Abstract
Background and Objectives
The objective of this study was to propose a clustering approach to identify migraine subgroups and test the clinical usefulness of the approach by providing prognostic information for electroacupuncture treatment selection.
Methods
Participants with migraine without aura (MWoA) were asked to complete a daily headache diary, self-rating depression and anxiety, and quality-of-life questionnaires. Whole-brain functional connectivities (FCs) were assessed on resting-state functional MRI (fMRI). By integrating clinical measurements and fMRI data, partial least squares correlation and hierarchical clustering analysis were used to cluster participants with MWoA. Multivariate pattern analysis was applied to validate the proposed subgrouping strategy. Some participants had an 8-week electroacupuncture treatment, and the response rate was compared between different MWoA subgroups.
Results
In study 1, a total of 97 participants (age of 28.2 ± 1.0 years, 70 female participants) with MWoA and 77 healthy controls (HCs) (age of 26.8 ± 0.1 years, 61 female participants) were enrolled (dataset 1), and 2 MWoA subgroups were defined. The participants in subgroup 1 had a significantly lower headache frequency (times/month of 4.4 ± 1.1) and significantly higher self-ratings of depression (depression score of 49.5 ± 2.3) when compared with participants in subgroup 2 (times/month of 7.0 ± 0.6 and depression score of 43.4 ± 1.2). The between-group differences of FCs were predominantly related to the amygdala, thalamus, hippocampus, and parahippocampal area. In study 2, 33 participants with MWoA (age of 30.9 ± 2.0 years, 28 female participants) and 23 HCs (age of 29.8 ± 1.1 years, 13 female participants) were enrolled as an independent dataset (dataset 2). The classification analysis validated the effectiveness of the 2-cluster solution of participants with MWoA in datasets 1 and 2. In study 3, 58 participants with MWoA were willing to receive electroacupuncture treatment and were assigned to different subgroups. Participants in different subgroups exhibited different response rates (p = 0.03, OR CI 0.086–0.93) to electroacupuncture treatment (18% and 44% for subgroups 1 and 2, respectively).
Discussion
Our study proposed a novel clustering approach to define distinct MWoA subgroups, which could be useful for refining the diagnosis of participants with MWoA and guiding individualized strategies for pain prophylaxis and analgesia.
Introduction
Migraine is a common neurologic disease, and it is characterized by recurring moderate-to-severe asymmetrical headaches.1 Researchers have acknowledged that migraine is a complex disease with heterogeneous clinical symptoms,2 including interindividual variability of disability,3 attack frequency,4 and psychological states.5 What drives such dramatic heterogeneity of migraine across individuals remains elusive.
The definition of homogeneous migraine subgroups that react differently to medications and treatments would have immediate applications in precision medicine. To date, some researchers have attempted to explore the phenotypic or neurologic markers of participant subgroups and observed some encouraging evidence.6 For example, Drysdale et al.7 defined subgroups by clustering participants with depression according to their brain functional networks and identified individuals most likely to benefit from targeted neurostimulation therapies. The same principle would work for classifying participants with migraine considering that advanced neuroimaging techniques have proven helpful in assessing abnormal brain functional and structural alterations in migraine.8 Previously, one study subclassified migraine into 2 subgroups and reported between-group differences in their clinical measurements.9 However, this study did not investigate whether patient subgroups had distinct treatment efficacy in migraine therapy.
Acupuncture reduces migraine attacks and is recommended as an effective alternative to drugs.10 Functional MRI (fMRI) studies pointed out that acupuncture might have a therapeutic effect by modulating pain processing pathways on migraine,11 and variability in acupuncture treatment efficacy might be associated with the variation of the individual's brain functional network architecture.12
In this study, we hypothesized that: (1) participants with migraine could be subclassified into different subgroups based on clinical measurements and brain neuroimaging data; (2) participants in different subgroups have distinct abnormal functional connectivity (FC) patterns compared with controls, and (3) participants in different subgroups may have different treatment outcomes. To achieve our aims, we proposed a novel processing pipeline for identifying migraine subgroups, validated the proposed subgrouping strategy using 2 datasets, and verified the clinical usefulness of the strategy by providing prognostic information for treatment selection. Specifically, in study 1, we adopted the partial least squares correlation (PLSC) analysis and a hierarchical clustering algorithm to divide participants with migraine into different clusters. In study 2, we assessed the external validity of the clustering strategy. In study 3, we evaluated whether the clustering strategy could be clinically valuable in providing prognostic information for an 8-week electroacupuncture treatment.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
Participants gave written informed consent, and the study aims and experimental procedures were fully explained to all of them. All studies were conducted based on the Declaration of Helsinki where the Institutional Review Board of the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine and West China Hospital approved the studies. Electroacupuncture treatment was listed in the Chinese Clinical Trial Registry (ChiCTR-IOR-15006648, June 23, 2015). The first participant was enrolled on July 17, 2015.
Study 1: Identifying Subgroups of Migraine Without Aura
Participants
We consecutively recruited participants with migraine without aura (MWoA) from the Department of Acupuncture and Neurology at Chengdu University of Traditional Chinese Medicine. The inclusion criteria were based on the third edition of the International Classification of Headache Disorders.2 When we enrolled a participant with MWoA who met the inclusion criteria, we chose her or his siblings, schoolmates, colleagues, or families as healthy controls (HCs). Potential participants needed to attend a screening appointment so we can check whether they are suitable to take part in the project as mentioned in the protocol.
The exclusion criteria included the following13: (1) individuals with any physical disease, including hepatitis, brain tumors, and epilepsy, based on medical records and clinical evaluations; (2) those with additional comorbid chronic pain disorders (e.g., fibromyalgia and tension-type headache); (3) those with a psychiatric disorder or brain disease; (4) those pregnant; (5) those using prescription drugs for migraine in the previous 3 months; (6) those with nicotine, alcohol, or drug abuse; and (7) those with claustrophobia.
Clinical Measurements and Imaging Acquisition
Participants with MWoA were instructed to report migraine duration during recruitment and complete a daily headache diary, including the headache frequency, Zung self-rating anxiety scale (SAS),14 average pain intensity, and Zung self-rating depression scale (SDS).15 The average pain intensity for each migraine attack was assessed using a numerical rating scale (NRS) going from 0 (zero pain) to 10 (the worst pain ever). The SAS and SDS are both 20-item surveys rated by respondents with scores ranging from 20 to 80. They evaluate physiologic and psychological symptoms that are assayed by participants based on how each one pertained to them in the past week.14,15 Moreover, the quality-of-life questionnaire (MSQ) pertaining to migraine, which is a patient-reported outcome assay with scores ranging from 0 to 100, assessed the quality of life of participants with MWoA across 3 essential aspects in the past 4 weeks: role function–preventive (RP), role function–restrictive (RR), and emotional function (EF).16
After clinical measurements, participants with MWoA underwent MRI scanning in the migraine's interictal phase (i.e., a minimum of 72 hours after a migraine attack). Resting-state brain functional images and high-resolution T1 scans were acquired using a 3.0T Signa GE scanning machine that had an 8-channel phase head coil located at the MR Research Center of the West China Hospital, Chengdu, China (eMethods, links.lww.com/WNL/C921).
Whole-Brain FC Estimation
Whole-brain FCs were estimated with the GRETNA toolbox.17 By using the Human Brainnetome Atlas,18 the whole brain was segment into 246 regions of interest (ROI) (eTable 1, links.lww.com/WNL/C921). After data preprocessing (eMethods), the blood oxygen level–dependent time series of every ROI were extracted and averaged, and Pearson correlation coefficients were computed between these time series to form a 246 × 246 correlation matrix containing 30,135 unique FC features. A Fisher Z transformation was used to convert all correlation coefficients. Linear regression was performed to adjust for the influences of sex and age, resulting in the adjusted FC features.4
PLSC Analysis
PLSC was used to investigate the relationship between 30,135 FC features and 8 clinical measurements (i.e., migraine duration, headache frequency, NRS, SAS, SDS, MSQ_EF, MSQ_RP, and MSQ_RR).19 This resulted in a set of orthogonal latent variables that can be used to identify connectivity patterns representing the optimal covariance between functional data and behavioral variables19 (eMethods and eFigure 1, links.lww.com/WNL/C921). Every latent variable is associated with (1) a clinical salience vector, indicating the contribution weight of every clinical measurement on the FC features–symptom association; and (2) a brain salience vector, indicating that the contribution weight of each FC feature contributes to the FC features–symptom association. Brain scores, a measure of the projections of FC features on their brain saliences, were calculated for each participant.20 A large absolute brain score demonstrates a strong contribution of individual FC features to the brain-symptom correlation, and a score close to zero implies a weak contribution.19
Hierarchical Clustering
Hierarchical clustering analysis21 was performed to assign participants with MWoA with similar patterns of brain scores into different subgroups. A dissimilarity matrix was calculated to describe the Euclidean distance between every pair of participants with MWoA. Ward minimum variance method was then applied to minimize the total within-cluster variance. Clustering analysis was performed using the pdist.m, linkage.m, and cluster.m functions in MATLAB. Without making any a priori assumption of the number of clusters, we repeatedly performed the clustering analysis using 2–8 clusters of participants with MWoA and used the variance ratio criterion to determine the optimal cluster number.22 The variance ratio criterion represents the ratio of the between-cluster variance to the within-cluster variance, with a higher variance ratio score (VRS) reflecting better clustering performance. The same hierarchical clustering analysis was also performed on the clinical measurements of participants with MWoA. Permutation tests (5,000 times) were performed to estimate the statistical significance (please see eMethods, links.lww.com/WNL/C921 for technical details).
Comparisons Between MWoA Subgroups Identified by Hierarchical Clustering
Between-group comparisons were performed to reveal their possible differences in clinical measurements and whole-brain FC patterns. Two-sample t tests were used, and the threshold for statistical significance was p < 0.001. False discovery rate was used to correct the multiple comparisons. The matrix of between-subgroup differences of FCs was summed over rows to show the strength of each ROI (please see eMethods, links.lww.com/WNL/C921 for technical details).
Study 2: Cross-Validation of the 2-Cluster Solution of Participants With MWoA
Clinical Measurements, Imaging Acquisition, and Data Analysis for Dataset 2
An independent dataset in study 2 (dataset 2) was recruited as outpatients from the Department of Acupuncture and Neurology (West China Hospital). The exclusion and inclusion criteria for participants with MWoA and HCs were identical to those in study 1. All clinical measurements and imaging acquisition were identical to study 1 (eMethods, links.lww.com/WNL/C921).
Cross-Validation of the 2-Cluster Solution
We adopted a multivariate pattern analysis (MVPA) procedure by applying the support vector machine (SVM) on 30,135 FC features to classify MWoA, with and without clustering (eFigures 2 and 3, links.lww.com/WNL/C921), and HCs. This analysis could quantify the performance of the clustering procedure by comparing classification performance with and without clustering in dataset 1, for which the results could be verified in dataset 2 (please see eMethods for technical details).
When the classifier was trained on HCs and MWoA without clustering, the large interindividual heterogeneity of patients would force the classifier to learn individual-level features rather than class-specific features.23,24 Hence, it would lead to poor classification performance in isolating MWoA from HCs. Because the hierarchical clustering would minimize migraine heterogeneity within each MWoA subgroup, when the classifier was trained on HCs and MWoA in a subgroup, classification performance (HCs vs MWoA in a subgroup) could be improved. The area under the receiver operating characteristic curve (AUC), κ coefficient, and accuracy were calculated. The classification accuracy was defined as the fraction of predictions the model got right and calculated by dividing the number of correct predictions by the total number of predictions (i.e., the total number of participants). Permutation tests (5,000 times) were performed to estimate the statistical significance (please see eMethods, links.lww.com/WNL/C921 for technical details).
Study 3: The Diverse Responsiveness of MWoA Subgroups to Electroacupuncture Treatment
Participants, Electroacupuncture Treatment, and Clinical Measurements
In study 3, some participants with MWoA in datasets 1 and 2 were willing to receive electroacupuncture treatment. After 4 weeks of baseline clinical evaluation, participants with MWoA received electroacupuncture treatment thrice (30 minutes each time) per week for 8 weeks, that is, a total of 24 sessions (eFigure 4, links.lww.com/WNL/C921). The details about the acupoints selected are described in our previous study.10 In brief, 4 acupoints were used per treatment. All participants with MWoA received electroacupuncture on 2 compulsory acupoints (GB8 and GB20), and the other 2 acupoints were selected based on syndrome distinction of meridians in the headache area (included BL60, GB34, GB40, LI4, LR3, SI3, SJ5, and ST44).10 Sterile disposable acuneedles, each 25–40 mm long and 0.25 mm wide, were used. Acupuncturists, who were blinded to the classification of participants based on the clustering approach used, received treatment unilaterally by switching from the left and to the right points.10 Every acupoint was used to achieve the De-qi feeling (such as distention, numbness, soreness, or radiating, which shows effectual acupuncture).10 We used the HANS acupoint nerve stimulator (model LH 200A; Han Institute, TENS, Nanjing, China) after acuneedle insertion. The stimulation frequency was set to 2/100 Hz (changing every 3 seconds), and the stimulus strength ranged from 0.1 to 1.0 mA until the participant was at ease.10 All participants with MWoA could not ingest prophylactic drugs. However, in instances of excruciating headache, participants with MWoA were given permission to ingest ibuprofen (300-mg sustained-release capsules).10 Detailed information about drug intake was noted. After 8 weeks of electroacupuncture treatment, clinical measurements for the following 4 weeks were evaluated again. Participants with MWoA with reduced headache frequency by at least 50% were considered responders.
Responsiveness to Electroacupuncture Treatment in Different MWoA Subgroups
Based on the imaging-based cluster centroid of the 2 MWoA subgroups in dataset 1, the brain scores of each participant in study 3 were calculated and then used to assign the participant with MWoA to one of the MWoA subgroups. Within each MWoA subgroups, the electroacupuncture response rate was measured as a ratio between the number of responders and the total number of participants with MWoA. The difference in the electroacupuncture response rate between participants in subgroups 1 and 2 was assessed using the χ2 test.
Electroacupuncture Response Rate Predictions With and Without MWoA Subgroup Labels
Studies suggested that acupuncture treatment outcomes in MWoA could be predicted from priori brain functional network.12,25 To determine whether the MWoA subgroup labels could improve the identification of individuals who are most likely to benefit from electroacupuncture treatment, we performed an MVPA to predict electroacupuncture responders and nonresponders based on whole-brain FCs before electroacupuncture treatment or based on the combination of whole-brain FCs and MWoA subgroup labels (eMethods and eFigure 5, links.lww.com/WNL/C921). The AUC, κ coefficient, and accuracy were calculated. Permutation tests (5,000 times) were performed to estimate the statistical significance. The same analysis was also performed to assess whether the differential electroacupuncture response might be explained by simpler measurements.
Data Availability
The MATLAB codes of the MVPA analysis are available on github (github.com/Jixin-Liu/Study-migraine-heterogeneity-using-fMRI.git). Additional data related to this article will be provided on reasonable request.
Results
Study 1: MWoA Subgroups Identified by Clustering Analysis
Demographics and Clinical Measurements
In total, 110 participants with MWoA and 90 HCs were recruited. Nine participants were excluded for missing data in the headache diary. Four participants and 7 HCs were excluded because of incomplete MRI scans. Six HCs were excluded because of schedule conflicts. Thus, 97 participants with MWoA with an average age of 28.2 ± 1.0 years and 77 HCs with an average age of 26.8 ± 0.1 years were selected (dataset 1 and Table 1). There was no significant difference between the 2 groups for age and sex. The headache frequency was 6.5 ± 0.5 times per month, and the average pain intensity was 5.6 ± 0.2. The average SAS and SDS scores were 46.4 ± 0.9 and 44.7 ± 1.1, respectively. The average RR, RP, and EF scores were 61.3 ± 1.9, 71.0 ± 2.0, and 71.9 ± 2.0, respectively (Table 1).
Table 1.
Demographic Characteristics of All Participants and Clinical Measurements of Participants With MWoA in Dataset 1

Hierarchical Clustering
PLSC analysis revealed 8 orthogonal latent variables and brain scores in each participant (eTables 2 and 3, links.lww.com/WNL/C921). When we used the 8 clinical measurements to cluster participants with MWoA similarly for all cluster solutions, the VRSs (Figure 1A, black) were much lower than those when the brain scores (Figure 1A, red) were used. The VRS that monotonically decreased with the increase in cluster number was maximal with a 2-cluster solution. For the 2-cluster solution, VRSs were larger than the chance level when brain scores and clinical measurements were used (p = 0.001, CI 0–0.002, Figure 1B). Moreover, imaging-based clusters had a significantly higher VRS than clinical-based clusters (p = 0.005, CI 0.002–0.008, Figure 1B).
Figure 1. Clustering Performance.
(A) The comparison of hierarchical clustering performance between the use of brain scores and clinical measurements. (B) Statistical comparison of the clustering performance when 2 clusters were considered.
Comparisons Between Imaging-Based 2 MWoA Subgroups and HCs in Dataset 1
As summarized in Table 2, no significant differences in demographics were found. The comparison of clinical measurements between imaging-based 2 MWoA subgroups in dataset 1 showed that participants in subgroup 1 had a lower headache frequency and MSQ-RP score and higher SDS when compared with the participants in subgroup 2.
Table 2.
Comparison of Demographic Characteristics and Clinical Measurements Between Different MWoA Subgroups in Dataset 1
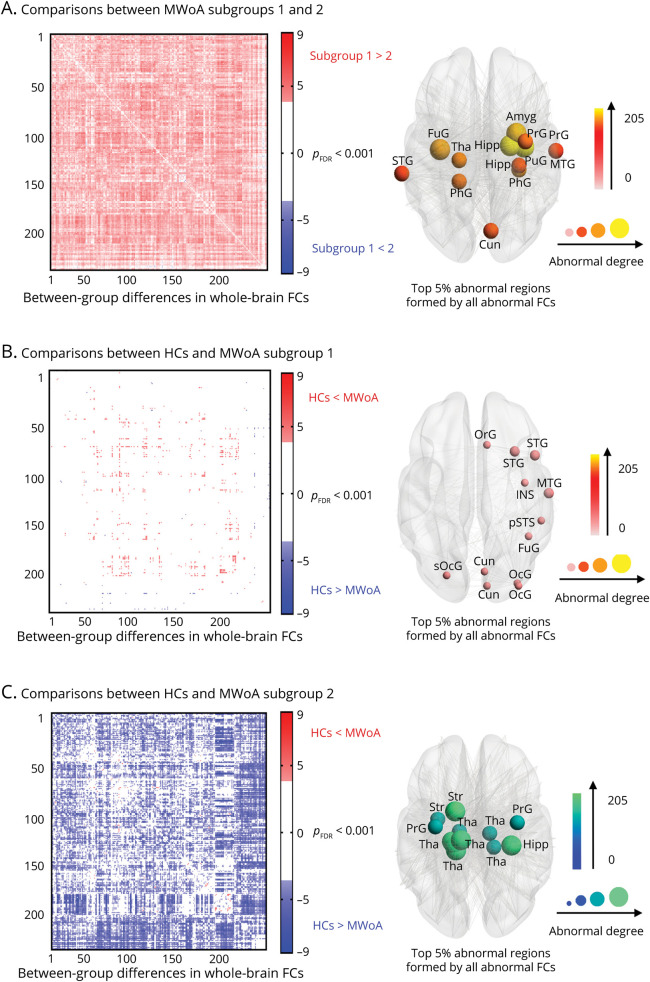
The comparison of brain FCs between imaging-based MWoA subgroups in dataset 1 showed that participants in subgroup 1 exhibited higher FCs than those in subgroup 2 (marked in red in Figure 2A), and the between-group differences of FCs were mainly related to the thalamus, amygdala, hippocampus, and the parahippocampal area (the top 12 ROIs, i.e., 5% of 246 ROIs, the same hereinafter, Figure 2A). In addition, participants in subgroup 1 exhibited higher FCs than HCs (marked in red in Figure 2B), and participants in subgroup 2 showed lower FCs than HCs (marked in blue in Figure 2C).
Figure 2. Abnormal FCs in Different MWoA Subgroups in Dataset 1.
Heat maps depicting a pattern of FCs for between-group differences (pFDR < 0.001). The size of the node indicates the degree of the between-group differences. The top 5% of abnormal brain regions formed by the abnormal FCs were exhibited. (A) The comparison between participants in the 2 subgroups. (B) The comparison between participants with MWoA in subgroup 1 and HCs. (C) The comparison between participants with MWoA in subgroup 2 and HCs. FC = functional connectivity; FDR = false discovery rate; HC = healthy control; MWoA = migraine without aura.
Study 2: Cross-Validation of MWoA Subgroups
Demographics and Clinical Measurements for Dataset 2
In dataset 2, 37 participants with MWoA and 27 HCs were recruited. Four patients with MWoA were excluded because of an incomplete headache diary. Four HCs were excluded because of discomfort or scheduling issues. Thus, 33 participants with MWoA with an average age of 30.9 ± 2.0 years and 23 HCs with an average age of 29.8 ± 1.1 years were selected from the Affiliated Hospital of Chengdu University of Traditional Chinese Medicine (eTable 4, links.lww.com/WNL/C921). There was no significant difference between the 2 groups for age and sex. Based on the imaging-based cluster centroid of the 2 MWoA subgroups in dataset 1, 15 participants with MWoA in dataset 2 were assigned to subgroup 1 and 18 participants with MWoA were assigned to subgroup 2 (eTable 4). For whole-brain FCs, participants in different MWoA subgroups showed similar between-group differences as those in dataset 1 (eFigure 6).
Classification Performance With and Without Clustering
In dataset 1 (study 1), when classifiers were trained on all participants without clustering, MWoA and HCs were correctly classified with an AUC of 0.92, a κ coefficient of 0.84, and an accuracy of 0.92 (all p = 0.0008, CI 0–0.0018; 5,000 permutations) (eFigure 7A, links.lww.com/WNL/C921). When classifiers were trained on participants after clustering, MWoA in subgroup 1 and HCs were discriminated with an AUC of 0.86, a κ coefficient of 0.80, and an accuracy of 0.90 (all p = 0.0008, CI 0–0.0018; 5,000 permutations) (eFigure 7A). MWoA in subgroup 2 and HCs were more accurately classified with an AUC of 0.94, a κ coefficient of 0.89, and an accuracy of 0.96 (all p = 0.006, CI 0–0.0015; 5,000 permutations) (eFigure 7A).
In dataset 2, when classification models from dataset 1 were used, all MWoA and HCs were classified with an AUC of 0.76, a κ coefficient of 0.52, and an accuracy of 0.77 (eFigure 7B, links.lww.com/WNL/C921). However, MWoA in subgroup 1 and HCs were poorly discriminated with an AUC of 0.51, a κ coefficient of 0.012, and an accuracy of 0.55 (eFigure 7B). MWoA in subgroup 2 and HCs were accurately classified with an AUC of 0.87, a κ coefficient of 0.74, and an accuracy of 0.91 (eFigure 7B). No canners' effects were observed between datasets 1 and 2 (please see eResults, links.lww.com/WNL/C921 for details).
Study 3: The Diverse Responsiveness of Imaging-Based MWoA Subgroups to Electroacupuncture Treatment
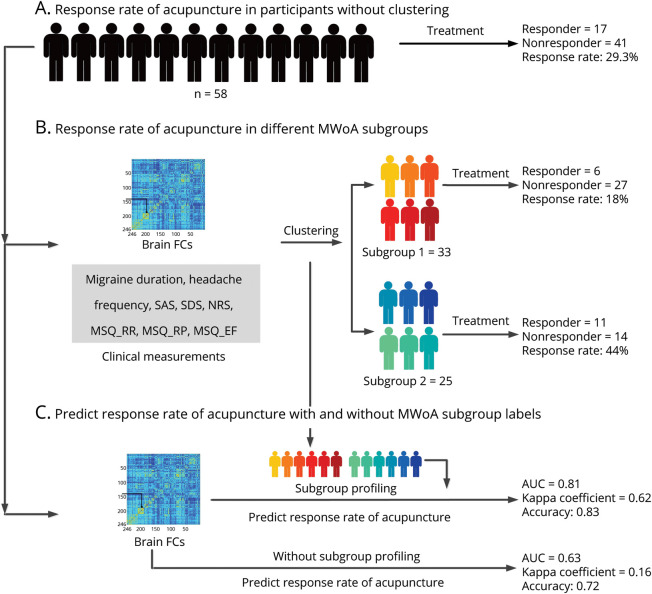
Only 58 participants with MWoA in datasets 1 and 2 were willing to receive electroacupuncture treatment (eTables 5–7, links.lww.com/WNL/C921), and there were 17 electroacupuncture responders (response rate = 29.3%, 17/58, Figure 3A). When the imaging-based cluster centroid was used, 33 participants were assigned to subgroup 1, and 6 electroacupuncture responders were found (response rate = 18%, 6/33, Figure 3B); 25 participants were assigned to subgroup 2 and 11 electroacupuncture responders were found (response rate = 44%, 11/25, Figure 3B). Participants in subgroup 2 had a significantly higher electroacupuncture response rate than those in subgroup 1 (p = 0.03, OR CI 0.086–0.93).
Figure 3. Electroacupuncture Response.
The diverse acupuncture responsiveness of an 8-week electroacupuncture treatment in different MWoA subgroups. (A) The response rate from all participants with MWoA. (B) The response rates from participants in different MWoA subgroups. (C) The prediction of electroacupuncture responders and non-responders based on whole-brain FCs before acupuncture treatment with and without subgroup labels of patients. AUC = area under the receiver operating characteristic curve; EF = emotional function; FC = functional connectivity; MSQ = Migraine-Specific Quality-of-Life Questionnaire; MWoA = migraine without aura; NRS = numerical rating scale; RP = role function–preventive; RR = role function–restrictive; SAS = self-rating anxiety scale; SDS = self-rating depression scale.
To determine whether the differential response might be explained by other measures, the 8 clinical measurements and 30,135 brain FCs were also used to cluster participants in study 3 into different subgroups. Unfortunately, all participants with MWoA in study 3 were assigned to the same group (please see eResults, links.lww.com/WNL/C921 for details).
Classification Performance
When the classifiers were trained on whole-brain FCs before electroacupuncture treatment without MWoA subgroup labels, electroacupuncture responders and nonresponders were correctly classified with an AUC of 0.63 (p = 0.024, CI 0.018–0.03), a κ coefficient of 0.16 (p = 0.16, CI 0.15–0.17), and an accuracy of 0.72 (p = 0.0028, CI 0.001–0.0047). However, when MWoA subgroup labels were added as discriminative features, the AUC, κ coefficient, and accuracy increased to 0.81 (p = 0.0012, CI 0–0.0025), 0.62 (p = 0.0008, CI 0–0.0018), and 0.83 (p = 0.0006, CI 0–0.0015), respectively (Figure 3C).
When the SVM classifier was trained on all 8 clinical measurements, electroacupuncture responders and nonresponders were not correctly classified with an AUC of 0.54, a κ coefficient of 0.13, and accuracy of 0.67.
Discussion
In this study, we identified a preliminary novel approach for identifying MWoA subgroups based on the association between clinical measurements and brain neuroimaging data. We tested the validity of this clustering approach using 2 independent datasets and illustrated its clinical values by comparing response rates with the 8-week electroacupuncture treatment of participants in different MWoA subgroups. This demonstrates that personalized treatment approaches to migraine, based on clinical and neuroimaging data, may be attainable.
As one of the most disabling neurologic diseases, migraine affects approximately 12% of the population worldwide.26,27 Due to its heterogeneity, the treatment outcomes vary tremendously among individuals.28 This situation could be improved if migraine can be more effectively diagnosed and treated, and a more targeted treatment to disease would improve the treatment outcomes for individuals.29,30 Indeed, some studies have defined MWoA subgroups according to clinical categorization schemes based on pain intensity or depression/anxiety levels.4,31,32 However, this strategy cannot fully capture MWoA heterogeneity because existing classifications based on clinical criteria may not delineate MWoA with neurobiologically distinct characteristics.33 This shortcoming calls for a novel method to integrate measurements from multiple categories, which would help provide a more comprehensive characterization of individual differences in participants with MWoA. In this study, combining clinical measurements with brain imaging data, we have obtained a series of latent components that linked a large set of clinical measurements to whole-brain FC patterns in participants with MWoA. The interindividual variability in the expression of these components captured differences among participants with MWoA in their clinical measurements and brain FCs. Clustering on these latent components, all participants with MWoA could be divided into 2 subgroups. Participants in subgroup 1 had higher self-ratings of depression and abnormal higher brain FCs, and participants in subgroup 2 had higher headache frequencies and abnormal lower brain FCs. These results were largely replicated in an independent dataset. Therefore, our study provided a novel processing pipeline to define distinct MWoA subgroups, which could help improve our understanding of clinically relevant neurobiological heterogeneity in migraine.
In our findings, the differences of brain FCs between participants in subgroups 1 and 2 were mainly centralized in the thalamus, amygdala, hippocampus, and the parahippocampus. As one of the supraspinal structures that receives projections from the spinal cord, the thalamus plays an important role in pain management by relaying ascending nociceptive information to other brain regions.34 Although still under investigation, the role of the thalamus in central sensitization, photophobia, and allodynia in migraine has been well documented.35-38 Considering these studies, the distinct pattern of thalamus-related FCs in our study may be associated with different pain processing and modulation in MWoA subgroups 1 and 2. Subcortical brain structures such as the amygdala and hippocampus are key regions for emotional and memory processing. The amygdala plays a crucial role in automatic management of memory and emotional stimuli,39 while the hippocampus regulates learned behavior and memory and pain-related attention and anxiety.40 These areas are also implicated in migraine.41,42 For example, Chen et al.43 observed abnormal FCs of the amygdala in individuals with chronic and episodic migraine. Schwedt et al.44 reported greater hippocampus activation while experiencing thermal pain stimuli in participants with MWoA than in HCs, and the strength of activation in the hippocampus was correlated to headache frequency. Therefore, different maladaptive states of neural activity related to the amygdala and hippocampus in different MWoA subgroups may be associated with between-group differences in depression level and headache frequencies.
In addition to improving our understanding of clinically relevant neurobiological heterogeneity in migraine, this study has prognostic potential in elaborating the treatment performance for participants with MWoA. Our results found that electroacupuncture was more effective in relieving pain in participants in subgroup 2 than in subgroup 1. Participants in subgroup 1 had higher depression scores but a lower response rate than participants in subgroup 2. Indeed, several studies pointed out that depression was related to difficulty in revising negative expectations after receiving unexpectedly positive information.45 Because positive and negative expectations were closely related to participants' analgesic response to subsequent placebo and active medical treatments,46,47,48 our results may suggest that depressive symptoms in participants in subgroup 1 could result in negative expectations about pain or treatment outcomes, thus preventing them from experiencing clinical benefits. In addition, we found that acupuncture responders and nonresponders were more accurately identified when the classifiers were trained on brain FCs before acupuncture treatment with MWoA subgroup labels than those without labels. Hence, MWoA subgroup labels were also a relevant feature for a priori prediction of electroacupuncture response in participants with MWoA and may help develop more efficient treatment approaches to migraine.
There are several issues that should be discussed in future research. The classification performance improved when the analysis was performed on clustered participants with MWoA in subgroup 2 than unclustered participants. This result may suggest that brain FC features of MWoA in subgroup 2 were informative for classification and could be generalized into independent datasets. However, poor classification performance was obtained for participants with MWoA in subgroup 1, which could be associated with the fact that there were fewer abnormal brain FCs in MWoA in subgroup 1 than in subgroup 2 (Figure 2B vs Figure 2C). Because the classification analysis was performed based on the differences in FCs between MWoA and HCs, fewer significant between-group differences would make it challenging to maximize the classification margins in SVM, which would result in poor classification performance. In addition, the number of patients in subgroup 1 was limited. Therefore, caution is warranted in interpreting abnormal FCs in MWoA in subgroup 1.
In addition, 8 clinical measurements in this study could not fully capture the clinical characteristics of participants with MWoA. Standardized procedures to comprehensively collect clinical measurements should be implemented. This will be crucial for refining our clustering method to identify MWoA subgroups with distinct migraine symptoms and behaviors. To avoid studying participants with MWoA during their premonitory phase, the moment elapsed from the time of the MRI until the following migraine attack should be evaluated. For collecting resting-state data, future studies should use a static visual stimulus (e.g., an X) to stare at or a boring movie without words or content to prevent participants from overthinking during the resting state.49 Given that the numbers of participants are not equal between participants with MWoA and HCs, further studies with a larger sample size are required to have a matched participant design and verify the clinical usefulness of our clustering method.
We proposed a novel processing pipeline to subclassify participants with MWoA into 2 different subgroups based on clinical and neuroimaging data, and participants in different subgroups have distinct abnormal FC patterns and electroacupuncture response rate. Our preliminary study may be useful to identify migraineurs who are most likely to benefit from electroacupuncture therapy.
Glossary
- AUC
area under the receiver operating characteristic curve
- EF
emotional function
- FC
functional connectivity
- fMRI
functional MRI
- HC
healthy control
- MSQ
Migraine-Specific Quality-of-Life Questionnaire
- MVPA
multivariate pattern analysis
- MWoA
migraine without aura
- NRS
numerical rating scale
- PLSC
partial least squares correlation
- ROI
region of interest
- RP
role function–preventive
- RR
role function–restrictive
- SAS
self-rating anxiety scale
- SDS
self-rating depression scale
- SVM
support vector machine
- VRS
variance ratio score
Appendix. Authors

Study Funding
This work was supported by the National Key Research and Development Project (No. 2019YFC1709701), the National Natural Science Foundation of China (No. 81973962, 81871330, 32071061, and 31822025), the First-Class Disciplines Development supported by Chengdu University of Traditional Chinese Medicine (No. CZYJC1901), the Fundamental Research Funds for the Central Universities (No. JB211203), and the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (No. ZYYCXTD-D-202003).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Silberstein SD. Migraine. Lancet. 2004;363(9406):381-391. doi: 10.1016/S0140-6736(04)15440-8 [DOI] [PubMed] [Google Scholar]
- 2.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. doi: 10.1177/0333102417738202 [DOI] [PubMed] [Google Scholar]
- 3.Lipton RB, Tepper SJ, Reuter U, et al. Erenumab in chronic migraine: patient-reported outcomes in a randomized double-blind study. Neurology. 2019;92(19):e2250-e2260. doi: 10.1212/wnl.0000000000007452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mu J, Chen T, Quan S, Wang C, Zhao L, Liu J. Neuroimaging features of whole‐brain functional connectivity predict attack frequency of migraine. Hum Brain Mapp. 2020;41(4):984-993. doi: 10.1002/hbm.24854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pistoia F, Salfi F, Saporito G, et al. Behavioral and psychological factors in individuals with migraine without psychiatric comorbidities. J Headache Pain. 2022;23(1):131. doi: 10.1186/s10194-022-01485-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dworkin RH, Edwards RR. Phenotypes and treatment response: it's difficult to make predictions, especially about the future. Pain. 2017;158(2):187-189. doi: 10.1097/j.pain.0000000000000771 [DOI] [PubMed] [Google Scholar]
- 7.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi: 10.1038/nm.4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May A. New insights into headache: an update on functional and structural imaging findings. Nat Rev Neurol. 2009;5(4):199-209. doi: 10.1038/nrneurol.2009.28 [DOI] [PubMed] [Google Scholar]
- 9.Schwedt TJ, Si B, Li J, Wu T, Chong CD. Migraine subclassification via a data-driven automated approach using multimodality factor mixture modeling of brain structure measurements. Headache. 2017;57(7):1051-1064. doi: 10.1111/head.13121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Chen J, Li Y, et al. The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Intern Med. 2017;177(4):508-515. doi: 10.1001/jamainternmed.2016.9378 [DOI] [PubMed] [Google Scholar]
- 11.Ma P, Dong X, Qu Y, et al. A narrative review of neuroimaging studies in acupuncture for migraine. Pain Res Manag. 2021;2021:9460695. doi: 10.1155/2021/9460695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster NE, Vertosick EA, Lewith G, et al. Identifying patients with chronic pain who respond to acupuncture: results from an individual patient data meta-analysis. Acupunct Med. 2021;39(2):83-90. doi: 10.1177/0964528420920303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu J, Ma S, Mu J, et al. Integration of white matter network is associated with interindividual differences in psychologically mediated placebo response in migraine patients. Hum Brain Mapp. 2017;38(10):5250-5259. doi: 10.1002/hbm.23729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371-379. doi: 10.1016/s0033-3182(71)71479-0 [DOI] [PubMed] [Google Scholar]
- 15.Zung WW. A self-rating depression scale. Arch Gen Psychiatry. 1965;12(1):63-70. doi: 10.1001/archpsyc.1965.01720310065008 [DOI] [PubMed] [Google Scholar]
- 16.Rendas-Baum R, Bloudek LM, Maglinte GA, Varon SF. The psychometric properties of the Migraine-Specific Quality of Life Questionnaire version 2.1 (MSQ) in chronic migraine patients. Qual Life Res. 2013;22(5):1123-1133. doi: 10.1007/s11136-012-0230-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;30:386. doi: 10.3389/fnhum.2015.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan L, Li H, Zhuo J, et al. The human brainnetome atlas: a new brain atlas based on connectional architecture. Cereb Cortex. 2016;26(8):3508-3526. doi: 10.1093/cercor/bhw157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56(2):455-475. doi: 10.1016/j.neuroimage.2010.07.034 [DOI] [PubMed] [Google Scholar]
- 20.Mu J, Wang Q, Dun W, et al. The effects of long-term menstrual pain on pain empathy in women with primary dysmenorrhea. Pain. 2021;162(7):2051-2059. doi: 10.1097/j.pain.0000000000002205 [DOI] [PubMed] [Google Scholar]
- 21.Randriamihamison N, Vialaneix N, Neuvial P. Applicability and interpretability of Ward's hierarchical agglomerative clustering with or without contiguity constraints. J Classif. 2021;38(2):363-389. doi: 10.1007/s00357-020-09377-y [DOI] [Google Scholar]
- 22.Tomašev N, Radovanović M. Clustering evaluation in high-dimensional data. In: Unsupervised Learning Algorithms. Springer; 2016:71-107. [Google Scholar]
- 23.Hu L, Iannetti GD. Neural indicators of perceptual variability of pain across species. Proc Natl Acad Sci USA. 2019;116(5):1782-1791. doi: 10.1073/pnas.1812499116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goswami S, Mehta S, Sahrawat D, Gupta A, Gupta R. Heterogeneity loss to handle intersubject and intrasubject variability in cancer. arXiv preprint. 2020. arXiv:200303295. [Google Scholar]
- 25.Tu Y, Zeng F, Lan L, et al. An fMRI-based neural marker for migraine without aura. Neurology. 2020;94(7):e741-e751. doi: 10.1212/wnl.0000000000008962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feigin VL, Vos T, Alahdab F, et al. Burden of neurological disorders across the US from 1990-2017: a global burden of disease study. JAMA Neurol. 2021;78(2):165-176. doi: 10.1001/jamaneurol.2020.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodick DW. Migraine. Lancet. 2018;391(10127):1315-1330. doi: 10.1016/s0140-6736(18)30478-1 [DOI] [PubMed] [Google Scholar]
- 28.Martelletti P, Schwedt TJ, Lanteri-Minet M, et al. My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain. 2018;19(1):115. doi: 10.1186/s10194-018-0946-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gewandter JS, Mcdermott MP, Mbowe O, et al. Navigating trials of personalized pain treatments: we're going to need a bigger boat. Pain. 2019;160(6):1235-1239. doi: 10.1097/j.pain.0000000000001504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis KD. Imaging vs quantitative sensory testing to predict chronic pain treatment outcomes. Pain. 2019;160(1):S59-S65. doi: 10.1097/j.pain.0000000000001479 [DOI] [PubMed] [Google Scholar]
- 31.Yu D, Yuan K, Zhao L, et al. White matter integrity affected by depressive symptoms in migraine without aura: a tract‐based spatial statistics study. NMR Biomed. 2013;26(9):1103-1112. doi: 10.1002/nbm.2924 [DOI] [PubMed] [Google Scholar]
- 32.Schwedt TJ, Chong CD, Wu T, Gaw N, Fu Y, Li J. Accurate classification of chronic migraine via brain magnetic resonance imaging. Headache. 2015;55(6):762-777. doi: 10.1111/head.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashina M, Terwindt GM, Al-Karagholi AM, et al. Migraine: disease characterisation, biomarkers, and precision medicine. Lancet. 2021;397(10283):1496-1504. doi: 10.1016/s0140-6736(20)32162-0 [DOI] [PubMed] [Google Scholar]
- 34.Vartiainen N, Perchet C, Magnin M, et al. Thalamic pain: anatomical and physiological indices of prediction. Brain. 2016;139(3):708-722. doi: 10.1093/brain/awv389 [DOI] [PubMed] [Google Scholar]
- 35.Samaira Y, Anders H, Rodrigo N, Messoud A. Current understanding of thalamic structure and function in migraine. Cephalalgia. 2019;39(13):1675-1682. doi: 10.1177/0333102418791595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodkinson DJ, Wilcox SL, Veggeberg R, et al. Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci. 2016;36(30):8026-8036. doi: 10.1523/jneurosci.1038-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amin FM, Hougaard A, Magon S, et al. Altered thalamic connectivity during spontaneous attacks of migraine without aura: a resting-state fMRI study. Cephalalgia. 2018;38(7):1237-1244. doi: 10.1177/0333102417729113 [DOI] [PubMed] [Google Scholar]
- 38.Tu Y, Fu Z, Zeng F, et al. Abnormal thalamo-cortical network dynamics in migraine. Neurology. 2019;92(23):e2706-e2716. doi: 10.1212/wnl.0000000000007607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nat Rev Neursci. 2010;11:773-782. doi: 10.1038/nrn2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu MG, Chen J. Roles of the hippocampal formation in pain information processing. Neurosci Bull. 2009;25(5):237-266. doi: 10.1007/s12264-009-0905-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jassar H, Nascimento TD, Kaciroti N, et al. Impact of chronic migraine attacks and their severity on the endogenous μ-opioid neurotransmission in the limbic system. Neuroimage Clin. 2019;23:101905. doi: 10.1016/j.nicl.2019.101905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu H-Y, Chou K-H, Lee P-L, et al. Hippocampus and amygdala volume in relation to migraine frequency and prognosis. Cephalalgia. 2017;37(14):1329-1336. doi: 10.1177/0333102416678624 [DOI] [PubMed] [Google Scholar]
- 43.Chen Z, Chen X, Liu M, Dong Z, Ma L, Yu S. Altered functional connectivity of amygdala underlying the neuromechanism of migraine pathogenesis. J Headache Pain. 2017;18(1):7. doi: 10.1186/s10194-017-0722-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwedt TJ, Chong CD, Chiang C-C, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia. 2014;34(12):947-958. doi: 10.1177/0333102414526069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jepma M, Koban L, van Doorn J, Jones M, Wager TD. Behavioural and neural evidence for self-reinforcing expectancy effects on pain. Nat Hum Behav. 2018;2(11):838-855. doi: 10.1038/s41562-018-0455-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zunhammer M, Ploner M, Engelbrecht C, Bock J, Kessner SS, Bingel U. The effects of treatment failure generalize across different routes of drug administration. Sci Transl Med. 2017;9(393):eaal2999. doi: 10.1126/scitranslmed.aal2999 [DOI] [PubMed] [Google Scholar]
- 47.Kessner S, Wiech K, Forkmann K, Ploner M, Bingel U. The effect of treatment history on therapeutic outcome: an experimental approach. JAMA Intern Med. 2013;173(15):1468-1469. doi: 10.1001/jamainternmed.2013.6705 [DOI] [PubMed] [Google Scholar]
- 48.Liu J, Mu J, Chen T, Ming Z, Tian J. White matter tract microstructure of the mPFC-amygdala predicts interindividual differences in placebo response related to treatment in migraine patients. Hum Brain Mapp. 2018;40(1):284-292. doi: 10.1002/hbm.24372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vanderwal T, Kelly C, Eilbott J, Mayes LC, Castellanos FX. Inscapes: a movie paradigm to improve compliance in functional magnetic resonance imaging. Neuroimage. 2015;15:222-232. doi: 10.1016/j.neuroimage.2015.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The MATLAB codes of the MVPA analysis are available on github (github.com/Jixin-Liu/Study-migraine-heterogeneity-using-fMRI.git). Additional data related to this article will be provided on reasonable request.