Abstract
Mice deficient in the transmembrane protein tyrosine phosphatase CD45 exhibit a block in thymocyte development. To determine whether the block in thymocyte development was due to the inability to dephosphorylate the inhibitory phosphorylation site (Y505) in p56lck (Lck), we generated CD45-deficient mice that express transgenes for the Lck Y505F mutation and the DO11.10 T-cell antigen receptor (TCR). CD4 single-positive T cells developed and accumulated in the periphery. Treatment with antigen resulted in thymocyte apoptosis and the loss of transgenic-TCR-bearing cells. Peripheral CD45-deficient T cells from the mice expressing both transgenes responded to antigen by increasing CD69 expression, interleukin-2 production, and proliferation. These results indicate that thymocyte development requires the dephosphorylation of the inhibitory site in Lck by CD45.
CD45 is a transmembrane protein tyrosine phosphatase uniquely expressed by cells of the immune system (38). For T cells, CD45 is required for T-cell antigen receptor (TCR) signal transduction (17, 31). CD45-deficient T cells are impaired in their ability to initiate signaling in response to either antigen or TCR cross-linking. The inefficient TCR-mediated signaling in CD45-deficient cells correlates with an eight- to ninefold increase in tyrosine phosphorylation at the inhibitory site in the Src family member p56lck (Lck) (10, 34). These results suggest that CD45 regulates Lck by dephosphorylating the inhibitory site. Since it is thought that Lck is the primary kinase responsible for initiating signals through the antigen receptor, the inability to activate Lck in CD45-deficient cells appears to account for the inefficient signaling through the TCR. However, while all CD45-deficient T-cell lines exhibit increased tyrosine phosphorylation of Lck at the inhibitory site, there is discordance with regard to kinase activity; some cell lines have increased kinase activity (8, 12, 13), while others have decreased kinase activity (10, 14, 26, 27, 33, 34). The basis for this difference is unknown. Furthermore, the paradox of inefficient signaling through the TCR despite enhanced Lck kinase activity is unexplained. These contradictory findings raise the possibility that the block in TCR signaling in CD45-deficient cells is not due to the failure to dephosphorylate the inhibitory site in Lck but is attributable to a different mechanism. In principle, expression of Lck Y505F in CD45-deficient cells should rescue TCR signaling. However, expression of Lck Y505F in CD45-deficient cell lines results in rapid internalization of the TCR, thereby preventing an assessment of the ability to rescue signaling (13).
CD45-deficient mice exhibit a block in thymocyte development at the stage at which cells express both CD4 and CD8 coreceptor proteins. This is the stage in thymocyte development at which newly rearranged TCRs are expressed and signaling thresholds are determined to eliminate cells that are autoreactive or fail to generate sufficient signals. Cells that signal appropriately develop further to express either CD4 or CD8 and are released to the periphery, where they recognize antigen in the context of major histocompatibility complex class II or class I molecules, respectively. As such there are subsequently few mature T cells in the periphery of CD45-deficient mice (9, 15). As predicted by studies of cell lines, the small number of T cells that accumulate in the periphery fail to efficiently signal through the TCR. The phenotype of CD45-deficient mice is consistent with the idea that CD45 is required for TCR signaling. However, whether this is due to the regulation of Lck by CD45 has not been determined.
The importance of Lck in thymocyte development and TCR function has been demonstrated by the development of transgenic and gene-ablated mice. Mutation of the inhibitory tyrosine phosphorylation site to phenylalanine (Y505F) results in a constitutively active kinase (4, 5). Expression of the Lck Y505F mutant as a transgene driven by the lck proximal promoter results in a block in thymocyte development due to premature signaling and a subsequent failure to rearrange the TCR β-chain (1, 2, 18). Developing thymocytes do not progress past the CD4+ CD8+ double-positive stage and do not up-regulate expression of the CD3 component of the TCR. However, development can be rescued by the simultaneous transgenic expression of a TCR β-chain which, obviously, has already been rearranged (7). Further, expression of the Lck Y505F transgene overcomes the block in thymocyte development observed in Rag-deficient mice and permits progression from CD4− CD8− double-negative to CD4+ CD8+ double-positive cells (6). The generation of Lck-deficient mice has demonstrated that Lck expression is critical for signal transduction through the pre-TCR that is required during the development of CD4+ CD8+ double-positive thymocytes from their CD4− CD8− double-negative precursors (7, 24, 30). These experiments have emphasized that Lck plays a role in the TCR signaling that is required for thymocyte development.
To examine whether the regulation by CD45 of the inhibitory tyrosine phosphorylation site in Lck is critical to thymocyte development, we generated CD45-deficient mice that express Lck Y505F under the control of the lck proximal promoter (1). To overcome the block in thymocyte development caused by Lck Y505F, the DO11.10 TCR was also expressed (25). Expression of Lck Y505F in the presence of the DO11.10 TCR relieves the requirement for CD45 in thymocyte development. Our results indicate that the inhibitory site in Lck is a critical substrate of CD45 in vivo. Dephosphorylation of Lck Y505 is necessary for the signaling through the TCR that is required for thymocyte development and response to antigen.
MATERIALS AND METHODS
Antibodies.
Anti-CD3ɛ (500A2), anti-CD4 (GK1.5), and anti-CD8 (53-6.72) were obtained from the American Type Culture Collection (Rockville, Md.). FcBlock (anti-CD16/CD32 2.4G2); fluorescein isothiocyanate-conjugated anti-CD3 (145-2C11), anti-CD8 (53-6.72), anti-CD45 (30F11), anti-CD69 (H1.2F3), anti-I-Ab (AF6-120.1), anti-I-Ad (AMS-32.1), and annexin V; CyChrome-conjugated anti-CD4 (RM4-5); and biotin-conjugated anti-CD3 (145-2C11), anti-CD4 (GK1.5), and anti-Vβ8 (F23.1) were purchased from Pharmingen (San Diego, Calif.). Biotin-conjugated clonotypic anti-DO11.10 TCR (KJ1-26) has been described previously (25). Streptavidin–R-phycoerythrin was obtained from Gibco BRL (Gaithersburg, Md.). Rabbit anti-Lck antiserum was prepared against the peptide EVRDPLVYEGS LPPASPLQDN as described previously (22). Protein A-Sepharose was purchased from Sigma (St. Louis, Mo.). Monoclonal antiphosphotyrosine (4G10) was purchased from Upstate Biotechnology (Lake Placid, N.Y.). Horseradish peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) and goat anti-rabbit Ig were purchased from Caltag (San Francisco, Calif.) and Cappel (Durham, N.C.), respectively. Streptavidin was purchased from Jackson ImmunoResearch (West Grove, Pa.).
Mice.
The CD45-deficient mice, mice expressing the Y505F mutant of Lck under the control of the lck proximal promoter, and mice bearing the DO11.10 α/β TCR specific for the peptide encompassing residues 323 to 339 of chicken ovalbumin (OVA peptide 323–339) presented by I-Ed have been previously described (2, 15, 25). To generate the mice of all genotypes used in this study, matings of mice hemizygous for the Lck Y505F transgene and heterozygous for CD45 with mice hemizygous for the DO11.10 TCR transgene and homozygous for CD45 deficiency were established. All individual experiments used littermates from a single set of parents. Mice were hemizygous for the transgenes and expressed the major histocompatibility complex class II protein necessary for positive selection of the DO11.10 TCR, I-Ed. Genotypes were monitored by PCR as described elsewhere (24, 25) and were confirmed by flow cytometry. Mice were maintained in the Washington University animal facility under specific-pathogen-free conditions in accordance with institutional guidelines. All experiments were performed with mice of 4 to 6 weeks of age.
Immunoblot analysis.
Thymocytes were collected, incubated on ice from 2 to 4 h, washed in phosphate-buffered saline (pH 7.4), and lysed in 1 ml of lysis buffer (1.5% Nonidet P-40 [NP-40], 0.45% sodium deoxycholate, 25 mM Tris-HCl [pH 8], 150 mM NaCl, 0.2 mg of aprotinin/ml, 2 mM leupeptin, 5 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF, 10 mM Na4P2O7, 10 mM Na2MoO4, 50 μM phenylarsine oxide, 200 μM pervanadate, 5 mM EGTA, and 10% glycerol, with 1 mg of OVA peptide/ml). After incubation on ice for 15 min, lysates were precleared with 10 μl of a 10% solution of Pansorbin cells (Calbiochem, La Jolla, Calif.) and centrifuged at 12,000 × g and 4°C for 15 min. Antiserum to CD3ɛ, CD4, CD8, or Lck was added, and lysates were agitated for 30 min to 2 h at 4°C. Twenty-five microliters of a 50% slurry of protein A-Sepharose (Sigma) was added, and lysates were agitated at 4°C for 2 to 3 h. The Sepharose was pelleted by centrifugation, washed four times with lysis buffer, boiled in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 15% β-mercaptoethanol, and resolved on an SDS–12% polyacrylamide gel. Proteins were transferred to NitroPlus 2000 membranes (MSI) by using a Bio-Rad Transblot apparatus at 90 V for 2 h with transfer buffer (20 mM glycine, 2.5 mM Tris-OH, 20% methanol, and 50 mM Na3VO4). Membranes were blocked for 2 to 18 h in PBS (pH 7.4)–0.05% Tween (PBS-T) containing 3% bovine serum albumin (Sigma). Antibodies were diluted in PBS-T (antiphosphotyrosine monoclonal 4G10 was diluted 1:3,000; antiserum to Lck was diluted 1:1,000) and incubated with the membranes for 1 h at room temperature. Following incubation with the primary antibody, the membranes were washed twice with NP-40 wash buffer (10 mM Tris [pH 8.0]–150 mM NaCl with 1% NP-40) and once with PBS-T for 5 min; developed with the appropriate horseradish peroxidase-conjugated secondary antibodies (either goat anti-mouse IgG or goat anti-rabbit IgG), diluted 1:5,000 in PBS-T, for 30 min at room temperature; and then washed as before. Blots were visualized by enhanced chemiluminescence (ECL, Amersham, Arlington Heights, Ill.) according to the manufacturer’s instructions. For quantitation, images were scanned and quantitated by Storm PhosphorImager analysis (Molecular Dynamics Inc., Sunnyvale, Calif.).
GST fusion protein.
The cDNA encoding the Lck SH2 domain was ligated in frame to DNA encoding glutathione S-transferase (GST) and was a gift from Andy Chan (Washington University, St. Louis, Mo.). The Lck SH2 domain-GST fusion protein was produced in Escherichia coli BL21. The fusion protein was induced in log-phase bacterial cultures by addition of 100 μM isopropyl-1-thio-β-d-galactoside, and culture incubation was continued at room temperature overnight with vigorous shaking. Bacteria were pelleted and lysed by rapid freezing and thawing followed by sonication in a buffer containing 50 mM HEPES (pH 7.4), 300 mM NaCl, 0.5 mM EDTA, 0.1% β-mercaptoethanol, 20 μg of aprotinin/ml, 2 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1% Triton X-100. Debris was removed by centrifugation, and lysates were agitated with glutathione-agarose beads (Sigma) for 1 h at 4°C. The beads were washed twice in a buffer containing 20 mM HEPES (pH 7.4), 25 mM NaCl, 0.1% β-mercaptoethanol, 0.5% Triton X-100, and 10% glycerol and twice in PBS. Bound GST fusion proteins were eluted in buffer containing 50 mM Tris-HCl (pH 8.0) and 10 mM glutathione and exhaustively dialyzed against PBS. The concentration and purity were assessed by SDS-PAGE. Purified GST fusion proteins coupled to glutathione beads were added to cell lysates, and associated proteins were resolved as described above.
Thymocyte stimulation by antibody cross-linking.
Single-cell suspensions of thymocytes were prepared and resuspended at a density of 108 cells/ml in PBS. Biotinylated anti-CD3ɛ (2C11) and anti-CD4 (GK1.5) antibodies were added to the cells at a final concentration of 10 μg/ml. The cells were incubated on ice for 10 min, washed in PBS, and resuspended to the original concentration in PBS. Cross-linking was performed by addition of streptavidin to a final concentration of 10 μg/ml. Cells were incubated at 37°C for 5 min, pelleted, and lysed as described above.
Kinase assays.
Immune complexes prepared as described above were washed twice with lysis buffer, once with 10 mM NaHPO4 (pH 7.4)–150 mM NaCl, and once with kinase reaction buffer (25 mM HEPES [pH 7.4], 150 mM NaCl, 5 mM MgCl2, 5 mM MnCl2, 1 mM CaCl2, 0.05 mg of aprotinin/ml, 0.5 mM leupeptin, 10 mM NaF, 10 mM Na2MoO4, 200 μM pervanadate, 20 mM ATP, and 1 mM dithiothreitol). Immunoprecipitates were resuspended in 15 μl of a kinase buffer containing 10 μCi of [γ-32P]ATP (6,000 Ci/mmol; Amersham) and 10 μg of acid- and heat-denatured enolase and incubated for 15 min at room temperature. Reactions were stopped by the addition of SDS-PAGE sample buffer and analyzed by gel electrophoresis as described above. After transfer of proteins to nitrocellulose, radiolabel incorporation was quantitated by Storm PhosphorImager analysis (Molecular Dynamics).
Flow-cytometric analysis.
Cells (5 × 105) were prepared as described above, washed twice with staining buffer (PBS containing 0.02% bovine serum albumin and 0.01% NaN3), preincubated with FcBlock, and stained with 0.5 μg of fluorochrome- or biotin-conjugated antibody for 30 min on ice. Cells were washed once with staining buffer, restained with streptavidin-phycoerythrin as appropriate, rewashed, gravity filtered through a 35-μm-pore-size cell strainer cap (Falcon, Lincoln Park, N.J.), and analyzed on a FACScan flow cytometer and with CellQuest software (Becton Dickinson, Franklin Lakes, N.J.). A minimum of 15,000 events were collected for each sample (50,000 for three-color analysis).
In vivo peptide treatment.
OVA peptide was injected as described elsewhere (25). Briefly, either OVA peptide 323–339 or OVA peptide 324–334 was administered in 250 μl of a sterile 100 μM solution by intraperitoneal injection. Twenty hours postinjection, thymi from treated mice were prepared and analyzed by flow cytometry as described above, with three-color analysis with annexin V, KJ1-26, and propidium iodide, in accordance with the manufacturer’s instructions for staining with annexin V.
Splenocyte stimulation.
Splenocytes (4 × 105) were prepared as described above and treated with OVA peptides as described elsewhere (25). Briefly, splenocytes were incubated with serial dilutions of OVA peptide 323–339 or OVA peptide 324–334 and examined at 12 h by flow cytometry as described above, using two-color analysis with anti-CD69 and KJ1-26. In one series, 100-μl volumes were harvested at 24 h for interleukin-2 (IL-2) production by a cytotoxic T-lymphocyte bioassay. The second series was labeled with 0.4 μCi of [3H]thymidine (3,000 Ci/mmol; Amersham) at 48 h. Cultures were harvested after overnight labeling to assay for cellular proliferation.
RESULTS
Dysregulation of Lck in CD45-deficient thymocytes.
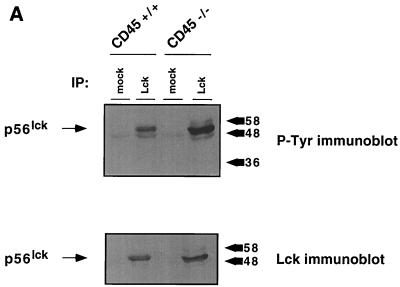
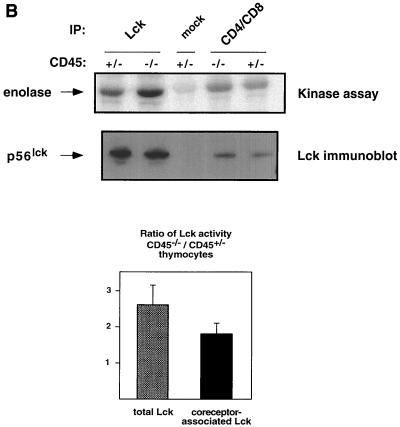
CD45-deficient T cells are blocked in TCR-mediated signaling and exhibit increased tyrosine phosphorylation at the negative regulatory site of Lck (Y505) (10, 33, 34). Since Lck is required to initiate TCR signaling (36), it seems likely that the increased phosphorylation at the inhibitory site in CD45-deficient cells accounts for the inability to signal through the TCR. Despite this increase in phosphorylation at the inhibitory site, the effect on kinase activity is unclear. Some CD45-deficient T cells exhibit increased Lck kinase activity, while others exhibit a decreased level of activity (10, 13). To determine whether CD45-deficient thymocytes also exhibit dysregulation of Lck, we immunoprecipitated Lck either directly or through its association with CD4 and CD8. Lck showed increased phosphotyrosine content (Fig. 1A) as observed in other CD45-deficient mouse T-cell lines (11, 35). Interestingly, in contrast to the decreased Lck kinase activity observed in most CD45-deficient T-cell lines (10, 17, 26, 28, 33), kinase activity increased 1.5- to 2.5-fold as judged by phosphorylation of the exogenous substrate enolase after normalization for protein loading (Fig. 1B). Since Lck kinase activity is increased in CD45-deficient thymocytes, it is possible that an increase in phosphorylation at the inhibitory site does not account for the block in thymocyte development.
FIG. 1.
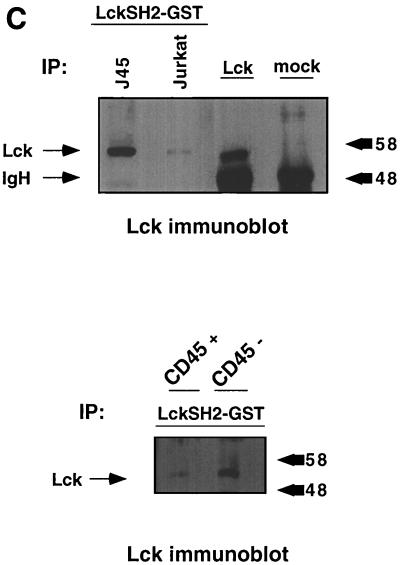
Lck kinase activity is increased in CD45-deficient thymocytes despite increased phosphorylation at the inhibitory site. (A) Thymocytes from CD45-deficient mice or their normal littermates were lysed and immunoprecipitated (IP) with anti-Lck antiserum CD4 and CD8 monoclonal antibodies together or with isotype-matched control antibodies (mock). Immunoprecipitates were separated by SDS-PAGE and immunoblotted with either anti-Lck antiserum or antiphosphotyrosine (P-Tyr) monoclonal antibody. The numbers on the right represent relative molecular weight (in thousands). (B) In vitro kinase reaction using enolase as an exogenous substrate for Lck immunoprecipitated from CD45-expressing or -deficient thymocytes. Lck kinase activity was determined for total cellular Lck or Lck bound to CD4 and CD8. The bar graph summarizes the results of three separate experiments showing fold increases and standard errors of the mean after normalization for Lck protein. (C) A GST-Lck SH2 domain fusion protein was used to isolate Lck phosphorylated at Y505. Lysates were agitated with the GST-Lck SH2 domain fusion protein coupled to beads, and bound proteins were eluted in sample buffer and separated by SDS-PAGE. Lck was visualized by immunoblot analysis with anti-Lck antiserum. Upper panel, Jurkat cells and the CD45-deficient Jurkat derivative J.45; lower panel, thymocytes from CD45-expressing and -deficient thymocytes.
The increased Lck kinase activity observed in thymocytes from the CD45-deficient mice suggests that in contrast to the results obtained in CD45-deficient cells, the increase in Lck tyrosine phosphorylation may not be due to increased tyrosine phosphorylation at the inhibitory site. Rather, the increased tyrosine phosphorylation may be at the autophosphorylation site, the site that potentiates kinase activity. To examine this issue, we used a GST fusion protein containing the SH2 domain of Lck to isolate Lck phosphorylated at the inhibitory site. Since the SH2 domain binds to the phosphorylated Y505 inhibitory site, interaction of Lck with the GST-SH2 domain fusion protein provides a measure of those Lck molecules phosphorylated at the inhibitory site. The fusion protein does not bind to the autophosphorylation site. This technique has previously been used to demonstrate increased tyrosine phosphorylation at the inhibitory site in CD45-deficient cells (34). To verify the technique, the GST-SH2 domain fusion protein was used to isolate Lck from the CD45-deficient Jurkat cell line J.45 (16). As expected, there was more Lck isolated from J.45 cells by using the GST-SH2 domain fusion protein than was isolated from Jurkat cells (Fig. 1C), indicating increased tyrosine phosphorylation at the inhibitory site in the absence of CD45. Similar results were obtained with thymocyte lysates from CD45-deficient mice and littermate controls. Thus, despite the increase in kinase activity in the CD45-deficient thymocytes, there is increased phosphorylation at the inhibitory site.
Expression of Lck Y505F rescues thymocyte maturation.
To examine whether the increase in phosphorylation at the negative inhibitory site in Lck accounts for the block in thymocyte development, we expressed the Lck Y505F transgene in CD45 exon 6-deficient mice bearing a transgenic DO11.10 TCR (25).
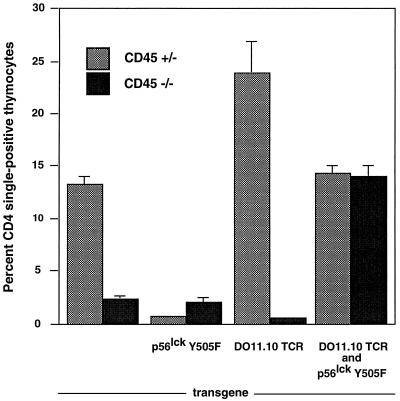
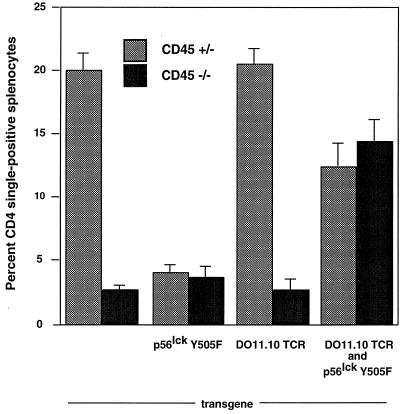
The Lck Y505F mutation by itself results in an active kinase and causes a block in thymocyte development due to premature signaling and a failure to rearrange the TCR β-chain (3). As expected, Lck kinase activity is increased in thymocytes bearing the Lck Y505F transgene (data not shown). However, the developmental block in Lck Y505F-transgenic mice can be overcome by simultaneously expressing a TCR transgene (6). These results were confirmed in subsequent experiments (Fig. 2). Similar to a previous report, mice with a functional allele of CD45 expressing Lck Y505F showed a loss of CD4 single positives; however, some CD8 single-positive thymocytes developed (4). CD4 single-positive cells were increased in the thymi of CD45-heterozygous mice expressing the DO11.10 transgene compared to their level in the thymi of normal mice. In contrast, CD45-deficient animals with either the DO11.10 TCR or Y505F Lck transgene produced negligible numbers of CD4 single-positive thymocytes. However, simultaneous expression of the DO11.10 TCR and Y505F Lck transgenes in CD45-deficient mice resulted in an increased proportion of CD4 single-positive cells in the thymus (Fig. 2). The proportions are similar to that observed in normal mice, despite the absence of any statistically significant change in total thymocyte number in mice of any genotype (data not shown). Staining with antibodies to CD3, the DO11.10 TCR, and the DO11.10 β-chain Vβ8 indicated that all were expressed on CD4 single-positive thymocytes regardless of the genotype (Table 1 and data not shown). Mice expressing the Lck Y505F transgene continued to exhibit the development of CD8 single-positive thymocytes in the absence of CD45. However, these cells did not express the DO11.10 transgene, and they expressed only low levels of CD3 (data not shown).
FIG. 2.
Rescue of CD4+ CD8+ double-positive thymocyte development in CD45-deficient Lck Y505F- and DO11.10 TCR-transgenic mice. Two-color cytometric analysis for CD4 and CD8 expression was performed on primary thymocytes. Percentages of CD4 single-positive thymocytes and standard errors of the mean for each genotype are graphed. Each data set contained 25,000 gated events. Data shown are representative results of from three to six experiments.
TABLE 1.
Percentages of DO11.10 TCRhi CD4+ CD8− thymocytesa
| Transgene(s) | % of TCRhi CD4+ CD8− thymocytes with genotype:
|
|
|---|---|---|
| CD45+/− | CD45−/− | |
| DO11.10 | 81.3 | 27.2 |
| DO11.10 and Lck Y505F | 68.4 | 58.9 |
Thymocytes were analyzed by three-color fluorescent flow cytometry for CD4, CD8, and DO11.10 TCR expression. Events were gated for CD4+ CD8− cells, and the percentage of DO11.10 TCRhi cells within the gate was determined.
Antigen-induced apoptosis in CD45-deficient, Lck Y505F-, DO11.10 TCR-transgenic mice.
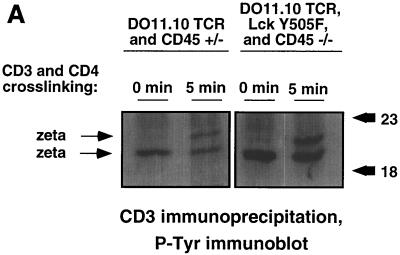
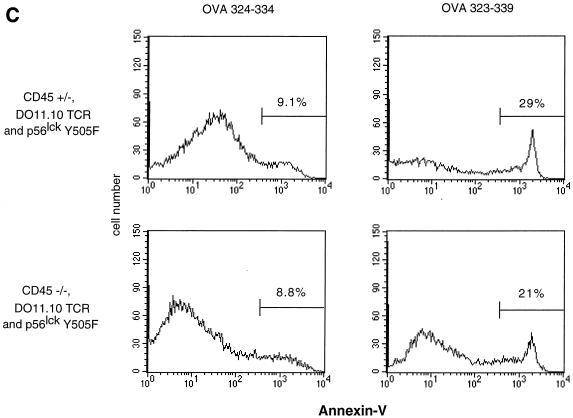
The occurrence of thymocyte maturation suggested functional reconstitution of TCR signaling in CD45-deficient mice expressing Lck Y505F and DO11.10 transgenes (21). This is supported by the demonstration of the ability to induce increased tyrosine phosphorylation of the TCR ζ-chain in response to antigen receptor cross-linking in DO11.10 and Lck Y505 transgene-expressing CD45-deficient thymocytes (Fig. 3A). The TCR ζ-chain exhibits basal tyrosine phosphorylation, and increased phosphorylation upon activation results in the appearance of a more slowly migrating form on SDS-PAGE gels. This indicates that the expression of Lck Y505F was having a direct effect on TCR signaling rather than altering thymocyte development through other mechanisms. To confirm this observation, we injected the antigenic OVA peptide 323–339, which is recognized by the DO11.10 TCR. Engagement of OVA peptide 323–339 by the DO11.10 TCR increases signaling during development and induces the elimination of these potentially autoreactive cells by thymic apoptosis and the rapid loss of transgenic TCR-bearing thymocytes (25). Mice were injected with either OVA peptide 323–339 or the nonantigenic OVA peptide 324–334 control (which binds H-2d class II molecules but does not stimulate the DO11.10 TCR). Thymocytes were harvested 20 h postinjection and analyzed by three-color flow cytometry with KJ1-26 anti-clonotypic antibody, annexin V, and propidium iodide. Consistent with previous results (25), thymocytes from animals with a functional allele of CD45 treated with OVA peptide 323–339 exhibited a dramatic decrease in the number of live cells expressing high levels of the DO11.10 TCR (Fig. 3B). These thymocytes also exhibited a concomitant increase in apoptosis of the remaining TCR-bearing thymocytes, as measured by annexin V binding (Fig. 3C). Genotype-matched littermates treated with the control OVA peptide 324–334 retained their TCRhi thymocytes and did not display increased annexin V staining. CD45-deficient, TCR-transgenic mice lacking the Lck Y505F transgene showed no change in TCR expression levels or annexin V binding in response to treatment with either peptide (data not shown). However, antigen-treated thymocytes from mice bearing both transgenes on the CD45-deficient background responded with a decrease in TCR expression and increased annexin V staining. Interestingly, a portion of the Lck Y505- and DO11.10-expressing CD45-deficient thymocytes responded by increasing TCR expression, perhaps indicating a selection of previously unresponsive thymocytes due to diminished TCR signaling. These results indicate that the transgenic antigen receptor in these animals is functional and retains its specificity.
FIG. 3.
Functional thymocyte antigen receptor in the absence of CD45 expression. (A) Thymocytes from CD45-deficient mice or littermate controls were activated, lysed, and immunoprecipitated with monoclonal anti-CD3 (500A2). Immunoprecipitates from equal number of cells were separated by SDS-PAGE and immunoblotted with antiphosphotyrosine (P-Tyr) monoclonal antibody. The numbers on the right represent relative molecular weights (in thousands). (B) Antigenic (OVA 323–339) or nonantigenic (OVA 324–334) peptide was administered by intraperitoneal injection. Thymocytes from treated mice were analyzed 20 h postinjection by three-color flow cytometry with annexin V, KJ1-26 (anti-DO11.10 TCR), and propidium iodide. KJ1-26 stained cells that were propidium iodide negative. (C) Annexin V staining of KJ1-26-positive, propidium iodide-negative cells. The analysis included 35,000 initial events. Data shown are representative of results from three separate experiments.
Accumulation of mature CD45-deficient Lck Y505F T cells in the periphery.
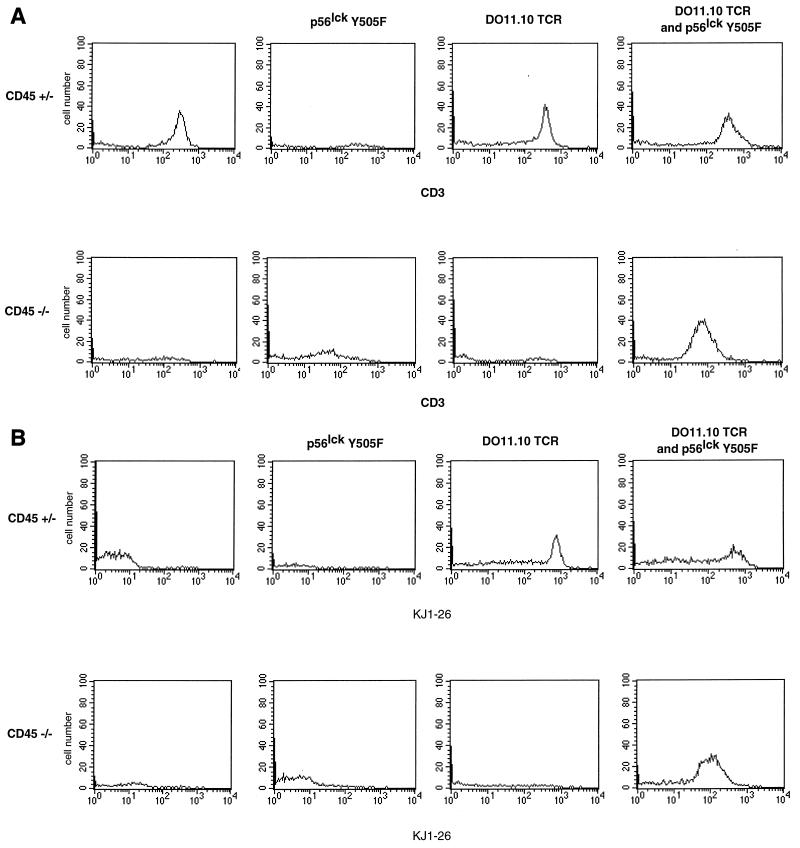
To determine whether reconstitution of thymic development was reflected in the periphery, we analyzed splenocytes by two-color flow cytometry. As in the thymus, CD4 single-positive T cells accumulated in the periphery of CD45-deficient animals bearing the DO11.10 TCR and Lck Y505F transgenes in proportions similar to those found in normal mice and in contrast to the proportions evident in CD45-deficient mice bearing only the transgenic DO11.10 TCR (Fig. 4). Additional three-color analysis permitted a comparison of antigen receptor expression levels on CD4 single-positive splenocytes. CD4 single-positive T cells from mice with a functional CD45 allele and the DO11.10 transgene expressed CD3, the TCR clonotype, and Vβ8. However, in the CD45-deficient animals, only those mice that expressed both the DO11.10 and Lck Y505F transgenes had peripheral T cells expressing the antigen receptor (Fig. 5). As in the thymus, only the combination of the TCR and Lck Y505F transgenes on the CD45-deficient background gave rise to mature CD4 single-positive, antigen receptor-bearing T cells.
FIG. 4.
Accumulation of mature peripheral T cells in CD45-deficient Lck Y505F- and DO11.10 TCR-transgenic mice. Two-color cytometric analysis for CD4 and CD8 expression was performed on primary splenocytes. Percentages of CD4 single-positive splenocytes and standard errors of the mean for each genotype are graphed. Each data set contained 25,000 gated events. Data shown are representative of results of from three to six experiments.
FIG. 5.
Appearance of peripheral CD4 single-positive T cells in CD45-deficient Lck Y505F- and DO11.10 TCR-transgenic mice. (A) CD3 expression on CD4 single-positive splenocytes. Shown are the results of a three-color cytometric analysis of CD4, CD8, and CD3. Data were gated for CD4 single-positive cells. The analysis included 50,000 initial events. Data shown are representative of results of from three to six separate experiments. (B) Clonotypic TCR expression on CD4 single-positive splenocytes. Shown are the results of a three-color cytometric analysis of CD4, CD8, and KJ1-26 (anti-DO11.10 TCR). Data were gated as described above. The analysis included 50,000 initial events. Data shown are representative of results of from three to six separate experiments.
Functional TCR in CD45-deficient, Lck Y505F-transgenic cells.
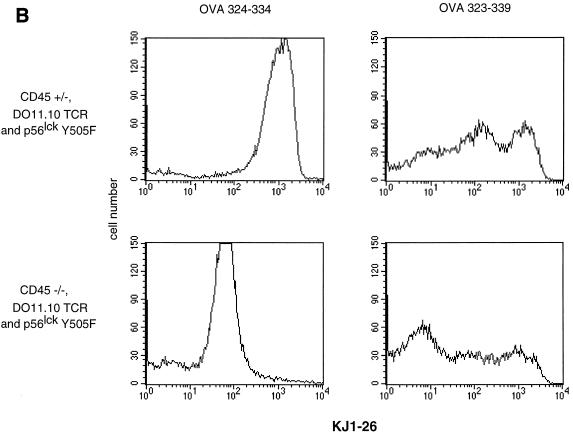
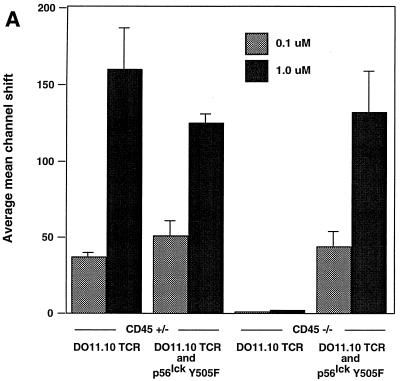
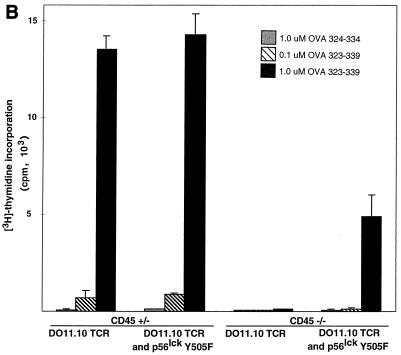
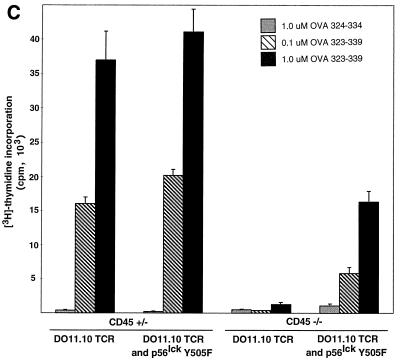
To determine whether the transgenic TCR expressed by peripheral CD45-deficient, Lck Y505F-transgenic T cells was able to transduce a specific signal in response to antigen, cells were treated with serial dilutions of either OVA peptide 323–339 or OVA peptide 324–334. The cells were then examined at 12 h for the induction of the early T-cell activation marker CD69. Treatment of DO11.10 TCR-expressing splenocytes with OVA peptide 323–339 led to a dose-dependent increase of CD69 expression on DO11.10 TCR-positive splenocytes that contained a functional allele of CD45 or were CD45 deficient and expressed Lck Y505F (Fig. 6A). CD45-deficient splenocytes lacking the Lck Y505F transgene did not respond to OVA peptide 323–339 (Fig. 6A). Similarly, the CD45-bearing and -deficient cells with both transgenes produced IL-2, whereas the CD45-deficient cells that lacked the Lck Y505F transgene did not produce IL-2 (Fig. 6B). Furthermore, [3H]thymidine labeling of splenocyte cultures revealed a dose-dependent proliferation at 72 h in the CD45-bearing and -deficient animals with both the Lck Y505F and DO11.10 transgenes (Fig. 6C). The truncated OVA peptide 324–334 did not elicit any response in cells of any genotype, consistent with the retention of antigen specificity by the transgenic TCR. The decreased proliferation observed in the CD45-deficient, double-transgenic splenocytes may reflect the lower level of TCR expression or may be due to a decrease in lck proximal promoter activity in the periphery, leading to a reduction in levels of the Lck Y505F mutant. However, Lck immunoprecipitated from splenocytes of Lck Y505F-transgenic mice exhibited increased kinase activity (data not shown), consistent with persistent peripheral expression of the transgene. These results are consistent with the presence of a functional and regulated TCR despite the absence of CD45.
FIG. 6.
Functional TCR in peripheral T cells in the absence of CD45 expression. (A) Increased CD69 expression in response to antigen. Splenocytes (4 × 105) were incubated with serial dilutions of an antigenic (OVA 323–339) or a nonantigenic (OVA 324–334) peptide and examined at 12 h by two-color flow cytometry with anti-CD69 and KJ1-26 (anti-DO11.10 TCR). Data are plotted as mean channel shifts, upon treatment with antigenic peptide, of CD69 expression beyond the basal level on DO11.10 TCR-expressing T cells. The analysis included 25,000 initial events. Data represent the averages and standard errors of the mean of values from three separate experiments. (B) IL-2 production in response to antigen. Supernatants from splenocyte cultures treated with antigenic or nonantigenic peptide were harvested at 24 h. IL-2 production was assayed by proliferation of cytotoxic T-lymphocyte line cells as measured by [3H]thymidine incorporation. Standard deviations within triplicate wells are shown. Data shown are representative of results of three separate experiments. (C) Proliferation in response to antigen. Splenocyte cultures were treated with antigenic or nonantigenic peptide, [3H]thymidine was added at 48 h, and cells were harvested at 72 h. Standard deviations of triplicate wells are shown. Data shown are representative of results of three separate experiments.
DISCUSSION
We showed that the generation of CD45-deficient mice expressing transgenes for both the Lck Y505F mutation and the DO11.10 TCR rescues thymocyte development and reconstitutes antigen-specific TCR signaling. T cells from these mice develop in the thymus, express antigen receptor and coreceptor, and accumulate in the periphery in normal proportions. The specific antigen peptide induces thymocyte apoptosis and in peripheral T cells induces activation and proliferation.
Our results provide evidence that the block in TCR signaling in CD45-deficient T cells is due to the inability to dephosphorylate the inhibitory site in Lck. Previously, it has been documented that the absence of CD45 in lymphocyte cell lines correlates with an increase in phosphotyrosine at the inhibitory site in Lck (10, 33, 34). Our present study demonstrates that CD45 regulation of the inhibitory phosphotyrosine is critical to thymocyte development. The presence of Lck Y505F permits CD45-independent TCR signaling. The failure of CD45-deficient thymocytes to develop past the CD4+ CD8+ double-positive stage is not relieved by the presence of a transgenic TCR, although the proportion of double-negative thymocytes does decrease (29). Since positive selection requires a signal through the antigen receptor, our data support the idea that the primary role for CD45 in the development of mature T cells is the dephosphorylation of Lck Y505.
Despite the observed increase in phosphorylation at the inhibitory site in Lck, some CD45-deficient cell lines exhibit enhanced kinase activity (12, 40). In agreement with this, we observe elevated Lck kinase activity in thymocytes from the CD45-deficient mouse (Fig. 1B). Increased Lck kinase activity was also recently observed in a separate line of CD45-deficient mice (11). Yet we demonstrated that despite the increased Lck kinase activity in CD45-deficient thymocytes, there is increased phosphorylation at the inhibitory site (Fig. 1C). How can there be increased Src family kinase activity in the presence of increased phosphorylation at the inhibitory site? A potential answer is that this reflects the pool of Src kinases being measured. Experiments that measure the phosphorylation state of the inhibitory site examine the total pool of a particular Src kinase in the cell. In contrast, assays of kinase activity will reflect changes in the subset of kinases associated with engaged receptors. Thus, in CD45-deficient thymocytes, CD45 functions to dephosphorylate the inhibitory site during steady state of the entire Lck pool. Our data support the notion that dephosphorylation of the inhibitory site by CD45 is essential for signaling through the TCR. However, Src family kinases function to regulate receptors other than antigen receptors, such as integrins. If CD45 functions to negatively regulate those receptors, elevation of kinase activity will be observed in the absence of CD45. In support of this idea, it has been demonstrated that CD45 functions in macrophages as a negative regulator of Src family kinases associated with β2 integrin-mediated adhesion (32). The increased Lck kinase activity in CD45-deficient thymocytes may be due to the lack of negative regulation of Lck by CD45 associated with receptors other than antigen receptors. Thus, it has been proposed that CD45 can function simultaneously as a positive and negative regulator of Src family kinase activity depending on whether CD45 is excluded from associating with engaged receptors, e.g., antigen receptors, or associates with engaged receptors, e.g., integrins (37).
Creating high-affinity binding sites for the SH2 and SH3 domains (20, 23) can activate Src family kinases. However, Src family kinases associated with the antigen-receptor cascade are not likely to be activated by an upstream mechanism. An Src family kinase is thought to be the initial kinase in the TCR signaling cascade, and thus dephosphorylation of the inhibitory site is essential (39). This may not be the case for Src family kinases associated with other receptors, for example integrins. Nonetheless, our data indicate that dephosphorylation of the inhibitory site is essential for signaling through the TCR. This supports the idea that CD45 can simultaneously function as a positive and negative regulator of Src family kinases depending on differential assortment with various receptors.
In T cells, Lck associates with the CD4 and CD8 TCR coreceptors, at which it is thought to function in TCR signal transduction (39). This is supported by the absence of TCR signaling in Lck-deficient cells lines (36), as well as in the very small number of peripheral T cells isolated from Lck-deficient mice (24). Lck expression is also required for signal transduction through the pre-TCR involved in the development of CD4+ CD8+ double-positive thymocytes from their CD4− CD8− double-negative precursors (7). Overexpression of kinase-dead mutants of Lck acts as a dominant-negative mutation on the pre-TCR signal and abolishes allelic exclusion at the TCR β-locus, blocking thymocyte development at the double-negative stage and preventing the expression of TCR α- and β-chains (7, 19). Mice bearing the activated Lck Y505F transgene are blocked at the CD4+ CD8+ double-positive stage during thymocyte development and exhibit little TCR α- or β-chain rearrangement. This developmental block is overcome (in contrast to the situation in the kinase-dead transgenics) by expression of a transgenic TCR β-chain (1). Thus, Lck influences both the development and activation of T cells.
Up-regulation of Lck kinase activity has been shown to correlate with a decrease in the surface expression of the TCR (13). Our data support this observation. There was decreased DO11.10 TCR expression in Lck Y505F-transgenic CD45-deficient thymocytes and splenocytes (Fig. 3A and 5). This suggests that activated Lck causes internalization of the antigen receptor. The results of T-cell line studies involving Lck Y505F and tyrosine kinase inhibitors are consistent with the increased TCR expression observed in Lck-deficient mice (24). This may explain the decrease in the absolute level of DO11.10 TCR expression that we observed in the thymi and spleens of animals expressing Lck Y505F along with the transgenic TCR (Fig. 3A and 4A). The decrease in TCR levels in these mice was more pronounced in the CD45-deficient mice, consistent with results previously observed in studies using cell lines (13). Further, the decreased surface expression of TCR may account for the decreased proliferative response seen during stimulation of splenocytes from these mice. This reduced proliferation may also reflect a loss of Lck Y505F transgene expression in the periphery due to decreased activity of the lck proximal promoter, although we observed increased Lck kinase activity in the splenocytes of Lck transgene-bearing mice, consistent with persistent expression of the Lck transgene (data not shown).
The Lck Y505F transgene is sufficient for the reconstitution of DO11.10 antigen receptor signaling. This suggests that the Y505 site of Lck is a critical substrate of CD45 in vivo. Furthermore, our data indicate that dephosphorylation of Lck Y505 is the specific mechanism for the positive regulation of the TCR complex and that the failure to dephosphorylate Y505 in CD45-deficient mice is the cause for the block in thymocyte development.
ACKNOWLEDGMENTS
This research was supported by grants from the National Institutes of Health. J.R.S. is supported by the Division of Biology and Biomedical Sciences, Washington University. M.L.T. and K.M.M. are investigators of the Howard Hughes Medical Institute.
We thank our colleagues for comments and suggestions during the course of this research. We especially thank Paul Allen and Andy Chan for gifts of reagents and help with the ζ-chain and Y505 phosphorylation experiments, respectively.
REFERENCES
- 1.Abraham K M, Levin S D, Marth J D, Forbush K A, Perlmutter R M. Delayed thymocyte development induced by augmented expression of p56lck. J Exp Med. 1991;173:1421–1432. doi: 10.1084/jem.173.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham K M, Levin S D, Marth J D, Forbush K A, Perlmutter R M. Thymic tumorigenesis induced by overexpression of p56lck. Proc Natl Acad Sci USA. 1991;88:3977–3981. doi: 10.1073/pnas.88.9.3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham N, Miceli M C, Parnes J R, Veillette A. Enhancement of T-cell responsiveness by the lymphocyte-specific tyrosine kinase p56lck. Nature. 1991;350:62–66. doi: 10.1038/350062a0. [DOI] [PubMed] [Google Scholar]
- 4.Abraham N, Veillette A. Activation of p56lck through mutation of a regulatory carboxy-terminal tyrosine residue requires intact sites of autophosphorylation and myristylation. Mol Cell Biol. 1990;10:5197–5206. doi: 10.1128/mcb.10.10.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amrein K E, Sefton B M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci USA. 1988;85:4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson S J, Abraham K M, Nakayama T, Singer A, Perlmutter R M. Inhibition of T-cell receptor β-chain gene rearrangement by overexpression of the non-receptor protein tyrosine kinase p56lck. EMBO J. 1992;11:4877–4886. doi: 10.1002/j.1460-2075.1992.tb05594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson S J, Levin S D, Perlmutter R M. Protein tyrosine kinase p56lck controls allelic exclusion of T-cell receptor β-chain genes. Nature. 1993;365:552–554. doi: 10.1038/365552a0. [DOI] [PubMed] [Google Scholar]
- 8.Burns C M, Sakaguchi K, Appella E, Ashwell J D. CD45 regulation of tyrosine phosphorylation and enzyme activity of src-family kinases. J Biol Chem. 1994;269:13594–13600. [PubMed] [Google Scholar]
- 9.Byth K F, Conroy L A, Howlett S, Smith A J H, May J, Alexander D R, Holmes N. CD45-null transgenic mice reveal a positive regulatory role for CD45 in early thymocyte development, in the selection of CD4+ CD8+ thymocytes, and in B cell maturation. J Exp Med. 1996;183:1707–1718. doi: 10.1084/jem.183.4.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahir McFarland E D, Hurley T R, Pingel J T, Sefton B M, Shaw A, Thomas M L. Correlation between Src-family member regulation by the protein tyrosine phosphatase, CD45, and transmembrane signaling through the T-cell receptor. Proc Natl Acad Sci USA. 1993;90:1402–1406. doi: 10.1073/pnas.90.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Oro U, Ashwell J D. The CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol. 1999;162:1879–1883. [PubMed] [Google Scholar]
- 12.D’Oro U, Sakaguchi K, Appella E, Ashwell J D. Mutational analysis of Lck in CD45-negative T cells: dominant role of tyrosine 394 phosphorylation in kinase activity. Mol Cell Biol. 1996;16:4996–5003. doi: 10.1128/mcb.16.9.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Oro U, Vacchio M S, Weissman A M, Ashwell J D. Activation of the Lck tyrosine kinase targets cell surface T cell antigen receptors for lysosomal degradation. Immunity. 1997;7:619–628. doi: 10.1016/s1074-7613(00)80383-0. [DOI] [PubMed] [Google Scholar]
- 14.Hurley T R, Hyman R, Sefton B M. Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol Cell Biol. 1993;13:1651–1656. doi: 10.1128/mcb.13.3.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishihara K, Penninger J, Wallace V A, Kundig T M, Kawai K, Wakeham A, Timms E, Pfeffer K, Ohashi P S, Thomas M L, Furlonger C, Paige C J, Mak T W. Normal B lymphocyte development but impaired T cell maturation in CD45-exon 6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 16.Koretzky G A, Picus J, Schultz T, Weiss A. Tyrosine phosphatase CD45 is required for T-cell antigen receptor and CD2-mediated activation of a protein tyrosine kinase and interleukin 2 production. Proc Natl Acad Sci USA. 1991;88:2037–2041. doi: 10.1073/pnas.88.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koretzky G A, Picus J, Thomas M L, Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyl inositol pathway. Nature. 1990;346:66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- 18.Levin S D, Abraham K M, Anderson S J, Forbush K A, Perlmutter R M. The protein tyrosine p56lck regulates thymocyte development independently of its interaction with CD4 and CD8 coreceptors. J Exp Med. 1993;178:245–255. doi: 10.1084/jem.178.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levin S D, Anderson S J, Forbush K A, Perlmutter R M. A dominant-negative transgene defines a role for p56lck in thymopoiesis. EMBO J. 1993;12:1671–1680. doi: 10.1002/j.1460-2075.1993.tb05812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Brodeur S R, Gish G, Songyang Z, Cantley L C, Laudano A P, Pawson T. Regulation of c-Src tyrosine kinase activity by the Src SH2 domain. Oncogene. 1993;8:1119–1126. [PubMed] [Google Scholar]
- 21.Loh D Y, Sha W C, Nelson C A, Newberry R D, Kranz D M, Russell J H. Positive and negative selection of T lymphocytes. Cold Spring Harbor Symp Quant Biol. 1989;54:147–151. doi: 10.1101/sqb.1989.054.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Marth J D, Lewis D B, Wilson C B, Gearn M E, Krebs E G, Perlmutter R M. Regulation of pp56lck during T cell activation: functional implications for the src-like protein tyrosine kinases. EMBO J. 1987;6:2727–2734. doi: 10.1002/j.1460-2075.1987.tb02566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee C H, Kuriyan J, Miller W T. Activation of the Src-family tyrosine kinase Hck by SH3 domain displacement. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 24.Molina T J, Kishihara K, Siderorski D P, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige C J, Hartmann K-U, Veillette A, Davidson D, Mak T W. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 25.Murphy K M, Heimberger A B, Loh D Y. Induction by antigen of intrathymic apoptosis of CD4+ CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 26.Mustelin T, Coggeshall K M, Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci USA. 1989;86:6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ostergaard H L, Schackelford D A, Hurley T R, Johnson P, Hyman R, Sefton B M, Trowbridge I S. Expression of CD45 alters phosphorylation of the lck-encoded tyrosine protein kinase in murine lymphoma T-cell lines. Proc Natl Acad Sci USA. 1989;86:8959–8963. doi: 10.1073/pnas.86.22.8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ostergaard H L, Trowbridge I S. Coclustering CD45 with CD4 or CD8 alters the phosphorylation and kinase activity of p56lck. J Exp Med. 1990;172:347–350. doi: 10.1084/jem.172.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penninger J, Wallace V, Kishihara K, Mak T. The role of p56lck and p59fyn tyrosine kinases and CD45 protein tyrosine phosphatase in T-cell development and clonal selection. Immunol Rev. 1993;135:183–214. doi: 10.1111/j.1600-065x.1993.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 30.Perlmutter R M, Levin S D, Appleby M W, Anderson S J, Alberola-Ila J. Regulation of lymphocyte function by protein phosphorylation. Annu Rev Immunol. 1993;11:451–499. doi: 10.1146/annurev.iy.11.040193.002315. [DOI] [PubMed] [Google Scholar]
- 31.Pingel J T, Thomas M L. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989;58:1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roach T I A, Slater S E, Koval M, White L, Cahir McFarland E D, Okumura M, Thomas M L, Brown E J. CD45 regulates Src-family member kinase activity associated with macrophage integrin-mediated adhesion. Curr Biol. 1997;7:408–417. doi: 10.1016/s0960-9822(06)00188-6. [DOI] [PubMed] [Google Scholar]
- 33.Shiroo M, Goff L, Biffen M, Shivnan E, Alexander D. CD45 tyrosine phosphatase-activated p59fyn couples the T cell antigen receptor to pathways of diacylglycerol production, protein kinase C activation and calcium influx. EMBO J. 1992;11:4887–4897. doi: 10.1002/j.1460-2075.1992.tb05595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sieh M, Bolen J B, Weiss A. CD45 specifically modulates binding of Lck to a phosphopeptide encompassing the negative regulatory tyrosine of Lck. EMBO J. 1993;12:315–321. doi: 10.1002/j.1460-2075.1993.tb05659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stone J D, Conroy L A, Byth K F, Hederer R A, Howlett S, Takemoto Y, Holmes N, Alexander D R. Aberrant TCR-mediated signaling in CD45-null thymocytes involves dysfunctional regulation of Lck, Fyn, TCR-ζ, and ZAP-70. J Immunol. 1997;158:5773–5782. [PubMed] [Google Scholar]
- 36.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 37.Thomas, M. L., and E. J. Brown. Positive and negative regulation of Src-family kinases by the protein tyrosine phosphatase CD45. Immunol. Today, in press. [DOI] [PubMed]
- 38.Trowbridge I S, Thomas M L. CD45: an emerging role as a protein tyrosine phosphatase required for lymphocyte activation and development. Annu Rev Immunol. 1994;12:85–116. doi: 10.1146/annurev.iy.12.040194.000505. [DOI] [PubMed] [Google Scholar]
- 39.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 40.Yanagi S, Sugawara H, Kurosaki M, Sabe H, Yamamura H, Kurosaki T. CD45 modulates phosphorylation of both autophosphorylation and negative regulatory tyrosines of Lyn in B cells. J Biol Chem. 1996;271:30487–30492. doi: 10.1074/jbc.271.48.30487. [DOI] [PubMed] [Google Scholar]