Abstract
Abundant formation of endogenous supersulfides, which include reactive persulfide species and sulfur catenated residues in thiols and proteins (supersulfidation), has been observed. We found here that supersulfides catalyze S-nitrosoglutathione (GSNO) metabolism via glutathione-dependent electron transfer from aldehydes by exploiting alcohol dehydrogenase 5 (ADH5). ADH5 is a highly conserved bifunctional enzyme serving as GSNO reductase (GSNOR) that down-regulates NO signaling and formaldehyde dehydrogenase (FDH) that detoxifies formaldehyde in the form of glutathione hemithioacetal. C174S mutation significantly reduced the supersulfidation of ADH5 and almost abolished GSNOR activity but spared FDH activity. Notably, Adh5C174S/C174S mice manifested improved cardiac functions possibly because of GSNOR elimination and consequent increased NO bioavailability. Therefore, we successfully separated dual functions (GSNOR and FDH) of ADH5 (mediated by the supersulfide catalysis) through the biochemical analysis for supersulfides in vitro and characterizing in vivo phenotypes of the GSNOR-deficient organisms that we established herein. Supersulfides in ADH5 thus constitute a substantial catalytic center for GSNO metabolism mediating electron transfer from aldehydes.
Supersulfides regulate NO signaling by accelerating NO metabolism that is mediated by electron transfer from formaldehyde.
INTRODUCTION
Production of a large quantity of reactive persulfide species including cysteine persulfide (CysSSH) and glutathione persulfide (GSSH) was recently noted in cells and tissues and was demonstrated to play critical roles in the regulation of electrophilic signaling (1–5). Supersulfides, which we currently define as hydropersulfide (RSSH) species and polysulfides with sulfur catenation (RSSnR, n > 1, R = hydrogen or alkyl), have been recognized as universal bioactive metabolites that occur in cells and tissues at submillimolar to millimolar levels (1, 5). Additional sulfur atoms in supersulfides confer a unique redox property, which forms a molecular basis for their biological roles: They behave as either nucleophiles or electrophiles depending on the context. Supersulfides exist in cells as low–molecular weight metabolites, such as CysSSH and GSSH, and as proteins with supersulfidation at cysteine residue side chains (1–6).
Alcohol dehydrogenase 5 (ADH5) is a member of the alcohol dehydrogenase family and is designated as a class III alcohol dehydrogenase. ADH5, which is highly conserved among both eukaryotic and prokaryotic organisms, is also known as S-nitrosoglutathione reductase (GSNOR) and contains an active center zinc ion (fig. S1) (7, 8). ADH5 is a major enzyme that reductively degrades S-nitrosoglutathione (GSNO) to generate hydroxylamine (NH2OH) and glutathione (GSH/GSSG) using reduced form of nicotinamide adenine dinucleotide (NADH), and ADH5 is thereby involved indirectly in nitric oxide (NO) signal regulation (Fig. 1A) (7, 8). Disruption of the Adh5 gene in mice consistently enhanced NO activity; most Adh5-null mice phenotypes were rescued by inhibiting NO synthetase activity (9–12). ADH5 has another important function as a GSH-dependent formaldehyde dehydrogenase (FDH) (13, 14). This enzyme, which uses oxidized form of nicotinamide adenine dinucleotide (NAD+) as an electron acceptor, oxidizes the substrate formaldehyde to produce formate in the form of GSH conjugates. S-Hydroxymethylglutathione (HM-SG, also called glutathione hemithioacetal) is generated by means of the nonenzymatic Michael addition of formaldehyde to GSH, followed by enzymatic oxidation to S-formylglutathione by ADH5. S-Formylglutathione is hydrolyzed to formate (Fig. 1A) (13, 14). Although these two activities of ADH5 have been studied separately, the detailed regulatory mechanisms of GSNOR and FDH activities and their relationships remain to be elucidated.
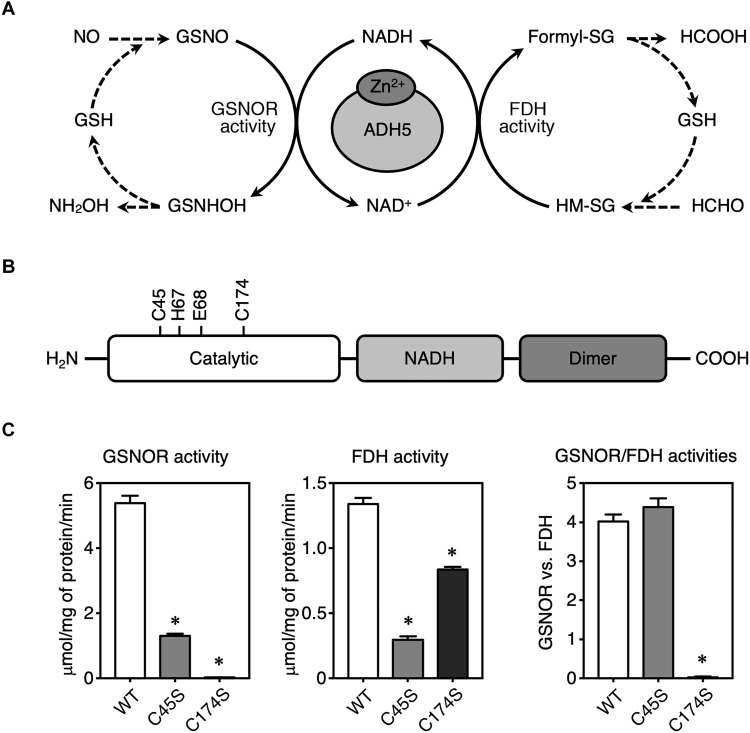
Fig. 1. Illustration of the GSNOR and FDH activities of recombinant human WT ADH5 protein and Cys mutants of ADH5.
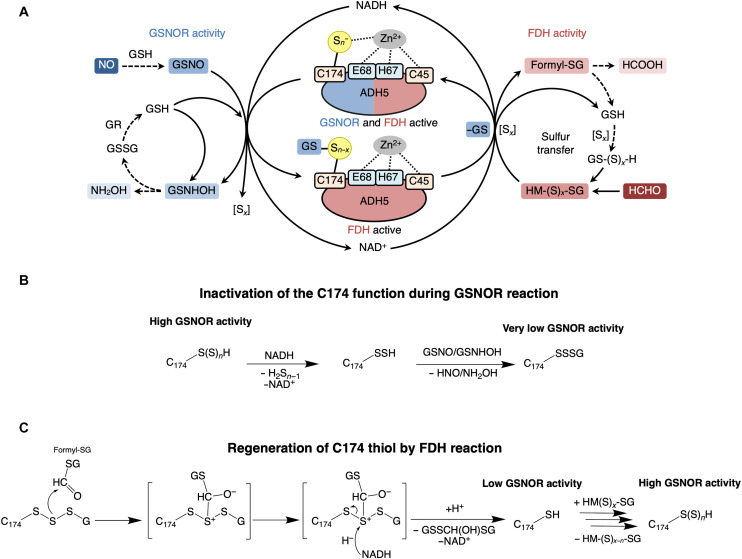
(A) Schematic showing reactions catalyzed by ADH5 with dual enzymatic activities—GSNOR and FDH. NH2OH, hydroxylamine; formyl-SG, S-formylglutathione; HCOOH, formic acid; HCHO, formaldehyde. (B) Domain structure of human ADH5 (hADH5) protein. (C) Measurement of enzymatic activities of recombinant WT hADH5 and mutants of hADH5. GSNOR (left) and FDH (middle) activities of ADH5 (WT, C45S, and C174S) proteins. The ratio of GSNOR activities to FDH activities was calculated for each ADH5 protein (right). The results are presented as means ± SD of three independent enzyme assays. *P < 0.001 versus WT; one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test.
Several groups developed Adh5-deficient mice, which have been used to clarify NO functions in vivo and thereby to investigate the pathophysiological relevance of ADH5. Adh5-null mice reportedly had increased rates of spontaneous and diethylnitrosamine-induced hepatocellular carcinomas, which were prevented by inhibiting NO production (11, 15, 16). Adh5-null mice also reportedly developed steatohepatitis more rapidly than wild-type (WT) mice (17). A series of studies with Adh5-null mice (called GSNOR knockout mice in these studies) showed that ADH5 altered NO-dependent smooth muscle tone and subsequently modulated pathophysiological events in airways and vasculature. Therefore, ADH5 has emerged as a therapeutic and preventive target for pulmonary, cardiovascular, and gastrointestinal diseases (9, 18–20). However, a simple loss of function of ADH5 will not be able to clarify the functional relationship between GSNOR and FDH activities of ADH5. To determine whether GSNOR and FDH activities are differentially regulated and whether the dual activities are functionally coupled to each other, biochemistry- and chemistry-based enzymology of ADH5 must be investigated in combination with mutation studies both in vitro and in vivo, which would demonstrate the molecular mechanisms of the two distinct enzymatic activities of this unique protein. Because we found earlier that ADH5 especially at its catalytic zinc-coordinating Cys174 residue is heavily supersulfidated in cells (5), we aimed here to thoroughly understand the regulatory mechanisms of the dual functions of ADH5—GSNOR and FDH—with a particular focus on protein supersulfidation.
Here, we unequivocally identified supersulfides to constitute the active center of ADH5, which thereby catalyze NO and formaldehyde metabolism via formation of GSSH-derived hemithioacetal. This demonstration is the first to verify that protein-bound supersulfides without any apparent redox cofactors can serve as a unique catalyst for the NAD+/NADH-coupled two-way redox reactions of simultaneous NO metabolism and aldehyde metabolism. Moreover, the unique supersulfide catalysis that we discovered herein allowed us to separate the dual functions, i.e., GSNOR from FDH, of ADH5, so that we could establish various GSNOR-selective deficient mutants including mice in vivo. The present findings thus may promote not only better understanding of physiological relevance of supersulfide-mediated NO signal regulation and formaldehyde metabolism but also the translational application in disease prevention and therapeutics.
RESULTS
Characterization of human ADH5 with serine substitution at Cys45 or Cys174
Human ADH5 (hADH5) protein contains two zinc ions, each in a single subunit, with each ion having either a catalytic or a structural function (21). Amino acid sequences of ADH5 and its orthologs are highly conserved among various organisms from prokaryotes to eukaryotes, including Saccharomyces cerevisiae, mouse, and human (fig. S1). To investigate the biological relevance of catalytic zinc-coordinating Cys residues (Cys45 and Cys174), we prepared WT hADH5 protein and two mutant proteins in which Cys residues were replaced by serine (C45S and C174S) (Fig. 1B and fig. S2), and we measured the GSNOR and FDH activities of these three proteins (Fig. 1C). We measured these GSNOR and FDH activities of the recombinant hADH5 proteins by monitoring NADH consumption and production in the presence of GSNO and HM-SG (GS hemithioacetal), respectively. As Fig. 1C shows, the C45S mutant exhibited lower activities of both GSNOR and FDH compared with the WT protein. The C174S mutant, although it had almost no GSNOR activity, did have appreciable FDH activity (Fig. 1C). Calculation of the GSNOR/FDH activity ratios showed that the C174S mutant had obviously unbalanced ADH5 dual activities (Fig. 1C, right). Thus, GSNOR activity of ADH5 absolutely requires Cys174.
Protein supersulfidation of WT ADH5 and C174S mutant ADH5
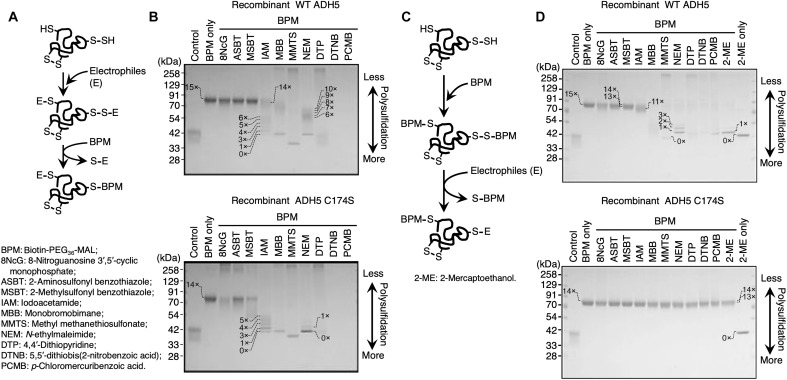
The specific requirement for Cys174 in the production of GSNOR activity led us to hypothesize a unique contribution of Cys174 to redox reactions catalyzed by ADH5. We recently found that the persulfide dioxygenase (ethylmalonic encephalopathy protein 1, ETHE1), an Fe2+-containing enzyme that oxidatively metabolizes GSSH to GSH, was extensively supersulfidated and that its enzymatic activity was regulated by the supersulfidation of Cys247 of ETHE1 (3). We then analyzed the supersulfidation status of WT ADH5 and C174S mutant ADH5 by using the original and modified polyethylene glycol (PEG)–conjugated maleimide (MAL)–labeling gel shift assay (PMSA) (Fig. 2) (3–5). The original PMSA was thus performed with recombinant WT ADH5 and C174S mutant ADH5, which were first reacted with various electrophiles (E), followed by the labeling with biotin-PEG36-MAL (BPM). The labeled proteins were heat-denatured in the presence or absence of 5% 2-mercaptoethanol (2-ME) and subjected to SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie Brilliant Blue (CBB) staining (Fig. 2, A and B). In parallel, we conducted the modified PMSA, in which the WT and C174S mutant proteins were reacted initially with BPM and subsequently treated with various electrophiles, after which they were subjected to the SDS-PAGE (Fig. 2, C and D). Because of the bipolar nucleophilic and electrophilic properties of each sulfur residue in the supersulfides, strong electrophiles attack the sulfur atoms and remove PEGylation sites from the supersulfidated Cys residues. Almost half of the electrophiles that we tested removed PEGylation sites from WT ADH5, whereas PEGylation in ADH5 C174S was less affected by the electrophiles than the WT, which suggests that WT ADH5, but not ADH5 C174S, contains a substantial number of supersulfide residues. On the basis of the profile obtained from the original PMSA, the C174S ADH5 mutant was found to be more susceptible to the electrophilic treatments (Fig. 2B), than was observed on the modified PMSA (Fig. 2D). In the modified PMSA, BPM reacts thoroughly with various Cys polysulfide residues to eliminate per-/polysulfide moieties and lastly obtaining stable BPM-Cys (simple thiol) adducts that are resistant to both alkylating and reducing agents. It is recently reported that the MAL-based alkylating agents have a high tendency to cleave polysulfide chains (5, 22, 23). It is thus suggested that the modified PMSA tends to underestimate the levels of supersulfides. Together, the PMSA we conducted herein indicates that the C174S mutant ADH5 is much less supersulfidated than the WT ADH5.
Fig. 2. Supersulfidation of recombinant hADH5 protein and its C174S mutant.
(A) Schematic showing the reaction mechanism in the original PMSA. In the initial step of the reaction, both thiol (─SH) and polysulfide [─(S)n-H] groups of the Cys residues in a protein are alkylated by the electrophiles (E). In the second reaction step, the PEGylation probe BPM selectively attacks alkylated polysulfides at proximal sulfur atoms. Polysulfide-rich proteins acquire PEGylation and demonstrate low gel mobility. (B) Detection of protein supersulfidation in WT ADH5 (top) and C174S mutant (bottom) proteins by using the original PMSA. Numbers on the gels and blots indicate expected numbers of PEG moieties in the protein, which were deduced from the mobility shift distance. Proteins were detected by means of CBB staining. 8NcG, 8-nitroguanosine 3′,5′-cyclic monophosphate; ASBT, 2-aminosulfonyl benzothiazole; MSBT, 2-methylsulfonyl benzothiazole; IAM, iodoacetamide; MBB, monobromobimane; MMTS, methyl methanethiosulfonate; NEM, N-ethylmaleimide; DTP, 4,4′-dithiopyridine; DTNB, 5,5′-dithiobis(2-nitrobenzoic acid); PCMB, p-chloromercuribenzoic acid. (C) Schematic drawing for the modified PMSA. In the initial step of the reaction, both thiol (─SH) and polysulfide [─(S)n-H] groups of the Cys residues in a protein are PEGylated by means of the PEGylation probe BPM. In the second reaction step, strong electrophilic compounds, but not weak electrophilic compounds, selectively attack PEGylated polysulfides at proximal sulfur atoms, which results in the removal of PEG moieties. Polysulfide-rich proteins lose PEGylation and exhibit high gel mobility by reacting with strong electrophiles. (D) Detection of protein supersulfidation in WT hADH5 (top) and C174S mutant (bottom) hADH5 proteins by using the modified PMSA.
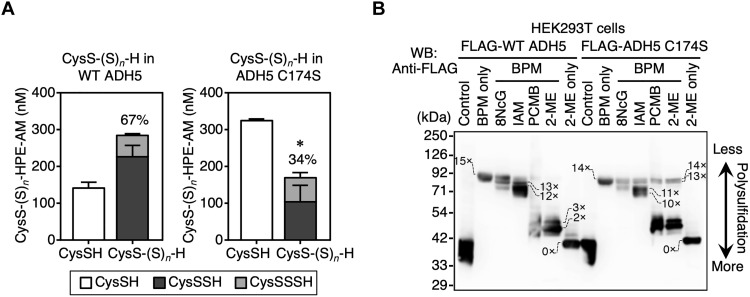
To verify the potential catalytic role of Cys174 supersulfidation, we analyzed supersulfide contents in recombinant WT ADH5 and C174S mutant ADH5 by using liquid chromatography–electrospray ionization–tandem mass spectrometry (LC-ESI-MS/MS) with β-(4-hydroxyphenyl)ethyl iodoacetamide (HPE-IAM) as a trapping agent for hydropolysulfides. HPE-IAM has been reported to preserve most of the supersulfides (5, 22, 23). More than 67% of Cys residues were supersulfidated in WT ADH5 (Fig. 3A and fig. S3), whereas ADH5 C174S had 34% supersulfidation (Fig. 3A and fig. S3). It is therefore confirmed that the degrees of supersulfidation are much lower in the C174S mutant than in the WT ADH5, suggesting that persulfidation of Cys174 residue most likely triggers to induce persulfidation of the other Cys residues of the proteins. In addition, given the results with ETHE1 (3), we expected that Cys174-dependent supersulfidation would sustain the GSNOR activity of ADH5, which may then be critically involved in the maintenance of supersulfidation of the whole ADH5 protein.
Fig. 3. Quantification of protein supersulfidation of recombinant ADH5 and detection of protein supersulfidation of ADH5 in mammalian cells.
(A) Quantification of supersulfidated Cys residues in recombinant ADH5 (WT and C174S) by LC-ESI-MS/MS. Results are presented as means ± SD (n = 4). *P < 0.01 (unpaired Student’s t test). (B) Detection of protein supersulfidation in WT ADH5 and C174S mutant ADH5 proteins by using the modified PMSA with proteins expressed in HEK293T cells. Proteins were detected by means of immunoblot analysis with anti-FLAG antibody. Numbers on the gels and blots indicate expected numbers of PEG moieties in the protein, which were deduced from the mobility shift distance. WB, Western blotting.
The original PMSA (Fig. 2B) then revealed a profile in ADH5 polysulfidation similar to the LC-MS/MS analysis (Fig. 3A). Meanwhile, it should be noted that, because the recombinant ADH5 used for the PMSA was purified and stored in the presence of reducing agents, many per-/polysulfide residues are removed by these reductants, rendering BPM-treated proteins resistant to both electrophilic and nucleophilic degradation (Fig. 2). This phenomenon was most evidently observed with the C174S mutant protein (Fig. 2D, bottom).
When FLAG-tagged WT ADH5 and ADH5 C174S were expressed in human embryonic kidney 293T (HEK293T) cells, the PEGylated form of WT ADH5 disappeared but the PEGylated form of ADH5 C174S remained, according to p-chloromercuribenzoic acid (PCMB) and 2-ME addition after BPM treatment (Fig. 3B). This PMSA assay with the ADH5 WT and C174S mutant proteins expressed in HEK293T cells suggests that the mutant ADH5 has lower levels of per-/polysulfidated cysteine than WT. However, in contrast to recombinant ADH5 mutant (Fig. 2, B and D, bottom), the mutant in HEK293T cells has more per-/polysulfidated residues (Fig. 3B, right part). The apparent distinct trends in terms of electrophilic susceptibility can be explained by the fact that the ADH5 protein expressed in HEK293T cells was not pretreated with any reducing agents before the PMSA. The existing persulfides on ADH5, formed in supersulfide-rich molecular environment of HEK293T cells, were better preserved for analysis, thereby showing a high susceptibility to the electrophiles (Fig. 3B).
Regulatory mechanisms of GSNOR and FDH activities of ADH5 protein
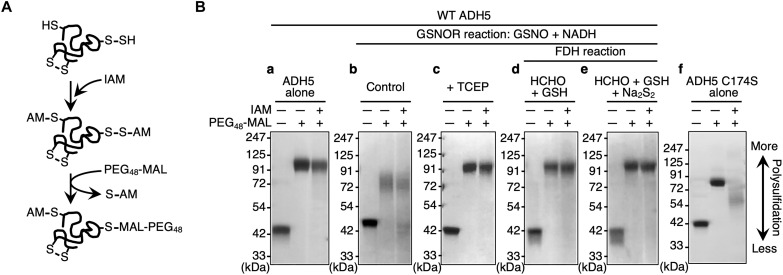
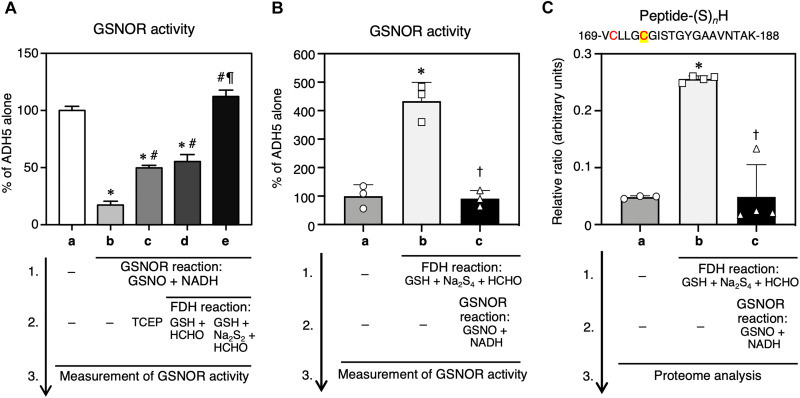
We examined the mechanism that regulates the supersulfidation of ADH5 by correlating its supersulfidation with its GSNOR activity. We used the original PMSA to estimate the degree of protein supersulfidation (Fig. 4A). Iodoacetamide (IAM) was used to block hydrosulfide and hydropolysulfide residues in proteins. The acetamide-blocked proteins were subsequently reacted with PEG48-MAL, which specifically attacks nucleophilic polysulfides, and resulted in PEGylation of the polysulfidated proteins. We found that WT ADH5 polysulfidation decreased after ADH5 underwent the GSNOR reaction with GSNO and NADH (Fig. 4B, compare a and b). In parallel with the reduced degree of polysulfidation, the GSNOR activity of WT ADH5 decreased significantly after ADH5 underwent the GSNOR reaction (Fig. 5A). When the reductant tris(2-carboxyethyl)phosphine (TCEP) was added after the GSNOR reaction, the post-GSNOR changes in the PMSA profile were significantly reversed, which indicated recovery of supersulfidation (Fig. 4B, compare b and c) accompanied by partial recovery of GSNOR activity (Fig. 5A). A similar recovery of supersulfidation and GSNOR activity of WT ADH5 occurred after a subsequent FDH reaction and addition of its substrate (HM-SG) formed with formaldehyde and GSH (fig. S4), after the GSNOR reaction (Fig. 4B, compare b and d; Fig. 5A). In addition, GSNOR activity was fully restored after addition of excessive persulfide (Na2S2) to the substrate reaction mixture (formaldehyde + GSH) to form HM-S-SG for FDH (Fig. 4B, d and e; fig. S4). Although the PMSA profile did not reflect the effect of Na2S2 supplementation (Fig. 4B, compare d and e), we assume that Na2S2 has further increased supersulfidation of ADH5 and led to full recovery of GSNOR activity. Thus, coupling the GSNOR reaction with the FDH reaction, particularly in the presence of persulfide donors, maintains the supersulfidation status and GSNOR activity of ADH5.
Fig. 4. Regulation of GSNOR by GSNOR/FDH reaction-dependent protein supersulfidation.
(A) Schematic showing the reaction of the original PMSA. This PMSA used PEG48-MAL that selectively attacks polysulfides alkylated by IAM at proximal sulfur atoms. Polysulfide-rich proteins thus acquire PEGylation and demonstrate low gel mobility. (B) Detection of supersulfidation of recombinant WT hADH5 before and after GSNOR and FDH reactions by using the original PMSA.
Fig. 5. Correlation of active site supersulfidation with GSNOR activity of ADH5.
Recombinant WT ADH5 was immobilized on Sepharose beads and then underwent sequential GSNOR and FDH reactions in the presence of the indicated substrates. (A) Effects of ADH5 enzyme reactions on the GSNOR activity of recombinant ADH5. The ADH5 protein was first treated with the GSNOR reaction mixtures (GSNO plus NADH) for 60 min at 37°C and then subjected to sequential FDH reactions, followed by measurement of GSNOR activity. The FDH reaction mixture included 1 mM NAD+ and HM-SG (100 μM GSH + 500 μM formaldehyde), HM-(S)n-SG (100 μM GSH + 500 μM formaldehyde + 50 μM Na2S2), or a reducing agent (1 mM TCEP). (B) Effects of ADH5 enzyme reactions on the GSNOR activity of recombinant ADH5. The ADH5 protein was first treated with the FDH reaction mixtures containing 100 μM NAD+ and a substrate cocktail of HM-(S)n-SG (10 μM GSH + 50 μM formaldehyde + 5 μM Na2S4) and then subjected to sequential GSNOR reactions, followed by measurement of GSNOR activity. (C) High-precision sulfur proteome analysis of supersulfidated Cys residues of ADH5. The recombinant ADH5 that were treated with sequential FDH and GSNOR reactions, after which supersulfides formed at the GSNOR catalytic center was identified via the sulfur proteome. A peptide fragment containing Cys residues C170 (red) and C174 (red and yellow) is shown in the panel headline. The ADH5 alone (a) served as a control for other reactions (b to e). Results are presented as means ± SD (n = 3 to 4). *P < 0.001 versus ADH5 alone (A-a, B-a, and C-a), #P < 0.001 versus GSNOR (A-b), ¶P < 0.001 versus GSNOR + FDH (GSH + HCHO) (A-d), †P < 0.001 versus FDH (B-b and C-b); one-way ANOVA with Tukey’s multiple comparison test.
To further clarify the role of Cys174 supersulfides in GSNOR activity, we have capitalized on high-precision sulfur proteome that we have established earlier (5). We then correlated the extent of polysulfidation of C174 Zn2+-binding active site of ADH5 and its GSNOR activity by using sulfidation and de-sulfidation processes catalytically introduced with repeated FDH and GSNOR reactions. Such a catalytic repetition (or enzyme recycling reaction) can be conducted in the same manner as in Fig. 5A. Consequently, we observed a significant correlation of GSNOR activity versus the Cys174 polysulfidation, as determined by the quantitative sulfur proteome analysis (Fig. 5, B and C, and fig. S5). The FDH reaction used in Fig. 5 was found to become effective in terms of activation (or reactivation) of GSNOR activity only in the presence of NAD+ but not with substrates alone (e.g., formaldehyde, polysulfides, and glutathione-related compounds) for the enzyme reactions without NAD+ (fig. S6, A and B). Also, we have used the mass spectrometry to measure the amount and length of the polysulfide chain attached to Cys174, which is biologically functioning as a supersulfide catalyst. This revealed that Cys174-SSH is a major and predominant form of catalytic ligand for Zn2+ prosthetic group (Fig. 5C and fig. S5). Although Cys174 trisulfide (CysSSSH) could not be identified in our present MS analysis, more extensive polysulfidation might be feasible. The number of sulfur atoms in Cys polysulfide chain (CysSSnH) is likely to be underestimated by the IAM-based proteome analysis, as reported earlier (5, 22, 23), and thus we expect that longer chains of polysulfides are present. However, our supersulfide analysis with HPE-IAM (which is expected to preserve longer polysulfide chains), as was initially performed and shown, indicated a nicely consistent result of predominant detection of Cys174 persulfide rather than its trisulfide CysSSSH (Fig. 3A). Therefore, it is highly conceivable that the Cys174 persulfide can serve as an effective catalyst in GSNOR and ADH5 reactions.
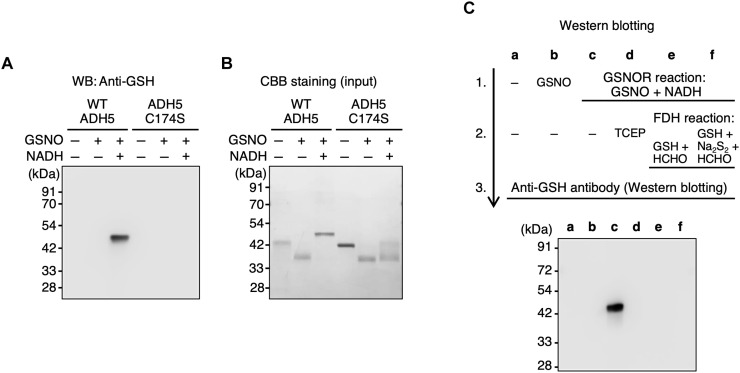
Because Cys174 is critical for both GSNOR activity and supersulfidation of ADH5 (Figs. 1 and 2) and supersulfidation of ADH5 correlates with its GSNOR activity (Figs. 4 and 5), we hypothesized that Cys174 was modified during the GSNOR reaction, which resulted in reduction of supersulfidation and decreased GSNOR activity. As we expected, ADH5 was glutathionylated after undergoing the GSNOR reaction in a Cys174-dependent manner (Fig. 6, A and B, and fig. S7). Moreover, this glutathionylation was reversed by reductive treatment with TCEP or a subsequent FDH reaction, with recovery of both supersulfidation and GSNOR activity (Fig. 6C). These results strongly suggest that Cys174 is glutathionylated when ADH5 undergoes the GSNOR reaction, which results in inhibition of the supersulfidation and GSNOR activity of ADH5.
Fig. 6. Glutathionylation of recombinant ADH5 protein and its C174S mutant.
(A) Immunoblot analysis to detect glutathionylation of WT ADH5 and C174S mutant ADH5 after the GSNOR reaction. (B) Proteins were detected by means of CBB staining. (C) Immunoblot analysis to detect glutathionylation of WT ADH5 after the GSNOR and FDH reactions. The ADH5 alone (lane a) served as a control for other reactions (lanes b to f).
Because of a relative shift of molecular weight (by about 2 kDa) apparently observed with WT ADH5, the glutathionylation is likely to occur not only on the Cys174 but also other residues. Such an extra-glutathionylation was detected by our proteome analysis (fig. S7), which is thought to be induced by a product GSSG or by an intermediate product GSNHOH generated during the GSNOR reaction, as was discussed below. It is also noted, however, that the electrophoretic mobility of the ADH5 might be changed, when the protein became unfolded by glutathionylation, and thus apparently behaved as if it was a larger protein than was originally under a nonreducing electrophoretic condition.
Specifics of the intracellular function of ADH5 C174S
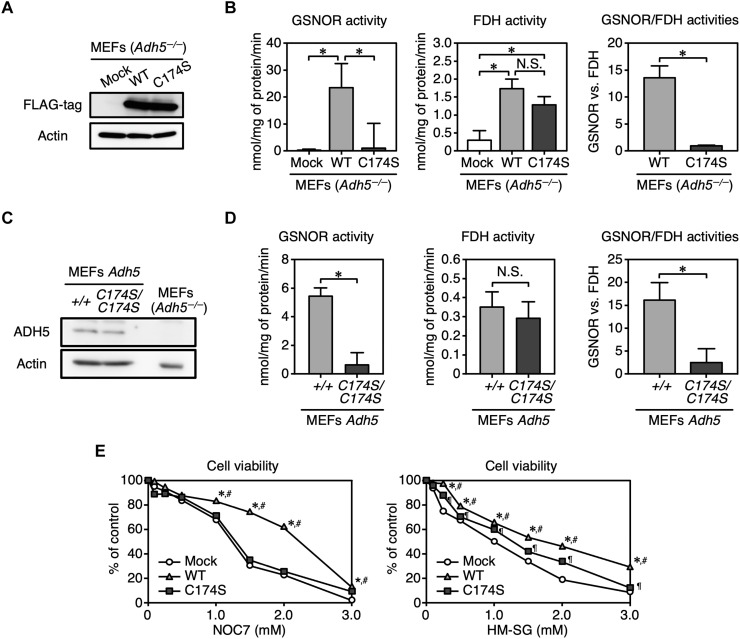
To investigate the intracellular function of ADH5 C174S, we transiently overexpressed FLAG-tagged WT ADH5 and C174S mutant ADH5 in Adh5−/− mouse embryonic fibroblasts (MEFs) (Fig. 7A). We determined the GSNOR and FDH activities in lysates of MEFs by measuring NADH consumption and production in the presence of GSNO and HM-SG, respectively. We found no GSNOR or FDH activity in the mock transfectant of Adh5−/− MEFs. WT ADH5-expressing cells had measurable GSNOR and FDH activities, whereas ADH5 C174S-expressing cells selectively showed FDH activity (Fig. 7B, left and middle). To verify the effect of the C174S mutation on the dual functions of endogenous ADH5, we introduced a point mutation in the mouse Adh5 gene by means of the CRISPR-Cas9 system and generated a mouse line carrying the Adh5 mutant allele encoding ADH5 C174S. WT (Adh5+/+) and Adh5C174S/C174S primary MEFs were established from littermate embryos of Adh5C174S/+ mating pairs. The abundance of ADH5 C174S in Adh5C174S/C174S MEFs was comparable to that of WT ADH5 in WT MEFs (Fig. 7C). GSNOR activity was markedly reduced in Adh5C174S/C174S MEFs compared with WT MEFs, whereas FDH activities of the two MEFs were similar (Fig. 7D, left and middle). These data are all consistent with the above mentioned results obtained for the recombinant ADH5 proteins (see Fig. 1C). Similar to the FDH activity of recombinant ADH5 proteins, while there was no significant difference in the FDH activity between WT and C174S ADH5, the GSNOR activity of ADH5 C174S was completely lost, which resulted in marked reduction in the GSNOR/FDH ratio for cell lysates containing ADH5 C174S compared with those containing WT ADH5 (Fig. 7, B and D, right).
Fig. 7. Characterization of ADH5 C174S in mammalian cells.
(A) Immunoblot analysis to detect FLAG-tagged WT ADH5 and C174S mutant ADH5 that were transiently expressed in Adh5−/− MEFs. (B) Measurement of GSNOR (left) and FDH (middle) activities in lysates of Adh5−/− MEFs expressing WT ADH5 or C174S mutant ADH5. Right: The ratio of GSNOR activities to FDH activities for each ADH5 protein expressed in Adh5−/− MEFs. Data are means ± SD (n = 3). *P < 0.001, one-way ANOVA with Tukey’s multiple comparisons test. N.S., not significant. (C) Immunoblot analysis of WT and mutant ADH5 proteins expressed in Adh5+/+ and Adh5C174S/C174S MEFs. (D) Measurement of GSNOR (left) and FDH (middle) activities in lysates of Adh5+/+ and Adh5C174S/C174S MEFs. Right: The ratio of GSNOR activities to FDH activities for each sample. Data are means ± SD, n = 3. *P < 0.001, unpaired Student’s t test. (E) Viability of MEFs after NOC7 or HM-SG treatment. Data are means ± SD, n = 4. *P < 0.001 versus Mock Adh5−/−, #P < 0.001 versus Adh5−/−-expressing C174S mutant, ¶P < 0.01 versus Mock Adh5−/−, two-way ANOVA with Tukey’s multiple comparisons test.
To evaluate the functional significance of the altered enzymatic activities of ADH5 C174S, we studied the cellular resistance to NO and formaldehyde. Adh5−/− MEFs were highly sensitive to the NO donor NOC7, which causes an increased intracellular concentration of NO and subsequently of GSNO. Introduction of WT ADH5 to Adh5−/− MEFs significantly improved cell viability after NOC7 and HM-SG treatment (Fig. 7E). The sensitivity of ADH5 C174S-expressing cells to the NO donor was almost the same as that of Adh5−/− cells, whereas the viability of ADH5 C174S-expressing cells after HM-SG treatment was appreciably higher than that of Adh5−/− cells (Fig. 7E). Thus, Cys174 can make a significant contribution to the GSNOR activity of ADH5 in cells.
Conservation of a Cys residue essential for GSNOR activity of ADH5 proteins in various species
ADH5 is very well conserved among species; in particular, the catalytic zinc-coordinating amino acids are completely conserved from yeast to human (fig. S1). Cys174 of hADH5 corresponds to Cys179 in the yeast ADH5 ortholog Sfa1. Because previous studies reported that Sfa1 also has both GSNOR and FDH activities (8, 24), we investigated the functional significance of Cys179 in Sfa1 by testing the activity of Sfa1 C179S. Recombinant WT Sfa1 and Sfa1 C179S had GSNOR and FDH activities (fig. S8) that were similar to those of hADH5 and its C174S mutant. While the FDH activity of the recombinant protein was affected significantly by the C179S mutation, its GSNOR activity was attenuated more remarkably and almost disappeared. We then studied the potential contribution of Cys179 to Sfa1 function in vivo. To this end, we prepared SFA1-deficient yeast (Δsfa1), and we stably introduced WT Sfa1 and Sfa1 C179S into these SFA1-deficient yeasts (fig. S9A). We prepared yeast cell lysates and measured GSNOR and FDH activities in these lysates (fig. S9B). The SFA1-deficient yeast showed no detectable GSNOR or FDH activities. WT Sfa1-expressing yeast exhibited measurable activities of both GSNOR and FDH, whereas Sfa1 C179S-expressing yeast lacked GSNOR activity but retained FDH activity. These findings were consistent with results obtained for the recombinant hADH5 proteins. We also investigated the significance of Sfa1 Cys179 to survival of yeast under stress conditions by analyzing growth inhibition induced by GSNO and formaldehyde exposure. SFA1-deficient yeast showed increased sensitivity to GSNO and formaldehyde treatment. Introduction of WT Sfa1 completely reversed this increased sensitivity (fig. S9C). In contrast, C179S-expressing yeast showed growth that was comparable to that of WT Sfa1-expressing yeast in the presence of formaldehyde but demonstrated growth suppression with GSNO treatment, similar to SFA1-deficient yeast (fig. S9C). The results from these yeast experiments are all consistent with those obtained for the function of Cys174 in human and mice ADH5 (Figs. 1C and 7), which suggest that ADH5 and its orthologs use dual enzymatic activities that are regulated via a mechanism that may depend on supersulfidation maintained by the zinc-coordinating Cys residue.
Cardiac function in Adh5C174S/C174S mice
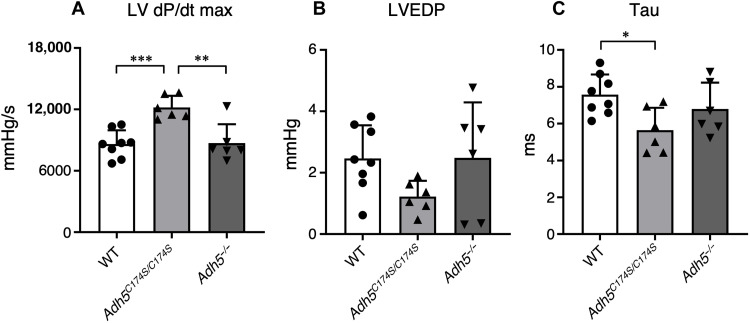
The major organs affected by GSNOR activity include the heart, where NO that activates its downstream guanosine 3′,5′-monophosphate (cGMP) and nitrosative signal pathways reportedly had potent beneficial effects that prevented and ameliorated cardiovascular diseases. As an intriguing result, Adh5−/− mice demonstrated alterations in cardiovascular function, i.e., reduced peripheral vascular tone accompanied by increased cardiac output and attenuation of cardiac contractility in response to β-adrenergic agonists (12). Here, we investigated basal cardiac functions of Adh5C174S/C174S mice and compared them with those of control WT mice by using a real-time left ventricular (LV) pressure analysis (Fig. 8 and fig. S10). Adh5C174S/C174S mice showed a significant increase in the maximal rate of LV pressure development (LV dP/dtmax; Fig. 8A) and significant decrease in the time constant of isovolumic relaxation (Tau; Fig. 8C), as compared with WT and ADH5 knockout mice. LV end-diastolic pressure (LVEDP) tended to be reduced in Adh5C174S/C174S mice, although the difference did not reach statistical significance (Fig. 8B). These results suggest an improvement in cardiac function in Adh5C174S/C174S mice. As another intriguing result, in this experimental setting, Adh5−/− mice did not apparently differ from the WT mice in terms of heart weight, heart rate, and other cardiac parameters among WT, Adh5C174S/C174S, and Adh5−/− mice (table S1). These results suggest that basal cardiac functions are efficiently improved in Adh5C174S/C174S mice because of reduced GSNOR activity. We theorize that selective reduction in GSNOR activity of ADH5 results in the increased bioavailability of GSNO and NO, particularly for cardiac tissue and systemic vasculature.
Fig. 8. Cardiac functions in ADH5 mutant mice and proposed supersulfide-mediated enzymatic reactions of ADH5.
Improved cardiac functions of Adh5C174S/C174S mice. Basal LV pressures in Adh5+/+ (WT) (n = 8), Adh5C174S/C174S (n = 6), and Adh5−/− (n = 6) mice. (A) The maximal rate of LV pressure rise (LV dP/dtmax). (B) LVEDP. (C) The time constant of isovolumic relaxation (Tau). *P < 0.05, **P < 0.01, ***P < 0.001; one-way ANOVA with Tukey’s multiple comparisons test.
Meanwhile, the phenotypes that we observed with Adh5C174S/C174S mice are not seen with Adh5-deficient mice, even if they lacked GSNOR function and may enhance GSNO bioavailability. The inconsistent phenotypes of Adh5 C174S mutant versus Adh5 knockout mice may be explained by the attenuated metabolism of formaldehyde, which was identified by our mass spectrometry analysis. We thus found that the amount of formaldehyde was increased significantly by ADH5 knockout; whereas the GSSH level was inversely decreased as shown in fig. S11. The detrimental effect of formaldehyde on cardiac functions was previously reported to be either dependent or independent of NO signaling pathways (25). Therefore, the increased formation of formaldehyde may counteract and abolish the enhanced beneficial effects of NO (because of reduced GSNOR activity), possibly through formaldehyde-induced impairment of antioxidant and cytoprotective effects that is mediated by supersulfides in general, which we previously demonstrated (1, 5). As we just described above, the phenotypic change of Adh5C174S/C174S mice, i.e., improved cardiac functions as determined by LV dP/dtmax, LVEDP, and Tau, is most likely to be caused by the canonical NO-cGMP signal pathway, similar to the previous reports with Adh5 knockout mice and based on the well-known effects of NO on the cardiac functions (26). Regarding NO bioavailability, GSNO should serve as a substrate for GSNOR to indirectly eliminate NO via scavenging of its nitrosative derivative of GSH (GSNO). Thus, it is conceivable that the cardiac functions controlled by a canonical NO signaling pathway are enhanced by the GSNOR nullification; whereas it will be cancelled by formaldehyde accumulated in Adh5 knockout mice at least in the normal rearing condition.
DISCUSSION
Our study here determined that supersulfide is required for the regulation of NO signaling. ADH5, which is ubiquitously expressed in various organisms, is a unique protein with dual enzymatic functions, GSNOR and FDH, which contribute to NO signal regulation and formaldehyde detoxification, respectively (7, 8, 13, 27, 28). By rigorous separation of GSNOR from FDH in the ADH5 enzyme activities, GSNOR activity is found to be conferred and sustained by supersulfidation of ADH5. As schematized in Fig. 9A (also see Fig. 1), ADH5 loses supersulfidation when it catalyzes the GSNOR reaction (Fig. 9B), which results in reduced GSNOR activity, whereas ADH5 regains supersulfidation when it catalyzes the FDH reaction, which restores GSNOR activity (Fig. 9C). Thus, coupling of the dual enzymatic activities of ADH5 underlies the supersulfide-dependent NO signal regulation by ADH5 (Fig. 9A). Briefly, we suggest herein that Cys174 supersulfides enhance GSNOR activity. During GSNOR reaction, Cys174 loses supersulfides (Sx) and becomes glutathionylated (Fig. 9B). The coupled FDH reaction can restore GSNOR catalytic activity by removing glutathionylation of Cys174 (Fig. 9C). The additional presence of supersulfides in FDH reaction (HM-(S)x-G) can further enhance GSNOR catalytic activity by restoring Cys174 supersulfidation (Fig. 9C).
Fig. 9. Proposed mechanisms of regulation of the GSNOR and FDH activities of ADH5 via protein supersulfidation.
(A) ADH5 protein contains a zinc ion to which four residues (Cys45, His67, Glu68, and Cys174) coordinate. ADH5 contributes to NO signal regulation and formaldehyde detoxification by GSNOR and FDH activities using NADH and NAD+ respectively. Cys174 supersulfides enhance GSNOR activity. During the GSNOR reaction Cys174 loses supersulfides (Sx) and becomes glutathionylated. The coupled FDH reaction can restore GSNOR catalytic activity by removing the glutathionylation of Cys174. The additional presence of supersulfides in FDH reaction [HM-(S)x-G] can further enhance GSNOR catalytic activity by restoring Cys174 supersulfidation. [Sx], supersulfides; GR, glutathione reductase; GSNHOH, glutathione N-hydroxysulfenamide; HM-(S)x-G, S-hydroxymethylglutathionesupersulfide; formyl-SG, S-formylglutathione. (B) Inactivation of the C174 catalytic function by GSNOR and regeneration by FDH reaction. Proposed mechanism of inactivation of the C174 function during GSNOR reaction. Cys174 (C174) supersulfides are reduced during the GSNOR reaction and resulting persulfide (C174-SSH) is glutathionylated by GSNO or by GSNHOH to form C174-SSSG, which inhibits GSNOR activity. (C) Proposed mechanism of C174 thiol regeneration by FDH reaction. In the first step of the FDH reaction, HM-SG donates two electrons to NAD+, producing formyl-SG and NADH. Formyl-SG reacts with the nucleophilic sulfur in the C174-SSSG. The resulting intermediate is reduced via hydride (H−) donation by NADH to regenerate free cysteine (C174-SH), which supports GSNOR catalytic activity. The GSNOR catalytic activity is further enhanced by the supersulfidation of Cys174 by HM(S)x-SG.
We have identified a critical Cys residue (Cys174 in mice/humans and Cys179 in yeast) that is a ligand for a zinc ion at the active center of ADH5 and that is needed for supersulfidation and maintenance of GSNOR activity. We successfully developed herein the Adh5C174S/C174S mice, which allowed us to precisely characterize the in vivo phenotypes of the GSNOR knockout mutant without affecting FDH activity of ADH5. Therefore, a novel concept emerging from this study is that a particular protein supersulfide residue has a critical catalytic function via a redox-coupling reaction with NAD+/NADH and GSH, which is also dynamically regulated and makes an essential contribution to sustainability of the active site Cys thiol against electrophilic and oxidative stress. These data provide the first and robust evidence verifying that the protein (ADH5)-bound supersulfide can serve as a versatile catalyst for regulation and metabolism of NO and formaldehyde.
Our earlier study indicated that the activity of the persulfide dioxygenase ETHE1, a major enzyme that metabolizes endogenous glutathione polysulfide, is maintained by means of supersulfidation of the Cys247 residue (3). Similar to Cys174 in ADH5, this Cys247 is located near the iron ion at the active center of ETHE1 (29). This finding clearly supports the interpretation that supersulfidation of the Cys residue that is functioning as a ligand for the active site zinc ion sustains the catalytic activity of ADH5. Other metal-dependent proteins and enzymes are also likely controlled by supersulfidation of Cys residues that coordinate metal ions at active centers.
Our enzymatic analysis indicated that addition of the reducing agent TCEP or completion of the FDH reaction with HM-SG as a substrate reverses the post-GSNOR glutathionylation at Cys174 and partially restores supersulfidation and GSNOR activity. An additional excessive sulfur supply leads to full recovery of GSNOR activity. These results strongly suggest that two regulatory steps, glutathionylation and supersulfidation, control GSNOR activity (Fig. 9A). In view of the potent redox-active property of supersulfides, supersulfide-linked glutathionylation may be more readily reversed (i.e., deglutathionylated) by the coupling reducing reaction than is a simple disulfide-linked glutathionylation. Thus, supersulfidation appears to facilitate both regulatory steps of GSNOR activity. We propose that supersulfidation achieves the functional and catalytic reversibility of proteins via conferring stability and sustainability to protein thiols when they are exposed to redox-based perturbation (30). That supersulfide likely functions not only as an important enzyme catalyst but also as a crucial component that reinforces the reductive potential of substrate (formaldehyde) for the FDH reaction is intriguing. In other words, the electron supply from the canonical substrate GSH hemithioacetal (HM-GSH) may not be sufficient for the FDH reaction to regain the GSNOR activity by rescuing the auto-inactivated GSNOR by its Cys174 glutathionylation. Thus, additional (or nucleophilic) reactions should be necessary to make the Cys174 fully active again (as schematized in Fig. 9C). Nevertheless, only GSNO, but not other GSH-related compounds such as GSSG, GSSSG, and GSSSSG, could serve as the substrate for GSNOR (fig. S6B). Therefore, an effective and steady NADH supply via FDH reaction will be achievable for GSNOR, as long as the enzyme can use a supersulfidated substrate such as GSH hemithioacetal (HM-GSSH), that has much greater nucleophilicity than does HM-GSH (fig. S12) (31, 32). The GSNO metabolism catalyzed by ADH5 is unequivocally elucidated with various organisms and especially in vivo in mice in our current study. However, because the amount of NADH supply in the cytosol is somehow limited as a result of a high ratio of NAD+/NADH by hundreds or so (33), it remains to be fully understood how much extent GSNOR of ADH5 is biologically functional in various organisms including humans.
ADH5 disruption in mice reportedly resulted in various phenotypes, which are closely associated with the pathogenesis of various disorders such as bronchial asthma and cardiac repair mechanisms after myocardial infarction (10, 12, 18, 34, 35). Many researchers believe that the NO signal is enhanced because of the reduced GSNOR activity, as in Adh5-deficient mice. Nevertheless, FDH activity is also eliminated in the same Adh5-deficient mice. Several important FDH functions have been established. For example, ADH5 is not only a major detoxification enzyme but also a source of formate for de novo purine biosynthesis via metabolizing formaldehyde through FDH activity (36). Synthetic lethal phenotypes occur with null mutations of Adh5 and Fancd2 in mice and appear to be caused by defective detoxification of endogenously produced formaldehyde and consequent DNA damage related to defective DNA repair (37). These findings raise a simple question: Are Adh5-null phenotypes caused by a deficiency in GSNOR activity or FDH activity? In this context, we made a notable discovery: ADH5 C174S selectively lacks GSNOR activity but retains FDH activity, which was observed in vitro with recombinant proteins as well as in vivo in MEFs and yeasts. Notably, the enhanced cardiac functions observed in Adh5C174S/C174S mice can be interpreted as being part of a phenotype caused by suppression of GSNOR activity, which is likely to increase the bioavailability of GSNO and NO and confer functional benefits on the heart and vasculature. Additional analysis of Adh5C174S/C174S mice will enable us to determine the contributions of the two distinct enzymatic activities of ADH5 to pathophysiological processes in vivo. It is well documented that excessive or dysregulated NO production causes impairment of the NO-dependent smooth muscle tone and subsequent pathophysiological events in airways and vasculature, including chronic lung diseases (e.g., bronchial asthma and pulmonary emphysema), cardiovascular ischemia, sepsis, etc. Therefore, the unique supersulfide catalysis, which we found to be critically involved in efficient GSNOR expression, greatly helped us to establish various GSNOR-deficient organisms and thereby may promote to gain better understanding of physiological relevance of supersulfide-dependent NO signal regulation in vivo.
In conclusion, this study revealed that protein-bound supersulfides serve as unique catalysts for the NAD+/NADH-coupled redox reactions of NO and aldehyde metabolisms. Thus, appropriate regulation of GSNOR function via ADH5 supersulfidation is likely to be translationally applicable to the therapeutic and preventive measures for various NO-related pathological conditions such as pulmonary, cardiovascular, gastrointestinal, and neurodegenerative diseases.
MATERIALS AND METHODS
Plasmids
For studies of the expression of recombinant hADH5, the full-length cDNA clone of hADH5 (pCMV6-Entry-hADH5) was purchased from OriGene Technologies (Rockville, MD). Polymerase chain reaction (PCR) amplification of hADH5 cDNA was performed with pCMV6-Entry-hADH5 as a template by using a primer set [hADH5 forward (Fw) and reverse (Rv); table S1], and the resultant amplicon was digested with XhoI and cloned into the XhoI site of pET-15b (N-terminal 6-His tag; Novagen, Madison, WI) to generate pET-15b-hADH5. To produce pET-15b-hADH5 C45S and pET-15b-hADH5 C174S, site-directed mutagenesis was performed by using the PrimeSTAR Mutagenesis Basal kit (Takara Bio, Shiga, Japan) with pET-15b-hADH5 as a template and primers sets (hADH5 C45S Fw and Rv, hADH5 C174S Fw and Rv; table S2), according to the manufacturer’s instructions. The plasmids were verified via DNA sequencing.
To construct mammalian expression vectors of WT ADH5 (pPyCAGIP-FLAG-hADH5) and the C174S mutant ADH5 (pPyCAGIP-FLAG-hADH5 C174S), XhoI fragments of pET-15b-hADH5 and pET-15b-hADH5 C174S were cloned into the XhoI site of the pPyCAGIP-FLAG vector (a gift from M. Nakao) (38).
For studies of the expression of the yeast ADH5 ortholog Sfa1 in the bacterium Escherichia coli and the yeast S. cerevisiae, plasmids were constructed by using Gateway technology (Thermo Fisher Scientific, Rockland, IL). pDONR-Sfa1 (WT) was generated by means of a BP reaction with pDONR221 (entry vector) and by using PCR-amplified fragments with primers [sfa1 Fw and sfa1 Rv(-stop); table S2] from a genomic DNA. pDONR-Sfa1 (C179S) was prepared by means of the QuikChange method with primers (Sfa1 C179S Fw and Sfa1 C179S Rv; table S3) and pDONR-Sfa1 (WT). pET53-Sfa1 (WT) and pET53-Sfa1 (C179S) were constructed via an LR reaction from pET53-DEST (C-terminal StrepII tag for E. coli expression; Novagen). pAG415GPD-Sfa1 (WT) and pAG4l3-Sfa1 (C179S) were constructed via an LR reaction from pAG415GPD-ccdB-HA [C-terminal hemagglutinin (HA) tag for S. cerevisiae expression, purchased from the AddGene repository] and pAG413GPD-ccdB-HA (C-terminal HA tag for S. cerevisiae expression, purchased from the AddGene repository), respectively.
Purification of recombinant proteins
Recombinant hADH5 was purified according to a previously described method with modifications (39, 40). Briefly, bacteria of the E. coli BL21 (DE3) strain transformed with pET-15b-hADH5, pET-15b-hADH5 C45S, or pET-15b-hADH5 C174S were grown at 30°C to an optical density of 0.6 to 0.7 at 600 nm (OD600), after which 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma-Aldrich, St. Louis, MO) was added to the culture. After 18 hours at 15°C, cells were harvested via centrifugation; resuspended in 20 mM Tris-HCl buffer (pH 8.0) containing 10 mM 2-ME (Wako Pure Chemical, Osaka Japan), 250 mM NaCl, lysozyme (1 mg/ml), and protein inhibitor cocktail (Nacalai Tesque, Kyoto, Japan); and lysed by sonication. The cell lysates were centrifuged at 10,000g for 20 min, and the supernatants were loaded onto a column packed with nickel nitrilotriacetic acid agarose (Qiagen, Valencia, CA) equilibrated with 20 mM Tris-HCl buffer (pH 8.0) containing 10 mM 2-ME and 250 mM NaCl. The hADH5 protein was eluted with 200 mM imidazole and then dialyzed with 20 mM Tris-HCl (pH 8.0) containing 10 mM 2-ME, 10% glycerol, and 10 μM ZnSO4 at 4°C. After desalting by using a NAP-5 column with 20 mM Tris-HCl buffer (pH 8.0) containing 1 mM TCEP (Nacalai Tesque), proteins were stored at −80°C. Protein concentration was determined by using the Protein Assay CBB Solution (Nacalai Tesque), and protein purity was confirmed via SDS-PAGE with CBB staining.
To prepare recombinant Sfa1, bacteria of the E. coli strain Rosetta2 (DE3) pLysS transformed with pET53-Sfa1 (WT) or pET53-Sfa1 (C179S) were grown at 37°C in LB medium containing ampicillin (100 μg/ml) and chloramphenicol (35 μg/ml). When the OD600 value has reached 0.6, 0.5 mM IPTG was added to the culture medium to induce gene expression. After cultivation for 18 hours at 17°C, the cells were harvested and suspended in ice-cold buffer A (100 mM Tris-HCl, 150 mM NaCl, 10 μM ZnSO4, 5% glycerol, and 10 mM 2-ME (pH 8.0)] and disrupted by sonication under cooling. After centrifugation (10,000g for 20 min), the soluble fraction of the supernatant was loaded with Strep-Tactin Sepharose (IBA Lifesciences, Goettingen, Germany) and washed with 20 column volumes of buffer A. The recombinant StrepII-tagged Sfa1 (WT and C179S) samples were eluted with buffer A containing 2.5 mM desthiobiotin (IBA Lifesciences). Protein concentration was determined by using the Bradford method with bovine serum albumin as the standard protein.
Measurement of GSNOR and FDH activities
Both GSNOR and FDH activities were measured spectrophotometrically at 30°C by monitoring the formation (FDH) or consumption (GSNOR) of NADH at 340 nm, using a molar absorption coefficient (6220 M−1 cm−1) of NADH at 340 nm (8) (UV-1850, SHIMADZU, Tokyo, Japan). A typical sample assay for GSNOR activity contained the following in a final volume of 0.5 ml: 200 μM NADH (Nacalai Tesque), 250 μM GSNO (Dojindo Laboratories, Kumamoto, Japan), and enzyme (crude enzyme: 10 to 70 μg, purified enzyme: 20 to 300 ng) in 25 mM Tris-HCl (pH 7.5). A sample assay for FDH activity contained the following in a final volume of 0.5 ml: 1 mM NAD+ (Nacalai Tesque), 1 mM HM-SG, and enzyme (crude enzyme: 10 to 50 μg, purified enzyme: 20 to 100 ng) in 25 mM Tris-HCl (pH 7.5). HM-SG was prepared by chemical reaction of 10 mM GSH (Wako) with 50 mM formaldehyde (Wako) for 10 min in the dark. To prepare HM-SG persulfide and polysulfides, i.e., HM-S-SG and HM-(S)n-SG, respectively, 10 mM GSH was incubated with 5 mM Na2S2 (Dojindo) or Na2S4 (Dojindo) for 5 min at 37°C and then with 50 mM formaldehyde at room temperature for 10 min in the dark. In some FDH assay, different concentrations of Na2S4 and incubation times were used before conducting the GSNOR reaction of ADH5, as follows.
We used a solid-phase segregation method using magnetic Sepharose beads, which can reversibly capture the ADH5 to completely separate the cofactors/substrates and products from two reactions, i.e., FDH and GSNOR reactions. This method thus allowed us to elucidate the role of Cys174 supersulfides in the GSNOR activity. Specifically, to analyze the effects of GSNOR and FDH reactions on the GSNOR activity of ADH5, the His-tagged protein was immobilized on the Ni-Mag Sepharose (GE Healthcare, Little Chalfont, England) in 20 mM Tris-HCl buffer (pH 7.5), for complete removal of cofactors (NAD+/NADH) and substrates such as GSNO or substrate cocktails containing various concentrations of HM-S-SG and HM-(S)n-SG and their products, before or after the reactions of GSNOR and FDH reaction. For the GSNOR reaction, for example, the WT ADH5 protein was incubated in 25 mM Tris-HCl buffer including GSNO (2 mM) and NADH (4 mM) for 1 hour at 37°C. The protein-loaded beads were captured using the magnetic rack and washed three times with 20 mM Tris-HCl buffer (pH 7.5). After being liberated from a magnetic field, the ADH5 proteins (75 nM) were then treated with the reaction mixture of FDH (various concentrations of NAD+ and HM-SG or HM-(S)n-SG) in 20 mM Tris-HCl buffer (pH 7.5) or with a reducing agent (1 mM TCEP) in 20 mM Tris-HCl buffer for 30 min at 37°C. The protein-loaded beads were captured again with the magnetic field and washed three times with 20 mM Tris-HCl buffer, after which the GSNOR activity was determined, as follows. Briefly, the ADH5 (loaded on beads) recovered after washing was incubated in the reaction mixture with 250 μM GSNO and 200 μM NADH in 20 mM Tris-HCl buffer at room temperature, which was terminated at 1 min after initiating the GSNOR reaction. Then, after the magnetic removal of the ADH5 protein, the NADH consumption of the resultant reaction mixture collected was measured with spectrophotometer. Through this solid-phase separation assay, we determined the relative GSNOR activity for each enzyme reaction as a percentage of that of ADH5 alone without any treatments of FDH and TCEP reactions.
Analysis of protein polysulfidation via the PMSA
Supersulfidation of the ADH5 protein was detected by using two different versions of the PMSA (i.e., the original and modified PMSA) which were described previously (3–5). In the original PMSA, purified recombinant ADH5 protein was applied to the PD SpinTrap G-25 column (GE Healthcare) equilibrated with radioimmunoprecipitation assay (RIPA) buffer [10 mM Tris-HCl, 1% NP-40, 0.1% sodium deoxycholate, 0.1% SDS, and 150 mM NaCl (pH 7.4)] to remove reductants. The proteins were quantified (0.3 mg/ml) by Bradford protein assay and incubated in RIPA buffer containing various electrophiles (3 mM each), including IAM (Nacalai Tesque), monobromobimane (Nacalai Tesque), 5,5′-dithiobis(2-nitrobenzoic acid) (Nacalai Tesque), 4,4′-dithiodipyridine (Nacalai Tesque), methyl methanethiosulfonate (Sigma-Aldrich), 2-methylsulfonyl benzothiazole (Dojindo), 2-aminosulfonyl benzothiazole (Dojindo), PCMB (Sigma-Aldrich), N-ethylmaleimide (Wako), and 8-nitroguanosine 3′,5′-cyclic monophosphate (8NcG), prepared according to the method previously reported (41), for 1 hour at 37°C. The mixtures were subsequently incubated with 2.5 mM BPM for 1 hour at 37°C. The proteins were heat-denatured in the presence or absence of 5% 2-ME and subjected to SDS-PAGE and CBB staining.
For the modified PMSA analysis of recombinant ADH5 proteins, the samples adjusted under the same conditions as the original PMSA method were incubated in RIPA buffer containing 1 mM BPM (Dojindo) for 1 hour at 37°C. The mixture was subsequently incubated with various electrophiles (3 mM each) used for the original PMSA at 37°C for 1 hour, after which the proteins were heat-denatured in the presence or absence of 5% 2-ME and subjected to SDS-PAGE and CBB staining. For modified PMSA analysis with lysates of mammalian cells, HEK293T cells expressing ADH5 (WT or C174S) were washed with ice-cold phosphate-buffered saline (PBS) (pH 7.4) and collected with ice-cold RIPA buffer plus 1 mM TCEP and proteinase inhibitor cocktail (Nacalai Tesque). Cells were homogenized by 20 passages through a 26-gauge needle with a 1-ml syringe on ice and then centrifuged at 20,000g for 25 min at 4°C. The supernatant was collected and incubated for 1 hour at 37°C in the presence of 1 mM TCEP, after which the sample was stored at −80°C until use. After TCEP was removed with the PD SpinTrap G-25 column, cell lysate proteins (3 mg/ml) were analyzed by the modified PMSA method described above. The ADH5 protein was detected by using Western blotting with an anti-FLAG-tag (described below).
To analyze the effects of the GSNOR and FDH reactions on protein polysulfidation, the original PMSA was modified (3). WT ADH5 protein was incubated in 25 mM Tris-HCl buffer including 2 mM GSNO and 4 mM NADH for 1 hour at 37°C. For the FDH reaction, the sample mixture was subsequently incubated with 1.67 mM HM-SG [formaldehyde (HCHO) + GSH), HM-(S)n-SG (HCHO + GSH + Na2S2] or 1 mM TCEP for 1 hour at 37°C to induce the FDH reaction. After the incubation, samples were desalted using the PD SpinTrap G-25 column, and the resultant eluate, containing protein fraction, was used as a sample in the original PMSA analysis. Briefly, the eluate was incubated in RIPA buffer with 3 mM IAM for 1 hour at 37°C, diluted three times with RIPA buffer, and then reacted with 2.5 mM PEG48-MAL (Quanta BioDesign, Powell, OH) in RIPA buffer for 1 hour at 37°C. After the proteins were heat-denatured in the absence of 5% 2-ME, they were subjected to SDS-PAGE and CBB staining.
LC-ESI-MS/MS analysis for protein supersulfidation in recombinant ADH5
The LC-ESI-MS/MS analysis was performed according to the method we previously reported (5). Briefly, the purified recombinant WT and C174S ADH5 proteins were applied to a PD SpinTrap G-25 column equilibrated with 10 mM Hepes buffer (pH 7.5) to remove reductants, after which proteins (0.7 mg/ml) were alkylated with 6 mM HPE-IAM (Molecular Biosciences, Boulder, CO) for 5 min at 37°C. The alkylated proteins (150 μg/ml) were digested with pronase (1 mg/ml; Merck, Darmstadt, Germany) in 40 mM sodium acetate buffer (pH 5.5) containing known amounts of isotope-labeled internal standards for 7 hours at 37°C to produce Cys-HPE-IAM or cysteine polysulfide-HPE-IAM adducts. After digestion, 0.1% formic acid was added to terminate the digestion. Following centrifugation (14,000g for 10 min), 10 μl of the resultant supernatants (1.5 μg/ml) was injected into LC-ESI-MS/MS (LCMS-8050; Shimadzu) coupled to the Nexera UHPLC system (Shimadzu). Cys-HPE-IAM and cysteine polysulfide-HPE-IAM adducts were separated by Nexera UHPLC with a reversed-phase column (YMC-Triart C18 column, 50 mm by 2.0 mm inner diameter; YMC, Kyoto, Japan) under the following elution conditions: mobile phase A (0.1% formic acid) with a linear gradient of mobile phase B (0.1% formic acid in methanol) from 5 to 90% for 15 min at a flow rate of 0.2 ml/min at 40°C. MS spectra were obtained at the ESI temperature of 300°C, desolvation line temperature of 250°C, and heat block temperature of 400°C; the nebulizer, heating, and drying nitrogen gas flows were set at 3, 10, and 10 liters/min, respectively. The HPE-IAM adducts of Cys, CysSSH, and cysteine trisulfide were identified and quantified by means of multiple reaction monitoring. For multiple reaction monitoring, the following transitions were used: CysS-HPE-IAM adduct: mass/charge ratio (m/z) 298.9 → 121.0, collision energy (CE) –29 V; CysSS-HPE-IAM adduct: m/z 330.8 → 121.0, CE −29 V; CysSSS-HPE-IAM adduct: m/z 362.8 → 121.0, CE –29 V; [13C]CysS-HPE-IAM adduct: m/z 299.9 → 121.0, CE −29 V; CysS34S-HPE-IAM adduct: m/z 332.8 → 121.0, CE −29 V; CysS34S34S-HPE-IAM adduct: m/z 366.8 → 121.0, CE −29 V.
Proteome analyses of cysteine residues in recombinant ADH5
Recombinant ADH5 (WT) were applied to a PD SpinTrap G-25 column equilibrated with 10 mM Hepes buffer (pH 7.5) to remove reductants, after which proteins (0.3 mg/ml) were incubated with 1 mM NAD+ (Nacalai Tesque) and 1.5 mM HM-(S)n-SG in 25 mM Tris-HCl (pH 7.5) at 37°C for 1 hour. To prepare HM-(S)n-SG, 10 mM GSH was incubated with 5 mM Na2S4 (Dojindo) for 5 min at 37°C and then with 50 mM formaldehyde at room temperature for 10 min in the dark. Then, the sample was incubated with 2 mM GSNO and 4 mM NADH in 20 mM Tris-HCl buffer (pH 7.5) at 37°C for 1 hour. The samples were alkylated with 5 mM IAM in 0.02% ProteaseMAX surfactant at 37°C for 10 min, followed by digestion with Trypsin Gold (27 μg/ml) at 37°C for 3 hours and then analyzed using liquid chromatography–electrospray ionization–quadrupole time-of-flight tandem mass spectrometry (LC–ESI–Q-TOF MS/MS). LC–ESI–Q-TOF analysis was performed by using 6545XT AdvanceBio LC/Q-TOF (Agilent Technologies) connected to the Agilent HPLC-Chip system (Agilent). The modification analysis of the active center cysteines (Cys170 and Cys174) was performed by means of Agilent MassHunter BioConfirm software. The digestion efficiency of the protein by trypsin was normalized with VDEFVTHNLSFDEINK peptide (m/z 636.3109). The modification levels in VCLLGCGISTGYGAAVNTAK peptide, which includes the active center cysteine residues, were detected by monitoring at m/z 692.6509 [for CysS-S-AM (AM, IAM adduct)] and m/z 858.0153 (for CysS-SG). The supersulfide levels of the fragment containing the active center cysteines were evaluated as the relative ratio of the intensity of one persulfide fragment (peptide-CysS-AM/CysSS-AM) plus both persulfide fragment (peptide-CysSS-AM/CysSS-AM) to the total intensity of fragment peptide. In terms of a technical issue of IAM alkylation of supersulfide-bound peptides/proteins (23), we considered that HPE-IAM used in our study should be appropriate for the quantitative analysis for protein-bound CysSnSH, because its HPE moiety could hinder the tautomerization of polymeric sulfurs to prevent the de-sulfidation of the IAM adducts, as shown in the aforementioned paper (23). Even for the IAM alkylation of the Cys174 persulfide, it can be still applicable to at least determine the relative quantity of CysSSH formed at the catalytic site of GSNOR; although it may be underestimated due to the polysulfur rearrangement, as was suggested earlier (23).
Cell culture
HEK293T cells and MEFs were cultured in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) (Sigma-Aldrich) supplemented with 10% fetal bovine serum (MP Biomedicals, Solon, OH) and 1% penicillin-streptomycin under standard cell culture conditions (37°C, 5% CO2, humidified).
Transient overexpression of FLAG-tagged WT ADH5 and ADH5 C174S in HEK293T cells
Transient transfection of the expression plasmid was performed by using the Lipofectamine 2000 kit (Thermo Fisher Scientific), according to the manufacturer’s instructions. HEK293T cells were seeded in 24-well plates (1.2 × 104 cells per well). Cells were transfected with 2.0 μg per well of pPyCAGIP-FLAG-hADH5 or 3.0 μg per well of pPyCAGIP-FLAG-hADH5 C174S, harvested after 48 hours, and used for the PMSA.
Animals
Adh5−/− mice (42) in the C57BL6/J genetic background were used in this study. Adh5C174S/C174S mice were generated by using CRISPR-Cas9 genome editing technology. Cas9 mRNA, guide RNA (5′-AAA GTC TGC CTT CTA GGT TG-3′), and targeting oligonucleotide (5′-TCC TTC GGC CCC TTT GGA TAA AGT CTG CCT TCT CGG CTC TGG TAT TTC AAC TGG CTA CGG GGC TGC TGT GAA CA-3′) were introduced into fertilized eggs obtained from the BDF1 mating pairs, which are obtained from the cross between C57BL/6J (B6) female x DBA/2J (D2) male, by electroporation according to protocols reported previously (43). The founder mice were crossed with C57BL/6J WT mice, and the mutation was transmitted to the germ line. By crossing Adh5C174S/+ mice after twice backcrossing into the C57BL/6J genetic background, we obtained Adh5C174S/C174S mice together with control WT mice. All mice were kept under specific pathogen–free conditions and treated according to the regulations of the Standards for Human Care and Use of Laboratory Animals of Tohoku University and the Guidelines for Proper Conduct of Animal Experiments of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan. The Tohoku University Committee for Laboratory Animal Research approved all animal experiments (#2019MdA-072-03).
Establishment of Adh5−/− MEFs expressing FLAG-tagged WT ADH5 or ADH5 C174S
Adh5−/− MEFs were established from embryos at E13.5 and immortalized by lentiviral introduction of SV40 large T antigen. To prepare WT ADH5-expressing and C174S mutant ADH5-expressing MEF cells, Adh5−/− MEF cells were seeded onto 24-well plates at a density of 2 × 104 cells per well and incubated at 37°C in 5% CO2. After 24 hours, the cells were transfected with 4 μg of pPyCAGIP-FLAG/empty, pPyCAGIP-FLAG-hADH5, or pPyCAGIP-FLAG-hADH5 C174S by using the Lipofectamine 2000 kit. After 4 hours, the culture medium was replaced with fresh culture medium. The cells were then incubated for 44 hours at 37°C and used for enzyme and cell viability assays (described below).
For the enzyme assay, cells were washed three times with ice-cold PBS; harvested with ice-cold Tris-HCl buffer (pH 8.0) including 0.5 mM EDTA, 0.1% NP-40, and proteinase inhibitor cocktail; and disrupted by sonication. After centrifugation at 15,000g for 20 min at 4°C, the supernatant was collected, and the protein concentration was determined by means of the bicinchoninic acid method.
Establishment of primary Adh5+/+ and Adh5C174S/C174S MEFs
The primary Adh5+/+ and Adh5C174S/C174S MEFs were established from embryos of Adh5+/+ and Adh5C174S/C174S mice, respectively, obtained from Adh5C174S/+ mating pairs. For the enzyme assay, cells were processed in a manner similar to that described above.
Preparation of yeast strains
The yeast strain S. cerevisiae on a BY4741 background was used in this study. To construct an SFA1-disrupted strain (Δsfa1), an integration cassette was amplified by PCR with primers (sfa1 deletion Fw and sfa1 deletion Rv; table S2) and pFA6a-5FLAG-hphMX6 (purchased from the AddGene repository). PCR fragments were integrated into the genome by transformation with the LiAc/SS carrier DNA/PEG method (44). Screening for hygromycin resistance was performed, and the correct integration event was verified by PCR using chromosomal DNA.
pAG415GPD-Sfa1 (WT) or pAG413GPD-Sfa1 (C179S) was transfected into Δsfa1 cells by using the LiAc/SS carrier DNA/PEG method (44). Stable transformants were selected by means of auxotrophic markers on synthetic complete medium without leucine and histidine (SC-LH). SC-LH contains 2% glucose, 0.67% yeast nitrogen base without amino acids (Difco Laboratories, Detroit, MI), and 0.2% dropout supplement without leucine and histidine (Takara Bio). The centromere-based low–copy number plasmids pRS415 and pRS413 (Stratagene, La Jolla, CA) were used for complementing the auxotrophic markers.
WT cells, Δsfa1 cells, and Δsfa1 cells transformed with pAG415GPD-Sfa1 (WT) or pAG413GPD-Sfa1 (C179S) were cultured at 30°C during the late exponential phase (OD600 = 3.0 to 4.0) in SC-LH, after which they were harvested and disrupted by using YeastBuster (Millipore, Bedford, MA) containing a protein inhibitor cocktail. The supernatants were collected and were used for Western blotting and enzyme assays.
Western blotting
Samples were heat-denatured and separated via SDS-PAGE, followed by transfer to polyvinylidene fluoride membranes (Immobilon-P) (Merck Millipore, Watford, UK). Membranes were blocked with Blocking One (Nacalai Tesque) or Tris-buffered saline-Tween (TTBS) containing 3% skim milk (Nacalai Tesque), after which they were incubated with antibodies in TTBS containing 5% skim milk overnight at 4°C. Antibodies used in this study included the following: anti-DDDDK (FLAG)-tag (Medical & Biological Laboratories Co., Ltd., Nagoya, Japan), anti–β-actin (Santa Cruz Biotechnology, Santa Cruz, CA), anti-ADH5 prepared as we described elsewhere (29), anti-protein S-glutathionylation (Virogen, Watertown, MA), anti-yeast 3-phosphoglycerate kinase (Pgk1) (clone: 22C5D8; Abcam, Cambridge, UK), and anti-HA (Medical & Biological Laboratories Co., Ltd.). Membranes were washed three times with TTBS and then incubated with a horseradish peroxidase–conjugated secondary antibody for 1 hour at room temperature. After the membranes were washed again three times with TTBS, immunoreactive bands were detected via a chemiluminescence reagent (ECL Prime Western Blotting Detection Reagent; GE Healthcare) with a luminescent image analyzer (ImageQuant LAS 500; GE Healthcare).
Cell viability assay
Cell viability was determined by using the sulforhodamine B (SRB) assay as described by Vichai and Kirtikara (45). Cells were treated with NOC7 (0 to 3.0 mM) (Dojindo) or HM-SG (0 to 3.0 mM) in serum-free DMEM. After incubation for 2 hours at 37°C, the medium was replaced with fresh serum-free DMEM and incubation continued for 22 hours. Cells were washed with ice-cold PBS, fixed with 2% ice-cold trichloroacetic acid for 1 hour at 4°C, and then rinsed with distilled water. Cells were then stained in 200 μl of 1% acetic acid including 0.4% SRB for 30 min at room temperature. The plates were rinsed four times with 1% acetic acid, then 500 μl of 10 mM Tris solution (pH 10.5) was added to each well, and the plates were shaken for 5 min to solubilize the protein-bound dye. Absorbance at 570 nm, with the reference at 655 nm, was measured with a plate reader (iMark; Bio-Rad, Hercules, CA). Cell viability was converted into percent absorbance relative to untreated cells.
Growth inhibition by GSNO and formaldehyde in yeast
Yeast cells were cultured in SC-LH at 0.01 of OD600. After 3 hours at 30°C, GSNO (5 mM) or formaldehyde (0.5 mM) was added to the culture, and the culture was started again at 30°C. The OD600 of the culture was measured after 18 hours for GSNO and 48 hours for formaldehyde. Growth rate was converted into percent growth relative to untreated controls.
Measurement of cardiac functions by catheterization
Cardiac catheterization was performed as described previously (46) with slight modifications. After 15- to 22-week-old male and female WT, Adh5−/−, and Adh5C174S/C174S mice received isoflurane anesthesia, a micromanometer catheter (Millar 1.4F, SPR 671) (Millar Instruments, Houston, TX) was inserted into the left ventricle via the left carotid artery, and steady-state LV hemodynamic parameters were analyzed by using LabChart software (AD Instruments, Colorado Springs, CO). Cardiac parameters measured in the mice are shown in table S3. Regarding the effect of the difference in gender on cardiac functions in terms of separation of two distinct activities of ADH5 (i.e., GSNOR versus FDH), the number of female and male mice used is three to five for each gender in three groups, except that two female Adh5 knockout mice were available for this experiment. No significant differences in the three parameters of cardiac functions that we examined under normal breeding conditions were reported previously between the genders in WT and Adh5 knockout mice (12, 47, 48), however.
Statistical analysis
Results are presented as means ± SD of at least three independent measurements unless otherwise specified. For statistical comparisons, we used unpaired Student’s t test or one-way analysis of variance (ANOVA) or two-way ANOVA followed by Tukey’s multiple comparisons test, with significance set at P < 0.05.
Acknowledgments
We thank J. B. Gandy for excellent editing of the manuscript before submission.
Funding: This work was supported, in part, by Transformative Research Areas, Scientific Research [(S), (A), (B), (C), Challenging Exploratory Research] from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, to T.A. (18H05277, 21H05258, 21H05263, and 22K19397), H.M. (21H05264, 21H05258, 21A303, and 21H04799), M.N. (21H05269, 21H05258, 22K19395, and 22H02772), Akiyuki Nishimura (21H05269), S.K. (22K06148), Akira Nishimura (21K05504), K.S. (22K20714), T.M. (22K06893), M.M. (19K07341 and 23K06145), T.I. (20K07306 and 23K06386), M.J. (23K14341), and S.O. (23K14333); Japan Science and Technology Agency (JST), CREST to M.N. and T.A. (JPMJCR2024); and a grant from the Japan Agency for Medical Research and Development (AMED) to H. Motohashi and T. Akaike (JP21zf0127001).
Author contributions: H.M. and T.A. designed the study; S.K., Akira Nishimura, M.M.A., M.M., K.S., T.M., M.J., T.I., S.O., U.B., M.N., Akiyuki Nishimura, H.M., and T.A. performed research; S.K., Akira Nishimura, M.M.A., M.M., K.S., T.M., M.J., T.I., S.O., U.B., M.N., Akiyuki Nishimura, H.M., and T.A. analyzed data; and S.K., Akira Nishimura, M.M.A., M.N., Akiyuki Nishimura, H.M., and T.A. wrote the paper.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S12
Tables S1 to S3
REFERENCES AND NOTES
- 1.Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., Suematsu M., Motohashi H., Fujii S., Matsunaga T., Yamamoto M., Ono K., Devarie-Baez N. O., Xian M., Fukuto J. M., Akaike T., Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. U.S.A. 111, 7606–7611 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ono K., Akaike T., Sawa T., Kumagai Y., Wink D. A., Tantillo D. J., Hobbs A. J., Nagy P., Xian M., Lin J., Fukuto J. M., Redox chemistry and chemical biology of H2S, hydropersulfides, and derived species: Implications of their possible biological activity and utility. Free Radic. Biol. Med. 77, 82–94 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung M., Kasamatsu S., Matsunaga T., Jung M., Kasamatsu S., Matsunaga T., Akashi S., Ono K., Nishimura A., Morita M., Hamid H. A., Fujii S., Kitamura H., Sawa T., Ida T., Motohashi H., Akaike T., Protein polysulfidation-dependent persulfide dioxygenase activity of ethylmalonic encephalopathy protein 1. Biochem. Biophys. Res. Commun. 480, 180–186 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Kasamatsu S., Nishimura A., Morita M., Matsunaga T., Hamid H. A., Akaike T., Redox signaling regulated by cysteine persulfide and protein polysulfidation. Molecules 21, 1721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akaike T., Ida T., Wei F. Y., Nishida M., Kumagai Y., Alam M. M., Ihara H., Sawa T., Matsunaga T., Kasamatsu S., Nishimura A., Morita M., Tomizawa K., Nishimura A., Watanabe S., Inaba K., Shima H., Tanuma N., Jung M., Fujii S., Watanabe Y., Ohmuraya M., Nagy P., Feelisch M., Fukuto J. M., Motohashi H., Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 8, 1177 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dóka É., Pader I., Bíró A., Johansson K., Cheng Q., Ballagó K., Prigge J. R., Pastor-Flores D., Dick T. P., Schmidt E. E., Arnér E. S., Nagy P., A novel persulfide detection method reveals protein persulfide- and polysulfide-reducing functions of thioredoxin and glutathione systems. Sci. Adv. 2, e1500968 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen D. E., Belka G. K., du Bois G. C., S-Nitrosoglutathione is a substrate for rat alcohol dehydrogenase class III isoenzyme. Biochem. J. 331, 659–668 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu L., Hausladen A., Zeng M., Que L., Heitman J., Stamler J. S., A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410, 490–494 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Liu L., Yan Y., Zeng M., Zhang J., Hanes M. A., Ahearn G., McMahon T. J., Dickfeld T., Marshall H. E., Que L. G., Stamler J. S., Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 116, 617–628 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Que L. G., Liu L., Yan Y., Whitehead G. S., Gavett S. H., Schwartz D. A., Stamler J. S., Protection from experimental asthma by an endogenous bronchodilator. Science 308, 1618–1621 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei W., Li B., Hanes M. A., Whitehead G. S., Gavett S. H., Schwartz D. A., Stamler J. S., S-Nitrosylation from GSNOR deficiency impairs DNA repair and promotes hepatocarcinogenesis. Sci. Transl. Med. 2, 19ra13 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beigi F., Gonzalez D. R., Minhas K. M., Sun Q. A., Foster M. W., Khan S. A., Treuer A. V., Dulce R. A., Harrison R. W., Saraiva R. M., Premer C., Schulman I. H., Stamler J. S., Hare J. M., Dynamic denitrosylation via S-nitrosoglutathione reductase regulates cardiovascular function. Proc. Natl. Acad. Sci. U.S.A. 109, 4314–4319 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koivusalo M., Baumann M., Uotila L., Evidence for the identity of glutathione-dependent formaldehyde dehydrogenase and class III alcohol dehydrogenase. FEBS Lett. 257, 105–109 (1989). [DOI] [PubMed] [Google Scholar]
- 14.Koivusalo M., Lapatto R., Uotila L., Purification and characterization of S-formylglutathione hydrolase from human, rat and fish tissues. Adv. Exp. Med. Biol. 372, 427–433 (1995). [DOI] [PubMed] [Google Scholar]
- 15.Wei W., Yang Z., Tang C. H., Liu L., Targeted deletion of GSNOR in hepatocytes of mice causes nitrosative inactivation of O6-alkylguanine-DNA alkyltransferase and increased sensitivity to genotoxic diethylnitrosamine. Carcinogenesis 32, 973–977 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang C. H., Wei W., Hanes M. A., Liu L., Hepatocarcinogenesis driven by GSNOR deficiency is prevented by iNOS inhibition. Cancer Res. 73, 2897–2904 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goto M., Kitamura H., Alam M. M., Ota N., Haseba T., Akimoto T., Shimizu A., Takano-Yamamoto T., Yamamoto M., Motohashi H., Alcohol dehydrogenase 3 contributes to the protection of liver from nonalcoholic steatohepatitis. Genes Cells 20, 464–480 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Que L. G., Yang Z., Stamler J. S., Lugogo N. L., Kraft M., S-Nitrosoglutathione reductase: An important regulator in human asthma. Am. J. Respir. Crit. Care Med. 180, 226–231 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima B., Forrester M. T., Hess D. T., Stamler J. S., S-Nitrosylation in cardiovascular signaling. Circ. Res. 106, 633–646 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marozkina N. V., Wei C., Yemen S., Wallrabe H., Nagji A. S., Liu L., Morozkina T., Jones D. R., Gaston B., S-Nitrosoglutathione reductase in human lung cancer. Am. J. Respir. Cell Mol. Biol. 46, 63–70 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanghani P. C., Bosron W. F., Hurley T. D., Human glutathione-dependent formaldehyde dehydrogenase. Structural changes associated with ternary complex formation. Biochemistry 41, 15189–15194 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Bogdándi V., Ida T., Sutton T. R., Bianco C., Ditrói T., Koster G., Henthorn H. A., Minnion M., Toscano J. P., van der Vliet A., Pluth M. D., Feelisch M., Fukuto J. M., Akaike T., Nagy P., Speciation of reactive sulfur species and their reactions with alkylating agents: Do we have any clue about what is present inside the cell? Br. J. Pharmacol. 176, 646–670 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schilling D., Barayeu U., Steimbach R. R., Talwar D., Miller A. K., Dick T. P., Commonly used alkylating agents limit persulfide detection by converting protein persulfides into thioethers. Angew. Chem. Int. Ed. Engl. 61, e202203684 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernández M. R., Biosca J. A., Parés X., S-Nitrosoglutathione reductase activity of human and yeast glutathione-dependent formaldehyde dehydrogenase and its nuclear and cytoplasmic localisation. Cell. Mol. Life Sci. 60, 1013–1018 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q., Tian P., Zhai M., Lei X., Yang Z., Liu Y., Liu M., Huang H., Zhang X., Yang X., Zhao Y., Meng Z., Formaldehyde regulates vascular tensions through nitric oxide-cGMP signaling pathway and ion channels. Chemosphere 193, 60–73 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Lundberg J. O., Gladwin M. T., Weitzberg E., Strategies to increase nitric oxide signalling in cardiovascular disease. Nat. Rev. Drug Discov. 14, 623–641 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Lee U., Wie C., Fernandez B. O., Feelisch M., Vierling E., Modulation of nitrosative stress by S-nitrosoglutathione reductase is critical for thermotolerance and plant growth in Arabidopsis. Plant Cell 20, 786–802 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen R., Sun S., Wang C., Li Y., Liang Y., An F., Li C., Dong H., Yang X., Zhang J., Zuo J., The Arabidopsis PARAQUAT RESISTANT2 gene encodes an S-nitrosoglutathione reductase that is a key regulator of cell death. Cell Res. 19, 1377–1387 (2009). [DOI] [PubMed] [Google Scholar]
- 29.Pettinati I., Brem J., McDonough M. A., Schofield C. J., Crystal structure of human persulfide dioxygenase: Structural basis of ethylmalonic encephalopathy. Hum. Mol. Genet. 24, 2458–2469 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dóka É., Ida T., Dagnell M., Abiko Y., Luong N. C., Balog N., Takata T., Espinosa B., Nishimura A., Cheng Q., Funato Y., Miki H., Fukuto J. M., Prigge J. R., Schmidt E. E., Arnér E. S. J., Kumagai Y., Akaike T., Nagy P., Control of protein function through oxidation and reduction of persulfidated states. Sci. Adv. 6, eaax8358 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimura E., Shionoya M., Hoshino A., Ikeda T., Yamada Y., A model for catalytically active zinc (II) ion in liver alcohol dehydrogenase: A novel “hydride transfer” reaction catalyzed by zinc (II)-macrocyclic polyamine complexes. J. Am. Chem. Soc. 114, 10134–10137 (1992). [Google Scholar]
- 32.Plapp B. V., Savarimuthu B. R., Ferraro D. J., Rubach J. K., Brown E. N., Ramaswamy S., Horse liver alcohol dehydrogenase: Zinc coordination and catalysis. Biochemistry 56, 3632–3646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson D. H., Lund P., Krebs H. A., The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 103, 514–527 (1967). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster M. W., Hess D. T., Stamler J. S., Protein S-nitrosylation in health and disease: A current perspective. Trends Mol. Med. 15, 391–404 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatzistergos K. E., Paulino E. C., Dulce R. A., Takeuchi L. M., Bellio M. A., Kulandavelu S., Cao Y., Balkan W., Kanashiro-Takeuchi R. M., Hare J. M., S-Nitrosoglutathione reductase deficiency enhances the proliferative expansion of adult heart progenitors and myocytes post myocardial infarction. J. Am. Heart Assoc. 4, e001974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae S., Chon J., Field M. S., Stover P. J., Alcohol dehydrogenase 5 is a source of formate for de novo purine biosynthesis in HepG2 cells. J. Nutr. 147, 499–505 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pontel L. B., Rosado I. V., Burgos-Barragan G., Garaycoechea J. I., Yu R., Arends M. J., Chandrasekaran G., Broecker V., Wei W., Liu L., Swenberg J. A., Crossan G. P., Patel K. J., Endogenous formaldehyde is a hematopoietic stem cell genotoxin and metabolic carcinogen. Mol. Cell 60, 177–188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aoto T., Saitoh N., Sakamoto Y., Watanabe S., Nakao M., Polycomb group protein-associated chromatin is reproduced in post-mitotic G1 phase and is required for S phase progression. J. Biol. Chem. 283, 18905–18915 (2008). [DOI] [PubMed] [Google Scholar]
- 39.Sanghani P. C., Stone C. L., Ray B. D., Pindel E. V., Hurley T. D., Bosron W. F., Kinetic mechanism of human glutathione-dependent formaldehyde dehydrogenase. Biochemistry 39, 10720–10729 (2000). [DOI] [PubMed] [Google Scholar]
- 40.Hurley T. D., Edenberg H. J., Bosron W. F., Expression and kinetic characterization of variants of human beta 1 beta 1 alcohol dehydrogenase containing substitutions at amino acid 47. J. Biol. Chem. 265, 16366–16372 (1990). [PubMed] [Google Scholar]
- 41.Sawa T., Zaki M. H., Okamoto T., Akuta T., Tokutomi Y., Kim-Mitsuyama S., Ihara H., Kobayashi A., Yamamoto M., Fujii S., Arimoto H., Akaike T., Protein S-guanylation by the biological signal 8-nitroguanosine 3′,5′-cyclic monophosphate. Nat. Chem. Biol. 3, 727–735 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Deltour L., Foglio M. H., Duester G., Metabolic deficiencies in alcohol dehydrogenase Adh1, Adh3, and Adh4 null mutant mice. Overlapping roles of Adh1 and Adh4 in ethanol clearance and metabolism of retinol to retinoic acid. J. Biol. Chem. 274, 16796–16801 (1999). [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto M., Yamashita Y., Takemoto T., Electroporation of Cas9 protein/sgRNA into early pronuclear zygotes generates non-mosaic mutants in the mouse. Dev. Biol. 418, 1–9 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Gietz R. D., Schiestl R. H., High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat. Protoc. 2, 31–34 (2007). [DOI] [PubMed] [Google Scholar]
- 45.Vichai V., Kirtikara K., Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 1, 1112–1116 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Nishida M., Sawa T., Kitajima N., Ono K., Inoue H., Ihara H., Motohashi H., Yamamoto M., Suematsu M., Kurose H., van der Vliet A., Freeman B. A., Shibata T., Uchida K., Kumagai Y., Akaike T., Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 8, 714–724 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulandavelu S., Dulce R. A., Murray C. I., Bellio M. A., Fritsch J., Kanashiro-Takeuchi R., Arora H., Paulino E., Soetkamp D., Balkan W., Van Eyk J. E., Hare J. M., S-Nitrosoglutathione reductase deficiency causes aberrant placental S-nitrosylation and preeclampsia. J. Am. Heart Assoc. 11, e024008 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casin K. M., Fallica J., Mackowski N., Veenema R. J., Chan A., St Paul A., Zhu G., Bedja D., Biswal S., Kohr M. J., S-Nitrosoglutathione reductase is essential for protecting the female heart from ischemia-reperfusion injury. Circ. Res. 123, 1232–1243 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S12
Tables S1 to S3