Abstract
Engineered nanostructures are materials with promising properties, enabled by precise design and fabrication, as well as size-dependent effects. Biomedical applications of nanomaterials in disease-specific prevention, diagnosis, treatment, and recovery monitoring require precise, specific, and sophisticated approaches to yield effective and long-lasting favorable outcomes for patients. In this regard, carbon nanofibers (CNFs) have been indentified due to their interesting properties, such as good mechanical strength, high electrical conductivity, and desirable morphological features. Broadly speaking, CNFs can be categorized as vapor-grown carbon nanofibers (VGCNFs) and carbonized CNFs (e.g., electrospun CNFs), which have distinct microstructure, morphologies, and physicochemical properties. In addition to their physicochemical properties, VGCNFs and electrospun CNFs have distinct performances in biomedicine and have their own pros and cons. Indeed, several review papers in the literature have summarized and discussed the different types of CNFs and their performances in the industrial, energy, and composites areas. Crucially however, there is room for a comprehensive review paper dealing with CNFs from a biomedical point of view. The present work therefore, explored various types of CNFs, their fabrication and surface modification methods, and their applications in the different branches of biomedical engineering.
Keywords: Carbon nanofibers, Electrospinning, Chemical vapor deposition, Biomedical applications
1. Introduction
Engineered nanostructures are fascinating materials with promising properties, enabled by precise design and fabrication, as well as size-dependent effects. Material properties at nanoscale can be modulated with precision to control the bulk properties effectively. Due to these characteristics, nanomaterials are widely used as alternatives to conventional materials to confer new properties and to improve or change specific properties [1,2]. Biomedical applications of nanomaterials in disease-specific prevention, diagnosis, treatment, and recovery monitoring require precise, specific, and sophisticated approaches to yield effective and long-lasting favorable outcomes for patients [3,4]. Engineered nanomaterials can satisfy these demands due to their precise design and fabrication as well as their effective integration with biological systems [5,6].
Various nanostructures and nanomaterials have been used in different branches of material science and biomedical engineering over the last decades. Based on the shape and dimensions, nanomaterials are divided into zero-dimensional (nanoparticles), one-dimensional (nanowires, nanofibers, and nanotubes), and two-dimensional (nanolayers, coating, and thin films) [7,8]. One-dimensional nanomaterials have attracted significant attention recently due to their high aspect ratio, good cohesion with extracellular matrix (ECM), and resemblance to ECM [9]. Among one-dimensional candidates for biomedical applications, nanofibers have shown promising performances since they can be fabricated from a wide variety of substances and surface modification chemistries can be readily applied [9–11].
Carbon nanofibers (CNFs) have interesting properties, such as good mechanical strength and high electrical conductivity, along with desirable morphological features [12,13]. Due to their high aspect ratio, it is possible to synthesize homogenous nanocomposites with a low amount (below 0.1 vol%) of CNFs [12]. The large surface area can be applied to adsorb various sensing and therapeutic agents in the diagnosis and therapy concepts, respectively. The electrical conductivity and mechanical properties of CNFs can accommodate the fabrication of sophisticated biosensors and electroconductive scaffolds with structural integrity for tissue engineering applications [14,15]. Although CNFs have lower crystallinity, electrical conductivity, mechanical strength, and smaller surface areas than carbon nanotubes (CNTs), they have been shown to offer lower toxicity, fabrication costs, and simple dispersion.
Moreover, the defects in the CNFs structures can serve as active sites for reactions and bioconjugations [16,17]. Depending on the fabrication method and the stacking manner of graphene layers, CNFs can have various forms, morphologies, and microstructures, as shown in Fig. 1. Broadly speaking, CNFs can be categorized as vapor-grown carbon nanofibers (VGCNFs) and electrospun CNFs, which have distinct microstructure, morphologies, and physicochemical properties [12]. VGCNFs are synthesized based on the catalysis methods and have more similarities to CNTs in terms of microstructure, crystallinity, mechanical and electrical properties. On the other hand, spun CNFs have an amorphous microstructure and lower mechanical properties, and electrical conductivity [18,19].
Fig. 1.

Schematic structure of (a) graphene platelets, (b) graphene fishbones, (c) graphene ribbons and (d) cup-stacked CNFs and (e) amorphous CNF without graphene layers. Reproduced with permission from Ref. [23].
Apart from their physicochemical properties, VGCNFs and spun CNFs have distinct performances in biomedicine and have their own pros and cons. Some review papers in the literature summarize and discuss different types of CNFs and their performance in the industrial, energy, and composites area [20–22], but there is room for a comprehensive review paper dialing with the CNFs from a biomedical point of view. Therefore, various types of CNFs, their fabrication and surface modification methods, and their applications in the different branches of biomedical engineering will be discussed in this review.
2. Carbon nanofibers
2.1. Synthesis
CNFs fabrication techniques can be classified into chemical vapor deposition (CVD)-based and carbonization of precursor nanofibers. The precursors nanofibers can be fabricated by template-based and spinning-based methods (force spinning, melt spinning, and electrospinning) following the carbonization process through the heat treatment. Moreover, naturally occurring fibers from cellulose, chitin, lignin, and chitosan can be converted to CNFs through the direct carbonization process [24,25]. Fabrication of CNFs using CVD dates back to 1889 and the synthesis of CNFs from hydrocarbon gas on metal crucibles. These nanomaterials are also called vapor-grown carbon fibers (VGCFs). Since then, various studies have been conducted to develop and optimize the synthesis method, and different hydrocarbons, such as carbon monoxide, benzene, acetylene, methane, and ethane, as the precursors and iron, cobalt, and nickel, as the catalysts have been evaluated [26]. In this method, the decomposition of hydrocarbon molecules on the metal catalyst takes place at a temperature range of 700–1200 K and CNFs form through the diffusion of the carbon atoms (Fig. 2). It has been shown that the catalyst type determines the structure of the fabricated CNFs, for instance using Fe results in thick CNFs with a multi-walled CNT core, while Ni produces cup-stacked-type CNFs [23]. CNFs fabricated based on CVD methods possess excellent electrical conductivity due to the high graphitization temperature for various applications. Despite their fascinating properties, VGCFs require high production costs and hydrophobic surfaces, which diminishes their widespread applications [12].
Fig. 2.

CNFs CVD instrumentation and growth mechanism.
The template-assisted fabrication technique uses porous membranes, hydrogels, or aerogels characterised with well-defined porosity and channels as the template for the desired precursor materials. Using the porous membrane, the precursor materials pass through the channels via physical forces (e.g., piston pressure or ultrasonic waves) and fibers with diameters that match the pore sizes are obtained. The resulting fibers are converted to CNFs through the carbonization process using heat treatment. The template-based techniques are relatively simple and CNFs diameter depends on the membrane’s pore size. For instance, Deeney et al. [27] used an Anodisc (anodized alumina) membrane with a 200 nm pore size as the template and beverage-related precursors to fabricate CNFs (Fig. 3). They reported that the obtained CNFs have diameter and length in accordance with membrane length and pore size. Despite its simplicity, it is not possible to fabricate fibers with long fiber lengths and micron-scale lengths are yielded.
Fig. 3.

Schematic representation of template-assisted CNFs fabrication using membrane with defined pore size and length. Reproduced with permission from Ref. [27].
Hydrogel/aerogels-based CNFs, also known as carbon gel, consist of three steps: cross-linking polymer chains, drying or solvent evaporation, and heat treatment or carbonization. The cross-linking can be conducted using physical, chemical, or enzymatic methods. The drying step is critical since it must preserve the porous architecture and pore size/morphology of the structure. Freeze drying and supercritical drying are commonly used drying technologies. Liang et al. [28] fabricated macroscopic monolithic carbon gel containing CNF based on ultrathin Te nanowires (TeNWs) dispersion in glucose solution. The resulting dispersion was converted to a robust monolithic gel-like Te/C structure using a heat treatment at 180 °C for 12–48 h after the etching and washing to remove TeNWs (Fig. 4a). The resulted structure is carbon gen with uniform CNFs (Fig. 4b).
Fig. 4.

Carbon gel fabrication overview. (a) Schematic process representing the sole solution and subsequent gel formation. (b) Macroscopic image of the resulting 12 L monolithic wet gel. (c) SEM micrograph with two different magnifications from the internal architecture of the carbon gel. Reproduced with permission from Ref. [28].
The spinning methods are based on the extrusion of polymeric precursors from a spinneret under the influence of a force (Centrifugal force for the force spinning (Fig. 5a), the electric field for electrospinning (Fig. 5b), and hot air blowing for melt spinning (Fig. 5c) [29]. The polymeric precursors can be dissolved in proper solvent/cosolvent or heated to melt in order to provide proper fluidity to pass through the spinneret. In the force spinning, the molten or solution of polymer pass through orifices under centrifugal force, and micro/nanofibers will form depending on the process parameters, such as rotational speed of the spinneret, orifices diameter and shape, collection system, and temperature (Table 1) [30]. In melt blowing spinning, the molten polymer extrudes from an orifice and is blown into continuous fibers by hot and high-velocity air and collected on a rotary drum. The morphology and diameter of the fibers depend on the airflow, air temperature, extruder morphology and temperature, and polymer feeding rate [31,32].
Fig. 5.

Various spinning-based CNFs fabricating methods. (a) Force spinning, (b) Electrospinning, and (c) Melt blown spinning.
Table 1.
Polymers used in electrospun carbon nanofibers fabrication as the host precursors or guest precursors.
| Precursors | Chemical structure | Electrospinning condition | Functionalities | Pros | Cons |
|---|---|---|---|---|---|
| PAN |

|
4–10 wt% in DMF, 10–30 kV | Carbon source for CNFs and their hybrids | High carbon yield (>50 %) and spinnability | Typically only DMF is used as solvent |
| Pitch | – | 30–40 wt% THF/DMF, 18–25 kV | Carbon source for CNFs | High carbon yield (60 % at 1000 °C) | Low spinnability |
| Lignin |

|
20–35 wt% in DMF/water, 6–26 kV | Carbon source for CNFs | Large surface area | Large diameter; low carbon yield of 20–40 % |
| Polyimide resin |

|
10–20 wt% in N-methyl, Pyrrolidone THF/metha ol/DMAc, 8–25 kV | Carbon source for CNFs, separator for LIB | High conductivity of asprepared CNFs | Complex fabrication process |

| |||||
| PVDF |

|
7–25 wt% in DMAc/DMF/acetone, 15–20 kV | Separator and gel electrolyte for LIB | High mechanical properties | Require Dehydrofluorina ion for carbonization |
| PVP |

|
4–10 wt% in ethanol/DMF, 10–25 kV | Carbon source for metal oxide/CNF composites and template for neat metal oxide fibers | Soluble in various solvent | Low carbon yield of 15 % |
| PVA |

|
8–10 wt% in water, 8–35 kV | Carbon source for metal oxide/CNF composites | Water soluble Good processability | Low carbon yield of 3–10 % |
| PMMA |

|
8–10 wt% in DMF, 10–20 kV | Scaffold for neat metal oxide fibers and sacrificial phase for voids | Compatible with other host polymer, such as PAN | Difficult to be fully removed, requiring 100 °C |
| PS |

|
5–35 wt% in DMF/THF/chloroform, 15–25 kV | Scaffold for neat metal oxide fibers an sacrificial phase for voids | Easy to be decomposed at ~450 °C | Poor compatibility with host polymer, beads are easy to form |
Abbreviations: PAN: Polyacrylonitrile, DMF: Dimethylformamide, CNFs: Carbon nanofibers, THF: Tetrahydrofuran, LIBs: lithium-ion batteries, PVDF: Polyvinylidene difluoride, DMAc: Dimethylacetamide, PVP: Polyvinylpyrrolidone, PVA: Poly(vinyl alcohol), PMMA: Poly(methyl methacrylate), PS: Polystyrene.
Unlike mechanically-driven spinning, a high voltage electric field is the electrospinning method’s main driving force of micro/nanofiber formation. The polymer solution or molten is charged using the applied electric field and is fed through the nozzle, pass through the spinning zone, and collected on the collector Fig. 5b). This technique is more sophisticated and effective in fabricating nanofibers, especially CNFs, with tuned diameter, morphology, and architecture. Moreover, it is possible to fabricate more complicated CNFs, such as hollow CNFs and aligned CNFs, using electrospinning. The fabricated nanofibers’ morphology, diameter, and architecture depend on various polymers, processes, and environmental parameters.
In the carbonization methods, the type of polymeric precursor has determinant effects, along with the process parameters. Various types of polymers can be utilized to fabricate CNFs (Table 1). Some of these polymers (known as host precursors) are used as the carbon source and converted to CNFs using the proper heat treatment, such as polyacrylonitrile (PAN) and polyimide (PI); on the other hand, some other (known as guest precursors) are removed through the heat treatment(s) and leave specific features in the finished CNFs, such as polystyrene (PS) and polymethyl methacrylate (PMMA). PAN has been extensively used due to its relatively high carbon yield (>50 %), good processability, spinnability, and fabulous mechanical properties.
Fabricated electrospun PAN nanofibers are converted to CNFs using a two-step heat treatment, stabilization, and carbonization process. During the stabilization process, the cyclization and dehydrogenation process occurs at 200–280 °C in air, which results in the formation of a ‘ladder’ structure consisting of a series of connected C—N aromatic rings, as shown in Fig. 6. The stabilization is critical to preserve the nanofiber structure during the high-temperature carbonization [33]. The carbonization is usually carried out at a high temperature (>800 °C) in an inert atmosphere (nitrogen or argon), where the aromatic structure is grown and the heteroatoms like nitrogen are eliminated (Fig. 6) [34–36]. Other polymeric precursors can be converted to CNFs with a similar two-step heat treatment, although the exact processing conditions may differ.
Fig. 6.
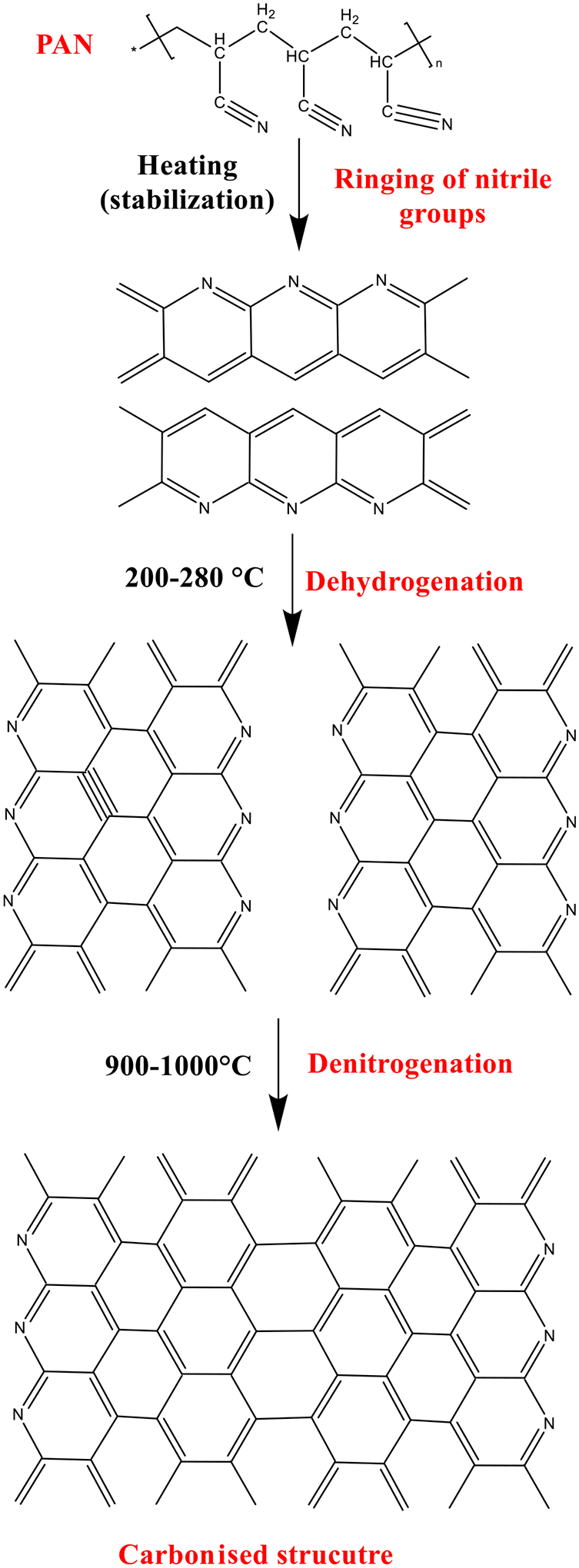
Structural changes of PAN during the heat treatment process.
Although the CVD-based synthesis methods provide CNFs with high graphitization and electrical conductivity, their production cost is remarkably higher than the spinning-based method. Moreover, using electrospinning, it is possible to synthesize CNFs with different morphology (solid, porous, and core/shell structured fibers) and architecture (random, aligned, and hybrid fibers) in a wide range of nanofibers diameter (10 nm to 10 μm). On the other hand, the electrospinning technique provides a mat/sheet of CNFs and must be crashed/powdered to be applied in nanocomposite applications and it is hard to obtain well dispersed, fine, and discrete CNF. The CVD-based methods have advantages in nanocomposite applications. For biomedical applications, the electrospun CNFs have priority over the CVD-based CNFs since they have lower toxicity issues. The possible toxicity of CVD-based CNFs can be attributed to the usage of metallic catalysts, which are not required in spinning-based methods.
2.2. Surface modifications
Improving the surface properties of CNFs, such as colloidal stabilization, constitutes an important research interest due to the propensity of CNFs to exist in aggregations, thus limiting their tissue engineering applications i.e., in polymer reinforcement [37]. Furthermore, the absence of sufficiently polar functional groups on the surface of CNFs may lead to weak interface bonding strength with polymers when employed in composite fabrication [38]. Klein et al. [39] also emphasized that improving the surface properties of CNF is crucial since these surface properties tend to dominate at the nanoscale because of the high surface-to-volume ratio of the CNF. Additionally, surface modification is necessary to enhance the interfacial adhesion and dispersion state of CNFs in polymer matrixes [40]. In composite fabrication, the surface modification of CNFs also can improve the mechanical properties of polymer-based composites. This is because the effective stress transfer from the polymer matrices to CNFs is optimized due to the presence of new covalent bonds between polymer matrices and the modified CNF surface [41]. CNF surface modification may also be employed to enhance its wettability [42]. In all cases of surface modification of CNFs, the objective is to modify surface chemistry as a pathway to enhance either the cell attachment sites, surface specificity, selectivity, or surface stability, depending on the CNF application of interest (i.e., biological, composite applications, etc.) [39]. It must be emphasized that the modification of the CNF mainly serves to change the surface of the CNF without altering the fiber morphology [43]. Recognizing the importance of surface modification of CNFs for improving their properties, several approaches have been explored in the literature and are discussed in the subsequent sections (Fig. 7).
Fig. 7.

Routine surface modification methods of carbon nanofibers.
2.2.1. Thermal or heat treatment
The CNF surface modification via thermal treatment for the addition of oxygenated functional groups may also be undertaken. For instance, in the study by Smolka et al. [44], the surface properties of CNFs were improved via oxidation in the air (i.e., dry oxidation) at the temperature of 800 °C while imposing a vacuum pressure. This study showed that the oxidation of the CNF surface led to improvements in CNF hydrophilicity and decreased the carbon content at the surface and at near-surface sites for enhanced biocompatibility and improved electrical conduction. Similarly, surface activation of CNF via surface modification was also demonstrated by Liu et al. [45]. The study showed that oxidation in atmospheres containing 5 % v/v O2 and Argon facilitated the introduction of C—O and O—H functional groups on the surface of the CNF. Such dry oxidation for the surface modification of CNF may also be achieved via ozonation [46]. This ozonation also facilitates the removal of residual amorphous carbons on the surface of the CNF while simultaneously leading to the generation and attachment of oxygenated functional groups, such as carboxylic, alcoholic, etc., via the 1,3 dipolar cycloadditions of ozone across double bonds [46]. Thermal treatments of CNFs may also be employed in introducing amine functionality to the surface of CNFs via a hydrothermal process [47].
For instance, Tshwenya et al. [47] showed that at a temperature of 180 °C for 6 h CNF could be modified by using 3 poly (propylene imine) as the amine group source. The now modified CNF, containing the amine functional groups, was shown to demonstrate anionic diode behavior rather than the cationic diode behavior that characterizes pristine CNFs when immersed in an aqueous electrolyte solution [47]. This observation indicated that the charge properties of CNF may also be modified via surface modification techniques.
In some cases, surface modification via oxidation-based thermal treatments may be integrated with other surface modification strategies to introduce unique properties to the CNF surface, such as enhanced heavy metal ion adsorption from aqueous solutions [48]. For instance, in the study by Thamer et al. [48], the covalent bonding of melamine, and poly (m-phenylene diamine) with oxidized CNFs (O-CNFs) was employed to produce melamine-functionalized (m-O-CNF) and poly (m-phenylene diamine)-functionalized (p-O-CNF) CNFs, respectively. The study demonstrated that the treatment of the O-CNF with melamine and poly (m-phenylene diamine) led to a ~twofold and ~4 fold increase in the adsorption capacity of the o-CNF. However, the functionalization of the CNFs surface via the addition of oxygen groups was also reported to impact their electronic conductivity, with higher levels of oxidation correlating with lower electronic conductivity [49]. These observations suggest that such oxidation-based surface modifications must be carefully modulated to avoid compromising CNF structural integrity and conductive properties.
2.2.2. Acid treatment
Surface modification of CNFs may also be achieved using oxidizing acids (i.e., wet oxidation) Klein, Melechko [39]. The surface modification using acids is also referred to as “Wet etch treatment” Klein, Melechko [39]. Such acid treatments typically utilize acids such as nitric acid and sulfuric acid to introduce oxygenated functional groups to the surface of CNFs. For instance, in the study by Din et al. [50], surface modification of CNFs was achieved via treatment using nitric acid solution [50]. The study showed that after the surface modification of CNFs via nitric acid treatment, C-OH and C=O functionalities were detected on the surface. Notably, oxidation approaches may facilitate the addition of several oxygenated functional groups to the CNF surface, as shown in Fig. 7.
Fig. 7 shows that oxidation may lead to the addition of C=O, OH, COOH, and COO− functional groups to the surface of the CNF. Din et al. [50] also reported that the oxidation using nitric acid facilitated a ~36 % increase in the BET surface area of the CNFs while also facilitating the addition of functional groups to the CNF structure.
Another study also employed a similar strategy of utilizing nitric acid in the modification of the surface of the CNF as an approach to improve the resultant anti-wear properties when used in the preparation of CNF/polytetrafluoroethylene (PTFE) composites. Nitric acid treatment was also shown to enhance the anti-wear property of the CNF (30 wt%)/PTFE composite when subjected to a 200 N load [51], with Lim et al. [52] showing that such nitric acid treatments may also be used in the recovery of free edges that may have degraded when platelet-type, graphitized CNFs are subjected to the annealing process. Crucially, however, such acid treatments are capable of destroying the structural integrity of CNF [53]. This is because acid treatments may lead to excessive oxidations, leading to a severely oxidized surface laden with defects in the inner layers of CNFs, and destruction of its fibrous structure [49].
Notably, the acid treatment and thermal treatment approaches discussed above are based on the direct attachment of functional groups to the surface or the chemical modification of the surface using the oxygen-containing groups via sidewall defects [41]. Generally speaking, introducing oxygen-containing hydrophilic groups to CNF surfaces also facilitates their dispersion in polar solvents and enhances secondary reactions with other functional groups, thus leading to further alterations of CNF surface chemistry and properties [37,54,55]. Furthermore, it is important to acknowledge that although the well-known acid treatments can efficiently facilitate improved hydrophilicity of CNFs via the introduction of oxygen-containing groups, such as hydroxyl and carboxyl groups, on the CNF surface, it may not support dispersal in electrolyte or protein solutions. This is due to rapid sedimentation after a short period [56].
2.2.3. Plasma polymerization
Other CNF surface modification techniques such as plasma polymerization [57] may also be employed. This technique involves the formation of high-molecular-weight deposits (polymers) via activating monomers using an energetic plasma species [58]. The plasma source (i.e., UV) induces radical formation, which may recombine to form polymers [58]. The plasma polymerization technique was demonstrated in the study Pastine, Okawa [57], where surface modification via UV-triggered attachment of perfluoroarylazides was undertaken. It was shown that surface modification of CNF with hydrophilic, hydrophobic, or polymerizable small molecules led to surface functionalization such that superhydrophobic surfaces containing superhydrophilic regions were present. In another study by Guadalupe et al., [59], it was shown that CNF surface modification for enhanced hydrophobicity and dispersal properties was achievable via plasma polymerization using methyl methacrylate as the monomer. The effect of utilizing this modified CNF as a reinforcement for polymethyl methacrylate (PMMA) was also explored. This study showed that the adding up to 8 wt% of the modified CNF facilitated an 8 % and a 178 % increase in the thermal stability and Younǵs modulus, respectively, compared to PMMA with no CNFs. A similar study showed that plasma surface modification of the CNF coupled with ultrasonication enhanced the dispersion property of CNFs in the CNF/polycarbonate composite [60]. In another study by Gao et al. [61], the surface of CNFs was modified via plasma polymerization for improved dispersion properties, as well as improved tensile strength and Young’s modulus of composites of the polycarbonate matrix, compared to when pure CNFs, were dispersed in a polycarbonate matrix.
2.2.4. Surface modification via silane
In a study, surface modification of CNF was achieved via functionalization with 3-glycidoxypropyltrimethoxysilane prior to its dispersion into epoxy resin. The study showed that the surface modification improved dispersion and interfacial interaction properties [37]. Surface modification may also be achieved via surface graft polymerization with acrylic acid using a KMnO4/H2SO4 redox-induced system [62]. This approach facilitates a ~twofold increase in surface carboxylic acid groups for enhanced adhesion via stronger interfacial bonds when mixed with an epoxy matrix [62].
2.2.5. Chemical vapor deposition of thin film coatings
CVD is a technique that facilitates the deposition of solid material from a vapor via chemical reactions occurring on or in the vicinity of a normally heated surface, and is generally classified based on the operating conditions and the physical characteristics of the vapor [63]. The CVD surface modification approach synthesizes thin films on the CNF surface by polymerizing vapor phase monomeric reactants and their subsequent deposition [64]. The properties of the films are typically tunable, thus permitting the development of CNF with unique characteristics [64]. According to Klein et al. study [39], CVD may be employed for film deposition on the surface of CNFs to impact different functionalities. The deposited films may be either dielectric, metals, metallic oxides, or polymers. For instance, in the study by Shi et al. [65], pyrrole was deposited as thin films on CNTs for surface modification. In another study [66], a silicon oxide film was deposited via CVD for surface modification by a novel self-aligned thin-film deposition technique such that the surface coating composition was stoichiometric SiO2.
2.2.6. Non-covalent functionalization for CNF surface modification
The non-covalent functionalization approach involves CNFs surface modification using supramolecular complexations and is conducted through the Van der Waals forces, hydrogen bonds, electrostatic forces, and π-stacking interactions [67]. These non-covalent functionalizations may be referred to as ‘non-invasive’ as they do not destroy the structure of the CNFs and involve ‘wrapping’ CNF with another material [67]. Polymer wrapping is an example of such non-covalent functionalization (Fig. 7) [46]. The polymer wrapping facilitates the maintenance of the Sp2 conjugated structure [46], with the dispersion of the CNFs in a given polymer now dependent on the miscibility of the polymer chain employed in wrapping the CNFs and the polymer matrix [46]. Generally speaking, the effectiveness of the non-covalent functionalization surface modification is dependent on the strength of the bonds between the CNFs and the polymer wrapping that arises due to van der Waals forces and π-π stacking between CNFs and polymer chains containing aromatic rings, respectively [46]. For instance, non-covalent functionalization which involves supramolecular complexation and employs Van der Waals forces, hydrogen bonds, electrostatic force, and π-stacking interactions.
2.2.7. Electrochemical modification of CNF surface
The electrochemical surface modification approach involves the deposition of materials to decorate the surface of the CNFs, as shown in Fig. 8a [68]. In the study by Meng et al. [68], electrochemical modification of the CNFs surface was achieved electrochemically via a dynamic liquid membrane. This study demonstrated the possibility of introducing the functional groups of –OH and –COOH to the surface of the CNFs, thus effectively enhancing the CNFs’ electrical conductivity and hydrophilicity. In another study, similar electrochemical treatments were employed to facilitate the alteration of the surface oxidation state via electron transfer. Such electron transfers enabled the functionalization of the surface for enhanced roughness, although unfavorable effects on the fiber strength were also reported [69].
Fig. 8.

(a) Fundamentals of surface functionalization of CNFs via electrochemical approach (b) Illustration of mass transport during electrochemical surface modification of CNF electrode. (c) Highlighting the interactions at surface of the modified CNF. ([68] Copyright © (2020) American Chemical Society.)
Surface modification by introducing oxygenated functional groups (i.e. NiO) may also be possible via nickel surface coating, which increases surface polarity and surface free energy for improved fracture toughness and interfacial bonding when employed in composite systems based on the phenolic matrix [70].
This section of this study has explored some major functionalization strategies for the surface modification of CNF and the associated improvement in their usefulness in tissue engineering applications. Several surface modification approaches, such as ozone, and plasma treatment were discussed in this section. This section showed the viability of employing these CNF functionalization techniques for the introduction of different functional groups such as hydroxyl, carboxylic, etc. on the CNF surface could alter essential properties (i.e., the BET area, elasticity, abrasion properties, etc.) of the materials when the modified CNF was introduced.
3. Biomedical applications
In recent years, electrospun nanofibers have emerged as attractive materials for numerous biomedical areas, including controlled drug delivery systems, biosensing, wound healing (as dressing), and tissue engineering (TE) [71,72]. Fabricating the nanofibers via the electrospinning technique provides considerable advantages compared to other techniques (e.g., drawing, freeze-drying, phase separation, template synthesis, and self-assembly) [73,74]. High surface-to-volume ratio, narrower diameter, high porosity, enhanced mechanical strength, tuneability, simplicity, and economical properties highlight using the electrospinning method [75–78]. Furthermore, this method has the ability to control and optimize parameters such as tip collector distance, fiber direction, and voltage [75,76]. In TE, the electrospun nanofibers may be used as scaffolds for mimicking the native nanoscale entities of the three-dimensional (3D) tissue extracellular matrix (ECM) to give rise to adhesion, proliferation, and differentiation of the cells and eventually regeneration of the damaged tissue [79]. Various natural (i.e., derived from natural sources) or synthetic scaffolds (i.e., synthesized from synthetic materials) have been electrospun by modulating the parameters in the electrospinning process [80]. Carbon-based nanomaterials (CNMs), including CNFs, CNTs, and graphene-based nanocarbons (the latter two are allotropes of carbon) [81,82], have attracted much interest for biomedical applications, especially in TE, due to their unique electrical conductivity, chemical, thermal, mechanical, and structural properties [83,84]. The electrospinning technique has also contributed to fabricating CNMs, including CNF, which can be referred to as electrospun CNF (ECNF) [74]. In this regard, the current review gives an insight into the biomedical applications of ECNFs in biosensors, and TE for skin, bone, neural, and musculoskeletal tissues.
3.1. Biosensors
Biosensors are analytical tools with applications in several fields, such as biomedical, to detect the presence and concentration of an analyte (e.g., glucose, cancer biomarkers, drugs, metabolites, toxins, etc.) [85,86]. These devices convert physiological or biological signals to electrical signals that represent the concentration of a given biochemical agent [87,88]. The construction of biosensors is commonly composed of a biorecognition element (BRE) as a bioreceptor, a transducer (electrode), and a detector for processing to get a signal output [86,89,90]. Bioreceptors can be enzymes, antibodies, nucleic acids, aptamers, microorganisms, cells, tissues, or even synthetic molecules (e.g., molecularly imprinted polymers (MIPs) which are immobilized on transducer as depicted in Fig. 9 [91–93]. Different transducer types include electrochemical, optical, piezoelectric, mechanical, acoustic, calorimetric, or magnetic. The most frequently used type is the electrochemical biosensors (comprising amperometry/voltammetry, potentiometry, and impedometry techniques) owing to cost-effectiveness, simplicity, and sensitivity [86,91]. Cyclic voltammetry (CV), differential pulse voltammetry (DPV), linear sweep voltammetry (LSV), and square wave voltammetry (SWV) are among the electrochemical amperometric biosensors [94].
Fig. 9.

Schematic illustration of bioreceptors and transducer structures in a biosensor. Figure created with BioRender.com.
The sensitivity and detection limit of the biosensor are essentially determined by bioreceptors and transducers [95]. Different samples from humans (e.g., blood, urine, saliva, sweat, etc.), food (e.g., milk, juice, etc.), and environment (e.g., air, water, soil, etc.) can be used in biosensors [95,96].
Biosensors can provide simple, specific, reliable, accurate, cost-effective, and real-time results [96]. CNMs such as ECNFs could be applied in fabricating the biosensors to improve their performance (e.g., sensitivity) [97–99] due to being functionalizable, biocompatible, sensitive, and cost-effective, and possessing electrocatalytic activities, mechanical strength, and high surface area to the volume [85,100–102]. Adabi et al. [103] demonstrated that the diameter of ECNFs ranging from 75 to 80 nm has desired performance and conductivity and can serve as nanoelectrodes.
ECNF-based biosensors have been applied in the electrochemical detection of various biomedical agents such as H2O2, glucose, dopamine (DA), drugs, etc. An H2O2 biosensor was created from the carboxylic acid group-functionalized ECNF (FCNF) combined with hydroxyapatite nanostructures coated on a polished Au electrode and subsequent immobilization of cytochrome c on its surface (Cyt c/FCNFs-HA/Au electrode). This composite has exhibited a good electrocatalytic activity (at an applied potential of −0.05 V), a fast response time (5 s), and a limit of detection (LOD) of 0.3 μM [100]. A wide linear range has been achieved by diverse H2O2 biosensors such as electrospun nanoporous CNF-platinum nanoparticle/glassy carbon electrode (GCE) (with 2 linear regions: 10 μM-9.38 mM and 9.38–74.38 mM) [101], electrospun cobalt nanoparticles/CNFGCE (up to 5 mM) [104], and electrospun nitrogen-doped CNFs (0.01–0.71 mM) [105]. A glucose biosensor as electrospun nickel–cobalt(II) oxide (Ni-CoO) loaded CNF indicated a wide linear range (0.25–600 μM), a low LOD (0.03 μM), and good stability [106].
In a study by Wang et al. [107], an enzyme-based biosensor utilizing FCNF (CNFs were prepared by electrospinning of PAN) was developed for detecting glucose. In their study, glucose oxidase (GOD) was immobilized on a Prussian blue–FCNF modified GCE (PB-FCNF/GCE) and represented a rapid response time (5 s), a low LOD (0.5 μM), a wide linear range (0.02–12 mM), high sensitivity (35.94 μA cm−2 mM−1), and good selectivity towards glucose in a related study by Li et al. [98], an enzyme-based biosensor has been designed by employing ECNFs (laccase-Nafion-ECNFs on the GCE) for the determination of catechol. This composition displayed good repeatability, reproducibility, stability, and selectivity. Another enzyme-based biosensor (electrospun Cu/CNFs/laccase(Lac)/Nafion/GCE) has been developed by Fu et al. [108] for catechol biosensing. Their investigation also exhibited good repeatability, reproducibility, stability, and selectivity with a rapid response (within 5 s), a wide linear range (9.95 × 10−6–9.76 × 10−3 M), and a low LOD (1.18 μM).
An electrospun Pd nanoparticle-loaded CNFs (Pd/CNFs) nanocomposite for detecting DA, uric acid (UA), and ascorbic acid (AA) demonstrated good sensitivity, selectivity, stability, and reproducibility [109]. A fast response, high sensitivity, wide linear range, low LOD, good stability and selectivity were obtained by silver-platinum/electrospun nanoporous CNFs-modified GCE (AgPt/pCNF/GCE), another biosensor for determining DA [102]. It is demonstrated that an ECNF-modified carbon paste electrode (CPE) has led to a low LOD of 0.02–11.47 μM, a wide linear range of 20 nM, as well as a high sensitivity of 10nA μM−1 toward dihydronicotinamide adenine dinucleotide (NADH) [110]. Electrospun CPE/Pd/CNF/methyl (trioctyl)ammonium bis(trifluoromethylsulfo nyl)imide cobalt/Nafion [111] and ECNFs/screen printed electrode (SPE) [112] are ECNF-based biosensors established for the determination of the pemetrexed (PTX), an anti-cancer drug and tramadol, an opioid pain medication, respectively. These biosensors generated good stability with high sensitivity and selectivity. For detecting xanthine (Xa), a purine base, an ECNF-modified CPE was fabricated by Tang et al. [113]. This biosensor’s linear range and LOD were 0.03–21.19 μM and 20 nM, respectively. In addition, ECNF-CPE presented good stability, selectivity, and sensitivity. Cai et al. [114] developed a pressure sensor (FeOCN) based on the ECNF consisting of ferrosoferric oxide (Fe3O4). A wide working range (0–4.9 kPa), a rapid response time (0.43 s), an ultralow LOD (6 Pa.), a high sensitivity (0.545 kPa−1), and good stability were acquired in this work.
Table 2 summarizes different characteristics related to various carbon-based biosensors such as different electrochemical detection methods, selectivity, linear range (LR), response time, the limit of detection (LOD), and sensitivity for the detection of biomarker/biomedical agents. An ideal biosensor represents the rapid response, low detection limit, and high sensitivity and selectivity for the intended target [86].
Table 2.
Summary of different characteristics related to various carbon-based biosensors for the detection of biomarker/biomedical agents.
| Biomarker/biomedical agents | Biosensor composition | Method(s) | Selectivity | LR | ResponseTime (s) | LOD | Sensitivity | Real sample | Key findings | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ascorbic acid (AA) | Electrospun Pd/CNFs | CV, DPV | DA, UA, AA | 0.05–4 mM | – | 15 μM | – | Urine |
|
[109] |
| Carcinoembryonic antigen (CEA) | CNTs/PDDA/HRP/ConA/HRP-Ab2 | CV, DPV, EIS | CEA | 0.05–5 to 5–200 ng mL−1 | – | 0.018 ng mL−1 | – | Serum |
|
[115] |
| Catechol | Laccase–Nafion–ECNFs on GCE | CV, chronoamperometry | Catechol | 1–1310 μM | 2 | 0.63 μM | 41 μA mM−1 | Water |
|
[98] |
| Catechol | Electrospun Cu/CNFs/laccase(Lac)/Nafion/GCE | CV | Catechol | 9.95 × 10−6–9.76 × 10−3 M | Within 5 | 1.18 μM | 33.1 μA mM−1 | – |
|
[108] |
| Dopamine (DA) | Electrospun Pd/CNFs | CV, DPV | DA, UA, AA | 0.5–160 μM | – | 0.2 μM | – | Urine |
|
[109] |
| Dopamine | ZnO NF/CF | CV, DPV | DA | 6–20 μM | – | 0.402 μM | – | – |
|
[116] |
| Dopamine | Electrospun Ag-Pt/pCNF/GCE | CV, DPV | DA | 10–500 μM | – | 0.11 μM | – | – |
|
[102] |
| Glucose | NiMoO4/CNF membrane | CV, EIS | Glucose | 0.0003–4.5 mM | 5 | 50 nM | 301.77 μA cm−2 mM−1 | Human blood serum |
|
[117] |
| Glucose | Electrospun GOD/PB-FCNF/GCE | CV, chronoamperometry | Glucose | 0.02–12 mM | 5 | 0.5 μM | 35.94 μA cm−2 mM−1 | – |
|
[107] |
| Glucose | PdNPs-ERGO-GOD/GCE | CV, chronoamperometry | Glucose | 25 μM-4.9 mM | Within 5 | 0.56 μM | – | Human blood serum |
|
[118] |
| Glucose | Cu2O NCs/r-GO/GCE | LSV | Glucose | 5.0–9595 μM | Less than 3 | 1.0 μM | 23.058 μA mM−1 | Human blood |
|
[119] |
| Glucose | Electrospun Ni-CoO/CNF | CV, EIS | – | 0.25–600 μM | – | 0.03 μM | – | Serum |
|
[106] |
| Glucose | NiCo/TiO2/CNFAs | CV, EIS | Glucose | 1–7658 μM | Within 5 | 0.6 μM | 975.3 μA cm−2 mM−1 | Human blood |
|
[120] |
| Glucose | Ni-NaA/CPE | CV, DPV, chronoamperometry | Glucose | 0.04–6.54 mM | Less than 60 | 1.98 × 10−3 mM | 225.8 μA cm−2 mM−1 | Blood serum |
|
[121] |
| H2O2 | Electrospun Cyt c/FCNFs-HA/Au | CV, chronoamperometry | H2O2 | 2.0 μM-8.7 mM | 5 | 0.3 μM | – | Blood |
|
[100] |
| H2O2 | Electrospun nanoporous CNF-PtNP/GCE | CV, chronoamperometry | H2O2 | 2 linear regions: 10 μM-9.38 mM and 9.38–74.38 mM | – | 1.9 μM | – | – |
|
[101] |
| H2O2 | Electrospun Co-NC/CNFGCE | CV, chronoamperometry | H2O2 | Up to 5 mM | − | 10 μM | 300 μA cm−2 mM−1 | Juice, milk |
|
[104] |
| H2O2 | Electrospun N-doped CNFs | CV | – | 0.01–0.71 mM | Within 30 | 0.62 μM | 357 μA cm−2 mM−1 | Milk |
|
[105] |
| NADH | Electrospun CNF-CPE | CV, DPV, EIS | NADH, AA | 0.02–11.47 μM | 5 | 20 nM | 10nA μM−1 | – |
|
[110] |
| Pemetrexed (PTX)* | Electrospun CPE/Pd/CNF/[M3OA] + [NTF2]-/Nafion | SWV, CV | PTX | 1.00–35.0 nM | – | 0.33 nM | – | Cancerous human plasma, pharmaceutical,health urine samples |
|
[111] |
| Pressure | Electrospun FeOCN | – | – | 0–4.9 kPa | 0.43 | 6 Pa. | 0.545 kPa−1 | Wrist pulse, phonation, breathing, finger bending |
|
[114] |
| Tramadol | Electrospun CNFs/SPE | CV, SWV | Tramadol | 0.05–100 nM | – | 0.016 nM | – | Urine |
|
[112] |
| Uric acid (UA) | Electrospun Pd/CNFs | CV, DPV | DA, UA, AA | 2–200 μM | – | 0.7 μM | – | Urine |
|
[109] |
| Xanthine (Xa) | Electrospun CNF-CPE | CV, DPV, EIS | Xa | 0.03–21.19 μM | – | 20 nM | – | Human urine, fish |
|
[113] |
Abbreviations: CV: cyclic voltammetry; DPV: differential pulse voltammetry; EIS: electrochemical impedance spectroscopy; SWV: square wave voltammetry; LSV: linear sweep voltammetry; LR: Linear range; LOD: Limit of detection; Pd/CNF: Palladium/Carbon nanofiber; CNTs/PDDA/HRP/ConA/HRP-Ab2: Carbon nanotubes/poly(diallyldimethylammonium chloride)/horseradish peroxidaselabeled/concanavalin/horseradish peroxidase-labeled carcinoembryonic secondary antibodies; ECNF: Electrospun CNF; GCE: Glassy carbon electrode; ZnO NF/CF: Zinc oxide nanofiber/carbon fiber; Ag-Pt/pCNFs: Silver-Platinum/Electrospun nanoporous CNFs; NiMoO4: Nickel molybdate; GOD/PB–FCNF: Glucose oxidase/Prussian Blue-Functionalized CNF; PdNPs-ERGO: Pd Nanoparticles-Electrochemically Reduced Glucose oxidase; Cu2O NCs/r-GO: Copper(I) oxide Nanoclusters/reduced-Graphene oxide; Ni-CoO: Nickel-Cobalt(II) oxide; NiCo/TiO2/C NFAs: Nickel-Cobalt/Titanium dioxide Carbon core–shell nanofiber arrays; Ni-NaA/CPE: Nickel-NaA nanozeolite/Carbon paste electrode; POx: Pyranose oxidase; CNTPE: Carbon nanotube-modified carbon paste electrode; Cyt c: Cytochrome c; HA/Au: Hydroxyapatite/Gold; PtNP: Platinum Nanoparticles; Co-NC: Cobalt Nanoparticles; N-doped CNFs: Nitrogen-doped carbon nanofibers; SWCNT: Single-walled carbon nanotubes; [M3OA] + [NTF2]: methyl (trioctyl)ammonium bis(trifluoromethylsulfonyl)imide cobalt; FeOCN: Ferrosoferric oxide (Fe3O4) carbon nanofibers; SPE: screen printed electrode.
anti-cancer drug.
3.2. Tissue engineering
Tissue enginnering (TE) aims to regenerate diseased or injured tissues and organs via three main factors known as “the TE triad” including cells (e.g., stem cells or differentiated cells), inductive agents (e.g., growth factors or small molecules), and scaffolds (natural, or synthetic) [122–124]. It is essential to fabricate scaffolds as ECM without side effects, cytotoxicity, or immunological rejection [125–127]. The major roles of ECM components are to provide the growth factors, induce signaling pathways, and direct cell-ECM communication [72]. A wide range of natural and synthetic nanofiber materials have been investigated in the TE field for different tissue types such as skin, bone, musculoskeletal, neural, and so forth [128–130]. Naskar et al. [131] fabricated VGCNFs reinforced nonmulberry silk protein fibroin films via solvent evaporation technique as an option for tissue regenerations (e.g., muscle, bone, and nerve tissue). They demonstrated that VGCNFs endow the composite films with outstanding electrical conductivity, tensile modulus, hydrophilicity, and thermal stability, providing better fibroblast cell growth and proliferation. Additionally, many studies have been conducted to investigate the potential application of electrospun scaffolds in this field [76]. For instance, as mentioned, ECNFs owing to significant mechanical properties, are promising tools for bone TE [132,133].
3.2.1. Skin tissue engineering/wound healing
The skin is the body’s largest organ, and it protects against foreign substances and organisms [134]. Serious wounds for any reason (e.g., burns, diabetes, infections, etc.) could result in loss of the reparative and regenerative capabilities of the skin cells [135]. Skin TE provides replacement therapy and rapid healing via ideal wound dressing, limiting the risk of infections that could negatively impact skin tissue regeneration [136]. Several natural (e.g., collagen, elastin, fibronectin, chitosan, etc.) and synthetic ((e.g. poly (ethylene glycol) (PEG), poly (lactic acid) (PLA), polyglycolide (PGA), poly (lactic-co-glycolic acid) (PLGA), poly-ε-caprolactone (PCL), etc.) polymers have been utilized for the skin TE [134,137]. Salesa et al. [138] developed a calcium alginate/VGCNFs composite with antibacterial activity for the first time to address the antibiotic resistance problems. This composite is not only cost-effective, but also shows no cytotoxicity on human keratinocytes (HaCaT cells). The same group incorporated 1D hydrophobic VGCNFs and 2D hydrophilic GO nanosheets into alginate films to assess their effects on HaCaT cell adhesion. They demonstrated that this composite showed no cytotoxicity but did not improve cell adhesion [139]. In another study, copper and zinc distributed in VGCNFs and grown on an activated carbon fiber substrate (Cu-Zn-ACF/CNFs) inhibited the growth of the Gram-negative bacteria (Escherichia coli, Gram-positive Staphylococcus aureus, and Methicillin Resistance Staphylococcus aureus), with high efficiency. In this study, this composite was suggested as an antibiotic wound dressing [140]. Li et al. [141] also designed a potential hemostatic patch (superhydrophobic VGCNFs coating). This VGCNFs gauze promoted rapid fibrin growth and clotting, and reduced bacteria attachment in an in vitro study.
3.2.2. Bone tissue engineering
Bone TE (BTE) is an emerging therapeutic approach to regenerate and repair bone defects [142]. Principally, in the case of critical-sized bone defects in which the bone cannot heal spontaneously, TE might offer an effective treatment strategy [143,144]. Introducing CNMs into biomedical applications has found prominent opportunities for BTE [145]. CNTs/CNFs are considered reinforcing agents for polymers such as PCL, PLA, and so on to enhance or improve their mechanical, biocompatibility, and cellular responses and resemble the nanoscale architecture of natural ECM as well [146].
CNFs have been shown to enhance the adhesion, and proliferation of osteoblasts, induce alkaline phosphatase (ALP), and Ca2+ secretions (osteoconductivity effects), and possess excellent mechanical strength three times more than that of native bone tissue with Young’s modulus of 2 TPa and the tensile strength similar to the bone [147–150]. Besides, these materials have unique chemical, electrical, morphological, and biological characteristics [143]. Zhou et al. [151] developed ECNFs through the carbonization of aligned electrospun PAN nanofibers bundles. They showed that with increasing the final carbonization temperature, the CNFs became more graphitic and structurally ordered, and higher anisotropic electrical conductivities. Yang et al. [152] fabricated electrospun PAN-based ECNFs containing bioactive glass nanoparticles (ECNFs/BG) by combining electrospinning and sol–gel techniques to evaluate their osteocompatibility. In this study, a sol–gel solution was produced by triethyl phosphate (TEP), calcium nitrate tetrahydrate (Ca(NO3)2), and tetraethoxysilane (TEOS). Then, the resulting sol–gel solution with different compositions (68S, 77S, and 86S) was added to a PAN solution in N,N-dimethylformamide (DMF) to obtain BG solutions. These composites indicated an improved formation of apatite (in vitro biomineralization) and MC3T3-E1 osteoblasts proliferation. However, ECNF/BG(68 s) was the best substrate for the adhesion and proliferation of MC3T3-E1 cells by having the highest silica solubility, and the ability to form apatite (Fig. 10). Remarkably, reinforced compressive strength and elastic modulus of 45S5 Bioglass scaffolds (ranging from 2.08 to 4.56 MPa and 111.50 to 266.59 MPa, respectively) synthesized by the sol–gel technique have been achieved by adding 0.25 wt% MWCNTs [153].
Fig. 10.

Well-attached on all the ECNF membranes (pure ECNF and different ECNF/BG composites) and spindle-shaped MC3T3-E1 cells along the fiber direction were observed by scanning electron microscope (SEM) on different days. Although the cell number increased with the culture time, ECNF/BG(68 s) had the highest cell density and the largest spreading area. Reproduced with permission from Ref. [152].
Another study by Samadian et al. [154] exhibited that tailoring the mineralized electrospun PAN-based ECNFs (M–ECNFs) by three artificial neural networks (ANN) models, each with different training algorithms for data analysis to fabricate electro-conductive scaffolds, possesses the potential application in BTE. The mentioned features are essential criteria for achieving an ideal bone regeneration scaffold. Notably, in contrast to CNTs, ECNFs display non-cytotoxic characteristics and could be considered safe and promising materials for bone, orthopedic, and dental TE and prosthetic, or implant applications [128,147]. Wu et al. [155] synthesized 3D electrospun PAN-based ECNFs/hydroxyapatite (HAp) composites (Fig. 11) with strong interfacial bonding sites (strong interaction of HAp with the ECNFs through coordination bonds) and high mechanical strength that have potential in BTE. Furthermore, the carboxylic groups-activated ECNF surfaces made them hydrophilic.
Fig. 11.

Synthesis of ECNFs/HAp composites scaffold for bone tissue engineering. (a) Schematic representation of the experiment, (b) SEM micrograph of pristine ECNFs after mineralization, (c) SEM micrograph of NaOH-treated ECNFs after mineralization, and (d) TEM micrograph of mineralized ECNFs. Reproduced with permission from Ref. [155].
Another 3-D CNF/HAp scaffold for bone tissue regeneration was produced via carbonization of bacterial cellulose (BC) nanofibers by Wan et al. [156]. In their work, nitric acid (HNO3)-surface treated CNFs encouraged the mineralization (HAp nucleation and growth) and changed the morphology of HAp crystals developed on CNFs (rod-like vs needle-like) (Fig. 12). Electro-conductive ECNFs can also provide substrates for bone regeneration objectives. It is believed that the existence of external electrical stimulation (e.g., direct current field (DCF), electromagnetic field (EMF), inductive coupling (IC), and capacitive coupling electrical field (CCEF)) for scaffolds is a potential osteogenic factor to regenerate bone. DCF is more popular because of its simplicity, and site-specificity. This factor can induce mineralization, proliferation, and differentiation of bone cells. In this regard, electro-conductive ECNFs-medicated DCF increased MG-63 cell growth and their osteogenic activity [147]. Since proliferation, differentiation, and mineralization of osteoblast cells could be encouraged by silica nanoparticles (SNPs), they are considered other osteoinductive agents [157]. ECNFs/SNPs have also been reported to improve attachment, viability, and proliferation of MG-63 cells in a related study [143]. Table 3 summarizes different carbon-based scaffolds applied for BTE.
Fig. 12.

Transmission electron microscopy (TEM) images and selected area diffraction (SAED) patterns of various CNFs/HAp hybrids after 7 days of mineralization (a, b) asprepared CNFs, (c, d) CNFs oxidized in 3 M HNO3, (inset of d) energy-dispersive X-ray spectroscopy (EDS) pattern of CNFs oxidized in 3 M HNO3, (e, f) CNFs oxidized in 10 M HNO3, and (inset of f) high magnification of f. Reproduced with permission from Ref. [156].
Table 3.
Summary of carbon-based scaffolds for bone tissue engineering.
| Scaffold composition | Cells (in vitro) | Key findings | Ref. |
|---|---|---|---|
| CNF compacts & PLGA casts of nanophase carbon fibers | Human osteoblasts |
|
[158] |
| Electrospun ECNFs/HA | MG-63 |
|
[33] |
| Electro-conductive electrospun CNFs-medicated DCF | MG-63 |
|
[147] |
| ECNFs/SNPs | MG-63 |
|
[143] |
| Electro-conductive electrospun CNFs/Fe2O3 | MG-63 |
|
[133] |
| Electro-conductive ECNFs/AuNP | MG-63 |
|
[159] |
| Electrospun β-TCP/ECNFs membranes | PDLCs |
|
[160] |
| Electrospun β-TCP@ECNFs membranes | MG-63 |
|
[161] |
| Thermal-based electrospun ECNF/BG | MC3T3-E1 |
|
[162] |
| Sol-gel based Electrospun CNF/BG | MC3T3-E1 |
|
[152] |
| Electrospun CNF/BG | Rat BM-MSCs |
|
[163] |
| BC nanofibers-derived 3-D CNF/HAp | – |
|
[156] |
| Electrospun ECNF/HAp | – |
|
[164] |
| Sol-gel based ECNF-Si/Ca | Normal human skin fibroblastsNHOst |
|
[165] |
| PP/CNF-HANR | Saos-2 |
|
[166] |
Abbreviations: PLGA: poly(lactic-co-glycolic acid; HA/HAp: Hydroxyapatite; DCF: Direct current field; SNPs: Silica nanoparticles; Fe2O3: Ferrous sulfate; AuNP: Gold nanoparticle; β-TCP: β-tricalcium phosphate; BG: Bioglass; BC: Bacterial cellulose; ECNF-Si/Ca: Electrospun CNF-silica/calcium; PP/CNF–HANR: Polypropylene/carbon nanofiber-hydroxyapatite nanorod; PLA: poly(lactic acid); PCL: poly-ε-caprolactone; g-C3N4: Graphitic carbon nitride; MWCNTs: Multiwalled carbon nanotubes; f-MWCNT/Chitosan/B-GP: Functionalized multiwalled carbon nanotubes/chitosan/β-Glycerophosphate; MG-63: Human osteoblastic cell line; PDLCs: Human periodontal ligament cells; MC3T3-E1: Mouse osteoblastic cell line; BM-MSCs: Bone marrow-derived primary MSCs; NHOst: Normal human osteoblasts; Saos-2: Human osteoblast cell line; HNO3: Nitric acid; ALP: Alkaline phosphatase.
3.2.3. Neural tissue engineering
TE could provide a promising strategy for the repair of the injured central nervous system (CNS) and peripheral nervous system (PNS). In this way, it is possible to treat neurodegenerative diseases such as Alzheimer’s and Parkinson’s diseases, traumatic brain injury (TBI), spinal cord injury (SCI), and peripheral nerve injury (PNI) by promoting neuronal growth [167]. PNI is a health problem mainly related to trauma characterized by serious loss of sensory, or motor control in affected patients that may lead to lifelong disability and disturbing quality of life [79,168]. The self-regeneration and repair of damaged neural cells of peripheral nerves can be achieved if the nerve gap is less than 5 mm (by re-extension of the axon over the gap) [169], but greater defects may limit self-regeneration potential [170]. Hence, it is beneficial to utilize appropriate biomaterials as scaffolds in order to provide a 3D cell growth microenvironment, regenerate and treat the damaged nerves [72]. An ideal scaffold for NTE must exhibit an appropriate biomimetic structure, including physiochemical, mechanical, and electrical features along with being biocompatible, porous, and biodegradable [169,171]. It should be noted that it is essential to employ scaffolds without any or fewer neuroinflammatory effects [172].
It is practical to use neurotrophic factors in a company with scaffolds. As an example, Liu et al. [173] developed the electrospun aligned scaffolds based on regenerated silk fibroin (RSF) that were loaded with brain-derived neurotrophic factor (BDNF) and VEGF (dual-factor). They concluded that the resulting scaffolds could promote nerves regeneration for damaged peripheral nerve in vitro and in vivo studies. Other neurotrophic factors that could play essential roles in neural tissue regeneration include nerve growth factor (NGF), nerve growth factor (NT-3), glial cell line-derived neurotrophic factor (GDNF), and acidic and basic fibroblast growth factor (aFGF, bFGF) [174].
Other synthetic materials to fabricate neural tissue scaffolds are based on CNMs, including graphene, CNT, and CNFs [175]. Due to their natural conductivity features that are necessary for neural action potential, graphene and CNT have been chiefly utilized in NTE [176]. Nevertheless, the results of a previous study showed that polycarbonate urethane/VGCNFs composite as neural implant could decrease glial scar formation by limiting astrocyte functions. This substrate had positive interactions with neurons as well [177]. Another recent study describes that using electrospun PCL/VGCNFs as NGC was suitable for PC12 cell (rat pheochromocytoma cells) attachment and proliferation [178]. In light of the advantages of the electrospinning technique, its exploitation in fabricating nanofibrous scaffolds has been designed for NTE to act as both regenerative potential and a vehicle for therapeutic delivery to a neural lesion [79]. Mirzaei et al. [179] fabricated ECNFs and evaluated the effects of ECNFs alignment on the morphology and behavior of neural cells. They observed that the ECNFs alignment induced neural biomarkers and changed the morphology of cells from spherical to stretched cells aligned with the CNFs axis. Table 4 Summarizes major carbon-based nanofibrous scaffolds applied in NTE applications.
Table 4.
Summary of carbon-based scaffolds for neural tissue engineering.
| Scaffold composition | Cells (in vitro) | Key findings | Ref. |
|---|---|---|---|
| PCU:CN | Rat astrocytes |
|
[177] |
| Electrospun PCL/CNF (conduit) | PC12 cells |
|
[178] |
| Electrospun CNFs (random and aligned topographies) | hEnSCs |
|
[179] |
Abbreviations: PCU:CN: Polycarbonate urethane: carbon nanofiber; PCL: Poly(ε-caprolactone); SWCNT/PLLA: Single-walled carbon-nanotube/Poly(l-Lactic acid); PLGA/CNTPD: Polylactide-co-glycolide/carbon nanotube-dopamine; CS-PU/fMWCNT: Chitosan-polyurethane/functionalized multiwall carbon nanotubes; PPy: polypyrrole; Gr/SF: Graphene-silk fibroin; rGONR: reduced graphene oxide nanoribbon; SiO2: Silicon dioxide; rGONMs: reduced graphene oxide nanomeshes; GOF: Graphene oxide foam; PC12: Rat pheochromocytoma cells; hEnSCs: Human endometrial stem cells; OEC: Olfactory ensheathing glial cells; S42: Schwann cells; L929: Mouse fibroblast cell line; SCs; Schwann cells; BV2: Murine microglial cell line; hNSCs: human neural stem cells; NE-4C: Neuroectodermal stem cells.
3.2.4. Musculoskeletal tissue engineering
The musculoskeletal system contains bones (the skeleton), muscles, cartilage, tendons, ligaments, and joints [180,181]. BTE has been discussed in section 3.2.2. Many tissues of an adult human body, such as cardiac tissue, have limited self-regeneration potential after injuries. Hence, TE could be an appealing option to be employed in these conditions [182]. For myocardial TE, several studies have also utilized CNMs. Stout et al. [183] assessed the effects of PLGA-VGCNFs composites on human cardiomyocytes. This cardiovascular patch was cytocompatible and increased cardiomyocyte adhesion and proliferation in the dead tissue zone. Typically, incorporating VGCNFs into polymeric scaffolds, such as PLGA, improves or increases their mechanical conductivity, cytocompatibility, and other properties [181,184]. Interestingly, along with electrospun CS-PVA-MWCNT scaffold, electrical stimulation and small molecules such as CHIR99021 (CHIR) have been applied to induce cardiac differentiation of unrestricted somatic stem cells (USSCs) for cardiac TE purposes [185]. Electrical stimulation and conductive scaffolds (e.g., CNF/CNT) are important factors for the attachment and growth of certain cells, especially skeletal muscle, cardiac, neural cells, and osteoblasts [128,185]. Most studies on CNMs for musculoskeletal engineering are developed by utilizing graphene, and CNTs [186]. For meniscal TE, Gopinathan et al. [187] designed customizable functionalized 3D PCL scaffolds reinforced with VGCNFs. They showed its mechanical integrity, electrical properties, and cytocompatibility in primary meniscal cells.
4. Clinical and preclinical studies
To the best of our knowledge, CNMs, including CNFs, ECNFs, and CNTs, have not yet been used clinically, although a plethora of in vitro studies suggests these materials’ TE potential. The important reason behind it is safety issues [188]. However, few in vivo studies (animal experiments) are being conducted based on in vitro findings. Herein, we mentioned certain in vivo studies. In vivo study for VGCNFs gauze was performed on rat back-bleeding models and promoted coagulation and reduced blood loss as a blood-repelling CNFs gauze. CNF gauze was applied to the rat skin for skin compatibility tests, with no itching or erythema observed after 12 h [141]. New ectopic bone formation was reported in a study that a 3D thin electrospun carbon fiber web (TCFW) and recombinant human bone morphogenetic protein 2 (rhBMP-2) as a growth factor was implanted into the dorsal muscle of a ddY mouse (ddY stands for Deutschland, Denken, and Yoken). It showed good bone-tissue compatibility without necrosis or significant inflammatory responses. The rhBMP-2/TCFW composite was also implanted into the rat ileum with critical-sized defects and repaired the orthotopic defect [189]. In another similar study, the ECNF sheets functionalized by HA and bovine serum albumin (BSA) as a growth factor were biocompatible, with fewer inflammatory cells implanted subcutaneously in a rabbit model. It is suggested that CNF/HA/BSA sheets as periosteum-like membranes have the potential for BTE [190].
In another recent survey, the osteoconductive effects (with the highest new bone formation (61.3 ± 4.2 %)) of ECNFs decorated with HA crystal (CNFs/HA) have been exhibited in a rat’s femur defect [33]. Another study reported the in vivo biocompatibility of porous ultra-short single-walled carbon nanotubes (US-tubes)-reinforced poly (propylene fumarate) (PPF) nanocomposites in a New Zealand rabbit model. The nanocomposite scaffolds were implanted into subcutaneous pockets (in the dorsum of the rabbits), and femoral condyles (to analyze soft and hard tissue responses). High bone tissue ingrowth, reduced inflammatory cell density, and increased connective tissue organization were observed 12 weeks after implantation. They have suggested that US-tube/PPF scaffolds are osteoconductive, and bioactive, assisting osteogenesis [191].
Farzamfar et al. [178] showed peripheral nerve regeneration using a conductive electrospun PCL/VGCNFs conduit as a neural guidance channel. This conduit exhibited no toxicity or immunogenic reactions after being implanted into a rat model of sciatic nerve defect. This study suggested that it could be a promising strategy for treating PNI in the clinic. In vivo study by Gopinathan et al. [187] for nanocomposite 3D PCL scaffold with VGCNFs in New Zealand white rabbits. In this study, subcutaneous implantation of nanocomposite was performed to evaluate its in vivo biocompatibility and biotoxicity to be employed as a meniscus scaffold. Their study demonstrated that nanocomposites had no toxicity. Concerning the growing number of in vitro and in vivo works pertinent to CNMs and their findings, it is anticipated that these materials, in particular, ECNFs, hold promise to be employed in clinical studies in the future.
5. Concluding remarks and future perspective
Nanomaterials and nanotechnology have inevitable impacts on modern devices and procedures in various fields of biomedicine. Carbon-based nanomaterials have shown promising and fascinating performance in diagnosing, treating, and recovering different diseases. Among them, CNFs have attracted significant attention due to their outstanding physicochemical properties. It has been shown that the synthesis method of CNFs (CVD growth and carbonization) has critical effects on the physicochemical properties and, subsequently, the performance of CNFs in the intended application. Although the non-spinning-based synthesis methods (e.g., CVD) provide CNFs with higher mechanical strength and electrical conductivity, they suffer from high synthesis costs. On the other hand, the carbonization-based methods (e.g., electrospinning) offer lower synthesis costs and toxicological issues but suffer from lower graphitization, electrical properties, mechanical strength, and more structural defects. However, the structural defects can be exploited for biomolecules conjugation/attachment and are an advantage from the biomedical point of view.
A critical issue in applying CNFs for biomedical applications and the clinical translation, except for biosensors, is the biopersistence, biodegradation, and the in vivo fate of CNFs. It has been reported that immune cells (e.g., neutrophils and macrophages) and some enzymes (e.g., peroxidases) can degrade carbon-based nanomaterials [192]. Moreover, the studies implied that the type of surface functional groups, structural defects, and oxygen percentage determine the biodegradation profile and biopersistence of CNFs [193]. In this concept, it can be assumed that the electrospun CNFs are more biodegradable than the CVD-based CNFs due to more structural defects and amorphous nature. However, comprehensive studies should be carried out using the big data-based experimental methods, such as structure–activity relationship (QSAR), artificial neural network (ANN), and design of experiment (DOE) approaches to precisely assess the biodegradation and biopersistence of each type of CNFs and the effect of the involved synthesis parameter and post-treatments. Nevertheless, the authors believe that CNFs have the potential to be considered innovative and enabling structures beneficial for various fileds of biomedicine.
Acknowledgment
The authors gratefully acknowledge the research council of the Hamadan University of Medical Sciences for the support. This publication has been supported by RUDN University Strategic Academic Leadership Program (R. Luque).
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- [1].Al-Mubaddel FS et al. , Engineered nanostructures: A review of their synthesis, characterization and toxic hazard considerations, Arabian J. Chem 10 (2017) S376–S388. [Google Scholar]
- [2].Stupp SI, Introduction: Functional Nanostructures, ACS Publications, 2005, pp. 1023–1024. [Google Scholar]
- [3].Singh SK, Kulkarni PP, Dash D, Biomedical applications of nanomaterials: an overview, Bio-Nanotechnology (2013) 1–32. [Google Scholar]
- [4].Alshamrani M, Broad-spectrum theranostics and biomedical application of functionalized nanomaterials, Polymers 14 (6) (2022) 1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Dolai J, Mandal K, Jana NR, Nanoparticle size effects in biomedical applications, ACS Appl. Nano Mater 4 (7) (2021) 6471–6496. [Google Scholar]
- [6].Aflori M, Smart nanomaterials for biomedical applications—A review, Nanomaterials 11 (2) (2021) 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yin Y, Talapin D, The chemistry of functional nanomaterials, Chem. Soc. Rev 42 (7) (2013) 2484–2487. [DOI] [PubMed] [Google Scholar]
- [8].Singh M, Goyal M, Devlal K, Size and shape effects on the band gap of semiconductor compound nanomaterials, J. Taibah Univ. Sci 12 (4) (2018) 470–475. [Google Scholar]
- [9].Lu X, Wang C, Wei Y, One-dimensional composite nanomaterials: Synthesis by electrospinning and their applications, Small 5 (21) (2009) 2349–2370. [DOI] [PubMed] [Google Scholar]
- [10].Gong S, Cheng W, One-dimensional nanomaterials for soft electronics, Adv. Electron. Mater 3 (3) (2017) 1600314. [Google Scholar]
- [11].Yi X, Shi X, Gao H, A universal law for cell uptake of one-dimensional nanomaterials, Nano Lett 14 (2) (2014) 1049–1055. [DOI] [PubMed] [Google Scholar]
- [12].Zhang B et al. , Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage, Prog. Mater Sci 76 (2016) 319–380. [Google Scholar]
- [13].Sharma R, Kar KK, Characteristics of carbon nanofibers, in: Handbook of Nanocomposite Supercapacitor Materials I, 2020, pp. 215–245. [Google Scholar]
- [14].Yadav D, Amini F, Ehrmann A, Recent advances in carbon nanofibers and their applications–a review, Eur. Polym. J 138 (2020) 109963. [Google Scholar]
- [15].Wang J et al. , Tubular carbon nanofibers: Synthesis, characterization and applications in microwave absorption, Carbon 152 (2019) 255–266. [Google Scholar]
- [16].Su DS, Schlögl R, Nanostructured carbon and carbon nanocomposites for electrochemical energy storage applications, ChemSusChem 3 (2) (2010) 136–168. [DOI] [PubMed] [Google Scholar]
- [17].Qie L et al. , Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability, Adv. Mater 24 (15) (2012) 2047–2050. [DOI] [PubMed] [Google Scholar]
- [18].Zhou X et al. , Carbon nanofiber-based three-dimensional nanomaterials for energy and environmental applications, Mater. Adv 1 (7) (2020) 2163–2181. [Google Scholar]
- [19].Mahanta NK et al. , Thermal conductivity of carbon nanofiber mats, Carbon 48 (15) (2010) 4457–4465. [Google Scholar]
- [20].Al-Saleh MH, Sundararaj U, Review of the mechanical properties of carbon nanofiber/polymer composites, Compos. A Appl. Sci. Manuf 42 (12) (2011) 2126–2142. [Google Scholar]
- [21].Zhang L et al. , A review: carbon nanofibers from electrospun polyacrylonitrile and their applications, J. Mater. Sci 49 (2) (2014) 463–480. [Google Scholar]
- [22].Feng L, Xie N, Zhong J, Carbon nanofibers and their composites: a review of synthesizing, properties and applications, Materials 7 (5) (2014) 3919–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Martin-Gullon I et al. , Differences between carbon nanofibers produced using Fe and Ni catalysts in a floating catalyst reactor, Carbon 44 (8) (2006) 1572–1580. [Google Scholar]
- [24].Fang W et al. , Manufacture and application of lignin-based carbon fibers (LCFs) and lignin-based carbon nanofibers (LCNFs), Green Chem 19 (8) (2017) 1794–1827. [Google Scholar]
- [25].Gaminian H et al. , Capacitance performance boost of cellulose-derived carbon nanofibers via carbon and silver nanoparticles, Cellulose 26 (4) (2019) 2499–2512. [Google Scholar]
- [26].De Jong KP, Geus JW, Carbon nanofibers: catalytic synthesis and applications, Catalysis Reviews 42 (4) (2000) 481–510. [Google Scholar]
- [27].Deeney C et al. , Template-assisted synthesis of luminescent carbon nanofibers from beverage-related precursors by microwave heating, Molecules 24 (8) (2019) 1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Liang HW et al. , Macroscopic-scale template synthesis of robust carbonaceous nanofiber hydrogels and aerogels and their applications, Angew. Chem. Int. Ed 51 (21) (2012) 5101–5105. [DOI] [PubMed] [Google Scholar]
- [29].Mingjun C et al. , An example of industrialization of melt electrospinning: Polymer melt differential electrospinning, Adv. Ind. Eng. Polymer Res 2 (3) (2019) 110–115. [Google Scholar]
- [30].Sarkar K et al. , Electrospinning to forcespinning™, Mater. Today 13 (11) (2010) 12–14. [Google Scholar]
- [31].Gupta B, Edwards J, Textile materials and structures for topical management of wounds, in: Advanced Textiles for Wound Care 2019, Elsevier. p. 55–104. [Google Scholar]
- [32].Brochocka A et al. , Multifunctional polymer composites produced by melt-blown technique to use in filtering respiratory protective devices, Materials 13 (3) (2020) 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Samadian H et al. , Osteoconductive and electroactive carbon nanofibers/hydroxyapatite nanocomposite tailored for bone tissue engineering: in vitro and in vivo studies, Sci. Rep 10 (1) (2020) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Rahaman MSA, Ismail AF, Mustafa A, A review of heat treatment on polyacrylonitrile fiber, Polym. Degrad. Stab 92 (8) (2007) 1421–1432. [Google Scholar]
- [35].Zhu D et al. , Study of carbon films from PAN/VGCF composites by gelation/crystallization from solution, Carbon 40 (3) (2002) 363–373. [Google Scholar]
- [36].Laffont L et al. , An EELS study of the structural and chemical transformation of PAN polymer to solid carbon, Carbon 42 (12–13) (2004) 2485–2494. [Google Scholar]
- [37].Nie Y, Hübert T, Surface modification of carbon nanofibers by glycidoxysilane for altering the conductive and mechanical properties of epoxy composites, Compos. A Appl. Sci. Manuf 43 (8) (2012) 1357–1364. [Google Scholar]
- [38].Hung P-Y et al. , Surface modification of carbon fibre using graphene–related materials for multifunctional composites, Compos. B Eng 133 (2018) 240–257. [Google Scholar]
- [39].Klein KL, et al. , Surface characterization and functionalization of carbon nanofibers 103(6) (2008) 061301. [Google Scholar]
- [40].Cai Z, et al. , Effects of surface modification of carbon nanofibers on the mechanical properties of polyamide 1212 composites 132(6) (2015). [Google Scholar]
- [41].Nie Y, Surface Silanization of Carbon Nanofibers and Nanotubes for Altering the Properties of Epoxy Composites, BAM Federal Institute for Materials Research and Testing, Berlin, 2012. [Google Scholar]
- [42].Zhang ZJ, Chen XY, Carbon nanofibers derived from bacterial cellulose: Surface modification by polydopamine and the use of ferrous ion as electrolyte additive for collaboratively increasing the supercapacitor performance 519 (2020) 146252. [Google Scholar]
- [43].Abu Ghalia M, Dahman Y, 15 - Synthesis and Utilization of Natural Fiber-reinforced Poly (lactic acid) bionanocomposites, in: Jawaid M, Md Tahir P, Saba N (Eds.), Lignocellulosic Fibre and Biomass-Based Composite Materials, Woodhead Publishing, 2017, pp. 313–345. [Google Scholar]
- [44].Smolka W et al. , Structure and biological properties of surface-engineered carbon nanofibers, J. Nanomater 2019 (2019) 4146190. [Google Scholar]
- [45].Liu HY et al. , Surface modification of vapor-grown carbon nanofibers in radio frequency plasma, Chinese J. Chem. Phys 20 (6) (2007) 759. [Google Scholar]
- [46].Nayak L, Rahaman M, Giri R, Surface modification/functionalization of carbon materials by different techniques: an overview, in: Rahaman M, Khastgir D, Aldalbahi AK (Eds.), Carbon-Containing Polymer Composites, Springer Singapore, Singapore, 2019, pp. 65–98. [Google Scholar]
- [47].Tshwenya L et al. , Surface modified carbon nanomats provide cationic and anionic rectifier membranes in aqueous electrolyte media, Electrochim. Acta 354 (2020) 136750. [Google Scholar]
- [48].Thamer BM et al. , Fabrication of functionalized electrospun carbon nanofibers for enhancing lead-ion adsorption from aqueous solutions, Sci. Rep 9 (1) (2019) 19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sebastián D et al. , The effect of the functionalization of carbon nanofibers on their electronic conductivity, Carbon 48 (15) (2010) 4421–4431. [Google Scholar]
- [50].Ud Din I, et al. , Surface modification of carbon nanofibers by HNO3 treatment. Ceram. Int, 2016. 42(1, Part A): 966–970. [Google Scholar]
- [51].Shi Y et al. , The effect of surface modification on the friction and wear behavior of carbon nanofiber-filled PTFE composites, Wear 264 (11) (2008) 934–939. [Google Scholar]
- [52].Lim S et al. , Surface modification of carbon nanofiber with high degree of graphitization, J. Phys. Chem. B 108 (5) (2004) 1533–1536. [Google Scholar]
- [53].Kim SW et al. , Surface modifications for the effective dispersion of carbon nanotubes in solvents and polymers, Carbon 50 (1) (2012) 3–33. [Google Scholar]
- [54].Gromov A et al. , Covalent amino-functionalisation of single-wall carbon nanotubes, J. Mater. Chem 15 (32) (2005) 3334–3339. [Google Scholar]
- [55].Karippal JJ et al. , Effect of amine functionalization of CNF on electrical, thermal, and mechanical properties of epoxy/CNF composites, Polym. Bull 65 (8) (2010) 849–861. [Google Scholar]
- [56].Dai J et al. , pH/photothermal dual-responsive drug delivery and synergistic chemo-photothermal therapy by novel porous carbon nanofibers, Chem. Eng. J 397 (2020) 125402. [Google Scholar]
- [57].Pastine SJ et al. , A facile and patternable method for the surface modification of carbon nanotube forests using perfluoroarylazides, J. Am. Chem. Soc 130 (13) (2008) 4238–4239. [DOI] [PubMed] [Google Scholar]
- [58].Gan JA, Berndt CC, 4 - Plasma surface modification of metallic biomaterials, in: Surface Coating and Modification of Metallic Biomaterials, Wen C, Editor 2015, Woodhead Publishing. p. 103–157. [Google Scholar]
- [59].Guadalupe N-V-M et al. , Surface modification of carbon nanofibers (CNFs) by plasma polymerization of methylmethacrylate and its effect on the properties of PMMA/CNF nanocomposites, J e-Polymers 8 (1) (2008). [Google Scholar]
- [60].He P et al. , Surface modification and ultrasonication effect on the mechanical properties of carbon nanofiber/polycarbonate composites, Compos. A Appl. Sci. Manuf 37 (9) (2006) 1270–1275. [Google Scholar]
- [61].Gao Y, et al. , Effects of surface modification, carbon nanofiber concentration, and dispersion time on the mechanical properties of carbon-nanofiber–polycarbonate composites 103(6) (2007) 3792–3797. [Google Scholar]
- [62].Xu Z et al. , Surface modification of carbon fiber by redox-induced graft polymerization of acrylic acid, J. Appl. Polym. Sci 108 (3) (2008) 1887–1892. [Google Scholar]
- [63].Mittal M, Sardar S, Jana A, Chapter 7 - Nanofabrication techniques for semiconductor chemical sensors, in: Handbook of Nanomaterials for Sensing Applications, Hussain CM and Kailasa SK, Editors. 2021, Elsevier. p. 119–137. [Google Scholar]
- [64].Chen N, et al. , Polymer thin films and surface modification by chemical vapor deposition: recent progress 7(1) (2016) 373–393. [DOI] [PubMed] [Google Scholar]
- [65].Shi D, et al. , Plasma deposition of Ultrathin polymer films on carbon nanotubes 81(27) (2002) 5216–5218. [Google Scholar]
- [66].Whittaker JD, et al. , Self-aligned mechanical attachment of carbon nanotubes to silicon dioxide structures by selective silicon dioxide chemical-vapor deposition 83(25) (2003) 5307–5309. [Google Scholar]
- [67].Meng L, Fu C, Lu Q, Advanced technology for functionalization of carbon nanotubes, Prog. Nat. Sci 19 (7) (2009) 801–810. [Google Scholar]
- [68].Meng L, Zeng Y, Zhu D, Dynamic liquid membrane electrochemical modification of carbon nanotube fiber for electrochemical microfabrication, ACS Appl. Mater. Interfaces 12 (5) (2020) 6183–6192. [DOI] [PubMed] [Google Scholar]
- [69].Vedrtnam A, Sharma SP, Study on the performance of different nano-species used for surface modification of carbon fiber for interface strengthening, Compos. A Appl. Sci. Manuf 125 (2019) 105509. [Google Scholar]
- [70].Park S-J, Jang Y-S, Interfacial characteristics and fracture toughness of electrolytically Ni-plated carbon fiber-reinforced phenolic resin matrix composites, J. Colloid Interface Sci 237 (1) (2001) 91–97. [DOI] [PubMed] [Google Scholar]
- [71].Kang MS et al. , Advanced techniques for skeletal muscle tissue engineering and regeneration, Bioengineering 7 (3) (2020) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Xie X et al. , Electrospinning nanofiber scaffolds for soft and hard tissue regeneration, J. Mater. Sci. Technol (2020). [Google Scholar]
- [73].Liu H et al. , Electrospinning of nanofibers for tissue engineering applications, J. Nanomater 2013 (2013). [Google Scholar]
- [74].Lee B-S, Yu W-R, Electrospun carbon nanofibers as a functional composite platform: a review of highly tunable microstructures and morphologies for versatile applications, Funct. Compos. Struct 2 (1) (2020) 012001. [Google Scholar]
- [75].Parham S et al. , Electrospun nano-fibers for biomedical and tissue engineering applications: A comprehensive review, Materials 13 (9) (2020) 2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ghaderpour A et al. , Altering the characterization of nanofibers by changing the electrospinning parameters and their application in tissue engineering, drug delivery, and gene delivery systems, Polym. Adv. Technol 32 (5) (2021) 1924–1950. [Google Scholar]
- [77].Stocco TD et al. , Nanofibrous scaffolds for biomedical applications, Nanoscale 10 (26) (2018) 12228–12255. [DOI] [PubMed] [Google Scholar]
- [78].Gupta KC et al. , Nanofibrous scaffolds in biomedical applications, Biomater. Res 18 (1) (2014) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Rahmati M et al. , Electrospinning for tissue engineering applications, Prog. Mater Sci (2020) 100721. [Google Scholar]
- [80].Liang D, Hsiao BS, Chu B, Functional electrospun nanofibrous scaffolds for biomedical applications, Adv. Drug Deliv. Rev 59 (14) (2007) 1392–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Scaffaro R et al. , Nanocarbons in electrospun polymeric nanomats for tissue engineering: A review, Polymers 9 (2) (2017) 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wan S, Bi H, Sun L, Graphene and carbon-based nanomaterials as highly efficient adsorbents for oils and organic solvents, Nanotechnol. Rev 5 (1) (2016) 3–22. [Google Scholar]
- [83].Maiti D et al. , Carbon-based nanomaterials for biomedical applications: a recent study, Front. Pharmacol 9 (2019) 1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Alshehri R et al. , Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity: miniperspective, J. Med. Chem 59 (18) (2016) 8149–8167. [DOI] [PubMed] [Google Scholar]
- [85].Mondal K, Sharma A, Recent advances in electrospun metal-oxide nanofiber based interfaces for electrochemical biosensing, RSC Adv 6 (97) (2016) 94595–94616. [Google Scholar]
- [86].Luka G et al. , Microfluidics integrated biosensors: A leading technology towards lab-on-a-chip and sensing applications, Sensors 15 (12) (2015) 30011–30031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Soleymani L, Li F, Mechanistic challenges and advantages of biosensor miniaturization into the nanoscale, ACS Sensors 2 (4) (2017) 458–467. [DOI] [PubMed] [Google Scholar]
- [88].Chamorro-Garcia A, Merkoçi A, Nanobiosensors in diagnostics. Nanobiomedicine 3 (2016) 1849543516663574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Bhalla N et al. , Introduction to biosensors, Essays Biochem 60 (2016) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Naresh V, Lee N, A review on biosensors and recent development of nanostructured materials-enabled biosensors, Sensors 21 (4) (2021) 1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Purohit B et al. , Biosensor nanoengineering: Design, operation, and implementation for biomolecular analysis, Sensors Int (2020) 100040. [Google Scholar]
- [92].Hasan A, et al. Recent advances in application of biosensors in tissue engineering BioMed Res. Int 2014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Cleeton C et al. , Electrospun nanofibers for drug delivery and biosensing, ACS Biomater. Sci. Eng 5 (9) (2019) 4183–4205. [DOI] [PubMed] [Google Scholar]
- [94].Hu J, Zhang Z, Application of electrochemical sensors based on carbon nanomaterials for detection of flavonoids, Nanomaterials 10 (10) (2020) 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bunyakul N, Baeumner AJ, Combining electrochemical sensors with miniaturized sample preparation for rapid detection in clinical samples, Sensors 15 (1) (2015) 547–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Senf B, Yeo W-H, Kim J-H, Recent advances in portable biosensors for biomarker detection in body fluids, Biosensors 10 (9) (2020) 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hu J, Zhang Z, Application of electrochemical sensors based on carbon nanomaterials for detection of flavonoids, Nanomaterials 10 (10) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Li D et al. , A catechol biosensor based on electrospun carbon nanofibers, Beilstein J. Nanotechnol 5 (1) (2014) 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Vamvakaki V, Tsagaraki K, Chaniotakis N, Carbon nanofiber-based glucose biosensor, Anal. Chem 78 (15) (2006) 5538–5542. [DOI] [PubMed] [Google Scholar]
- [100].Cui K et al. , Architecture of electrospun carbon nanofibers–hydroxyapatite composite and its application act as a platform in biosensing, Sens. Actuators, B 160 (1) (2011) 435–440. [Google Scholar]
- [101].Li Y et al. , Nanoporous carbon nanofibers decorated with platinum nanoparticles for non-enzymatic electrochemical sensing of H2O2, Nanomaterials 5 (4) (2015) 1891–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Huang Y et al. , Electrospun carbon nanofibers decorated with Ag–Pt bimetallic nanoparticles for selective detection of dopamine, ACS Appl. Mater. Interfaces 6 (15) (2014) 12449–12456. [DOI] [PubMed] [Google Scholar]
- [103].Adabi M et al. , Performance of electrodes synthesized with polyacrylonitrile-based carbon nanofibers for application in electrochemical sensors and biosensors, Mater. Sci. Eng., C 48 (2015) 673–678. [DOI] [PubMed] [Google Scholar]
- [104].Riaz MA et al. , Hierarchically porous carbon nanofibers embedded with cobalt nanoparticles for efficient H2O2 detection on multiple sensor platforms, Sens. Actuators, B 319 (2020) 128243. [Google Scholar]
- [105].Lyu Y-P et al. , Hydrothermal and plasma nitrided electrospun carbon nanofibers for amperometric sensing of hydrogen peroxide, Microchim. Acta 185 (8) (2018) 1–7. [DOI] [PubMed] [Google Scholar]
- [106].Mei Q et al. , Electrospinning of highly dispersed Ni/CoO carbon nanofiber and its application in glucose electrochemical sensor, J. Electroanal. Chem 847 (2019) 113075. [Google Scholar]
- [107].Wang L et al. , Controllable growth of Prussian blue nanostructures on carboxylic group-functionalized carbon nanofibers and its application for glucose biosensing, Nanotechnology 23 (45) (2012) 455502. [DOI] [PubMed] [Google Scholar]
- [108].Fu J et al. , Laccase biosensor based on electrospun copper/carbon composite nanofibers for catechol detection, Sensors 14 (2) (2014) 3543–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Huang J et al. , Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode, Biosens. Bioelectron 24 (4) (2008) 632–637. [DOI] [PubMed] [Google Scholar]
- [110].Liu Y, Hou H, You T, Synthesis of carbon nanofibers for mediatorless sensitive detection of NADH, Electroanalysis 20 (15) (2008) 1708–1713. [Google Scholar]
- [111].Afzali M et al. , A novel voltammetric sensor based on palladium nanoparticles/carbon nanofibers/ionic liquid modified carbon paste electrode for sensitive determination of anti-cancer drug pemetrexed, J. Mol. Liq 282 (2019) 456–465. [Google Scholar]
- [112].Jahromi Z et al. , A rapid and selective electrochemical sensor based on electrospun carbon nanofibers for tramadol detection, Microchem. J 157 (2020) 104942. [Google Scholar]
- [113].Tang X et al. , A nonenzymatic sensor for xanthine based on electrospun carbon nanofibers modified electrode, Talanta 83 (5) (2011) 1410–1414. [DOI] [PubMed] [Google Scholar]
- [114].Cai C et al. , A flexible and highly sensitive pressure sensor based on three-dimensional electrospun carbon nanofibers, RSC Adv 11 (23) (2021) 13898–13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Yang P et al. , Sandwich-type amperometric immunosensor for cancer biomarker based on signal amplification strategy of multiple enzyme-linked antibodies as probes modified with carbon nanotubes and concanavalin A, J. Electroanal. Chem 732 (2014) 38–45. [Google Scholar]
- [116].Yang C et al. , Ultra-long ZnO/carbon nanofiber as free-standing electrochemical sensor for dopamine in the presence of uric acid, J. Mater. Sci 54 (24) (2019) 14897–14904. [Google Scholar]
- [117].Rani SD et al. , NiMoO4 nanoparticles decorated carbon nanofiber membranes for the flexible and high performance glucose sensors, Sens. Actuators, B 312 (2020) 127886. [Google Scholar]
- [118].Qin Y et al. , In situ synthesis of highly loaded and ultrafine Pd nanoparticles-decorated graphene oxide for glucose biosensor application, J. Mater. Chem 22 (47) (2012) 24821–24826. [Google Scholar]
- [119].Mei L-P et al. , Nonenzymatic amperometric sensing of glucose using a glassy carbon electrode modified with a nanocomposite consisting of reduced graphene oxide decorated with Cu 2 O nanoclusters, Microchim. Acta 182 (9) (2015) 1701–1708. [Google Scholar]
- [120].Wang J et al. , Quasi-aligned nanorod arrays composed of Nickel-Cobalt nanoparticles anchored on TiO2/C nanofiber arrays as free standing electrode for enzymeless glucose sensors, J. Alloy. Compd 821 (2020) 153510. [Google Scholar]
- [121].Hassaninejad-Darzi SK, Rahimnejad M, Mirzababaei SN, Electrocatalytic oxidation of glucose onto carbon paste electrode modified with nickel hydroxide decorated NaA nanozeolite, Microchem. J 128 (2016) 7–17. [Google Scholar]
- [122].O’brien FJ,, Biomaterials & scaffolds for tissue engineering, Mater. Today 14 (3) (2011) 88–95. [Google Scholar]
- [123].Zhang Y, et al. , Stem cell-friendly scaffold biomaterials: applications for bone tissue engineering and regenerative medicine. Front. Bioeng. Biotechnol, 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gorain B et al. , Carbon nanotube scaffolds as emerging nanoplatform for myocardial tissue regeneration: A review of recent developments and therapeutic implications, Biomed. Pharmacother 104 (2018) 496–508. [DOI] [PubMed] [Google Scholar]
- [125].Eltom A, Zhong G, Muhammad A, Scaffold techniques and designs in tissue engineering functions and purposes: a review, Adv. Mater. Sci. Eng 2019 (2019). [Google Scholar]
- [126].Garg T et al. , Scaffold: a novel carrier for cell and drug delivery, Crit. Rev. Ther. Drug Carrier Syst 29 (1) (2012). [DOI] [PubMed] [Google Scholar]
- [127].Calori IR et al. , Polymer scaffolds as drug delivery systems, Eur. Polym. J 129 (2020) 109621. [Google Scholar]
- [128].Vasita R, Katti DS, Nanofibers and their applications in tissue engineering, Int. J. Nanomed 1 (1) (2006) 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Narayanan N et al. , Polymeric electrospinning for musculoskeletal regenerative engineering, Regener. Eng. Transl. Med 2 (2) (2016) 69–84. [Google Scholar]
- [130].Mazzoni E et al. , Bioactive materials for soft tissue repair, Front. Bioeng. Biotechnol 9 (2021) 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Naskar D et al. , Carbon nanofiber reinforced nonmulberry silk protein fibroin nanobiocomposite for tissue engineering applications, ACS Appl. Mater. Interfaces 9 (23) (2017) 19356–19370. [DOI] [PubMed] [Google Scholar]
- [132].Moulefera I et al. , Electrospun carbon nanofibers from biomass and biomass blends—current trends, Polymers 13 (7) (2021) 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Nekounam H et al. , Electro-conductive carbon nanofibers containing ferrous sulfate for bone tissue engineering, Life Sci (2021) 119602. [DOI] [PubMed] [Google Scholar]
- [134].Chaudhari AA et al. , Future prospects for scaffolding methods and biomaterials in skin tissue engineering: a review, Int. J. Mol. Sci 17 (12) (2016) 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Xie X et al. , Electrospinning nanofiber scaffolds for soft and hard tissue regeneration, J. Mater. Sci. Technol 59 (2020) 243–261. [Google Scholar]
- [136].Sylvester MA, Amini F, Tan CK, Electrospun nanofibers in wound healing, Mater. Today:. Proc 29 (2020) 1–6. [Google Scholar]
- [137].Mohamed A, Xing MM, Nanomaterials and nanotechnology for skin tissue engineering, Int. J. Burns Trauma 2 (1) (2012) 29. [PMC free article] [PubMed] [Google Scholar]
- [138].Salesa B et al. , Carbon nanofibers in pure form and in calcium alginate composites films: New cost-effective antibacterial biomaterials against the life-threatening multidrug-resistant Staphylococcus epidermidis, Polymers 11 (3) (2019) 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Salesa B, Llorens-Gámez M, Serrano-Aroca Á, Study of 1D and 2D carbon nanomaterial in alginate films, Nanomaterials 10 (2) (2020) 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Ashfaq M, Verma N, Khan S, Copper/zinc bimetal nanoparticles-dispersed carbon nanofibers: a novel potential antibiotic material, Mater. Sci. Eng., C 59 (2016) 938–947. [DOI] [PubMed] [Google Scholar]
- [141].Li Z et al. , Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion, Nat. Commun 10 (1) (2019) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Chocholata P, Kulda V, Babuska V, Fabrication of scaffolds for bone-tissue regeneration, Materials 12 (4) (2019) 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Nekounam H et al. , Silica nanoparticles-incorporated carbon nanofibers as bioactive biomaterial for bone tissue engineering, Diam. Relat. Mater 115 (2021) 108320. [Google Scholar]
- [144].Wei S et al. , Biodegradable materials for bone defect repair, Mil. Med. Res 7 (1) (2020) 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Eivazzadeh-Keihan R et al. , Carbon based nanomaterials for tissue engineering of bone: Building new bone on small black scaffolds: A review, J. Adv. Res 18 (2019) 185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Cai Q, et al. , Carbon nanomaterials for implant dentistry and bone tissue engineering, in: Nanobiomaterials in Clinical Dentistry 2019, Elsevier. p. 429–468. [Google Scholar]
- [147].Samadian H et al. , Electro-conductive carbon nanofibers as the promising interfacial biomaterials for bone tissue engineering, J. Mol. Liq 298 (2020) 112021. [Google Scholar]
- [148].Aoki K et al. , The use of electrospun organic and carbon nanofibers in bone regeneration, Nanomaterials 10 (3) (2020) 562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Zanello LP et al. , Bone cell proliferation on carbon nanotubes, Nano Lett 6 (3) (2006) 562–567. [DOI] [PubMed] [Google Scholar]
- [150].Arshad SN, Naraghi M, Chasiotis I, Strong carbon nanofibers from electrospun polyacrylonitrile, Carbon 49 (5) (2011) 1710–1719. [Google Scholar]
- [151].Zhou Z et al. , Development of carbon nanofibers from aligned electrospun polyacrylonitrile nanofiber bundles and characterization of their microstructural, electrical, and mechanical properties, Polymer 50 (13) (2009) 2999–3006. [Google Scholar]
- [152].Yang Q et al. , Osteocompatibility characterization of polyacrylonitrile carbon nanofibers containing bioactive glass nanoparticles, Carbon 56 (2013) 288–295. [Google Scholar]
- [153].Touri R, et al. , The use of carbon nanotubes to reinforce 45S5 bioglass-based scaffolds for tissue engineering applications. BioMed Res. Int 2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Samadian H et al. , Effective parameters on conductivity of mineralized carbon nanofibers: an investigation using artificial neural networks, RSC Adv 6 (113) (2016) 111908–111918. [Google Scholar]
- [155].Wu M et al. , Biomimetic synthesis and characterization of carbon nanofiber/hydroxyapatite composite scaffolds, Carbon 51 (2013) 335–345. [Google Scholar]
- [156].Wan Y et al. , Preparation and mineralization of three-dimensional carbon nanofibers from bacterial cellulose as potential scaffolds for bone tissue engineering, Surf. Coat. Technol 205 (8–9) (2011) 2938–2946. [Google Scholar]
- [157].Waisi BI, Al-Jubouri SM, McCutcheon JR, Fabrication and characterizations of silica nanoparticle embedded carbon nanofibers, Ind. Eng. Chem. Res 58 (11) (2019) 4462–4467. [Google Scholar]
- [158].Price RL et al. , Nanometer surface roughness increases select osteoblast adhesion on carbon nanofiber compacts, J. Biomed. Mater. Res. Part A 70 (1) (2004) 129–138. [DOI] [PubMed] [Google Scholar]
- [159].Nekounam H et al. , Simple and robust fabrication and characterization of conductive carbonized nanofibers loaded with gold nanoparticles for bone tissue engineering applications, Mater. Sci. Eng., C 117 (2020) 111226. [DOI] [PubMed] [Google Scholar]
- [160].Liu H et al. , The biological properties of carbon nanofibers decorated with β-tricalcium phosphate nanoparticles, Carbon 48 (8) (2010) 2266–2272. [Google Scholar]
- [161].Liu H et al. , β-Tricalcium phosphate nanoparticles adhered carbon nanofibrous membrane for human osteoblasts cell culture, Mater. Lett 64 (6) (2010) 725–728. [Google Scholar]
- [162].Cheng D et al. , Thermal-based regulation on biomineralization and biological properties of bioglass nanoparticles decorated PAN-based carbon nanofibers, RSC Adv 6 (1) (2016) 428–438. [Google Scholar]
- [163].Han B et al. , Improved bioactivity of PAN-based carbon nanofibers decorated with bioglass nanoparticles, J. Biomater. Sci. Polym. Ed 25 (4) (2014) 341–353. [DOI] [PubMed] [Google Scholar]
- [164].Rajzer I et al. , Carbon nanofibers produced from modified electrospun PAN/hydroxyapatite precursors as scaffolds for bone tissue engineering, Mater. Sci. Eng., C 32 (8) (2012) 2562–2569. [Google Scholar]
- [165].Smolka W et al. , Carbon nanofibers coated with silicon/calcium-based compounds for medical application, J. Nanomater 2019 (2019). [Google Scholar]
- [166].Liao CZ et al. , The development, fabrication, and material characterization of polypropylene composites reinforced with carbon nanofiber and hydroxyapatite nanorod hybrid fillers, Int. J. Nanomed 9 (2014) 1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Lee Y-S, Livingston Arinzeh T, Electrospun nanofibrous materials for neural tissue engineering, Polymers 3 (1) (2011) 413–426. [Google Scholar]
- [168].Dong R, et al. , MSC-derived exosomes-based therapy for peripheral nerve injury: a novel therapeutic strategy. BioMed Res. Int 2019 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Shrestha S et al. , Electrodeless coating polypyrrole on chitosan grafted polyurethane with functionalized multiwall carbon nanotubes electrospun scaffold for nerve tissue engineering, Carbon 136 (2018) 430–443. [Google Scholar]
- [170].Isaacs J, Browne T, Overcoming short gaps in peripheral nerve repair: conduits and human acellular nerve allograft, Hand 9 (2) (2014) 131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Kabiri M et al. , Cytocompatibility of a conductive nanofibrous carbon nanotube/poly (L-Lactic acid) composite scaffold intended for nerve tissue engineering, EXCLI J 14 (2015) 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wang ZZ, Sakiyama-Elbert SE, Matrices, scaffolds & carriers for cell delivery in nerve regeneration, Exp. Neurol 319 (2019) 112837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Liu Q et al. , Dual-factor loaded functional silk fibroin scaffolds for peripheral nerve regeneration with the aid of neovascularization, RSC Adv 6 (9) (2016) 7683–7691. [Google Scholar]
- [174].Doblado LR, Martínez-Ramos C, Pradas MM, Biomaterials for neural tissue engineering, Front. Nanotechnol 3 (2021) 21. [Google Scholar]
- [175].Nazeri N, Karimi R, Ghanbari H, The effect of surface modification of polylactide-co-glycolide/carbon nanotube nanofibrous scaffolds by laminin protein on nerve tissue engineering, J. Biomed. Mater. Res. Part A 109 (2) (2021) 159–169. [DOI] [PubMed] [Google Scholar]
- [176].Song S et al. , Additive manufacturing of nerve guidance conduits for regeneration of injured peripheral nerves, Front. Bioeng. Biotechnol 8 (2020) 1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].McKenzie JL et al. , Decreased functions of astrocytes on carbon nanofiber materials, Biomaterials 25 (7–8) (2004) 1309–1317. [DOI] [PubMed] [Google Scholar]
- [178].Farzamfar S et al. , A novel polycaprolactone/carbon nanofiber composite as a conductive neural guidance channel: an in vitro and in vivo study, Prog. Biomater 8 (4) (2019) 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Mirzaei E et al. , The differentiation of human endometrial stem cells into neuron-like cells on electrospun PAN-derived carbon nanofibers with random and aligned topographies, Mol. Neurobiol 53 (7) (2016) 4798–4808. [DOI] [PubMed] [Google Scholar]
- [180].Weaver CM, Peacock M, Skeletal changes across the life span, in: Basic and Applied Bone Biology 2019, Elsevier. p. 189–202. [Google Scholar]
- [181].Shin YC et al. , Nanocomposite scaffolds for myogenesis revisited: Functionalization with carbon nanomaterials and spectroscopic analysis, Appl. Spectrosc. Rev 53 (2–4) (2018) 129–156. [Google Scholar]
- [182].Tenreiro MF et al. , Next generation of heart regenerative therapies: progress and promise of cardiac tissue engineering, NPJ Regener. Med 6 (1) (2021) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Stout DA et al. , Growth characteristics of different heart cells on novel nanopatch substrate during electrical stimulation, Bio-Med. Mater. Eng 24 (6) (2014) 2101–2107. [DOI] [PubMed] [Google Scholar]
- [184].Esrafilzadeh D et al. , High-performance multifunctional graphene-PLGA fibers: Toward biomimetic and conducting 3D scaffolds, Adv. Funct. Mater 26 (18) (2016) 3105–3117. [Google Scholar]
- [185].Abedi A et al. , Concurrent application of conductive biopolymeric chitosan/polyvinyl alcohol/MWCNTs nanofibers, intracellular signaling manipulating molecules and electrical stimulation for more effective cardiac tissue engineering, Mater. Chem. Phys 258 (2021) 123842. [Google Scholar]
- [186].Oftadeh MO et al. , Sequential application of mineralized electroconductive scaffold and electrical stimulation for efficient osteogenesis, J. Biomed. Mater. Res. Part A 106 (5) (2018) 1200–1210. [DOI] [PubMed] [Google Scholar]
- [187].Gopinathan J et al. , Carbon nanofiber amalgamated 3D poly-ε-caprolactone scaffold functionalized porous-nanoarchitectures for human meniscal tissue engineering: in vitro and in vivo biocompatibility studies, Nanomed. Nanotechnol. Biol. Med 14 (7) (2018) 2247–2258. [DOI] [PubMed] [Google Scholar]
- [188].Saito N et al. , Safe clinical use of carbon nanotubes as innovative biomaterials, Chem. Rev 114 (11) (2014) 6040–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Aoki K et al. , A thin carbon-fiber web as a scaffold for bone-tissue regeneration, Small 5 (13) (2009) 1540–1546. [DOI] [PubMed] [Google Scholar]
- [190].Abd El-Aziz A et al. , Preparation and characterization of carbon nanofibrous/hydroxyapatite sheets for bone tissue engineering, Mater. Sci. Eng., C 76 (2017) 1188–1195. [DOI] [PubMed] [Google Scholar]
- [191].Sitharaman B et al. , In vivo biocompatibility of ultra-short single-walled carbon nanotube/biodegradable polymer nanocomposites for bone tissue engineering, Bone 43 (2) (2008) 362–370. [DOI] [PubMed] [Google Scholar]
- [192].Kurapati R et al. , Degradation of single-layer and few-layer graphene by neutrophil myeloperoxidase, Angew. Chem. Int. Ed 57 (36) (2018) 11722–11727. [DOI] [PubMed] [Google Scholar]
- [193].Reina G et al. , Promises, facts and challenges for graphene in biomedical applications, Chem. Soc. Rev 46 (15) (2017) 4400–4416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


