Abstract
Objective
To derive a comprehensive implementation framework for clinical AI models within hospitals informed by existing AI frameworks and integrated with reporting standards for clinical AI research.
Materials and Methods
(1) Derive a provisional implementation framework based on the taxonomy of Stead et al and integrated with current reporting standards for AI research: TRIPOD, DECIDE-AI, CONSORT-AI. (2) Undertake a scoping review of published clinical AI implementation frameworks and identify key themes and stages. (3) Perform a gap analysis and refine the framework by incorporating missing items.
Results
The provisional AI implementation framework, called SALIENT, was mapped to 5 stages common to both the taxonomy and the reporting standards. A scoping review retrieved 20 studies and 247 themes, stages, and subelements were identified. A gap analysis identified 5 new cross-stage themes and 16 new tasks. The final framework comprised 5 stages, 7 elements, and 4 components, including the AI system, data pipeline, human-computer interface, and clinical workflow.
Discussion
This pragmatic framework resolves gaps in existing stage- and theme-based clinical AI implementation guidance by comprehensively addressing the what (components), when (stages), and how (tasks) of AI implementation, as well as the who (organization) and why (policy domains). By integrating research reporting standards into SALIENT, the framework is grounded in rigorous evaluation methodologies. The framework requires validation as being applicable to real-world studies of deployed AI models.
Conclusions
A novel end-to-end framework has been developed for implementing AI within hospital clinical practice that builds on previous AI implementation frameworks and research reporting standards.
Keywords: AI framework, AI implementation, machine learning, artificial intelligence, healthcare framework
INTRODUCTION
Modern healthcare is underpinned by the translation of research findings into clinical practice. Regulatory practices in most countries aim to minimize the risks associated with introducing new technologies such as drugs and medical devices. Honest and accurate appraisal of new technologies is also encouraged by clinical researchers adhering to reporting standards.1,2 However, despite prolific growth in research into artificial intelligence (AI) based decision support technologies over recent years,3 particularly diagnostic and prognostic prediction models, translation into clinical practice has been slow4,5 and the numbers of AI-based systems of this type implemented into routine care remain very low.6,7 In a recent scoping review, just 45 of these AI systems had been implemented over 10 years,8 compared to over 15 000 published research papers on AI in healthcare in 2020 alone.3
The reasons for slow uptake are multiple, including lack of clinician trust in often-unexplainable and opaque “black-box” AI methods,9–13 consumer fears over data privacy,14,15 health inequity concerns about potential underlying data biases13,16 and underdeveloped or absent government regulation.15,17 For healthcare organizations, unlike the step-wise, systematic process for introducing new drugs into clinical practice,18 no equivalent approach exists for introducing AI interventions into hospitals. In contrast, researchers are developing, or have already released, standards for reporting studies relevant to each evolutionary stage of AI-based interventions, from retrospective evaluation of AI model performance (TRIPOD2,19; TRIPOD-AI20) through to prospective pilot evaluations (DECIDE-AI21) and large-scale clinical trials (CONSORT-AI22). These standards require researchers to fully disclose how they have developed and evaluated AI-based interventions. Integrating these standards within a clinical intervention implementation framework could provide a more systematic end-to-end clinical AI implementation framework, more akin to the process for introducing drugs that healthcare organizations are used to. In this paper we derive such a framework, intended for application within hospital care settings and to be used by a wide audience of stakeholders involved in developing, testing, deploying, funding, and governing AI-based decision support technologies.
Background
We define implementation by extending the Cambridge dictionary definition23 as the act of starting to use a plan or system to change or incorporate a new intervention into clinical practice. We define AI as computer programs that learn from and can make predictions based on data, including machine learning/deep learning models. Theoretical clinical intervention implementation frameworks24–28 attempt to identify key stages, tasks, and contextual factors that warrant consideration. Nilsen defines a framework as, “a structure, overview, outline, system or plan consisting of various descriptive categories… and the relations between them that are presumed to account for a phenomenon.”29 An example of a parallel operational clinical implementation framework is the US Food and Drug Administration’s (FDA) Drug Development Process.18 Such a framework is needed in identifying clear steps and transparent evaluation gateways that provide a systematic pathway for organizations to minimize the risks associated with incorporating new drugs into clinical practice.
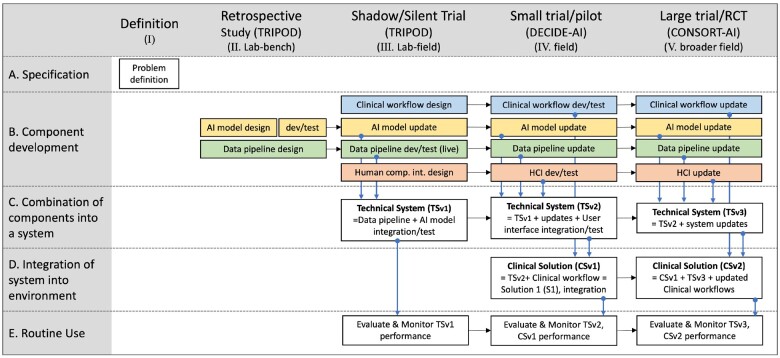
There is currently no equivalent widely acknowledged framework for implementing AI interventions into clinical practice, yet the systematic methodology to evaluate clinical AI implementation at multiple stages exists, as reported in Vasey et al’s Decide-AI reporting standard (Figure 1).21 Aligned to the serial stages (see Table 1) are the evaluation reporting standards of TRIPOD,2,19 TRIPOD-AI,20 DECIDE-AI,21 and CONSORT(-AI),22 herein referred to as the AI reporting standards. The standards are founded on long-serving, widely used (>10 000 citations) and effective30 intervention evaluation methodologies.1,2 Based on Gama et al’s review of existing theoretical AI implementation frameworks, none of the identified AI implementation frameworks explicitly integrate these standards.
Figure 1.
Provisional staged clinical AI implementation (SALIENT) framework. Adapted from Stead et al 12 and aligned with the TRIPOD, DECIDE-AI, and CONSORT-AI reporting guideline 39–42,44 stages and tasks. The colored boxes refer to solution components (see Element B). Blue for the clinical workflow, yellow for the AI model, green for the data pipeline, and red for the human computer interface. HCI: human computer interface; dev: development.
Table 1.
Translations of Stead et al’s levels of evaluation taxonomy to the research evaluation stage-based terminology.
| Implementation stage | Stead et al’s stage (level of evaluation) | Research evaluation stage and associated reporting standard |
|---|---|---|
| I | I. Definition | Definition (from Stead et al) |
| II | II. Lab-bench | Retrospective study (TRIPOD)2,19 |
| III | III. Lab-field | Shadow/silent study (TRIPOD)2,19 |
| IV | IV. Field | Small trial/pilot (DECIDE-AI)21 |
| V | V. Broader field | Large trial/RCT (CONSORT-AI)22,43 |
RCT: randomized controlled trial.
Prior clinical implementation frameworks generally fit into 2 of Nilsen’s 5 framework categories: Determinant and process models.29 Determinant frameworks identify themes or domains that can influence implementation outcomes, such as Greenhalgh et al’s nonadoption, abandonment, scale-up, spread, and sustainability (NASSS) framework.27 It has 7 key domains including clinical condition or context, technology, value proposition, adopters, organization, wider system, and embedding and adaption over time. It was derived through qualitative evaluation of technology implementation case studies complemented by a review of other frameworks. The NASSS framework and other similar conceptual or theme-based frameworks, such as the modified RE-AIM framework by Bakken et al,26 Damschroder et al’s Consolidated Framework For Implementation Research (CFIR)25 and Beil et al’s ethical pathway framework31 are positioned from a wide range of perspectives although none focus on AI implementation and most omit or remain unclear about the complete implementation cycle, including: (1) the start to finish staged sequence; (2) identification of key intervention components and associated tasks; and (3) progression and ultimate integration of all components into an end-to-end technical and clinical intervention.
Process models, which usually specify stages in the process of translating research into practice,29 redress some of these deficiencies. In the case of Sendak et al,32 a pathway consisting of 3 primary stages is proposed based on their experience: (1) design and develop; (2) evaluate and validate; (3) diffuse and scale. Van De Sande et al also proposed a step-by-step approach with 5 phases and 16 steps based on synthesis of data from a literature review.33 The phases are quite different to those of Sendak et al and others,34 and none are aligned with the AI reporting standards.
In summary, there are many implementation frameworks self-derived or derived from practice and prior literature that provide a wide range of differing perspectives and pathways for supporting healthcare organizations to implement AI. However, for the introduction of AI, as with the process for implementing other clinical interventions such as new drugs, staged evaluation of the intervention is central; yet none of the frameworks mentioned above are founded on this common approach. We hypothesized that by deriving a staged AI implementation framework directly aligned with the AI reporting standards and grounded in a well-established theory of translating clinical informatics interventions into practice, a more systematic staged approach would emerge. Because the AI reporting standards are limited by their focus on evaluation, we also sought to augment the derived framework with elements from prior AI frameworks.
Objective
This study had 3 objectives: (1) Derive a provisional end-to-end clinical AI implementation framework that integrates the TRIPOD, DECIDE-AI, and CONSORT-AI reporting standards with an informatics translation theory; (2) Conduct a scoping review of clinical AI implementation framework studies to capture essential themes and stages; and (3) Refine the provisional framework by incorporating important missing elements identified from the scoping review.
MATERIALS AND METHODS
Derivation of a provisional clinical AI implementation framework
The AI reporting standards and associated item lists provide a foundation for a framework but lack process and structure. We therefore reviewed prior theoretical frameworks (AHV, IAS) for possible candidates that could align with the standards, identifying these through scrutinizing articles found within 3 review papers and further snowballing.35–37 We searched for frameworks that: (1) had similar stages to those of the AI reporting standards; and (2) were sufficiently flexible to support the development and implementation of AI solution elements derived from the AI reporting standards, which included the AI algorithm, data pipeline, human-computer interface, and clinical workflow. During candidate appraisal, determinant models were excluded because they did not support stages and would be radically modified by their addition.25–28,38–41 Process models did have stages,33,42 but they were fixed and varied in number and content from the AI reporting standards stages. Trying to retrofit a new set of stages would have violated those original frameworks. One exception was Stead et al’s taxonomy for translating medical informatics interventions from the laboratory to the field.24
Stead et al’s process framework considers how different components, technical and clinical, need to be developed and integrated, and in which of 5 evaluation stages these tasks need to occur: (I) Definition; (II) Laboratory—bench; (III) Laboratory—field; (IV) Remoter field—validity; and (V) Remoter field—efficacy. Stead et al identified 5 key elements in developing and implementing interventions: (A) Specification; (B) Component development; (C) Combination of components into a system; (D) Integration of system into environment; and (E) Routine use. We chose this as our baseline framework because of its clinical orientation, end-to-end nature, flexibility to incorporate solution components and close stage-alignment with the AI reporting standards.
While the stages of the Stead taxonomy are designed for any clinical informatics intervention, we intuited that analyzing the items in the current AI reporting standards may identify specific components and tasks that could be aligned with each stage for AI-based interventions. Accordingly, we used the following method to derive the provisional framework (see Supplementary Appendix SA for more details and examples):
Step 1: Define the baseline implementation stages: Map each implementation stage, as reported in the DECIDE-AI guideline,21 to the similar stage in the Stead taxonomy (see Table 1).
Step 2: Identify the intervention components and their associated implementation tasks: For each reporting item specified in TRIPOD, DECIDE-AI, and CONSORT-AI19,21,22,43 identify existing or create new components and component tasks and assign the implementation stage based on the mapping identified in Step 1, as exemplified in Table 2. This step was initially performed by AV and then using his draft task list, RS repeated the task creation independently. The final harmonized task set was agreed by consensus (RS, AV).
Table 2.
Example of translating TRIPOD reporting item 9 to a component and component task.
| TRIPOD report item 9: | Describe how missing data were handled (eg, complete-case analysis, single imputation, and multiple imputation) with details of any imputation method |
| Task(s) created: | Define and handle missing data (imputation) |
| Component created: | Data pipeline |
| Stage: | Retrospective and silent tracking |
Step 3: Consolidate similar components and component tasks identified in Step 2 into a final reduced task and component set.
Step 4: Back- or forward-fill missing tasks across stages as, in some instances, tasks are identified in one stage and required in earlier or later stages but the latter stage reporting standards make no provision for them. For example, CONSORT-AI item 4b (Extension) is, “Describe how the AI intervention was integrated into the trial setting, including any onsite or offsite requirements.”22 A stage V task generated from this item is the data pipeline component task, “Develop real-time data capture/transform capability.” However, this task is required in both the silent study stage (III) and the small pilot trial stage (IV), where real-time data are also required, and hence this task is copied backwards across the earlier stages.
Step 5: Identify the components that make up technical systems (TS) and clinical solutions (CS) at each stage (I to V) which apply to Stead et al’s elements C (Combination of components into a system) and D (Integration of system into environment). Finally, element E (Routine use) incorporates the evaluation and performance monitoring tasks for both the technical system and overall clinical solution.
Scoping review of clinical AI implementation framework studies
The scoping review consisted of a comprehensive systematic search for existing AI implementation frameworks, with analysis limited to identification of themes and stages reported in the identified frameworks.44 It was reported according to the PRISMA Extension for Scoping Review (PRISMA-ScR) guidelines.45 No formal quality assessment of the papers was performed, although the source and derivation of the frameworks were reported.
Search strategy
Five databases (Pubmed/Medline, EMBASE, Web of Science, CINAHL, and IEEExplore) were searched up to November 25, 2022 for titles and abstracts published in English using keywords and synonyms for: (1) AI or “artificial intelligence” or “machine learning”; AND (2) framework or “step-by-step” or roadmap; AND (3) implement* or deploy* or adopt*. For nonclinical databases, a “medic* OR clinic*” search phrase was appended with an AND statement (See Supplementary Appendix SB for complete search queries).
Study selection
All studies proposing a framework for implementing AI into clinical practice were included unless solely focused on imaging applications or a single AI solution, eg, a specific information technology (IT) infrastructure or a specific clinical task, such as sepsis prediction (full eligibility details in Supplementary Appendix SC). Covidence software46 supported a 2-stage screening process: (1) Screening of abstracts and titles by 3 independent reviewers (AHV, PL, or VK) with conflicts agreed by 3-way consensus (AHV, VK, KD); and (2) Full-text review conducted by 2 independent reviewers (AHV, KD), with selection agreed by 3-way consensus (AHV, RJS, KD).
Data extraction
Data from each paper were extracted into an Excel template and comprised study metadata, objective, clinical setting, theoretical underpinnings, methods for deriving the framework, and details relating to themes and stages. (See Supplementary Appendix SD for listing of data elements extracted).
Refinement of provisional implementation framework
A gap analysis was performed (AV, KD) to identify lack of concordance between the themes and stages extracted from each paper in the review and the stages, components and tasks of the provisional framework (see Supplementary Appendix SE for further mapping details). Missing or partially mapped elements were grouped and assigned to one of: (1) new stage; (2) new cross-stage element, where the missing element was applicable across more than one stage; (3) new component; or (4) new component task. The purpose of this step was to augment SALIENT with prior framework themes and stages that it was missing, generating a more comprehensive and useful final AI implementation framework.
RESULTS
Derivation of provisional implementation framework
The outputs of the 5-step process were used to derive our provisional implementation framework, titled the staged clinical AI implementation (SALIENT) framework (Figure 1). This comprised 5 implementation stages, labeled I to V, positioned across the top of Figure 1 and 5 elements, labeled A to E, positioned down the left-hand side. Element A, specification, describes preparatory work to clearly articulate the problem definition and proposed intervention (hereafter termed solution) specification. Element B comprises the development of 4 essential solution components: (1) AI model; (2) data pipeline; (3) human-computer interface (HCI); and (4) clinical workflow. Component development is divided into 3 engineering steps: (1) design; (2) develop and test; and (3) update. The stage-timing of these steps depends on the solution requirements at each stage for each component. For example, at the retrospective stage (II), the AI model is designed, developed, and validated using static datasets, whereas development and testing of the data pipeline using live or near-live data is only required at the silent study stage (III).
Element C of SALIENT combines solution components into functioning systems over 3 stages. Firstly, the technical system (TSv1) comprising the AI model and data pipeline are integrated for the silent study stage (III). In Stage IV, the HCI must also be integrated (TSv2) so that evaluation of clinician-computer interactions can be performed. Following further iterations and refinement of the system in response to these study results, the final technical system is completed for the large trial or roll-out in Stage V. Element D of SALIENT marks the coming together of the overall solution when the system is integrated into the live, routine clinical practice environment. The clinical solution (CSv1) must be ready at stage IV, comprising the technical solution (TSv2) and the clinical workflows, which is then updated ready for final trial and rollout in stage V. Element E, routine use, denotes all tasks required for normal continuous operation of the solution.
Table 3 identifies the components and tasks across each element (A to E) in the framework. For example, there are 4 tasks (AM01-04) itemized for the AI model component within element B (Component development) and 11 tasks identified for Element E (routine use). The individual reporting standard items (TRIPOD, DECIDE-AI, CONSORT-AI) accounting for each component task are specified in the respective, color-coded stage column (II/III [pink], IV [grey] and V [purple]). As previously noted, sometimes a task is needed in an earlier or later stage, but a relevant reporting item is missing in that stage. Where a task has been copied backward to an earlier stage (denoted by ‡) or copied forward to a later stage (denoted by §), the originating stage color is preserved in the earlier or later stage cell in the table so that one can see from which stage the task was derived.
Table 3.
Implementation tasks (left hand column) mapped to each reporting guideline item (right-hand 3 columns) and allocated to the provisional SALIENT AI framework elements (A, B, C, D, E) and components.
| SALIENT framework: components and tasks | TRIPOD Stages II/III | DECIDE—AI Stage IV | CONSORT-AI Stage V | |
|---|---|---|---|---|
| Framework element A: specifications | ||||
| Component: problem definition (PD) | ||||
| P1 | Rationale for change, background, context | 3a | 2 | 2a; 2a(i) |
| P2 | Intended use | 2 | 2a; 2a(i) | |
| Framework element B: component development | ||||
| Component: artificial intelligence model (AM) | ||||
| AM01 | Select and define data elements (predictors) required and units | 7a; 15a | 4b | 5(ii) |
| AM02 | Select/develop AI model(s) versions (including comparators) and internally validate; specify how predictions are calculated | 10b; 10c; 15a | 4a | 5(i)] |
| AM03 | Define and implement model calibration/fine tuning process | 10d | § | 4b(ext) |
| AM04 | Define and execute AI model update process and code management | 10e | 11; 17 | 5(i); 25(ext) |
| Component: data pipeline (DP) | ||||
| DP01 | Identify input data capture method (automated/manual) Clarify/specify measurement methods/units for capture | 7a | 4b | § |
| DP02 | Identify source systems for data elements | ‡ | 4b | § |
| DP03 | Add/extend systems for any new data element entry | ‡ | 4b | § |
| DP04 | Identify eligible patients: inclusion/exclusion criteria + minimum data requirements/patient level sample size | 5b; 8 | 3a; 9a | 4a(ii); 7a; 14a |
| DP05 | Identify and select data elements required | 5c; 7a | 4b | 5(ii) |
| DP06 | Transform data: Clean poor quality data (eg, outlier/invalid data)/Define and handle missing data (imputation)/Define and handle feature/predictor transformations/Perform other necessary data preprocessing steps | 9; 10a; 7a | 4b; 9a | 5(ii); 5(iii) |
| DP07 | Develop end-to-end data-pipeline for: AI models, comparative models, patient outcome, and risk group identification | 6a; 7a; 11 | 4b; IV | 6a; 6b |
| DP08 | Develop real-time data capture/transform capability | ‡ | ‡ | 4b(ext) |
| DP09 | Build or procure retrospective data set for AI training/validation/test | 4a | 4a | |
| DP10 | Define and execute pipeline update process and code management | ‡ | 11; 17 | § |
| Component: human-computer interface (HCI) | ||||
| HC1 | Define and develop content, layout and format of interface for users, including level of user customization | ‡ | 4c | § |
| HC2 | Specify update frequency, design, develop, and test real-time HC interface system | ‡ | 4c | 4b(ext); 5(iv); 5(v); 9 |
| HC3 | Conduct preclinical human factors evaluation | 7 | ||
| HC4 | Define and execute HC interface update process and code management | ‡ | 11; 17 | § |
| Component: clinical workflows (CW) | ||||
| CW01 | Identify target patients: eligibility, location(s), settings, sample sizes | 4b; 5a; 5b; 5c; 8 | 2a; 3a | 4a; 4a(i); 4b; 14a; 7a |
| CW02 | Identify target patient outcomes and subgroup definitions | 6a; 11 | IV | 6a; 6b |
| CW03 | Identify participant clinicians (users) | ‡ | 3b; 9b | § |
| CW04 | Reengineer clinical workflow/care pathways to include AI information; Clarify/specify decision making process incorporating the AI; Establish user agreement with AI system | 2b; 5a; 5b; 10b; 12 | 4b(ext); 5(vi) | |
| CW05 | Develop, test, execute processes to capture failures and feedback: user errors and feedback, software/AI malfunctions, patient harms/risks | ‡ | 6a; 13a; 13b | 5(vi); 19(ext); 19 |
| CW06 | Develop, test, and execute staff training in new process and AI system training | ‡ | 3c | 5(iv) |
| CW07 | Conduct pre/postclinical risk-assessment and mitigation plan | 6b; 16 | ||
| Framework element C: combination of components into a system | ||||
| Technical system (TS) | ||||
| TS01 | Integrate data pipeline with AI model output | ‡ | ‡ | 4b(ext) |
| TS02 | Integrate human-computer interface and changes to AI model/data pipeline | 4c | 4b(ext); 5(iv); 5(v); 9 | |
| TS03 | Integrate any changes from human-computer interface/AI model/data pipeline | 11; 17 | 5(i); 25(ext) | |
| Framework element D: integration of system into environment | ||||
| Clinical solution (CS) | ||||
| CS01 | Integrate clinical workflows with technical system (TS02) | 2b; 5a; 5b; 10b; 12 | 4b(ext); 5(vi) | |
| CS02 | Integrate updates to clinical workflows and the technical system (TS03) | 2b; 5a; 5b; 10b; 12 | 4b(ext); 5(vi) | |
| Framework element E: routine use | ||||
| Evaluation and monitoring (EM) | ||||
| EM1 | Select models for comparative evaluation | 3a | ||
| EM2 | Report patient population characteristics (dataset shift) including treatments received; also report patient flow, exclusions and outcome/risk group results | 4a; 5c; 11; 13a; 13b | 4a; 9a | 12b; 13; 13a; 15; 16 |
| EM3 | Define and report metrics for AI model validation, evaluation and comparison and any updated models | 10d; 16; 17 | § | § |
| EM4 | Define and report errors: user, system malfunctions and AI model errors, safety | ‡ | 6a; 10a; 13 | 19(ext) |
| EM5 | Define and report AI system safety evaluation, harm and unintended effects | ‡ | 6b; 13a | 19 |
| EM6 | Define and report HCI usage, use-variation and usability evaluation (eg, NASA’s Task load index47) | 7; 10a; 13a, 14a; 14b | 5(vi) | |
| EM7 | Define and report AI system biases | ‡ | 8 | 20 |
| EM8 | Define and report data missingness | 10a; 13b | 9a | § |
| EM9 | Define and report clinical results (outcomes by subgroup) | V; VII; VIII | 12a; 13; 13a; 17a; 17b | |
| EM10 | Report deeper analyses of results (by outcome), eg, univariate associations | 14a; 14b | § | 12b; 18 |
| EM11 | Report differences to prior phase (setting, outcome, predictors, results) | 12; 13c; 19a | § | § |
Note that the reporting guideline items are referenced exactly as they appear in the guideline papers and can be alpha-numeric (eg, 8, or 9a), Roman numerals (eg, VII, 5[vi]) and extensions, denoted “ext.” Each SALIENT stage is color-coded: White means no guideline element is applicable; pink for retrospective and silent trial stages II and III (TRIPOD40,44); grey for pilot/trial stage IV (DECIDE-AI39); purple for large-trial/roll-out stage V (CONSORT-AI41,42).
Task missing: copy task backward from later project stage.
Task missing: copy task forward from previous project stage. See text for more details.
Scoping review of clinical AI implementation framework studies
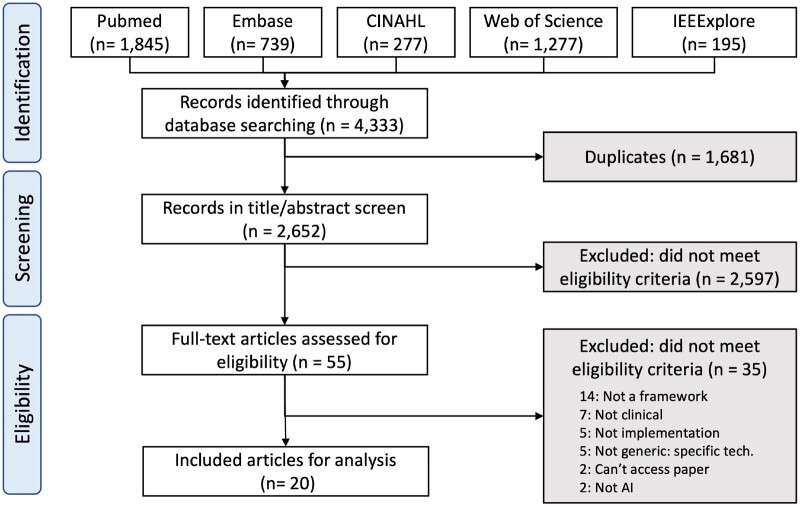
From 4333 retrieved abstracts, 1681 duplicates were removed, leaving 2652 for screening from which 20 full-text articles19–38 were included for analysis (Figure 2).
Figure 2.
PRISMA-ScR flowchart for study selection.
Study characteristics
All 20 studies were published between 2019 and 2022, with 70% (n = 14) published in 2021–22 (see Table 4). Ten studies were from the United States, 3 from Canada, 2 from the Netherlands, 2 from Europe and one each from Australia, United Kingdom, and Sweden. Half of the studies (n = 10) were frameworks targeting specific domains: (1) specific clinical disciplines including oncology,48,49 radiology (but not entirely focused on imaging processing or interpretation),50 or paediatrics51; (2) evaluation52,53; (3) ethics48,54; (4) governance50,55; (5) regulation56; and (6) safety.57 Of the other 10 generic framework papers, 5 were process frameworks (stage-based)33,42,58–60 with 3 to 7 (median 5) stages, and 5 were determinant frameworks (theme-based) with 3 to 7 themes (median 5).35,38,61–63
Table 4.
Study characteristics.
| Author, year, country | Source of framework, stage/dimensions identified | |
|---|---|---|
| Stage-based (process) frameworks: | ||
| 1 | van de Sande et al 2022,33 Netherlands | Source: Literature review; author creation. |
| Phases: (1) Preparation; (2) Model development; (3) Assessment of AI performance and reliability; (4) Clinically testing AI; (5) Implementing and governing of AI. | ||
| 16 substeps identified | ||
| 2 | de Hond et al 2022,59 Netherlands (AIPM) | Source: Multiple prior models + scoping review (72 papers). |
| Phases: (1) Preparation, collection and checking of the data; (2) Development of the AIPM; (3) Validation of the AIPM; (4) Development of the software application; (5) Impact assessment of the AIPM with software; (6) Implementation and use in daily healthcare practice. | ||
| 27 substage steps and a further 6 phase overarching topics. | ||
| 3 | Sendak et al 2020,42 United States | Source: Prior models58,64 + own experience. |
| Phases: 3 unnamed. | ||
| 8 substeps | ||
| 4 | Wiens et al 2019,58 United States | Source: Author created. |
| Phases: (1) Choosing the right problem; (2) Developing a useful solution; (3) Considering ethical implications; (4) Rigorously evaluating the model; (5) Reporting results; (6) Deploying responsibly; (7) Making it to market | ||
| 5 | Assadi et al 2022,60 Canada | Source: Narrative review and expert consensus, based on systems engineering and software development. |
| Phases: (1) Inception; (2) Preparation; (3) Development; (4) Integration. | ||
| 3 substeps (technical systems, human, environment) identified per phase. | ||
| Theme-based (determinant) frameworks: | ||
| 1 | Gama et al 2020,35 Sweden | Source: Scoping review (7 papers), derived from NASSS.27 |
| Themes: Same as NASSS: (1) Condition or illness; (2) Technology; (3) Value proposition; (4) Adopter system; (5) Organization(s); (6) Wider context; (7) Interaction and mutual adaptation between domains. | ||
| 22 subdomains and 7 new subdomains | ||
| 2 | Truong et al 2019,38 Canada | Source: Meetings with subject matter experts from 4 hospitals. |
| Themes: (1) Data; (2) Trust; (3) Ethics; (4) Readiness; (5) Expertise; (6) Buy-in; (7) Regulatory strategy. | ||
| 3 | Salwei et al 2022,61 United States | Source: Self-created and non-AI case study. |
| Themes: (1) Integrate AI into work system; (2) Integrate AI into clinical workflow; (3) Support decision making. | ||
| 4 | Oala et al 2021,62 Europe | Source: Consensus findings, self-created. |
| Themes: (1) Technical validation; (2) Clinical evaluation; (3) Regulatory assessment | ||
| 5 | Siala and Wang, 2022,63 United Kingdom (SHIFT-AI) | Source: Literature review (253 papers) and inductive thematic analysis. |
| Themes; (1) Sustainable AI; (2) Human-centered AI; (3) Inclusive AI; (4) FAIR AI; (5) Transparent AI. | ||
| 14 subthemes identified | ||
| Targeted frameworks: | ||
| 1 | Park et al 2020,52 United States | Targeting: Evaluating AI in healthcare. |
| Source: Mapping of study designs to drug/medial trial phases. | ||
| Phases: (1) Discovery and invention; (2) Safety and dosage; (3) Efficacy and side effects; (4) Therapeutic efficacy; (5) Safety and effectiveness. | ||
| 2 | Reddy et al 2021 (TEHAI),53 Australia | Targeting: Evaluating real-world artificial intelligence systems. |
| Source: Literature review (6 papers) and consensus inclusion with panel. Components: (1) Capability; (2) Utility; (3) Adoption. | ||
| 15 subcomponents | ||
| 3 | Hantel et al 2022 (A4R-OAI),48 United States | Targeting: Ethically deployed oncology AI. |
| Source: Based on accountability for reasonableness framework. | ||
| Principles: (1) Relevance; (2) Publicity; (3) Revision; (4) Empowerment; (5) Enforcement. | ||
| 4 | Bedoya et al 2022 (ABCDS),55 United States | Targeting: Governance. |
| Source: Software development cycle, FDA regulatory best practice and self-created and implemented governance methodology. | ||
| Phases: (1) Model development; (2) Silent evaluation; (3) Effectiveness evaluation; (4) General deployment. | ||
| Further 16 subphase steps. | ||
| 5 | Bazoukis et al 2022,56 United States | Targeting: Integrated regulatory framework. |
| Source: Self-created. | ||
| Themes: (1) Regulatory challenges; (2) Oversight and regulation; (3) Safety and efficacy surveillance; (4) Accountability; (5) Liability; (6) Equity and inclusion; (7) Transparency; (8) Education; (9) Patient engagement; (10) Cybersecurity and privacy; (11) Ethics and fairness; (12) Financial incentives. | ||
| 6 | Char et al 2020,54 United States | Targeting: Ethical AI implementation. |
| Source: Literature review (83 papers) and author created. | ||
| Stages: (1) Conception; (2) Development; (3) Calibration; (4) Initial implementation; (5) Subsequent implementations. | ||
| 7 | Davahli et al 2021,57 United States | Targeting: Safety control system framework. |
| Source: Multiattribute value model approach, systematic review (67 papers), 10 interviews, 2 surveys. | ||
| Domains: (1) Safety policy; (2) Incentives for clinicians; (3) Clinician and patient training; (4) Communication and interaction; (5) Planning of actions; (6) Control on actions. | ||
| 13 second level attributes. | ||
| 8 | Nagaraj et al 2020,51 Canada | Targeting: Pediatric care. |
| Source: Self-created. | ||
| Stages: (1) Clinical use-case design; (2) Data acquisition and preparation; (3) Model development; (4) Model validation; (5) User validation; (6) Clinical integration; (7) Legal, privacy, and ethical considerations. | ||
| 5 further substeps. | ||
| 9 | Daye et al 2022,50 United States | Targeting: Radiology/governance. |
| Source: Self-created. | ||
| Steps: (1) Who decides which tool to implement; (2) What should be considered when assessing a tool for implementation; (3) How should each application be implemented in clinical practice; (4) How should tools be monitored and maintained for implementation. | ||
| Step 3 has 3 substeps. | ||
| 10 | Tsopra et al 2021,49 Europe | Targeting: Clinical validation of AI technologies for prediction in oncology. |
| Source: The ITFoC (Information Technology for the Future of Cancer) consortium, a multidisciplinary group from 6 European countries, self-created. | ||
| Principles: (1) Specify the intended use of AI; (2) Clearly specify the target population; (3) Specify the timing of AI evaluation; (4) Specify the datasets used for AI evaluation; (5) Specify the procedures used to ensure data safety; (6) Specify the metrics used for measuring AI performance; (7) Specify the procedures to ensure AI explainability. | ||
Framework names in parenthesis, if provided.
Five studies proposed frameworks without a stated methodology,50,51,56,58,61 8 utilized literature reviews,33,35,53,54,57,59,60,63 and 7 derived their framework from prior frameworks.35,48,52,55,57,59,60 Four studies employed a consensus method,49,53,60,62 2 used subject matter expert interviews,38,57 and 2 were based on authors’ own experiences.42,55 Two studies utilized 3 of the above methods57,60 and 4 studies utilized 2.35,53,55,59 Given the recency of all frameworks, assessing study acceptance using citation counts was not performed.
Refinement of the provisional SALIENT framework
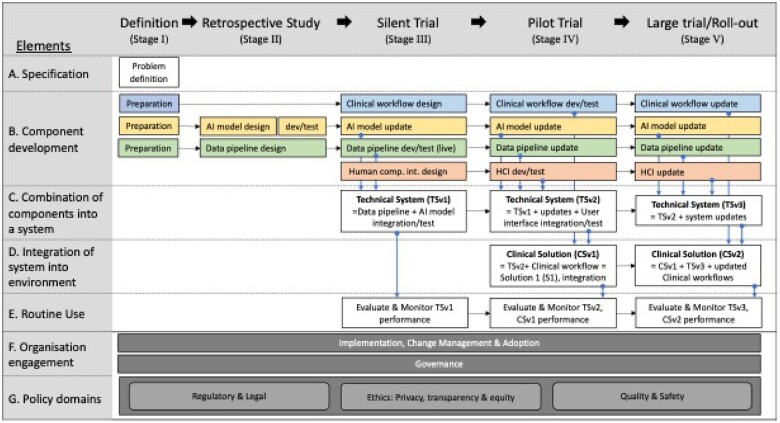
Of the 247 stages and themes (including subelements) extracted from the 20 included papers, 37% (n = 92) could be fully mapped to the provisional SALIENT framework, 40% (n = 98) could be partially mapped and 23% (n = 57) could not be mapped at all (see Supplementary Appendix SE for complete mapping). The gap analysis consolidated the partial and unmapped elements and informed the inclusion into SALIENT of 5 new cross-stage themes, 3 Stage I (Definition) component additions, and 16 new component tasks.
Two of the cross-stage themes—(1) Implementation, change management, and adoption33,38,42,48,51,53,56,58–60; and (2) Governance33,42,50,53,56—were housed in a new SALIENT element, “F. Organisation engagement.” These 2 cross-stage themes were informed by prior framework findings. Implementation, change management, and adoption required (1) the clear identification and engagement of all stakeholders, including not just clinicians and data scientists, but patients, ethicists, social scientists, managers, and legal experts33,38,48,51,56,58–60; (2) use of broad communication strategies, especially regarding stakeholder roles and responsibilities38,42,51; and (3) planning to generate long-term clinical buy-in and adoption, especially for nondevelopment sites with possibly different clinical workflows.38,42,51,53,59 The second cross-stage theme, Governance, involves the arrangements for providing program oversight, deciding on final AI model selection, timing and readiness for implementation, and ensuring various governance standards (see below), are known and upheld.33,42,50,53,56
Three other cross-stage themes were grouped into a new SALIENT element, “G. Policy domains,” comprising (1) Regulatory and legal33,38,48,51,56,57,60; (2) Ethics, including privacy, transparency, and equity33,38,48,49,51,54–60; and (3) Quality and safety.33,38,50,54,56–59 The first domain is awareness by all concerned of the relevant jurisdictional legal and regulatory evaluation and approval frameworks prior to AI implementation.33,38,48,56,57,60 Healthcare organizations and their clinicians need to understand who assumes liability and accountability for using AI model outputs in making clinical decisions.51,56,57 The second domain of ethics has 3 components: (1) Data privacy, including compliance with privacy laws, mandating consideration of data ownership, data traceability, right to privacy, and cyber security protections to prevent breaches33,38,48,49,51,53,54,56,58–60; (2) Transparency in relation to who generates the AI model and who uses model outputs, including the degree of clinician autonomy (assistive or autonomous AI), locked or adaptive (continuously learning) AI model,38,48,54 and scope,33,38,57–59 interpretability and auditability38,48,49,53,54,59 of the AI model; and (3) Healthcare equity including assessments and monitoring of model fairness and bias across all stages to protect minority populations.38,51,53–56,58–60 The third domain of quality and safety includes: (1) automated systems to detect data shift and where necessary retire, retrain or upgrade AI models33,50,54,58,59; (2) quality management systems to monitor for clinical practice updates that might disrupt AI model inputs or corrupt AI model accuracy; (3) systems for logging and tracing clinician decisions in response to model outputs59; (4) risk management strategies and safety surveillance for capturing adverse events related to AI-based decisions and determining agreed accuracy thresholds for the timely recall of AI models if becoming unreliable30,53,56,57; and (5) safety incentive programs to promote the judicial use by clinicians of AI rather than blind reliance.57
The stage I definition element was also expanded to include 6 preparation tasks for the AI model, clinical workflow and data pipeline components, 10 other new tasks were integrated into existing components, and all tasks, respective sources and applicable stages are shown in Table 5, with the finalized SALIENT framework depicted in Figure 3.
Table 5.
New tasks informed by findings from the scoping review of prior AI implementation frameworks, grouped by the SALIENT AI framework component (column 1, blue text).
| Revised SALIENT framework: components and tasks | Stages II and III | Stage IV | Stage V | |
|---|---|---|---|---|
| Framework element A: specifications | ||||
| Preparation—data pipeline | ||||
| PDP01 | Identify, collect, and prepare data for development and validation, including training data54,58–60 | |||
| PDP02 | Establish interoperability and align to clinical coding standards (eg, ICD codes)33,49,59 | |||
| PDP03 | Investigate and establish IT hardware and storage capability60 | |||
| Preparation—AI model | ||||
| PAM01 | Search for and evaluate existing AI models33,42 | |||
| PAM02 | Perform a cost benefit analysis and feasibility assessment of using AI27,35,53,59 | |||
| Preparation—clinical workflow | ||||
| PCW01 | Create a plan to evaluate the success of the implementation38,53 | |||
| Framework element B: component development | ||||
| Data pipeline (DP) | ||||
| DP11 | Stress test infrastructure59 | x | x | x |
| DP12 | Scalability assessment: assess changes to data sources and input protocols27,35 | x | x | |
| Artificial intelligence model (AM) | ||||
| AM05 | Devise means and document interpretability of AI model outputs38,48,49,53,59 | x | x | |
| AM06 | Define, publish, and update AI model fact label defining standardized communication of AI model information to end users33,38,57–59 | x | x | x |
| AM07 | Externally validate the model and assess generalizability33,49,53,58,59 | x | x | |
| AM08 | Check for, report on, and apply methods to reduce overfitting59 | x | x | |
| AM09 | Stress test AI model software59 | x | ||
| Human-computer interface (HC) | ||||
| HC5 | Stress test HC interface software59 | x | ||
| Framework element E: routine use | ||||
| Evaluation and monitoring | ||||
| EM12 | Monitor and track data shift and AI quality27,33,35,50,53,54,58,59 | x | ||
| EM13 | Log AI decisions for traceability59 | x | x | |
Applicable stages for each task, according to the TRIPOD, DECIDE-AI, and CONSORT-AI defined stages, are marked with an “x” in the appropriate columns, ie, for stage II/III (blue column), stage IV (amber column), and stage V (green column). Note that preparation tasks (PDP, PAM, PCW) are only applicable to stage I (Definition), which is not marked here.
ICD: International Classification of Diseases; IT: information technology; AI: artificial intelligence.
Figure 3.
Final clinical AI implementation framework (SALIENT). The colored boxes refer to solution components (see Element B). Blue for the clinical workflow, yellow for the AI model, green for the data pipeline, and red for the human computer interface. The dark grey shaded boxes identify the cross-stage elements (F and G). HCI: human computer interface; dev: development.
DISCUSSION
Our overarching aim was to develop a comprehensive, end-to-end clinical AI implementation framework that was integrated with current reporting standards for clinical AI research and informed by contemporary theories of staged AI implementation. Existing stage and theme-based frameworks were deemed inadequate in demarcating solution components or associated tasks and none incorporated the reporting standards. Our pragmatic staged approach, adapted from Stead et al, sought to fill this gap by addressing the what (components), when (stages), and how (tasks) of AI implementation, while the who (organization) and why (policy domains) were captured through the 5 cross-stage elements. In this way, SALIENT encompasses the “organisation,” “adopters,” and “wider systems domains” of the NASSS framework,27 the process implementation domain of the CFIR,25 and the “ethics,” “buy-in,” and “regulatory strategy” themes of Beil et al31 and Truong et al.38
The fact that 70% of AI implementation studies in our scoping review appeared within the last 2 years, with no studies prior to 2019, suggests AI framework theory has lagged behind the early adopters who deployed AI systems prior to 201965–68 and had to confront new challenges unaided by a fully developed implementation framework.7,69,70 Many of the subsequent frameworks found in our review were informed by these early experiences48,56,60,61,63 and a quarter specifically targeted emerging areas of common concern, including regulatory requirements,56 ethical concerns,48,54 and governance,50,55 which were captured in the new SALIENT cross-stage elements F and G.
The SALIENT framework is unique in several ways. Firstly, it includes both theme and stage elements, whereas all frameworks except one59 are either process or determinant. Secondly, SALIENT stands alone in mapping and integrating all elements of the reporting standards applicable to studies of AI development and evaluation. van de Sande et al33 and de Hond et al59 integrated some elements of these standards, and some are mentioned in 3 other frameworks.50,53,61 Crossnohere et al assessed the coverage of 14 descriptive and reporting clinical AI implementation frameworks across 5 content domains (transparency, reproducibility, ethics, effectiveness, and engagement) and showed CONSORT-AI and DECIDE-AI together covered 17 of 25 (68%) content items.71 By integrating these reporting standards, clinicians can be assured that AI implementation based on SALIENT is grounded in rigorous evaluation methodologies. Thirdly, by adapting Stead et al’s clinical informatics translation approach, SALIENT provides full visibility of the end-to-end solution scope including its intrinsic components, how and when they integrate, and the underlying implementation tasks.
This stand-alone implementation framework study has an associated companion study72 in which the utility of the SALIENT framework is validated by applying it to studies of deployed AI models for predicting sepsis in hospitalized patients, identified in a systematic review, and mapping the barriers, facilitators and key implementation decisions reported in these studies to the SALIENT framework. This companion study found that SALIENT had full coverage of all the stages and components of implementing sepsis AI prediction systems which need to be considered and accounted for.
Strengths and limitations
As far as we know, SALIENT is the only clinical AI implementation framework that conceptualizes all important tasks and solution components as one integrated schema (Figure 3). It provides immediately actionable insights, in for the form of checklists of component tasks for each implementation stage, for both AI developers and healthcare leaders wanting to successfully deploy clinical AI in real time and at a whole-of-organization level. SALIENT allows both clinicians and technologists to drill down, with a high level of structured detail missing in other guidance reports,30 to task-level responsibilities for each stage of implementation and for each component of the overall AI solution.
SALIENT is limited in that it attempts to present a generalizable and purpose-agnostic conceptualization of real-world AI implementation. Consequently, it cannot provide high-level granular detail for each task and theme relevant to specific AI applications, although each theme is extensively cited with primary sources that provide more information about specific areas of regulatory compliance,56 ethical concerns,48,54 governance,50,55 and patient and public involvement,73,74 all of which may vary across different jurisdictions.75 While SALIENT has been mapped to systematically retrieved studies of implemented sepsis prediction models (see companion paper), it requires further validation as a framework capable of meaningful application to real-world studies of deployed purpose-specific AI models.
CONCLUSIONS
This study has generated a novel end-to-end framework for implementing clinical AI within hospitals which has integrated existing theoretical frameworks with current reporting standards for research related to AI models. Its use may help healthcare organizations to navigate the steps required to successfully implement AI in clinical practice.
Supplementary Material
ACKNOWLEDGMENTS
External review was conducted with thanks by Dr. Amith Shetty, Clinical Director at the NSW Ministry of Health.
Contributor Information
Anton H van der Vegt, Centre for Health Services Research, The University of Queensland, Brisbane, Australia.
Ian A Scott, Department of Internal Medicine and Clinical Epidemiology, Princess Alexandra Hospital, Brisbane, Australia.
Krishna Dermawan, Centre for Information Resilience, The University of Queensland, St Lucia, Australia.
Rudolf J Schnetler, School of Information Technology and Electrical Engineering, The University of Queensland, St Lucia, Australia.
Vikrant R Kalke, Patient Safety and Quality, Clinical Excellence Queensland, Queensland Health, Brisbane, Australia.
Paul J Lane, Safety Quality & Innovation, The Prince Charles Hospital, Queensland Health, Brisbane, Australia.
FUNDING
AHV was funded through a Queensland Government, Advanced Queensland Industry Research Fellowship grant. The Queensland Government had no role within this research.
AUTHOR CONTRIBUTIONS
AHV and IAS conceptualized the review. AHV, PJL, VRK, and KD conducted the title/abstract screening and full text review. KD and AHV performed the quality assessments all data extraction and tabular data collation. AHV derived the proposed framework and AHV, RJS, KD, and IAS performed the task mapping, gap analysis and SALIENT framework update. AHV and IAS drafted the manuscript with revisions and feedback from PJL and VRK. Suzanne Bakken.
SUPPLEMENTARY MATERIAL
Supplementary material is available at Journal of the American Medical Informatics Association online.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests with respect to this publication.
DATA AVAILABILITY
There are no new data associated with this article.
REFERENCES
- 1. Amital H, Aamar S, Rubinow A. Bilateral septic arthritis of the hip: does etanercept play a role? A case report. Ann Intern Med 2001; 134 (8): 663–94. [DOI] [PubMed] [Google Scholar]
- 2. Collins GS, Reitsma JB, Altman DG, Moons KGM. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur J Clin Invest 2015; 45 (2): 204–14. [DOI] [PubMed] [Google Scholar]
- 3. OECD. AI and health: related scientific research by country. https://oecd.ai/en/dashboards/policy-areas/PA11. Published 2022. Accessed December 13, 2022.
- 4. Schootman M, Wiskow C, Loux T, et al. Evaluation of the effectiveness of an automated sepsis predictive tool on patient outcomes. J Crit Care 2022; 71: 154061. [DOI] [PubMed] [Google Scholar]
- 5. Cabitza F, Rasoini R, Gensini GF. Unintended consequences of machine learning in medicine. J Am Med Assoc 2017; 318 (6): 517–8. [DOI] [PubMed] [Google Scholar]
- 6. Triantafyllidis AK, Tsanas A. Applications of machine learning in real-life digital health interventions: review of the literature. J Med Internet Res 2019; 21 (4): e12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. He J, Baxter SL, Xu J, Xu J, Zhou X, Zhang K. The practical implementation of artificial intelligence technologies in medicine. Nat Med 2019; 25 (1): 30–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sharma M, Savage C, Nair M, Larsson I, Svedberg P, Nygren JM. Artificial intelligence applications in health care practice: scoping review. J Med Internet Res 2022; 24 (10): e40238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sujan MA, White S, Habli I, Reynolds N. Stakeholder perceptions of the safety and assurance of artificial intelligence in healthcare. Saf Sci 2022; 155: 105870. [Google Scholar]
- 10. Joshi M, Mecklai K, Rozenblum R, Samal L. Implementation approaches and barriers for rule-based and machine learning-based sepsis risk prediction tools: a qualitative study. JAMIA Open 2022; 5 (2): ooac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jocelyn Chew HS, Achananuparp P. Perceptions and needs of artificial intelligence in health care to increase adoption: scoping review. J Med Internet Res 2022; 24 (1): e32939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fujimori R, Liu K, Soeno S, et al. Acceptance, barriers, and facilitators to implementing artificial intelligence-based decision support systems in emergency departments: quantitative and qualitative evaluation. JMIR Form Res 2022; 6 (6): e36501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrison S, Despotou G, Arvanitis TN. Hazards for the implementation and use of artificial intelligence enabled digital health interventions, a UK perspective. Stud Health Technol Inform 2022; 289: 14–7. [DOI] [PubMed] [Google Scholar]
- 14. Richardson JP, Curtis S, Smith C, et al. A framework for examining patient attitudes regarding applications of artificial intelligence in healthcare. Digit Heal 2022; 8: 205520762210890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hashiguchi TCO, Oderkirk J, Slawomirski L. Fulfilling the promise of artificial intelligence in the health sector: let’s get real. Value Health 2022; 25 (3): 368–73. [DOI] [PubMed] [Google Scholar]
- 16. Rajkomar A, Hardt M, Howell MD, Corrado G, Chin MH. Ensuring fairness in machine learning to advance health equity. Ann Intern Med 2018; 169 (12): 866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goirand M, Austin E, Clay-Williams R. Implementing ethics in healthcare AI-based applications: a scoping review. Sci Eng Ethics 2021; 27 (5): 61. [DOI] [PubMed] [Google Scholar]
- 18. U.S. Food & Drug Administration. The Drug Development Process. https://www.fda.gov/patients/learn-about-drug-and-device-approvals/drug-development-process#:~:text=Drugs undergo laboratory and animal testing to answer basic questions about safety. Drugs are tested on people, they are safe and effective. FDA review teams thoroughly examine, or not to approve it. Accessed April 11, 2023.
- 19. Moons KGM, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015; 162 (1): W1–73. [DOI] [PubMed] [Google Scholar]
- 20. Collins GS, Dhiman P, Andaur Navarro CL, et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open 2021; 11 (7): e048008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vasey B, Nagendran M, Campbell B, et al. Reporting guideline for the early stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. BMJ 2022; 377: e070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu X, Rivera SC, Moher D, Calvert MJ, Denniston AK; SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. BMJ 2020; 370: m3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cambridge Dictionary. Meaning of implementation in english. https://dictionary.cambridge.org/dictionary/english/implementation. Accessed September 19, 2022.
- 24. Stead WW, Haynes RB, Fuller S, et al. Designing medical informatics resource projects to increase what is learned. J Am Med Inform Assoc 1994; 1 (1): 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009; 4 (1): 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bakken S, Ruland CM. Translating clinical informatics interventions into routine clinical care: how can the RE-AIM framework help? J Am Med Inform Assoc 2009; 16 (6): 889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Greenhalgh T, Wherton J, Papoutsi C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res 2017; 19 (11): e367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reed JE, Howe C, Doyle C, Bell D. Successful healthcare improvements from translating evidence in complex systems (SHIFT-Evidence): simple rules to guide practice and research. Int J Qual Health Care 2019; 31 (3): 238–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015; 10 (1): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Plint AC, Moher D, Morrison A, et al. Does the CONSORT checklist improve the quality of reports of randomised controlled trials? A systematic review. Med J Aust 2006; 185 (5): 263–7. [DOI] [PubMed] [Google Scholar]
- 31. Beil M, Proft I, van Heerden D, Sviri S, van Heerden PV. Ethical considerations about artificial intelligence for prognostication in intensive care. Intensive Care Med Exp 2019; 7 (1): 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sendak MP, Arcy JD, Kashyap S, et al. A path for translation of machine learning products into healthcare delivery. EMJ Innov 2020; 10: 19-00172. [Google Scholar]
- 33. Van De Sande D, Van Genderen ME, Smit JM, et al. Developing, implementing and governing artificial intelligence in medicine: a step-by-step approach to prevent an artificial intelligence winter. BMJ Heal Care Informatics 2022; 29 (1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verma AA, Murray J, Greiner R, et al. Implementing machine learning in medicine. CMAJ 2021; 193 (34): E1351–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gama F, Tyskbo D, Nygren J, Barlow J, Reed J, Svedberg P. Implementation frameworks for artificial intelligence translation into health care practice: scoping review. J Med Internet Res 2022; 24 (1): e32215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reed JE, Green S, Howe C. Translating evidence in complex systems: a comparative review of implementation and improvement frameworks. Int J Qual Health Care 2019; 31 (3): 173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Segar V, Ang PK, Foteff C, Ng K. A review of implementation frameworks to operationalize health technology assessment recommendations for medical technologies in the Singapore setting. Int J Technol Assess Health Care 2021; 37 (1): e56. [DOI] [PubMed] [Google Scholar]
- 38. Truong T, Gilbank P, Johnson-Cover K, Ieraci A. A framework for applied AI in healthcare. Stud Health Technol Inform 2019; 264: 1993–4. [DOI] [PubMed] [Google Scholar]
- 39. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. Electron Heal Rec Challenges Des Implement 2013; 10 (6): 135–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kitson A, Harvey G, McCormack B. Enabling the implementation of evidence based practice: a conceptual framework. Qual Health Care 1998; 7 (3): 149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larrabee JH, Rycroft-Malone J. Using the best evidence to change practice the PARIHS framework – a framework for guiding the implementation of evidence-based practice against a background of rising health. J Nurs Care Qual 2004; 19 (4): 297–304. [DOI] [PubMed] [Google Scholar]
- 42. Sendak MP, Ratliff W, Sarro D, et al. Real-world integration of a sepsis deep learning technology into routine clinical care: implementation study. JMIR Med Inform 2020; 8 (7): e15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schulz KF, Altman DG, Moher D; for the CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 2010; 7 (3): e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009; 26 (2): 91–108. [DOI] [PubMed] [Google Scholar]
- 45. Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4 (1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Veritas Health Innovation. Covidence. 2022.
- 47. Hart SG, Staveland LE. Development of NASA-TLX (Task Load Index): results of empirical and theoretical research. In: Hancock PA, Meshkati N, eds. Human Mental Workload. Amsterdam: North Holland Press; 1988. [Google Scholar]
- 48. Hantel A, Clancy DD, Kehl KL, et al. A process framework for ethically deploying artificial intelligence in oncology. J Clin Oncol 2022; 40 (34): 3907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tsopra R, Fernandez X, Luchinat C, et al. A framework for validating AI in precision medicine: considerations from the European ITFoC consortium. BMC Med Inform Decis Mak 2021; 21 (1): 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Daye D, Wiggins WF, Lungren MP, et al. Implementation of clinical artificial intelligence in radiology: who decides and how? Radiology 2022; 305 (3): 555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nagaraj S, Harish V, McCoy LG, et al. From clinic to computer and back again: practical considerations when designing and implementing machine learning solutions for pediatrics. Curr Treat Options Peds 2020; 6 (4): 336–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Park Y, Jackson GP, Foreman MA, Gruen D, Hu J, Das AK. Evaluating artificial intelligence in medicine: phases of clinical research. JAMIA Open 2020; 3 (3): 326–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reddy S, Rogers W, Makinen VP, et al. Evaluation framework to guide implementation of AI systems into healthcare settings. BMJ Heal Care Informatics 2021; 28 (1): 100444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Char DS, Abràmoff MD, Feudtner C. Identifying ethical considerations for machine learning healthcare applications. Am J Bioeth 2020; 20 (11): 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bedoya AD, Economou-Zavlanos NJ, Goldstein BA, et al. A framework for the oversight and local deployment of safe and high-quality prediction models. J Am Med Inform Assoc 2022; 29 (9): 1631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bazoukis G, Hall J, Loscalzo J, Antman EM, Fuster V, Armoundas AA. The inclusion of augmented intelligence in medicine: a framework for successful implementation. Cell Reports Med 2022; 3 (1): 100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davahli MR, Karwowski W, Fiok K, Wan T, Parsaei HR. Controlling safety of artificial intelligence‐based systems in healthcare. Symmetry (Basel) 2021; 13 (1): 102–25. [Google Scholar]
- 58. Wiens J, Saria S, Sendak M, et al. Do no harm: a roadmap for responsible machine learning for health care. Nat Med 2019; 25 (9): 1337–40. [DOI] [PubMed] [Google Scholar]
- 59. de Hond AAH, Leeuwenberg AM, Hooft L, et al. Guidelines and quality criteria for artificial intelligence-based prediction models in healthcare: a scoping review. NPJ Digit Med 2022; 5 (1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Assadi A, Laussen PC, Goodwin AJ, et al. An integration engineering framework for machine learning in healthcare. Front Digit Heal 2022; 4: 932411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Salwei ME, Carayon P. A sociotechnical systems framework for the application of artificial intelligence in health care delivery. J Cogn Eng Decis Mak 2022; 16 (4): 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Oala L, Murchison AG, Balachandran P, et al. Machine learning for health: algorithm auditing & quality control. J Med Syst 2021; 45 (12): 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siala H, Wang Y. SHIFTing artificial intelligence to be responsible in healthcare: a systematic review. Soc Sci Med 2022; 296: 114782. [DOI] [PubMed] [Google Scholar]
- 64. Greene SM, Reid RJ, Larson EB. Implementing the learning health system: from concept to action. Ann Intern Med 2012; 157 (3): 207–10. [DOI] [PubMed] [Google Scholar]
- 65. Shimabukuro DW, Barton CW, Feldman MD, Mataraso SJ, Das R. Effect of a machine learning-based severe sepsis prediction algorithm on patient survival and hospital length of stay: a randomised clinical trial. BMJ Open Resp Res 2017; 4 (1): e000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brown SM, Jones J, Kuttler KG, Keddington RK, Allen TL, Haug P. Prospective evaluation of an automated method to identify patients with severe sepsis or septic shock in the emergency department. BMC Emerg Med 2016; 16 (1): 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McCoy A, Das R. Reducing patient mortality, length of stay and readmissions through machine learning-based sepsis prediction in the emergency department, intensive care unit and hospital floor units. BMJ Open Qual 2017; 6 (2): e000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yin J, Ngiam KY, Teo HH. Role of artificial intelligence applications in real-life clinical practice: systematic review. J Med Internet Res 2021; 23 (4): e25759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sendak M, Gao M, Nichols M, Lin A, Balu S. Machine learning in health care: a critical appraisal of challenges and opportunities. eGEMs (Wash DC) 2019; 7 (1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shaw J, Rudzicz F, Jamieson T, Goldfarb A. Artificial intelligence and the implementation challenge. J Med Internet Res 2019; 21 (7): e13659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Crossnohere NL, Elsaid M, Paskett J, Bose-Brill S, Bridges JFP. Guidelines for artificial intelligence in medicine: literature review and content analysis of frameworks. J Med Internet Res 2022; 24 (8): e36823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van der Vegt AH, Scott IA, Dermawan K, Schnetler RJ, Kalke VR, Lane PJ. Deployment of machine learning algorithms to predict sepsis: systematic review and application of the SALIENT clinical AI implementation framework. J Am Med Inform Assoc 2023; ocad075. 10.1093/jamia/ocad075 [DOI] [PMC free article] [PubMed]
- 73. Schwartz JM, Moy AJ, Rossetti SC, Elhadad N, Cato KD. Clinician involvement in research on machine learning-based predictive clinical decision support for the hospital setting: a scoping review. J Am Med Inform Assoc 2021; 28 (3): 653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Banerjee S, Alsop P, Jones L, Cardinal RN. Patient and public involvement to build trust in artificial intelligence: a framework, tools, and case studies. Patterns 2022; 3 (6): 100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Donnelly D-L. First do no harm: legal principles regulating the future of artificial intelligence in health care in South Africa. Potchefstroom Electron Law J 2022; 25: 1–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
There are no new data associated with this article.