Abstract
Colony-stimulating factor 1 (CSF-1) triggers the activation of intracellular proteins in macrophages through selective assembly of signalling complexes. The separation of multimeric complexes of the CSF-1 receptor (CSF-1R) by anion-exchange chromatography enabled the enrichment of low-stoichiometry complexes. A significant proportion of the receptor in CSF-1-stimulated cells that neither possessed detectable tyrosine kinase activity nor formed complexes was separated from the receptor pool displaying autokinase activity that formed chromatographically distinct multimeric complexes. A small pool of CSF-1R formed a multimeric complex with phosphatidylinositol-3 kinase (PI-3 kinase), SHP-1, Grb2, Shc, c-Src, Cbl, and a significant number of tyrosine-phosphorylated proteins in CSF-1-stimulated cells. The complex showed a considerable amount of CSF-1R complex-associated kinase activity. A detectable level of the complex was also present in untreated cells. PI-3 kinase in the multimeric complex displayed low lipid kinase activity despite the association with several proteins. The major pool of activated CSF-1R formed transient multimeric complexes with distinctly different tyrosine-phosphorylated proteins, which included STAT3 but also PI-3 kinase, Shc, SHP-1, and Grb2. A significant level of lipid kinase activity was detected in PI-3 kinase in the latter complexes. The different specific enzyme activities of PI-3 kinase in these complexes support the notion that the activity of PI-3 kinase is modulated by its association with CSF-1R and other associated cellular proteins. Specific structural proteins associated with the separate CSF-1R multimeric complexes upon CSF-1 stimulation and the presence of the distinct pools of the CSF-1R were dependent on the integrity of the microtubular network.
Macrophage colony-stimulating factor 1 (CSF-1) is a lineage-specific growth factor required for the survival, proliferation, and differentiation of mononuclear phagocytes (51). The biological effects of CSF-1 are mediated through a single class of high-affinity CSF-1 receptor (CSF-1R) encoded by the c-fms proto-oncogene (48). The mature glycosylated form of the CSF-1R, expressed as a 165-kDa transmembrane glycoprotein, has a structural domain arrangement characteristic of a family of tyrosine kinase receptors, members of which include platelet-derived growth factor receptor (PDGFR), c-Kit, and Flt3/FLK3 receptor (43).
In the absence of CSF-1, the CSF-1R is present in an aggregated or a dynamic interactive state (27). CSF-1 binding results in a conformational change to the receptor subunits which causes the clustered CSF-1 receptors to form noncovalent dimers, thus activating the receptor tyrosine kinase (27). The activation initiates a cascade of signalling events leading to the transient phosphorylation of primarily cytosolic proteins (47). In parallel with these events, the activated ligand-bound receptor is rapidly lost from the cell surface as a consequence of internalization via clathrin-coated pits (31) before being degraded in a chloroquine-sensitive lysosomal compartment (15). Although the ligand and receptor initially share the same endocytic pathway, our recent study suggests that they may be targeted to separate compartments at later stages of degradation in some populations of macrophages (23). The receptor is downmodulated following dephosphorylation and internalization, but the importance of these events in attenuating the biological signal remains unclear (27).
The activation of the CSF-1R upon ligand binding leads to the transphosphorylation of specific tyrosine residues in the cytoplasmic domain of the receptor, and their requirement for CSF-1 signal transduction has been investigated by mutagenesis (6, 45, 54). The sites that have been mapped include Tyr697, Tyr706, and Tyr721 in the kinase insert domain of the murine CSF-1R and Tyr807 in the kinase domain. Many of these tyrosine phosphorylation sites serve as binding sites for Src homology 2 (SH2)-containing proteins that relay and amplify the signal from the receptor to the nucleus along specific intracellular signalling pathways (13, 52). Tyr559 in the juxtamembrane domain of the receptor is a binding site for Src family members (2). The adapter protein Grb2 associates with Tyr697, which enables the nucleotide exchange factor Sos1, constitutively bound to Grb2, to activate Ras (28, 54). Tyr706 was identified as a site required for the activation of the STAT1 transcription factor (34), while Tyr721 regulates the CSF-1-induced activity of phosphatidylinositol-3 kinase (PI-3 kinase) through the binding of the regulatory p85 subunit of PI-3 kinase (40).
CSF-1 induces responses in macrophages ranging from early morphological changes, which include membrane ruffling, filopodium formation, cell spreading, and cytoskeletal reorganization, to more long-term effects associated with survival, proliferation, and differentiation of the cell (5). The biological effects elicited by the growth factor are regulated by signalling pathways. The stimulation of such pathways triggers the phosphorylation and activation of intracellular proteins which selectively assemble into signalling complexes. The localization of these complexes in the cell has been found to be essential to signal transmission by an extracellular stimulus (37). CSF-1R expressed in primary bone marrow-derived macrophages or CSF-1-dependent macrophage cell lines has not been found to associate with many signalling proteins in stoichiometric amounts on ligand activation (7, 24, 28). The discovery of new potential binding partners for CSF-1R by the sensitive yeast two-hybrid technique (7) and the recent demonstration of a transient association between the tyrosine phosphatase SHP-1, p130 tyrosine-phosphorylated protein, BIT, and the CSF-1R in CSF-1-stimulated macrophages (53) emphasize not only the existence of yet-to-be discovered binding proteins but also the need to progress to methods that allow for more sensitive detection of transient complexes. Given our previous observation that a complex formed between CSF-1R, PI-3 kinase, and several tyrosine-phosphorylated proteins in CSF-1-treated macrophages is stable to anion-exchange chromatography (24), we fractionated cell lysate by anion-exchange chromatography to enrich for other CSF-1R complexes present in small amounts in the cell. We report the separation of chromatographically stable multimeric complexes of CSF-1R with distinct tyrosine-phosphorylated proteins, some of which have been identified to be signalling molecules previously not shown to form a complex with the activated CSF-1R. The study also examines the different pools of two of the signalling molecules, PI-3 kinase and SHP-1, in detail and their interaction with other tyrosine-phosphorylated proteins, in particular their association with chromatographically separate pools of the activated CSF-1R. The characterization of the various pools of signalling molecules may provide further understanding of the regulation of activity of the signalling proteins through specific assembly of complexes.
MATERIALS AND METHODS
Reagents.
The following antibodies were obtained from commercial sources: monoclonal antiphosphotyrosine antibody 4G10 (anti-pTyr) conjugated to horseradish peroxidase (HRP), rabbit polyclonal antibody against the p85α subunit of PI-3 kinase (anti-p85α), and rabbit polyclonal antibody against SHP-1 (Upstate Biotechnology, Lake Placid, N.Y.); rabbit polyclonal antibodies anti-Cbl, anti-Shc, and anti-c-Src and goat polyclonal antibody anti-PI-3 kinase p110α (Santa Cruz Biotechnology, Santa Cruz, Calif.); monoclonal antibodies antidynamin and anti-Grb2 as well as polyclonal antibodies anti-STAT3 and anti-TYK2 (Transduction Laboratories); and mouse anticlathrin (ICN Biochemicals Inc., Costa Mesa, Calif.). Polyclonal anti-CSF-1R antibody, used for immunoprecipitations, was raised in our laboratory as described previously (24). A second polyclonal antibody to the kinase domain of the murine CSF-1R (28), used for immunoblotting membranes, was a gift from L. Rohrschneider (Fred Hutchinson Cancer Research Center, Seattle, Wash.). The reagents cytochalasin D and nocodazole were purchased from ICN Biochemicals (Aurora, Ohio) and Sigma, respectively. Purified human recombinant CSF-1 and PDGF were gifts from Chiron, Emeryville, Calif., and Amgen Pharmaceuticals, Boulder, Colo., respectively.
Cell culture conditions.
The CSF-1-dependent murine macrophage cell line BAC1.2F5 was grown in 15-cm-diameter tissue culture plates as described previously (24). The cells were seeded at a density of 105 cells/ml and cultured until subconfluent. BAC1.2F5 cells were rendered quiescent by reculturing them in growth medium lacking L-cell-conditioned medium for 18 to 20 h prior to stimulation with CSF-1 at 5,000 U/ml at 37°C for the times indicated in Results. Experiments involving depolymerizing agents were carried out by pretreating BAC1.2F5 cells with nocodazole (40 μg/ml) or cytochalasin D (2.5 μM) for 60 min at 37°C prior to stimulation with growth factor.
Immunoprecipitations.
Untreated or CSF-1-treated BAC1.2F5 cells were placed on ice and washed twice in ice-cold phosphate-buffered saline prior to solubilization in lysis buffer containing 25 mM Tris-Cl (pH 7.5), 137 mM NaCl, 1 mM EDTA, 1% Triton X-100, 10 μg of aprotinin per ml, 1 μM leupeptin, 1 μM pepstatin, 0.1 mM pefabloc, 50 mM sodium fluoride, 50 mM β-glycerophosphate, and 1 mM sodium vanadate at 4°C. Extract containing 2 mg of protein was precleared before immunoprecipitation with specific antibodies overnight at 4°C followed by incubation with protein A-Sepharose for a further 1 h. Immunoprecipitated proteins were resolved on a sodium dodecyl sulfate (SDS)–10% polyacrylamide gel and transferred to a nitrocellulose membrane for immunoblotting. Proteins recognized by the primary antibody were visualized with HRP-conjugated secondary antibodies and enhanced chemiluminescence reagents (Amersham Corp.). Blots were reprobed with other primary antibodies after removal of bound antibody by incubation in 62.5 mM Tris-Cl (pH 6.7)–0.1 M 2-mercaptoethanol–2% SDS (60°C, 30 min).
Column chromatography.
Untreated or CSF-1-treated (2 or 30 min at 37°C) BAC1.2F5 cells were lysed in solubilization buffer as described above. Subsequent steps, including the column chromatography, were carried out at 4°C. Approximately 3 mg of total protein extract was dialyzed against 100 ml of buffer A (10 mM Tris-Cl [pH 7.5], 40 mM β-glycerophosphate, 1 mM dithiothreitol, 1 mM EGTA, 0.1 mM sodium vanadate). The extract was then centrifuged at 15,000 × g for 5 min to remove unsolubilized material and loaded onto a 1-ml MonoQ anion-exchange Econo column (Bio-Rad) equilibrated in buffer A. The column was washed in 10 volumes of buffer A at a flow rate of 1 ml/min. The unadsorbed fractions were pooled for analysis. A gradient of 0 to 400 mM NaCl was then applied, and 1-ml fractions collected over 30 min for analysis. The unadsorbed material and every third fraction collected over the NaCl gradient were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with specific antibodies as indicated in Results. Equal volumes of the unadsorbed fraction (0.3 ml) and eluted fractions from the salt gradient were immunoprecipitated with the specific antibodies, and the immunoprecipitates were analyzed by immunoblotting the membrane with specific antibodies.
In vitro kinase activity.
Equal volumes (0.3 ml) of fractions following the separation of BAC1.2F5 lysates on the MonoQ anion-exchange column were immunoprecipitated with anti-CSF-1R and assayed for in vitro CSF-1R kinase activity. The kinase assay was carried out by resuspending the protein A-Sepharose in 20 μl of kinase buffer (25 mM HEPES [pH 7.5], 10 mM MnCl2, 0.1 mM sodium vanadate, 50 mM sodium fluoride, 50 mM β-glycerophosphate, 1 μM leupeptin, 1 μM pepstatin, and 0.1 mM pefabloc) and incubating in the presence of 10 μCi of [γ-32P]ATP (4,000 Ci/mmol) at 30°C for 15 min. The reaction was terminated by adding SDS loading buffer, and the samples were analyzed by separation on SDS-PAGE. The polyacrylamide gel was incubated in 5 M KOH at 55°C for 60 min in order to remove background contributed by serine phosphorylation (10). The gel was dried, and results were analyzed by autoradiography.
PI-3 kinase assay.
Equal volumes (0.4 ml) of the fractions obtained from chromatographic separation of cell lysate were immunoprecipitated with anti-p85α, and the immunoprecipitates were assayed for PI-3 kinase activity in vitro as described previously (23, 39).
RESULTS
CSF-1R complexes detected by immunoprecipitation.
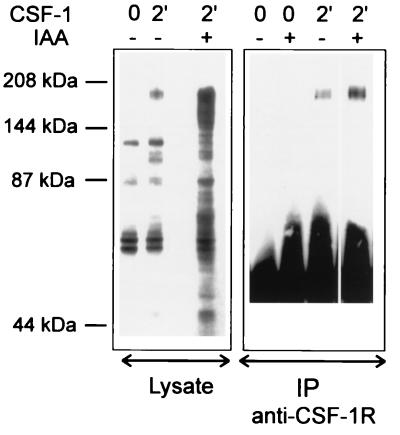
CSF-1 stimulation of macrophages has been shown to result in the assembly of different complexes involving the CSF-1R (19, 24, 28, 54). However, not all signal transduction complexes formed can be demonstrated by coimmunoprecipitation studies, especially when antireceptor antibodies are used for the immunoprecipitation (7, 24, 53). We were unable to detect significant association of tyrosine-phosphorylated proteins with the CSF-1R in anti-CSF-1R immunoprecipitates of lysates prepared from CSF-1-treated BAC1.2F5 cells (Fig. 1), consistent with observations made by others (3, 12, 39, 58), and attempts to increase the sensitivity of the assay by pretreating the macrophages with iodoacetic acid (IAA) prior to CSF-1 treatment also failed to show associated tyrosine-phosphorylated proteins (Fig. 1). In agreement with the earlier work, we did observe an overall increase in the tyrosine phosphorylation of cellular proteins (Fig. 1). This effect has been shown to be due, at least in part, to inhibition of receptor internalization (27) but may also be a consequence of inactivation of protein tyrosine phosphatases through carboxymethylation of the cysteine residue at the active site (61) by IAA. These observations might suggest that only a small population of CSF-1R physically associates with intracellular signalling molecules and therefore the complexes cannot be detected in CSF-1R immunoprecipitates from crude cell lysate. In the following experiments, we demonstrated that enrichment of CSF-1R by column chromatography allows us to detect the presence of multimeric CSF-1R-containing complexes. Furthermore, association with different intracellular proteins gives rise to several chromatographically distinct CSF-1R-containing complexes.
FIG. 1.
Tyrosine-phosphorylated proteins in CSF-1R immunoprecipitates. Quiescent BAC1.2F5 cells were treated with 8 mM IAA for 15 min at 37°C where indicated before stimulation with CSF-1 for 2 min at 37°C. Lysates (left panel) or anti-CSF-1R immunoprecipitates (IP) of the lysates (right panel) were resolved by SDS-PAGE, and proteins were transferred to nitrocellulose. The immunoblots were probed with anti-pTyr (4G10)–HRP. The positions of prestained molecular mass markers are indicated.
Fractionation of CSF-1R, signalling proteins, and other tyrosine-phosphorylated proteins from cell lysates on anion-exchange chromatography.
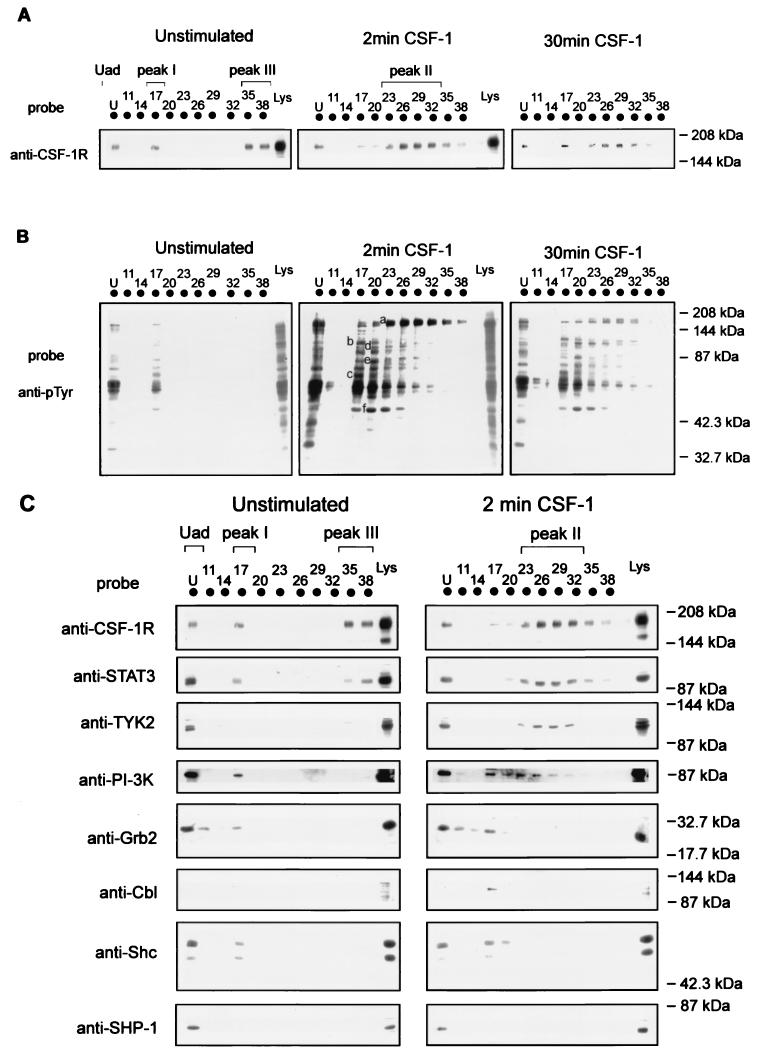
Solubilized lysates prepared from untreated or CSF-1-treated BAC1.2F5 cells were applied to an anion-exchange column, and protein fractions were eluted with an NaCl gradient. The fractions were then analyzed for the presence of CSF-1R. In untreated BAC1.2F5 cell lysate, CSF-1R fractionated into three pools: one pool present in the unadsorbed fraction, a second distinct pool eluting at approximately 90 mM NaCl (fraction 17 [Fr 17]), and a third pool, containing the majority of the immunoreactivity to CSF-1R, eluting at 330 to 370 mM NaCl (Fr 35 to 38) (Fig. 2A). Fractions eluting beyond Fr 38 did not contain detectable levels of CSF-1R (data not shown).
FIG. 2.
Distribution of CSF-1R, tyrosine-phosphorylated proteins, and signalling proteins in untreated and CSF-1-stimulated lysates following anion-exchange column chromatography. (A) Solubilized lysate of untreated or CSF-1-treated (2 or 30 min at 37°C) BAC1.2F5 cells was applied to a MonoQ anion-exchange column (Bio-Rad), and protein fractions were eluted with a 0 to 0.4 M NaCl gradient. Aliquots of 1-ml fractions and the initial lysate (Lys) were analyzed by SDS-PAGE, and protein was transferred to nitrocellulose which was immunoblotted with anti-CSF-1R. The pools containing CSF-1R are indicated, in order of their elution from the ion-exchange column, as Uad (unadsorbed), peak I (Fr 17), peak II (Fr 23 to 32; evident on CSF-1 treatment), and peak III (Fr 35 to 38). (B) The membrane was reprobed with anti-pTyr (4G10)–HRP. Tyrosine-phosphorylated CSF-1R (band a) and other tyrosine-phosphorylated proteins (bands b, c, d, e, and f) enriched in specific fractions are marked. (C) The immunoblots of fractions from the separation of untreated or 2-min-CSF-1-treated BAC1.2F5 cell lysates (Lys) were also probed with antibodies to specific signalling proteins as indicated. The fractionations were representative of eight experiments.
Stimulation of the macrophages with CSF-1 (2 min) resulted in a change in the distribution of CSF-1R from that observed with untreated cell lysate. The most significant change was a shift in the immunoreactivity of CSF-1R to a broad peak over a range of NaCl concentrations (170 to 300 mM NaCl) (Fr 23 to 32) (Fig. 2A). For ease of discussion, we have named the pools containing CSF-1R, in order of their elution from the ion-exchange column, Uad (unadsorbed), peak I (Fr 17), peak II (Fr 23 to 32; evident only on CSF-1 treatment), and peak III (Fr 35 to 38). The amount of detectable CSF-1R in Uad remained relatively unchanged on CSF-1 stimulation, while the CSF-1R in peak I diminished slightly. Extension of the incubation time with CSF-1 to 30 min showed the distribution of the fractionated CSF-1R to follow a profile similar to that of 2-min-stimulated cell lysate, but the level of CSF-1R immunoreactivity was considerably diminished (Fig. 2A), consistent with the internalization of a large proportion of the CSF-1R (32). The fractionations demonstrated in Fig. 2A are representative of eight experiments, and approximately 90 to 96% of the CSF-1R loaded onto the column was recovered following fractionation. These observations suggest that the CSF-1R distributes in particular pools which change with time following CSF-1 binding. Peak II was a transient pool which appeared initially on CSF-1 stimulation but, relative to peak I, rapidly disappeared on further stimulation. The decrease in the level of receptor in peak II corresponded to the time course of internalization and degradation of the CSF-1R (27); longer incubation with CSF-1 (4 h) led to the appearance of a separate pool of receptor in peak III, similar to the distribution in untreated cells (data not shown).
Having established the profile of fractionation of the CSF-1R, we next probed the membrane with anti-pTyr to determine the tyrosine phosphorylation status of the CSF-1R in the various peaks and to monitor the distribution of other proteins that are tyrosine phosphorylated upon CSF-1 stimulation (Fig. 2B). Again, the fractionations were representative of eight experiments, as mentioned above, and close to 100% recovery of tyrosine-phosphorylated proteins in the initial lysate was achieved following fractionation. In unstimulated cells, CSF-1R (p165) in both Uad and peak I was found to be tyrosine phosphorylated and presumably represents the basal level of phosphorylation of the receptor in growth-arrested cells (Fig. 2B). In contrast, the main immunoreactive pool of CSF-1R present in peak III (Fig. 2A) showed no detectable tyrosine phosphorylation (Fig. 2B). CSF-1 stimulation resulted in a significant change in the profile of tyrosine-phosphorylated CSF-1R as well as in those of other tyrosine-phosphorylated proteins. Apart from the tyrosine-phosphorylated CSF-1R (p165), which was enriched in Fr 23 to 32 (Fig. 2B, band a), specific tyrosine-phosphorylated proteins were enriched in particular fractions. For example, p120 (band b) and p65 (band c) were enriched in Fr 17, p100 (bands d) in Fr 20 to 23, and p80 (band e) and p44 (band f) in Fr 20 following the fractionation of 2-min-CSF-1-treated cell lysate (Fig. 2B). Fr 17 (peak I), with a high proportion of tyrosine-phosphorylated proteins, contained only 1% of the total solubilized protein in the cell lysate. On the other hand, 60% of the total protein was present in the Uad fraction, which also contained a separate pool of tyrosine-phosphorylated proteins. Most tyrosine-phosphorylated proteins observed in the untreated and CSF-1-stimulated crude cell lysates were fractionated within the NaCl gradient used (Fig. 2B). The level of tyrosine phosphorylation of most of the proteins fractionated from 30-min-CSF-1-treated cell lysate decreased significantly relative to that of 2-min-CSF-1-treated cells (Fig. 2B), but the profiles of the distributions of tyrosine-phosphorylated proteins fractionated along the gradient did not differ. The observations that the extent of the tyrosine phosphorylation of the proteins did not always parallel the elution profile suggests that the tyrosine phosphorylation status of proteins alone was not responsible for the change in the chromatographic distribution of the CSF-1R and other proteins following CSF-1 stimulation (see Discussion).
In order to identify some of the tyrosine-phosphorylated proteins resolved in Fig. 2B, we next determined the distribution of signalling proteins previously shown to be activated or recruited to the CSF-1R upon CSF-1 stimulation (Fig. 2C). Approximately 90 to 95% of the signalling proteins in the initial lysate were recovered following fractionation, as also found for the CSF-1R above, and most of the detectable immunoreactivity to the signalling proteins eluted within the NaCl concentration range shown. Significant levels of immunoreactivity to most signalling proteins screened (STAT3, TYK2, PI-3 kinase [p85α], Grb2, Shc, and SHP-1) were present in the Uad peak on analysis of either untreated or CSF-1-treated cell lysates (Fig. 2C). However, differences in the distribution of proteins along the NaCl gradient were observed. Proteins involved in the JAK/STAT pathway of activated macrophages, namely, STAT3 and TYK2 (33), showed a significant change in distribution following CSF-1 stimulation. These proteins coeluted with a significant proportion of the adsorbed pool of tyrosine-phosphorylated CSF-1R in peak II (Fig. 2C). PI-3 kinase, previously reported to be activated in ligand-stimulated macrophages (40), also showed a significant change in its distribution on CSF-1 stimulation by fractionating over a broad NaCl concentration range (Fr 17 to 32), which included peak I and peak II (Fig. 2C). PI-3 kinase detected in peak I (Fr 17) with Grb2 and Shc (Fig. 2C) was present in both unstimulated and CSF-1-stimulated cells. Detectable levels of Cbl, a molecule identified to be a negative regulator (36), cofractionated with these proteins in peak I only on CSF-1 stimulation. It is interesting that the cofractionation of a pool of tyrosine-phosphorylated CSF-1R, PI-3 kinase, Cbl, Shc, and Grb2 in CSF-1-stimulated macrophages eluting at 90 mM NaCl was associated with the formation of a multimeric complex which is stable to anion-exchange chromatography (24). The low levels of SHP-1 present in fractions eluted along the NaCl gradient were evident only on immunoprecipitation with anti-SHP-1 antibody (see below). Many of the signalling proteins known to be activated on CSF-1 stimulation comigrated with tyrosine-phosphorylated bands in the adsorbed fractions (data not shown), reflecting the degree of enrichment of the activated or participating pool of signalling molecules.
Activated CSF-1R forms chromatographically distinct multimeric complexes.
Having established first that the chromatographic distributions of the CSF-1R and signalling proteins change on CSF-1 stimulation and second that distinct pools of tyrosine-phosphorylated proteins cochromatograph with separate pools of tyrosine-phosphorylated CSF-1R, we next explored the hypothesis that the distribution was partly a consequence of formation of multimeric complexes containing CSF-1R. We therefore examined anti-CSF-1R immunoprecipitates of the column fractions for specific signalling proteins.
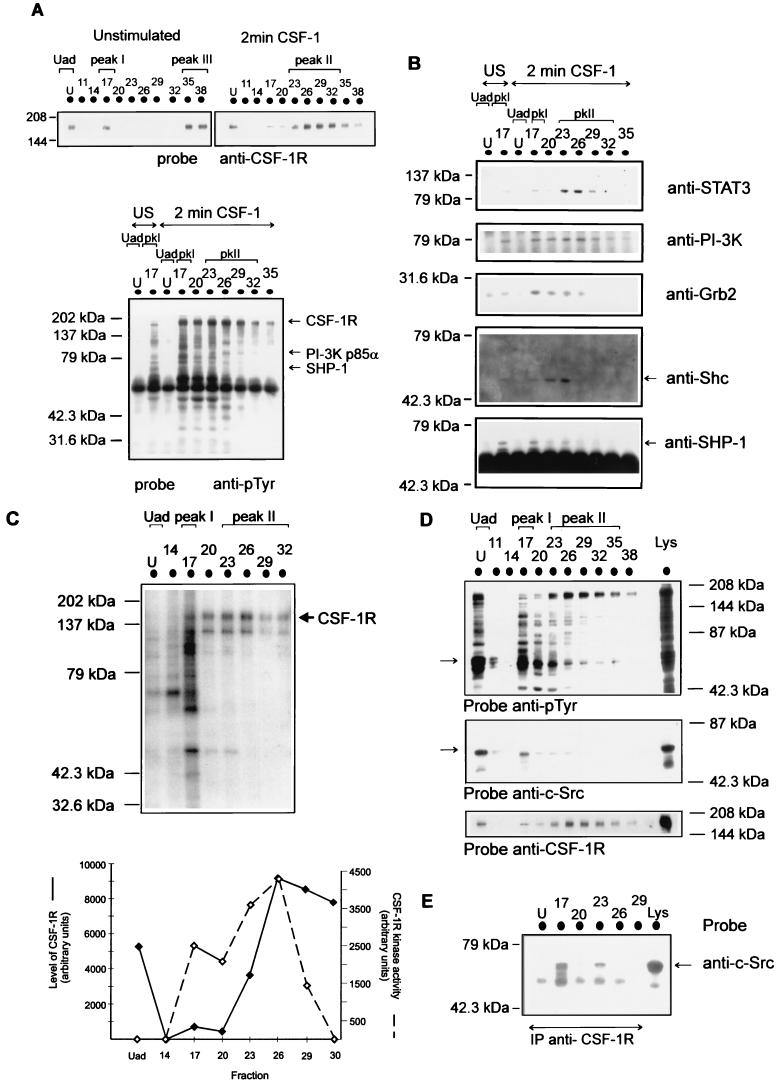
For Fig. 3A, anti-CSF-1R immunoprecipitates of equal volumes of the fractions containing the CSF-1R (Fig. 2A) were initially probed for tyrosine-phosphorylated proteins. We have included again the distribution of CSF-1R in these fractions for convenience. In contrast to the results in Fig. 1, where tyrosine-phosphorylated proteins were not readily detected in anti-CSF-1R immunoprecipitates of CSF-1-treated whole-cell lysate, analysis of immunoprecipitates from the column fractions of CSF-1-treated cell lysate revealed the coimmunoprecipitation of tyrosine-phosphorylated proteins with the tyrosine-phosphorylated CSF-1R (Fig. 3A) in selected fractions (Fr 17 to 32). Hence, an important outcome of the anion-exchange chromatography has been the considerable enrichment of certain pools of receptor forming chromatographically stable multimeric complexes with tyrosine-phosphorylated proteins. The Uad pool of CSF-1R, which was chromatographically separate from the CSF-1R pools in the adsorbed fractions (peak I and peak II), did not coimmunoprecipitate with tyrosine-phosphorylated proteins (Fig. 3A) contained in the same fraction (Fig. 2B).
FIG. 3.
Separation of multimeric complexes of CSF-1R containing distinct signalling proteins. (A) Fractions following the separation of untreated or CSF-1-treated (2 min at 37°C) BAC1.2F5 lysate by anion-exchange chromatography that were identified as containing CSF-1R by immunoblotting (top panel) were immunoprecipitated with anti-CSF-1R. The immunoprecipitates were resolved by SDS-PAGE and transferred to nitrocellulose. The membrane was initially probed with anti-pTyr (4G10)–HRP. The arrows indicate the positions of CSF-1R, PI-3 kinase p85α, and SHP-1. The pools of fractions identified as containing CSF-1R are indicated as Uad, peak I (pKI), and peak II. U, Uad; US, unstimulated. (B) The membrane was then stripped and reprobed with anti-STAT3, anti-PI-3 kinase p85α, anti-Grb2, anti-Shc, and anti-SHP-1 as indicated. (C) The CSF-1R immunoprecipitates of fractions from CSF-1-treated lysate fractionation were also assayed for in vitro kinase activity with [γ-32P]ATP. The proteins were resolved by SDS-PAGE, and the gel was incubated with 5 M KOH at 55°C for 30 min. The dried gel was subjected to autoradiography. The arrow indicates the position of the mature glycosylated form of the CSF-1R (p165). The kinase activity of CSF-1R from the densitometric analysis of p165 and the level of CSF-1R from the densitometric analysis of the immunoblot of the fractions with anti-CSF-1R are shown for each fraction (bottom panel). (D and E) Immunoblots of the fractions following the separation of CSF-1-treated (2 min at 37°C) BAC1.2F5 lysate (Lys) (D) and CSF-1R immunoprecipitates (IP) of the fractions (E) were probed with anti-pTyr or anti-c-Src as indicated.
The fractionated tyrosine-phosphorylated CSF-1R from CSF-1-stimulated lysate coimmunoprecipitated with distinct tyrosine-phosphorylated proteins. The pool of receptor in peak I, although minor, associated with a significant number of highly tyrosine-phosphorylated proteins (Fig. 3A), representing a significant proportion of the tyrosine-phosphorylated proteins present in this fraction (Fig. 2B). The extent of tyrosine phosphorylation of this pool of receptor following immunoprecipitation was also consistently high in proportion to the level of receptor (Fig. 3A). Tyrosine kinases associating with the CSF-1R and being activated during immunoprecipitation with anti-CSF-1R (see below) may provide an explanation for the increased tyrosine phosphorylation. A low level of this complex was also present in the untreated cell lysate fractionation (Fig. 3A, peak I). The later-eluting fractions along peak II containing significant levels of the CSF-1R were also found to coimmunoprecipitate with fewer but distinctly different tyrosine-phosphorylated proteins (Fig. 3A).
It would appear that the CSF-1R-associated complexes identified after column fractionation must be relatively stable, since they survive both column chromatography and subsequent immunoprecipitations. As further support for this concept, Fr 17 (peak I) from CSF-1-treated lysate was reapplied to a Sephacryl S200 gel filtration column, which enabled the fractionation of proteins in the range of 5,000 to 250,000 Da. Almost all of the tyrosine-phosphorylated proteins eluted in the void volume together with the tyrosine-phosphorylated CSF-1R (data not shown). The lack of the expected fractionation, particularly of low-molecular-weight tyrosine-phosphorylated proteins, suggests that most of the proteins in this fraction, at least, were associated in a multimeric complex(es) (see Discussion).
Having examined the distribution of tyrosine-phosphorylated proteins in the anti-CSF-1R immunoprecipitates from the column chromatography, we decided to screen these same CSF-1R immunoprecipitates for signalling proteins. The Uad pool of CSF-1R did not coimmunoprecipitate detectable levels of signalling proteins in either untreated or CSF-1-stimulated cells (Fig. 3B), even though it contained a significant level of these proteins (Fig. 2C). However, signalling molecules eluted along the gradient were found to immunoprecipitate with anti-CSF-1R (Fig. 3B). STAT3 coimmunoprecipitated with CSF-1R in peak II (Fr 23 to 26); this complex is also relatively stable given that it is maintained following elution at 250 mM NaCl. PI-3 kinase was present throughout the CSF-1R immunoprecipitates from peak II but also in those from peak I, a profile correlating with its detection over a broad range of fractions (Fig. 2C). Examination of the CSF-1R immunoprecipitates for other signalling proteins revealed that the multimeric complex of CSF-1R in early-eluting fractions also contained Shc, Grb2, and Cbl (data not shown) and SHP-1 (Fig. 3B). A detectable level of the multimeric complex of CSF-1R in peak I was also observed on fractionation of untreated lysate. The interactions of CSF-1R with PI-3 kinase and SHP-1 are described below in more detail.
Kinase activity of CSF-1R forming multimeric complexes.
We next attempted to establish the correlation between the formation of multimeric complexes of the CSF-1R and the intrinsic kinase activity of the CSF-1R in the separate pools. The CSF-1R present in the unadsorbed fraction (Uad) neither showed CSF-1R kinase activity (Fig. 3C) nor associated with signalling proteins to form multimeric complexes (Fig. 3A). Almost all of the kinase activity was in the CSF-1R pool present in the adsorbed fractions, and the degree of kinase activity within specific adsorbed fractions varied. Peak II showed the maximal level of kinase activity, which correlates with the fact that these fractions contain the maximal level of CSF-1R (Fig. 2A and 3C). The major phosphorylated protein, p165 (Fig. 3C), migrating as a broad band, corresponds to the mature form of the CSF-1R. The phosphorylated band at p130 is consistent with the nonglycosylated form of the CSF-1R (48). In contrast, peak 1 (Fr 17), while containing a considerable amount of kinase activity in the CSF-1R immunoprecipitates, showed p165 to be a minor phosphorylated protein in comparison to other phosphorylated proteins in this fraction, such as p130, p120, p60, and p45 (Fig. 3C). We suggest that it is more likely that an associated kinase(s) contributes significantly to the kinase activity observed in this fraction. Having first established that the profile of tyrosine-phosphorylated proteins from the separation of CSF-1-stimulated lysate was consistent with previous fractionations (Fig. 3D and 2B), we then probed the immunoblot with anti-c-Src (Fig. 3D). A small proportion of tyrosine-phosphorylated c-Src present in peak I (Fig. 3D) associated with CSF-1R (Fig. 3E), while a large proportion of c-Src present in the Uad fraction did not coimmunoprecipitate with the CSF-1R (Fig. 3E).
The SHP-1 multimeric complex with CSF-1R is distinct from the complex with tyrosine-phosphorylated p140.
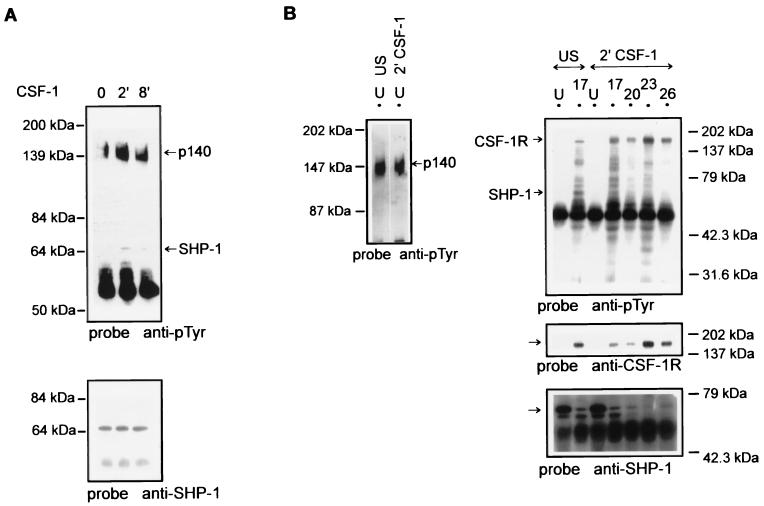
The tyrosine phosphatase SHP-1 has previously been shown to be activated in macrophages upon CSF-1 stimulation (9, 58). However, stable interactions between the CSF-1R and SHP-1 have not been demonstrated (9, 53). We have shown above that fractionation of CSF-1R pools by chromatography allowed the detection of CSF-1R complexes with signalling proteins upon CSF-1 stimulation (Fig. 3B), which could not be demonstrated by immunoprecipitating whole-cell lysate. Probing of CSF-1R immunoprecipitates with anti-SHP-1 showed that the phosphatase coimmunoprecipitated in early-eluting CSF-1R pools (Fig. 3B). A tyrosine-phosphorylated band, p65, comigrated with SHP-1 in the CSF-1R immunoprecipitates (Fig. 3A). The same complex was also found to contain PI-3 kinase, Shc, Grb2, and a number of yet-unidentified or novel tyrosine-phosphorylated proteins.
We further analyzed this complex by immunoprecipitation with anti-SHP-1 antibody. Consistent with observations by others, anti-SHP-1 immunoprecipitates of total lysate of untreated or CSF-1-treated BAC1.2F5 cells showed SHP-1 to be tyrosine phosphorylated on CSF-1 stimulation but failed to show significant association of tyrosine-phosphorylated proteins except p140 (Fig. 4A) (9). Immunoprecipitation of fractions (1 ml) from the separation of untreated or CSF-1-treated cell lysate with anti-SHP-1 showed that tyrosine-phosphorylated protein p140 coimmunoprecipitated with SHP-1 in the Uad fraction (Fig. 4B). Analysis of anti-SHP-1 immunoprecipitates of equal volumes of fractions (0.3 ml) identified the Uad fraction as containing the major pool of SHP-1 which was not tyrosine phosphorylated to a significant extent. Longer exposure showed that tyrosine-phosphorylated p140 coimmunoprecipitated with SHP-1 (data not shown), as described above. In contrast, a low level of SHP-1 in peak I and early-eluting peak II (Fr 17 to 26) coimmunoprecipitated with CSF-1R and other tyrosine-phosphorylated proteins in CSF-1-stimulated cells (Fig. 4B). A detectable level of the multimeric complex was also found in untreated-lysate fractionation but was limited to peak I (Fig. 4B). Hence, the two complexes of SHP-1, namely, SHP-1–CSF-1R and SHP-1–p140, appear in chromatographically separate pools, which may reflect the regulatory role of p140 described recently (53). The two immunoreactive bands recognized by anti-SHP-1 are probably due to the heterogeneity of SHP-1 caused by alternate splicing rather than to cross-reactivity to SHP-2. SHP-2 migrated with slower mobility than SHP-1 in total cell lysate and did not cross-react with either of the two bands of SHP-1 in anti-SHP-1 immunoprecipitates of the fractions (data not shown).
FIG. 4.
Specific pools of SHP-1 form multimeric complexes with CSF-1R and other tyrosine-phosphorylated proteins following CSF-1 stimulation. (A) Total extract of untreated or CSF-1-treated BAC1.2F5 cells was immunoprecipitated with anti-SHP-1, and the immunoblot was probed with anti-pTyr (4G10). The membrane was then stripped and reprobed with anti-SHP-1. The arrows indicate the positions of the tyrosine-phosphorylated proteins p140 and SHP-1. (B) Left panel, the unadsorbed fraction (U) (1 ml) following the separation of unstimulated (US) or CSF-1-treated (2 min at 37°C) BAC1.2F5 lysate was immunoprecipitated with anti-SHP-1. The immunoprecipitates were resolved by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was probed with anti-pTyr (4G10)–HRP. The arrow indicates the position of the tyrosine-phosphorylated p140. Right panel, equal volumes (0.3 ml) of the adsorbed and unadsorbed fractions following the separation of untreated or CSF-1-treated (2 min at 37°C) BAC1.2F5 lysate were immunoprecipitated with anti-SHP-1. The immunoblot of the samples was initially probed with anti-pTyr (4G10)–HRP and then stripped and reprobed with anti-CSF-1R and anti-SHP-1. The arrows indicate the positions of CSF-1R and SHP-1.
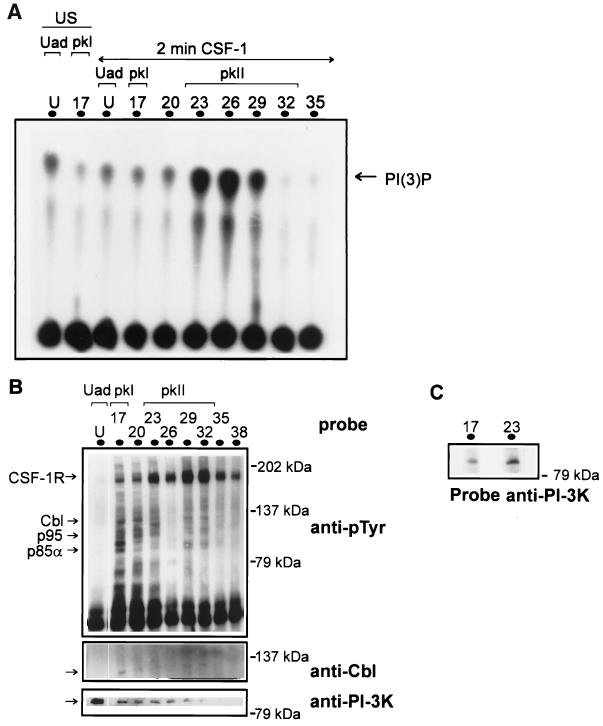
Separation of PI-3 kinase complexes containing different levels of lipid kinase activity.
PI-3 kinase p85α was observed in immunoprecipitates of a number of fractions eluted along the NaCl gradient on fractionation of CSF-1-treated lysate (Fig. 3B). Given the allosteric regulation of PI-3 kinase by tyrosine-phosphorylated proteins (42), we decided to analyze the fractions for enzymatic activity by determining in vitro lipid kinase activity in immunoprecipitates of PI-3 kinase (Fig. 5A). The main pool of PI-3 kinase present in the Uad fraction and the less significant pool present in peak I contained a basal level of activity in untreated and CSF-1-treated cell lysate fractionations. CSF-1 stimulation resulted in a significant increase in activity of only selected pools of PI-3 kinase corresponding to peak II (Fr 29 to 32). The obvious explanation that these fractions contained higher levels of the protein was eliminated by probing either the fractions (Fig. 2C) or the PI-3 kinase immunoprecipitates of the fractions (Fig. 5B) with anti-p85α.
FIG. 5.
PI-3 kinase displays different levels of lipid kinase activity in the multimeric complexes. (A) Fractions following the separation of unstimulated (US) or CSF-1-treated (2 min at 37°C) BAC1.2F5 lysate were immunoprecipitated with anti-PI-3 kinase p85α and the in vitro lipid kinase activity was estimated by thin-layer chromatography. The arrow indicates the position of PI-3 phosphate. pKI, peak I. (B) The immunoprecipitates of PI-3 kinase p85α were also resolved by SDS-PAGE, and the immunoblots were probed with anti-pTyr (4G10), anti-Cbl, and anti-PI-3 kinase p85α. The positions of tyrosine-phosphorylated CSF-1R, p95, Cbl, and PI-3 kinase p85α are indicated. (C) Immunoprecipitates of PI-3 kinase p110α from fractions 17 (peak I) and 23 (peak II) were resolved by SDS-PAGE, and the proteins were transferred to a nitrocellulose membrane. The membrane was probed with anti-PI-3 kinase p85α.
PI-3 kinase immunoprecipitates of the fractions were next analyzed for associated proteins. The Uad pool, which contained only a basal level of lipid kinase activity, failed to coimmunoprecipitate other tyrosine-phosphorylated proteins (Fig. 5B). Unlike that in the Uad pool, PI-3 kinase p85α in the adsorbed pool was itself tyrosine phosphorylated and was found to coimmunoprecipitate with a significant number of tyrosine-phosphorylated proteins (Fig. 5B). Despite the formation of a complex, the PI-3 kinase in peak I failed to show significant lipid kinase activity (Fig. 5A). The multimeric complex of PI-3 kinase isolated from this pool containing highly tyrosine-phosphorylated proteins, including Cbl, Shc, an unidentified p95, and CSF-1R (Fig. 5B), corresponds to that characterized previously (24). In contrast, PI-3 kinase in later-eluting fractions (Fr 23 to 29 of peak II) contained maximal levels of PI-3 kinase activity (Fig. 5A). A similar observation was made on examination of lipid kinase activity in anti-pTyr immunoprecipitates of the adsorbed fractions (data not shown). We excluded the possibility that the low lipid kinase activity in peak I was due to the absence of the catalytic subunit p110α. Coimmunoprecipitation of the regulatory subunit p85α with the catalytic subunit p110α in Fr 17 and Fr 23, representative of peak I and peak II, respectively (Fig. 5C), suggests other reasons for the modulation in activity. PI-3 kinase in the adsorbed fractions coimmunoprecipitated with distinctly different tyrosine-phosphorylated proteins, with CSF-1R being the most prominent tyrosine-phosphorylated protein present (Fig. 5B). A correlation between the fractions that contained the maximal level of tyrosine-phosphorylated CSF-1R or CSF-1R kinase activity (Fig. 5B and 3C) and those that exhibited maximal lipid kinase activity (Fig. 5A) was observed. Hence, not all interactions of PI-3 kinase with tyrosine-phosphorylated proteins contribute to an increase in lipid kinase activity.
Multimeric complexes of CSF-1R contain structural proteins.
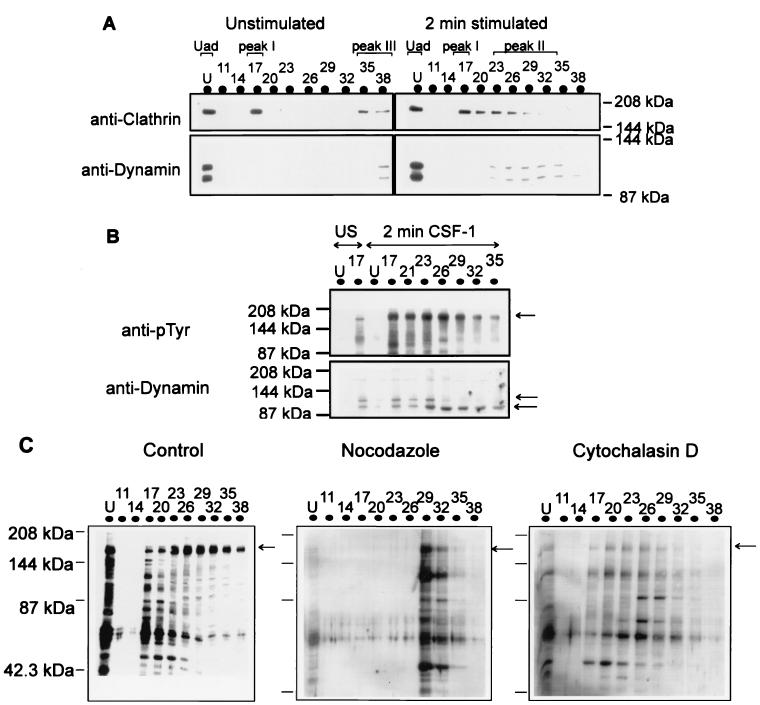
The recruitment of CSF-1R and signalling proteins to specific subcellular sites upon CSF-1 stimulation (1, 5, 8) may involve interactions with structural protein components. We investigated the distribution of specific cytoskeletal and marker proteins in the fractions following anion-exchange chromatography of lysate. Clathrin, a structural protein which forms scaffolds in clathrin-coated pits and coated vesicles (41), appeared in fractions (Fig. 6A) which also contained the CSF-1R following the fractionation of untreated cell lysate (Uad, peak I, and peak III in Fig. 2A). CSF-1 stimulation resulted in a redistribution of anticlathrin immunoreactivity in the adsorbed pool over a range of NaCl concentrations, with the maximal immunoreactivity detected in fractions at low NaCl concentrations (peak I). These fractions overlapped with a part of the pool of tyrosine-phosphorylated CSF-1R (Fig. 2B). Paxillin, a focal adhesion protein, was present in Fr 17 on fractionation of untreated lysate and was found to increase in amount in the same fraction on CSF-1 stimulation (data not shown). Dynamin, a GTPase that is involved in the invagination of clathrin-coated pits to form vesicles (41), displayed a distribution different from that of clathrin following CSF-1 stimulation. Although a significant pool of dynamin was detected in the Uad pool in both untreated and CSF-1-treated cells, the observation of interest was the distribution of the adsorbed pool of dynamin upon ligand stimulation (Fig. 6A). The maximal immunoreactivity to dynamin in the adsorbed fractions appeared in fractions different from those containing the maximal level of clathrin but parallel to those containing the maximal level of CSF-1R (Fig. 2A). Lower levels of dynamin were detected in early-eluting fractions on longer exposure, which were previously found to contain clathrin. The meaning of the recognition of two proteins by antidynamin antibody is not clear at present but may reflect isoforms of dynamin. Therefore, different structural proteins eluted with distinct pools of tyrosine-phosphorylated CSF-1R following CSF-1 stimulation. Furthermore, the presence of dynamin in CSF-1R immunoprecipitates of fractions from peak II (Fig. 6B) and of clathrin in CSF-1R immunoprecipitates of fractions from peak I (data not shown) on CSF-1 stimulation suggests the formation of multimeric complexes with specific structural proteins. Although the two proteins recognized by antidynamin antibody are present in detectable levels in CSF-1R complexes in early-eluting fractions, only the faster-migrating form was evident in the receptor complexes eluted at higher NaCl concentrations (Fig. 6B). The formation and distribution of the multimeric complexes were dependent on the integrity of the sorting mechanism. Fractionation of lysate from cells pretreated with agents that affected the polymerization of microtubules, such as nocodazole, prior to CSF-1 stimulation showed a lack of distribution of tyrosine-phosphorylated proteins over a range of NaCl concentrations (Fig. 6C). This suggests an absence of formation of the chromatographically distinct CSF-1R multimeric complexes in nocodazole-treated cells, consistent with the disruption of the sorting process (25). Analysis of the fractions from the separation of control lysate showed a broad elution profile of tyrosine-phosphorylated proteins, consistent with previous fractionations (Fig. 2B). Agents that affected the polymerization of the actin cytoskeleton, such as cytochalasin D, had little effect on the distribution of tyrosine-phosphorylated proteins, although the extent of tyrosine phosphorylation of specific proteins in peak I (Fr 17) was diminished (Fig. 6C) relative to that in the corresponding pool in control CSF-1-stimulated lysate fractionation (Fig. 6C).
FIG. 6.
Structural proteins are distributed in pools containing specific CSF-1R multimeric complexes. (A) Solubilized lysate of untreated or CSF-1-treated (2 min at 37°C) BAC1.2F5 cells was applied to a MonoQ anion-exchange column and eluted with a 0 to 0.4 M NaCl gradient. Aliquots of protein fractions eluted from the column were analyzed by Western blotting by probing with anticlathrin or antidynamin. U, unadsorbed fraction. (B) CSF-1R immunoprecipitates of the fractions were probed with antidynamin. US, unstimulated. (C) Lysate of BAC1.2F5 cells treated with nocodazole (40 μg/ml) or cytochalasin D (2.5 μM) or left untreated prior to stimulation with CSF-1 for 2 min at 37°C was fractionated on an anion-exchange column, and the immunoblot of the fractions was probed with anti-pTyr.
DISCUSSION
The binding of a ligand to transmembrane receptors initiates the activation of a number of signal transduction cascades. In order for a distinct biological signal to be relayed in an organized and coordinated manner, several regulatory mechanisms exist to ensure the correct localization and sequence of activation of specific target proteins (37). The downmodulation of the biological signal also requires the continuation of the sorting process, perhaps involving the recruitment and interaction of other proteins (35, 46). The transient interactions between proteins at specific subcellular localization sites require a degree of organization in modules, which may consist of the selective assembly of large complexes of proteins (20, 37, 55). Apart from the recruitment of active enzymes into signalling networks, many modules exist to facilitate the positioning of substrates close to their activators (20, 55, 60). Adapter, anchoring, and scaffolding proteins play a pivotol role in the specificity of assembly of such signalling networks (37). A well-characterized functioning module, for example, is found in yeast, where a molecular scaffold (Ste 5) physically organizes elements of the mitogen-activated protein kinase cascade (18). Also, the capacity of B-Raf to activate the mitogen-activated protein kinase cascade in response to nerve growth factor was recently shown to correlate with the formation of a stable association with another scaffolding protein, HSP90 (20).
The discovery of new potential binding partners for CSF-1R by use of the sensitive yeast two-hybrid technique (7) emphasizes not only the existence of yet-to-be discovered binding proteins but also the need to progress to methods which allow for more sensitive detection of complexes isolated from physiologically relevant cell populations. A major factor governing the sensitivity of coimmunoprecipitation studies is the absolute number of receptors expressed on the cell surface (7, 38). CSF-1R, however, is expressed in high numbers (60,000 per cell) on the surface of BAC1.2F5 cells (32), suggesting that a more reasonable explanation is the low stoichiometry of individual complexes within the cell. Several factors may contribute to the inability to detect receptor complexes. The receptor participates in a number of different signalling pathways, and hence any individual signalling complex may be present in small amounts. Second, a rapid turnover of signalling protein complexes is suggested by the presence of stoichiometrically minor autophosphorylation sites on the receptor (40, 54). The presence of two different molecules competing for the same site on the receptor, as recently demonstrated (7), may also further limit the detection of any one complex.
The yeast two-hybrid technique is limited by the fact that it can detect only proteins which directly interact with the receptor and will miss important indirect associations. In addition, this approach can only demonstrate potential interactions and should be supported with direct evidence of such associations. We recently showed that a signalling complex isolated from Triton-solubilized lysates of macrophages by using anti-PI-3 kinase antibodies was stable enough to be reisolated following anion-exchange chromatography (24). This suggested that such a method might provide a general approach to isolating receptor complexes present in small amounts in the cell. In this study we have demonstrated chromatographically separate multimeric complexes of CSF-1R with specific signalling proteins which could not be detected by coimmunoprecipitation from whole-cell lysates.
Enrichment and fractionation of subpopulations of CSF-1R forming multimeric complexes.
The CSF-1R solubilized from growth-arrested macrophages chromatographically resolved into distinct pools which changed on CSF-1 stimulation of macrophages. In addition, we found that distinct tyrosine-phosphorylated proteins fractionated with the separate pools of activated CSF-1R, with a considerable enrichment of proteins in these fractions (Fig. 2A). Although many of the observed tyrosine-phosphorylated proteins correspond to known signalling proteins (such as Cbl [p120], PI-3 kinase [p85], and Shc [p56 and p46] [Fig. 2A and B]; c-Src [p60] [Fig. 3D]; and Erk 1 and 2 [p44 and p42] [data not shown]), many others remain to be identified. We are currently using this method as a step to purify novel proteins involved in CSF-1 signalling. Signalling proteins previously found to be activated in response to CSF-1 or recruited to the CSF-1R complex (28, 33, 40, 54, 56, 58) showed distinct distribution profiles on fractionation of CSF-1-stimulated lysates. Similar observations have been made on fractionation of nerve growth factor-stimulated PC12 cell lysate, where coelution of B-Raf with HSP90 was associated with the formation of a functional complex required for the activation of the mitogen-activated protein kinase pathway (20). The considerable enrichment of signalling proteins participating in CSF-1-mediated responses by anion-exchange chromatography makes this approach a potentially useful tool for analysis.
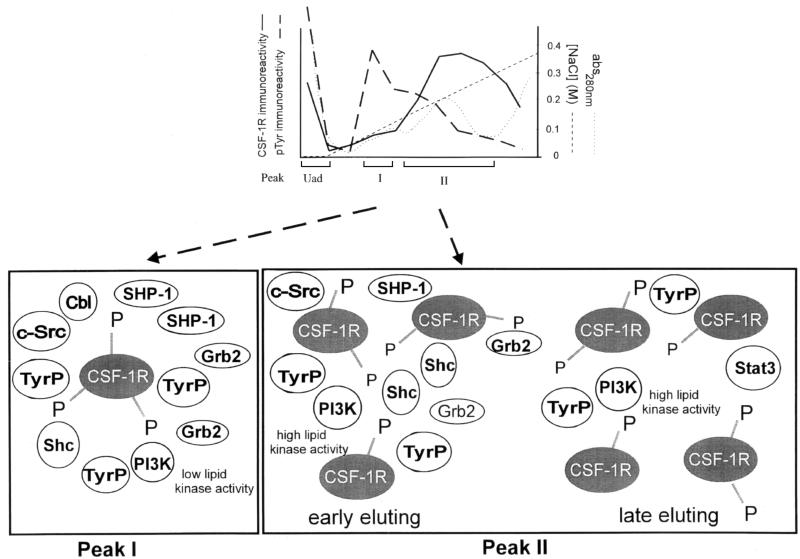
Our hypothesis for the change in the chromatographic distribution of the CSF-1R and specific signalling proteins on CSF-1 stimulation is that it occurs as a consequence of formation of stable protein interactions. The observations summarized in Fig. 7 show that a significant proportion of the CSF-1R (Uad pool) neither possessed intrinsic kinase activity nor formed complexes with other tyrosine-phosphorylated proteins. On the other hand, a chromatographically separate CSF-1R pool that eluted at progressively higher salt concentrations formed multimeric complexes with distinct signalling proteins. This result is significant, since it demonstrates that the interactions between the receptor and signalling proteins are not simply nonspecific associations; otherwise, a significant association of these proteins would be expected to occur maximally in the Uad pool under lower-ionic-strength conditions. A small pool of CSF-1R eluting at low ionic strength (peak I) was associated with Grb2, Shc, PI-3 kinase, SHP-1, c-Src, Cbl, PLC-γ2 (data not shown), and other highly tyrosine-phosphorylated proteins (Fig. 7). This multimeric complex contained a considerable amount of CSF-1R-associated tyrosine kinase activity but a relatively low receptor autophosphorylation activity. The receptor-associated kinase activity is most likely due to kinases associated with the CSF-1R, such as c-Src or other members of the Src family such as Fyn (2, 10). An interesting relevant observation was the presence of paxillin in the early-eluting multimeric complex (data not shown), which was present in detectable levels in untreated cells but increased on CSF-1 stimulation together with other signalling proteins. The complex characterized in peak I is consistent with recruitment of signalling proteins to focal complexes (14, 17) recently identified in macrophages which result from cell-to-substratum contacts (1). While this paper was in preparation, a report published by Yeung et al. (59) suggested that many signalling proteins recruited to a preexisting cytosolic complex on CSF-1 stimulation are closely associated with the actin cytoskeleton. This observation may relate to the multimeric complex characterized in peak I of our study, which was found to interact with a small pool of CSF-1R. The CSF-1R multimeric complexes of most interest were those that appeared transiently on CSF-1 stimulation in peak II (Fig. 7). This transient pool of receptor, representing a significant proportion of the activated receptor, formed multimeric complexes predominantly with Shc, STAT3, and PI-3 kinase but also with minor amounts of Grb2 and SHP-1. We have previously shown that signalling proteins involved in the JAK/STAT pathway, STAT3 and TYK2, are activated by CSF-1R in macrophages as well as in fibroblasts ectopically expressing CSF-1R (33). We demonstrate in this study that STAT3 in fact forms a close association with the activated receptor. These observations are summarized in Fig. 7.
FIG. 7.
Schematic representation of CSF-1R containing multimeric complexes fractionated from CSF-1-treated macrophage cell lysate. CSF-1R resolved into separate pools (Uad, peak I, and peak II) following the fractionation of CSF-1-treated BAC1.2F5 macrophage cell lysate on an anion-exchange column. A large pool of CSF-1R (Uad) that neither showed an appreciable amount of intrinsic tyrosine kinase activity nor formed multimeric complexes was separated from the activated pool of CSF-1R that formed chromatographically stable multimeric complexes. These complexes contained distinct signalling proteins and tyrosine-phosphorylated (TyrP) proteins. A low level of CSF-1R present in the adsorbed pool, peak I, formed multimeric complexes with signalling proteins PI-3 kinase, Grb2, Cbl, c-Src, Shc, and SHP-1 and a significant number of other highly TyrP proteins. A transient pool of CSF-1R which resolved as a broad peak (peak II) formed a range of complexes with specific signalling proteins. The multimeric complex of CSF-1R in peak II contained predominantly PI-3 kinase, STAT3, and Shc but also minor amounts of SHP-1, c-Src, Grb2, and other TyrP proteins. A large pool of PI-3 kinase (Uad) with a basal level of lipid kinase activity did not form multimeric complexes despite the presence of a significant amount of TyrP proteins. PI-3 kinase present in a number of fractions along the gradient formed a range of complexes with CSF-1R, but not all complexes contained active lipid kinase. The early-eluting PI-3 kinase in peak I showed minimal activity, while PI-3 kinase in peak II contained a significant level of enzymatic activity. The assembly of chromatographically separate complexes may reflect the various CSF-1-mediated signalling events activated in macrophages.
CSF-1R forms a multimeric complex containing SHP-1.
Analysis of chromatographically separated lysate from CSF-1-treated macrophages revealed the existence of a multimeric complex between SHP-1, CSF-1R, and other proteins which could not be detected by coimmunoprecipitation from whole-cell lysate. Analysis of the chromatographically enriched complex also reveals features that are not readily observable by using the former approach. The tyrosine-phosphorylated SHP-1 present in the complex with CSF-1R represented a relatively small proportion of the total pool of SHP-1 and was chromatographically separate from the major pool of SHP-1 present in the Uad fraction. The latter pool coimmunoprecipitated with the tyrosine-phosphorylated protein p140, which was recently found to consist of two transmembrane glycoproteins, PIR-B/p91A and BIT (53). The CSF-1R contained in the complex eluting at low ionic strength (peak I) was not tyrosine phosphorylated to a significant extent compared to other proteins, which appeared to be strongly tyrosine phosphorylated. This observation could reflect the intrinsic specificity of SHP-1 for sequences present around the CSF-1R autophosphorylation sites or, alternatively, that the enzymatic activity of SHP-1 is constrained by its tight binding within such a complex. Characterization of this complex may therefore give valuable information as to how the specificity of SHP-1 for its substrates is determined, which is of key importance to a better understanding of CSF-1 signalling, since the motheaten mouse has provided strong evidence for the importance of SHP-1 in this process (9, 53).
PI-3 kinase displaying different specific enzyme activities in different multimeric complexes.
We have previously shown that PI-3 kinase is a major binding partner for the CSF-1R, being the only protein identifiable as specifically binding to the receptor in anti-CSF-1R immunoprecipitates prepared from [35S]methionine-labelled cells (24). Consistent with this, approximately 85% of the receptor in BAC1.2F5 cells can be isolated in anti-PI-3 kinase immunoprecipitates (22a). In the present study, we observed that PI-3 kinase was widely distributed throughout the column profile when lysates prepared from activated macrophages were analyzed and that PI-3 kinase in these pools was associated with the CSF-1R. The large number of chromatographically distinct complexes between the receptor and PI-3 kinase may reflect the participation of PI-3 kinase in multiple CSF-1-mediated events. We have previously shown that PI-3 kinase is involved in the CSF-1-dependent activation of p70s6 kinase (16) and in pathways of CSF-1 degradation in some populations of macrophages (23). When the PI-3 kinase activities in the various pools were measured, we found that not all of the complexes containing tyrosine-phosphorylated PI-3 kinase and receptor were enzymatically active (Fig. 7). Most of the PI-3 kinase activity was found in complexes that contained predominantly tyrosine-phosphorylated CSF-1R (peak II). In contrast, the complex of tyrosine-phosphorylated PI-3 kinase, CSF-1R, Cbl, Grb2, Shc, SHP-1, and other tyrosine-phosphorylated proteins (24) did not have lipid kinase activity significantly above the basal level (Fig. 7). Hence, column chromatography has enabled the separation of distinct PI-3 kinase multimeric complexes participating in CSF-1-mediated events. It is not yet clear whether the PI-3 kinase present in either of the complexes binds directly to the receptor. However, what is known so far about the regulation of PI-3 kinase suggests that the active pool is the result of either direct interaction of the SH2 domains of the p85α regulatory subunit with phosphorylated tyrosine 721 of the CSF-1R (40) or occupation of both SH2 domains by more than one protein (42). We are currently investigating these alternatives.
Significance of the chromatographically distinct multimeric complexes of CSF-1R.
Our hypothesis is that the chromatographic behavior of the solubilized CSF-1R and signalling proteins is related to the formation of stable interactions with distinct proteins involved in different intracellular signalling events. These interactions would include scaffolding and structural proteins. In this regard, it is interesting that clathrin, dynamin, and paxillin cochromatograph with distinct pools of the CSF-1R upon CSF-1 stimulation but, more significantly, that some were found to associate with the CSF-1R pools. A small pool of CSF-1R associating with paxillin formed multimeric complexes consistent with focal complexes, as discussed above. Signalling proteins Shc, Cbl, SHP-1, PI-3 kinase, and Grb2 were identified in this complex with a significant number of other highly tyrosine-phosphorylated proteins, reflecting the extent of kinase activity in the complex. We did not detect immunoreactivity to anti-Fak in this peak. The recent report of the activation of the related adhesion focal tyrosine kinase by CSF-1 in monocyte-macrophages (17) may provide an explanation for the lack of detection of Fak in our study. A large proportion of the activated CSF-1R, representing a chromatographically separate but transient pool, formed multimeric complexes with distinctly different tyrosine-phosphorylated proteins that included signalling proteins STAT3, Shc, PI-3 kinase, and minor levels of SHP-1 and Grb2. This transient pool of activated CSF-1R coimmunoprecipitated with dynamin. Although dynamin guanosine triphosphatases have been implicated in scission of clathrin-coated vesicles from the plasma membrane during endocytosis (41), recent studies also suggest that other isoforms of dynamin participate in the formation of distinct transport vesicles from the trans-Golgi network (22). The presence of the active pool of PI-3 kinase in the complex containing CSF-1R and dynamin in our study is particularly interesting given the role of PI-3 kinase in PDGFR-mediated endocytosis (21, 46), where endosomes containing PI-3 kinase were found to be associated with microtubule network (25). Other signalling proteins have also been implicated in receptor-mediated endocytosis (57). The formation of the distinct multimeric complexes of the CSF-1R is dependent on the integrity of the sorting mechanism of which the microtubular network forms an integral part. There is a considerable amount of literature on the localization and recruitment of specific signalling proteins into structures such as postendocytic vesicles (11, 49, 50), focal complexes (1, 17), and caveoli (4, 29). Marker proteins for caveoli in macrophages have not been identified, but it would be of considerable interest if the complexes described in this study could be identified with those present in such organelles. We have attempted to address the importance of studying specific pools of signalling proteins and their regulation in CSF-1-mediated signalling through interaction with other proteins, including scaffolding proteins. Although we have characterized pools with respect to CSF-1R, PI-3 kinase, and SHP-1 in some detail, understanding of the nature of the specific interactions that distinguish the pools requires further study.
ACKNOWLEDGMENTS
We are grateful to Heung-Chin Cheng for helpful comments on the manuscript. We also thank Graeme Guy, Ulrike Novak, and Peter Vadiveloo for helpful discussions.
REFERENCES
- 1.Allen W E, Jones G E, Pollard J W, Ridley A J. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. J Cell Sci. 1997;110:707–720. doi: 10.1242/jcs.110.6.707. [DOI] [PubMed] [Google Scholar]
- 2.Alonso G, Koegl M, Mazurenko N, Courtneidge S A. Sequence requirements for binding of Src family tyrosine kinases to activated growth factor receptors. J Biol Chem. 1995;270:9840–9848. doi: 10.1074/jbc.270.17.9840. [DOI] [PubMed] [Google Scholar]
- 3.Baccarini M, Sabatini D M, App H, Rapp U R, Stanley E R. Colony stimulating factor-1 (CSF-1) stimulates temperature dependent phosphorylation and activation of the Raf-1 proto-oncogene product. EMBO J. 1990;9:3649–3657. doi: 10.1002/j.1460-2075.1990.tb07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohuslav J, Horejsi V, Hansmann C, Stockl J, Weidle U H, Majdic O, Bartke I, Knapp W, Stockinger H. Urokinase plasminogen activator receptor, β2-integrins, and Src-kinases within a single receptor complex of human monocytes. J Exp Med. 1995;181:1381–1390. doi: 10.1084/jem.181.4.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boocock C A, Jones G E, Stanley E R, Pollard J W. Colony-stimulating factor-1 induces rapid behavioural responses in the mouse macrophage cell line, BAC1.2F5. J Cell Sci. 1989;93:447–456. doi: 10.1242/jcs.93.3.447. [DOI] [PubMed] [Google Scholar]
- 6.Bourette R P, Myles G M, Carlberg K, Chen A R, Rohrschneider L R. Uncoupling of the proliferation and differentiation signals mediated by the murine macrophage colony-stimulating factor receptor expressed in myeloid FDC-P1 cells. Cell Growth Differ. 1995;6:631–645. [PubMed] [Google Scholar]
- 7.Bourette R P, Myles G M, Choi J-L, Rohrschneider L R. Sequential activation of phosphatidylinositol 3-kinase and phospholipase C-γ2 by the M-CSF receptor is necessary for differentiation signaling. EMBO J. 1997;16:5880–5893. doi: 10.1093/emboj/16.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen B D-M, Kuhn III C, Lin H-S. Receptor-mediated binding and internalization of colony-stimulating factor (CSF-1) by mouse peritoneal exudate macrophages. J Cell Sci. 1984;70:147–166. doi: 10.1242/jcs.70.1.147. [DOI] [PubMed] [Google Scholar]
- 9.Chen H E, Chang S, Trub T, Neel B G. Regulation of colony-stimulating factor 1 receptor signaling by the SH2 domain-containing tyrosine phosphatase SHPTP1. Mol Cell Biol. 1996;16:3685–3697. doi: 10.1128/mcb.16.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Courneidge S A, Dhand R, Pilat D, Twamley G M, Waterfield M D, Roussel M F. Activation of Src family kinases by colony stimulating factor-1, and their association with its receptor. EMBO J. 1993;12:943–950. doi: 10.1002/j.1460-2075.1993.tb05735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Guglielmo G M, Baass P C, Ou W J, Posner B I, Bergeron J J M. Compartmentalization of Shc, Grb2 and mSos, and hyperphosphorylation of Raf-1 by EGF but not insulin in liver parenchyma. EMBO J. 1994;13:4269–4277. doi: 10.1002/j.1460-2075.1994.tb06747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downing J R, Margolis B L, Zilberstein A, Ashmun R A, Ullrich A, Sherr C J, Schlessinger J. Phospholipase C-gamma, a substrate for PDGF receptor kinase, is not phosphorylated on tyrosine during the mitogenic response to CSF-1. EMBO J. 1989;8:3345–3350. doi: 10.1002/j.1460-2075.1989.tb08496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downing J R, Rettenmier C W, Sherr C J. Ligand-induced tyrosine kinase activity of colony-stimulating factor-1 receptor in murine macrophage cell line. Mol Cell Biol. 1988;8:1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ganju R K, Hatch W C, Avraham H, Ona M A, Druker B, Avraham S, Groopman J E. RAFTK, a novel member of the focal adhesion kinase family, is phosphorylated and associates with signaling molecules upon activation of mature T lymphocytes. J Exp Med. 1997;185:1055–1063. doi: 10.1084/jem.185.6.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guilbert L J, Stanley E R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986;261:4024–4032. [PubMed] [Google Scholar]
- 16.Hamilton J A, Byrne R, Whitty G, Vadiveloo P K, Marmy N, Pearson R B, Christy E, Jaworowski A. Effects of wortmannin and rapamycin on CSF-1-mediated responses in macrophages. Int J Biochem Cell Biol. 1998;30:271–283. doi: 10.1016/s1357-2725(97)00111-8. [DOI] [PubMed] [Google Scholar]
- 17.Hatch W C, Ganju R K, Hiregowdara D, Avraham S, Groopman J E. The related adhesion focal tyrosine kinase (RAFTK) is tyrosine phosphorylated and participates in colony-stimulating factor-1/macrophage colony-stimulating factor signaling in monocyte-macrophages. Blood. 1998;91:3967–3973. [PubMed] [Google Scholar]
- 18.Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- 19.Husson H, Mograbi B, Schmid-Antomarchi H, Fischer S, Rossi B. CSF-1 stimulation induces the formation of a multiprotein complex including CSF-1 receptor, c-Cbl, PI 3-kinase, Crk-II and Grb2. Oncogene. 1997;14:2331–2338. doi: 10.1038/sj.onc.1201074. [DOI] [PubMed] [Google Scholar]
- 20.Jaiswal R K, Weissinger E, Kolch W, Landreth G E. Nerve growth factor-mediated activation of the mitogen-activated protein (MAP) kinase cascade involves a signaling complex containing B-Raf and HSP90. J Biol Chem. 1996;271:23626–23629. doi: 10.1074/jbc.271.39.23626. [DOI] [PubMed] [Google Scholar]
- 21.Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth factor receptor trafficking. J Biol Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- 22.Jones S M, Howell K E, Henley J R, Cao H, McNiven M A. Role of dynamin in the formation of transport vesicles from the trans-Golgi network. Science. 1998;279:573–577. doi: 10.1126/science.279.5350.573. [DOI] [PubMed] [Google Scholar]
- 22a.Kanagasundaram, V. Unpublished data.
- 23.Kanagasundaram V, Christy E, Hamilton J A, Jaworowski A. Different pathways of colony stimulating factor-1 degradation in macrophage populations revealed by wortmannin sensitivity. Biochem J. 1998;330:197–202. doi: 10.1042/bj3300197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanagasundaram V, Jaworowski A, Hamilton J A. Association between phosphatidylinositol-3 kinase, Cbl and other tyrosine phosphorylated proteins in CSF-1 stimulated macrophages. Biochem J. 1996;320:68–77. doi: 10.1042/bj3200069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapeller R, Chakrabarti R, Cantley L, Fay F, Corvera S. Internalization of activated platelet-derived growth factor receptor-phosphatidylinositol-3′ kinase complexes: potential interactions with microtubule cytoskeleton. Mol Cell Biol. 1993;13:6052–6063. doi: 10.1128/mcb.13.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan D R, Whitman M, Schaffhausen B, Pallas D C, White M, Cantley L, Roberts T M. Common elements in growth factor stimulation and oncogenic transformation: 85 kd phosphoprotein phosphotidylinositol kinase activity. Cell. 1987;50:1021–1029. doi: 10.1016/0092-8674(87)90168-1. [DOI] [PubMed] [Google Scholar]
- 27.Li W, Stanley E R. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991;10:277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lioubin M N, Myles G M, Carlberg K, Bowtell D, Rohrschneider L R. SHC, GRB2, SOS1, and a 150-kilodalton tyrosine-phosphorylated protein form complexes with Fms in hematopoietic cells. Mol Cell Biol. 1994;14:5682–5691. doi: 10.1128/mcb.14.9.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P, Ying Y, Ko Y-G, Anderson R G W. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- 30.Lowenstein E J, Daly R J, Batzer A G, Li W, Margolis B, Lammers R, Ullrich A, Skolnik E Y, Bar Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein Grb2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 31.Manger R, Najita L, Nichols E J, Hakomori S, Rohrschneider L. Cell surface expression of the McDonough strain of feline sarcoma virus fms gene product (gp140fms) Cell. 1984;39:327–337. doi: 10.1016/0092-8674(84)90011-4. [DOI] [PubMed] [Google Scholar]
- 32.Morgan C, Pollard J W, Stanley E R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. 1987;130:420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- 33.Novak U, Harpur A G, Paradiso L, Kanagasundaram V, Jaworowski A, Wilks A F, Hamilton J A. CSF-1 induced Stat1 and Stat3 activation is accompanied by phosphorylation of Tyk2 in macrophage and Tyk2 and Jak1 in fibroblasts. Blood. 1995;86:2948–2956. [PubMed] [Google Scholar]
- 34.Novak U, Nice E, Hamilton J A, Paradiso L. Requirement for Y706 of the murine (or Y708 of the human) CSF-1 receptor for Stat1 activation in response to CSF-1. Oncogene. 1996;13:2607–2613. [PubMed] [Google Scholar]
- 35.Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 36.Ota Y, Samelson L E. The product of the proto-oncogene c-cbl: a negative regulator of the Syk tyrosine kinase. Science. 1997;276:418–420. doi: 10.1126/science.276.5311.418. [DOI] [PubMed] [Google Scholar]
- 37.Pawson T, Scott J D. Signaling through scaffold, anchoring, and adaptor proteins. Science. 1997;278:2075–2080. doi: 10.1126/science.278.5346.2075. [DOI] [PubMed] [Google Scholar]
- 38.Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, Graziani A, Panayotou G, Comoglio P M. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- 39.Reedijk M, Liu X, Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990;10:5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reedijk M, Liu X, van der Geer P, Letwin K, Waterfield M D, Hunter T, Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3′-kinase SH2 domains: a model for SH2-mediated receptor target interactions. EMBO J. 1992;11:1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robinson M S. The role of clathrin, adaptors and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 42.Rordorf-Nikolic T, van Horn D J, Chen D, White M F, Backer J M. Regulation of phosphatidylinositol 3′-kinase by tyrosyl phosphoproteins. J Biol Chem. 1995;270:3662–3666. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- 43.Rosnet O, Birnbaum D. Hematopoietic receptors of class III receptor-type tyrosine kinases. Crit Rev Oncogen. 1993;4:595–613. [PubMed] [Google Scholar]
- 44.Roussel M F, Dull T J, Rettenmier C W, Ralph P, Ullrich A, Sherr C J. Transforming potential of the c-fms proto-oncogene (CSF-1 receptor) Nature. 1987;325:549–552. doi: 10.1038/325549a0. [DOI] [PubMed] [Google Scholar]
- 45.Roussel M F, Shurtleff S A, Downing J R, Sherr C J. A point mutation at tyrosine 809 in the human colony-stimulating factor 1 receptor impairs mitogenesis without abrogating tyrosine kinase activity, association with phosphatidylinositol 3-kinase, or induction of fos and junB genes. Proc Natl Acad Sci USA. 1990;87:6738–6742. doi: 10.1073/pnas.87.17.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scaife R, Gout I, Waterfield M D, Margolis R L. Growth factor-induced binding of dynamin to signal transduction proteins involves sorting to distinct and separate proline-rich dynamin sequences. EMBO J. 1994;13:2574–2582. doi: 10.1002/j.1460-2075.1994.tb06547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sengupta A, Liu W-K, Yeung Y G, Yeung D C Y, Frackelton A R, Jr, Stanley E R. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor-1. Proc Natl Acad Sci USA. 1988;85:8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sherr C J, Rettenmier C W, Sacca R, Roussel M F, Look A T, Stanley E R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985;41:665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- 49.Sorkin A, Eriksson A, Heldin C-H, Westermark B, Claesson-Welsh L. Pool of ligand-bound platelet-derived growth factor β-receptors remain activated and tyrosine phosphorylated after internalization. J Cell Physiol. 1993;156:373–382. doi: 10.1002/jcp.1041560221. [DOI] [PubMed] [Google Scholar]
- 50.Sorkin A, Waters C M. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 51.Stanley E R, Guilbert L J, Tushinski R J, Bartelmez S H. CSF-1-A mononuclear phagocyte lineage-specific hematopoietic growth factor. J Cell Biochem. 1983;21:151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- 52.Tapley P, Kazlauskas A, Cooper J A, Rohrschneider L R. Macrophage colony-stimulating factor-induced tyrosine phosphorylation of c-fms proteins expressed in FDC-P1 and BALB/c-3T3 cells. Mol Cell Biol. 1990;10:2528–2538. doi: 10.1128/mcb.10.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Timms J F, Carlberg K, Gu H, Chen H, Kamatkar S, Nadler M J S, Rohrschneider L R, Neel B G. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol Cell Biol. 1998;18:3838–3850. doi: 10.1128/mcb.18.7.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Geer P, Hunter T. Mutation of Tyr697, a GRB2-binding site, and Tyr721, a PI 3-kinase binding site, abrogates signal transduction by the murine CSF-1 receptor expressed in Rat-2 fibroblasts. EMBO J. 1993;12:5161–5172. doi: 10.1002/j.1460-2075.1993.tb06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Hoek M, Allen C S, Parsons S J. Phosphotyrosine phosphatase activity associated with c-Src in large multimeric complexes isolated from adrenal medullary chromaffin cells. Biochem J. 1997;326:271–277. doi: 10.1042/bj3260271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y, Yeung Y-G, Langdon W Y, Stanley E R. c-Cbl is transiently tyrosine-phosphorylated, ubiquitinated, and membrane-targeted following CSF-1 stimulation of macrophages. J Biol Chem. 1996;271:17–20. doi: 10.1074/jbc.271.1.17. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Moran M F. Requirement for the adaptor protein Grb2 in EGF receptor endocytosis. Science. 1996;272:1935–1938. doi: 10.1126/science.272.5270.1935. [DOI] [PubMed] [Google Scholar]
- 58.Yeung Y G, Berg K L, Pixley F J, Angeletti R H, Stanley E R. Protein tyrosine phosphatase-1C is rapidly phosphorylated in tyrosine in macrophages in response to colony stimulating factor-1. J Biol Chem. 1992;267:23447–23450. [PubMed] [Google Scholar]
- 59.Yeung Y-G, Wang Y, Einstein D B, Lee P S W, Stanley E R. Colony-stimulating factor-1 stimulates the formation of multimeric cytosolic complexes of signaling proteins and cytoskeletal components in macrophages. J Biol Chem. 1998;273:17128–17137. doi: 10.1074/jbc.273.27.17128. [DOI] [PubMed] [Google Scholar]
- 60.Zanke B W, Rubie E A, Winnett E, Chan J, Randall S, Parsons M, Boudreau K, McInnis M, Yan M, Templeton D J, Woodgett J R. Mammalian mitogen-activated protein kinase pathways are regulated through formation of specific kinase-activator complexes. J Biol Chem. 1996;271:29876–29881. doi: 10.1074/jbc.271.47.29876. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Z Y, Davis J P, Van Etten R L. Covalent modification and active-site directed inactivation of a low molecular weight phosphotyrosyl protein phosphatase. Biochemistry. 1992;31:1701–1711. doi: 10.1021/bi00121a018. [DOI] [PubMed] [Google Scholar]