Abstract
Introduction
Tracheobronchopathia osteochondroplastica (TO) is a relatively rare benign tracheobronchial disease, which is often misdiagnosed or missed. To date, there is no specific treatment for TO. The aim of this study was to investigate the clinical manifestations, imaging features, bronchoscopy results, pathological findings, and diagnostic points of TO.
Patients and methods
A total of 33 patients diagnosed with TO were enrolled. Clinical data were collected using retrospective methods in the period from January 2021 and November 2022. Descriptive analysis was used.
Results
Patients included 17 (51.5%) male and 16 (48.5%) female, with a median age of 54 years. The main clinical manifestations included cough in 15 cases, fever in 6 cases, chest tightness in 4 cases, haemoptysis in 3 cases, and chest pain in 4 cases. The time from the onset of symptoms to diagnosis was 1 week to 96 months. Some patients were diagnosed with other lung diseases, including 16 patients with tuberculosis, 2 patients with lung cancer, 3 patients with nontuberculous mycobacteriosis, 3 patients with tuberculous pleurisy, 2 patients with bronchiectasis, and 1 patient with pneumonia. Chest computed tomography (CT) scan demonstrated calcified nodules in 10 (30.3%) patients. In bronchoscopy, entire tracheal involvement was found in 21 (63.6%) patients, 12 (36.4%) patients were found to have involvement of only part of the trachea. The patients were divided into three groups according to the bronchoscopic presentation, the largest proportion was stage II (19/33), followed by stage I (8/33) and stage III (6/33). Histopathological findings showed squamous metaplasia, cartilaginous, and bony tissues.
Conclusion
TO is a slowly progressing disease with non-specific clinical symptoms and a low positive rate of imaging diagnosis, making it susceptible to misdiagnosis and missed diagnosis. The disease needs to be diagnosed by combining imaging features, fiberoptic bronchoscopy, and pathological findings.
Keywords: benign tracheobronchial disease, tuberculosis, misdiagnosis, bronchoscopy
Introduction
Tracheobronchopathia osteochondroplastica (TO) is a rare tracheobronchial lesion of unknown etiology. It is an idiopathic benign disease, also known as tracheobronchial cystic fibrous osteochondritis and osteoformative tracheomalacia, in which granular nodules of varying size and number are seen protruding into the lumen of the airway, the nodules generally occur in the anterolateral wall of the trachea and the proximal bronchi, these nodules originate from cartilage, and the acoustic hilum, supraglottis, and posterior tracheal wall are usually unaffected.1 The disease is relatively rare, with an estimated prevalence of between 0.01% and 0.80%,2 while a recent study showed a prevalence of 0.09%.3 Because of the variable clinical presentation of this disease and the lack of specificity of symptoms, it is prone to underdiagnosis and misdiagnosis, especially when combined with other respiratory diseases such as bronchial tuberculosis. Although diagnostic methods including chest computed tomography (CT) are also widely used, bronchoscopy remains the recognized standard for confirming the diagnosis of TO. To date, there is no specific treatment for TO. Patients are often diagnosed decades after the onset of the disease. There were few domestic and foreign reports on TO and most cases currently being reported were sporadic.
In order to improve the understanding of the clinical and pathological aspects of TO and reduce its misdiagnosis and missed diagnosis rates, we selected 33 TO patients who underwent comprehensive examinations at the hospital as the research subjects. The differential diagnosis, treatment, and prognosis of the disease were explored by analyzing the clinical manifestations, imaging features, fiberoptic bronchoscopy, and pathological findings of the patients.
Methods
Study Design
This is a retrospective observational study on the visits of patients with TO in Wuhan Pulmonary Hospital. Informed consent was waived because the study was retrospective in nature and all data were anonymous. This study was approved by the Medical Ethics Committee of Wuhan Pulmonary Hospital, and the approval number was Wuhan Pulmonary Hospital Ethics (2018) No. 05.
Participant and Data Collection
Among 18,708 patients who had undergone bronchoscopic examination in Wuhan Pulmonary Hospital between January 2021 and November 2022. The diagnosis of To in 33 patients was made by a bronchoscopist with more than 3 years of experience in bronchoscopy and two pathologists with more than 5 years of experience in diagnostic pathology. Medical records of these patients including demographic characteristics (age, sex, symptoms, associated lung diseases, smoking history, reason for bronchoscopy), radiological results, and bronchoscopic and histopathological findings were extracted from patient medical records held at Wuhan Pulmonary Hospital.
The categories of subjects were classified based on criteria developed in a large Chinese cohort study using characteristic bronchoscopic image. TO was divided into the following three stages: stage I (early stage, also a mild grade), which consisted of plaque-like infiltrations of soft, yellow-whitish lesions scattered in the lumen mucosa, accompanied by a change of mucosa hyperemia edema; stage II (middle stage, also a moderate grade), which showed diffuse cartilaginous nodules and sessile spicules protruding into the lumen with a cobblestone or stalactite appearance; and stage III (late stage, also a severe grade), which indicates deformation and rigid narrowing of the airway that may cause obstruction.4
Statistical Analysis
Continuous variables were described as medians with interquartile range (IQRs) and categorical variables were expressed as frequency (n) and percentage (%).
Results
Characteristics of the Study Patients
During the study period, 33 patients with confirmed TO were included. The median age of the patients was 54 years old (IQR, 47–61), ranging from 32 years to 81 years, and 17 of the 33 patients (51.5%) were male.
The baseline clinical characteristics in our cohort are summarized in Table 1. The most frequent manifestation was chronic cough (n=15, 45.5%), and other clinical symptoms include fever (n=6, 18.2%), increased chest tightness (n=4, 12.1%), haemoptysis (n=3, 9.1%), and chest pain (n=4, 12.1%), with some patients being asymptomatic (n=6, 18.2%). The time from the onset of symptoms to diagnosis was 1 week to 96 months. Pulmonary tuberculosis was found in some patients (n=16, 48.5%) and 2 (6.1%) patients were diagnosed with lung cancer. Five patients were former smokers (15.2%). More than half of the patients (n=23, 69.7%) visited the hospital to evaluate lung nodules or masses suspected of pulmonary tuberculosis or non-tuberculosis mycobacterial pulmonary disease and 1 (3.0%) patients was referred to the hospital because of suspected lung tumor.
Table 1.
Baseline Clinical Characteristics of 33 Patients
| Variables | No. (%) or Median (IQR) |
|---|---|
| Age (yr), median (Q1, Q3) | 54 (47, 61) |
| Sex, n (%) | |
| Male | 17 (51.5) |
| Female | 16 (48.5) |
| Signs and symptoms, n (%) | |
| Cough | 15 (45.5) |
| Fever | 6 (18.2) |
| Chest tightness | 4 (12.1) |
| Haemoptysis | 3 (9.1) |
| Chest pain | 4 (12.1) |
| Asymptomatic | 6 (18.2) |
| Associated lung diseases, n (%) | |
| Lung cancer | 2 (6.1) |
| Pulmonary tuberculosis | 16 (48.5) |
| Nontuberculous mycobacterial diseases | 3 (9.1) |
| Tuberculous pleurisy | 3 (9.1) |
| Bronchiectasis | 2 (6.1) |
| Pneumonia | 1 (3.0) |
| Smoking history, n (%) | |
| Never | 26 (78.8) |
| Former | 5 (15.2) |
| Present | 2 (6.1) |
| Reason for bronchoscopy, n (%) | |
| Suspected pulmonary tuberculosis or non tuberculosis | 23 (69.7) |
| Suspected lung tumor | 1 (3.0) |
| Assessment of bronchial tuberculosis treatment outcomes | 7 (21.2) |
| Interventional treatment of bronchial lesion | 1 (3.0) |
| Mediastinal occupancy | 1 (3.0) |
Chest CT results
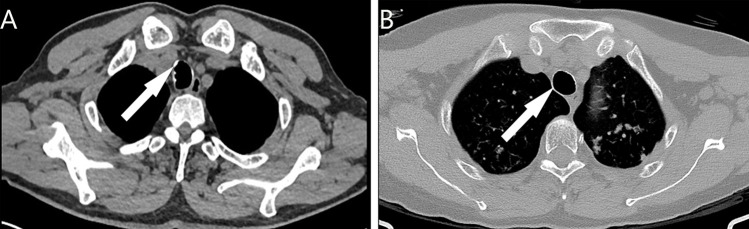
Chest CT results are presented in Table 2, 33 patients underwent chest CT scan in our hospital. As shown in Figure 1, lesions and calcified nodules were present in 10 patients, whereas the other 23 individuals did not show significant abnormalities.
Table 2.
Radiologic, and Histopathologic Tests
| Variables | No. (%) |
|---|---|
| Chest CT, n (%) | |
| Calcified nodules | 10 (30.3) |
| Normal | 23 (69.7) |
| Involved sites on bronchoscopy, n (%) | |
| Entire trachea | 21 (63.6) |
| Extending to both main bronchi | 5 (15.2) |
| Extending to both main bronchi and involving lower bronchi | 5 (15.2) |
| Partially involved only on the trachea | 12 (36.4) |
| Involved upper trachea | 3 (9.1) |
| Involved upper and middle trachea | 3 (9.1) |
| Involved middle and lower trachea | 4 (12.1) |
| Involved lower trachea | 2 (6.1) |
| Bronchoscopic stage, n (%) | |
| Stage I | 8 (24.2) |
| Stage II | 19 (57.6) |
| Stage III | 6 (18.2) |
| Histopathologic findings, n (%) | |
| Cartilaginous | 15 (45.5) |
| Bony tissues | 18 (54.5) |
| Squamous metaplasia | 3 (9.1) |
Figure 1.
Chest CT results of TO demonstrated calcified nodules in the anterolateral wall protruding into the tracheal lumen. (A) Calcified nodules in the anterior wall (arrow). (B) Calcified nodules in the lateral wall (arrow).
Bronchoscopic Results
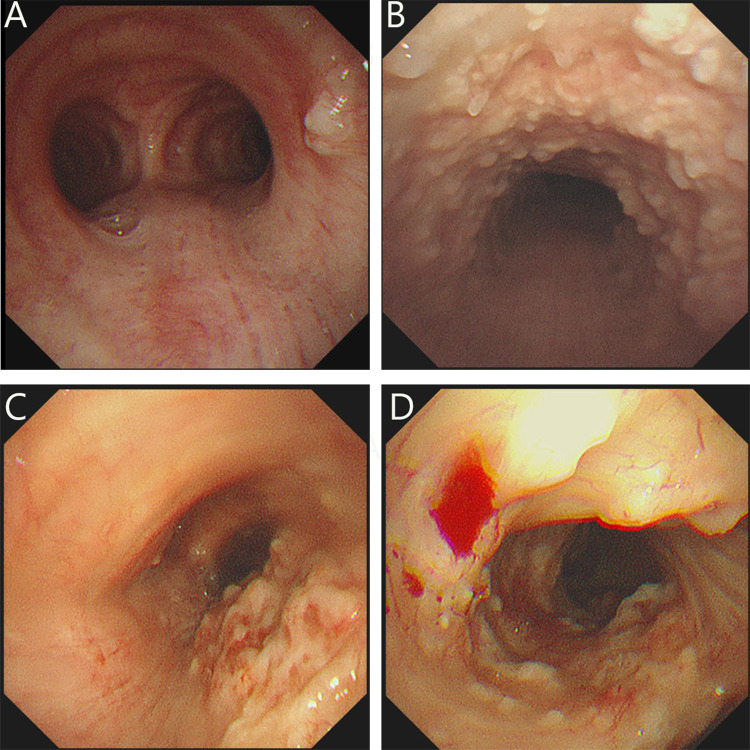
The main bronchoscopic and histopathologic results are represented in Table 2, and all patients underwent bronchoscopy in our hospital. Among all patients, multiple small irregular granular calcified nodules were observed in the anterolateral wall of the trachea projecting into the lumen, with nodules distributed along the cartilaginous ring, and the size of individual nodules was mostly 1–10 mm. The entire trachea was involved in 21 (63.6%) patients, 5 (15.2%) patients showed involvement of both main bronchi. In 5 (15.2%) patients, the involvement extended to both main bronchi and lower bronchi. Twelve (36.4%) patients showed involvement in only one part of the trachea patients: upper trachea in 3 patients, upper and middle trachea in 3 patients, middle and lower trachea in 4 patients, and lower trachea in 2 patients, no segmental bronchial involvement was found. In light of the bronchoscopic manifestations, all patients were categorized as three groups as shown in Figure 2: stage I (n=8, 24.2%), with relatively scattered nodules and normal inter-nodal mucosa (Figure 2A); stage II (n=19, 57.6%), with numerous nodules diffusely distributed on the mucosal surface of the lumen (Figure 2B); stage III (n=6, 18.2%), with widely fused nodules, resulting in different degrees of deformation and narrowing of the lumen (Figure 2C and D).
Figure 2.
Bronchoscopic results of TO in different stages. (A) Stage I: Scattered nodular protrusions in the lower part of the trachea near the bulge; (B) Stage II: Diffuse distribution of nodular protrusions on the anterior and lateral walls of the trachea; (C and D) Stage III: Deformation and narrowing of the lumen.
Histopathologic Findings
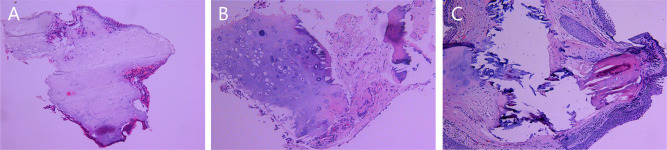
The histopathologic results of 33 subjects were obtained, as shown in Table 2. Histopathologic findings (Figure 3) included presented cartilaginous (n=15, 45.5%), bony tissues (n=18, 54.5%), and squamous metaplasia (n=3, 9.1%) in the bronchial submucosa.
Figure 3.
Histopathological findings of TO illustrateTracheal mucosa intact. (A) Nodular cartilaginous tissue with calcification; (B) Nodular cartilaginous tissue and mature bone tissue with calcification; (C) Squamous epithelial metaplasia of the tracheal mucosa and nodular mature cartilage and bone tissue were seen in the interstitium.
Treatment and Outcome
Therapeutic management and follow-up data were available for all 33 patients, and the median follow-up time for all patients was 11 months. None of the patients had received specific treatment for TO. Two patients TO with lung cancer received surgical treatment, chemotherapy, and targeted gene therapy, and are currently alive. Sixteen TO patients with tuberculosis received regular anti-tuberculosis treatment, of which 14 patients eventually achieved clinical cure and 2 patients failed treatment but had symptomatic relief. The remaining patients received antibiotics, expectorants, cough suppressants, and other symptomatic treatments with remission. Twenty-one patients underwent bronchoscopy reexamination, and the results showed that all patients had stable TO condition without significant worsening.
Discussion
To is a rare benign tracheobronchial lesion characterized by nodular hyperplasia of the submucosal bone or cartilage of tracheobronchial. TO was first identified at autopsy by RoRitanky in 1855 and was first described pathologically and histologically by Wilks et al in 1857.5 There is no certainty regarding its incidence, and to date, more than 600 cases of TO have been reported worldwide.6 With the deepening of research and the widespread use of bronchoscopy, more and more reports have been made. It is generally accepted that the course of TO is relatively long, and the age of predilection is usually older than 50 years, with no significant gender differences and occasional reports of adolescents and children.6–8 The average age of the data in this study was 54.8 years old, with no significant gender difference, which is consistent with the literature reports. The clinical symptoms of TO are non-specific, and usually include coughing, expectoration, wheezing, and other symptoms. This is due to the irregularity of the tracheal cavity caused by the lesion, which increases the sensitivity of air torrent and cough reflex, as well as the accumulation of sputum caused by the lack of normal ciliated columnar epithelium.9,10 It had also been shown11 that about 52.5% of TO patients were asymptomatic. Notably, 16 cases (48.5%) in this group were combined with pulmonary tuberculosis, and a few other patients were accompanied by nontuberculous mycobacterial lung disease, lung cancer, and bronchiectasis, which can themselves cause respiratory symptoms, leading to an inevitable overlap of symptoms between diseases.
The pathogenesis of TO is unknown, and it is speculated that it may be the result of submucosal elastic fibrosis producing elastic cartilage, which then calcifies and ossifies, or the proliferation of tracheal cartilage rings, forming exophytic osteochondral warts that subsequently ossify.12,13 Osteogenic protein-2 is involved in this process together with transforming growth factor-β1.14 A recent study8 suggested that airway stem cell dysfunction can induce new bone formation, which may be one of the mechanisms of TO formation. The causative factors of TO may include chronic infection, congenital airway developmental abnormalities, amyloidosis, silicosis, and IgA deficiency.5,6 In this study, 78.8% of patients (26/33) were non-smokers, suggesting that smoking is not associated with the development of TO. Of interest, the combination of pulmonary tuberculosis in 16 patients and nontuberculous mycobacterial lung disease in 3 cases may suggest chronic airway infection as a causative factor for TO, as described in the literature. Twenty-three patients (69.7%) in the study were referred to our hospital because of suspected pulmonary nodules or masses at an outside hospital, and bronchoscopy was performed in this group of patients with the primary purpose of obtaining deep respiratory secretions for mycobacterium tuberculosis pathogenesis or for simultaneous endoscopic treatment of the lesion and assessment of treatment outcome. In contrast, TO is found incidentally during bronchoscopy. Although the combination of TO with tuberculosis was clearly identified by bronchoscopic biopsy, it was not possible to determine the chronology of the development of TO and tuberculosis, making it difficult to clarify the causal relationship between TO and tuberculosis. There are few reports on the combination of TO with TB. Mittal, S, etc., found that a patient with laryngeal tuberculosis had a secondary TO during the course of treatment, and concluded that the cause of the etiology of granulomatous diseases such as tuberculosis infection can lead to the development of TO.15 Another report showed that old TB and active TB may lead to TO,16 but whether mycobacterium tuberculosis is a direct causative factor of TO needs to be confirmed by further studies.
The CT presentation of TO has its own specific features, and the chest CT scan can show specific submucosal multiple calcified nodules. This imaging sign can also exclude recurrent polychondritis, which is mostly a thickening and deformation of cartilage without intracanalicular calcified nodules.17 Some literature reported that the diagnostic sensitivity of CT for TO is about 60–70%,7 but also up to 97%,2 or only about 30%.8 In practice, imaging physicians often miss the diagnosis of this disease, and only 30.3% of the 33 cases in our group who had chest CT proposed the diagnosis of TO. On the one hand, imaging physicians pay more attention to lung images and tend to ignore tracheobronchial luminal lesions, and on the other hand, some patients with TO have small and sparsely distributed bronchial nodules, which are easily missed by CT. However, CT is still the most reliable diagnostic imaging method for TO and also facilitates the follow-up of TO patients.
Bronchoscopy is considered to be the gold standard for the diagnosis of TO.7,18 TO is typically characterized by the presence of numerous cartilaginous and/or bony nodules on the submucosa of the tracheobronchial tree, protruding into the lumen. On appearance, the nodules are cobble-stone, stalactite caves, and the nodules are mostly found in the anterior and lateral walls of the trachea. The lesions usually do not involve the posterior wall because there is no cartilaginous ring in the posterior wall of the trachea. Depending on the severity of the lesion, the nodules may have a needle-like, beaded, stalactite-like, or cobblestone-like appearance.19 TO can also occur in pharynx,10,20 but no pharyngeal involvement was seen in any of our cases. The nodules are usually 1–10 mm in diameter, and in later stages of the disease the nodules may fuse into sheets and cause deformation, stiffness, and luminal narrowing of the tracheal wall. In this paper, all 33 cases showed typical multiple nodules of different sizes on bronchoscopy. Overall, 63.6% (21/33) involved only the entire trachea, while 36.4% (12/33) involved both the main bronchus and the upper and middle bronchi starting from the trachea, and there were no cases where only the bronchus was involved and the trachea was exempted, suggesting that the lesions started in the trachea and then extended to the proximal bronchus. The nodules were all located in the anterior and lateral walls and distributed along the cartilaginous ring without involving the posterior wall, which is consistent with the literature. According to the degree of disease development, the bronchoscopic manifestations can be divided into three stages:4 stage I, where the nodules are scattered and the inter-nodal mucosa is normal; stage II, where a large number of nodules are diffusely distributed on the mucosal surface of the lumen; and stage III, where the nodules are extensively fused, resulting in different degrees of deformation and narrowing of the lumen. In this study, stage II was predominant, about 57.6%.
Since bronchoscopic nodules are hard and generally not easy to clamp for biopsy, it has been suggested that a typical bronchoscopic presentation can confirm the diagnosis without pathologic biopsy.21 However, submucosal biopsy is thought to identify other airway diseases, such as amyloidosis, endobronchial nodular disease, tuberculosis-like calcification, recurrent polychondritis, and tracheobronchial papilloma.2,7,22 A single-center study in India suggested that a bronchoscopic biopsy of TPO lesions should be performed to rule out other aetiologies.3 Biopsies were taken from all our study data, and the pathological histomorphology showed submucosal nodular hyperplasia of cartilage and/or mature bone tissue, and 9.1% surface epithelial squamous metaplasia. The histomorphology of TO needs to be distinguished from calcification caused by other diseases, such as calcification of tuberculous necrotic material and granulomatous polyangiitis, which usually do not have ossification, whereas TO often has mature bone tissue and even bone marrow formation on the basis of calcification. Nodular hyperplasia of cartilage is seen in TO, which needs to be distinguished from chondromatous malformations and normal bronchial cartilage. Chondromatous malformations are well-defined nodules on CT, with fat, smooth muscle, and fibrous components within the cartilage, and respiratory epithelial components can be seen. In normal bronchial cartilage, there are no nodular hyperplasia. Pathological diagnosis is difficult to differentiate in combination with bronchoscopic manifestations.
There is no clear uniform opinion about the treatment of TO. Since complete removal of the nodule is not possible, its treatment depends on disease symptoms, the presence or absence of infection, and the severity of airway obstruction or deformation. Treatment modalities include symptomatic treatment, hormone inhalation,23 etc., and bronchoscopic laser ablation, cryotherapy, and surgical treatment for those with severe tracheobronchial stenosis or airway obstruction.9,19,24 The interpretation of this study was limited by retrospective design study performed in a single hospital and a small study population.
Conclusions
In summary, TO is a rare benign tracheobronchial disease that occurs in people over 50 years of age. Clinical manifestations are non-specific, manifesting as one or more non-specific respiratory symptoms such as cough and hemoptysis, especially when combined with other respiratory diseases because the symptoms overlap and the diagnosis is easily missed. CT of the chest has a diagnostic role, but typical manifestations on bronchoscopy combined with pathological histological patterns are necessary for a definitive diagnosis. Most patients with TO generally do not receive specific treatment, and those with severe airway narrowing require endoscopic or surgical treatment. Clinicians’ awareness of the disease is crucial, at least bearing in mind that bronchoscopy is necessary to exclude the possibility of TO when coughing, wheezing, hemoptysis, and other respiratory symptoms are evident in the absence of conventional clinical management.
Funding Statement
This work was supported by Health Commission of Hubei Province Scientific Research Project (WJ2019H320) and the Foundation of Wuhan Pulmonary Hospital (YNZZ202216).
Ethical Statement
The study was approved by the medical ethics committee of Wuhan Pulmonary Hospital, and the approval number was Wuhan Pulmonary Hospital Ethics (2018) No. 05. Informed consent was waived because the study was retrospective in nature and all data were anonymous. This study complied with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflicts of interest to declare for this work.
References
- 1.Juanola Pla J, Rejon Cabezas T, Ortega Castillo MP. Tracheobronchopathia osteochondroplastica. Arch Bronconeumol. 2020;56(3):172. doi: 10.1016/j.arbres.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 2.Dumazet A, Launois C, Lebargy F, et al. Tracheobronchopathia osteochondroplastica: clinical, bronchoscopic, and comorbid features in a case series. BMC Pulm Med. 2022;22(1):423. doi: 10.1186/s12890-022-02225-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nair AA, Gupta R, Irodi A, et al. Tracheobronchopathia osteochondroplastica - to biopsy or not to biopsy? A relook at the rare disease. J Bronchology Interv Pulmonol. 2023. doi: 10.1097/LBR.0000000000000931 [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Wu N, Huang HD, et al. A clinical study of tracheobronchopathia osteochondroplastica: findings from a large Chinese cohort. PLoS One. 2014;9(7):e102068. doi: 10.1371/journal.pone.0102068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ning JY, Zhang LQ, Guo H, et al. 骨化性气管支气管病的临床病理学观察 [Clinic pathologic studies of cases with tracheobronchopathia osteochondroplastica]. Zhonghua Bing Li Xue Za Zhi. 2018;47(11):857–858. Chinese. doi: 10.3760/cma.j.issn.0529-5807.2018.11.008 [DOI] [PubMed] [Google Scholar]
- 6.Guo R, Zhou M, Wei X, et al. Clinical characteristics of six cases of tracheobronchopathia osteochondroplastica. Can Respir J. 2020;2020:8685126. doi: 10.1155/2020/8685126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ulasli SS, Kupeli E. Tracheobronchopathia osteochondroplastica: a review of the literature. Clin Respir J. 2015;9(4):386–391. doi: 10.1111/crj.12166 [DOI] [PubMed] [Google Scholar]
- 8.Simsek PO, Ozcelik U, Demirkazik F, et al. Tracheobronchopathia osteochondroplastica in a 9-year-old girl. Pediatr Pulmonol. 2006;41(1):95–97. doi: 10.1002/ppul.20311 [DOI] [PubMed] [Google Scholar]
- 9.Dharia I, Bielamowicz S. Surgical management in tracheobronchopathia osteochondroplastica: a case study. Laryngoscope. 2021;131(3):E911–E913. doi: 10.1002/lary.29095 [DOI] [PubMed] [Google Scholar]
- 10.Han J, Wei JG, Gao XZ, et al. 骨化性气管支气管病18例临床病理学分析 [Tracheobronchopathia osteochondroplastica: a clinicopathological analysis of 18 cases]. Zhonghua Bing Li Xue Za Zhi. 2019;48(4):303–306. Chinese. doi: 10.3760/cma.j.issn.0529-5807.2019.04.008 [DOI] [PubMed] [Google Scholar]
- 11.Cho HK, Jeong BH, Kim H. Clinical course of tracheobronchopathia osteochondroplastica. J Thorac Dis. 2020;12(10):5571–5579. doi: 10.21037/jtd-20-1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jindal S, Nath A, Neyaz Z, et al. Tracheobronchopathia osteochondroplastica--A rare or an overlooked entity? J Radiol Case Rep. 2013;7(3):16–25. doi: 10.3941/jrcr.v7i3.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pounder DJ, Pieterse AS. Tracheopathia osteoplastica: a study of the minimal lesion. J Pathol. 1982;138(3):235–239. doi: 10.1002/path.1711380305 [DOI] [PubMed] [Google Scholar]
- 14.Tajima K, Yamakawa M, Katagiri T, et al. Immunohistochemical detection of bone morphogenetic protein-2 and transforming growth factor beta-1 in tracheopathia osteochondroplastica. Virchows Arch. 1997;431(5):359–363. doi: 10.1007/s004280050111 [DOI] [PubMed] [Google Scholar]
- 15.Mittal S, Jain A, Arava S, et al. Tracheobronchopathia osteochondroplastica following laryngeal tuberculosis. Lung India. 2017;34(5):483–485. doi: 10.4103/lungindia.lungindia_54_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sousa M, Silva J, Rodrigues B. Coincident Tuberculosis and Tracheobronchopathia Osteochondroplastica in a Patient. Arch Bronconeumol. 2017;53(6):343. doi: 10.1016/j.arbres.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 17.Devaraja K, Sagar P, Chirom AS. Tracheobronchopathia osteochondroplastica: awareness is the key for diagnosis and management. BMJ Case Rep. 2017;2017. doi: 10.1136/bcr-2017-220567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakash UB. Tracheobronchopathia osteochondroplastica. Semin Respir Crit Care Med. 2002;23(2):167–175. doi: 10.1055/s-2002-25305 [DOI] [PubMed] [Google Scholar]
- 19.Luo T, Zhou H, Meng J. Clinical characteristics of tracheobronchopathia osteochondroplastica. Respir Care. 2019;64(2):196–200. doi: 10.4187/respcare.05867 [DOI] [PubMed] [Google Scholar]
- 20.Paaske PB, Tang E. Tracheopathia osteoplastica in the larynx. J Laryngol Otol. 1985;99(3):305–310. doi: 10.1017/S0022215100096742 [DOI] [PubMed] [Google Scholar]
- 21.Kirbaş G, Dağli CE, Tanrikulu AC, et al. Unusual combination of tracheobronchopathia osteochondroplastica and AA amyloidosis. Yonsei Med J. 2009;50(5):721–724. doi: 10.3349/ymj.2009.50.5.721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chrysikos S, Karampitsakos T, Tzouvelekis A, et al. Coexistence of tracheobronchopathia osteochondroplastica and sarcoidosis: accidental finding or something more? J Bronchology Interv Pulmonol. 2019;26(1):e7–e8. doi: 10.1097/LBR.0000000000000521 [DOI] [PubMed] [Google Scholar]
- 23.Zhang XB, Zeng HQ, Cai XY, et al. Tracheobronchopathia osteochondroplastica: a case report and literature review. J Thorac Dis. 2013;5(5):E182–E184. doi: 10.3978/j.issn.2072-1439.2013.08.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jabbardarjani HR, Radpey B, Kharabian S, et al. Tracheobronchopathia osteochondroplastica: presentation of ten cases and review of the literature. Lung. 2008;186(5):293–297. doi: 10.1007/s00408-008-9088-4 [DOI] [PubMed] [Google Scholar]