Abstract
The hearing healthcare industry is evolving rapidly. A framework addressing provision options in contemporary hearing care could assist clinician and client navigate their options to find the most appropriate solution for each individual. A PRISMA approach was used followed by mapping, validation, and thematic analysis to produce a framework to better describe and discuss service and product delivery options in contemporary hearing care. No frameworks were identified to advise matching needs with current provision options in audiological care. Charting, mapping, and thematic analysis of the validation criteria and hearing care literature produced three core domains: Service, Channel, and Technology/Device. The framework developed in this review allows for an understanding of where innovation is occurring in hearing healthcare and differentiates between changes to technology, channel, and service. New questions open up such as whether one model is more effective than another or which model of hearing help is best for which type of person. This framework allows for the disambiguation of hearing health services, hearing loss technology, and the channel in which services and technology are delivered. It has potential to be a versatile and valuable addition to the industry of hearing healthcare.
Keywords: framework, hearing care, devices, self-fit
The hearing healthcare industry is evolving rapidly. New hearing technology solutions are continually being introduced. Innovation in new ways of providing devices and service is being driven by the COVID pandemic that is globally restricting the ability of clinicians to reach clients face to face. These and other changes have the potential to impact how care is practiced, received, evaluated, and governed as a professional and as a consumer. With these positive changes, however, comes a consequence of potential confusion for the consumer, client, and clinician to successfully navigate these options and choose the most appropriate solution to address their needs. 1 Frameworks are analytical tools that can provide great value in enabling discussion of a new or challenging topic. In line with this, a framework addressing provision options in contemporary hearing care could assist both clinician and client navigate their options and needs to find the most appropriate solution for everyone.
In 2021, there are many providers exploring new and emerging provision options. Modern options include the use of online audiology services, teleaudiology, 2 and provision of hearing aids that do not require clinic-based consultations. For clarity, in this article, a hearing aid is a hearing instrument that requires regulatory approval as a medical device, while hearing device refers collectively to any type of amplification device. There is also renewed and new interest in sales channels using modes of direct-to-consumer (DTC), meaning bought without attending a clinic, and the newly created over-the-counter (OTC), meaning bought without a prescription, respectively. The OTC sales channel is a DTC channel. However, DTC is not always OTC. There is also an increasing variety in the types of hearing devices (e.g., hearables and PSAPs) 3 to incorporate into business operating models. Service options have been explored as independent on-line clinician service (The Hearing Collective 4 and Tuned 5 ), blended offering interchangeable online and face-to-face services (Blamey Saunders hears, see Saunders et al., 2019 6 ), and online-only services with device provision (Listen Lively Corp 7 ). Provision of hearing aids has also included DTC by mail-order and nonclinical outlet sales channels for providing hearing aids: Lively, Blamey Saunders hears, Lexie, 8 Bose, 9 and other hearing technology such as hearables (e.g., Nuheara 10 ). In the United States, a self-fitting hearing aid classification has been created by the Food and Drug Administration (FDA), 9 11 challenging how clinical hearing services are considered. Medical device regulatory-approved hearing aids are no longer the only type of device designed to assist in customizing the hearing experience, although they are, by definition, the only devices that can be described as correcting hearing loss. Hearing devices and care, however, have been available in nontraditional, consumer-focused models for at least 30 years, highlighting that the changes occurring in the hearing care industry were already evolving organically.
The ubiquitous use of the internet, communication technologies, and home delivery services means that geographical access is no longer the barrier to hearing aid uptake that it has long been assumed to be. DTC models have been available as mail order pre-set hearing aids in the United States, Japan, and in Australia for many years. 12 13 14 15 The arrival of OTC legislation will allow a consumer to buy an FDA-approved hearing aid without a prescription, clinician, or consultation, thereby shifting the onus of choice to the consumer. The consumer currently has more options in choosing hearing care products and services than ever before. Despite this, market surveys of the last few years do not show a significant increase in population uptake of hearing devices despite greater accessibility. 16 17 Variation in how and where a consumer chooses to acquire their hearing device, what category of device, and how they wish to be supported creates a potentially confusing array of choices to the clinician and prospective customer. The growing diversity of hearing care services and device provision may, however, provide opportunity in overcoming barriers affecting those who would benefit from hearing help but who do not have hearing devices. Bridging the gap between opportunity to assist hearing difficulties and provision should be easier than ever before.
Recent attempts to better understand the factors behind a persistently low uptake of hearing care have explored clinically defined need in combination with self-perception and acceptance of need. 17 Mapping client needs and degree of acceptance of hearing difficulty to create a suitability framework allows improved clarity on the characteristics of the total potential hearing care market, including those without hearing aids representing a larger proportion than those who do. The emphasis on traditional care providing traditional hearing devices bundled with diagnostic and rehabilitative services has indeed been discussed as a narrowed focus on a minority portion of the market, 18 19 in agreement with this framework approach. If our collective goal in hearing care is to increase the uptake of hearing devices and services, breaking beyond only those currently seeking and utilizing hearing help, it will be imperative for providers and hearing care professionals to be clearly and fully informed on how to match the devices and services to the needs of those with hearing difficulty as effectively as possible. Additionally, service providers will need to be able to inform people with hearing difficulty, clearly and fully, how to choose and use the full range of options now accessible to best serve the client's needs and preferences within the context of client centered care.
The last decade has seen increasing change in hearing healthcare, from legislative changes accommodating self-fit hearing aids and the newly added OTC category in the United States, a revival of DTC hearing aid provision, improved application of online services including teleaudiology, and growth in the number of categories of hearing devices now available. This scoping review searches for frameworks assisting the selection and application of options currently available with the aim of guiding and supporting individuals to make the best choices for their needs.
METHODS
The qualitative framework developed by Ritchie et al was a foundation for the method applied in this article. 20 The research team developed a research question and overall protocol that was divided into five key steps that followed the PRISMA-ScR method and the final stage was further divided into two stages for data summary and then synthesis using a priori themes. As per guidelines for a PRISMA-ScR, 21 22 a five-stage framework was followed tor the conduct of the scoping review. This involves (1) identifying the review question; (2) identifying the relevant studies; (3) selecting the studies; (4) charting the data; and (5) collating, summarizing, and reporting the results. Given the objective to address new and emerging provision options in hearing care, the findings were validated with regard to whether they could recognize a set of contemporary provision models as criteria.
Research Question
A framework is a valuable tool to guide best practices where recommendations or guidelines are unclear or lacking. A key issue with new models of care, especially those that act independently of clinical service, is the lack of clarity or confidence by those in the hearing care industry. Clarity on how to define and assure suitability for nontraditional service models is of importance to the practicing clinician. To this end, the research question was chosen to be “What frameworks exist to advise how to match client needs with current options available in audiological care.”
Data Sources and Search Strategy
The search for relevant literature was performed in May 2021, in three electronic databases: PubMed, Scopus, and Google Scholar. The databases were chosen to allow a wide range of disciplines and types of articles to be identified. Given the focus on nontraditional, new innovations in models of hearing care delivery, articles other than research literature was thought to potentially be relevant and so no restriction on subject or type of article was imposed. No date restriction was applied at the time of performing the search enquiry.
The search query consisted of three components: “Framework,” “Audiology or Hearing,” and “Service or Provision or Care.” Given the research question, “Framework” was chosen as a single compulsory search term, whereas both “Audiology” and “Hearing” will identify literature pertinent to hearing care services, especially among gray literature that will be included in the eligibility criteria. The third and final search term element was chosen to help distinguish clinical practice frameworks and guidelines that the research question seeks as opposed to pedagogical literature. The terms “Services,” “Provision,” or “Care” were selected to indicate the delivery of services.
Scoping reviews offer a preliminary assessment of available literature, 23 to identify whether further attention or investigation is warranted. 24 The search strategy was designed to allow identification of any relevant material pertinent to the research question, conscious of the possibility that no framework may yet exist due to the relatively young stage of market evolution of nontraditional hearing care provision, though relevant material for the development of one may be. In the interest of available resources for screening, and the relevance of articles found, screening of a limited selection of first hits per search was adopted. 25 26
Eligibility Criteria
Following PRISMA 21 as a guide, the authors screened all identified articles for duplications, content eligibility, relevance, and finally full-text screening, removing duplications found. The remaining article titles, keywords, and abstracts were screened for content eligibility. Conscious of the potential paucity of relevant literature, broad description, or reference of the key search terms was eligible for inclusion. Articles deemed irrelevant to delivery of hearing care services were excluded. Full text of the remaining articles was screened. Those which met the inclusion criteria were agreed before proceeding to the next stage (articles and PRISMA stages are shown in Table 1 ).
Table 1. Articles reviewed for scoping from various sources.
| Source | Title | Authors | Year | Prisma stage |
|---|---|---|---|---|
| Previously identified gray literature (comprising industry documentation, reports, and publications) | Teleaudiology | Audiology Australia | 2020 | Eligibility |
| Adult rehabilitation and hearing aids: adult patients with severe-to-profound unilateral sensorineural hearing loss | American Academy of Audiology | Screening | ||
| Hearing aid fitting standard for adult and geriatric patients | Audiology Practice Standards Organization | 2021 | Screening | |
| General audiology intake standards | Audiology Practice Standards Organization | 2020 | Screening | |
| Audiology Australia Professional Practice Standards - Part B Clinical standards | Audiology Australia | 2013 | Screening | |
| Service delivery framework for hearing services in Australia | Department of Health | 2018 | Eligibility | |
| National Practice Standards for Hearing Care Practitioners | National Practice Standards for Hearing Care Practitioners | 2016 | Eligibility | |
| Tele-audiology: an opportunity for expansion of hearing healthcare services in Australia | Eikelboom RH, Bennet R, and Brennan M | 2021 | Qualitative synthesis | |
| Roadmap for hearing health | Hearing Health Sector Committee | 2019 | Qualitative synthesis | |
| The audiologists guide to hearing aids, PSAPs, hearables and OTC devices | American Academy of Audiology | 2018 | Qualitative synthesis | |
| PubMed | Preterm birth: causes, consequences, and Prevention | Institute of Medicine (US) Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman RE, Butler AS, editors | 2007 | Screening |
| Miscommunication in doctor-patient communication | McCabe R and Healey PGT | 2018 | Screening | |
| The journey to interprofessional collaborative practice: Are we there yet? | Golom FD and Schreck JS | 2018 | Screening | |
| Clinical practice guideline: tinnitus | Tunkel DE, Bauer CA, Sun GH, Rosenfeld RM, Chandrasekhar SS, Cunningham ER Jr, Archer SM, Blakley BW, Carter JM, Granieri EC, Henry JA, Hollingsworth D, Khan FA, Mitchell S, Monfared A, Newman CW, Omole FS, Phillips CD, Robinson SK, Taw MB, Tyler RS, Waguespack R, and Whamond EJ | 2014 | Screening | |
| Early intervention for children with autism spectrum disorder under 3 years of age: recommendations for practice and research | Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D, Mailloux Z, Smith Roley S, Wagner S, Fein D, Pierce K, Buie T, Davis PA, Newschaffer C, Robins D, Wetherby A, Stone WL, Yirmiya N, Estes A, Hansen RL, McPartland JC, and Natowicz MR | 2015 | Screening | |
| Clinical measures of bulbar dysfunction in ALS | Yunusova Y, Plowman EK, Green JR, Barnett C, and Bede P | 2019 | Screening | |
| Nurses' perceived barriers to bedside handover and their implication for clinical practice | Tobiano G, Whitty JA, Bucknall T, and Chaboyer W | 2017 | Screening | |
| Physical health and mental illness: listening to the voice of carers | Happell B, Wilson K, Platania-Phung C, and Stanton R | 2017 | Screening | |
| Hearing impairment and cognitive energy: the framework for understanding effortful listening (FUEL) | Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BW, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, Naylor G, Phillips NA, Richter M, Rudner M, Sommers MS, Tremblay KL, and Wingfield A | 2016 | Eligibility | |
| Child and adolescent health from 1990 to 2015: findings from the Global Burden of Diseases, Injuries, and Risk Factors 2015 Study | Global Burden of Disease Child and Adolescent Health Collaboration | 2017 | Screening | |
| Scopus | Optimization of computer-aided English translation teaching based on network teaching platform | Gu S and Li X | 2022 | Screening |
| Systematic review of outcome domains and instruments used in designs of clinical trials for interventions that seek to restore bilateral and binaural hearing in adults with unilateral severe to profound sensorineural hearing loss ('single-sided deafness') | Katiri R, Hall DA, Killan CF, Smith S, Prayuenyong P, and Kitterick PT | 2021 | Screening | |
| Conductive hearing loss during development does not appreciably alter the sharpness of cochlear tuning | Ye Y, Ihlefeld A, and Rosen MJ | 2021 | Screening | |
| Estimating multiple latencies in the auditory system from auditory steady-state responses on a single EEG channel | Wang L, Noordanus E, and van Opstal AJ | 2021 | Screening | |
| Are need for affect and cognition culture dependent? Implications for global public health campaigns: a cross-sectional study | Zhang M, Zhu B, Yuan C, Zhao C, Wang J, Ruan Q, Han C, Bao Z, Chen J, Arceneaux KV, Wielen RV, and Siegle GJ | 2021 | Screening | |
| Community-based organizations' perspectives on improving health and social service integration | Agonafer EP, Carson SL, Nunez V, Poole K, Hong CS, Morales M, Jara J, Hakopian S, Kenison T, Bhalla I, Cameron F, Vassar SD, and Brown AF | 2021 | Screening | |
| Sensitivity to haptic sound-localisation cues | Fletcher MD, Zgheib J, and Perry SW | 2021 | Screening | |
| Factors affecting the implementation of evidence-based progressive tinnitus management in Department of Veterans Affairs Medical Centers | Zaugg TL, Thielman EJ, Carlson KF, Tuepker A, Elnitsky C, Drummond KL, Schmidt CJ, Newell S, Kaelin C, Choma C, and Henry JA | 2021 | Screening | |
| The experiences of and teaching strategies for deaf and hard of hearing foreign language learners: a systematic review of the literature | Kang KY and Scott JA | 2021 | Screening | |
| Stakeholder consensus for decision making in eye-gaze control technology for children, adolescents and adults with cerebral palsy service provision: findings from a Delphi study | Karlsson P, Griffiths T, Clarke MT, Monbaliu E, Himmelmann K, Bekteshi S, Allsop A, Pereksles R, Galea C, and Wallen M | 2021 | Screening | |
| Google Scholar | The curriculum of practice: a conceptual framework for speech-language therapy and audiology practice with a black African first language clientele | Pillay M, Kathard H, and Samuel H | 1997 | Eligibility |
| Interventional audiology: moving from concept to practice: enhancing communication in adults with dementia and age-related hearing loss | Mamo S, Oh E, and Lin F | 2017 | Screening | |
| Patient complexity charge matrix for audiology services: a new perspective on unbundling | Windmill I, Bishop C, Elkins A, Johnson, and Sturdivant M | 2016 | Screening | |
| Speech-language pathology and audiology: transformation in teaching, research and service delivery | Uys I and Hugo R | 1997 | Eligibility | |
| Fitting audiology within the population health perspective | Fitzpatrick E, Johnston J, Angus D, and Durieux-Smith J | 2006 | Screening | |
| Telehealth in audiology: the need and potential to reach underserved communities | Swanepoel D, Clark J, and Koekemoer D | 2010 | Screening | |
| Measuring quality in audiology a global framework | Davis A | 2008 | Eligibility | |
| Survey of ENT services in Africa: need for a comprehensive intervention | Fagan J and Jacobs M | 2009 | Eligibility | |
| Diagnostic audiology | Sanders J | 1990 | Screening | |
| iManage program: decision coaching guide to promote audiology care | Preminger J and Galloway L | 2021 | Screening |
Data Characterization
Full-text review of articles deemed eligible for inclusion was conducted. The final articles identified and deemed to be relevant to research question and purpose proceeded to qualitative synthesis whereby the context and information provided in the article were analyzed.
Data Summary and Synthesis
Data analysis was conducted in two stages: in the first stage, data were gathered from the qualitative synthesis to identify relevance to the research question; the second stage consisted of a qualitative framework approach with a priori themes. 20 Table 2 describes the priori themes derived from a sample of modern, nontraditional, commercial hearing care delivery models. The a priori themes act as validation criteria to support determining if content derived from data analysis is pertinent to the research question. Stage 1 thus consisted of finding relevant literature in support of the themes in Table 2 and the research question, and stage 2 consisted of second-order analysis to develop descriptive conclusions and interpretations.
Table 2. Validation criteria composed of currently operating hearing care provision models.
| Provider | Year launched | Country | Primary product | System |
|---|---|---|---|---|
| Blamey Saunders hears | 2011 | Australia | Self-fit hearing aids | Blended model |
| Lively | Est. 2016 | America | DTC hearing aids | Teleaudiology model |
| The Hearing Collective | 2018 | Australia | Teleaudiology services | Unbundled teleaudiology model |
| Nuheara | 2016 | America | Hearables | DTC |
| Bose | 2021 | America | Self-fit hearing aids | DTC |
| Lexie (@Walgreen) | 2021 | America | Hearing aids | DTC |
FINDINGS
A PRISMA approach was used 24 followed by a qualitative framework approach with a priori themes to validate the information gathered ( Tables 1 and 2 ). 20 A search of three databases—SCOPUS, PubMed, and Google Scholar—for literature appropriate to the research question proved unsuccessful in identifying any existing frameworks, with limited literature appropriate to address the research question. Only three articles succeeded to qualitative synthesis, 27 28 29 containing minor content relevant to a framework; however, the broader contents within these articles were deemed to be relevant if a framework was to be produced. The qualitative framework approach showed a poor level of compatibility with the validation criteria provided in the a priori themes of modern hearing care service models. A gap between available literature and praxis in meeting client needs in contemporary audiological care was therefore found (see Table 3 ).
Table 3. Validation outcomes illustrating thematic analysis and mapping of core domains.
| Provider | Framework problem | Thematic domain |
|---|---|---|
| Blamey Saunders hears | DTC acquisition Flexible support provision Self-fit validation |
Channel Services Technology |
| Lively | DTC acquisition Teleaudiology |
Channel Services |
| The Hearing Collective | Uncoupled technology Unbundled teleaudiology |
Technology Services |
| Nuheara | DTC acquisition Non-audiological Validation Hearables |
Channel Technology Technology |
| Bose | DTC acquisition Self-fit validation Non-audiological support |
Channel Technology Services |
| Lexie (@Walgreen) | DTC acquisition | Channel |
Eligibility Screening
Ten articles were provided from professional, government, and independent associations related to audiological/hearing care in Australia or the United States.
After checking for duplicates, screening using the remaining search terms “Framework” and “Service(s),” or “Provision” led to the exclusion of 34 articles that provided no relevant context to any of these key terms; all 30 articles were from database searches and 4 were from the gray literature (see Table 1 and Fig. 1 ). 30 31 32 Eligibility analysis was used to look at the remaining six articles and led to the further exclusion of three gray literature articles. 2 33 34
Figure 1.
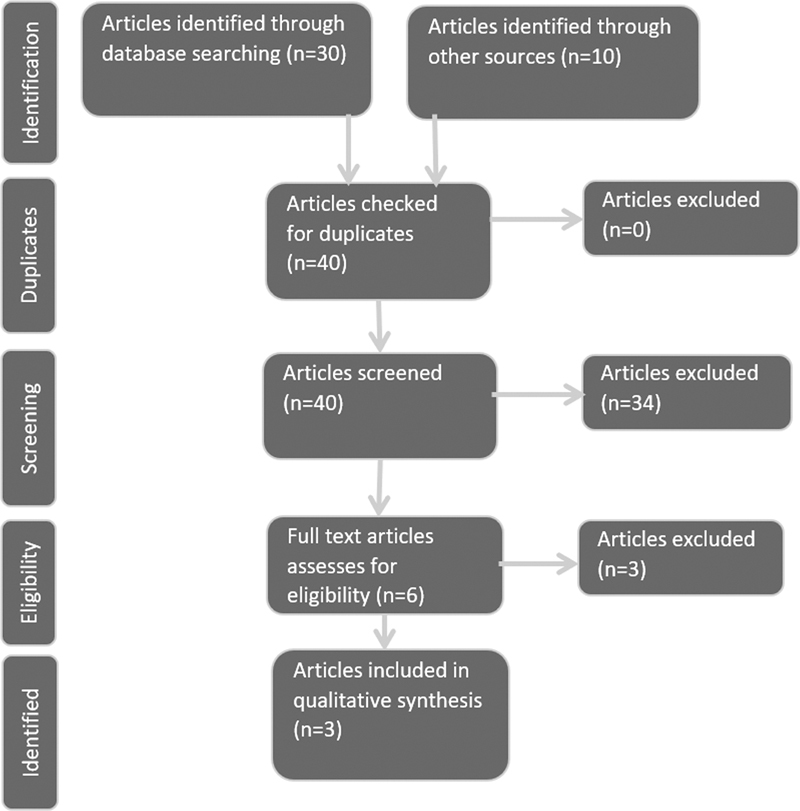
PRISMA approach flow diagram illustrating the scoping, and screening process (adapted from Liberati et al 20 ).
Among the excluded articles, reference to a framework was found to be in context of the ICF framework 35 or to a suggested services framework that would be appropriate to the research question; however, this does not yet exist. 2 34 The final article excluded at this stage noted a recommendation for a service delivery framework noting the need for one is being recognized. 2
Data Analysis
Three articles succeeded to qualitative synthesis using the PRISMA protocol. 21 27 28 29 All three recognized the potential impact or considerations in service delivery/device provision. Two of these documents discussed the need for a framework or guidance on service provision, 27 28 while the remaining article did not address any frameworks or need for service and product provision guidelines. The final article succeeded the qualitative synthesis as it contained the most in-depth discussion regarding considerations on suitability, service support, and potential models of care. 29 Despite the lack of discussion of a framework, the content of the OTC guidelines of the American Academy of Audiology ultimately contained the greatest relevance for what would be most valued in a framework. Overall, though the value of a framework to support service delivery was acknowledged, none of the articles were able to offer one.
Second-Order Analysis
No frameworks were identified that answered the research question and so interpretation and descriptive conclusions were the greatest value that could be derived from the literature that was identified from the scoping review methodology. The qualitative framework approach allowed analysis of the literature identified with a priori themes. The a priori themes composed of validation criteria from nontraditional commercial hearing care business models, allowing pertinent discussion for the goal of providing clarity and direction to benefit all of the industry of audiological care (see Table 2 ). Incompatibilities found between the validation criteria and hearing care literature were then mapped by thematic analysis to three core domains: Service, Channel, and Technology/Device (see Fig. 2 ).
Figure 2.
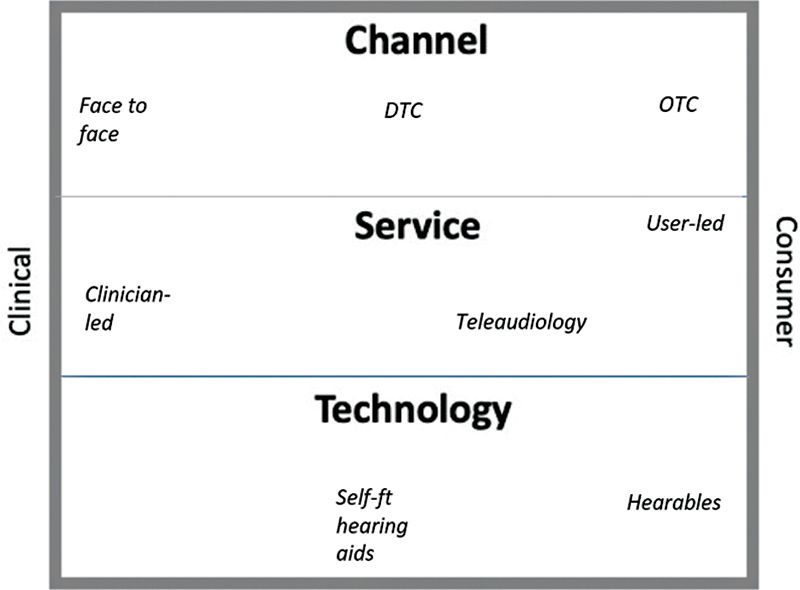
Hearing care landscape framework.
Services
Services were mentioned in all three articles, but referred mainly to fitting of hearing aids, diagnostic/testing services, and post-fitting/rehabilitation services. The use of teleaudiology for all audiology services was thoroughly recognized by Eikelboom et al (2021), 27 as expected given this document specifically addresses teleaudiology in audiological practice. Though a thorough recognition of all audiological services potentially delivered using teleaudiology as clinician-led, facilitator-assisted, or self-led is present, there were fundamental conflicts and gaps identified during validation. There are a growing number of providers offering multiple forms of service delivery, often allowing interchangeability between the three modes of service delivery. Consideration in changing modes of service delivery was not a focus in these articles. The articles do not attempt to identify those individuals who may need or benefit from more rigorous diagnostic evaluation, either to seek information on barriers to success, such as abnormal loudness growth, or any underlying medical conditions.
The American Academy of Audiology's document providing guidance on OTC was identified by qualitative synthesis. 29 The primary focus of this document was to offer considerations on providing and supporting the various types of devices currently available in hearing care. While services were not the subject of the document, service considerations were recognized and discussed in light of their use in the support of hearing aid devices sold to the consumer. Hearing device support thus provides relevant content for the research question asked here. The common practice of bundling client services and hearing aid purchases was considered according to whether diagnostic services were grouped with device-fitting services or not. Audiological services for rehabilitation and non–device-related support, however, were not recognized as candidates for unbundling in this article.
The final article identified in qualitative synthesis described practice standards for hearing care. 33 Services were mentioned many times throughout this document; however, the context provided no relevance to guidance on combined device and service selection and suitability. The focus was instead to guide professional conduct and behavior.
Validation of these articles highlighted a focus on clinic-based services as the primary model for hearing care. There was no recognition of flexibility of service provision as demonstrated by a blended service model. In a blended model assessment, fitting, verification, and/or validation of a hearing aid fitting, and rehabilitation support, can occur interchangeably between clinic, self-led care, and with teleaudiology support (e.g., Blamey Saunders hears blended care model). In an unbundled model of hearing care, the provision of a hearing device can be independent of any rehabilitation or diagnostic services offered or used (e.g., Bose). There will, however, be a requirement to address warranty and/or device services, as would be normal in a consumer model. The “Hearing Collective” demonstrated that unbundled, remote clinical services are a viable offering. The online hearing aid provider Lively retails online, dispatching pre-programmed hearing aids akin to older mail-order of pre-set hearing aid provision models in the United States and Japan, and then provides centralized telehealth support as required, presumably factored into the price. The fitting process is performed independent of the client's presence or input, by the clinician, prior to the client receiving the aid, where support services are available post “fitting” where the format of the service may be client led; however, the adjustments themselves may very much be clinician led as per their clinical judgment. The variation in service offerings in the provision models of Bose, Lively, and The Hearing Collective are not well recognized among the literature identified.
Verifying a hearing aid fitting is a clinically described part of audiological services that has been tied to reimbursement in Australian services. The rationale behind using verification of a hearing aid fitting for a user was born out of assuring a standard of practice. However, it was also used to justify exclusion of discussing validation methods for self-fit hearing aids as they do not require verification. 27 The Australian government provides a rigorous reimbursement system that asks for evidence of measurement of objective benefit following fitting of a hearing aid, such as verification testing results. This verification procedure may indicate a prioritization of clinical economic influences over audiological ones. However, self-reporting-based methods for validating fitting of any hearing device type was noted implying confusion over the role technology has in affording various service options in hearing care. Separation of technology from services in the literature analyzed here appears to have limited the capacity to recognize flexibility of service models available and consequently shows limited recognition that an innovative service model may be strongly underpinned by technology (e.g., Blamey Saunders hears).
In summary, validation of the articles addressing current service options showed that interchangeability of services, suitability of client objectives for different service delivery modes, and the full range of audiological services in an unbundled model were not well recognized by the current literature.
Channel
Hearing aids have predominantly been sold to end users by hearing care professionals (clinicians), through specialist audiology (or hearing and vision) clinics. Hearing aid manufacturers traditionally have sold their products to clinics, making clinicians the primary customer, rather than the end user. Recent advancements in the hearables market, DTC models, and the introduction of OTC legislation in the United States have challenged the notion of who the hearing device manufacturers should consider as their primary customer and how end users can buy their hearing care products. The retailer Costco, for example, is a major hearing aid supplier and customer for hearing aid manufacturers. As in many industry sectors, the end user and the customer are not the same. Hearing device selection, suitability, and end user management are therefore more important than ever for hearing care professionals and users alike. The three articles identified provided little recognition of the options available to users for buying their devices; only the document focusing on OTC explicitly referred to “other platforms” to buy devices in the context of unbundled services. 29 However, in advising where and how hearing devices can be purchased, only the “audiologist” was offered for hearing aids while recognizing “nonclinical outlets” for other types of hearing amplification instruments.
The validation criteria used in this review includes various DTC sales channel models. In a DTC model, the retail company is selling directly to the user, which is also the case in the OTC model which affords further freedom for the consumer by negating the requirement for a prescription. Most recently, Lexie Hearing has explored the versatility of sales channels, uncoupling the hearing aid from fixed clinical services. 36 This is demonstrated with their recent engagement with Walgreens shopping outlets supporting sales of their products in a “nonclinical outlet.” In Australia, DTC has been available from the manufacturer and hearing care provider Blamey Saunders hears, where hearing aids were offered in a self-fit system allowing users to follow an entirely independent hearing care journey or choose to use teleaudiology and/or clinic-based services; thus, offering clinic and DTC-based sales channels with one business model. Notably, this was a technology-driven and customer-care-focused model. The ability of one manufacturer and provider to offer “audiological” and “nonclinical outlets” is not discussed in the literature analyzed. To do so would require recognition of bespoke technology in signal processing strategy, fitting software, user-interface, database, and all appropriate system components, such as that used by Blamey Saunders hears. 13 15 The sales channel options for a hearing device are therefore a product of the possibilities afforded by the functionality incorporated in the technology.
The business model for Lively, an online hearing aid provider, and the new self-fit hearing aid provider Bose are also part of the validation criteria used in this review. Lively is an online hearing aid sales and services model, with a pre-purchase remote consultation with an audiologist, following an online warble tone hearing test. As such, it is an online implementation of a clinician-led model. The Bose' self-fit hearing aid system does not supply clinician services, offering the only bespoke self-fit hearing aid available in the United States at the time of writing this review. The Bose model shows technology specifically developed for the end user to self-fit the hearing aid, rather than for a clinician to use. Given there is legislation specific to recognizing self-fit hearing aids in the United States, it would be appropriate to have this category recognized in service guidelines. The literature on these models highlighted a lack of recognition for DTC models for hearing aids as well as a gap in discussing the sales channel independently of service offerings with hearing devices.
Technology
The current hearing device market consists of devices with the sometimes-overlapping descriptors of hearing aids, hearing-assistive devices (also referred to as ALDs), and hearables which are also described as PSAPs (personal sound amplification products). Some of these products are marketed and labeled as having an intended use to compensate for hearing loss, while others have an intended use to personalize the individual hearing experience. All hearing devices are intended to provide benefit to users and thus are clinically relevant, but regulatory bodies typically categorize devices as providing therapeutic benefit for hearing loss or not and classify those that do as regulated medical devices (i.e., hearing aids). Hearing care providers benefit from these classifications when selecting, supporting, and understanding suitability of the device for their clients. Users should also benefit from these classifications by knowing the intended use of the hearing devices and knowing that hearing aids intended to help with their hearing loss have met the requirements of the regulatory body. This scoping review found a basic level of guidance on suitability and selection of OTC devices, which currently comprises hearables, ALDs, and PSAPs. The majority of articles that provided guidance focused on clinic-based services, 30 32 33 37 providing negligible discussion around device selection or suitability.
Clinical validation of a hearing aid fitting can be tested in a variety of ways. Assurance that the user deems the device to be beneficial, however, is commercially important to support the user's choice to buy the product and clinically important to assure benefit is being achieved thus supporting the likelihood the user will commit to using the product. Commercially available self-fit hearing aids have thus far shown two different approaches to validating a device fitting. Blamey Saunders hears and Bose use methods that are performed independent of clinical assistance or clinic-based tools. In the case of Blamey Saunders hears, an aided speech perception test is performed with the fitted device and the increase in score over the unaided speech perception test is used as a measure of hearing aid benefit. 38 This provides an objective measure in answer to validation of a fitting that is both clinically and personally relevant. In the case of Bose, the fitting method consists of two fine-tuning wheels that are adjusted by the user to maximize perceived sound quality and satisfaction. 39 Validation is, therefore, ultimately a subjective conclusion on the user's part of their experience with their hearing aids. These perceptual methods contrast with the most common clinical verification, where the device output matches the prescription as determined by the chosen prescription formula in a hearing aid test box. Clinical verification asks about the functional performance of a device: while it may be intended as a question assuring clinical best practice fittings are used, its application bears no consequence on whether the prescribed fitting used is the best choice for the client. This verification method uses equipment that is typically designed for clinic-only use. This fundamental difference in approaches to device fitting validation suggests that a clearer understanding of the value and versatility of fitting technology and methods for hearing devices could be a positive addition for clinicians to successfully and confidently navigate the various hearing device options available today. Clarification on these would further allow a clinician to better decide which products they can use, with which available equipment and support services and which clients, ultimately offered in which modes of service. The Hearing Collective uncoupled technology from (fixed) service provision models thus requiring an understanding of the technology objectives, potential use of various hearing devices, and clinical tools available to best suit the user's needs. The open market of hearing device and service provision is aimed at the user who decides which technology they will purchase (e.g., to visit Walgreens to acquire a Lexie hearing aid) to shop online for a hearable device like Nuheara's products or a self-fit hearing aid purchased online (e.g., Bose) or from a clinic with extensive service provision (e.g., Blamey Saunders hears). This is analogous to normal consumer choices. The literature analyzed in this review did not portray the user's active role in choosing the technology.
Mapping a Framework
A framework is an analytical tool to enable discussion of a topic. No frameworks were identified in this scoping exercise that could effectively advise matching needs with current provision options available in audiological care. The research question was therefore unable to be answered. However, analyzing the literature identified highlighted 14 points of conflict regarding current hearing care models (see Table 3 ). The problems identified were thematically mapped to the three core domains; Services, Channel, and Technology, together offering a framework by which any new or existing hearing care product and/or provision model can be recognized and evaluated (see Fig. 2 ). In this proposed framework, any product, service, or model of care can describe their core elements with respect to a relative positioning of clinic(ian) centric to consumer centric. A Blamey Saunders hears hearing aid purchased online provided as part of a blended service model may be described as a clinical technology device (i.e., a hearing aid, bought via a DTC consumer channel), supported with consumer-led (teleaudiology) services. A hearable such as a Nuheara product is a consumer technology device, bought in a consumer channel as OTC, with no audiology services offered. However, the user could seek independent clinical services either face to face in a clinic or via teleaudiology for assistance (where available) with their device. In other words, this framework can be a tool to describe what (technology), where (channel), and how (service) a user chooses their hearing care.
DISCUSSION
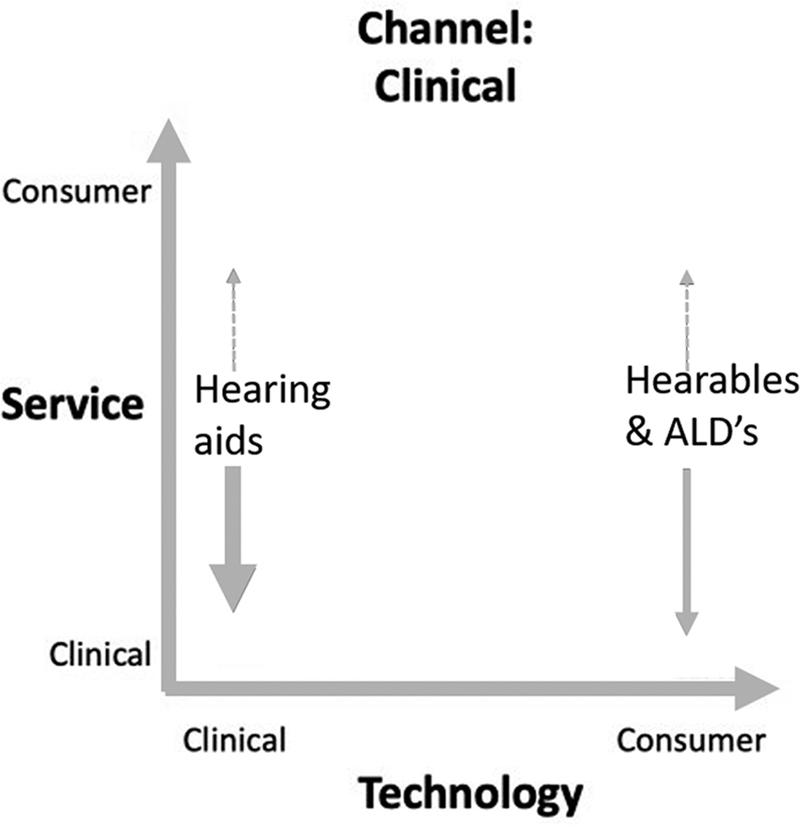
Historically, hearing care has been dominated by clinic-based care, making traditional channel, service, and technology a clinician-centered care rather than a client-centered care. Hearing aids and ALDs have until recently been the predominant choices of technology available in clinic-based hearing care, with hearing aids being the primary device. Both types of devices have the potential to show growth in pairing consumer-led services with these clinical technologies while remaining in a clinic sales channel (see Fig. 3 ). Today, all major manufacturers of hearing aids support consumer-led service options in the platforms made available for clients. The use of these platforms to enable and offer consumer-led support services is no longer a question of access but of provider and client choice.
Figure 3.
Clinic sales channel presentation using proposed framework.
Technology Is an Enabler for Innovation in Service, Channel, and Device
The 2020/2021 “lockdown” approach to COVID has challenged clinicians to adopt new tools that allow access to clients in nontraditional ways (e.g., teleaudiology to overcome barriers created by restrictions on mobility and contact). Recent estimates continue to recognize a majority of potential hearing device users not yet doing so. 17 There is also increasing recognition that nontraditional care may be better suited to help meet the needs of a greater proportion of the market and reduce the number of people remaining unassisted. 18 19 Technology has been fundamental in enabling the creation of various fitting methods and service support systems, in turn leading to new sales channels. It is therefore imperative that all participants in hearing care, clinician and client alike, can navigate the array of options available between services, channel, and technology to best suit each person's needs.
According to the framework proposed here, hearing devices (i.e., technology) are spread across a spectrum from clinic-led to consumer led, with regulated hearing aids sitting on the clinical side, against hearables and ALDs on the consumer side. This spectrum also covers supporting the client needs with clinical assistance on one end and nonclinical hearing support on the other. Traditionally, hearing need has been defined medically by level of clinically measured hearing loss as defined by the audiogram. The audiogram, or amount of hearing loss expressed in decibels of hearing loss (dB HL) at each frequency, also defines the loss of audibility at each frequency and the amount of gain needed to restore audibility to what is considered clinically normal. Not all hearing need is defined by the audiogram and has been instrumental in designing innovative hearing technology such as self-fit hearing aid fitting systems along with new amplification strategies found in hearables. Adding to the complexity of understanding the breadth of hearing device technologies is that many of the technologies are similar, with both clinical and consumer products having directional microphones, noise reduction, wireless streaming, amplification (gain), and other audio processing features, although amplification strategies vary. Even the look can be similar, with some regulated hearing aids looking like hearables and hearables looking like traditional hearing aids.
The FDA and other regulatory bodies differentiate hearing aids from consumer hearables by their intended use. Hearing aids are defined as products that are intended to compensate for impaired hearing. Any device that is labeled, marketed, and designed to compensate for hearing impairment is a medical device. Devices that are not labeled, marketed, and designed to compensate for hearing impairment are consumer products. So, two products could be the same, with the same look and the same features, but if one is intended to compensate for hearing loss and one is intended to correct the hearing of anyone regardless of their hearing ability, then they lie on opposite ends of the spectrum.
So, to differentiate between clinical and consumer devices, the intended use is the defining factor. Whether a device looks like a hearing aid or whether it has traditional features for helping with speech in noise do not determine where on the spectrum products will sit. Furthermore, the channel in which a hearing device is distributed does not determine where it sits on the technology spectrum of products.
By unlinking technology considerations from service and delivery channel considerations, we see that different business models in the hearing field can and are being defined. In many places in the world, a regulated medical device hearing aid can be sold in a consumer store or online without the assistance of a healthcare professional, along with unregulated nonmedical devices, hearable, sold in an audiologist's clinic. A hearing aid can be fit without any service or support by a healthcare practitioner, while a hearing care practitioner's service could be provided to someone purchasing a consumer product.
Using the Framework
This framework allows for the disambiguation of hearing health services, hearing loss technology, and the channel in which services and technology are delivered. By separating these three factors explicitly, one can see that changes in one factor do not necessarily require changes in another. For example, the introduction of a consumer hearing device such as a hearable or the introduction of a hearing aid in a consumer delivery channel does not inherently remove the possibility for audiological services to be provided. Each solution provided to someone seeking hearing help can be a blend of these three factors, with each factor ranging across the spectrum from purely clinical at one end to purely consumer at the other—essentially a mix-and-match of technology, service, and channel. This framework helps define the different options available to someone seeking hearing help and clearly differentiate different approaches.
If one considers only the pure clinical and pure consumer options for each of the three factors, then eight archetypes of solutions exist. For clarity in defining each archetype, the consumer and clinical options will be denoted with a Co and Ci, respectively. Technology, channel, and service will be denoted by T, C, and S, respectively, such that CoT represents consumer technology and CiC represents the clinical channel. The combination of each three together into archetype can then be represented as follows for the purely clinical solution: CiT-CiC-CiS. If one of the factors is the consumer option instead of the clinical one, a Co replaces the C, such as CiT-CoC-CoS. Fig. 2 shows how this can be easily represented. We will now discuss the eight fundamental archetypes in this framework and provide examples of them in current hearing solutions, if they exist.
CiT-CiC-CiS: Clinical Technology, Clinical Channel, Clinical Service
The archetype represents the most traditional method for providing hearing solutions: a hearing aid provided at a clinic and fit by a clinician. This is the most common solution provided in many countries.
CoT-CiC-CoS: Consumer Technology, Clinical Channel, Consumer Service
This archetype represents consumer technology, such as a hearable or ALD, provided in a clinic but without any fitting or support by a clinician. Offering ALDs in hearing clinics is not a new concept but offering hearables—an alternative to hearing aids—in a clinic is not as common. A lack of clarity on who is a better candidate for a hearable rather than a hearing aid may be one reason for why this model is uncommon. Typically, in a hearing healthcare clinic, hearing aids are recommended to clients who present with a hearing loss—the client either accepts a recommendation of a hearing aid or leaves with no solution. More consumer-like solutions, such as hearables, could be offered to someone who needs hearing aids but rejects them or for those for whom hearing aids are not an appropriate solution (e.g., they have a normal audiogram but difficulty with speech in noise).
CoT-CiC-CiS: Consumer Technology, Clinical Channel, Clinical Service
This archetype represents consumer technology, such as a hearable or ALD, provided in a clinic supported by the services of a clinician. While most consumer products, such as hearables, are not designed to need clinician support, there are hearable products that allow the user to adjust the product settings to their hearing ability or needs, and some users may benefit from the assistance and counseling of a clinician. A clinician may charge for this service or bundle charges for support into the price of the product, similar to how service charges are bundled into the price for hearing aids.
CiT-CoC-CoS : Clinical Technology, Consumer Channel, Consumer Service
This archetype represents hearing aids obtained in a retail channel, which could be a pharmacy, an electronics store or online, without any support provided by a clinician. Bose selling hearing aids directly to consumers in pharmacies with a self-fitting system is one example of this model. There are currently many hearing devices available online or through mail order, some promoted as self-fit hearing aids thus fit this model. However, the distinction between what the device in question is regulated and registered as, rather than the chosen marketing messages, determines if the same device would fit as consumer technology rather than clinical technology in this framework. This should highlight that the potential use of a technology should be the key factor but the chosen path to making the technology available may impact how well this is understood.
CiT-CiC-CoS: Clinical Technology, Clinical Channel, Consumer Service
This archetype represents hearing aids provided by a clinician but without any clinician support. Such a hearing aid could be preset to a mild amount of gain for people who do not need more sophisticated products or for people who cannot afford a more expensive device that requires expensive services from the clinician.
CiT-CoC-CiS: Clinical Technology, Consumer Channel, Clinical Service
This archetype represents hearing aids that have clinician support where the technology is obtained through a retail channel. The company Lively has this business model (see Fig. 4 ), where consumers order hearing aids online and receive audiologist support through teleaudiology services.
Figure 4.

Consumer sales channel according to the proposed framework showing relative positioning of various models.
CoT-CiC-CoS: Consumer Technology, Clinical Channel, Consumer Service
This archetype represents consumer devices, such as hearables or ALDs, provided in a clinic, any services that are provided are not defined by the clinician though are not mutually exclusive of them. This could be for someone who needs hearing help but not a hearing aid or who rejects a recommendation for a hearing aid yet is open to other solutions.
CoT-CoC-CiS: Consumer Technology, Consumer Channel, Clinical Service
This archetype represents consumer devices that are obtained in a consumer channel but receive clinical service. Unlike other CiS archetypes where the cost of clinician's service is bundled into the price of the hearing device, a consumer technology sold in a consumer channel is presumably designed to not require the assistance of a clinician. The consumer would then presumably seek clinician support for the product due to some need for assistance in using the product. Unless the clinicians were at a not-for-profit or government services program, this service would be an additional cost to the consumer.
SUMMARY
The consumer channel for hearing care shows versatility in services and technology. While traditional hearing aids are medical devices, placed in a clinician-based model of care, innovation in the technology involved in the client journey has facilitated creation of self-fitting, impacting compatibility with different support service structures. At Blamey Saunders hears, the fitting software IHearYou is user-centric/consumer-driven by design thinking, while allowing clinical functionality should that be required. This design resulted from the goal of delivering a blended service model with a client-centered choice of clinical or consumer channel (see Fig. 4 ). Bose has also created technology that allows a consumer-centric fitting process. Bose, however, has opted not to provide clinical functionality as per the consumer-led services their product targets. The newly created OTC category will add to the innovation in hearing care that has already been building around the world for some years now.
FUTURE DIRECTIONS
The framework developed in this review allows for an understanding of where innovation is occurring in hearing healthcare and differentiates between changes to technology, channel, and service. Under this framework, common questions and concerns are no longer relevant, such as whether online distribution channels eliminate opportunities for clinical services or whether hearables replace the need for hearing healthcare clinics. With different options available for technology, channels, and services and the ability to mix and match, a variety of different options exist for people who need hearing help. New questions arise, such as whether one model is more effective than another or which model of hearing help is best for which type of person.
Enabling these discussions benefits the user and clinician while also inviting all stakeholders to be able to be part of a productive conversation where each role has different objectives. For example, the user and clinician can be supported in decision-making to assure the most appropriate solution is sought, while policy makers and entrepreneurs can also identify gaps and potential for innovation and analysts are also able to understand the landscape of the hearing healthcare sector. Each stakeholder is then also able to gain new perspectives such as a clinician looking to understand how new innovations may affect their profession. This framework has potential to be a versatile and valuable addition to the industry of hearing healthcare.
LIMITATIONS
The inclusion criteria yielded surprisingly few articles from academic sources. The addition of nonacademic sources was therefore needed and yet the level of rigor in the gray literature would not have matched any academic material. A recommendation for continuation of this project would therefore be to design selection criteria primarily for gray literature on account of the varying readerships and purposes of documentation in this group.
This scoping review has produced a framework aimed at facilitating discussion and acknowledgment of current and emerging models of hearing care technology and services available. While the proposed framework can be applied globally, the nature of this review as a scoping exercise means a larger study would be needed to qualify its validity to the global market. Doing so is beyond the aim of this scoping review.
The final limitation to note for this review is the time frame of the scoping amid a period of fast pace of change. Since the beginning of writing this review, more models of DTC hearing devices, both hearing aids and hearables, have continued to enter and change the market. The Hearing Collective is no longer operational. However, an American counterpart has since presented the same service model, like wise Bose has announced they will be closing their hearing aid division. There are other self-fit hearing aid products that certainly do and will match the framework position held at the time of writing. Likewise further publications have and will continue to be published that add to the story addressed here. While the outcomes of this review still likely stands amid the changes that continue to arrive, it is not possible to say so confidently without continually repeating the exercise performed here. This in itself further supports the need for a forward thinking, inclusive framework that will cater to the changes we cannot yet see coming.
CONCLUSION
The last decade has seen vast growth in hearing healthcare, from new service models to device types and creation of sales channels globally. There is also a large portion of the hearing care market which is not actively using hearing care highlighting a need to innovate and address how best to apply contemporary care with the modern consumer. This scoping review found no frameworks available for assisting the selection and application of current hearing care provision options. A new framework was therefore created with the aim to guide and support individuals to make the best choices for their needs. The available literature showed a lack of acknowledgment of interchangeable service options, uncoupling of technology, services, and sales channels, and the user as active in choosing technology. Together these findings show a tendency to overlook the need for client and clinician to fully understand which technology will be the best client fit. The framework produced here can be a tool to describe what (technology), where (channel), and how (service) a user chooses their hearing care. This framework has potential to be a versatile and valuable addition to hearing healthcare. While innovations may start with user choice and flexibility afforded by technology design, it is in the challenging times that progress can really be determined.
Footnotes
Conflict of Interest None declared.
References
- 1.Office of the Attorney General Attorney General Bonta Issues Consumer Alert on Hearing Aids Sold Online or Over-the-Counter [Press release]State of California department of Justice Office of the attorney General, DOJ: August 4, 2021. 2021. Accessed August 10, 2021 at:https://oag.ca.gov/news/press-releases/attorney-general-bonta-issues-consumer-alert-hearing-aids-sold-online-or-over
- 2.Audiology Australia Teleaudiology [Position Statement]. Audiology Australia, Suite 7, 476 Canterbury Road, Forest Hill, VIC 3131 2020. Accessed February 12, 2021 at:https://audiology.asn.au/Tenant/C0000013/AudA%20Position%20Statement%20Teleaudiology%202020%20Final(1).pdf
- 3.Staab W, Hosford-Dunn H.The Big Fat PSAP Book (Kindle Edition), Hearing Health & Technology Matters 2017. Accessed May 15, 20223 at:https://hearing-health-matters-store.myshopify.com/products/the-big-fat-psap-book
- 4.The Hearing Collective Hearing Collective Services. The Hearing Collective © 2018. Accessed March 13, 2021 at: https://www.hearingcollective.com/services [See also: Penno, Kat. Wow Woman in Wearabletech: Kathryn 'Kat' Penno, Founder and Audiologist at Hearing Collective. Edited by Marija Butkovic. Women of Wearables, 2020. Accessed May 20, 2020 at: https://www.womenofwearables.com/new-blog/wow-woman-in-wearable-tech-kathryn-penno-hearing-collective]
- 5.Tuned. Tuned care ©. V.2.16 2022. Accessed February 3, 2022 at:https://www.tunedcare.com/
- 6.Saunders E, Brice S, Alimoradian R. IGI Global; 2019. Goldstein and Stephens revisited and extended to a telehealth model of hearing aid optimization; pp. 33–62. [Google Scholar]
- 7.Lively Hearing Corp Listen Lively. Listen Lively© 2019. Accessed March 31, 2021 at:https://www.listenlively.com/
- 8.Cooling G.Lexie Hearing, Probably The Best Direct to Consumer Hearing Aids We Have Seen. [Blog]. The hearing KnowJanuary 18, 2021. Accessed January 21, 2021 at:https://www.hearingaidknow.com/lexie-hearing-direct-to-consumer-hearing-aids
- 9.Strom K.Bose Announces First Self-Fitting Hearing Aid: SoundControl. The Hearing Review 2021. Accessed June 2, 2021 at:https://www.hearingreview.com/inside-hearing/industry-news/bose-announces-first-self-fitting-hearing-aid-soundcontrol
- 10.Nuheara Limited About Nuheara. Nuheara Ltd© 2016. Accessed May 21, 2021 at:https://www.nuheara.com/usa/
- 11.Auditory Insight FDA OTC Rule and Medicare Legislation; Hearing Aid Market Development Impact [Ed Nancy Williams]Auditory Insight 2021; Research Note: Q4 2021
- 12.Kochkin S. A comparison of consumer satisfaction, subjective benefit, and quality of life changes associated with traditional and direct-mail hearing aid use. Hearing Review. 2014;21(01):16–26. [Google Scholar]
- 13.Saunders E.From the bionic ear to the 'audiologist in your pocket [Press Release]. Science in Publics, 2015Accessed March 31, 2021 at:https://www.scienceinpublic.com.au/media-releases/incus-launch
- 14.Rion Ltd About Rion: History Rion Ltd; 2020. Accessed May 13, 2021 at:https://www.rion.co.jp/english/corporate/history.html [Google Scholar]
- 15.Beckett R C, Blamey P J, Saunders S. IGI Global; 2016. Optimizing hearing aid utilisation using telemedicine tools; pp. 72–85. [Google Scholar]
- 16.Powers T, Rogin C. MarkeTrak 10: Hearing aids in an era of disruption and DTC/OTC devices. The Hearing Review. 2019;26(08):12–20. [Google Scholar]
- 17.Edwards B. Emerging technologies, market segments, and MarkeTrak 10 insights in hearing health technology. Semin Hear. 2020;41(01):37–54. doi: 10.1055/s-0040-1701244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swanepoel D W. eHealth technologies enable more accessible hearing care. Semin Hear. 2020;41(02):133–140. doi: 10.1055/s-0040-1708510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstein B E. Unpacking the OTC Hearing Aid Act: How about the 12% with normal hearing and self-rated hearing difficulties? Hear J. 2021;74(05):22–23. [Google Scholar]
- 20.Ritchie J, Lewis J, McNaughton C, Nicholls C, Ormston R. London, UK: Sage; 2013. Qualitative Research Practice: A Guide for Social Science Students and Researchers. [Google Scholar]
- 21.Liberati A, Altman D G, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters M, Godfrey C, McInerney P, Baldini Soares C, Khalil H, Parker D. The Joanna Briggs Institute; 2017. Scoping Reviews (2020 version) [Google Scholar]
- 23.Grant M J, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J. 2009;26(02):91–108. doi: 10.1111/j.1471-1842.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 24.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(01):19–32. [Google Scholar]
- 25.Pham M T, Rajić A, Greig J D, Sargeant J M, Papadopoulos A, McEwen S A. A scoping review of scoping reviews: advancing the approach and enhancing the consistency. Res Synth Methods. 2014;5(04):371–385. doi: 10.1002/jrsm.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brice S, Almond H. Health professional digital capabilities frameworks: a scoping review. J Multidiscip Healthc. 2020;13:1375–1390. doi: 10.2147/JMDH.S269412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eikelboom R H, Bennett R, Brennan M. Ear Science Institute; 2021. Tele-audiology: an opportunity for expansion of hearing healthcare services in Australia. Review of Telehealth services; p. 87. [Google Scholar]
- 28.Hearing Health Sector Committee (HHSC) Roadmap for Hearing Health 2019. Accessed February 21, 2021 at:https://www1.health.gov.au/internet/main/publishing.nsf/content/CDFD1B86FA5F437CCA2583B7000465DB/$File/Roadmap%20for%20Hearing%20Health.pdf
- 29.American Academy of Audiology (AmAcAud) The audiologists guide to hearing aids, PSAPs, hearables and OTC devices [Professional Guide] American Academy of Audiology; 2018. Accessed November 21, 2020 at:https://mk0audiologyotvgk5ps.kinstacdn.com/wp-content/uploads/2021/05/20180130_AuD_Guide_OTC.pdf [Google Scholar]
- 30.Audiology Australia Audiology Australia Professional Practice Standards - Part B Clinical Standards. Part B Clinical Standards [Clinical Standards]Audiology Australia, Suite 7, 476 Canterbury Road, Forest Hill, VIC 3131. 2013. Accessed October 15, 2020 at:https://audiology.asn.au/Tenant/C0000013/Position%20Papers/Member%20Resources/Clinical%20Standards%20partb%20-%20whole%20document%20July13%201.pdf
- 31.American Academy of Audiology (AmAcAud) American Academy of Audiology Clinical Practice Guidelines: Adult patients with severe-to-profound unilateral sensorineural hearing loss. Clinical Practice Guidelines, American Academy of Audiology [Clinical Practice Guidelines] 2015. Accessed November 21, 2020 at:https://mk0audiologyotvgk5ps.kinstacdn.com/wp-content/uploads/2021/05/PractGuidelineAdultsPatientsWithSNHL.pdf [DOI] [PubMed]
- 32.Audiology Practice Standards Organization (ASPO) “Hearing aid fitting standard for adult & geriatric patients” [Clinical standards] ASPO; 2021. Accessed March 21, 2021 at:https://www.audiologystandards.org/standards/display.php?id=102 [Google Scholar]
- 33.Australian Healthcare Associates (AHA) National Practice Standards for Hearing Care Practitioners [Standards] AHA, Level 6, 140 Bourke St, Melbourne; 2016. Accessed June 23, 2020 at:https://www.ahaconsulting.com.au/wp-content/uploads/2016/05/Hearing-Care-Practitioner-Standards_draft-for-consultation.pdf [Google Scholar]
- 34.Hearing Services Program Service Delivery Framework for hearing services in Australia [Government Document] Department of Health; Hearing Services Program, Australian Government; 2018. Accessed November 21, 2020 at:https://hearingservices.gov.au/wps/wcm/connect/hso/b84bb811-ca26-4943-9b76-41484eb54e25/Service+Delivery+Framework+for+hearing+services+in+Australia+Sept2018.pdf?MOD=AJPERES&CONVERT_TO=url&CACHEID=b84bb811-ca26-4943-9b76-41484eb54e25 [Google Scholar]
- 35.Australian Digital Health Agency (ADHA) Framework for action: How Australia will deliver the benefits of digitally enabled health and care. Australia's National Digital Health Strategy [Report] ADHA; 2018. Accessed March 13, 2021 at:https://www.digitalhealth.gov.au/about-us/national-digital-health-strategy-and-framework-for-action [Google Scholar]
- 36.Hearing Health Technology Matters (HHTM) Lexie Hearing Makes Hearing Aids Available for Sale at Walgreens Stores HHTM; 2021. Accessed May 15, 2021 at:https://hearinghealthmatters.org/hearingnewswatch/2021/lexie-hearing-aids-walgreens-stores/ [Google Scholar]
- 37.Audiology Practice Standards Organization (ASPO) General Audiology Intake Standards [Clinical standards] APSO; 2020. Accessed March 21, 2021 at:https://www.audiologystandards.org/standards/display.php?id=101 [Google Scholar]
- 38.Blamey P. Hershey, PA: IGI Global; 2019. The Expected Benefit of Hearing Aids in Quiet as a Function of Hearing Thresholds; pp. 63–85. [Google Scholar]
- 39.Sabin A T, Van Tasell D J, Rabinowitz B, Dhar S. Validation of a self-fitting method for over-the-counter hearing aids. Trends Hear. 2020;24(24):2.331216519900589E15. doi: 10.1177/2331216519900589. [DOI] [PMC free article] [PubMed] [Google Scholar]


