ABSTRACT
The unsuitable disposal of plastic wastes has caused serious environmental pollution, and finding a green manner to address this problem has aroused wide concern. Plastic wastes, especially polyolefin wastes, are rich in carbon and hydrogen, and chemical recycling shows distinct advantages in their conversion into olefins and realizes a closed-loop cycling of plastic wastes. Plastic wastes should be labeled before disposal. The necessity for, and methods of, pretreatment are introduced in this paper and the whole recycling process of polyolefin wastes is also summarized. As the core technology pyrolysis, including thermal, catalytic and solvolysis processes, is introduced in detail due to its potential for future development. We also briefly describe the feasible strategies of pyrolytic oil refining and life cycle assessment of the chemical recycling process. In addition, suggestions and perspectives concerning the industrial improvement of polyolefin chemical recycling are proposed.
Keywords: polyolefins, pretreatment, chemical recovery, product refining, life cycle assessment
This paper gives an overview of the whole chemical recycling process of polyolefin wastes, including the pretreatment of feedstocks, chemical recovery, product refining to olefins, and LCA analysis of chemical recycling.
INTRODUCTION
Compared with steel, wood and cement, plastic exhibits excellent advantages, such as durability, light weight, strong plasticity, energy efficiency and other properties which enable it to be widely used in construction, transportation, electrical and electronic, agriculture, packaging, medical and other industries. By 2022, the total global plastic output had reached 367 million tons [1], and 50% of plastic products are estimated to end up in the waste stream after being used only once or entering the circulation domain for a short period of time [2]. The service life of several plastic products, such as packaging, bags, and utensils, is less than one month [3]. Household plastic waste streams mainly include five types of plastics, namely, polyethylene (PE), polypropylene (PP), polyvinyl chloride (PVC), polystyrene (PS) and polyethylene-terephthalate (PET), which account for 74% of all plastic wastes [4]. Given their biological and chemical inertness, the degradation period of these plastic products in the natural environment is long, and can be further prolonged due to the addition of antioxidants and stabilizers during production. As a result, plastic wastes, especially packaging bags, accumulate in the natural environment or landfills, which causes severe environmental pollution, such as air, groundwater and marine pollution [5]. Although biodegradable polymers can be one of the solutions to the problem of plastic wastes, only 2.6 million tons of biodegradable polymers are estimated to be produced by 2023 (less than 1% of the demand for plastic) [6]. Furthermore, their production costs, mechanical properties, and chemical durability are incomparable to those of petroleum-based plastics. In addition, the degradation rate of biodegradable polymers in the natural environment remains poor. Therefore, the recovery and high-value utilization of plastic waste is the most important means to transform wastes into valuable materials and solve the problem of ecological and environmental pollution [7].
Given the increased amount of plastic waste, several recycling/recovery methods, including energy recovery, mechanical recycling and chemical recycling, have been proposed. Energy recovery by incineration is a suitable solution taking advantage of the high energy content of plastics. However, this process leads to the possible emission of toxic compounds and a large amount of carbon dioxide. Compared with energy recovery, mechanical recycling and chemical recycling of plastic wastes have attracted considerable attention in the context of carbon emission reduction. Mechanical recycling is widely used in the treatment of single-component plastic wastes, but it is difficult to use in the recovery of plastics with high performance, multiple functions and complicated components. Chemical recycling can depolymerize plastic wastes into their monomers, which can then be used as feedstocks to produce virgin plastics. However, chemical recycling of plastic wastes comes with a number of challenges, such as how to collect and sort low-value plastic wastes, difficulty of obtaining pyrolysis products with properties that meet refinery requirements and the lack of a suitable business model. However, from the perspective of sustainable development, the advantage of chemical recycling will promote its rapid development in the coming decades.
Plastic waste management aims at using a hierarchical approach. As shown in Fig. 1, plastic wastes must be labeled as clean, polluted, single, mixed, composite, etc., followed by appropriate pretreatments like sorting, compressing, crushing and pelletizing to produce raw materials. Targeted recycling schemes should be selected for different labeled plastic wastes. Severely polluted and mixed plastic wastes with extremely complex compositions are suitable for energy recovery. Clean and single plastic wastes are preferred in the production of flakes or granules through mechanical recycling. Chemical recycling is an effective method for the closed-loop cycling of slightly polluted mixed or composite plastic wastes.
Figure 1.

Recycling strategies of plastic wastes.
In theory, chemical recycling approaches can be used in the infinite recycling of polymers into monomers. However, notable technical and economic challenges exist for the fully circular economy of commodity plastics, in particular polyolefins. In terms of thermodynamic factors, saturated PE and PP are generated by the addition of polymerization reactions; however, numerous polymerization reactions require an extremely large Gibbs free-energy change for the complete reconversion of polymers to monomers [7]. As a result, a number of undesirable side reactions with low activation energy occur. Thus, the recovery of monomers from waste polyolefins through pyrolysis produces complex mixtures that require further processing.
In the past several decades, reviews have summarized the chemical recovery of polyolefin wastes, and most researchers have used pure resin as feedstocks, ignoring the source and composition complexity of actual polyolefin waste streams. This review aims to provide insights into the whole process of chemical recycling, including pretreatment, chemical recovery, and product refining to olefins and highlights the main progress in the chemical recovery of polyolefin wastes. The green properties of the chemical recycling of polyolefin wastes are explained through LCA analysis, and its future development direction is proposed. This review can help researchers determine the direction for their research, and practitioners to select appropriate technical solutions in order to establish the chemical recycling industry chain of polyolefin wastes.
PRETREATMENT OF POLYOLEFIN WASTES
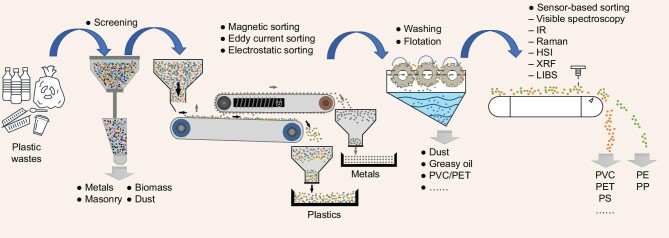
The pretreatment of polyolefin wastes is an essential step of chemical recycling, although it brings additional costs. On the one hand, this process is advantageous in improving the stability of unit operation; on the other hand, it enhances the quality of pyrolysis products and maintains the economic feasibility of the related processing plants, such as those plants used for pyrolysis and refining. The primary pretreatment of polyolefin wastes includes the removal of extraneous materials and nonpolyolefin plastics (Fig. 2).
Figure 2.
Chemical recycling pretreatment scheme.
The removal of extraneous materials involves screening, sorting, washing and flotation, and the size of polyolefin wastes must be uniform in order to efficiently optimize removal [8]. Metals can be removed by magnetic, electrostatic and eddy current separation techniques. The washing process includes coarse/rough and fine washing. Most of the sediment and water-soluble impurities adhering to polyolefin wastes can be removed by coarse washing, and alkali or acid washings are typical methods of fine washing that improves the cleaning quality. However, 2–3 m3 water is required to clean 1 t of polyolefin waste in the latest generation of wash plants, which brings about additional costs and a substantial quantity of wastewater [9]. Table 1 shows the technical principles and characteristics of these methods [10–13].
Table 1.
Brief introduction of the main sorting methods.
| Method | Technical principles | Characteristics | Ref. |
|---|---|---|---|
| Drum screen | The input material is loosened and redistributed by the rotary motion, which exposes enclosed particles and separates the undersize particles from fine to coarse. | Low separation efficiency; impurities can be coated by two-dimensional (2D) films; adhesive wet impurities are difficult to remove; screening efficiency of 70%–90%; approximately 15% degree of filling in drum screen. | [10] |
| Magnetic sorting | Change the motion state of feedstocks by the difference in magnetic induction forces in the magnetic field. | Can only remove ferromagnetic metals in feedstocks. The most efficient separation for grain class (0.32–0.1 mm). |
[11] |
| Electrostatic sorting | Based on the electrical properties of each component in a high-voltage electric field. | Used for sorting conductive, semiconductive and nonconductive materials; can also be used to sort different types of plastics. The diameter of feedstocks is 0.04–1 mm. Requires more than 10 000 V static voltage and dry materials. |
[12] |
| Eddy current sorting | Conductors can generate induced currents in high-frequency alternating magnetic field, generate a magnetic field in the opposite direction of the original one, and finally form a repulsive force. | Used for sorting nonferrous metals with particle size > 2 mm; iron must be removed first; high-strength permanent magnet materials effectively save electricity. | Open data |
| Flotation | Utilizes the difference in floatability of fine-grained materials to achieve separation. | Suitable for sorting plastic mixtures with little or no density contrast of components; the product purity can reach over 90%, and the recovery rate is 80%–99%. Suitable range of particle size depends on the flotation device. Inclusion of additional chemicals poses a risk of water pollution. |
[13] |
| Wind separation | Based on the difference in suspension speed between materials and impurities. | Always combined with cyclone separator; low sorting efficiency of 40%–70%. Susceptible to moisture content, bag breakage rate, and entanglement conditions and uniformity; needs a uniform particle size. |
Open data |
| Visible spectroscopy | Based on visible spectra and performed in the wavelength range 400–700 nm or adopted in digital imaging. | Low separation efficiency; always used for color separation of clean monomaterial steam. | [17] |
| IR spectroscopy | Based on the difference in characteristic peaks of different functional groups in near-IR (NIR) (780–1100 nm) and middle IR (MIR) (1100–2526 nm) bands. | High detection efficiency, online nondestructive testing and harmless to humans; MIR is unaffected by black plastics. Susceptible to environmental interference. |
[18,19] |
| HSI | Based on image data technology of many narrow bands; it combines imaging technology with spectral technology to detect 2D geometric space and 1D spectral information of targets and obtain continuous and narrow band image data with a high spectral resolution. | Rich spectral information, high spatial and spectral resolution, numerous bands and abundant information; reduction in analysis time and objective analysis; high dimensionality of HSI data. | [20,21] |
| Raman spectroscopy | The frequency of the interaction between photons and molecular changes and the light signal scattered from the sample surface are collected to obtain relevant information on the detected molecules. | High detection efficiency; overcomes the shortcomings of NIR. Background fluorescence of Raman often overshadows several characteristic peaks. |
[22] |
| X-ray fluorescence | Identification of plastic species based on X-ray spectrum of characteristic atoms in plastic. | High sorting efficiency for halogen-containing plastics. Easily affected by mutual element interference and superimposed peaks. |
[23] |
| LIBS | Plasma is formed on the sample surface by focusing the ultrashort pulse laser, and the plasma emission spectrum is analyzed to determine the material composition and content of the sample. | Sensitive to specific chemical bonds and has high accuracy and wide range of identifiability. Easily affected by similar element composition and additives in plastic. |
[19] |
Nonpolyolefin polymers, such as PVC, PET and acrylonitrile butadiene styrene, should be removed to reduce their effect on pyrolysis. This process usually includes flotation and sensor-based separation. Flotation is a simple and effective method to separate different kinds of plastics. The quality of separation is related to reagent selection and varies with the mixture type; it depends not only on the hydrophobicity of the plastic but also on the size, density and shape of particles [14]. Plastic particles with small size, lamellar shape and low density show desirable floatability [15,16]. Sensor-based sorting is an automated sorting method based on the differences in spectral properties of plastics, including visible spectroscopy, infrared (IR) spectroscopy, hyperspectral imaging (HSI), Raman spectroscopy, X-ray fluorescence and laser-induced breakdown spectroscopy (LIBS). Table 1 shows the technical principles and characteristics of these methods [17–23]. The interference of noise, background factors and extraneous materials can affect the quality of collected data and thus the sorting accuracy for actual nonpolyolefin wastes. In terms of sorting practices, multiple technologies are usually needed to improve the efficiency of separation.
A number of laboratory studies have focused on the sorting technology of plastic wastes; thus, the detailed content will not be elaborated further in this study. The chemical recycling technology of plastic wastes, which is currently in the pilot or industrial demonstration stage, requires high-quality feedstocks. Thus, it is necessary to pretreat the feedstocks to meet the feeding requirements. The Hydro-PRT® developed by Mura requires a polyolefin content higher than 80% and PVC, PET, moisture, and contaminant (paper, metals and organic waste) contents lower than 0.5%, 5%, 5% and 5%, respectively.
The energy consumption and cost of pretreatment technology, which depends on the properties of feedstocks, are relatively limited. Using the plastic waste excavated from landfills as an example, the comprehensive cost of sorting plastic waste in order to get impurity and moisture content below 5% is approximately 600–800 RMB/t. The price of sorted miscellaneous films can reach 1000–1500 RMB/t. In addition, given the limitations on word count requirements in this special issue, only feasible disposal methods are provided for reference.
CHEMICAL RECOVERY PROCESS
Typical chemical recovery methods of plastic wastes include pyrolysis and gasification. Plastic pyrolysis involves the heating of raw material in a pyrolysis reactor to break the C–C and C–H bonds in the absence of oxygen and its conversion into small-molecule products dominated by hydrocarbons [24]. As plastic wastes are derived from the petrochemical industry, their conversion back into petroleum fractions or other petrochemical products has a high atomic efficiency. Gasification of waste polyolefins leads to the production of a stream made up of mainly H2 and CO. In general, the production of liquid hydrocarbons or syngas from polyolefin wastes by adjusting the reaction temperature is technically feasible, and both products can be further used to prepare olefins, which contributes to the circular economy by returning plastic wastes back into the cycle loop.
Thermal pyrolysis
Thermal pyrolysis of polyolefins mainly follows the free-radical reaction mechanism, which involves three stages: chain initiation, propagation and termination [25]. First, the initial thermal shock causes the fracture of any C–C bonds along the PE chain, which results in the formation of primary free radicals and reduction of the molecular weight of polymers (Fig. 3a) [25]. In the second stage, new radicals are generated due to H-abstraction reactions between primary free radicals and hydrocarbons and are further converted into olefins through the decomposition of C–H bonds. The obtained olefins cause the formation of aromatics through cyclization and aromatization reactions. In addition, several free radicals that undergo isomerization reactions stabilize and may break down into a large amount of liquid olefins and new radicals through further β-scission. In the termination stage, interactions occur by disproportionation or recombination of existing radicals, which results in the formation of H2, CH4, alkanes and short-chain alkenes.
Figure 3.
Proposed reaction mechanisms of polyolefins during thermal pyrolysis: (a) free radical reaction and (b) carbonium ion reaction. Reproduced from ref. [25].
Numerous studies have reported the pyrolysis characteristics of polyolefins, and the reviews of product distributions are summarized in Table 2 [26–39]. The factors affecting the pyrolysis of plastic wastes mainly include reactor type, feedstock, reaction temperature, residence time and pressure. The complex behavior of polyolefin wastes with low thermal conductivity, sticky nature, and low softening and melting temperatures during pyrolysis brings a great challenge for the development of suitable reactors. In general, two crucial principles, such as improving the heat transfer rates and lowering the residence time, are preferred in the design of pyrolysis reactors, which help in the production of more liquid oil and inhibit the secondary reactions that form more char or gaseous products. Fixed bed reactors have a low heat transfer rate and long residue time and noncontinuous operations limit their widespread application [40]. Meanwhile, these reactors cannot maintain uniform temperature profiles, especially in the pyrolysis of large volumes of polyolefin wastes. Fluidized bed reactors allow strong heat and mass transfer, and they are suitable for continuous isothermal pyrolysis with relatively low residence time and operating cost [41]. Park et al. [42,43] designed a fluidized bed reactor to prepare aromatic hydrocarbons from PE and PP and observed that the aromatic selectivity in pyrolysis oil was as high as 92%. Salaudeen et al. [44] developed an internally circulating fluidized bed reactor to reduce the residence time of plastic wastes and improve the selectivity of olefins and demonstrated its validity. However, several disadvantages, such as particle size limitations and attrition of bed material during operation, have been recorded. Compared with fluidized bed reactors, conical spouted bed reactors have lower attrition and bed segregation due to the tight interaction between phases, and the substantially increased collision rate between particles decreases the agglomeration. Furthermore, this type of reactor can adapt to applications with a wide particle size distribution, large particles and different particle densities [45]. Meanwhile, the residence time of feedstocks in conical spouted bed reactors is reduced to approximately 20 ms, which can effectively avoid the occurrence of secondary reactions and reduce the formation of coke. Orozco et al. [46] confirmed that these reactors are suitable for the pyrolysis of plastic wastes given their hydrodynamic behavior and versatility. However, the complex construction process and a variety of pumps in the system result in high investment costs. Screw kiln reactors can operate in continuous mode and control the residence time of feedstocks by changing the rotation speed of the screw. During pyrolysis, an inert gas is fed into the reactor to ensure the absence of oxygen in the system, which contributes to the transport of pyrolysis steam [46]. In addition, solid carriers can be placed in the reactor to further improve the efficiency of heat and mass transfer, which results in the closer interaction between feedstocks. However, screw kiln reactors have the risk of plugging, possible heat transfer difficulties at large scales and poor mixing in the radial direction [47]. Compared with the above conventional reactors, microwave reactors have numerous advantages. Microwave energy can be applied directly to feedstocks through molecular interactions with the electromagnetic field, which increases the heating rate of the pyrolysis process. The low dielectric constant of plastics can reduce the efficiency of microwave heating; thus, a microwave-absorbent material should be mixed with plastic wastes during pyrolysis. Zhou et al. [48] designed a 200 kg/day laboratory-scale continuous microwave pyrolysis reactor, used high-temperature SiC balls to heat HDPE chips and confirmed that the reactor can effectively improve heat transfer efficiency and achieve fast pyrolysis. The different heating efficiencies of each material limits the industrial application of this technology. In addition, molten salt reactors [38], induced plasma reactors [39] and screw extruder reactors used for polyolefin pyrolysis have been developed. Screw extruder reactors are normally used for densification, viscosity reduction and transportation of polyolefin wastes. A single-screw extruder is the most mature type of extruder at the moment, and it is widely used in polymer extrusion processes with the advantages of high speed, high performance, stable extrusion and low equipment cost. The screws of a twin-screw extruder (TSE) typically engage with each other and rotate in the same or opposite directions. By comparison, the corotating TSE offers higher capacity and better thermal management and cleaning capabilities, and Sinopec has applied it as visbreaking equipment for the chemical recovery of plastic wastes. The plastic wastes enter the extruder through a feeder and are rapidly heated to a melt at a temperature of 350oC. These plastics are further heated to 500oC after entering the coking reactor, and the polymer chain is split into smaller monomers in an oxygen-free environment. The combination process of visbreaking and coking developed by Sinopec has improved the yield of pyrolysis oil to a certain extent.
Table 2.
Product distributions in the thermal pyrolysis of plastics.
| Yield of product (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Feedstock | Reactor | T (oC) | Residence time or Feed rate | Pressure | Oil | Wax | Gas | Residue | Ref. |
| HDPE | Fixed bed reactor | 550 | — | — | 70 (oil + wax) | — | 22 | 7 | [26] |
| PP | Fixed bed reactor | 525 | — | Atmospheric | 76.4 | 4.2 | 10.1 | 2.6 | [27] |
| PP | Fixed bed reactor | 525 | — | Vacuum | 70.8 | 20.5 | 5.1 | 2.4 | [27] |
| HDPE:LDPE:PP = 25:35:40 | Fixed bed reactor | 500 | 60 min | — | 30.7 (oil + wax) | — | 67.9 | 1.4 | [28] |
| PE | Fluidized bed reactor | 500–600 | 12.4–20.4 s | — | 81.2–28.5 | — | 8.2–56.8 | 10.6–14.7 | [29] |
| HDPE | Fluidized bed reactor | 640–850 | 3–4 g·min−1 | — | 68.5–16.2 | — | 33.5–89.1 | — | [30] |
| PP | Fluidized bed reactor | 510 | 14 g·min−1 | — | 10.5 | 86.6 | 2.7 | 0.17 | [31] |
| PE | Fluidized bed reactor | 510 | 16 g·min−1 | — | 30.1 | 63.1 | 6.5 | 0.26 | [31] |
| Mixed municipal plastic wastes | Screw kiln reactor | 500 | 5 g·min−1 | — | 5.5 | 93.2 | 1.3 | — | [32] |
| PP | Screw kiln reactor | 500–700 | 10 min | — | 50–20 | — | 6–80 | — | [33] |
| LDPE | Screw kiln reactor | 500–550 | 1.5 g·min−1 | — | 84.6–79.3 | 14.4–13.6 | 1.0–7.1 | — | [34] |
| HDPE | Conical spouted bed reactor | 500–700 | 1 g·min−1 | — | 31.4–49.3 | 67–11.3 | 1.6–39.4 | — | [35] |
| PE | Conical spouted bed reactor | 450–600 | 1 g·min−1 | — | 20–49 (oil + gas) | 80–51 | — | — | [36] |
| PP | Conical spouted bed reactor | 450–600 | 1 g·min−1 | — | 8–50 (oil + gas) | 92–50 | — | — | [36] |
| HDPE | Microwave reactor | 500 | — | — | 74.7 (oil + wax) | — | 13.3 | 12.0 | [37] |
| PP | Microwave reactor | 600 | — | — | 83.9 (oil + wax) | — | 15.7 | 0.4 | [37] |
| HDPE | Molten salt pyrolysis reactor | 408–423 | — | — | 94–92 (oil + wax) | — | 6∼8 | — | [38] |
| PP | Molten salt pyrolysis reactor | 362–417 | — | — | 98–96 (oil + wax) | — | 2∼3 | — | [38] |
| LDPE | Induced plasma reactor | 550 | 30 min | — | 56.9 | — | 37.8 | 5.3 | [39] |
| LDPE | Screw extruder reactor + Coking reactor | 500 | 30 min | — | 93.8 (oil) | — | 5.1 | 1.1 | Sinopec |
The pyrolysis of polyolefin wastes is a typical endothermic reaction, and temperature is one of the most significant operating parameters affecting the distribution and quality of products. The appropriate increase in temperature favors the production of liquid oil and gases, while an excessively high temperature will lead to further cleavage of macromolecular pyrolysis products to small-molecular gases. The aliphatic content in the oil sharply decreases, and those of benzene, toluene and xylene (BTX) aromatics increase at high temperatures because of the enhancement of Diels–Alder and dehydrogenation reactions [49]. During the pyrolysis of polyolefin wastes, the target product, such as wax, light olefin or BTX, can be obtained by reactor design and adjusting the operating parameters [50]. The yield of the target product waxes increases under temperatures less than 500°C and short residence times of volatiles [40]. The production of light olefins requires higher reaction temperatures in the range of 800°C to 950°C. During processing, the target temperature should be reached rapidly to facilitate the deep cracking of polyolefin wastes, and the residence time of volatiles should be kept to a minimum in order to inhibit other side reactions. BTX are usually formed by the synergistic effect of the Diels–Alder reaction of small molecular olefins in primary pyrolysis products and the dehydrogenation reaction at temperatures above 700°C [50]. However, the aromatization of olefins and condensation of monocyclic aromatics into polycyclic aromatics (PAHs) are sensitive to the residence time, which results in the production of low-yield BTX by direct thermal pyrolysis; in addition, controlling the generation of PAHs in the process is difficult [51].
Pressure and residence time are also significant operating parameters for the pyrolysis of polyolefin wastes. Pressurized conditions are conducive to aromatization and oligomerization reactions, which promote the formation of aromatic hydrocarbons [51]. Nevertheless, these reactions decrease the selectivity of light aromatics and facilitate the generation of heavy aromatics as coke precursors at elevated pressures [45]. Residence time shows similar effects on the pyrolysis of polyolefin wastes under reaction temperatures. Long residence times promote the conversion of polyolefin wastes into light hydrocarbons and noncondensable gases. It also increases the chance of secondary reactions, such as further cracking of the primary products and isomerization, cyclization and aromatization reactions [28].
To a certain extent, the quality of pyrolysis oil can be improved by adjusting the reaction parameters. However, the pyrolysis of polyolefin wastes follows the rule of random chain breaking, which results in a complex and wide range of pyrolysis oils (C5–C60). The liquid products need to be further upgraded before replacing traditional fuels in chemical production. Thus, finding a mechanism on how to effectively increase the content of valuable components in pyrolysis oil is a key scientific problem that must be solved for the direct preparation of high-quality liquid fuel from waste polyolefins.
Catalytic pyrolysis
The catalytic pyrolysis of polyolefin wastes is a thermal cracking process that selectively breaks polymer molecular chains, in which the reaction process and product distribution are tunable with different catalysts (Fig. 4) [45]. With the addition of catalysts, the cracking of long-chain substrates is accelerated at low reaction temperatures and short reaction times [52], which result in concentrated product distribution and energy savings. At present, catalysts with acid sites and pores [53], such as zeolites (ZSM-5, HY, beta, HUSY, etc.), activated carbon, fluid catalytic cracking (FCC) equilibrium catalysts and metal oxides, have been used to increase the yield of targeted products and change the mechanism of catalytic pyrolysis (Table 3) [54–60]. Specifically, the acidity strength of catalysts contributes to the cracking and aromatization of polymers, which determines the reaction process and yield of targeted products. To a certain extent, the porous structure of catalysts affects the molecular weight of the corresponding intermediates, and a large pore size results in long-chain products [61].
Figure 4.
Catalytic pyrolysis of polyolefin wastes to high-value products. Reproduced from ref. [45].
Table 3.
Catalytic performance of different plastics under different reaction conditions.
| Yield of product (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Feedstock | Catalyst | Reactor | T (°C) | Alkenes | Alkanes | Gasolines | Aromatics | Ref. |
| PE | HZSM-5 | Fixed bed (in-situ) | 600 | 28 | 34 | — | 27 | [54] |
| PE | HZSM-5 | Fixed bed (ex-situ) | 600 | 80 | 7 | — | 11 | [54] |
| HDPE | Hβ | Batch reactor | 480 | 65 | 30 | 83 | 5 | [55] |
| HDPE | HY | Batch reactor | 480 | 30 | 45 | 60 | 25 | [55] |
| HDPE | HZSM-5 | Batch reactor | 480 | 50 | 25 | 66 | 25 | [55] |
| HDPE | Al-MCM-41 | Fixed bed | 450 | 51 | 37 | 56 | 4 | [56] |
| PE | HY | Fixed bed | 400 | 31.7 | 34.9 | — | 2 | [57] |
| PE | HY | Fixed bed | 500 | 34.2 | 30.8 | — | 2.3 | [57] |
| HDPE | FCC equilibrium catalyst | Batch reactor | 350 | 52.6 | 34.4 | 47.7 | 13.0 | [58] |
| LDPE | Activated carbons | Fixed bed | 500 | — | 71.8 | — | 28.2 | [59] |
| LDPE | Al2O3 + M-clay | Batch reactor | 30–600 | 17 | 60 | 70 | 14 | [60] |
Typically, zeolites play a substantial role in the catalytic pyrolysis of polyolefin wastes (Fig. 5a) [55]. HZSM-5 zeolite has been applied in the conversion of PE into liquid fuels [54]. The yields of alkene and alkane reached 80% and 7%, respectively, during ex-situ catalytic pyrolysis, and 28% alkenes and 34% alkanes were produced during in situ catalytic pyrolysis. The aromatic yield was 27% during the in situ catalytic pyrolysis of PE, and it decreased to 11% when PE during ex-situ pyrolization. In the catalytic pyrolysis of PE using HZSM-5, the olefins generated from PE decomposition can be aromatized, and the aromatization reactions released free hydrogen atoms [54]. The hydrogen atoms contribute to the thermal decomposition of PE and the saturation of olefins into alkanes. In situ catalytic pyrolysis can promote the aromatization of olefins inside the catalyst pores and the alkane yield was higher because of more hydrogen atoms for the saturation reactions. During ex-situ catalytic pyrolysis, catalytic cracking of PE was the predominant reaction, resulting in high olefin yields in liquids. Wang et al. [55] observed that HZSM-5/HY/Hβ catalysts showed high alkane and alkene selectivity but low aromatic selectivity in liquids during the catalytic pyrolysis of HDPE at 480°C. Aguado et al. [56] reported a similar conclusion during the catalytic pyrolysis of LDPE with Al-MCM-41.
Figure 5.
(a) Typical zeolites used for LDPE catalytic pyrolysis. Reproduced from ref. [55]; (b) the proposed reaction route of converting polyolefin wastes into transportation jet fuel over activated carbon. Reproduced from ref. [59]; (c) structure illustration of FCC catalysts. Reproduced from ref. [63].
Activated carbons, which possess a large surface area and porosity, have excellent application prospects in the catalytic pyrolysis of polyolefins [62]. Zhang et al. [59] employed different activated carbons in the deconstruction of LDPE and proved that catalysts with weak and relevant strong acidity favored jet fuel-range alkanes and aromatics, respectively (Fig. 5b). In addition, the selectivity of alkanes and aromatics reached 71.8% and 28.2%, respectively, the aromatization of alkanes was enhanced and more H2 was released at higher temperatures.
FCC catalysts are composed of active components (generally zeolites, such as Y and ZSM-5 zeolites) and the matrix (clay, alumina, silica, etc.) (Fig. 5c) [63]. Given the high density of Brønsted acids, the catalyst is active for cracking and aromatization. Mesopores in the FCC catalyst allow large molecules to access the inside of the catalyst for precracking and provide Lewis acid sites for aromatization. Therefore, it has considerable potential for the conversion of plastic wastes into high-value-added products, such as diesel oil and gasoline [63].
Considering the different mechanisms of acid and base sites, metal oxides or metal salts exhibit high activity in the catalytic pyrolysis of polyolefins; CuCO3 contributes 94% yield of liquid hydrocarbons [64]. Compared with a single catalyst, a narrower product distribution from the deconstruction of LDPE was obtained under the relay catalysis mode using Al2O3 catalysts followed by ZSM-5 at 550°C. During the process, polymers penetrated the large pores of the Al2O3 catalyst for cracking into lighter hydrocarbons, which can enter the micropores of ZSM-5 for further reformation, which contributed to C5–C12 alkanes/olefins with a selectivity up to 77% [60]. The synergy of the two catalysts narrowed the production distribution. Recent studies have reported the strategy of converting PE into high-value products under mild conditions. Tennakoon et al. [65] designed a catalyst with a mesoporous shell/active site/core (mSiO2/Pt/SiO2) structure, and the active site of platinum nanoparticles was located at the bottom of the mesopore in the silica shell. The long chain of HDPE moved in the hole, and the active site can catalyze its hydrogenolysis reaction. The small molecular products were desorbed from the end of the long chain and escaped from the hole. At 300°C, 24 h and 1.38 MPa H2, HDPE was converted into small molecular alkane segments with narrow chain lengths, and the products can be used for the preparation of diesel and lubricating oil. Duan et al. [66] reported the low-temperature conversion of PE into olefins. They mixed the PE feed with rationally designed ZSM-5 zeolite nanosheets at 280°C with flowing hydrogen as a carrier gas and obtained a yield of up to 74.6% light hydrocarbons (C1–C7) in which 83.9% of these products were C3–C6 olefins with almost undetectable coke formation. In addition, Li et al. [67] designed a tandem catalytic conversion of LDPE into naphtha with beta zeolite and silicalite–1 encapsulated Pt nanoparticles as catalysts, which provided an 89.5% yield of naphtha and a 96.8% selectivity of C5–C9 hydrocarbons under mild conditions. These new strategies can realize the high value-added utilization of polyolefin wastes, but the reaction time must be extended to improve the yield of the target product.
In summary, the acidity, pore size and active metal of catalysts have significant effects on the yield and composition of catalytic pyrolysis oil from plastic wastes. Catalytic pyrolysis involves a free-radical mechanism and carbocation reaction mechanism. During the ex-situ reaction, plastic is first thermally cracked in a single-pyrolysis reactor, and the vapor follows the free-radical mechanism. Random scission occurs to generate free radicals along the carbon chains, and a molecule with an unsaturated end and another with a terminal free radical are formed with further cleavage. Through H-abstraction reactions, straight-chain dienes, alkenes and alkanes are obtained from the radical fragments [68]. Using solid acid catalysts, the in-situ reaction mechanism of the pyrolysis of polyolefins follows a carbonium ion mechanism (Fig. 3b) [25]. The cracking of polymer chains begins with protonation of Brønsted acid sites or hydride abstract on the surface Lewis acid sites to form carbocationic intermediates, followed by β-scission and skeletal rearrangement to form short-chain hydrocarbons and more isomers. With the catalysis of solid acid, cyclization, aromatization and alkylation can occur to form aromatics and significant amounts of H2.
From the results of the above research, the selection of catalysts for the upcycling of plastic wastes plays a critical role in catalytic pyrolysis. Catalysts drive isomerization, oligomerization cracking, and hydrogen transfer and have a large influence on product distribution. The conversion efficiency of feedstocks and the selectivity of the target product are mostly determined by the acid strength and porosity of catalysts. Microporous zeolites (HZSM-5, HY, Hβ, etc.) can considerably increase the yield of gaseous products during polyolefin pyrolysis, which results in a low yield of liquid products. Mesoporous zeolites (MCM-41, SAHA, etc.) have a higher activity in the selective production of liquid fuels than microporous catalysts, but they show lower hydrothermal stability, mechanical stability and acidity. Compared with zeolite and FCC catalysts, activated carbons have the lowest acidity but have the advantages of a simple preparation process, low cost and high thermal stability. In addition, to improve the efficiency of the catalytic pyrolysis process, most methods for modification of catalysts have been carried out, such as acid leaching and thermal and wet impregnation [69]. Furthermore, the synergy of different catalysts can tune product distribution through a combination of high porosity, high acidity, and hydrogenation properties to prevent deactivation and increase catalyst reuse.
Although the addition of catalysts shows great advantages over thermal pyrolysis, the pyrolysis oil obtained from catalytic pyrolysis of waste polyolefins is still a complex hydrocarbon mixture, and its carbon numbers range from C6 to above C25. Thus, pyrolysis oil needs further upgrading steps, such as catalytic cracking and hydrogenation, before they can be used as fuels. In addition, issues, such as how to prevent catalyst deactivation due to carbon deposition and the ability to recycle catalysts in order to reduce costs, still need further research.
Solvolysis
Catalytic pyrolysis of polyolefin wastes can considerably improve the selectivity of target materials, which provides the possibility for further application of liquid products. However, given the poor thermal conductivity and high melting viscosity of plastics, the catalyst is prone to coke deposition and deactivation during pyrolysis. In general, compared with solvent-free pyrolysis, PE depolymerization can be promoted substantially in the presence of solvents because of the improvement in mass transfer and heat transfer rates. Meanwhile, the solvent effect may change the distribution of pyrolysis products by changing the thermal degradation mechanism. The thermal degradation of HDPE in the presence of hydrogen-donating solvents follows the free radical mechanism, as in the case of cracking in the absence of solvents. The solvent-free pyrolysis of PE contributes to the production of alkanes, and the yield of olefins is high in solvent pyrolysis. The radicals generated from solvents can follow two pathways during pyrolysis. One of the pathways is hydrogen atom abstraction from the polymer chains by solvent radicals, which leads to secondary radicals and regeneration of the corresponding solvent molecules. This effect, in turn, favors HDPE chain β-scissions and thus an increase in HDPE conversion. The other pathway is the dehydrogenation reaction of solvents [70]. Serrano's team studied the pyrolysis characteristics of HDPE in hydrogen donor solvents, such as tetrahydronaphthalene, decalin and phenol, under noncatalytic conditions and observed that the presence of solvents promoted the hydrogen transfer reaction from solvents to HDPE radicals and from molecular chains to solvent radicals, which aided in the conversion of HDPE and production of α-olefins [70–72]. Ellis et al. [73] and Jia et al. [74] reported that PE was degraded into transportation fuels and waxes through a tandem catalytic cross-alkane metathesis method with n-pentane or n-hexane, and 73%–98% of these products were converted into liquid hydrocarbon oils at 150°C–200°C within 15 h to 72 h. Conk et al. [75] improved the process and directly converted HDPE or LDPE into propylene through a series of reactions. Specifically, the method involved a small extent of desaturation of each PE chain by a dehydrogenation catalyst, followed by steady breakdown of the chains into propylene through catalytic isomerization and metathesis reactions with ethylene. The propylene yield was as high as 80%. In addition, to improve the catalytic decomposition efficiency, Jia et al. [76] reported the depolymerization of HDPE in various liquid-phase solvents with the Ru/C catalyst. Under optimal conditions (220°C, 2 MPa H2), the yields of jet-fuel and lubricant-range hydrocarbons reached 60.8% and 31.6% within 1 h, respectively. Zhang et al. [77] reported a new strategy that combines endothermic cleavage of the polymer C–C bonds with the exothermic alkylation reaction of the cracking products and realizes the full conversion of polyolefins into liquid iso-alkanes (C6 to C10) at temperatures below 100°C within 4 h. In addition, several researchers have noted that solvents play a very important role in direct coal liquefaction [78,79]. The physical role of solvents is to facilitate heat transfer and dissolve coal, hydrogen and liquefaction products. The chemical action of solvents is to provide activated hydrogen for coal, transfer H2 to coal through the hydrogen shuttle action and promote the cleavage of the Caryl–Calkyl bond. A well-designed solvent system and suitable catalyst can realize the highly selective depolymerization of polyolefins under mild conditions. In addition, in several studies, polyolefin wastes have been dissolved in solvent oils, such as vacuum gas oil (VGO), light cycle oil (LCO) and heavy cycle oil, to realize the catalytic pyrolysis of these wastes based on existing catalytic cracking or hydrocracking processes in the oil refining field. This process changes the solid−solid reaction to liquid−solid reaction, which improves the yield of pyrolysis oil and selectivity of target products. Kohli et al. [80] investigated the thermal cracking of heavy crude and vacuum residues with polyolefin wastes at 420°C and 6 MPa H2. They demonstrated that the yield of the middle distillate fraction was maximized by minimizing the retrogressive polymerization reactions during the reaction process. Moreover, the addition of polyolefin wastes to vacuum residue feeds helped to reduce coke formation and increase metal and asphaltene removal activities. Rodríguez et al. [81] studied the cocracking of HDPE dissolved in VGO under conditions similar to those of the industrial FCC unit. High conversions of feedstocks and yield of naphtha and concentration of light olefins were observed in this previous study due to the synergistic effect. However, these researches only analyzed the influence of operating parameters, such as pyrolysis temperature, residence time and catalyst addition, on the product distribution during copyrolysis of plastics and solvent oils. The synergy mechanism between the two still needs further exploration.
Gasification
The gasification of waste polyolefins is expected to increase the yield of gaseous products or syngas and reduce the yield of the main undesirable byproducts, such as tar and char. Compared with the pyrolysis technology, a remarkable advantage of gasification is the greater flexibility in jointly valorizing plastic wastes with other raw materials. The composition of gaseous products produced by plastic gasification is closely related to the gasifying agent. Air gasification has been widely used in experiments and pilot projects, its main purpose being for energy production. Steam gasification is an effective protocol for producing syngas with rich H2 from waste polyolefins. However, the large formation of tar during the steam gasification process remains a challenge. Currently, the strategy of combining pyrolysis with steam reforming has gained increasing attention. The gas products produced in the process are free of tar, which solves the main problem of the gasification process.
Gasification mainly involves the following steps: pyrolysis of feedstocks, cracking and reforming reactions in the gas phase, and heterogeneous char gasification. The pyrolysis process involves a series of complex endothermic chemical reactions. The low thermal conductivity and sticky nature of waste polyolefins reduce their thermal decomposition kinetics and easily lead to the formation of fused plastic agglomerates. Thus, in the gasifier design, heat transfer rates should be increased as much as possible. Meanwhile, waste polyolefins can be almost completely converted to volatiles at high heating rates. Therefore, the heterogeneous char gasification step can usually be ignored in the overall conversion. At temperatures above 800°C, the main pyrolysis mechanism of waste polyolefins mainly follows an end-chain scission, such as direct, 1,5-radical transfer and multiple step-radical transfer scissions [82]. The homogeneous gasification process involves a large number of reactions, such as steam reforming of hydrocarbons, methane reforming, dry reforming of hydrocarbons, Boudouard reaction and water–gas shift reaction. The balance of these reactions is greatly related to the type of gasifying agent, equivalence ratio or steam-to-carbon ratio (S/C) and temperature. Arena et al. [83] investigated the air gasification of PE in a bubbling fluidized bed using olivine as a catalyst and observed that olivine can greatly improve the efficiency of the gasification process and reduce the content of tar in gas products. The contents of H2, CO and CH4 in the gas product were 30.1–29.1, 18.4–20.9 and 3.4–1.5 vol.%, respectively. Wilk and Hofbauer [84] developed a dual fluidized bed reactor and studied the steam gasification of mixed waste polyolefins. At 850°C and S/C ratio of 2, the main gaseous product compositions were 46 vol.% H2, 22 vol.% CO and 16 vol.% CH4.
Syngas is the main product of the waste polyolefin gasification process, and it can be used to prepare olefins by Fischer-Tropsch synthesis and methanol-to-olefin technology. Olefins can be further used as feedstock to produce new plastic products, which realize the chemical recycling of polyolefin wastes.
Other chemical recovery strategies
Several novel strategies, such as photocatalysis and electrocatalysis, for degrading plastic wastes at ambient temperature and pressure have been reported. During the photocatalytic process, photogenerated electrons (e−) and holes (h+) are generated when photon energy irradiates the catalyst. On the one hand, plastic waste can be oxidized by h+ [85]. On the other hand, plastic wastes can also be decomposed by hydroxyl and superoxide radicals produced from the reaction of e− and h+ with O2 or H2O [86]. During the electrocatalytic process, oxidation and reduction reactions occur at two electrodes that are linked via an exterior electric circuit and the in-between electrolyte [87]. The externally applied potential triggers chemical reactions, which enable the conversion of plastic wastes into valuable chemicals under mild conditions. Although these methods are more energy saving and environmentally friendly and coincide with the concept of sustainable development, the reaction process requires a relatively long time period, and the conversion efficiency of plastic wastes is very low compared with that under violent conditions such as those involving pyrolysis, catalytic pyrolysis, solvolysis and gasification. In addition, these technologies are still a distance away from industrialization.
REFINING OF PYROLYSIS OIL
The properties and composition of polyolefin pyrolysis oil have been analyzed by a number of researchers. The oil obtained in the slow pyrolysis of HDPE at 430°C has a composition similar to that of VGO, and the composition of HDPE pyrolysis oil is similar to that of LCO above 460°C [88]. Quesada et al. [89] analyzed the composition of pyrolysis oil of PE and PP at 500°C and reported that the composition of PE pyrolysis oil mainly included 1-alkenes and n-alkanes in the range of C7 to C35. The PP pyrolysis oil at 405°C was enriched in the naphtha range hydrocarbons with a preponderance of diesel range hydrocarbons (C6–C16: ∼50%; C13–C16: 33.04%), and the distributions of paraffinic, olefinic and naphthenic hydrocarbons were 66.55%, 25.7% and 7.58%, respectively [90]. Sinopec collected and analyzed several pyrolysis oils of polyolefin waste (Table 4). These pyrolysis oils showed a wide distillation range, covered all fractions from gasoline to heavy oil, and contained more than 30% naphthene/olefins. Based on the above properties, pyrolysis oil from polyolefin wastes is a promising feedstock for the production of liquid transportation fuels or polymer monomers (C2H4 and C3H6). The refining of pyrolysis oil is similar to that of petroleum-based feedstock. In consideration of the economy of chemical recycling of polyolefin wastes, catalytic cracking and steam cracking are usually used to refine pyrolysis oil to olefins.
Table 4.
Composition and properties of pyrolysis oil from actual polyolefin wastes and hydrotreated pyrolysis oil.
| Samples* | Oil 1# | Oil 2# | Oil 3# | Hydrotreated oil 1 | Hydrotreated oil 2 | Hydrotreated oil 3 |
|---|---|---|---|---|---|---|
| Density/(20°C, kg·m−3) | 830.9 | 815.5 | 823.6 | 819.2 | 817.2 | 803.4 |
| w(S)/(mg·kg−1) | 336.0 | 310.0 | 576.0 | 36.0 | 94.0 | 27.0 |
| w(N)/(mg·kg−1) | 629.0 | 421.0 | 2600.0 | 7.0 | 19.0 | 60.0 |
| w(Cl)/(mg·kg−1) | 84.0 | 90.0 | 182.0 | <1.0 | <1.0 | <1.0 |
| w(Si)/(mg·kg−1) | 46.0 | 71.0 | 451.0 | <1.0 | <1.0 | <1.0 |
| w(O)/wt.% | 0.2 | 0.3 | 0.5 | <0.2 | <0.2 | <0.2 |
| w(C)/wt.% | 87.4 | 88.1 | 86.3 | 85.5 | 86.6 | 86.0 |
| w(H)/wt.% | 12.6 | 11.9 | 12.7 | 13.9 | 13.4 | 14.0 |
| Hydrocarbon composition/wt.% | ||||||
| Paraffins | 25.4 | 14.3 | 18.6 | 60.4 | 48.0 | 60.2 |
| Naphthene/olefins | 52.3 | 39.7 | 63.0 | 23.3 | 20.8 | 19.0 |
| Aromatics | 22.3 | 46.0 | 18.4 | 16.2 | 31.2 | 20.8 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 |
*The sample is the actual polyolefin waste, but the pyrolysis temperature and residence time are different from each other.
As shown in Table 4, high concentrations of contaminants (i.e. heteroatoms and metals) and olefins results in uncertainty during catalytic cracking and steam cracking. N, S, O, Cl, Si, and metals are the most prominent pollutants in polyolefin wastes, which can lead to serious problems, such as corrosion, fouling, increased coke formation, and downstream catalyst poisoning during the steam cracking process [91]. In addition, olefins in the steam cracker have a considerable influence on coke formation and the fouling of heat exchanger surfaces [92]. The hydrotreatment step prior to catalytic cracking and steam cracking can efficiently decrease the concentration of unsaturated hydrocarbons and remove heteroatoms and metals [93]. Our previous work (Table 4) also proved this conclusion. Therefore, hydrotreatment of pyrolysis oil prior to catalytic cracking and steam cracking is an essential step to avoid unfavorable influences. The energy consumption of the hydrogenation unit varies depending on the raw materials. The energy consumption of the diesel hydrogenation unit of Zhongke Refining and Petrochemical Company Limited is 2.77 kgEo/t, and that of the residue oil hydrogenation unit of Sinopec Maoming Petrochemical Company is 8.47 kgEo/t. However, no energy consumption data are available for the commercial operation of pyrolysis oil hydrogenation units.
The conversion of pyrolysis oil from polyolefin wastes into low-carbon olefins is one of the essential steps to realize the chemical recycling of polyolefin wastes. As the core conversion process in a refinery, catalytic cracking has an irreplaceable position in the deep processing of heavy oils to light oils and low-carbon olefins. It has the advantages of wide adaptability of raw materials, high conversion rate of heavy oil, high yield of light oil, flexible product scheme, and low operating pressure and investment [94]. Table 5 summarizes several catalytic cracking processes of polyolefin pyrolysis oil [95–99]. A competitive olefin yield can be obtained by optimizing the conditions of catalytic cracking, such as increasing space–time and temperature and selecting an appropriate catalyst. After separation, these mixed olefins can be repolymerized as feedstocks into virgin polyolefins. The energy consumption of catalytic cracking units accounts for 25%–34% of the total energy consumption of refineries. The energy consumption of the 4.2 million tons/a catalytic cracking unit of Zhongke Refining and Petrochemical Company Limited is 31.69 kgEo/t.
Table 5.
Brief introduction of catalytic cracking of polyolefin pyrolysis oil.
| Feeds | Conditions | Outputs | References |
|---|---|---|---|
| HEPE wax | Catalyst: HZSM-5 zeolite Temperature: 550oC |
C2–C3 olefins:62.9 wt.%, ethylene: 10.6 wt.%, propylene: 35.6 wt.%, butene: 16.7 wt.% | [95] |
| Pyrolysis oil of polyolefin wastes | Catalyst: FCC equilibrium catalyst Temperature: 520oC |
Ethylene:3.9 wt.%, propylene: 15.5 wt.%, gasoline: 30.5 wt.% | [96] |
| HEPE wax | Catalyst: FCC equilibrium catalyst Temperature: 500–560oC |
Naphtha:23.8–31.1 wt.%, LPG fractions: 13.7–18.1 wt.% | [97] |
| HDPE waxes (20 wt.%) and VGO | Catalyst: FCC equilibrium catalyst Temperature: 500–560oC Catalyst to oil mass ratio: 3–7 gcat/gfeed−1 Contact time: 6 s |
Ethylene: 0.8–4.5 wt.%, propylene: 3.1–6.7 wt.% | [98] |
| PE, PP waxes and HVGO* | Catalyst: FCC equilibrium catalyst Temperature: 525oC Catalyst to oil mass ratio: 12 gcat/gfeed−1 Contact time: 6 s |
For PE wax, ethylene: 1.7–2.0 wt.%, propylene: 7.1–10.2 wt.%, liquid org. phase: 48.9–53.9 wt.%. For PE wax and HVGO, ethylene: 1.8–2.1 wt.%, propylene: 7.1–10.8 wt.%, liquid org. phase: 49.9–52.9 wt.% For PP wax, ethylene: 1.2–2.1 wt.%, propylene:4.3–9.5 wt.%, liquid org. phase: 50.7–67.6 wt.% For PP wax and HVGO, ethylene: 1.7–1.9 wt.%, propylene: 5.4–9.1 wt.%, liquid org. phase: 52.9–56.9 wt.% |
[99] |
*HVGO is the abbreviation of vacuum gas oil.
As the leading technology used in refineries, steam cracking has great potential to produce ethylene, propylene, 1,3-butadiene, etc., with the use of pyrolysis oil from polyolefin wastes [100]. In theory, the pyrolysis oil from polyolefin wastes can be directly used in steam crackers without unit modification or replacement within the refinery complex [101]. Given the large-scale operation of industrial steam crackers, co-feeding of pyrolysis oil and fossil fuel blends can reduce the contaminant levels and improve the ethylene yield [91,102]. Furthermore, as shown in the section on the pretreatment of polyolefin wastes, the systematic separation of polyolefin waste from other polymers is important to reduce the main sources of contaminants, such as nitrogen, chlorine and oxygen. As shown in Table 4, the properties of hydrogenated pyrolysis oil are similar to those of crude oil fractions. The energy consumption data of its steam cracking unit are the same as those of the current mainstream cracking unit in refineries, that is, the combustion energy consumption of olefins (ethylene + propylene) ranges from 400 kgEo/t olefins to 430 kgEo/t olefins.
Currently, numerous companies have invested considerable effort in polyolefin chemical recycling. The pyrolysis process is the main technology for most plants. Most petrochemical companies have attempted to refine pyrolysis oil to chemicals and/or fuels. Plastic Energy developed the Thermal Anaerobic Conversion technology to convert polyolefins to pyrolysis oil (850 L/t polyolefin wastes) and offered it to SABIC. Pyrolysis oil is further refined to a certified circular polymer consisting of PE and PP by SABIC [103]. In addition, Quantafuel has developed pyrolysis technology and achieved 85% pyrolysis oil yield. Its products can be used for BASF’s integrated chemical production network. With HDPE as an example, the yield (virgin plastics/waste HDPE) can reach 65% with the cooperation of Quantafuel and BASF [104]. In addition, ExxonMobil is collaborating with Plastic Energy regarding an advanced recycling plant in Notre Dame de Gravenchon, France; this plant is expected to process 25 000 metric tons of plastic waste per year by 2023, with the potential for further expansion to 33 000 metric tons of annual capacity [105]. Sinopec developed a continuous pyrolysis technology to convert polyolefin wastes to pyrolysis oil and liquefied petroleum gas with a total yield of 88 wt.%, and is building a pyrolysis unit to process 10 000 tons of polyolefin waste per year followed by refining of pyrolysis oil to virgin polyolefins in Tahe Refining and Chemical Company. Other petrochemical companies, such as Chevron and Eastman Chemical, have prepared patent portfolios in the field of refining pyrolysis oil.
Existing refining technologies have become the preferred method for refining and chemical enterprises to treat pyrolysis oil. The cooperation of plastic recycling, pretreatment, and pyrolysis plants and petrochemical enterprises will become the mainstream to realize the chemical recycling of polyolefin wastes in the future.
LIFE CYCLE ASSESSMENT (LCA) OF POLYOLEFIN CHEMICAL RECYCLING
LCA applications have become increasingly popular to address the environmental footprints of polyolefin recycling. Given the differences in calculation models and boundary conditions, CO2 emissions differ substantially from each other. Rem et al. [106] indicated that the absolute CO2 emissions of polyolefin waste pretreatment were 112–119 kg CO2 eq./kg polyolefin waste. Jeswani et al. [107] analyzed the life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste and indicated that two-thirds of the total impact (before the credits) came from the pyrolysis process and 26% from waste collection and sorting. Schwarz et al. [108] indicated that waste streams with high polyolefin contents showed better environmental results with chemical recycling (gasification and pyrolysis to waxes and feedstock) than incineration with energy recovery. In addition, pyrolysis of polyolefins to monomers resulted in a CO2 reduction of more than 80% compared with that obtained during incineration (1.5–2.9 kg CO2 eq./kg polymer) [109]. Li et al. [96] designed several chemical recovery/recycling routes for polyolefin wastes and quantified their environmental impact through LCA. As shown in Table 6, the combustion of byproduct oil in the refinery contributed the most to the overall impacts [107]. In addition, CO2 emissions of the refining process, including refining of oil and polymerization of olefins, were several times higher than those of polyolefin waste pretreatment and pyrolysis. Furthermore, compared with chemical recycling of oils and polyolefins, chemical recycling of polyolefins had more advantages in the reduction of CO2 emissions. The results also showed that the CO2 emission reduction of polyolefin chemical recycling (pyrolysis→hydrotreatment of oil→steam cracking for olefins→polymerization) was more than 65.3% compared with that of incineration, which indicated good environmental performance and green characteristics.
Table 6.
CO2 emissions during the whole process of chemical recycling and incineration (kg CO2 eq./kg polyolefin wastes).
| Scheme | Pretreatment* | Pyrolysis | Refining of oil | Polymerization | Combustion of by-product oil | Total |
|---|---|---|---|---|---|---|
| For polyolefins | 0.059 | 0.15 | 0.77 | 0.26 | 1.19 | 2.43 |
| For polyolefins and oils | 0.059 | 0.15 | 0.62 | 0.059 | 1.69 | 2.58 |
| Incineration | 6.86 |
*Pretreatment CO2 emission was estimated from the data of Jeswani et al. [107].
CONCLUSION AND PERSPECTIVES
This paper reviewed the chemical recycling process of polyolefin wastes, including moderate pretreatment, pyrolysis, gasification, and refining of pyrolysis oil for olefins, and brief LCA applications were used to address the environmental footprints.
Appropriate pretreatment is necessary to provide relatively pure feedstock for the pyrolysis and refining process, and a low sorting efficiency can affect the economic and environmental performance. Thermal pyrolysis is one of the most effective chemical recovery methods for polyolefin wastes, but the wide product distribution and high impurity content limit its further application. Catalytic pyrolysis and solvolysis can improve the product distribution of pyrolysis oils to a certain extent. However, the development of cheap, efficient, and stable catalysts is still a key research direction in the future. Catalytic and steam cracking are essential processes in the production of olefins, which are the monomers of plastics from pyrolysis oil, and realization of the closed-loop recycling of polyolefin wastes. Compared with incineration, chemical recycling of polyolefin wastes shows good environmental performance and green characteristics, and it will play an essential role in the future of plastic recycling.
Facing the current chemical recycling challenge of polyolefin wastes, the following problems should be solved in the future:
New plastics should be developed to decrease or avoid the application of additives, and the application of composite plastics should be decreased.
The management of plastic wastes should aim at the use of a hierarchical approach. Targeted recycling schemes should be selected for different labeled plastic wastes.
The influence of impurities on pyrolysis or catalytic pyrolysis should be clarified, and antipollution catalysts should be developed. The heat, mass and momentum transfer of polyolefin fluids with a high viscosity in the reactor should be further studied.
More attention should be given to the development of high-value utilization technology for polyolefin wastes to avoid the destruction of polymer molecular chains. New technologies that can efficiently depolymerize polyolefin plastics into high-quality fuels with high light-component content and relatively concentrated carbon numbers under mild conditions should be developed.
The cost of chemical recycling of plastic wastes needs to be reduced further. For the realization of closed-loop recycling of plastic wastes, authorities should rely on existing facilities in refineries to further process pyrolysis products. This model is not only conducive to achieving carbon emission reduction in refineries but also transforming them from polluters to pollution controllers.
We should accelerate the low-carbon certification of pyrolysis technology and its products to promote the development of chemical recovery technology.
Contributor Information
Liang Zou, Sinopec Research Institute of Petroleum Processing Co., Ltd, Beijing 100083, China.
Run Xu, Sinopec Research Institute of Petroleum Processing Co., Ltd, Beijing 100083, China.
Hui Wang, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China; 2060 Research Institute, ShanghaiTech University, Shanghai 201210, China.
Zhiqiang Wang, Sinopec Research Institute of Petroleum Processing Co., Ltd, Beijing 100083, China.
Yuhan Sun, Shanghai Advanced Research Institute, Chinese Academy of Sciences, Shanghai 201210, China; 2060 Research Institute, ShanghaiTech University, Shanghai 201210, China.
Mingfeng Li, Sinopec Research Institute of Petroleum Processing Co., Ltd, Beijing 100083, China.
FUNDING
This work was supported by the China Petroleum & Chemical Corporation (120040), the National Key Research and Development Program of China (2022YFA1504800), the National Natural Science Foundation of China (22108289 and 21905291) and the Shanghai Institute of Cleantech Innovation (E244831E01).
Conflict of interest statement. None declared.
REFERENCES
- 1. Zanella D, Romagnoli M, Malcangi Set al. The contribution of high-resolution GC separations in plastic recycling research. Anal Bioanal Chem 2023; 415: 2343–55. 10.1007/s00216-023-04519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang SH. Plastic waste as pyrolysis feedstock for plastic oil production: a review. Sci Total Environ 2023; 877: 162719. 10.1016/j.scitotenv.2023.162719 [DOI] [PubMed] [Google Scholar]
- 3. Hahladakis JN, Velis CA, Weber Ret al. An overview of chemical additives present in plastics: migration, release, fate and environmental impact during their use, disposal and recycling. J Hazard Mater 2018; 344: 179–99. 10.1016/j.jhazmat.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 4. Ryberg MW, Hauschild MZ, Wang Fet al. Global environmental losses of plastics across their value chains. Resour Conserv Recycl 2019; 151: 104459. 10.1016/j.resconrec.2019.104459 [DOI] [Google Scholar]
- 5. Matjašič T, Simčič T, Medvešček Net al. Critical evaluation of biodegradation studies on synthetic plastics through a systematic literature review. Sci Total Environ 2021; 752: 141959. 10.1016/j.scitotenv.2020.141959 [DOI] [PubMed] [Google Scholar]
- 6. Biopolymers . Plastics Today. https://www.plasticstoday.com/biopolymers/present-and-future-trends-biodegradable-polymers (18 July 2023, date last accessed). [Google Scholar]
- 7. Yeung CWS, Teo JYQ, Loh XJet al. Polyolefins and polystyrene as chemical resources for a sustainable future: challenges, advances, and prospects. ACS Mater Lett 2021; 3: 1660–76. 10.1021/acsmaterialslett.1c00490 [DOI] [Google Scholar]
- 8. Singh N, Hui D, Singh Ret al. Recycling of plastic solid waste: a state of art review and future applications. Compos Part B-Eng 2017; 115: 409–22. 10.1016/j.compositesb.2016.09.013 [DOI] [Google Scholar]
- 9. Soto JM, Martín-Lara MA, Blázquez Get al. Novel pre-treatment of dirty post-consumer polyethylene film for its mechanical recycling. Process Saf Environ Prot 2020; 139: 315–24. 10.1016/j.psep.2020.04.044 [DOI] [Google Scholar]
- 10. Möllnitz S, Küppers B, Curtis Aet al. Influence of pre-screening on down-stream processing for the production of plastic enriched fractions for recycling from mixed commercial and municipal waste. Waste Manage 2021; 119: 365–73. 10.1016/j.wasman.2020.10.007 [DOI] [PubMed] [Google Scholar]
- 11. Qin Y, Yao Z, Ruan Jet al. Motion behavior model and multistage magnetic separation method for the removal of impurities from recycled waste plastics. ACS Sustainable Chem Eng 2021; 9: 10920–8. 10.1021/acssuschemeng.1c03580 [DOI] [Google Scholar]
- 12. Louati H, Zouzou N, Tilmatine Aet al. Experimental investigation of an electrostatic adhesion device used for metal/polymer granular mixture sorting. Powder Technol 2021; 391: 301–10. 10.1016/j.powtec.2021.06.019 [DOI] [Google Scholar]
- 13. Wang C-Q, Wang H, Fu J-Get al. Flotation separation of waste plastics for recycling — a review. Waste Manage (Oxford) 2015; 41: 28–38. 10.1016/j.wasman.2015.03.027 [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Zhang Y, Wang C. Surface modification and selective flotation of waste plastics for effective recycling — a review. Sep Purif Technol 2019; 226: 75–94. 10.1016/j.seppur.2019.05.052 [DOI] [Google Scholar]
- 15. Pita F, Castilho A. Separation of plastics by froth flotation. The role of size, shape and density of the particles. Waste Manage (Oxford) 2017; 60: 91–9. 10.1016/j.wasman.2016.07.041 [DOI] [PubMed] [Google Scholar]
- 16. Jiang H, Zhang Y, Wang H. Surface reactions in selective modification: the prerequisite for plastic flotation. Environ Sci Technol 2020; 54: 9742–56. 10.1021/acs.est.9b07861 [DOI] [PubMed] [Google Scholar]
- 17. Serranti S, Bonifazi G. 2 – Techniques for separation of plastic wastes. In: Pacheco-Torgal F, Khatib J, Colangelo Fet al. (eds.). Use of Recycled Plastics in Eco-efficient Concrete. Cambrige: Woodhead Publishing, 2019, 9–37. [Google Scholar]
- 18. Signoret C, Caro-Bretelle AS, Lopez-Cuesta JMet al. Alterations of plastics spectra in MIR and the potential impacts on identification towards recycling. Resour Conserv Recycl 2020; 161: 104980. 10.1016/j.resconrec.2020.104980 [DOI] [Google Scholar]
- 19. Neo ERK, Yeo Z, Low JSCet al. A review on chemometric techniques with infrared, Raman and laser-induced breakdown spectroscopy for sorting plastic waste in the recycling industry. Resour Conserv Recycl 2022; 180: 106217. 10.1016/j.resconrec.2022.106217 [DOI] [Google Scholar]
- 20. Faltynkova A, Johnsen G, Wagner M. Hyperspectral imaging as an emerging tool to analyze microplastics: a systematic review and recommendations for future development. Microplast Nanoplast 2021; 1: 13. 10.1186/s43591-021-00014-y [DOI] [Google Scholar]
- 21. Lv W, Wang X. Overview of hyperspectral image classification. J Sensors 2020; 2020: 4817234. 10.1155/2020/4817234 [DOI] [Google Scholar]
- 22. Dong M, Zhang Q, Xing Xet al. Raman spectra and surface changes of microplastics weathered under natural environments. Sci Total Environ 2020; 739: 139990. 10.1016/j.scitotenv.2020.139990 [DOI] [PubMed] [Google Scholar]
- 23. Abubaker SA, Chaqmaqchee FA, Taha AH. Identification and characterization of different types of plastics wastes using X-ray diffraction and X-ray fluorescence techniques. ARO Sci J Koya Univ 2021; 9: 22–5. [Google Scholar]
- 24. Kartik S, Balsora HK, Sharma Met al. Valorization of plastic wastes for production of fuels and value-added chemicals through pyrolysis — a review. Therm Sci Eng Prog 2022; 32: 101316. 10.1016/j.tsep.2022.101316 [DOI] [Google Scholar]
- 25. Hussain I, Ganiyu SA, Alasiri Het al. A state-of-the-art review on waste plastics-derived aviation fuel: unveiling the heterogeneous catalytic systems and techno-economy feasibility of catalytic pyrolysis. Energ Convers Manage 2022; 274: 116433. 10.1016/j.enconman.2022.116433 [DOI] [Google Scholar]
- 26. Al-Salem SM. Thermal pyrolysis of high density polyethylene (HDPE) in a novel fixed bed reactor system for the production of high value gasoline range hydrocarbons (HC). Process Saf Environ Prot 2019; 127: 171–9. 10.1016/j.psep.2019.05.008 [DOI] [Google Scholar]
- 27. Parku GK, Collard F-X, Görgens JF. Pyrolysis of waste polypropylene plastics for energy recovery: influence of heating rate and vacuum conditions on composition of fuel product. Fuel Process Technol 2020; 209: 106522. 10.1016/j.fuproc.2020.106522 [DOI] [Google Scholar]
- 28. Papuga SV, Gvero PM, Vukic LM. Temperature and time influence on the waste plastics pyrolysis in the fixed bed reactor. Therm Sci 2016; 20: 731–41. 10.2298/TSCI141113154P [DOI] [Google Scholar]
- 29. Zhao D, Wang X, Miller JBet al. The chemistry and kinetics of polyethylene pyrolysis: a process to produce fuels and chemicals. Chem Sus Chem 2020; 13: 1764–74. 10.1002/cssc.201903434 [DOI] [PubMed] [Google Scholar]
- 30. Mastral FJ, Esperanza E, Berrueco Cet al. Fluidized bed thermal degradation products of HDPE in an inert atmosphere and in air–nitrogen mixtures. J Anal Appl Pyrol 2003; 70: 1–17. 10.1016/S0165-2370(02)00068-2 [DOI] [Google Scholar]
- 31. Predel M, Kaminsky W. Pyrolysis of mixed polyolefins in a fluidised-bed reactor and on a pyro-GC/MS to yield aliphatic waxes. Polym Degrad Stab 2000; 70: 373–85. 10.1016/S0141-3910(00)00131-2 [DOI] [Google Scholar]
- 32. Al-Salem SM, Yang Y, Wang Jet al. Pyro-oil and wax recovery from reclaimed plastic wastes in a continuous auger pyrolysis reactor. Energies 2020; 13: 2040. 10.3390/en13082040 [DOI] [Google Scholar]
- 33. Kodera Y, Ishihara Y, Kuroki T. Novel process for recycling waste plastics to fuel gas using a moving-bed reactor. Energ Fuel 2006; 20: 155–8. 10.1021/ef0502655 [DOI] [Google Scholar]
- 34. Serrano DP, Aguado J, Escola JMet al. Conversion of low density polyethylene into petrochemical feedstocks using a continuous screw kiln reactor. J Anal Appl Pyrol 2001; 58–59: 789–801. 10.1016/S0165-2370(00)00153-4 [DOI] [Google Scholar]
- 35. Elordi G, Olazar M, Lopez Get al. Product yields and compositions in the continuous pyrolysis of high-density polyethylene in a conical spouted bed reactor. Ind Eng Chem Res 2011; 50: 6650–9. 10.1021/ie200186m [DOI] [Google Scholar]
- 36. Arabiourrutia M, Elordi G, Lopez Get al. Characterization of the waxes obtained by the pyrolysis of polyolefin plastics in a conical spouted bed reactor. J Anal Appl Pyrol 2012; 94: 230–7. 10.1016/j.jaap.2011.12.012 [DOI] [Google Scholar]
- 37. Undri A, Rosi L, Frediani Met al. Efficient disposal of waste polyolefins through microwave assisted pyrolysis. Fuel 2014; 116: 662–71. 10.1016/j.fuel.2013.08.037 [DOI] [Google Scholar]
- 38. Stelmachowski M. Feedstock recycling of waste polymers by thermal cracking in molten metal: thermodynamic analysis. J Mater Cycles Waste Manag 2014; 16: 211–8. 10.1007/s10163-013-0178-x [DOI] [Google Scholar]
- 39. Gabbar HA, Aboughaly M, Stoute CAB. DC thermal plasma design and utilization for the low density polyethylene to diesel oil pyrolysis reaction. Energies 2017; 10: 784. 10.3390/en10060784 [DOI] [Google Scholar]
- 40. Uçar S, Özkan AR, Karagöz S. Co-pyrolysis of waste polyolefins with waste motor oil. J Anal Appl Pyrol 2016; 119: 233–41. 10.1016/j.jaap.2016.01.013 [DOI] [Google Scholar]
- 41. Jahirul MI, Rasul MG, Schaller Det al. Transport fuel from waste plastics pyrolysis – a review on technologies, challenges and opportunities. Energ Convers Manage 2022; 258: 115451. 10.1016/j.enconman.2022.115451 [DOI] [Google Scholar]
- 42. Park K-B, Jeong Y-S, Kim J-S. Activator-assisted pyrolysis of polypropylene. Appl Energ 2019; 253: 113558. 10.1016/j.apenergy.2019.113558 [DOI] [Google Scholar]
- 43. Park K-B, Jeong Y-S, Guzelciftci Bet al. Characteristics of a new type continuous two-stage pyrolysis of waste polyethylene. Energy 2019; 166: 343–51. 10.1016/j.energy.2018.10.078 [DOI] [Google Scholar]
- 44. Salaudeen SA, Al-Salem SM, Sharma Set al. Pyrolysis of high-density polyethylene in a fluidized bed reactor: pyro-wax and gas analysis. Ind Eng Chem Res 2021; 60: 18283–92. 10.1021/acs.iecr.1c03373 [DOI] [Google Scholar]
- 45. Peng Y, Wang Y, Ke Let al. A review on catalytic pyrolysis of plastic wastes to high-value products. Energ Convers Manage 2022; 254: 115243. 10.1016/j.enconman.2022.115243 [DOI] [Google Scholar]
- 46. Orozco S, Alvarez J, Lopez Get al. Pyrolysis of plastic wastes in a fountain confined conical spouted bed reactor: determination of stable operating conditions. Energ Convers Manage 2021; 229: 113768. 10.1016/j.enconman.2020.113768 [DOI] [Google Scholar]
- 47. Campuzano F, Brown RC, Martinez JD. Auger reactors for pyrolysis of biomass and wastes. Renew Sust Energ Rev 2019; 102: 372–409. 10.1016/j.rser.2018.12.014 [DOI] [Google Scholar]
- 48. Zhou N, Dai L, Lv Yet al. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem Eng J 2021; 418: 129412. 10.1016/j.cej.2021.129412 [DOI] [Google Scholar]
- 49. Jung S-H, Cho M-H, Kang B-Set al. Pyrolysis of a fraction of waste polypropylene and polyethylene for the recovery of BTX aromatics using a fluidized bed reactor. Fuel Process Technol 2010; 91: 277–84. 10.1016/j.fuproc.2009.10.009 [DOI] [Google Scholar]
- 50. Lopez G, Artetxe M, Amutio Met al. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew Sust Energ Rev 2017; 73: 346–68. 10.1016/j.rser.2017.01.142 [DOI] [Google Scholar]
- 51. Shoinkhorova T, Cordero-Lanzac T, Ramirez Aet al. Highly selective and stable production of aromatics via high-pressure methanol conversion. ACS Catal 2021; 11: 3602–13. 10.1021/acscatal.0c05133 [DOI] [Google Scholar]
- 52. Zhang Z, Gora-Marek K, Watson JSet al. Recovering waste plastics using shape-selective nano-scale reactors as catalysts. Nat Sustain 2019; 2: 39–42. 10.1038/s41893-018-0195-9 [DOI] [Google Scholar]
- 53. Gaurh P, Pramanik H. A novel approach of solid waste management via aromatization using multiphase catalytic pyrolysis of waste polyethylene. Waste Manage 2018; 71: 86–96. 10.1016/j.wasman.2017.10.053 [DOI] [PubMed] [Google Scholar]
- 54. Xue Y, Johnston P, Bai X. Effect of catalyst contact mode and gas atmosphere during catalytic pyrolysis of waste plastics. Energ Convers Manage 2017; 142: 441–51. 10.1016/j.enconman.2017.03.071 [DOI] [Google Scholar]
- 55. Wang Y, Zhang Y, Fan Het al. Elucidating the structure-performance relationship of typical commercial zeolites in catalytic cracking of low-density polyethylene. Catal Today 2022; 405–406: 135–43. 10.1016/j.cattod.2022.06.024 [DOI] [Google Scholar]
- 56. Aguado J, Serrano DP, San Miguel Get al. Feedstock recycling of polyethylene in a two-step thermo-catalytic reaction system. J Anal Appl Pyrol 2007; 79: 415–23. 10.1016/j.jaap.2006.11.008 [DOI] [Google Scholar]
- 57. Chen Z, Zhang X, Che Let al. Effect of volatile reactions on oil production and composition in thermal and catalytic pyrolysis of polyethylene. Fuel 2020; 271: 117308. 10.1016/j.fuel.2020.117308 [DOI] [Google Scholar]
- 58. Aisien FA, Aisien ET. Production and characterization of liquid oil from the pyrolysis of waste high-density polyethylene plastics using spent fluid catalytic cracking catalyst. Sustainable Chemistry for Climate Action 2023; 2: 100020. 10.1016/j.scca.2023.100020 [DOI] [Google Scholar]
- 59. Zhang Y, Duan D, Lei Het al. Jet fuel production from waste plastics via catalytic pyrolysis with activated carbons. Appl Energ 2019; 251: 113337. 10.1016/j.apenergy.2019.113337 [DOI] [Google Scholar]
- 60. Dai L, Zhou N, Lv Yet al. Chemical upcycling of waste polyolefinic plastics to low-carbon synthetic naphtha for closing the plastic use loop. Sci Total Environ 2021; 782: 146897. 10.1016/j.scitotenv.2021.146897 [DOI] [Google Scholar]
- 61. González-García P. Activated carbon from lignocellulosics precursors: a review of the synthesis methods, characterization techniques and applications. Renew Sust Energ Rev 2018; 82: 1393–414. 10.1016/j.rser.2017.04.117 [DOI] [Google Scholar]
- 62. Yang Z, Lei H, Zhang Yet al. Production of renewable alkyl-phenols from catalytic pyrolysis of Douglas fir sawdust over biomass-derived activated carbons. Appl Energ 2018; 220: 426–36. 10.1016/j.apenergy.2018.03.107 [DOI] [Google Scholar]
- 63. Vollmer I, Jenks MJF, Mayorga Gonzalez Ret al. Plastic waste conversion over a refinery waste catalyst. Angew Chem Int Ed 2021; 60: 16101–8. 10.1002/anie.202104110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Singh MV, Kumar S, Sarker M. Waste HD-PE plastic, deformation into liquid hydrocarbon fuel using pyrolysis-catalytic cracking with a CuCO3 catalyst. Sustain Energ Fuels 2018; 2: 1057–68. 10.1039/C8SE00040A [DOI] [Google Scholar]
- 65. Tennakoon A, Wu X, Paterson ALet al. Catalytic upcycling of high-density polyethylene via a processive mechanism. Nat Catal 2020; 3: 893–901. 10.1038/s41929-020-00519-4 [DOI] [Google Scholar]
- 66. Duan J, Chen W, Wang Cet al. Coking-resistant polyethylene upcycling modulated by zeolite micropore diffusion. J Am Chem Soc 2022; 144: 14269–77. 10.1021/jacs.2c05125 [DOI] [PubMed] [Google Scholar]
- 67. Li L, Luo H, Shao Zet al. Converting plastic wastes to naphtha for closing the plastic loop. J Am Chem Soc 2023; 145: 1847–54. 10.1021/jacs.2c11407 [DOI] [PubMed] [Google Scholar]
- 68. Zhang X, Lei H, Yadavalli Get al. Gasoline-range hydrocarbons produced from microwave-induced pyrolysis of low-density polyethylene over ZSM-5. Fuel 2015; 144: 33–42. 10.1016/j.fuel.2014.12.013 [DOI] [Google Scholar]
- 69. Miandad R, Barakat MA, Aburiazaiza ASet al. Catalytic pyrolysis of plastic waste: a review. Process Saf Environ Prot 2016; 102: 822–38. 10.1016/j.psep.2016.06.022 [DOI] [Google Scholar]
- 70. Serrano DP, Aguado J, Vicente Get al. Effects of hydrogen-donating solvents on the thermal degradation of HDPE. J Anal Appl Pyrol 2007; 78: 194–9. 10.1016/j.jaap.2006.07.001 [DOI] [Google Scholar]
- 71. Vicente G, Aguado J, Serrano DPet al. HDPE chemical recycling promoted by phenol solvent. J Anal Appl Pyrol 2009; 85: 366–71. 10.1016/j.jaap.2008.10.007 [DOI] [Google Scholar]
- 72. Aguado J, Serrano DP, Vicente Get al. Enhanced production of α-olefins by thermal degradation of high-density polyethylene (HDPE) in decalin solvent: effect of the reaction time and temperature. Ind Eng Chem Res 2007; 46: 3497–504. 10.1021/ie060975d [DOI] [Google Scholar]
- 73. Ellis LD, Orski SV, Kenlaw GAet al. Tandem heterogeneous catalysis for polyethylene depolymerization via an olefin-intermediate process. ACS Sustainable Chem Eng 2021; 9: 623–8. 10.1021/acssuschemeng.0c07612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jia X, Qin C, Friedberger Tet al. Efficient and selective degradation of polyethylenes into liquid fuels and waxes under mild conditions. Sci Adv 2016; 2: e1501591. 10.1126/sciadv.1501591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Conk RJ, Hanna S, Shi JXet al. Catalytic deconstruction of waste polyethylene with ethylene to form propylene. Science 2022; 377: 1561–6. 10.1126/science.add1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jia C, Xie S, Zhang Wet al. Deconstruction of high-density polyethylene into liquid hydrocarbon fuels and lubricants by hydrogenolysis over Ru catalyst. Chem Catal 2021; 1: 437–55. 10.1016/j.checat.2021.04.002 [DOI] [Google Scholar]
- 77. Zhang W, Kim S, Wahl Let al. Low-temperature upcycling of polyolefins into liquid alkanes via tandem cracking-alkylation. Science 2023; 379: 807–11. 10.1126/science.ade7485 [DOI] [PubMed] [Google Scholar]
- 78. Qin X, Liu Q, Yu Jet al. Study on structure and properties of remained solids from liquefaction of Naomaohu coal in tetrahydronaphthalene. Fuel 2023; 339: 127348. 10.1016/j.fuel.2022.127348 [DOI] [Google Scholar]
- 79. Wang X-B, Bai J-K, Zhang X-Bet al. The mechanism and rate constant of hydrogen transfer from solvent radicals to coal-based model compounds in direct coal liquefaction. J Anal Appl Pyrol 2022; 167: 105637. 10.1016/j.jaap.2022.105637 [DOI] [Google Scholar]
- 80. Kohli K, Prajapati R, Maity SKet al. Hydrocracking of heavy crude/residues with waste plastic. J Anal Appl Pyrol 2019; 140: 179–87. 10.1016/j.jaap.2019.03.013 [DOI] [Google Scholar]
- 81. Rodríguez E, Gutiérrez A, Palos Ret al. Co-cracking of high-density polyethylene (HDPE) and vacuum gasoil (VGO) under refinery conditions. Chem Eng J 2020; 382: 122602. 10.1016/j.cej.2019.122602 [DOI] [Google Scholar]
- 82. Lopez G, Artetxe M, Amutio Met al. Recent advances in the gasification of waste plastics. A critical overview. Renew Sust Energ Rev 2018; 82: 576–96. 10.1016/j.rser.2017.09.032 [DOI] [Google Scholar]
- 83. Arena U, Zaccariello L, Mastellone ML. Fluidized bed gasification of waste-derived fuels. Waste Manage 2010; 30: 1212–9. 10.1016/j.wasman.2010.01.038 [DOI] [PubMed] [Google Scholar]
- 84. Wilk V, Hofbauer H. Conversion of mixed plastic wastes in a dual fluidized bed steam gasifier. Fuel 2013; 107: 787–99. 10.1016/j.fuel.2013.01.068 [DOI] [Google Scholar]
- 85. Zheng K, Wu Y, Hu Zet al. Progress and perspective for conversion of plastic wastes into valuable chemicals. Chem Soc Rev 2023; 52: 8–29. 10.1039/D2CS00688J [DOI] [PubMed] [Google Scholar]
- 86. Ouyang Z, Yang Y, Zhang Cet al. Recent advances in photocatalytic degradation of plastics and plastic-derived chemicals. J Mater Chem A 2021; 9: 13402–41. 10.1039/D0TA12465F [DOI] [Google Scholar]
- 87. Novaes LFT, Liu J, Shen Yet al. Electrocatalysis as an enabling technology for organic synthesis. Chem Soc Rev 2021; 50: 7941–8002. 10.1039/D1CS00223F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Palos R, Gutiérrez A, Vela FJet al. Assessing the potential of the recycled plastic slow pyrolysis for the production of streams attractive for refineries. J Anal Appl Pyrol 2019; 142: 104668. 10.1016/j.jaap.2019.104668 [DOI] [Google Scholar]
- 89. Quesada L, Calero M, Martín-Lara MÁet al. Production of an alternative fuel by pyrolysis of plastic wastes mixtures. Energ Fuels 2020; 34: 1781–90. 10.1021/acs.energyfuels.9b03350 [DOI] [Google Scholar]
- 90. Ahmad I, Khan MI, Khan Het al. Pyrolysis study of polypropylene and polyethylene into premium oil products. Int J Green Energ 2015; 12: 663–71. 10.1080/15435075.2014.880146 [DOI] [Google Scholar]
- 91. Kusenberg M, Eschenbacher A, Djokic MRet al. Opportunities and challenges for the application of post-consumer plastic waste pyrolysis oils as steam cracker feedstocks: to decontaminate or not to decontaminate? Waste Manage 2022; 138: 83–115. 10.1016/j.wasman.2021.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kopinke FD, Zimmermann G, Reyniers GCet al. Relative rates of coke formation from hydrocarbons in steam cracking of naphtha. 2. paraffins, naphthenes, mono-, di-, and cycloolefins, and acetylenes. Ind Eng Chem Res 1993; 32: 56–61. 10.1021/ie00013a009 [DOI] [Google Scholar]
- 93. Izaddoust S, Hita I, Zambrano Net al. Fuel production via catalytic cracking of pre-hydrotreated heavy-fuel oil generated by marine-transport operations. Fuel 2022; 325: 124765. 10.1016/j.fuel.2022.124765 [DOI] [Google Scholar]
- 94. Gao JS. 8 Catalytic cracking. In: Xu CM, Yang CH (eds.). Petroleum Refining Engineering. Beijing: Petroleum Industry Press, 2009, 294–9. [Google Scholar]
- 95. Artetxe M, Lopez G, Amutio Met al. Light olefins from HDPE cracking in a two-step thermal and catalytic process. Chem Eng J 2012; 207–208: 27–34. 10.1016/j.cej.2012.06.105 [DOI] [Google Scholar]
- 96. Li M, Cai Z, Zou Let al. Exploration on chemical recovery technology of plastic wastes in Sinopec. China Plastics 2021; 35: 64–76. [Google Scholar]
- 97. Rodríguez E, Palos R, Gutiérrez Aet al. Effect of the FCC equilibrium catalyst properties and of the cracking temperature on the production of fuel from HDPE pyrolysis waxes. Energ Fuels 2019; 33: 5191–9. 10.1021/acs.energyfuels.9b00993 [DOI] [Google Scholar]
- 98. Rodríguez E, Palos R, Gutiérrez Aet al. Towards waste refinery: co-feeding HDPE pyrolysis waxes with VGO into the catalytic cracking unit. Energ Convers Manage 2020; 207: 112554. 10.1016/j.enconman.2020.112554 [DOI] [Google Scholar]
- 99. Lovás P, Hudec P, Jambor Bet al. Catalytic cracking of heavy fractions from the pyrolysis of waste HDPE and PP. Fuel 2017; 203: 244–52. 10.1016/j.fuel.2017.04.128 [DOI] [Google Scholar]
- 100. Ragaert K, Delva L, Van Geem K. Mechanical and chemical recycling of solid plastic waste. Waste Manage 2017; 69: 24–58. 10.1016/j.wasman.2017.07.044 [DOI] [PubMed] [Google Scholar]
- 101. Palos R, Gutiérrez A, Vela FJet al. Waste refinery: the valorization of waste plastics and end-of-life tires in refinery units. A review. Energ Fuels 2021; 35: 3529–57. 10.1021/acs.energyfuels.0c03918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kusenberg M, Roosen M, Zayoud Aet al. Assessing the feasibility of chemical recycling via steam cracking of untreated plastic waste pyrolysis oils: feedstock impurities, product yields and coke formation. Waste Manage 2022; 141: 104–14. 10.1016/j.wasman.2022.01.033 [DOI] [PubMed] [Google Scholar]
- 103. News & Media . Saudi Basic Industry Corporation. https://www.sabic.com/en/news/17390-sabic-pioneersfirst-production-of-certified-circular-polymers (18 July 2023, date last accessed).
- 104. Media . BASF Group. https://www.basf.com/cn/en/media/news-releases/global/2021/05/p-21–189.html (18 July 2023, date last accessed).
- 105. News . Exxonmobil Corporation. https://corporate.exxonmobil.com/News/Newsroom/News-releases/2021/1029_ExxonMobil-earns-6_8-billion-in-third-quarter-2021 (18 July 2023, date last accessed).
- 106. Rem P, Olsen S, Welink J-Het al. Carbon dioxide emission associated with the production of plastics — a comparison of production from crude oil and recycling for the Dutch case. Environ Eng Manag J 2009; 8: 975–80. 10.30638/eemj.2009.142 [DOI] [Google Scholar]
- 107. Jeswani H, Krüger C, Russ Met al. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Sci Total Environ 2021; 769: 144483. 10.1016/j.scitotenv.2020.144483 [DOI] [PubMed] [Google Scholar]
- 108. Schwarz AE, Ligthart TN, Boukris Eet al. Sources, transport, and accumulation of different types of plastic litter in aquatic environments: a review study. Mar Pollut Bull 2019; 143: 92–100. 10.1016/j.marpolbul.2019.04.029 [DOI] [PubMed] [Google Scholar]
- 109. Schwarz AE, Ligthart TN, Godoi Bizarro Det al. Plastic recycling in a circular economy; determining environmental performance through an LCA matrix model approach. Waste Manage 2021; 121: 331–42. 10.1016/j.wasman.2020.12.020 [DOI] [PubMed] [Google Scholar]