Abstract
Hydrogels are three-dimensional polymer networks that are hydrophilic and capable of retaining a large amount of water. Hydrogels also can act as vehicles for the controlled delivery of active compounds. Bio-polymers are polymers that are derived from natural sources. Hydrogels prepared from biopolymers are considered non-toxic, biocompatible, biodegradable, and cost-effective. Therefore, bio-polymeric hydrogels are being extensively synthesized and used all over the world. Hydrogels based on biopolymers finds important applications in the agricultural field where they are used as soil conditioning agents as they can increase the water retention ability of soil and can act as a carrier of nutrients and other agrochemicals. Hydrogels are also used for the controlled delivery of fertilizer to plants. In this review, bio-polymeric hydrogels based on starch, chitosan, guar gum, gelatin, lignin, and alginate polymer have been discussed in terms of their synthesis method, swelling behavior, and possible agricultural application. The urgency to address water scarcity and the need for sustainable water management in agriculture necessitate the exploration and implementation of innovative solutions. By understanding the synthesis techniques and factors influencing the swelling behavior of these hydrogels, we can unlock their full potential in fostering sustainable agriculture and mitigating the challenges posed by an ever-changing environment.
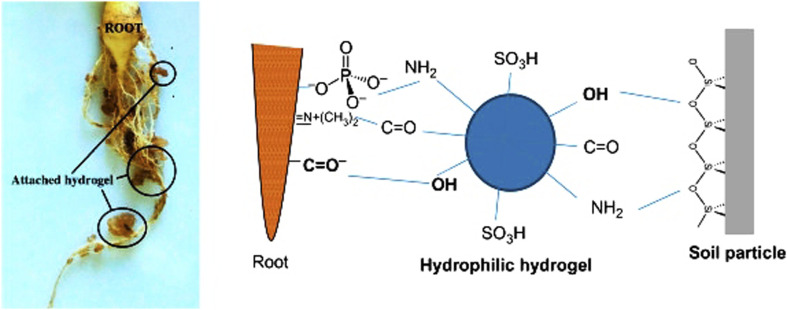
Hydrogels are three-dimensional polymer networks that are hydrophilic and capable of retaining a large amount of water.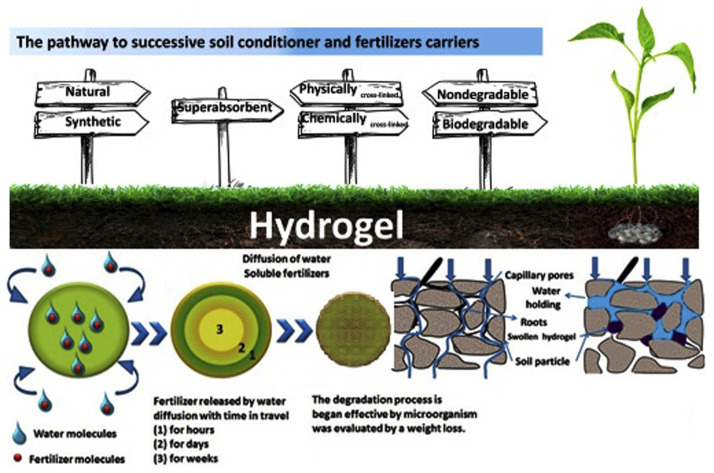
1. Introduction
1.1. Agriculture issues and hydrogels
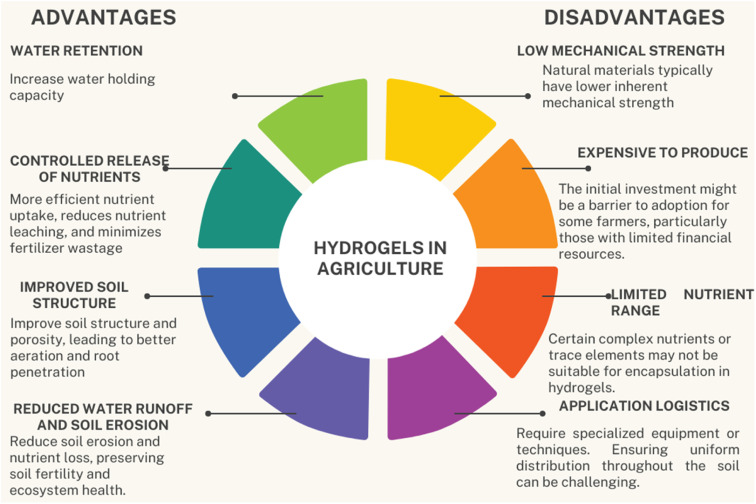
Desertification is a process of land degradation due to natural causes or human activities which decrease the productivity of vegetative plants. Many regions of the world are facing the problem of desertification. Climate change in the past decades has resulted in temperature variation, low rainfall, and an increased rate of drought, which have reduced crop yield. In addition, a large amount of water is lost via evaporation and runoff, which reduces the uptake of water by plants. A large amount of fresh water is used for agricultural purposes. Thereby agriculture is the most drought-sensitive area of the world's economy. The world's population is increasing continuously, and the demand for food and other necessities is also increasing. So, there is a serious threat of food shortage in the near future due to reduced crop production.1 According to an estimation, by 2050, about 6 billion people around the world could be affected by water shortage. Hydrological drought has posed serious impacts on crop yield, which have compelled researchers to discover fresh approaches in this regard.2 Various technologies have been invented to tackle hydrological drought, such as drip irrigation systems and low-pressure micro-sprinklers. But these technologies are mostly applied for high-value crops and are not economical. Moreover, these techniques also require a high operational budget and good expertise of cultivators. The gels (hydrogels) which could retain a large amount of water have become a popular area of interest for researchers.3 Hydrogels increase the soil's ability to hold water while lowering evaporation, leaching process, and runoff. They also increase plants' uptake of water and its availability, mainly during drought conditions, by enhancing aeration, porosity, soil structure, and biological activity of soil. With the use of hydrogels, there is a reduction in soil erosion and compaction. Hydrogels improve crop growth and also increase the yield and quality of the crops. They are cost-effective as they conserve water energy and labor costs. Overuse of hydrogels can cause a reduction of oxygen supply to plants' roots and can also lead to waterlogging. Moreover, biopolymeric hydrogels have low mechanical strength and are less sustainable.3,4 Hydrogels have many advantages in agricultural practices but also have a few disadvantages, which are summarized below (Fig. 1).
Fig. 1. Advantages and disadvantages of biopolymeric hydrogels in agriculture.
The urgency for conducting this review arises from the growing imperative to improve water utilization efficiency within the agricultural sector. Conventional irrigation methods frequently result in the inefficient utilization of water resources, the occurrence of runoff, and the deterioration of soil quality, hence exacerbating environmental issues. This study seeks to offer valuable insights to academics, policymakers, and practitioners in the agricultural sector by conducting a comprehensive assessment of synthesis methods and examining the factors that impact the degree of swelling in hydrogels based on biopolymers.
This review aims to provide insight into the various applications of hydrogels derived from biopolymers in the field of agriculture. Hydrogels possess the capacity to significantly transform agricultural methodologies, ranging from the regulation of soil moisture levels to the controlled discharge of nutrients and agrochemicals. The hydrogels possess characteristics that are in line with the worldwide endeavors to establish a more environmentally friendly and sustainable agriculture industry. Insufficient research has been conducted to thoroughly examine the complex interactions between biopolymer-based hydrogels and various plant species. The investigation of the impact of hydrogels on plant development, nutrient absorption, and total crop productivity under different environmental circumstances is a significant area of research that requires further exploration. Although a wide range of hydrogel formulations have been produced, there is still no consensus in the research community about the optimal biopolymer type, concentration, and application method for various agricultural practices and crop kinds. The comprehensive understanding of the long-term effects of employing biopolymer-based hydrogels on soil characteristics and the broader ecosystem remains incomplete. Conducting research that investigates the potential impacts on soil microbiology, soil structure, and ecological equilibrium is of utmost importance. The majority of studies pertaining to hydrogels derived from biopolymers are typically carried out at the laboratory level. It is imperative to undertake an examination of the obstacles and potential adjustments necessary for the proper implementation of large-scale operations in the field. This examination should encompass considerations of cost-effectiveness and logistical aspects.
1.2. Nature of hydrogels and their method of preparation
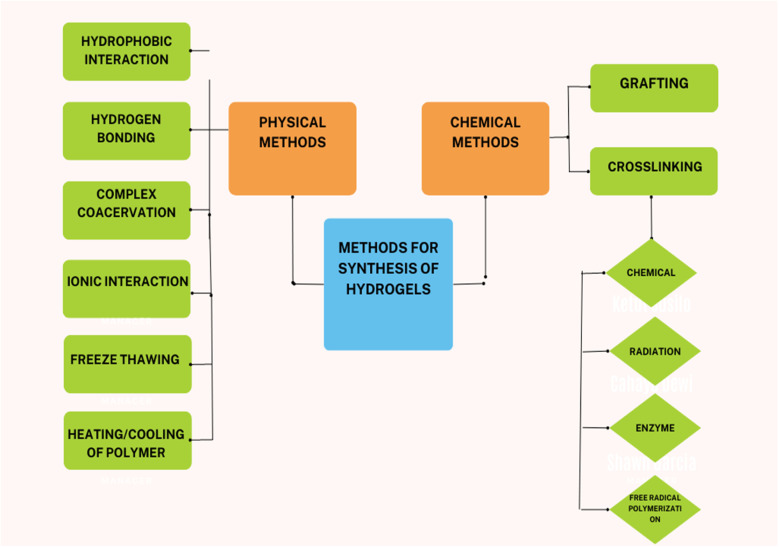
Hydrogels are a 3-dimensional polymer network that is hydrophilic and capable of retaining a large amount of water. The hydrogels, when placed in water, swell up due to the uptake of water without dissolving in it. The polymeric chains in the hydrogel are cross-linked via chemical bonds or any other physical interaction, such as van der Waals interaction or hydrogen bonding (Fig. 2). To formulate the hydrogel; various chemical methods are used for polymerization. These include graft polymerization and cross-linking polymerization (chemical cross-linking, radiation cross-linking, free radical polymerization, and enzyme cross-linking).5 Hydrogels are sensitive to environmental stimuli such as pH, concentration of material, and temperature, exhibit good transport ability, and allow controlled release of materials. Moreover, hydrogels are biocompatible and can be easily modified. Hydrogels exhibit a wide range of applications in agriculture, food, biomedical field, cosmetics, engineering, and construction. In agriculture, hydrogels have their application as soil conditioners and nutrient reservoirs. It means hydrogels can increase the water-holding capacity of the soil and can also act as a material that can encapsulate agrochemicals for their controlled release.6
Fig. 2. Methods for the preparation of hydrogels.
1.3. Classification of hydrogels
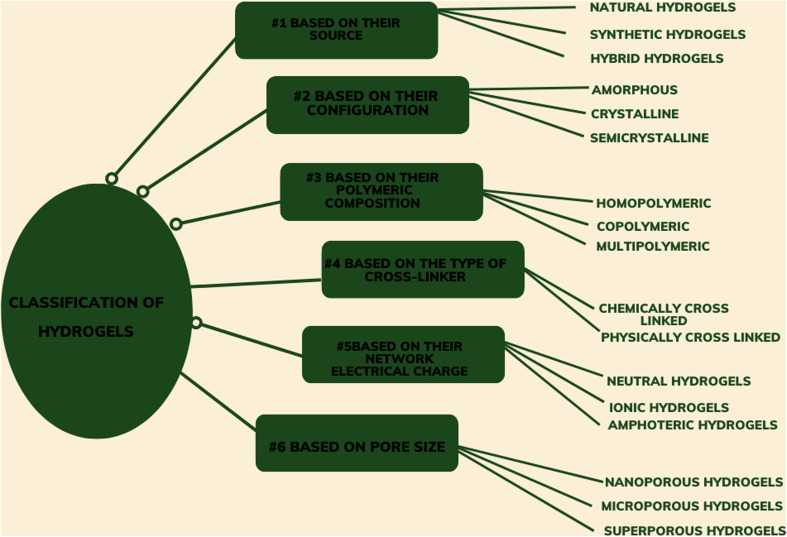
Based on their structure, hydrogels can be amorphous, crystalline, or semi-crystalline. Hydrogels can be made by cross-linking one type of polymer (homo-polymeric hydrogel) or two different types of polymer (co-polymeric hydrogel). Some hydrogels contain more than two types of polymer chains cross-linked together; these are called multi-polymeric hydrogels. Based on their source, the hydrogel can be classified as natural, synthetic, or hybrid. Natural hydrogels are formed by the cross-linking of natural polymers or biopolymers, whereas synthetic hydrogels contain synthetic polymer chains cross-linked to each other. Hybrid hydrogels contain both natural and synthetic polymeric chains cross-linked with one another.7 The details of the classifications of hydrogels are summarized in Fig. 3.
Fig. 3. Classification of hydrogels.
1.4. Biopolymer based hydrogels
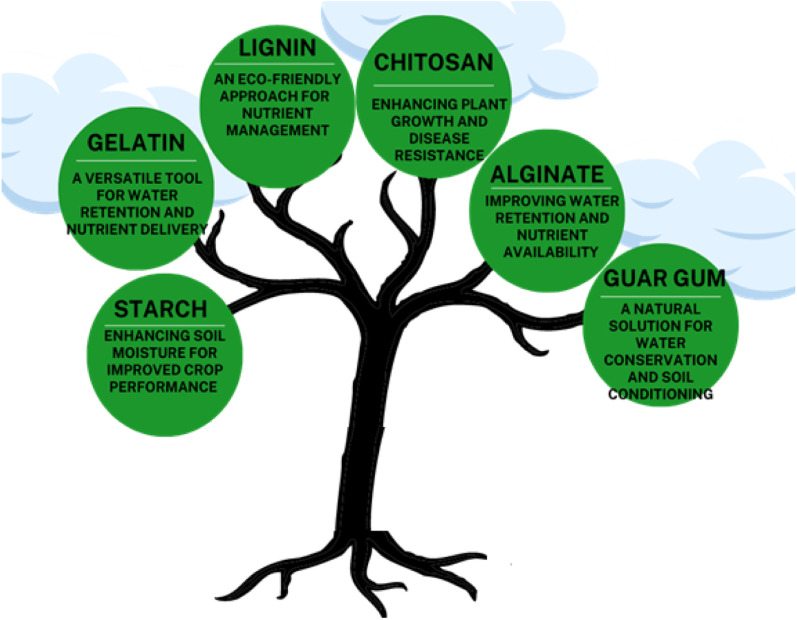
Biopolymers, also called natural polymers, are the type of polymers that can be derived from natural sources. They can be obtained from green plants, fungi, animals, bacteria, and other microorganisms. Bio-polymers are excellent candidates for the formulation of hydrogels. Bio-polymeric hydrogels have several advantages over synthetic hydrogels as they offer good biodegradability and biocompatibility. Bio-polymeric hydrogels are more eco-friendly as compared to synthetic hydrogels. In addition, the use of natural polymers for hydrogel formulation provides a way to utilize our natural resources effectively. Natural hydrogels are a potential tool for agricultural applications as they can retain a large amount of water and allow the controlled release of fertilizers and other agrochemicals. In recent year, the use of natural hydrogels have increased, and researchers are working to find new approaches in this regard, especially to increase their mechanical strength and reduce their production cost.1 In this review, a brief study on bio-polymeric hydrogel regarding their synthesis, water absorption, swelling behavior, and their applications in the agricultural field is discussed. Natural hydrogels based on starch, chitosan, guar gum, alginate, lignin, and gelatin (Fig. 4) have been discussed.
Fig. 4. Common bio-polymeric hydrogels.
Starch, chitosan, guar gum, alginate, gelatin, and lignin are some of the examples of biopolymers8 are presented in Table 1.
Various bio-polymers and their sources.
| Sr. no. | Bio-polymers | Source |
|---|---|---|
| 1 | Starch | Cereals, grains, and potatoes |
| 2 | Chitosan | Shells of crustacean |
| 3 | Guar gum | Guar seeds (Cyamopsis tetragonoloba plant seeds) |
| 4 | Alginate | Brown algae |
| 5 | Gelatin | Hydrolysis of collagen (derived from the connective tissues of animals) |
| 6 | Lignin | Support tissues of most plants |
2. Starch based hydrogels
Starch belongs to an important class of bio-polymers called carbohydrates. It is a polysaccharide in which glucose molecules are joined together by α-1,4 and α-1,6 glycosidic linkage. Native starch is a combination of two polymers, amylose, and amylopectin polymer. Amylose is the unbranched or linear polymeric form of starch, whereas amylopectin is a branched or non-linear form of starch. Potatoes, grains, and cereals are the main source of starch.9 This natural polymer is of special interest to the scientist because it is biodegradable and non-toxic. In addition, starch is an abundant and inexpensive polymer. All these properties make starch a potential candidate for the formulation of the hydrogel. Starch hydrogels are formed by itself cross-linking or with other polymers. Starch-based polymeric hydrogels exhibit good water-holding capacity and biodegradability, which make them a potential candidate for agricultural use.10 Various methods for the formulation of starch-based superabsorbent hydrogels have been reported. Starch-based hydrogel has been prepared using native cassava starch and polyacrylonitrile (PAN) by alkaline hydrolysis. Sodium hydroxide hydrolyzes the starch and PAN to form polyacrylonitrile, which is a superabsorbent hydrogel (SAH). The effect of this hydrogel on Zea may (maize seedling) has been evaluated. It has been observed that starch–PAN hydrogel not only increases the water absorption capacity of the soil but also has a positive effect on plant growth (Table 2).11 Another important method for the preparation of pH-sensitive starch-polyacrylate hydrogel using graft polymerization is the grafting of 2-hydroxy ethyl methacrylate and acrylic acid on the starch backbone. The reaction is carried out using N,N′-methylene bisacrylamide (cross-linker), and ammonium persulfate (initiator). This particular hydrogel shows a pH-sensitive reversible response at a pH of 2 and 8. Due to the pH sensitivity, it can be used for the controlled delivery of bioactive material and agrochemicals.12 SAH has been prepared by gamma irradiating the mixture of starch and carboxymethyl cellulose (CMC). SAH showed good water absorption even in high electrolyte concentrations. Best absorption was shown when starch was taken 30% to CMC (Table 2). The hydrogel formulated by cross-linking CMC and starch showed less sensitivity to ionic concentration in water than CMC hydrogel.13 Starch-based hydrogels have also been formulated by chemical cross-linking of waxy corn starch and potato starch individually using succinic anhydride (cross-linker). These hydrogels possess good water absorption capacity both in distilled water and saline solution. The effect of these hydrogels on the growth of corn plants has been investigated. These hydrogels (Table 2) have been shown to have a positive effect on the growth of corn plants, especially in the early stage of their growth.14 Gamma irradiation has also been used to synthesize nano-hydrogel based on starch. In this method, starch nanoparticles are prepared by acid hydrolysis followed by ethanol precipitation. After that, nano-hydrogel is prepared by gamma irradiating the starch nano-particle. Starch nano-hydrogel prepared by gamma irradiation has less swelling ability than those nano-hydrogel prepared by other methods. However, gamma-irradiated nano-hydrogel has a more stable structure than non-irradiated nano-hydrogel.15 Starch-based semi-interpenetrating superabsorbent hydrogel has also been formulated by the graft polymerization technique. Composite hydrogels were prepared using starch (St) grafted polyvinyl alcohol (PVA) and poly(acrylic acid-co-acrylamide) (AA-co-AM) hydrogel with nano-cellulose (NC). These composite hydrogels exhibit much more swelling ability than hydrogel itself. This composite also showed pH-sensitive swelling behavior and good swelling ability in saline medium, which make it an excellent candidate for agricultural use.16 Starch hydrogels are also prepared by physically blending the amylose and amylopectin polymer and grafting them with acrylamide (Fig. 5).
Details of starch-based hydrogels.
| Polymer | Synthesis method and cross-linker used | Swelling degree (SD)/water absorption | Agricultural application | Reference |
|---|---|---|---|---|
| Native cassava starch & polyacrylonitrile | Alkaline hydrolysis (macro-alkoxide initiated cross-linking) | 682 g g−1 in distilled water (maximum SD) | Water absorption facilitates plant growth | 11 |
| Starch grafter with acrylic acid and 2-hydroxy ethyl methacrylate | Graft polymerization | 95 g g−1 at pH = 8 (maximum SD) | Controlled delivery materials (agrochemicals) | 12 |
| Starch and carboxymethyl cellulose (CMC) | Radiation-induced polymerization (gamma irradiation) | 100–350 g g−1 in distilled water | Enhance the water absorption capacity of soil | 13 |
| Potato starch (PS), waxy corn starch (WCS) | Chemical cross-linking (succinic anhydride-cross linker) | PS-260 g g−1 WCS-199 g g−1 (in distilled water) | Improves the water absorption capacity of the soil, improves the early growth of the plant | 14 |
| PS- 18 g g−1, WCS-38 g g−1 (in saline solution-0.9% NaCl) | ||||
| Starch nanoparticles | Radiation-induced polymerization | 256–365% in distilled water | Increase water holding ability of soil | 15 |
| Gamma irradiation | ||||
| Starch, poly(acrylic acid-co-acrylamide) and polyvinyl alcohol | Graft polymerization | 772–922 g g−1 in distilled water | 16 | |
| Amylose & amylopectin (starch)/acrylamide | Graft polymerization (grafting by ultrasonic heating) | 1500–4500% in distilled water | Superabsorbent hydrogel for water retention in soil | 17 |
| Starch | Chemical cross-linking (itaconic acid-cross linker) | 650 g g−1 in distilled water (maximum SD) | Increase the water retention ability of soil | 18 |
| Starch and N,N-dimethylacrylamide | Redox polymerization (N,N-methylene-bis-acrylamide-cross linker) | 1115–2629% in distilled water | Water absorption, controlled release of urea/fertilizer | 19 |
| Starch (composite with zeolite NPs) | Chemical cross-linking (N,N-methylene-bis-acrylamide-cross linker) | 900–1150 g g−1 in distilled water | Water retention, controlled release of zinc ion in soil | 20 |
| Starch and chitosan | Glutaraldehyde, glyoxal | — | Controlled release of herbicides | 21 |
| Starch, 2-acrylamido-2-methylpropansulfonic acid, and acrylamide | Radical initiated polymerization | 158.3 g g−1 in tap water | Enhance water absorption capacity, promote plant growth | 22 |
| 50 g g−1 in saline water | ||||
| 141.2 g g−1 in urea solution |
Fig. 5. Schematic presentation of amylose/amylopectin synergistic regulation of gel microstructure, reproduced from ref. 17, under the terms of the Creative Commons CC BY license, 2021.
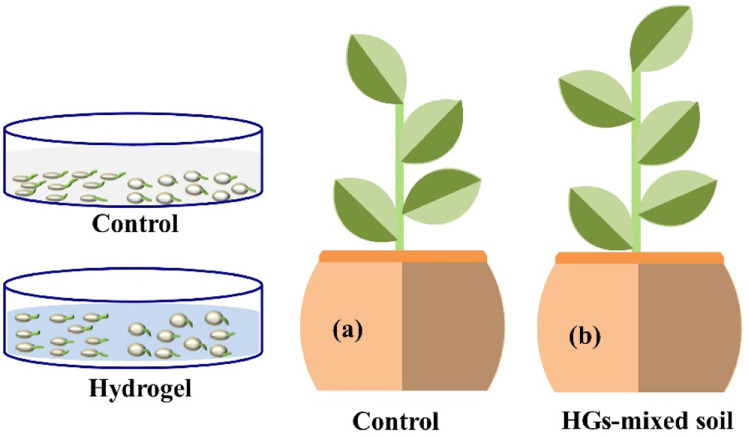
Results obtained by the analysis of these hydrogels (Table 2) indicated that an increase in the proportion of amylopectin results in the enhancement of the grafting ratio. The best absorption of water occurs when amylopectin is taken 70% proportion to amylose.17 A starch-based biodegradable superabsorbent hydrogel can be synthesized by chemically cross-linking the starch with itaconic acid. The prepared hydrogel exhibits a porous structure which led to its great water retention capability. Swelling kinetics of this hydrogel indicated that as the amount of cross-linker (itaconic acid) is increased, water absorption by the hydrogel occurs at a fast rate. In addition, starch–itaconic acid superabsorbent hydrogel shows very good degradability.18 Another environmentally friendly, starch-based hydrogels have been prepared by redox polymerization of starch and N,N-dimethylacrylamide method for controlled release of urea. Cross-linker used for this method is N,N-methylene-bis-acrylamide (MBA). These hydrogels showed great water absorption ability and have expressed different urea release behavior at different pH values (Table 2).19 Starch and zeolite-nanoparticle-based hydrogel can be prepared using an alkaline hydrolysis technique followed by a chemical cross-linking reaction, using MBA as a cross-linker and ammonium persulfate as an initiator. It has been observed that the amount of zeolite nano-particles in the hydrogel influences its water absorption capacity. Maximum water absorption by the hydrogel takes place when 10% of zeolite nano-particles are used. The release of Zn ion from starch–zeolite nano-composite hydrogel has also been investigated. It has been observed that the release of zinc ions from the composites increases as the amount of zeolite in composite hydrogel increases.20 Starch–chitosan hydrogels have potential applications in the agriculture field as a control release material for herbicides. These hydrogels have been synthesized by chemical cross-linking method, using glutaraldehyde and glyoxal as cross-linking agents.21 Another biodegradable and cost-effective hydrogel has been synthesized using starch (from rice-cooked wastewater), 2-acrylamido-2-methylpropansulfonic acid, and acrylamide. This hydrogel was found to have good swelling ability in water, urea, and saline solutions. In addition, this hydrogel proved to promote the growth of plant,22 as depicted in Fig. 6.
Fig. 6. Illustration of the plant (Capsicum frutescens) growth in both (a) control and (b) hydrogel (HGs)-mediated soils for 30 days, reproduced with permission from ref. 22 copyright, Elsevier, 2022.
It could be concluded that starch-based hydrogel could be used as a potential candidate for agricultural application. The synthetic method of the starch-based hydrogels, their swelling behavior, and their potential applications in the agricultural field are given below (Table 2).
3. Chitosan based hydrogels
Chitosan (CS) is a biopolymer obtained from chitin which is a polysaccharide. CS is a copolymer of N-acetyl-d-glucosamine and d-glucosamine, which are joined to each other by β-1,4 glycosidic linkage. CS is derived naturally from crustaceans like prawns, crabs, and shrimp shells. CS based hydrogels find diverse applications in the field of agriculture. CS derivatives exhibit antimicrobial properties in plants. CS formulations can increase plant growth and allow controlled release of agrochemicals.23 Many methods have been proposed regarding the formulation of CS based hydrogels. CS coated hydrogel beads have been prepared using NPK fertilizers as inner core material. The NPK core was coated with CS as the inner coating, whereas the outer coating of beads was done by using poly(acrylic acid-co-acrylamide). These hydrogel beads not only possess good water retention ability and biodegradability but also have the potential for controlled release of fertilizer.24 CS microspheres were synthesized by encapsulating urea, and these microspheres have shown good water uptake and controlled release of urea. These microspheres were fabricated in two steps. First, encapsulation of urea in CS was done by emulsification technique; then, chemical cross-linking was carried out using genipin.25 CS based hydrogel has also been synthesized by using the chemical cross-linking method, and the release of agrochemical-dicyandiamide from the hydrogel was investigated. It was observed that the release of dicyandiamide occurred by quasi-Fickian diffusion. The results of this experiment indicated that these hydrogels have the potential ability for the slow release of agrochemicals (dicyandiamide).26 CS based hydrogel coated fertilizers have been formulated by immersing the fertilizer granules in CS and poly(vinyl alcohol) (PVA) solution and cross-linking them with glutaraldehyde. Then hydrogels were prepared by immersing these CS/PVA fertilizer hydrogel in acrylic acid and acrylamide solution by inverse polymerization method. For this purpose, MBA was used as a cross-linker. Fertilizer release from this hydrogel was evaluated. It was observed that the release of fertilizer follows both Ritger–Peppas and Korsmeyer–Peppas models, and release occurs by pseudo-Fickian diffusion.27 A superabsorbent hydrogel has been prepared by crosslinking CS with EDTA and urea. This hydrogel could have diverse applications in agriculture as it can not only absorb large amounts of water but also allows the controlled release of urea and metal ion resent in the soil as these metal ions can bind with EDTA. Moreover, this hydrogel is biodegradable due to the presence of CS, which is a bio-polymer.28 Another CS based superabsorbent hydrogel was synthesized by cross-linking of CS with high acetyl gellan gum (HAGG) via ionic bond formation. It was observed that water sorption by the hydrogel decreases by decreasing the HAGG. The best hydrogel was obtained when CS and HAGG were used in a ratio of 1 : 4. Fertilizer release from this particular hydrogel was also investigated. Results indicated that fertilizer release occurs in a period of 8 h, and the period of release depends on the quantity of fertilizer used.29 Silk fibroin (SF), CS, and gelatin based hydrogel have been synthesized for the slow and controlled release of urea. This type of hydrogel was formulated by blending CS with gelatin and silk fibroin (CS/gelatin/SF). Urea release from the formulated hydrogel has also been observed. CS present in the hydrogel was found to influence the release of urea from the hydrogel. There is a slow release of urea in CS/Gelatin/SF hydrogel than in SF/gelatin hydrogel.30 Graft polymerization technique has been used for the formulation of CS hydrogel with cellulose and acrylic acid (Fig. 7). First, a CS–cellulose hybrid was prepared then grafting of acrylic acid on the prepared hybrid was carried out. The formulated hydrogel was found to possess good mechanical strength and water absorption capacity in distilled water. Although, in saline solution, there is much less water absorption by the hydrogel. Results of the experiment indicated that these hydrogels could prove to be an effective tool for agricultural application due to their excellent water holding capacity and controlled fertilizer release ability.31
Fig. 7. Illustration of the steps involved in the coupling of cellulose and chitosan into the crosslinked backbone and successive graft polymerization of acrylic acid from this backbone, reproduced with permission from ref. 31 copyright, Elsevier, 2016.
Another method for the formulation of CS-based hydrogel was proposed by the researchers. CS and starch were polymerized to each other using ionotropic gelation and neutralization techniques, and fertilizer release from the formulated hydrogel was observed. Fertilizer release by this hydrogel was 70–93% after 14 days of loading. These hydrogels are expected to be a good material for the controlled release of fertilizer and other agrochemicals.32 Superabsorbent hydrogels based on polyacrylamide (PAAm) and Na-alginate (Alg) or CS by γ-rays induced polymerization have also been proposed. This hydrogel was found to have great water absorption capacity. The gel content and water absorbency follow the order PAAm/Alg > PAAm/Alg/CS > PAAm/CS. The impact of this hydrogel on the growth of maize was determined. Results of this experiment showed that formulated hydrogel could increase the growth of maize plants to 1.5 times the untreated soil (Fig. 8). The response of plants towards different hydrogels used followed the order PAAm/Alg > PAAm/Alg/CS > PAAm/CS. The superabsorbent hydrogel could be used as a soil conditioning material.33
Fig. 8. Effect of swollen hydrogels on the growth of maize plants (B) growth of maize cob yield size. (a) Untreated soil (control), (b) soil treated with PAAm/Alg, (c) soil treated with PAAm/CS, and (d) soil treated with PAAm/Alg/CS, reproduced with permission from ref. 33, copyright, Elsevier, 2017.
CS based superabsorbent hydrogel has also been synthesized by grafting CS with poly(acrylic acid-co-acrylamide) via the free radical graft co-polymerization method and then making its composite with ground basalt. For this purpose, MBA was used as a cross-linker. Hydrogel composite prepared by this method was found to exhibit good mechanical strength and water absorption capacity. These composite hydrogels were found to be more stable than the hydrogel (CS/poly(acrylic acid-co-acrylamide)). The impact of this hydrogel composite on the growth of eggplant was examined, which showed its positive impact on the growth of plants. It could be concluded that this hydrogel composite is an effective soil conditioning material.34 CS and PVA hydrogel formulated by freeze–thawing procedure proved to have good mechanical properties and water holding capacity. This hydrogel could have a positive effect on the germination of okra plants and allow the controlled release of fertilizer.35 CS based hydrogels have been prepared by mild oxidation and grafting technique. Investigation of swelling degree and water retention by both hydrogels showed that grafted hydrogel has more swelling ability than oxidized CS hydrogel. Moreover, urea release from these formulated hydrogels has also been estimated. These chitosan-based hydrogel can have potential applications in agricultural fields due to their water retention ability and ability to deliver fertilizer in a controlled way to the plants.36 CS and starch hydrogel macro-beads were developed by the dripping technique, using sodium tripolyphosphate as a cross-linker found to have their application in the delivery of growth-promoting bacteria for plants. Formulation of these macro-beads involved ionotropic gelation and neutralization process. These macro-beads have the potential to be used as bio-fertilizer.37 Superabsorbent hydrogel based on CS has been synthesized, and their water holding capacity was evaluated, which was found to be 1250 g g−1. The hydrogel was prepared by using the chemical cross-linking method, in which CS and urea were cross-linked together using citric acid as a cross-linker.38 A soil conditioning material (hydrogel) was formulated by cross-linking CS with salicylaldehyde (cross-linker). Urea fertilizer was also added to the reaction mixture to make its blended hydrogel. Formulated hydrogel proved to be very beneficial for plant growth as it could increase plant growth by 70% and can retain large amounts of water in soil.39 Hydrogel based on CS, PVA, and gelatin has been prepared and loaded with inulin to protect the chili plant from Phytophthora capsici. It was observed that this bio-degradable hydrogel has good water absorption capacity and could be used successfully for inulin release to protect chili plants from Phytophthora capsici, which is a plant pathogen.40 CS and starch hydrogel films have been prepared using citric acid and glycerol. For this purpose, titanium dioxide (TiO2) solution with calcium carbonate (CaCO3) was added to the reaction mixture. This hydrogel mulching is bio-degradable and can be used to promote the growth of invasive (short-cycled) plants. These films could also be used as an effective material to promote fertilizer-free plant growth.41 CS hydrogel beads have also been synthesized by blending them with starch via the ionotropic gelation method. These beads were found to have the ability for the controlled delivery of fertilizer. Fertilizer-loaded CS/starch hydrogel beads allow more than 70% of fertilizer release within 16 days.42 CS-co-polyacrylamide–TiO2 hydrogel developed by chemical crosslinking method using glutaraldehyde as a cross-linker can be effectively used as a soil conditioning material. The effect of this hydrogel on the growth of the soya bean plant was observed. Results of the experiment indicated that this hydrogel has positive effects on the growth of plant.43 Eco-friendly hydrogel based on CS and starch has been synthesized by cross-linking starch and CS with citric acid and has been found to exhibit good water holding capacity. It was observed that hydrogel with desirable characteristics could be obtained when starch and CS are used in equal proportion.44 The swelling properties of CS-based hydrogel, along with their synthetic method and agricultural applications, have been discussed below (Table 3).
Details of chitosan-based hydrogels.
| Polymer | Synthesis method & cross-linker used | Swelling degree (SD)/water absorption | Agricultural application | Reference |
|---|---|---|---|---|
| Chitosan, poly(acrylic acid-co-acrylamide) | Spray coating followed by chemical cross-linking (N,N-methylene-bis-acrylamide-cross linker) | 40–70 g g−1 in distilled water | Enhance water retention, controlled release of fertilizer | 24 |
| Chitosan | Chemical cross-linking (genipin-cross linker) | 112–145% in distilled water | Increased water uptake by the plant, controlled release of urea | 25 |
| Chitosan | Chemical cross-linking (glyoxal-cross linker) | — | Slow release of agrochemicals (dicyandiamide/DCD) | 26 |
| Chitosan, polyvinyl alcohol, acrylamide, and acrylic acid | Reverse suspension polymerization (chemical cross-linking) (glutaraldehyde and N,N-methylene-bis-acrylamide-cross linker) | 120–230 g g−1 in deionized water | Enhance water absorption capacity of the soil, controlled fertilizer release | 27 |
| Chitosan | Chemical cross-linking (EDTA–urea-cross linker) | 238–570 g g−1 in distilled water | Water absorption, controlled release of urea and metal ions | 28 |
| Chitosan and high acetyl gellan gum (HAGG) | Cross-linking via ionic bond formation | 219 g g−1 in pure water | Water retention, controlled release of fertilizer | 29 |
| Chitosan, silk fibroin and gelatin | Blended hydrogel (no intermolecular interaction) | 5.32 parts per hundred of polymer | Slow release of urea | 30 |
| Chitosan–cellulose and acrylic acid | Graft polymerization | 390 g g−1 in distilled water and 39.5 g g−1 in saline solution (0.9% NaCl) | Increase water absorption capacity of soil | 31 |
| Controlled release of fertilizer | ||||
| Chitosan and starch | Inotropic gelation technique | — | Controlled release of agrochemicals | 32 |
| Chitosan, sodium alginate, and polyacrylamide | Radiation-induced polymerization (gamma-radiations) | 397 g g−1 in distilled water (maximum absorption) | Water retention, soil conditioning, increase plant growth | 33 |
| Chitosan, poly(acrylic acid-co-acrylamide (ground basalt for making composite) | Graft free radical co-polymerization | ∼600 g g−1 at pH = 3 | Soil conditioning enhances plant growth and pH-sensitive water absorption | 34 |
| Chitosan and polyvinyl alcohol | Freeze thawing method | 58.3% in distilled water (with 6 wt% chitosan) | Water retention, increased plant germination, and controlled release of fertilizer | 35 |
| Chitosan | Graft polymerization and mild oxidation | ∼2500% in distilled water | Water regulation, controlled urea release | 36 |
| Chitosan and starch | Ionotropic gelation and neutralization | 143–235% in distilled water | Water retention, bio-fertilizer | 37 |
| Chitosan | Chemical cross-linking (citric acid-cross linker) | 1250 g g−1 in distilled water | Water absorption | 38 |
| Chitosan | Chemical cross-linking salicylaldehyde-cross linker) | 154% in distilled water | Water absorption, controlled fertilizer release, enhanced plant growth | 39 |
| Chitosan, gelatin and PVA | Chemical cross-linking | 10–12 times than its mass | Controlled delivery of agrochemical (inulin), water absorption | 40 |
| Chitosan ad starch | Chemical cross-linking (glycerol & citric acid-cross linker) | — | Biodegradable mulch for invasive plants increases plant growth | 41 |
| Chitosan and starch | Ionotropic gelation | 120–162% in distilled water | Controlled release of fertilizer, water absorption | 42 |
| Chitosan-co-polyacrylamide–TiO2 | Chemical cross-linking (glutaraldehyde-cross linker) | 364.76% without TiO2 maximum swelling | Soil conditioner, plant growth promoter | 43 |
| 66.45–100% in distilled water | ||||
| Chitosan and starch | Chemical cross-linking (citric acid-cross linker) | ∼120% (almost the same in distilled water, glucose solution, and urea) | Increase water absorption capacity of soil | 44 |
4. Guar gum based hydrogels
Guar gum is a biopolymer with many desirable characteristics. It is a biodegradable, non-toxic polymer derived from guar seeds of the Cyamopsis tetragonoloba plant. Guar gum consists of galactose and mannose monomer units, so it is also called galactomannan. Chemically, the backbone of guar gum polymer is made up of d-mannose, which are linked to each other via β1→4 linkage, whereas d-galactose molecules are attached to every second unit of mannose by α1→6 (on the backbone).45 Guar gum can develop hydrogel bonding with water molecules. Guar gum-based hydrogel synthesized in recent years has many applications in the biomedical field, agricultural field, and food industry.46 Various methods for the synthesis of guar gum based agricultural hydrogel have been proposed by the researchers.
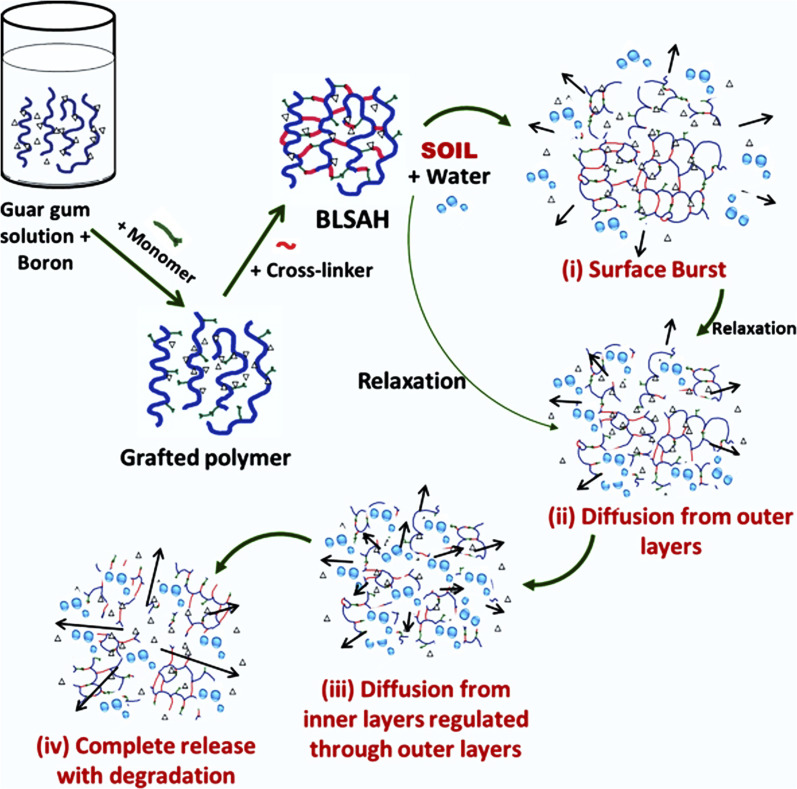
Guar gum based superabsorbent hydrogel has been synthesized by using graft co-polymerization technique. For this purpose, partially neutralized acrylic acid (NaAA) was grafted on guar gum using MBA as a cross-linker. Formulated hydrogels not only exhibit good water retention ability but are also very stable and eco-friendly.47 Another important hydrogel was prepared by using guar gum and acrylic acid using the graft co-polymerization method. In this experiment, hexamine was utilized as cross-linking agent. The hydrogel was pH sensitive and exhibited great swelling capacity and water absorption ability. Maximum absorption occurs at neutral pH. This hydrogel could be used as an excellent material for agricultural applications, especially in arid areas, for water retention.48 Guar gum based hydrogel formulated by grafting acrylamide on guar gum backbone could be used as a soil conditioning material. This hydrogel could enhance the water absorption capacity of soil for better growth of plants and have different water absorption in different mediums. However, the swelling ability of the hydrogel was found to be influenced by the amount of water, cross-linker concentration, and monomer-backbone ratio.49 Researchers have synthesized a guar gum based hydrogel composite by graft polymerization of guar gum with methyl methacrylate using boric acid as a cross-linker. Effect of pH and conc. of monomer, cross-linker, and guar gum on the water absorption capacity of the prepared hydrogel was instigated. The developed hydrogel was found to have very good water absorption ability and could be an effective tool for water retention in soil that face water deficiency.50 Another hydrogel was developed by grafting acrylic acid on guar gum using ethylene glycol di-methacrylic acid as a cross-linker (GG/AA/EGDMA). This hydrogel was found to have good porosity and water holding capacity. Moreover, this hydrogel is biodegradable, having a half-life of about 77 days. This hydrogel appeared to be a promising material for soil conditioning.51 Microwave radiations have also been used to synthesize guar gum based hydrogel by grafting guar gum with acrylic acid using a free-radical emulsifier. This technique is called free-emulsion graft polymerization. The hydrogel is thermally stable and biodegradable. The water absorption capacity of this hydrogel was quite good, and maximum swelling was observed at a pH of 5.0–7.4.52 Boron-loaded guar gum hydrogel has been formulated and investigated by the researchers for the controlled delivery of nutrients, particularly boron (Fig. 9). First, boron was loaded into guar gum, and then hydrogel was synthesized using ethylene-glycol-di-methacrylate (cross-linker) and benzoyl peroxide (initiator). It was observed that about 60% of the boron is released in 5 days.53
Fig. 9. Illustration of synthesis of boron-loaded guar gum hydrogel and boron release mechanism, reproduced with permission from ref. 53 copyright, John Wiley and Sons, 2022.
A hydrogel synthesized by grafting methyl methacrylate on guar gum to evaluate its impact on the growth of sugar cane crops. It was found that this hydrogel can retain a large amount of water and hence can be used to increase sugar cane yield, especially under water stress conditions.54 An eco-friendly hydrogel composite based on guar gum has been formulated by using gamma irradiations. For this purpose, guar gum, polyacrylamide, pectin, and ZnO were cross-linked via gamma irradiation. The composite was observed to have good biodegradability and water holding capacity. Results indicated that the water absorption capacity of the prepared composite increased by increasing the concentration of ZnO. This composite hydrogel could be used as an effective soil conditioning material in the agriculture sector.55Table 4 shows the synthetic method, water holding capacity, and potential applications of guar gum-based hydrogels used in agriculture.
Details of guar gum-based hydrogels.
| Polymers | Synthesis method & cross-linker used | Swelling degree (SD)/water absorption | Agricultural application | Reference |
|---|---|---|---|---|
| Guar gum, partially neutralized acrylic acid | Graft co-polymerization (N,N′-methylenebisacrylamide-cross linker) | 1107 g g−1 in distilled water | Water retention, pH stability (pH = 4–11) | 47 |
| 88 g g−1 in saline solution (0.9 wt%) | ||||
| Guar gum, acrylic acid | Graft co-polymerization (hexamine-cross linker) | 5307% (pH ∼7) maximum swelling | Water absorption, pH sensitive behavior | 48 |
| Guar gum, acrylamide | Graft polymerization | ∼600 g g−1 in distilled water (maximum absorption) | Enhance the water absorption capacity of soil | 49 |
| 400–500 g g−1 in saline solution (maximum absorption) | ||||
| Guar gum, methyl methacrylate | Graft polymerization (boric acid-cross-linker) | 3650% in distilled water | Excellent water absorption ability (soil conditioner) | 50 |
| Guar gum, acrylic acid | Graft polymerization (ethylene glycol di-methacrylic acid-cross linker) | 806 g g−1 in distilled water | Soil conditioning material | 51 |
| 77 g g−1 in saline solution (0.9 wt% NaCl) | ||||
| Guar gum, acrylic acid | Free-emulsion graft-polymerization | 120% at pH = 7 (distilled water) | Increase water absorption capacity of soil | 52 |
| Guar gum (loaded with boron) | Graft polymerization (ethylene-glycol-di-methacrylate-cross linker) | 300–375 g g−1 (at pH = 9) | Controlled delivery of micronutrients, water retention | 53 |
| 80–120 g g−1 in distilled water | ||||
| Guar gum, methyl methacrylate | Graft polymerization (polyethylene glycol-cross linker) | ∼110 g g−1 in distilled water | Soil conditioning material, enhances crop yield | 54 |
| Guar gum, polyacrylamide, pectin | Radiation polymerization (gamma irradiation) | 600–1050 g g−1 in distilled water | Enhance water holding capacity of soil | 55 |
5. Gelatin based hydrogels
Gelatin is a natural polymer that is derived from collagen protein. Collagen is obtained naturally from the skin and bones of animals. Chemically, gelatin consists of proline, glycine, and 4-hydroxy proline. Gelatin can be acidic or basic depending upon the method used for its extraction. Gelatin can form hydrogen bonding with other materials and is hydrophilic. Gelatin found wide application in the food, medicine, and cosmetic industries. Gelling characteristics of gelatin can be modified according to demand by cross-linking. Gelatin has been used for making hydrogels for the past few decades.56 Gelatin hydrogels have special applications in the biomedical field, cosmetic industry, and agriculture. Synthesis and application of some of the gelatin based agricultural hydrogel have been discussed below.
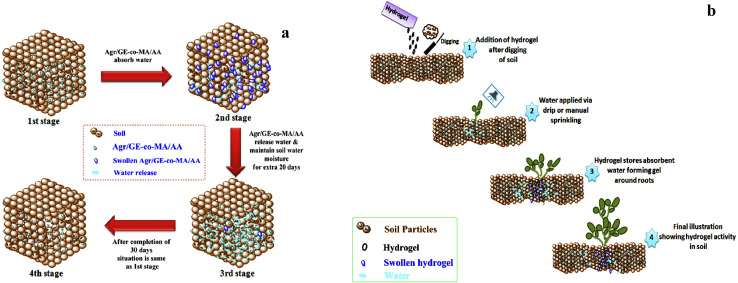
Gelatin based superabsorbent hydrogel composites have been synthesized by graft polymerization of acrylic acid on gelatin backbone using kaolin powder. In this experiment, MBA was utilized as a cross-linker. It was observed that the concentration of gelatin polymer, monomer, cross-linker, and kaolin influenced the water absorption capacity of the hydrogel. Results indicated that these hydrogel composites exhibit good swelling ability or water retention capacity. The swelling behavior of the hydrogel was sensitive to different values of pH.57 Gelatin based hydrogel has been used for the controlled delivery of fertilizer to the plants. One of the methods for the development of this type of hydrogel includes the crosslinking of gelatin loaded with ammonium nitrate (AN) using glutaraldehyde as cross-linking agent. The impact of the formulated hydrogel (gelatin/AN) on cucumber was investigated. The results indicated that gelatin/AN hydrogel could be used as a promising controlled-release fertilizer material for agricultural use.58 Gelatin hydrogel prepared from the co-polymerization of gelatin and agar agar with acrylic acid and methyl acrylate can be used as a satisfactory soil conditioning agent. This hydrogel can retain a large amount of water (Fig. 10a) and is biodegradable, non-toxic, and economical; thus, it could be used as an eco-friendly soil conditioning agent (Fig. 10b).59
Fig. 10. (a) Schematic presentation of water retention in the soil through agar agar/gelatin/methyl acrylate/acrylic acid (Agr/GE-co-MA/AA hydrogel) (b) demonstrates hydrogel used in the agriculture field for the function of plant water assimilation, reproduced from ref. 59 under the terms of the Creative Commons CC BY license, 2020.
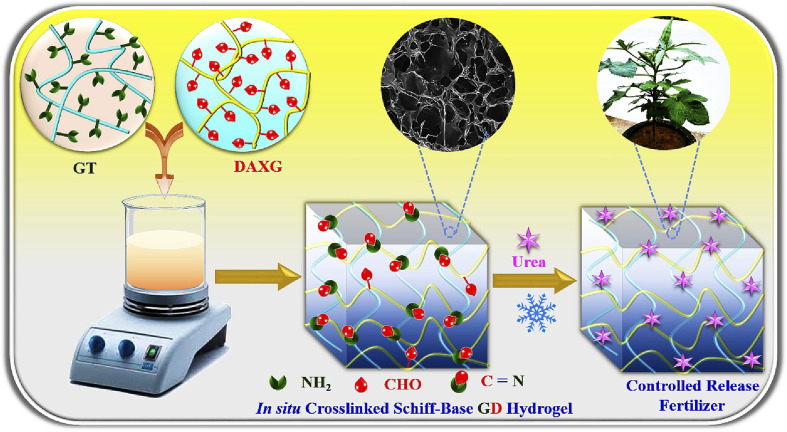
Alg and gelatin based hydrogel could be effectively used for the controlled release of urea fertilizer. Researchers have formulated urea loaded gelatin/Alg hydrogels by using calcium chloride as a cross-linker. The hydrogel was analyzed, and the results of the analysis showed that hydrogel with desirable characteristics was obtained when a 0.5 : 1 ratio of gelatin and Alg was used. This hydrogel allows the controlled release of urea (and other blended nutrients) in an environmentally friendly way.60 Modified gelatin hydrogel films loaded with urea and wood ash have been synthesized and investigated. Chemical cross-linking of gelatin with glutaraldehyde was done for this purpose. This hydrogel composite could be used as a cost-effective slow-release fertilizer for plants. The best release of urea (54.43%) from the formulated hydrogel films was observed when the ratio of urea to gelatin (U/G) = 2.22 g, wood ash to U/G ratio = 1.50 g, and glutaraldehyde was used in a concentration of 2.47 mL g−1. Moreover, this hydrogel composite has good water holding capacity and also shows a positive impact on the growth of plants.61 Gelatin based hydrogel was prepared by chemical cross-linking of gelatin and di-aldehyde xanthan gum. This hydrogel can be used as a control release fertilizer material when blended with the fertilizer (Fig. 11). The hydrogel not only acts as a carrier of fertilizer but can also hold a large amount of water to improve plant growth in water stress condition.62
Fig. 11. Schematic representation of the synthesis of gelatin (GT)-dialdehyde xanthan gum (DAXG) Schiff-base hydrogels (GD) as urea-controlled release fertilizers (CRFs) in agriculture, reproduced with permission from ref. 62 copyright, Elsevier, 2023.
Chemical cross-linking of urea with polylactic acid and CS produced a hydrogel composite that exhibited excellent water holding ability. The blending of this composite hydrogel with urea ensures the slow release of nitrogen in the soil. Hence, this gelatin based hydrogel composite could be used as an efficient soil conditioning tool for agricultural purposes.63 Potential applications of gelatin-based hydrogels in agricultural fields, along with their synthetic method and water holding capacity, are given below (Table 5).
Details of gelatin-based hydrogels.
| Polymers | Synthesis method & cross-linker used | Swelling degree (SD)/water absorption | Agricultural applications | References |
|---|---|---|---|---|
| Gelatin, acrylic acid, kaolin powder | Graft polymerization (methylenebisacrylamide-cross linker) | 85–90 g g−1 at pH = 8 | Enhance the water retention capacity of soil (pH-responsive behavior) | 57 |
| 55 g g−1 in NaCl solution (maximum) | ||||
| Gelatin | Chemical cross-linking (glutaraldehyde-cross linker) | 218% in distilled water (maximum) | Controlled release of fertilizer enhances the water absorption capacity of soil | 58 |
| Gelatin and agar | Chemical cross-linking | 636.1% in deionized water (maximum) | Excellent water holding capacity | 59 |
| Gelatin, sodium alginate | Ionic gelation method (calcium chloride-cross linker) | 13.95 g g−1 in deionized water (maximum) | Controlled release of urea and other nutrients | 60 |
| Gelatin | Chemical cross-linking (glutaraldehyde-cross linker) | 228–251% in deionized water | Controlled release of urea and other nutrients and fertilizer, water retention, enhanced plant growth | 61 |
| Gelatin, di-aldehyde xanthan gum | Chemical cross-linking | — | Allow controlled release of fertilizers, improves water holding capacity of soil | 62 |
| Gelatin, polylactic acid and chitosan | Chemical cross-linking | 4448% at 30 °C in deionized water | Excellent water retention ability allow the slow release of nitrogen and other useful nutrients | 63 |
6. Lignin based hydrogels
Lignin is a bio-polymer derived naturally from the cell wall of plant cells. It is a carbohydrate in which cellulose micro-fibers are arranged in the form of layers. Chemically, it is formed from phenylpropanoid precursors commonly called monolignols. The monolignols include coniferyl alcohol, p-coumaryl alcohol, and sinapyl alcohol. The relative proportion of monolignols varies in various plants. These monolignols are connected via C–C (5–5, β-5, β-1y β–β) linkage and (O-4, α-O-4, 4-O-5) ether linkages. Most of the linkage in natural lignin is β-O-4 linkage. Lignin finds important applications in medicine and agriculture. Lignin can be used to make adhesives, coatings, polyols and emulsifying agents, etc.64 Lignin based hydrogels are used in the agricultural sector as soil conditioning material and for other purposes. Synthesis method and possible applications of lignin based agricultural hydrogel have been discussed in this section.
Lignin based superabsorbent hydrogel has been prepared by mixing it with cellulose and then chemically cross-linking them with epichlorohydrin, which is a cross-linker. Swelling property and controlled release behavior of the hydrogel was instigated. Polyphenols were loaded into the hydrogel to examine their release pattern. It was observed that polyphenols released from the hydrogel depend upon the lignin content of the hydrogel matrix. Results showed that release of polyphenols increases by increasing the lignin content of the hydrogel. Maximum swelling was observed when cellulose and lignin were mixed in a proportion of 0.125 g and 0.375 g, respectively 65. Lignin based water absorbent hydrogel prepared by cross-linking lignin and poly(ethylene glycol) diglycidyl ether in an alkali solution. Formulated hydrogel exhibits good swelling ability and is biodegradable. The swelling behavior of the hydrogel was investigated in distilled water, CaCl2, and NaCl solutions. Maximum swelling of hydrogel was observed in distilled water. This hydrogel proved to be an effective material to be used for plants under water stress conditions.66 Another method proposed for the synthesis of lignin hydrogel includes the use of waste from the pulp and paper industry by a co-polymerization technique using acrylic acid and red liquor (industrial waste). This hydrogel was not only cost-effective but is also very environmentally friendly as it is biodegradable and reduces waste pollution. This hydrogel possesses the characteristics of water retention and slow release of material (if blended with the hydrogel). Hence it is a potential agent for agricultural application.67 Lignin and PVA hydrogel has been developed by using epichlorohydrin as a cross-linker which can be used as a water holding agent in agriculture. This hydrogel exhibits great water absorption capacity, which ranges from 92–456 g g−1. It was concluded that the swelling ability of hydrogel is enhanced if high molecular weight poly-vinyl alcohol is used. Thus, this lignin based hydrogel is a potential candidate for agricultural use.68 An agricultural hydrogel has been prepared using lignosulfonate (L), konjaku flour (KJ), and Alg. This hydrogel increases the water and nutrient holding capacity of soil as it prevents the nutrient from leaching. The impact of this hydrogel on the growth of tobacco plants was examined. The photosynthetic ability of tobacco plants was found to be enhanced by the application of this hydrogel in soil. Commercial acrylamide hydrogels and L/KJ/Alg hydrogels, as depicted in Fig. 12, may both lengthen the growth cycle of the tobacco plant by 2–4 days and 9–14 days, respectively, during periods of intense drought. It is evident that the water retained by acrylamide hydrogels cannot be utilized for plant growth, despite the fact that their water absorption is significantly more than that of L/KJ/Alg hydrogels.69
Fig. 12. Illustration of different treatments of tobacco growth after stopping water supply. M: L/KJ/Alg hydrogel treatment; S: acrylamide hydrogel, CK: untreated soil, reproduced with permission from ref. 69 copyright, Elsevier, 2019.
Another important method for the development of agricultural hydrogel based on lignin was proposed by the researchers to increase the water retention ability of the silt loam soil. Alkali solution of lignin was cross-linked with poly(ethylene glycol) di-glycidyl ether. This hydrogel is a promising material to improve water retention of soil in drought conditions.70Table 6 describes some of the important hydrogels based on lignin, their synthetic method, swelling behavior, and agricultural applications.
Details of lignin-based hydrogels.
| Polymers | Synthesis method & cross-linker used | Swelling degree (SD)/water absorption | Agricultural applications | References |
|---|---|---|---|---|
| Lignin and cellulose | Chemical cross-linking (epichlorohydrin-cross linker) | 3061% (maximum) in a water–ethanol mixture (ethanol : water = 1 : 19) | Controlled release of polyphenols (agrochemical) enhances the water absorption capacity of soil | 65 |
| Lignin | Chemical cross-linking (using alkali) | 34 g g−1 in distilled water (maximum) | Increase the water retention ability of soil | 66 |
| Lignin (waste from the paper and pulp industry) | Co-polymerization (chemical cross-linking using N,N-methylene-bis-acrylamide) | 280 g g−1 in deionized water (maximum) | Increase water holding ability of soil, slow release of agrochemicals | 67 |
| Lignin, poly-vinyl alcohol | Chemical cross-linking (epichlorohydrin-cross linker) | 92–456 g g−1 in distilled water | Enhance the water absorption capacity of soil | 68 |
| Lignosulfonate, konjaku flour, and sodium alginate | Chemical cross-linking in the liquid phase (anhydrous calcium chloride solution-cross linker) | 41.13 g g−1 in distilled water (maximum) | Improve the photosynthetic ability of plants (tobacco plant), water retention in soil | 69 |
| Lignin (alkali lignin) | Chemical cross-linking (poly(ethylene glycol) di-glycidyl ether-cross linker) | 2013% in deionized water, 825% in 0.9% NaCl solution, and 1092% in tap water | Increase water retention capacity of soil | 70 |
7. Alginate based hydrogels
Alginate is a natural polymer mainly derived from brown seaweed. It is a linear polysaccharide and is a co-polymer formed by the combination of l-guluronic acid and d-mannuronic acid monomers via 1,4-glycosidic linkage. It is also called alginic acid or algin. Sodium alginate is the most commonly used alginate.71 Alginate is an important bio-polymer that contains many desirable characteristics and found a wide range of applications in the agriculture sector, pharmaceutical industry, and food industry. Alginate is also used to formulate hydrogels, and the hydrogels based on alginate are sustainable, non-toxic, biodegradable, and environmentally friendly.72 Although alginate hydrogels are mostly used for biomedical purposes, these hydrogels also find their application in the agricultural field. In this section, a brief discussion has been done on the fabrication of alginate based agricultural hydrogel and their possible application.
Alginate based hydrogel has been synthesized by the chemical crosslinking of PVA and Alg using glutaraldehyde as cross-linking agent. It was observed that as the concentration of cross-linker (glutaraldehyde) increases, the swelling degree of this hydrogel decreases. The swelling behavior of this hydrogel was investigated in acidic and alkaline media. The results indicated that the swelling ability of hydrogel was enhanced with the increase in the amount of acid. The swelling rate of hydrogel examined in different salt solutions showed. The swelling rate of hydrogel reduces as the concentration of salt increases in the solution. This hydrogel could be used as an effective material for the retention of water in soil.73 A cost-effective method to prepare alginate based hydrogel beads by the ionic cross-linking of Alg using calcium chloride. These hydrogel beads exhibit pH-sensitive swelling behavior and could be used for enhancing the water holding capacity of the soil. The effect of cross-linking time, concentration of cross-linker, pH, and temperature on the swelling degree of beads was studied. Results showed that the swelling degree decreases as the cross-linking period of the hydrogel increases. Moreover, it was also observed that a change in temperature does not have a considerable impact on the swelling capacity of the hydrogel.74 To increase the water absorption capacity of the soil and control the delivery of nutrients to the plants, another important hydrogel has been synthesized. This hydrogel was formulated by the physical cross-linking of hydroxyl-propyl cellulose (HPC), carboxymethyl cellulose (CMC), and Alg by using aluminum sulfate (cross-linker). This hydrogel is a potential candidate to be used as a soil conditioning agent in agricultural sector.75 Alginate based hydrogel beads prepared by cross-linking Alg with calcium chloride could be effectively used as a substitute for synthetic hydrogel. These beads are non-toxic and biodegradable and can improve the water retention ability of soil. The water retention ability of soil is significantly influenced by the type of soil used for farming. However, the results of the experiment show that these hydrogel beads can be used by farmers to improve water retention in the soil, especially in water stress conditions (Fig. 13).76
Fig. 13. Illustration of effect of hydrogel substrate supplementation on lettuce plants subjected to drought for 7 days; C: substrate without hydrogel supplementation, 1% w/w or 5% w/w-supplemented substrates. CW: set of plants grown without hydrogel were continually watered along the experiment, reproduced with permission from ref. 76 copyright, Elsevier, 2020.
Hydrogel developed by graft polymerization of phosphorylated alginate with polyacrylamide exhibits good water absorption ability and allows the controlled delivery of phosphorous to the plants. Phosphorylated alginate samples were prepared by the reaction of Alg and PVA with di-ammonium phosphate and mono-ammonium phosphate. Afterward, these phosphorylated alginate were grafted individually with polyacrylamide. Release of phosphorous from di-ammonium phosphate and mono-ammonium phosphate hydrogel sample was observed in 45 days which was 57% and 77%, respectively.77 Alginate based hydrogel beads have also been synthesized by using alginate derived from kelp industrial waste using calcium chloride as a cross-linker. These beads do not exhibit good water retention properties but also allow controlled release of fertilizer and useful nutrients. Moreover, these hydrogel beads help to control the pH of the soil as well.78 Some of the alginate-based hydrogels used in agriculture have been discussed in Table 7, along with the method of their synthesis and agricultural applications.
Details of alginate-based hydrogels.
| Polymers | Synthesis method & cross-linker used | Swelling degree (SD)/water absorption | Agricultural applications | References |
|---|---|---|---|---|
| Sodium alginate and polyvinyl alcohol | Chemical cross-linking (glutaraldehyde-cross linker) | 50–400% (in different salt solutions) | Enhance water retention capacity of soil | 73 |
| Sodium alginate | Ionic cross-linking method (calcium chloride-cross linker) | >1000% (pH = 8, 30 °C) | Excellent material for increasing the water retention ability of soil | 74 |
| Sodium alginate, HPC, and CMC | Physical cross-linking (ionic interaction) aluminum sulfate-cross linker | 1585% (maximum) | Slow release of fertilizer and nutrients and increase moisture holding capacity of soil | 75 |
| Sodium alginate | Ionic cross-linking method (calcium chloride-cross linker) | >55 g g−1 in distilled water, >36 g g−1 in tap water | Improve moisture holding ability of soil | 76 |
| Sodium alginate and polyacrylamide | Graft polymerization | 6–8 g g−1 in distilled water | Controlled release of phosphorous | 77 |
| Alginate from kelp industrial waste | Ionic cross-linking method (calcium chloride-cross linker) | 0.6–1.8 g g−1 in deionized water | Improve water retention ability of soil, allow controlled release of fertilizers, and control pH of soil | 78 |
8. Mechanism of hydrogels as a soil conditioner
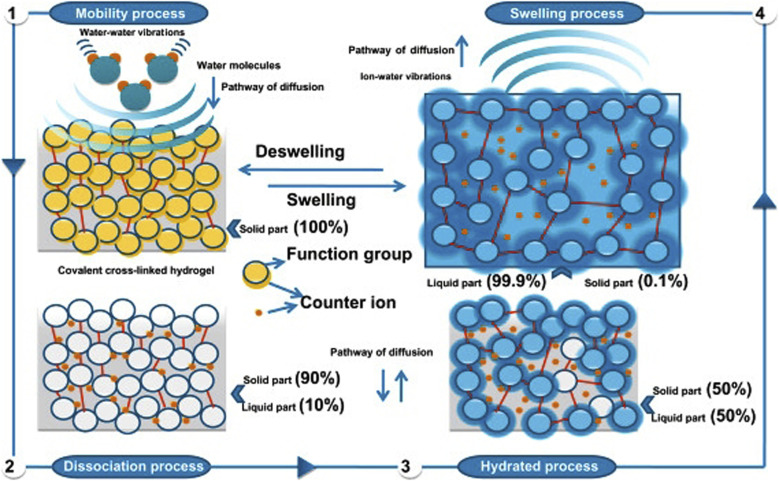
To further explain how hydrogels function as soil conditioners, it's crucial to comprehend how they absorb and hold water (Fig. 14). One factor that supports the use of water and swelling hydrogels as soil conditioners is their capacity to convey nutrients and allow additional irrigation, which results in a gradual release of water and nutrients to the plants. The cross-linked density and the number of hydrophilic groups both affect the water-holding capacity. Water–water vibrations allow for unrestricted movement, which increases water's solubility power. The water molecule tries to dissolve the hydrogel by cleaving the strong covalent bonds that are cross-linked by its vibrational modes, but it only succeeds in cleaving hydrogen bonds. This is an indication of alterations in hydrogen bonding because it permits the water molecules to be restricted inside the pores of the hydrogel's network structure (swelling process). So the osmotically active presence of mobile polar molecules is what causes swelling.1,79
Fig. 14. The swelling mechanism of hydrogels, reproduced from ref. 1, copyright, Elsevier, 2020.
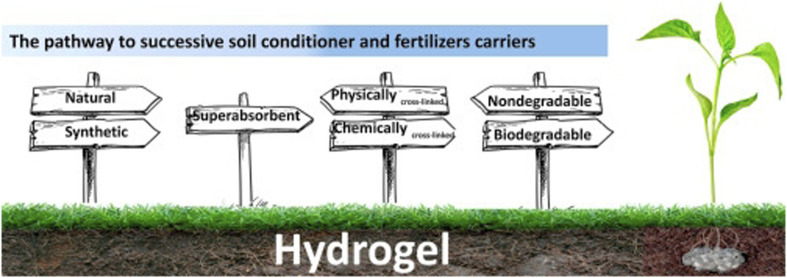
The majority of hydrophilic hydrogels can be applied during planting or coated on the seed itself, and they are primarily used in agriculture as nutrient carriers and soil conditioners. Fig. 15 depicts the process leading to fertilizer carriers and soil conditioners. The preparation of soil conditioner should take into account the basic criteria, as shown in Fig. 15. When choosing a hydrogel as a soil conditioner, at least three factors must be taken into account such as superabsorbent, biodegradable, and chemically covalently cross-linked.1,80–82
Fig. 15. Aspects to choose the ideal hydrogel for use as a soil conditioner, reproduced from ref. 1, copyright, Elsevier, 2020.
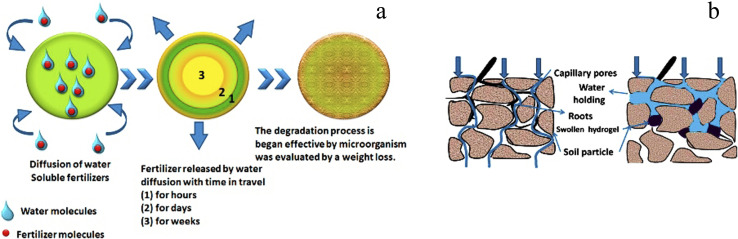
Water is trapped in the gaps between soil granules as it comes into touch with the land. When hydrogel is present, it uses osmosis to take water into its network as the soil becomes moist. The process of photosynthesis is carried out by chlorophyll, which converts water and carbon dioxide (CO2) into carbohydrates and oxygen. Plants with access to plenty of water have higher chlorophyll levels, which boost agricultural yield. Hydrogels have the effect of expanding the area available for air and water infiltration in the soil. This particular soil has the perfect texture for gardening. The hydrogel will quickly release water into the environment from its internal polymer network once the soil has dried. When required, the water is released from a hydrogel network and returned to the soil. The soil structure became stable as a result of soil–water interactions generated by swollen hydrogel.83,84 This is also observed with the nutrient and fertilizer leaching from agricultural soils. Hydrogel is used in fertilizer formulations to lessen fertilizer leaching. Because of the way fertilized hydrogel behaves, fertilizer can be kept in place and released gradually. As a result of less leaching, crop output increases, and fertilizer requirements decrease. Fertilizers loaded into hydrogel have been demonstrated to discharge at a rate that is half that of fertilizer applied in water alone.85–87Fig. 16a shows hydrogel as a slow-release technique for water and dissolved fertilizers in soil. The diffusion mechanism, which starts with a deswelling process where water is diffused and carries fertilizer via the porous network to the outside, is typically how fertilizers are released into the environment.88–90
Fig. 16. (a) An illustration of the complete fertilizer release procedure from hydrogel (b) the impact of polysaccharide hydrogel on the soil texture for plant growth, reproduced from ref. 1, copyright, Elsevier, 2020.
Ammonium (NH41+) and nitrate (NO31−) are nitrogen fertilizers that encourage the production of N2O, which contributes more to global warming than CO2 emissions. The current challenge is to maximize nitrogen retention while reducing methane (CH4) and N2O emission. The majority of the nutrients from dissolved fertilizers are retained by soil conditioner hydrogel, which also reduces the requirement for nutrients in agricultural soils, as well as nitrogen leaching and N2O emission.91–93 The pores of soil are mostly filled with water after irrigation or rainfall, and the water escapes downhill. Soil is ideal for aeration but cannot hold water. Polysaccharide hydrogel, a soil conditioner, aids in holding water, which creates ideal growing conditions for plant roots (Fig. 16b).
Fig. 17 demonstrates how tightly the hydrogel adheres to the roots, making it possible for the roots of the plant to access nutrients. Ions and nutrients are transported more quickly by soil conditioner polysaccharide hydrogel adaption of plant roots. The plasma membrane, made up of phospholipids, proteins, and steroid compounds, covers the surface of the root. Assisting in increasing the active transport of nutrients and water to plants by negatively charged clay surfaces with cationic functional groups (Al3+, Fe3+, and Ca2+), hydrogel increased the contact between soil particles and roots through a hydrogen bond or dipole–dipole interaction. Reduced crop output can result from fertilizers' and herbicides' detrimental effects on non-target plants. As a result, biodegradable polysaccharide hydrogel-based agrochemicals have been developed for use in safe and effective ways in agricultural settings. Various works on the subject of polysaccharides use them as elicitors, with chitosan being one of the most significant as a plant defense-booster.94,95
Fig. 17. The hydrogel adheres to the roots tightly and improves soil-root contact, reproduced from ref. 1, copyright, Elsevier, 2020.
Polysaccharides, such as chitosan and chitin, have been shown to trigger plant defensive reactions against a variety of phytopathogens, including plant viruses and fungi. Currently, several experimental findings have demonstrated that chitosan helps prevent viral infections.96 The effects of the growth media and the absence of phytotoxicity of the polysaccharide hydrogel have been documented.97,98 It's crucial to evaluate the phytotoxicity of hydrogels to spot any growth inhibition. Root surface area and length increased in plants exposed to soil conditioner hydrogel. The rate of seed germination and seedling survival can be increased by seed coating.99,100 A slower release of nutrients into the soil is made possible by nano-fertilizers, which are soluble fertilizers that have been loaded or encapsulated by hydrogel nanoparticles. A biodegradable hydrogel that has been nano-fertilized quickly spreads into roots over time. The nanoparticle quickly diffused into the roots of the plants by symplastic and apoplastic routes before moving to the aerial sections of the plants, including the stems and leaves, via xylem tissue.101,102 It is desirable to wrap fertilizer with a clever biodegradable hydrogel to increase its efficacy. The fertilizer will perform better and be more sophisticated, for example, making itself available to roots and plants for a long time and encouraging effective root uptake control, which could be influenced by the soil's P, N, and K content, and allowing roots easy access to the fertilizer. The crop yield will rise, and the compaction will go.95,103
9. Urgency of hydrogels in agricultural applications
Due to several urgent issues facing modern agriculture, hydrogels are becoming increasingly important in agricultural applications. To effectively solve these urgent concerns, it is essential to continue researching, developing, and using hydrogel technology in agricultural applications. The following reasons underline the significance and urgency of using hydrogel in agriculture.
9.1. Water shortage
Water scarcity is a major issue in many regions of the world, and agriculture uses a significant amount of fresh water. Hydrogels, which can absorb and hold significant amounts of water, can be used to boost the effectiveness of how much water is used in agricultural systems, which will help to solve the problem. Hydrogels make it possible to utilize limited water resources more efficiently by lowering water loss from evaporation and runoff.
9.2. Resistance to drought
The frequency and intensity of drought episodes are increasing due to climate change, which hurts crop productivity. By creating a water reserve in the root zone and enabling plants to access water when it is dry, hydrogels can assist plants in withstanding drought conditions. This increases crop resilience to drought and aids in maintaining crop yield in the face of water constraints.
9.3. Degrading of the soil
Global issues include soil degradation, which includes erosion, compaction, and nutrient loss. Hydrogels can assist in reducing the rate of soil deterioration by improving soil fertility and structure. By raising soil water-holding capacity, lowering erosion by stabilizing soil surfaces, and increasing plant nutrient availability, they combat soil deterioration and safeguard arable land.
9.4. Sustainable agriculture
Environmental concerns and the need to feed a growing world population are driving up demand for sustainable agriculture methods. A more environmentally friendly method of managing nutrients and irrigation is provided by hydrogels. Hydrogels support the objectives of sustainable agriculture by decreasing runoff and leaching, reducing water and fertilizer usage, and encouraging effective resource use.
9.5. Food security
Food security is a major issue on a worldwide scale. By increasing crop yields, boosting agribusiness productivity, and strengthening crop resilience, hydrogels have the potential to help ensure global food security. Hydrogels can boost agricultural output and help feed a growing population by maximizing the water and nutrient availability of plants.
9.6. Environmental impact
Soil erosion and water contamination from fertilizer runoff are only two examples of how conventional farming practices can have a negative influence on the environment. By lessening soil erosion and nutrient loss, hydrogels can lessen these negative environmental effects. Furthermore, the compositions of their biodegradable products further lessen the long-term environmental risks connected to their use.
10. Potential environmental hazards of hydrogels and their mitigation
Although hydrogels have many useful applications, it's vital to take into account any potential environmental risks. These potential hydrogel environmental issues are listed below.
10.1. Non-biodegradability
Many hydrogels take a long time to degrade because they are not easily biodegradable. Hydrogels can build up in soil or water bodies if they are not properly managed after usage, which could hurt the environment. Non-biodegradable hydrogel buildup has the potential to disturb natural ecosystems and alter the balance of the organisms that live there.
10.2. Water bodies' contamination
Hydrogel contamination can result from improper disposal or unintentional leakage into water bodies. Non-water-soluble hydrogels can build up in water systems, compromising the water's quality and perhaps endangering aquatic life. This may hurt biodiversity and aquatic ecology.
10.3. Natural materials displacement
The use of hydrogels may occasionally cause natural materials to be replaced. For instance, overuse of hydrogels in soil may substitute for organic matter or other naturally occurring soil elements, thereby changing the soil's natural makeup and nutrient cycling procedures.
10.4. Pollution risk from microplastics
Some hydrogels are made of synthetic polymers that, over time, can break down into microplastics. Microplastics are little particles that can get into soil and water sources and endanger living things and ecosystems. Although the long-term implications of microplastic pollution on the environment are still being researched, their presence raises questions about both ecological and health effects.
It is critical to take into account the following steps to reduce these potential environmental risks.
10.5. Biodegradable substitutes
Focus should be placed on creating biodegradable hydrogel materials that degrade more quickly in the environment, hence lowering their long-term persistence.
10.6. Responsible disposal and waste management
It is important to appropriately dispose of hydrogels by following suggested rules. To prevent their discharge into the environment, hydrogels that are not water-soluble should be collected and disposed of in the proper waste management systems.
10.7. Regulating and assessing risks
Governments and regulatory organizations should evaluate the environmental concerns related to the use of hydrogels and put in place the necessary rules and regulations to ensure that they are used safely and responsibly. This entails assessing their possible effects on ecosystems, encouraging sustainable use, and making sure that appropriate waste disposal procedures are followed.
10.8. Studies on the long-term effects on the environment
To fully comprehend the long-term environmental implications of using hydrogel, more research is required. Studying their fate in various habitats, possible accumulation in organisms, and the ecological effects of their presence are all included in this.
The potential dangers connected with hydrogels in the environment can be reduced while still reaping the benefits of their agricultural uses by addressing these environmental concerns and implementing proper usage and disposal methods.
11. Conclusion and future perspective
Biopolymers are biodegradable polymers that are found abundantly in nature and are considered non-toxic, biocompatible, and cost-effective. These biopolymers are being extensively used in making hydrogels. Hydrogels made from biopolymers are being used in various all over the world. One of the major applications of bio-polymeric hydrogels is their use in the agricultural field as a soil conditioning agent. Natural polymers are crosslinked to create these hydrogels, which have a three-dimensional network structure that can contain and absorb a lot of water. With fewer evaporation losses and more water available to plants, hydrogels can significantly enhance soil water retention. This quality is especially important in dry and drought-prone areas where agriculture has serious difficulties as a result of water scarcity. Bio-polymer based hydrogels are an effective and biodegradable substitute for synthetic hydrogels to boost the soil's capacity to retain water. Hydrogel engineering allows for a regulated release of nutrients, providing plants with a steady supply of essential elements. As the hydrogel matrix degrades or is in reaction to environmental cues like moisture or temperature, nutrients can be gradually supplied by adding fertilizers. This technique improves nitrogen use efficiency while reducing environmental contamination and fertilizer leaching. Hydrogel-coated seed covers can aid in seedling establishment and germination. The hydrogel coating offers a barrier around the seed to shield it from desiccation and encourage water absorption. The hydrogel can also contain bioactive substances, growth regulators, or advantageous microbes to promote seed germination, root growth, and overall plant growth. Hydrogels can increase agricultural yields by enhancing soil moisture levels, nutrient availability, and plant protection. By preserving ideal moisture levels and nutrient supplies, they can also assist plants in withstanding abiotic challenges such as drought, salinity, and extremely high temperatures. Hydrogels can create a better microclimate for plant growth and serve as a physical barrier against environmental stressors. Hydrogels made of bio-polymers are more environmentally friendly and biodegradable than those made of synthetic materials like petrochemicals. Utilizing biocompatible and renewable polymers lessens their potential for long-term buildup in soil and their negative effects on the environment. This fits with the desire to lessen reliance on non-renewable resources and the rising demand for sustainable agriculture practices. Hydrogels made from bio-polymers have a lot of potential for use in agriculture in the future. They are useful tools for boosting agricultural productivity, sustainability, and resilience in the face of a variety of challenges because of their capacity to retain water, control the release of nutrients, have seed coating properties, provide crop protection, be environmentally sustainable, and have the potential to increase yield.
Therefore, the purpose of this review is to assist academics, researchers, and industry experts in developing hydrogels based on biopolymers that have gained unique significance in agricultural applications (soil conditioners, fertilizer, and pesticide carriers).
Informed consent statement
This study was not performed on humans.
Data availability
This study did not report any data.
Conflicts of interest
The authors declare no conflict of interest.
Supplementary Material
Acknowledgments
The authors received no specific funding for this work.
Biographies
Biography
Dure Najaf Iqbal.

Dr Dure Najaf Iqbal completed the doctorate in chemistry with distinction at the Lahore College for Women University, Lahore in 2014. She is currently working as an associate professor of chemistry in Department of Chemistry, University of Lahore. Dr Dure was fortune enough to avail HEC Scholarship under indigenous scholarship scheme Batch-IV and international research support initiative program for six months in University of Bristol under IRSIP. She worked with Prof. Christine Wills at University of Bristol. She has contributed to several national and international publications and conferences with impact factor 100+. Her current research interests are organic synthesis, polymer chemistry, FR polymers and hydrogels.
Biography
Mahmood Ahmed.

Dr Mahmood Ahmed is an Assistant Professor of Chemistry, in the Department of Chemistry, University of Education, Lahore-Pakistan. He received his PhD degree in Chemistry from University of the Punjab, Lahore-Pakistan. He has more than 20 years of professional, research and teaching experience. His expertise lies in analytical, pharmaceutical and medicinal chemistry. He also worked in the pharmaceutical industry, where he did a quality by design (QbD) based project for manufacturing of haemodialysis in Pakistan & Malaysia. He has published 102 research/review articles as principle, corresponding and co-author in peer reviewed journals. He is on the reviewer board of various journals from Elsevier, RSC, ACS, Springer-Nature, MDPI, Taylor & Francis, and Hindawi. He is an academic editor of Journal of Chemistry, and International Journal of Analytical Chemistry.
References
- Ghobashy M. M., in Hydrogels based on natural polymers, Elsevier, 2020, pp. 329–356 [Google Scholar]
- Li S. Hernandez S. Salazar N. Sustainability. 2023;15:848. [Google Scholar]
- Patra S. K. Poddar R. Brestic M. Acharjee P. U. Bhattacharya P. Sengupta S. Pal P. Bam N. Biswas B. Barek V. Int. J. Polym. Sci. 2022;2022:4914836. [Google Scholar]
- Pradeep M. Senthilkumar S. Kumar A. R. Manivannan S. Water Energy Int. 2023;66:15–18. [Google Scholar]
- Peppas N. A. and Hoffman A. S., in Biomaterials science, Elsevier, 2020, pp. 153–166 [Google Scholar]
- Tomadoni B., Casalongué C. and Alvarez V. A., Polymers for Agri-food applications, 2019, pp. 99–125 [Google Scholar]
- Bustamante-Torres M. Romero-Fierro D. Arcentales-Vera B. Palomino K. Magaña H. Bucio E. Gels. 2021;7:182. doi: 10.3390/gels7040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur M., Sharma A., Chandel M. and Pathania D., in Green Functionalized Nanomaterials for Environmental Applications, Elsevier, 2022, pp. 259–281 [Google Scholar]
- Apriyanto A. Compart J. Fettke J. Plant Sci. 2022:111223. doi: 10.1016/j.plantsci.2022.111223. [DOI] [PubMed] [Google Scholar]
- Lertsarawut P. Rattanawongwiboon T. Tangthong T. Laksee S. Kwamman T. Phuttharak B. Romruensukharom P. Suwanmala P. Hemvichian K. Polymers. 2021;13:1314. doi: 10.3390/polym13081314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekebafe L. Ogbeifun D. Okieimen F. Am. J. Polym. Sci. 2011;1:6–11. [Google Scholar]
- Sadeghi M. Soleimani F. J. Biomater. Nanobiotechnol. 2012;3:310–314. [Google Scholar]
- Fekete T. Borsa J. Takács E. Wojnárovits L. Chem. Cent. J. 2017;11:1–10. doi: 10.1186/s13065-017-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak V., Effect of Starch-based Hydrogel on Early Growth of Corn, MS thesis, Purdue University, 2018 [Google Scholar]
- Puspita I., Winarti C., Maddu A. and Kurniati M., 2019
- Olad A. Doustdar F. Gharekhani H. Colloids Surf., A. 2020;601:124962. [Google Scholar]
- Luo H. Dong F. Wang Q. Li Y. Xiong Y. Molecules. 2021;26:3999. doi: 10.3390/molecules26133999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora A. Karak N. Eur. Polym. J. 2022;176:111430. [Google Scholar]
- Dudu T. E. Alpaslan D. MANAS J. Eng. 2022;10:116–124. [Google Scholar]
- Manafzadeh R. and Rashidinejad F., Preparation and Investigating the Superabsorbent Properties of Starch/Zeolite Nanocomposite in Order to Release the Zinc Element from it, available at SSRN: https://ssrn.com/abstract=4067321
- Supare K. Mahanwar P. J. Polym. Environ. 2022;30:2448–2461. [Google Scholar]
- Kolya H. Kang C.-W. Int. J. Biol. Macromol. 2023;226:1477–1489. doi: 10.1016/j.ijbiomac.2022.11.260. [DOI] [PubMed] [Google Scholar]
- Michalik R. Wandzik I. Polymers. 2020;12:2425. doi: 10.3390/polym12102425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L. Liu M. Carbohydr. Polym. 2008;72:240–247. [Google Scholar]
- Hussain M. R. Devi R. R. Maji T. K. Iran. Polym. J. 2012;21:473–479. [Google Scholar]
- Minet E. P. O'Carroll C. Rooney D. Breslin C. McCarthy C. Gallagher L. Richards K. Chemosphere. 2013;93:2854–2858. doi: 10.1016/j.chemosphere.2013.08.043. [DOI] [PubMed] [Google Scholar]
- Noppakundilograt S. Pheatcharat N. Kiatkamjornwong S. J. Appl. Polym. Sci. 2015;132:41249. [Google Scholar]
- Narayanan A. Dhamodharan R. Carbohydr. Polym. 2015;134:337–343. doi: 10.1016/j.carbpol.2015.08.010. [DOI] [PubMed] [Google Scholar]
- Sabadini R. C. Martins V. C. Pawlicka A. Cellulose. 2015;22:2045–2054. [Google Scholar]
- Rattanamanee A. Niamsup H. Srisombat L.-o. Punyodom W. Watanesk R. Watanesk S. J. Polym. Environ. 2015;23:334–340. [Google Scholar]
- Essawy H. A. Ghazy M. B. Abd El-Hai F. Mohamed M. F. Int. J. Biol. Macromol. 2016;89:144–151. doi: 10.1016/j.ijbiomac.2016.04.071. [DOI] [PubMed] [Google Scholar]
- Perez J. J. Francois N. J. Carbohydr. Polym. 2016;148:134–142. doi: 10.1016/j.carbpol.2016.04.054. [DOI] [PubMed] [Google Scholar]
- Elbarbary A. M. Abd El-Rehim H. A. El-Sawy N. M. Hegazy E.-S. A. Soliman E.-S. A. Carbohydr. Polym. 2017;176:19–28. doi: 10.1016/j.carbpol.2017.08.050. [DOI] [PubMed] [Google Scholar]
- Said M. Atassi Y. Tally M. Khatib H. J. Polym. Environ. 2018;26:3937–3948. [Google Scholar]
- Nayan N. H. M. Hamzah M. S. A. Tahir A. A.-h. M. Rajali A. A. A. Muslih E. F. Mazlan R. J. Sci. Technol. 2018;10:21–27. [Google Scholar]
- León O. Muñoz-Bonilla A. Soto D. Ramirez J. Marquez Y. Colina M. Fernández-García M. J. Polym. Environ. 2018;26:728–739. [Google Scholar]
- Perez J. Francois N. Maroniche G. A. Borrajo M. P. Pereyra M. Creus C. M. Carbohydr. Polym. 2018;202:409–417. doi: 10.1016/j.carbpol.2018.07.084. [DOI] [PubMed] [Google Scholar]
- Narayanan A. Kartik R. Sangeetha E. Dhamodharan R. Carbohydr. Polym. 2018;191:152–160. doi: 10.1016/j.carbpol.2018.03.028. [DOI] [PubMed] [Google Scholar]
- Iftime M. M. Ailiesei G. L. Ungureanu E. Marin L. Carbohydr. Polym. 2019;223:115040. doi: 10.1016/j.carbpol.2019.115040. [DOI] [PubMed] [Google Scholar]
- López-Velázquez J. C. Rodríguez-Rodríguez R. Espinosa-Andrews H. Qui-Zapata J. A. García-Morales S. Navarro-López D. E. Luna-Bárcenas G. Vassallo-Brigneti E. C. García-Carvajal Z. Y. J. Chem. Technol. Biotechnol. 2019;94:3495–3504. [Google Scholar]
- Brandelero R. P. H. Alfaro A. Marques P. Brandelero E. Rev. Virtual Quim. 2019;11:686–698. [Google Scholar]
- Perez Bravo J. J. François N. J. J. Polym. Environ. 2020;28:2681–2690. [Google Scholar]
- Ritonga H. Basri M. I. Rembon F. S. Soil Sci. Annu. 2020;71:194–204. [Google Scholar]
- Peidayesh H. Ahmadi Z. Khonakdar H. A. Abdouss M. Chodák I. Polym. Adv. Technol. 2020;31:1256–1269. [Google Scholar]
- Mudgil D. Barak S. Khatkar B. S. J. Food Sci. Technol. 2014;51:409–418. doi: 10.1007/s13197-011-0522-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur S. Sharma B. Verma A. Chaudhary J. Tamulevicius S. Thakur V. K. Int. J. Polym. Anal. Charact. 2018;23:621–632. [Google Scholar]
- Wang W. B. Wang A. Q. Adv. Mater. Res. 2010;96:177–182. [Google Scholar]
- Kaith B. S. Sharma R. Kalia S. Bhatti M. S. RSC Adv. 2014;4:40339–40344. [Google Scholar]
- Chandrika K. P. Singh A. Sarkar D. J. Rathore A. Kumar A. J. Appl. Polym. Sci. 2014;131:41060. [Google Scholar]
- Rathore K. Loonker S. Int. J. ChemTech Res. 2017;10:177–184. [Google Scholar]
- Thombare N. Mishra S. Siddiqui M. Jha U. Singh D. Mahajan G. R. Carbohydr. Polym. 2018;185:169–178. doi: 10.1016/j.carbpol.2018.01.018. [DOI] [PubMed] [Google Scholar]
- Behera P. Sethy T. R. Sahoo P. K. Int. J. Nanomater. Nanotechnol. Nanomed. 2018;4(2):9–13. [Google Scholar]
- Thombare N. Mishra S. Shinde R. Siddiqui M. Jha U. Biopolymers. 2021;112:e23418. doi: 10.1002/bip.23418. [DOI] [PubMed] [Google Scholar]
- Songara J. C. Patel J. N. J. Indian Chem. Soc. 2021;98:100220. [Google Scholar]
- Sayed A. Mohamed M. M. Abdel-Raouf M. Adel G. J. Inorg. Organomet. Polym. 2022;32:4589–4600. [Google Scholar]
- Deshmukh K., Ahamed M. B., Deshmukh R., Pasha S. K., Bhagat P. and Chidambaram K., in Biopolymer composites in electronics, Elsevier, 2017, pp. 27–128 [Google Scholar]
- Pourjavadi A. Hosseinzadeh H. Sadeghi M. J. Compos. Mater. 2007;41:2057–2069. [Google Scholar]
- Pulat M. Saglam N. Y. Eurasia Proc. Sci. Technol. Eng. Math. 2018:17–24. [Google Scholar]
- Chaudhary J. Thakur S. Sharma M. Gupta V. K. Thakur V. K. Biomolecules. 2020;10:939. doi: 10.3390/biom10060939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnoosong W. Rungcharoenthong P. Sangjan S. Key Eng. Mater. 2021;889:98–103. [Google Scholar]
- Manzoor E. Majeed Z. Nawazish S. Akhtar W. Baig S. Baig A. Fatima Bukhari S. M. Mahmood Q. Mir Z. Shaheen S. Agriculture. 2022;12:1743. [Google Scholar]
- Das S. Dalei G. Sci. Total Environ. 2023;875:162660. doi: 10.1016/j.scitotenv.2023.162660. [DOI] [PubMed] [Google Scholar]
- Yuan W. Li S. Guan H. Zhang S. Zhang Y. Zhang M. Yu Y. Chen X. Polymers. 2023;15:997. doi: 10.3390/polym15040997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásquez-Garay F. Carrillo-Varela I. Vidal C. Reyes-Contreras P. Faccini M. Teixeira Mendonça R. Sustainability. 2021;13:2697. [Google Scholar]
- Ciolacu D. Oprea A. M. Anghel N. Cazacu G. Cazacu M. Mater. Sci. Eng., C. 2012;32:452–463. [Google Scholar]
- Mazloom N. Khorassani R. Zohuri G. H. Emami H. Whalen J. Clean: Soil, Air, Water. 2019;47:1900101. [Google Scholar]
- Meng Y. Liu X. Li C. Liu H. Cheng Y. Lu J. Zhang K. Wang H. Int. J. Biol. Macromol. 2019;135:815–820. doi: 10.1016/j.ijbiomac.2019.05.195. [DOI] [PubMed] [Google Scholar]
- Rohde L. E. Clausell N. Ribeiro J. P. Goldraich L. Netto R. Dec G. W. DiSalvo T. G. Polanczyk C. A. Int. J. Cardiol. 2005;102:71–77. doi: 10.1016/j.ijcard.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Song B. Liang H. Sun R. Peng P. Jiang Y. She D. Int. J. Biol. Macromol. 2020;144:219–230. doi: 10.1016/j.ijbiomac.2019.12.082. [DOI] [PubMed] [Google Scholar]
- Adjuik T. A., Nokes S. E., Montross M. D. and Wendroth O., in 2021 ASABE Annual International Virtual Meeting, American Society of Agricultural and Biological Engineers, 2021, p. 1 [Google Scholar]
- Ji D. Park J. M. Oh M. S. Nguyen T. L. Shin H. Kim J. S. Kim D. Park H. S. Kim J. Nat. Commun. 2022;13:3019. doi: 10.1038/s41467-022-30691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Cano B. Mendoza-Meneses C. J. García-Trejo J. F. Macías-Bobadilla G. Aguirre-Becerra H. Soto-Zarazúa G. M. Feregrino-Pérez A. A. Molecules. 2022;27:4248. doi: 10.3390/molecules27134248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumara L. R. Demappa T. Turk. J. Pharm. Sci. 2019;16:252. doi: 10.4274/tjps.galenos.2018.92408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULAT M. OZUKAYA D. Eurasia Proc. Sci. Technol. Eng. Math. 2019;6:32–38. [Google Scholar]
- Palem R. R. Rao K. M. Shimoga G. Saratale R. G. Shinde S. K. Ghodake G. S. Lee S.-H. Int. J. Biol. Macromol. 2021;178:464–476. doi: 10.1016/j.ijbiomac.2021.02.195. [DOI] [PubMed] [Google Scholar]
- Tomadoni B. Salcedo M. F. Mansilla A. Y. Casalongué C. Alvarez V. A. Eur. Polym. J. 2020;137:109953. [Google Scholar]
- Al Rohily K. El-Hamshary H. Ghoneim A. Modaihsh A. ACS Omega. 2020;5:32919–32929. doi: 10.1021/acsomega.0c03740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Merwe R. d. T. Goosen N. J. Pott R. W. M. Gels. 2022;8:548. doi: 10.3390/gels8090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Loos M. Feringa B. L. van Esch J. H. Eur. J. Org Chem. 2005;2005:3615–3631. [Google Scholar]
- Idumah C. I. Nwuzor I. C. Odera R. S. Curr. Res. Green Sustainable Chem. 2021;4:100143. [Google Scholar]
- El Bouchtaoui F.-Z. Ablouh E.-H. Mhada M. Kassem I. Salim M. H. Mouhib S. Kassab Z. Sehaqui H. El Achaby M. Int. J. Biol. Macromol. 2022;221:398–415. doi: 10.1016/j.ijbiomac.2022.08.194. [DOI] [PubMed] [Google Scholar]
- Mushtaq F. Raza Z. A. Batool S. R. Zahid M. Onder O. C. Rafique A. Nazeer M. A. Int. J. Biol. Macromol. 2022;218:601–633. doi: 10.1016/j.ijbiomac.2022.07.168. [DOI] [PubMed] [Google Scholar]
- Buchmann C. Bentz J. Schaumann G. Soil Tillage Res. 2015;154:22–33. [Google Scholar]
- Buchmann C., 2018
- Rashidzadeh A. Olad A. Reyhanitabar A. Polym. Bull. 2015;72:2667–2684. [Google Scholar]
- Wang J. Liu S. Qin Y. Chen X. Xing R. e. Yu H. Li K. Li P. Chin. J. Oceanol. Limnol. 2017;35:1086–1093. [Google Scholar]
- Davidson D. W. Verma M. S. Gu F. X. SpringerPlus. 2013;2:1–9. doi: 10.1186/2193-1801-2-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaviv A. Raban S. Zaidel E. Environ. Sci. Technol. 2003;37:2251–2256. doi: 10.1021/es011462v. [DOI] [PubMed] [Google Scholar]
- Ramli R. A. Polym. Chem. 2019;10:6073–6090. [Google Scholar]
- Kassem I. Ablouh E.-H. El Bouchtaoui F.-Z. Kassab Z. Hannache H. Sehaqui H. El Achaby M. Prog. Org. Coat. 2022;162:106575. [Google Scholar]
- Lenka S. Lenka N. K. Singh A. B. Singh B. Raghuwanshi J. Environ. Sci. Pollut. Res. 2017;24:4603–4612. doi: 10.1007/s11356-016-8189-5. [DOI] [PubMed] [Google Scholar]
- Malla G. Bhatia A. Pathak H. Prasad S. Jain N. Singh J. Chemosphere. 2005;58:141–147. doi: 10.1016/j.chemosphere.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Bley H. Gianello C. Santos L. d. S. Selau L. P. R. Rev. Bras. Cienc. Solo. 2017;41:e0160142. [Google Scholar]
- Katiyar D. Hemantaranjan A. Singh B. Indian J. Plant Physiol. 2015;20:1–9. [Google Scholar]
- Oladosu Y. Rafii M. Y. Arolu F. Chukwu S. C. Salisu M. A. Fagbohun I. K. Muftaudeen T. K. Swaray S. Haliru B. S. Horticulturae. 2022;8:605. [Google Scholar]
- Terry L. A. Joyce D. C. Postharvest Biol. Technol. 2004;32:1–13. [Google Scholar]
- Rudzinski W. E. Dave A. M. Vaishnav U. H. Kumbar S. G. Kulkarni A. R. Aminabhavi T. Des. Monomers Polym. 2002;5:39–65. [Google Scholar]
- Chen Y.-C. Chen Y.-H. Sci. Total Environ. 2019;655:958–967. doi: 10.1016/j.scitotenv.2018.11.264. [DOI] [PubMed] [Google Scholar]
- Zheng L. Seidi F. Wu W. Pan Y. Xiao H. Int. J. Biol. Macromol. 2023;235:123701. doi: 10.1016/j.ijbiomac.2023.123701. [DOI] [PubMed] [Google Scholar]
- Yang W. Qu Z. Feng G. Yang Y. Song R. Chil. J. Agric. Res. 2023;83:94–106. [Google Scholar]
- Sun D. Hussain H. I. Yi Z. Siegele R. Cresswell T. Kong L. Cahill D. M. Plant Cell Rep. 2014;33:1389–1402. doi: 10.1007/s00299-014-1624-5. [DOI] [PubMed] [Google Scholar]
- Jakhar A. M. Aziz I. Kaleri A. R. Hasnain M. Haider G. Ma J. Abideen Z. NanoImpact. 2022;27:100411. doi: 10.1016/j.impact.2022.100411. [DOI] [PubMed] [Google Scholar]
- Gebre M. G. Earl H. J. Front. Plant Sci. 2021;12:581127. doi: 10.3389/fpls.2021.581127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This study did not report any data.