Abstract
Prolactinomas are the most common pituitary tumor histotype, with microprolactinomas being prevalent in women and macroprolactinomas in men. Hyperprolactinemia is among the most common causes of hypogonadotropic hypogonadism in both sexes, prompting medical advice for hypogonadism (infertility, oligo-amenorrhea, impotence, osteoporosis/osteopenia) in both sexes, and for signs and symptoms of mass effects (hypopituitarism, visual loss, optic chiasm compression, cranial nerve deficits, headaches) predominantly in men. Diagnostic workup involves a single prolactin measurement and pituitary imaging, but some laboratory artifacts (ie, the “hook effect” and macroprolactin) can complicate or delay the diagnosis. The treatment of choice for prolactinomas is represented by dopamine agonists, mainly cabergoline, which are able to induce disease control, restore fertility in both sexes, and definitively cure one-third of patients, thus permitting treatment discontinuation. Pregnancy and menopause may promote spontaneous prolactin decline and anticipate cabergoline discontinuation in women. Surgery and/or radiotherapy are indicated in case of resistance to cabergoline not overcome by the increase in drug dose up to the maximally tolerated or the patient's personal choice of surgery. The evidence of resistance to cabergoline in invasive and proliferative tumors may indicate biological aggressiveness, thus requiring alternative therapeutic approaches mainly based on temozolomide use as monotherapy or combined with radiotherapy. In uncontrolled patients, new medical approaches (alternative hormonal treatments, cytotoxic drugs, peptide receptor radionuclide therapy, mTOR/Akt inhibitors, tyrosine kinase inhibitors, or immunotherapy) may be offered but the experience collected to date is still very scant. This article reviews different facets of prolactinomas and discusses approaches to the condition in more common clinical situations.
Keywords: prolactin, hyperprolactinemia, pituitary tumor, dopamine agonists, cabergoline, treatment withdrawal, pregnancy, menopause
CASE 1
In February 2019, a male patient aged 40 years was referred to a neurologist because of intense and recurrent headaches. Among the examinations performed to investigate the headaches, the brain magnetic resonance imaging (MRI) scans showed an intrasellar pituitary microadenoma 8 × 6 × 8 mm in size (tumor volume, 1.96 cm3). The patient was then referred for endocrinological advice. Medical history revealed that the patient was the father of 2 children aged 4 years and 2 years, respectively. Decreased libido and erectile dysfunction occurred over the past year. At the first endocrinological assessment, pituitary function investigation revealed hyperprolactinemia (prolactin [PRL], 900 µg/L; normal range, 5-20 µg/L) and concomitant hypogonadotropic hypogonadism (FSH, 1.1 IU/mL; LH, 1.3 IU/mL; testosterone, 175 ng/dL). A concomitant seminal fluid examination revealed the presence of mild oligospermia, whereas at the dual-energy x-ray absorptiometry scan bone mineral density was normal. Therefore, the diagnosis of pituitary microadenoma with hyperprolactinemia was given, and cabergoline therapy was started at the dose of 1 mg/wk. The clinical course was indolent over the following 6 months and responsiveness to cabergoline was acceptable, with a significant decrease in PRL levels (180 µg/L, Δ = −80%), together with a reduction in the headache intensity and frequency and an improvement in libido and erectile function. Therefore, given that PRL levels were progressively decreasing, the treatment with cabergoline was maintained at the same dose.
CASE 2
In December 2009, a woman aged 26 years was referred to endocrinological consultation for infertility. Medical history revealed oligo-amenorrhea and mild hirsutism (Ferriman–Gallway score = 9) over the past 10 years, associated with progressive weight gain in the past 3 years. At the gynecological ultrasounds performed over several years, the ovaries displayed a multifollicular aspect with no clear evidence of polycystic ovary syndrome, whereas hormonal assessment revealed normal androgen levels associated with mild hyperprolactinemia (up to 36 µg/L; normal range, 5 -25 µg/L). The patient had received treatment with oral contraceptives for 2 years, from 2004 to 2006, with rapid relapse of the clinical syndrome after the oral contraceptives were withdrawn. At the first endocrinological assessment, the patient reported amenorrhea over the past 9 months, recurrent headaches, and moderate hyperprolactinemia (PRL, 126.7 µg/L). A pituitary MRI scan was performed and revealed a microadenoma of 8 mm maximal diameter. The patient's facial characteristics (prominent frontal and zygomatic bones, enlarged nose, and mandibular prognathism) were suggestive of concomitant GH hypersecretion, but GH and IGF-I levels were within the normal range (GH, 0.3 µg/L; IGF-I, 206 µg/L). Therefore, the diagnosis of pituitary microadenoma with hyperprolactinemia was carried out, and cabergoline therapy was started at the dose of 0.5 mg/wk. The clinical course was indolent over the years and responsiveness to cabergoline was optimal, with complete PRL normalization (PRL, 18 µg/L), and a 50% shrinkage in pituitary tumor size, associated with full recovery of the clinical syndrome. In November 2016, the pituitary MRI scan revealed a 50% increase in maximal tumor diameter, from 3 to 6 mm, suggesting tumor regrowth. PRL levels slightly increased to 28 µg/L, thus requiring an increase in cabergoline dose to 1 mg/wk. One year later, PRL remained stable at 27 µg/L and pituitary MRI scans revealed a 25% decrease in maximal tumor diameter, but the patient reported persistent headache and asthenia. In September 2018, the patient was discovered to be pregnant; therefore, cabergoline was promptly withdrawn. The patient underwent regular endocrinological controls during gestation; no impairment in the visual field was recorded, and a term delivery without maternal and fetal complications occurred in March 2019. The patient skipped postpartum controls for 1 year during breastfeeding and required new endocrinological consultation only in March 2020, when the first PRL assessment 4 months after cessation of breastfeeding result was as high as 30.7 µg/L.
CASE 3
In November 2004, a male patient aged 35 years was referred to ophthalmologic consultation because of the occurrence of visual disturbances, particularly the narrowing of the visual field. Concomitant visual field examination revealed bitemporal hemianopsia. Medical history revealed that the patient was the father of 2 children aged 6 years and 4 years, respectively. Decreased libido and frequent headaches occurred over the past 3 years and were associated with weight gain in the past 5 years. A brain MRI scan was then performed to evaluate the optic chiasm region, and a pituitary tumor 30 × 25 mm in size with intrasellar, suprasellar, and right parasellar extension, partially obliterating the pontine cistern, was seen. At the first endocrinological assessment, pituitary function investigation revealed severe hyperprolactinemia (PRL, 8040 µg/L; normal range, 5-20 µg/L) and hypogonadotropic hypogonadism (FSH < 0.1 IU/mL, LH <0.1 IU/mL, testosterone, 103 ng/dL), whereas the remaining pituitary function was preserved despite pituitary compression by the tumor mass. A concomitant seminal fluid examination revealed the presence of azoospermia, whereas a dual-energy x-ray absorptiometry scan revealed the presence of osteopenia. Therefore, the diagnosis of a PRL-secreting pituitary tumor was determined, and in February 2005 the patient underwent transsphenoidal surgery as first-line treatment to decompress the optic chiasm. The report of the histological examination lay for invasive prolactinoma with chromophobic cells, sparsely granulated pattern, characterized by numerous mitoses (mitotic index = 3-7 M/10 HPF) and Ki-67%/MIB-1 proliferation index as high as 10%. At the 1-month postoperative evaluation, PRL was 386 µg/L, and pituitary MRI scans detected a macroadenoma 32 × 20 × 28 mm in size (tumor volume, 9.318 cm3), invading the cavernous sinuses bilaterally and the subarachnoid space of the pontine cistern. Aggressive biological behavior resulting from rapid tumor regrowth and histological characteristics suggested the diagnosis of an atypical pituitary adenoma according to World Health Organization classification (1). On the basis of this evidence, cabergoline therapy was promptly started at the dose of 1 mg/wk and progressively increased to 2 mg/wk during follow-up. Testosterone replacement therapy was simultaneously started.
Accounting for approximately 40% of the entire cohort of pituitary tumors, prolactinomas are the most common hormone-secreting pituitary adenomas; infertility and gonadal and sexual dysfunction are the most relevant clinical features in both sexes (2). Prolactinomas are the main pathologic cause of PRL excess, albeit several different conditions may induce the increase in PRL levels and should be excluded before a diagnosis is made (Table 1). In clinical practice, microprolactinomas (<10 mm in size) are more frequent than macroprolactinomas (>10 mm in size) and occur more frequently in women, in whom the disturbances of the menstrual cycle and infertility generally prompt rapid medical advice leading to an early diagnosis, as in clinical case 2. Conversely, in men the rate of macroprolactinomas with visual field defects, as in clinical case 3, and hypopituitarism at first presentation is higher (3) and the mean age at diagnosis is at least 10 years delayed compared with women (2-5), given that the most important components of the clinical syndrome are the decrease of libido and/or erectile dysfunction, as in clinical case 3.
Table 1.
Causes of hyperprolactinemia
| Physiologic | Pathologic |
|---|---|
| Coitus | Pituitary stalk section |
| Physical exercise | Primary hypothyroidism |
| Lactation | Granuloma |
| Pregnancy | Infiltrative diseases: sarcoidosis, histiocytosis |
| Sleep | Cranial/hypothalamic radiation |
| Stress | Trauma |
| Pituitary disease | |
| Idiopathic hyperprolactinemia | |
| Acromegaly | |
| Hypophysitis | |
| Macroadenoma (impingement of pituitary stalk) | |
| Macroprolactinemia | |
| Prolactinoma | |
| Surgerya | |
| Traumaa | |
| Rathke's cleft cysta | |
| Hypothalamic: tumorsa: craniopharyngioma, germinoma, hypothalamic metastases, meningioma | |
| Systemic disease | |
| Surgery, herpes zoster, chest or spine lesions | |
| Chronic kidney insufficiency | |
| Liver insufficiency | |
| Liver cirrhosis | |
| Epilepsy/seizure | |
| Polycystic ovarian syndrome | |
| Breast and nipple stimulation | |
| Drugs | |
| Anaesthetics (benzodiazepines, ketamine, propofol) | |
| Anticonvulsants (phenytoin) | |
| Antidepressants (amoxapine, imipramine, amitriptyline, fluoxetine) | |
| H2 antihistamines (cimetidine, ranitidine) | |
| Antihypertensive (α-metildopa, reserpine, verapamil) | |
| Dopamine receptor blockers (metoclopramide, sulpiride, domperidone) | |
| Estrogens/oral contraceptives/oral contraceptives withdrawal) | |
| Neuroleptics/antipsychotics (perphenazine, fluphenazine, flupenthixol, promazine, haloperidol, loxapine, chlorpromazine, pimozide, risperidone) | |
| Opiates (methadone, morphine, heroin) | |
Via stalk effect.
In adults, the estimated prevalence of prolactinomas accounts for 60 to 100 per million population (3, 6, 7). According to a recent review study including different patient series (8), where standardized incidence rates for pituitary tumors range from 4 to 7.39 cases per 100,000/y, prolactinomas have been reported to represent 40% to 66% of all pituitary tumors (8). Patient age has been shown to differently influence incidence rates in men and women: indeed, between the age of 20 and 50 years, the ratio between women and men is estimated to be 10:1, whereas after the sixth decade of life, the frequency of prolactinomas is similar in both sexes (8-11). This difference may reflect that in young females the clinical syndrome resulting from hypogonadism (infertility and oligo-amenorrhea) prompts early medical advice, particularly in case of pregnancy desire. Conversely, in elderly patients, including postmenopausal women, and in men, the diagnosis is due predominantly to mass effects from large tumors. In pediatric and adolescent ages, prolactinomas, albeit rare, represent about 50% of all pituitary tumors (12, 13).
THE INTERPLAY BETWEEN PROLACTIN AND FERTILITY
Hyperprolactinemia is the most common cause of secondary hypogonadism and infertility in both sexes. In infertile women, PRL excess as the main cause of infertility has been found to vary from 7% to 20% of cases, being lower than that reported in women with amenorrhea and/or galactorrhea, but at least 10-fold higher than that of the general population (14). The impact of PRL excess on the reproductive axis reflects its peculiar actions at both the central and peripheral levels (15). At the central level, PRL modulates the reproductive axis by directly suppressing kisspeptin secretion, thus lowering GnRH activation and gonadotropins secretion, so promoting hypogonadism and infertility (16, 17). At the peripheral level, PRL plays a direct inhibitory effect on sexual hormone synthesis and secretion. In women, PRL inhibits estrogen and progesterone synthesis (18-21). In men, PRL receptors have been identified on Leydig cells, Sertoli cells, and epithelial cells of efferent ducts, suggesting a potential role for PRL in promoting steroidogenesis, spermatogenesis, and secretory/adsorptive functions of male reproductive organs (22). In infertile men, the prevalence of PRL increase as the main cause of infertility is still unknown; nevertheless, endocrine disorders, including hyperprolactinemia, reportedly account for only 2% to 4% of cases of male infertility (14).
APPROACH TO PATIENT IDENTIFICATION
Predisposition
Although most pituitary adenomas occur in a sporadic setting, prolactinomas may arise from germline genetic mutations predisposing to pituitary tumor development in the context of some inherited syndromes (23). The association with genetic defects may result in more aggressive clinical behavior and poor responsiveness to standard treatment with dopamine agonists (DA). Particularly, in the context of multiple endocrine neoplasia type 1 (MEN-1) and type 4 (MEN-4), and familial isolated pituitary adenomas, prolactinomas are the most frequent pituitary tumor histotype. Overall, the efficacy of DA has been documented in 51.5% of prolactinomas within MEN-1 (Table 2), but responsiveness to DA has been not consistently reported across studies (24-28), varying from a scant (24, 25) to a good response to DA (26-28), and from an aggressive (24, 25) to an indolent tumor clinical behavior leading to occasional tumor growth without clinical consequences (26-28). To a similar extent, in the context of MEN-4 and familial isolated pituitary adenomas, prolactinomas are larger, more invasive, and less responsive to DA than their sporadic counterparts (29-32), suggesting a more aggressive clinical behavior as compared with sporadic tumors. In the context of Carney complex (CNC), prolactinomas are rare, but histology may reveal somatomammotroph hyperplasia leading to the development of GH- and/or PRL-secreting adenomas. In fact, concomitant GH and PRL hypersecretion has been shown to occur in up to 64% of patients with CNC (33). Concomitant GH and PRL hypersecretion may occur independently in CNC, and the development of acromegaly in patients with a known prolactinoma is rare.
Table 2.
Responsiveness to treatment with dopamine agonists in patients with prolactinomas in the context of a multiple endocrine neoplasia type 1
| Author, year (reference) | Patients | Prolactinomas (%) | Micro | Macro | Dopamine agonist responders (%) |
|---|---|---|---|---|---|
| Burgess, 1996 (24) | 165 | 18a | 7 | 5 | 7 (58.3)b |
| Verges, 2002 (25) | 324 | 84 (26) | 13 | 71 | 35 (42) |
| de Laat, 2015 (26) | 323 | 50 (15.5) | 30 | 20 | 31 (59.6) |
| Goroshi, 2016 (27) | 18 | 8 (44.4) | 3 | 5 | 8 (100) |
| Vannucci, 2017 (28) | 22 | 7 (31.8) | 5 | 2 | 5 (71.4) |
| Total | 852 | 167 (19.6) | 58 (34.7) | 103 (61.7) | 86 (51.5) |
Six patients with normal pituitary scan.
Calculated on 12 patients with tumor evidence at pituitary scan.
Altogether, these findings suggest that for those individuals with prolactinomas who are at risk for carrying a genetic mutation already diagnosed in a relative, genetic screening should be offered after appropriate counseling and an explanation of the potential benefits deriving from early diagnosis (29).
Clinical Presentation at Diagnosis
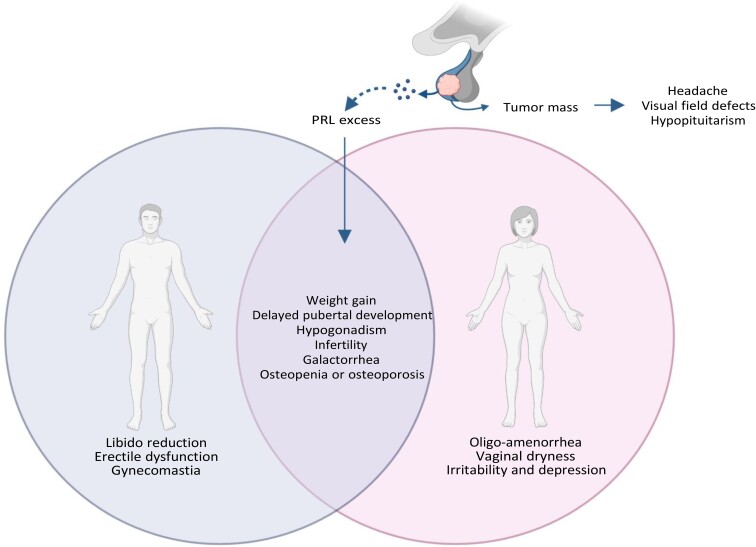
Clinical features of prolactinomas are summarized in Fig. 1. The pituitary adenoma per se may exert several compressive mass effects, resulting in headache, visual field defects, and hypopituitarism (34, 35). Prolactin excess results in both sexes in weight gain, delayed pubertal development, hypogonadism, infertility, galactorrhea, and osteopenia or osteoporosis (34, 35). Increased PRL levels underpin infertility in 7% to 20% of women and 2% to 4% of men (14). Other signs and symptoms are gender-related and include, in men, libido reduction, erectile dysfunction, and gynecomastia and, in women, oligo-amenorrhea, vaginal dryness, irritability, and depression (34, 35). Particularly in women, the classical amenorrhea–galactorrhea syndrome generally encourages rapid medical consultation, as in clinical case 2, whereas weak symptoms of impotence and decreased libido in men might be frequently underestimated, as in clinical case 1, leading to a diagnostic delay (35), as in clinical case 3. However, different pathogenesis in men and women has also been hypothesized because rapidly growing prolactinomas with increased markers of cellular proliferation have been reported to occur more frequently in men (9, 36). These findings have raised the question of whether prolactinomas are more aggressive in men than in women, but the available evidence is still controversial. In men, prolactinomas are usually large and invasive, with signs and symptoms of hypogonadism and mass effects being the most frequent clinical features (37). Additionally, according to the last World Health Organization classification (38), lactotroph tumors in men have a high probability of recurrence because of increased mitotic count and Ki-67 leading to elevated proliferative activity, or plurihormonal PIT-1–positive immunostaining. The latter identifies tumors composed of a monomorphous population of poorly differentiated cells displaying various levels of immunoreactivity for several pituitary tropines, including PRL and GH, β-TSH, and α-subunit, and likely belonging to the acidophilic lineage of adenomas (38). These PIT-1 immunoreactive adenomas belonging to the acidophilic lineage display an intrinsic aggressive behavior and a high degree of invasiveness, low rates of disease-free survival, and a high propensity for recurrence (38).
Figure 1.
Clinical characteristics of patients with prolactinoma. Pituitary tumor per se exerts several compressive mass effects, leading to headache, visual field defects, and hypopituitarism. Prolactin excess results in both sexes in weight gain, delayed pubertal development, hypogonadism, infertility, galactorrhea, and osteopenia or osteoporosis. Other signs and symptoms are gender-related and include in men libido reduction, erectile dysfunction, and gynecomastia; in women, oligo-amenorrhea, vaginal dryness, irritability, and depression. Created with Biorender.com.
Besides the hypogonadism-related signs and symptoms, PRL excess may exert extragonadal systemic effects. Given the direct actions of PRL and dopaminergic tone on pancreatic β cells and adipocytes, hyperprolactinemia may also induce an unfavorable metabolic profile. Increased food intake and weight gain in patients with prolactinomas have been shown to promote altered body composition, insulin resistance, impaired glucose tolerance, and adverse lipid profile, leading to visceral obesity and metabolic syndrome in approximately one-third of patients (39-45). However, the potential impact of hyperprolactinemia-induced hypogonadism on body composition and metabolic profile cannot be excluded. Indeed, men with testosterone and dihydrotestosterone levels in the lower quartiles have been found to have a 2-fold higher risk of developing obesity and metabolic syndrome (46). Similarly, in 40 premenopausal hyperprolactinemic women, in whom FSH, LH, and estrogen levels were in the normal range, but their pulsatile secretion was decreased because of hyperprolactinemia, PRL excess has been found associated with hyperinsulinemia and insulin resistance independently of body weight and leptin and adiponectin levels (44). The altered body composition, characterized by increased fat mass and reduced lean mass (39, 40), together with concomitant secondary hypogonadism, contribute to decreased bone density from the direct and indirect (ie, hyperprolactinemia-induced hypogonadism) effects of prolactin on bone physiology, early alterations in bone turnover markers, reduced bone mass mainly in trabecular rather than cortical bone, delayed peak bone mass acquisition, and high risk of vertebral fractures in both sexes (47-54).
DIAGNOSTIC APPROACH
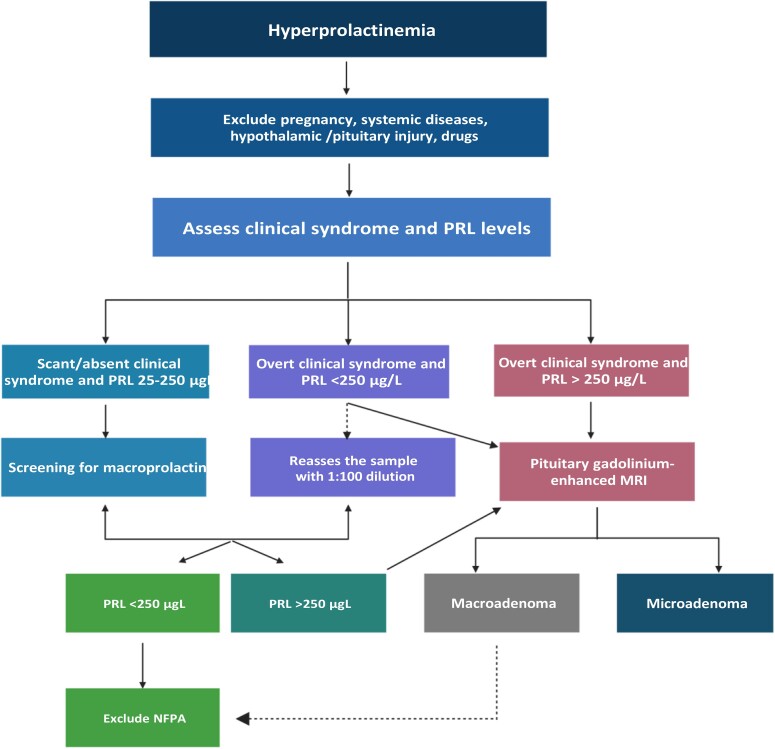
The diagnostic workup for hyperprolactinemia is shown in Fig. 2. As reported in Table 1, a number of physiologic (pregnancy, breastfeeding, stress, exercise, food intake, and sleep) and pathologic conditions (chronic kidney and liver failure, primary hypothyroidism, compression of the pituitary stalk by a non–PRL-secreting pituitary tumor or different parasellar mass, and granulomatous infiltration of the hypothalamus), as well as several drugs (mainly antidepressants, dopamine receptor blockers, dopamine synthesis inhibitors, oral contraceptives, gastrointestinal medications, neuroleptics, and antipsychotics) can induce symptomatic hyperprolactinemia (34, 55). Before a correct diagnosis of hyperprolactinemia is made, such conditions must be investigated and excluded. Attention should be paid to medical history, concomitant medications, and biochemical assessment (34, 55). This is particularly true in the case of modest PRL elevations, which only occasionally may mask the presence of a prolactinoma (34), as in clinical case 2. To ascertain the diagnosis of a PRL-secreting pituitary tumor, a single measurement of serum PRL without excessive venipuncture stress is strongly recommended (34). Breast examination in patients with galactorrhea should not be performed immediately before PRL assessment because any nipple stimulation might result in PRL increase. A level above the upper limit of normal (25 μg/L in women, 20 μg/L in men) raises diagnostic suspicion (34, 55). Conversely, dynamic testing of PRL secretion based on the administration of TRH, L-dopa, nomifensine, and domperidone does not find clinical application currently (34). In uncertain conditions, sampling can be repeated after overnight fasting on a different day in 2 to 3 samples in time course at 15- to 20-minute intervals to minimize the effect of PRL pulsatile secretion (34). PRL levels >250 μg/L generally confirm the diagnosis of prolactinoma, albeit PRL increase over 200 μg/L can be also seen in case of non–PRL-secreting tumor mass, such as nonfunctioning pituitary tumors (34, 55). In presence of a serum PRL level >500 μg/L, the diagnosis of macroprolactinoma can be ruled in (34). Once other causes of hyperprolactinemia (see previous section) have been excluded, a radiological confirmation with pituitary imaging, mainly based on gadolinium-enhanced MRI is required (55). Over the past 20 years, pituitary MRI has progressively replaced computed tomography with IV contrast enhancement because the latter is less effective than MRI in visualizing small tumors on the one hand and in shaping the extension of large or giant tumors on the other (55). Nowadays, a pituitary computed tomography scan is recommended only when MRI is unavailable or contraindicated, such as in patients with cardiac pacemakers, implanted cardiac defibrillators; internal pacing wires; clips for cerebral, carotid, or aortic aneurysm; cochlear implants; any implant held in by magnet; Swan-Ganz catheter; and pregnancy (55). In the latter circumstance, the use of pituitary MRI is not recommended except in the case of clinical confirmation of tumor growth suggested by sudden impairment in the visual field (34), and MRI should be performed without gadolinium after the second trimester of pregnancy (34). In those patients with macroadenomas impinging the optic chiasm, a visual field examination is recommended, whereas visual testing is not mandatory for patients with microadenomas (55).
Figure 2.
Diagnostic workup for hyperprolactinemia. Scarceness or absence of the peculiar clinical syndrome of PRL excess in patients with PRL levels <250 μg/L should suggest the screening for macroprolactin before performing a pituitary MRI (left). In patients with overt clinical syndrome of PRL excess and PRL values >250 μg/L, the diagnosis of a prolactinoma can be ruled in and a pituitary gadolinium-enhanced MRI is strongly recommended (right), to investigate for the presence of a microadenoma or a macroadenoma. Discrepancy between PRL levels and tumor size, particularly in case of tumor size > 3 cm, should suggest a potential hook effect and requires the reassessment of the sample with serum dilution (at least 1:100, middle). Created with Biorender.com.
CHALLENGES IN DIAGNOSIS
The “Hook Effect”
Diagnostic workup for prolactinomas may be tangled by several challenges. The maximal diameter of the prolactinoma reportedly correlates with PRL levels at baseline (9). Therefore, in case of discrepancy between a very large pituitary tumor (>3 cm in size) and a modestly increased PRL level, a 1:100 serum sample dilution is recommended to investigate and overcome a laboratory artifact responsible for falsely low PRL levels, namely the “hook effect” (55). This phenomenon commonly occurs with the use of some immunoassays, such as the 2-site monoclonal “sandwich” assay (56). The term “hook effect” refers to the typical shape of the binding curve, which goes up as long as the analyte concentrations gradually increase in the sample, but at some critical point exceeding the capacity of the assay components, it turns down (56). The hook effect may therefore mask a prolactinoma and suggest the incorrect diagnosis of a nonfunctioning pituitary tumor (56).
Of note, current assay methodologies are mainly based on 2-site immunometric assays, which are more sensitive and specific than previous competitive assays but are also susceptible to some interferences such as the high-dose hook effect (57). Sandwich assays, generally performed in 2 steps or by diluting the samples, are able to avoid this interference, so now the high-dose hook effect persists in a very small minority of PRL assays (57). Nevertheless, consideration of the hook effect should be taken in PRL assay recommendations (57).
Macroprolactin
Macroprolactin is an isoform of PRL with greater molecular weight and reduced biological activity. In patients with asymptomatic hyperprolactinemia, the assessment for macroprolactin can help endocrinologists in preventing incorrect diagnoses leading to inappropriate treatments, and in discriminating true hyperprolactinemia requiring a proper therapeutic approach (55, 58). Although more than 80% of circulating PRL is monomeric (23 kDa), serum can also contain a covalently bound dimer and a larger polymeric form, known as “big prolactin” (50 kDa) and “big-big prolactin” (150 kDa), respectively (55, 58). In most cases, macroprolactin is composed of a complex formed by an IgG and a monomeric PRL (59, 60); consequent hyperprolactinemia results from low renal PRL clearance and reduced dopaminergic tone (61). Macroprolactin is quite common; it has been reported to cause hyperprolactinemia in approximately 20% of cases (62). Therefore, screening for macroprolactin with the use of polyethylene glycol should be routinely offered, before investigating alternative causes of PRL excess, in patients with asymptomatic hyperprolactinemia (55, 62). The overall prevalence of signs and symptoms of PRL excess in patients with macroprolactin is generally lower than that observed in patients with monomeric hyperprolactinemia (63). Indeed, menstrual disturbances, including both oligomenorrhea and amenorrhea, as well as galactorrhea and a combination of menstrual disturbances and galactorrhea have been reported to occur in 24%, 13%, and 2% of patients with macroprolactin, respectively, presumably resulting from concomitant disorders, such as polycystic ovary syndrome and pituitary tumors, compared with 26%, 29%, and 34% of those with monomeric hyperprolactinemia, respectively (63). This suggests a potential overlap in the clinical presentation mainly in terms of infertility features (63). Of note, more than 60% of patients with macroprolactin reported no peculiar signs and symptoms of PRL excess (63). The coexistence of macroprolactin and pituitary incidentalomas may result in a further diagnostic pitfall leading to an improper diagnosis of prolactinoma (61), but the absence of a clinical syndrome specifically ascribable to hyperprolactinemia may assist in a better diagnostic definition (63).
Diagnosis at Menopausal Age
Menopause is associated with a physiologic decline of the stimulatory effects of estrogens on PRL secretion and lactotroph cell proliferation and a physiological reduction in circulating PRL levels (64). Therefore, in postmenopausal women, when fertility does not represent a foremost concern, the lack of the classical amenorrhea–galactorrhea syndrome from PRL excess may result in the underestimation of the exact prevalence of prolactinomas (64). This is particularly true for women with microprolactinomas, although at least one-third of those diagnosed at menopausal age have reported secondary amenorrhea in their medical history (64-66), suggesting that a scrupulous investigation of these symptoms during fertile age might prompt an earlier diagnosis (64). Most patients (92%) with prolactinomas diagnosed after menopause have been found to harbor a pituitary macroadenoma, or even a giant pituitary tumor (65-67), leading predominantly to signs and symptoms of mass effect, such as headache and visual loss, with pituitary apoplexy occurring in approximately 5% of cases (65-67). The evidence of large tumor size and frequent invasiveness of prolactinomas in postmenopausal women has raised the question of whether these tumors are biologically comparable with those of male patients (64), likewise characterized by rapid growth rate and increased markers of cellular proliferation (9, 36). However, a lower estrogen receptor α expression, together with low estrogen production, may per se promote lactotroph cell proliferation and trigger the development of large, invasive pituitary tumors in postmenopausal women (68).
Aggressive and Malignant Prolactinomas
Lactotroph tumors are the second-most frequent aggressive and malignant tumors (68). According to the last World Health Organization classification of pituitary tumors (38), pituitary adenomas have been renamed with the term pituitary neuroendocrine tumors (PitNET) in replacement of adenomas, and specific clinical, pathological, and radiological characteristics have been proposed to identify aggressive and malignant PitNET, including prolactinomas. In this light, attention should be paid to tumor invasiveness and proliferation. Invasion is defined on the basis of histological and/or radiological evidence of cavernous or sphenoid sinus invasion, whereas proliferation is defined on the basis of a mitotic count >2/10 HPF, Ki-67 ≥ 3%, and >10 p53 strongly positive nuclei/10 HPF (69). Lactotroph tumors developing in patients aged younger than 20 years, mainly in males, and/or with a genetic predisposition have generally a poor prognosis because of a larger size, more frequent invasiveness, and overt resistance to DA (68), leading to a high risk of recurrence and malignancy. Densely granulated PIT-1–positive PRL-secreting pituitary tumors, mixed somatotroph–lactotroph tumors, and pluri-hormonal PIT-1–positive acidophilic stem cell tumors (tumors of lactotroph differentiation often associated with hyperprolactinemia) are generally more invasive than sparsely granulated variants and less responsive to conventional medical therapy with DA, with overall reduced cure rates (38). The expression of proliferation markers (Ki-67 expression ≥3%, mitotic count >2) is also correlated with tumor invasiveness and proliferation (68). Low estrogen receptor α expression and high vascular endothelial growth factor (VEGF) and epidermal growth factor expression are similarly correlated to tumor aggressiveness, and the expression of some adhesion molecules such as E-cadherin, matrix metalloproteinase 9, and abnormalities in chromosomes 1, 11, and 19 has also been correlated with tumor aggressiveness (38, 68). As for all PitNET subtypes, according to pathological and/or radiological findings (69), lactotroph tumors can be classified based on a 5 degree-scale as: noninvasive and nonproliferative (grade 1a); noninvasive and proliferative (grade 1b); invasive and nonproliferative (grade 2a); invasive and proliferative (grade 2b); and metastatic tumor (grade 3). Based on this classification, grade 2b (aggressive) lactotroph tumors have a 20 times higher risk of progression compared with grade 1a tumors (69).
As for aggressive lactotroph tumors, malignant prolactinomas are the second most frequent malignant pituitary tumors after ACTH-secreting pituitary carcinomas (70). The exact incidence of PRL-secreting carcinomas is yet to be clearly defined, as with that of all pituitary carcinomas, which are known to be very rare (<0.2% of all pituitary tumors). According to the recent European Society surveys (71, 72), malignant prolactinomas have been reported to account for approximately 9% of patients with aggressive and malignant pituitary tumors. The definition of malignant prolactinomas parallels that of all pituitary carcinomas and requires the confirmation of distant cerebrospinal, meningeal, and/or systemic metastases (70).
BACK TO THE CASES
Case 1
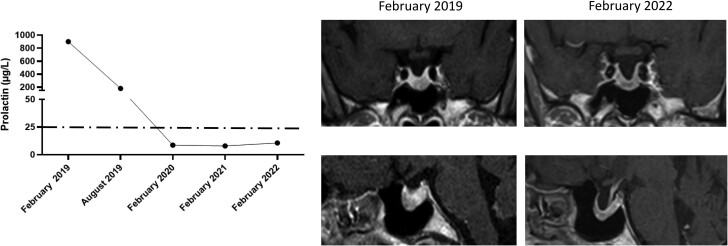
At the endocrinological assessment performed after 12 months of continuous treatment with cabergoline, PRL levels were fully normalized (PRL 8.6 µg/L) and pituitary MRI revealed a slight reduction in pituitary tumor volume (1.71 cm3, Δ = −13%). The pituitary evaluation revealed the restoration of normal gonadotropins and testosterone levels, which were associated with satisfactory libido and erectile function. Therefore, cabergoline was maintained at the same dose and the addition of testosterone replacement treatment was not required. Pituitary evaluation performed over the years revealed the persistence of normoprolactinemia while on treatment with cabergoline, which is associated with a further reduction in pituitary tumor volume (1.43 cm3, Δ = −27% compared with baseline). At the last endocrinological evaluation performed in February 2022, PRL levels were 10.6 µg/L and pituitary MRI revealed a further shrinkage in tumor volume (1.14 cm3, Δ = −42% compared with baseline). Now, the patient is receiving cabergoline at the dose of 1.0 mg/wk, PRL being 6.7 µg/L. Occasional headaches persist, whereas clinical symptoms of decreased libido and erectile dysfunction did not recur after starting cabergoline treatment. The clinical history of this patient is shown in Fig. 3.
Figure 3.
Biochemical and radiological evolution of clinical case 1. The left panel shows changes in PRL levels from diagnosis to the last follow-up. The right panel shows changes in tumor size from the first available MRI to the last follow-up.
Case 2
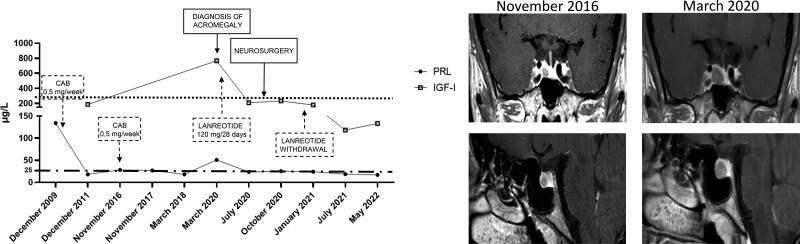
At the endocrinological consultation in March 2020, evident facial disfigurement was associated with macroglossia, soft-tissue swelling, and arthralgia, suggesting a diagnosis of acromegaly. Because of this suspicion, hormonal and MRI evaluations were immediately performed. As expected, IGF-I was 3.9× the upper limit of normal (ULN), and pituitary tumor was increased 2-fold in maximal tumor diameter (9 mm vs 4.5 mm), whereas PRL was 30.7 µg/L, confirming the diagnosis of acromegaly. Metabolic, cardiologic, and respiratory complications and colon polyps were excluded. Because of the COVID-19 pandemics, transsphenoidal surgery treatment was not possible at that time because of the restrictive measures limiting or delaying routine nonurgent clinical procedures and was therefore postponed, considering that no peculiar symptoms ascribable to tumor mass effect were complained by the patient except for headaches. In fact, treatment with the somatostatin analogue lanreotide 120 mg every 28 days was started. IGF-I was normalized (0.9× ULN) after 3 months of medical therapy, but the clinical syndrome associated with acromegaly persisted. Lanreotide was continued at the same dose until September 2020, when neurosurgery was feasible because of the restarting of routine procedures in Italian hospitals. The report of the histological examination lay for pituitary GH-secreting tumor, immunohistochemically characterized by diffuse positivity for GH and focal for LH, rare cells immunoreactive for PRL, and Ki-67%/MIB-1 proliferation index <1%. One month later, the postoperative assessment showed full PRL normalization (23 µg/L) but persistently elevated GH (12.6 µg/L) and IGF-I (1.2×ULN); therefore, lanreotide was restarted at the dose of 90 mg every 28 days. In January 2021, biochemical and radiological complete remission of acromegaly was documented and lanreotide was withdrawn. Five months later, normal IGF-I values (118 µg/L, 0.4× ULN) were still recorded. Now, acromegaly remission is still confirmed by IGF-I 0.4× ULN (133 µg/L), with evidence of secondary empty sella on MRI, and no medical treatment is required to maintain disease control in this patient.
To date, PRL levels are still within the normal range. The clinical history of this patient is shown in Fig. 4.
Figure 4.
Biochemical and radiological evolution of clinical case 2. The left panel shows changes in PRL levels from diagnosis to the last follow-up. The right panel shows changes in tumor size from the first available MRI to the last follow-up.
Case 3
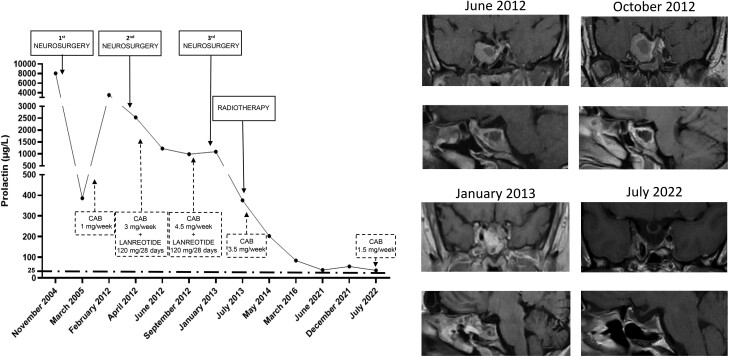
Despite the important reduction in pituitary tumor size during postsurgical treatment with cabergoline (7.030 cm3, D = −24.5%), PRL normalization was never achieved during follow-up. In February 2012, the patient’s PRL level rapidly rose to 3500 µg/L, and a significant increase in tumor size was registered (tumor volume = 20.827 cm3, Δ = +66%), the tumor being still located at the cavernous sinus level, totally surrounding the right sinus and leading to the paresis of the III, IV, and VI cranial nerves. Because of uncontrolled hyperprolactinemia and worsening neurological symptoms, the patient voluntarily underwent a second transsphenoidal surgery to achieve tumor debulking and cranial nerve decompression in March 2012. The second histological examination revealed an atypical prolactinoma with a sparsely granulated pattern, Ki-67%/MIB-1 proliferation index of 7%, and adenoma pleomorphism with “dot-like” reactivity for PRL at immunohistochemistry. Postoperative pituitary function assessment revealed the persistence of hyperprolactinemia (PRL, 2523 µg/L), and concomitant secondary hypocortisolism, for which proper replacement therapy was started. To counteract the biological aggressiveness, high-dose (3 mg/week) cabergoline therapy was resumed, and the somatostatin analogue lanreotide was added at the dose of 120 mg every 28 days to limit residue growth. Despite this combined therapy, PRL did not normalize (PRL, 1225 µg), requiring a further increase in cabergoline dose to 4.5 mg/wk. Despite high-dose cabergoline, PRL persisted at very high levels (PRL 988 µg/L) and a dramatic headache together with sudden visual loss occurred, suggesting the potential regrowth of the tumor. In December 2012, 9 months after the second neurosurgery, the patient underwent the third transsphenoidal surgery, immediately followed by fractional stereotaxic radiotherapy (25 fractions of 1.8 Gy each, for a total dose of 45 Gy, isodose 92.8%), which was performed on a lesion of 2.638 cm3, confirming the rapid tumor regrowth after the third surgical procedure. After radiotherapy, cabergoline monotherapy was still required at the dose of 3.5 mg/wk, PRL being 376 µg/L. After radiotherapy, secondary hypocortisolism and hypogonadism were confirmed, whereas somatotropic and thyrotropic axes were still preserved. High-dose cabergoline therapy was continued with a modest gradual reduction in PRL levels. As expected, 1 year after radiotherapy secondary hypothyroidism was diagnosed and L-thyroxine replacement was started, although IGF-I levels were still within the normal range. PRL below 100 µg/L (84.7 µg/L) was achieved in March 2016 (ie, only 3 years after radiotherapy), with a slow and continuous PRL decrease under cabergoline therapy being observed in the following years. Although never normalized, PRL nadir (38.8 µg/L) was registered in June 2021. Concomitantly, progressive tumor shrinkage was observed after radiotherapy, reaching maximal efficacy in July 2022 (tumor volume, 0.619 cm3, Δ = −76.5%). Now, the patient is receiving cabergoline at the dose of 1.5 mg/wk, PRL being 37 µg/L. Replacement therapies were constantly adjusted according to hormonal evaluations during follow-up. Particularly, testosterone replacement treatment allowed the complete recovery of signs and symptoms of hypogonadism, confirming the relevant benefits provided by close monitoring and proper therapy. Clinical symptoms definitively disappeared, and the patient complained of headaches only occasionally. Because prolonged high-dose cabergoline therapy was administered, echocardiography evaluations were regularly performed to study valves' function and early detect possible insufficiency. Although high-dose cabergoline was administered for 10 years, no valvular dysfunction occurred in this patient. At the last follow-up in July 2022, 9 years after radiotherapy, GH deficiency was documented. The clinical history of this patient is shown in Fig. 5.
Figure 5.
Biochemical and radiological evolution of clinical case 3. The left panel shows changes in PRL levels from diagnosis to the last follow-up. The right panel shows changes in tumor size from the first available MRI to the last follow-up.
APPROACH TO PATIENT MANAGEMENT
Before DA became accessible for medical therapy, surgery and/or radiation therapy were the treatment approaches of choice for prolactinomas (73). The introduction of DA in the therapeutic algorithm for prolactinomas totally changed the natural course of these tumors, offering a successful treatment strategy that progressively substituted surgery and radiotherapy in routine clinical management (73). DA, mainly cabergoline, are now recommended as the treatment of choice to lower PRL levels, decrease tumor size, and restore gonadal function for patients harboring PRL-secreting tumors, regardless of tumor size (2, 3, 32, 73). However, multimodal therapy also including surgery and/or radiotherapy may be required in patients resistant to DA or for those with aggressive prolactinomas (73).
Indications to Treat Patients With Hyperprolactinemia
The goals of treatment for prolactinomas include PRL normalization, decrease in tumor size and restoration of gonadal function (2, 3, 34, 55). In patients with prolactinomas, effective treatments result in relief from the effects of tumor mass, including hypopituitarism, visual field defects, headaches, and cranial nerve palsy; and relief from the effects of PRL excess, including hypogonadism, infertility, and osteopenia/osteoporosis (2). Noteworthy, asymptomatic patients with microprolactinomas do not categorically require treatment (2, 34) because these tumors rarely grow over years (74). Conversely, independently of tumor size, the presence of a clinical syndrome of PRL excess support the introduction of PRL-lowering therapy (2, 4). However, hypogonadal premenopausal women with microadenomas without pregnancy desire may benefit from oral contraceptives/sex hormone replacement instead of dopamine agonists (34). Caution should be paid to periodic evaluation of PRL levels and potential growth of microadenomas following treatment with oral estrogen therapy, albeit no clear evidence of tumor enlargement after estrogen therapy has been provided so far (34).
The presence of a macroadenoma represents per se a strong indication of treatment, given the known propensity of these tumors to grow (2). Additional indications for treatment include tumor invasiveness and compression of adjacent structures, such as pituitary stalk or optic chiasm (2, 34).
Medical Treatment of Prolactinomas: Dopamine Agonists
Effects on PRL excess, tumor mass and gonadal status
DAs represent the recommended treatment to achieve therapeutic goals (2, 5, 34). The use of DA is based on the intrinsic property of dopamine to bind type 2 dopamine receptors (D2DR), which are G-coupled receptor proteins whose activation is able to suppress PRL secretion in the pituitary lactotrophs, as well as PRL gene expression and lactotroph proliferation (75). Among DA, bromocriptine and cabergoline are the most commonly used compounds in clinical practice worldwide, whereas pergolide, quinagolide, and lisuride are less frequently used or no longer available (34). Cabergoline is strongly recommended as the treatment of choice for prolactinomas of any size, given the proven greater efficacy over different DA either in PRL normalization and tumor shrinkage (34, 76-78), although head-to-head comparison studies among different DA formulations are still limited. In patients with microprolactinomas and macroprolactinomas, cabergoline has been reported to induce PRL normalization and tumor shrinkage in 95% and 80%, respectively (79). Regardless of tumor size, PRL normalization has been accomplished in 76% of patients treated with bromocriptine, 87% of those treated with pergolide and 89% of those treated with cabergoline (79). While on cabergoline, tumor shrinkage has been shown to occur in 60% of patients previously treated with other DA (77). A similar biochemical and tumoral effectiveness of cabergoline has been documented also in patients of pediatric and adolescent ages (2, 12).
Following treatment with cabergoline, amenorrhea, infertility, galactorrhea, and sexual dysfunction have been reported to improve in 82%, 53%, 86%, and 67%, respectively, of women with prolactinomas (34, 55). The rapid amelioration of women's fertility justifies the advice to use mechanical contraception in patients without pregnancy desire (34, 55). Hypogonadism may persist only in a minority of women with prolactinomas and may require replacement treatment (34, 55). Symptomatic hyperprolactinemic hypogonadal women may be cautiously administered estrogens/progesterone therapy but careful periodic observation is recommended, although a negative outcome on tumor size has not been documented to date (80). In men receiving cabergoline, hypogonadism, seminal fluid parameters (sperm count and volume), decreased libido and erectile dysfunction have been reported to improve in 60%, 100%, and 61% of cases, respectively (81-83). However, restoration of normal testosterone may not be sufficient to correct sexual dysfunction and seminal abnormalities, thus requiring testosterone replacement therapy (34, 55, 81-83). In these patients, attention should be paid to specific side effects of improper testosterone replacement treatment, such as aggressiveness, hypersexuality, polycythemia, and prostate enlargement, generally caused by excessive dosages and thus compelling proper dose adjustment (83). While on testosterone replacement therapy, potential testosterone aromatization to estrogen may occur, virtually stimulating the proliferation and hyperplasia of lactotroph cells in the pituitary and inducing resistance to dopamine agonists (80). In case of persistent hypogonadism despite PRL normalization following treatment with dopamine agonists, clomiphene citrate may represent an option to improve sperm quality and restore fertility (80).
Effects on Metabolic Profile
Additional extrapituitary and extragonadal effects of medical therapy with cabergoline mainly include a metabolic gain (41). Long-term treatment with cabergoline has been demonstrated to ameliorate or even recover metabolic consequences of PRL excess and hypogonadism (39, 40, 84-86), such as altered body composition, insulin resistance, impaired glucose tolerance, and adverse lipid profile, which is known to trigger visceral obesity and metabolic syndrome (39-45). Long-term treatment with cabergoline has been shown to significantly reduce body weight, body mass index, and waist circumference, thus improving visceral obesity (84-86). However, independently of the reduction in body weight, the impact on visceral obesity is supported by the direct beneficial effects of DA on glucose metabolism, insulin resistance, and lipid fractions. Indeed, both bromocriptine and cabergoline have been reported to induce a significant improvement in glucose metabolism and insulin resistance after 6 months of treatment for prolactinomas (39, 40, 84-86). Particularly, cabergoline significantly reduced fasting insulin and homeostatic model assessment for insulin resistance in prolactinomas, predominantly when used at doses higher than 0.5 mg/wk (43, 84). The positive impact on insulin metabolism is strengthened by the significant improvement seen also in insulin secretion and peripheral sensitivity (43). That this favorable action can be directly attributed to cabergoline rather than to weight loss is confirmed by the evidence that cabergoline dose was the best predictor of the percent decrease in fasting insulin (85). In male patients with concomitant hypogonadism, which is known to negatively affect insulin metabolism, fasting insulin and indices of insulin resistance, secretion, and sensitivity were found to improve after long-term treatment with cabergoline and further ameliorated by testosterone replacement treatment (86). Even in case of resistance to conventional cabergoline doses requiring a high-dose treatment protocol (dose range, 2-7 mg/wk; median, 3 mg/wk), despite PRL normalization occurring only in one-half of the patients, fasting insulin and indices of insulin secretion and peripheral sensitivity significantly improved regardless from a concomitant amelioration of fasting glucose and body mass index, whereas pituitary surgery failed to achieve a similar outcome on insulin metabolism (87). The improvement seen in the insulin profile parallels the amelioration of lipid fractions (40, 43, 84, 86, 88-90). The reduction of total and low-density lipoprotein cholesterol and triglycerides, together with the increase in high-density lipoprotein cholesterol occurring after treatment with either bromocriptine or cabergoline, has been shown to be independent of changes in body weight (40, 43, 84, 86, 88-90). As for insulin metabolism, in male patients with hyperprolactinemic and concomitant hypogonadism, treatment with cabergoline has been demonstrated to significantly reduce total and low-density lipoprotein cholesterol and triglycerides, without a further amelioration of lipids after testosterone replacement therapy (43). Conversely, the use of a high-dose cabergoline schedule did not remarkably improve lipid metabolism, whereas pituitary surgery was able to significantly reduce total cholesterol and triglycerides (87), suggesting that lipid fractions might be affected more markedly by the rapid correction of PRL excess rather than by DA.
The direct consequence of the metabolic advantage induced by DA is a reduction by 20% in the prevalence of metabolic syndrome (43, 45, 84, 86).
Safety and Tolerability
Over the years, the longer half-life and the reduced incidence of side effects contributed to progressively enhancing the use of cabergoline in preference to other DA in clinical practice. Adverse effects of cabergoline are generally less frequent, less severe, and of shorter duration compared with other DA (2), and mainly include nausea or vomiting, headache, dizziness, vertigo, and arterial hypotension (2, 91). Noteworthy, DA use has been found to be associated, albeit rarely, with the occurrence of compulsive behavior and psychosis. Particularly, even at low doses, bromocriptine has been reported to induce mania in postpartum women and induce psychotic reactions in patients with preexisting mental disorders (91). Conversely, similar findings have been very occasionally reported following treatment with cabergoline (91). Based on this evidence, the use of DA to achieve normal PRL levels in patients with antipsychotic-induced hyperprolactinemia is still questionable because DA might exacerbate underlying psychosis (34).
Given the high binding affinity of cabergoline for serotonin receptor subtype 2B, whose activation reportedly promotes fibroblast proliferation and mitogenesis at the cardiac valve level (92, 93), over the past 15 years several independent studies (94-115) have investigated the potential association between the use of cabergoline and the development of clinically relevant cardiac valve disease in patients with prolactinomas, as previously demonstrated in patients with Parkinson disease (116-118). Noteworthy, the experience collected in patients with Parkinson disease has demonstrated that cardiac valve disease occurred in 29% to 39% of patients, usually receiving cabergoline at doses as high as 3 to 5 mg/d and mean weekly doses up to 25 mg and taking median cumulative doses ranging from 2600 to 6700 mg (116-118). Conversely, standard doses of cabergoline routinely used for patients with prolactinomas do not exceed 2 mg/wk in most cases, and only a minority of patients require higher doses to achieve disease control. A recent meta-analysis investigating the prevalence of cardiac valvulopathy did not confirm a similar association and found no significant increase in the prevalence of any valvular disease in patients with hyperprolactinemia receiving cabergoline at standard doses compared with controls, apart from a slight increase in mild (ie, nonclinically relevant) tricuspid regurgitation (119). Based on these findings and considering that the cabergoline dose threshold at which valve abnormalities can occur is yet to be fully elucidated, a standard transthoracic echocardiogram before starting treatment with cabergoline (or pergolide) is still recommended (120). Nevertheless, given that the evidence of clinically relevant valve disease is nearly absent, and that generally the use of cabergoline at doses ≤2 mg/wk is not associated with pathological changes in leaflet thickness, restriction, or retraction (120), a transthoracic echocardiogram should be repeated every 5 years in patients treated with cabergoline doses ≤2 mg/wk, or yearly in those receiving higher doses (120). Cabergoline duration and cumulative dose have been shown to exert no significant impact on cardiac valvulopathy in prolactinomas (121).
Resistant and Aggressive Prolactinomas
According to international guidelines (34), resistance to DA occurs in case of failure to achieve PRL normalization and at least ≥50% reduction in tumor size at maximally tolerated doses. Following treatment with DA, some patients may display discordant responses because PRL normalization may be not associated with a reduction in tumor size or vice versa (34); other patients may display partial responsiveness and require higher than standard doses of DA to achieve a satisfactory response (34). However, the definition of resistance to DA is still open to question because there is no full consensus about the definition of the DA dose to which the drug should be up-titrated before classifying a patient as resistant to DA (122), and specifically, if this dose should be ≥2.0 mg/wk for cabergoline and ≥15 mg/d for bromocriptine (122).
Primary resistance to DA in prolactinomas is rare, accounting for 20% to 30% of patients treated with bromocriptine and nearly 10% to 20% of those receiving cabergoline (122, 123). Secondary resistance to DA has been seldom reported in patients with prolactinomas, but its occurrence has been generally linked to poor prognosis and potential aggressive or malignant progression of the pituitary tumor (124-130). Several molecular mechanisms may cause the development of resistance to DA (131). Among them, genetic variants, intracellular signalling alterations, and the expression of the small single-stranded noncoding RNA molecules microRNA, which function in RNA silencing and posttranscriptional regulation of gene expression, may lead to a decreased number of D2DR, a decreased affinity of D2DR for DA, or altered signal transduction rising resistance to DA (131). Particularly, a decreased mRNA stability and synthesis of D2DR resulting from genetic alterations, such as the gene variant NcoI T+, has been reported to cause resistance to DA (132). On the other hand, estrogens may affect the balance between the short (D2S) and long (D2L) isoforms of D2DR, increasing the expression of the latter and thus limiting the effectiveness of DA (133-135). Intracellular signalling may be negatively affected by cytoskeleton proteins, such as Filamin-A (136) or β-arrestins (137, 138), known to act as scaffolds for signalling molecules involved in D2DR signal transduction in lactotroph tumors and in dopaminergic neurotransmission. More recently, resistance to DA has been found associated with an increased expression of miR-93-5p (139, 140) and miR-1299 (141), or a reduced expression of miR-145 (142).
Resistance to DA may require different therapeutic approaches to be overcome. In patients resistant to a certain DA, dose escalation to maximally tolerated doses is recommended (34), as in clinical case 3. Cabergoline doses as high as 12 mg/wk have been needed to overthrow resistance (143). Alternatively, the switch from different DA to cabergoline may increase responsiveness to medical therapy (34). Indeed, 6-month treatment with cabergoline at a dose range of 0.5 to 3 mg/wk has been shown to induce PRL normalization in nearly 63% and tumor shrinkage in more than 44% of patients with proven resistance to long-term bromocriptine or quinagolide (144), thus demonstrating that patients unresponsive to different DA may benefit from chronic administration of cabergoline. Successful disease control resulted in the restoration of gonadal function in 70% of patients (144).
Nevertheless, some patients may require a multimodal therapeutic approach, including the association of medical therapy and surgery, ultimately combined with radiotherapy, particularly in presence of aggressive tumors (34, 145), as in clinical case 3. Such a treatment strategy has successfully controlled 56% of patients (130). Resistance to cabergoline represents the first clear indication for surgery in prolactinomas (146), together with pituitary apoplexy, intolerance to DA, persistent chiasmal compression despite optimal medical therapy, cerebrospinal fluid leak while on DA, and psychiatric conditions in macroadenomas (146). Interestingly, surgical debulking has been found to prompt a significant decrease in PRL levels while reducing the weekly cabergoline dose by 50% (147). In experienced hands, initial remission rates with surgery were approximately 40% and 75% for macroprolactinomas and microprolactinomas, respectively, and long-term remission rates were approximately 65% and 80% for macroprolactinomas and microprolactinomas, respectively (35). However, the improvement of surgical techniques over the years has progressively increased the successful use of neurosurgery also as a first-line treatment in prolactinomas, resulting as effective as cabergoline in patients with enclosed adenomas not located laterally from the gland and not invasive to the cavernous sinus (148). In these patients, the surgical approach could increase cure rates and reduce the requirement of dopamine agonists for those not cured by first-line surgery, also limiting medical costs and side effects of chronic DA treatment (149). Conversely, tumors invasive to the cavernous sinus have been reported to be associated with a higher risk of venous bleeding and a lower remission rate compared with enclosed tumors (148). So far, surgery (83%) has been shown to provide similar outcomes compared with DA (91%) in terms of achievement of biochemical control in patients with microprolactinomas, but not in those with macroprolactinomas, in whom the rate of biochemical control is higher in medically treated (77%) compared with surgically treated (60%) patients, according to a recent meta-analysis (148). Altogether, this evidence supports the use of surgery as a viable first-line therapeutic choice for patients with enclosed and not invasive prolactinomas and suggests an adequate tailoring of treatments based on tumor characteristics, size, and invasiveness.
Radiotherapy, in any form, is now considered a third-line treatment and is reserved for those patients with macroadenomas resistant to either medical therapy or surgery, as in clinical case 3, mainly because of the long latency to therapeutic efficacy and the frequent occurrence of hypopituitarism (34).
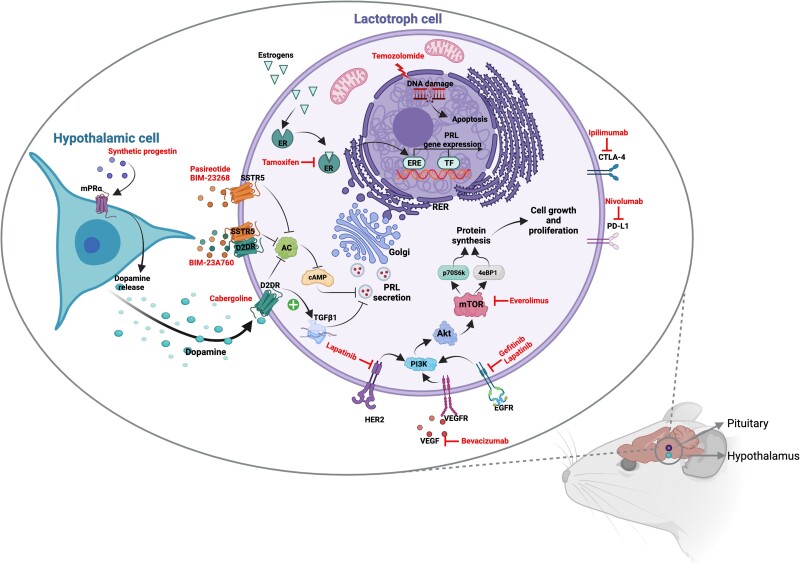
Aggressive and malignant prolactinomas refractory to all treatment strategies may benefit from the administration of the oral alkylating chemotherapeutic agent temozolomide (Fig. 6), whose efficacy in terms of tumor growth control accounts for up to 50% of patients with prolactinomas (150). According to the European Society survey of 166 patients, including 38 with aggressive lactotroph tumors, the use of temozolomide in aggressive prolactinomas resulted in complete regression in 5%, partial regression in 45%, stable disease in 26%, and tumor progression in 24% of cases (71). Temozolomide is now recommended as first-line chemotherapy for aggressive pituitary tumors and pituitary carcinomas following documented tumor growth (151). Responsiveness to temozolomide may be predicted by the evaluation of O(6)-methylguanine methyl transferase (MGMT) status by immunohistochemistry by expert neuropathologists, as high MGMT expression is generally suggestive of a lack of response (152). Indeed, tumor regression has been documented in 46% of tumors with low MGMT expression, but only in 23.5% of those with high MGMT expression (151). The first evaluation of the response to temozolomide should be done after the first 3 cycles (151). If radiological progression is demonstrated, temozolomide treatment should be stopped (152). Conversely, in patients proven to respond to first-line temozolomide after 3 cycles, treatment can be continued for at least 6 months or a longer time if a sustained therapeutic benefit is observed (151). As for all chemotherapeutic agents, close monitoring of hematological parameters, liver function tests, and careful clinical observation should be performed for potential adverse effects (eg, fatigue, nausea, vomiting) (151). The effectiveness of temozolomide has been shown to increase when combined with radiotherapy given its radio-sensitizing properties, thus offering a further therapeutic option for those individuals with aggressive or malignant prolactinomas (150, 151). Indeed, tumor regression has been documented in 71% of patients receiving temozolomide and radiotherapy combined treatment, but only in 34% of those receiving temozolomide monotherapy (151). The combination of temozolomide and radiotherapy can be offered to patients with rapid tumor growth in whom maximal doses of radiotherapy have not been reached (152). However, disease progression has been reported to occur in 38% of cases despite treatment with temozolomide (152). A second trial of 3 cycles of temozolomide can be attempted in patients developing recurrence following the initial response to the drug (151). Alternatively, a trial with other systemic cytotoxic therapy can be proposed in patients with rapid tumor progression while on temozolomide (151).
Figure 6.
Alternative medical treatment in resistant prolactinoma: molecular mechanisms explored in murine models. Temozolomide elicits cytotoxicity through the methylation of DNA guanine and adenine residues, leading to single- and double-stranded DNA breaks. Prolonged exposure to estrogens induces the growth of lactotroph cells and PRL synthesis, this latter by binding the estrogen receptors (ER) α and β, abundantly expressed on lactotroph cells, and by activating the estrogen responsive elements (ERE) and other transcription factors (TF). Tamoxifen, by competing with estrogens at the receptor site and blocking the promotional role of estrogens, has been shown to prevent tumor growth and suppress PRL synthesis in the rat pituitary gland. Synthetic progestins have been recently investigated as potential treatments for resistant prolactinomas because of their capability to bind the membrane progesterone receptors, by mediating nongenomic progesterone effects, and inducing a rapid DA release by hypothalamic dopaminergic neurons. The consequent binding of DA to D2DR in the rat pituitary lactotrophs might activate TGFβ1 transcription and decreases the adenylate cyclase (AC) and cAMP levels, leading to the inhibition of PRL secretion. Similarly, somatostatin analogues, with high binding affinity to SSTR5, BIM-23268 and pasireotide, and chimeric compounds, binding SSTR5/D2DR, BIM-23A760, have been demonstrated to reduce PRL synthesis by reducing AC and cAMP levels in rat pituitary cell lines. Everolimus, the mTOR inhibitor, by decreasing the downstream molecules p70S6k and 4eBP1, induces cell proliferation inhibition as a consequence of reduced protein synthesis. The blocking of vascular endothelial growth factor (VEGF) by bevacizumab and of epidermal growth factor receptor (EGFR) and epidermal growth factor receptor 2 (HER2) tyrosine kinase phosphorylation by lapatinib and gefitinib inhibits the activation of proliferative signalling. Finally, the blocking of PD-L1 with nivolumab and of CTLA4 with ipilimumab can prevent the immune escape of lactotroph tumor cells. Created with Biorender.com.
Management After Treatment Withdrawal
In selected cases, treatment with DA for prolactinomas may result in a complete cure and can be definitively stopped. Specifically, after bromocriptine discontinuation, persistent normoprolactinemia reportedly ranged from 7% to 44% (153-159), indicating a wide variability in the long-term outcome after bromocriptine withdrawal. Recurrence of hyperprolactinemia generally occurred within 3 months after bromocriptine discontinuation (153-159). However, symptomatic tumor regrowth has been reported to occur in less than 10% of cases (160, 161) and to be influenced by the duration of previous DA treatment (157). Tumor re-expansion was successfully managed by bromocriptine restarting (161). A greater success has been demonstrated after cabergoline withdrawal (Table 3). Preliminary studies (162-164, 171, 176, 177) have reported the persistence of normoprolactinemia after cabergoline withdrawal to range from 10% to 31% of patients. In a prospective study, 36 to 48 months after cabergoline discontinuation, persistent normoprolactinemia was found in 19 of 25 (76%) patients with nontumoral hyperprolactinemia, 73 of 105 (70%) of those with microprolactinomas, and in 45 of 70 (64%) of those with macroprolactinomas (165). The median time to recurrence of hyperprolactinemia ranged from 12 to 18 months (165). The Kaplan-Maier estimate of disease recurrence at 5 years was significantly lower in patients with nontumoral hyperprolactinemia compared with those harboring a pituitary tumor, and in patients with no evidence of tumor remnant at MRI before cabergoline withdrawal compared with those with residual tumor (165). The role of tumor mass as the main determinant of long-term remission was confirmed by the finding that the maximal diameter during cabergoline treatment was the best predictor of the PRL level at the last follow-up visit after treatment discontinuation, with a 19% hazard rate for the recurrence of hyperprolactinemia for each millimeter increment in the maximal tumor diameter (165). Now, the achievement of a residual tumor maximal diameter smaller than 3.1 mm in size associated with nadir PRL levels <5.4 μg/L is considered the best predictor of long-term remission from hyperprolactinemia after cabergoline withdrawal (166).
Table 3.
Prevalence of long-term remission from hyperprolactinemia after DA withdrawal: an overview of the literature
| Author, year (reference) | Patient no. | Therapy | Duration (mo) | Follow-up (mo) | Remission (%) | |||
|---|---|---|---|---|---|---|---|---|
| NTHP | Micro | Macro | Overall | |||||
| Johnston, 1984 (153) | 15 | BRC | 42 | 12 | NA | NA | NA | 7 |
| Zarate, 1983 (158) | 16 | BRC | 24 | 24 | — | 17 | 100 | 37.5 |
| Moriondo, 1985 (154) | 36 | BRC | 24 | 30 | — | 22 | — | 22 |
| Wang, 1987 (156) | 24 | BRC | 24 | 12-48 | 40 | 8.7 | 4.3 | 10 |
| Rasmussen, 1987 (155) | 75 | BRC | 24-65 | 6 | NA | NA | NA | 44 |
| van’t Verlaat, 1991 (157) | 12 | BRC | 36-84 | 12 | — | — | 9 | 9 |
| Passos, 2002 (159) | 131 | BRC | 48 | 42 | — | 26 | 16 | 20.6 |
| Remission from bromocriptine | 309 | 2/5 (40%) | 28/133 (21%) | 17/108 (15.7%) | 19.1 | |||
| Ferrari, 1992 (162) | 127 | CAB | 40-84 | 12 | 8.1 | 7 | 10 | 7.9 |
| Muratori, 1997 (163) | 26 | CAB | 12 | 38-60 | — | 19 | — | 19 |
| Cannavò, 1999 (164) | 37 | CAB | 24 | 12 | — | 15.4 | 9.1 | 13.5 |
| Colao, 2003 (165) | 175 | CAB | 48 | 24-60 | 76 | 70 | 64 | 68.5 |
| Colao, 2007 (166) | 194 | CAB | 36-45 | 48 | 74 | 66 | 47 | 60 |
| Kharlip, 2009 (167) | 46 | CAB | 52 | 52 | 25 | 48 | 45 | 45.6 |
| Sala, 2016 (168) | 74 | CAB | 66-79 | 12 | — | 56 | 50 | 54 |
| Espinosa-Cárdenas, 2020 (169) | 50 | CAB | 60 | 132-408 | — | 18.7 | 38.3 | 32 |
| Kim, 2021 (170) | 44 | CAB | 12-120 | 12-97 | — | 78.9 | 63.6 | 75 |
| Remission from cabergoline | 773 | 43/93 (46.2%) | 238/479 (49.7%) | 119/253 (47%) | 48.5 | |||
| Biswas, 2005 (171) | 89 | Both | 37 | 12 | — | 36 | — | 36 |
| Guitelman, 2006 (172) | 100 | Both | 24-60 | 60 | — | 20 | 3 | 15 |
| Huda, 2010 (195) | 40 | Both | 108 | 12 | — | 22.5 | — | 22.5 |
| Barber, 2011 (173) | 60 | Both | 90 | 3-36 | — | 36 | 7 | 28.3 |
| Anagnostis, 2012 (174) | 26 | Both | 79 | 24 | — | 60 | 50 | 57.7 |
| Teixeira, 2017 (175) | 50 | Both | 118 ± 85 | — | — | 78 | 44.4 | 72 |
| Total | 1447 | 45/98 (45.9%) | 381/853 (44.6%) | 145/423 (34.3%) | ||||
Abbreviations: BRC, bromocriptine; CAB, cabergoline; DA, dopamine agonist; NA, not available; NTHP, nontumoral hyperprolactinemia.
Altogether, these findings guided the advice that, after therapy with DA for at least 2 years, a trial of progressive DA dose-lowering and discontinuation can be started if PRL levels are normal and the tumor volume is evidently shrunk (55).
As shown in Table 3, the application in clinical practice of the aforementioned criteria for DA discontinuation in prolactinomas has resulted in a substantial progressive increase in the long-term remission rate after treatment withdrawal up to 75% of patients (167-170, 172-175, 195). Nevertheless, the pooled proportion of patients with persisting normoprolactinemia after DA withdrawal was 21% in a systematic review and meta-analysis (178), where remission rates were confirmed to be higher in idiopathic hyperprolactinemia (32%) and microprolactinomas (21%) compared with macroprolactinomas (16%).
In patients with evidence of recurrence of hyperprolactinemia after treatment discontinuation, DA must be restarted. However, a second withdrawal may be attempted if patients have received cabergoline for 2 additional years (179, 180). Evidence from previous studies has documented a successful outcome, defined as the achievement of normoprolactinemia, in 30% of cases (179, 180).
Altogether, these findings suggest that more than one-third of patients totally fulfilling the criteria for drug discontinuation may exhibit persistent normoprolactinemia over time. However, a careful follow-up after therapy withdrawal remains strongly recommended to identify as soon as possible the recurrence of hyperprolactinemia and/or tumor regrowth requiring restarting prompt treatment (55).
Management in Pregnancy
Given the great efficacy of DA in restoring fertility almost immediately after treatment starts, mechanical contraception should be recommended in women beginning DA therapy and without pregnancy desire (34, 55). For those women with macroprolactinomas wishing pregnancy, conception should be planned after the achievement of PRL normalization together with a marked reduction in tumor mass, primarily to minimize the risk of optic chiasm compression in case of tumor expansion during pregnancy (34, 55). The risk of tumor enlargement during pregnancy is modest and influenced by tumor size and previous treatments (181). In fact, tumor growth has been reported to occur in 2.4% of microadenomas and 4.7% of macroadenomas treated with surgery or radiotherapy before pregnancy compared with 21.0% of those with macroadenomas not receiving prior surgery or radiotherapy (181). In pregnant women, tumor re-expansion can be suspected in case of persistent or worsening headaches or abnormal visual field testing (181). In such cases, PRL cannot be used as a biomarker of disease activity because its levels beyond the threshold do not suggest unquestionably tumor growth and cannot prompt therapeutic concerns per se, unless PRL increases up to the levels measured at diagnosis, thus advising a pituitary MRI for a more accurate diagnostic assessment (182).
The low risk of tumor enlargement during pregnancy has provided the reason why women with prolactinomas must be instructed to withdraw DA as soon as pregnancy is confirmed (34, 55), as in clinical case 2. In fact, in humans, bromocriptine has been demonstrated to cross the placenta (181); data collected in animal models have confirmed a similar effect for cabergoline, but definitive confirmation in humans is still lacking (181). However, exposure to bromocriptine or cabergoline within the first 6 weeks of gestation has been found not to increase the risk for unfavorable maternal and foetal outcomes, including spontaneous abortions, ectopic pregnancies, trophoblastic disease, multiple pregnancies, or congenital malformations (181, 183, 184). To a similar extent, long-term follow-up studies (up to 12 years) on children born from mothers treated with bromocriptine or cabergoline before pregnancy have rarely reported abnormalities in physical or mental development (185, 186).
The use of DA during pregnancy is not officially approved, with the only exception of bromocriptine, whose use is approved for conception (187). Nevertheless, in selected patients with macroadenomas who become pregnant while on DA and who have not had prior therapy, it may be cautious to continue DA throughout the pregnancy, especially if the tumor is invasive or abutting the optic chiasm (34). In patients experiencing symptomatic growth of a prolactinoma during pregnancy treatment restarting is recommended, and bromocriptine is the therapy of choice (34, 187), based on the large experience over years not documenting any increased risk for maternal and foetal outcomes (184). The experience of using cabergoline throughout pregnancy is still scant and limited to a few studies, but resulting in at-term delivery in most cases, whereas preterm delivery and intrauterine death occurred in less than 7% of cases (185, 186).
After pregnancy, an absolute restriction of breastfeeding is not mandatory because it has been demonstrated to be not associated with an increased risk of tumor expansion (184, 188). However, in patients harboring large residual tumors abutting the optic chiasm, breastfeeding should be adequately considered and tailored based on tumor characteristics, invasiveness, and size. Nevertheless, DA cannot be used until the desired breastfeeding has been completed (34).
Noteworthy, pregnancy may contribute to triggering spontaneous remission from hyperprolactinemia (184, 187, 189-194). As shown in Table 4, approximately 39% of patients with prolactinomas have been reported to experience a spontaneous fall in PRL levels following pregnancy and breastfeeding (185, 187, 189-194), with permanent discontinuation of cabergoline being reported in up to 66% of patients with microprolactinoma, 70% of those with macroprolactinoma, and in 100% of women with nontumoral hyperprolactinemia (184). Older maternal age, lower PRL at diagnosis, and tumor size at diagnosis have been found to be the main determinants for the achievement of normoprolactinemia (193). A role for vascular and molecular mechanisms has been hypothesized to explain the naturally occurring remission from hyperprolactinemia after pregnancy. The acute decrease in blood pressure and the hemodynamic changes directly caused by the delivery and placental expulsion could lead to the autoinfarction of prolactinomas, inducing a decrease in PRL secretion (184). On the other hand, the expression of estrogen receptors, known to affect lactotroph growth and differentiation, may play a role in the reduction in tumor size after pregnancy (196-199).
Table 4.
Spontaneous remission from hyperprolactinemia after pregnancy: overview of literature
| Author, year (reference) | Patient no. | Remission rate (%) |
|---|---|---|
| Crosignani, 1989 (189) | 54 | 17 |
| Crosignani, 1992 (190) | 176 | 29 |
| Jeffcoate, 1996 (191) | 70 | 35 |
| Huda, 2010 (195) | 40 | 10 |
| Auriemma, 2013 (184) | 91 | 68 |
| Domingue, 2014 (192) | 73 | 41 |
| Araujo, 2017 (193) | 25 | 12 |
| O'Sullivan, 2020 (194) | 47 | 25 |
| Sant’ Anna, 2020 (186) | 194 | 10 |
| Total | 770 | 27.8 |
Management in Menopause
In postmenopausal women, prolactinomas are rarely diagnosed (64, 200-202). With the beginning of menopause, the physiological state of hypogonadism is generally associated with the spontaneous decline in the stimulatory effects of estrogens on PRL secretion and lactotroph cell proliferation, resulting in the reduction of circulating PRL levels (64, 200). Consequently, in postmenopausal women, microprolactinomas can remain undiagnosed for a long time because they lack specific features of hormonal hypersecretion (201, 202). In absence of signs and symptoms of PRL excess and given the reportedly low risk of enlargement for microprolactinomas, postmenopausal women may not require medical treatment with DA (64). To a similar extent, patients with microprolactinomas diagnosed at fertile age and passing through menopause may be successfully discontinued from DA. Indeed, spontaneous remission from hyperprolactinemia after DA withdrawal has been reported to occur in two-thirds of postmenopausal women, mainly those with microprolactinomas, whereas the observed recurrence rate was 33% of patients (203-206).
However, most women of menopausal age harbor macroadenomas or giant tumors, responsible for a clinical picture mainly characterized by signs and symptoms of mass effects rather than PRL excess (65-67). Headache and visual loss have been reported to be the most common features at presentation; pituitary apoplexy from very large tumors has been experienced in approximately 5% of cases (66, 67, 207). Therefore, in postmenopausal women with macroprolactinomas, treatment with DA should be cautiously maintained to counteract tumor enlargement (64). In selected patients with macroprolactinomas not abutting the optic chiasm and stable in size while on low DA doses, a trial of DA withdrawal can be contemplated, but with careful monitoring of tumor progression (64).
The use of DA during menopause might be further supported by the evidence that these compounds could improve cardiometabolic and bone health in these patients, as already documented in men with prolactinomas and in women with hypothalamic amenorrhea (41, 207, 208). In this light, the choice to discontinue DA should be carefully weighed considering their beneficial effects at peripheral levels, regardless of PRL and tumor control (64).
AREAS OF UNCERTAINTY AND FUTURE LANDSCAPE
Despite the remarkable effectiveness of DA in the therapeutic management of prolactinomas, patients with aggressive or malignant tumors are still orphans of successful drugs able to overcome resistance to standard treatments or disease recurrence.
Aside from surgery, radiotherapy, and chemotherapy with temozolomide, alternative treatment schedules have been investigated or are now tested in clinical and preclinical models, but the experience is still too scant to draw definitive conclusions. Among them, alternative hormonal treatments, cytotoxic drugs, peptide receptor radionuclide therapy, mTOR/Akt inhibitors, tyrosine kinase inhibitors (TKIs), and immunotherapy appeared to offer promising results, as shown in Fig. 6.
The rationale for the use of selective estrogen receptor modulators (Fig. 6) in prolactinomas rose from the evidence that estrogens affect PRL homeostasis in the hypothalamus, anterior pituitary, and posterior pituitary (209, 210). Prolonged exposure and high doses of estrogens result in an increase in PRL levels; such effects are mediated by estrogen receptors α and β, which are expressed on lactotroph cells and induce cell proliferation in cultured lactotrophs (209, 211). In vitro tamoxifen has been shown to prevent tumor growth and suppress PRL synthesis in the rat pituitary gland (210, 212). In patients with giant, invasive, resistant prolactinomas, the use of selective estrogen receptor modulators, such as tamoxifen and raloxifene, has been found to be associated with a decrease in PRL levels by at least 20% of baseline values (213-215), and an additive effect to bromocriptine (213, 214) or cabergoline (215) has been documented. Nevertheless, high heterogeneity in the achievement of complete biochemical control, defined as PRL normalization, has been reported, varying from 0% (213) to 58.3% (214) and to 71% (215); data about tumor outcomes were not available.
Different steroid hormones, mainly progesterone, have been recently investigated as potential treatments for resistant prolactinomas (216). Whereas in rats, the binding of nuclear progesterone receptors reportedly triggers classical, genomic pathways able to prevent the hypertrophic and hyperplastic effects of estrogens by reducing the number of proliferating cells and increasing cell death (217), the activation of membrane progesterone receptors (Fig. 4), belonging to the progestin and adipoQ receptor family and mediating nongenomic progesterone effects, has been proposed to induce a rapid DA release in hypothalamic dopaminergic neurons (217). The consequent binding of DA to D2DR in the rat pituitary lactotrophs might activate TGFβ1 and decrease cAMP levels, thus inhibiting PRL secretion (216). The simultaneous mPRα activation in the lactotroph cell membrane might induce the inhibition of the adenylyl cyclase activity and cAMP levels, the phosphorylation of ERK, and the activation of TGFβ1, resulting in the inhibition of PRL secretion (217). These promising preclinical results in rats are still waiting for clinical confirmation in humans.
However, in patients with DA-resistant prolactinomas, a higher expression of somatostatin receptors (SSTRs, Fig. 6) has been demonstrated compared with those that are DA-sensitive (218). SSTR5 and SSTR1 have been found to be highly expressed in DA-resistant tumors compared with SSTR2 and SSTR3 (218, 219). Interestingly, in DA-sensitive adenomas, SSTR expression was neglectable compared with D2DR expression (218). At high concentrations, selective binding of SSTR5 by BIM-23268, but not that of SSTR2 by octreotide or BIM-23197, has been demonstrated to inhibit PRL secretion with similar and not additive effects to DA (218). The in vitro investigation of pasireotide-mediated PRL suppression has led to the conclusion that pasireotide potently inhibited PRL secretion in primary cultures of GH and PRL cosecreting adenomas (220) but was almost ineffective in DA-resistant prolactinomas (219), whereas the use of the chimeric SSTR-DR compound BIM-23A760 was associated with an approximately 20% decrease in PRL levels, suggesting partial effectiveness of this drug (219).
This evidence has encouraged the clinical application of treatment with the first-generation somatostatin analogue octreotide (221, 222) and the novel somatostatin analogue pasireotide (223, 224) as alternative therapeutic approaches for resistant prolactinomas, but with discordant results. Indeed, the addition of octreotide LAR to cabergoline has been reported to induce a remarkable tumor shrinkage but only modest inhibition of PRL secretion in selected patients (221, 222), as in clinical case 3. Anecdotal cases reporting the use of pasireotide monotherapy in resistant aggressive prolactinomas, however, have documented the achievement of long-term biochemical and tumoral control (223, 224), suggesting the potential application of pasireotide in the therapeutic algorithm of resistant prolactinomas.
The known expression of SSTRs and the evidence for uptake of radiolabeled somatostatin analogues, such as 68Ga-DOTATATE (225), in prolactinomas have also fostered the investigation of peptide receptor radionuclide therapy effectiveness in aggressive prolactinomas. Treatments with 111Ind-DTPA-octreotide (226, 227), 68Ga-DOTATATE (228), and 17Lu-DOTATOC (227) in patients resistant to multiple surgeries, radiotherapy and medical therapy with temozolomide have been reported to variably reduce tumor volume and PRL levels in some cases (226, 227), or to be almost ineffective in other cases (228), leading to the conclusion that proper identification of candidates to peptide receptor radionuclide therapy and timing of treatment is mandatory to increase the therapeutic efficacy of this promising approach.
The modest and partial effectiveness of hormonal and peptide receptor treatments in aggressive and malignant prolactinomas provided the basis to extend research studies to other alternative therapeutic strategies, sharing the oncological experience.
Cytotoxic chemotherapies have been tested in PRL-secreting carcinomas, but with no evident success. Several chemotherapy protocols, based on the use of carboplatin (229) or associations of lomustine/5-fluorouracil (229), lomustine/procarbazine/etoposide (228), carboplatin/etoposide (230), 5FU/oxaliplatin (225), and cisplatin/procarbazine/lomustine/vincristine (231), have been shown to produce a modest tumoral response, sometimes requiring permanent discontinuation because of hematological toxicity (232).
To a similar extent, mTOR inhibitors (Fig. 6) have been proposed for aggressive prolactinomas after the activation of the PI3K/AKT/mTOR pathway has been demonstrated in murine GH3 cell lines and in PRL-secreting pituitary tumors (233). In GH3 cell lines, both cabergoline and the mTOR inhibitor everolimus monotherapies have been shown to inhibit cell proliferation and PRL secretion, but combined treatment has been found to produce a synergistic effect only on the suppression of PRL levels but not on cell proliferation (233). At immunohistochemistry evaluation, elevated phosphorylated (p-)AKT, p-4EBP1, and p-S6 have been demonstrated in tumor tissues (233). The administration of everolimus in association with cabergoline in a patient with an aggressive prolactinoma resulted in PRL decrease and tumor regression in 5 months, followed by tumor stabilization and PRL reincrease for 12 months (233).
The effectiveness of the antiangiogenic anti-VEGF agent bevacizumab (Fig. 6) in aggressive or malignant prolactinomas is yet to be investigated. In pituitary adenoma tissue samples, western blot analysis has demonstrated high expression of VEGF in approximately 59% of 197 different pituitary tumors, mainly in PRL, ACTH, FSH-secreting and nonfunctioning pituitary adenomas (234). In aggressive ACTH-secreting (234, 235) or nonfunctioning (236) adenomas resistant to multiple surgical, chemotherapy, and radiotherapy approaches, the use of bevacizumab as monotherapy (236) or in combination with temozolomide (233) has resulted in remarkable hormonal reduction (233, 236) and tumor stabilization (236), raising the question of whether similar results can be expected also in prolactinomas.
After epidermal growth factor receptor 2 (HER2)/ErbB2 overexpression has been demonstrated in murine models of prolactinoma and ErbB receptor ligands have been shown to regulate PRL gene expression (237), the use of the TKI (Fig. 6) gefitinib, an epidermal growth factor receptor antagonist (237), and lapatinib, a dual TKI of both epidermal growth factor receptor/ErbB1 and HER2 (238), has been tested on cell proliferation and PRL secretion in animal and human models. Suppressed cell proliferation (237, 238), blocked PRL gene expression (238), inhibited PRL mRNA expression and secretion (238), and reversed EGF-mediated somatotroph-lactotroph phenotype switching (238) have been demonstrated following treatment with gefitinib or lapatinib. Based on this evidence, clinical trials investigating the effects of targeted TKI treatment in aggressive resistant (239) or malignant (240) prolactinomas have been proposed. Tumor shrinkage (239) or stabilization (240) were reported, with PRL levels not always paralleling tumor outcomes, suggesting the potential application of such target therapy in well-selected patients with aggressive prolactinomas or carcinomas.
Last, a high expression of programmed death-ligand 1 (Fig. 6) has been demonstrated in functioning pituitary tumors, mainly PRL-secreting, and has been correlated with aggressive behavior (241). In clinical practice, the association of anti–programmed death-ligand 1 nivolumab and anti-CTLA4 ipilimumab has been reported to induce a significant reduction in pituitary tumor volume and the dominant liver metastasis and hormonal values in a patient with ACTH-secreting carcinoma (242). The evidence about the use of immune checkpoint inhibitors in patients with prolactinomas is limited to 4 cases, in which the use of immune checkpoint inhibitors resulted in complete/partial radiological response in 50% and complete biochemical response in 33% (243).
Conclusions
The approach to the patient with prolactinoma is uncomplicated in most cases. The peculiar clinical picture, mainly characterized by hypogonadism concerns in both sexes, together with signs and symptoms of mass effect in presence of a macroadenoma, suggests the diagnosis of a PRL-secreting pituitary tumor, and a single PRL assessment together with a pituitary MRI are sufficient to carry out the correct diagnosis. Nevertheless, some pitfalls, namely the hook effect and macroprolactin, may tangle and confound the diagnostic workup. Similarly, in menopausal age, the lack of fertility concerns may mask the presence of a prolactinoma, leading to a diagnostic delay. Therapeutic management of prolactinomas is primarily based on using cabergoline as first-line treatment and often sole medical therapy required to control PRL excess and tumor mass and restore fertility. In patients treated with cabergoline for at least 2 years and achieving residual tumor maximal diameter <3.1 mm in size and nadir PRL levels <5.4 μg/L, cabergoline can be totally curative and definitively withdrawn, with long-term remission being seen in approximately 40% of cases and disease relapse occurring within the first 12 months after treatment discontinuation in most patients. Some physiologic conditions in women, such as menopause and pregnancy, may prompt a spontaneous decline in PRL levels, allowing treatment withdrawal. However, 10% to 20% of prolactinomas display resistance to cabergoline, which may be overcome by increasing the drug dose to the maximally tolerated or offering pituitary surgery and radiotherapy. Resistance to cabergoline is a common feature of aggressive or malignant prolactinomas, defined on the basis of tumor invasiveness and proliferation. Aggressive and malignant prolactinomas generally require a combination of medical therapy with surgery and radiotherapy. Unfortunately, intrinsic biological aggressiveness because of a high proliferation rate in some cases may limit the effectiveness of the multimodal approach compelling the use of alternative therapeutic strategies. Temozolomide is now considered the treatment of choice for patients with aggressive or malignant prolactinomas, but responsiveness to treatment appears to be influenced by the MGMT expression in the tumor. The effectiveness of temozolomide can be enhanced by combining this chemotherapy with radiotherapy, but this approach is now reserved for patients with rapid tumor growth in whom maximal doses of radiotherapy have not been reached. In case of ineffectiveness of all available therapeutic strategies, new medical approaches, including alternative hormonal treatments, cytotoxic drugs, peptide receptor radionuclide therapy, mTOR/Akt inhibitors, tyrosine kinase inhibitors, or immunotherapy, may be considered but the experience collected to date is still too scant to draw definitive conclusions. Future studies will clarify the potential applications of such treatments, driving endocrinologists in the choice of the best-tailored therapy for patients with aggressive and malignant prolactinomas.
Abbreviations
- CNC
Carney complex
- D2DR
type 2 dopamine receptor
- DA
dopamine agonist
- HER2
epidermal growth factor receptor 2
- MEN
multiple endocrine neoplasia
- MGMT
O(6)-methylguanine methyl transferase
- MRI
magnetic resonance imaging
- PitNET
pituitary neuroendocrine tumor
- PRL
prolactin
- SSTR
somatostatin receptor
- TKI
tyrosine kinase inhibitor
- ULN
upper limit of normal; VEGF vascular endothelial growth factor
Contributor Information
Renata S Auriemma, Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Università Federico II di Napoli, 80131 Naples, Italy.
Rosa Pirchio, Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Università Federico II di Napoli, 80131 Naples, Italy.
Claudia Pivonello, Dipartimento di Sanità Pubblica, Università Federico II di Napoli, 80131 Naples, Italy.
Francesco Garifalos, Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Università Federico II di Napoli, 80131 Naples, Italy; Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Unità di Andrologia e Medicina della Riproduzione e Sessualità Maschile e Femminile (FERTISEXCARES), Università Federico II di Napoli, 80131 Naples, Italy.
Annamaria Colao, Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Università Federico II di Napoli, 80131 Naples, Italy; Unesco Chair for Health Education and Sustainable Development, “Federico II” University, 80131 Naples, Italy.
Rosario Pivonello, Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Università Federico II di Napoli, 80131 Naples, Italy; Dipartimento di Medicina Clinica e Chirurgia, Sezione di Endocrinologia, Unità di Andrologia e Medicina della Riproduzione e Sessualità Maschile e Femminile (FERTISEXCARES), Università Federico II di Napoli, 80131 Naples, Italy; Unesco Chair for Health Education and Sustainable Development, “Federico II” University, 80131 Naples, Italy.
Funding
None.
Author Contributions
The work submitted for publication is original and has not been published elsewhere for print or electronic publication consideration. The authors affirm that each person listed as the authors participated in the work in a substantive manner. R.S.A. provided substantial contributions to the conception and design of the work, the acquisition, analysis, and interpretation of literature, and to the drafting of the manuscript. R. Pirchio, C.P., and F.G. provided substantial contributions to the acquisition and interpretation of literature and to the drafting of the figures. A.C. and R. Pivonello provided substantial contributions in revising the manuscript critically for important intellectual content. R. Pivonello provided the final approval of the version to be published. All authors consent to the investigation of any improprieties that may be alleged regarding the Work. Each author further releases and holds harmless the Endocrine Society from any claim or liability that may arise therefrom.
Disclosures
A.C. has been Principal Investigator of Research Studies for Novartis, Ipsen, Pfizer, Lilly, Merck, and Novo Nordisk; consultant for Novartis, Ipsen, Pfizer; and received honoraria from Novartis, Ipsen, and Pfizer beyond the confines of this work. R. Pivonello has been Principal Investigator of Clinical and/or Translational Research Studies for Novartis, HRA Pharma, Ipsen, Shire, Corcept Therapeutics, Cortendo AB, Janssen Cilag, Camurus, Strongbridge, and Pfizer; co-investigator of research studies for Pfizer; received research grants from Novartis, Pfizer, Ipsen, HRA Pharma, Shire, IBSA, and Strongbridge Biopharma; has been an occasional consultant for Novartis, Ipsen, Pfizer, Shire, HRA Pharma, Cortendo AB, Ferring, Strongbridge Biopharma, Recordati, Corcept Therapeutics, Crinetics Pharmaceuticals, ARH Healthcare, and Biohealth Italia; and has received fees and honoraria for presentations from Novartis, Shire, Pfizer, and Recordati beyond the confines of this work. R.S.A., R. Pirchio, C.P., and F.G. have nothing to declare.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. De Lellis RA, Lloyd RV, Heitz PU, Eng C, Eng C, Machado De Avila RA. Pathology and Genetics of Tumours of Endocrine Organs. IARC Press; 2004. [Google Scholar]
- 2. Gillam MP, Molitch ME, Lombardi G, Colao A. Advances in the treatment of prolactinomas. Endocr Rev. 2006;27(5):485‐534. [DOI] [PubMed] [Google Scholar]
- 3. Colao A, Lombardi G. Growth-hormone and prolactin excess. Lancet. 1998;352(9138):1455‐1461. [DOI] [PubMed] [Google Scholar]
- 4. Schlechte JA. Clinical practice prolactinoma. N Engl J Med. 2003;349(21):2035‐2041. [DOI] [PubMed] [Google Scholar]
- 5. Cunnah D, Besser M. Management of prolactinomas. Clin Endocrinol. 1991;34(3):231‐235. [DOI] [PubMed] [Google Scholar]
- 6. Ciccarelli A, Daly AF, Beckers A. The epidemiology of prolactinomas. Pituitary. 2005;8(1):3‐6. [DOI] [PubMed] [Google Scholar]
- 7. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91(12):4769‐4775. [DOI] [PubMed] [Google Scholar]
- 8. Vroonen L, Daly AF, Beckers A. Epidemiology and management challenges in prolactinomas. Neuroendocrinology. 2019;109(1):20‐27. [DOI] [PubMed] [Google Scholar]
- 9. Colao A, Sarno AD, Cappabianca P, et al. Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur J Endocrinol. 2003;148(3):325‐331. [DOI] [PubMed] [Google Scholar]
- 10. Mindermann T, Wilson CB. Age-related and gender-related occurrence of pituitary adenomas. Clin Endocrinol (Oxf). 1994;41(3):359‐364. [DOI] [PubMed] [Google Scholar]
- 11. Kars M, Souverein PC, Herings RM, et al. Estimated age- and sex-specific incidence and prevalence of dopamine agonist-treated hyperprolactinemia. J Clin Endocrinol Metab. 2009;94(8):2729‐2734. [DOI] [PubMed] [Google Scholar]
- 12. Colao A. Pituitary tumours in childhood. In: New MI [ed] Endotext.org; 2004.
- 13. Colao A, Loche S. Prolactinomas in children and adolescents. Endocr Dev. 2009;17:146‐159. [DOI] [PubMed] [Google Scholar]
- 14. Maiter D. Mild hyperprolactinemia in a couple: what impact on fertility? Ann Endocrinol (Paris). 2022;83(3):164‐167. [DOI] [PubMed] [Google Scholar]
- 15. Auriemma RS, Del Vecchio G, Scairati R, et al. The interplay between prolactin and reproductive system: focus on uterine pathophysiology. Front Endocrinol (Lausanne). 2020;11:594370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shibli-Rahhal A, Schlechte J. Hyperprolactinemia and infertility. Endocrinol Metab Clin North Am. 2011;40(4):837‐846. [DOI] [PubMed] [Google Scholar]
- 17. Donato J Jr, Frazão R. Interactions between prolactin and kisspeptin to control reproduction. Arch Endocrinol Metab. 2016;60(6):587‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demura R, Ono M, Demura H, Shizume K, Oouchi H. Prolactin directly inhibits basal as well as gonadotropin-stimulated secretion of progesterone and 17 beta-estradiol in the human ovary. J Clin Endocrinol Metab. 1982;54(6):1246‐1250. [DOI] [PubMed] [Google Scholar]
- 19. Corenblum B, Pairaudeau N, Shewchuk AB. Prolactin hypersecretion and short luteal phase defects. Obstet Gynecol. 1976;47(4):486‐488. [PubMed] [Google Scholar]
- 20. Maslar IA, Ansbacher R. Effect of short-duration progesterone treatment on decidual prolactin production by cultures of proliferative human endometrium. Fertil Steril. 1988;50(2):250‐254. [DOI] [PubMed] [Google Scholar]
- 21. Walters CA, Daly DC, Chapitis J, et al. Human myometrium: a new potential source of prolactin. Am J Obstet Gynecol. 1983;147(6):639‐644. [DOI] [PubMed] [Google Scholar]
- 22. Dabbous Z, Atkin SL. Hyperprolactinaemia in male infertility: clinical case scenarios. Arab J Urol. 2018;16(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Coopmans EC, Korbonits M. Molecular genetic testing in the management of pituitary disease. Clin Endocrinol (Oxf). 2022;97(4):424‐435. [DOI] [PubMed] [Google Scholar]
- 24. Burgess JR, Shepherd JJ, Parameswaran V, Hoffman L, Greenaway TM. Prolactinomas in large kindred with multiple endocrine neoplasia type 1: clinical features and inheritance pattern. J Clin Endocrinol Metab. 1996;81(5):1841‐1845. [DOI] [PubMed] [Google Scholar]
- 25. Vergès B, Boureille F, Goudet P, et al. Pituitary disease in MEN type 1 [MEN1]: data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87(2):457‐465. [DOI] [PubMed] [Google Scholar]
- 26. de Laat JM, Dekkers OM, Pieterman CR, et al. Long-term natural course of pituitary tumors in patients with MEN1: results from the DutchMEN1 study group (DMSG). J Clin Endocrinol Metab. 2015;100(9):3288‐3296. [DOI] [PubMed] [Google Scholar]
- 27. Goroshi M, Bandgar T, Lila AR, et al. Multiple endocrine neoplasia type 1 syndrome: single centre experience from western India. Fam Cancer. 2016;15(4):617‐624. [DOI] [PubMed] [Google Scholar]
- 28. Vannucci L, Marini F, Giusti F, Ciuffi S, Tonelli F, Brandi ML. MEN1 In children and adolescents: data from patients of a regional referral center for hereditary endocrine tumors. Endocrine. 2018;59(2):438‐448. [DOI] [PubMed] [Google Scholar]
- 29. Alrezk R, Hannah-Shmouni F, Stratakis CA. MEN4 And CDKN1B mutations: the latest of the MEN syndromes. Endocr Relat Cancer. 2017;24(10):T195‐T208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev. 2013;34(2):239‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Daly AF, Tichomirowa MA, Petrossians P, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010;95(11):E373‐E383. [DOI] [PubMed] [Google Scholar]
- 32. Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J. Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J Clin Endocrinol Metab. 1997;82(7):2102‐2107. [DOI] [PubMed] [Google Scholar]
- 33. Kamilaris CDC, Faucz FR, Voutetakis A, Stratakis CA. Carney complex. Exp Clin Endocrinol Diabetes. 2019;127(2-3):156‐164. [DOI] [PubMed] [Google Scholar]
- 34. Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA; Endocrine Society . Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273‐288. [DOI] [PubMed] [Google Scholar]
- 35. Colao A. Pituitary tumours: the prolactinoma. Best Pract Res Clin Endocrinol Metab. 2009;23(5):575‐596. [DOI] [PubMed] [Google Scholar]
- 36. Berezin M, Shimon I, Hadani M. Prolactinoma in 53 men: clinical characteristics and modes of treatment (male prolactinoma). J Endocrinol Invest. 1995;18(6):436‐440. [DOI] [PubMed] [Google Scholar]
- 37. Duskin-Bitan H, Shimon I. Prolactinomas in males: any differences? Pituitary. 2020;23(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 38. Lopes MBS. The 2017 World Health Organization classification of tumors of the pituitary gland: a summary. Acta Neuropathol. 2017;134(4):521‐535. [DOI] [PubMed] [Google Scholar]
- 39. Naliato EC, Violante AH, Caldas D, et al. Body fat in nonobese women with prolactinoma treated with dopamine agonists. Clin Endocrinol (Oxf). 2007;67(6):845‐852. [DOI] [PubMed] [Google Scholar]
- 40. Naliato EC, Violante AH, Gaccione M, et al. Body fat in men with prolactinoma. J Endocrinol Invest. 2008;31(11):985‐990. [DOI] [PubMed] [Google Scholar]
- 41. Auriemma RS, De Alcubierre D, Pirchio R, Pivonello R, Colao A. The effects of hyperprolactinemia and its control on metabolic diseases. Expert Rev Endocrinol Metab. 2018;13(2):99‐106. [DOI] [PubMed] [Google Scholar]
- 42. Posawetz AS, Trummer C, Pandis M, et al. Adverse body composition and lipid parameters in patients with prolactinoma: a case–control study. BMC Endocr Disord. 2021;21(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Auriemma RS, Granieri L, Galdiero M, et al. Effect of cabergoline on metabolism in prolactinomas. Neuroendocrinology. 2014;98(4):299‐310. [DOI] [PubMed] [Google Scholar]
- 44. Atmaca A, Bilgici B, Ecemis GC, Tuncel OK. Evaluation of body weight, insulin resistance, leptin and adiponectin levels in premenopausal women with hyperprolactinemia. Endocrine. 2013;44(3):756‐761. [DOI] [PubMed] [Google Scholar]
- 45. dos Santos Silva CM, Barbosa FR, Lima GA. et al. et al. BMI And metabolic profile in patients with prolactinoma before and after treatment with dopamine agonists. Obesity (Silver Spring). 2011;19(4):800‐805. [DOI] [PubMed] [Google Scholar]
- 46. Hsu B, Cumming RG, Naganathan V, et al. Associations between circulating reproductive hormones and SHBG and prevalent and incident metabolic syndrome in community-dwelling older men: the Concord Health and Ageing in Men project. J Clin Endocrinol Metab. 2014;99(12):E2686‐E2691. [DOI] [PubMed] [Google Scholar]
- 47. Seriwatanachai D, Thongchote K, Charoenphandhu N, et al. Prolactin directly enhances bone turnover by raising osteoblastexpressed receptor activator of nuclear factor κB ligand/osteoprotegerin ratio. Bone. 2008;42(3):535‐546. [DOI] [PubMed] [Google Scholar]
- 48. Di Somma C, Colao A, Di Sarno A, et al. Bone marker and bone density responses to dopamine agonist therapy in hyperprolactinemic males. J Clin Endocrinol Metab. 1998;83(3):807‐813. [DOI] [PubMed] [Google Scholar]
- 49. Schlechte J, el-Khoury G, Kathol M, Walkner L. Forearm and vertebral bone mineral in treated and untreated hyperprolactinemic amenorrhea. J Clin Endocrinol Metab. 1987;64(5):1021‐1026. [DOI] [PubMed] [Google Scholar]
- 50. Colao A, Di Somma C, Loche S, et al. Prolactinomas in adolescents: persistent bone loss after 2 years of prolactin normalization. Clin Endocrinol (Oxf). 2000;52(3):319‐327. [DOI] [PubMed] [Google Scholar]
- 51. Mazziotti G, Porcelli T, Mormando M, et al. Vertebral fractures in males with prolactinoma. Endocrine. 2011;39(3):288‐293. [DOI] [PubMed] [Google Scholar]
- 52. Mazziotti G, Mancini T, Mormando M, et al. High prevalence of radiological vertebral fractures in women with prolactin secreting pituitary adenomas. Pituitary. 2011;14(4):299‐306. [DOI] [PubMed] [Google Scholar]
- 53. Kayath MJ, Lengyel AM, Vieira JG. Prevalence and magnitude of osteopenia in patients with prolactinoma. Brazilian Journal of Medical and Biological Research. 1993;26(9):933‐941. [PubMed] [Google Scholar]
- 54. Mills EG, Yang L, Nielsen MF, Kassem M, Dhillo WS, Comninos AN. The relationship between bone and reproductive hormones beyond estrogens and androgens. Endocr Rev. 2021;42(6):691‐719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65(2):265‐273. [DOI] [PubMed] [Google Scholar]
- 56. Haddad RA, Giacherio D, Barkan AL. Interpretation of common endocrine laboratory tests: technical pitfalls, their mechanisms and practical considerations. Clin Diabetes Endocrinol. 2019;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Raverot V, Perrin P, Chanson P, Jouanneau E, Brue T, Raverot G. Prolactin immunoassay: does the high-dose hook effect still exist? Pituitary. 2022;25(4):653‐657. [DOI] [PubMed] [Google Scholar]
- 58. Biagetti B, Ferrer Costa R, Alfayate Guerra R, et al. Macroprolactin: from laboratory to clinical practice. Endocrinol Diabetes Nutr (Engl Ed). 2022;69(1):63‐69. [DOI] [PubMed] [Google Scholar]
- 59. Shimatsu A, Hattori N. Macroprolactinemia: diagnostic, clinical, and pathogenic significance. Clin Dev Immunol. 2012;2012:167132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cavaco B, Leite V, Santos MA, Arranhado E, Sobrinho LG. Some forms of big big prolactin behave as a complex of monomeric prolactin with an immunoglobulin G in patients with macroprolactinemia or prolactinoma. J Clin Endocrinol Metab. 1995;80(8):2342‐2346. [DOI] [PubMed] [Google Scholar]
- 61. Glezer A, Bronstein MD. Approach to the patient with persistent hyperprolactinemia and negative sellar imaging. J Clin Endocrinol Metab. 2012;97(7):2211‐2216. [DOI] [PubMed] [Google Scholar]
- 62. Che Soh NAA, Yaacob NM, Omar J, et al. Global prevalence of macroprolactinemia among patients with hyperprolactinemia: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(21):8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vilar L, Fleseriu M, Bronstein MD. Challenges and pitfalls in the diagnosis of hyperprolactinemia. Arq Bras Endocrinol Metabol. 2014;58(1):9‐22. [DOI] [PubMed] [Google Scholar]
- 64. Auriemma RS, Pirchio R, Pivonello R, Colao A. Hyperprolactinemia after menopause: diagnosis and management. Maturitas. 2021;151:36‐40. [DOI] [PubMed] [Google Scholar]
- 65. Maor Y, Berezin M. Hyperprolactinemia in postmenopausal women. Fertil Steril. 1997;67(4):693‐696. [DOI] [PubMed] [Google Scholar]
- 66. Shimon I, Bronstein MD, Shapiro J, Tsvetov G, Benbassat C, Barkan A. Women with prolactinomas presented at the postmenopausal period. Endocrine. 2014;47(3):889‐894. [DOI] [PubMed] [Google Scholar]
- 67. Santharam S, Tampourlou M, Arlt W, et al. Prolactinomas diagnosed in the postmenopausal period: clinical phenotype and outcomes. Clin Endocrinol (Oxf). 2017;87(5):508‐514. [DOI] [PubMed] [Google Scholar]
- 68. Trouillas J, Delgrange E, Wierinckx A, et al. Clinical, pathological, and molecular factors of aggressiveness in lactotroph tumours. Neuroendocrinology. 2019;109(1):70‐76. [DOI] [PubMed] [Google Scholar]
- 69. Trouillas J, Jaffrain-Rea ML, Vasiljevic A, Raverot G, Roncaroli F, Villa C.. How to classify the pituitary neuroendocrine tumors (PitNET)s in 2020. Cancers (Basel). 2020;12(2):514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chanson P, Maiter D. The epidemiology, diagnosis and treatment of prolactinomas: the old and the new. Best Pract Res Clin Endocrinol Metab. 2019;33(2):101290. [DOI] [PubMed] [Google Scholar]
- 71. McCormack A, Dekkers OM, Petersenn S. et al. et al. ESE survey collaborators. Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol. 2018;178(3):265‐276. [DOI] [PubMed] [Google Scholar]
- 72. Burman P, Trouillas J, Losa M, et al. ESE survey collaborators . Aggressive pituitary tumours and carcinomas, characteristics and management of 171 patients. Eur J Endocrinol. 2022;187(4):593‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Auriemma RS, Pirchio R, De Alcubierre D, Pivonello R, Colao A. Dopamine agonists: from the 1970s to today. Neuroendocrinology. 2019;109(1):34‐41. [DOI] [PubMed] [Google Scholar]
- 74. Schlechte J, Dolan K, Sherman B, Chapler F, Luciano A. The natural history of untreated hyperprolactinemia: a prospective analysis. J Clin Endocrinol Metab. 1989;68(2):412‐418. [DOI] [PubMed] [Google Scholar]
- 75. Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22(6):724‐763. [DOI] [PubMed] [Google Scholar]
- 76. Biller BM, Colao A, Petersenn S, Bonert VS, Boscaro M. Prolactinomas, Cushing's disease and acromegaly: debating the role of medical therapy for secretory pituitary adenomas. BMC Endocr Disord. 2010;10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Colao A, Di Sarno A, Landi ML, et al. Macroprolactinoma shrinkage during cabergoline treatment is greater in naive patients than in patients pretreated with other dopamine agonists: a prospective study in 110 patients. J Clin Endocrinol Metab. 2000;85(6):2247‐2252. [DOI] [PubMed] [Google Scholar]
- 78. Di Sarno A, Landi ML, Cappabianca P, et al. Resistance to cabergoline as compared with bromocriptine in hyperprolactinemia: prevalence, clinical definition, and therapeutic strategy. J Clin Endocrinol Metab. 2001;86(11):5256‐5261. [DOI] [PubMed] [Google Scholar]
- 79. Colao A, Di Sarno A, Pivonello R, Di Somma C, Lombardi G. Dopamine receptor agonists for treating prolactinomas. Expert Opin Investig Drugs. 2002;11(6):787‐800. [DOI] [PubMed] [Google Scholar]
- 80. Bonert V. Do nothing but observe microprolactinomas: when and how to replace sex hormones? Pituitary. 2020;23(3):307‐313. [DOI] [PubMed] [Google Scholar]
- 81. De Rosa M, Zarrilli S, Vitale G, et al. Six months of treatment with cabergoline restores sexual potency in hyperprolactinemic males: an open longitudinal study monitoring nocturnal penile tumescence. J Clin Endocrinol Metab. 2004;89(2):621‐625. [DOI] [PubMed] [Google Scholar]
- 82. De Rosa M, Ciccarelli A, Zarrilli S, et al. The treatment with cabergoline for 24 months normalises seminal fluid quality in hyperprolactinaemic males. Clin Endocrinol (Oxf). 2006;64(3):307‐313. [DOI] [PubMed] [Google Scholar]
- 83. Salenave S, Trabado S, Maione L, Brailly-Tabard S, Young J. Male acquired hypogonadotropic hypogonadism: diagnosis and treatment. Ann Endocrinol (Paris). 2012;73(2):141‐146. [DOI] [PubMed] [Google Scholar]
- 84. Ciresi A, Amato MC, Guarnotta V, Lo Castro F, Giordano C. Higher doses of cabergoline further improve metabolic parameters in patients with prolactinoma regardless of the degree of reduction in prolactin levels. Clin Endocrinol (Oxf). 2013;79(6):845‐852. [DOI] [PubMed] [Google Scholar]
- 85. Bhansali A, Walia R, Dutta P, et al. Efficacy of cabergoline on rapid escalation of dose in men with macroprolactinomas. Indian J Med Res. 2010;131:530‐535. [PubMed] [Google Scholar]
- 86. Auriemma RS, Galdiero M, Vitale P, et al. Effect of chronic cabergoline treatment and testosterone replacement on metabolism in male patients with prolactinomas. Neuroendocrinology. 2015;101(1):66‐81. [DOI] [PubMed] [Google Scholar]
- 87. Pirchio R, Auriemma RS, Solari D, et al. Effects of pituitary surgery and high-dose cabergoline therapy on metabolic profile in patients with prolactinoma resistant to conventional cabergoline treatment. Front Endocrinol (Lausanne). 2021;12:769744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Holt RI, Barnett AH, Bailey CJ. Bromocriptine: old drug, new formulation and new indication. Diabetes Obes Metab. 2010;12(12):1048‐1057. [DOI] [PubMed] [Google Scholar]
- 89. Pala NA, Laway BA, Misgar RA, Dar RA. Metabolic abnormalities in patients with prolactinoma: response to treatment with cabergoline. Diabetol Metab Syndr. 2015;7:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Schwetz V, Librizzi R, Trummer C, et al. Treatment of hyperprolactinaemia reduces total cholesterol and LDL in patients with prolactinomas. Metab Brain Dis. 2017;32(1):155‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Webster J. A comparative review of the tolerability profiles of dopamine agonists in the treatment of hyperprolactinaemia and inhibition of lactation. Drug Saf. 1996;14:228‐238. [DOI] [PubMed] [Google Scholar]
- 92. Redfield MM, Nicholson WJ, Edwards WD, Tajik AJ. Valve disease associated with ergot alkaloid use: echocardiographic and pathologic correlations. Ann Intern Med. 1992;117(1):50‐52. [DOI] [PubMed] [Google Scholar]
- 93. Jähnichen S, Horowski R, Pertz HH. Agonism at 5-HT2B receptors is not a class effect of the ergolines. Eur J Pharmacol. 2005;513:225‐228. [DOI] [PubMed] [Google Scholar]
- 94. Lancellotti P, Livadariu E, Markov M, et al. Cabergoline and the risk of valvular lesions in endocrine disease. Eur J Endocrinol. 2008;159(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 95. Devin JK, Lakhani VT, Byrd BF, Blevins LS. Prevalence of valvular heart disease in a cohort of patients taking cabergoline for management of hyperprolactinemia. Endocr Pract. 2008;14(6):672‐677. [DOI] [PubMed] [Google Scholar]
- 96. Kars M, Delgado V, Holman ER, et al. Aortic valve calcification and mild tricuspid regurgitation, but no clinical heart disease after 8 years of dopamine agonist therapy for prolactinomas. J Clin Endocrinol Metab. 2008;93(9):3348‐3356. [DOI] [PubMed] [Google Scholar]
- 97. Colao A, Galderisi M, Di Sarno A, et al. Increased prevalence of tricuspid regurgitation in patients with prolactinomas chronically treated with cabergoline. J Clin Endocrinol Metab. 2008;93(10):3777‐3784. [DOI] [PubMed] [Google Scholar]
- 98. Wakil A, Rigby AS, Clark AL, Kallvikbacka-Bennett A, Atkin SL. Low dose of cabergoline for hyperprolactinemia is not associated with clinically significant valvular heart disease. Eur J Endocrinol. 2008;159(4):R11‐R14. [DOI] [PubMed] [Google Scholar]
- 99. Bogazzi F, Buralli S, Manetti L, et al. Treatment with low doses of cabergoline is not associated with increased prevalence of cardiac valve regurgitation in patients with hyperprolactinemia. Int J Clin Pract. 2008;62(12):1864‐1869. [DOI] [PubMed] [Google Scholar]
- 100. Herring N, Szmigielski C, Becher H, Karavitaki N, Wass JA. Valvular heart disease and the use of cabergoline for the treatment of prolactinomas. Clin Endocrinol (Oxf). 2009;70(1):104‐108. [DOI] [PubMed] [Google Scholar]
- 101. Vallette S, Serri K, Rivera J, et al. Long-term cabergoline therapy is not associated with valvular heart disease in patients with prolactinomas. Pituitary. 2009;12(3):153‐157. [DOI] [PubMed] [Google Scholar]
- 102. Nachtigall L, Valassi E, Lo J, et al. Gender effects on cardiac valvular function in hyperprolactinaemic patients receiving cabergoline: a retrospective study. Clin Endocrinol (Oxf). 2010;72(1):53‐58. [DOI] [PubMed] [Google Scholar]
- 103. Tan T, Cabrita IZ, Hensman D, et al. Assessment of cardiac valve dysfunction in patients receiving cabergoline treatment for hyperprolactinaemia. Clin Endocrinol. 2010;73(3):369‐374. [DOI] [PubMed] [Google Scholar]
- 104. Boguszewski CL, dos Santos CM, Sakamoto KS, Marini LC, de Souza AM, Azevedo M. A comparison of cabergoline and bromocriptine on the risk of valvular heart disease in patients with prolactinomas. Pituitary. 2012;15(1):44‐49. [DOI] [PubMed] [Google Scholar]
- 105. Delgado V, Biermasz NR, van Thiel SW, et al. Changes in heart valve structure and function in patients treated with dopamine agonists for prolactinomas, a 2-year follow-up study. Clin Endocrinol. 2012;77(1):99‐105. [DOI] [PubMed] [Google Scholar]
- 106. Elenkova A, Shabani R, Kalinov K, Zacharieva S. Increased prevalence of subclinical cardiac valve fibrosis in patients with prolactinomas on long-term bromocriptine and cabergoline treatment. Eur J Endocrinol. 2012;167(1):17‐25. [DOI] [PubMed] [Google Scholar]
- 107. Halperin I, Aller J, Varela C, et al. No clinically significant valvular regurgitation in long-term cabergoline treatment for prolactinomas. Clin Endocrinol. 2012;77(2):275‐280. [DOI] [PubMed] [Google Scholar]
- 108. Córdoba-Soriano JG, Lamas-Oliveira C, Hidalgo-Olivares VM, Tercero-Martínez A, Barambio-Ruíz M, Salas-Nieto J. Valvular heart disease in hyperprolactinemic patients treated with low doses of cabergoline. Rev Esp Cardiol. 2013;66(5):410‐412. [DOI] [PubMed] [Google Scholar]
- 109. Auriemma RS, Pivonello R, Perone Y, et al. Safety of long-term treatment with cabergoline on cardiac valve disease in patients with prolactinomas. Eur J Endocrinol. 2013;169(3):359‐366. [DOI] [PubMed] [Google Scholar]
- 110. Drake WM, Stiles CE, Howlett TA, Toogood AA, Bevan JS, Steeds RP; UK Dopamine Agonist Valvulopathy Group . A cross-sectional study of the prevalence of cardiac valvular abnormalities in hyperprolactinemic patients treated with ergot-derived dopamine agonists. J Clin Endocrinol Metab. 2014;99(1):90‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vroonen L, Lancellotti P, Garcia MT, et al. Prospective, long-term study of the effect of cabergoline on valvular status in patients with prolactinoma and idiopathic hyperprolactinemia. Endocrine. 2017;55(1):239‐245. [DOI] [PubMed] [Google Scholar]
- 112. Drake WM, Stiles CE, Bevan JS, et al. A follow-up study of the prevalence of valvular heart abnormalities in hyperprolactinemic patients treated with cabergoline. J Clin Endocrinol Metab. 2016;101(11):4189‐4194. [DOI] [PubMed] [Google Scholar]
- 113. Khare S, Lila AR, Patil R, et al. Long-term cardiac (valvulopathy) safety of cabergoline in prolactinoma. Indian J Endocrinol Metab. 2017;21(1):154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Budayr A, Tan TC, Lo JC, et al. Cardiac valvular abnormalities associated with use and cumulative exposure of cabergoline for hyperprolactinemia: the CATCH study. BMC Endocr Disord. 2020;20(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Stiles CE, Lloyd G, Bhattacharyya S, et al. Incidence of cabergoline-associated valvulopathy in primary care patients with prolactinoma using hard cardiac endpoints. J Clin Endocrinol Metab. 2021;106(2):e711‐e720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Van Camp G, Flamez A, Cosyns B, Weytjens C, Muyldermans L, Van ZM. Treatment of Parkinson's disease with pergolide and relation to restrictive valvular heart disease. Lancet. 2004;363:1179‐1183. [DOI] [PubMed] [Google Scholar]
- 117. Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med. 2007;356(1):29‐38. [DOI] [PubMed] [Google Scholar]
- 118. Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G. Valvular heart disease and the use of dopamine agonists for Parkinson's disease. N Engl J Med. 2007;356(1):39‐46. [DOI] [PubMed] [Google Scholar]
- 119. Stiles CE, Tetteh-Wayoe ET, Bestwick J, Steeds RP, Drake WM. A meta-analysis of the prevalence of cardiac valvulopathy in hyperprolactinemic patients treated with cabergoline. J Clin Endocrinol Metab. 2018;104(2):523‐538. [DOI] [PubMed] [Google Scholar]
- 120. Steeds RP, Stiles CE, Sharma V, Chambers JB, Lloyd G, Drake W. Echocardiography and monitoring patients receiving dopamine agonist therapy for hyperprolactinaemia: a joint position statement of the British Society of Echocardiography, the British Heart Valve Society and the Society for Endocrinology. Echo Res Pract. 2019;6(1):G1‐G8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Auriemma RS, Pivonello R, Ferreri L, Priscitelli P, Colao A. Cabergoline use for pituitary tumors and valvular disorders. Endocrinol Metab Clin North Am. 2015;44(1):89‐97. [DOI] [PubMed] [Google Scholar]
- 122. Maiter D. Management of dopamine agonist-resistant prolactinoma. Neuroendocrinology. 2019;109(1):42‐50. [DOI] [PubMed] [Google Scholar]
- 123. Souteiro P, Karavitaki N. Dopamine agonist resistant prolactinomas: any alternative medical treatment? Pituitary. 2020;23(1):27‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Delgrange E, Crabbe J, Donckier J. Late development of resistance to bromocriptine in a patient with macroprolactinoma. Horm Res. 1998;49(5):250‐253. [DOI] [PubMed] [Google Scholar]
- 125. Behan LA, Draman MS, Moran C, et al. Secondary resistance to cabergoline therapy in a macroprolactinoma: a case report and literature review. Pituitary. 2011;14(4):362‐366. [DOI] [PubMed] [Google Scholar]
- 126. Breidahl HD, Topliss DJ, Pike JW. Failure of bromocriptine to maintain reduction in size of a macroprolactinoma. BMJ. 1983;287(6390):451‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. McCall D, Hunter SJ, Cooke RS, Herron B, Sheridan B, Atkinson AB. Unusual late development of dopamine agonist resistance in two women with hyperprolactinaemia associated with transition from micro to macroadenoma. Clin Endocrinol (Oxf). 2007;66(1):149‐150. [DOI] [PubMed] [Google Scholar]
- 128. Alberiche Ruano M, Boronat Cortés M, Ojeda Pino A, et al. Acquired resistance to cabergoline: progression from initially responsive micro to macroprolactinoma. Pituitary. 2010;13(4):380‐382. [DOI] [PubMed] [Google Scholar]
- 129. Sbardella E, Farah G, Fathelrahman A, et al. A macroprolactinoma becoming resistant to cabergoline and developing atypical pathology. Endocrinol Diabetes Metab Case Rep. 2016;2016:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Eshkoli T, Fraenkel M, Zaid D, et al. Resistant prolactinomas: a case series of 26 patients. Endocrine. 2022;77(2):349‐356. [DOI] [PubMed] [Google Scholar]
- 131. Pivonello C, Patalano R, Negri M, et al. Resistance to dopamine agonists in pituitary tumors: molecular mechanisms. Front Endocrinol (Lausanne). 2022;12:791633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Filopanti M, Barbieri AM, Angioni AR, et al. Dopamine D2 receptor gene polymorphisms and response to cabergoline therapy in patients with prolactin-secreting pituitary adenomas. Pharmacogenomics J. 2008;8(5):357‐363. [DOI] [PubMed] [Google Scholar]
- 133. Pasqualini C, Bojda F, Kerdelhue B. Direct effect of estradiol on the number of dopamine receptors in the anterior pituitary of ovariectomized rats. Endocrinology. 1986;119(6):2484‐2489. [DOI] [PubMed] [Google Scholar]
- 134. Guivarc’h D, Vincent JD, Vernier P. Alternative splicing of the D2 dopamine receptor messenger ribonucleic acid is modulated by activated sex steroid receptors in the MMQ prolactin cell line. Endocrinology. 1998;139(10):4213‐4221. [DOI] [PubMed] [Google Scholar]
- 135. Oomizu S, Boyadjieva N, Sarkar DK. Ethanol and estradiol modulate alternative splicing of dopamine D2 receptor messenger RNA and abolish the inhibitory action of bromocriptine on prolactin release from the pituitary gland. Alcohol Clin Exp Res. 2003;27(6):975‐980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Peverelli E, Mantovani G, Vitali E, et al. Filamin-A is essential for dopamine d2 receptor expression and signaling in tumorous lactotrophs. J Clin Endocrinol Metab. 2012;97(3):967‐977. [DOI] [PubMed] [Google Scholar]
- 137. Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-Arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261‐273. [DOI] [PubMed] [Google Scholar]
- 138. Gatto F, Feelders R, van der Pas R, et al. Β-Arrestin 1 and 2 and G protein-coupled receptor kinase 2 expression in pituitary adenomas: role in the regulation of response to somatostatin analogue treatment in patients with acromegaly. Endocrinology. 2013;154(12):4715‐4725. [DOI] [PubMed] [Google Scholar]
- 139. Wu Z, Cai L, Lu J, et al. MicroRNA-93 mediates cabergoline-resistance by targeting ATG7 in prolactinoma. J Endocrinol. 2018;240(1):1‐13. [DOI] [PubMed] [Google Scholar]
- 140. Hu B, Mao Z, Du Q, et al. miR-93-5p targets Smad7 to regulate the transforming growth factor-β1/Smad3 pathway and mediate fibrosis in drug-resistant prolactinoma. Brain Res Bull. 2019;149:21‐31. [DOI] [PubMed] [Google Scholar]
- 141. Xiao Z, Wang Z, Hu B. et al. et al. MiR-1299 promotes the synthesis and secretion of prolactin by inhibiting FOXO1 expression in drug-resistant prolactinomas. Biochem Biophys Res Commun. 2019;520(1):79‐85. [DOI] [PubMed] [Google Scholar]
- 142. Jian M, Du Q, Zhu D, et al. Tumor suppressor miR-145-5p sensitizes prolactinoma to bromocriptine by downregulating TPT1. J Endocrinol Invest. 2019;42(6):639‐652. [DOI] [PubMed] [Google Scholar]
- 143. Ono M, Miki N, Kawamata T, et al. Prospective study of high-dose cabergoline treatment of prolactinomas in 150 patients. J Clin Endocrinol Metab. 2008;93(12):4721‐4727. [DOI] [PubMed] [Google Scholar]
- 144. Colao A, Di Sarno A, Sarnacchiaro F, et al. Prolactinomas resistant to standard dopamine agonists respond to chronic cabergoline treatment. J Clin Endocrinol Metab. 1997;82(3):876‐883. [DOI] [PubMed] [Google Scholar]
- 145. Cozzi R, Ambrosio MR, Attanasio R, et al. Italian Association of Clinical Endocrinologists (AME) and International Chapter of Clinical Endocrinology (ICCE). Position statement for clinical practice: prolactin-secreting tumors. Eur J Endocrinol. 2022;186(3):P1‐P33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Klibanski A. Clinical practice. Prolactinomas. N Engl J Med. 2010;362(13):1219‐1226. [DOI] [PubMed] [Google Scholar]
- 147. Vroonen L, Jaffrain-Rea ML, Petrossians P, et al. Prolactinomas resistant to standard doses of cabergoline: a multicenter study of 92 patients. Eur J Endocrinol. 2012;167(5):651‐662. [DOI] [PubMed] [Google Scholar]
- 148. Zamanipoor Najafabadi AH, Zandbergen IM, de Vries F, et al. Surgery as a viable alternative first-line treatment for prolactinoma patients. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2020;105(3):e32‐e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Mamelak A. Surgery as a first-line option for prolactinomas. Expert Rev Endocrinol Metab. 2022;17(6):485‐498. [DOI] [PubMed] [Google Scholar]
- 150. Lasolle H, Ilie MD, Raverot G. Aggressive prolactinomas: how to manage? Pituitary. 2020;23(1):70‐77. [DOI] [PubMed] [Google Scholar]
- 151. Raverot G, Burman P, McCormack A, et al. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1‐G24. [DOI] [PubMed] [Google Scholar]
- 152. Das L, Rai A, Salunke P, et al. Temozolomide nonresponsiveness in aggressive prolactinomas and carcinomas: management and outcomes. J Endocr Soc. 2022;6(2):bvab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Johnston DG, Hall K, Kendall-Taylor P, Patrick D, Watson M, Cook DB. Effect of dopamine agonist withdrawal after long-term therapy in prolactinomas. Studies with high-definition computerised tomography. Lancet. 1984;324(8396):187‐192. [DOI] [PubMed] [Google Scholar]
- 154. Moriondo P, Travaglini P, Nissim M, Conti A, Faglia G. Bromocriptine treatment of microprolactinomas: evidence of stable prolactin decrease after drug withdrawal. J Clin Endocrinol Metab. 1985;60(4):764‐772. [DOI] [PubMed] [Google Scholar]
- 155. Rasmussen C, Bergh T, Wide L. Prolactin secretion and menstrual function after long-term bromocriptine treatment. Fertil Steril. 1987;48(4):550‐554. [DOI] [PubMed] [Google Scholar]
- 156. Wang C, Lam KS, Ma JT, Chan T, Liu MY, Yeung RT. Long-term treatment of hyperprolactinaemia with bromocriptine: effect of drug withdrawal. Clin Endocrinol (Oxf). 1987;27(3):363‐371. [DOI] [PubMed] [Google Scholar]
- 157. van't Verlaat JW, Croughs RJ. Withdrawal of bromocriptine after long-term therapy for macroprolactinomas; effect on plasma prolactin and tumour size. Clin Endocrinol (Oxf). 1991;34(3):175‐178. [DOI] [PubMed] [Google Scholar]
- 158. Zárate A, Canales ES, Cano C, Pilonieta CJ. Follow-up of patients with prolactinomas after discontinuation of long-term therapy with bromocriptine. Acta Endocrinol (Copenh). 1983;104(2):139‐142. [DOI] [PubMed] [Google Scholar]
- 159. Passos VQ, Souza JJ, Musolino NR, Bronstein MD. Long-term follow-up of prolactinomas: normoprolactinemia after bromocriptine withdrawal. J Clin Endocrinol Metab. 2002;87(8):3578‐3582. [DOI] [PubMed] [Google Scholar]
- 160. Thorner MO, Perryman RL, Rogol AD, et al. Rapid changes of prolactinoma volume after withdrawal and reinstitution of bromocriptine. J Clin Endocrinol Metab. 1981;53(3):480‐483. [DOI] [PubMed] [Google Scholar]
- 161. Orrego JJ, Chandler WF, Barkan AL. Rapid re-expansion of a macroprolactinoma after early discontinuation of bromocriptine. Pituitary. 2000;3(3):189‐192. [DOI] [PubMed] [Google Scholar]
- 162. Ferrari C, Paracchi A, Mattei AM, et al. Cabergoline in the long-term therapy of hyperprolactinemic disorders. Acta Endocrinol. 1992;126(6):489‐494. [DOI] [PubMed] [Google Scholar]
- 163. Muratori M, Arosio M, Gambino G, et al. Use of cabergoline in the long-term treatment of hyperprolactinemic and acromegalic patients. J Endocrinol Invest. 1997;20:537‐546. [DOI] [PubMed] [Google Scholar]
- 164. Cannavò S, Curto L, Squadrito S, et al. Cabergoline: a first-choice treatment in patients with previously untreated prolactin-secreting pituitary adenoma. J Endocrinol Invest. 1999;22(5):354‐359. [DOI] [PubMed] [Google Scholar]
- 165. Colao A, Di Sarno A, Cappabianca P, Di Somma C, Pivonello R, Lombardi G. Withdrawal of long-term cabergoline therapy for tumoral and nontumoral hyperprolactinemia. N Engl J Med. 2003;349:2023‐2033. [DOI] [PubMed] [Google Scholar]
- 166. Colao A, Di Sarno A, Guerra E, et al. Predictors of remission of hyperprolactinaemia after long-term withdrawal of cabergoline therapy. Clin Endocrinol (Oxf). 2007;67(3):426‐433. [DOI] [PubMed] [Google Scholar]
- 167. Kharlip J, Salvatori R, Yenokyan G, Wand GS. Recurrence of hyperprolactinemia after withdrawal of long-term cabergoline therapy. J Clin Endocrinol Metab. 2009;94(7):2428‐2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Sala E, Bellaviti Buttoni P, Malchiodi E, et al. Recurrence of hyperprolactinemia following dopamine agonist withdrawal and possible predictive factors of recurrence in prolactinomas. J Endocrinol Invest. 2016;39(12):1377‐1382. [DOI] [PubMed] [Google Scholar]
- 169. Espinosa-Cárdenas E, Sánchez-García M, Ramírez-Rentería C, Mendoza-Zubieta V, Sosa-Eroza E, Mercado M. High biochemical recurrence rate after withdrawal of cabergoline in prolactinomas: is it necessary to restart treatment? Endocrine. 2020;70(1):143‐149. [DOI] [PubMed] [Google Scholar]
- 170. Kim K, Park YW, Kim D, et al. Biochemical remission after cabergoline withdrawal in hyperprolactinemic patients with visible remnant pituitary adenoma. J Clin Endocrinol Metab. 2021;106(2):e615‐e624. [DOI] [PubMed] [Google Scholar]
- 171. Biswas M, Smith J, Jadon D, et al. Long-term remission following withdrawal of dopamine agonist therapy in subjects with microprolactinomas. Clin Endocrinol (Oxf). 2005;63:26‐31. [DOI] [PubMed] [Google Scholar]
- 172. Guitelman M. Long-term follow-up of prolactinomas: should dopamine agonist treatment be life-long? Front Horm Res. 2006;35:88‐101. [DOI] [PubMed] [Google Scholar]
- 173. Barber TM, Kenkre J, Garnett C, Scott RV, Byrne JV, Wass JA. Recurrence of hyperprolactinaemia following discontinuation of dopamine agonist therapy in patients with prolactinoma occurs commonly especially in macroprolactinoma. Clin Endocrinol (Oxf). 2011;75(6):819‐824. [DOI] [PubMed] [Google Scholar]
- 174. Anagnostis P, Adamidou F, Polyzos SA, Efstathiadou Z, Karathanassi E, Kita M. Long term follow-up of patients with prolactinomas and outcome of dopamine agonist withdrawal: a single center experience. Pituitary. 2012;15(1):25‐29. [DOI] [PubMed] [Google Scholar]
- 175. Teixeira M, Souteiro P, Carvalho D. Prolactinoma management: predictors of remission and recurrence after dopamine agonists withdrawal. Pituitary. 2017;20(4):464‐470. [DOI] [PubMed] [Google Scholar]
- 176. Pereira AM. Update on the withdrawal of dopamine agonists in patients with hyperprolactinemia. Curr Opin Endocrinol Diabetes Obes. 2011;18(4):264‐268. [DOI] [PubMed] [Google Scholar]
- 177. Di Sarno A, Landi ML, Marzullo P, et al. The effect of quinagolide and cabergoline, two selective dopamine receptor type 2 agonists, in the treatment of prolactinomas. Clin Endocrinol (Oxf). 2000;53:53‐60. [DOI] [PubMed] [Google Scholar]
- 178. Dekkers OM, Lagro J, Burman P, Jørgensen JO, Romijn JA, Pereira AM. Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95(1):43‐51. [DOI] [PubMed] [Google Scholar]
- 179. Kwancharoen R, Auriemma RS, Yenokyan G, Wand GS, Colao A, Salvatori R. Second attempt to withdraw cabergoline in prolactinomas: a pilot study. Pituitary. 2014;17(5):451‐456. [DOI] [PubMed] [Google Scholar]
- 180. Vilar L, Albuquerque JL, Gadelha PS, et al. Second attempt of cabergoline withdrawal in patients with prolactinomas after a failed first attempt: is it worthwhile? Front Endocrinol (Lausanne). 2015;6(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Molitch ME. Endocrinology in pregnancy: management of the pregnant patient with a prolactinoma. Eur J Endocrinol. 2015;172(5):R205‐R213. [DOI] [PubMed] [Google Scholar]
- 182. Karaca Z, Tanriverdi F, Unluhizarci K, Kelestimur F. Pregnancy and pituitary disorders. Eur J Endocrinol. 2010;162(3):453‐475. [DOI] [PubMed] [Google Scholar]
- 183. Glezer A, Bronstein MD. Prolactinomas in pregnancy: considerations before conception and during pregnancy. Pituitary. 2020;23(1):65‐69. [DOI] [PubMed] [Google Scholar]
- 184. Auriemma RS, Perone Y, Di Sarno A, et al. Results of a single-center observational 10-year survey study on recurrence of hyperprolactinemia after pregnancy and lactation. J Clin Endocrinol Metab. 2013;98(1):372‐379. [DOI] [PubMed] [Google Scholar]
- 185. Huang W, Molitch ME. Pituitary tumors in pregnancy. Endocrinol Metab Clin North Am. 2019;48(3):569‐581. [DOI] [PubMed] [Google Scholar]
- 186. Sant’ Anna BG, Musolino NRC, Gadelha MR, et al. A Brazilian multicentre study evaluating pregnancies induced by cabergoline in patients harboring prolactinomas. Pituitary. 2020;23(2):120‐128. [DOI] [PubMed] [Google Scholar]
- 187. Pivonello R, De Martino MC, Auriemma RS, et al. Pituitary tumors and pregnancy: the interplay between a pathologic condition and a physiologic status. J Endocrinol Invest. 2014;37(2):99‐112. [DOI] [PubMed] [Google Scholar]
- 188. Ikegami H, Aono T, Koizumi K, Koike K, Fukui H, Tanizawa O. Relationship between the methods of treatment for prolactinomas and the puerperal lactation. Fertil Steril. 1987;47(5):867‐869. [DOI] [PubMed] [Google Scholar]
- 189. Crosignani PG, Mattei AM, Scarduelli C, Cavioni V, Boracchi P. Is pregnancy the best treatment for hyperprolactinaemia? Hum Reprod. 1989;4(8):910‐912. [DOI] [PubMed] [Google Scholar]
- 190. Crosignani PG, Mattei AM, Severini V, Cavioni V, Maggioni P, Testa G. Long-term effects of time, medical treatment and pregnancy in 176 hyperprolactinemic women. Eur J Obstet Gynecol Reprod Biol. 1992;44(3):175‐180. [DOI] [PubMed] [Google Scholar]
- 191. Jeffcoate WJ, Pound N, Sturrock ND, Lambourne J. Long-term follow-up of patients with hyperprolactinaemia. Clin Endocrinol (Oxf). 1996;45(3):299‐303. [DOI] [PubMed] [Google Scholar]
- 192. Domingue ME, Devuyst F, Alexopoulou O, Corvilain B, Maiter D. Outcome of prolactinoma after pregnancy and lactation: a study on 73 patients. Clin Endocrinol (Oxf). 2014;80(5):642‐648. [DOI] [PubMed] [Google Scholar]
- 193. Araujo B, Belo S, Carvalho D. Pregnancy and tumor outcomes in women with prolactinoma. Exp Clin Endocrinol Diabetes. 2017;125(10):642‐648. [DOI] [PubMed] [Google Scholar]
- 194. O'Sullivan SM, Farrant MT, Ogilvie CM, Gunn AJ, Milsom SR. An observational study of pregnancy and post-partum outcomes in women with prolactinoma treated with dopamine agonists. Aust N Z J Obstet Gynaecol. 2020;60(3):405‐411. [DOI] [PubMed] [Google Scholar]
- 195. Huda MS, Athauda NB, Teh MM, Carroll PV, Powrie JK. Factors determining the remission of microprolactinomas after dopamine agonist withdrawal. Clin Endocrinol (Oxf). 2010;72(4):507‐511. [DOI] [PubMed] [Google Scholar]
- 196. Gittoes NJ, McCabe CJ, Sheppard MC, Franklyn JA. Estrogen receptor beta mRNA expression in normal and adenomatous pituitaries. Pituitary. 1999;1(2):99‐104. [DOI] [PubMed] [Google Scholar]
- 197. Heaney AP, Fernando M, Melmed S. Functional role of estrogen in pituitary tumor pathogenesis. J Clin Invest. 2002;109(2):277‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Burdman JA, Pauni M, Heredia Sereno GM, Bordón AE. Estrogen receptors in human pituitary tumors. Horm Metab Res. 2008;40(8):524‐527. [DOI] [PubMed] [Google Scholar]
- 199. Leng L, Zhang Y. Effects of an estrogen receptor antagonist on proliferation, prolactin secretion and growth factor expression in the MMQ pituitary prolactinoma cell line. J Clin Neurosci. 2011;18(12):1694‐1698. [DOI] [PubMed] [Google Scholar]
- 200. Cocks Eschler D, Javanmard P, Cox K, Geer EB. Prolactinoma through the female life cycle. Endocrine. 2018;59(1):16‐29. [DOI] [PubMed] [Google Scholar]
- 201. Pekić S, Medic Stojanoska M, Popovic V. Hyperprolactinemia/prolactinomas in the postmenopausal period: challenges in diagnosis and management. Neuroendocrinology. 2019;109(1):28‐33. [DOI] [PubMed] [Google Scholar]
- 202. Greenman Y. Prolactinomas and menopause: any changes in management? Pituitary. 2020;23(1):58‐64. [DOI] [PubMed] [Google Scholar]
- 203. Karunakaran S, Page RC, Wass JA. The effect of the menopause on prolactin levels in patients with hyperprolactinaemia. Clin Endocrinol (Oxf). 2001;54(3):295‐300. [DOI] [PubMed] [Google Scholar]
- 204. Mallea-Gil MS, Manavela M, Alfieri A, et al. Prolactinomas: evolution after menopause. Arch Endocrinol Metab. 2016;60(1):42‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Santharam S, Fountas A, Tampourlou M, et al. Impact of menopause on outcomes in prolactinomas after dopamine agonist treatment withdrawal. Clin Endocrinol (Oxf). 2018;89(3):346‐353. [DOI] [PubMed] [Google Scholar]
- 206. Indirli R, Ferrante E, Sala E, Giavoli C, Mantovani G, Arosio M. Cabergoline withdrawal before and after menopause: outcomes in microprolactinomas. Horm Cancer. 2019;10(2-3):120‐127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Greenspan SL, Oppenheim DS, Klibanski A. Importance of gonadal steroids to bone mass in men with hyperprolactinemic hypogonadism. Ann Intern Med. 1989;110(7):526‐531. [DOI] [PubMed] [Google Scholar]
- 208. Biller BM, Coughlin JF, Saxe V, Schoenfeld D, Spratt DI, Klibanski A. Osteopenia in women with hypothalamic amenorrhea: a prospective study. Obstet Gynecol. 1991;78(6):996‐1001. [PubMed] [Google Scholar]
- 209. Kohama SG, Bethea CL. Steroid regulation of tyrosine hydroxylase messenger ribonucleic acid in dopaminergic subpopulations of monkey hypothalamus. Endocrinology. 1995;136(4):1790‐1800. [DOI] [PubMed] [Google Scholar]
- 210. de Quijada M, Timmermans HA, Lamberts SW. Tamoxifen suppresses both the growth of prolactin-secreting pituitary tumours and normal prolactin synthesis in the rat. J Endocrinol. 1980;86(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 211. Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80(4):1523‐1631. [DOI] [PubMed] [Google Scholar]
- 212. Nagy I, Valdenegro CA, MacLeod RM. Effect of antiestrogens on pituitary prolactin production in normal and pituitary tumor-bearing rats. Neuroendocrinology. 1980;30(6):389‐395. [DOI] [PubMed] [Google Scholar]
- 213. Lamberts SW, Verleun T, Oosterom R. Effect of tamoxifen administration on prolactin release by invasive prolactin-secreting pituitary adenomas. Neuroendocrinology. 1982;34(5):339‐342. [DOI] [PubMed] [Google Scholar]
- 214. Völker W, Gehring WG, Berning R, Schmidt RC, Schneider J, von zur Mühlen A. Impaired pituitary response to bromocriptine suppression: reversal after bromocriptine plus tamoxifen. Acta Endocrinol (Copenh). 1982;101(4):491‐500. [DOI] [PubMed] [Google Scholar]
- 215. Choudhary C, Hamrahian AH, Bena JF, Recinos P, Kennedy L, Dobri G. The effect of raloxifene on serum prolactin level in patients with prolactinoma. Endocr Pract. 2019;25(7):684‐688. [DOI] [PubMed] [Google Scholar]
- 216. Camilletti MA, Abeledo-Machado A, Faraoni EY, Thomas P, Díaz-Torga G. New insights into progesterone actions on prolactin secretion and prolactinoma development. Steroids. 2019;152:108496. [DOI] [PubMed] [Google Scholar]
- 217. Piroli GG, Cassataro J, Pietranera L, et al. Progestin regulation of galanin and prolactin gene expression in oestrogen-induced pituitary tumours. J Neuroendocrinol. 2001;13(3):302‐309. [DOI] [PubMed] [Google Scholar]
- 218. Jaquet P, Ouafik L, Saveanu A, et al. Quantitative and functional expression of somatostatin receptor subtypes in human prolactinomas. J Clin Endocrinol Metab. 1999;84(9):3268‐3276. [DOI] [PubMed] [Google Scholar]
- 219. Fusco A, Gunz G, Jaquet P, et al. Somatostatinergic ligands in dopamine-sensitive and -resistant prolactinomas. Eur J Endocrinol. 2008;158(5):595‐603. [DOI] [PubMed] [Google Scholar]
- 220. Hofland LJ, van der Hoek J, van Koetsveld PM, et al. The novel somatostatin analog SOM230 is a potent inhibitor of hormone release by growth hormone- and prolactin-secreting pituitary adenomas in vitro. J Clin Endocrinol Metab. 2004;89(4):1577‐1585. [DOI] [PubMed] [Google Scholar]
- 221. Sosa-Eroza E, Espinosa E, Ramírez-Rentería C, Mendoza V, Arreola R, Mercado M. Treatment of multiresistant prolactinomas with a combination of cabergoline and octreotide LAR. Endocrine. 2018;61(2):343‐348. [DOI] [PubMed] [Google Scholar]
- 222. Fusco A, Lugli F, Sacco E, et al. Efficacy of the combined cabergoline and octreotide treatment in a case of a dopamine-agonist resistant macroprolactinoma. Pituitary. 2011;14(4):351‐357. [DOI] [PubMed] [Google Scholar]
- 223. Lasolle H, Vasiljevic A, Borson-Chazot F, Raverot G. Pasireotide: a potential therapeutic alternative for resistant prolactinoma. Ann Endocrinol (Paris). 2019;80(2):84‐88. [DOI] [PubMed] [Google Scholar]
- 224. Coopmans EC, van Meyel SWF, Pieterman KJ, et al. Excellent response to pasireotide therapy in an aggressive and dopamine-resistant prolactinoma. Eur J Endocrinol. 2019;181(2):K21‐K27. [DOI] [PubMed] [Google Scholar]
- 225. Xiao J, Zhu Z, Zhong D, Ma W, Wang R. Improvement in diagnosis of metastatic pituitary carcinoma by 68Ga DOTATATE PET/CT. Clin Nucl Med. 2015;40(2):e129‐e131. [DOI] [PubMed] [Google Scholar]
- 226. Priola SM, Esposito F, Cannavò S, et al. Aggressive pituitary adenomas: the dark side of the moon. World Neurosurg. 2017;97:140‐155. [DOI] [PubMed] [Google Scholar]
- 227. Giuffrida G, Ferraù F, Laudicella R, et al. Peptide receptor radionuclide therapy for aggressive pituitary tumors: a monocentric experience. Endocr Connect. 2019;8(5):528‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Bengtsson D, Schrøder HD, Andersen M, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689‐1698. [DOI] [PubMed] [Google Scholar]
- 229. Kaltsas GA, Mukherjee JJ, Plowman PN, Monson JP, Grossman AB, Besser GM. The role of cytotoxic chemotherapy in the management of aggressive and malignant pituitary tumors. J Clin Endocrinol Metab. 1998;83(12):4233‐4238. [DOI] [PubMed] [Google Scholar]
- 230. Petterson T, MacFarlane IA, MacKenzie JM, Shaw MD. Prolactin secreting pituitary carcinoma. J Neurol Neurosurg Psychiatry. 1992;55(12):1205‐1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Lasolle H, Cortet C, Castinetti F, et al. Temozolomide treatment can improve overall survival in aggressive pituitary tumors and pituitary carcinomas. Eur J Endocrinol. 2017;176(6):769‐777. [DOI] [PubMed] [Google Scholar]
- 232. Pernicone PJ, Scheithauer BW, Sebo TJ, et al. Pituitary carcinoma: a clinicopathologic study of 15 cases. Cancer. 1997;79(4):804‐812. [DOI] [PubMed] [Google Scholar]
- 233. Zhang D, Way JS, Zhang X, et al. Effect of everolimus in treatment of aggressive prolactin-secreting pituitary adenomas. J Clin Endocrinol Metab. 2019;104(6):1929‐1936. [DOI] [PubMed] [Google Scholar]
- 234. Wang Y, Li J, Tohti M, et al. The expression profile of Dopamine D2 receptor, MGMT and VEGF in different histological subtypes of pituitary adenomas: a study of 197 cases and indications for the medical therapy. J Exp Clin Cancer Res. 2014;33(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Osterhage K, Rotermund R, Droste M, et al. Bevacizumab in aggressive pituitary adenomas—experience with 3 patients. Exp Clin Endocrinol Diabetes. 2021;129(3):178‐185. [DOI] [PubMed] [Google Scholar]
- 236. Touma W, Hoostal S, Peterson RA, Wiernik A, SantaCruz KS, Lou E. Successful treatment of pituitary carcinoma with concurrent radiation, temozolomide, and bevacizumab after resection. J Clin Neurosci. 2017;41:75‐77. [DOI] [PubMed] [Google Scholar]
- 237. Vlotides G, Siegel E, Donangelo I, Gutman S, Ren SG, Melmed S. Rat prolactinoma cell growth regulation by epidermal growth factor receptor ligands. Cancer Res. 2008;68(15):6377‐6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Fukuoka H, Cooper O, Mizutani J, et al. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol Endocrinol. 2011;25(1):92‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Cooper O, Mamelak A, Bannykh S, et al. Prolactinoma ErbB receptor expression and targeted therapy for aggressive tumors. Endocrine. 2014;46(2):318‐327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Cooper O, Bonert VS, Rudnick J, et al. EGFR/ErbB2-targeting lapatinib therapy for aggressive prolactinomas. J Clin Endocrinol Metab. 2021;106(2):e917‐e925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Wang PF, Wang TJ, Yang YK, et al. The expression profile of PD-L1 and CD8+ lymphocyte in pituitary adenomas indicating for immunotherapy. J Neurooncol. 2018;139(1):89‐95. [DOI] [PubMed] [Google Scholar]
- 242. Lin AL, Jonsson P, Tabar V, et al. Marked response of a hypermutated ACTH-secreting pituitary carcinoma to Ipilimumab and Nivolumab. J Clin Endocrinol Metab. 2018;103(10):3925‐3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Ilie MD, Vasiljevic A, Jouanneau E, Raverot G. Immunotherapy in aggressive pituitary tumors and carcinomas: a systematic review. Endocr Relat Cancer. 2022;29(7):415‐426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.