Abstract
Background and Objectives
Primary CNS lymphoma (PCNSL), a rare CNS malignancy, is usually treated with high-dose methotrexate in the first-line setting, typically followed by consolidation therapy. Due to the broad range of currently available treatments for PCNSL, comparability in long-term follow-up studies is limited, and data are scattered across small studies.
Methods
In this study, we report the long-term survival of patients with newly diagnosed immunocompetent PCNSL, enrolled in a phase II trial from June 2005 to September 2011. Patients were treated using rituximab, methotrexate, vincristine, and procarbazine (R-MVP) chemotherapy followed by high-dose chemotherapy (HDC) and autologous stem cell transplant (ASCT) in those with partial or complete response to R-MVP. In a post hoc analysis, clinical and imaging features were evaluated in those still alive.
Results
26 of 32 patients underwent HDC-ASCT consolidation. Of them, 3 patients died of treatment-related toxicity and 2 due to disease progression within 1 year of ASCT. None of the remaining 21 patients had disease progression with a median follow-up of 12.1 years and were included in the analysis. Compared with the post–HDC-ASCT assessment, at the last follow-up, there was no significant difference in the median Karnofsky Performance Status (80 [range: 60–100] vs 90 [range: 70–100]), the median Neurologic Assessment in Neuro-Oncology score (1 [range: 0–4] vs 1 [range: 0–5]), and leukoencephalopathy score (1 [range: 0–3] vs 1 [range: 1–4]).
Discussion
Long-term follow-up demonstrated that treatment was well tolerated in most patients enrolled in this study, with stable leukoencephalopathy on imaging and stable clinical performance status. Disease recurrence was not observed beyond 2 years after HDC-ASCT consolidation.
Introduction
Primary CNS lymphoma (PCNSL) is a rare extranodal lymphoma confined to the CNS, including the brain, spine, CSF, and eye. Treatment is based on high-dose methotrexate chemotherapy with leucovorin rescue.1 This is accompanied by varying supportive chemotherapy agents, including rituximab, vincristine, and procarbazine (R-MVP) and other regimens that may include temozolomide, etoposide, and cytarabine. However, recurrence on these treatments remain high, and typically, a consolidation therapy is used to prevent disease relapse.2 While whole-brain radiation therapy has been used in the past, it can cause permanent cognitive dysfunction, and consequently, other consolidation options for disease in remission have been introduced, including nonmyeloablative chemotherapy or high-dose chemotherapy followed by autologous stem cell transplant (HDC-ASCT).3 Myeloablative chemotherapy is used to maximize effectiveness. However, HDC-ASCT carries its own significant treatment-related risks, most concerningly infections during pancytopenia.
Because PCNSL is a rare disease, data on long-term follow-up for consolidation therapies are scattered across a few small studies.4–6 A prospective study using R-MVP chemotherapy followed by HDC-ASCT was performed at Memorial Sloan Kettering Cancer Center in the early 2000s.7 The goal of this study was to perform a post hoc long-term follow-up on these patients, 10 years after the last patient was registered to report long-term recurrence risk. We also assess long-term functional and neurologic status and leukoencephalopathy on MRI.
Methods
Patients
Enrollment process, inclusion and exclusion criteria, chemotherapy, and HDC-ASCT protocols for this phase 2 study have been previously published.7 In brief, from June 2005 to September 2011, 33 newly diagnosed immunocompetent PCNSL were enrolled at MSKCC (clinicaltrials.gov: NCT00596154). Eligible patients had non-Hodgkin PCNSL with radiographic evidence on MRI that was histologically confirmed, through brain biopsy, CSF cytology/flow cytometry, or vitreous/uveal biopsy, with no evidence of systemic lymphoma on both CT of the chest, abdomen, and pelvis and bone marrow biopsy. CSF involvement was not an exclusion criterion. Enrollment age was 18–72 years. Patients were excluded if HIV testing yielded positive results, if prior radiation or chemotherapy of the treatment for PCNSL was used, and if another active malignancy was present.
Chemotherapy and HDC-ASCT
Patients received 5 doses of rituximab (500 mg/m2), methotrexate (3.5 gm/m2), procarbazine (100 mg/m2), and vincristine (1.4 mg/m2). An MRI of the brain with and without contrast was obtained after 5 doses of R-MVP; patients with progressive disease (PD) were removed from the study, patients with partial response (PR) or stable disease (SD) received 2 more doses of R-MVP, and patients with complete response (CR) preceded to consolidation. For patients who received 2 additional R-MVP doses, a repeat MRI of the brain was completed after dose 7, and they were taken off study for SD/PD or proceeded to consolidation for PR/CR.
Consolidation therapy consisted of HDC with thiotepa (250 mg/m2), busulfan (3.2 mg/kg), and cyclophosphamide (60 mg/kg), followed by ASCT. Patients had undergone peripheral blood stem cell harvesting early in their R-MVP cycles.7 None of the patients received radiation. Patients were then followed up with periodic MRI scans every 3 months for the first 2 years after consolidation and then every 4 months for the third and fourth years, and finally, every 6 months onward, without further treatment offered unless there was evidence of progression.
Follow-up Measurements
Long-term follow-up status including disease status, functional status, and leukoencephalopathy were collected in this post hoc analysis. Patients' current survival and disease status were determined through a chart review. If the patient had not undergone a follow-up in >2 years, patients were contacted through mail to their last known address or called to discuss current functional status and disease status.
The patients' Karnofsky Performance Status (KPS) scale was assessed at enrollment, post-ASCT, and at follow-up visits. If a KPS scale was not explicitly stated in the note of the patient's last follow-up, it was derived from the examination stated and from the patient's examination narrative regarding current employment status, walking assistance, ability to live independently, or any noted cognitive complaints.
The Neurologic Assessment in Neuro-Oncology (NANO) score was evaluated on post-ASCT examinations and patient's most recent examination.8 This is an individual rating based on routine neurologic examination findings of neurologic deficits within 9 domains, with a maximal total potential score of 23, which would indicate the highest number of neurologic deficits.
The T2/FLAIR sequences of the post-ASCT and most recent MRI brain scan were reviewed and graded on a modified Fazekas scale of 0–5: 0, no white matter abnormality; 1, minimal patchy white matter foci; 2, start of confluence of white matter disease; 3, large confluent areas; 4, confluence of white matter abnormalities with cortical and subcortical involvement; and 5, diffuse leukoencephalopathy.7,9
Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method. The association between age younger than 60 years, response to MTX, receipt of ASCT, and survival was evaluated using univariable and multivariable Cox proportional hazards regression models. The NANO score and KPS were collected at initial diagnosis, after completion of trial therapy (post-ASCT), and at the last follow-up (current). The modified Faszekas scale scores were collected after completion of trial therapy (post-ASCT) and at the last follow-up (current). The Student t test was used to compare age differences at study enrollment between patients with low (≤2) and high NANO scores (>2) at the last follow-up and between patients with low (<2) and high modified Fazekas scale scores (≥2) at the last follow-up.
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Institutional Review Board. Every patient signed an informed consent before any trial investigations and treatments. The trial was registered with clinicaltrials.gov (registration number: NCT00596154).
Results
Patient Characteristics
Initially, 33 patients were enrolled to the original study between June 2005 and September 2011. Of these 33 patients, 1 patient was removed before treatment initiation due to systemic involvement (Figure 1). The remaining 32 patients received the intended study treatment. One patient developed disease progression during R-MVP and was taken off study. Two did not undergo HDC-ASCT based on physician decision, and 3 withdrew consent. Ultimately, 26 (81%) patients received stem cell transplant (Table 1). The median age of the original study population was 52 years with a range of 24–68 years. The median age of those receiving HDC-ASCT was 51 years. The median KPS was 80 in both groups. Approximately 47% of all participants and 46% of patients who received ASCT were women. There were no major differences between patients enrolled in the original study and those receiving HDC-ASCT (Table 1).
Figure 1. CONSORT Flow Diagram.
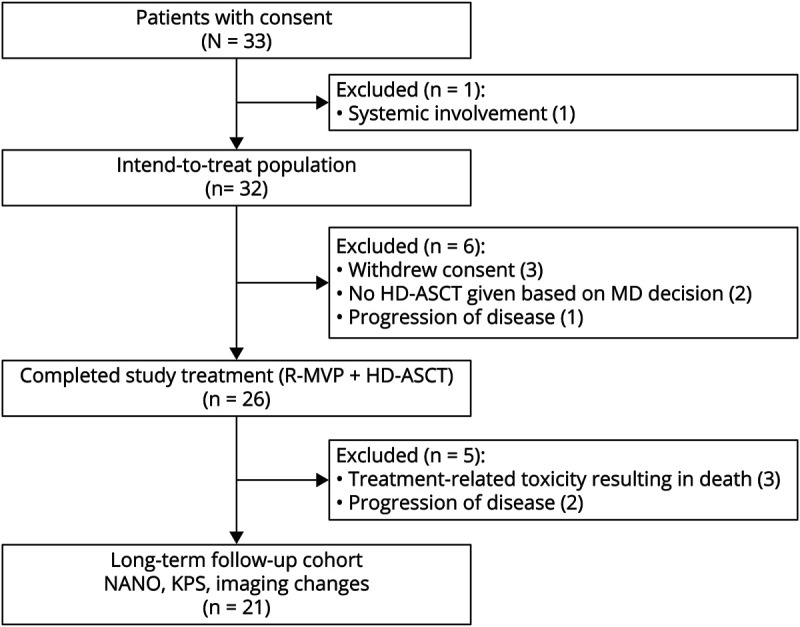
Table 1.
Patient Characteristics

Progression-Free Survival and Overall Survival
Of the 26 patients who received HDC-ASCT, 3 died within the first 2 years due to treatment-related toxicity (experiencing Steven–Johnson syndrome, septic shock, and graft vs host disease, respectively). Two additional patients developed disease progression and subsequently died 6 and 9 months after completion of HDC-ASCT.
At a median follow-up of 12.1 years (range: 5–15.5 years), the median PFS and median OS of the 32 patients receiving the intended trial therapy was not reached (Figure 2A). The 5-year, 10-year and 15-year PFS was 78%, 74.7%, and 74.7, respectively. The 5-year, 10-year, and 15-year OS was 81.3%, 81.3%, and 74.5%, respectively. Among the 26 transplanted patients, the 5-year PFS and OS were 80.8% and remained unchanged at 10 and 15 years (Figure 2B). In the transplanted patients, no disease recurrence or death was observed beyond 2 years from ASCT.
Figure 2. Progression-Free Survival and Overall Survival.
(A) Kaplan–Meier curves showing progression-free survival (PFS) and overall survival (OS) for all patients who received the intended trial treatment (n = 32). (B) Kaplan–Meier curves showing PFS and OS for transplanted patients (n = 26).
Of note, the 3 eligible patients who withdrew consent and did not proceed to HDC-ASCT received cytarabine consolidation; 1 died and 2 developed disease progression (at 1.2 and 7.4 years post-RMVP). Univariate and multivariate analyses showed a trend but no significant difference to better overall survival in patients younger than 60 years, CR to methotrexate induction therapy, and those who received an ASCT (Table 2).
Table 2.
Association Between Age, Response to Methotrexate Induction, Receipt of ASCT, and Overall Survival
KPS and NANO Scores
Of the 21 patients who underwent HDC-ASCT and were still alive, the median KPS at original study enrollment was 90 (range: 60–100), after HDC-ASCT was 80 (range: 60–100), and on the most recent follow-up was 90 (range: 70–100) (Figure 3A). From the time of HDC-ASCT to the last follow-up, the KPS improved in 13 patients, remained the same in 5, and decreased in 3. For this same group of patients, the median NANO score was 1 at registration (range: 0–6) and after HDC-ASCT (range: 0–4) and 0 at the last follow-up (range: 0–5) (Figure 3B). From the time of HDC-ASCT to the last follow-up, the NANO score reduced in 7 patients consistent with a clinical improvement, remained the same in 6, and increased in 8 by a median of 1 point (range: 1–2). Patients with higher NANO scores of 3–5 at the last follow-up were older at trial enrollment (n = 5; median age: 65.15 (range: 52.7–67.6)) compared with those with low NANO scores of 0–2 (n = 16; median age: 49 (range: 24.3–64.6); p = 0.017 (t test)).
Figure 3. Clinical Parameters During and After HDC-ASCT.
(A) The Karnofsky Performance Scale (KPS) at initial enrollment into the trial (initial), after completion of high-dose chemotherapy with stem cell rescue (post-ASCT), and at the most recent follow-up (current) are displayed. (B) The Neurologic Assessment in Neuro-Oncology (NANO) score is a standardized assessment of neurologic function based on the standard domains in the neurologic examinationA8. The scale increases for each domain/severity of abnormality on examination. NANO scores at initial enrollment into the trial (initial), after completion of high-dose chemotherapy with stem cell rescue (post-ASCT), and at the most recent follow-up (current) are displayed.
Leukoencephalopathy Scores
Five patients experienced no changes in the extend of leukoencephalopathy (Figure 4, A and C). In 10 patients, the modified Fazekas leukoencephalopathy scale increased by 1 point (0 to 1 in 5 patients, 1 to 2 in 3 patients, 2 to 3 in 1 patient, and 3 to 4 in 1 patient [Figure 4B]), and only 1 patient experienced a worsening by 2 points (from 1 to 3). The median modified Fazekas leukoencephalopathy scale on the post–HDC-ASCT MRI scan was 1 (range: 0–3) and remained stable at a median of 1 (range: 1–4) at the most recent follow-up (Figure 4C). While some patients did experience mild worsening of their leukoencephalopathy (Figure 4C), they remained functionally independent with KPS scores ≥70. Patients with higher leukoencephalopathy scores of ≥2 at the last follow-up were older at trial enrollment (n = 8; median age: 62.7 years [range: 33.4–67.6]) compared with those with low scores of <2 (n = 13; median age: 47.5 years [range: 24.3–65.2]; p = 0.0281 [t test]). There was no significant correlation between the NANO score and modified Fazekas scale.
Figure 4. Leukoencephalopathy During and After HDC-ASCT.
(A) Fluid-attenuated inversion recovery (FLAIR) MRI of a representative patient who developed only minimal leukoencephalopathy throughout his treatment and during long-term follow-up. (B) FLAIR MRI of a representative patient with a higher degree of leukoencephalopathy. (C) Displayed are the leukoencephalopathy scores of the study participants on FLAIR MRI after completion of high-dose chemotherapy with stem cell rescue (post-ASCT) and at the most recent follow-up (current). Modified Fazekas score: 0: no white matter abnormalities; 1: mild patchy white matter foci; 2: beginning of confluence of white matter disease; 3: large confluent areas; 4: confluence of white matter abnormalities with cortical and subcortical involvement; and 5: diffuse leukoencephalopathy.
Discussion
The median time to relapse in PCNSL has been reported between 10 and 18 months, and most relapses occur within the first 2 years of initial diagnosis.2 Younger patients (younger than 60 years) receiving a methotrexate-based chemotherapy regimen followed by whole-brain radiation consolidation were found to have a median overall survival of 9.5 years.4 Moreover, relapsing disease has been observed more than 5 years after initial diagnosis.10 Following the wide implementation of HDC-ASCT consolidation, long-term follow-up data in PCNSL are sparse. Two large, 2-arm phase 2 trials have reported 2-year progression-free survival rates of 72%6 and 87%5 in those receiving HDC-ASCT. However, long-term data are still maturing. In this study, we report the long-term follow-up of a prospective phase 2 study of patients with PCNSL treated with R-MVP followed by HDC-ASCT consolidation. Our data show that the median PFS and OS in patients who received HDC-ASCT were not reached at a median follow-up of 12.1 years. The PFS and OS was 80% at 5 and 10 years. Of those who did not progress within the first 2 years of HDC-ASCT, none experienced disease recurrence or death.
We observed that patients tolerated HDC-ASCT well from a functional standpoint, with stable median KPS and NANO score from enrollment to the most recent follow-up and no change in the median leukoencephalopathy score. Of interest, patients with poorer NANO and leukoencephalopathy scores at long-time follow-up were older study participants. Clinical studies comparing neurocognitive parameters in patients treated with radiation or HDC-ASCT consolidation reported overall better cognitive functionality for those receiving HDC-ASCT,5,6 although patients with PCNSL who have undergone ASCT still have been shown to demonstrate decreased performance in attention and memory tasks on follow-up testing 3 years after treatment completion.11 Leukoencephalopathy develops in approximately 50% of patients with PCNSL, particularly after radiation, and there has been a strong correlation between imaging changes, including atrophy, and neurocognitive impairment.12
Our data suggest that a methotrexate-based chemotherapy regimen followed by thiotepa-based HDC-ASCT offer a curative treatment option for patients with adequate organ function and age younger than 72 years. There are some limitations to this study, including the small sample size and single institutional experience. Our findings may be compared with long-term data from larger multicenter phase 2 trials for which long-term follow-up is still maturing.5,6 Overall, our findings indicate that treatment was well tolerated without significant long-term effects on imaging and clinical performance status and excellent long-term disease control.
Glossary
- ASCT
autologous stem cell transplant
- CR
complete response
- FLAIR
fluid-attenuated inversion recovery
- HDC
high-dose chemotherapy
- KPS
Karnofsky Performance Status
- NANO
Neurologic Assessment in Neuro-Oncology
- OS
overall survival
- PCNSL
Primary CNS lymphoma
- PD
progressive disease
- PFS
progression-free survival
- PR
partial response
- R-MVP
rituximab, methotrexate, vincristine, and procarbazine
- SD
stable disease
Appendix. Authors

Footnotes
Infographic: NPub.org/ig1017
Study Funding
This research was supported by grants from the NIH (1R01NS080944–01 to I.K.M., P30-CA008748), the Lymphoma Research Foundation Career Development Award (C.G), and Cycle for Survival Equinox Innovation Award (C.G).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Grommes C, Deangelis LM. Primary CNS lymphoma. J Clin Oncol. 2017;35(21):2410-2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jahnke K, Thiel E, Martus P, et al. Relapse of primary central nervous system lymphoma: clinical features, outcome and prognostic factors. J Neurooncol. 2006;80(2):159-165. [DOI] [PubMed] [Google Scholar]
- 3.Grommes C, Rubenstein JL, Deangelis LM, Ferreri AJM, Batchelor TT. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro Oncol. 2019;21(3):296-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gavrilovic IT, Hormigo A, Yahalom J, Deangelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24(28):4570-4574. [DOI] [PubMed] [Google Scholar]
- 5.Houillier C, Taillandier L, Dureau S, et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol. 2019;37(10):823-833. [DOI] [PubMed] [Google Scholar]
- 6.Ferreri AJM, Cwynarski K, Pulczynski E, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017;4(11):e510-e523. [DOI] [PubMed] [Google Scholar]
- 7.Omuro A, Correa DD, Deangelis LM, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood. 2015;125(9):1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nayak L, DeAngelis LM, Brandes AA, et al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. AJR Am J Roentgenol. 1987;149(2):351-356. [DOI] [PubMed] [Google Scholar]
- 10.Nayak L, Hedvat C, Rosenblum MK, Abrey LE, DeAngelis LM. Late relapse in primary central nervous system lymphoma: clonal persistence. Neuro Oncol. 2011;13(5):525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa DD, Braun E, Kryza-Lacombe M, et al. Longitudinal cognitive assessment in patients with primary CNS lymphoma treated with induction chemotherapy followed by reduced-dose whole-brain radiotherapy or autologous stem cell transplantation. J Neurooncol. 2019;144(3):553-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka R, Morii K, Sano M, et al. Long-term survivors of primary central nervous system lymphoma. Jpn J Clin Oncol. 2017;47(2):101-107. [DOI] [PubMed] [Google Scholar]






