Abstract
Objective:
To lay out the argument that exercise impacts neurobiological targets common to both mood and cognitive functioning, and thus more research should be conducted on its use as an alternative or adjunctive treatment in late-life depression.
Method:
This narrative review summarizes the literature on cognitive impairment in late-life depression, describes the structural and functional brain changes and neurochemical changes that are linked to both cognitive impairment and mood disruption, and explains how exercise targets these same neurobiological changes and can thus provide an alternative or adjunctive treatment for cognitive impairment in late-life depression.
Results:
Cognitive impairment is common in late-life depression and predicts recurrence of depression, poor response to antidepressant treatment, and overall disability. Traditional depression treatment with medication, psychotherapy, or both, is not effective in fully reversing cognitive impairment for most depressed older adults. Physical exercise is an ideal treatment candidate based on evidence that it 1) is an effective treatment for depression, 2) enhances cognitive functioning in normal aging and in other patient populations, and 3) targets many of the neurobiological mechanisms that underlie mood and cognitive functioning. The limited existing clinical trials of exercise for cognitive impairment in depression are mixed but overall support this contention.
Conclusions:
Although limited, existing evidence suggests exercise may be a viable alternative or adjunctive treatment to address cognitive impairment in late-life depression, and thus more research in this area is warranted. Moving forward, additional research is needed in large, diverse samples to translate the growing research findings into clinical practice.
Keywords: physical activity, geriatric depression, cognition, mood, antidepressant, brain Topic Areas: depression, exercise
Depression is one of the leading causes of disability and can be particularly pernicious in older adults (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018; Vaughan, Corbin, & Goveas, 2015). A meta-analysis estimated the prevalence of current clinical depression in adults aged 50 and older to be 3.29%, with a higher rate of 19.47% for depressive symptoms (Volkert, Schulz, Harter, Wlodarczyk, & Andreas, 2013). Depression, especially in older adults, is associated with functional decline, decreased quality of life, higher healthcare utilization, increased economic burden, and increased mortality (Menchetti, Cevenini, De Ronchi, Quartesan, & Berardi, 2006). The impact of late-life depression (LLD) on daily functioning, quality of life, and other outcomes is attributable at least in part to cognitive impairment (Alexopoulos et al., 2005). Unfortunately, traditional depression treatments—pharmacotherapy and psychotherapy—are generally ineffective in resolving the cognitive impairment that often accompanies mood disorders (Butters et al., 2008; Reppermund, Ising, Lucae, & Zihl, 2009). As such, there is increasing focus on alternative interventions that might better target the underlying brain changes in LLD, thereby benefiting both mood and cognitive functioning (Morimoto & Alexopoulos, 2013). Physical exercise is a prime candidate for such an intervention. We will argue that research in this area is an important focus for the field to improve outcomes in depressed older adults.
In this narrative review, we will 1) briefly summarize the literature on cognitive impairment in LLD, 2) describe the structural and functional brain changes and neurochemical changes that are linked to both cognitive impairment and mood disruption, 3) explain how exercise targets these same brain changes and can thus provide an alternative or adjunctive treatment for cognitive impairment in LLD, and 4) summarize the existing literature on exercise for cognitive impairment in depression, including LLD.
Cognitive Functioning in Late-life Depression
Depression is a heterogeneous disorder characterized by a variety of affective, somatic, interpersonal, and cognitive symptoms. Attention, memory, and other cognitive complaints are not required for diagnosis but are nonetheless common in depressed individuals (Srisurapanont et al., 2018). Despite the discrepancy that can occur between subjective cognitive complaints and actual performance in depression, there is a large body of literature documenting objective cognitive deficits in this population (Zlatar, Moore, Palmer, Thompson, & Jeste, 2014; Zlatar, Muniz, Galasko, & Salmon, 2018). Depressed adults most often show signs of cognitive weaknesses or impairment in the domains of attention, executive functioning, learning and memory, and psychomotor speed (Koenig, Bhalla, & Butters, 2014; Morimoto & Alexopoulos, 2013).
A number of studies report a relationship between subclinical depressive symptoms and cognitive dysfunction, particularly in the area of executive or cognitive control (Dotson, Resnick, & Zonderman, 2008; Elderkin-Thompson, Mintz, Haroon, Lavretsky, & Kumar, 2006; Shimada et al., 2014). A recent meta-analysis (Dotson et al., 2020) examined cognitive control deficits in both clinical and subclinical depression across the lifespan. Across 73 studies, a total of 16,806 participants between the ages of seven and 97 were included. The meta-analysis showed that both major depression and subclinical depressive symptoms were associated with cognitive control deficits, though the relationship was weaker in subthreshold depression. The study also found that the relationship was stronger in later stages of the lifespan. In fact, effect sizes were not significant in studies that only included child, adolescent, or young adult (age ≤ 25 years) samples. The finding that older adults are more vulnerable to depression-related changes in cognitive functioning is supported by other studies as well (Dotson et al., 2008; Dotson et al., 2014; Lockwood, Alexopoulos, & van Gorp, 2002).
Cognitive dysfunction in LLD predicts both short-term and long-term adverse consequences. Studies have shown that the combination of cognitive impairment and depression in older adults is associated with an increased relapse relate, a poorer response to antidepressant treatment, and greater overall disability (Alexopoulos et al., 2000; J. R. Sneed et al., 2007). Executive dysfunction, in particular, increases the risk for functional disability in LLD (Gansler, Suvak, Arean, & Alexopoulos, 2015). Considering that normal aging is associated with declines in executive functioning, this suggests the combination of age and depression makes depressed older adults especially vulnerable to functional decline.
Prognosis of Cognitive Impairment in Late-life Depression
The prognosis of LLD with cognitive impairment varies. One possible outcome is the development of dementia. Depression in older adults confers a nearly twofold increase in dementia risk relative to non-depressed seniors (Cherbuin, Kim, & Anstey, 2015; Diniz, Butters, Albert, Dew, & Reynolds, 2013; Ownby, Crocco, Acevedo, John, & Loewenstein, 2006). Moreover, in a longitudinal study that followed adults age 55 and older for up to 20 years, each episode of depression was associated with a 14% increase in risk for all-cause dementia (Dotson, Beydoun, & Zonderman, 2010). The link between depression and subsequent dementia is generally explained in one of three ways: Depression is a prodrome of dementia, depression lowers the threshold at which dementia is manifested, or depression-related brain changes contribute to the development of dementia (Jorm, 2001; Ownby et al., 2006). There is evidence for each of these hypotheses.
Another possible outcome is that cognitive impairment resolves with the remission of LLD. This is sometimes referred to as “pseudodementia” since the cognitive difficulties can be mistaken for a neurodegenerative disease (Connors, Quinto, & Brodaty, 2018). A recent study found that 29% of older adults with remitted depression showed signs of cognitive improvement at follow-up (Siddarth, Funes, Laird, Ercoli, & Lavretsky, 2020). However, these patients still have a high risk for developing irreversible dementia in the future (Alexopoulos, Meyers, Young, Mattis, & Kakuma, 1993; Connors et al., 2018).
The most common outcome of LLD with cognitive impairment is persistent cognitive problems after affective symptoms have remitted (Lee, Potter, Wagner, Welsh-Bohmer, & Steffens, 2007; Majer et al., 2004; Murphy & Alexopoulos, 2004; Nebes et al., 2003; Semkovska et al., 2019). Meta-analysis has shown that deficits in selective attention, working memory, and long-term memory persist in remission from a major depressive episode and worsen with repeated episodes (Semkovska et al., 2019). Cognitive impairment persists for as long as four years in longitudinal studies (Kohler, Thomas, Barnett, & O’Brien, 2010). One study (Bhalla et al., 2006) found that in remitted LLD, a staggering 94% of the patients who were cognitively impaired at baseline remained impaired one year later. More recently, analysis of data from two clinical trials revealed that less than a third of patients showed cognitive improvement after their mood remitted (Siddarth et al., 2020). Reports of persistent executive dysfunction (Koenig et al., 2015; Nakano et al., 2008) are particularly troubling in light of the aforementioned relationship between executive deficits and functional disability. Indeed, there is evidence that persistent cognitive deficits in remitted depression predicts disability, even in younger adults (Woo, Rosenblat, Kakar, Bahk, & McIntyre, 2016).
Risk factors such as lower baseline cognitive function, older age, later age of depression onset, and greater vascular burden are associated with worse cognitive outcomes in depression (Barch et al., 2012; Koenig et al., 2014). Conversely, lower baseline depression severity, depression onset prior to age 60, and better social functioning are associated with cognitive improvement after depression remission (Siddarth et al., 2020). The large proportion of older adults whose cognitive impairment persists after depression remission points to the need for depression treatments that address both affective and cognitive symptoms. Neurobiological contributors common to mood disruption and cognitive decline are potential treatment targets.
Overlapping Neurobiological Contributors in Depression and Cognitive Impairment
Several recent reviews provide excellent summaries of our current understanding of neurobiological mechanisms underlying LLD (Rutherford, Taylor, Brown, Sneed, & Roose, 2017; Weisenbach & Kumar, 2014). Cognitive impairment and mood disturbance in LLD may be caused by common neurobiological contributors. Indeed, converging evidence from affective neuroscience studies show disruptions in distributed but integrated neural circuits that underlie both emotional and cognitive functioning in LLD (Butters et al., 2008; Linnemann & Lang, 2020).
Structural and functional alterations associated with depressive symptoms have been identified within the frontolimbic network, including the prefrontal and cingulate cortices, medial temporal lobes, and limbic regions (Drevets, Price, & Furey, 2008; Du et al., 2014). Specifically, the orbitofrontal cortex, dorsolateral prefrontal cortex (PFC), anterior cingulate, hippocampus, putamen, and caudate are frequently implicated in the depression literature and in many cases are disproportionately impacted at older ages (Ballmaier et al., 2004; Dotson, Davatzikos, Kraut, & Resnick, 2009; Naismith, Norrie, Mowszowski, & Hickie, 2012). These regions are highly interconnected and interact to form the neural circuitry that govern both emotional and cognitive functioning (Aziz & Steffens, 2017; Crocco, Castro, & Loewenstein, 2010; Kempton et al., 2011; Pandya, Altinay, Malone, & Anand, 2012), including memory, executive functions, attention, and processing speed. For example, hippocampal atrophy, a hallmark of memory dysfunction, is a common finding in depression (Sexton, Mackay, & Ebmeier, 2013). In fact, an ENIGMA Consortium meta-analysis found that hippocampal volume reduction in depression was the most robust finding across a number of brain regions (Schmaal et al., 2016). Stress-related glucocorticoid overproduction (hypercortisolemia), via dysregulation of the hypothalamic-pituitary-adrenal axis, promotes hippocampal cell injury and death and subsequent memory deficits in depression (Herbert & Lucassen, 2016; Keller et al., 2017).
Mood and cognitive dysfunction relate not just to changes to frontolimbic areas of the brain, but also to disruption of structural and functional networks made up of these distinct brain regions (Price & Drevets, 2010). Neuroimaging studies have identified altered structural and functional connectivity in frontal-executive and corticolimbic pathways in LLD (Rashidi-Ranjbar, Miranda, Butters, Mulsant, & Voineskos, 2020). Reduced white matter integrity disrupts communication, particularly along frontolimbic circuits, resulting in a “disconnection” between cortical and subcortical brain regions (Liao et al., 2013), thereby yielding mood and executive dysregulation. Negative affectivity in LLD is associated with resting state activity differences in the dorsomedial and ventromedial PFC, as well as changes in the connections of these regions to the amygdala and the posterior cingulate cortex (Steffens, Wang, Manning, & Pearlson, 2017). Aberrant connectivity is also observed in the executive control, default mode, and salience network that mediate internally oriented and self-referential thought and cognitive functions such as attention and cognitive control (Kaiser, Andrews-Hanna, Wager, & Pizzagalli, 2015; W. Li et al., 2017).
Neurovascular changes have also been linked to both cognitive impairment and mood disorders. The importance of vascular pathology in LLD has long been recognized. The “vascular depression” hypothesis was introduced by Alexopoulos and colleagues (1997) over 20 years ago, bringing into focus the role of cerebrovascular disease in predisposing, precipitating, and perpetuating depression in older adults. Neuroimaging evidence is consistent with this hypothesis. White matter hyperintensities, which are typically associated with ischemic damage (Lin, Wang, Lan, & Fan, 2017), occur more often in depressed compared to nondepressed older adults (Greenstein et al., 2010; Joel R. Sneed, Rindskopf, Steffens, Krishnan, & Roose, 2008) and are associated with subthreshold depressive symptoms as well (Dotson, Zonderman, Kraut, & Resnick, 2013; Kirton, Resnick, Davatzikos, Kraut, & Dotson, 2014). Diffusion tensor imaging studies reveal altered white matter connectivity in depression. LLD is also associated with impaired arterial function and abnormal wall structure (Greenstein et al., 2010). These white matter changes in LLD are associated with slower processing speed and executive dysfunction (Respino et al., 2019; Ye et al., 2017).
Overlapping neuromolecular changes also occur in depression and cognitive impairment. The inflammatory immune response, if prolonged, causes a number of adverse changes to the central nervous system. According to the “inflammaging” hypothesis, aging is a process of chronic inflammation that increases mortality risk in older adults (Franceschi & Campisi, 2014). The release of proinflammatory cytokines impact serotonergic, noradrenergic, and dopaminergic neuronal circuits that mediate both depression and cognitive functions such as processing speed, executive functions, and memory (Miller & Raison, 2016; Ownby, 2010; Rutherford et al., 2017; Teixeira, Barbosa, Diniz, & Kummer, 2010). Additionally, studies have demonstrated differences in the circulation of neurotrophic factors between older adults with LLD and healthy controls. For example, significant reductions of brain-derived neurotrophic factor (BDNF) and glial cell-line derived neurotrophic factor levels have been observed in adults with late-onset depression compared to matched controls (Diniz et al., 2010; Teixeira et al., 2010). Polymorphisms in the BDNF gene have also been linked to depression risk and response to antidepressant treatment (Amare, Schubert, & Baune, 2017; Nestor et al., 2019). BDNF has a potent effect on synapses and a strong role in learning and memory (Ownby, 2010).
These neurobiological alterations often interrelate. For example, decreased BDNF expression and signaling results in reduced neuroplasticity associated with neuronal atrophy and reduced synaptic plasticity, particularly in the medial PFC and hippocampus (Diniz et al., 2014; Duman & Aghajanian, 2012). Changes in proinflammatory processes and neurotrophic function contribute to hippocampal volume reduction in depression (Audet & Anisman, 2013; Frodl et al., 2007). Moreover, prolonged hypercortisolemia and vascular pathology have both been linked to proinflammatory changes associated with functional changes in brain networks (Butters et al., 2008; Maier, Makwana, & Hare, 2015).
In sum, several neurobiological mechanisms appear to work in concert to mediate mood and cognitive function. Studies suggest stress-related, neurodegenerative, inflammatory, and vascular processes contribute to dysregulation in multiple parallel and synergistic neural circuits that underscore cognitive impairment in LLD. The overlapping neural underpinnings of mood and cognitive functioning are natural treatment targets for LLD with cognitive impairment.
Exercise and Neurobiological Changes in Late-life Depression
Exercise may be an ideal treatment for LLD with cognitive impairment because of its impact on neurobiological mechanisms that are important for both mood and cognitive functioning. Over the past few decades, exercise has come to be recognized as an effective treatment for mood symptoms in depression equivalent to psychological and pharmacological treatments. The literature on the mood benefits of exercise has been summarized in a number of reviews and meta-analyses (Kvam, Kleppe, Nordhus, & Hovland, 2016; Md Zemberi, Ismail, & Abdullah, 2020; Zhang, Xiang, Li, & Pan, 2021) and will not be repeated here, rather, we highlight the neurobiological mechanisms that might underlie these benefits. It should be noted that the literature not only supports the benefits of exercise—planned, structured physical activity that is intended to improve or maintain physical fitness—but also physical activity in general, that is, any intentional movement that expends energy.
Exercise is a particularly powerful treatment because it improves mood symptoms while also providing protection from age-related brain atrophy (Colcombe et al., 2003), contributing to neuroplasticity in the hippocampus, PFC, and anterior cingulate (Kandola, Ashdown-Franks, Hendrikse, Sabiston, & Stubbs, 2019), and inducing volumetric increases in both grey and white matter regions that overlap with depression-related volume reductions (Gujral, Aizenstein, Reynolds, Butters, & Erickson, 2017). For example, in contrast to hippocampal volume reduction in depression (Sexton et al., 2013), high cardiorespiratory fitness, the ability of the heart, lungs, and vascular system to supply the muscles with oxygen during sustained physical activity, is associated with larger hippocampal volumes among older adults (Erickson, Leckie, & Weinstein, 2014; Gujral et al., 2017; Kvam et al., 2016). Even low-intensity exercise such as training in coordination and balance has been shown to increase hippocampal volume over time (Niemann, Godde, & Voelcker-Rehage, 2014). Increased hippocampal tissue density (Kleemeyer et al., 2016) and vascular plasticity (Maass et al., 2015) driven by exercise may underlie these macro-level volume changes.
While depression is generally associated with volume reductions in prefrontal regions (Bora, Harrison, Davey, Yucel, & Pantelis, 2012), a recent meta-analysis of randomized controlled trials revealed that, compared to control interventions, exercise interventions significantly increase gray matter volume bilaterally in the medial and superior frontal gyri, as well as the inferior frontal gyrus, left cingulate gyrus, and right anterior cingulate (Zheng et al., 2019). Aerobic exercise have also been linked to larger dorsolateral PFC (Jonasson et al., 2017) and superior frontal volumes among older adults (Bugg & Head, 2011). Thus, exercise appears to promote structural brain integrity across the hippocampus and regions of the PFC relevant to depression.
Both structured exercise and everyday physical activity also counteract white matter damage in depression (Reppermund et al., 2014; Respino et al., 2019), preserving white matter integrity across a number of frontolimbic white matter tracts important for emotion regulation, including the corpus callosum and uncinate fasciculus (Loprinzi, Harper, & Ikuta, 2020; Strommer et al., 2020). A systematic review of studies examining physical fitness or physical activity concluded that overall, higher physical fitness and higher physical activity levels are associated with better white matter structure in older adults, including greater white matter volumes, reduced volume and severity of white matter lesions, and improved measures of white matter microstructure (Sexton et al., 2016).
Exercise promotes functional connectivity in the brain, which is important in light of the disrupted functional connectivity reported in LLD (Tadayonnejad & Ajilore, 2014). For example, a systematic review examining the effects of aerobic exercise on structure and functioning within the default mode network reported that aerobic exercise interventions increased functional connectivity between the hippocampus, cingulate cortex, and other medial temporal areas of the default mode network (M. Y. Li et al., 2017). Non-aerobic exercise including stretching, toning, and yoga has also been found to improve functional connectivity in the default mode network (Gothe, Khan, Hayes, Erlenbach, & Damoiseaux, 2019; Voss et al., 2010).
Exercise appears to counteract reductions in BDNF that are reported in depression. Aerobic exercise, and potentially strength training, facilitates BDNF expression (Szuhany, Bugatti, & Otto, 2015). The effect is observable after a single exercise session, but is greater in long-term exercise interventions (Szuhany et al., 2015). It should be noted that while animal studies consistently show that exercise can elevate BDNF in the brain (Voss, Vivar, Kramer, & van Praag, 2013), meta-analyses of human studies have produced mixed results when examining whether exercise increases serum or plasma BDNF levels in depression. There is evidence that polymorphisms in the BDNF gene moderate the antidepressant effect of exercise (Dotson et al., 2016).
Overall, the widespread benefits of exercise on the aging brain, particularly in areas negatively impacted by depression (Figure 1), point towards the importance of continuing to study exercise interventions for LLD. Most research focuses on aerobic exercise, which requires more energy expenditure that leads to vascular changes and higher oxygen and glucose consumption (Stimpson, Davison, & Javadi, 2018), but other exercise modalities have brain benefits as well.
Figure 1.
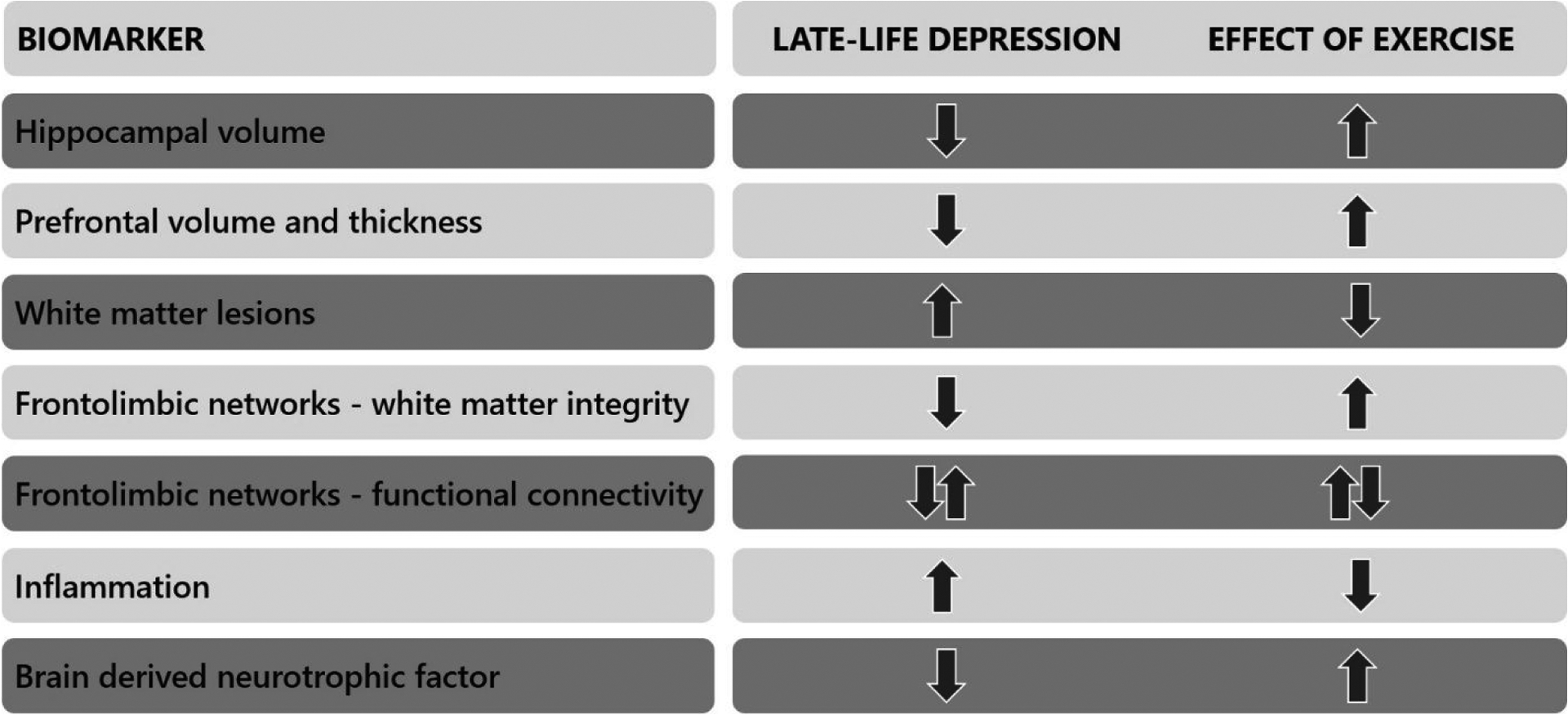
Summary of neurobiological changes in late-life depression that may be reversed by physical exercise.
Exercise Effects on Cognitive Functioning
Despite the longstanding use of exercise to improve mood in depression, less focus has been put on specifically prescribing exercise for cognitive impairment in LLD. Exercise, especially aerobic exercise, has been shown to improve cognitive function in healthy individuals across the lifespan and among different patient populations (Berwid & Halperin, 2012). Physical activity and long-term exercise have been shown to improve cognitive functioning, preserve cognitive functioning over time, and even prevent or delay dementia in older adults (Sofi et al., 2011). A meta-analysis of 39 randomized controlled trials found that in people over the age of 50, physical exercise improved cognitive functioning, including executive function, memory, and working memory (Northey, Cherbuin, Pumpa, Smee, & Rattray, 2018). Aerobic exercise, resistance training, multicomponent training, and tai chi were all found to be effective. Interventions that included 45–60 minute sessions of moderate or vigorous aerobic or resistance activities showed the strongest effects. Other studies have shown that resistance training improves spatial learning and memory, associative memory, cognitive control, and selective attention in older adults (Cassilhas et al., 2007; Liu-Ambrose, Nagamatsu, Voss, Khan, & Handy, 2012). It has been suggested that multicomponent training, involving aerobic and resistance training, may be more beneficial for global cognition than aerobic training alone (Saez de Asteasu, Martinez-Velilla, Zambom-Ferraresi, Casas-Herrero, & Izquierdo, 2017).
The body of literature examining the cognitive benefits of exercise for depression is relatively small and characterized by mixed findings. Although this is a narrative review, we systematically searched the literature in February 2021 using PubMed and PsycInfo to identify studies with this focus. Briefly, the PubMed search terms were “exercise AND cogn* AND depress*[Title] AND Humans[Mesh] NOT “review”[Publication Type] NOT meta-analysis[Title]”. The PsycInfo search terms were “exercise AND cogn* AND TI depress*”, and for Methodology we excluded literature review, meta-analysis, and systematic review. We then repeated each search substituting “cogn*” with “memory,” “executive,” “speed,” “attention,” “language,” and “visu*”. We will summarize the relevant studies that this systematic search identified.
A recent study (Olson, Brush, Ehmann, & Alderman, 2017) randomized depressed outpatients to an eight-week intervention involving three 45-minute sessions per week of either aerobic exercise or light-intensity stretching. Participants in the aerobic exercise group demonstrated significant improvements in cognitive control, as measured by reduced reaction time on a flanker task, which consists of a set of response inhibitions tasks that require selective attention and inhibition control. The aerobic exercise group also showed an increase in the amplitude of the event-related potential N2 component on incongruent trials. These findings suggest enhanced conflict monitoring and cognitive control—an aspect of executive functioning—after engaging in regular aerobic exercise. In a longer 12-week exercise intervention, Greer, Grannemann, Chansard, Karim, and Trivedi (2015) showed that aerobic exercise improved performance across several cognitive domains in depressed individuals, including psychomotor speed, visuospatial memory, and executive function. For individuals who exercised the most, improvements were also noted in spatial working memory. Similarly, aerobic exercise interventions led to greater improvement in cognitive functions including processing speed, working memory, and visual memory compared to control interventions in other studies (Krogh, Videbech, Thomsen, Gluud, & Nordentoft, 2012; Oertel-Knöchel et al., 2014), including a study that examined the acute effects of 30 minutes of aerobic exercise (Kubesch et al., 2003). Moreover, Buschert et al. (2019) found significant improvements in reaction time and immediate memory in depressed inpatients who participated in an average of ten 30-minute aerobic exercise sessions compared to the active controls who underwent occupational therapy. No differences in executive function were noted post-intervention. In a large sample of 3,658 participants with either subsyndromal or clinical depression, self-reported physical activity was positively associated with processing speed and verbal fluency in men (Joutsenniemi et al., 2013). Another study of self-reported physical activity found lower physical activity levels were associated with worse global cognitive functioning (Sanchez-Carro et al., 2021).
Despite these positive findings, other studies have failed to find an effect. For example, a four-month study comparing aerobic exercise, strength training, and relaxation training did not find an effect of either exercise intervention on cognitive functioning (Krogh, Saltin, Gluud, & Nordentoft, 2009). A recent meta-analysis in this area (Sun, Lanctot, Herrmann, & Gallagher, 2018) included 9 studies and, consistent with a previous meta-analysis (Brondino et al., 2017), failed to find a significant benefit of exercise for cognition in major depression in the overall analysis. However, positive findings were found when (a) exercise was combined with cognitive training and (b) in lower-intensity interventions, presumably due to the significantly better treatment adherence that was also found in the meta-analysis. Importantly, the authors noted several limitations to their findings. First, the mean dosage of exercise used in the included studies (131 minutes) was below the World Health Organization guidelines of 150 minutes of moderate intensity exercise per week (World Health Organization, 2019). In addition, cognitive impairment was not an inclusion criterion for any study in the meta-analysis, resulting in a general lack of cognitive impairment at baseline. This left little room for improvement (i.e., a ceiling effect), and research suggests that those with greater cognitive impairment may benefit more from exercise interventions (Smith et al., 2010). Overall, the lack of scientifically rigorous studies in this field suggests these results should be interpreted with discretion.
Even less research has addressed the impact of exercise on cognition in depressed older adults in particular. In an intervention study, Neviani et al. (2017) randomly assigned 121 depressed older adults to an (a) antidepressant medication group, (b) antidepressant plus non-aerobic exercise group, or (c) antidepressant plus progressive aerobic exercise group. After the six-month intervention, older adults in the antidepressant plus progressive aerobic exercise group showed significant improvements in Montreal Cognitive Assessment total scores as well as visuospatial/executive and language subdomains. Of note, disability scores improved after the intervention as well. Participants in the non-aerobic exercise group did not show significant cognitive differences compared the control group. Benefits of exercise have been shown in shorter interventions as well. Depressed middle-aged to older adults who spent four months in an aerobic exercise intervention showed greater improvements in memory and executive functions than their peers who received antidepressant medication (Khatri et al., 2001). Another study found that 12 weeks of exergames improved cognitive functioning in older adults with subsyndromal depression (Rosenberg et al., 2010). In contrast, a small pilot study that compared six older depressed inpatients who completed a 4-week exercise program involving aerobic, strength, and coordination exercises to six who received relaxation training did not find any changes in cognitive functioning (Heissel et al., 2015). The small sample size, short time frame, or the severity of depression in the inpatient sample might all contribute to the null results. It should be noted that at least one study suggests an acute benefit of aerobic exercise on inhibitory control but not on immediate memory or working memory (Vasques, Moraes, Silveira, Deslandes, & Laks, 2011).
Studies that examine self-report of physical activity provide supporting evidence that exercise might benefit cognitive functioning in LLD. In a recent cross-sectional study examining data from 2,604 adults age 60 and older from the National Health and Nutrition Examination Survey, (Hu, Smith, Imm, Jackson, & Yang, 2019), verbal fluency and processing speed were preserved even in moderately to severely depressed older adults who maintained a sufficient level of physical activity (≥ 150 min/week moderate-to-vigorous physical activity). Similarly, two studies with middle aged or older samples with depression found positive relationships between higher self-reported exercise frequency and cognitive functioning (Pitts et al., 2020; Yuan, Fu, Liu, & Fang, 2020).
Overall, the research in this area is limited but the emerging evidence supporting the procognitive effects of exercise in LLD is promising.
Future Directions
Physical exercise has the potential to be an accessible and effective treatment for cognitive impairment in LLD. Overall, there is a strong body of literature supporting the benefits of exercise as a treatment for affective symptoms in depression and as a positive modifier of cognitive and brain aging in non-depressed older adults (Chen et al., 2020; Kvam et al., 2016; Md Zemberi et al., 2020; Quigley, MacKay-Lyons, & Eskes, 2020; Zhang et al., 2021). Studies specifically examining the impact of exercise on cognitive impairment in LLD are more limited, but emerging studies are encouraging. Much more work is needed in this area in order to translate the growing research findings into clinical practice. Clinical translation will need to address issues of motivation and adherence, since depressed mood and related symptoms, such as loss of interest and energy, fatigue, and low self-worth are common barriers to participation in exercise (Firth et al., 2016; Knapen, Vancampfort, Morien, & Marchal, 2015).
Large randomized clinical trials are needed, focused on the impact of different forms of exercise on impairment in different cognitive functions in LLD. Although aerobic exercise interventions are most common, studies have shown that other forms of exercise can benefit mood, cognitive functioning, and brain health, including resistance training, yoga, and tai chi (Gordon et al., 2018; Gothe et al., 2019; Wayne et al., 2014). Future studies should seek to determine which forms of exercise, or combination of forms, is most effective for ameliorating cognitive impairment in LLD. Similarly, there is evidence that exercise can benefit a variety of cognitive functions (Northey et al., 2018) but more research is needed to understand whether different forms of exercise are differentially beneficial for specific cognitive deficits in LLD. Executive dysfunction is particularly important to target since executive abilities are affected in normal aging as well as depression, and are predictive of functional decline (Gansler et al., 2015).
It is important for future studies to consider the demographic and clinical heterogeneity within the population of depressed older adults. Clinically, there is growing evidence that cognitive and brain changes, response to treatment, and clinical outcomes differ depending on the severity of specific dimensions of depressive symptoms, such as affective, somatic, and cognitive symptoms (Lugtenburg, Zuidersma, Oude Voshaar, & Schoevers, 2016; Majd, Saunders, & Engeland, 2020; McLaren et al., 2017; Schouten et al., 2019). There is also preliminary evidence that the antidepressant effect of exercise varies across symptom dimension in older adults (Dotson et al., 2016; Murri et al., 2018). Thus, future studies should seek to determine whether there are subtypes of LLD based on clinical presentation that are more responsive to the mood and cognitive enhancing effects of exercise.
Also important is the impact of sex, race/ethnicity, socioeconomic status, and other aspects of diversity on both the efficacy and the feasibility of implementing exercise as an intervention for cognitive impairment in LLD. Demographic factors have been shown to impact risk for and outcomes of depression, trajectories of cognitive and brain aging, as well as access to and participation in structured exercise (Komulainen et al., 2008; Vyas et al., 2020). In order for this area of research to be relevant to the increasingly diverse population of older adults, it is critical for studies to consider aspects of diversity in their design and analysis (Dotson & Duarte, 2020).
Neuroimaging studies will be helpful in identifying the neurobiological mechanisms underlying exercise-related improvements in cognitive functioning in LLD. Although a systematic review concluded that exercise produces changes in cortical activity, endocrine response, and oxidative stress in depression (Schuch et al., 2016) and there is evidence that cardiorespiratory fitness is associated with cortical thickness in LLD (Gujral et al., 2019), it is still unclear whether these changes mediate the relationship between exercise and cognitive functioning. Alternatively, or perhaps in conjunction, it is possible that exercise-related reductions in depression lead to improved cognitive functioning. For example, in a sample of older adults, some with mild cognitive impairment, Vance et al. (2016) used structural equation modeling to show a path from higher self-reported physical activity to lower depressive symptoms, which in turn related to better cognitive ability. Clarifying the mechanism underlying any cognitive enhancing effects is important, as it will aid in identifying other treatments for cognitive impairment in LLD that can target those mechanisms.
Finally, future studies should examine the benefits of combined interventions for cognitive impairment in LLD. For example, there is evidence suggesting the combination of cognitive and exercise training is more beneficial than either intervention alone (Karssemeijer et al., 2017). Such interventions can involve either simultaneous cognitive challenge and physical exercise (such as performing a cognitive feat while walking), or a program that includes both cognitive training and physical exercise activities performed asynchronously. In addition, omega-3 fatty acids, antioxidants such as Vitamin E, and polyphenols have been shown to enhance the effects of exercise on cognition and BDNF-related synaptic plasticity in non-human animal subjects (Gomez-Pinilla & Nguyen, 2012; Sakr, Abbas, & El Samanoudy, 2015; Wu, Ying, & Gomez-Pinilla, 2008), though limited studies have been conducted in humans (Bischoff-Ferrari et al., 2020; Schattin et al., 2019).
Conclusion
Depression in older adults is a risk factor for a number of negative outcomes, in part due to comorbid cognitive impairment that is common in this population. Traditional treatments often result in improved mood yet lingering cognitive and brain changes that put depressed older adults at risk for recurrence of depression, functional decline, and dementia. Treatments are needed that target the underlying neurobiological changes that contribute to both cognitive impairment and mood disruption. Exercise appears to have potential as an alternative or adjunctive treatment given its known impact on depression, on cognitive functioning in older adults and in other patient populations, and on many of the neurobiological mechanisms linked to depression and cognitive functioning. Additional research in this area in large, diverse samples has the potential to tremendously impact the health and well-being of the growing population of older adults.
Acknowledgements
Conflict of interest:
VMD is owner of CerebroFit, LLC.
Funding sources:
VMD is supported by the National Institutes of Health (AG054046-04). HRB and ZT are supported by the Health Resources and Service Administration (D40HP33346). AMG is supported by the Georgia State University Brains & Behavior graduate student fellowship.
References
- Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, & Gunning-Dixon F (2005). Executive dysfunction and the course of geriatric depression. Biological Psychiatry, 58(3), 204–210. doi: 10.1016/j.biopsych.2005.04.024 [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, & Charlson M (1997). ‘Vascular depression’hypothesis. Archives of General Psychiatry, 54(10), 915–922. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Kalayam B, Kakuma T, Gabrielle M, … Hull J (2000). Executive dysfunction and long-term outcomes of geriatric depression. Archives of General Psychiatry, 57(3), 285–290. doi: 10.1001/archpsyc.57.3.285 [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Meyers BS, Young RC, Mattis S, & Kakuma T (1993). The course of geriatric depression with “reversible dementia”: a controlled study. American Journal of Psychiatry, 150(11), 1693–1699. doi: 10.1176/ajp.150.11.1693 [DOI] [PubMed] [Google Scholar]
- Amare AT, Schubert KO, & Baune BT (2017). Pharmacogenomics in the treatment of mood disorders: Strategies and Opportunities for personalized psychiatry. EPMA Journal, 8(3), 211–227. doi: 10.1007/s13167-017-0112-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet M-C, & Anisman H (2013). Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Frontiers in Cellular Neuroscience, 7, 68–68. doi: 10.3389/fncel.2013.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz R, & Steffens D (2017). Overlay of late-Life depression and cognitive impairment. Focus: The Journal of Lifelong Learning in Psychiatry, 15(1), 35–41. doi: 10.1176/appi.focus.20160036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballmaier M, Sowell ER, Thompson PM, Kumar A, Narr KL, Lavretsky H, … Toga AW (2004). Mapping brain size and cortical gray matter changes in elderly depression. Biological Psychiatry, 55(4), 382–389. [DOI] [PubMed] [Google Scholar]
- Barch DM, D’Angelo G, Pieper C, Wilkins CH, Welsh-Bohmer K, Taylor W, … Sheline YI (2012). Cognitive improvement following treatment in late-life depression: relationship to vascular risk and age of onset. American Journal of Geriatric Psychiatry, 20(8), 682–690. doi: 10.1097/JGP.0b013e318246b6cb [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwid OG, & Halperin JM (2012). Emerging support for a role of exercise in attention deficit/hyperactivity disorder intervention planning. Current Psychiatry Reports, 14(5), 543–551. doi: 10.1007/s11920-012-0297-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, … Becker JT (2006). Persistence of neuropsychologic deficits in the remitted state of late-life depression. American Journal of Geriatric Psychiatry, 14(5), 419–427. doi: 10.1097/01.JGP.0000203130.45421.69 [DOI] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, Vellas B, Rizzoli R, Kressig RW, da Silva JAP, Blauth M, … Group, D.-H. R. (2020). Effect of vitamin D supplementation, omega-3 fatty acid supplementation, or a strength-training exercise program on clinical outcomes in older adults: the DO-HEALTH randomized clinical trial. JAMA, 324(18), 1855–1868. doi: 10.1001/jama.2020.16909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Davey CG, Yucel M, & Pantelis C (2012). Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychological Medicine, 42(4), 671–681. doi: 10.1017/s0033291711001668 [DOI] [PubMed] [Google Scholar]
- Brondino N, Rocchetti M, Fusar-Poli L, Codrons E, Correale L, Vandoni M, … Politi P (2017). A systematic review of cognitive effects of exercise in depression. Acta Psychiatrica Scandinavica, 135(4), 285–295. doi: 10.1111/acps.12690 [DOI] [PubMed] [Google Scholar]
- Bugg JM, & Head D (2011). Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiology of Aging, 32(3), 506–514. doi: 10.1016/j.neurobiolaging.2009.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschert V, Prochazka D, Bartl H, Diemer J, Malchow B, Zwanzger P, & Brunnauer A (2019). Effects of physical activity on cognitive performance: A controlled clinical study in depressive patients. European Archives of Psychiatry and Clinical Neuroscience, 269(5), 555–563. doi: 10.1007/s00406-018-0916-0 [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF 3rd, … Becker JT (2008). Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience, 10(3), 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassilhas RC, Viana VA, Grassmann V, Santos RT, Santos RF, Tufik S, & Mello MT (2007). The impact of resistance exercise on the cognitive function of the elderly. Medicine & Science in Sports & Exercise, 39(8), 1401–1407. doi: 10.1249/mss.0b013e318060111f [DOI] [PubMed] [Google Scholar]
- Chen FT, Hopman RJ, Huang CJ, Chu CH, Hillman CH, Hung TM, & Chang YK (2020). The Effect of Exercise Training on Brain Structure and Function in Older Adults: A Systematic Review Based on Evidence from Randomized Control Trials. J Clin Med, 9(4). doi: 10.3390/jcm9040914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Kim S, & Anstey KJ (2015). Dementia risk estimates associated with measures of depression: a systematic review and meta-analysis. BMJ Open, 5(12), e008853. doi: 10.1136/bmjopen-2015-008853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, & Kramer AF (2003). Aerobic fitness reduces brain tissue loss in aging humans. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 58(2), 176–180. doi: 10.1093/gerona/58.2.m176 [DOI] [PubMed] [Google Scholar]
- Collaborators, G. D. a. I. I. a. P. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet, 392(10159), 1789–1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors MH, Quinto L, & Brodaty H (2018). Longitudinal outcomes of patients with pseudodementia: a systematic review. Psychological Medicine, 1–11. doi: 10.1017/S0033291718002829 [DOI] [PubMed] [Google Scholar]
- Crocco EA, Castro K, & Loewenstein DA (2010). How late-life depression affects cognition: neural mechanisms. Current Psychiatry Reports, 12(1), 34–38. doi: 10.1007/s11920-009-0081-2 [DOI] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, & Reynolds CF 3rd. (2013). Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. British Journal of Psychiatry, 202(5), 329–335. doi: 10.1192/bjp.bp.112.118307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Reynolds CF 3rd, Begley A, Dew MA, Anderson SJ, Lotrich F, … Butters MA (2014). Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: a longitudinal study. Journal of Psychiatric Research, 49, 96–101. doi: 10.1016/j.jpsychires.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Talib LL, Mendonca VA, Gattaz WF, & Forlenza OV (2010). Serum brain-derived neurotrophic factor level is reduced in antidepressant-free patients with late-life depression. World Journal of Biological Psychiatry, 11(3), 550–555. doi: 10.3109/15622970903544620 [DOI] [PubMed] [Google Scholar]
- Dotson VM, Beydoun MA, & Zonderman AB (2010). Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology, 75(1), 27–34. doi: 10.1212/WNL.0b013e3181e62124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Davatzikos C, Kraut MA, & Resnick SM (2009). Depressive symptoms and brain volumes in older adults: a longitudinal magnetic resonance imaging study. Journal of Psychiatry & Neuroscience, 34(5), 367. [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, & Duarte A (2020). The importance of diversity in cognitive neuroscience. Annals of the New York Academy of Sciences, 1464(1), 181–191. doi: 10.1111/nyas.14268 [DOI] [PubMed] [Google Scholar]
- Dotson VM, Hsu FC, Langaee TY, McDonough CW, King AC, Cohen RA, … Life Study, G. (2016). Genetic moderators of the impact of physical activity on depressive symptoms. Journal of Frailty & Aging, 5(1), 6–14. doi: 10.14283/jfa.2016.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, McClintock SM, Verhaeghen P, Kim JU, Draheim AA, Syzmkowicz SM, … Wit L (2020). Depression and cognitive control across the lifespan: A systematic review and meta-analysis. Neuropsychology Review. doi: 10.1007/s11065-020-09436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Resnick SM, & Zonderman AB (2008). Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. American Journal of Geriatric Psychiatry, 16(4), 318–330. doi: 10.1097/JGP.0b013e3181662a9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Szymkowicz SM, Kirton JW, McLaren ME, Green ML, & Rohani JY (2014). Unique and interactive effect of anxiety and depressive symptoms on cognitive and brain function in young and older adults. Journal of Depression and Anxiety, Suppl 1. doi: 10.4172/2167-1044.S1-003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Zonderman AB, Kraut MA, & Resnick SM (2013). Temporal relationships between depressive symptoms and white matter hyperintensities in older men and women. International Journal of Geriatric Psychiatry, 28(1), 66–74. doi: 10.1002/gps.3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, & Furey ML (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Structure & Function, 213(1–2), 93–118. doi: 10.1007/s00429-008-0189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du M, Liu J, Chen Z, Huang X, Li J, Kuang W, … Bi F (2014). Brain grey matter volume alterations in late-life depression. Journal of Psychiatry & Neuroscience, 39(6), 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, & Aghajanian GK (2012). Synaptic dysfunction in depression: potential therapeutic targets. Science, 338(6103), 68–72. doi: 10.1126/science.1222939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, & Kumar A (2006). Executive dysfunction and memory in older patients with major and minor depression. Archives of Clinical Neuropsychology, 21(7), 669–676. doi: 10.1016/j.acn.2006.05.011 [DOI] [PubMed] [Google Scholar]
- Erickson KI, Leckie RL, & Weinstein AM (2014). Physical activity, fitness, and gray matter volume. Neurobiology of Aging, 35 Suppl 2, S20–28. doi: 10.1016/j.neurobiolaging.2014.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth J, Rosenbaum S, Stubbs B, Gorczynski P, Yung AR, & Vancampfort D (2016). Motivating factors and barriers towards exercise in severe mental illness: a systematic review and meta-analysis. Psychol Med, 46(14), 2869–2881. doi: 10.1017/S0033291716001732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, & Campisi J (2014). Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. The Journals of Gerontology: Series A, 69(Suppl_1), S4–S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- Frodl T, Schüle C, Schmitt G, Born C, Baghai T, Zill P, … Meisenzahl EM (2007). Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Archives of General Psychiatry, 64(4), 410–416. doi: 10.1001/archpsyc.64.4.410 [DOI] [PubMed] [Google Scholar]
- Gansler DA, Suvak M, Arean P, & Alexopoulos GS (2015). Role of executive dysfunction and dysexecutive behavior in late-life depression and disability. American Journal of Geriatric Psychiatry, 23(10), 1038–1045. doi: 10.1016/j.jagp.2015.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, & Nguyen TT (2012). Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutritional Neuroscience, 15(3), 127–133. doi: 10.1179/1476830511Y.0000000035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon BR, McDowell CP, Hallgren M, Meyer JD, Lyons M, & Herring MP (2018). Association of efficacy of resistance exercise training with depressive symptoms: Meta-analysis and metaregression analysis of randomized clinical trials. JAMA Psychiatry, 75(6), 566–576. doi: 10.1001/jamapsychiatry.2018.0572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothe NP, Khan I, Hayes J, Erlenbach E, & Damoiseaux JS (2019). Yoga effects on brain health: A systematic review of the current literature. Brain Plasticity, 5(1), 105–122. doi: 10.3233/BPL-190084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein AS, Paranthaman R, Burns A, Jackson A, Malik RA, Baldwin RC, & Heagerty AM (2010). Cerebrovascular damage in late-life depression is associated with structural and functional abnormalities of subcutaneous small arteries. Hypertension, 56(4), 734–740. [DOI] [PubMed] [Google Scholar]
- Greer TL, Grannemann BD, Chansard M, Karim AI, & Trivedi MH (2015). Dose-dependent changes in cognitive function with exercise augmentation for major depression: Results from the TREAD study. European Neuropsychopharmacology, 25(2), 248–256. doi: 10.1016/j.euroneuro.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Gujral S, Aizenstein H, Reynolds CF 3rd, Butters MA, & Erickson KI (2017). Exercise effects on depression: Possible neural mechanisms. General Hospital Psychiatry, 49, 2–10. doi: 10.1016/j.genhosppsych.2017.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujral S, Aizenstein H, Reynolds CF 3rd, Butters MA, Grove G, Karp JF, & Erickson KI (2019). Exercise for depression: A feasibility trial exploring neural mechanisms. American Journal of Geriatric Psychiatry, 27(6), 611–616. doi: 10.1016/j.jagp.2019.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissel A, Vesterling A, White SA, Kallies G, Behr D, Arafat AM, … Budde H (2015). Feasibility of an Exercise Program for Older Depressive Inpatients. 28(4), 163–171. doi: 10.1024/1662-9647/a000134 [DOI] [Google Scholar]
- Herbert J, & Lucassen PJ (2016). Depression as a risk factor for Alzheimer’s disease: Genes, steroids, cytokines and neurogenesis - What do we need to know? Frontiers in Neuroendocrinology, 41, 153–171. doi: 10.1016/j.yfrne.2015.12.001 [DOI] [PubMed] [Google Scholar]
- Hu L, Smith L, Imm KR, Jackson SE, & Yang L (2019). Physical activity modifies the association between depression and cognitive function in older adults. Journal of Affective Disorders, 246, 800–805. doi: 10.1016/j.jad.2019.01.008 [DOI] [PubMed] [Google Scholar]
- Jonasson LS, Nyberg L, Kramer AF, Lundquist A, Riklund K, & Boraxbekk C-J (2017). Aerobic exercise intervention, cognitive performance, and brain structure: Results from the physical influences on brain in aging (PHIBRA) study. Frontiers in Aging Neuroscience, 8. doi: 10.3389/fnagi.2016.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF (2001). History of depression as a risk factor for dementia: an updated review. Australian and New Zealand Journal of Psychiatry, 35(6), 776–781. doi: 10.1046/j.1440-1614.2001.00967.x [DOI] [PubMed] [Google Scholar]
- Joutsenniemi K, Tuulio-Henriksson A, Elovainio M, Härkänen T, Sainio P, Koskinen S, … Partonen T (2013). Depressive symptoms, major depressive episodes and cognitive test performance-what is the role of physical activity? Nord J Psychiatry, 67(4), 265–273. doi: 10.3109/08039488.2012.736533 [DOI] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, & Pizzagalli DA (2015). Large-scale network dysfunction in major depressive disorder: A meta-analysis of resting-state functional connectivity. JAMA Psychiatry, 72(6), 603–611. doi: 10.1001/jamapsychiatry.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandola A, Ashdown-Franks G, Hendrikse J, Sabiston CM, & Stubbs B (2019). Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neuroscience & Biobehavioral Reviews, 107, 525–539. doi: 10.1016/j.neubiorev.2019.09.040 [DOI] [PubMed] [Google Scholar]
- Karssemeijer EGA, Aaronson JA, Bossers WJ, Smits T, Olde Rikkert MGM, & Kessels RPC (2017). Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Research Reviews, 40, 75–83. doi: 10.1016/j.arr.2017.09.003 [DOI] [PubMed] [Google Scholar]
- Keller J, Gomez R, Williams G, Lembke A, Lazzeroni L, Murphy GM Jr., & Schatzberg AF (2017). HPA axis in major depression: cortisol, clinical symptomatology and genetic variation predict cognition. Molecular Psychiatry, 22(4), 527–536. doi: 10.1038/mp.2016.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempton MJ, Salvador Z, Munafò MR, Geddes JR, Simmons A, Frangou S, & Williams SC (2011). Structural neuroimaging studies in major depressive disorder. Meta-analysis and comparison with bipolar disorder. Archives of General Psychiatry, 68(7), 675–690. doi: 10.1001/archgenpsychiatry.2011.60 [DOI] [PubMed] [Google Scholar]
- Khatri P, Blumenthal JA, Babyak MA, Craighead WE, Herman S, Baldewicz T, … Krishnan KR (2001). Effects of exercise training on cognitive functioning among depressed older men and women. Journal of Aging and Physical Activity, 9(1), 43–57. [Google Scholar]
- Kirton JW, Resnick SM, Davatzikos C, Kraut MA, & Dotson VM (2014). Depressive symptoms, symptom dimensions, and white matter lesion volume in older adults: a longitudinal study. American Journal of Geriatric Psychiatry, 22(12), 1469–1477. doi: 10.1016/j.jagp.2013.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleemeyer MM, Kuhn S, Prindle J, Bodammer NC, Brechtel L, Garthe A, … Lindenberger U (2016). Changes in fitness are associated with changes in hippocampal microstructure and hippocampal volume among older adults. Neuroimage, 131, 155–161. doi: 10.1016/j.neuroimage.2015.11.026 [DOI] [PubMed] [Google Scholar]
- Knapen J, Vancampfort D, Morien Y, & Marchal Y (2015). Exercise therapy improves both mental and physical health in patients with major depression. Disabil Rehabil, 37(16), 1490–1495. doi: 10.3109/09638288.2014.972579 [DOI] [PubMed] [Google Scholar]
- Koenig AM, Bhalla RK, & Butters MA (2014). Cognitive functioning and late-life depression. Journal of the International Neuropsychological Society, 20(5), 461–467. doi: 10.1017/s1355617714000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig AM, DeLozier IJ, Zmuda MD, Marron MM, Begley AE, Anderson SJ, … Butters MA (2015). Neuropsychological functioning in the acute and remitted States of late-life depression. Journal of Alzheimer’s Disease, 45(1), 175–185. doi: 10.3233/JAD-148006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S, Thomas AJ, Barnett NA, & O’Brien JT (2010). The pattern and course of cognitive impairment in late-life depression. Psychological Medicine, 40(4), 591–602. doi: 10.1017/S0033291709990833 [DOI] [PubMed] [Google Scholar]
- Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, … Rauramaa R (2008). BDNF is a novel marker of cognitive function in ageing women: the DR’s EXTRA Study. Neurobiology of Learning and Memory, 90(4), 596–603. doi: 10.1016/j.nlm.2008.07.014 [DOI] [PubMed] [Google Scholar]
- Krogh J, Saltin B, Gluud C, & Nordentoft M (2009). The DEMO trial: a randomized, parallel-group, observer-blinded clinical trial of strength versus aerobic versus relaxation training for patients with mild to moderate depression. J Clin Psychiatry, 70(6), 790–800. doi: 10.4088/jcp.08m04241 [DOI] [PubMed] [Google Scholar]
- Krogh J, Videbech P, Thomsen C, Gluud C, & Nordentoft M (2012). DEMO-II trial. Aerobic exercise versus stretching exercise in patients with major depression-a randomised clinical trial. PLoS One, 7(10), e48316. doi: 10.1371/journal.pone.0048316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubesch S, Bretschneider V, Freudenmann R, Weidenhammer N, Lehmann M, Spitzer M, & Grön G (2003). Aerobic Endurance Exercise Improves Executive Functions in Depressed Patients. The Journal of Clinical Psychiatry, 64(9), 1005–1012. doi: 10.4088/JCP.v64n0905 [DOI] [PubMed] [Google Scholar]
- Kvam S, Kleppe CL, Nordhus IH, & Hovland A (2016). Exercise as a treatment for depression: A meta-analysis. Journal of Affective Disorders, 202, 67–86. doi: 10.1016/j.jad.2016.03.063 [DOI] [PubMed] [Google Scholar]
- Lee JS, Potter GG, Wagner HR, Welsh-Bohmer KA, & Steffens DC (2007). Persistent mild cognitive impairment in geriatric depression. International Psychogeriatrics, 19(1), 125–135. doi: 10.1017/S1041610206003607 [DOI] [PubMed] [Google Scholar]
- Li MY, Huang MM, Li SZ, Tao J, Zheng GH, & Chen LD (2017). The effects of aerobic exercise on the structure and function of DMN-related brain regions: a systematic review. International Journal of Neuroscience, 127(7), 634–649. doi: 10.1080/00207454.2016.1212855 [DOI] [PubMed] [Google Scholar]
- Li W, Wang Y, Ward BD, Antuono PG, Li SJ, & Goveas JS (2017). Intrinsic inter-network brain dysfunction correlates with symptom dimensions in late-life depression. Journal of Psychiatric Research, 87, 71–80. doi: 10.1016/j.jpsychires.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Huang X, Wu Q, Yang C, Kuang W, Du M, … Gong Q (2013). Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. Journal of Psychiatry & Neuroscience, 38(1), 49–56. doi: 10.1503/jpn.110180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wang D, Lan L, & Fan Y (2017). Multiple Factors Involved in the Pathogenesis of White Matter Lesions. Biomed Res Int, 2017, 9372050. doi: 10.1155/2017/9372050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnemann C, & Lang UE (2020). Pathways connecting late-life depression and dementia. Frontiers in Pharmacology, 11, 279. doi: 10.3389/fphar.2020.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, & Handy TC (2012). Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiology of Aging, 33(8), 1690–1698. doi: 10.1016/j.neurobiolaging.2011.05.010 [DOI] [PubMed] [Google Scholar]
- Lockwood KA, Alexopoulos GS, & van Gorp WG (2002). Executive dysfunction in geriatric depression. American Journal of Psychiatry, 159(7), 1119–1126. doi: 10.1176/appi.ajp.159.7.1119 [DOI] [PubMed] [Google Scholar]
- Loprinzi PD, Harper J, & Ikuta T (2020). The effects of aerobic exercise on corpus callosum integrity: systematic review. Physician and Sportsmedicine, 1–7. doi: 10.1080/00913847.2020.1758545 [DOI] [PubMed] [Google Scholar]
- Lugtenburg A, Zuidersma M, Oude Voshaar RC, & Schoevers RA (2016). Symptom dimensions of depression and 3-year incidence of dementia: Results from the Amsterdam Study of the Elderly. Journal of Geriatric Psychiatry and Neurology, 29(2), 99–107. doi: 10.1177/0891988715606235 [DOI] [PubMed] [Google Scholar]
- Maass A, Duzel S, Goerke M, Becke A, Sobieray U, Neumann K, … Duzel E (2015). Vascular hippocampal plasticity after aerobic exercise in older adults. Molecular Psychiatry, 20(5), 585–593. doi: 10.1038/mp.2014.114 [DOI] [PubMed] [Google Scholar]
- Maier SU, Makwana AB, & Hare TA (2015). Acute stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain’s decision circuits. Neuron, 87(3), 621–631. doi: 10.1016/j.neuron.2015.07.005 [DOI] [PubMed] [Google Scholar]
- Majd M, Saunders EFH, & Engeland CG (2020). Inflammation and the dimensions of depression: A review. Frontiers in Neuroendocrinology, 56, 100800. doi: 10.1016/j.yfrne.2019.100800 [DOI] [PubMed] [Google Scholar]
- Majer M, Ising M, Kunzel H, Binder EB, Holsboer F, Modell S, & Zihl J (2004). Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychological Medicine, 34(8), 1453–1463. doi: 10.1017/s0033291704002697 [DOI] [PubMed] [Google Scholar]
- McLaren ME, Szymkowicz SM, O’Shea A, Woods AJ, Anton SD, & Dotson VM (2017). Vertex-wise examination of depressive symptom dimensions and brain volumes in older adults. Psychiatry Research: Neuroimaging, 260, 70–75. doi: 10.1016/j.pscychresns.2016.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md Zemberi NFN, Ismail MM, & Abdullah M (2020). Exercise Interventions as the Primary Treatment for Depression: Evidence from a Narrative Review. Malays J Med Sci, 27(5), 5–23. doi: 10.21315/mjms2020.27.5.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menchetti M, Cevenini N, De Ronchi D, Quartesan R, & Berardi D (2006). Depression and frequent attendance in elderly primary care patients. Gen Hosp Psychiatry, 28(2), 119–124. doi: 10.1016/j.genhosppsych.2005.10.007 [DOI] [PubMed] [Google Scholar]
- Miller AH, & Raison CL (2016). The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nature Reviews Immunology, 16(1), 22–34. doi: 10.1038/nri.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto SS, & Alexopoulos GS (2013). Cognitive deficits in geriatric depression: clinical correlates and implications for current and future treatment. Psychiatric Clinics of North America, 36(4), 517–531. doi: 10.1016/j.psc.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CF, & Alexopoulos GS (2004). Longitudinal association of initiation/perseveration and severity of geriatric depression. American Journal of Geriatric Psychiatry, 12(1), 50–56. [PubMed] [Google Scholar]
- Murri MB, Ekkekakis P, Menchetti M, Neviani F, Trevisani F, Tedeschi S, … Amore M (2018). Physical exercise for late-life depression: Effects on symptom dimensions and time course. Journal of Affective Disorders, 230, 65–70. doi: 10.1016/j.jad.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Naismith SL, Norrie LM, Mowszowski L, & Hickie IB (2012). The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Progress in Neurobiology, 98(1), 99–143. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Baba H, Maeshima H, Kitajima A, Sakai Y, Baba K, … Arai H (2008). Executive dysfunction in medicated, remitted state of major depression. Journal of Affective Disorders, 111(1), 46–51. doi: 10.1016/j.jad.2008.01.027 [DOI] [PubMed] [Google Scholar]
- Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, & Reynolds CF 3rd. (2003). Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. Journal of Psychiatric Research, 37(2), 99–108. doi: 10.1016/s0022-3956(02)00085-7 [DOI] [PubMed] [Google Scholar]
- Nestor PG, O’Donovan K, Lapp HE, Hasler VC, Boodai SB, & Hunter R (2019). Risk and protective effects of serotonin and BDNF genes on stress-related adult psychiatric symptoms. Neurobiology of Stress, 11, 100186. doi: 10.1016/j.ynstr.2019.100186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neviani F, Belvederi Murri M, Mussi C, Triolo F, Toni G, Simoncini E, … Neri M (2017). Physical exercise for late life depression: Effects on cognition and disability. International Psychogeriatrics, 29(7), 1105–1112. doi: 10.1017/S1041610217000576 [DOI] [PubMed] [Google Scholar]
- Niemann C, Godde B, & Voelcker-Rehage C (2014). Not only cardiovascular, but also coordinative exercise increases hippocampal volume in older adults. Frontiers in Aging Neuroscience, 6, 170. doi: 10.3389/fnagi.2014.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northey JM, Cherbuin N, Pumpa KL, Smee DJ, & Rattray B (2018). Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. British Journal of Sports Medicine, 52(3), 154–160. doi: 10.1136/bjsports-2016-096587 [DOI] [PubMed] [Google Scholar]
- Oertel-Knöchel V, Mehler P, Thiel C, Steinbrecher K, Malchow B, Tesky V, … Hänsel F (2014). Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. European Archives of Psychiatry and Clinical Neuroscience, 264(7), 589–604. doi: 10.1007/s00406-014-0485-9 [DOI] [PubMed] [Google Scholar]
- Olson RL, Brush CJ, Ehmann PJ, & Alderman BL (2017). A randomized trial of aerobic exercise on cognitive control in major depression. Clinical Neurophysiology, 128(6), 903–913. doi: 10.1016/j.clinph.2017.01.023 [DOI] [PubMed] [Google Scholar]
- Organization”, W. H. (2019). Global action plan on physical activity 2018–2030: More active people for a healthier world: World Health Organization. [Google Scholar]
- Ownby RL (2010). Neuroinflammation and cognitive aging. Current Psychiatry Reports, 12(1), 39–45. doi: 10.1007/s11920-009-0082-1 [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, & Loewenstein D (2006). Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Archives of General Psychiatry, 63(5), 530–538. doi: 10.1001/archpsyc.63.5.530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya M, Altinay M, Malone DA Jr., & Anand A (2012). Where in the brain is depression? Current Psychiatry Reports, 14(6), 634–642. doi: 10.1007/s11920-012-0322-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts BL, Wen V, Whealin JM, Fogle BM, Southwick SM, Esterlis I, & Pietrzak RH (2020). Depression and cognitive dysfunction in older US Military veterans: Moderating effects of BDNF Val66Met polymorphism and physical exercise. The American Journal of Geriatric Psychiatry, 28(9), 959–967. doi: 10.1016/j.jagp.2020.02.001 [DOI] [PubMed] [Google Scholar]
- Price JL, & Drevets WC (2010). Neurocircuitry of mood disorders. Neuropsychopharmacology, 35(1), 192–216. doi: 10.1038/npp.2009.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley A, MacKay-Lyons M, & Eskes G (2020). Effects of Exercise on Cognitive Performance in Older Adults: A Narrative Review of the Evidence, Possible Biological Mechanisms, and Recommendations for Exercise Prescription. J Aging Res, 2020, 1407896. doi: 10.1155/2020/1407896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidi-Ranjbar N, Miranda D, Butters MA, Mulsant BH, & Voineskos AN (2020). Evidence for structural and functional alterations of frontal-executive and corticolimbic circuits in late-life depression and relationship to mild cognitive impairment and dementia: A systematic review. Frontiers in Neuroscience, 14(253). doi: 10.3389/fnins.2020.00253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppermund S, Ising M, Lucae S, & Zihl J (2009). Cognitive impairment in unipolar depression is persistent and non-specific: further evidence for the final common pathway disorder hypothesis. Psychol Med, 39(4), 603–614. doi: 10.1017/S003329170800411X [DOI] [PubMed] [Google Scholar]
- Reppermund S, Zhuang L, Wen W, Slavin MJ, Trollor JN, Brodaty H, & Sachdev PS (2014). White matter integrity and late-life depression in community-dwelling individuals: diffusion tensor imaging study using tract-based spatial statistics. British Journal of Psychiatry, 205(4), 315–320. doi: 10.1192/bjp.bp.113.142109 [DOI] [PubMed] [Google Scholar]
- Respino M, Jaywant A, Kuceyeski A, Victoria LW, Hoptman MJ, Scult MA, … Gunning FM (2019). The impact of white matter hyperintensities on the structural connectome in late-life depression: Relationship to executive functions. Neuroimage: Clinical, 23, 101852. doi: 10.1016/j.nicl.2019.101852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg D, Depp CA, Vahia IV, Reichstadt J, Palmer BW, Kerr J, … Jeste DV (2010). Exergames for subsyndromal depression in older adults: A pilot study of a novel intervention. The American Journal of Geriatric Psychiatry, 18(3), 221–226. doi: 10.1097/JGP.0b013e3181c534b5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford BR, Taylor WD, Brown PJ, Sneed JR, & Roose SP (2017). Biological aging and the future of geriatric psychiatry. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 72(3), 343–352. doi: 10.1093/gerona/glw241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez de Asteasu ML, Martinez-Velilla N, Zambom-Ferraresi F, Casas-Herrero A, & Izquierdo M (2017). Role of physical exercise on cognitive function in healthy older adults: A systematic review of randomized clinical trials. Ageing Research Reviews, 37, 117–134. doi: 10.1016/j.arr.2017.05.007 [DOI] [PubMed] [Google Scholar]
- Sakr HF, Abbas AM, & El Samanoudy AZ (2015). Effect of vitamin E on cerebral cortical oxidative stress and brain-derived neurotrophic factor gene expression induced by hypoxia and exercise in rats. Journal of Physiology and Pharmacology, 66(2), 191–202. [PubMed] [Google Scholar]
- Sanchez-Carro Y, Portella MJ, Leal-Leturia I, Salvat-Pujol N, Etxandi M, de Arriba-Arnau A, … Lopez-Garcia P (2021). Age at illness onset and physical activity are associated with cognitive impairment in patients with current diagnosis of major depressive disorder. J Affect Disord, 279, 343–352. doi: 10.1016/j.jad.2020.10.032 [DOI] [PubMed] [Google Scholar]
- Schattin A, Baier C, Mai D, Klamroth-Marganska V, Herter-Aeberli I, & de Bruin ED (2019). Effects of exergame training combined with omega-3 fatty acids on the elderly brain: a randomized double-blind placebo-controlled trial. BMC Geriatr, 19(1), 81. doi: 10.1186/s12877-019-1084-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, … Hibar DP (2016). Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Molecular Psychiatry, 21(6), 806–812. doi: 10.1038/mp.2015.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouten RW, Harmse VJ, Dekker FW, van Ballegooijen W, Siegert CEH, & Honig A (2019). Dimensions of depressive symptoms and their association with mortality, hospitalization, and quality of life in dialysis patients: A cohort study. Psychosomatic Medicine, 81(7), 649–658. doi: 10.1097/PSY.000000000000723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, & Stubbs B (2016). Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. Journal of Psychiatric Research, 77, 42–51. doi: 10.1016/j.jpsychires.2016.02.023 [DOI] [PubMed] [Google Scholar]
- Semkovska M, Quinlivan L, O’Grady T, Johnson R, Collins A, O’Connor J, … Gload T (2019). Cognitive function following a major depressive episode: a systematic review and meta-analysis. Lancet Psychiatry, 6(10), 851–861. doi: 10.1016/S2215-0366(19)30291-3 [DOI] [PubMed] [Google Scholar]
- Sexton CE, Betts JF, Demnitz N, Dawes H, Ebmeier KP, & Johansen-Berg H (2016). A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage, 131, 81–90. doi: 10.1016/j.neuroimage.2015.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton CE, Mackay CE, & Ebmeier KP (2013). A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. American Journal of Geriatric Psychiatry, 21(2), 184–195. doi: 10.1016/j.jagp.2012.10.019 [DOI] [PubMed] [Google Scholar]
- Shimada H, Park H, Makizako H, Doi T, Lee S, & Suzuki T (2014). Depressive symptoms and cognitive performance in older adults. Journal of Psychiatric Research, 57, 149–156. doi: 10.1016/j.jpsychires.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Siddarth P, Funes CM, Laird KT, Ercoli L, & Lavretsky H (2020). Predictors of cognitive improvement following treatment for late-life depression. Journal of Geriatric Psychiatry and Neurology, 891988720915515. doi: 10.1177/0891988720915515 [DOI] [PubMed] [Google Scholar]
- Smith PJ, Blumenthal JA, Hoffman BM, Cooper H, Strauman TA, Welsh-Bohmer K, … Sherwood A (2010). Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosomatic Medicine, 72(3), 239–252. doi: 10.1097/PSY.0b013e3181d14633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Rindskopf D, Steffens DC, Krishnan KRR, & Roose SP (2008). The vascular depression subtype: evidence of internal validity. Biological Psychiatry, 64(6), 491–497. doi: 10.1016/j.biopsych.2008.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneed JR, Roose SP, Keilp JG, Krishnan KR, Alexopoulos GS, & Sackeim HA (2007). Response inhibition predicts poor antidepressant treatment response in very old depressed patients. American Journal of Geriatric Psychiatry, 15(7), 553–563. doi: 10.1097/JGP.0b013e3180302513 [DOI] [PubMed] [Google Scholar]
- Sofi F, Valecchi D, Bacci D, Abbate R, Gensini GF, Casini A, & Macchi C (2011). Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. Journal of Internal Medicine, 269(1), 107–117. doi: 10.1111/j.1365-2796.2010.02281.x [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Mok YM, Yang YK, Chan HN, Della CD, Zainal NZ, … Kalita P (2018). Cognitive complaints and predictors of perceived cognitive dysfunction in adults with major depressive disorder: Findings from the Cognitive Dysfunction in Asians with Depression (CogDAD) study. Journal of Affective Disorders, 232, 237–242. doi: 10.1016/j.jad.2018.02.014 [DOI] [PubMed] [Google Scholar]
- Steffens DC, Wang L, Manning KJ, & Pearlson GD (2017). Negative affectivity, aging, and depression: results from the Neurobiology of Late-Life Depression (NBOLD) Study. The American Journal of Geriatric Psychiatry, 25(10), 1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson NJ, Davison G, & Javadi AH (2018). Joggin’ the Noggin: Towards a Physiological Understanding of Exercise-Induced Cognitive Benefits. Neurosci Biobehav Rev, 88, 177–186. doi: 10.1016/j.neubiorev.2018.03.018 [DOI] [PubMed] [Google Scholar]
- Strommer JM, Davis SW, Henson RN, Tyler LK, Cam CAN, & Campbell KL (2020). Physical activity predicts population-level age-related differences in frontal white matter. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(2), 236–243. doi: 10.1093/gerona/gly220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Lanctot K, Herrmann N, & Gallagher D (2018). Exercise for cognitive symptoms in depression: A systematic review of interventional studies. The Canadian Journal of Psychiatry, 63(2), 115–128. doi: 10.1177/0706743717738493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuhany KL, Bugatti M, & Otto MW (2015). A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. Journal of Psychiatric Research, 60, 56–64. doi: 10.1016/j.jpsychires.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadayonnejad R, & Ajilore O (2014). Brain network dysfunction in late-life depression: a literature review. Journal of Geriatric Psychiatry and Neurology, 27(1), 5–12. doi: 10.1177/0891988713516539 [DOI] [PubMed] [Google Scholar]
- Teixeira AL, Barbosa IG, Diniz BS, & Kummer A (2010). Circulating levels of brain-derived neurotrophic factor: correlation with mood, cognition and motor function. Biomarkers in Medicine, 4(6), 871–887. doi: 10.2217/bmm.10.111 [DOI] [PubMed] [Google Scholar]
- Vance DE, Marson DC, Triebel KL, Ball KK, Wadley VG, & Cody SL (2016). Physical Activity and Cognitive Function in Older Adults: The Mediating Effect of Depressive Symptoms. J Neurosci Nurs, 48(4), E2–e12. doi: 10.1097/jnn.0000000000000197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasques PE, Moraes H, Silveira H, Deslandes AC, & Laks J (2011). Acute exercise improves cognition in the depressed elderly: the effect of dual-tasks. Clinics (Sao Paulo), 66(9), 1553–1557. doi: 10.1590/s1807-59322011000900008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan L, Corbin AL, & Goveas JS (2015). Depression and frailty in later life: a systematic review. Clinical Interventions in Aging, 10, 1947–1958. doi: 10.2147/CIA.S69632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkert J, Schulz H, Harter M, Wlodarczyk O, & Andreas S (2013). The prevalence of mental disorders in older people in Western countries - a meta-analysis. Ageing Res Rev, 12(1), 339–353.doi: 10.1016/j.arr.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, Kim JS, … Kramer AF (2010). Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Vivar C, Kramer AF, & van Praag H (2013). Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences, 17(10), 525–544. doi: 10.1016/j.tics.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas CM, Donneyong M, Mischoulon D, Chang G, Gibson H, Cook NR, … Okereke OI (2020). Association of race and ethnicity with late-life depression severity, symptom burden, and care. JAMA Network Open, 3(3), e201606. doi: 10.1001/jamanetworkopen.2020.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne PM, Walsh JN, Taylor-Piliae RE, Wells RE, Papp KV, Donovan NJ, & Yeh GY (2014). Effect of tai chi on cognitive performance in older adults: systematic review and meta-analysis. Journal of the American Geriatrics Society, 62(1), 25–39. doi: 10.1111/jgs.12611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenbach SL, & Kumar A (2014). Current understanding of the neurobiology and longitudinal course of geriatric depression. Current Psychiatry Reports, 16(9), 463. [DOI] [PubMed] [Google Scholar]
- Woo YS, Rosenblat JD, Kakar R, Bahk WM, & McIntyre RS (2016). Cognitive Deficits as a Mediator of Poor Occupational Function in Remitted Major Depressive Disorder Patients. Clinical Psychopharmacology and Neuroscience, 14(1), 1–16. doi: 10.9758/cpn.2016.14.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, & Gomez-Pinilla F (2008). Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience, 155(3), 751–759. doi: 10.1016/j.neuroscience.2008.05.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, Su F, Gong L, Shu H, Liao W, Xie C, … Bai F (2017). Divergent roles of vascular burden and neurodegeneration in the cognitive decline of geriatric depression patients and Mild Cognitive Impairment patients. Frontiers in Aging Neuroscience, 9, 288. doi: 10.3389/fnagi.2017.00288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan M, Fu H, Liu R, & Fang Y (2020). Effect of Frequency of Exercise on Cognitive Function in Older Adults: Serial Mediation of Depression and Quality of Sleep. Int J Environ Res Public Health, 17(3). doi: 10.3390/ijerph17030709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Xiang K, Li S, & Pan HF (2021). Physical activity and depression in older adults: the knowns and unknowns. Psychiatry Res, 297, 113738. doi: 10.1016/j.psychres.2021.113738 [DOI] [PubMed] [Google Scholar]
- Zheng G, Ye B, Zheng Y, Xiong Z, Xia R, Qiu P, … Chen L (2019). The effects of exercise on the structure of cognitive related brain regions: A meta-analysis of functional neuroimaging data. International Journal of Neuroscience, 129(4), 406–415. doi: 10.1080/00207454.2018.1508135 [DOI] [PubMed] [Google Scholar]
- Zlatar ZZ, Moore RC, Palmer BW, Thompson WK, & Jeste DV (2014). Cognitive complaints correlate with depression rather than concurrent objective cognitive impairment in the successful aging evaluation baseline sample. Journal of Geriatric Psychiatry and Neurology, 27(3), 181–187. doi: 10.1177/0891988714524628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar ZZ, Muniz M, Galasko D, & Salmon DP (2018). Subjective cognitive decline correlates with depression symptoms and not with concurrent objective cognition in a clinic-based sample of older adults. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 73(7), 1198–1202. doi: 10.1093/geronb/gbw207 [DOI] [PMC free article] [PubMed] [Google Scholar]


