Abstract
In this study, antioxidative methanolic leaf extract (MeOH-SIS) of Urtica dioica was characterized for anti-diabetic activity. The extract was purified on a column to yield seven homogenous fractions (F1–F7) which were further determined for DPPH radical scavenging activity. MeOH-SIS and the fraction F1 (selected based on % yield and activity) were evaluated for their in vitro α-amylase and α-glucosidase inhibitory activity. The results showed inhibition of both enzymes in a dose dependent manner and F1 exhibited relatively higher inhibition than its mother extract MeOH-SIS. GC–MS analyses of both the extracts identified 24 major compounds among which 10 were previously described as bioactive compounds. Among all, 5 compounds demonstrated to have quality pharmacokinetics profiles and were examined for possible binding affinity against the active sites of α-amylase and α-glucosidase using molecular docking. The binding interaction of 2R-acetoxymethyl-1,3,3-trimethyl-4 T-(3-methyl-2-buten-1-yl)-1 T-cyclohexanol within the active sites of the target receptors was found to be significant among others, and can be developed as a potential inhibitor of α-amylase and α-glucosidase. The leaf extract can be utilized to develop food additive for the control and management of oxidative stress induced diabetes.
Keywords: Green leafy vegetable, Urtica dioica, Sikkim Himalayan region, Anti-diabetic activity, Antioxidants
Introduction
Green leafy vegetables have been contributing a very significant role in human health since ancient times. Being considered as one of the important sources of carbohydrates, fats, vitamins, essential amino acids and proteins, dietary fibers and minerals, some of them are equally important as nutraceuticals. Due to the gaining popularity of nutraceuticals and functional foods, researchers are characterizing the bioactive metabolites associated with lesser studied green leafy vegetables and fruits. Several plant derived bioactive compounds have been characterized and assessed for biological activity to explore their applications as functional food additives (Kalai et al. 2022; Khalid et al. 2023; Ibrahim et al. 2022). Their secondary metabolites exhibit a wide range of biological functions including antioxidative property, and are proven to be useful in overcoming oxidative stress and other associated disorders (Bhat and Al-Daihan 2014; Sharma et al. 2022). Beyond antioxidant potential leafy vegetables have been reported for other health benefits for their application as functional food additive includes anti-diabetic, anti-inflammatory, immunomodulatory, antibacterial, and anti-cancer properties (Khalid et al. 2023). Nonetheless, the pharmacological significance and phyto-constituents of most such leafy vegetables is still unknown.
Leafy vegetables are an integral part of the usual diet in Sikkim, a small Himalayan state in the north eastern region of India, where most of the vegetables are considered to be medicinally relevant. Urtica dioica (or stinging nettle), a perennial herb and a member of the family Urticaceae is used as an important leafy vegetable in this region. Locally it is known as “Sisnu” and is consumed as sour soup, curry, and as a vegetable complement (Sharma et al. 2022). U. dioica, since long years, has been used as diuretic and to treat joint pain, gout, arthritis and other rheumatic aliments (Hall and Bravo-Clouzet 2013). In modern clinical treatments, leaves of this plant are applied in the management of cardiovascular and diabetic symptoms (Chrubasik et al. 2007; Kianbakht et al. 2013). However, most of its pharmacological properties remain speculative and await scientific and clinical validation.
In this investigation, the methanolic extract of U. dioica leaves, which was found to have significant antioxidant activity in our earlier study was further purified into its sub fractions and evaluated for DPPH radical scavenging and in vitro antidiabetic (α-amylase and α-glucosidase inhibiting activity) activity. Gas Chromatography–mass spectrometry (GC–MS) methodology has been applied for identification of bioactive compounds in medicinal plants having numerous health promoting potentials (Malheiro et al. 2016; Ralte et al. 2022). GC–MS has been applied as a reliable technique for identifying phytochemicals including flavonoids, alkaloids, organic acids, lipid-based nutraceuticals, amino acids, etc. (Babatunde et al. 2019; Ralte et al. 2022; Ramya 2022). The most active fraction of U. dioica leaves extract was characterized using GC–MS, in silico pharmacokinetics and molecular docking. The characterization of bioactive constituents associated with U. dioica leaves along with its functional properties can lead to its popularization as functional food in the Himalayan region. Further, their active extracts can be applied as functional food additive for enhancing specific health benefits in other foods.
Materials and methods
Chemicals
Methanol (MeOH), hexane (HEX), ethyl acetate (EtOAC), 2,2-diphenyl-1-picrylhydrazyl (DPPH), Starch, 3, 5-Dinitrosalicylic acid (DNS), Di-sodium hydrogen phosphate, sodium carbonate (Na2CO3), Sodium-di-hydrogen phosphate, α-amylase, α-glucosidase, p-nitrophenyl-α-D-glucoside (PNPG), Folin–Ciocalteu reagent are procured from Sigma Aldrich.
Sampling and metabolites extraction
Fresh leaves of Urtica dioica were procured from the local market (Lal Bazaar, Gangtok). The leaves were cleaned by washing by tap water followed by oven-drying (40° C). The dried leaves were powdered using a mixer grinder and the leaf powder (100 g) was extracted with methanol (500 mL). The solution was kept for 72 h in dark with shaking being done at every 12 h. The extracts were collected by filtration using filter papers (Whatman No. 1), followed by concentration in a Rota evaporator (R 100, Buchi). The methanolic extract (1.25 g) was further purified by chromatography on a silica gel column (75 × 3 cm) using approximately 2 L of hexane: ethyl acetate (7:3) as eluent and 7 homogenous fraction groups were collected.
DPPH radical scavenging activity of different fractions
DPPH Radical scavenging activities of different fractions of SIS-MeOH were carried out as per methodology described by Chourasia et al. (2022). Briefly, extracts (200 μL) having defined concentration were added to test tube containing 2 mL of 0.16 mM DPPH solution. The content was kept for incubation (30 min) followed by measuring the absorbance at 517 nm by a spectrophotometer (Shimadzu, Kyoto, Japan). The calculation of DPPH radical scavenging potential was done by the equation given below:
Scavenging potential (%) = {1 − (Sabs − Babs/Cabs)} × 100.
Where, Sabs-absorbance of the extract, Cabs-absorbance of the control (DPPH) and Babs-absorbance of extract blank.
α-Amylase inhibitory assay
α-Amylase inhibitory assay of MeOH SIS and F1 extracts was carried by following the method explained by Ibrahim et al. (2014). Plant extract was added to 250 µL α-amylase (prepared in 0.02 M sodium phosphate buffer) followed by incubation for 10 min at 25ºC. Further, 250 µL of 1% starch solution was added to the mixture and followed by the incubation at 25 ºC for 10 min. To this solution mixture, 500 µL DNS was added and kept for incubation for 5 min in boiling water followed by addition of distilled water (5 mL). For sample blank 250 µL sodium phosphate buffer (0.02 M) was added instead of α-amylase and starch. Absorbance of the content was recorded at 540 nm using a spectrophotometer.
α-Glucosidase inhibitory assay
Inhibition of α-glucosidase by sample extract was assayed following the method explained by (Ibrahim et al. 2018). Briefly, 50 μL of α-glucosidase (1 U/mL) was preincubated with 100 μL sample extract and 100 μL potassium phosphate buffer (0.1 M, pH 6.9) for 10 min at 37 °C. Further, 50 mL of 5 mM p-nitrophenyl-α-D-glucoside (PNPG) was added to the preincubated mixture, vortexed and incubated for reaction at 37 °C for 30 min. The reaction was stopped by addition of 0.1 M sodium carbonate (1 mL) and the absorbance was measured at 405 nm in a spectrophotometer. Inhibition of α-glucosidase activity was assessed using the equation given below:α- glucosidase inhibition (%) = [(A − B) − (C − D)/(A − B)] × 100
Where, A is the absorbance in the presence of α-glucosidase and absence of the sample (sample substituted by buffer), B-absorbance in the absence of the enzyme and sample (buffer only), C-absorbance of mixture of enzyme and the sample, and D-absorbance in the presence of the sample and absence of the enzyme (α-glucosidase substituted by buffer).
Gas Chromatography–Mass Spectrometry (GC–MS)
The chemical constituents of MeOH extract of U. Dioica leaves and its fraction F1 were subjected to a Perkin Elmer Model: Clarus 680/600C GC–MS equipped with an Elite 5MS column (60.0 mm × 0.25 mm ID). 1 μL of the sample was injected and run for 39 min. The analysis conditions were: 1 min at 60 °C, ramp 7° C/min to 200 °C, hold 3 min, ramp 10 °C/min to 300 °C, hold 5 min. Helium (He) was used as the carrier gas and split ratio was 10:1. The MS scan was performed with EI (+ ve mode) within the range 50–600 Da. The peaks were identified on comparing the spectra with the National Institute Standard and Technique (NIST) library.
Drug likeliness and Pharmacokinetics
The ADMET (absorption, distribution, metabolism, elimination and toxicity) properties of the identified compounds were predicted using ADMETlab 2.0 (Xiong et al. 2021). The ‘simplified molecular input line entry system’ (SMILES) codes of the compounds were used as input for the program. The important ADMET predictions include drug likeliness (Lipinski’s rule), water solubility, human intestinal absorption (HIA), blood brain barrier penetration (BBB), human ether-a-go-go related gene (hERG) blockers, hepatotoxicity (HT), drug induced liver injury (DILI), Ames mutagenicity, and toxicophore prediction. The compounds displaying desirable ADMET features were selected and used in molecular docking.
Molecular Docking
Briefly, the crystallographic 3D structures of human pancreatic alpha amylase (PAM) and human maltase glucoamylase (MGA) were retrieved from the protein data bank using PDB identifiers 3BAI and 3CTT, respectively. The structures were selected based on the best available resolution in the database. Protein structures were optimized upon removing the heteroatoms and water molecules, and adding polar hydrogens and Kollman charges using Autodock tools. Similarly, chemical structures of the selected compounds were retrieved from PubChem and minimized prior to docking which was performed using CB Dock web server that uses the popular docking program Autodock Vina (Liu et al. 2020). The docked complexes were visualized in Discovery Studio Visualizer (DSV) 2020 and receptor ligand interactions were analyzed. The docking results were also compared to a reference inhibitor and anti-diabetic drug, acarbose.
Statistical analysis
The data of all the experiments (performed in triplicates) in the present study were presented as mean ± standard deviation. The statistical analyses were accomplished by subjecting the experimental data to ANOVA (one way analysis of variance) using Minitab 19 software with a confidence of 95% (P < 0.05).
Results and discussion
The significance of ample vegetable consumption in the promotion of human health has been known since years. Studies on green leafy vegetables on the other hand deserve special attention as they have potential in development of functional foods. They have been proposed to have various antioxidant compounds and some of them have been shown to reduce the risk of oxidative stress mediated metabolic disorders such as type 2 diabetes (Brouwer-Brolsma et al. 2020). The leaf extract used in this study was one among several organic extracts which have shown promising antioxidant activity in our earlier study (Sharma et al. 2022). Characterization of active constituents and bioactivity of the extract as well as its fractions can lead to their application as functional food additives. The methanolic (MeOH-SIS) extract (1.25 g) was purified on a column to yield 7 homogenous fractions namely F1 (173 mg), F2 (94 mg), F3 (36 mg), F4 (125 mg), F5 (17 mg), F6 (41 mg) and F7 (56 mg). The percentage yields of each fraction have been presented in Fig. 1A. The fractions were evaluated for their antioxidant potential using DPPH radical scavenging activity assay, and fractions F1 and F6 demonstrated highest antioxidant activity as compared to other fractions. Overall antioxidant activity of the MeOH extract was 20.92% and the activity of the fractions ranged from 17.63 to 41.47% (Fig. 1B). Based on the % yield and DPPH radical scavenging activity, fraction F1 was selected for further study. Activity of F1 fraction was found to be 1.89-fold higher than parent extract.
Fig. 1.
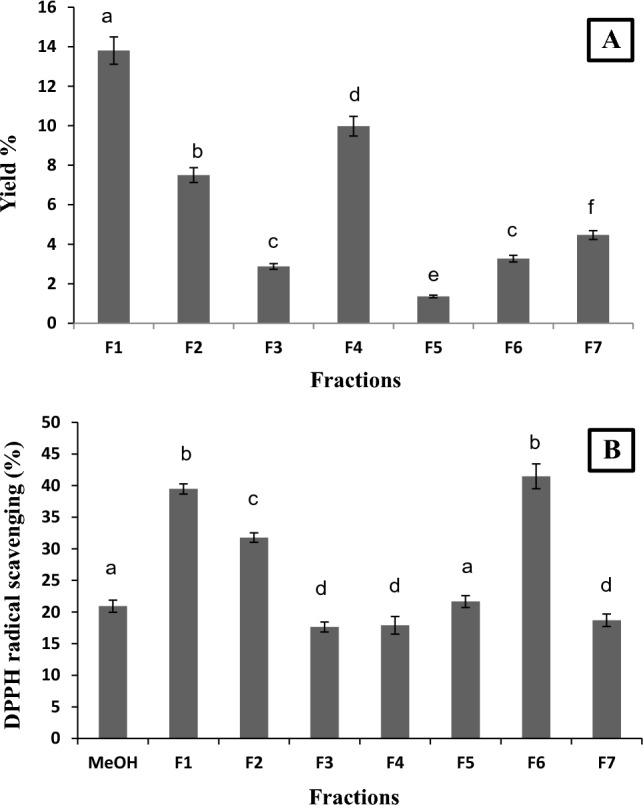
Percentage yield A and DPPH scavenging activity B of obtained fractions (F1–F7) from MeOH-SIS crude organic extract. Data in the graphs not sharing common alphabets (a, b, c, d, e) in respective figure are significantly different (p < 0.05). F1–F7: Seven homogenous fraction groups eluted using hexane: ethyl acetate (7:3)
The antioxidative phytoconstituents (for example phenolic acids and flavonoids) are reported to scavenge the excess production of reactive oxygen species (ROS). In addition, they can neutralize the affects of ROS on the β-cells which secrete insulin and facilitate glucose uptake into muscle, brain, liver, adipose tissue and other glucose recipient organs, thereby preventing the oxidative stress induced diabetes (Sekhon-Loodu and Rupasinghe 2019). The carbohydrate metabolizing enzymes such as α-glucosidase and α-amylase influence an increase in postprandial blood glucose level in diabetic patients and are recognized as important markers of hyperglycemia or diabetes. Inhibition of these digestive enzymes may result in lowering of blood glucose level and reduced risk of diabetes (Poovitha and Parani 2016). Many of the phytoconstituents having antioxidant potential have been suggested to inhibit α-amylase and α-glucosidase, contributing in the treatment of diabetes (Pieczykolan et al. 2021). It is therefore pertinent to explore leafy vegetables that can be utilized in reducing blood-glucose levels and others diabetic biomarkers. The parental MeOH-SIS and fraction F1 were evaluated for in vitro α-amylase and α-glucosidase inhibition. The extracts demonstrated inhibition of both the enzymes in a concentration dependent manner. The α-glucosidase inhibitory potential of MeOH-SIS and F1 fraction was studied from an initial concentration of 0.25 mg/ml up to 1.25 mg/ml. The α-glucosidase followed the increasing trend of inhibitory effect ranging from 9.36-46.06% and 15.76-87.74% for MeOH-SIS and F1 fraction, respectively (Fig. 2). Similarly, α-amylase inhibition was studied at an increasing concentration from 0.375-0.625 mg/ml (Fig. 3). α-amylase inhibition was observed in the range of 4.51-27.66% for MeOH-SIS and 8.71-43.71% for fraction F1. IC50 values of the F1 fraction exhibited 1.9-fold higher inhibitions in comparison to MeOH-SIS. The difference in enzyme inhibitory activity could be attributed to the ability of different solvents to extract specific compounds based on their physiochemical properties such as polarity, chemical nature of the extracted compounds, and the presence or absence of interfering substances (Boeing et al. 2014). The active extracts were subjected to GC–MS-based identification of major constituents. The GC–MS chromatograms demonstrated presence of several prominent peaks with considerable height and area based on which 24 major peaks (12 each from MeOH-SIS and F1) were selected and identified. Ten of the GC–MS-based identified compounds have been reported to have functional properties including antioxidant properties, and others were identified in several active extracts of many plants (Table 1). Some of the peaks were identified as common in both the extracts which include 3-methyl-2-(2-oxopropyl) furan, hentriacontane, Z,Z-6,28-heptatriactontadien-2-one and cyclotrisiloxane, hexamethyl.
Fig. 2.
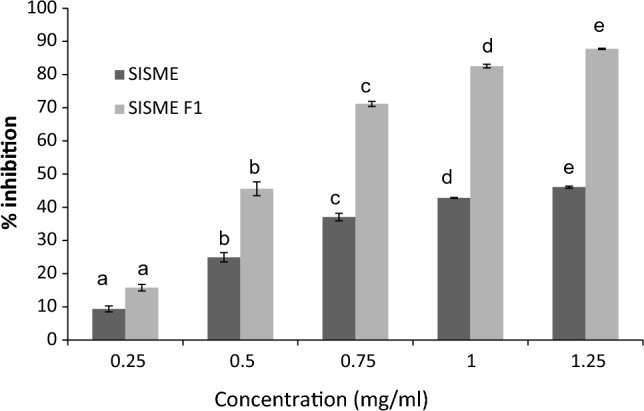
α-Glucosidase inhibiting activity of the MeOH-SIS extract (SISME) and F1 fraction (SISME F1). Data in the graphs not sharing common alphabets (a, b, c, d, e) within same extract in respective figure are significantly different (p < 0.05)
Fig. 3.

α-Amylase inhibiting activity of the MeOH-SIS extract (SISME) and F1 fraction (SISME F1). Data not sharing common alphabets (a, b, c, d, e) within same extract in respective figure are significantly different (p < 0.05)
Table 1.
Major phyto-constituents identified from the U. dioica MeOH extract and its fraction F1 using GC–MS
| Retention time (Rt) | Area (%) | Name of the compound(s) | MW (g/mol) | Chemical formula | Bioactivity | References |
|---|---|---|---|---|---|---|
| SISNU MeOH Extract | ||||||
| 8.023 | 1.556 | 3-Methyl-2-(2-oxopropyl)furan | 138.16 | C8H10O2 | Antioxidant & antimicrobial | Ralte et al. 2022 |
| 8.629 | 5.507 | Rhodoviolascin | 596.9 | C42H60O2 | Antioxidant | Black et al. 2020 |
| 10.930 | 1.113 | 2-Hexenoic acid, (E)- | 114.14 | C6H10O2 | Flavoring agent | Malherio et al. 2016 |
| 13.060 | 1.075 | Oxalic acid, ethyl neopentyl ester | 188.22 | C9H16O4 | Antiobesity | Kalayoglu et al. 2013 |
| 17.882 | 0.902 | 1H-indene, 1-ethylidene- | 142.20 | C11H10 | NR | Joshi 2018 |
| 22.774 | 1.380 | Hentriacontane | 436.8 | C31H64 | anti-inflammatory, antitumor & antimicrobial | Khajuria et al. 2017 |
| 27.416 | 2.507 | Neophytadiene | 278.5 | C20H38 | Antimicrobial & antioxidant | Pratama et al. 2019 |
| 31.308 | 3.684 | Z,Z-6,28-heptatriactontadien-2-one | 530.9 | C37H70O | NR | Ralte et al. 2022 |
| 32.878 | 3.047 | 2R-acetoxymethyl-1,3,3-trimethyl-4 T-(3-methyl-2-buten-1-yl)-1t-cyclohexanol | 282.4 | C17H30O3 | Antimicrobial & antioxidant | Naine et al. 2016 |
| 33.459 | 4.525 | 1,3,6,10-Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl)-, (S-(E,Z,E,E))- | 272.5 | C20H32 | NR | Thimmegowda et al. 2013 |
| 38.701 | 4.513 | Cyclotrisiloxane, hexamethyl- | 222.46 | C6H18O3Si3 | NR | Ahmed et al. 2020 |
| 38.826 | 2.437 | Arsenous acid, tris (trimethylsilyl) ester | 342.49 | C9H27AsO3Si3 | NR | Falowo et al. 2017 |
| SISNU F1 Fraction | ||||||
| 8.023 | 0.917 | 3-Methyl-2-(2-oxopropyl)furan | 138.16 | C8H10O2 | Antioxidant & antimicrobial | Ralte et al. 2022 |
| 9.264 | 1.265 | Meso-2,5-dimethyl-3,4-hexanediol | 146.23 | C8H18O2 | NR | Zhang et al. 2022 |
| 22.774 | 2.097 | Hentriacontane | 596.9 | C42H60O2 | anti-inflammatory, antitumor & antimicrobial | Khajuria et al. 2017 |
| 27.416 | 1.198 | Z,Z-6,28-heptatriactontadien-2-one | 530.9 | C37H70O | NR | Ralte et al. 2022 |
| 28.787 | 4.719 | Methyl 11-methyl-dodecanoate | 228.37 | C14H28O2 | NR | Ralte et al. 2022 |
| 31.088 | 4.584 | 11,14-Octadecadienoic acid, methyl ester | 294.5 | C19H34O2 | NR | Siswadi & Saragih 2021 |
| 31.163 | 3.775 | Methyl 8,11,14-heptadecatrienoate | 278.4 | C18H30O2 | NR | Manjari et al. 2014 |
| 31.313 | 6.213 | Phytol | 296.5 | C20H40O | Antioxidant, antimicrobial & immune-modulating | Islam et al. 2018 |
| 36.095 | 2.553 | Alpha-tocopheryl acetate | 472.7 | C31H52O3 | Antioxidant | Sunarić et al. 2017 |
| 38.706 | 2.048 | Tris(tert-butyldimethylsilyloxy)arsane | 468.7 | C18H45AsO3Si3 | NR | Babatunde et al. 2019 |
| 32.998 | 0.954 | 2,4,4-Trimethyl-3-hydroxymethyl-5A-(3-methyl-but-2-enyl)-cyclohexene | 222.37 | C15H26O | NR | Anupama et al. 2014 |
| 36.800 | 0.945 | Cyclotrisiloxane, hexamethyl- | 222.46 | C6H18O3Si3 | NR | Ahmed et al. 2020 |
Assessment of the ADME features has been playing a considerable role in developing bioceutical (pharmaceutical or nutraceutical) and the same reportedly has accounted for the failure of majority of test compounds in the clinical phases. Aqueous solubility of proposed molecules is an excellent predictor of their absorption; better solubility correlates with better absorption of the compound. However, the real duodenal contents contain more detergents than water and favours drugs that are moderately or less soluble in water (Lipinski 2008). For any bioactive compounds to be used as bioceuticals they need to be absorbed in the blood stream or mucosal surface before reaching to the target tissue. Poor solubility, intestinal transit, and the inability to permeate the intestinal wall are some of the limiting factors in the absorption of active molecules. In addition, the lack of ability to infiltrate the blood brain barrier (BBB) is the reason why few treatments that demonstrate activity in other organs fail to reach the brain. The Caco-2 permeability has been considered as another important predictor of intestinal absorption of small molecules. Caco-2 cells are human colon adenocarcinoma cell lines which have been used as a model of human intestinal absorption of bioceuticals (Padhi et al. 2021; van Breemen and Li 2005).
The extent to which a candidate drug can cause harm to the living cells/organisms is referred to as drug toxicity and this remains one of the most crucial reasons for most drug failure in final stages. Early prediction and identification of toxicity parameters is therefore critical in the process of drug design and development. The voltage gated potassium channels encoded by the gene hERG are an essential component of cardiac depolarization and re-polarization, and play a central role in regulation of heart’s action and resting potential. Any blockade in this channel may result into long QT syndrome, Torsade de Pointes (TdP) that in turn may lead to cardiotoxicity with severe fatal irregularities (Witchel Witchel and Harry 2011). Therefore, well-grounded appraisal of hERG liability is indispensable in order to minimize the cardiotoxic risk of bioactive drug or nutraceutical candidates.
Many drugs carry one or more reactive moieties which are otherwise known as toxicophore(s) and can participate in a toxic response. Toxicophores have been known to precipitate drug-induced liver damage (DILI) in susceptible patients as most prescribed medications are metabolized by human liver. Therefore, DILI and hepatotoxicity of bioactive molecules are major concerns and are the main reason for drug withdrawl from the pharmaceutical market (Boelsterli and Kashimshetty 2010). Carcinogenicity remains as one of the major setbacks in the bioceutical development process (Meunier and Larrey 2019). It is important to assess the ability of the proposed compounds to cause damage to the DNA and impede host cellular metabolic process. As such, many approved drugs have been identified as carcinogens to humans and withdrawn from the market. In addition, with the vast available experimental data on the Ames mutagenicity of known chemical compounds it is now possible to identify the DNA reactive impurities from their chemical structure (Synder and Smith 2005). Among the identified compounds, ADME and T profiles of 2R-acetoxymethyl-1,3,3-trimethyl-4t-(3-methyl-2-buten-1-yl)-1 T-cyclohexanol (1), methyl 11-methyl-dodecanoate (2), 11,14-octadecadienoic acid methyl ester (3), methyl 8,11,14-heptadecatrienoate (4), and 2,4,4-trimethyl-3-hydroxymethyl-5A-(3-methyl-but-2-enyl)-cyclohexene (5) were found to be satisfactory and demonstrated the potential for application in the nutraceutical industry (Table 2). Further, the chemical structures of selected compounds were used as ligands to examine their binding affinity against the active sites of human PAM and MGA using molecular docking. Molecular docking has become a widely used computational approach to screen and predict the intermolecular affinity and interactions between a receptor protein and a set of small molecules in modern nutraceutical development (Talukdar et al. 2021). The compound (1) and (5) demonstrated notable binding free energy (− 7.0 and − 6.7 kcal/mol, respectively) against human PAM. Similarly, the binding free energy of (4) (− 7.2 kcal/mol) was found to be substantial against human MGA which was followed by compound (3) (− 6.6 kcal/mol). The binding free energies of the compounds were similar to that of acarbose. Above all, binding interaction of compound (1) against both the target sites was significant. The docking results are given in Table 3. In every case, the interactions were supported by several non-covalent forces of attraction, including hydrogen bonds, electrostatic and hydrophobic interactions. The hydrogen bonds are known to confer stability and structural integrity to the receptor-ligand complexes. The hydrophobic interactions on the other hand intercalate the binding affinity of the ligand within the binding pockets of the target receptors (Rahman et al. 2016). Overall findings of the study suggest that the leaves of U. dioica are a rich source of antioxidant compounds that could further exert significant α-amylase and α-glucosidase inhibitory activity. Owing to the drug likeliness and pharmacokinetics, compound (1) which was reported as an experimentally proven natural antioxidant that has shown significant binding affinity and interactions towards the active sites of α-amylase and α-glucosidase. The study can lead to development of dried extracts and its fraction (F1) as functional food additives having potential blood glucose lowering property provided that their efficacy and potency must be evaluated in animal models and clinical settings. In addition, the leafy vegetable from the Himalayan region can be popularised as one of the functional foods for the management of oxidative stress induced diabetes.
Table 2.
Representative ADMET profiles of selected compounds used in this study predicted using ADMETlab 2.0
| Name of the compound | Solubility (log S) | HIA | BBB | Caco-2 | hERG | H-HT | DILI | Ames mutagenicity | Carcinogenicity | Toxicophore | Drug likeliness | Golden triangle |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2R-ACETOXYMETHYL-1,3,3-TRIMETHYL-4 T-(3-METHYL-2-BUTEN-1-YL)-1 T-CYCLOHEXANOL | − 4.407 | 0.012 | 0.942 | − 4.509 | 0.009 | 0.253 | 0.037 | 0.004 | 0.048 | 0 | Accepted | Accepted |
| METHYL 11-METHYL-DODECANOATE | − 5.554 | 0.002 | 0.798 | − 4.501 | 0.062 | 0.044 | 0.292 | 0.006 | 0.103 | 0 | Accepted | Accepted |
| 11,14-OCTADECADIENOIC ACID, METHYL ESTER | − 5.925 | 0.041 | 0.145 | − 4.794 | 0.166 | 0.269 | 0.031 | 0.002 | 0.059 | 0 | Accepted | Accepted |
| METHYL 8,11,14-HEPTADECATRIENOATE | − 5.538 | 0.083 | 0.018 | − 4.804 | 0.061 | 0.87 | 0.014 | 0.002 | 0.074 | 0 | Accepted | Accepted |
| 2,4,4-TRIMETHYL-3-HYDROXYMETHYL-5A-(3-METHYL-BUT-2-ENYL)-CYCLOHEXENE | − 4.998 | 0.007 | 0.937 | − 4.321 | 0.007 | 0.701 | 0.016 | 0.002 | 0.052 | 0 | Accepted | Accepted |
Values in the range 0–0.3: excellent, 0.3–0.7: medium and 0.7–1: poor except for log S (− 4–0.5 log mol/L, proper), Caco-2 (> − 5.15, excellent) and Toxicophore (0-negative, 1-positive)
HIA human intestinal absorption, BBB blood brain barrier penetration, Caco-2 human colon adenocarcinoma cell lines permeability, hERG human ether-a-go-go related gene blockers, H-HT hepatotoxicity, DILI drug induced liver injury
Table 3.
Binding free energy and 2D representation of receptor–ligand interactions between the target enzymes and test compounds used in this study
Conclusion
The present study investigated the α-amylase and α-glucosidase inhibitory potentials of an antioxidative U. dioica leaf extract and a purified fraction F1. The fraction F1 exhibited higher inhibition of both the enzymes than the mother extract. Five compounds (one from MeOH-SIS-and four from fraction F1) among others were identified using GC–MS and demonstrated quality drug likeliness and pharmacokinetics features, and molecular docking of the compounds predicted 2R-acetoxymethyl-1,3,3-trimethyl-4 T-(3-methyl-2-buten-1-yl)-1 T-cyclohexanol as having substantial binding affinity and interaction against α-amylase and α-glucosidase. Further investigations to prove its efficacy and potency need to be done in animal models. The plant extract as well as F1 fraction can have potential application as food additive for the management of oxidative stress induced diabetes.
Acknowledgements
We acknowledge Institute of Bioresource and Sustainable Development and Department of Biotechnology, Ministry of Science and Technology, Govt. of India for the funding for conducting the research work.
Author’s contribution
SS-Conducted the experiments, performed the data analyses and Writing-original draft; SP-performed the data analyses, Writing—original draft, review & editing; RC-performed the data analyses, Writing—original draft; SD-performed the data analyses; SP-Conceptualization, and Writing—review & editing. DS-Conceptualization, Project administration, review & editing. The final manuscript was read and approved by all contributors.
Funding
The research work was supported by Department of Biotechnology, Govt. of India.
Data availability
All datasets generated for this study are included in the article/supplementary material.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed M, Peiwen Q, Gu Z, Liu Y, Sikandar A, Hussain D, et al. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.) Sci Reports. 2020;10(1):1–10. doi: 10.1038/s41598-019-57092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anupama N, Madhumitha G, Rajesh KS. Role of dried fruits of Carissa carandas as anti-inflammatory agents and the analysis of phytochemical constituents by GC–MS. Biomed Res Int. 2014;2014:512369. doi: 10.1155/2014/512369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babatunde DE, Otusemade GO, Efeovbokhan VE, Ojewumi ME, Bolade OP, Owoeye TF. Chemical composition of steam and solvent crude oil extracts from Azadirachta indica leaves. Chem Data Collect. 2019;20:100208. doi: 10.1016/j.cdc.2019.100208. [DOI] [Google Scholar]
- Bhat RS, Al-Daihan S. Phytochemical constituents and antibacterial activity of some green leafy vegetables. Asian Pac J Trop Biomed. 2014;4(3):189–193. doi: 10.1016/S2221-1691(14)60230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black HS, Boehm F, Edge R, Truscott TG. The benefits and risks of certain dietary carotenoids that exhibit both anti-and pro-oxidative mechanisms—a comprehensive review. Antioxidants. 2020;9(3):264. doi: 10.3390/antiox9030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeing JS, Barizão ÉO, e Silva BC, Montanher PF, de Cinque AV, Visentainer JV. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: application of principal component analysis. Chem Cent J. 2014;8(1):1–9. doi: 10.1186/s13065-014-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelsterli UA, Kashimshetty R. Idiosyncratic drug-induced liver injury: mechanisms and susceptibility factors. In: McQueen CA, editor. Comprehensive toxicology. 2. Netherlands: Elsevier; 2010. pp. 383–402. [Google Scholar]
- Brouwer-Brolsma EM, Brandl B, Buso MEC, Skurk T, Manach C. Food intake biomarkers for green leafy vegetables, bulb vegetables, and stem vegetables: a review. Genes Nutr. 2020;15(1):1–12. doi: 10.1186/s12263-020-00667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourasia R, Kumari R, Singh SP, Sahoo D, Rai AK. Characterization of native lactic acid bacteria from traditionally fermented chhurpi of Sikkim Himalayan region for the production of chhurpi cheese with enhanced antioxidant effect. LWT Food Sci Technol. 2022;154:112801. doi: 10.1016/j.lwt.2021.112801. [DOI] [Google Scholar]
- Chrubasik JE, Roufogalis BD, Wagner H, Chrubasik S. A comprehensive review on the stinging nettle effect and efficacy profiles. Part II: urticae radix. Phytomedicine. 2007;14(7–8):568–579. doi: 10.1016/j.phymed.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Falowo AB, Muchenje V, Hugo A, Aiyegoro OA, Fayemi PO. Antioxidant activities of Moringa oleifera L. and Bidens pilosa L. leaf extracts and their effects on oxidative stability of ground raw beef during refrigeration storage. CYTA J Food. 2017;15(2):249–256. doi: 10.1080/19476337.2016.1243587. [DOI] [Google Scholar]
- Hall J, Bravo-Clouzet R. Anti-Inflammatory Herbs for Arthritis. In: Watson RR, Preedy VR, editors. Bioact food as dietary intervention arthritis and related inflammatory diseases. Cambridge: Academic Press; 2013. pp. 619–631. [Google Scholar]
- Ibrahim MA, Bester MJ, Neitz AW, Gaspar ARM. Rational in silico design of novel α-glucosidase inhibitory peptides and in vitro evaluation of promising candidates. Biomed Pharmacother. 2018;107:234–242. doi: 10.1016/j.biopha.2018.07.163. [DOI] [PubMed] [Google Scholar]
- Ibrahim MA, Koorbanally NA, Islam MS. Antioxidative activity and inhibition of key enzymes linked to type-2 diabetes (α-Glucosidase and α-Amylase) by khaya senegalensis. Acta Pharm. 2014;64(3):311–324. doi: 10.2478/acph-2014-0025. [DOI] [PubMed] [Google Scholar]
- Ibrahim RYM, Hammad HBI, Gaafar AA, Saber AA. The possible role of the seaweed Sargassum vulgare as a promising functional food ingredient minimizing aspartame-associated toxicity in rats. Int J Environ Health Res. 2022;32:752–771. doi: 10.1080/09603123.2020.1797642. [DOI] [PubMed] [Google Scholar]
- Islam MT, Ali ES, Uddin SJ, Shaw S, Islam MA, Ahmed MI, et al. Phytol: a review of biomedical activities. Food Chem Toxicol. 2018;121:82–94. doi: 10.1016/j.fct.2018.08.032. [DOI] [PubMed] [Google Scholar]
- Joshi RK. Volatile Constituents of Emilia sonchifolia from India. Nat Prod Commun. 2018;13(10):1355–1356. [Google Scholar]
- Kalai FZ, Dakhlaoui S, Hammami M, Mkadmini K, Ksouri R, Isoda H. Phenolic compounds and biological activities of different organs from aerial part of Nitraria retusa (Forssk.) Asch.: effects of solvents. Int J Food Prop. 2022;25:1524–1538. doi: 10.1080/10942912.2022.2087673. [DOI] [Google Scholar]
- Kalayoglu M, Singer M (2013) U.S. Patent No. US20130178525A1. Washington, DC: U.S. Patent and Trademark Office
- Khalid W, Iqra AF, Rahim MA, Rehman AA, Rasul HF, Arshad MS, Ambreen S, Zubair M, Safdar S, Al-Farga A, Refai M. Industrial applications of kale (Brassica oleracea var. sabellica) as a functional ingredient: a review. Int J Food Prop. 2023;26(1):489–501. doi: 10.1080/10942912.2023.2168011. [DOI] [Google Scholar]
- Khajuria V, Gupta S, Sharma N, Kumar A, Lone NA, Khullar M, Dutt P, Sharma PR, Bhagat A, Ahmed Z. Anti-inflammatory potential of hentriacontane in LPS stimulated RAW 264.7 cells and mice model. Biomed Pharmacother. 2017;92:175–186. doi: 10.1016/j.biopha.2017.05.063. [DOI] [PubMed] [Google Scholar]
- Kianbakht S, Khalighi-Sigaroodi F, Dabaghian FH. Improved glycemic control in patients with advanced type 2 diabetes mellitus taking Urtica dioica leaf extract: a randomized double-blind placebo-controlled clinical trial. Clin Lab. 2013;59(9–10):1071–1076. doi: 10.7754/clin.lab.2012.121019. [DOI] [PubMed] [Google Scholar]
- Lipinski CA. ADMET screen. encycl cancer 74–77. In: Schwab M, editor. Encyclopedia of cancer. Heidelberg: Springer; 2008. pp. 74–77. [Google Scholar]
- Liu Y, Grimm M, Dai W-t, Hou M-c, Xiao ZX, Cao Y. CB-Dock: a web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol Sin. 2020;41(1):138–144. doi: 10.1038/s41401-019-0228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malheiro R, Casal S, Cunha SC, Baptista P, Pereira JA. Identification of leaf volatiles from olive (Olea europaea) and their possible role in the ovipositional preferences of olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae) Phytochemistry. 2016;121:11–19. doi: 10.1016/j.phytochem.2015.10.005. [DOI] [PubMed] [Google Scholar]
- Manjari MS, Karthi S, Ramkumar G, Muthusamy R, Natarajan D, Shivakumar MS. Chemical composition and larvicidal activity of plant extracts from Clausena dentata (Willd) (Rutaceae) against dengue, malaria, and filariasis vectors. Parasitol Res. 2014;113(7):2475–2481. doi: 10.1007/s00436-014-3896-7. [DOI] [PubMed] [Google Scholar]
- Meunier L, Larrey D. Drug-induced liver injury: biomarkers, requirements, candidates, and validation. Front Pharmacol. 2019;10:1482. doi: 10.3389/fphar.2019.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naine SJ, Devi CS, Mohanasrinivasan V, Doss CGP, Kumar DT. Binding and molecular dynamic studies of sesquiterpenes (2R-acetoxymethyl-1,3,3-trimethyl-4t-(3-methyl-2-buten-1-yl)-1t-cyclohexanol) derived from marine Streptomyces sp. VITJS8 as potential anticancer agent. Appl Microbiol Biotechnol. 2016;100(6):2869–2882. doi: 10.1007/s00253-015-7156-2. [DOI] [PubMed] [Google Scholar]
- Padhi S, Masi M, Chourasia R, Rajashekar Y, Rai AK, Evidente A. ADMET profile and virtual screening of plant and microbial natural metabolites as SARS-CoV-2 S1 glycoprotein receptor binding domain and main protease inhibitors. Eur J Pharmacol. 2021;890:173648. doi: 10.1016/j.ejphar.2020.173648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieczykolan A, Pietrzak W, Gawlik-Dziki U, Nowak R. Antioxidant, anti-inflammatory, and anti-diabetic activity of phenolic acids fractions obtained from Aerva lanata (L.) Juss. Mol. 2021;26(12):3486. doi: 10.3390/molecules26123486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovitha S, Parani M. In vitro and in vivo α-amylase and α-glucosidase inhibiting activities of the protein extracts from two varieties of bitter gourd (Momordica charantia L.) BMC Complement Altern Med. 2016;16(1):1–8. doi: 10.1186/s12906-016-1085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratama OA, Tunjung WAS, Sutikno DBS. Bioactive compound profile of melon leaf extract (Cucumis melo L. ‘Hikapel’) infected by downy mildew. Biodiversitas J Biol Divers. 2019;20(11):3448–3453. doi: 10.13057/biodiv/d201143. [DOI] [Google Scholar]
- Rahman A, Hoque MM, Khan MA, Sarwar MG, Halim MA. Non-covalentinteractions involving halogenated derivatives of capecitabine and thymidylatesynthase: a computational approach. Springerplus. 2016;5:146. doi: 10.1186/s40064-016-1844-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralte L, Khiangte L, Thangjam NM, Kumar A, Singh YT. GC–MS and molecular docking analyses of phytochemicals from the underutilized plant, Parkia timoriana revealed candidate anti-cancerous and anti-inflammatory agents. Sci Reports. 2022;12(1):1–21. doi: 10.1038/s41598-022-07320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya R. GC–MS Analysis of bioactive compounds in ethanolic Leaf extract of Hellenia speciosa (J.Koenig) S.R Dutta. Appl Biochem Biotechnol. 2022;194:176–186. doi: 10.1007/s12010-021-03742-2. [DOI] [PubMed] [Google Scholar]
- Sekhon-Loodu S, Rupasinghe HPV. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Front Nutr. 2019;6:53. doi: 10.3389/fnut.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Padhi S, Kumari M, Patnaik S, Sahoo D. Antioxidant potential of selected wild edible leafy vegetables of Sikkim Himalayan region: effects of cooking methods and gastrointestinal digestion on activity. Front Nutr. 2022;9:598. doi: 10.3389/fnut.2022.861347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siswadi S, Saragih GS. Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R.Br. AIP Conf Proc. 2021;2353(1):030098. doi: 10.1063/5.0053057. [DOI] [Google Scholar]
- Sunarić S, Lalić J, Spasić A. Simultaneous determination of alpha-tocopherol and alpha-tocopheryl acetate in dairy products, plant milks and health supplements by using spe and hplc method. Food Anal Methods. 2017;10(12):3886–3901. doi: 10.1007/s12161-017-0943-x. [DOI] [Google Scholar]
- Talukdar R, Padhi S, Rai AK, Masi M, Evidente A, Jha DK, et al. Isolation and characterization of an endophytic fungus Colletotrichum coccodes producing tyrosol from Houttuynia cordata Thunb. Using ITS2 RNA secondary structure and molecular docking study. Front Bioeng Biotechnol. 2021;9:484. doi: 10.3389/fbioe.2021.650247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmegowda SC, Naidoo YNJR, Naidoo G. Chemical composition of the essential oil from the leaves of Endostemon obtusifolius (E.Mey. ex Benth.) N.E.Br. BiochemPharmacol. 2013;2:4. [Google Scholar]
- Van Breemen RB, Li Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin Drug Metab Toxicol. 2005;1(2):175–185. doi: 10.1517/17425255.1.2.175. [DOI] [PubMed] [Google Scholar]
- Witchel HJ, Harry Witchel CJ. Drug-induced hERG Block and Long QT Syndrome. Cardiovasc Ther. 2011;29(4):251–259. doi: 10.1111/j.1755-5922.2010.00154.x. [DOI] [PubMed] [Google Scholar]
- Xiong G, Wu Z, Yi J, Fu L, Yang Z, Hsieh C, et al. ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021;4(W1):W5–W14. doi: 10.1093/nar/gkab255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JW, Feng YX, Du YS, Lu XX, Zheng Y, Wang D, et al. Composition and insecticidal activity of Elsholtzia kachinensis Prain, a traditional vegetable and herbal medicine. J Oleo Sci. 2022;71(7):1075–1084. doi: 10.5650/jos.ess21245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Not applicable.



