Abstract
Background
Converging lines of evidence confirmed neuroinflammation’s role in autism spectrum disorder (ASD) etiological pathway. A disintegrin and metalloproteinase 8 (ADAM8) play major roles in inflammatory and allergic processes in various diseases.
Aim
This study aimed to investigate ADAM8 plasma levels in autistic children compared to healthy controls. Also, to discover the association between ADAM8, disease severity, and neuroinflammation in ASD.
Methodology
This case–control study included children with ASD (n=40) and aged-matched healthy controls (n=40). The plasma levels of the ADAM 8 were determined using enzyme-linked immunosorbent assay (ELISA). The assessment of ASD severity and social and sensory behaviors were categorized as mild, moderate and severe. Correlations among ADAM8 plasma levels and ASD severity scores [Childhood Autism Rating Scale (CARS), Social Responsiveness Scale (SRS) and Short Sensory Profile (SSP)] were obtained by Spearman correlation coefficient (r).
Results
ASD children (n=40), including severe autism (n=21) and mild-to-moderate autism (n=19), showed significantly (p ≤ 0.05) lower plasma levels of ADAM8 [4683 (2885–5229); 4663 (4060–5000); 4632 (2885–5229)], respectively, than those of healthy controls [5000 (4047–5000)] [median (IQR) pg/mL]. However, there was no significant difference between the ADAM8 levels of children with severe and mild-to-moderate autism (p = 0.71). Moreover, ADAM8 plasma levels were not significantly correlated with the severity of ASD measured by behavioral scales [CARS (r= −0.11, p=0.55), SRS (r=0.11, p= 0.95), SSP (r=−0.23, p=0.23)].
Conclusion
The low ADAM8 plasma levels in children with ASD possibly indicated that ADAM8 might be implicated in the pathogenesis of ASD but not in the severity of the disease. These results should be interpreted with caution until additional studies are carried out with larger populations to decide whether the reduction in plasma ADAM8 levels is a mere consequence of ASD or if it plays a pathogenic role in the disease.
Keywords: autism spectrum disorder, neuroinflammation, a disintegrin and metalloprotease 8, neurodevelopmental disorder
Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impaired social interactions with repetitive stereotypical behaviors.1 The etiopathology of ASD is unclear, and no diagnostic or predictive biomarkers have yet been discovered. A diagnosis is achieved using behavioral assessments using various ASD screening and diagnostic methods, including the Diagnostic and Statistical Manual of Mental Disorders, fifth Edition (DSM-V), Childhood Autism Rating Scale (CARS), Social Responsiveness Scale (SRS) and Short Sensory Profile (SSP).2–5 A sudden rise in the prevalence of ASD has encouraged an increase in research studies on the fundamental neuropathologic routes of this disorder. An increasing body of research states that neuroinflammation and dysregulated immune responses are essential pathological processes involved in the etiological pathway of ASD.6–9
Neuroinflammation is primarily considered a continuous process in which the increasing activation of microglia evokes and expands cytokine signaling that continues beyond a transient neuroprotective stage, thus creating an advanced pathological state.10,11 Increased indications associate neuroinflammation as a primary source in neuropsychiatric and neurodevelopmental disorders such as ASD and schizophrenia.12–14 Several studies have revealed that inflammatory cytokines were raised in blood serum and cerebrospinal fluid (CSF) in children with ASD.15,16 Recently, evidence concerning the contribution of various factors of neuroinflammation in postmortem brain tissues of autistic children and its possible pathogenic and therapeutic mechanisms in ASD.7 However, exploring the role of neuroinflammation in the progress of ASD is essential both as a marker for disease diagnosis and as a target for therapeutic intervention.
The inherent immunity is intimately associated with inflammatory routes and leads to numerous bacterial and viral infections.17 Researchers have reported several inflammasomes, which play a significant role in the manifestation and progress of different major diseases such as ischemic Parkinson’s disease and stroke.18,19
A disintegrin and metalloproteinase 8 (ADAM8) is a transmembrane protein, a member of a disintegrin and metalloproteases (ADAMs) group that mediates cell adhesion and cell migration through the cleavage of membrane-bound cytokines, growth factors, and receptors.20 ADAM8 is expressed mainly in the immune system cells, dendritic cells, eosinophils, neutrophils and monocytes.21 ADAM8 has been involved in various biological activities, including cell–cell and cell–matrix connections, neurogenesis and muscle growth.22 The individual expression pattern of ADAM8 showed possible roles in neuropathology and immunology.23 Nevertheless, the molecular mechanism of ADAM8 is still not clear. ADAM 8 was discovered under many pathological disorders; hence, it plays a vital role in inflammation and tumor-related diseases.19,24 Inflammatory stimuli such as tumor necrosis factor (TNF-α), Interferon- c (IFN-c) and Lipopolysaccharide (LPS) activate ADAM8, which supports its function in regulating inflammation.25 The contribution of ADAM 8 in different diseases shows its effectiveness as a prognostic and diagnostic biomarker.26 Therefore, ADAM8 is a possible attractive drug target in variable conditions.20 Earlier studies also revealed that ADAM8 plays a significant role in allergic and neuroinflammation.19,21 However, it is still not clear whether ADAM8 acts in a pro-inflammatory or anti-inflammatory mode.20,27
ADAM8 inhabits a vital position in encouraging the manifestation of inflammatory diseases ADAM8 can trigger the production of several inflammatory factors and eventually cause tissue injury in many diseases, including rheumatoid arthritis and tracheal inflammation.28
ADAM8 is expressed in the mammalian central nervous system (CNS), mainly in oligodendrocytes and neurons.23 Moreover, ADAM 8 contributes to the myelination and plasticity of proteins, demonstrating that ADAM8 plays a key role in the brain and regulation of neuroinflammation. However, limited research about ADAM8 in the CNS has been reported. Hisa et al29 revealed that ADAM8 expression was low in the brain of normal adult mice. However, the concentration of TNFα and ADAM 8 increased significantly in the mouse model of neurodegeneration. Recently, Zheng et al30 reported that ADAM10 and ADAM17 regulate synaptic functions, neuroinflammation, immunity and brain development, which are involved in the pathogenesis of ASD.
Since ADAM8 plasma levels in ASD subjects have never been reported earlier, thus considering the crucial role of ADAM8 in the brain development and regulation of neuroinflammation, it has been hypothesized that ADAM8 could play a pathogenic role in ASD patients. Thus, the main objective of the current study was to examine the role of ADAM8 in subjects with ASD compared to age-matched healthy controls and to establish the connection between ADAM8 and the severity of the disease in autistic children.
Methods
Participants
This case–control study was carried out from October 2021 to February 2022 at Autism Research and Treatment Center (ARTC), King Saud University. Our participants included 80 children aged 3–13 years, including forty children (37 male+ 03 female) with ASD from the ARTC and 40 healthy children (25 male +15 female) from the pediatric clinic at King Khalid University Hospital. The ASD diagnosis was performed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-V). Subjects associated with fragile X syndrome, epileptic seizures, obsessive-compulsive disorder, affective disorders, or any other neurological or psychiatric diseases were excluded from the study. The Institutional Review Committee of the college of medicine at King Saud University approved this study. Informed written consent was signed by the parents or the legal guardians of all the enrolled participants. All procedures followed the Helsinki Declaration for human investigations. The severity of ASD, as well as social and sensory performances, were measured in all children with ASD by using the CARS, SRS and SSP scales, respectively.31
Childhood Autism Rating Scale (CARS)
The CARS score was measured as a scale for ASD severity.2 CARS assesses the child on a scale from 1 to 4 in each of 15 dimensions, including the ability to relate to people, emotional response, imitation, body use, object use, listening response, fear or nervousness, verbal communication, nonverbal communication, activity level, level and reliability of intellectual response, adaptation to changes, visual response, taste, smell and touch responses and general impressions. A total score of at least 30 strongly suggests the presence of ASD. Children who score between 30 and 36.5 have mild-to-moderate ASD, while those with scores between 37 and 60 have severe ASD.
Social Responsiveness Scale (SRS)
The SRS is a validated test of interpersonal behavior, communication, and stereotypical traits in ASD.3 It is used as a diagnostic tool, distinguishing clinically significant ASD from varying levels of social impairment in other psychiatric disorders. It consists of 5 subscales: social awareness, social cognition, social communication, social motivation, and autistic mannerisms. Total SRS scores range from 0 to 195, corresponding to significant social impairment observed in individuals with ASD. A score of 76 or higher is considered severe and is strongly associated with a clinical diagnosis of ASD. A score between 60 and 75 is in the mild-to-moderate range of social impairment.
The Short Sensory Profile (SSP)
The SSP is a 38-item questionnaire designed for children aged 3–14 years; it provides quick information about the sensory processing skills of autistic children. Each item on the SSP is measured on a 5-point Likert scale. Domain scores were measured in tactile, taste/smell, and movement sensitivity, seeking sensation, auditory filtering, low energy levels, and visual/auditory sensitivity. Domain scores and overall sensory responses were categorized as typical performance, the probable difference from typical performance, or a definite difference from typical performance. Scores less than 142 indicate severe performance (definite difference from typical performance), scores between 142 and 152 indicate mild-to-moderate performance (probable difference from typical performance), and scores between 153 and 190 indicate typical performance. The SSP has been used in many studies.4
Blood Sample Collection
A 3 mL blood sample was collected from each child while fasting in morning in test tubes containing EDTA. Blood samples were immediately centrifuged at 4°C at 3000 rpm for 20 minutes to collect plasma samples, which were stored in a freezer at −80°C until analysis. The ADAM 8 concentrations were measured in the plasma of all subjects using a commercially available sandwich ELISA kit (Cusabio Biotech Co. Ltd., Wuhan, China). All biochemical analyses were performed in duplicate, and mean values were reported. No significant cross-reactivity or interference was observed.
Statistical Analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS 21.0 for Windows; SPSS, Chicago, IL, USA). The parametric data were presented as mean and standard deviation (SD), while nonparametric data were presented as Median and IQR levels. The t-test was used for the comparison of parametric data. In contrast, the Mann–Whitney U-test was used to compare nonparametric data of ADAM 8 protein levels between the ASD and healthy groups. The Spearman’s correlation coefficient ‘r’ was employed to determine correlations between severity variables (SRS, CARS, and SSP). The p-values less than 0.05 were considered statistically significant.
Results
The general features of the study participants, along with plasma levels of ADAM8 [median (interquartile range=IQR) pg/mL] of the ASD group with different severity (mild-moderate or severe) and healthy controls, are summarized in Table 1.
Table 1.
General Characteristics, Plasma Levels of ADAM 8, in Children with ASD and Healthy Controls and Their Association with ASD Severity
| Age (Years) Median (IQ Range) | ADAM8 pg/mL Median (IQ Range) | p-value* | CARS Score | |
|---|---|---|---|---|
| Children with ASD, n = 37 (34 male+ 03 female) | 6 (3–12) | 4683 (2885–5229) | <0.01* | > 30 |
| Children with severe ASD (n =21) | -- | 4663 (4060–5000) | <0.05* 0.71 |
> 36.5 |
| Children with mild to moderate ASD (n =16) | -- | 4632 (2885–5229) | <0.01* | < 36.5 |
| Healthy children, n= 40 (25 male + 15 female) | 11 (3–13) p= <0.05* |
5000 (4047–5000) | <0.05* |
Notes: *Comparing ASD children with control subjects (p-value ≤ 0.05 was considered statistically significant).
Three children were excluded for scoring below 30 points in CARS. The remaining ASD children (n = 37, CARS score >30) with severe ASD (n=21, CARS score >37) and mild-to-moderate ASD (n=16, CARS score >30) exhibited significantly (p ˂0.05) lower plasma levels of ADAM8 [4683 (2885–5229); 4663 (4060–5000); 4632 (2885–5229)], respectively, than those of healthy controls [5000 (4047–5000)] [median (IQR) pg/mL]. However, there was no significant difference between the ADAM 8 levels of children with severe and mild-to-moderate autism (p = 0.71).
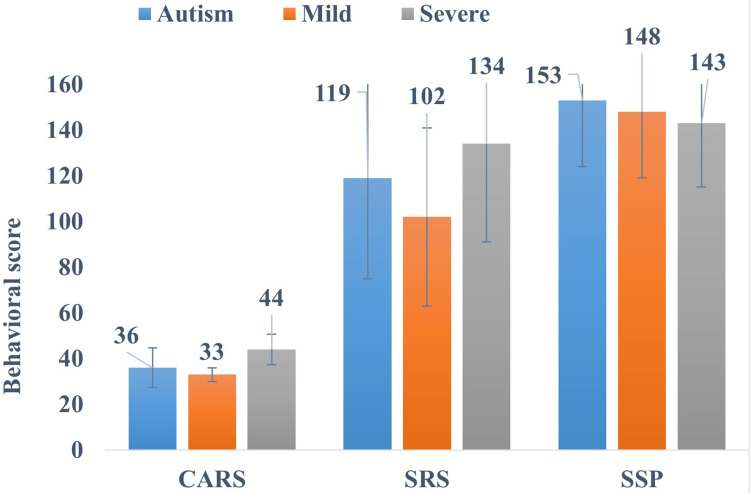
The severity of ASD in children was further categorized according to their measured CARS scores. SRS and SSP scores recorded the social and sensory functions of ASD children. Overall, ASD children with severe and mild-to-moderate autism show 36 (8.6), 44 (6.7) and 33 (3.0) [mean (SD)] CARS scores, respectively (p ˂0.05), which strongly recommend the severity of ASD.
The Mean (SD) values of total SRS scores of all the ASD, severe and mild-to-moderate ASD categories were found to be 119 (44), 134 (43) and 102 (39), respectively, which are considered severe and are strongly linked with a clinical diagnosis of ASD. Also, the SSP measured sensory processing dysfunction and showed a mean (SD) score of 153 (29) in children with ASD (Figure 1). Moreover, children with severe and moderate ASD showed 143±28 and 148±29 SSP scores, respectively, which are associated with mild-to-moderate presentation, clearly different from a typical presentation for ASD. Mean scores of behavioral rating scales (SRS, CARS, and SSP) along with subcategories of severity are also presented in Table 2.
Figure 1.
Behavioral scales (CARS, SRS and SSP) scores in children with ASD and their association with ASD severity.
Table 2.
Plasma Levels of ADAM 8 in Subcategories According to Behavioral Tests (CARS, SRS, SSP) in ASD Children
| ASD Subcategories | Mean Plasma Levels of ADAM 8 (pg\mL) | SD |
|---|---|---|
| CARS | ||
| Mild to Moderate | 4332 | 1274 |
| Severe | 4041 | 1141 |
| SRS | ||
| Mild to moderate | 4822 | 1066 |
| Severe | 4136 | 1081 |
| SSP | ||
| Typical | 4377 | 1133 |
| Atypical | 4203 | 1097 |
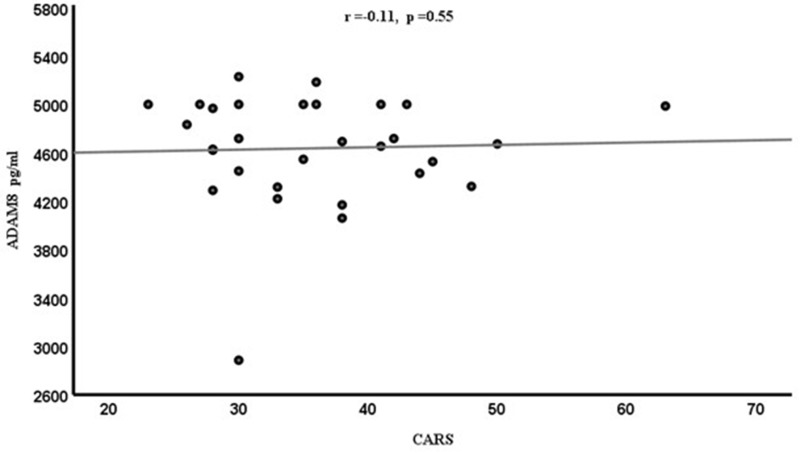
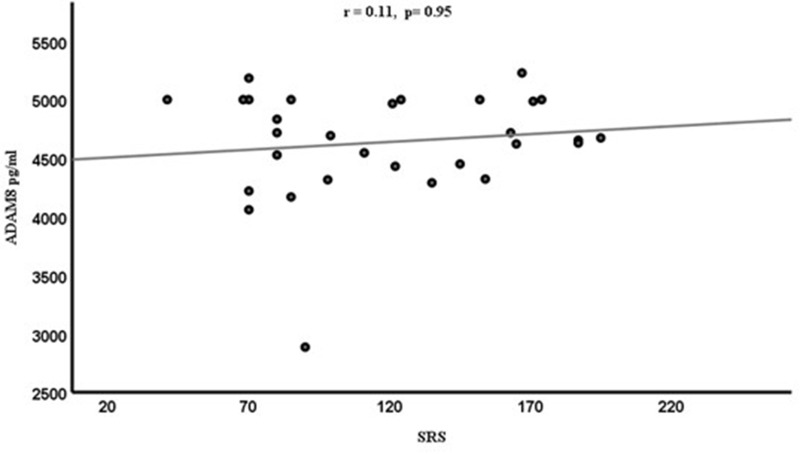
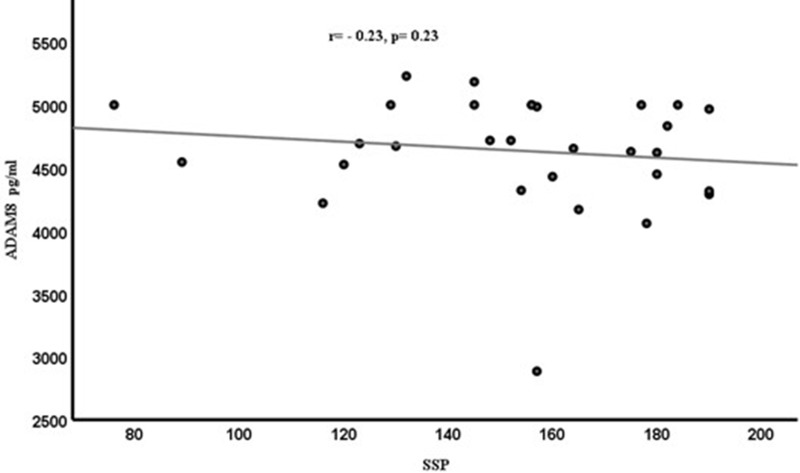
Spearman correlation coefficient (r) was calculated to determine the relationships between ADAM8 levels and different variables (CARS, SRS, SSP). However, the resulting graphs showed no significant correlations between ADAM8 levels and variable behavioral scales [CARS (r=−0.11, p=0.55) in Figure 2, SRS (r=0.11, p=0.95) in Figure 3 and SSP (r=−0.23, p=0.23)] in Figure 4 scores among the ASD subjects. Although ASD severity scores were not significantly correlated with ADAM8 levels, the subcategories of ASD according to severity showed a significant decrease in ADAM8 in severe ASD compared to healthy control and between mild ASD and severe ASD groups (Table 1). Also, all behavioral tests (Table 2) showed higher plasma levels of ADAM 8 in mild-to-moderate ASD compared to severe ASD, but the correlations were not significant.
Figure 2.
Correlation between the plasma levels of ADAM8 (pg/mL) and the CARS score in ASD children.
Figure 3.
Correlation between the plasma levels of ADAM8 (pg/mL) and the SRS score in ASD children.
Figure 4.
Correlation between the plasma levels of ADAM8 (pg/mL) and the SSP score in ASD children.
Discussion
Autism spectrum disorder (ASD) is a neurodevelopmental disorder with many etiologies and mechanisms leading to abnormal brain development. The neurological cause of ASD is still not well known, and the role of neuroinflammation in ASD must be investigated. Moreover, no disease markers for the diagnosis of ASD have been confirmed. However, the discovery of biomarkers in patients with ASD with variable scales of clinical appearance (ie, mild, moderate and severe) will give a better understanding of the pathogenesis of the disease and help suitable early diagnostic approaches and therapies for this disorder.
Neuroinflammation is a complex process in which the brain reacts to different stimuli, which is a significant cause of several diverse pathological disorders.32 In recent years, various study groups proposed possible neuroinflammation as a critical etiological cause of ASD.6,7
Several research groups have studied a range of immune cell effectors in ASD. Still, very few have investigated molecules supporting immune cell migration into the CNS in ASD patients and healthy controls.33 The role of neuroinflammation and cellular dysfunction in ASD suggested that abnormal neuroimmune interactions may occur in the CNS.34,35 The significant activation of astroglia and microglia with abnormal concentrations of cytokines, chemokines and immunoglobulin were detected in ASD.36–38 These results advised immune system alterations, which may involve neuroinflammation and possible neurotransmitter imbalances in ASD.39
Despite extensive research, the pathogenic mechanism of ASD has not been explained yet. It was assumed that mitochondrial dysfunction, oxidative stress, abnormal protein folding and apoptotic mechanisms might be reasons for neurodegeneration in relationship with neuroinflammatory mechanisms, which could stimulate the cascade of incidents leading to ASD.15 Moreover, the link between neuroinflammation, mast cell activation and seizures through the secretion of pro-inflammatory mediators and regulation of the blood–brain barrier (BBB) permeability was also proposed.40
ADAM8 is exhibited primarily in immune system cells, such as monocytes, neutrophils, eosinophils, dendritic and B cells; thus, it could play a pathogenic role in inflammation and autoimmune disorders.21 The expression pattern of ADAM8 and its capability to process proteins responsible for neurodevelopment, myelination and plasticity support the crucial role of this protein in the brain.29
ADAM8 was found to be linked to Astro/microgliosis and confirmed to be neuroprotective in murine models of neurodegeneration. This shows a major role for ADAM8 in regulating neuroinflammation.41 Also, ADAM8 might be implicated in common basic cellular processes that also affect the nervous system, or it may influence specific neurodevelopmental mechanisms directly and contribute to the progress of ASD. In all conditions, ADAM8 would be suggested as a possible biomarker and therapeutic target for ASD.
Given the main role of ADAM8 in brain development and neuroinflammation, it occupies an important position in promoting the occurrence of inflammatory and allergic diseases.24,41
ADAM8 can increase the production of many inflammatory factors in several inflammatory diseases and ultimately cause tissue damage.28 Moreover, it was reported that ADAM8 plays an important role in encouraging the release of inflammatory factors such as TNFα, causing neuroinflammation in Parkinson’s and Alzheimer’s disease.29
To our knowledge, the current study is the first to explore the plasma ADAM8 values and their relation to behavioral, social and sensory symptoms in ASD children. Our results revealed that ADAM8 levels are considerably decreased in children with ASD compared to typically developing controls. The lower ADAM8 values in children with ASD may cause improper regulation of immune responses as well as the development of neuroinflammation associated with inflammatory mechanisms linked with microglial activation in ASD. This supported the idea that ADAM8 may be related to pathological and physiological development in ASD. On the other hand, the increase in ADAM8 levels in healthy children acts as an anti-inflammatory. Subsequently, it prevents bacterial endotoxin-induced production of proinflammatory mediators (such as IL-1, TNF, and nitric oxide) in macrophage cultures.42
Nevertheless, these results should be used with great care until additional studies are carried out with more subjects to confirm whether the reduction in ADAM8 concentration is a simple outcome of ASD or has a pathogenic role in the disease. Furthermore, it was impossible to find information in the literature about the ADAM8 levels in ASD or any other neurodevelopmental disorders to compare our results. However, previous studies determined the association of different biomarkers with neuroinflammation in subjects with ASD.43
The behaviors of autistic children are linked with complications in cognitive and social, and sensory reflections, which result in social and sensory impairments and cognitive deficiencies for these children and limit their daily activities. The different ASD behavioral severity scales (CARS, SRS and SSP) were designed to assess the behavioral signs in children with ASD. The search for potential biomarkers to decide the characteristics of ASD severity can offer us better information on ASD pathophysiology. Limited studies have reported correlations between plasma levels of different proteins and the severity of ASD symptoms.31,43
The present study evaluated the correlation of ADAM8 levels with CARS, SRS and SSP in children with ASD. Although plasma ADAM8 levels were higher in mild-to-moderate ASD children compared to severe ASD children, it is not significantly correlated with CARS, SRS and SSP scores, suggesting that ADAM8 may not be associated with the disease’s severity.
These results offer preliminary, direct information on new ADAM8 protein in ASD, which may involve in the early pathogenesis of ASD, provide possible biomarkers and lead to novel medical treatments. Our results are also accordant with the assumption that lower ADAM8 levels may result due to deregulated anti-inflammatory function in the brain of ASD. Higher ADAM8 concentrations in healthy controls act as an anti-inflammatory, which may provide immunity in healthy children compared to children with ASD. It also encourages the possible relationship between neurodevelopmental changes with low ADAM8 levels in ASD.
We may conclude that the fundamental biology of ADAM8 is complicated. As with various ADAM8 roles in controlling inflammatory responses, the pathogenic pathways are possibly involved in the pathophysiology of neuroinflammation in children with ASD.
Study Limitation
The limitations of this study include its small sample size and cross-sectional design; this may have resulted in missing the identification of significant changes between the ADAM 8 and behavioral scores (CARS, SRS, and SSP). Additional limitation of this study is that adaptive behavior was not evaluated in the control group. Thus, whether ADAM8 levels may be linked with behavioral characteristics in healthy controls remains to be clarified. Secondly, there were no information available on the health conditions including any immune-related diagnoses and co-morbidities of the ASD subjects. Therefore, ASD subjects were not analyzed based on the presence/absence of immune conditions, this could have been making a relationship between ADAM8 levels and ASD severity.
Conclusion
The ADAM8 plasma levels in children with ASD were significantly lower than those of healthy controls, which probably indicates that the ADAM8 protein might be implicated in the pathophysiology of ASD. However, the low ADAM 8 protein was not found to be related to the severity of ASD as ADAM8 did not correlate with cognitive, social, and sensory behaviors. The reason is still not known, and further research is required. Furthermore, our results recommended that ADAM8 may play a role in neuroinflammation in ASD. ADAM8 might act as a valuable protein for a forthcoming investigation into the ASD mechanisms at the molecular level or the detection of ASD biomarkers. Nevertheless, these results should be used with great care until additional studies are carried out with more subjects to confirm whether the reduction in ADAM8 concentration is a simple outcome of ASD or has a pathogenic role in the disease.
Acknowledgments
We thank Autism Research and Treatment Centre, Department of Physiology, Faculty of Medicine, King Saud University. King Abdul Aziz City for Science and Technology (KACST), and Vice Deanship of Research Chairs, at King Saud University, Kingdom of Saudi Arabia for financial support. This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology (KACST), Kingdom of Saudi Arabia (Project No. 08-MED 510-02).
Funding Statement
This study was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology (KACST), Kingdom of Saudi Arabia.
Ethical Approval
Ethical approval was obtained from the Ethics Committee of King Khalid Hospital at King Saud University, Riyadh, Kingdom of Saudi Arabia prior to initiation of the research work (No: 22/0122/IRB).
Disclosure
The authors declare that there is no conflict of interest.
References
- 1.Lord C, Elsabbagh M, Baird G, et al. Autism spectrum disorder. Lancet. 2018;392(10146):508–520. doi: 10.1016/S0140-6736(18)31129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schopler E, Van Bourgondien ME, Wellman GJ, Love SR. Childhood Autism Rating Scale (CARS2). Los Angeles, CA: Western Psychological Services; 2010. [Google Scholar]
- 3.Constantino JN. Social Responsiveness Scale, in Encyclopedia of Autism Spectrum Disorders. New York, NY: Springer New York; 2013. [Google Scholar]
- 4.Dunn W. Sensory Profile. San Antonio, TX: Psychological Corporation; 1999. [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5™, 5th Ed, in Diagnostic and Statistical Manual of Mental Disorders: DSM-5™. 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc.; 2013. [Google Scholar]
- 6.Matta SM, Hill-Yardin EL, Crack PJ. The influence of neuroinflammation in autism spectrum disorder. Brain Behav Immun. 2019;79:75–90. doi: 10.1016/j.bbi.2019.04.037 [DOI] [PubMed] [Google Scholar]
- 7.Liao X, Liu Y, Fu X, et al. Postmortem studies of neuroinflammation in autism spectrum disorder: a systematic review. Mol Neurobiol. 2020;57(8):3424–3438. doi: 10.1007/s12035-020-01976-5 [DOI] [PubMed] [Google Scholar]
- 8.Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17(4):389–401. doi: 10.1038/mp.2011.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology. 2017;42(1):284–298. doi: 10.1038/npp.2016.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Najjar S, Pearlman DM, Alper K, et al. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10:43. doi: 10.1186/1742-2094-10-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streit WJ, Mrak RE, Griffin WST. Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation. 2004;1(1):14. doi: 10.1186/1742-2094-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pardo CA, Vargas DL, Zimmerman AW. Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry. 2005;17(6):485–495. doi: 10.1080/02646830500381930 [DOI] [PubMed] [Google Scholar]
- 13.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33(3):195–201. doi: 10.1016/j.pediatrneurol.2005.03.014 [DOI] [PubMed] [Google Scholar]
- 14.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69(5 Pt 2):26r–33r. doi: 10.1203/PDR.0b013e318212c196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Ansary A, Al-Ayadhi L. Neuroinflammation in autism spectrum disorders. J Neuroinflammation. 2012;9(1):265. doi: 10.1186/1742-2094-9-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molloy CA, Morrow A, Meinzenderr J, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172(1–2):198–205. doi: 10.1016/j.jneuroim.2005.11.007 [DOI] [PubMed] [Google Scholar]
- 17.Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15(2):84–97. doi: 10.1038/nrn3638 [DOI] [PubMed] [Google Scholar]
- 18.Strowig T, Henao-Mejia J, Elinav E, et al. Inflammasomes in health and disease. Nature. 2012;481(7381):278–286. doi: 10.1038/nature10759 [DOI] [PubMed] [Google Scholar]
- 19.Lu H, Meng Y, Han X, et al. ADAM8 activates NLRP3 inflammasome to promote cerebral ischemia-reperfusion injury. J Healthc Eng. 2021;2021:3097432. doi: 10.1155/2021/3097432 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Knolle MD, Owen CA. ADAM8: a new therapeutic target for asthma. Expert Opin Ther Targets. 2009;13(5):523–540. doi: 10.1517/14728220902889788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuno O, Miyazaki E, Nureki S, et al. Elevated soluble ADAM8 in bronchoalveolar lavage fluid in patients with eosinophilic pneumonia. Int Arch Allergy Immunol. 2007;142(4):285–290. doi: 10.1159/000097359 [DOI] [PubMed] [Google Scholar]
- 22.Yoshiyama K, Higuchi Y, Kataoka M, et al. CD156 (human ADAM8): expression, primary amino acid sequence, and gene location. Genomics. 1997;41(1):56–62. doi: 10.1006/geno.1997.4607 [DOI] [PubMed] [Google Scholar]
- 23.Schlomann U, Wildeboer D, Webster A, et al. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002;277(50):48210–48219. doi: 10.1074/jbc.M203355200 [DOI] [PubMed] [Google Scholar]
- 24.Erbek SS, Hizal E, Erinanc H, et al. Expression of a disintegrin and metalloproteinase 8 by inflammatory cells in nasal polyps. Am J Rhinol Allergy. 2013;27(2):151. doi: 10.2500/ajra.2013.27.3859 [DOI] [PubMed] [Google Scholar]
- 25.Kataoka M, Yoshiyama K, Matsuura K, et al. Structure of the murine CD156 gene, characterization of its promoter, and chromosomal location*. J Biol Chem. 1997;272(29):18209–18215. doi: 10.1074/jbc.272.29.18209 [DOI] [PubMed] [Google Scholar]
- 26.Chung HW, Kim JJ, Choi JI, et al. A disintegrin and metalloproteinase 8 as a potential blood biomarker for early diagnosis of gastric cancer. Yonsei Med J. 2019;60(8):713–719. doi: 10.3349/ymj.2019.60.8.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen J, Jiang X, Duan Y, et al. ADAM8 in asthma. friend or foe to airway inflammation? Am J Respir Cell Mol Biol. 2013;49(6):875–884. doi: 10.1165/rcmb.2013-0168TR [DOI] [PubMed] [Google Scholar]
- 28.Alishahi M, Farzaneh M, Ghaedrahmati F, et al. NLRP3 inflammasome in ischemic stroke: as possible therapeutic target. Int J Stroke. 2019;14(6):574–591. doi: 10.1177/1747493019841242 [DOI] [PubMed] [Google Scholar]
- 29.Hsia HE, Tüshaus J, Brummer T, et al. Functions of ‘A disintegrin and metalloproteases (ADAMs)’ in the mammalian nervous system. Cell Mol Life Sci. 2019;76(16):3055–3081. doi: 10.1007/s00018-019-03173-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Verhoeff TA, Perez Pardo P, et al. The gut-brain axis in autism spectrum disorder: a focus on the metalloproteases ADAM10 and ADAM17. Int J Mol Sci. 2020;22(1):118. doi: 10.3390/ijms22010118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Mazidi S, Al-Ayadhi LY. Plasma levels of alpha and gamma synucleins in autism spectrum disorder: an indicator of severity. Med Princ Pract. 2021;30(2):160–167. doi: 10.1159/000513935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glass CK, Saijo K, Winner B, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Onore CE, Nordahl CW, Young GS, et al. Levels of soluble platelet endothelial cell adhesion molecule-1 and P-selectin are decreased in children with autism spectrum disorder. Biol Psychiatry. 2012;72(12):1020–1025. doi: 10.1016/j.biopsych.2012.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vargas DL, Nascimbene C, Krishnan C, et al. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- 35.Ashwood P, Enstrom A, Krakowiak P, et al. Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol. 2008;204(1–2):149–153. doi: 10.1016/j.jneuroim.2008.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heuer L, Ashwood P, Schauer J, et al. Reduced levels of immunoglobulin in children with autism correlates with behavioral symptoms. Autism Res. 2008;1(5):275–283. doi: 10.1002/aur.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Ayadhi LY, Mostafa GA. Elevated serum levels of macrophage-derived chemokine and thymus and activation-regulated chemokine in autistic children. J Neuroinflammation. 2013;10(1):846. doi: 10.1186/1742-2094-10-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ashwood P, Krakowiak P, Hertz-Picciotto I, et al. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25(1):40–45. doi: 10.1016/j.bbi.2010.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohly HH, Panja A. Immunological findings in autism. Int Rev Neurobiol. 2005;71:317–341. [DOI] [PubMed] [Google Scholar]
- 40.Theoharides TC, Zhang B. Neuro-inflammation, blood-brain barrier, seizures and autism. J Neuroinflammation. 2011;8(1):168. doi: 10.1186/1742-2094-8-168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartsch JW, Wildeboer D, Koller G, et al. Tumor necrosis factor-alpha (TNF-alpha) regulates shedding of TNF-alpha receptor 1 by the metalloprotease-disintegrin ADAM8: evidence for a protease-regulated feedback loop in neuroprotection. J Neurosci. 2010;30(36):12210–12218. doi: 10.1523/JNEUROSCI.1520-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dziegielewska KM, Andersen NA, Saunders NR. Modification of macrophage response to lipopolysaccharide by fetuin. Immunol Lett. 1998;60(1):31–35. doi: 10.1016/S0165-2478(97)00126-0 [DOI] [PubMed] [Google Scholar]
- 43.Hamed N, Elkhawad AO, Osman MA, et al. Determination of neuroinflammatory biomarkers in autistic and neurotypical Saudi children. Metab Brain Dis. 2019;34:1049–1060. doi: 10.1007/s11011-019-00420-5 [DOI] [PubMed] [Google Scholar]