Abstract
The most common form of congenital adrenal hyperplasia is 21-hydroxylase deficiency (21OHD), which in the classic (severe) form occurs in roughly 1:16 000 newborns worldwide. Lifelong treatment consists of replacing cortisol and aldosterone deficiencies, and supraphysiological dosing schedules are typically employed to simultaneously attenuate production of adrenal-derived androgens. Glucocorticoid titration in 21OHD is challenging as it must balance the consequences of androgen excess vs those from chronic high glucocorticoid exposure, which are further complicated by interindividual variability in cortisol kinetics and glucocorticoid sensitivity. Clinical assessment and biochemical parameters are both used to guide therapy, but the specific purpose and goals of each biomarker vary with age and clinical context. Here we review the approach to medication titration for children and adults with classic 21OHD, with an emphasis on how to interpret adrenal biomarker values in guiding this process. In parallel, we illustrate how an understanding of the pathophysiologic and pharmacologic principles can be used to avoid and to correct complications of this disease and consequences of its management using existing treatment options.
Keywords: congenital adrenal hyperplasia, 11-oxygenated androgens, 17-hydroxyprogesterone
Case Presentations
Case 1—Pediatric
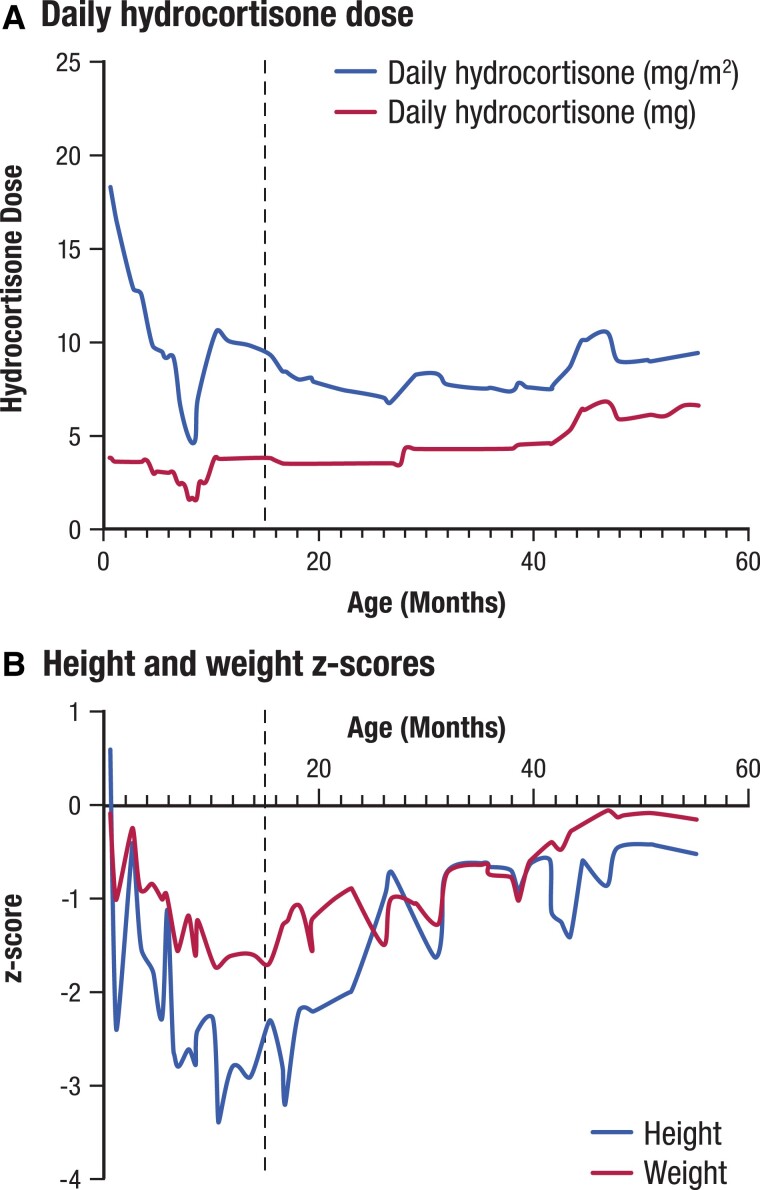
A 15-month-old male with congenital adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency (21OHD) presented with a history of failure to thrive, oversuppression of the hypothalamic-pituitary-adrenal (HPA) axis and repeated periods of hypoglycemia. He was born full term at 3.15 kg weight with an uncomplicated neonatal course. He developed salt-wasting and an adrenal crisis on day 5 of life with a serum sodium level of 127 mmol/L (normal range: 133-143 mmol/L) and a weight of 2.67 kg (a loss of 15.3% of birth weight). The newborn screening result on day 6 of life was positive for 21OHD, and confirmatory testing revealed markedly elevated 17-hydroxyprogesterone (17OHP), androstenedione (A4), adrenocorticotropic hormone (ACTH), and plasma renin activity (PRA) values (Table 1). At that time, the diagnosis of 21OHD was made, and he received treatment with 1.26 mg 3 times daily of hydrocortisone (as a 2 mg/mL suspension, 20 mg/m2/day), fludrocortisone 0.15 mg daily, and sodium chloride 4 mEq/kg/day. Weight at discharge was 2.925 kg (5th percentile, z-score −1.65) and length was 50.5 cm (22nd percentile, z-score −0.78). The patient's hydrocortisone dose and growth over time are presented in Figure 1.
Table 1.
Case 1 laboratory values
| Patient age | ACTH, pmol/L (pg/mL) | 17OHP, nmol/L (ng/dL) | A4, nmol/L (ng/dL) | Testosterone, nmol/L (ng/dL) | PRA, ng/mL/hr |
|---|---|---|---|---|---|
| 6 days | 153 (696) | 718 (23 700) | 86 (2450) | — | 46.7 |
| 5 weeks | 18 (80) | 36 (1180) | 1.3 (38) | 1.5 (43) | 8.7 |
| 3.5 months | <LLOD | 1.2 (38) | <LLOD | — | <LLOD |
| 8 months | — | 246a (8120a) | — | — | 11 |
| 9 months | 14 (63) | 542 (17 888) | 1.7 (48) | 0.7 (21) | 25 |
| 14 months | 2 (10) | 12 (402) | <LLOD | 0.1 (2.7) | 9.7 |
| 15 months | — | 49 (1630) | <LLOD | 0.1 (3) | 13 |
“–” indicates laboratory test was not performed at that visit.
Before morning hydrocortisone dose; 17OHP value after the morning dose was 143 nmol/L (4730 ng/dL).
Normal ranges are as follows: ACTH, 0-9 years, 7.2-63 pg/mL; 17OHP, 0-28 days, < 630 ng/dL; 17OHP, 6 months-9 years, < 110 ng/dL; A4, 1-7 days, 20-290 ng/dL; A4, 1 month-1 year, < 69 ng/dL; A4, 1-9 years, < 51 ng/dL; total testosterone, 0-5 months, 75-400 ng/dL; total testosterone, 6 months-9 years, < 7-20 ng/dL; PRA, 0-2 years, 1.4-7.8 ng/mL/h (from Mayo Clinic Laboratories Pediatric Catalog; https://www.mayocliniclabs.com/test-info/pediatric/refvalues/reference.php).
Abbreviations: 17OHP, 17-hydroxyprogesterone; A4, androstenedione; ACTH, adrenocorticotropic hormone; LLOD, lower limit of detection; PRA, plasma renin activity.
Figure 1.
Case 1 hydrocortisone dose and growth over time. The dotted line indicates measurements at first visit at our institution and a change in dosing regimen from reverse circadian to circadian hydrocortisone dosing.
At 3.5 months, he was noted to have failure to thrive (weight z-score −0.91 and height z-score −1.5). Follow-up serum 17OHP and A4 concentrations revealed oversuppression of the HPA axis, and PRA was not elevated (Table 1). His hydrocortisone dose formulation was switched from suspension to tablet at a dose of 0.83 mg administered every 8 hours at 7 Am, 3 Pm, and 11 Pm for a total daily dose of 7.1 mg/m2/day. The manipulation of a 5 mg tablet to achieve the 0.83 mg dose was to split in half a scored 5 mg tablet and then further divide the 2.5 mg half-tablet into 3 more pieces. Multiple manipulations of the hydrocortisone tablet made it highly unlikely that accurate dosing was being achieved. Growth failure continued despite reversal of HPA axis oversuppression (Fig. 1), as evidenced by markedly increased 17OHP concentration (Table 1). Hydrocortisone regimen was changed to a quarter tablet (1.25 mg) 3 times a day for a total daily dose 9.3 mg/m2/day. At 14 months of age, parents reported he had random and frequent episodes of lethargy and irritability associated with blood glucose <60 mg/dL (<3.3 mmol/L) determined by glucometer, which his mother treated with 1.25 mg additional doses of hydrocortisone. 17OHP, A4, and PRA remained well controlled, and evaluation for other causes of hypoglycemia was unrevealing.
At 15 months of age, he presented for a second opinion due to continued growth failure and hypoglycemia. At that time, his height was at the 1st percentile (z-score −2.3), and weight was at the 5th percentile (z-score −1.7). Initial evaluation showed elevated 17OHP, prepubertal testosterone, and undetectable A4 concentrations (Table 1).
Case 2—Adult
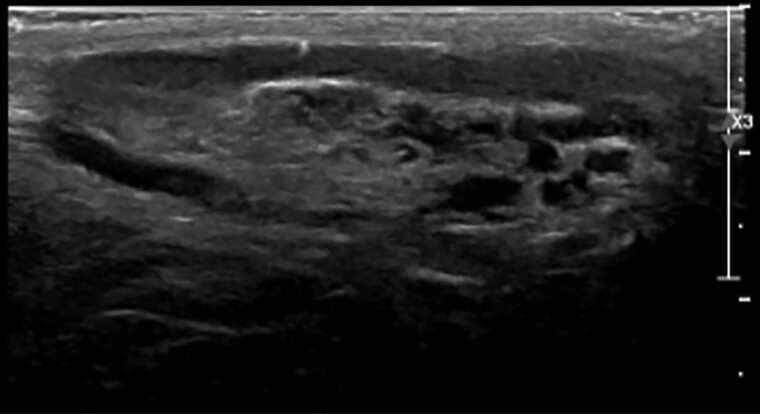
A 30-year-old male patient with classic 21OHD due to a homozygous I172N pathogenic variant in the CYP21A2 gene was referred for evaluation of infertility; his wife, who had normal hormonal evaluation, did not become pregnant despite frequent intercourse for 2 years. He was diagnosed with 21OHD at the age of 5 years due to precocious pubarche (Tanner Stage III for pubic hair), penile enlargement, accelerated growth velocity, and a bone age of 8 years with prepubertal testes size of 3 mL. There were no signs of salt wasting. He was treated with supraphysiological dosages of hydrocortisone 3 times a day. At age 16 years, having reached final adult height, which was 2 SD below his target height, he decided to stop his glucocorticoid (GC) treatment and had not taken any GC therapy since then. His testicular volumes at that time were 15 and 10 mL without palpable nodules. Scrotal ultrasound was not performed. At 30 years of age, he reported that during the 14 years without GC therapy he experienced no symptoms of adrenal insufficiency and that he did not require stress dosing during illness. On physical examination, he had a normal blood pressure and heart rate, slightly hyperpigmented skin, and mild gynecomastia. His testicular volumes were 10 and 8 mL and contained multiple firm, irregular palpable nodules. Ultrasound of the testes showed multinodular hypoechogenic lesions with a diameter of 2 to 3 cm with hyperechogenic reflections, all located in the rete testes (Fig. 2). Additional blood tests showed that his ACTH, A4, and 17OHP were significantly elevated (Table 2). He also had low gonadotropins and testosterone levels in the lower range with an A4/testosterone ratio of > 1. Estrogens were slightly elevated, most likely caused by the elevated adrenal androgen production and their aromatization to estrogens by aromatase, which led to suppression of the hypothalamic-pituitary-gonadal (HPG) axis.
Figure 2.
Ultrasound image of testicular adrenal rest tumor.
Table 2.
Case 2 laboratory values
| Analyte | Patient value | Normal rangea |
|---|---|---|
| 17OHP, nmol/L (ng/dL) | 270 (8911) |
<6.6 (<220) |
| A4, nmol/L (ng/dL) | 50 (1433) |
1.4-5.2 (40-150) |
| ACTH, pmol/L (pg/mL) | 33 (150) |
2.2-13 (10-60) |
| Testosterone, nmol/L (ng/dL) | 9.8 (282) |
10-38 (291-1100) |
| LH, IU/L | <0.1 | 2-9 |
| FSH, IU/L | <0.1 | 1-7 |
| Renin, ng/mL/hr | 8.4 | 0.2-3.6 |
Normal ranges are from American Board of Internal Medicine Test Reference Ranges, January 2022; https://www.abim.org/Media/bfijryql/laboratory-reference-ranges.pdf.
Abbreviations: 17OHP, 17-hydroxyprogesterone; A4, androstenedione; ACTH, adrenocorticotropic hormone; FSH, follicle-stimulating hormone; LH, luteinizing hormone.
Normal and Disordered Adrenal Steroidogenesis in 21OHD
Basic principles of steroidogenesis and congenital adrenal hyperplasia
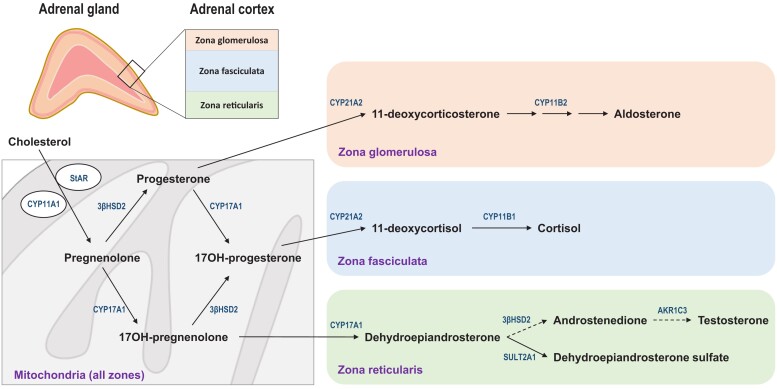
All steroids produced in the adrenal cortex derive from cholesterol, which is mobilized from a pool on the outer mitochondrial membrane. The steroidogenic acute regulatory protein (StAR) facilitates the transfer of this cholesterol to the side-chain cleavage enzyme (CYP11A1, P450 11A1), located on the inner mitochondrial membrane. Here, cholesterol is cleaved to pregnenolone, the first committed intermediate in human steroid biosynthesis. This first step is the point of quantitative regulation in steroidogenesis and the point of acute regulation, for example, by ACTH via its second messenger, cyclic adenosine monophosphate. Nascent pregnenolone then follows any of several pathways available in the specific adrenal zonae to aldosterone, cortisol, or dehydroepiandrosterone sulfate (DHEAS), the principal products of the zona glomerulosa, zona fasciculata, or zona reticularis, respectively (Fig. 3) (1). In the normal human adrenal, intermediates (precursors) do not significantly accumulate, and the production of theoretical side products, from aberrant combinations of enzyme activities off the major pathways, is minimal.
Figure 3.
Initiation of steroidogenesis and downstream metabolism in the 3 zones of the adrenal cortex. Cholesterol in the outer mitochondrial membrane is converted to pregnenolone via the side-chain cleavage enzyme CYP11A1 on the inner mitochondrial membrane in a process that requires the StAR for maximal efficiency. Nascent pregnenolone is either converted to progesterone via the 3βHSD2 or 17-hydroxylated via CYP17A1. In the zona glomerulosa, absence of CYP17A1 and presence of 21-hydroxylase (CYP21A2) and aldosterone synthase (CYP11B2) limits metabolism to the mineralocorticoids DOC and aldosterone. In the zona fasciculata, 17OHP is sequentially 21- and 11β-hydroxylated to cortisol, reactions that CYP21A2 and CYP11B1 catalyze, respectively. In the zona reticularis, pregnenolone metabolism is limited to CYP17A1, first to 17-hydroxypregneneolone and then to DHEA, the latter via the 17,20-lyase activity. Most DHEA is sulfonylated to DHEAS via the sulfotransferase SULT2A1, but lesser amounts of DHEA are converted to A4 and then via AKR1C3 to testosterone. Note that the pathways to cortisol and aldosterone—but not the pathway to A4—require CYP21A2. Abbreviations: 3βHSD2, 3β-hydroxysteroid dehydrogenase/isomerase type 2 enzyme; A4, androstenedione; AKR1C3, aldo-keto reductase type 1C3; DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; DOC, 11-deoxycorticosterone; StAR, steroidogenic acute regulatory protein.
In the various forms of CAH, a genetic deficiency in 1 of the enzymes required for cortisol biosynthesis creates several layers of dysregulation (2). The first consequence is “underfill,” the deficiency of cortisol and sometimes other steroids as well. The second consequence is “overflow,” the shunting of steroids into other pathways, due to mass action of ACTH-driven precursor accumulation. For example, elevated production of adrenal-derived androgens and/or mineralocorticoids is observed in various forms of CAH. The third consequence is “diversion,” the augmentation of aberrant or alternative pathways, which are minor in normal physiology but can become prominent in the presence of an enzyme deficiency with accumulation of intermediate steroids. These pathways can lead to either dead-end inactive products, traditional active steroids via alternate routes, or odd steroids with some biological activity. In most forms of CAH including 21OHD, all 3 forms of dysregulated steroidogenesis are observed. A prominent exception is congenital lipoid adrenal hyperplasia due to mutations in StAR and the similar syndrome rarely caused by CYP11A1 pathological variants, for which production of all steroids is reduced and no steroids are elevated (3).
Steroid 21-hydroxylase deficiency
Steroid 21-hydroxylase (CYP21A2, P450 21A2) occupies a central position in the biosynthesis of both aldosterone and cortisol. Because circulating aldosterone concentrations are generally 0.1 nmol/L or less, whereas plasma cortisol is closer to 100 nmol/L, aldosterone production is only impaired in complete enzyme deficiency. Alleles that retain 5% to 20% of enzyme activity give rise to nonclassic 21OHD, in which basic cortisol production is adequate to prevent clinical manifestations of adrenal insufficiency but still features slightly elevated adrenal precursors and androgen production. Classic 21OHD manifests with cortisol deficiency and much greater precursor accumulation (4, 5).
The many fates of 17-hydroxyprogesterone
The precursor steroid immediately before the CYP21A2 block in the cortisol pathway is 17OHP (Table 3), which has been traditionally used to establish the diagnosis of 21OHD. A basal or stimulated 17OHP >30 nmol/L (1000 ng/dL) establishes a diagnosis of 21OHD with very rare exceptions, and values in classic 21OHD are typically 10 to 100 times higher (6). Given its prominent position immediately prior to the 21-hydroxylation reaction, 17OHP has also been used as a key biomarker to monitor disease activity, particularly in children. Further upstream of 17OHP is progesterone, a very early intermediate in several pathways, and progesterone is increased in all forms of CAH except in congenital lipoid adrenal hyperplasia. The importance of progesterone as a biomarker in 21OHD is discussed in the section on female infertility.
Table 3.
Biomarkers in 21OHD: significance, uses, and limitations
| Steroid | Significance | Uses | Limitations |
|---|---|---|---|
| 17OHP | Immediately above block | Titrate GC | Varies widely from dose |
| 21dF | Purely adrenal steroid | Diagnosis | Mass spectrometry |
| A4 | Proximate 19-carbon steroid | Titrate GC | Also derives from gonads |
| Testosterone | Active androgen | Titrate GC | Also derives from gonads |
| 11OHA4 | Major adrenal product | Titrate GC | Limited data in management |
| 11KT | Purely adrenal androgen | Titrate GC | Limited data in management |
| Progesterone | Affects endometrium | Female fertility | Must be follicular phase |
| PRA/renin mass | Volume status | Titrate mineralocorticoid | Renal insufficiency |
Abbreviations: 11KT, 11-ketotestosterone; 11OHA4, 11β-hydroxyandrostenedione; 17OHP, 17-hydroxyprogesterone; 21dF, 21-deoxycortisol; 21OHD, 21-hydroxylase deficiency; A4, androstenedione; GC, glucocorticoid; PRA, plasma renin activity.
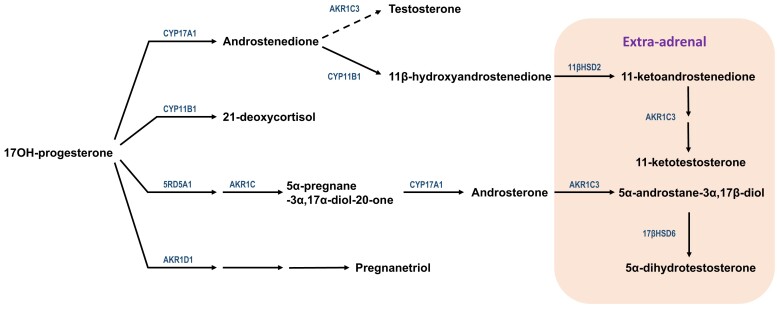
Rather than a dead-end product, 17OHP has at least 2 alternative conversion pathways besides 21-hydroxylation (Fig. 4). The first pathway is cleavage to A4—an “overflow” consequence—via the 17,20-lyase activity of CYP17A1 (P450 17A1), which is also the steroid 17-hydroxylase enzyme. Although the 17,20-lyase activity of human CYP17A1 with 17OHP as the substrate is poor, roughly 50 times less efficient than with 17-hydroxypregnenolone as the substrate (3), the accumulation of 17-hydroxysteroids drives A4 production as a proximate 19-carbon steroid.
Figure 4.
Potential fates of 17OHP in 21OHD. Top, canonical pathway to A4 via CYP17A1, though much less efficient than production of DHEA, can be driven by high concentrations of 17OHP that accumulate immediately prior to the enzymatic block. Some A4 is metabolized to testosterone via intra-adrenal and more by extra-adrenal AKR1C3 (dashed arrow angled up). The intra-adrenal 11β-hydroxylation of A4 via CYP11B1 yields 11OHA4 (arrow angled down), the first 11-oxygenated androgen product, which is oxidized outside the adrenal (shaded box, far right) via 11βHSD2 to 11KA4. Subsequently, 11KA4 is a much better substrate for AKR1C3 than A4, which yields 11KT. Second pathway from top, CYP11B1 also catalyzes the 11β-hydroxylation of 17OHP to 21dF. Third pathway from top, in the fetal adrenal and neonatal period, SRD5A1 and 1 or more AKR1C enzymes reduce 17OHP to 5α-pregnane-3α,17α-diol-20-one, the substrate that CYP17A1 cleaves to androsterone. Extra-adrenal metabolism of androsterone, most likely via AKR1C3 and the oxidative 3α-hydroxysteroid dehydrogenase activity of 17βHSD6 (shaded box, far right), affords DHT without the intermediacy of A4 or testosterone. Bottom pathway, 5β-reductase (AKR1D1) catalyzes the first of 3 steps in the catabolism of 17OHP to its major urinary metabolite, pregnanetriol. Abbreviations: 11βHSD2, 11β-hydroxysteroid dehydrogenase type 2; 11KA4, 11-ketoandrostenedione; 11OHA4, 11β-hydroxyandrostenedione; 17OHP, 17-hydroxyprogesterone; 17βHSD6, 17β-hydroxysteroid dehydrogenase type 6; 21dF, 21-deoxycortisol; 21OHD, 21-hydroxylase deficiency; A4, androstenedione; AKR1C3, aldo-keto reductase type 1C3; AKR1D1, aldo-keto reductase type 1D1 (5β-reductase); DHEA, dehydroepiandrosterone; DHEAS, dehydroepiandrosterone sulfate; DHT, 5α-dihydrotestosterone; SRD5A1, 5α-reductase type 1.
The second 17OHP metabolic pathway is 11-hydroxylation to 21-deoxycortisol (21dF), a steroid produced in only trace amounts from the normal adrenal and an example of the “diversion” consequence of the enzyme block. Despite its lower abundance than 17OHP, 21dF is a very discriminatory steroid, because 17OHP derives both from the adrenals and gonads, and 17OHP is elevated in some other forms of CAH. In contrast, the 11-hydroxysteroid 21dF is purely of adrenal origin, and 21dF is uniquely elevated in 21OHD (7). The theme that 11-hydroxylated steroids are exclusively of adrenal origin will recur for other metabolites later. Both 17OHP and 21dF have some GC activity (8) but not enough to rescue the phenotype in classic 21OHD.
At least in the fetal and neonatal adrenal, a third “diversion” pathway also occurs when 5α-reductase type 1 is expressed. The sequential 5α- and 3α-reduction 17OHP affords an excellent substrate for the 17,20-lyase activity of human CYP17A1 (9). The 19-carbon steroid product androsterone can be 17β-reduced in extra-adrenal tissues and 3α-oxidized in target tissues to DHT, the most potent known endogenous androgen. This alternative pathway to DHT bypasses A4 and testosterone as intermediates; hence the colloquial term “backdoor pathway” has been used to describe this flux to DHT (10). The several potential fates of 17OHP illustrates both the importance and the limitation of 17OHP as a barometer of disease control. When 17OHP is low, then little flux to androgens can occur; however, modest accumulation of 17OHP is not necessarily deleterious, particularly if the main flux of 17OHP in that patient is to 21dF and/or to catabolic pathways, primarily via 5β-reduction en route to its major urinary metabolite, pregnanetriol. In addition, circulating 17OHP varies markedly with time after GC dose, which adds another layer of complexity to interpretation.
Which adrenal androgens are important in 21OHD and how are these androgens produced?
If androgen biosynthesis followed the canonical adrenal pathway to 19-carbon steroids in the zona reticularis, one might predict that circulating DHEAS would be very high in classic 21OHD. Although DHEAS is often high-normal or modestly elevated in nonclassic 21OHD, DHEAS is not proportionately higher in classic 21OHD and is easily suppressed with GC treatment—even when A4 and testosterone are elevated. A4 varies less throughout the day than 17OHP and testosterone even less than A4. A4 is also a substrate for peripheral conversion to estrone and then estradiol, and estrogens are the primary if not sole mediator of bone age advancement. Because of the exquisite sensitivity of the skeleton to estrogens, bone age advancement can be observed even when estrogens cannot be reliably measured in the most sensitive clinical assays. Consequently, A4 has become a major biomarker of disease control in all forms of 21OHD.
In contrast to the limited intra-adrenal 17β-reduction of A4 to testosterone, A4 is an excellent substrate for the 11-hydroxylase enzyme CYP11B1 (P450 11B1). The product of this reaction, 11β-hydroxyandrostenedione (11OHA4), is the major unconjugated 19-carbon steroid in the circulation of adults with classic 21OHD (11) or untreated nonclassic 21OHD (12). In peripheral tissues, 11OHA4 is oxidized to 11-ketoandrostenedione, with the same enzyme 11β-hydroxysteroid dehydrogenase type 2 that converts cortisol to cortisone. The same aldo-keto reductase type 1C3 enzyme (also known as 17β-hydroxysteroid dehydrogenase type 5) that catalyzes the reduction of A4 to testosterone with poor efficiency in peripheral tissues also converts 11-ketoandrostenedione to the active androgen 11-ketotestosterone (11KT) and with substantially greater activity than the A4-to-testosterone reaction (Fig. 4) (13). Similar to the dominance of 11OHA4 over A4, 11KT is the most abundant active androgen in the circulation of many adults and children with classic 21OHD, roughly double the concentration of testosterone on average. In addition, 11KT is a substrate for the 5α-reductase, and the product appears to have similarly augmented androgen action as for DHT. Collectively, these 19-carbon steroids downstream of the CYP11B1-catalyzed 11β-hydroxylation are commonly referred to as “11-oxygenated androgens” (14).
Assessment of 19-carbon steroids as biomarkers in 21OHD is dependent on age and sex. In prepubertal children, no gonadal contribution to androgen production is present, and all androgens derive from the adrenal. The major active adrenal androgen is 11KT, and several studies are consistent with the model that 11KT is the androgen responsible for mediating pubic hair development, as most often occurs during adrenarche (15, 16). In children with classic 21OHD with discordance among classical biomarkers of disease (17OHP and A4), measurement of 11-oxygenated androgens might resolve the quandary of whether disease control is adequate (17). However, target concentration ranges for adequate treatment in children are not yet known. In women with classic 21OHD, the 17-ketosteroids A4 and 11OHA4 both utilize the aldo-keto reductase type 1C3 enzyme for conversion to their active androgen congeners testosterone and 11KT, respectively, and testosterone and 11KT generally correlate linearly (11). Elevation of 1 or more of these steroids—A4, testosterone, 11OHA4, or 11KT—is consistent with adrenal androgen excess, but the 11-oxygenated androgens 11OHA4 and 11KT are more specific for 21OHD, because these steroids derive only from the adrenals (18). A normal A4 and testosterone even with an elevated 17OHP suggests adequate disease control, yet treatment decisions as discussed later require integration of clinical factors in the decision, such as menstrual regularity, hirsutism, and acne, and whether pregnancy is being pursued (4). In men with classic 21OHD, testosterone and 11KT correlate inversely (11). Men with poor disease control produce 11KT via 11OHA4 from their adrenals in excess of testosterone and A4; adrenal-derived 11KT suppresses gonadotropins and in turn testicular testosterone biosynthesis. Men with 21OHD and good hormonal control produce little 11KT from their adrenals and more testosterone than A4 from their testes, similar to healthy men. Thus, both A4/testosterone and 11KT/testosterone ratios <0.5 are measures of good disease control in men with classic 21OHD (4).
Renin, electrolytes, and mineralocorticoids
Historically, prior to neonatal screening, patients with classic 21OHD were categorized as having either “salt-wasting” or “simple virilizing” disease, alluding to the residual aldosterone production. Clinically, this distinction was based on whether spontaneous salt-wasting crises occurred prior to adequate treatment, but all children with classic 21OHD waste variable sodium with intercurrent illness (19). Aldosterone measurements were historically of little utility because the newborn kidney is very resistant to mineralocorticoid action, circulating aldosterone is generally quite high though variable in healthy neonates, high 17OHP antagonizes aldosterone action, and cross-reactivity with other steroids in aldosterone immunoassays clouded interpretation. For these reasons, indirect measures of mineralocorticoid action are preferred, including blood pressure and heart rate—recognizing the challenges of accurate blood pressure measurement in small children—as well as serum potassium and PRA (activity or mass). An elevated PRA and decreased serum sodium levels, particularly when tachycardia and salt craving are also present, indicates salt-wasting 21OHD and inadequate mineralocorticoid replacement. Elevated serum potassium and inappropriate natriuesis are also consistent with relative mineralocorticoid deficiency. Adults with classic 21OHD are less prone to salt-wasting crises, particularly crises related to infectious diseases, than infants and small children given their larger body size and generally high sodium content of the diet in many countries (20). In addition, circulating progesterone can be 21-hydroxylated in the liver to 11-deoxycorticosterone, a mineralocorticoid, via the action the CYP3A4 and CYP2C19 that may partly compensate for the lack of mineralocorticoids (21). Nevertheless, attention to adequate mineralocorticoid replacement is important, particularly for adults treated with GCs other than hydrocortisone, as only hydrocortisone in replacement doses has some intrinsic mineralocorticoid activity. Chronic fatigue that is not present on awakening but gradually worsens through the day without improvement after a GC dose is suspicious of chronic volume depletion and underreplacement of mineralocorticoid. The dose of fludrocortisone acetate should be titrated to achieve normal standing blood pressure and serum potassium, and plasma renin is often used as a deciding factor when these 2 results are not convincingly abnormal. Hypertension and hypokalemia with suppressed plasma renin suggest fludrocortisone overtreatment.
Childhood Through Adolescence
From infancy through childhood there are many challenges in the monitoring and control of adrenal androgens in patients with classic 21OHD: the complex underlying physiological mechanisms regulating a dynamic HPA axis include circadian and ultradian hormonal profiles, the limitations of hydrocortisone, the recommended GC used to treat growing children, and the limited information provided from current modalities used to monitor disease control. The treatment goals are to replace deficient hormones, prevent adrenal crises, reduce adrenal androgens, achieve normal childhood growth and pubertal development, and prevent comorbidities.
Part of the shortcomings of current GC treatments is that they simply replace cortisol but are unable to closely mimic the pulsatility of the HPA axis and cortisol secretion. The HPA axis utilizes feed-forward and feedback loops to regulate GC hormone levels within the physiological range appropriate for system homeostasis and is characterized by discrete pulses of ACTH and cortisol secretion. Cortisol secretion has a classic circadian pattern: cortisol levels reach nadir at midnight, begin to rise around 2 Am, peak early in the morning (cortisol awakening response), and gradually decrease throughout the day (22, 23). The circadian cortisol secretion pattern is actually derived from a dynamic ultradian rhythm of discrete cortisol pulses that follow ACTH pulses (24–26), with larger and more frequent pulses occurring early in the morning (22, 26). In human beings, secretory pulses of cortisol occur every 80 to 110 minutes with a mean number of 12 ± 0.7 pulses (24, 26–29).
Pulsatile access of GCs to their receptors has been previously shown in vitro and in vivo in animal models to be of critical importance for gene regulation, nongenomic GC signaling, HPA axis regulation, and endocrine and behavioral responses (30–35). Pulsatile changes in plasma GC levels result in pulsatile transcription of target genes mediated by transient GC receptor activation, which rapidly responds to circulating hormone levels. Conversely, constant, nonoscillatory hormone levels result in continuous transcription, aberrant messenger RNA accumulation, abnormal protein levels, and GC resistance (34, 36). Dysregulation of GC pulsatility can influence gene transcription (32) and neuroendocrine responsiveness to GC challenge (31, 37) and alters electrophysiological properties of the hippocampus (38).
Endogenous cortisol production ranges between 5 and 11 mg/m²/day with a mean of 7 mg/m²/day and demonstrates a high rate of variability, both intra- and interindividually (26, 39, 40). Factors that can contribute to this wide variability include weight, age, sex, puberty status, cortisol-binding proteins, cortisol metabolism, and GC sensitivity (26, 41–44).
While cortisol replacement therapy is used in all forms of primary adrenal insufficiency, the treatment of children with classic 21OHD presents its own unique challenges, namely finding the right balance of delivering sufficient cortisol replacement and, at the same time, reducing overproduction of adrenal androgens (hyperandrogenemia) while avoiding the effects of GC excess (hypercortisolemia).
Hydrocortisone, adrenal biomarkers, and challenges in therapy and monitoring in children with classic 21OHD
The current 21OHD consensus guidelines recommend treating children with oral hydrocortisone, a short acting GC, at a dose of 10 to 15 mg/m2/day divided in 3 doses (6). The daily hydrocortisone dose given tends to be higher than the endogenous production rate because of its short half-life and partial destruction by both gastric acidity and first-pass metabolism (45). Long-acting GCs (dexamethasone, prednisone, prednisolone) are not recommended in growing children as they can negatively impact growth (6). The GC serves 2 purposes: to replace the cortisol deficiency and to exert negative feedback on the HPA axis, which lowers ACTH and the production of adrenal-derived androgens and cortisol precursors.
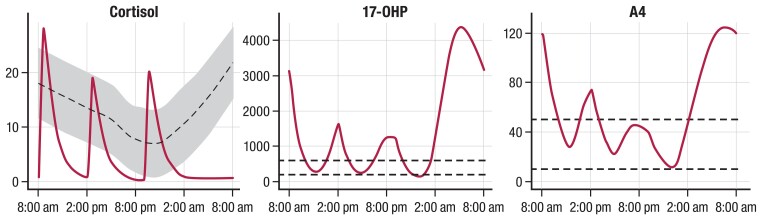
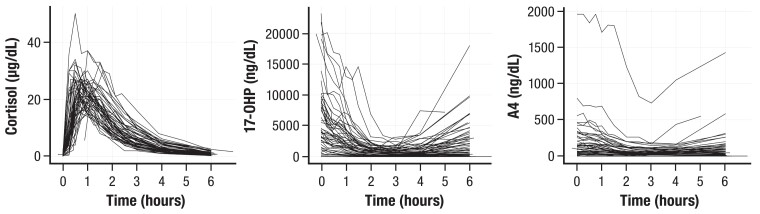
Because of these dual purposes, optimizing GC therapy remains a challenge, as children can still experience adverse outcomes during childhood related to the disease and/or treatment that can carry over into adulthood. One of the primary issues is hydrocortisone's rapid half-life, which is even shorter in children with 21OHD (median elimination half-life of 58 minutes, range: 41-105 minutes) (43, 46). In part, intermittent increases in adrenal sex steroids throughout the day can alter the activity of 11β-hydroxysteroid dehydrogenase isoenzymes and other enzymes that participate in cortisol metabolism and determine half-life (47–49). For these reasons, the recommended 3 times a day dosing schedule can be inadequate, leading to rapid swings in cortisol, 17OHP, and adrenal androgens. Children are inevitably exposed to alternating periods of hyper- and hypocortisolemia with resultant hyperandrogenemia throughout the day, thereby incurring risk for the deleterious effects of both these states (43, 50). Figure 5 shows the magnitude and the time over 24 hours that cortisol, 17OHP, and A4 concentrations remain above or below a target threshold, especially during the early morning hours, in a child with classic 21OHD treated with hydrocortisone (43, 46, 51). Therefore, unless a dose is administered around 3 Am, most of the evening hydrocortisone dose, which is typically administered around 8 Pm to 10 Pm in children with classic 21OHD, is cleared overnight, resulting in unopposed ACTH-stimulated adrenal androgen production and significant androgen exposure in the early morning hours when ACTH peaks (∼6 Am). Cortisol pharmacokinetic (PK)/pharmacodynamic (PD) studies (43, 46, 51) have shown that even while hydrocortisone given 4 times a day provides better adrenal biomarker profiles than 3 times a day, children with classic 21OHD still experience hypocortisolemia and resultant hyperandrogenemia, since most of a hydrocortisone dose is cleared within 4 to 5 hours (41, 43), which leads to rebounding of 17OHP and A4 to predose concentrations (Fig. 6).
Figure 5.
Simulated 24-hour hydrocortisone PK/PD hormone profiles in a prepubertal child with 21OHD. Simulated 24-hour cortisol, 17OHP, and A4 concentrations in a prepubertal child with 21OHD based on 3 times a day hydrocortisone dosing regimen using an integrated PK/PD model (46). The target range for cortisol, 17OHP, and A4 is shaded in gray. Dotted lines represent age- and sex-specific normal ranges. Abbreviations: 17OHP, 17-hydroxyprogesterone; 21OHD, 21-hydroxylase deficiency; A4, androstenedione; PK/PD, pharmacokinetic-pharmacodynamic.
Figure 6.
Rapid rebound of 17OHP and A4, and rapid decrease of cortisol to predose concentrations over 6 hours after the morning hydrocortisone dose in 67 children with 21OHD. Abbreviations: 17OHP, 17-hydroxyprogesterone; 21OHD, 21-hydroxylase deficiency; A4, androstenedione.
Periods of hypocortisolemia trigger increased production of ACTH with consequently increased adrenal production of progesterone, 17OHP, A4, testosterone, and estrogen through aromatization of increased androgen, leading to premature fusion of the growth plates, genital virilization, sexual precocity or precocious puberty, adrenal rest formation, and menstrual disorders with hyperandrogenism in adolescent females (52–56). Furthermore, low hydrocortisone dosing has been associated with increased rates of illness episodes and adrenal crises in children (57, 58). Chronic GC overexposure during childhood has untoward long-term effects such as short stature, weight gain, osteopenia, metabolic morbidity, and increased risk for cardiovascular disease in adulthood (59–66).
Additional challenges in management of children with classic 21OHD are the varied interindividual differences of cortisol PK regarding clearance and volume of distribution as well as PD response resulting in variable cortisol and adrenal androgen profiles in response to hydrocortisone therapy. Furthermore, intraindividual differences in hydrocortisone PK and PD occur over time. The endocrine milieu at puberty alters cortisol PK, because the increase in GH and insulin-like growth factor 1 during puberty enhance cortisol clearance (41). Moreover, obesity, insulin resistance, and fatty liver alter cortisol PK and increase the metabolic clearance of cortisol, as the primary site of cortisol metabolism in humans is the liver (67, 68).
The poor correlation between cortisol area under the curves (AUCs) with changes from baseline in AUCs for either 17OHP or A4 hinders prediction of the adrenal steroid response to a certain hydrocortisone dose, unless the individual's hydrocortisone PK and PD parameters have been determined (43). Variations in GC receptor and tissue sensitivities can further challenge optimization of hydrocortisone dosing.
Although guidelines for total daily GC dosing in children with 21OHD exist, evidence-based guidelines for optimal dosing distribution over the course of the day do not exist and as such vary among treating physicians. Higher doses in the evening (reverse-circadian) are sometimes employed to prevent the ACTH-stimulated adrenal androgen production in the early morning hours. Reverse circadian administration has been reported in 41% of 199 adults with 21OHD in the UK (69), in 28% of patients reported in a US cohort, and in 21% of children with 21OHD in the international I-CAH registry (52, 70). Increases in cortisol levels have been associated with poor sleep quality and increased sleep disturbances characterized by reduced sleep time and reduced rapid eye movement sleep in healthy adults (71) and in adults with Addison’s disease (72, 73). The efficacy of reverse circadian hydrocortisone treatment is debatable, as data are limited to studies of short duration with small sample sizes. Improved disease control was not observed in a retrospective study of 9 prepubertal children receiving reverse-circadian hydrocortisone dosing for 6 months (74). No differences were observed in disease control, sleep quality, and daytime activity between 3 different treatment schedules (high morning vs high evening vs equal doses) in an open-label, crossover, randomized trial of 15 children with classic 21OHD; however, important clinical outcomes such as growth and bone age maturation were not reported (75). Based on normal physiology, circulating cortisol concentrations are low at the initial part of sleep but increase at the end of the sleep period, peaking just minutes before the individual awakens. Reverse circadian hydrocortisone dosing has therefore the theoretical potential of poor sleep efficiency and awakening response (76–79). In contrast, a recent study of 39 patients with classic 21OHD found sleep results comparable after 3 weeks of using a higher evening dose vs 3 weeks of a higher morning dose with no washout period. Sleep was assessed by participants or parents using a daily sleeping score between 0 and 5, rather than formal sleep studies or validated sleep questionnaires (80). Potential adverse effects of high nocturnal cortisol levels include reduced sleep-entrained growth hormone secretion and deleterious metabolic effects (81).
The response to hydrocortisone therapy and disease control is traditionally evaluated by single measurements of 17OHP and/or A4 at clinic visits every 3 to 6 months, along with clinical parameters including growth velocity, weight, blood pressure, and pubertal development. Bone age is typically measured every 6 to 12 months, as bone maturation rate reflects the cumulative exposure of bones to androgen, primarily through aromatization to estrogens, over the interval. Like hemoglobin A1C in diabetes, bone age is an integrated but lagging indicator of antecedent control, which complements clinical signs and symptoms. It is important to keep in mind that while reduced or an accelerated height velocity, accelerated bone maturation, and physical features of androgen exposure generally indicate that adrenal androgens are not adequately suppressed, information regarding which specific aspect of the regimen (dose, timing, frequency) to adjust is lacking. One reason we lack evidence-based guidelines regarding specific therapeutic ranges to be targeted is that we do not have the ability of convenient real-time point-of-care measurements of adrenal steroid biomarkers at multiple times over 24 hours in order to determine meaningful exposure metrics (minimum plasma concentration, maximum plasma concentration, or AUC) that can be linked with short- or long-term outcomes such as quality of life, fatigue, bone maturation, growth, sleep patterns, endothelial function, or bone density. This deficiency represents a significant barrier to advancing our understanding of how to adjust GC dosing to improve clinical outcomes. PK/PD modeling based on 6-hour serial sampling of cortisol, A4, and 17OHP concentrations performed in an outpatient setting (43, 46) and 24-hour serial sampling of cortisol and 17OHP concentrations performed in an inpatient setting (51) have been developed and can predict the effect that changing the amount of dose, time of dose, and frequency of dose will have on an individual's adrenal steroid response over a 24 hour period. In the 6 hour serial sampling after the regular morning hydrocortisone dose, cortisol PK of half-life, clearance, volume of distribution, maximum concentration, time to maximum concentration, minimum concentration, and time to minimum concentration are determined as is the PD response to cortisol of 17OHP and A4 (maximum concentration, time to maximum concentration, minimum concentration, time to minimum concentration, and AUC) (43). PK/PD modeling has the potential to provide targeted individualized glucocorticoid dose adjustments and monitoring of disease control in children and adults with 21OHD. However, further studies are needed to determine the effect on long-term outcomes. Salivary measurements of cortisol, 17OHP, and A4 (82) can also potentially increase the feasibility of performing sparse, less invasive serial sampling over the course of the day to help guide the clinician's dosing recommendations.
The titration of fludrocortisone therapy is based on PRA, aiming for the age-specific normal range, serum sodium and potassium, and blood pressure. In infancy, decreased renal sensitivity to mineralocorticoid often requires salt supplementation during the first year of life and higher fludrocortisone dosing, leading to special considerations and close monitoring in the first 2 years of life (83). Hydrocortisone has ∼0.5% the mineralocorticoid activity of fludrocortisone per milligram, and 0.1 mg of fludrocortisone has similar GC activity as 1.5 mg hydrocortisone (4). Therefore, in addition to age-specific considerations, the interrelationship between these 2 medications needs to be considered in the management of children with 21OHD.
In general, there is good but variable correlation between 17OHP and A4 (17, 84, 85). However, whether A4 is superior to 17OHP for titrating therapy is debatable, and relevant data are lacking. A serum 17OHP level of 36 nmol/L (1200 ng/dL) is often used as the upper limit of acceptable range (52, 86), because normalization of 17OHP levels may reflect excessive hydrocortisone exposure (6). A4 is typically targeted at the upper limit of the age, sex, and Tanner stage laboratory-specific reference range (87).
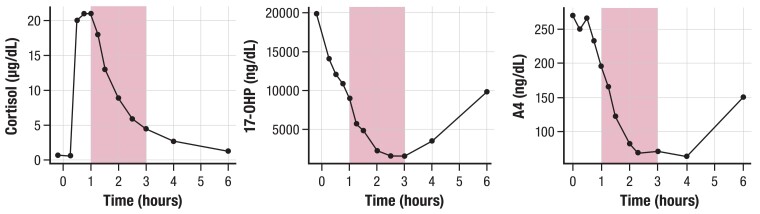
The timing of both 17OHP and A4 measurements relative to the last steroid dose should be documented for comparing across clinic visits, as circadian rhythm and the timing of GC intake influence steroid measurements (6). Sometimes in order to determine PD response to a hydrocortisone dose, patients may be instructed to schedule a blood draw for A4 and 17OHP measurements at 3 to 4 hours post-dose, which is the time of maximal suppression in response to the preceding hydrocortisone dose (43, 46, 84, 88). Alternatively, to gauge a patient's length of adrenal androgen response to a hydrocortisone dose, measurement of 17OHP and A4 concentrations may be taken at least 6 hours post-dose, when hydrocortisone exposure wanes due to its short half-life, and adrenal steroid production in most patients rebounds (43, 46) as seen in Figure 6. Measuring A4 and 17OHP concentrations at 8 Am to 9 Am, prior to the morning hydrocortisone dose, can reliably assess the HPA axis for oversuppression, as undetectable or very low adrenal biomarker concentrations 10 to 12 hours post-evening hydrocortisone dose suggests overtreatment and oversuppression of the HPA axis. Figure 7 depicts the wide variability of cortisol, 17OHP, and A4, even within a 2-hour time frame, in a patient who underwent a 6-hour serial sampling following the usual morning oral hydrocortisone dose. This variability illustrates why a random single, untimed measurement does not provide comprehensive assessment of a child's disease control.
Figure 7.
Wide variability of adrenal steroid concentrations within a 2-hour time frame after the morning hydrocortisone dose in a representative child with 21OHD. Observed cortisol, 17OHP, and A4 concentrations over 6 hours. Shaded area depicts a 2-hour time window, 1 to 3 hours post morning 6 mg hydrocortisone dose. Concentration levels of each adrenal steroid showed a > 60% change within the 2-hour time frame. Child's regular total daily hydrocortisone dose was 12 mg/m2/day. Abbreviations: 17OHP, 17-hydroxyprogesterone; 21OHD, 21-hydroxylase deficiency; A4, androstenedione.
Discordance between 17OHP and A4 concentrations can be observed, with 1 adrenal biomarker disproportionately high while the other remains in the normal range, all of which can confound the interpretation of disease control (17, 43, 46). In a large cohort of adults and children with 21OHD, Jha et al found discrepancy between 17OHP and A4 concentrations in 17% of more than 2700 laboratory assessments (17). Among those with discrepant biomarkers, elevated serum 17OHP with A4 within range was the most frequent finding (86%), with higher prevalence in children (92%). Among 46 patients who either had elevated 17OHP but A4 within range or vice versa, 65% of the patients were categorized as being in good clinical control. Among the 41 patients with elevated 17OHP but A4 within reference range, the study reported a median elevation of 11KT as high as 2.5 times relative to control; 13 of these 41 patients (28%) were in poor clinical control, indicating that abnormally high levels of 17OHP in the absence of concomitant high levels of A4 should not be dismissed. The study also found that 11β-hydroxytestosterone and less significantly 11KT were higher in patients in poor vs good clinical control, indicating potential advantages of 11-oxygenated androgens as biomarkers in assessing disease control (17). ACTH concentrations are not typically used in the monitoring of 21OHD but may be useful for the evaluation of HPA axis over- or undersuppression.
In summary, current monitoring of glucocorticoid treatment in children with 21OHD is far from perfect. Single measurements of adrenal biomarkers provide information about disease control for a point in time but do not provide complete information regarding disease control and androgen exposure over the course of the day or between clinic visits. Furthermore, clinical signs of over- or undertreatment do not always correlate with serum 17OHP and A4, as clinical features, which result from cumulative exposure to cortisol and androgens, can be slow in onset and regression.
Adverse clinical outcomes in children
Supraphysiologic hydrocortisone exposure during childhood remains a principal reason for treatment-related growth failure in 21OHD, as recent studies have found that higher hydrocortisone doses are associated with reduced adult height (89). The hydrocortisone effect on predicted adult height was first quantified by Sarafoglou et al, who showed that each 1 mg/m2/day increase in the average hydrocortisone dose during the growth period was associated with a 0.37 cm average decrease in the predicted adult height in children with 21OHD (59). Children with 21OHD were on average 7.4 cm shorter than their parents, and it was estimated that roughly 95% of that loss was associated with the negative effects of hydrocortisone. These findings demonstrate the overriding negative impact of excess hydrocortisone on skeletal growth, even after accounting for secular trends in height over the past 5 decades. Similarly, Pijnenburg-Kleizen et al found that each additional 1 mg/m2/day of hydrocortisone was associated with a reduction in adult height of 0.13 SD score (90). Increased cumulative hydrocortisone exposure during early years may have a detrimental effect on growth as well. Bomberg et al reported that children with “simple-virilizing” 21OHD fared the same as or better than their “salt-wasting” counterparts in final adult height, despite their later age of diagnosis and more advanced bone age (60).
Besides a major adverse effect of GCs on height outcome, excess of adrenal steroids also impairs adult height by causing prepubertal skeletal maturation and early puberty. Advanced prepubertal skeletal maturation attenuates height gain during puberty. In a retrospective cohort study of 496 children in France, advanced bone age at age 8 years had a strong impact on the risk of short adult stature, and almost no pubertal growth spurt was observed (91). A meta-analysis of >1000 patients with classic 21OHD found shorter than average adult height when adjusted for genetic potential or mid-parental height (−1.03 SD below mid-parental height or ∼7 cm) (92). Another important consideration is the use of mineralocorticoid in all children with classic 21OHD, which clinical practice guidelines recommend (6). Reports of the effect of mineralocorticoid on height outcomes are mixed, with some studies reporting a negative effect (93), no effect (59, 60), or better height outcomes (92); however, the last study noted that the better height outcome was more an effect of time of diagnosis, with mineralocorticoid use most likely an indirect marker for timing of presentation. Thus, delayed diagnosis of children can lead to growth acceleration and bone age maturation in the early years of life.
Children with 21OHD are at higher risk for earlier adiposity rebound and early-onset obesity and overweight (52, 94), which contributes to increased risk of obesity during adulthood and unfavorable cardiovascular profile (95–98). Increased risk of hypertension has also been reported in children with 21OHD (52, 62, 99–103). Some studies have implicated fludrocortisone as a contributor to the hypertension observed in children with classic 21OHD (62, 102, 103). Endothelial dysfunction and mild left ventricular diastolic dysfunction have been described in male adolescents with 21OHD (104, 105). A meta-analysis of 20 studies of 21OHD (n = 416 patients; 72% children) found an increased risk of insulin resistance, elevated blood pressure, and carotid intima thickness, although the quality of evidence was low (106). A longitudinal study of 58 patients with classic 21OHD with approximately 2000 visits spanning a median of 18.6 years found metabolic morbidity starting before puberty, which was associated with both GC and mineralocorticoid exposures (66).
21OHD is a disease of multiple hormonal imbalances with age- and sex-specific effects (2). Given the challenges of biochemical monitoring, age-specific review of symptoms that are suggestive of hyperandrogenism (early pubic hair, increased growth velocity, acne), hypercortisolism (weight gain, striae, slow growth rate), hypocortisolism (fatigue, weakness, decreased appetite, weight loss), and salt loss (ie, weight loss and failure-to-thrive in infants, salt-craving and postural hypotension) are essential in the management of children with classic 21OHD and in the prevention of comorbidities. There is an urgent need to further evaluate the performance of various steroid biomarkers of disease control in order to improve the management of 21OHD.
Female Fertility and the Use of Biomarkers in its Management
Female fertility in classic 21OHD is substantially reduced. Data from the UK showed that fertility is reduced to 0.25 children/woman in 21OHD vs 1.8 in the general population (107). There are 2 main reasons for reduced fertility. First, female patients with classic 21OHD experience virilization of their external genitalia already in utero, whereas the internal (Müllerian) structures are normal (2). Vaginoplasty allows normal menstrual blood flow, vaginal intercourse, and natural conception later in life. Surgical outcomes, however, are suboptimal, particularly in the most severe, null genotype (56). Patients with classic 21OHD report impaired sexual function and satisfaction resulting in reduced sexual confidence (108). Associated psychosexual challenges with reduced partnerships and sexual experiences are common as well as an impaired body image (56, 109, 110). Fewer women with classic 21OHD have been found to seek motherhood due to different gender behavior and low maternal interests (111, 112).
Second, cortisol deficiency elicits a counterregulatory increase in ACTH and a subsequent overproduction of GC precursors and adrenal androgens (2). GC precursors, in particular progesterone and also 17OHP, as well as adrenal androgens, both interfere with the HPG axis.
The integrated effects of elevated adrenal-derived androgens and progestins disturb the hormonal balance, which is crucial for ovulation, conception, implantation, and—in the absence thereof—normal menstruation. Regular menstruation is governed by tightly orchestrated changes in hormone concentrations, in particular ovarian estrogens in the follicular phase and progesterone in the luteal phase, through their interaction and feedback with pituitary gonadotropins. Chronically elevated adrenal-derived androgens and progestins as in women with poorly controlled 21OHD prevent ovulation and preparation of the endometrium for implantation of a conceptus. These effects are reflected by menstrual irregularities in 30% to 60% of women with 21OHD not receiving hormonal contraceptives (113, 114).
Importantly, however, fecundity is not impaired when patients have regular sexual intercourse and are adequately treated (107). Women with classic 21OHD can conceive at the same percentage as the general population if the delicate hormonal balances of the HPA and HPG axes are restored by adequate treatment. Striking the right hormonal balance enabling an ovulatory cycle and conception, however, is challenging in this patient population.
A common target for chronic therapy is to maintain 17OHP and A4 near the upper limit of normal (6). Normalization of these adrenal biomarkers, however, often requires supraphysiological GC doses and usually causes iatrogenic Cushing’s syndrome. For fertility optimization, however, we and others have observed that normalization or even suppression of 17OHP might be necessary. One explanation for this observation is that 17OHP binds to the progesterone receptor; furthermore, 17OHP adrenal-derived progesterone itself is also regularly increased in 21OHD patients. Elevated progesterone prevents endometrial growth in the follicular phase, alters cervical mucous, and thereby prevents conception (115, 116). Progesterone also reduces gonadotropin-releasing hormone pulse frequency at the hypothalamus and also acts directly at the pituitary level, which both interfere with ovulation (117–119). A French study implicated adrenal progesterone excess in 21OHD as the primary mechanism for altered luteinizing hormone (LH) pulsatility, with reduced LH pulse frequency and amplitude (120). The study also showed that women with classic 21OHD and good hormonal control have comparable LH pulsatility to healthy control women. Patients with elevated 17OHP, progesterone, testosterone, and A4 concentrations, on the other hand, had not only reduced LH pulse frequency but also wider pulses with reduced amplitude. In a plot analysis, progesterone concentrations were found to be sufficient to separate the 2 clusters of patients with normal LH pulsatility vs those with altered LH pulsatility. Testosterone concentrations in this study were not necessary to predict the 2 clusters of LH pulsatility, which emphasizes the importance of progesterone in influencing fertility in classic 21OHD. Therefore, progesterone appears to be the most important biomarker for fertility optimization in women with 21OHD, and progesterone concentrations should be below 2 nmol/L (0.6 ng/mL) based on clinical experience (107).
The effects of androgens on gonadotropin secretion are less studied, but moderately increased testosterone concentrations were shown to have suppressive effects on LH pulse frequency. Higher concentrations of testosterone impacted on both LH pulse frequency and amplitude (121–123). According to the previously cited French study in women with 21OHD, however, testosterone seems to contribute less than progesterone to infertility. The effects on the HPG axis could also be exerted via aromatization to estradiol, which interferes with gonadotropin-releasing hormone pulse frequency and can lead to anovulation. A4 itself is an excellent substrate for 11-hydroxylase, yielding 11OHA4, which is the most abundant androgen precursor in most patients with 21OHD (11). One study found that women with 21OHD and menstrual disturbances have elevated 11-oxygenated androgens, which correlated closely with other markers of hormonal control as 17OHP and A4 (113). As 11-oxygenated androgens are not produced by the human ovary, they might therefore be helpful in distinguishing adrenal and ovarian effects on fertility (18). The exact contributions of the 11-oxygenated androgens to HPG axis dysregulation and superiority to established biomarkers, however, has yet to be investigated in detail and in large cohorts. In summary, currently progesterone is considered the most impactful biomarker in women with classic 21OHD for maintaining fertility.
In order to achieve adequate suppression of serum progesterone during the follicular phase of the menstrual cycle, long-acting synthetic prednisolone might be superior compared to hydrocortisone alone, particularly for the latest daily dose. In 1 study, prednisolone given thrice daily in 8 hourly intervals or daytime hydrocortisone with bedtime prednisolone consistently achieved adequate progesterone suppression and pregnancies (107).
In very rare cases where adrenal-suppressive treatment with GCs could not achieve adequate disease control, bilateral adrenalectomy has been employed to successfully achieve pregnancy (124). Furthermore, adequate mineralocorticoid replacement seems important to achieve fertility, titrated to renin concentration or activity in the normal range. One study found that the addition of fludrocortisone helped to achieve fertility in females previously categorized as “simple virilising” patients (125). As dexamethasone traverses the placenta, it should not be given in women who desire to conceive.
Male Gonadal Function in 21OHD Patients
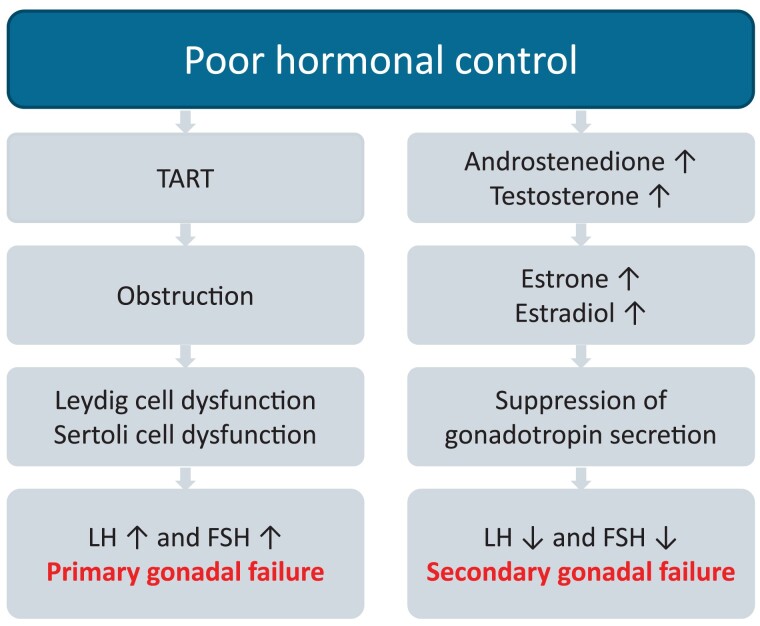
Gonadal dysfunction is one of the most important long-term complications and the most common cause of infertility in male patients with classic 21OHD (126, 127). Gonadal function can be impaired due to primary gonadal failure, mostly caused by testicular adrenal rest tumors (TART) and/or secondarily by suppression of the HPG axis, due to elevated androgen production and their aromatization to estrogens from TART and/or adrenals in poorly controlled patients (Fig. 8).
Figure 8.
Primary and secondary gonadal failure. Abbreviations: FSH, follicle-stimulating hormone; LH, luteinizing hormone; TART, testicular adrenal rest tumor.
TART
TART are a typical finding in adult males with 21OHD (128). The overall prevalence of TART in several well-designed studies ranged from 14% to 86% and depends mainly on age of the patient and severity of the disease but also on hormonal control and method of detection (128). TART are mainly found in adult patients, but some case reports and smaller studies in children report the presence of TART already in childhood with an increase in prevalence during adolescence (54, 129, 130). It is of clinical importance that TART are primarily detected in patients with classic 21OHD and null or nearly null alleles (128, 131). The presence of TART in nonclassic 21OHD is controversial and only anecdotally described (132–134). TART are associated with longstanding poor hormonal control (reported in about 58% of the cases) already during childhood and adolescence (128). Therefore, it is hypothesized that chronically elevated ACTH is an important etiological factor in the development of TART.
TART are typically found in both testes (77% of the cases) and are mainly located within the rete testes with a firm and multinodular character. Microscopically, TART resemble adrenocortical cells, with strings of fibrous tissue and in some cases also calcifications (128). The clear pathogenesis of TART is not yet unraveled. Gene and protein expression in TART shows similarity with normal and aberrant Leydig cells and adrenocortical cells, indicating both adrenal and testicular characteristics (135, 136). There is also evidence that TART tissue actively synthesizes steroids. Besides the production of 17OHP, 21dF, and progesterone, TART tissue can contribute to the production of adrenal androgens, including 11-oxygenated androgens (137–139).
Diagnosis of TART
Imaging and differentiation from Leydig cell tumors
Usually, only lesions of more than 2 cm can be detected by palpation due to their central location in the rete testes (140–142). Therefore, additional radiological techniques, preferably ultrasound, are needed to detect smaller lesions. A screening scrotal ultrasound at the end of adolescence or even earlier in poorly controlled boys with 21OHD is recommended, with periodic imaging through adulthood. On ultrasound (Fig. 2), the lesions show hypervascularity and have hypoechogenic features sometimes with hyperechogenic reflections (128). The discrimination of TART from Leydig cell tumors (LCTs) can be difficult either by palpation or imaging (143–145), and no biochemical markers are currently known that can distinguish between TART and LCTs (128). Some characteristics may help to make the correct diagnosis: bilateral firm, irregular testicular masses in patients with 21OHD, especially in those with poor hormonal control, are most likely TART, as LCTs are mostly present unilateral. LCTs often produce estrogen and cause gynecomastia, unlike TART that make androgens and 11-oxygenated androgens (139). On testicular biopsy, both tissues show characteristics of steroid-producing cells, but Reinke crystals can be present in LCTs yet are never found in TART (146, 147).
Biochemical evaluation
There are no biomarkers specific for TART. As longstanding TART can lead to primary gonadal failure with Sertoli cell dysfunction, inhibin B levels as a marker for Sertoli cell function are often decreased in patients with TART (132, 148). Paradoxically, gonadotropins may be within the normal range, which can be misleading (148). This finding can be explained by the presence of elevated adrenal androgens and their aromatization to estrogens leading to (partial) suppression of the HPG axis. Furthermore, especially in poor hormonal control, testosterone levels are within the (low) normal range but are mainly from adrenal origin. So even in patients with apparently normal gonadotropins and normal testosterone levels, primary gonadal failure cannot be excluded. To distinguish testosterone from testicular or adrenal origin, the use of serum A4/testosterone ratio has been suggested (114, 149). In poorly controlled patients, the androgens are predominately from adrenal origin, resulting in an A4/testosterone ratio >1, whereas in patients with optimal hormonal control, A4 levels are low with an A4/testosterone ratio of <0.5. Hormonal control can be monitored by measuring 17OHP, A4, and progesterone (6, 133). To avoid cross-reactivity, steroids are best measured by liquid chromatography-tandem mass spectrometry (150). Generally total testosterone is measured, but as total testosterone is decreased in obese patients due to low sex hormone-binding globulin concentrations, measurement or calculation of free and/or bioavailable testosterone can be necessary. In the future, a more detailed measurement of 11-oxygenated androgens may help to further evaluate hormonal control more accurately (17). Currently, 11-oxygenated androgen measurements are only performed in a few specialized laboratories.
Long-term consequences of TART
It is hypothesized that TART develop in 5 different stages starting as single nodular lesions that become hyperplastic and hypertrophic (151). Several nodules will coalesce to form a single confluent mass or a few multinodular tumors that will lead to obstruction of the seminiferous tubules. In longstanding TART, peritubular fibrosis and lymphocytic infiltration will lead to damage of the surrounding testes tissue (stage 5). Because the testis is confined to a capsule, the expanding mass raises intratesticular pressure and compromises blood flow to the tubules. Therefore, sub/infertility results from the obstruction of the seminiferous tubules and irreversible testes damage but also from direct toxic effects of adrenal androgens. Longstanding TART become more and more fibrotic and thereby lose the potential for shrinkage in response to treatment intensification. In this stage, intensified GC treatment is incompletely effective.
Treatment of TART
There are no clear guidelines to treat or even to prevent TART (6). As TART are associated with elevated ACTH as the most important growth-promoting factor, the first treatment goal is to optimize GC treatment thereby consistently lowering ACTH stimulation in an attempt to reduce the tumor size. A positive effect can be expected with hydrocortisone intensification in motivated adolescents with good compliance and without a history of longstanding TART. In adults, supraphysiologic dosages of dexamethasone, prednisolone (or prednisone/methylprednisolone), or combinations can be used. As clear dosage recommendations are not defined, treatment should be carefully titrated and monitored by measuring 17OHP and A4 levels. The lowest GC dosage that lowers ACTH sufficiently to achieve normalization of 17OHP throughout the day should be used and further titrated to achieve TART shrinkage. Most often, increased GC treatment is only given for several months until semen analysis has improved, because serious side effects commonly ensue with prolonged treatment intensification, such as weight gain, development of striae, and hypertension.
To restore fertility in patients with 21OHD and TART, treatment with mitotane has been described to reduce tumor size and consequently to improve sperm count. Mitotane is an adrenal toxic drug that is commonly used in adrenal carcinoma and Cushing’s syndrome. A first case report was published in 2014 describing successful 8-month treatment with mitotane, with shrinkage of TART tissues and improved sperm count (152). A recent study by Bachelot et al describes the treatment of mitotane in 5 adult patients with 21OHD and TART with a follow-up period of 1 to 5 years. TART size decreased in all patients with a complete disappearance in 2 patients and an improvement of semen quality in 2 patients (153). However, mitotane may cause irreversible chemical adrenalectomy and has severe adverse effects, making close monitoring necessary. Moreover, long-term outcomes in patients and offspring have not been studied yet. Therefore, this treatment is generally only recommended as last resort treatment following failure of GC intensification. One case report describes the use of human chorionic gonadotropin treatment in combination with recombinant follicle-stimulating hormone in a patient with 21OHD and TART, resulting in restored testicular function and fertility; however, studies in larger cohorts are lacking (154). Successful testicular sperm extraction for in vitro fertilization from a testis with TART has not been reported.
Testis sparing surgery has been described in several smaller cohorts but without significant improvement of semen quality after surgery (148, 155, 156). This failure might be explained by the longstanding presence of these TART, leading to irreversible damage of the surrounding testicular tissue, but surgery may also contribute to testicular damage. Whether testis-sparing surgery has beneficial effects in earlier-stage TART has not yet been studied. As TART can lead to irreversible damage and infertility, patients with 21OHD and TART should be informed about the consequences, and sperm cryopreservation should be considered at an early stage (126).
Hypogonadotropic hypogonadism
In poorly controlled male patients with 21OHD, elevated adrenal A4 will be aromatized to estrone and elevated testosterone is aromatized to estradiol. These estrogens, more so than the androgens themselves, suppress the HPG axis via negative feedback. In such patients without TART, small testes with volumes of up to 10 mL are found. A typical biochemical profile includes low LH and follicle-stimulating hormone levels and high A4; however, testosterone levels are within the low normal range yet from adrenal origin as described in the previous sections. In addition to the suppressive effects of estrogens, some studies also describe a suppressive effect of elevated progesterone on the HPG axis (157).
Most men with 21OHD do not suffer from clinical symptoms of hypogonadotropic hypogonadism, even those in whom the testosterone levels are below the reference. This paradox may be explained by the elevated 11-oxygenated androgens, especially 11KT, that are significantly elevated in 21OHD patients with poor hormonal control but are not yet measured routinely. Treatment consists of optimizing GC therapy, and in most cases hypogonadism eventually resolves (158, 159).
In summary, gonadal dysfunction is a common serious complication in males with classic 21OHD. Patients and parents should be informed already in childhood and adolescence about the consequences of medical nonadherence lax disease management, and those with poor hormonal control should be carefully monitored. Chronically elevated ACTH levels are the most important stimulating factor in the development of TART. The use of GCs mostly in supraphysiological dosages is currently the most effective approach to suppress ACTH levels and prevent or treat gonadal dysfunction but with high risk of iatrogenic Cushing’s syndrome from chronically intensified treatment.
Overtreatment and Consequences to Bone and Cardiometabolic Health
Supra- and nonphysiological replacement with GCs and mineralocorticoids can lead to several long-term negative outcomes, the most important being on bone and cardiometabolic health (160, 161). Low androgen levels secondary to supraphysiological GCs may also affect the long-term outcomes (162, 163). Moreover, the different GC regimens may have different outcomes, with hydrocortisone affecting bone mineral density (BMD) and body mass index (BMI) the least and dexamethasone the most, while prednisolone/prednisone is somewhere in between (164).
GC treatment is one of the most common causes of secondary osteoporosis and increased risk of fractures (165, 166). The direct and indirect effects of GCs on bone result in initial increased resorption and later decrease in bone formation, which cause microarchitectural distortion and increased fracture risk (167–169). Reduction of intestinal calcium absorption and increased renal calcium excretion can be an effect of GCs and result in secondary hyperparathyroidism. Osteoblast proliferation and differentiation are stimulated by gonadal and adrenal androgens in both sexes (170). Adrenal androgens have an impact on bone metabolism, especially during adrenarche, and particularly on cortical bone (171).
There have been conflicting results concerning BMD in patients with 21OHD, but the majority of studies have shown low BMD in all, or at least some, of the measured locations in both children and adults of both sexes (52, 65, 69, 163, 171–177). In a recent meta-analysis, individuals with 21OHD had decreased BMD in total body, lumbar spine, and femoral neck compared with matched controls (178). Some studies have reported a negative association with present or long-term GC dose and BMD (174–176, 179). Moreover, patients with nonclassic 21OHD had better BMD than those with classic 21OHD (171). However, BMD is not as important as fragility fractures, which are the principal cause of morbidity in osteoporosis. Nevertheless, the association between BMD and fractures is poor, especially in GC-induced osteoporosis (180).
Only a few studies have investigated fractures in 21OHD, and in a recent meta-analysis of patients receiving GC replacement therapy, the fracture rate was increased compared to matched controls (181); however, the difference was only significant for those with Addison’s disease or pituitary insufficiency. After this publication, an epidemiological study of fractures in all Swedish patients with 21OHD (n = 714) compared with matched controls (n = 71 400) was published (182). Despite the young mean age of 29.8 years, patients with 21OHD had more fractures compared to controls (odds ratio 1.61, 95% CI 1.35-1.91), and this increase was demonstrated for both sexes. Fragility fractures were increased in individuals with classic 21OHD, while those with nonclassic 21OHD had similar fracture rate as matched controls (182). Interestingly, fractures were significantly increased only in patients with 21OHD born before the introduction of neonatal screening (introduced in 1986 in Sweden) but not in those born afterwards (182). The finding was unexpected; however, those born before 1986 were the oldest in the cohort. In addition, early diagnosis and modern management of 21OHD in the younger patients may have a more beneficial effect on reducing fractures than prolonged androgen exposure in the older patients.
Cardiometabolic risk has been reported to be increased in patients with 21OHD (52, 66, 69, 102, 106, 162, 183–186). In a registry-based study of 588 patients with 21OHD, cardiovascular disease (odds ratio 2.7, 95% CI 1.9-3.9), hypertension, hyperlipidemia, atrial fibrillation, venous thromboembolism, obesity, diabetes (mainly type 2), and obstructive sleep disorder were increased compared to 58 800 matched controls (185). Females with all forms of 21OHD and patients with nonclassic 21OHD seemed generally more affected. Hypertension was especially prevalent in children (185). Another study showed that hypertension was more common in younger children than in adolescents (18.5% vs 4.9%) (102). High fludrocortisone doses could be the cause of hypertension in children less than 8 years, while BMI contributed thereafter (99, 102).
Most studies have demonstrated an increased BMI in subjects with 21OHD (52, 69, 102, 162, 186, 187); however, in adult females with 21OHD, lean mass and fat mass both increased concomitantly (162). A meta-analysis of 300 children/adolescents and 137 adults showed that subjects with 21OHD had higher Homeostatic Model Assessment for Insulin Resistance values compared to controls (106). Other studies have demonstrated that gestational diabetes, a risk factor for subsequent diabetes, was more common in pregnant women with 21OHD than in matched controls (111, 188). Moreover, diabetes was more common in both females and males above 40 years of age compared to matched controls (185). In contrast, type 1 diabetes frequency was not increased (189).
Studies measuring lipid levels have provided conflicting results (52, 66, 69, 127, 162, 184–186). The meta-analysis by Tamhane et al reported similar lipid levels in patients with 21OHD receiving GCs and/or mineralocorticoids compared to controls (106). Thus, it seems that the impact of 21OHD and GC replacement on plasma lipids are limited.
Increased heart rate has been reported in a few studies. In 1 study, mean 24-hour heart rate was higher in adults with 21OHD than in BMI-matched control subjects (77.1 vs 74.2 bpm) (190). In another study of adult males with 21OHD, increased 24-hour heart rate was found only in men ages 30 years or older (by 13 and 27 bpm during day and night, respectively), but not in younger men, compared to matched controls (184). It should be noted that the older men with 21OHD had higher BMI and total fat mass compared to controls. Increased heart rate is a risk factor for cardiovascular death, particular in men, independent of other risk factors (191, 192). Small increases of heart rate, within normal range, are associated with increased frequency of cardiovascular events (192).
Carotid intima-media thickness (CIMT) measured with ultrasound is a noninvasive way to evaluate subclinical atherosclerosis and is a well-established independent marker for future cardiovascular mortality as well as cardiovascular events, including myocardial infarction and stroke (193). Most studies investigating CIMT in patients with 21OHD have shown increased thickness compared to controls (63, 106, 194). These changes were observed even in children, adolescents, and young adults with 21OHD (63, 194). However, the increased CIMT was more pronounced in adults than in children and adolescents with 21OHD (106).
Mortality in patients with 21OHD has been demonstrated to be increased compared to controls (195, 196), mainly due to adrenal crises—but cardiovascular disease was the second most common cause of death (195). Most likely, cardiovascular disease will be even a more important cause of death when more patients with 21OHD reach older ages, as illustrated by the increased cardiometabolic risk profiles in younger people with 21OHD (66, 69, 185, 186).
In summary, both bone and cardiometabolic health is impaired in 21OHD, mainly due to supra- and nonphysiological GC and mineralocorticoid replacement. An annual bone and cardiovascular health assessment is recommended for patients with 21OHD. BMD measurement has been suggested to all patient upon transfer to adult care and then every 2 to 5 years (197), while blood pressure, lipids, hemoglobin A1c (or plasma glucose), BMI, and preferably also waist circumference may be done at least annually in adult patients. Optimization of the GC and mineralocorticoid replacement is key to prevent bone and cardiometabolic events, which is achieved by monitoring several parameters (Table 4).
Table 4.
Bone and cardiometabolic scenarios in adult patients with 21OHD and suggested clinical actions
| Scenario | Recommended clinical action |
|---|---|
| Decreased BMD | Optimize GC doses and regimens Optimize lifestylea Add calcium and vitamin D supplementation as appropriate Consider adding estrogen (females)b or testosterone (males) if low In older individuals consider bisphosphonate or denosumab |
| Osteoporotic fracture | Same as above Consider teriparatide |
| Increased BMI and/or waist circumference | Optimize GC doses and regimens Optimize lifestylea Consider weight reducing program Consider gastric-by-pass or equivalent if >40 kg/m2 |
| Increased blood pressure | Decrease fludrocortisone dose (if renin/PRA is suppressed or within reference limits) Optimize lifestylea Add an antihypertensive drug, eg, an ACE-inhibitor or ARB |
| Increased glucose level (due to insulin resistance) and/or type 2 diabetes | Optimize GC doses and regimens Optimize lifestylea Add metformin Consider other glucose-lowering medications |
| Increased lipid levels | Optimize lifestylea Add a statin |
Exercise, appropriate diet, no tobacco, alcohol in moderation.
Estrogen can be given as oral contraceptive pills in young fertile women. In young postmenopausal women hormone replacement therapy can be considered.
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMD, bone mineral density; BMI, body mass index; GC, glucocorticoid; PRA, plasma renin activity.
Back to the Patients
Case 1
The family was advised to treat hypoglycemia with carbohydrates instead of additional hydrocortisone doses. Additionally, it was advised that parents discontinue splitting hydrocortisone tablets and resume 2 mg/mL hydrocortisone suspension (pharmacy-compounded alcohol-free hydrocortisone) to allow for more precise dosing. The child’s failure to thrive does not appear to have been caused by salt wasting as his plasma renin activity was not significantly elevated but more likely due to dividing an already halved hydrocortisone tablet into 3 more pieces resulting in imprecise doses, which is well known to occur when manipulating tablets (198–201). Depending on the portions of expedient and active ingredient of the hydrocortisone contained in the one-third unscored portion of the 2.5 mg hydrocortisone tablet, it is possible that the infant received smaller amounts of hydrocortisone on some days than prescribed, resulting in hypoglycemia, and received higher hydrocortisone on other days, which during the evening could have resulted in nocturnal GH suppression and eventually poor growth (202). Although reliable suspensions are now available (203), caution should be used as variable dose accuracies have been reported in compounded preparations, and compounded preparations are not subject to regulatory health agency approval (204–206). An immediate-release granule formulation of hydrocortisone is also available for more precise dosing (207) and recently low-dose immediate-release hydrocortisone tablets (1, 2, 3, and 5 mg) in some European countries. The hydrocortisone dosing regimen was switched from 3 equal doses to a more circadian schedule of 2 mg at 6 Am, 1 mg at 2 Pm , and 0.5 mg at 10 Pm (8.5 mg/m2/day), and the fludrocortisone acetate dose was 0.1 mg/day. Follow-up labs demonstrated improved adrenal control, and hydrocortisone regimen remained unchanged through age 2 years 3 months. During this period, 17OHP was maintained between 15 and 38 nmol/L (500-1250 ng/dL), and PRA fluctuated between 5.1 and 14 ng/mL/hr (normal range: 1.4-7.8 ng/mL/hr) prior to mid-day dose. Additionally, his hypoglycemia resolved completely, and his linear growth underwent a marked acceleration.
His hydrocortisone doses were titrated from 7.4 to 10.5 mg/m2/day over the next 2 years to maintain 17OHP within the targeted range. With follow-up, he continued to have improved growth without acceleration of bone age. Height ultimately reached the 30th percentile (z-score −0.5) and weight reached the 44th percentile (z-score −0.15) by age 4 years 7 months. His bone age at that time was 4 years 3 months.
Case 2
In this patient the diagnosis of TART was made. Semen analysis showed azoospermia most likely due to obstruction of the seminiferous tubules. The results were discussed with the patient and his partner, and treatment with dexamethasone 0.5 mg per day was started. Within 3 months, A4 levels normalized, and the gonadotropins were within normal range. Ultrasound showed a decrease in TART size. The partner of the patient achieved pregnancy 9 months after resumption of GC treatment. After pregnancy was achieved, dexamethasone was replaced by hydrocortisone due to patient's weight gain and hypertension.
Conclusion
As described in the previous sections, specific biomarkers should be assessed when titrating conventional therapies for optimal outcomes in 21OHD. In children with growth failure, serial measurements of 17OHP, A4, and cortisol throughout the day provides insight to the PK and PD of hydrocortisone and might identify periods of over- and undertreatment, which suggest better hydrocortisone dosing distributions. Normalization of follicular-phase progesterone is critical for women attempting pregnancy, and PRA aids in the titration of fludrocortisone for all patients with classic 21OHD. Measurement of 11-oxygenated androgens might resolve quandaries of discrepant test results, and these biomarkers also gauge chronic disease control. New therapies in development (CRH-type 1 receptor antagonists crinecerfont and tildacerfont; CYP17A1 inhibitor abiraterone acetate) that target the HPA axis in order to control the excess production of adrenal androgens have shown promise (208–210). Biomarker measurements, in conjunction with clinical assessment, are essential to the proper titration of current and future therapies to optimize outcomes in 21OHD and to avoid the toxicities of overtreatment.
Acknowledgments
Editorial support was provided by Prescott Medical Communications Group (Chicago, IL, USA) with support from Neurocrine Biosciences, Inc.
Contributor Information
Kyriakie Sarafoglou, Department of Pediatrics, Division of Pediatric Endocrinology, University of Minnesota Medical School, Minneapolis, MN 55454, USA; Department of Experimental and Clinical Pharmacology, University of Minnesota College of Pharmacy, Minneapolis, MN 55455, USA.
Deborah P Merke, Department of Pediatrics, National Institutes of Health Clinical Center, Bethesda, MD 20892, USA; Eunice Kennedy Shriver National Institute of Child Health and Human Development, Bethesda, MD, USA.
Nicole Reisch, Medizinische Klinik and Poliklinik IV, Klinikum der Universität München, 80336 Munich, Germany.
Hedi Claahsen-van der Grinten, Department of Pediatrics, Amalia Children's Hospital, Radboud University Medical Center, 6500 HB, Nijmegen, The Netherlands.
Henrik Falhammar, Department of Molecular Medicine and Surgery, Karolinska Institutet, SE-17176, Stockholm, Sweden; Department of Endocrinology, Karolinska University Hospital, SE-17176, Stockholm, Sweden.
Richard J Auchus, Departments of Pharmacology and Internal Medicine, Division of Metabolism, Endocrinology and Diabetes, University of Michigan Medical School, Ann Arbor, MI 48109, USA.
Disclosures
K.S.: Research funding from Neurocrine Biosciences, Inc., Spruce Biosciences, and Adrenas Therapeutics; consulting for Eton Pharmaceuticals, Neurocrine Biosciences, Spruce Biosciences, Crinetics Pharmaceuticals.
D.P.M.: Unrelated research funds from Diurnal Limited and Neurocrine Biosciences, Inc. through the National Institutes of Health Cooperative Research and Development Agreement.
N.R.: Consulted for Neurocrine Biosciences, Inc., Spruce Biosciences, H Lundbeck A/S, Crinetics Pharmaceuticals, and Diurnal Limited.
H.C.: Consulted for Neurocrine Biosciences, Inc. and Spruce Biosciences.
H.F.: Consulted for Neurocrine Biosciences, Inc., Diurnal Limited, Roche Diagnostics International Ltd, and Adrenas Therapeutics.
R.J.A.: Research funding and consulting fees from Neurocrine Biosciences, Inc.; research funding from Spruce Biosciences and Diurnal, LTD; and consulting fees from OMass Therapeutics, Crinetics Pharmaceuticals, H Lundbeck A/S, Novo Nordisk, and Adrenas Therapeutics.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev. 2011;32(1):81‐151. 10.1210/er.2010-0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merke DP, Auchus RJ. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N Engl J Med. 2020;383(13):1248‐1261. 10.1056/NEJMra1909786 [DOI] [PubMed] [Google Scholar]
- 3. Auchus RJ. The classic and nonclassic congenital adrenal hyperplasias. Endocr Pract. 2015;21(4):383‐389. 10.4158/EP14474.RA [DOI] [PubMed] [Google Scholar]
- 4. Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, et al. Congenital adrenal hyperplasia-current insights in pathophysiology, diagnostics, and management. Endocr Rev. 2022; 43(1):91‐159. 10.1210/endrev/bnab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Auer MK, Nordenstrom A, Lajic S, Reisch N. Congenital adrenal hyperplasia. Lancet. 2023;401(10372):227‐244. 10.1016/S0140-6736(22)01330-7 [DOI] [PubMed] [Google Scholar]
- 6. Speiser PW, Arlt W, Auchus RJ, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2018;103(11):4043‐4088. 10.1210/jc.2018-01865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Costa-Barbosa FA, Carvalho VM, Nakamura OH, Bachega TA, Vieira JG, Kater CE. Zona fasciculata 21-hydroxysteroids and precursor-to-product ratios in 21-hydroxylase deficiency: further characterization of classic and non-classic patients and heterozygote carriers. J Endocrinol Invest. 2011;34(8):587‐592. 10.3275/7273 [DOI] [PubMed] [Google Scholar]
- 8. Pijnenburg-Kleizen KJ, Engels M, Mooij CF, et al. Adrenal steroid metabolites accumulating in congenital adrenal hyperplasia lead to transactivation of the glucocorticoid receptor. Endocrinology. 2015;156(10):3504‐3510. 10.1210/en.2015-1087 [DOI] [PubMed] [Google Scholar]
- 9. Gupta MK, Guryev OL, Auchus RJ. 5alpha-reduced C21 steroids are substrates for human cytochrome P450c17. Arch Biochem Biophys. 2003;418(2):151‐160. 10.1016/j.abb.2003.07.003 [DOI] [PubMed] [Google Scholar]
- 10. Reisch N, Taylor AE, Nogueira EF, et al. Alternative pathway androgen biosynthesis and human fetal female virilization. Proc Natl Acad Sci USA. 2019;116(44):22294‐22299. 10.1073/pnas.1906623116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Turcu AF, Nanba AT, Chomic R, et al. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur J Endocrinol. 2016;174(5):601‐609. 10.1530/eje-15-1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turcu AF, El-Maouche D, Zhao L, et al. Androgen excess and diagnostic steroid biomarkers for nonclassic 21-hydroxylase deficiency without cosyntropin stimulation. Eur J Endocrinol. 2020;183(1):63‐71. 10.1530/EJE-20-0129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barnard M, Quanson JL, Mostaghel E, Pretorius E, Snoep JL, Storbeck KH. 11-Oxygenated androgen precursors are the preferred substrates for aldo-keto reductase 1C3 (AKR1C3): implications for castration resistant prostate cancer. J Steroid Biochem Mol Biol. 2018;183:192‐201. 10.1016/j.jsbmb.2018.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turcu AF, Rege J, Auchus RJ, Rainey WE. 11-Oxygenated androgens in health and disease. Nat Rev Endocrinol. 2020;16(5):284‐296. 10.1038/s41574-020-0336-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rege J, Turcu AF, Kasa-Vubu JZ, et al. 11-Ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J Clin Endocrinol Metab. 2018;103(12):4589‐4598. 10.1210/jc.2018-00736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wise-Oringer BK, Burghard AC, O'Day P, et al. The unique role of 11-oxygenated C19 steroids in both premature adrenarche and premature pubarche. Horm Res Paediatr. 2020;93(7-8):460‐469. 10.1159/000513236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jha S, Turcu AF, Sinaii N, Brookner B, Auchus RJ, Merke DP. 11-Oxygenated androgens useful in the setting of discrepant conventional biomarkers in 21-hydroxylase deficiency. J Endocr Soc. 2021;5(2):bvaa192. 10.1210/jendso/bvaa192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Auer MK, Hawley JM, Lottspeich C, et al. 11-Oxygenated androgens are not secreted by the human ovary: in-vivo data from four different cases of hyperandrogenism. Eur J Endocrinol. 2022;187(6):K47‐K53. 10.1530/EJE-22-0518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frisch H, Battelino T, Schober E, Baumgartner-Parzer S, Nowotny P, Vierhapper H. Salt wasting in simple virilizing congenital adrenal hyperplasia. J Pediatr Endocrinol Metabol. 2001;14(9):1649‐1655. 10.1515/jpem.2001.14.9.1649 [DOI] [PubMed] [Google Scholar]
- 20. Reisch N, Willige M, Kohn D, et al. Frequency and causes of adrenal crises over lifetime in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2012;167(1):35‐42. 10.1530/EJE-12-0161 [DOI] [PubMed] [Google Scholar]
- 21. Gomes LG, Huang N, Agrawal V, Mendonca BB, Bachega TA, Miller WL. Extraadrenal 21-hydroxylation by CYP2C19 and CYP3A4: effect on 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2009;94(1):89‐95. 10.1210/jc.2008-1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33(1):14‐22. 10.1210/jcem-33-1-14 [DOI] [PubMed] [Google Scholar]
- 23. Pruessner JC, Wolf OT, Hellhammer DH, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539‐2549. [DOI] [PubMed] [Google Scholar]
- 24. Veldhuis JD, Iranmanesh A, Lizarralde G, Johnson ML. Amplitude modulation of a burstlike mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am J Physiol. 1989;257(1 Pt 1):E6‐14. [DOI] [PubMed] [Google Scholar]
- 25. Jasper MS, Engeland WC. Synchronous ultradian rhythms in adrenocortical secretion detected by microdialysis in awake rats. Am J Physiol. 1991;261(5 Pt 2):R1257‐R1268. [DOI] [PubMed] [Google Scholar]
- 26. Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab. 1993;76(6):1505‐1510. [DOI] [PubMed] [Google Scholar]
- 27. Lightman SL, Wiles CC, Atkinson HC, et al. The significance of glucocorticoid pulsatility. Eur J Pharmacol. 2008;583(2-3):255‐262. 10.1016/j.ejphar.2007.11.073 [DOI] [PubMed] [Google Scholar]
- 28. Follenius M, Simon C, Brandenberger G, Lenzi P. Ultradian plasma corticotropin and cortisol rhythms: time-series analyses. J Endocrinol Invest. 1987;10(3):261‐266. [DOI] [PubMed] [Google Scholar]
- 29. Lightman S, Terry JR. The importance of dynamic signalling for endocrine regulation and drug development: relevance for glucocorticoid hormones. Lancet Diabetes Endocrinol. 2014;2(7):593‐599. 10.1016/S2213-8587(13)70182-7 [DOI] [PubMed] [Google Scholar]
- 30. Deng Q, Riquelme D, Trinh L, et al. Rapid glucocorticoid feedback inhibition of ACTH secretion involves ligand-dependent membrane association of glucocorticoid receptors. Endocrinology. 2015;156(9):3215‐3227. 10.1210/EN.2015-1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sarabdjitsingh RA, Conway-Campbell BL, Leggett JD, et al. Stress responsiveness varies over the ultradian glucocorticoid cycle in a brain-region-specific manner. Endocrinology. 2010;151(11):5369‐5379. 10.1210/en.2010-0832 [DOI] [PubMed] [Google Scholar]
- 32. Sarabdjitsingh RA, Isenia S, Polman A, et al. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology. 2010;151(3):1177‐1186. 10.1210/en.2009-1119 [DOI] [PubMed] [Google Scholar]
- 33. Sarabdjitsingh RA, Jezequel J, Pasricha N, et al. Ultradian corticosterone pulses balance glutamatergic transmission and synaptic plasticity. Proc Natl Acad Sci USA. 2014;111(39):14265‐14270. 10.1073/pnas.1411216111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stavreva DA, Wiench M, John S, et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol. 2009;11(9):1093‐1102. 10.1038/ncb1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russell GM, Lightman SL. Can side effects of steroid treatments be minimized by the temporal aspects of delivery method? Expert Opin Drug Saf. 2014;13(11):1501‐1513. 10.1517/14740338.2014.965141 [DOI] [PubMed] [Google Scholar]
- 36. Biddie SC, Conway-Campbell BL, Lightman SL. Dynamic regulation of glucocorticoid signalling in health and disease. Rheumatology. 2012;51(3):403‐412. 10.1093/rheumatology/ker215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarabdjitsingh RA, Spiga F, Oitzl MS, et al. Recovery from disrupted ultradian glucocorticoid rhythmicity reveals a dissociation between hormonal and behavioural stress responsiveness. J Neuroendocrinol. 2010;22(8):862‐871. 10.1111/j.1365-2826.2010.02004.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10(6):459‐466. 10.1038/nrn2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Esteban NV, Yergey AL. Cortisol production rates measured by liquid chromatography/mass spectrometry. Steroids. 1990;55(4):152‐158. [DOI] [PubMed] [Google Scholar]
- 40. Linder BL, Esteban NV, Yergey AL, Winterer JC, Loriaux DL, Cassorla F. Cortisol production rate in childhood and adolescence. J Pediatr. 1990;117(6):892‐896. [DOI] [PubMed] [Google Scholar]
- 41. Charmandari E, Hindmarsh PC, Johnston A, Brook CG. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: alterations in cortisol pharmacokinetics at puberty. J Clin Endocrinol Metab. 2001;86(6):2701‐2708. [DOI] [PubMed] [Google Scholar]
- 42. Thomson AH, Devers MC, Wallace AM, et al. Variability in hydrocortisone plasma and saliva pharmacokinetics following intravenous and oral administration to patients with adrenal insufficiency. Clin Endocrinol (Oxf). 2007;66(6):789‐796. 10.1111/j.1365-2265.2007.02812.x [DOI] [PubMed] [Google Scholar]
- 43. Sarafoglou K, Zimmerman CL, Gonzalez-Bolanos MT, Willis BA, Brundage R. Interrelationships among cortisol, 17-hydroxyprogesterone, and androstenendione exposures in the management of children with congenital adrenal hyperplasia. J Investig Med. 2015;63(1):35‐41. 10.1097/JIM.0000000000000121 [DOI] [PubMed] [Google Scholar]
- 44. van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59(1):333‐357. 10.1210/rp.59.1.333 [DOI] [PubMed] [Google Scholar]
- 45. Oprea A, Bonnet NCG, Polle O, Lysy PA. Novel insights into glucocorticoid replacement therapy for pediatric and adult adrenal insufficiency. Ther Adv Endocrinol Metab. 2019;10:2042018818821294. 10.1177/2042018818821294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Al-Kofahi M, Ahmed MA, Jaber MM, et al. An integrated PK-PD model for cortisol and the 17-hydroxyprogesterone and androstenedione biomarkers in children with congenital adrenal hyperplasia. Br J Clin Pharmacol. 2021;87(3):1098‐1110. 10.1111/bcp.14470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Low SC, Chapman KE, Edwards CR, Wells T, Robinson IC, Seckl JR. Sexual dimorphism of hepatic 11 beta-hydroxysteroid dehydrogenase in the rat: the role of growth hormone patterns. J Endocrinol. 1994;143(3):541‐548. [DOI] [PubMed] [Google Scholar]
- 48. Gathercole LL, Lavery GG, Morgan SA, et al. 11beta-Hydroxysteroid dehydrogenase 1: translational and therapeutic aspects. Endocr Rev. 2013;34(4):525‐555. 10.1210/er.2012-1050 [DOI] [PubMed] [Google Scholar]
- 49. Paulsen SK, Pedersen SB, Jorgensen JO, et al. Growth hormone (GH) substitution in GH-deficient patients inhibits 11beta-hydroxysteroid dehydrogenase type 1 messenger ribonucleic acid expression in adipose tissue. J Clin Endocrinol Metab. 2006;91(3):1093‐1098. 10.1210/jc.2005-1694 [DOI] [PubMed] [Google Scholar]
- 50. Maguire AM, Ambler GR, Moore B, McLean M, Falleti MG, Cowell CT. Prolonged hypocortisolemia in hydrocortisone replacement regimens in adrenocorticotrophic hormone deficiency. Pediatrics. 2007;120(1):e164-171. 10.1542/peds.2006-2558 [DOI] [PubMed] [Google Scholar]
- 51. Melin J, Parra-Guillen ZP, Michelet R, et al. Pharmacokinetic/pharmacodynamic evaluation of hydrocortisone therapy in pediatric patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2020;105(4):e1729-e1740. 10.1210/clinem/dgaa071 [DOI] [PubMed] [Google Scholar]
- 52. Finkielstain GP, Kim MS, Sinaii N, et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2012;97(12):4429‐4438. 10.1210/jc.2012-2102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reichman DE, White PC, New MI, Rosenwaks Z. Fertility in patients with congenital adrenal hyperplasia. Fertil Steril. 2014;101(2):301‐309. [DOI] [PubMed] [Google Scholar]
- 54. Claahsen-van der Grinten HL, Dehzad F, Kamphuis-van Ulzen K, de Korte CL. Increased prevalence of testicular adrenal rest tumours during adolescence in congenital adrenal hyperplasia. Horm Res Paediatr. 2014;82(4):238‐244. [DOI] [PubMed] [Google Scholar]
- 55. Pierre P, Despert F, Tranquart F, et al. Adrenal rest tissue in gonads of patients with classical congenital adrenal hyperplasia: multicenter study of 45 French male patients. Ann Endocrinol (Paris). 2012;73(6):515‐522. [DOI] [PubMed] [Google Scholar]
- 56. Nordenstrom A, Frisen L, Falhammar H, et al. Sexual function and surgical outcome in women with congenital adrenal hyperplasia due to CYP21A2 deficiency: clinical perspective and the patients’ perception. J Clin Endocrinol Metab. 2010;95(8):3633‐3640. 10.1210/jc.2009-2639 [DOI] [PubMed] [Google Scholar]
- 57. Ali SR, Bryce J, Haghpanahan H, et al. Real-world estimates of adrenal insufficiency-related adverse events in children with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2021;106(1):e192‐e203. 10.1210/clinem/dgaa694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. El-Maouche D, Hargreaves CJ, Sinaii N, Mallappa A, Veeraraghavan P, Merke DP. Longitudinal assessment of illnesses, stress dosing, and illness sequelae in patients with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2018;103(6):2336‐2345. 10.1210/jc.2018-00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sarafoglou K, Addo OY, Turcotte L, et al. Impact of hydrocortisone on adult height in congenital adrenal hyperplasia—the Minnesota cohort. J Pediatr. 2014;164(5):1141‐1146 e1141. 10.1016/j.jpeds.2014.01.011 [DOI] [PubMed] [Google Scholar]
- 60. Bomberg EM, Addo OY, Kyllo J, et al. The relation of peripubertal and pubertal growth to final adult height in children with classic congenital adrenal hyperplasia. J Pediatr. 2015;166(3):743‐750. 10.1016/j.jpeds.2014.11.027 [DOI] [PubMed] [Google Scholar]
- 61. Speiser PW, Serrat J, New MI, Gertner JM. Insulin insensitivity in adrenal hyperplasia due to nonclassical steroid 21-hydroxylase deficiency. J Clin Endocrinol Metab. 1992;75(6):1421‐1424. [DOI] [PubMed] [Google Scholar]
- 62. Subbarayan A, Dattani MT, Peters CJ, Hindmarsh PC. Cardiovascular risk factors in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2014;80(4):471‐477. 10.1111/cen.12265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sartorato P, Zulian E, Benedini S, et al. Cardiovascular risk factors and ultrasound evaluation of intima-media thickness at common carotids, carotid bulbs, and femoral and abdominal aorta arteries in patients with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(3):1015‐1018. 10.1210/jc.2006-1711 [DOI] [PubMed] [Google Scholar]
- 64. Williams RM, Deeb A, Ong KK, et al. Insulin sensitivity and body composition in children with classical and nonclassical congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2010;72(2):155‐160. 10.1111/j.1365-2265.2009.03587.x [DOI] [PubMed] [Google Scholar]
- 65. Falhammar H, Filipsson H, Holmdahl G, et al. Fractures and bone mineral density in adult women with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(12):4643‐4649. 10.1210/jc.2007-0744 [DOI] [PubMed] [Google Scholar]
- 66. Torky A, Sinaii N, Jha S, et al. Cardiovascular disease risk factors and metabolic morbidity in a longitudinal study of congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2021;106(12):e5247-e5257. 10.1210/clinem/dgab133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mallappa A, Nella AA, Kumar P, et al. Alterations in hydrocortisone pharmacokinetics in a patient with congenital adrenal hyperplasia following bariatric surgery. J Endocr Soc. 2017;1(7):994‐1001. 10.1210/js.2017-00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ahmed A, Rabbitt E, Brady T, et al. A switch in hepatic cortisol metabolism across the spectrum of non alcoholic fatty liver disease. PLoS One. 2012;7(2):e29531. 10.1371/journal.pone.0029531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Arlt W, Willis DS, Wild SH, et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J Clin Endocrinol Metab. 2010;95(11):5110‐5121. 10.1210/jc.2010-0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bacila I, Freeman N, Daniel E, et al. International practice of corticosteroid replacement therapy in congenital adrenal hyperplasia: data from the I-CAH registry. Eur J Endocrinol. 2021;184(4):553‐563. 10.1530/EJE-20-1249 [DOI] [PubMed] [Google Scholar]
- 71. Balbo M, Leproult R, Van Cauter E. Impact of sleep and its disturbances on hypothalamo-pituitary-adrenal axis activity. Int J Endocrinol. 2010;2010:759234. 10.1155/2010/759234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lovas K, Husebye ES, Holasten F, Bjorvatn B. Sleep disturbances in patients with Addison's disease. Eur J Endocrinol. 2003;148(4):449‐456. [DOI] [PubMed] [Google Scholar]
- 73. Henry M, Thomas KGF, Ross IL. Sleep, cognition and cortisol in Addison's disease: a mechanistic relationship. Front Endocrinol (Lausanne). 2021;12:694046. 10.3389/fendo.2021.694046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dubinski I, Bechtold Dalla-Pozza S, Bidlingmaier M, Reisch N, Schmidt H. Reverse circadian glucocorticoid treatment in prepubertal children with congenital adrenal hyperplasia. J Pediatr Endocrinol Metabol: JPEM. 2021;34(12):1543‐1548. 10.1515/jpem-2021-0540 [DOI] [PubMed] [Google Scholar]
- 75. German A, Suraiya S, Tenenbaum-Rakover Y, Koren I, Pillar G, Hochberg Z. Control of childhood congenital adrenal hyperplasia and sleep activity and quality with morning or evening glucocorticoid therapy. J Clin Endocrinol Metab. 2008;93(12):4707‐4710. 10.1210/jc.2008-0519 [DOI] [PubMed] [Google Scholar]
- 76. Vgontzas AN, Chrousos GP. Sleep, the hypothalamic–pituitary–adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31(1):15‐36. 10.1016/S0889-8529(01)00005-6 [DOI] [PubMed] [Google Scholar]
- 77. Hirotsu C, Tufik S, Andersen ML. Interactions between sleep, stress, and metabolism: from physiological to pathological conditions. Sleep Sci. 2015;8(3):143‐152. 10.1016/j.slsci.2015.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267(9):1244‐1252. [PubMed] [Google Scholar]
- 79. Vgontzas AN, Zoumakis M, Bixler EO, et al. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metabol. 2003;88(5):2087‐2095. [DOI] [PubMed] [Google Scholar]
- 80. Schroder MAM, van Herwaarden AE, Span PN, et al. Optimizing the timing of highest hydrocortisone dose in children and adolescents with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2022;107(4):e1661‐e1672. 10.1210/clinem/dgab826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Plat L, Leproult R, L'Hermite-Baleriaux M, et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84(9):3082‐3092. 10.1210/jcem.84.9.5978 [DOI] [PubMed] [Google Scholar]
- 82. Adriaansen BPH, Kamphuis JS, Schroder MAM, et al. Diurnal salivary androstenedione and 17-hydroxyprogesterone levels in healthy volunteers for monitoring treatment efficacy of patients with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2022;97(1):36‐42. 10.1111/cen.14690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Neumann U, van der Linde A, Krone RE, et al. Treatment of congenital adrenal hyperplasia in children aged 0-3 years: a retrospective multicenter analysis of salt supplementation, glucocorticoid and mineralocorticoid medication, growth and blood pressure. Eur J Endocrinol. 2022;186(5):587‐596. 10.1530/EJE-21-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Charmandari E, Matthews DR, Johnston A, Brook CG, Hindmarsh PC. Serum cortisol and 17-hydroxyprogesterone interrelation in classic 21-hydroxylase deficiency: is current replacement therapy satisfactory? J Clin Endocrinol Metab. 2001;86(10):4679‐4685. 10.1210/jcem.86.10.7972 [DOI] [PubMed] [Google Scholar]
- 85. Juniarto AZ, Goossens K, Setyawati BA, Drop SL, de Jong FH, Faradz SM. Correlation between androstenedione and 17-hydroxyprogesterone levels in the saliva and plasma of patients with congenital adrenal hyperplasia. Singapore Med J. 2011;52(11):810‐813. [PubMed] [Google Scholar]
- 86. Dauber A, Kellogg M, Majzoub JA. Monitoring of therapy in congenital adrenal hyperplasia. Clin Chem. 2010;56(8):1245‐1251. 10.1373/clinchem.2010.146035 [DOI] [PubMed] [Google Scholar]
- 87. Soldin S, Brugnara C, Wong E. Androstenedione. AACC Press; 2003. [Google Scholar]
- 88. Fuqua JS, Rotenstein D, Lee PA. Duration of suppression of adrenal steroids after glucocorticoid administration. Int J Pediatr Endocrinol. 2010;2010(1):712549. 10.1155/2010/712549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Bonfig W, Pozza SB, Schmidt H, Pagel P, Knorr D, Schwarz HP. Hydrocortisone dosing during puberty in patients with classical congenital adrenal hyperplasia: an evidence-based recommendation. J Clin Endocrinol Metab. 2009;94(10):3882‐3888. 10.1210/jc.2009-0942 [DOI] [PubMed] [Google Scholar]
- 90. Pijnenburg-Kleizen KJ, Thomas CMG, Otten BJ, Roeleveld N, Claahsen-van der Grinten HL. Long-term follow-up of children with classic congenital adrenal hyperplasia: suggestions for age dependent treatment in childhood and puberty. J Pediatr Endocrinol &Metabol: JPEM. 2019;32(10):1055‐1063. 10.1515/jpem-2019-0006 [DOI] [PubMed] [Google Scholar]
- 91. Bretones P, Riche B, Pichot E, et al. Growth curves for congenital adrenal hyperplasia from a national retrospective cohort. J Pediatr Endocrinol &Metabol: JPEM. 2016;29(12):1379‐1388. 10.1515/jpem-2016-0156 [DOI] [PubMed] [Google Scholar]
- 92. Muthusamy K, Elamin MB, Smushkin G, et al. Clinical review: adult height in patients with congenital adrenal hyperplasia: a systematic review and metaanalysis. J Clin Endocrinol Metab. 2010;95(9):4161‐4172. 10.1210/jc.2009-2616 [DOI] [PubMed] [Google Scholar]
- 93. Patel L, Chandrashekhar SR, Gemmell I, et al. Gender dichotomy in long term growth trajectories of children with 21-hydroxylase deficiency congenital adrenal hyperplasia. Horm Res Paediatr. 2011;75(3):206‐212. 10.1159/000320700 [DOI] [PubMed] [Google Scholar]
- 94. Sarafoglou K, Forlenza GP, Yaw Addo O, et al. Obesity in children with congenital adrenal hyperplasia in the Minnesota cohort: importance of adjusting body mass index for height-age. Clin Endocrinol (Oxf). 2017;86(5):708‐716. 10.1111/cen.13313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mooij CF, Kroese JM, Claahsen-van der Grinten HL, Tack CJ, Hermus AR. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clin Endocrinol (Oxf). 2010;73(2):137‐146. 10.1111/j.1365-2265.2009.03690.x [DOI] [PubMed] [Google Scholar]
- 96. Improda N, Barbieri F, Ciccarelli GP, Capalbo D, Salerno M. Cardiovascular health in children and adolescents with congenital adrenal hyperplasia due to 21-hydroxilase deficiency. Front Endocrinol (Lausanne). 2019;10:212. 10.3389/fendo.2019.00212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bhullar G, Tanawattanacharoen VK, Yeh MY, et al. Early adiposity rebound predicts obesity and adiposity in youth with congenital adrenal hyperplasia. Horm Res Paediatr. 2021;93(11-12):609‐615. 10.1159/000514130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Whitaker RC, Pepe MS, Wright JA, Seidel KD, Dietz WH. Early adiposity rebound and the risk of adult obesity. Pediatrics. 1998;101(3):E5. [DOI] [PubMed] [Google Scholar]
- 99. Maccabee-Ryaboy N, Thomas W, Kyllo J, et al. Hypertension in children with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2016;85(4):528‐534. 10.1111/cen.13086 [DOI] [PubMed] [Google Scholar]
- 100. Roche EF, Charmandari E, Dattani MT, Hindmarsh PC. Blood pressure in children and adolescents with congenital adrenal hyperplasia (21-hydroxylase deficiency): a preliminary report. Clin Endocrinol (Oxf). 2003;58(5):589‐596. 10.1046/j.1365-2265.2003.01757.x [DOI] [PubMed] [Google Scholar]
- 101. Volkl TM, Simm D, Dotsch J, Rascher W, Dorr HG. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(12):4888‐4895. 10.1210/jc.2006-1069 [DOI] [PubMed] [Google Scholar]
- 102. Bonfig W, Roehl FW, Riedl S, et al. Blood pressure in a large cohort of children and adolescents with classic adrenal hyperplasia (CAH) due to 21-hydroxylase deficiency. Am J Hypertens. 2016;29(2):266‐272. 10.1093/ajh/hpv087 [DOI] [PubMed] [Google Scholar]
- 103. Bonfig W, Schwarz HP. Blood pressure, fludrocortisone dose and plasma renin activity in children with classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency followed from birth to 4 years of age. Clin Endocrinol (Oxf). 2014;81(6):871‐875. 10.1111/cen.12498 [DOI] [PubMed] [Google Scholar]
- 104. Harrington J, Pena AS, Gent R, Hirte C, Couper J. Adolescents with congenital adrenal hyperplasia because of 21-hydroxylase deficiency have vascular dysfunction. Clin Endocrinol (Oxf). 2012;76(6):837‐842. 10.1111/j.1365-2265.2011.04309.x [DOI] [PubMed] [Google Scholar]
- 105. Marra AM, Improda N, Capalbo D, et al. Cardiovascular abnormalities and impaired exercise performance in adolescents with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2015;100(2):644‐652. 10.1210/jc.2014-1805 [DOI] [PubMed] [Google Scholar]
- 106. Tamhane S, Rodriguez-Gutierrez R, Iqbal AM, et al. Cardiovascular and metabolic outcomes in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(11):4097‐4103. 10.1210/jc.2018-01862 [DOI] [PubMed] [Google Scholar]
- 107. Casteras A, De Silva P, Rumsby G, Conway GS. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin Endocrinol (Oxf). 2009;70(6):833‐837. 10.1111/j.1365-2265.2009.03563.x [DOI] [PubMed] [Google Scholar]
- 108. Dwiggins M, Brookner B, Fowler K, Veeraraghavan P, Gomez-Lobo V, Merke DP. Multidimensional aspects of female sexual function in congenital adrenal hyperplasia: a case-control study. J Endocr Soc. 2020;4(11):bvaa131. 10.1210/jendso/bvaa131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rapp M, Duranteau L, van de Grift TC, et al. Self- and proxy-reported outcomes after surgery in people with disorders/differences of sex development (DSD) in Europe (DSD-LIFE). J Pediatr Urol. 2021;17(3):353‐365. 10.1016/j.jpurol.2020.12.007 [DOI] [PubMed] [Google Scholar]
- 110. Sircili MH, Bachega TS, Madureira G, Gomes L, Mendonca BB, Dénes FT. Surgical treatment after failed primary correction of urogenital sinus in female patients with virilizing congenital adrenal hyperplasia: are good results possible? Front Pediatr. 2016;4:118. 10.3389/fped.2016.00118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hagenfeldt K, Janson PO, Holmdahl G, et al. Fertility and pregnancy outcome in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Hum Reprod. 2008;23(7):1607‐1613. 10.1093/humrep/den118 [DOI] [PubMed] [Google Scholar]
- 112. Frisen L, Nordenstrom A, Falhammar H, et al. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J Clin Endocrinol Metab. 2009;94(9):3432‐3439. 10.1210/jc.2009-0636 [DOI] [PubMed] [Google Scholar]
- 113. Auer MK, Paizoni L, Neuner M, et al. 11-Oxygenated androgens and their relation to hypothalamus-pituitary-gonadal-axis disturbances in adults with congenital adrenal hyperplasia. J Steroid Biochem Mol Biol. 2021;212:105921. 10.1016/j.jsbmb.2021.105921 [DOI] [PubMed] [Google Scholar]
- 114. Turcu AF, Mallappa A, Elman MS, et al. 11-Oxygenated androgens are biomarkers of adrenal volume and testicular adrenal rest tumors in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2017;102(8):2701‐2710. 10.1210/jc.2016-3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Reichman DE, White PC, New MI, Rosenwaks Z. Fertility in patients with congenital adrenal hyperplasia. Fertil Steril. 2014;101(2):301‐309. 10.1016/j.fertnstert.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 116. Labarta E, Martínez-Conejero JA, Alamá P, et al. Endometrial receptivity is affected in women with high circulating progesterone levels at the end of the follicular phase: a functional genomics analysis. Hum Reprod. 2011;26(7):1813‐1825. 10.1093/humrep/der126 [DOI] [PubMed] [Google Scholar]
- 117. Couzinet B, Young J, Brailly S, Chanson P, Thomas JL, Schaison G. The antigonadotropic activity of progestins (19-nortestosterone and 19-norprogesterone derivatives) is not mediated through the androgen receptor. J Clin Endocrinol Metab. 1996;81(12):4218‐4223. 10.1210/jcem.81.12.8954018 [DOI] [PubMed] [Google Scholar]
- 118. Soules MR, Steiner RA, Clifton DK, Cohen NL, Aksel S, Bremner WJ. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J Clin Endocrinol Metab. 1984;58(2):378‐383. 10.1210/jcem-58-2-378 [DOI] [PubMed] [Google Scholar]
- 119. Couzinet B, Young J, Kujas M, et al. The antigonadotropic activity of a 19-nor-progesterone derivative is exerted both at the hypothalamic and pituitary levels in women. J Clin Endocrinol Metab. 1999;84(11):4191‐4196. 10.1210/jcem.84.11.6162 [DOI] [PubMed] [Google Scholar]
- 120. Bachelot A, Chakhtoura Z, Plu-Bureau G, et al. Influence of hormonal control on LH pulsatility and secretion in women with classical congenital adrenal hyperplasia. Eur J Endocrinol. 2012;167(4):499‐505. 10.1530/eje-12-0454 [DOI] [PubMed] [Google Scholar]
- 121. Dewis P, Newman M, Ratcliffe WA, Anderson DC. Does testosterone affect the normal menstrual cycle? Clin Endocrinol (Oxf). 1986;24(5):515‐521. 10.1111/j.1365-2265.1986.tb03280.x [DOI] [PubMed] [Google Scholar]
- 122. Dunaif A. Do androgens directly regulate gonadotropin secretion in the polycystic ovary syndrome? J Clin Endocrinol Metab. 1986;63(1):215‐221. 10.1210/jcem-63-1-215 [DOI] [PubMed] [Google Scholar]
- 123. Serafini P, Silva PD, Paulson RJ, Elkind-Hirsch K, Hernandez M, Lobo RA. Acute modulation of the hypothalamic-pituitary axis by intravenous testosterone in normal women. Am J Obstet Gynecol. 1986;155(6):1288‐1292. 10.1016/0002-9378(86)90161-4 [DOI] [PubMed] [Google Scholar]
- 124. MacKay D, Nordenstrom A, Falhammar H. Bilateral adrenalectomy in congenital adrenal hyperplasia: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2018;103(5):1767‐1778. 10.1210/jc.2018-00217 [DOI] [PubMed] [Google Scholar]
- 125. Hoepffner W, Schulze E, Bennek J, Keller E, Willgerodt H. Pregnancies in patients with congenital adrenal hyperplasia with complete or almost complete impairment of 21-hydroxylase activity. Fertil Steril. 2004;81(5):1314‐1321. 10.1016/j.fertnstert.2003.10.024 [DOI] [PubMed] [Google Scholar]
- 126. Claahsen-van der Grinten HL, Stikkelbroeck N, Falhammar H, Reisch N. Management of endocrine disease: gonadal dysfunction in congenital adrenal hyperplasia. Eur J Endocrinol. 2021;184(3):R85‐R97. 10.1530/EJE-20-1093 [DOI] [PubMed] [Google Scholar]
- 127. Bouvattier C, Esterle L, Renoult-Pierre P, et al. Clinical outcome, hormonal status, gonadotrope axis, and testicular function in 219 adult men born with classic 21-hydroxylase deficiency. A French national survey. J Clin Endocrinol Metab. 2015;100(6):2303‐2313. 10.1210/jc.2014-4124 [DOI] [PubMed] [Google Scholar]
- 128. Engels M, Span PN, van Herwaarden AE, Sweep F, Stikkelbroeck N, Claahsen-van der Grinten HL. Testicular adrenal rest tumors: current insights on prevalence, characteristics, origin, and treatment. Endocr Rev. 2019;40(4):973‐987. 10.1210/er.2018-00258 [DOI] [PubMed] [Google Scholar]
- 129. Kim MS, Goodarzian F, Keenan MF, et al. Testicular adrenal rest tumors in boys and young adults with congenital adrenal hyperplasia. J Urol. 2017;197(3 Part 2):931‐936. 10.1016/j.juro.2016.09.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chihaoui M, Kanoun F, Chaker F, et al. Testicular adrenal rest tumours in young adult males with congenital adrenal hyperplasia: prevalence and impact on testicular function. Andrologia. 2016;48(1):45‐50. 10.1111/and.12416 [DOI] [PubMed] [Google Scholar]
- 131. Reisch N, Flade L, Scherr M, et al. High prevalence of reduced fecundity in men with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2009;94(5):1665‐1670. 10.1210/jc.2008-1414 [DOI] [PubMed] [Google Scholar]
- 132. Engels M, Gehrmann K, Falhammar H, et al. Gonadal function in adult male patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(3):285‐294. 10.1530/EJE-17-0862 [DOI] [PubMed] [Google Scholar]
- 133. Claahsen-van der Grinten HL, Speiser PW, Ahmed SF, et al. Congenital adrenal hyperplasia—current insights in pathophysiology, diagnostics and management. Endocr Rev. 2021;43(1):91‐159. 10.1210/endrev/bnab016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Falhammar H, Nystrom HF, Ekstrom U, Granberg S, Wedell A, Thoren M. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2012;166(3):441‐449. 10.1530/EJE-11-0828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Smeets EEJW, Span PN, van Herwaarden AE, et al. Molecular characterization of testicular adrenal rest tumors in congenital adrenal hyperplasia: lesions with both adrenocortical and Leydig cell features. J Clin Endocrinol Metabol. 2015;100(3):E524‐E530. 10.1210/jc.2014-2036 [DOI] [PubMed] [Google Scholar]
- 136. Engels M, Span PN, Mitchell RT, et al. GATA transcription factors in testicular adrenal rest tumours. Endocr Connect. 2017;6(8):866‐875. 10.1530/EC-17-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Turcu AF, Rege J, Chomic R, et al. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100(6):2283‐2290. 10.1210/jc.2015-1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Turcu AF, Nanba AT, Auchus RJ. The rise, fall, and resurrection of 11-oxygenated androgens in human physiology and disease. Horm Res Paediatr. 2018;89(5):284‐291. 10.1159/000486036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schroder MAM, Turcu AF, O'Day P, et al. Production of 11-oxygenated androgens by testicular adrenal rest tumors. J Clin Endocrinol Metab. 2022;107(1):e272‐e280. 10.1210/clinem/dgab598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Avila NA, Premkumar A, Merke DP. Testicular adrenal rest tissue in congenital adrenal hyperplasia: comparison of MR imaging and sonographic findings. AJR Am J Roentgenol. 1999;172(4):1003‐1006. 10.2214/ajr.172.4.10587136 [DOI] [PubMed] [Google Scholar]
- 141. Yilmaz R, Sahin D, Aghayev A, et al. Sonography and magnetic resonance imaging characteristics of testicular adrenal rest tumors. Pol J Radiol. 2017;82:583‐588. 10.12659/PJR.901986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Stikkelbroeck NM, Otten BJ, Pasic A, et al. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2001;86(12):5721‐5728. 10.1210/jcem.86.12.8090 [DOI] [PubMed] [Google Scholar]
- 143. Inaba H, Suzuki S, Shigematsu S, et al. Leydig cell tumor and malignant lymphoma in a patient with nonclassical 21-hydroxylase deficiency. Intern Med. 2009;48(8):601‐605. 10.2169/internalmedicine.48.1733 [DOI] [PubMed] [Google Scholar]
- 144. Santoriello A, Benevento R, Petronella P, Perna G, Canonico S. Congenital adrenal hyperplasia and Leydig cell tumor of testis. Case report and review of literature. Ann Ital Chir. 2010;81(6):445‐448. [PubMed] [Google Scholar]
- 145. Entezari P, Kajbafzadeh AM, Mahjoub F, Vasei M. Leydig cell tumor in two brothers with congenital adrenal hyperplasia due to 11-beta hydroxylase deficiency: a case report. Int Urol Nephrol. 2012;44(1):133‐137. 10.1007/s11255-010-9890-9 [DOI] [PubMed] [Google Scholar]
- 146. Ashley RA, McGee SM, Isotaolo PA, Kramer SA, Cheville JC. Clinical and pathological features associated with the testicular tumor of the adrenogenital syndrome. J Urol. 2007;177(2):546‐549. 10.1016/j.juro.2006.09.041 [DOI] [PubMed] [Google Scholar]
- 147. Wang Z, Yang S, Shi H, et al. Histopathological and immunophenotypic features of testicular tumour of the adrenogenital syndrome. Histopathology. 2011;58(7):1013‐1018. 10.1111/j.1365-2559.2011.03861.x [DOI] [PubMed] [Google Scholar]
- 148. Claahsen-van der Grinten HL, Otten BJ, Takahashi S, et al. Testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia: evaluation of pituitary-gonadal function before and after successful testis-sparing surgery in eight patients. J Clin Endocrinol & Metabol. 2007;92(2):612‐615. 10.1210/jc.2006-1311 [DOI] [PubMed] [Google Scholar]
- 149. Auchus RJ. Management considerations for the adult with congenital adrenal hyperplasia. Mol Cell Endocrinol. 2015;408:190‐197. 10.1016/j.mce.2015.01.039 [DOI] [PubMed] [Google Scholar]
- 150. Kulle A, Krone N, Holterhus PM, et al. Steroid hormone analysis in diagnosis and treatment of DSD: position paper of EU COST action BM 1303 “DSDnet.” Eur J Endocrinol. 2017;176(5):P1‐P9. 10.1530/EJE-16-0953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Claahsen-van der Grinten HL, Otten BJ, Stikkelbroeck MM, Sweep FC, Hermus AR. Testicular adrenal rest tumours in congenital adrenal hyperplasia. Best Pract Res Clin Endocrinol Metab. 2009;23(2):209‐220. 10.1016/j.beem.2008.09.007 [DOI] [PubMed] [Google Scholar]
- 152. Bry-Gauillard H, Cartes A, Young J. Mitotane for 21-hydroxylase deficiency in an infertile man. N Engl J Med. 2014;371(21):2042‐2044. 10.1056/NEJMc1410041 [DOI] [PubMed] [Google Scholar]
- 153. Bachelot A, Lapoirie M, Dulon J, Leban M, Renard Penna R, Touraine P. Effects of mitotane on testicular adrenal rest tumors in congenital adrenal hyperplasia due to 21-hydroxylase deficiency: a retrospective series of five patients. Eur J Endocrinol. 2021;184(3):365‐371. 10.1530/EJE-20-0787 [DOI] [PubMed] [Google Scholar]
- 154. Rohayem J, Tuttelmann F, Mallidis C, Nieschlag E, Kliesch S, Zitzmann M. Restoration of fertility by gonadotropin replacement in a man with hypogonadotropic azoospermia and testicular adrenal rest tumors due to untreated simple virilizing congenital adrenal hyperplasia. Eur J Endocrinol. 2014;170(4):K11‐K17. 10.1530/EJE-13-0449 [DOI] [PubMed] [Google Scholar]
- 155. Claahsen-van der Grinten HL, Otten BJ, Hermus ARMM, Sweep FCGJ, Hulsbergen-van de Kaa CA. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil Steril. 2008;89(3):597‐601. 10.1016/j.fertnstert.2007.03.051 [DOI] [PubMed] [Google Scholar]
- 156. Tiryaki T, Aycan Z, Hucumenoglu S, Atayurt H. Testis sparing surgery for steroid unresponsive testicular tumors of the congenital adrenal hyperplasia. Pediatr Surg Int. 2005;21(10):853‐855. 10.1007/s00383-005-1547-x [DOI] [PubMed] [Google Scholar]
- 157. Abbe CR, Page ST, Thirumalai A. Male contraception. Yale J Biol Med. 2020;93(4):603‐613. [PMC free article] [PubMed] [Google Scholar]
- 158. Tiitinen A, Valimaki M. Primary infertility in 45-year-old man with untreated 21-hydroxylase deficiency: successful outcome with glucocorticoid therapy. J Clin Endocrinol Metab. 2002;87(6):2442‐2445. 10.1210/jcem.87.6.8616 [DOI] [PubMed] [Google Scholar]
- 159. Kalachanis I, Rousso D, Kourtis A, Goutzioulis F, Makedos G, Panidis D. Reversible infertility, pharmaceutical and spontaneous, in a male with late onset congenital adrenal hyperplasia, due to 21-hydroxylase deficiency. Arch Androl. 2002;48(1):37‐41. [DOI] [PubMed] [Google Scholar]
- 160. Nordenstrom A, Lajic S, Falhammar H. Clinical outcomes in 21-hydroxylase deficiency. Curr Opin Endocrinol Diabetes Obes. 2021;28(3):318‐324. 10.1097/MED.0000000000000625 [DOI] [PubMed] [Google Scholar]
- 161. Nordenstrom A, Lajic S, Falhammar H. Long-term outcomes of congenital adrenal hyperplasia. Endocrinol Metab (Seoul). 2022;37(4):587‐598. 10.3803/EnM.2022.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Falhammar H, Filipsson H, Holmdahl G, et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2007;92(1):110‐116. 10.1210/jc.2006-1350 [DOI] [PubMed] [Google Scholar]
- 163. Falhammar H, Filipsson Nystrom H, Wedell A, Brismar K, Thoren M. Bone mineral density, bone markers, and fractures in adult males with congenital adrenal hyperplasia. Eur J Endocrinol. 2013;168(3):331‐341. 10.1530/EJE-12-0865 [DOI] [PubMed] [Google Scholar]
- 164. Whittle E, Falhammar H. Glucocorticoid regimens in the treatment of congenital adrenal hyperplasia: a systematic review and meta-analysis. J Endocr Soc. 2019;3(6):1227‐1245. 10.1210/js.2019-00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000;15(6):993‐1000. 10.1359/jbmr.2000.15.6.993 [DOI] [PubMed] [Google Scholar]
- 166. van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int. 2002;13(10):777‐787. 10.1007/s001980200108 [DOI] [PubMed] [Google Scholar]
- 167. Chappard D, Legrand E, Basle MF, et al. Altered trabecular architecture induced by corticosteroids: a bone histomorphometric study. J Bone Miner Res. 1996;11(5):676‐685. 10.1002/jbmr.5650110516 [DOI] [PubMed] [Google Scholar]
- 168. Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102(2):274‐282. 10.1172/JCI2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Dalle Carbonare L, Arlot ME, Chavassieux PM, Roux JP, Portero NR, Meunier PJ. Comparison of trabecular bone microarchitecture and remodeling in glucocorticoid-induced and postmenopausal osteoporosis. J Bone Miner Res. 2001;16(1):97‐103. 10.1359/jbmr.2001.16.1.97 [DOI] [PubMed] [Google Scholar]
- 170. Kasperk CH, Wakley GK, Hierl T, Ziegler R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J Bone Miner Res. 1997;12(3):464‐471. 10.1359/jbmr.1997.12.3.464 [DOI] [PubMed] [Google Scholar]
- 171. El-Maouche D, Collier S, Prasad M, Reynolds JC, Merke DP. Cortical bone mineral density in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Clin Endocrinol (Oxf). 2015;82(3):330‐337. 10.1111/cen.12507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Zimmermann A, Sido PG, Schulze E, et al. Bone mineral density and bone turnover in Romanian children and young adults with classical 21-hydroxylase deficiency are influenced by glucocorticoid replacement therapy. Clin Endocrinol (Oxf). 2009;71(4):477‐484. 10.1111/j.1365-2265.2008.03518.x [DOI] [PubMed] [Google Scholar]
- 173. Sciannamblo M, Russo G, Cuccato D, Chiumello G, Mora S. Reduced bone mineral density and increased bone metabolism rate in young adult patients with 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2006;91(11):4453‐4458. 10.1210/jc.2005-2823 [DOI] [PubMed] [Google Scholar]
- 174. Espinosa Reyes TM, Leyva Gonzalez G, Dominguez Alonso E, Falhammar H. Bone mass in young patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm Res Paediatr. 2021;94(1-2):1‐8. 10.1159/000515833 [DOI] [PubMed] [Google Scholar]
- 175. Riehl G, Reisch N, Roehle R, Claahsen van der Grinten H, Falhammar H, Quinkler M. Bone mineral density and fractures in congenital adrenal hyperplasia: findings from the DSD-LIFE study. Clin Endocrinol (Oxf). 2020;92(4):284‐294. 10.1111/cen.14149 [DOI] [PubMed] [Google Scholar]
- 176. Hagenfeldt K, Martin Ritzen E, Ringertz H, Helleday J, Carlstrom K. Bone mass and body composition of adult women with congenital virilizing 21-hydroxylase deficiency after glucocorticoid treatment since infancy. Eur J Endocrinol. 2000;143(5):667‐671. 10.1530/eje.0.1430667 [DOI] [PubMed] [Google Scholar]
- 177. Halper A, Sanchez B, Hodges JS, et al. Bone mineral density and body composition in children with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2018;88(6):813‐819. 10.1111/cen.13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rangaswamaiah S, Gangathimmaiah V, Nordenstrom A, Falhammar H. Bone mineral density in adults with congenital adrenal hyperplasia: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2020;11:493. 10.3389/fendo.2020.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Chakhtoura Z, Bachelot A, Samara-Boustani D, et al. Impact of total cumulative glucocorticoid dose on bone mineral density in patients with 21-hydroxylase deficiency. Eur J Endocrinol. 2008;158(6):879‐887. 10.1530/EJE-07-0887 [DOI] [PubMed] [Google Scholar]
- 180. Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. 2020;16(8):437‐447. 10.1038/s41574-020-0341-0 [DOI] [PubMed] [Google Scholar]
- 181. Li L, Bensing S, Falhammar H. Rate of fracture in patients with glucocorticoid replacement therapy: a systematic review and meta-analysis. Endocrine. 2021;74(1):29‐37. 10.1007/s12020-021-02723-z [DOI] [PubMed] [Google Scholar]
- 182. Falhammar H, Frisen L, Hirschberg AL, Nordenskjold A, Almqvist C, Nordenstrom A. Increased prevalence of fractures in congenital adrenal hyperplasia: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2022;107(2):e475‐e486. 10.1210/clinem/dgab712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Falhammar H, Filipsson H, Holmdahl G, et al. Increased liver enzymes in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr J. 2009;56(4):601‐608. 10.1507/endocrj.k08e-312 [DOI] [PubMed] [Google Scholar]
- 184. Falhammar H, Filipsson Nystrom H, Wedell A, Thoren M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur J Endocrinol. 2011;164(2):285‐293. https://doi.org/EJE-10-0877 [DOI] [PubMed] [Google Scholar]
- 185. Falhammar H, Frisen L, Hirschberg AL, et al. Increased cardiovascular and metabolic morbidity in patients with 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Clin Endocrinol Metab. 2015;100(9):3520‐3528. 10.1210/JC.2015-2093 [DOI] [PubMed] [Google Scholar]
- 186. Falhammar H, Claahsen-van der Grinten H, Reisch N, et al. Health status in 1040 adults with disorders of sex development (DSD): a European multicenter study. Endocr Connect. 2018;7(3):466‐478. 10.1530/EC-18-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Bachelot A, Golmard JL, Dulon J, et al. Determining clinical and biological indicators for health outcomes in adult patients with childhood onset of congenital adrenal hyperplasia. Eur J Endocrinol. 2015;173(2):175‐184. 10.1530/EJE-14-0978 [DOI] [PubMed] [Google Scholar]
- 188. Hirschberg AL, Gidlof S, Falhammar H, et al. Reproductive and perinatal outcomes in women with congenital adrenal hyperplasia: a population-based cohort study. J Clin Endocrinol Metab. 2021;106(2):e957‐e965. 10.1210/clinem/dgaa801 [DOI] [PubMed] [Google Scholar]
- 189. Falhammar H, Frisen L, Hirschberg AL, Nordenskjold A, Almqvist C, Nordenstrom A. Increased risk of autoimmune disorders in 21-hydroxylase deficiency: a Swedish population-based national cohort study. J Endocr Soc. 2019;3(5):1039‐1052. 10.1210/js.2019-00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Mooij CF, Kroese JM, Sweep FC, Hermus AR, Tack CJ. Adult patients with congenital adrenal hyperplasia have elevated blood pressure but otherwise a normal cardiovascular risk profile. PLoS One. 2011;6(9):e24204. 10.1371/journal.pone.0024204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kannel WB, Kannel C, PaffenbargerRS, Jr., Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J. 1987;113(6):1489‐1494. 10.1016/0002-8703(87)90666-1 [DOI] [PubMed] [Google Scholar]
- 192. Mensink GB, Hoffmeister H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur Heart J. 1997;18(9):1404‐1410. 10.1093/oxfordjournals.eurheartj.a015465 [DOI] [PubMed] [Google Scholar]
- 193. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7(10):1025‐1038. 10.1016/j.jcmg.2013.11.014 [DOI] [PubMed] [Google Scholar]
- 194. Wasniewska M, Balsamo A, Valenzise M, et al. Increased large artery intima media thickness in adolescents with either classical or non-classical congenital adrenal hyperplasia. J Endocrinol Invest. 2013;36(2):12‐15. 10.3275/8194 [DOI] [PubMed] [Google Scholar]
- 195. Falhammar H, Frisen L, Norrby C, et al. Increased mortality in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(12):E2715‐E2721. 10.1210/jc.2014-2957 [DOI] [PubMed] [Google Scholar]
- 196. Jenkins-Jones S, Parviainen L, Porter J, et al. Poor compliance and increased mortality, depression and healthcare costs in patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178(4):309‐320. 10.1530/EJE-17-0895 [DOI] [PubMed] [Google Scholar]
- 197. Falhammar H, Thoren M. Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine. 2012;41(3):355‐373. 10.1007/s12020-011-9591-x [DOI] [PubMed] [Google Scholar]
- 198. Madathilethu J, Roberts M, Peak M, Blair J, Prescott R, Ford JL. Content uniformity of quartered hydrocortisone tablets in comparison with mini-tablets for paediatric dosing. BMJ Paediatr Open. 2018;2(1):e000198. 10.1136/bmjpo-2017-000198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Watson C, Webb EA, Kerr S, Davies JH, Stirling H, Batchelor H. How close is the dose? Manipulation of 10 mg hydrocortisone tablets to provide appropriate doses to children. Int J Pharm. 2018;545(1-2):57‐63. 10.1016/j.ijpharm.2018.04.054 [DOI] [PubMed] [Google Scholar]
- 200. Abu-Geras D, Hadziomerovic D, Leau A, et al. Accuracy of tablet splitting and liquid measurements: an examination of who, what and how. J Pharm Pharmacol. 2017;69(5):603‐612. 10.1111/jphp.12671 [DOI] [PubMed] [Google Scholar]
- 201. Verrue C, Mehuys E, Boussery K, Remon JP, Petrovic M. Tablet-splitting: a common yet not so innocent practice. J Adv Nurs. 2011;67(1):26‐32. 10.1111/j.1365-2648.2010.05477.x [DOI] [PubMed] [Google Scholar]
- 202. Al-Rayess H, Fleissner K, Jaber M, Brundage RC, Sarafoglou K. Manipulation of hydrocortisone tablets leads to iatrogenic Cushing syndrome in a 6-year-old girl with CAH. J Endocr Soc. 2020;4(8):bvaa091. 10.1210/jendso/bvaa091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Sarafoglou K, Gonzalez-Bolanos MT, Zimmerman CL, Boonstra T, Yaw Addo O, Brundage R. Comparison of cortisol exposures and pharmacodynamic adrenal steroid responses to hydrocortisone suspension vs. commercial tablets. J Clin Pharmacol. 2015;55(4):452‐457. 10.1002/jcph.424 [DOI] [PubMed] [Google Scholar]
- 204. Barillas JE, Eichner D, Van Wagoner R, Speiser PW. Iatrogenic Cushing syndrome in a child with congenital adrenal hyperplasia: erroneous compounding of hydrocortisone. J Clin Endocrinol Metab. 2018;103(1):7‐11. 10.1210/jc.2017-01595 [DOI] [PubMed] [Google Scholar]
- 205. Gudeman J, Jozwiakowski M, Chollet J, Randell M. Potential risks of pharmacy compounding. Drugs R D. 2013;13(1):1‐8. 10.1007/s40268-013-0005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Neumann U, Burau D, Spielmann S, et al. Quality of compounded hydrocortisone capsules used in the treatment of children. Eur J Endocrinol. 2017;177(2):239‐242. 10.1530/EJE-17-0248 [DOI] [PubMed] [Google Scholar]
- 207. Neumann U, Whitaker MJ, Wiegand S, et al. Absorption and tolerability of taste-masked hydrocortisone granules in neonates, infants and children under 6 years of age with adrenal insufficiency. Clin Endocrinol (Oxf). 2018;88(1):21‐29. 10.1111/cen.13447 [DOI] [PubMed] [Google Scholar]
- 208. Sarafoglou K, Barnes CN, Huang M, et al. Tildacerfont in adults with classic congenital adrenal hyperplasia: results from two phase 2 studies. J Clin Endocrinol Metab. 2021;106(11):e4666‐e4679. 10.1210/clinem/dgab438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Auchus RJ, Sarafoglou K, Fechner PY, et al. Crinecerfont lowers elevated hormone markers in adults with 21-hydroxylase deficiency congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2022;107(3):801‐812. 10.1210/clinem/dgab749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Auchus RJ, Buschur EO, Chang AY, et al. Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2014;99(8):2763‐2770. 10.1210/jc.2014-1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.