Abstract
Facemasks are important tools for fighting against disease spread, including Covid-19 and its variants, and some may be treated with per- and polyfluoroalkyl substances (PFAS). Nine facemasks over a range of prices were analyzed for total fluorine and PFAS. The PFAS compositions of the masks were then used to estimate exposure and the mass of PFAS discharged to landfill leachate. Fluorine from PFAS accounted only for a small fraction of total fluorine. Homologous series of linear perfluoroalkyl carboxylates and the 6:2 fluorotelomer alcohol indicated a fluorotelomer origin. Inhalation was estimated to be the dominant exposure route (40%–50%), followed by incidental ingestion (15%–40%) and dermal (11%–20%). Exposure and risk estimates were higher for children than adults, and high physical activity substantially increased inhalation exposure. These preliminary findings indicate that wearing masks treated with high levels of PFAS for extended periods of time can be a notable source of exposure and have the potential to pose a health risk. Despite modeled annual disposal of ~29–91 billion masks, and an assuming 100% leaching of individual PFAS into landfill leachate, mask disposal would contribute only an additional 6% of annual PFAS mass loads and less than 11 kg of PFAS discharged to U.S. wastewater.
Keywords: PFAS, facemasks, Covid-19, total fluorine, LC-qTOF, GC-MS, exposure, landfill
Graphical Abstract
INTRODUCTION
Facemasks are important tools to combat the spread of Sars-CoV-2 and its variants and as protection during wildfires.1,2 Types of facemasks range from homemade to medical grade masks.3,4 Characterizations of facemasks reveal the presence of chemicals including hydrocarbons,5,6 phthalates,7,8 organophosphate ester compounds,9,10 amides, paraffins, olefins, polyethylene terephthalate oligomers,6 and microplastics.11
Facemasks are designed to not only prevent inhalation of particles or pathogens but also to repel fluids (e.g., bodily).4 The repellency factor indicates the potential presence of perand polyfluoroalkyl substances (PFAS), which are known components of specialty, water-repellant fabrics, such as firefighter turnout gear, jackets,12–14 and surgical gowns.15 While there are numerous reports of PFAS in consumer products,16–19 except for a news story on unnamed PFAS and a cross-linker used in textile treated with PFAS in facemasks,20 there is no published information on the presence of PFAS in facemasks. Facemasks treated with PFAS have the potential to act as sources of human exposure to PFAS from dermal absorption, inhalation of gas-phase PFAS, and ingestion of particulate-phase PFAS. While estimates showed low daily intakes and inhalation risk of organophosphate ester and phthalates due to wearing facemasks,7,9,10 there is no exposure assessment for PFAS in facemasks yet to our knowledge.
Facemasks, particularly single-use surgical masks, are ultimately disposed to landfills or combusted in incineration facilities. Estimates of mask disposal presented below are based on consumer use, although an unknown fraction of these masks is used in hospitals and are burned in dedicated medical-waste incinerators. The U.S. EPA estimates that about 50% of all municipal solid waste (MSW) is landfilled and about 12% is combusted, with the balance treated biologically or recycled.21 While the overall MSW recycling rate is 24%, we are not aware of any recycling of single-use facemasks.21 The presence of PFAS in landfill leachate is well documented and results from PFAS release as water infiltrates through PFAS-treated consumer products disposed in landfills.16−19,22 On the basis of a recent survey, ~1.1 billion facemask wastes were generated per week in the U.S.23 However, the mass of PFAS released to landfill leachate as a result of facemask disposal has yet to be characterized.
The objective of this study was to characterize PFAS associated with different types of facemasks. Nine masks were collected and characterized for their total fluorine using particle-induced gamma emission (PIGE)16,24 and for nonvolatile and volatile PFAS by liquid chromatography quadrupole time-of-flight mass spectrometry (LC-qTOF) and gas chromatography MS (GC-MS), respectively. On the basis of the PFAS analysis, exposure and environmental implications are discussed. It is important to emphasize that this study does not discourage the public from wearing facemasks, particularly during an active pandemic. Rather, the study results are intended to aid the public in making informed decisions regarding the types of facemasks to wear and to encourage manufacturers to consider the chemicals that are incorporated into facemasks.
MATERIALS AND METHODS
Materials.
Water and solvents for analyses are listed in the Supporting Information (SI). Target chemicals and surrogate standards are provided in Tables S1−S3.
Samples.
Nine facemasks were collected and manually separated into their respective layers, if composed of multiple layers. The material composition was based on information provided on the website of the facemasks (see SI). Packaging for two samples, RC-4 and RC-5, indicated stain resistant chemical. There were four types of facemasks: a surgical, single-use disposable mask (SUD); an N95 mask (N95); six reusable cloth masks (RC-1 to RC-6); and a specialty mask advertised to firefighters (FF) (Table 1). No homemade cloth facemasks were collected.
Table 1.
Facemasks by Type, Price per Unit, Total Fluorine, and Summed Concentrations of Nonvolatile and Volatile PFASa
| Type of facemaskb | Price per unitc | Total fluorine (nmol F/cm2)d | Nonvolatile PFAS (nmol F/cm2) | Volatile PFAS (nmol F/cm2)e |
|---|---|---|---|---|
| SUD | $ | <LOD | 0.0016 ± 0.00043 | <LOD |
| N95 | $ | <LOD | 0.00054 | <LOD |
| RC-1 | $ | <LOD | 0.0048f | <LOD |
| RC-2 | $ $ $ | 7100h | 0.0050f | 0.079h |
| RC-3 | $ | <LOD | 0.0055 | <LOD |
| RC-4 | $ | 7600h | 0.010f | 0.85g |
| RC-5 | $ $ | 17,000f | 0.0063f | 1.2f |
| RC-6 | $ $ | 40,000 ± 18,000 | 0.024 ± 0.0041 | 0.90 ± 0.057 |
| FF | $ $ $ $ | 640f | 0.042f | 4.7f |
Samples SUD and RC-6 were analyzed in triplicates, and the data are provided as average ± standard error.
SUD = surgical single-use disposable; N95 = N95; RC = reusable cloth facemasks; FF = facemask advertised to firefighters.
Price per unit (US dollars): $ = <1−14; $ $ = 14−28; $ $ $ = 28–42; $ $ $ $ = 42–56, as of Dec 2021.
LOD = limit of detection (6.8 nmol F/cm2); LOQ = limit of quantification (20 nmol F/cm2).
LOD = 0.00027–0.047 nmol F/cm2.
Total fluorine or PFAS was measured in all three layers.
Total fluorine or PFAS was measured in two of three layers.
Total fluorine or PFAS was measured in one of three layers.
Total Fluorine Analysis.
Samples were analyzed for total fluorine using PIGE,12 with fluorine signal normalized to Ar.25 See SI for details on PIGE analysis, including the conversion to nmol F/cm2 and method limit of detection (LOD) and limit of quantitation (LOQ).
PFAS Analysis.
Extraction and analysis of PFAS were performed using the method described in Muensterman et al.14 with further details given in the SI. Briefly, facemasks were cut into pieces using methanol-rinsed scissors. Nonvolatile PFAS were determined by spiking the textiles with 31 mass-labeled surrogate standards and extracting with methanol. Nonvolatile PFAS extracts were spiked with two mass-labeled internal standards and analyzed for 50 target and 4886 suspect nonvolatile PFAS by LC-qTOF. For volatile PFAS, methanol was added to samples and spiked with 10 surrogate standards, followed by sonication at ambient temperature. Extracts were cleaned using solid phase extraction, spiked with a mass-labeled internal standard, and analyzed for 15 target and 24 suspect volatile PFAS by GC-MS. Whole method LOD and LOQ were determined using a previous method,26 while accounting for potential false positives arising from addition of volatile PFAS surrogate standards.27 See SI and Tables S4–S7 for additional details on extraction methods, analyses, method performance, LODs, and LOQs.
PFAS Exposure Estimation.
Exposure estimates for children (2 years old) and adults (women and men, 18 years old) were based on the total PFAS concentration for each mask type, assuming 10 h of wear time per day, via inhalation, dermal contact, and incidental ingestion routes. The 10-h exposure duration was selected based on time spent at daycare (children), work (adults), and in public where facemask wearing may be mandated or chosen. Exposure modeling for the inhalation and incidental ingestion exposure routes was preformed using ConsExpo,28 an online tool developed by the Danish National Institute for Public Health and the Environment. Model inputs are provided in Table S8. Modeling was performed on SUD, RC-6, and FF as representative facemasks. Because the FF mask had three layers and PFAS can potentially migrate through fabric, the maximum value for each PFAS across the layers was applied, and the PFAS concentrations were summed. The reference dose was selected a priori for 6:2 fluorotelomer alcohol (FTOH) from the Danish Ministry of the Environment, which identified a no observed adverse effect level of 5000 μg/kg-bw/day for male rats.29 A reference dose of 5 μg/kg-day was derived by applying a safety factor of 1000 to account for the conversion from animals to humans, human variation in sensitivity, and conversion from subchronic to chronic exposure.30 The reference dose was chosen for a single PFAS (6:2 FTOH) to reduce complexity for this preliminary assessment because it was detected at the highest concentration in the facemasks.
Estimate of PFAS Mass Release to Leachates.
The methanol-extractable PFAS content of the various masks was used to estimate a range of potential PFAS release to landfill leachate. Two cases were evaluated, a “likely case” and an “extreme case” (Table S9). In the “likely case”, 60% of the U.S. population was assumed to wear facemasks. Also, the mask disposal rate is 3.6 facemasks per week,23 and 1% of the methanol-extractable PFAS is released to leachate. The corresponding values for the “extreme case” are 100% facemask use, seven facemasks disposed per week, and 100% of the methanol-extractable PFAS released to leachate. In both cases, children under five, comprising 6% of the U.S. population,31 are assumed to not wear facemasks. Given the absence of recycling, 81% of facemasks are estimated to be disposed in landfills. The annual leachate volume was estimated to be 61.5 million m3.18
RESULTS AND DISCUSSION
Total Fluorine.
Total fluorine was quantifiable in five of nine facemasks and ranged from less than LOD to 40,000 nmol F/cm2 (Table 1). In facemasks with multiple layers (RC-2, RC-4, RC-5, and FF), total fluorine was the highest in the outer layer (Table S10). The least expensive facemasks (SUD and N95) were less than LOD (Table 1 and Table S10). The most expensive facemask (FF) gave the lowest total fluorine among facemasks with quantifiable levels of total fluorine. Total fluorine in this study was among the highest measured in consumer products including textiles, papers, cosmetics, and food packaging.16,32−37 No correlation was observed between the price of facemasks and total fluorine, total fluorine and PFAS, or the price of facemasks and PFAS. The contribution of PFAS to total fluorine was insignificant (Table 1) and consistent with other measurements for textiles.14,32 The gap in fluorine between total fluorine and PFAS from facemask to facemask is likely due to the presence of fluoropolymers, such as side-chain fluoropolymers,38 in some of these facemasks.
PFAS in Facemasks.
Nonvolatile PFAS were found in all facemasks, and volatile PFAS were found in five facemasks. Summed PFAS concentrations ranged from 15 to 2900 μg/m2 (Figure 1). The SUD and N95 masks gave the lowest measured total PFAS, with FF the highest total PFAS (Figure 1), yet the total fluorine of FF was the lowest among the facemasks (Table 1). The frequency of detection among the RC masks were similar (Figure 1). Detailed PFAS data per layer are provided in the Tables S11 and S12.
Figure 1.
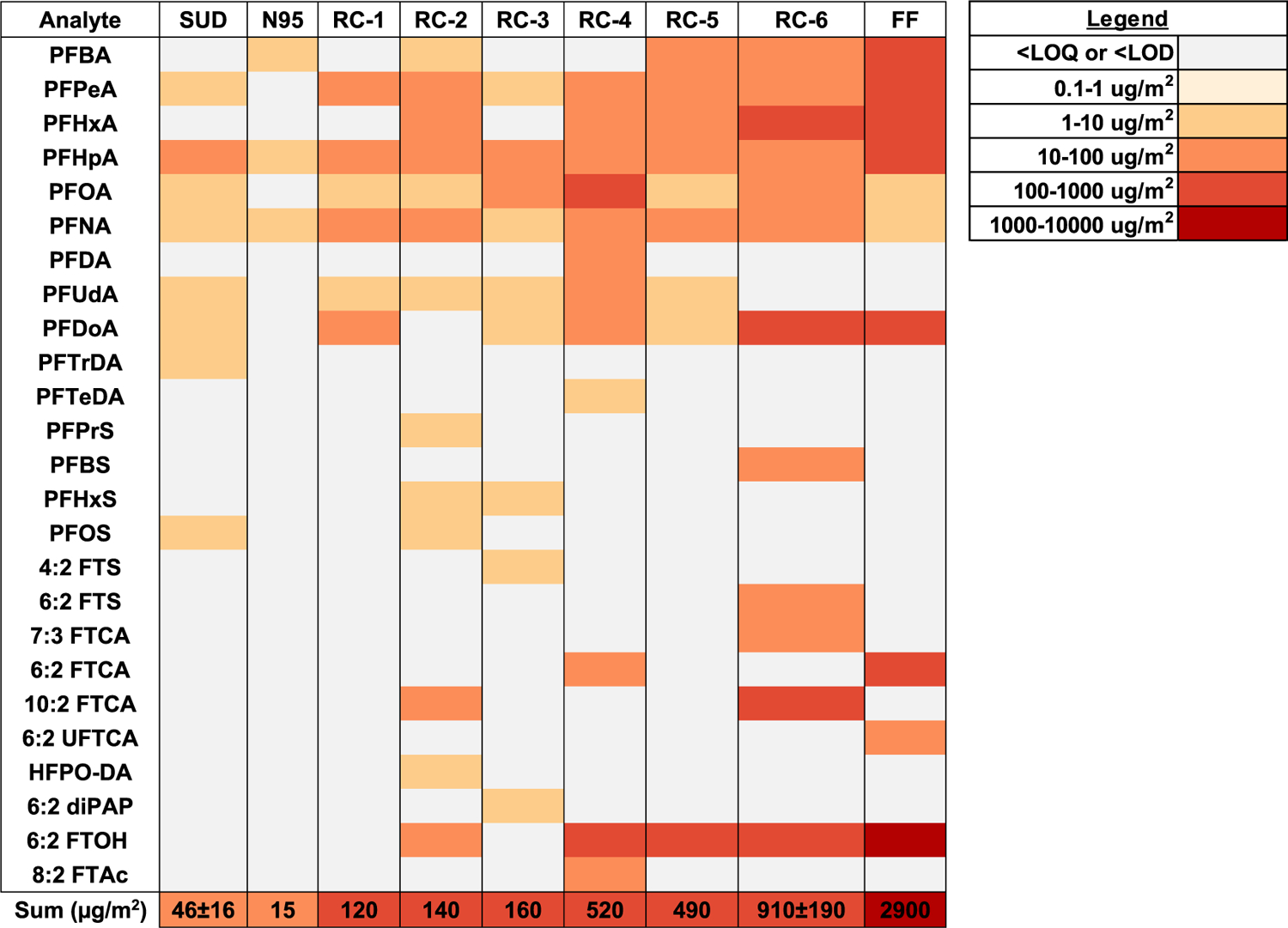
Heat map of nonvolatile and volatile PFAS (μg/m2) in facemasks. (Only PFAS with at least one greater than LOQ concentration was included.) Layer concentration summed for samples with multiple layers (RC-1, RC-2, RC-4, RC-5, and FF). Average concentrations ± standard error for n = 3 replicates of SUD and RC-6. A similar heat map in units of ng/g is provided in the Figure S1.
Of the nonvolatile PFAS, perfluoroalkyl carboxylates (PFCAs) gave the highest detection frequency, followed by fluorotelomer-based PFAS, and perfluoroalkyl sulfonates (PFSAs) (Figure 1, Figure S2, Table S11). The highest summed PFCAs on facemasks were generally similar or greater than those reported for other various textiles and car seats14,32,39–45 but lower than that of outdoor clothes and treated textiles,45,46 while PFSAs were less than those that included older (1988) textiles32,44 or were in the same range as that of outdoor clothing, furniture textiles, and jackets.39,40 The C5 and C6 PFCAs (e.g., PFHxA, PFHpA, respectively) were detected at the highest concentration, with the exception of C11 in RC-6 (PFDoA = 140 μg/m2, Table S11). Members of the PFCAs appeared as homologous series, and only the linear isomers were detected (Table S11), indicating a fluorotelomer origin. In RC-6, the concentration of 10:2 FTCA (110 μg/m2) was at comparable levels with PFHxA and PFDoA. Members of the PFSAs were infrequently detected and not as a homologous series (Table S11, Figure S2). Nonvolatile PFAS suspect screening revealed tentative identification of only three PFAS (Table S13).
Of the volatile PFAS, 6:2 FTOH was found on nine layers associated with five facemasks, while 8:2 FTAc was found in one layer (Figure 1, Table S12). The highest 6:2 FTOH concentration was for FF-O (1200 μg/m2, Table S12), and the 6:2 FTOH concentration exceeded those of individual nonvolatile PFAS (Table S11). The concentrations of FTOHs in the samples were generally similar to levels found in outdoor jackets and treated textiles,19,32,39,41,44–46 lower than that of older (1988) jackets and firefighter turnout gear,14,19,32 and higher than that of car seats and household linens.40,42 The PFAS found in the facemasks could originate from sources such as PFAS-impacted water used in manufacturing, PFAS in components to maintain or operate machinery,47 or as a result of intentional addition of side-chain fluoropolymers.13,42,48 If repellency characteristics are needed in facemasks, PFAS alternatives such as silicone- and hyperbranched polymers-based repellant and hydrocarbon-based wax may be considered.49,50
Preliminary Estimates of Human Exposure and Risk.
Inhalation, based on the octanol−air partition coefficient of 6:2 FTOH, was estimated to be the dominant exposure route, accounting for over 40% (children) and 50% (adults) of total median exposure to PFAS in facemasks (Table 2). Of the total exposure related to facemasks, incidental ingestion accounted for over 40% (children), 15% (women), and 30% (men). The lowest exposures were for the dermal route, which accounted for 11%–20% of total exposure to PFAS from facemasks. High physical activity increased inhalation exposure estimates to over 70% (children), 700% (women), and 400% (men) more than the summed ingestion and dermal exposure routes. Total estimated exposures exceeded the reference dose for 6:2 FTOH of 5 μg/kg-day for a child wearing the FF mask at moderate physical activity level and via the inhalation exposure route for both children and adults wearing it at a high physical activity level for an extended period of time. These preliminary findings indicate that wearing masks treated with high levels of PFAS for extended periods of time can be a notable source of exposure and have the potential to pose a health risk.
Table 2.
Estimated Exposure (μg/kg-day) Wearing a Facemask for 10 h/daya
| Inhalation | ||||||
|---|---|---|---|---|---|---|
| Population | Mask sample | Moderate activity High activity | Incidental ingestion | Dermal | Total exposureb | |
| Median Exposure Estimates | ||||||
| Childc | SUD | 0.10 (0.02) | 0.24 (0.05) | 0.10 (0.02) | 0.04 (0.01) | 0.23 (0.05) |
| RC-6 | 1.10 (0.22) | 2.70 (0.54) | 1.20 (0.24) | 0.51 (0.10) | 2.81 (0.56) | |
| FF | 2.90 (0.58) | 7.20 (1.44) | 3.00 (0.60) | 1.14 (0.23) | 7.04 (1.41) | |
| Womand | SUD | 0.04 (0.01) | 0.13 (0.03) | 0.03 (0.01) | 0.01 (0.002) | 0.08 (0.02) |
| RC-6 | 0.48 (0.10) | 1.50 (0.30) | 0.11 (0.02) | 0.15 (0.03) | 0.74 (0.15) | |
| FF | 1.20 (0.24) | 3.40 (0.68) | 0.29 (0.06) | 0.33 (0.07) | 1.82 (0.36) | |
| Mand | SUD | 0.05 (0.01) | 0.14 (0.03) | 0.03 (0.01) | 0.01 (0.002) | 0.09 (0.02) |
| RC-6 | 0.54 (0.11) | 1.50 (0.30) | 0.30 (0.06) | 0.13 (0.03) | 0.97 (0.19) | |
| FF | 1.40 (0.28) | 4.00 (0.80) | 0.79 (0.16) | 0.30 (0.06) | 2.49 (0.50) | |
| 99th Percentile Exposure Estimates | ||||||
| Childc | SUD | 0.13 (0.03) | 0.34 (0.07) | 0.14 (0.03) | 0.05 (0.01) | 0.32 (0.06) |
| RC-6 | 1.50 (0.30) | 3.80 (0.76) | 1.60 (0.32) | 0.65 (0.13) | 3.75 (0.75) | |
| FF | 3.90 (0.78) | 10.0 (2.00) | 4.10 (0.82) | 1.45 (0.29) | 9.45 (1.89) | |
| Womand | SUD | 0.10 (0.02) | 0.27 (0.05) | 0.16 (0.03) | 0.01 (0.003) | 0.27 (0.05) |
| RC-6 | 1.10 (0.22) | 3.30 (0.66) | 0.25 (0.05) | 0.21 (0.04) | 1.56 (0.31) | |
| FF | 2.80 (0.56) | 7.50 (1.50) | 0.65 (0.13) | 0.46 (0.09) | 3.91 (0.78) | |
| Mand | SUD | 0.10 (0.02) | 0.28 (0.06) | 0.06 (0.01) | 0.01 (0.003) | 0.17 (0.03) |
| RC-6 | 1.10 (0.22) | 3.10 (0.62) | 0.60 (0.12) | 0.18 (0.04) | 1.88 (0.38) | |
| FF | 2.90 (0.58) | 8.10 (1.62) | 1.60 (0.32) | 0.41 (0.08) | 4.91 (0.98) | |
The hazard index (unitless) is provided in parentheses, and bold values indicate exceedance of the reference dose (5 μg/kg-day). Median and 99th percentiles reflect population body weight variability.
With moderate activity level.
2 to <3 years old.
18 to <19 years.
The exposure models are based on assumptions that are sources of uncertainty. Concentrations of PFAS were based on results of the methanol extraction, which may underestimate PFAS, such as FTOHs, released from side-chain fluoropolymers51,52 and thus may underestimate exposure, but methanol-extractable concentrations may be a better estimate for the bioavailable fraction. Another assumption that contributed to a possible exposure underestimation is the room volume, which was required to be a minimum of 0.001 m3, when the estimated volume of air between the mask and face was an order of magnitude lower (~0.0002 m3). The ventilation rate is the number of total air exchanges per hour, which was assumed to occur every 10 breaths based on 30 breaths/min for children and 20 breaths/min for adults. A textile−air partition coefficient was not available for 6:2 FTOH; therefore, the octanol−air partition coefficient from EpiSuite as reported by ChemSpider53 was utilized. Exposure scenarios were chosen to represent realistic exposure for children and adults wearing facemasks at school and work, so the scenario overestimates exposure for those who wear one for fewer hours per day. Finally, 100% absorption for each exposure route was assumed, which may overestimate exposure for some of the PFAS.
Landfill and Wastewater Environment Implications.
In the “likely case”, it is estimated that ~28 billion masks will be disposed in U.S. landfills annually while the pandemic persists and will result in ~0.11 kg PFAS/year released to leachate. About 90% of the PFAS release to landfill leachate due pandemic-related mask use can be attributed to SUD, with 10% to N95 masks. This PFAS mass release is insignificant relative to the 600 kg PFAS/year that was estimated to be released in U.S. landfill leachate as collected for wastewater treatment in 2017.18 In the “extreme case”, ~91 billion masks will be disposed with an estimated 37 kg PFAS/year released to leachate. The “extreme case” is an upper bound of PFAS released from landfills to leachate as it assumes 100% of methanol-extractable PFAS is released to leachate. This value is uncertain as there is not a good understanding of the relationship between methanol-extractable PFAS and the release of PFAS in solid waste to leachate under landfill-relevant conditions. While the same PFAS measured in facemasks were measured in landfill leachates prior to the Covid-19 pandemic,18 even in the “extreme case” the mass of PFAS release is only 6% of the current estimated total mass of PFAS mass released as landfill leachate. Thus, the concentration of PFAS in leachate is not predicted to be significantly impacted by facemask disposal. The model assumed no degradation of precursors, such as side-chain fluoropolymers,42,49 which are known to degrade to nonvolatile PFAS.54–56 The FF facemask was not included in the calculations because their use is limited. The RC facemasks were not included in the landfill mass release calculations since these facemasks are designed for reuse. Assuming an RC facemask lifetime of one year, it is estimated that 0.7–2.2 billion RC facemasks will be used annually in the “likely case” and “extreme case” scenarios. However, laundering of RC facemasks will likely transfer the PFAS to municipal waste-water,57,58 resulting in an estimated 0.04 (“likely case”) to 11 kg (“extreme case”) PFAS input to wastewater treatment plants.
Supplementary Material
ACKNOWLEDGMENTS
Support for M.A.B. and F.B.D.C. is provided, in part, by the North Carolina Policy Collaboratory. Support for C.C.C. is provided, in part, by Grant R01ES028311 from the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH), by Grants 84025201 and R839482 from the U.S. Environmental Protection Agency (EPA), and by Grant MICL02565 from the National Institute of Food and Agriculture (NIFA) of the U.S. Department of Agriculture (USDA). Support for G.F.P. is provided, in part, by Grant PHYS-2011890 (Nuclear Science Laboratory) from the National Science Foundation (NSF). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the North Carolina Policy Collaboratory, NIEHS, NIH, EPA, NIFA, USDA, and NSF. Oregon State University in Corvallis, Oregon, is located within the traditional homelands of the Mary’s River or Ampinefu Band of Kalapuya. Following the Willamette Valley Treaty of 1855, Kalapuya people were forcibly removed to reservations in Western Oregon. Today, living descendants of these people are a part of the Confederated Tribes of Grand Ronde Community of Oregon (grandronde.org) and the Confederated Tribes of the Siletz Indians (ctsi.nsn.us).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.estlett.2c00019
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.estlett.2c00019.
Detailed experimental methods; list of target and suspect PFAS analytes; method precision, accuracy, LOD, and LOQ of target PFAS analytes; surrogate standard recoveries; target analyte concentrations; model parameters for landfill leachate and exposure estimations; results from exposure estimations; and results from suspect screening of nonvolatile PFAS (PDF)
The authors declare no competing financial interest.
Contributor Information
Derek J. Muensterman, Department of Chemistry, Oregon State University, Corvallis, Oregon 97331, United States.
Liliana Cahuas, Department of Chemistry, Oregon State University, Corvallis, Oregon 97331, United States.
Ivan A. Titaley, Department of Environmental and Molecular Toxicology, Oregon State University, Corvallis, Oregon 97331, United States.
Christopher Schmokel, Department of Chemistry, Oregon State University, Corvallis, Oregon 97331, United States.
Florentino B. De la Cruz, Department of Civil, Construction, and Environmental Engineering, North Carolina State University, Raleigh, North Carolina 27695-7908, United States
Morton A. Barlaz, Department of Civil, Construction, and Environmental Engineering, North Carolina State University, Raleigh, North Carolina 27695-7908, United States
Courtney C. Carignan, Department of Food Science and Human Nutrition and Department of Pharmacology and Toxicology, Michigan State University, East Lansing, Michigan 48824, United States
Graham F. Peaslee, Department of Physics, University of Notre Dame, Notre Dame, Indiana 46556, United States
Jennifer A. Field, Department of Environmental and Molecular Toxicology, Oregon State University, Corvallis, Oregon 97331, United States
REFERENCES
- (1).Santana FN; Fischer SL; Jaeger MO; Wong-Parodi G Responding to Simultaneous Crises: Communications and Social Norms of Mask Behavior during Wildfires and COVID-19. Environ. Res. Lett 2020, 15 (11), 111002. [Google Scholar]
- (2).Stone SL; Sacks J; Lahm P; Clune A; Quinn T; D’Alessandro M; Radonovich L; Hutson M; Mirabelli M Wildfire Smoke: A Guide for Public Health Officials; EPA-452/R-21–901; California Air Resources Board, California Office of Environmental Health Hazard Assessment, U.S. Centers for Disease Control and Prevention, U.S. Forest Service, U.S. Environmental Protection Agency, 2021; p 88. [Google Scholar]
- (3).O’Dowd K; Nair KM; Forouzandeh P; Mathew S; Grant J; Moran R; Bartlett J; Bird J; Pillai SC Face Masks and Respirators in the Fight Against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13 (15), 3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chua MH; Cheng W; Goh SS; Kong J; Li B; Lim JYC; Mao L; Wang S; Xue K; Yang L; Ye E; Zhang K; Cheong WCD; Tan BH; Li Z; Tan BH; Loh XJ Face Masks in the New COVID-19 Normal: Materials, Testing, and Perspectives. Research 2020, 2020, 7286735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Jung S; Lee S; Dou X; Kwon EE Valorization of Disposable COVID-19 Mask through the Thermo-Chemical Process. Chem. Eng. J 2021, 405, 126658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Favela KA; Hartnett MJ; Janssen JA; Vickers DW; Schaub AJ; Spidle HA; Pickens KS Nontargeted Analysis of Face Masks: Comparison of Manual Curation to Automated GCxGC Processing Tools. J. Am. Soc. Mass Spectrom 2021, 32 (4), 860–871. [DOI] [PubMed] [Google Scholar]
- (7).Wang X; Okoffo ED; Banks AP; Li Y; Thomas KV; Rauert C; Aylward LL; Mueller JF Phthalate Esters in Face Masks and Associated Inhalation Exposure Risk. J. Hazard. Mater 2022, 423, 127001. [DOI] [PubMed] [Google Scholar]
- (8).Min K; Weng X; Long P; Ma M; Chen B; Yao S Rapid In-Situ Analysis of Phthalates in Face Masks by Desorption Corona Beam Ionization Tandem Mass Spectrometry. Talanta 2021, 231, 122359. [DOI] [PubMed] [Google Scholar]
- (9).Fernández-Arribas J; Moreno T; Bartrolí R; Eljarrat E COVID-19 Face Masks: A New Source of Human and Environmental Exposure to Organophosphate Esters. Environ. Int 2021, 154, 106654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Liu R; Mabury SA Single-Use Face Masks as a Potential Source of Synthetic Antioxidants to the Environment. Environ. Sci. Technol. Lett 2021, 8 (8), 651–655. [Google Scholar]
- (11).Li L; Zhao X; Li Z; Song K COVID-19: Performance Study of Microplastic Inhalation Risk Posed by Wearing Masks. J. Hazard. Mater 2021, 411, 124955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Peaslee GF; Wilkinson JT; McGuinness SR; Tighe M; Caterisano N; Lee S; Gonzales A; Roddy M; Mills S; Mitchell K Another Pathway for Firefighter Exposure to Per- and Polyfluoroalkyl Substances: Firefighter Textiles. Environ. Sci. Technol. Lett 2020, 7 (8), 594–599. [Google Scholar]
- (13).Holmquist H; Schellenberger S; van der Veen I; Peters GM; Leonards PEG; Cousins IT Properties, Performance and Associated Hazards of State-of-the-Art Durable Water Repellent (DWR) Chemistry for Textile Finishing. Environ. Int 2016, 91, 251–264. [DOI] [PubMed] [Google Scholar]
- (14).Muensterman DJ; Titaley IA; Peaslee GF; Minc LD; Cahuas L; Rodowa AE; Horiuchi Y; Yamane S; Fouquet TNJ; Kissel JC; Carignan CC; Field JA Disposition of Fluorine on New Firefighter Turnout Gear. Environ. Sci. Technol 2022, 56 (2), 974–983. [DOI] [PubMed] [Google Scholar]
- (15).Liu X; Guo Z; Folk EE; Roache NF Determination of Fluorotelomer Alcohols in Selected Consumer Products and Preliminary Investigation of Their Fate in the Indoor Environment. Chemosphere 2015, 129, 81–86. [DOI] [PubMed] [Google Scholar]
- (16).Ritter EE; Dickinson ME; Harron JP; Lunderberg DM; DeYoung PA; Robel AE; Field JA; Peaslee GF PIGE as a Screening Tool for Per- and Polyfluorinated Substances in Papers and Textiles. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At 2017, 407, 47–54. [Google Scholar]
- (17).Kim M; Li LY; Grace JR; Benskin JP; Ikonomou MG Compositional Effects on Leaching of Stain-Guarded (Perfluoroalkyl and Polyfluoroalkyl Substance-Treated) Carpet in Landfill Leachate. Environ. Sci. Technol 2015, 49 (11), 6564–6573. [DOI] [PubMed] [Google Scholar]
- (18).Lang JR; Allred BM; Field JA; Levis JW; Barlaz MA National Estimate of Per- and Polyfluoroalkyl Substance (PFAS) Release to U.S. Municipal Landfill Leachate. Environ. Sci. Technol 2017, 51 (4), 2197–2205. [DOI] [PubMed] [Google Scholar]
- (19).Rewerts JN; Morré JT; Massey Simonich SL; Field JA In-Vial Extraction Large Volume Gas Chromatography Mass Spectrometry for Analysis of Volatile PFASs on Papers and Textiles. Environ. Sci. Technol 2018, 52 (18), 10609–10616. [DOI] [PubMed] [Google Scholar]
- (20).Mowbray J Exclusive: Chemical cocktail found in face masks, April 1, 2021. Ecotextile News. https://www.ecotextile.com/2021040127603/dyes-chemicals-news/exclusive-chemical-cocktail-found-in-face-masks.html (accessed 2021−07 −22).
- (21).Advancing Sustainable Materials Management: 2018 Fact Sheet; Facts and Figures about Materials, Waste and Recycling; EPA 530-F-20–009; United States Environmental Protection Agency: Washington, DC, 2020; p 25. [Google Scholar]
- (22).Lang JR; Allred BM; Peaslee GF; Field JA; Barlaz MA Release of Per- and Polyfluoroalkyl Substances (PFASs) from Carpet and Clothing in Model Anaerobic Landfill Reactors. Environ. Sci. Technol 2016, 50 (10), 5024–5032. [DOI] [PubMed] [Google Scholar]
- (23).Selvaranjan K; Navaratnam S; Rajeev P; Ravintherakumaran N Environmental Challenges Induced by Extensive Use of Face Masks during COVID-19: A Review and Potential Solutions. Environ. Chall 2021, 3, 100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rodowa AE; Christie E; Sedlak J; Peaslee GF; Bogdan D; DiGuiseppi B; Field JA Field Sampling Materials Unlikely Source of Contamination for Perfluoroalkyl and Polyfluoroalkyl Substances in Field Samples. Environ. Sci. Technol. Lett 2020, 7 (3), 156–163. [Google Scholar]
- (25).Wilkinson JT; McGuinness SR; Peaslee GF External Beam Normalization Measurements Using Atmospheric Argon Gamma Rays. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At 2020, 484, 1–4. [Google Scholar]
- (26).Vial J; Jardy A Experimental Comparison of the Different Approaches To Estimate LOD and LOQ of an HPLC Method. Anal. Chem 1999, 71 (14), 2672–2677. [Google Scholar]
- (27).Cahuas L; Titaley IA; Field JA Mass-Labeled Fluorotelomer Alcohol Fragmentation Gives “False Positive” for Nonlabeled Fluorotelomer Alcohols with Implications for Consumer Product Analysis. J. Am. Soc. Mass Spectrom 2022, 33 (2), 399–403. [DOI] [PubMed] [Google Scholar]
- (28).Delmaar JE; Schuur AG ConsExpo Web. Consumer Exposure Models - Model Documentation; RIVM Report 2017–0197; National Institute for Public Health and the Environment, 2017. DOI: 10.21945/RIVM-2017-0197 [DOI] [Google Scholar]
- (29).Kjølholt J; Jensen AA; Warming M Short-Chain Polyfluoroalkyl Substances (PFAS). A Literature Review of Information on Human Health Effects and Environmental Fate and Effect Aspects of Short-Chain PFAS; Environmental project 1707; The Danish Environmental Protection Agency: Copenhagen, Denmark, 2015. [Google Scholar]
- (30).Reference Dose (RfD): Description and Use in Health Risk Assessments. United States Environmental Protection Agency. https://www.epa.gov/iris/reference-dose-rfd-description-and-use-health-risk-assessments (accessed 2021−12 −27).
- (31).QuickFacts: United States. U.S. Census Bureau. https://www.census.gov/quickfacts/fact/table/US/PST045219 (accessed 2021− 12 −17).
- (32).Robel AE; Marshall K; Dickinson M; Lunderberg D; Butt C; Peaslee G; Stapleton HM; Field JA Closing the Mass Balance on Fluorine on Papers and Textiles. Environ. Sci. Technol 2017, 51 (16), 9022–9032. [DOI] [PubMed] [Google Scholar]
- (33).Whitehead HD; Venier M; Wu Y; Eastman E; Urbanik S; Diamond ML; Shalin A; Schwartz-Narbonne H; Bruton TA; Blum A; Wang Z; Green M; Tighe M; Wilkinson JT; McGuinness S; Peaslee GF Fluorinated Compounds in North American Cosmetics. Environ. Sci. Technol. Lett 2021, 8 (7), 538–544. [Google Scholar]
- (34).Schultes L; Peaslee GF; Brockman JD; Majumdar A; McGuinness SR; Wilkinson JT; Sandblom O; Ngwenyama RA; Benskin JP Total Fluorine Measurements in Food Packaging: How Do Current Methods Perform? Environ. Sci. Technol. Lett 2019, 6 (2), 73–78. [Google Scholar]
- (35).Schultes L; Vestergren R; Volkova K; Westberg E; Jacobson T; Benskin JP Per- and Polyfluoroalkyl Substances and Fluorine Mass Balance in Cosmetic Products from the Swedish Market: Implications for Environmental Emissions and Human Exposure. Environ. Sci. Process. Impacts 2018, 20 (12), 1680–1690. [DOI] [PubMed] [Google Scholar]
- (36).Schaider LA; Balan SA; Blum A; Andrews DQ; Strynar MJ; Dickinson ME; Lunderberg DM; Lang JR; Peaslee GF Fluorinated Compounds in U.S. Fast Food Packaging. Environ. Sci. Technol. Lett 2017, 4 (3), 105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Manso AR; McIntosh AB; Peaslee GF; Gauthier J; Hagel K; Heilborn L; Jedele A; McCarthy M; Pajouhafsar Y; Salas E; Zarrella A; Wakhle A; Yennello S Implementing PIXE and PIGE at the Texas A&M University Cyclotron Institute. AIP Conf. Proc 2018, 2160 (1), 050023. [Google Scholar]
- (38).Henry BJ; Carlin JP; Hammerschmidt JA; Buck RC; Buxton LW; Fiedler H; Seed J; Hernandez O A Critical Review of the Application of Polymer of Low Concern and Regulatory Criteria to Fluoropolymers. Integr. Environ. Assess. Manag 2018, 14 (3), 316–334. [DOI] [PubMed] [Google Scholar]
- (39).van der Veen I; Hanning A-C; Stare A; Leonards PEG; de Boer J; Weiss JM The Effect of Weathering on Per- and Polyfluoroalkyl Substances (PFASs) from Durable Water Repellent (DWR) Clothing. Chemosphere 2020, 249, 126100. [DOI] [PubMed] [Google Scholar]
- (40).Vestergren R; Herzke D; Wang T; Cousins IT Are Imported Consumer Products an Important Diffuse Source of PFASs to the Norwegian Environment? Environ. Pollut 2015, 198, 223–230. [DOI] [PubMed] [Google Scholar]
- (41).Gremmel C; Frömel T; Knepper TP Systematic Determination of Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Outdoor Jackets. Chemosphere 2016, 160, 173–180. [DOI] [PubMed] [Google Scholar]
- (42).Wu Y; Miller GZ; Gearhart J; Peaslee G; Venier M Side-Chain Fluorotelomer Based Polymers in Children Car Seats. Environ. Pollut 2021, 268, 115477. [DOI] [PubMed] [Google Scholar]
- (43).Washburn ST; Bingman TS; Braithwaite SK; Buck RC; Buxton LW; Clewell HJ; Haroun LA; Kester JE; Rickard RW; Shipp AM Exposure Assessment and Risk Characterization for Perfluorooctanoate in Selected Consumer Articles. Environ. Sci. Technol 2005, 39 (11), 3904–3910. [DOI] [PubMed] [Google Scholar]
- (44).Herzke D; Olsson E; Posner S Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Consumer Products in Norway − A Pilot Study. Chemosphere 2012, 88 (8), 980–987. [DOI] [PubMed] [Google Scholar]
- (45).Berger U; Herzke D Per- and Polyfluorinated Alkyl Substances (PFAS) Extracted from Textile Samples. Organohalogen Compd. 2006, 68, 2023–2026. [Google Scholar]
- (46).Liu X; Guo Z; Krebs KA; Pope RH; Roache NF Concentrations and Trends of Perfluorinated Chemicals in Potential Indoor Sources from 2007 through 2011 in the US. Chemosphere 2014, 98, 51–57. [DOI] [PubMed] [Google Scholar]
- (47).Glüge J; Scheringer M; Cousins IT; DeWitt JC; Goldenman G; Herzke D; Lohmann R; Ng CA; Trier X; Wang Z An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22 (12), 2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Dinglasan-Panlilio MJA; Mabury SA Significant Residual Fluorinated Alcohols Present in Various Fluorinated Materials. Environ. Sci. Technol 2006, 40 (5), 14471453. [DOI] [PubMed] [Google Scholar]
- (49).Schellenberger S; Hill PJ; Levenstam O; Gillgard P; Cousins IT; Taylor M; Blackburn RS Highly Fluorinated Chemicals in Functional Textiles Can Be Replaced by Re-Evaluating Liquid Repellency and End-User Requirements. J. Clean. Prod 2019, 217, 134–143. [Google Scholar]
- (50).Holmquist H; Roos S; Schellenberger S; Jönsson C; Peters G What Difference Can Drop-in Substitution Actually Make? A Life Cycle Assessment of Alternative Water Repellent Chemicals. J. Clean. Prod 2021, 329, 129661. [Google Scholar]
- (51).Washington JW; Naile JE; Jenkins TM; Lynch DG Characterizing Fluorotelomer and Polyfluoroalkyl Substances in New and Aged Fluorotelomer-Based Polymers for Degradation Studies with GC/MS and LC/MS/MS. Environ. Sci. Technol 2014, 48 (10), 5762–5769. [DOI] [PubMed] [Google Scholar]
- (52).Larsen BS; Stchur P; Szostek B; Bachmura SF; Rowand RC; Prickett KB; Korzeniowski SH; Buck RC Method Development for the Determination of Residual Fluorotelomer Raw Materials and Perflurooctanoate in Fluorotelomer-Based Products by Gas Chromatography and Liquid Chromatography Mass Spectrometry. J. Chromatogr. A 2006, 1110 (1), 117–124. [DOI] [PubMed] [Google Scholar]
- (53).Search and Share Chemistry. ChemSpider. http://www.chemspider.com/ (accessed 2021−12 −23).
- (54).Dinglasan MJA; Ye Y; Edwards EA; Mabury SA Fluorotelomer Alcohol Biodegradation Yields Poly- and Perfluorinated Acids. Environ. Sci. Technol 2004, 38 (10), 2857–2864. [DOI] [PubMed] [Google Scholar]
- (55).Wang N; Szostek B; Buck RC; Folsom PW; Sulecki LM; Capka V; Berti WR; Gannon JT Fluorotelomer Alcohol BiodegradationDirect Evidence That Perfluorinated Carbon Chains Breakdown. Environ. Sci. Technol 2005, 39 (19), 7516–7528. [DOI] [PubMed] [Google Scholar]
- (56).Li L; Liu J; Hu J; Wania F Degradation of Fluorotelomer-Based Polymers Contributes to the Global Occurrence of Fluorotelomer Alcohol and Perfluoroalkyl Carboxylates: A Combined Dynamic Substance Flow and Environmental Fate Modeling Analysis. Environ. Sci. Technol 2017, 51 (8), 4461–4470. [DOI] [PubMed] [Google Scholar]
- (57).Furthering the Understanding of the Migration of Chemicals from Consumer Products; Commission for Environmental Cooperation, 2017. [Google Scholar]
- (58).Zheng G; Salamova A Are Melamine and Its Derivatives the Alternatives for Per- and Polyfluoroalkyl Substance (PFAS) Fabric Treatments in Infant Clothes? Environ. Sci. Technol 2020, 54 (16), 10207–10216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


