Abstract
Background:
Despite evidence supporting the cardiovascular and cognitive benefits of intensive blood pressure (BP) management, older adults have the lowest rates of BP control. We determined the association between age and therapeutic inertia (TI) in the Systolic Blood Pressure Intervention Trial (SPRINT), and if frailty, cognitive function, or gait speed moderate or mediate these associations.
Methods:
We performed a secondary analysis of SPRINT of participant-visits with BP above randomized treatment goal. We categorized baseline age as <60, 60 to <70, 70 to <80, and ≥80 years, and TI as no antihypertensive medication intensification per participant-visit. Generalized estimating equations generated odds ratios (OR) for TI associated with age, stratified by treatment group based on nested models adjusted for baseline frailty index (FI) score (fit[FI≤0.10], less fit[0.10<FI≤0.21], frail[0.21<FI]), cognitive function by Montreal Cognitive Assessment, and gait speed (participants ≥75 years), separately.
Results:
Participants 60 to <70, 70 to <80, and ≥80 years had a higher prevalence of TI in both treatment groups vs. participants <60 years (Standard: 59.7%, 60.5%, 60.1% vs. 56.0%; 29,527 participant-visits; Intensive: 55.1%, 57.2%, 57.8% vs. 53.8%; 47,129 participant-visits). The adjusted OR for TI comparing participants ≥80 vs. <60 years was 1.32 (95% confidence interval [CI] 1.14–1.53) and 1.25 (95% CI 1.11–1.41) in the standard and intensive treatment group, respectively. Adjustment for frailty, cognitive function, or gait speed did not attenuate the association nor demonstrate effect modification (all pinteraction>0.10).
Conclusion:
Older age is associated with greater TI independent of physical or cognitive function, implying age-bias in hypertension management.
Keywords: hypertension, age, disparities, therapeutic inertia, functional status, frailty
Graphical Abstract
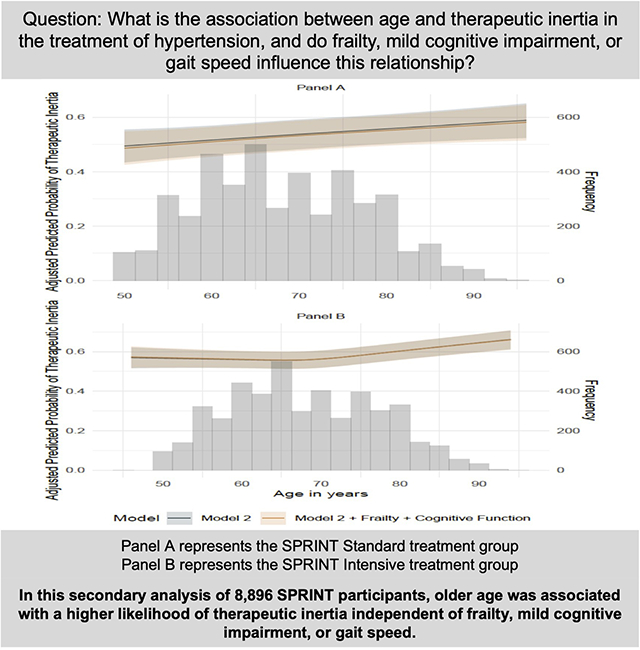
Introduction
Hypertension remains a leading modifiable risk factor for cardiovascular disease (CVD) and cognitive impairment, and disproportionately affects older adults in the United States (US).1 Recent data from the National Health and Nutrition Examination Surveys (NHANES) show that blood pressure (BP) control rates have declined overall and to a greater degree among US adults ≥75 years.2,3 Among adults ≥75 years taking antihypertensive medications, rates of controlled BP are 47.7% compared to 73.5% among those age 45–64 years.2 The strong evidence of CVD and cognitive benefit, higher awareness, and treatment rates for hypertension among older adults have failed to translate into better BP control.3–6
Therapeutic inertia (TI), the phenomenon of not initiating or intensifying a medication regimen despite indication, is recognized as a major barrier to achieving greater BP control.7–9 Moreover, TI is common among older adults with hypertension, occurring in 89.6% of outpatient visits between 2015 and 2018 based on the National Ambulatory Medical Care Survey.10 There is little data on the association between age and TI in hypertension, and whether the interplay between age and physical or cognitive function impacts treatment intensification decisions in older adults. Clinician apprehension of the risk-benefit tradeoff of intensive BP control among older adults may contribute to TI.11 Assumptions about age-related frailty and risks associated with treatment intensification have been cited as a rationale for the perceived low clinical benefit of intensive BP control.12 However analyses from the HYpertension in the Very Elderly Trial (HYVET) and Systolic Blood Pressure Intervention Trial (SPRINT) both found that the benefit of intensive BP reduction is not attenuated by age or frailty.5,13 Moreover, in the pre-specified analysis of SPRINT participants age ≥75 years at baseline, the beneficial effect of intensive treatment was greater than the overall trial population with no appreciable effect on serious adverse events (SAEs), injurious falls, or detriment to health related quality of life.5,14,15
Clinician concern about the potential risk of SAEs with intensive BP control may impact the appropriate use of antihypertensive medications among older adults, particularly among those with limited functionality or cognitive impairment, leading to greater TI. However, it remains unclear if TI among older adults is driven by clinician decision making based on age alone, or a combination of age and physical and cognitive function. Using SPRINT, we assessed the association between age and TI, and if baseline frailty, cognitive function, and gait speed impacted this relationship.
Methods
All data and materials have been made publicly available at the National Heart, Lung, and Blood Institute and can be accessed at https://biolincc.nhlbi.nih.gov/studies/sprint/.
Study Population and Sample
The institutional review board approved of the primary study protocol for each SPRINT clinical site with written informed consent from each study participant. The University of Utah Institutional Review Board deemed this secondary analysis to be exempt.
The SPRINT rationale, design, and results have been previously published.16–19 SPRINT was a multicenter, randomized trial which recruited and enrolled adults age ≥50 years with a high risk of CVD and a systolic BP (SBP) 130–180 mmHg depending on the number of antihypertensive medications between November 2010 and March 2013 to intensive treatment (SBP goal <120 mmHg) or standard (SBP goal <140 mmHg) treatment.
As TI can only occur at participant-visits where SBP is above goal, we included only participant-visits where the participant’s measured SBP above the assigned treatment goal. In the final analytical sample of participant-visits, there were 47,129 participant-visits included in the intensive treatment group (4,561 unique participants) and 29,527 participant-visits in the standard treatment group (4,335 unique participant). Additional details regarding the study population can be found in the Supplemental Methods.
Outcome measure – Therapeutic Inertia
We calculated a therapeutic intensity score (TIS) for each participant’s antihypertensive regimen recorded at each participant-visit where SBP was ≥120 mmHg and ≥140 mmHg in the intensive and standard treatment groups, respectively, to capture the intensity of each medication regimen. The TIS is a summary measure of medication regimen intensity.9,20 A modified TIS (mTIS) based on the 2017 American College of Cardiology/American Heart Association BP Guideline-defined maximum dose for each medication, which has been previously described, was used in the current analysis.21 Additional details can be found in the Supplemental Methods.
Covariates
Frailty
Frailty was determined at baseline and categorized by a Frailty Index (FI), a validated measure of deficit accumulation.22 The FI was modeled after previous indices from the African American Health Study and HYVET, including 36 deficit domains of cognitive function, self-rated health and depression, laboratory measurements, BP, and comorbid conditions.13,23 We categorized the FI into categories of fit (FI≤0.10), less fit (0.10<FI≤0.21), or frail (FI>0.21), as in previous work. The distribution of FI was similar between randomized treatment groups overall and by age (Figure S1–S2).24,25
Cognitive Function
A Montreal Cognitive Assessment (MoCA) was measured at baseline in SPRINT. The MoCA is a validated screening tool for cognitive impairment and offers a summative score (0–30) of executive function, memory, language, visuospatial, orientation, and attention.26 We classified lower cognitive function at baseline into race- and education-specific MoCA screening thresholds for mild cognitive impairment (referred to as cognitive function).27,28 Race- and education-specific thresholds for cognitive function based on MoCA have been previously validated for non-Hispanic White, non-Hispanic Black, and Hispanic adults with education levels categorized as ≤12 years, 13–16 years, and >16 years.27
Gait speed
Gait speed was measured using a timed 4-meter walk-test only among participants ≥75 years at baseline. Participants performed this task twice from a standing start and were allowed to use an assistive walking device. Only the faster of the two times were used in the current analysis, dichotomized as ≥0.8 meters/second (m/s) vs. <0.8 m/s.
Other study measures
All sociodemographic data for each SPRINT participant were collected at baseline. Clinical, laboratory, and antihypertensive medication data were collected at baseline then every 3 months by trained study personnel.16,17 As treatment-related SAEs can impact treatment intensification decisions and therefore the occurrence of therapeutic inertia, we included the following treatment-related SAEs in our analysis: orthostatic hypotension with symptoms, electrolyte abnormalities, syncope, injurious fall, acute kidney injury, hypotension, and bradycardia. Treatment-related SAEs that occurred in the one month prior to each visit were included as a covariate.16,17
Statistical Analysis
Baseline characteristics were calculated and compared between baseline age categories (<60 60 to <70, 70 to <80, ≥80). To assess the association between baseline age and TI and whether this association was attenuated or potentiated after adjustment for baseline frailty and cognitive function, separately, we constructed a series of nested multivariable generalized estimating equation (GEE) models accounting for within-subject correlation to calculate odds-ratios (OR) and 95% confidence intervals (CI). Model 1 included baseline age and month of follow-up. Model 2 additionally adjusted for baseline sociodemographic, participant-visit vitals, recent laboratory measurements, comorbid conditions, and medication use at each participant-visit (Table S1–S2; Supplemental Methods). We subsequently added frailty and cognitive function, separately (Models 3 and 4, respectively).27,28 Model 5 included variables in Model 2 plus MoCA measured continuously. Our final model included Model 2 plus frailty and categorized cognitive function (Model 6).
To determine if effect modification was present, we repeated Model 2 within subgroups of frailty and cognitive function, seperately.27,28 We tested for interactions using likelihood ratio tests.
A marginal standardization method was used to estimate an average model-based multivariable-adjusted probability of TI during the first 48 months of follow-up by age, within levels of frailty and cognitive function, separately.27,28 Additionally, we evaluated participant factors at baseline associated with TI stratified by age using Model 6 (described above).
As only participants ≥75 years at baseline in SPRINT had a timed 4-meter walk test performed, we repeated the above analysis within the subgroup age ≥75 years divided into tertiles using Model 2 plus baseline gait speed (≥0.8 m/s vs. <0.8 m/s).
Because the protocol dictated SBP thresholds at which to intensify treatment in each treatment group, three sensitivity analyses were conducted using more strict TI definitions: a) two consecutive visits at or above goal for both the intensive and standard treatment groups, b) ≥140 mmHg at two consecutive visits or ≥160 mmHg at a single visit in the standard treatment group only, and c) SBP 10 mmHg above randomized target for both treatment groups. We also re-defined FI as fit (FI≤0.10), less fit (0.10<FI≤0.30), or frail (FI>0.30) for all models.
Statistical significance was determined for p-values<0.05 and all analyses were performed as complete cases due to minimal missing data using R v.4.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
Among included participants in the standard treatment group, 882 (20.3%), 1,599 (36.9%), 1,316 (30.4%), and 538 (12.4%) were <60, 60 to <70, 70 to <80, ≥80 years, respectively. In the intensive treatment group, 965 (21.2%), 1,656 (36.3%), 1,369 (30.0%), and 571 (12.5%) were <60, 60 to <70, 70 to <80, ≥80 years, respectively. In both the standard and intensive treatment groups, participants <60 years were more likely to be self-reported non-Hispanic Black or Hispanic, employed, uninsured, not have a usual source of healthcare, current smokers, and have a higher BMI compared to participants 60 to <70, 70 to <80, and ≥80 years (Table S1–S2).
Over the course of 48 months follow-up, the prevalence of TI was 56.0%, 59.7%, 60.5%, and 60.1% among participants <60, 60 to <70, 70 to <80, and ≥80 years, respectively, among 29,527 included participant-visits in the standard treatment group (Table S3). There were 47,129 included participant visits in the intensive treatment group, and the overall prevalence of TI was 53.8%, 55.1%, 57.2%, and 57.8% among participants <60, 60 to <70, 70 to <80, and ≥80 years, respectively (Table S3).
Association between age and therapeutic inertia
Following full adjustment (Model 6), SPRINT participants 60 to <70, 70 to <80, and ≥80 years in the standard treatment group were more likely to experience TI vs. participants <60 years (OR 1.12 95% CI 1.02–1.23, 1.24 95% CI 1.09–1.40, and 1.32 95% CI 1.14–1.53, respectively; Table 1). Addition of baseline frailty and cognitive function, separately and together, did not change the association between age and TI and were not independently associated with TI (Table 1).
Table 1.
The degree to which the association of age and therapeutic inertia is attenuated/modified after adjusted for frailty and race- and education-specific MoCA threshold for cognitive function over the first 48 months of follow-up in SPRINT
| Variable | Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
Model 4 OR (95% CI) |
Model 5 OR (95% CI) |
Model 6 OR (95% CI) |
|---|---|---|---|---|---|---|
| Standard treatment group | ||||||
| Main Exposure | ||||||
| Age categories, years | ||||||
| < 60 | Ref | Ref | Ref | Ref | Ref | Ref |
| 60 to <70 | 1.15 (1.07–1.24) | 1.11 (1.01–1.22) | 1.12 (1.02–1.23) | 1.11 (1.01–1.21) | 1.10 (1.01–1.21) | 1.12 (1.02–1.23) |
| 70 to <80 | 1.21 (1.12–1.30) | 1.23 (1.09–1.38) | 1.24 (1.10–1.40) | 1.22 (1.08–1.37) | 1.21 (1.08–1.37) | 1.24 (1.09–1.40) |
| ≥ 80 | 1.21 (1.11–1.33) | 1.31 (1.14–1.52) | 1.32 (1.14–1.53) | 1.30 (1.13–1.51) | 1.30 (1.12–1.50) | 1.32 (1.14–1.53) |
| Effect Modifiers | ||||||
| Frailty | ||||||
| Fit (FI ≤ 0.10) | - | - | Ref | - | - | Ref |
| Less fit (FI 0.10 < FI ≤ 0.21) | - | - | 1.00 (0.92–1.09) | - | - | 1.00 (0.91–1.09) |
| Frail (FI > 0.21) | - | - | 1.12 (1.00–1.24) | - | - | 1.11 (0.99–1.24) |
| Cognitive function a | ||||||
| No cognitive impairment | - | - | - | Ref | - | Ref |
| Cognitive impairment | - | - | - | 1.03 (0.97–1.10) | - | 1.02 (0.95–1.09) |
| As a continuous variable | - | - | - | - | 0.99 (0.99–1.00) | - |
| Covariates | No | Yes | Yes | Yes | Yes | Yes |
| Intensive treatment group | ||||||
| Main Exposure | ||||||
| Age categories, years | ||||||
| < 60 | Ref | Ref | Ref | Ref | Ref | Ref |
| 60 to <70 | 1.04 (0.98–1.12) | 1.02 (0.95–1.11) | 1.02 (0.94–1.11) | 1.02 (0.94–1.11) | 1.02 (0.94–1.11) | 1.02 (0.94–1.10) |
| 70 to <80 | 1.13 (1.05–1.21) | 1.06 (0.96–1.18) | 1.06 (0.96–1.17) | 1.06 (0.96–1.18) | 1.06 (0.96–1.17) | 1.05 (0.95–1.17) |
| ≥ 80 | 1.21 (1.12–1.31) | 1.27 (1.12–1.42) | 1.26 (1.12–1.42) | 1.26 (1.12–1.42) | 1.26 (1.11–1.42) | 1.25 (1.11–1.41) |
| Effect Modifiers | ||||||
| Frailty | ||||||
| Fit (FI ≤ 0.10) | - | - | Ref | - | - | Ref |
| Less fit (FI 0.10 < FI ≤ 0.21) | - | - | 1.06 (0.99–1.14) | - | - | 1.06 (0.98–1.14) |
| Frail (FI > 0.21) | - | - | 1.01 (0.93–1.11) | - | - | 1.00 (0.91–1.10) |
| Cognitive function a | ||||||
| No cognitive impairment | - | - | - | Ref | - | Ref |
| Cognitive impairment | - | - | - | 1.02 (0.97–1.08) | - | 1.02 (0.97–1.08) |
| As a continuous variable | - | - | - | - | 1.00 (0.99–1.00) | - |
| Covariates | No | Yes | Yes | Yes | Yes | Yes |
FI: frailty index; MOCA: Montreal Cognitive Assessment
Race- and education-specific cut-points as follows: Non-Hispanic White participants ≤12 years of education: <22, 13–16 years of education: <24, >16 years of education: <25; Non-Hispanic Black participants ≤12 years of education: <19, 13–16 years of education: <23, >16 years of education: <23; Hispanic participants, ≤12 years of education: <23, 13–16 years of education: <24, >16 years of education: <24
Model 1 includes baseline age and month of follow-up as fixed effects.
Model 2 includes variables in Model 1 plus a suite of covariates provided in Table 1 above.
Model 3 includes variables in Model 2 plus frailty as defined by a frailty index.
Model 4 includes variables in Model 2 plus race- and education- specific MoCA thresholds (cognitive impairment vs. no cognitive impairment).
Model 5 includes variables in Model 2 plus MoCA as a continuous variable.
Model 6 includes all variables in Model 2 plus frailty and race- and education- specific MoCA thresholds (cognitive impairment vs. no cognitive impairment).
In the intensive treatment group, TI was more likely only among SPRINT participants ≥80 years vs. participants <60 years (1.25 95% CI 1.11–1.41; Table 1). There was a trend for a greater likelihood of TI among participants 60 to <70 and 70 to <80 years vs. <60 years (OR 1.02 95% CI 0.94–1.10 and 1.05 95% CI 0.95–1.17; Table 1). Neither the addition of frailty or cognitive function changed the association between age and TI (Table 1).
The predicted probability of TI in the standard and intensive treatment groups increased with greater baseline age (Figure 1). In both the treatment groups, the predicted probability of TI by frailty (Figure 2) and cognitive function (Figure 3) increased overall with greater baseline age.
Figure 1. Predicted prevalence of therapeutic inertia by age in the standard and intensive treatment groups of SPRINT by spline regression.
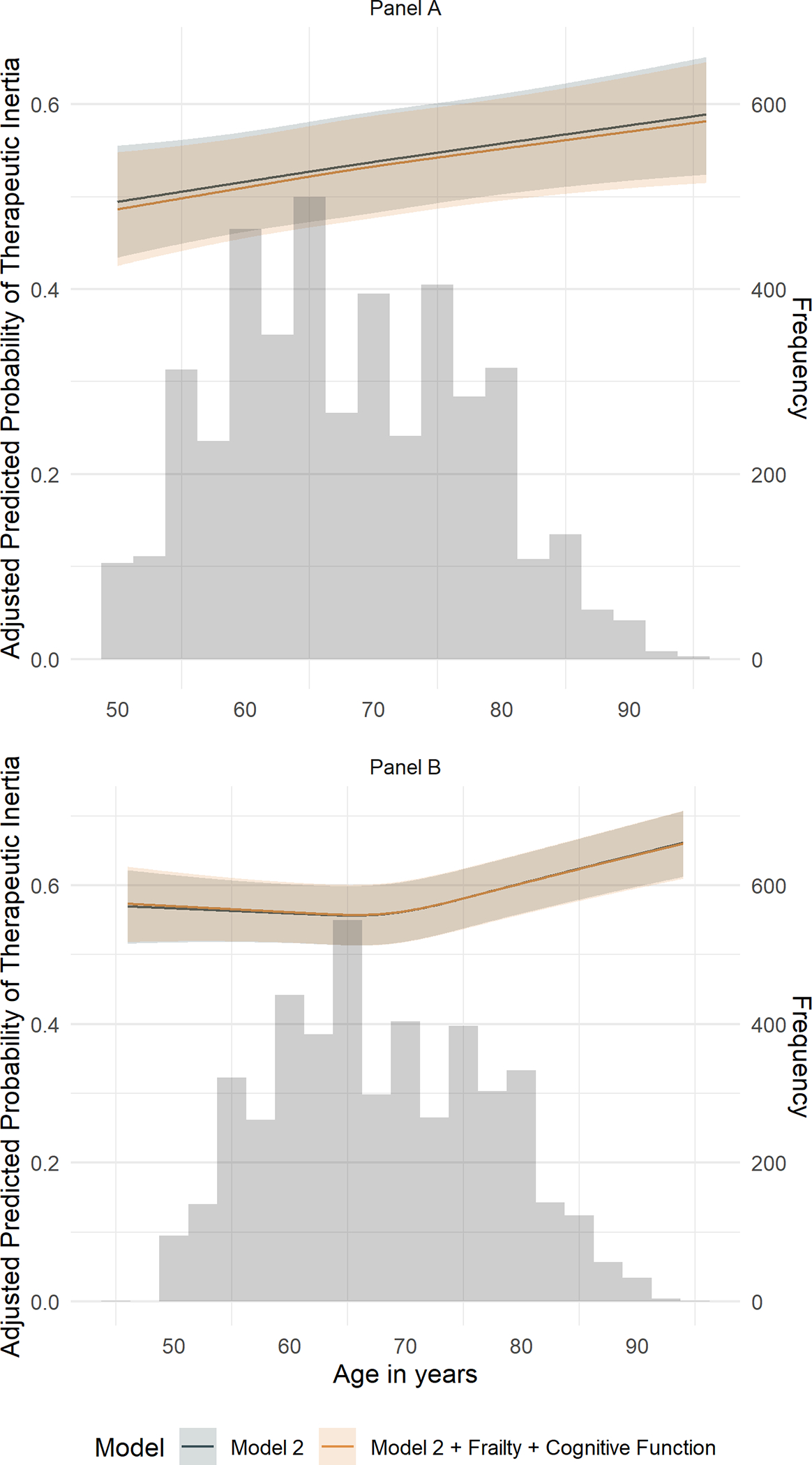
Predicted probability of therapeutic inertia with 95% asymptotic confidence bands shown for participants randomized to the standard treatment group on the top (Panel A) and intensive treatment group on the bottom (Panel B) through 48 months after initial enrollment using a cubic spline regression with knots at quartiles of participant ages averaged across the observed values of all other covariates in the GEE model. The underlying histogram represents the baseline age distribution included in the current analysis.
Figure 2. Predicted prevalence of therapeutic inertia by age in the standard and intensive treatment groups of SPRINT by spline regression stratified by frailty.
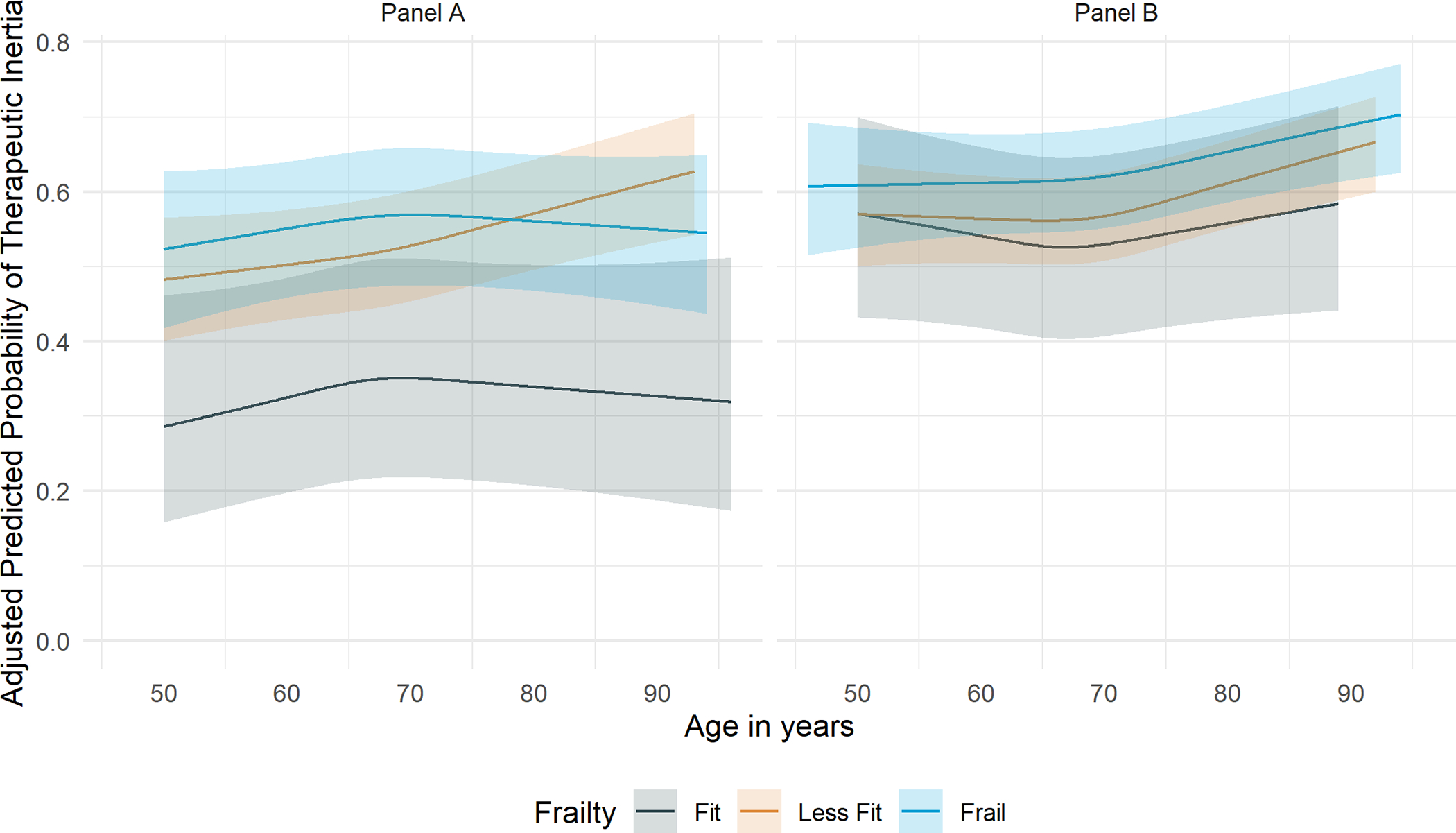
Predicted probability of therapeutic inertia with 95% asymptotic confidence bands shown for participants randomized to the standard treatment group on the left (Panel A) and intensive treatment group on the right (Panel B) through 48 months after initial enrollment using a cubic spline regression with knots at quartiles of participant ages averaged across the observed values of all other covariates in the GEE model.
Figure 3. Predicted prevalence of therapeutic inertia by age in the standard and intensive treatment groups of SPRINT by spline regression stratified by race- and education-specific MoCA threshold for cognitive function.
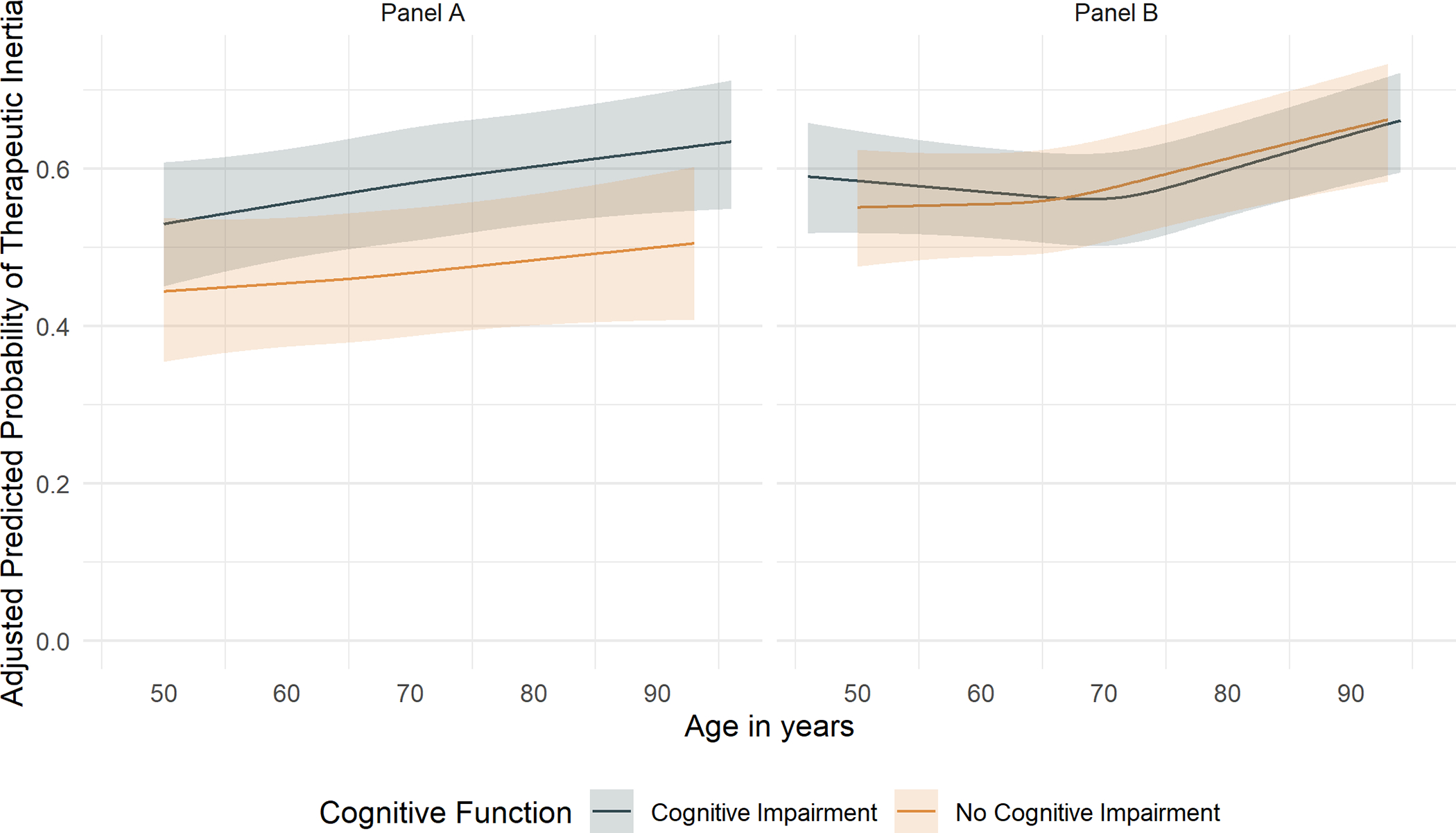
Predicted probability of therapeutic inertia with 95% asymptotic confidence bands shown for participants randomized to the standard treatment group on the left (Panel A) and intensive treatment group on the right (Panel B) through 48 months after initial enrollment using a cubic spline regression with knots at quartiles of participant ages averaged across the observed values of all other covariates in the GEE model.
After stratification, frailty did not demonstrate significant effect modification between age and TI in either the standard treatment group (p-for-interaction = 0.31 [Table S4]) or intensive treatment group (p-for-interaction = 0.39 [Table S4]), Additionally, cognitive function failed to show significant effect modification on the association between age and TI in the standard (p-for-interaction = 0.24 [Table S4]) and intensive treatment groups (p-for-interaction = 0.88 [Table S4]).
Among SPRINT participants ≥75 years old, gait speed was not associated with TI, nor changed the association between age and TI or demonstrate effect modification in the standard and intensive treatment groups (Table 2, Table S4).
Table 2.
The degree to which the association of age and therapeutic inertia is attenuated/modified after adjusted for gait speed among participants ≥75 years over the first 48 months of follow-up in SPRINT
| Variable | Model 1 OR (95% CI) |
Model 2 OR (95% CI) |
Model 3 OR (95% CI) |
|---|---|---|---|
| Standard treatment group | |||
| Main Exposure | |||
| Age categories, years | |||
| Tertile 1 – [75,78) | Ref | Ref | Ref |
| Tertile 2 – [78–82) | 1.01 (0.90–1.14) | 1.06 (0.93–1.21) | 1.05 (0.92–1.20) |
| Tertile 3 – [82,96] | 1.07 (0.95–1.21) | 1.19 (1.03–1.38) | 1.19 (1.02–1.38) |
| Effect Modifiers | |||
| ≥ 0.8 m/s | - | - | Ref |
| < 0.8 m/s | - | - | 0.90 (0.79–1.03) |
| Covariates | No | Yes | Yes |
| Intensive treatment group | |||
| Main Exposure | |||
| Age categories, years | |||
| Tertile 1 – [75–78) | Ref | Ref | Ref |
| Tertile 2 – [78,82) | 1.05 (0.95–1.15) | 1.12 (1.02–1.24) | 1.12 (1.01–1.24) |
| Tertile 3 – [82,94] | 1.10 (1.00–1.22) | 1.23 (1.10–1.39) | 1.22 (1.08–1.38) |
| Effect Modifiers | |||
| ≥ 0.8 m/s | - | - | Ref |
| < 0.8 m/s | - | - | 0.95 (0.85–1.05) |
| Covariates | No | Yes | Yes |
Model 1 includes baseline age and month of follow-up as fixed effects.
Model 2 includes variables in Model 1 plus a suite of covariates provided in Table 1 above.
Model 3 includes variables in Model 2 plus gait speed (≥0.8 m/s, <0.8 m/s)
Factors associated with therapeutic inertia by age
In both treatment groups, and in all age groups, greater time (in months) after randomization and higher mTIS were associated with a higher likelihood of TI (Figure S3–S4). Participants randomized to either standard or intensive treatment, irrespective of age, were associated with a lower likelihood of TI if they were taking an ACEI/ARB, CCB, or beta-blocker, and for each 10 mm Hg SBP above goal.
Sensitivity analysis
In sensitivity analyses with more strict TI definitions, TI was lower in both the standard and intensive treatment groups compared to the primary analysis (Table S3). Though the prevalence of TI was lower, no meaningful change was demonstrated in the association between age and TI in either treatment group, nor after the addition of frailty and MCI separately and together or gait speed after restricting TI to either a) two consecutive visits at or above goal for both treatment groups, b) ≥140 mmHg at two consecutive visits or ≥160 mmHg at a single visit in the standard treatment group only, and c) SBP 10 mmHg above randomized target for both treatment groups (Table S5–S10). The association between age and TI was unchanged after re-defining FI as fit (FI≤0.10), less fit (0.10<FI≤0.30), or frail (FI>0.30; Table S11).
Discussion
In this secondary analysis of SPRINT, there are several important findings regarding the association between age and TI. First, the overall prevalence of TI was high among all age groups. Among participants ≥80 years in the standard and intensive treatment groups TI was present in 60.1% and 57.8% of participant-visits, respectively, with a graded association between older age and a higher likelihood of TI. Second, the estimated associations between each age group and TI were unchanged after adjustment for frailty, cognitive function, or gait speed. Third, the association between age and TI was not appreciably different within levels of frailty, cognitive function, or gait speed. These findings suggest age alone is associated with greater TI independent of physical or cognitive function within the context of protocolized care in a clinical trial setting. Taken together, these results imply that undertreatment of elevated BP in older adults may be largely driven by age-bias, without consideration of a patient’s physical or cognitive function. Understanding real-world decision making in the treatment of older adults with hypertension and drivers of age-based discrimination in withholding appropriate antihypertensive medication is key to overcoming TI, reducing CVD, and addressing disparities in hypertension care.
Mounting clinical trial and observational evidence strongly supports intensive BP targets among older adults. The Strategy of Blood Pressure Intervention in the Elderly Hypertensive Patients (STEP) trial randomized 8,511 adults aged 60–80 years to a BP goal of 110 to <130 mmHg or 130 to 150 mmHg. Participants randomized to the intensive treatment group had a lower composite outcome event rate (hazard ratio [HR] 0.74; 95% CI 0.60–0.92).6 This was concordant with SPRINT, which demonstrated a lower event from for the composite outcome in the intensive treatment group (HR 0.66; 95% CI, 0.51–0.85) among participants ≥75 years old at baseline.5 Additionally, intensive BP control has been associated with a reduced risk of MCI.28,29 Clinical hesitation to initiate or intensify antihypertensive medication may contribute to age-based disparities and insufficient treatment of hypertension among older adults.
The high prevalence of antihypertensive TI in older adults is likely multifactorial. One common concern is that the patient may not survive long enough to incur benefits from intensive treatment, compared to the perceived risk of SAEs. However, older adults derive benefit from intensive BP control over a relatively short time course. A recent meta-analysis of 38,779 patients demonstrated the prevention of one stroke for every 200 patients after 1.7 years of treatment among older participants.30 Clinician concern that intensive BP reduction may result in more SAEs is not without merit.31 Hypotension-related SAEs, such as syncope, have been demonstrated in a meta-analysis of intensive BP target among adults ≥60 years (relative risk 1.52; 95% CI 1.22–2.07), however, other research has shown that the risk of falling and orthostatic hypotension were either similar or less prevalent among participants treated to intensive BP targets.32,33 Though concerns regarding SAEs may warrant caution in the use of antihypertensive medications among older adults, assessment of a patient’s function and cognition may help clinicians determine appropriate antihypertensive medication intensification.12 However, this consideration was not evident in the current analysis, in which greater TI was increasingly prevalent with participant age irrespective of SAEs or functional status. Reliance on age as a proxy for determining the risk-benefit of BP management, despite available SAE and physical and cognitive functional data, suggests that clinicians would benefit from more comprehensive training in the management of BP in older adults, particularly given its high prevalence and risk for CVD.
Due to the progressive increase in the degree of heterogeneity in both cognitive and physical function with chronological age, age alone is a rudimentary and non-individualized decision heuristic for appropriate antihypertensive use and may lead to broad undertreatment for older adults. A framework focused on assessing the benefit of intensive BP control with a standardized approach to BP measurement, a defined threshold for intervention, and a consistent medication regimen may offer a more reliable approach for shared-decision making and appropriately treating older adults with hypertension.34,35 Providing clinicians with a framework for managing antihypertensive therapy among older adults, and patients with education on antihypertensive benefits and risks, may reduce TI and improve BP control.
Study Limitations
There are limitations to the current analysis. First, there may be residual confounding. Though we relied on validated measures of frailty, cognitive function, and gait speed to capture functionality, this approach may fail to capture a participant’s complete functionality. Additionally, the MoCA score threshold used to categorize cognitive function is not an adjudicated approach to formally diagnose mild cognitive impairment. While the current analysis includes events that may preclude antihypertensive intensification and thus can be classified as appropriate inaction (i.e., treatment-related SAEs), there are other factors that were not accounted for (e.g., medication contraindication, new co-morbid conditions) that may influence TI. Older adults are more at risk of isolated systolic hypertension, and low diastolic BP may preclude intensification.36 However, we found a similar distribution of isolated systolic hypertension and low diastolic BP by treatment group and TI (Table S12). Finally, all interactions occurred within the context of an RCT among a participant-population that may be healthier than the average population which could limit generalizability to a less-functional patient population receiving community-based care.
Perspectives
Older adults with hypertension have higher rates of undertreatment with antihypertensive medication suggesting an age-bias in the management of older adults with hypertension. In the current analysis of SPRINT, TI was associated with increased age and was minimally impacted by frailty, cognitive function, or gait speed. The current analysis indicates that chronological age may be the predominant driver of TI among older adults without consideration of physical or cognitive function. These results suggest that implicit age-bias is evident with respect to the benefit/risk of more intensive SBP control among older adults irrespective of physical or cognitive functional status. Increased attention to counter this age-bias is warranted.
Supplementary Material
What is new?
In the United States, older adults face lower rates of blood pressure (BP) control, despite representing a large proportion of adults with hypertension. Understanding if insufficient BP control is driven by age or functional status can improve targeted interventions to improve BP control.
What is relevant?
In this secondary analysis of SPRINT participants, older age was associated with a higher likelihood of therapeutic inertia independent of frailty, mild cognitive impairment, or gait speed.
Clinical/Pathophysiological Implications?
Undertreatment of elevated BP in older adults may be largely driven by age without consideration of a patient’s physical or cognitive function.
Sources of Funding:
1. Drs. Zheutlin and Stulberg are supported by 1R38HL143605–01.
2. Dr. Bress is supported by 1R01AG065805, K01HL133468, and R01HL139837.
3. Dr. Pajewski is supported by R01AG055606 and R01AG065805.
Nonstandard abbreviations and acronyms
- BP
blood pressure
- TI
therapeutic inertia
- FI
frailty index
- SAE
serious adverse events
- SPRINT
Systolic Blood Pressure Intervention Trial
- TIS
therapeutic intensity score
- mTIS
modified therapeutic intensity score
- MoCA
Montreal Cognitive Assessment
Footnotes
Disclosures:
1. The remaining authors have nothing to disclose relevant to the current manuscript.
References
- 1.Frieden TR, Jaffe MG. Saving 100 million lives by improving global treatment of hypertension and reducing cardiovascular disease risk factors. J Clin Hypertens. 2018;20:208–211. doi: 10.1111/jch.13195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muntner P, Miles MA, Jaeger BC, Hannon Iii L, Hardy ST, Ostchega Y, Wozniak G, Schwartz JE. Blood Pressure Control Among US Adults, 2009 to 2012 Through 2017 to 2020. Hypertension. 2022;79:1971–1980. doi: 10.1161/HYPERTENSIONAHA.122.19222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB, Colantonio LD. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. doi: 10.1001/jama.2020.14545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakhuja S, Colvin CL, Akinyelure OP, Jaeger BC, Foti K, Oparil S, Hardy ST, Muntner P. Reasons for Uncontrolled Blood Pressure Among US Adults: Data From the US National Health and Nutrition Examination Survey. Hypertension. 2021;78:1567–1576. doi: 10.1161/HYPERTENSIONAHA.121.17590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williamson JD, Supiano MA, Applegate WB, Berlowitz DR, Campbell RC, Chertow GM, Fine LJ, Haley WE, Hawfield AT, Ix JH, et al. Intensive vs Standard Blood Pressure Control and Cardiovascular Disease Outcomes in Adults Aged >/=75 Years: A Randomized Clinical Trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, Yang J, Jiang Y, Xu X, Wang TD, et al. Trial of Intensive Blood-Pressure Control in Older Patients with Hypertension. N Engl J Med. 2021;385:1268–1279. doi: 10.1056/NEJMoa2111437 [DOI] [PubMed] [Google Scholar]
- 7.Carey RM, Muntner P, Bosworth HB, Whelton PK. Prevention and Control of Hypertension. Journal of the American College of Cardiology. 2018;72:1278–1293. doi: doi: 10.1016/j.jacc.2018.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon DL, Sharma G, Sandesara PB, Yang E, Braun LT, Mensah GA, Sperling LS, Deedwania PC, Virani SS. Therapeutic Inertia in Cardiovascular Disease Prevention: Time to Move the Bar. J Am Coll Cardiol. 2019;74:1728–1731. doi: 10.1016/j.jacc.2019.08.014 [DOI] [PubMed] [Google Scholar]
- 9.Berlowitz DR. Clinical inertia and the 2017 ACA/AHA guideline. J Clin Hypertens. 2018;20:1392–1394. doi: 10.1111/jch.13373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu N, Chiu L, Aggarwal R, Raber I, Bhatt DL, Mukamal KJ. Trends in Blood Pressure Treatment Intensification in Older Adults With Hypertension in the United States, 2008 to 2018. Hypertension. 2022:101161hypertensionaha12219882. doi: 10.1161/hypertensionaha.122.19882 [DOI] [PubMed] [Google Scholar]
- 11.Alhawassi TM, Krass I, Pont LG. Prevalence, prescribing and barriers to effective management of hypertension in older populations: a narrative review. J Pharm Policy Pract. 2015;8:24. doi: 10.1186/s40545-015-0042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benetos A, Petrovic M, Strandberg T. Hypertension Management in Older and Frail Older Patients. Circ Res. 2019;124:1045–1060. doi: 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 13.Warwick J, Falaschetti E, Rockwood K, Mitnitski A, Thijs L, Beckett N, Bulpitt C, Peters R. No evidence that frailty modifies the positive impact of antihypertensive treatment in very elderly people: an investigation of the impact of frailty upon treatment effect in the HYpertension in the Very Elderly Trial (HYVET) study, a double-blind, placebo-controlled study of antihypertensives in people with hypertension aged 80 and over. BMC Med. 2015;13:78. doi: 10.1186/s12916-015-0328-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, Gure TR, Kimmel PL, Kirchner K, Morisky DE, et al. Effect of Intensive Blood-Pressure Treatment on Patient-Reported Outcomes. N Engl J Med. 2017;377:733–744. doi: 10.1056/NEJMoa1611179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berlowitz DR, Foy C, Conroy M, Evans GW, Olney CM, Pisoni R, Powell JR, Gure TR, Shorr RI. Impact of Intensive Blood Pressure Therapy on Concern about Falling: Longitudinal Results from the Systolic Blood Pressure Intervention Trial (SPRINT). J Am Geriatr Soc. 2020;68:614–618. doi: 10.1111/jgs.16264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrosius WT, Sink KM, Foy CG, Berlowitz DR, Cheung AK, Cushman WC, Fine LJ, Goff DC Jr., Johnson KC, Killeen AA, et al. The design and rationale of a multicenter clinical trial comparing two strategies for control of systolic blood pressure: the Systolic Blood Pressure Intervention Trial (SPRINT). Clin Trials. 2014;11:532–546. doi: 10.1177/1740774514537404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson KC, Whelton PK, Cushman WC, Cutler JA, Evans GW, Snyder JK, Ambrosius WT, Beddhu S, Cheung AK, Fine LJ, et al. Blood Pressure Measurement in SPRINT (Systolic Blood Pressure Intervention Trial). Hypertension. 2018;71:848–857. doi: 10.1161/HYPERTENSIONAHA.117.10479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cushman WC, Ringer RJ, Rodriguez CJ, Evans GW, Bates JT, Cutler JA, Hawfield A, Kitzman DW, Nasrallah IM, Oparil S, et al. Blood Pressure Intervention and Control in SPRINT. Hypertension. 2022;79:2071–2080. doi: 10.1161/HYPERTENSIONAHA.121.17233 [DOI] [PubMed] [Google Scholar]
- 20.Rose AJ, Berlowitz DR, Manze M, Orner MB, Kressin NR. Comparing methods of measuring treatment intensification in hypertension care. Circ Cardiovasc Qual Outcomes. 2009;2:385–391. doi: 10.1161/CIRCOUTCOMES.108.838649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheutlin AR, Mondesir FL, Derington CG, King JB, Zhang C, Cohen JB, Berlowitz DR, Anstey DE, Cushman WC, Greene TH, et al. Analysis of Therapeutic Inertia and Race and Ethnicity in the Systolic Blood Pressure Intervention Trial: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2022;5:e2143001. doi: 10.1001/jamanetworkopen.2021.43001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pajewski NM, Williamson JD, Applegate WB, Berlowitz DR, Bolin LP, Chertow GM, Krousel-Wood MA, Lopez-Barrera N, Powell JR, Roumie CL, et al. Characterizing Frailty Status in the Systolic Blood Pressure Intervention Trial. J Gerontol A Biol Sci Med Sci. 2016;71:649–655. doi: 10.1093/gerona/glv228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc. 2014;62:721–726. doi: 10.1111/jgs.12735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoover M, Rotermann M, Sanmartin C, Bernier J. Validation of an index to estimate the prevalence of frailty among community-dwelling seniors. Health Rep. 2013;24:10–17. [PubMed] [Google Scholar]
- 25.Blodgett J, Theou O, Kirkland S, Andreou P, Rockwood K. Frailty in NHANES: Comparing the frailty index and phenotype. Archives of Gerontology and Geriatrics. 2015;60:464–470. doi: 10.1016/j.archger.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 26.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 27.Gaussoin SA, Pajewski NM, Chelune G, Cleveland ML, Crowe MG, Launer LJ, Lerner AJ, Martindale-Adams J, Nichols LO, Ogrocki PK, et al. Effect of intensive blood pressure control on subtypes of mild cognitive impairment and risk of progression from SPRINT study. J Am Geriatr Soc. 2022;70:1384–1393. doi: 10.1111/jgs.17583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson JD, Pajewski NM, Auchus AP, Bryan RN, Chelune G, Cheung AK, Cleveland ML, Coker LH, Crowe MG, Cushman WC, et al. Effect of Intensive vs Standard Blood Pressure Control on Probable Dementia: A Randomized Clinical Trial. JAMA. 2019;321:553–561. doi: 10.1001/jama.2018.21442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pajewski NM, Berlowitz DR, Bress AP, Callahan KE, Cheung AK, Fine LJ, Gaussoin SA, Johnson KC, King J, Kitzman DW, et al. Intensive vs Standard Blood Pressure Control in Adults 80 Years or Older: A Secondary Analysis of the Systolic Blood Pressure Intervention Trial. J Am Geriatr Soc. 2020;68:496–504. doi: 10.1111/jgs.16272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho VS, Cenzer IS, Nguyen BT, Lee SJ. Time to benefit for stroke reduction after blood pressure treatment in older adults: A meta-analysis. J Am Geriatr Soc. 2022;70:1558–1568. doi: 10.1111/jgs.17684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bress AP, Kramer H, Khatib R, Beddhu S, Cheung AK, Hess R, Bansal VK, Cao G, Yee J, Moran AE, et al. Potential Deaths Averted and Serious Adverse Events Incurred From Adoption of the SPRINT (Systolic Blood Pressure Intervention Trial) Intensive Blood Pressure Regimen in the United States: Projections From NHANES (National Health and Nutrition Examination Survey). Circulation. 2017;135:1617–1628. doi: 10.1161/CIRCULATIONAHA.116.025322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss J, Freeman M, Low A, Fu R, Kerfoot A, Paynter R, Motu’apuaka M, Kondo K, Kansagara D. Benefits and Harms of Intensive Blood Pressure Treatment in Adults Aged 60 Years or Older: A Systematic Review and Meta-analysis. Ann Intern Med. 2017;166:419–429. doi: 10.7326/M16-1754 [DOI] [PubMed] [Google Scholar]
- 33.Juraschek SP, Hu JR, Cluett JL, Ishak A, Mita C, Lipsitz LA, Appel LJ, Beckett NS, Coleman RL, Cushman WC, et al. Effects of Intensive Blood Pressure Treatment on Orthostatic Hypotension : A Systematic Review and Individual Participant-based Meta-analysis. Ann Intern Med. 2021;174:58–68. doi: 10.7326/M20-4298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowling CB, Lee A, Williamson JD. Blood Pressure Control Among Older Adults With Hypertension: Narrative Review and Introduction of a Framework for Improving Care. Am J Hypertens. 2021;34:258–266. doi: 10.1093/ajh/hpab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backman WD, Levine SA, Wenger NK, Harold JG. Shared decision-making for older adults with cardiovascular disease. Clin Cardiol. 2020;43:196–204. doi: 10.1002/clc.23267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


