Abstract
Objective:
To estimate the rate of perinatal transmission of hepatitis C virus (HCV), to identify risk factors for perinatal transmission of HCV, and to determine the viremic threshold for perinatal transmission.
Methods:
This was a prospective, multicenter observational study of pregnant individuals less than 24 weeks’ gestation screened for HCV from 2012 through 2018 in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Individuals found to be HCV antibody positive were followed throughout the pregnancy. Neonates were followed for evidence of perinatal transmission at 2–6 months (HCV RNA testing) and at 18–24 months (HCV RNA and antibody testing) of life. The primary outcome was perinatal transmission defined as positive testing at either follow-up time point.
Results:
109,379 individuals were screened for HCV. Of those who screened positive 772/1224 (63.1%) participants enrolled and 432/772 (56%) participants had data available to assess primary outcome. The overall rate of perinatal transmission was 6.0% (26/432, 95% confidence interval (CI) 4.0 – 8.7%). All infected infants were from mothers with demonstrable viremia. In viremic participants (n=314), the perinatal transmission rate was 8.0% (95% CI 5.2 – 11.5%). Risk factors for perinatal transmission included HCV RNA >106 IU/mL (adjusted odds ratio (aOR) 8.22, 95% CI 3.16 – 21.4) and vaginal bleeding reported at any time prior to delivery (aOR 3.26, 95% CI 1.32 – 8.03). A viremic threshold for perinatal transmission could not be established.
Conclusion:
Perinatal transmission of HCV was limited to viremic individuals. High viral loads and antepartum bleeding were associated with perinatal transmission.
Precis:
Perinatal transmission of hepatitis C virus only occurred in the setting of maternal viremia, and risk factors included a viral load of >106 IU/mL and antepartum vaginal bleeding.
Introduction
Hepatitis C virus (HCV) is a global health problem. In the United States (U.S.), the incidence in the general population is increasing. In 2015, the incidence of acute HCV was 0.8/100,000 persons, representing a 294% increase between 2010 and 20151. This increase is mirrored in the pregnant population, with estimates of prevalence in the obstetric population increasing 161% in a similar time frame. In 2017, HCV accounted for approximately 100,000 cases out of 31 million births2. Accordingly, the WHO has called for action to support eradication of the virus by 20303.
Eradication of HCV among reproductive-aged individuals is important, because a cure eliminates infection not only in the adult but also in their future children. To that end, several professional societies have recommended universal screening of pregnant individuals for HCV4–7, as identification is necessary for treatment to occur.
At present, there is controversy regarding management of HCV in pregnancy given the lack of quality evidence regarding the epidemiology and risk factors for perinatal transmission of HCV. The rate of perinatal transmission has been reported to be between 1 and 10%. A recent meta-analysis estimated perinatal transmission to be 5.8% in HIV-negative women infected with HCV8. Maternal viremia has been demonstrated to be of importance for perinatal transmission, particularly at higher viral loads10–12. Coinfection with HIV is understood to be a risk factor for perinatal transmission9, as is intravenous drug use (IVDU)13,14. Prolonged rupture of membranes and maternal blood exposure at time of delivery have also been identified as possible risk factors for perinatal transmission10,15–16.
Nevertheless, these data are non-contemporary, derived from studies with small study populations, or have very few infected neonates, and therefore have not been considered compelling enough to modify obstetric practice patterns for the prevention of perinatal transmission. Thus, the objectives of our study were to estimate the rate of HCV perinatal transmission in a contemporary population, identify risk factors for perinatal transmission, and determine the threshold of viremia below which transmission does not occur. The overarching goal of this work was to identify those individuals at highest risk of transmission. An understanding of modifiable risk factors for perinatal transmission of HCV is critical to the appropriate design of future intervention trials to prevent perinatal transmission.
Methods
This was a prospective observational study of pregnant individuals screened for HCV at one of 45 hospitals participating in the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network’s “An Observational Study of HCV in Pregnancy” from 2012 through 2018. During this period, 38 participating hospitals were included from the MFMU Network, a nationwide network of academic (n=33) and non-academic (n=5) institutions across the U.S. This study also included 7 hospitals in the U.S. and Puerto Rico from the NICHD HIV Studies (HIVS) Network. Prior to initiation of the observational study, approval by the Institutional Review Boards (IRB) was obtained at all participating hospitals.
Pregnant individuals with a singleton pregnancy who were HCV-antibody positive (signal to cutoff ≥ 5) at the time of screening (i.e., less than 24 weeks’ gestation) and who enrolled for prospective assessment at less than 28 weeks’ gestation were included in the study. Additional details on HCV screening for this study have been previously published.8 Individuals were excluded from the study if they planned termination of pregnancy, had known major fetal anomalies or demise, intended to deliver outside of a participating hospital, or had participated in this study in a prior pregnancy. Individuals were also excluded if they were unwilling to commit to 18 months of follow up for the infant, or if they had evidence of acute CMV (i.e., they were potentially eligible for the concurrently enrolling MFMU CMV trial).
Demographic and medical history were collected at enrollment via patient interview and medical record abstraction. Maternal blood samples were collected at enrollment (less than 28 weeks’ gestation), 24 to 28 weeks’ gestation, 32 to 36 weeks’ gestation, and delivery. Neonatal blood samples were collected at 2 to 6 months and 18 to 24 months of life. All study biospecimens were shipped to the central laboratory at the University of Washington Molecular Virology Laboratory in Seattle, WA. Tests run by the central laboratory included maternal HCV PCR (all visits), genotype (enrollment visit only), ALT, and AST (post-enrollment visits) and neonatal HCV PCR (both visits) and antibody (18 to 24 month visit only). After delivery, neonatal follow-up and clinical result data at 2 to 6 months and 18 to 24 months of life were collected via patient interview or medical record abstraction.
The primary outcome for this study was perinatal transmission of HCV, defined as either an infant who demonstrated HCV RNA at 2 to 6 months of life or was found to be HCV RNA or antibody positive at 18 to 24 months of life. If HCV RNA and antibody results at 18 to 24 months of life were discordant, a confirmatory test was run on the positive sample. If HCV RNA was positive and HCV antibody was negative at 18 to 24 months, then confirmatory testing consisted of repeating the HCV RNA test to ensure it was positive. Likewise, if HCV antibody was positive and HCV RNA was negative at 18 to 24 months, then HCV antibody test was repeated to confirm the positive result. If the participant was lost to follow-up and did not have enough data from the study samples to define the primary outcome, then clinical laboratory results at 18 months or later defined the primary outcome.
A sample size of 500 seropositive viremic pregnant individuals was chosen to provide 80% power to detect an odds ratio as low as 3.0 for any risk factor that was 40% prevalent, assuming a transmission rate of 6%10. We estimated approximately 67% of participants seropositive for HCV at enrollment would be RNA positive (viremic)17 and assumed perinatal transmission was most likely to occur in viremic participants. Thus, we estimated a target enrollment of 750 participants seropositive for HCV to achieve our desired sample of 500 who were viremic.
Participants demonstrating HCV perinatal transmission were compared to participants without perinatal transmission. Descriptive summary statistics were calculated for baseline characteristics. Participants were compared using Wilcoxon rank sum test for continuous variables and Chi-Square or Fisher’s exact test for categorical variables, as appropriate. Odds ratios and 95% confidence intervals (CI) were estimated using logistic regression with perinatal transmission status as the dependent variable.
For descriptive purposes, maternal HCV viral loads, ALT, and AST at each visit were summarized by transmission status. For statistical analyses, geometric means were calculated for skewed values and only results from the central laboratory were included in longitudinal analysis (i.e., ALT and AST local laboratory results at enrollment were excluded). The association between gestational age and the laboratory values were assessed using generalized linear models assuming a first-order auto-regressive correlation structure to account for repeated measurements. Statistical models included perinatal transmission status as an independent variable and an interaction term for transmission and gestational age to compare trends between perinatal transmission statuses using the Wald test. If the interaction term was not statistically significant, then the overall trend was reported.
Potential risk factors included HCV RNA titer greater than 106 IU/mL (based on the highest value prior to delivery), antepartum bleeding (defined as any bleeding prior to delivery), planned pre-labor cesarean, any IVDU during the current pregnancy, premature rupture of membranes, duration of membrane rupture at least 6 hours, internal fetal monitoring, breastfeeding, any infections during the current pregnancy (including HIV, hepatitis B or D, UTI or pyelonephritis, pneumonia, syphilis, gonorrhea, chlamydia, trichomonas vaginalis, bacterial vaginosis, or herpes), maternal age, smoking, parity, household status, income, education, employment, insurance status, and fetal sex. Risk factors associated with perinatal transmission were determined using backward elimination with statistically significant variables retained in the model. The final model included all variables found to be significant from the backward elimination and any statistically significant two-way interactions among variables that remained in the model. As a planned sensitivity analysis, the final model was re-run with random intercepts for hospital to ensure that hospital differences did not change the result.
Area under the receiver operating characteristic curves (AUC-ROC) were created to evaluate the sensitivity, specificity, and likelihood ratios of maternal viral load at each visit (at enrollment, 24–28 weeks, 32–36 weeks, and delivery). The viremic threshold for perinatal transmission was defined as the viremic cutoff with sensitivity of 100% (zero false negative)
Nominal two-sided p-values are reported. A p-value less than 0.05 was considered statistically significant. No adjustments were made for multiple comparisons. Statistical analyses were performed using SAS statistical software version 9.4.
Results
In all, 109,379 individuals were screened for HCV, some based upon universal screening efforts (n=106842, 97.7%) and some based upon referrals (those with known risk factors for HCV or history of HCV) (n=2537, 2.3%). Among those universally screened for HCV, 301 (0.28%, 95% CI 0.25 – 0.32%) screened positive, and among referrals, 923 (36.4%, 95% CI 34.5 – 38.3%) screened positive. Of the 1224 participants who screened positive, 1072 were eligible, and 772/1224 (63.1%) participants provided consent for the prospective study. Among those eligible and screened positive, the most common reasons for exclusion were refusal of consent (n=108), lost contact (n=93), and unwilling to commit to follow-up (n=77). Among all enrolled HCV cases (n=772), 11 had missing or indeterminate samples for viral load at baseline, rendering 548/761 (72.0%) viremic. Primary outcome data were available on 432/772 (56.0%). The most common reasons identified for the difference between enrollment and the final cohort numbers were lost contacts, parental refusal of sample collection, or infant placed in foster care or adopted (Figure 1).
Figure 1:
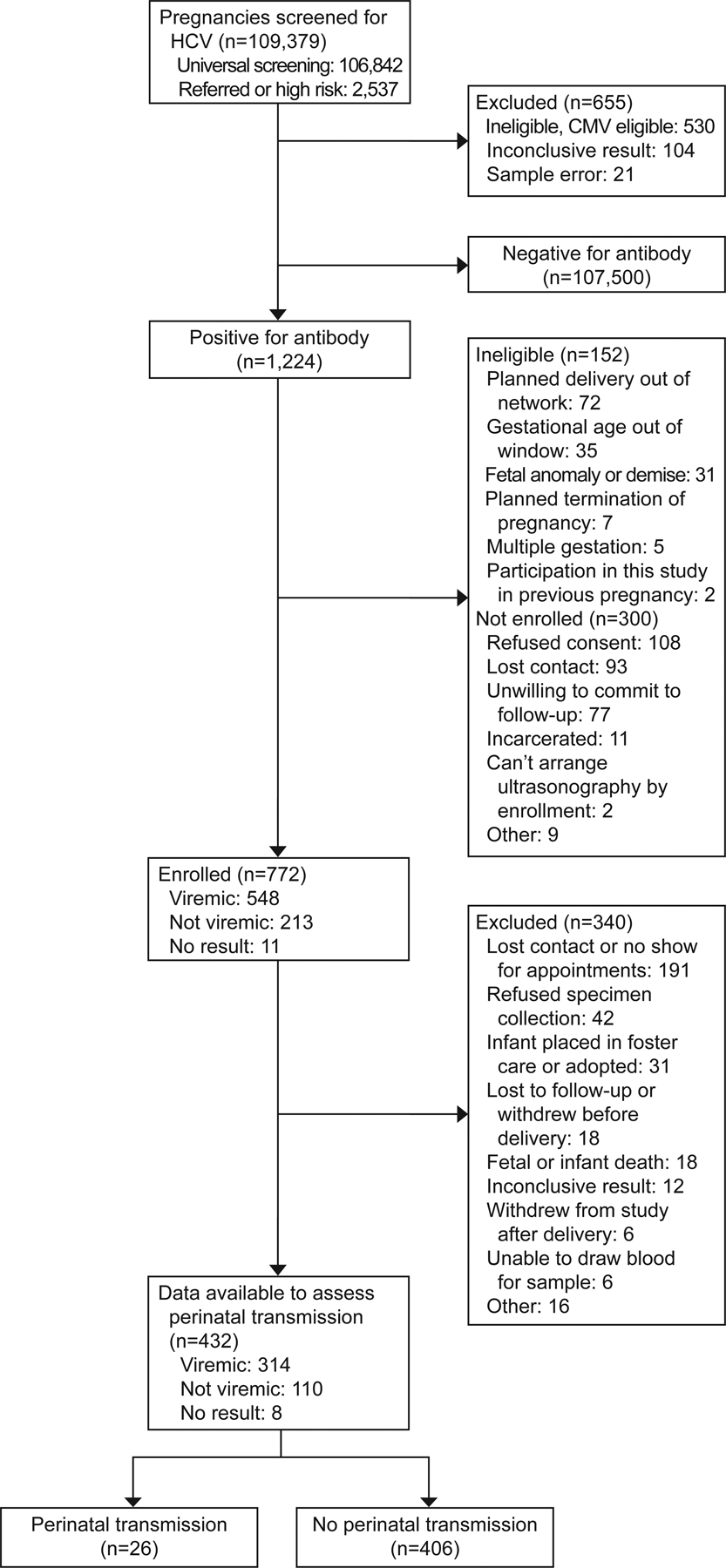
Analysis cohort. HCV, hepatitis C virus; CMV, cytomegalovirus.
The study population (n=432) primarily identified as non-Hispanic White (77.8%), with a high prevalence of tobacco (78.7%) and injection drug use (26.4%). Of those participants with baseline HCV PCR and primary outcome data, 314/424 (74.1%) were found to be viremic at baseline (average ± standard deviation gestational age at baseline: 19.7 weeks ± 4.5) (Table 1). The proportion who were viremic was similar between those with and without primary outcome (314/424 [74.1%] vs 234/337 [69.4%], p=0.16).
Table 1.
Demographics
| Characteristic | Perinatal transmission (n=26) |
No perinatal transmission (n=406) |
p-value | Overall (n=432) |
|---|---|---|---|---|
| Maternal age (years) | 29.0 ± 4.9 | 28.7 ± 5.1 | 0.59 | 28.7 ± 5.1 |
| Non-Hispanic White | 22 (84.6) | 314 (77.3) | 0.39 | 336 (77.8) |
| BMI (kg/m2) | 23.3 (21.3 – 30.9) | 25.0 (21.8 – 30.0) | 0.41 | 24.9 (21.8 – 30.0) |
| Nulliparous | 9 (34.6) | 120 (29.6) | 0.58 | 129 (29.9) |
| HIV | 2 (7.7) | 5 (1.2) | 0.06 | 7 (1.6) |
| Married or living with partner | 10 (38.5) | 184 (45.3) | 0.50 | 194 (44.9) |
| Employed | 8 (30.8) | 146 (36.0) | 0.59 | 154 (35.7) |
| Less than high school education | 15 (57.7) | 231 (57.0) | 0.95 | 246 (57.1) |
| Private insurance | 4 (15.4) | 51 (12.6) | 0.76 | 55 (12.7) |
Data are mean ± standard deviation, median (IQR), or n (%), unless otherwise specified.
Number of missing values: BMI (n=4), education (n=1).
Maternal HCV viral loads during pregnancy across gestational age were greater in those who transmitted HCV compared to those who did not (Geometric mean 411 vs 49 IU/mL increase per one week gestation, Geometric mean ratio 8.36, 95% CI 5.33 – 13.1). Conversely, ALT decreased with gestational age (Geometric mean 0.995 IU/mL decrease per one week gestation, 95% CI 0.993 – 0.998) and was similar between perinatal transmission statuses (p-value=0.33). There were no statistically significant trends with AST and gestational age (p-value=0.09).
Of the 432 infants for whom data were available to assess the primary outcome, 26 (6.0% (95% CI 4.0 – 8.7%)) demonstrated perinatal transmission. 25/26 infants were born to viremic participants. 1 infant was born to a participant with indeterminate results for viremia. In viremic participants, the perinatal transmission rate was 8.0% (25/314, 95% CI 5.2 – 11.5%). Among those with determinant HCV genotype results (285/314), the predominant HCV genotype in viremic participants was genotype 1 (165/285, 57.9%). The rates of perinatal transmission were not statistically different for those with genotype 1 versus other genotypes (16/165 [9.7%] vs. 7/120 [5.8%], p=0.24). Among viremic, the rates of perinatal transmission were not statistically different for those universally screened versus referrals (3/53 [5.7%] vs. 22/261 [8.4%], p=0.78).
Risk factors that remained in our final model were HCV RNA >106 IU/mL (aOR 8.22, 95% CI 3.16 – 21.4) and vaginal bleeding reported at any time prior to delivery (aOR 3.26, 95% CI 1.32 – 8.03) (Table 2). Results were similar with random intercepts for hospital (data not shown).
Table 2.
Risk factors for perinatal transmission of HCV
| Risk Factor | Perinatal transmission (n=26) |
No perinatal transmission (n=406) |
OR (95% CI) | aOR (95% CI), Final Model |
|---|---|---|---|---|
| HCV RNA titer >10^6 IU/mL | 20 (76.9) | 128 (31.7) | 7.19 (2.82 – 18.33) | 8.22 (3.16 – 21.39) |
| Antepartum bleeding | 9 (34.6) | 72 (17.7) | 2.46 (1.05 – 5.73) | 3.26 (1.32 – 8.03) |
| Planned pre-labor cesarean | 4 (15.4) | 72 (17.7) | 0.84 (0.28 – 2.52) | |
| Any self-reported injection drug use during current pregnancy | 6 (23.1) | 108 (26.6) | 0.83 (0.32 – 2.12) | |
| Premature rupture of membranes | 9 (34.6) | 108 (27.1) | 1.43 (0.62 – 3.30) | |
| Duration of membrane rupture ≥ 6 hours | 9 (36.0) | 131 (33.9) | 1.10 (0.47 – 2.55) | |
| Internal fetal monitoring | 6 (23.1) | 79 (19.5) | 1.24 (0.48 – 3.18) | |
| Breastfeeding | 13 (52.0) | 219 (57.3) | 0.81 (0.36 – 1.81) | |
| Any infections during current pregnancy | 14 (53.8) | 163 (40.1) | 1.74 (0.78 – 3.86) | |
| Maternal age (per 1 year) | 29.0 ± 4.9 | 28.7 ± 5.1 | 1.01 (0.94 – 1.10) | |
| Tobacco use | 21 (80.8) | 319 (78.6) | 1.15 (0.42 – 3.13) | |
| Nulliparous | 9 (34.6) | 120 (29.6) | 1.26 (0.55 – 2.91) | |
| Household status: Own/Rent | 9 (34.6) | 222 (54.7) | 0.44 (0.19 – 1.01) | |
| Annual income: less than $10,000 | 10 (45.5) | 171 (48.0) | 0.90 (0.38 – 2.14) | |
| Less than 12 years of schooling | 15 (57.7) | 231 (57.0) | 1.03 (0.46 – 2.29) | |
| Employed | 8 (30.8) | 146 (36.0) | 0.79 (0.34 – 1.87) | |
| Private insurance | 4 (15.4) | 51 (12.6) | 1.27 (0.42 – 3.82) | |
| Fetal sex: male | 13 (50.0) | 202 (49.8) | 1.01 (0.46 – 2.23) |
Data are mean ± standard deviation or n (%), unless otherwise specified.
Number of missing values: HCV RNA titer (n=2), rupture of membranes (n=7), duration of membrane rupture (n=20), fetal monitoring (n=1), breastfeeding (n=25), income (n=54), education (n=1).
Adjusted model included HCV RNA titer and any antepartum bleeding.
The distribution of the highest viral load prior to delivery in participants who did and did not transmit to their neonates is illustrated in Figure 2. All but one transmission who demonstrated indeterminate viral load occurred in participants with viremia, but not all participants with high viral loads demonstrated perinatal transmission. The lowest viral load associated with perinatal transmission was 12,244 IU/mL (4.1 log10 IU/mL), aside from the transmission which occurred in a participant with indeterminate viral load. The AUC-ROC ranged from 0.75 to 0.83 for viral loads assessed at enrollment, 24–28 weeks, 32–36 weeks, and delivery. Viral loads at 24–28 weeks’ gestation yielded the greatest AUC-ROC for perinatal transmission (0.83, 95% CI 0.75–0.91) (Figure 3). Using a cutoff value of 1,111,754 IU/mL (6.0 log10 IU/mL) at 24–28 weeks yields 80% sensitivity and 77% specificity with a positive likelihood ratio of 3.5 and a negative likelihood ratio of 0.26. Planned pre-labor cesarean was not associated with perinatal transmission (4/76 [5.3%] vs 22/356 [6.2%], OR 0.84, 95% CI 0.28 – 2.5).
Figure 2.
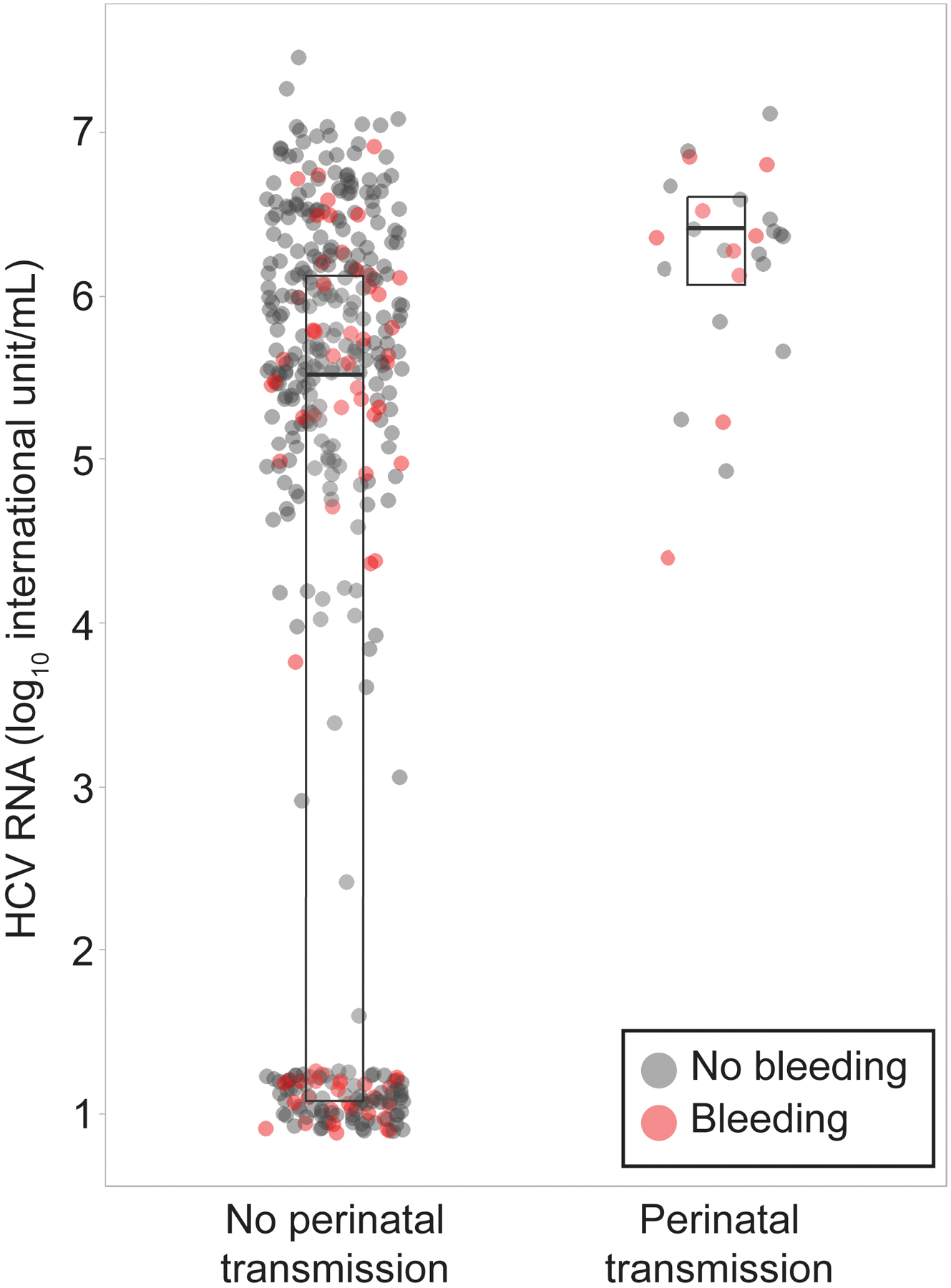
Maternal hepatitis C virus (HCV) RNA by perinatal transmission status and antepartum bleeding. Middle line is the median, lower line is the first quartile, and the upper line is the third quartile.
Figure 3.
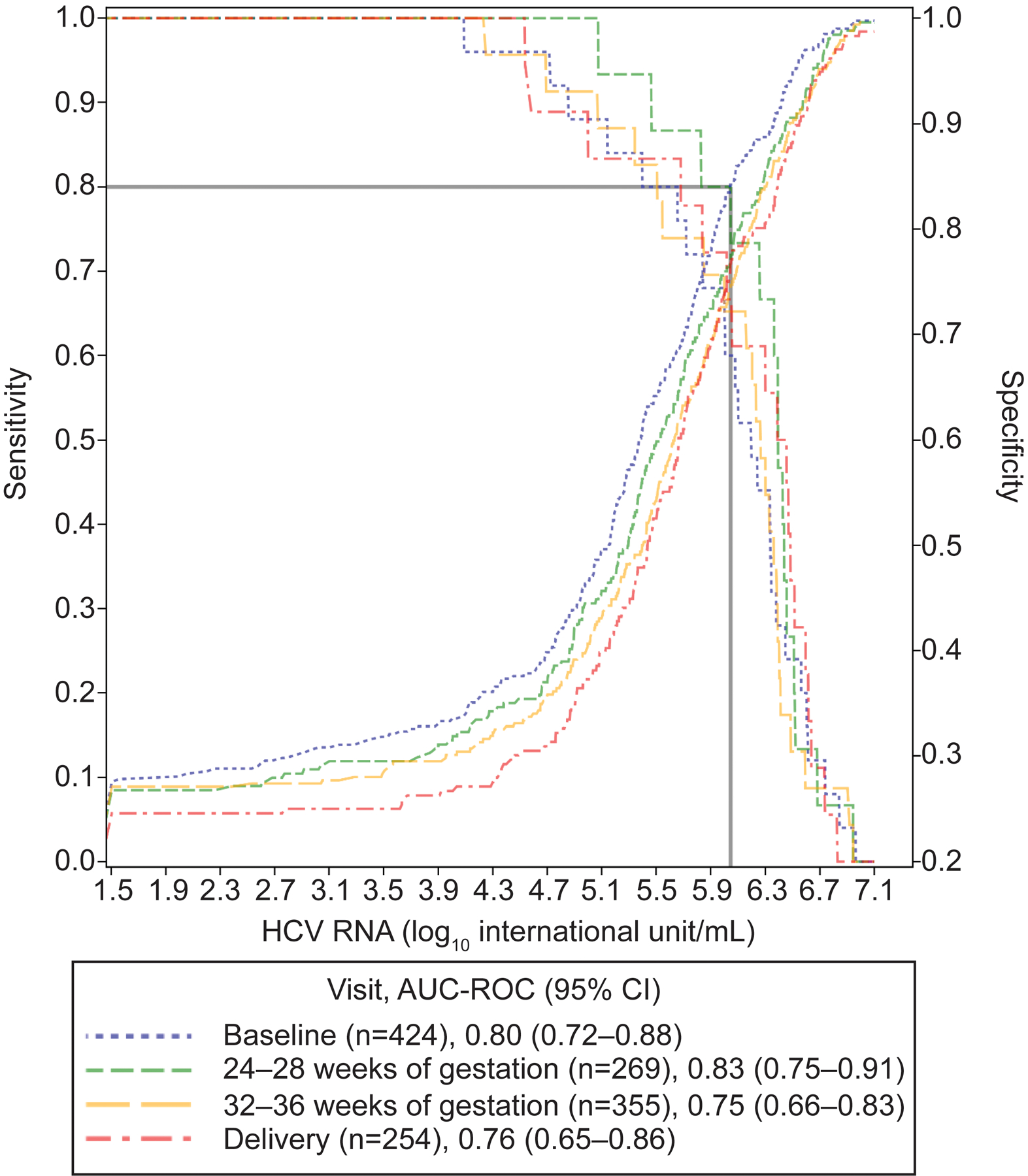
Sensitivity and specificity of maternal viral loads for perinatal transmission. HCV, hepatitis C virus; AUC, area under the curve; ROC, receiver operating characteristic.
Discussion
Our study has shown that in a contemporary cohort the rate of HCV perinatal transmission is 8.0% in viremic individuals, acknowledging that missing data due to loss to follow up limits the precision and potentially the accuracy of the estimates of perinatal HCV transmission. Risk factors for perinatal transmission were HCV RNA viral loads >106 IU/mL and vaginal bleeding reported at any time prior to delivery. This work also showed that when viral load is not detected by PCR, transmission .did not occur.
Participants who demonstrated perinatal transmission had higher HCV viral loads during pregnancy through delivery compared to participants who did not demonstrate perinatal transmission. As the participant demonstrating the highest viral load did not demonstrate perinatal transmission, an absolute viremic threshold for perinatal transmission was not established. In participants for whom viral load was established, the lowest viral load associated with transmission was 12,244 IU/mL (4.1 log10 IU/mL). When interpreting the association of transmission with viral load >106, it is important to note that only 20/148 participants with this viral load transmitted. As well, transmission occurred as low as 12,244 IU/mL (4.1 log10 IU/mL) suggesting that viral load alone, though associated with transmission, is likely influenced by additional factors. None the less, risk factors for perinatal transmission that emerged from this work included higher viral loads during pregnancy and vaginal bleeding occurring at any time prior to delivery. Planned pre-labor cesarean was not associated with perinatal transmission. HCV genotype was also not associated with perinatal transmission in this cohort.
The findings from this study are important in the counseling of patients with HCV. For example, if a patient is HCV antibody positive, but demonstrates no viremia, we can counsel that the best evidence suggests that the patient will not transmit HCV to their child Our findings are different from the existing literature19 in that internal fetal monitoring, prolonged ruptured membranes, and duration of membrane rupture > 6 hours did not significantly affect perinatal transmission. We suggest that intrapartum management does not need to be modified in this population, though further research would be necessary to be definitive. We have identified that with viremia and/or bleeding complicating pregnancy, the urgency for postnatal follow up of those children is heightened. This subset of neonates needs to be evaluated for evidence of perinatal transmission and linkage to care for childhood treatment should be prioritized.
There were several strengths of this study. This contemporary cohort included the largest numbers of participants screened for HCV from U.S. hospitals, found to be HCV positive, and prospectively followed through pregnancy. The demographics for this study population mirrored national data with respect to those who carry a diagnosis of HCV18. This is also the largest study of infected infants where such conclusions can be derived.
In spite of those strengths, we were limited by loss to follow up, and this requires significant discussion. The MFMU has a research structure which has typical follow up rates even at several years of follow up of greater than 90 percent20. This historic success rendered us confident that we would have excellent ability to identify and follow even complex populations. The MFMU is also known to be a generalizable population, but this cohort was not easily ascertained in this network for reasons we do not fully understand. In spite of the prevalence of HCV anticipated in the MFMU network during a time coexistent with the opioid epidemic, it took from 2012–2019 to identify and enroll the patients in this cohort. The length of time affected our ability to enroll an optimal study size and feasibility was thus also affected. We encountered difficulty enrolling participants once found to be HCV positive (enrolling only 63.1%), most commonly due to refusal of consent and unwillingness to commit to follow up, which was a similar pattern of loss to follow up encountered during the study.
This study’s loss to follow up rate of 44.0% (i.e., the number of infants without primary outcome data) challenged feasibility and spoke to the complexity of circumstances in this study population. When not associated with research, loss to follow up rates in this population have been shown to be 94%21, suggesting that the research environment was much more successful than standard care. While each participant was offered some monetary incentive for participation, we have learned that future studies are likely to be more successful with a keener understanding of barriers to follow up so that study design and incentives can be tailored to improve retention. Critical to success of future studies would be patient engagement in the study design, perhaps in the form of a patient advisory board to influence both study design as well as study monitoring.
Given recent consensus in recommendations from the Centers for Disease Control and Prevention (CDC), United States Preventive Services Task Force (USPSTF), American College of Obstetricians and Gynecologists (ACOG), and Society for Maternal-Fetal Medicine (SMFM) for universal screening for HCV in pregnancy, there is an urgency to define best practices for pregnant people with HCV. This work suggests that the greatest effects in terms of reducing perinatal transmission would be decreasing viral loads in birthing persons prior to delivery. There is an ongoing trial to determine safety, tolerability, and outcomes of direct acting antivirals for treatment of HCV in pregnancy22. If direct acting antivirals are found to be safe in pregnancy and improve childhood outcomes, it stands to reason that treatment to cure either before pregnancy or prior to delivery may effectively eliminate perinatal transmission of HCV. In the meantime, efforts to optimize systems of care to combat loss to follow up of this complex population stand to make a significant public health effects by prioritizing linkage to care in those affected by HCV.
Supplementary Material
Acknowledgments:
The authors thank Francee Johnson, RN, BSN, for protocol development and coordination between clinical research centers; Catherine Y. Spong, M.D. for protocol development; and Elizabeth Thom, Ph.D. for protocol development and oversight.
Funding:
Supported by grants (HD27915, HD53097, HD40500, HD40544, HD27869, HD40560, HD34208, HD40485, HD87230, HD68258, HD68282, HD40545, HD40512, HD68268, HD34116, HD87192, HD36801) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH), all components of the National Institutes of Health (NIH), under Award Numbers UM1AI068632-15 (IMPAACT LOC), UM1AI068616-15 (IMPAACT SDMC) and UM1AI106716-15 (IMPAACT LC), and by NICHD contract number HHSN275201800001I. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Centers for Disease Control and Prevention.
Financial Disclosure
Mona Prasad served on the medical advisory board for Gilead. Brenna Hughes disclosed receiving payment from UptoDate and the Johns Hopkins DSMB. Ana Puga disclosed that she is cochair of the Broward County Perinatal HIV Network (no compensation) and a full-time employee of ViiV Healthcare since 2018. Torri D. Metz disclosed receiving UptoDate royalties for two topics on trial of labor after cesarean. Her institution received payment from Gestvision for her being a site PI for a preeclampsia point-of-care test (institution received money to conduct study (ended August 2020]) and from Pfizer for being a site PI for a Phase III respiratory syncytial virus (RSV) vaccine trial (institution received money to conduct the study). She has been a member of the medical advisory board for Pfizer, a site PI for a COVID-19 vaccination trial, and has served on the Board of Directors for the Society of Maternal-Fetal Medicine. Geeta Swamy reports money was paid to her from GlaxoSmithKline, Pfizer, and WebMD/Medscape. Andrew Wiznia received payment for a consultantcy with Janssen Pharmaceuticals, where he has been a chairperson of the Independent Data Safety Monitoring Board. He has also received payment from Merck for a consultancy and protocol development. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal’s requirements for authorship.
Footnotes
Presented at the Society for Maternal-Fetal Medicine Annual Meeting, held virtually, February 3, 2022.
References
- 1.Campbell CA, Canary L, Smith N, Teshale E, Ryerson AB, Ward JW. State HCV incidence and policies related to HCV preventive and treatment services for persons who inject drugs—United States, 2015–2016. MMWR. Morbidity and mortality weekly report 2017. May 12;66(18):465. DOI: 10.15585/mmwr.mm6618a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rossi RM, Wolfe C, Brokamp R, McAllister JM, Wexelblatt S, Warshak CR, Hall ES. Reported prevalence of maternal hepatitis C virus infection in the United States. Obstetrics & Gynecology. 2020. Feb 1;135(2):387–95. DOI: 10.1097/AOG.0000000000003644 [DOI] [PubMed] [Google Scholar]
- 3.Dhiman RK, Premkumar M. Hepatitis C virus elimination by 2030: conquering mount improbable. Clinical Liver Disease. 2020. Dec;16(6):254. DOI: 10.1002/cld.978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Owens DK, Davidson KW, Krist AH, Barry MJ, Cabana M, Caughey AB, Donahue K, Doubeni CA, Epling JW, Kubik M, Ogedegbe G. Screening for hepatitis C virus infection in adolescents and adults: US Preventive Services Task Force recommendation statement. Jama. 2020. Mar 10;323(10):970–5. DOI: 10.1001/jama.2020.1123 [DOI] [PubMed] [Google Scholar]
- 5.Schillie S, Wester C, Osborne M, Wesolowski L, Ryerson AB. CDC recommendations for hepatitis C screening among adults—United States, 2020. MMWR Recommendations and Reports. 2020. Apr 3;69(2):1. DOI: 10.15585/mmwr.rr6902a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2021/05/routine-hepatitis-c-virus-screening-in-pregnant-individuals. Accessed 4/24/2022.
- 7.https://www.smfm.org/publications/391-smfm-consult-series-56-hepatitis-c-in-pregnancyupdated-guidelines. Accessed 4/24/2022.
- 8.Prasad M, Saade GR, Sandoval G, Hughes BL, Reddy UM, Mele L, Salazar A, Varner MW, Gyamfi-Bannerman C, Thorp JM Jr, Tita ATN, Swamy GK, Chien EK, Case BM, Peaceman AM, El-Sayed YY, Iams JD, Gibbs RS, Sibai B, Wiese N, Kamili S, Macones GA. Hepatitis C virus antibody screening in a cohort of pregnant women: identifying seroprevalence and risk factors. Obstetrics and gynecology. 2020. Apr;135(4):778. DOI: 10.1097/AOG.0000000000003754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benova L, Mohamoud YA, Calvert C, Abu-Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clinical infectious diseases. 2014. Sep 15;59(6):765–73. DOI: 10.1093/cid/ciu447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, Alter MJ. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. The Journal of infectious diseases. 2005. Dec 1;192(11):1880–9. DOI: 10.1086/497701 [DOI] [PubMed] [Google Scholar]
- 11.Ceci O, Margiotta M, Marello F, Francavilla R, Loizzi P, Francavilla A, Mautone A, Impedovo L, Ierardi E, Mastroianni M, Bettocchi S. Vertical transmission of hepatitis C virus in a cohort of 2,447 HIV-seronegative pregnant women: a 24-month prospective study. Journal of pediatric gastroenterology and nutrition. 2001. Nov 1;33(5):570–5. DOI: 10.1097/00005176-200111000-00011 [DOI] [PubMed] [Google Scholar]
- 12.Ohto H, Terazawa S, Sasaki N, Sasaki N, Hino K, Ishiwata C, Kako M, Ujiie N, Endo C, Matsui A, Okamoto H. Transmission of hepatitis C virus from mothers to infants. New England Journal of Medicine. 1994. Mar 17;330(11):744–50. DOI: 10.1056/NEJM199403173301103 [DOI] [PubMed] [Google Scholar]
- 13.Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, Vierucci A. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Bmj. 1998. Aug 15;317(7156):437–41. DOI: 10.1136/bmj.317.7156.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Azzari C, Resti M, Moriondo M, Ferrari R, Lionetti P, Vierucci A. Vertical transmission of HCV is related to maternal peripheral blood mononuclear cell infection. Blood, The Journal of the American Society of Hematology. 2000. Sep 15;96(6):2045–8. [PubMed] [Google Scholar]
- 15.Spencer JD, Latt N, Beeby PJ, Collins E, Saunders JB, McCaughan GW, Cossart YE. Transmission of hepatitis C virus to infants of human immunodeficiency virus‐negative intravenous drug‐using mothers: rate of infection and assessment of risk factors for transmission. Journal of viral hepatitis. 1997. Nov;4(6):395–409. DOI: 10.1046/j.1365-2893.1997.00073.x [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Tejedor A, Maiques-Montesinos V, Diago-Almela VJ, Pereda-Perez A, Alberola-Cuñat V, López-Hontangas JL, Perales-Puchalt A, Perales A. Risk factors for vertical transmission of hepatitis C virus: a single center experience with 710 HCV-infected mothers. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2015. Nov 1;194:173–7. DOI: 10.1016/j.ejogrb.2015.09.009 [DOI] [PubMed] [Google Scholar]
- 17.https://www.who.int/news-room/fact-sheets/detail/hepatitis-c. Accessed 4/24/2022.
- 18.Patrick SW, Dupont WD, McNeer E, McPheeters M, Cooper WO, Aronoff DM, Osmundson S, Stein BD. Association of Individual and Community Factors With Hepatitis C Infections Among Pregnant People and Newborns. In JAMA Health Forum 2021. Oct 1 (Vol. 2, No. 10, pp. e213470–e213470). American Medical Association.. doi: 10.1001/jamahealthforum.2021.3470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Shabrawi MHF, Kamal NM, Mogahed EA, Elhusseini MA, Aljabri MF. Perinatal transmission of hepatitis C virus: an update. Arch Med Sci. 2019. Mar 15;16(6):1360–1369. doi: 10.5114/aoms.2019.83644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, Reddy UM, Wapner RJ, Thorp JM Jr, Saade G, Tita AT, Rouse DJ, Sibai B, Iams JD, Mercer BM, Tolosa J, Caritis SN, VanDorsten JP. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. New England Journal of Medicine. 2017. Mar 2;376(9):815–25. DOI: 10.1056/NEJMoa1606205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jarlenski M, Chen Q, Ahrens KA, Allen L, Austin AE, Chappell C, Donohue JM, Hammerslag L, Lanier P, McDuffie MJ, Talbert J, Tang L, Krans EE; Medicaid Outcomes Distributed Research Network (MODRN). Postpartum Follow-up Care for Pregnant Persons With Opioid Use Disorder and Hepatitis C Virus Infection. Obstet Gynecol. 2022. May 1;139(5):916–918.. Epub 2022 Apr 5. doi: 10.1097/AOG.0000000000004760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.https://clinicaltrials.gov/ct2/show/NCT05140941?term=STORC&draw=2&rank=1. Accessed 4/24/2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


