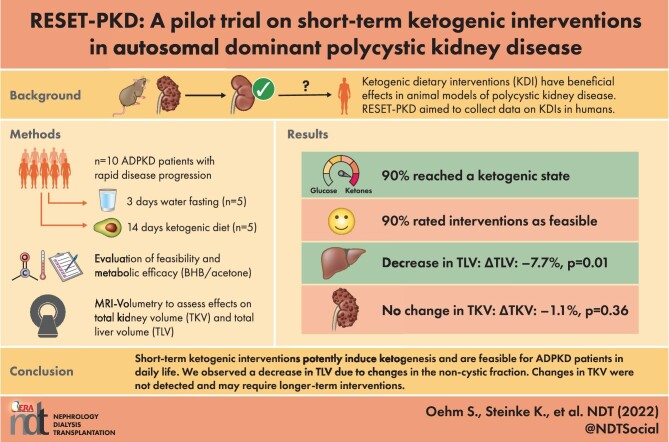
ABSTRACT
Background
Ketogenic dietary interventions (KDI) have been shown to be effective in animal models of polycystic kidney disease (PKD), but data from clinical trials are lacking.
Methods
Ten autosomal dominant PKD (ADPKD) patients with rapid disease progression were enrolled at visit V1 and initially maintained a carbohydrate-rich diet. At V2, patients entered one of the two KDI arms: a 3-day water fast (WF) or a 14-day ketogenic diet (KD). At V3, they resumed their normal diet for 3–6 weeks until V4. At each visit, magnetic resonance imaging kidney and liver volumetry was performed. Ketone bodies were evaluated to assess metabolic efficacy and questionnaires were used to determine feasibility.
Results
All participants [KD n = 5, WF n = 5; age 39.8 ± 11.6 years; estimated glomerular filtration rate 82 ± 23.5 mL/min/1.73 m2; total kidney volume (TKV) 2224 ± 1156 mL] were classified as Mayo Class 1C–1E. Acetone levels in breath and beta-hydroxybutyrate (BHB) blood levels increased in both study arms (V1 to V2 average acetone: 2.7 ± 1.2 p.p.m., V2 to V3: 22.8 ± 11.9 p.p.m., P = .0006; V1 to V2 average BHB: 0.22 ± 0.08 mmol/L, V2 to V3: 1.88 ± 0.93 mmol/L, P = .0008). Nine of 10 patients reached a ketogenic state and 9/10 evaluated KDIs as feasible. TKV did not change during this trial. However, we found a significant impact on total liver volume (ΔTLV V2 to V3: −7.7%, P = .01), mediated by changes in its non-cystic fraction.
Conclusions
RESET-PKD demonstrates that short-term KDIs potently induce ketogenesis and are feasible for ADPKD patients in daily life. While TLV quickly changed upon the onset of ketogenesis, changes in TKV may require longer-term interventions.
Keywords: ADPKD, fasting, ketogenic diet, ketosis, nutrition, polycystic kidney disease
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What is already known about this subject?
Ketogenic dietary interventions (KDIs) have been shown to be effective in several animal models of polycystic kidney disease (PKD), inhibiting cyst growth, fibrosis and PKD-associated signaling pathways. These beneficial effects were mediated by ketosis.
Recent retrospective work has pointed towards beneficial effects of dietary interventions in patients with autosomal dominant PKD (ADPKD).
What this study adds?
In RESET-PKD, 10 ADPKD patients with rapid disease progression performed a 3-day water fast or a 14-day ketogenic diet and were examined biochemically, using patient-reported outcome questionnaires and through magnetic resonance imaging–based kidney and liver volumetry.
RESET-PKD provides clear evidence of patient-reported feasibility, including a detailed analysis of ketogenesis of two KDIs in ADPKD.
Short-term KDIs have an impact on total liver volume (TLV), probably due to emptying of liver glycogen stores, while total kidney volume is not affected.
What impact this may have on practice or policy?
This study provides first prospective in-human data with monitored ketosis concerning feasibility, efficacy and safety of ketogenic dietary interventions in ADPKD, and can now serve as the foundation for larger randomized trials.
The effects on TLV should be further studied in patients with severe polycystic liver disease.
INTRODUCTION
There is increasing evidence that alterations in the energy metabolism of cyst lining cells—especially increased glucose dependency and defects in fatty acid oxidation—may underlie the pathogenesis of autosomal dominant polycystic kidney disease (ADPKD) [1–3]. Dietary interventions have been shown to be surprisingly effective in several polycystic kidney disease (PKD) animal models, where they lead to a strong decrease in cyst growth [4, 5]. The positive effects of mild food reduction were most likely mediated by ketosis, as a ketogenic state—regardless of whether it was induced by a time-restricted diet, a ketogenic diet (KD) or a short-term water fast (WF)—resulted in significantly inhibited cyst growth, fibrosis and PKD-associated signaling pathways in different animal models, even when the state of ketosis was only induced for a short period of time [6].
Ketogenic dietary interventions (KDI) are high-fat, low-carbohydrate (CHO) and moderate-protein diets which mimic a fasting state [7]. KDIs have been used as an effective tool for the treatment of obesity and childhood epilepsy and could potentially be beneficial in several other diseases [8, 9]. A recent retrospective case series indicated safety, feasibility and positive effects of KDIs in patients with ADPKD for the first time [10]. Most recently, the results of a 1-year behavioral weight loss study in obese ADPKD patients supported therapeutic feasibility of weight loss interventions and hinted towards possible positive effects such as slowing of kidney growth [11]. However, no trials investigating the effects of ketosis per se in patients with ADPKD have been performed. Therefore, this proof-of-principle trial aimed to provide prospectively collected data on the short-term effects of KDIs in ADPKD patients.
MATERIALS AND METHODS
Study design and participants
This trial was designed and conducted as a non-randomized, non-blinded, single-center study at the University Hospital Cologne. Approval was obtained from the institutional review board of the University Hospital Cologne. ADPKD patients aged 18–60 years with an estimated glomerular filtration rate (eGFR) ≥45 mL/min/1.73 m2 and rapidly progressing disease defined as the presence of at least one of the following criteria were enrolled after having obtained written informed consent: Mayo Class 1C–1E, truncating PKD1 mutation, early onset of hypertension (before 35 years of age), early onset of urological complications (before 35 years of age), historical eGFR decline >2.5 mL/min/1.73 m2/year or a PROPKD score >6. Main exclusion criteria were current tolvaptan therapy, body mass index (BMI) <18 or >35 kg/m2, diabetes mellitus, alcohol addiction, vegan or vegetarian lifestyle, known circumstances prohibiting the use of a ketogenic diet {e.g. liver damage [AST/ALT >3× upper limit norm (ULN), AP >6× ULN, bilirubin ≥3 mg/dL], pyruvate carboxylase deficiency, defects of gluconeogenesis, defects of ketolysis/ketoneogenesis, hyperinsulinism, defects of fatty acid oxidation}, allergies and intolerances to components of a KD, eating disorders, participation in a weight loss program or taking weight loss promoting medication in the last 6 months, KD for more than 1 month in the last 12 months, chronic renal replacement therapy, previous kidney transplantation, pregnancy or contraindications to magnetic resonance imaging (MRI) scans. Patients were recruited from the German AD(H)PKD cohort (ClinicalTrials.gov Identifier: NCT02497521) or through the patients’ advocacy organization “Familiäre Zystennieren e.V.” If assessed eligible at the screening visit by medical history, physical examination and laboratory parameters, participants were enrolled after having obtained written informed consent. The study was conducted in accordance with the Declaration of Helsinki and the good clinical practice guidelines by the International Conference on Harmonization. The study is registered on www.clinicaltrials.gov (NCT04472624). The study protocol will be provided upon request.
Study structure: visits and intervals
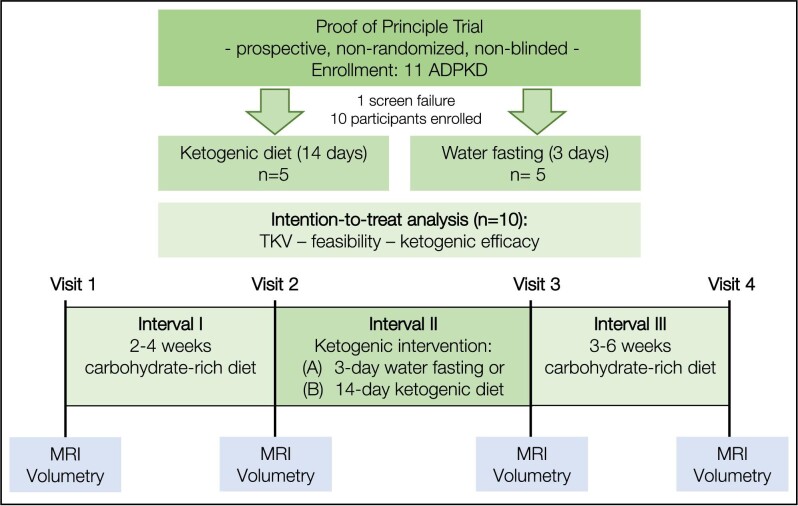
Four study visits (V1–V4) were conducted as part of the study (Fig. 1). Each visit included blood and urine tests, bioimpedance measurements, measurements of anthropometric parameters, measurements of ketosis in fingerstick blood, urine and breath, and an MRI abdomen for kidney and liver volumetry. Between V1 and V2 (Interval 1, I1), patients continued to eat according to their usual dietary habits, i.e. a high-CHO diet, for a minimum of 13 and a maximum of 28 days. After V2, the KDI was started within 7 days and the KDI was finished by V3 (Interval 2, I2). Patients could choose whether they wanted to achieve the ketogenic metabolic state by WF for 3 days or by a KD for 14 days. V3 was conducted after a maximum of 72 h upon termination of the diet. Between V3 and V4 (Interval 3, I3), participants switched back to their standard ad libitum diet for a minimum of 20 and a maximum of 43 days.
Figure 1:
Five ADPKD patients were enrolled in each study arm. Interval 1: patients adhered to their normal CHO-rich diet for 2–4 weeks. Interval 2: patients underwent one of the two KDIs. Interval 3: patients returned to their normal CHO-rich eating habits for 3–6 weeks. Acetone measurements in breath, BHB in capillary blood and urinary ketone bodies concentration were measured regularly. MRI-based volumetry was performed at visits. Safety: blood and urine tests were performed at all visits.
During I1 and I3, patients measured the extent of ketosis in breath, urine and fingertip blood at least three times, while in I2, during the KDIs, ketosis measurements were performed twice a day (in the morning before breakfast and in the evening before going to bed). Patients were provided with a diet diary for daily documentation of hunger, well-being and (although discouraged) potential additional foods consumed as well as results of the daily ketosis measurements. After completion of the KDIs, a dedicated questionnaire (Supplementary data, Table S5) was used to assess feasibility and tolerability of the KDIs.
Dietary interventions
Patients in the WF study arm limited oral intake to ad libitum amount of water and a low-salt broth once a day for a period of 3 days; in the KD study arm, patients consumed a very high-fat, low-CHO diet for a period of 14 days according to individual dietary plans. KD was based on a fat:protein:CHO ratio of 10:4:1 (in grams) and calorie requirements were calculated individually for each patient with the Mifflin-St. Jeor equation [12]. Ten percent of the fat calories were provided as medium-chain triglycerides (for example MCT oil). Patients were supplied with the required food items. Patients in the KD group were advised to consume at least 20 kcal/kg body weight, but preferably 25 kcal/kg body weight daily. In addition, regular phone calls ascertained patients’ well-being and monitored adherence to the protocol. Patients in the KD arm were instructed to refrain from additional intake of non-ketogenic foods during the intervention. Both groups of patients received ketogenic snacks in case they desired food in between scheduled meals or experienced undesired symptoms during WF, respectively. Patients were advised to eat blueberries in case of blood beta-hydroxybutyrate (BHB) levels >3.5 mmol/L or breath acetone levels > 40 p.p.m. and/or malaise or symptoms of ketosis (e.g. brain fog, “keto flu”).
Study assessments
Baseline demographic and clinical data including comorbidities and medication were assessed at the screening visit. Anthropometric data (body weight, waist circumference, height, body composition) were recorded at all study visits. Vital parameters (blood pressure, heart rate) were assessed at each study visit. Acetone concentrations were measured using a portable breath analyzer (ACE KETOSCAN mini, ACE Instruments, 83395 Freilassing, Germany). BHB measurements in fingerstick blood were performed with a portable ketone meter (GlucoMen areo 2 K, Berlin-Chemie AG, 12489 Berlin, Germany). Urine ketones were measured using urine dipsticks (Ketostix, Ascensia Diabetes Care Deutschland GmbH, 51377 Leverkusen, Germany). The MRIs were performed by the in-house Department of Radiology on a 1.5-T system (Ingenia, Philips Healthcare, Best, The Netherlands). For assessment of total kidney volume (TKV) and total liver volume (TLV), kidney and liver boundaries of each patient were manually traced in axial T2 SPIR scans by a radiologist using Intellispace Discovery (Philips, Best, The Netherlands). The renal hilum was excluded from the kidney outline while the gallbladder and the main portal vein were excluded from the liver outline. A second reader was employed to additionally segment the kidneys and liver of each patient to estimate inter-reader variability in TKV and TLV. Both readers were blinded to patient information and previous tracing results.
To evaluate cyst fraction in both organs, the T2 map was overlayed with each volume. Using Intellispace Discovery, each voxel within each volume was classified in either cystic or non-cystic depending on a cut-off value of 250 ms. This cut-off appeared to sufficiently differentiate tissue depending on its water content in a previous study [13] and allows to approximate cyst fraction.
The T1 mDIXON sequence which is included in our research protocol facilitates the assessment of fat content of liver tissue using chemical shift imaging. This allowed to check for potential fatty liver disease such as non-alcoholic fatty liver disease (NAFLD). Additionally, morphologic sequences were used to find potential fibrotic or cirrhotic changes of liver tissue.
Clinical chemistry measurements on blood and urine samples were performed by the in-house central laboratory (University Hospital Cologne). Body composition was evaluated using a Tanita BC 418 MA scale (Tanita Europe BV, 1101 BE Amsterdam, The Netherlands).
Outcomes
RESET-PKD (NCT044726249) was designed as a pilot trial analyzing pre-defined exploratory endpoints on feasibility and safety of short-term KDIs as well as their impact on TKV and TLV. Regarding TKV, we focused on the relative change in TKV between V2 and V3 (assessed using MRI volumetry). This analysis was complemented by the absolute and relative differences of TKV/height-adjusted TKV between study visits (i.e. V1 vs V2, V1 vs V3, V1 vs V4, V2 vs V3, V2 vs V4, V3 vs V4); the same timepoints were compared regarding TLV, anthropometric parameters (weight, height, body composition, BMI, waist circumference) and blood pressure. To allow for the detection of potential safety signals a panel of blood and urine values including kidney function, lipids and liver values were examined (Supplementary data, Tables S1 and S2), and feeling of hunger, discomforts and problems with general well-being were analyzed from the diet diary (Supplementary data, Table S2). The biochemical efficacy of the KDIs was assessed as follows: absolute and relative differences in self-measurements of ketosis parameters (at least three measurements in I1, at least twice daily, fasting in the morning and before bedtime in the evening in I2, at least three measurements in I3). Most investigators agree that normal values for BHB on standard Western diets are 0.1–0.5 mmol/L [14, 15]. A non-linear relationship between acetone in breath and BHB in plasma has been described in adults [16]. Therefore, we defined the cutoffs for the metabolic endpoints as follows: for the KD group: acetone level ≥10 p.p.m. or a BHB level ≥0.8 mmol/L in ≥75% of home measurements (excluding the first 3 days of KD); WF group: acetone level ≥10 p.p.m. or a BHB level ≥0.8 mmol/L in ≥75% of home measurements, or alternatively, a ketogenic state (BHB level ≥0.8 mmol/L or acetone level ≥10 p.p.m.) in at least one measurement in either method on 2/3 days during the WF. Feasibility was assessed using a questionnaire that contained 17 questions directly related to the KDI. Patients could rate each question on a scale from −4 to +4 with −4 representing low and +4 high feasibility (Supplementary data, Fig. S6 and Table S5). An average score of ≥0 was required to consider the KDI feasible. Both the metabolic endpoint and the feasibility endpoint had to be reached to meet the combined feasibility endpoint. The self-reported feeling of hunger was recorded regularly (I1 and I3: once each as total value for the entire interval, I2: daily) in the study diary and linked to numbers from 1 (not hungry at all) to 4 (very hungry). Stool, urine and blood samples were frozen for future analyses after patients had given specific informed consent for biobanking.
Statistical analyses
Unless otherwise stated, paired two-tailed t-tests were used for the statistical analyses, and error bars represent standard deviations. The P-values given refer to tests comparing measurements at V2 and V3, i.e. at the start and end of the intervention interval, unless other comparisons are explicitly mentioned. Statistical testing was not adjusted for multiple comparisons considering the exploratory nature of this study; therefore, the results should be interpreted as exploratory.
RESULTS
RESET-PKD enrolled 10 ADPKD patients with indicators of rapid progression in two KDI arms (KD and WF). Out of 11 patients screened, 10 patients were enrolled, one patient did not meet the inclusion criteria due to BMI >35 kg/m² (Fig. 1). The mean age of patients was 39.8 ± 11.6 years while mean eGFR was 82.2 ± 23.5 mL/min/1.73 m2; 80% of all patients included were male. All patients were in Mayo Classes 1C, 1D or 1E. Mean TKV was 2224 ± 1156 mL without significant differences between the groups. 90% of patients reported urological symptoms (60% before the age of 35 years). All patients were diagnosed with arterial hypertension at the time of inclusion (70% before the age of 35 years). Mean BMI was 25.5 ± 2.3 kg/m² with no significant differences between the two study groups (P = .5426). All patients completed the study. Notably, therapy with tolvaptan was an exclusion criterion to avoid its effects as a confounder in small subgroups. All participants had been offered tolvaptan but had rejected this therapy due to concerns regarding polyuria. Baseline characteristics are shown in Table 1.
Table 1:
Patient demographics and clinical characteristics. Baseline characteristics of the study cohort and subgroups.
| Characteristics | All | KD | WF |
|---|---|---|---|
| n | 10 | 5 | 5 |
| Men, n (%) | 8 (80) | 3 (60) | 5 (100) |
| Age (years) | 39.8 ± 11.6 | 40.6 ± 13.4 | 39.0 ± 11.0 |
| eGFR (mL/min/1.73 m²) | 82.2 ± 23.5 | 84.8 ± 21.7 | 79.6 ± 27.4 |
| TKV (mL) | 2224 ± 1156 | 1752 ± 893 | 2696 ± 1286 |
| Mayo Class 1C, n (%) | 4 (40) | 3 (60) | 1 (20) |
| Mayo Class 1D, n (%) | 5 (50) | 2 (40) | 3 (60) |
| Mayo Class 1E, n (%) | 1 (10) | 0 (0) | 1 (20) |
| CKD1, n (%) | 4 (40) | 3 (60) | 1 (20) |
| CKD2, n (%) | 5 (50) | 1 (20) | 4 (80) |
| CKD3a, n (%) | 1 (10) | 1 (20) | 0 (0) |
| BMI (kg/m²) | 25.5 ± 2.3 | 25.1 ± 2.8 | 26 ± 1.9 |
| Weight (kg) | 84.4 ± 12.6 | 78.1 ± 12.2 | 90.7 ± 10.5 |
| Urological symptoms, n (%) | 9 (90) | 5 (100) | 4 (80) |
| Urological symptoms <35 years, n (%) | 6 (60) | 3 (60) | 3 (60) |
| Arterial hypertension, n (%) | 10 (100) | 5 (100) | 5 (100) |
| Arterial hypertension <35 years, n (%) | 7 (70) | 3 (60) | 4 (80) |
| Positive family history, n (%) | 8 (80) | 4 (80) | 4 (80) |
| Serum glucose V1 (mg/dL) | 83 ± 6.9 | 85 ± 6.9 | 81 ± 7 |
| BHB I1 (mmol/L)a | 0.22 ± 0.08 | 0.17 ± 0.08 | 0.28 ± 0.15 |
| Serum uric acid V1 (mg/dL) | 6.2 ± 1.3 | 6.0 ± 1.4 | 6.4 ± 1.4 |
| Serum total cholesterol V1 (mg/dL) | 176.3 ± 26.5 | 181.2 ± 33.7 | 171.4 ± 19.6 |
| Systolic blood pressure at V1 (mmHg) | 130.6 ± 12.7 | 136.8 ± 4.1 | 124.4 ± 15.8 |
Data are given as mean ± SD unless otherwise indicated.
aAverage values for I1 between V1 and V2 are shown.
CKD = chronic kidney disease.
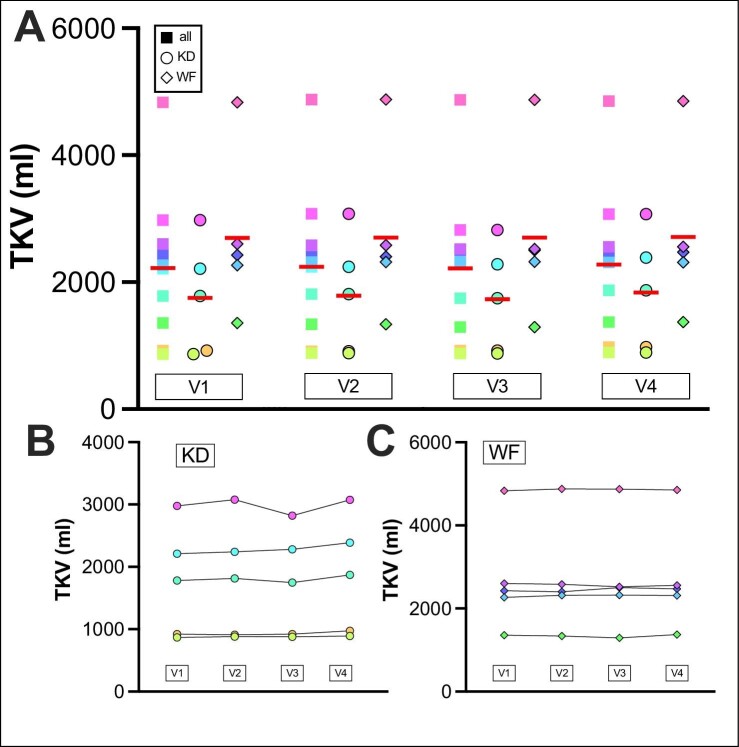
Total kidney volume
Neither KDI resulted in differences in TKV (Fig. 2, Supplementary data, Fig. S1). One patient showed a TKV reduction upon KD by 258 mL (8.38%) and a return to the baseline volume at the final study visit and this reduction was confirmed by a second reader (Supplementary data, Table S6). This patient was consistently ketogenic after Day 3 of the KD (22/22 measurements) and showed the highest in-group acetone levels (mean: 34.3 p.p.m., peak: 82 p.p.m.; mean rest of KD group: 16.1 p.p.m., peak rest of KD group: 49 p.p.m.).
Figure 2:
TKV of patients was monitored at all visits (V1–V4). TKV is shown for (A) all patients, (B) the KD group and (C) the WF group. Red line indicates mean. V2 to V3: interval from start to end of KDIs. All: ΔTKV V1 to V2: +0.75%, SEM: 0.52%, ΔTKV V2 to V3: −1.06%, SEM 1.1%, ΔTKV V3 to V4: +3.28%, SEM 1.13%, TKV V2 to V3 P = .3765. KD: ΔTKV V1 to V2: +1.47%: SEM: 0.69%, ΔTKV V2 to V3: −1.88%, SEM: 1.87%, ΔTKV V3 to V4: +5.54%, SEM: 1.25%, TKV V2 to V3 P = .3605. WF: ΔTKV V1 to V2: +0.03%, SEM: 0.69%, ΔTKV V2 to V3: −0.23%, SEM: 1.28%, ΔTKV V3 to V4: +1.02%, SEM: 1.30%, TKV V2 to V3 P = .9893. SEM: standard error of the mean.
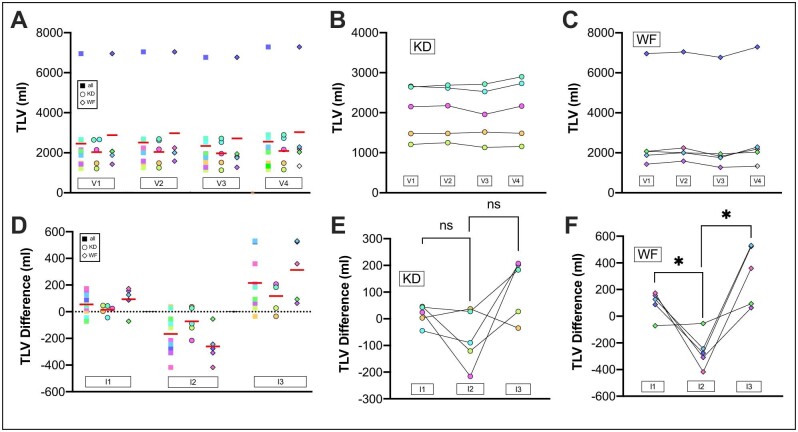
Total liver volume
Sixty percent of patients had more than 10 liver cysts at baseline while no patient showed radiological evidence of NAFLD (Supplementary data, Table S7). TLV decreased significantly from V2 to V3 in all 10 participants (P = .0067) and the WF group (P = .0116), but not in the KD group (P = .2012). We also found a significant difference in ΔTLV comparing the change in I1 and I2 in all 10 participants (P = .0114, Fig. 3). This reduction again reached statistical significance for the WF group (P = .0251) but not for the KD group (P = .1616). Volumetries of a second blinded reader showed very good agreement between the two readings (Supplementary data, Fig. S5) and reproduced the statistically significant findings (Supplementary data, Table S6). All but one patient who reached efficient ketosis experienced a reduction of TLV at the end of the KDI. No significant differences in liver cyst volume (KD P = .3345, WF P = .1845) or cyst fraction were found after the KDIs (KD P = .2545, WF P = .1854). However, we found a significant reduction in liver parenchyma in the WF group (P = .0123) while the trend to lower values in the KD group did not reach statistical significance (P = .144, Supplementary data, Fig. S7, and Tables S8 and S9). The switch back to the CHO-rich ad libitum diet was accompanied by a rebound in TLV (Fig. 3). The ratio of TLV/BW decreased significantly after the KDIs in the whole study cohort and the WF group but was not significantly changed in the KD group (all: P = .0482, V2/V3: KD P = .6351, V2/V3: WF P = .0453; Supplementary data, Fig. S8).
Figure 3:
TLV. TLV was monitored at all visits by MRI. (A) TLV values for all patients at visits. V2 to V3 all P = .0067. (B) TLV values for KD group. V2 to V3 KD P = .2012. (C) TLV values for the WF group. V2 to V3 WF P = 0.0116. (D) TLV differences of the different intervals are shown. The intervals correspond to the periods between the two surrounding visits, for example, I1 between V1 and V2. ΔTLV V1 to V2: +2.9%, SEM: 1.4%, ΔTLV V2 to V3: −7.7%, SEM 2.4%%, ΔTLV V3 to V4: +9.3%, SEM 2.9%; I1 to I2 all: P = .0114, I2 to I3 all: P = .0036. (E) TLV interval differences for KD group are shown. I1 to I2 KD P = .1616, I2 to I3 KD P = .0834. (F) TLV interval differences for WF group are shown. I1 to I2 WF P = .0251, I2 to I3 WF P = .0125.
Effects on body weight and blood pressure
Both KDIs induced a significant weight loss (Supplementary data, Fig. S2). Loss of body water and loss of fat mass contributed equally (Supplementary data, Table S4). Blood pressure values did not show any significant changes (Supplementary data, Table S4). However, two patients in the KD group reported lower blood pressure values in their home measurements. In one of those patients, antihypertensive medication had to be paused upon the start of the ketogenic diet due to orthostasis. All anthropometric parameters are provided in Supplementary data, Table S4.
Biochemical efficacy as a readout of adherence
KDIs induced a significant increase in acetone and BHB levels during the KDIs in both groups (Fig. 4). Glucose levels showed a significant drop only in the WF group after the KDI (Supplementary data, Table S1, all: P = .0111, KD: P = .4929, WF: P = .0003).
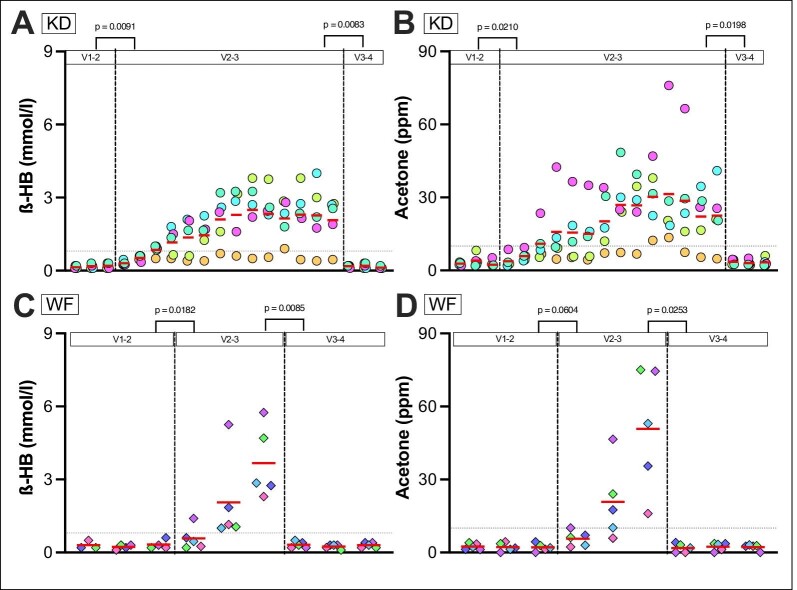
Figure 4:
Metabolic endpoints of KD group (A and B) and WF group (C and D). Self-measurements of finger-stick BHB (A, C) and acetone levels in breath (B, D) are shown. Daily mean values are shown (separate morning and evening values are provided in Supplementary data, Fig. S3). The red line represents the mean. The dotted grey line (A–D) is set at a cutoff of 10 p.p.m. for breath acetone and 0.8 mmol/L BHB, respectively. For one patient in the WF group, no BHB measurements are available in I1 because he accidentally measured glucose values instead. The visit measurements (see Supplementary data, Table S1) show significant increases in BHB and acetone levels in both groups from V2 to V3. Mean I1 to I2 P-values acetone: all P = .0006, KD P = .0210, WF P = .0224. Mean I1 to I2 P-values BHB: all P = .0008, KD P = .0091, WF P = .0604. PPM = parts per million.
Self-reported feasibility
Five of five patients in the KD group rated KDIs as feasible with an average score per question of 0.39 points. In the WF group, 1/5 patients reached a score below the feasibility threshold (score: −0.58), while the other four participants reached an average score of 0.75. In total, 90% of patients reached the pre-defined feasibility threshold (Fig. 5A). The individual components of the feasibility questionnaire are provided in Supplementary data, Table S5 while the individual score for each question can be found in Supplementary data, Fig. S6.
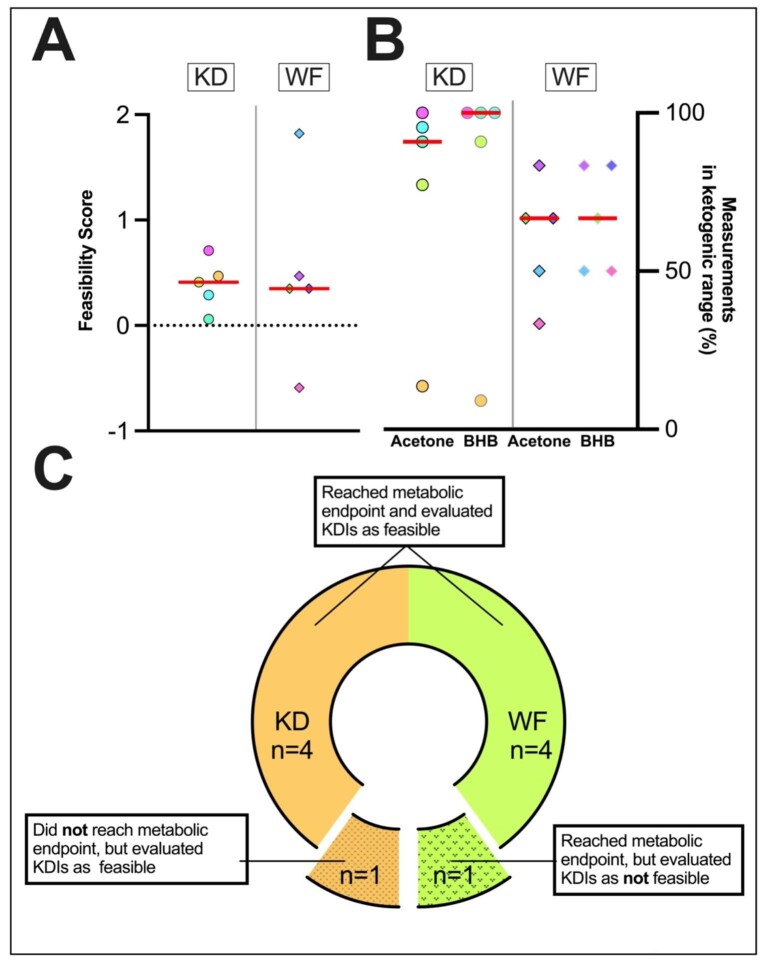
Figure 5:
Patient-reported feasibility of the KDIs. (A) Average feasibility score for individual patients, assessed with a self-designed feasibility questionnaire. Patients scored 17 questions focusing on feasibility from −4 (very negative) to +4 (very positive). Feasibility was defined as an average score per question ≥0. Nine of 10 of patients rated KDIs as feasible. (B) Percentage of measurements in the ketogenic range for individual patients in home measurements are shown. The metabolic endpoint was defined as: for the KD group acetone level ≥10 p.p.m. or a BHB level ≥0.8 mmol/L in ≥75% of home measurements (excluding the first 3 days of KD);—WF group: acetone level ≥10 p.p.m. or a BHB level ≥0.8 mmol/L in ≥75% of home measurements, or alternatively, a ketogenic state (BHB level ≥0.8 mmol/L or acetone level ≥10 p.p.m.) in at least one measurement in either method on 2/3 days during the WF). Five of 5 patients in the WF group and 4/5 patients in the KD group reached the metabolic endpoint. The only patient who did not reach the metabolic endpoint is represented by the yellow dot. (C) Combined feasibility endpoint: 8/10 patients reached the metabolic endpoint and rated KDIs as feasible.
The KD group showed no significant changes in feeling of hunger levels (I1 vs I2: P = .7592), while the WF group showed a significant increase in hunger (I1 vs I2: P = .0299), which was most pronounced on Day 1 of the intervention (Supplementary data, Fig. S4). Except for predefined rationed doses of blueberries, and an extra salt broth consumed once by a KD group patient, no intake of additional food was reported. Sixty percent of patients in the KD group said they would like to incorporate aspects of the KD into their future eating habits, and 100% in the WF group could see themselves fasting again in the future.
Combined feasibility endpoint
Actual feasibility is only assured if patient-reported feasibility is reached in the setting of actual efficient ketogenesis. Five of five patients (WF) and 4/5 patients (KD) reached the pre-defined metabolic endpoint (Fig. 5B). One patient in the KD group did not reach ketosis. In total, 90% of patients reached the metabolic endpoint and/or the self-reported feasibility endpoint while 80% of all patients reached both the metabolic endpoint and rated KDIs as feasible (Fig. 5C).
Safety and general changes in lab values
All lab values and safety-relevant symptoms are summarized in Supplementary data, Tables S1–S3. Only mild changes in lab values were recorded. Briefly, to report the most relevant findings reaching statistical significance, a mild decrease in pCO2 and HCO3− could be observed in the WF group. Serum bilirubin and AP levels showed a significant mild increase only upon WF. Cholesterol and low-density lipoprotein-cholesterol (LDL-C) increased significantly only in the KD group (KD: V2/V3 total cholesterol P = .0151, V2/V3 LDL-C: P = .0287; WF: total cholesterol: P = .1022, LDL-c: P = .0522) while high-density lipoprotein-cholesterol was not altered significantly (Table 2). Cholesterol and LDL-C returned to baseline after switching back to a high-CHO diet. Insulin-like growth factor 1 values decreased significantly only in the WF group. We observed a significant increase in uric acid levels in both groups (uric acid V2/V3 all: P = .0003, KD: P = .0289, WF: P = .0115; Table 2), but no gout attacks or symptomatic kidney stones. Two patients reported self-limited palpitations. One of them had previously experienced cardiac arrhythmias on a standard high-CHO diet as well and patient history suggested an atrioventricular nodal reentry tachycardia. Three of 10 patients consumed blueberries after reaching a BHB value >3.5 mmol/L a total of four times, consistently resulting in the intended slight reductions in ketosis.
Table 2:
Cholesterol and uric acid data are shown.
| All | WF | KD | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | V1 | V2 | V3 | V4 | P | V1 | V2 | V3 | V4 | P | V1 | V2 | V3 | V4 | P |
| Cholesterol, total (mg/dL) | 176.3 ± 26.5 | 179 ± 21.8 | 198.5 ± 27.6 | 184.9 ± 26.8 | .0022 | 171.4 ± 19.6 | 175.4 ± 15.5 | 190 ± 17.3 | 180 ± 18.8 | .1022 | 181.2 ± 33.7 | 182.6 ± 28.3 | 207 ± 35.2 | 189.8 ± 34.7 | .0151 |
| HDL-C (mg/dL) | 54.0 ± 11.8 (9)a | 56.6 ± 10.9 | 55.5 ± 12.0 | 57.5 ± 9.4 | .5879 | 51.8 ± 8.4 | 54.2 ± 12.3 | 52.8 ± 10.1 | 55.6 ± 7.7 | .6757 | 56.8 ± 16.2 (4)a | 59.0 ± 10.1 | 58.2 ± 14.3 | 59.4 ± 11.4 | .7853 |
| LDL-C (mg/dL) | 105.9 ± 30.2 (9)a | 109.7 ± 26.5 | 127.4 ± 31.3 | 114.4 ± 31.3 | .0180 | 107.4 ± 21.8 | 106.6 ± 23.4 | 120.6 ± 20.8 | 107.8 ± 22.3 | .0522 | 104 ± 42.2 (4)a | 112.8 ± 31.7 | 134.2 ± 40.7 | 121 ± 40.0 | .0287 |
| Triglycerides (mg/dL) | 85.9 ± 26.2 | 82.1 ± 36.9 | 101.1 ± 42.2 | 85.1 ± 41.2 | .1592 | 80.4 ± 16.8 | 94.4 ± 45.6 | 108.8 ± 46.1 | 108.8 ± 45.8 | .5820 | 91.4 ± 34.4 | 69.8 ± 24.9 | 93.4 ± 41.7 | 61.4 ± 17.8 | .0772 |
| Uric acid (mg/dL) | 6.2 ± 1.3 | 6.2 ± 1.3 | 10.1 ± 2.0 | 6.1 ± 1.7 | .0003 | 6.4 ± 1.4 | 6.7 ± 1.4 | 10.7 ± 1.4 | 6.9 ± 1.9 | .0115 | 6.0 ± 1.4 | 5.7 ± 1.2 | 9.4 ± 2.4 | 5.2 ± 0.9 | .0289 |
Data are given as mean ± SD. Given P-values refer to tests comparing measurements at V2 and V3.
aIn case of missing values for individual patients the number of measurements is indicated in brackets.
HDL = high-density lipoprotein.
Statistically significantly different values are written in bold.
DISCUSSION
RESET-PKD is the first prospective interventional trial to combine exploratory analyses of metabolic efficacy (ketone bodies), feasibility and efficacy (TKV/TLV) of short-term controlled ketogenic metabolism in patients with ADPKD. Following the promising data on the beneficial effects of ketosis in PKD animal models [4–6], the present study was designed to include patients who could benefit most, i.e. rapid progressors. Although the inclusion criteria were defined inclusively, so that patients with individual clinical criteria of rapid disease progression could have been included, all patients fulfilled the Mayo classification criterion as primary reason for inclusion (i.e. 100% in classes 1C/1D/1E).
Short-term KDIs did not show an acute effect on TKV in either arm. Indeed, dietary interventions in small animals are expected to result in earlier responses than in humans. Interestingly, one patient in the KD group showed a TKV reduction by 8.4% after KD with a return to baseline at the final study visit. This patient was consistently ketogenic throughout the KD and reached peak values for the KD group in acetone measurements. A recent study on intermittent fasting and caloric restriction (CR) in obese ADPKD patients showed positive effects with reduction of body weight and reduction of adipose lipid stores correlating with slowed kidney growth [11]. However, the study did not measure ketone bodies and considering the type of intervention efficient ketosis is not expected. CR without limiting CHO intake hardly induces ketosis and in intermittent fasting a single daily CHO-containing meal interrupts ketogenesis. In studies examining non-ADPKD patients, similar dietary regimens only intermittently lead to very low levels of ketosis [17, 18]. Nevertheless, it is indeed possible that low ketone body levels potentially reached may have contributed to their findings. Whether longer-lasting KDIs have beneficial effects on TKV should be further investigated in larger studies. A randomized controlled clinical trial on this topic (KETO-ADPKD, NCT04680780) is currently ongoing and another study has been announced [19]. TLV measurements showed a significant decrease in 8/10 patients. However, after returning to a CHO-rich diet, there was a clear-cut, prompt rebound. Glucose restriction as in ketosis results in a depletion of liver glycogen stores [20]. Considering that we only found significant changes in the non-cystic liver parenchyma this is likely to be the reason for the reversible TLV changes. In non-ADPKD patients, reductions in liver volume due to low-calorie diets have been widely described and are commonly exploited in bariatric surgery [21, 22]. However, a final conclusion on this topic and the effects of KDIs on cystic and non-cystic liver parenchyma in ADPKD will require analyses of larger cohorts and longer-term intervention in patients with severe polycystic liver disease (PLD), also considering the fact that our study included a high proportion of patients with a very low liver cyst fraction. Consequently, it is worth further investment in this regard taking into account the complete lack of efficient therapeutic options for PLD. Rebounds of TLV have also been described after discontinuation of disease-modifying drug therapy with somatostatin analogues in PLD [23].
Hunger occurred significantly more often in the WF group, while an increased feeling of fullness was occasionally reported in the KD group. Two patients reported self-limited palpitations. Ketogenic diets can lead to prolonged QT time with an increased risk of cardiac arrhythmias [24, 25]. Under ketosis, regular electrocardiogram checks should be considered for patients at risk. Apart from this, no safety-relevant physical complaints, in particular no gout attacks, no kidney stones and no hypoglycemia, were observed. We observed a statistically significant increase in total cholesterol and LDL-C in the KD group and an almost statistically significant increase of LDL-C in the WF group. It is known that KDs and WF can lead to at least transient increases in LDL-C and total cholesterol, most likely through depletion of adipose lipid stores and—for KD—additionally increased intake of fatty acids [26–30]. While cholesterol levels normalize after cessation of fasting, ketogenic diets have historically shown inconsistent effects on cholesterol and LDL-C levels [27, 31, 32]. However, potential increases in total cholesterol and LDL-C may normalize on longer-term ketogenic diets [33, 34]. KDs are known to have several beneficial effects on cardiovascular disease (CVD) risk, such as improvements in body weight, insulin resistance, blood pressure, HbA1c levels or inflammatory markers [35–39]. Besides, the increase in LDL-C is mainly due to large LDL particles, not the more atherogenic small dense LDL particles [40]. However, elevated LDL-C levels are a clearly defined cardiovascular risk factor in clinical practice, regardless of their subtyping, and chronic kidney disease is a state of increased cardiovascular risk in general [41]. Consequently, prospective long-term studies are needed to draw a definitive conclusion on the effects of a prolonged ketogenic diet on cardiovascular risk in ADPKD patients.
Furthermore, there was a significant increase in uric acid resulting in a hyperuricemia in both groups after the KDIs. Increases in uric acid under ketogenic metabolism and fasting have already been described multiple times [42, 43]. One of the reasons for hyperuricemia is competition between BHB and uric acid for the same kidney transport sites [44]. Uric acid levels returned to baseline after resumption of a CHO-rich diet in both groups, while no gout attacks or kidney stones were observed. Whether the increase in uric acid is clinically meaningful will require larger longer-term trials. Patients at risk should be monitored during KDIs and appropriate measures, e.g. prescription of citrate, may be considered.
We also detected a significant increase in serum bilirubin levels in our WF group (all men). Such increases upon fasting are known [45, 46] and considered not to be clinically relevant. The KDIs led to a significant weight loss and a reduction in body fat that persisted even after returning to a CHO-rich diet. Beneficial effects of KDIs, such as improved body weight and anti-inflammatory effects, have been discussed to outweigh the possible adverse effects on CVD risk associated with cholesterol increases [36–39] and have a protective role in NAFLD [47]. Regarding ADPKD, a recent study indicated that weight reduction in overweight patients may slow the rate of kidney growth compared with historical data and obesity has been shown to be associated with disease progression [11, 48]. Previously reported effects of KDIs on blood pressure were not observed in our trial which may be a consequence of the short period. However, blood pressure medication had to be stopped in one patient due to a symptomatic blood pressure decrease upon starting KD.
In total, 80% of all patients reached the combined feasibility and metabolic endpoint. This is in line with recent studies indicating good feasibility of KDIs in ADPKD patients [10, 49]. Besides, Hopp et al. recently reported good feasibility in their 1-year weight-loss trial in ADPKD patients [11]. Some side effects of KDs, like the “keto flu,” occur mainly in the beginning. Therefore, the feasibility of KDs might be even better with longer intake and adaptation to the diet. Taken together there appears to be general good acceptance of dietary interventions among ADPKD patients.
This study has several limitations: most importantly, the small number of participants needs to be considered when interpreting the results of statistical testing. Second, there was a gender imbalance, with 80% of participants being male, which limits the comparability of our data. The KDIs were of short duration. Whether longer-term KDIs thus have a more significant effect, e.g. on TKV, remains unclear. The BHB and acetone cutoffs were based on a limited amount of data. The aim of the present study was (i) to investigate dietary interventions that can be accessible for a wide community which would not be possible if aiming for deep ketosis and (ii) stay clearly in the ketogenic range. Most investigators agree that normal BHB values are between 0.1 and 0.5 mmol/L [14, 15]. Since we were not aiming for deep ketosis, we therefore defined the ketosis range from a BHB value of 0.8 mmol/L, which is significantly above these values and should roughly correspond to an acetone level of 10 p.p.m. [16]. Also, the manufacturer recommendations indicate a target ketosis range between
10 and 40 p.p.m. with the cutoff to ketosis indicated as low as 5 p.p.m. acetone in breath [50].
In conclusion, in our proof-of-principle trial, short-term KDIs in ADPKD are safe, feasible and triggered ketosis effectively but did not show an acute impact on TKV. Larger studies are required to further investigate the potential beneficial effects of KDIs in ADPKD.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the patients involved in the RESET-PKD study for their participation. Furthermore, Cornelia Böhme and Jasmin Garha provided excellent assistance within the Study Center and Israa Karimov supported the generation of the biosamples.
Contributor Information
Simon Oehm, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
Konstantin Steinke, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
Johannes Schmidt, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
Sita Arjune, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
Polina Todorova, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
Christoph Heinrich Lindemann, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
Fabian Wöstmann, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
Franziska Meyer, University of Cologne, Faculty of Medicine and University Hospital, Institute of Diagnostic and Interventional Radiology, Cologne, Germany.
Florian Siedek, University of Cologne, Faculty of Medicine and University Hospital, Institute of Diagnostic and Interventional Radiology, Cologne, Germany.
Thomas Weimbs, Department of Molecular, Cellular and Developmental Biology and Neuroscience Research Institute, University of California Santa Barbara, Santa Barbara, CA, USA.
Roman-Ulrich Müller, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany; Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD), University of Cologne, Faculty of Medicine and University Hospital Cologne, Cologne, Germany.
Franziska Grundmann, University of Cologne, Faculty of Medicine and University Hospital, Department 2 of Internal Medicine and Center for Molecular Medicine, Cologne, Germany.
FUNDING
This project received funding through the Koeln Fortune Program (Faculty of Medicine, University of Cologne) to F.G. R.-U.M. was supported by the Ministry of Science North Rhine-Westphalia (Nachwuchsgruppen.NRW 2015–2021) and the German Research Foundation (CRU 329, DFG MU 3629/6-1, DFG DI 1501/9, DFG MU 3629/3-1).
AUTHORS’ CONTRIBUTIONS
S.O., T.W., R.-U.M. and F.G. conceptualized the study. S.O., K.S., P.T., C.H.L., F.W., F.M. and F.S. performed the investigation. S.O., K.S., R-U.M., F.G. and S.A. were involved in data curation, analysis, validation, and visualization. J.S. performed statistical analysis. R.-U.M. and F.G. acquired funding. S.O., K.S. and S.A. wrote the original manuscript. All authors edited and reviewed the final manuscript.
DATA AVAILABILITY STATEMENT
The complete individual data underlying this article cannot be shared publicly to protect data privacy of the trial participants. The data will be shared on reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
The Dept 2 of Internal Medicine received research funding from Otsuka Pharmaceuticals and ThermoFisherScientific outside the scope of this study. R.-U.M. is a member of the Scientific Advisory Board at Santa Barbara Nutrients. T.W. is an inventor on issued and pending patents of the University of California, Santa Barbara related to PKD, is a shareholder of Santa Barbara Nutrients, Inc., and holds a managerial position, is a scientific advisor and shareholder of Chinook Therapeutics, received research funding from Chinook Therapeutics, and received speaker fees from Sanofi Genzyme.
REFERENCES
- 1. Chiaravalli M, Rowe I, Mannella Vet al. 2-Deoxy-d-glucose ameliorates PKD progression. J Am Soc Nephrol 2016;27:1958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Menezes LF, Lin CC, Zhou Fet al. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 2016;5:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rowe I, Chiaravalli M, Mannella Vet al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat Med 2013;19:488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kipp KR, Rezaei M, Lin Let al. A mild reduction of food intake slows disease progression in an orthologous mouse model of polycystic kidney disease. Am J Physiol Renal Physiol 2016;310:F726–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Warner G, Hein KZ, Nin Vet al. Food restriction ameliorates the development of polycystic kidney disease. J Am Soc Nephrol 2016;27:1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Torres JA, Kruger SL, Broderick Cet al. Ketosis ameliorates renal cyst growth in polycystic kidney disease. Cell Metab 2019;30:1007–23e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huffman J, Kossoff EH.. State of the ketogenic diet(s) in epilepsy. Curr Neurol Neurosci Rep 2006;6:332–40. [DOI] [PubMed] [Google Scholar]
- 8. Longo VD, Mattson MP.. Fasting: molecular mechanisms and clinical applications. Cell Metab 2014;19:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Paoli A, Rubini A, Volek JSet al. Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 2013;67:789–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Strubl S, Oehm S, Torres JAet al. Ketogenic dietary interventions in autosomal dominant polycystic kidney disease—a retrospective case series study: first insights into feasibility, safety and effects. Clin Kidney J. 2021;15:1079–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hopp K, Catenacci VA, Dwivedi Net al. Weight loss and cystic disease progression in autosomal dominant polycystic kidney disease. iScience 2022;25:103697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mifflin MD, St Jeor ST, Hill LAet al. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr 1990;51:241–7. [DOI] [PubMed] [Google Scholar]
- 13. Siedek F, Grundmann F, Weiss Ket al. Magnetic resonance kidney parenchyma-T2 as a novel imaging biomarker for autosomal dominant polycystic kidney disease. Invest Radiol 2020;55:217–25. [DOI] [PubMed] [Google Scholar]
- 14. Burstal RJ, Reilly JR, Burstal B.. Fasting or starving? Measurement of blood ketone levels in 100 fasted elective and emergency adult surgical patients at an Australian tertiary hospital. Anaesth Intensive Care 2018;46:463–7. [DOI] [PubMed] [Google Scholar]
- 15. Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999;15:412–26. [DOI] [PubMed] [Google Scholar]
- 16. Musa-Veloso K, Likhodii SS, Cunnane SC.. Breath acetone is a reliable indicator of ketosis in adults consuming ketogenic meals. Am J Clin Nutr 2002;76:65–70. [DOI] [PubMed] [Google Scholar]
- 17. Liu B, Hutchison AT, Thompson CHet al. Effects of intermittent fasting or calorie restriction on markers of lipid metabolism in human skeletal muscle. J Clin Endocrinol Metab 2021;106:e1389–99. [DOI] [PubMed] [Google Scholar]
- 18. Stekovic S, Hofer SJ, Tripolt Net al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab Cell Metab 2019;30:462–76.e6. [DOI] [PubMed] [Google Scholar]
- 19. Testa F, Marchiò M, D'Amico Ret al. GREASE II. A phase II randomized, 12-month, parallel-group, superiority study to evaluate the efficacy of a modified Atkins diet in autosomal dominant polycystic kidney disease patients. PharmaNutrition 2020;13:100206. [Google Scholar]
- 20. de Cabo R, Mattson MP.. Effects of intermittent fasting on health, aging, and disease. N Engl J Med 2019;381:2541–51. [DOI] [PubMed] [Google Scholar]
- 21. Romeijn MM, Kolen AM, Holthuijsen DDBet al. Effectiveness of a low-calorie diet for liver volume reduction prior to bariatric surgery: a systematic review. Obes Surg 2021;31:350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Wissen J, Bakker N, Doodeman HJet al. Preoperative methods to reduce liver volume in bariatric surgery: a systematic review. Obes Surg 2016;26:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hogan MC, Masyuk T, Bergstralh Eet al. Efficacy of 4 years of octreotide long-acting release therapy in patients with severe polycystic liver disease. Mayo Clin Proc 2015;90:1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bank IM, Shemie SD, Rosenblatt Bet al. Sudden cardiac death in association with the ketogenic diet. Pediatr Neurol 2008;39:429–31. [DOI] [PubMed] [Google Scholar]
- 25. Best TH, Franz DN, Gilbert DLet al. Cardiac complications in pediatric patients on the ketogenic diet. Neurology 2000;54:2328–30. [DOI] [PubMed] [Google Scholar]
- 26. Horne BD, Muhlestein JB, Lappe DLet al. Randomized cross-over trial of short-term water-only fasting: metabolic and cardiovascular consequences. Nutr Metab Cardiovasc Dis 2013;23:1050–7. [DOI] [PubMed] [Google Scholar]
- 27. Kirkpatrick CF, Bolick JP, Kris-Etherton PMet al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol 2019;13:689–711.e1. [DOI] [PubMed] [Google Scholar]
- 28. Krause BR, Hartman AD.. Adipose tissue and cholesterol metabolism. J Lipid Res 1984;25:97–110. [PubMed] [Google Scholar]
- 29. Retterstol K, Svendsen M, Narverud Iet al. Effect of low carbohydrate high fat diet on LDL cholesterol and gene expression in normal-weight, young adults: a randomized controlled study. Atherosclerosis 2018;279:52–61. [DOI] [PubMed] [Google Scholar]
- 30. Savendahl L, Underwood LE.. Fasting increases serum total cholesterol, LDL cholesterol and apolipoprotein B in healthy, nonobese humans. J Nutr 1999;129:2005–8. [DOI] [PubMed] [Google Scholar]
- 31. Dashti HM, Mathew TC, Hussein Tet al. Long-term effects of a ketogenic diet in obese patients. Exp Clin Cardiol 2004;9:200–5. [PMC free article] [PubMed] [Google Scholar]
- 32. Kwiterovich PO Jr, Vining EP, Pyzik Pet al. Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children. JAMA 2003;290:912–20. [DOI] [PubMed] [Google Scholar]
- 33. Groesbeck DK, Bluml RM, Kossoff EH.. Long-term use of the ketogenic diet in the treatment of epilepsy. Dev Med Child Neurol 2006;48:978–81. [DOI] [PubMed] [Google Scholar]
- 34. Yilmaz U, Edizer S, Kose Met al. The effect of ketogenic diet on serum lipid concentrations in children with medication resistant epilepsy. Seizure 2021;91:99–107. [DOI] [PubMed] [Google Scholar]
- 35. Cicero AF, Benelli M, Brancaleoni Met al. Middle and long-term impact of a very low-carbohydrate ketogenic diet on cardiometabolic factors: a multi-center, cross-sectional, clinical study. High Blood Press Cardiovasc Prev 2015;22:389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Forsythe CE, Phinney SD, Fernandez MLet al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008;43:65–77. [DOI] [PubMed] [Google Scholar]
- 37. Mansoor N, Vinknes KJ, Veierod MBet al. Effects of low-carbohydrate diets v. low-fat diets on body weight and cardiovascular risk factors: a meta-analysis of randomised controlled trials. Br J Nutr 2016;115:466–79. [DOI] [PubMed] [Google Scholar]
- 38. Volek JS, Phinney SD, Forsythe CEet al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009;44:297–309. [DOI] [PubMed] [Google Scholar]
- 39. Wood TR, Hansen R, Sigurethsson AFet al. The cardiovascular risk reduction benefits of a low-carbohydrate diet outweigh the potential increase in LDL-cholesterol. Br J Nutr 2016;115:1126–8. [DOI] [PubMed] [Google Scholar]
- 40. Westman EC, Yancy WS Jr, Olsen MKet al. Effect of a low-carbohydrate, ketogenic diet program compared to a low-fat diet on fasting lipoprotein subclasses. Int J Cardiol 2006;110:212–6. [DOI] [PubMed] [Google Scholar]
- 41. Boren J, Chapman MJ, Krauss RMet al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2020;41:2313–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldfinger S, Klinenberg E Jr, Seegmiller JE. Renal retention of uric acid induced by infusion of beta-hydroxybutyrate and acetoacetate. N Engl J Med 1965;272:351–5. [DOI] [PubMed] [Google Scholar]
- 43. Oglodek E, Pilis Prof W. Is water-only fasting safe? Glob Adv Health Med 2021;10:21649561211031178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Palmer BF, Clegg DJ.. Starvation ketosis and the kidney. Am J Nephrol 2021;52:467–78. [DOI] [PubMed] [Google Scholar]
- 45. Griffin PM, Elliott SL, Manton KJ.. Fasting increases serum bilirubin levels in clinically normal, healthy males but not females: a retrospective study from phase I clinical trial participants. J Clin Pathol 2014;67:529–34. [DOI] [PubMed] [Google Scholar]
- 46. Meyer BH, Scholtz HE, Schall Ret al. The effect of fasting on total serum bilirubin concentrations. Br J Clin Pharmacol 1995;39:169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Watanabe M, Tozzi R, Risi Ret al. Beneficial effects of the ketogenic diet on nonalcoholic fatty liver disease: a comprehensive review of the literature. Obes Rev 2020;21:e13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nowak KL, Steele C, Gitomer Bet al. Overweight and obesity and progression of ADPKD. Clin J Am Soc Nephrol 2021;16:908–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Testa F, Marchiò M, Belli Met al. A pilot study to evaluate tolerability and safety of a modified Atkins diet in ADPKD patients. PharmaNutrition 2019;9:100154. [Google Scholar]
- 50. https://asset.re-in.de/add/160267/c1/-/de/002204885IN00/IN_ACE-Keto-Messgeraet-Ketoscan-mini-Keto-Messgeraet.pdf (20 December 2022, date last accessed). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete individual data underlying this article cannot be shared publicly to protect data privacy of the trial participants. The data will be shared on reasonable request to the corresponding author.