Abstract
The PI3K/AKT/mTOR (PAM) signaling pathway is a highly conserved signal transduction network in eukaryotic cells that promotes cell survival, cell growth, and cell cycle progression. Growth factor signalling to transcription factors in the PAM axis is highly regulated by multiple cross-interactions with several other signaling pathways, and dysregulation of signal transduction can predispose to cancer development. The PAM axis is the most frequently activated signaling pathway in human cancer and is often implicated in resistance to anticancer therapies. Dysfunction of components of this pathway such as hyperactivity of PI3K, loss of function of PTEN, and gain-of-function of AKT, are notorious drivers of treatment resistance and disease progression in cancer. In this review we highlight the major dysregulations in the PAM signaling pathway in cancer, and discuss the results of PI3K, AKT and mTOR inhibitors as monotherapy and in co-administation with other antineoplastic agents in clinical trials as a strategy for overcoming treatment resistance. Finally, the major mechanisms of resistance to PAM signaling targeted therapies, including PAM signaling in immunology and immunotherapies are also discussed.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12943-023-01827-6.
Keywords: PI3K/AKT/mTORC pathway, Pan PI3K inhibitors, Isoform-specific PI3K inhibitors, Dual PI3K/mTOR inhibitors, AKT inhibitors, Allosteric mTOR inhibitors, ATP-competitive mTOR inhibitors, Bi-steric mTOR inhibitors, PDK1 inhibitors, Cancer
The PAM pathway in cancer
Introduction
The PAM signaling pathway is a highly conserved major transduction network in all higher eukaryotic cells that promotes cell survival, growth, and proliferation in response to external stimuli [1–3]. The two major functional proteins in this pathway are PI3K and AKT [4–7]. Importantly, external growth factors signaling to transcription factors in the PAM pathway is a highly regulated process involving extensive cross-talk with other cell signaling networks [8–10]. Dysregulation of the PAM pathway is known to drive cancer development and progression [11–13]. Indeed, PAM pathway aberration occurs in approximately 50% of tumors [14], and is the most commonly activated pathway in human cancer [15–17]. In addition, PAM pathway hyperactivation in cancer frequently underpins the development of treatment resistance [11, 14, 18]. Aberrant expression or mutation of many components of this pathway are known to be related to human oncogenesis [19, 20]. Thence, activation of membrane receptors (RTK or GPCR), induction of oncogenes upstream of PI3K [21], mutations or amplifications of kinases such as PIK3CA, reduced expression or inactivation of tumor suppressor PTEN [22], and/or mutations such as amplification and gain-of-function missense mutations in AKT oncogene [23], can possibly lead to the onset and/or progression of cancer [11, 24, 25]. Furthermore, overactivity of PAM pathway also promotes epithelial-mesenchymal transition (EMT) and metastasis through its remarkable impact on cell migration [26, 27]. Herein we highlight the biology and biochemistry of the PAM axis, describe its major dysregulations in cancer, show its main crosstalks with other signaling pathways, and discuss PI3K-, AKT-, mTOR-, and PDK1-targeted inhibitors, emphasizing on their mechanisms of therapeutic resistance. Thus, our manuscript is comprehensive on the whole PAM signaling pathway including its major effectors AKT and mTOR in biology and disease. For more detailed reviews on PI3K inhibitors targeting cancer stroma with a focus on immune modulation we refer readers to Okkenhaug et al. 2016 [28], and Vanhaesebroeck et al. 2022 [29]. Additional information on past and future PI3K inhibitors only we refer the reader to Castel et al. 2021 [30]. Moreover, while our manuscript focuses on multiple PI3K-driven cancers, for a specific perspective on the relevance of the PI3K pathway in estrogen receptor (ER) + breast cancer, and the crosstalk between ER and the PI3K pathway in breast cancer have been extensively reviewed by Vasan et al. 2019 [31]. Furthermore, since the present work does not discuss in details the role of PI3K pathway on metabolism, we direct the reader for this specific topic to Vasan and Cantley 2022 [32]. In this review, we first focus on major differences of the PAM axis between normal cells and cancer cells, and then, describe current PI3K, AKT, mTORC1/mTORC2, and PDK1 inhibitors, by summarising active and completed trials in a wide range of cancers.
PAM signaling in cancer
RTK overactivation in cancer
Growth factor-mediated induction of RTKs [33] or GPCRs [34] usually initiates the canonical pathway that engenders the activation of AKT, resulting in plasma membrane localization and induction of one, or more isoforms of the class I PI3K family [35] (Fig. 1a) (Supplementary information 1). Alterations in epidermal growth factor receptor (EGFR) [36, 37], fibroblast growth factor receptor (FGFR) [38, 39], platelet-derived growth factor receptor (PDGFR) [40, 41], vascular endothelial growth factor receptor (VEGFR) [42, 43], hepatocyte growth factor (HGF) [44, 45], leukocyte receptor tyrosine kinase (LTK) [46, 47], and insulin receptor (INSR) [48, 49] family genes make a significant contribution to treatment failure mainly through the activation of PAM pathway (Fig. 1b) (Supplementary information 1).
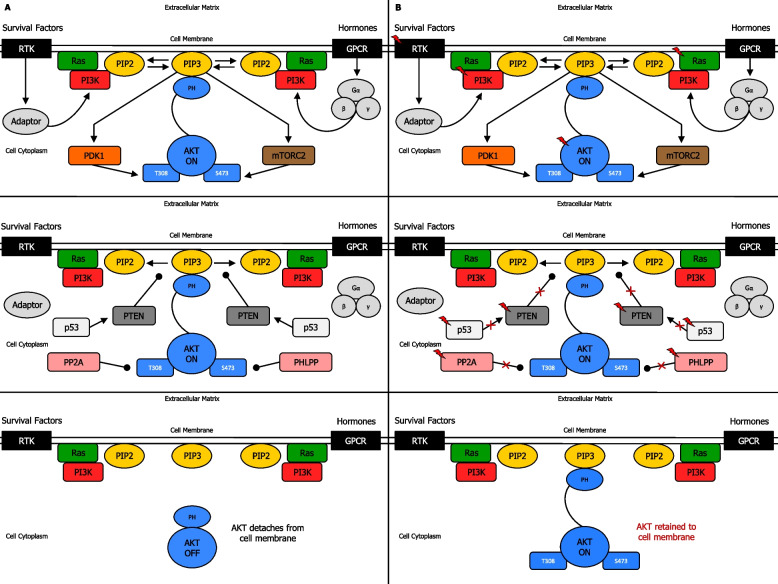
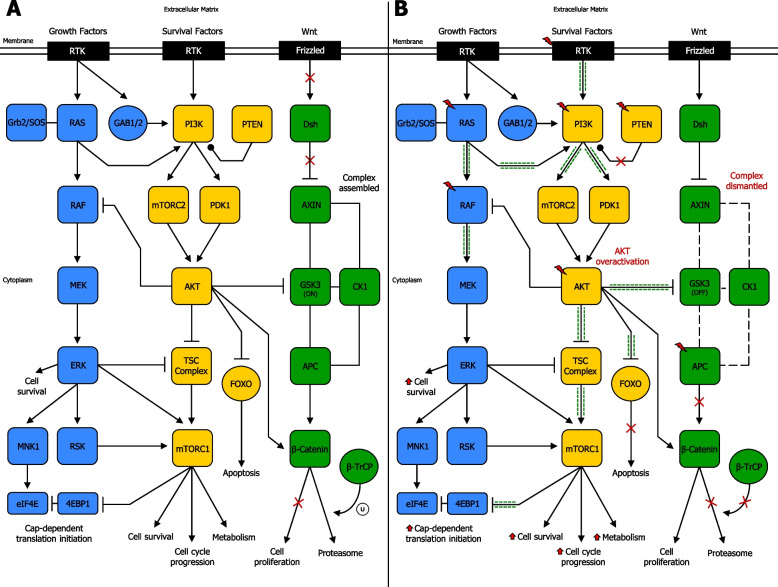
Fig. 1.
Biochemical mechanism of PI3K, PTEN and AKT regulation. A Mechanism of PI3K, PTEN and AKT regulation in normal cells. Induction of RTK or GPCR results in the activation of Ras-regulated PI3K, which interacts with PIP2, and produces PIP3 at the plasma membrane. Inactive AKT in the cytoplasmic matrix is recruited to cell membrane and binds PIP3 through a PH binding domain. This drives phosphorylation of T308 by PDK1, and phosphorylation of S473 by mTORC2, leading to complete activation of AKT (above). Signal termination is determined by loss of PI3K-PIP2 interaction, via inhibition by (PIP3) PTEN protein phosphatase, (AKT) PP2A protein phosphatase, and (AKT) PHLPP protein phosphatase, leading to AKT detaching from the cell membrane. Due to DNA damage response, p53 activates PTEN, whose function reduces PAM-induced cell proliferation (middle). AKT then shifts to off-mode in the cytoplasm (below). B Mechanism of PI3K, PTEN and AKT regulation in cancer cells. Mutations in RTK, Ras, PI3K, AKT (above), PTEN protein phosphatase, p53, (AKT) PP2A protein phosphatases and (AKT) PHLPP protein phosphatases may occur, resulting in AKT retention to cell membrane (middle). AKT then remains in on-mode in the cytoplasm (below), leading to dysregulation of PAM pathway signal transduction, and possibly cancer onset and/or progression (below). Activation (phosphorylation or non-phosphorylation) is shown with arrowhead lines, whereas dephosphorylation is indicated with roundhead lines. Red lightning symbol shows mutation for a particular gene in the PAM pathway. Red crosses emphasise signaling blockage. P: phosphoryl group
PI3K mutation and amplification in cancer
Among the numerous types of PI3K, only class I can exert lipid phosphorylation following growth stimulation [50]. PI3K (class I) is a heterodimer comprising two distinct subunits: the regulatory subunit p85, and the catalytic subunit p110 [51, 52]. Specifically, the regulatory subunit p85 of PI3K can bind to phosphorylated tyrosine residues on the activated RTKs through its Src homology 2 (SH2) domain. Subsequently, PI3K catalytic subunit p110 can form a complete active PI3K enzyme [53] (Fig. 1a) (Supplementary information 1). PIK3CA activating mutation, encoding the p110α catalytic subunit of PI3K, is the most commonly mutated oncogene detected across tumor lineages [15, 54]. Indeed, PIK3CA is usually either mutated or amplified in several human cancers [55], including colorectal cancer (CRC) [56], breast [57], lung [58], gastric [59], prostate [60], and cervical cancer [61, 62]. Most mutations are found close to spots E545K (exon 9) and H1047 (exon 20). The hotpots E542K and E545K, present in the helical phosphatidylinositol (inositol phospholipid) kinase homology domain, decrease suppression of p110α by the p85 regulatory subunit, whereas H1047, which is adjacent to the end of the catalytic domain, enhances the p110α-lipid membrane interaction [63, 64]. Mutations in p110β, p110γ and p110δ subunits are rather uncommon; however, their overexpression can easily promote oncogenicity in cultured cells [65]. PIK3CA mutations are present in head and neck squamous cell cancer (HNSCC) [66], gastric cancer [67], gallbladder cancer [68], and melanoma [69]. Besides, mutations of PIK3CA E542K and PIK3CA E545K are known to endorse proliferation and glycolysis of cervical cancer [64]. Also, PIK3CA mutation leads to prostate cancer in mice and correlates with poor prostate cancer prognosis. Notably, PIK3CA mutation and PTEN loss coexist in prostate cancer patients and synergistically can cooperate in vivo to accelerate carcinogenesis and cancer progression via PAM pathway hyperactivation [70, 71]. PIK3CA mutations are frequently associated with FGFR3 mutations in metastatic non-muscle invasive bladder cancer [72]. In line with this, mutations in PAM pathway are detected in 25% of osteosarcoma patients. In fact, PIK3CA and mTOR are critical for survival and proliferation of osteosarcoma cells [73]. Mutations in PI3K family genes, especially PIK3CA or PIK3R1 are often present in glioblastoma multiforme [74], testicular germ cell tumors [75], and Ewing’s sarcoma [76], resulting in alteration pattern of PAM pathway. Mutations of PIK3CA, PIK3R1, and PIK3R2 are frequently detected in small-cell lung cancer (SCLC), non-small-cell lung cancer (NSCLC) [77], hepatocellular cancer [78], and ovarian serous cystadenocarcinoma [79]. Similarly, dysregulation of PAM pathway in CRC is mainly due to mutations in PIK3CA, and to a lesser extent PIK3R1 and PIK3R2. In addition, CRC PIK3CA mutations are generally associated with KRAS mutations [80, 81]. The genetic alterations of PAM pathway in renal cancer are also due to PIK3CA, PIK3R1, and PIK3R2 mutations. Moreover, PIK3R1 can regulate EMT, as well as stem-like phenotype, of renal cell carcinoma cells via the AKT/GSK3β/β-catenin signaling pathway [82, 83]. Oesophageal squamous cell carcinoma present genetic alterations of PI3K family genes and PTEN, especially somatic mutations of PIK3CA, PIK3CG, PIK3C2A, and PIK3C2G [84]. Furthermore, mutations in PAM pathway genes such as PIK3CA, PIK3CG, PIK3C2G, PIK3C3, PIK3R1, and PIK3R2 are often detected in poorly differentiated thyroid cancer and anaplastic thyroid cancer [85]. Thus, mutations in the PAM pathway can affect RTKs and growth factors, as well as Ras and PI3K p110 subunits, resulting in abnormal signaling activity (Fig. 1b).
PTEN inactivation or loss in cancer
PI3K/PIP3 signal termination is mainly attained by tumor suppressor PTEN, which exerts dephosphorylation on PIP3, thereby switching it back to PIP2. Thus, PTEN acts as an essential negative regulator of the PAM pathway affecting cell growth survival, whereas loss of PTEN results in the sustained output of these intracellular signalings [86] (Fig. 1a) (Supplementary information 1). PTEN loss-of-function mutations are present in a several tumors [87, 88]. PTEN loss frequently occurs in primary and metastatic breast cancer leading to hyperactivation of PAM pathway, and consequently, enhancing cell proliferation [89, 90]. Both PTEN downregulation and PAM pathway activation are related to anti-estrogen therapy resistance [91]. Mutations in PTEN are often detected in gastric cancer [67]. Besides, overexpressed [92] and/or amplified [93] PRL-3 downregulates the expression of PTEN through dephosphorylation [94], and as a result indirectly increases signals through PAM pathway [95] in human gastric cancer [93]. Mutations in PTEN are observed in CRC too [80]. Notably, loss of TGF-β signalling results in PRL-3 upregulation and PAM pathway activation, which can promote EMT and tumor aggressiveness in primary CRC [96]. Mutations in PTEN are also reported in bladder cancer since loss of PTEN combined with altered TP53 determines a negative effect, enhancing tumor progression [97]. The PAM pathway can also function as a pro-survival factor in leukemia stem cells, and thus, genetic aberrations in PTEN are likely to be detected in leukemia. In fact, PTEN regulates the activity of hematopoietic stem cell via a niche-dependent mechanism, as well as leukemogenesis and hematopoiesis [98]. PTEN loss-of-function alterations, especially deletion, are also detected in brain cancer [87], glioblastoma multiforme [99], anaplastic/poorly differentiated thyroid cancer [85], SCLC, NSCLC [77], melanoma [69], oesophageal cancer [100], gallbladder cancer [68], pancreatic cancer [101], renal cell carcinoma [102], prostate cancer [103], testicular germ cell tumors [75], cervical cancer [104], ovarian cancer [105], and many types of sarcoma [106, 76], leading to the typical pathological effects of PAM pathway (Fig. 1b).
AKT overactivation in cancer
Phosphorylated phosphatidylinositol lipids on the inner face of plasma membrane can directly bind intracellular proteins which contain PH or FYVE zinc finger domains. Indeed, PIP3 binds AKT and PDK1, and as a result, they can accumulate near the membrane [107]. Once activated, AKT migrates from plasma membrane to cytoplasm and nucleus, where many substrates are located [108] (Fig. 1a) (Supplementary information 1). Mutations in AKT genes are rather infrequent in human cancers [1]. However, gain-of-function missense mutations and amplification in genes that encode one of the three isoforms of oncogenic protein AKT, known to be implicated in regulating cell survival, proliferation, growth, apoptosis, and glycogen metabolism [109], have been reported [23]. Notably, the most frequent mutation is AKT1 point mutation in the PH domain where glutamic acid at residue 17 is replaced with lysine (E17K) at residue 17, resulting in enhanced activity of AKT1 by inducing its constitutive localization to the plasma membrane [110]. Moreover, other activating mutations include E49K (AKT1) substitution occurring in the PH domain, and G171R (AKT3) substitution occurring in the kinase domain [111]. Indeed, activated p-AKT levels are significantly increased in cancer cell lines due to these point mutations, and levels of p-AKT correlates with sensitivity to AKT inhibition [112]. Furthermore, high-resolution sequencing studies in breast cancer have also reported further somatic variants in the AKT PH domain [113, 114]. However, due to the relative infrequency of AKT mutations, their significance as drivers of oncogenesis has not been thoroughly clarified. In fact, changes in AKT activity normally occur through the activating mutations or amplifications upstream AKT, such as in PIK3CA or in PTEN, growth factor or cytokine receptors, and intracellular oncogenes like Ras, which lead to enhanced expression and activity of one, two or all three isoforms of AKT [1]. Unlike AKT mutations, AKT gene amplifications are more frequent, and have been detected in breast [115], colon, gastric, ovarian, pancreatic, oesophageal and thyroid cancers, with major amplifications usually involving the AKT2 isoform [116]. In addition, AKT post-translational modification, such as lysine modifications, tyrosine phosphorylation, O-GlcNAcylation, acetylation, and sumoylation are important in retaining AKT hyperactivation in cancers, even in conditions where normal PI3K and PTEN activity persists [1, 117, 118]. AKT is elevated in a subset of premalignant breast lesions. Indeed, p-AKT is overexpressed in 33% ductal carcinoma in situ lesions and in 38% of invasive breast cancers, where most tumors (79%) express the oestrogen receptor [119]. Additionally, phosphorylation of AKT at Ser473 can promote breast cancer metastasis [120], and increased AKT1 activity has been observed in 40% of breast cancers [121]. Moreover, AKT overactivation has been observed in several other types of cancers [77, 122, 123, 80, 78, 82, 75] (Fig. 1b) (Supplementary information 1).
AKT protein targets in cancer
AKT phosphorylation of downstream substrates determines the regulation of distinct cellular functions [124] (Fig. 2a) (Supplementary information 1). In cancer cells, AKT overactivation due to mutation of AKT or mutations upstream the PAM pathway, can trigger phosphorylation on substrates, determining either blockage or enhancement of their activities [125]. Indeed, in cancer, AKT can exert numerous important functions: 1) increases phosphorylation on BAD, thereby inhibiting apoptosis, and thus, increasing cell survival [126]; 2) enhances phosphorylation on IKKα, contributing to cell survival and proliferation [127]; 3) increases phosphorylation, and thus, inhibits the transcriptional functions of FOXO, contributing to cell survival, proliferation, growth [128] and reprogramming cell metabolism [129]; 4) increases MDM2 phosphorylation, thereby regulating and inhibiting p53 response, promoting cell survival and proliferation, leading to tumorigenesis [130]; 5) enhances phosphorylation on Chk1, endorsing cell survival and proliferation [131]; 6) increases phosphorylation, and thus, inhibition of p21 and p27, thereby increasing cell proliferation [132]; 7) enhances phosphorylation on GSK3 leading to increase in cell proliferation and growth, as well as boosting cellular anabolism [133]; and 8) increases phosphorylation on TSC2, and consequently, reduces inhibition of mTORC1, resulting in enhanced cell growth and cell metabolism [134] (Fig. 2b) (Supplementary information 1).
Fig. 2.
AKT signaling network targets and regulates critical cellular substrates. A AKT regulation of targeted proteins in normal cells. AKT phosphorylation of downstream substrates determines regulation of distinct cellular functions. There are several AKT cytoplasmic targets, including BAD, IKKα, FOXO, MDM2, CHK1, p21, p27, GSK-3, and TSC2, representing crucial signaling nodes that interlink AKT signaling with supplementary cellular regulatory circuits. In normal conditions, PAM pathway moderately promotes essential cellular functions such as survival, proliferation, growth and metabolism. B AKT regulation of targeted proteins in cancer cells. Mutations in RTK, Ras, PI3K, PTEN protein phosphatase, AKT, and/or other proto-oncogenes, may occur, resulting in AKT overexpression, leading to enhanced inhibition of BAD, FOXO, CHK1, p21, p27, GSK3, and TSC2, as well as increased activity of IKKα, MDM2, with consequently higher survival, increased proliferation, enhanced growth and boosted metabolism. Activation (phosphorylation or non-phosphorylation) is shown with arrowhead lines, inhibition (phosphorylation or non-phosphorylation) is indicated with blocked lines, and dephosphorylation, carried out by phosphatases, is displayed with roundhead lines. Red lightning symbol shows mutation for a particular gene in the PAM pathway. Red crosses emphasise signaling blockage, whereas green dash-dotted lines (adjacent to arrowhead lines) highlight signaling enhancement. P: phosphoryl group
AKT-mediated GSK3 inhibition in cancer
AKT-mediated GSK3 inhibition is determined by AKT phosphorylation on GSK3 NH2-terminus, forming an intramolecular pseudo-substrate that obstructs the phosphate-binding pocket, and consequently, suppresses substrate availability to GSK3 [135] (Fig. 3a) (Supplementary information 1). GSK3 closely interacts with the PAM pathway and can function both as a tumor promoter and tumor suppressor. In fact, abnormal expression of GSK3 can interfere with the advancement and evolution of tumor through dysregulation of cell cycle, apoptosis, and senescence, as well as resistance to chemotherapy and radiotherapy [133]. GSK3 is a signal integrator that often acts at the intersection of several biochemical pathways. Indeed, a GSK3-associated signaling transduction pathway that is often involved in human tumor is the EGFR/Ras/PI3K/PTEN/AKT/GSK3/mTORC1 axis, which exerts an important role in natural cell growth, and is frequently overactivated with mutations mainly occurring in PI3K (PIK3CA), Ras, and PTEN [136]. Notably, AKT can directly phosphorylate, inactivate and target GSK3 for degradation [137]. Consequently, inactivation, or low levels of active GSK-3, can lead to dysregulation of multiple signaling pathways. In fact, when mTOR and TSC2 are not inactivated by GSK3, the mTORC1 complex results active, leading to translation of several growth-regulating mRNAs [138]. Importantly, the WNT/β-catenin is the major pathway regulated by GSK3. The WNT/β-catenin axis is also critical in cellular proliferation and in EMT, which is essential for epithelial tumor metastasis. GSK3, when present in its active form, phosphorylates β-catenin, resulting in the proteasome degradation of β-catenin; and thus, several important genes required for cell proliferation are not transcribed. Conversely, AKT phosphorylation suppresses GSK3, thereby allowing β-catenin to shuttle from the cytoplasm into the nucleus to function as transcription factor, and consequently, leading to cell proliferation [139] (Fig. 3b) (Supplementary information 1).
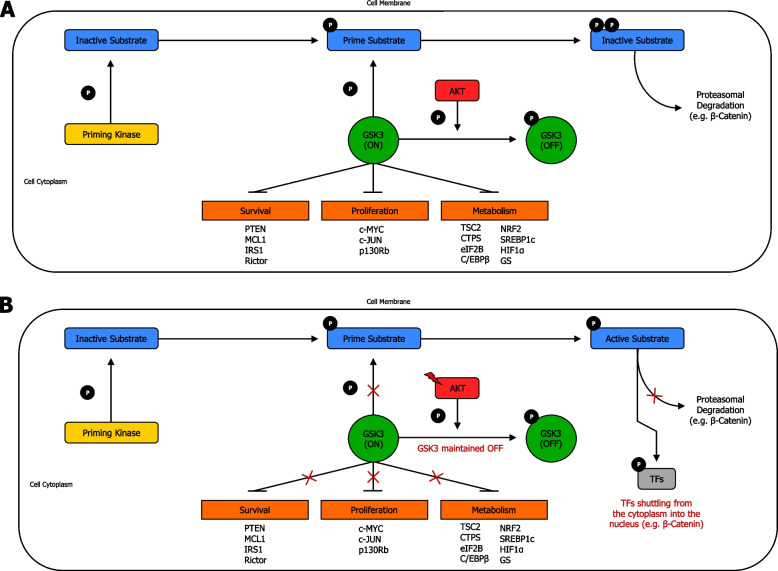
Fig. 3.
AKT-mediated GSK3 phosphorylation and regulation. A AKT-mediated GSK3 regulation in normal cells. AKT-mediated GSK3 regulation is exerted by AKT phosphorylation on GSK3 amino-terminus, thereby creating an intramolecular pseudo-substrate that occludes the phosphate-binding pocket, and inhibits substrate accessibility to GSK3. When in active (on) form, GSK3 can only recognise and phosphorylate substrates previously phosphorylated by a priming kinase. Conversely, when in inactive (off) form, GSK3 results blocked due to AKT phosphorylation, and thus, its access to primed substrates is denied. Some GSK3 substrates, with their corresponding cellular function are shown. B AKT-mediated GSK3 regulation in cancer cells. Mutations in AKT can enhance phosphorylation, and thus, inactivation of GSK3. Consequently, inactivation or limited amount of active GSK3 can lead to dysregulation of several signal transduction, resulting in cancer onset and/or progression. Reduction or absence of phosphorylation, and thence decreased proteasomal degradation of molecules (e.g. β-catenin) can arise from excessive inhibition of GSK3 by AKT phosphorylation, which can lead to increased survival, enhanced proliferation, and boosted metabolism. Red lightning symbol shows mutation for a particular gene in the PAM pathway. TFs: transcription factors. Phosphorylation is shown with arrowhead lines, whereas inhibition is indicated with blocked lines. Red crosses emphasise signaling blockage. P: phosphoryl group
AKT-induced FOXO regulation in cancer
Several gene targets mainly involved in response to different insulin and insulin-like growth factor 1 (IGF1) signaling are regulated by FOXO transcription factor family [140]. FOXO-targeted genes are associated with activation of apoptosis, cell cycle blockage, growth inhibition, and tissue-specific metabolic changes [141] (Fig. 4a) (Supplementary information 1). In cancer, overactivation of AKT induces continuous phosphorylation of FOXO and binding of FOXO to 14-3-3 protein, which consequently results in durable FOXO nuclear export. FOXO3 then undergoes ubiquitination in the cytoplasm, and thus is degraded by the proteasome. This AKT-mediated activity causes stable blockage of FOXO expression, thereby promoting cell survival (e.g. due to BIM inactivation), cell proliferation (e.g. due to p21 inactivation), cell growth (e.g. due to ATG4B inactivation) [142], and reduction of tissue-specific metabolic changes (e.g. due to LPL inactivation) [143]. Thus, due to its pro-apoptotic activity, FOXO can also function as tumor suppressor in several types of cancer [144] (Fig. 4b).
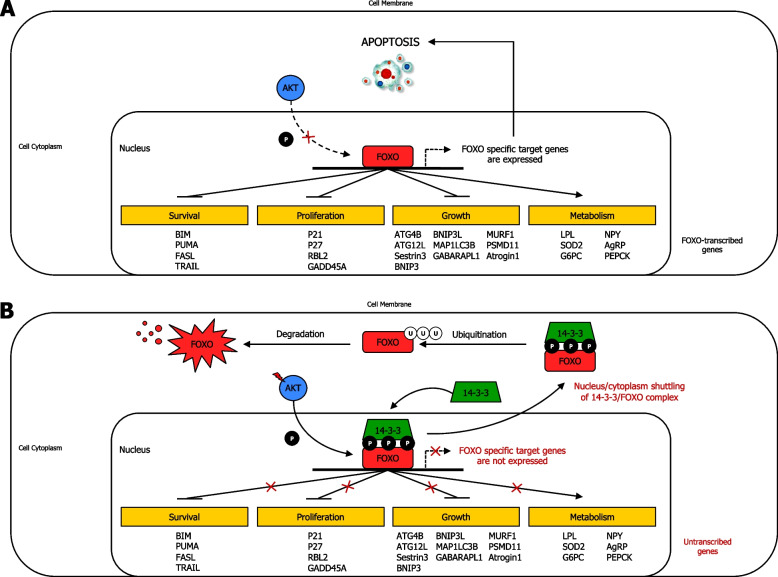
Fig. 4.
AKT-induced FOXO phosphorylation and regulation. A AKT-induced FOXO phosphorylation and regulation in normal cells. In normal condition, AKT exerts an ordinary moderate FOXO phosphorylation, which allows FOXO to transcribe its target genes. B AKT-induced FOXO phosphorylation and regulation in cancer cells. Mutations upstream AKT and/or AKT, with its consequent overexpression, can increase FOXO phosphorylation by AKT, resulting in binding of 14–3-3 adapter protein to FOXO, and leading to 14–3-3/FOXO complex being shuttled from nucleus to cytoplasm, thereby inhibiting the expression of FOXO gene targets. Therefore, excessive inhibition of FOXO by AKT phosphorylation can ultimately increase survival, enhance proliferation, increase growth, and suppress cell metabolism. Activation (phosphorylation or non-phosphorylation), interactions, and nucleus/cytoplasm shuttling are shown with arrowhead lines, moderate or possible phosphorylation is indicated with dotted-arrowhead lines, and inhibition is displayed with blocked lines. Red lightning symbol shows mutation for a particular gene in the PAM pathway. Red crosses emphasise signaling blockage. P: phosphoryl group. Survival: Cell survival; Proliferation: Cell proliferation; Growth: Cell growth; Metabolism: tissue-specific metabolic changes
AKT-mediated regulation of mTORC1 and TSC2 in cancer
Cell growth is mainly regulated through AKT-mediated activation of the protein kinase mTORC1 [145]. Activation of mTORC1 occurs through nutrient- and AKT-induced inhibitory phosphorylation of TSC2 [146], which acts in a molecular complex (known as the TSC complex) that also incorporates TSC1 and TBC1D7 [145]. mTORC1 exerts a dual role: a promoting downstream effector of PAM signaling pathway, and an inhibiting regulator with remarkable negative feedback effects on the induction of AKT by cell surface receptors (RTKs or GPCRs) [147] (Fig. 5a) (Supplementary information 1). In cancer cells, mutations [23] and/or gene amplifications in AKT [116], or mutations upstream genes in the PAM pathway, especially PI3K genes [15] and PTEN [148], can potentially result in AKT-induced overactivation of mTORC1, leading to increased cell survival, growth, proliferation, and metabolism in cancer cells. Moreover, altered mTORC1 activation sends critical signals that enhance tumor cells to metastasize, and invade new healthy tissues [149]. Thus, mutations in PAM pathway allow TSC complex to be released from Rheb, and consequently, Rheb turns into GTP loaded, leading to activation of mTORC1, recruited by Rag proteins [150] (Fig. 5b).
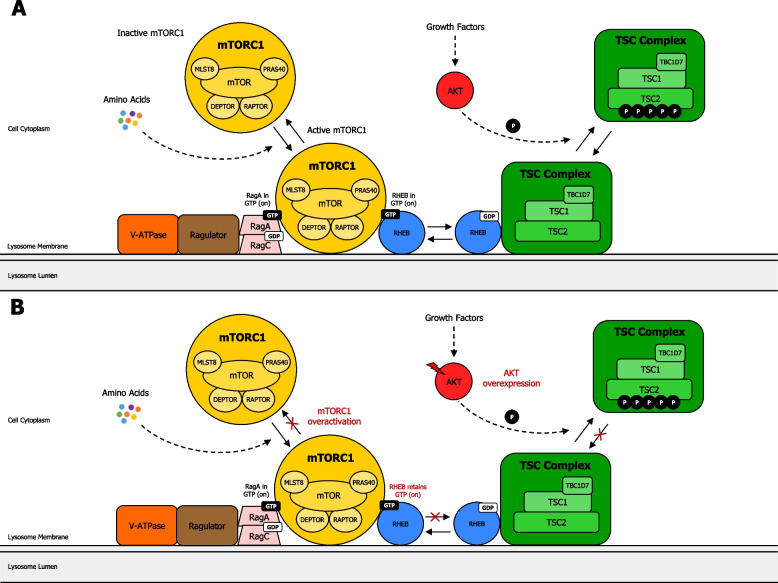
Fig. 5.
Regulation of mTORC1 through the TSC complex. A Regulation of mTORC1 through the TSC complex in normal cells. The signal integration model of mTORC1 is regulated by growth factors and amino acids. Rag heterodimer (RagA and RagC) interacts with Ragulator and V-ATPase on the lysosome membrane. Amino acids then allow connection of mTORC1 to Rag heterodimer/Ragulator/V-ATPase complex. The TSC complex maintains Rheb in the GDP-bound state. Growth factor-induced AKT phosphorylates TSC2, leading to dissociation from the lysosomal membrane, promoting Rheb to become GTP loaded, and thus, activating mTORC1. B Regulation of mTORC1 through the TSC complex in cancer cells. Mutations in AKT or upstream genes in the PAM pathway, can potentially lead to overactivation of mTORC1, due to TSC complex being released from Rheb. Consequently, Rheb becomes GTP loaded, resulting in activation of mTORC1, recruited by Rag proteins. This dysregulation may promote the onset and/or progress of cancer, resulting in enhanced cell survival, proliferation, growth, and metabolism in cancer cells. Activation of mTORC1 potentially sends critical signals that engender tumor cells to metastasize and invade new tissues. mTORC1: mechanistic target of rapamycin complex 1; DEPTOR: DEP domain-containing mTOR-interacting protein; MLST8: mammalian lethal with SEC13 protein 8; PRAS40: proline-rich AKT1 substrate 1; RAPTOR: regulatory-associated protein of mTOR; RHEB: Ras homolog enriched in brain; TBC1D7: TBC1 Domain Family Member 7; TSC2: tuberous sclerosis complex 2. Activation (phosphorylation or non-phosphorylation) is shown with arrowhead lines or dotted-arrowhead lines. Red lightning symbol shows mutation for a particular gene in the PAM pathway. Red crosses emphasise signaling blockage. P: phosphoryl group
PAM Signaling network in cancer
Feedback mechanisms in cancer
The PAM pathway is regulated by negative and positive feedback to ensure that stimulation signal transductions are captured and delivered transiently [151] (Fig. 6a) (Supplementary information 1). In cancer, mutations in RTK [152], PI3K [153], AKT [23], mTORC2 [154], and/or mTORC1 genes [155] can alter the PAM negative feedback signal transduction. Likewise, mutations in NF-κB [156], PRL-3 [92] and/or PTEN [157] can dysregulate the PAM positive feedback loop. Thus, PAM pathway mutations in either negative or positive feedback loops can potentially lead to the onset and/or progression of cancer [158, 145] (Fig. 6b).
Fig. 6.
The PAM signaling pathway and its downstream functions. A PAM pathway downstream functions in normal cells. PI3K activation occurs by growth factor-induced receptors or through interaction with scaffolding adaptors, including IRS1/2 proteins. PI3K is then recruited to its substrate PIP2, promoting generation of PIP3. Inactive AKT in the cytoplasmic matrix binds to PIP3 on the cell membrane, allowing phosphorylation by PDK1 and mTORC2, leading to complete activation of AKT, which subsequently phosphorylates several downstream targets, including multiple sites on TSC2, which forms a functional complex with TSC1 (TSC Complex). AKT-induced phosphorylation on TSC2 hampers the ability of TSC Complex to act as a GAP toward the small GTPase Rheb, endorsing Rheb-GTP accumulation. As a result, Rheb-GTP remarkably activates mTORC1, which phosphorylates and activates S6K. In the negative PAM feedback loop, mTORC1 and S6K1 directly phosphorylate IRS1/2, impeding PI3K activation. In addition, mTORC1 blocks GRB10-mediated growth factor-induced receptor signaling to PI3K. Conversely, in the positive PAM feedback loop, AKT phosphorylates IKKα, which indirectly activates transcription factor NF-κB, allowing PTEN phosphatase inhibition. Besides, PRL-3 phosphatase can also inhibit PTEN phosphatase. PAM downstream functions include cell survival, metabolism, anabolism, catabolism, and cell cycle progression. B PAM pathway downstream functions in cancer cells. Mutations in RTK, PI3K, AKT, PTEN, and possibly other genes, may occur. Overactivation of AKT strongly enhances phosphorylation on TSC2, which further hampers the ability of the TSC Complex to act as a GAP toward the small GTPase Rheb, thereby remarkably endorsing Rheb-GTP accumulation. Thus, dysregulation of PAM pathway signal transduction, due to mutations and/or inevitable alterations in the negative feedback loop or positive feedback loop, can possibly lead to cancer onset and/or progression. This results in enhanced PAM downstream functions, such as increased cell survival, boosted metabolism, enhanced anabolism, reduced catabolism, and increased cell cycle progression. Activation (phosphorylation or non-phosphorylation) is shown with arrowhead lines or dotted-arrowhead lines, inhibition (phosphorylation or non-phosphorylation) is indicated with blocked lines, and dephosphorylation is displayed with roundhead lines. Red lightning symbol shows mutation for a particular gene in the PAM pathway. Red crosses emphasise signaling blockage, whereas green dash-dotted lines (adjacent to arrowhead lines or blocked lines) highlight signaling enhancement. Red upper-arrows show increases, whereas blue lower-arrows indicate reduction. Red upper-arrows show increases, whereas blue lower-arrows indicate reduction. P: phosphoryl group
Major PAM pathway cross-regulation with Ras/ERK and Wnt/GSK3/β-catenin pathways
The PAM pathway frequently cross-regulates with the Ras/ERK pathway [159] (Fig. 7a) (Supplementary information 1). In human tumors, mutations in genes encoding effector molecules of PAM pathway and Ras/ERK pathway commonly coexist. Indeed, PI3K genes and Ras genes mutations, PI3K genes and BRAF mutations, or PTEN and BRAF mutations often occur together in numerous cancer types. Interestingly, concurrent mutations in these two pathways can abrogate the dependence on a single pathway in tumor cells due to the induction of molecules whose function integrates the effects of both these signalling axes. In fact, 4E-BP1, a repressor of mRNA translation, is a crucial integrator of PAM pathway and Ras/ERK pathway, acting as a key mediator of their effects on malignant transformation [160]. Importantly, mTOR persistently restrains the tumor-suppressive function of 4E-BP1 via phosphorylation thereby releasing 4E‐BPs from eIF4E and enabling cap-dependent translation initiation in cancer [161, 162]. In breast tumor cells, which overexpress ErbB-2, PI3K inhibition causes an activation of Ras/ERK pathway, as a consequence of ErbB receptor induction. Treatment with ErbB-2 inhibitors or MEK inhibitors enhances the activity of PI3K inhibitors, leading to reduced proliferation and improved anticancer efficacy, in comparison to a single agent [163]. Further studies have described the cross-regulation between PAM pathway and Ras/ERK pathway in cancer (Fig. 7b) (Supplementary information 1). The PAM pathway also often cross-regulates with the Wnt/GSK3/β-catenin pathway [164] (Fig. 7a) (Supplementary information 1). In cancer, AKT is usually overexpressed, and consequently, GSK3 is often further inactivated [133]. Besides, AKT can directly phosphorylate (Ser552) and activate β-catenin, triggering its nuclear translocation, enhancing its transcriptional activity [139], resulting in uncontrolled, cell proliferation and playing a critical role in cancer invasion and development [165]. Likewise, AKT expression can also be regulated by Wnt/β-catenin signaling. In fact, β-catenin is able to increase AKT activation in colorectal tumors [166]. Moreover, GSK3 can phosphorylate TSC complex [167]. Interestingly, TSC complex, in turn has been reported to regulate β-catenin signaling activity through GSK3, and this crosstalk promotes degradation of β-catenin. In fact, mutations of the TSC complex in tuberous sclerosis-patients are often associated with incidence of cancers such as subependymal giant cell astrocytoma (SEGA). Higher levels of β-catenin have been detected in tumor tissues of SEGA patients, and this augmentation was associated with translocation of β-catenin from cytoplasm to nucleus, with upregulation of target proto-oncogene c-Myc [168]. Thus, when TSC2 complex is not disabled by GSK3, the mTORC1 complex is effective and can lead to translation and proliferation of several growth-regulating mRNAs [138]. More studies have focussed on the cross-regulation between PAM pathway and Wnt/GSK3/β-catenin pathway in cancer [169] (Fig. 7b) (Supplementary information 1).
Fig. 7.
Network of signaling cross-regulation between PAM pathway and RAS/ERK pathway or Wnt/GSK3/β-catenin pathway. A Simplified cross-regulation between PAM pathway and RAS/ERK pathway or Wnt/GSK3/β-catenin pathway in normal cells. Numerous cross-talk points occur between PAM pathway and RAS/ERK pathway or Wnt/GSK3/β-catenin pathway, leading to ordinary cell survival and proliferation, cell cycle progression, cell metabolism, apoptosis, and other cellular functions. B Simplified cross-regulation between PAM pathway and RAS/ERK pathway or Wnt/GSK3/β-catenin pathway in cancer cells. Mutations in RTK, RAS, PI3K, PTEN, AKT, APC, and possibly other genes, may occur, resulting in cross-talk dysregulations between PAM pathway and RAS/ERK pathway or Wnt/GSK3/β-catenin pathway. This can lead to enhanced PAM downstream signaling, such as increased cell survival and proliferation, enhanced cell cycle progression, boosted cell metabolism, reduced apoptosis, and dysregulation of other important cellular functions. Activation (phosphorylation or non-phosphorylation) is shown with arrowhead lines, inhibition (phosphorylation or non-phosphorylation) is indicated with blocked lines, interaction is displayed with continuous lines, disassociation is shown with dotted lines, and dephosphorylation, carried out by phosphatases, is indicated with roundhead lines. Red lightning symbol shows mutation for a particular gene in the PAM pathway. Red crosses emphasise signaling blockage, whereas green dash-dotted lines (adjacent to arrowhead lines or blocked lines) highlight signaling enhancement. Red upper-arrows show increases. U: ubiquitination
Major cross-regulation between PAM pathway and other pathways
Apart from MEK pathway and Wnt pathway, there are other signaling pathways interacting directly with PAM pathway, including NF-κB pathway [170], G-protein pathway [171], integrin pathway [172], intrinsic apoptotic pathway [173], and p53 pathway [174, 175] (Fig. 8). In cancer cells, mutations in RTK and PI3K genes, as well as Ras genes, PTEN, AKT, and/or other proto-oncogenes can occur, leading to PAM signaling pathway overexpression. AKT overactivation can trigger phosphorylation of substrates, determining either blockage or enhancement of their activities. In addition, signaling cross-talks between PAM pathway and other pathways exert a significant role in the dysregulation of cellular functions in tumor. Importantly, dysregulation of PAM signaling pathway can often result in stronger AKT-induced inhibition of pro-apoptotic proteins, including FOXO [128], BAD [126], BAX [176], BAK [173], and/or enhanced activity of anti-apoptotic proteins such as XIAP [177]. Besides, this condition can lead to AKT-induced higher activity of mTORC1 [134], IKKα [127], and MDM2 [130], as well as AKT-induced phosphorylation and consequent inhibition of GSKβ [133]. Moreover, PI3K-mediated increased activity of PKC [178], and Rac [179], as well as Ras-induced overexpression of CDC42 [180], may occur. Altogether, these altered transductions signaling can thereafter result in uncontrolled survival, proliferation, growth and boosted metabolism in cancer cells (Fig. 9).
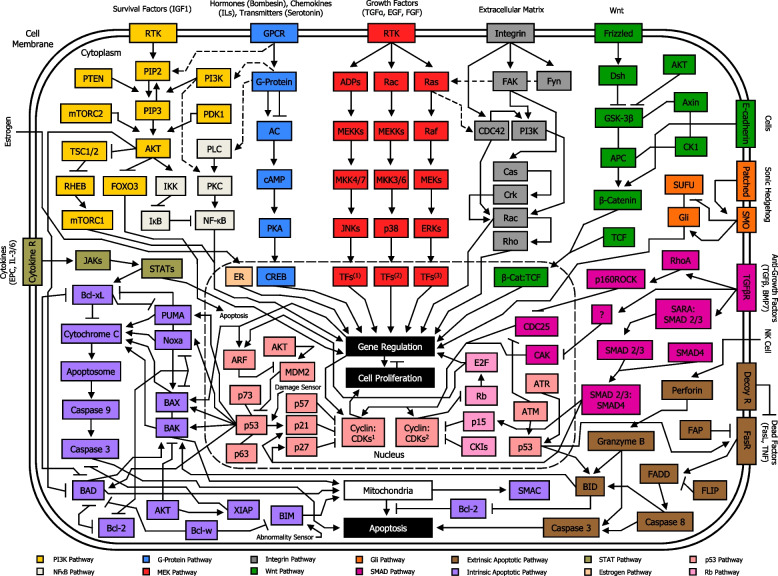
Fig. 8.
Simplified overview of PAM signaling pathway location among other major signal transduction pathways in the network circuit of a normal cell. Each component of a signaling pathway is termed according to the role it plays with respect to the initial stimulus, such as ligands (e.g. IGF1), receptors (e.g. RTK), and effectors (e.g. PI3K). In normal cells, PAM pathway moderately promotes essential cellular functions such as survival, proliferation, growth and metabolism. Signaling cross-talks between PAM pathway and other pathways play a major role in the ultimate function of the cell. PI3K pathway, NF-κB pathway, G-Protein pathway, MEK pathway, Integrin pathway, Wnt pathway, Gli pathway, SMAD pathway, extrinsic apoptotic pathway, intrinsic apoptotic pathway, STAT pathway, Estrogen pathway, p53 pathway, and Rb pathway are shown. Nuclear membrane is delimited by a long-dashed circular dotted line. Arrowhead lines: activation (phosphorylation or non-phosphorylation). Blocked lines: inhibition (phosphorylation or non-phosphorylation). Continuous lines: interaction. Dotted arrowhead lines: cross-talk activation (phosphorylation or non-phosphorylation) between pathways. Dotted blocked lines: cross-talk inhibition (phosphorylation or non-phosphorylation) between pathways. PI3K: PI3K/Ras complex. MEKs: MEK1/2. ERKs: ERK1/2. TFs1: Transcription factors Jun, ATF2, RNPK, p53, NFAT4, Shc. TFs2: Transcription factors CHOP, ATF2, MNK, MSK, MEF2, Elk-1. TFs3: Transcription factors Elk-1, Ets-2, RSK, MNK, MSK, cPLA2, Fos, Myc. FAK: FAK/Src complex. Fyn: Fyn/Shc complex. Dsh: Dishevelled. Cyclin:CDKs1: Cyclin A:CDK1, Cyclin A:CDK2, Cyclin B:CDK1, Cyclin E:CDK2. Cyclin:CDKs2: Cyclin D:CDK4, Cyclin D:CDK6. CKIs: Cyclin-dependent kinase inhibitors (p16, p18, p19)
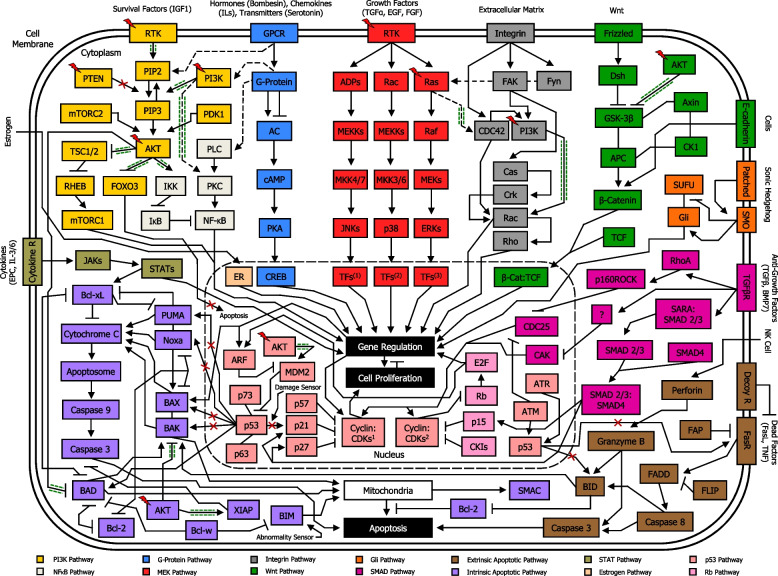
Fig. 9.
Simplified overview of PAM signaling pathway location among other major signal transduction pathways in the network circuit of a cancer cell. Each component of a signaling pathway is termed according to the role it plays with respect to the initial stimulus, such as ligands (e.g. IGF1), receptors (e.g. RTK), and effectors (e.g. PI3K). In cancer cells, mutations in RTK, Ras, PI3K, PTEN protein phosphatase, AKT, and/or other proto-oncogenes, may occur, resulting in PAM signaling pathway overexpression. This condition can lead to stronger AKT-induced inhibition of pro-apoptotic proteins such as FOXO, BAD, BAX, BAK, NOXA, PUMA, as well as enhanced activity of anti-apoptotic proteins such as XIAP, and amplified activity of mTORC1, IKKα, PKC, CDC42, Rac, GSKβ, and MDM2, with consequently uncontrolled survival, proliferation, growth and boosted metabolism. Signaling cross-talk between PAM pathway and other pathways play a major role in the dysregulation of functions in a cancer cell. PI3K pathway, NF-κB pathway, G-Protein pathway, MEK pathway, Integrin pathway, Wnt pathway, Gli pathway, SMAD pathway, extrinsic apoptotic pathway, intrinsic apoptotic pathway, STAT pathway, Estrogen pathway, p53 pathway, and Rb pathway are shown. Nuclear membrane is delimited by a long-dashed circular dotted line. Arrowhead lines: activation (phosphorylation or non-phosphorylation). Blocked lines: inhibition (phosphorylation or non-phosphorylation). Continuous lines: interaction. Dotted arrowhead lines: cross-talk activation (phosphorylation or non-phosphorylation) between pathways. Dotted blocked lines: cross-talk inhibition (phosphorylation or non-phosphorylation) between pathways. Red lightning symbol shows mutation for a particular gene in the PAM pathway. Red crosses: signaling blockage. Green dash-dotted lines (adjacent to arrowhead lines or blocked lines or dotted arrowhead lines): signaling enhancement. PI3K: PI3K/Ras complex. MEKs: MEK1/2. ERKs: ERK1/2. TFs1: Transcription factors Jun, ATF2, RNPK, p53, NFAT4, Shc. TFs2: Transcription factors CHOP, ATF2, MNK, MSK, MEF2, Elk-1. TFs3: Transcription factors Elk-1, Ets-2, RSK, MNK, MSK, cPLA2, Fos, Myc. FAK: FAK/Src complex. Fyn: Fyn/Shc complex. Dsh: Dishevelled. Cyclin:CDKs1: Cyclin A:CDK1, Cyclin A:CDK2, Cyclin B:CDK1, Cyclin E:CDK2. Cyclin:CDKs2: Cyclin D:CDK4, Cyclin D:CDK6. CKIs: Cyclin-dependent kinase inhibitors (p16, p18, p19)
Epigenetic regulation of PAM pathway in cancer
Accumulating evidence suggests that epigenetic alterations are as crucial as genetic mutations in dysregulating PAM signaling pathway in different types of cancer [181–184]. In particular, DNA methylation [185], histone post-translational modifications [186], and non-coding RNAs modulation [187, 188], are three main epigenetic mechanisms that affect PAM pathway and have been associated with cancer. Accordingly, loss of PTEN tumor suppressor gene expression due to aberrant promoter hypermethylation has been implicated in the development of gastric [189], colorectal [190], and endometrial cancers [191]. Similarly, promoter hypermethylation coupled with transcriptional silencing has been documented in loci coding for negative regulators of AKT, including tumor suppressor genes SCGB3A1 in NSCLC [192] and PPP2R2B in breast cancer [193], therefore contributing to cancer onset/progression. In turn, AKT hyperactivation in cancer cells brings further dysregulation in various epigenetic players participating in the PAM pathway, such as DNA maintenance methyltransferase DNMT1 [194], histone acetyltransferase CBP/p300 complex [195], histone H3K27 methyltransferase EZH2 [196], and histone H3K4 methyltransferase and demethylase KMT2D and KDM5A, respectively [31]. It could be argued that the imbalance in histone acetylation and methylation at chromatin loci regulated by the PAM pathway frequently occurs during cancer progression. Indeed, exposure of thyroid cancer cell lines to AKT inhibitor B2311 drastically reduces H3K9ac and H3K4me3 levels, both transcription activation marks; and increases levels of H3K27me3, a well-known transcription repression mark, at the promoter of tumor suppressor tshr gene, leading to downregulation of tshr [197]. The epigenetic signature of different cancers is also linked to the impact of multiple long and short noncoding RNAs in the PAM pathway. In fact, distinct miRNAs trigger PAM pathway by targeting PTEN mRNA, including miR-21 in gastric cancer [198], miR-20b and miR-301a-3p in esophageal carcinoma [199], and miR-421 in NSCLC [200]. Stepwise investigation reveales cooperative roles of distinct components of the epigenetic machinery to induce cancer cell proliferation through PAM pathway. For instance, altered chromatin topology and DNA methylation pattern at the imprinted IGF2-AS locus provoke significant decreased transcription of the long noncoding IGF-AS RNA in prostate [201] and CRC [202], as well as in cervical intraepithelial neoplasia [203]. Moreover, patients with low IGF2-AS abundance likely develop larger tumor size. In contrast, overtranscription of IGF2-AS inhibits breast cancer cell proliferation, thereby retarding tumor malignancy and progression in vivo. Mechanistically, IGF2-AS inhibits the expression of its sense-cognate igf2 gene in an epigenetic DNMT1-dependent manner, leading to the inactivation of downstream PAM pathway [204]. Thus, identification and understanding of epigenetic changes, such as DNA methylation, histone post-translational modifications, and non-coding RNA-mediated transcriptional silencing, occurring along the PAM pathway may hold a great promise for improving personalized medicine to a larger number of cancer patients.
Clinical developments in PAM inhibitors
PI3K Inhibitors
Along the PAM pathway PI3K is a major drug target for cancer treatment since its hyperactivity is remarkably correlated with human tumor progression, enhanced tumor microvessel formation, and increased number of invasive cancer cells [205]. A strenuous effort has been committed to improve inhibitors targeting PI3K signaling. Indeed, several pharmaceutical companies have developed drug inhibitors of PI3K during the last decades [206]. Notwithstanding some inhibitors have been approved by the Food and Drug Administration (FDA), there is still concern regarding the development of resistance, sensitivity markers, and toxicology [207]. Importantly, PI3K inhibitors are classified into three main groups: pan-PI3K inhibitors (pan-PI3Ki), isoform-specific PI3K inhibitors (IS PI3Ki), and dual PI3K/mTOR inhibitors (dual PI3K/mTORi) [208] (Fig. 10).
Fig. 10.
PI3K-, AKT-, mTOR- and PDK1-targeted inhibitors along the PAM pathway in cancer cells. PI3K inhibitors are divided into Pan-PI3K inhibitors (Pan-PI3Ki), Dual PI3K/mTOR inhibitors (Dual PI3K/mTORi), and Isoform-Specific PI3K inhibitors (IS PI3Ki), named Isoform-Specific PI3Kα inhibitors (IS PI3Kαi), Isoform-Specific PI3Kβ inhibitors (IS PI3Kβi), Isoform-Specific PI3Kγ inhibitors (IS PI3Kγi), and Isoform-Specific PI3Kδ inhibitors (IS PI3Kδi). Overall AKT inhibitors are referred as AKTi. mTOR inhibitors are divided into allosteric (non-competitive) mTOR inhibitors (A-NC mTORi), ATP-competitive mTOR inhibitors (ATP-C mTORi), and Bi-Steric mTOR inhibitors (Bi-Steric mTORi). PDK1 inhibitors are referred as PDK1i. Substrate activation (phosphorylation) along the PAM pathway is shown with arrowhead lines. Substrate inhibition along the PAM pathway, exerted by Pan-PI3Ki, Dual PI3K/mTORi, IS PI3Kαi, IS PI3Kβi, IS PI3Kγi, IS PI3Kδi, AKTi, A-NC mTORi, ATP-C mTORi, Bi-Steric mTORi, and PDK1i, is shown with blocked lines. Ⓐ: FDA-approved inhibitor
Pan-PI3K Inhibitors
Pan-PI3Ki suppress the cataytic activity of all four PI3K class I isoforms: PI3Kα, PI3Kβ, PI3Kγ, and PI3Kδ, encoded by PIK3CA, PIK3CB, PIK3CG, and PIK3CD, respectively. Thus, these drugs are normally effective in tumors producing high level of PIP3, regardless of the type of PI3K gene or PTEN alterations implicated. Potentially, pan-PI3Ki provide a broader range of activity by comprising numerous molecular targets, although exists increasing risk of on-target and off-target toxicity [209]. Several pan-PI3Ki have been in clinical development, but only copanlisib, a potent and selective agent with targeted activity predominantly against PI3K p110α and p110δ isoforms, has reached significant effectiveness in clinical trials [210]. Indeed, copanlisib has exhibited remarkable clinical benefits in patients with relapsed follicular lymphoma (FL) who have received ≥ 2 prior systemic therapies, and thus has been FDA-approved for use in this cohort (Supplementary information 2) (Supplementary figure 1). The safety profile of copanlisib in FL patients is favourable or acceptable. Treatment-related adverse events of any grade include hyperglycaemia, leukopenia, decreased energy, diarrhea, hypertension, neutropenia, nausea, thrombocytopenia, and infections. Serious adverse reactions comprise pneumonia (8%), pneumonitis (5%), and hyperglycaemia (5%) [211]. Clinical anticancer activity of copanlisib has been shown as monotherapy in hematological malignancies [212–215] and in advanced solid tumors [216, 217], as well as in combination with either ibrutinib [218], gemcitabine [219], bendamustine [220], rituximab [221], or rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone [220], in hematological malignancies, and in combination with either refametinib [222], gemcitabine [223], or gemcitabine + cisplatin [224], in advanced solid tumors. Besides, antineoplastic activity has been observed with other pan-PI3Ki including buparlisib as monotherapy/in combination in hematological malignancies and advanced solid tumors, and pictilisib, pilaralisib and sonolisib as monotherapy/in combination with other drugs in advanced solid tumors (Supplementary information 2) (Supplementary table 1).
Isoform-Specific PI3K Inhibitors
IS PI3Ki have been established to target cancer types dependent on either PI3Kα, PI3Kβ, PI3Kγ, or PI3Kδ isoforms. Conventionally, these drugs show a wider therapeutic index, and lesser off-target-based toxicologic effects due to the reduced expression of the diverse PI3K isoforms in non-cancerous cells. Notably, PI3Kα and PI3Kβ isoforms are ubiquitously expressed, whereas PI3Kγ and PI3Kδ isoforms are predominantly restrained to leukocytes [225]. Among all IS PI3Ki, only three have been approved by the FDA: alpelisib, duvelisib, and idelalisib. Alpelisib, a potent and specific drug with targeted efficacy against PI3Kα isoform, in combination with fulvestrant, has displayed significant clinical benefits in hormone receptor (HR) + /HER2- PIK3CA-mutated advanced or metastatic breast cancer patients, and as a result has been FDA-approved for use in this cohort (Supplementary information 2) (Supplementary figure 1). The safety profile of this combination in breast cancer patients is favourable or manageable. Grade 1–2 adverse events include hyperglycemia, diarrhea, rash, nausea, fatigue, diarrhea, anemia, stomatitis, reduced appetitie, vomiting, anorexia, and alopecia [226, 227]. Clinical antitumor activity of alpelisib has been shown as monotherapy in breast cancer [228] and other advanced solid tumors [229, 230], as well as in combination with either trastuzumab-emtansine [231], fulvestrant [232–235], letrozole [236–239], nab-paclitaxel [240], olaparib [241], or trastuzumab + LJM716 [242], in breast cancer, and in combination with either imatinib [243], BGJ398 [244], binimetinib [245], olaparib [246], cetuximab + intensity modulated radiation therapy [247], everolimus + exemestane [248], or cetuximab + encorafenib [249, 250], in advanced solid tumors. Duvelisib, a potent and selective agent with targeted efficacy against PI3Kγ and PI3Kδ isoforms, has exhibited remarkable clinical benefits in relapsed or refractory chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) patients after ≥ 2 prior therapies, and in relapsed or refractory FL patients after ≥ 2 prior systemic therapies, and thus has been FDA-approved for use in these cohorts (Supplementary information 2) (Supplementary figure 1). The safety profile of duvelisib in CLL, SLL, and FL patients is favourable or tolerable. The most common adverse events include diarrhea, neutropenia, pyrexia, anaemia, nausea, and cough. In CLL/SLL patients only, grade ≥ 3 immune-related toxicities consist of colitis (12%), pneumonitis (3%), increased alanine aminotransferase (ALT) (3%), and enhanced aspartate transferase (AST) (3%); in addition, the most frequently reported serious adverse event is pneumonia (15%) [251]. Clinical anticancer activity of duvelisib has been shown as monotherapy [252–258], as well as in combination with either rituximab + bendamustine [259], or fludarabine + cyclophosphamide + rituximab [260], in several hematological malignancies. Idelalisib, a specific drug with targeted efficacy against PI3Kδ isoform, has displayed significant clinical benefits as monotherapy in SLL and FL patients who have received ≥ 2 prior systemic therapies, and in combination with rituximab in CLL patients for whom rituximab singly would be considered appropriate therapy due to co-morbidities. As a result, idelalisib has been FDA-approved for use in these cohorts [261, 262] (Supplementary information 2) (Supplementary figure 1). The safety profile of idelalisib in SLL, FL and CLL patients is favourable or acceptable. The most frequent ≥ grade 3 adverse events after idelalisib monotherapy in SLL and FL patients include decreased neutrophils (25%), increased ALT (18%), pneumonia (16%), diarrhea (14%), and enchanced AST (12%) [261]; whereas the incidence of ≥ grade 3 adverse events after combination of idelalisib and rituximab in CLL patients comprise neutropenia (34%), thrombocytopenia (10%), anemia (5%), elevation in transaminases (5%), and diarrhea (4%) [262]. Clinical antitumor activity of idelalisib has been shown as monotherapy [263–269], in different hematological malignancies, and in combination with either rituximab [270–275], bendamustine [270], tirabrutinib [276], ofatumumab [277], or rituximab + bendamustine [270, 278], in chronic lymphocytic leukemia, as well as in combination with obinutuzumab in Waldenström’s macroglobulinemia [279]. Notably, dual inhibitor of PI3Kδ/casein kinase-1-ε umbralisib received its first FDA approval in 2021 for the treatment of relapsed or refractory marginal zone lymphoma (MZL) patients who had received ≥ 1 prior anti-CD20-based regimen, and relapsed or refractory FL patients who had received ≥ 3 prior lines of systemic therapy [280]. Nonetheless, due to safety concerns the FDA withdrew its approval in 2022. Several clinical studies have shown anticancer activity of umbralisib, both as monotherapy [281] and in combination [282], in different hematological malignancies. Besides, antineoplastic activity has been observed with other IS PI3Ki such as PI3Kαi taselisib and serabelisib as monotherapy/in combination with other drugs in advanced solid tumors, PI3Kβi GSK2636771 and AZD8186 as monotherapy/in combination with other agents in advanced solid tumors, PI3Kβ/δi acalisib as monotherapy in hematological malignancies, PI3Kγi eganelisib in combination in advanced solid tumors, PI3Kγ/δi tenalisib as monotherapy in hematological malignancies, PI3Kδi linperlisib as monotherapy in hematological malignancies, and PI3Kδi parsaclisib as monotherapy in hematological malignancies and in combination in advanced solid tumors (Supplementary information 2) (Supplementary table 1).
Dual PI3K/mTOR Inhibitors
Dual PI3K/mTORi are mostly effective against all PI3K isoforms, as well as mTORC1/mTORC2, leading to suppression of the three crucial intersections of the PAM signaling pathway. There are several ongoing clinical trials using dual PI3K/mTORi, including apitolisib, dactolisib and paxalisib as monotherapy in advanced solid tumors, gedatolisib and samotolisib as monotherapy/in combination with other drugs in advanced solid tumors, and voxtalisib monotherapy/in combination in hematological malignancies and advanced solid tumors. However, none of them has reached FDA approval (Supplementary information 2) (Supplementary table 1).
AKT Inhibitors
Numerous drugs can specifically inhibit AKT proteins, thereby impeding overactivation of downstream proteins in PAM signalling pathway. Indeed, there are several ongoing clinical trials using AKT inhibitors, such as capivasertib, ipatasertib and M2698 as monotherapy/in combination with other drugs in advanced solid tumors, afuresertib and perifosine as monotherapy/in combination with other agents in advanced solid tumors and in combination only in hematological malignancies, and MK-2206 as monotherapy/in combination in advanced solid tumors and hematological malignancies, and TAS-117 as monotherapy in advanced solid tumors. However, none of these agents has been approved by the FDA (Fig. 10). Notably, capivasertib, a potent and selective inhibitor of all three AKT isoforms [283], is the most prominent agent against AKT in advanced solid tumors, particularly in breast cancer [284]. Indeed, in the recent phase 3 CAPItello-291 study, combination of capivasertib and fulvestrant doubled PFS compared to fulvestrant singly in HR + , HER2- breast cancer patients who have developed resistance to aromatase inhibitors and CDK4/CDK6 inhibitors [285, 286]. Thus, capivasertib represents a new valuable treatment option for these patients and is expected to receive FDA approval in due time [227] (Supplementary information 2) (Supplementary table 2).
mTOR Inhibitors
mTOR inhibitors were the first PAM-targeting drugs to advance to the clinic [287]. Induction of mTORC1 enhances the formation of proteins, lipids, nucleotides, and decreases autophagy, resulting in cell survival, proliferation, and growth; whereas activation of mTORC2 regulates protein kinases, including AKT, leading to cell survival, and proliferation [288, 289]. Therefore, both mTORC1 and mTORC2 functions provide an important rationale for targeting mTOR complexes in tumor, although the effectiveness of some mTORC inhibitors may be compromised by a compensatory feedback loop leading to AKT activation [290]. There are three types of mTOR inhibitors: first-generation allosteric (non-competitive) mTOR inhibitors (allosteric mTORi), which inhibit mTORC1 only; second-generation ATP-competitive mTOR inhibitors (ATP-competitive mTORi), which suppress both mTORC1 and mTORC2, and third-generation bi-steric mTOR inhibitors (bi-steric mTORi), which inhibit mTORC1 only (Fig. 10).
Allosteric mTOR Inhibitors
Allosteric (non-competitive) mTORi act against mTORC1. Since allosteric inhibitors can only exert their function towards mTORC1 they cannot avoid the feedback loop-based induction of AKT determined by the suppression of mTORC1 [291]. Besides, allosteric mTORi can modestly reduce p4E-BP1 levels through the inhibition of (4E-BP1) phosphorylation, and consequently, cannot effectively restrain eIF4E-mediated cap-dependent translation initiation in cancer [161]. Therefore, these agents exert a weaker PAM signalling inhibition, compared to ATP-competitive mTORi, resulting in decreased antitumor activity. The first-generation allosteric mTORi includes rapamycin and its analogues, commonly known as rapalogs, which only exert a specifical inhibition towards mTORC1 [291]. Everolimus, a selective agent with targeted efficacy against mTORC1, has exhibited moderate clinical benefits in several types of cancers. Accordingly, everolimus has been FDA-approved as monotherapy in neuroendocrine tumor (NET) patients, TSC-associated SEGA patients, renal TSC-associated angiomyolipoma adult patients, and advanced-stage renal cell carcinoma (RCC) patients, as well as in combination with lenvatinib in advanced-stage RCC patients who have received a prior antiangiogenic therapy, and in combination with exemestane in postmenopausal HR + /HER2 breast cancer patients with recurrence or progression following prior therapy with letrozole or anastrozole (Supplementary information 2) (Supplementary figure 1). The safety profile of everolimus in NET, SEGA, angiomyolipoma, RCC, and breast cancer patients is acceptable. Grade 3–4 drug-related adverse events after everolimus monotherapy in NET patients include stomatitis (9%), diarrhoea (7%), infections (7%), anaemia (4%), fatigue (3%), and hyperglycaemia (3%). The most common grade 3–4 treatment-emergent adverse event after combination of everolimus and lenvatinib in RCC patients are constipation (37%) and diarrhea (20%) [4, 292]. Clinical anticancer activity of everolimus has been shown as monotherapy in advanced solid tumors [293–298], and in combination with either exemestane [299–301], letrozole [302], exemestane + ribociclib [303], exemestane + xentuzumab [304], letrozole + trastuzumab [305], or paclitaxel + trastuzumab [306], in breast cancer, as well as in combination with either ribociclib [307], lenvatinib [292], letrozole [308], paclitaxel [309], alpelisib + exemestane [248], or letrozole + metformin [310], in advanced solid tumors. Temsirolimus, an ester of sirolimus (rapamycin), is an intravenous-administered drug that establishes a complex with FKBP12, which is then integrated into mTORC1, but not mTORC2, thereby inhibiting mTORC1 [311], and consequently suppressing the generation of proteins implicated in cell cycle [312] and angiogenesis [313]. Temsirolimus has displayed significant clinical benefits as monotherapy in RCC patients, and thus has been FDA-approved for use in this cohort (Supplementary information 2) (Supplementary figure 1). The safety profile of temsirolimus in RCC patients is acceptable. The most common temsirolimus-induced grade 3–4 adverse reactions include hypertriglyceridemia (44%), anemia (20%), hypophosphatemia (18%), lymphocytopenia (16%), hyperglycemia (16%), asthenia (11%), dyspnea (9%), neutropenia (5%), rash (5%), and pain (5%) [314]. Clinical antitumor activity of temsirolimus has been shown as monotherapy in hematological malignancies [315, 316], and RCC [287], in combination with either lenalidomide [317], etoposide + cyclophosphamide [318], or rituximab + cladribine [319], in advanced solid tumors, and in combination with either perifosine [320], capecitabine [321], bevacizumab [322], or chemotherapy [323], in advanced solid tumors. Besides, antineoplastic activity has been observed with other agents such as nab-sirolimus as monotherapy in advanced solid tumors, as well as rapamycin and ridaforolimus as monotherapy/in combination with other drugs in advanced solid tumors (Supplementary information 2) (Supplementary table 3).
ATP-competitive mTOR inhibitors
ATP-competitive mTORi (also known as active-site mTORi) can act against both mTORC1 and mTORC2, avoiding the feedback loop-based induction of AKT determined by the suppression of mTORC1 [291]. Notably, ATP-competitive mTORi remarkably reduce p4E-BP1 levels through the inhibition of (4E-BP1) phosphorylation, and as a result, can effectively prevent eIF4E-mediated cap-dependent translation initiation in cancer. Hence, ATP-competitive mTORi actively promote dephosphorylation on Thr46 (4E-BP1), thereby re-establishing its endogenous functions of growth suppression and pro-apoptosis [161]. Therefore, these inhibitors induce a stronger PAM signalling inhibition compared to allosteric mTORi, leading to enhanced antitumor activity [291]. This difference is mainly due to the fact that allosteric mTORi, which represent the first-generation mTORi, suppress mTORC1 indirectly by binding to FKBP12; while ATP-competitive mTORi, which represents the second-generation mTORi, suppress both mTORC1 and mTORC2 by inhibiting the mTOR kinase directly [324]. In fact, in regard to allosteric mTORi, there is a catalytic cleft within the FKBP12-rapamycin-binding (FRB) domain of mTOR, enabling limited access to 4E-BP1 as a substrate; whereas ATP-competitive mTORi are able to bind deeper inside the catalytic cleft, thereby abolishing the capacity to phosphorylate 4E-BP1 [161]. There are several ongoing clinical trials using ATP-competitive mTORi including sapanisertib as monotherapy in hematological malignancies and advanced solid tumors, as well as in combination in advanced solid tumors, especially breast cancer, onatasertib and AZD8055 as monotherapy in advanced solid tumors, vistusertib monotherapy in hematological malignancies and in combination in advanced solid tumors, and OSI 027 monotherapy in hematological malignancies. However, mainly due to toxicity, none of them has reached approval by the FDA (Supplementary information 2) (Supplementary table 3).
Bi-steric mTOR inhibitors
Since the clinical benefits of ATP-competitive mTORi are obstacled by toxicity, a third-generation mTORi named bi-steric mTORi (also known as RapaLinks) that selectively inhibit mTORC1 and not mTORC2 has recently been designed [325]. These inhibitors, which contain a rapamycin-like core moiety covalently linked to an mTOR active-site inhibitor [326], are termed bi-steric due to their simultaneous engagement of the allosteric FRB domain and orthosteric catalytic domain of mTOR, in order to deepen the suppression of mTORC1 while also retaining selectivity for mTORC1 over mTORC2 [325]. Importantly, bi-steric mTORi such as RMC-4627, have shown potent and selective inhibition of 4E-BP1 phosphorylation leading to tumor regression in B-cell acute lymphoblastic leukemia xenografts [327] and breast cancer xenografts [325, 326]. Besides, bi-steric mTORi RMC-6272-mediated inhibition of mTORC1 in ER + /HER2- breast cancer has displayed significant efficacy in hormone therapy-resistant acquired patient-derived xenografts, and in patient-derived xenografts from CDK4/6 inhibitor-resistant patients [328]. Notably, these compounds cause less relief of AKT-dependent feedback inhibition of RTK expression, which notoriously results in RTK receptor reactivation-induced adaptive resistance, and toxicity in comparison to ATP-competitive mTORi. Also, bi-steric mTORi display a longer dwell-time on target compared to other types of mTORi, and thus can be regularly used in intermittent dosing schedules [327, 325]. Interestingly, bi-steric mTORi RMC-5552 [329] in combination with Ras inhibitors has exhibited clinical anticancer activity with a favourable safety profile in relapsed or refractory RAS-mutated solid tumor patients [330]. Thus, according to these pre-clinical and clinical data bi-steric mTORi can be considered a major candidate to be used as mTORi in future cancer treatments (Supplementary information 2) (Supplementary table 3).
PDK1 Inhibitors
PDK1, also known as PDPK1, is a crucial regulator of PAM signalling pathway due to its phosphorylation on AKT. Indeed, PDK1 can exert a potential role in developing chemoresistance in various types of malignancy [331]. Thus, it is reasonable to suggest that PDK1 inhibition, singly or in combination with other PAM inhibitors, could contribute to the enhancement of antitumor efficacy in different types of human cancer (Fig. 10). Several PDK1 inhibitors have shown great potential as anticancer drugs in vitro and in vivo, but none of them has yet reached the clinic. This is mainly due to the fact that PDK1 drug discovery efforts have been somehow obstracted by the remarkable attention given to other more characterized kinases such as PI3K, AKT and mTOR. Nevertheless, an increasing number of patent applications have reported on putative PDK1 inhibitors since its discovery [332] (Supplementary information 2).
Resistance
The PAM signaling pathway involves numerous feedback loops, compensatory pathways, and crosstalk nodes with other signal transduction axes that hamper the inhibition of PI3K, AKT, mTORC1, mTORC2, and PDK1 in cancer. Indeed, recent studies have reported that short administration of drug inhibitor therapies can determine feedback loop inductions that consecutively reduce the overall response rate (ORR). Additionally, chronic administration of inhibitor-based therapies can lead to the accumulation of slow-cycling cells that can possibly gain genetic mutation contributing to drug resistance [333]. The major mechanisms of resistance to PAM signalling-targeted inhibitors are revised below.
Mechanisms of resistance to PI3K Inhibitors
Even though PAM-targeted agents, particularly PI3K drug inhibitors, have demonstrated significant therapeutic activity in human cancer, acquired and intrinsic resistance has hindered their clinical efficacy [334–336]. Thus, a rationale for alternative clinical strategies could be provided by an accurate understanding of the biochemical mechanisms whereby resistance to PI3Ki occur. Various targeted therapies can induce several possible mechanisms of resistance to PI3Ki as described below.
RTK Reactivation
RTKs are often induced following treatment with specific inhibitors, and as a result, they promote the activation of the PAM and Ras/MEK/ERK signalling cascades [163, 337]. This feature is determined by the loss of the suppression that AKT exerts on mTORC1 and FOXO, leading to RTK transcription, and reinduction of these signal transduction axes [338, 163]. When the PAM signaling pathway is activated, AKT-induced phosphorylation and blockage of transcription factor FOXO in the cytoplasm reduces the activity of FOXO molecules, and consequently, ceases the induction of FOXO target genes related to the promotion of cell cycle arrest or apoptosis [339]. Concurrently, this mechanism hampers the capacity of FOXO to regularly transcribe different RTKs, representing an indirect feedback mechanism that limits extracellular stimuli-mediated induction of RTKs. Conversely, when PI3K is inhibited, AKT-induced FOXO phosphorylation is suppressed, enhancing the expression of FOXO in the nucleus, which leads to the stimulation of RTKs and partial restoration of PIP3 activity. Thus, PAM signaling induction cannot be thoroughly suppressed, since PIP3 level is preserved, resulting in cell proliferation.
PI3K p110β is the major PI3K isoform that drives PI3K-mediated signaling in PTEN-null cancers [340]. PI3K p110β inhibitor AZD8186 treatment of PTEN-null cells remarkably reduces PI3K signal transduction and cancer cell survival. Accordingly, specific inhibition of PI3K p110α in these tumor cells displays no effect since their type of cancer only depend on PI3K p110β signaling. Nevertheless, downregulation of AKT and mTOR in these tumor cells is transient, since mTOR downregulation leads to FOXO de-repression, and consequently, RTK transcription, resulting in PI3K p110α-induced AKT signal transduction cascade. Hence, targeted suppression with an IS PI3Ki (e.g. p110β) induces AKT and mTOR signaling due to the reinduction of the alternative PI3K isoform (e.g. p110α). Notably, this reciprocal activation has been successfully abrogated through the concurrent inhibition of PI3K p110α and PI3K p110β, leading to a more significant anticancer efficacy compared to monotherapy with either PI3K inhibitors [341].
Clinical studies with PI3Kα inhibitor alpelisib have provided evidence that activation of alternative signaling pathways may contribute to primary resistance or early emergence of resistance in cancer patients. Gene expression profile analysis of paired pre-treatment and on-treatment tumor samples, collected from patients treated with alpelisib as a single agent or in combination with an aromatase inhibitor, has shown that ESR1 and its target gene PGR are among the most highly induced genes upon PI3K inhibition. This supports the notion that ER mRNA increases during PI3K inhibition, and suggests that activation of this compensatory pathway may decrease antitumor efficacy [342]. Besides, recent studies have also demonstrated that ESR1 activating mutations expand in number and allele fraction after combination treatment of alpelisib and aromatase inhibitor in HR + metastatic breast cancer patients, and their presence is associated with resistance [239] Table 1.
Table 1.
Pre-existing and acquired mutations implicated in clinical resistance to PAM inhibitors
| Mutations implicated in clinical resistance to PAM inhibitors | ||||||
|---|---|---|---|---|---|---|
| PI3K Inhibitors | ||||||
| Agent | Type of mutations | Genes mutated | P/A prior to treatment | Type of resistance | Disease setting | References |
| Alpelisib | ESR1 activating mutations | ESR1 E380Q | P | Primary/Secondary | PIK3CA-mutated metastatic breast cancer | 239 |
| ESR1 L363Q | P | |||||
| ESR1 F461V | P | |||||
| ESR1 H524L | P | |||||
| ESR1 Y537N | P | |||||
| ESR1 Y537C | P | |||||
| ESR1 D538G | P | |||||
| Alpelisib | PTEN copy number loss and loss of function mutations | PTEN D97H | P | Primary/Secondary | PIK3CA-mutated metastatic breast cancer | 239, 351 |
| PTEN L108H | P | |||||
| PTEN A126S | P | |||||
| PTEN R130* | P | |||||
| PTEN M134I | A | |||||
| PTEN | A | |||||
| L139Nfs*3 | A | |||||
| PTEN T167P | A | |||||
| PTEN Q214R | A | |||||
| PTEN E242G | A | |||||
| PTEN S339fs | A | |||||
| K342_splice | A | |||||
| Idelalisib | PIK3R1 inactivating mutation | PIK3R1 | A | Secondary | CLL | 352 |
| Idelalisib | MAP2K1, BRAF and KRAS activating mutations | MAP2K1 Q56P | A | Primary | CLL | 361 |
| MAP2K1 | A | |||||
| E203K | A | |||||
| KRAS G13D | A | |||||
| KRAS Q22K* | A | |||||
| BRAF G469A | A | |||||
| BRAF | A | |||||
| N581_splice | A | |||||
| BRAF V600E | A | |||||
| BRAF K601E | A | |||||
| Idelalisib | BIRC3 inactivating mutation | BIRC3 | A | Secondary | CLL | 352 |
| mTOR Inhibitors | ||||||
| Agent | Type of mutations | Genes mutated | P/A prior to treatment | Type of resistance | Disease setting | References |
| Everolimus | Loss-of-binding/drug resistance mutation | MTOR F2108L | A | Secondary | Metastatic ATC | 375 |
This table includes mutations and copy number changes in primary patient samples following clinical treatment with PAM inhibitors. Expression level changes in clinical samples and resistance mutations generated in cell lines or ex vivo culture models are not included
A/P Mutation absent/present, P Present (mutation present prior to treatment), A Absent (mutation absent prior to treatment), ATC Anaplastic thyroid cancer
IGF1R upregulation has been identified as a potential resistance mechanism to PI3Kδ inhibition in CLL patients without activating MAPK pathway mutations. Indeed, IGF1R expression is elevated, both at baseline and at the time of disease progression, in RNA samples of patients who develop resistance to idelalisib compared to a set of previously untreated CLL samples. This indicates a potential role for IGF1R signalling in resistance to PI3Kδ inhibitors [343]. An expanded analysis of idelalisib-refractory patients, pooled from three clinical trials, has further demonstrated IGF1R overexpression in 87.5% of patients, whose paired RNA from treatment initiation and refractory time point were available [344]. Notably, this analysis has confirmed an enrichment for MAPK pathway variants in the primary refractory subset, further discussed below; whereas IGF1R upregulation was present at the point of secondary resistance. This emphasizes the importance of understanding the specific molecular pathways involved in different settings of resistance to define strategies to overcome treatment challenges.
Acquired mutation and amplification of PI3K genes
Acquired mutation and/or amplification of PIK3CA or PIK3CB, which often result in enhanced overall PI3K activity, are notorious to increase resistance to PI3K-targeted inhibitors [345, 346]. Notably, phosphorylation of PI3K regulatory subunit p85 also plays an important role in developing resistance against PI3K inhibitors. Besides, resistance to PI3K inhibition is also conferred by the existence of a regulatory loop between PI3K p85 and Src [347]. When PTEN is absent, cancer cell proliferation mainly depends on PI3K p110β isoform activity [348, 349]. PTEN loss singly is unable to generate resistance to pictilisib, a class I PI3K inhibitor; nevertheless, amphiregulin can significantly increase the resistance, leading to enhanced EGFR signaling [347]. Moreover, it has been shown that continuous mTORC1 activity positively correlates with intrinsic resistance to PI3K p110α inhibitors. In fact, growth factors including IGF1 and neuregulin 1 are known to induce mTOR, thereby mediating PI3K p110α inhibitor resistance [350].
Analysis of tumor biopsies from a PIK3CA-mutated metastatic breast cancer patient, enrolled on a study of alpelisib, has demonstrated the clinical relevance of progressive loss of PTEN expression and consequent gain of dependency on PI3K p110β isoform [351]. In line with this, evidence that dependence on PI3K p110β isoform is of clinical relevance has also emerged from the analysis of multiple tumor biopsies from a patient with PIK3CA-mutated metastatic breast cancer, enrolled in a human study of alpelisib. This patient first achieved a long clinical response and then suffered a relapse with new lung metastases. At the time of death, after metastatic sites were analyzed, all lesions displayed a copy loss of PTEN not present in the pre-treatment sample. Besides, metastases that progressed on therapy had additionally gained various PTEN alterations, which consequently, resulted in its loss of expression. The same mechanism has been identified in a longitudinal analysis of tumor and plasma circulating tumor DNA from patients treated with alpelisib and an aromatase inhibitor. Indeed, loss-of-function PTEN mutations were observed in 25% of patients with resistance [239] Table 1. Recently, an acquired mutation in the PIK3R1 gene has been reported in a CLL patient who became treatment-refractory to idelalisib after 4.4 years [352] Table 1.
Other mechanism of resistance
Other mechanism of resistance such as insulin signalling and PI3K reactivation [353, 354], altered cell metabolism [355, 356], interactions between PAM pathway and other pathways [357, 358], and cellular plasticity [359, 360] can contribute to drug tolerance (Supplementary information 3).
Constitutive mutational MAPK pathway activation has been identified as a clinical mechanisms of primary resistance in CLL. In a recent analysis of patient samples collected from trials involving various PI3K inhibitors, but mostly idelalisib, 60% of CLL patients who had no initial response to therapy have shown activating MAPK pathway mutations, which are specifically found in MAP2K1, KRAS and BRAF [361] Table 1. Recently, a study focusing on acquired resistance to idelalisib in CLL has revealed, among other alterations, the acquisition of a BIRC3 mutation, suggesting the activation of the NF-κB pathway as another potential bypass mechanism [352] Table 1. Also, there is emerging evidence for the role of secreted factors, primarily IL-6, in the development of resistance to PI3K inhibitors, with significant clinical relevance in primary and secondary resistance to idelalisib in MZL patients [362].
Mechanisms of resistance to AKT Inhibitors
Resistance to AKT inhibitors has been the object of several studies in oncology research [363, 364]. AKT inhibition has been reported to activate phosphorylation and expression of several RTKs, and these RTK signaling can lead to the attenuation of their anticancer activity in breast cancer cells, suggesting that combinatorial suppression of AKT activity and HER kinase activity exerts a more significant efficacy compared to mono-agent therapies [365]. Interestingly, AKTE17K mutation is considered a promising biomarker since clinical data report a correlation between the presence of this alteration and a response to AKT inhibitor capivasertib [366]. Importantly, upregulation of AKT3, which is epigenetically regulated by extra terminal domain proteins and bromodomain proteins, has been found to confer AKT inhibitor MK-2206 resistance in breast cancer, providing a rationale for developing future drug therapies targeting AKT3 in order to evade this acquired resistance [367]. Mutations that confer resistance to one allosteric AKT inhibitor do not necessarily determine resistance to other AKT allosteric inhibitors. In fact, clinically relevant activating AKT1Q79K mutation can confer resistance to miransertib, but not to MK-2206, further emphasising the importance of understanding AKT genotype for an appropriate treatment selection [368].
Mechanisms of resistance to mTORC Inhibitors
Various mechanisms are involved in the failure of first-generation allosteric mTORi [369]. Rapalogs are known to inhibit only some of the mTORC1 functions since they are FKBP12-dependent allosteric inhibitors. In addition, rapamycin generally exerts its activity against unstable mTORC1 substrates including ribosomal protein S6K, instead of stable substrates such as eIF4E-binding protein 4E-BP1, and consequently, only exerts a partial inhibition of the mTORC1-mediated protein synthesis [370]. Since 4E-BP1 is the substrate whereby mTORC1 regulates cell proliferation, the inadequate inhibition of this strong and stable molecule can be considered a plausible explanation of the modest antiproliferative efficacy of rapamycin on tumor cells [371, 372]. Another mechanism of escaping rapalogs inhibition is the compensatory activation of various signal transduction pathways due to mTORC1-induced suppression of negative feedback loops. The major effect is determined by PAM signaling activation, since rapalogs mitigate the mTORC1-induced negative feedback inhibition of insulin and IGF1 receptor mitogenic signalling [373]. Indeed, S6K activates phosphorylation of insulin receptor substrate 1 (IRS1), thereby leading to IRS1 suppression, or IRS1 degradation, or IRS1 binding to IGF1 receptor. Thus, rapamycin treatment enhances the level of IRS1 and induces the PAM axis by insulin and IGF1 receptor signaling [374]. Further studies have described other mechanisms involved in the failure of allosteric mTORi (Supplementary information 3).
Clinically, allosteric mTOR inhibitors have shown activity in various malignancies with mTOR-pathway activating mutations, and in some cases additional mutations within the pathway have been linked to acquired resistance. For instance, a patient with metastatic anaplastic thyroid cancer who had an exceptional response to everolimus lasting 18 months prior to treatment was found to have a nonsense mutation in the tumor suppressor gene TSC2, leading to mTOR pathway activation, and at progression acquired a mutation in MTOR that conferred resistance to allosteric mTOR inhibition. Importantly, the acquired MTOR mutation did not confer resistance to ATP-competitive mTOR inhibitors [375] Table 1. Several studies have demonstrated an enrichment for mTOR pathway mutations in responders or exceptional responders to these agents. Absence of mTOR pathway dependence may conversely be considered a mechanism of primary resistance. Examples include a study of rapalog-treated metastatic RCC patients, which identified an enrichment for MTOR, TSC1 and TSC2 mutations in responders [376], a report of a single exceptional responder to pazopanib and everolimus with urothelial carcinoma who was found to have two activating mutations in MTOR [377], and two studies of patients with perivascular epithelioid cell tumors (PEComas) who responded to sirolimus, some of which demonstrated loss of TSC1/TSC2 or TSC2 aberrations [378, 379]. As a counterpoint it should be noted that a substantial proportion of responders in some of the above-mentioned studies [376] had no identifiable alterations in mTOR pathway genes. Likewise, in the setting of postmenopausal nonsteroidal aromatase inhibitor-resistant ER + HER2- breast cancer patients treated with everolimus and exemestane, comprehensive sequencing analysis did not identify predictive biomarkers, although patients with PIK3CA exon 9 mutations experienced a quantitative benefit compared to patients with PIK3CA exon 20 mutations [380].
Several mechanisms of resistance can hamper the efficiency of second-generation ATP-competitive mTORi [369]. Some kinase domain mutations, including the methionine 2327 isoleucine substitution (M2327I), enhance mTOR catalytic activity, and therefore, both mTORC1/mTORC2 activeness. Consequently, concentration of ATP-competitive mTORi (e.g. AZD8055) necessary to suppress mTORC1 and mTORC2 substrates must be higher, compared to those required for wild-type mTOR kinase [326]. Tumor cells can also gain resistance to ATP-competitive mTORi by exerting downregulation of 4E-BPs, such as EIF4E-BP1, and EIF4E-BP2. This results in an enhanced EIF4E/4E-BPs ratio, which limits the suppressive action of these agents on the translation of EIF4E-susceptible mRNAs, and thus, decreases their anticancer efficacy [381, 382]. Interestingly, there is an inverse correlation between 4E-BP1 expression and Snail level in tumor cell lines and in clinical biospecimens. Indeed, Snail can suppress 4E-BP1 transcription by binding to three E-boxes located in the human 4E-BP1 promoter. Accordingly, ectopic expression of Snail in tumor cell lines remarkably inhibits 4E-BP1 expression, and consequently, reduces the anti-proliferative effect of ATP-competitive mTORi [383]. Also, ATP-competitive mTORi are more efficacious than rapalogs in numerous tumor models since they can act against both mTORC1 and mTORC2, avoiding the feedback loop-based induction of AKT caused by the suppression of mTORC1 [373]. Nevertheless, ATP-competitive mTORi induce overactivation of the Ras/MEK/ERK pathway through PI3K-independent feedback loops. Indeed, in multiple myeloma cells, torkinib induces ERK through an mTORC1/eIF-4E/Raf pathway [384]. More studies have reported different mechanisms involved in escaping ATP-competitive mTORi (Supplementary information 3). Interestingly, third-generation bi-steric mTORi can drive cancer regression more significantly than ATP-competitive mTORi, due to their potent and selective inhibition of 4E-BP1 phosphorylation, while minimizing adaptive resistance due to the relief of feedback inhibition of RTK expression and signaling, as well as undesirable toxicity and glucose intolerance [369]. Thus, bi-steric mTORi may be useful for treating cancers that are driven by activated mTORC1 [325, 327].
PAM signaling in immunology and immunotherapy
PAM signaling has emerged as a dominant regulator of the immune response. Indeed, PAM pathway-mediated control of the immune system is finely-tuned, allowing for precise mobilization or suppression of immune cell subsets through tightly-regulated signalings. The role of the PAM axis in immunoregulation and the possible roles for PAM-directed therapies in combination with immunotherapy are outlined below.
Effects of PAM signaling on the immune system and tumor microenvironment
PAM signaling plays crucial roles in immune cell maturation, differentiation, recruitment, and survival. However, each layer of the PAM signaling axis exerts its own unique control over the constituent subsets of the immune system.
PI3K, especially in the leukocyte-predominant PI3Kδ and PI3Kγ isoforms, is an important regulator of immune homeostasis. PI3Kδ is unique among the PI3K isoforms in its near-monopoly over B-cell receptor signaling, more specifically in CLL and mantle cell lymphoma (MCL). Several studies have shown that, in explanted human regulatory T-cells (Tregs), murine Tregs, and murine models of inactivated PI3Kδ, immunosuppressive Tregs are exquisitely reliant on PI3Kδ [385–387]. Consequently, inhibition of PI3Kδ can suppress the innate and adaptive immune system, predisposing patients to serious infections, including Pneumocystis jirovencii and cytomegalovirus. Accordingly, the observation that immunosuppressive Tregs are also uniquely dependent on PI3Kδ may begin to explain why autoimmune pneumonitis and colitis often accompany PI3Kδ inhibition [388]. Like PI3Kδ, the PI3Kγ isoform is active in lymphocytes. PI3Kγ and its regulatory subunits PIK3R5 and PIK3R6 are uniquely overexpressed within the myeloid compartment, where PI3Kγ serves as the principal catalytic PI3K [389]. Direct inhibition of PI3Kγ has been shown to diminish the immunosuppressive myeloid phenotype, notably shifting myeloid cells away from the immunosuppressive M2-like phenotype and towards the immunostimulatory M1-like phenotype [389–391]. Both PI3Kδ and PI3Kγ have also been implicated in the activity of myeloid-derived suppressor cells (MDSCs), and blocking the two isoforms impairs MDSC immunosuppression [392].
AKT plays key roles in both innate and adaptive immunity. In particular, T-cell receptor (TCR) signaling promotes T-cell survival and cell cycle progression through the activation of AKT-dependent transcriptional programs [393]. AKT1/2 promotes terminal differentiation of CD8 + T-cells and blunts the development of central memory and effector memory CD8 + T-cell populations [394, 395]. Additionally, AKT is also important in CD4 + T-cells, where it guides T helper (Th) cell differentiation in an isoform-specific manner [396]. T-cell activation via TCR and CD28 co-stimulation promotes downstream induction of T helper type 1 (Th1) cell-mediated cytokines IL-2 and IFN-γ in an AKT-dependent manner. Notably, regulation of T helper type 2 (Th2) cell-induced cytokines IL-4 and IL-5 is AKT-independent. AKT has also been shown to control Treg homeostasis through inhibitory phosphorylation of FOXO1, which in turn promotes Treg suppression [397]. Titration of this signaling axis modulates immune tolerance, implying that upstream inhibition of PI3K may disrupt tumor immune tolerance [398]. Additionally, it has recently been shown that inhibitory signaling through PD-1 restricts activation of Treg-mediated immunosuppression in an AKT-dependent manner [399]. Moreover, AKT is also known to modify myeloid cell fate and survival through its regulation of β-catenin, NF-κB signaling, and cytokine production [400]. Furthermore, isoform-dependent AKT signaling is a key determinant of M1/M2 macrophage polarization [401, 402], and AKT is required for the maturation of bone marrow-derived dendritic cells [403]. mTOR-mediated regulation of the immune system is complex and is comprehensively reviewed elsewhere [404, 405]. mTOR is the catalytic domain of the complementary mTORC1 and mTORC2 complexes. These complexes co-regulate cellular metabolism, the primary mechanism by which mTOR regulates the immune system, as mobilizing immune cells is energetically expensive and requires metabolic reconfiguration. mTORC1, in particular, has been shown to regulate memory T-cell differentiation [406] and establish the function of immunosuppressive Tregs [407]. Therefore, even though rapamycin-induced inhibition of mTORC1 promotes Treg development from naïve T-cells [408] and subsequent Treg expansion [409, 410], rapamycin can still promote autoimmunity through the suppression of Treg function [407]. The role of mTORC2 is more subtle, but it has been shown to play an inhibitory role in the generation of CD8 + memory T-cells, driven by mTORC2-induced inhibition of FOXO-mediated IL-15R expression [411]. Importantly, mTORC1 and mTORC2 are also directly involved in regulating innate immunity. mTORC1 controls monocyte/macrophage-mediated inflammation by regulating production of inflammatory cytokines via inhibition of NF-κB (downstream PI3Kγ) [412]. Again, mTORC2 plays a complementary role, modifying chemotaxis in mast cells and neutrophils. Besides, mTORC2 downregulates IL-12 in dendritic cells, due to inhibition of FOXO1 [413], and plays an important role in IL-4-dependent, alternative activation of M2 macrophages [414, 415]. Cancer cell-autonomous activation of PAM signaling has been shown to promote expression of immunosuppressive cytokines, chemokines, and immune checkpoints, which can be reversed through pharmacologic inhibition of PAM signaling [416]. mTOR regulates the expression of inflammatory cytokines such as IL-10, IL-12, TGF-β, and TNF [417, 418]. Activation of neoplastic PAM signaling also promotes expression of vascular endothelial growth factor (VEGF), an important mediator of angiogenic signaling that also acts as a chemoattractant of immunosuppressive MDSCs [419] and Tregs [420]. Reciprocally, inhibition of mTOR with everolimus, has been shown to partially phenocopy the anti-angiogenic effect of VEGF inhibitors [421]. Moreover, due to the role of PAM and especially mTOR in metabolic regulation, PAM inhibition within nutrient-constrained tumor microenvironments can disrupt the balance between CD4 + /CD8 + T-cells, Tregs/T helper 17 (Th17) cells, and M1/M2 macrophages [417]. Finally, many studies have associated activated PAM signaling with increased PD-L1 expression [422, 423], and PAM inhibition has been shown to be capable of reducing PD-L1 expression in PTEN-driven breast and CRC cells [424].
Targeting PAM Signaling alongside immunotherapy
Over the last decade, the treatment of cancer has been revolutionized by the introduction of immunotherapies. New agents that target the PD-1/PD-L1 and CTLA-4 immune checkpoints, known as immune checkpoint inhibitors (ICIs), and cellular therapies designed to seek out cancer cells, have demonstrated remarkable efficacy. Yet, despite these successes, many cancers do not respond or respond incompletely to immunotherapy, underscoring the importance of better understanding the mechanisms underlying the resistance to these agents.
Immune checkpoint inhibitors (ICIs)
Many of the mechanisms that underlie ICI treatment failure, including defective neoantigen presentation, T-cell resistance, excess immunosuppressive cytokines, and intratumoral inhibitory MDSCs or Tregs [425], are associated with PAM signaling; and thus, may be reversible with PAM inhibition. Indeed, there is increasing evidence that targeting the PAM pathway can reduce the production of immunosuppressive cytokines and restrict the proliferation and penetration of immunosuppressive Tregs and MDSCs, thereby boosting the anti-tumor effects of immune checkpoint blockade [389, 426, 427]. Activation of oncogenic pathways like PAM and MAPK promote transcriptional upregulation of neoplastic PD-L1. Accordingly, therapies targeting PAM and upstream kinase signaling have been shown to reduce intrinsic neoplastic PD-L1 expression. In PTEN-driven murine models of melanoma, the efficacy of both anti-PD-1 and anti-CTLA-4 antibodies has been improved by co-inhibition of PI3K [427]. In syngeneic and genetically-engineered mouse models of lung cancer, inhibition of mTOR bolsters the effects of PD-1 blockade [422]. Similar results have been reported with anti-PD-1 and anti-CTLA-4 therapies in breast cancer [428]. Combining PAM inhibition with ICI treatment decreases Treg populations and enhances the CD8 + memory T-cell response in murine models and in vitro patient cell assays [429]. Many early-phase clinical studies have been testing the aforementioned principles by assessing the tolerability and efficacy of combined PAM inhibition and immune checkpoint inhibition, primarily in advanced solid tumors, and lymphomas [430, 206]. Some of these clinical trials are highlighted below. Phase 1/2 clinical trial NCT03131908 is studying the combination of PD-1 inhibitor pembrolizumab and PI3Kβ inhibitor GSK2636771 in PD-1 refractory patients with metastatic melanoma and PTEN loss [431]. Besides, phase 1/2 clinical trial NCT04688658 is focussing on the combination of PD-1 inhibitor nivolumab and PI3Kδγ inhibitor duvelisib in patients with unresectable melanoma that have already progressed on PD-1 therapy, with clinical response in at least one patient in preliminary results [432]. Moreover, phase 2 clinical trial NCT03484819 is evaluating the combination of nivolumab and PI3Kαδ inhibitor copanlisib in relapsed/refractory diffuse large B-cell lymphoma or primary mediastinal large B-cell lymphoma. This clinical study is also trying to characterize the effects of this combination on the tumor microenvironment and immune response [433]. Furthermore, phase 1 clinical trial (NCT03884998) is investigating the the combination of nivolumab and pan-PI3K inhibitor copanlisib in patients with Richter’s transformation, with to date a favorable safety profile [434].
Chimeric antigen receptor (CAR) T-cell therapy
Another emerging pillar of immunotherapy is cellular therapy, particularly chimeric antigen receptor (CAR) T-cell therapy, where synthetically-engineered receptors direct pre-harvested T-cells to cancer cells expressing a select antigen. There is evidence that PAM-directed therapies could serve as a novel modifier to CAR-T cell therapy, possibly at the point of treatment [435], but primarily as a cell culture additive during T-cell expansion [430, 436]. Mechanistically, it is reported that anti-CD3/CD28 stimulation of T-cell receptor during CAR T-cell manufacturing process induces PAM signaling, resulting in terminal T-cell differentiation and decreased CAR T-cell persistence [430]. Earlier studies associate PAM activation with CAR T-cell decreased persistence and showed that PI3K inhibition with the non-selective PI3K inhibitor LY294002 was able to restrict CAR T-cell differentiation. This enables the maintenance of a less-differentiated state and ultimately improving persistence in vivo [437]. Subsequently, other groups have shown that targeted PI3K inhibition, focusing on the PI3Kγ and PI3Kδ isoforms, is capable of decreasing the expression of T-cell exhaustion markers PD-1 and Tim-3 while upregulating the lymph node homing marker CD62L [438], decreasing the expression of immune checkpoints, and increasing the yield of T-stem cell memory and central memory CD8 + CAR T-cells [439, 440]. These initial observations in CAR T-cell therapy may also be applicable to the growing field of bispecific antibodies, some of which have recently been approved for the treatment of B-cell non-Hodgkin's lymphomas.
Conclusion
PI3K inhibition is considered a major target for antitumor therapy. Unfortunately, past and present clinical trials using PI3Ki have exhibited modest anticancer activity mainly due to resistance to PI3K inhibition and insufficient target inhibition at tolerated doses. Indeed, over the last few decades, pan-PI3Ki and dual PI3K/mTORi, not only have demonstrated limited efficacy but also are associated with significant side effects. As a result, few PI3Ki have been approved by the FDA. Since selective isoforms of PI3K perform pivotal functions in the biology of specific cancer subtypes, the present-day focus is centered on the development of IS PI3Ki. Of late, IS PI3Ki have shown promising results in several clinical trials, largely exhibiting better target specificity and toxicity profiles as compared to conventional pan-PI3Ki and/or dual PI3K/mTORi. PAM pathway inhibitors have produced limited therapeutic efficacy and significant treatment-related toxicity particularly when combined with standard treatments or other targeted agents. Hence, most PAM pathway inhibitors are unsuitable for adoption as mainstream oncological treatment strategies. For this reason, combination therapy with two or more agents in conjunction with other therapeutic strategies, such as surgery, hormonal therapy, and other antitumor drugs, has been the trend in recent years. In line with this, combination of PI3Ki with ncRNAs, or inhibitors of crucial signaling nodes in other cross-interacting pathways, may represent a future approach to effectively suppress the PAM pathway, whilst minimizing the risk of developing drug resistance and occurrence of adverse events. In future studies it would be ideal to identify stable biomarkers for patient stratification, according to cancer types and genetic profiles, to better benefit from PI3K inhibition. The mechanism exerted by PI3Ki has not been thoroughly elucidated, and will require further studies to properly delineate its pros and cons as part of the endeavour to personalize oncological therapeutics. The design of novel PI3K inhibitors structurally-based on different binding sites would undoubtedly improve specificity, and reduce toxicity of IS PI3Ki. Therefore, demystifying the mechanisms of PI3K signaling and PI3K inhibition is essential to improve combination strategy, and patient selection, which would in turn enhance the efficacy of these cancer drugs.
Inhibition of AKT can potentially exert remarkable antineoplastic effects, although dose-limiting toxicity and insufficient knowledge of the different isoforms have impeded its successful pharmacological usage. Indeed, pharmacodynamic markers of AKT inhibitors have shown incomplete target modulation. Nowadays, there are no approved biomarkers that can predict therapeutic responses to specific AKT inhibitors before using a particular agent. Thus, it is not possible to predict which patients can have adverse effects from AKT inhibition. Targeting AKT is considered a critical research area in clinical oncology, and possibly, in future studies, AKT inhibitors in combination with synergistic cytotoxic drugs could probably improve their clinical efficacy.
Several classes of mTORi have been developed, but only everolimus and temsirolimus have obtained approval as therapy for human tumors. The clinical application of these agents is impeded by resistance determined through a variety of molecular mechanisms common to the different class of drugs, suggesting that co-targeting alternative pathways may be a more feasible strategy than improving the action of mTORi. Importantly, pre-clinical and clinical data suggest that novel bi-steric mTORi can drive cancer regression more significantly compared to other mTORi, by enhancing inhibition of 4E-BP1 phosphorylation, and reducing adaptive resistance due to the relief of feedback inhibition of RTK expression. Thus, bi-steric mTORi may be the most promising inhibitors in treating activated mTORC1-driven cancers. Also, developing novel therapeutic combinations could lead to the identification of molecular factors resistant to each class of drugs to improve the selection of patients who may benefit from a specific treatment. Besides, optimisation of treatment sequence using different agents may be useful to delay or overcome resistance to mTOR inhibitors.
Additionally, PAM signaling exert a fine control over the immune system-mediated functions, including antitumor response. Ongoing studies are evaluating the impact of PAM-targeted treatments on cancer response, immune reaction and their synergy with immune-based cancer therapies.
The potential of PAM inhibitors clearly depends on the combinatorial strategies. However, the main difficulties are: 1) defining the ideal combination for each patient, and 2) managing toxicity of combination approaches that necessitate innovative scheduling. In addition, the biomarker development is challenged by the complex interactions of the pathway and in general multiple co-existing alterations that help to maintain cancer survial. Finally, we must also improve our understanding of the effects determined by PAM signaling pathway inhibitors on the tumour microenvironment for plausible drug combinations with specific immunotherapies.
Supplementary Information
Additional file 1: Supplementary figure 1.
Additional file 2: Supplementary information 1.
Additional file 3: Supplementary information 2.
Additional file 4: Supplementary information 3.
Additional file 5: Supplementary table 1.
Additional file 6: Supplementary table 2.
Additional file 7: Supplementary table 3.
Acknowledgements
H.E. is supported by a NUS Medicine Postdoctoral Fellowship funded by the Ministry of Education (MOE) and the Social Sciences Research Council (SSRC) of Singapore. D.B.L.T. is supported by grants from the Singapore National Medical Council (A-0006179-00-00—NMRC OF YIRG) and from the Singapore Ministry of Education (A-0006237-00-00—MOE GAP Funding). BG is supported by the Medecin-Chercheur Parrainage program of Gustave Roussy Cancer Center. The mTOR inhibitor research in the laboratory of D.A.F. is supported by a Reach Grant from Alex’s Lemonade Stand Foundation.
Abbreviations
- CAR
Chimeric antigen receptor
- CLL
Chronic lymphocytic leukemia
- CRC
Colorectal cancer
- EGFR
Epidermal growth factor receptor (ERBB)
- EMT
Epithelial-mesenchymal transition
- ER
Estrogen receptor
- FDA
Food and Drug Administration
- FGFR
Fibroblast growth factor receptor
- FRB
FKBP12-rapamycin-binding
- HGF
Hepatocyte growth factor
- HNSCC
Head and neck squamous cell cancer
- HR
Hormone receptor
- ICIs
Immune checkpoint inhibitors
- IGF1
Insulin-like growth factor 1
- INSR
Insulin receptor
- IRS1
Insulin receptor substrate 1
- LTK
Leukocyte receptor tyrosine kinase
- MCL
Mantle cell lymphoma
- MDSCs
Myeloid-derived suppressor cells
- MCL
Mantle cell lymphoma
- MZL
Marginal zone lymphoma
- NET
Neuroendocrine tumor
- NSCLC
Non-small-cell lung cancer
- ORR
Overall response rate
- PAM
PI3K/AKT/mTOR
- PDGFR
Platelet-derived growth factor receptor
- RCC
Renal cell carcinoma
- SCLC
Small-cell lung cancer
- SEGA
Subependymal giant cell astrocytoma
- SLL
Small lymphocytic lymphoma
- TCR
T-cell receptor
- Tregs
Regulatory T-cells
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
Authors’ contributions
A.G and A.P.K. conceptualized the manuscript topic; A.G., J.S., D.A.F., and A.P.K. collated literature for the manuscript; A.G. wrote the manuscript; A.S.C.F., H.Y.L., K.C.H.Y., W.J., R.H.J., H.E., M.G.N., P.M., B.G., M.H.K., R.D.B., J.S.P., D.C., C.P., D.B.L.T., G.S., V.C., K.H.L., N.R.J.S, E.T., M.S.D., J.R.B., P.D., J.S., D.A.F., and A.P.K. edited the manuscript; A.G. designed the figures; A.G., E.T., M.S.D., J.R.B., P.D., J.S., D.A.F and A.P.K. finalized the manuscript. All authors have read and agreed to the submitted version of the manuscript.
Funding
A.P.K. was supported by a grant from the Singapore Ministry of Education (MOE-T2EP30120-0016).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/1/2023
Supplementary information 2 should be updated.
References
- 1.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian LY, Smit DJ, Jücker M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int J Mol Sci. 2023;24(3):2652. [DOI] [PMC free article] [PubMed]
- 3.Ahmad I, Hoque M, Alam SSM, Zughaibi TA, Tabrez S. Curcumin and Plumbagin Synergistically Target the PI3K/Akt/mTOR Pathway: A Prospective Role in Cancer Treatment. Int J Mol Sci. 2023;24(7):6651. [DOI] [PMC free article] [PubMed]
- 4.Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol. 2018;15(5):273–291. doi: 10.1038/nrclinonc.2018.28. [DOI] [PubMed] [Google Scholar]
- 5.Mohan CD, Srinivasa V, Rangappa S, Mervin L, Mohan S, Paricharak S, et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE. 2016;11(4):e0153155. doi: 10.1371/journal.pone.0153155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JH, Chinnathambi A, Alharbi SA, Shair OHM, Sethi G, Ahn KS. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol Res. 2019;150:104504. doi: 10.1016/j.phrs.2019.104504. [DOI] [PubMed] [Google Scholar]
- 7.Tewari D, Patni P, Bishayee A, Sah AN. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: A novel therapeutic strategy. Semin Cancer Biol. 2022;80:1–17. doi: 10.1016/j.semcancer.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20(2):74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang MH, Lee JH, Ko JH, Jung SH, Sethi G, Ahn KS. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules. 2019;24(8):1584. [DOI] [PMC free article] [PubMed]
- 10.Siveen KS, Ahn KS, Ong TH, Shanmugam MK, Li F, Yap WN, et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget. 2014;5(7):1897–1911. doi: 10.18632/oncotarget.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4(12):988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Kim C, Um JY, Sethi G, Ahn KS. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers (Basel). 2019;11(2):254. [DOI] [PMC free article] [PubMed]
- 13.Ong PS, Wang LZ, Dai X, Tseng SH, Loo SJ, Sethi G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front Pharmacol. 2016;7:395. doi: 10.3389/fphar.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46(6):372–383. doi: 10.3109/07853890.2014.912836. [DOI] [PubMed] [Google Scholar]
- 15.Lawrence MS, Stojanov P, Mermel CH, Robinson JT, Garraway LA, Golub TR, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505(7484):495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turdo A, D'Accardo C, Glaviano A, Porcelli G, Colarossi C, Colarossi L, et al. Targeting Phosphatases and Kinases: How to Checkmate Cancer. Front Cell Dev Biol. 2021;9:690306. doi: 10.3389/fcell.2021.690306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yuan Y, Long H, Zhou Z, Fu Y, Jiang B. PI3K-AKT-Targeting Breast Cancer Treatments: Natural Products and Synthetic Compounds. Biomolecules. 2023;13(1):93. [DOI] [PMC free article] [PubMed]
- 18.Zhu K, Wu Y, He P, Fan Y, Zhong X, Zheng H, et al. PI3K/AKT/mTOR-Targeted Therapy for Breast Cancer. Cells. 2022;11(16):2508. [DOI] [PMC free article] [PubMed]
- 19.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 20.Yu L, Wei J, Liu P. Attacking the PI3K/Akt/mTOR signaling pathway for targeted therapeutic treatment in human cancer. Semin Cancer Biol. 2022;85:69–94. doi: 10.1016/j.semcancer.2021.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Zhou BP, Hu MC, Miller SA, Yu Z, Xia W, Lin SY, et al. HER-2/neu blocks tumor necrosis factor-induced apoptosis via the Akt/NF-kappaB pathway. J Biol Chem. 2000;275(11):8027–8031. doi: 10.1074/jbc.275.11.8027. [DOI] [PubMed] [Google Scholar]
- 22.Dillon RL, White DE, Muller WJ. The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene. 2007;26(9):1338–1345. doi: 10.1038/sj.onc.1210202. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Kwok-Shing Ng P, Kucherlapati M, Chen F, Liu Y, Tsang YH, et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell. 2017;31(6):820–32.e3. doi: 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko JH, Um JY, Lee SG, Yang WM, Sethi G, Ahn KS. Conditioned media from adipocytes promote proliferation, migration, and invasion in melanoma and colorectal cancer cells. J Cell Physiol. 2019;234(10):18249–18261. doi: 10.1002/jcp.28456. [DOI] [PubMed] [Google Scholar]
- 25.Ashrafizadeh M, Zarrabi A, Hushmandi K, Kalantari M, Mohammadinejad R, Javaheri T, et al. Association of the Epithelial-Mesenchymal Transition (EMT) with Cisplatin Resistance. Int J Mol Sci. 2020;21(11):4002. [DOI] [PMC free article] [PubMed]
- 26.Deng J, Bai X, Feng X, Ni J, Beretov J, Graham P, et al. Inhibition of PI3K/Akt/mTOR signaling pathway alleviates ovarian cancer chemoresistance through reversing epithelial-mesenchymal transition and decreasing cancer stem cell marker expression. BMC Cancer. 2019;19(1):618. doi: 10.1186/s12885-019-5824-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Y, Wang Y, Zhou C, Mei W, Zeng C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front Oncol. 2022;12:819128. doi: 10.3389/fonc.2022.819128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in Cancer: Impact on Tumor Cells, Their Protective Stroma, Angiogenesis, and Immunotherapy. Cancer Discov. 2016;6(10):1090–1105. doi: 10.1158/2159-8290.CD-16-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanhaesebroeck B, Perry MWD, Brown JR, André F, Okkenhaug K. PI3K inhibitors are finally coming of age. Nat Rev Drug Discov. 2021;20(10):741–769. doi: 10.1038/s41573-021-00209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castel P, Toska E, Engelman JA, Scaltriti M. The present and future of PI3K inhibitors for cancer therapy. Nat Cancer. 2021;2(6):587–597. doi: 10.1038/s43018-021-00218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol. 2019;30(Suppl_10):x3–x11. doi: 10.1093/annonc/mdz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasan N, Cantley LC. At a crossroads: how to translate the roles of PI3K in oncogenic and metabolic signalling into improvements in cancer therapy. Nat Rev Clin Oncol. 2022;19(7):471–485. doi: 10.1038/s41571-022-00633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 34.Law NC, White MF, Hunzicker-Dunn ME. G protein-coupled receptors (GPCRs) That Signal via Protein Kinase A (PKA) Cross-talk at Insulin Receptor Substrate 1 (IRS1) to Activate the phosphatidylinositol 3-kinase (PI3K)/AKT Pathway. J Biol Chem. 2016;291(53):27160–27169. doi: 10.1074/jbc.M116.763235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol. 2010;11(5):329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 36.Lyu H, Han A, Polsdofer E, Liu S, Liu B. Understanding the biology of HER3 receptor as a therapeutic target in human cancer. Acta Pharm Sin B. 2018;8(4):503–510. doi: 10.1016/j.apsb.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidorov M, Dighe P, Woo RWL, Rodriguez-Brotons A, Chen M, Ice RJ, et al. Dual Targeting of EGFR and MTOR Pathways Inhibits Glioblastoma Growth by Modulating the Tumor Microenvironment. Cells. 2023;12(4):547. [DOI] [PMC free article] [PubMed]
- 38.Babina IS, Turner NC. Advances and challenges in targeting FGFR signalling in cancer. Nat Rev Cancer. 2017;17(5):318–332. doi: 10.1038/nrc.2017.8. [DOI] [PubMed] [Google Scholar]
- 39.Brown LM, Ekert PG, Fleuren EDG. Biological and clinical implications of FGFR aberrations in paediatric and young adult cancers. Oncogene. 2023;42(23):1875–1888. doi: 10.1038/s41388-023-02705-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 41.Pandey P, Khan F, Upadhyay TK, Seungjoon M, Park MN, Kim B. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed Pharmacother. 2023;161:114491. doi: 10.1016/j.biopha.2023.114491. [DOI] [PubMed] [Google Scholar]
- 42.He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):425. doi: 10.1038/s41392-021-00828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mabeta P, Steenkamp V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int J Mol Sci. 2022;23(24):15585. [DOI] [PMC free article] [PubMed]
- 44.Ding X, Ji J, Jiang J, Cai Q, Wang C, Shi M, et al. HGF-mediated crosstalk between cancer-associated fibroblasts and MET-unamplified gastric cancer cells activates coordinated tumorigenesis and metastasis. Cell Death Dis. 2018;9(9):867. doi: 10.1038/s41419-018-0922-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cecchi F, Rex K, Schmidt J, Vocke CD, Lee YH, Burkett S, et al. Rilotumumab Resistance Acquired by Intracrine Hepatocyte Growth Factor Signaling. Cancers (Basel). 2023;15(2):460. [DOI] [PMC free article] [PubMed]
- 46.Chen Y, Takita J, Choi YL, Kato M, Ohira M, Sanada M, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455(7215):971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 47.Cooper AJ, Sequist LV, Johnson TW, Lin JJ. LTK fusions: A new target emerges in non-small cell lung cancer. Cancer Cell. 2022;40(1):23–25. doi: 10.1016/j.ccell.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;12(3):159–169. doi: 10.1038/nrc3215. [DOI] [PubMed] [Google Scholar]
- 49.Martínez Báez A, Castro Romero I, Chihu Amparan L, Castañeda JR, Ayala G. The Insulin Receptor Substrate 2 Mediates the Action of Insulin on HeLa Cell Migration via the PI3K/Akt Signaling Pathway. Curr Issues Mol Biol. 2023;45(3):2296–2308. doi: 10.3390/cimb45030148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27(41):5486–5496. doi: 10.1038/onc.2008.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114(Pt 8):1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 52.Kim C, Lee JH, Ko JH, Chinnathambi A, Alharbi SA, Shair OHM, et al. Formononetin Regulates Multiple Oncogenic Signaling Cascades and Enhances Sensitivity to Bortezomib in a Multiple Myeloma Mouse Model. Biomolecules. 2019;9(7):262. [DOI] [PMC free article] [PubMed]
- 53.Castellano E, Downward J. RAS Interaction with PI3K: More Than Just Another Effector Pathway. Genes Cancer. 2011;2(3):261–274. doi: 10.1177/1947601911408079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Triscott J, Rubin MA. Prostate Power Play: Does Pik3ca Accelerate Pten-Deficient Cancer Progression? Cancer Discov. 2018;8(6):682–685. doi: 10.1158/2159-8290.CD-18-0369. [DOI] [PubMed] [Google Scholar]
- 55.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 56.Voutsadakis IA. KRAS mutated colorectal cancers with or without PIK3CA mutations: Clinical and molecular profiles inform current and future therapeutics. Crit Rev Oncol Hematol. 2023;186:103987. doi: 10.1016/j.critrevonc.2023.103987. [DOI] [PubMed] [Google Scholar]
- 57.de Godoy BLV, Moschetta-Pinheiro MG, Chuffa LGA, Pondé NF, Reiter RJ, Colombo J, et al. Synergistic actions of Alpelisib and Melatonin in breast cancer cell lines with PIK3CA gene mutation. Life Sci. 2023;324:121708. doi: 10.1016/j.lfs.2023.121708. [DOI] [PubMed] [Google Scholar]
- 58.Daher S, Zer A, Tschernichovsky R, Yacobi R, Barshack I, Tsabari S, et al. Driver mutation characteristics of phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) in advanced non-small cell lung cancer. Lung Cancer. 2023;178:229–236. doi: 10.1016/j.lungcan.2023.02.023. [DOI] [PubMed] [Google Scholar]
- 59.Yao J, You Q, Zhang X, Zhang Y, Xu J, Zhao X, et al. PIK3CA somatic mutations as potential biomarker for immunotherapy in elder or TP53 mutated gastric cancer patients. Clin Genet. 2023;103(2):200–208. doi: 10.1111/cge.14260. [DOI] [PubMed] [Google Scholar]
- 60.Herberts C, Murtha AJ, Fu S, Wang G, Schönlau E, Xue H, et al. Activating AKT1 and PIK3CA Mutations in Metastatic Castration-Resistant Prostate Cancer. Eur Urol. 2020;78(6):834–844. doi: 10.1016/j.eururo.2020.04.058. [DOI] [PubMed] [Google Scholar]
- 61.Jiang W, Ouyang X, Ji Z, Shi W, Wu Y, Yao Q, et al. The PIK3CA-E545K-SIRT4 signaling axis reduces radiosensitivity by promoting glutamine metabolism in cervical cancer. Cancer Lett. 2023;556:216064. doi: 10.1016/j.canlet.2023.216064. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Nie J, Ma X, Wei Y, Peng Y, Wei X. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol Cancer. 2019;18(1):26. doi: 10.1186/s12943-019-0954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ikenoue T, Kanai F, Hikiba Y, Obata T, Tanaka Y, Imamura J, et al. Functional analysis of PIK3CA gene mutations in human colorectal cancer. Cancer Res. 2005;65(11):4562–4567. doi: 10.1158/0008-5472.CAN-04-4114. [DOI] [PubMed] [Google Scholar]
- 64.Jiang W, He T, Liu S, Zheng Y, Xiang L, Pei X, et al. The PIK3CA E542K and E545K mutations promote glycolysis and proliferation via induction of the β-catenin/SIRT3 signaling pathway in cervical cancer. J Hematol Oncol. 2018;11(1):139. doi: 10.1186/s13045-018-0674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Croessmann S, Sheehan JH, Lee KM, Sliwoski G, He J, Nagy R, et al. C2 Domain Deletions Hyperactivate Phosphoinositide 3-kinase (PI3K), Generate Oncogene Dependence, and Are Exquisitely Sensitive to PI3K. Clin Cancer Res. 2018;24(6):1426–1435. doi: 10.1158/1078-0432.CCR-17-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cochicho D, Esteves S, Rito M, Silva F, Martins L, Montalvão P, et al. PIK3CA Gene Mutations in HNSCC: Systematic Review and Correlations with HPV Status and Patient Survival. Cancers (Basel). 2022;14(5):1286. [DOI] [PMC free article] [PubMed]
- 67.Fang WL, Huang KH, Lan YT, Lin CH, Chang SC, Chen MH, et al. Mutations in PI3K/AKT pathway genes and amplifications of PIK3CA are associated with patterns of recurrence in gastric cancers. Oncotarget. 2016;7(5):6201–6220. doi: 10.18632/oncotarget.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li M, Liu F, Zhang F, Zhou W, Jiang X, Yang Y, et al. Genomic ERBB2/ERBB3 mutations promote PD-L1-mediated immune escape in gallbladder cancer: a whole-exome sequencing analysis. Gut. 2019;68(6):1024–1033. doi: 10.1136/gutjnl-2018-316039. [DOI] [PubMed] [Google Scholar]
- 69.Jiang N, Dai Q, Su X, Fu J, Feng X, Peng J. Role of PI3K/AKT pathway in cancer: the framework of malignant behavior. Mol Biol Rep. 2020;47(6):4587–4629. doi: 10.1007/s11033-020-05435-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pearson HB, Li J, Meniel VS, Fennell CM, Waring P, Montgomery KG, et al. Identification of Pik3ca Mutation as a Genetic Driver of Prostate Cancer That Cooperates with Pten Loss to Accelerate Progression and Castration-Resistant Growth. Cancer Discov. 2018;8(6):764–779. doi: 10.1158/2159-8290.CD-17-0867. [DOI] [PubMed] [Google Scholar]
- 71.Tortorella E, Giantulli S, Sciarra A, Silvestri I. AR and PI3K/AKT in Prostate Cancer: A Tale of Two Interconnected Pathways. Int J Mol Sci. 2023;24(3):2046. [DOI] [PMC free article] [PubMed]
- 72.López-Knowles E, Hernández S, Malats N, Kogevinas M, Lloreta J, Carrato A, et al. PIK3CA mutations are an early genetic alteration associated with FGFR3 mutations in superficial papillary bladder tumors. Cancer Res. 2006;66(15):7401–7404. doi: 10.1158/0008-5472.CAN-06-1182. [DOI] [PubMed] [Google Scholar]
- 73.Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci U S A. 2014;111(51):E5564–E5573. doi: 10.1073/pnas.1419260111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brennan CW, Verhaak RG, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feldman DR, Iyer G, Van Alstine L, Patil S, Al-Ahmadie H, Reuter VE, et al. Presence of somatic mutations within PIK3CA, AKT, RAS, and FGFR3 but not BRAF in cisplatin-resistant germ cell tumors. Clin Cancer Res. 2014;20(14):3712–3720. doi: 10.1158/1078-0432.CCR-13-2868. [DOI] [PubMed] [Google Scholar]
- 76.Moore JB, Loeb DM, Hong KU, Sorensen PH, Triche TJ, Lee DW, et al. Epigenetic reprogramming and re-differentiation of a Ewing sarcoma cell line. Front Cell Dev Biol. 2015;3:15. doi: 10.3389/fcell.2015.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pérez-Ramírez C, Cañadas-Garre M, Molina M, Faus-Dáder MJ, Calleja-Hernández M. PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics. 2015;16(16):1843–1862. doi: 10.2217/pgs.15.122. [DOI] [PubMed] [Google Scholar]
- 78.Kitagawa M, Liao PJ, Lee KH, Wong J, Shang SC, Minami N, et al. Dual blockade of the lipid kinase PIP4Ks and mitotic pathways leads to cancer-selective lethality. Nat Commun. 2017;8(1):2200. doi: 10.1038/s41467-017-02287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuo KT, Mao TL, Jones S, Veras E, Ayhan A, Wang TL, et al. Frequent activating mutations of PIK3CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174(5):1597–1601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jin J, Shi Y, Zhang S, Yang S. PIK3CA mutation and clinicopathological features of colorectal cancer: a systematic review and Meta-Analysis. Acta Oncol. 2020;59(1):66–74. doi: 10.1080/0284186X.2019.1664764. [DOI] [PubMed] [Google Scholar]
- 81.Atanasova VS, Riedl A, Strobl M, Flandorfer J, Unterleuthner D, Weindorfer C, et al. Selective Eradication of Colon Cancer Cells Harboring PI3K and/or MAPK Pathway Mutations in 3D Culture by Combined PI3K/AKT/mTOR Pathway and MEK Inhibition. Int J Mol Sci. 2023;24(2):1668. [DOI] [PMC free article] [PubMed]
- 82.Lin A, Piao HL, Zhuang L, Sarbassov dD, Ma L, Gan B. FoxO transcription factors promote AKT Ser473 phosphorylation and renal tumor growth in response to pharmacologic inhibition of the PI3K-AKT pathway. Cancer Res. 2014;74(6):1682–93. doi: 10.1158/0008-5472.CAN-13-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Badoiu SC, Greabu M, Miricescu D, Stanescu-Spinu II, Ilinca R, Balan DG, et al. PI3K/AKT/mTOR Dysregulation and Reprogramming Metabolic Pathways in Renal Cancer: Crosstalk with the VHL/HIF Axis. Int J Mol Sci. 2023;24(9):8391. [DOI] [PMC free article] [PubMed]
- 84.Lin DC, Hao JJ, Nagata Y, Xu L, Shang L, Meng X, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46(5):467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016;126(3):1052–1066. doi: 10.1172/JCI85271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chow LM, Baker SJ. PTEN function in normal and neoplastic growth. Cancer Lett. 2006;241(2):184–196. doi: 10.1016/j.canlet.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 87.Fusco N, Sajjadi E, Venetis K, Gaudioso G, Lopez G, Corti C, et al. Alterations and Their Role in Cancer Management: Are We Making Headway on Precision Medicine? Genes (Basel). 2020;11(7):719. [DOI] [PMC free article] [PubMed]
- 88.Vidotto T, Melo CM, Lautert-Dutra W, Chaves LP, Reis RB, Squire JA. Pan-cancer genomic analysis shows hemizygous PTEN loss tumors are associated with immune evasion and poor outcome. Sci Rep. 2023;13(1):5049. doi: 10.1038/s41598-023-31759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–96. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 90.Bergholz JS, Wang Q, Ramseier M, Prakadan S, Wang W, Fang R, et al. PI3Kβ controls immune evasion in PTEN-deficient breast tumours. Nature. 2023;617(7959):139–146. doi: 10.1038/s41586-023-05940-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller TW, Pérez-Torres M, Narasanna A, Guix M, Stål O, Pérez-Tenorio G, et al. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69(10):4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stebbing J, Lit LC, Zhang H, Darrington RS, Melaiu O, Rudraraju B, et al. The regulatory roles of phosphatases in cancer. Oncogene. 2014;33(8):939–953. doi: 10.1038/onc.2013.80. [DOI] [PubMed] [Google Scholar]
- 93.Ooki A, Yamashita K, Kikuchi S, Sakuramoto S, Katada N, Waraya M, et al. Therapeutic potential of PRL-3 targeting and clinical significance of PRL-3 genomic amplification in gastric cancer. BMC Cancer. 2011;11:122. doi: 10.1186/1471-2407-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li Q, Bai Y, Lyle LT, Yu G, Amarasinghe O, Nguele Meke F, et al. Mechanism of PRL2 phosphatase-mediated PTEN degradation and tumorigenesis. Proc Natl Acad Sci U S A. 2020;117(34):20538–20548. doi: 10.1073/pnas.2002964117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H, Quah SY, Dong JM, Manser E, Tang JP, Zeng Q. PRL-3 down-regulates PTEN expression and signals through PI3K to promote epithelial-mesenchymal transition. Cancer Res. 2007;67(7):2922–2926. doi: 10.1158/0008-5472.CAN-06-3598. [DOI] [PubMed] [Google Scholar]
- 96.Jiang Y, Liu XQ, Rajput A, Geng L, Ongchin M, Zeng Q, et al. Phosphatase PRL-3 is a direct regulatory target of TGFbeta in colon cancer metastasis. Cancer Res. 2011;71(1):234–244. doi: 10.1158/0008-5472.CAN-10-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23(6):675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Valent P. Targeting of leukemia-initiating cells to develop curative drug therapies: straightforward but nontrivial concept. Curr Cancer Drug Targets. 2011;11(1):56–71. doi: 10.2174/156800911793743655. [DOI] [PubMed] [Google Scholar]
- 99.Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou YA, Zhang T, Zhao JB, Wang XP, Jiang T, Gu ZP, et al. The adenovirus-mediated transfer of PTEN inhibits the growth of esophageal cancer cells in vitro and in vivo. Biosci Biotechnol Biochem. 2010;74(4):736–740. doi: 10.1271/bbb.90787. [DOI] [PubMed] [Google Scholar]
- 101.Zhu W, Shao Y, Yang M, Jia M, Peng Y. Asparaginyl endopeptidase promotes proliferation and invasiveness of prostate cancer cells via PI3K/AKT signaling pathway. Gene. 2016;594(2):176–182. doi: 10.1016/j.gene.2016.08.049. [DOI] [PubMed] [Google Scholar]
- 102.Lee JS, Kim HS, Kim YB, Lee MC, Park CS. Expression of PTEN in renal cell carcinoma and its relation to tumor behavior and growth. J Surg Oncol. 2003;84(3):166–172. doi: 10.1002/jso.10302. [DOI] [PubMed] [Google Scholar]
- 103.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 104.Hsieh SM, Maguire DJ, Lintell NA, McCabe M, Griffiths LR. PTEN and NDUFB8 aberrations in cervical cancer tissue. Adv Exp Med Biol. 2007;599:31–36. doi: 10.1007/978-0-387-71764-7_5. [DOI] [PubMed] [Google Scholar]
- 105.Takei Y, Saga Y, Mizukami H, Takayama T, Ohwada M, Ozawa K, et al. Overexpression of PTEN in ovarian cancer cells suppresses i.p. dissemination and extends survival in mice. Mol Cancer Ther. 2008;7(3):704–11. doi: 10.1158/1535-7163.MCT-06-0724. [DOI] [PubMed] [Google Scholar]
- 106.Grossmann AH, Layfield LJ, Randall RL. Classification, molecular characterization, and the significance of pten alteration in leiomyosarcoma. Sarcoma. 2012;2012:380896. doi: 10.1155/2012/380896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 108.Kim SW, Kim SM, Bae H, Nam D, Lee JH, Lee SG, et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate. 2013;73(3):296–305. doi: 10.1002/pros.22574. [DOI] [PubMed] [Google Scholar]
- 109.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 110.Kumar A, Purohit R. Cancer associated E17K mutation causes rapid conformational drift in AKT1 pleckstrin homology (PH) domain. PLoS ONE. 2013;8(5):e64364. doi: 10.1371/journal.pone.0064364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Parikh C, Janakiraman V, Wu WI, Foo CK, Kljavin NM, Chaudhuri S, et al. Disruption of PH-kinase domain interactions leads to oncogenic activation of AKT in human cancers. Proc Natl Acad Sci U S A. 2012;109(47):19368–19373. doi: 10.1073/pnas.1204384109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park ES, Rabinovsky R, Carey M, Hennessy BT, Agarwal R, Liu W, et al. Integrative analysis of proteomic signatures, mutations, and drug responsiveness in the NCI 60 cancer cell line set. Mol Cancer Ther. 2010;9(2):257–267. doi: 10.1158/1535-7163.MCT-09-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Network CGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stephens PJ, Tarpey PS, Davies H, Van Loo P, Greenman C, Wedge DC, et al. The landscape of cancer genes and mutational processes in breast cancer. Nature. 2012;486(7403):400–404. doi: 10.1038/nature11017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Basu A, Lambring CB. Akt Isoforms: A Family Affair in Breast Cancer. Cancers (Basel). 2021;13(14):3445. [DOI] [PMC free article] [PubMed]
- 116.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 117.Yang WL, Wu CY, Wu J, Lin HK. Regulation of Akt signaling activation by ubiquitination. Cell Cycle. 2010;9(3):487–497. doi: 10.4161/cc.9.3.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chan CH, Jo U, Kohrman A, Rezaeian AH, Chou PC, Logothetis C, et al. Posttranslational regulation of Akt in human cancer. Cell Biosci. 2014;4(1):59. doi: 10.1186/2045-3701-4-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol. 2006;19(2):238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 120.Qiao M, Iglehart JD, Pardee AB. Metastatic potential of 21T human breast cancer cells depends on Akt/protein kinase B activation. Cancer Res. 2007;67(11):5293–5299. doi: 10.1158/0008-5472.CAN-07-0877. [DOI] [PubMed] [Google Scholar]
- 121.Bellacosa A, Testa JR, Moore R, Larue L. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol Ther. 2004;3(3):268–275. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- 122.McCubrey JA, Steelman LS, Abrams SL, Lee JT, Chang F, Bertrand FE, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279. doi: 10.1016/j.advenzreg.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 123.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8(4):1168–1171. [PubMed] [Google Scholar]
- 124.Agarwal AK. How to explain the AKT phosphorylation of downstream targets in the wake of recent findings. Proc Natl Acad Sci U S A. 2018;115(27):E6099–E6100. doi: 10.1073/pnas.1808461115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tsai PJ, Lai YH, Manne RK, Tsai YS, Sarbassov D, Lin HK. Akt: a key transducer in cancer. J Biomed Sci. 2022;29(1):76. doi: 10.1186/s12929-022-00860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kizilboga T, Baskale EA, Yildiz J, Akcay IM, Zemheri E, Can ND, et al. Bag-1 stimulates Bad phosphorylation through activation of Akt and Raf kinases to mediate cell survival in breast cancer. BMC Cancer. 2019;19(1):1254. doi: 10.1186/s12885-019-6477-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bai D, Ueno L, Vogt PK. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer. 2009;125(12):2863–2870. doi: 10.1002/ijc.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813(11):1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 129.Nakae J, Oki M, Cao Y. The FoxO transcription factors and metabolic regulation. FEBS Lett. 2008;582(1):54–67. doi: 10.1016/j.febslet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 130.Chibaya L, Karim B, Zhang H, Jones SN. Mdm2 phosphorylation by Akt regulates the p53 response to oxidative stress to promote cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2021;118(4):e2003193118. [DOI] [PMC free article] [PubMed]
- 131.Song N, Che X, Xu L, Qu J, Zheng H, Hou K, et al. A novel function of hepatocyte growth factor in the activation of checkpoint kinase 1 phosphorylation in colon cancer cells. Mol Cell Biochem. 2017;436(1–2):29–38. doi: 10.1007/s11010-017-3075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Guo Q, Xiong Y, Song Y, Hua K, Gao S. ARHGAP17 suppresses tumor progression and up-regulates P21 and P27 expression via inhibiting PI3K/AKT signaling pathway in cervical cancer. Gene. 2019;692:9–16. doi: 10.1016/j.gene.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 133.Duda P, Akula SM, Abrams SL, Steelman LS, Martelli AM, Cocco L, et al. Targeting GSK3 and Associated Signaling Pathways Involved in Cancer. Cells. 2020;9(5):1110. [DOI] [PMC free article] [PubMed]
- 134.Chiang YJ, Liao WT, Ho KC, Wang SH, Chen YG, Ho CL, et al. CBAP modulates Akt-dependent TSC2 phosphorylation to promote Rheb-mTORC1 signaling and growth of T-cell acute lymphoblastic leukemia. Oncogene. 2019;38(9):1432–1447. doi: 10.1038/s41388-018-0507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hermida MA, Dinesh Kumar J, Leslie NR. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul. 2017;65:5–15. doi: 10.1016/j.jbior.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 136.Davis NM, Sokolosky M, Stadelman K, Abrams SL, Libra M, Candido S, et al. Deregulation of the EGFR/PI3K/PTEN/Akt/mTORC1 pathway in breast cancer: possibilities for therapeutic intervention. Oncotarget. 2014;5(13):4603–4650. doi: 10.18632/oncotarget.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 138.Stambolic V, Woodgett JR. Mitogen inactivation of glycogen synthase kinase-3 beta in intact cells via serine 9 phosphorylation. Biochem J. 1994;303(Pt 3):701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu C, Li Y, Semenov M, Han C, Baeg GH, Tan Y, et al. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 140.Jiang Y, Yan F, Feng Z, Lazarovici P, Zheng W. Signaling Network of Forkhead Family of Transcription Factors (FOXO) in Dietary Restriction. Cells. 2019;9(1):100. [DOI] [PMC free article] [PubMed]
- 141.Webb AE, Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci. 2014;39(4):159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17(1):104. doi: 10.1186/s12943-018-0856-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kodani N, Nakae J. Tissue-Specific Metabolic Regulation of FOXO-Binding Protein: FOXO Does Not Act Alone. Cells. 2020;9(3):702. [DOI] [PMC free article] [PubMed]
- 144.Fu Z, Tindall DJ. FOXOs, cancer and regulation of apoptosis. Oncogene. 2008;27(16):2312–2319. doi: 10.1038/onc.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168(6):960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Inoki K, Li Y, Zhu T, Wu J, Guan KL. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol. 2002;4(9):648–657. doi: 10.1038/ncb839. [DOI] [PubMed] [Google Scholar]
- 147.Xu F, Na L, Li Y, Chen L. Roles of the PI3K/AKT/mTOR signalling pathways in neurodegenerative diseases and tumours. Cell Biosci. 2020;10(1):54. doi: 10.1186/s13578-020-00416-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 148.Mayer IA, Arteaga CL. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu Rev Med. 2016;67:11–28. doi: 10.1146/annurev-med-062913-051343. [DOI] [PubMed] [Google Scholar]
- 149.Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature. 2012;485(7396):55–61. doi: 10.1038/nature10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sekulić A, Hudson CC, Homme JL, Yin P, Otterness DM, Karnitz LM, et al. A direct linkage between the phosphoinositide 3-kinase-AKT signaling pathway and the mammalian target of rapamycin in mitogen-stimulated and transformed cells. Cancer Res. 2000;60(13):3504–3513. [PubMed] [Google Scholar]
- 151.Shah OJ, Hunter T. Turnover of the active fraction of IRS1 involves raptor-mTOR- and S6K1-dependent serine phosphorylation in cell culture models of tuberous sclerosis. Mol Cell Biol. 2006;26(17):6425–6434. doi: 10.1128/MCB.01254-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 153.Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, et al. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11(8):2875–2878. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- 154.Tian T, Li X, Zhang J. mTOR Signaling in Cancer and mTOR Inhibitors in Solid Tumor Targeting Therapy. Int J Mol Sci. 2019;20(3):755. [DOI] [PMC free article] [PubMed]
- 155.Yang H, Jiang X, Li B, Yang HJ, Miller M, Yang A, et al. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature. 2017;552(7685):368–373. doi: 10.1038/nature25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li Z, Yang Z, Passaniti A, Lapidus RG, Liu X, Cullen KJ, et al. A positive feedback loop involving EGFR/Akt/mTORC1 and IKK/NF-kB regulates head and neck squamous cell carcinoma proliferation. Oncotarget. 2016;7(22):31892–31906. doi: 10.18632/oncotarget.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haddadi N, Lin Y, Travis G, Simpson AM, Nassif NT, McGowan EM. PTEN/PTENP1: 'Regulating the regulator of RTK-dependent PI3K/Akt signalling', new targets for cancer therapy. Mol Cancer. 2018;17(1):37. doi: 10.1186/s12943-018-0803-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27(41):5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 159.Shafei MA, Forshaw T, Davis J, Flemban A, Qualtrough D, Dean S, et al. BCATc modulates crosstalk between the PI3K/Akt and the Ras/ERK pathway regulating proliferation in triple negative breast cancer. Oncotarget. 2020;11(21):1971–1987. doi: 10.18632/oncotarget.27607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.She QB, Halilovic E, Ye Q, Zhen W, Shirasawa S, Sasazuki T, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell. 2010;18(1):39–51. doi: 10.1016/j.ccr.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang Z, Feng X, Molinolo AA, Martin D, Vitale-Cross L, Nohata N, et al. 4E-BP1 Is a Tumor Suppressor Protein Reactivated by mTOR Inhibition in Head and Neck Cancer. Cancer Res. 2019;79(7):1438–1450. doi: 10.1158/0008-5472.CAN-18-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chiu H, Buono R, Jackson LV, Herzog LO, Mallya S, Conn CS, et al. Reduced eIF4E function impairs B-cell leukemia without altering normal B-lymphocyte function. iScience. 2021;24(7):102748. doi: 10.1016/j.isci.2021.102748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30(22):2547–2557. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fukumoto S, Hsieh CM, Maemura K, Layne MD, Yet SF, Lee KH, et al. Akt participation in the Wnt signaling pathway through Dishevelled. J Biol Chem. 2001;276(20):17479–17483. doi: 10.1074/jbc.C000880200. [DOI] [PubMed] [Google Scholar]
- 165.Fang D, Hawke D, Zheng Y, Xia Y, Meisenhelder J, Nika H, et al. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J Biol Chem. 2007;282(15):11221–11229. doi: 10.1074/jbc.M611871200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dihlmann S, Kloor M, Fallsehr C, von Knebel DM. Regulation of AKT1 expression by beta-catenin/Tcf/Lef signaling in colorectal cancer cells. Carcinogenesis. 2005;26(9):1503–1512. doi: 10.1093/carcin/bgi120. [DOI] [PubMed] [Google Scholar]
- 167.Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126(5):955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 168.Jozwiak J, Kotulska K, Grajkowska W, Jozwiak S, Zalewski W, Oldak M, et al. Upregulation of the WNT pathway in tuberous sclerosis-associated subependymal giant cell astrocytomas. Brain Dev. 2007;29(5):273–280. doi: 10.1016/j.braindev.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 169.Pecoraro C, Faggion B, Balboni B, Carbone D, Peters GJ, Diana P, et al. GSK3β as a novel promising target to overcome chemoresistance in pancreatic cancer. Drug Resist Updat. 2021;58:100779. doi: 10.1016/j.drup.2021.100779. [DOI] [PubMed] [Google Scholar]
- 170.Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401(6748):86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- 171.Tilton B, Andjelkovic M, Didichenko SA, Hemmings BA, Thelen M. G-Protein-coupled receptors and Fcgamma-receptors mediate activation of Akt/protein kinase B in human phagocytes. J Biol Chem. 1997;272(44):28096–28101. doi: 10.1074/jbc.272.44.28096. [DOI] [PubMed] [Google Scholar]
- 172.Wu C, You J, Fu J, Wang X, Zhang Y. Phosphatidylinositol 3-Kinase/Akt Mediates Integrin Signaling To Control RNA Polymerase I Transcriptional Activity. Mol Cell Biol. 2016;36(10):1555–1568. doi: 10.1128/MCB.00004-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kennedy SG, Kandel ES, Cross TK, Hay N. Akt/Protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol Cell Biol. 1999;19(8):5800–5810. doi: 10.1128/mcb.19.8.5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3(11):973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 175.Wijnen R, Pecoraro C, Carbone D, Fiuji H, Avan A, Peters GJ, et al. Cyclin Dependent Kinase-1 (CDK-1) Inhibition as a Novel Therapeutic Strategy against Pancreatic Ductal Adenocarcinoma (PDAC). Cancers (Basel). 2021;13(17):4389. [DOI] [PMC free article] [PubMed]
- 176.Kale J, Kutuk O, Brito GC, Andrews TS, Leber B, Letai A, et al. Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Rep. 2018;19(9):e45235. [DOI] [PMC free article] [PubMed]
- 177.Merlo P, Cecconi F. XIAP: inhibitor of two worlds. EMBO J. 2013;32(16):2187–2188. doi: 10.1038/emboj.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18(4):R125–R147. doi: 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- 179.Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017;45:62–71. doi: 10.1016/j.ceb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arias-Romero LE, Chernoff J. Targeting Cdc42 in cancer. Expert Opin Ther Targets. 2013;17(11):1263–1273. doi: 10.1517/14728222.2013.828037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fattahi S, Amjadi-Moheb F, Tabaripour R, Ashrafi GH, Akhavan-Niaki H. PI3K/AKT/mTOR signaling in gastric cancer: Epigenetics and beyond. Life Sci. 2020;262:118513. doi: 10.1016/j.lfs.2020.118513. [DOI] [PubMed] [Google Scholar]
- 182.Faleiro I, Roberto VP, Demirkol Canli S, Fraunhoffer NA, Iovanna J, Gure AO, et al. DNA Methylation of PI3K/AKT Pathway-Related Genes Predicts Outcome in Patients with Pancreatic Cancer: A Comprehensive Bioinformatics-Based Study. Cancers (Basel). 2021;13(24):6354. [DOI] [PMC free article] [PubMed]
- 183.Marini F, Giusti F, Palmini G, Perigli G, Santoro R, Brandi ML. Genetics and Epigenetics of Parathyroid Carcinoma. Front Endocrinol (Lausanne) 2022;13:834362. doi: 10.3389/fendo.2022.834362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Riquelme I, Pérez-Moreno P, Mora-Lagos B, Ili C, Brebi P, Roa JC. Long Non-Coding RNAs (lncRNAs) as Regulators of the PI3K/AKT/mTOR Pathway in Gastric Carcinoma. Int J Mol Sci. 2023;24(7):6294. [DOI] [PMC free article] [PubMed]
- 185.Sergeeva A, Davydova K, Perenkov A, Vedunova M. Mechanisms of human DNA methylation, alteration of methylation patterns in physiological processes and oncology. Gene. 2023;875:147487. doi: 10.1016/j.gene.2023.147487. [DOI] [PubMed] [Google Scholar]
- 186.Cavalieri V. The Expanding Constellation of Histone Post-Translational Modifications in the Epigenetic Landscape. Genes (Basel). 2021;12(10):1596. [DOI] [PMC free article] [PubMed]
- 187.Dexheimer PJ, Cochella L. MicroRNAs: From Mechanism to Organism. Front Cell Dev Biol. 2020;8:409. doi: 10.3389/fcell.2020.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yin Q, Ma H, Bamunuarachchi G, Zheng X, Ma Y. Long Non-Coding RNAs, Cell Cycle, and Human Breast Cancer. Hum Gene Ther. 2023;34(11–12):481–494. doi: 10.1089/hum.2023.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kang YH, Lee HS, Kim WH. Promoter methylation and silencing of PTEN in gastric carcinoma. Lab Invest. 2002;82(3):285–291. doi: 10.1038/labinvest.3780422. [DOI] [PubMed] [Google Scholar]
- 190.Goel A, Arnold CN, Niedzwiecki D, Carethers JM, Dowell JM, Wasserman L, et al. Frequent inactivation of PTEN by promoter hypermethylation in microsatellite instability-high sporadic colorectal cancers. Cancer Res. 2004;64(9):3014–3021. doi: 10.1158/0008-5472.can-2401-2. [DOI] [PubMed] [Google Scholar]
- 191.Salvesen HB, MacDonald N, Ryan A, Jacobs IJ, Lynch ED, Akslen LA, et al. PTEN methylation is associated with advanced stage and microsatellite instability in endometrial carcinoma. Int J Cancer. 2001;91(1):22–26. doi: 10.1002/1097-0215(20010101)91:1<22::aid-ijc1002>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 192.Yu Y, Yin D, Hoque MO, Cao B, Jia Y, Yang Y, et al. AKT signaling pathway activated by HIN-1 methylation in non-small cell lung cancer. Tumour Biol. 2012;33(2):307–314. doi: 10.1007/s13277-011-0266-2. [DOI] [PubMed] [Google Scholar]
- 193.Stefansson OA, Esteller M. Epigenetic modifications in breast cancer and their role in personalized medicine. Am J Pathol. 2013;183(4):1052–1063. doi: 10.1016/j.ajpath.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 194.Estève PO, Chang Y, Samaranayake M, Upadhyay AK, Horton JR, Feehery GR, et al. A methylation and phosphorylation switch between an adjacent lysine and serine determines human DNMT1 stability. Nat Struct Mol Biol. 2011;18(1):42–48. doi: 10.1038/nsmb.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868(1):123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338(6113):1465–1469. doi: 10.1126/science.1227604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.D'Agostino M, Sponziello M, Puppin C, Celano M, Maggisano V, Baldan F, et al. Different expression of TSH receptor and NIS genes in thyroid cancer: role of epigenetics. J Mol Endocrinol. 2014;52(2):121–131. doi: 10.1530/JME-13-0160. [DOI] [PubMed] [Google Scholar]
- 198.Yang SM, Huang C, Li XF, Yu MZ, He Y, Li J. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology. 2013;306:162–168. doi: 10.1016/j.tox.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 199.Zarrilli G, Galuppini F, Angerilli V, Munari G, Sabbadin M, Lazzarin V, et al. miRNAs Involved in Esophageal Carcinogenesis and miRNA-Related Therapeutic Perspectives in Esophageal Carcinoma. Int J Mol Sci. 2021;22(7):3640. [DOI] [PMC free article] [PubMed]
- 200.Xu YJ, Wei RS, Li XH, Li Q, Yu JR, Zhuang XF. MiR-421 promotes lipid metabolism by targeting PTEN via activating PI3K/AKT/mTOR pathway in non-small cell lung cancer. Epigenomics. 2022;14(3). 10.2217/epi-2021-0229. [DOI] [PubMed]
- 201.Chen Q, Sun T, Wang F, Gong B, Xie W, Ma M, et al. Long Noncoding RNA IGF2AS is Acting as an Epigenetic Tumor Suppressor in Human Prostate Cancer. Urology. 2019;124:310.e1. doi: 10.1016/j.urology.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 202.Liu B, Liu Y, Zhou M, Yao S, Bian Z, Liu D, et al. Comprehensive ceRNA network analysis and experimental studies identify an IGF2-AS/miR-150/IGF2 regulatory axis in colorectal cancer. Pathol Res Pract. 2020;216(10):153104. doi: 10.1016/j.prp.2020.153104. [DOI] [PubMed] [Google Scholar]
- 203.Gomih A, Smith JS, North KE, Hudgens MG, Brewster WR, Huang Z, et al. DNA methylation of imprinted gene control regions in the regression of low-grade cervical lesions. Int J Cancer. 2018;143(3):552–560. doi: 10.1002/ijc.31350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Zhang Y, Yan H, Jiang Y, Chen T, Ma Z, Li F, et al. Long non-coding RNA IGF2-AS represses breast cancer tumorigenesis by epigenetically regulating IGF2. Exp Biol Med (Maywood) 2021;246(4):371–379. doi: 10.1177/1535370220966253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu R, Chen Y, Liu G, Li C, Song Y, Cao Z, et al. PI3K/AKT pathway as a key link modulates the multidrug resistance of cancers. Cell Death Dis. 2020;11(9):797. doi: 10.1038/s41419-020-02998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sirico M, D'Angelo A, Gianni C, Casadei C, Merloni F, De Giorgi U. Current State and Future Challenges for PI3K Inhibitors in Cancer Therapy. Cancers (Basel). 2023;15(3):703. [DOI] [PMC free article] [PubMed]
- 207.Yu M, Chen J, Xu Z, Yang B, He Q, Luo P, et al. Development and safety of PI3K inhibitors in cancer. Arch Toxicol. 2023;97(3):635–650. doi: 10.1007/s00204-023-03440-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hillmann P, Fabbro D. PI3K/mTOR Pathway Inhibition: Opportunities in Oncology and Rare Genetic Diseases. Int J Mol Sci. 2019;20(22):5792. [DOI] [PMC free article] [PubMed]
- 209.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, et al. BAY 80–6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther. 2013;12(11):2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 211.Markham A. Copanlisib: First Global Approval. Drugs. 2017;77(18):2057–2062. doi: 10.1007/s40265-017-0838-6. [DOI] [PubMed] [Google Scholar]
- 212.Patnaik A, Appleman LJ, Tolcher AW, Papadopoulos KP, Beeram M, Rasco DW, et al. First-in-human phase I study of copanlisib (BAY 80–6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin's lymphomas. Ann Oncol. 2016;27(10):1928–1940. doi: 10.1093/annonc/mdw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Dreyling M, Morschhauser F, Bouabdallah K, Bron D, Cunningham D, Assouline SE, et al. Phase II study of copanlisib, a PI3K inhibitor, in relapsed or refractory, indolent or aggressive lymphoma. Ann Oncol. 2017;28(9):2169–2178. doi: 10.1093/annonc/mdx289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lenz G, Hawkes E, Verhoef G, Haioun C, Thye Lim S, Seog Heo D, et al. Single-agent activity of phosphatidylinositol 3-kinase inhibition with copanlisib in patients with molecularly defined relapsed or refractory diffuse large B-cell lymphoma. Leukemia. 2020;34(8):2184–2197. doi: 10.1038/s41375-020-0743-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Dreyling M, Santoro A, Mollica L, Leppä S, Follows GA, Lenz G, et al. Phosphatidylinositol 3-Kinase Inhibition by Copanlisib in Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2017;35(35):3898–3905. doi: 10.1200/JCO.2017.75.4648. [DOI] [PubMed] [Google Scholar]
- 216.Doi T, Fuse N, Yoshino T, Kojima T, Bando H, Miyamoto H, et al. A Phase I study of intravenous PI3K inhibitor copanlisib in Japanese patients with advanced or refractory solid tumors. Cancer Chemother Pharmacol. 2017;79(1):89–98. doi: 10.1007/s00280-016-3198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Damodaran S, Zhao F, Deming DA, Mitchell EP, Wright JJ, Gray RJ, et al. Phase II Study of Copanlisib in Patients With Tumors With PIK3CA Mutations: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol Z1F. J Clin Oncol. 2022;40(14):1552–1561. doi: 10.1200/JCO.21.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Qualls D, Lam HYJ, Whiting K, Kumar A, Matasar MJ, Owens C, et al. A phase 1 trial of copanlisib plus ibrutinib in relapsed/refractory mantle cell lymphoma. Blood Adv. 2022:5262–6. [DOI] [PMC free article] [PubMed]
- 219.Yhim HY, Kim T, Kim SJ, Shin HJ, Koh Y, Kim JS, et al. Combination treatment of copanlisib and gemcitabine in relapsed/refractory PTCL (COSMOS): an open-label phase I/II trial. Ann Oncol. 2021;32(4):552–559. doi: 10.1016/j.annonc.2020.12.009. [DOI] [PubMed] [Google Scholar]
- 220.Matasar MJ, Dreyling M, Leppä S, Santoro A, Pedersen M, Buvaylo V, et al. Feasibility of Combining the Phosphatidylinositol 3-Kinase Inhibitor Copanlisib With Rituximab-Based Immunochemotherapy in Patients With Relapsed Indolent B-cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21(11):e886–e894. doi: 10.1016/j.clml.2021.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Matasar MJ, Capra M, Özcan M, Lv F, Li W, Yañez E, et al. Copanlisib plus rituximab versus placebo plus rituximab in patients with relapsed indolent non-Hodgkin lymphoma (CHRONOS-3): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22(5):678–689. doi: 10.1016/S1470-2045(21)00145-5. [DOI] [PubMed] [Google Scholar]
- 222.Ramanathan RK, Von Hoff DD, Eskens F, Blumenschein G, Richards D, Genvresse I, et al. Phase Ib Trial of the PI3K Inhibitor Copanlisib Combined with the Allosteric MEK Inhibitor Refametinib in Patients with Advanced Cancer. Target Oncol. 2020;15(2):163–174. doi: 10.1007/s11523-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim RD, Alberts SR, Peña C, Genvresse I, Ajavon-Hartmann A, Xia C, et al. Phase I dose-escalation study of copanlisib in combination with gemcitabine or cisplatin plus gemcitabine in patients with advanced cancer. Br J Cancer. 2018;118(4):462–470. doi: 10.1038/bjc.2017.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tan ES, Cao B, Kim J, Al-Toubah TE, Mehta R, Centeno BA, et al. Phase 2 study of copanlisib in combination with gemcitabine and cisplatin in advanced biliary tract cancers. Cancer. 2021;127(8):1293–1300. doi: 10.1002/cncr.33364. [DOI] [PubMed] [Google Scholar]
- 225.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9(8):550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 226.Narayan P, Prowell TM, Gao JJ, Fernandes LL, Li E, Jiang X, et al. FDA Approval Summary: Alpelisib Plus Fulvestrant for Patients with HR-positive, HER2-negative, PIK3CA-mutated, Advanced or Metastatic Breast Cancer. Clin Cancer Res. 2021;27(7):1842–1849. doi: 10.1158/1078-0432.CCR-20-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Alves CL, Ditzel HJ. Drugging the PI3K/AKT/mTOR Pathway in ER+ Breast Cancer. Int J Mol Sci. 2023;24(5):4522. [DOI] [PMC free article] [PubMed]
- 228.Savas P, Lo LL, Luen SJ, Blackley EF, Callahan J, Moodie K, et al. Alpelisib Monotherapy for PI3K-Altered, Pretreated Advanced Breast Cancer: A Phase II Study. Cancer Discov. 2022;12(9):2058–2073. doi: 10.1158/2159-8290.CD-21-1696. [DOI] [PubMed] [Google Scholar]
- 229.Juric D, Rodon J, Tabernero J, Janku F, Burris HA, Schellens JHM, et al. Phosphatidylinositol 3-Kinase α-Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J Clin Oncol. 2018;36(13):1291–1299. doi: 10.1200/JCO.2017.72.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ando Y, Iwasa S, Takahashi S, Saka H, Kakizume T, Natsume K, et al. Phase I study of alpelisib (BYL719), an α-specific PI3K inhibitor, in Japanese patients with advanced solid tumors. Cancer Sci. 2019;110(3):1021–1031. doi: 10.1111/cas.13923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Jain S, Santa-Maria CA, Rademaker A, Giles FJ, Cristofanilli M, Gradishar WJ. Phase I study of Alpelisib (BYL-719) and T-DM1 in HER2-positive metastatic breast cancer after trastuzumab and taxane therapy. (Abstract). J Clin Oncol. 2017;35(15):1026. [DOI] [PubMed]
- 232.Juric D, Janku F, Rodón J, Burris HA, Mayer IA, Schuler M, et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2019;5(2):e184475. doi: 10.1001/jamaoncol.2018.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Turner S, Chia S, Kanakamedala H, Hsu WC, Park J, Chandiwana D, et al. Effectiveness of Alpelisib + Fulvestrant Compared with Real-World Standard Treatment Among Patients with HR+, HER2-, PIK3CA-Mutated Breast Cancer. Oncologist. 2021;26(7):e1133–e1142. doi: 10.1002/onco.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380(20):1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 235.André F, Ciruelos EM, Juric D, Loibl S, Campone M, Mayer IA, et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: final overall survival results from SOLAR-1. Ann Oncol. 2021;32(2):208–217. doi: 10.1016/j.annonc.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 236.Mayer IA, Abramson VG, Formisano L, Balko JM, Estrada MV, Sanders ME, et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2- Metastatic Breast Cancer. Clin Cancer Res. 2017;23(1):26–34. doi: 10.1158/1078-0432.CCR-16-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Munster PM, Hamilton EP, Estevez LG, De Boer RH, Mayer, IA, et al. Ph IB study of LEE011 and BYL719 in combination with letrozole in ER+, HER2- breast cancer. (Abstract). J Clin Oncol. 2014;32(26):143.
- 238.Mayer IA, Prat A, Egle D, Blau S, Fidalgo JAP, Gnant M, et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB) Clin Cancer Res. 2019;25(10):2975–2987. doi: 10.1158/1078-0432.CCR-18-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Razavi P, Dickler MN, Shah PD, Toy W, Brown DN, Won HH, et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat Cancer. 2020;1(4):382–393. doi: 10.1038/s43018-020-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sharma P, Abramson VG, O'Dea A, Nye L, Mayer I, Pathak HB, et al. Clinical and Biomarker Results from Phase I/II Study of PI3K Inhibitor Alpelisib plus Nab-paclitaxel in HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2021;27(14):3896–3904. doi: 10.1158/1078-0432.CCR-20-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Batalini F, Xiong N, Tayob N, Polak M, Eismann J, Cantley LC, et al. Phase 1b Clinical Trial with Alpelisib plus Olaparib for Patients with Advanced Triple-Negative Breast Cancer. Clin Cancer Res. 2022;28(8):1493–1499. doi: 10.1158/1078-0432.CCR-21-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Jhaveri K, Drago JZ, Shah PD, Wang R, Pareja F, Ratzon F, et al. A Phase I Study of Alpelisib in Combination with Trastuzumab and LJM716 in Patients with PIK3CA-Mutated HER2-Positive Metastatic Breast Cancer. Clin Cancer Res. 2021;27(14):3867–3875. doi: 10.1158/1078-0432.CCR-21-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Pantaleo MA, Heinrich MC, Italiano A, Valverde C, Schöffski P, Grignani G, et al. A multicenter, dose-finding, phase 1b study of imatinib in combination with alpelisib as third-line treatment in patients with advanced gastrointestinal stromal tumor. BMC Cancer. 2022;22(1):511. doi: 10.1186/s12885-022-09610-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hyman DM, Tran B, Jaime JC, Garralda E, Machiels JPH, Schellens JHM, et al. Phase Ib study of BGJ398 in combination with BYL719 in patients (pts) with select advanced solid tumors. (Abstract). J Clin Oncol. 2016;34(15):2500.
- 245.Juric D, Soria JC, Sharma S, Banerji U, Azaro A, Desai J, et al. A phase 1b dose-escalation study of BYL719 plus binimetinib (MEK162) in patients with selected advanced solid tumors. (Abstract). J Clin Oncol. 2014;32(15):9051.
- 246.Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol. 2019;20(4):570–580. doi: 10.1016/S1470-2045(18)30905-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dunn LA, Riaz N, Fury MG, McBride SM, Michel L, Lee NY, et al. A Phase 1b Study of Cetuximab and BYL719 (Alpelisib) Concurrent with Intensity Modulated Radiation Therapy in Stage III-IVB Head and Neck Squamous Cell Carcinoma. Int J Radiat Oncol Biol Phys. 2020;106(3):564–570. doi: 10.1016/j.ijrobp.2019.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Curigliano G, Martin M, Jhaveri K, Beck JT, Tortora G, Fazio N, et al. Alpelisib in combination with everolimus ± exemestane in solid tumours: Phase Ib randomised, open-label, multicentre study. Eur J Cancer. 2021;151:49–62. doi: 10.1016/j.ejca.2021.03.042. [DOI] [PubMed] [Google Scholar]
- 249.van Geel RMJM, Tabernero J, Elez E, Bendell JC, Spreafico A, Schuler M, et al. A Phase Ib Dose-Escalation Study of Encorafenib and Cetuximab with or without Alpelisib in Metastatic. Cancer Discov. 2017;7(6):610–619. doi: 10.1158/2159-8290.CD-16-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tabernero J, Van Geel R, Guren TK, Yaeger RD, Spreafico A, Faris JE, et al. Phase 2 results: Encorafenib (ENCO) and cetuximab (CETUX) with or without Alpelisib (ALP) in patients with advanced BRAF-mutant colorectal cancer (BRAFm CRC). (Abstract). J Clin Oncol. 2016;34(15):3544.
- 251.Blair HA. Duvelisib: First Global Approval. Drugs. 2018;78(17):1847–1853. doi: 10.1007/s40265-018-1013-4. [DOI] [PubMed] [Google Scholar]
- 252.Horwitz SM, Koch R, Porcu P, Oki Y, Moskowitz A, Perez M, et al. Activity of the PI3K-δ, γ inhibitor duvelisib in a phase 1 trial and preclinical models of T-cell lymphoma. Blood. 2018;131(8):888–898. doi: 10.1182/blood-2017-08-802470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Izutsu K, Kato K, Kiyoi H, Yamamoto G, Shimada K, Akashi K. Phase I study of duvelisib in Japanese patients with relapsed or refractory lymphoma. Int J Hematol. 2020;112(4):504–509. doi: 10.1007/s12185-020-02929-3. [DOI] [PubMed] [Google Scholar]
- 254.Flinn IW, O'Brien S, Kahl B, Patel M, Oki Y, Foss FF, et al. Duvelisib, a novel oral dual inhibitor of PI3K-δ, γ, is clinically active in advanced hematologic malignancies. Blood. 2018;131(8):877–887. doi: 10.1182/blood-2017-05-786566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zheng Z, Gao Y, Song Y, Qian Y, Jing H, Liu T, et al. Efficacy and safety of duvelisib, a phosphoinositide 3 kinase (PI3K) δ and γ inhibitor, in Chinese patients (pts) with relapsed/refractory follicular lymphoma (R/R FL): A singlearm, open-label, multicenter, phase II clinical trial. (Abstract). J Clin Oncol. 2021;39(15):e19532.
- 256.Flinn IW, Miller CB, Ardeshna KM, Tetreault S, Assouline SE, Mayer J, et al. DYNAMO: A Phase II Study of Duvelisib (IPI-145) in Patients With Refractory Indolent Non-Hodgkin Lymphoma. J Clin Oncol. 2019;37(11):912–922. doi: 10.1200/JCO.18.00915. [DOI] [PubMed] [Google Scholar]
- 257.Flinn IW, Hillmen P, Montillo M, Nagy Z, Illés Á, Etienne G, et al. The phase 3 DUO trial: duvelisib vs ofatumumab in relapsed and refractory CLL/SLL. Blood. 2018;132(23):2446–2455. doi: 10.1182/blood-2018-05-850461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Davids MS, Kuss BJ, Hillmen P, Montillo M, Moreno C, Essell J, et al. Efficacy and Safety of Duvelisib Following Disease Progression on Ofatumumab in Patients with Relapsed/Refractory CLL or SLL in the DUO Crossover Extension Study. Clin Cancer Res. 2020;26(9):2096–2103. doi: 10.1158/1078-0432.CCR-19-3061. [DOI] [PubMed] [Google Scholar]
- 259.Flinn IW, Cherry MA, Maris MB, Matous JV, Berdeja JG, Patel M. Combination trial of duvelisib (IPI-145) with rituximab or bendamustine/rituximab in patients with non-Hodgkin lymphoma or chronic lymphocytic leukemia. Am J Hematol. 2019;94(12):1325–1334. doi: 10.1002/ajh.25634. [DOI] [PubMed] [Google Scholar]
- 260.Davids MS, Fisher DC, Tyekucheva S, McDonough M, Hanna J, Lee B, et al. A phase 1b/2 study of duvelisib in combination with FCR (DFCR) for frontline therapy for younger CLL patients. Leukemia. 2021;35(4):1064–1072. doi: 10.1038/s41375-020-01010-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Miller BW, Przepiorka D, de Claro RA, Lee K, Nie L, Simpson N, et al. FDA approval: idelalisib monotherapy for the treatment of patients with follicular lymphoma and small lymphocytic lymphoma. Clin Cancer Res. 2015;21(7):1525–1529. doi: 10.1158/1078-0432.CCR-14-2522. [DOI] [PubMed] [Google Scholar]
- 262.Shah A, Mangaonkar A. Idelalisib: A Novel PI3Kδ Inhibitor for Chronic Lymphocytic Leukemia. Ann Pharmacother. 2015;49(10):1162–1170. doi: 10.1177/1060028015594813. [DOI] [PubMed] [Google Scholar]
- 263.Brown JR, Byrd JC, Coutre SE, Benson DM, Flinn IW, Wagner-Johnston ND, et al. Idelalisib, an inhibitor of phosphatidylinositol 3-kinase p110δ, for relapsed/refractory chronic lymphocytic leukemia. Blood. 2014;123(22):3390–3397. doi: 10.1182/blood-2013-11-535047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Kahl BS, Spurgeon SE, Furman RR, Flinn IW, Coutre SE, Brown JR, et al. A phase 1 study of the PI3Kδ inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL) Blood. 2014;123(22):3398–3405. doi: 10.1182/blood-2013-11-537555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Flinn IW, Kahl BS, Leonard JP, Furman RR, Brown JR, Byrd JC, et al. Idelalisib, a selective inhibitor of phosphatidylinositol 3-kinase-δ, as therapy for previously treated indolent non-Hodgkin lymphoma. Blood. 2014;123(22):3406–3413. doi: 10.1182/blood-2013-11-538546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fukuhara N, Kinoshita T, Yamamoto K, Nagai H, Izutsu K, Yamamoto G, et al. Phase 1b study to investigate the safety and tolerability of idelalisib in Japanese patients with relapsed/refractory follicular lymphoma and chronic lymphocytic leukemia. Jpn J Clin Oncol. 2020;50(12):1395–1402. doi: 10.1093/jjco/hyaa153. [DOI] [PubMed] [Google Scholar]
- 267.Gopal AK, Fanale MA, Moskowitz CH, Shustov AR, Mitra S, Ye W, et al. Phase II study of idelalisib, a selective inhibitor of PI3Kδ, for relapsed/refractory classical Hodgkin lymphoma. Ann Oncol. 2017;28(5):1057–1063. doi: 10.1093/annonc/mdx028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, et al. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370(11):1008–1018. doi: 10.1056/NEJMoa1314583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Fjordén K, Ekberg S, Kuric N, Smedby KE, Lagerlöf I, Larsen TS, et al. Idelalisib in relapsed/refractory diffuse large B-cell lymphoma: results from a Nordic Lymphoma Group phase II trial. Br J Haematol. 2021;196(2):437–40. [DOI] [PubMed]
- 270.Coutre SE, Flinn IW, de Vos S, Barrientos JC, Schreeder MT, Wagner-Johnson ND, et al. Idelalisib in Combination With Rituximab or Bendamustine or Both in Patients With Relapsed/Refractory Chronic Lymphocytic Leukemia. Hemasphere. 2018;2(3):e39. doi: 10.1097/HS9.0000000000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.O'Brien SM, Lamanna N, Kipps TJ, Flinn I, Zelenetz AD, Burger JA, et al. A phase 2 study of idelalisib plus rituximab in treatment-naïve older patients with chronic lymphocytic leukemia. Blood. 2015;126(25):2686–2694. doi: 10.1182/blood-2015-03-630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sharman JP, Coutre SE, Furman RR, Cheson BD, Pagel JM, Hillmen P, et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J Clin Oncol. 2019;37(16):1391–1402. doi: 10.1200/JCO.18.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ghia P, Pluta A, Wach M, Lysak D, Kozak T, Simkovic M, et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J Clin Oncol. 2020;38(25):2849–2861. doi: 10.1200/JCO.19.03355. [DOI] [PubMed] [Google Scholar]
- 275.Eyre TA, Preston G, Kagdi H, Islam A, Nicholson T, Smith HW, et al. A retrospective observational study to evaluate the clinical outcomes and routine management of patients with chronic lymphocytic leukaemia treated with idelalisib and rituximab in the UK and Ireland (RETRO-idel) Br J Haematol. 2021;194(1):69–77. doi: 10.1111/bjh.17475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Danilov AV, Herbaux C, Walter HS, Hillmen P, Rule SA, Kio EA, et al. Phase Ib Study of Tirabrutinib in Combination with Idelalisib or Entospletinib in Previously Treated Chronic Lymphocytic Leukemia. Clin Cancer Res. 2020;26(12):2810–2818. doi: 10.1158/1078-0432.CCR-19-3504. [DOI] [PubMed] [Google Scholar]
- 277.Jones JA, Robak T, Brown JR, Awan FT, Badoux X, Coutre S, et al. Efficacy and safety of idelalisib in combination with ofatumumab for previously treated chronic lymphocytic leukaemia: an open-label, randomised phase 3 trial. Lancet Haematol. 2017;4(3):e114–e126. doi: 10.1016/S2352-3026(17)30019-4. [DOI] [PubMed] [Google Scholar]
- 278.Zelenetz AD, Barrientos JC, Brown JR, Coiffier B, Delgado J, Egyed M, et al. Idelalisib or placebo in combination with bendamustine and rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia: interim results from a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2017;18(3):297–311. doi: 10.1016/S1470-2045(16)30671-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tomowiak C, Poulain S, Herbaux C, Perrot A, Mahé B, Morel P, et al. Obinutuzumab and idelalisib in symptomatic patients with relapsed/refractory Waldenström macroglobulinemia. Blood Adv. 2021;5(9):2438–2446. doi: 10.1182/bloodadvances.2020003895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Dhillon S, Keam SJ. Umbralisib: First Approval. Drugs. 2021;81(7):857–866. doi: 10.1007/s40265-021-01504-2. [DOI] [PubMed] [Google Scholar]
- 281.Fowler NH, Samaniego F, Jurczak W, Ghosh N, Derenzini E, Reeves JA, et al. Umbralisib, a Dual PI3Kδ/CK1ε Inhibitor in Patients With Relapsed or Refractory Indolent Lymphoma. J Clin Oncol. 2021;39(15):1609–1618. doi: 10.1200/JCO.20.03433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chavez JC, Goldschmidt N, Samaniego F, Wrobel T, Cavallo F, Fonseca G, et al. The combination of Umbralisib plus Ublituximab is active in patients with relapsed or refractory marginal zone lymphoma (MZL): results from the phase 2 global unity-NHL trial. (Abstract). Blood. 2021;138(1):45.
- 283.Jones RH, Casbard A, Carucci M, Cox C, Butler R, Alchami F, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020;21(3):345–357. doi: 10.1016/S1470-2045(19)30817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Sidaway P. Capivasertib delays disease progression. Nat Rev Clin Oncol. 2023. 10.1038/s41571-023-00790-x. Online ahead of print. [DOI] [PubMed]
- 285.Turner N. Capivasertib Doubles PFS in Some Breast Cancers. Cancer Discov. 2023;13(2):250. doi: 10.1158/2159-8290.CD-NB2022-0078. [DOI] [PubMed] [Google Scholar]
- 286.Turner NC, Oliveira M, Howell SJ, Dalenc F, Cortes J, Gomez Moreno HL, et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2023;388(22):2058–2070. doi: 10.1056/NEJMoa2214131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 288.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 289.Mao B, Zhang Q, Ma L, Zhao DS, Zhao P, Yan P. Overview of Research into mTOR Inhibitors. Molecules. 2022;27(16):5295. [DOI] [PMC free article] [PubMed]
- 290.Machl A, Wilker EW, Tian H, Liu X, Schroeder P, Clark A, et al. M2698 is a potent dual-inhibitor of p70S6K and Akt that affects tumor growth in mouse models of cancer and crosses the blood-brain barrier. Am J Cancer Res. 2016;6(4):806–818. [PMC free article] [PubMed] [Google Scholar]
- 291.Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, et al. The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science. 2011;332(6035):1317–1322. doi: 10.1126/science.1199498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 293.Lau DK, Tay RY, Yeung YH, Chionh F, Mooi J, Murone C, et al. Phase II study of everolimus (RAD001) monotherapy as first-line treatment in advanced biliary tract cancer with biomarker exploration: the RADiChol Study. Br J Cancer. 2018;118(7):966–971. doi: 10.1038/s41416-018-0021-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Escudier B, Molinie V, Bracarda S, Maroto P, Szczylik C, Nathan P, et al. Open-label phase 2 trial of first-line everolimus monotherapy in patients with papillary metastatic renal cell carcinoma: RAPTOR final analysis. Eur J Cancer. 2016;69:226–235. doi: 10.1016/j.ejca.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 295.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Fazio N, Buzzoni R, Delle Fave G, Tesselaar ME, Wolin E, Van Cutsem E, et al. Everolimus in advanced, progressive, well-differentiated, non-functional neuroendocrine tumors: RADIANT-4 lung subgroup analysis. Cancer Sci. 2018;109(1):174–181. doi: 10.1111/cas.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 299.Jerusalem G, de Boer RH, Hurvitz S, Yardley DA, Kovalenko E, Ejlertsen B, et al. Everolimus Plus Exemestane vs Everolimus or Capecitabine Monotherapy for Estrogen Receptor-Positive, HER2-Negative Advanced Breast Cancer: The BOLERO-6 Randomized Clinical Trial. JAMA Oncol. 2018;4(10):1367–1374. doi: 10.1001/jamaoncol.2018.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366(6):520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tesch H, Stoetzer O, Decker T, Kurbacher CM, Marmé F, Schneeweiss A, et al. Efficacy and safety of everolimus plus exemestane in postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative locally advanced or metastatic breast cancer: Results of the single-arm, phase IIIB 4EVER trial. Int J Cancer. 2019;144(4):877–885. doi: 10.1002/ijc.31738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Fan Y, Sun T, Shao Z, Zhang Q, Ouyang Q, Tong Z, et al. Effectiveness of adding Everolimus to the first-line treatment of advanced breast cancer in premenopausal women who experienced disease progression while receiving selective estrogen receptor modulators: a phase 2 randomized clinical trial. JAMA Oncol. 2021;7(10):e213428. [DOI] [PMC free article] [PubMed]
- 303.Bardia A, Hurvitz SA, DeMichele A, Clark AS, Zelnak A, Yardley DA, et al. Phase I/II Trial of Exemestane, Ribociclib, and Everolimus in Women with HR. Clin Cancer Res. 2021;27(15):4177–4185. doi: 10.1158/1078-0432.CCR-20-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Schmid P, Sablin MP, Bergh J, Im SA, Lu YS, Martínez N, et al. A phase Ib/II study of xentuzumab, an IGF-neutralising antibody, combined with exemestane and everolimus in hormone receptor-positive, HER2-negative locally advanced/metastatic breast cancer. Breast Cancer Res. 2021;23(1):8. doi: 10.1186/s13058-020-01382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ballhausen A, Wheler JJ, Karp DD, Piha-Paul SA, Fu S, Pant S, et al. Phase I Study of Everolimus, Letrozole, and Trastuzumab in Patients with Hormone Receptor-positive Metastatic Breast Cancer or Other Solid Tumors. Clin Cancer Res. 2021;27(5):1247–1255. doi: 10.1158/1078-0432.CCR-20-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Toi M, Shao Z, Hurvitz S, Tseng LM, Zhang Q, Shen K, et al. Efficacy and safety of everolimus in combination with trastuzumab and paclitaxel in Asian patients with HER2+ advanced breast cancer in BOLERO-1. Breast Cancer Res. 2017;19(1):47. doi: 10.1186/s13058-017-0839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bautista F, Paoletti X, Rubino J, Brard C, Rezai K, Nebchi S, et al. Phase I or II Study of Ribociclib in Combination With Topotecan-Temozolomide or Everolimus in Children With Advanced Malignancies: Arms A and B of the AcSé-ESMART Trial. J Clin Oncol. 2021;39(32):3546–3560. doi: 10.1200/JCO.21.01152. [DOI] [PubMed] [Google Scholar]
- 308.Slomovitz BM, Jiang Y, Yates MS, Soliman PT, Johnston T, Nowakowski M, et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J Clin Oncol. 2015;33(8):930–936. doi: 10.1200/JCO.2014.58.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Jun T, Hahn NM, Sonpavde G, Albany C, MacVicar GR, Hauke R, et al. Phase II Clinical and Translational Study of Everolimus ± Paclitaxel as First-Line Therapy in Cisplatin-Ineligible Advanced Urothelial Carcinoma. Oncologist. 2022;27(6):432–e52. doi: 10.1093/oncolo/oyab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Soliman PT, Westin SN, Iglesias DA, Fellman BM, Yuan Y, Zhang Q, et al. Everolimus, Letrozole, and Metformin in Women with Advanced or Recurrent Endometrioid Endometrial Cancer: A Multi-Center, Single Arm. Phase II Study Clin Cancer Res. 2020;26(3):581–7. doi: 10.1158/1078-0432.CCR-19-0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Harding MW. Immunophilins, mTOR, and pharmacodynamic strategies for a targeted cancer therapy. Clin Cancer Res. 2003;9(8):2882–6. [PubMed] [Google Scholar]
- 312.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–45. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 313.Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003;9(13):4641–52. [PubMed] [Google Scholar]
- 314.Kwitkowski VE, Prowell TM, Ibrahim A, Farrell AT, Justice R, Mitchell SS, et al. FDA approval summary: temsirolimus as treatment for advanced renal cell carcinoma. Oncologist. 2010;15(4):428–35. doi: 10.1634/theoncologist.2009-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Korfel A, Schlegel U, Herrlinger U, Dreyling M, Schmidt C, von Baumgarten L, et al. Phase II Trial of Temsirolimus for Relapsed/Refractory Primary CNS Lymphoma. J Clin Oncol. 2016;34(15):1757–63. doi: 10.1200/JCO.2015.64.9897. [DOI] [PubMed] [Google Scholar]
- 316.Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770–8. doi: 10.1016/S0140-6736(15)00667-4. [DOI] [PubMed] [Google Scholar]
- 317.Major A, Kline J, Karrison TG, Fishkin PAS, Kimball AS, Petrich AM, et al. Phase I/II clinical trial of temsirolimus and lenalidomide in patients with relapsed and refractory lymphomas. Haematologica. 2021;107(7):1608–18. [DOI] [PMC free article] [PubMed]
- 318.Tasian SK, Silverman LB, Whitlock JA, Sposto R, Loftus JP, Schafer ES, et al. Temsirolimus combined with cyclophosphamide and etoposide for pediatric patients with relapsed/refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium trial (TACL 2014-001). Haematologica. 2022;107(10):2295–303. [DOI] [PMC free article] [PubMed]
- 319.Inwards DJ, Fishkin PA, LaPlant BR, Drake MT, Kurtin PJ, Nikcevich DA, et al. Phase I trial of rituximab, cladribine, and temsirolimus (RCT) for initial therapy of mantle cell lymphoma. Ann Oncol. 2014;25(10):2020–4. doi: 10.1093/annonc/mdu273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Kaley TJ, Panageas KS, Pentsova EI, Mellinghoff IK, Nolan C, Gavrilovic I, et al. Phase I clinical trial of temsirolimus and perifosine for recurrent glioblastoma. Ann Clin Transl Neurol. 2020;7(4):429–36. doi: 10.1002/acn3.51009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Trivedi ND, Armstrong S, Wang H, Hartley M, Deeken J, Ruth He A, et al. A phase I trial of the mTOR inhibitor temsirolimus in combination with capecitabine in patients with advanced malignancies. Cancer Med. 2021;10(6):1944–54. doi: 10.1002/cam4.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Hobday TJ, Qin R, Reidy-Lagunes D, Moore MJ, Strosberg J, Kaubisch A, et al. Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors. J Clin Oncol. 2015;33(14):1551–6. doi: 10.1200/JCO.2014.56.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Mascarenhas L, Chi YY, Hingorani P, Anderson JR, Lyden ER, Rodeberg DA, et al. Randomized Phase II Trial of Bevacizumab or Temsirolimus in Combination With Chemotherapy for First Relapse Rhabdomyosarcoma: A Report From the Children's Oncology Group. J Clin Oncol. 2019;37(31):2866–74. doi: 10.1200/JCO.19.00576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–93. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Lee BJ, Boyer JA, Burnett GL, Thottumkara AP, Tibrewal N, Wilson SL, et al. Selective inhibitors of mTORC1 activate 4EBP1 and suppress tumor growth. Nat Chem Biol. 2021;17(10):1065–74. doi: 10.1038/s41589-021-00813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, et al. Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature. 2016;534(7606):272–6. doi: 10.1038/nature17963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lee BJ, Mallya S, Dinglasan N, Fung A, Nguyen T, Herzog LO, et al. Efficacy of a Novel Bi-Steric mTORC1 Inhibitor in Models of B-Cell Acute Lymphoblastic Leukemia. Front Oncol. 2021;11:673213. doi: 10.3389/fonc.2021.673213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Meng D, Zhao X, Yang YC, Navickas A, Helland C, Goodarzi H, et al. A bi-steric mTORC1-selective inhibitor overcomes drug resistance in breast cancer. Oncogene. 2023;42(28):2207–17. [DOI] [PMC free article] [PubMed]
- 329.Burnett GL, Yang YC, Aggen JB, Pitzen J, Gliedt MK, Semko CM, et al. Discovery of RMC-5552, a Selective Bi-Steric Inhibitor of mTORC1, for the Treatment of mTORC1-Activated Tumors. J Med Chem. 2023;66(1):149–69. doi: 10.1021/acs.jmedchem.2c01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Burris HA, Ulahannan SV, Haura EB, Ignatius Ou SI, Capasso A, Munster PN, et al. The bi-steric mTORC1-selective inhibitor RMC-5552 in tumors with activation of mTOR signaling: Preclinical activity in combination with RAS(ON) inhibitors in RAS-addicted tumors, and initial clinical findings from a single agent phase 1/1b study. (Abstract). Journal of Clinical Oncology. 2022;40(16):3098.
- 331.Emmanouilidi A, Falasca M. Targeting PDK1 for Chemosensitization of Cancer Cells. Cancers (Basel). 2017;9(10):140. [DOI] [PMC free article] [PubMed]
- 332.Sestito S, Rapposelli S. A patent update on PDK1 inhibitors (2015-present) Expert Opin Ther Pat. 2019;29(4):271–82. doi: 10.1080/13543776.2019.1597852. [DOI] [PubMed] [Google Scholar]
- 333.Marine JC, Dawson SJ, Dawson MA. Non-genetic mechanisms of therapeutic resistance in cancer. Nat Rev Cancer. 2020;20(12):743–56. doi: 10.1038/s41568-020-00302-4. [DOI] [PubMed] [Google Scholar]
- 334.Bertucci A, Bertucci F, Gonçalves A. Phosphoinositide 3-Kinase (PI3K) Inhibitors and Breast Cancer: An Overview of Current Achievements. Cancers (Basel). 2023;15(5):1416. [DOI] [PMC free article] [PubMed]
- 335.Belli C, Repetto M, Anand S, Porta C, Subbiah V, Curigliano G. The emerging role of PI3K inhibitors for solid tumour treatment and beyond. Br J Cancer. 2023;128(12):2150–62. doi: 10.1038/s41416-023-02221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Raith F, O'Donovan DH, Lemos C, Politz O, Haendler B. Addressing the Reciprocal Crosstalk between the AR and the PI3K/AKT/mTOR Signaling Pathways for Prostate Cancer Treatment. Int J Mol Sci. 2023;24(3):2289. [DOI] [PMC free article] [PubMed]
- 337.Chakrabarty A, Sánchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109(8):2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.O'Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66(3):1500–8. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Muranen T, Selfors LM, Worster DT, Iwanicki MP, Song L, Morales FC, et al. Inhibition of PI3K/mTOR leads to adaptive resistance in matrix-attached cancer cells. Cancer Cell. 2012;21(2):227–39. doi: 10.1016/j.ccr.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Costa C, Ebi H, Martini M, Beausoleil SA, Faber AC, Jakubik CT, et al. Measurement of PIP3 levels reveals an unexpected role for p110β in early adaptive responses to p110α-specific inhibitors in luminal breast cancer. Cancer Cell. 2015;27(1):97–108. doi: 10.1016/j.ccell.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Mukherjee R, Vanaja KG, Boyer JA, Gadal S, Solomon H, Chandarlapaty S, et al. Regulation of PTEN translation by PI3K signaling maintains pathway homeostasis. Mol Cell. 2021;81(4):708–23.e5. doi: 10.1016/j.molcel.2021.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Bosch A, Li Z, Bergamaschi A, Ellis H, Toska E, Prat A, et al. PI3K inhibition results in enhanced estrogen receptor function and dependence in hormone receptor-positive breast cancer. Sci Transl Med. 2015;7(283):283ra51. doi: 10.1126/scitranslmed.aaa4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Scheffold A, Jebaraj BMC, Bloehdorn J, Tausch E, Bahlo J, Robrecht S, et al. High IGF1R Expression Is Associated with Worse Prognosis in CLL and Impacts Response to PI3K-δ Inhibitor Treatment. Blood. 2017;130(641):390. [Google Scholar]
- 344.Tausch E, Ljungström V, Agathangelidis A, Zapatka M, Scarfò L, Jebaraj BMC, et al. Secondary resistance to idelalisib is characterized by upregulation of IGF1R rather than by MAPK/ERK pathway mutations. Blood. 2022;139(22):3340–4. doi: 10.1182/blood.2021014550. [DOI] [PubMed] [Google Scholar]
- 345.Huw LY, O'Brien C, Pandita A, Mohan S, Spoerke JM, Lu S, et al. Acquired PIK3CA amplification causes resistance to selective phosphoinositide 3-kinase inhibitors in breast cancer. Oncogenesis. 2013;2:e83. doi: 10.1038/oncsis.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Nakanishi Y, Walter K, Spoerke JM, O'Brien C, Huw LY, Hampton GM, et al. Activating Mutations in PIK3CB Confer Resistance to PI3K Inhibition and Define a Novel Oncogenic Role for p110β. Cancer Res. 2016;76(5):1193–203. doi: 10.1158/0008-5472.CAN-15-2201. [DOI] [PubMed] [Google Scholar]
- 347.Han MW, Ryu IS, Lee JC, Kim SH, Chang HW, Lee YS, et al. Phosphorylation of PI3K regulatory subunit p85 contributes to resistance against PI3K inhibitors in radioresistant head and neck cancer. Oral Oncol. 2018;78:56–63. doi: 10.1016/j.oraloncology.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 348.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454(7205):776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Edgar KA, Wallin JJ, Berry M, Lee LB, Prior WW, Sampath D, et al. Isoform-specific phosphoinositide 3-kinase inhibitors exert distinct effects in solid tumors. Cancer Res. 2010;70(3):1164–72. doi: 10.1158/0008-5472.CAN-09-2525. [DOI] [PubMed] [Google Scholar]
- 350.Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, et al. mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Transl Med. 2013;5(196):196ra99. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Juric D, Castel P, Griffith M, Griffith OL, Won HH, Ellis H, et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature. 2015;518(7538):240–4. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Yadav S, Khalsa J, Murali I, Furman RR, Kay NE, Braggio E, et al. Potential Drivers of Acquired Resistance to Idelalisib in CLL Patients. Blood. 2022;140(641):12373–4. [Google Scholar]
- 353.Khan KH, Wong M, Rihawi K, Bodla S, Morganstein D, Banerji U, et al. Hyperglycemia and Phosphatidylinositol 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin (PI3K/AKT/mTOR) Inhibitors in Phase I Trials: Incidence, Predictive Factors, and Management. Oncologist. 2016;21(7):855–60. doi: 10.1634/theoncologist.2015-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Barbour LA, Shao J, Qiao L, Leitner W, Anderson M, Friedman JE, et al. Human placental growth hormone increases expression of the p85 regulatory unit of phosphatidylinositol 3-kinase and triggers severe insulin resistance in skeletal muscle. Endocrinology. 2004;145(3):1144–50. doi: 10.1210/en.2003-1297. [DOI] [PubMed] [Google Scholar]
- 355.Koh KX, Tan GH, Hui Low SH, Mohd Omar MF, Han MJ, Iacopetta B, et al. Acquired resistance to PI3K/mTOR inhibition is associated with mitochondrial DNA mutation and glycolysis. Oncotarget. 2017;8(66):110133–44. doi: 10.18632/oncotarget.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Bhola NE, Jansen VM, Koch JP, Li H, Formisano L, Williams JA, et al. Treatment of Triple-Negative Breast Cancer with TORC1/2 Inhibitors Sustains a Drug-Resistant and Notch-Dependent Cancer Stem Cell Population. Cancer Res. 2016;76(2):440–52. doi: 10.1158/0008-5472.CAN-15-1640-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Müller U, et al. JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell. 2012;22(6):796–811. doi: 10.1016/j.ccr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 358.Le X, Antony R, Razavi P, Treacy DJ, Luo F, Ghandi M, et al. Systematic Functional Characterization of Resistance to PI3K Inhibition in Breast Cancer. Cancer Discov. 2016;6(10):1134–47. doi: 10.1158/2159-8290.CD-16-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Arozarena I, Wellbrock C. Phenotype plasticity as enabler of melanoma progression and therapy resistance. Nat Rev Cancer. 2019;19(7):377–91. doi: 10.1038/s41568-019-0154-4. [DOI] [PubMed] [Google Scholar]
- 360.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14(10):611–29. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Murali I, Kasar S, Naeem A, Tyekucheva S, Khalsa JK, Thrash EM, et al. Activation of the MAPK pathway mediates resistance to PI3K inhibitors in chronic lymphocytic leukemia. Blood. 2021;138(1):44–56. doi: 10.1182/blood.2020006765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Arribas AJ, Napoli S, Cascione L, Sartori G, Barnabei L, Gaudio E, et al. Resistance to PI3Kδ inhibitors in marginal zone lymphoma can be reverted by targeting the IL-6/PDGFRA axis. Haematologica. 2022;107(11):2685–97. doi: 10.3324/haematol.2021.279957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Alves CL, Ehmsen S, Terp MG, Portman N, Tuttolomondo M, Gammelgaard OL, et al. Co-targeting CDK4/6 and AKT with endocrine therapy prevents progression in CDK4/6 inhibitor and endocrine therapy-resistant breast cancer. Nat Commun. 2021;12(1):5112. doi: 10.1038/s41467-021-25422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Takagi S, Onishi T, Takashima T, Shibahara K, Mori M. Acquired AKT-inhibitor Resistance Is Mediated by ATP-binding Cassette Transporters in Endometrial Carcinoma. Anticancer Res. 2023;43(6):2501–7. doi: 10.21873/anticanres.16417. [DOI] [PubMed] [Google Scholar]
- 365.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19(1):58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Hyman DM, Smyth LM, Donoghue MTA, Westin SN, Bedard PL, Dean EJ, et al. AKT Inhibition in Solid Tumors With AKT1 Mutations. J Clin Oncol. 2017;35(20):2251–9. doi: 10.1200/JCO.2017.73.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Stottrup C, Tsang T, Chin YR. Upregulation of AKT3 Confers Resistance to the AKT Inhibitor MK2206 in Breast Cancer. Mol Cancer Ther. 2016;15(8):1964–74. doi: 10.1158/1535-7163.MCT-15-0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Shi H, Hong A, Kong X, Koya RC, Song C, Moriceau G, et al. A novel AKT1 mutant amplifies an adaptive melanoma response to BRAF inhibition. Cancer Discov. 2014;4(1):69–79. doi: 10.1158/2159-8290.CD-13-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Alammar H, Nassani R, Alshehri MM, Aljohani AA, Alrfaei BM. Deficiency in the Treatment Description of mTOR Inhibitor Resistance in Medulloblastoma, a Systematic Review. Int J Mol Sci. 2021;23(1):464. [DOI] [PMC free article] [PubMed]
- 370.Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Res. 2016;5:F1000 Faculty Rev-2078.
- 371.Ebi H, Costa C, Faber AC, Nishtala M, Kotani H, Juric D, et al. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proc Natl Acad Sci U S A. 2013;110(52):21124–9. doi: 10.1073/pnas.1314124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov. 2015;14(4):261–78. doi: 10.1038/nrd4505. [DOI] [PubMed] [Google Scholar]
- 373.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1(3):248–59. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature. 2004;431(7005):200–5. doi: 10.1038/nature02866. [DOI] [PubMed] [Google Scholar]
- 375.Wagle N, Grabiner BC, Van Allen EM, Amin-Mansour A, Taylor-Weiner A, Rosenberg M, et al. Response and acquired resistance to everolimus in anaplastic thyroid cancer. N Engl J Med. 2014;371(15):1426–33. doi: 10.1056/NEJMoa1403352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Kwiatkowski DJ, Choueiri TK, Fay AP, Rini BI, Thorner AR, de Velasco G, et al. Mutations in TSC1, TSC2, and MTOR Are Associated with Response to Rapalogs in Patients with Metastatic Renal Cell Carcinoma. Clin Cancer Res. 2016;22(10):2445–52. doi: 10.1158/1078-0432.CCR-15-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Wagle N, Grabiner BC, Van Allen EM, Hodis E, Jacobus S, Supko JG, et al. Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov. 2014;4(5):546–53. doi: 10.1158/2159-8290.CD-13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol. 2010;28(5):835–40. doi: 10.1200/JCO.2009.25.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: clinical and molecular correlates. Int J Cancer. 2013;132(7):1711–7. doi: 10.1002/ijc.27800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Hortobagyi GN, Chen D, Piccart M, Rugo HS, Burris HA, Pritchard KI, et al. Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From BOLERO-2. J Clin Oncol. 2016;34(5):419–26. doi: 10.1200/JCO.2014.60.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Alain T, Morita M, Fonseca BD, Yanagiya A, Siddiqui N, Bhat M, et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer Res. 2012;72(24):6468–76. doi: 10.1158/0008-5472.CAN-12-2395. [DOI] [PubMed] [Google Scholar]
- 382.Mallya S, Fitch BA, Lee JS, So L, Janes MR, Fruman DA. Resistance to mTOR kinase inhibitors in lymphoma cells lacking 4EBP1. PLoS ONE. 2014;9(2):e88865. doi: 10.1371/journal.pone.0088865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Wang J, Ye Q, Cao Y, Guo Y, Huang X, Mi W, et al. Snail determines the therapeutic response to mTOR kinase inhibitors by transcriptional repression of 4E-BP1. Nat Commun. 2017;8(1):2207. doi: 10.1038/s41467-017-02243-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Hoang B, Benavides A, Shi Y, Yang Y, Frost P, Gera J, et al. The PP242 mammalian target of rapamycin (mTOR) inhibitor activates extracellular signal-regulated kinase (ERK) in multiple myeloma cells via a target of rapamycin complex 1 (TORC1)/eukaryotic translation initiation factor 4E (eIF-4E)/RAF pathway and activation is a mechanism of resistance. J Biol Chem. 2012;287(26):21796–805. doi: 10.1074/jbc.M111.304626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Ahmad S, Abu-Eid R, Shrimali R, Webb M, Verma V, Doroodchi A, et al. Differential PI3Kdelta Signaling in CD4(+) T-cell Subsets Enables Selective Targeting of T Regulatory Cells to Enhance Cancer Immunotherapy. Cancer Res. 2017;77(8):1892–904. doi: 10.1158/0008-5472.CAN-16-1839. [DOI] [PubMed] [Google Scholar]
- 386.Dong S, Harrington BK, Hu EY, Greene JT, Lehman AM, Tran M, et al. PI3K p110δ inactivation antagonizes chronic lymphocytic leukemia and reverses T cell immune suppression. J Clin Invest. 2019;129(1):122–36. doi: 10.1172/JCI99386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Chellappa S, Kushekhar K, Munthe LA, Tjønnfjord GE, Aandahl EM, Okkenhaug K, et al. The PI3K p110δ Isoform Inhibitor Idelalisib Preferentially Inhibits Human Regulatory T Cell Function. J Immunol. 2019;202(5):1397–405. doi: 10.4049/jimmunol.1701703. [DOI] [PubMed] [Google Scholar]
- 388.Tarantelli C, Argnani L, Zinzani PL, Bertoni F. PI3Kδ Inhibitors as Immunomodulatory Agents for the Treatment of Lymphoma Patients. Cancers (Basel). 2021;13(21):5535. [DOI] [PMC free article] [PubMed]
- 389.Schmid MC, Avraamides CJ, Dippold HC, Franco I, Foubert P, Ellies LG, et al. Receptor tyrosine kinases and TLR/IL1Rs unexpectedly activate myeloid cell PI3kγ, a single convergent point promoting tumor inflammation and progression. Cancer Cell. 2011;19(6):715–27. doi: 10.1016/j.ccr.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Kaneda MM, Cappello P, Nguyen AV, Ralainirina N, Hardamon CR, Foubert P, et al. Macrophage PI3Kγ Drives Pancreatic Ductal Adenocarcinoma Progression. Cancer Discov. 2016;6(8):870–85. doi: 10.1158/2159-8290.CD-15-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature. 2016;539(7629):443–7. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120(1):16–25. doi: 10.1038/s41416-018-0333-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Guerau-de-Arellano M, Piedra-Quintero ZL, Tsichlis PN. Akt isoforms in the immune system. Front Immunol. 2022;13:990874. doi: 10.3389/fimmu.2022.990874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Abu Eid R, Friedman KM, Mkrtichyan M, Walens A, King W, Janik J, et al. Akt1 and -2 inhibition diminishes terminal differentiation and enhances central memory CD8. Oncoimmunology. 2015;4(5):e1005448. doi: 10.1080/2162402X.2015.1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Abu Eid R, Ahmad S, Lin Y, Webb M, Berrong Z, Shrimali R, et al. Enhanced Therapeutic Efficacy and Memory of Tumor-Specific CD8 T Cells by. Cancer Res. 2017;77(15):4135–45. doi: 10.1158/0008-5472.CAN-16-1925. [DOI] [PubMed] [Google Scholar]
- 396.Ouyang S, Zeng Q, Tang N, Guo H, Tang R, Yin W, et al. Akt-1 and Akt-2 Differentially Regulate the Development of Experimental Autoimmune Encephalomyelitis by Controlling Proliferation of Thymus-Derived Regulatory T Cells. J Immunol. 2019;202(5):1441–52. doi: 10.4049/jimmunol.1701204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Ouyang W, Liao W, Luo CT, Yin N, Huse M, Kim MV, et al. Novel Foxo1-dependent transcriptional programs control T(reg) cell function. Nature. 2012;491(7425):554–9. doi: 10.1038/nature11581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Luo CT, Liao W, Dadi S, Toure A, Li MO. Graded Foxo1 activity in Treg cells differentiates tumour immunity from spontaneous autoimmunity. Nature. 2016;529(7587):532–6. doi: 10.1038/nature16486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Tan CL, Kuchroo JR, Sage PT, Liang D, Francisco LM, Buck J, et al. PD-1 restraint of regulatory T cell suppressive activity is critical for immune tolerance. J Exp Med. 2021;218(1):e20182232. [DOI] [PMC free article] [PubMed]
- 400.Zhang Y, Wang X, Yang H, Liu H, Lu Y, Han L, et al. Kinase AKT controls innate immune cell development and function. Immunology. 2013;140(2):143–52. doi: 10.1111/imm.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Vergadi E, Ieronymaki E, Lyroni K, Vaporidi K, Tsatsanis C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J Immunol. 2017;198(3):1006–14. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 402.Rückerl D, Jenkins SJ, Laqtom NN, Gallagher IJ, Sutherland TE, Duncan S, et al. Induction of IL-4Rα-dependent microRNAs identifies PI3K/Akt signaling as essential for IL-4-driven murine macrophage proliferation in vivo. Blood. 2012;120(11):2307–16. doi: 10.1182/blood-2012-02-408252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Miao Y, Jiang M, Qi L, Yang D, Xiao W, Fang F. BCAP Regulates Dendritic Cell Maturation Through the Dual-Regulation of NF-κB and PI3K/AKT Signaling During Infection. Front Immunol. 2020;11:250. doi: 10.3389/fimmu.2020.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Jones RG, Pearce EJ. MenTORing Immunity: mTOR Signaling in the Development and Function of Tissue-Resident Immune Cells. Immunity. 2017;46(5):730–42. doi: 10.1016/j.immuni.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Weichhart T, Hengstschläger M, Linke M. Regulation of innate immune cell function by mTOR. Nat Rev Immunol. 2015;15(10):599–614. doi: 10.1038/nri3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460(7251):108–12. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499(7459):485–90. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol. 2006;177(2):944–9. doi: 10.4049/jimmunol.177.2.944. [DOI] [PubMed] [Google Scholar]
- 409.Scottà C, Esposito M, Fazekasova H, Fanelli G, Edozie FC, Ali N, et al. Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4(+)CD25(+)FOXP3(+) T regulatory cell subpopulations. Haematologica. 2013;98(8):1291–9. doi: 10.3324/haematol.2012.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177(12):8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 411.Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, Wen J, et al. mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J Clin Invest. 2015;125(5):2090–108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29(4):565–77. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 413.Brown J, Wang H, Suttles J, Graves DT, Martin M. Mammalian target of rapamycin complex 2 (mTORC2) negatively regulates Toll-like receptor 4-mediated inflammatory response via FoxO1. J Biol Chem. 2011;286(52):44295–305. doi: 10.1074/jbc.M111.258053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Hallowell RW, Collins SL, Craig JM, Zhang Y, Oh M, Illei PB, et al. mTORC2 signalling regulates M2 macrophage differentiation in response to helminth infection and adaptive thermogenesis. Nat Commun. 2017;8:14208. doi: 10.1038/ncomms14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Huang SC, Smith AM, Everts B, Colonna M, Pearce EL, Schilling JD, et al. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity. 2016;45(4):817–30. doi: 10.1016/j.immuni.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Kaymak I, Williams KS, Cantor JR, Jones RG. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell. 2021;39(1):28–37. doi: 10.1016/j.ccell.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Mafi S, Mansoori B, Taeb S, Sadeghi H, Abbasi R, Cho WC, et al. mTOR-Mediated Regulation of Immune Responses in Cancer and Tumor Microenvironment. Front Immunol. 2021;12:774103. doi: 10.3389/fimmu.2021.774103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Ostrand-Rosenberg S, Fenselau C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J Immunol. 2018;200(2):422–31. doi: 10.4049/jimmunol.1701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13(12):871–82. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Lane HA, Wood JM, McSheehy PM, Allegrini PR, Boulay A, Brueggen J, et al. mTOR inhibitor RAD001 (everolimus) has antiangiogenic/vascular properties distinct from a VEGFR tyrosine kinase inhibitor. Clin Cancer Res. 2009;15(5):1612–22. doi: 10.1158/1078-0432.CCR-08-2057. [DOI] [PubMed] [Google Scholar]
- 422.Lastwika KJ, Wilson W, Li QK, Norris J, Xu H, Ghazarian SR, et al. Control of PD-L1 Expression by Oncogenic Activation of the AKT-mTOR Pathway in Non-Small Cell Lung Cancer. Cancer Res. 2016;76(2):227–38. doi: 10.1158/0008-5472.CAN-14-3362. [DOI] [PubMed] [Google Scholar]
- 423.Mittendorf EA, Philips AV, Meric-Bernstam F, Qiao N, Wu Y, Harrington S, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–70. doi: 10.1158/2326-6066.CIR-13-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Song M, Chen D, Lu B, Wang C, Zhang J, Huang L, et al. PTEN loss increases PD-L1 protein expression and affects the correlation between PD-L1 expression and clinical parameters in colorectal cancer. PLoS ONE. 2013;8(6):e65821. doi: 10.1371/journal.pone.0065821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.O'Donnell JS, Massi D, Teng MWL, Mandala M. PI3K-AKT-mTOR inhibition in cancer immunotherapy, redux. Semin Cancer Biol. 2018;48:91–103. doi: 10.1016/j.semcancer.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 426.Hirsch E, Ciraolo E, Franco I, Ghigo A, Martini M. PI3K in cancer-stroma interactions: bad in seed and ugly in soil. Oncogene. 2014;33(24):3083–90. doi: 10.1038/onc.2013.265. [DOI] [PubMed] [Google Scholar]
- 427.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, et al. Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer Discov. 2016;6(2):202–16. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Yan C, Yang J, Saleh N, Chen SC, Ayers GD, Abramson VG, et al. Inhibition of the PI3K/mTOR Pathway in Breast Cancer to Enhance Response to Immune Checkpoint Inhibitors in Breast Cancer. Int J Mol Sci. 2021;22(10):5207. [DOI] [PMC free article] [PubMed]
- 429.Isoyama S, Mori S, Sugiyama D, Kojima Y, Tada Y, Shitara K, et al. Cancer immunotherapy with PI3K and PD-1 dual-blockade via optimal modulation of T cell activation signal. J Immunother Cancer. 2021;9(8):e002279. [DOI] [PMC free article] [PubMed]
- 430.Chandrasekaran S, Funk CR, Kleber T, Paulos CM, Shanmugam M, Waller EK. Strategies to Overcome Failures in T-Cell Immunotherapies by Targeting PI3K-δ and -γ. Front Immunol. 2021;12:718621. doi: 10.3389/fimmu.2021.718621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Tawbi HA, Peng W, Phillips S, Milton DR, Amaria RN, Diab A, et al. Safety results from phase I/II study of the PI3Kβ inhibitor GSK2636771 (G) in combination with pembrolizumab (P) in patients (pts) with PD-1 refractory metastatic melanoma (MM) and PTEN loss. (Abstract). J Clin Oncol. 2020;38(15):e22000.
- 432.Jin J, Rohatgi A, Gabrielle A, Rose A, Wilson M, Bruno TC, et al. Interim results from a phase I/II study of duvelisib PI3Kδγ inhibitor and nivolumab in patients with advanced unresectable melanoma who have progressed on anti-PD1 therapy. (Abstract). J Clin Oncol. 2023;41(16):9540.
- 433.Bennani NN, Atherton P, Micallef I, Colgan JP, Thanarajasingam G, Nowakowski G, et al. A Phase II Study of Nivolumab in Patients with Relapsed or Refractory Peripheral T-Cell Lymphoma. Blood. 2019;134(624):467. [Google Scholar]
- 434.Shouse G, Siddiqi T, Popplewell LL, Muir A, Melgar I, Orand K, et al. A Phase I Trial of PI3Kαδ Inhibitor Copanlisib in Combination with Nivolumab in Patients with Richter's Transformation (RT) or Transformed Non-Hodgkin Lymphoma (tNHL) Blood. 2022;140:6633–4. [Google Scholar]
- 435.Huye LE, Nakazawa Y, Patel MP, Yvon E, Sun J, Savoldo B, et al. Combining mTor inhibitors with rapamycin-resistant T cells: a two-pronged approach to tumor elimination. Mol Ther. 2011;19(12):2239–48. doi: 10.1038/mt.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Stock S, Kluever AK, Endres S, Kobold S. Enhanced chimeric antigen receptor T cell therapy through co-application of synergistic combination partners. Biomedicines. 2022;10(2):307. [DOI] [PMC free article] [PubMed]
- 437.Zheng W, O'Hear CE, Alli R, Basham JH, Abdelsamed HA, Palmer LE, et al. PI3K orchestration of the in vivo persistence of chimeric antigen receptor-modified T cells. Leukemia. 2018;32(5):1157–67. doi: 10.1038/s41375-017-0008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Stock S, Übelhart R, Schubert ML, Fan F, He B, Hoffmann JM, et al. Idelalisib for optimized CD19-specific chimeric antigen receptor T cells in chronic lymphocytic leukemia patients. Int J Cancer. 2019;145(5):1312–24. doi: 10.1002/ijc.32201. [DOI] [PubMed] [Google Scholar]
- 439.Funk CR, Wang S, Chen KZ, Waller A, Sharma A, Edgar CL, et al. PI3Kδ/γ inhibition promotes human CART cell epigenetic and metabolic reprogramming to enhance antitumor cytotoxicity. Blood. 2022;139(4):523–37. doi: 10.1182/blood.2021011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Dwyer CJ, Arhontoulis DC, Rangel Rivera GO, Knochelmann HM, Smith AS, Wyatt MM, et al. Ex vivo blockade of PI3K gamma or delta signaling enhances the antitumor potency of adoptively transferred CD8. Eur J Immunol. 2020;50(9):1386–99. doi: 10.1002/eji.201948455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary figure 1.
Additional file 2: Supplementary information 1.
Additional file 3: Supplementary information 2.
Additional file 4: Supplementary information 3.
Additional file 5: Supplementary table 1.
Additional file 6: Supplementary table 2.
Additional file 7: Supplementary table 3.
Data Availability Statement
Not applicable.