Abstract
A benign peripheral nerve sheath tumor (bPNST) is a rare lesion associated with peripheral nerval structures. Symptoms may be heterogeneous, complicating diagnosis finding. Additionally, management concepts of bPNST may vary. In some cases, initial misdiagnosis leads to mistreatment resulting in severe functional deficits and chronic pain syndromes. Therefore, we analyzed patients treated for bPNST in our specialized institution with a primary focus on prior misdiagnosis and possible mistreatment. Patients with bPNSTs (schwannomas, neurofibromas, hybrid nerve sheath tumors, and perineuriomas) treated at the Neurosurgical Department between January 1, 2015, and July 31, 2021, were included. Assessment of demographics, tumor entity, tumor location, symptoms, the interval between the onset of symptoms and surgery, involved medical specialties, and outpatients’ treatment, with particular focus on initial misdiagnosis and inappropriate medical treatment, was performed. Eighty-five patients were included in the final analysis with schwannoma being the most prevalent histopathological diagnosis (schwannoma (75.3%, n=64), neurofibroma (12.9%, n=11), hybrid nerve sheath tumor (5.9%, n=5), and perineurioma (5.9%, n=5)). An incorrect primary diagnosis was detected in 44.7% (n=38), leading to suboptimal or insufficient treatment in these cases. Of those, 28.9% (n=11/38) were treated suboptimal, while 18.5% (n=7/38) underwent unnecessary invasive diagnostics. Inappropriate surgery based on prior misdiagnosis, which led to severe neurological deficits in all these cases, was reported in 26.3% (n=10/38). For the first time, our data shows the quantity and impact of incorrect initial diagnosis in bPNST causing a delay in causative treatment or resulting in unnecessary or potentially harmful treatment.
Keywords: Benign peripheral nerve sheath tumors, Diagnostics, Mistreatment, Misdiagnosis
Introduction
A benign peripheral nerve sheath tumor (bPNST) is a rare lesion of neuroectodermal origin, directly associated with peripheral nerve structures [1]. Clinical presentation of bPNSTs includes asymptomatic palpable lesions, painful palpable lesions, and lesions accompanied by neurological deficits [2–5]. The most common bPNSTs are schwannomas and neurofibromas. Their localization can cause various symptoms such as local swelling, motor deficits, hypoesthesia, and neuropathic pain due to the ongoing pressure on the unaffected nerve fascicles or the loss of function of the affected fascicles themselves. These symptoms can sometimes be misinterpreted as radicular symptoms or other frequently symptomatic conditions, such as joint and muscle pain without a specific cause [2, 3, 6–8].
Diagnostic workup includes neurological examination, magnetic resonance imaging (MRI), ultrasound, and electrophysiological testing. Especially high-resolution nerve sonography is more and more commonly used for peripheral neuropathies [9–12].
However, one major symptom may be a local swelling or a palpable lump that may lead some clinicians to make wrong assumptions regarding tumor origin when careful neurologic assessment is not performed. Differential diagnosis of local swelling includes pathologic lymph nodes, metastasis, soft tissue tumors of non-nervous origin, and others [8, 13].
Patients with the symptom of local swelling may be transferred to different medical subspecialties leading to various diagnostic and therapeutic pathways. For example, neurologists, orthopedics, plastic and reconstructive surgeons, general surgeons, thoracic surgeons, and neurosurgeons may be involved in the treatment of soft tissue masses.
A nervous origin might not be assumed initially, leading to treatment according to the respective guidelines. Consequently, the initial diagnosis and treatment may not be performed by someone experienced in treating benign peripheral nerve lesions, which promotes delayed diagnosis and nerve damage [14].
We aimed to evaluate the rate of primary misdiagnosis and mistreatment in patients suffering from deep-seated bPNSTs. Furthermore, we aimed to identify causative factors for mistreatment.
Methods
Study design
A retrospective analysis of our prospective dataset “peripheral nerve lesion” was performed for patients treated at our institution between January 1, 2015, and July 31, 2021.
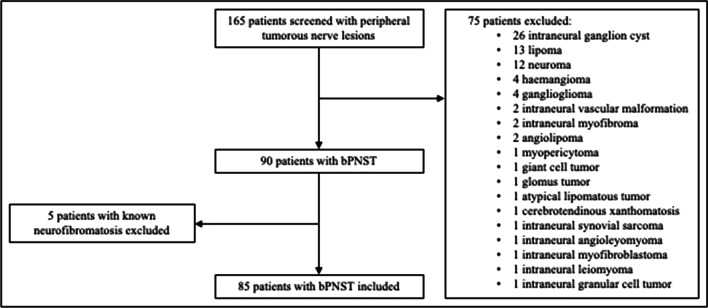
Patients with sporadic, deep-seated bPNSTs (schwannomas, neurofibromas, hybrid nerve sheath tumors (HNST), and perineuriomas) involving the extremities and the lumbar or cervical nerve plexus were included. Therefore, patients with cutaneous tumors were excluded from analysis. Furthermore, patients with other benign intraneural tumors without nerve sheet origin and malignant nerve sheath tumors and patients with known neurofibromatosis were also excluded (Fig. 1).
Fig. 1.
Flow-chart: inclusion and exclusion of patients. bPNST, benign peripheral nerve sheath tumor
Ethics
The study was conducted following the STROBE guidelines after approval by the Institutional Review Board (Medical Faculty, University of Duisburg-Essen, Registration number: 18–7955-BO).
Evaluated parameters
Demographics, tumor entity, tumor location (upper and lower extremity, lumbar or cervical nerve plexus), symptoms, the interval between the onset of symptoms and surgery, involved medical specialties, outpatients’ treatment, diagnostic before surgery (MRI, computed tomography (CT), ultrasound and electrophysiology), possible false diagnosis, and inappropriate medical treatment and mismanagement were evaluated.
Definition of “misdiagnosis”
The Department of Neuropathology confirmed the final diagnosis of bPNST after surgical treatment at the Department of Neurosurgery and Spine Surgery.
A misdiagnosis occurred, if the final diagnosis was inconsistent with the diagnosis which was set up by the doctor who treated the patient at first. The assessment of misdiagnosis was made based on the external treatment records and each patient’s medical history.
Preoperative assumption of a bPNST different from the final diagnosis was not defined as misdiagnosis.
Definition of “suboptimal treatment” and “inappropriate surgical treatment”
Suboptimal treatment was assumed in symptomatic patients, if symptoms remained the same or progressed for more than 6 months under conservative treatment and without surgical treatment.
Inappropriate surgical treatment was present, if the patient was surgically treated not due to the bPNST, but because of an accompanying disc prolapse or a nerve entrapment syndrome, for example, without relieve of the symptoms.
Mistreatment was present, if the surgical treatment of the bPNST did not follow the current treatment standard for deep-seated nerval tumors (microsurgical tumor resection with neurophysiological monitoring) [15].
Statistical analysis
Data were analyzed using SPSS 25.0 (Statistical Package for the Social Sciences, SPSS Inc., Chicago, IL, USA). Metric data were described by mean, standard deviation, and nominal data by frequency and valid percent. p-values <0.05 in two-sided testing were considered significant.
Demographic, clinical, and radiographic parameters were analyzed univariately regarding their association or correlation with misdiagnosis or suboptimal treatment and malpractice. Therefore, Pearson’s χ2 statistics or Fisher’s exact test was used for dichotomous variables.
Results
Study population
Over the observed period, 165 patients with peripheral nerve tumors were surgically treated at our department by one neurosurgeon specialized in peripheral nerve surgery. Of those, 80 patients were excluded as they did not meet the inclusion criteria. Our final analysis included 85 patients (44 males, 51.8%) with a mean age of 49.4 ± 16.1 years (range: 13–84 years). Time from the beginning of the symptoms until surgery was 22.9 ± 42.6 months, ranging from 0 months to 348 months (Table 1).
Table 1.
Patients’ demographics and tumor characteristics
| Demographics | |
| Age (years) | 49.4 ± 16.1 |
| Sex | |
| Females | 41 (48.2%) |
| Males | 44 (51.8%) |
| Tumor entities | |
| Schwannoma | 64/85 (75.3%) |
| Neurofibroma | 11/85 (12.9%) |
| Perineurioma | 5/85 (5.9%) |
| Hybrid nerve sheet tumor | 5/85 (5.9%) |
| Tumor location | |
| Upper limb/cervico-brachial plexus | 33/85 (38.8%) |
| Lower limb/lumbo-sacral plexus | 46/85 (54.1%) |
| Other location | 6/85 (7.1%) |
| Presenting symptom | |
| Pain only | 21/85 (24.7%) |
| Palpable mass and self-induced pain | 14/85 (16.5%) |
| Palpable mass only | 15/85 (17.6%) |
| Neurological deficits | 22/85 (25.9%) |
| By chance | 13/85 (15.3%) |
| Hoffmann Tinel’s sign | 50/85 (58%) |
| Symptom duration until surgery (months) | 22.9 ± 42.6 |
Tumor entity and tumor location
Histopathological analysis revealed 64 schwannomas (75.3%), 11 neurofibromas (12.9%), five HNSTs (5.9%), and five perineuriomas (5.9%). Most cases affected the lower limb and lumbosacral plexus (54.1%, n=46) or the upper limb and cervicobrachial plexus (38.8%, n=31). Five bPNTs involved the thoracic nerves and one the supraorbital nerve (Table 1).
Presenting symptoms
A palpable symptomless mass, discovered by self-examination, was present in 17.6%. Pain as the presenting symptom was described in 24.7%, whereas in 16.5%, a self-induced pain by touching the palpable mass was complained. In 58%, a Hoffmann Tinel’s sign could be triggered by touching or tapping. Neurological deficits such as hypoesthesia, dysesthesia, or motor deficits were detectable in 25.9%. Of those, 86.3% complained about sensory deficits, while 13.6% showed motor deficits. In 15.3%, the tumor was diagnosed by chance during another examination (Table 1).
Physicians consulted
The patients visited a general practitioner at first in 84.7%, while 5.9% went to a neurologist. Additionally, 5.9% presented to an orthopedic, and 3.5% chose their gynecologist as their primary consultant.
Further referral to a specialist was generally based on the location of the mass (extremities, abdomen, and pelvis) or the leading symptom (neurological deficits, pain). Thus, referral to a general surgeon was made in 16.5%, while 21.2% were referred to an orthopedic based on their symptoms. Furthermore, 11.6% were transferred to a neurologist. An otolaryngologist was visited in 3.6%. However, the majority of 42.4% were transferred to a neurosurgeon (Table 2).
Table 2.
Overview of the involved medical society and the diagnostics
| Physicians consulted at first | |
| General practitioner | 72 (84.7%) |
| Neurologist | 5 (5.9%) |
| Orthopedics | 5 (5.9%) |
| Gynecologist | 3 (3.5%) |
| Referral to specialist | |
| Neurosurgeon | 36 (42.4%) |
| Orthopedics | 18 (21.2%) |
| General surgeon | 14 (16.5%) |
| Neurologist | 11 (12.9%) |
| Otolaryngologist | 3 (3.6%) |
| Others | 3 (3.6%) |
| Diagnostic workup | |
| MRI | 82 (96.4%) |
| CT | 1 (1.2%) |
| Ultrasound | 1 (1.2%) |
| Clinical examination | 1 (1.2%) |
| MRI according to the symptoms | |
| Palpable mass | 14/15 (93.3%) |
| Palpable mass and self-induced pain | 13/14 (92.9%) |
| Pain | 21/21 (100%) |
| Neurological deficits | 21/22 (95.5%) |
| By chance | 13/13 (100%) |
MRI magnetic resonance imaging, CT computed tomography
Diagnostic imaging
Magnetic resonance imaging as the primary diagnostic tool was performed in 96.4%. However, MRI was often initially focused on the spine, especially in cases of unclear radiating pain as primary symptom (31.7%). There was only one case where a CT scan was used as the initial diagnostic tool and one case where ultrasound was used at first (Table 2).
Misdiagnosis
An initial incorrect diagnosis was made in 44.7% of patients. In most of the cases, typical spinal disorders were suspected (34.2%), such as spinal canal stenosis or herniated disc of the cervical spine (46.2%) or the lumbar spine (53.8%). A sarcoma was suspected in 23.7%, while a nerve entrapment syndrome was wrongly diagnosed in 13.2% and a malignant lymph node in 10.5%. The other cases (18.4%) consisted of a shoulder-arm syndrome, a malignant peripheral nerve tumor, an idiopathic foot drop paresis, a dental root problem, and a hallux valgus (Table 3).
Table 3.
Type of misdiagnosis and mistreatment
| Misdiagnosis | Suspected diagnosis | Number of Cases |
| Cervical spine syndrome | 6/38 (15.8%) | |
| Lumbar spine syndrome | 7/38 (18.4%) | |
| Sarcoma | 9/38 (23.7%) | |
| Nerve entrapment syndrome | 5/38 (13.2%) | |
| Malignant lymph node | 4/38 (10.5%) | |
| Others | 7/38 (18.4%) | |
| Total amount of cases | 38/85 (44.7%) | |
| Mistreatment | Prolonged conservative treatment with irreparable damage | 11/38 (28.9%) |
| Unnecessary invasive diagnostic (CT-guided biopsy) | 7/38 (18.5%) | |
| Wrong surgical treatment based on misdiagnosis | 10/38 (26.3%) | |
| Inadequate surgical technique used | 10/38 (26.3%) |
Suboptimal treatment or inappropriate surgical treatment
Prolonged conservative treatment was present in 28.9%, resulting in pronounced neurological handicaps in two cases and neuropathic pain syndrome in five patients.
In 18.5%, an unnecessary invasive diagnostic (CT-guided biopsy) was performed before the definitive therapy due to a suspected diagnosis of a malignant retroperitoneal tumor in a general surgery department. Two patients persistently suffered from neuropathic pain after a CT-guided biopsy, but we did not find any case with permanent neurological deficit related to these biopsies. In all these cases, the biopsy confirmed the correct diagnosis.
Furthermore, an unnecessary surgical treatment unrelated to the bPNST was performed in 26.3%. These were carpal tunnel release, cubital tunnel release, hallux valgus surgery, cervical disc surgery, dental surgery, spinal neurinoma surgery, and shoulder arthroscopy.
Surgical removal or partial removal of the bPNST was performed using an inappropriate surgical technique (non-microsurgical and without intraoperative electrophysiological testing) in 26.3%. This led to severe motor and sensory deficits in seven patients and resulted in revision surgery in every case (Table 3).
Misdiagnosis and inappropriate surgical treatment and their correlation
Misdiagnosis and mistreatment showed a significant correlation with the treating specialist. Patients referred to a neurosurgeon by the general practitioner under the suspected diagnosis of a bPNST showed significantly lower rates of misdiagnosis and mistreatment than the other specialists (p<0.001).
Furthermore, patients with neurological deficits were misdiagnosed and mistreated significantly more often than patients without neurological deficits (p=0.003, p<0.001, respectively).
Patients receiving MRI of the cervical spine or the lumbar spine as an initial diagnostic tool showed significantly higher rates of misdiagnosis and mistreatment in comparison to patients receiving MRI of another area, which was usually the tumor-bearing region (p-value for MRI of the cervical spine: 0.039 for both; p-value for MRI of the lumbar spine: 0.010 for both).
Furthermore, MRI of the cervical spine was significantly more often performed in patients with neurological deficits than in patients without neurological deficits (p=0.007). This significant difference was not detected for patients receiving MRI of the lumbar spine (p=0.275) (Table 4).
Table 4.
Univariate analyses correlating misdiagnosis and mistreatment with clinical and radiographic parameters
| p-value | ||
| Misdiagnosis | Mistreatment | |
| Referred to (neurosurgeon vs. another specialist) | <0.001 | <0.001 |
| First symptom (with vs. without neurological deficit) | 0.003 | <0.001 |
| MRI cervical spine (present vs. absent) | 0.039 | 0.039 |
| MRI lumbar spine (present vs. absent) | 0.010 | 0.010 |
| MRI (cervical spine) | MRI (lumbar spine) | |
| First symptom (with vs. without neurological deficit) | 0.007 | 0.275 |
vs. versus
Illustrative case
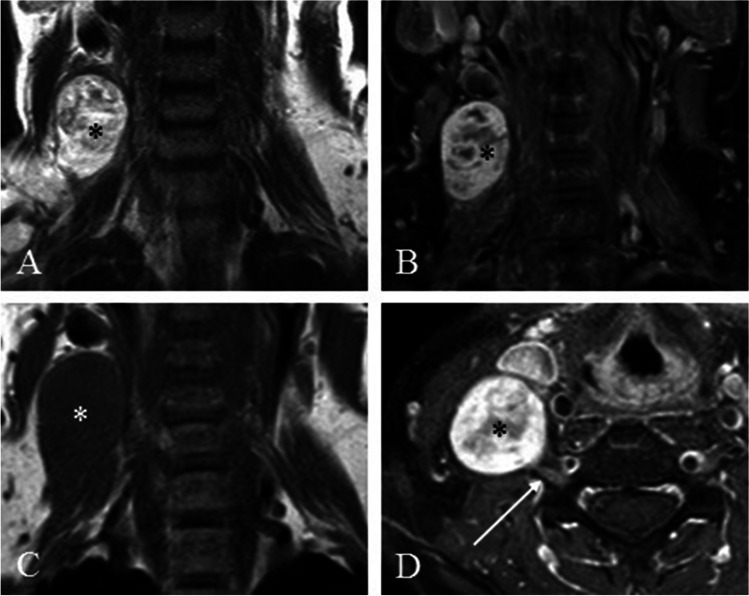
A 52-year-old female patient noticed an elastic swelling on the right side of her neck. After consultation, her general practitioner indicated an MRI of the neck. It showed a smoothly circumscribed paired-spinous mass with apparent contrast enhancement. The diagnosis of “lymph node metastasis” was proposed by the radiologist in charge. Treatment in an Otolaryngologist Department and tumor removal of the mass followed. Intraoperatively, the C6 root was cut for total tumor removal. Histopathological examination revealed the diagnosis of schwannoma. Postoperatively, there was a severe weakness of arm flexion with denervation of the biceps muscle, a disturbance of the fine motor function of the hand, a loss of sensitivity in the area supplied by the C6 root, and a pronounced neuropathic pain in the entire right arm. The patient was referred to our department eight months after surgery. A sural nerve graft was performed, and the patient partially recovered. At last follow-up, seven months after surgery, the patient presented with a weakness of the biceps muscle, the fist, and hypoesthesia of the hand. However, the neuropathic pain symptom entirely disappeared (Fig. 2).
Fig. 2.
Illustrative case showing the preoperative MRI of the schwannomma (*) misdiagnosed as malignant lymph node resulting in a biopsy. A T2-weighted MRI without contrast showing well encapsulated tumor (*), C T1-TSE weighted MRI without contrast, B, D T1-weighted MRI with contrast enhancement of the tumor (*) in direct contact to the CX nerve root (→)
Discussion
A bPNST is a lesion directly associated with peripheral nervous structures and of neuroectodermal origin [1]. Symptom presentation varies depending on their localization, including local swelling, motor deficits, hypoesthesia, and pain [2, 3, 6–8].
A nervous origin might not be assumed at first due to its rarity, leading to treatment under a suspected different diagnosis. Consequently, misdiagnosis and resulting suboptimal or insufficient treatment with possible severe complications might occur.
For the first time, our study presents data on rates of initial misdiagnosis and mistreatment in patients with bPNST. In our series, 44.7% of patients with sporadic bPNST presenting to our specialized institution had an initial misdiagnosis that led to inappropriate surgical treatment in most of these patients (71.1%).
Symptom presentation
Thus, in our group of patients, we could find extremely painful, completely painless large, and very small tumors. Hypoesthesia was also commonly reported. The literature only rarely mentions severe or pronounced symptoms such as paralysis or complete loss of sensitivity, and we also found this confirmed by our patients [2, 4]. Some individuals reported about radiating pain to the corresponding extremities, which may explain the relatively high number of spinal MRI. Symptom presentation of perineurioma differs from other bPNSTs, presenting as a mononeuropathy of gradual onset and slow progression, resulting in progressive neurological deficits like hypoesthesia or, much more frequently, a muscular weakness but, at least in our patients, never a positive Hoffmann Tinel’s sign [10, 11]. In 58% of the other patients, a Hoffmann Tinel’s sign was triggered by touching or tapping. This corresponds with the information in the literature that the Hoffmann Tinel’s sign is highly suggestive for a bPNST and should lead to further diagnostics [15–17].
Diagnosis of bNPST
Diagnostic workup of a bPNST includes MRI, neurosonography, and electrophysiology. MRI is obligatory to confirm or exclude the suspected diagnosis [13, 18]. While MRI of the corresponding region was performed for visible and/or palpable tumors of the extremities, MRI of the spine was performed more frequently for symptomatic but not visible tumors or in cases of radiating symptoms.
In our study, MRI of the spine was performed in all patients with tumors of the sciatic nerve, although they consistently reported triggerable pain. For example, pain was reproducible while sitting on the edge of a hard chair. Consequently, a non-pathological MRI of the spine often led to a delay of definitive diagnosis, especially if conservative therapies were first initiated.
Even though neurosonography is a quick and straightforward method, especially because the nerval tumor origin can be quickly recognized, this method was used only in one case as an initial diagnostic tool. Modern high-resolution nerve sonography makes it possible to visualize the carrier nerve in detail. An experienced examiner can easily make a diagnosis of bPNST. The technique of high-resolution nerve sonography is used in many neurological centers [14, 19].
The situation can be more difficult in cases of retroperitoneal tumors. In those mostly asymptomatic cases, the diagnosis of a retroperitoneal mass as an incidental finding depends on a focused examination of specialized consultants.
Differential diagnosis includes retroperitoneal sarcoma, liposarcoma, or tumors of ovarian origin. Therefore, these patients are often treated appropriately in specialized sarcoma or gynecological centers. A CT-guided biopsy is performed before therapy planning as an additional diagnostical tool according to their guidelines.
Causes of misdiagnosis
We were able to identify four main causes resulting in misdiagnosis of bPNST. Firstly, variation of different symptoms and especially failure to recognize and interpret specific symptoms (for example, a positive Hoffmann Tinel’s sign) may lead to misdiagnosis concerning a non-pathological MRI of the spine. Practitioners might then stop further diagnostic workup.
Secondly, symptom presentation of bPNST might be similar or mimic symptoms of more common diseases. In particular, symptoms similar to radicular symptoms (pain radiating to an extremity) caused by degenerative spinal disorders and symptoms similar to those of nerve entrapment syndromes need to be mentioned. In our study, spinal MRI was often performed in cases of radicular pain. Misdiagnosis was significantly higher in those patients. Therefore, practitioners might be misguided by the presenting symptoms.
Thirdly, bPNST may occur in every region of the body. Radiologists might interpret findings according to their professional experience and knowledge and the frequency and probability of the presented findings. Therefore, one might diagnose a cervical disc prolapse in cases with radiating pain, a malignant lymph node in the axilla or the neck, or a retroperitoneal sarcoma instead of a rare bPNST. In our study, bPNST of the brachial or lumbar plexus were misdiagnosed as malignant lymph nodes or sarcoma. In those cases, anatomical relation to the plexus was not detected or misinterpreted; therefore, bPNST as a differential diagnosis was neglected. While the incidence of bPNSTs in patients with neurofibromatosis type I is well studied, usable results on the frequency of sporadic bPNST do not exist in detail. In literature, the incidence of 10–15% of treated soft tissue tumors is often quoted [2, 3, 6–8].
Lastly, in the German medical system, general practitioners are the first consultants of the patients and are essential for organizing further therapy at a specialized center (84.7% in our study). Therefore, they significantly impact treatment with their “gatekeeper function.” Furthermore, diagnosis and therapy depend on a specialized consultant. We could show that patients referred to a neurosurgeon had significantly lower rates of misdiagnosis and mistreatment. However, it is understandable that medical specialization leads to an influenced interpretation of findings due to the low frequency of diagnoses made in the corresponding field [20–22].
Causes of mistreatment
Our data show the prolonged time for a symptomatic patient to receive a definite diagnosis and adequate therapy in many cases. Failure to recognize a nerve tumor as the cause of pain or sensation deficits leads, at best, only to delayed therapy or, in more serious cases, to the development of a chronic pain syndrome or severe neurological deficits [14, 23]. However, chronic pain and the missing explanation of individual symptoms can lead to a significant psychological burden [24]. Additionally, patients with neurologic deficits were at particular risk for misdiagnosis and mistreatment. Moreover, MRI of the spine did not prevent misdiagnosis as in 76% of the patients with spinal MRI, an unnecessary or insufficient therapy was detected. Two patients that underwent spinal surgery had abnormalities that were believed to be the cause of the problems complained of or had problems that were also explained by spinal pathology.
Another reason for mistreatment is the result of a priorly wrong diagnosis. We found that 26.3% of the patients underwent surgery under an incorrect diagnosis. This includes differential diagnoses such as lipoma, malignant lymph node, or a soft tissue tumor.
The misinterpretation of a symptom and/or the radiological imaging may lead to unnecessary or inappropriate surgical treatment unrelated to the actual disease or to a more radical surgical treatment [25].
This resulted in severe neurological deficits that had to be treated with nerve interposition devices during revision surgery. In the remaining patients, revision surgery was also performed to remove remaining tumor tissue or to perform neurolysis.
Furthermore, a CT-guided biopsy bears the risk of nerve-damaging and is a permanent cause for discussion between the different disciplines. Nevertheless, biopsy or macroscopic removal is performed for diagnostic purposes according to the guidelines of the different oncological, gynecological, or visceral surgical societies [26–28].
Complete resection of schwannomas is standard of care and possible without loss of function [29–32]. Preservation of function must be the primary goal of surgery, especially if these benign tumors have not caused any neurological deficits before surgery [4, 31, 33]. After complete, subcapsular removal of the schwannoma, recurrence is very rare [9, 31].
Removal of a neurofibroma can be more challenging for the surgeon as these often do not have a clear capsule, and injury of the fascicles is more likely [4]. This makes it even more relevant to use a consistent microsurgical technique in these procedures, supported by a surgical microscope, stimulating forceps and, the appropriate microsurgical instrumentation to avoid unnecessary postoperative neurological damage [34–36].
The treatment of perineuriomas has changed over time. A biopsy of a thickened and non-functional fascicle including an epineurotomy is more and more common, due to the slowly progressive course and the lack of delimitation. Complete removal is not recommended, as this would ultimately necessitate nerve replacement with poor neurological outcomes [10, 11, 37].
However, the correct diagnosis did not lead to sufficient therapy in several cases. In our analysis, 26.3% of the patients were treated insufficiently, or surgical treatment was not performed in the recommended manner. Treatment by an experienced nerve surgeon showed significantly lower rates of mistreatment compared with treatment by other specialists. Performing a closed biopsy to confirm the diagnosis of a benign nerve tumor should be the exception and not the norm to avoid unnecessary nerve injury [38].
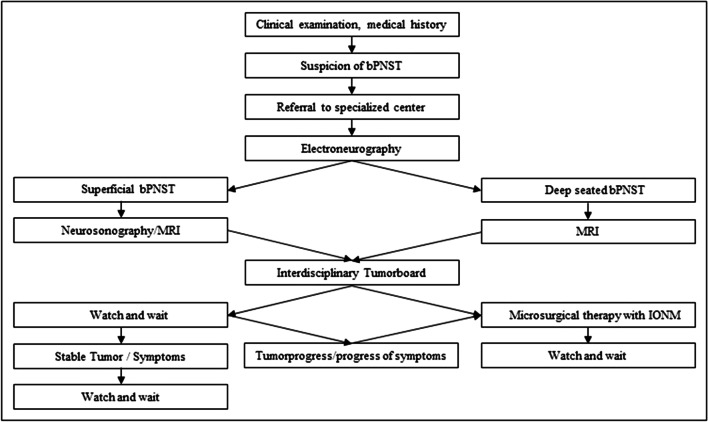
Approach for optimization in clinical management
Our approach for optimization is based on our experience (Fig. 3). Neurological examination and its interpretation have to be performed with care. It is an important part of diagnosis. Our data supports the relevance of the clinical examination and the Tinel’s sign as an excellent diagnostic tool for the diagnosis of bPNST [39].
Fig. 3.
Flow-chart: diagnostical and therapeutical approach of treatment of bPNST. bPNST, benign peripheral nerve sheath tumor; IONM, intraoperative neuromonitoring
Furthermore, the range of diagnostic tools should be widened in daily practice. High-resolution nerve sonography is a promising and straightforward tool to diagnose superficial bPNST, while MRI should be performed in cases of deep-seated bPNST [40–43].
Practitioners should be aware of bPNST as differential diagnosis in patients presenting with a mass located nearby a peripheral nerve, or in patients presenting with neuropathic pain or sensory deficit in a peripheral nerve supply area, and/or motor deficits [44].
Thereupon, interdisciplinary discussion and management in a specialized center may reduce the rate of misdiagnosis and inappropriate treatment. Additionally, rising number of interdisciplinary guidelines may help to keep this rare entity in mind and, therefore, to reduce the number of misdiagnosis and mistreatment in the future.
Limitations
The present study has several limitations that must be acknowledged. The data were retrospectively analyzed from our prospective dataset “peripheral nerve lesion.” Furthermore, the number of patients included at a single center was limited due to the rarity of bPNST. Therefore, statistics might be influenced by the small sample size and collection bias of a specialized center of peripheral nerve surgery. The German medical system is different from other countries, and the results may not be representative for other regions.
Conclusions
Tumors of peripheral nerves are commonly benign and can be removed without functional deficits in most cases. However, there is a high risk of initial misdiagnosis due to their rarity leading to a consecutive mistreatment with potential severe consequences. Therefore, treating disciplines have to keep these rare differential diagnoses in mind. Treatment should be performed only at a specialized center.
Abbreviations
- bPNST
benign peripheral nerve sheath tumor
- CT
computed tomography
- HNST
hybrid nerve sheet tumor
- IONM
intraoperative neuromonitoring
- MRI
magnetic resonance imaging
Author contribution
Anne-Kathrin Uerschels: writing the manuscript, acquisition of data, analysis and interpretation of data, study concept and design.
Nora F. Dengler: revising the manuscript, analysis and interpretation of data.
Mehdi Chihi: statistics, analysis and interpretation of data, revising the manuscript.
Annika Lenkeit: revising the manuscript, analysis and interpretation of data.
Thiemo F. Dinger: revising the manuscript, analysis and interpretation of data.
Ramazan Jabbarli: revising the manuscript, analysis and interpretation of data.
Ulrich Sure: revising the manuscript, analysis and interpretation of data.
Karsten H. Wrede: revising the manuscript, analysis and interpretation of data.
Oliver Gembruch: writing the manuscript, acquisition of data, analysis and interpretation of data, study concept and design.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Data are available upon request.
Declarations
Ethical approval
The study has been conducted in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Institutional Review Board (Medical Faculty, University of Duisburg-Essen, Registration number: 18–7955-BO).
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stone JJ, Boland JM, Spinner RJ. Analysis of peripheral nerve schwannoma pseudocapsule. World Neurosurg. 2018;119:e986–e990. doi: 10.1016/j.wneu.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Ramcharan R, Midha R. Clinical presentation and physical examination. Neurosurg Clin N Am. 2004;15:125–132. doi: 10.1016/j.nec.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Lwu S, Midha R. Clinical examination of brachial and pelvic plexus tumors. Neurosurg Focus. 2007;22:E5. doi: 10.3171/foc.2007.22.6.6. [DOI] [PubMed] [Google Scholar]
- 4.Gosk J, Gutkowska O, Mazurek P, Koszewicz M, Ziolkowski P. Peripheral nerve tumours: 30-year experience in the surgical treatment. Neurosurg Rev. 2015;38:511–520. doi: 10.1007/s10143-015-0620-8. [DOI] [PubMed] [Google Scholar]
- 5.Yonezawa H, Miwa S, Yamamoto N, et al. Structural origin and surgical complications of peripheral schwannomas. Anticancer Res. 2020;40:6563–6570. doi: 10.21873/anticanres.14681. [DOI] [PubMed] [Google Scholar]
- 6.Ahlawat S, Blakeley JO, Rodriguez FJ, Fayad LM. Imaging biomarkers for malignant peripheral nerve sheath tumors in neurofibromatosis type 1. Neurology. 2019;93:e1076–e1084. doi: 10.1212/WNL.0000000000008092. [DOI] [PubMed] [Google Scholar]
- 7.Paradowski B, Bilinska M, Sasiadek M, Jelen M. Plexiform neurofibromas of the brachial plexuses. Neurology. 2005;64:1943. doi: 10.1212/01.WNL.0000163857.23551.2C. [DOI] [PubMed] [Google Scholar]
- 8.Hans VH, Surov A, Kornhuber M, Behrmann C. Schwannomatosis. Neurology. 2009;72:1188. doi: 10.1212/01.wnl.0000339391.48966.ab. [DOI] [PubMed] [Google Scholar]
- 9.Stone JJ, Spinner RJ. Go for the gold: a "plane" and simple technique for resecting benign peripheral nerve sheath tumors. Oper Neurosurg. 2020;18:60–68. doi: 10.1093/ons/opz034. [DOI] [PubMed] [Google Scholar]
- 10.Uerschels AK, Krogias C, Junker A, Sure U, Wrede KH, Gembruch O. Modern treatment of perineuriomas: a case-series and systematic review. BMC Neurol. 2020;20:55. doi: 10.1186/s12883-020-01637-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brand C, Pedro MT, Pala A, et al. Perineurioma: a rare entity of peripheral nerve sheath tumors. J Neurol Surg A Cent Eur Neurosurg. 2022;83:1–5. doi: 10.1055/s-0041-1726110. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez FJ, Folpe AL, Giannini C, Perry A. Pathology of peripheral nerve sheath tumors: diagnostic overview and update on selected diagnostic problems. Acta Neuropathol. 2012;123:295–319. doi: 10.1007/s00401-012-0954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Datir A, James SL, Ali K, Lee J, Ahmad M, Saifuddin A. MRI of soft-tissue masses: the relationship between lesion size, depth, and diagnosis. Clin Radiol. 2008;63:373–378. doi: 10.1016/j.crad.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 14.Vetrano IG, Acerbi F, Marucci G, Nazzi V. The effect of ozone injection within a common peroneal nerve schwannoma: a mistreatment due to a misdiagnosis. Surg Neurol Int. 2020;11:413. doi: 10.25259/SNI_296_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347–350. doi: 10.1016/j.cps.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Kim DH, Murovic JA, Tiel RL, Moes G, Kline DG. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246–255. doi: 10.3171/jns.2005.102.2.0246. [DOI] [PubMed] [Google Scholar]
- 17.Kokkalis ZT, Stavropoulos NA, Mavrogenis AF, Panagopoulos A, Soucacos PN. Benign peripheral nerve tumors. Injury. 2019;50(Suppl 5):S77–s83. doi: 10.1016/j.injury.2019.10.054. [DOI] [PubMed] [Google Scholar]
- 18.Pham K, Ezuddin NS, Pretell-Mazzini J, Subhawong TK. Small soft tissue masses indeterminate at imaging: histological diagnoses at a tertiary orthopedic oncology clinic. Skeletal Radiol. 2019;48:1555–1563. doi: 10.1007/s00256-019-03205-0. [DOI] [PubMed] [Google Scholar]
- 19.Maeshima S, Nakamura H, Nishikawa M, et al. Retroperitoneal schwannomas simulating adrenal tumors. Clin Imaging. 1992;16:121–124. doi: 10.1016/0899-7071(92)90125-S. [DOI] [PubMed] [Google Scholar]
- 20.Vieira R, Navarro de Oliveira LC, Martins RM, Falcao-Junior PC. Schwannoma associated with breast cancer mimicking axillary metastasis. Breast J. 2020;26:2412–2413. doi: 10.1111/tbj.14090. [DOI] [PubMed] [Google Scholar]
- 21.Lopez Gonzalez FJ, Garcia Alfonso L, Enriquez Rodriguez AI, Torres Rivas HE. Pleural schwannoma mimicking metastatic rectal carcinoma. Arch Bronconeumol. 2019;55:110–111. doi: 10.1016/j.arbres.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Aran T, Guven S, Gocer S, Ersoz S, Bozkaya H. Large retroperitoneal schwannoma mimicking ovarian carcinoma: case report and literature review. Eur J Gynaecol Oncol. 2009;30:446–448. [PubMed] [Google Scholar]
- 23.Richter KK, Premkumar R, Yoon HS, Mercer P. Laparoscopic adrenalectomy for a rare 14-cm adrenal schwannoma. Surg Laparosc Endosc Percutan Tech. 2011;21:e339–e343. doi: 10.1097/SLE.0b013e31823ac4d4. [DOI] [PubMed] [Google Scholar]
- 24.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397:2082–2097. doi: 10.1016/S0140-6736(21)00393-7. [DOI] [PubMed] [Google Scholar]
- 25.Hsiao H-L, Li C-C, Lin H-C, Yeh H-C, Huang C-H, Wu W-J. Adrenal schwannoma treated with laparoscopic adrenalectomy: a case report. Kaohsiung J Med Sci. 2008;24:553–557. doi: 10.1016/S1607-551X(09)70016-6. [DOI] [PubMed] [Google Scholar]
- 26.German Guideline Program in Oncology (German Cancer Society GCA, AWMF). Interdisciplinary evidenced-based practice guideline for the early detection, diagnosis, treatment and follow-up of breast cancer. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/S3_Guideline_Breast_Cancer.pdf. Accessed 27 March 2022
- 27.German Guideline Program in Oncology (German Cancer Society GCA, AWMF). S3-Leitlinie Diagnostik, Therapie und Nachsorge des Hodgkin Lymphoms bei erwachsenen Patienten. https://www.awmf.org/uploads/tx_szleitlinien/018-029OLl_S3_Hodgkin-Lymphom_Erwachsene_Diagnostik_Therapie_Nachsorge_2022-03.pdf. Accessed 27 March 2022
- 28.German Guideline Program in Oncology (German Cancer Society GCA, AWMF). S3-Leitlinie Adulte Weichgewebesarkome. https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Adulte_Weichgewebesarkome/LL_Weichgewebesarkome_Leitlinienreport_1.0.pdf. Accessed 27 March 2022
- 29.Kim DH, Murovic JA, Tiel RL, Kline DG. Operative outcomes of 546 Louisiana State University Health Sciences Center peripheral nerve tumors. Neurosurg Clin N Am. 2004;15:177–192. doi: 10.1016/j.nec.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Donner TR, Voorhies RM, Kline DG. Neural sheath tumors of major nerves. J Neurosurg. 1994;81:362–373. doi: 10.3171/jns.1994.81.3.0362. [DOI] [PubMed] [Google Scholar]
- 31.Russell SM. Preserve the nerve: microsurgical resection of peripheral nerve sheath tumors. Neurosurgery. 2007;61:113–117. doi: 10.1227/01.neu.0000289724.89588.bc. [DOI] [PubMed] [Google Scholar]
- 32.Muramatsu K, Tani Y, Seto T, et al. Schwannoma in the extremity: clinical features and microscopic intra-capsular enucleation. J Rural Med. 2021;16:184–190. doi: 10.2185/jrm.2021-020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granlund AS, Sorensen MS, Jensen CL, Bech BH, Petersen MM. Clinical outcome after surgery on schwannomas in the extremities. World J Orthop. 2021;12:760–767. doi: 10.5312/wjo.v12.i10.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flores LP. The importance of the preoperative clinical parameters and the intraoperative electrophysiological monitoring in brachial plexus surgery. Arq Neuropsiquiatr. 2011;69:654–659. doi: 10.1590/S0004-282X2011000500015. [DOI] [PubMed] [Google Scholar]
- 35.Ducic I, Barrett DM, Al-Attar A. Benign peripheral nerve tumors: treatment algorithm and reconstructive options. Ann Plast Surg. 2009;63:156–161. doi: 10.1097/SAP.0b013e318189389e. [DOI] [PubMed] [Google Scholar]
- 36.Li X, Zhong G, Xu X, et al. Surgical strategies for peripheral nerve schwannoma based on the intraoperative neurophysiological monitoring. Laparoscopic, Endoscopic and Robotic Surgery. 2019;2:65–69. doi: 10.1016/j.lers.2019.06.001. [DOI] [Google Scholar]
- 37.Wilson TJ, Howe BM, Stewart SA, Spinner RJ, Amrami KK. Clinicoradiological features of intraneural perineuriomas obviate the need for tissue diagnosis. J Neurosurg. 2018;129:1034–1040. doi: 10.3171/2017.5.JNS17905. [DOI] [PubMed] [Google Scholar]
- 38.Pendleton C, Spinner RJ. Image-guided percutaneous biopsy of peripheral nerve tumors of indeterminate nature: risks and benefits. Acta Neurochir. 2020;162:1425–1429. doi: 10.1007/s00701-020-04257-1. [DOI] [PubMed] [Google Scholar]
- 39.Ogose A, Hotta T, Morita T, et al. Tumors of peripheral nerves: correlation of symptoms, clinical signs, imaging features, and histologic diagnosis. Skeletal Radiol. 1999;28:183–188. doi: 10.1007/s002560050498. [DOI] [PubMed] [Google Scholar]
- 40.Holzgrefe RE, Wagner ER, Singer AD, Daly CA. Imaging of the peripheral nerve: concepts and future direction of magnetic resonance neurography and ultrasound. J Hand Surg Am. 2019;44:1066–1079. doi: 10.1016/j.jhsa.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Wu G, Liu L, Mei Z, Li X. Diffusion-weighted MR is useful to assess peripheral nerve invasion of soft tissue tumor. Medicine. 2022;101:e29779. doi: 10.1097/MD.0000000000029779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krogias C, Gold R, Schelle T, et al. Teaching NeuroImages: sonographic detection of intraneural perineurioma in therapy-refractory carpal tunnel syndrome. Neurology. 2017;88:e85–e86. doi: 10.1212/WNL.0000000000003692. [DOI] [PubMed] [Google Scholar]
- 43.Telleman JA, Stellingwerff MD, Brekelmans GJ, Visser LH. Nerve ultrasound: a useful screening tool for peripheral nerve sheath tumors in NF1? Neurology. 2017;88:1615–1622. doi: 10.1212/WNL.0000000000003870. [DOI] [PubMed] [Google Scholar]
- 44.Sughrue ME, Levine J, Barbaro NM. Pain as a symptom of peripheral nerve sheath tumors: clinical significance and future therapeutic directions. J Brachial Plex Peripher Nerve Inj. 2008;3:6. doi: 10.1186/1749-7221-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request.