Abstract
Plant growth-promoting rhizobacteria benefit plants by stimulating their growth or protecting them against phytopathogens. Rhizobacteria must colonize and persist on plant roots to exert their benefits. However, little is known regarding the processes by which rhizobacteria adapt to different plant species, or behave under alternating host plant regimes. Here, we used experimental evolution and whole-population whole-genome sequencing to analyse how Bacillus subtilis evolves on Arabidopsis thaliana and tomato seedlings, and under an alternating host plant regime, in a static hydroponic setup. We observed parallel evolution across multiple levels of biological organization in all conditions, which was greatest for the two heterogeneous, multi-resource, spatially structured environments at the genetic level. Species-specific adaptation at the genetic level was also observed, possibly caused by the selection stress imposed by different host plants. Furthermore, a trade-off between motility and biofilm development was supported by mutational changes in motility- and biofilm-related genes. Finally, we identified several condition-specific and common targeted genes in different environments by comparing three different B. subtilis biofilm adaptation settings. The results demonstrate a common evolutionary pattern when B. subtilis is adapting to the plant rhizosphere in similar conditions, and reveal differences in genetic mechanisms between different host plants. These findings will likely support strain improvements for sustainable agriculture.
Keywords: adaptation, Bacillus subtilis, experimental evolution, plant–bacteria interaction, trade-off
Data Summary
Sequencing data associated with this article are available in the CNGB Sequence Archive (CNSA) [1] of the China National GeneBank DataBase (CNGBdb) [2] under accession numbers CNP0002416 and CNP0003952. Strain data for the DK1042 ancestor are available under accession number CNP0002416.
Impact Statement.
For rhizobacteria to benefit plant growth and protect against phytopathogens, bacteria must colonize and persist on plant roots. Understanding how rhizobacteria adapt to different plant species will assist strain development in sustainable agriculture. To explore the rhizobacterial adaptation process for different plant species and alternating host regimes, B. subtilis was experimentally evolved on A. thaliana or tomato roots, or an alternating host regime. Both parallel and species-specific adaptation were revealed at the genetic level. Analysis of the trade-off between motility and biofilm formation revealed several condition-specific and commonly targeted genes based on experimentally evolving B. subtilis biofilms.
Introduction
In natural environments, plant growth and yield depend on a plethora of interactions within the complex and dynamic communities of bacteria and fungi [3]. The rhizosphere provides a niche for the mutualistic relationships between plants and micro-organisms in the surrounding soil. Plants exude up to 20 % of fixed carbon and 15 % of nitrogen into their environment [4, 5], and these exudates shape the microbiome. Importantly, the amount and composition of exudates vary depending on plant species, stage of development and environmental stresses [6]. Exudates contribute to rhizobiome formation by recruiting plant growth-promoting rhizobacteria (PGPR) and serving as nutrients [7], and PGPR benefit plant growth and enhance plant tolerance to different abiotic and biotic stresses [8]. Bacillus subtilis is a soil-dwelling, biofilm-forming, Gram-positive PGPR commonly found in association with plants and their rhizosphere [9]. This bacterial species aids plants in several direct and indirect ways [10]. Some B. subtilis strains have been applied as biocontrol agents within agriculture, but the effects vary immensely under field conditions [11, 12]. To exert their beneficial effects, PGPR must colonize and persist on the root surface, which mainly depends on their ability to form biofilms.
Each plant species has a distinct effect on its rhizobiome and this is partly driven by plant-secreted exudates. Root exudates not only provide a nutritional source for soil micro-organisms, but also serve as a signal to attract or repel specific groups of microbes [13]. For example, diverse sugars and organic acids induce biofilm formation of B. subtilis in vitro or directly on the plant via different molecular mechanisms. Spo0A is a central transcriptional regulator in B. subtilis involved in biofilm development [14]. Biofilm formation is triggered when moderate levels of phosphorylated Spo0A (Spo0A-P) are present within a cell. The level of Spo0A-P is controlled by five histidine kinases (KinA−E), which respond to different environmental signals [15]. Chen and colleagues found that B. subtilis biofilm formation on tomato roots is dependent on KinD and small signalling molecules, possibly l-malic acid, released by the roots to stimulate B. subtilis biofilm in vitro [16]. Unlike in tomato, deletion of kinCD only has a moderate effect on biofilm formation on Arabidopsis thaliana roots [17], suggesting that the kinase requirement of B. subtilis for root colonization varies between plant species. In the case of A. thaliana, biofilm formation by B. subtilis is triggered by plant polysaccharides pectin, arabinogalactan and xylan, which act both as environmental cues and substrates for matrix synthesis [17]. Moreover, the disaccharide sucrose that is abundantly secreted from plant roots activates a signalling cascade to trigger solid surface motility and promote rhizosphere colonization by B. subtilis [18].
Interestingly, a B. subtilis strain isolated from banana rhizosphere and a Bacillus amylolicefaciens strain isolated from cucumber rhizosphere colonize their original plant hosts more efficiently than the non-host plant [19], demonstrating species-specific colonization. Additionally, organic acids detected exclusively in one plant species induce chemotaxis and biofilm formation of the corresponding bacterial isolate from that given host [19]. Chemotaxis and swarming motility are important for plant colonization [19, 20]; when defective in either, the ability of B. subtilis to efficiently colonize tomato seedlings was reduced in a gnotobiotic system [20]. Similarly, mutants lacking flagellum production were unable to colonize the roots of A. thaliana in a static hydroponic setup and tomato in a gnotobiotic system [20, 21].
In addition to testing specific mutants, experimental evolution provides a powerful tool for revealing the molecular mechanisms related to plant interactions because microbes adapt to the plant root niche [22]. Experimentally evolved clones of Bacillus thuringiensis from A. thaliana roots displayed improved root colonization ability and enhanced swarming, but impaired swimming, in addition to altered bacterial differentiation and pathogenicity [23]. In a similar setup, when B. subtilis was adapted to A. thaliana roots, evolved isolates demonstrated elevated root colonization and impaired swimming and swarming motility [24], indicating a possible biofilm–motility trade-off. Pseudomonas protegens , adapted to A. thaliana rhizosphere under axenic sandy conditions, exhibited enhanced swimming motility and impaired swarming motility [25]. Although these results highlight the existence of an evolutionary trade-off between biofilm development and motility, it remains largely unknown how such mechanisms supervene from an evolutionary perspective.
Since a crop rotation regime has a great influence on soil multifunctionality and bacterial community in agroecosystems [26], we wondered whether a plant-colonizing bacterium adapts differently in an alternating host regime compared with using the same plant throughout the experiment. In addition, we questioned what portion of bacterial adaptation on a plant root will be host-specific. To explore these questions, we employed experimental evolution combined with whole-population metagenome sequencing to analyse how B. subtilis evolves on A. thaliana and tomato seedlings, as well as in an alternating host regime of these two species in a static hydroponic setup. We observed parallel evolution across multiple levels of biological organization and a higher gene-level parallelism in populations evolved in an alternating host regime. We also observed species-specific adaptation at the genetic level, which was potentially provoked by specific host plant-imposed selection, either due to root exudates, plant polysaccharides, or certain stress conditions. Additionally, motility–biofilm trade-off was revealed in the mutational landscape of related genes, in addition to reduced swimming and swarming motility. Finally, we identified several condition-specific and shared mutated genes of B. subtilis when evolved in different biofilm environments.
Methods
Bacterial strains and experimental methods
We used identical materials and methods for tomato and two host-cycling evolution setups as described previously for the A. thaliana selection approach [27], including the bacterial strain and culture conditions, seedling preparation, experimental evolution and morphological diversification experiments.
Experimental evolution
To initiate experimental evolution we used A. thaliana Col-0 seedlings at 14–16 days old and tomato Col-0 seedlings at 8–10 days old. Seedlings were placed in 100 ml reagent bottles containing 27 ml of MSNg and inoculated with 3 ml of B. subtilis DK1042 bacterial culture adjusted to OD600=0.2, resulting in a starting OD600 of 0.02. To ensure that roots but not sprouts or leaves provided surfaces for colonization, seedlings were pulled through a sterile nylon mesh floating on top of the liquid medium, leaving only the roots submerged in the medium. Seedlings were incubated for 48 h under static conditions in a plant chamber with a day/night cycle of 16 h light at 24 °C and 8 h dark at 20 °C. We conducted two mono-host evolution experiments, one using only A . thaliana (Bs_root_ A ) and another with T omato (Bs_root_ T ), as well as two host-cycling evolution experiments, which either started on A . thaliana (Bs_root_ A T) or T omato (Bs_root_ T A) and switched plant species after every third transfer. Therefore, four different evolution experiments, each with five parallel lineages (A to E) were passaged on both A. thaliana and tomato seedlings for 20 transfers, spanning 40 days (Fig. 1). Consequently, every 2 days, a previously colonised plant seedling was transferred to a new sterile seedling, allowing only bacterial cells attached to the new root to continue in the experiment, enabling selection for a continuous cycle of dispersal, chemotaxis towards the plant root and biofilm formation on the root. To keep track and ensure sterile handling of seedlings during evolution, two controls without initial inoculant were included, in which either sterile seedlings or 10 % of the MSNg medium were passaged. To follow the development of populations and test for contamination, bacterial cells colonising old roots, as well as the two controls, were plated on lysogeny broth (LB, Lennox; Carl Roth, Karlsruhe, Germany; 10 g l-1 tryptone, 5 g l-1 yeast extract, 5 g l-1 NaCl) agar medium after initial colonisation and after transfer 5, 10, 17 and 20.
Fig. 1.
Evolution of B. subtilis on different plant roots. (a) Experimental evolution scheme of B. subtilis adapting to plant seedlings (created with BioRender.com). (b) Four evolution experiments were conducted, either as mono-evolution experiments with only A. thaliana or tomato seedlings, or cycling evolution experiments with plant species switched after every third transfer. Sequenced population samples are indicated by arrows. (c) Root colonization ability was measured as c.f.u. g−1 of root for T1, T5, T10, T17 and T20 in all lineages (n=3, *P<0.05, **P<0.01, ***P<0.001). (d) Dynamic frequency changes of different morphotypes in the evolution experiment averaged for all five populations in each condition (n=5). Results are means and error bars represent standard error in (c) and (d).
Colony-forming units (c.f.u.) quantification
In order to isolate biofilms from seedlings for quantification, roots were detached from sprouts, carefully washed in sterile MilliQ water and vortexed vigorously for 3 min in 1 ml of 0.9 % NaCl in the presence of small glass beads (0.75–1.00 mm; Carl Roth, Karlsruhe, Germany). The resulting cell suspensions were plated for c.f.u. quantification and preserved as frozen stocks for subsequent comparison and DNA sequencing.
Motility assays
A 20 ml volume of either 0.3 or 0.7 % lysogeny broth (LB) agar plates was used to test swimming and swarming motility. In both cases, plates were dried for 20 min before bacterial cultures were applied to the centre of plates. A 2 µl volume of overnight culture of ancestor or evolved isolates adjusted to an OD600 of 5.0 were spotted in the middle of the plate. To maintain similar humidity across all plates, multiple stacking was avoided. Plates were incubated at 30 °C and images were taken for swimming (after 6, 8 and 24 h) and swarming (after 8, 10 and 24 h) using a Lumix DC T290 camera (Panasonic) equipped with a DC Vario-ELMAR lens (Leica). The area covered by bacterial cells at a given time point as well as the area of the whole agar plate were measured using Fiji ImageJ 1.52 p. The capacity for swimming and swarming motility was calculated by dividing the covered area by the total area.
Sequencing and variant calling
Archived samples from each experimental evolution environment were revived via cultivation in LB medium at 37℃ for 16 h. Genomic DNA was extracted from harvested cells of each culture using a EURx Bacterial and Yeast Genomic DNA kit (EURx, Gdansk, Poland).
For whole-population genome sequencing of the evolved populations and ancestor strain samples, an MGIEasy PCR-free Library Prep Set (MGI Tech, Shenzhen, PR China) was used for library preparation, and 150 bp ×2 pair-end sequencing was performed on a DNBSEQ-Tx sequencer (MGI Tech) following the manufacturer’s procedures [28, 29]. More than 200× depth coverage of raw data was achieved for all population samples for further polymorphism calling.
To remove low-quality data, filtering was performed using SOAPnuke (version 1.5.6) [30]. Therefore, reads including >50 % of bases with quality score <12, reads including >10 % of unknown bases (N) and reads containing adaptor contamination were removed. Clean data were normalized to 200× depth for all samples to ensure similar variant calling sensitivity. Mutations were identified using breseq (version 0.35.7) with default parameters and a -p option for population samples [31, 32]. The default parameters only reported mutations if they included at least two reads from each strand and reached a frequency of at least 5 % in the population. Mutations also present in ancestor strains were removed for further analysis.
Genotype inferences from populations and Muller plots
To generate Muller plots, the Lolipop (version 0.6) package (https://github.com/cdeitrick/lolipop) was used with default parameters [33, 34]. These methods predict genotypes and lineages based on the shared trajectories of mutations over time to test their probability of nonrandom genetic linkage. Muller plots were then manually coloured by the presence of shared mutated genes or genotypes. Genotypes and their frequencies were used for genotype diversity calculation in R (version 4.2.2) using the vegan (version 2.6–4) package.
Jaccard index calculation
Jaccard index (J) for a pair of evolved lineages was calculated using formula (1). For a pair of lineages, each contains mutated gene sets G1 and G2, respectively. J is the number of genes mutated in both lineages divided by the total number of genes mutated in lineage 1 or lineage 2. J ranges from 0 to 1, with 1 indicating two lineages sharing exactly the same set of mutated genes and 0 indicating no mutated genes shared in the two lineages. This value was calculated for all pairs of evolved lineages, both within and between evolved environments.
| (1) |
Results
B. subtilis showed rapid improvement and diversification during evolution on plant roots
We wondered whether plant-colonizing bacteria adapt differently in an alternating host regime compared with a setup in which the same plant is used throughout the experiment. To investigate this, we explored adaptation of B. subtilis on the roots of two different plant species and compared the results with those of an experiment in which plant hosts were sequentially alternated. Evolved clones from the Bs_root_A setup have been examined in detail previously [27]. To evaluate the improvements in root colonization during experimental evolution, c.f.u. g−1 of roots was measured after transfer 1, 5, 10, 17 and 20 (T1, T5, T10, T17 and T20, respectively). As expected [23, 24, 27], root colonization ability improved significantly in all evolving lineages and conditions, by an average of 9.10-fold, 7.41-fold, 25.29-fold and 11.98-fold after 20 transfers in Bs_root_A, Bs_root_T, Bs_root_AT and Bs_root_TA, respectively (Fig. 1).
Furthermore, morphological diversification was also observed in all evolving lineages and conditions in CFU assays with morphotypes termed as Wrinkle (WR), Snow (SN) and Smooth (SM) [27], with colony morphology of the WR variant comparable to that of the ancestor. To investigate how the distribution of the distinct morphotypes developed during experimental evolution, overnight-grown cultures of frozen stocks of evolved populations from root 5, 10, 17 and 20 as well as from the ancestral strain (transfer 0) were prepared and plated on LB-agar. Different morphological variants were identified and counted to calculate the ratio of a given morphotype at each timepoint for each lineage. In all four conditions, WR was the most abundant in all lineages throughout the experiment (Fig. 1). SN appeared in all lineages at T5, reaching an average frequency of 21.78%, 15.48%, 23.81% and 21.28% in Bs_root_A, Bs_root_T, Bs_root_AT and Bs_root_TA, respectively (Fig. 1), but it was below the detection limit in some lineages at T10 and T17, then raised again in all lineages at T20. SM was observable in 11 of 20 lineages at T10 and only absent in one lineage at T17 and T20. The three distinct morphotypes coexisted until the end of the evolution experiment, reaching average highly similar ratios of ~80% WR, 17% SN and 3% SM in all four evolution experiments, revealing an astounding degree of parallelism at the morphotypic level.
Evolved isolates showed impaired motility
In a soil environment, bacterial cells must actively move towards plant roots during the initial phase of root colonization, as well as when recolonizing [21]. We hypothesized that, in the static hydroponic setup, which selects for regular root recolonization, including chemotaxis, biofilm formation and dispersal, motility would improve significantly during the evolution experiment, resulting in better root colonization. To examine this, evolved end-point isolates from Bs_root_A and Bs_root_T were tested for swimming and swarming motility by spotting 2 µl of an overnight culture on 0.3 and 0.7 % LB agar, respectively. Contrary to our expectations, none of the seven tested isolates evolved on A. thaliana or the five tested isolates evolved on tomato showed improvements in either swimming or swarming (Fig. 2). Instead, most isolates displayed a significantly smaller swimming radius after 6 and 8 h compared with the ancestor, although all isolates colonized the entire plate after 24 h, thus mobility was preserved. Importantly, other forms of motility, such as sliding [35, 36], might mask the effect of swimming after 24 h. Moreover, all isolates exhibited either delayed, reduced or even impaired swarming abilities. In future experiments, the frequencies of altered motility phenotype need to be assayed for each condition to clearly conclude selection against motility trait.
Fig. 2.

Evolved isolates showed impaired motility. Swimming (a) and swarming (b) motility of evolved isolates from Bs_root_A (lime green, seven isolates) and Bs_root_T (soft orange, five isolates) compared with the ancestor (black). Notably, swarming motility of several isolates was similar and low, and hence indistinguishable in (b). Results are means and error bars represent the standard error; each isolate was tested in triplicate, and the ancestor was repeated nine times. Tested isolates included two SN from lineage A and E of Bs_root_A, two SM from lineage A and E of Bs_root_A, three WR from lineage A, D and E of Bs_root_A, and five WR from lineage A (1), B (2) and C (2) of Bs_root_T.
Mutation spectrum and genetic diversity
Mutations provide the ultimate source of diversity, allowing selection to act and evolution to take place. To understand the genetic basis underlying the observed phenotypic diversification, impaired motility, and the dynamic landscape of evolution, we performed longitudinal whole-population genome sequencing of 79 B. subtilis population samples (1 population sample was lost during freezer maintenance) and the ancestor strain, using the DNBSEQ-Tx platform [28, 29]. More than 200× coverage depth of raw data was achieved for each sample. Mutations, including single-nucleotide polymorphisms (SNPs) and short insertions and deletions (indels), were identified using the breseq pipeline (version 0.35.7) [31, 32]. The default parameters only identified mutations if they appeared in at least two reads from each strand and reached a frequency of at least 5 % in the population. This analysis revealed 117, 106, 104 and 134 mutations in the evolved lineages from the Bs_root_A, Bs_root_T, Bs_root_AT and Bs_root_TA conditions, respectively (Table 1). No significant differences in mutation numbers were identified between the evolution conditions (Kruskal–Wallis test, P=0.1277), although the mutation spectra were slightly different between the selection setups (Table 1). When the filtering step excluded mutations below 10 % frequency, the cyclic-evolved conditions were found to contain more mutations than the mono-evolved conditions (P=0.0005162, t=−4.2419, df=17.542 via two-tailed t-test), suggesting that the mono-evolved conditions contained many low-frequency mutations, especially the Bs_root_A setup. Only 4 fixed mutations were present in all 20 lineages, possibly due to the short transfer times used in the experiment.
Table 1.
Mutation type and number
|
Category |
Bs_root_A |
Bs_root_T |
Bs_root_AT |
Bs_root_TA |
|---|---|---|---|---|
|
Pseudogene |
0 |
1 |
0 |
0 |
|
Non-coding gene* |
0 |
1 |
0 |
0 |
|
Coding gene |
81 |
91 |
88 |
104 |
|
Synonymous |
15 |
15 |
19 |
19 |
|
Non-synonymous |
57 |
58 |
47 |
58 |
|
Nonsense |
0 |
1 |
3 |
6 |
|
InDel** |
9 |
17 |
19 |
21 |
|
Intergenic*** |
36 |
13 |
16 |
30 |
|
No. of mutations |
117 |
106 |
104 |
134 |
|
No. of mutations (>10 %) |
50 |
69 |
80 |
92 |
|
Fixed mutations |
1 |
0 |
2 |
1 |
Note: there was no overlap between each mutation type. *, non-coding genes include tRNA, ncRNA and repeat region (IS); **, InDel indicates small insertions or deletions in coding gene regions; ***, intergenic refers to all kinds of mutations occurring in intergenic regions.
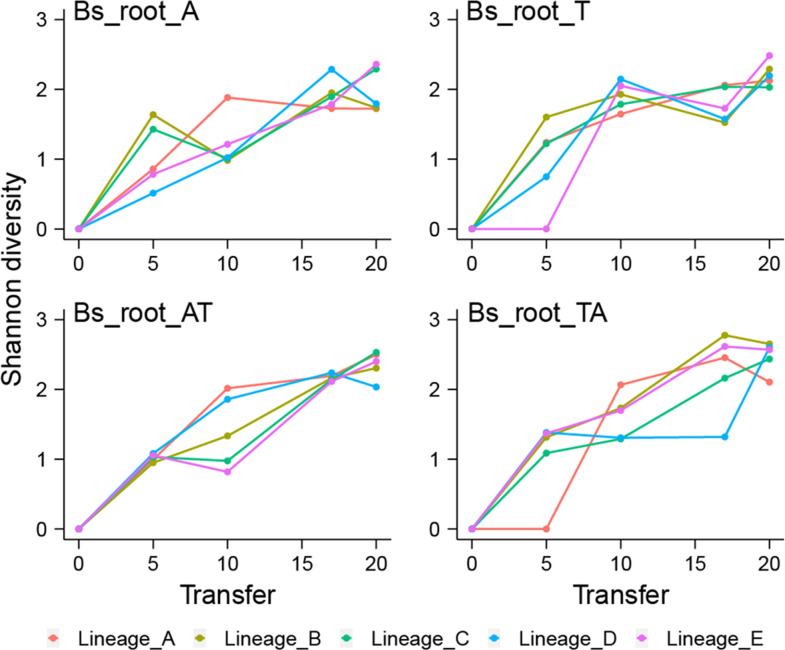
Although there was no significant difference in the number of mutations between the evolution conditions, we predicted that populations evolved under cyclic conditions would show greater genetic diversity than mono-evolved populations at the end of the experiment due to the higher complexity and fluctuating nutrient composition caused by the alternating host plants. Therefore, the genotype diversities of endpoint samples were compared between conditions. Indeed, genotype diversity was significantly lower in mono-evolved conditions than in cyclic-evolved conditions (P=0.01133, t=−2.9044, df=14.315 via two-tailed t-test), and lowest in the Bs_root_A condition (Fig. 3; P=0.1049, 0.03864 and 0.02767 via two-tailed t-test compared with Bs_root_T, Bs_root_AT and Bs_root_TA, respectively).
Fig. 3.
Genotype diversity. Dynamic distribution of genotype alpha diversity (Shannon method) in each population of four adaptation models over time. Genotypes and their frequencies are from genotype and genealogy analysis using Lolipop.
Ecological differentiation and parallel evolution
To infer the ecological differentiation in each lineage and visualize the changes in lineage frequencies from shared, nested mutation trajectories over time, we utilized the Lolipop software package developed by Cooper and colleagues [33, 34]. An average of 16±1.10, 15.6±2.15, 14.6±1.85 and 17.4±2.15 genotypes were obtained in Bs_root_A, Bs_root_T, Bs_root_AT and Bs_root_TA conditions, respectively, with multiple mutations in each genotype. Consistent with the number of mutations, there were no significant differences in genotype numbers between evolved conditions (Kruskal–Wallis test, P=0.3129). Overall, the number of genotypes increased continuously in all conditions, although it fell in some lineages at certain time points, consistently with the detected genotype diversity in each condition. Notably, neither genotype number nor diversity reached a peak within the time frame of the experiment (Fig. 3).
Each of the lineages was dominated by a few genotypes containing one or more mutations in certain genes. In general, sinR, pstBA, yuxH, sigD and eight flagellum-related genes were the most selected, demonstrating a high parallel evolution at gene and function levels within and between different evolved conditions (Fig. 4). Diverse functional genes were mutated, among which bacterial motility-related genes were targeted most frequently, indicating high parallelism at the functional level across all environments. To further compare genetic similarity between conditions, we determined the Jaccard index (J) of all pairs of lineages within each condition. The Jaccard index is commonly used for measuring similarity, indicating how likely it is for the same gene to be mutated in two separate lineages [37, 38], ranging from 0 to 1. Again, we observed lower genetic similarity in mono-evolved conditions than in cyclic-evolved conditions, and the lowest genetic similarity within the Bs_root_A condition, significantly lower than in Bs_root_T (P=0.0249, t=−2.4471, df=17.998 via two-tailed t-test) and the other two cyclic-evolved conditions (P<0.001 and <0.05, respectively; Fig. 5).
Fig. 4.
Evolutionary dynamics of B. subtilis during plant root colonization. Each shade or colour represents a different genotype and the vertical area corresponds to genotype frequency, inferred by Lolipop. Genotypes under selective pressure in certain conditions are highlighted in different colours and with annotations within or near shadows, while other genotypes are in different levels of grey without annotation. Nested genotypes of the highlighted genotype are highlighted with the same colour, except for nested genotypes, which also contain genes under selective pressure. Notably, some genotypes are less abundant in the figure because their frequencies were similar to their nested genotypes (e.g. fliI and yuxH in lineage A of Bs_root_A, pstBA and yuxH in lineage B and C of Bs_root_A). Different colours represent different genotypes containing certain targeted genes, flagellar-related genes including fliF, fliI, fliR, flhB, fliG, fliM, fliY and hag. Genotypes containing more than one targeted gene are coloured to match the gene displayed in the front.
Fig. 5.
Parallelism. Degree of parallelism within and between each evolved environment was estimated using the Jaccard index. Asterisks indicate significant differences between different environment combinations (*P<0.05, ***P<0.001, by Student’s unpaired two-tailed t-test). Boxes indicate Q1−Q3, lines indicate the median, and circles indicate the J value of each pair within (blue) or between (pink) evolved conditions.
Subsequently, we compared the genetic similarity between different evolved conditions. The similarity between the two cyclic-evolved conditions was much higher than that between the two mono-evolved conditions (P=2.628e-12, t=−10.022, df=38.695 via two-tailed t-test), and the similarity between Bs_root_A and two cyclic conditions was also much lower than the similarity between Bs_root_T and two cyclic conditions (Fig. 5). These results strongly indicate that the selection pressure imposed by the A. thaliana was much weaker than observed with tomato, and the tomato root was potentially the main selective factor in the two cyclic-evolved conditions.
We observed high parallelism from root colonization ability and morphotype to functional pathway and gene levels, as well as extensive parallelism at the level of individual nucleotides (Supplementary Material 1, available in the online version of this article). Strikingly, 18 of 20 lineages contained a frameshift in the flhB gene required for flagellum and nanotube assembly [39]. Another 8 bp deletion found in the yuxH gene, involved in c-di-GMP regulation in B. subtilis [40, 41], was detected in 15 lineages. Additional high-parallelism mutations at the nucleotide level, present in more than 10 lineages and belonging to non-synonymous mutations, occurred in hag and sigD genes. Notably, all mutations mentioned above reached a high frequency from 12.9 –68.53 % in the evolution conditions. We did not identify these mutations in the ancestor strain stock below a frequency of 5 %, but we cannot rule out that they were already acquired in the overnight culture used to start the experimental evolution.
B. subtilis adapts specifically towards different plant species
The lower mutation number (frequency >10 %), lower genotype diversity and lower genetic similarity of Bs_root_A strongly imply that the adaptation strategies between Bs_root_A and the other three conditions differed. Therefore, we focused on genes with a mutation frequency >10 % and categorized them based on Encyclopedia of Genes and Genomes (KEGG) pathways. Overall, bacterial motility proteins, genetic information processing and metabolism-related genes were mutated more frequently and reached high frequencies, accounting for 61.36 % of mutated genes (frequency >10 %).
As mentioned above, bacterial motility proteins were the most selected in our experiment. Among the revealed motility protein genes, 8 of 11 were flagellar structure protein-coding genes (Fig. 6) [42], and could be categorized into filament-coding genes (flagellin, hag), basal body-coding genes (fliF, fliG, fliM and fliY) and flagellar type III secretion (T3S) apparatus protein genes (fliI, fliR, and flhB) [42]. Although bacterial motility genes were targeted frequently in all conditions, fliI and fliR were only mutated in Bs_root_A, and mutations in fliG, fliY and hag were only absent in Bs_root_A. FliI is a flagellar-specific ATPase functioning in the export of flagellar proteins [43], and FliR is part of the T3S apparatus [44] required for flagellum and nanotube assembly. Another gene required for flagellum and nanotube assembly, flhB [39], was mutated in all evolved conditions. FliG is involved directly in flagellar rotation [45], FliY interacts with the chemotaxis system and controls the direction of flagellar rotation [42, 46] and hag encodes the flagellin monomer protein Hag, which is also essential for flagellar assembly [47, 48]. The sigma factor-encoding gene sigD, which regulates flagella, motility, chemotaxis and autolysis in B. subtilis [48–50], was mutated in all except Bs_root_A samples, while yuxH (pdeH) encoding a phosphodiesterase that degrades c-di-GMP was mutated in all conditions and at relatively high frequencies. c-di-GMP is a second messenger that regulates diverse cellular processes in bacteria, including bacterial motility, biofilm formation, cell–cell signalling and host colonization [40, 41]. Overall, species-specific and common mutations in both flagellar structural proteins and motility regulator proteins were identified, which were induced by species-specific evolution stress imposed by different plant species and the same static hydroponic setup.
Fig. 6.
Parallelism across different experimental setups. (a) Genes with mutation frequency >10 % in this study. (b) Mutated genes shared across different experimental setups. Each column represents one replicate lineage. In each lineage, the colour represents the highest frequency of mutations in that particular gene. Detailed information for these genes and mutations in (a) can be found in Supplementary Material 1.
Mutations in biofilm development gene sinR, encoding a DNA-binding protein that regulates the expression of epsA-O and tapA-sipW-tasA operons involved in matrix production [51, 52], was only detected transiently with low frequency (maximum 7.19 %) in Bs_root_T (Figs 4 and 6 and Supplementary Material 1), while it was observed more frequently and with higher frequency in the other conditions, suggesting an A. thaliana-specific mutation.
Another suspected A. thaliana-specific mutated gene, pstBA, encoding a phosphate transport system ATP-binding protein, was only absent in the Bs_root_T sample, and transiently with low frequency in the two cyclic-evolved conditions, but it was detected at high frequency in three of five lineages in Bs_root_A. The pstBA gene belongs to the five-gene pst operon, and it encodes a member of the PhoP–PhoR two-component signal transduction system [53–55] responsible for phosphate uptake under nutrient stress in B. subtilis [55], while the pst operon is alkali-inducible [53].
Discussion
We explored whether plant-colonizing bacteria adapt differently in an alternating host regime compared with the same plant used throughout an experiment, and whether a bacterial species adapts differently to different plant hosts. To do this, experimental evolution and whole-population WGS sequencing were employed to analyse how B. subtilis evolves on A. thaliana and tomato seedlings, and when these two plant hosts were alternated in a static hydroponic setup [27]. The results did not provide a simple binary answer to the first question, and we observed species-specific adaptation patterns at the genetic level.
Similar to several previous studies [23, 24, 27], B. subtilis showed rapid improvement in root colonization ability, albeit at varying rates, as three distinct morphotypes during adaptation in all evolved lineages and conditions. Additionally, the three distinct morphotypes coexisted until the end of the evolution experiment, reaching highly similar ratios in all four evolution experiments. More significantly, complementary effects were observed during root colonization of A. thaliana seedlings through mixing of the three morphotypes from Bs_root_A [27]. Both root colonization improvement and morphotypic diversification displayed a remarkable degree of parallelism across all evolved conditions. Furthermore, all but one randomly selected isolate from Bs_root_A and Bs_root_T showed impaired swimming and swarming motility. Populations from two host cyclic-evolved conditions shared numerous mutated genes with Bs_root_T, also suggesting similar trade-offs in the alternating plant setup. Analysis of the genetic basis underlying the two levels of parallelism (morphotypes and altered motility) revealed bacterial motility proteins, genetic information processing and metabolism-related genes to be mutated more frequently and reaching high frequencies in all evolved conditions. Besides parallelism at phenotypic and functional levels, mutational resemblance was observed in certain genes, even at the individual nucleotide level. Two genes, flhB and yuxH, showed striking parallelism at both gene and individual nucleotide levels; 18 of 20 lineages contained a frameshift in the flhB gene and 15 lineages contained an 8 bp deletion in yuxH. The flhB gene is required for flagellum and nanotube assembly and yuxH is involved in c-di-GMP regulation in B. subtilis [40, 41], and both may contribute to adaptation of B. subtilis to plant roots by affecting motility. However, reintroduction of single mutations alone or in combination will be necessary in the future to precisely measure the influence of these mutations on fitness. Herein, we identified repeated changes across multiple levels of biological organization from phenotypic functional pathways and genes to individual nucleotides [37], demonstrating the overlapping evolutionary patterns employed by B. subtilis when adapting to A. thaliana and tomato roots, as well as roots in an alternating regime.
The static hydroponic setup used in our study selects for regular root recolonization by bacterial cells on different plant species, including bacterial traits of chemotaxis, biofilm formation and dispersal [27]. Different histidine kinases influence the phosphorylation level of the global regulator Spo0A [15] when B. subtilis forms a biofilm on the roots of A. thaliana [16] and tomato [17], and different Bacillus species have distinct host plant colonization abilities [19]. Based on previous results, we hypothesized that B. subtilis adaptation to A. thaliana or tomato will display distinct gene-level attributes underpinning species-specific adaptation. Although parallelism was identified at phenotypic and nucleotide change levels, obvious differences were detected in the number of mutations (at a frequency >10 %), genotype diversity, mutated genes and genetic similarity between and among different experimental conditions.
Fewer mutations (at a frequency >10 %), lower genotype diversity and lower within-conditions genetic similarity were observed in mono-evolved conditions, especially in Bs_root_A. The difference between mono-evolved and cyclic-evolved conditions could be explained by a more heterogeneous, multi-resource, spatially structured environment created by host cycling, suggesting that environmental heterogeneity might have an important influence on adaptation [34, 37, 56]. When Pseudomonas fluorescens was evolved in five different environments varying in terms of resource type and arrangement, more mutations and a higher degree of parallelism were observed at the gene level in the most heterogeneous environment [37]. Similarly, evolution of Pseudomonas aeruginosa populations on plastic beads that favour a biofilm lifestyle exhibited higher morphological diversity and mutation rates than planktonic cultures [57]. Consistent with previous studies, the degree of parallel evolution was significantly higher in B. subtilis populations evolved in a heterogeneous, multi-resource, spatially structured environment in the present work.
Bs_root_A populations harboured the fewest mutations and showed the least similarity among these, even lower than Bs_root_T samples. Additionally, genetic similarity between the two mono-evolved conditions was much lower than between the two cyclic-evolved conditions, and similarity between Bs_root_A and the two cyclic conditions was also much lower than that between Bs_root_T and the two cyclic conditions. The lowest genetic similarity within Bs_root_A suggests that the fitness landscape in the A. thaliana evolving environment might have been more rugged than in tomato [58]. Furthermore, several specific mutated genes were also observed, which were present or absent exclusively in one plant species. These results imply that the adaptation patterns of B. subtilis to roots of A. thaliana and tomato differed, and that A. thaliana exerted less influence on the adaptation process in the two cyclic-evolved conditions. A potential key difference between A. thaliana and tomato that might alter bacterial evolution is the composition and amount of exudates. In our experimental system, B. subtilis was evolved in minimal salt medium (MSM; see Methods), in which bacterial cells are dependent on carbon sources provided by the plant for their growth. The composition and amount of exudates in A. thaliana [59] and tomato [60] vary depending on growth stage.
We found that the most targeted genes were related to motility, while all lineages displayed improved root colonization. Motility and biofilm formation are considered to be inversely regulated lifestyles during which bacteria express genes necessary for either motility or biofilm matrix production, but not both simultaneously [61, 62]. Motility is vital for many bacteria, as this process enables them to explore resources and supports the dispersal of progeny [63]. Motility is important for the effective root colonization of different plant species by B subtilis under different conditions [18–21]. It is not surprising that the loss of motility is repeatedly evolved in populations growing in well-mixed liquid media where motility is not essential [37], and where the production of proteins for motility, primarily for flagella, is costly in terms of energy and resources [64]. A motility–biofilm trade-off has been suggested for B. subtilis evolved on A. thaliana roots under shaking conditions [24]. Surprisingly, all mutations in flagellar structure protein-coding genes were non-synonymous SNPs or InDels, suggesting that these mutations may cause loss of function of flagella. Notably, a 78 bp duplication (23 amino acids, residues 133–155) occurred in hag in three evolved conditions (12 lineages) and reached a high frequency, which would strongly affect the structure and function of flagellin, and therefore cell motility. Additionally, widespread and high-frequency mutations in sinR encoding a biofilm master regulator [65], sigD-encoding sigma factor D [48–50], which regulates flagella, motility, chemotaxis and autolysis of B. subtilis , yuxH, which is involved in c-di-GMP regulation in B. subtilis [40, 41], and pstBA, which is responsible for phosphate uptake under nutrient stress in B. subtilis [55], were all predicted to regulate the motility–biofilm balance in B. subtilis , indicating that pleiotropy is widespread and can influence the adaptation of B. subtilis .
Finally, we compared the genetic similarities and differences in specific biological functions, including aerotaxis, chemotaxis, motility (regulation) and biofilm development, when B. subtilis was evolved under different experimental conditions, including a static air–medium pellicle biofilm transfer mode (Bs_pellicle) [66], an A. thaliana root colonization study under a shaking hydroponic setup (Bs_root) [24] and plant root colonization under a static hydroponic setup (Bs_root_ATTA; Fig. 6b). Both common and condition-specific genes were found to carry mutations. We found that hemAT was mutated in both Bs_pellicle and Bs_root_ATTA systems, although less frequently in Bs_root_ATTA. Importantly, both experiments were performed under static conditions, where an oxygen gradient is generated in the culture, and HemAT is a key oxygen sensor that is important during pellicle formation [50]. The lower frequency of mutation in hemAT detected in Bs_root_ATTA suggests lower selection for aerotaxis in cells that colonize the submerged parts of plant roots compared with cells localized at the air–medium interface. Interestingly, the sigma factor-encoding gene sigD was only mutated in conditions with tomato serving as host in Bs_root_ATTA, suggesting that it is a tomato-specific mutational target gene. Flagella-related genes were found to be mutated in all three conditions, although mutations were detected in different genes and with different frequencies. Furthermore, the c-di-GMP-degrading phosphodiesterase-encoding pdeH was mutated in all three conditions, and more frequently in Bs_root_ATTA. The c-di-GMP second messenger modulates diverse cellular activities in bacteria, including bacterial motility, biofilm formation, cell–cell signalling and host colonization [40, 41]. However, previous studies on c-di-GMP signalling have mostly focused on Gram-negative bacteria. The sinR gene appears to be a target in plant-specific conditions because it only lacked mutations in Bs_pellicle samples. Another possible static condition-specific target similar to hemAT is pstBA, which is involved in high-affinity phosphate uptake in B. subtilis [55].
In conclusion, our study revealed parallel evolution across multiple levels of biological organization from phenotypic to individual nucleotide levels, while the degree of parallel evolution at the gene level was significantly higher in populations evolved in a heterogeneous, multi-resource, spatially structured environment. We also observed species-specific adaptation at the genetic level, possibly caused by selection stress imposed by different host plants. Additionally, a strong trade-off between motility and growth as well as biofilm formation was observed under the static selection conditions applied herein. Finally, we identified several condition-specific and common mutated genes by comparing genetic similarities and differences between three studies.
Supplementary Data
Funding information
This project was supported by the China National GeneBank (CNGB), the Danish National Research Foundation (DNRF137) for the Center for Microbial Secondary Metabolites, and Novo Nordisk Foundation within the INTERACT project of the Collaborative Crop Resiliency Programme (NNF19SA0059360).
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: Bs_root_A, Bacillus subtilis evolved on Arabidopsis thaliana root only; Bs_root_AT, Bacillus subtilis evolved on Arabidopsis thaliana and Tomato root in an alternating regime and started on Arabidopsis thaliana root; Bs_root_T, Bacillus subtilis evolved on Tomato root only; Bs_root_TA, Bacillus subtilis evolved on Arabidopsis thaliana and Tomato root in an alternating regime and started on Tomato root; CFU, colony forming unit; InDel, short insertions and deletions; OD, optical density; PGPR, plant growth-promoting rhizobacteria; SM, smooth; SN, snow; SNP, single nucleotide polymorphism; WR, wrinkled.
All supporting data, code and protocols have been provided within the article or through supplementary data files. One supplementary file is available with the online version of this article.
References
- 1.Guo X, Chen F, Gao F, Li L, Liu K, et al. CNSA: a data repository for archiving omics data. Database. 2020;2020:baaa055. doi: 10.1093/database/baaa055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen FZ, You LJ, Yang F, Wang LN, Guo XQ, et al. CNGBdb: China National GeneBank DataBase. Hereditas. 2020;42:799–809. doi: 10.16288/j.yczz.20-080. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt JE, Bowles TM, Gaudin ACM. Using ancient traits to convert soil health into crop yield: impact of selection on maize root and rhizosphere function. Front Plant Sci. 2016;7:373. doi: 10.3389/fpls.2016.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haichar FEZ, Heulin T, Guyonnet JP, Achouak W. Stable isotope probing of carbon flow in the plant holobiont. Curr Opin Biotechnol. 2016;41:9–13. doi: 10.1016/j.copbio.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 5.Venturi V, Keel C. Signaling in the Rhizosphere. Trends Plant Sci. 2016;21:187–198. doi: 10.1016/j.tplants.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Sasse J, Martinoia E, Northen T. Feed your friends: do plant exudates shape the root microbiome? Trends Plant Sci. 2018;23:25–41. doi: 10.1016/j.tplants.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Lareen A, Burton F, Schäfer P. Plant root-microbe communication in shaping root microbiomes. Plant Mol Biol. 2016;90:575–587. doi: 10.1007/s11103-015-0417-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vives-Peris V, de Ollas C, Gómez-Cadenas A, Pérez-Clemente RM. Root exudates: from plant to rhizosphere and beyond. Plant Cell Rep. 2020;39:3–17. doi: 10.1007/s00299-019-02447-5. [DOI] [PubMed] [Google Scholar]
- 9.Arnaouteli S, Bamford NC, Stanley-Wall NR, Kovács ÁT. Bacillus subtilis biofilm formation and social interactions. Nat Rev Microbiol. 2021;19:600–614. doi: 10.1038/s41579-021-00540-9. [DOI] [PubMed] [Google Scholar]
- 10.Blake C, Christensen MN, Kovács ÁT. Molecular aspects of plant growth promotion and protection by Bacillus subtilis . Mol Plant Microbe Interact. 2021;34:15–25. doi: 10.1094/MPMI-08-20-0225-CR. [DOI] [PubMed] [Google Scholar]
- 11.Peng G, McGregor L, Lahlali R, Gossen BD, Hwang SF, et al. Potential biological control of clubroot on canola and crucifer vegetable crops. Plant Pathol. 2011;60:566–574. doi: 10.1111/j.1365-3059.2010.02400.x. [DOI] [Google Scholar]
- 12.Wei F, Hu X, Xu X. Dispersal of Bacillus subtilis and its effect on strawberry phyllosphere microbiota under open field and protection conditions. Sci Rep. 2016;6:22611. doi: 10.1038/srep22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badri DV, Weir TL, van der Lelie D, Vivanco JM. Rhizosphere chemical dialogues: plant-microbe interactions. Curr Opin Biotechnol. 2009;20:642–650. doi: 10.1016/j.copbio.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Hamon MA, Lazazzera BA. The sporulation transcription factor Spo0A is required for biofilm development in Bacillus subtilis . Mol Microbiol. 2001;42:1199–1209. doi: 10.1046/j.1365-2958.2001.02709.x. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Shao W, Perego M, Hoch JA. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis . Mol Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Cao S, Chai Y, Clardy J, Kolter R, et al. A Bacillus subtilis sensor kinase involved in triggering biofilm formation on the roots of tomato plants. Mol Microbiol. 2012;85:418–430. doi: 10.1111/j.1365-2958.2012.08109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauregard PB, Chai Y, Vlamakis H, Losick R, Kolter R. Bacillus subtilis biofilm induction by plant polysaccharides. Proc Natl Acad Sci. 2013;110:E1621–30. doi: 10.1073/pnas.1218984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian T, Sun B, Shi H, Gao T, He Y, et al. Sucrose triggers a novel signaling cascade promoting Bacillus subtilis rhizosphere colonization. ISME J. 2021;15:2723–2737. doi: 10.1038/s41396-021-00966-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Wang D, Liu Y, Li S, Shen Q, et al. Effects of different plant root exudates and their organic acid components on chemotaxis, biofilm formation and colonization by beneficial rhizosphere-associated bacterial strains. Plant Soil. 2014;374:689–700. doi: 10.1007/s11104-013-1915-6. [DOI] [Google Scholar]
- 20.Gao S, Wu H, Yu X, Qian L, Gao X. Swarming motility plays the major role in migration during tomato root colonization by Bacillus subtilis SWR01. Biological Control. 2016;98:11–17. doi: 10.1016/j.biocontrol.2016.03.011. [DOI] [Google Scholar]
- 21.Allard-Massicotte R, Tessier L, Lécuyer F, Lakshmanan V, Lucier J-F, et al. Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio. 2016;7:e01664-16. doi: 10.1128/mBio.01664-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenski RE. What is adaptation by natural selection? Perspectives of an experimental microbiologist. PLoS Genet. 2017;13:e1006668. doi: 10.1371/journal.pgen.1006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Alstrup M, Pang JKY, Maróti G, Er-Rafik M, et al. Adaptation of Bacillus thuringiensis to plant colonization affects differentiation and toxicity. mSystems. 2021;6:e0086421. doi: 10.1128/mSystems.00864-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordgaard M, Blake C, Maróti G, Hu G, Wang Y, et al. Experimental evolution of Bacillus subtilis on Arabidopsis thaliana roots reveals fast adaptation and improved root colonization. iScience. 2022;25:104406. doi: 10.1016/j.isci.2022.104406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li E, Zhang H, Jiang H, Pieterse CMJ, Jousset A, et al. Experimental-evolution-driven identification of Arabidopsis Rhizosphere competence genes in pseudomonas protegens. mBio. 2021;12:e0092721. doi: 10.1128/mBio.00927-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li M, Guo J, Ren T, Luo G, Shen Q, et al. Crop rotation history constrains soil biodiversity and multifunctionality relationships. Agric Ecosyst Environ. 2021;319:107550. doi: 10.1016/j.agee.2021.107550. [DOI] [Google Scholar]
- 27.Blake C, Nordgaard M, Maróti G, Kovács ÁT. Diversification of Bacillus subtilis during experimental evolution on Arabidopsis thaliana and the complementarity in root colonization of evolved subpopulations. Environ Microbiol. 2021;23:6122–6136. doi: 10.1111/1462-2920.15680. [DOI] [PubMed] [Google Scholar]
- 28.Chen A, Liao S, Cheng M, Ma K, Wu L, et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell. 2022;185:1777–1792. doi: 10.1016/j.cell.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Han L, Wei X, Liu C, Volpe G, Zhuang Z, et al. Cell transcriptomic atlas of the non-human primate Macaca fascicularis . Nature. 2022;604:723–731. doi: 10.1038/s41586-022-04587-3. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Chen Y, Shi C, Huang Z, Zhang Y, et al. SOAPnuke: a MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience. 2018;7:1–6. doi: 10.1093/gigascience/gix120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deatherage DE, Barrick JE. Identification of mutations in laboratory-evolved microbes from next-generation sequencing data using breseq. Methods Mol Biol. 2014;1151:165–188. doi: 10.1007/978-1-4939-0554-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barrick JE, Colburn G, Deatherage DE, Traverse CC, Strand MD, et al. Identifying structural variation in haploid microbial genomes from short-read resequencing data using breseq. BMC Genomics. 2014;15:1039. doi: 10.1186/1471-2164-15-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scribner MR, Santos-Lopez A, Marshall CW, Deitrick C, Cooper VS. Parallel evolution of tobramycin resistance across species and environments. mBio. 2020;11:e00932-20. doi: 10.1128/mBio.00932-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harris KB, Flynn KM, Cooper VS. Polygenic adaptation and clonal interference enable sustained diversity in experimental Pseudomonas aeruginosa populations. Mol Biol Evol. 2021;38:5359–5375. doi: 10.1093/molbev/msab248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovács ÁT, Grau R, Pollitt EJG. Surfing of bacterial droplets: Bacillus subtilis sliding revisited. Proc Natl Acad Sci. 2017;114:E8802. doi: 10.1073/pnas.1710371114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hölscher T, Kovács ÁT. Sliding on the surface: bacterial spreading without an active motor. Environ Microbiol. 2017;19:2537–2545. doi: 10.1111/1462-2920.13741. [DOI] [PubMed] [Google Scholar]
- 37.Bailey SF, Rodrigue N, Kassen R. The effect of selection environment on the probability of parallel evolution. Mol Biol Evol. 2015;32:1436–1448. doi: 10.1093/molbev/msv033. [DOI] [PubMed] [Google Scholar]
- 38.Bailey SF, Blanquart F, Bataillon T, Kassen R. What drives parallel evolution?: How population size and mutational variation contribute to repeated evolution. Bioessays. 2017;39:1–9. doi: 10.1002/bies.201600176. [DOI] [PubMed] [Google Scholar]
- 39.Kuhlen L, Johnson S, Zeitler A, Bäurle S, Deme JC, et al. The substrate specificity switch FlhB assembles onto the export gate to regulate type three secretion. Nat Commun. 2020;11:1296. doi: 10.1038/s41467-020-15071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Y, Chai Y, Guo J, Losick R. Evidence for cyclic Di-GMP-mediated signaling in Bacillus subtilis. J Bacteriol. 2012;194:5080–5090. doi: 10.1128/JB.01092-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao X, Mukherjee S, Matthews PM, Hammad LA, Kearns DB, et al. Functional characterization of core components of the Bacillus subtilis cyclic-di-GMP signaling pathway. J Bacteriol. 2013;195:4782–4792. doi: 10.1128/JB.00373-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mukherjee S, Kearns DB. The structure and regulation of flagella in Bacillus subtilis . Annu Rev Genet. 2014;48:319–340. doi: 10.1146/annurev-genet-120213-092406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ibuki T, Imada K, Minamino T, Kato T, Miyata T, et al. Common architecture of the flagellar type III protein export apparatus and F- and V-type ATPases. Nat Struct Mol Biol. 2011;18:277–282. doi: 10.1038/nsmb.1977. [DOI] [PubMed] [Google Scholar]
- 44.Kuhlen L, Abrusci P, Johnson S, Gault J, Deme J, et al. Author correction: structure of the core of the type III secretion system export apparatus. Nat Struct Mol Biol. 2018;25:743. doi: 10.1038/s41594-018-0095-8. [DOI] [PubMed] [Google Scholar]
- 45.Ward E, Kim EA, Panushka J, Botelho T, Meyer T, et al. Organization of the flagellar switch complex of Bacillus subtilis. J Bacteriol. 2019;201:e00626-18. doi: 10.1128/JB.00626-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sircar R, Greenswag AR, Bilwes AM, Gonzalez-Bonet G, Crane BR. Structure and activity of the flagellar rotor protein FliY: a member of the CheC phosphatase family. J Biol Chem. 2013;288:13493–13502. doi: 10.1074/jbc.M112.445171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaVallie ER, Stahl ML. Cloning of the flagellin gene from Bacillus subtilis and complementation studies of an in vitro-derived deletion mutation. J Bacteriol. 1989;171:3085–3094. doi: 10.1128/jb.171.6.3085-3094.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hölscher T, Schiklang T, Dragoš A, Dietel A-K, Kost C, et al. Impaired competence in flagellar mutants of Bacillus subtilis is connected to the regulatory network governed by DegU. Environ Microbiol Rep. 2018;10:23–32. doi: 10.1111/1758-2229.12601. [DOI] [PubMed] [Google Scholar]
- 49.Márquez LM, Helmann JD, Ferrari E, Parker HM, Ordal GW, et al. Studies of σD-dependent functions in Bacillus subtilis . J Bacteriol. 1990;172:3435–3443. doi: 10.1128/jb.172.6.3435-3443.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hölscher T, Bartels B, Lin Y-C, Gallegos-Monterrosa R, Price-Whelan A, et al. Motility, chemotaxis and aerotaxis contribute to competitiveness during bacterial pellicle biofilm development. J Mol Biol. 2015;427:3695–3708. doi: 10.1016/j.jmb.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearns DB. A field guide to bacterial swarming motility. Nat Rev Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- 53.Atalla A, Schumann W. The pst operon of Bacillus subtilis is specifically induced by alkali stress. J Bacteriol. 2003;185:5019–5022. doi: 10.1128/JB.185.16.5019-5022.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allenby NEE, O’Connor N, Prágai Z, Carter NM, Miethke M, et al. Post-transcriptional regulation of the Bacillus subtilis pst operon encoding a phosphate-specific ABC transporter. Microbiology. 2004;150:2619–2628. doi: 10.1099/mic.0.27126-0. [DOI] [PubMed] [Google Scholar]
- 55.Moreno-Letelier A, Olmedo G, Eguiarte LE, Martinez-Castilla L, Souza V. Parallel evolution and horizontal gene transfer of the pst operon in firmicutes from oligotrophic environments. Int J Evol Biol. 2011;2011:781642. doi: 10.4061/2011/781642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van den Bergh B, Swings T, Fauvart M, Michiels J. Experimental design, population dynamics, and diversity in microbial experimental evolution. Microbiol Mol Biol Rev. 2018;82:e00008-18. doi: 10.1128/MMBR.00008-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flynn KM, Dowell G, Johnson TM, Koestler BJ, Waters CM, et al. Evolution of ecological diversity in biofilms of Pseudomonas aeruginosa by altered cyclic diguanylate signaling. J Bacteriol. 2016;198:2608–2618. doi: 10.1128/JB.00048-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferretti L, Weinreich D, Tajima F, Achaz G. Evolutionary constraints in fitness landscapes. Heredity. 2018;121:466–481. doi: 10.1038/s41437-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chaparro JM, Badri DV, Bakker MG, Sugiyama A, Manter DK, et al. Root exudation of phytochemicals in arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS One. 2013;8:e55731. doi: 10.1371/journal.pone.0055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kravchenko LV, Azarova TS, Leonova-Erko EI, Shaposhnikov AI, Makarova NM, et al. Tomato root exudates and their effect on the growth and antifungal activity of Pseudomonas strains. Mikrobiology. 2003;72:48–53. [PubMed] [Google Scholar]
- 61.Guttenplan SB, Kearns DB. Regulation of flagellar motility during biofilm formation. FEMS Microbiol Rev. 2013;37:849–871. doi: 10.1111/1574-6976.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Ditmarsch D, Boyle KE, Sakhtah H, Oyler JE, Nadell CD, et al. Convergent evolution of hyperswarming leads to impaired biofilm formation in pathogenic bacteria. Cell Rep. 2013;4:697–708. doi: 10.1016/j.celrep.2013.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wadhwa N, Berg HC. Bacterial motility: machinery and mechanisms. Nat Rev Microbiol. 2022;20:161–173. doi: 10.1038/s41579-021-00626-4. [DOI] [PubMed] [Google Scholar]
- 64.Keegstra JM, Carrara F, Stocker R. The ecological roles of bacterial chemotaxis. Nat Rev Microbiol. 2022;20:491–504. doi: 10.1038/s41579-022-00709-w. [DOI] [PubMed] [Google Scholar]
- 65.Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- 66.Dragoš A, Lakshmanan N, Martin M, Horváth B, Maróti G, et al. Evolution of exploitative interactions during diversification in Bacillus subtilis biofilms. FEMS Microbiol Ecol. 2018;93 doi: 10.1093/femsec/fix155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.