Abstract
Bioactive peptides (BAPs) have been found to promote health through various mechanisms. Among them, antimicrobial peptides are gaining recognition as promising novel treatments. This study aims to generate BAPs from bovine colostrum whey using the proteolytic activity of Lactobacillus rhamnosus C25 and to evaluate their potential antibacterial efficacy, including their ability to synergistic efficacy against resistant bacteria. Bioactive peptides were successfully generated from lactobacillus culture proteases that were cultivated through batch fermentation. The resulting peptide fractions were then evaluated for their antibacterial efficacy against a selection of strains, including E. coli ATCC25922, S. aureus MTCC1144, Acinetobacter baumannii ATCC 17978, as well as clinically isolated resistant strains of E. coli (ESBL 1384), Acinetobacter 1379, and S. aureus (MRSA 1418). Notably, the peptide fractions with a molecular weight of < 10 kDa (0–10 kDa) significantly increased the membrane permeability of both E. coli (70.30 ± 0.41%) and S. aureus (63.04 ± 0.31%) as assessed by the crystal violet assay. The checkerboard method was utilized to perform synergistic tests with peptides and antibiotics. The peptide fractions with a molecular weight of (< 10 kDa) demonstrated synergistic effects with several antibiotics, including gentamycin, Rifampicin, Levofloxacin, Ciprofloxacin, and Chloramphenicol, against the resistant ESBL 1384 strain, as indicated by ΣFICI values of 0.55, 0.53, 0.52, 0.54, and 0.52, respectively. Furthermore, the HT-29 cell line remained completely unaffected by both peptide fractions. These findings suggest that the < 10 kDa peptide fraction possesses significant antibacterial efficacy against both reference and ESBL 1384 resistant bacterial strain. Additionally, both MRSA 1418 and Acinetobacter 1379 displayed resistance to all fractions tested. To summarize the findings of this study, colostrum whey peptides with a broad spectrum of antimicrobial activity can be efficiently produced through fermentation. This method could prove valuable for both the pharmaceutical and food industries.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13197-023-05776-2.
Keywords: Lactobacillus rhamnosus (C25), Antimicrobial resistance, Antimicrobial peptide, Mode of action, Pathogen
Introduction
The food and beverage industry offers a wealth of opportunities for creating healthy and functional foods. One increasingly popular functional ingredient is bovine colostrum (BC), which boasts numerous health benefits (Chae et al. 2017). Cow mammary secretions also contain various antimicrobial components, including leukocytes, lactoferrin (LF), lysozyme (LZM), and the inhibitory mechanism catalyzed by lactoperoxidase (LP) (Korhonen 1973). In 2017, the World Health Organization (WHO) was prompted to create a list of priority microorganisms for research and development of treatments. Bacterial resistance has become an urgent global health crisis and has led to the loss of millions of lives in recent years (WHO 2017). In our study, we examine existing regulatory mechanisms and explore the potential use of antimicrobial peptides (AMPs) as a strategy in the fight against pathogenic or extensively resistant bacteria. To the best of our knowledge, this is the first article that emphasizes the potential of colostrum whey-derived AMPs fraction in combating pathogenic and resistant bacteria. In a separate study, researchers evaluated the antibacterial effectiveness of lactoferrin and peptide precursors against MRSA both in vitro and in vivo (Yamauchi et al. 1993). The proteolytic enzymes are found in utmost lactic acid bacteria (LAB). Enzymes such as proteinases and peptidases, which are involved in the proteolysis process, break down large protein molecules in milk into smaller peptides and free amino acids through enzymatic proteolysis. Proteolytic enzymes produced by bacteria such as Lactococcus lactis ssp. cremoris FT4, Lactobacillus helveticus, and Lactobacillus delbrueckii ssp. bulgaricus SS1 play a crucial role in the production of BAPs during milk fermentation (Gobbetti et al. 2002). In recent years, numerous antimicrobial combinations have been studied for their efficacy against Gram-negative infections that are difficult to treat, such as MDR and XDR. Synergistic effects have been observed when using clinically utilized AMPs like colistin in combination with rifampin, or other AMPs, carbapenems, or beta-lactams (Hilchie et al. 2013). In our study, our aim was to characterize the fermented colostrum whey peptide fractions with molecular weights < 10 kDa (0–10) and < 5 kDa (0–5) using RP-HPLC followed by particle analysis. Additionally, we assessed the percentage of bacterial strain inhibition. The permeabilization of peptides in the bacterial cell membranes was analyzed using ONPG permeability and crystal violet assays. In addition, the 0–10 kDa peptide fraction was used to rupture bacterial cells, and the mode of action was observed using SEM and fluorescence microscopy. We evaluated the cytotoxicity of the peptide fractions using an MTT assay on the HT-29 cell line. Additionally, we investigated the antibacterial activity of bovine colostrum whey samples against a range of pathogenic and opportunistic bacterial strains. Furthermore, the present study highlights the effective utilization of antimicrobial peptides (AMPs) produced from fermented colostrum whey. These peptides were generated using a low cost-effective technique and both peptide fractions exhibited antibacterial activity in vitro.
Material and methods
Bacterial strains and chemicals
In our laboratory, we previously isolated proteolytic Lactobacillus casei NCDC17, L. plantarum C6 (NCBI Accession no. KF806536), and L. rhamnosus C25 (KF806538) from cheddar cheese (FFFBAP, Dairy Microbiology Division, Indian Council of Agriculture Research-National Dairy Research Institute, Karnal, India). We assessed the proteolytic activity of the L. rhamnosus C25 strain and found that the diameter of the clear zone was correlated with its proteolytic activity (Moulay et al. 2006). The clinical isolates used in this study were S. aureus (MRSA 1418) isolated from human pus, ESBL 1384 and Acinetobacter 1379 obtained from human urine, as well as the indicator microorganisms Escherichia coli (ATCC 25922), Staphylococcus aureus (MTCC1144), and Acinetobacter baumannii (ATCC 17978). The cultures were sourced from the American Type Culture Collection and Microbial Type Culture Collection. All pathogens were grown in Brain Heart Infusion broth at 37 °C. Cow colostrum was procured from the National Dairy Research Institute in India. HPLC quality acetonitrile, methanol, and trifluoroacetic acid (TFA), as well as antibiotics were purchased from Sigma Aldrich in India. Brain heart infusion (BHI), Muller Hinton broth, agar powder, and MRS broth media were purchased from Hi-media Labs, India.
Isolation of colostrum whey
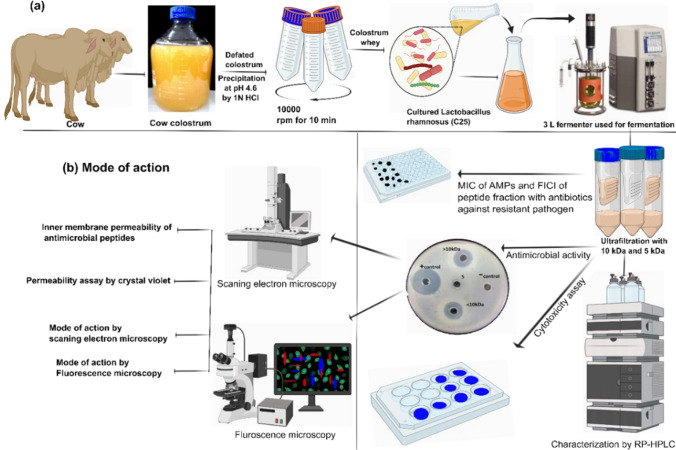
Cow colostrum was defatted through a centrifuge at 10,000 rpm for 10 min at 4 °C. The cow colostrum was acid precipitated at pH 4.6 with 1 molL−1 HCl, followed by centrifugation at 4500 rpm for 10 min at 4 °C to separate the whey from the caseins (Korhonen 1973) (Fig. 1).
Fig. 1.
a The flow diagram shows the antimicrobial susceptibility assay. The cow colostrum defatted by centrifugation, and after that, fermentation via Lactobacillus rhamnosus C25, followed by separation of peptides fractions by different-sized MWCO (10, 5 and 3 kDa) ultrafiltration, and tested the antimicrobial activity, MIC and FICI of antimicrobial peptides. Peptide fractions were characterized using the C18 column of RP-HPLC. b Mode of action. Description of the data analysis; detailed information is provided in the “Materials and methods” section
Batch fermentation conducted for antimicrobial peptides production by L. rhamnosus (C25)
The fermenter (3-L New Brunswick BioFlo fermenter) was autoclaved for 30 min at 121 °C. To prepare the inoculum, the L. rhamnosus cells (shown in Fig. 2) were first cultured in MRS broth for 18 h at 37 °C in an incubator, and adapted once before each fermentation cycle. Next, a pre-culture was established in a 20 mL test tube, and once the cells entered the logarithmic phase, they were used to create the final inoculum. The final inoculum was prepared in a 250 mL Erlenmeyer flask using an optimal culture medium, with an initial OD600 of 0.1. The cells were inoculated into the fermenter at a rate of 5–8% v/v after they had reached the logarithmic phase. The initial OD600 of the fermenter contents was approximately 0.1, and fermentation was conducted for 48 h at a temperature of 37 °C while maintaining a rotation speed of 100 rpm. After modifying the inoculum's optical density (OD) and the initial pH of the culture medium, L. rhamnosus (C25) was cultured. Following 18 h of cell growth, measurements were taken for the OD, temperature, and pH. A pH of 5 was maintained in the fermenter by adding 1 M NaOH as needed. The fermentation vessel was sampled at a suitable interval under sterile conditions, and the level of growth was evaluated by measuring the OD600 (Modiri et al. 2021).
Fig. 2.
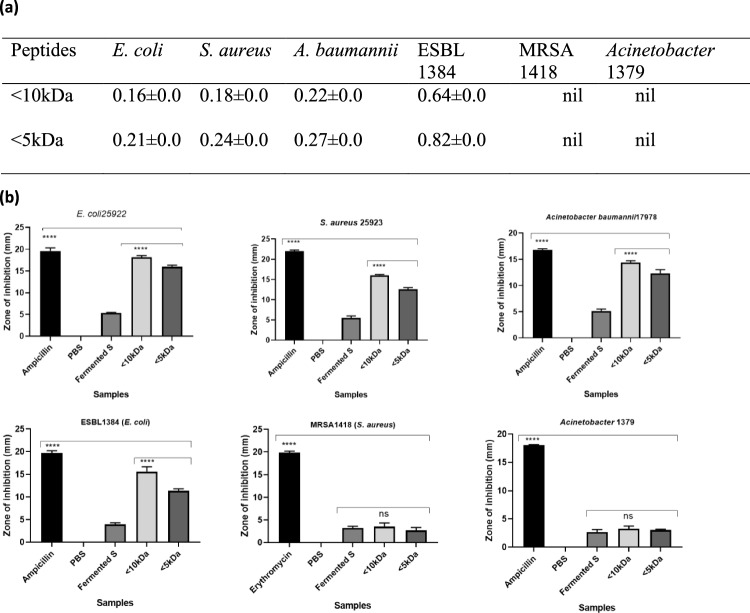
MIC of AMPs against a range of microbes. Average MIC (mg/mL) values were calculated from at least three independent experiments mean ± SD. b Antimicrobial activity of fermented cow colostrum whey derived peptides fractions of < 10 and < 5 kDa peptides against different bacterial strains. Showed highest significant difference (p < 0.001) data are the mean ± SD of at least three independent experiments
Separation peptide fractions from fermented colostrum whey
To extract bacterial cells from fermented colostrum whey, the mixture was centrifuged at 7000 rpm for 15 min at room temperature. Ultrafiltration membranes with MWCO (molecular weight cut-off) of 5 and 10 kDa, specifically Amicon tubes from Millipore (USA), were used to isolate two fractions from the supernatant: one with molecules smaller than < 5 kDa (0–5), and another with molecules smaller than < 10 kDa (0–10). To adjust the pH 7.0, a solution of 1 mol/L sodium hydroxide was used. The peptide fractions were then lyophilized and stored at − 20 °C for subsequent investigation.
Peptide fractions characterization via RP-HPLC and particle sizer
Colostrum whey peptides produced after fermentation were analyzed by RP-HPLC using a C18 column (Trujillo et al. 2000). The mobile phases, consisting of TFA (trifluoroacetic acid) at 0.1 percent (v/v) in deionized water and 0.1% (v/v) in 100 percent pure ACN (acetonitrile), were employed. Peptide separation was achieved using a linear gradient of solvent A (100%) for 5 min, followed by a gradient eluent of solvent B: 0–5 min (0%), 5–10 min (0–50%), 10–30 min (50–65%), 30–35 min (65–100%), 35–40 min (100%), and 40–45 min (100–0%). Throughout, the flow rate was kept constant at 0.6 mL/min, and the absorption spectrum of the eluent was measured using UV at 214 nm (Panchal et al. 2020). The particle size and PDI of the fermented and unfermented cow colostrum whey were measured by a nano-meter analyzer (Malvern Nanoparticle Analyzer). Prior to measurement, the freeze-dried fermented and unfermented powder was diluted 100-fold or 10-fold with 0.1 moL/L sodium phosphate buffer (pH 7.0) to avoid multiple scattering during measurements.
Antibiotic resistance profiling
A disc diffusion experiment was used to investigate the pattern of antibiotic susceptibility. MRSA 1418, ESBL 1384, and Acinetobacter 1379 resistant and reference strain S. aureus MTCC1144, E. coli ATCC25922, and Acinetobacter baumannii 17978 cultured in BHI broth for 18 h were diluted in sterile physiological saline to achieve comparable turbidity of 0.5 McFarland (MF) (1 × 106 CFU/mL). Solidified Mueller Hinton agar plate seeded with 50 μL of 0.5 MF test isolates. The antibiotics disc were incubated at 37 °C for 18–24 h on the surface of Solidifed agar. The findings were calculated in accordance with the Clinical and Laboratory Standards Institute (CLSI) recommendations available at (https://clsi.org/media/1632/m07a10_sample.pdf). When the CLSI breakpoint was unavailable, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria (https://www.eucast.org/clinicalbreakpoints) were used. Based on the antibiotic susceptibility test, the multiple antibiotic resistance (MAR) index was calculated by dividing the number of antibiotics against which the tested isolate was resistant by the total number of antibiotics tested.
Antimicrobial activity of colostrum whey-derived peptide fractions
The peptide content of the fractions was assessed using the Bicinchoninic acid (BCA) technique (Smith et al. 1985). To evaluate the antimicrobial activity of peptides, we tested them against various pathogens, including S. aureus (MRSA 1418), E. coli (ESBL 1384), and Acinetobacter 1379, as well as the wild strains E. coli (ATCC 25922), S. aureus (MTCC1144), and A. baumannii (ATCC17978). The pathogens were grown overnight and 100 µL (1 × 106 CFU/mL) of the culture were added to BHI medium plates. The wells were added 100 µl of AMPs 28 mg/mL each well received fractions of < 5 and < 10 kDa, was incubated at 37 °C for 12 h. The microbiological effects of various AMP fractions were assessed using the diameter of the inhibitory zone formed around the wells (Mohammadi et al. 2018).
Evaluation of antimicrobial inhibition (%) by peptides fraction
The antibacterial activity of the < 10 kDa and < 5 kDa peptide fractions was assessed using a microplate test developed by Dasari et al. (2014). As a result, overnight grown culture (E. coli, S. aureus, A. baumannii, and resistant ESBL 1384 were washed two times with sterile PBS before resuspended in MHB to (1 × 104 CFU/mL). Then, on a sterile 96-microwell plate, 50 μL of < 10 kDa and < 5 kDa fractions (varied concentrations) and combined with 50 μL of each bacterium and 100 μl of MHB were added for 18 h at 37 °C. The optical density at 600 nm was measured at the end of the incubation period. Following the equation, the % of bacterial inhibition was calculated:
where, ODcontrol and ODsample are the optical densities of the control samples, respectively.
Minimum inhibitory concentration (MIC) of antimicrobial peptides
The bacterial strains were cultivated for 16 h and adjusted to 1 × 104 CFU/ml. The peptide fractions solution was produced at serially diluted to a fold dilution (120, 60, 30, 15, 7.5, 3.75, 1.87 mg/mL). In the 96-well plates, 50 μl of the twofold diluted peptide samples and 50 μl of bacterial cells were mixed. After hours of incubation at 37 °C, the combination's absorbance values (OD600) were measured. BHI medium (100 µl) without bacterial culture was utilised as a negative control, while media containing the microbial culture was used as a positive control. The MIC value was determined as the lowest peptide concentration that stopped observable bacterial growth (Mohammadi et al. 2018).
Permeabilization (ONPG assay) and permeability (crystal violat assay)
The purpose of the procedure was to determine the permeability of the inner membrane of E. coli, to evaluate the effect of peptides on the permeability of the inner membrane, β-galactosidase activity was measured using ONPG as a substrate, as described by Li et al. (2016). The E. coli cells were grown in BHI medium until they reached the logarithmic phase and then were centrifuged at 5000 g for 5 min. The bacterial cells were subsequently diluted in 10 mM PBS to achieve an OD600 of 0.05, which contained 1.5 mM ONPG. Peptides concentrations were 8× MIC, and data were collected from 0 to 90 min at OD420. The crystal violet test was used to assess the change in membrane permeability (Xue et al. 2015) by using a microplate assay. After 12 h of incubation, the untreated cells and peptide (8× MIC) treated cells was centrifuged, After being washed twice with PBS, the cells were resuspended in a crystal violet solution (2 mg/mL) and incubated at 37 °C for 30 min. Subsequently, the supernatant was centrifuged to determine the optical density at 600 nm. The crystal violet solution’s OD value was estimated to be 100%. The proportion of crystal violet absorption was stated as follows:
Peptides exhibiting the greatest impact on growth parameters and crystal violet uptake percentage of < 10 kDa fraction were selected for further investigation to identify potential antimicrobial peptides (AMPs) and assess their effect on the bacterial cell wall’s integrity.
Synergistic effect of AMPs with antibiotics against bacteria
The chequerboard method, based on the broth microdilution method and cation-adjusted Mueller–Hinton broth, was used to determine the MIC of antibiotics in conjunction with peptides (Rodríguez-Hernandez et al. 2006). In brief, the concentration ranges were determined using the MIC of the peptides and each antibiotic. The fractional inhibitory concentration index was used to assess the interaction of the < 10 kDa peptide (A) with each antibiotic (B) (FICI). FICI = FICA + FICB = (MICA in combination/MICA alone) + (MICB in combination/ MICB alone) is the equation used to generate this index. FICI was interpreted as follows: FICI 0.5 synergy, 0.5 < FICI 1 partial synergy, 1 < FICI 4 additive effect or indifference, 4 < FICI antagonism (Rodríguez-Hernandez et al. 2006).
Cytotoxicity on HT-29 cell line by MTT assay
The cytotoxicity of each fractions was determined using MTT assays, as previously published by Gomes et al. (2015). In summary, HT-29 cells were grown overnight in 96-well plates with 10% foetal calf serum and DMEM at 37 °C with 5% CO2 at 37 °C. Peptide dilutions were made in DMEM in two-fold serial dilutions. AMPs were introduced to the plates and incubated for 20–24 h at 37 °C with 5% CO2. Following that, 40 µl of MTT solution was put into each well, and the plates were incubated overnight at 37 °C for 4 h. Next, 150 µl of DMSO was added to the wells, the plates were agitated for 10 min, and absorbance at 492 nm was recorded using a microplate reader.
Mode of action by SEM and fluorescence microscopy
As reported by Juba et al. (2015), E. coli were resuspended in PBS (pH 7.2) to an OD600 of 0.5 in the mid-log phase in MHB medium. The bacterial cells were incubated at 37 °C for 6 h with < 10 kDa fraction at 8× MIC. Bacterial cells were collected using centrifugation, followed by washing with PBS and preservation in 2.5% glutaraldehyde. Samples were then rinsed with PBS and dehydrated using graded ethanol for 15 min (50%, 70%, 90%, and 100%). The bacterial specimens were gold coated, and examined using a SEM (Hitachi S-4800; Hitachi, Tokyo, Japan). For fluorescence microscopy, bacterial suspensions (1 × 104 CFU per mL) and 8× MIC of < 10 kDa fraction were incubated at 37 °C for 6 h (Chen et al. 2014). The cells were harvested by centrifugation at 6000 rpm and suspended in 1 mL of water. The cells were then stained with 10 µl propidium iodide for 15 min, followed by counter-staining with 10 µl of DAPI (4′,6-diamidino-2-phenylindole) for 5 min in the dark.
Results and discussion
Characterization of peptide fractions by RP-HPLC and particle sizer
The colostrum whey fermented peptides with a molecular weight of less than 5 and 10 kilodaltons were analyzed using RP-HPLC, and their chromatograms are presented in Figure 5a and b in supplementary file S1. The chromatogram of the < 10 kDa peptide fraction showed peaks with retention times ranging from 7.9 to 30 min, with the majority of peaks detected between 10 and 30 min (Figure 5a). Similarly, the chromatogram of the < 5 kDa peptide fraction displayed peaks with retention times ranging from 6.1 to 23 min (Figure 5b). Peaks were observed throughout the retention time range of 5 to 30 min. Panchal (2020) reported that fermented goat milk fractions exhibited RP-HPLC retention times ranging from 15 to 35 min. Regarding particle size and polydispersity index (PDI), Figure 5c and d illustrate the particle size distribution by intensity of cow colostrum whey, both fermented and unfermented. The unfermented whey showed a relatively high z-average diameter and polydispersity index (951.3 nm and 0.351, respectively) (Figure 5c, supplementary file S1). The fermented whey, on the other hand, displayed a smaller particle size (475.5 nm) and higher polydispersity index (0.687) (Figure 5d). This decrease in particle size could be due to the protease activity of lactobacillus, which hydrolyzes whey proteins into shorter peptides and amino acids. The polymer dispersity index (PDI) provides information on the molecular weight distribution of a polymer, as demonstrated in Figure 5c and d (supplementary file S1). The more uniform the molecular weight distribution, the smaller is the PDI value (Ai et al. 2019). The PDI of fermented and unfermented colostrum whey were 0.687 and 0.351, respectively. These results indicated that the fermentation by Lactobacillus rhamnosus to breakdown the whey proteins into peptides.
Antibacterial susceptibility assay
The current studys workflow is illustrated in Fig. 1a and b. The majority of the findings indicate a significant prevalence of multidrug resistance, especially among pathogens such as MRSA 1418, ESBL 1384, and Acinetobacter 1379. In our study, we identified ESBL 1384 from urine samples and the strain exhibited a resistance phenotype of 27.26%, showing resistance to six out of 22 antibiotics and intermediate susceptibile to 12 antibiotics tested (Figure 1a, d in supplementary file S1), with a MAR index of 0.27 (Table 1 in supplementary file S1). It’s important to note that MRSA can cause infections both in the community and hospital settings. Kumari et al. (2008) reported a methicillin resistance rate of 60% among S. aureus isolates from respiratory tract specimens in eastern Nepal. Our study found that MRSA 1418 was highly resistant, with an observed resistance rate of 81.80% (Figure 1b, d in supplementary file S1), and a MAR index of 0.81 (Table 1 in supplementary file S1). Similarly, our findings on the prevalence of Acinetobacter spp. were higher than the reported 3.9% of LRTI cases in Western Nepal (Tamang et al. 2005). Our current findings indicate that Acinetobacter 1379 is resistant to 63.6% of the antibiotics tested (Figure 1c, d in supplementary file S1), with a MAR index of 0.63 (Table 1 in supplementary file S1). In contrast, wild-type strains such as S. aureus MTCC 1144, E. coli ATCC25922, and A. baumannii ATCC17978 were found to be susceptible to almost all antibiotics, except for a few in which they showed intermediate susceptibility.
Antimicrobial activity and MIC of colostrum whey derived peptide fractions
Colostrum whey contains bioactive proteins like immunoglobulin, lactoferrin, lactoperoxidase, and lysozyme that release more bioactive peptides at high protein concentrations, positively affecting physiological functions. Peptide fractions of 10 kDa, 5 kDa, and 3 kDa were prepared from the fermentate to study the immunomodulatory and antimicrobial activity of colostrum whey peptides. Peptides with a molecular weight of less than 10 kDa and low peptide content showed greater antimicrobial activity against diarrheagenic E. coli pathogens. LCMS/MS analysis identified 56 novel peptides, including 16 peptide sequences with antimicrobial activity (Kashyap et al. 2022). During our investigation, it was observed that L. rhamnosus C25 exhibited the highest proteolytic activity when compared to other probiotics such as L. casei NCDC 17 and L. plantarum (C6) (refer to Figure 2 in supplementary file S1). Similarly, in a study conducted by Bartkiene et al. (2020), it was demonstrated that fermented bovine colostrum exhibited antibacterial inhibitory action, with the largest inhibition zone observed against K. pneumoniae. During our research, we separated the supernatant and peptide fractions (< 5 and < 10 kDa) and tested them against both Gram-positive and Gram-negative bacteria, all of which were effectively suppressed (Figure 3 in the supplementary file S1 for more details). Our findings indicated that the AMPs of < 10 kDa demonstrated a significantly higher antibacterial activity (p < 0.001) in comparison to < 5 kDa and fermented supernatant (‘S’) fraction. Among these peptides, those with a molecular weight of < 10 kDa demonstrated the strongest inhibition zone against E. coli (17.63 mm), followed by S. aureus (16.11 mm), A. baumannii (14.8 mm), and ESBL 1384 (15.21 mm). However, as illustrated in Fig. 2a and b, MRSA 1418 and Acinetobacter 1379 exhibited an extreme level of resistance to these peptides. Another study has demonstrated that, Kashk is a Middle Eastern fermented dairy product made from sour milk and/or dairy waste. Its water-soluble peptide fraction was studied using liquid chromatography-mass spectrometry and showed significant antibacterial activity against Staphylococcus aureus. The peptide fraction also demonstrated skin healing activity, accelerating wound closure in vitro by approximately 44% in the presence of TNF-α (Folliero et al. 2022). Other similar study on fermented goat milk derived, antimicrobial peptides (AMPs) are a promising new class of drugs produced by fermenting goat milk using Lactobacillus rhamnosus. Ultrafiltration membranes were used to separate the peptide fractions by size, and those with molecular weights under 5 kDa had the highest antibacterial activity against E. faecalis, E. coli, and S. typhi. Six AMPs were selected using molecular docking to evaluate their potent MurD ligase inhibitors, and peptides IGHFKLIFSLLRV and KSFCPAPVAPPPPT were identified as high-potent antimicrobial peptides (Iram et al. 2022). Our findings suggest that bacteria attempt to adapt to the conditions created by the system, resulting in a disruption of their growth rate and inhibition of maximal growth (Fig. 2a and b). The MIC values of < 10 kDa and < 5 kDa were determined using the Clinical and Laboratory Standards Institute (CLSI) microdilution method. Mokhtari et al. (2023) research showed that, bioactive peptides were produced by hydrolyzing casein protein with trypsin (pH = 8). Antioxidant activity and antimicrobial activity were evaluated, and minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) were determined. Peptides had the greatest effect on S. typhimurium at a concentration of 3 mg/ml − 1, but had no effect on S. aureus (P < 0.05). The lowest MIC and MBC were observed with B. cereus (3.412 and 7.725 μg/ml, respectively), while the highest were observed with S. typhimurium (8.515 and 8.555 μg/ml, respectively).The MIC values of all peptide fractions were measured against various test pathogens. The MIC for < 10 kDa peptides was (0.16 ± 0.465) mg/ml against E. coli, (0.18) mg/ml against S. aureus, (0.22) mg/ml against Acinetobacter baumannii, and (0.64 ± 0) mg/ml against ESBL 1384. The < 5 kDa fractions exhibited MIC values of (0.21 ± 0.116) mg/ml, (0.24 ± 0) mg/ml, (0.27 ± 0) mg/ml, and (0.82 ± 0) mg/ml against the same pathogens. Both fractions showed significantly higher efficacy against E. coli compared to other fractions. The results also showed that these two fractions had some inhibited effect on S. aureus, A. baumannii, and ESBL 1384 (Fig. 2a). Overall, < 10 kDa fractions demonstrated greater pathogen susceptibility, with E. coli being the most sensitive. At least three independent experiments were conducted to calculate the MIC values.
Antibacterial inhibition (%) of peptide fractions
According to our results, the < 10 kDa peptide fractions exhibited 74.5% inhibition against E. coli at a concentration of 50 mg/ml of peptides. Similarly, the < 5 kDa peptides inhibited approximately 62.5% of E. coli at the same concentration (Table 2 in supplementary file S1). Aguilar-Toalá et al. (2020) reported that the peptide fractions in the 3–10 kDa range inhibited S. enterica serovar typhimurium K1028 and L. monocytogenes 10403S over 50% of the time, while the < 3 kDa peptide fraction inhibited all bacterial strains over 50% of the time.
Synergistic effects of peptide fraction with antibiotics
The combination of penicillin, streptomycin, and isracidin has been found to be effective against antibiotic-resistant S. aureus (Lahov and Regelson 1996). However, there has been limited research conducted on investigating the synergistic antibacterial activity of milk-derived peptides. One study found that lactoferricin-derived peptides P2-15 and P2-27 exhibited a synergistic antibacterial effect against P. aeruginosa when combined with erythromycin antibiotics (Sánchez-Gómez et al. 2011). According to the researchers, LFcin B caused disorganization and permeabilization of both the cell wall and membrane, thereby allowing antibiotics to penetrate the cell and cause injury (Longhi et al. 2009). Our study found that the synergistic effects of food grade peptides with other antibiotics against pathogenic bacteria have been underutilized. There is a need for further research on the potential of such combinations. In our study, we evaluated the antibacterial activity of the < 10 kDa peptide fraction in combination with eight standard antibiotics (levofloxacin, chloramphenicol, rifampicin, amoxicillin, tetracycline, vancomycin, gentamicin, and ciprofloxacin) using the broth microdilution technique and calculated the fractional inhibitory concentration index (FICI). The < 10 kDa peptide fractions were mixed with conventional antibiotics using the checkerboard approach to investigate the synergistic effect of these combinations on the antibacterial efficacy of both peptides and individual antibiotics, respectively. The increase in antibacterial efficacy as a result of synergism is reported by computing the FIC indices, which show whether the peptide antibiotic combination was synergistic (FIC ≤ 0.5), additive (FIC 0.5 < FIC ≤ 1), indifferent (1 < FIC ≤ 4), or antagonistic (FIC > 4) effect, respectively (Odds 2003). In our results, we observed a significant decrease in MIC values when combining antibiotics with < 10 kDa peptide fractions. The combinations of < 10 kDa peptide fractions with eight antibiotics resulted in an additive or indifferent effect on the methicillin-resistant bacteria MRSA 1418 and Acinetobacter 1379, as shown by the FIC index of 1.5 to 1.98 (Table 1). Only the combination of < 10 kDa peptides–Rifampicin, Levofloxacin, Ciprofloxacin, and Chloramphenicol—displayed a synergistic effect for the clinically isolated resistant strain of EBSL 1384, with a FICI index of 0.53, 0.52, 0.54, and 0.52, when compared to its individual MIC, the synergistic impact of < 10 kDa peptides fractions—Rifampicin combination resulted in a 40.62% decrease in the MIC value of < 10 kDa peptides. The < 10 kDa peptides reduced the MIC values of levofloxacin, ciprofloxacin, chloramphenicol, rifampicin, and gentamicin by 34.32%, 42.18%, 35.93%, 40.6%, and 39%, respectively (Table 1). The MIC values of these antibiotics were reduced by different percentages, namely 18.51% (levofloxacin), 12.90% (ciprofloxacin), 17.20% (chloramphenicol), 13.9% (rifampicin), and 16.6% (gentamicin), as depicted in Figure 4 in supplementary file S1. Among the combinations, only the < 10 kDa–chloramphenicol showed a synergistic effect, as indicated by FIC values of 0.52 against the Gram-negative ESBL 1384. However, with the < 10 kDa peptides, an additive effect was observed, leading to enhanced antibacterial activity of amoxicillin and tetracycline with reported FIC indices of 1.6 and 1.98, respectively (Table 1). The remaining combinations had additive or indifferent effects, with FIC values in the range of (0.5 < FIC ≤ 1) or (1 < FIC ≤ 4).
Table 1.
Synergistic effect of colostrum whey derived peptide fractions with antibiotics including the fractional inhibitory concentrations index (FICI) against tested bacterial species
| Bacterial strains | Antibiotics | MICs alone antibiotics (µg/mL) | Alone peptides (mg/mL) | Combination effect Peptide Antibiotics (mg/mL) (µg/mL) |
ΣFICI |
|---|---|---|---|---|---|
| ESBL (E. coli) | Vancomycin | 128.4 | 0.64 |
0.470 12 |
0.82 |
| Gentamicin | 24 | 0.64 |
0.250 4 |
0.55 | |
| Amoxicillin | 170.8 | 0.64 |
0.850 64.2 |
1.6 | |
| Rifampicin | 28.6 | 0.64 |
0.260 4 |
0.53 | |
| Levofloxacin | 21.6 | 0.64 |
0.220 4 |
0.52 | |
| Ciprofloxacin | 31 | 0.64 |
0.270 4 |
0.54 | |
| Chloramphenicol | 46.5 | 0.64 |
0.230 8 |
0.52 | |
| Tetracycline | 250 | 0.64 |
0.950 125 |
1.98 | |
| S. aureus (MRSA) | Vancomycin | 228 | 180 |
180 160 |
1.7 |
| Gentamicin | 250 | 180 |
180 171.2 |
1.68 | |
| Azithromycin | 170 | 180 |
180 141 |
1.82 | |
| Amoxicillin | 128 | 180 |
180 85.5 |
1.66 | |
| Levofloxacin | 171.8 | 180 |
180 100 |
1.64 | |
| Chloramphenicol | 220 | 180 |
180 171 |
1.77 | |
| Rifampicin | 64.4 | 180 |
180 32.2 |
1.5 | |
| Clarithromycin | 228.6 | 180 |
180 196.3 |
1.85 | |
| Acinetobacter 1379 | Vancomycin | 32 | 180 |
0.64 16 |
1.5 |
| Gentamicin | 256 | 180 |
0.64 176.4 |
1.68 | |
| Azithromycin | 128 | 180 |
0.64 87.5 |
1.68 | |
| Amoxicillin | 114 | 180 |
0.64 112 |
1.98 | |
| Ciprofloxacin | 128 | 180 |
0.64 71 |
1.55 | |
| Tobramycin | 18 | 180 |
0.64 6.75 |
1.37 | |
| Meropenem | 526 | 180 |
0.64 262.5 |
1.49 | |
| Tetracycline | 250 | 180 |
0.64 210 |
1.84 |
The data in each column represents three independent experiments (P < 0.05)
Evaluation of inner membrane and permeability assay
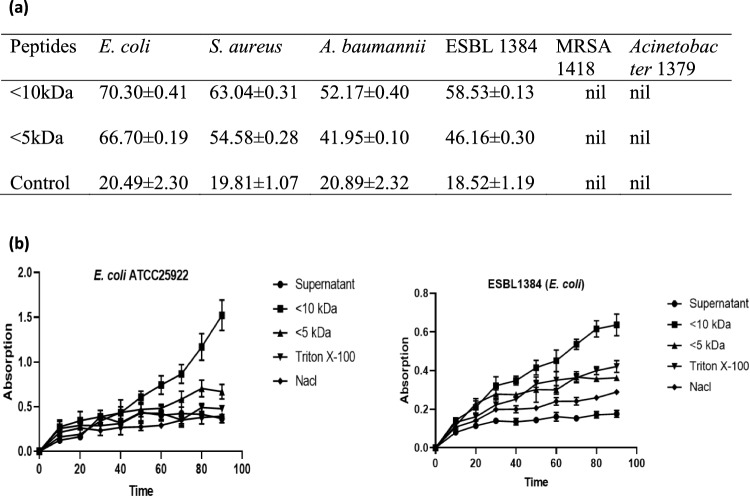
According to Hou et al. (2018), the permeabilization of the outer membrane of E. coli by Cp1 bovine casein-derived peptide was concentration-dependent. At 1× MIC and 1/2 MIC concentrations, both Cp1 and melittin were able to permeabilize the inner membrane to ONPG, indicating their significant membrane-permeabilizing potential with concentration-dependent effects. The ONPG assays were utilized in this study to assess the peptides inner membrane permeability. ONPG can interact with cytoplasmic beta-galactosidase to produce ortho-nitrophenol, which is absorbed at 420 nm, as shown in Fig. 3b. As a positive control, 1% Triton-X 100 was used, while 0.5% saline was used as the negative control. At 5× MIC of both < 10 kDa and < 5 kDa peptide fractions, the permeability of the inner membrane was rapidly enhanced, with both peptides showing a consistent upward trend. In our study, we found that the inner membrane permeability of the < 10 kDa peptides was even higher at 8× MIC. The alteration in membrane permeability was assessed by measuring the uptake of crystal violet, a dye that has limited penetration of the outer membrane but can enter cells with damaged membranes. Aguilar-Toalá et al. (2020) discovered that antibacterial peptide fractions derived from chia seeds, specifically the < 3 kDa fraction, substantially increased membrane permeability in E. coli (71.49% crystal violet uptake) and L. monocytogenes (80.10%). Our results presented in Fig. 3a demonstrate that the < 10 kDa peptide fractions derived from fermented cow colostrum whey exhibit the highest antibacterial efficacy against the three bacterial strains tested (E. coli, S. aureus, and A. baumannii), including the resistant bacteria ESBL1384. These fractions showed significant improvements in membrane permeability, with the highest increase observed in E. coli (70.30 ± 0.41% crystal violet uptake) and S. aureus (63.04 ± 0.31%). Based on their antibacterial activity, the peptide treatment within the < 5 kDa fractions was chosen for E. coli (66.70 ± 0.19% uptake) and S. aureus (54.58 ± 0.28%) (Fig. 3a). To investigate their impact on bacterial cell wall integrity, scanning electron microscopy (SEM) was used.
Fig. 3.
a Values are mean ± standard deviation of triplicate determinations. Crystal violet uptake (%) by bacterial strains after the treatment with < 10 kDa and < 5 kDa peptide fractions. b Inner membrane permeability of the < 10 kDa peptides fraction. Hydrolysis of ONPG due to release of cytoplasmic β-gaIactosidase in E. coli (ATCC) cells treated with peptides at 8× MIC was measured spectroscopically at an absorbance of 420 nm as a function of time. Triton-X 100 (1%) was used as a positive control, while 0.5% saline was used as a negative control
SEM and fluorescence microscopy
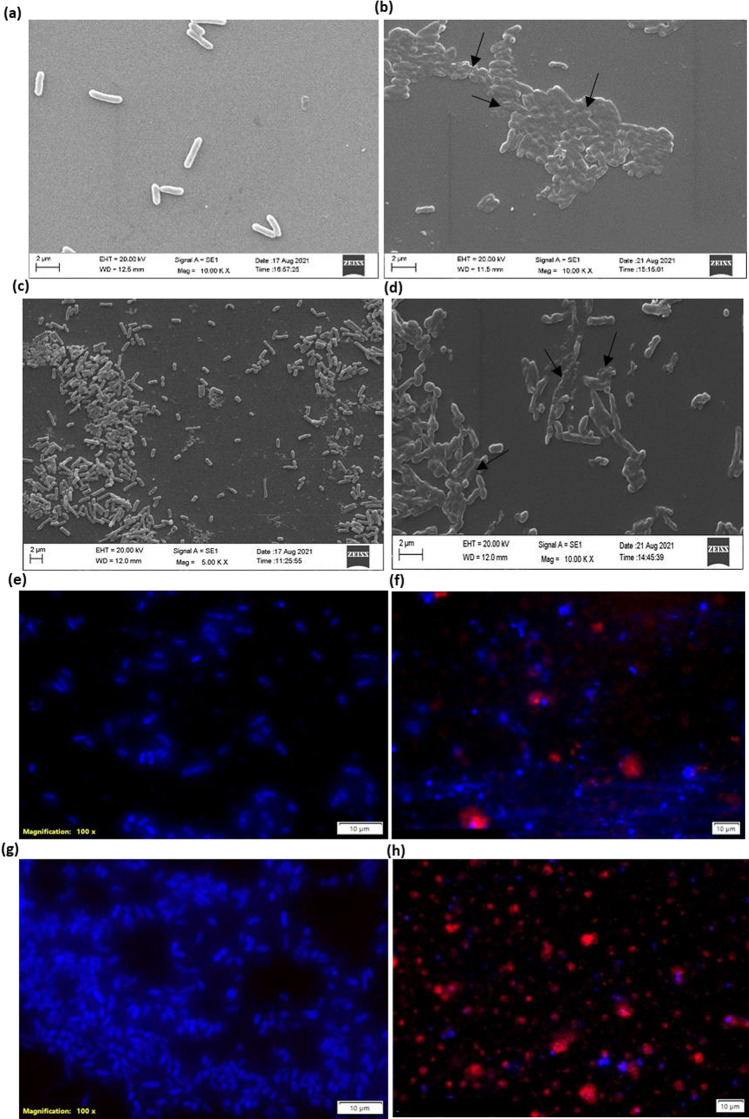
To assess its impact on bacterial cell integrity, we selected the < 10 kDa fraction with the highest antibacterial activity. The SEM and fluorescence microscopy images showed that the control bacteria's untreated cells had a smooth surface with an intact morphology and homogeneous structures. Conversely, the peptide-treated cells had numerous indentations, significant deformations, wrinkling of the membrane, and the loss of intracellular components (Fig. 4a–h). It is possible that electrostatic contact between the peptides and bacterial membrane components led to the formation of holes or temporary transmembrane channels, which could explain the observed effects. In a study by Aguilar-Toalá et al. (2020), it was found that the peptides in the AF-MW 3 kDa fraction have different mechanisms of action against Gram-negative and Gram-positive bacteria, possibly due to variations in their ability to penetrate the microorganisms cytoplasm and differences in their membrane structure. The fermented milk beverage kefir was shown to have activity against E. coli and S. aureus by Gao et al. (2019), where the peptides caused distortion of the cell shape and loss of cell integrity due to permanent membrane damage. Based on the SEM images presented in Fig. 4a and c, it can be inferred that the untreated E. coli cells had a smooth and intact surface, indicating their healthy state with no visible signs of cell lysis or debris. However, treatment with only < 10 kDa peptides at 8× MIC for 3 h resulted in rupture and debris formation in the E. coli cells, as shown in Fig. 4b. Furthermore, the combination of < 10 kDa fractions with levofloxacin exhibited a synergistic effect and demonstrated increased inhibition against the resistant ESBL 1384 strain, as seen in Fig. 4d. Conversely, this treatment also caused significant morphological changes in the bacteria, such as partial blebbing and budding, extracellular debris, and abnormal morphologies. Furthermore, evidence of damage to the bacterial membrane was observed by co-staining the bacterial cells with two fluorescent nucleic acid dyes. Specifically, DAPI, a membrane-permeable dye that emits blue fluorescence, was used to stain the bacterial cells, while propidium iodide (PI), a dye that only penetrates disrupted non-living cells and produces red fluorescence, was used to identify damaged cells. The E. coli bacterial cultures were treated with < 10 kDa peptide fractions at 8× MIC for 3 h before being examined using fluorescence microscopy after co-staining with DAPI and PI. The untreated E. coli cells emitted a blue fluorescence, indicating their viability, as shown in Fig. 4e and g. However, treatment with < 10 kDa peptide fractions resulted in inhibition of the bacteria, as evidenced by the red fluorescence, indicating membrane damage, as shown in Fig. 4f. The combination of levofloxacin and the < 10 kDa peptide fraction exhibited the highest inhibition of the ESBL 1384 strain, as shown in Fig. 5h, with red fluorescence indicating membrane damage. Specifically, the addition of < 10 kDa peptides led to significant membrane permeabilization in E. coli cells, as shown by the red fluorescence in the fluorescence microscopy images. The investigation clearly demonstrated that treatment with < 10 kDa peptides enhances the permeability of Gram-negative membranes. Moreover, the results suggest that the bactericidal effect of < 10 kDa peptides on E. coli is directly accomplished through membrane permeabilization and damage.
Fig. 4.
SEM micrographs of untreated (a and c) and treated (b and d) cells of Escherichia coli ATCC 25922, respectively. Cells were treated with < 10 kDa peptides fraction (8× MIC) obtained from the fermented cow colostrum whey. Arrows indicate the possible damage. Fluorescence images of untreated e and g E. coli after 6 h incubation with < 10 kDa fraction stained with DAPI and PI dyes. Blue fluorescence indicates unaltered membrane integrity. f and h Red fluorescence indicates membrane disintegration (permeable membrane) (blue—live cells; red—dead cells). The marker corresponds to 10 µm (Color figure online)
Fig. 5.
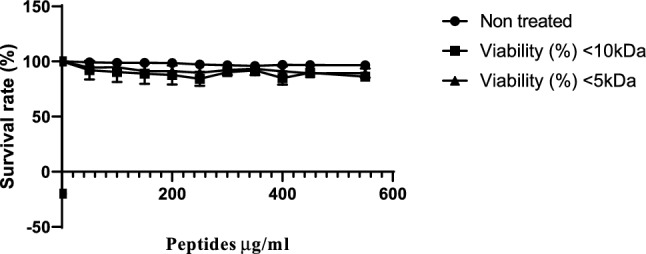
Cytotoxicity of colostrum whey derived peptides fraction on HT-29 cells
Cytotoxicity activity
Cytotoxicity is a crucial metric for assessing peptide toxicity in mammalian cells, as reported by Xu et al. (2015). Their investigation revealed that Cp1 had a modest cytotoxicity activity, indicating that it exhibited excellent cell selectivity. However, our findings suggest that the two peptide fractions tested have cytotoxic effects on the HT-29 cell line, as shown in Fig. 5. The < 10 and < 5 kDa peptide fractions exhibited minimal cytotoxic effects at doses ranging from 50 to 550 μg/ml. These peptides showed significant viability activity, as evidenced by cell survival rates of 96.02%, 97.63%, 94.01%, 93.52%, 93.31%, 94.89%, 92.64%, 90.46%, 91.78%, and 88.74% at concentrations of 0.50, 0.10, 0.15, 0.20, 0.25, 0.30, 0.35, 0.40, 0.45, 0.50, and 0.55 mg/ml, respectively, as depicted in Fig. 5. Our study demonstrated that the < 10 kDa and < 5 kDa peptide fractions had low cytotoxicity activities, indicating that these peptides possessed high cell selectivity.
Conclusion
In this study, L. rhamnosus (C25) was used to ferment cow colostrum whey in order to release peptides with potent antimicrobial activity. The ultrafiltered colostrum whey fermentate fractions exhibited greater antibacterial activity against several pathogenic bacteria, including E. coli (ATCC 25922), S. aureus (MTCC 1144), A. baumannii (ATCC 17978), and clinically isolated resistant strains ESBL 1384, Acinetobacter 1379, and MRSA 1418. The isolated colostrum whey fermented fraction (< 10 kDa) showed the highest antimicrobial activity against E. coli (ATCC 25922), followed by S. aureus (MTCC 1144). In addition, the peptides examined in this study demonstrated diverse mechanisms of action with a broad spectrum of activity against both Gram-positive and Gram-negative strains, which is attributed to the peptide fraction’s ability to interact with the bacterial membrane and/or disrupt bacterial metabolism. However, while several antibiotics exhibited intermediate activity against drug-resistant bacteria, they showed good synergistic combinations with the peptide fraction. On the other hand, some antibiotics that were resistant to bacteria did not show any synergistic effects with the peptide fraction against resistant bacteria. Further research is necessary to uncover the specific mechanism of action of these peptides, establish the relationship between peptide structure and activity, and validate their biological activities for applications in functional food development, dietary supplements, and medication to treat various human ailments. Additionally, research is needed to explore their potential as preservatives for a broad range of foods, as well as their effectiveness in disease and crop protection.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are very grateful to ICAR-National Dairy Research Institute, Karnal, and Haryana, India for institutional funding and facilities to perform the presented work.
Author contributions
DI: methodology, data creation, conceptualization and data validation. MSS: data validation, writing. SM: writing methodology. AKP: discussion and writing, review & editing. SV: conceptualization, supervision, editing, project administration.
Funding
Financial support from the parent Institute ICAR-National Dairy Research Institute.
Data availability
The data and material that support our finding are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors have declared no conflict of interest.
Consent to participate
The contribution of all the authors have been mentioned with credits.
Consent for publication
The presented manuscript has not been submitted and published anywhere.
Ethical approval
The project has been approved under the risk category 2 experiments requiring BSL-2 biocontainment facility by IBSC (11-2021/IRC D-61).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aguilar-Toalá JE, Deering AJ, Liceaga AM. New insights into the antimicrobial properties of hydrolysates and peptide fractions derived from chia seed (Salvia hispanica L.) Probiotics Antimicrob Proteins. 2020;12(4):1571–1581. doi: 10.1007/s12602-020-09653-8. [DOI] [PubMed] [Google Scholar]
- Ai M, Tang T, Zhou L, Ling Z, Guo S, Jiang A. Effects of different proteases on the emulsifying capacity, rheological and structure characteristics of preserved egg white hydrolysates. Food Hydrocoll. 2019;87:933–942. doi: 10.1016/j.foodhyd.2018.09.023. [DOI] [Google Scholar]
- Bartkiene E, Lele V, Sakiene V, Zavistanaviciute P, Ruzauskas M, Stankevicius A, et al. Fermented, ultrasonicated, and dehydrated bovine colostrum: changes in antimicrobial properties and immunoglobulin content. J Dairy Sci. 2020;103(2):1315–1323. doi: 10.3168/jds.2019-16357. [DOI] [PubMed] [Google Scholar]
- Chae A, Aitchison A, Day AS, Keenan JI. Bovine colostrum demonstrates anti-inflammatory and antibacterial activity in in vitro models of intestinal inflammation and infection. J Funct Foods. 2017;28:293–298. doi: 10.1016/j.jff.2016.11.016. [DOI] [Google Scholar]
- Chen J, Peng H, Wang X, Shao F, Yuan Z, Han H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale. 2014;6(3):1879–1889. doi: 10.1039/C3NR04941H. [DOI] [PubMed] [Google Scholar]
- Dasari S, Shouri RND, Wudayagiri R, Valluru L. Antimicrobial activity of Lactobacillus against microbial flora of cervicovaginal infections. Asian Pac J Trop Dis. 2014;4(1):18–24. doi: 10.1016/S2222-1808(14)60307-8. [DOI] [Google Scholar]
- Folliero V, Lama S, Franci G, Giugliano R, D’Auria G, Ferranti P, Galdiero M, Stiuso P. Casein-derived peptides from the dairy product kashk exhibit wound healing properties and antibacterial activity against Staphylococcus aureus: Structural and functional characterization. Food Res Int. 2022;153:110949. doi: 10.1016/j.foodres.2022.110949. [DOI] [PubMed] [Google Scholar]
- Gao X, Chen Y, Chen Z, Xue Z, Jia Y, Guo Q, Ma Q, Zhang M, Chen H. Identification and antimicrobial activity evaluation of three peptides from laba garlic and the related mechanism. Food Funct. 2019;10:4486–4496. doi: 10.1039/C9FO00236G. [DOI] [PubMed] [Google Scholar]
- Gobbetti M, Ferranti P, Smacchi E, Goffedi F, Addeo F. Production of angiotensin-I converting enzyme-inhibitory peptides in fermented milk started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactobacillus lactis subsp. cremoris FT4. Appl Environ Microbiol. 2002;66:3898–3904. doi: 10.1128/AEM.66.9.3898-3904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A, Mano J, Queiroz J, Gouveia I. Incorporation of antimicrobial peptides on functionalized cotton gauzes for medical applications. Carbohydr Polym. 2015;127:451–461. doi: 10.1016/j.carbpol.2015.03.089. [DOI] [PubMed] [Google Scholar]
- Hilchie AL, Wuerth K, Hancock RE. Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol. 2013;9(12):761–768. doi: 10.1038/nchembio.1393. [DOI] [PubMed] [Google Scholar]
- Hou J, Liu Z, Cao S, Wang H, Jiang C, Hussain MA, Pang S. Broad-Spectrum antimicrobial activity and low cytotoxicity against human cells of a peptide derived from bovine αS1-casein. Molecules. 2018;23(5):1220. doi: 10.3390/molecules23051220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iram D, Kindarle UA, Sansi MS, Meena S, Puniya AK, Vij S. Peptidomics-based identification of an antimicrobial peptide derived from goat milk fermented by Lactobacillus rhamnosus (C25) J Food Biochem. 2022 doi: 10.1111/jfbc.14450. [DOI] [PubMed] [Google Scholar]
- Juba ML, Porter DK, Williams EH, Rodriguez CA, Barksdale SM, Bishop BM. Helical cationic antimicrobial peptide length and its impact on membrane disruption. Biochim Biophys Acta (BBA)-Biomembr. 2015;1848:1081–1091. doi: 10.1016/j.bbamem.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Kashyap R, Narayan KS, Vij S. Identification of antibacterial and immunomodulatory bioactive peptides generated from buffalo (Bubalus bubalis) colostrum whey fermented by Lactobacillus rhamnosus C25: LC-MS/MS-based analysis. J Funct Foods. 2022;95:105158. doi: 10.1016/j.jff.2022.105158. [DOI] [Google Scholar]
- Korhonen, H. 1973. Untersuchungen zur Bakterizidie der Milch und Immunisierung der hovinen Milchdriise. Meijeritiet. Aikakausk. (Finnish J. Dairy Sci.) 32: (3) 1–158.
- Kumari N, Mohapatra TM, Singh YI. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in a tertiary-care hospital in Eastern Nepal. J Nepal Med Assoc. 2008;47(170):53–56. doi: 10.31729/jnma.309. [DOI] [PubMed] [Google Scholar]
- Lahov E, Regelson W. Antibacterial and immunostimulating casein-derived substances from milk: casecidin, isracidin peptides. Food Chem Toxicol. 1996;34:131–145. doi: 10.1016/0278-6915(95)00097-6. [DOI] [PubMed] [Google Scholar]
- Li W, Tan T, Xu W, Xu L, Dong N, Ma D, Shan A. Rational design of mirror-like peptides with alanine regulation. Amino Acids. 2016;48:403–417. doi: 10.1007/s00726-015-2094-y. [DOI] [PubMed] [Google Scholar]
- Longhi C, Marazzato M, Conte MP, Iebba V, Schippa S, Seganti L, Comanducci A. Effect of lactoferricin on fluoroquinolone susceptibility of uropathogenic Escherichia coli. J Antibiot. 2009;62:109–111. doi: 10.1038/ja.2008.22. [DOI] [PubMed] [Google Scholar]
- Modiri S, Kermanshahi RK, Soudi MR, Dad N, Ebadi M, Zahiri HS, Noghabi KA. Growth optimization of Lactobacillus acidophilus for production of antimicrobial peptide acidocin 4356: scale up from flask to lab-scale fermenter. Iran J Biotechnol. 2021;19(3):e2686. doi: 10.30498/ijb.2021.218725.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Taheri B, Momenzadeh N, Salarinia R, Nabipour I, Farshadzadeh Z, Bargahi A. Identification and characterization of novel antimicrobial peptide from hippocampus comes by In Silico and experimental studies. Mar Biotechnol. 2018;20(6):718–728. doi: 10.1007/s10126-018-9843-3. [DOI] [PubMed] [Google Scholar]
- Mokhtari R, Rezaei M, Fard MK, Dirandeh E. Evaluation of antimicrobial and antioxidant activities of casein-derived bioactive peptides using trypsin enzyme. J Food Qual. 2023 doi: 10.1155/2023/1792917. [DOI] [Google Scholar]
- Moulay M, Aggad H, Benmechernene Z, Guessas B, Henni DE, Kihal M. Cultivable lactic acid bacteria isolated from Algerian raw goat’s milk and their proteolytic activity. World J Dairy Food Sci. 2006;1(1):12–18. [Google Scholar]
- Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52(1):1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- Panchal G, Hati S, Sakure A. Characterization and production of novel antioxidative peptides derived from fermented goat milk by L. fermentum. LWT. 2020;119:108887. doi: 10.1016/j.lwt.2019.108887. [DOI] [Google Scholar]
- Rodríguez-Hernandez MJ, Saugar J, Docobo-Perez F, de La Torre BG, Pachón-Ibañez ME, et al. Studies on the antimicrobial activity of cecropin A-melittin hybrid peptides in colistin-resistant clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother. 2006;58:95–100. doi: 10.1093/jac/dkl145. [DOI] [PubMed] [Google Scholar]
- Sánchez-Gómez S, Japelj B, Jerala R, Moriyón I, Fernández Alonso M, Leiva J, Blondelle SE, Andrä J, Brandenburg K, Lohner K, de Tejada GM. Structural features governing the activity of lactoferricin-derived peptides that act in synergy with antibiotics against Pseudomonas aeruginosa in vitro and in vivo. Antimicrob Agents Chemother. 2011;55:218–228. doi: 10.1128/AAC.00904-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PE, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano M, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tamang MD, Dey S, Makaju RK, Jha BK, Shivananda PG, Bhramadatan KN. Bacterial aetiology of lower respiratory tract infection in patients attending Manipal Teaching Hospital. J Nepal Assoc Med Lab Sci. 2005;7:15–19. [Google Scholar]
- Trujillo AJ, Casals I, Guamis B. Analysis of major ovine milk proteins by reversed-phase high-performance liquid chromatography and flow injection analysis with electrospray ionization mass spectrometry. J Chromatogr A. 2000;870(1–2):371–380. doi: 10.1016/S0021-9673(99)01097-3. [DOI] [PubMed] [Google Scholar]
- WHO . Critically important antimicrobials for human medicine: ranking of antimicrobial agents for risk management of antimicrobial resistance due to non-human use. 5. Geneva: World Health Organization; 2017. [Google Scholar]
- Xu L, Chou S, Wang J, Shao C, Li W, Zhu X, Shan A. Antimicrobial activity and membrane-activemechanism of tryptophan zipper-like β-hairpin antimicrobial peptides. Amino Acids. 2015;47:2385–2397. doi: 10.1007/s00726-015-2029-7. [DOI] [PubMed] [Google Scholar]
- Xue J, Michael Davidson P, Zhong Q. Antimicrobial activity of thyme oil co-nanoemulsified with sodium caseinate and lecithin. Int J Food Microbiol. 2015;210:1–8. doi: 10.1016/j.ijfoodmicro.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Tomita M, Giehl TJ, Ellison RT. Antibacterial activity oflactoferrin and a pepsin-derived lactoferrin peptide fragment. Infect Immun. 1993;61(2):719–728. doi: 10.1128/iai.61.2.719-728.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and material that support our finding are available from the corresponding author upon reasonable request.
Not applicable.