Abstract
Objective
The prognosis for acute exacerbation of chronic obstructive pulmonary disease (AECOPD) is not optimistic, and severe AECOPD leads to an increased risk of mortality. Prediction models help distinguish between high‐ and low‐risk groups. At present, many prediction models have been established and validated, which need to be systematically reviewed to screen out more suitable models that can be used in the clinic and provide evidence for future research.
Methods
We searched PubMed, EMBASE, Cochrane Library and Web of Science databases for studies on risk models for AECOPD mortality from their inception to 10 April 2022. The risk of bias was assessed using the prediction model risk of bias assessment tool (PROBAST). Stata software (version 16) was used to synthesize the C‐statistics for each model.
Results
A total of 37 studies were included. The development of risk prediction models for mortality in patients with AECOPD was described in 26 articles, in which the most common predictors were age (n = 17), dyspnea grade (n = 11), altered mental status (n = 8), pneumonia (n = 6) and blood urea nitrogen (BUN, n = 6). The remaining 11 articles only externally validated existing models. All 37 studies were evaluated at a high risk of bias using PROBAST. We performed a meta‐analysis of five models included in 15 studies. DECAF (dyspnoea, eosinopenia, consolidation, acidemia and atrial fibrillation) performed well in predicting in‐hospital death [C‐statistic = 0.91, 95% confidence interval (CI): 0.83, 0.98] and 90‐day death [C‐statistic = 0.76, 95% CI: 0.69, 0.82] and CURB‐65 (confusion, urea, respiratory rate, blood pressure and age) performed well in predicting 30‐day death [C‐statistic = 0.74, 95% CI: 0.70, 0.77].
Conclusions
This study provides information on the characteristics, performance and risk of bias of a risk model for AECOPD mortality. This pooled analysis of the present study suggests that the DECAF performs well in predicting in‐hospital and 90‐day deaths. Yet, external validation in different populations is still needed to prove this performance.
Keywords: AECOPD, mortality, pooled analysis, prediction models
We conducted a meta‐analysis of AECOPD mortality risk prediction models. The results showed that DECAF performs well in predicting in‐hospital death [C‐statistic = 0.91, 95%CI: 0.83–0.98] and 90‐day death [C‐statistic = 0.76, 95%CI: 0.69–0.82] and CURB‐65 performs well in predicting 30‐day death [C‐statistic = 0.74, 95%CI: 0.70–0.77].

1. INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a slowly progressing incurable respiratory disease that causes morbidity and mortality worldwide. In many countries, the prevalence of COPD has increased steeply with age, with the highest prevalence amongst those aged >60 years. 1 The Global Burden of Disease Study estimated that the global incidence of COPD was 3.9% in 2017, with 41.9 deaths from COPD per 100 000 people worldwide per year, representing the highest case fatality among chronic respiratory diseases. 2 Acute exacerbation of COPD (AECOPD) is defined as an acute worsening of respiratory symptoms that necessitate additional therapy. 3 The economic burden of treatment for COPD exacerbations accounts for the largest proportion of the cost of the disease. Studies have shown that hospitalization for AECOPD is independently associated with mortality. 4 Mortality after AECOPD ranges from 3.6% of short‐term mortality (within 90 days) to 31% of long‐term mortality (between 90 days and 2 years), and the mortality rate of patients admitted to intensive care units (ICUs) is as high as 29%. 5 Therefore, early assessment of the prognosis of patients with AECOPD and timely adjustment of treatment options can help reduce mortality and combat negative emotions.
A clinical risk prediction model is a mathematical equation that relates multiple predictive factors to disease diagnosis or prognosis. 6 As a quantitative tool for risk and benefit assessment, the prediction model can distinguish between low‐risk and high‐risk populations, which is helpful in upgrading the treatment plan or prescribing palliative treatment for a high‐risk population. We have found that mortality from AECOPD is associated with multiple independent predictors, such as age, low body mass index and heart failure, among others. 5 Notably, the multidimensional scoring system can better predict subsequent survival than a single predictor. 7 Previous studies have constructed research maps of prognostic models for patients with COPD but have not limited disease stage and prognosis. 8 Therefore, knowledge on prediction models for mortality in patients with AECOPD is limited. In addition, the efficacy and accuracy of these prognostic models differ; thus, strict review and screening are required for clinical application. This study aimed to systematically review prediction models for the risk of mortality from AECOPD to help clinical decision‐makers select appropriate prediction models.
2. METHODS
The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO), and the registration number is CRD42022328505.
2.1. Search strategy
PubMed, EMBASE, Cochrane Library, and Web of Science databases were searched from their inception to 10 April 2022. The search terms applied were as follows: (‘acute exacerbation of chronic obstructive pulmonary disease’ OR ‘AECOPD’ OR ‘acute exacerbation of COPD’ OR ‘exacerbation of COPD’ OR ‘COPD exacerbation’) AND (‘predict*’ OR ‘progn*’ OR ‘score’ OR ‘risk calculation’ OR ‘risk assessment’ OR ‘risk factor’ OR ‘model’ OR ‘machine learning’ OR ‘artificial intelligence’ OR ‘algorithm’ OR ‘deep learning’ OR ‘regression’) AND (‘death’ OR ‘mortality’ OR ‘survival’). Additionally, we manually searched for references and relevant articles to identify additional studies. The detailed retrieval strategies and steps are presented in Table S1.
2.2. Study selection
We included articles written in English that developed or validated prediction models for mortality risk in patients with AECOPD. Meanwhile, studies with incomplete data, duplicate publications, conference abstracts and study protocols were excluded.
2.3. Data extraction
Two reviewers (ZLJ and SYL) independently screened the literature and collected data, including author information, year of publication, country, research type, prediction results, sample size, predictors, model discrimination and calibration, modelling method and methods for handling missing data. In cases of disagreement, decisions were made following discussion with a third investigator (YX).
2.4. Assessment of risk of bias
The prediction model risk of bias assessment tool (PROBAST) 9 (Table S2) was used to assess the quality of the included studies, with 20 questions in four key domains: participants, predictors, outcome and analysis. Each question was answered with ‘yes/probably yes’, ‘no/probably no’ and ‘no information’. Moreover, the evaluation results of each domain were judged using ‘low’, ‘high’ or ‘unclear’. Assessments were performed independently by two investigators (ZLJ and JXX), and in cases of disagreement, decisions were made following discussion with a third investigator (YX).
2.5. Statistical analysis
A descriptive analysis was used to summarize the general findings of the predictive models, and the frequencies of the variables were calculated. In addition, a random‐effects meta‐analysis using STATA software (version 16) was used to synthesize C‐statistics from multiple studies validating the same model. 10 Between‐study heterogeneity was quantified using the I 2 statistic. If I 2 was >50%, the studies were considered statistically heterogeneous. The STATA command is listed in Table S3. The meta‐analysis was summarized in a forest plot showing pooled performance.
3. RESULTS
3.1. Study selection
A total of 4376 pieces of literature were obtained through database searching, and 37 pieces of literature 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 were finally included after the screening. The process and results are shown in Figure 1. A list of excluded studies and reasons for exclusion are provided in Table S4. This study included 26 studies 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 that developed models with or without validation and 11 studies 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 that only validated models. Fifteen studies 30 , 32 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 were finally included for quantitative statistical analysis.
FIGURE 1.
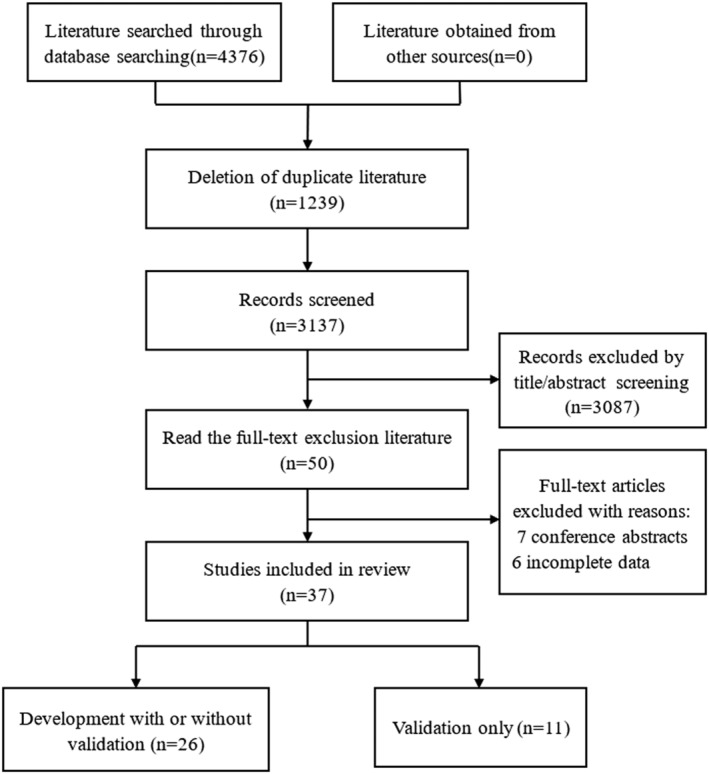
Literature screening flow chart.
3.2. Characteristics of the 26 studies that developed models with or without validation
We found 26 studies 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 describing the development of risk prediction models for mortality in patients with AECOPD. In‐hospital death (n = 15) was the most common predictor of mortality. The prediction models were mainly built in the United States (n = 5), Spain (n = 5) and China (n = 3). The sample sizes ranged from 61 to 150 035 for the development cohort and 284 to 149 646 for the validation cohort. The characteristics of the model building are listed in Table 1. Additional details are provided in Tables S5 and S6. The prediction models were built with different situations: six emergency departments, three ICUs, one primary care and 16 studies 13 , 14 , 15 , 16 , 17 , 18 , 19 , 22 , 25 , 26 , 27 , 30 , 33 , 34 , 35 , 36 did not specifically address a particular hospitalization setting, which we summarized as an inpatient setting. Internal validation of the model was performed using bootstrapping (n = 9), random splitting (n = 7) and a combination of methods (n = 2). The two most frequently used modelling methods were logistic regression (n = 20) and classification and regression tree (n = 3). Many studies did not report how to handle missing values, and imputation (n = 6) was used for the few models for which this was performed. Four studies 18 , 33 , 34 , 35 assessed the calibration of the model using the Hosmer–Lemeshow test and calibration plot, and the Hosmer–Lemeshow test was the most frequently used calibration method. Many studies adopted the sum score (n = 14) to represent the model, and four studies 11 , 13 , 31 , 34 reported this equation. As shown in Figure 2, among the 26 prediction models, the most commonly used predictors were age (n = 17), dyspnoea grade (n = 11), altered mental status (n = 8), pneumonia (n = 6) and blood urea nitrogen (BUN, n = 6).
TABLE 1.
Characteristics of the 26 studies that developed models with or without validation.
| Inpatient setting (n = 16) | Emergency department (n = 6) | Intensive care unit (n = 3) | Primary care (n = 1) | Overall (n = 26) | |
|---|---|---|---|---|---|
| Internal validation | |||||
| Random splitting | 3 | 3 | 1 | 0 | 7 |
| Bootstrapping | 6 | 1 | 1 | 1 | 9 |
| Cross‐validation | 0 | 0 | 0 | 0 | 0 |
| Combination of methods | 1 | 1 | 0 | 0 | 2 |
| None | 6 | 1 | 1 | 0 | 8 |
| Modelling method | |||||
| Logistic regression | 11 | 5 | 3 | 1 | 20 |
| Generalized linear model | 1 | 0 | 0 | 0 | 1 |
| Classification and regression tree | 2 | 1 | 0 | 0 | 3 |
| Machine learning | 1 | 0 | 0 | 0 | 1 |
| More than one method | 1 | 0 | 0 | 0 | 1 |
| Not reported | 0 | 0 | 0 | 0 | 0 |
| Handling of missing data | |||||
| Imputation | 4 | 1 | 0 | 1 | 6 |
| No missing values | 0 | 0 | 0 | 0 | 0 |
| Inappropriate handling | 2 | 0 | 0 | 0 | 2 |
| Not reported | 10 | 5 | 3 | 0 | 18 |
| Model discrimination | |||||
| C‐statistic | 16 | 6 | 3 | 1 | 26 |
| None | 0 | 0 | 0 | 0 | 0 |
| Model calibration | |||||
| Hosmer–Lemeshow test | 3 | 1 | 1 | 0 | 5 |
| Calibration plot | 1 | 0 | 0 | 1 | 2 |
| Hosmer–Lemeshow test and Calibration plot | 4 | 0 | 0 | 0 | 4 |
| Other | 4 | 0 | 0 | 0 | 4 |
| None | 4 | 5 | 2 | 0 | 11 |
| Model presentation | |||||
| Sum score | 9 | 4 | 1 | 0 | 14 |
| Decision tree | 1 | 1 | 0 | 0 | 2 |
| Nomogram | 1 | 1 | 0 | 0 | 2 |
| Equation | 2 | 0 | 1 | 1 | 4 |
| More than one method | 1 | 0 | 0 | 0 | 1 |
| None | 2 | 0 | 1 | 0 | 3 |
FIGURE 2.
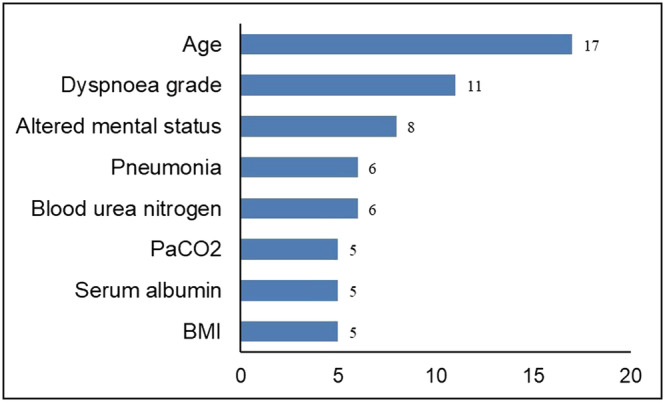
Predictors in 26 risk prediction models for AECOPD mortality. AECOPD, acute exacerbation of chronic obstructive pulmonary disease; BMI, body mass index; PaCO2, partial pressure of carbon dioxide.
3.3. Characteristics of the 11 studies that only validated the models
As shown in Table 2, 11 articles 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 were the only externally validated existing models, and the sample size ranged from 100 to 3321. Three studies 39 , 40 , 43 were from the United Kingdom. The application occasion for two studies 40 , 42 was the emergency room, and three studies 39 , 42 , 43 dealt with missing data using imputation. All studies used C‐statistics to express discrimination, and the Hosmer–Lemeshow test was the most frequently used calibration method. The primary outcome was in‐hospital mortality. Additional details are provided in Tables S7 and S8.
TABLE 2.
Characteristics of the 11 studies that only validated the models.
| Inpatient setting (n = 8) | Emergency department (n = 2) | Intensive care unit (n = 1) | Overall (n = 11) | |
|---|---|---|---|---|
| Handling of missing data | ||||
| Imputation | 2 | 1 | 0 | 3 |
| No missing values | 0 | 1 | 0 | 1 |
| Inappropriate handling | 0 | 0 | 0 | 0 |
| Not reported | 6 | 0 | 1 | 7 |
| Model calibration | ||||
| Hosmer–Lemeshow test | 3 | 0 | 0 | 3 |
| Calibration plot | 0 | 1 | 0 | 1 |
| Hosmer–Lemeshow test and Calibration plot | 0 | 1 | 0 | 1 |
| Other | 2 | 0 | 0 | 2 |
| None | 3 | 0 | 1 | 4 |
3.4. Risk of bias assessment
We evaluated 37 studies 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 for the risk of bias using the PROBAST checklist, and all studies were at a high risk of bias, as shown in Figure 3. The main sources of risk were failure to correctly assess predictive model performance (n = 29), insufficient sample size (n = 27), selection of predictors using univariate analysis (n = 17), inappropriate data sources (n = 17), lack of internal validation (n = 8), continuous predictors handled inappropriately (n = 7) and missing data not handled appropriately (n = 2).
FIGURE 3.
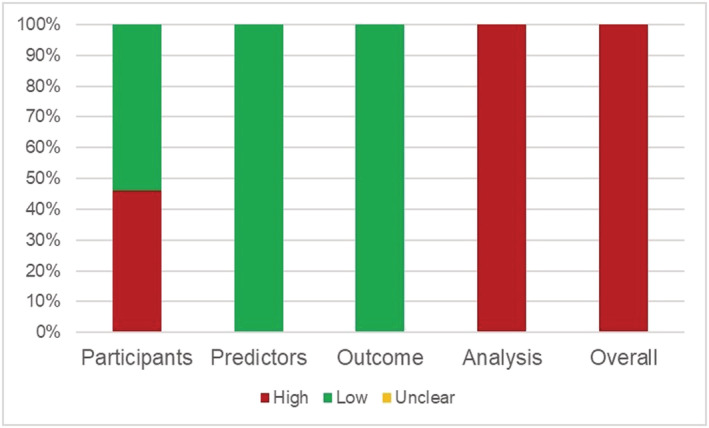
Risk of bias assessment in 37 studies.
3.5. Statistical analysis
We performed a meta‐analysis of the C‐statistics of the five models included in the 15 studies. 30 , 32 , 34 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 More details are provided in Table S9. The results are shown in Figures 4, 5, 6, 7. The pooled C‐statistics of BAP‐65 (BUN, altered mental status, pulse, age), CURB‐65 (confusion, urea, respiratory rate, blood pressure, and age), and DECAF (dyspnoea, eosinopenia, consolidation, acidemia and atrial fibrillation) in predicting in‐hospital mortality were 0.71 (95% confidence interval [CI]: 0.67, 0.74), 0.74 (95% CI: 0.70, 0.77) and 0.91 (95% CI: 0.83, 0.98) respectively. Those for predicting 30‐day mortality were 0.71 (95% CI: 0.68, 0.75), 0.74 (95% CI: 0.70, 0.77) and 0.73 (95% CI: 0.61, 0.86), respectively. Further, those for predicting 90‐day mortality were 0.65 (95% CI: 0.59, 0.72), 0.67 (95% CI: 0.56, 0.77) and 0.76 (95% CI: 0.69, 0.82), respectively. The pooled C‐statistic of NEWS (respiratory rate, oxygen saturation, temperature, systolic blood pressure, pulse rate and level of consciousness) in predicting in‐hospital mortality was 0.72 (95% CI: 0.63, 0.81); the performance of CODEX (comorbidity, obstruction, dyspnoea and previous severe exacerbations) in predicting 90‐day mortality was 0.71 (95% CI: 0.65, 0.76), and that for 1‐year mortality was 0.67 (95% CI: 0.65, 0.70).
FIGURE 4.
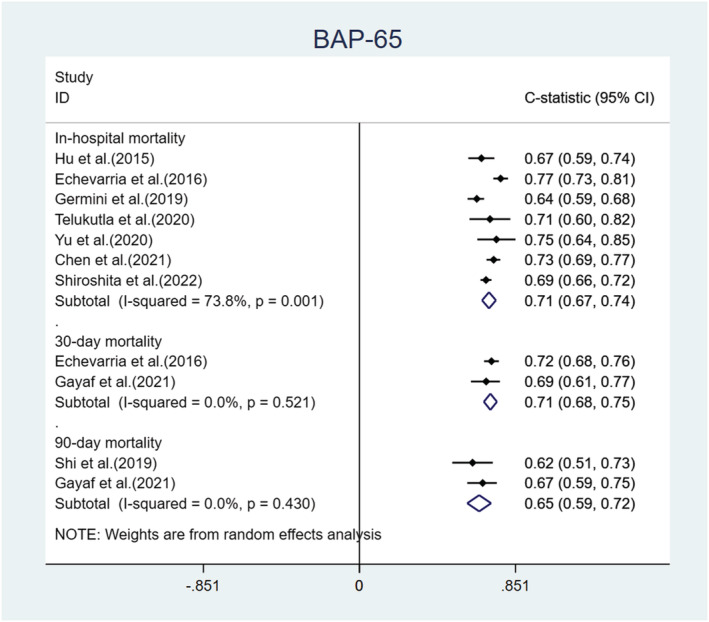
Forest plot showing C‐statistics of BAP‐65 scores in predicting in‐hospital, 30‐day, and 90‐day mortalities.
FIGURE 5.
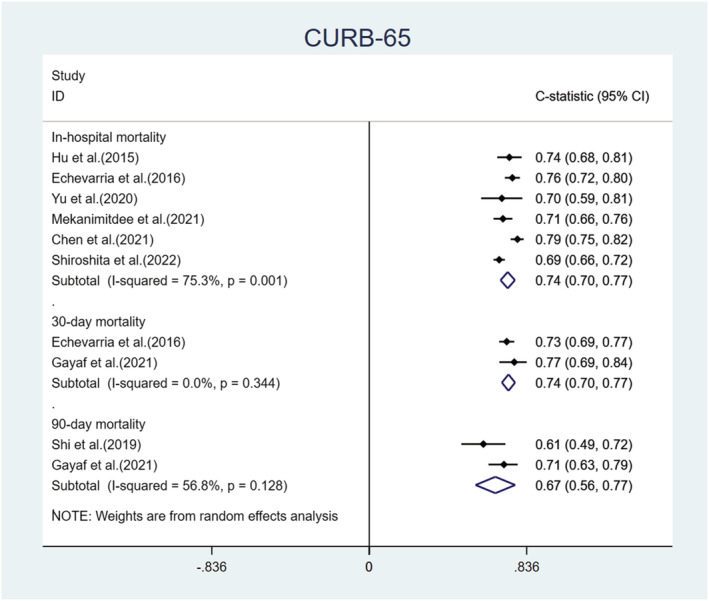
Forest plot showing C‐statistics of CURB‐65 scores in predicting in‐hospital, 30‐day, and 90‐day mortalities.
FIGURE 6.
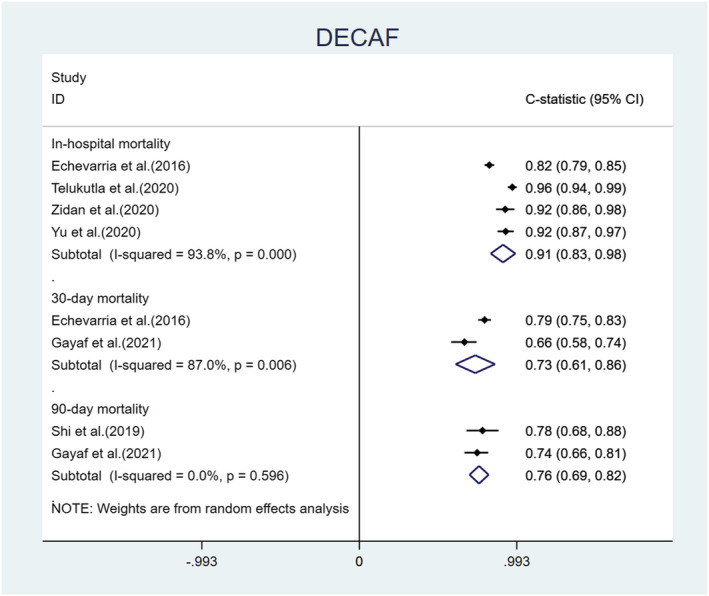
Forest plot showing C‐statistics of DECAF scores in predicting in‐hospital, 30‐day, and 90‐day mortalities.
FIGURE 7.
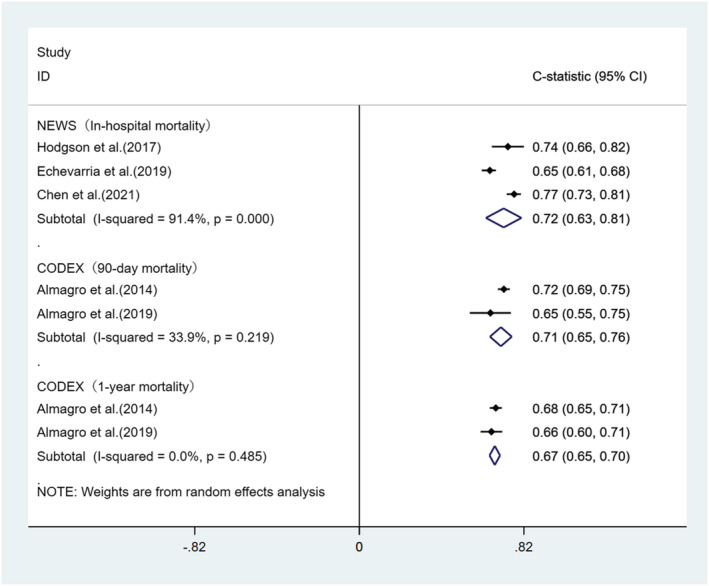
Forest plot showing C‐statistics of NEWS scores in predicting in‐hospital mortality and CODEX scores in predicting 90‐day and 1‐year mortalities.
4. DISCUSSION
This systematic review of risk prediction models for mortality in patients with AECOPD included 37 studies, presenting 26 studies of the model development processes and 11 studies to validate the models. Although a certain number of models can be chosen, there is a high risk of bias for all model developments or validations. Age, dyspnoea grade, altered mental status, pneumonia and urea nitrogen were the most frequently used predictors to develop risk models for mortality from AECOPD. We also performed a meta‐analysis on the external validation of the five models, and the most external validation scales were BAP‐65, CURB‐65 and DECAF.
The present study revealed methodological flaws during model building and validation, which were also reflected in the assessment of the risk of bias. The performance of the model was typically demonstrated by discrimination and calibration, with all studies providing C‐statistics; however, only eight studies 18 , 26 , 31 , 33 , 34 , 35 , 40 , 42 provided calibration plots. Calibration assessed by the Hosmer–Lemeshow test has limited applicability for assessing poorer calibration and is sensitive to the number of groups and sample size. The sample size of 27 studies 11 , 12 , 15 , 17 , 20 , 22 , 23 , 24 , 25 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 36 , 37 , 38 , 39 , 40 , 42 , 43 , 44 , 45 , 46 , 47 was insufficient. Moreover, studies have suggested a minimum of 20 events per independent variable (EPV) for model development, 48 and an EPV for model validation should be greater than or equal to 100. Seventeen studies 12 , 15 , 17 , 18 , 20 , 21 , 22 , 23 , 24 , 26 , 27 , 28 , 29 , 30 , 31 , 34 , 35 employed univariate analysis to screen for predictors that would miss important variables and, therefore, have a high risk of bias. Moreover, there were 17 retrospective cohort studies 14 , 16 , 18 , 19 , 21 , 26 , 28 , 31 , 32 , 33 , 34 , 36 , 37 , 40 , 41 , 42 , 43 with a high risk of bias because data from retrospective studies are often inconsistently measured and recorded. 49 Most studies perform internal validation during model development, which provides more accurate estimates of model performance. The dichotomization of continuous predictors should be avoided because it leads to a loss of information and reduces the model's predictive ability. 50 Missing data need to be handled appropriately, with only six studies 31 , 34 , 36 , 39 , 42 , 43 in this study dealing with missing values using multiple imputations, which outperformed other methods in controlling bias and precision. 51 Many studies were completed before the publication of PROBAST. Thus, the assessment of previous studies using the new evaluation criteria may be too stringent.
This study found that age, degree of dyspnoea, altered mental status, pneumonia and BUN were important predictors in the models. Consistent with previous reports, 5 , 52 age was a significant predictor of mortality. As age increases, the quality of life and the physical and functional status of various organs continue to decline, resulting in older people being more susceptible to various diseases and a gradually increasing mortality rate. Studies showed that the dyspnoea grade was independently associated with in‐hospital mortality in AECOPD, 53 and the dyspnoea grade predicted survival more closely than it did according to the percentage of predicted forced expiratory volume in the first second (FEV1). 54 Most of the included studies used the British Medical Research Council (MRC) scale, modified MRC scale and extended MRC dyspnoea score to assess dyspnoea grade. The altered mental status evaluation was mainly performed using the Glasgow Coma Scale (GCS), which indicates acute cardiopulmonary impairment. 20 The GCS was first used to evaluate patients with a head injury and is widely used to evaluate patients' mental health. 55 Studies have shown that the GCS is independently associated with the death of patients with AECOPD in the ICU. 5 It is estimated that approximately 18% of hospitalized patients with AECOPD have concomitant pneumonia. 56 Pneumonia is common in patients with AECOPD and is associated with higher mortality. 57 BUN is a key factor reflecting the intricate interrelationship between patients' nutritional status, protein metabolism and renal status, and high BUN levels can help identify patients with more severe clinical conditions. 58 The BUN has also been considered an important marker of poor prognosis in respiratory diseases, 59 and in AECOPD, it may reflect intravascular volume depletion from poor oral intake and hyperventilation in the days before admission. 14
The DECAF, BAP‐65 and CODEX indices were specifically developed to predict the risk of death from AECOPD. DECAF used the expectation–maximization algorithm for the imputation of missing data and used the bootstrap method for internal validation. Although its EPV is <20 and screening of predictors using univariate analysis causes some bias to the model, the DECAF score has been consistently shown to be a good predictive model because of the simplicity of the measured variables and its external validation in multiple national populations. 60 The C‐statistic of the DECAF score was 0.91 for in‐hospital deaths and 0.73 for 30‐day deaths in this study, with large heterogeneity that may be related to the fact that the study participants were from different regions and records, where data were inconsistently collected. The BAP‐65 score is also a simpler and more convenient model. Although the model was not assessed for calibration, extensive external validation demonstrated good predictive performance of BAP‐65 with a pooled C‐statistic of 0.71 for both in‐hospital and 30‐day mortalities. The CODEX score is suitable for predicting long‐term mortality in AECOPD (such as 90‐day and 1‐year mortalities); however, its predictive ability is weak. CURB‐65 was originally established to assess the severity of community‐acquired pneumonia. 61 However, it has also been largely validated in the AECOPD population, with a pooled C‐statistic of 0.74 for both in‐hospital and 30‐day deaths, outperforming BAP‐65. The NEWS is often used to assess the severity of acute diseases to remind clinicians of the deterioration of the disease. It is less commonly used in AECOPD, and additional external validation is needed. Compared with BAP‐65, CURB‐65 and CODEX, DECAF performed best at predicting 90‐day mortality, and further external validation is needed to validate the predictive ability of this model.
One strength of this study was the systematic description of the methodological characteristics during the development and validation of the risk prediction model for AECOPD mortality. A meta‐analysis of the C‐statistics was performed using commonly used external validation models. We also performed a risk of bias assessment of the included studies using the PROBAST. Our limitation is that only English literature was included, and it is possible that high‐quality studies in other languages were missed. Moreover, we did not conduct a meta‐analysis of the calibration of the model because the reports and data were too few to perform that analysis.
5. CONCLUSION
This study provides information on the characteristics, performance and risk of bias of a risk model for AECOPD mortality. Despite the development of many models, the number of models that have undergone extensive external validation and can be applied clinically is poor. In addition, the safety, clinical effectiveness and cost‐effectiveness of the models should be considered. The meta‐analysis of the present study suggests that the DECAF performs well in predicting in‐hospital and 90‐day mortalities. However, external validation in different populations is still needed to support this.
AUTHOR CONTRIBUTIONS
Yang Xie and Xuanlin Li would answer for the design and conception of the article, Zile Ji, Siyuan Lei and Jiaxin Xu would answer for the collection and assembly of materials; Zile Ji, Siyuan Lei and Xuanlin Li would answer for data interpretation and analysis; Zile Ji drafted the manuscript; Xuanlin Li, Siyuan Lei, Jiaxin Xu and Yang Xie revised it. All authors reviewed and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
ETHICS STATEMENT
Not applicable.
Supporting information
Table S1: Search strategy(2022.4.10).
Table S2: PROBAST: Assessment of Risk of Bias.
Table S3: The STATA command.
Table S4: Characteristics of excluded studies.
Table S5: Basic characteristics of the 26 studies that developed models (whether validated or not).
Table S6: Methodological characteristics of the 26 studies that developed models (whether validated or not).
Table S8: Methodological of characteristics of the 11 studies that only validated the models.
Table S9: Data from 15 studies used for meta‐analysis
ACKNOWLEDGMENTS
We would like to thank all the authors whose articles have been used in this systematic review.
Ji Z, Li X, Lei S, Xu J, Xie Y. A pooled analysis of the risk prediction models for mortality in acute exacerbation of chronic obstructive pulmonary disease. Clin Respir J. 2023;17(8):707‐718. doi: 10.1111/crj.13606
Funding information This work was supported by the National Key R&D Program (2018YFC1704806); Henan Science and Technology Research Project (212102311128); Natural Science Foundation of Henan Youth Fund (212300410056); Henan Province Priority and Advantage Discipline Construction Engineering Projects‐Traditional Chinese Medicine (STG‐ZYX01‐202102); Henan Medical Science and Technology Research Program (LHGJ20220586); and Henan Province Second Batch of Top‐notch Chinese Medicine Talent Projects (2021 No.15).
DATA AVAILABILITY STATEMENT
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Global Initiative for Chronic Obstructive Lung Disease . (updated 2022). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Accessed April 23, 2022. https://goldcopd.org/2022-gold-reports-2/
- 2. GBD Chronic Respiratory Disease Collaborators . Prevalence and attributable health burden of chronic respiratory diseases, 1990‐2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. 2020;8(6):585‐596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370(9589):786‐796. doi: 10.1016/S0140-6736(07)61382-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soler‐Cataluña JJ, Martínez‐García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925‐931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann am Thorac Soc. 2013;10(2):81‐89. doi: 10.1513/AnnalsATS.201208-043OC [DOI] [PubMed] [Google Scholar]
- 6. Ranstam J, Cook JA, Collins GS. Clinical prediction models. Br J Surg. 2016;103(13):1886. doi: 10.1002/bjs.10242 [DOI] [PubMed] [Google Scholar]
- 7. Celli BR, Cote CG, Marin JM, et al. The body‐mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005‐1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 8. Bellou V, Belbasis L, Konstantinidis AK, Tzoulaki I, Evangelou E. Prognostic models for outcome prediction in patients with chronic obstructive pulmonary disease: systematic review and critical appraisal. BMJ. 2019;367:l5358. doi: 10.1136/bmj.l5358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolff RF, Moons KGM, Riley RD, et al. PROBAST: a tool to assess the risk of bias and applicability of prediction model studies. Ann Intern Med. 2019;170(1):51‐58. doi: 10.7326/M18-1376 [DOI] [PubMed] [Google Scholar]
- 10. Debray TP, Damen JA, Snell KI, et al. A guide to systematic review and meta‐analysis of prediction model performance. BMJ. 2017;356:i6460. doi: 10.1136/bmj.i6460 [DOI] [PubMed] [Google Scholar]
- 11. Khilnani GC, Banga A, Sharma SK. Predictors of mortality of patients with acute respiratory failure secondary to chronic obstructive pulmonary disease admitted to an intensive care unit: a one year study. BMC Pulm Med. 2004;4(1):12. doi: 10.1186/1471-2466-4-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roche N, Zureik M, Soussan D, Neukirch F, Perrotin D, Urgence BPCO (COPD Emergency) Scientific Committee . Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Eur Respir J. 2008;32(4):953‐961. doi: 10.1183/09031936.00129507 [DOI] [PubMed] [Google Scholar]
- 13. Mohan A, Bhatt SP, Mohan C, Arora S, Luqman‐Arafath TK, Guleria R. Derivation of a prognostic equation to predict in‐hospital mortality and requirement of invasive mechanical ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci. 2008;50(4):335‐342. [PubMed] [Google Scholar]
- 14. Tabak YP, Sun X, Johannes RS, Gupta V, Shorr AF. Mortality and need for mechanical ventilation in acute exacerbations of chronic obstructive pulmonary disease: development and validation of a simple risk score. Arch Intern Med. 2009;169(17):1595‐1602. doi: 10.1001/archinternmed.2009.270 [DOI] [PubMed] [Google Scholar]
- 15. Tsimogianni AM, Papiris SA, Stathopoulos GT, Manali ED, Roussos C, Kotanidou A. Predictors of outcome after exacerbation of chronic obstructive pulmonary disease. J Gen Intern Med. 2009;24(9):1043‐1048. doi: 10.1007/s11606-009-1061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asiimwe AC, Brims FJ, Andrews NP, et al. Routine laboratory tests can predict in‐hospital mortality in acute exacerbations of COPD. Lung. 2011;189(3):225‐232. doi: 10.1007/s00408-011-9298-z [DOI] [PubMed] [Google Scholar]
- 17. Steer J, Gibson J, Bourke SC. The DECAF score: predicting hospital mortality in exacerbations of chronic obstructive pulmonary disease. Thorax. 2012;67(11):970‐976. doi: 10.1136/thoraxjnl-2012-202103 [DOI] [PubMed] [Google Scholar]
- 18. Tabak YP, Sun X, Johannes RS, Hyde L, Shorr AF, Lindenauer PK. Development and validation of a mortality risk‐adjustment model for patients hospitalized for exacerbations of chronic obstructive pulmonary disease. Med Care. 2013;51(7):597‐605. doi: 10.1097/MLR.0b013e3182901982 [DOI] [PubMed] [Google Scholar]
- 19. Lindenauer PK, Grosso LM, Wang C, et al. Development, validation, and results of a risk‐standardized measure of hospital 30‐day mortality for patients with exacerbation of chronic obstructive pulmonary disease. J Hosp Med. 2013;8(8):428‐435. doi: 10.1002/jhm.2066 [DOI] [PubMed] [Google Scholar]
- 20. Quintana JM, Esteban C, Unzurrunzaga A, et al. Predictive score for mortality in patients with COPD exacerbations attending hospital emergency departments. BMC Med. 2014;12(1):66. doi: 10.1186/1741-7015-12-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Batzlaff CM, Karpman C, Afessa B, Benzo RP. Predicting 1‐year mortality rate for patients admitted with an acute exacerbation of chronic obstructive pulmonary disease to an intensive care unit: an opportunity for palliative care. Mayo Clin Proc. 2014;89(5):638‐643. doi: 10.1016/j.mayocp.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roche N, Chavaillon JM, Maurer C, Zureik M, Piquet J. A clinical in‐hospital prognostic score for acute exacerbations of COPD. Respir Res. 2014;15(1):99. doi: 10.1186/s12931-014-0099-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zidan MH, Rabie AK, Megahed MM, Abdel‐Khaleq MY. The usefulness of the DECAF score in predicting hospital mortality in acute exacerbations of chronic obstructive pulmonary disease. Egypt J Chest Dis Tu. 2015;64(1):75‐80. doi: 10.1016/j.ejcdt.2014.11.006 [DOI] [Google Scholar]
- 24. Esteban C, Arostegui I, Garcia‐Gutierrez S, et al. A decision tree to assess short‐term mortality after an emergency department visit for an exacerbation of COPD: a cohort study. Respir Res. 2015;16(1):151. doi: 10.1186/s12931-015-0313-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duenk RG, Verhagen C, Bronkhorst EM, et al. Development of the ProPal‐COPD tool to identify patients with COPD for proactive palliative care. Int J Chron Obstruct Pulmon Dis. 2017;12:2121‐2128. doi: 10.2147/COPD.S140037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sakamoto Y, Yamauchi Y, Yasunaga H, et al. Development of a nomogram for predicting in‐hospital mortality of patients with exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:1605‐1611. doi: 10.2147/COPD.S129714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Esteban C, Castro‐Acosta A, Alvarez‐Martínez CJ, Capelastegui A, López‐Campos JL, Pozo‐Rodriguez F. Predictors of one‐year mortality after hospitalization for an exacerbation of COPD. BMC Pulm Med. 2018;18(1):18. doi: 10.1186/s12890-018-0574-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jain SS, Sarkar IN, Stey PC, Anand RS, Biron DR, Chen ES. Using demographic factors and comorbidities to develop a predictive model for ICU mortality in patients with acute exacerbation COPD. AMIA Annu Symp Proc. 2018;2018:1319‐1328. [PMC free article] [PubMed] [Google Scholar]
- 29. Arostegui I, Legarreta MJ, Barrio I, et al. A computer application to predict adverse events in the short‐term evolution of patients with exacerbation of chronic obstructive pulmonary disease. JMIR Med Inform. 2019;7(2):e10773. doi: 10.2196/10773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu X, Zhu GP, Cai TF, Zheng JY. Establishment of risk prediction model and risk score for in‐hospital mortality in patients with AECOPD. Clin Respir J. 2020;14(11):1090‐1098. doi: 10.1111/crj.13246 [DOI] [PubMed] [Google Scholar]
- 31. Alameda C, Matía ÁC, Casado V. Predictors for mortality due to acute exacerbation of COPD in primary care: derivation of a clinical prediction rule in a multicentre cohort study. Eur J Gen Pract. 2021;27(1):211‐220. doi: 10.1080/13814788.2021.1959547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen L, Chen L, Zheng H, Wu S, Wang S. Emergency admission parameters for predicting in‐hospital mortality in patients with acute exacerbations of chronic obstructive pulmonary disease with hypercapnic respiratory failure. BMC Pulm Med. 2021;21(1):258. doi: 10.1186/s12890-021-01624-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dong F, Ren X, Huang K, Wang Y, Jiao J, Yang T. Development and validation of risk prediction model for in‐hospital mortality among patients hospitalized with acute exacerbation chronic obstructive pulmonary disease between 2015 and 2019. Front Med (Lausanne). 2021;8:630870. doi: 10.3389/fmed.2021.630870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mekanimitdee P, Morasert T, Patumanond J, Phinyo P. The MAGENTA model for individual prediction of in‐hospital mortality in chronic obstructive pulmonary disease with acute exacerbation in resource‐limited countries: a development study. PLoS ONE. 2021;16(8):e0256866. doi: 10.1371/journal.pone.0256866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hartley T, Lane ND, Steer J, et al. The noninvasive ventilation outcomes (NIVO) score: prediction of in‐hospital mortality in exacerbations of COPD requiring assisted ventilation [published correction appears in Eur Respir J. 2021 Nov 11;58(5)]. Eur Respir J. 2021;58(2):2004042. doi: 10.1183/13993003.04042-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shiroshita A, Kimura Y, Shiba H, et al. Predicting in‐hospital death in pneumonic COPD exacerbation via BAP‐65, CURB‐65 and machine learning. ERJ Open Res. 2022;8(1):00452‐02021. doi: 10.1183/23120541.00452-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Almagro P, Soriano JB, Cabrera FJ, et al. Short‐ and medium‐term prognosis in patients hospitalized for COPD exacerbation: the CODEX index. Chest. 2014;145(5):972‐980. doi: 10.1378/chest.13-1328 [DOI] [PubMed] [Google Scholar]
- 38. Hu G, Zhou Y, Wu Y, Yu Y, Liang W, Ran P. The pneumonia severity index as a predictor of in‐hospital mortality in acute exacerbation of chronic obstructive pulmonary disease. PLoS ONE. 2015;10(7):e0133160. Published 2015 Jul 17. doi: 10.1371/journal.pone.0133160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Echevarria C, Steer J, Heslop‐Marshall K, et al. Validation of the DECAF score to predict hospital mortality in acute exacerbations of COPD. Thorax. 2016;71(2):133‐140. doi: 10.1136/thoraxjnl-2015-207775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hodgson LE, Dimitrov BD, Congleton J, Venn R, Forni LG, Roderick PJ. A validation of the national early warning score to predict outcome in patients with COPD exacerbation. Thorax. 2017;72(1):23‐30. doi: 10.1136/thoraxjnl-2016-208436 [DOI] [PubMed] [Google Scholar]
- 41. Almagro P, Martínez‐Camblor P, Miravitlles M, et al. External validation and recalculation of the CODEX index in COPD patients. A 3CIAplus cohort study. COPD. 2019;16(1):8‐17. doi: 10.1080/15412555.2018.1484440 [DOI] [PubMed] [Google Scholar]
- 42. Germini F, Veronese G, Marcucci M, et al. Validation of the BAP‐65 score for prediction of in‐hospital death or use of mechanical ventilation in patients presenting to the emergency department with an acute exacerbation of COPD: a retrospective multi‐center study from the Italian Society of Emergency Medicine (SIMEU). Eur J Intern Med. 2019;61:62‐68. doi: 10.1016/j.ejim.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 43. Echevarria C, Steer J, Bourke SC. Comparison of early warning scores in patients with COPD exacerbation: DECAF and NEWS score. Thorax. 2019;74(10):941‐946. doi: 10.1136/thoraxjnl-2019-213470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shi QF, Sheng Y, Zhu N, et al. The v‐DECAF score can predict 90‐day all‐cause mortality in patients with COPD exacerbation requiring invasive mechanical ventilation. Clin Respir J. 2019;13(7):438‐445. doi: 10.1111/crj.13028 [DOI] [PubMed] [Google Scholar]
- 45. Telukutla SR, Vidya TA, Nellaiappa Ganesan SK. BAP 65 and DECAF scores in predicting outcomes in acute exacerbation of COPD: a prospective observational study. J Clin Diagn Res. 2020;14(11):1‐4. doi: 10.7860/JCDR/2020/45490.14176 [DOI] [Google Scholar]
- 46. Zidan M, Gharraf H, Wahdan B. A comparative study of DECAF score and modified DECAF score in predicting hospital mortality rates in acute exacerbation of chronic obstructive pulmonary disease. Egypt J Chest Dis Tu. 2020;69(3):532‐541. doi: 10.4103/ejcdt.ejcdt_203_19 [DOI] [Google Scholar]
- 47. Gayaf M, Karadeniz G, Güldaval F, Polat G, Türk M. Which one is superior in predicting 30 and 90 days mortality after COPD exacerbation: DECAF, CURB‐65, PSI, BAP‐65, PLR, NLR. Expert Rev Respir Med. 2021;15(6):845‐851. doi: 10.1080/17476348.2021.1901584 [DOI] [PubMed] [Google Scholar]
- 48. Courvoisier DS, Combescure C, Agoritsas T, Gayet‐Ageron A, Perneger TV. Performance of logistic regression modeling: beyond the number of events per variable, the role of data structure. J Clin Epidemiol. 2011;64(9):993‐1000. doi: 10.1016/j.jclinepi.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 49. Riley RD, Ensor J, Snell KI, et al. External validation of clinical prediction models using big datasets from e‐health records or IPD meta‐analysis: opportunities and challenges. BMJ. 2016;353:i3140. doi: 10.1136/bmj.i3140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127‐141. doi: 10.1002/sim.2331 [DOI] [PubMed] [Google Scholar]
- 51. Vergouwe Y, Royston P, Moons KG, Altman DG. Development and validation of a prediction model with missing predictor data: a practical approach. J Clin Epidemiol. 2010;63(2):205‐214. doi: 10.1016/j.jclinepi.2009.03.017 [DOI] [PubMed] [Google Scholar]
- 52. Stone RA, Lowe D, Potter JM, Buckingham RJ, Roberts CM, Pursey NJ. Managing patients with COPD exacerbation: does age matter? Age Ageing. 2012;41(4):461‐468. doi: 10.1093/ageing/afs039 [DOI] [PubMed] [Google Scholar]
- 53. Steer J, Norman EM, Afolabi OA, Gibson GJ, Bourke SC. Dyspnoea severity and pneumonia as predictors of in‐hospital mortality and early readmission in acute exacerbations of COPD. Thorax. 2012;67(2):117‐121. doi: 10.1136/thoraxjnl-2011-200332 [DOI] [PubMed] [Google Scholar]
- 54. Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5‐year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434‐1440. doi: 10.1378/chest.121.5.1434 [DOI] [PubMed] [Google Scholar]
- 55. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81‐84. doi: 10.1016/s0140-6736(74)91639-0 [DOI] [PubMed] [Google Scholar]
- 56. Stefan MS, Shieh MS, Pekow PS, Hill N, Rothberg MB, Lindenauer PK. Trends in mechanical ventilation among patients hospitalized with acute exacerbations of COPD in the United States, 2001 to 2011. Chest. 2015;147(4):959‐968. doi: 10.1378/chest.14-1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Søgaard M, Madsen M, Løkke A, Hilberg O, Sørensen HT, Thomsen RW. Incidence and outcomes of patients hospitalized with COPD exacerbation with and without pneumonia. Int J Chron Obstruct Pulmon Dis. 2016;11:455‐465. doi: 10.2147/COPD.S96179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Arihan O, Wernly B, Lichtenauer M, et al. Blood urea nitrogen (BUN) is independently associated with mortality in critically ill patients admitted to ICU. PLoS ONE. 2018;13(1):e0191697. Published 2018 Jan 25. doi: 10.1371/journal.pone.0191697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ito A, Ishida T, Tokumasu H, et al. Prognostic factors in hospitalized community‐acquired pneumonia: a retrospective study of a prospective observational cohort. BMC Pulm Med. 2017;17(1):78. doi: 10.1186/s12890-017-0424-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shen MH, Qiu GQ, Wu XM, Dong MJ. Utility of the DECAF score for predicting survival of patients with COPD: a meta‐analysis of diagnostic accuracy studies. Eur Rev Med Pharmacol Sci. 2021;25(11):4037‐4050. doi: 10.26355/eurrev_202106_26045 [DOI] [PubMed] [Google Scholar]
- 61. Lim WS, van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58(5):377‐382. doi: 10.1136/thorax.58.5.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Search strategy(2022.4.10).
Table S2: PROBAST: Assessment of Risk of Bias.
Table S3: The STATA command.
Table S4: Characteristics of excluded studies.
Table S5: Basic characteristics of the 26 studies that developed models (whether validated or not).
Table S6: Methodological characteristics of the 26 studies that developed models (whether validated or not).
Table S8: Methodological of characteristics of the 11 studies that only validated the models.
Table S9: Data from 15 studies used for meta‐analysis
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


