Abstract
Ongoing investigations of targeted therapeutic agents and their increased clinical applications, together with research in genomics and proteomics, have explored a variety of novel approaches for treatment of lung cancer, and ‘molecular subtypes’ have been defined based on specific actionable genetic aberrations. Liquid biopsies, including circulating tumor DNA (ctDNA) testing, are of value for cancer diagnosis and comprehensive genomic profiling, such as the identification of cancer subtypes and major genetic alterations in cancer cells. The case of a 66-year-old male patient with newly-diagnosed driver mutation-negative advanced non-small cell lung cancer (NSCLC) who underwent conventional therapy is described in the present report. The patient underwent regular monitoring, including continuous ctDNA analysis, imaging and assessment of tumor marker levels such as carcinoembryonic antigen (CEA). The patient initially presented with deep vein thrombosis which affected both lower extremities and without any symptoms in the lung, with a positron emission tomography scan identifying irregular pulmonary nodules in the right lower lobe and enlarged right supraclavicular lymph nodes. Subsequent ultrasound-guided fine-needle aspiration with nodule biopsy indicated advanced unresectable disease at stage IIIB based on the Tumor-Node-Metastasis staging system by the American Joint Committee on Cancer. Next-generation sequencing of tumor tissue and peripheral blood confirmed driver mutation-negative genes, including epidermal growth factor receptor, rat sarcoma, ALK receptor tyrosine kinase, ROS1 proto-oncogene receptor tyrosine kinase and rearrangement during transfection (RET). After 5 years of chemoradiotherapy and surveillance of ctDNA and CEA levels, detectable kinesin family member 5B (KIF5B)-RET fusion in ctDNA and rising CEA levels prompted early scans, which identified disease progression. The patient subsequently received the oral RET inhibitor pralsetinib, with treatment being currently ongoing for ≥17 months without detectable KIF5B-RET ctDNA or elevated CEA levels, with an ongoing minor response and stable disease based on Response Evaluation Criteria in Solid Tumors v1.1 on imaging. The present case illustrates the potential role of on-therapy circulating tumor biomarker monitoring as a non-traumatic method to evaluate therapy response and detect early disease progression in patients with advanced NSCLC. Integration of circulating tumor biomarker testing into the management of patients with advanced NSCLC requires additional prospective studies to actively assess and elucidate optimal treatment strategies.
Keywords: NSCLC, ctDNA, RET fusion, pralsetinib, case report
Introduction
During the past decades, the management of lung cancer has been markedly improved with the application of targeted therapy. Despite targeted drug therapies having resulted in notable therapeutic benefits and prolongation of survival on the whole, the extent of benefit of targeted therapies is not uniform. The rearrangement during transfection (RET) proto-oncogene encodes a transmembrane receptor tyrosine kinase, which functions as the receptor for the growth factors of the glial cell line-derived neurotropic factor family (1). The binding of glial cell line-derived neurotrophic factor family ligands facilitates RET kinase activation, which triggers the activation of signaling pathways associated with cell proliferation, including the MAPK and PI3K signaling pathways (1). Fusions in RET, such as KIF5B-RET, CCDC6-RET and NCOA4-RET consist of a type of aberration that can result in kinase activation. RET fusions have been reported in different types of malignancies, including 20–40% of sporadic cases of papillary thyroid carcinoma and 1.4–2.5% of non-small cell lung cancer (NSCLC) (2–5). Fragile sites in RET are relatively conservative, and the chromosomal breakpoints of RET often occur within intron 11 (6). Kinesin family member 5B (KIF5B), described in the present case report, is the most common RET fusion partner gene accounting for 60–80% of all rearrangements (7,8). Similar to other types of oncogenic RET fusions observed, KIF5B-RET proteins are likely to form a homodimer through the coiled-coil domain of KIF5B, which causes aberrant activation of the kinase function of RET in a manner similar to KIF5B-ALK receptor tyrosine kinase fusions (9). The two selective RET inhibitors approved by the China National Medical Products Administration, pralsetinib and selpercatinib, have markedly changed the treatment landscape for RET fusion-positive NSCLC between 2021 and 2022 (10). Unique genetic characteristics and clinicopathological features have been observed in patients with NSCLC harboring RET fusions (11).
Both histological and molecular subtyping have become increasingly important as predictors of benefits of treatment in lung cancer (12). Further evidence has emerged which demonstrates the importance of the molecular re-subtyping of different histology in predicting treatment benefits for patients with lung cancer (13). There has been a marked paradigm shift in the diagnosis and individualized clinical management of advanced NSCLC (14). Although tumor tissue sampling is the gold standard for molecular testing, ≤20% of samples from patients with advanced stage NSCLC may not be adequate in quantity and quality for pathological diagnosis and genomic profiling (15). Circulating biomarkers such as circulating tumor DNA (ctDNA), carcinoembryonic antigen (CEA) and cancer antigen 125 have emerged as valuable surrogates for auxiliary diagnosis and monitoring of treatment response in solid tumors (16). Prior studies have demonstrated that ctDNA is superior to CEA in terms of the detection of residual disease and early recurrence (17–19). Both of the aforementioned biomarkers are easily acquired, exhibit a rapid turnover and have high patient compliance, which has increased their clinical use in monitoring treatment efficacy and cancer progression. However, their roles still require further elucidation.
The case of a patient with advanced NSCLC negative for EGFR, RAS, BRAF, ALK, ROS1 and RET driver mutations is described in the present report.
Case report
A 66-year-old male patient with no notable past medical and family history initially presented at the Qingdao Municipal Hospital (Qingdao, China) in April 2016 with deep vein thrombosis in both lower extremities without pulmonary symptoms reported. A positron emission tomography scan demonstrated a 9×16 mm fluorodeoxyglucose (FDG)-avid right lower lobe (RLL) irregular pulmonary nodule with an FDG-avid 12×20 mm right supraclavicular lymph node. An ultrasound-guided fine-needle aspiration with pleural and nodule biopsies confirmed unresectable metastatic adenocarcinoma at stage IIIB (cT1N3M0; Tumor-Node-Metastasis staging system by AJCC; Fig. 1) (20). Analysis of tumor tissue and peripheral blood using next-generation sequencing (NGS) revealed the absence of driver mutations in EGFR, RAS, BRAF, ALK, ROS1 and RET genes (Shanghai Topgen Biomedical Technology Co., Ltd.). The KAPA HyperPlus Kit (cat. no. 7962428001; Roche Sequencing Solutions, Inc.) that enables rapid construction of DNA libraries was used to prepare DNA samples for sequencing. The Agilent 2100 bioanalyzer (Agilent Technologies, Inc.) uses capillary electrophoresis on a microchip device (LabChip 7500; Caliper Life Sciences, Inc.), and is capable of quality check after library dilution. Sequencing was performed on a NextSeq 550 System (Illumina, Inc.) in 75 bp paired-end mode for short libraries using a NextSeq 500/550 High Output Kit v2.5 and a total of 150 cycles (cat. no. 20024907; Illumina, Inc.) with a loading concentration of 1.4 pM final library. Brain magnetic resonance imaging was negative for intracranial disease. At the time of diagnosis of metastatic disease, the patient was asymptomatic and the physical exam was unremarkable with the exception of decreased breath sounds at the right lung base.
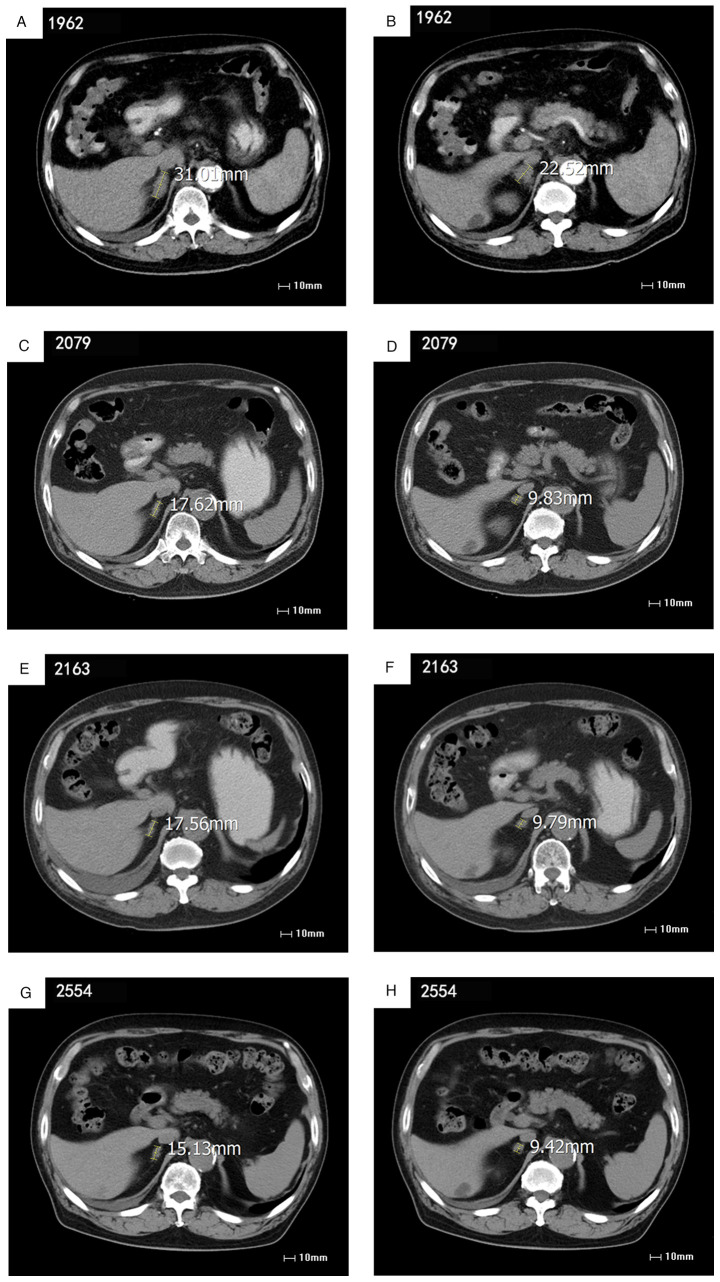
Figure 1.
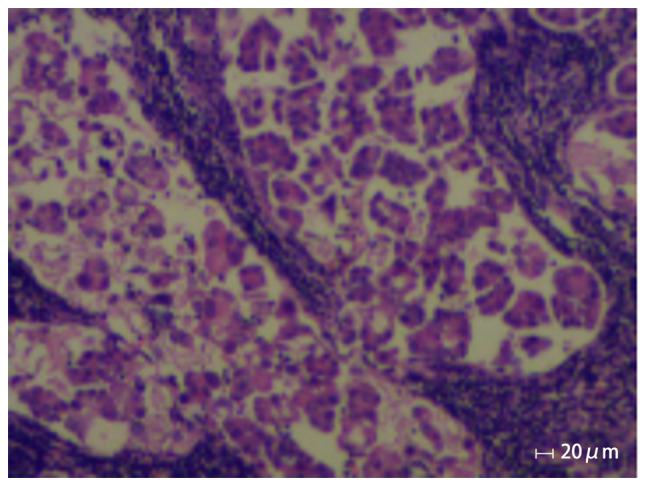
Histopathology showing cancer cells in the lymphoid tissue from the right supraclavicular fossa since the first visit in April 2016. Epithelioid cells are present in scattered nests and micropapillary structures, and focal calcifications, cytological atypia and mitotic figures occur in the lymphoid tissue from the right supraclavicular fossa.
In May 2016, four cycles (each 21 days) of induction cisplatin (75 mg/m2) and pemetrexed (600 mg/m2) chemotherapy were performed, with endostatin (30 mg/kg) added in cycle 3 until pathological response. In June 2016, involved-field radiotherapy alone was administered for palliative care with a dose of 60 Gy/30 fractions delivered in 2.0 Gy/day fractions, including the mediastinal regions and the supraclavicular lymph drainage area, after which both lesions continued to shrink. Subsequently maintenance with pemetrexed (600 mg/m2 on day 5) and endostatin (30 mg/kg from days 1 to 7) (21-day cycle, for 4 cycles) was performed in October 2016. Stable disease was achieved until July 2018. The detection of a 6-mm pulmonary nodule in the right lower lobe on the follow-up CT, along with a comprehensive evaluation of treatment efficacy, suggested gradual disease progression. Additionally, subsequent systemic chemotherapy with carboplatin (400 mg/m2 on day 1) and lipo-paclitaxel (240 mg/m2 on day 2) was performed, followed by carboplatin (400 mg/m2 on day 2), lipo-paclitaxel (240 mg/m2; on day 2) and bevacizumab (500 mg/kg; on day 1) until stable disease in September 2018. Due to grade III neutropenia (21) developing during subsequent treatment, the dose and regimen were adjusted to bevacizumab (500 mg/kg on day 0) and lipo-paclitaxel (180 mg/m2 on day 3). In February 2019, a follow-up CT demonstrated an enlarged 1.2 cm lesion in the RLL, which indicated metastatic disease. Concurrent chemoradiotherapy was considered for the patient and intensity modulated radiation therapy was prescribed at a dose of 60 Gy/30 fractions, administered as five fractions per week for 6 weeks. The chemotherapy schedule consisted of bevacizumab (500 mg/kg on day 1) and lipo-paclitaxel (180 mg/m2 on day 2), and was repeated every 4 weeks for four cycles. A follow-up CT identified right-sided pleural effusion in September 2019. Due to gastrointestinal bleeding, potentially due to bevacizumab intolerance, the regimens were adjusted to anlotinib (12 mg/kg daily) alone from November 2020 to August 2021. Admission and hospitalization occurred after 2 weeks of discomfort, including fatigue and anorexia. A follow-up CT of the metastatic abdominal lymph node demonstrated no change from earlier images; however, advanced adenocarcinoma at stage IV (cT1N3Mx) was confirmed (Fig. 2A and B).
Figure 2.
Imaging assessments of abdominal lymph node metastasis during treatment. The numeric value adjacent to the image number represents the time interval in days from the first visit. (A and B) No significant changes from earlier images of biopsy-confirmed RET-fusion-positive stage IV NSCLC prior to pralsetinib treatment. (C and D) Stable disease with pralsetinib during treatment. (E and F) Stable disease with pralsetinib on-treatment. Red arrows indicate changes in the size of abdominal lymph node metastasis. (A) and (B) demonstrated no significant changes from earlier images of biopsy-confirmed RET-fusion-positive stage IV NSCLC prior to pralsetinib treatment. (C-H) demonstrate stable disease with pralsetinib on-treatment. Size in (A-H) show changes in size of the left adrenal metastasis. CT, computed tomography; NSCLC, non-small cell lung cancer.
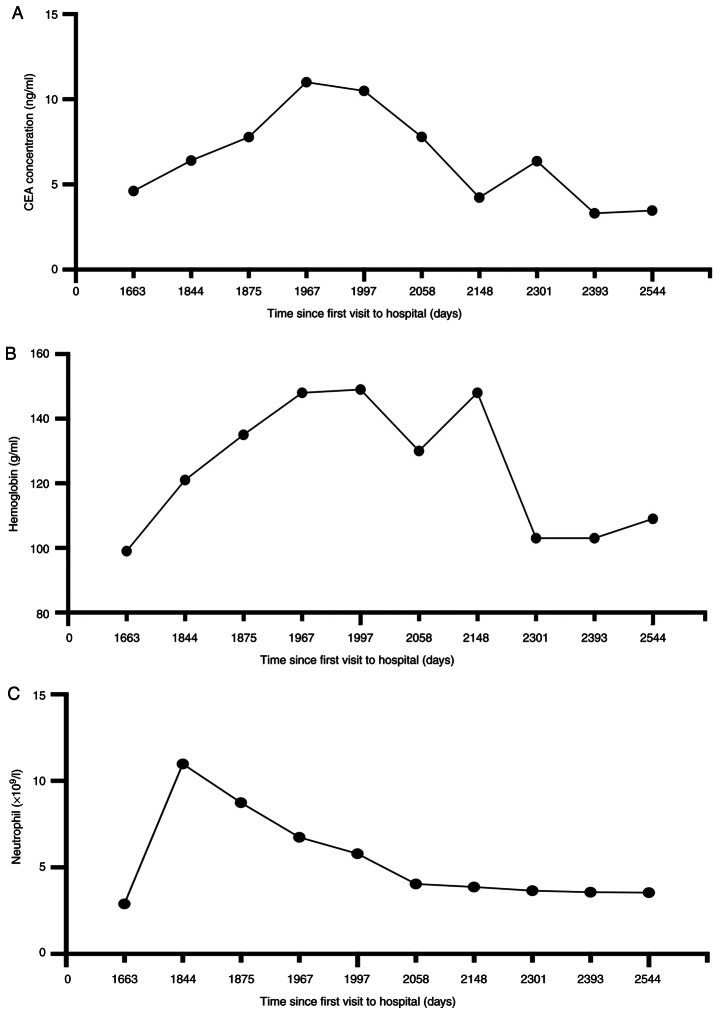
From November 2020 onwards, the effectiveness of systemic chemotherapy was assessed through the dynamic monitoring of serum CEA levels; this was conducted continuously for a duration of 891 days. Between May and August 2021, the CEA levels rose from 7.0 to ~10 ng/ml, which suggested progressive disease (Fig. 3A). Peripheral blood ctDNA test using NGS as aforementioned (Topgen Bio-Pharm Co, Ltd.) revealed a KIF5B-RET fusion with KIF5B exon 15 fused to RET exon 12 at a 7.4% variant allele fraction (VAF). VAF is the percentage of sequence reads observed matching a specific DNA variant divided by the overall coverage at that locus. Additionally, a TP53 G244D substitution with 4.7% VAF and a glutamate ionotropic receptor NMDA type subunit 2A (GRIN2A) F613V substitution with 2.0% VAF were also identified (Table I).
Figure 3.
Changes in carcinoembryonic antigens, neutrophil, and hemoglobin during pralsetinib treatment. (A) Changes in CEA since November 2021. Elevated levels of CEA pretreatment and decreased levels posttreatment were demonstrated during post-line treatment since November 2020. (B and C) The neutrophil count (×109/l) and hemoglobin level (g/l) were measured before and after treatment with pralsetinib, starting from 1,994 days after the initial visit. CEA, carcinoembryonic antigen.
Table I.
Next generation sequencing results acquired from the patient involved in this case.
| Days since first visit in April 2016 | Results | Sample type |
|---|---|---|
| 20 | Negative for EGFR, ALK and other driver genes. EGFR (−); ALK (−) | Peripheral blood |
| 1,969 | Detectable kinesin family member 5B-rearranged during transfection fusion; VAF 7.4% | Peripheral blood |
| TP53 c.731G>A (p.G244D); VAF 4.7% | ||
| GRIN2A c.1837T>G (p.F613V); VAF 2.0% | ||
| 2,343 | GRIN2A c.1516G>C (p.F183I); VAF 0.6% | Peripheral blood |
VAF, variant allele fraction; ALK, ALK receptor tyrosine kinase; GRIN2A, glutamate ionotropic receptor NMDA type subunit 2A.
In September 2021, the patient received RET inhibitor pralsetinib orally at a reduced dose of 300 mg once a day. An abdominal CT scan and testing of the conventional hematological clinical indicators, such as routine blood tests, liver function and renal function, obtained 23 days after the initiation of therapy, showed a minor response [stable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) v1.1; Fig. 2C and D] (17). The patient continued to take pralsetinib orally until April 2022 for a total of 8 months of treatment, and CEA levels continued to decrease during follow-up tests. An abdominal CT scan of the metastatic abdominal lymph node demonstrated no change from the last images (Fig. 2E and F). At the request of the patient, ctDNA was monitored at periodic intervals, the RET fusion was no longer detected by ctDNA assessment in December 2022, and only GRIN2A F183I with 0.6% VAF was observed, and ~13 months after the initiation of therapy, the CEA level dropped from 12 ng/ml in September 2021 to 3 ng/ml in November 2022 (Table I). The patient generally tolerated treatment well with certain clinically relevant adverse reactions observed, including a decreasing peripheral neutrophil count and a slightly decreased peripheral lymphocyte count, as well as an elevation of aspartate aminotransferase (AST) and grade II edema (22), which required a dose reduction to 300 mg (Fig. 3; Table II).
Table II.
Results of blood, liver and kidney function indicators for this patient.
| Days since first visit in April 2016 | |||||
|---|---|---|---|---|---|
|
|
|||||
| Function indicator | 1994 | 2079 | 2163 | 2554 | Reference range |
| Albumin, g/l | 28a | 39a | 39a | 41 | 40-55 |
| Prealbumin, mg/l | 281 | 413 | 436b | 376 | 200-430 |
| Aspartate aminotransferase, U/l | 30 | 38 | 58b | 35 | 15-45 |
| Gamma glutamyl transferase, U/l | 41 | 64b | 67b | 44 | 10-60 |
| Leucine aminopeptidase, U/l | 55 | 80b | 73b | 51 | 30-70 |
| α-L-fucosidase, U/l | 37 | 51b | 57b | 32 | 0-40 |
| Blood urea nitrogen, mmol/l | 9.9b | 10.4b | 11.5b | 18.4b | 3.6–9.5 |
| Creatinine, µmol/l | 115b | 117b | 125b | 152b | 57-111 |
| Cystatin, mg/l | 1.7b | 1.5b | 1.5b | 2.1b | 0.6–1.0 |
| Lymphocyte count, ×109/l | 0.9a | 3.0 | 2.6 | 3.1 | 1.1–3.2 |
| Lymphocyte, % | 15a | 39 | 37 | 43 | 20-50 |
| Red blood cells, 1012/l | 5.2 | 4.0a | 4.7 | 4.3a | 4.3–5.8 |
| Mean corpuscular volume, fl | 81a | 104 | 100b | - | 82-100 |
| Mean corpuscular hemoglobin concentration, pg | 25a | 32 | 31 | 26a | 27-34 |
| Mean corpuscular hemoglobin concentration, g/l | 309a | 310a | 314a | 288a | 316-354 |
| Red blood cell volume distribution width, fl | 63b | 75b | 54b | 59b | 37-54 |
Value below reference interval;
value above reference interval.
At the time of submission of the present report, the patient continued treatment with undetectable levels of KIF5B-RET ctDNA and exhibited a decreasing serum CEA level in the plasma for >17 months after the initiation of pralsetinib treatment, with an ongoing minor response and stable disease according to RECIST v1.1 on imaging, with a performance status (PS) score of 0–1 (23–25) (Fig. 2G and H).
The present study was performed in accordance with the Helsinki Declaration (2013 revision) and ethical standards approved by the Qingdao Municipal Hospital. The patient provided written consent for the publication of the case report and associated images.
Discussion
In the era of precision medicine and personalized cancer treatment, the demand for tumor molecular profiling is steadily increasing. The potential uses of liquid biopsy, such as ctDNA testing, have been identified in the context of diagnostics, prediction of prognosis and therapeutic efficacy, relapse monitoring and resistance detection (26–28). Implementing ctDNA testing for cancer diagnosis and monitoring in clinical application fosters research in patient management, despite the challenges and expectations it poses. The case of a patient with advanced NSCLC negative for EGFR, RAS, BRAF, ALK, ROS1 and RET driver mutations was described in the present report. Periodic on-treatment CEA surveillance and ctDNA reevaluation in the asymptomatic patient led to early identification of further disease progression, which supported prompt therapeutic decision-making.
Chemotherapy is considered to be the standard of care for the first-line treatment of patients with advanced-stage, either IIIB or IV, NSCLC with a good PS (ECOG PS<2) (23,24). A study previously reported that the combination of pemetrexed plus cisplatin was associated with significantly improved survival compared with gemcitabine plus cisplatin in patients with adenocarcinoma, with median survival time 12.6 vs. 10.9 months, respectively and P=0.03 (29). Due to its limited use in clinical practice, chemotherapy remains the main treatment option for patients with RET-rearranged advanced NSCLC. Multiple studies have shown that chemotherapy in patients with RET fusions yields an overall response rate (ORR) ranging from 26 to 50%, with a median progression-free survival (PFS) time of 5 to 9.2 months for first-line treatment and 2.8 to 5.2 months for second-line treatment (30–32). In a study of patients NSCLC and KIF5B-RET fusions compared with patients with non-KIF5B-RET fusions, PFS was assessed (7.8 vs. 11.2 months; P=0.847; hazard ratio, 0.902) (30). Although the survival benefit in RET fusion-positive NSCLC is limited, chemotherapy regimens containing pemetrexed may be relatively more effective in treating the condition. The present case study indicated the benefit of treatment, and revealed a >17-month PFS without detectable KIF5B-RET ctDNA or reduced CEA levels and a >7-year overall survival time since first-line chemotherapy with pemetrexed plus cisplatin was initiated in April 2016. At the time of submission of the present report, the patient continued to be on 300 mg pralsetinib with ongoing imaging-assessed stable disease according to RECIST v1.1 (25).
Targeted treatments based on driver genes, such as EGFR, ALK, RET and ROS1, provide a more precise option for the treatment of advanced NSCLC, and driver gene tests have become an essential component of clinical practice for the stratification of patients who are most likely to benefit from specific tyrosine kinase inhibitors (33). The phase I/II ARROW trial of pralsetinib in patients with advanced RET fusion-positive NSCLC reported an ORR of 63% and a complete response of 6%. Data from the Chinese subgroup indicated that pralsetinib was associated with an ORR of 56% and a disease control rate of 97% in patients in whom platinum-based therapy failed, ~50% of whom had experienced ≥3 lines of systemic treatment, and the median PFS time was 16.5 months (34,35). Clinically relevant adverse reactions in <15% of patients who received pralsetinib in the ARROW trial included changes in certain laboratory abnormalities, including chemistry indices such as increased AST and decreased albumin, and hematology indices such as decreased neutrophils and hemoglobin (35).
A previous retrospective study reported that cell-free ctDNA testing to detect fusion events could predict therapy response despite assay limitations (31). It has been reported that the detection rate of fusions in metastatic NSCLC is high at the initial onset of disease progression, and changes in RET fusion levels according to ctDNA testing are associated with tumor burden and treatment process (36,37). The detection rates of high frequency RET fusions such as KIF5B-RET and coiled-coil domain containing 6-RET in ctDNA could be improved by NGS that has higher sensitivity, which can benefit patients whose tissue or cytology samples are unavailable (38,39). There is also an increasing number of publications which have reported the application of circulating biomarkers, such as ctDNA and CEA, for real-time monitoring for early detection of disease progression in advanced NSCLC (16,17). Early reduction of circulating biomarkers after initiation of systemic therapy has been reported to be a predictive marker of clinical benefit in advanced tumors (27,28). In a study of 28 patients with metastatic NSCLC who received immune checkpoint inhibitor (anti-PD-1 or anti-PD-L1) therapy, most patients who demonstrated a long-term benefit from immunotherapy rapidly achieved a drop in ctDNA levels of 50–100%, and the initial ctDNA response occurred a median of 42.5 days earlier than the initial radiographic response (40). In a retrospective study of 40 patients with lung cancer treated with curative intent, detection of ctDNA posttreatment preceded radiographic progression in 72% of patients, by a median of 5.2 months (26). Early reduction in ctDNA after initiation of systemic therapy has been reported to be a predictive marker of clinical benefit in numerous advanced tumors (41).
For the patient in the present case study, continuous circulating biomarker monitoring of ctDNA and CEA resulted in the early detection of disease progression in the absence of symptoms and molecular re-subtyping after multiple lines of therapy, enabled a prompt adjustment in therapy, which the patient continued to receive at the time of this report. Dynamic monitoring of CEA has been performed since post-line treatment. The change in CEA level combined with CT imaging is of great significance for the management of the disease throughout the disease course. In genetic testing, there may be heterogeneity among samples from different disease stages, as well as heterogeneity between primary and metastatic lesions (42). Previous studies have reported that the mutation status of driver genes, including EGFR, ALK, BRAF, KRAS, ROS1 and RET, may change in patients with advanced NSCLC throughout the chemotherapy period (43,44). RET fusion was detected in ctDNA after the patient developed distant metastasis, which may also be the acquired RET fusion induced by platinum-based chemotherapy. However, the mechanism of chemotherapy-induced mutation status of driver genes requires further study in NSCLC. Furthermore, the dosage of pralsetinib was reduced due to an adverse reaction, but the treatment was not interrupted based on changes in certain laboratory parameters. In addition, targeted therapy with a RET inhibitor such as prelsetinib resulted in a reduction of metastatic lesion volume and a concomitant decrease in VAF value. As NGS provides a near random sample of the DNA molecules, VAF is thus a surrogate measure of the proportion of DNA molecules in the original specimen carrying the variant (45). A retrospective cohort analysis of 561 patients with advanced solid cancers, including NSCLC, pancreatic, prostate, colon and breast cancer, reported that there was a positive association between maximal VAF levels with the diameter or volume of all lesions, and in patients with undetectable ctDNA, a lower ctDNA VAF was associated with improved survival (46). Further investigation is necessary to elucidate the relationship between tumor mutation burden and prognosis, as well as its effect on ctDNA levels. In addition, it is unclear whether the prognostic value of ctDNA mutations and abundance is affected by chemotherapy or targeted therapy, despite previous findings which suggest baseline ctDNA levels can serve as a prognostic indicator (47). The lack of available tumor tissue during liquid biopsy prevented NGS from detecting genetic information in the tumor tissue, which was a limitation of the present case report; further exploration of NGS remains necessary for future research endeavors.
In conclusion, the case of a patient with advanced NSCLC which was negative for EGFR, RAS, BRAF, ALK, ROS1 and RET driver mutations at initial diagnosis, and only RET-fusion-positive after conventional treatment is described in the current report. ctDNA and CEA surveillance in the patient prompted early imaging which resulted in improved therapeutic decision-making. The current report provides further evidence of the potential benefit of serial tests or different combinations of tests during treatment as a useful and effective method to evaluate treatment efficacy and detect relapse (15,16). Further research is required to define the optimal integration of the scheme for management throughout the disease course in patients with advanced KIF5B-RET fusion-positive NSCLC, and to demonstrate the subsequent clinical decision-making that is informed by the scheme presented in the current report.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- RET
rearranged during transfection
- CEA
carcinoembryonic antigen
- RLL
right lower lobe
- NGS
next-generation sequencing
- ctDNA
circulating tumor DNA
- PFS
progression-free survival
- ORR
overall response rate
- VAF
variant allele fraction
Funding Statement
Funding: No funding was received.
Availability of data and materials
The raw data of NGS can be accessed using accession number PRJNA985255 in SRA of NCBI (https://www.ncbi.nlm.nih.gov/sra).
Authors' contributions
YB and CX drafted the manuscript. YB, XZ and HL collected data and served as scientific advisors. CX and HL performed data analysis and the literature review. YB, HL and CX conducted the final critical review of the paper. YB, XZ and HL confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study involving a human patient was reviewed and approved by the Qingdao Municipal Hospital (approval no. 20220616; Qingdao, China). Written informed consent was obtained from the patient, and all procedures were conducted in accordance with the Declaration of Helsinki. Shanghai Topgen Biomedical Technology Co., Ltd. had a College of American Pathologists certificate (approval no. 8671637-01) for assessing human samples using NGS genetic testing.
Patient consent for publication
The patient provided written consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Houvras YL. Completing the Arc: Targeted inhibition of RET in medullary thyroid cancer. J Clin Oncol. 2012;30:200–202. doi: 10.1200/JCO.2011.38.7639. [DOI] [PubMed] [Google Scholar]
- 2.Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European journal of endocrinology prize lecture. Eur J Endocrinol. 2006;155:645–653. doi: 10.1530/eje.1.02289. [DOI] [PubMed] [Google Scholar]
- 3.Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: Next-Generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23:1988–1997. doi: 10.1158/1078-0432.CCR-16-1679. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Guo L, Liu Y, Dong L, Yang L, Chen L, Liu K, Shao Y, Ying J. Potential unreliability of uncommon ALK, ROS1, and RET genomic breakpoints in predicting the efficacy of targeted therapy in NSCLC. J Thorac Oncol. 2021;16:404–418. doi: 10.1016/j.jtho.2020.10.156. [DOI] [PubMed] [Google Scholar]
- 5.Zhang K, Chen H, Wang Y, Yang L, Zhou C, Yin W, Wang G, Mao X, Xiang J, Li B, et al. Clinical characteristics and molecular patterns of RET-Rearranged lung cancer in Chinese patients. Oncol Res. 2019;27:575–582. doi: 10.3727/096504018X15344979253618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi M, Dillon LW, Pramanik S, Nikiforov YE, Wang YH. DNA breaks at fragile sites generate oncogenic RET/PTC rearrangements in human thyroid cells. Oncogene. 2010;29:2272–2280. doi: 10.1038/onc.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li AY, McCusker MG, Russo A, Scilla KA, Gittens A, Arensmeyer K, Mehra R, Adamo V, Rolfo C. RET fusions in solid tumors. Cancer Treat Rev. 2019;81:101911. doi: 10.1016/j.ctrv.2019.101911. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi K, Soda M, Togashi Y, Suzuki R, Sakata S, Hatano S, Asaka R, Hamanaka W, Ninomiya H, Uehara H, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 9.Kohno T, Ichikawa H, Totoki Y, Yasuda K, Hiramoto M, Nammo T, Sakamoto H, Tsuta K, Furuta K, Shimada Y, et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen P, Liu Y, Wen Y, Zhou C. Non-small cell lung cancer in China. Cancer Commun (Lond) 2022;42:937–970. doi: 10.1002/cac2.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu Z, Ye B, Wang K, Zhou P, Zhao S, Li W, Tian P. Unique genetic characteristics and clinical prognosis of female patients with lung cancer harboring RET fusion gene. Sci Rep. 2020;10:10387. doi: 10.1038/s41598-020-66883-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pikor LA, Ramnarine VR, Lam S, Lam WL. Genetic alterations defining NSCLC subtypes and their therapeutic implications. Lung Cancer. 2013;82:179–189. doi: 10.1016/j.lungcan.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Shim HS, Choi YL, Kim L, Chang S, Kim WS, Roh MS, Kim TJ, Ha SY, Chung JH, Jang SJ, et al. Molecular testing of lung cancers. J Pathol Transl Med. 2017;51:242–254. doi: 10.4132/jptm.2017.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan BA, Hughes BG. Targeted therapy for non-small cell lung cancer: Current standards and the promise of the future. Transl Lung Cancer Res. 2015;4:36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim C, Tsao MS, Le LW, Shepherd FA, Feld R, Burkes RL, Liu G, Kamel-Reid S, Hwang D, Tanguay J, et al. Biomarker testing and time to treatment decision in patients with advanced non small-cell lung cancer. Ann Oncol. 2015;26:1415–1421. doi: 10.1093/annonc/mdv208. [DOI] [PubMed] [Google Scholar]
- 16.Janse van Rensburg HJ, Spiliopoulou P, Siu LL. Circulating biomarkers for therapeutic monitoring of anti-cancer agents. Oncologist. 2022;27:352–362. doi: 10.1093/oncolo/oyac047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy MJ, Crown J. Circulating Tumor DNA as a biomarker for monitoring patients with solid cancers: Comparison with standard protein biomarkers. Clin Chem. 2022;68:1381–1390. doi: 10.1093/clinchem/hvac121. [DOI] [PubMed] [Google Scholar]
- 18.Kotani D, Oki E, Nakamura Y, Yukami H, Mishima S, Bando H, Shirasu H, Yamazaki K, Watanabe J, Kotaka M, et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat Med. 2023;29:127–134. doi: 10.1038/s41591-022-02115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao H, Chen KZ, Hui BG, Zhang K, Yang F, Wang J. Role of circulating tumor DNA in the management of early-stage lung cancer. Thorac Cancer. 2018;9:509–515. doi: 10.1111/1759-7714.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim W, Ridge CA, Nicholson AG, Mirsadraee S. The 8th lung cancer TNM classification and clinical staging system: Review of the changes and clinical implications. Quant Imaging Med Surg. 2018;8:709–718. doi: 10.21037/qims.2018.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaba H, Fukuda H, Yamamoto S, Ohashi Y. Reliability at the National Cancer Institute-Common Toxicity Criteria version 2.0. Gan To Kagaku Ryoho. 2004;31:1187–1192. (In Japanese) [PubMed] [Google Scholar]
- 22.Brodovicz KG, McNaughton K, Uemura N, Meininger G, Girman CJ, Yale SH. Reliability and feasibility of methods to quantitatively assess peripheral edema. Clin Med Res. 2009;7:21–31. doi: 10.3121/cmr.2009.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azam F, Latif MF, Farooq A, Tirmazy SH, AlShahrani S, Bashir S, Bukhari N. Performance status assessment by using ECOG (Eastern Cooperative Oncology Group) score for cancer patients by oncology healthcare professionals. Case Rep Oncol. 2019;12:728–736. doi: 10.1159/000503095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly CM, Shahrokni A. Moving beyond Karnofsky and ECOG performance status assessments with new technologies. J Oncol. 2016;2016:6186543. doi: 10.1155/2016/6186543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Chaudhuri AA, Chabon JJ, Lovejoy AF, Newman AM, Stehr H, Azad TD, Khodadoust MS, Esfahani MS, Liu CL, Zhou L, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov. 2017;7:1394–1403. doi: 10.1158/2159-8290.CD-17-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arrieta O, Villarreal-Garza C, Martínez-Barrera L, Morales M, Dorantes-Gallareta Y, Peña-Curiel O, Contreras-Reyes S, Macedo-Pérez EO, Alatorre-Alexander J. Usefulness of serum carcinoembryonic antigen (CEA) in evaluating response to chemotherapy in patients with advanced non small-cell lung cancer: A prospective cohort study. BMC Cancer. 2013;13:254. doi: 10.1186/1471-2407-13-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo YS, Zheng MY, Huang MF, Miao CC, Yang LH, Huang TW, Chou YT. Association of divergent carcinoembryonic antigen patterns and lung cancer progression. Sci Rep. 2020;10:2066. doi: 10.1038/s41598-020-59031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 30.Shen T, Pu X, Wang L, Yu Z, Li J, Zhang Y, Liang X, Chen H, Xu C, Song Z, Wang W. Association between RET fusions and efficacy of pemetrexed-based chemotherapy for patients with advanced NSCLC in China: A multicenter retrospective study. Clin Lung Cancer. 2020;21:e349–e354. doi: 10.1016/j.cllc.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, Camidge R, Narayanan V, Doebele RC, Besse B, et al. Targeting RET in patients with RET-Rearranged lung cancers: Results from the global, multicenter RET registry. J Clin Oncol. 2017;35:1403–1410. doi: 10.1200/JCO.2016.70.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drilon A, Bergagnini I, Delasos L, Sabari J, Woo KM, Plodkowski A, Wang L, Hellmann MD, Joubert P, Sima CS, et al. Clinical outcomes with pemetrexed-based systemic therapies in RET-rearranged lung cancers. Ann Oncol. 2016;27:1286–1291. doi: 10.1093/annonc/mdw163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.König D, Savic Prince S, Rothschild SI. Targeted therapy in advanced and metastatic non-small cell lung cancer. An update on treatment of the most important actionable oncogenic driver alterations. Cancers (Basel) 2021;13:804. doi: 10.3390/cancers13040804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q, Wu Y, Chang J, Wang H, Fan Y, Wang K, Wu G, Nian W, Sun Y, Sun M, et al. Efficacy and safety of pralsetinib in Chinese patients with advanced RET fusion+ non-small cell lung cancer after platinum-based chemotherapy. J Thorac Oncol. 2021;16:216–227. S100. doi: 10.1016/j.jtho.2021.01.1629. [DOI] [Google Scholar]
- 35.Subbiah V, Cassier PA, Siena S, Garralda E, Paz-Ares L, Garrido P, Nadal E, Vuky J, Lopes G, Kalemkerian GP, et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion-positive solid tumors from the phase 1/2 ARROW trial. Nat Med. 2022;28:1640–1645. doi: 10.1038/s41591-022-01931-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Supplee JG, Milan MSD, Lim LP, Potts KT, Sholl LM, Oxnard GR, Paweletz CP. Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer. 2019;134:96–99. doi: 10.1016/j.lungcan.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Rolfo C, Mack PC, Scagliotti GV, Baas P, Barlesi F, Bivona TG, Herbst RS, Mok TS, Peled N, Pirker R, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): A statement paper from the IASLC. J Thorac Oncol. 2018;13:1248–1268. doi: 10.1016/j.jtho.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 38.Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, Kushima R. RET-rearranged non-small-cell lung carcinoma: A clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571–1578. doi: 10.1038/bjc.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin C, Wang S, Xie W, Chang J, Gan Y. The RET fusion gene and its correlation with demographic and clinicopathological features of non-small cell lung cancer: A meta-analysis. Cancer Biol Ther. 2015;16:1019–1028. doi: 10.1080/15384047.2015.1046649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg SB, Narayan A, Kole AJ, Decker RH, Teysir J, Carriero NJ, Lee A, Nemati R, Nath SK, Mane SM, et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res. 2018;24:1872–1880. doi: 10.1158/1078-0432.CCR-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanz-Garcia E, Zhao E, Bratman SV, Siu LL. Monitoring and adapting cancer treatment using circulating tumor DNA kinetics: Current research, opportunities, and challenges. Sci Adv. 2022;8:eabi8618. doi: 10.1126/sciadv.abi8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caswell DR, Swanton C. The role of tumour heterogeneity and clonal cooperativity in metastasis, immune evasion and clinical outcome. BMC Med. 2017;15:133. doi: 10.1186/s12916-017-0900-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi X, Huang F, Chen A, Wu Z, Huang Q, Liang Y, Zhou Q, Mo H, Li X, Zhang J. The impact of chemotherapy on EGFR mutation status in non-small-cell lung cancer: A meta-analysis. Open J Gen. 2017;7:117–129. doi: 10.4236/ojgen.2017.74010. [DOI] [Google Scholar]
- 44.Pich O, Muiños F, Lolkema MP, Steeghs N, Gonzalez-Perez A, Lopez-Bigas N. The mutational footprints of cancer therapies. Nat Genet. 2019;51:1732–1740. doi: 10.1038/s41588-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strom SP. Current practices and guidelines for clinical next-generation sequencing oncology testing. Cancer Biol Med. 2016;13:3–11. doi: 10.20892/j.issn.2095-3941.2016.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsiehchen D, Espinoza M, Gerber DE, Beg MS. Clinical and biological determinants of circulating tumor DNA detection and prognostication using a next-generation sequencing panel assay. Cancer Biol Ther. 2021;22:455–464. doi: 10.1080/15384047.2021.1963166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parikh AR, Mojtahed A, Schneider JL, Kanter K, Van Seventer EE, Fetter IJ, Thabet A, Fish MG, Teshome B, Fosbenner K, et al. Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clin Cancer Res. 2020;26:1877–1885. doi: 10.1158/1078-0432.CCR-19-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data of NGS can be accessed using accession number PRJNA985255 in SRA of NCBI (https://www.ncbi.nlm.nih.gov/sra).