledge of the researchers have brought up the benefits of intranasal vaccines. The paradigm of clinical evaluation and the timeline for approval of vaccines has been modernized based on scientific improvements and an increased number of diseased people. Even after the growth of intranasal vaccines, there are still many hurdles to overcome in the development process of vaccines.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Feng J, Li Q. How to ensure vaccine safety: an evaluation of China’s vaccine regulation system. Vaccine. 2021;39:5285–5294. doi: 10.1016/j.vaccine.2021.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canoui E, Launay O. History and principles of vaccination. Rev Mal Respir. 2019;36:74–81. doi: 10.1016/j.rmr.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Omer SB. Why and how vaccines work. Cell. 2020;183:290–295. doi: 10.1016/j.cell.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shukla VV, Shah RC. Vaccinations in primary care. Indian J Pediatr. 2018;85:1118–1127. doi: 10.1007/s12098-017-2555-2. [DOI] [PubMed] [Google Scholar]
- 5.Rappuoli R, Pizza M, Del Giudice G, De Gregorio E. Vaccines, new opportunities for a new society. Proc Natl Acad Sci U S A. 2014;111:12288–12293. doi: 10.1073/pnas.1402981111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saleh A, Qamar S, Tekin A, Singh R, Kashyap R. Vaccine development throughout history. Cureus. 2021;13:e16635. doi: 10.7759/cureus.16635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zakir F, Islam F, Jabeen A, Sivagurunathan Moni S. Vaccine development: a historical perspective. Biomed Res. 2019;30:452–455. [Google Scholar]
- 8.Das S, Kar SS, Samanta S, Banerjee J, Giri B, Dash SK. Immunogenic and reactogenic efficacy of Covaxin and Covishield: a comparative review. Immunol Res. 2022;70:289–315. doi: 10.1007/s12026-022-09265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broom D. From cholera to COVID: a brief history of vaccines. World Economic Forum 2022 Apr 27. [cited 2022 Sep 20]. Available from: https://www.weforum.org/agenda/2022/04/from-cholera-to-covid-a-brief-history-of-vaccines/
- 10.Jadhav VR, Aher JS, Bhagare AM, Dhaygude AC. COVID-19 era: what’s impact of the lockdown on India’s environment? J Chem Environ Sci Its Appl. 2020;7:1–6. [Google Scholar]
- 11.Batra S, Sharma S, Verma P, Arora N. COVID-19: an Insight on the third respiratory global emergency of the century. Coronaviruses. 2021;2:339–345. [Google Scholar]
- 12.Kciuk M, Mujwar S, Rani I, et al. Computational bioprospecting guggulsterone against ADP ribose phosphatase of SARS-CoV-2. Molecules. 2022;27:8287. doi: 10.3390/molecules27238287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigues CM, Plotkin SA. Impact of vaccines; health, economic and social perspectives. Front Microbiol. 2020;11:1526. doi: 10.3389/fmicb.2020.01526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han X, Xu P, Ye Q. Analysis of COVID-19 vaccines: types, thoughts, and application. J Clin Lab Anal. 2021;35:e23937. doi: 10.1002/jcla.23937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.COVID19 Vaccine Tracker [Internet] [place unknown]: VIPER Group COVID19 Vaccine Tracker Team; 2022. [cited 2022 Sep 20]. Available from: https://covid19.trackvaccines.org/ [Google Scholar]
- 16.Sharma M, Mordani S. Covaxin, Covishield granted market approval in India but conditions apply. India Today [Internet] 2022. Jan 27, [cited 2022 Sep 20]. Available from: https://www.indiatoday.in/coronavirus-outbreak/vaccine-updates/story/covaxin-covishield-granted-regular-market-approval-india-1905218-2022-01-27 .
- 17.Kumar A, Shrivastava S, Tiwari P. Management of adverse events post-COVID-19 vaccination with Covaxin and Covishield: a literature review. One Health Bull. 2022;2:6 [Google Scholar]
- 18.ChAd36-SARS-CoV-S COVID-19 vaccine (recombinant): summary of product characteristics (SmPC) [Internet] Shamirpet: Bharat Biotech International Ltd; 2022. [cited 202 Sep 20]. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=OTAwOQ== [Google Scholar]
- 19.Bharat Biotech. Restricted use in emergency situation of COVID-19 SARS-CoV-2 vaccine [Internet] Hyderabad: Bharat Biotech; 2019. [cited 2022 Nov 24]. Available from: http://www.bharatbiotech.com . [Google Scholar]
- 20.Sharun K, Dhama K. India’s role in COVID-19 vaccine diplomacy. J Travel Med. 2021;28:taab064. doi: 10.1093/jtm/taab064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Module 1: administrative/legal information: trivalent seasonal influenza virus-like particle (VLP) vaccine: summary of product characteristics [Internet] Ahmedabad: CADILA Pharmaceuticals Limited; 2018. [cited 2022 Nov 24]. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NDE3NA== [Google Scholar]
- 22.Summary of product characteristics [Internet] Ghatkesar: Dano Vaccines and Biologicals Private Limited; 2010. [cited 2022 Nov 24]. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=NDE3Ng== [Google Scholar]
- 23.Kakar A, Gogia A, Solanki SK, Jadiya A, Singh D. Safety and immunogenicity of SURE RAB(TM), an inactivated cell culture derived rabies vaccine: a comparative, phase III clinical trial. Indian J Med Microbiol. 2021;39:63–66. doi: 10.1016/j.ijmmb.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Arora A, Nagpal M, Aggarwal G. Microneedle mediated vaccine delivery: a comprehensive review. J Pharm Technol Res Manag. 2017;5:163–184. [Google Scholar]
- 25.Okuyan O, Elgormus Y, Sayili U, Dumur S, Isik OE, Uzun H. The effect of virus-specific vaccination on laboratory infection markers of children with acute rotavirus-associated acute gastroenteritis. Vaccines (Basel) 2023;11:580. doi: 10.3390/vaccines11030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priyanka, Chopra H, Choudhary OP. mRNA vaccines as an armor to combat the infectious diseases. Travel Med Infect Dis. 2023;52:102550. doi: 10.1016/j.tmaid.2023.102550. [DOI] [PubMed] [Google Scholar]
- 27.Ella R, Babji S, Ciarlet M, Blackwelder WC, Vadrevu KM. A randomized, open-labelled, non-inferiority phase 4 clinical trial to evaluate the immunogenicity and safety of the live, attenuated, oral rotavirus vaccine, ROTAVAC(R) in comparison with a licensed rotavirus vaccine in healthy infants. Vaccine. 2019;37:4407–4413. doi: 10.1016/j.vaccine.2019.05.069. [DOI] [PubMed] [Google Scholar]
- 28.Lahariya C. A brief history of vaccines & vaccination in India. Indian J Med Res. 2014;139:491–511. [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharya S. Expunging variola: the control and eradication of smallpox in India, 1947-1977. Hyderabad: Orient Blackswan; 2006. [Google Scholar]
- 30.Madhavi Y. Vaccine policy in India. PLoS Med. 2005;2:e127. doi: 10.1371/journal.pmed.0020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.History [Internet] Hyderabad: Biological E. Limited; c2020. [cited 2022 Nov 24]. Available from: https://www.biologicale.com/history.html . [Google Scholar]
- 32.Chakraborty C, Agoramoorthy G. India’s cost-effective COVID-19 vaccine development initiatives. Vaccine. 2020;38:7883–7884. doi: 10.1016/j.vaccine.2020.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doherty M, Buchy P, Standaert B, Giaquinto C, Prado-Cohrs D. Vaccine impact: benefits for human health. Vaccine. 2016;34:6707–6714. doi: 10.1016/j.vaccine.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 34.World Data Bank. World development indicators [Internet] Washington (DC): The World Bank; 2017. [cited 2022 Sep 20]. Available from: https://datatopics.worldbank.org/world-development-indicators/ [Google Scholar]
- 35.Hooda SK. Out-of-pocket payments for healthcare in India: who have affected the most and why? J Health Manag. 2017;19:1–15. [Google Scholar]
- 36.Wagstaff A, Flores G, Smitz MF, Hsu J, Chepynoga K, Eozenou P. Progress on impoverishing health spending in 122 countries: a retrospective observational study. Lancet Glob Health. 2018;6:e180–e192. doi: 10.1016/S2214-109X(17)30486-2. [DOI] [PubMed] [Google Scholar]
- 37.Nandi A, Shet A. Why vaccines matter: understanding the broader health, economic, and child development benefits of routine vaccination. Hum Vaccin Immunother. 2020;16:1900–1904. doi: 10.1080/21645515.2019.1708669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chatterjee A, Chakravarty A. Neurological complications following COVID-19 vaccination. Curr Neurol Neurosci Rep. 2023;23:1–14. doi: 10.1007/s11910-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benefits and risks of vaccines [Internet] Columbia (SC): South Carolina Department of Health and Environmental Control; 2022. [cited 2022 Sep 20]. Available from: https://scdhec.gov/benefits-risks-vaccines . [Google Scholar]
- 40.Koller CN, Schwerzmann CJ, Lang AS, Alexiou E, Krishnakumar J. Addressing different needs: the challenges faced by India as the largest vaccine manufacturer while conducting the world’s biggest COVID-19 vaccination campaign. Epidemiologia (Basel) 2021;2:454–470. doi: 10.3390/epidemiologia2030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asundi A, O’Leary C, Bhadelia N. Global COVID-19 vaccine inequity: the scope, the impact, and the challenges. Cell Host Microbe. 2021;29:1036–1039. doi: 10.1016/j.chom.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milstien JB, Batson A, Wertheimer AI. Vaccines and drugs: characteristics of their use to meet public health goals [Internet] Washington (DC): World Bank; 2005. [cited 2022 Sep 20]. Available from: https://www.researchgate.net/publication/228615171_Vaccines_and_Drugs_Characteristics_of_Their_Use_to_Meet_Public_Health_Goals . [Google Scholar]
- 44.Wynaden D, Landsborough I, McGowan S, Baigmohamad Z, Finn M, Pennebaker D. Best practice guidelines for the administration of intramuscular injections in the mental health setting. Int J Ment Health Nurs. 2006;15:195–200. doi: 10.1111/j.1447-0349.2006.00423.x. [DOI] [PubMed] [Google Scholar]
- 45.Rodger MA, King L. Drawing up and administering intramuscular injections: a review of the literature. J Adv Nurs. 2000;31:574–582. doi: 10.1046/j.1365-2648.2000.01312.x. [DOI] [PubMed] [Google Scholar]
- 46.Kilic E, Kalay R, Kilic C. Comparing applications of intramuscular injections to dorsogluteal or ventrogluteal regions. J Exp Integr Med. 2014;4:171–174. [Google Scholar]
- 47.Biswas S. Covid-19 nasal vaccine: are these the better solution?: all you need to know. Mint [Internet] 2022. May 28, [cited 2022 Oct 5]. Available from: https://www.livemint.com/science/health/covid19-nasal-vaccine-are-these-the-better-solution-all-you-need-to-know-11653731602540.html .
- 48.Administer the vaccine(s) [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2021. [cited 2022 Oct 5]. Available from: https://www.cdc.gov/vaccines/hcp/admin/administer-vaccines.html . [Google Scholar]
- 49.Clark EM, Pippin MM. Safe and effective administration of vaccines and epinephrine autoinjection. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 50.Vela Ramirez JE, Sharpe LA, Peppas NA. Current state and challenges in developing oral vaccines. Adv Drug Deliv Rev. 2017;114:116–131. doi: 10.1016/j.addr.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renukuntla J, Vadlapudi AD, Patel A, Boddu SH, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447:75–93. doi: 10.1016/j.ijpharm.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010;18:771–786. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 53.Birkhoff M, Leitz M, Marx D. Advantages of intranasal vaccination and considerations on device selection. Indian J Pharm Sci. 2009;71:729–731. [Google Scholar]
- 54.Waltz E. How nasal-spray vaccines could change the pandemic. Nature. 2022;609:240–242. doi: 10.1038/d41586-022-02824-3. [DOI] [PubMed] [Google Scholar]
- 55.Tiboni M, Casettari L, Illum L. Nasal vaccination against SARS-CoV-2: synergistic or alternative to intramuscular vaccines? Int J Pharm. 2021;603:120686. doi: 10.1016/j.ijpharm.2021.120686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.CanSinoBIO. CanSinoBIO’s Convidecia air receives approval in China [Internet] Tianjin: CanSinoBIO; 2022. [cited 2022 Oct 5]. Available from: https://www.cansinotech.com/html/1/179/180/1100.html . [Google Scholar]
- 57.Bharat Biotech. Bharat Biotech’s iNCOVACC world’s first intra nasal vaccine receives approval for emergency use in India [Internet] Hyderabad: Bharat Biotech; 2022. [cited 2022 Oct 5]. Available from: https://www.bharatbiotech.com/images/press/Bharat-Biotech-iNCOVACC-Worlds-First-Intra-Nasal-Vaccine-Receives-Approval.pdf . [Google Scholar]
- 58.Choudhary OP, Priyanka, Mohammed TA, Singh I. Intranasal COVID-19 vaccines: is it a boon or bane? Int J Surg. 2021;94:106119. doi: 10.1016/j.ijsu.2021.106119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Latpate S, Salunkhe K, Pagar P. Regulatory aspects for vaccines in India and us an overview. Int J Res Eng Sci. 2022;10:238–241. [Google Scholar]
- 60.Tahamtan A, Charostad J, Hoseini Shokouh SJ, Barati M. An overview of history, evolution, and manufacturing of various generations of vaccines. J Arch Mil Med. 2017;5:e12315 [Google Scholar]
- 61.Annual report 2016-17: organization and infrastructure [Internet] New Delhi: Ministry of Health and Family Welfare; 2018. [cited 2022 Oct 5]. Available from: https://main.mohfw.gov.in/sites/default/files/1201617.pdf . [Google Scholar]
- 62.Howard N, Walls H, Bell S, Mounier-Jack S. The role of National Immunisation Technical Advisory Groups (NITAGs) in strengthening national vaccine decision-making: a comparative case study of Armenia, Ghana, Indonesia, Nigeria, Senegal and Uganda. Vaccine. 2018;36:5536–5543. doi: 10.1016/j.vaccine.2018.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sathiaraj E. Better nutrition for a better future [Internet] Geneva: James Lind Institute; 2021. [cited 2022 Oct 5]. Available from: https://www.jli.edu.in/blog/roles-and-responsibilities-ofcdsco/#:~:text=The%20central%20authorities%20are%20responsible,of%20bringing%20about20the%20uniformity . [Google Scholar]
- 64.Announcement: Indian Council of Medical Research: reference list [Internet] New Delhi: Indian Council of Medical Research; 2017. [cited 2022 Oct 5]. Available from: http://www.icmr.nic.in/ [Google Scholar]
- 65.National regulatory agencies [Internet] New Delhi: World Health Organization South-East Asia Region; [date unknown]. [cited 2022 Oct 5]. Available from: https://www.who.int/southeastasia/activities/national-regulatory-agencies#:~:text=NRAs%20are%20national%20regulatory%20agencies,quality%20and%20safety%20and%20efficacy . [Google Scholar]
- 66.Regulation of vaccines: building on existing drug regulatory authorities [Internet] Geneva: World Health Organization, Department of Vaccines and Other Biologicals; 1999. [cited 2022 Oct 5]. Available from: https://apps.who.int/iris/bitstream/handle/10665/65968/WHO_V-B_99.10_eng.pdf;jsessionid=6AB4805A2DA3FA6411F5E9C9DF49797A?sequence=1 . [Google Scholar]
- 67.Goetz KB, Pfleiderer M, Schneider CK. First-in-human clinical trials with vaccines: what regulators want. Nat Biotechnol. 2010;28:910–916. doi: 10.1038/nbt0910-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kondal A, Krishna GV, Bansal D. Clinical trial regulations in India: progress and challenges arising from recent amendments to schedule Y of the Drugs and Cosmetics (D&C) Act 1940 (D&C Rules 1945) Pharm Med. 2016;30:1–13. [Google Scholar]
- 69.Ethics committee [Internet] New Delhi: Central Drugs Standard Control Organization; 2022. [cited 2022 Oct 31]. Available from: https://cdsco.gov.in/opencms/opencms/en/Clinical-Trial/Ethics-Committee/ [Google Scholar]
- 70.How are vaccines developed?: what are the stages? [Internet] Mumbai: HDFC ERGO; 2022. [cited 2022 Sep 25]. Available from: https://www.hdfcergo.com/blogs/health-insurance/how-are-vaccines-developed-what-are-the-stages . [Google Scholar]
- 71.Thanuja MB, Maddi R. Comparison of regulatory approval process for vaccines development and manufacturing in India & USA. UPI J Pharm Med Health Sci. 2021;4:18–24. [Google Scholar]
- 72.Central Drug Standard Control Organization (CDSCO) user manual for e-governance solution for CDSCO: version 1.0 [Internet] Pune: Centre for Development of Advanced Computing; 2017. [cited 2022 Oct 25]. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/SUGAM_user_manual.pdf . [Google Scholar]
- 73.Kumar D, Rahi S, Rana A. Comparative study of updated clinical trial regulations in India with respect to Australia, Europe, Japan and US. Int J Drug Regul Aff. 2021;9:48–61. [Google Scholar]
- 74.Walter EB, Moody MA. Vaccine development: steps to approval of an investigational vaccine. N C Med J. 2021;82:141–144. doi: 10.18043/ncm.82.2.141. [DOI] [PubMed] [Google Scholar]
- 75.World Health Organization. Research ethics committees: basic concepts for capacity-building. Geneva: World Health Organization; 2009. [Google Scholar]
- 76.Kadam R, Karandikar S. Ethics committees in India: facing the challenges! Perspect Clin Res. 2012;3:50–56. doi: 10.4103/2229-3485.96444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.New Drugs and Clinical Trial Rules, 2019: NDCTR_G.S.R. 227(E) [Internet] New Delhi: Central Drugs Standard Control Organization; 2019. [cited 2022 Oct 25]. Available from: https://cdsco.gov.in/opencms/opencms/system/modules/CDSCO.WEB/elements/download_file_division.jsp?num_id=OTI4MA== [Google Scholar]
- 78.Dellepiane N, Pagliusi S Registration Experts Working Group. Challenges for the registration of vaccines in emerging countries: differences in dossier requirements, application and evaluation processes. Vaccine. 2018;36:3389–3396. doi: 10.1016/j.vaccine.2018.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knipe DM, Levy O, Fitzgerald KA, Muhlberger E. Ensuring vaccine safety. Science. 2020;370:1274–1275. doi: 10.1126/science.abf0357. [DOI] [PubMed] [Google Scholar]
- 80.Gustafson CE, Kim C, Weyand CM, Goronzy JJ. Influence of immune aging on vaccine responses. J Allergy Clin Immunol. 2020;145:1309–1321. doi: 10.1016/j.jaci.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salerno-Goncalves R, Sztein MB. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 2006;14:536–542. doi: 10.1016/j.tim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 82.Zimmermann P, Curtis N. Factors that influence the immune response to vaccination. Clin Microbiol Rev. 2019;32:e00084–e00018. doi: 10.1128/CMR.00084-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.World Health Organization. Ten threats to global health in 2019 [Internet] Geneva: World Health Organization; 2019. [cited 2022 Oct 20]. Available from: https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019 . [Google Scholar]
- 84.Neumann-Bohme S, Varghese NE, Sabat I, et al. Once we have it, will we use it?: a European survey on willingness to be vaccinated against COVID-19. Eur J Health Econ. 2020;21:977–982. doi: 10.1007/s10198-020-01208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lazarus JV, Ratzan SC, Palayew A, et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]