Abstract
Background and Objectives
Traumatic brain injury (TBI) may be a chronic condition carrying risk of future sequelae; few prospective studies examine long-term postinjury outcomes. We examined the prevalence of functional, cognitive, and psychiatric change outcomes from 1 to 7 years postinjury.
Methods
Transforming Research and Clinical Knowledge in TBI LONG (TRACK-TBI LONG) participants were prospectively enrolled within 24 hours of injury and followed up to 1 year postinjury; a subset participated in long-term follow-up from 2 to 7 years postinjury. Reliable change thresholds for the Brief Test of Adult Cognition by Telephone General Composite (cognition) and Brief Symptom Inventory (BSI)–18 (psychiatric) were derived from orthopedic trauma controls (OTCs). Multiple assessments were completed (postinjury baseline assessment and 2 or 3 visits 2–7 years postinjury) within a sample subset. Change was assessed for functional outcome (Glasgow Outcome Scale–Extended [GOSE]) and self-report/informant report of decline. Prevalence ratios for outcomes classified as stable, improved, and declined were reported individually and collectively. The Fisher exact test and log-binomial regression models examined factors associated with decline and improvement.
Results
Of the sample (N = 1,264; mild TBI [mTBI], Glasgow Coma Scale [GCS] 13–15, n = 917; moderate-to-severe TBI [msTBI], GCS 3–12, n = 193; or OTC n = 154), “stable” was the most prevalent outcome. Functional outcome showed the highest rates of decline, regardless of TBI severity (mild = 29%; moderate/severe = 23%). When measures were collectively considered, rates of decline included mTBI (21%), msTBI (26%), and OTC (15%). Age and preinjury employment status were associated with functional decline (per 10 years; relative risk [RR] 1.16, 95% CI 1.07–1.25, p < 0.001; higher in retired/disabled/not working vs full-time/part-time; RR 1.81, 95% CI 1.33–2.45, respectively) in the mTBI group. Improvement in functional recovery 2–7 years postinjury was associated with higher BSI scores (per 5 points; RR 1.11, 95% CI 1.04–1.18, p = 0.002) and GOSE score of 5–7 (GOSE = 8 as reference; RR 2.64, 95% CI 1.75–3.97, p < 0.001). Higher BSI scores and identifying as Black (RR 2.28, 95% CI 1.59–3.25, p < 0.001) were associated with a greater likelihood of improved psychiatric symptoms in mTBI (RR 1.21, 95% CI 1.14–1.29, p < 0.001). A greater likelihood of cognitive improvement was observed among those with higher educational attainment in msTBI (per 4 years; RR 2.61, 95% CI 1.43–4.79, p = 0.002).
Discussion
Function across domains at 1-year postinjury, a common recovery benchmark, undergoes change across the subsequent 6 years. Results support consideration of TBI as a chronic evolving condition and suggest continued monitoring, rehabilitation, and support is required to optimize long-term independence and quality of life.
Introduction
Traumatic brain injury (TBI) has traditionally been considered a discrete medical event, which produces injury-related, time-limited sequelae that improve over a period of recovery and remain static thereafter.1,2 This perspective has undergone a gradual paradigm shift to a more recent appreciation that it may represent a dynamic condition with evolving processes that can manifest well after the injury and initial period of recovery.3-6 With growing evidence that TBI represents a chronic process that involves ongoing interactions between neurologic and non-neurologic pathologic processes,4,7,8 it is critical to gain a better understanding of changes in function across multiple domains (cognitive, psychiatric, and functional outcomes) beyond the conventionally assessed period of injury recovery and to identify factors that influence these long-term changes.
Although TBI is associated with an increased incidence of neurologic disease, non-neurologic disease, and mortality,4,7,8 not all who experience TBI will inevitability experience longer-term decline after injury. Preliminary evidence from the Traumatic Brain Injury Model Systems suggests that individuals who received inpatient rehabilitation for TBI experience varied courses of change in functional independence between 1 year and 5 years postinjury, with substantial subgroups improving, remaining stable, or deteriorating.9 As part of a follow-up study in the same cohort at 10 years postinjury, trends in change were not congruent across outcome domains, with only 53.5% reporting consistent direction of change across the various domains of function assessed. Taken together, this emphasizes the importance of investigating individual patterns of change and simultaneously considering varied domains of function when assessing long-term outcomes beyond 1-year post-TBI.
Based on the conventional notion that recovery stabilizes and remains static beyond 6 months or a year, previous studies of TBI were designed to cease follow-up at these early time points, resulting in limited data on longer-term outcomes. Within the multicenter Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) cohort of level 1 trauma center patients, notable rates of persistent functional limitations were recorded across the spectrum of TBI (mild to severe) at 1-year postinjury.10,11 Investigating outcomes beyond 1-year postinjury in this large-scale, prospective sample provides an opportunity for a greater understanding of ongoing needs in this population, including understanding the prevalence of decline that would indicate a need to initiate new clinical services and aid in further refining comprehensive models of monitoring, assessment, and long-term support across the life span.12
The objectives of this study were to: (1) examine the prevalence and patterns of change (stable, improved, and declined) in cognitive, psychiatric, and functional outcomes from a postinjury baseline assessment (i.e., Brief Test of Adult Cognition by Telephone [BTACT] at 6 months and other measures at 1-year postinjury) to 7-year postinjury, annually, across TBI severity; (2) investigate the dynamic nature of change from baseline to 7-year postinjury over multiple annual assessments (i.e., consistency of change; within-measure across time point); and (3) identify which sociodemographic, injury, situation, and medical comorbidity factors influence long-term decline and improvement. We anticipated that long-term change would occur in all directions, be adversely influenced by age, insurance status, and the presence of comorbid health conditions.
Methods
Study Design and Participants
The TRACK-TBI prospectively recruited participants between February 26, 2014, and July 27, 2018, within 24 hours of injury at 18 level 1 trauma centers in the United States. Once enrolled, follow-up assessments were conducted at 2 weeks and at 3, 6, and 12 months postinjury. Inclusion criteria for the TBI group were clinically suspected TBI (head CT scan ordered by treating clinician) and confirmation of TBI (at least 1 of the following present: objective evidence brain injury [e.g., positive head CT] and evidence of altered consciousness consistent with the American Congress of Rehabilitation Medicine definition of TBI).13 Inclusion criteria for orthopedic trauma controls (OTCs) were presentation for orthopedic injuries and no signs of head trauma or TBI. Exclusions for all participants were nonsurvivable physical trauma, current pregnancy, being in police custody, a history of debilitating mental health disorder or neurologic disease, and non-English or Spanish speaking.
For this study (i.e., TRACK-TBI LONG), participants from the initial TRACK-TBI study were considered eligible if they were not known to have died by 1 year postinjury, were at least 17 years of age, and had a known admission Glasgow Coma Scale (GCS) score. Long-term follow-up assessments by phone began on January 4, 2019, for participants who were 2 or more years postinjury. Participants were required to have completed a postinjury baseline (cognitive assessment at 6 months and/or functional and psychiatric outcomes at 12 months postinjury) and at least 1 long-term follow-up assessment between 2 and 7 years postinjury. Follow-up studies were conducted on an anniversary of ±3 months of injury. A summary of those who were eligible for the current TRACK-LONG follow-up study from the parent TRACK-TBI study according to follow-up appointments by year postinjury and number followed up is summarized in eTable 1 (links.lww.com/WNL/C910). Those with GCS scores of 13–15 were classified as having mild TBI (mTBI) and those with GCS scores of 3–12 were classified as having moderate-to-severe TBI (msTBI). Consistent with several injury severity criteria, participants with GCS scores between 13 and 15 with positive intracranial findings on CT were classified as having an mTBI.14
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the institutional review board of each enrolling institution. Participants or their legally authorized representatives completed written informed consent.
Outcome Measures
Brief Test of Adult Cognition by Telephone
The BTACT is a neurocognitive measure that primarily assesses memory and executive functions.15,16 Scores on 6 subtests are standardized as z scores based on age-corrected and education-corrected normative data and averaged across the 6 subtests to form the BTACT Composite.17,18 Reliable change thresholds for the BTACT Composite were derived from the distribution of change among the OTC group between 6 months and any follow-up visit within 7-year postinjury and based on the 10th and 90th percentiles of change in the OTC group. This approach yielded the following thresholds for reliable change: −1.3 z (decline) and +0.8 z (improvement).
Glasgow Outcome Scale–Extended
The Glasgow Outcome Scale–Extended (GOSE) measures disruptions to various aspects of functional outcome due to traumatic injuries based on participant (or informant) report and interviewer observation.19,20 For this study, we included functional limitations due to TBI and concurrent peripheral injuries. Total scores on the GOSE range from 1 to 8 and reflect broad qualitatively different outcomes. An increase or decrease of 1 point in the total GOSE score was considered as the threshold for change in this study.
Brief Symptom Inventory–18
The Brief Symptom Inventory (BSI)–18 measures symptoms of psychological distress across 3 dimensions—anxiety, depression, and somatization.21 Eighteen symptoms are rated on a 5-point Likert scale (0 = none at all to 4 = extremely) and summed to yield a Global Severity Index (BSI-GSI) (range 0–72). Eighty percent reliable change thresholds for the BSI-GSI (i.e., 10th and 90th percentiles of the OTC distribution derived from assessments between 12 months and any follow-up visit through 7-year postinjury) were established as ±11 points.
Self-reported Perceptions of Function Interview
An interview was introduced for the 2- to 7-year postinjury assessments that inquired about self-reported and informant-reported perceptions of decline across various domains. Questions assessed the perspective of both participants and informants about participant function in the following areas: new-onset parkinsonism, mobility/walking, new-onset cognitive difficulties or diagnoses, completion of functional daily activities, physical function, and emotional function (eTable 2, links.lww.com/WNL/C910). Due to some variability in the direction of change across response items (i.e., some items allow for endorsement of improvement where others provide options for decline only), individual items were coded as “declined” and “not declined”; participants were considered declined if self-rating or informant rating reflected decline in any domain.
Statistical Analysis
Characteristics of the subsamples with vs without outcome data available for this study were compared with Mann-Whitney U tests and Fisher exact tests. Inverse probability weighting was conducted to reflect the characteristics of the full sample meeting inclusion criteria (eTable 3, links.lww.com/WNL/C910). Weighting was based on a boosted regression algorithm that estimates participant propensity for having completed any of the year 2 to year 7 primary outcome assessments during the 7-year postinjury study duration. Statistical weights used in the analysis were inverted based on the propensity estimates and included in all analyses to reduce the influence of bias due to confounding factors, such as inability to complete a measure due to greater levels of impairment.
To complete the first study objective, prevalence estimates of change (stable, improved, and declined) were calculated for each measure individually from postinjury baseline assessment to each long-term follow-up at 2–7 years postinjury by dividing the number of those who exceeded change thresholds for that measure in the given direction by the total number of participants assessed. Global patterns of change across measures within time points were also calculated for the 3 outcomes classifiable in all directions (stable, improved, and declined; i.e., GOSE, BSI, and BTACT). Specifically, participants were classified as globally “stable” if exhibiting no change on any of the measures, “improved” if improved on at least 1 measure and stable on the others, and “declined” if declined on at least 1 measure and stable across all measures within a time point. Participants were characterized as “variable” if a discrepant pattern was recorded (e.g., improved on the BSI-18, declined on the BTACT). The prevalence of each category per year calculated represents rate of change from the postinjury baseline assessment to that specific year and are not cumulative (i.e., each year from 2 to 7 years is independent). The average rate of decline from postinjury baseline assessment to each year 2–7 years postinjury was also calculated and presented as “all.” Those who died over the course of the study were classified as declined on GOSE and interview at the single subsequent assessment period. Because select aspects of the subjective perceptions of the function interview inquire about decline only, this outcome was not included as part of the within-time across measure classification because it would bias the direction of classification because it does not contain the option “improve.”
For study objective 2, multiple long-term assessments were completed (n = 571; n = 225 with postinjury baseline assessment and 2 visits 2–7 years postinjury; n = 346 with postinjury baseline assessment and 3 visits 2–7 years postinjury) within a subset of the sample. For within measure, across time point analyses, prevalence rates of change for the 3 primary outcome measures (BTACT, GOSE, and BSI) were calculated separately based on the following classifications: “stable” if change not recorded at any point; “improved” if improvement recorded across any follow-up assessment with no decline; “declined” if decline recorded across any follow-up assessment with no improvement; and “variable” if both improvement and decline recorded at follow-up assessments (all possible patterns of change are summarized in eTables 4 and 5, links.lww.com/WNL/C910). Only those with complete data and having the same measure completed over multiple time points recorded were included, and list-wise deletion of participants due to missing information (i.e., completed 2 or 3 visits, but not a particular measure) occurred at less than a 5% rate. Given that select aspects of the subjective perceptions of function interview inquire specifically about decline only, change on this measure was classified as either “declined” if decline was reported at any follow-up or “not declined” if no decline was reported.
For study objective 3, the Fisher exact test for categorical variables and log-binomial regression models were performed to examine the influence of patient-specific factors on decline (vs stable/improved) and improvement (vs stable/declined). Factors of interest included age, sex, race, insurance type, education, employment status, living situation, injury characteristics, aspects of medical history, all representing status at the time of injury. In addition, associations between functional outcome (GOSE score) and psychiatric status (BSI score) at 2 weeks postinjury with long-term decline and improvement were also examined. Associated risk ratios and CIs were calculated for each of the tests. These analyses were conducted within the subset of the sample with multiple 2- to 7-year time points to focus on predicting robust (consistent) patterns of change. Statistical significance for logistic regression analyses was evaluated at the 0.05 level (2-tailed), with a 5% false discovery rate computed for consideration of multiple comparisons.22 Boosted regression modeling was completed using the Windows version of the TWANG Shiny App software package developed by RAND Corporation23 and SAS statistical software, version 9.4 (SAS Institute, Cary, NC). Data that support the findings of this study are available from the corresponding author on reasonable request.
Data Availability
Investigators interested in the long-term outcome data from the TRACK-TBI LONG study can submit a Data Collaboration Request to the TRACK-TBI Executive Committee through the following website (tracktbi.ucsf.edu/collaboration-opportunities).
Results
Sample Characteristics
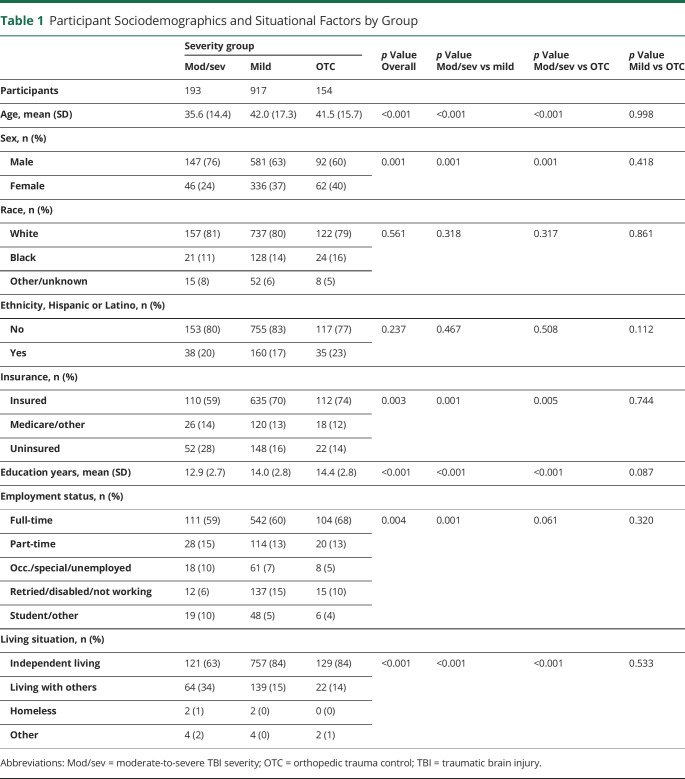
Of the eligible participants with TBI from the TRACK-TBI acute injury study (N = 2,996), the primary analyses focused on 1,264 participants (917 mTBI, 193 msTBI; 154 OTCs for reliable change calculations) who met inclusion criteria (eFigure 1, links.lww.com/WNL/C910). eTable 3 summarizes the characteristics of the subsamples who met (n = 1,264) vs those who did not meet (n = 1,397) inclusion criteria and sample differences before and after weighting. For those who met inclusion criteria, the mean age at injury was 41.0 (SD = 16.8) years; most of the sample identified as male (65%), White (80%), and non-Hispanic (81%). Statistically significant differences between the mTBI, msTBI, and OTC analysis groups were observed across sociodemographic (e.g., race, employment status, and living situation), medical history (e.g., prior TBI, cardiovascular disease), and injury factors (e.g., cause of injury; Table 1 and eTable 6).
Table 1.
Participant Sociodemographics and Situational Factors by Group
Rates of Change From Postinjury Baseline Assessment to Each Long-term Follow-Up
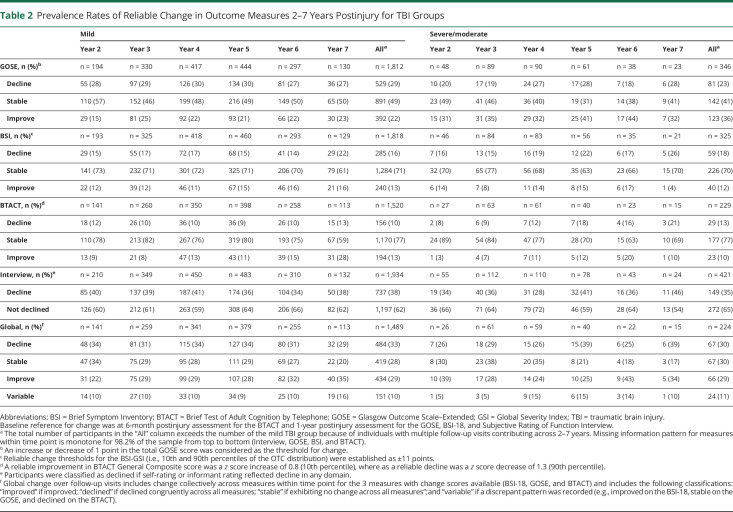
Prevalence rates of change (stable, improved, and declined) for individual outcomes at each long-term follow-up are summarized in Table 2 and eTable 7 (links.lww.com/WNL/C910). In general, “stable” was the most frequent change outcome for individual measures from the postinjury baseline assessment to 7 years postinjury, and rates of change were broadly comparable across year of follow-up. The highest rate of decline across all follow-up years was for functional outcome (GOSE ranged from 18% to 30%). Although direct year-to-year comparisons would not be appropriate due to differences in sample composition across years, a general trend toward higher rates of cognitive decline was observed further from injury up to 7-year postinjury for the msTBI group (8%–21%). When compared with the msTBI group (i.e., GOSE; 23% overall decline), there was generally a slightly higher rate of decline in functional outcome among the mTBI group (29% overall) when aggregated across years. Conversely, higher rates of improvement in functional outcome were observed for the msTBI group (36%) compared with those observed for the mTBI group (22%). Rates of decline across functional outcome, cognition, and psychiatric symptoms were higher for mTBI and msTBI groups compared with OTCs across all domains. For the self/informant interview, in which declined vs not declined was recorded, the rate of decline ranged from 28% to 46% of the sample across years and TBI severity. Aggregate (across 2–7 years) rates of decline for mTBI (38%) and msTBI (35%) were notably higher compared with those for OTCs (23%) (Table 2 and eTable 7).
Table 2.
Prevalence Rates of Reliable Change in Outcome Measures 2–7 Years Postinjury for TBI Groups
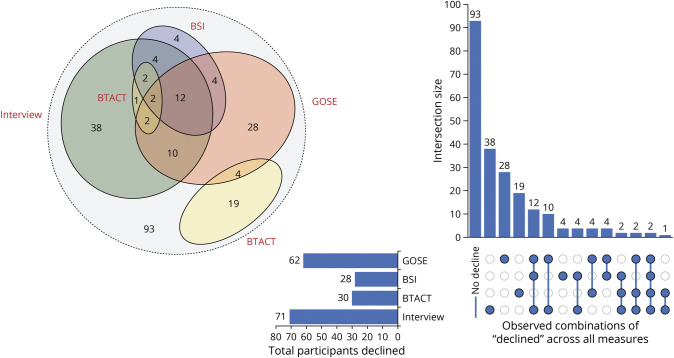
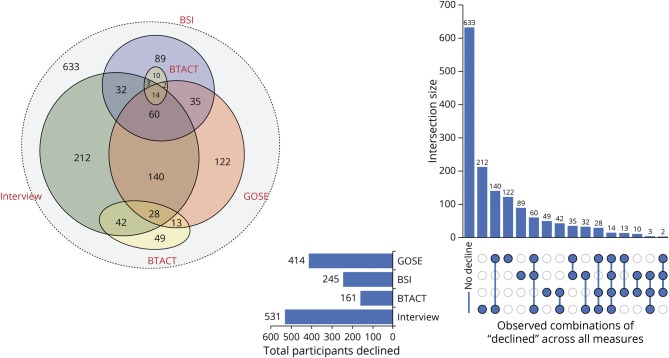
When the 3 primary measures were simultaneously considered (BTACT, GOSE, and BSI) within the same follow-up, rates of improved, declined, and stable outcomes were broadly comparable within and between mTBI and msTBI groups. The aggregate rates of declined outcome were slightly lower among the msTBI group (30%) when compared with those the mTBI group (33%), and both were higher compared with those among OTCs (20%). A gradual incremental decline in stability for all measures collectively was observed for both mTBI (34%–29%) and msTBI groups (30%–17%) from 2 to 7 years. This pattern indicates that the longer the postinjury the less likely individuals are to experience a static and established clinical outcome, regardless of TBI severity. A relatively small percentage of participants with TBI (5%–16%) exhibited variable change outcome across measures within time point, in which one area declined and another measure improved (e.g., cognitive functions improve while psychiatric symptoms worsen; Figures 1 and 2).
Figure 1. Area-Proportional Venn Diagram and Upset Plot Displaying Areas of Decline Across Potential Combinations in the Moderate-to-Severe TBI Group Aggregated Across Postinjury Years 2–7.
BSI = Brief Symptom Inventory; BTACT = Brief Test of Adult Cognition by Telephone; GOSE = Glasgow Outcome Scale–Extended; TBI = traumatic brain injury.
Figure 2. Area-Proportional Venn Diagram and Upset Plot Displaying Areas of Decline Across Potential Combinations in the Mild TBI Group Aggregated Across Postinjury Years 2–7.
BSI = Brief Symptom Inventory; BTACT = Brief Test of Adult Cognition by Telephone; GOSE = Glasgow Outcome Scale–Extended; TBI = traumatic brain injury.
Rates of Change for Each Outcome From the Postinjury Baseline Assessment to 7-Year Postinjury
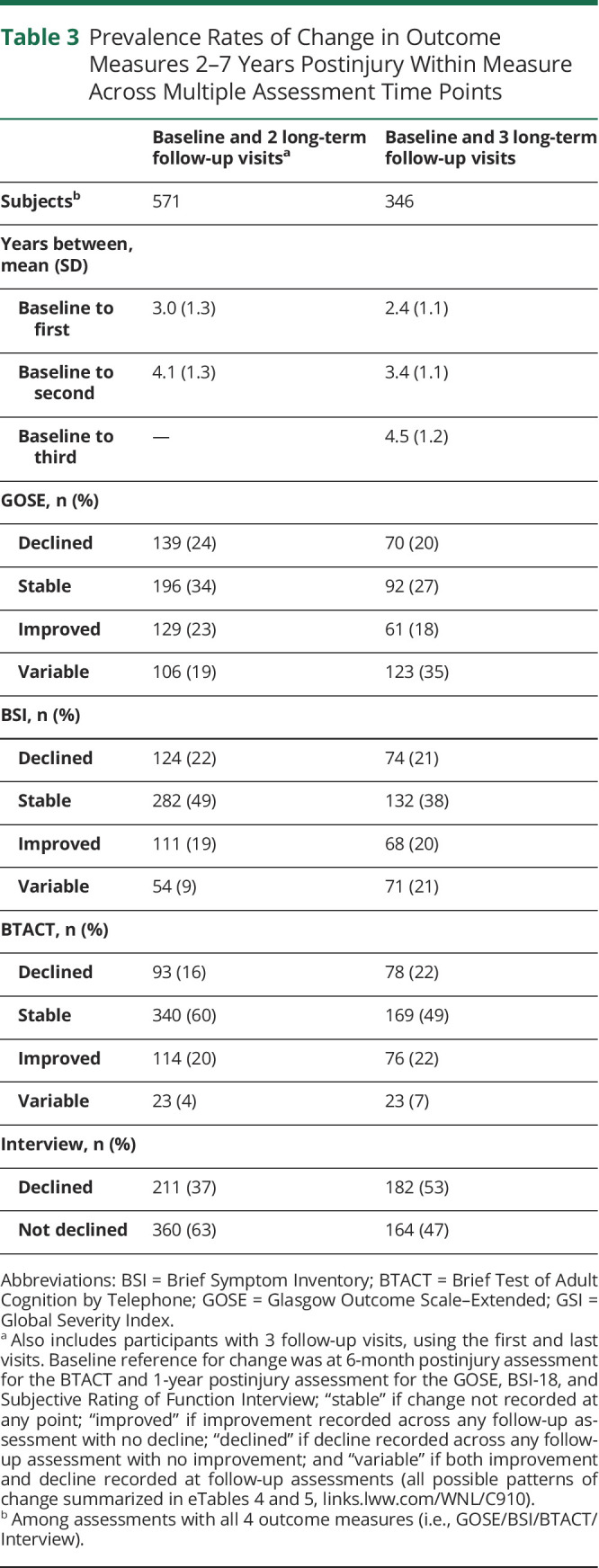
In contrast with that listed in Table 2, which characterizes patterns of change from postinjury baseline assessment to each individual long-term follow-up, Table 3 summarizes the rates of longitudinal patterns of change over time for each individual outcome. Similar to the data summarized earlier, the outcome with the greatest prevalence of consistent decline was interview-reported function (i.e., 36%–51%; Table 3). The rates of improved and declined across all domains of function were generally consistent between those with 2 or 3 follow-ups. Compared with those with 2 visits (postinjury baseline assessment and a single visit 2–7 postinjury assessment), a slight increase in the prevalence of interview-reported functional decline was observed with greater number of assessments (postinjury baseline assessment and 2 or 3 follow-ups).
Table 3.
Prevalence Rates of Change in Outcome Measures 2–7 Years Postinjury Within Measure Across Multiple Assessment Time Points
Factors Influencing Decline and Improvement From Baseline Postinjury Assessment to 7-Year Postinjury
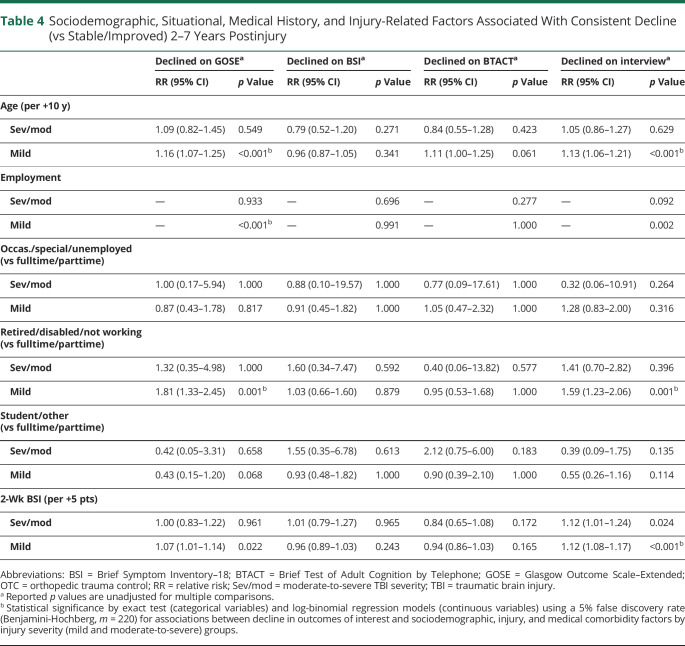
After correction for a 5% false discovery rate with multiple comparisons, decline in the level of functional independence was significantly associated with age (per 10 years; relative risk [RR] 1.16, 95% CI 1.07–1.25, p < 0.001) and preinjury employment status (higher in retired/disabled/not working; RR 1.81, 95% CI 1.33–2.45, p < 0.001; Table 4 and eTable 8, links.lww.com/WNL/C910) in the mTBI group. Self-reported and informant-reported decline were also significantly associated with age (RR 1.13, 95% CI 1.06–1.21, p < 0.001) and preinjury employment status (higher in retired/disabled/not working; RR 1.59, 95% CI 1.23–2.06, p < 0.001). Higher levels of psychiatric symptomology at 2-week postinjury was associated with a greater risk of self-reported and informant-reported decline across 2–7 years postinjury in the mTBI group (per increase in 5 points on the BSI at 2 weeks postinjury; RR 1.12, 95% CI 1.08–1.17, p < 0.001). Sociodemographic, injury, situational, and medical comorbidity factors were not significantly associated with a greater risk of decline in any outcome area for the msTBI group after correction for multiple comparisons.
Table 4.
Sociodemographic, Situational, Medical History, and Injury-Related Factors Associated With Consistent Decline (vs Stable/Improved) 2–7 Years Postinjury
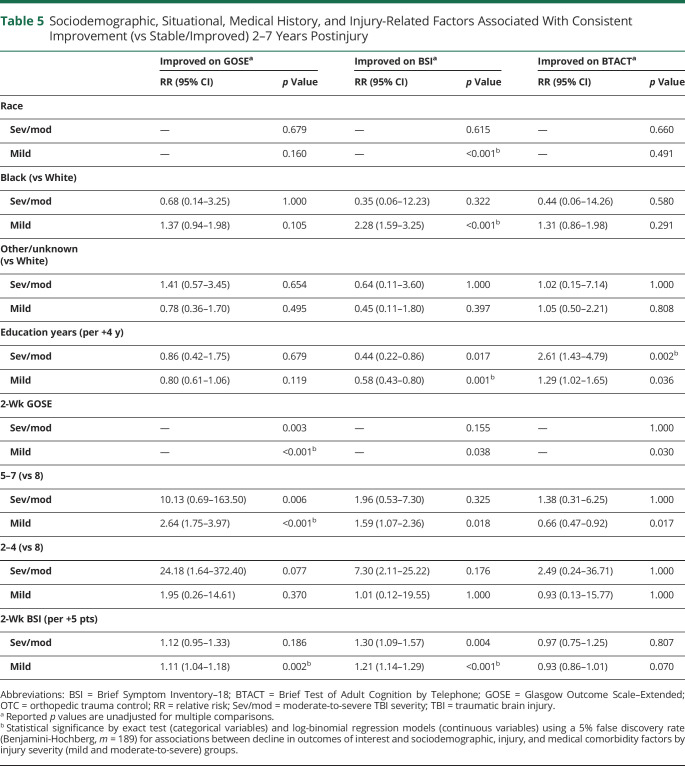
Within the mTBI group, a greater likelihood of improvement in functional outcome over 2–7 years postinjury was associated with having greater functional limitations at 2 weeks (Table 5 and eTable 9, links.lww.com/WNL/C910). Specifically, compared with those with a GOSE score of 8, a greater increase in GOSE scores over 2–7 years postinjury were observed among those with 2-week GOSE scores of 5–7 (RR 2.64, 95% CI 1.75–3.97, p < 0.001), but not 2-week GOSE scores of 2–4 (RR 1.95, 95% CI 0.26–14.61, p = 0.370). Similarly, among the mTBI group, higher BSI scores at 2 weeks were significantly associated with an increased likelihood of improvement in GOSE scores between years 2–7 postinjury (RR 1.11, 95% CI 1.04–1.18, p = 0.002). Among the mTBI group, a greater likelihood of improved BSI scores between 2 and 7 years postinjury was associated with identifying as Black (compared with identifying as White; RR 2.28, 95% CI 1.59–3.25, p < 0.001) and endorsing higher psychiatric symptoms (i.e., higher BSI scores) at 2 weeks postinjury (RR 1.21, 95% CI 1.14–1.29, p < 0.001). Conversely, higher education was associated with a decreased likelihood of improvement in psychiatric symptomology between 2 and 7 years postinjury (RR 0.58, 95% CI 0.43–0.80, p = 0.001) in the mTBI group. Among those who sustained a moderate/severe TBI, higher education was associated with a greater likelihood of cognitive improvement on the BTACT between 2 and 7 years postinjury (per 4 years of education; RR 2.61, 95% CI 1.43–4.79, p = 0.002).
Table 5.
Sociodemographic, Situational, Medical History, and Injury-Related Factors Associated With Consistent Improvement (vs Stable/Improved) 2–7 Years Postinjury
Discussion
In this prospective longitudinal study of US level 1 trauma center patients, reliable change was observed for both improvement and decline from the postinjury baseline assessment (i.e., cognition at 6 months and other measures at 1-year postinjury) out to 2–7 years post-TBI across multiple domains of function. Regardless of TBI severity, long-term follow-up evaluation at different times postinjury indicate that individuals continue to experience changes in psychiatric status, cognition, and functional outcomes; furthermore, select patient-specific characteristics (age and employment status) were associated with a higher late decline, particularly among those who experienced mTBI. Continued decline through 7 years postinjury was notably higher in the TBI group (32%) compared with OTCs (20%) when collectively considering multiple domains of function. An increased likelihood of improvement 2–7 years postinjury was associated with worse functional outcome and psychiatric status subacutely postinjury, indicating opportunity for further recovery and resolution of sequelae beyond 1 year. Taken together, these findings support conceptualizing TBI as a chronic condition for many patients, which requires routine follow-up, medical monitoring, responsive care, and support, adapting to their evolving needs many years after injury.
In this study, the lowest degree of stability was observed in the level of functional outcome as assessed by the GOSE, with approximately half of the sample experiencing either improvement or decline, regardless of the length of time postinjury at follow-up. Those in the mTBI group showed slightly higher rates of decline compared with those with msTBI, likely due to the msTBI group having lower levels of function at 1 year, leading to floor effects.10,11 However, these findings suggest that a nontrivial proportion of those with a “mild” injury (28% compared with OTC 17%) require continued follow-up and potentially modified support (longer-term rehabilitation and community services/resources) several years postinjury to address new-onset functional limitations.
Examination of trends among those who completed long term follow-up visits between 2 and 7 years postinjury revealed significant and notable trends in race, ethnicity, sex, and education. Factors such as identifying as Black, Hispanic, male, nonprivate health insurance, or lower educational attainment was associated with a greater likelihood of being lost to follow-up from the original acute injury and recovery period to long-term assessment. These trends are generally consistent with findings from a prior systemic review, which also showed associations between similar factors and various aspects of healthcare utilization after TBI.24 While this study attempted to account for bias associated with differences in study retention using inverse probability weighting, the above noted patient factors reflect target areas for prioritization to mitigate barriers to equitable TBI research representation and healthcare delivery.
Rates of stability and change reported in this study differ to some extent from those reported in the TBI-Model Systems (TBIMS) study. Specifically, the TBIMS sample displayed much higher rates of stable functional outcome (approximately 76%) from 1 to 5 years postinjury than the present sample. In addition, we reported generally comparable rates of improvement and decline, whereas the TBIMS study has consistently reported higher levels of improvement than decline.9,25,26 Differences in these rates may reflect variations in the methods used to measure functional independence, characterize change, cohort composition (i.e., more patients with msTBI in the TBIMS), and/or approach to enrollment. Specifically, this study prospectively enrolled patients within 24 hours of presentation to a level 1 trauma center irrespective of whether or not they subsequently received follow-up or rehabilitation services, when compared with the TBIMS study, which only enrolled participants receiving inpatient rehabilitation. Future studies are needed to investigate the potential moderating influence of rehabilitation services, particularly within a level 1 trauma center population.
Prior studies have also shown that cognitive difficulties and psychiatric symptomology are commonly associated with and may exacerbate functional limitations after TBI.8,27,28 In this study, functional decline was the most prevalent outcome displaying change and frequently occurred in the absence of reliable change in cognitive and psychiatric functioning. This pattern may reflect differences in demarcating change across measures (reliable change scores were only available for BTACT and BSI) or that factors independent of cognitive and psychiatric decline can also adversely affect functional independence. In contrast with previous findings,8,28-31 this study did not observed greater rates of decline among those with a history of comorbid health conditions, and future studies are needed to identify factors not currently considered that may influence worse longer-term outcomes after TBI.
Within this study, age and employment status were selectively associated with decline in functional independence for persons with mTBI. Older adults are at greater risk of TBI and worse outcomes (i.e., functional outcome) after injury compared with those at younger ages.32-35 This study adds to these previous findings, indicating that older adults, particularly those not working at the time of injury, are at higher risk of long-term functional decline in the first 7 years postinjury and may require closer monitoring and community support. A number of other potential risk factors, such as history of psychiatric disorder, cardiovascular disease, headache disorder, and prior TBI had unadjusted p values of <0.05 for various outcome domains (Table 5), but did not survive correction for multiple comparisons. Further investigations into these individual risk factors with greater detail is required to understand their potential incremental contribution to risk of late decline.
Among those with mTBI, worse functional outcome and psychiatric status at 2 weeks postinjury was associated with an increased likelihood of improvement in these domains 2–7 years postinjury. The observed association also indicates that the prospect of continued functional improvement and symptom resolution is possible and greater among those who are experiencing higher levels of postinjury sequelae during the subacute to 1-year postinjury window of recovery. Persistent functional limitations and symptom endorsement up to 1 year postinjury among patients with mTBI have been reported.10,36,37 Current findings underscore the importance of continued intervention and counseling patients that continued resolution of difficulties between 2 and 7 years postinjury can occur, particularly among those experiencing the greatest levels of disruption during the subacute to later stages of recovery.
Relatedly, a higher likelihood of improvement in psychiatric symptoms 2–7 years postinjury was observed among individuals identifying as Black. Given prior investigations within this cohort have reported greater sleep-related and trauma-related symptomology among Black identifying individuals from the subacute to 1-year recovery period,38,39 the increased likelihood of improvement between 2 and 7 years may also reflect the trend of later symptom resolution among those with higher levels of initial difficulties. Among those with moderate-to-severe TBI, a higher likelihood of meaningful cognitive improvement was observed among those with a history of higher educational attainment. While also correlated with a number of health behaviors and outcomes,40 educational attainment is commonly regarded as a proxy for cognitive reserve in other clinical populations (e.g., Alzheimer disease and related dementias, stroke), consistent with the current findings in patients with TBI.41,42
While the study design did not allow for an annual follow-up of every participant enrolled prospectively in the parent TRACK-TBI protocol, inverse probability weighting was used to reduce bias that may have occurred between the final sample included in the analysis and those who were not followed up to ultimately approximate results for the entire sample of interest. Initial inclusion for the TRACK-TBI study involved presenting to a level 1 trauma center within 24 hours, and as such, the generalizability of these findings from the current long-term follow-up study to other populations is unclear. The benchmark for reliable change for BSI and BTACT was defined by the 10th/90th percentile of OTCs; because some of these individuals had long-lasting impairments due to injury, this may have contributed to underestimating the prevalence of change when compared with using a general community/noninjured control sample. Relatedly, peripheral injury sources of disruptions to functional ratings on the GOSE and perceptions of function interview are not able to be differentiated from TBI-related contributions. Relatively lower rates of decline observed for cognitive function may be because baseline cognition was measured at 6 months and those with more severe injury may have been still experiencing recovery in this domain; however, a lower rate of cognitive decline would be consistent with a smaller prospective study of long-term cognitive changes after moderate-to-severe TBI.43
Within this study of patients with TBI with a wide range of severities prospectively enrolled at level 1 trauma centers within 24 hours of injury and followed up for 2–7 years, results dispute the notion that TBI is a discrete isolated medical event with a finite static functional outcome after a relatively short period of upward recovery. Rather, individuals continue to exhibit improvement and decline across a range of domains. These findings highlight the need to embrace conceptualization of TBI as a chronic condition to establish systems of care that provide continued follow-up and monitoring with treatment and supports that adapt to evolving patient needs regardless of the directions of change, placing greater emphasis on functional independence with improvement and implement greater levels of support with decline.
Glossary
- BSI
Brief Symptom Inventory
- BTACT
Brief Test of Adult Cognition by Telephone
- GCS
Glasgow Coma Scale
- GOSE
Glasgow Outcome Scale–Extended
- GSI
Global Severity Index
- mTBI
mild TBI
- msTBI
moderate-to-severe TBI
- OTC
orthopedic trauma control
- RR
relative risk
- TBI
traumatic brain injury
- TBIMS
TBI-Model Systems
- TRACK-TBI
Transforming Research and Clinical Knowledge in TBI
Appendix 1. Authors
Appendix 2. Coinvestigators
Footnotes
Editorial, page 287
Study Funding
The study was funded by National Institute of Neurological Disorders and Stroke (NINDS) grant number U01 NS086090 and the National Football League (NFL) Scientific Advisory Board. The study also received funding from (CDMRP) TBI Endpoints Development (TED) Initiative (grant number W81XWH-14-2-0176). B.L. Brett's effort was also supported by National Institute on Aging grant number K23 AG073528-01 and NINDS grant number L30 NS113158-02. L. Nelson's effort was also supported by NINDS grant numberR01 NS110856. The manuscript's contents are solely the responsibility of the authors; they do not necessarily represent the official views of the NIH and are not necessarily endorsed by the US Department of Defense or NFL.
Disclosure
B.L. Brett reports grants from the National Institute on Aging and National Institute of Neurological Disorders and Stroke (NINDS). N. Temkin reported receiving grants from the US Department of Defense (DoD) and NIH/NINDS during the conduct of the study. M. Stein reported receiving grants from the NIH subcontract from University of California, San Francisco (UCSF) during the conduct of the study; other from Oxeia Biopharmaceuticals Stock options outside the submitted work. J.T. Giacino reported receiving support from the UCSF subcontract during the conduct of the study. M.A. McCrea reported receiving grants from the UCSF, Research subaward to Medical College of Wisconsin during the conduct of the study. Y.G. Bodien reported receiving grants from NINDS, DoD, James S. McDonnell Foundation, Tiny Blue Dot, and National Institute on Disability, Independent Living, and Rehabilitation Research during the conduct of the study. J. Corrigan reported receiving grants from the NIH during the conduct of the study. R. Diaz-Arrastia reported receiving grants from the NIH and DoD during the conduct of the study; owing stock in BrainBox and Nia Therapeutics; and personal fees from Pinteon Therapeutics, MesoScale Discoveries, and Ischemix outside the submitted work. L. Nelson reported receiving grants from the NINDS, Centers for Disease Control and Prevention, DoD, and Advancing a Healthier Wisconsin outside the submitted work. Go to Neurology.org/N for full disclosures.
References
- 1.Lj C, Jd C, Pm P, et al. Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J Rehabil Med. 2004;43(suppl):84-105. doi: 10.1080/16501960410023859 [DOI] [PubMed] [Google Scholar]
- 2.Pagulayan KF, Temkin NR, Machamer J, Dikmen SS. A longitudinal study of health-related quality of life after traumatic brain injury. Arch Phys Med Rehabil. 2006;87(5):611-618. doi: 10.1016/j.apmr.2006.01.018 [DOI] [PubMed] [Google Scholar]
- 3.Masel BE, DeWitt DS. Traumatic brain injury: a disease process, not an event. J Neurotrauma. 2010;27(8):1529-1540. doi: 10.1089/neu.2010.1358 [DOI] [PubMed] [Google Scholar]
- 4.Izzy S, Chen PM, Tahir Z, et al. Association of traumatic brain injury with the risk of developing chronic cardiovascular, endocrine, neurological, and psychiatric disorders. JAMA Netw Open. 2022;5(4):e229478. doi: 10.1001/jamanetworkopen.2022.9478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrigan JD, Hammond FM. Traumatic brain injury as a chronic health condition. Arch Phys Med Rehabil. 2013;94(6):1199-1201. doi: 10.1016/j.apmr.2013.01.023 [DOI] [PubMed] [Google Scholar]
- 6.McInnes K, Friesen CL, MacKenzie DE, Westwood DA, Boe SG. Mild traumatic brain injury (mTBI) and chronic cognitive impairment: a scoping review. PLoS One. 2017;12(4):e0174847. doi: 10.1371/journal.pone.0174847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrigan JD, Cuthbert JP, Harrison-Felix C, et al. US population estimates of health and social outcomes 5 years after rehabilitation for traumatic brain injury. J Head Trauma Rehabil. 2014;29(6):E1. doi: 10.1097/HTR.0000000000000020 [DOI] [PubMed] [Google Scholar]
- 8.Kumar RG, Ketchum JM, Corrigan JD, Hammond FM, Sevigny M, Dams-O'Connor K. The longitudinal effects of comorbid health burden on functional outcomes for adults with moderate to severe traumatic brain injury. J Head Trauma Rehabil. 2020;35(4):E372. doi: 10.1097/HTR.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hammond FM, Grattan KD, Sasser H, et al. Five years after traumatic brain injury: a study of individual outcomes and predictors of change in function. Neurorehabilitation. 2004;19(1):25-35. doi: 10.3233/NRE-2004-19104 [DOI] [PubMed] [Google Scholar]
- 10.Nelson LD, Temkin NR, Dikmen S, et al. Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: a transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) study. JAMA Neurol. 2019;76(9):1049-1059. doi: 10.1001/jamaneurol.2019.1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCrea MA, Giacino JT, Barber J, et al. Functional outcomes over the first year after moderate to severe traumatic brain injury in the prospective, longitudinal TRACK-TBI study. JAMA Neurol. 2021;78(8):982-992. doi: 10.1001/jamaneurol.2021.2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Traumatic Brain Injury: A Roadmap for Accelerating Progress. National Academies Press; 2022. doi: 10.17226/25394 [DOI] [PubMed] [Google Scholar]
- 13.Kay T, Harrington DE, Adams R, et al. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86-87. [Google Scholar]
- 14.Silverberg ND, Iverson GL; ACRM Mild TBI Definition Expert Consensus Group and the ACRM Brain Injury Special Interest Group Mild TBI Task Force. Expert panel survey to update the American Congress of Rehabilitation Medicine definition of mild traumatic brain injury. Arch Phys Med Rehabil. 2021;102(1):76-86. doi: 10.1016/j.apmr.2020.08.022 [DOI] [PubMed] [Google Scholar]
- 15.Hughes ML, Agrigoroaei S, Jeon M, Bruzzese M, Lachman ME. Change in cognitive performance from midlife into old age: findings from the Midlife in the United States (MIDUS) study. J Int Neuropsychol Soc. 2018;24(8):805-820. doi: 10.1017/S1355617718000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lachman ME, Agrigoroaei S, Tun PA, Weaver SL. Monitoring cognitive functioning: psychometric properties of the brief test of adult cognition by telephone. Assessment. 2014;21(4):404-417. doi: 10.1177/1073191113508807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiBlasio CA, Sima A, Kumar RG, et al. Research letter: performance of the brief test of adult cognition by telephone in a national sample. J Head Trauma Rehabil. 2021;36(4):E233. doi: 10.1097/HTR.0000000000000664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nelson LD, Barber JK, Temkin NR, et al. Validity of the brief test of adult cognition by telephone in level 1 trauma center patients six months post-traumatic brain injury: a TRACK-TBI study. J Neurotrauma. 2021;38(8):1048-1059. doi: 10.1089/neu.2020.7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the Extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573-585. doi: 10.1089/neu.1998.15.573 [DOI] [PubMed] [Google Scholar]
- 20.Wilson L, Boase K, Nelson LD, et al. A manual for the Glasgow Outcome Scale-Extended interview. J Neurotrauma. 2021;38(17):2435-2446. doi: 10.1089/neu.2020.7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derogatis L. Brief Symptom Inventory 18 (BSI-18): Administration, Scoring, and Procedures Manual. Pearson; 2001. [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289-300. [Google Scholar]
- 23.Griffin BA, Ridgeway G, Morral AR, et al. Toolkit for Weighting and Analysis of Nonequivalent Groups (TWANG). RAND Corporation; 2014. Accessed October 9, 2022 rand.org/statistics/twang. [Google Scholar]
- 24.Gao S, Kumar RG, Wisniewski SR, Fabio A. Disparities in health care utilization of adults with traumatic brain injuries are related to insurance, race, and ethnicity: a systematic review. J Head Trauma Rehabil. 2018;33(3):E40-E50. doi: 10.1097/HTR.0000000000000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond FM, Malec JF, Corrigan JD, et al. Patterns of functional change five to ten years after moderate-severe traumatic brain injury. J Neurotrauma. 2021;38(11):1526-1534. doi: 10.1089/neu.2020.7499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond FM, Perkins SM, Corrigan JD, et al. Functional change from five to fifteen years after traumatic brain injury. J Neurotrauma. 2021;38(7):858-869. doi: 10.1089/neu.2020.7287 [DOI] [PubMed] [Google Scholar]
- 27.Wilson L, Horton L, Kunzmann K, et al. Understanding the relationship between cognitive performance and function in daily life after traumatic brain injury. J Neurol Neurosurg Psychiatry. 2021;92(4):407-417. doi: 10.1136/jnnp-2020-324492 [DOI] [PubMed] [Google Scholar]
- 28.Malec JF, Ketchum JM, Hammond FM, et al. Longitudinal effects of medical comorbidities on functional outcome and life satisfaction after traumatic brain injury: an individual growth curve analysis of NIDILRR traumatic brain injury model system data. J Head Trauma Rehabil. 2019;34(5):E24. doi: 10.1097/HTR.0000000000000459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mikolić A, van Klaveren D, Groeniger JO, et al. Differences between men and women in treatment and outcome after traumatic brain injury. J Neurotrauma. 2021;38(2):235-251. doi: 10.1089/neu.2020.7228 [DOI] [PubMed] [Google Scholar]
- 30.Starkey NJ, Duffy B, Jones K, et al. Sex differences in outcomes from mild traumatic brain injury eight years post-injury. PLoS One. 2022;17(5):e0269101. doi: 10.1371/journal.pone.0269101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dams-O'Connor K, Ketchum JM, Cuthbert JP, et al. Functional outcome trajectories following inpatient rehabilitation for TBI in the United States: a NIDILRR TBIMS and CDC Interagency Collaboration. J Head Trauma Rehabil. 2020;35(2):127-139. doi: 10.1097/HTR.0000000000000484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mosenthal AC, Lavery RF, Addis M, et al. Isolated traumatic brain injury: age is an independent predictor of mortality and early outcome. J Trauma Acute Care Surg. 2002;52(5):907-911. [DOI] [PubMed] [Google Scholar]
- 33.Susman M, DiRusso SM, Sullivan T, et al. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J Trauma Acute Care Surg. 2002;53(2):219-224. [DOI] [PubMed] [Google Scholar]
- 34.Gardner RC, Dams-O'Connor K, Morrissey MR, Manley GT. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma. 2018;35(7):889-906. doi: 10.1089/neu.2017.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Vlegel M, Mikolić A, Lee Hee Q, et al. Health care utilization and outcomes in older adults after traumatic brain injury: a CENTER-TBI study. Injury. 2022;53(8):2774-2782. doi: 10.1016/j.injury.2022.05.009 [DOI] [PubMed] [Google Scholar]
- 36.Theadom A, Parag V, Dowell T, et al. Persistent problems 1 year after mild traumatic brain injury: a longitudinal population study in New Zealand. Br J Gen Pract. 2016;66(642):e16-e23. doi: 10.3399/bjgp16X683161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Losoi H, Silverberg ND, Wäljas M, et al. Recovery from mild traumatic brain injury in previously healthy adults. J Neurotrauma. 2016;33(8):766-776. doi: 10.1089/neu.2015.4070 [DOI] [PubMed] [Google Scholar]
- 38.Wickwire EM, Albrecht JS, Capaldi VF II, et al. Trajectories of insomnia in adults after traumatic brain injury. JAMA Netw Open. 2022;5(1):e2145310. doi: 10.1001/jamanetworkopen.2021.45310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein MB, Jain S, Giacino JT, et al. Risk of posttraumatic stress disorder and major depression in civilian patients after mild traumatic brain injury: a TRACK-TBI study. JAMA Psychiatry. 2019;76(3):249-258. doi: 10.1001/jamapsychiatry.2018.4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zajacova A, Lawrence EM. The relationship between education and health: reducing disparities through a contextual approach. Annu Rev Public Health. 2018;39:273-289. doi: 10.1146/annurev-publhealth-031816-044628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shin M, Sohn MK, Lee J, et al. Effect of cognitive reserve on risk of cognitive impairment and recovery after stroke. Stroke. 2020;51(1):99-107. doi: 10.1161/STROKEAHA.119.026829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thow ME, Summers MJ, Saunders NL, Summers JJ, Ritchie K, Vickers JC. Further education improves cognitive reserve and triggers improvement in selective cognitive functions in older adults: the Tasmanian Healthy Brain Project. Alzheimers Dement. 2018;10:22-30. doi: 10.1016/j.dadm.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hicks AJ, Spitz G, Rowe CC, Roberts CM, McKenzie DP, Ponsford JL. Does cognitive decline occur decades after moderate to severe traumatic brain injury? A prospective controlled study. Neuropsychol Rehabil. 2022;32(7):1530-1549. doi: 10.1080/09602011.2021.1914674 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Investigators interested in the long-term outcome data from the TRACK-TBI LONG study can submit a Data Collaboration Request to the TRACK-TBI Executive Committee through the following website (tracktbi.ucsf.edu/collaboration-opportunities).