Abstract
In the current scenario, the use of synthetic fertilizers is at its peak, which is an expensive affair, possesses harmful effects to the environment, negatively affecting soil fertility and beneficial soil microfauna as well as human health. Because of this, the demand for natural, chemical-free, and organic foods is increasing day by day. Therefore, in the present circumstances use of biofertilizers for plant growth-promotion and microbe-based biopesticides against biotic stresses are alternative options to reduce the risk of both synthetic fertilizers and pesticides. The plant growth promoting rhizobacteria (PGPR) and microbial biocontrol agents are ecologically safe and effective. Owning their beneficial properties on plant systems without harming the ecosystem, they are catching the widespread interest of researchers, agriculturists, and industrialists. In this context, the genus Stenotrophomonas is an emerging potential source of both biofertilizer and biopesticide. This genus is particularly known for producing osmoprotective substances which play a key role in cellular functions, i.e., DNA replication, DNA-protein interactions, and cellular metabolism to regulate the osmotic balance, and also acts as effective stabilizers of enzymes. Moreover, few species of this genus are disease causing agents in humans that is why; it has become an emerging field of research in the present scenario. In the past, many studies were conducted on exploring the different applications of Stenotrophomonas in various fields, however, further researches are required to explore the various functions of Stenotrophomonas in plant growth promotion and management of pests and diseases under diverse growth conditions and to demonstrate its interaction with plant and soil systems. The present review discusses various plant growth and biocontrol attributes of the genus Stenotrophomonas in various food crops along with knowledge gaps. Additionally, the potential risks and challenges associated with the use of Stenotrophomonas in agriculture systems have also been discussed along with a call for further research in this area.
Keywords: crop protection, ecofriendly, ecosystem, organic, osmoprotective, PGPR
Introduction
In recent years, the harmful effects of pesticides and synthetic fertilizers on humans, animals as well as on the whole ecosystem have led to the expansion of novel beneficial microbes. There are likely many undiscovered microorganisms in unexplored plants and soils that may play a crucial role in promoting plant growth through their various activities. The bacterial genus Stenotrophomonas is referred to as a potential PGPR with advantageous effects because of its capacity to produce siderophores, the ability to solubilize phosphate, and the generation of phytohormones and spermidine (Ulrich et al., 2021). This genus belongs to the family Xanthomonadaceae as an emended description of the Lysobacteraceae family (Cutiño-Jiménez et al., 2020). The Lysobacteraceae (Xanthomonadaceae) family covers a diverse group of bacteria, which includes Pseudoxanthomonas, Stenotrophomonas, Xanthomonas, and Xylella, these are closely related bacterial genera that form a phylogroup referred to as XSXP (Bansal et al., 2021). The Xanthomonadaceae family also contains plant pathogenic bacteria namely Xanthomonas and Xylella, which are reported to cause economic losses in several crops (Parte, 2018). On the other hand, this family also included the PGPR including Pseudomonas geniculata and S. rhizophilia with medical, environmental, and biotechnological significance (Cutiño-Jiménez et al., 2020). The Stenotrophomonas maltophilia was earlier described as Pseudomonas maltophilia in the year 1961 (An and Berg, 2018; Wang et al., 2018a,b).
Stenotrophomonas species are Gram-negative and associated with wide a range of habitats, including animals as well as plant hosts (Hayward et al., 2010). Additionally, this bacterium is cosmopolitan and ubiquitous that found in an environmental habitat range, including extreme ones, although naturally it is associated with plant’s rhizosphere and mainly contributed to the elemental cycling of sulphur and nitrogen, and also degrades complex compounds and pollutants, and promotes the growth of plants and their health (An and Berg, 2018; Pérez-Martínez et al., 2020). Moreover, the bacterium S. maltophilia is the first member of this genus which is a predominant species observed in plants, water, soil, animals, and humans (Wang et al., 2018b). Stenotrophomonas maltophilia has a sequenced genome with a genome size of approximately 4.8 Mbp with a G + C content of 66.7% (Crossman et al., 2008). The whole genome sequence analysis of S. indicatrix BOVIS40 yielded a 4.42 Mb genome size with ~66.4% G + C content (Adeleke et al., 2021). Bansal et al. (2021) suggested that, in light of deep phylotaxonomy genomics findings along with published polyphasic data, XSXP phylogroup warrants reunification and need to consider Xylella, Stenotrophomonas, and Pseudoxanthomonas as synonyms of Xanthomonas.
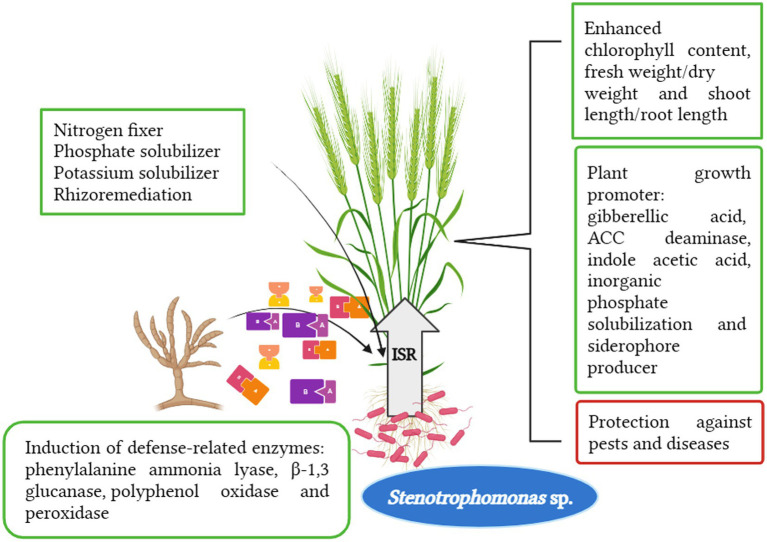
Many researchers reported the benefits of the genus Stenotrophomonas for plant systems (Figure 1). The genus Stenotrophomonas colonizes extreme manmade niches in space shuttles, hospitals, and clean rooms (An and Berg, 2018). Stenotrophomonas bacteria are becoming more researchable because of their potential use as effective bioinoculants for promoting plant growth and managing several diseases of food crops. This aspect is of growing biotechnological interest (Ulrich et al., 2021). Nowadays, this genus has become a research opportunity as a multidrug-resistant human pathogen, which does not commonly infect healthy humans but it may be associated with high morbidity and mortality in severely immunocompromised and debilitated patients by causing several infectious diseases (Flores-Treviño et al., 2019). These bacteria can also be recovered from polymicrobial infections, most especially from the respiratory tract (lungs) of cystic fibrosis patients (Brooke, 2014). Additionally, it has biodefense capacity against plant pathogenic fungi and bacteria as well as resistance to biotic and abiotic stress, anti-quorum sensing, and anti-biofilm bioactivities (Ulrich et al., 2021). The closely related S. rhizophilia provides a substitute for biotechnological applications without any harm to human health (An and Berg, 2018).
Figure 1.
Multi-fold action of Stenotrophomonas spp. in plant system.
The role of Stenotrophomonas in human infectious diseases has been discussed previously (Berg and Martinez, 2015; Anđelković et al., 2019; Gil-Gil et al., 2020; Dadashi et al., 2023), but to date, there have been no reviews on its significance of plant disease management and plant growth promotion. In this review, the growth and biocontrol traits of the genus Stenotrophomonas in several food crops, as well as the probable risks and challenges associated with the use of Stenotrophomonas in agriculture systems and potential future research, have been discussed.
Biocontrol potential of the genus Stenotrophomonas
Efficiency against plant pathogens
In recent years, the use of antagonistic microorganisms to manage plant diseases has been regarded as a viable alternative to synthetic fungicides. The effects of beneficial microorganisms on growth improvement and higher disease tolerance have been studied in many cropping systems. Microbial biocontrol agents have been shown to have a high possibility of enhancing the yield of food crops by increasing phosphate solubilization, fixing nitrogen, or restricting diseases (Parnell et al., 2016). The genus Stenotrophomonas is used as a potential microbial biocontrol agent against many plant pathogens hampering commercial crops (Mahaffee and Kloepper, 1997; Germida and Siciliano, 2001; Mehnaz et al., 2001; Berg et al., 2002). In addition to their other plant growth-promoting characteristics, Stenotrophomonas spp. exhibited antagonistic patterns against pathogenic oomycetes, fungi, bacteria, and insect pests. There are several Stenotrophomonas spp. that may interact well with plants, especially S. maltophilia and S. rhizophilia (Ryan et al., 2009). However, still, no report is available on the plant pathogenic nature of Stenotrophomonas and its related species.
According to de Oliveira-Garcia et al. (2003), S. maltophilia produces various kinds of pili that are involved in the development of complex biofilms as well as adherence to surfaces. The potential of S. maltophilia to interact with other microbes on the plant’s surface may be influenced by both adhesion and biofilm formation (Elvers et al., 2001). S. maltophilia strains has S. maltophilia fimbriae 1 (SMF1) fimbriae, which is made up of fimbrin subunits that are significantly similar to numerous pathogenic Escherichia coli and the Pseudomonas aeruginosa fimbria in terms of amino-terminal of amino acid sequence. The genome of S. maltophilia strains includes genes that encode type I and type IV pili, type I pili involved in adhesion and the early phases of biofilm formation. Type IV pili are also involved in adherence, auto aggregation, twitching motility, and biofilm formation. This might imply that invasion strategies of Stenotrophomonas spp. in plants form biofilms and exhibit twitching motility. S. maltophilia has the mannose-6-phosphate (Man) gene and lipopolysaccharide/exopolysaccharide-coupled biosynthetic genes (rmlA, rmlC, and xanB), which encode enzymes involved in the synthesis of lipopolysaccharides (LPS) and exopolysaccharides (Huang et al., 2006).
The biocontrol ability of Stenotrophomonas spp. has been proved in numerous crops. Through several mechanisms, these bacteria suppress the growth of phytopathogens. The novel antifungal substances maltophilin (Jakobi et al., 1996) and xanthobaccin (Nakayama et al., 1999) have been identified, and the majority of S. maltophilia isolates exhibit antifungal activity against various pathogens in vitro (Minkwitz and Berg, 2001). The strains of S. maltophilia generate a wide range of proteases, chitinases, glucanases, RNases, DNases, lipases, and laccases, and they have an incredibly high hydrolytic potential (Tandavanitj et al., 1989; Galai et al., 2009). The biocontrol capability of S. maltophilia includes both chitinolytic and proteolytic activities. Through the breakage of fungal cell walls, chitinases may protect plants against phytopathogens, but they may also play a part in activating induced defense systems (Mastretta et al., 2006). It is necessary to determine the precise roles played by the several exoenzymes involved in the antagonistic activity of Stenotrophomonas spp., thus making it possible that additional bacterial products may similarly control plant disease by inducing plant defenses.
The competition for iron is another essential factor in preventing pathogens. Siderophores produced by various pathogens such as ferrichrome, which is produced by several fungi, including the phytopathogenic Ustilago maydis can be efficiently captured by Stenotrophomonas (Ardon et al., 1997). The sequenced Stenotrophomonas spp. genomes feature large amounts of volatile organic compounds (VOCs), outer membrane proteins, and TonB-dependent receptors (TBDRs). Moreover, Stenotrophomonas spp. may release VOCs that act as inter- and intracellular communication signals and adversely influence the development of several pathogens (Wheatley, 2002). Several distinct VOCs produced by S. maltophilia and S. rhizophila inhibited the development of Rhizoctonia solani, which causes severe damage to economically significant crops and trees worldwide. Phenylethanol and dodecanal are the VOCs that have been identified in this study (Kai et al., 2007). However, the specific mechanism by which these secondary metabolites affect the pathogen is unknown and needs further research.
Further, Stenotrophomonas spp. release many hydrolytic enzymes that are important in the inhibition of plant diseases, including lipases, chitinases, RNAses, DNAses, and proteases (Berg et al., 2010). S. maltophilia MB9, a marine-isolated strain, was successful in preventing large numbers of phytopathogens including R. solani, F. oxysporum, and Curvularia sp. by producing dodecanoic acid, a broad-spectrum antifungal antibiotic (John and Thangavel, 2017). In a dual culture laboratory study, Stenotrophomonas spp. displayed significant zones of inhibition against R. solani, Verticillium dahlia, and Sclerotinia sclerotiorum (Wolf et al., 2002). S. maltophilia strains obtained from the rhizosphere of the brinjal crop in Egypt demonstrated inhibitory effects against Ralstonia solanacearum, a potato brown rot pathogen. The isolates were useful for decreasing symptoms in potatoes grown in soil in addition to being beneficial in vitro (Messiha et al., 2007).
According to Schmidt et al. (2012), a S. rhizophila strain (DSM14405T) promoted plant development by changing the rhizosphere microbiome. It was observed that the strain was successful in colonizing both the root and the shoots of cotton and sweet pepper. Based on molecular profiling using single-strand conformation polymorphism (SSCP), the rhizosphere fungal microbiome appears to be affected by S. rhizophila DSM14405T. In another study, S. maltophilia C3-derived chitinase inhibited conidial development and germ tube extension in Bipolaris sorokiniana (Zhang et al., 2001). Amino acid sequencing, polyacrylamide gel electrophoresis, and purification indicated that the isolate produced at least two distinct chitinases with potential antifungal activity (Zhang et al., 2001). Kobayashi et al. (2002) isolated a protein and the responsible gene from S. maltophilia 34S1 having chitinolytic and antifungal activities. The release of elicitor molecules by hydrolytic enzymes, in particular, contributes to the activation of plant defense systems in addition to the degradation of pathogenic cell structures. In the other study treatment of wheat plants with S. maltophilia SPB-9 during the in vivo experiment led to a rise in defense enzymes (Singh and Jha, 2017).
There have also been reports of Stenotrophomonas species developing increased disease resistance to plant pathogenic viruses. S. maltophilia HW2, which was isolated from cucumber, improved the plant’s resistance to the cucumber green mottled mosaic virus. HW2 application on cucumber may slow down virus replication and prevent the expression of viral protein genes. It also induced the expression of antioxidant enzyme genes and defense-related genes (Li et al., 2016). The most recent research on the biocontrol effectiveness of Stenotrophomonas species used against different plant diseases is summarized in Table 1. However, further research is needed to fully understand the mechanisms of action and to optimize (toxicity, formulation, consortium development) the use of this bacterium in agriculture.
Table 1.
Biocontrol efficiency of Stenotrophomonas spp. against plant pathogens.
| Stenotrophomonas spp. | Pathogen | Hosts | Reference |
|---|---|---|---|
| S. rhizophila | Colletotrichum gloeosporioides | Mango | Reyes-Perez et al. (2019) |
| S. maltophilia E38 | Ralstonia solanacearum | Tobacco | Li et al. (2023) |
| S. maltophilia CR71 | Botrytis cinerea | Tomato | Rojas-Solís et al. (2018) |
| S. maltophilia B8 | Fusarium oxysporum f.sp. cepae | Garlic | Dewi et al. (2023) |
| S. maltophilia UN1512 | Colletotrichum nymphaeae | Strawberry | Alijani et al. (2020) |
| S. rhizophila | Fusarium proliferatum | Muskmelon | Rivas-Garcia et al. (2018) |
| S. maltophilia PPB3 | Sclerotium rolfsii | Tomato | Sultana and Motaher Hossain (2022) |
| Stenotrophomonas sp. BHU-S7 (AgNPs) | S. rolfsii | chickpea | Mishra et al. (2017) |
| S. rhizophila |
Leptosphaeria maculans, Leptosphaeria biglobosa |
Rapeseed | Schmidt et al. (2021) |
| S. maltophilia UPMKH2 | Pyricularia oryzae | Rice | Badri Fariman et al. (2022) |
| S. maltophilia | Magnaporthe grisea | Rice | Etesami and Alikhani (2016) |
| S. rhizophila | Fusarium oxysporum | Cucumber | Wang et al. (2022) |
| Stenotrophomonas sp. TRM2 | Bipolaris sorokiniana | Wheat | Villa-Rodríguez et al. (2019) |
| S. rhizophila KM01, KM02 | Pythium ultimum | Chilli | Lara-Capistran et al. (2020) |
| S. maltophilia | Colletotrichum musae | Banana | Damasceno et al. (2019) |
| S. rhizophila 88bfp | Athelia rolfsii | Turfgrass | Ünal et al. (2019) |
| S. chelatiphaga | Pseudomonas tolaasii, Ewingella americana | Button mushroom | Aslani et al. (2018) |
| S. maltophilia S23, S24, S26 and S28 | Fusarium oxysporum f. sp. lycopersici | Tomato | Aydi-Ben-Abdallah et al. (2020) |
| Stenotrophomonas sp. S81-1 | Ganoderma boninense | Oil palm | Fadly et al. (2021) |
| S. maltophilia JVB5 | Various pathogens | Sunflower | Adeleke et al. (2022) |
| Stenotrophomonas sp. P7T6–4 | Rhizoctonia solani | Tomato | Hadi et al. (2020) |
| S. rhizophila 88bfp | Fusarium cerealis | Turfgrass | Senocak et al. (2020) |
| S. maltophilia TD 1 | Fusarium solani | Citrus | Ezrari et al. (2021) |
| Stenotrophomonas sp. AG3 | Macrophomina phaseolina | soybean | Santos et al. (2021) |
| S. rhizophila | Xylella fastidiosa | Olive | Mourou et al. (2022) |
| S. maltophilia strain A1w2 | Xanthomonas oryzae pv. oryzae | Rice | Rahma et al. (2022) |
| S. maltophilia Sg3 | Cucumber Mosaic Virus | Tobacco | Khalimi et al. (2020) |
Entomopathogenic effect against insect pests
Plant defense against attack by insect pests is associated with a number of phytohormones. Jasmonic acid (JA) is the main phytohormone that supports plant defense against insect pests (War et al., 2012). According to research by Pangesti et al. (2015), the JA signaling pathway is critical for Arabidopsis thaliana rhizobacteria-triggered ISR against Mamestra brassicae (cabbage moth). It is well known that plants need proteinase inhibitors (PIs) in order to protect themselves against insect pests. Studies revealed that PGPR-mediated ISR is associated with elevated expression of plant responsive genes that encode for PIs and elevated activity of defense-related enzymes (Harun-Or-Rashid and Chung, 2017). In plants that have received the PGPR treatment, defense responses may be generated more rapidly in the case of a pest attack. Inoculating tobacco plants with S. rhizophilia may induce JA accumulation and increase the transcript level of JA sensitive genes, resulting in the induction of systemic resistance in tobacco plants against Spodoptera litura (tobacco cutworm) (Ling et al., 2022). The biocontrol ability of Stenotrophomonas spp. against insect pests is less recorded and further research on integrated management of serious insect pests is required. However, the summary of recent research on Stenotrophomonas spp. biocontrol efficiency applied against insect and nematode pests is provided in Table 2. Further research on Stenotrophomonas spp. against insects is needed to fully understand its capabilities and limitations. However, the promising results of recent studies suggest that Stenotrophomonas may be a useful lever in integrated pest management for a variety of crops (Table 3).
Table 2.
Entomopathogenic effect of Stenotrophomonas spp. against insect pests.
| Stenotrophomonas spp. | Insect | Scientific name | Reference |
|---|---|---|---|
| S. tumulicola T5916-2-1b | Aphids |
Aphis punicae, Aphis illinoisensis |
Baazeem et al. (2022) |
| S. maltophilia | Termites |
Coptotermes heimi, Heterotermes indicola |
Jabeen et al. (2018) |
| S. maltophilia W 2–7 | Root-Knot Nematode | Meloidogyne spp. | Vishnu and Nisha (2020) |
| S. rhizophila | Tobacco cutworm | Spodoptera litura | Ling et al. (2022) |
| S. maltophilia | Potato beetle | Leptinotarsa decemlineata | Aktas et al. (2022) |
Table 3.
The effect of Stenotrophomonas spp. on host plant.
| Host/ Source | Strain | Homology to the reference strain | Accession no. | Colonization status | Action | Reference |
|---|---|---|---|---|---|---|
| Rhizosphere of tomato plants | Two oxalotrophic strains (OxA and OxB) | S. maltophilia | - | Endophytically | Strain OxA and OxB protected host from the damage caused by high doses of oxalic acid Protected host from S. sclerotiorum and B. cinerea infections. Moreover, Callose deposition induced by OxA and OxB was required for protection against phytopathogens Inoculation of theses bacteria induced the production of phenolic compounds and the expression of PR-1 Both isolates exerted a protective effect against fungal pathogens in Arabidopsis mutants affected in the synthesis pathway of salicylic acid (sid2-2) and jasmonate perception (coi1) Moreover, B. cinerea and S. sclerotiorum mycelial growth was reduced in culture media containing cell wall polysaccharides from leaves inoculated with each bacterial strain |
Marina et al. (2019) |
| Equisetum arvense | ES2 | S. maltophilia | KY486848 | Endophytically | Greatest ability to synthesize indole-3-acetic acid (IAA)-like compounds, Highest metabolic activity based on the Biolog GEN III test. |
Woźniak et al. (2019) |
| Zea mays | ZR5 | Stenotrophomonas sp. | KY486808 | |||
| Arctium lappa | AR4 | S. maltophilia | KY486847 | |||
| Pistacia atlan-tica L. | Sm25 | S. maltophilia IAM12423T | KU693275 | Endophytically | Siderophore production Plant growth promotion |
Etminani and Harighi (2018) |
| Sm97 | S. maltophilia IAM12423T | KU693276 | ||||
| Nodule-associated bacteria (NAB) were isolated from wild legume nodules | LXo13 | Stenotrophomonas sp. | – | – | The most frequent PGP activity identified among the strains isolated from wild legumes was IAA synthesis Two bacteria, Stenotrophomonas sp. |
Tapia-García et al. (2020) |
| LX010a | ||||||
| Rhizospheric isolates | NGB-15 | Stenotrophomonas sp. strain KG-16-3 | LC322228 | Rhizospheric | Phosphate solubilization Plant growth promoting activity over the control (increased shoot and root fresh and dry biomass) |
Youseif (2018) |
| NGB-18 | S. maltophilia strain E136 | LC322229 | ||||
| Corn Rhizosphere | L,o.INCA-FRr1 | S. rhizophila | – | Rhizospheric | Ability to perform the FBN, Solubilization of phosphorus, potassium and antagonistic activity against Fusarium oxysporum |
Pérez-Martínez et al. (2020) |
| INCA-FRc24 | S. pavanii | – | ||||
| Aerial parts of poplar | Stenotrophomonas strain 169 | Stenotrophomonas strain Fa6 (AY131216) | CP061204 | Endophytically | The root weight of the inoculated plants was three times higher than that of the untreated plants, Showing a significant increase in both root and shoot length of the inoculated in vitro plants Tolerance of plants to abiotic stresses Production of siderophores and the ability to mobilize phosphates, as well as the production of the plant growth-promoting substances IAA and polyamines |
Ulrich et al. (2021) |
| 11 wild plants (rhizosphere and phyllosphere) | Stenotrophomonas sp. TmP43c | S. rhizophila | – | rhizosphere | Highest number of psychrotolerant strains, Higher phosphate solubilization activity |
Vega-Celedón et al. (2021) |
| Sugarcane rhizosphere | COA2 | S. maltophilia | MN527324 | Rhizospheric | Phosphate solubilizing, Siderophore Production IAA production Ammonia Production ACC production Antagonistic activity Increased the activity of SOD, CAT, PAL, CHI, and GLU enzyme Nitrogen fixative |
Singh et al. (2020) |
| Sunflower root endosphere | SAMN18138830 | S. indicatrix BOVIS40 | JAGENA000000000 | Endospheric | Plant growth promoter IAA production Siderophore production Phosphate solubilizing Exopolysaccharide Amylase, Cellulase, Xylanase, Mannanase and Protease enzymes enhancer |
Adeleke et al. (2021) |
| Strawberry leaves | UN1512 | S. maltophilia | MT448956 | Endophytically | Antagonistic effect against C. nymphaeae in dual culture Secreted protease, chitinase, pectinase, siderophore, IAA, and gibberellin Produced volatile compounds (Benzothiazole, Cyclooctatetraene-1-carboxaldehyde, Carbonic acid, octadecyl phenyl ester, Benzaldehyde, 2,5-bis, Estragole, Benzaldehyde) Growth promoter |
Alijani et al. (2020) |
| Oil-free soil | SR1 | S. maltophilia-SR1 | MH634684 | Rhizospheric | Highest plant growth promoter Upregulated nitrate, nitrate reductase, total nitrogen, and nonenzymatic and enzymatic antioxidants in plants Suppressed oxidative and nitrosative stress Produced indoleacetic acid and ammonia as well as phosphate solubilization |
Bashandy et al. (2020) |
| Tomatillo (Physalis ixocarpa) roots | CR71 | S. maltophilia | MF992168 | Endophytically | Promoted the shoot and root length, chlorophyll content, and total fresh weight of tomato Biocontrol of B. cinerea through the production of potent volatiles such as dimethyl disulphide |
Rojas-Solís et al. (2018) |
| Salt-resistant Carex distans (distant sedge) roots | SRS1 | S. rhizophila DSM14405 | – | Endophytically | Increased the induction of plant genes related to abscisic acid and auxin signaling Enhanced plant growth |
Manh Tuong et al. (2022) |
| Mucuna utilis var. capitata L. (Safed Kaunch) | RMC6 | S. maltophilia | HM480495 | Endophytically | Antagonistic against Fusarium udum Plant growth promoter (IAA, phosphate solubilization and ACC deaminase, siderophore production) |
Aeron et al. (2020) |
| Soil Contaminated by Industrial Effluent | S25 | S. maltophilia | KY651248 | rhizosphere | As-reducing capability Produced Hydrogen Cyanide, Nitrogen Fixation, and Auxin Plant growth promoter |
Huda et al. (2022) |
Plant growth promoting activity of Stenotrophomonas spp.
Biofertilizers are a reliable source to promote plant growth without harming the soil, plants and environment (Reddy et al., 2020). The plant growth promoting rhizobacteria could be a viable substitute to overcome the load of synthetic fertilizers which are used indiscriminately in the agricultural sector as well as to enhance farmers income because they are cost-effective and durable (Pérez-Martínez et al., 2020). In this context, the genus Stenotrophomonas is characterized as promising plant growth promoting bacterium, which are reported as inducers and protectors against biotic and abiotic stresses (Table 3).
In addition to that, the three isolates of the genus Stenotrophomonas isolated from healthy tomato plants were able to produce indole-3-acetic acid; two of these strains had phosphate solubilization ability (Ben Abdallah et al., 2018). S. maltophilia P9 was isolated from the algal biomass and identified as a potential pectinase producing with biotechnological significance (Sharma and Sharma, 2018). In a study, Woźniak et al. (2019) reported that Stenotrophomonas strain ES2 promotes growth under in vitro conditions. Seed treated wheat plants with rhizospheric S. maltophilia SBP-9 originated under salt stress conditions, showed improved plant growth, such as increased shoot and root length, along with balanced chlorophyll content compared to controls (Singh and Jha, 2017). Similar results were reported with another strain of S. maltophilia BJ01 by Alexander et al. (2020), they found that peanut crop showed improved growth and enhanced photosynthetic pigments and growth hormones under salt stress conditions. In a recent study, Nigam et al. (2022) reported that Stenotrophomonas sp. were effective in increased growth, protein accumulation, osmotic adjustment, and Ascorbate peroxidase (PAX) activity in soybean and spinach cultivars under salt stress conditions.
In another study, Bashandy et al. (2020) isolated S. maltophilia-SR1 from oil free soils and applied it to soil contaminated by oily wastewater, reporting that the strain successfully used several aromatic hydrocarbons, including benzene, toluene, and xylene, as its sole carbon source and showed plant growth promoting (PGP) properties (indoleacetic acid (IAA), and phosphate solubilization). The mode of action of the arsenic-resistant S. maltophilia S255 isolated by Huda et al. (2022) appears to involve several mechanisms such as auxin and hydrogen cyanide production, phosphate solubilization, and nitrogen fixation. These mechanisms may enhance plant growth and improve nutrient uptake, which could be beneficial in agriculture. However, it is important to note that these findings were obtained from a glasshouse study, and further research is needed to confirm the efficacy of this bacterium in large multilocated field trials. It is possible that the efficacy of the arsenic-resistant S. maltophilia S255 could vary depending on the origin of the isolates and that native isolates should be considered for recommendation. Therefore, future research should focus on investigating the efficacy of this bacterium in different regions and under various environmental conditions. It is also important to evaluate the safety and potential risks associated with the use of this bacterium as a growth promoter in agriculture.
Stenotrophomonas in nitrogen fixation
Nitrogen-fixing microorganisms use a complex enzyme system called nitrogenase to convert atmospheric elemental nitrogen into plant-usable forms (Masson-Boivin and Sachs, 2018). Nonsymbiotic nitrogen fixation occurs among various genera, including Acetobacter, Arthrobacter, Azotobacter, Bacillus, Clostridium, Diazotrophicus, Pseudomonas, and Stenotrophomonas (Fitton et al., 2019), while symbiotic nitrogen fixation occurs among the members of Rhizobiaceae family along with leguminous plants (Dinnage et al., 2019). Beneficial soil microorganisms, including PGPR, are responsible for fixing a large portion of the elemental nitrogen that enters the soil under natural conditions (Tang et al., 2020). Thus, biological nitrogen fixation via plant-microbe interactions is a major factor in the manufacturing of organic fertilizers (Singh et al., 2023).
Recent research has shown that the S. maltophilia strain UPMKH2 can increase rice yield and productivity by providing the plant with a steady supply of nitrogen (Badri Fariman et al., 2022). As a nitrogen source, these bacteria can produce plant growth regulators like IAA, and ACC deaminase (precursor of ethylene), which aid plants in absorbing nutrients and expanding their tissues (Sarkar et al., 2018). In addition to maize, peanuts, rice, sugarcane, and wheat and have all been shown to benefit from S. maltophilia’s nitrogen-fixing abilities (Wang et al., 2018a) as PGPR. Different PGP-traits and nitrogenase activities were confirmed with nitrogenase (nifH) gene amplification after strains of S. maltophilia-COA2 were selected and identified by sequencing their 16S rRNA gene.
To combat sugarcane diseases and cut down on nitrogen fertilizer use, researchers have looked into S. maltophilia -COA2 for the first time (Singh et al., 2020). Cerezer et al. (2014) evaluated the nitrogen-fixing capabilities of the atmosphere and symptomatic Stenotrophomonas spp. It was discovered that Stenotrophomonas isolates have the ability to fix atmospheric nitrogen in the soil, leading researchers to speculate that the reduction of nifH clusters of genes is a conservation of energy adaptation of Stenotrophomonas during its evolution from a free-living to an opportunistic pathogenic form. Inoculating foxtail millet with the nitrogen-fixing strain S. rhizophila EU-FEN-32 as part of a microbial consortium resulted in greater increases in growth and physiological parameters compared to both synthetic fertilizer and the untreated control (Kaur et al., 2023).
Endophytic association with S. maltophilia is beneficial to anti-fungal activity and plant growth (phytohormone induction, N2 fixation) (Rojas-Solís et al., 2018a). In addition, S. pavanii, a Gram-negative, non-motile, and spore-less species, fixes N2 in sugarcane (Ramos et al., 2011). Recent research has shown that common nitrogen fixers, such as rhizobia, do not always colonize or infect the plant roots of leguminous plants but instead typically coexist with Stenotrophomonas in other plants. Synergistic processes for nodule formation and enhanced nitrogen fixation capabilities have been postulated when PGPR, like Stenotrophomonas species, interact with Rhizobium (Abd-Alla et al., 2019). The ability to form nodules in the roots of Robinia pseudoacacia has been attributed in part to the horizontal transfer of essential nodulation and nitrogen-fixation genes from rhizobia to other Gammaproteobacteria (Stenotrophomonas) and Betaproteobacteria (Burkholderia) (Abbott and Peleg, 2015). These nitrogen-fixing Stenotrophomonas species can be evaluated in other cropping systems for their potential role in nitrogen fixation and any other side effects on beneficial microbes found in the soil ecosystem.
Stenotrophomonas in phosphorous solubilization
One of the main macroelements that plants require for growth and development is phosphorous, but due to its poor solubility, most of the phosphorous in the soil is unreachable to plants. Phosphorous cannot be utilized by plants because it quickly precipitates in the soil as insoluble combinations with a variety of cations, including Mg, Ca, Al, and Fe. Because P ions are strong ligands, they frequently unite with metal ions to create complexes (Shen et al., 2011). Despite their numerous disadvantages, chemical phosphorous fertilizers are frequently recommended for agricultural soils with a phosphorous deficiency. Due to a confluence of environmental factors and the excessive use of synthetic fertilizers, soil fertility is declining (Ali et al., 2021).
Phosphorus-solubilizing microorganisms are being investigated as an alternative way to address these issues and meet the phosphorous requirements of crop plants. Although many phosphorous solubilizing microorganisms have been identified (Kishore et al., 2015), the majority of them are not well suited to the environmental factors that lead to the production of available phosphorous in the field. Similar to this, Stenotrophomonas is a potent phosphorus solubilizing bacterial genus that can release an adequate amount of phosphorus in solution from insoluble rock phosphates and calcium phosphate (CP) (Amri et al., 2023). Xiao et al. (2009) used the National Botanical Research Institute Phosphate (NBRIP) medium to isolate S. maltophilia YC from Chinese phosphate mines. The isolate efficiently produced 180.5 mg/L when Tricalcium phosphate (TCP) was the only source of soluble P. The medium’s pH decreased from its initial value of 7.0 to its lowest value of 4.3 after 4 days of incubation. According to the high performance liquid chromatography (HPLC) findings, the isolates were gluconic acid producers during the P-solubilization procedure. The isolate was found to solubilize phosphorus most successfully when fed a diet of maltose and ammonium nitrogen, according to the researchers (Paul and Sinha, 2017).
Singh and Jha (2017) also discovered S. maltophilia with P-solubilizing potential in the rhizosphere soil of Sorghum bicolor. The organism only made a tiny amount of soluble P (10.73 2.34 mg/mL) using TCP. On the other hand, S. maltophilia MB9 was discovered to be effective at producing noticeable zones of solubilization on TCP-containing agar plates after being successfully isolated from a marine environment (John and Thangavel, 2017). Stenotrophomonas maltophilia AVP27, which was isolated from the chilli rhizosphere, produced significant zones of P solubilization (Kumar and Audipudi, 2015). TCP significantly increased the isolate’s ability to produce soluble P, as determined by quantitative techniques. Stenotrophomonas maltophilia MTP 42 was found to produce 362 mg/mL of soluble P in the rhizosphere soil of Coleus forskohlii (Patel and Saraf, 2017). It has been found that Stenotrophomonas sp. RC5 was discovered in the rhizosphere of ray grass (Lolium perenne), where it synthesizes carboxyl and hydroxyl ions to chelate cations or lower the pH to release P. In the periplasm, the direct oxidation path produces the organic acids. P ions are released due to the substitution of H+ for Ca2+ that occurs during the excretion of these organic acids, which acidifies the microbial cells and the surrounding environment (Barra et al., 2019). These Stenotrophomonas species can be used in various crops for phosphate solubilization and plant growth improvement, but further research is needed to determine any side effects.
Stenotrophomonas in potassium solubilization
Although nitrogen and phosphorus have been extensively studied in relation to the success of exotic species invasions (Mudau et al., 2007; Sinha and Tandon, 2020), the role of potassium in such invasions has received much less attention. However, K is the second most abundant nutrient in leaves, after nitrogen, and the most abundant cation in plant cells. Soil is home to a wide range of potassium-solubilizing bacteria (KSB), as has been demonstrated by numerous studies (Han and Lee, 2005; Kumar et al., 2015; Meena et al., 2015; Sun et al., 2020). Bacillus, Pantoea, Paenibacillus, Pseudomonas, Rahnella, and Stenotrophomonas, are some of the KSB genera that have been the subject of research in the past (Bahadur et al., 2019; Adeleke and Babalola, 2022). Stenotrophomonas maltophilia MB1, MB5, MB6, and MB9 potassium solubilization and biocontrol activities like production of ACC deaminase, siderophore, and yield enhancing strains are isolated from marine environments, in which MB9 is the most potent and dominant strain after application of S. maltophilia MB1, MB5, MB6, and MB9 in crops (John and Thangavel, 2017).
In another study, S. maltophilia RSD6 has been successfully isolated from the rhizospheric soil of Oryza sativa, and can be used as an alternative to agrochemicals (Nevita et al., 2018). Bacillus spp., Burkholderia spp., Pseudomonas spp., and Stenotrophomonas spp., were all isolated from tea rhizosphere soil and proven to be effective KSB strains (Gopi et al., 2020). Only Streptomyces alboviridis, S. rhizophila, and Nocardiopsis alba out of a panel of Actinobacteria strains studied were able to dissolve potassium from mica. By Pérez-Martínez et al. (2020) 15 Stenotrophomonas strains were isolated from the maize rhizosphere, and two of these were able to soluble potassium sources, while another six showed antagonisms against the pathogen. Acidic and neutral pH usually led to more K release, whereas alkalinity conditions only made Stenotrophomonas sp. INCA-FRr1 release more K (Verma et al., 2016). These Stenotrophomonas species can be utilized in food crops to enhance crop growth after multilocation and large-scale field trials, however, more potential KSB strains of Stenotrophomonas that have both disease control and plant growth promotion activity should be identified in future research so that they could be used as multi-fold agents.
Stenotrophomonas in phytohormones production
Apical dominance, cell elongation, cell division, tissue differentiation, and intracellular communication are just some of the physiological processes that are influenced by phytohormones, also known as plant growth regulators, which are substances synthesized by plants and act as signaling molecules (Tshikhudo et al., 2023). There are five broad categories based on their structural make-up and how they interact with plants’ physiological processes. Auxins, gibberellins, cytokinins, ethylene, and abscisic acid make up the big five. In order to combat the harmful effects of environmental stress, plants will often keep their levels of endogenous hormones constant (Kumar et al., 2012). Phytohormones are produced by a diverse group of bacteria found in plants and soil. Plants’ responses to hormones are crucial to their development and growth. Phytohormones play a significant role in mitigating both biotic and abiotic stress. Plant growth is controlled by a variety of hormones, including gibberellins, auxins, and cytokinins, and these hormones have been linked to developmental processes in plants (Wani et al., 2016). Here we describe how Stenotrophomonas affects plant growth and development. Gibberellic acid (GA), ethylene, and indole acetic acid (IAA) were the plant growth regulator traits found in S. maltophilia (Singh and Jha, 2017). Deconjugation of gibberellin-glucosyl conjugates secreted from the roots stimulates plant growth (Jędrzejuk et al., 2023). Roots contain inactive 3-deoxy gibberellins, which are converted by bacteria and fungi into their active forms, GA1, GA3, and GA4 (Salazar-Cerezo et al., 2018).
Many aspects of plant development, such as differentiation and cell division, organogenesis, tropic responses, and gene regulation, are controlled by plant growth regulators like IAA (Ryu and Patten, 2008). Many different rhizobacterial strains have been shown to significantly increase plant growth by producing IAA. Due to their consistent release of IAA at minimal concentrations, some strains are also considered to be particularly effective at accelerating plant growth (Tsavkelova et al., 2007). Plant-associated Stenotrophomonas species, like many others, were found to effectively produce IAA in the medium used for crop cultivation, with or without the addition of tryptophan. IAA production in 16 Stenotrophomonas isolates (both clinical and environmental) was studied by Suckstorff and Berg (2003) and every single isolate tested positive for IAA production.
A study conducted in Germany with S. maltophilia e-p19 had an IAA concentration of 5.2 mg/mL, while S. maltophilia e-a23 had a concentration of 0.7 mg/mL. Isolates found to be part of environmental clusters were also found to produce more IAA than clinical cluster isolates (Hassan and Bano, 2016). In another study, IAA was found to be produced by S. maltophilia BE-25, which was isolated from the root of a banana plant. With or without tryptophan, IAA production from the isolate was sufficient, according to a thin layer and high-performance liquid chromatography analysis (Ambawade and Pathade, 2013). Stenotrophomonas maltophilia, isolated from the forest soil, also produced IAA in higher amounts (50.4 ± 0.9 g/mL) (Amri et al., 2023). The isolate was also capable of producing gibberellic acid, another plant growth regulator. Stenotrophomonas maltophilia, obtained from the rhizosphere of Cenchrus ciliaris, was also studied for its ability to promote plant development in the presence of tryptophan under salt stress conditions (Hassan and Bano, 2016). Their findings suggest that S. maltophilia IAA production is crucial to the induction of salt tolerance. However, their actual efficacy on a large field scale requires further attention for their commercialization.
Stenotrophomonas in siderophore production
Due to its importance in respiration, DNA synthesis, heme formation, and other biochemical reactions, iron deficiency can stunt the development of plants (Shetty and Corson, 2020). Iron is present in high concentrations in the Earth’s crust, but its bioavailability is limited due to the insoluble nature of the Fe3+ ion (Behnke and LaRoche, 2020). Common minerals’ hydroxide and oxide phases tend to accumulate iron, rendering it unavailable to plants and other living things. Stenotrophomonas is one of the PGPR that can make a siderophore, which is utilized to remove iron from insoluble mineral phases. According to their chemical makeup and coordination site, these low molecular weight (500–1,000 Da) ferric ion chelating compounds can be grouped into three groups: the catecholate, the carboxylic type, and the hydroxamate type (Pan et al., 2022). Although many pathogenic organisms view the production of siderophores as a virulence factor, plant-associated organisms view it as a growth-promoting trait because it facilitates iron uptake and prevents the spread of plant pathogenic microorganisms.
In addition to PGPR strains, it has been reported that several plant-associated Stenotrophomonas strains also produce siderophores. Like many other strains of S. maltophilia, SPB-9 was found to produce an orange color zone on chrome azurol S (CAS) agar plates (Singh and Jha, 2017). Hydroxamate-type siderophores are produced by S. maltophilia MTP-42, which has been found in the rhizosphere of Coleus forskohlii (Patel and Saraf, 2017). Ghavami et al. (2017) studied the siderophore production by S. chelatiphaga LPM-5 T and reported that in the CAS liquid medium, the isolate was found to produce 12.66% siderophore. In a spectrophotometric test performed by Wilson et al. (2016), positive results indicated that the isolate generated a carboxylic siderophore. Siderophores are essential for preventing the invasion of pathogenic fungi and also help plants absorb more iron. By limiting the amount of ferric ions available, the growth of pathogenic fungal strains is stifled (Kloepper et al., 1980). Several strains of Stenotrophomonas were found to have the ability to produce siderophores, which were then shown to inhibit the growth of pathogenic fungi. Stenotrophomonas can scavenge molecules of the siderophore class that are produced by other microorganisms, such as the phytopathogenic fungus Ustilago (Sun et al., 2022). TonB-dependent outer membrane protein receptors (TBDRs) have been reported to be present in the genome of S. maltophilia, and their primary function is the active transport of the iron-siderophore complex. This superior iron uptake capacity suggested that they could pose a threat to other organisms as they evolved into endophytes or rhizosphere residents.
Exopolysaccharides (EPS) production by Stenotrophomonas spp.
Bacterial EPS supplies protection from different environmental stresses, such as predation, desiccation, and the effects of antibiotics (Limoli et al., 2015). EPSs have an important role in the aggregation of bacterial cells and supply carbon when the substrate is in low concentration (Banerjee et al., 2021). The biosynthesis of EPS by bacterial cells depends upon environmental and nutritional conditions (Nouha et al., 2018). Different microorganisms utilize various sources of carbon and nitrogen and differ in their mineral requirements, pH, and temperature, which are important factors for maximum EPS production (Aeron et al., 2020). Brevibacillus parabrevis (V4) and S. maltophilia (c6) were the two nodule endophytic isolates with the highest EPS production capability (among C1-C13 and V1-V7) (Abd-Alla et al., 2018). This research suggests that Brevibacillus parabrevis (V4) and S. maltophilia (c6) can generate a high yield of EPS when fed a diet rich in sucrose. Stenotrophomonas maltophilia (c6) does not use date molasses, lactose, galactose, or glucose as carbon sources for EPS production. Potassium nitrate stimulated EPS production in S. maltophilia (c6) and glycine did the same for B. parabrevis (V4) (Castellane et al., 2015). It is worth noting that S. maltophilia (c6) EPS yield and growth are suppressed by Fe3O4 (25–200 g/mL) and Fe2O3 (20–100 g/mL) NPs at varying concentrations. Stenotrophomonas maltophilia strain WR-C isolated from a clogged septic tank system that consistently formed biofilms on sand grains produced EPS, and caused clogging in the sand column (Abd-Alla et al., 2018).
Stenotrophomonas in rhizoremediation
Plant enzymes initiate the degradation of substances during phytoremediation, while the local microbial population carries it out during natural attenuation or bioaugmentation. It has been reported in several studies that certain microbes in the rhizosphere of plants used for phytoremediation or of plants growing from surrounding vegetation on a contaminated site play a significant role in the degradation of pollutants. This process, known as rhizoremediation, involves the rhizobacterial community (Rane et al., 2022). Sometimes, bacteria in the rhizosphere are essential to the decomposition process. There is widespread agreement that microbial bioremediation processes are a useful method for cleaning up polluted areas. This catabolic plasticity plays a crucial role in the breakdown of xenobiotic compounds and the conversion or accumulation of environmental pollutants.
In order to understand metabolic and regulatory networks and to provide novel pathways and microorganisms that will be useful for future applications, genome-based global studies are on the rise. After analyzing their genomes, scientists discovered that different strains of Stenotrophomonas can produce enzymes that aid in the breakdown of polychlorinated hydrocarbons and metals. Significant roles for Stenotrophomonas spp. in the breakdown of geosmin, hexahydro-1, 3, 5-trinitro-1, 3, 5–triazine (RDX), keratin, macrocyclic hydrocarbon, nitrophenol, and phenanthrene (Gao et al., 2013). Species of Stenotrophomonas have been found effective for bioremediation of agricultural soil by removing various chemical pesticides and insecticides, in addition to a wide range of environmental pollutants. The species will be useful in reducing the need for harmful chemicals in farms. A strain of S. acidaminiphila isolated by (Uniyal et al., 2016) from the rhizosphere soil of Zea mays was found to degrade fipronil, a common insecticide. A novel fipronil degrading pathway for the isolate has been proposed, and it has been suggested that the isolate could be used for bioremediation of fipronil-contaminated soil. Pourbabaee et al. (2017) also reported that S. maltophilia degraded diazinon (a pesticide). They also provided an analysis of the pesticide’s likely degradation pathway based on FTIR. A mineralization pathway involving dechlorination, hydroxylation, and carboxylation processes was proposed based on genome annotation of the DDT degradation gene and GC–MS analysis of metabolites (Pan et al., 2016).
Zaffar et al. (2018) reported that a S. maltophilia EM-1 strain degrades the organochlorinated pesticide endosulfan. Endosulfan was the only sulphur source used by the bacteria. According to gas chromatography mass spectrometry testing, the bacteria are capable of metabolizing endosulfan into safer compounds like endosulfan diol. Organophosphorus insecticides belonging to the groups O, O-dialkyl phosphate, and O, O-dialkyl phosphorothioate were degraded by Stenotrophomonas sp. G1, which was isolated from the sludge of a chlorpyrifos manufacturer plant (Deng et al., 2015). Two Stenotrophomonas isolates, S. maltophilia MHF ENV20 and S. maltophilia MHF ENV23, were found to degrade chlorpyrifos, cypermethrin, and fenvalerate (Fulekar, 2014). It has also been reported that S. maltophilia M1 degrades the nemato-pesticide methomyl (Oxime carbamates). The strain was discovered at a site where methomyl was used for irrigation. A 5 kb plasmid (PMb) containing the gene responsible for methomyl degradation was confirmed after the transformation of the plasmid DNA into Escherichia coli (Mohamed, 2009).
In a symbiotic association with strain Stenotrophomonas sp. W16, the rate at which fomesafen is degraded in the soil increases from 29.17 to 57.87%. To aid in the bioremediation of herbicide-contaminated farming soil, this study presents a novel fomesafen-degrading rhizobium that may be used in conjunction with legumes in symbiotic systems to break down the chemical (Chen et al., 2023). Pesticides often have residual concentrations that are above regulatory thresholds. Wherever this is a problem, getting chemical-free agricultural soils fit for growing eco-friendly crops is a major obstacle. Since bioremediation is a greener, cheaper, and more effective method than physical and chemical methods, it can be used to exploit the microbial metabolism of native microorganisms for degradation.
Potential risks and challenges of using Stenotrophomonas spp. in agriculture
Stenotrophomonas is a novel, multi-antibiotic resistant, opportunistic plant-associated bacterium on a global scale. It is reported that this bacterium remains associated with several plants. Moreover, it is used in bioremediation techniques and sustainable agricultural practices as a microbial biocontrol or anti-stress agent for crops. Several studies have demonstrated the immense potential of Stenotrophomonas spp. in agriculture; they may enhance plant growth and germination while suppressing plant diseases (Messiha et al., 2007).
On the other hand, S. maltophilia is also a novel human pathogen that can cause infectious diseases in humans. The only Stenotrophomonas species known to cause human illness is S. maltophilia; however, isolates of this species vary widely in terms of phylogeny and phenotype (Roscetto et al., 2008). This is probably related to the plethora of environmental niches occupied by this bacterium; the vast majority of infections are likely due to contact with distinct environmental sources. In fact, S. maltophilia epidemics are uncommon and are induced by contaminated origin, such as water sources (Park et al., 2008). S. maltophilia strains are undoubtedly equally capable of infecting people but only affect those with underlying illnesses, not the general population (Lira et al., 2017). The innate resistance of S. maltophilia to many first-line antimicrobials, including beta-lactams, macrolides, tetracycline, chloramphenicol, and quinolones, is the primary cause of the increase in S. maltophilia infections (Crossman et al., 2008). Additionally, S. maltophilia isolates may quickly evolve resistance to newer antibiotics via mutation; the underlying processes are unknown but are likely to be the consequence of excess production of intrinsic efflux transporters (Gould and Avison, 2006). Insufficient information exists regarding the pathogenicity of these organisms to humans. It is further demonstrated by the phenotypic and phylogenetic diversity analysis that there is a difference between pathogenic and other strains, but it is unquestionably a PGPR. Field tests and other direct applications cannot be conducted with opportunistic pathogens.
However, since S. rhizophila is not recognized as a human pathogen, these strains are interesting choices for biological control and stress resistance in plants because they often live endophytically. Stenotrophomonas pavanii is a recently discovered bacterium that can fix nitrogen from the atmosphere. The S. pavanii strain obtained from sugar cane is widely used in organic agriculture (Ramos et al., 2011). S. pavanii is a unique species of PGPR, and it is thought to focus on improving development and yield in many crops.
The diversity of Stenotrophomonas strains makes it difficult to distinguish characteristics because of their advantageous interactions with plants and their facultatively harmful infections with humans (Berg and Martinez, 2015). Differentiation of characteristics is crucial for future agricultural applications as well as for our understanding of infection risks and associated epidemiological issues. Predicting beneficial pathogen threats to human health is one of the current difficulties for agricultural biotechnology. Therefore, there is room to develop a new aspect of Stenotrophomonas spp. uses and importance in agriculture.
Future remarks
The genus Stenotrophomonas has been shown to possess a number of functional properties, and the research performed so far has demonstrated that it can be used to manage pests and pathogens as well as promote plant growth. However, there are still gaps in research that must be filled. For example, few species of Stenotrophomonas are human pathogens that can cause serious diseases in humans; however, further research is needed to discriminate between human pathogenic and non-pathogenic strains of Stenotrophomonas spp. and their deployment in crop improvement and ex-situ conservation. Using the novel molecular tools, the human pathogenic strains of Stenotrophomonas can be engineered into non-pathogenic strains so that they could be used in food crops for growth enhancement as well as for management of pests and diseases.
Although many studies were conducted in the past on exploring the applications of Stenotrophomonas in crop protection and crop improvement, further investigations are required to explore the various functions of Stenotrophomonas in plant growth promotion under diverse growth conditions and to demonstrate their interaction with the plant and soil systems under various environmental conditions. Further, advanced molecular techniques should be developed to identify and discriminate between human pathogenic and non-pathogenic strains of the genus Stenotrophomonas, so that non-pathogenic strains can be deployed in diversified cropping systems. Besides, molecular mechanisms underlying Stenotrophomonas-plant interactions are poorly understood and need to be carried out using advanced omics approaches such as transcriptomics, proteomics, and metabolomics. Further, as an opportunistic mycoparasite, research into its induction and regulation of enzyme expression is needed in order to improve its biocontrol abilities and to come up with potential commercial bio-fungicides. Besides, identifying various physiological traits to enhance industrial application of Stenotrophomonas as an alternative strategy for producing antibiotics and enzymes could be other areas for future research.
Conclusion
In conclusion, the use of beneficial microorganisms for crop health management has been studied in many cultivated crops. In this regard, Stenotrophomonas species have been proven to be efficient microbial biocontrol agents and have exhibited both antagonistic activity against phytopathogens and entomopathogenic activity against insect pests. The biocontrol capability of Stenotrophomonas spp. includes both chitinolytic and proteolytic activities, which may protect plants against phytopathogens but also play an important role in activating plant defense systems. In particular, research conducted worldwide demonstrates that various Stenotrophomonas species are effective in managing many pests and diseases of food crops, as well as promoting plant growth. The potential strains of Stenotrophomonas spp. for crop health improvement identified by the researchers can be commercialized among farmers after their validation under field conditions in larger scale/multilocation trials. However, more work is needed to determine the cost–benefit ratio for economically commercializing bio-fertilizers and biopesticides based on Stenotrophomonas species. Furthermore, Stenotrophomonas species-based bio-products must be evaluated for regulatory risk parameters before they can be given to the farmers.
Author contributions
AK, LR, and VK: conceptualization and writing—original draft preparation. AKP and NR: writing—review and editing. KC and A: review and editing. All authors contributed to the article and approved the submitted version.
Funding
AKP received funding from Department of Science and Technology (DST), Science and Engineering Research Board, Government of India (SRG/2021/000299) through Start-up Research Grant.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank the reviewers and editors for contributing to improving the manuscript.
References
- Abbott I. J., Peleg A. Y. (2015). Stenotrophomonas, Achromobacter, and nonmelioid Burkholderia species: antimicrobial resistance and therapeutic strategies. Semin. Respir. Crit. Care Med. 36, 99–110. doi: 10.1055/s-0034-1396929, PMID: [DOI] [PubMed] [Google Scholar]
- Abd-Alla M. H., Bashandy S. R., Nafady N. A., Hassan A. A. (2018). Enhancement of exopolysaccharide production by Stenotrophomonas maltophilia and Brevibacillus parabrevis isolated from root nodules of Cicer arietinum L. and Vigna unguiculata L. (Walp.) plants. Rendiconti Lincei. 29, 117–129. doi: 10.1007/s12210-018-0671-1 [DOI] [Google Scholar]
- Abd-Alla M. H., Nafady N. A., Bashandy S. R., Hassan A. A. (2019). Mitigation of effect of salt stress on the nodulation, nitrogen fixation and growth of chickpea (Cicer arietinum L.) by triple microbial inoculation. Rhizosphere 10:100148. doi: 10.1016/j.rhisph.2019.100148 [DOI] [Google Scholar]
- Adeleke B. S., Ayangbeno A. S., Babalola O. O. (2022). Effect of endophytic bacterium, Stenotrophomonas maltophilia JVB5 on sunflower. Plant Prot. Sci. 58, 185–198. doi: 10.17221/171/2021-PPS [DOI] [Google Scholar]
- Adeleke B. S., Ayangbenro A. S., Babalola O. O. (2021). Genomic assessment of Stenotrophomonas indicatrix for improved sunflower plant. Curr. Genet. 67, 891–907. doi: 10.1007/s00294-021-01199-8, PMID: [DOI] [PubMed] [Google Scholar]
- Adeleke B. S., Babalola O. O. (2022). Meta-omics of endophytic microbes in agricultural biotechnology. Biocatal. Agric. Biotechnol. 42:102332. doi: 10.1016/j.bcab.2022.102332 [DOI] [Google Scholar]
- Aeron A., Dubey R. C., Maheshwari D. K. (2020). Characterization of a plant-growth-promoting non-nodulating endophytic bacterium (Stenotrophomonas maltophilia) from the root nodules of Mucuna utilis var. capitata L. (Safed Kaunch). Can. J. Microbiol. 66, 670–677. doi: 10.1139/cjm-2020-0196, PMID: [DOI] [PubMed] [Google Scholar]
- Aktas C., Ruzgar D., Gurkok S., Gormez A. (2022). Purification and characterization of Stenotrophomonas maltophilia chitinase with antifungal and insecticidal properties. Prep. Biochem. Biotechnol., 1–10. doi: 10.1080/10826068.2022.2142942 [DOI] [PubMed] [Google Scholar]
- Alexander A., Singh V. K., Mishra A. (2020). Halotolerant PGPR Stenotrophomonas maltophilia BJ01 induces salt tolerance by modulating physiology and biochemical activities of Arachis hypogaea. Front. Microbiol. 11, –568289. doi: 10.3389/fmicb.2020.568289, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. S., Kornaros M., Manni A., Al-Tohamy R., El-Shanshoury A. E. R. R., Matter I. M., et al. (2021). Advances in microorganisms-based biofertilizers: major mechanisms and applications. Biofertilizers 1, 371–385. doi: 10.1016/B978-0-12-821667-5.00023-3 [DOI] [Google Scholar]
- Alijani Z., Amini J., Ashengroph M., Bahramnejad B. (2020). Volatile compounds mediated effects of Stenotrophomonas maltophilia strain UN1512 in plant growth promotion and its potential for the biocontrol of Colletotrichum nymphaeae. Physiol. Mol. Plant Pathol. 112:101555. doi: 10.1016/j.pmpp.2020.101555 [DOI] [Google Scholar]
- Ambawade M., Pathade G. (2013). Production of indole acetic acid (IAA) by Stenotrophomonas maltophilia BE25 isolated from roots of banana (Musa spp.) | semantic scholar. Int. J. Sci. Res. 4, 2644–2650. [Google Scholar]
- Amri M., Rjeibi M. R., Gatrouni M., Mateus D. M. R., Asses N., Pinho H. J. O., et al. (2023). Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms 11:783. doi: 10.3390/MICROORGANISMS11030783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S. Q., Berg G. (2018). Stenotrophomonas maltophilia. Trends Microbiol. 26, 637–638. doi: 10.1016/j.tim.2018.04.006, PMID: [DOI] [PubMed] [Google Scholar]
- Anđelković M. V., Janković S. M., Kostić M. J., Živković Zarić R. S., Opančina V. D., Živić M., et al. (2019). Antimicrobial treatment of Stenotrophomonas maltophilia invasive infections: systematic review. J. Chemother. 31, 297–306. doi: 10.1080/1120009X.2019.1620405, PMID: [DOI] [PubMed] [Google Scholar]
- Ardon O., Weizman H., Libman J., Shanzer A., Chen Y., Hadar Y. (1997). Iron uptake in Ustilago maydis: studies with fluorescent ferrichrome analogues. Microbiology 143, 3625–3631. doi: 10.1099/00221287-143-11-3625, PMID: [DOI] [PubMed] [Google Scholar]
- Aslani M. A., Harighi B., Abdollahzadeh J. (2018). Screening of endofungal bacteria isolated from wild growing mushrooms as potential biological control agents against brown blotch and internal stipe necrosis diseases of agaricus bisporus. Biol. Control 119, 20–26. doi: 10.1016/j.biocontrol.2018.01.006 [DOI] [Google Scholar]
- Aydi-Ben-Abdallah R., Jabnoun-Khiareddine H., Daami-Remadi M. (2020). Fusarium wilt biocontrol and tomato growth stimulation, using endophytic bacteria naturally associated with solanum sodomaeum and S. bonariense plants. Egypt. J. Biol. Pest Control. 30, 1–13. doi: 10.1186/s41938-020-00313-1 [DOI] [Google Scholar]
- Baazeem A., Alotaibi S. S., Khalaf L. K., Kumar U., Zaynab M., Alharthi S., et al. (2022). Identification and environment-friendly biocontrol potential of five different bacteria against Aphis punicae and Aphis illinoisensis (Hemiptera: Aphididae). Front. Microbiol. 13:961349. doi: 10.3389/fmicb.2022.961349, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badri Fariman A., Abbasiliasi S., Akmar Abdullah S. N., Mohd Saud H., Wong M. Y. (2022). Stenotrophomonas maltophilia isolate UPMKH2 with the abilities to suppress rice blast disease and increase yield a promising biocontrol agent. Physiol. Mol. Plant Pathol. 121:101872. doi: 10.1016/j.pmpp.2022.101872 [DOI] [Google Scholar]
- Bahadur I., Maurya R., Roy P., Kumar A. (2019). Potassium-solubilizing bacteria (KSB): a microbial tool for K-solubility, cycling, and availability to plants. In: Kumar A., Meena V. (eds) Plant Growth Promoting Rhizobacteria for Agricultural Sustainability. Springer, Singapore. [Google Scholar]
- Banerjee A., Sarkar S., Govil T., González-Faune P., Cabrera-Barjas G., Bandopadhyay R., et al. (2021). Extremophilic exopolysaccharides: biotechnologies and wastewater remediation. Front. Microbiol. 12:2349. doi: 10.3389/FMICB.2021.721365/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal K., Kumar S., Kaur A., Singh A., Patil P. B. (2021). Deep phylo-taxono genomics reveals Xylella as a variant lineage of plant associated Xanthomonas and supports their taxonomic reunification along with Stenotrophomonas and Pseudoxanthomonas. Genomics 113, 3989–4003. doi: 10.1016/j.ygeno.2021.09.021, PMID: [DOI] [PubMed] [Google Scholar]
- Barra P. J., Pontigo S., Delgado M., Parra-Almuna L., Duran P., Valentine A. J., et al. (2019). Phosphobacteria inoculation enhances the benefit of P–fertilization on Lolium perenne in soils contrasting in P–availability. Soil Biol. Biochem. 136:107516. doi: 10.1016/j.soilbio.2019.06.012 [DOI] [Google Scholar]
- Bashandy S. R., Abd-Alla M. H., Dawood M. F. A. (2020). Alleviation of the toxicity of oily wastewater to canola plants by the N2-fixing, aromatic hydrocarbon biodegrading bacterium Stenotrophomonas maltophilia-SR1. Appl. Soil Ecol. 154:103654. doi: 10.1016/j.apsoil.2020.103654 [DOI] [Google Scholar]
- Behnke J., LaRoche J. (2020). Iron uptake proteins in algae and the role of iron starvation-induced proteins (ISIPs). Eur. J. Phycol. 55, 339–360. doi: 10.1080/09670262.2020.1744039 [DOI] [Google Scholar]
- Ben Abdallah R. A., Jabnoun-Khiareddine H., Nefzi A., Daami-Remadi M. (2018). Evaluation of the growth-promoting potential of endophytic bacteria recovered from healthy tomato plants. J. Hortic. 5:234. doi: 10.4172/2376-0354.1000234 [DOI] [Google Scholar]
- Berg G., Egamberdieva D., Lugtenberg B., Hagemann M. (2010). “Symbiotic plant–microbe interactions: stress protection, plant growth promotion, and biocontrol by Stenotrophomonas” in Symbioses and Stress. Cellular Origin, Life in Extreme Habitats and Astrobiology. eds. Seckbach J., Grube M. (Dordrecht: Springer; ) [Google Scholar]
- Berg G., Martinez J. L. (2015). Friends or foes: can we make a distinction between beneficial and harmful strains of the Stenotrophomonas maltophilia complex? Front. Microbiol. 6:241. doi: 10.3389/fmicb.2015.00241, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg G., Roskot N., Steidle A., Eberl L., Zock A., Smalla K. (2002). Plant-dependent genotypic and phenotypic diversity of antagonistic rhizobacteria isolated from different verticillium host plants. Appl. Environ. Microbiol. 68, 3328–3338. doi: 10.1128/AEM.68.7.3328-3338.2002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke J. S. (2014). New strategies against Stenotrophomonas maltophilia: a serious worldwide intrinsically drug-resistant opportunistic pathogen. Expert Rev. Anti-Infect. Ther. 12, 1–4. doi: 10.1586/14787210.2014.864553 [DOI] [PubMed] [Google Scholar]
- Castellane T. C. L., Otoboni A. M. M. B., Lemos E. G. M. (2015). Characterization of exopolysaccharides produced by rhizobia species. Rev. Bras. Cienc. Solo. 39, 1566–1575. doi: 10.1590/01000683rbcs20150084, PMID: 36104575 [DOI] [Google Scholar]
- Cerezer V. G., Bando S. Y., Pasternak J., Franzolin M. R., Moreira-Filho C. A. (2014). Phylogenetic analysis of Stenotrophomonas spp. isolates contributes to the identification of nosocomial and community-acquired infections. Biomed. Res. Int. 2014:2014: 151405. doi: 10.1155/2014/151405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Gao Y., Shi G., Li J., Fan G., Yang C., et al. (2023). Enhanced degradation of fomesafen by a rhizobial strain Sinorhizobium sp. W16 in symbiotic association with soybean. Appl. Soil Ecol. 187:104847. doi: 10.1016/j.apsoil.2023.104847 [DOI] [Google Scholar]
- Crossman L. C., Gould V. C., Dow J. M., Vernikos G. S., Okazaki A., Sebaihia M., et al. (2008). The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 9, 1–13. doi: 10.1186/gb-2008-9-4-r74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutiño-Jiménez A. M., Menck C. F. M., Cambas Y. T., Díaz-Pérez J. C. (2020). Protein signatures to identify the different genera within the Xanthomonadaceae family. Braz. J. Microbiol. 51, 1515–1526. doi: 10.1007/s42770-020-00304-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadashi M., Hajikhani B., Nazarinejad N., Nourisepehr N., Yazdani S., Hashemi A., et al. (2023). Global prevalence and distribution of antibiotic resistance among clinical isolates of Stenotrophomonas maltophilia: a systematic review and meta-analysis. J. Glob. Antimicrob. Resist. doi: 10.1016/j.jgar.2023.02.018, PMID: [DOI] [PubMed] [Google Scholar]
- Damasceno C. L., Duarte E. A. A., dos Santos L. B. P. R., de Oliveira T. A. S., de Jesus F. N., de Oliveira L. M., et al. (2019). Postharvest biocontrol of anthracnose in bananas by endophytic and soil rhizosphere bacteria associated with sisal (Agave sisalana) in Brazil. Biol. Control 137:104016. doi: 10.1016/j.biocontrol.2019.104016 [DOI] [Google Scholar]
- de Oliveira-Garcia D., Dall’Agnol M., Rosales M., Azzuz A. C. G. S., Alcántara N., Martinez M. B., et al. (2003). Fimbriae and adherence of Stenotrophomonas maltophilia to epithelial cells and to abiotic surfaces. Cell. Microbiol. 5, 625–636. doi: 10.1046/j.1462-5822.2003.00306.x, PMID: [DOI] [PubMed] [Google Scholar]
- Deng S., Chen Y., Wang D., Shi T., Wu X., Ma X., et al. (2015). Rapid biodegradation of organophosphorus pesticides by Stenotrophomonas sp. G1. J. Hazard. Mater. 297, 17–24. doi: 10.1016/j.jhazmat.2015.04.052, PMID: [DOI] [PubMed] [Google Scholar]
- Dewi R. R., Rahmah S. M., Taruna A., Aini L. Q., Fernando I., Abadi A. L., et al. (2023). The effectiveness comparison between application of indigenous arbuscular mycorrhizal fungal community and Stenotrophomonas maltophilia to suppress Fusarium wilt incidence on local garlic plant (Lumbu hijau). AGRIVITA J. Agric. Sci. 45, 131–146. doi: 10.17503/agrivita.v45i1.3970 [DOI] [Google Scholar]
- Dinnage R., Simonsen A. K., Barrett L. G., Cardillo M., Raisbeck-Brown N., Thrall P. H., et al. (2019). Larger plants promote a greater diversity of symbiotic nitrogen-fixing soil bacteria associated with an Australian endemic legume. J. Ecol. 107, 977–991. doi: 10.1111/1365-2745.13083 [DOI] [Google Scholar]
- Elvers K. T., Leeming K., Lappin-Scott H. M. (2001). Binary culture biofilm formation by Stenotrophomonas maltophilia and fusarium oxysporum. J. Ind. Microbiol. Biotechnol. 26, 178–183. doi: 10.1038/sj.jim.7000100, PMID: [DOI] [PubMed] [Google Scholar]
- Etesami H., Alikhani H. A. (2016). Suppression of the fungal pathogen Magnaporthe grisea by Stenotrophomonas maltophilia, a seed-borne rice (Oryza sativa L.) endophytic bacterium. Arch. Agron. Soil Sci. 62, 1271–1284. doi: 10.1080/03650340.2016.1139087 [DOI] [Google Scholar]
- Etminani F., Harighi B. (2018). Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from wild pistachio trees. Plant Pathol. J. (Faisalabad) 34, 208–217. doi: 10.5423/PPJ.OA.07.2017.0158, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezrari S., Mhidra O., Radouane N., Tahiri A., Polizzi G., Lazraq A., et al. (2021). Potential role of rhizobacteria isolated from citrus rhizosphere for biological control of citrus dry root rot. Plan. Theory 10:872. doi: 10.3390/plants10050872, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadly F., Lisnawita, Safni I., Lubis K., Nurliana (2021). The potency of antagonistic microbes as plant growth-promoting on oil palm seedling infected with basal stem rot disease (Ganoderma boninense). Conf. Ser.: Earth Environ. Sci. 782:042060. doi: 10.1088/1755-1315/782/4/042060 [DOI] [Google Scholar]
- Fitton N., Bindi M., Brilli L., Cichota R., Dibari C., Fuchs K., et al. (2019). Modelling biological N fixation and grass-legume dynamics with process-based biogeochemical models of varying complexity. Eur. J. Agron. 106, 58–66. doi: 10.1016/j.eja.2019.03.008 [DOI] [Google Scholar]
- Flores-Treviño S., Bocanegra-Ibarias P., Camacho-Ortiz A., Morfín-Otero R., Salazar-Sesatty H. A., Garza-González E. (2019). Stenotrophomonas maltophilia biofilm: its role in infectious diseases. Expert Rev. Anti-Infect. Ther. 17, 877–893. doi: 10.1080/14787210.2019.1685875, PMID: [DOI] [PubMed] [Google Scholar]
- Fulekar M. H. (2014). Rhizosphere bioremediation of pesticides by microbial consortium and potential microorganism. Int. J. Curr. Microbiol. Appl. Sci. 3, 235–248. Available at: http://www.ijcmas.com [Google Scholar]
- Galai S., Limam F., Marzouki M. N. (2009). A new Stenotrophomonas maltophilia strain producing laccase. Use in decolorization of synthetics dyes. Appl. Biochem. Biotechnol. 158, 416–431. doi: 10.1007/s12010-008-8369-y, PMID: [DOI] [PubMed] [Google Scholar]
- Gao S., Seo J. S., Wang J., Keum Y. S., Li J., Li Q. X. (2013). Multiple degradation pathways of phenanthrene by Stenotrophomonas maltophilia C6. Int. Biodeterior. Biodegradation 79, 98–104. doi: 10.1016/j.ibiod.2013.01.012, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germida J. J., Siciliano S. D. (2001). Taxonomic diversity of bacteria associated with the roots of modern, recent and ancient wheat cultivars. Biol. Fertil. Soils 33, 410–415. doi: 10.1007/s003740100343 [DOI] [Google Scholar]
- Ghavami N., Alikhani H. A., Pourbabaei A. A., Besharati H. (2017). Effects of two new siderophore-producing rhizobacteria on growth and iron content of maize and canola plants. J. Plant Nutr. 40, 736–746. doi: 10.1080/01904167.2016.1262409 [DOI] [Google Scholar]
- Gil-Gil T., Martínez J. L., Blanco P. (2020). Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: a review of current knowledge. Expert Rev. Anti-Infect. Ther. 18, 335–347. doi: 10.1080/14787210.2020.1730178, PMID: [DOI] [PubMed] [Google Scholar]
- Gopi K., Jinal H. N., Prittesh P., Kartik V. P., Amaresan N. (2020). Effect of copper-resistant Stenotrophomonas maltophilia on maize (Zea mays) growth, physiological properties, and copper accumulation: potential for phytoremediation into biofortification. Int. J. Phytoremediation 22, 662–668. doi: 10.1080/15226514.2019.1707161, PMID: [DOI] [PubMed] [Google Scholar]
- Gould V. C., Avison M. B. (2006). SmeDEF-mediated antimicrobial drug resistance in Stenotrophomonas maltophilia clinical isolates having defined phylogenetic relationships. J. Antimicrob. Chemother. 57, 1070–1076. doi: 10.1093/jac/dkl106, PMID: [DOI] [PubMed] [Google Scholar]
- Hadi M. N., Taheri P., Tarighi S. (2020). Biological control of tomato damping-off caused by Rhizoctonia solani by using native antagonistic bacteria. Plant Archives 20, 4169–4180. [Google Scholar]
- Han H. S., Lee K. D. (2005). Phosphate and potassium solubilizing bacteria effect on mineral uptake, soil availability and growth of eggplant. Res. J. Agric. Biol. Sci. 1, 176–180. [Google Scholar]
- Harun-Or-Rashid M., Chung Y. R. (2017). Induction of systemic resistance against insect herbivores in plants by beneficial soil microbes. Front. Plant Sci. 8:1816. doi: 10.3389/fpls.2017.01816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan T. U., Bano A. (2016). Comparative effects of wild type Stenotrophomonas maltophilia and its indole acetic acid-deficient mutants on wheat. Plant Biol. (Stuttg.) 18, 835–841. doi: 10.1111/plb.12477, PMID: [DOI] [PubMed] [Google Scholar]
- Hayward A. C., Fegan N., Fegan M., Stirling G. R. (2010). Stenotrophomonas and Lysobacter: ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. J. Appl. Microbiol. 108, 756–770. doi: 10.1111/j.1365-2672.2009.04471.x, PMID: [DOI] [PubMed] [Google Scholar]
- Huang T. P., Somers E. B., Wong A. C. L. (2006). Differential biofilm formation and motility associated with lipopolysaccharide/exopolysaccharide-coupled biosynthetic genes in Stenotrophomonas maltophilia. J. Bacteriol. 188, 3116–3120. doi: 10.1128/JB.188.8.3116-3120.2006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda N., Tanvir R., Badar J., Ali I., Rehman Y. (2022). Arsenic-resistant plant growth promoting Pseudoxanthomonas mexicana S254 and Stenotrophomonas maltophilia S255 isolated from agriculture soil contaminated by industrial effluent. Sustainability 14:10697. doi: 10.3390/su141710697 [DOI] [Google Scholar]
- Jabeen F., Hussain A., Manzoor M., Younis T., Rasul A., Qazi J. I. (2018). Potential of bacterial chitinolytic, Stenotrophomonas maltophilia, in biological control of termites. Egypt. J. Biol. Pest Control. 28, 1–10. doi: 10.1186/s41938-018-0092-6 [DOI] [Google Scholar]
- Jakobi M., Winkelmann G., Kaiser D., Kempter C., Jung G., Berg G., et al. (1996). Maltophilin: a new antifungal compound produced by Stenotrophomonas maltophilia R3089. J. Antibiot. 49, 1101–1104. doi: 10.7164/antibiotics.49.1101, PMID: [DOI] [PubMed] [Google Scholar]
- Jędrzejuk A., Kuźma N., Orłowski A., Budzyński R., Gehl C., Serek M. (2023). Mechanical stimulation decreases auxin and gibberellic acid synthesis but does not affect auxin transport in axillary buds; it also stimulates peroxidase activity in petunia × atkinsiana. Molecules 28:2714. doi: 10.3390/molecules28062714, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- John N., Thangavel M. (2017). Stenotrophomonas maltophilia: a novel plant growth promoter and biocontrol agent from marine environment. Int. J. Adv. Res. 5, 207–214. doi: 10.21474/IJAR01/3797 [DOI] [Google Scholar]
- Kai M., Effmert U., Berg G., Piechulla B. (2007). Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen rhizoctonia solani. Arch. Microbiol. 187, 351–360. doi: 10.1007/s00203-006-0199-0, PMID: [DOI] [PubMed] [Google Scholar]
- Kaur T., Devi R., Kumar S., Kour D., Yadav A. N. (2023). Synergistic effect of endophytic and rhizospheric microbes for plant growth promotion of foxtail millet (Setaria italica L.). Natl. Acad. Sci. Lett. 46, 27–30. doi: 10.1007/s40009-022-01190-y [DOI] [Google Scholar]
- Khalimi K., Temaja I. G. R. M., Suprapta D. N. (2020). Systemic resistance induced by Stenotrophomonas maltophilia sg3 against cucumber mosaic virus in tobacco plant. Int. J. Agric. Biol. 23, 1–6. doi: 10.17957/IJAB/15.1271 [DOI] [Google Scholar]
- Kishore N., Pindi P. K., Reddy S. R. (2015). “Phosphate-solubilizing microorganisms: a critical review” in Plant Biology and Biotechnology: Plant Diversity, Organization, Function and Improvement. eds. Bahadur B., Venkat Rajam M., Sahijram L., Krishnamurthy K. (New Delhi: Springer; ) [Google Scholar]
- Kloepper J. W., Leong J., Teintze M., Schroth M. N. (1980). Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 286, 885–886. doi: 10.1038/286885a0 [DOI] [Google Scholar]
- Kobayashi D. Y., Reedy R. M., Bick J. A., Oudemans P. V. (2002). Characterization of a chitinase gene from Stenotrophomonas maltophilia strain 34S1 and its involvement in biological control. Appl. Environ. Microbiol. 68, 1047–1054. doi: 10.1128/AEM.68.3.1047-1054.2002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A. A., Mishra P., Kumari K., Panigrahi K. C. S. (2012). Environmental stress influencing plant development and flowering. Front. Biosci. (Schol. Ed.) 4, 1315–1324. doi: 10.2741/s333, PMID: [DOI] [PubMed] [Google Scholar]
- Kumar A., Bahadur I., Maurya B. R., Raghuwanshi R., Meena V. S., Singh D. K., et al. (2015). Does a plant growth-promoting rhizobacteria enhance agricultural sustainability. J. Pure Appl. Microbiol. 9, 715–724. [Google Scholar]
- Kumar N. P., Audipudi A. V. (2015). Exploration of a novel plant growth promoting bacteria Stenotrophomonas maltophilia AVP27 isolated from the Chilli Rhizosphere soil. Int. J. Eng. Res. Generic Sci. 3, 265–276. (www.ijergs.org [Google Scholar]
- Lara-Capistran L., Zulueta-Rodriguez R., Castellanos-Cervantes T., Reyes-Perez J. J., Preciado-Rangel P., Hernandez-Montiel L. G. (2020). Efficiency of marine bacteria and yeasts on the biocontrol activity of Pythium ultimum in ancho-type pepper seedlings. Agronomy 10:408. doi: 10.3390/agronomy10030408 [DOI] [Google Scholar]
- Li H., Huang W., Xu L., Zhou X., Liu H., Cheng Z. (2016). Stenotrophomonas maltophilia HW2 enhanced cucumber resistance against cucumber green mottle mosaic virus. J. Plant Biol. 59, 488–495. doi: 10.1007/s12374-016-0246-6 [DOI] [Google Scholar]
- Li Y., Qi G., Xie Z., Li B., Wang R., Tan J., et al. (2023). The endophytic root microbiome is different in healthy and ralstonia solanacearum-infected plants and is regulated by a consortium containing beneficial endophytic bacteria. Microbiol. Spectr. 11, 02031–02022. doi: 10.1128/spectrum.02031-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli D. H., Jones C. J., Wozniak D. J. (2015). Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 3:10.1128/microbiolspec.MB-0011-2014. doi: 10.1128/microbiolspec.MB-0011-2014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S., Zhao Y., Sun S., Zheng D., Sun X., Zeng R., et al. (2022). Enhanced anti-herbivore defense of tomato plants against Spodoptera litura by their rhizosphere bacteria. BMC Plant Biol. 22:254. doi: 10.1186/s12870-022-03644-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira F., Berg G., Martínez J. L. (2017). Double-face meets the bacterial world: the opportunistic pathogen Stenotrophomonas maltophilia. Front. Microbiol. 8:2190. doi: 10.3389/fmicb.2017.02190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffee W. F., Kloepper J. W. (1997). Temporal changes in the bacterial communities of soil, rhizosphere, and endorhiza associated with field-grown cucumber (Cucumis sativus L.). Microb. Ecol. 34, 210–223. doi: 10.1007/s002489900050, PMID: [DOI] [PubMed] [Google Scholar]
- Manh Tuong H., Garcia Mendez S., Vandecasteele M., Willems A., Luo D., Beirinckx S., et al. (2022). Stenotrophomonas sp. SRS1 promotes growth of Arabidopsis and tomato plants under salt stress conditions. Plant Soil 473, 547–571. doi: 10.1007/s11104-022-05304-9 [DOI] [Google Scholar]
- Marina M., Romero F. M., Villarreal N. M., Medina A. J., Gárriz A., Rossi F. R., et al. (2019). Mechanisms of plant protection against two oxalate-producing fungal pathogens by oxalotrophic strains of Stenotrophomonas spp. Plant Mol. Biol. 100, 659–674. doi: 10.1007/s11103-019-00888-w, PMID: [DOI] [PubMed] [Google Scholar]
- Masson-Boivin C., Sachs J. L. (2018). Symbiotic nitrogen fixation by rhizobia — the roots of a success story. Curr. Opin. Plant Biol. 44, 7–15. doi: 10.1016/j.pbi.2017.12.001, PMID: [DOI] [PubMed] [Google Scholar]
- Mastretta C., Barac T., Vangronsveld J., Newman L., Taghavi S., Lelie D. v. d. (2006). Endophytic bacteria and their potential application to improve the phytoremediation of contaminated environments. Biotechnol. Genet. Eng. Rev. 23, 175–188. doi: 10.1080/02648725.2006.10648084, PMID: [DOI] [PubMed] [Google Scholar]
- Mehnaz S., Mirza M. S., Haurat J., Bally R., Normand P., Bano A., et al. (2001). Isolation and 16S rRNA sequence analysis of the beneficial bacteria from the rhizosphere of rice. Can. J. Microbiol. 47, 110–117. doi: 10.1139/w00-132, PMID: [DOI] [PubMed] [Google Scholar]
- Meena R. K., Singh R. K., Singh N. P., Meena S. K., Meena V. S. (2015). Isolation of low temperature surviving plant growth–promoting rhizobacteria (PGPR) from pea (Pisum sativum L.) and documentation of their plant growth promoting traits. Biocatal. Agric. Biotechnol. 4, 806–811. doi: 10.1016/j.bcab.2015.08.006 [DOI] [Google Scholar]
- Messiha N. A. S., van Diepeningen A. D., Farag N. S., Abdallah S. A., Janse J. D., van Bruggen A. H. C. (2007). Stenotrophomonas maltophilia: a new potential biocontrol agent of Ralstonia solanacearum, causal agent of potato brown rot. Eur. J. Plant Pathol. 118, 211–225. doi: 10.1007/s10658-007-9136-6 [DOI] [Google Scholar]
- Minkwitz A., Berg G. (2001). Comparison of antifungal activities and 16S ribosomal DNA sequences of clinical and environmental isolates of Stenotrophomonas maltophilia. J. Clin. Microbiol. 39, 139–145. doi: 10.1128/JCM.39.1.139-145.2001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S., Singh B. R., Naqvi A. H., Singh H. B. (2017). Potential of biosynthesized silver nanoparticles using Stenotrophomonas sp. BHU-S7 (MTCC 5978) for management of soil-borne and foliar phytopathogens. Sci. Rep. 7, 1–15. doi: 10.1038/srep45154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. S. (2009). Degradation of methomyl by the novel bacterial strain Stenotrophomonas maltophilia M1. Electron. J. Biotechnol. 12, 1–6. doi: 10.2225/vol12-issue4-fulltext-11 [DOI] [Google Scholar]
- Mourou M., Hanani A., D’onghia A. M., Davino S. W., Balestra G. M., Valentini F. (2022). Antagonism and antimicrobial capacity of epiphytic and endophytic bacteria against the phytopathogen Xylella fastidiosa. Agronomy 12:1266. doi: 10.3390/agronomy12061266 [DOI] [Google Scholar]
- Mudau F. N., Soundy P., Du Toit E. S. (2007). Nitrogen, phosphorus, and potassium nutrition increases growth and total polyphenol concentrations of bush tea in a shaded nursery environment. HortTechnology 17, 107–110. doi: 10.21273/HORTTECH.17.1.107 [DOI] [Google Scholar]
- Nakayama T., Homma Y., Hashidoko Y., Mizutani J., Tahara S. (1999). Possible role of xanthobaccins produced by Stenotrophomonas sp. strain SB-K88 in suppression of sugar beet damping-off disease. Appl. Environ. Microbiol. 65, 4334–4339. doi: 10.1128/AEM.65.10.4334-4339.1999, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevita T., Sharma G. D., Pandey P. (2018). Composting of rice-residues using lignocellulolytic plant-probiotic Stenotrophomonas maltophilia, and its evaluation for growth enhancement of Oryza sativa L. J. Environ. Sustain. 1, 185–196. doi: 10.1007/S42398-018-0017-Z [DOI] [Google Scholar]
- Nigam B., Dubey R. S., Rathore D. (2022). Protective role of exogenously supplied salicylic acid and PGPB (Stenotrophomonas sp.) on spinach and soybean cultivars grown under salt stress. Sci. Hortic. 293:110654. doi: 10.1016/j.scienta.2021.110654 [DOI] [Google Scholar]
- Nouha K., Kumar R. S., Balasubramanian S., Tyagi R. D. (2018). Critical review of EPS production, synthesis and composition for sludge flocculation. J. Environ. Sci. 66, 225–245. doi: 10.1016/j.jes.2017.05.020, PMID: [DOI] [PubMed] [Google Scholar]
- Pan X., Lin D., Zheng Y., Zhang Q., Yin Y., Cai L., et al. (2016). Biodegradation of DDT by Stenotrophomonas sp. DDT-1: characterization and genome functional analysis. Sci. Rep. 6, 1–10. doi: 10.1038/srep21332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Qin R., Hou M., Xue J., Zhou M., Xu L., et al. (2022). The interactions of polyphenols with Fe and their application in Fenton/Fenton-like reactions. Sep. Purif. Technol. 300:121831. doi: 10.1016/j.seppur.2022.121831 [DOI] [Google Scholar]
- Pangesti N., Pineda A., Dicke M., van Loon J. J. A. (2015). Variation in plant-mediated interactions between rhizobacteria and caterpillars: potential role of soil composition. Plant Biol. 17, 474–483. doi: 10.1111/plb.12265, PMID: [DOI] [PubMed] [Google Scholar]
- Park Y. S., Kim S. Y., Park S. Y., Kang J. H., Lee H. S., Seo Y. H., et al. (2008). Pseudo-outbreak of Stenotrophomonas maltophilia bacteremia in a general ward. Am. J. Infect. Control 36, 29–32. doi: 10.1016/j.ajic.2006.12.013, PMID: [DOI] [PubMed] [Google Scholar]
- Parnell J. J., Berka R., Young H. A., Sturino J. M., Kang Y., Barnhart D. M., et al. (2016). From the lab to the farm: An industrial perspective of plant beneficial microorganisms. Front. Plant Sci. 7:1110. doi: 10.3389/fpls.2016.01110, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parte A. C. (2018). LPSN - list of prokaryotic names with standing in nomenclature (bacterio.Net), 20 years on. Int. J. Syst. Evol. Microbiol. 68, 1825–1829. doi: 10.1099/ijsem.0.002786, PMID: [DOI] [PubMed] [Google Scholar]
- Patel T., Saraf M. (2017). Exploration of novel plant growth promoting bacteria Stenotrophomonas maltophilia MTP42 isolated from the rhizospheric soil of coleus forskohlii. Int. J. Curr. Microbiol. Appl. Sci. 6, 944–955. doi: 10.20546/ijcmas.2017.611.111 [DOI] [Google Scholar]
- Paul D., Sinha S. N. (2017). Isolation and characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa KUPSB12 with antibacterial potential from river ganga, India. Ann. Agrar. Sci. 15, 130–136. doi: 10.1016/j.aasci.2016.10.001 [DOI] [Google Scholar]
- Pérez-Martínez S., Oudot M., Hernández I., Nápoles M. C., Pérez-Martínez S. (2020). Isolation and characterization of Stenotrophomonas associated to maize (Zea Mays L.) rhizosphere. Cultivos Tropicales 41:e03 Available at: http://ediciones.inca.edu.cu [Google Scholar]
- Pourbabaee A. A., Soleymani S., Farahbakhsh M., Torabi E. (2017). Biodegradation of diazinon by the Stenotrophomonas maltophilia PS: pesticide dissipation kinetics and breakdown characterization using FTIR. Int. J. Environ. Sci. Technol. 15, 1073–1084. doi: 10.1007/S13762-017-1452-6 [DOI] [Google Scholar]
- Rahma H., Nurbailis N., Busniah M., Kristina N., Larasati Y. (2022). The potential of endophytic bacteria to suppress bacterial leaf blight in rice plants. Biodiversitas 23, 775–782. doi: 10.13057/biodiv/d230223 [DOI] [Google Scholar]
- Ramos P. L., Van Trappen S., Thompson F. L., Rocha R. C. S., Barbosa H. R., de Vos P., et al. (2011). Screening for endophytic nitrogen-fixing bacteria in Brazilian sugar cane varieties used in organic farming and description of Stenotrophomonas pavanii sp. nov. Int. J. Syst. Evol. Microbiol. 61, 926–931. doi: 10.1099/ijs.0.019372-0, PMID: [DOI] [PubMed] [Google Scholar]
- Rane N. R., Tapase S., Kanojia A., Watharkar A., Salama E. S., Jang M., et al. (2022). Molecular insights into plant–microbe interactions for sustainable remediation of contaminated environment. Bioresour. Technol. 344:126246. doi: 10.1016/j.biortech.2021.126246, PMID: [DOI] [PubMed] [Google Scholar]
- Reddy G. C., Goyal R. K., Puranik S., Waghmar V., Vikram K. V., Sruthy K. S. (2020). Biofertilizers toward sustainable agricultural development. In: Varma A., Tripathi S., Prasad R. (eds) Plant Microbe Symbiosis. Springer, Cham. [Google Scholar]
- Reyes-Perez J. J., Hernandez-Montiel L. G., Vero S., Noa-Carrazana J. C., Quiñones-Aguilar E. E., Rincón-Enríquez G. (2019). Postharvest biocontrol of Colletotrichum gloeosporioides on mango using the marine bacterium Stenotrophomonas rhizophila and its possible mechanisms of action. J. Food Sci. Technol. 56, 4992–4999. doi: 10.1007/s13197-019-03971-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Garcia T., Murillo-Amador B., Nieto-Garibay A., Chiquito-Contreras R. G., Rincon-Enriquez G., Hernandez-Montiel L. G. (2018). Effect of ulvan on the biocontrol activity of Debaryomyces hansenii and Stenotrophomonas rhizophila against fruit rot of Cucumis melo L. Agronomy 8:273. doi: 10.3390/agronomy8120273 [DOI] [Google Scholar]
- Rojas-Solís D., Zetter-Salmón E., Contreras-Pérez M., Rocha-Granados M. C., Macías-Rodríguez L., Santoyo G. (2018). Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal. Agric. Biotechnol. 13, 46–52. doi: 10.1016/j.bcab.2017.11.007 [DOI] [Google Scholar]
- Roscetto E., Carlomagno M. S., Casalino M., Colonna B., Zarrilli R., Di Nocera P. P. (2008). PCR-based rapid genotyping of Stenotrophomonas maltophilia isolates. BMC Microbiol. 8, 1–9. doi: 10.1186/1471-2180-8-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan R. P., Monchy S., Cardinale M., Taghavi S., Crossman L., Avison M. B., et al. (2009). The versatility and adaptation of bacteria from the genus Stenotrophomonas. Nat. Rev. Microbiol. 7, 514–525. doi: 10.1038/nrmicro2163, PMID: [DOI] [PubMed] [Google Scholar]
- Ryu R. J., Patten C. L. (2008). Aromatic amino acid-dependent expression of indole-3-pyruvate decarboxylase is regulated by tyrr in Enterobacter cloacae UW5. J. Bacteriol. 190, 7200–7208. doi: 10.1128/JB.00804-08, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar-Cerezo S., Martínez-Montiel N., García-Sánchez J., Pérez-y-Terrón R., Martínez-Contreras R. D. (2018). Gibberellin biosynthesis and metabolism: a convergent route for plants, fungi and bacteria. Microbiol. Res. 208, 85–98. doi: 10.1016/j.micres.2018.01.010, PMID: [DOI] [PubMed] [Google Scholar]
- Santos A. P., Muratore L. N., Solé-Gil A., Farías M. E., Ferrando A., Blázquez M. A., et al. (2021). Extremophilic bacteria restrict the growth of Macrophomina phaseolina by combined secretion of polyamines and lytic enzymes. Biotechnol. Rep. 32:674. doi: 10.1016/j.btre.2021.e00674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A., Pramanik K., Mitra S., Soren T., Maiti T. K. (2018). Enhancement of growth and salt tolerance of rice seedlings by ACC deaminase-producing Burkholderia sp. MTCC 12259. J. Plant Physiol. 231, 434–442. doi: 10.1016/j.jplph.2018.10.010, PMID: [DOI] [PubMed] [Google Scholar]
- Schmidt C. S., Alavi M., Cardinale M., Müller H., Berg G. (2012). Stenotrophomonas rhizophila DSM14405 T promotes plant growth probably by altering fungal communities in the rhizosphere. Biol. Fertil. Soils 48, 947–960. doi: 10.1007/s00374-012-0688-z [DOI] [Google Scholar]
- Schmidt C. S., Mrnka L., Lovecká P., Frantík T., Fenclová M., Demnerová K., et al. (2021). Bacterial and fungal endophyte communities in healthy and diseased oilseed rape and their potential for biocontrol of sclerotinia and Phoma disease. Sci. Rep. 11:3810. doi: 10.1038/s41598-021-81937-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senocak A. A., Ünal F., Yildirir M., Kullanımı B. M. Y. B. (2020). The use of Turkish bacterial strains for the biological control of Fusarium cerealis which causes root and crown rot in turfgrass. Türkiye Biyolojik Mücadele Dergisi 11, 23–33. [Google Scholar]
- Sharma P., Sharma N. (2018). Molecular identification, production and optimization of pectinase by using Stenotrophomonas maltophilia P9 isolated from algal biomass of Himachal Pradesh. Int. J. Curr. Microbiol. App. Sci. 7, 670–680. doi: 10.20546/ijcmas.2018.701.082 [DOI] [Google Scholar]
- Shen J., Yuan L., Zhang J., Li H., Bai Z., Chen X., et al. (2011). Phosphorus dynamics: from soil to plant. Plant Physiol. 156, 997–1005. doi: 10.1104/pp.111.175232, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty T., Corson T. W. (2020). Mitochondrial heme synthesis enzymes as therapeutic targets in vascular diseases. Front. Pharmacol. 11:1015. doi: 10.3389/FPHAR.2020.01015/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Singh R. K., Li H. B., Guo D. J., Sharma A., Verma K. K., et al. (2023). Nitrogen fixation and phytohormone stimulation of sugarcane plant through plant growth promoting diazotrophic pseudomonas. Biotechnol. Genet. Eng. Rev. doi: 10.1080/02648725.2023.2177814, PMID: [DOI] [PubMed] [Google Scholar]
- Singh R. K., Singh P., Li H. B., Guo D. J., Song Q. Q., Yang L. T., et al. (2020). Plant-PGPR interaction study of plant growth-promoting diazotrophs Kosakonia radicincitans BA1 and Stenotrophomonas maltophilia COA2 to enhance growth and stress-related gene expression in saccharum spp. J. Plant Interact. 15, 427–445. doi: 10.1080/17429145.2020.1857857 [DOI] [Google Scholar]
- Singh R. P., Jha P. N. (2017). The PGPR Stenotrophomonas maltophilia SBP-9 augments resistance against biotic and abiotic stress in wheat plants. Front. Microbiol. 8:1945. doi: 10.3389/fmicb.2017.01945, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D., Tandon P. K. (2020). An overview of nitrogen, phosphorus and potassium: key players of nutrition process in plants, Mishra K., Tandon P.K., Srivastava S. (eds) Sustainable Solutions for Elemental Deficiency and Excess in Crop Plants. Springer, Singapore [Google Scholar]
- Suckstorff I., Berg G. (2003). Evidence for dose-dependent effects on plant growth by Stenotrophomonas strains from different origins. J. Appl. Microbiol. 95, 656–663. doi: 10.1046/j.1365-2672.2003.02021.x, PMID: [DOI] [PubMed] [Google Scholar]
- Sultana F., Motaher Hossain M. (2022). Assessing the potentials of bacterial antagonists for plant growth promotion, nutrient acquisition, and biological control of southern blight disease in tomato. PLoS One 17:267253. doi: 10.1371/journal.pone.0267253, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Ou Q., Wang N., Guo Z., Ou Y., Li N., et al. (2020). Isolation and identification of potassium-solubilizing bacteria from Mikania micrantha rhizospheric soil and their effect on M. micrantha plants. Glob. Ecol. Conserv. 23:e01141. doi: 10.1016/J.GECCO.2020.E01141 [DOI] [Google Scholar]
- Sun Y., Wu J., Shang X., Xue L., Ji G., Chang S., et al. (2022). Screening of siderophore-producing bacteria and their effects on promoting the growth of plants. Curr. Microbiol. 79, 1–12. doi: 10.1007/S00284-022-02777-W/METRICS [DOI] [PubMed] [Google Scholar]
- Tandavanitj S., Ishida S., Okutani K. (1989). Isolation and characterization of an extracellular mucopolysaccharide produced by a marine strain of Pseudomonas. Nippon Suisan Gakkaishi 55, 2015–2019. doi: 10.2331/suisan.55.2015 [DOI] [Google Scholar]
- Tang S., Liao Y., Xu Y., Dang Z., Zhu X., Ji G. (2020). Microbial coupling mechanisms of nitrogen removal in constructed wetlands: a review. Bioresour. Technol. 314:123759. doi: 10.1016/j.biortech.2020.123759, PMID: [DOI] [PubMed] [Google Scholar]
- Tapia-García E. Y., Hernández-Trejo V., Guevara-Luna J., Rojas-Rojas F. U., Arroyo-Herrera I., Meza-Radilla G., et al. (2020). Plant growth-promoting bacteria isolated from wild legume nodules and nodules of Phaseolus vulgaris L. trap plants in central and southern Mexico. Microbiol. Res. 239:126522. doi: 10.1016/j.micres.2020.126522, PMID: [DOI] [PubMed] [Google Scholar]
- Tsavkelova E. A., Cherdyntseva T. A., Klimova S. Y., Shestakov A. I., Botina S. G., Netrusov A. I. (2007). Orchid-associated bacteria produce indole-3-acetic acid, promote seed germination, and increase their microbial yield in response to exogenous auxin. Arch. Microbiol. 188, 655–664. doi: 10.1007/s00203-007-0286-x, PMID: [DOI] [PubMed] [Google Scholar]
- Tshikhudo P. P., Ntushelo K., Mudau F. N. (2023). Sustainable applications of endophytic bacteria and their physiological/biochemical roles on medicinal and herbal plants: review. Microorganisms 11:453. doi: 10.3390/microorganisms11020453, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich K., Kube M., Becker R., Schneck V., Ulrich A. (2021). Genomic analysis of the endophytic Stenotrophomonas strain 169 reveals features related to plant-growth promotion and stress tolerance. Front. Microbiol. 12:687463. doi: 10.3389/fmicb.2021.687463, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünal F., Aşkın A., Koca E., Yıldırır M., Bingöl M. Ü. (2019). Mycelial compatibility groups, pathogenic diversity and biological control of Sclerotium rolfsii on turfgrass. Egypt. J. Biol. Pest Control. 29, 1–7. doi: 10.1186/s41938-019-0144-6 [DOI] [Google Scholar]
- Uniyal S., Paliwal R., Sharma R. K., Rai J. P. N. (2016). Degradation of fipronil by Stenotrophomonas acidaminiphila isolated from rhizospheric soil of Zea mays. 3 Biotech 6, 1–10. doi: 10.1007/S13205-015-0354-X/FIGURES/6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega-Celedón P., Bravo G., Velásquez A., Cid F. P., Valenzuela M., Ramírez I., et al. (2021). Microbial diversity of psychrotolerant bacteria isolated from wild flora of Andes mountains and Patagonia of Chile towards the selection of plant growth-promoting bacterial consortia to alleviate cold stress in plants. Microorganisms 9, 1–28. doi: 10.3390/microorganisms9030538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma P., Yadav A. N., Khannam K. S., Kumar S., Saxena A. K., Suman A. (2016). Molecular diversity and multifarious plant growth promoting attributes of bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J. Basic Microbiol. 56, 44–58. doi: 10.1002/jobm.201500459, PMID: [DOI] [PubMed] [Google Scholar]
- Villa-Rodríguez E., Parra-Cota F., Castro-Longoria E., López-Cervantes J., de los Santos-Villalobos S. (2019). Bacillus subtilis TE3: a promising biological control agent against Bipolaris sorokiniana, the causal agent of spot blotch in wheat (Triticum turgidum L. subsp. durum). Biol. Control 132, 135–143. doi: 10.1016/j.biocontrol.2019.02.012 [DOI] [Google Scholar]
- Vishnu J. S., Nisha M. S. (2020). Management of root-knot nematode in tomato using antagonistic bacteria Stenotrophomonas maltophilia strain w 2-7. Indian J. Nematol. 50, 71–78. [Google Scholar]
- Wang L., Xi N., Lang D., Zhou L., Zhang Y., Zhang X. (2022). Potential biocontrol and plant growth promotion of an endophytic bacteria isolated from Glycyrrhiza uralensis seeds. Egypt. J. Biol. Pest Control. 32, 1–16. doi: 10.1186/s41938-022-00556-0 [DOI] [Google Scholar]
- Wang S., Xu Y., Li Z. (2018a). Nitrogen utilization and transformation of Stenotrophomonas maltophilia W-6 with nitrogen-fixing ability. bioRxiv 1–9. doi: 10.1101/337386 [DOI] [Google Scholar]
- Wang Y., He T., Shen Z., Wu C. (2018b). Antimicrobial resistance in Stenotrophomonas spp. Microbiol. Spectr. 409–423. doi: 10.1128/microbiolspec.arba-0005-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wani S. H., Kumar V., Shriram V., Sah S. K. (2016). Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4, 162–176. doi: 10.1016/j.cj.2016.01.010, PMID: 36844040 [DOI] [Google Scholar]
- War A. R., Paulraj M. G., Ahmad T., Buhroo A. A., Hussain B., Ignacimuthu S., et al. (2012). Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 7, 1306–1320. doi: 10.4161/psb.21663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley R. E. (2002). The consequences of volatile organic compound mediated bacterial and fungal interactions. Anton. Leeuw. Int. J. Gen. Mol. Microbiol. 81, 357–364. doi: 10.1023/A:1020592802234, PMID: [DOI] [PubMed] [Google Scholar]
- Wilson B. R., Bogdan A. R., Miyazawa M., Hashimoto K., Tsuji Y. (2016). Siderophores in iron metabolism: from mechanism to therapy potential. Trends Mol. Med. 22, 1077–1090. doi: 10.1016/j.molmed.2016.10.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf A., Fritze A., Hagemann M., Berg G. (2002). Stenotrophomonas rhizophila sp. nov., a novel plant-associated bacterium with antifungal properties. Int. J. Syst. Evol. Microbiol. 52, 1937–1944. doi: 10.1099/00207713-52-6-1937, PMID: [DOI] [PubMed] [Google Scholar]
- Woźniak M., Gałązka A., Tyśkiewicz R., Jaroszuk-ściseł J. (2019). Endophytic bacteria potentially promote plant growth by synthesizing different metabolites and their phenotypic/physiological profiles in the biolog gen iii microplate™ test. Int. J. Mol. Sci. 20:5283. doi: 10.3390/ijms20215283, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C. Q., Chi R. A., He H., Zhang W. X. (2009). Characterization of tricalcium phosphate solubilization by Stenotrophomonas maltophilia YC isolated from phosphate mines. J. Cent. South Univ. 16, 581–587. doi: 10.1007/s11771-009-0097-0 [DOI] [Google Scholar]
- Youseif S. H. (2018). Genetic diversity of plant growth promoting rhizobacteria and their effects on the growth of maize plants under greenhouse conditions. Ann. Agric. Sci. 63, 25–35. doi: 10.1016/j.aoas.2018.04.002 [DOI] [Google Scholar]
- Zaffar H., Sabir S. R., Pervez A., Naqvi T. A. (2018). Kinetics of Endosulfan biodegradation by Stenotrophomonas maltophilia EN-1 isolated from pesticide-contaminated soil. Soil Sediment Contam. Int. J. 27, 267–279. doi: 10.1080/15320383.2018.1470605 [DOI] [Google Scholar]
- Zhang Z., Yuen G. Y., Sarath G., Penheiter A. R. (2001). Chitinases from the plant disease biocontrol agent, Stenotrophomonas maltophilia C3. Phytopathology 91, 204–211. doi: 10.1094/PHYTO.2001.91.2.204, PMID: [DOI] [PubMed] [Google Scholar]