Abstract
Hantaan virus (HTNV), the prototype virus of hantavirus, could escape innate immunity by restraining type I interferon (IFN) responses. It is largely unknown whether there existed other efficient anti-hantaviral tactics in host cells. Here, we demonstrate that the stimulator of interferon genes (STING) strengthens the host IFN-independent anti-hantaviral immunity. HTNV infection activates RIG-I through IRE1-XBP 1-mediated ER stress, which further facilitates the subcellular translocation and activation of STING. During this process, STING triggers cellular autophagy by interacting with Rab7A, thus restricting viral replication. To note, the anti-hantaviral effects of STING are independent of canonical IFN signaling. Additionally, neither application of the pharmacological antagonist nor the agonist targeting STING could improve the outcomes of nude mice post HTNV challenge in vivo. However, the administration of plasmids exogenously expressing the mutant C-terminal tail (ΔCTT) STING, which would not trigger the type I IFN responses, protected the nude mice from lethal HTNV infection. In summary, our research revealed a novel antiviral pathway through the RIG-I-STING-autophagy pathway, which offered novel therapeutic strategies against hantavirus infection.
Keywords: Hantaan virus (HTNV), IRE1, RIG-I, STING, Autophagy, Innate immunity
Highlights
-
•
HTNV infection activates STING through IRE1-RIG-I-dependent signaling.
-
•
STING exerts anti-hantaviral effects by enhancing autophagy instead of the type I IFN responses.
-
•
Administration of the CTT-truncated STING (△STING) might be a promising antiviral strategy against HTNV infection without triggering excessive inflammation.
1. Introduction
Hantaviruses (family Hantaviridae, order Bunyavirales) are enveloped, negative-sense RNA viruses. The hantaviral genome contains S (Small), M (Medium), and L (Large) segments, which encode the nucleocapsid protein (NP), glycoproteins precursor (GPC, cleavage into the N-terminal G1/Gn and C-terminal G2/Gc by the cellular signal peptidase complex), and the RNA-dependent polymerase protein (RdRp), respectively (Muyangwa et al., 2015). In humans, hantavirus infection causes two clinical syndromes, namely hemorrhagic fever with renal syndrome (HFRS) and hantavirus cardiopulmonary syndrome (HPS). HFRS most commonly occurs in Eurasia, caused by the Hantaan virus (HTNV), Seoul virus (SEOV), Dobrava virus (DOBV), and Puumala virus (PUUV), with a case-fatality rate of 15%. In contrast, HPS is caused by the Andes virus (ANDV) and the Sin Nombre virus (SNV) in America, with a mortality rate of 40% (Meier et al., 2021). Approximately 200,000 cases of HFRS are documented annually around the world, with more than 90% of HFRS cases occurring in Eurasia, among which China is the most seriously affected country (Jiang et al., 2017; Saavedra et al., 2021; Lu et al., 2021). HTNV, the prototypical hantavirus, is the etiological agent in severe HFRS patients. Considering, no effective therapeutics are available against HTNV infection (Liu et al., 2019b), it is of great importance to understand the interactions between HTNV and the host is essential to develop novel antiviral agents.
The innate immune system is the first line of defense against pathogenic microorganisms (Brubaker et al., 2015). Pathogen-associated molecular patterns (PAMPs) from evading pathogens, such as lipids, proteins, and nucleic acids, are recognized by the pathogen recognition receptors (PRRs) of host immune or tissue cells. They rapidly activate a series of anti-microbial signaling events characterized by type I interferons (IFNs) and other inflammatory responses (Kumar et al., 2011). The RIG-I-like receptors (RLRs), including RIG-I, MDA5, and LGP2, participate in sensing RNA virus infection to establish host antiviral immunity (Yoneyama et al., 2004). Type I IFN responses are crucial for defending against hantaviral infection (Matthys and Mackow, 2012). Hantaviruses employ various strategies to escape immune response and successfully replicate in host target cells (Wang et al., 2011). Therefore, investigating IFN-independent antiviral effects is clinically relevant. Our previous research shows that HTNV infection-induced autophagy exerts antiviral effects (Wang et al., 2019), which may be an important interferon-independent mechanism. Previous studies indicated that HTNV could trigger innate antiviral responses through RIG-I signaling, which is independent of the mitochondrial antiviral signaling protein (MAVS), but the specific molecular mechanism is unclear (Wang et al., 2019). Recently, studies have shown that RIG-I can bind and activate STING to exert an IFN-independent antiviral effect (Zevini et al., 2017; Zhang et al., 2022), but its relationship with HTNV infection remains poorly understood.
Stimulator of interferon genes (STING), is also known as TMEM173 (transmembrane protein 173), MITA (mediator of IRF3 activation), ERIS (endoplasmic reticulum IFN stimulator), or MPYS (N-terminal methionine-proline-tyrosine-serine plasma membrane tetraspanner). It is encoded by the gene Tmem173 and plays an essential role in host defense against a broad range of viruses (Liu et al., 2019a). It comprises three functional domains, namely the cytosolic N-terminal four-span transmembrane domain (TM 1–TM 4), the central cyclic GMP-AMP (cGAMP) binding domain (CBD) and the cytoplasmic C-terminal tail (CTT) domain (Shang et al., 2019; Zhang et al., 2020). The canonical function of STING is to trigger type I IFN responses post DNA virus infection or in the tumor microenvironment (Sun et al., 2013). The pathogen-derived DNAs (e.g., DNA viruses, retroviruses) or self-DNAs (e.g., mitochondria DNA/mtDNA, nuclear DNA) can be detected by cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS), which are further catalyzed into the second messenger cyclic GMP-AMP (cGAMP) from adenosine triphosphate (ATP) and guanosine triphosphate (GTP) (Woodward et al., 2010; Sun et al., 2013). Subsequently, cGAMP activates the endoplasmic reticulum (ER)-resident protein STING and promotes its traffic from the ER to Golgi (Gonugunta et al., 2017), during which STING recruits TANK-binding kinase (TBK1) through its CTT domain and drives the interferon regulatory factor 3 (IRF3) or IRF7-associated IFN responses (Zhang et al., 2019; Tanaka and Chen, 2012; Banete et al., 2018). Additionally, STING also activates the inhibitors of nuclear factor kappa-B (NF-κB) kinase (IKK) and NF-κB (IκB), leading to the expression of multiple inflammatory cytokines, including TNF, IL-1β, and IL-6 (Decout et al., 2021). Moreover, STING activation induces non-canonical autophagy (Liu et al., 2019a; Gui et al., 2019), which is independently of TBK1 as well as ULK1, Beclin1, and ATG9a (Rong et al., 2022). Our previous research demonstrated that hantavirus Gn-induced mitophagy inhibits type I IFN responses by degrading MAVS in the early stage, and NP inhibits Gn-induced mitophagy at late stage of infection (Wang et al., 2019). Although the functions of STING in DNA virus infection have been well documented (Hu et al., 2016; Singh et al., 2022; Li et al., 2022), the role of STING in RNA virus infection remains largely unclear (Ni et al., 2018), especially in hantavirus infection. STING also interacts with RIG-I and MAVS, which are key components of the RNA sensing pathway (Ishikawa and Barber, 2008).
In this study, we demonstrate that STING strengthens host autophagy-dependent anti-hantaviral immunity. STING induced the autophagy flux by interacting with Rab7A, which facilitated the clearance of viral proteins and the degradation of STING itself. Intriguingly, the anti-hantaviral effects exerted by RIG-I and STING post HTNV infection were irrelevant to the canonical IFN responses in vitro and in vivo. Our findings may help to develop novel clinical prophylaxis and therapy strategies against HFRS and other hemorrhagic fever diseases.
2. Materials and methods
2.1. Cell cultures
Human embryonic kidney 293T cell (HEK293T) (ATCC, Cat# CRL-3216), Vero E6 (ATCC, Cat# CCL-81), mouse brain-derived endothelial cells 3 (Bend3) (ATCC, Cat# CRL-2299), HeLa (ATCC, Cat# CCL-2) and human hepatoma (Huh7) cells were cultured in Dulbecco's modified Eagle's medium (DMEM, HyClone, Utah, USA, Cat# SH30809.01), supplemented with 10% fetal bovine serum (FBS, Biological Industries, Cat# 04-001-1ACS) and 1% penicillin-streptomycin (Solarbio Life Sciences) at 37 °C with 5% CO2. Human umbilical vein endothelial cells (HUVECs) from ScienCell (Cat# 8000) were cultured in endothelial cell medium (ECM, ScienCell, Cat# 1001) at 37 °C with 5% CO2. THP-1 cells (ATCC, Cat# TIB-202) were cultured in RPMI-1640 medium (CORNING, Jiang Su, Cat# 10040051) with 10% FBS and 1% penicillin-streptomycin at 37 °C with 5% CO2.
To generate the murine bone marrow-derived macrophages (mBMDMs), the femur and tibia were removed from the sacrificed adult mice. The bones were first rinsed with sterile phosphate-buffered saline (PBS) containing 0.1% (v/v) penicillin-streptomycin (P/S) solution. Subsequently, the bone marrow was flushed with Roswell Park Memorial Institute 1640 (RPMI 1640, Hyclone) containing 10% FBS and 0.1% P/S and filtered with the cell strainer (70 mm). Cells were resuspended with RPMI 1640 after centrifugation, and then primed with macrophage colony-stimulating factor (M-CSF, 20 ng/mL, PeproTech) with medium exchange every other day for four days to generate mBMDMs.
2.2. Virus infection
HTNV (strain 76-118) was preserved in our lab, and propagated in Vero E6 cells. EV71 (strain 87-2008 Xi'an Shaanxi, GenBank accession no. HM003207.1) was obtained from the Xi'an Centre for Disease Control and Prevention (Xi'an, Shaanxi, China). JEV wild-type strain P3 strain was propagated in the mosquito cell line C6/36. SINV was preserved in our lab. Cells were seeded in 6-well plates and cultured to a density about 50%–60%. Then the Cells were transfected with Flag-STING or Flag-△CTT, and infected with EV71 (MOI = 1), JEV (MOI = 1) and SINV (MOI = 1) for 2 h. After that, the viruses were removed and the cells were cultured in containing 10% FBS DMED.
2.3. Antibodies and reagents
All the antibodies and reagents applied in this article are listed in the Supplementary Table S1.
2.4. Plasmids
The full-length human STING sequence were amplified from the cDNA of HUVECs, and STING was cloned into pcDNA3.1-Flag, pLVX-IRES-ZsGreen 1, and pcDNA6.2-CMV-EGFP vector, respectively. For the construction of truncations (TM, CBD, △CTT, △TM) and site-directed mutagenesis (R238A, S366A, and R238A & S366A) of STING, PCR-based cloning was performed with a kit from Stratagene according to the manufacturer's protocol. Then the truncations and mutagenesis of STING were cloned into pcDNA3.1-Flag. Primers used for PCR, mutagenesis and truncations are shown in Supplementary Table S2. pCMA-Rab5A-Myc (Cat# HG14013-CM), pCMA-Rab7A-Myc (Cat# HG16168-CM) and pCMA-LMAN1/ERGIC-53-HA (Cat# HG16166-CY) were purchased from Sino Biological (Beijing, China). pcDNA3.1-Myc-NP, pcDNA3.1-RFP-LC3, pcDNA3.1-GFP-LC3 and pCDH-RIG-I-Myc were constructed in our lab.
2.5. Transfection
Cells were transfected with the indicated plasmids using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA, USA, Cat# 92008) or Hieff Trans Liposomal Transfection Reagent (Yeasen, Shanghai, China, Cat# 40802ES03) for 24 h, according to the manufacturer's instructions. siRNA oligomers were purchased from Gene Pharma (Suzhou, China) and Sangon Biotech (Shanghai, China), and transfected using Lipofectamine RNAi MAX (Invitrogen, Carlsbad, CA, USA, Cat# 13778-150) for 24 h, according to the manufacturer's instructions. The siRNA sequences used are shown in Supplementary Table S3.
2.6. qRT-PCR
Total RNA was extracted using E.Z.N.A.® Total RNA Kit Ⅰ (omega, Georgia, USA, Cat# R6834-02) according to the manufacturer's instructions. The concentration of RNAs was measured with Bio Tek Epoch, and RNA was reverse transcribed into first-strand complementary DNA (cDNA) using HiScript® II Q RT SuperMix for qPCR (+gDNA wiper) (Vazyme, Nanjing, China, Cat# R223-01) according to the manufacturer's protocol. Quantitative real-time polymerase chain reaction (qRT-PCR) assays were performed in a CFX96 Touch Real-Time PCR Detection System (BioRad, CA, USA) using 2×qPCR SmArt Mix (SYBR Green) (Low Rox) (DIYIBIO, Shanghai, China, Cat# DY20302). The relative abundance of gene expression was normalized to GAPDH. The primer sequences used in qRT-PCR were listed in Supplementary Table S4.
2.7. Immunofluorescence assays (IFA)
Cells were fixed with ice-cold 4% paraformaldehyde (PFA) for 15 min at 25 °C, after washing three times with phosphate-buffered saline (PBS) and permeabilized with 0.5% (v/v) Triton X-100 for 15 min. After blocking with 3% bovine serum albumin (BSA) (Yeasen, Cat# 36101ES76) for 30 min at 25 °C. After washing three times with PBS, the cells were incubated with specific primary antibody for HTNV NP (1:50 dilution), STING, RIG-I, Rab5A, Rab7A, ERGIC-53 overnight at 4 °C. Subsequently, the cells were washed three times with PBS and incubated with the secondary antibodies Alexa Fluor 488-conjugated goat anti-mouse IgG, Cy3-conjugated Donkey anti-rabbit IgG, Cy3-conjugated Donkey anti-mouse IgG and Cy5-conjugated Donkey anti-rabbit IgG for 1 h at 25 °C. After washing three times, nuclei were stained with Hoechst under dark conditions for 5 min at 25 °C. Finally, the cells were mounted with the Anti-Fade Mountant Medium (Sangon, Cat# E675011-0010). Samples were imaged using a confocal fluorescence microscope (A1R-HD25, Nikon).
2.8. Immunoblot assays
The immunoblot protocol was similar to our prior study (Wang et al., 2019). Briefly, cultured cells with the indicated treatment were harvested and lysed with cell lysis buffer (Beyotime, Shanghai, China, Cat# P0013C) containing protease inhibitor cocktail (TargetMol, Cat# C0001) on ice for 30 min. Supernatants were collected by centrifugation at 12,000 ×g for 20 min at 4 °C, and the protein concentration was determined using a Pierce™ BCA Protein Assay Kit (Thermo scientific, MA, USA, Cat# 23225). The whole cell lysates in lysis buffer were mixed with SDS-loading buffer (GenScript, Nanjing, China, Cat# M00676-250) and denatured at 95 °C for 10 min. The proteins were separated by SDS-PAGE and then transferred to polyvinylidene fluoride membranes (PVDF) (Immobilon, Ireland, Cat# ISEQ00010), followed by blocking with 5% Difco™ Skim Milk (BD, Cat# 232100) in 1× TBST. The membranes were incubated with indicated primary antibodies present in Supplementary Table S1 overnight, followed by incubation with secondary Abs labeled with infrared dyes. The signals on the PVDF membrane were visualized using the Odyssey Infrared Imaging System (LI-COR Biosciences). The band intensity was quantified by Image J software.
2.9. Co-immunoprecipitation
After HEK293T cells were transfected with the indicated plasmids for 36 h, cells were washed with ice-cold PBS and lysed with cell lysis buffer for Western and IP (Beyotime, Cat# P0013) containing protease inhibitor cocktail on ice for 15 min. The cells were scraped from the plate and transferred to microcentrifuge tubes on ice for 15 min, and the supernatants were collected by centrifugation at 12,000 ×g for 20 min at 4 °C. A small amount of supernatant was saved as input. The remaining supernatant was incubated with the anti-Flag magnetic beads (bimake, TX, USA, Cat# B26102), anti-Myc magnetic beads (bimake, Cat# B26302) or protein A/G agarose beads (bimake, Cat# B23202) incubated-antibodies overnight at 4 °C. Once the binding step was complete, the magnetic beads were collected, and the supernatant was removed. The magnetic beads were washed with TBS buffer to remove all nonspecifically bound proteins. Then, the collected beads were boiled at 95 °C for 10 min in 50 μL water and 10 μL 5× SDS protein-loading buffer and analyzed with immunoblot.
2.10. Lentivirus packing and infection
For overexpression of STING in cells that are difficult to transfect plasmids with liposomes, the human STING was cloned into the pLVX-IRSE-ZsGreen 1 vector using specific primers. The primers used are shown in Supplementary Table S2. Lentiviral particles were produced by co-transfecting HEK293T cells with transfer plasmid (empty vector or pLVX-IRSE-ZsGreen-STING), psPAX2 packaging plasmid and pMD2.G envelope plasmid with Hieff Trans Liposomal Transfection Reagent (Yeasen, Shanghai, China). After 6 h of post-transfection, media was changed with DMEM (HyClone, Cat# SH30809.01) containing 10% FBS (FBS, Cat# 04-001-1ACS). Supernatants were collected at 48 h and 72 h and then centrifuged at 2000 ×g for 15 min. The supernatants were aliquoted and stored at −80 °C until use. For transfection, cells were seeded in a 12-well or 6-well plate and incubated with lentivirus containing 4 μg/mL polybrene (Solarbio, Cat# H8761). A total of 48 h, the culture medium was replaced with a fresh medium containing 2 μg/mL puromycin (Solarbio, Cat# H8761).
2.11. Animal experiments
Six-to-eight-week-old female nude mice were obtained from the Experimental Animal Center of Fourth Military Medical University. All mice were maintained in pathogen-free barrier facilities. The mice were intraperitoneally injected with PBS or HTNV (4 × 104 TCID50/g), respectively. The cages were assigned randomly to PBS, PBS + HTNV, HTNV + H-151 and HTNV + DMXAA groups after the HTNV infection. Mice received every two days dose of H-151 (10 mg/kg) or DMXAA (25 mg/kg) starting at day 2 after HTNV infection and were euthanized at day 3 or day 6 post infection (dpi). Different tissues, including heart, livers, spleens, lung and kidneys were collected. Half of each tissue were fixed in 4% PFA for IFA assays or hematoxylin and eosin (H&E) staining, and the other half of the tissues were used for immunoblotting or qRT-PCR assays. Moreover, we measured the survival or body weight study. To assess the therapeutic potential of the non-classical function of STING, the mice were intraperitoneally inoculated with PBS and HTNV (4 × 104 TCID50/g), and the tail vein inoculated with empty vector, wild-type (WT) and △CTT STING (20 mg/kg), follow-up experiments as described above.
2.12. Histological analyses
The Histological analyses protocol was similar to our prior study (Wang et al., 2019). Briefly, formalin-fixed paraffin-embedded (FFPE) tissue samples were sectioned and placed on slides. Sections were first deparaffinized and rehydrated, and then stained with hematoxylin and eosin. Finally, slides were scanned with the Panoramic MIDI (3DHISTECH) and analyzed using Case Viewer 2.3 software. For immunofluorescence analysis, sections were stained with anti-STING and anti-HTNV NP antibodies (1A8 prepared by our lab), and then stained with fluorescently labeled secondary antibodies. Cell nuclei were stained with DAPI, and then confocal microscopy detection was performed.
2.13. Statistical analysis
Statistical analyses are described in the figure legends. Data were expressed as mean ± SEM. Statistical significance was determined using GraphPad Prism (GraphPad Software, Version 8.0.2). Significant differences between two groups were determined by two-tailed unpaired t-test, while the multiple comparisons were performed by one-way ANOVA. ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns, not significant.
3. Results
3.1. The reaction of STING during HTNV infection
To investigate the role of STING protein in the HTNV life cycle, human umbilical vein endothelial cells (HUVECs), which are HTNV target cells in vivo, were infected with HTNV at a MOI of 1. Subsequently, cell lysates were analyzed by immunoblot at different time points post-infection. The results showed that the total STING protein was significantly loss with increasing viral replication at 36 h post-infection (hpi) (Fig. 1A and B), but significant increases in mRNA levels of STING (Fig. 1C). Previous studies indicated that the dengue virus (DENV) NS2B3 protease complex specifically cleaves human STING protein but not mouse STING, leading to STING degradation (Aguirre et al., 2012). The inter-species differences in the degradation of human and mouse STING proteins caused by HTNV infection were investigated. The protein levels of STING in mouse brain-derived endothelial cells 3 (Bend3) were detected after HTNV infection, revealing STING loss from 36 hpi of viral infection (Supplementary Fig. S1A). Similar results were observed in HTNV-infected THP-1 cells (a human monocyte cell line) (Supplementary Fig. S1B).
Fig. 1.
The reaction of STING during HTNV infection. A–C Human umbilical vein endothelial cells (HUVECs) were mock-infected or HTNV-infected (MOI = 1) for indicated time points. A The protein levels of HTNV NP and STING were detected by immunoblotting. B The grey values of the protein level of STING bands from three independent immunoblot through the software Image J and carry out statistical analysis. C the mRNA levels of STING was determined by qRT-PCR with GAPDH as the internal control. D–F HUVECs were infected with HTNV at indicated MOI for 36 h, and the lysates were harvested for immunoblotting analysis of HTNV NP and STING. E The grey values of the protein level of STING bands from three independent immunoblot through the software Image J and carry out statistical analysis. F The qRT-PCR analysis for STING mRNA levels in HTNV-infected HUVECs for indicated MOI. G HUVECs were mock-infected or HTNV-infected (MOI = 1) for indicated time points. Cells were fixed and permeabilized for immunofluorescence using STING (red) antibody and HTNV NP (green) antibody, respectively. Nuclei were stained with DAPI. The scale bar is shown as 20 μm. H HUVECs were mock-infected or HTNV-infected at an MOI of 1, after 24 h, cells were treated with autophagy inhibitor chloroquine (CQ) (20 μmol/L), bafilomycin A1 (BAFA1) (100 nmol/L), 3-methyladenine (3-MA) (25 μmol/L) and proteasome inhibitor MG132 (10 μmol/L). The lysates were harvested for immunoblot analysis. I Quantification of the protein level of STING from (H). Data are expressed as mean ± SEM (n = 3). Statistical significance was calculated using Student's t-test or one-way ANOVA; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns, not significant.
To further clarify the relationship between STING reaction and HTNV infection, we next detected STING protein after HTNV infection with different MOI in HUVECs. At lower viral titers (MOI from 0.01 to 0.1) STING protein was almost unchanged, while STING protein was decreased with higher viral titers (MOI = 1) at 36 h post infection (Fig. 1D and E). Interestingly, the relative mRNA expression level of STING was negatively correlated with the protein level at high titers (MOI = 1) in HUVECs (Fig. 1F). Similar results were observed in HTNV-infected Bend3 and THP-1 cells (Supplementary Fig. S1C and S1D). Consistent with the immunoblotting results, the immunofluorescence also revealed that HTNV infection led to a significant decrease in STING in HUVECs (Fig. 1G). Collectively, these results suggest that HTNV infection leads to the loss of STING protein.
The ubiquitin-proteasome system and autophagy-lysosome degradation are the two major intracellular protein degradation pathways in eukaryotes (Cohen-Kaplan et al., 2016). To explore the mediating pathways responsible for STING protein loss in HTNV infection, we treated mock- and HTNV-infected HUVECs (MOI = 1) were treated with chloroquine (CQ), bafilomycin A1 (BAFA1), 3-methyladenine (3-MA) or MG132. The proteasome inhibitor MG132 failed to inhibit STING protein degradation after HTNV infection (Fig. 1H and I). Conversely, the autophagy inhibitor CQ, BAFA1 and 3-MA successfully inhibited STING protein degradation during HTNV infection (Fig. 1H and I), indicating that HTNV infection might result in the autophagic degradation of STING. Taken together, these results indicated that STING degradation following HTNV infection was primarily mediated by autophagy.
3.2. STING inhibits HTNV and a broad range of RNA virus replication
To further investigate the role of STING in HTNV replication, STING-specific small interfering RNAs (siRNA) were used to knock down STING expression in HUVECs, and its ablation efficiency was confirmed by immunoblot and qRT-PCR at 24 hpi (Supplementary Figs. S2A and S2B). Subsequently, the STING-silenced HUVECs were infected with HTNV for 36 h at an MOI of 1. We found that STING knockdown remarkably facilitated HTNV NP production (Fig. 2A) and promoted viral RNA replication (Fig. 2B). Moreover, exogenously STING expression decreased HTNV NP production (Fig. 2C) and inhibited viral RNA replication (Fig. 2D). Similar results were observed in human hepatoma (Huh7) cells (Supplementary Figs. S2C and S2D), which were STING-deficient cells. In addition, both hSTING (human STING) and mSTING (mouse STING) could inhibit HTNV replication in a dose-dependent manner (Supplementary Figs. S2E and S2F), indicating that the inhibitory effect of STING on virus replication is highly evolutionarily conserved. Next, the pharmacological STING antagonist H-151 was used to inhibit its activity by covalently binding to STING at the transmembrane cysteine residue 91, blocking STING palmitoylation. Mouse bone marrow-derived macrophages (mBMDMs) were treated with H-151 and then infected with HTNV at an MOI of 1, and the results indicated that blocking STING palmitoylation facilitates HTNV replication (Fig. 2E–G). STING agonist DMXAA treatment inhibits virus replication (Fig. 2E–G).
Fig. 2.
STING inhibits HTNV and a broad range of RNA virus replication. A, B Human umbilical vein endothelial cells (HUVECs) were transfected with 150 nmol/L STING-specific siRNA (siSTING-1, siSTING-2, siSTING-3) or a negative control sequence (NC) for 24 h and then infected with HTNV (MOI = 1) for 36 h. Cell lysates were harvested for immunoblot (A) or qRT-PCR (B) analysis. C, D HUVECs were transfected with Flag-STING (3 μg/well) for 24 h and then infected with HTNV (MOI = 1) for 36 h. C Cell lysates were used for detecting the protein level of HTNV NP through immunoblotting. D The mRNA levels of HTNV S were detected through qRT-PCR with GAPDH as the internal control. E–G Mouse bone marrow-derived macrophages were treated with STING antagonist H-151 (0.5 μmol/mL) and agonist DMXAA (20 μmol/mL) for 24 h and then infected with HTNV (MOI = 1) for 36 h. E Cells were harvested for analysis of HTNV NP and STING protein expression and HTNV S mRNA level. F The grey values of the protein level of HTNV NP bands from three independent immunoblot through the software Image J and carry out statistical analysis. G The mRNA levels of HTNV S were detected through qRT-PCR with GAPDH as the internal control. H Schematic diagrams of human STING protein. I Huh7 cells were transfected with empty vector, WT, TM, CBD, △CTT, and △TM of STING for 24 h and then infected with HTNV (MOI = 1) for 36 h. Cell lysates were used for detecting the protein level of HTNV NP through immunoblot. J The mRNA levels of HTNV S were detected through qRT-PCR with GAPDH as the internal control. K Huh7 cells were transfected with empty vector, WT, △CTT of STING for 24 h and then infected with VSV-GFP, SINV, JEV, and EV71 for other 24 h. Cell lysates were used for detecting the protein level of RNA virus through immunoblot. Data are expressed as mean ± SEM (n = 3). Statistical significance was calculated using Student's t-test or one-way ANOVA; ∗P < 0.05; ∗∗∗P < 0.001.
STING is an evolutionarily conserved transmembrane protein that localizes to the endoplasmic reticulum (ER) membrane. The STING proteins Nematostella vectensis (NvSTING) and Xenopus tropicalis (XtSTING) do not encode type I IFNs. STING comprises a four-span transmembrane domain, a connector region and a cytosolic ligand-binding domain (LBD). To investigate which domain within STING responsible for the antiviral activity, different truncated STING plasmids were constructed, namely TM (1–139 aa), CBD (139–340 aa), △CTT (1–340 aa), and △TM (139–379 aa) (Fig. 2H). Huh7 cells were transfected with truncated plasmids and then infected with HTNV (MOI = 1). We found that △CTT restrained HTNV NP production (Fig. 2I) and inhibited viral RNA replication to a similar level as the WT STING (Fig. 2J). The findings suggested that inhibition of the HTNV replication is dependent on the STING conserved domain but not the interferon-inducing domain. Furthermore, to confirm whether STING can inhibit a broad range of RNA virus replication, Huh7 cells were transfected with Flag-STING (WT) and △CTT and then infected with vesicular stomatitis virus-green fluorescent protein (VSV-GFP) (Lawson et al., 1995), Sindbis virus (SINV), Japanese encephalitis virus (JEV), and Enterovirus 71 (EV71). We found that SINV, JEV and EV71 replication were inhibited in Huh7 cells with WT and △CTT STING overexpression. In contrast, VSV-GFP replication was almost unaffected (Fig. 2K), suggesting that the antiviral effect of STING is not attributed to its CTT domain and interferon induction. Collectively, these findings suggested that the inhibitive properties of STING on HTNV and a broad range of RNA virus replication are independent of its CTT domain.
3.3. STING inhibits HTNV replication through an IFN-independent pathway
The classical cGAS-STING-IFN pathway is known for its pivotal role in response to DNA virus infection. Recent studies reported that STING plays an important role in RNA virus infection (Ni et al., 2018; Webb and Fernandez-Sesma, 2022; Franz et al., 2018; Citil Dogan et al., 2017). We also confirmed that STING exerts anti-HTNV effect, although its specific mechanism is remains unclear. To further clarify the relationship between STING and IFN production after HTNV infection, we constructed three STING mutants, R238A, S366A, and R238A/S366A double mutation (Fig. 3A). The R238A mutation could disrupt 2′3′-cGAMP binding to STING, while the S366A mutation disables STING phosphorylation, thereby disrupting IRF3 recruitment (Yamashiro et al., 2020). These mutants were transfected into Huh7 cells and stimulated with HTNV and STING-specific agonists 2′3′-cGAMP. As expected, 2′3′-cGAMP stimulation could trigger IFN-β responses only in WT-STING over-expression group, but not the STING-mutant over-expression group (Fig. 3B-ⅰ, B-ⅱ). Similar phenomenon was also observed during HTNV infection, as shown in (Fig. 3B-ⅲ). These results reveal that the antiviral properties of STING are type I IFN-independent. The replication of HTNV was assessed by the HTNV-NP protein levels and HTNV-S RNA levels, and the results suggested that overexpression of R238A, S366A, and ΔCTT in Huh7 cells could restrain HTNV NP production (Fig. 3C and D) and inhibit viral RNA replication (Fig. 3E). In addition, STING inhibits HTNV replication independently of the CTT domain. HTNV infection failed to induce IFN-β production in △CTT-overexpressing Huh 7 cells, while IFN, ISGs (Mx1, ISG56) and chemokines (IL-1β) were significantly increased in WT STING-overexpressing Huh 7 cells (Fig. 3F). These results indicate that STING inhibition is HTNV replication independent of interferon. Type I IFN production requires the coordinated of multiple STING downstream molecules, such as TBK1 and the IRF3.
Fig. 3.
STING inhibits HTNV replication through an IFN-independent pathway. A Schematic diagrams of human STING mutagenesis. B Huh7 cells were transfected with 3 μg/well empty vector or mutagenesis (R238A, S366A, R238A & S366A) of STING for 24 h and then mock-treated (ⅰ), cGAMP-treated (ⅱ) or HTNV-infected (MOI = 1) (ⅲ) for 12 h or 36 h. The mRNA levels of IFN-β were detected through qRT-PCR with GAPDH as the internal control. C–E Huh7 cells were transfected with empty vector or mutagenesis (R238A, S366A, R238A & S366A, △CTT) of STING (3 μg/well) for 24 h and then HTNV-infected (MOI = 1) 36 h. C Cell lysates were harvested for immunoblotting analysis. D The grey values of the protein level of HTNV NP bands from three independent immunoblot through the software Image J and carry out statistical analysis. E The mRNA levels of HTNV S were detected through qRT-PCR with GAPDH as the internal control. F Huh7 cells were transfected with empty vector or truncations (TM, CBD, △CTT, △TM) of STING (3 μg/well) for 24 h and then HTNV-infected (MOI = 1) 36 h. The mRNA levels of Mx1 (ⅰ), ISG56 (ⅱ), IFN-β (ⅲ) and IL-1β (Ⅳ) were detected through qRT-PCR with GAPDH as the internal control. G Huh7 cells were transfected with TBK1/IRF3-specific siRNA or NC (150 nmol/L) for 24 h and then transfected with Flag-STING for 24 h, and subsequently infected with HTNV (MOI = 1) for 36 h. Cell lysates were harvested for immunoblotting analysis. H Huh7 cells were transfected with TBK1/IRF3-specific siRNA or NC (150 nmol/L) for 24 h and then infected with HTNV (MOI = 1) for 36 h. The mRNA levels of IFN-β were detected through qRT-PCR with GAPDH as the internal control. I HUVECs were treated with H-151 and DMXAA for 24 h and then infected with HTNV (MOI = 1) for 36 h. The mRNA levels of IFN-β were detected through qRT-PCR with GAPDH as the internal control. J Huh7 cells were seeded in a 12-well plate and incubated with sh-TBK1 lentivirus containing 4 μg/mL polybrene. A total of 48 h, the culture medium was replaced with a fresh medium containing 2 μg/mL puromycin. The cells screened by purinomycin were transfected with Flag-STING and then infected with HTNV (MOI = 1) for 36 h. Cell lysates were used for detecting the protein level of HTNV NP and TBK1 through immunoblotting. K Vero E6 cells were infected with lentivirus vector or pLVX-IRES-ZsGreen-STING for 24 h and then mock-infected or HTNV-infected at an MOI of 1. After 36 h. Immunoblot analysis was used to quantify viral protein (HTNV NP) expression. Data are expressed as mean ± SEM (n = 3). Statistical significance was calculated using Student's t-test or one-way ANOVA; ∗∗∗P < 0.001; ns, not significant.
To further confirm whether the antiviral effect of STING depends on its downstream molecules, we knocked down the above molecules before and after STING overexpression and tested their effects on virus replication. Knockdown of IRF3 or TBK1 promoted HTNV replication in the absence of STING overexpression (Fig. 3G), which may be associated with reduced IFN production (Fig. 3H and I). However, the knockdown of these molecules did not reverse the anti-HTNV effect of STING in the presence of STING overexpression (Fig. 3G). Similar results were observed in TBK1 stable knocked out Huh7 cells (Fig. 3J). Moreover, STING overexpression by lentivirus still inhibited HTNV replication in Vero E6 cells, an interferon-deficient green monkey kidney epithelial cell line (Fig. 3K), suggesting that the antiviral effect of STING is independent of the above molecules. Collectively, STING exerts an IFN-independent anti-hantaviral effect.
3.4. STING-mediated autophagy restricts HTNV replication
The above-mentioned findings indicated that STING could inhibit HTNV replication independent of classical cGAS-STING-IFN signaling. However, the mechanism underlying the antiviral effects of STING remains unknown. In recent years, many studies have shown that STING-induced autophagy plays a crucial role in antiviral responses (Zhang et al., 2021). To investigate whether STING-induced autophagy participate in the suppression of HTNV replication, mock-infected and HTNV-infected (MOI = 1) cells were treated with different inhibitors of protein degradation. The autophagy inhibitors BAFA1 and proteasome inhibitor MG132 were used (Fig. 4A). The results revealed that BAFA1 could reverse STING inhibition of HTNV replication and result in higher levels of LC3B-II and p62 accumulate in overexpression STING cells compared to the vector cells. However, MG132 failed to rescue STING-induced inhibition of HTNV replication, and no change in the levels of LC3B-II and p62 expression was found (Fig. 4B and C). Similar results were observed in Vero E6 cells (Supplementary Figs. S3A and S3B), indicating that STING-induced autophagy may inhibit HTNV replication. BAFA1 could also reverse STING inhibition of SINV and EV71 replication (Fig. 4D), indicating that STING-induced autophagy may inhibit a broad range of RNA virus replication.
Fig. 4.
STING-mediated autophagy restricts HTNV replication. A Diagram of the assay for (B). B Huh7 cells were transfected with Flag-hSTING (3 μg/well) for 24 h and then infected with HTNV (MOI = 1) for 36 h, and subsequently treated with BAFA1 or MG132 for 4 h or 8 h. HTNV NP and autophagy-related protein (p62 and LC3B) were detected by immunoblotting. C The grey values of the protein level of LC3B-Ⅰ/LC3B-Ⅱ bands from three independent immunoblot through the software Image J and carry out statistical analysis. Statistical significance was calculated using Student's t-test; ∗∗∗P < 0.001. D Huh7 cells were transfected with WT or △CTT of STING (3 μg/well) for 24 h and then infected with VSV-GFP, SINV, and EV71 for 24 h, and subsequently treated with BAFA1 for 4 h. Viral proteins were detected by immunoblotting. E Huh7 cells were transfected with 3 μg/well empty vector or truncations (WT, TM, CBD, △CTT, △TM) of STING for 24 h, and the protein levels of p62 and LC3B were detected by immunoblotting. F Huh7 cells were transfected with 3 μg/well empty vector, WT and truncations mutagenesis (R238A, S366A and △CTT) of STING for 24 h and then infected with HTNV (MOI = 1) for 36 h. The protein levels of HTNV NP and LC3B were detected by immunoblotting.
We have demonstrated that ΔCTT effectively suppresses HTNV replication, and STING (WT) also induces autophagy (Figs. 2G, 4A and B). STING-induced autophagy does not require its CTT domain, as evidenced by Daniorerio STING (DrSTING), Nematostella vectensis STING (NvSTING), and Xenopus tropicalis STING (XtSTING) proteins exhibit a solid ability to induce autophagy. To confirm the relationship between STING-induced autophagy and HTNV replication, WT STING and mutants were overexpressed in Hela cells. The immunoblot results revealed that WT STING overexpression and the truncated ΔCTT could induce higher levels of LC3B-II accumulation and p62 degradation, while TM (1–139 aa), CBD (139–340 aa), ΔTM (139–379 aa) failed to induce autophagy (Fig. 4E). Confocal microscopy also revealed that overexpression of WT and ΔCTT of STING significantly increased the levels of LC3B puncta formation in HeLa cells (Supplementary Fig. S3C). Furthermore, the R238A and S366A mutants of STING significantly increased LC3B-II accumulation in HeLa cells (Fig. 4F). Conjointly, these results suggest that STING-induced autophagy could inhibit HTNV replication.
3.5. STING trafficking from ER to late endosome participate in viral protein degradation
HTNV replication inhibition by STING is dependent on STING-induced autophagy but independent of IFN antiviral response. The mechanism underlying STING-activated autophagy to restrict HTNV replication remains largely unclear. Therefore, HUVECs were infected with HTNV, and STING trafficking post-infection was monitored by confocal microscopy after co-staining STING with the early endosome marker Rab5A, late endosome/lysosome marker Rab7A, ERGIC-53, and lysosome tracker. The results showed that STING was trafficked to ERGIC (Fig. 5A) and colocalized with the HTNV NP as well as the late endosome/lysosome marker Rab7A (Fig. 5B) and lysosome tracker (Fig. 5C) but not Rab5A (Supplementary Fig. S4A). Moreover, co-immunoprecipitation (co-IP) experiments also indicated that STING interacted with Rab7A (Fig. 5D) but not Rab5A (Supplementary Fig. S4B) in HEK293T cells. Co-IP experiments in HTNV-infected HUVECs and transfected HEK293 cells showed that STING interacted with HTNV NP (Fig. 5E, Supplementary Fig. S4C). These findings indicated that STING might promote the fusion of late endosomes and lysosomes to promote HTNV protein degradation.
Fig. 5.
STING trafficking from ER to late endosome participate in viral protein degradation. A HUVECs were mock-infected or HTNV-infected (MOI = 1) for 24 h, and incubated with mouse anti-STING and rabbit anti-ERGIC-53 antibodies, following stained with Cy3-conjugated anti-mouse and FITC-conjugated anti-rabbit secondary antibodies. Nuclei were stained with DAPI. Scale bar, 10 μm. B HUVECs were mock-infected or HTNV-infected (MOI = 1) for 24 h, and incubated with mouse anti-STING and rabbit anti-Rab7A antibodies, following stained with Cy3-conjugated anti-mouse and FITC-conjugated anti-rabbit secondary antibodies. Nuclei were stained with DAPI. Scale bar, 10 μm. C HUVECs were mock-infected or HTNV-infected (MOI = 1) for 24 h, and incubated with Lysosome Tracker, mouse anti-1A8 and rabbit anti-STING antibodies, following stained with F488-conjugated anti-mouse and Cy5-conjugated anti-rabbit secondary antibodies, respectively. Nuclei were stained with DAPI. Scale bar, 10 μm. D HEK293T cells were single or co-transfected with Flag-STING (10 μg/well) or Myc-Rab7A (10 μg/well) for 36 h. The cell lysates were immunoprecipitated with anti-Flag antibody, and detected by immunoblotting with anti-Flag or anti-Myc antibody. E HEK293T cells were single or co-transfected with Flag-STING or Myc-NP for 36 h. The cell lysates were immunoprecipitated with anti-Flag antibody, and detected by immunoblotting with anti-Flag or anti-Myc antibody. F–H HVECs were infected with HTNV (MOI = 1) for 24 h and then treated with BAF for 4 h. HTNV NP, STING and LC3B were detected by immunoblotting (F). The grey values of the protein level of HTNV NP bands (G) and LC3B-Ⅰ/LC3B-Ⅱ bands (H) from three independent immunoblot through the software Image J and carry out statistical analysis. Data are expressed as mean ± SEM (n = 3). Statistical significance was calculated using Student's t-test; ∗P < 0.05; ∗∗P < 0.01.
Recently, studies reported that blocked STING trafficking using brefeldin A (BFA) specifically inhibits STING-dependent signaling (Gonugunta et al., 2017). Hence, we performed experiments to determine whether blocking STING trafficking would affect the viral replication. HUVECs were infected with HTNV and then treated with BFA. We found that STING shifted from ER localization to Golgi localization during infection (Fig. 5C). Blocking the translocation process with inhibitors inhibited STING-mediated autophagy disabling its anti-HTNV effect (Fig. 5F). In addition, inhibiting translocation can inhibit the localization of STING and lysosome, further indicating the key role of translocation in promoting autophagic flow. Collectively, STING trafficking from the ER to endosomes was required for ERGIC-53-induced formation of autophagosomes and fusion with lysosomes to degrade viral protein and inhibit viral replication.
3.6. IRE1-XBP1-RIG-I signaling initiates STING-mediated autophagy post HTNV infection
Intriguingly, it has been reported that FMDV infection does not activate general ER unfolded protein responses but specifically activates the EIF2AK3-mediated integrated stress response (Dasgupta et al., 2018). The ER stress response generally activates three mechanisms, namely the protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK), inositol-requiring enzyme 1 (IRE1) and activating transcription factor 6 (ATF6) (Di Conza and Ho, 2020) (Fig. 6A). Recent studies have shown the relationship between the ER stress sensor IRE1α and the RIG-I pathway, which triggers an inflammatory response upon detection of viral RNAs (Lencer et al., 2015). HTNV replication included viral RNA and protein synthesis in the ER, so we hypothesized that STING-mediated autophagy might be initiated by one of the three signaling pathways. To test this hypothesis, HUVECs were treated with the ERN1-specific inhibitor 4-methylumbelliferone 8-carbaldehyde (4μ8c) (Cross et al., 2012). The results suggest that blocking ERN1-XBP1 signaling significantly increases HTNV NP production (Fig. 6B) and facilitates viral RNA replication (Supplementary Fig. S5A), and inhibits IFN production (Supplementary Fig. S5B). ATF4-specific siRNA to knock down the expression of ATF4 in HUVECs to investigate the involvement of other branches of ER unfolded protein responses in autophagy induction (Supplementary Fig. S5C). No difference in autophagy induction and STING protein degradation was observed after ATF4 knockdown (Fig. 6C). Similar results were observed in ATF6 knockdown HUVECs (Fig. 6C, Supplementary Fig. S5D). Moreover, the knockout of ATF4 and ATF6 had no effect on autophagy (Fig. 6C). Collectively, these findings revealed that HTNV infection activates the ERN1-XBP1 signaling-mediated integrated stress response to inactivate STING-induced autophagy.
Fig. 6.
IRE1-XBP1-RIG-I signaling initiates STING-mediated autophagy post HTNV infection. A ER stress signaling pathway. B HUVECs were treated with 4μ8c or DMSO for 24 h and then infected with HTNV (MOI = 1) for 36 h. The protein levels of HTNV NP and STING were detected by immunoblotting. C HUVECs treated with 150 nmol/L ATF4 or ATF6-specific siRNA or a negative control sequence (NC) for 24 h and then infected with HTNV (MOI = 1) for 36 h. The protein levels of HTNV NP, STING and LC3B were detected by immunoblotting. D HUVECs treated with 150 nmol/L cGAS or STING-specific siRNA or a negative control sequence (NC) for 24 h and then infected with HTNV (MOI = 1) for 36 h. The protein levels of HTNV NP and STING were detected by immunoblotting. E HUVECs treated with 150 nmol/L TLR3, TLR4 or RIG-I-specific siRNA or a negative control sequence (NC) for 24 h and then infected with HTNV (MOI = 1) for 36 h. The protein levels of HTNV NP, LC3B and STING were detected by immunoblotting. F HUVECs treated with cGAS, STING, TLR3, TLR4 or RIG-I-specific siRNA or a negative control sequence (NC) for 24 h and then infected with HTNV (MOI = 1) for 36 h. The mRNA levels of HTNV S were detected through qRT-PCR with GAPDH as the internal control. G HUVECs were mock-infected or HTNV-infected (MOI = 1) for 24 h, and incubated with rabbit anti-RIG-I and mouse anti-STING antibodies, following stained with Cy3-conjugated anti-mouse and FITC-conjugated anti-rabbit secondary antibodies. Nuclei were stained with DAPI. Scale bar, 10 μm. H HUVECs were mock-infected or HTNV-infected (MOI = 1) for 36 h. The cell lysates were immunoprecipitated with anti-STING or IgG antibody, and detected by immunoblotting with anti-STING or anti-RIG-I antibody. Data are expressed as mean ± SEM (n = 3). Statistical significance was calculated using Student's t-test or one-way ANOVA; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001; ns, not significant.
The above studies demonstrated that the role of STING in inhibiting HTNV replication, but the activation mechanism was still obscure. Canonical STING is best known for its pivotal role in the detection of foreign DNA-induced IFN responses, such as DNA viruses. We wondered whether upstream nucleotide sensors are required for the STING-induced autophagy response during HTNV infection. To test this hypothesis, the expression of cGAS was silenced in HUVECs using small interfering RNAs (siRNA), and the ablation efficiency of cGAS was confirmed by immunoblot and qRT-PCR (Fig. 6D, Supplementary Fig. S5E). Subsequently, the cGAS-silenced HUVECs were infected with HTNV for 36 h at an MOI of 1. cGAS knock-down did not affect the replication of HTNV, and HTNV infection still led to a decrease in STING protein (Fig. 6D), which suggests that cGAS does not play an essential role in the STING-mediated anti-HTNV effects, nor does it induce STING activation and degradation.
Previous studies have shown that HTNV can activate PRRs such as RIG-I, toll-like receptor 3 (TLR3), toll-like receptor 4 (TLR4), melanoma differentiation-associated gene 5 (MDA5), etc (Handke et al., 2009; Zhang et al., 2014; Jiang et al., 2008). The above PRRs may be the key factors in activating STING. Interestingly, recent studies shows that RIG-I anti-HTNV does not depend on classical RIG-I-MAVS-IFN (Kell et al., 2020), and some studies have found that RIG-I can bind to STING (Zhang et al., 2022; Nazmi et al., 2012; Triantafilou et al., 2022). Therefore, we hypothesized that RIG-I participated in the activation of STING to exert an antiviral effect in HTNV-infected cells. For this purpose, RIG-I, TLR3, and TLR4 were knocked down in HUVECs (Supplementary Figs. S5F–S5H), which were infected with HTNV at an MOI of 1. We found that HTNV infection did not induce autophagy and STING degradation in RIG-I knockdown cells (Fig. 6E). In contrast, the knockdown of TLR3 and TLR4 had no effect on HTNV-induced STING degradation and autophagy (Fig. 6E). Consistently, knockdown of RIG-I remarkably facilitated HTNV NP production and promoted viral RNA replication (Fig. 6E and F). Confocal microscopy analysis revealed that RIG-I colocalized with STING in HTNV-infected HUVECs (Fig. 6G). Moreover, coimmunoprecipitation experiments indicated that RIG-I could bind to STING in HUVECs. RIG-I bound to STING to a greater extent upon infection of HUVECs with HTNV (Fig. 6H). These findings suggested that RIG-I was required for STING-mediated antiviral effect during HTNV infection, while cGAS was not.
3.7. STING enhances anti-hantaviral immunity responses in vivo
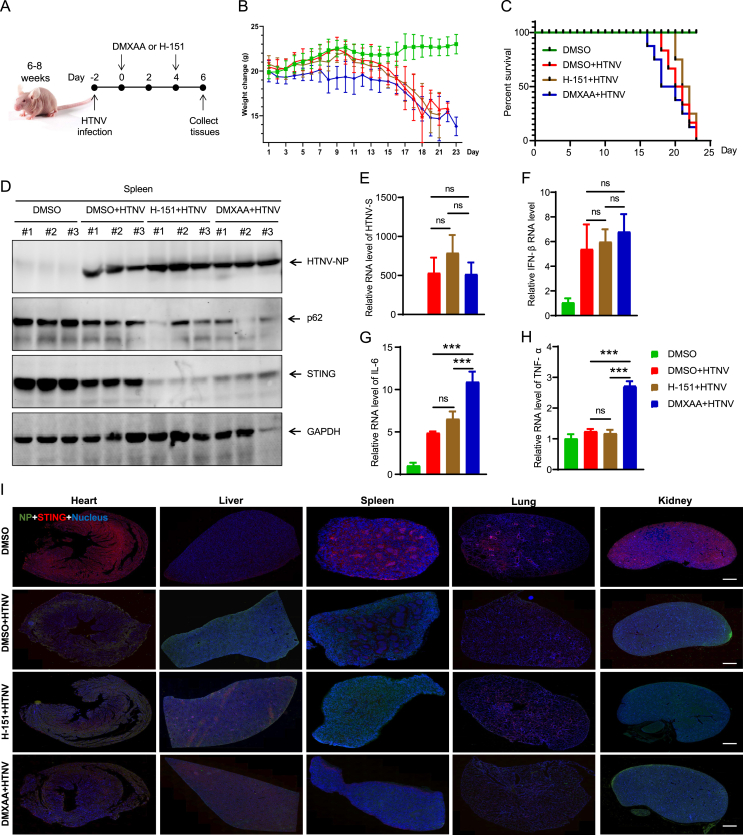
Next, to investigate the role of STING during HTNV infection in vivo. We used nude mice as the HTNV-infected animal model. The mice were intraperitoneally injected with PBS or HTNV, respectively. Mice received every two days dose of STING antagonist H-151 starting 2 days after HTNV infection and were euthanized at 6 days post-infection (dpi) (Fig. 7A). The body weight changes of the infected mice were monitored over time. Compared to treatment with vehicle, no significant improvement in body weight and survival was observed with H-151 treatment after infection (Fig. 7B and C). Consistently, viral replication was similar in the presence or absence of H-151 in the mice spleen from mice, as evidenced by the immunoblotting and qRT-PCR results (Fig. 7D and E). Furthermore, the immunofluorescence assays of multiple tissues also indicated similar HTNV replication was similar in mice alveolar vessels, kidney cortexes, splenic cords, cardiomyocytes, and hepatocytes (Fig. 7I). The levels of IFN and pro-inflammatory genes (IL-6, TNF-α) were also unaffected by H-151 treatment compared to the vehicle-treated mice. Additionally, histological examination of multiple tissues revealed no significant change in tissue damage in H-151-treated mice at 6 dpi (Supplementary Fig. S6A). These data showed that H-151 has no obvious therapeutic effect in vivo.
Fig. 7.
STING enhances anti-hantaviral immunity responses in vivo.A Nude mice (6–8 weeks) were intraperitoneally inoculated with HTNV (4 × 104 TCID50/g per mouse) and intraperitoneal administration of vehicle, H-151 (10 mg/kg), or DMXAA (25 mg/kg) (starting at 2 day after infection). Eight mice were involved in each group. B,C The mice weighed daily (B) and survival was recorded daily (C) after HTNV infection. D The immunoblot analysis for viral proteins, p62 and STING protein expression in different nude mice spleen. E–H The qRT-PCR analysis for viral RNA HTNV S, IFN-β, IL-6 and TNF-α in different nude mice spleen. I The immunofluorescence assays for HTNV NP (green) and STING (red) in different nude mice tissues at day 3 post infection. Scale bars, 1000 μm. Data are expressed as mean ± SEM (n = 8). Statistical significance was calculated using Student's t-test or one-way ANOVA; ∗∗∗P < 0.001; ns, not significant.
Using the same methods as the H-151 experiment, mice were also treated with the STING agonist DMXAA. The results showed similar effects on body weight, survival, and HTNV replication as with the antagonist H-151 (Fig. 7B–D and I). In contrast, chemokines (IL-6, TNF-α) and IFN were significantly increased in DMXAA-treated mice compared to vehicle-treated mice (Fig. 7F–H). Accordingly, DMXAA treatment induced serious inflammatory responses in multiple tissues (Supplementary Fig. S6A). These data show that STING has a unique and critical function in eliciting type I IFN and inflammatory responses in HTNV infection, which is related to excessive tissue damage, but not with the peak of viral replication.
Finally, the therapeutic potential of STING via the non-classical function during HTNV infection was investigated in vivo. The WT, △CTT of STING and the negative control sequence were cloned into pCDNA6.2-EGFP, respectively (Fu et al., 2021). Nude mice were intraperitoneally injected with PBS or HTNV, respectively. Next, mice were intravenously injected with the vector, WT and △CTT every 2 days (Fig. 8A). The mice were then monitored to evaluate body weight and survival relative after infection. Compared to the vector, treatment with △CTT not only significantly attenuated weight loss after infection and markedly increased the overall survival rate (Fig. 8B and C) but also reduced the severity and decreased the levels of type I IFNs and other cytokines (Fig. 8F–I), while viral replication was significantly decreased (Fig. 8D and E). However, treatment with WT markedly reduced the overall survival rate and body weight compared to the vector (Fig. 8B and C). Especially, treatment with WT markedly increased pathological damage and raised the levels of type I IFNs and other cytokines compared to the vector or △CTT (Fig. 8F–I). Therefore, this may be the main factor determining the survival rate and low weight. These results suggested that △CTT facilitates autophagy and enhances anti-hantaviral immunity responses in vivo.
Fig. 8.
△CTT facilitates autophagy and enhances anti-hantaviral immunity responses in vivo.A Nude mice (6–8 weeks) were intraperitoneally inoculated with HTNV (4 × 104 TCID50/g per mouse) and intraperitoneal administration of vector, WT, or △CTT (starting at 2 day after infection). Eight mice were involved in each group. B,C The mice weighed daily (B) and survival was recorded daily (C) after HTNV infection. D The immunoblot analysis for viral proteins, p62 and STING protein expression in different nude mice tissues. E–I The qRT-PCR analysis for viral RNA HTNV S, IFN-β and ISGs in different nude mice tissues. J The immunofluorescence assays for HTNV NP (green) and STING (red) in different nude mice tissues at day 3 post infection. Scale bars, 1000 μm. Data are expressed as mean ± SEM (n = 8). Statistical significance was calculated using Student's t-test or one-way ANOVA; ∗P < 0.05; ∗∗∗P < 0.001; ns, not significant.
4. Discussion
Innate immunity is the first line of defense against the invasion of pathogenic microorganisms. Type I IFN-centered innate antiviral responses by RIG-I-mediated signaling plays a key role in the body's resistance to hantavirus infection (Matthys and Mackow, 2012). Our previous research found that HTNV can effectively inhibit the production of type I IFN in the early stage of infection (within 24 h), which is beneficial to the proliferation of the virus. In the late stage of infection (after 24 h), it induces a large amount of type I IFN that cannot effectively inhibit virus replication (Ma et al., 2017; Geimonen et al., 2002). Recent studies have shown that autophagy plays an essential role in the regulation of the innate immune response process (Choi et al., 2018), and our previous study found that HTNV infection can dynamically regulate changes in autophagic flux (Wang et al., 2019). In this study, we found that STING strengthens host autophagy-dependent anti-hantaviral immunity through IRE1-RIG-I-STING pathway. STING polymerization or translocation is necessary for the HTNV-induced ER stress response and autophagy. However, it does not require IFN response activation (Fig. 9). Physiologically, neither application of the pharmacological STING antagonist H-151 nor the agonist DMXAA could improve the outcome post HTNV challenge in vivo. The △CTT of STING is essential in reducing damage caused by a viral infection and in defending against HTNV infection. Our work demonstrated that the IRE1-RIG-I-STING-autophagy signaling could inhibit the replication of HTNV and indicated that induction of autophagy in response to RNA virus infection might be an evolutionarily conserved function of STING.
Fig. 9.
The working model of the STING-induced anti-hantaviral immunity responses. HTNV infection activates RIG-I through IRE1-XBP-dependent signaling. RIG-I interacts with STING via the △CTT domain within STING, leading to STING trafficking from the endoplasmic reticulum to the Golgi apparatus and inducing autophagy. STING localized on late endosomes interacts with Rab7A and fuses with lysosomes, leading to reduced expression of STING and antiviral effects. △CTT STING can serve as a novel therapeutic strategies against hantavirus infection in mice. The black arrow indicates that HTNV infection does not work, while the red arrow indicates that HTNV infection is working.
Host cells detect intracellular pathogen-associated molecular patterns (PAMPs) such as viral nucleic acid or bacterial cell wall components, including lipopolysaccharide or flagellar proteins, resulting in the induction of anti-pathogen genes (Kumar et al., 2011). The presence of PAMPs in the cytosol is sensed by cytosolic PRRs. For example, viral RNA can be detected by membrane-bound toll-like receptors (TLRs) or by TLR-independent intracellular DExD/H box RNA helicases referred to as RIG-I or melanoma differentiation-associated antigen 5 (MDA5, also referred to as IFIH1 and helicard) (Takeda and Akira, 2005). Previous studies have shown that PRR molecules, such as RIG-I, MDA5, TLR3, and TLR4, can recognize hantavirus infection and induce type I IFN response in A549, Huh7, HaCaT, HUVECs and other cell lines/primary cells (Handke et al., 2009; Zhang et al., 2014; Ma et al., 2017). Most of the current studies concur with the above conclusions and argue that the recognition of HTNV by pattern recognition receptors initiates an innate immune response to exert antiviral effects. However, increased HTNV replication in HUVCs treated with 4μ8c, which is an ERN1-specific inhibitor, suggests that the IRE1-XBP1-dependent ER stress triggers a STING-dependent IFN response during infection. Although the three gene fragments of hantavirus form a closed “pan handle” through their complementary sequences at their respective 5′ and 3′, avoiding the formation of 5′ triphosphate (ppp) and escaping the recognition of PRRs such as RIG-I (Wang et al., 2011). Previous studies found that the unfolded protein response (UPR), which generates endogenous RLR ligands through IRE-1 endonuclease cleavage of cellular RNAs, triggers type IIFN production (Eckard et al., 2014). Our findings suggest a new potential mechanism by which HTNV or RNA virus infection exerts antiviral effects by activating innate immune responses through ER stress. In this model, HTNV induces the IRE1-dependent signaling to activate endogenous RIG-I ligands. Inhibition or deletion of key molecules in this pathway blocks signal transmission and could promote virus replication.
Numerous publications were analyzed, revealing that DNA virus infection leads to STING conformational changes and modifications, which is required for its trafficking and activating. However, there are few studies on any post-translational modifications nor the degradation of STING by RNA virus infection (Li et al., 2021; Ji et al., 2022). On the contrary, infection of some RNA viruses was seen to up-regulate the expression of STING at both the mRNA and protein levels (Nazmi et al., 2012). HTNV, the prototype virus of hantavirus, could lead to loss of STING protein in a viral load-dependent manner, while the mRNA levels of STING remarkable increased.
Although studies have reported that cGAS can recognize RNA (Slavik et al., 2021), we found that knockdown of cGAS in HUVEC cells did not impact viral replication and STING degradation, indicating that changes in STING protein caused by HTNV infection are not related to cGAS. Recently, studies revealed the relationship between the RLR/MAVS pathway of RNA sensing and the cGAS/STING pathway that detects foreign or aberrant DNA extensively collaborate to coordinate innate immune responses against invading pathogens (Liu et al., 2016). In this study, we found that HTNV infection activates the cellular pattern recognition receptor RIG-I. RIG-I transmits signals to the ER-anchored adaptor protein STING, which specifically activates autophagy polymerization or translocation-dependent and finally leads to the degradation of STING itself. STING plays an essential role in RNA viruses infection, such as human rhinovirus (HRV), SARS-CoV-2, vesicular stomatitis virus (VSV), Sendai virus (SeV), dengue virus (DENV), and West Nile virus (WNV), etc (Ishikawa et al., 2009; Aguirre et al., 2017; Schoggins et al., 2014; Franz et al., 2018; Webb and Fernandez-Sesma, 2022; Han et al., 2022). However, exactly how STING are regulates the immune responses in RNA virus infection is remains largely unknown.
Macroautophagy (hereafter referred to as autophagy) is an evolutionarily conserved eukaryotic degradation process involving the cellular recycling of multiple cytoplasmic under components during physiological conditions or pathological stress, such as starvation, pathogen invasion, and oxidative stimulation (Choi et al., 2018). As a homeostatic control mechanism, increased autophagy levels exert protective effects against stress by maintaining cellular biosynthetic capacity and ATP supply. We previously demonstrated that hantavirus Gn-induced mitophagy inhibits type I IFN responses by degrading MAVS at the early stage, and NP disturbs Gn-induced mitophagy at late stage of infection. The canonical autophagy pathway is initiated by the activation of the Unc-51-like kinase 1 (ULK1) complex that activates components of the class III PI3K (PI3KC3) complex I by phosphorylation (Zhang et al., 2021). Recently, studies reported that STING activation inducing non-canonical autophagy is independent of its TBK1 activity as well as ULK1, BECN1, and ATG9a (Rong et al., 2022). However, the WD repeat domain, phosphoinositide-interacting 2 (WIPI2), ATG5, and ATG7 are required for STING-induced autophagosome formation (Gui et al., 2019). Furthermore, HTNV promoted the STING translocation from ER to ERGIC and lysosomes, which is dependent on Rab7A. HTNV-induced autophagy, STING polymerization or translocation are essential processes for the HTNV-induced ER stress response and autophagy. Moreover, the activation of STING also induces V-ATPase-dependent MAP1LC3 lipidation to single-membrane perinuclear vesicles through ATG16L1 (Fischer et al., 2020). Still, further research is required to fully understand the underlying mechanism.
The cGAS-STING pathway reportedly play a fundamental role in the production of IFNs and pro-inflammatory cytokines in response to DNA derived from invading microbial pathogens (Kumar, 2019), STING overexpression significantly upregulated IFNs, pro-inflammatory cytokines (IL-1β), and interferon-stimulated genes (ISGs) (for example, Mx1 and ISG56) upon HTNV infection (Fig. 3D). Our study provides a foundation for understanding the mechanism linking STING-induced autophagy and HTNV. HTNV infection activates the IRE1-XBP1-RIG-I signaling-mediated ER stress response to induce STING-dependent autophagy to protect against viral infection. This indicates that STING has potential importance in the prevention and treatment of HTNV. Although the cellular experiments showed that treatment with H-151 or DMXAA clearly promoted or inhibited viral replication, respectively. H-151- or DMXAA-treated mice displayed no effect on virus replication in vivo. These results indicated that the polymerization of STING plays an important role in antiviral immunity. Notably, H-151 did not exhibit a statistically significant HTNV inhibition in nude mice models, but there was a trend toward increased viral infection. STING activation by DMXAA did not exert an obvious antiviral effect but induced higher inflammation. Considering that STING-mediated inhibition of HTNV is coordinated by numerous elements, these antiviral factors are likely influenced by other factors in the complex environment of the body. In addition, HTNV has developed multiple strategies to evade the host immune response. Our results showed that STING is essential for protection against HTNV. To further evaluate the therapeutic potential antiviral effect of STING in vivo, WT or △CTT were constructed to investigate its therapeutic effect in nude mice. Consistent with the cellular findings, WT STING significantly inhibited viral replication but produced higher levels of interferons and inflammatory factors in vivo, leading to lower survival and significant body weight loss. △CTT can not only inhibit virus replication, but also eliminate interferon and inflammatory factors caused by the CTT domain of STING, thus preventing death due to excessive inflammation.
5. Conclusions
In conclusion, this study described an IFN-independent antiviral mechanism that inhibits hantavirus replication by degrading viral proteins through the IRE1-RIG-I-mediated STING-autophagy pathway in eukaryotic cells. This non-canonical role of STING has therapeutic potential and is particularly important not only for HTNV replication but possibly for other RNA viruses as well.
Ethics statement
All animal protocols were reviewed and approved by the First Affiliated Hospital of Air Force Medical University Animal Care and Use Committee (Approval Number: # KY20194020) and were performed in strict accordance with the Animal Ethics Procedures and Guidelines of the People's Republic of China.
Author contributions
Kerong Wang: conceptualization, data curation, formal analysis, investigation, methodology, software, visualization, writing-original draft, writing-review & editing. Jian Zhang: data curation, formal analysis, investigation, methodology, software, validation. Yongheng Yang: data curation, investigation, methodology, validation. Yue Si: investigation, methodology. Ziqing Zhou: methodology. Xudong Zhu: methodology. Sushan Wu: conceptualization. He Liu: methodology. Hui Zhang: methodology. Liang Zhang: conceptualization, supervision. Linfeng Cheng: conceptualization, supervision. Wei Ye: conceptualization, supervision. Xin Lv: conceptualization, supervision. Yingfeng Lei: conceptualization, supervision. Xijing Zhang: conceptualization, supervision. Shilin Cheng: methodology. Lixin Shen: conceptualization, resources. Fanglin Zhang: conceptualization, project administration, supervision, visualization, funding acquisition, writing-review & editing. Hongwei Ma: conceptualization, project administration, supervision, visualization, writing-review & editing.
Conflict of interest
The authors declare that they have no competing interests.
Acknowledgements
The authors thank Professor Chenyu Zhang of Nanjing University for providing pCDNA6.2-EGFP. The present study was supported by grants from the National Natural Science Foundation of China (No. 31970148, 82172272 and 82202367), and the Key Research and Development Program of Shaanxi (2021ZDLSF01-02 and 2021ZDLSF01-05).
Footnotes
Appendix A
Supplementary data to this article can be found online at https://doi.org/10.1016/j.virs.2023.06.006.
Contributor Information
Lixin Shen, Email: shenlx@nwu.edu.cn.
Fanglin Zhang, Email: flzhang@fmmu.edu.cn.
Hongwei Ma, Email: mahongwei0720@sina.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- Aguirre S., Luthra P., Sanchez-Aparicio M.T., Maestre A.M., Patel J., Lamothe F., Fredericks A.C., Tripathi S., Zhu T., Pintado-Silva J., Webb L.G., Bernal-Rubio D., Solovyov A., Greenbaum B., Simon V., Basler C.F., Mulder L.C., García-Sastre A., Fernandez-Sesma A. Dengue virus NS2B protein targets cGAS for degradation and prevents mitochondrial DNA sensing during infection. Nat. Microbiol. 2017;2 doi: 10.1038/nmicrobiol.2017.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre S., Maestre A.M., Pagni S., Patel J.R., Savage T., Gutman D., Maringer K., Bernal-Rubio D., Shabman R.S., Simon V., Rodriguez-Madoz J.R., Mulder L.C., Barber G.N., Fernandez-Sesma A. DENV inhibits type I IFN production in infected cells by cleaving human STING. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banete A., Seaver K., Bakshi D., Gee K., Basta S. On taking the STING out of immune activation. J. Leukoc. Biol. 2018;10:1002. doi: 10.1002/JLB.2MIR0917-383R. [DOI] [PubMed] [Google Scholar]
- Brubaker S.W., Bonham K.S., Zanoni I., Kagan J.C. Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Bowman J.W., Jung J.U. Autophagy during viral infection - a double-edged sword. Nat. Rev. Microbiol. 2018;16:341–354. doi: 10.1038/s41579-018-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citil Dogan A., Wayne S., Bauer S., Ogunyemi D., Kulkharni S.K., Maulik D., Carpenter C.F., Bahado-Singh R.O. The Zika virus and pregnancy: evidence, management, and prevention. J. Matern. Fetal Neonatal Med. 2017;30:386–396. doi: 10.3109/14767058.2016.1174210. [DOI] [PubMed] [Google Scholar]
- Cohen-Kaplan V., Livneh I., Avni N., Cohen-Rosenzweig C., Ciechanover A. The ubiquitin-proteasome system and autophagy: coordinated and independent activities. Int. J. Biochem. Cell Biol. 2016;79:403–418. doi: 10.1016/j.biocel.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Cross B.C., Bond P.J., Sadowski P.G., Jha B.K., Zak J., Goodman J.M., Silverman R.H., Neubert T.A., Baxendale I.R., Ron D., Harding H.P. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta Y., Golovine K., Nieborowska-Skorska M., Luo L., Matlawska-Wasowska K., Mullighan C.G., Skorski T. Drugging DNA repair to target T-ALL cells. Leuk. Lymphoma. 2018;59:1746–1749. doi: 10.1080/10428194.2017.1397662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decout A., Katz J.D., Venkatraman S., Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat. Rev. Immunol. 2021;21:548–569. doi: 10.1038/s41577-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Conza G., Ho P.C. ER stress responses: an emerging modulator for innate immunity. Cells. 2020;9:695. doi: 10.3390/cells9030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckard S.C., Rice G.I., Fabre A., Badens C., Gray E.E., Hartley J.L., Crow Y.J., Stetson D.B. The SKIV2L RNA exosome limits activation of the RIG-I-like receptors. Nat. Immunol. 2014;15:839–845. doi: 10.1038/ni.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer T.D., Wang C., Padman B.S., Lazarou M., Youle R.J. STING induces LC3B lipidation onto single-membrane vesicles via the, V-ATPase and ATG16L1-WD40 domain. J. Cell Biol. 2020;219 doi: 10.1083/jcb.202009128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz K.M., Neidermyer W.J., Tan Y.J., Whelan S.P.J., Kagan J.C. STING-dependent translation inhibition restricts RNA virus replication. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E2058–e2067. doi: 10.1073/pnas.1716937115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z., Zhang X., Zhou X., Ur-Rehman U., Yu M., Liang H., Guo H., Guo X., Kong Y., Su Y., Ye Y., Hu X., Cheng W., Wu J., Wang Y., Gu Y., Lu S.F., Wu D., Zen K., Li J., Yan C., Zhang C.Y., Chen X. In vivo self-assembled small RNAs as a new generation of RNAi therapeutics. Cell Res. 2021;31:631–648. doi: 10.1038/s41422-021-00491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geimonen E., Neff S., Raymond T., Kocer S.S., Gavrilovskaya I.N., Mackow E.R. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. U. S. A. 2002;99:13837–13842. doi: 10.1073/pnas.192298899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonugunta V.K., Sakai T., Pokatayev V., Yang K., Wu J., Dobbs N., Yan N. Trafficking-mediated STING degradation requires sorting to acidified endolysosomes and can be targeted to enhance anti-tumor response. Cell Rep. 2017;21:3234–3242. doi: 10.1016/j.celrep.2017.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui X., Yang H., Li T., Tan X., Shi P., Li M., Du F., Chen Z.J. Autophagy induction via STING trafficking is a primordial function of the cGAS pathway. Nature. 2019;567:262–266. doi: 10.1038/s41586-019-1006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L., Zheng Y., Deng J., Nan M.L., Xiao Y., Zhuang M.W., Zhang J., Wang W., Gao C., Wang P.H. SARS-CoV-2 ORF10 antagonizes STING-dependent interferon activation and autophagy. J. Med. Virol. 2022;94:5174–5188. doi: 10.1002/jmv.27965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handke W., Oelschlegel R., Franke R., Krüger D.H., Rang A. Hantaan virus triggers TLR3-dependent innate immune responses. J. Immunol. 2009;182:2849–2858. doi: 10.4049/jimmunol.0802893. [DOI] [PubMed] [Google Scholar]
- Hu M.M., Yang Q., Xie X.Q., Liao C.Y., Lin H., Liu T.T., Yin L., Shu H.B. Sumoylation promotes the stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–569. doi: 10.1016/j.immuni.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Ishikawa H., Barber G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H., Ma Z., Barber G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461:788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., Wang Y., Zhou L., Lu J., Bao S., Shen Q., Wang X., Liu Y., Zhang W. E3 ubiquitin ligases: the operators of the ubiquitin code that regulates the RLR and cGAS-STING pathways. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232314601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H., Wang P.Z., Zhang Y., Xu Z., Sun L., Wang L.M., Huang C.X., Lian J.Q., Jia Z.S., Li Z.D., Bai X.F. Hantaan virus induces toll-like receptor 4 expression, leading to enhanced production of beta interferon, interleukin-6 and tumor necrosis factor-alpha. Virology. 2008;380:52–59. doi: 10.1016/j.virol.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Jiang H., Zheng X., Wang L., Du H., Wang P., Bai X. Hantavirus infection: a global zoonotic challenge. Virol. Sin. 2017;32:32–43. doi: 10.1007/s12250-016-3899-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell A.M., Hemann E.A., Turnbull J.B., Gale M., Jr. RIG-I-like receptor activation drives type I IFN and antiviral signaling to limit Hantaan orthohantavirus replication. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H., Kawai T., Akira S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- Kumar V. A STING to inflammation and autoimmunity. J. Leukoc. Biol. 2019;106:171–185. doi: 10.1002/JLB.4MIR1018-397RR. [DOI] [PubMed] [Google Scholar]
- Lawson N.D., Stillman E.A., Whitt M.A., Rose J.K. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U. S. A. 1995;92:4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lencer W.I., Deluca H., Grey M.J., Cho J.A. Innate immunity at mucosal surfaces: the IRE1-RIDD-RIG-I pathway. Trends Immunol. 2015;36:401–409. doi: 10.1016/j.it.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Feng L., Luo W.W., Lei C.Q., Li M., Shu H.B. The RNA-binding protein LUC7L2 mediates MITA/STING intron retention to negatively regulate innate antiviral response. Cell Discov. 2021;7:46. doi: 10.1038/s41421-021-00277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., He M., Wang Z., Duan Z., Guo Z., Wang Z., Gong R., Chu T., Cai J., Gao B. STING signaling activation inhibits HBV replication and attenuates the severity of liver injury and HBV-induced fibrosis. Cell. Mol. Immunol. 2022;19:92–107. doi: 10.1038/s41423-021-00801-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D., Wu H., Wang C., Li Y., Tian H., Siraj S., Sehgal S.A., Wang X., Wang J., Shang Y., Jiang Z., Liu L., Chen Q. STING directly activates autophagy to tune the innate immune response. Cell Death Differ. 2019;26:1735–1749. doi: 10.1038/s41418-018-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Ma H., Shu J., Zhang Q., Han M., Liu Z., Jin X., Zhang F., Wu X. Vaccines and therapeutics against Hantaviruses. Front. Microbiol. 2019;10:2989. doi: 10.3389/fmicb.2019.02989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Goulet M.L., Sze A., Hadj S.B., Belgnaoui S.M., Lababidi R.R., Zheng C., Fritz J.H., Olagnier D., Lin R. RIG-I-mediated STING upregulation restricts herpes simplex virus 1 infection. J. Virol. 2016;90:9406–9419. doi: 10.1128/JVI.00748-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D.H., Jiang H., Lian J.Q. Hantavirus infection during pregnancy. Virol. Sin. 2021;36:345–353. doi: 10.1007/s12250-020-00300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Han P., Ye W., Chen H., Zheng X., Cheng L., Zhang L., Yu L., Wu X., Xu Z., Lei Y., Zhang F. The long noncoding RNA NEAT1 exerts antihantaviral effects by acting as positive feedback for RIG-I signaling. J. Virol. 2017;91 doi: 10.1128/JVI.02250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthys V., Mackow E.R. Hantavirus regulation of type I interferon responses. Adv. Virol. 2012;2012 doi: 10.1155/2012/524024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier K., Thorkelsson S.R., Quemin E.R.J., Rosenthal M. Hantavirus replication cycle-an updated structural virology perspective. Viruses. 2021;13:1561. doi: 10.3390/v13081561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyangwa M., Martynova E.V., Khaiboullina S.F., Morzunov S.P., Rizvanov A.A. Hantaviral proteins: structure, functions, and role in Hantavirus infection. Front. Microbiol. 2015;6:1326. doi: 10.3389/fmicb.2015.01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A., Mukhopadhyay R., Dutta K., Basu A. STING mediates neuronal innate immune response following Japanese encephalitis virus infection. Sci. Rep. 2012;2:347. doi: 10.1038/srep00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni G., Ma Z., Damania B. cGAS and STING: at the intersection of DNA and RNA virus-sensing networks. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong Y., Zhang S., Nandi N., Wu Z., Li L., Liu Y., Wei Y., Zhao Y., Yuan W., Zhou C., Xiao G., Levine B., Yan N., Mou S., Deng L., Tang Z., Liu X., Kramer H., Zhong Q. STING controls energy stress-induced autophagy and energy metabolism via STX17. J. Cell Biol. 2022;221 doi: 10.1083/jcb.202202060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra F., Díaz F.E., Retamal-Díaz A., Covián C., González P.A., Kalergis A.M. Immune response during hantavirus diseases: implications for immunotherapies and vaccine design. Immunology. 2021;163:262–277. doi: 10.1111/imm.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins J.W., Macduff D.A., Imanaka N., Gainey M.D., Shrestha B., Eitson J.L., Mar K.B., Richardson R.B., Ratushny A.V., Litvak V., Dabelic R., Manicassamy B., Aitchison J.D., Aderem A., Elliott R.M., García-Sastre A., Racaniello V., Snijder E.J., Yokoyama W.M., Diamond M.S., Virgin H.W., Rice C.M. Pan-viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature. 2014;505:691–695. doi: 10.1038/nature12862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang G., Zhang C., Chen Z.J., Bai X.C., Zhang X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature. 2019;567:389–393. doi: 10.1038/s41586-019-0998-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.S., Vidhyasagar V., Yang S., Arna A.B., Yadav M., Aggarwal A., Aguilera A.N., Shinriki S., Bhanumathy K.K., Pandey K., Xu A., Rapin N., Bosch M., Decoteau J., Xiang J., Vizeacoumar F.J., Zhou Y., Misra V., Matsui H., Ross S.R., Wu Y. DDX41 is required for cGAS-STING activation against DNA virus infection. Cell Rep. 2022;39 doi: 10.1016/j.celrep.2022.110856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavik K.M., Morehouse B.R., Ragucci A.E., Zhou W., Ai X., Chen Y., Li L., Wei Z., Bähre H., König M., Seifert R., Lee A.S.Y., Cai H., Imler J.L., Kranzusch P.J. cGAS-like receptors sense RNA and control 3'2'-cGAMP signalling in Drosophila. Nature. 2021;597:109–113. doi: 10.1038/s41586-021-03743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L., Wu J., Du F., Chen X., Chen Z.J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–791. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17:1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Chen Z.J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 2012;5 doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M., Ramanjulu J., Booty L.M., Jimenez-Duran G., Keles H., Saunders K., Nevins N., Koppe E., Modis L.K., Pesiridis G.S., Bertin J., Triantafilou K. Human rhinovirus promotes STING trafficking to replication organelles to promote viral replication. Nat. Commun. 2022;13:1406. doi: 10.1038/s41467-022-28745-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Vaheri A., Weber F., Plyusnin A. Old World hantaviruses do not produce detectable amounts of dsRNA in infected cells and the 5' termini of their genomic RNAs are monophosphorylated. J. Gen. Virol. 2011;92:1199–1204. doi: 10.1099/vir.0.029405-0. [DOI] [PubMed] [Google Scholar]
- Wang K., Ma H., Liu H., Ye W., Li Z., Cheng L., Zhang L., Lei Y., Shen L., Zhang F. The glycoprotein and nucleocapsid protein of Hantaviruses manipulate autophagy flux to restrain host innate immune responses. Cell Rep. 2019;27:2075–2091.e5. doi: 10.1016/j.celrep.2019.04.061. [DOI] [PubMed] [Google Scholar]
- Webb L.G., Fernandez-Sesma A. RNA viruses and the cGAS-STING pathway: reframing our understanding of innate immune sensing. Curr. Opin. Virol. 2022;53 doi: 10.1016/j.coviro.2022.101206. [DOI] [PubMed] [Google Scholar]
- Woodward J.J., Iavarone A.T., Portnoy D.A. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science. 2010;328:1703–1705. doi: 10.1126/science.1189801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro L.H., Wilson S.C., Morrison H.M., Karalis V., Chung J.J., Chen K.J., Bateup H.S., Szpara M.L., Lee A.Y., Cox J.S., Vance R.E. Interferon-independent STING signaling promotes resistance to HSV-1 in vivo. Nat. Commun. 2020;11:3382. doi: 10.1038/s41467-020-17156-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Zevini A., Olagnier D., Hiscott J. Crosstalk between cytoplasmic RIG-I and STING sensing pathways. Trends Immunol. 2017;38:194–205. doi: 10.1016/j.it.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Shang G., Gui X., Zhang X., Bai X.C., Chen Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394–398. doi: 10.1038/s41586-019-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Kang R., Tang D. The STING1 network regulates autophagy and cell death. Signal Transduct. Target. Ther. 2021;6:208. doi: 10.1038/s41392-021-00613-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Qin X., Yang Y., Zhu X., Zhao S., Zhang Z., Su Q., Zhao Z., Yin X., Meng X., Zhang Z., Li Y. STING1 is essential for an RNA-virus triggered autophagy. Autophagy. 2022;18:816–828. doi: 10.1080/15548627.2021.1959086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Bai X.C., Chen Z.J. Structures and mechanisms in the cGAS-STING innate immunity pathway. Immunity. 2020;53:43–53. doi: 10.1016/j.immuni.2020.05.013. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Liu B., Ma Y., Yi J., Zhang C., Zhang Y., Xu Z., Wang J., Yang K., Yang A., Zhuang R., Jin B. Hantaan virus infection induces CXCL10 expression through TLR3, RIG-I, and MDA-5 pathways correlated with the disease severity. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/697837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.