Abstract
Background
Children with congenital heart disease (CHD) often have inactive lifestyles and motor skill deficits beginning in infancy. The least active infants continue to be the least active children at school age. Enhancing physical activity and motor development in infancy, at the time of CHD treatment, may prevent inactive lifestyle habits.
Methods
All children being treated, through surgery or catheterization, for congenital heart disease are eligible if they are 3 to 72 months of age at enrollment. The Peabody Motor Development Scales (Version 2) and 7-day accelerometry (Actigraph GT9X Link) assess motor skills and physical activity prior to treatment and 7 weeks, 6 months and 12 months post-treatment. Participants are randomized 3:1 to intervention:control. Until 7 weeks post-treatment, intervention activities focus on regaining pre-treatment mobility and midline crossing. From 7 weeks to 6 months post-treatment, the intervention is individualized to each child’s assessment results and is parent-led, delivered at home and play-based.
Conclusion
This feasibility study will provide essential data for a randomized controlled trial to evaluate play-based, parent-delivered interventions optimized to support age-appropriate physical activity and motor skills among young children with CHD. Preliminary intervention efficacy data will inform an evidence-based sample size calculation, optimize intervention timing, and identify hypotheses on the motor skill—physical activity connection and the impact of play-based, parent-led interventions during recovery from CHD treatment. Long-term, the goal is to optimize motor skill and active lifestyles among young children with CHD, enabling their healthy growth and development and enhancing childhood quality of life.
Trial registration
Clinical trials registration: NCT04619745.
Introduction
Active play is critically important for young children [1–5] as it is the foundation for childhood socialization, provides emotional, psychological and cognitive benefits [5] and is essential for childhood health [6], and biological and psychosocial development [1, 5]. Children with simple or complex congenital heart defects (CHD) are often less active [7], unable to achieve the 180 mins of daily activity recommended for the optimal health of young children [8], even when these children have age-appropriate motor skills [9, 10]. Highly inactive infants with CHD become the most inactive school age children [11], suggesting that an effective intervention to enhance active play should target children with CHD in infancy.
Although most older children and adults with simple or complex CHD are more sedentary than healthy peers [12–14], a small proportion of these patients lead active lifestyles [15, 16] and exercise [17], fitness [18, 19], or movement skill training [17] improves their performance. Therefore, their sedentary behavior is unlikely to result from physiological limitations of the cardiac diagnosis. The hypoactive lifestyles [14] among children with simple or complex CHD are suggested to result from limited self-efficacy for physical activity [20], parental overprotection [21] or perceptions of the child as fragile [14, 17]. Uncertainty naturally leads to caution, and eventually a more sedentary lifestyle. Given the difficulty in altering established habits, few interventions to enhance active lifestyles among older children with CHD have demonstrated long-term benefits [18]. Interventions that target young children are required so that active lifestyles can be established before sedentary lifestyle habits emerge.
The development of an intervention targeting physical activity in very young children with CHD would be highly novel, with several theoretical issues identified. Most importantly, it is necessary to determine whether parents would be willing to have their children participate. It is unclear whether parents of younger infants and those with less severe forms of CHD would be willing to enroll in a 6-month intervention at the time of treatment (i.e., recruitment feasibility). There is also evidence needed to support the feasibility, and parent and healthcare professional acceptance of completing recruitment and baseline assessments within the 4-to-6 week interval between scheduling of treatment and treatment date. Data on intervention efficacy and compliance, and the willingness of patients to be randomized to either intervention or control conditions are required to calculate an appropriate randomized controlled trial (RCT) sample size. Data on the time to contact, enroll, and assess patients, as well as the time required to develop and implement each child’s intervention are required in addition to data on withdrawal and ineligibility rates.
To prepare for an RCT examining the efficacy of a 6-month intervention during the treatment and post-treatment recovery phase of infants with CHD, this study is evaluating the feasibility of participant recruitment, data collection procedures and outcome measures, the acceptability and suitability of the intervention, and the resources required. We are also gathering preliminary efficacy evidence of response to the intervention, and professional and parent perceptions of study burden. This feasibility trial is also designed to reveal unforeseen problems and provide an opportunity to find appropriate solutions that would optimize the RCT design. We hypothesize that it will be feasible to recruit patients, collect data and perform the outcome measures of the intervention. We also hypothesize that parents will not feel burdened by the study. Furthermore, we hypothesize that the 6-month, home-based, parent-led, kinesiologist-designed physical activity program, completed immediately after surgical or catheterization treatment, may enable young children with CHD to achieve the recommended 180 minutes of daily physical activity.
Methods
Study design
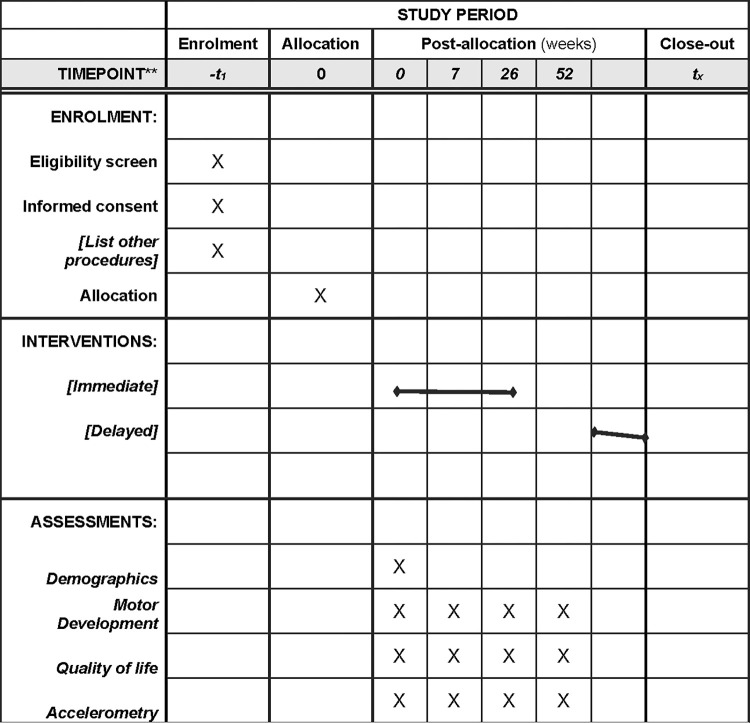
This hypothesis generating, feasibility study (Fig 1) is designed to include comprehensive measures of motor skill and physical activity, uniquely intervene at a very young age, and target the high-risk status for sedentary lifestyles of children with CHD. Long-term, the goal is to enable the design of a randomized, controlled trial to evaluate play-based, parent-delivered interventions optimized to support age-appropriate physical activity and motor skills among young children with CHD. Ethics approval for this study protocol (S1 File) is granted by the Research Ethics Board at the Children’s Hospital of Eastern Ontario (CHEO) (REB file #20/67X).
Fig 1. Schedule of enrollment, interventions, and assessments.
Participants
All children receiving elective treatment via cardiac surgery or catheterization intervention for CHD at the Children’s Hospital of Eastern Ontario (CHEO) are eligible if they are 3 to 72 months of age. Patients with genetic conditions or physical disabilities affecting motor development, lack of independent limb movement, receiving medical care that is incompatible with the study assessments, or receiving emergency treatment are excluded. Total planned enrollment is 56 children, allowing for a sufficient sample (n = 48) to have 80% power to detect a difference in daily physical activity of 20% with alpha = 0.10 and allowing for a 15% drop-out rate.
Scheduled treatment procedures are reviewed weekly to identify eligible patients. Families are contacted by a member of the circle of care 4 to 6 weeks prior to the treatment (surgery or catheterization) date. Those willing to speak with the researcher are provided detailed information about the study. Families consenting to the child’s study participation provide written informed consent and complete the baseline assessment during the first study visit, with families choosing to have the visit in conjunction with the pre-treatment clinic visit or on a separate day. Given the young age of the participants, assent is assumed through the children’s willingness to perform the study activities. Some CHD diagnoses are more common in males, so recruitment of females is enhanced to target equal sex representation in the study sample. The cardiologist responsible for the child’s care approves their enrolment into the study and their continued participation prior to each study visit. The cardiologist also specifies any medically necessary activity restrictions that apply to the child. The approach and enrollment of patients is documented according to the CONSORT guidelines [22].
Assessment of outcomes
All children receive standard of care for their treatment procedure and complete four study visits: 1 to 2 weeks prior to treatment, and 7 weeks, 6 months, and 12 months after treatment. After the first study visit, participants are randomized to either the intervention or control study group. Participants are randomly assigned, in random blocks of 4 and 8, in a 3:1 ratio to either the intervention (n = 36) or control (n = 12, standard care but no intervention) study group. Standard care includes the monitoring of heart function and treatment recovery but does not include physiotherapy or any support for motor development or physical activity.
The feasibility outcomes assessed address four aspects of study design:
Participant recruitment (# of patients treated per month, randomization to control or intervention, clarity of inclusion/exclusion criteria, ability of healthcare professionals to facilitate pre-treatment recruitment, reasons for refusal/ineligibility),
Data collection procedures (% of patients with completed pre-treatment data, # of days available prior to treatment for baseline data collection, % of parents able to complete child accelerometer wear for 7 days, % of control and intervention who complete all data sessions, frequency of missing data, study time and burden),
Intervention delivery (% retention and follow up, % compliant with intervention and rate of adherence, intervention time and burden), and
Required resources (staff time required to identify/consent/follow patients, space available for baseline testing, kinesiologist time to create/support interventions).
Preliminary efficacy data are collected from all study participants at or after each 1-hour study visit. The researcher conducting the study assessments is blind to study group allocation. The medical history of each participant is compiled from medical records (e.g., diagnosis, type and time of repair, length of hospital stay(s)). Parents complete four questionnaires: a) demographic questionnaire (age, gender, family income, parent education), b) an assessment of their child’s quality of life (Pediatric Quality of Life (PedsQL [23] or Infant Toddler Quality of Life (ITQOL-47)54), c) the Social Skills Checklist [24] for their child, and d) the Parenting Stress Index: short form [25]. 100mm visual analogue scales are used to evaluate parent perceptions of the child’s activity and skill relative to peers (above/below), activity and skill relative to optimal levels (above/below), and the perceived activity implications of the child’s CHD diagnosis (uncertain/clear) and treatment (very limited/none). Children 5 years of age or older also complete the PedsQL child report. All parents who do not complete the study and 10 randomly selected parents from those with complete data are asked to partake in a semi-structured exit interview, where they are asked about their perceptions, barriers, and facilitators to study completion. At each study visit, all child participants complete the Peabody Developmental Motor Scales-2 (test-retest ICC = 0.97 [26]) [27]. Children are asked to perform a series of play-based activities (building blocks, jumping, etc.) based on activities that could be completed by children of similar chronological age. The difficulty of the activities is then adjusted until the actual skills of the child being assessed are determined.
At the end of each study visit, children are given a tri-axial Actigraph GT9X Link accelerometer to wear on a waistband under the child’s outer garments, over the right hip, in the mid axillary line [28]. The daily physical activity of independently mobile children is measured over 7 consecutive days, with parents recording wear, nap, and sleep times, and reasons for device removal on a log sheet. At least 3 weekdays and 1 weekend day of valid data (at least 5 hours of recorded movement data excluding sleep and nap times [29]) is required [30]. The accelerometers measure activity intensity and established cut points [31] are used to calculate daily minutes of sedentary and light+moderate+vigorous activity.
Intervention
Each child in the intervention group is provided with 6 months of parent-led, home and play-based activity plans that follow a standardized format and are individualized to each child’s age and previous visit assessments. The in-hospital intervention begins once the child returns to the regular hospital ward from the ICU, where the kinesiologist provides daily play activities that are focused on maintaining or regaining range of motion (upper and lower limbs) and supporting midline crossing (e.g., hand clapping, reaching for toys). The kinesiologist collaborates with parents to ensure accurate performance and implementation of the in-hospital program. From discharge to week 7, lower body mobility activities are combined with the in-hospital activities, since upper body weight bearing and lifting activities are restricted for children undergoing surgical treatment until the week 7 evaluation (for sternal healing). The kinesiologist contacts parents weekly to provide support, adjust the child’s physical activity, and assess adherence.
If physical activity is unrestricted after the week 7 assessment, progressively more difficult, individualized, parent-led, home and play-based activity plans (see S2 File) are provided every four weeks until the 6-month study visit. Each plan reflects the child’s baseline and week 7 assessment results and focuses on developing daily activity habits and age-appropriate movement skills. The kinesiologist demonstrates the planned activities and educates parents on implementing the activities through active play, as well as contacts parents every 2 weeks to provide support and obtain feedback on the child’s progress. At each contact, the kinesiologist assesses the ability of parents to properly lead the play-based activities and monitors home-based adherence to assess intervention fidelity.
Data analyses
Feasibility outcomes (participant recruitment, data collection procedures, intervention delivery, RCT resources) are calculated as descriptive statistics and assessed relative to criteria established prior to study implementation. Based on our previous intervention trials with children with CHD, it is established that recruitment is considered feasible if at least 60% of families are willing to enroll and less than 20% of patients are ineligible, missed or withdrawn. Inclusion criteria are revised if more than 5% of patients required a cardiologist consult to determine eligibility. Data collection procedures are feasible if at least 80% of participants completed pre-treatment data, including accelerometer wear, and the scores from parents and healthcare professionals indicated that both the time and burden of data collection are acceptable (> 60 mm on visual analogue scale). Less than 20% of participants are to have missing data or data collection sessions during study participation. The intervention is feasible if less than 20% of families withdraw prior to study completion, and at least 70% of families complete the activities at least 3 times per week. The resources to conduct the study are feasible if adequate space is available for 100% of baseline testing sessions and the Research Coordinator has sufficient time to identify, consent, assess and intervene with all children receiving CHD treatment.
Frequency tabulations and descriptive statistics (mean±SD or median [q1, q3] as appropriate) are used to describe study participants. Logistic regression, adjusted for age, sex and group (intervention/control) is used for the preliminary assessment of study visit impact (1/2/3/4) on achievement of age-appropriate movement skills (Peabody Total Score < 1 SD from mean) or physical activity (180 mins/day). Groups are compared at each time point (chi-square). To examine group differences on changes in a) physical activity and b) motor skills, repeated measures ANOVA are used. Group (intervention/control) and time (baseline, 6 months, 12 months) are the within-repeated measures factor, with age, sex, and season as covariates. These analyses are not powered to determine statistical effects but will provide descriptive statistics for the feasibility study. Change in physical activity and motor skill effect sizes by study group indicating potential intervention impact, will be used to inform the required sample size for a fully powered multi-site randomized controlled trial and confirmed outcome variable changes are in the expected direction.
Discussion
Pediatric CHD research has historically focused on reducing mortality. Now that even those with complex CHD survive to adulthood, the importance of promoting quality of life and decreasing secondary morbidity risk has dramatically increased. Through our unique and innovative research, we are striving to ensure that children with CHD are supported to not only survive, but thrive. Clinicians and families caring for children with CHD know they often are less skilled and less active than their peers. This study is designed to target the creation of active lifestyle habits among our youngest patients utilizing parent-led, home and play-based interventions modeled on similar interventions successfully used in older children [18, 19]. The feasibility and efficacy data generated through this project will enable the optimal design of a full-scale multi-site randomized controlled trial evaluating the impact of this physical activity intervention timed for the expected change in children’s physical activity as they are treated for their CHD. The novel aspects of this research include the comprehensive motor skill and physical activity measures, participants’ young age, and the high-risk status for inactive lifestyles of children with CHD. The lack of existing data on active play among infants during recovery from CHD treatment currently limits our ability to evaluate intervention effectiveness in an appropriately powered trial.
Limitations
Known limitations for this research included the variability that occurs within standardized assessment protocols, the limited capacity of young children for completing assessment tasks, and the potential for recruitment bias among families choosing to enroll in a physical activity study. Our study team’s demonstrated skill in implementing the proposed assessments with very young children [3, 10] is expected to mitigate the impact of assessment and technical problems. To minimize recruitment bias, all eligible children treated at CHEO, either through surgery or catheterization, are approached to participate. Approaching all children decreases the risk of bias from active parents being more likely to volunteer for a physical activity study. The standardized template for creating activity plans and the database of activities linked to assessment results enable intervention delivery, with data on the required kinesiologist support informing the potential of the intervention as a standard of CHD treatment.
Conclusions
This study is designed to provide essential feasibility data for a randomized controlled trial to evaluate play-based, parent-delivered interventions optimized to support age-appropriate physical activity and motor skills among young children with CHD. Preliminary intervention efficacy data will inform an evidence-based sample size calculation, optimize intervention timing, and identify hypotheses on the motor skill—physical activity connection and the impact of play-based, parent-led interventions during recovery from CHD treatment. Long-term, the goal is to optimize motor skill and active lifestyles among young children with CHD, enabling their healthy growth and development and enhancing childhood quality of life [32].
Supporting information
(DOC)
(PDF)
(PDF)
Acknowledgments
The authors are very grateful for the support of participating families who engaged in this research at the time of their children’s treatment for congenital heart disease. The collaboration of treatment staff, including Tara Girolamo and Jocelyne Bill, is greatly appreciated. The collection of study data would not have been possible without the contributions of Miranda DiGasparro, Angelica Blais and Jenna Yaraskavitch.
Data Availability
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.
Funding Statement
Funding for this research was provided by the Canadian Institutes for Health Research (grant # 202104PJT‐461792‐CID‐CECC‐158683) and the Heart and Stroke Foundation of Canada (grant # G‐20‐ 0028713) to Principal Investigator Dr. Longmuir. The study was prospectively registered in ClinicalTrials.gov (NCT04619745). The trial has been approved by the Research Ethics Board of the Children’s Hospital of Eastern Ontario (REB file #20/67X, version 1, 2021‐11‐16) The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ginsburg KR MD, MSEd, Health the C on C and the C on PA of C and F, Committee on Communications and the Committee on Psychosocial Aspects of Child and Family Health. The Importance of Play in Promoting Healthy Child Development. Pediatrics. 2007;119: 182–188. Available: file:///V:/HALO/HALO Staff/Longmuir/New Literature B/Am Acad Ped Importance of Play 2012.pdf17200287 [Google Scholar]
- 2.Bar-Or O, Rowland TW. Habitual activity and energy expenditure in the healthy child. Pediatric Exercise Medicine: From Physiologic Principles to Health Care Application. Champaign, IL: Human Kinetics; 2004. pp. 64–67. [Google Scholar]
- 3.Klavora P. Foundations of Exercise Science. Toronto, Ontario: Sport Books Publisher; 2004. [Google Scholar]
- 4.Centers for Disease Control, Centers for Disease Control and Prevention. How Much Physical Activity Do Children Need? /www.cdc.gov/physicalactivity/everyone/guidelines/children.html, editor. Centers for Disease Control; 2022. Available: http://www.cdc.gov/physicalactivity/everyone/guidelines/children.html [Google Scholar]
- 5.Timmons BW, Naylor PJ, Pfeiffer KA. Physical activity for preschool children—how much and how? Can J Public Health. 2007;98 Suppl 2: S122–34. doi: 10.1139/H07-112 [DOI] [PubMed] [Google Scholar]
- 6.Public Health Agency of Canada. The Benefits of Physical Activity: For Children/Youth. Public Health Agency of Canada. Public Health Agency of Canada; 2005. Available: http://www.phac-aspc.gc.ca/hp-ps/hl-mvs/pa-ap/02paap-eng.php [Google Scholar]
- 7.Warnes CA, Liberthson R, Danielson GK, Dore A, Harris L, Hoffman JIE, et al. Task force 1: The changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37: 1170–1175. doi: 10.1016/s0735-1097(01)01272-4 [DOI] [PubMed] [Google Scholar]
- 8.Tremblay MS, Chaput JP, Adamo KB, Aubert S, Barnes JD, Choquette L, et al. Canadian 24-Hour Movement Guidelines for the Early Years (0–4 years): An Integration of Physical Activity, Sedentary Behaviour, and Sleep. BMC Public Health. 2017;17. doi: 10.1186/s12889-017-4859-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telama R. Tracking of physical activity from childhood to adulthood: A review. Eur J Obes. 2009;3: 187–195. doi: 10.1159/000222244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longmuir PE, Turner JAP, Rowe RD, Olley PM. Postoperative exercise rehabilitation benefits children with congenital heart disease. Clin Invest Med. 1985;8: 232–8. Available: http://www.ncbi.nlm.nih.gov/pubmed/4042466 [PubMed] [Google Scholar]
- 11.Longmuir PE, Wang S, Timmons BW, Mondal T, Cinanni NL, Di Cristofaro NA, et al. Inactive Lifestyles among Young Children with Innocent Murmurs or Congenital Heart Disease Regardless of Disease Severity or Treatment. Can J Cardiol. 2022;38: 59–67. doi: 10.1016/j.cjca.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 12.Hovels-Gurich HH, Konrad K, Skorzenski D, Nacken C, Minkenberg R, Messmer BJ, et al. Long-term neurodevelopmental outcome and exercise capacity after corrective surgery for tetralogy of Fallot or ventricular septal defect in infancy. Ann Thorac Surg. 2006;81: 958–966. doi: 10.1016/j.athoracsur.2005.09.010 [DOI] [PubMed] [Google Scholar]
- 13.Massin MM, Hovels-Gurich HH, Gerard P, Seghaye MC, Hövels-Gürich HH, Gérard P, et al. Physical activity patterns of children after neonatal arterial switch operation. Ann Thorac Surg. 2006;81: 665–670. doi: 10.1016/j.athoracsur.2005.07.034 [DOI] [PubMed] [Google Scholar]
- 14.Reybrouck T, Mertens L. Physical performance and physical activity in grown-up congenital heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12: 498–502. Available: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16210938 doi: 10.1097/01.hjr.0000176510.84165.eb [DOI] [PubMed] [Google Scholar]
- 15.Mccrindle BW, Williams R V, Mital S, Clark BJ, Jennifer L, Klein G, et al. Reduced physical activity levels after the Fontan procedure: Related to exercise capacity or own health perception? 2007. doi: 10.1136/adc.2006.105239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Voss C, Duncombe SL, Dean PH, De Souza AM, Harris KC. Physical activity and sedentary behavior in children with congenital heart disease. J Am Heart Assoc. 2017;6: e004665. doi: 10.1161/JAHA.116.004665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes J, Curran TJ, Camil L, Rabideau N, Fulton DR, Gauthier NS, et al. Impact of cardiac rehabilitation on the exercise function of children with serious congenital heart disease. Pediatrics. 2005;116: 1339–1345. doi: 10.1542/peds.2004-2697 [DOI] [PubMed] [Google Scholar]
- 18.Longmuir PE, Tremblay MS, Goode RC. Postoperative exercise training develops normal levels of physical activity in a group of children following cardiac surgery. Pediatr Cardiol. 1990;11: 126–30. doi: 10.1007/BF02238841 [DOI] [PubMed] [Google Scholar]
- 19.Longmuir PE, Tyrrell PN, Corey M, Faulkner G, Russell L, Mccrindle BW, et al. Home-based rehabilitation enhances daily physical activity and motor skill in children who have undergone the fontan procedure. Pediatr Cardiol. 2013;34: 1130–1151. doi: 10.1007/s00246-012-0618-8 [DOI] [PubMed] [Google Scholar]
- 20.Bar-Mor G, Bar-Tal Y, Krulik T, Zeevi B. Self-efficacy and physical activity in adolescents with trivial, mild, or moderate congenital heart malformations. Cardiol Young. 2000;10: 561–566. [DOI] [PubMed] [Google Scholar]
- 21.Duppen N, Takken T, Hopman MTE, Ten Harkel ADJ, Dulfer K, Utens EMWJ, et al. Systematic review of the effects of physical exercise training programmes in children and young adults with congenital heart disease. Int J Cardiol. 2013;168: 1779–1787. Available: doi: 10.1016/j.ijcard.2013.05.086 [DOI] [PubMed] [Google Scholar]
- 22.The CONSORT Group. The CONSORT Statement (Consolidated Standards of Reporting Trials). CONSORT web site. 2010. Available: http://www.consort-statement.org/ [Google Scholar]
- 23.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: Reliability and Validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in Healthy and Patient Populations. Med Care. 2001;39: 800–12. doi: 10.1097/00005650-200108000-00006 [DOI] [PubMed] [Google Scholar]
- 24.Raat H, Landgraf JM, Oostenbrink R, Moll HA, Essink-Bot M-L. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16: 445–60. doi: 10.1007/s11136-006-9134-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Project DATA. Social Skills Checklist. St. Louis: University of Washington; 2007. Available: https://www.z2systems.com/neon/resource/msha/files/Mora_-_Social_Skills_Checklist.pdf [Google Scholar]
- 26.Folio MR, Fewell RR. Peabody Developmental Motor Scales Second Edition. Second. Toronto, Canada: Pearson; 2000. Available: https://www.pearsonassessments.com/footer/about.html [Google Scholar]
- 27.Abidin RR. Parenting Stress Index. Odessa, Florida: Psychological Assessment Resources, Inc.; 1990. [Google Scholar]
- 28.Griffiths A, Toovey T, Morgan PE, Spittle AJ. Psychometric properties of gross motor assessment tools for children: a systematic review. BMJ Open. 2018;8: e021734. doi: 10.1136/bmjopen-2018-021734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adolph AL, Puyau MR, Vohra FA, Nicklas TA, Zakeri IF, Butte NF. Validation of uniaxial and triaxial accelerometers for the assessment of physical activity in preschool children. J Phys Act Health. 2012;9: 944–53. doi: 10.1123/jpah.9.7.944 [DOI] [PubMed] [Google Scholar]
- 30.Pfeiffer KA, Dowda M, McIver KL, Pate RR. Factors related to objectively measured physical activity in preschool children. Pediatr Exerc Sci. 2009;21: 196–208. doi: 10.1123/pes.21.2.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costa S, Barber SE, Cameron N, Clemes SA. Calibration and validation of the ActiGraph GT3X+ in 2–3 year olds. J Sci Med Sport. 2014;17: 617–622. doi: 10.1016/j.jsams.2013.11.005 [DOI] [PubMed] [Google Scholar]
- 32.Karsdorp PA, Everaerd W, Kindt M, Mulder BJM. Psychological and cognitive functioning in children and adolescents with congenital heart disease: A meta-analysis. J Pediatr Psychol. 2007;32: 527–541. doi: 10.1093/jpepsy/jsl047 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(PDF)
Data Availability Statement
No datasets were generated or analysed during the current study. All relevant data from this study will be made available upon study completion.