Abstract
Exosomes derived from endothelial cells and Schwann cells have been employed as novel treatments of neurological diseases, including peripheral neuropathy. Exosomal cargo plays a critical role in mediating recipient cell function. In this study, we thus performed a comprehensive proteomic analysis of exosomes derived from healthy mouse dermal microvascular endothelial cells (EC-Exo) and healthy mouse Schwann cells (SC-Exo). We detected 1,817and 1,579 proteins in EC-Exo and SC-Exo, respectively. Among them, 1506 proteins were present in both EC-Exo and SC-Exo, while 311 and 73 proteins were detected only in EC-Exo and SC-Exo, respectively. Bioinformatic analysis revealed that EC-Exo enriched proteins were involved in neurovascular function, while SC-Exo enriched proteins were related to lipid metabolism. Western blot analysis of 14 enriched proteins revealed that EC-Exo contained proteins involved in mediating endothelial function such as delta-like 4 (DLL4) and endothelial NOS (NOS3), whereas SC-Exo had proteins involved in mediating glial function such as apolipoprotein A-I (APOA1) and phospholipid transfer protein (PLTP). Collectively, the present study identifies differences in the cargo protein profiles of EC-Exo and SC-Exo, thus providing new molecular insights into their biological functions for the treatment of peripheral neuropathy.
Introduction
Schwann cells are the most abundant cells in the peripheral nervous system (PNS) and play a vital role in the maintenance of peripheral nerve function [1, 2]. Microvascular endothelial cells maintain neurovascular function through crosstalk among endothelium, Schwann cells, and nerve fibers [3, 4]. Dysfunction of this communication is involved in the development of peripheral nerve damage [5, 6].
Exosomes are nano-size biovesicles (~30-200nm) released from nearly all cells and they play critical roles in mediating intercellular communication. Emerging evidence shows that exosomes have therapeutic effects on neurodegenerative diseases [7, 8]. We have demonstrated that exosomes derived from endothelial cells (EC-Exo) and Schwann cells (SC-Exo) ameliorate peripheral neuropathy caused by diabetes and chemotherapy [9, 10]. The therapeutic effect of exosomes on neurological diseases is likely impacted by transferring exosomal cargo biological materials into recipient cells, consequently leading to changes of recipient cell function [11, 12]. Exosomal cargo materials contain proteins, RNAs, and lipids, which are from the original cells. Although both EC-Exo and SC-Exo have therapeutic effects on peripheral neuropathy, the molecular mechanisms underlying the beneficial effects of these two exosomes may differ. Until now, only a few studies have investigated the protein profiles of EC-Exo and SC-Exo [13–16]. Knowledge of their cargo contents will provide new insights into the molecular mechanisms of these respective exosomes. This may permit further development of exosome-based treatment for neuropathy by engineering their respective molecular content, e.g. incorporating specific cargo proteins to enhance neurovascular and myelin function. In the present study, we thus analyzed protein profiles of EC-Exo derived from mouse dermal microvascular endothelial cells and of SC-Exo derived from Schwann cells.
Materials and methods
Primary cell culture
Primary mouse dermal microvascular endothelial cells were purchased from Cell Biologics (C57-6064), which were isolated from skin tissues of C57BL/6 mice. These cells are well characterized morphologically and phenotypically (VE-cadherin; AF1002; CD31/PECAM-1 positive). Primary Schwann cells were purchased from ScienCell (M1700-57), which were isolated from postnatal day 8 C57BL/6 mouse sciatic nerves. These cells exhibit Schwann cell phenotype marker proteins of S100, GFAP and CD9.
Isolation of EC-Exo and SC-Exo
Mouse primary dermal microvascular endothelial cells and Schwann cells at passage 3–5 were cultured using complete culture medium (M168, cell biologics and 1701 ScienCell, respectively). When the cells reach to 60%~80% confluence, the exosome-depleted fetal bovine serum (FBS) medium (SF-4Z0-500, Cell Systems) was replaced and cultured for an additional 48 hours. The supernatant was then collected. Using a differentiation ultracentrifugation approach, exosomes were isolated from the supernatant according to our published protocol [10]. Briefly, the supernatant was passed through a 0.22 μm filter to remove dead cells and large debris. A 10,000g centrifugation for 30 min was performed to further remove small debris. Ultracentrifugation was performed at 100,000g (Optima XE-100 Ultracentrifuge, SW 32 Ti Rotor) for 2 hours and the pellet was resuspended with sterilized phosphate-buffered saline (PBS). The concentration and size distribution of exosomes were quantified using the NanoSight NS300 system (Malvern Panalytical). The exosomes were further verified by transmission electron microscopy (TEM, JEOL JEM- 1400). Western blot analysis with antibodies Alix, CD9, CD63, CD81 and Calnexin were used to confirm exosome marker proteins.
Proteomics analysis
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [17] partner repository with the dataset identifier PXD041547 and 10.6019/PXD041547.
Two individual replicates of EC-Exo or SC-Exo were used for proteomics analysis and each EC-Exo and SC-Exo biological replicate was isolated from supernatant pooled from 3 independent cell cultures.
Total proteins in the exosomes were extracted according to published protocols [9, 10]. Briefly, the exosomes were lysed in 50 μl RIPA buffer with 1% protease inhibitor cocktail (Sigma-Aldrich) and incubated at 4°C for 30 min and followed by gentle mixing on ice for 15 min. The protein concentrations were determined by a bicinchoninic acid assay (BCA, Piece). The samples were submitted to the proteomics core facility of Wayne State University for exosomal cargo protein analysis. Briefly, 30 μg of each sample was heated at 95°C for 5 min with the addition of 2% lithium dodecyl sulfate. Samples were resuspended in 20 mM triethylammonium bicarbonate (TEAB) buffer, then reduced with 5 mM DL-Dithiothretol (DTT) and alkylated with 15 mM iodoacetamide (IAA) under standard conditions. Excess IAA was quenched with an additional 5 mM DTT. Next, the S-Trap precipitation protocol (Protifi) was performed, followed by an overnight digestion at 37°C in 40 mM TEAB and sequencing-grade trypsin (Promega). The next day, the peptides were separated by reversed-phase chromatography (Acclaim PepMap100 C18 column, Thermo Scientific), followed by ionization with the Nanospray Flex Ion Source (Thermo Scientific), and introduced into a Q Exactive mass spectrometer (Thermo Scientific). Abundant species were fragmented with high-energy collision-induced dissociation (HCID).
The raw data analysis was performed using Proteome Discoverer 2.4 (Thermo Scientific) which incorporated the Sequest algorithm (Thermo Scientific). The Uniprot_Mus_Compl_20181221 database was searched for mouse protein sequences and a reverse decoy protein database was run simultaneously for false discovery rate (FDR) determination. The data files were loaded into Scaffold (Proteome Software) for distribution.
Sequest was searched with a fragment ion mass tolerance of 0.02 Da and a parent ion tolerance of 10 PPM. Carbamidomethylation of cysteine was specified in Sequest as a fixed modification. Deamidation of asparagine and glutamine, oxidation of methionine, and acetylation of the n-terminus were specified in Sequest as variable modifications.
Bioinformatics analysis
Protein stoichiometry in differences of EC-Exo and SC-Exo was analyzed using R programming (V4.2.1). Differential Enrichment analysis of Proteomics data (DEP) package (V1.16.00), general and standard approaches for analysis of proteomics, were applied for the differential protein analysis. Outliers from the protein profiles were eliminated from the analysis. First, the read count of each peptide was normalized using the variance stabilizing normalization (VSN) method. Then, the missing values from the protein profiles were identified and imputed with the k-nearest neighbor (KNN) approach. Proteins detected in both replicates of EC-Exo or SC-Exo were used for analysis. Next, a moderated t-tests built in the DEP package based on FDR calculated a statistical significance between EC-Exo and SC-Exo based on FDR determination. The log fold change >2 and p<0.05 were used to make a volcano visual plot. The differential proteins were separated into three subgroups: EC-Exo specific, SC-Exo specific, and differentially expressed based on their read count.
Protein enrichment analysis was performed on each subgroup using the Database for Annotation, Visualization and Integrated Discovery (DAVID) Bioinformatics Resources (V 6.8). Top gene ontology (GO) annotations including biological processes (BP), cellular components (CC), molecular function (MF) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway for each subgroup were visualized by GraphPad Prime (V 9.0). For further the pathway analysis, Ingenuity Pathway Analysis (IPA, QIAGEN) database was used. The Canonical Pathways as well as Diseases and Biological Function tools from IPA Core analysis were performed on all significant proteins from each subgroup. The significant cut-off was set to Z-score > 1.3. The present study was not associated with cancer, so the cancer related pathways and biological functions were eliminated from the results. However, the pathways and functions that mediated cellular function and cell cycle were kept. All the significant proteins were identified and compared with ExoCarta database which is an online source specific on exosome protein profiles. The core proteins from each subgroup were identified based on their enrichment in the top GO terms and pathways and were validated by the Western Blot.
Western blot analysis of selected proteins
EC-Exo and SC-Exo (n = 3/group) were homogenized in lysis buffer and the protein concentrations were determined using a bicinchoninic acid assay [10]. Western blot was performed according to published protocols [10]. Briefly, 30 μg of protein for each sample were subjected to electrophoresis and transferred to PVDF membrane. The membrane was incubated with primary antibodies followed by horseradish peroxidase (HRP)-conjugated secondary antibodies (1:1000) along with molecular weight markers. An enhanced chemiluminescence development kit was employed for detection. A list of individual primary and secondary antibodies used in the present study is provided in S1 Table. Statistical significance was determined using a student’s t test.
Results
Characterization of EC-Exo and SC-Exo
We first characterized EC-Exo and SC-Exo isolated from the supernatant of cultured ECs and SCs by a differential ultra-centrifugation approach [10]. Nanoparticle tracking analysis (NTA) revealed that the particle number and size of EC-Exo and SC-Exo were 3.0x109/particles/ml and 3.6x109/particles/ml with a mean size of 142±3.3 nm and 162±3.0 nm, respectively (Fig 1A). TEM analysis showed typical exosomal morphology (Fig 1B). Western blot analysis showed that EC-Exo and SC-Exo contained exosome marker proteins Alix, CD9, CD63 and CD81, but not Calnexin which is a negative control for an exosome protein marker (Fig 1C).
Fig 1.
Characterization of EC-Exo and SC-Exo by (A) Particle size distribution analysis of EC-Exo and SC-Exo using the NanoSight analyzer, (B) Representative transmission electron micrographs (TEM) of EC-Exo and SC-Exo. Scale bar = 200nm. (C) Western blots analysis showing the expression of Alix, CD9, CD63, CD81 and Calnexin in EC-Exo and SC-Exo, respectively. (D) Venn diagram displaying common and unique proteins in EC-Exo and SC-Exo. EC-Exo, exosomes derived from endothelial cells. SC-Exo, exosomes derived from Schwann cells.
Protein profiles in EC-Exo and SC-Exo
To examine stoichiometry differences in cargo protein compositions between EC-Exo and SC-Exo, we then performed a proteome analysis by means of label-free quantitative LC–MS.
Protein identifications were accepted if they had greater than 99.0% probability to achieve a false discovery rate (FDR) less than 1.0% and contained at least 2 identified peptides. Based on these criteria, there were 1,817 and 1,579 proteins in EC-Exo and SC-Exo, respectively. Among the detected proteins, approximately 97% proteins (1,766 in EC-Exo and 1,544 in SC-Exo) have been reported in the exosome protein database, Exocarta. Although there were 1,506 proteins that were shared between EC-Exo and SC-Exo, there were 311 and 73 proteins that were highly enriched in EC-Exo and in SC-Exo, respectively (Fig 1D), which reflects the different nature of their parent cells between endothelial cells and Schwann cells.
Bioinformatics analysis of EC-Exo enriched proteins
To further characterize EC-Exo and SC-Exo enriched proteins, we first performed GO analysis of EC-Exo enriched proteins (S2 Table). The top 10 GO terms ranked according to their significance level (p<0.05) are listed in Fig 2. Functional annotation to GO parent terms “cellular components” assigned the largest fraction of identified proteins to the category of “extracellular” exosome (Fig 2), indicating that our procedures were reliable and effective to isolate exosomes. Additionally, cellular component identified proteins that were significantly enriched in cytoplasm (Fig 2A). Cytoplasmic proteins are mainly involved energy production, metabolism, and protein biosynthesis [18]. The biological processes are mainly related to endocytosis and cell-cell adhesion, as well as angiogenesis (Fig 2B). In terms of molecular functions, proteins were enriched in nucleotide binding and protein binding (Fig 2C). Binding proteins on the exosome surface may facilitate exosomes to target recipient cells [19].
Fig 2. Analysis of EC-Exo enriched proteins.
GO functional analysis showed the 10 most significantly (p<0.05) enriched proteins in cellular component (A), biological process (B), molecular function (C). KEGG pathway enrichment analysis showed pathways that these proteins are involved (D). The x axis shows the negative of the log-base-10 of the p- value.
In addition, the KEGG pathway enrichment analysis revealed that these proteins are related to Notch signaling and tight junction pathways (Fig 2D). The Notch signaling pathway mediates angiogenesis and axonal regeneration [20, 21], while the tight junction pathway plays a crucial role in angiogenesis and control the permeability of blood vessels [22].
Using IPA, we further analyzed key signaling pathways associated with EC-Exo enriched proteins. A total of 81 enriched canonical pathways were identified in these proteins by using the -log(p-value) >1.3 threshold. The top 20 signaling pathways are shown in Fig 3. The signaling pathways involved in angiogenesis and vascular function such as T helper cell type 1 (Th1)/T helper cell type II (Th2) and Peroxisome Proliferator Activated Receptor Alpha (PPARa)/Retinoid X Receptor Alpha (RXRa) activation and Notch signaling were highly ranked. Th1/Th2 cytokines control angiogenesis, while PPARa/RXRa signaling regulates vascular and inflammatory responses [23]. In addition, the protein kinase A (PKA) signaling pathway is involved in regulation of synaptic plasticity and vascular tone.
Fig 3. Canonical pathway analysis of EC-Exo enriched proteins using IPA.
The top 20 most significant pathways are presented. The x-axis shows the negative log of p-value. IPA, ingenuity pathway analysis.
Table 1 lists EC-Exo enriched proteins that are involved in vascular function such as angiogenesis and vasculogenesis. These proteins included proteins in the Notch pathway, delta-like protein 4 (DLL4) and presenilin-1 (PSEN1), as well as stromal cell-derived factor 1 (CXCL12), Ephrin-B2 (EFNB2), matrix metalloproteinase-14 (MMP14), protein kinase C alpha type (PRKCA) and semaphorin-3C (SEMA3c). In addition to vascular function, these EC-Exo enriched proteins are also involved in axonal guidance signaling and growth (Table 2).
Table 1. Exosome proteins associated with vascular function.
| EC-Exo enriched proteins | ||
| Protein ID | Gene name | Protein name |
| P15116 | CDH2 | Cadherin-2 |
| Q91WQ3 | YARS1 | Tyrosine—tRNA ligase, cytoplasmic |
| Q8VCC6 | CCM2 | Cerebral cavernous malformations protein 2 |
| P48678 | LMNA | Prelamin-A |
| Q9JI71 | DLL4 | Delta-like protein 4 |
| Q60805 | MERTK | Tyrosine-protein kinase Mer |
| Q62181 | SEMA3C | Semaphorin-3C |
| P52795 | EFNB2 | Ephrin-B2 |
| Q811D0 | DLG1 | Disks large homolog 1 |
| Q9Z0J1 | RECK | Reversion-inducing cysteine-rich protein with Kazal motifs |
| P83741 | WNK | Serine/threonine-protein kinase WNK1 |
| Q60751 | IGF1R | Insulin-like growth factor 1 receptor |
| P28862 | MMP3 | Stromelysin-1 |
| Q08857 | CD36 | Platelet glycoprotein 4 |
| Q01102 | SELP | P-selectin |
| Q9JKK1 | STX6 | Syntaxin-6 |
| Q8K019 | BCLAF1 | Bcl-2-associated transcription factor 1 |
| P16382 | IL4R | Interleukin-4 receptor subunit alpha |
| Q9D154 | SERPINE1 | Plasminogen activator inhibitor 1 |
| P70372 | ELAV1 | ELAV-like protein 1 |
| Q61288 | ACVR1 | Activin receptor type-1 |
| P23242 | GJA1 | Gap junction alpha-1 protein |
| Q3MI99 | CCBE1 | Collagen and calcium-binding EGF domain-containing protein 1 |
| P40224 | CXCL12 | Stromal cell-derived factor 1 |
| O08599 | STXBP2 | Syntaxin-binding protein 2 |
| P98156 | VLDLR | Very low-density lipoprotein receptor |
| Q8CFG0 | SULF2 | Extracellular sulfatase Sulf-2 |
| Q62433 | NDRG1 | Protein NDRG1 |
| Q8BVF7 | APH1A | Gamma-secretase subunit APH-1A |
| Q8R4G6 | MGAT5 | Alpha-1,6-mannosylglycoprotein 6-beta-N-acetylglucosaminyltransferase A |
| P49769 | PSEN1 | Presenilin-1 |
| Q9R0C8 | VAV3 | Guanine nucleotide exchange factor VAV3 |
| Q9Z1B3 | PLCB1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-1 |
| Q8R3B1 | PLCD1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase delta-1 |
| Q9QUH0 | GLRX | Glutaredoxin-1 |
| Q62443 | NPTX1 | Neuronal pentraxin-1 |
| P31230 | AIMP1 | Aminoacyl tRNA synthase complex-interacting multifunctional protein 1 |
| Q64455 | PTPRJ | Receptor-type tyrosine-protein phosphatase eta |
| Q8VE98 | CD276 | CD276 antigen |
| P11103 | PARP1 | Poly [ADP-ribose] polymerase 1 |
| P58022 | LOXL2 | Lysyl oxidase homolog 2 |
| P20444 | PRKCA | Protein kinase C alpha type |
| P56546 | CTBP2 | C-terminal-binding protein 2 |
| P27808 | MGAT1 | Alpha-1,3-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase |
| P41241 | CSK | Tyrosine-protein kinase CSK |
| P16110 | LGALS3 | Galectin-1 |
| O89110 | CASP8 | Caspase-8 |
| P53690 | MMP14 | Matrix metalloproteinase-14 |
| P09470 | ACE | Angiotensin-converting enzyme |
| P70313 | NOS3 | Nitric oxide synthase, endothelial |
| Q9CQI3 | GMFB | Glia maturation factor beta |
| Q9WUD1 | STUB1 | STIP1 homology and U box-containing protein 1 |
| SC-Exo enriched proteins | ||
| Protein ID | Gene name | Protein name |
| P23927 | CRYAB | Alpha-crystallin B chain |
| Q8R0X7 | SGPL1 | Sphingosine-1-phosphate lyase 1 |
| Q99K41 | EMILIN1 | EMILIN-1 |
| P47877 | IGFBP2 | Insulin-like growth factor-binding protein2 |
| Q8C4U3 | SFRP1 | Secreted frizzled-related protein 1 |
| Q9R045 | ANGPTL2 | Angiopoietin-related protein 2 |
| P35441 | THBS1 | Thrombospondin-1 |
| Q9R182 | ANGPTL3 | Angiopoietin-related protein 3 |
| O55188 | DMP1 | Dentin matrix acidic phosphoprotein 1 |
| O35474 | EDIL3 | EGF-like repeat and discoidin I-like domain-containing protein 3 |
| Q61738 | ITGA7 | Integrin alpha-7 |
| Q04207 | RELA | Transcription factor p65 |
| P14602 | HSPB1 | Heat shock protein beta-1 |
| Q9WVJ9 | EFEMP2 | EGF-containing fibulin-like extracellular matrix protein 1 |
| O35945 | ALDH1A7 | Aldehyde dehydrogenase, cytosolic 1 |
| P37889 | FBLN2 | Fibulin-2 |
| P18242 | CATHEPSIN | Cathepsin |
Exosome proteins associated with vascular function. The table shows a list of proteins that are enriched in EC-Exo and SC-Exo.
Table 2. Exosome proteins associated with neural function.
| EC-Exo enriched proteins | ||
| Protein ID | Gene name | Protein name |
| Q7TT50 | CDC42B | Serine/threonine-protein kinase MRCK beta |
| P52800 | EFNB2 | Ephrin-B2 |
| F8VQB6 | MYO10 | Unconventional myosin |
| P40224 | CXCL12 | Stromal cell-derived factor 1 |
| P15116 | CDH2 | Cadherin-2 |
| P98156 | VLDLR | Very low-density lipoprotein receptor |
| Q62443 | NPTX1 | Neuronal pentraxin-1 |
| P35285 | RAB22A | Ras-related protein Rab-22A |
| Q62448 | EIF4G2 | Eukaryotic translation initiation factor 4 gamma 2 |
| P28740 | KIF2A | Kinesin-like protein KIF2A |
| Q60751 | IGF1R | Insulin-like growth factor 1 receptor |
| P11103 | PARP1 | Poly [ADP-ribose] polymerase 1 |
| Q9WV80 | SNX1 | Sorting nexin-12 |
| Q9ES28 | ARHGEF7 | Rho guanine nucleotide exchange factor 7 |
| Q8BL66 | EEA1 | Early endosome antigen 1 |
| P22777 | SERPINE1 | Plasminogen activator inhibitor 1 |
| O70493 | SNX12 | Sorting nexin-12 |
| B0V2N1 | PTPRS | Receptor-type tyrosine-protein phosphatase S |
| P49769 | PSEN1 | Presenilin-1 |
| P23242 | GJA1 | Gap junction alpha-1 protein |
| Q60780 | GAS7 | Growth arrest-specific protein 7 |
| Q9ES28 | ARHGEF7 | Rho guanine nucleotide exchange factor 7 |
| Q03137 | EPHA4 | Ephrin type-A receptor 4 |
| O88447 | KLC1 | Kinesin light chain 1 |
| P53690 | MMP14 | Matrix metalloproteinase-14 |
| P28862 | MMP3 | Matrix metalloproteinase-3 |
| Q8VD65 | PIK3R4 | Phosphoinositide 3-kinase regulatory subunit 4 |
| Q9Z1B3 | PLCB1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-1 |
| Q8R3B1 | PLCD1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase delta-1 |
| Q8CIH5 | PLCG2 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-2 |
| P20444 | PRKCA | Protein kinase C alpha type |
| Q62181 | SEMA3C | Semaphorin-3C |
| Q76KF0 | SEMA6D | Semaphorin-6D |
| Q6PHZ2 | CAMK2D | Calcium/calmodulin-dependent protein kinase type II subunit delta |
| Q80X90 | FLNB | Filamin-B |
| SC-Exo enriched proteins | ||
| Protein ID | Gene name | Protein name |
| O35945 | ALDH1a7 | Aldehyde dehydrogenase, cytosolic 1 |
| O08917 | FLOT1 | Flotillin-1 |
| P18872 | GNAO1 | Guanine nucleotide-binding protein G(o) subunit alpha |
| Q62059 | VCAN | Versican core protein |
| O08989 | MRAS | Ras-related protein M-Ras |
| Q07235 | SERPINE2 | Plasminogen activator inhibitor 1 |
| P35441 | THBS1 | Thrombospondin-1 |
| Q04207 | RELA | Prolow-density lipoprotein receptor-related protein 1 |
| P18872 | GNAO1 | Guanine nucleotide-binding protein G(o) subunit alpha |
Exosome proteins associated with neural function. The table shows a list of proteins that are enriched in EC-Exo and SC-Exo.
Bioinformatics analysis of SC-Exo enriched proteins
Compared to EC-Exo, there were 73 enriched proteins in SC-Exo (S3 Table). GO analysis of cellular components showed that enriched SC-Exo proteins were related to extracellular exosome, extracellular region, and extracellular space (Fig 4A). These proteins significantly enriched biological processes and molecular function were cell adhesion, response to calcium ion, and calcium ion binding proteins (Fig 4B and 4C), which is in line with the supportive role of SCs in peripheral nerve function [24, 25]. The KEGG pathway analysis showed that these proteins are involved in the endoplasmic reticulum pathways via protein processing. The endoplasmic reticulum pathway is critical for Schwann cells to synthesize myelin protein and to maintain myelin structure (Fig 4D) [26].
Fig 4. Analysis of EC-Exo enriched proteins.
GO enrichment analysis showed the 10 most significantly (p<0.05) enriched proteins in cellular component (A), biological process (B), molecular function (C). KEGG pathway enrichment analysis showed pathways that these proteins are involved (D). The x-axis shows the negative of the log-base-10 of the p- value.
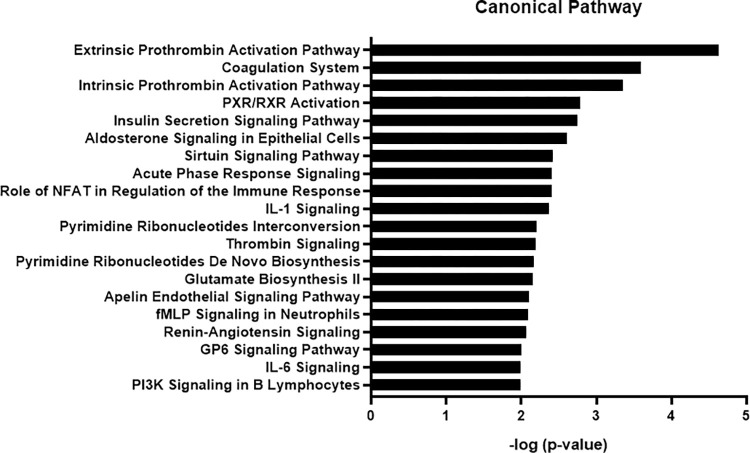
IPA showed that the top 20 canonical pathways in SC-Exo enriched proteins include the insulin secretion pathway and pregnane X receptor (PXR)/retinoid x receptor (RXR) signaling (Fig 5). Disrupting insulin signaling such as insulin-like growth factor-binding protein 2 (IGFBP2) in SCs, impairs myelination and induces a sensory neuropathy [27]. RXRr accelerates remyelination [28]. In addition, the PI3K signaling pathway modulates axonal outgrowth and myelination [29]. Moreover, the extrinsic/intrinsic prothrombin activation pathways regulate Schwann cell supported neuronal regeneration [30]. Furthermore, interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells [31].
Fig 5. Canonical pathway analysis of SC-Exo enriched proteins using IPA.
The top 20 most significant pathways are presented. The x-axis shows the negative log of p- value.
In contrast to EC-Exo, SC-Exo were enriched with proteins involved in metabolism, synthesis, and transportation of lipids. These proteins included glia-derived nexin (SERPINE2), cytochrome b5 (CYB5A), angiopoietin-related protein 3 (ANGPTL3), adipocyte enhancer-binding protein 1(AEBP1) and IGFBP2 (Table 3). Additionally, SC-Exo were also enriched with proteins involved in peripheral nerve fiber growth and axonal guidance, such as flotillin-1 (FLOT1), thrombospondin-1 (THBS1), Ras-related protein M-Ras (MRAS) and guanine nucleotide-binding protein G(o) subunit alpha (GNAO1) (Table 2). Thus, these data suggest that SC-Exo could mediate myelination and axonal growth.
Table 3. Exosome proteins associated with lipid metabolism process.
| EC-Exo enriched proteins | ||
| Protein ID | Gene name | Protein name |
| P41233 | ABCA1 | ATP-binding cassette sub-family A member 1 |
| Q64343 | ABCG1 | ATP-binding cassette sub-family G member 1 |
| P47856 | GFPT1 | Glutamine—fructose-6-phosphate aminotransferase [isomerizing] 1 |
| P48678 | LMNA | Prelamin-A/C |
| P06795 | ABCB1 | Multidrug resistance protein 1B |
| Q9Z1B3 | PLCB1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase beta-1 |
| Q8R3B1 | PLCD1 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase delta-1 |
| Q62077 | PLCG2 | 1-phosphatidylinositol 4,5-bisphosphate phosphodiesterase gamma-1 |
| Q08857 | CD36 | Platelet glycoprotein 4 |
| P11103 | PARP1 | Poly [ADP-ribose] polymerase 1 |
| P98156 | VLDLR | Very low-density lipoprotein receptor |
| P70372 | ELAVL1 | ELAV-like protein 1 |
| Q9JIZ9 | PLSCR3 | Phospholipid scramblase 3 |
| P62331 | ARF6 | ADP-ribosylation factor 6 |
| SC-Exo enriched proteins | ||
| Protein ID | Gene name | Protein name |
| Q9Z0K8 | VNN1 | Pantetheinase |
| Q640N1 | AEBP1 | Adipocyte enhancer-binding protein 1 |
| Q8R0X7 | SGPL1 | Sphingosine-1-phosphate lyase 1 |
| Q8BLF1 | NCEH1 | Neutral cholesterol ester hydrolase 1 |
| P97742 | CPT1A | Carnitine O-palmitoyltransferase 1, liver isoform |
| Q9Z0J0 | NPC2 | NPC intracellular cholesterol transporter 2 |
| Q9R045 | ANGPTL1 | Angiopoietin-related protein 2 |
| Q04207 | RELA | Transcription factor p65 |
| Q9QXP7 | C1QTNF1 | Complement C1q tumor necrosis factor-related protein 1 |
| P56395 | CYB5A | Cytochrome b5 |
| Q8R0X7 | SGPL1 | Sphingosine-1-phosphate lyase 1 |
| O35945 | ALDH1A7 | Aldehyde dehydrogenase, cytosolic 1 |
| P51660 | HSD17B4 | Peroxisomal multifunctional enzyme type 2 |
| Q80TA6 | MTMR12 | Myotubularin-related protein 12 |
| Q07235 | SERPINE2 | Glia-derived nexin |
| P47877 | IGFBP2 | Insulin-like growth factor-binding protein 2 |
| Q9Z0J0 | NPC2 | NPC intracellular cholesterol transporter 2 |
| Q9R182 | ANGPTL3 | Angiopoietin-related protein 3 |
| Q99L43 | CDS2 | Phosphatidate cytidylyltransferase 2 |
| O08917 | FLOT1 | Flotillin-1 |
| Q640N1 | AEBP1 | Adipocyte enhancer-binding protein 1 |
| Q99KQ4 | NAMPT | Nicotinamide phosphoribosyltransferase |
| Q99LJ1 | FUCA1 | Tissue alpha-L-fucosidase |
| O08710 | TG | Thyroglobulin |
| O35474 | EDIL3 | EGF-like repeat and discoidin I-like domain-containing protein 3 |
| Q64521 | GPD2 | Glycerol-3-phosphate dehydrogenase, mitochondrial |
| Q00623 | APOA1 | Apolipoprotein A-I |
| P50428 | ARSA | Arylsulfatase A |
| Q60931 | VDAC3 | Voltage-dependent anion-selective channel protein 3 |
| P26443 | GLUD1 | Glutamate dehydrogenase 1 |
| P18242 | CATHEPSIN | Cathepsin |
| P11276 | FN1 | Fibronectin |
| P55065 | PLTP | Phospholipid transfer protein |
Exosome proteins associated with lipid metabolism. The table shows a list of proteins that are enriched in EC-Exo and SC-Exo.
Bioinformatics analysis of the common proteins between EC-Exo and SC-Exo
We then examined protein stoichiometry for differences among shared 1,506 common proteins between EC-Exo and SC-Exo. We found 47 and 8 abundant proteins in EC-Exo and in SC-Exo than in SC-Exo and EC-Exo, respectively, based on a threshold of p<0.05 with two-fold change (Fig 6, S4 and S5 Tables). The EC-Exo abundant proteins included disintegrin and metalloproteinase domain-containing protein 9 (ADAM9), CD2-associated protein (CD2AP), and cAMP-dependent protein kinase catalytic subunit beta (PRKACB), which mediate axon guidance and neurovascular function. There were multiple glycoproteins in SC-Exo that regulate myelin sheath formation, maintenance and degeneration, such as apolipoprotein A-I (APOA1), collagen alpha-2(I) chain (COL1A2), fibulin-2 (FBLN2), fibronectin (FN1), and cation-independent mannose-6-phosphate receptor (IGF2R).
Fig 6. Volcano plot showing p-values (-log 10) versus protein ratio of (log2 EC-Exo vs.SC-Exo).
Red: EC-Exo abundant proteins (fold-change>2, p-value<0.05), Blue: SC-Exo abundant proteins (fold-change <-2, p-value <0.05), grey: no significant change. A few selected differentially abundant proteins are labeled.
Western blot analysis to confirm the result of the proteomic experiment
Using Western blot analysis, we further measured 20 proteins selected based on differences between EC-Exo and SC-Exo. Among them, Western blot (Fig 7, S1 Raw images) confirmed EC-Exo relatively enriched proteins: angiotensin-converting enzyme (ACE), delta-like protein 4 (DLL4), glia maturation factor-β (GMFB), filamin-B (FLNB), endothelial nitric oxides synthase (NOS3), and STIP1 homology and U box-containing protein 1 (STUB1). For SC-Exo relatively enriched proteins were APOA1, FBLN2, SERPINE2, glutamate dehydrogenase 1 (GLUD1), CATHEPSIN, FN1, phospholipid transfer protein (PLTP). and Voltage-dependent anion-selective channel protein 3 (VDAC3).
Fig 7. Western blot analysis of selective enriched proteins in EC-Exo and SC-Exo.
Representative Western blot images (A) and their relatively quantitative data (B) showed protein levels in EC-Exo and SC-Exo. TSG101 protein was used as a reference protein. n = 3/group. Data represent mean±SE. *p-value <0.01.
Discussion
The present study demonstrated that EC-Exo and SC-Exo have different protein cargo profiles, with EC-Exo enriched proteins involved in vascular function and SC-Exo enriched proteins involved in regulation of myelin related lipid metabolism, while cargo proteins shared by EC-Exo and SC-Exo could modulate axonal guidance and growth. These data provide evidence to support that EC-Exo and SC-Exo contribute to the roles of endothelial and Schwann cells in the growth and maintenance of peripheral nerves.
Comprehensive and comparative analysis of the composition and function of exosomal cargo proteins derived from healthy mouse endothelial cells and Schwann cells have not been reported. Mouse dermal microvascular endothelial cells are major components of skin blood vessels and provide nutrients to the epidermal nerve tissue, and closely mimic endothelial cell function in the PNS. A detailed comparative analysis between the two types of exosomes may provide increased insight into their biological function, and is critical for treatment of peripheral nerve damage, such as peripheral neuropathy. Dysfunction of communication between endothelial cells and Schwann cells is involved in the development of peripheral nerve damage [5, 6]. We and others have demonstrated that individual EC-Exo and SC-Exo treatment have therapeutic effects on peripheral neuropathy [9, 32]. The present study also provides information to potentially generate engineered exosomes by combining specific cargo proteins which regulate neurovascular and myelin functions from EC-Exo and SC-Exo. This may further advance exosome-based treatment for neuropathy.
The present study is consistent with and extends previous proteomics findings of exosomes derived from endothelial and Schwann cells [13, 15]. Wei et.al reported that exosomes derived from rat primary Schwann cells contain 12 proteins that mediate neuronal function, in which 11 of the 12 proteins were detected by the present study [13]. Li et.al revealed abundant levels of TGFβ2 and Octamer-binding transcription factor 4 in EVs isolated from the rat spontaneously immortalized Schwann cell line RSC96 [33]. Boyer et.al found that EVs derived from rat aortic endothelial cells contain proteins that modulate smooth muscle cell phenotype and protein synthesis [15].
Within EC-Exo enriched proteins, we observed that several key signaling pathways are related to angiogenesis, vasculogenesis, and axonal growth, which may contribute to the functional role of ECs. For example, several proteins were found to associate with the Notch signaling pathway. DLL4 is a transmembrane ligand for Notch receptors and endothelial DLL4 deficiency impairs arterial relaxation [34]. DLL4-containing EC exosomes participate in angiogenesis through interaction with recipient endothelial cells [35]. Of interest, NOS3 is a regulator of Notch signaling, and plays an important role in regulating vascular relaxation and blood flow by activating the soluble guanylate cyclase (sGC)-cGMP-PKG pathway and impairing vascular redox environment [36, 37].
In addition, several EC-Exo abundant proteins, such as ADAM9, CD2AP and PRKACB are related to axon guidance and growth. Filamin-B (FLNB) are expressed in endothelial cells and play an essential role in vascular development and angiogenesis [38]. Moreover, Glia maturation factor beta (GMFB) is a growth factor for both glia and neurons. It stimulates axon regeneration in transected rat sciatic nerve [39]. We speculate that the expression of these enriched proteins in EC-Exo may improve the microenvironment and enhance neurovascular remodeling in peripheral nerve damage.
SC-Exo have been mainly evaluated in the context of peripheral nerve repair [40–42]. We found that the enriched proteins in SC-Exo were mainly annotated to the extracellular exosome, calcium ion binding, and cell adhesion which strongly influence nerve regeneration and myelination [42, 43]. The enriched signaling pathway is involved in myelination and axonal regeneration, such as the insulin secretion pathway and the PI3K signaling pathway. Insulin signaling is essential for SC myelination by activating the PI3K/AKT pathway and lipid metabolism [27]. GLUD1 is a mitochondrial matrix enzyme and involved in insulin secretion [44].
SC-Exo enriched proteins were associated with lipid metabolism, including synthesis and transport of lipid. Myelin contains a high proportion of lipids [45]. The lipid synthesis and fatty acid are required for myelination and peripheral nerve function [27, 45]. Peripheral nerve myelin is affected in lipid metabolism disorder [46]. APOA1 is expressed in myelinating sciatic nerve and involved in myelin synthesis by the local transport of lipids [47]. It also increases neurite outgrowth and neuronal regeneration by restricting inflammatory response and enhances angiogenesis by synthesizing cell surface ATP [48]. Moreover, PLTP, a crucial modulator of lipoprotein (HDL) metabolism, may be involved in the maintenance of the functional and structural integrity of myelin and regulates axonal guidance and sprouting [49, 50]. SC-Exo enriched proteins are involved in nerve regeneration, such as cathepsins and serpine2. Others have shown that SC-Exo increase axonal growth of DRG neurons and promote regeneration of damaged peripheral nerve [32, 40, 51]. Fibronectin, a major extracellular matrix, regulates remyelination and promotes Schwann cell growth. Loss of fibronectin in SCs impairs their directional migration affecting the alignment of the axons [52]. Furthermore, VDAC3 may increase the efficiency of bioenergetic metabolism and protect mitochondria from oxidative stress which are critical for proper peripheral nerve function [53]. We thus speculate that lipid- and axon-related proteins in the SC-Exo cargo may enhance myelin, and axonal formation and function.
The present study revealed that EC-Exo and SC-Exo shared many proteins related to axonal guidance and nerve growth, suggesting that these cargo proteins could contribute to the therapeutic effect of EC-Exo and SC-Exo on peripheral neuropathy [9, 32].
The present study has limitations including a sample size with two replicates of each proteomics analysis and functional analysis, although each individual exosome sample was isolated from the supernatant pooled from three biological replicates. Our data also suggest that protein stoichiometry between different exosome cargo based on proteomics analysis needs to be further confirmed using Western blot analysis or other more quantitative approaches. In addition, the relatively restrained threshold in the present study may exclude certain proteins from the analysis, which leads to only a small number of proteins with significant differences between EC-Exo and SC-Exo. However, the excluded cargo proteins could potentially contribute to exosome function in recipient cells. It should be emphasized that additional experiments are warranted to investigate the effect of specific individual cargo proteins on neurovascular and myelin functions.
In summary, our proteomic analysis indicates that proteins involved in neurovascular function were abundant in the EC-Exo cargo, while SC-Exo cargo had more lipid metabolism proteins that regulate nerve myelination formation and function. The present study provides molecular insight into the therapeutic benefit of both SC-Exo and EC-Exo, and may lead to generation of exosomes whose protein content is engineered to enhance their therapeutic benefit for neuropathy.
Supporting information
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank the Wayne State Proteomics Lab Paul Stemmer’s Proteomics Laboratory at Wayne State University, Detroit for their expertise in performing the proteomics measurements. We would like to thank Julie Landschoot-Ward and Amy Kemper for their technical assistance.
Data Availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD041547 and 10.6019/PXD041547. All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) RO1 DK124377 (L.W.). The funder had no role in study design, data collect and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lehmann HC, Hoke A. Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neurol Disord Drug Targets. 2010;9(6):801–6. Epub 2010/09/30. doi: 10.2174/187152710793237412 ; PubMed Central PMCID: PMC4053445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch-Queralt M, Fledrich R, Stassart RM. Schwann cell functions in peripheral nerve development and repair. Neurobiol Dis. 2023;176:105952. Epub 2022/12/10. doi: 10.1016/j.nbd.2022.105952 . [DOI] [PubMed] [Google Scholar]
- 3.Meng DH, Zou JP, Xu QT, Wang JY, Yu JQ, Yuan Y, et al. Endothelial cells promote the proliferation and migration of Schwann cells. Ann Transl Med. 2022;10(2):78. Epub 2022/03/15. doi: 10.21037/atm-22-81 ; PubMed Central PMCID: PMC8848405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mompeo B, Engele J, Spanel-Borowski K. Endothelial cell influence on dorsal root ganglion cell formation. J Neurocytol. 2003;32(2):123–9. Epub 2004/01/07. doi: 10.1023/b:neur.0000005597.28053.2a:neur.0000005597.28053.2a. . [DOI] [PubMed] [Google Scholar]
- 5.Roustit M, Loader J, Deusenbery C, Baltzis D, Veves A. Endothelial Dysfunction as a Link Between Cardiovascular Risk Factors and Peripheral Neuropathy in Diabetes. J Clin Endocrinol Metab. 2016;101(9):3401–8. doi: 10.1210/jc.2016-2030 ; PubMed Central PMCID: PMC5010566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapouly C, Yao Q, Vandierdonck S, Larrieu-Lahargue F, Mariani JN, Gadeau AP, et al. Impaired Hedgehog signalling-induced endothelial dysfunction is sufficient to induce neuropathy: implication in diabetes. Cardiovasc Res. 2016;109(2):217–27. Epub 2015/12/10. doi: 10.1093/cvr/cvv263 . [DOI] [PubMed] [Google Scholar]
- 7.Hill AF. Extracellular Vesicles and Neurodegenerative Diseases. J Neurosci. 2019;39(47):9269–73. Epub 2019/11/22. doi: 10.1523/JNEUROSCI.0147-18.2019 ; PubMed Central PMCID: PMC6867808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howitt J, Hill AF. Exosomes in the Pathology of Neurodegenerative Diseases. J Biol Chem. 2016;291(52):26589–97. Epub 2016/11/18. doi: 10.1074/jbc.R116.757955 ; PubMed Central PMCID: PMC5207170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Li C, Qin Y, Cepparulo P, Millman M, Chopp M, et al. Small extracellular vesicles ameliorate peripheral neuropathy and enhance chemotherapy of oxaliplatin on ovarian cancer. J Extracell Vesicles. 2021;10(5):e12073. Epub 2021/03/18. doi: 10.1002/jev2.12073 ; PubMed Central PMCID: PMC7931803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Chopp M, Szalad A, Lu X, Zhang Y, Wang X, et al. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes. 2020;69(4):749–59. Epub 2020/01/10. doi: 10.2337/db19-0432 ; PubMed Central PMCID: PMC7085247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider A, Simons M. Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res. 2013;352(1):33–47. Epub 2012/05/23. doi: 10.1007/s00441-012-1428-2 ; PubMed Central PMCID: PMC3602607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai CP, Breakefield XO. Role of exosomes/microvesicles in the nervous system and use in emerging therapies. Front Physiol. 2012;3:228. Epub 2012/07/04. doi: 10.3389/fphys.2012.00228 ; PubMed Central PMCID: PMC3384085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Z, Fan B, Ding H, Liu Y, Tang H, Pan D, et al. Proteomics analysis of Schwann cell-derived exosomes: a novel therapeutic strategy for central nervous system injury. Mol Cell Biochem. 2019;457(1–2):51–9. Epub 20190304. doi: 10.1007/s11010-019-03511-0 ; PubMed Central PMCID: PMC6548868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Shen M, Wang X, Zhang S, Yu S, Chen G, et al. Comparative proteomic analysis of primary schwann cells and a spontaneously immortalized schwann cell line RSC 96: a comprehensive overview with a focus on cell adhesion and migration related proteins. J Proteome Res. 2012;11(6):3186–98. Epub 20120503. doi: 10.1021/pr201221u . [DOI] [PubMed] [Google Scholar]
- 15.Boyer MJ, Kimura Y, Akiyama T, Baggett AY, Preston KJ, Scalia R, et al. Endothelial cell-derived extracellular vesicles alter vascular smooth muscle cell phenotype through high-mobility group box proteins. J Extracell Vesicles. 2020;9(1):1781427. Epub 2020/09/19. doi: 10.1080/20013078.2020.1781427 ; PubMed Central PMCID: PMC7480479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song WP, Gu SJ, Tan XH, Gu YY, Song WD, Zeng JY, et al. Proteomic analysis and miRNA profiling of human testicular endothelial cell-derived exosomes: the potential effects on spermatogenesis. Asian J Androl. 2022;24(5):478–86. doi: 10.4103/aja202190 ; PubMed Central PMCID: PMC9491036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Riverol Y, Bai J, Bandla C, Garcia-Seisdedos D, Hewapathirana S, Kamatchinathan S, et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50(D1):D543–D52. doi: 10.1093/nar/gkab1038 ; PubMed Central PMCID: PMC8728295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.M MA, Dunstan RH, M MM, V KS, T KR. Analysis of Cytoplasmic and Secreted Proteins of Staphylococcus aureus Revealed Adaptive Metabolic Homeostasis in Response to Changes in the Environmental Conditions Representative of the Human Wound Site. Microorganisms. 2020;8(7). Epub 20200720. doi: 10.3390/microorganisms8071082 ; PubMed Central PMCID: PMC7409162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Statello L, Maugeri M, Garre E, Nawaz M, Wahlgren J, Papadimitriou A, et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS One. 2018;13(4):e0195969. Epub 20180424. doi: 10.1371/journal.pone.0195969 ; PubMed Central PMCID: PMC5918169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akil A, Gutierrez-Garcia AK, Guenter R, Rose JB, Beck AW, Chen H, et al. Notch Signaling in Vascular Endothelial Cells, Angiogenesis, and Tumor Progression: An Update and Prospective. Front Cell Dev Biol. 2021;9:642352. Epub 20210216. doi: 10.3389/fcell.2021.642352 ; PubMed Central PMCID: PMC7928398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El Bejjani R, Hammarlund M. Notch signaling inhibits axon regeneration. Neuron. 2012;73(2):268–78. doi: 10.1016/j.neuron.2011.11.017 ; PubMed Central PMCID: PMC3690129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallez Y, Huber P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta. 2008;1778(3):794–809. Epub 2007/10/27. doi: 10.1016/j.bbamem.2007.09.003 . [DOI] [PubMed] [Google Scholar]
- 23.Plutzky J. The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ Res. 2011;108(8):1002–16. doi: 10.1161/CIRCRESAHA.110.226860 . [DOI] [PubMed] [Google Scholar]
- 24.Roche PH, Figarella-Branger D, Daniel L, Bianco N, Pellet W, Pellissier JF. Expression of cell adhesion molecules in normal nerves, chronic axonal neuropathies and Schwann cell tumors. J Neurol Sci. 1997;151(2):127–33. doi: 10.1016/s0022-510x(97)00110-x . [DOI] [PubMed] [Google Scholar]
- 25.Chernov AV, Dolkas J, Hoang K, Angert M, Srikrishna G, Vogl T, et al. The calcium-binding proteins S100A8 and S100A9 initiate the early inflammatory program in injured peripheral nerves. J Biol Chem. 2015;290(18):11771–84. Epub 20150319. doi: 10.1074/jbc.M114.622316 ; PubMed Central PMCID: PMC4416877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saher G, Quintes S, Mobius W, Wehr MC, Kramer-Albers EM, Brugger B, et al. Cholesterol regulates the endoplasmic reticulum exit of the major membrane protein P0 required for peripheral myelin compaction. J Neurosci. 2009;29(19):6094–104. doi: 10.1523/JNEUROSCI.0686-09.2009 ; PubMed Central PMCID: PMC6665514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackett AR, Strickland A, Milbrandt J. Disrupting insulin signaling in Schwann cells impairs myelination and induces a sensory neuropathy. Glia. 2020;68(5):963–78. Epub 20191123. doi: 10.1002/glia.23755 ; PubMed Central PMCID: PMC7067678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang JK, Jarjour AA, Nait Oumesmar B, Kerninon C, Williams A, Krezel W, et al. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14(1):45–53. Epub 20101205. doi: 10.1038/nn.2702 ; PubMed Central PMCID: PMC4013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu S, Jia J, Zhou H, Zhang C, Liu L, Liu J, et al. PTEN modulates neurites outgrowth and neuron apoptosis involving the PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2019;20(5):4059–66. Epub 20190910. doi: 10.3892/mmr.2019.10670 ; PubMed Central PMCID: PMC6797942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pompili E, Fabrizi C. Thrombin in peripheral nerves: friend or foe? Neural Regen Res. 2021;16(6):1223–4. doi: 10.4103/1673-5374.300446 ; PubMed Central PMCID: PMC8224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vela JM, Molina-Holgado E, Arevalo-Martin A, Almazan G, Guaza C. Interleukin-1 regulates proliferation and differentiation of oligodendrocyte progenitor cells. Mol Cell Neurosci. 2002;20(3):489–502. doi: 10.1006/mcne.2002.1127 . [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Chopp M, Szalad A, Lu X, Zhang Y, Wang X, et al. Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type II Diabetic Mice. Diabetes. 2020. doi: 10.2337/db19-0432 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Liang Y, Pan K, Li H, Yu M, Guo W, et al. Schwann cells secrete extracellular vesicles to promote and maintain the proliferation and multipotency of hDPCs. Cell Prolif. 2017;50(4). doi: 10.1111/cpr.12353 ; PubMed Central PMCID: PMC6529079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basu S, Barbur I, Calderon A, Banerjee S, Proweller A. Notch signaling regulates arterial vasoreactivity through opposing functions of Jagged1 and Dll4 in the vessel wall. Am J Physiol Heart Circ Physiol. 2018;315(6):H1835–H50. Epub 20180831. doi: 10.1152/ajpheart.00293.2018 ; PubMed Central PMCID: PMC6336965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharghi-Namini S, Tan E, Ong LL, Ge R, Asada HH. Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci Rep. 2014;4:4031. Epub 20140207. doi: 10.1038/srep04031 ; PubMed Central PMCID: PMC3916896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bosse K, Hans CP, Zhao N, Koenig SN, Huang N, Guggilam A, et al. Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J Mol Cell Cardiol. 2013;60:27–35. Epub 20130411. doi: 10.1016/j.yjmcc.2013.04.001 ; PubMed Central PMCID: PMC4058883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tawa M, Shimosato T, Nakagawa K, Okamura T, Ohkita M. Soluble Guanylate Cyclase-Mediated Relaxation in Aortas from Rats with Renovascular Hypertension. Pharmacology. 2022;107(3–4):235–40. Epub 20211220. doi: 10.1159/000520655 . [DOI] [PubMed] [Google Scholar]
- 38.Del Valle-Perez B, Martinez VG, Lacasa-Salavert C, Figueras A, Shapiro SS, Takafuta T, et al. Filamin B plays a key role in vascular endothelial growth factor-induced endothelial cell motility through its interaction with Rac-1 and Vav-2. J Biol Chem. 2010;285(14):10748–60. Epub 20100128. doi: 10.1074/jbc.M109.062984 ; PubMed Central PMCID: PMC2856282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harman K, Katnick J, Lim R, Zaheer A, de la Torre JC. Glia maturation factor beta stimulates axon regeneration in transected rat sciatic nerve. Brain Res. 1991;564(2):332–5. doi: 10.1016/0006-8993(91)91472-d . [DOI] [PubMed] [Google Scholar]
- 40.Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795–806. Epub 2013/09/17. doi: 10.1002/glia.22558 . [DOI] [PubMed] [Google Scholar]
- 41.Lopez-Leal R, Court FA. Schwann Cell Exosomes Mediate Neuron-Glia Communication and Enhance Axonal Regeneration. Cell Mol Neurobiol. 2016;36(3):429–36. doi: 10.1007/s10571-015-0314-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ching RC, Kingham PJ. The role of exosomes in peripheral nerve regeneration. Neural Regen Res. 2015;10(5):743–7. doi: 10.4103/1673-5374.156968 ; PubMed Central PMCID: PMC4468764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilson ER, Della-Flora Nunes G, Weaver MR, Frick LR, Feltri ML. Schwann cell interactions during the development of the peripheral nervous system. Dev Neurobiol. 2021;81(5):464–89. Epub 20200505. doi: 10.1002/dneu.22744 ; PubMed Central PMCID: PMC7554194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luczkowska K, Stekelenburg C, Sloan-Bena F, Ranza E, Gastaldi G, Schwitzgebel V, et al. Hyperinsulinism associated with GLUD1 mutation: allosteric regulation and functional characterization of p.G446V glutamate dehydrogenase. Hum Genomics. 2020;14(1):9. Epub 20200306. doi: 10.1186/s40246-020-00262-8 ; PubMed Central PMCID: PMC7060525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poitelon Y, Kopec AM, Belin S. Myelin Fat Facts: An Overview of Lipids and Fatty Acid Metabolism. Cells. 2020;9(4). Epub 20200327. doi: 10.3390/cells9040812 ; PubMed Central PMCID: PMC7226731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chrast R, Saher G, Nave KA, Verheijen MH. Lipid metabolism in myelinating glial cells: lessons from human inherited disorders and mouse models. J Lipid Res. 2011;52(3):419–34. Epub 20101109. doi: 10.1194/jlr.R009761 ; PubMed Central PMCID: PMC3035679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeBlanc AC, Foldvari M, Spencer DF, Breckenridge WC, Fenwick RG, Williams DL, et al. The apolipoprotein A-I gene is actively expressed in the rapidly myelinating avian peripheral nerve. J Cell Biol. 1989;109(3):1245–56. doi: 10.1083/jcb.109.3.1245 ; PubMed Central PMCID: PMC2115768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez-Pecchi V, Valdes S, Pons V, Honorato P, Martinez LO, Lamperti L, et al. Apolipoprotein A-I enhances proliferation of human endothelial progenitor cells and promotes angiogenesis through the cell surface ATP synthase. Microvasc Res. 2015;98:9–15. Epub 20141108. doi: 10.1016/j.mvr.2014.11.003 . [DOI] [PubMed] [Google Scholar]
- 49.Albers JJ, Vuletic S, Cheung MC. Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism. Biochim Biophys Acta. 2012;1821(3):345–57. Epub 20110628. doi: 10.1016/j.bbalip.2011.06.013 ; PubMed Central PMCID: PMC3192936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vuletic S, Jin LW, Marcovina SM, Peskind ER, Moller T, Albers JJ. Widespread distribution of PLTP in human CNS: evidence for PLTP synthesis by glia and neurons, and increased levels in Alzheimer’s disease. J Lipid Res. 2003;44(6):1113–23. Epub 20030401. doi: 10.1194/jlr.M300046-JLR200 . [DOI] [PubMed] [Google Scholar]
- 51.Zhou M, Hu M, He S, Li B, Liu C, Min J, et al. Effects of RSC96 Schwann Cell-Derived Exosomes on Proliferation, Senescence, and Apoptosis of Dorsal Root Ganglion Cells In Vitro. Med Sci Monit. 2018;24:7841–9. doi: 10.12659/MSM.909509 ; PubMed Central PMCID: PMC6228118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baron-Van Evercooren A, Kleinman HK, Seppa HE, Rentier B, Dubois-Dalcq M. Fibronectin promotes rat Schwann cell growth and motility. J Cell Biol. 1982;93(1):211–6. doi: 10.1083/jcb.93.1.211 ; PubMed Central PMCID: PMC2112101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reina S, Nibali SC, Tomasello MF, Magri A, Messina A, De Pinto V. Voltage Dependent Anion Channel 3 (VDAC3) protects mitochondria from oxidative stress. Redox Biol. 2022;51:102264. Epub 20220212. doi: 10.1016/j.redox.2022.102264 ; PubMed Central PMCID: PMC8857518. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD041547 and 10.6019/PXD041547. All other relevant data are within the paper and its Supporting Information files.