Highlights
-
•
Cells infected with HSV-2 release migrasomes containing HSV-2 virions.
-
•
HSV-2 in the isolated migrasomes can be transmitted to uninfected cells and cause productive infection.
-
•
It is the first time that migrasomes have been found to play a role in virus spread.
Dear Editor,
Migrasomes are newly discovered cellular organelles with diameters of 0.5–3 μm which have been found to be produced by normal and cancer cells, and distributed in various organs of animals (Ma et al., 2015) and in human sera (Zhao et al., 2019). Migrasomes are present inside the cavities of pulmonary alveoli, blood vessels and lymph capillaries (Zhang et al., 2020), and can be captured by surrounding cells with their cargoes internalized. The biological roles of migrasomes have been reported in cell-to-cell communication (Zhu et al., 2021), organ morphogenesis during zebrafish embryonic development (Jiang et al., 2019), embryonic angiogenesis (Zhang et al., 2022a) and maintenance of cellular homeostasis (Jiao et al., 2021). Furthermore, migrasomes have been used as a diagnostic marker of early renal system injury in patients with diabetic-nephropathy (Liu et al., 2020). Recently, Zhang et al. reported that Chikungunya virus nsP1 can induce the formation of migrasomes (Zhang et al., 2022b); however, whether migrasomes play a role in virus infections remains unknown. It is unclear whether cells infected with viruses can release migrasomes containing virions. If so, it is unknown whether such migrasomes act as intermediate carriers for virus transmission. Herpes simplex virus type 2 (HSV-2), a typical member of the α-herpesvirus subfamily, can infect genital epithelium to cause herpes (Kimberlin and Whitley, 2007). HSV-2 infection also increases the risk of HIV-1 acquisition and transmission (Baeten et al., 2004; Freeman et al., 2006). Currently known modes of HSV-2 transmission include virus-cell and cell-to-cell spread mediated by the virus and virus-infected cells, respectively (Sattentau, 2008). Whether HSV-2 uses other mechanisms to spread remains elusive. Considering the characteristics of migrasomes and the fact that HSV-2 spread is predominantly dependent on cell-to-cell contact, we speculated that HSV-2 may take advantage of migrasome as a "Trojan horse" to be transmitted from cell to cell as one of the spread routes (Schematic diagram in Fig. 1A).
Fig. 1.
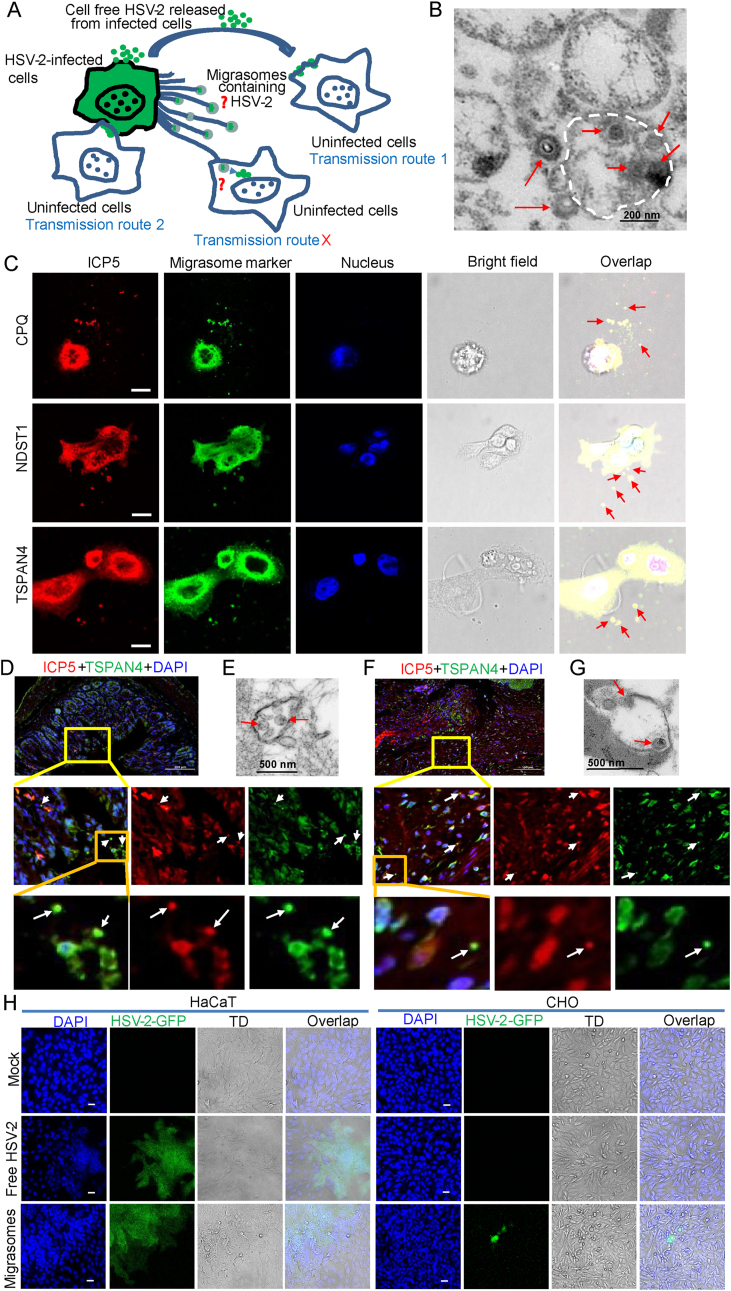
Migrasomes facilitate HSV-2 cell-to-cell spread. A Schematic diagram of HSV-2 transmission routes. B TEM image of HSV-2 virions in an isolated migrasome fraction. Scale bar, 200 nm. C Colocalization of migrasome markers CPQ, NDST1 and TSPAN4 with HSV-2 ICP5 in HSV-2-infected HaCaT cells was detected under fluorescence microscope. HaCaT cells infected with HSV-2 were fixed with polyformaldehyde at 20 hpi, followed by immunofluorescence assay. Scale bar, 10 μm. D TSPAN4 and HSV-2 ICP5 in ultra-thin sections of mouse intestinal tissues were assessed by immunofluorescence. Scale bar, 100 μm. E Migrasomes containing HSV-2 in ultra-thin sections of mouse intestinal tissues were observed under TEM. Scale bar, 500 nm. F TSPAN4 and HSV-2 ICP5 in ultra-thin sections of mouse cervical tissues were assessed by immunofluorescence. Scale bar, 100 μm. G Migrasomes containing HSV-2 in ultra-thin sections of mouse cervical tissues were observed under TEM. Scale bar, 500 nm. H Fluorescence images of HSV-2 infected HaCaT or CHO cells. Scale bar, 50 μm. One representative experiment out of three is shown. All experimental details were provided in Supplementary Materials.
To test this hypothesis, HSV-2 was used as a model to infect HaCaT cells in the culture dishes pretreated with fibronectin (FN). Migrasomes were collected at 20 h post infection (hpi) and purified by ultracentrifugation (Supplementary Fig. S1). The contents of the isolated migrasomes were observed under electron microscopy. As shown in Fig. 1B, HSV-2 virions were found in the migrasomes. Considering the conventional use of tetraspanin-4 (TSPAN4), carboxypeptidase Q (CPQ) and bifunctional heparan sulfate N-deacetylase/N-sulfotransferase 1 (NDST1) for migrasome detection (Bassani and Cingolani, 2012; Huang et al., 2019), HaCaT cells infected with HSV-2 were fixed with polyformaldehyde at 20 hpi, followed by immunofluorescence assay. Colocalization of the migrasome markers TSPAN4, CPQ and NDST1 with the major capsid protein ICP5 of HSV-2 in the extracellular vesicle was detected using immunofluorescence (indicated by the red arrows in Fig. 1C). In addition, ultrathin sections of HSV-2 infected mouse tissues were observed under transmission electron microscope (TEM) and fluorescence microscope. Six to eight-week-old female C57BL/6J mice were challenged intravaginally (i.vag.) with HSV-2 (G strain). The mice were sacrificed when genital ulceration and severe inflammation were observed at 7 dpi. The intestinal and cervical tissues were collected for TEM analysis and immunofluorescence staining. TEM images showed that HSV-2 virions exist in the migrasome-like structure of mouse intestinal (Fig. 1E) and cervical (Fig. 1G) tissues. The colocalization of TSPAN4 and ICP5 in the vesicle was detected in the mouse intestinal (indicated by white arrows in Fig. 1D) and cervical tissues (indicated by white arrows in Fig. 1F). These findings support our hypothesis that HSV-2 virions can be encapsulated in migrasomes.
We then assessed whether HSV-2 in the isolated migrasomes could be transmitted to uninfected cells and cause productive infection. HSV-2-GFP was used to infect HaCaT cells in the culture dish pretreated with fibronectin (FN). Migrasomes were subsequently isolated at 20 hpi and purified by ultracentrifugation. Cell-free HSV-2-GFP supernatant was filtered with 0.45 μm filter and the purified migrasomes were added into culture dishes coated with HSV-2 permissive cells (HaCaT) or non-permissive cells (CHO). As shown in Fig. 1H, both cell-free HSV-2-GFP and purified migrasomes resulted in the infection of HaCaT cells with green plaques and syncytia being observed. No green fluorescence was observed in HSV-2 non-permissive CHO cells following the infection of cell-free HSV-2-GFP. In contrast, although no plaque or syncytium formation was detected, green fluorescence was seen in individual CHO cells co-cultured with purified migrasomes, suggesting that HSV-2-GFP spread to CHO cells likely via migrasomes, which could lead to productive infection.
In conclusion, the results of this study suggest a novel mechanism of HSV-2 cell-to-cell spread mediated by migrasomes, providing a potential intervention target against HSV-2 transmission. To our knowledge, this is the first time that migrasomes have been found to play a role in virus spread.
Footnotes
This work was supported by the National Natural Science Foundation of China (31970172 and 82171736), the National Key Research and Development Program of China (2022YFC2304300), the Emergency Prevention and Control Capacity Program for New Severe Infectious diseases of National Institute for Viral Disease Control and Prevention, and the 135 Strategic Program of Chinese Academy of Sciences. We thank the Center for Instrumental Analysis and Metrology of Wuhan Institute of Virology for technique supports of electronic microscopy (Ms. Pei Zhang) and confocal microscopy (Dr. Ding Gao), and the Center for Experimental Animals of Wuhan Institute of Virology for assistance on animal study. All animal experiments were done in accordance with guidelines and protocols approved by the Institutional Review Board (IRB) of Wuhan Institute of Virology, Chinese Academy of Sciences. The authors declare that they have no conflict of interest. Qinxue Hu is an editorial board member for Virologica Sinica and was not involved in the editorial review or the decision to publish this article. All the data generated during the current study are included in the manuscript and the supplementary material.
The following are the Supplementary data to this article.
figs1.
Contributor Information
Yalan Liu, Email: liuyl@wh.iov.cn.
Qinxue Hu, Email: qhu@wh.iov.cn.
References
- Baeten J.M., McClelland R.S., Corey L., Overbaugh J., Lavreys L., Richardson B.A., Wald A., Mandaliya K., Bwayo J.J., Kreiss J.K. Vitamin A supplementation and genital shedding of herpes simplex virus among HIV-1-infected women: a randomized clinical trial. J. Infect. Dis. 2004;189:1466–1471. doi: 10.1086/383049. [DOI] [PubMed] [Google Scholar]
- Bassani S., Cingolani L.A. Tetraspanins: interactions and interplay with integrins. Int. J. Biochem. Cell Biol. 2012;44:703–708. doi: 10.1016/j.biocel.2012.01.020. [DOI] [PubMed] [Google Scholar]
- Freeman E.E., Weiss H.A., Glynn J.R., Cross P.L., Whitworth J.A., Hayes R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- Huang Y., Zucker B., Zhang S., Elias S., Zhu Y., Chen H., Ding T., Li Y., Sun Y., Lou J., Kozlov M.M., Yu L. Migrasome formation is mediated by assembly of micron-scale tetraspanin macrodomains. Nat. Cell Biol. 2019;21:991–1002. doi: 10.1038/s41556-019-0367-5. [DOI] [PubMed] [Google Scholar]
- Jiang D., Jiang Z., Lu D., Wang X., Liang H., Zhang J., Meng Y., Li Y., Wu D., Huang Y., Chen Y., Deng H., Wu Q., Xiong J., Meng A., Yu L. Migrasomes provide regional cues for organ morphogenesis during zebrafish gastrulation. Nat. Cell Biol. 2019;21:966–977. doi: 10.1038/s41556-019-0358-6. [DOI] [PubMed] [Google Scholar]
- Jiao H., Jiang D., Hu X., Du W., Ji L., Yang Y., Li X., Sho T., Wang X., Li Y., Wu Y.T., Wei Y.H., Hu X., Yu L. Mitocytosis, a migrasome-mediated mitochondrial quality-control process. Cell. 2021;184:2896–2910 e2813. doi: 10.1016/j.cell.2021.04.027. [DOI] [PubMed] [Google Scholar]
- Kimberlin D.W., Whitley R.J. In: Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Cambridge University Press; 2007. Antiviral therapy of HSV-1 and -2. [PubMed] [Google Scholar]
- Liu Y., Li S., Rong W., Zeng C., Zhu X., Chen Q., Li L., Liu Z.H., Zen K. Podocyte-released migrasomes in urine serve as an indicator for early podocyte injury. Kidney Dis. 2020;6:422–433. doi: 10.1159/000511504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L., Li Y., Peng J., Wu D., Zhao X., Cui Y., Chen L., Yan X., Du Y., Yu L. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015;25:24–38. doi: 10.1038/cr.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- Zhang C., Li T., Yin S., Gao M., He H., Li Y., Jiang D., Shi M., Wang J., Yu L. Monocytes deposit migrasomes to promote embryonic angiogenesis. Nat. Cell Biol. 2022;24:1726–1738. doi: 10.1038/s41556-022-01026-3. [DOI] [PubMed] [Google Scholar]
- Zhang N., Gao S., Zhang L. Chikungunya virus nsP1 induces migrasome formation. J. Infect. 2022;85:e158–e161. doi: 10.1016/j.jinf.2022.07.025. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang J., Ding Y., Zhang J., Xu Y., Xu J., Zheng S., Yang H. Migrasome and tetraspanins in vascular homeostasis: concept, present, and future. Front. Cell Dev. Biol. 2020;8:438. doi: 10.3389/fcell.2020.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Lei Y., Zheng J., Peng J., Li Y., Yu L., Chen Y. Identification of markers for migrasome detection. Cell Discov. 2019;5:27. doi: 10.1038/s41421-019-0093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M., Zou Q., Huang R., Li Y., Xing X., Fang J., Ma L., Li L., Yang X., Yu L. Lateral transfer of mRNA and protein by migrasomes modifies the recipient cells. Cell Res. 2021;31:237–240. doi: 10.1038/s41422-020-00415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.