Abstract
Background
The present study aimed to assess variables associated to ICU-mortality among patients admitted to surgical intensive care unit in Ethiopia.
Methods
A Hospital-based retrospective follow-up study was conducted on all patients who were admitted to the surgical intensive care unit. Data were extracted from patients’ charts with a pretested data extraction tool, entered into Epi-data 4.6.0, and analyzed with STATA- 14. Bivariate and multivariate Cox proportional hazards regression models were fitted.
Results
Of the total study participants (388), 148 (38.1%) patients admitted to the surgical intensive care unit died during the follow-up period with a median survival time of 11 days. Potassium level < 3.5 mmol/L (adjusted hazard ratio ( AHR): 3.46, 95% CI (1.83 6.55), potassium level > 5.0 mmol/L (AHR:2.41, 95% CI (1.29–4.51), hypoxia (AHR:1.66, 95% CI (1.10–2.48), Glasgow Coma Scale (GCS) score < 9 (AHR: 4.06, 95% CI (1.51–10.89), mechanical ventilation (AHR:12, 95%CI (3–45), absence of thromboprophylaxis (AHR:10.8,95% CI (6.04–19.29), absence of enteral feeding (AHR:3.56, 95% CI (2.20–5.78) were variables associated with ICU-mortality among patients admitted to surgical intensive care unit.
Conclusions
The overall ICU-mortality of patients admitted to our surgical intensive care unit was higher compared to patients admitted to similar intensive care unit in developed countries. The variables associated to ICU-mortality among patients admitted to surgical intensive care unit were abnormal serum potassium level, lower GCS score, mechanical support, hypoxia, absence of thromboprophylaxis, and enteral feeding.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-023-02230-w.
Keywords: Incidence, Intensive care unit, Mortality, Predictors, Surgical
Background
The intensive care unit (ICU) is where patients that need critical care are managed. The surgical intensive care unit (SICU) is one of the acute care facilities with a focus on surgical conditions [1]. Each year, 310 million major surgical procedures are performed worldwide [2]. Nearly seven million patients experienced serious morbidity during the perioperative phase, yet only 0.5% of surgeries result in death [3, 4]. According to the intensive care over nations (ICON) audit, the likelihood of death is statistically significantly correlated with per-country income [5].
The risk of death in critical care units is determined by a number of measures, including the Simplified Acute Physiology Score (SAPS) and the Acute Physiology and Chronic Health Evaluation (APACHE) score [6].
In affluent nations, the ICU-mortality in surgical intensive care units ranges from 9.3 to 26.2% [7, 8] while in undeveloped nations, varies from 27 to 53.6% [9–12]. Between 35.4 and 46.3% of patients in surgical intensive care units in Ethiopia die, while 36.5 to 47% of patients experience complications, with organ failure being the most frequent [13–15].
Trauma from a road traffic accident (RTA) was the most common cause of admission to a surgical intensive care unit (SICU) in Ethiopia, and surgical cases in general accounted for 22.1% of intensive care unit admissions, with males having a higher admission rate than females [16]. Developing quality indicators, monitoring resources, conducting audits, and making adjustments should all be requirements for providing high-quality care [17].
Patients usually get vasopressor support and mechanical ventilation in the surgical intensive care unit (SICU) [5, 18, 19].
Infection, trauma, and a lack of essential medications and supplies contribute to a greater ICU-mortality among patients referred to surgical intensive care units [14, 19]. Resources (personnel, equipment, drugs), patients’ prior medical histories, and other factors can also have a direct impact on ICU-mortality in critical care units in hospitals around the world [20].
The nation’s first critical care unit was established in Addis Abeba’s Tikur Anbessa Hospital more than 30 years ago, and since then, both public and private hospitals have begun to offer more intense care services. The country’s earlier studies focused on the admission trends, indications, and risk factors for ICU-mortality in the medical critical care unit [21, 22].
This study aimed to investigate variables associated to ICU-mortality among patients admitted to surgical intensive care unit in Ethiopia.
Methods
Study design, period, and setting
A single-centered retrospective follow-up study was conducted, from September 19/2019- April 30/2022 G.C, at the University of Gondar Comprehensive Specialized Hospital. University of Gondar Comprehensive Specialized Hospital is providing services for more than 7 million people. On September 19, 2019 (G.C.), the surgical intensive care service was launched with four beds, two mechanical ventilators, one defibrillator, and four non-invasive hemodynamic monitoring gadgets. Ten functional beds, four mechanical ventilators with functional monitoring, a staff of three anesthesiologists, one resident in general surgery who rotates monthly, one general practitioner, and 12 critical care nurses make up the surgical intensive care unit (SICU) at the moment. The area provides critical care services for surgical, trauma and orthopedics, and obstetric cases.
Study population and data source
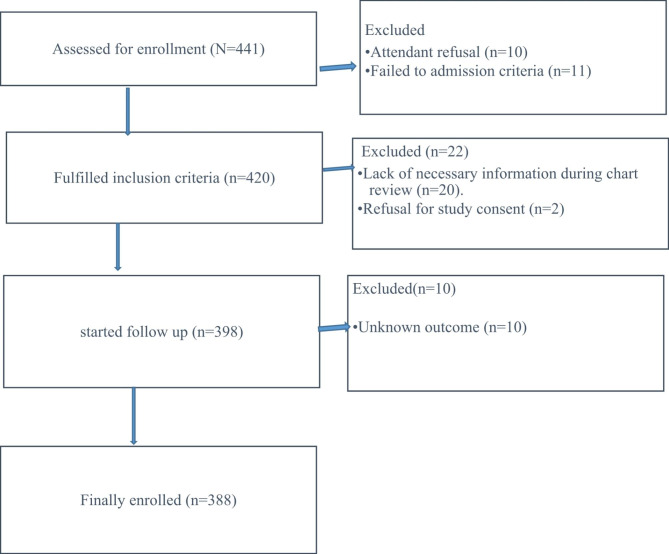
The study included all patients who met the requirements for admission to the SICU, including those who needed or were likely to need advanced respiratory support, needed support for two or more organ systems, or had chronic impairment of one or more organ systems who also needed support for an acute reversible failure of another organ. Patients whose outcomes were unknown were excluded from the study and reported with, ‘‘The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies’’ [23](Fig. 1). A pretested structured questionnaire that included the chart numbers of patients, date of admission, socio-demographic characteristics, source of admission, diagnosis at admission, admission category, vital signs at admission, presence of comorbidity, length of stay, intervention in surgical intensive care unit, and outcome was used to collect the data. The University of Gondar’s School of Medicine’s institutional review board (IRB) gave its approval to this study with the reference/number/ SoM/12/02/2022. The study was carried out in accordance with the Declaration of Helsinki. Documentation of informed consent was waived by our institutional review board, University of Gondar, School of Medicine.
Fig. 1.
STROBE diagram shows the study participants who were included and excluded in the study (STROBE: Strengthening the reporting of observational studies in epidemiology)
Dependent and independent variables
The outcome variable/dependent variable was ICU-mortality in the surgical intensive care unit (number of patients in the surgical intensive care unit of the setting (University of Gondar) who passed away), while the independent variables were age, sex, residency, type of surgery (elective surgery, emergency surgery, and traumatic injury), operated, not-operated, cancer, cardiac illness, hypertension, diabetes mellitus (DM), asthma, chronic obstructive pulmonary disease (COPD), oxygen saturation (SpO2), temperature, glasgow coma scale (GCS) score, creatinine, serum glutamic pyruvic transaminase (SGPT) level, serum glutamic-oxaloacetic transaminase (SGOT), random blood sugar (RBS), hemoglobin (Hgb), mechanical ventilation, vasopressors, blood transfusion, administration of thromboprophylaxis, feeding, and fluid administration, cardiac arrest, anemia, arrhythmia, infection, and aspiration.
Statistical analyses
Epi-Data version 4.6.0 was used to enter the data, and STATA version 14 was used for the analysis. Categorical variables were expressed as proportions and numerical descriptive statistics were expressed as mean with standard deviation (SD) or median with interquartile range (IQR).
The log-rank test and Kaplan-Meier survival curve were fitted to the ICU-mortality of patients in surgical intensive care unit to evaluate whether there was a difference in the ICU-mortality among the participants. To find the variables associated with the ICU-mortality, bivariate and multivariate survival analyses were performed. Variables with a p-value of 0.25 in the bivariable analyses were candidates for the multivariable analyses. An adjusted hazard ratio (AHR) with a 95% confidence interval was used to assess the strength of the association between the outcome and independent variables. In multivariable analyses, an independent variable was considered statistically significant if its p-value was < 0.05.
Results
Demographic characteristics and admission patterns of the study participants
Among the 441 participants, 388 (87.98%) patients admitted in surgical intensive care unit were included in the final analysis. Two hundred eighty of the study participants were males, and the majority of participants, 257 (66.2%), were from rural areas. Nearly half (49.7%) of the participants were aged between 15 and 30 years and had a 49.3% of ICU-mortality (Table 1).
Table 1.
Socio-demographic characteristics and ICU-mortality of patients in SICU, (N = 388)
| Variables | Categories | Total (N = 388) | Outcome | |
|---|---|---|---|---|
| Dead (n = 148) | Alive (n = 240) | |||
| Sex | Female | 108 (27.8%) | 32 (21.6%) | 76 (31.7%) |
| Male | 280 (72.2%) | 116 (78.4%) | 164 (68.3%) | |
|
Age (years) |
15–30 | 193 (49.7%) | 73 (49.3%) | 120 (50%) |
| 31–45 | 84 (21.6%) | 38 (25.7%) | 46 (19.2%) | |
| 46–64 | 82 (21.1%) | 25 (16.9%) | 57 (23.7%) | |
| ≥ 65 | 29 (7.5%) | 12 (8.1%) | 17 (7.1%) | |
| Residence | Urban | 131 (33.8%) | 48 (32.4%) | 83 (34.6%) |
| Rural | 257 (66.2%) | 100 (67.6%) | 157 (65.4%) | |
Key: - SICU = Surgical Intensive Care Unit
Of the total patients enrolled in the study, patients who came from emergency departments to surgical intensive care unit experienced the highest ICU-mortality, 54% (Table 2).
Table 2.
Admission characteristics and ICU-mortality of patients in SICU, (N = 388)
| Variables | Categories | Total (N = 388) | Outcome | |
|---|---|---|---|---|
| Dead (n = 148) | Alive (n = 240) | |||
| Source of admission | Emergency department | 203 (52.3%) | 80 (54%) | 123 (51%) |
| Inpatient | 22 (5.7%) | 4 (2.7%) | 18 (7.5%) | |
| Operation room | 121 (31.2%) | 50 (33.8%) | 71 (29.7%) | |
| PACU | 42 (10.8%) | 14 (9.5%) | 28 (11.8%) | |
| Urgency cases | Emergency cases | 336 (86.6%) | 140 (94.6%) | 196 (81.7%) |
| Elective cases | 52 (13.4%) | 8 (5.4%) | 44 (18.3%) | |
| Admission unit | Surgery | 151 (38.9%) | 56 (37.8%) | 95 (39.6%) |
| Gynecology/Obstetrics | 13 (3.4%) | 5 (3.4%) | 8 (3.3%) | |
| Orthopedics and trauma | 224 (57.7%) | 87 (58.8%) | 137 (57.1%) | |
Key: PACU = Post- Anesthesia Care Unit; SICU = Surgical Intensive Care Unit
The commonest indication for admission to SICU was a head injury, 128 (33%). Septic shock was the leading cause of death for patients in surgical intensive care unit, 63 (42.6%). Among patients admitted to SICU, 38 (9.58%) had different types of comorbidities. The commonest complication was aspiration pneumonia (Table 3).
Table 3.
Indications for ICU admission, co-morbidities, complications, and ICU-mortality of patients in SICU, (N = 388)
| Variables | Categories | Total (N = 388) |
Outcome | |
|---|---|---|---|---|
| Dead (n = 148 ) | Alive (n = 240) | |||
| Indication | Peritonitis | 24 (6.2%) | 5 (3.4%) | 19 (7.9%) |
| Septic shock | 114 (29.4%) | 63 (42.6%) | 51 (21.2%) | |
| Head injury | 128 (33%) | 37 (25%) | 91 (37.9%) | |
| Hemorrhagic shock | 22 (5.7%) | 7 (4.7%) | 15 (6.3%) | |
| Thoracoabdominal injury | 49 (12.6%) | 14 (9.5%) | 35 (14.6%) | |
| ARDS | 51 (13.1%) | 22 (14.9%) | 29 (12.1%) | |
| Comorbidity | Yes | 38 (9.8%) | 12 (8.1%) | 26 (10.8%) |
| No | 350 (90.2%) | 136 (91.9%) | 214 (89.2%) | |
| Type of comorbidity (N = 38) | Hypertension | 8 (21.1%) | 4 (2.7%) | 4 (1.7%) |
| HIV | 5 (13.2%) | 3 (2%) | 2 (0.8%) | |
| Cancer | 21 (55.3%) | 3 (2%) | 18 (7.5%) | |
| Others (Diabetic, asthmatic.) | 4 (10.5%) | 2 (1.4%) | 2 (0.8%) | |
| Readmission | Yes | 3 (0.8%) | 0 (0%) | 3 (1.2%) |
| No | 385 (99.2%) | 148 (100%) | 237 (98.8%) | |
| Complications | Yes | 176 (45.4%) | 93 (62.8%) | 83 (34.6%) |
| No | 212 (54.6%) | 55 (37.2%) | 157 (65.4%) | |
|
Complications during ICU stay (N = 176) |
Arrhythmia | 34 (19.3%) | 23 (15.5%) | 11 (4.6%) |
| Aspiration pneumonia | 87 (49.4%) | 33 (22.3%) | 54 (22.5%) | |
| Cardiac arrest | 12 (6.8%) | 10 (6.8%) | 2 (0.8%) | |
| Sepsis | 24 (13.6%) | 14 (9.5%) | 10 (4.2%) | |
| AKI | 19 (10.8%) | 16 (10.9%) | 3 (1.3%) | |
Key: ARDS = Acute Respiratory Distress Syndrome; HIV = Human Immune Virus; AKI = Acute Kidney Injury; ICU = Intensive Care Unit; SICU = Surgical Intensive Care Unit
More than half of the patients admitted to the SICU had a GCS score of < 9 and one-fourth of the patients had hypotension episodes at admission (Table 4).
Table 4.
Vital signs characteristics at admission and ICU-mortality of patients in SICU, (N = 388)
| Variables | Categories | Total (N = 388) |
Outcome | ||
|---|---|---|---|---|---|
| Dead (n = 148) | Alive (n = 240) | ||||
| Systolic blood pressure (mmHg) | < 90 | 100 (25.8%) | 85 (57.4%) | 15 (6.3%) | |
| 90–140 | 267 (68.8%) | 50 (33.8%) | 217 (90.4%) | ||
| > 140 | 21 (5.4%) | 13 (8.8%) | 8 (3.3%) | ||
| Heart rate (bpm) | < 60 | 17 (4.4%) | 9 (6.1%) | 8 (3.2%) | |
| 60–100 | 100 (25.8%) | 19 (12.8%) | 81 (33.8%) | ||
| > 100 | 271 (69.8%) | 120 (81.1%) | 151 (63%) | ||
| Respiratory rate (breath per minute) | < 12 | 6 (1.6%) | 2 (1.4%) | 4 (1.7%) | |
| 12–20 | 30 (7.8%) | 5 (3.4%) | 25 (10.4%) | ||
| > 20 | 352 (90.7%) | 141 (95.2%) | 211 (87.9%) | ||
| Temperature (oc) | < 36.5 | 92 (23.7%) | 48 (32.4%) | 44 (18.3%) | |
| 36.5–37.5 | 124 (32.0%) | 21 (14.2%) | 103 (42.9%) | ||
| > 37.5 | 172 (44.3%) | 79 (53.4%) | 93 (38.8%) | ||
| Saturation (SPO2) (%) | < 90 | 88 (22.7%) | 66 (44.6%) | 22 (9.2%) | |
| > 90 | 300 (77.3%) | 82 (55.4%) | 218 (90.8%) | ||
| Glasgow Coma Scale | Sever (3–8) | 195 (50.3%) | 135 (91.2%) | 60 (25%) | |
| Moderate (9–13) | 53 (13.6%) | 7 (4.7%) | 46 (19.2%) | ||
| Mild (14–15) | 140 (36.1%) | 6 (4.1%) | 134 (55.8%) | ||
Key:- SICU = Surgical Intensive Care Unit; mmHg = millimeters of mercury; bpm = beats per minute; oc= degrees centigrade
More than one–fourth of the participants had higher levels of potassium ( hyperkalemia) and sodium (hypernatremia) (27.1%), (28.8%) with an ICU-mortality of 83 (56.1%) and 43 (32.4%), respectively (Table 5).
Table 5.
Investigations charactersitics and ICU-mortality of patients in SICU, (N = 388)
| Variables | Categories | Total (N = 388) | Outcome | |
|---|---|---|---|---|
| Dead (n = 148) | Alive (n = 240) | |||
| Hemoglobin (g/dl) | Anemia | 321 (82.7%) | 133 (89.9%) | 188 (78.3%) |
| Normal | 67 (17.3%) | 15 (10.1%) | 52 (21.7%) | |
| Random blood sugar (mg/dl) | < 70 | 41 (8.9%) | 34 (23%) | 7 (2.9%) |
| 70–160 | 139 (35.8%) | 23 (15.5%) | 116 (48.3%) | |
| > 160 | 208 (53.6%) | 91 (61.5%) | 117 (48.8%) | |
| Creatinine (mg/dl) | < 0.7 | 39 (10%) | 18 (12.2%) | 21 (8.8%) |
| 0.7–1.4 | 224 (58.8%) | 34 (23%) | 190 (79.2%) | |
| ≥ 1.5 | 125 (32.2%) | 96 (64.8%) | 29 (12%) | |
| SGPT (U/L) | ≤ 32 | 145 (37.4%) | 27 (18.2%) | 118 (49.2%) |
| > 32 | 243 (62.6%) | 121 (81.8%) | 122 (50.8%) | |
| SGOT (U/L) | ≤ 32 | 96 (24.7%) | 21 (14.2%) | 75 (31.2%) |
| > 32 | 292 (75.3%) | 127 (85.8%) | 165 (68.8%) | |
| Sodium (mEq/L) | < 135 | 98 (25.3%) | 62 (41.9%) | 36 (15%) |
| 135–145 | 178 (45.9%) | 38 (25.7%) | 140 (58.3%) | |
| > 145 | 112 (28.8%) | 48 (32.4%) | 64 (26.7%) | |
| Potassium (mEq/L) | < 3.5 | 49 (12.6%) | 29 (19.6%) | 20 (8.3%) |
| 3.5-5.0 | 234 (60.3%) | 36 (24.3%) | 198 (82.5%) | |
| > 5.0 | 105 (27.1) % | 83 (56.1%) | 22 (9.2%) | |
Key: - SICU = Surgical Intensive Care Unit; SGPT = Serum Glutamic-Pyruvic Transaminase; SGOT = Serum Glutamic-Oxaloacetic Transaminase; g/dl = grams per deciliter; mg/dl = milligrams per deciliter; mEq/L = milliEquivalents per Liter; U/L = Units per Liter
Among patients admitted to the unit, two hundred fifty-nine patients were supported by mechanical ventilation. Below 50% of the patients were on vasopressors and seven patients were treated with dialysis (Table 6).
Table 6.
Interventions characteristics and ICU-mortality of patients in SICU, (N = 388)
| Variables | Category | Total (N = 388) | Outcome | |
|---|---|---|---|---|
| Dead (n = 148) | Alive (n = 240) | |||
| Mechanical ventilation | Yes | 259 (66.8%) | 145 (98%) | 114 (47.5%) |
| No | 129 (33.2%) | 3 (2%) | 126 (52.5%) | |
| Vasopressor | Yes | 140 36.1%) | 95 (64.2%) | 45 (18.8%) |
| No | 248 (63.9%) | 53 (35.8%) | 195 (81.2%) | |
| Transfusions | Yes | 158 (40.7%) | 85 (57.4%) | 73 (30.4%) |
| No | 230 (59.3%) | 63 (42.6%) | 167 (69.6%) | |
| Dialysis | Yes | 7 (1.8%) | 4 (2.7%) | 3 (1.2%) |
| No | 381 (98.2%) | 144 (97.3%) | 237 (98.8%) | |
| Thromboprophylaxis | Yes | 163 (42%) | 56 (37.8%) | 107 (44.6%) |
| No | 225 (68%) | 92 (62.2%) | 133 (55.4%) | |
| Feeding | Yes | 140 (36.1%) | 36 (24.3%) | 104 (43.3%) |
| No | 248 (63.9) % | 112 (75.7%) | 136 (56.7%) | |
| Fluid | Yes | 380 (97.9%) | 147 (99.3%) | 233 (97.1%) |
| No | 8 (2.1%) | 1 (0.7%) | 7 (2.9%) | |
| Reoperation | Yes | 13 (3.6%) | 5 (3.4%) | 8 (3.3%) |
| No | 375 (96.4%) | 143 (96.6%) | 232 (96.7%) | |
Key: SICU = Surgical Intensive Care Unit
Variables associated with ICU-mortality of patients admitted to surgical intensive care unit
The overall median follow-up time of patients at the intensive care unit was 264 (95% CI: 192,408) hours with a minimum and maximum follow-up time of 1 and 1152 h, respectively. In this study, 148 (38.1%) of the study participants died during the follow-up period in the surgical intensive care unit (SICU).
All variables entered into the bivariable Cox proportional hazard regression model. Trauma, the presence of complications, mechanical ventilation use, vasopressor support, blood transfusion, enteral feeding, reoperation, thromboprophylaxis, heart rate, oxygen saturation (SPO2), systolic blood pressure, temperature, SGPT, SGOT, creatinine, potassium level, sodium level, GCS, hemoglobin level, random blood sugar were fitted to multivariable Cox proportional hazard regression. Low GCS score, abnormal potassium level, hypoxia, mechanical ventilation, absence of enteral feeding, and absence of thromboprophylaxis were variables associated with ICU-mortality among patients admitted to the SICU (Table 7).
Table 7.
Bivariable and multivariable Cox regression analysis of variables associated with ICU-mortality among patients in the SICU (N = 388)
| Variables | Categories | Outcome | CHR (95%CI) | AHR (95%CI) | p-value | |
|---|---|---|---|---|---|---|
| Dead (n = 148 ) |
Alive (n = 240) |
|||||
| Glasgow coma scale | Sever | 135 | 60 | 14.43 (6.36–32.73)* | 4.06(1.51–10.89) | 0.005 |
| Moderate | 7 | 46 | 2.50 (0.84-7.47)* | 0.97(0.30–3.15) | ||
| Mild | 6 | 134 | 1 | 1 | ||
| Mechanical ventilation | Yes | 145 | 114 | 4 (1–15)* | 12 (3–45) | 0.002 |
| No | 3 | 126 | 1 | 1 | ||
| Potassium | < 3.5 | 29 | 20 | 3.98 (2.43–6.51)* | 3.46(1.83–6.55) | < 0.001 |
| 3.5-5.0 | 36 | 198 | 1 | 1 | ||
| > 5.0 | 83 | 22 | 6.50 (4.39–9.63)* | 2.41(1.29–4.51) | 0.005 | |
| Saturation (Spo2) | < 90 | 66 | 22 | 4.26 (3.06–5.94)* | 1.66(1.10–2.48) | 0.014 |
| >=90 | 82 | 218 | 1 | 1 | ||
| Feeding | Yes | 36 | 104 | 1 | 1 | |
| No | 112 | 136 | 4.29(2.88–6.39)* | 3.56(2.20–5.78) | < 0.001 | |
| Thromboprophylaxis | Yes | 56 | 107 | 1 | 1 | |
| No | 92 | 133 | 7.07(4.62–10.81)* | 10.8(6.0419.29) | < 0.001 | |
Key: - * Significant (P-value < 0.05); CHR = Crude Hazard Ratio; AHR = Adjusted Hazard Ratio; 1 = reference categories, SICU = Surgical Intensive Care Unit
Discussion
This study was conducted to identify variables associated with ICU-mortality among patients admitted to the surgical intensive care unit at a Comprehensive Specialized Hospital in Ethiopia.
In this study, emergency cases and males had a higher admission rate to the surgical intensive care unit. This study is inline in studies conducted in Ireland, Jordan, and India [24–26].
This could be explained by the higher incidence of trauma (violence) in males and the insufficient time for optimisation in emergency patients.
Trauma was the leading cause of admission to surgical intensive care unit (SICU) which was in line with studies performed in Western Kenya, Aqaba-Jordan, Tribhuvan University, Nepal, and Ethiopia [24, 27–29], respectively. However, other studies in Ethiopia, Nigeria, Malawi, Ireland, and United States [14, 21, 26, 30, 31] showed that the leading cause of admission to SICU was complications after acute abdomen and thoracoabdominal injuries. Early warning scores have been established as clinical prognostication tools to identify patients who are fast deteriorating [32].
The ICU-mortality of patients admitted to the surgical intensive care unit was 38.1% (95% CI: 33.3–42.1). This finding is consistent with the findings of other studies conducted in Nigeria (34.6%) [10], Uganda (37.6%) [33], Tanzania (41.4%) [34], the Republic of Congo (37.4%) [35], St. Paul’s Hospital Millennium Medical College (40.2%)[14], and Jimma (39.8%) [19]. This similarity can be explained by the fact that the places have comparable resources and population demographics.
However, this study showed a higher frequency of ICU-mortality of patients in SICU than studies conducted in Mekele (Ethiopia) (27%) [36], three Scandinavian countries (9.1%) [37], Spain (5.63%) [38], United States (11.2%) [39], Turkey (15.8%) [40], Brazil (22.9%) [41], and Greece (27.3%) [42]. The reason might be due to a lack of necessary equipment (like arterial blood gas (ABG)-analyzer machine, portable dialysis machine, and portable x-ray service), pre-hospital care, or a general lack of resources in the area of the current study.
This result, however, is lower than studies carried out in Turkey (50%) [43], Egypt (58%) [44], Jimma (52.8%) [45], and Brazil (89.1%) [46]. This study also lower compared to another study conducted in Western Kenya showed that 30-day mortality was 57.3% [11]. This can be explained that their report was 30-day mortality.
In our study, the median length of ICU stay was 264 h (11 days) and in line with studies conducted at St. Paul’s Hospital Millennium Medical College [14], Belgium, Nigeria, Tanzania, Malawi, and Kenya [5, 21, 30, 47, 48], respectively. However, it is much longer than studies performed in Kenya and Tanzania [29, 47], respectively. The reason could be due to the scarcity of necessary resources in a setting like a high-dependency unit.
The hazard of death among patients with a GCS score < 9 was higher as compared to those who had a higher GCS score. This finding is in agreement with other studies conducted in Boston, Greece, Turkey [40, 43], Spain [49], Egypt, and Ethiopia.
Patients who were on mechanical ventilation had a higher risk of ICU-mortality. This finding is in line with studies done at Saint Paul Hospital Millennium Medical College (Ethiopia), Kenya, and Brazil [29, 50, 51], respectively. The possible reason might be related to an increased severity of these patients. To be added, mecanichal ventilation leads to increased risk of ventilator-associated pneumonia and other nosocomial infection [52, 53].
The risk of death among patients with hypoxia had higher as compared with patients without hypoxia, in line with previous studies [54–56]. As a result, patients may benefit from prompt management of hypoxia by the use of oxygen with higher FiO2 up to 100% oxygen or assist with mechanical ventilation accordingly [57].
This study also found that hypokalemia was another variable associated with ICU-mortality among patients admitted to SICU. This study is in line with the studies conducted in Japan, China [58], and Boston[59]. Hyperkalemia was also another variable associated with ICU-mortality of patients in SICU, in line with other similar studies conducted in the Netherlands, Boston, US [59–61], respectively. The evidence suggested that potassium is an important determinant of myocardial function and hypokalemia can lead to arrhythmia and sudden cardiac death [62, 63].
The hazards of death among patients who didn’t get thromboprophylaxis had 10.53 times higher as compared with patients getting thromboprophylaxis as already highlighted by previous studies [64, 65]. Due to their immobility, use of mechanical ventilation, and central catheters, patients in the intensive care unit (ICU) had a higher risk of thrombotic events. However, the requirement for thromboprophylaxis decreased the dangers of death in the ICU [66].
Interestingly, enteral nutrition seems to represent a preventive factor of ICU-mortality among patients admitted to the SICU, as the risk of death among patients receiving enteral nutrition was decreased by 77% compared with those who didn’t receive enteral nutrition. In fact, it is known that enteral nutrition provides both macro and micronutrients, which help to sustain gut integrity through the stimulation of blood flow in intraepithelial cells, and promote immune functions which decreases the risk of infection [67, 68].
Limitations of the study
Using secondary data may limit the researcher to measure all possible predictors like body mass index (BMI), pain management, and sedation were not assessed because of a lack of organized documentation. Physiologic scores such as acute physiological and chronic health evaluation (APACHE) and sequential organ failure assessment (SOFA) were not obtained. Additionally, the small sample size, collected in a single-center, as well as the lack of generalisability, and changes in practice over time must be highlighted as other relevant limations of the study.
Finally, we used as primary outcome ICU-mortality, as we were unable to provide 28-days mortality.
Conclusions
The overall ICU-mortality of patients admitted to surgical intensive care unit at academic hospital in Ethiopia was higher compared to patients admitted in similar intensive care unit type in developed countries. Septic shock was the leading cause of death. Hypokalemia, hyperkalemia, lower GCS score(< 9), mechanical ventilation, hypoxia, absence of thromboprophylaxis, and absence of enteral feeding were variables associated with ICU-mortality among patients admitted to surgical intensive care unit.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate the University of Gondar’s assistance in making patient charts available to us during the data collection period.
Authors’ contributions
This work was carried out in collaboration among all authors. M. M. Zewudie and D.Y.Melesse contributed to the conception the review and interpreted the literatures based on the level of evidence and revised the manuscript. T. D. Filatie, M. E. Zeleke, participate in reviewing preparation of the manuscript. Both authors participate in preparation and critical review of the manuscripts. In addition, all authors read and approved the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data Availability
The data sets used and analyzed during the study are available from the corresponding author/primary investigator upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by an institutional review board (IRB) of the University of Gondar, School of Medicine with ref/number/ SoM/12/02/2022. This study was performed by the Declaration of Helsinki. Documentation of informed consent was waived by our institutional review board, University of Gondar, School of Medicine.
Consent for publication
Not applicable.
Conflict of interest
The authors declares no conflict of interest in preparing this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. The Lancet. 2010;376(9749):1339–46. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobson GP. Trauma of major surgery: a global problem that is not going away. Int J Surg (London England) 2020;81:47–54. doi: 10.1016/j.ijsu.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, Gawande AA. An estimation of the global volume of surgery: a modelling strategy based on available data. The Lancet. 2008;372(9633):139–44. doi: 10.1016/S0140-6736(08)60878-8. [DOI] [PubMed] [Google Scholar]
- 4.Bickler SW, Spiegel DA, Weiser TG, Regenbogen SE, Thompson KD. An estimation of the global volume of surgery: a modelling strategy based on available data. Commentary. Lancet (British edition) 2008, 372(9633). [DOI] [PubMed]
- 5.Vincent J-L, Marshall JC, Ñamendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. The lancet Respiratory medicine. 2014;2(5):380–6. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 6.Baker S, Xiang W, Atkinson I. Continuous and automatic mortality risk prediction using vital signs in the intensive care unit: a hybrid neural network approach. Sci Rep. 2020;10(1):21282. doi: 10.1038/s41598-020-78184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rhodes A, Moreno RP. Intensive care provision: a global problem. Revista Brasileira de terapia intensiva. 2012;24(4):322–5. doi: 10.1590/S0103-507X2012000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent JL, Marshall JC, Namendys-Silva SA, François B, Martin-Loeches I, Lipman J, Reinhart K, Antonelli M, Pickkers P, Njimi H, et al. Assessment of the worldwide burden of critical illness: the intensive care over nations (ICON) audit. The Lancet Respiratory medicine. 2014;2(5):380–6. doi: 10.1016/S2213-2600(14)70061-X. [DOI] [PubMed] [Google Scholar]
- 9.Abate SM, Assen S, Yinges M, Basu B. Survival and predictors of mortality among patients admitted to the intensive care units in southern Ethiopia: a multi-center cohort study. Annals of Medicine and Surgery. 2021;65:102318. doi: 10.1016/j.amsu.2021.102318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilori IU, Kalu QN. Intensive care admissions and outcome at the University of Calabar Teaching Hospital, Nigeria. J Crit Care. 2012;27(1):105e101–104. doi: 10.1016/j.jcrc.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Lalani HS, Waweru-Siika W, Mwogi T, Kituyi P, Egger JR, Park LP, Kussin PS. Intensive care outcomes and mortality prediction at a National Referral Hospital in Western Kenya. 2018, 15(11):1336–43. [DOI] [PubMed]
- 12.Tesema HG, Lema GF. Patterns of admission and clinical outcomes among patients admitted to Medical Intensive Care Unit of a teaching and Referral Hospital. Northwest Ethiopia. 2021;10:2164956121989258. doi: 10.1177/2164956121989258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosissa D, Alemu S, Rad MH, Yesuf EA. Outcomes of Surgical Patients admitted to the Intensive Care Unit of Jimma University Medical Center. Health Sci J. 2021;15(5):1–4. [Google Scholar]
- 14.Abebe K, Negasa T, Argaw F. Surgical admissions and treatment outcomes at a tertiary hospital intensive care unit in Ethiopia: a two-year review. Ethiop J health Sci 2020, 30(5). [DOI] [PMC free article] [PubMed]
- 15.Seyoum N, Biluts H, Zemenfes D, Chane W, Seme A. Review of morbidity and mortality among patients adimitted to the Surgical Intensive Care Unit at Tikur Anbessa Specialized Teaching Hospital, Ethiopia. Ethiop Med J. 2014;52(2):77–85. [PubMed] [Google Scholar]
- 16.Abebe K, Negasa T, Argaw F. Surgical admissions and treatment outcomes at a Tertiary Hospital Intensive Care Unit in Ethiopia: a two-year review. Ethiop J health Sci. 2020;30(5):725–32. doi: 10.4314/ejhs.v30i5.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray B, Samaddar D, Todi S, Ramakrishnan N, John G, Ramasubban S. Quality indicators for ICU: ISCCM guidelines for ICUs in India. Indian J Crit care medicine: peer-reviewed official publication Indian Soc Crit Care Med. 2009;13(4):173. [PMC free article] [PubMed] [Google Scholar]
- 18.Onyekwulu F, Anya S. Pattern of admission and outcome of patients admitted into the Intensive Care Unit of University of Nigeria Teaching Hospital Enugu: a 5–year review. Niger J Clin Pract. 2015;18(6):775–9. doi: 10.4103/1119-3077.163291. [DOI] [PubMed] [Google Scholar]
- 19.Mosissa D, Alemu S, Rad MH, Yesuf EA. Outcomes of Surgical Patients admitted to the Intensive Care Unit of Jimma University Medical Center. Health Sci J 2021:1–4.
- 20.Kwizera A, Dünser M, Nakibuuka J. National intensive care unit bed capacity and ICU patient characteristics in a low income country. BMC Res Notes. 2012;5(1):475. doi: 10.1186/1756-0500-5-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilori IU, Kalu QN. Intensive care admissions and outcome at the University of Calabar Teaching Hospital, Nigeria. J Crit Care. 2012;27(1):105. doi: 10.1016/j.jcrc.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Smith Z, Ayele Y, McDonald P. Outcomes in critical care delivery at Jimma University Specialised Hospital, Ethiopia. Anaesth Intensive Care. 2013;41(3):363–8. doi: 10.1177/0310057X1304100314. [DOI] [PubMed] [Google Scholar]
- 23.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 24.El-Nabulsi BA, Holy M, Al-Suleihat A, Smadi S. Appropriateness of admissions to intensive care unit. JRMS. 2005;12(2):6–9. [Google Scholar]
- 25.Abubakar AS, Ojo E, El-Nafaty A, Edomwonyi N. An audit of one-year intensive care practice in a developing country. internet J Anesthesiology. 2008;18(2):1–5. [Google Scholar]
- 26.Fowler AL, Cullivan O, Sibartie S, O’Shea A, Waldron R, Khan I, Khan W, Barry KM. Utilisation of critical care services for surgical patients in a model three hospital. Ir J Med Sci (1971-) 2019;188(4):1137–42. doi: 10.1007/s11845-019-01981-1. [DOI] [PubMed] [Google Scholar]
- 27.Acharya SP, Bhattarai A, Bhattarai B. An audit of an intensive care unit of a tertiary care hospital. JNMA: J Nepal Med Association. 2018;56(212):759. doi: 10.31729/jnma.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Betemariam K, Hagos G. Pattern of admission to surgical intensive care unit of Tikur Anbessa Hospital for mechanical ventilatory support. Ethiop J Health Dev. 2001;15(3):193–5. [Google Scholar]
- 29.Lalani HS, Waweru-Siika W, Mwogi T, Kituyi P, Egger JR, Park LP, Kussin PS. Intensive care outcomes and mortality prediction at a national referral hospital in western Kenya. Annals of the American Thoracic Society. 2018;15(11):1336–43. doi: 10.1513/AnnalsATS.201801-051OC. [DOI] [PubMed] [Google Scholar]
- 30.Size M, Borgstein ES, Haisma H. One-year audit of admissions to the intensive care unit of the queen Elizabeth Central Hospital, Blantyre. Malawi Med J. 2005;17(1):12–4. doi: 10.4314/mmj.v17i1.10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lissauer ME, Galvagno SM, Jr, Rock P, Narayan M, Shah P, Spencer H, Hong C, Diaz JJ. Increased ICU resource needs for an academic emergency general surgery service. Crit Care Med. 2014;42(4):910–7. doi: 10.1097/CCM.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 32.Durantez-Fernández C, Martín-Conty JL, Medina-Lozano E, Mohedano-Moriano A, Polonio-López B, Maestre-Miquel C, Viñuela A, López-Izquierdo R, Sánchez Bermejo R, Martín-Rodríguez F. Early detection of intensive care needs and mortality risk by use of five early warning scores in patients with traumatic injuries: an observational study. Intensive and Critical Care Nursing. 2021;67:103095. doi: 10.1016/j.iccn.2021.103095. [DOI] [PubMed] [Google Scholar]
- 33.Ttendo SS, Was A, Preston MA, Munyarugero E, Kerry VB, Firth PG. Retrospective descriptive study of an Intensive Care Unit at a Ugandan Regional Referral Hospital. World J Surg. 2016;40(12):2847–56. doi: 10.1007/s00268-016-3644-5. [DOI] [PubMed] [Google Scholar]
- 34.Sawe HR, Mfinanga JA, Lidenge SJ, Mpondo BC, Msangi S, Lugazia E, Mwafongo V, Runyon MS, Reynolds TA. Disease patterns and clinical outcomes of patients admitted in intensive care units of tertiary referral hospitals of Tanzania. BMC Int health Hum rights. 2014;14:26. doi: 10.1186/1472-698X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elombila M, Monkessa CMME, Otiobanda GF, Mbaki HBE, Outsouta GN, Ngala MAN. Epidemiology of Mortality in Polyvalent Intensive Care Unit at University Hospital of Brazzaville. Open J Emerg Med. 2018;6(4):112–21. [Google Scholar]
- 36.Gidey K, Hailu A, Bayray A. Pattern and outcome of medical intensive care unit admissions to ayder comprehensive specialized hospital in tigray, ethiopia. Ethiop Med J 2017, 56(1).
- 37.Strand K, Walther SM, Reinikainen M, Ala-Kokko T, Nolin T, Martner J, Mussalo P, Søreide E, Flaatten HK. Variations in the length of stay of intensive care unit nonsurvivors in three scandinavian countries. Crit Care. 2010;14(5):R175. doi: 10.1186/cc9279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barea-Mendoza JA, Chico-Fernández M, Quintana-Díaz M, Pérez-Bárcena J, Serviá-Goixart L, Molina-Díaz I, Bringas-Bollada M, Ruiz-Aguilar AL, Ballesteros-Sanz M, Llompart-Pou JA. Risk factors Associated with mortality in severe chest trauma patients admitted to the ICU. J Clin Med. 2022;11(1):266. doi: 10.3390/jcm11010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michetti CP, Fakhry SM, Brasel K, Martin ND, Teicher EJ, Newcomb A. Trauma ICU Prevalence Project: the diversity of surgical critical care. Trauma Surg acute care open. 2019;4(1):e000288. doi: 10.1136/tsaco-2018-000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duran M, ULUDAG Ö. Mortality analysis of hospitalized trauma patients in the intensive care unit. J Surg Med. 2020;4(11):994–7. [Google Scholar]
- 41.Pogorzelski GF, Silva TA, Piazza T, Lacerda TM, Netto FAS, Jorge AC, Duarte PA. Epidemiology, prognostic factors, and outcome of trauma patients admitted in a brazilian intensive care unit. Open access emergency medicine: OAEM. 2018;10:81. doi: 10.2147/OAEM.S162695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papadimitriou-Olivgeris M, Panteli E, Koutsileou K, Boulovana M, Zotou A, Marangos M, Fligou F. Predictors of mortality of trauma patients admitted to the ICU: a retrospective observational study☆. Brazilian J Anesthesiology. 2021;71:23–30. doi: 10.1016/j.bjane.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Süt N, Memiş D. Intensive care cost and survival analyses of traumatic brain injury. Ulus Travma Acil Cerrahi Derg. 2010;16(2):149–54. [PubMed] [Google Scholar]
- 44.Beniameen M, Abbas N, Mashhour K, ElGohary T. 32 predictors of Mortality among Head Trauma Patients reaching ICU. Ann Emerg Med. 2017;70(4):13–S14. [Google Scholar]
- 45.Mosissa D, Alemu S, Rad MH, Yesuf EA. Outcomes of Surgical Patients admitted to the Intensive Care Unit of Jimma University Medical Center. Health Sci J 2021:0–0.
- 46.Quevedo JBIEG, de Lima Escobal AP, de Pinto DM, Silveira NP, Mocellin LP. Clinical, sociodemographic profile and predictors of death in intensive care unit.
- 47.Sawe HR, Mfinanga JA, Lidenge SJ, Mpondo BC, Msangi S, Lugazia E, Mwafongo V, Runyon MS, Reynolds TA. Disease patterns and clinical outcomes of patients admitted in intensive care units of tertiary referral hospitals of Tanzania. BMC Int health Hum rights. 2014;14(1):1–8. doi: 10.1186/1472-698X-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wangara AA, Hunold KM, Leeper S, Ndiawo F, Mweu J, Harty S, Fuchs R, Martin IB, Ekernas K, Dunlop SJ. Implementation and performance of the south african triage scale at kenyatta national hospital in nairobi, kenya. Int J Emerg Med. 2019;12(1):1–8. doi: 10.1186/s12245-019-0221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.González-Robledo J, Martín-González F, Moreno-García M, Sánchez-Barba M, Sánchez-Hernández F. Prognostic factors associated with mortality in patients with severe trauma: from prehospital care to the intensive care unit. Med Intensiva (English Edition) 2015;39(7):412–21. doi: 10.1016/j.medin.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Toufen Junior C, Franca SA, Okamoto VN, Salge JM, Carvalho CRR. Infection as an independent risk factor for mortality in the surgical intensive care unit. Clinics. 2013;68:1103–8. doi: 10.6061/clinics/2013(08)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alban RF, Nisim AA, Ho J, Nishi GK, Shabot MM. Readmission to surgical intensive care increases severity-adjusted patient mortality. J Trauma Acute Care Surg. 2006;60(5):1027–31. doi: 10.1097/01.ta.0000218217.42861.b7. [DOI] [PubMed] [Google Scholar]
- 52.Fialkow L, Farenzena M, Wawrzeniak IC, Brauner JS, Vieira SRR, Vigo A, Bozzetti MC. Mechanical ventilation in patients in the intensive care unit of a general university hospital in southern Brazil: an epidemiological study. Clinics. 2016;71:144–51. doi: 10.6061/clinics/2016(03)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bouadma L, Sonneville R, Garrouste-Orgeas M, Darmon M, Souweine B, Voiriot G, Kallel H, Schwebel C, Goldgran-Toledano D, Dumenil A-S. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015;43(9):1798–806. doi: 10.1097/CCM.0000000000001091. [DOI] [PubMed] [Google Scholar]
- 54.STGdgeuabDGSHEBJLFBTPJRAT S. Hypoxemia in the ICU: prevalence, treatment, and outcome. Ann Intensiv Care. 2018;8:1–11. doi: 10.1186/s13613-018-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sutton AD, Bailey M, Bellomo R, Eastwood GM, Pilcher DV. The association between early arterial oxygenation in the ICU and mortality following cardiac surgery. Anaesth Intensive Care. 2014;42(6):730–5. doi: 10.1177/0310057X1404200608. [DOI] [PubMed] [Google Scholar]
- 56.Wahyuningsih I, Islamiati N, Ubaidillah Z, Pratiwi ID. Literature study: factors affecting prognostics of head injury patients. East Asian Journal of Multidisciplinary Research (EAJMR) 2022;1(1):13–26. [Google Scholar]
- 57.Bitterman H. Bench-to-bedside review: oxygen as a drug. Crit Care (London England) 2009;13(1):205. doi: 10.1186/cc7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Q, Xu F, Fan L, Xiong L, Li H, Cao S, Lin X, Zheng Z, Yu X, Mao H. Serum potassium levels and its variability in incident peritoneal dialysis patients: associations with mortality. PLoS ONE. 2014;9(1):e86750. doi: 10.1371/journal.pone.0086750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McMahon GM, Mendu ML, Gibbons FK, Christopher KB. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38(11):1834–42. doi: 10.1007/s00134-012-2636-7. [DOI] [PubMed] [Google Scholar]
- 60.Xie Y, Pittaluga S, Jaffe ES. The histological classification of diffuse large B-cell lymphomas. Seminars in hematology: 2015: Elsevier; 2015: 57–66. [DOI] [PMC free article] [PubMed]
- 61.Hessels L, Hoekstra M, Mijzen LJ, Vogelzang M, Dieperink W, Lansink AO, Nijsten MW. The relationship between serum potassium, potassium variability and in-hospital mortality in critically ill patients and a before-after analysis on the impact of computer-assisted potassium control. Crit Care. 2015;19(1):1–11. doi: 10.1186/s13054-014-0720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Basnet S, Dhital R, Tharu B. Influence of abnormal potassium levels on mortality among hospitalized heart failure patients in the US: data from National Inpatient Sample. 2019, 9(2):103–7. [DOI] [PMC free article] [PubMed]
- 63.Makinouchi R, Machida S, Matsui K, Shibagaki Y, Imai N. Severe hypokalemia in the emergency department: a retrospective, single-center study. 2022, 5(3):e594. [DOI] [PMC free article] [PubMed]
- 64.Al-Hameed FM, Al-Dorzi HM, Qadhi AI, Shaker A, Al-Gahtani FH, Al-Jassir FF, Zahir GF, Al-Khuwaitir TS, Addar MH, Al-Hajjaj MS. Thromboprophylaxis and mortality among patients who developed venous thromboembolism in seven major hospitals in Saudi Arabia. Annals of thoracic medicine. 2017;12(4):282. doi: 10.4103/atm.ATM_101_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim W, Meade M, Lauzier F, Zarychanski R, Mehta S, Lamontagne F, Dodek P, McIntyre L, Hall R, Heels-Ansdell D. Failure of anticoagulant thromboprophylaxis: risk factors in medical-surgical critically ill patients. Crit Care Med. 2015;43(2):401–10. doi: 10.1097/CCM.0000000000000713. [DOI] [PubMed] [Google Scholar]
- 66.Lentine KL, Flavin KE, Gould MK. Variability in the use of thromboprophylaxis and outcomes in critically ill medical patients. Am J Med. 2005;118(12):1373–80. doi: 10.1016/j.amjmed.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 67.Lentsck MH, Oliveira RR. Risk factors for death of trauma patients admitted to an Intensive Care Unit. 2020, 28:e3236. [DOI] [PMC free article] [PubMed]
- 68.Ojo O, Ojo OO, Feng Q, Boateng J. The Effects of Enteral Nutrition in critically ill patients with COVID-19: a systematic review and Meta-analysis. 2022, 14(5). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets used and analyzed during the study are available from the corresponding author/primary investigator upon reasonable request.