Abstract
In several neuronal cell systems, fibroblast-derived growth factor (FGF) and nerve growth factor (NGF) act as neurogenic agents, whereas epidermal growth factor (EGF) acts as a mitogen. The mechanisms responsible for these different cellular fates are unclear. We report here that although FGF, NGF, and EGF all activate mitogen-activated protein (MAP) kinase (extracellular signal-related kinase [ERK]) in rat hippocampal (H19-7) and pheochromocytoma (PC12) cells, the activation of ERK by the neurogenic agents FGF and NGF is dependent upon protein kinase Cδ (PKCδ), whereas ERK activation in response to the mitogenic EGF is independent of PKCδ. Antisense PKCδ oligonucleotides or the PKCδ-specific inhibitor rottlerin inhibited FGF- and NGF-induced, but not EGF-induced, ERK activation. In contrast, EGF-induced ERK activation was inhibited by the phosphatidylinositol-3-kinase inhibitor wortmannin, which had no effect upon FGF-induced ERK activation. Rottlerin also inhibited the activation of MAP kinase kinase (MEK) in response to activated Raf, but had no effect upon c-Raf activity or ERK activation by activated MEK. These results indicate that PKCδ functions either downstream from or in parallel with c-Raf, but upstream of MEK. Inhibition of PKCδ also blocked neurite outgrowth induced by FGF and NGF in PC12 cells and by activated Raf in H19-7 cells, indicating a role for PKCδ in the neurogenic effects of FGF, NGF, and Raf. Interestingly, the PKCδ requirement is apparently cell type specific, since FGF-induced ERK activation was independent of PKCδ in NIH 3T3 murine fibroblasts, in which FGF is a mitogen. These data demonstrate that PKCδ contributes to growth factor specificity and response in neuronal cells and may also promote cell-type-specific differences in growth factor signaling.
Although activation of mitogen-activated protein (MAP) kinases (extracellular signal-related kinase 1 [ERK1] and -2) by growth factors can lead to a variety of cellular fates, including growth and differentiation, the mechanism by which specificity is determined is not known. One explanation for the observed differences involves the duration of ERK activation (32). For example, epidermal growth factor (EGF) induces transient activation of ERKs and stimulates proliferation of pheochromocytoma (PC12) cells, while fibroblast-derived growth factor (FGF) and nerve growth factor (NGF) stimulate prolonged ERK activation and induce cellular differentiation (46). Another potential explanation is that there are differences in the intracellular signals that couple growth factors to ERKs. Several molecules exclusive of the linear Ras, c-Raf, MAP kinase kinase (MEK), and MAP kinase pathways have been shown to mediate ERK activation (4, 13, 41, 47) and among these are members of the protein kinase C (PKC) family.
To date, 11 members of the PKC superfamily have been identified (reviewed in references 9, 22, and 36). The PKCs have been classified into three groups based upon their ability to be activated by Ca2+ and diacylglycerol (DAG). The classical PKCs (cPKCs) are activated by both Ca2+ and DAG and include the α, βI, βII, and γ isoforms. The Ca2+-independent but DAG-dependent isoforms (θ, η, δ, and ɛ) comprise the novel PKCs (nPKCs). Finally, the atypical PKCs (aPKCs), ζ, ι/λ, and μ, are both Ca2+ and DAG independent. Various PKCs have been shown to mediate or modulate the activation of ERKs by growth factors, hormones, and phorbol esters (3, 31, 41, 45). Many of these PKCs act as potentiators of cell cycle progression.
Interestingly, PKCδ differs from other closely related PKCs, such as PKCɛ. PKCδ has a distinct subcellular localization (15) and mediates tetradecanoyl phorbol acetate-induced differentiation of murine myeloid progenitor cells into macrophages (34) as well as the secretory response of antigen-stimulated rat basophilic RBL-2H3 cells (34, 44). Overexpression of PKCδ leads to growth arrest in vascular smooth muscle, capillary endothelial, NIH 3T3, and CHO cells (14, 20, 33, 48), a function often associated with differentiation. Furthermore, N-myc-induced transformation in a rat neuroblastoma cell line results in a decrease in PKCδ expression (2). Consistent with these studies, PKCδ blocks cellular transformation by Src and has been postulated to be a tumor suppressor (29). Finally, PKCδ, but not PKCɛ, is translocated to the membrane in response to NGF in PC12 cells (38). Thus, PKCδ appears to have a growth-inhibiting and differentiating function in a variety of cell types.
In the present study, we have investigated the role of PKCδ in the activation of MAP kinase and the induction of neurite outgrowth in PC12 and H19-7 cells. The conditionally immortalized H19-7 cell line was generated by transducing rat E17 hippocampal cells with a retroviral vector expressing a temperature-sensitive simian virus 40 large T antigen (10). At the nonpermissive temperature, when T is inactivated, H19-7 cells differentiate upon stimulation by FGF, but not upon exposure to EGF (10, 25, 26). In contrast to PC12 cells, H19-7 cells lack the Trk receptor and therefore are nonresponsive to NGF (11).
We now show that PKCδ is required for ERK activation by FGF and other differentiating factors in both H19-7 and PC12 cells. Preincubation of cells with rottlerin, a PKC inhibitor with specificity for the δ isoform (17), blocks activation of MEK but not Raf, suggesting that PKCδ acts either downstream or in parallel with Raf. Finally, rottlerin inhibits neurite outgrowth in response to activated Raf in H19-7 cells and FGF or NGF in PC12 cells. The results presented here suggest that PKCδ is required for the activation of MEK by FGF or NGF in neuronal cells, and this pathway may account for some of the selective effects of differentiating versus growth-promoting factors.
MATERIALS AND METHODS
Materials.
Receptor-grade EGF was purchased from Biomedical Technologies, Inc. (Stoughton, Mass.). Basic FGF was purchased from Research Diagnostics, Inc. (Flanders, N.J.). Phorbol 12, 13-dibutyrate (PDBu), phorbol 12-myristate 13-acetate, phosphatidylserine, wortmannin, myelin basic protein (MBP), peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG), and peroxidase-conjugated goat anti-mouse IgG were purchased from Sigma Chemicals (St. Louis, Mo.). Chelerythrine chloride, Gö 6976, and rottlerin were purchased from Calbiochem (La Jolla, Calif.). The MEK inhibitor PD098059 was a gift from Alan Saltiel (Parke-Davis). Anti-MAP kinase antiserum (Ab283) was developed as previously described (25). Monoclonal antibodies 12CA5 and 9E10 against the hemagglutinin (HA) and myc epitopes, respectively, were purchased from BabCo (Emeryville, Calif.). High-affinity rat anti-HA monoclonal antibody (3F10), grade II NGF, and peroxidase-conjugated, affinity-purified sheep anti-rat Fab Ig were purchased from Boehringer Mannheim (Indianapolis, Ind.). Anti-active MAP kinase polyclonal antibody was purchased from Promega (Madison, Wis.). Phospho-specific MEK 1/2 (Ser 217/221) polyclonal antibody was purchased from New England BioLabs (Beverly, Mass.). Monoclonal antibody M5 against the FLAG epitope and X-Omat film were purchased from Eastman Kodak Co. (New Haven, Conn.). Polyclonal MEK antibody (C-18) was purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). The PKC monoclonal antibody sampler kit was purchased from Transduction Labs (Lexington, Ky.). Polyclonal rabbit anti-PKCδ was from Zymed (South San Francisco, Calif.). Protein G-Sepharose (4 Fast Flow) was purchased from Pharmacia Biotech AB (Uppsala, Sweden). Protein A-Sepharose was supplied by Jeffrey Bluestone (University of Chicago). Enhanced chemiluminescence reagents and [γ-32P]ATP (6,000 Ci/mmol) were purchased from DuPont/NEN Research Products (Boston, Mass.). The purified, kinase-dead MEK (MEK K97A) was a gift from Angus MacNichol (University of Chicago). The activated MEK-2E and HA-tagged mouse ERK2 constructs were described previously (25). The FLAG-Raf construct was a gift from Andrey Shaw (Washington University). The myc-PKCδ plasmid was a gift from Peter Parker (ICRF, London, United Kingdom). Plasmid DNAs were prepared by CsCl-ethidium bromide gradient centrifugation as previously described (25) or by purification through columns according to the manufacturer’s instructions (Qiagen, Chatsworth, Calif.).
Cell culture.
The immortalized H19-7 cells were generated from embryonic rat hippocampal cells as previously described (10). A subclone of H19-7 stably expressing an oncogenic Raf-estrogen receptor (ER) fusion protein, ΔRaf-1:ER, was previously described (25). PC12 cells were grown on tissue culture plates coated with poly-l-lysine in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 5% horse serum and supplemented with antibiotics (50 U of penicillin per ml, 50 μg of streptomycin per ml) at 37°C in a 95% air–5% CO2 atmosphere. Quiescent cells were obtained by starving with 2% FBS and 1% horse serum overnight. NIH 3T3 cells were grown on tissue culture plates in DMEM containing 10% fetal calf serum (FCS) supplemented with antibiotics (50 U of penicillin per ml, 50 μg of streptomycin per ml) at 37°C in a 95% air–5% CO2 atmosphere. Quiescent cells were obtained by starving with 0.5% FCS overnight.
Transient transfections.
Approximately 106 cells were seeded on 100-mm-diameter plates and incubated overnight. The medium was changed to serum-free OptiMem (Gibco/BRL), and cells were transfected with a total of 10 μg of plasmid DNA and 40 μl of TransIt LT-1 according to the manufacturer’s protocol (Pan Vera Corp., Madison, Wis.). Ten percent of the total plasmid DNA consisted of pGreen Lantern-1 (Gibco/BRL), and the percentage of green fluorescent protein-expressing cells was scored to normalize transfection efficiency between groups. Cells were made quiescent for 24 h prior to treatment and harvesting. PC12 cells were transfected as described above, except that Effectene transfection reagent (Qiagen) was used as per the manufacturer’s protocol.
Treatment of cells with ODNs.
H19-7 or PC12 cells were seeded in six-well poly-l-lysine-coated plates to near confluency. H19-7 cells were switched to N2 medium at 39°C, and PC12 cells were switched to 1% FCS–0.5% horse serum before oligonucleotide (ODN) addition. The antisense sequences used were 5′ GAAGGAGATGCGCTGGAA 3′ for PKCδ and 5′ GCCATTGAACACTACCAT 3′ for PKCɛ (12). The antisense sequence for PKCδ is based on nucleotides 10 to 27 of the murine coding sequence, while the sequence for PKCɛ is based on the start codon plus the next 15 downstream nucleotides. The appropriate sense sequence was used as a control. ODNs were added daily to a final concentration of 30 μM, and then the cells were incubated for 3 days (H19-7) or 7 days (PC12) prior to growth factor stimulation.
In vitro Raf and HA-ERK2 kinase assays.
FLAG-Raf or HA-ERK2 was overexpressed in H19-7 cells and pretreated with rottlerin or vehicle. Cells were then treated with or without FGF followed by two washings with phosphate-buffered saline (PBS). Cells were then lysed with 1% Triton-based lysis buffer (TLB) containing 1% Triton X-100, 50 mM Tris-HCl [pH 7.5], 40 mM β-glycerophosphate, 100 mM NaCl, 50 mM NaF, 2 mM EDTA, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 μg of aprotinin per ml, 1 μg of leupeptin per ml, and 20 mM ρ-nitrophenyl phosphate. The cell debris was removed by centrifugation (14,000 rpm for 10 min [Biofuge Fresco; Heraeus Instruments] at 4°C), and protein concentrations were determined by a Bio-Rad protein assay (Bio-Rad, Hercules, Calif.) with bovine serum albumin as a standard. Lysates were precleared with 50 μl of protein G-Sepharose for 30 min at 4°C. The M5 and 12CA5 monoclonal antibodies against the FLAG and HA epitopes, respectively, were coupled to protein G-Sepharose beads by adding 20 μg of M5 or 12CA5 to 1 ml of a 50:50 slurry of protein G-Sepharose in TLB overnight at 4°C. One hundred microliters of the antibody-protein G-Sepharose complex was added to 500 μg of cellular lysate protein, and this mixture was incubated for 3 h at 4°C. Immune complexes were then washed three times with TLB and two times in kinase buffer (1× kinase buffer is 25 mM HEPES [pH 7.4], 10 mM MgCl2, 1 mM MnCl2, 1 mM dithiothreitol, and 0.2 mM sodium vanadate). The final pellet was resuspended with 1× (vol/vol) kinase buffer, and reactions were started by addition of 2 μM ATP, 5 μCi of [γ-32P]ATP, and 100 ng of purified MEK K97A or MBP and carried out for 30 min at 30°C. Reactions were stopped by addition of 6× concentrated sample buffer and boiling for 5 min at 100°C. Beads were pelleted by centrifugation (14,000 rpm for 5 min), and supernatants were loaded onto a 10% acrylamide separation gel. Proteins were transferred to nitrocellulose and subjected to autoradiography.
Cellular fractionations.
H19-7 cells were starved overnight prior to treatment. Cells were lysed by addition of a hypotonic buffer (40 mM HEPES, 4 mM EDTA, 2 mM EGTA, 10 mM dithiothreitol, 1 mM sodium vanadate, 1 mM PMSF, 1 μg of aprotinin per ml, 1 μg of leupeptin per ml, 20 mM ρ-nitrophenyl phosphate) and incubated for 30 min at 4°C. The cells were then sonicated twice with a Sonifier cell disruptor model W140 (Heat Systems-Ultrasonics, Inc., Plainview, N.Y.) at setting 5. Membranes were pelleted by centrifugation (100,000 rpm for 1 h at 4°C) in an Optima TLX ultracentrifuge by using the TLA 120.1 rotor and polycarbonate centrifuge tubes (8 by 34 mm) (Beckman, Palo Alto, Calif.). The supernatant was collected as the cytosolic fraction, and the pellets were washed twice with PBS. The final pellet was resuspended in TLB and incubated with agitation for 1 h at 4°C. Membranes were collected as the supernatant following centrifugation (14,000 rpm for 5 min at 4°C). Protein concentrations in each fraction were quantitated by Bio-Rad protein assay, and equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Western analysis.
Cell extracts (10 to 20 μg) were resolved on a 10% acrylamide separation gel by SDS-PAGE. Proteins were transferred to a nitrocellulose membrane. Membrane blocking, washing, antibody incubation, and detection by enhanced chemiluminescence were performed as previously described (26). When antibodies against phospho-specific peptides were used, blots were stripped by washing six times for 5 min each with Tris-buffered saline (TBS)-Tween (0.1%) at room temperature, 30 min at 55°C with stripping buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 100 mM 2-mercaptoethanol), and finally six times for 5 min each with TBS-Tween at room temperature. The stripped blots were then reprobed with the corresponding pan-ERK or pan-MEK antibody to ensure equal protein loading.
RESULTS
PKCδ is activated by FGF but not EGF in H19-7 cells.
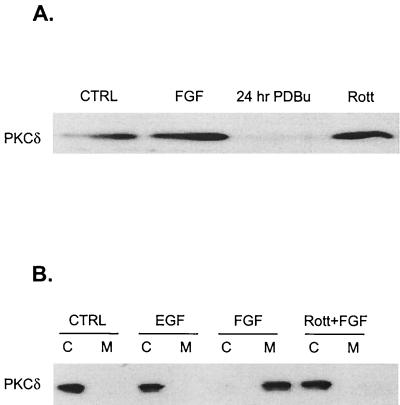
Since PKCδ is activated in response to differentiating agents in PC12 cells, as shown by membrane translocation (38), we initially determined whether PKCδ is similarly expressed and activated in a conditionally immortalized rat hippocampal cell line, H19-7. As shown in Fig. 1A, PKCδ was detected in H19-7 cells by immunoblotting with an anti-PKCδ antibody. As in other cells, chronic pretreatment of H19-7 cells with PDBu led to proteolytic degradation of PKCδ. However, no change in PKCδ expression was observed in response to rottlerin, a bisindolylmaleimide kinase inhibitor with selectivity for PKCδ (30). The 50% inhibitory concentrations (IC50s) of rottlerin were 3 to 6 μM for PKCδ; 30 to 42 μM for PKCα, -β, and -γ; and 80 to 100 μM for PKCɛ, -η, and -ζ (17). Upon treatment of H19-7 cells with the differentiating agent FGF, PKCδ was activated, as monitored by translocation from the cytosol to the membrane. In contrast, no membrane translocation occurred in response to EGF or when cells were pretreated with the kinase inhibitor rottlerin prior to FGF treatment (Fig. 1B). Consistent with these results, FGF but not EGF stimulated transiently transfected PKCδ kinase activity (7). These results indicate that PKCδ is activated in response to differentiating signals in H19-7 cells.
FIG. 1.
PKCδ is expressed in H19-7 cells and is selectively activated by neurogenic factors in H19-7 and PC12 cells. (A) H19-7 cells in N2 medium at 39°C were either untreated (CTRL), stimulated with 10 ng of FGF per ml for 10 min, pretreated with 400 nM PDBu for 24 h, or pretreated with 5 μM rottlerin (Rott) for 5 h. Cells were lysed, and equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-PKCδ antibody. (B) H19-7 cells were either untreated (CTRL), stimulated with 10 ng of EGF per ml, stimulated with 10 ng of FGF per ml, or pretreated with 5 μM rottlerin for 6 h prior to stimulation with 10 ng of FGF per ml for 10 min. The cells were lysed and fractionated into cytosolic (C) and membrane (M) fractions and immunoblotted with anti-PKCδ antibody as described in Materials and Methods.
PKCδ inhibitors block ERK activation by FGF, NGF, and Raf in neuronal cells.
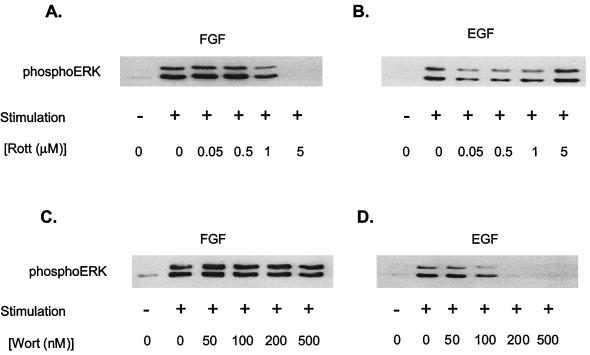
The inhibition of FGF-induced PKCδ activation by rottlerin is associated with an inhibition of MAP kinase (ERK1 and ERK2) activity. Pretreatment of H19-7 cells with 5 μM rottlerin completely suppressed FGF-induced phosphorylation of the conserved TEY motif within the activation loop in ERK (6), as shown by immunoblotting with an anti-phospho-ERK antibody (Fig. 2A). This dose of rottlerin is within the range of concentrations that inhibit PKCδ, but not other known PKC isozymes, and the effect was observed for at least 2 h following FGF treatment (7). In contrast, no inhibition of EGF-induced MAP kinase activation was observed at a comparable dose of rottlerin (Fig. 2B). When cells were analyzed for phosphatidylinositol-3-kinase (PI-3-kinase) involvement, the opposite pattern was obtained. Pretreatment of cells with ≥200 nM wortmannin, an inhibitor of PI-3-kinase, resulted in complete inhibition of EGF-stimulated MAP kinase activity but had no effect on FGF-stimulated MAP kinase activity (Fig. 2C and D). These results indicate that FGF and EGF activate MAP kinase by distinct signaling pathways and are consistent with a role for PKCδ in the activation of ERKs by FGF.
FIG. 2.
Effects of rottlerin and wortmannin on FGF- and EGF-induced ERK activation in H19-7 cells. (A) H19-7 cells in N2 medium at 39°C were pretreated with the indicated dose of rottlerin and then stimulated with 10 ng of FGF per ml for 10 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-phospho-ERK antibody. (B) Cells were treated as for panel A except that 10 ng of EGF per ml rather than FGF was used for stimulation. (C) H19-7 cells in N2 medium at 39°C were pretreated with the indicated dose of wortmannin for 15 min and then stimulated with 10 ng of FGF per ml for 10 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-phospho-ERK antibody. (D) Cells were treated for panel C, except that 10 ng of EGF per ml rather than FGF was used for stimulation.
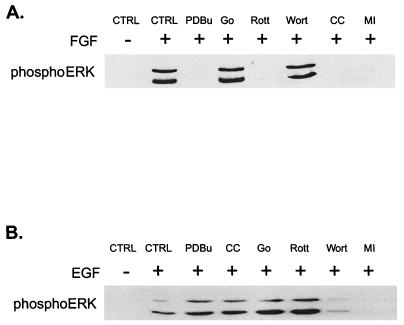
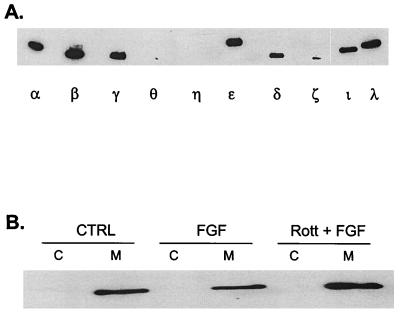
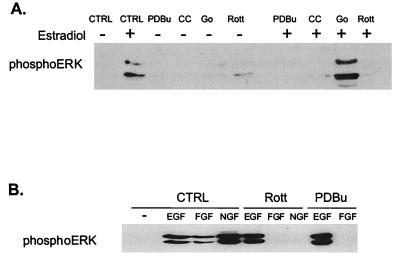
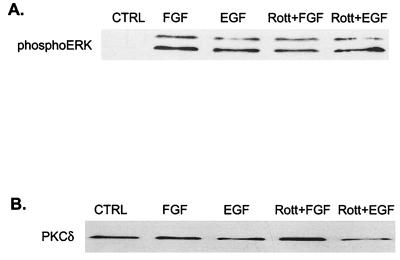
The requirement for PKC in ERK activation by FGF was verified by using other inhibitors of PKC activity. PDBu down-regulation, which inactivates all but the aPKCs, blocked activation of ERK1 and -2 by FGF (Fig. 3A). Similarly, pretreatment of cells with chelerythrine chloride, a nonspecific PKC inhibitor, also prevented FGF stimulation of MAP kinase. In contrast, an inhibitor of the Ca2+-dependent classic isozymes (Gö 6976) (16) had no effect, indicating that neither the α, β, nor γ PKC isozymes are involved. Whereas the MEK inhibitor PD98059 blocked ERK stimulation by both FGF and EGF, none of the inhibitors other than wortmannin suppressed EGF stimulation of ERK (Fig. 3B). Taken together, these results implicate one of the nPKCs in the FGF signaling pathway. Of the four nPKCs tested, H19-7 cells express only the δ and ɛ forms (Fig. 4A). Furthermore, PKCδ but not PKCɛ is translocated to the membrane in response to FGF (Fig. 4B). In conjunction with the dose response for inhibition by rottlerin, these studies suggest that PKCδ is the enzyme required for activation of MAP kinase in these cells.
FIG. 3.
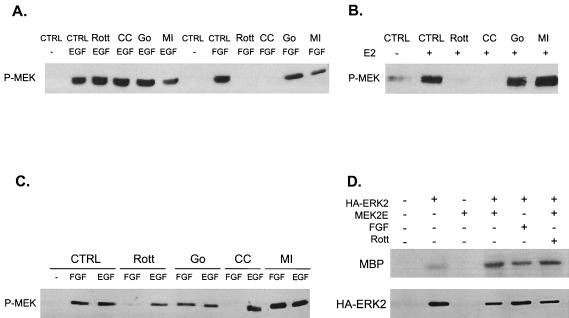
Effects of various inhibitors on FGF- and EGF-induced ERK activation in H19-7 cells. (A) H19-7 cells in N2 medium at 39°C were either untreated (CTRL) or were pretreated with 400 nM PDBu for 24 h, 1 μM Gö 6976 (Go) for 2 h, 5 μM rottlerin (Rott) for 6 h, 200 nM wortmannin (Wort) for 15 min, 1 μM chelerythrine chloride (CC) for 2 h, or 30 μM MEK inhibitor PD98059 (MI) for 15 min. Cells were then stimulated with 10 ng of FGF per ml for 10 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and then immunoblotted with anti-phospho-ERK antibody. (B) Cells were treated as in for panel A, except that they were stimulated with 10 ng of EGF per ml rather than FGF.
FIG. 4.
PKCɛ is expressed in H19-7 cells and is constitutively associated with the membrane fraction. (A) Expression of PKC isozymes in H19-7 cells. H19-7 cells were lysed, and equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide). Samples were immunoblotted with antibodies to PKCα, PKCβ, PKCγ, PKCθ, PKCη, PKCɛ, PKCδ, PKCζ, PKCι, and PKCλ. (B) H19-7 cells were either untreated (CTRL) or were stimulated with 10 ng of FGF per ml or pretreated with 5 μg of rottlerin for 6 h prior to stimulation with 10 ng of FGF per ml for 10 min (Rott + FGF). The cells were lysed and fractionated into cytosolic (C) and membrane (M) fractions and immunoblotted with anti-PKCɛ antibody as described in Materials and Methods.
A role for PKC in the activation of ERKs is not limited to FGF-treated DC12 cells or to H19-7 cells. In a subclone of H19-7 cells stably expressing a fusion protein between Raf and the ER (ΔRaf-1:ER), 1 μM estradiol activates Raf and is sufficient for neuronal differentiation (25). Down-regulation of PKC by chronic exposure to PDBu or pretreatment with the nonspecific PKC inhibitor chelerythrine chloride blocked ERK activation by Raf in ΔRaf-1:ER cells stimulated with 1 μM estradiol (Fig. 5A). As in the case of FGF, the inhibitor of the cPKCs (Gö 6976) had no significant effect on Raf-activated MAP kinase, but the PKCδ inhibitor rottlerin completely blocked activation. Similarly, PDBu down-regulation or pretreatment with rottlerin also inhibited stimulation of ERK by NGF or FGF in PC12 cells (Fig. 5B). Surprisingly, even though NIH 3T3 cells express PKCδ, rottlerin had no effect on ERK activation by FGF or EGF in these cells (Fig. 6). Taken together, these data suggest that PKCδ is required for FGF, NGF, or Raf stimulation of MAP kinase in both PC12 and H19-7 cells, and the effect is cell type specific.
FIG. 5.
Effects of various inhibitors on ERK activation in ΔRaf-1:ER and PC12 cells. (A) ΔRaf-1:ER cells were either untreated (CTRL) or were pretreated with 400 nM PDBu for 24 h, 1 μM Gö 6976 (Go) for 2 h, 5 μM rottlerin (Rott) for 6 h, or 1 μM chelerythrine chloride (CC) for 2 h and then stimulated with (+) or without (−) 1 μM estradiol for 30 min. After cell lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide) and immunoblotted with anti-phospho-ERK antibody. (B) PC12 cells were either untreated (CTRL) or were pretreated with 400 nM PDBu (PDBu) for 24 h or 5 μM rottlerin (Rott) for 6 h and then stimulated with 10 ng of EGF, FGF, or NGF per ml for 10 min. Samples were then processed as for panel A.
FIG. 6.
Rottlerin does not block FGF- or EGF-induced ERK activation in NIH 3T3 cells. (A) Effect of FGF or EGF on ERK phosphorylation. NIH 3T3 cells were either untreated (CTRL) or were stimulated with 10 ng of EGF or FGF per ml for 10 min in the presence or absence of 5 μM rottlerin (Rott). Cells were lysed, resolved by SDS-PAGE (10% polyacrylamide), and then immunoblotted with anti-phospho-ERK. (B) Expression of PKCδ in NIH 3T3 cells. Cells were treated as for panel A, lysed, resolved by SDS-PAGE (10% polyacrylamide), and then immunoblotted with anti-PKCδ antibody.
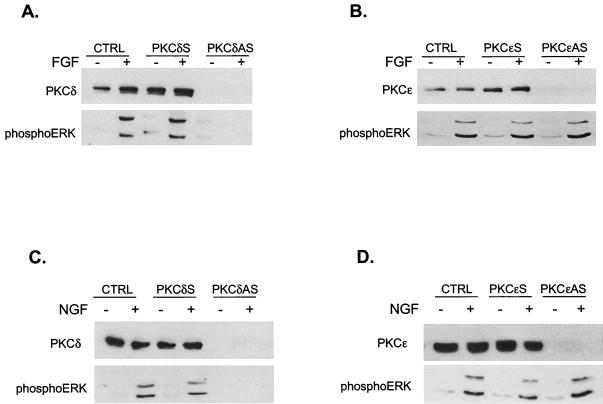
A role for PKCδ was directly confirmed by using an antisense oligonucleotide approach. H19-7 cells were shifted to 39°C in serum-free N2 medium prior to the addition of antisense or sense PKCδ or PKCɛ oligonucleotides. Cells were left untreated or stimulated with FGF and then assayed for expression of the respective PKC isozymes as well as ERK activity. As shown in Fig. 7, the antisense oligonucleotides were able to completely block expression of PKCδ (Fig. 7A) or PKCɛ (Fig. 7B). However, only antisense PKCδ prevented phosphorylation and thus activation of ERK1 and -2 by FGF. A similar inhibition of ERK activation by NGF was observed in PC12 cells pretreated with PKCδ (Fig. 7C) but not PKCɛ antisense (Fig. 7D) ODNs. The same effect was seen in FGF-treated PC12 cells (7). These results confirm that rottlerin is acting as a specific inhibitor of PKCδ which can play a key role in the activation of MAP kinase by neuronal differentiating factors.
FIG. 7.
PKCδ but not PKCɛ is required for FGF-induced ERK activation. (A) Antisense (AS) PKCδ phosphorothioate ODNs block PKCδ expression and ERK activation in H19-7 cells. Cells were pretreated with either sense or antisense PKCδ ODNs as described in Materials and Methods and then either left untreated (CTRL) or were stimulated with 10 ng of FGF per ml for 10 min. Cells were then lysed, and the lysates were resolved by SDS-PAGE (10% polyacrylamide) and assayed for PKCδ expression by immunoblotting with anti-PKCδ antibody. MAP kinase activation was assayed by immunoblotting with anti-phospho-ERK antibody. (B) Antisense PKCɛ phosphorothioate oligonucleotides block PKCɛ expression, but do not inhibit ERK activation in H19-7 cells. Cells were pretreated with either sense or antisense PKCɛ oligonucleotides as described in Materials and Methods and then either were left untreated or were stimulated with 10 ng of FGF per ml for 10 min. Cells were then lysed, and the lysates were resolved by SDS-PAGE (10% polyacrylamide) and assayed for PKCɛ expression by immunoblotting with anti-PKCɛ antibody. MAP kinase activation was assayed by immunoblotting with anti-phospho-ERK antibody. (C) Antisense PKCδ phosphorothioate oligonucleotides block PKCδ expression and ERK activation by NGF in PC12 cells. PC12 cells were treated and analyzed as for panel A, except that 50 ng of NGF per ml was used instead of FGF. (D) Antisense PKCɛ phosphorothioate oligonucleotides block PKCɛ expression but do not inhibit ERK activation by NGF in PC12 cells. PC12 cells were treated and analyzed as for panel B, except that 50 ng of NGF per ml was used instead of FGF.
Rottlerin does not inhibit c-Raf activation or Raf kinase activity.
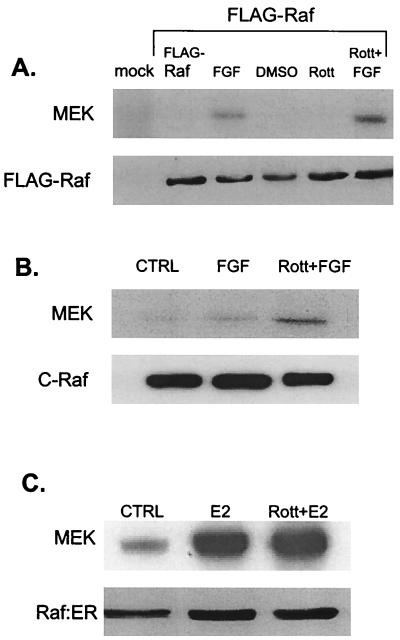
At what point in the pathway is PKCδ acting? The observation that rottlerin suppresses ERK stimulation by constitutively activated Raf suggests that the inhibitory step is downstream of Raf. To investigate this possibility further, we focused on H19-7 cells and determined whether Raf kinase activity is suppressed by rottlerin by using three different approaches. First, H19-7 cells were transfected with FLAG-tagged c-Raf. After exposure of cells to the PKCδ inhibitor prior to stimulation, Raf was immunoprecipitated with anti-FLAG antibody and then assayed for kinase activity with kinase-dead MEK as a substrate. As shown in Fig. 8A, pretreatment with rottlerin actually enhanced c-Raf activity in response to FGF. Second, we directly assayed endogenous c-Raf that was immunoprecipitated from H19-7 cells treated with FGF in the presence or absence of rottlerin (Fig. 8B). The results clearly indicate that c-Raf kinase activity is not suppressed by rottlerin and in fact seems to be further stimulated, probably due to suppression of feedback inhibition by downstream effectors (49). To ensure that the suppression of ERK activity by rottlerin is not due to modulation of Raf kinase activity, we determined the effect of rottlerin on ΔRaf-1:ER by immunoprecipitating the Raf fusion protein with anti-ER antibody from estradiol-stimulated cells. As shown in Fig. 8C, rottlerin had no significant effect on ΔRaf-1:ER activity. These results indicate that PKCδ is required for activation of the signaling pathway downstream of Raf.
FIG. 8.
Rottlerin (Rott) does not block FGF-induced c-Raf, FLAG-Raf, or estradiol-induced ΔRaf-1:ER kinase activity. (A) H19-7 cells were transfected with either control vector or an expression vector for FLAG-tagged c-Raf. The cells were then left untreated (CTRL), pretreated with 5 μM rottlerin for 6 h, and/or stimulated with 10 ng of FGF per ml for 10 min. Following treatment, cells were lysed, and FLAG-Raf was immunoprecipitated with anti-FLAG antibody. The samples were resolved by SDS-PAGE (10% polyacrylamide) and assayed for Raf kinase activity by using inactive MEK as a substrate as described in Materials and Methods. The immunoprecipitated Raf was quantitated by immunoblotting with anti-FLAG antibody. DMSO, dimethyl sulfoxide. (B) H19-7 cells were left untreated, pretreated with 5 μM rottlerin for 6 h, and/or stimulated with 10 ng of FGF per ml for 10 min. Following treatment, cells were lysed, and endogenous c-Raf was immunoprecipitated with anti-c-Raf antibody. The samples were resolved by SDS-PAGE (10% polyacrylamide) and assayed for Raf kinase activity by using inactive MEK as a substrate as described in Materials and Methods. The immunoprecipitated Raf was quantitated by immunoblotting with anti-c-Raf antibody. (C) ΔRaf-1:ER cells were either untreated or were stimulated with 1 μM estradiol for 30 min. The cells were then lysed, and the ΔRaf-1:ER was immunoprecipitated with anti-ER antibodies and assayed for Raf kinase activity by using inactive MEK as a substrate as described in Materials and Methods. Immunoprecipitated ΔRaf-1:ER was quantitated by immunoblotting with anti-ER antibody.
PKCδ is required for MEK activation.
Since rottlerin inhibits ERK activity, it is likely that either activation of MEK or activation of ERK is being suppressed. To monitor the activation state of MEK, we analyzed MEK phosphorylation at the key serine residues in the activation loop by using an anti-phospho-MEK antibody. The results indicate that rottlerin and chelerythrine chloride block MEK activation by FGF but not EGF in both H19-7 and PC12 cells (Fig. 9). Consistent with our previous observations, the inhibitor of the cPKCs had no effect. Similar results were obtained when ΔRaf-1:ER cells stimulated with estradiol or PC12 cells stimulated with FGF or EGF in the presence or absence of the PKC inhibitors were evaluated (Fig. 9B and C). To determine whether activation of ERK by MEK requires PKCδ, H19-7 cells were cotransfected with expression vectors for constitutively activated MEK (MEK-2E) and HA-ERK2. Cells were either untreated or exposed to rottlerin, and then the HA-ERK2 was immunoprecipitated and assayed for kinase activity with MBP as a substrate. As shown in Fig. 9D, rottlerin had no significant effect on the activation of ERK by MEK-2E. However, rottlerin was able to inhibit FGF-induced ERK activation in the same experiment (7). Taken together, these results indicate that PKCδ is required for the phosphorylation and activation of MEK.
FIG. 9.
Activation of MEK but not ERK requires PKCδ. (A) PKCδ inhibitors block MEK activation by FGF but not EGF in H19-7 cells. Cells were either untreated (CTRL) or were pretreated with 1 μM Gö 6976 (Go) for 2 h, 5 μM rottlerin (Rott) for 6 h, 1 μM chelerythrine chloride (CC) for 2 h, or 30 μM MEK inhibitor PD98059 (MI) for 15 min. Cells were then stimulated with 10 ng of either FGF or EGF per ml for 10 min. After cell lysis, equal protein aliquots were resolved by SDS-PAGE (10% polyacrylamide). MEK activation was assayed by immunoblotting with anti-phospho-MEK (P-MEK) antibody. (B) PKCδ inhibitors block MEK activation by estradiol in ΔRaf-1:ER cells. Cells were pretreated as for panel A and then exposed to 1 μM estradiol for 30 min. Samples were resolved by SDS and assayed for MEK activation as for panel A. (C) PKCδ inhibitors block MEK activation by FGF but not EGF in PC12 cells. PC12 cells were treated and processed as for panel A. (D) Rottlerin does not block activation of ERK by constitutively activated MEK. H19-7 cells were mock transfected or cotransfected with an expression vector for HA-ERK2 and MEK-2E, a constitutively activated MEK. Cells were then left untreated or were pretreated with 5 μM rottlerin for 6 h or stimulated with 10 ng of FGF per ml as indicated. Following treatment, cells were lysed, and HA-ERK was immunoprecipitated with anti-HA antibody. The immunoprecipitated HA-ERK was assayed for kinase activity by using MBP as a substrate as described in Materials and Methods, and the reaction products were resolved by SDS-PAGE (10% polyacrylamide). The immunoprecipitated ERK was quantitated by immunoblotting.
PKCδ mediates neurite outgrowth.
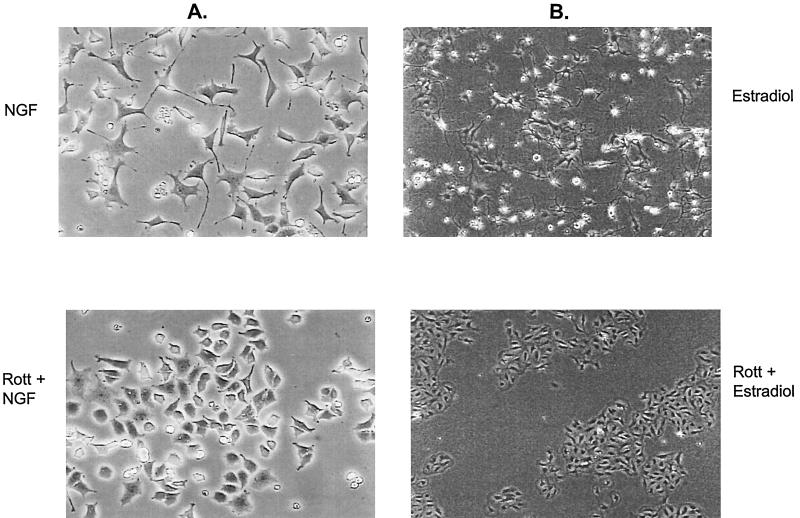
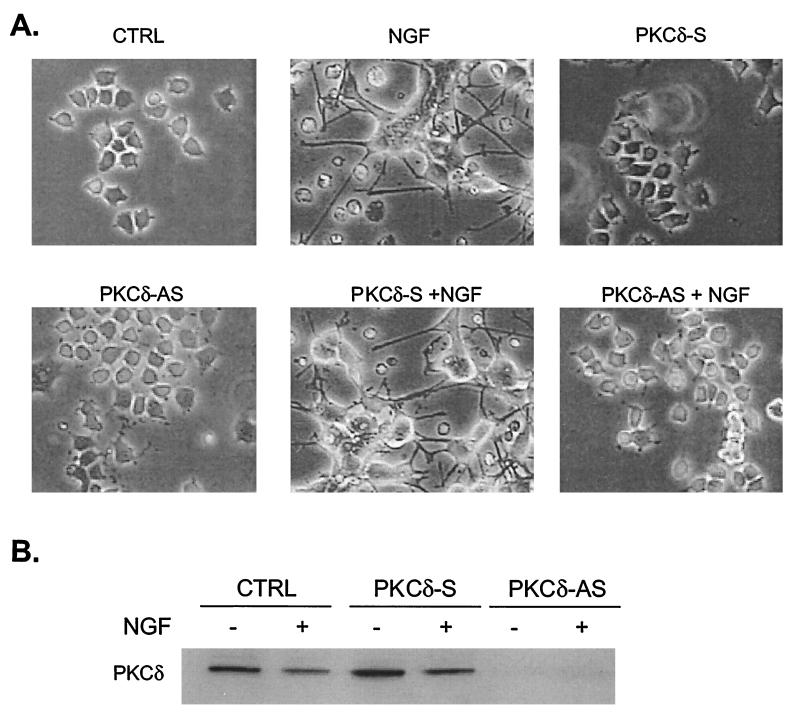
The MAP kinase signaling pathway plays a critical role in neuronal differentiation by FGF and NGF in PC12 cells (8, 39) and by activated Raf in H19-7 cells (25, 26). To test whether PKCδ is also necessary for these processes, PC12 cells were stimulated with NGF in the presence or absence of rottlerin, and the cells were monitored for morphological differentiation. As shown in Fig. 10A, rottlerin inhibited neurite outgrowth in NGF-treated cells. Pretreatment with rottlerin also inhibited FGF-induced neurite outgrowth in PC12 cells (7). Similarly, rottlerin prevented estradiol-induced neurite outgrowth in ΔRaf-1:ER cells (Fig. 10B), consistent with its ability to block ERK activation. In contrast, rottlerin did not block differentiation of H19-7 cells by FGF (7), consistent with our previous results demonstrating that FGF can promote neuronal differentiation via an MEK-independent pathway (26). The role of PKCδ in neurite outgrowth was confirmed by the use of antisense PKCδ oligonucleotides. As shown in Fig. 11A, pretreatment of PC12 cells with antisense but not sense PKCδ oligonucleotides suppressed neurite outgrowth in response to 5 days of NGF treatment. Under these conditions, the antisense oligonucleotides still blocked expression of PKCδ (Fig. 11B). These results indicate that PKCδ is an important physiological regulator of neurite outgrowth in at least two cell systems.
FIG. 10.
Rottlerin blocks neurite outgrowth in NGF-induced PC12 and estradiol-activated ΔRaf-1:ER cells. (A) PC12 cells untreated or pretreated with 5 μM rottlerin (Rott) and then exposed to 50 ng of NGF per ml for 4 days. Original magnification, ×100. (B) ΔRaf-1:ER cells untreated or pretreated with 5 μM rottlerin and then exposed to 1 μM estradiol for 1 day. Original magnification, ×40.
FIG. 11.
PKCδ antisense oligonucleotides block neurite outgrowth in NGF-induced PC12 cells. (A) PC12 cells either untreated (CTRL) or pretreated with sense (PKCδ-S) or antisense (PKCδ-AS) ODNs as described in Materials and Methods and then stimulated with 50 ng of NGF per ml (+ NGF) for 5 days as indicated. Original magnification, ×100. (B) Immunoblots of PKCδ from cells treated as described for panel A. Cell lysates were collected and resolved by SDS-PAGE (10% polyacrylamide) and assayed for PKCδ expression by immunoblotting with anti-PKCδ antibody.
DISCUSSION
This paper describes the surprising finding that PKCδ is required for activation of the MAP kinase cascade by neurogenic factors FGF and NFG in two different neuronal cell systems: hippocampal H19-7 and pheochromocytoma PC12 cells. In contrast, EGF, which acts as a mitogen in these cells, activates MAP kinase by a different, wortmannin-sensitive pathway. This effect is cell type specific, since FGF does not require PKCδ for MAP kinase induction in NIH 3T3 fibroblasts. Finally, PKCδ is also required for neurite outgrowth in response to neurogenic agents that are dependent upon MEK for differentiation. Moreover, the mechanism by which PKCδ activates this pathway has not been previously described. Surprisingly, our data suggest that PKCδ acts downstream of or in parallel with Raf and upstream of MEK in the FGF signaling cascade.
Many of the experiments presented here utilized the pharmacological agent rottlerin, a bisindolylmaleimide kinase inhibitor, as a potent inhibitor of PKCδ. Rottlerin also inhibits the activity of CaM kinase III (eEF2) at a similar concentration (IC50, 5.3 μM). Since a 6-h pretreatment with rottlerin was necessary to fully block mitogen-induced ERK activation, we cannot rule out the possibility that rottlerin might also be acting indirectly through effects on protein translation. It should be noted, however, that similar pretreatment times have been utilized to suppress PKCδ activity in other cell systems (29). In addition, other lines of evidence confirm that PKCδ is required for MAP kinase activation. First, the nonselective PKC inhibitors PDBu and chelerythrine chloride produced the same results as those seen with rottlerin. In contrast, Gö 6976, a PKC inhibitor with specificity for the Ca2+-dependent α and βI isoforms, did not mimic the effects of rottlerin. Second, we utilized an antisense strategy to demonstrate that blocking expression of the δ but not the ɛ isoform yielded the same effects as pretreatment with rottlerin. Taken together, these results indicate that PKCδ is necessary for ERK activation by FGF and NGF as well as neurite outgrowth induced by activated Raf or NGF in neuronal cells.
Some progress has been made recently in elucidating the signaling pathways mediated by FGF. Upon binding to the receptor, FGF interacts with heparin proteoglycan sulfates to promote dimerization and activation (40). Receptor tyrosine kinases undergo autophosphorylation upon stimulation; some of the phosphorylation sites act as anchors for other signaling molecules, and other sites within the catalytic domain are required for activity. There are at least seven autophosphorylation sites in FGFR1. A C-terminal site (Y766) recruits phospholipase Cγ (PLCγ) and has been implicated in neurite outgrowth of cultured cerebellar neurons (19). However, a recent analysis of FGFR1 and FGFR3 chimeras demonstrated that the juxtamembrane region is primarily responsible for neurite outgrowth in PC12 cells; the PLCγ binding site induced only a very modest increase in neurite outgrowth (28). Elimination of all of these phosphorylation sites except the two within the catalytic domain does not prevent FGF from activating the MAP kinase cascade or inducing PC12 cell differentiation (35). Instead, FGFR interacts via the juxtamembrane domain with a lipid-linked docking protein, termed SNT/FRS2, which forms a complex with Grb2, Sos (the activator of Ras), and the tyrosine phosphatase Shp2 upon FGF stimulation (24). This complex appears to be the primary mechanism by which FGF stimulates MAP kinase and PC12 cell differentiation (18). Interestingly, SNT/FRS2 also interacts with the NGF receptor and mediates NGF-induced PC12 cell differentiation, consistent with a common signaling mechanism. The observation that EGF does not signal via the SNT/FRS2 adapter protein and activates ERK independently of PKCδ, whereas FGF and NGF both require PKCδ, suggests that PKCδ may also be a component of this common neuronal differentiation signaling pathway.
Several lines of evidence have supported a role for PKC in PC12 cell neurite outgrowth. PLCγ activation by FGFR1 has been reported to promote MAP kinase activation (21). Similarly, mutational analysis of the Trk receptor suggests that the PLCγ binding site is required for signaling (37, 43). Since PLCγ generates DAG, an activator of PKC, these studies are consistent with a role for PKC. More direct evidence comes from microinjection studies using a PKC-neutralizing antibody (1) and bryostatin-mediated down-regulation of PKC (42) to inhibit neurite outgrowth. Using the first variable domains of PKCδ and PKCɛ as inhibitors, Messing and coworkers concluded that PKCɛ, but not PKCδ, is required for enhancement of NGF responses by phorbol esters and ethanol in PC12 cells (23). Surprisingly, they did not observe any inhibition of NGF-induced MAP kinase activation or neurite outgrowth by the PKCδ fragment, presumably due to incomplete inhibition of PKCδ activity. Weinstein and coworkers have noted that PKCδ is selectively translocated in response to NGF but not EGF in PC12 cells, whereas PKCα, -β, and -ɛ are not changed (38), consistent with our observations with H19-7 cells. However, the studies reported herein provide the first direct evidence that PKCδ is the specific PKC isozyme responsible for neurite outgrowth in PC12 cells.
Very recently, PKCδ (27) and PKCζ (5, 27) have been shown to be activated by a wortmannin-sensitive, PI-3-kinase-dependent pathway via phosphorylation of PKC by PDK1. Surprisingly, although EGF stimulated ERK by a wortmannin-sensitive pathway, the activation was not suppressed by PKC inhibitors. However, we cannot rule out the possibility that the aPKCζ might play a role in the EGF signaling pathway. In contrast, the PKCδ-dependent ERK activation that we have observed in response to FGF or NGF is wortmannin insensitive. Thus, it appears that PDK1 is not a key regulator of PKC or ERK activation by FGF or NGF in the neuronal cells.
Previous studies of nonneuronal cells have shown that Raf can be activated by PKCs. Cooper and colleagues demonstrated that constitutively activated PKCɛ and PKCα can activate Raf, and the phorbol ester-sensitive PKCs are required for EGF stimulation of Raf in NIH 3T3 and COS cells (3). Interestingly, our results indicate that the phorbol ester-sensitive PKCs are not required for EGF stimulation of MAP kinase in either PC12 or H19-7 cells. Recently, Parker and coworkers (41) showed by transfection studies with constitutively active PKCs that PKCα and PKCη and presumably other classic and novel PKC isozymes can activate Raf in COS cells. In contrast to these studies, using a similar approach, Ueda et al. (47) concluded that PKCδ, but not PKCα or PKCɛ, could activate MEK in a Raf-dependent manner in COS cells. While the reason for this discrepancy is not clear, these studies demonstrate that PKCδ as well as other PKCs can activate Raf.
The mechanism by which PKCδ regulates MEK activation remains to be elucidated. The only PKC that has been shown to activate MEK directly is PKCζ (41), and this signaling pathway is Raf independent. The role of Raf in the action of PKCδ in H19-7 and PC12 cells is not completely clear. In H19-7 cells, FGF activates c-Raf, and dominant-negative c-Raf blocks activation of ERK (25, 26). The data presented here show that PKCδ is not required for c-Raf activation or Raf kinase activity. Although we cannot rule out a role for PKCδ in B-Raf activation, the data clearly demonstrate that activation of MEK by c-Raf or B-Raf requires PKCδ. It appears that ERK activation by MEK does not require PKCδ, since rottlerin treatment did not significantly alter ERK activation by constitutively activated MEK. One possible mechanism by which PKCδ might activate MEK is through direct phosphorylation of MEK or indirect phosphorylation via a mechanism such as inactivation of phosphatases. Alternatively, PKCδ may act as a scaffolding protein that brings MAP kinase and its activators together in the appropriate cellular location.
The difference between the EGF and FGF-NGF signaling pathways in the activation of MEK in PC12 and H19-7 cells may be a contributing factor in the different physiological responses to these stimuli. Several lines of evidence have shown that activation of MEK is both required and sufficient for neurite outgrowth in PC12 cells (8, 39). In both PC12 and H19-7 cells, EGF activates a transient MAP kinetics signal, whereas that from FGF is prolonged, and the difference between the actions of EGF and FGF has been ascribed to the difference in the kinetics (32). However, it has recently been shown that Rap1 is required for the prolonged ERK activation, but not for the initial MAP kinase signal or for the neurite outgrowth (50). Thus, it is likely that the mechanism responsible for the initial activation of MEK and ERKs by FGF relative to EGF, which we have now shown involves PKCδ, could regulate the signaling cascade leading to neuronal differentiation.
ACKNOWLEDGMENTS
We thank Larry Hill and Jane Booker for assistance with the preparation of the manuscript.
This work was supported by National Institutes of Health grants NS33858 (M.R.R.) and CA46677 (D.A.F.), Pharmacological Sciences Training grant 5 T32 GM 07151-24 (K.C.C.), American Cancer Society grant BE-243 (D.A.F.), a Research Centers in Minority Institutions (RCMI) award from the Division of Research Resources (D.A.F.), a National Institutes of Health grant (RR 03037) to Hunter College (D.A.F.), and a gift from the Cornelius Crane Trust for Eczema Research (M.R.R.).
REFERENCES
- 1.Altin J G, Wetts R, Riabowol K T, Bradshaw R A. Testing the in vivo role of protein kinase C and c-Fos in neurite outgrowth by microinjection of antibodies into PC12 cells. Mol Biol Cell. 1992;3:323–333. doi: 10.1091/mbc.3.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernards R. N-myc disrupts protein kinase C-mediated signal transduction in neuroblastoma. EMBO J. 1991;10:1119–1125. doi: 10.1002/j.1460-2075.1991.tb08052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco M T, Moscat J, Rapp U, Cooper G M. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chao T-S O, Byron K L, Lee K-M, Villereal M, Rosner M R. Activation of MAP kinase by calcium-dependent and calcium-independent pathways. J Biol Chem. 1992;267:19876–19883. [PubMed] [Google Scholar]
- 5.Chou M M, Hou W, Johnson J, Graham L K, Lee M H, Chen C-S, Newton A C, Schaffhausen B S, Toker A. Regulation of protein kinase Cζ by PI 3-kinase and PDK-1. Curr Biol. 1998;8:1069–1077. doi: 10.1016/s0960-9822(98)70444-0. [DOI] [PubMed] [Google Scholar]
- 6.Cobb M H, Xu S, Cheng M, Ebert D, Robbins D, Goldsmith E, Robinson M. Structural analysis of the MAP kinase ERK2 and studies of MAP kinase regulatory pathways. Adv Pharmacol. 1996;36:49–65. doi: 10.1016/s1054-3589(08)60576-1. [DOI] [PubMed] [Google Scholar]
- 7.Corbit, K., and M. R. Rosner. Unpublished observations.
- 8.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 9.Dekker L V, Parker P J. Protein kinase C—a question of specificity. Trends Biochem Sci. 1994;19:73–77. doi: 10.1016/0968-0004(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 10.Eves E M, Tucker M S, Roback J D, Downen M, Rosner M R, Wainer B H. Immortal rat hippocampal cell lines exhibit neuronal and glial lineages and neurotrophin gene expression. Proc Natl Acad Sci USA. 1992;89:4373–4377. doi: 10.1073/pnas.89.10.4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eves, E. M., W. Xiong, and M. R. Rosner. Unpublished data.
- 12.Fleming I, MacKenzie S J, Vernon R G, Anderson N G, Houslay M D, Kilgour E. Protein kinase C isoforms play differential roles in the regulation of adipocyte differentiation. Biochem J. 1998;333:719–727. doi: 10.1042/bj3330719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost J A, Steen H, Shapiro P, Lewis T, Ahn N, Shaw P E, Cobb M H. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukumoto S, Nishizawa Y, Hosoi M, Koyama H, Yamakawa K, Ohno S, Morii H. Protein kinase C delta inhibits the proliferation of vascular smooth muscle cells by suppressing G1 cyclin expression. J Biol Chem. 1997;272:13816–13822. doi: 10.1074/jbc.272.21.13816. [DOI] [PubMed] [Google Scholar]
- 15.Goodnight J A, Mischak H, Kolch W, Mushinski J F. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. Isoform-specific association with microfilaments, Golgi, endoplasmic reticulum, and nuclear and cell membranes. J Biol Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- 16.Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller H J, Johannes F J. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase C isoenzymes. FEBS Lett. 1996;392:77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 17.Gschwendt M, Muller H J, Kielbassa K, Zang R, Kittstein W, Rincke G, Marks F. Rottlerin, a novel protein kinase inhibitor. Biochem Biophys Res Commun. 1994;199:93–98. doi: 10.1006/bbrc.1994.1199. [DOI] [PubMed] [Google Scholar]
- 18.Hadari Y R, Kouhara H, Lax I, Schlessinger J. Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor-induced PC12 cell differentiation. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hall H, Williams E J, Moore S E, Walsh F S, Prochiantz A, Doherty P. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr Biol. 1996;6:580–587. doi: 10.1016/s0960-9822(02)00544-4. [DOI] [PubMed] [Google Scholar]
- 20.Harrington E O, Loffler J, Nelson P R, Kent K C, Simons M, Ware J A. Enhancement of migration by protein kinase Calpha and inhibition of proliferation and cell cycle progression by protein kinase Cdelta in capillary endothelial cells. J Biol Chem. 1997;272:7390–7397. doi: 10.1074/jbc.272.11.7390. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Mohammadi M, Rodrigues G A, Schlessinger J. Reduced activation of RAF-1 and MAP kinase by a fibroblast growth factor receptor mutant deficient in stimulation of phosphatidylinositol hydrolysis. J Biol Chem. 1995;270:5065–5072. doi: 10.1074/jbc.270.10.5065. [DOI] [PubMed] [Google Scholar]
- 22.Hug H, Sarre T F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hundle B, McMahon T, Dadgar J, Chen C H, Mochly-Rosen D, Messing R O. An inhibitory fragment derived from protein kinase Cepsilon prevents enhancement of nerve growth factor responses by ethanol and phorbol esters. J Biol Chem. 1997;272:15028–15035. doi: 10.1074/jbc.272.23.15028. [DOI] [PubMed] [Google Scholar]
- 24.Kouhara H, Hadari Y R, Spivak-Kroizman T, Schilling J, Bar-Sagi D, Lax I, Schlessinger J. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 25.Kuo W-L, Abe M, Rhee J, Eves E M, McCarthy S A, Yan M, Templeton D J, McMahon M, Rosner M R. Raf, but not MEK or ERK, is sufficient for differentiation of hippocampal neuronal cells. Mol Cell Biol. 1996;16:1458–1470. doi: 10.1128/mcb.16.4.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuo W-L, Chung K-C, Rosner M R. Differentiation of central nervous system neuronal cells by fibroblast-derived growth factor requires at least two signaling pathways: roles for Ras and Src. Mol Cell Biol. 1997;17:4633–4643. doi: 10.1128/mcb.17.8.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Good J A, Ziegler W H, Parekh D B, Alessi D R, Cohen P, Parker P J. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 28.Lin H-Y, Xu J, Ischenko I, Ornitz D M, Halegoua S, Hayman M J. Identification of the cytoplasmic regions of fibroblast growth factor (FGF) receptor 1 which play important roles in induction of neurite outgrowth in PC12 cells by FGF-1. Mol Cell Biol. 1998;18:3762–3770. doi: 10.1128/mcb.18.7.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Z, Hornia A, Jiang Y-W, Zang Q, Ohno S, Foster D A. Tumor promotion by depleting cells of protein kinase Cδ. Mol Cell Biol. 1997;17:3418–3428. doi: 10.1128/mcb.17.6.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Z, Liu D, Hornia A, Devonish W, Pagano M, Foster D A. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacKenzie S, Fleming I, Houslay M D, Anderson N G, Kilgour E. Growth hormone and phorbol esters require specific protein kinase C isoforms to activate mitogen-activated protein kinases in 3T3-F442A cells. Biochem J. 1997;324:159–165. doi: 10.1042/bj3240159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 33.Mischak H, Goodnight J A, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz M G, Blumberg P M, Pierce J H, Mushinski J F. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 34.Mischak H, Pierce J H, Goodnight J, Kazanietz M G, Blumberg P M, Mushinski J F. Phorbol ester-induced myeloid differentiation is mediated by protein kinase C-alpha and -delta and not by protein kinase C-beta II, -epsilon, -zeta, and -eta. J Biol Chem. 1993;268:20110–20115. [PubMed] [Google Scholar]
- 35.Mohammadi M, Dikic I, Sorokin A, Burgess W H, Jaye M, Schlessinger J. Identification of six novel autophosphorylation sites on fibroblast growth factor receptor 1 and elucidation of their importance in receptor activation and signal transduction. Mol Cell Biol. 1996;16:977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 37.Obermeier A, Bradshaw R A, Seedorf K, Choidas A, Schlessinger J, Ullrich A. Neuronal differentiation signals are controlled by nerve growth factor receptor/Trk binding sites for SHC and PLC gamma. EMBO J. 1994;13:1585–1590. doi: 10.1002/j.1460-2075.1994.tb06421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Driscoll K R, Teng K K, Fabbro D, Greene L A, Weinstein I B. Selective translocation of protein kinase C-delta in PC12 cells during nerve growth factor-induced neuritogenesis. Mol Biol Cell. 1995;6:449–458. doi: 10.1091/mbc.6.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pang L, Sawada T, Decker S, Saltiel A. Inhibition of MAP kinase kinase blocks the differentiation of PC-12 cells induced by nerve growth factor. J Biol Chem. 1995;270:13585–13588. doi: 10.1074/jbc.270.23.13585. [DOI] [PubMed] [Google Scholar]
- 40.Schlessinger J, Lax I, Lemmon M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 1995;83:357–360. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 41.Schönwasser D C, Marais R M, Marshall C J, Parker P J. Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Singh K R, Taylor L K, Campbell X Z, Fields A P, Neet K E. A bryostatin-sensitive protein kinase C required for nerve growth factor activity. Biochemistry. 1994;33:542–551. doi: 10.1021/bi00168a020. [DOI] [PubMed] [Google Scholar]
- 43.Stephens R M, Loeb D M, Copeland T D, Pawson T, Greene L A, Kaplan D R. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma1 to mediate NGF response. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- 44.Szallasi Z, Denning M F, Chang E Y, Rivera J, Yuspa S H, Lehel C, Olah Z, Anderson W B, Blumberg P M. Development of a rapid approach to identification of tyrosine phosphorylation sites: application to PKC delta phosphorylated upon activation of the high affinity receptor for IgE in rat basophilic leukemia cells. Biochem Biophys Res Commun. 1995;214:888–894. doi: 10.1006/bbrc.1995.2370. [DOI] [PubMed] [Google Scholar]
- 45.Tian Y, Smith R D, Balla T, Catt K J. Angiotensin II activates mitogen-activated protein kinase via protein kinase C and Ras/Raf-1 kinase in bovine adrenal glomerulosa cells. Endocrinology. 1998;139:1801–1809. doi: 10.1210/endo.139.4.5865. [DOI] [PubMed] [Google Scholar]
- 46.Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells: comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288:351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C delta activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271:23512–23519. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Ono Y, Taniyama Y, Hazama K, Igarashi K, Ogita K, Kikkawa U, Nishizuka Y. Cell division arrest induced by phorbol ester in CHO cells overexpressing protein kinase C-delta subspecies. Proc Natl Acad Sci USA. 1992;89:10159–10163. doi: 10.1073/pnas.89.21.10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss R H, Mada E A, Ramirez A. MEK inhibition augments Raf activity, but has variable effects on mitogenesis, in vascular smooth muscle cells. Am J Physiol. 1998;274:C1521–C1529. doi: 10.1152/ajpcell.1998.274.6.C1521. [DOI] [PubMed] [Google Scholar]
- 50.York R D, Yao H, Dillon T, Ellig C L, Eckert S P, McCleskey E W, Stork P J. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]