Abstract
The clinical value of fractional flow reserve and non-hyperaemic pressure ratios are well established in determining an indication for percutaneous coronary intervention (PCI) in patients with coronary artery disease (CAD). In addition, over the last 5 years we have witnessed a shift towards the use of physiology to enhance procedural planning, assess post-PCI functional results, and guide PCI optimisation. In this regard, clinical studies have reported compelling data supporting the use of longitudinal vessel analysis, obtained with pressure guidewire pullbacks, to better understand how obstructive CAD contributes to myocardial ischaemia, to establish the likelihood of functionally successful PCI, to identify the presence and location of residual flow-limiting stenoses and to predict long-term outcomes. The introduction of new functional coronary angiography tools, which merge angiographic information with fluid dynamic equations to deliver information equivalent to intracoronary pressure measurements, are now available and potentially also applicable to these endeavours. Furthermore, the ability of longitudinal vessel analysis to predict the functional results of stenting has played an integral role in the evolving field of simulated PCI. Nevertheless, it is important to have an awareness of the value and challenges of physiology-guided PCI in specific clinical and anatomical contexts. The main aim of this European Association of Percutaneous Cardiovascular Interventions clinical consensus statement is to offer up-to-date evidence and expert opinion on the use of applied coronary physiology for procedural PCI planning, disease pattern recognition and post-PCI optimisation.
Introduction
Intracoronary physiological assessment is acknowledged as a valuable strategy to identify the presence of flow-limiting epicardial stenoses in patients with chronic coronary syndromes (CCS) and to determine an indication for percutaneous coronary interventions (PCI)1. Yet, its role in procedural planning and in assessing the functional results of PCI is less clear.
In practice, it had been previously assumed that once the presence of flow-limiting disease was confirmed with a single pressure guidewire measurement, PCI guided by angiography should lead to effective restoration of vessel conductance. However, studies with physiological post-PCI evaluation based on fractional flow reserve (FFR) and non-hyperaemic pressure ratios (NHPR) demonstrate that this supposition is not correct and that relying on angiographic guidance alone can be associated with suboptimal functional results post-PCI in many cases2,3,4.
This document addresses the use of coronary physiology to plan and guide PCI using physiological tools, providing evidence from the literature, expert opinion from operators, and revisiting available tools. The document provides an expert consensus on how to use commercially available physiology tools for these purposes. It has been reviewed and approved by the European Society of Cardiology (ESC) Clinical Guidelines Committee to ensure that no conflict exists with available guidelines. Many of the topics discussed herein pertain to the use of intracoronary pressure guidewires, which are the most widely used devices for physiological assessment in the cardiac catheterisation laboratory. Functional coronary angiography (FCA) tools, which merge angiographic information with fluid dynamic equations to deliver information equivalent to intracoronary pressure measurements and which constitute a valuable alternative to invasive tools in planning coronary revascularisation, are also discussed. A detailed and updated revision of the use of NHPR and FFR in different clinical scenarios is provided in Supplementary Appendix 1.
From confirming the indication to planning and guiding PCI
The limitations of coronary angiography in characterising the flow-limiting effect of coronary stenoses can be overcome by non-invasive and/or adjunctive invasive physiological assessments of coronary artery disease (CAD), the value of which are supported by evidence-based guideline recommendations5. When prior evidence of myocardial ischaemia is not available, FFR or instantaneous wave-free ratio (iFR) are recommended by the guidelines to assess the haemodynamic relevance of intermediate-grade coronary stenoses. FFR can also be considered in patients with multivessel disease (MVD) undergoing PCI5.
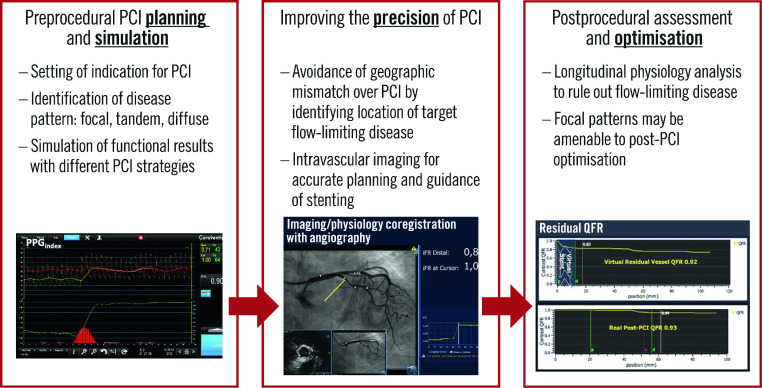
Over the last decade, criticism concerning the use of angiography alone to guide revascularisation decisions has been extended to the decision of when an optimal functional result of the intervention has been achieved. Such criticism is supported by suboptimal functional results identified in vessels despite a satisfactory angiographic PCI result2,3. Prior and recent observations have shown that such functionally suboptimal PCI results carry prognostic relevance (Supplementary Table 1)4,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25. Physiological guidance could contribute to improved PCI results in at least 3 ways: 1) improving preprocedural planning and simulation, 2) improving intraprocedural precision of PCI in addressing flow-limiting disease, and 3) guiding procedural optimisation of suboptimal PCI results (Central illustration).
Central illustration. Applications of physiology in planning and guiding PCI procedures.
iFR: instantaneous wave-free ratio; PCI: percutaneous coronary intervention; PPG: pullback pressure gradient; QFR: quantitative flow ratio
Despite the limited number of available studies in this field, there are now rational grounds to consider using physiological tools to plan effective PCI strategies to ideally remove all flow-limiting stenoses, to verify that functionally successful PCI has been achieved, and to contribute to procedural optimisation. Table 1 provides key information on the physiological guidance of PCI.
Table 1. Objectives of physiological planning and guidance of PCI.
| Pre-PCI physiology assessment | ||
|---|---|---|
| Objective | Technique | Benefit |
| Determining the indication for PCI | Pressure wire or FCA to identify the presence of flow-limiting disease | – Management of significant stenoses may confer prognostic or symptom benefit – Deferral of non-flow-limiting disease avoids unnecessary PCI |
| Identifying patterns of flow-limiting disease | Longitudinal physiological vessel analysis to identify focal, tandem, and diffuse patterns of flow-limiting disease | – Assists in gauging effectiveness of PCI – Aids with planning the length of stent and/or number of stents – Reconsider PCI when a suboptimal result is anticipated |
| Simulate impact of stenting in specific locations | NHPR and imaging-based functional assessments to simulate relief of stenosis virtually | – Plan effectiveness of PCI before stent deployment – Allows for several simulations prior to PCI |
| Facilitating precision stent deployment | Correlating physiology and angiography using either coregistration technologies or visual assessment, use of concomitant intracoronary imaging | – Avoidance of geographical miss during stenting, missing flow-limiting lesions – Accurate sizing of balloons and stents |
| Post-PCI physiology assessment | ||
| Objective | Technique | Benefit |
| Ensuring an optimal functional result of PCI | Physiological measurements in PCI target vessel after satisfactory angiographic result, jailed side-branch interrogation | – Early identification of residual flow-limiting disease in the PCI target vessel after the intervention |
| Identifying potential targets of functional optimisation of PCI | Post-PCI longitudinal vessel analysis | – Establishing the cause of suboptimal functional PCI results – Establishing the feasibility and mode of PCI optimisation |
| Assessing the impact of PCI optimisation | Repeat physiological measurements after physiology-based optimisation | – Residual disease not amenable to PCI may identify need for directed medical therapy or surgical revascularisation |
| FCA: functional coronary angiography; NHPR: non-hyperaemic pressure ratio; PCI: percutaneous coronary intervention | ||
Technological developments in applied coronary physiology in PCI
HYPERAEMIC AND NON-HYPERAEMIC PRESSURE INDICES
FFR is the most widely used intracoronary tool to define flow-limiting epicardial stenoses, with randomised clinical trials and observational studies supporting deferral26,27,28 or performance26,29 of revascularisation based on FFR values, predominately in patients with CCS. Contrast-based FFR, which relies upon submaximal myocardial hyperaemia induced by iodinated contrast administration, has been proposed as an adenosine-free alternative to FFR30. NHPR is increasingly utilised in clinical practice owing to a more favourable side-effect profile, reduced cost and procedure time, and ease of use compared with pharmacological hyperaemia. This facilitates multiple intraprocedural measurements either in different vessels or at different stages of the PCI procedure. Guidance by iFR, a widely utilised NHPR, was non-inferior to FFR guidance of revascularisation for major adverse cardiac events (MACE) at 1-year31,32 and 5-year follow-ups33. Other diastolic NHPR correlate closely with the iFR, with a similar ischaemic cut-off of ≤0.8934. All NHPR have demonstrated significant association with the risk of 2-year vessel-oriented composite endpoints35.
In the extended scenario of planning and guiding PCI, the use of pressure guidewires can be subject to specific caveats related to physiological assessment in complex clinical and anatomical scenarios (Table 2). Additional information detailing potential pitfalls in physiological assessment and considerations regarding the use of guide catheters, guide catheter extensions and microcatheters is provided in Supplementary Appendix 1.
Table 2. Challenges and pitfalls related to physiological assessment.
| Patient factors | ||
|---|---|---|
| Challenges and pitfalls | Comment | Solution |
| Presence of significant ostial CAD | Identified with ventricularisation or “damping” pressure waveform on engagement, not assessable with some FCA modalities | Reposition guide catheter to a more proximal position, ensure coaxiality, consider downsizing guide catheter, use an additional guidewire or septal wire to control catheter tip position |
| Haemodynamic crosstalk in presence of tandem lesions | Sequential lesions along a vessel invariably physiologically influence the other lesions in the vessel | Consider simulated planning prior to undertaking PCI on 1 or 2 stenoses based on longitudinal vessel analysis obtained with functional coronary angiography, NHPR, or FFR |
| Procedural factors | ||
| Challenges and pitfalls | Comment | Solution |
| Pressure wire drift | Offset of pressure reading during the procedure | Repeat procedure, use drift correction/re-normalise, or use image-based technique |
| Pseudostenosis caused by intracoronary wires | Straightening of tortuous vessels by intra-coronary wire may generate introsusceptions with haemodynamic relevance | Consider FCA as an alternative to intracoronary pressure guidewires |
| Ventricularisation/damping of aortic pressure | Guiding catheter engaged abutting the vascular lumen or in significant ostial lesions | Reposition guide catheter, ensure coaxiality, consider downsizing guide catheter, equalisation of pressure guidewire is advised |
| Use of guide catheter with side holes | False estimate of Pa at the tip of the guiding catheter | Advise not to use catheter with side holes for intracoronary assessment. If needed for clinical reasons, ensure catheter disengagement and use of IV adenosine. |
| Use of guide catheter extensions | May cause ventricularisation of Pa, if introduced after pressure equalisation, may modify Pa and affect measurements | Perform pressure equalisation with extension within guide catheter, withdraw guide extension at the time of physiological assessment |
| Use of coronary microcatheters | If used after pressure equalisation, may modify Pa and affect measurements | Perform pressure equalisation with microcatheter within guide catheter before measurements |
| Wire whipping | Pressure wire signal degrading through contact with vascular lumen | Repositioning of the pressure wire often resolves this issue |
| Transient microvascular dysfunction associated with the procedure | Rotational atherectomy and PCI tools generating microparticles may cause transient microvascular dysfunction, affecting pressure indices | Consider FCA as an alternative to intracoronary assessment if transient microvascular dysfunction is assumed or suspected |
| FFR measurement in region without stable hyperaemia | Whilst the software reports the lowest physiological reading, these might not be in stable hyperaemia and therefore overestimate lesion severity | Measure FFR in stable hyperaemia, and if needed adjust measurement points manually |
| CAD: coronary artery disease; IV: intravenous; FCA: functional coronary angiography; FFR: fractional flow reserve; NHPR: non-hyperaemic pressure ratio; Pa: aortic pressure; PCI: percutaneous coronary intervention | ||
FUNCTIONAL CORONARY ANGIOGRAPHY
The success of FFR and NHPR as indices to estimate functional stenosis severity has led to the development of new FCA technologies. These can derive similar information from an invasive coronary angiogram or non-invasive computed tomography coronary angiography (CTCA), with both approaches demonstrating good correlation with wire-based FFR. Computed tomography-derived FFR (FFRCT) is obtained by merging a three-dimensional (3D) reconstruction of vessels from CTCA with computational fluid dynamics36, thereby providing a reliable estimate of invasive FFR37. Similarly, several FFR-equivalent measurements can also be derived from invasive coronary angiograms38,39,40,41; these are supported by prospective validation studies. Quantitative flow ratio (QFR) is the only angiography-based physiological index that has been prospectively validated and has already been demonstrated to be associated with improved clinical outcomes when used to decide upon coronary revascularisation, compared with conventional angiography42. A distinct advantage of all FCA modalities is that they provide longitudinal vessel analysis, allowing accurate length measurements and localisation of flow-limiting disease to vessel anatomy. Detailed information on potential limitations of imaging-based functional coronary analysis is provided in Supplementary Table 2.
LONGITUDINAL PHYSIOLOGICAL VESSEL ANALYSIS
Customarily, indices of coronary physiology have been reported as a single value in the distal segment of the interrogated coronary artery, reflecting cumulative pressure losses along the entire length of the epicardial vessel. Longitudinal assessment, utilising a pressure wire pullback, adds an additional dimension to the physiological analysis. Pullback manoeuvres can be performed manually43 with either FFR or NHPR44,45. Accurate length measurements to the order of millimetres can be obtained by using either motorised pullback46 or dedicated software that tracks the radiopaque wire tip during pullback47. Dedicated software is available to provide stable FFR, iFR, and resting full-cycle ratio (RFR) pullback curves, free of fluctuations due to the Venturi effect, where a drop in intracoronary pressure is seen as the sensor crosses the stenosis (Supplementary Figure 1).
Prior to PCI, longitudinal vessel assessment informs on the distribution of pressure losses along the epicardial vessel and can differentiate focal and/or diffuse patterns of flow-limiting disease (Figure 1). Longitudinal vessel analysis allows for the identification of focal and diffuse patterns of flow-limiting disease, either subjectively by visual inspection of the pullback curve or objectively by using the pressure pullback gradient index (PPGindex)45, which can be performed manually with high inter- and intraoperator reproducibility48. Differentiation of diffuse from focal disease is also feasible with an automated algorithm that analyses the instantaneous FFR gradient per time unit, the dFFR(t)/dt index49. Both the PPGindex and dFFR(t)/dt can be derived from QFR, and their prognostic relevance have been documented with post-PCI clinical outcomes49,50. The criteria used to define focal, tandem, and diffuse patterns, as well as supporting studies, can be found in Table 3 and Supplementary Table 3, respectively2,3,45,50,51,52,53,54,55,56,57,58,59,60,61. The current definitions of focal and diffuse disease are largely qualitative, and research to formally define these lesion characteristics is awaited. The ability to discriminate between patterns of CAD carries immediate and relevant clinical implications; a focal pattern is associated with an optimal physiological result after PCI, with consequent good prognosis and relief of angina. On the contrary, a diffuse pattern of disease is associated with suboptimal post-PCI results and prognosis and more residual anginal symptoms62.
Figure 1. Patterns of flow-limiting disease identified in longitudinal vessel analysis before and after PCI.
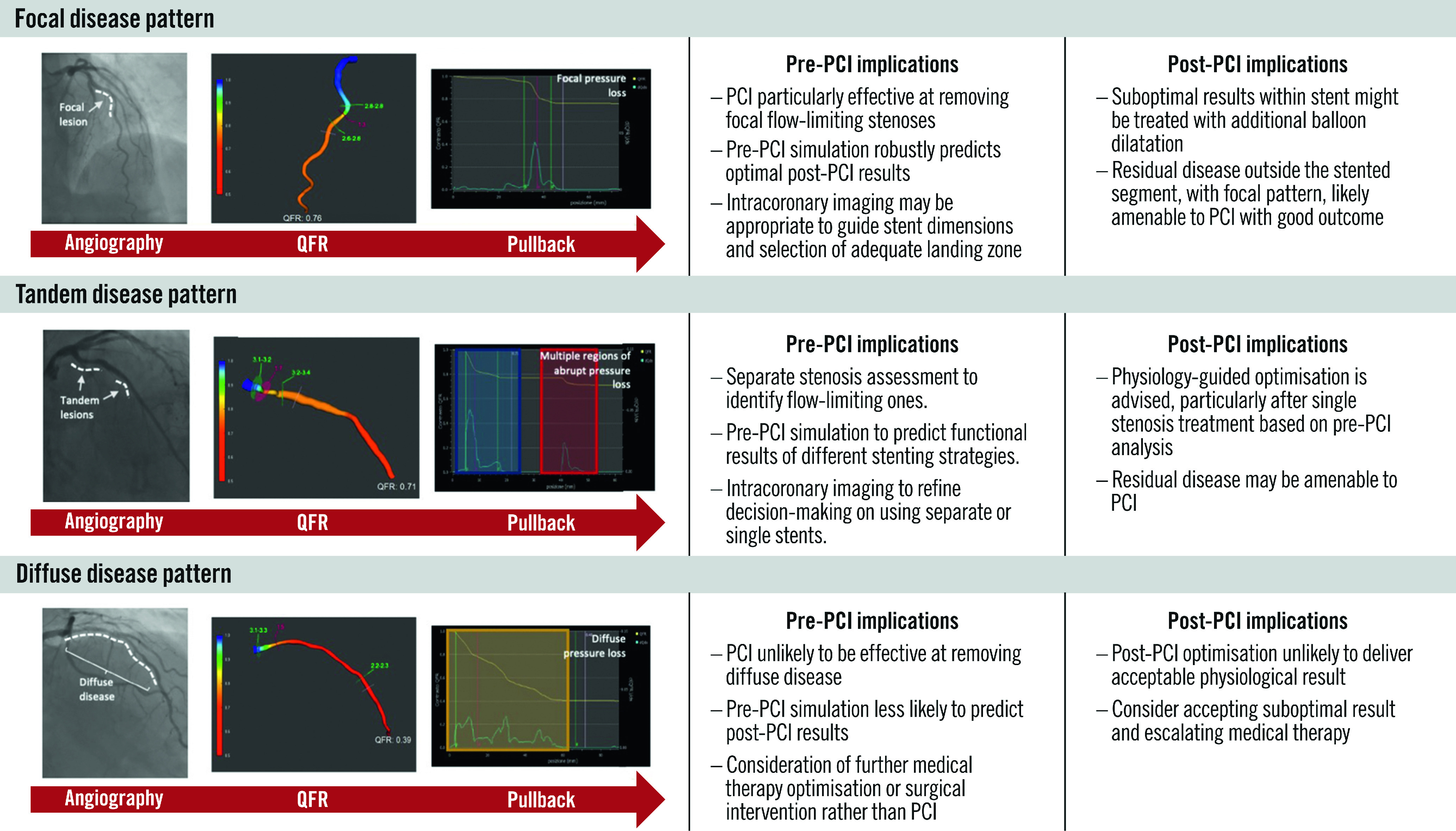
PCI: percutaneous coronary intervention; QFR: quantitative flow ratio
Table 3. Criteria used to define focal, tandem and diffuse patterns of obstructive coronary disease.
| Pre-PCI physiology assessments | |||
|---|---|---|---|
| Pattern | FFR | NHPR | FCA |
| Focal | FFR ≤0.80 at distal segment, abrupt single point of pressure loss. | iFR/RFR/DFR ≤0.89 at distal segment, with abrupt single point of ≥0.03 index units over ≤15 mm segment. | QFR/vFFR/FFRCT ≤0.80 at distal segment, with single region of pressure loss of>0.05 in <10 mm segment. |
| Tandem | FFR ≤0.80 at distal segment, presence of ≥2 abrupt change of FFR values. | iFR/RFR/DFR ≤0.89, at distal segment, with presence of ≥2 abrupt changes of index values. | QFR/vFFR/FFRCT ≤0.80 at distal vessel with presence of ≥2 abrupt changes of pressure loss. |
| Diffuse* | FFR ≤0.80 at distal segment with progressive and linear loss in FFR values over length of vessel. | iFR/RFR/DFR ≤0.89 at distal segment, with progressive and linear loss in pressure over length of vessel during iFR pullback. | Progressive and linear loss in QFR/vFFR/FFRCT values over length of vessel during longitudinal analysis. |
| Post-PCI physiology assessments | |||
| Pattern | FFR | NHPR | FCA |
| Focal | FFR with abrupt pressure loss at the stented site or elsewhere within the treated vessel. | Abrupt drop of iFR/RFR/DFR values at the stented site or elsewhere within the treated vessel. | Abrupt drop of QFR/vFFR values at the stented site or elsewhere within the treated vessel. |
| Tandem | Abrupt drop of FFR values at the level of an untreated tandem stenosis. | Abrupt drop of iFR/RFR/DFR values at the level of an untreated tandem stenosis. | Abrupt drop of QFR/vFFR values at the level of an untreated tandem stenosis. |
| Diffuse* | Progressive and linear loss in FFR values over length of stented vessel during pullback. | Progressive and linear loss in iFR/RFR/DFR values over length of stented vessel during pullback. | Progressive and linear loss in QFR/vFFR values over length of stented vessel. |
| *These criteria are clinical consensus statements and further validation is warranted. DFR: diastolic hyperaemia-free ratio; FCA: functional coronary angiography; FFR: fractional flow reserve; FFRCT: computed tomography-derived FFR; iFR: instantaneous wave-free ratio; NHPR: non-hyperaemic pressure ratio; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; RFR: resting full-cycle ratio; vFFR: virtual FFR | |||
After PCI, longitudinal physiological vessel interrogation may identify residual focal pressure gradients inside or outside the stent which might be amenable to additional stent post-dilatation or PCI2. Alternatively, a diffuse pattern of residual disease after PCI may discourage operators from further vessel instrumentation.
COREGISTRATION WITH ANGIOGRAPHY
Merging longitudinal vessel physiology with the coronary angiogram allows for accurate localisation of flow-limiting atherosclerotic disease and facilitates procedural planning47. The coregistered map provides signposts with a clear distribution of regions of pressure loss, enabling optimal localisation of specific target lesions that might benefit from PCI, and the implementation of length measurements (Supplementary Figure 2). This technology can be used in conjunction with iFR and is particularly useful when forming strategies for intervention on tandem lesions and interrogating areas of diffuse disease.
SIMULATION OF FUNCTIONAL PCI RESULTS
Despite consistent evidence showing that suboptimal values of physiological indices after PCI are associated with poorer outcomes, there is a relative paucity of evidence regarding whether they can be used to guide PCI optimisation and, ultimately, to improve patient outcomes. Since the aim of PCI is the elimination of ischaemia-generating lesions, predicting the haemodynamic results of a given strategy before embarking on stenting appears to be a rational approach to avoid suboptimal results of the intervention. The concept of in silico simulation of PCI to predict functional results of an intervention is appealing, as it allows for both procedural planning and modelling of post-PCI physiology prior to undertaking the procedure.
There are different approaches to simulate functional PCI results from baseline longitudinal vessel analysis (Figure 2). A simple mathematical approach to estimate the impact of PCI based on pullback curves can be followed: predicted NHPR (NHPRpred)=pre-PCI NHPR (lowest value) + ∑intention-to-treat NHPR gradient(s). Pioneering studies with iFR pullback have demonstrated its ability to predict post-PCI results63. Subsequently, this has also been confirmed for other NHPR such as the RFR and diastolic pressure ratio61. Subtraction of the flow-limiting effect of one or more coronary stenoses can be readily performed with current software versions of iFR coregistration with angiography31. From the perspective of FCA, a dedicated virtual stenting tool has been developed for FFRCT64. The FFRCT Planner (HeartFlow) has been shown to be accurate and precise at predicting post-PCI FFR65. Analysis with QFR and virtual FFR (vFFR) provides an estimate of post-PCI values after treatment of a given stenosis (residual QFR and vFFR), which has been shown to predict post-PCI FFR25,66,67. Figure 3 shows a pullback curve analysis performed with iFR and coregistration, including simulated stenting at a specific location.
Figure 2. Approaches for simulating functional PCI results based on longitudinal vessel analysis.

3D-QCA: three-dimensional quantitative coronary angiography; CFD: computational fluid dynamics; CTCA: computed tomography coronary angiography; FFR: fractional flow reserve; FFRCT: computed tomography-derived FFR; iFR: instantaneous wave-free ratio; LAD: left anterior descending artery; NHPR: non-hyperaemic pressure ratio; PCI: percutaneous coronary intervention; QFR: quantitative flow ratio; RCA: right coronary artery
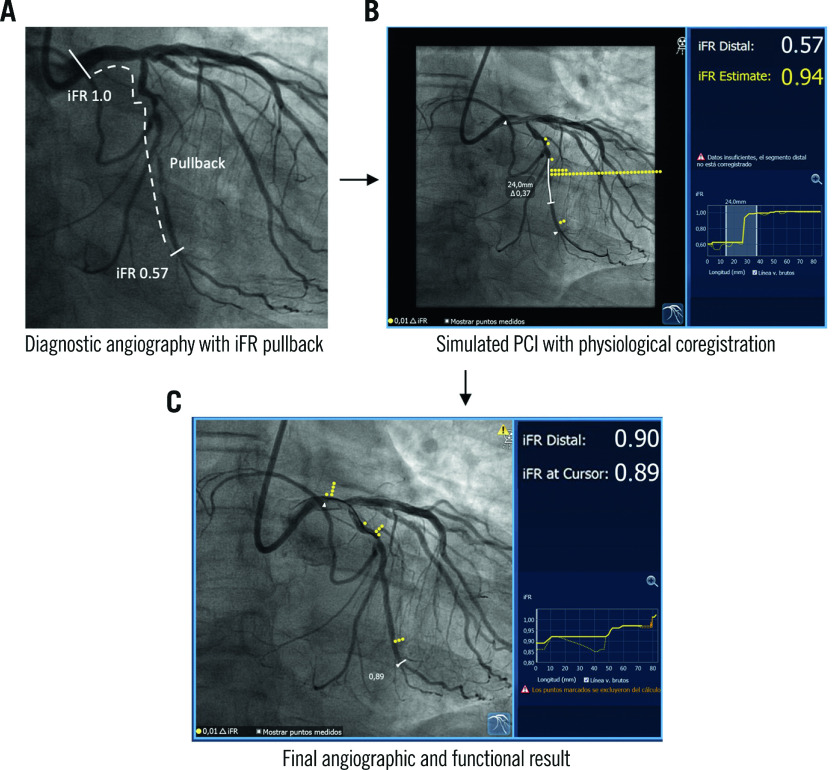
Figure 3. Simulation of functional PCI results using coregistration of physiology and the coronary angiogram.
A) Angiography of the left circumflex artery interrogated with longitudinal physiological assessment. B) The presence of flow-limiting disease in the vessel is demonstrated by a distal iFR of 0.57. Longitudinal vessel analysis revealed a focal disease phenotype. iFR coregistration with angiography allows accurate localisation of flow-limiting disease in the coronary anatomy. The PCI simulation suggests adequate functional results of the intervention, with a predicted post-PCI iFR of 0.94. C) Final co-registered angiographic-physiology result shown, with final iFR of 0.90. iFR: instantaneous wave-free ratio; PCI: percutaneous coronary intervention
CORONARY PRESSURE WIRES AND MEASURING TOOLS
Continued improvement in pressure guidewire technology has facilitated the accurate interrogation and measurement of complex coronary anatomies. Contemporary guidewire-based pressure indices are fitted with sensors that use either electrical or fibre-optic signal transmission. Advances in manufacturing processes have resulted in an improved accuracy and stability of the sensor, thus, minimising the drift phenomenon with workhorse guidewire-like characteristics, resulting in optimised torque control and manoeuvrability. The latter is critical for the safe wiring of the target vessel prior to the measurement of pressure loss and PCI. Microcatheter-based pressure measurement technology requires a 0.014” guidewire, according to anatomy or operator preference, but requires a 0.020” shaft profile for the microcatheter, which may interfere with measurements, especially in small vessels or severe stenoses, and can be difficult to navigate around tight angulations. The currently available and pending devices for invasive functional assessments are shown in Supplementary Table 4.
Physiological assessment of post-PCI results
Post-PCI intracoronary pressure measurements can identify residual flow-limiting disease, differentiate residual focal lesions from diffuse disease and provide prognostic information. Whilst a higher post-PCI FFR is associated with a lower incidence of adverse clinical outcomes, the cut-off point for an optimal PCI result is still debated68. A post-PCI FFR ≥0.90 has been associated with a significantly lower risk of repeat PCI and MACE in a systematic review of 7,470 patients69. The most robust recent data, obtained in a patient-level meta-analysis of 5,869 vessels treated with modern drug-eluting stents, reported optimal post-PCI FFR cut-off values of 0.86 for target vessel failure and 0.80 for the composite of cardiac death or target vessel myocardial infarction70. For NHPR, a post-PCI iFR ≥0.95 was associated with improved patient outcomes in the DEFINE PCI study3. An optimal cut-off for post-PCI distal coronary pressure/aortic pressure (Pd/Pa) ratio of>0.96 has also been proposed23.
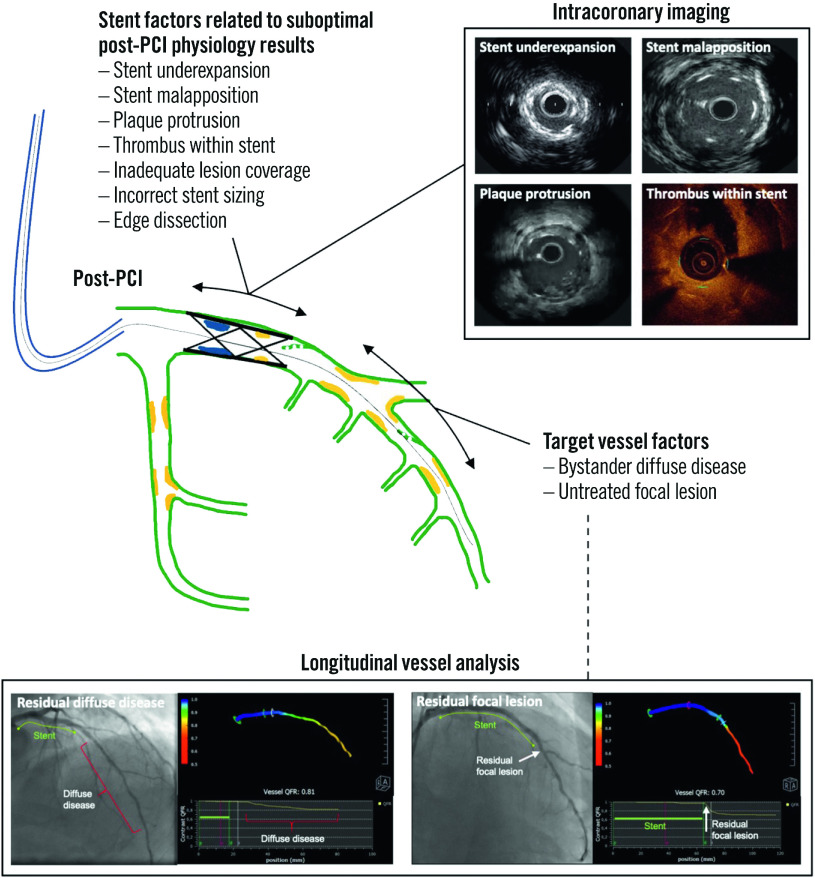
Contemporary reports indicate that post-PCI physiology results might remain below the clinical revascularisation thresholds of ≤0.80 for FFR and <0.90 for NHPR in approximately 24-36% of cases2,3,71. Potential mechanisms of suboptimal post-PCI physiology results are outlined in Figure 4. Additional interventions guided by post-PCI coronary physiology assessments can improve the final result2,10,71. Caution should be exerted to avoid overtreatment, for example when the identified cause of suboptimal functional results entails treatment of long coronary segments or aggressive lesion manipulation. Intracoronary imaging may contribute to a better understanding of the cause of suboptimal functional results and to safer decision-making. Furthermore, it has to be kept in mind that cardiovascular risk may differ between patients despite having achieved an optimal functional result; this is largely dictated by the extension and characteristics of underlying atherosclerosis.
Figure 4. Mechanisms of suboptimal post-PCI physiological results.
PCI: percutaneous coronary intervention; QFR: quantitative flow ratio
While functionally suboptimal PCI results are associated with adverse clinical outcomes and can be related to residual treatable disease, they may more frequently be an epiphenomenon of diffuse atherosclerosis that cannot be adequately addressed with revascularisation. This may explain why larger relative increases in FFR after stenting (typically achieved through treatment of focal, physiologically severe lesions) are associated with lower rates of target vessel failure, reduced incidence of angina and improved quality of life14,21,72. As not all residual flow-limiting disease is amenable to treatment with PCI, achieving optimal post-PCI FFR or NHPR target values can be challenging, or frequently impossible, in many patients73. Beyond the pattern of coronary artery disease, there are additional anatomical factors that may contribute to lower pressure-based indices. Evolving evidence suggests that post-PCI FFR values are consistently lower in left anterior descending (LAD) versus non-LAD vessels and might require vessel-specific optimal thresholds, adding a further level of complexity2,22. The lower LAD values likely occur because of the interplay of several factors, including the higher prevalence of intramuscular coronary tracts observed in the LAD (Supplementary Figure 3), the impact of hydrostatic effects relating to coronary anatomy and the resultant height of the pressure wire sensor above or below the aortic pressure transducer (typically causing a hydrostatic offset of around 3.6 mmHg)74, and higher coronary flow rates due to the larger myocardial mass subtended by the LAD (high flow across long segments of mild residual diffuse atheroma can generate an appreciable pressure gradient). Based on this, normal FFR values for the LAD have recently been shown to be 0.92, compared to 0.96 in non-LAD vessels75. Finally, in all anatomical locations, when NHPR are used, residual reactive hyperaemia induced by intervention-related vessel manipulation may render false positive measurements if performed immediately after stent implantation or contrast/saline injections.
Complementary role of combined imaging and physiology in PCI planning and guidance
Whilst the objective of PCI is the removal of flow-limiting disease, long-term results of interventions are strongly influenced by procedural factors that are better addressed with intracoronary imaging techniques, such as intravascular ultrasound (IVUS) and optical coherence tomography (OCT) − both of which provide detail on CAD patterns and guidance for PCI. A detailed description of these techniques and on their use in planning, guiding and optimising PCI is available elsewhere76. The results of comprehensive vessel imaging post-PCI compared with FFR and its effect on long-term outcomes have been reported77. In addition, there is growing interest in whether the presence of vulnerable atheroma in non-flow-limiting lesions might be associated with a higher risk of future events78.
Merging information based on the presence and location of flow-limiting stenoses provided by physiology, with information on plaque characteristics and distribution, and vessel dimensions derived from imaging might aid in the selection of PCI devices and strategies in terms of lesion preparation, plaque-free landing zone for PCI, and the selection of stent diameter and length, all important aspects which ultimately improve long-term procedural results. Triregistration, with physiology and intracoronary imaging in conjunction with angiography, is currently available for IVUS and iFR. Coregistration with angiography is also feasible with OCT. Intracoronary imaging-derived physiology, such as OCT-derived FFR, currently lacks validation and is not available for clinical use.
In addition, there is extensive evidence suggesting that PCI optimisation with intravascular imaging improves long-term procedural outcomes2,76,79,80,81,82,83,84. This benefit is independent of the modest increase in FFR values noted in image-based PCI optimisation studies79,85,86. The synergistic use of intracoronary imaging and post-PCI longitudinal vessel pullback can be used to investigate causes of focal pressure loss after PCI and assist with decisions on how they could be rectified86. Imaging may also highlight the existence of high-risk morphological features of coronary disease − both at baseline and post-PCI − with prognostic implications, even when these are not ischaemia-generating87, such as in instances of malapposition or significant edge dissection.
Integrating available tools and knowledge into algorithms for physiology-based planning and optimisation
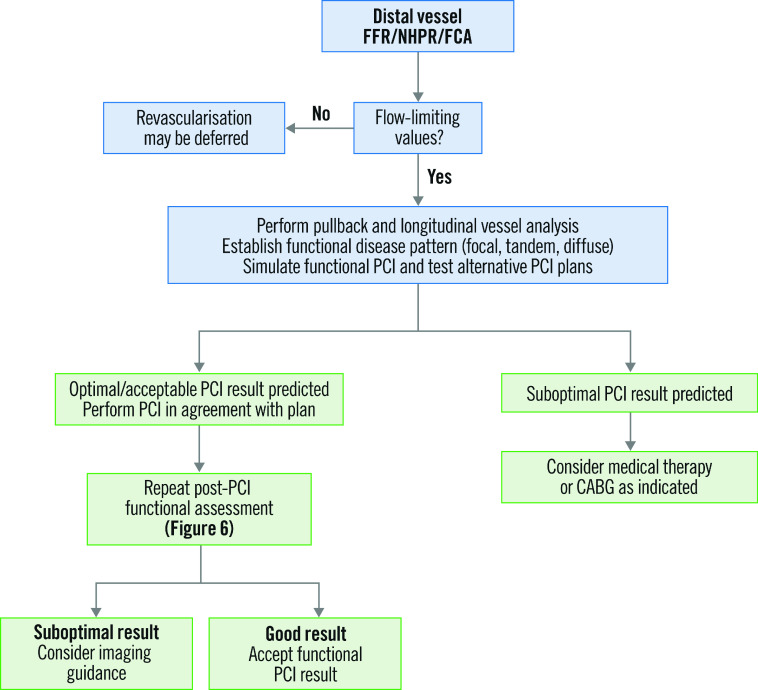
The points discussed thus far can be tentatively integrated into algorithms for PCI planning and post-PCI evaluation. It is suggested that a comprehensive virtual analysis of the target vessel be performed prior to PCI, to support the indication for PCI and to assist in procedural planning and strategising, with the support of simulation if available. Following PCI, the result should be interrogated using a combination of physiology and/or imaging techniques, with post-PCI optimisation carried out when residual focal or tandem pressure loss remains. Suggested algorithms for comprehensive lesion assessment using pre- and post-PCI physiology are shown in Figure 5 and Figure 6, respectively.
Figure 5. Proposed algorithm for pre-PCI physiology assessment and planning of PCI based on physiological interrogation.
CABG: coronary artery bypass graft; FCA: functional coronary angiography; FFR: fractional flow reserve; NHPR: non-hyperaemic pressure ratio; PCI: percutaneous coronary intervention
Figure 6. Proposed algorithm for postprocedural assessment and optimisation of functional PCI results based on physiological interrogation.
PCI: percutaneous coronary intervention
Applied coronary physiology in specific PCI scenarios
In addition to the general principles discussed above, the use of physiology to plan and guide PCI in specific scenarios is deserving of a separate discussion. Supplementary Appendix 2 outlines applied coronary physiology for the following contexts: multivessel coronary artery disease, vessels with tandem lesions or diffusely diseased vessels, lesions in vessels providing collaterals to chronically occluded arteries, patients with acute coronary syndromes, bifurcation lesions and jailed side branches, left main stem stenosis, coronary lesions in patients with aortic stenosis, native vessel or surgical graft stenosis in patients with prior coronary artery bypass graft, and in vessels with myocardial bridge − all in greater detail.
Future directions and outlook
The use of physiology in procedural planning and simulation of PCI is an area of growing interest, though, as yet, supported only by a relatively small evidence base. Upcoming trials in the field will provide valuable evidence on the use of invasive physiology and FCA for this purpose. Whilst it is generally accepted that PCI is an appropriate therapy for patients with focal CAD, the optimal treatment strategy for patients with haemodynamically significant lesions in the presence of diffuse disease is a subject of ongoing investigation.
Prospective studies are also being conducted to assess the use of coregistration technology on stent deployment and patient outcomes. The DEFINE GPS (Distal Evaluation of Functional Performance With Intravascular Sensors to Assess the Narrowing Effect: Guided Physiologic Stenting; ClinicalTrials.gov: NCT04451044) and iLARDI (Usefulness of the Use of Co-registration Strategy With iFR in Long and/or Diffuse Coronary Lesions; ClincalTrials.gov: NCT04283734) investigators aim to assess the impact of iFR coregistration (SyncVision; Philips) on guiding PCI and influencing the numbers and lengths of implanted stents, and in DEFINE GPS, whether this might influence the rate of MACE. The PPG Global Registry involves a prospective evaluation of the impact of the PPG index on clinical decision-making for PCI (or not) and related outcomes (ClinicalTrials.gov: NCT04789317). Future studies should better define the role of microvascular dysfunction in relation to PCI outcomes. Collectively, these studies will add significant value in objectively assessing the utility of pre-PCI simulation and planning using physiological indices.
Conclusions
The raison d’être of PCI is to abolish flow-limiting stenoses and ischaemia in order to improve patient symptoms and/or prognosis. In conjunction with angiographic findings, physiology determined by FFR, NHPR and FCA assists in weighing important decisions in procedural planning. Emerging studies hint at the application of in silico PCI simulation prior to intervening on a patient. This offers a novel perspective on planning the effectiveness of an intervention and could enable the adoption of revascularisation strategies associated with the highest physiological gain. Furthermore, identifying patients with little physiological gain after PCI, despite optimisation, is highly important, as they are at high risk of premature stent failure.
Physiology in the post-PCI setting facilitates procedural optimisation through descriptive longitudinal vessel analyses of stented segments, offering clarity on the presence of residual flow-limiting disease. These technologies, in combination with the ability to coregister anatomy with physiology and intravascular imaging, which allows for the identification of different disease phenotypes, have revolutionised PCI in the modern era. Clinical trials assessing the effectiveness of these tools on clinical outcomes are eagerly awaited.
Supplementary data
Guiding catheters, guide catheter extensions and microcatheters.
Applied coronary physiology in specific PCI scenarios.
Studies supporting the prognostic impact of functional PCI results.
Limitations related to image-based functional coronary analysis.
Supporting studies used to define focal, tandem and diffuse disease patterns.
Currently available and pending devices for invasive functional coronary assessment.
Correction of intracoronary pressure pullback curves with dedicated software addressing fluctuations caused by the Venturi effect.
Longitudinal iFR mapping coregistered with coronary angiography.
Pre- and post-PCI functional test in the presence of myocardial bridge.
Acknowledgments
Acknowledgements
We are indebted to Silvio Capuano for their technical support in preparing the supplementary tables for this document.
Conflict of interest statement
J. Escaned reports speaker or advisory board member fees from Abbott, Boston Scientific, Medis, and Philips. C. Berry is employed by the University of Glasgow, which holds consultancy and research agreements for his work with Abbott Vascular, AstraZeneca, Boehringer Ingelheim, Causeway Therapeutics, Coroventis, Genentech, GSK, HeartFlow, Menarini, Neovasc, Novartis, Siemens Healthcare, and Valo Health. B. De Bruyne reports receiving consultancy fees from Boston Scientific and Abbott Vascular; research grants from Coroventis Research, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular; and owning equity in Siemens, GE HealthCare, Philips, HeartFlow, Edwards Lifesciences, Bayer, Sanofi, and Celyad. C. Collet reports receiving research grants from Biosensor, Coroventis Research, Medis Medical Imaging, Pie Medical Imaging, CathWorks, Boston Scientific, Siemens, HeartFlow, and Abbott Vascular; and consultancy fees from HeartFlow, OpSens, Abbott Vascular, and Philips/Volcano. J.M. Lee received institutional research grants from Abbott Vascular, Boston Scientific, Terumo Corporation, Philips/Volcano, Medis Medical Imaging, and Zoll Medical. Y Appelman received speaker fees from Abbott Vascular. Si. Biscaglia received unrestricted research grants and speaker's fees from SMT, Medis, Abbott, and Insight Lifetech. P.P. Buszman is employed by the American Heart of Poland, which holds research agreements with Meril Life, and received speakers’ honoraria from Novartis. G. Campo received institutional research grants from Medis, GE HealthCare, Siemens Healthcare, Abbott Vascular, Sahajanand Medical Technologies, and Insight Lifetech. D. Collison has received consultancy and speaker fees from Abbott. A. Jeremias reports consultancy fees from Philips/Volcano, Abbott Vascular, Boston Scientific, and ACIST Medical Systems. Z. Piróth has received speakers’ fees from Abbott, Boston Scientific, and Opsens. L. Raposo has received honoraria and research grants from Philips/Volcano, St. Jude Medical (now Abbott Vascular) and HeartFlow, as well as consultancy fees from Boston Scientific. T. Rudolph received speaker honoraria from Abbott Vascular, Philips, Neovasc, AstraZeneca, Bayer, Pfizer, Philips/Volcano, Zoll Medical, and RainMed. G. Sarno has received a research grant from Boston Scientific (to the institution); and personal fees from Abbott Vascular, Boston Scientific, and Pfizer/BMS. D. Dudek reports participation in company-sponsored speaker’s bureaus for Abbott, Boston Scientific, Bracco, Philips, and Siemens Healthcare; and unrestricted grants from Abbott, Boston Scientific, Bracco, Philips, and Siemens Healthcare. The other authors have no conflicts of interest to declare.
Abbreviations
- ACS
acute coronary syndrome
- CAD
coronary artery disease
- CCS
chronic coronary syndrome
- CTCA
computed tomography coronary angiography
- FCA
functional coronary angiography
- FFR
fractional flow reserve
- FFRCT
computed tomography-derived fractional flow reserve
- iFR
instantaneous wave-free ratio
- IVUS
intravascular ultrasound
- MACE
major adverse cardiovascular event
- MVD
multivessel disease
- NHPR
non-hyperaemic pressure ratio
- OCT
optical coherence tomography
- PCI
percutaneous coronary intervention
- PPGindex
pressure pullback gradient index
- QFR
quantitative flow ratio
- RFR
resting full-cycle ratio
- vFFR
virtual fractional flow reserve
Contributor Information
Javier Escaned, Hospital Clínico San Carlos IdISCC, Complutense University of Madrid, Madrid, Spain.
Colin Berry, Institute of Cardiovascular & Medical Sciences, BHF Glasgow Cardiovascular Research Centre, University of Glasgow, Glasgow, UK.
Bernard De Bruyne, Cardiovascular Center Aalst, OLV Clinic, Aalst, Belgium; Department of Cardiology, Lausanne University Center Hospital, Lausanne, Switzerland.
Asad Shabbir, Hospital Clínico San Carlos IdISCC, Complutense University of Madrid, Madrid, Spain.
Carlos Collet, Cardiovascular Center Aalst, OLV Clinic, Aalst, Belgium.
Joo Myung Lee, Division of Cardiology, Department of Internal Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea.
Yolande Appelman, Amsterdam UMC, Department of Cardiology, Amsterdam Cardiovascular Sciences, Amsterdam, the Netherlands.
Emanuele Barbato, Cardiovascular Center Aalst, OLV Clinic, Aalst, Belgium; Division of Cardiology, Department of Advanced Biomedical Sciences, Federico II University of Naples, Naples, Italy.
Simone Biscaglia, Cardiology Unit, Azienda Ospedaliero Universitaria di Ferrara, Cona, Italy.
Piotr P. Buszman, Andrzej Frycz Modrzewski Kraków University, Kraków, Poland; American Heart of Poland, Ustroń, Poland.
Gianluca Campo, Cardiology Unit, Azienda Ospedaliero Universitaria di Ferrara, Cona, Italy.
Alaide Chieffo, Interventional Cardiology Unit, IRCCS San Raffaele Scientific Institute, Milan, Italy.
Allen Jeremias, St. Francis Hospital & Heart Center, Roslyn, NY, USA.
Valeria Paradies, Department of Cardiology, Maasstad Hospital, Rotterdam, the Netherlands.
Zsolt Piróth, Gottsegen National Cardiovascular Center, Budapest, Hungary.
Luís Raposo, Unidade de Intervenção Cardiovascular, Serviço de Cardiologia, Hospital de Santa Cruz, Centro Hospitalar de Lisboa Ocidental, Lisboa, Portugal.
Ariel Roguin, Hillel Yaffe Medical Center, Hadera, Israel; Faculty of Medicine, Technion, Haifa, Israel.
Tanja Rudolph, Heart and Diabetes Center North Rhine-Westphalia, Bad Oeynhausen, Germany.
Giovanna Sarno, Cardiology, Department of Medical Sciences and Uppsala Clinical Research Center, Uppsala University, Uppsala, Sweden.
Sayan Sen, Imperial College Healthcare NHS Trust, Hammersmith Hospital, London, UK.
Gabor G. Toth, Department of Cardiology, Medical University of Graz, Graz, Austria.
Eric Van Belle, Department of Interventional Cardiology for Coronary, Valves and Structural Heart Diseases, Institut Coeur Poumon, Lille, France; Department of Cardiology, Institut Pasteur de Lille, Lille, France.
Frederick M. Zimmermann, Department of Cardiology, Catharina Hospital, Eindhoven, the Netherlands.
Dariusz Dudek, Interventional Cardiology Unit, Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy; Institute of Cardiology, Jagiellonian University Medical College, Kraków, Poland.
Giulio Stefanini, Department of Biomedical Sciences, Humanitas University, Pieve Emanuele, Milan, Italy; Humanitas Research Hospital IRCCS, Rozzano, Milan, Italy.
Giuseppe Tarantini, Humanitas Research Hospital IRCCS, Rozzano, Milan, Italy; University of Padua Medical School, Padua, Italy.
References
- Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, Prescott E, Storey RF, Deaton C, Cuisset T, Agewall S, Dickstein K, Edvardsen T, Escaned J, Gersh BJ, Svitil P, Gilard M, Hasdai D, Hatala R, Mahfoud F, Masip J, Muneretto C, Valgimigli M, Achenbach S, Bax JJ ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- Collison D, Didagelos M, Aetesam-Ur-Rahman M, Copt S, McDade R, McCartney P, Ford TJ, McClure J, Lindsay M, Shaukat A, Rocchiccioli P, Brogan R, Watkins S, McEntegart M, Good R, Robertson K, O’Boyle P, Davie A, Khan A, Hood S, Eteiba H, Berry C, Oldroyd KG. Post-stenting fractional flow reserve vs coronary angiography for optimization of percutaneous coronary intervention (TARGET-FFR). Eur Heart J. 2021;42:4656–68. doi: 10.1093/eurheartj/ehab449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremias A, Davies JE, Maehara A, Matsumura M, Schneider J, Tang K, Talwar S, Marques K, Shammas NW, Gruberg L, Seto A, Samady H, Sharp A, Ali ZA, Mintz G, Patel M, Stone GW. Blinded Physiological Assessment of Residual Ischemia After Successful Angiographic Percutaneous Coronary Intervention: The DEFINE PCI Study. JACC Cardiovasc Interv. 2019;12:1991–2001. doi: 10.1016/j.jcin.2019.05.054. [DOI] [PubMed] [Google Scholar]
- Pijls NH, Klauss V, Siebert U, Powers E, Takazawa K, Fearon WF, Escaned J, Tsurumi Y, Akasaka T, Samady H, De Bruyne Fractional Flow Reserve (FFR) Post-Stent Registry Investigators. Coronary pressure measurement after stenting predicts adverse events at follow-up: a multicenter registry. Circulation. 2002;105:2950–4. doi: 10.1161/01.cir.0000020547.92091.76. [DOI] [PubMed] [Google Scholar]
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- Bech GJ, Pijls NH, De Bruyne, Peels KH, Michels HR, Bonnier HJ, Koolen JJ. Usefulness of fractional flow reserve to predict clinical outcome after balloon angioplasty. Circulation. 1999;99:883–8. doi: 10.1161/01.cir.99.7.883. [DOI] [PubMed] [Google Scholar]
- Klauss V, Erdin P, Rieber J, Leibig M, Stempfle HU, König A, Baylacher M, Theisen K, Haufe MC, Sroczynski G, Schiele T, Siebert U. Fractional flow reserve for the prediction of cardiac events after coronary stent implantation: results of a multivariate analysis. Heart. 2005;91:203–6. doi: 10.1136/hrt.2003.027797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam CW, Hur SH, Cho YK, Park HS, Yoon HJ, Kim H, Chung IS, Kim YN, Kim KB, Doh JH, Koo BK, Tahk SJ, Fearon WF. Relation of fractional flow reserve after drug-eluting stent implantation to one-year outcomes. Am J Cardiol. 2011;107:1763–7. doi: 10.1016/j.amjcard.2011.02.329. [DOI] [PubMed] [Google Scholar]
- Ito T, Tani T, Fujita H, Ohte N. Relationship between fractional flow reserve and residual plaque volume and clinical outcomes after optimal drug-eluting stent implantation: insight from intravascular ultrasound volumetric analysis. Int J Cardiol. 2014;176:399–404. doi: 10.1016/j.ijcard.2014.07.115. [DOI] [PubMed] [Google Scholar]
- Agarwal SK, Kasula S, Hacioglu Y, Ahmed Z, Uretsky BF, Hakeem A. Utilizing Post-Intervention Fractional Flow Reserve to Optimize Acute Results and the Relationship to Long-Term Outcomes. JACC Cardiovasc Interv. 2016;9:1022–31. doi: 10.1016/j.jcin.2016.01.046. [DOI] [PubMed] [Google Scholar]
- Piroth Z, Toth GG, Tonino PAL, Barbato E, Aghlmandi S, Curzen N, Rioufol G, Pijls NHJ, Fearon WF, Jüni P, De Bruyne. Prognostic Value of Fractional Flow Reserve Measured Immediately After Drug-Eluting Stent Implantation. Circ Cardiovasc Interv. 2017;10:e005233. doi: 10.1161/CIRCINTERVENTIONS.116.005233. [DOI] [PubMed] [Google Scholar]
- Li SJ, Ge Z, Kan J, Zhang JJ, Ye F, Kwan TW, Santoso T, Yang S, Sheiban I, Qian XS, Tian NL, Rab TS, Tao L, Chen SL. Cutoff Value and Long-Term Prediction of Clinical Events by FFR Measured Immediately After Implantation of a Drug-Eluting Stent in Patients With Coronary Artery Disease: 1- to 3-Year Results From the DKCRUSH VII Registry Study. JACC Cardiovasc Interv. 2017;10:986–95. doi: 10.1016/j.jcin.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Kasula S, Agarwal SK, Hacioglu Y, Pothineni NK, Bhatti S, Ahmed Z, Uretsky B, Hakeem A. Clinical and prognostic value of poststenting fractional flow reserve in acute coronary syndromes. Heart. 2016;102:1988–94. doi: 10.1136/heartjnl-2016-309422. [DOI] [PubMed] [Google Scholar]
- Lee JM, Hwang D, Choi KH, Rhee TM, Park J, Kim HY, Jung HW, Hwang JW, Lee HJ, Jang HJ, Kim SH, Song YB, Cho YK, Nam CW, Hahn JY, Shin ES, Kawase Y, Matsuo A, Tanaka N, Doh JH, Koo BK, Matsuo H. Prognostic Implications of Relative Increase and Final Fractional Flow Reserve in Patients With Stent Implantation. JACC Cardiovasc Interv. 2018;11:2099–109. doi: 10.1016/j.jcin.2018.07.031. [DOI] [PubMed] [Google Scholar]
- Azzalini L, Poletti E, Demir OM, Ancona MB, Mangieri A, Giannini F, Carlino M, Chieffo A, Montorfano M, Colombo A, Latib A. Impact of Post-Percutaneous Coronary Intervention Fractional Flow Reserve Measurement on Procedural Management and Clinical Outcomes: The REPEAT-FFR Study. J Invasive Cardiol. 2019;31:229–34. [PubMed] [Google Scholar]
- Jensen LO, Thayssen P, Thuesen L, Hansen HS, Lassen JF, Kelbaek H, Junker A, Hansen KN, Boetker HE, Krusell LR, Pedersen KE. Influence of a pressure gradient distal to implanted bare-metal stent on in-stent restenosis after percutaneous coronary intervention. Circulation. 2007;116:2802–8. doi: 10.1161/CIRCULATIONAHA.107.704064. [DOI] [PubMed] [Google Scholar]
- Shin D, Lee SH, Lee JM, Choi KH, Hwang D, Lee HJ, Jang HJ, Kim HK, Kwak JJ, Ha SJ, Song YB, Shin ES, Doh JH. Prognostic Implications of Post-Intervention Resting Pd/Pa and Fractional Flow Reserve in Patients With Stent Implantation. JACC Cardiovasc Interv. 2020;13:1920–33. doi: 10.1016/j.jcin.2020.05.042. [DOI] [PubMed] [Google Scholar]
- Biscaglia S, Tebaldi M, Brugaletta S, Cerrato E, Erriquez A, Passarini G, Ielasi A, Spitaleri G, Di Girolamo, Mezzapelle G, Geraci S, Manfrini M, Pavasini R, Barbato E, Campo G. Prognostic Value of QFR Measured Immediately After Successful Stent Implantation: The International Multicenter Prospective HAWKEYE Study. JACC Cardiovasc Interv. 2019;12:2079–88. doi: 10.1016/j.jcin.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Zhang R, Xu B, Dou K, Guan C, Zhao Y, Wang X, Zou T, Qiao Z, Xie L, Wang H, Yuan S, Song L, Tu S, Wang Y, Wijns W. Post-PCI outcomes predicted by pre-intervention simulation of residual quantitative flow ratio using augmented reality. Int J Cardiol. 2022;352:33–9. doi: 10.1016/j.ijcard.2022.01.054. [DOI] [PubMed] [Google Scholar]
- Patel MR, Jeremias A, Maehara A, Matsumura M, Zhang Z, Schneider J, Tang K, Talwar S, Marques K, Shammas NW, Gruberg L, Seto A, Samady H, Sharp ASP, Ali ZA, Mintz G, Davies J, Stone GW. 1-Year Outcomes of Blinded Physiological Assessment of Residual Ischemia After Successful PCI: DEFINE PCI Trial. JACC Cardiovasc Interv. 2022;15:52–61. doi: 10.1016/j.jcin.2021.09.042. [DOI] [PubMed] [Google Scholar]
- Fournier S, Ciccarelli G, Toth GG, Milkas A, Xaplanteris P, Tonino PAL, Fearon WF, Pijls NHJ, Barbato E, De Bruyne. Association of Improvement in Fractional Flow Reserve With Outcomes, Including Symptomatic Relief, After Percutaneous Coronary Intervention. JAMA Cardiol. 2019;4:370–4. doi: 10.1001/jamacardio.2019.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D, Lee JM, Lee HJ, Kim SH, Nam CW, Hahn JY, Shin ES, Matsuo A, Tanaka N, Matsuo H, Lee SY, Doh JH, Koo BK. Influence of target vessel on prognostic relevance of fractional flow reserve after coronary stenting. EuroIntervention. 2019;15:457–64. doi: 10.4244/EIJ-D-18-00913. [DOI] [PubMed] [Google Scholar]
- Hakeem A, Ghosh B, Shah K, Agarwal S, Kasula S, Hacioglu Y, Bhatti S, Ahmed Z, Uretsky B. Incremental Prognostic Value of Post-Intervention Pd/Pa in Patients Undergoing Ischemia-Driven Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2019;12:2002–14. doi: 10.1016/j.jcin.2019.07.026. [DOI] [PubMed] [Google Scholar]
- Kogame N, Takahashi K, Tomaniak M, Chichareon P, Modolo R, Chang CC, Komiyama H, Katagiri Y, Asano T, Stables R, Fath-Ordoubadi F, Walsh S, Sabaté M, Davies JE, Piek JJ, van Geuns, Reiber JHC, Banning AP, Escaned J, Farooq V, Serruys PW, Onuma Y. Clinical Implication of Quantitative Flow Ratio After Percutaneous Coronary Intervention for 3-Vessel Disease. JACC Cardiovasc Interv. 2019;12:2064–75. doi: 10.1016/j.jcin.2019.08.009. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Mejía-Rentería H, Escaned J, Doh JH, Lee JM, Hwang D, Yuasa S, Choi KH, Jang HJ, Jeon KH, Lee J, Nam CW, Shin ES, Koo BK. Prediction of functional results of percutaneous coronary interventions with virtual stenting and quantitative flow ratio. Catheter Cardiovasc Interv. 2022;100:1208–17. doi: 10.1002/ccd.30451. [DOI] [PubMed] [Google Scholar]
- Zimmermann FM, Ferrara A, Johnson NP, van Nunen, Escaned J, Albertsson P, Erbel R, Legrand V, Gwon HC, Remkes WS, Stella PR, van Schaardenburgh, Bech GJ, De Bruyne, Pijls NH. Deferral vs. performance of percutaneous coronary intervention of functionally non-significant coronary stenosis: 15-year follow-up of the DEFER trial. Eur Heart J. 2015;36:3182–8. doi: 10.1093/eurheartj/ehv452. [DOI] [PubMed] [Google Scholar]
- Ahn JM, Park DW, Shin ES, Koo BK, Nam CW, Doh JH, Kim JH, Chae IH, Yoon JH, Her SH, Seung KB, Chung WY, Yoo SY, Lee JB, Choi SW, Park K, Hong TJ, Lee SY, Han M, Lee PH, Kang SJ, Lee SW, Kim YH, Lee CW, Park SW, Park SJ IRIS-FFR Investigators. Fractional Flow Reserve and Cardiac Events in Coronary Artery Disease: Data From a Prospective IRIS-FFR Registry (Interventional Cardiology Research Incooperation Society Fractional Flow Reserve). Circulation. 2017;135:2241–51. doi: 10.1161/CIRCULATIONAHA.116.024433. [DOI] [PubMed] [Google Scholar]
- Tonino PA, De Bruyne, Pijls NH, Siebert U, Ikeno F, van’ t, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee, MacCarthy PA, Fearon WF FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- Xaplanteris P, Fournier S, Pijls NHJ, Fearon WF, Barbato E, Tonino PAL, Engstrøm T, Kääb S, Dambrink JH, Rioufol G, Toth GG, Piroth Z, Witt N, Fröbert O, Kala P, Linke A, Jagic N, Mates M, Mavromatis K, Samady H, Irimpen A, Oldroyd K, Campo G, Rothenbühler M, Jüni P, De Bruyne FAME 2 Investigators. Five-Year Outcomes with PCI Guided by Fractional Flow Reserve. N Engl J Med. 2018;379:250–9. doi: 10.1056/NEJMoa1803538. [DOI] [PubMed] [Google Scholar]
- Leone AM, Martin-Reyes R, Baptista SB, Amabile N, Raposo L, Franco Pelaez, Trani C, Cialdella P, Basile E, Zimbardo G, Burzotta F, Porto I, Aurigemma C, Rebuzzi AG, Faustino M, Niccoli G, Abreu PF, Slama MS, Spagnoli V, Telleria Arrieta, Amat Santos, de la, Lopez Palop, Crea F. The Multi-center Evaluation of the Accuracy of the Contrast MEdium INduced Pd/Pa RaTiO in Predicting FFR (MEMENTO-FFR) Study. EuroIntervention. 2016;12:708–15. doi: 10.4244/EIJV12I6A115. [DOI] [PubMed] [Google Scholar]
- Götberg M, Christiansen EH, Gudmundsdottir IJ, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Öhagen P, Olsson H, Omerovic E, Calais F, Lindroos P, Maeng M, Tödt T, Venetsanos D, James SK, Kåregren A, Nilsson M, Carlsson J, Hauer D, Jensen J, Karlsson AC, Panayi G, Erlinge D, Fröbert O iFR-SWEDEHEART Investigators. Instantaneous Wave-free Ratio versus Fractional Flow Reserve to Guide PCI. N Engl J Med. 2017;376:1813–23. doi: 10.1056/NEJMoa1616540. [DOI] [PubMed] [Google Scholar]
- Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle, Krackhardt F, Bojara W, Going O, Härle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Canas Silva, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria-Barrero E, Meuwissen M, Piek JJ, van Royen, Sezer M, Di Mario, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J. Use of the Instantaneous Wave-free Ratio or Fractional Flow Reserve in PCI. N Engl J Med. 2017;376:1824–34. doi: 10.1056/NEJMoa1700445. [DOI] [PubMed] [Google Scholar]
- Götberg M, Berntorp K, Rylance R, Christiansen EH, Yndigegn T, Gudmundsdottir IJ, Koul S, Sandhall L, Danielewicz M, Jakobsen L, Olsson SE, Olsson H, Omerovic E, Calais F, Lindroos P, Maeng M, Venetsanos D, James SK, Kåregren A, Carlsson J, Jensen J, Karlsson AC, Erlinge D, Fröbert O. 5-Year Outcomes of PCI Guided by Measurement of Instantaneous Wave-Free Ratio Versus Fractional Flow Reserve. J Am Coll Cardiol. 2022;79:965–74. doi: 10.1016/j.jacc.2021.12.030. [DOI] [PubMed] [Google Scholar]
- Van’t Veer, Pijls NHJ, Hennigan B, Watkins S, Ali ZA, De Bruyne, Zimmermann FM, van Nunen, Barbato E, Berry C, Oldroyd KG. Comparison of Different Diastolic Resting Indexes to iFR: Are They All Equal? J Am Coll Cardiol. 2017;70:3088–96. doi: 10.1016/j.jacc.2017.10.066. [DOI] [PubMed] [Google Scholar]
- Lee JM, Choi KH, Park J, Hwang D, Rhee TM, Kim J, Park J, Kim HY, Jung HW, Cho YK, Yoon HJ, Song YB, Hahn JY, Nam CW, Shin ES, Doh JH, Hur SH, Koo BK. Physiological and Clinical Assessment of Resting Physiological Indexes. Circulation. 2019;139:889–900. doi: 10.1161/CIRCULATIONAHA.118.037021. [DOI] [PubMed] [Google Scholar]
- Kim KH, Doh JH, Koo BK, Min JK, Erglis A, Yang HM, Park KW, Lee HY, Kang HJ, Kim YJ, Lee SY, Kim HS. A novel noninvasive technology for treatment planning using virtual coronary stenting and computed tomography-derived computed fractional flow reserve. JACC Cardiovasc Interv. 2014;7:72–8. doi: 10.1016/j.jcin.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Driessen RS, Danad I, Stuijfzand WJ, Raijmakers PG, Schumacher SP, van Diemen, Leipsic JA, Knuuti J, Underwood SR, van de, van Rossum, Taylor CA, Knaapen P. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J Am Coll Cardiol. 2019;73:161–73. doi: 10.1016/j.jacc.2018.10.056. [DOI] [PubMed] [Google Scholar]
- Gosling RC, Morris PD, Silva Soto, Lawford PV, Hose DR, Gunn JP. Virtual Coronary Intervention: A Treatment Planning Tool Based Upon the Angiogram. JACC Cardiovasc Imaging. 2019;12:865–72. doi: 10.1016/j.jcmg.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papafaklis MI, Muramatsu T, Ishibashi Y, Lakkas LS, Nakatani S, Bourantas CV, Ligthart J, Onuma Y, Echavarria-Pinto M, Tsirka G, Kotsia A, Nikas DN, Mogabgab O, van Geuns, Naka KK, Fotiadis DI, Brilakis ES, Garcia-Garcia HM, Escaned J, Zijlstra F, Michalis LK, Serruys PW. Fast virtual functional assessment of intermediate coronary lesions using routine angiographic data and blood flow simulation in humans: comparison with pressure wire - fractional flow reserve. EuroIntervention. 2014;10:574–83. doi: 10.4244/EIJY14M07_01. [DOI] [PubMed] [Google Scholar]
- Li J, Gong Y, Wang W, Yang Q, Liu B, Lu Y, Xu Y, Huo Y, Yi T, Liu J, Li Y, Xu S, Zhao L, Ali ZA, Huo Y. Accuracy of computational pressure-fluid dynamics applied to coronary angiography to derive fractional flow reserve: FLASH FFR. Cardiovasc Res. 2020;116:1349–56. doi: 10.1093/cvr/cvz289. [DOI] [PubMed] [Google Scholar]
- Xu B, Tu S, Qiao S, Qu X, Chen Y, Yang J, Guo L, Sun Z, Li Z, Tian F, Fang W, Chen J, Li W, Guan C, Holm NR, Wijns W, Hu S. Diagnostic Accuracy of Angiography-Based Quantitative Flow Ratio Measurements for Online Assessment of Coronary Stenosis. J Am Coll Cardiol. 2017;70:3077–87. doi: 10.1016/j.jacc.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Xu B, Tu S, Song L, Jin Z, Yu B, Fu G, Zhou Y, Wang J, Chen Y, Pu J, Chen L, Qu X, Yang J, Liu X, Guo L, Shen C, Zhang Y, Zhang Q, Pan H, Fu X, Liu J, Zhao Y, Escaned J, Wang Y, Fearon WF, Dou K, Kirtane AJ, Wu Y, Serruys PW, Yang W, Wijns W, Guan C, Leon MB, Qiao S, Stone GW FAVOR III China study group. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): a multicentre, randomised, sham-controlled trial. Lancet. 2021;398:2149–59. doi: 10.1016/S0140-6736(21)02248-0. [DOI] [PubMed] [Google Scholar]
- Ando H, Takashima H, Suzuki A, Sakurai S, Kumagai S, Kurita A, Waseda K, Amano T. Impact of lesion characteristics on the prediction of optimal poststent fractional flow reserve. Am Heart J. 2016;182:119–24. doi: 10.1016/j.ahj.2016.09.015. [DOI] [PubMed] [Google Scholar]
- Nijjer SS, Sen S, Petraco R, Mayet J, Francis DP, Davies JE. The Instantaneous wave-Free Ratio (iFR) pullback: a novel innovation using baseline physiology to optimise coronary angioplasty in tandem lesions. Cardiovasc Revasc Med. 2015;16:1 67–71. doi: 10.1016/j.carrev.2015.01.006. [DOI] [PubMed] [Google Scholar]
- Collet C, Sonck J, Vandeloo B, Mizukami T, Roosens B, Lochy S, Argacha JF, Schoors D, Colaiori I, Di Gioia, Kodeboina M, Suzuki H, Van ‘t, Bartunek J, Barbato E, Cosyns B, De Bruyne. Measurement of Hyperemic Pullback Pressure Gradients to Characterize Patterns of Coronary Atherosclerosis. J Am Coll Cardiol. 2019;74:1772–84. doi: 10.1016/j.jacc.2019.07.072. [DOI] [PubMed] [Google Scholar]
- Matsuo A, Shimoo S, Takamatsu K, Tsuji Y, Kyodo A, Mera K, Koide M, Isodono K, Tsubakimoto Y, Sakatani T, Inoue K, Fujita H. Visualization of the improvement of myocardial perfusion after coronary intervention using motorized fractional flow reserve pullback curve. Cardiovasc Interv Ther. 2018;33:99–108. doi: 10.1007/s12928-016-0448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashioka D, Shiono Y, Kubo T, Kitabata H, Nishi T, Terada K, Emori H, Takahata M, Wada T, Shimamura K, Matsuo Y, Ino Y, Tanaka A, Hozumi T, Akasaka T. The inter-study reproducibility of instantaneous wave-free ratio and angiography coregistration. J Cardiol. 2020;75:507–12. doi: 10.1016/j.jjcc.2019.09.016. [DOI] [PubMed] [Google Scholar]
- Sonck J, Mizukami T, Johnson NP, Nagumo S, Gallinoro E, Candreva A, Mileva N, Munhoz D, Shinke T, Svanerud J, Barbato E, De Bruyne, Collet C. Development, validation, and reproducibility of the pullback pressure gradient (PPG) derived from manual fractional flow reserve pullbacks. Catheter Cardiovasc Interv. 2022;99:1518–25. doi: 10.1002/ccd.30064. [DOI] [PubMed] [Google Scholar]
- Lee SH, Shin D, Lee JM, Lefieux A, Molony D, Choi KH, Hwang D, Lee HJ, Jang HJ, Kim HK, Ha SJ, Kwak JJ, Park TK, Yang JH, Song YB, Hahn JY, Doh JH, Shin ES, Nam CW, Koo BK, Choi SH, Gwon HC. Automated Algorithm Using Pre-Intervention Fractional Flow Reserve Pullback Curve to Predict Post-Intervention Physiological Results. JACC Cardiovasc Interv. 2020;13:2670–84. doi: 10.1016/j.jcin.2020.06.062. [DOI] [PubMed] [Google Scholar]
- Shiono Y, Kubo T, Honda K, Katayama Y, Aoki H, Satogami K, Kashiyama K, Taruya A, Nishiguchi T, Kuroi A, Orii M, Kameyama T, Yamano T, Yamaguchi T, Matsuo Y, Ino Y, Tanaka A, Hozumi T, Nishimura Y, Okamura Y, Akasaka T. Impact of functional focal versus diffuse coronary artery disease on bypass graft patency. Int J Cardiol. 2016;222:16–21. doi: 10.1016/j.ijcard.2016.07.052. [DOI] [PubMed] [Google Scholar]
- van Beek, van Steenbergen, Vervaat FE, Mulders BCJH, van Straten, van Nunen, Wijnbergen IF. Single center experience in the treatment of hemodynamically significant diffuse coronary artery disease of the left anterior descending. Int J Cardiol. 2022;352:40–4. doi: 10.1016/j.ijcard.2022.01.048. [DOI] [PubMed] [Google Scholar]
- Biscaglia S, Uretsky B, Barbato E, Collet C, Onuma Y, Jeremias A, Tebaldi M, Hakeem A, Kogame N, Sonck J, Escaned J, Serruys PW, Stone GW, Campo G. Invasive Coronary Physiology After Stent Implantation: Another Step Toward Precision Medicine. JACC Cardiovasc Interv. 2021;14:237–46. doi: 10.1016/j.jcin.2020.10.055. [DOI] [PubMed] [Google Scholar]
- Candreva A, Mizukami T, Sonck J, Munhoz D, Nagumo S, Di Gioia, Gallinoro E, Mileva N, Bartunek J, Wyffels E, Barbato E, De Bruyne, Perera D, Collet C. Hyperemic hemodynamic characteristics of serial coronary lesions assessed by pullback pressure gradients. Catheter Cardiovasc Interv. 2021;98:E647–54. doi: 10.1002/ccd.29868. [DOI] [PubMed] [Google Scholar]
- Warisawa T, Howard JP, Cook CM, Ahmad Y, Doi S, Nakayama M, Goto S, Yakuta Y, Karube K, Seike F, Uetani T, Murai T, Kikuta Y, Shiono Y, Kawase Y, Shun-Shin MJ, Kaihara T, Higuma T, Ishibashi Y, Matsuda H, Nishina H, Matsuo H, Escaned J, Akashi YJ, Davies JE. Inter-observer differences in interpretation of coronary pressure-wire pullback data by non-expert interventional cardiologists. Cardiovasc Interv Ther. 2021;36:289–97. doi: 10.1007/s12928-020-00673-3. [DOI] [PubMed] [Google Scholar]
- Matsuo A, Kasahara T, Ariyoshi M, Irie D, Isodono K, Tsubakimoto Y, Sakatani T, Inoue K, Fujita H. Utility of angiography-physiology coregistration maps during percutaneous coronary intervention in clinical practice. Cardiovasc Interv Ther. 2021;36:208–18. doi: 10.1007/s12928-020-00668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarsini R, Fezzi S, Pesarini G, Del Sole, Venturi G, Mammone C, Marcoli M, Gambaro A, Tavella D, Pighi M, Ribichini F. Impact of physiologically diffuse versus focal pattern of coronary disease on quantitative flow reserve diagnostic accuracy. Catheter Cardiovasc Interv. 2022;99:736–45. doi: 10.1002/ccd.30007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Chu J, Hou H, Lai Y, Tu S, Chen F, Yao Y, Ye Z, Gao Y, Mao Y, Zhuang S, Liu X. Clinical implication of QFR in patients with ST-segment elevation myocardial infarction after drug-eluting stent implantation. Int J Cardiovasc Imaging. 2021;37:755–66. doi: 10.1007/s10554-020-02068-0. [DOI] [PubMed] [Google Scholar]
- Biscaglia S, Uretsky BF, Tebaldi M, Erriquez A, Brugaletta S, Cerrato E, Quadri G, Spitaleri G, Colaiori I, Di Girolamo, Scoccia A, Zucchetti O, D’Aniello E, Manfrini M, Pavasini R, Barbato E, Campo G. Angio-Based Fractional Flow Reserve, Functional Pattern of Coronary Artery Disease, and Prediction of Percutaneous Coronary Intervention Result: a Proof-of-Concept Study. Cardiovasc Drugs Ther. 2022;36:645–53. doi: 10.1007/s10557-021-07162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Dai N, Lee SH, Choi KH, Lefieux A, Molony D, Hwang D, Kim HK, Jeon KH, Lee HJ, Jang HJ, Ha SJ, Park TK, Yang JH, Song YB, Hahn JY, Choi SH, Doh JH, Shin ES, Nam CW, Koo BK, Gwon HC, Ge J, Lee JM. Physiological Distribution and Local Severity of Coronary Artery Disease and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc Interv. 2021;14:1771–85. doi: 10.1016/j.jcin.2021.06.013. [DOI] [PubMed] [Google Scholar]
- Kőszegi Z, Berta B, Tóth GG, Tar B, Üveges Á, Ágoston A, Szücs A, Szabó GT, Barta J, Szük T, Czuriga D, Komócsi A, Ruzsa Z. Anatomical Assessment vs. Pullback REsting full-cycle rAtio (RFR) Measurement for Evaluation of Focal and Diffuse CoronarY Disease: Rationale and Design of the “READY Register”. Front Cardiovasc Med. 2021 Dec 8;:784220. doi: 10.3389/fcvm.2021.784220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori H, Kawase Y, Mizukami T, Tanigaki T, Hirata T, Kikuchi J, Ota H, Sobue Y, Miyake T, Kawamura I, Okubo M, Kamiya H, Hirakawa A, Kawasaki M, Nakagawa M, Tsuchiya K, Suzuki Y, Ito T, Terashima M, Kondo T, Suzuki T, Escaned J, Matsuo H. Comparisons of Nonhyperemic Pressure Ratios: Predicting Functional Results of Coronary Revascularization Using Longitudinal Vessel Interrogation. JACC Cardiovasc Interv. 2020;13:2688–98. doi: 10.1016/j.jcin.2020.06.060. [DOI] [PubMed] [Google Scholar]
- Collet C, Collison D, Mizukami T, McCartney P, Sonck J, Ford T, Munhoz D, Berry C, De Bruyne, Oldroyd K. Differential Improvement in Angina and Health-Related Quality of Life After PCI in Focal and Diffuse Coronary Artery Disease. JACC Cardiovasc Interv. 2022;15:2506–18. doi: 10.1016/j.jcin.2022.09.048. [DOI] [PubMed] [Google Scholar]
- Nijjer SS, Sen S, Petraco R, Escaned J, Echavarria-Pinto M, Broyd C, Al-Lamee R, Foin N, Foale RA, Malik IS, Mikhail GW, Sethi AS, Al-Bustami M, Kaprielian RR, Khan MA, Baker CS, Bellamy MF, Hughes AD, Mayet J, Francis DP, Di Mario, Davies JE. Pre-angioplasty instantaneous wave-free ratio pullback provides virtual intervention and predicts hemodynamic outcome for serial lesions and diffuse coronary artery disease. JACC Cardiovasc Interv. 2014;7:1386–96. doi: 10.1016/j.jcin.2014.06.015. [DOI] [PubMed] [Google Scholar]
- Modi BN, Sankaran S, Kim HJ, Ellis H, Rogers C, Taylor CA, Rajani R, Perera D. Predicting the Physiological Effect of Revascularization in Serially Diseased Coronary Arteries. Circ Cardiovasc Interv. 2019;12:e007577. doi: 10.1161/CIRCINTERVENTIONS.118.007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonck J, Nagumo S, Norgaard BL, Otake H, Ko B, Zhang J, Mizukami T, Maeng M, Andreini D, Takahashi Y, Jensen JM, Ihdayhid A, Heggermont W, Barbato E, Mileva N, Munhoz D, Bartunek J, Updegrove A, Collinsworth A, Penicka M, Van Hoe, Leipsic J, Koo BK, De Bruyne, Collet C. Clinical Validation of a Virtual Planner for Coronary Interventions Based on Coronary CT Angiography. JACC Cardiovasc Imaging. 2022;15:1242–55. doi: 10.1016/j.jcmg.2022.02.003. [DOI] [PubMed] [Google Scholar]
- van Diemen, de Winter, Schumacher SP, Bom MJ, Driessen RS, Everaars H, Jukema RA, Somsen YB, Popelkova L, van de, van Rossum, van de, de Haan, Marques KM, Lemkes JS, Appelman Y, Nap A, Verouden NJ, Opolski MP, Danad I, Knaapen P. Residual Quantitative Flow Ratio to Estimate Post-Percutaneous Coronary Intervention Fractional Flow Reserve. J Interv Cardiol. 2021;2021:4339451. doi: 10.1155/2021/4339451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaniak M, Neleman T, Ziedses des, Masdjedi K, van Zandvoort, Kochman J, den Dekker, Wilschut JM, Diletti R, Kardys I, Zijlstra F, Van Mieghem, Daemen J. Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting. J Clin Med. 2022;11:1397. doi: 10.3390/jcm11051397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NP, Tóth GG, Lai D, Zhu H, Açar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino, Domínguez-Franco AJ, Dupouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jiménez-Navarro MF, Katritsis DG, Kocaman SA, Koo BK, López-Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodés-Cabau J, Sedlis SP, Takeishi Y, Tonino PA, Van Belle, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne, Gould KL. Prognostic Value of Fractional Flow Reserve: Linking Physiologic Severity to Clinical Outcomes. J Am Coll Cardiol. 2014;64:1641–54. doi: 10.1016/j.jacc.2014.07.973. [DOI] [PubMed] [Google Scholar]
- Rimac G, Fearon WF, De Bruyne, Ikeno F, Matsuo H, Piroth Z, Costerousse O, Bertrand OF. Clinical value of post-percutaneous coronary intervention fractional flow reserve value: A systematic review and meta-analysis. Am Heart J. 2017;183:1–9. doi: 10.1016/j.ahj.2016.10.005. [DOI] [PubMed] [Google Scholar]
- Hwang D, Koo BK, Zhang J, Park J, Yang S, Kim M, Yun JP, Lee JM, Nam CW, Shin ES, Doh JH, Chen SL, Kakuta T, Toth GG, Piroth Z, Johnson NP, Pijls NHJ, Hakeem A, Uretsky BF, Hokama Y, Tanaka N, Lim HS, Ito T, Matsuo A, Azzalini L, Leesar MA, Neleman T, van Mieghem, Diletti R, Daemen J, Collison D, Collet C, De Bruyne. Prognostic Implications of Fractional Flow Reserve After Coronary Stenting: A Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5:e2232842. doi: 10.1001/jamanetworkopen.2022.32842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uretsky BF, Agarwal SK, Vallurupalli S, Al-Hawwas M, Hasan R, Miller K, Hakeem A. Prospective Evaluation of the Strategy of Functionally Optimized Coronary Intervention. J Am Heart Assoc. 2020;9:e015073. doi: 10.1161/JAHA.119.015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi T, Piroth Z, De Bruyne, Jagic N, Möbius-Winkler S, Kobayashi Y, Derimay F, Fournier S, Barbato E, Tonino P, Jüni P, Pijls NHJ, Fearon WF. Fractional Flow Reserve and Quality-of-Life Improvement After Percutaneous Coronary Intervention in Patients With Stable Coronary Artery Disease. Circulation. 2018;138:1797–804. doi: 10.1161/CIRCULATIONAHA.118.035263. [DOI] [PubMed] [Google Scholar]
- van Bommel, Masdjedi K, Diletti R, Lemmert ME, van Zandvoort, Wilschut J, Zijlstra F, de Jaegere, Daemen J, van Mieghem. Routine Fractional Flow Reserve Measurement After Percutaneous Coronary Intervention. Circ Cardiovasc Interv. 2019;12:e007428. doi: 10.1161/CIRCINTERVENTIONS.118.007428. [DOI] [PubMed] [Google Scholar]
- Härle T, Luz M, Meyer S, Kronberg K, Nickau B, Escaned J, Davies J, Elsässer A. Effect of Coronary Anatomy and Hydrostatic Pressure on Intracoronary Indices of Stenosis Severity. JACC Cardiovasc Interv. 2017;10:764–73. doi: 10.1016/j.jcin.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Fournier S, Keulards DCJ, van ‘t, Colaiori I, Di Gioia, Zimmermann FM, Mizukami T, Nagumo S, Kodeboina M, El Farissi, Zelis JM, Sonck J, Collet C, Pijls NHJ, De Bruyne. Normal values of thermodilution-derived absolute coronary blood flow and microvascular resistance in humans. EuroIntervention. 2021;17:e309–16. doi: 10.4244/EIJ-D-20-00684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Räber L, Mintz GS, Koskinas KC, Johnson TW, Holm NR, Onuma Y, Radu MD, Joner M, Yu B, Jia H, Meneveau N, de la, Escaned J, Hill J, Prati F, Colombo A, di Mario, Regar E, Capodanno D, Wijns W, Byrne RA, Guagliumi G ESC Scientific Document Group. Clinical use of intracoronary imaging. Part 1: guidance and optimization of coronary interventions. An expert consensus document of the European Association of Percutaneous Cardiovascular Interventions. Eur Heart J. 2018;39:3281–300. doi: 10.1093/eurheartj/ehy285. [DOI] [PubMed] [Google Scholar]
- Burzotta F, Leone AM, Aurigemma C, Zambrano A, Zimbardo G, Arioti M, Vergallo R, De Maria, Cerracchio E, Romagnoli E, Trani C, Crea F. Fractional Flow Reserve or Optical Coherence Tomography to Guide Management of Angiographically Intermediate Coronary Stenosis: A Single-Center Trial. JACC Cardiovasc Interv. 2020;13:49–58. doi: 10.1016/j.jcin.2019.09.034. [DOI] [PubMed] [Google Scholar]
- Ferraro RA, van Rosendael, Lu Y, Andreini D, Al-Mallah MH, Cademartiri F, Chinnaiyan K, Chow BJW, Conte E, Cury RC, Feuchtner G, de Araújo, Hadamitzky M, Kim YJ, Leipsic J, Maffei E, Marques H, Plank F, Pontone G, Raff GL, Villines TC, Lee SE, Al’Aref SJ, Baskaran L, Cho I, Danad I, Gransar H, Budoff MJ, Samady H, Stone PH, Virmani R, Narula J, Berman DS, Chang HJ, Bax JJ, Min JK, Shaw LJ, Lin FY. Non-obstructive high-risk plaques increase the risk of future culprit lesions comparable to obstructive plaques without high-risk features: the ICONIC study. Eur Heart J Cardiovasc Imaging. 2020;21:973–80. doi: 10.1093/ehjci/jeaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meneveau N, Souteyrand G, Motreff P, Caussin C, Amabile N, Ohlmann P, Morel O, Lefrançois Y, Descotes-Genon V, Silvain J, Braik N, Chopard R, Chatot M, Ecarnot F, Tauzin H, Van Belle, Belle L, Schiele F. Optical Coherence Tomography to Optimize Results of Percutaneous Coronary Intervention in Patients with Non-ST-Elevation Acute Coronary Syndrome: Results of the Multicenter, Randomized DOCTORS Study (Does Optical Coherence Tomography Optimize Results of Stenting). Circulation. 2016;134:906–17. doi: 10.1161/CIRCULATIONAHA.116.024393. [DOI] [PubMed] [Google Scholar]
- Tian NL, Gami SK, Ye F, Zhang JJ, Liu ZZ, Lin S, Ge Z, Shan SJ, You W, Chen L, Zhang YJ, Mintz G, Chen SL. Angiographic and clinical comparisons of intravascular ultra-sound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10:1409–17. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Mintz GS, Ahn CM, Kim JS, Kim BK, Ko YG, Kang TS, Kang WC, Kim YH, Hur SH, Hong BK, Choi D, Kwon H, Jang Y, Hong MK IVUS-XPL Investigators. Effect of Intravascular Ultrasound-Guided Drug-Eluting Stent Implantation: 5-Year Follow-Up of the IVUS-XPL Randomized Trial. JACC Cardiovasc Interv. 2020;13:62–71. doi: 10.1016/j.jcin.2019.09.033. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Kim D, Kim BK, Ahn CM, Kim JS, Ko YG, Choi D, Hong MK, Jang Y. Acute and one-year clinical outcomes of pre-stenting intravascular ultrasound: a patient-level meta-analysis of randomised clinical trials. EuroIntervention. 2021;17:202–11. doi: 10.4244/EIJ-D-20-00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong SJ, Zhang JJ, Mintz GS, Ahn CM, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Kan J, Pan T, Gao X, Ge Z, Chen SL, Hong MK. Improved 3-Year Cardiac Survival After IVUS-Guided Long DES Implantation: A Patient-Level Analysis From 2 Randomized Trials. JACC Cardiovasc Interv. 2022;15:208–16. doi: 10.1016/j.jcin.2021.10.020. [DOI] [PubMed] [Google Scholar]
- Shin DH, Hong SJ, Mintz GS, Kim JS, Kim BK, Ko YG, Choi D, Jang Y, Hong MK. Effects of Intravascular Ultrasound-Guided Versus Angiography-Guided New-Generation Drug-Eluting Stent Implantation: Meta-Analysis With Individual Patient-Level Data From 2,345 Randomized Patients. JACC Cardiovasc Interv. 2016;9:2232–39. doi: 10.1016/j.jcin.2016.07.021. [DOI] [PubMed] [Google Scholar]
- Wijns W, Shite J, Jones MR, Lee SW, Price MJ, Fabbiocchi F, Barbato E, Akasaka T, Bezerra H, Holmes D. Optical coherence tomography imaging during percutaneous coronary intervention impacts physician decision-making: ILUMIEN I study. Eur Heart J. 2015;36:3346–55. doi: 10.1093/eurheartj/ehv367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neleman T, van Zandvoort, Tovar Forero, Masdjedi K, Ligthart JMR, Witberg KT, Groenland FTW, Cummins P, Lenzen MJ, Boersma E, Nuis RJ, den Dekker, Diletti R, Wilschut J, Zijlstra F, Van Mieghem, Daemen J. FFR-Guided PCI Optimization Directed by High-Definition IVUS Versus Standard of Care: The FFR REACT Trial. JACC Cardiovasc Interv. 2022;15:1595–607. doi: 10.1016/j.jcin.2022.06.018. [DOI] [PubMed] [Google Scholar]
- Kedhi E, Berta B, Roleder T, Hermanides RS, Fabris E, IJsselmuiden AJJ, Kauer F, Alfonso F, von Birgelen, Escaned J, Camaro C, Kennedy MW, Pereira B, Magro M, Nef H, Reith S, Al Nooryani, Rivero F, Malinowski K, De Luca, Garcia Garcia, Granada JF, Wojakowski W. Thin-cap fibroatheroma predicts clinical events in diabetic patients with normal fractional flow reserve: the COMBINE OCT-FFR trial. Eur Heart J. 2021;42:4671–79. doi: 10.1093/eurheartj/ehab433. [DOI] [PubMed] [Google Scholar]
- Van Belle, Gil R, Klauss V, Balghith M, Meuwissen M, Clerc J, Witzenbichler B, Cercek M, Vlachojannis M, Lang I, Commeau P, Vincent F, Testa L, Wasek W, Debry N, Kische S, Gabrielli G, Sardella G. Impact of Routine Invasive Physiology at Time of Angiography in Patients With Multivessel Coronary Artery Disease on Reclassification of Revascularization Strategy: Results From the DEFINE REAL Study. JACC Cardiovasc Interv. 2018;11:354–65. doi: 10.1016/j.jcin.2017.11.030. [DOI] [PubMed] [Google Scholar]
- Escaned J, Collet C, Ryan N, De Maria, Walsh S, Sabate M, Davies J, Lesiak M, Moreno R, Cruz-Gonzalez I, Hoole SP, Ej West, Piek JJ, Zaman A, Fath-Ordoubadi F, Stables RH, Appleby C, van Mieghem, van Geuns, Uren N, Zueco J, Buszman P, Iniguez A, Goicolea J, Hildick-Smith D, Ochala A, Dudek D, Hanratty C, Cavalcante R, Kappetein AP, Taggart DP, van Es, Morel MA, de Vries, Onuma Y, Farooq V, Serruys PW, Banning AP. Clinical outcomes of state-of-the-art percutaneous coronary revascularization in patients with de novo three vessel disease: 1-year results of the SYNTAX II study. Eur Heart J. 2017;38:3124–34. doi: 10.1093/eurheartj/ehx512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van’t Veer, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee, MacCarthy PA, De Bruyne FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–84. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Banning AP, Serruys P, De Maria, Ryan N, Walsh S, Gonzalo N, Jan van, Onuma Y, Sabate M, Davies J, Lesiak M, Moreno R, Cruz-Gonzalez I, Hoole SP, Piek JJ, Appleby C, Fath-Ordoubadi F, Zaman A, Van Mieghem, Uren N, Zueco J, Buszman P, Iniguez A, Goicolea J, Hildick-Smith D, Ochala A, Dudek D, de Vries, Taggart D, Farooq V, Spitzer E, Tijssen J, Escaned J. Five-year outcomes after state-of-the-art percutaneous coronary revascularization in patients with de novo three-vessel disease: final results of the SYNTAX II study. Eur Heart J. 2022;43:1307–16. doi: 10.1093/eurheartj/ehab703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon WF, Zimmermann FM, De Bruyne B, Piroth Z, van Straten AHM, Szekely L, Davidavičius G, Kalinauskas G, Mansour S, Kharbanda R, Östlund-Papadogeorgos N, Aminian A, Oldroyd KG, Al-Attar N, Jagic N, Dambrink JE, Kala P, Angerås O, MacCarthy P, Wendler O, Casselman F, Witt N, Mavromatis K, Miner SES, Sarma J, Engstrøm T, Christiansen EH, Tonino PAL, Reardon MJ, Lu D, Ding VY, Kobayashi Y, Hlatky MA, Mahaffey KW, Desai M, Woo YJ, Yeung AC, Pijls NHJ FAME 3 Investigators. Fractional Flow Reserve-Guided PCI as Compared with Coronary Bypass Surgery. N Engl J Med. 2022;386(2):128–37. doi: 10.1056/NEJMoa2112299. [DOI] [PubMed] [Google Scholar]
- Thuesen AL, Riber LP, Veien KT, Christiansen EH, Jensen SE, Modrau I, Andreasen JJ, Junker A, Mortensen PE, Jensen LO. Fractional Flow Reserve Versus Angiographically-Guided Coronary Artery Bypass Grafting. J Am Coll Cardiol. 2018;72:2732–43. doi: 10.1016/j.jacc.2018.09.043. [DOI] [PubMed] [Google Scholar]
- Toth GG, De Bruyne, Kala P, Ribichini FL, Casselman F, Ramos R, Piroth Z, Fournier S, Piccoli A, Van Mieghem, Penicka M, Mates M, Nemec P, Van Praet, Stockman B, Degriek I, Barbato E. Graft patency after FFR-guided versus angiography-guided coronary artery bypass grafting: the GRAFFITI trial. EuroIntervention. 2019;15:e999–e1005. doi: 10.4244/EIJ-D-19-00463. [DOI] [PubMed] [Google Scholar]
- Glineur D, Grau JB, Etienne PY, Benedetto U, Fortier JH, Papadatos S, Laruelle C, Pieters D, El Khoury, Blouard P, Timmermans P, Ruel M, Chong AY, So D, Chan V, Rubens F, Gaudino MF. Impact of preoperative fractional flow reserve on arterial bypass graft anastomotic function: the IMPAG trial. Eur Heart J. 2019;40:2421–8. doi: 10.1093/eurheartj/ehz329. [DOI] [PubMed] [Google Scholar]
- Toth GG, Mizukami T, Collet C, Thuesen AL, Casselman F, Jensen LO, De Bruyne, Barbato E. Impact of Functional Severity of Coronary Artery Disease on Arterial Versus Venous Graft Patency. JACC Cardiovasc Interv. 2022;15:1098–100. doi: 10.1016/j.jcin.2022.03.026. [DOI] [PubMed] [Google Scholar]
- Hara H, Gao C, Kogame N, Ono M, Kawashima H, Wang R, Morel MA, O’Leary N, Sharif F, Möllmann H, Reiber JHC, Sabaté M, Zaman A, Wijns W, Onuma Y, Serruys PW. A randomised controlled trial of the sirolimus-eluting biodegradable polymer ultra-thin Supraflex stent versus the everolimus-eluting biodegradable polymer SYNERGY stent for three-vessel coronary artery disease: rationale and design of the Multivessel TALENT trial. EuroIntervention. 2020;16:e997–e1004. doi: 10.4244/EIJ-D-20-00772. [DOI] [PubMed] [Google Scholar]
- Collet C, Miyazaki Y, Ryan N, Asano T, Tenekecioglu E, Sonck J, Andreini D, Sabate M, Brugaletta S, Stables RH, Bartorelli A, de Winter, Katagiri Y, Chichareon P, De Maria, Suwannasom P, Cavalcante R, Jonker H, Morel MA, Cosyns B, Kappetein AP, Taggart DT, Farooq V, Escaned J, Banning A, Onuma Y, Serruys PW. Fractional Flow Reserve Derived From Computed Tomographic Angiography in Patients With Multivessel CAD. J Am Coll Cardiol. 2018;71:2756–69. doi: 10.1016/j.jacc.2018.02.053. [DOI] [PubMed] [Google Scholar]
- Collet C, Onuma Y, Andreini D, Sonck J, Pompilio G, Mushtaq S, La Meir, Miyazaki Y, de Mey, Gaemperli O, Ouda A, Maureira JP, Mandry D, Camenzind E, Macron L, Doenst T, Teichgräber U, Sigusch H, Asano T, Katagiri Y, Morel MA, Lindeboom W, Pontone G, Lüscher TF, Bartorelli AL, Serruys PW. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur Heart J. 2018;39:3689–98. doi: 10.1093/eurheartj/ehy581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini D, Modolo R, Katagiri Y, Mushtaq S, Sonck J, Collet C, De Martini, Roberto M, Tanaka K, Miyazaki Y, Czapla J, Schoors D, Plass A, Maisano F, Kaufmann P, Orry X, Metzdorf PA, Folliguet T, Färber G, Diamantis I, Schönweiß M, Bonalumi G, Guglielmo M, Ferrari C, Olivares P, Cavallotti L, Leal I, Lindeboom W, Onuma Y, Serruys PW, Bartorelli AL SYNTAX III REVOLUTION Investigators. Impact of Fractional Flow Reserve Derived From Coronary Computed Tomography Angiography on Heart Team Treatment Decision-Making in Patients With Multivessel Coronary Artery Disease: Insights From the SYNTAX III REVOLUTION Trial. Circ Cardiovasc Interv. 2019;12:e007607. doi: 10.1161/CIRCINTERVENTIONS.118.007607. [DOI] [PubMed] [Google Scholar]
- Pijls NH, De Bruyne, Bech GJ, Liistro F, Heyndrickx GR, Bonnier HJ, Koolen JJ. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery: validation in humans. Circulation. 2000;102:2371–7. doi: 10.1161/01.cir.102.19.2371. [DOI] [PubMed] [Google Scholar]
- Warisawa T, Howard JP, Kawase Y, Tanigaki T, Omori H, Cook CM, Ahmad Y, Francis DP, Akashi YJ, Matsuo H, Davies JE. Difference in functional assessment of individual stenosis severity in serial coronary lesions between resting and hyperemic pressure-wire pullback: Insights from the GIFT registry. Int J Cardiol. 2020;312:10–5. doi: 10.1016/j.ijcard.2020.05.001. [DOI] [PubMed] [Google Scholar]
- Kim HL, Koo BK, Nam CW, Doh JH, Kim JH, Yang HM, Park KW, Lee HY, Kang HJ, Cho YS, Youn TJ, Kim SH, Chae IH, Choi DJ, Kim HS, Oh BH, Park YB. Clinical and physiological outcomes of fractional flow reserve-guided percutaneous coronary intervention in patients with serial stenoses within one coronary artery. JACC Cardiovasc Interv. 2012;5:1013–8. doi: 10.1016/j.jcin.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Ahn JM, Nakayoshi T, Hashikata T, Kashiyama K, Arashi H, Kweon J, Van’t Veer, Lyons J, Fearon WF. Impact of Serial Coronary Stenoses on Various Coronary Physiologic Indices. Circ Cardiovasc Interv. 2022;15:e012134. doi: 10.1161/CIRCINTERVENTIONS.122.012134. [DOI] [PubMed] [Google Scholar]
- Kikuta Y, Cook CM, Sharp ASP, Salinas P, Kawase Y, Shiono Y, Giavarini A, Nakayama M, De Rosa, Sen S, Nijjer SS, Al-Lamee R, Petraco R, Malik IS, Mikhail GW, Kaprielian RR, Wijntjens GWM, Mori S, Hagikura A, Mates M, Mizuno A, Hellig F, Lee K, Janssens L, Horie K, Mohdnazri S, Herrera R, Krackhardt F, Yamawaki M, Davies J, Takebayashi H, Keeble T, Haruta S, Ribichini F, Indolfi C, Mayet J, Francis DP, Piek JJ, Di Mario, Escaned J, Matsuo H, Davies JE. Pre-Angioplasty Instantaneous Wave-Free Ratio Pullback Predicts Hemodynamic Outcome In Humans With Coronary Artery Disease: Primary Results of the International Multicenter iFR GRADIENT Registry. JACC Cardiovasc Interv. 2018;11:757–67. doi: 10.1016/j.jcin.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Rubimbura V, Guillon B, Fournier S, Amabile N, Chi Pan, Combaret N, Eeckhout E, Kibler M, Silvain J, Wijns W, Schiele F, Muller O, Meneveau N, Adjedj J. Quantitative flow ratio virtual stenting and post stenting correlations to post stenting fractional flow reserve measurements from the DOCTORS (Does Optical Coherence Tomography Optimize Results of Stenting) study population. Catheter Cardiovasc Interv. 2020;96:1145–53. doi: 10.1002/ccd.28615. [DOI] [PubMed] [Google Scholar]
- Guan S, Gan Q, Han W, Zhai X, Wang M, Chen Y, Zhang L, Li T, Chang X, Liu H, Hong W, Li Z, Tu S, Qu X. Feasibility of Quantitative Flow Ratio Virtual Stenting for Guidance of Serial Coronary Lesions Intervention. J Am Heart Assoc. 2022;11:e025663. doi: 10.1161/JAHA.122.025663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der, Janssens GN, de Waard, Everaars H, Broyd CJ, Beijnink CWH, van de, Nijveldt R, Cook CM, Petraco R, Ten Cate, von Birgelen, Escaned J, Davies JE, van Leeuwen, van Royen. Temporal Changes in Coronary Hyperemic and Resting Hemodynamic Indices in Nonculprit Vessels of Patients With ST-Segment Elevation Myocardial Infarction. JAMA Cardiol. 2019;4:736–44. doi: 10.1001/jamacardio.2019.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ntalianis A, Sels JW, Davidavicius G, Tanaka N, Muller O, Trana C, Barbato E, Hamilos M, Mangiacapra F, Heyndrickx GR, Wijns W, Pijls NH, De Bruyne. Fractional flow reserve for the assessment of nonculprit coronary artery stenoses in patients with acute myocardial infarction. JACC Cardiovasc Interv. 2010;3:1274–81. doi: 10.1016/j.jcin.2010.08.025. [DOI] [PubMed] [Google Scholar]
- Musto C, De Felice, Rigattieri S, Chin D, Marra A, Nazzaro MS, Cifarelli A, Violini R. Instantaneous wave-free ratio and fractional flow reserve for the assessment of nonculprit lesions during the index procedure in patients with ST-segment elevation myocardial infarction: The WAVE study. Am Heart J. 2017;193:63–9. doi: 10.1016/j.ahj.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Thim T, Götberg M, Fröbert O, Nijveldt R, van Royen, Baptista SB, Koul S, Kellerth T, Bøtker HE, Terkelsen CJ, Christiansen EH, Jakobsen L, Kristensen SD, Maeng M. Non-culprit Stenosis Evaluation Using Instantaneous Wave-Free Ratio in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc Interv. 2017;10:2528–35. doi: 10.1016/j.jcin.2017.07.021. [DOI] [PubMed] [Google Scholar]
- Cuculi F, De Maria, Meier P, Dall’Armellina E, de Caterina, Channon KM, Prendergast BD, Choudhury RP, Forfar JC, Kharbanda RK, Banning AP. Impact of microvascular obstruction on the assessment of coronary flow reserve, index of microcirculatory resistance, and fractional flow reserve after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1894–904. doi: 10.1016/j.jacc.2014.07.987. [DOI] [PubMed] [Google Scholar]
- De Bruyne, Pijls NH, Bartunek J, Kulecki K, Bech JW, De Winter, Van Crombrugge, Heyndrickx GR, Wijns W. Fractional flow reserve in patients with prior myocardial infarction. Circulation. 2001;104:157–62. doi: 10.1161/01.cir.104.2.157. [DOI] [PubMed] [Google Scholar]
- Smits PC, Abdel-Wahab M, Neumann FJ, Boxmade Klerk, Lunde K, Schotborgh CE, Piroth Z, Horak D, Wlodarczak A, Ong PJ, Hambrecht R, Angerås O, Richardt G, Omerovic E Compare-Acute Investigators. Fractional Flow Reserve-Guided Multivessel Angioplasty in Myocardial Infarction. N Engl J Med. 2017;376:1234–44. doi: 10.1056/NEJMoa1701067. [DOI] [PubMed] [Google Scholar]
- Engstrøm T, Kelbæk H, Helqvist S, Høfsten DE, Kløvgaard L, Holmvang L, Jørgensen E, Pedersen F, Saunamäki K, Clemmensen P, De Backer, Ravkilde J, Tilsted HH, Villadsen AB, Aarøe J, Jensen SE, Raungaard B, Køber L DANAMI-3—PRIMULTI Investigators. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3—PRIMULTI): an open-label, randomised controlled trial. Lancet. 2015;386:665–71. doi: 10.1016/s0140-6736(15)60648-1. [DOI] [PubMed] [Google Scholar]
- Puymirat E, Cayla G, Simon T, Steg PG, Montalescot G, Durand-Zaleski I, le Bras, Gallet R, Khalife K, Morelle JF, Motreff P, Lemesle G, Dillinger JG, Lhermusier T, Silvain J, Roule V, Labèque JN, Rangé G, Ducrocq G, Cottin Y, Blanchard D, Charles Nelson, De Bruyne, Chatellier G, Danchin N FLOWER-MI Study Investigators. Multivessel PCI Guided by FFR or Angiography for Myocardial Infarction. N Engl J Med. 2021;385:297–308. doi: 10.1056/NEJMoa2104650. [DOI] [PubMed] [Google Scholar]
- Piróth Z, Boxma-de Klerk, Omerovic E, Andréka P, Fontos G, Fülöp G, Abdel-Wahab M, Neumann FJ, Richardt G, Abdelghani M, Smits PC. The Natural History of Non-culprit Lesions in STEMI: An FFR Substudy of the Compare-Acute Trial. JACC Cardiovasc Interv. 2020;13:954–61. doi: 10.1016/j.jcin.2020.02.015. [DOI] [PubMed] [Google Scholar]
- Denormandie P, Simon T, Cayla G, Steg PG, Montalescot G, Durand-Zaleski I, le Bras A, le Breton H, Valy Y, Schiele F, Cuisset T, Vanzetto G, Levesque S, Goube P, Nallet O, Angoulvant D, Roubille F, Charles Nelson A, Chatellier G, Danchin N, Puymirat E. Compared Outcomes of ST-Segment-Elevation Myocardial Infarction Patients With Multivessel Disease Treated With Primary Percutaneous Coronary Intervention and Preserved Fractional Flow Reserve of Nonculprit Lesions Treated Conservatively and of Those With Low Fractional Flow Reserve Managed Invasively: Insights From the FLOWER-MI Trial. Circ Cardiovasc Interv. 2021;14:e011314. doi: 10.1161/CIRCINTERVENTIONS.121.011314. [DOI] [PubMed] [Google Scholar]
- Leone AM, Liuzzo G. No blossom for fractional flow reserve in FLOWER-MI. Eur Heart J. 2021;42:2971–2. doi: 10.1093/eurheartj/ehab425. [DOI] [PubMed] [Google Scholar]
- Leone AM, Cialdella P, Lassandro Pepe, Basile E, Zimbardo G, Arioti M, Ciriello G, D’Amario D, Buffon A, Burzotta F, Porto I, Aurigemma C, Niccoli G, Rebuzzi AG, Trani C, Crea F. Fractional flow reserve in acute coronary syndromes and in stable ischemic heart disease: clinical implications. Int J Cardiol. 2019;277:42–6. doi: 10.1016/j.ijcard.2018.08.024. [DOI] [PubMed] [Google Scholar]
- Van Belle, Baptista SB, Raposo L, Henderson J, Rioufol G, Santos L, Pouillot C, Ramos R, Cuisset T, Calé R, Teiger E, Jorge E, Belle L, Machado C, Barreau D, Costa M, Hanssen M, Oliveira E, Besnard C, Costa J, Dallongeville J, Pipa J, Sideris G, Fonseca N, Bretelle C, Guardado J, Lhoest N, Silva B, Barnay P, Sousa MJ, Leborgne L, Silva JC, Vincent F, Rodrigues A, Seca L, Fernandes R, Dupouy P PRIME-FFR Study Group. Impact of Routine Fractional Flow Reserve on Management Decision and 1-Year Clinical Outcome of Patients With Acute Coronary Syndromes: PRIME-FFR (Insights From the POST-IT [Portuguese Study on the Evaluation of FFR-Guided Treatment of Coronary Disease] and R3F [French FFR Registry] Integrated Multicenter Registries - Implementation of FFR [Fractional Flow Reserve] in Routine Practice). Circ Cardiovasc Interv. 2017;10:e004296. doi: 10.1161/CIRCINTERVENTIONS.116.004296. [DOI] [PubMed] [Google Scholar]
- Sels JW, Tonino PA, Siebert U, Fearon WF, Van’t Veer, De Bruyne, Pijls NH. Fractional flow reserve in unstable angina and non-ST-segment elevation myocardial infarction experience from the FAME (Fractional flow reserve versus Angiography for Multivessel Evaluation) study. JACC Cardiovasc Interv. 2011;4:1183–9. doi: 10.1016/j.jcin.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Burzotta F, Lassen JF, Lefèvre T, Banning AP, Chatzizisis YS, Johnson TW, Ferenc M, Rathore S, Albiero R, Pan M, Darremont O, Hildick-Smith D, Chieffo A, Zimarino M, Louvard Y, Stankovic G. Percutaneous coronary intervention for bifurcation coronary lesions: the 15th consensus document from the European Bifurcation Club. EuroIntervention. 2021;16:1307–17. doi: 10.4244/EIJ-D-20-00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo BK, Kang HJ, Youn TJ, Chae IH, Choi DJ, Kim HS, Sohn DW, Oh BH, Lee MM, Park YB, Choi YS, Tahk SJ. Physiologic assessment of jailed side branch lesions using fractional flow reserve. J Am Coll Cardiol. 2005;46:633–7. doi: 10.1016/j.jacc.2005.04.054. [DOI] [PubMed] [Google Scholar]
- Kumsars I, Narbute I, Thuesen L, Niemelä M, Steigen TK, Kervinen K, Sondore D, Holm NR, Lassen JF, Christiansen EH, Maeng M, Jegere S, Juhnevica D, Erglis A Nordic-Baltic PCI study group. Side branch fractional flow reserve measurements after main vessel stenting: a Nordic-Baltic Bifurcation Study III substudy. EuroIntervention. 2012;7:1155–61. doi: 10.4244/EIJV7I10A186. [DOI] [PubMed] [Google Scholar]
- Chen SL, Ye F, Zhang JJ, Xu T, Tian NL, Liu ZZ, Lin S, Shan SJ, Ge Z, You W, Liu YQ, Qian XS, Li F, Yang S, Kwan TW, Xu B, Stone GW. Randomized Comparison of FFR-Guided and Angiography-Guided Provisional Stenting of True Coronary Bifurcation Lesions: The DKCRUSH-VI Trial (Double Kissing Crush Versus Provisional Stenting Technique for Treatment of Coronary Bifurcation Lesions VI). JACC Cardiovasc Interv. 2015;8:536–46. doi: 10.1016/j.jcin.2014.12.221. [DOI] [PubMed] [Google Scholar]
- Omori H, Kawase Y, Hara M, Tanigaki T, Okamoto S, Hirata T, Kikuchi J, Ota H, Sobue Y, Miyake T, Kawamura I, Okubo M, Kamiya H, Tsuchiya K, Suzuki T, Pijls NHJ, Matsuo H. Feasibility and safety of jailed-pressure wire technique using durable optical fiber pressure wire for intervention of coronary bifurcation lesions. Catheter Cardiovasc Interv. 2019;94:E61–6. doi: 10.1002/ccd.28106. [DOI] [PubMed] [Google Scholar]
- Warisawa T, Cook CM, Rajkumar C, Howard JP, Seligman H, Ahmad Y, El Hajj, Doi S, Nakajima A, Nakayama M, Goto S, Vera-Urquiza R, Sato T, Kikuta Y, Kawase Y, Nishina H, Petraco R, Al-Lamee R, Nijjer S, Sen S, Nakamura S, Lerman A, Matsuo H, Francis DP, Akashi YJ, Escaned J, Davies JE. Safety of Revascularization Deferral of Left Main Stenosis Based on Instantaneous Wave-Free Ratio Evaluation. JACC Cardiovasc Interv. 2020;13:1655–64. doi: 10.1016/j.jcin.2020.02.035. [DOI] [PubMed] [Google Scholar]
- Cerrato E, Echavarria-Pinto M, D’Ascenzo F, Gonzalo N, Quadri G, Quirós A, de la, Tomassini F, Barbero U, Nombela-Franco L, Nuñez-Gil I, Biondi-Zoccai G, Macaya C, Varbella F, Escaned J. Safety of intermediate left main stenosis revascularization deferral based on fractional flow reserve and intravascular ultrasound: A systematic review and meta-regression including 908 deferred left main stenosis from 12 studies. Int J Cardiol. 2018;271:42–8. doi: 10.1016/j.ijcard.2018.04.032. [DOI] [PubMed] [Google Scholar]
- Bech GJ, Droste H, Pijls NH, De Bruyne, Bonnier JJ, Michels HR, Peels KH, Koolen JJ. Value of fractional flow reserve in making decisions about bypass surgery for equivocal left main coronary artery disease. Heart. 2001;86:547–52. doi: 10.1136/heart.86.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilos M, Muller O, Cuisset T, Ntalianis A, Chlouverakis G, Sarno G, Nelis O, Bartunek J, Vanderheyden M, Wyffels E, Barbato E, Heyndrickx GR, Wijns W, De Bruyne. Long-term clinical outcome after fractional flow reserve-guided treatment in patients with angiographically equivocal left main coronary artery stenosis. Circulation. 2009;120:1505–12. doi: 10.1161/CIRCULATIONAHA.109.850073. [DOI] [PubMed] [Google Scholar]
- Yong AS, Daniels D, De Bruyne, Kim HS, Ikeno F, Lyons J, Pijls NH, Fearon WF. Fractional flow reserve assessment of left main stenosis in the presence of downstream coronary stenoses. Circ Cardiovasc Interv. 2013;6:161–5. doi: 10.1161/CIRCINTERVENTIONS.112.000104. [DOI] [PubMed] [Google Scholar]
- Fearon WF, Yong AS, Lenders G, Toth GG, Dao C, Daniels DV, Pijls NHJ, De Bruyne. The impact of downstream coronary stenosis on fractional flow reserve assessment of intermediate left main coronary artery disease: human validation. JACC Cardiovasc Interv. 2015;8:398–403. doi: 10.1016/j.jcin.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Ahmad Y, Götberg M, Cook C, Howard JP, Malik I, Mikhail G, Frame A, Petraco R, Rajkumar C, Demir O, Iglesias JF, Bhindi R, Koul S, Hadjiloizou N, Gerber R, Ramrakha P, Ruparelia N, Sutaria N, Kanaganayagam G, Ariff B, Fertleman M, Anderson J, Chukwuemeka A, Francis D, Mayet J, Serruys P, Davies J, Sen S. Coronary Hemodynamics in Patients With Severe Aortic Stenosis and Coronary Artery Disease Undergoing Transcatheter Aortic Valve Replacement: Implications for Clinical Indices of Coronary Stenosis Severity. JACC Cardiovasc Interv. 2018;11:2019–31. doi: 10.1016/j.jcin.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelis JM, Tonino PAL, Pijls NHJ, De Bruyne, Kirkeeide RL, Gould KL, Johnson NP. Coronary Microcirculation in Aortic Stenosis: Pathophysiology, Invasive Assessment, and Future Directions. J Interv Cardiol. 2020;2020:4603169. doi: 10.1155/2020/4603169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijf JD, Wijns W, Jukema JW, Atsma DE, de Roos, Lamb HJ, Stokkel MP, Dibbets-Schneider P, Decramer I, De Bondt, van der, Vanhoenacker PK, Bax JJ. Relationship between noninvasive coronary angiography with multi-slice computed tomography and myocardial perfusion imaging. J Am Coll Cardiol. 2006;48:2508–14. doi: 10.1016/j.jacc.2006.05.080. [DOI] [PubMed] [Google Scholar]
- Hwang D, Jeon KH, Lee JM, Park J, Kim CH, Tong Y, Zhang J, Bang JI, Suh M, Paeng JC, Na SH, Cheon GJ, Cook CM, Davies JE, Koo BK. Diagnostic Performance of Resting and Hyperemic Invasive Physiological Indices to Define Myocardial Ischemia: Validation With 13N-Ammonia Positron Emission Tomography. JACC Cardiovasc Interv. 2017;10:751–60. doi: 10.1016/j.jcin.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Mejía-Rentería H, Nombela-Franco L, Paradis JM, Lunardi M, Lee JM, Amat-Santos IJ, Veiga Fernandez, Kalra A, Bansal EJ, de la, Rodés-Cabau J, Ribichini FL, Escaned J Collaborators. Angiography-based quantitative flow ratio versus fractional flow reserve in patients with coronary artery disease and severe aortic stenosis. EuroIntervention. 2020;16:e285–92. doi: 10.4244/EIJ-D-19-01001. [DOI] [PubMed] [Google Scholar]
- Kleczynski P, Dziewierz A, Rzeszutko L, Dudek D, Legutko J. Quantitative flow ratio for evaluation of borderline coronary lesions in patients with severe aortic stenosis. Rev Esp Cardiol (Engl Ed) 2022;75:472–8. doi: 10.1016/j.rec.2021.04.008. [DOI] [PubMed] [Google Scholar]
- Sejr-Hansen M, Christiansen EH, Ahmad Y, Vendrik J, Westra J, Holm NR, Thim T, Seligman H, Hall K, Sen S, Terkelsen CJ, Eftekhari A. Performance of quantitative flow ratio in patients with aortic stenosis undergoing transcatheter aortic valve implantation. Catheter Cardiovasc Interv. 2022;99:68–73. doi: 10.1002/ccd.29518. [DOI] [PubMed] [Google Scholar]
- Gohmann RF, Pawelka K, Seitz P, Majunke N, Heiser L, Renatus K, Desch S, Lauten P, Holzhey D, Noack T, Wilde J, Kiefer P, Krieghoff C, Lücke C, Gottschling S, Ebel S, Borger MA, Thiele H, Panknin C, Horn M, Abdel-Wahab M, Gutberlet M. Combined cCTA and TAVR Planning for Ruling Out Significant CAD: Added Value of ML-Based CT-FFR. JACC Cardiovasc Imaging. 2022;15:476–86. doi: 10.1016/j.jcmg.2021.09.013. [DOI] [PubMed] [Google Scholar]
- Michail M, Ihdayhid AR, Comella A, Thakur U, Cameron JD, McCormick LM, Gooley RP, Nicholls SJ, Mathur A, Hughes AD, Ko BS, Brown AJ. Feasibility and Validity of Computed Tomography-Derived Fractional Flow Reserve in Patients With Severe Aortic Stenosis: The CAST-FFR Study. Circ Cardiovasc Interv. 2021;14:e009586. doi: 10.1161/CIRCINTERVENTIONS.120.009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahwala UK, Brilakis ES, Byrne J, Davies JE, Ward MR, Weaver JC, Bhindi R. Applicability and Interpretation of Coronary Physiology in the Setting of a Chronic Total Occlusion. Circ Cardiovasc Interv. 2019;12:e007813. doi: 10.1161/CIRCINTERVENTIONS.119.007813. [DOI] [PubMed] [Google Scholar]
- Iqbal MB, Shah N, Khan M, Wallis W. Reduction in myocardial perfusion territory and its effect on the physiological severity of a coronary stenosis. Circ Cardiovasc Interv. 2010;3:89–90. doi: 10.1161/CIRCINTERVENTIONS.109.904193. [DOI] [PubMed] [Google Scholar]
- Kurisu S, Mitsuba N, Ishibashi K, Kato Y, Dohi Y, Nishioka K, Kihara Y. A pitfall of fractional flow reserve associated with the presence of collateral circulation. Intern Med. 2011;50:2811–3. doi: 10.2169/internalmedicine.50.6104. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Kawase Y. Physiological impact of CTO recanalization assessed by coronary pressure measurement: a case report. Catheter Cardiovasc Interv. 2013;82:E459–64. doi: 10.1002/ccd.24842. [DOI] [PubMed] [Google Scholar]
- Sachdeva R, Agrawal M, Flynn SE, Werner GS, Uretsky BF. Reversal of ischemia of donor artery myocardium after recanalization of a chronic total occlusion. Catheter Cardiovasc Interv. 2013;82:E453–8. doi: 10.1002/ccd.25031. [DOI] [PubMed] [Google Scholar]
- Sachdeva R, Uretsky BF. The effect of CTO recanalization on FFR of the donor artery. Catheter Cardiovasc Interv. 2011;77:367–9. doi: 10.1002/ccd.22806. [DOI] [PubMed] [Google Scholar]
- Fournier S, Toth GG, De Bruyne, Johnson NP, Ciccarelli G, Xaplanteris P, Milkas A, Strisciuglio T, Bartunek J, Vanderheyden M, Wyffels E, Casselman F, Van Praet, Stockman B, Degrieck I, Barbato E. Six-Year Follow-Up of Fractional Flow Reserve-Guided Versus Angiography-Guided Coronary Artery Bypass Graft Surgery. Circ Cardiovasc Interv. 2018;11:e006368. doi: 10.1161/CIRCINTERVENTIONS.117.006368. [DOI] [PubMed] [Google Scholar]
- Toth G, De Bruyne, Casselman F, De Vroey, Pyxaras S, Di Serafino, Van Praet, Van Mieghem, Stockman B, Wijns W, Degrieck I, Barbato E. Fractional flow reserve-guided versus angiography-guided coronary artery bypass graft surgery. Circulation. 2013;128:1405–11. doi: 10.1161/CIRCULATIONAHA.113.002740. [DOI] [PubMed] [Google Scholar]
- Spadaccio C, Glineur D, Barbato E, Di Franco, Oldroyd KG, Biondi-Zoccai G, Crea F, Fremes SE, Angiolillo DJ, Gaudino M. Fractional Flow Reserve-Based Coronary Artery Bypass Surgery: Current Evidence and Future Directions. JACC Cardiovasc Interv. 2020;13:1086–96. doi: 10.1016/j.jcin.2019.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes NH, Paulitsch Fda, Gois AF, Pereira AC, Stolf NA, Dallan LO, Ramires JA, Hueb WA. Impact of number of vessels disease on outcome of patients with stable coronary artery disease: 5-year follow-up of the Medical, Angioplasty, and bypass Surgery study (MASS). Eur J Cardiothorac Surg. 2008;33:349–54. doi: 10.1016/j.ejcts.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Tarantini G, Barioli A, Nai Fovino, Fraccaro C, Masiero G, Iliceto S, Napodano M. Unmasking Myocardial Bridge-Related Ischemia by Intracoronary Functional Evaluation. Circ Cardiovasc Interv. 2018;11:e006247. doi: 10.1161/CIRCINTERVENTIONS.117.006247. [DOI] [PubMed] [Google Scholar]
- Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Iliceto S. Left Anterior Descending Artery Myocardial Bridging: A Clinical Approach. J Am Coll Cardiol. 2016;68:2887–99. doi: 10.1016/j.jacc.2016.09.973. [DOI] [PubMed] [Google Scholar]
- Waterbury TM, Tarantini G, Vogel B, Mehran R, Gersh BJ, Gulati R. Non-atherosclerotic causes of acute coronary syndromes. Nat Rev Cardiol. 2020;17:229–41. doi: 10.1038/s41569-019-0273-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Guiding catheters, guide catheter extensions and microcatheters.
Applied coronary physiology in specific PCI scenarios.
Studies supporting the prognostic impact of functional PCI results.
Limitations related to image-based functional coronary analysis.
Supporting studies used to define focal, tandem and diffuse disease patterns.
Currently available and pending devices for invasive functional coronary assessment.
Correction of intracoronary pressure pullback curves with dedicated software addressing fluctuations caused by the Venturi effect.
Longitudinal iFR mapping coregistered with coronary angiography.
Pre- and post-PCI functional test in the presence of myocardial bridge.