This cross-sectional study investigates how the phenotypic features of patients vary across genetic burden for primary open-angle glaucoma.
Key Points
Question
How do phenotypic features of patients vary across genetic burden for primary open-angle glaucoma (POAG)?
Findings
In a population-based cross-sectional study including 407 667 participants and 14 171 POAG cases, individuals at higher risk of glaucoma were identified using a genome-wide polygenic risk score. Higher polygenic risk was associated with more advanced disease (higher cup-disc ratio, intraocular pressure, thinner retinal nerve fiber layers/ganglion cell complex layers, or greater medication requirements, laser, or surgery treatment).
Meaning
Polygenic risk for POAG identified individuals at higher risk for POAG, supporting polygenic risk score stratification to identify individuals at higher risk of severe disease, potentially informing health care resource allocation and clinical decisions.
Abstract
Importance
Better understanding of primary open-angle glaucoma (POAG) genetics could enable timely screening and promote individualized disease risk prognostication.
Objective
To evaluate phenotypic features across genetic burden for POAG.
Design, Setting, and Participants
This was a cross-sectional, population-based study conducted from 2006 to 2010. Included participants were individuals from the UK Biobank aged 40 to 69 years. Individuals with non-POAG forms of glaucoma were excluded from the analysis. Data were statistically analyzed from October 2022 to January 2023.
Main Outcomes and Measures
POAG prevalence based on structural coding, self-reports, and glaucoma-related traits.
Results
Among 407 667 participants (mean [SD] age, 56.3 [8.1] years; 219 183 majority sex [53.8%]) were 14 171 POAG cases. Area under receiver operating characteristic curve for POAG detection was 0.748 in a model including polygenic risk score (PRS), age, sex, and ancestry. POAG prevalence in the highest decile of PRS was 7.4% (3005 of 40 644) vs 1.3% (544 of 40 795) in lowest decile (P < .001). A 1-SD increase in PRS was associated with 1.74 times higher odds of POAG (95% CI, 1.71-1.77), a 0.61-mm Hg increase in corneal-compensated intraocular pressure (IOP; 95% CI, 0.59-0.64), a −0.09-mm Hg decrease in corneal hysteresis (95% CI, −0.10 to −0.08), a 0.08-mm Hg increase in corneal resistance factor (95% CI, 0.06-0.09), and a −0.08-diopter decrease in spherical equivalent (95% CI, −0.11 to −0.07; P < .001 for all). A 1-SD increase in PRS was associated with a thinning of the macula-region retinal nerve fiber layer (mRNFL) of 0.14 μm and macular ganglion cell complex (GCC) of 0.26 μm (P < .001 for both). In the subset of individuals with fundus photographs, a 1-SD increase in PRS was associated with 1.42 times higher odds of suspicious optic disc features (95% CI, 1.19-1.69) and a 0.013 increase in cup-disc ratio (CDR; 95% CI, 0.012-0.014; P < .001 for both). A total of 22 of 5193 fundus photographs (0.4%) in decile 10 had disc hemorrhages, and 27 of 5257 (0.5%) had suspicious optic disc features compared with 9 of 5158 (0.2%) and 10 of 5219 (0.2%), respectively, in decile 1 (P < .001 for both). CDR in decile 10 was 0.46 compared with 0.41 in decile 1 (P < .001).
Conclusion and Relevance
Results suggest that PRS identified a group of individuals at substantially higher risk for POAG. Higher genetic risk was associated with more advanced disease, namely higher CDR and corneal-compensated IOP, thinner mRNFL, and thinner GCC. Associations with POAG PRS and corneal hysteresis and greater prevalence of disc hemorrhages were identified. These results suggest that genetic risk is an increasingly important parameter for risk stratification to consider in clinical practice.
Introduction
Primary open-angle glaucoma (POAG), the most common form of glaucoma, is a highly heritable complex disease.1,2 POAG heritability is estimated to be approximately 70%3 and a population-based study demonstrated that first-degree relatives of patients with POAG had a 9-fold increased risk of developing glaucoma.4,5 Although genome-wide association studies (GWAS) have identified at least 127 disease risk loci to date, POAG genetic architecture remains incompletely explained and individual POAG genetic risk variants have relatively small effects and poor predictive value.6
For complex diseases, polygenic risk scores (PRSs) can be used to measure the cumulative risk from contributions of many disease-associated DNA variants reflecting aggregate genetic risk. Accurate, generalizable PRSs can potentially inform clinical practice and influence disease-screening recommendations, as previously demonstrated in other common complex disease processes such as coronary heart disease, prostate cancer, and breast cancer.7,8,9,10 Prior POAG genetic risk scores and multitrait analysis of GWAS (MTAG)–derived PRSs for POAG have been generated, demonstrating that higher POAG genetic risk is associated with a higher risk of advanced glaucoma, higher intraocular pressure (IOP), earlier age of diagnosis and increased probability of disease progression in early-stage disease; furthermore, POAG PRSs have been shown to modulate the effect of myocilin variants.11,12,13 Prior glaucoma-related PRSs used in many of these studies have either been derived primarily from variants associated with glaucoma-related traits or a small number of disease-associated genetic variants. A genome-wide PRS for glaucoma that can be used to better understand the cumulative genetic burden for POAG as well as ocular features that may be associated with higher genetic risk for POAG could be used to help guide glaucoma management decisions.
The purpose of our study was to use available data in the UK Biobank (UKBB) to understand the association of background polygenetic risk for POAG with disease diagnosis as well as ocular and image-based features within a large population. Our results may contribute to a better understanding of how a POAG PRS may be associated with POAG disease features and ultimately be incorporated into individualized disease risk prognostication.
Methods
The UKBB Data Set
We used the UKBB data set, a prospective cohort study of 502 506 UK residents aged 40 to 69 years. The data set includes detailed genotypic and phenotypic information on all participants. Participant ancestry predicted from participant genotype was evaluated instead of race and ethnicity. Over 130 000 participants underwent eye examinations, including cornea-corrected IOP, corneal hysteresis (CH), and corneal resistance factor (CRF) using the Ocular Response Analyzer (Reichert) and autorefraction using the RC-5000 (Tomey). The National Research Ethics Service Committee NorthWest–Haydock approved the study, and it was conducted in accordance with the Declaration of Helsinki. All participants provided written informed consent. Participants did not receive financial incentive to participate in this study. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Assessment of POAG
Individuals with POAG were identified by the International Classification of Diseases, Ninth Revision (ICD-9) and Tenth Revision (ICD-10), diagnosis codes for POAG (ICD-9: 365.2; ICD-10: H40.1, H40.8, H40.9) from UKBB data field 41271/41270 or if they self-reported POAG (SR-POAG) on the eye problems/disorders (UKBB data field 6148) or noncancer illness fields (UKBB data field 20002), henceforth referred to in this article as ICD/SR-POAG. Individuals without glaucoma were identified if they had no ICD-9/ICD-10 diagnosis for POAG, no SR-POAG, no glaucomatous features on fundus photographs (cup-disc ratio [CDR] < 0.7 and no hemorrhage or suspicious optic disc features), medication-adjusted cornea-corrected IOP less than 21 mm Hg, and no history of glaucoma treatment (eg, glaucoma medications, glaucoma surgery, or laser). Individuals with non-POAG forms of glaucoma (eg, primary angle-closure glaucoma, secondary forms of glaucoma) were excluded from the analysis. The ICD/SR-POAG case vs control definition was used for the area under the receiver operating curve (AUROC) analysis; the remainder of the analysis included the entire cohort.
Fundus Photographs
Fundus photographs (FPs) were obtained for a subset of participants using the 3-dimensional (3-D) optical coherence tomography (OCT) 1000 Mark II (Topcon) and stored as .png image files. These images were evaluated by trained and certified ophthalmic image graders of the Network of Ophthalmic Reading Centres UK for a measurement of CDR and the presence of disc hemorrhage or other suspicious optic disc features (eg, notch, inferior rim thinning). FP assessments were made masked to POAG PRS. FPs assessed to be ungradable were excluded. Per Warwick et al,14 evidence of POAG on FP, henceforth referred to as FP-POAG in this article, was present if the vertical CDR (vCDR) was greater than 0.7 or if there was evidence of hemorrhage or suspicious optic disc features. Similarly, control individuals were identified if they had no ICD-9/ICD-10 diagnosis for POAG, no SR-POAG, no glaucomatous features on FPs (vCDR < 0.7, no hemorrhage or suspicious optic disc features), medication-adjusted cornea-corrected IOP less than 21 mm Hg, and no history of glaucoma treatment (eg, glaucoma medications, glaucoma surgery, or laser).
Assessment of Ocular Factors
Cornea-compensated IOP, CH, and CRF were obtained from UKBB data fields 5254, 5262, and 5256 for the right eye and 5264, 5257, and 5265 for the left eye. Information on cornea-corrected IOP-lowering medication use was obtained from UKBB data field 20003; pretreatment cornea-corrected IOP was imputed by dividing cornea-corrected IOP by 0.7 for those taking any IOP-lowering medication.15 Cornea-corrected IOP less than 5 mm Hg or greater than 60 mm Hg was excluded from the analysis. Spherical power and cylindrical power were obtained from UKBB data fields 5084 and 5085 for the right eye and 5087 and 5086 for the left eye. Spherical equivalent was calculated by adding half the cylindrical power to the spherical power. CRF, CH, and spherical equivalent greater than 3 SDs away from the mean were excluded from the analysis.
Assessment of Glaucoma Medications and Glaucoma Surgery
Individuals using glaucoma medications were identified if they reported glaucoma medication use (UKBB data field 20003) (eTable 1 in Supplement 1). Individuals who had previously undergone surgery or laser treatment for glaucoma were identified if they reported previous surgery or laser treatment for glaucoma (UKBB data fields 5326 and 5327).
OCT
Spectral-domain OCT scans of the macula were obtained on a subset of participants, and 3-D macular volume scans were also obtained (512 horizontal A-scans/B-scans; 128 B-scans in a 6 × 6-mm raster pattern). All OCT images were stored in .fda image files without prior analysis of macular thickness. The Topcon Advanced Boundary Segmentation algorithm was used to automatically segment all scans, using dual-scale gradient information to allow for automated segmentation of the inner and outer retinal boundaries and retinal sublayers.16,17 Segmented boundaries include the internal limiting membrane (ILM), retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), inner plexiform layer (IPL), inner nuclear layer (INL), external limiting membrane, photoreceptor inner segment/outer segment, retinal pigment epithelium (RPE), Bruch membrane, and the choroid-scleral interface (CSI). The thickness of each sublayer was calculated as the difference between boundaries of interest and averaging across all scans. The location of the fovea was determined by calculating the minimum thickness of the 3 inner-most segments across all B-scans and identifying the location where this thickness value approached 0. All B-scans obtained before this location were used to calculate mean thickness in the superior quadrants, whereas the numbers after were used to calculate inferior-quadrant thickness values. Sublayers include the RNFL, GCL, IPL, ganglion cell complex (GCC, defined as the total thickness of RNFL, GCL, and IPL), INL, outer plexiform layer, photoreceptor segment, RPE, and CSI (eTable 2 in Supplement 1).
The software provides an image quality score and segmentation indicators, which were used for quality control. Segmentation indicators included the ILM indicator, a measure of the minimum localized edge strength around the ILM boundary across the scan, which can be used to identify blinks, scans that contain regions of signal fading, and segmentation errors. We excluded all images with image quality less than 40 and images representing the poorest 10% using the ILM indicator.18 To exclude outliers, we also excluded any image with a layer thickness greater than 2.5 SDs away from the mean.
POAG PRS Calculation
The PRS for POAG was computed using GWAS summary statistics from the largest cross-ancestry meta-analysis,6 after exclusion of the UKBB cohort.19 Participants’ imputed genetic data were used as previously described.20 To predict the ancestral background of participants using ancestral labels from the 1000 Genomes Project Phase 3 reference panel, principal component analysis to linkage disequilibrium–pruned (r2 < 0.1 in 200kb windows) genetic markers with minor allele frequency greater than 1% and the k-nearest neighbors algorithm were used.13 The Lassosum method, a regression-based model that shrinks the variants via variable selection and retains the best set of variants by adjusting the tuning parameters, was used to compute the PRS using 9 705 359 imputed variants from 408 463 participants.21 Parameter settings included a sample of 5000 and cluster of cl. Calculated PRSs were normalized to a mean of 0 and SD of 1 within each ancestry group.
Statistical Analysis
For both cases and controls, participant-level cornea-corrected IOP, CH, CRF, spherical equivalent, FP features, and OCT values were calculated for the more severely affected eye. We defined the worse eye as the eye with the larger CDR if FP was available, thinner GCC if FP was not available, and higher cornea-corrected IOP if neither FP nor GCC were available. If data were available for only 1 eye, data for that eye were used. As visual field data were not available, higher vCDR, cornea-corrected IOP, thinner mRNFL and GCC, and greater requirements for medication, laser, and/or surgery to treat glaucoma were used as a proxy for more advanced disease.
Statistical analyses were performed from October 2022 to January 2023 using R, version 4.0.4 and RStudio, version 1.4.1106 (R Project for Statistical Computing). Mean and SD values were calculated for demographic and ocular characteristics. Mean and frequency values were compared across groups using 2-tailed t tests and χ2 tests or Fisher exact tests for continuous and categorical variables, respectively. We used logistic regression models adjusted for age, age2, sex, and ancestry to evaluate associations between PRS and POAG diagnosis, as well as PRS and glaucoma features on FP. Linear regression models adjusted for age, age2, sex, and ancestry were used to estimate associations between POAG PRS and ocular factors (cornea-corrected IOP, CH, CRF, spherical equivalent), POAG PRS and retinal thicknesses, and POAG PRS and CDRs. P values were 2-sided. For retinal thickness analyses with 9 nonoverlapping retinal layers, the threshold for significance was defined using a Bonferroni adjustment (P < .05/9 = .006).
Results
Study Population
Among the 407 667 UKBB participants (mean [SD] age, 56.3 [8.1] years; 188 484 male sex [46.2%]; 219 183 female sex [53.8%]) included in this analysis, 14 171 (3.5%) were identified as ICD/SR-POAG cases. A total of 87 812 participants (21.5%) had ocular data, including cornea-corrected IOP, CH, CRF, and spherical equivalent; 44 450 participants had FPs; and 37 851 participants had OCTs available for analysis. Of the 44 450 individuals with gradable FPs, 710 (1.6%) were identified as FP-POAG cases. Additionally, of the 44 411 individuals with gradable FPs, 1559 (3.5%) were ICD/SR-POAG cases, and 188 were identified as both FP-POAG and ICD/SR-POAG cases. Further study population characteristics can be found in eTable 3 in Supplement 1.
POAG PRS Performance
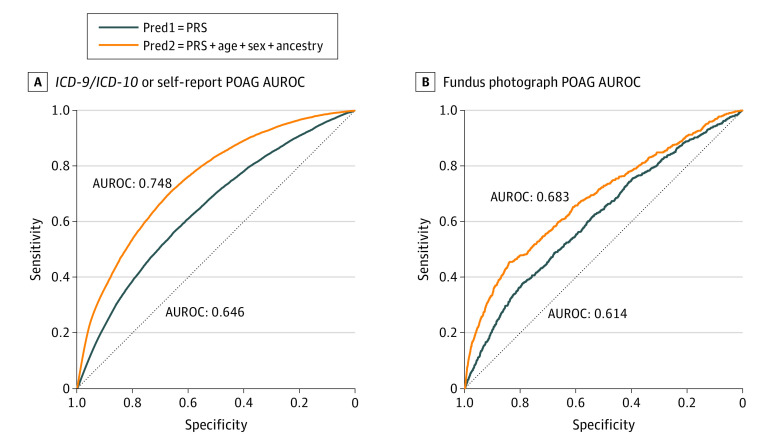
A POAG PRS was computed for the 14 171 ICD/SR-POAG cases and 393 496 controls. Individuals with ICD/SR-POAG had higher mean (SD) PRS for POAG compared with those without ICD/SR-POAG (0.50 [1.02] vs −0.02 [0.99]; P < .001). The AUROC for ICD/SR-POAG case detection was 0.646 for PRS alone and 0.748 with the addition of age, sex, and inferred ancestry (Figure 1). The prevalence of ICD/SR-POAG in the entire cohort was 3.5% (14 171 of 407 667); this prevalence increased progressively with each ICD/SR-POAG PRS decile (Figure 2). The prevalence of ICD/SR-POAG in decile 10 (those at highest genetic risk) was more than 5 times the prevalence of ICD/SR-POAG in decile 1 (those at lowest genetic risk; 1.3% [544 of 40 795] vs 7.4% [3005 of 40 644]). ICD/SR-POAG prevalence was higher with increased genetic risk at all ages; this outcome was most pronounced in older individuals (Figure 3). In a logistic regression model adjusting for age, age2, and sex, a 1-SD increase in PRS was associated with 1.74 times higher odds of ICD/SR-POAG (adjusted odds ratio [aOR], 1.74; 95% CI, 1.71-1.77; P < .001).
Figure 1. Area Under the Receiver Operating Characteristic Curve (AUROC) for International Classification of Diseases, Ninth (ICD-9) and Tenth (ICD-10) Revision/Self-Report Primary Open-Angle Glaucoma (POAG).
Pred1 indicates predictive model 1; pred2, predictive model 2; PRS, polygenic risk score.
Figure 2. Primary Open-Angle Glaucoma (POAG) Prevalence per POAG Polygenic Risk Score (PRS) Decile.
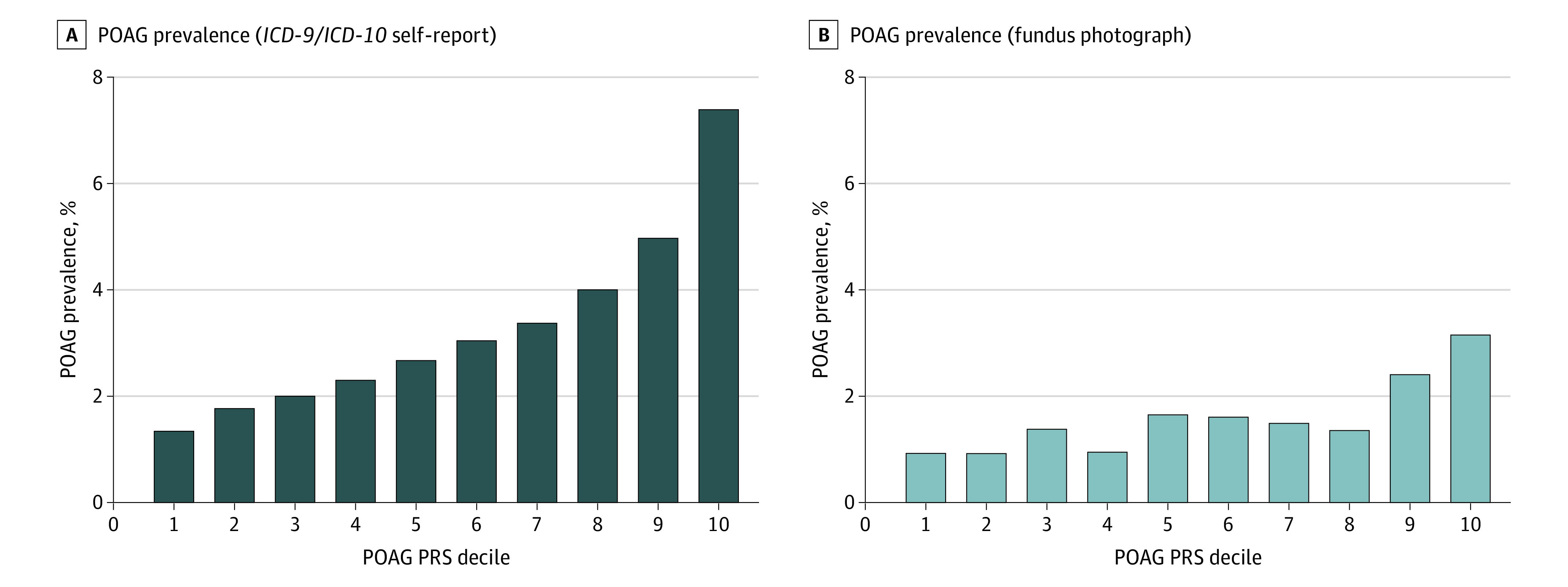
ICD-9 indicates International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision.
Figure 3. Primary Open-Angle Glaucoma (POAG) Prevalence by POAG Polygenic Risk Score (PRS) and Age.
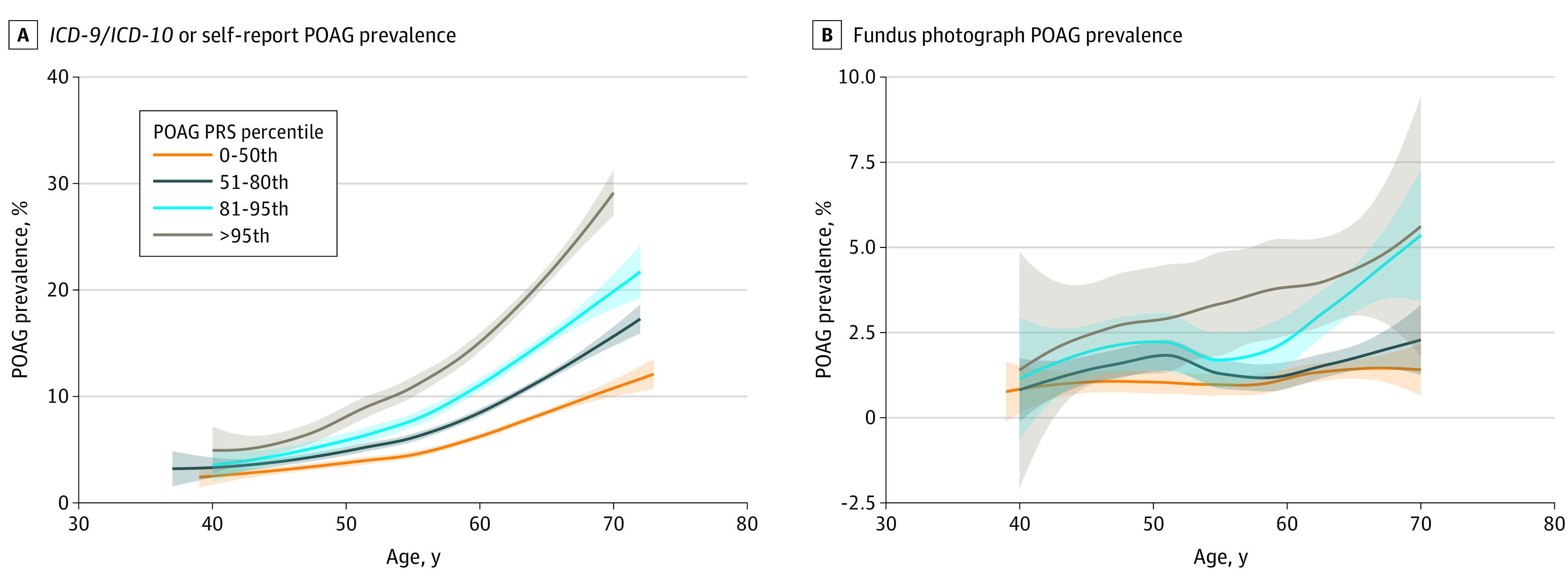
ICD-9 indicates International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision.
Similarly, in the subset of 44 411 individuals with available FPs, individuals at higher POAG genetic risk were more likely to have FP-POAG. AUROC for FP-POAG case detection was 0.614 for PRS alone and 0.683 with the addition of age, sex, and inferred ancestry (Figure 1). The prevalence of FP-POAG among individuals with available FPs was 1.6% (703 of 44 411). Although there was some variability, there was a progressive increase of FP-POAG prevalence from decile 1 to decile 10 (Figure 2), and FP-POAG prevalence increased with genetic risk at all ages (Figure 3). In an adjusted logistic regression, a 1-SD increase in PRS was associated with 1.46 times higher odds of FP-POAG (aOR, 1.46; 95% CI, 1.36-1.58; P < .001).
Use of Glaucoma Medications or Prior Glaucoma Surgery
Among the 407 667 participants included in this analysis, 4299 (1.05%) reported glaucoma medication use. A subset (n = 5617) had available data on previous glaucoma surgery or laser use; of this subset, 148 (2.63%) reported previous glaucoma surgery or laser use. Glaucoma medication use and previous glaucoma surgery or laser use increased with PRS decile (eFigure 1 in Supplement 1). In adjusted logistic regression, a 1-ICD/SD increase in PRS was associated with 1.95 times higher odds of glaucoma medication use (aOR, 1.95; 95% CI, 1.89-2.01; P < .001) and 1.67 times higher odds of previous glaucoma surgery or laser (aOR, 1.67; 95% CI, 1.42-1.97; P < .001).
Ocular Factors
A total of 87 512 individuals in this analysis had complete ocular data. Mean (SD) medication-adjusted cornea-corrected IOP was 16.60 (4.17) mm Hg, mean (SD) CH was 10.40 (1.91) mm Hg, mean (SD) CRF was 10.55 (1.98) mm Hg, and mean (SD) spherical equivalent was −0.21 (2.34) diopter (D). Higher POAG PRS decile was associated with higher medication-adjusted cornea-corrected IOP and CRF and lower spherical equivalent and CH (eFigure 2 and eTable 4 in Supplement 1). In adjusted models, a 1-SD increase in PRS was associated with 0.61 mm Hg higher cornea-corrected IOP (95% CI, 0.59-0.64; P < .001), −0.09 mm Hg lower CH (95% CI, −0.10 to −0.08, P < .001), 0.08 mm Hg higher CRF (95% CI, 0.06-0.09; P < .001), and a 0.08 D more myopic spherical equivalent (95% CI, −0.11 to −0.07; P < .001) (Table).
Table. Ocular Factors Logistic Regression per 1-Point Increase in Primary Open Angle Glaucoma Polygenic Risk Scorea.
| Variable | B (95% CI) | P value | Adjusted β (95% CI) | P value |
|---|---|---|---|---|
| Intraocular pressure, mm Hg | 0.61 (0.59 to 0.64) | <.001 | 0.62 (0.59 to 0.64) | <.001 |
| Corneal hysteresis, mm Hg | −0.09 (−0.10 to −0.08) | <.001 | −0.09 (−0.10 to −0.08) | <.001 |
| Corneal resistance factor, mm Hg | 0.08 (0.06 to 0.09) | <.001 | 0.08 (0.06 to 0.09) | <.001 |
| Spherical equivalent, diopter | −0.09 (−0.11 to −0.07) | <.001 | −0.08 (−0.10 to −0.07) | <.001 |
Adjusted model includes age, age2, sex, and ancestry as covariates.
Additionally, 4.0% of individuals (3464 of 87 512) had cornea-corrected IOP greater than 24 mm Hg, and 0.9% of individuals (824 of 87 512) had cornea-corrected IOP greater than 30 mm Hg. The prevalence of eyes with high cornea-corrected IOP greater than 24 mm Hg and 30 mm Hg increased with PRS decile. A total of 179 of 8737individuals (2.1%) in decile 1 had cornea-corrected IOP greater than 24 mm Hg, compared with 672 of 8737 (7.7%) in decile 10 (P < .001), and 40 of 8602 individuals (0.5%) in decile 1 had cornea-corrected IOP greater than 30 mm Hg, compared with 168 of 8737 (1.9%) in decile 10 (P < .001).
Imaging Features
Of the 44 411 FPs available for analysis, 111 (0.3%) had a hemorrhage on the disc, 126 (0.3%) had glaucomatous optic disc features, and 315 (0.7%) had a vCDR greater than 0.7. Mean (SD) vCDR was 0.43 (0.10). Prevalence of optic disc hemorrhage and glaucomatous optic disc features were highest in POAG PRS decile 10. A total of 22 of 5193 FPs (0.4%) in decile 10 had a hemorrhage on the optic disc, compared with 9 of 5158 (0.2%) in decile 1 (P = .07). Similarly, 27 of 5257 FPs (0.5%) in decile 10 had a suspicious optic disc feature, compared with 10 of 5219 (0.2%) in decile 1 (P = .03). vCDR increased progressively with POAG PRS decile (0.46 in decile 10 vs 0.41 in decile 1; P < .001) (eFigure 3 in Supplement 1). In an adjusted logistic regression, a 1-SD increase in PRS was associated with 1.42 times higher odds of glaucomatous optic disc features (aOR, 1.42; 95% CI, 1.19-1.69; P < .001). The association between PRS and optic disc hemorrhage did not reach significance (aOR, 1.19; 95% CI, 0.99-1.43; P = .07). In an adjusted linear regression, a 1-SD increase in PRS was associated with a 0.013 increase in vCDR (adjusted B = 0.013; 95% CI, 0.012-0.014; P < .001).
eTable 5 in Supplement 1 summarizes the mean thicknesses for each retina layer from 37 818 available OCTs. A 1-SD increase in POAG PRS was associated with a 0.14-μm thinner RNFL (95% CI, −0.19 to −0.1), a 0.05-μm thinner GCL (95% CI, −0.08 to −0.02), a 0.06-μm thinner IPL (95% CI, −0.09 to −0.04), and a 0.26-μm thinner GCC (95% CI, −0.34 to −0.17; P < .001 for all) (eTable 6 in Supplement 1). Similarly, decreases in INL (adjusted β = −0.03; 95% CI, −0.05 to −0.01; P = .003) were observed per 1-SD increase in POAG PRS (eTable 6 in Supplement 1). Although most layers had consistent changes in superior- and inferior-layer thickness per 1-SD increase in POAG PRS, the inferior RNFL had an adjusted β value that was more than double that of the superior RNFL (−0.2 μm vs −0.1 μm; P < .001 for both) (eTable 6 in Supplement 1). Among individuals with ICD/SR-POAG, those in decile 10 of POAG PRS had thinner inferior RNFL compared with those in decile 1 of POAG PRS (35.9 μm vs 39.2 μm; P < .001).
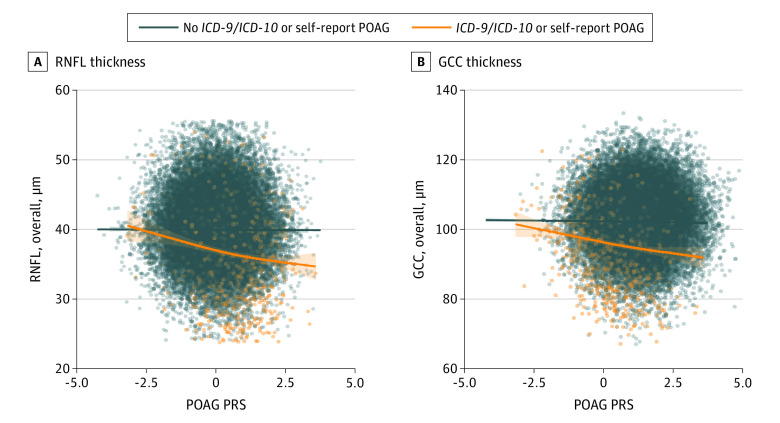
The association between PRS and RNFL and GCC thickness (eFigure 4 in Supplement 1) appeared to be largely driven by individuals with POAG. When our cohort was stratified by ICD/SR-POAG vs controls, individuals with ICD/SR-POAG had an association between PRS and thinner RNFL (adjusted β = −0.88 μm; 95% CI, −1.21 to −0.55; P < .001) whereas controls had no association. Similarly, individuals with ICD/SR-POAG had an association between PRS and thinner GCC (adjusted β = −1.4 μm; 95% CI, −1.98 to −0.23; P < .001), whereas controls had a diminished association (adjusted β = −0.14 μm; 95% CI, −0.23 to −0.05; P = .002) (Figure 4). These findings were replicated when stratified by FP-POAG case vs control.
Figure 4. Association Between Retinal Nerve Fiber Layer (RNFL) and Ganglion Cell Complex (GCC) Thickness With Primary Open-Angle Glaucoma (POAG) Polygenic Risk Score (PRS) for Individuals With POAG vs Controls.
ICD-9 indicates International Classification of Diseases, Ninth Revision; ICD-10, International Classification of Diseases, Tenth Revision.
Discussion
We were able to identify individuals at substantially higher risk of glaucoma using a genome-wide PRS. The risk increased across deciles and in all age groups, with the outcome most pronounced in older individuals. The PRS was associated with having more advanced disease, specifically higher vCDR and cornea-corrected IOP, thinner mRNFL and GCC, and greater requirements for medication, laser, and/or surgery to treat glaucoma. We also identified novel associations with background genetic risk and CH, greater prevalence of disc hemorrhages, and preponderance for decreased inferior RNFL thickness.
The prevalence of POAG in the highest PRS decile was more than 5 times the prevalence of POAG in the lowest decile, indicating that those with high PRS are truly at risk of developing glaucoma. Although there is limited work on this topic, a prior study11 using MTAG-derived PRSs that was constructed based on glaucoma disease status, vCDR, and IOP found that individuals in the top PRS decile had 14.9 times higher risk of POAG compared with those in the lowest decile. Overall, the AUROC using the PRS was somewhat useful but not likely high enough for population-based screening. Adding age, sex, and inferred ancestry increased the AUROC and resulted in similar findings to those reported previously with traditionally used risk factors (age, sex, and SR family history).11 Differences in performance of our PRS and prior literature are likely due to differing methods of PRS calculation used in each study as well as the definition of glaucoma. Prior MTAG-derived PRSs have been tested using data sets with clinically confirmed glaucoma cases,11 although here we applied a different PRS derived using Lassosum-penalized regression in a population where there was no systematic confirmation of case status, and our PRS still performed well.
Our results suggest that the AUROC for POAG case detection was similar for individuals with available FPs and for those without FPs. The prevalence of POAG in both individuals with FPs and without FPs progressively increased with each POAG PRS decile. This suggests that the usage of ICD diagnosis codes and SR-POAG is a valid way to identify individuals with glaucoma when assessing the utility of PRS in large population-based or registry studies. Indeed, prior studies have found high accuracy of ICD codes for the diagnosis of glaucoma.22,23,24
Those with higher PRS scores likely had more advanced disease, specifically, they had higher vCDR and cornea-corrected IOP, thinner mRNFL and GCC, higher glaucoma medication use, and were more likely to have prior glaucoma surgery and/or laser procedures. A previous study assessing an IOP-based PRS constructed from single-nucleotide variants demonstrated similar results in a sample of White European study participants from the UKBB cohort, with higher PRS associated with higher likelihood of increased IOP.25 A prior PRS stratification that assessed IOP using a registry of patients in Australia and New Zealand also demonstrated a significant association between high genetic risk groups having a higher maximum IOP as compared with lower genetic risk groups and demonstrated that treatment intensity (including the number of medications used and number of glaucoma operations) increased with higher PRS.26 Even with elevated treatment intensity, patients with higher PRS may have worse visual outcomes. In a longitudinal cohort study of individuals with early or suspected glaucoma, Siggs et al27 found that people in the top 5% of their MTAG-derived PRSs had a greater likelihood of visual field progression despite receiving significantly more eye drops and laser trabeculoplasty procedures. Due to higher medication, surgery, and laser use, the true strength of association between PRSs and glaucoma severity may be underestimated.
Thinning of the RNFL has been shown in the literature to be associated with progressive functional loss.28,29 Our results showed that higher PRS was associated with thinner macular RNFL, particularly in the inferior sector. Among individuals with glaucoma, those with higher polygenic risk for POAG had thinner inferior RNFL. It is possible that individuals without inferior thinning have nongenetic or not yet identified genetic causes of glaucoma. This association may also be a result of more frequent inferior RNFL thinning in early glaucoma. Prior studies have found that the AUROC tends to be greater for the inferior area compared with other quadrants, suggesting that the inferior area of the optic nerve is most affected in glaucoma.30,31 Although the inferior RNFL seems to undergo the most thinning in glaucoma, this region may also have a greater capacity for thinning before visual field loss, making it an optimal parameter for detecting early glaucoma. A previous retrospective cross-sectional study32 of 108 glaucoma study participants found that in the inferior quadrant, a greater percentage of RNFL thinning is required to detect functional loss of vision compared with the superior quadrant. Further work is required to understand imaging phenotypes associated with POAG genetic risk and how these may be combined to improve risk stratification.
We demonstrated a novel association between higher PRS and prevalence of disc hemorrhages on FP. It is possible that our result did not reach statistical significance owing to the rarity of this event and the possibility that eyes with disc hemorrhage secondary to causes other than glaucoma may have been included. Although the exact mechanism underlying disc hemorrhages remains unclear, multiple studies have demonstrated a strong association between disc hemorrhage and glaucoma progression.33 This suggests that accumulated genetic risk burden may predispose individuals to glaucoma visual field progression. Although is it not possible to assess progression rates in a population-based study, prior work has demonstrated an association between PRS and visual field progression in patients with glaucoma.27 The association between higher PRS and prevalence of disc hemorrhages may point to alternate ischemic or vascular etiology in high PRS glaucoma compared with glaucoma associated with low PRS. POAG is a complex disease with both genetic and environmental factors; therefore, disc hemorrhages may represent specific biological pathways that may help us better elucidate the mechanism of disease.
Higher PRS was also associated with lower CH in our study. Although central corneal thickness has been used classically to assess glaucoma risk, the association with higher PRS and CH here suggests that increased clinical attention should also be given to measuring CH.34 In separate unpublished analyses, our groups found that central corneal thickness did not correlate with POAG genetic risk in study participants from the Ocular Hypertension Treatment Study, suggesting that CH may be a better marker of POAG risk (unpublished data). Although the association between CH and glaucoma has been less thoroughly examined in previous literature, multiple studies have demonstrated that CH is strongly associated with glaucoma presence, risk of progression, and effectiveness of certain treatments.35,36,37,38,39,40,41 Even in patients with glaucoma and well-controlled intraocular pressure, lower CH was associated with a higher risk of global visual field progression.42 Low CH has also been found to be a risk factor for central visual field progression, which is a major concern for vision-related quality of life.43,44 It has been proposed that low CH may be associated with glaucoma progression because CH measurements may indirectly provide information about the characteristics of posterior ocular tissue extracellular matrix that make an eye more susceptible to glaucomatous damage.42 Our findings thus reinforce the clinical significance of CH in the diagnosis and management of glaucoma, especially in patients with higher PRS.
Strengths and Limitations
This study has several strengths, including its use of genetically inferred ancestry, large sample size, and exploration of viable glaucoma endophenotypes using IOP and OCT-derived retinal layer thicknesses. We also used not only diagnosis and SR-based definitions of glaucoma, but we also explored FP-based definitions of glaucoma. We were also able to demonstrate that individuals with the highest POAG PRS also had the lowest CH and highest myopia, the latter factors increasing propensity for developing severe disease.
However, this study is subject to several limitations that should be considered. First, 95.7% of the UKBB participants that met the inclusion criteria for our study are of European ancestry. In addition, although we used cross-ancestry summary statistics to construct our PRS, these weights are derived from prior GWAS with mostly European participants. Although the prior GWAS found that the majority of POAG loci had generally consistent effects across different ancestries, this highlights an issue of equity in representation in data.6 Further investigation is required to improve the generalizability of our PRS. Second, UKBB participants are aged 40 to 69 years. The prevalence of POAG increases with age, and people older than 80 years are at highest risk of having POAG.45 Despite the younger population and likely lower prevalence of POAG in the UKBB cohort, we observed a large effect size. Third, our data set is subject to influence from possible inaccuracies in medication self-reporting and medical documentation. These inaccuracies likely explain the limited overlap between study participants with ICD/SR-POAG and FP-POAG. However, the size of the data set likely diminishes this effect. Fourth, this study uses macular RNFL thicknesses, although in clinical practice, peripapillary RNFL are more often used. Fifth, we used a definition of POAG with lower specificity than other population-based studies; despite this limitation, our PRS performed well. Our definition of ICD/SR-POAG inferred that all self-reported cases of glaucoma had POAG, which may not be true, but we compensated by using alternative definitions and objective endophenotypes. Sixth, we used a vCDR cutoff of 0.7 to categorize FP-POAG. This may have resulted in false categorization of some individuals with large optic discs as having FP-POAG. Conversely, it is possible that this cutoff may have missed some true POAG cases. Finally, this study included individuals with ICD codes for POAG; diagnosis codes of secondary causes of glaucoma including exfoliation syndrome glaucoma and pigmentary glaucoma were excluded. Similarly, this data set and its conclusions may not apply to a population with normal tension glaucoma.
Conclusions
This cross-sectional investigation identified individuals at higher risk of POAG and found that higher PRS was associated with markers for more severe disease. We also identified associations of POAG PRS with optic disc hemorrhages and corneal hysteresis. This study supports the increasing clinical importance of PRS risk stratification to identify individuals at higher risk of severe disease to help inform health care resource allocation and clinical decision-making. Continuing to investigate the genetic markers contributing to our PRS may further our understanding of glaucoma pathology and reveal biomarkers useful for treatment development and disease monitoring.
eTable 1. Glaucoma Medications
eTable 2. Retinal Layer Boundaries of Interest
eTable 3. Study Population Characteristics
eTable 4. Ocular Factors by POAG PRS Decile
eTable 5. Retinal Layer Thicknesses
eTable 6. Association of Retinal Layer Thicknesses per 1 Point Increase in PRS
eFigure 1. Use of Glaucoma Medications or Prior Glaucoma Surgery/Laser per POAG PRS Decile
eFigure 2. Ocular Factors (SE, CHF, CRF, IOPcc) per POAG PRS Decile
eFigure 3. Fundus Photograph Characteristics (Hemorrhage On Disc, Suspicious Optic Disc Features, and Cup-to-Disc Ratio) by POAG PRS Decile
eFigure 4. Figure 4. Retinal Nerve Fiber Layer (RNFL) and Ganglion Cell Complex (GCC) Layer Thickness Among Individuals With Primary Open-Angle Glaucoma (POAG), Decile 1 vs Decile 10 of POAG Polygenic Risk Score (PRS)
Nonauthor Collaborators. UK Biobank Eye and Vision Consortium
Data Sharing Statement
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711-1720. doi: 10.1016/S0140-6736(04)16257-0 [DOI] [PubMed] [Google Scholar]
- 2.Greco A, Rizzo MI, De Virgilio A, Gallo A, Fusconi M, de Vincentiis M. Emerging concepts in glaucoma and review of the literature. Am J Med. 2016;129(9):1000.e7-1000.e13. doi: 10.1016/j.amjmed.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 3.Wang K, Gaitsch H, Poon H, Cox NJ, Rzhetsky A. Classification of common human diseases derived from shared genetic and environmental determinants. Nat Genet. 2017;49(9):1319-1325. doi: 10.1038/ng.3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khawaja AP, Viswanathan AC. Are we ready for genetic testing for primary open-angle glaucoma? Eye (Lond). 2018;32(5):877-883. doi: 10.1038/s41433-017-0011-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfs RCW, Klaver CC, Ramrattan RS, van Duijn CM, Hofman A, de Jong PT. Genetic risk of primary open-angle glaucoma—population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1640-1645. doi: 10.1001/archopht.116.12.1640 [DOI] [PubMed] [Google Scholar]
- 6.Gharahkhani P, Jorgenson E, Hysi P, et al. ; NEIGHBORHOOD consortium; ANZRAG consortium; Biobank Japan project; FinnGen study; UK Biobank Eye and Vision Consortium; GIGA study group; 23 and Me Research Team . Genome-wide meta-analysis identifies 127 open-angle glaucoma loci with consistent effect across ancestries. Nat Commun. 2021;12(1):1258. doi: 10.1038/s41467-020-20851-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knowles JW, Ashley EA. Cardiovascular disease: the rise of the genetic risk score. PLoS Med. 2018;15(3):e1002546. doi: 10.1371/journal.pmed.1002546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grossman DC, Curry SJ, Owens DK, et al. ; US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319(18):1901-1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 9.Maas P, Barrdahl M, Joshi AD, et al. Breast cancer risk from modifiable and nonmodifiable risk factors among white women in the US. JAMA Oncol. 2016;2(10):1295-1302. doi: 10.1001/jamaoncol.2016.1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seibert TM, Fan CC, Wang Y, et al. ; PRACTICAL Consortium . Polygenic hazard score to guide screening for aggressive prostate cancer: development and validation in large-scale cohorts. BMJ. 2018;360:j5757. doi: 10.1136/bmj.j5757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig JE, Han X, Qassim A, et al. ; NEIGHBORHOOD consortium; UK Biobank Eye and Vision Consortium . Multitrait analysis of glaucoma identifies new risk loci and enables polygenic prediction of disease susceptibility and progression. Nat Genet. 2020;52(2):160-166. doi: 10.1038/s41588-019-0556-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan BJ, Bailey JC, Igo RP Jr, et al. Association of a primary open-angle glaucoma genetic risk score with earlier age at diagnosis. JAMA Ophthalmol. 2019;137(10):1190-1194. doi: 10.1001/jamaophthalmol.2019.3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zebardast N, Sekimitsu S, Wang J, et al. ; International Glaucoma Genetics Consortium . Characteristics of p.Gln368Ter myocilin variant and influence of polygenic risk on glaucoma penetrance in the UK Biobank. Ophthalmology. 2021;128(9):1300-1311. doi: 10.1016/j.ophtha.2021.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warwick AN, Curran K, Hamill B, et al. ; UKBB Eye and Vision Consortium . UK Biobank retinal imaging grading: methodology, baseline characteristics and findings for common ocular diseases. Eye (Lond). 2022. Published online November 3, 2022. doi: 10.1038/s41433-022-02298-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Aschard H, Kang JH, et al. ; Modifiable Risk Factors for Glaucoma Collaboration . Intraocular pressure, glaucoma, and dietary caffeine consumption: a gene-diet interaction study from the UK Biobank. Ophthalmology. 2021;128(6):866-876. doi: 10.1016/j.ophtha.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keane PA, Grossi CM, Foster PJ, et al. ; UK Biobank Eye Vision Consortium . Optical coherence tomography in the UK Biobank Study—rapid automated analysis of retinal thickness for large population-based studies. PLoS One. 2016;11(10):e0164095. doi: 10.1371/journal.pone.0164095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Q, Reisman CA, Wang Z, et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. Opt Express. 2010;18(20):21293-21307. doi: 10.1364/OE.18.021293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Currant H, Hysi P, Fitzgerald TW, et al. Genetic variation affects morphological retinal phenotypes extracted from UK Biobank optical coherence tomography images. PLoS Genet. 2021;17(5):e1009497. doi: 10.1371/journal.pgen.1009497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvard University . Ayellet Segrè laboratory. Accessed October 20, 2022. https://segrelab.meei.harvard.edu/data/
- 20.Kolli A, Sekimitsu S, Wang J, et al. Background polygenic risk modulates the association between glaucoma and cardiopulmonary diseases and measures: an analysis from the UK Biobank. Br J Ophthalmol. Published online March 31, 2022. doi: 10.1136/bjophthalmol-2021-320305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak TSH, Porsch RM, Choi SW, Zhou X, Sham PC. Polygenic scores via penalized regression on summary statistics. Genet Epidemiol. 2017;41(6):469-480. doi: 10.1002/gepi.22050 [DOI] [PubMed] [Google Scholar]
- 22.Biggerstaff KS, Frankfort BJ, Orengo-Nania S, et al. Validity of code based algorithms to identify primary open angle glaucoma (POAG) in Veterans Affairs (VA) administrative databases. Ophthalmic Epidemiol. 2018;25(2):162-168. doi: 10.1080/09286586.2017.1378688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cai CX, Michalak SM, Stinnett SS, Muir KW, Fekrat S, Borkar DS. Effect of ICD-9 to ICD-10 transition on accuracy of codes for stage of diabetic retinopathy and related complications: results from the CODER Study. Ophthalmol Retina. 2021;5(4):374-380. doi: 10.1016/j.oret.2020.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of international classification of diseases, ninth revision, clinical modification billing codes for common ophthalmic conditions. JAMA Ophthalmol. 2013;131(1):119-120. doi: 10.1001/jamaophthalmol.2013.577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao XR, Huang H, Kim H. Polygenic risk score is associated with intraocular pressure and improves glaucoma prediction in the UK Biobank Cohort. Transl Vis Sci Technol. 2019;8(2):10. doi: 10.1167/tvst.8.2.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qassim A, Souzeau E, Siggs OM, et al. An intraocular pressure polygenic risk score stratifies multiple primary open-angle glaucoma parameters including treatment intensity. Ophthalmology. 2020;127(7):901-907. doi: 10.1016/j.ophtha.2019.12.025 [DOI] [PubMed] [Google Scholar]
- 27.Siggs OM, Qassim A, Han X, et al. Association of high polygenic risk with visual field worsening despite treatment in early primary open-angle glaucoma. JAMA Ophthalmol. 2022;141(1):73-77. doi: 10.1001/jamaophthalmol.2022.4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sayah DN, Mazzaferri J, Descovich D, Costantino S, Lesk MR. The association between ocular rigidity and neuroretinal damage in glaucoma. Invest Ophthalmol Vis Sci. 2020;61(13):11. doi: 10.1167/iovs.61.13.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renard JP, Fénolland JR, Giraud JM. Glaucoma progression analysis by spectral-domain optical coherence tomography (SD-OCT). J Fr Ophtalmol. 2019;42(5):499-516. doi: 10.1016/j.jfo.2019.03.001 [DOI] [PubMed] [Google Scholar]
- 30.Kanamori A, Nakamura M, Escano MFT, Seya R, Maeda H, Negi A. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135(4):513-520. doi: 10.1016/S0002-9394(02)02003-2 [DOI] [PubMed] [Google Scholar]
- 31.Zangwill LM, Bowd C, Berry CC, et al. Discriminating between normal and glaucomatous eyes using the Heidelberg retina tomograph, GDx nerve fiber analyzer, and optical coherence tomograph. Arch Ophthalmol. 2001;119(7):985-993. doi: 10.1001/archopht.119.7.985 [DOI] [PubMed] [Google Scholar]
- 32.Alasil T, Wang K, Yu F, et al. Correlation of retinal nerve fiber layer thickness and visual fields in glaucoma: a broken stick model. Am J Ophthalmol. 2014;157(5):953-959. doi: 10.1016/j.ajo.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee EJ, Kee HJ, Han JC, Kee C. Evidence-based understanding of disc hemorrhage in glaucoma. Surv Ophthalmol. 2021;66(3):412-422. doi: 10.1016/j.survophthal.2020.09.001 [DOI] [PubMed] [Google Scholar]
- 34.Belovay GW, Goldberg I. The thick and thin of the central corneal thickness in glaucoma. Eye (Lond). 2018;32(5):915-923. doi: 10.1038/s41433-018-0033-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simcoe MJ, Khawaja AP, Hysi PG, Hammond CJ; UK Biobank Eye and Vision Consortium . Genome-wide association study of corneal biomechanical properties identifies over 200 loci providing insight into the genetic etiology of ocular diseases. Hum Mol Genet. 2020;29(18):3154-3164. doi: 10.1093/hmg/ddaa155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freeman EE, Roy-Gagnon MH, Descovich D, Massé H, Lesk MR. The heritability of glaucoma-related traits corneal hysteresis, central corneal thickness, intraocular pressure, and choroidal blood flow pulsatility. PLoS One. 2013;8(1):e55573. doi: 10.1371/journal.pone.0055573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang L, Zhang R, He LY. Corneal hysteresis and glaucoma. Int Ophthalmol. 2019;39(8):1909-1916. doi: 10.1007/s10792-018-1011-2 [DOI] [PubMed] [Google Scholar]
- 38.Deol M, Taylor DA, Radcliffe NM. Corneal hysteresis and its relevance to glaucoma. Curr Opin Ophthalmol. 2015;26(2):96-102. doi: 10.1097/ICU.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medeiros FA, Meira-Freitas D, Lisboa R, Kuang TM, Zangwill LM, Weinreb RN. Corneal hysteresis as a risk factor for glaucoma progression: a prospective longitudinal study. Ophthalmology. 2013;120(8):1533-1540. doi: 10.1016/j.ophtha.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Moraes CVG, Hill V, Tello C, Liebmann JM, Ritch R. Lower corneal hysteresis is associated with more rapid glaucomatous visual field progression. J Glaucoma. 2012;21(4):209-213. doi: 10.1097/IJG.0b013e3182071b92 [DOI] [PubMed] [Google Scholar]
- 41.Zhang B, Shweikh Y, Khawaja AP, Gallacher J, Bauermeister S, Foster PJ; UKBiobank Eye and Vision Consortium . Associations with corneal hysteresis in a population cohort: results from 96 010 UK Biobank participants. Ophthalmology. 2019;126(11):1500-1510. doi: 10.1016/j.ophtha.2019.06.029 [DOI] [PubMed] [Google Scholar]
- 42.Susanna BN, Ogata NG, Jammal AA, Susanna CN, Berchuck SI, Medeiros FA. Corneal biomechanics and visual field progression in eyes with seemingly well-controlled intraocular pressure. Ophthalmology. 2019;126(12):1640-1646. doi: 10.1016/j.ophtha.2019.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kamalipour A, Moghimi S, Eslani M, et al. A prospective longitudinal study to investigate corneal hysteresis as a risk factor of central visual field progression in glaucoma. Am J Ophthalmol. 2022;240:159-169. doi: 10.1016/j.ajo.2022.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blumberg DM, De Moraes CG, Prager AJ, et al. Association between undetected 10-2 visual field damage and vision-related quality of life in patients with glaucoma. JAMA Ophthalmol. 2017;135(7):742-747. doi: 10.1001/jamaophthalmol.2017.1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang N, Wang J, Chen B, Li Y, Jiang B. Prevalence of primary angle closure glaucoma in the last 20 years: a meta-analysis and systematic review. Front Med (Lausanne). 2021;7:624179. doi: 10.3389/fmed.2020.624179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Glaucoma Medications
eTable 2. Retinal Layer Boundaries of Interest
eTable 3. Study Population Characteristics
eTable 4. Ocular Factors by POAG PRS Decile
eTable 5. Retinal Layer Thicknesses
eTable 6. Association of Retinal Layer Thicknesses per 1 Point Increase in PRS
eFigure 1. Use of Glaucoma Medications or Prior Glaucoma Surgery/Laser per POAG PRS Decile
eFigure 2. Ocular Factors (SE, CHF, CRF, IOPcc) per POAG PRS Decile
eFigure 3. Fundus Photograph Characteristics (Hemorrhage On Disc, Suspicious Optic Disc Features, and Cup-to-Disc Ratio) by POAG PRS Decile
eFigure 4. Figure 4. Retinal Nerve Fiber Layer (RNFL) and Ganglion Cell Complex (GCC) Layer Thickness Among Individuals With Primary Open-Angle Glaucoma (POAG), Decile 1 vs Decile 10 of POAG Polygenic Risk Score (PRS)
Nonauthor Collaborators. UK Biobank Eye and Vision Consortium
Data Sharing Statement