Abstract
L6 myoblasts stably transfected with a GLUT4 cDNA harboring an exofacial myc epitope tag (L6-GLUT4myc myoblasts) were used to study the role of protein kinase B alpha (PKBα)/Akt1 in the insulin-induced translocation of GLUT4 to the cell surface. Surface GLUT4myc was detected by immunofluorescent labeling of the myc epitope in nonpermeabilized cells. Insulin induced a marked translocation of GLUT4myc to the plasma membrane within 20 min. This was prevented by transient transfection of a dominant inhibitory construct of phosphatidylinositol (PI) 3-kinase (Δp85α). Transiently transfected cells were identified by cotransfection of green fluorescent protein. A constitutively active PKBα, created by fusion of a viral Gag protein at its N terminus (GagPKB), increased the cell surface density of GLUT4myc compared to that of neighboring nontransfected cells. A kinase-inactive, phosphorylation-deficient PKBα/Akt1 construct with the mutations K179A (substitution of alanine for the lysine at position 179), T308A, and S473A (AAA-PKB) behaved as a dominant-negative inhibitor of insulin-dependent activation of cotransfected wild-type hemagglutinin (HA)-tagged PKB. Furthermore, AAA-PKB markedly inhibited the insulin-induced phosphorylation of cotransfected BAD, demonstrating inhibition of the endogenous PKB/Akt. Under the same conditions, AAA-PKB almost entirely blocked the insulin-dependent increase in surface GLUT4myc. PKBα with alanine substitutions T308A and S473A (AA-PKB) or K179A (A-PKB) alone was a less potent inhibitor of insulin-dependent activation of wild-type HA-PKB or GLUT4myc translocation than was AAA-PKB. Cotransfection of AAA-PKB with a fourfold DNA excess of HA-PKB rescued insulin-stimulated GLUT4myc translocation. AAA-PKB did not prevent actin bundling (membrane ruffling), though this response was PI 3-kinase dependent. Therefore, it is unlikely that AAA-PKB acted by inhibiting PI 3-kinase signaling. These results outline an important role for PKBα/Akt1 in the stimulation of glucose transport by insulin in muscle cells in culture.
Translocation of GLUT4 from an intracellular compartment to the plasma membrane largely accounts for the stimulation of glucose transport by insulin in skeletal muscle (16, 31, 38), cardiac muscle (48), and adipose cells (23, 24). Two insulin-responsive cell lines also express this transporter: L6 rat skeletal myotubes (34, 40) and 3T3-L1 mouse adipocytes (24). Transfection of a molecularly engineered form of this transporter containing an exofacial epitope tag between the first and second transmembrane domains allows for the detection of surface transporters in intact cells. GLUT4 molecules with an exofacial epitope tag have been heterologously expressed in rat adipose cells (44, 51), 3T3-L1 adipocytes (26), CHO cells (12, 26), H9c2 cardiomyocytes (55), and rat 3Y1 cells (22). We have recently shown that stable expression of GLUT4myc in L6 myoblasts (L6-GLUT4myc myoblasts) mimics the response to insulin seen with endogenous GLUT4 in differentiated myotubes (29, 60).
Insulin-induced translocation of GLUT4 to the plasma membrane requires the activity of phosphatidylinositol (PI) 3-kinase (47) in rat adipocytes (43, 45), 3T3-L1 adipocytes (8, 9, 21, 27, 39, 51), L6 muscle cells (53), and rat skeletal muscle (62). Moreover, treatment of intact 3T3-L1 adipocytes with a cell-permeant PI 3,4,5-triphosphate [PI (3,4,5)-P3] compound, which is converted into a product of PI 3-kinase once inside the cell, partly rescued the inhibition of insulin-stimulated glucose transport by wortmannin (25). It is unclear how the lipid products of PI 3-kinase relay the insulin signal to the glucose transporters, but the serine/threonine kinase protein kinase B (PKB)/Akt interacts with the lipid products of PI 3-kinase (19), and activation of PKB/Akt by insulin is prevented by inhibitors of PI 3-kinase (1). To date, three isoforms of PKB/Akt have been identified: PKBα, -β, and -γ (Akt1, -2, and -3) (17). In skeletal muscle and L6 muscle cells, PKBα and PKBγ, but not PKBβ, are stimulated by insulin (59). Full activation of PKB/Akt by insulin requires hierarchical phosphorylation on two residues, Thr308 (Thr309 and Thr305 in the case of PKBβ and -γ, respectively) and Ser473 (Ser474 in the case of PKBβ; PKBγ lacks an equivalent site) by 3-phosphoinositide-dependent protein kinase 1 (PDK-1) and PDK-2, respectively (1–3, 14, 50).
Recent reports have suggested that activation of PKB/Akt may mediate the stimulation of glucose transport by insulin, since stable overexpression of wild-type PKBα/Akt1 or constitutively active mutants of PKBα/Akt1 increased glucose transport and translocation of GLUT4 to levels similar to or greater than those achieved with insulin in rat adipocytes (52), 3T3-L1 adipocytes (33, 56), and L6 muscle cells (20, 56). Stimulation of glucose uptake was also observed in 3T3-L1 adipocytes expressing a conditionally active membrane-targeted PKBα/Akt1-mutant estrogen receptor fusion protein that displays PKB activity only upon addition of tamoxifen (32). Finally, treatment of isolated rat adipocytes with insulin increased the presence of PKBβ/Akt2 protein on immunopurified GLUT4 vesicles (7, 37), leading to phosphorylation of GLUT4 vesicle proteins (37). Definitive proof that PKB/Akt participates in insulin-dependent GLUT4 translocation, however, requires demonstration that ablation of the kinase activity precludes the arrival of GLUT4 at the cell surface. Two recent reports approached this question in rat adipocytes or 3T3-L1 adipocytes but yielded opposite results. In the first study, transient overexpression of a kinase-inactive mutant of PKBα/Akt1 (with a substitution of alanine for lysine at position 179 [K179A]) in isolated rat adipocytes reduced the sensitivity and the maximal response of the GLUT4 response to insulin (11). The second study showed that adenovirus-driven overexpression of a phosphorylation-deficient PKBα/Akt1 mutant (T308A S473A) did not alter the stimulation of glucose transport and GLUT4 translocation by insulin, despite markedly inhibiting endogenous PKBα/Akt1 activation (30). Hence, the necessity for the stimulation of GLUT4 translocation in two adipose cell types is controversial.
In the present study, we examined the possible role of PKB in the stimulation of GLUT4 translocation by insulin in muscle cells. L6 myoblasts expressing GLUT4myc were transiently transfected with a PKBα/Akt1 construct that combines the mutations seen in the kinase-inactive and phosphorylation-deficient mutants of PKBα/Akt1. We demonstrate that this construct has a dominant inhibitory action and that it markedly inhibits insulin-induced GLUT4 translocation without inhibiting insulin-stimulated, PI 3-kinase-dependent actin remodeling.
MATERIALS AND METHODS
Materials.
Minimum essential medium α, fetal bovine serum, and other tissue culture reagents were purchased from Life Technologies/GIBCO (Burlington, Ontario, Canada). Human insulin (Humulin R) was obtained from Eli Lilly Canada Inc. (Toronto, Ontario, Canada). pcDNA3 was purchased from Invitrogen. pEGFP was purchased from Clontech (Palo Alto, Calif.). pEBG-mBAD and antibodies to BAD (Bcl-associated death promoter) and phospho(Ser136)-BAD proteins were purchased from New England Biolabs (Mississauga, Ontario, Canada). Effectene transfection kits and plasmid DNA purification columns were purchased from Qiagen (Mississauga, Ontario, Canada). Restriction enzymes, ligase, and polymerase were purchased from New England Biolabs. Oligonucleotides were purchased from Life Technologies/GIBCO. Protein A-Sepharose and protein G-Sepharose were from Pharmacia (Uppsala, Sweden). Polyclonal anti-Akt1 (raised to 20 C-terminal amino acids), rabbit polyclonal anti-protein kinase C zeta (anti-PKCζ; raised to 20 C-terminal amino acids), and monoclonal anti-myc (9E10) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-hemagglutinin (anti-HA) antibody (HA.11) was purchased from Babco (Berkeley, Calif.). Indocarbocyanine (Cy3)-conjugated goat anti-mouse immunoglobulin G (IgG) and horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, Pa.). PKB/Akt substrate peptide (Crosstide) was from Upstate Biotechnology (Lake Placid, N.Y.). Enhanced chemiluminescence (ECL) reagent and [γ-32P]ATP (6,000 Ci/mmol) were purchased from Amersham (Oakville, Ontario, Canada). Wortmannin and myelin basic protein were obtained from Sigma (St. Louis, Mo.). Microcystin and okadaic acid were from Biomol (Plymouth Meeting, Pa.). Rhodamine-phalloidin and Antifade mounting compound were purchased from Molecular Probes (Leiden, The Netherlands). All electrophoresis equipment and polyvinylidene difluoride membranes were purchased from Bio-Rad (Mississauga, Ontario, Canada). All other reagents were of the highest analytical grade.
Plasmids, cells, and transfections.
GLUT4myc cDNA was constructed by inserting the human c-myc epitope (14 amino acids) into the first ectodomain of GLUT4, subcloned into the pCXN2 vector, and stably transfected into L6 myoblasts (26, 29). The open reading frame of the bovine PKB cDNA was cloned into the eukaryotic expression vector pcDNA3 (Invitrogen) and in the process was fused to an N-terminal HA tag (recognized by the HA.11 antibody). HA-PKB K179A was obtained by mutagenesis as described earlier (6) and was employed as a template for additional point mutations using the pALTER-1 plasmid (Promega). The oligonucleotides 5′-TCC CCC AGT TCG ACT ACT CGG CTA GCG CGA CGG CC-3′ and 5′-TCC CCC AGT TCG CCT ACT CGG CTA GCG CGA CGG CC-3′ were used to insert the T308A and S473A changes, respectively. Mutations were confirmed by DNA sequencing. The construct pcDNA3 GagPKB (6) was a kind gift from Paul J. Coffer (University Hospital, Utrecht, The Netherlands). The construct pSG5p85αΔSH2-N, commonly referred to as Δp85α, the dominant-negative mutant of the type I PI 3-kinase (46), was a kind gift from Julian Downward (Imperial Cancer Research Fund, United Kingdom), and the cDNA insert was subcloned into pcDNA3 for experimentation. The wild-type, HA-tagged PKCζ construct subcloned into plasmid pCDNA3 was a kind gift from Robert Farese (University of South Florida).
Parental L6 cells and L6-GLUT4myc myoblasts were maintained in minimal essential medium-α supplemented with 10% fetal bovine serum in a humidified atmosphere containing 5% CO2 and 95% air at 37°C (41). Transfections were performed in six-well plates according to the Effectene product manual (Qiagen). L6 myoblasts were seeded at a density of 2 × 105 cells/well and incubated overnight. DNA complexes were made at an 8:1 enhancer/DNA ratio in all cases. However, in transfections that utilized less than 1 μg of DNA per well, the Effectene reagent was used at 6 μl per condition. DNA was introduced to the cells at the start of the day for 5 h and then removed, and the cells were maintained for ca. another 43 h until experimentation. For single-cell analysis of GLUT4myc translocation or actin rearrangements, PKB or Δp85α constructs were cotransfected with 0.4 μg of pEGFP into L6-GLUT4myc myoblasts grown on coverslips as indicated in the figure legends. For PKB/Akt activity assays or expression of glutathione S-transferase (GST)–BAD protein, transfections were also performed in six-well plates as described in the figure legends.
Immunoprecipitation and assay of PKB/Akt kinase activity.
Immunoprecipitation of HA-PKB and the kinase assay were performed as described previously (49). Cells were disrupted by addition of a lysis buffer containing 50 mM HEPES (pH 7.6), 150 mM NaCl, 10% (vol/vol) glycerol, 1% (vol/vol) Triton X-100, 30 mM sodium pyrophosphate, 10 mM NaF, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 1 mM Na3VO4, 1 mM dithiothreitol (DTT), and 100 nM okadaic acid. For each condition, HA antibody (3 μg) was precoupled (16 h) to a mixture of protein A- and protein G-Sepharose beads. These anti-HA–bead complexes were washed twice with phosphate-buffered saline (PBS) and once with lysis buffer (4°C). HA-PKB was immunoprecipitated by incubating 200 μg of total cellular protein with the anti-HA–bead complexes for 2 to 3 h with constant rotation (4°C). HA-PKB immunocomplexes were washed four times with 1 ml of wash buffer (25 mM HEPES [pH 7.8], 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100, 0.1% [wt/vol] bovine serum albumin [BSA], 1 M NaCl, 1 mM DTT, 1 mM phenylmethylsulfonyl fluoride, 1 μM microcystin, and 100 nM okadaic acid) and twice with 1 ml of kinase buffer (50 mM Tris-HCl [pH 7.5], 10 mM MgCl2, and 1 mM DTT). The immunocomplexes were incubated with constant agitation for 30 min at 30°C with 30 μl of reaction mixture (kinase buffer containing 5 μM ATP, 2 μCi of [γ-32P]ATP, and 100 μM Crosstide). Following the reaction, 30 μl of the supernatant was transferred onto Whatman p81 filter paper and washed four times for 10 min each time with 3 ml of 175 mM phosphoric acid and once with distilled water for 5 min. The filters were air dried and then subjected to liquid scintillation counting.
Immunoprecipitation and assay of atypical PKC activity.
Immunoprecipitation of PKCζ and assay of its kinase activity were performed as described above for PKB with some modifications. Two types of assays were used: immunoprecipitation of endogenous PKCζ from L6 myotubes, and immunoprecipitation of wild-type HA-tagged PKCζ expressed in L6 myoblasts (generated by transfecting 0.4 μg of HA-PKCζ or pcDNA3 vector into L6 myoblasts and assaying activity 48 h later). Cell lysates were prepared from transfected or untransfected cells by using the lysis buffer described above. To isolate wild-type PKCζ, anti-PKCζ antibody (C-20) or an irrelevant rabbit IgG was precoupled to protein A-Sepharose beads by incubating 2 μg of antibody per condition with 40 μl of the protein A-Sepharose beads (100 mg/ml) for a minimum of 2 h. To isolate HA-tagged PKCζ, anti-HA antibody was precoupled to a 1:1 mixture of protein A- and protein G-Sepharose beads. Antibody-bead complexes were washed twice with ice-cold PBS and once with ice-cold lysis buffer. PKCζ was isolated and washed as described above for Akt. The PKCζ immunocomplex was then incubated with constant agitation for 10 min at 30°C with 30 μl of reaction mixture (kinase buffer containing 25 μM ATP, 5 μCi of [γ-32P]ATP, and 5 μM myelin basic protein). Following the reaction, 30 μl of the supernatant was transferred onto Whatman p81 filter paper and washed four times for 10 min each time with 3 ml of 175 mM phosphoric acid and once with distilled water for 5 min. The filters were air dried and then subjected to liquid scintillation counting.
Immunoblotting.
Equal amounts of proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrotransferred onto polyvinylidene difluoride filters as described previously (49). Immunoreactive bands were visualized either with HRP-conjugated sheep anti-mouse IgG for monoclonal antibodies or with horseradish peroxidase-conjugated goat anti-rabbit IgG for polyclonal antibodies (as indicated in the figure legends), using an ECL detection technique. Developed films were scanned and quantitated by using NIH Image software (National Institutes of Health, Bethesda, Md.). Cellular protein content was measured by the bicinchoninic acid method (5).
Indirect immunofluorescence and measurement of GLUT4myc translocation.
Subconfluent L6-GLUT4myc myoblasts were grown on 25-mm-diameter glass coverslips in six-well plates and transfected as described above. Cells were deprived of serum in culture medium for 5 h prior to incubation with 100 nM insulin (20 min, 37°C). The following steps were performed at 4°C unless indicated otherwise. Cells were rinsed once with PBS prior to being incubated with 3% paraformaldehyde in PBS for 3 min and then PBS plus 100 mM glycine for 10 min. The cells were blocked with PBS containing 5% goat serum and 3% BSA (PBS-BSA plus GS) for 30 min. To detect GLUT4myc, coverslips were incubated with anti-myc antibody (9E10, 1:100) for 60 min, rinsed four times with PBS-BSA plus GS, and incubated with secondary antibody (Cy3-conjugated goat anti-mouse IgG, 1:1,000) for 30 min. The cells were rinsed four to six times in PBS and fixed in 3% paraformaldehyde for 30 min while shifting from 4°C to room temperature. The cells were incubated with PBS plus 100 mM glycine for 10 min, rinsed with PBS, and then rinsed with water before the coverslips were mounted and immediately analyzed by fluorescence microscopy with a Leica TCS 4D laser confocal fluorescence microscope.
Actin filament visualization.
Subconfluent L6-GLUT4myc myoblasts were grown on 25-mm-diameter glass coverslips in six-well plates and transfected as described above. Cells were deprived of serum in culture medium for a total of 5 h prior to incubation with 100 nM insulin (5 min, 37°C) or, where indicated, 100 nM wortmannin (15-min pretreatment before addition of insulin and maintained during the insulin incubation). The cells were rinsed once with PBS (4°C) and then fixed with 3% paraformaldehyde for 30 min at room temperature followed by a 10-min wash in PBS plus 100 mM glycine. The cells were permeabilized with 0.1% (vol/vol) Triton X-100 in PBS for 10 min and rinsed twice with PBS. The cells were incubated with 0.2 U of rhodamine-phalloidin for 30 min prior to being rinsed four to six times with PBS and then with water before being mounted and immediately analyzed by fluorescence microscopy with a Leica inverted fluorescence microscope.
RESULTS
AAA-PKB acts as a dominant-negative inhibitor of PKBα/Akt1.
A dominant-negative PKBα/Akt1 was created by substituting alanine residues at the two major regulatory phosphorylation sites of PKBα/Akt1 (Thr308 and Ser473) and the phosphate transfer residue in the catalytic site (Lys179) (AAA-PKB) (25a). To demonstrate that AAA-PKB was kinase inactive, HA epitope-tagged AAA-PKB and wild-type PKB (HA-AAA-PKB and HA-PKB, respectively) were transiently expressed in L6-GLUT4myc myoblasts, independently. After 48 h, cells were either treated with 100 nM insulin for 10 min or left untreated. HA-AAA-PKB and HA-PKB were immunoprecipitated from cell lysates with an anti-HA epitope monoclonal antibody, and the samples were processed for an in vitro PKB/Akt kinase assay. HA-PKB immunoprecipitated from insulin-treated cells showed a mean increase in kinase activity ± standard error (SE) of 18-fold ± 3-fold relative to untreated (basal) cells (Fig. 1A). In contrast, the kinase activity associated with HA-AAA-PKB was not elevated by insulin, as predicted (Fig. 1A).
FIG. 1.
AAA-PKB acts as a dominant-negative mutant of PKB. (A) L6-GLUT4myc myoblasts grown in six-well plates were transiently transfected with HA-tagged wild-type PKBα/Akt1 (HA-PKB; 0.4 μg per well) or with HA-tagged AAA-PKBα/Akt1 (HA-AAA-PKB; 0.4 μg per well) and incubated for 48 h in culture. Cells were serum deprived for 5 h and were left untreated (basal) or treated with 100 nM insulin for 10 min (insulin). Cell lysates were prepared, HA-tagged proteins were immunoprecipitated with anti-HA antibodies (3 μg), and PKB kinase activity in the immune complexes was measured as described in Materials and Methods. The basal activity of HA-PKB was assigned a value of 1.0; all other activities were expressed relative to this value. The results represent the means ± SE of data from three independent experiments. (B) Cells were transfected with HA-PKB (0.4 μg per well) in combination either with pcDNA3 vector alone (4 μg per well) or with untagged AAA-PKB in pcDNA3 (4 μg per well) as indicated. Cells were treated with insulin as indicated, and lysates were prepared and processed for the PKB/Akt kinase assay as described for panel A. The basal activity of HA-PKB cotransfected with pcDNA3 was assigned a value of 1.0; all other activities were expressed relative to this value. The results represent the means ± SE of data from five independent experiments.
To determine the effect of mutant AAA-PKB on HA-PKB activation, the cDNA of AAA-PKB (untagged) was cotransfected with the HA-PKB construct at a 10:1 DNA ratio. Forty-eight hours later, cells were either treated with 100 nM insulin for 10 min or left untreated. The in vitro PKB kinase activity of immunoprecipitated HA-PKB in untreated cells cotransfected with HA-PKB and a 10-fold excess empty vector was assigned a value of 1, and all other measurements were expressed relative to this value. Insulin stimulated HA-PKB activity by 19-fold ± 4-fold (Fig. 1B). In contrast, the insulin stimulation of HA-PKB was almost completely prevented by coexpression of AAA-PKB (amounting to only a 7.9% ± 3.9% stimulation by insulin relative to that of HA-PKB plus pcDNA3 alone). Hence, in L6 muscle cells, AAA-PKB acts as a dominant-negative PKBα/Akt1.
BAD, a proapoptotic protein, is one of only a few well-characterized substrates of PKB. Growth factors stimulate phosphorylation of BAD on Ser136 by a PKB-dependent mechanism (13, 42), and this response can be monitored with antibodies that specifically recognize phosphorylated Ser136 on BAD. L6-GLUT4myc myoblasts were transiently transfected with a GST-BAD fusion construct. Insulin treatment led to a rapid elevation in Ser136 phosphorylation of BAD (by 5.5-fold ± 0.4-fold) (Fig. 2). Cotransfection of AAA-PKB and GST-BAD markedly diminished the ability of insulin to stimulate Ser136 phosphorylation of BAD (attaining an increase in phosphorylation of only 2.2-fold ± 0.8-fold). This suggests that AAA-PKB can effectively inhibit the activation of endogenous PKB molecules by insulin. Together, the results shown in Fig. 1 and 2 indicate that AAA-PKB is a dominant-negative inhibitor of the activation of wild-type PKB by insulin.
FIG. 2.
Insulin-stimulated phosphorylation of GST-BAD is inhibited by coexpression of AAA-PKB. L6-GLUT4myc myoblasts grown in six-well plates were transiently transfected with pEBG-mBAD (0.4 μg per well) in combination with either pcDNA3 vector alone or HA-AAA-PKB (0.4 μg per well) and incubated for 48 h in culture. Cells were serum deprived for 5 h and were left untreated (−) or treated with 100 nM insulin (+) for 10 min. Cells were lysed in detergent-containing buffer as described in Materials and Methods and then immunoblotted (IB) with anti-BAD (α-BAD; 1:500) or anti-phospho(Ser136)-BAD (α-pBAD; 1:500) antibodies. (A) Representative immunoblots for GST-BAD protein (BAD, upper panel) or Ser136-phosphorylated GST-BAD (pBAD, lower panel). The positions of molecular mass markers are indicated on the right side of the gel in kilodaltons. (B) Autoradiographs of four experiments were densitometrically scanned, and the results were plotted as phosphorylated BAD (pBAD)/BAD protein ratios for each set of conditions (insulin-treated [insulin] or untreated [basal] cells) relative to the ratio calculated for basal cells transfected with pEG-mBAD and pcDNA3, with the latter being assigned a value of 1.
Characterization of a single-cell assay for GLUT4myc translocation in L6 myoblasts.
L6-GLUT4myc myoblasts were used to establish a single-cell assay for GLUT4 translocation. The myc epitope is on the first extracellular segment of GLUT4, facilitating recognition of cell surface transporters by extracellular labeling without the need to perform subcellular fractionation, exofacial photolabeling, or plasma membrane lawn preparation. We recently reported that L6-GLUT4myc myoblasts respond to insulin by translocating GLUT4myc to the plasma membrane in a wortmannin-sensitive manner (60). Insulin caused a twofold increase in cell surface content of GLUT4myc and in glucose transport activity in L6-GLUT4myc myoblasts (60). Moreover, in these cells about 50% of the GLUT4myc protein was located intracellularly in a unique compartment enriched in the insulin-regulated aminopeptidase vp165 and relatively depleted of GLUT1 (56a). Thus, in L6 myoblasts, ectopic expression of GLUT4 generates an insulin-responsive phenotype.
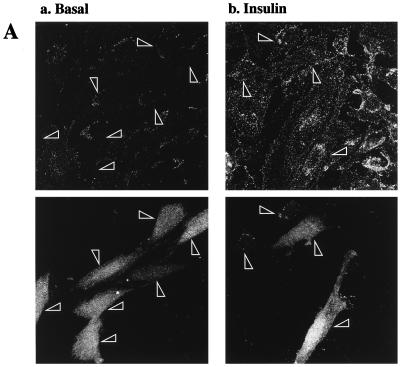
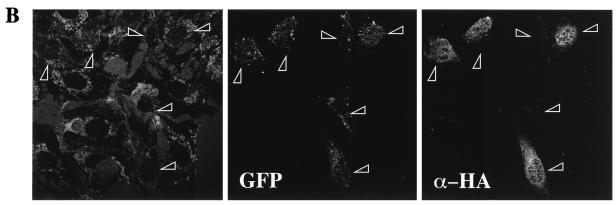
To determine the effects of transient transfection of various cDNA constructs on the translocation of GLUT4myc to the cell surface, cotransfection with green fluorescent protein (GFP) cDNA was performed to facilitate recognition of transfected cells. Essentially, its use alleviated the need to permeabilize the cells to identify the expression of the transfected proteins. However, preliminary experiments were necessary to rule out the possibility that GFP expression itself interferes with insulin-stimulated GLUT4 translocation. Transfection of GFP alone (Fig. 3A, lower panels) did not alter cell surface GLUT4myc levels in either unstimulated cells (Fig. 3A, upper left panel) or cells treated with 100 nM insulin for 20 min (upper right panel) compared to untransfected neighboring cells. We next determined that GFP expression was a good indicator of coexpression of other DNA constructs. Equal amounts of GFP and HA-PKB DNA constructs were cotransfected into L6 myoblasts and processed for indirect immunofluorescence of the HA epitope. The left panel in Fig. 3B indicates the total number of cells present in the entire field of one representative experiment. The other panels in Fig. 3B illustrate that all of the GFP-positive cells (middle panel) in this field of cells were also positive for HA-PKB expression (right panel).
FIG. 3.
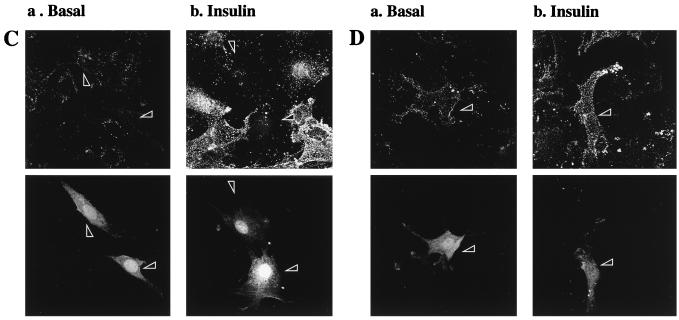
Transient transfection of Δp85α PI 3-kinase inhibits, and GagPKB potentiates, insulin-stimulated translocation of GLUT4myc in muscle cells. L6-GLUT4myc myoblasts were transiently transfected with GFP (0.4 μg) (A) and cotransfected with HA-PKB (0.4 μg) (B), dominant-negative Δp85α (0.4 μg) (C), or GagPKB (0.4 μg) (D) and incubated for 48 h in culture. (A) Cells were left untreated (basal) or treated with 100 nM insulin for 20 min (insulin) and then processed for cell surface GLUT4myc detection with anti-myc antibody (1:100) followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells (arrowheads) is shown. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of the transfected cells. Results shown are representative of at least five experiments. (B) Cells were fixed and processed for indirect immunofluorescence with anti-HA antibody (α-HA; 1:1,000) followed by detection with a Cy3-conjugated goat anti-mouse antibody. The panel on the left shows the number of cells in the field seen by phase-contrast microscopy. The middle and right panels show the GFP-positive cells and the HA-PKB-positive cells, respectively, in the same field. The results indicate complete overlap of GFP- and HA-PKB-positive cells. Results shown are representative of three experiments. (C) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with Δp85α. Results shown are representative of three experiments. (D) Cells were treated as described for panel A. The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b). Arrowheads indicate the positions of cells transfected with GagPKB. Results shown are representative of three experiments.
Inhibition of insulin-stimulated GLUT4 translocation by dominant-negative PI 3-kinase in L6-GLUT4myc myoblasts.
Expression of a dominant-negative construct of the p85α regulatory subunit of PI 3-kinase lacking the region that binds to the p110 catalytic subunit (Δp85α) inhibits insulin-induced GLUT4 translocation in rat fat cells and 3T3-L1 adipocytes (35, 45). Transient transfection of Δp85α in L6 myoblasts did not alter the level of cell surface GLUT4myc in untreated (basal) cells compared to that in untransfected neighboring cells (Fig. 3C, panel a). However, Δp85α transfection completely blocked the insulin-stimulated translocation of GLUT4myc to the cell surface (Fig. 3C, panel b). This experiment confirmed the dependence of GLUT4myc translocation on PI 3-kinase activity in L6 myoblasts.
A constitutively active PKBα/Akt1 stimulates GLUT4myc translocation.
Expression of constitutively active forms of PKBα/Akt1 in L6 muscle cells results in an increase in glucose transport and in the amount of GLUT4 present at the plasma membrane (20, 56). Here we coexpressed GFP with a constitutively active PKBα/Akt1 that has a viral Gag protein with its N-terminal membrane-targeting myristoylation motif fused in frame to the N terminus of PKBα (6). The resulting PKB protein is located almost exclusively at the plasma membrane and has a very high basal activity level (57). Expression of GagPKB augmented the cell surface density of GLUT4myc in the absence of insulin treatment (Fig. 3D, panel a) compared to the surrounding, untransfected cells. Insulin treatment of cells expressing GagPKB slightly increased the translocation of GLUT4myc to the cell surface compared to neighboring, untransfected cells (Fig. 3D, panel b). Expression of wild-type, HA-tagged PKBα/Akt1 had no effect on either basal or insulin-dependent surface exposure of GLUT4 (results not shown).
Expression of AAA-PKB inhibits GLUT4 translocation.
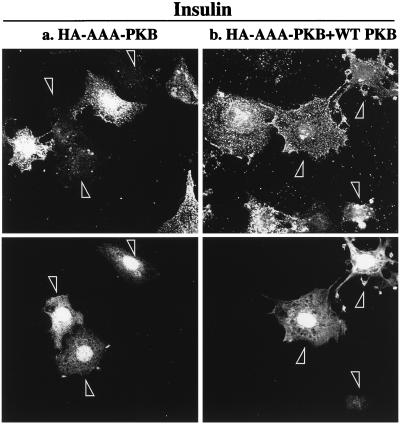
The elevation of surface GLUT4myc levels in the L6 myoblasts expressing GagPKB suggested that this kinase might be part of the signal transduction pathway utilized by insulin to stimulate glucose transport. To further investigate the role of PKBα/Akt1 in the stimulation of glucose transport by insulin, cells were transiently transfected with HA-AAA-PKB, the dominant-negative inhibitor of PKB activation by insulin. The effect of HA-AAA-PKB on insulin-induced translocation of GLUT4 to the cell surface is illustrated in Fig. 4 (upper panels). The transfected cells were identified by their expression of GFP (shown in the lower panels). HA-AAA-PKB had no detectable effect on the basal amount of GLUT4myc on the cell surface compared to that of the surrounding, untransfected cells (Fig. 4a). However, insulin could not stimulate GLUT4myc translocation in cells that expressed HA-AAA-PKB compared to that of the surrounding, untransfected cells (Fig. 4b).
FIG. 4.
AAA-PKB inhibits insulin-induced translocation of GLUT4myc in muscle cells. L6-GLUT4myc myoblasts were cotransfected with GFP and HA-AAA-PKB (0.4 μg each) and incubated for 48 h in culture. Cells were serum deprived for 5 h, left untreated or treated with 100 nM insulin for 20 min (insulin), and then processed for cell surface GLUT4myc detection with anti-myc (1:100) antibody followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells is shown (arrowheads). The upper panels show the cell surface GLUT4myc density under basal conditions (a) or with insulin treatment (b) for three separate experiments. Arrowheads indicate the positions of cells transfected with HA-AAA-PKB in the upper panels. Results shown are representative of at least five experiments.
AAA-PKB is a more effective inhibitor of GLUT4 translocation than AA-PKB or A-PKB.
Two kinase-inactive mutants of PKBα/Akt1 have been suggested to have dominant inhibitory activity over the activation of endogenous PKB by insulin or other growth factors. One of these mutants has a K179A substitution (A-PKB) and has been referred to as kinase dead since it cannot bind ATP or phosphorylate substrates (11, 18). A nonactivatable mutant was also generated by alanine substitutions T308A and S473A (AA-PKB) (30). This mutant cannot receive activating inputs from the upstream kinases PDK-1 and PDK-2, and so it cannot be stimulated by growth factor receptor signals. We compared the effectiveness of A-PKB and AA-PKB in inhibiting the stimulation of GLUT4myc translocation and the activation of HA-PKB by insulin in L6-GLUT4myc myoblasts with that of AAA-PKB. L6-GLUT4myc myoblasts were transfected with 0.4 μg of either AAA-PKB, AA-PKB, or A-PKB cDNA and then processed for GLUT4myc translocation as described in the legend to Fig. 4. The intensity of the fluorescent label of cell surface GLUT4myc was quantitated by using NIH Image software. The pixel intensity of GLUT4myc staining per cell was measured in similar numbers of transfected and nontransfected cells. A value of 100% was assigned to nontransfected, insulin-stimulated cells within each field. The pixel intensity of the transfected cells in the same field was then calculated as a fraction of this value. At least three experiments using each of the PKB/Akt mutants were quantitated, and the averaged results are shown in Fig. 5A. The pixel intensity in parallel coverslips of unstimulated cells transfected with pcDNA3 and GFP only was also measured and is indicated by the dotted line. Insulin caused a 1.9-fold increase in GLUT4myc above that in unstimulated cells (Fig. 5A). This degree of stimulation was not affected by the transfection of pcDNA3. AAA-PKB significantly reduced the ability of insulin to recruit GLUT4myc to the cell surface, so that the hormone caused only a 0.6-fold increase (above that in unstimulated cells). Transfected AAA-PKB was significantly more effective than either AA-PKB or A-PKB at inhibiting GLUT4 translocation. In fact, neither AA-PKB nor A-PKB affected the increase in surface GLUT4myc in response to insulin relative to untransfected or pcDNA3-transfected cells (Fig. 5A).
FIG. 5.
Comparison of AAA-PKB, AA-PKB, and A-PKB with regard to insulin-stimulated GLUT4myc translocation and activation of HA-PKB by insulin. (A) L6-GLUT4myc myoblasts were cotransfected with GFP (0.4 μg) and HA-AAA-PKB, HA-AA-PKB, or HA-A-PKB (0.4 μg each) and incubated for 48 h in culture. Cells were serum deprived for 5 h, left untreated (basal) or treated with 100 nM insulin for 20 min (insulin), and then processed for detection of cell surface GLUT4myc with anti-myc antibody (1:100) followed by Cy3-conjugated goat anti-mouse antibody as indicated in Materials and Methods. The intensity of the fluorescent label of cell surface GLUT4myc was quantitated by using NIH Image soft ware. The pixel intensity of GLUT4myc staining per cell was measured in similar numbers of transfected and nontransfected cells. A value of 100% was assigned to nontransfected, insulin-stimulated cells within each field. The pixel intensity of the transfected cells in the same field was then calculated as a fraction of this value. The pixel intensity in parallel coverslips of unstimulated cells transfected with pcDNA3 and GFP only was also measured and is indicated by the dotted line. The results are means ± SE of data from at least three independent experiments under each set of conditions. (B) L6-GLUT4myc myoblasts were cotransfected with HA-PKB (0.4 μg) and empty pcDNA3 vector, untagged AAA-PKB, AA-PKB, or A-PKB (4.0 μg each) and incubated as described for panel A prior to analysis. Cells were left untreated (basal) or treated with 100 nM insulin for 10 min (insulin). Cell lysates were prepared, HA-tagged proteins were immunoprecipitated with anti-HA antibody (3 μg), and PKB kinase activity in the immune complexes was measured as described in Materials and Methods. The basal activity of HA-PKB cotransfected with pcDNA3 was assigned a value of 1.0. All other activities were expressed relative to this value. The results represent the means ± SE of data from three experiments under each set of conditions.
The relative potency of these constructs in inhibiting wild-type HA-PKB activity was determined. HA-PKB (0.4 μg of DNA) was cotransfected along with a 10-fold excess (4 μg) of either pcDNA3 or one of the three untagged versions of the mutant constructs (AAA-PKB, AA-PKB, or A-PKB). The insulin-dependent stimulation of HA-PKB activity in cells transfected with pcDNA3 alone was assigned a value of 100%. The insulin-stimulated activity of HA-PKB was almost completely abolished by AAA-PKB (to 7.5% ± 2.8%) and was less effectively decreased by AA-PKB (to 19% ± 10%) and A-PKB (27% ± 9.5%). These data demonstrate that, under similar experimental conditions, AAA-PKB is a more effective inhibitor of PKB activation by insulin than either A-PKB or AA-PKB.
Coexpression of wild-type PKBα with AAA-PKB restores GLUT4 translocation.
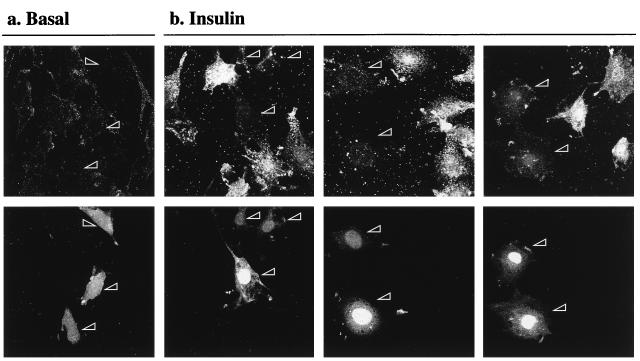
To further investigate whether the inhibitory action of AAA-PKB was realized through its action on the PKB pathway, cells were cotransfected with HA-AAA-PKB and HA-PKB at a DNA ratio of 1:4, with the rationale that excess wild-type PKB would restore insulin-stimulated GLUT4 translocation. The results of these experiments are shown in Fig. 6. Cells expressing GFP are identified by arrowheads. As seen above, insulin could not stimulate the translocation of GLUT4myc in cells that expressed HA-AAA-PKB (Fig. 6a). However, HA-AAA-PKB had no inhibitory effect on insulin-induced GLUT4myc translocation in cells that were cotransfected with four times more HA-PKB cDNA (Fig. 6b). These data strongly support the hypothesis that AAA-PKB prevented GLUT4myc translocation by inhibiting insulin activation of endogenous PKB.
FIG. 6.
Coexpression of wild-type PKB with AAA-PKB rescues the inhibition of insulin-stimulated GLUT4 translocation. L6-GLUT4myc myoblasts were cotransfected with GFP (0.4 μg), HA-AAA-PKB (0.4 μg), and pcDNA3 (1.6 μg) (a) or with GFP (0.4 μg), HA-AAA-PKB (0.4 μg), and HA-PKB (1.6 μg) (b) and incubated for 48 h in culture. Cells were serum deprived for 5 h, left untreated or treated with 100 nM insulin for 20 min, and then processed for cell surface GLUT4myc detection as indicated in Materials and Methods. Untreated cells are not shown. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells is shown (arrowheads). The upper panels show the cell surface GLUT4myc density for the transfection of AAA-PKB plus pcDNA3 vector alone (a) or the transfection of AAA-PKB with excess wild-type (WT) PKB (b). Arrowheads indicate the positions of transfected cells. Results shown are representative of at least three experiments.
PI 3-kinase-dependent actin reorganization is not affected by AAA-PKB.
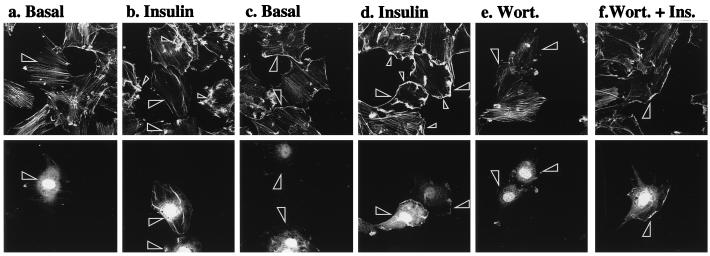
It is conceivable that the inhibition of GLUT4 translocation was a consequence of the ability of AAA-PKB to sequester PI 3-kinase products, independent of its ability to inhibit PKB activation. To examine this possibility, we determined the effect of AAA-PKB on insulin-induced actin filament reorganization, a phenomenon that requires activation of PI 3-kinase in L6 muscle cells and 3T3-L1 adipocytes (39, 54). By comparison, the reorganization of the actin cytoskeleton stimulated by platelet-derived growth factor is also PI 3-kinase dependent but is also known to be PKBα independent in porcine aortic endothelial cells (15), pig aortic endothelial cells (61), and SKF5 neuroectodermal cells (58). To explore the relationship between PKB/Akt and actin reorganization in muscle cells, L6-GLUT4myc myoblasts cotransfected with HA-AAA-PKB and GFP were treated for 5 min with insulin or were left untreated. Cellular filamentous actin was then labeled with rhodamine-conjugated phalloidin. Under basal conditions, phalloidin labeled a large number of distinct actin filaments (Fig. 7a). Insulin treatment resulted in a dramatic reorganization of actin, manifested as extensive bundling of actin structures at the cell periphery and in distinctive actin spikes within the cells (Fig. 7b, small arrowheads). In transfected cells (indicated by large arrowheads), Δp85α had no effect on the organization of actin in unstimulated cells but was able to completely block the insulin-stimulated bundling of actin at the cell periphery and the formation of actin spikes (Fig. 7a and b). Cells expressing HA-AAA-PKB (Fig. 7c and d, arrowheads) did not have altered actin filament organization in unstimulated cells. Nor did HA-AAA-PKB interfere with insulin-induced actin bundling compared with that observed in untransfected neighboring cells (Fig. 7c and d). The actin filament reorganization induced by insulin was prevented by wortmannin in all cells regardless of the presence or absence of AAA-PKB (Fig. 7e and f). These results demonstrate that activation of PKBα/Akt1 is not required for actin bundling and furthermore suggest that the inhibitory actions of AAA-PKB on GLUT4myc translocation are due to the ability of this mutant to prevent the activation of endogenous PKB and are not attributable to inhibition of PI 3-kinase.
FIG. 7.
PI 3-kinase-dependent actin remodeling is not affected by AAA-PKB. L6-GLUT4myc myoblasts were cotransfected with GFP (0.4 μg) and Δp85α (0.4 μg) (a and b) or with GFP (0.4 μg) and AAA-PKB (0.4 μg) (c to f) and incubated for 48 h in culture. Cells were serum deprived for 5 h and left untreated (basal) or treated with 100 nM insulin for 5 min (insulin). Cells in panels e and f were pretreated with wortmannin for 15 min before treatment with insulin (or no further treatment). All cells were processed for detection of actin with rhodamine-phalloidin as indicated in Materials and Methods. Each pair of panels (upper and lower) shows the same field of cells. In the lower panels, GFP fluorescence in transfected cells is shown (arrowheads). The upper panels show filamentous actin in untreated cells (basal) (a and c), cells pretreated with wortmannin (Wort.) (e), insulin-treated cells (insulin) (b and d), or wortmannin pretreated, insulin-treated cells (Wort. + Ins.) (f). Arrowheads indicate the positions of transfected cells. Results shown are representative of at least three experiments.
Measurement of atypical PKC activity.
L6 muscle cells express atypical protein PKCζ (28) as detected with a polyclonal antibody that also reacts with the λ isoform. To assess whether PKCζ was activated by insulin, the endogenous enzyme was immunoprecipitated from parental L6 myotubes with the same antibody. In vitro kinase activity of the immunoprecipitates toward myelin basic protein was measured. Immunoprecipitates from control cells showed substantial activity above the background measured in irrelevant-IgG immunoprecipitates. The background activity was only 7% of the activity in PKCζ immunoprecipitates. Immunoprecipitates from L6 myotubes treated with insulin for 5 min did not show any further increase in PKCζ activity relative to immunoprecipitates from control cells (control, 1.00; insulin, 0.96 ± 0.08 [mean ± SE of data from six independent experiments]). The PKCζ activity of HA-tagged wild-type PKCζ transfected into L6-GLUT4myc myoblasts was also determined. The transfected cells were incubated with insulin for 5 min, and HA-tagged proteins were immunoprecipitated for in vitro PKCζ activity assays. As in the case of the endogenous kinase, the exogenous enzyme was already active in immunoprecipitates from control cells (the background amounted to only 25% of the activity) and insulin stimulation did not increase the activity further (control, 1.00; insulin, 0.98 ± 0.005 [mean ± standard deviation of two independent experiments]). Similar results were obtained when cells were incubated with insulin for up to 15 min. Thus, contrary to a previous report (4), we could not demonstrate insulin-dependent activation of PKCζ in parental L6 myotubes or in L6-GLUT4myc myoblasts.
DISCUSSION
Inhibition of GLUT4myc translocation by AAA-PKB.
The PI 3-kinase dependence of insulin stimulation of glucose transporter translocation has been clearly demonstrated in a variety of insulin-responsive tissues and cell lines (47). How the PI 3-kinase lipid products mediate this translocation is still a point of debate. Recent evidence indicates that the PH domain of the serine/threonine kinase PKBα/Akt1 binds PI (3,4)-P2 and PI (3,4,5)-P3 in membranes and that full stimulation of this enzyme by insulin requires phosphorylation by the upstream kinases PDK1 and PDK2 (2, 3, 50). Hence, the question of whether PKBα/Akt1 activation is required for the stimulation of glucose uptake by insulin and other metabolic actions of the hormone has been raised. Processes such as amino acid uptake, glycogen synthesis, and glucose uptake were elevated in cells expressing constitutively or conditionally active versions of PKBα/Akt1 (20, 32, 33, 52). The use of a conditionally active PKBα/Akt1 chimera was the most informative of these approaches because the kinase could be activated by tamoxifen with a time course closely resembling that of the activation of the endogenous PKBα/Akt1 by insulin (32). Although these studies provide important insight into the function of PKBα/Akt1, they do not directly prove the participation of PKBα/Akt1 in the insulin-dependent regulation of these metabolic functions.
In the present study, we examined the participation of PKBα/Akt1 in the regulation of insulin-stimulated GLUT4 translocation in L6-GLUT4myc myoblasts by transiently expressing the AAA-PKBα/Akt1 mutant, which cannot be phosphorylated by PDK1 or PDK2 and is itself kinase inactive. We demonstrated that in L6-GLUT4myc myoblasts this mutant acts as a dominant-negative inhibitor of insulin-stimulated PKBα/Akt1, since it could block the activation of HA-tagged wild-type PKBα/Akt1 in cotransfection experiments as well as insulin-stimulated phosphorylation of ectopically expressed BAD protein. These two approaches are complementary; the first demonstrates that AAA-PKB can completely inhibit activation of PKB when the mutant is in excess of the wild-type enzyme, while the second approach shows that AAA-PKB can markedly inhibit the activation of endogenous PKB under a defined set of transfection conditions. Therefore, the AAA-PKB mutant was tested for its ability to interfere with insulin-dependent GLUT4myc translocation under the same conditions. Measuring the insulin-stimulated arrival of GLUT4myc at the cell surface, we observed marked inhibition of this action of insulin in cells transfected with AAA-PKB (Fig. 4 and 5A). Importantly, expression of excess wild-type PKB could reverse the inhibitory effect of AAA-PKB (Fig. 6). This latter result suggests that the AAA-PKB construct did not exert nonspecific effects, e.g., irreversibly binding to upstream or downstream signaling molecules and blocking their function.
To underscore the suggestion that AAA-PKB does not inhibit PI 3-kinase activation or signaling, we analyzed the effects of AAA-PKB on the insulin-dependent rearrangement of actin filaments in L6 myoblasts. Insulin stimulates actin bundling under the plasma membrane (referred to as membrane ruffling) in a PI 3-kinase-dependent manner (39). In this study, we showed that inhibition of PI 3-kinase by wortmannin or by expression of a dominant-negative Δp85α inhibitory molecule of PI 3-kinase abolished insulin-stimulated actin rearrangements in L6-GLUT4myc myoblasts. Recently, actin rearrangements associated with membrane ruffling were shown to be PKB/Akt independent in other cells treated with platelet-derived growth factor (15, 58, 61). We observed that AAA-PKB did not prevent the wortmannin-sensitive actin bundling elicited by insulin. These results extend PKB independence to insulin-induced actin bundling and demonstrate that PI 3-kinase functions normally in the presence of AAA-PKB. Thus, AAA-PKB likely prevented GLUT4myc translocation by inhibiting the activation of endogenous PKB/Akt.
Effect of other PKB mutants on GLUT4 translocation.
Attempts to inhibit the endogenous PKB/Akt activation and assess glucose transport regulation were made in two recent studies with different types of adipose cells. In the first study, cDNA encoding kinase-inactive A-PKB was introduced into rat adipocytes by electroporation and its effects on the appearance of cotransfected HA-tagged GLUT4 were tested after 20 h (11). In the second study, a cDNA encoding AA-PKB was introduced into 3T3-L1 adipocytes by adenovirus gene transfer and its effects were tested after 48 h (30). A-PKB caused a decrease in the sensitivity of insulin-stimulated HA-GLUT4 translocation and a partial (20%) reduction in the maximal mobilization of HA-GLUT4 to the cell surface of rat adipocytes (11). In contrast, AA-PKB did not prevent the stimulation of glucose transport by insulin or the translocation of the endogenous GLUT4 to the cell surface, despite preventing stimulation of protein synthesis by insulin (30). Unfortunately, due to experimental limitations, the former study could not demonstrate that the A-PKB mutant prevented PKB/Akt activation in rat adipocytes (11). On the other hand, AA-PKB was shown to significantly inhibit a large portion of the endogenous PKB/Akt activity on the basis of the reduced kinase activity remaining in the supernatant of lysates from cells transfected with HA-AA-PKB following the quantitative removal of HA-AA-PKB by immunoprecipitation (30). While this evidence is compelling, it is important to point out that approximately 15% of the insulin-stimulated activity of the endogenous PKB remained even at the highest dose of AA-PKB infection in that study (30). This represented a threefold increase in PKB activity above basal values. It is conceivable that this residual activation of PKB sufficed to stimulate glucose transport. The results from our study support this possibility. AA-PKB caused about an 80% inhibition of cotransfected HA-PKB but did not inhibit GLUT4myc translocation in L6 GLUT4myc myoblasts (Fig. 5). A more drastic inhibition of HA-PKB activity by AAA-PKB correlated with a substantial inhibition of GLUT4myc translocation. A-PKB was even less effective than AA-PKB at inhibiting HA-PKB activity, and like AA-PKB it was also unable to inhibit GLUT4myc translocation. We interpret these collective results to indicate that PKB activity may have to be inhibited substantially in order to result in an inhibition of GLUT4 translocation.
How is it envisaged that different PKB mutant forms have different dominant-negative effects? PKB forms multimeric complexes (possibly trimers) that are held in an inactive conformation by intermolecular interactions (10). PKB has no autokinase activity; its activation depends on phosphorylation of Thr308 and Ser473 by PDK1 and PDK2, which releases the inhibitory effect on a partnering PKB molecule (10). We predict that AAA-PKB would function as a complete inhibitor when two molecules of AAA-PKB complex with one wild-type PKB molecule. In this model, a complex consisting of two molecules of the other mutant, AA-PKB, and one wild-type PKB molecule could still retain up to one-third of its activity. That is, PDK phosphorylation of the wild-type PKB molecule in such a complex would derepress the activity of one of the AA-PKBs, allowing it to engage substrates.
The mechanism described above lends support to the conclusion that the different PKB mutants reported in the literature and in the present study lead to different degrees of inhibition of wild-type PKB and that only when a nearly complete inhibition of the endogenous PKB is achieved is glucose transport inhibited. In addition, it is possible that different cell types have different levels of requirement for PKB in the stimulation of glucose transport, and part of this may be due to the requirement for specific PKB isoforms. In L6 muscle cells and skeletal muscle, the PKBβ/Akt2 isoform is not activated by insulin to any measurable extent (59), whereas the PKBβ/Akt2 isoform is more robustly activated by insulin than is PKBα/Akt1 in rat adipocytes (59). In addition to inhibiting the endogenous PKBα/Akt1 in 3T3-L1 adipocytes to a large but incomplete degree, the AA-PKB mutant reduced the endogenous activity of PKBβ/Akt2 also to an incomplete degree (a twofold stimulation in response to insulin was still observed) (36). Clearly, sorting out the cell-specific, isoform-specific, and activity threshold requirements of PKB will be essential to our understanding of the precise mechanism of action of insulin.
While the manuscript was under review, Kasuga and colleagues reported compelling evidence that a dominant-negative version of the atypical PKCλ reduced the insulin-stimulated glucose uptake in 3T3-L1 cells by 50% (36). The possible contribution of atypical PKC to GLUT4 translocation in L6-GLUT4myc myoblasts remains to be determined. However, we were unable to show significant stimulation by insulin of either endogenous atypical PKCζ/λ or transfected HA-tagged PKCζ, even though a significant level of basal activity was measured. These results may underscore cell type-dependent engagements of the PKB and PKC pathways.
It is quite possible that both types of targets of PI 3-kinase, namely, the PKBs and atypical PKCs, contribute to the full translocation of GLUT4 and stimulation of glucose uptake. The relative participation of each pathway may be cell context dependent, and this context may include the relative amounts and proportions of the different isoforms within the two families. Ambient factors such as the state of differentiation of the cell or tissue and the action of concomitant stimuli may also be important. It is conceivable that PKBs and PKCs provide the cells with a certain degree of redundancy and that their interplay confers fine tuning of the insulin response.
In conclusion, the results presented here strongly suggest that PKBα/Akt1 participates in the stimulation of GLUT4 translocation in muscle cells. Conversely, stimulation of actin remodeling by insulin, which requires activation of PI 3-kinase, could be dissociated from the activation of PKBα/Akt1. The PKBα/Akt1 mutant described here has potent dominant inhibitory actions that will be useful in explorations of the regulation of other physiological responses.
ACKNOWLEDGMENTS
This work was supported by a grant from the Medical Research Council of Canada to A.K. (MT7307). Q.W. was supported by a fellowship from the Eli Lilly/Banting & Best Diabetes Centre Research Personnel Awards Program.
We thank Gary Sweeney for helpful discussions. We are also grateful to Paul Coffer for the GagPKB construct, Julian Downward for the Δp85α construct, Robert Farese for the Ha-PKCζ construct, and Richard Roth for advice on the PKB in vitro kinase assay.
Q.W. and R.S. contributed equally to this study.
REFERENCES
- 1.Alessi D R, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings B A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. doi: 10.1016/s0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 3.Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R, Reese C B, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 4.Bandyopadhyay G, Standaert M L, Galloway L, Moscat J, Farese R V. Evidence for involvement of protein kinase C (PKC)-zeta and noninvolvement of diacylglycerol-sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- 5.Brown R E, Jarvis K L, Hyland K J. Protein measurement using bicinchoninic acid: elimination of interfering substances. Anal Biochem. 1989;180:136–139. doi: 10.1016/0003-2697(89)90101-2. [DOI] [PubMed] [Google Scholar]
- 6.Burgering B M, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. doi: 10.1038/376599a0. [DOI] [PubMed] [Google Scholar]
- 7.Calera M R, Martinez C, Liu H, Jack A K, Birnbaum M J, Pilch P F. Insulin increases the association of Akt-2 with Glut4-containing vesicles. J Biol Chem. 1998;273:7201–7204. doi: 10.1074/jbc.273.13.7201. [DOI] [PubMed] [Google Scholar]
- 8.Cheatham B, Vlahos C J, Cheatham L, Wang L, Blenis J, Kahn C R. Phosphatidylinositol 3-kinase activation is required for insulin stimulation of pp70 S6 kinase, DNA synthesis, and glucose transporter translocation. Mol Cell Biol. 1994;14:4902–4911. doi: 10.1128/mcb.14.7.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke J F, Young P W, Yonezawa K, Kasuga M, Holman G D. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem J. 1994;300:631–635. doi: 10.1042/bj3000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffer P J, Jin J, Woodgett J R. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cong L N, Chen H, Li Y, Zhou L, McGibbon M A, Taylor S I, Quon M J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 12.Czech M P, Chawla A, Woon C W, Buxton J, Armoni M, Tang W, Joly M, Corvera S. Exofacial epitope-tagged glucose transporter chimeras reveal COOH-terminal sequences governing cellular localization. J Cell Biol. 1993;123:127–135. doi: 10.1083/jcb.123.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datta S R, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg M E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 14.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher A M. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- 16.Douen A G, Ramlal T, Rastogi S, Bilan P J, Cartee G D, Vranic M, Holloszy J O, Klip A. Exercise induces recruitment of the “insulin-responsive glucose transporter”: evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265:13427–13430. [PubMed] [Google Scholar]
- 17.Downward J. Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol. 1998;10:262–267. doi: 10.1016/s0955-0674(98)80149-x. [DOI] [PubMed] [Google Scholar]
- 18.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 19.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 20.Hajduch E, Alessi D R, Hemmings B A, Hundal H S. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes. 1998;47:1006–1013. doi: 10.2337/diabetes.47.7.1006. [DOI] [PubMed] [Google Scholar]
- 21.Herbst J J, Andrews G C, Contillo L G, Singleton D H, Genereux P E, Gibbs E M, Lienhard G E. Effect of the activation of phosphatidylinositol 3-kinase by a thiophosphotyrosine peptide on glucose transport in 3T3-L1 adipocytes. J Biol Chem. 1995;270:26000–26005. doi: 10.1074/jbc.270.43.26000. [DOI] [PubMed] [Google Scholar]
- 22.Imanaka T, Hayashi H, Kishi K, Wang L, Ishii K, Hazeki O, Katada T, Ebina Y. Reconstitution of insulin signaling pathways in rat 3Y1 cells lacking insulin receptor and insulin receptor substrate-1. Evidence that activation of Akt is insufficient for insulin-stimulated glycogen synthesis or glucose uptake in rat 3Y1 cells. J Biol Chem. 1998;273:25347–25355. doi: 10.1074/jbc.273.39.25347. [DOI] [PubMed] [Google Scholar]
- 23.James D E, Brown R, Navarro J, Pilch P F. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988;333:183–185. doi: 10.1038/333183a0. [DOI] [PubMed] [Google Scholar]
- 24.James D E, Strube M, Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989;338:83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- 25.Jiang T, Sweeney G, Rudolf M T, Klip A, Traynor-Kaplan A, Tsien R. Membrane-permeant esters of phosphatidylinositol 3,4,5-triphosphate. J Biol Chem. 1998;273:11017–11024. doi: 10.1074/jbc.273.18.11017. [DOI] [PubMed] [Google Scholar]
- 25a.Jin, J., and J. R. Woodgett. Unpublished data.
- 26.Kanai F, Nishioka Y, Hayashi H, Kamohara S, Todaka M, Ebina Y. Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J Biol Chem. 1993;268:14523–14526. [PubMed] [Google Scholar]
- 27.Katagiri H, Asano T, Ishihara H, Inukai K, Shibasaki Y, Kikuchi M, Yazaki Y, Oka Y. Overexpression of catalytic subunit p110α of phosphatidylinositol 3-kinase increases glucose transport activity with translocation of glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1996;271:16987–16990. doi: 10.1074/jbc.271.29.16987. [DOI] [PubMed] [Google Scholar]
- 28.Khayat Z, Tsakiridis T, Ueyama A, Somwar R, Ebina Y, Klip A. The rapid stimulation of glucose transport by mitochondrial uncoupling depends in part on cytosolic Ca2+ and Ca2+-sensitive protein kinase C. Am J Physiol. 1998;275:C1487–C1497. doi: 10.1152/ajpcell.1998.275.6.C1487. [DOI] [PubMed] [Google Scholar]
- 29.Kishi K, Muromoto N, Nakaya Y, Miyata I, Hagi A, Hayashi H, Ebina Y. Bradykinin directly triggers GLUT4 translocation via an insulin-independent pathway. Diabetes. 1998;47:550–558. doi: 10.2337/diabetes.47.4.550. [DOI] [PubMed] [Google Scholar]
- 30.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, Kasuga M. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klip A, Volchuk A, He L, Tsakiridis T. The glucose transporters of skeletal muscle. Semin Cell Dev Biol. 1996;7:229–237. [Google Scholar]
- 32.Kohn A D, Barthel A, Kovacina K S, Boge A, Wallach B, Summers S A, Birnbaum M J, Scott P H, Lawrence J C, Jr, Roth R A. Construction and characterization of a conditionally active version of the serine/threonine kinase Akt. J Biol Chem. 1998;273:11937–11943. doi: 10.1074/jbc.273.19.11937. [DOI] [PubMed] [Google Scholar]
- 33.Kohn A D, Summers S A, Birnbaum M J, Roth R A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 34.Koivisto U M, Martinez-Valdez H, Bilan P J, Burdett E, Ramlal T, Klip A. Differential regulation of the GLUT-1 and GLUT-4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J Biol Chem. 1991;266:2615–2621. [PubMed] [Google Scholar]
- 35.Kotani K, Carozzi A J, Sakaue H, Hara K, Robinson L J, Clark S F, Yonezawa K, James D E, Kasuga M. Requirement for phosphoinositide 3-kinase in insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 1995;209:343–348. doi: 10.1006/bbrc.1995.1509. [DOI] [PubMed] [Google Scholar]
- 36.Kotani K, Ogawa W, Matsumoto M, Kitamura T, Sakaue H, Hino Y, Miyake K, Sano W, Akimoto K, Ohno S, Kasuga M. Requirement of atypical protein kinase Cλ for insulin stimulation of glucose uptake but not for Akt activation in 3T3-L1 adipocytes. Mol Cell Biol. 1998;18:6971–6982. doi: 10.1128/mcb.18.12.6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kupriyanova T A, Kandror K V. Akt-2 binds to Glut4-containing vesicles and phosphorylates their component proteins in response to insulin. J Biol Chem. 1999;274:1458–1464. doi: 10.1074/jbc.274.3.1458. [DOI] [PubMed] [Google Scholar]
- 38.Marette A, Richardson J M, Ramlal T, Balon T W, Vranic M, Pessin J E, Klip A. Abundance, localization, and insulin-induced translocation of glucose transporters in red and white muscle. Am J Physiol. 1992;263:C443–C452. doi: 10.1152/ajpcell.1992.263.2.C443. [DOI] [PubMed] [Google Scholar]
- 39.Martin S S, Haruta T, Morris A J, Klippel A, Williams L T, Olefsky J M. Activated phosphatidylinositol 3-kinase is sufficient to mediate actin rearrangement and GLUT4 translocation in 3T3-L1 adipocytes. J Biol Chem. 1996;271:17605–17608. doi: 10.1074/jbc.271.30.17605. [DOI] [PubMed] [Google Scholar]
- 40.Mitsumoto Y, Burdett E, Grant A, Klip A. Differential expression of the GLUT1 and GLUT4 glucose transporters during differentiation of L6 muscle cells. Biochem Biophys Res Commun. 1991;175:652–659. doi: 10.1016/0006-291x(91)91615-j. [DOI] [PubMed] [Google Scholar]
- 41.Mitsumoto Y, Klip A. Developmental regulation of the subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J Biol Chem. 1992;267:4957–4962. [PubMed] [Google Scholar]
- 42.Nunez G, del Peso L. Linking extracellular survival signals and the apoptotic machinery. Curr Opin Neurobiol. 1998;8:613–618. doi: 10.1016/s0959-4388(98)80089-5. [DOI] [PubMed] [Google Scholar]
- 43.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor, wortmannin. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 44.Quon M J, Butte A J, Zarnowski M J, Sesti G, Cushman S W, Taylor S I. Insulin receptor substrate 1 mediates the stimulatory effect of insulin on GLUT4 translocation in transfected rat adipose cells. J Biol Chem. 1994;269:27920–27924. [PubMed] [Google Scholar]
- 45.Quon M J, Chen H, Ing B L, Liu M-L, Zarnowski M J, Yonezawa K, Kasuga M, Cushman S W, Taylor S I. Roles of 1-phosphatidylinositol 3-kinase and ras in regulating translocation of GLUT4 in transfected rat adipose cells. Mol Cell Biol. 1995;15:5403–5411. doi: 10.1128/mcb.15.10.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Viciana P, Warne P H, Khwaja A, Marte B M, Pappin D, Das P, Waterfield M D, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- 47.Shepherd P R, Withers D J, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333:471–490. doi: 10.1042/bj3330471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slot J W, Geuze H J, Gigengack S, James D E, Lienhard G E. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc Natl Acad Sci USA. 1991;88:7815–7819. doi: 10.1073/pnas.88.17.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Somwar R, Sweeney G, Ramlal T, Klip A. Stimulation of glucose and amino acid transport and activation of the insulin signalling pathways by insulin lispro in L6 skeletal muscle cells. Clin Ther. 1998;20:125–140. doi: 10.1016/s0149-2918(98)80040-4. [DOI] [PubMed] [Google Scholar]
- 50.Stokoe D, Stephens L R, Copeland T, Gaffney P R J, Reese C B, Painter G F, Holmes A B, McCormic F, Hawkins P T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science. 1997;277:567–570. doi: 10.1126/science.277.5325.567. [DOI] [PubMed] [Google Scholar]
- 51.Tanti J F, Gremeau T, Grillo S, Calleja V, Klippel A, Williams L T, Obberghen E V, LeMarchand-Brustel Y. Overexpression of a constitutively active form of phosphatidylinositol 3-kinase is sufficient to promote GLUT4 translocation in adipocytes. J Biol Chem. 1996;271:25227–25232. doi: 10.1074/jbc.271.41.25227. [DOI] [PubMed] [Google Scholar]
- 52.Tanti J F, Grillo S, Gremeaux T, Coffer P J, Van Obberghen E, Le Marchand-Brustel Y. Potential role of protein kinase B in glucose transporter 4 translocation in adipocytes. Endocrinology. 1997;138:2005–2010. doi: 10.1210/endo.138.5.5136. [DOI] [PubMed] [Google Scholar]
- 53.Tsakiridis T, McDowell H E, Walker T, Downes P C, Hundal H S, Vranic M, Klip A. Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport and glucose transporters in L6 skeletal muscle cells. Endocrinology. 1995;136:4315–4322. doi: 10.1210/endo.136.10.7664650. [DOI] [PubMed] [Google Scholar]
- 54.Tsakiridis T, Wang Q, Taha C, Grinstein S, Downey G, Klip A. Involvement of the actin network in insulin signalling. Soc Gen Physiol Ser. 1997;52:257–271. [PubMed] [Google Scholar]
- 55.Tsiani E, Bogdanovic E, Sorisky A, Nagy L, Fantus I G. Tyrosine phosphatase inhibitors, vanadate and pervanadate, stimulate glucose transport and GLUT translocation in muscle cells by a mechanism independent of phosphatidylinositol 3-kinase and protein kinase C. Diabetes. 1998;47:1676–1686. doi: 10.2337/diabetes.47.11.1676. [DOI] [PubMed] [Google Scholar]
- 56.Ueki K, Yamamoto-Honda R, Kaburagi Y, Yamauchi T, Tobe K, Burgering B M, Coffer P J, Komuro I, Akanuma Y, Yazaki Y, Kadowaki T. Potential role of protein kinase B in insulin-induced glucose transport, glycogen synthesis, and protein synthesis. J Biol Chem. 1998;273:5315–5322. doi: 10.1074/jbc.273.9.5315. [DOI] [PubMed] [Google Scholar]
- 56a.Ueyama, A., K. Yaworsky, Q. Wang, Y. Ebina, and A. Klip. Unpublished data.
- 57.van Weeren P C, de Bruyn K M, de Vries-Smits A M, van Lint J, Burgering B M T. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 58.van Weering D H J, de Rooij J, Marte B, Downward J, Bos J L, Burgering B M T. Protein kinase B activation and lamellipodium formation are independent phosphoinositide 3-kinase-mediated events differentially regulated by endogenous Ras. Mol Cell Biol. 1998;18:1802–1811. doi: 10.1128/mcb.18.4.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Walker K S, Deak M, Paterson A, Hudson K, Cohen P, Alessi D R. Activation of protein kinase B beta and gamma isoforms by insulin in vivo and by 3-phosphoinositide-dependent protein kinase-1 in vitro: comparison with protein kinase B alpha. Biochem J. 1998;331:299–308. doi: 10.1042/bj3310299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Q H, Khayat Z, Kishi K, Ebina Y, Klip A. GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Lett. 1998;427:193–197. doi: 10.1016/s0014-5793(98)00423-2. [DOI] [PubMed] [Google Scholar]
- 61.Welch H, Eguinoa A, Stephens L R, Hawkins P T. Protein kinase B and rac are activated in parallel within a phosphatidylinositide 3OH-kinase-controlled signaling pathway. J Biol Chem. 1998;273:11248–11256. doi: 10.1074/jbc.273.18.11248. [DOI] [PubMed] [Google Scholar]
- 62.Yeh J I, Gulve E A, Rameh L, Birnbaum M J. The effects of wortmannin on rat skeletal muscle. Dissociation of signaling pathways for insulin- and contraction-activated hexose transport. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.5.2107. [DOI] [PubMed] [Google Scholar]