Abstract
Hydrocotyle bonariensis is an edible herb, that is also used for traditional medical purposes. It is high in antioxidants, phenols, and flavonoids. However, there is limited information on the nutritional composition and the mechanisms by which nutritional and functional constituents of H. bonariensis affect human metabolism. With an aim to identify gaps in evidence to support the mainstream use of H. bonariensis for health and as a functional food, this review summarises current knowledge of the taxonomy, habitat characteristics, nutritional value and health-related benefits of H. bonariensis and its extracts. Ethno-medical practices for the plant are supported by pharmacological studies, yet animal model studies, clinical trials and food safety assessments are needed to support the promotion of H. bonariensis and its derivatives as superfoods and for use in the modern pharmaceutical industry.
Keywords: Functional food, Nutraceutical, Phytochemicals, Pharmacological, Therapeutic plant, Superfood
Introduction
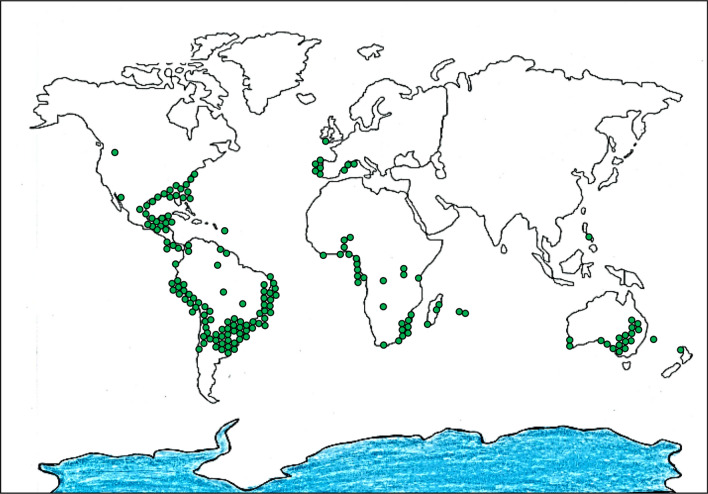
Hydrocotyle bonariensis (H. bonariensis) is a wild species of large leaf marsh pennywort, previously assigned to the family Apiaceae, but now placed within the family Araliaceae of the order of Apiales (Nicolas and Plunkett 2009). The plant grows predominately in wetlands of temperate and tropical regions (Florinsiah et al. 2013; Masoumian et al. 2011) and is well distributed across many regions, including the southeastern part of the United States, Central America, South America, the Caribbean and sub-saharan Africa (Fig. 1). H. bonariensis can also be found in Asian countries, including China, India, Sri Lanka, Indonesia and Malaysia (Goh 2007). Regionally, H. bonariensis is known by an array of common names, such as pegaga embun in Malaysia (Ajani et al. 2009), karo in Nigeria (Ajani et al. 2012) as well as redondita de agua, paragüita, and tembladerilla in South America (Ouviña et al. 2009). The plant is semi-aquatic and can grow in soils ranging from inland damp, wet trenches and edges of ponds to saline beach dunes (Evans and Whitney 1992). A recent study showed the ability of H. bonariensis to successfully thrive in multiple habitats is mainly because of high phenotypic plasticity (Chiarello and Joesting 2018). H. bonariensis contains several bioactive components, including alkaloids, tannins, flavonoids, saponins, and phenolic compounds (Go et al. 2017). Among the bioactive components, phenolic acids and flavonoids are the core phenolics that constitute the total antioxidant content (Wojdyło et al. 2007). The antioxidant contents of H. bonariensis are reported to be higher than those of several other nutraceutical herbs such as Barringtonia racemose (powder-puff tree), Kaempferia galanga (aromatic ginger), Piper sarmentosum (wild betel) and Cosmos caudatus (Ulam raja) (Sumazian et al. 2010). Although the plant is rich in bioactive compounds, has a high antioxidant content and is easy to grow, H. bonariensis is not widely consumed in modern diets. To promote this plant as healthy food and for use in value-added nutraceutical and potentially pharmaceutical products, there is a need to collate the relevant information for H. bonariensis. Here, we considered the phytochemical and pharmacological value of H. bonariensis, also the taxonomy, habitat characteristics and reports of use as a functional food. Next, we discuss the prospects for the development of novel nutriceuticals and plant-based pharmaceuticals based on H. bonariensis and its derivatives. All information on H. bonariensis was retrieved from online searches using Google Scholar, Web of Science, ScienceDirect and PubMed. Global databases, including Integrated Taxonomic Information System (http://www.itis.gov), PopSet (https://ncbi.nlm.nih.gov/popset) and PubChem (https://pubchem.ncbi.nlm.nih.gov/) were used to validate the scientific names, define the evolution of H. bonariensis and verify chemical descriptions of phytochemicals present in the plant.
Fig. 1.
Distribution of H. bonariensis across the world (Data
source: Global Biodiversity Information Facility, https://www.gbif.org/species/3034611)
Taxonomy, molecular phylogeny and ecology of H. bonariensis
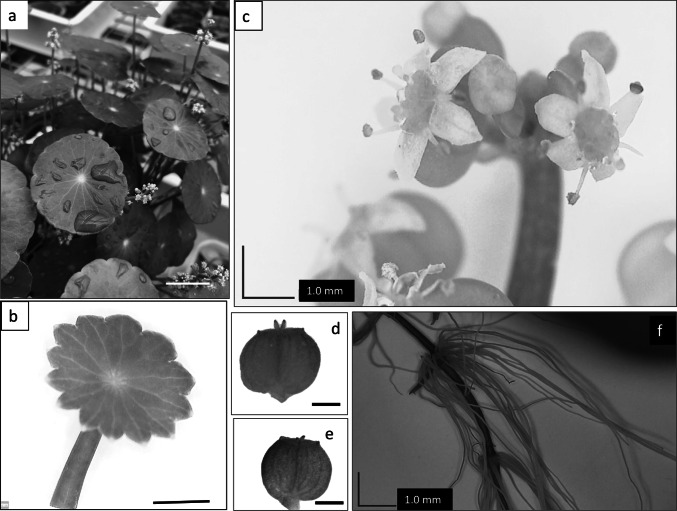
J. P. Tournefort was the first to introduce the genus Hydrocotyle (Hylander 1945). ‘Hydrocotyle’ is derived from the Greek words 'hydro', for water and ‘kotyle’ for dish or plate, referring to the aquatic habitat and dish or plate-shaped leaf of the species (Bandara et al. 2011). The genus was later validated and expanded by C. Linnaeus in 1753 (Konstantinova and Yembaturova 2010). The species name bonariensis was derived from Buenos Aires, Argentina, where the plant is native and was first identified. H. bonariensis is a semiaquatic perennial herb with long stolons (runners), from which roots, leaves and inflorescences are produced at nodes (Fig. 2a). Nodes with an independent root system can act as physiologically independent units known as ramets and can be propagated vegetatively (Evans 1992). The leaves are circular to widely elliptical with palmate venation, arranged in small clusters of one to five glossy leaflets, 1.2 to 3 cm in diameter and attached by a petiole of 2 to 20.5 cm long (Fig. 2b). Inflorescences are produced at the nodes and opposite to the leaf (Fig. 2c). The inflorescence is umbelliferous, about 5.08 to 7.62 cm long, with clusters of white fragrant flowers. The hermaphroditic flower contains five sepals, five petals, six stamens and two separate carpels with an inferior ovary surmounted by a style and a stigma (Fig. 2c). The fruit emerges as a green schizocarp turning brown when mature (Fig. 2d, e) and containing brown to black seeds. Although seed production occurs throughout the growing season, the seedling survival rate is reported to be less than 1% and hence vegetative reproduction through stolon (Fig. 2f) is the primary mode of reproduction for this plant (Evans 1992).
Fig. 2.
Hydrocotyle bonariensis a Mature plant with inflorescence (bar = 40 mm), b Immature leaf (bar = 30 mm), c Compound umbel inflorescence (bar = 1 mm), d Indehiscent schizocarp (bar = 1 mm), e Mature schizocarp (bar = 10 mm), f Stolon with lateral roots (bar = 1 mm)
A few molecular phylogenetic and taxonomic studies have been undertaken to assess the evolutionary position of the Hydrocotyle taxa (Nicolas and Plunkett 2009; Danderson et al. 2018; Perkins 2019). The studies have assisted in the re-categorisation of Hydrocotyle from the family Apiaceae to the family Araliaceae in the order of Apiales based on chloroplast markers (Nicolas and Plunkett 2009; Danderson et al. 2018; Perkins 2019). There is little molecular phylogenetic or genomic information currently available for H. bonariensis, with only ten DNA sequence accessions of H. bonariensis in the National Center for Biotechnology Information (NCBI) database at the present time (Table 1). H. bonariensis can be found in two main distinct ecological environments; coastal sand dune and inland habitats. H. bonariensis growing in coastal sand dune environments is exposed to harsh growing conditions, including relatively higher temperatures of air and sand, higher intensity of sunlight due to lack of shading plants, and high salinity (Hesp 1991; Haddad and Mazzafera 1999). H. bonariensis in inland habitats have more favourable conditions of growth, such as the shade of a canopy and freshwater irrigation. In addition, inland soils are mainly loamy and consist of smaller particle sizes that hold more water and nutrients (e.g. organic matter, potassium, nitrate, and ammonium) compared to large-sized sandy soils (Chiarello and Joesting 2018). As a result, H. bonariensis grown on inland soil has been reported to have greater leaf area, petiole length, petiole thickness, petiole fresh weight, and abaxial stomata density compared to those grown on coastal sand dunes (Chiarello and Joesting 2018). In order to survive in sand dune habitats, H. bonariensis undergoes phenotypic adjustment, adopting a strategy of leaf inclination to withstand extreme weather conditions (Chiarello and Joesting 2018; Joesting et al. 2012). The capacity of H. bonariensis to thrive in this environment suggests that the plant has good potential for cultivation in saline agriculture. However, such studies appear to be lacking. It would also be interesting to know how if and how such stress affects phytonutrient and active compound content in H. bonariensis. Because there is a general lack of information on diverse plant accessions and their diversity, there is also a lack of understanding in terms of nutrient variability, flavour, growth characteristics.
Table 1.
GenBank sequence data of H. bonariensis (http://www.ncbi.nlm.nih.gov/)
| Accession number | Sequence type | Size (bp) | Source | Description |
|---|---|---|---|---|
| KJ773564.1 | DNA | 1323 | Chloroplast | Hydrocotyle bonariensis ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) gene, partial cds |
| KJ772838.1 | DNA | 651 | Chloroplast | Hydrocotyle bonariensis maturase K (matK) gene, partial cds |
| MH780008.1 | DNA | 914 | Chloroplast | Hydrocotyle bonariensis tRNA-Leu (trnL) gene and trnL-trnF intergenic spacer, partial sequence |
| MH757450.1 | DNA | 456 | Nuclear | Hydrocotyle bonariensis external transcribed spacer and 18S ribosomal RNA gene, partial sequence |
| KP057627.1 | DNA | 568 | Chloroplast | Hydrocotyle bonariensis isolate P069 tRNA-Leu (trnL) gene, partial sequence |
| KP057626.1 | DNA | 564 | Chloroplast | Hydrocotyle bonariensis isolate P008 tRNA-Leu (trnL) gene, partial sequence |
| JQ937322.1 | DNA | 308 | Chloroplast | Hydrocotyle bonariensis psbA-trnH intergenic spacer, complete sequence |
| GQ243831.1 | DNA | 884 | Chloroplast | Hydrocotyle bonariensis voucher Ware s.n. tRNA-Asp (trnD) gene, partial sequence; trnD-trnY intergenic spacer, tRNA-Tyr (trnY) gene, trnY-trnE intergenic spacer, tRNA-Glu (trnE) gene, and trnE-trnT intergenic spacer, complete sequence; and tRNA-Thr (trnT) gene, partial sequence |
| AF077894.1 | DNA | 619 | Nuclear | Hydrocotyle bonariensis internal transcribed spacer 1, partial sequence; 5.8S ribosomal RNA gene, complete sequence and internal transcribed spacer 2, partial sequence |
| GQ244102.1 | DNA | 1007 | Chloroplast | Hydrocotyle bonariensis voucher Ware s.n. rpl16 gene, intron |
Traditional and current uses of H. bonariensis in food and medicine
In Indonesia, Malaysia and Taiwan, the leaves and stalks of H. bonariensis are consumed as a fresh salad (known in the Malay language as “ulam”), fresh juice, as a fermented vegetable, as a condiment sprinkled on food and as a herbal tea (Reihani and Azhar 2012). H. bonariensis is also recognised as a wild edible plant in Texas, U.S.A. (https://medivetus.com/botanic/hydrocotyle-bonariensis-beach-pennywort-edible-and-medicinal-uses/). Other than that, H. bonariensis is also recognised for being an excellent source of bioactive phytochemicals for traditional treatments of diseases. For example, indigenous people in the United States use this herb as an emetic, diuretic and laxative (Evans 1992), while the locals in South America use the aerial part of the plants for treating cutaneous erythema (Ouviña et al. 2009). In addition, H. bonariensis is used among local people in Western Nigeria for treatment of tuberculosis and ophthalmic diseases, for pain relief due to rheumatism and arthritis, as well as for longevity and enhancement of brain capacity (Masoumian et al. 2011). As there are only anecdotal sources, it is difficult to determine how widely the plant is used or cultivated for such use, but reports for other pennywort species suggest that overcollection from their natural habitats is an issue of concern and there is a need for more economically viable and sustainable solutions, such as farming on a larger scale (Hashim 2011).
Potential of H. bonariensis as a functional food and nutraceutical
Functional foods are foods either whole, fortified, enriched or enhanced forms of food that provide health benefits beyond basic nutrition (Hasler 2002). Functional foods have high global demand, with the revenue generated by the functional food market worldwide expected to rise substantially from about 174.75 billion U.S. dollars in 2019 to over 275.77 billion U.S. dollars in 2025 (Global functional food market revenue 2019−2025). From the nutritional aspect, H. bonariensis leaves contain high amounts of choline (3.20 mg/L) compared to other pennyworts such as Centella asiatica and H. sibthorpioides (Maulidiani et al. 2012). Choline is an essential nutrient involved in the production of acetylcholine, a neurotransmitter which is associated with memory, muscle and liver functions (Wallace et al. 2018). According to the Food and Nurition Board of the U.S.A. Institute of Medicine, the recommended daily intake for choline has been set at 425 mg per day for women and 550 mg per day for men (Zeisel and Da Costa 2009). Humans can produce choline via the hepatic phosphatidylethanolamine N-methyltransferase pathway in the liver, but the amount naturally synthesised is not sufficient to meet human needs (Jacobs et al. 2010). Hence, dietary intake of food rich in choline is essential to prevent deficiency (Wallace et al. 2018).
Also, essential micronutrients such as calcium (Ca) (60%), magnesium (Mg) (35%), iron (Fe) (3%), zinc (Zn) (1%) and copper (Cu) (1%) calculated as the percentage of the dry weight of leaves were also found to be higher in H. bonariensis compared to Centella asiatica and H. sibthorpioides (Monyn et al. 2016). These minerals are essential for human health and thus deficiencies in the minerals can lead to certain health risks (Mehri 2020). For example, a dietary Ca deficiency was reported to be epidemiologically linked to bone diseases (Almaghamsi et al. 2018), Mg deficiency may increase risks of atherosclerosis and endothelial dysfunction (Kostov and Halacheva 2018), Cu (Myint et al. 2018) and Fe (Gooding et al. 2019) deficiencies are common causes of anaemia, and Zn deficiency is associated with hypogeusia (Gooding et al. 2019). Eating a well-balanced diet, such as by consuming foods rich in Ca, Mg, Cu, Fe, and Zn can fulfil nutritional needs and prevent mineral deficiencies and so the inclusion of H. bonariensis in the diet can address this need.
Several parts of the plant, including roots, petioles and leaves (Haida et al. 2021) of H. bonariensis were reported to have a high amount of polysaccharides (1.00 mass/mass ratio) which can serve as a source of dietary fibre (Dantas-Santos et al. 2012). Plant-derived fibre, which primarily comprises plant-cell wall polysaccharides, is important in the diet for human health as a source of energy and to minimize risk factors of chronic diseases (Lovegrove et al. 2017). The fresh aerial part of H. bonariensis was reported to have relatively higher total reducing sugar (1.94 mg g−1FW) and hydrolysed sugar (3.84 mg g−1FW), total phenolics (0.96 mg GAE g−1FW) and total flavonoids (13.79 mg CE g−1FW) and antioxidant activities (45.45% 2,2-diphenyl-1-picrylhydrazyl inhibition) compared to Centella asiatica (Haida et al. 2021). Polyphenols such as flavonoids inhibit or delay oxidative damage of cells by scavenging the free radicals (peroxide or hydroperoxide) and thus reduce the risk of degenerative diseases (Chandra et al. 2014). Yet, despite the widespread traditional use, other than these sparse reports, there is a distinct lack of information on the nutritional and health-related content of H. bonariensis, which is surely worthy of attention. Studies to document the nutritional profile would enable accurate comparisons with other herbs and could lead to more widespread cultivation and use. While H. bonariensis is noted as a traditional herb among several communities around the world, it appears that its use is very localised to those specific communities and it has yet to reach sufficient interest for export trade as either a food or medicinal ingredient. The potential for wider use and even export will require enhancing production by larger-scale cultivation, including indoor cultivation. A recent study showed indoor cultivation of H. bonariensis under red and blue LED light with a higher blue irradiance (R: B = 83: 65) can enhance plant growth and biomass by twofold compared to plants grown under natural light conditions (Nair et al. 2021). Other than large scale cultivation, it is also important to develop post-harvest processes that retain health-promoting phytochemicals in the end-product to better serve the export potential for this herb. There is only one report on post-harvest processing of H. bonariensis, in which leaves were dried using a multi-chamber dehumidified-air drying system with an airflow rate of 50 L/min and temperature at 50 °C (Go et al. 2017). Leaves dried with this system retained 84.9% of the phenolic and 66.5% of the flavonoid contents, comparing favourably with commercial methods requiring higher temperatures, such as hot air drying, drum drying and spray drying (Go et al. 2017). Further research on processing and shelf-life for food and nutraceutical use will be of great value to develop markets for H. bonariensis products.
Potential of H. bonariensis for human health use
Phytochemicals have protective qualities for human health. Few studies have been conducted to analyse and quantify the phytochemical content in H. bonariensis, with the majority examining leaf tissue extracts (Table 2). A total of sixteen bioactive compounds have been reported for H. bonariensis, mainly belonging to three major groups of phytochemicals: saponins, flavonols and triterpenes (Table 2). Table 3 shows the chemical structures of phytochemicals present in H. bonariensis, constructed using ChemDraw® program (https://www.perkinelmer.com/product/chemdraw-professional-chemdrawpro). Previous studies using in-vitro and in-vivo approaches have demonstrated that the phytochemical compounds reported in leaves and roots of H. bonariensis have unique biological and pharmacological functionalities such as antioxidants and for the treatment of health-related conditions including inflammation, cataract development, arthritis and cancer (Table 4).
Table 2.
Various extraction methods used to isolate phytochemicals from different plant parts of H. bonariensis
| Plant part | Extraction method | Phytochemicals | Quantity | References |
|---|---|---|---|---|
| Leaf | 100% aqueous | Tannic acid | 0.38 µ mol−1 | Marino et al. (2009) |
| Leaf | 100% aqueous | Flavonoids | 3.32 mg/g DW | Sumazian et al. (2010) |
| Phenols | 9.3 mg/g DW | |||
| Ascorbic acid | 0.70 mg/g FW | |||
| Root | 70% aqueous methanol | Saponins | Not determined | Tabopda et al. (2012) |
| Leaf | 70% aqueous methanol | Phenols | 28.6 mg GAE/100 g DW | Maulidiani et al. (2014) |
| Flavonoids | 0.759 mg/100 g of extract | |||
| Triterpenes glycosides | Not determined | |||
| Leaf | Hexane | Saponins | 47.20 ± 1.600% FW | Obaseki et al. (2016) |
| Phenols | 1.07 ± 0.003 mg(GE)/g | |||
| Flavonoids | 1.36 ± 0.042 mg(QE)/g | |||
| Tannins | 18.74 ± 0.050% FW | |||
| Terpenoids | Not determined | |||
| Sterols | Not determined | |||
| Leaf | Ethylacetate: methanol | Flavonoids (quercetin) | Not determined | Ajani et al. (2017) |
FW Fresh weight, DW Dry weight
Table 3.
Chemical descriptions of phytochemicals present in H. bonariensis
| Group | Chemical structure |
Molecular formula/ (Molecular weight) |
Chemical Name (Common name, if available) |
Reference (PubChem CID, if available) |
|---|---|---|---|---|
| Saponins |
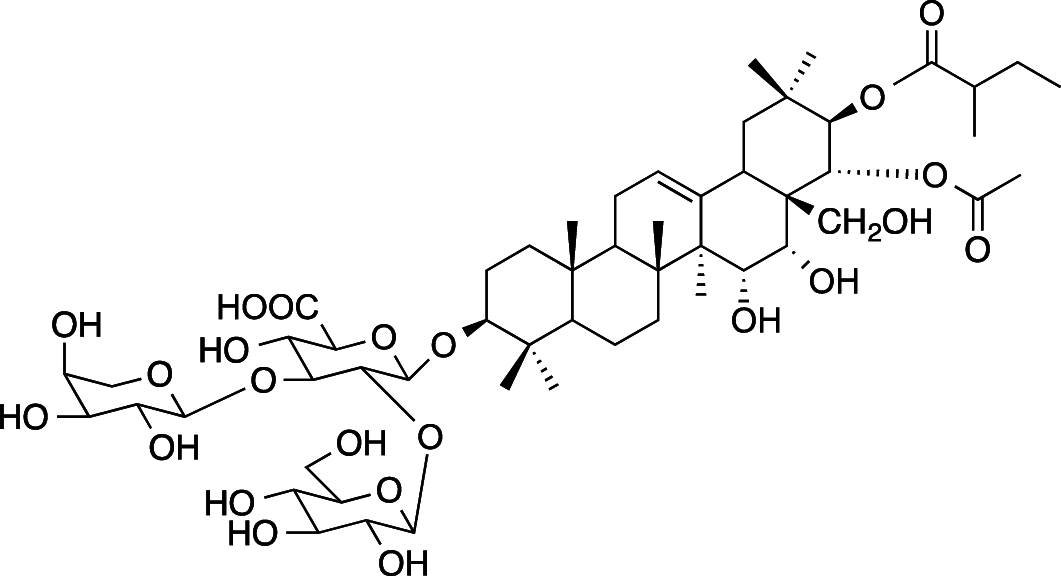
|
C54H86O23 (1103.26 g/mol) |
3-O-{β-D-glucopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-D-glucuronopyranosyl}-21-O-(2-methylbutyroyl)-22-O-acetyl-R1-barrigenol (Common name: bonarienoside A) |
Tabopda et al. (2012) (PubChem CID: 56,951,544) |
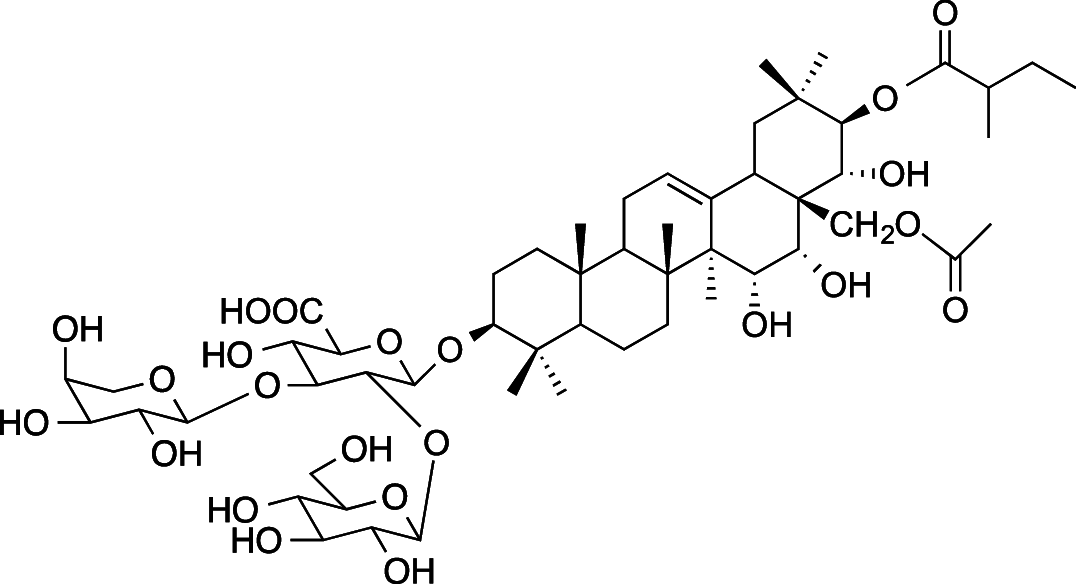
|
C54H86O23 (1103.26 g/mol) |
3-O-{β-D-glucopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-D-glucuronopyranosyl}-21-O-(2-methylbutyroyl)-28-O-acetyl-R1-barrigenol (Common name: bonarienoside B) |
Tabopda et al. (2012) (PubChem CID: 56,951,656) | |
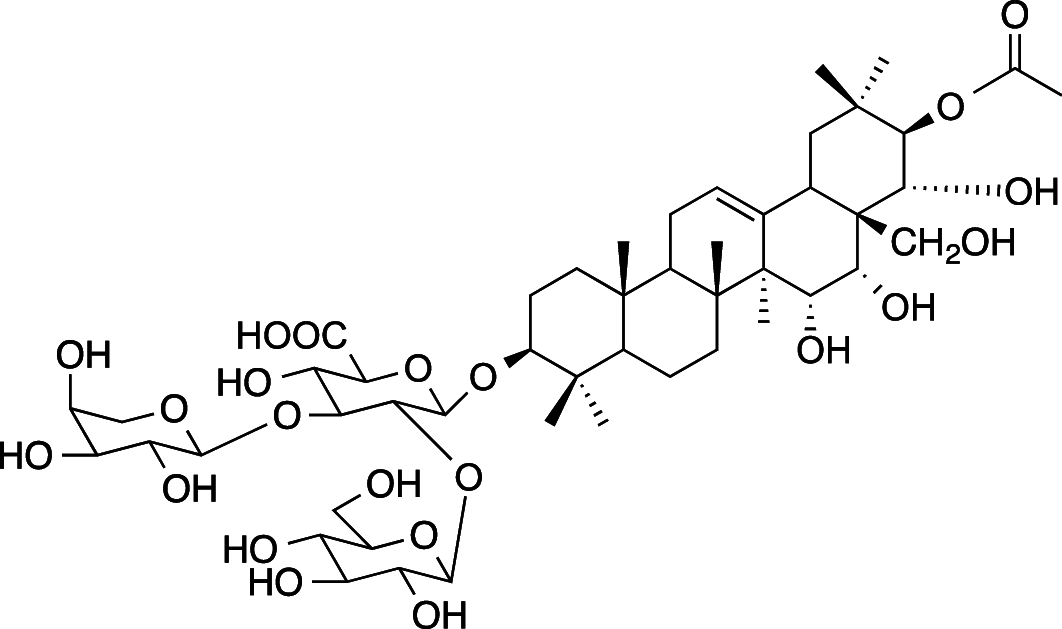
|
C49H78O22 (1019.14 g/mol) |
3-O-{β-D-glucopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-D-glucuronopyranosyl}-21-O-acetyl-R1-barrigenol (Common name: bonarienoside C) |
Tabopda et al. (2012) (PubChem CID: 56,951,657) | |
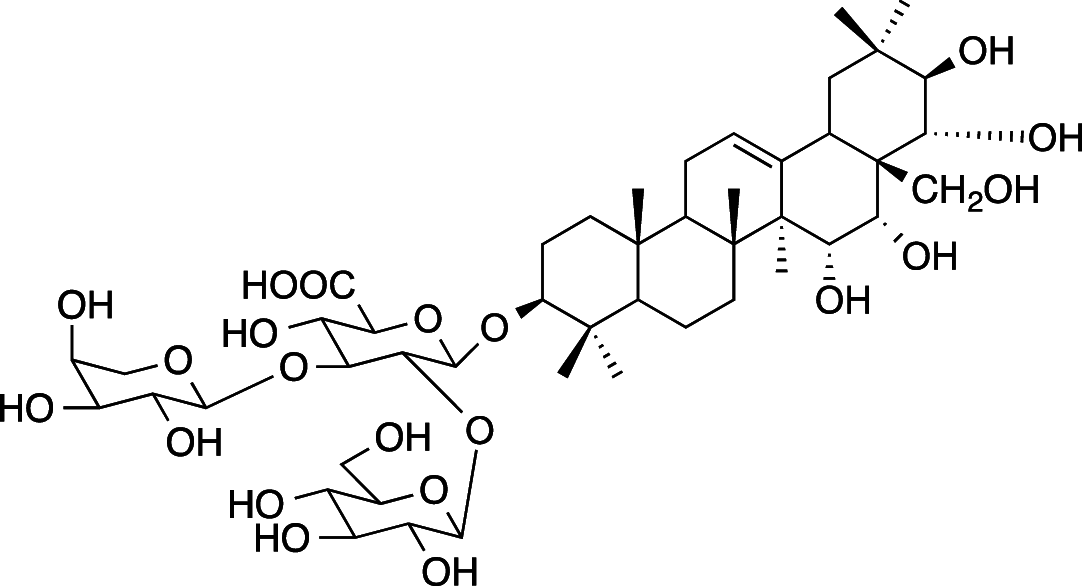
|
C47H76O21 (977.10 g/mol) |
3-O-{β-D-glucopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-D-glucuronopyranosyl}-R1-barrigenol (Common name: bonarienoside D) |
Tabopda et al. (2012) (PubChem CID: 56,949,673) | |
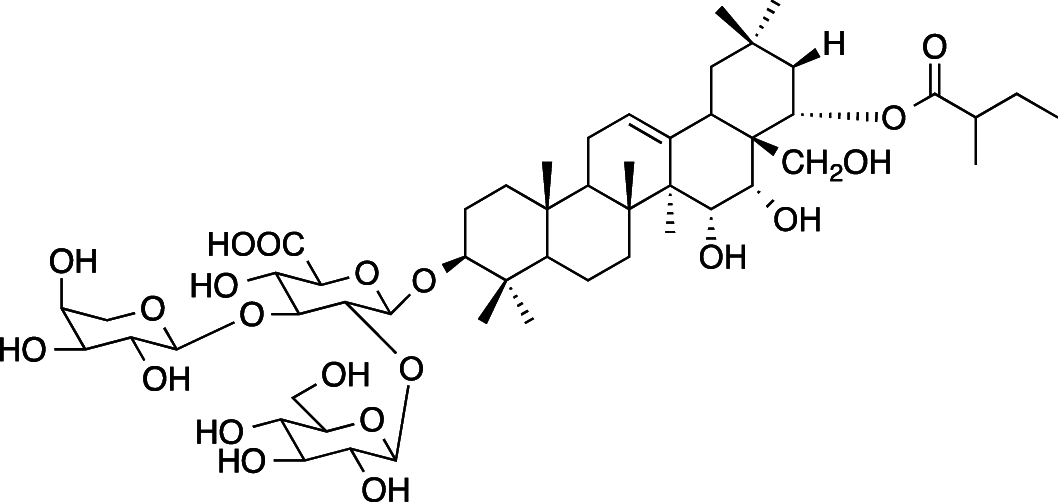
|
C52H84O21 (1045.22 g/mol) |
3-O-{β-D-glucopyranosyl-(1 → 2)-[α-l-arabinopyranosyl-(1 → 3)]-β-D-glucuronopyranosyl}-22-O-(2-methylbutyroyl)-A1-barrigenol (Common name: bonarienoside E) |
Tabopda et al. (2012) (PubChem CID: 56,949,674) | |
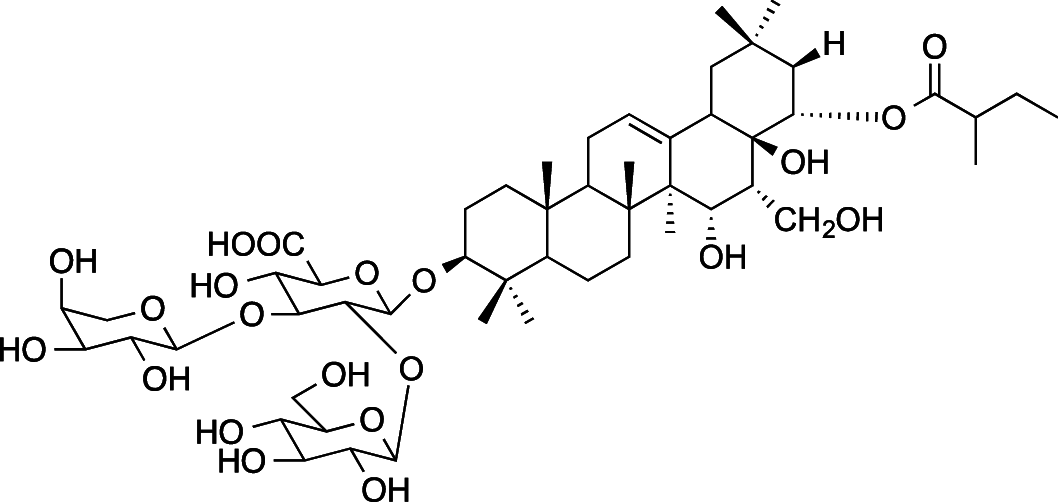
|
C52H84O21 (1045.22 g/mol) |
21-O-[2-methylbutanoyl]-3β, 15α, 16α, 21β, 22α, 28-hexahydroxyolean-12-ene 3-O-[α-L-arabinopyranosyl(1 → 3)]β-D-glucopyranosyl (1 → 2)-beta-D-glucuronopyranoside (Common name: saniculoside-R1) |
Tabopda et al. (2012); Schöpke et al. (1998) (PubChem CID: 9,491,771) |
|
| Flavonols |
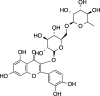
|
C27H30O16 (610.52 g/mol) |
Quercetin-rutinoside | Maulidiani et al. (2014) (PubChem CID: 124,221,768) |
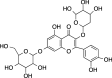
|
C26H28O16 (596.49 g/mol) |
Quercetin-3-O-pentosyl-7-O-hexoside | Maulidiani et al. (2014) (PubChem CID: 133,053,374) | |
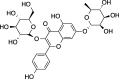
|
C27H30O15 (594.52 g/mol) |
Kaempferol-3-O-glucoside 7-O-rhamnoside | Maulidiani et al. (2014) (PubChem CID: 14,035,324) | |
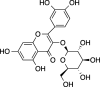
|
C21H20O12 (466.39 g/mol) |
Quercetin-3-O-glucoside |
Maulidiani et al. (2014) (PubChem CID: 5,280,804) |
|
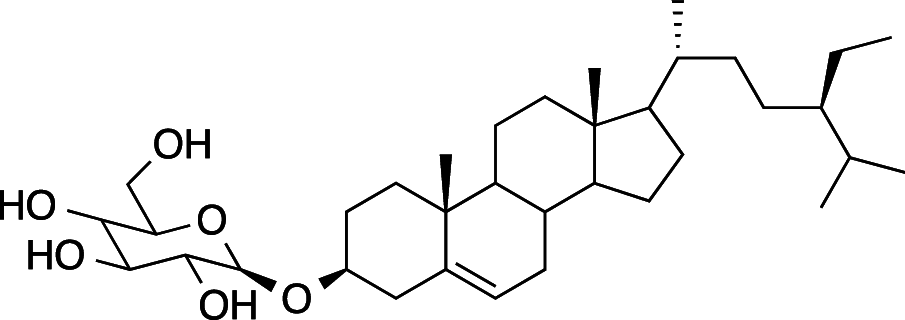
|
C35H60O6 (576.86 g/mol) |
3-O-β-D-glucopyranosyl-sitosterol | Ajani et al. (2017) (PubChem CID: 5,742,590) | |
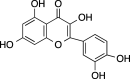
|
C15H10O7 (302.24 g/mol) |
Quercetin | Ajani et al. (2017) (PubChem CID: 5,280,343) | |
| Tripertenes |
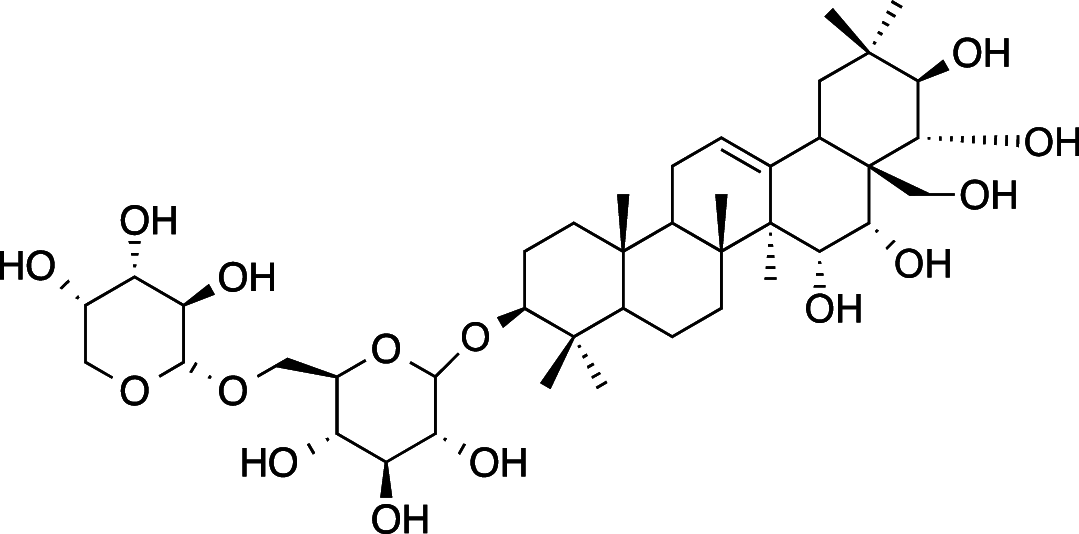
|
C41H68O15 (800.98 g/mol) |
3β,15α,16α,21β,22x,28-hexahydroxy-Δ12-oleanane-3-Ο-[α-L-arabinopyranosyl-(1 → 6)]-β-D-glucopyranoside (Common name: Ranuncoside I) |
Maulidiani et al. (2014); Greca et al. (1994) (PubChem CID: 101,672,524) |
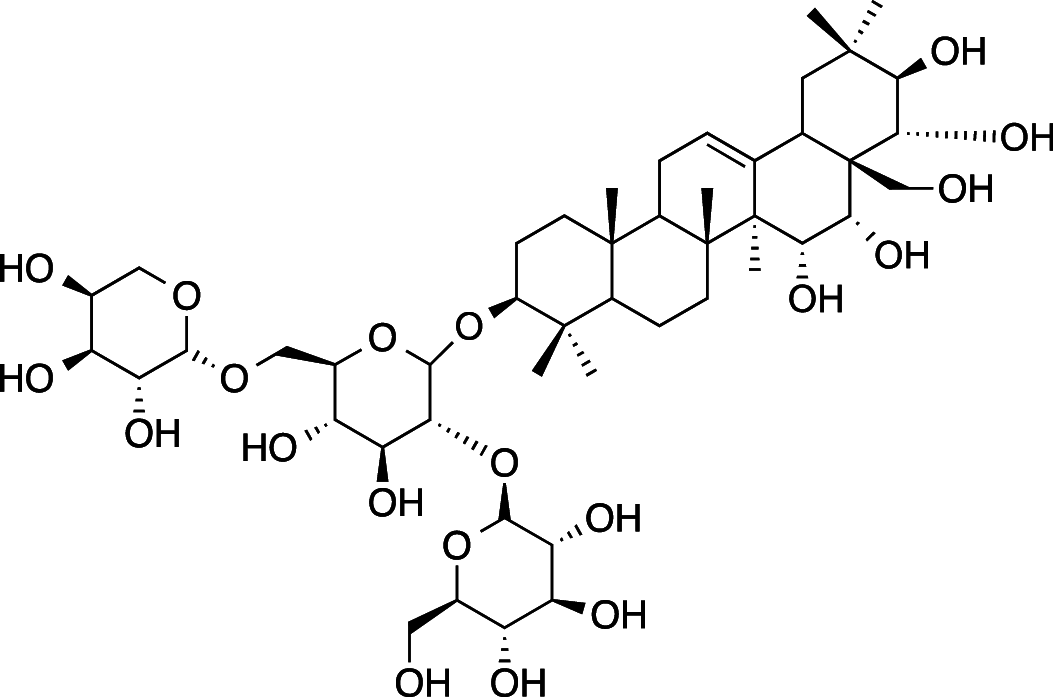
|
C47H78O20 (963.12 g/mol |
)3β,15α,16x,21β,22α,28-hexahydroxy-Δ12-oleanane-3-Ο-[α-L-arabinopyranosyl-(1 → 6)]-β-D-glucopyranosyl-(1 → 2)- β- D-glucopyranoside (Common name: Ranuncoside II) |
Maulidiani et al. (2014); Greca et al. (1994) (PubChem CID: 101,673,080) | |
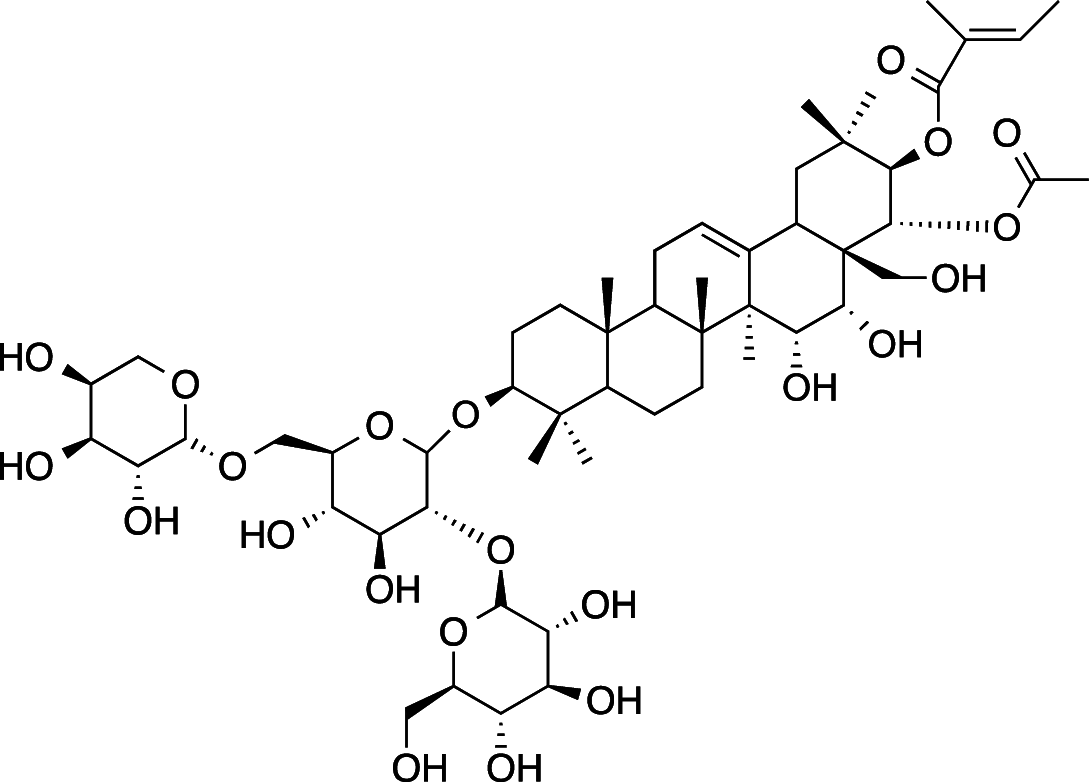
|
C54H86O22 (1087.26 g/mol) |
3β,15α,16α,21β,22α,28-hexahydroxy-Δ12-oleanane-21-Ο-tigloyl-22acetyl-3-Ο-[α-L-arabinopyranosyl-(1 → 6)][β-D-glucopyranosyl-(1 → 2)]-β-D-glucopyranoside (Common name: Ranuncoside IV) |
Maulidiani et al. (2014); Greca et al. (1994) (PubChem CID: 101,672,525) | |
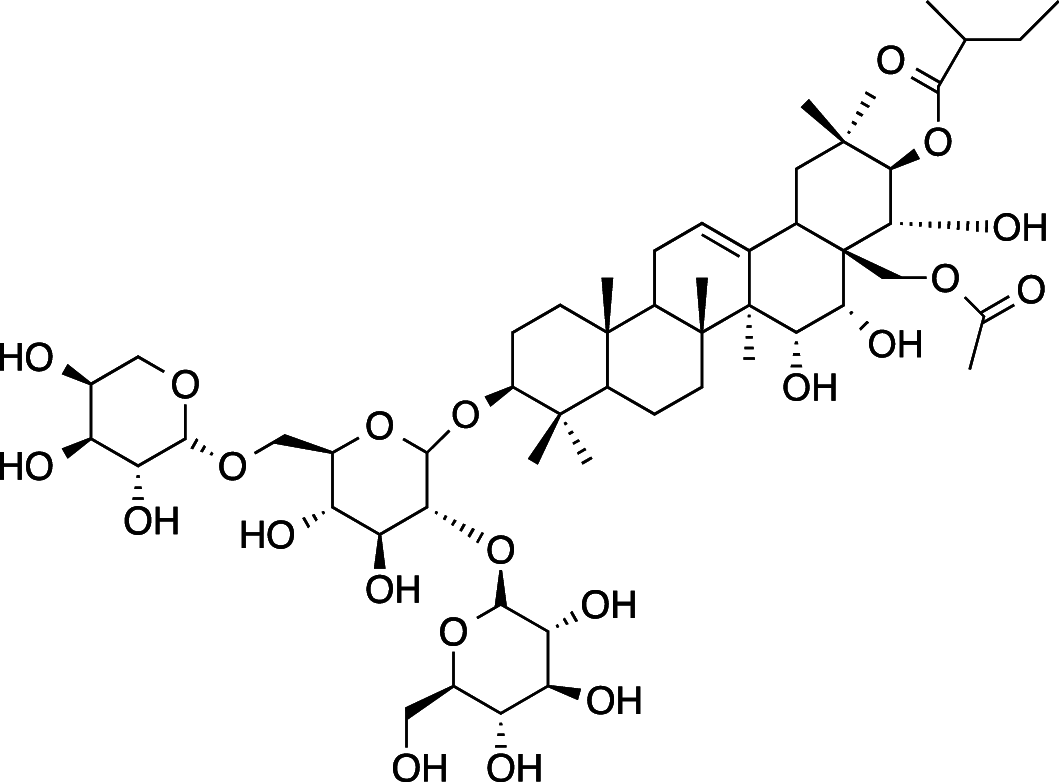
|
C54H88O22 (1089.28 g/mol) |
3β,15α,16α,21β,22α,28-hexahydroxy-Δ12-oleanane-21-Ο-[2-methylbutyroyl)-28-Ο-acetyl-3-Ο-[α-L-arabinopyranosyl-(1 → 6)][β-D-glucopyranosyl-(1 → 2)]- β- D-glucopyranoside (Common name: Ranuncoside V) |
Maulidiani et al. (2014); Greca et al. (1994) (PubChem CID: 101,672,526) |
Table 4.
Pharmacological activities of phytochemicals and extracts of H. bonariensis
| Phytochemicals | Biochemical analysis/ biological assays | Pharmacological activity | Reference |
|---|---|---|---|
| Not mentioneda |
1. Thiobarbituric acid reacting substances test 2. Reduced glutathione activity 3. Superoxide dismutase activity 4. Colorimteric assay of catalase |
Anticataract potential | Ajani et al. (2009) |
| Not mentioneda |
1. Triglyceride GPOPAP kit 2. Cholesterol CHODPAP kit 3. High Density Lipoprotein Cholesterol precipitant determination 4. Low Density Lipoprotein Cholesterol calculation 5. Atherogenic risk index 6. Thrombin physicochemical transformation |
Cardioprotective effects | Ajani et al. (2012) |
| Not mentioneda | Patch clamp experiments for IK currents recording | Potential for management of cardiac arrythmias | Kaboua et al. (2021a) |
| Saponins (bonarienoside derivatives) | MTT ([3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cytotoxicity assay | Anti-cancer potential | Tabopda et al. (2012) |
| Oleanane-type triterpenes | 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay | Antioxidant activity | Maulidiani et al. (2014) |
|
Flavonoids/ polyphenols |
1. DPPH radical scavenging assay 2. Metal chelating activity 3. Nitric oxide (NO) radical scavenging activity 4. Hydrogen peroxide (H2O2) scavenging activity |
Antioxidant activity | Ajani et al. (2017) |
| Not mentioneda | 12-O-Tetradecanoylphorbol-13 acetate (TPA)-induced ear edema in a mouse model | Anti-inflammatory effects | Ouviña et al. (2009) |
| Hexadecanoic acid methyl ester, falcarinol, phytol | Heat-induced albumin denaturation, human red blood cell (HRBC) membrane stabilization assays, haematological assessments, liver function tests | Anti-inflammatory effects | Obaseki et al. (2016) |
| Not mentioneda |
MTT cytotoxic assay Antichlamydial activity assays |
Inhibitory activity against Chlamydia trachomatis and Chlamydia pneumoniae | Entrocassi et al. (2021) |
| Dibenzylbutyrolactone lignans |
MTT cytotoxic assay Haemolytic activity test |
Inhibitory activity against Trypanosoma cruzi |
Souza et al. (2021) |
aNot mentioned as total leaf extracts were used
Anti-inflammatory activity
Scientific studies on H. bonariensis extracts suggest the plant to have anti-inflammatory potential, which could be developed as an alternative to synthetic anti-inflammatory drugs that present adverse effects when used in the long term (Wongrakpanich et al. 2018). For example, topical application of infusion and methanolic extracts from the total aerial part of H. bonariensis showed 30% to 58% inhibition of mouse ear oedema (a local swelling), suggesting the effectiveness of the plant extract to reduce inflammation in the mouse model (Ouviña et al. 2009). The potency of anti-inflammatory effects of H. bonariensis was further elucidated by evaluating the hexane extracts of the leaf containing active compounds hexadecenoic acid methyl ester, falcarinol and phytol (Obaseki et al. 2016). The hexane extract was tested in human red blood cell (HRBC) membrane stabilisation studies as the red blood cell membrane is similar to lysosomal membranes that influence the inflammatory process. The anti-inflammatory effect was observed with an increase in hexane extract concentration from 30.1% to 71.3% in membrane stabilisation (half maximal inhibitory concentration; IC50 of 117.37 μg/ml), suggesting that the plant extract has membrane stabilisation properties that may contribute to the potency of the anti-inflammatory agent (Obaseki et al. 2016). Membrane stabilisation is critical for limiting the anti-inflammatory response, as it prevents the release of lysosomal components such as proteases that cause more tissue inflammation and damage after extracellular release (Obaseki et al. 2016). The anti-inflammatory property of the plant was further assessed against protein denaturation, which is an important cause of inflammation. The inhibition protein denaturation assay confirmed the inhibitory effect of H. bonariensis on heat-induced bovine serum albumin (BSA) denaturation and was reported to act in a concentration-dependent manner, ranging from 26.50% to 69.23% with an in vitro IC50 value of 44.84 µg/ml compared to the standard non-steroidal anti-inflammatory drug diclofenac sodium (46.15% to 85.47% inhibition with an IC50 value of 10.68 µg/ml) (Obaseki et al. 2016). The anti-inflammatory activity of H. bonariensis was also evaluated using the arthritis-induced rat paw oedema model, which confirmed the potent anti-inflammatory effect at a dose of 250 mg/kg body weight (Obaseki et al. 2016).
Antioxidant activity
Oxidative stress is induced by the excessive production of reactive oxygen species (ROS) and/or a decrease in antioxidant defenses (Siddeeg et al. 2021). Excessive ROS can damage DNA, RNA, proteins and lipids, resulting in an increased risk for chronic diseases, including cancer, neurodegenerative, diabetes and cardiovascular diseases (White et al. 2014). Antioxidants such as polyphenols and flavonoids (Hunyadi 2019) can slow down or prevent oxidative damage by directly or indirectly scavenging and/or controlling ROS production (Lü et al. 2010). The mechanisms of action for antioxidants, including polyphenols and flavonoids, have been reported previously in the literature (Leopoldini et al. 2011). H. bonariensis possesses antioxidant activities, owing to the plant’s rich content of polyphenols and flavonoids (Ajani et al. 2017). Based on 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity, fractionated phenolic leaf extracts showed antioxidant potential through their ability to scavenge free radicals, leading to the disappearance of colour and therefore expressing high antioxidant activity (Maulidiani et al. 2014). Antioxidant properties were further assessed in fractionated extracts of H. bonariensis leaves, which were partitioned using different solvents of increasing polarity, namely hexane, chloroform, ethyl acetate and methanol (Ajani et al. 2017). The antioxidant activity assessment was conducted using DPPH radical scavenging assay, metal chelating activity, free radical (OH, H2O2 and NO) scavenging activity and reducing power assay. The tests showed that methanol and aqueous fractions had the highest free radical scavenging activity while the aqueous fraction had the highest metal chelating activity (35.5%) and free radical scavenging activity and reduced power activity compared to other fractionated extracts (Ajani et al. 2017).
Preventive activity against ophthalmic diseases
Potent antioxidant activities of H. bonariensis leaf extracts may be beneficial in reducing cataract progression by preventing oxidative damage in eye lens tissue. Cataract disease has resulted from increased peroxidation and reduced antioxidant enzymatic activity of catalase, superoxide dismutase and lower glutathione levels in eye lens tissue (Ajani et al. 2009). The oral treatment of lyophilised leaf extracts restored the activity of catalase and superoxide dismutase, increased glutathione levels, and simultaneously reduced the level of peroxidation in galactose-fed, weanling albino rat lens tissues (Ajani et al. 2009). Although the leaf extract administration has reduced the progression of cataracts, it has not reversed cataractogenesis. These results are promising for the use of H. bonariensis extracts as active ingredients for cataract prevention and/or treatment.
Preventive activity against cardiovascular diseases
The potential use of H. bonariensis extract as an active ingredient for therapeutic use for cataracts is further assessed to evaluate the possible cardiovascular disease (CVD) risk associated with its administration. An experimental animal model has shown that aqueous extract of H. bonariensis is effective in reducing CVD risk, which is often associated with increased dietary galactose that can result in cataract progression. Following the oral administration of the extract to cataract-affected weanling albino rats at 500 mg/kg and 1000 mg/kg, the plasma lipid profile, fibrinogen and platelet counts of galactose-fed rats showed low-dose H. bonariensis leaf extracts (500 mg/kg) to be more effective in reducing plasma fibrinogen and platelet counts than diets either supplemented with 1000 mg/kg leaf extract or with no leaf extract (Ajani et al. 2012). A recent study showed the potential of hydro-ethanolic leaf extract of H. bonariensis to selectively block the potassium current which controls cardiac repolarization (Kaboua et al. 2021a). This effect was comparable to the effects of class III anti-arrhythmics drugs, indicating the potential use of H. bonariensis in the management of cardiac arrhythmia.
Anticancer activity
H. bonariensis is also reported to have anticancer properties. The anti-tumour potential was reported in Tabopda et al. (2012), which showed that two plant saponin derivatives (Bonarienosides A and Bonarienosides B) isolated from the leaf extract displayed toxicity (IC50 24.1 and 24.0, 83.0 μM and 83.6 μM, respectively) against two colon cancer cell lines (HCT 116 and HT-29). Saponins have potent anticancer activities by inducing cell cycle arrest and apoptosis (Man et al. 2010). Interestingly, H. bonariensis extract does not show any cytotoxicity effect on normal cells. In a cellular toxicity study based on the survival of shrimp larvae, the hydroethanolic extract of H. bonariensis was considered non-toxic for average lethal concentrations (LC) ≥ 0.1 mg/ml as the obtained LC was 0.12 mg/ml, which is higher than the limit. An orally administered extract was also reported to be toxicologically safe in a mammal model, as negligible acute and subchronic toxicity signs were observed in the treated Wistar rats compared to the control batch (Kaboua et al. 2021b).
Antimicrobial activity
The exploration of H. bonariensis’ antimicrobial activity has been limited, however, antiviral, antibacterial, and antiprotozoal activity have been reported: Antiviral activity was demonstrated for a H. bonariensis methanol extract and water infusion against bovine viral diarrhea virus type 1 (Ruffa et al. 2004); A dicholoromethane extract of aerial parts of H. bonariensis was able to inhibit the growth of bacterial pathogens Chlamydia trachomatis (causative agent of sexually transmitted disease Chlamydia) and Chlamydia pneumoniae (causative agent of neonatal conjunctivitis and pneumonia) in LLC-MK2 or HeLa cells culture (Entrocassi et al. 2021); A methanol extract of H. bonariensis leaves was shown to inhibit the protozoan parasite Leishmania infantum with 100% lethality at 500 µg/ml concentration (Tempone et al. 2008). Also, hexane extract of the aerial part of H. bonariensis was reported to inhibit the protozoan parasite Trypanosoma cruzi with 100% lethality at 300 µg/ml concentration (Souza et al. 2021). The antimicrobial activity of H. bonariensis extracts may be related to the high flavonoid and polyphenol content: Flavonoids extracted from plants have been shown to have antiviral (Ninfali et al. 2020), antibacterial (Adamczak et al. 2020) and antifungal activities (Al Aboody and Mickymaray 2020), while polyphenols in plant extracts have been reported to have antimicrobial and antiviral properties that can be useful in the food industry (Olszewska et al. 2020). While there are no reports of the antimicrobial or anti-inflammatory effects of including H. bonariensis in the human diet, the health-promoting effects of foods rich in flavanols are widely reported (Barreca et al. 2021) therefore, it is likely that mechanisms of action of H. bonariensis flavonoid compounds will be a fruitful area of research.
As a rich source of several saponins, flavonols and triterpenes (Table 3), H. bonariensis possesses a promising health benefit potential. The chemical structures that we have generated using ChemDraw® (Table 3) may serve as a resource for predicting their molecular targets and studying molecular interactions. Isolating or synthesising these phytochemicals and testing their activity in in vitro and in vivo conditions will be useful for further exploration of the mechanism of each individual compound in promoting human health.
In-vitro studies for enhancement of phytochemical production in H. bonariensis
A diet enriched with health-promoting phytochemicals reduces disease incidence, thus it is recommended for general health, particularly to reduce the incidence of non-communicable diseases (Chang et al. 2016). From a pharmaceutical perspective, in most cases, plants are used to discover of novel compounds that are then more cost-effective to produce synthetically or semi-synthetically, as production directly from plant materials is often not sustainable (Zhao et al. 2019). However, several strategies have been explored to enhance the content of beneficial phytochemicals in crop plants, ranging from the exogenous application of light (Kim et al. 2021), chemical inducers (Alothman et al. 2009) to genetic manipulation of associated genes in metabolic pathways (Singh 2016). For phytochemical enhancement in H. bonariensis, Masoumian et al. (2011) have accelerated the flavonoid production in in vitro leaf-callus tissues by supplementing precursors of flavonoids such as phenylalanine, proline, glutamine and naringenin at different concentrations. H. bonariensis callus produced the highest flavonoid content when grown on media containing either 3 mg/l phenylalanine (11.4 mg/g dry weight), 4 mg/l proline (10.7 mg/g dry weight), 1 mg/l of glutamine (10.5 mg/g dry weight) or 4 mg/l naringenin (10.1 mg/g dry weight). Overall, the study suggested that an optimum concentration of flavonoid precursors can be used to elevate the phytochemical concentration in H. bonariensis. While there remains much room to improve the detailed understanding of plant secondary metabolite biosynthetic pathways, genomic, transcriptomic and metabolomic approaches are narrowing this gap in knowledge (Saito 2013; Nielson et al. 2019; Delfin et al. 2019). Nair et al. (2021) reported the enhancement of total antioxidant content, total phenol and total flavonoid content of H. bonariensis when plants are grown under red and blue LED with a higher level of blue irradiance compared to plants grown under natural light conditions. The study suggested that specific LED spectra can be used to increase the phytochemical content of H. bonariensis. Future research on the secondary metabolite biosynthesis, its genetic regulation and its association with photoreceptors in H. bonariensis would be useful to enhance its potential as a functional food and for pharmaceutical use.
Conclusion and future perspectives
The main phytochemical compounds found in H. bonariensis are saponins, phenols, triterpenes and flavonoids. A rich phytochemical content, robust growth characteristics and adaptability to extreme environmental conditions (Chiarello and Joesting 2018) make H. bonariensis worthy for inclusion as a mainstream edible functional food crop. H. bonariensis also has a great potential to be used in the pharmaceutical industry, as inferred from pharmacological activities. Prolonged traditional dietary and ethnomedicinal use suggest the plant is safe for consumption and pharmacological studies provide evidence of biological activity, however, much research is still needed to quantify the bioactive compounds of fresh and processed plant materials along with systematic animal and clinical trials to evaluate safety and efficacy both for edible and pharmacological use.
Research highlights
H. bonariensis has wide traditional and potential functional food uses.
Cell and animal studies show anti-inflammatory, antimicrobial, anti-tumoural and antioxidant activity.
Acknowledgements
CEBAR Research University Grants (RU006-2017 and RU006-2018) awarded to the University of Malaya under the Ministry of Education, Malaysia
Funding
CEBAR Research University Grants (RU006-2017 and RU006-2018).
Availability of data and material
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adamczak A, Ożarowski M, Karpiński TM. Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J Clin Med. 2020;9:109. doi: 10.3390/jcm9010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajani EO, Sabiu S, Zakari M, Olanipekun BE, Akintunde JK, Bamisaye FA. Antioxidant capacity of fractionated extract and structural elucidation of isolated compounds from leaf extract of Hydrocotyl bonariensis Comm. Ex Lam. JBAPN. 2017;7:166–177. doi: 10.1080/22311866.2017.1329667. [DOI] [Google Scholar]
- Ajani EO, Salakoa AA, Sharlie PD, Akinleyec WA, Adeoyea AO, Salaua BA, Adebawoa OO. Chemopreventive and remediation effect of Hydrocotyl bonariensis Comm. Ex. Lam J Ethnopharmacol. 2009;123:134–142. doi: 10.1016/j.jep.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Ajani EO, Salau BA, Adebayo OL, Adegbesan BO, Odufuwa KT. Administration of Hydrocotyl bonariensis aqueous leaves extract regulates cardiovascular disease risk factors in galactose model of experimental cataractogenesis. Sci Res Essays. 2012;7:1527–1530. doi: 10.5897/SRE12.191. [DOI] [Google Scholar]
- Al Aboody MS, Mickymaray S. Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics. 2020;9:45. doi: 10.3390/antibiotics9020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almaghamsi A, Almalki MH, Buhary BM. Hypocalcemia in pregnancy: A clinical review update. Oman Med J. 2018;33:453. doi: 10.5001/omj.2018.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alothman M, Bhat R, Karim AA. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends Food Sci Technol. 2009;20:201–212. doi: 10.1016/j.tifs.2009.02.003. [DOI] [Google Scholar]
- Bandara MS, Lee EL, Thomas JE. Gotu Kola (Centella asiatica L.): An under-utilized herb. Am J Plant Sci. 2011;5:20–31. [Google Scholar]
- Barreca D, Trombetta D, Smeriglio A, Mandalari G, Romeo O, Felice MR, Gattuso G, Nabavi SM. Food flavonols: Nutraceuticals with complex health benefits and functionalities. Trends Food Sci Technol. 2021 doi: 10.1016/j.tifs.2021.03.030. [DOI] [Google Scholar]
- Chandra S, Khan S, Avula B, Lata H, Yang MH, ElSohly MA, Khan IA. Assessment of total phenolic and flavonoid content, antioxidant properties, and yield of aeroponically and conventionally grown leafy vegetables and fruit crops: A comparative study. Evid Based Complement Alternat Med. 2014 doi: 10.1155/2014/253875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SK, Alasalvar C, Shahidi F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J Funct Foods. 2016;21:113–132. doi: 10.1016/j.jff.2015.11.034. [DOI] [Google Scholar]
- Chiarello SD, Joesting HM. Examination of phenotypic plasticity in Hydrocotyle bonariensis in response to two soil types. Bios. 2018;89:65–73. doi: 10.1893/0005-3155-89.2.65. [DOI] [Google Scholar]
- Danderson CA, Downie SR, Hermann M. Rampant polyphyly in the Arracacia clade (Apiaceae) and an assessment of the phylogenetic utility of 20 noncoding plastid loci. Mol Phylogenet Evol. 2018;118:286–305. doi: 10.1016/j.ympev.2017.10.006. [DOI] [PubMed] [Google Scholar]
- Dantos-santos N, Gomes DL, Costa LS, Cordeiro SL, Costa MSSP, Trindade ES, Franco CRC, Scortecci KC, Leite EL, Rocha HAO. Freshwater plants synthesize sulfated polysaccharides: Heterogalactans from water hyacinth (Eicchornia crassipes) Int J Mol Sci. 2012;13:961–976. doi: 10.3390/ijms13010961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfin JC, Watanabe M, Tohge T. Understanding the function and regulation of plant secondary metabolism through metabolomics approaches. Theor Exp Plant Physiol. 2019;31:127–138. doi: 10.1007/s40626-018-0126-1. [DOI] [Google Scholar]
- Entrocassi AC, Catalano AV, Ouviña AG, Wilson EG, López PG, Fermepin MR. In vitro inhibitory effect of Hydrocotyle bonariensis Lam. extracts over Chlamydia trachomatis and Chlamydia pneumoniae on different stages of the chlamydial life cycle. Heliyon. 2021;7:e06947. doi: 10.1016/j.heliyon.2021.e06947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JP (1992) Seedling establishment and genet recruitment in a population of a clonal dune perennial, Hydrocotyle bonariensis. In: Barrier Island Ecology of the Mid-Atlantic Coast. A Symposium. Atlanta, G. A, pp 75–84
- Evans JP, Whitney S. Clonal integration across a salt gradient by a nonhalophyte, Hydrocotyle bonariensis (Apiaceae) Am J Bot. 1992;79:1344–2147. doi: 10.2307/2445132. [DOI] [Google Scholar]
- Florinsiah L, Farida ZMY, Nur SN, Norfazlina MN, Suziana ZC, Rajab NF. Mutagenicity effect of Hydrocotyle bonariensis extracts in Salmonella/microsome assay. Int J Pharm Sci Rev Res. 2013;20:47–50. doi: 10.1002/(SICI)1099-1573(199908/09)13:53.0.CO. [DOI] [Google Scholar]
- Global functional food market revenue (2019–2025) https://www.statista.com/statistics/252803/global-functional-food-sales/. Accessed 9 Jul 2021
- Go SK, Chia SL, Tan CP, Chong GH. Effects of multi-stage dehumidified-air drying on the polyphenol content of Hydrocotyle bonariensis. Int Food Res J. 2017;24:589–593. [Google Scholar]
- Goh LG (2007) Effect of light intensity on the growth and chlorophyll content of pennywort (Hydrocotyle bonariensis comm. Ex lam.). Master thesis, University Putra Malaysia
- Gooding AJ, Packer CD, Pensiero AL. Zinc deficiency-induced hypogeusia in a patient with refractory iron-deficiency anemia: a case report. Cureus. 2019;11:e6365. doi: 10.7759/cureus.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greca MD, Florentino A, Monaco P, Previtera L. Oleanane glycosides from Hydrocotyle ranunculoides. Phytochemistry. 1994;36:1479–1483. doi: 10.1016/S0031-9422(00)89746-0. [DOI] [PubMed] [Google Scholar]
- Haddad CRB, Mazzafera P. Sodium chloride-induced leaf senescence in Hydrocotyle bonariensis Lam. and Foeniculum vulgare L. Braz Arch Biol Technol. 1999;42:1. doi: 10.1590/S1516-89131999000200005. [DOI] [Google Scholar]
- Haida Z, Maziah M, Hakiman M. Primary metabolites, phenolics content and antioxidant activities of Hydrocotyle bonariensis and Centella asiatica. Fundam Appl Agric. 2021;6(1):27–34. doi: 10.5455/faa.32251. [DOI] [Google Scholar]
- Hashim P. Centella asiatica in food and beverage applications and its potential antioxidant and neuroprotective effect. Int Food Res J. 2011;18:1215–1222. [Google Scholar]
- Hasler CM. Functional foods: benefits, concerns and challenges—a position paper from the American Council on Science and Health. J Nutr. 2002;132:3772–3781. doi: 10.1093/jn/132.12.3772. [DOI] [PubMed] [Google Scholar]
- Hesp PA. Ecological processes and plant adaptations on coastal dunes. J Arid Environ. 1991;21:165–191. doi: 10.1016/S0140-1963(18)30681-5. [DOI] [Google Scholar]
- Hunyadi A. The mechanism (s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med Res Rev. 2019;39:2505–2533. doi: 10.1002/med.21592. [DOI] [PubMed] [Google Scholar]
- Hylander N (1945) Nomenklatorische und systematische Studien über nordische Gefässpflanzen. Uppsala Univ Årsskr 7:5–337 (reference taken from Konstantinova & Yembaturova, 2010)
- Jacobs RL, Zhao Y, Koonen DP, Sletten T, Su B, Lingrell S, Cao G, Peake DA, Kuo MS, Proctor SD, Kennedy BP. Impaired de novo choline synthesis explains why phosphatidylethanolamine N-methyltransferase-deficient mice are protected from diet-induced obesity. J Biol Chem. 2010;285:22403–22413. doi: 10.1074/jbc.M110.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joesting HM, Sprague MO, Smith WK. Seasonal and diurnal leaf orientation, bifacial sunlight incidence, and leaf structure in the sand dune herb Hydrocotyle bonariensis. Environ Exp Bot. 2012;75:196–203. doi: 10.1016/j.envexpbot.2011.06.013. [DOI] [Google Scholar]
- Kaboua K, Mouzou A, Pakoussi T, Assih M, Chatelier A, Diallo A, Bois P, Bescond J. Hydrocotyle bonariensis Comm ex Lamm (Araliaceae) leaves extract inhibits IKs not IKr potassium currents: Potential implications for anti-arrhythmic therapy. J Tradit Complement Med. 2021 doi: 10.1016/j.jtcme.2021.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaboua K, Pakoussi T, Mouzou A, Assih M, Kadissoli B, Dossou-Yovo KM, Bois P. Toxicological evaluation of Hydrocotyle bonariensis Comm. ex Lamm (Araliaceae) leaves extract. Beni-Suef Univ J Basic Appl Sci. 2021;10:1–7. doi: 10.1186/s43088-021-00160-5. [DOI] [Google Scholar]
- Kim YJ, Kim HM, Kim HM, Lee HR, Jeong BR, Lee HJ, Kim HJ, Hwang SJ. Growth and phytochemicals of ice plant (Mesembryanthemum crystallinum L.) as affected by various combined ratios of red and blue LEDs in a closed-type plant production system. J Appl Res Med Aromat Plants. 2021;20:100267. doi: 10.1016/j.jarmap.2020.100267. [DOI] [Google Scholar]
- Konstantinova AI, Yembaturova EY. Structural traits of some species of Hydrocotyle (Araliaceae) and their significance for constructing the generic system. Plant Diver Evol. 2010;128:329–346. doi: 10.1127/1869-6155/2010/0128-0015. [DOI] [Google Scholar]
- Kostov K, Halacheva L. Role of magnesium deficiency in promoting atherosclerosis, endothelial dysfunction, and arterial stiffening as risk factors for hypertension. Int J Mol Sci. 2018;19:1724. doi: 10.3390/ijms19061724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopoldini M, Russo N, Toscano M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011;125:288–306. doi: 10.1016/j.foodchem.2010.08.012. [DOI] [Google Scholar]
- Lovegrove A, Edwards CH, De Noni I, Patel H, El SN, Grassby T, Zielke C, Ulmius M, Nilsson L, Butterworth PJ, Ellis PR, Shewry PR. Role of polysaccharides in food, digestion, and health. Crit Rev Food Sci Nutr. 2017;57:237–253. doi: 10.1080/10408398.2014.939263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü JM, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J Cell Mol Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man S, Gao W, Zhang Y, Huang L, Liu C. Chemical study and medical application of saponins as anti-cancer agents. Fitoterapia. 2010;81:703–714. doi: 10.1016/j.fitote.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Marino CD, Zuppardo LZL, Armando J, Ruggiero ADA, Moya HD. Analysis of the polyphenols content in medicinal plants based on the reduction of Cu (II)/Bicinchoninic complexes. J Agric Food Chem. 2009;57:11061–11066. doi: 10.1021/jf902197p. [DOI] [PubMed] [Google Scholar]
- Masoumian M, Arbakariya A, Syahida A, Maziah M. Effect of precursors on flavonoid production by Hydrocotyle bonariensis callus tissues. Afr J Biotechnol. 2011;10:32. doi: 10.5897/AJB10.1480. [DOI] [Google Scholar]
- Maulidiani H, Abas F, Khatiba A, Shaar K, Nordin HL. Chemical characterization and antioxidant activity of three medicinal Apiaceae species. Ind Crop Prod. 2014;55:238–247. doi: 10.1016/j.indcrop.2014.02.013. [DOI] [Google Scholar]
- Maulidiani H, Khatib A, Shaari K, Abas F, Shitan M, Kneer R, Neto V, Lajis NH. Discrimination of three pegaga (Centella) varieties and determination of growth-lighting effects on metabolites content based on the chemometry of 1H Nuclear Magnetic Resonance spectroscopy. J Agric Food Chem. 2012;60:410–417. doi: 10.1021/jf200270y. [DOI] [PubMed] [Google Scholar]
- Mehri A. Trace elements in human nutrition (ii)–an update. Int J Prev Med. 2020;11:2. doi: 10.4103/ijpvm.IJPVM_48_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyn ED, Bakayoko A, Bi FHT, Yao K, Kone MW. Knowledge level and mineral content of Hydrocotyle bonariensis Lam. (Araliaceae), a plant used by households in the district of Abidjan (Cote d'Ivoire) Int J Biol Chem Sci. 2016;10:2046–2061. doi: 10.4314/ijbcs.v10i5.9. [DOI] [Google Scholar]
- Myint ZW, Oo TH, Thein KZ, Tun AM, Saeed H. Copper deficiency anemia. Ann Hematol. 2018;97:1527–1534. doi: 10.1007/s00277-018-3407-5. [DOI] [PubMed] [Google Scholar]
- Nair I, Mazumdar P, Singh P, Rengasamy N, Harikrishna JA. Increasing the Ratio of Blue to Red Light Improves Growth and Phytochemical Content in Hydrocotyle bonariensis. Russ J Plant Physiol. 2021;68:337–346. doi: 10.1134/S1021443721020126. [DOI] [Google Scholar]
- Nicolas AN, Plunkett GM. The demise of subfamily Hydrocotyloideae (Apiaceae) and the re-alignment of its genera across the entire order Apiales. Mol Phylogenet Evol. 2009;53:134–151. doi: 10.1016/j.ympev.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Nielson E, Temporiti MEE, Cella R. Improvement of phytochemical production by plant cells and organ culture and by genetic engineering. Plant Cell Rep. 2019;38:1199–1215. doi: 10.1007/s00299-019-02415-z. [DOI] [PubMed] [Google Scholar]
- Ninfali P, Antonelli A, Magnani M, Scarpa ES. Antiviral properties of flavonoids and delivery strategies. Nutrients. 2020;12:2534. doi: 10.3390/nu12092534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaseki OE, Adesegun OI, Anyasor GN, Abebawo OO. Evaluation of the anti-inflammatory properties of the hexane extract of Hydrocotyle bonariensis Comm. Ex Lam. Leaves. Afr J Biotechnol. 2016;15:2759–2771. doi: 10.5897/AJB2016.15631. [DOI] [Google Scholar]
- Olszewska MA, Gędas A, Simões M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res Int. 2020 doi: 10.1016/j.foodres.2020.109214. [DOI] [PubMed] [Google Scholar]
- Ouviña A, Gorzalczany S, Acevedo C, Ferraro G. Topic anti-inflammatory activity of extracts from Hydrocotyle bonariensis Lam. (Apiaceae) Lat Am J Pharm. 2009;28:941–944. [Google Scholar]
- Perkins A. Molecular phylogenetics and species delimitation in annual species of Hydrocotyle (Araliaceae) from South Western Australia. Mol Phylogenet Evol. 2019;134:129–141. doi: 10.1016/j.ympev.2019.02.011. [DOI] [PubMed] [Google Scholar]
- Reihani SFS, Azhar ME. Antioxidant activity and total phenolic content in aqueous extracts of selected traditional Malay salads (Ulam) Int Food Res J. 2012;19:1439–1444. [Google Scholar]
- Ruffa MJ, Wagner ML, Suriano M, Vicente C, Nadinic J, Pampuro S, Salomón H, Campos RH, Cavallaro L. Inhibitory effect of medicinal herbs against RNA and DNA viruses. Antivir Chem Chemother. 2004;15:153–159. doi: 10.1177/095632020401500305. [DOI] [PubMed] [Google Scholar]
- Saito K. Phytocemical genomics- a new trend. Curr Opin Plant Biol. 2013;16:373–380. doi: 10.1016/j.pbi.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Schöpke Th, Janka M, Nimtz M, Wray V, HiIIer K. Saniculoside R-1: a new triterpenoid saponin from Sankula europaea. Planta Med. 1998;64:83–85. doi: 10.1055/s-2006-957377. [DOI] [PubMed] [Google Scholar]
- Siddeeg A, AlKehayez NM, Abu-Hiamed HA, Al-Sanea EA, Al-Farga AM. Mode of action and determination of antioxidant activity in the dietary sources: An overview. Saudi J Biol Sci. 2021;28:1633. doi: 10.1016/j.sjbs.2020.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S. Enhancing phytochemical levels, enzymatic and antioxidant activity of spinach leaves by chitosan treatment and an insight into the metabolic pathway using DART-MS technique. Food Chem. 2016;199:176–184. doi: 10.1016/j.foodchem.2015.11.127. [DOI] [PubMed] [Google Scholar]
- Souza DC, Costa-Silva TA, Morais TR, Brito JR, Ferreira EA, Antar GM, Sartorelli P, Tempone AG, Lago JH. Simplified Derivatives of Dibenzylbutyrolactone Lignans from Hydrocotyle bonariensis as Antitrypanosomal Candidates. Chem Biodivers. 2021;18:e2100515. doi: 10.1002/cbdv.202100515. [DOI] [PubMed] [Google Scholar]
- Sumazian Y, Syahida A, Hakiman M, Maziah M. Antioxidant activities, flavonoids, ascorbic acid and phenolic contents of Malaysian vegetables. J Med Plant Res. 2010;4:881–890. doi: 10.5897/JMPR10.011. [DOI] [Google Scholar]
- Tabopda TK, Mitaine-Offer AC, Miyamoto T, Tanaka C, Mirjolet JF, Duchamp O, Ngadjui BT, Lacaille-Dubois MA. Triterpenoid saponins from Hydrocotyle bonariensis Lam. Phytochemistry. 2012;73:142–147. doi: 10.1016/j.phytochem.2011.08.027. [DOI] [PubMed] [Google Scholar]
- Tempone AG, Sartorelli P, Teixeira D, Prado FO, Calixto IA, Lorenzi H, Melhem MS. Brazilian flora extracts as source of novel antileishmanial and antifungal compounds. Mem Inst Oswaldo Cruz. 2008;1035:443–449. doi: 10.1590/S0074-02762008000500006. [DOI] [PubMed] [Google Scholar]
- Wallace TC, Blusztajn JK, Caudill MA, Klatt KC, Natker E, Zeisel SH, Zelman KM. Choline: The underconsumed and underappreciated essential nutrient. Nutr Today. 2018;53:240–253. doi: 10.1097/NT.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PA, Oliveira R, Oliveira AP, Serafini MR, Araújo AA, Gelain DP, Moreira JC, Almeida JR, Quintans JS, Quintans-Junior LJ, Santos MR. Antioxidant activity and mechanisms of action of natural compounds isolated from lichens: a systematic review. Molecules. 2014;19:14496–14527. doi: 10.3390/molecules190914496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojdyło A, Oszmiański J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. doi: 10.1016/j.foodchem.2007.04.038. [DOI] [Google Scholar]
- Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. 2018;9:143. doi: 10.14336/AD.2017.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel SH, Da Costa KA. Choline: an essential nutrient for public health. Nutr Rev. 2009;67:615–623. doi: 10.1111/j.1753-4887.2009.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Jian X, Wu J, Huang W, Huang C, Luo J, Kong L. Elucidation of the biosynthesis pathway and heterologous construction of a sustainable route for producing umbelliferone. J Biol Eng. 2019;13:44. doi: 10.1186/s13036-019-0174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.