Abstract
Background
Previous studies found that increasing vegetable intake benefits are reduced after adjustment for socioeconomic factors. Using genetic variation as an instrumental variable for vegetable intake and socioeconomic status, we investigated the relationship between vegetable intake and ischemic cardio-cerebral vascular diseases and focused on whether socioeconomic status was a possible confounder.
Methods
From three independent genome-wide association studies, we extracted instrumental variables reflecting raw and cooked vegetable intake, which were used to perform Mendelian randomization analysis. To evaluate the effects of socioeconomic factors on vegetable intake, univariate and multivariate Mendelian randomization analyses were performed using single nucleotide polymorphisms representing education attainment and household income reported in the literature. We also performed outlier assessment and a series of sensitivity analyses to confirm the results.
Results
Genetically predicted raw and cooked vegetable intake were not associated with any ischemic cardio-cerebral vascular diseases and lipid components after Bonferroni correction. Univariate Mendelian randomized analysis revealed that raw vegetable intake was positively correlated with education attainment (β = 0.04, p = 0.029) and household income (β = 0.07, p < 0.001). Multivariate Mendelian randomized model showed a positive correlation between household income and raw vegetable intake (β = 0.06, p = 0.004). Socioeconomic status was closely associated with eating habits and lifestyle related to the risk of cardiovascular diseases.
Conclusion
Genetically determined raw and cooked vegetable intake was not associated with significant benefits in terms of ischemic cardio-cerebral vascular diseases while genetically determined socioeconomic status may have an impact on vegetable intake. Socioeconomic status, which was closely associated with other eating habits and lifestyle, may affect the association between vegetable intake and ischemic cardio-cerebral vascular diseases.
Keywords: vegetable intake, socioeconomic status, ischemic cardiovascular disease, ischemic cerebrovascular disease, Mendelian randomization
Introduction
Ischemic cardio-cerebral vascular diseases are the leading cause of death and decreased quality of life worldwide (1–3). Increased vegetable intake is widely recommended in the cardiovascular disease (CVD) field (4, 5). Many observational studies support the benefits of increased vegetable intake (6, 7), including lowering serum lipids and preventing chronic disease. However, the independent effects of raw and cooked vegetables on ischemic cardiovascular disease are poorly understood, and results vary in traditional epidemiological studies (8–12).
Socioeconomic status (SES), such as education attainment and household income, has a significant impact on the occurrence of cardiovascular disease (13). A large study found that those with primary education had an increased risk of cardiovascular disease, cardiovascular event mortality, and all-cause mortality compared with those with higher education (14). Patients with financial barriers are at higher risk of future CVD events (15). Socioeconomic status can limit access to fresh vegetables and fruits due to a lack of health literacy and high prices (16, 17). Several studies found that increasing vegetable intake benefits are reduced after adjustment for socioeconomic factors (8, 10, 18). And the risk of ischemic cardio-cerebral vascular disease is related to various lifestyle factors, including diet, smoking, drinking, physical inactivity, etc. (19–22), which are also closely related to education attainment and household income (14, 23, 24).
The Mendelian randomization (MR) approach has been widely used to assess the causal effects of risk factors on disease. Relying on pooled data from genome-wide association studies (GWAS) (25), the MR approach uses genetic variants, randomly assigned to individuals at conception, as instrumental variables (IVs) to analyze causal relationships between exposure and outcome. When performed carefully, MR analysis largely overcomes the limitations of confounders and avoids the bias of typical observational studies (26). Several previous Mendelian randomization studies (12, 27, 28) have found that vegetable intake did not reduce certain metabolic risk factors and the risk of some cardiovascular events. We aimed to estimate the effects of vegetable intake on ischemic cardio-cerebral vascular disease risk and serum lipids and to focus on the influence of socioeconomic status on vegetable intake, other eating habits, and lifestyle.
Methods
Study design
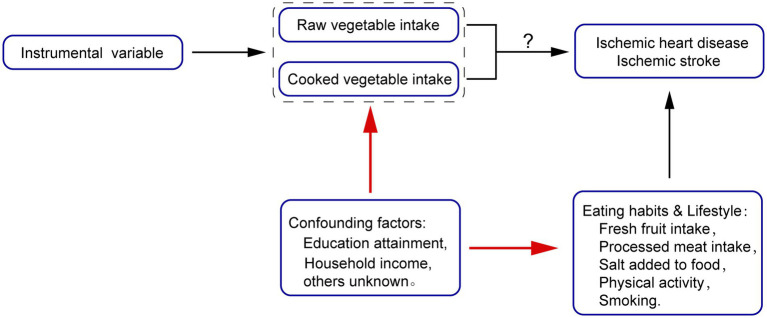
We designed a one-sample Mendelian Randomization study to evaluate how vegetable intake was related to ischemic heart disease and ischemic stroke. We selected angina, acute myocardial infarction, chronic ischemic heart disease, and cerebral infarction as our outcome variables. Considering the genetic connection between instrumental variables used for the intake of raw and cooked vegetables, both univariable and multivariable MR studies were used to assess the causal relationship between exposure and outcome. After discovering a lack of clear causal relationship between vegetable intake and ischemic cardiovascular events, we further conducted a multivariable Mendelian Randomization study to estimate the potential impact of socioeconomic status on vegetable intake, and we also attempted to prove that this impact is equally widespread in other dietary habits and lifestyle (Figure 1).
Figure 1.
Mendelian Randomization (MR) Model of our study.
Instrumental variable selection
Genetic instrumental variables reflecting vegetable intake were derived from three independent UK Biobank-based GWAS (Supplementary Table S1) (29–31). The measures of dietary intake, including vegetable intake, in UK Biobank are based on self-reported questionnaire data. We identified single nucleotide polymorphisms (SNPs) with a genome-wide significant p value (<5 × 10−8) associated with phenotypes. After removing duplicate SNPs, we obtained 39 SNPs reflecting raw vegetable intake, and 25 reflecting cooked vegetable intake. The SNP rs12629972 was excluded because it reflected both raw and cooked vegetable intake. We also excluded palindrome SNPs with intermediate allele frequencies (>0.42) or indel genetic variants. To account for the possibility that the three GWAS may have identified interrelated SNPs as reflecting vegetable intake, we used LDlink to remove SNPs in linkage disequilibrium (32). If a pair of SNPs had LD R2 > 0.01, the SNP with the higher p value was removed. Finally, we used PhenoScanner, a curated database of publicly available results from large-scale genetic association studies in humans (33), to check whether each SNP was associated with potential confounders (p < 5 × 10−8, proxies for European: R2 > 0.8). Rs11191193 was excluded because of its significant genetic association with educational attainment, and rs838133 because it was directly associated with total cholesterol. Finally, we used 18 SNPs reflecting raw vegetable/salad intake and 11 SNPs reflecting cooked vegetable intake for MR analysis (Supplementary Tables S2, S3). Supplementary Figures S1, S2 show the detailed selection process. Proxy SNPs (minimum linkage disequilibrium R2 = 0.8) were used for vegetable intake-associated SNPs that were unavailable in outcome datasets. Some studies have shown that these SNPs may influence people’s dietary preferences and alter genetically determined raw and cooked vegetable intake through senses such as olfaction and taste (34, 35).
In addition, in order to get a more definite conclusion, we also directly extracted the instrumental variables from MRC-IEU. The visualization results of the exposed GWAS can be obtained in Supplementary Figures S3, S4. 18 and 17 SNP were used to reflex raw and cooked vegetables, respectively. Considering that some of the SNPs may have never been reported or used, we calculated the F statistic () for each SNP to test for a weak instrumental variable bias. F statistics for all variables were over 10, and weak instrument bias was avoided in principle (Supplementary Tables S4, S5) (36).
Genetic associations with education attainment were obtained from the GWAS conducted under the auspices of the Social Science Genetic Association Consortium, which reported 74 genome-wide significant loci associated with educational attainment in people of European descent (n = 293,723) (37). Educational attainment was measured by the number of years of schooling completed (EduYears, mean = 14.3, SD = 3.6). 12 loci that were unavailable in the target data-set were removed and the remaining 62 loci have been retained (Supplementary Table S6). The SNPs used as instrumental variables related to household income were derived from Shi et al. (38). The same method was used to extract the genetic association of the 54 SNPs with education attainment and vegetable intake. Of 54 SNPs, 5 were unavailable and removed; the remaining 49 SNPs were retained to perform further analysis (Supplementary Table S7). SNPs associated with education attainment may affect years of education by altering neurodevelopment at different stages. For example, rs4500960 may be related to developmental biology, brain size, and cerebra core methodology, while rs61160187 is closely related to transcription factor binding and negative regulation of signal transmission (37). And SNPs associated with household income are associated with intracranial volume, infant head circumference, and level of cognitive ability (39).
Data sources for outcome of the Mendelian randomization analysis
The primary outcomes included ischemic cardio-cerebral vascular diseases diagnosed by the International Classification of Diseases version, Tenth Version, with the following ICD-10 codes: I20 (angina pectoris), I21 (acute myocardial infarction), I25 (chronic ischemic heart disease), and I63 (cerebral infarction). Data were acquired from the UK Biobank summary statistics curated in the MRC-IEU Open GWAS database or provided by Neale Lab (40). Serum lipid and lipoprotein levels were measured using the Nightingale high-throughput NMR metabolomics platform in 2020, and blood samples were provided by UK Biobank. GWAS results based on these metabolic biomarkers can be obtained from MRC-IEU (European, N = 115,078).
The raw and cooked vegetable intake as well as vegetarian alternatives intake used as outcome variables in MVMR were obtained from MRC-IEU. To illustrate the broad impact of education attainment and household income on IHD risk was not limited to the preferences for vegetable intake, we also obtained a series of outcome variables related to diet and lifestyle, including fresh fruit intake, processed meat intake, salt add to food and smoking (from MRC-IEU) and physical activity (from Within family GWAS consortium). Source and relevant information of result variables used in the study can be acquired from Supplementary Table S8.
Statistical analysis
We used the inverse-variance weighted (IVW) method as the main method to evaluate the causal effect of exposure and outcome. Due to the potential genetic association between raw and cooked vegetables, as well as between education and household income, we used the multivariate Mendelian method to analyze their independent effects. The SNPs used to conduct multivariable MR were combinations of instrumental variables of each exposure in univariable MR. We calculated the odds ratio (OR) and 95% confidence interval (CI). Cochrane’s Q-statistic was used to assess the heterogeneity of SNP effects (Supplementary Tables S15, S16). If significant heterogeneity was observed, a random-effects IVW model was applied. For sensitivity analyses, we used three complementary methods with different assumptions for valid estimates. MR-Egger lacks statistical power for assessment of causal effects and provides wider confidence intervals, but can detect horizontal pleiotropy by p value for its intercept term (Supplementary Table S14) (41). A weighted median method generates homogeneous causal estimates, although >50% of weights derived in the analysis arise from invalid instrumental variables (42). Mendelian Randomization Pleiotropy RESidual Sum and Outlier (MR-PRESSO) can identify outlying SNPs and correct for horizontal pleiotropy through their exclusion (Supplementary Table S13) (43). Additionally, we performed leave-one-out sensitivity analysis to identify potentially highly influential SNPs (Supplementary Figures S5–S12).
All statistical analyses were performed in R (version 4.1.3, R Foundation for Statistical Computing, Vienna, Austria), with MR analyses performed using the TwoSampleMR package (version 0.5.6) (44), MRPRESSO package (version 1.0) (43), and MVMR package (45). Statistical significance was set at a two-sided p value < 0.05. Bonferroni correction of the evidential threshold was based on the number of exposures (p value <0.05/the number of exposures).
Results
Association of genetically predicted vegetable intake with ischemic cardio-cerebral vascular disease and lipid profile
Genetically predicted raw and cooked vegetable intake (IVs from three UK biobank based GWAS) were not associated with any ischemic cardiovascular diseases according to IVW analysis (P>0.05) (Table 1). The multivariate MR (IVs from three UK biobank based GWAS) observed a correlation between cooked vegetable intake and an increased risk of Chronic ischemic heart disease (OR 1.02; 95% CI 1.00, 1.04; p = 0.042) (Table 1), but did not exceed the significance level of Bonferroni correction (p > 0.0125). IVW analysis also showed that genetically predicted raw and cooked vegetable intake (IVs from MRC-IEU) were not associated with any ischemic cardiovascular diseases (Supplementary Table S9; Supplementary Figures S13, S14).
Table 1.
Estimates given as odds ratios (ORs) and 95% confidence intervals for the effect of raw and cooked vegetable intake on ischemic cardio-cerebral vascular diseases.
| Outcomes | Method | Raw vegetable intake | Cooked vegetable intake | ||||||
|---|---|---|---|---|---|---|---|---|---|
| SNPs | OR | 95% CI | p-value | SNPs | OR | 95% CI | P-value | ||
| Angina pectoris | MR-Egger | 18 | 1.01 | 0.98 ~ 1.05 | 0.405 | 11 | 0.97 | 0.91 ~ 1.02 | 0.243 |
| Weighted median | 18 | 1.00 | 0.99 ~ 1.02 | 0.782 | 11 | 1.01 | 0.99 ~ 1.02 | 0.587 | |
| Inverse variance weighted | 18 | 1.01 | 0.99 ~ 1.02 | 0.421 | 11 | 1.00 | 0.98 ~ 1.01 | 0.692 | |
| MR-PRESSO(Outlier-corrected) | NA | NA | NA | NA | NA | NA | NA | NA | |
| MVMR | 18 | 1.00 | 0.99 ~ 1.01 | 0.915 | 11 | 1.00 | 0.98 ~ 1.01 | 0.659 | |
| Myocardial infarction | MR-Egger | 18 | 1.01 | 0.99 ~ 1.04 | 0.341 | 11 | 1.02 | 0.96 ~ 1.08 | 0.51 |
| Weighted median | 18 | 0.99 | 0.98 ~ 1.01 | 0.372 | 11 | 1.02 | 1.00 ~ 1.04 | 0.038 | |
| Inverse variance weighted | 18 | 1.00 | 0.99 ~ 1.01 | 0.909 | 11 | 1.01 | 1.00 ~ 1.02 | 0.18 | |
| MR-PRESSO(Outlier-corrected) | NA | NA | NA | NA | 11 | 1.01 | 1.00 ~ 1.03 | 0.05 | |
| MVMR | 18 | 0.99 | 0.98 ~ 1.00 | 0.202 | 11 | 1.01 | 1.00 ~ 1.03 | 0.056 | |
| Chronic ischaemic heart disease | MR-Egger | 18 | 1.04 | 1.00 ~ 1.08 | 0.067 | 11 | 1.04 | 0.95 ~ 1.14 | 0.443 |
| Weighted median | 18 | 1.00 | 0.98 ~ 1.02 | 0.786 | 11 | 1.02 | 0.99 ~ 1.04 | 0.229 | |
| Inverse variance weighted | 18 | 0.99 | 0.98 ~ 1.01 | 0.424 | 11 | 1.02 | 0.99 ~ 1.04 | 0.156 | |
| MR-PRESSO(Outlier-corrected) | NA | NA | NA | NA | NA | NA | NA | NA | |
| MVMR | 18 | 0.98 | 0.97 ~ 1.00 | 0.075 | 11 | 1.02 | 1.00 ~ 1.04 | 0.042 | |
| Cerebral infarction | MR-Egger | 18 | 1.01 | 0.99 ~ 1.02 | 0.573 | 11 | 0.99 | 0.95 ~ 1.03 | 0.582 |
| Weighted median | 18 | 1.00 | 0.99 ~ 1.01 | 0.701 | 11 | 1.00 | 0.99 ~ 1.01 | 0.933 | |
| Inverse variance weighted | 18 | 1.00 | 0.99 ~ 1.01 | 0.417 | 11 | 1.00 | 0.99 ~ 1.01 | 0.561 | |
| MR-PRESSO(Outlier-corrected) | NA | NA | NA | NA | NA | NA | NA | NA | |
| MVMR | 18 | 1.00 | 0.99 ~ 1.01 | 0.874 | 11 | 0.99 | 0.98 ~ 1.00 | 0.285 | |
IVW analysis showed that genetically predicted raw vegetable intake (IVs from three UK biobank based GWAS) was associated with reduced low-density lipoprotein cholesterol (LDL-C) (β −0.25; 95% CI −0.44, −0.07; p = 0.006) and apolipoprotein B (ApoB) (β −0.28; 95% CI −0.43, −0.12; p < 0.001) (Supplementary Tables S10, S11). MVMR analysis showed that raw vegetable intake was only associated with reduced ApoB (β −0.22; 95% CI −0.38, −0.06; p = 0.013), but did not exceed the significance level of Bonferroni correction (p > 0.0083) (Supplementary Tables S10, S11). Cooked vegetable intake did not significantly influence serum lipid components according to the IVW and MVMR analysis. Results of Mendelian randomization analysis of genetically predicted vegetable intake (IVs from MRC-EU) and lipid profiles are presented in Supplementary Tables S12; Supplementary Figures S15, S16.
Impact of social-economic conditions on vegetable intake
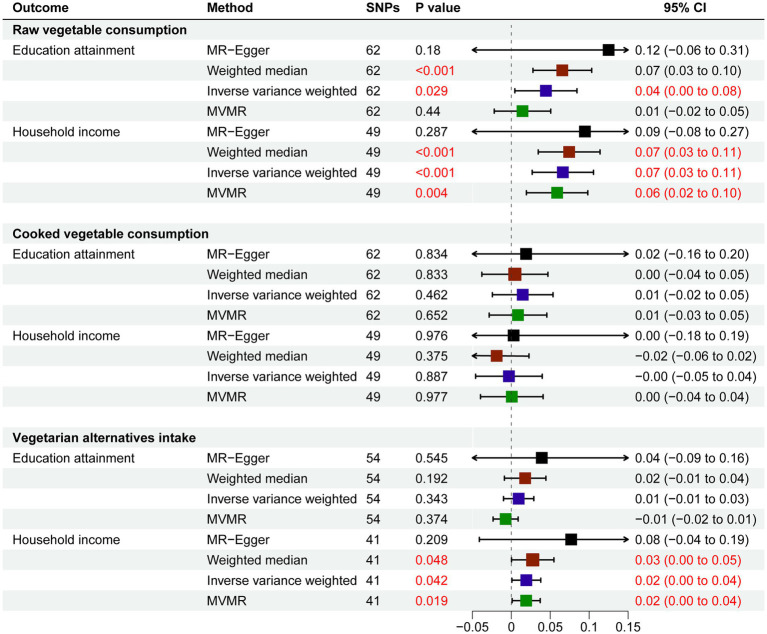
We conducted univariable MR analysis to explore the impact of education attainment and household income on raw and cooked vegetable intake. People with higher education attainment tended to eat more raw vegetables (IVW: β 0.04; 95% CI 0.00, 0.08; p = 0.029). Similarly, people with higher household income tended to eat more raw vegetables (IVW: β 0.07; 95% CI 0.03, 0.11; P<0.001) and vegetarian alternatives intake (IVW: β 0.02; 95% CI 0.00, 0.04; p = 0.042), while we did not find the impact of social-economic conditions on cooked vegetable intake (Figure 2).
Figure 2.
Estimates Given as Beta and 95% Confidence Intervals for the Effect of Education Attainment and Household Income on Vegetable Consumption.
Considering that education and income are related genetically and socially, we next performed MVMR. In the MVMR model, education attainment had a weakened influence on raw vegetable intake (β 0.01; 95% CI −0.02, 0.05; p = 0.440). Household income was still positively related to raw vegetable intake (β 0.06; 95% CI 0.02, 0.10; p = 0.004) and vegetarian alternatives intake (IVW: β 0.02; 95% CI 0.00, 0.04; p = 0.019) (Figure 2).
Impact of social-economic conditions on other eating habits and lifestyle
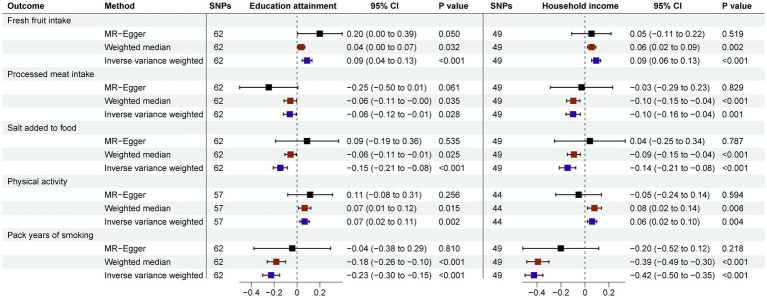
Finally, we conducted univariable MR analysis to explore the impact of education attainment and household income on other eating habits and lifestyle to show that socioeconomic status has an extremely broad impact on ischemic cardio-cerebral vascular diseases. People with higher education attainment and household income tended to eat more fresh fruit intake (IVW: β 0.09; p < 0.001) (IVW: β 0.09; p < 0.001), eat less processed meat (IVW: β −0.06; p = 0.028) (IVW: β −0.10; p = 0.001), add less salt to food (IVW: β −0.15; p < 0.001) (IVW: β −0.14; p < 0.001), have more physical activity (IVW: β 0.07; p = 0.002) (IVW: β 0.06; p = 0.004) and smoke less (IVW: β −0.23; p < 0.001) (IVW: β −0.42; p < 0.001; Figure 3).
Figure 3.
Estimates Given as Beta and 95% Confidence Intervals for the Effect of Education Attainment and Household Income on Eating Habits and Lifestyle.
Discussion
Our results showed that genetic variant-determined increases in raw or cooked vegetable intake did not confer a benefit in terms of ischemic cardio-cerebral vascular diseases. Increased raw vegetable intake may reduce LDL-C and ApoB levels. In addition, socioeconomic status, including income and education, have positive effects on raw vegetable intake in persons of European ancestry. Meanwhile, socioeconomic status had an extremely broad impact on other eating habits and lifestyle.
Worldwide dietary guidelines (4, 5) include recommendations for increased vegetable intake to reduce the risk of CVD, but the independent effects of raw versus cooked vegetables by different epidemiological studies have yielded inconsistent results. Leenders et al. (9) reported that vegetable intake was associated with lower CVD mortality, with a stronger association for raw vegetables. Miller et al. (10) showed that CVD incidence was only associated with high cooked vegetable intake. In a large prospective cohort study (8), total and raw vegetable intake was inversely associated with cardiovascular disease outcomes. Systematic reviews have shown that total vegetable intake is associated with reduced CVD incidence and stroke risk (46–48). A Mendelian randomization study (27) has found no evidence of an association between cooked and raw vegetable intake and coronary heart disease, heart failure, or atrial fibrillation. Since the Mendelian randomization method can reduce the unknown confounders, different results between conventional epidemiological studies and Mendelian randomization studies may be related to differences in study populations and research methods, and insufficient adjustment for confounding factors in conventional epidemiological studies. High-vegetable diets are generally lower in calories, fat, sodium, and glycemic load, which are well-established risk factors for ischemic cardio-cerebral vascular diseases (11, 49). It may be a lack of significance in merely increasing vegetable intake without controlling for other variables such as salt intake and total energy intake. And the impact of diet on health outcomes is complex. It is important to control for consistency in overall dietary patterns when assessing the health effects of single foods or nutrients (30).
LDL-C and other apolipoproteins play central roles in ischemic cardio-cerebral vascular disease occurrence and progression (50–52). Increased vegetable intake may improve plasma lipid profiles, potentially protecting against ischemic cardio-cerebral vascular diseases. Our findings indicated that increased vegetable intake did not significantly improve lipoproteins, except that raw vegetable intake may be associated with improved LDL-C and ApoB. Although increased vegetable intake has been associated with lower plasma LDL-C in observational studies (53, 54), randomized controlled trial results suggest that increased vegetable intake has negligible effects on plasma cholesterol component concentrations (55, 56). This suggests that although increased vegetable intake may have some beneficial influence on lipid profile, the benefits may be insufficient to yield clinical effects. There are several possible reasons for the different effects of raw and cooked vegetables on lipoproteins. Cooking vegetables may increase salt and fat intake, which are linked to CVD morbidity and mortality (57, 58). Cooking improves food safety and food digestibility but also impairs food quality, resulting in the loss of certain nutrients (59–61). Moreover, different types of vegetables may be eaten differently (8).
Low socioeconomic status has been associated with the development of ischemic cardio cerebral vascular disease and may confer comparable cardiovascular risks to traditional risk factors (62, 63). A prospective cohort study demonstrated that adults with low SES are at higher risk of CVD mortality and cardiovascular events than adults with high SES, partly mediated by lifestyle (64). The increased CVD burden in low SES populations may be due to a range of biological, behavioral, and psychosocial risk factors (13). In our screening of instrumental variables of vegetable intake, rs11191193 had significant genetic associations with educational attainment. Roos et al. (65) showed that household education level is an important determinant of raw vegetable intake. A large study found that individuals with primary school education had an increased risk of cardiovascular disease and cardiovascular mortality compared with those with higher education (14). An MR study (66) reported an inverse association between genetically determined educational attainment and CAD risk. And there is a strong association between genetically determined educational attainment and risk factors such as smoking, body mass index, and hypertension (66). One analysis suggested that most CHD risk among individuals with low education is due to behavioral and biological risk factors, with the main contributors being smoking, physical inactivity, and hypertension (23). The level of education may affect the ability to develop health literacy and access to healthy lifestyle and dietary recommendations (16, 67), leading to changes in vegetable intake as well as other lifestyle. Income levels have been consistently associated with CVD risk (14). Population analysis studies of atherosclerosis risk in communities have shown that living in deprived areas is associated with a higher incidence of coronary heart disease (68). The increased risk of CVD in low-income groups may be related to poor dietary choices and increased costs of healthy foods. In low-income areas with limited access, there are more fast food restaurants, fewer supermarkets, and fewer brand options, resulting in limited access to fresh fruits and vegetables (17, 69–71). Economic differences can affect not only the availability of resources but also the promotion or maintenance of a healthy lifestyle (72, 73).
Higher income and higher education attainment may be associated with improved health perceptions and lifestyle factors (74, 75), potentially explaining the positive conclusions of studies that did not fully adjust for socioeconomic status. In a large prospective cohort study (8), total and raw vegetable intake was inversely associated with cardiovascular disease outcomes. However, these associations significantly decreased after adjustment for potential confounders, such as socioeconomic status and lifestyle factors. Results from a cohort study (10) showed that higher vegetable intake was inversely associated with major cardiovascular disease, myocardial infarction, and cardiovascular mortality in models adjusted only for age, sex, and center. After multivariable adjustment including socioeconomic status, the association was significantly attenuated, and only non-cardiovascular mortality and total mortality remained significant. Similarly, in another study from China (18), higher vegetable intake was associated with lower CVD mortality risk in a minimally adjusted model, and the association was no longer significant after adjustment for factors such as socioeconomic status. Given the complex interrelationships between socioeconomic status, eating habits, lifestyle, and health outcomes, it is important to adjust for socioeconomic status to reduce bias.
Strength
Our study has several strengths. We investigated the independent effects of raw and cooked vegetable intake on ischemic cardio-cerebral vascular diseases and lipid profiles. Compared with traditional observational studies, MR studies reduce the effects of unknown confounders and reverse causality (30). Especially, by using the Mendelian randomization analysis, we examined the impact of socioeconomic factors on vegetable intake, other eating habits, and lifestyle, confirming that the effects of education and income on dietary habits and lifestyle are relatively common and may be extremely important confounders in previous studies.
Limitations
Our work had several limitations. Our selected GWAS for vegetable intake did not report specific intake characteristics, such as vegetable type. Therefore, we could not perform a stratified MR analysis by vegetable type, which would help draw more valuable causal inferences. Additionally, our MR study was restricted to individuals of European ancestry (76). Due to differences in genetic background and eating habits, similar studies should be conducted on a larger scale around the world. Moreover, the SNPs of the utilized GWAS were all self-reported, which may differ from the actual intake, potentially introducing some bias to the results. Individuals tend to report more healthy foods and less unhealthy foods, and people with a high BMI tend to report less food intake (77, 78). Diet changes over time may also be a potential limitation (79). Furthermore, we selected two sample datasets with a large proportion of the population overlapping, which may increase the bias in the direction of the observed association and the inflation of the Type I error rate and false-positive results (80). Finally, MR relies on assumptions that genetic tools are associated with exposure, independent of potential confounders and that genetic tools are associated with outcomes only through exposure. Although we speculated about the possible mechanism of action of the instrumental variable SNPs, the effect of the SNP on the function of the gene product is unknown and is based only on a statistical association between gene and apparent effect (81). We cannot completely avoid horizontal pleiotropic effects because it is difficult to determine the biological function of exact genetic variants. There remains a need for further high-quality GWAS and MR analyses.
Conclusion
Our results showed that genetically determined raw and cooked vegetable intake was not associated with significant benefits related to ischemic cardio-cerebral vascular disease. Genetically determined socioeconomic status may have an impact on vegetable intake. Additionally, socioeconomic status, which was closely associated with other eating habits and lifestyle, may affect the association between vegetable intake and ischemic cardio-cerebral vascular diseases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JH and ZH: design the study and draft the work. MX and JD: data collection and statistical analysis. Y-tZ: design the study and revise it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We want to acknowledge participants and investigators from the IEU Open GWAS Project and the creator of MRC-IEU UK Biobank GWAS pipeline. If there is no open systematic data platform provided by them, there will be no such research. We do thank Stephen Burgess and Deborah A. Lawlor, for their selfless help with data statistics. We acknowledge Tai-kang Yao and Zhi-fei Li for contributing his ideas and providing suggestions. We thanks to all the participating authors.
Glossary
Abbreviations
- ApoB
apolipoprotein B
- CI
confidence interval
- CVD
cardiovascular disease
- IEU
Integrative Epidemiology Unit
- IVs
instrumental variables
- IVW
inverse-variance weighted
- GWAS
genome-wide association studies
- LDL-C
low-density lipoprotein cholesterol
- MR
Mendelian randomization
- MVMR
multivariable Mendelian randomization
- OR
odds ratio
- SES
socioeconomic status
- SNPs
single nucleotide polymorphisms
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2023.1161175/full#supplementary-material
References
- 1.GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. (2018) 392:1736–88. doi: 10.1016/S0140-6736(18)32203-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celermajer DS, Chow CK, Marijon E, Anstey NM, Woo KS. Cardiovascular disease in the developing world: prevalences, patterns, and the potential of early disease detection. J Am Coll Cardiol. (2012) 60:1207–16. doi: 10.1016/j.jacc.2012.03.074 [DOI] [PubMed] [Google Scholar]
- 3.Thomas H, Diamond J, Vieco A, Chaudhuri S, Shinnar E, Cromer S, et al. Global atlas of cardiovascular disease 2000-2016: the path to prevention and control. Glob Heart. (2018) 13:143–63. doi: 10.1016/j.gheart.2018.09.511, PMID: [DOI] [PubMed] [Google Scholar]
- 4.National Health and Medical Research Council . Australian dietary guidelines. Available at: https://www.eatforhealth.gov.au/guidelines (2013).
- 5.United States Department of Agriculture . 2015 – 2020 dietary guidelines for Americans. 8th ed Available at: http://health.gov/dietaryguidelines/2015/guidelines/ (2015).
- 6.Wang DD, Li Y, Bhupathiraju SN, Rosner BA, Sun Q, Giovannucci EL, et al. Fruit and vegetable intake and mortality: results from 2 prospective cohort studies of US men and women and a meta-analysis of 26 cohort studies. Circulation. (2021) 143:1642–54. doi: 10.1161/CIRCULATIONAHA.120.048996, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alissa EM, Ferns GA. Dietary fruits and vegetables and cardiovascular diseases risk. Crit Rev Food Sci Nutr. (2017) 57:1950–62. doi: 10.1080/10408398.2015.1040487, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Feng Q, Kim JH, Omiyale W, Besevic J, Conroy M, May M, et al. Raw and cooked vegetable consumption and risk of cardiovascular disease: a study of 400,000 adults in UK biobank. Front Nutr. (2022) 9:831470. doi: 10.3389/fnut.2022.831470, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leenders M, Sluijs I, Ros MM, Boshuizen HC, Siersema PD, Ferrari P, et al. Fruit and vegetable consumption and mortality: European prospective investigation into cancer and nutrition. Am J Epidemiol. (2013) 178:590–602. doi: 10.1093/aje/kwt006 [DOI] [PubMed] [Google Scholar]
- 10.Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. (2017) 390:2037–49. doi: 10.1016/S0140-6736(17)32253-5 [DOI] [PubMed] [Google Scholar]
- 11.Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. (2017) 46:1029–56. doi: 10.1093/ije/dyw319, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobylecki CJ, Afzal S, Davey Smith G, Nordestgaard BG. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: a Mendelian randomization study. Am J Clin Nutr. (2015) 101:1135–43. doi: 10.3945/ajcn.114.104497, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, et al. Socioeconomic status and cardiovascular outcomes: challenges and interventions. Circulation. (2018) 137:2166–78. doi: 10.1161/CIRCULATIONAHA.117.029652, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodward M, Peters SA, Batty GD, Ueshima H, Woo J, Giles GG, et al. Socioeconomic status in relation to cardiovascular disease and cause-specific mortality: a comparison of Asian and Australasian populations in a pooled analysis. BMJ Open. (2015) 5:e006408. doi: 10.1136/bmjopen-2014-006408, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh PB, Yang J, Leigh S, Dorjee K, Parikh R, Sakellarios N, et al. The impact of financial barriers on access to care, quality of care and vascular morbidity among patients with diabetes and coronary heart disease. J Gen Intern Med. (2014) 29:76–81. doi: 10.1007/s11606-013-2635-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luepker RV, Rosamond WD, Murphy R, Sprafka JM, Folsom AR, McGovern PG, et al. Socioeconomic status and coronary heart disease risk factor trends. The Minnesota Heart Survey. Monograph. (1993) 88:2172–9. doi: 10.1161/01.CIR.88.5.2172, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Alwitt LF, Donley TD. Retail Stores in Poor Urban Neighborhoods. J Consum Aff. (1997) 31:139–64. doi: 10.1111/j.1745-6606.1997.tb00830.x [DOI] [Google Scholar]
- 18.Liu W, Hu B, Dehghan M, Mente A, Wang C, Yan R, et al. Fruit, vegetable, and legume intake and the risk of all-cause, cardiovascular, and cancer mortality: a prospective study. Clin Nutr. (2021) 40:4316–23. doi: 10.1016/j.clnu.2021.01.016, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. (2016) 133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson S, Mason A, Bäck M, Klarin D, Damrauer S, Michaëlsson K, et al. Genetic predisposition to smoking in relation to 14 cardiovascular diseases. Eur Heart J. (2020) 41:3304–10. doi: 10.1093/eurheartj/ehaa193, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bell S, Daskalopoulou M, Rapsomaniki E, George J, Britton A, Bobak M, et al. Association between clinically recorded alcohol consumption and initial presentation of 12 cardiovascular diseases: population based cohort study using linked health records. BMJ. (2017) 356:j909. doi: 10.1136/bmj.j909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piché M, Tchernof A, Després J. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126:1477–500. doi: 10.1161/CIRCRESAHA.120.316101 [DOI] [PubMed] [Google Scholar]
- 23.Kershaw KN, Droomers M, Robinson WR, Carnethon MR, Daviglus ML, Monique Verschuren WM. Quantifying the contributions of behavioral and biological risk factors to socioeconomic disparities in coronary heart disease incidence: the MORGEN study. Eur J Epidemiol. (2013) 28:807–14. doi: 10.1007/s10654-013-9847-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stirbu I, Looman C, Nijhof GJ, Reulings PG, Mackenbach JP. Income inequalities in case death of ischaemic heart disease in the Netherlands: a national record-linked study. J Epidemiol Community Health. (2012) 66:1159–66. doi: 10.1136/jech-2011-200924, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Hemani G, Tilling K, Davey Smith G. Orienting the causal relationship between imprecisely measured traits using GWAS summary data. PLoS Genet. (2017) 13:e1007081. doi: 10.1371/journal.pgen.1007081, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davey Smith G. Use of genetic markers and gene-diet interactions for interrogating population-level causal influences of diet on health. Genes Nutr. (2011) 6:27–43. doi: 10.1007/s12263-010-0181-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Q, Grant AJ, Yang Q, Burgess S, Bešević J, Conroy M, et al. Vegetable intake and cardiovascular risk: genetic evidence from Mendelian randomization. medRxiv. (2022) [Google Scholar]
- 28.Feng Q, Bešević J. Vegetable intake and metabolic risk factors: a Mendelian randomization study. medRxiv. (2022) [Google Scholar]
- 29.Cole JB, Florez JC, Hirschhorn JN. Comprehensive genomic analysis of dietary habits in UK biobank identifies hundreds of genetic associations. Nat Commun. (2020) 11:1467. doi: 10.1038/s41467-020-15193-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pirastu N, McDonnell C, Grzeszkowiak EJ, Mounier N, Imamura F, Merino J, et al. Using genetic variation to disentangle the complex relationship between food intake and health outcomes. PLoS Genet. (2022) 18:e1010162. doi: 10.1371/journal.pgen.1010162, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niarchou M, Byrne EM, Trzaskowski M, Sidorenko J, Kemper KE, McGrath JJ, et al. Genome-wide association study of dietary intake in the UK biobank study and its associations with schizophrenia and other traits. Transl Psychiatry. (2020) 10:51. doi: 10.1038/s41398-020-0688-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. (2015) 31:3555–7. doi: 10.1093/bioinformatics/btv402, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics. (2019) 35:4851–3. doi: 10.1093/bioinformatics/btz469, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaeger SR, McRae JF, Bava CM, Beresford MK, Hunter D, Jia Y, et al. A Mendelian trait for olfactory sensitivity affects odor experience and food selection. Curr Biol. (2013) 23:1601–5. doi: 10.1016/j.cub.2013.07.030, PMID: [DOI] [PubMed] [Google Scholar]
- 35.McRae JF, Jaeger SR, Bava CM, Beresford MK, Hunter D, Jia Y, et al. Identification of regions associated with variation in sensitivity to food-related odors in the human genome. Curr Biol. (2013) 23:1596–600. doi: 10.1016/j.cub.2013.07.031, PMID: [DOI] [PubMed] [Google Scholar]
- 36.Burgess S, Thompson SG. Avoiding bias from weak instruments in Mendelian randomization studies. Int J Epidemiol. (2011) 40:755–64. doi: 10.1093/ije/dyr036, PMID: [DOI] [PubMed] [Google Scholar]
- 37.Okbay A, Beauchamp JP, Fontana MA, Lee JJ, Pers TH, Rietveld CA, et al. Genome-wide association study identifies 74 loci associated with educational attainment. Nature. (2016) 533:539–42. doi: 10.1038/nature17671, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi X, Yuan W, Cao Q, Cui W. Education plays a crucial role in the pathway from poverty to smoking: a Mendelian randomization study. Addiction. (2022) 118:128–39. doi: 10.1111/add.16019, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Hill WD, Hagenaars SP, Marioni RE, Harris SE, Liewald DCM, Davies G, et al. Molecular genetic contributions to social deprivation and household income in UK biobank. Curr Biol. (2016) 26:3083–9. doi: 10.1016/j.cub.2016.09.035, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell R, Elsworth BL, Mitchell R, Raistrick CA, Paternoster L, Hemani G, et al. MRC-IEU UK biobank GWAS pipeline version 2. Bristol: University of Bristol. [Google Scholar]
- 41.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44:512–25. doi: 10.1093/ije/dyv080, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40:304–14. doi: 10.1002/gepi.21965, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. (2018) 50:693–8. doi: 10.1038/s41588-018-0099-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. elife. (2018) 7:7. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanderson E, Davey Smith G, Windmeijer F, Bowden J. An examination of multivariable Mendelian randomization in the single-sample and two-sample summary data settings. Int J Epidemiol. (2019) 48:713–27. doi: 10.1093/ije/dyy262, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhan J, Liu YJ, Cai LB, Xu FR, Xie T, He QQ. Fruit and vegetable consumption and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. (2017) 57:1650–63. doi: 10.1080/10408398.2015.1008980, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, et al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. (2019) 59:1071–90. doi: 10.1080/10408398.2017.1392288, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Oude Griep LM, Verschuren WM, Kromhout D, Ocké MC, Geleijnse JM. Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in the Netherlands. Eur J Clin Nutr. (2011) 65:791–9. doi: 10.1038/ejcn.2011.36, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Kanter M, Angadi S, Slavin J. Glycemic index, glycemic load, and cardiovascular disease and mortality. N Engl J Med. (2021) 385:378–80. doi: 10.1056/NEJMc2107926 [DOI] [PubMed] [Google Scholar]
- 50.Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of lipids on cardiovascular health: JACC health promotion series. J Am Coll Cardiol. (2018) 72:1141–56. doi: 10.1016/j.jacc.2018.06.046 [DOI] [PubMed] [Google Scholar]
- 51.Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. (2017) 38:2459–72. doi: 10.1093/eurheartj/ehx144, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, et al. Lipids, lipoproteins, and metabolites and risk of myocardial infarction and stroke. J Am Coll Cardiol. (2018) 71:620–32. doi: 10.1016/j.jacc.2017.12.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fornés NS, Martins IS, Hernan M, Velásquez-Meléndez G, Ascherio A. Frequency of food consumption and lipoprotein serum levels in the population of an urban area, Brazil. Rev Saude Publica. (2000) 34:380–7. doi: 10.1590/S0034-89102000000400011, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Djoussé L, Arnett DK, Coon H, Province MA, Moore LL, Ellison RC. Fruit and vegetable consumption and LDL cholesterol: the national heart, lung, and blood institute family heart study. Am J Clin Nutr. (2004) 79:213–7. doi: 10.1093/ajcn/79.2.213, PMID: [DOI] [PubMed] [Google Scholar]
- 55.John JH, Ziebland S, Yudkin P, Roe LS, Neil HA. Effects of fruit and vegetable consumption on plasma antioxidant concentrations and blood pressure: a randomised controlled trial. Lancet. (2002) 359:1969–74. doi: 10.1016/S0140-6736(02)98858-6, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Zino S, Skeaff M, Williams S, Mann J. Randomised controlled trial of effect of fruit and vegetable consumption on plasma concentrations of lipids and antioxidants. BMJ. (1997) 314:1787–91. doi: 10.1136/bmj.314.7097.1787, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: Cochrane systematic review and meta-analysis of randomised trials. BMJ. (2013) 346:f1325. doi: 10.1136/bmj.f1325, PMID: [DOI] [PubMed] [Google Scholar]
- 58.Unhapipatpong C, Shantavasinkul PC, Kasemsup V, Siriyotha S, Warodomwichit D, Maneesuwannarat S, et al. Tropical oil consumption and cardiovascular disease: an umbrella review of systematic reviews and meta analyses. Nutrients. (2021) 13:1549. doi: 10.3390/nu13051549, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palermo M, Pellegrini N, Fogliano V. The effect of cooking on the phytochemical content of vegetables. J Sci Food Agric. (2014) 94:1057–70. doi: 10.1002/jsfa.6478, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Lee S, Choi Y, Jeong HS, Lee J, Sung J. Effect of different cooking methods on the content of vitamins and true retention in selected vegetables. Food Sci Biotechnol. (2018) 27:333–42. doi: 10.1007/s10068-017-0281-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Boekel M, Fogliano V, Pellegrini N, Stanton C, Scholz G, Lalljie S, et al. A review on the beneficial aspects of food processing. Mol Nutr Food Res. (2010) 54:1215–47. doi: 10.1002/mnfr.200900608 [DOI] [PubMed] [Google Scholar]
- 62.Franks P, Winters PC, Tancredi DJ, Fiscella KA. Do changes in traditional coronary heart disease risk factors over time explain the association between socio-economic status and coronary heart disease? BMC Cardiovasc Disord. (2011) 11:28. doi: 10.1186/1471-2261-11-28, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stringhini S, Carmeli C, Jokela M, Avendaño M, Muennig P, Guida F, et al. Socioeconomic status and the 25 × 25 risk factors as determinants of premature mortality: a multicohort study and meta-analysis of 1·7 million men and women. Lancet. (2017) 389:1229–37. doi: 10.1016/S0140-6736(16)32380-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang YB, Chen C, Pan XF, Guo J, Li Y, Franco OH, et al. Associations of healthy lifestyle and socioeconomic status with mortality and incident cardiovascular disease: two prospective cohort studies. BMJ. (2021) 373:n604. doi: 10.1136/bmj.n604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roos EB, Hirvonen T, Mikkilä V, Karvonen S, Rimpelä M. Household educational level as a determinant of consumption of raw vegetables among male and female adolescents. Prev Med. (2001) 33:282–91. doi: 10.1006/pmed.2001.0882, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Zeng L, Ntalla I, Kessler T, Kastrati A, Erdmann J, Group UKBCCCW et al. Genetically modulated educational attainment and coronary disease risk. Eur Heart J. (2019) 40:2413–20. doi: 10.1093/eurheartj/ehz328, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cajita MI, Cajita TR, Han HR. Health literacy and heart failure: a systematic review. J Cardiovasc Nurs. (2016) 31:121–30. doi: 10.1097/JCN.0000000000000229, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. (2001) 345:99–106. doi: 10.1056/NEJM200107123450205 [DOI] [PubMed] [Google Scholar]
- 69.Giang T, Karpyn A, Laurison HB, Hillier A, Perry RD. Closing the grocery gap in underserved communities: the creation of the Pennsylvania fresh food financing initiative. J Public Health Manag Pract. (2008) 14:272–9. doi: 10.1097/01.PHH.0000316486.57512.bf, PMID: [DOI] [PubMed] [Google Scholar]
- 70.Block JP, Scribner RA, DeSalvo KB. Fast food, race/ethnicity, and income: a geographic analysis. Am J Prev Med. (2004) 27:211–7. doi: 10.1016/S0749-3797(04)00139-4, PMID: [DOI] [PubMed] [Google Scholar]
- 71.Block D, Kouba J. A comparison of the availability and affordability of a market basket in two communities in the Chicago area. Public Health Nutr. (2006) 9:837–45. doi: 10.1017/PHN2005924, PMID: [DOI] [PubMed] [Google Scholar]
- 72.Christine PJ, Auchincloss AH, Bertoni AG, Carnethon MR, Sánchez BN, Moore K, et al. Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: the multi-ethnic study of atherosclerosis (MESA). JAMA Intern Med. (2015) 175:1311–20. doi: 10.1001/jamainternmed.2015.2691, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Creatore MI, Glazier RH, Moineddin R, Fazli GS, Johns A, Gozdyra P, et al. Association of neighborhood walkability with change in overweight, obesity, and diabetes. JAMA. (2016) 315:2211–20. doi: 10.1001/jama.2016.5898, PMID: [DOI] [PubMed] [Google Scholar]
- 74.Bedford JL, Barr SI. Diets and selected lifestyle practices of self-defined adult vegetarians from a population-based sample suggest they are more 'health conscious'. Int J Behav Nutr Phys Act. (2005) 2:4. doi: 10.1186/1479-5868-2-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang-Claude J, Hermann S, Eilber U, Steindorf K. Lifestyle determinants and mortality in German vegetarians and health-conscious persons: results of a 21-year follow-up. Cancer Epidemiol Biomark Prev. (2005) 14:963–8. doi: 10.1158/1055-9965.EPI-04-0696, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Fry A, Littlejohns TJ, Sudlow C, Doherty N, Adamska L, Sprosen T, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank participants with those of the general population. Am J Epidemiol. (2017) 186:1026–34. doi: 10.1093/aje/kwx246, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Livingstone MB, Black AE. Markers of the validity of reported energy intake. J Nutr. (2003) 133:895s–920s. doi: 10.1093/jn/133.3.895S [DOI] [PubMed] [Google Scholar]
- 78.Tooze JA, Subar AF, Thompson FE, Troiano R, Schatzkin A, Kipnis V. Psychosocial predictors of energy underreporting in a large doubly labeled water study. Am J Clin Nutr. (2004) 79:795–804. doi: 10.1093/ajcn/79.5.795, PMID: [DOI] [PubMed] [Google Scholar]
- 79.Stevenson RJ. Psychological correlates of habitual diet in healthy adults. Psychol Bull. (2017) 143:53–90. doi: 10.1037/bul0000065, PMID: [DOI] [PubMed] [Google Scholar]
- 80.Pierce BL, Burgess S. Efficient design for Mendelian randomization studies: subsample and 2-sample instrumental variable estimators. Am J Epidemiol. (2013) 178:1177–84. doi: 10.1093/aje/kwt084, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimaldi KA, van Ommen B, Ordovas JM, Parnell LD, Mathers JC, Bendik I, et al. Proposed guidelines to evaluate scientific validity and evidence for genotype-based dietary advice. Genes Nutr. (2017) 12:35. doi: 10.1186/s12263-017-0584-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.