Abstract
Background
Regardless of its rare occurrence, Thymoma remains the most frequently encountered primary tumor of the anterior mediastinum comprising about 50% of all masses in the region. Surgical resection, via thymectomy, remains the mainstay treatment modality. In locally advanced and borderline resectable tumors, neoadjuvant chemotherapy (NACT) may be utilized to increase the chance of R0 resection, raising the question of its efficacy and safety.
Methods
Demographic and clinical data from patients who presented to a tertiary cancer center between January 2015–October 2021 with a diagnosis of thymoma and underwent curative surgical resection was collected. Computed tomography scan was used to delineate clinical staging, tumor size and to detect post-therapeutic variations in tumor burden. The response evaluation criteria in solid tumors (RECIST) was used to classify the effect of NACT on tumor burden. The pathological response was determined by measuring the percentage of necrotic tissue.
Results
A total of 23 patients were diagnosed with thymoma. Most patients were male with a mean age 46 (± 15) years at diagnosis. The most common clinical stage was stage II with 5 patients (22%). A total of 12 patients had NACT as compared to 11 patients who had upfront surgery. The mean change in tumor volume was 165 cm3 (p = 0.079) and the change in and maximum diameter was 1.53 ± 1.49 cm (p < 0.01). The effect of NACT on tumor burden based on RECIST criteria was minimal as 8 patients had stable disease. Based on pathological findings, the average necrotic portion of the tumor was 39.5% (p = 0.152). The overall survival rate is 95.65%, mean survival was 115 months (4–125). Recurrence occurred in 5 patients. The NACT group had a higher risk for recurrence (4; 33.3%) with a mean survival of 43.8 months compared to 59.6 months in those who did not receive induction therapy.
Conclusions
The exact role of induction chemotherapy in locally advanced thymoma patients remains controversial. NACT effect after utilizing radiological and pathological assessment tools was not found to significantly improve oncological outcomes compared to upfront surgery in locally advanced disease, with minimal radiologic and pathologic effect. To further demonstrate the impact of induction chemotherapy, we recommend multicentric collaborative studies.
Keywords: Thymoma, Neoadjuvant chemotherapy, Radiographic effect, Histopathological effect
Introduction
Tumors of the thymus gland, termed thymomas, are rare but remain the most frequently encountered primary tumor of the anterior mediastinum comprising about 75–80% of all masses in the region [1]. This constitutes Thymic epithelial tumors (TETs), originating from the epithelial lining of the thymus [2]. According to World Health Organization Classification, thymoma has 8 major subtypes (type A, atypical type A variant, Type AB, Type B1. Type B2, Type B3, micronodular thymoma with lymphoid stroma, and metaplastic thymoma) with the addition of three rare subtypes (microscopic thymoma; sclerosing thymoma, lipofibroadenoma) [3], while Masoka –Koga staging is based on tumor invasiveness [4] Surgical resection, via thymectomy, remains the mainstay treatment modality for early and recurrent cases [5]. In contrast, a multi-disciplinary approach of chemo-radiotherapy, followed by surgery is utilized in locally advanced or metastatic thymoma [6].
In locally advanced and borderline resectable tumors, Masaoka-Koga stage III/IVA, induction chemotherapy may be warranted to increase the rate of complete resection with negative margins [7].
Moreover, evidence on the outcomes of individuals with advanced illness who received induction treatment is inconsistent. Herein, we present comparison between two groups of patients who were diagnosed with thymoma and either underwent neoadjuvant therapy before surgical resection or had upfront surgery.
Methods
Relevant data from patients who were diagnosed to have thymoma and treated at King Hussein Cancer Center between January 2015–October 2021 was accessed after obtaining institutional review board (IRB#). Electronic medical records were utilized to obtain patients’ demographic data and clinical characteristics including; age, gender, smoking status, staging, treatment regimens, response, post-treatment, or post-operative events, and comorbidities. Immunological studies have been carried out to confirm co-existing Myasthenia Gravis (MG).
Radiological evaluation
Contrast-enhanced computed tomography (CT) scan was used to delineate clinical staging, tumor size and to detect post-neoadjuvant chemotherapy treatment effect on tumor burden. The response evaluation criteria in solid tumors (RECIST) was used to classify the effect of neoadjuvant chemotherapy (NACT) on tumor burden as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD). Comparison was made between pre-neoadjuvant therapy initiation and post-operatively. All images were evaluated by a single expert radiologist (Fig. 1).
Fig. 1.
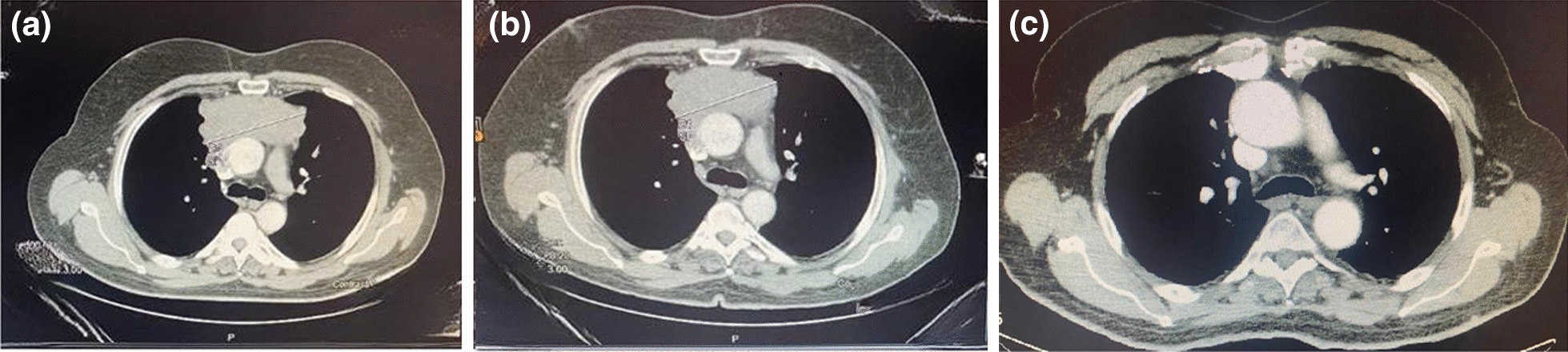
a Computed tomography (CT) of thymoma tumor pre-neoadjuvant chemotherapy. b Computed tomography (CT) of thymoma tumor post-neoadjuvant chemotherapy. c Chest computed tomography (CT) after surgery
Pathological evaluation
All histological specimens were received immediately postoperatively in formalin providing information about histological type, margins, regional lymph nodes, and the presence of lymphovascular invasion. The pathological response was determined by measuring the percentage of necrotic tissue (Fig. 2).
Fig. 2.

a Histological view of thymoma tissue. b Necrotic thymoma histology
Surgical approach
All patients underwent surgical resection of the thymoma either via open approaches utilizing the median sternotomy or thoracotomy. Some patients underwent minimally invasive with multiple or single port Video assisted thoracoscopic surgery (VATS).
Results
A total number of 23 patients were included in this retrospective analysis.
The majority of the patients were male with a mean age of 46 ± 15 at diagnosis. Clinical stage distribution was found to be: stage I (3; 13%), stage II (5; 22%), stage III (3; 13%), stage IV (12; 52%). Myasthenia Gravis was diagnosed in 22% of patients.
Patients who received NACT were distributed among stage I (1, 8%), stage III (2, 16%) and stage IVA (9, 39%). Chemotherapy regimen was a combination of doxorubicin, cisplatin, and cyclophosphamide. Eleven patients underwent VATS tumor resection with two in the NACT group (18%). Negative resection margins were found in 18 (78%) patients (Table 1). A single patient received definitive radiation and 12 cycles of chemotherapy outside our center, developed chemotherapy-induced heart failure, and died immediately postoperatively. No other patients reported severe toxicity. It was noted that those who had advanced clinical stage (Stage IV) were more likely to receive neoadjuvant therapy (p = 0.013) and those who had an open thymectomy procedure were more likely to have neoadjuvant therapy (p = 0.002) as shown on Table 2.
Table 1.
Patient characteristics
| Variable | Number of patients n = 23 |
|---|---|
| Age (years) | 46.28 (± 15.3) |
| Gender | |
| Male | 18 (78%) |
| Female | 5 (22%) |
| Comorbiditiesα | |
| Diabetes mellitus | 3 (13%) |
| Hypertension | 3 (13%) |
| Otherβ | 5 (22%) |
| Histologic subtypes | |
| A | 1 (4%) |
| AB | 4 (17%) |
| BI | 1 (4%) |
| BII | 8 (35%) |
| BIII | 9 (39%) |
| Staging of Masaoka | |
| Stage I | 3 (13%) |
| Stage II | 5 (22%) |
| Stage III | 3 (13%) |
| Stage IV | 12 (52%) |
| Myasthenia graves | 5 (22%) |
| Neoadjuvant chemotherapy | |
| Stage I | 1 (8%) |
| Stage III | 2 (16%) |
| Stage IIV | 9 (75%) |
| Surgical approach | |
| VATSΩ | 11 (48%) |
| Open | 12 (52%) |
| Negative resection margin (R0) | 18 (78%) |
α Two patients had diabetes mellitus and hypertension simultaneously
β Included: hyperlipidemia, carcinoid syndrome, heart failure, atrial fibrillation, Immune thrombocytopenic purpura, and epilepsy
Ω Video-assisted thoracoscopic surgery
Table 2.
Univariate analysis between those who received neoadjuvant therapy and those who did not
| Variable | Total N = 23 |
Neoadjuvant | p Value | |
|---|---|---|---|---|
| Yes N = 12 |
No N = 11 |
|||
| Gender | ||||
| Female | 5 | 3 (60%) | 2 (40%) | 0.99 |
| Male | 18 | 9 (50%) | 9 (50%) | |
| Negative resection margin | ||||
| No | 5 | 4 (80%) | 1 (20%) | 0.317 |
| Yes | 18 | 8 (44%) | 10 (56%) | |
| Smoking | ||||
| Missing | 1 | 0 | 1 | 0.99 |
| Ex-smoker | 4 | 2 (50%) | 2 (50%) | |
| No | 12 | 7 (58%) | 5 (42%) | |
| Yes | 6 | 3 (50%) | 3 (50%) | |
| Stage | ||||
| I | 3 | 1 (33%) | 2 (67%) | 0.013 |
| II | 5 | 0 | 5 (100%) | |
| III | 3 | 2 (67%) | 1 (33%) | |
| IV | 12 | 9 (75%) | 3 (25%) | |
| Histology | ||||
| A | 1 | 0 | 1 (9.1%) | 0.99 |
| AB | 4 | 2(16.7%) | 2 (18.2%) | |
| BI | 1 | 0 | 1 (100%) | |
| BII | 8 | 4 (50%) | 4 (50%) | |
| BIII | 9 | 6 (67%) | 3 (33%) | |
| Surgical approach | ||||
| Open | 12 | 10 (83%) | 2 (17%) | 0.002 |
| VATS | 11 | 2 (18%) | 9 (82%) | |
| Adjuvant therapy | ||||
| No | 8 | 5 (63%) | 3 (38%) | 0.667 |
| Yes | 15 | 7 (47%) | 8 (53%) | |
| Complications | ||||
| No | 17 | 8 (47%) | 9 (53%) | 0.640 |
| Yes | 6 | 4 (67%) | 2 (33%) | |
| Lymphovascular invasion | ||||
| Missing | 7 | 4 (57%) | 3 (43%) | 0.282 |
| No | 11 | 4 (36%) | 7 (64%) | |
| Yes | 5 | 4 (80%) | 1 (20%) | |
| Myasthenia gravis diagnosis | ||||
| No | 18 | 11 (61%) | 7 (39%) | 0.155 |
| Yes | 5 | 1 (20%) | 4 (80%) | |
| Current status | ||||
| Alive | 22 | 11 (50%) | 11 (50%) | 0.99 |
| Dead | 1 | 1 (100%) | 0 | |
| Recurrence | ||||
| No | 18 | 8 (44%) | 10 (56%) | 0.317 |
| Yes | 5 | 4 (80%) | 1 (20%) | |
| Cystic component | ||||
| Missing | 3 | 2 (67%) | 1 (33%) | 0.474 |
| No | 18 | 10 (56%) | 8 (44%) | |
| Yes | 2 | 0 | 2 (100%) | |
Radiological evaluation
The mean change in tumor volume and maximum diameter was 165 cm3 (p = 0.079) and 1.53 ± 1.49 cm (p < 0.01) respectively (Table 3). Tumor diameter stratified according to stage showed a variation of 2.0 ± 1.6 cm in stage IVa (p = 0.02) and 1 ± 1.35 cm in stage III (p = 0.08), respectively. The effect of NACT on tumor burden based on RECIST criteria was minimal as 80% (n = 8) of patients had SD and the remaining 2 patients had PR and PD.
Table 3.
Radiographic effect of NACT on thymic tumors
| Variable | Measurement | p-Value |
|---|---|---|
| Change in tumor volume (mean) | 165 cm3 | 0.079 |
| Change in maximum diameter | 1.53 cm ± 1.49 | < 0.01 |
| Tumor diameter | ||
| Stage III | 1 cm ± 1.35 | 0.08 |
| Stage IV | 2.0 cm ± 1.6 | 0.02 |
Pathological evaluation
Based on pathological findings, the average necrotic portion of the tumor was 39.5%. Although the portion of necrosis was evidently higher in stage IVA (56% vs 23%), these values were not statistically significant (p = 0.152) as shown in Table 4.
Table 4.
Histopathological effect of NACT on thymic tumors
| NACT effect on tumors | No. of patients N = 12 |
|---|---|
| Stable disease | 8 (66%) |
| Partial response | 1 (8%) |
| Progressive disease | 1 (8%) |
Survival analysis
There was only a single documented death in the pre-operative chemotherapy group. This patient underwent surgical resection one year after receiving definitive chemoradiation outside our center. Consequently, median survival could not be defined. A time-dependent survival analysis of surgical techniques encompassing overall survival (OS) and recurrence-free survival (RFS) was done. An overall survival rate of 95.65%, yielding a mean survival of 115 months (4–125). Recurrence occurred in 22% (n = 5). The NACT group had a higher risk for recurrence (4; 33%) with a mean survival of 43.8 months in comparison to 59.6 months in those who did not receive induction therapy (Fig. 3).
Fig. 3.
Kaplan Meier curves showing overall survival and recurrence free survival between those who had neoadjuvant therapy and those who did not receive neoadjuvant therapy
Discussion
We are presenting a study in which we retrospectively compared between two groups of thymoma patients. Although our study group number is small but we combined both radiological and pathological tools for better assessment of neoadjuvant chemotherapy effect on tumor burden, and we found that the radiographic and histopathological effect of NACT on thymic tumors is minimal especially on stage III with the greatest variation in tumor burden is in Stage IVa, and did not have any significant oncological outcome effect.
Thymoma, which is the most common anterior mediastinal tumor presents with chest pain, cough and dyspnea and can be accompanied by autoimmune disorders like myasthenia gravis and pure red cell aplasia. One literature described a patient with autoimmune chronic pancreatitis who presented with steatorrhea beside amenorrhea resulting from high gonadotrophin levels [8]. Thymectomy is an optimal choice for treatment but in some cases, the advancement of the tumor and the invasion into adjacent structures open the gate for multimodality treatment of neoadjuvant chemotherapy, surgery, and radiotherapy, in turn, to shrink tumor size and make it possible to resect the tumor with negative margins.
Locally invasive thymic tumors have been found to be responsive to platinum-based therapy with an 80–90% response rate [9], ranging from PAC (cisplatin, doxorubicin, and cyclophosphamide), ADOC (Adriamycin, cisplatin, vincristine, and cyclophosphamide) and CEVP-16 (cisplatin, epidoxorubicin, and etoposide). The response was determined based on two modalities in different studies, radiologic and pathological evaluation.
Radiologic evaluation using RECIST criteria; Berruti et al., reported an (83.3%) PR [8], Lucchi et Al., reported PR in 20 out of 30 patients (66%) and 2 of 30 (6%) CR [10]. On the other hand, Fornasiero reported 30% CR and one PR [11]. Although the mean change in tumor volume in our study was higher than that reported in the literature, it was not statistically significant. Suh et al., using 3D reconstructed imaging, described a significant change in tumor volume of 125 cm3 (p = 0.001) [12]. One paper found that radiologic evaluation was not a completely reliable method to judge tumor response [13]. Which has guided other papers to use the pathological evaluation response, indicating necrosis, cystic changes, hemorrhage, and calcification. A limitation of this method rose since the fact that all these histological findings can be found in untreated tumors which will make it difficult to distinguish a chemotherapy effect from untreated tumor findings [14].
Cisplatin-based regimens display minimal effect on achieving negative resection margins [12]. Similarly, although Berruti et al. reported a high response rate, only one of the six patients displayed negative surgical margins [15]. Other papers reported high R0 rates, Mineo et al. reported 51% [13] and Lucchi reported 23 cases of 30 means 76% [10]. Therefore, NACT may prove its utility in preoperative debulking of the tumor in preparation for surgical resection.
Predictor factors in invasive thymoma; combined p27 low, p21 low, and p53 expression proved a predictor of poor response to neoadjuvant chemotherapy p = 0.001 [16]. Histopathologically, Weissferdt Et al. found a higher tumor response rate in tumors that displayed an extensive lymphocytic component, WHO type B1 and B2 [14]. Additionally, pathological stage T3 and smaller tumor size of less than 5 cm have shown better response [17]. In contrast, although not statistically significant we found a higher portion of necrosis in stage IVA (56%) when compared to stage 3 (23%) (p = 0.152).
Reported side effects were nausea and vomiting, alopecia, leukopenia, cardiac toxicity, and infection [10]. Postoperative morbidity was higher in the NACT group vs. the surgery alone group (p < 0.03), expressing respiratory failure, pneumonia, extensive reconstruction (P = 0.05), and complete resection [13]. Mortality due to metastasis, myasthenia complications, myocardial ischemia, and pulmonary fibrosis [10, 13]. Speaking of myasthenia gravis, one literature did not report any deterioration due to chemotherapy and attributed it to effective medical therapy [10].
The literature illustrates no significant difference in overall survival among patients who underwent primary surgery vs neoadjuvant chemotherapy (p = 0.285) [12, 18, 19]. On the other hand, Yamada et al., who studied stage III patients, observed less favorable outcomes in patients given neoadjuvant chemotherapy [20]. Long-term survival was negatively affected by the triple combination of P27, P21, and P53 and incomplete resection (p < 0.0001) [10].
Lastly, we are aware that our definition of pathological response may result in an underestimation of the results. According to the literature, the pathological response does not rely entirely on necrosis as histopathological changes were found to encompass cystic changes, hemorrhage, histiocytic proliferation, and calcifications [21].
Our study has limitations, (1) it was conducted in single tertiary cancer center but King Hussein Cancer Center is considered one of the main cancer centers in the Middle East and the region, receiving multiple referrals from neighboring countries. (2) the study cohort was small and that can be explained by the rarity of the disease. (3) the retrospective design of the study which can only be utilized in such cases due to inability to perform randomization on such population.
Conclusions
Induction chemotherapy in locally advanced thymoma patients' theoretical role to increase the chance of R0 resection still remains controversial. Despite our small study group number, we found that the radiographic and histopathological effect of NACT on thymic tumors is minimal with the greatest variation in tumor burden is in Stage IVa. However, regardless of stage, NACT was not found to significantly improve oncological outcomes compared to upfront surgery. With all above-mentioned variable results, more studies are required to adjust guidelines for NACT, but due to the rare epidemiology of this tumor it will be difficult to induce a clinical trial but prospective institution experiences.
Acknowledgements
None.
Abbreviations
- TETs
Thymic epithelial tumors
- NACT
Neoadjuvant chemotherapy
- MG
Myasthenia Gravis
- CPG
Clinical Practice Guidelines
- CT
Computed tomography
- RECIST
Response evaluation criteria in solid tumors
- CR
Complete response
- PR
Partial response
- SD
Stable disease
- PD
Progressive disease
- VATS
Video assisted thoracoscopic surgery
- OS
Overall survival
- RFS
Recurrence-free survival
Author contributions
FAA and ZO wrote the first draft of the manuscript, performed the literature search guided by RAJ. FAA, AKH, TBS, HH, and MKAC performed data collection. MKAC provided the first revision of the manuscript. AG provided radiological consult and expertise. RAJ, AA-S, and AA-E were the surgical team who performed the procedures in the study. RAJ oversaw the study design, guided the literature search, and provide major revision. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by The Institutional Review Board at King Hussein Cancer Center with reference number 19KHCC145 on 31/Dec/2019. Waiver of consent was obtained due to the retrospective nature of the study.
Consent for publication
Waived.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y, et al. Thymoma and thymic carcinoma: Surgical resection and multidisciplinary treatment. Cancers. 2023;15(7):1953. doi: 10.3390/cancers15071953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okumura M, Shiono H, Minami M, et al. Clinical and pathological aspects of thymic epithelial tumors. Gen Thorac Cardiovasc Surg. 2008;56(1):10–16. doi: 10.1007/s11748-007-0177-8. [DOI] [PubMed] [Google Scholar]
- 3.Marx A, Detterback F, Marom EM, et al. Tumours of the thymus. In: WHO Classification of Tumours Editorial Board. Thoracic tumours [Internet]. Lyon (France): International Agency for Research on Cancer; 2021. 12 Sept 2021. (WHO classification of tumours series, 5th ed.; vol. 5). https://tumourclassification.iarc.who.int/chapters/35.
- 4.Detterbeck FC, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thor Oncol. 2011;6(7):S1710–S1716. doi: 10.1097/JTO.0b013e31821e8cff. [DOI] [PubMed] [Google Scholar]
- 5.Margaritora S, et al. Single-centre 40-year results of redo operation for recurrent thymomas. Eur J Cardio-Thor Surg. 2011;40(4):894–901. doi: 10.1016/j.ejcts.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Strobel P, Bauer A, Puppe B, Kraushaar T, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. J Clin Oncol. 2004;22(8):1501–1509. doi: 10.1200/JCO.2004.10.113. [DOI] [PubMed] [Google Scholar]
- 7.Macchiarini P, Chella A, Ducci F, et al. Neoadjuvant chemotherapy, surgery, and postoperative radiation therapy for invasive thymoma. Cancer. 1991;68(4):706–137. doi: 10.1002/1097-0142(19910815)68:4<706::AID-CNCR2820680407>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Roncari A, Gorzegno G, et al. Neoadjuvant chemotherapy with adriamycin, cisplatin, vincristine and cyclophosphamide (ADOC) in invasive thymomas: results in six patients. Ann Oncol. 1993;4(5):429–431. doi: 10.1093/oxfordjournals.annonc.a058527. [DOI] [PubMed] [Google Scholar]
- 9.Fornasiero A, Daniele O, Ghiotto C, et al. Chemotherapy for invasive thymoma. A 13-year experience. Cancer. 1991;68:30–33. doi: 10.1002/1097-0142(19910701)68:1<30::AID-CNCR2820680106>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Lucchi M, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thor Oncol. 2006;1(4):308–313. doi: 10.1016/S1556-0864(15)31586-0. [DOI] [PubMed] [Google Scholar]
- 11.Fornasiero A, Daniele O, Ghiotto C et al. Neoadjuvant treatment in locally advanced thymoma. In: 3rd International Congress on Neoadjuvant Chemotherapy: 1991; 90.
- 12.Suh JW, Park SY, Lee CY, et al. Neoadjuvant therapy for thymic neoplasms reduces tumor volume per 3D-reconstructed images but does not improve the complete resection rate. PLoS ONE. 2019;14(3):e0214291. doi: 10.1371/journal.pone.0214291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mineo TC, et al. New predictors of response to neoadjuvant chemotherapy and survival for invasive thymoma: a retrospective analysis. Ann Surg Oncol. 2010;17(11):3022–3029. doi: 10.1245/s10434-010-1134-9. [DOI] [PubMed] [Google Scholar]
- 14.Weissferdt A, Moran CA. The impact of neoadjuvant chemotherapy on the histopathological assessment of thymomas: a clinicopathological correlation of 28 cases treated with a similar regimen. Lung. 2013;191(4):379–383. doi: 10.1007/s00408-013-9465-5. [DOI] [PubMed] [Google Scholar]
- 15.Bretti S, Berruti A, Loddo C, et al. Multimodal management of stages III-IVa malignant thymoma. Lung Cancer. 2004;44:69–77. doi: 10.1016/j.lungcan.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Mineo TC, Mineo D, Onorati I, et al. New predictors of response to neoadjuvant chemotherapy and survival for invasive thymoma: a retrospective analysis. Ann Surg Oncol. 2010;17(11):3022–3029. doi: 10.1245/s10434-010-1134-9. [DOI] [PubMed] [Google Scholar]
- 17.Leuzzi G, Rocco G, Ruffini E, et al. Multimodality therapy for locally advanced thymomas: a propensity score-matched cohort study from the European Society of Thoracic Surgeons Database. J Thorac Cardiovasc Surg. 2016;151:47–57. doi: 10.1016/j.jtcvs.2015.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Leuzzi G, Alessandrini G, Sperduti I, et al. Induction therapy versus initial surgery in advanced thymic tumors: perioperative and oncological outcome. Thorac Cardiovasc Surg. 2017;65(3):234–243. doi: 10.1055/s-0035-1564890. [DOI] [PubMed] [Google Scholar]
- 19.Wei Y, Gu Z, Shen Y, et al; Members of the Chinese Alliance for Research in Thymomas. Preoperative induction therapy for locally advanced thymic tumors: a retrospective analysis using the ChART database. J Thorac Dis. 2016 [DOI] [PMC free article] [PubMed]
- 20.Yamada Y, Yoshino I, Nakajima J, et al. Surgical outcomes of patients with stage III thymoma in the Japanese Nationwide Database. Ann Thorac Surg. 2015;100:961–967. doi: 10.1016/j.athoracsur.2015.04.059. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari S, Hall KS, Luksch R, et al. Nonmetastatic Ewing family tumors: high-dose chemotherapy with stem cell rescue in poor responder patients. Results of the Italian Sarcoma Group/Scandinavian Sarcoma Group III protocol. Ann Oncol. 2011;22(5):1221–1227. doi: 10.1093/annonc/mdq573. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.



