Abstract
Background and Objective
Human plasma is used for the generation of several life-saving drugs and contains valuable antibodies from the immunoglobulin classes IgG, IgM and IgA. Purified intravenous IgG solutions (IVIGs) form the majority of plasma-derived medicine to treat patients with various forms of immunodeficiencies. In conventional IVIG manufacturing processes, immunoglobulin classes IgM and IgA are often discarded as contaminants, but these antibody classes have been proven to be effective for the treatment of acute bacterial infections. Considering the increase in demand for human plasma-derived products and the ethical value of the raw material, a more resource-saving usage of human plasma is needed. Intensive research over the last decades showed that adverse reactions to IVIGs depend on the presence of thrombogenic factors, partially unfolded proteins, non-specific activation of the complement system, and blood group specific antibodies. Therefore, new IVIG preparations with reduced risks of adverse reactions are desirable.
Method
A new manufacturing process that yields two biologics was established and quality attributes of the new IVIG solution (Yimmugo®) obtained from this process are presented.
Results
Here, we provide a biochemical characterization of Yimmugo®, a new 10% IVIG preparation. It is derived from human blood plasma by a combined manufacturing process, where IgM and IgA are retained for the production of a new biologic (trimodulin, currently under investigation in phase III clinical trials). Several improvements have been implemented in the manufacturing of Yimmugo® to reduce the risk of adverse reactions. Gentle and efficient mixing by vibration (called “vibromixing”) during a process step where proteins are at risk to aggregate was implemented to potentially minimize protein damage. In addition, a dedicated process step for the removal of the complement system activator properdin was implemented, which resulted in very low anticomplementary activity levels. The absence of measurable thrombogenic activity in combination with a very high degree of functional monomeric antibodies predict excellent efficacy and tolerability.
Conclusion
Yimmugo® constitutes a new high quality IVIG preparation derived from a novel manufacturing process that takes advantage of the full therapeutic immunoglobulin potential of human plasma.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40268-023-00430-w.
Key points
| Development of a novel manufacturing process for intravenous immunoglobulin solutions (IVIGs) from human plasma, where valuable antibodies from the IgA and IgM class are saved for a second drug. |
| Development of a gentle IVIG manufacturing process using mixing-by-vibration technology at a critical step, resulting in a drug with very low levels of aggregates. |
| Identification of the complement system protein properdin causing elevated anticomplementary activity and its removal from the IVIG solution by a dedicated ion exchange chromatography step. |
Introduction
IVIGs are derived from pooled human plasma from thousands of healthy donors and contain highly purified polyspecific IgG antibodies for the treatment of immunological disorders and autoimmune diseases [1]. These include replacement therapies for patients with primary immunodeficiencies (PID) and some forms of secondary immunodeficiencies (SID), as well as immunomodulatory therapies for the treatment of autoimmune diseases such as primary immune thrombocytopenia (ITP), Guillain–Barré syndrome, chronic inflammatory demyelinating polyneuropathy, and Kawasaki disease (comprehensively reviewed in [2]).
Human plasma predominantly contains IgG antibodies (⁓ 75% of immunoglobulins) and the antibody classes IgA (⁓ 15%) and IgM (⁓ 10%) [3]. Preparations containing IgA and IgM antibodies have been shown to be effective for the treatment and prevention of infection-induced sepsis in several patient subpopulations [4–6]. However, during manufacturing of standard IVIGs, IgA and IgM antibodies are often discarded as contaminants and are therefore lost for further usage. The worldwide annual production of plasma-derived antibody-containing drugs dramatically increased over the last decade. Since 2010, a 12% annual increase of production from ~ 107 metric tons in 2010 to ~ 211 metric tons in 2018 was recorded [7]. Demand is expected to increase even further. The main drivers for this expected rise are an increasing number of SIDs [7] and better availability for diagnostic tools for the detection of PIDs [8], which are currently underdiagnosed [9]. Thus, manufacturers are urged to improve their yield and to utilize the limited starting material in a resource-saving manner.
Since their first applications in the 1950s, the safety and efficacy of immunoglobulins for intravenous injections have improved dramatically [10]. These included aspects of purity, stability, safety, and tolerability. Nevertheless, IVIGs still bear potential risks to cause side effects, but much progress has been made in correlating these side effects to measurable quality attributes of the drug itself [11]. Some side effects have been associated with unwanted activation of the complement system [12–14]. This is thought to be, at least in part, caused by shear stress during large scale manufacturing, which results in structurally damaged antibodies that bind to components of the complement system in the absence of antigens, termed anticomplementary activity [15–17]. Another potential side effect associated with IVIGs are thromboembolic events [11]. These are linked to copurified protein contaminants that enhance clotting. For example, a correlation of residual amounts of factor XIa protein contaminations in IVIGs and thromboembolic events could be established [18, 19]. Hemolytic events based on remaining blood type specific antibodies are another concern for plasma derived drugs [20, 21]. This illustrates the need for improved manufacturing processes to obtain IVIGs with high potency and reduced risks for patients.
Here, we present a novel manufacturing approach that yields two drugs from the same starting material: Yimmugo® and trimodulin (human IgM, IgA, and IgG solution). Yimmugo® constitutes a new 10% IgG preparation for intravenous use, for which a biochemical and functional characterization is presented. A side fraction of the manufacturing process for Yimmugo® yields trimodulin, which contains the IgM and IgA antibodies, as well as a subset of IgG antibodies, and is currently investigated in clinical trials for the treatment of pulmonary infections [coronavirus disease 2019 (COVID-19): NCT05531149 and severe community-acquired pneumonia: NCT05722938]. Yimmugo® has been tested in phase III clinical trials, where excellent tolerability and excellent efficacy in protecting PID patients against infections was demonstrated [22, 23]; furthermore, good efficacy was demonstrated in modulating the peripheral blood platelet count in ITP patients [24]. Its manufacturing includes several innovative improvements. Use of industrial scale mixing-by-vibration (also called vibromixing) reduces shear stress at fast mixing rates, implementation of a dedicated step for the removal of the complement system activator properdin, utilization of two process steps to remove thrombogenic factors, and four highly effective and validated virus reduction steps lead to the production of a safe and effective state-of-the-art IVIG with excellent stability.
Material and Methods
Manufacturing Process
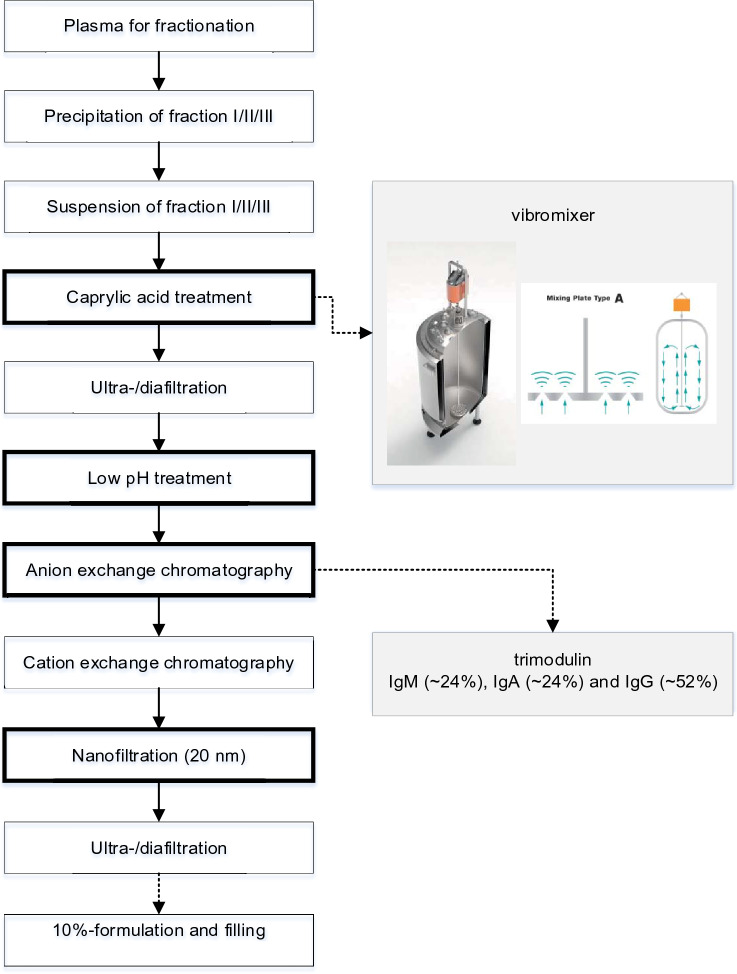
The manufacturing process for Yimmugo® is outlined in Fig. 1 and the functions of each step are summarized in online resource Table S1. The basic fractionation (step 1–4) results in the fraction I/II/III, which is the starting material for the production process described below. Up to step 10, the same manufacturing process is used for both Yimmugo® and trimodulin. Fraction I/II/III is suspended with sodium acetate buffer in order to solubilize proteins/immunoglobulins (step 5). The suspension is treated with caprylic acid. For this step, a vibromixer is in place to ensure efficient solution mixing and to reduce shear stress. The resulting precipitate is removed by depth filtration (step 6). The filtrate is concentrated and diafiltrated (step 7). The protein solution is subjected to a treatment at pH 4 and incubated at 37 °C (step 8). The intermediate is further purified by an anion exchange chromatography step (step 10), resulting in an unbound IgG-containing fraction flowing through the column, which can be further processed to Yimmugo®. The column bound proteins are eluted in a separate step and are further processed to obtain trimodulin. The unbound IgG containing fraction is subjected to a cation exchange chromatography step (step 11) and afterwards filtered through a nanometer filter (20 nm, step 12). After buffer exchange by diafiltration, the immunoglobulin solution is finally adjusted in terms of pH and final protein concentration and formulated by adding low amounts of polysorbate 80. Prior to storage, the drug substance is filtered (step 14) and packaged into the final shipping vials.
Fig. 1.
Overview of the manufacturing process. Boxes in bold are related to virus safety. Validation of virus inactivation will be published elsewhere in a separate study. The scheme of the Fundamix® vibromixer was kindly provided by Klaus Brekow from DrM, Dr. Mueller AG, Krefeld Germany
Analytical Methods
A total of 19 batches were used for the general biochemical characterization. For the extended biological characterization (antibody titers and in vitro functionality tests), at least three batches were used. All measurements that constitute mandatory release specifications by the authorities were performed according to procedures specified in the European Pharmacopoeia (Ph. Eur.) or official regulations, such as the code of federal regulations (CFR). All analytical methods are described in more detail in the supplementary methods section included in the online resources.
Results
We conducted a thorough analysis of Yimmugo’s® general properties for 19 manufactured batches that show all characteristics of a state-of-the-art sugar-free and glycine-stabilized 10% IVIG (Table 1). The observed variability of general properties between batches was low (CV% ≤ 2%), indicative of a stable and reproducible manufacturing process. Furthermore, the analysis revealed an IgG subclass distribution resembling typical values found in human plasma (Table 2) [25] and an overall high purity as judged by a its high gamma globulin content (Table 1).
Table 1.
Product characteristics
| Parameter | Unit | Mean (median) | SD (CV%) | N |
|---|---|---|---|---|
| Total protein | mg/ml | 100.8 (101.0) | 2.0 (1.9) | 19 |
| Gamma globulin | % of total protein | 100 (100) | 0.0 (0.0) | 19 |
| pH | 4.7 (4.7) | 0.1 (1.0) | 19 | |
| Osmolality | mOsmol/kg | 344.6 (345.0) | 4.2 (1.3) | 19 |
| Glycine | mM | 296.7 (296.0) | 5.1 (1.8) | 19 |
| Polysorbate 80 | µg/ml | 3.9 (4.0) | 1.1 (1.8) | 19 |
Table 2.
Immunoglobulin subclass distribution
| Parameter | Unit | Mean (median) | SD | N |
|---|---|---|---|---|
| IgG1 | % | 63.9 (64.3) | 1.8 | 19 |
| IgG2 | % | 30.4 (30.0) | 2.1 | 19 |
| IgG3 | % | 4.2 (4.3) | 0.5 | 19 |
| IgG4 | % | 1.4 (1.4) | 0.2 | 19 |
Purity
We optimized the manufacturing process to minimize protein contaminations associated with known side effects, which we grouped here into the categories IgA/IgM content, complement system activators, thromboembolic events, and hemolytic events (Table 3).
Table 3.
Accompanying proteins, properties, and activities
| Protein | Unit | Mean (median) | SD | N | Assay type |
|---|---|---|---|---|---|
| Albumin | % | < 0.35 | N/A | 8 | Nephelometry |
| Proteolytic activity | U/ml | < 3 | N/A | 8 | Chromogenic assay |
| Immunoglobulin class purity | |||||
| IgA | %# | 0.1 (0.1) | 0.1 | 19 | Nephelometry |
| IgM | %# | 0.1 (0.1) | 0.1 | 19 | Nephelometry |
| IgE | IU/ml | < 0.1 | N/A | 3 | ELISA |
| Complement system proteins | |||||
| Properdin | ng/mg protein | 0.8 (0.8) | 0.14 | 3 | ELISA |
| C3 | mg/ml | < 0.05 | N/A | 6 | Nephelometry |
| C3a | ng/ml | < 0.012 | N/A | 1 | ELISA |
| C5a | ng/ml | < 0.01 | N/A | 1 | ELISA |
| ACA | CH50/mg protein | 0.3 (0.3) | 0.2 | 19 | Complement-mediated hemolysis |
| Antibodies related to hemolysis (median only) | |||||
| Anti-A | 1× dilution | 16* | N/A | 19 | Direct hemagglutination |
| Anti-B | 1× dilution | 8* | N/A | 19 | Direct hemagglutination |
| Anti-D | 1× dilution | < 2* | N/A | 19 | Direct haemagglutination |
| Coagulation factors (related to thromboembolic events) | |||||
| Fibrinogen antigen | mg/ml | < 0.002 | N/A | 8 | Nephelometry |
| FII | % of norm | < 10 | N/A | 8 | Clotting assay |
| FVII | % of norm | < 5 | N/A | 8 | Clotting assay |
| FVIIa | mIU/ml | < 5 | N/A | 8 | Clotting assay |
| FIX | % of norm | < 5 | N/A | 8 | Clotting assay |
| FX | % of norm | < 10 | N/A | 8 | Clotting assay |
| FXI | % of norm | < 1 | N/A | 8 | Clotting assay |
| FXIa | mIU/ml | < 2 | N/A | 8 | Chromogenic assay |
| FXII | % of norm | < 1 | N.A. | 8 | Clotting assay |
| FXIII | % of norm | < 15 | N/A | 8 | Chromogenic assay |
| Prekallikrein | % of norm | < 15 | N/A | 8 | Chromogenic assay |
| Kallikrein | U/ml | < 0.2 | N/A | 8 | Chromogenic assay |
| Plasminogen | % of norm | < 5 | N/A | 8 | Chromogenic assay |
| Plasmin | IU/ml | < 0.2 | N/A | 8 | Chromogenic assay |
| NaPTT | Sec |
256.2 (252.0) |
18.9 | 19 | Clotting assay |
| NaPTT | % of ref. buffer |
101.2 (101) |
5.3 | 19 | Clotting assay |
| TGA (peak thrombin) | mIU FXIa/mL | < 1.5 | N/A | 19 | Fluorogenic assay |
| Prekallikrein activator | IU/ml | < 5 | N/A | 19 | Chromogenic assay |
#The % refers to the sum of IgA, IgM, and IgG measured by nephelometry. *Dilutions refer to prediluted solutions of 25 mg/ml (see Ph.Eur. 2.6.20 and Ph.Eur. 2.6.26). Final concentrations in the assay are: 12.5 mg/ml (1:2 dilution), 6.25 mg/ml (1:4 dilution), 3.125 mg/ml (1:8 dilution), 1.563 mg/ml (1:16), 0.781 mg/ml (1:32 dilution), and 0.391 mg/ml (1:64 dilution). For anti-D antibodies, no hemagglutination was observed at 12.5 mg/ml (1:2 dilution) and higher concentrations were not tested. The release testing is confirmatory as the manufacturing process starts from plasma donations that were screened for the absence of anti-D antibodies
IgA/IgM antibodies are removed during IVIG manufacturing to obtain a highly purified IgG solution; but they are valuable ingredients of trimodulin. IgA and IgM are separated from Yimmugo® at the anion exchange chromatography step (Fig. 1). We optimized loading conditions for this process step and found conditions where essentially all IgA/IgM antibodies are retained for the production of trimodulin (Fig. 2), leading to IgA/IgM levels in Yimmugo® of only 0.1% (Table 3). This value of 0.1% IgA is within the range of 0.01–0.22% IgA found for comparator commercial IVIGs (online resource Fig. S1) that are produced in a way where IgA/IgM antibodies are discarded during the manufacturing process. We calculated the step yield for this process step and found that > 90% of antibodies were recovered. The flow through, from which Yimmugo® is manufactured, contained ⁓ 72% of protein. The elution fraction, which is used to manufacture trimodulin, contained ⁓ 21% of the protein content (Fig. 2), which would be otherwise lost in a standard IVIG manufacturing process. The eluted IgG antibodies in the trimodulin branch contain similar levels of IgG2 and IgG3 subclasses and are enriched in IgG4 (⁓ 14% as compared with 1.4% in Yimmugo®), whereas IgG1 accounts for roughly 50% of IgG antibodies. This subclass distribution is expected because IgG4 antibodies tend to have lower isoelectric points [26] and therefore bind with higher affinity to the chromatography resins.
Fig. 2.
Immunoglobulin class separation during anion exchange chromatography within the Yimmugo® manufacturing process. a Scheme illustrating the split point of the manufacturing process. The solution containing IgA, IgM, and IgG antibodies is loaded onto a column. The flow through contains IgG antibodies while IgA and IgM and some IgG antibodies are retained and eluted in a separate step to manufacture trimodulin. Values in percent represent the yields based on the measured protein concentration from six batches. b–d Relative concentration of IgG (b), IgA (c), and IgM (d) in the input solution (load), the collected flow through (Yimmugo® branch), and the collected elution fraction (trimodulin branch). Presented values come from at least eight manufacturing batches. Displayed box plots encompass the 25th–75th percentiles, the middle line indicates the median, the small box indicates the mean, the whiskers extend to the top 5%/top 95% of the distribution of numbers and diagonal crosses represent the minimum and maximum of the sample set
Next, we assessed potential impacts on the complement system, as its activation may lead to side effects [12–14]. We therefore tested for potentially copurified components of the complement system and performed nephelometry and ELISA experiments to detect C3, C3a, and C5a [27]. We found no measurable contaminations by these complement system components (Table 3). In addition to protein contaminations, subvisible particles in IVIGs have been shown to non-specifically activate the complement system in a concentration-dependent manner [28]. Herein, subvisible particles in the range of ≥ 2 µm were shown to have the strongest impact. In addition to the compendial measurements of subvisible particles ≥ 10 µm and ≥ 25 µm, we analyzed the subvisible particle content ≥ 2 µm in our preparation and found that the concentration was in a range where no activation of the complement system was observable (online resource Table S2), as our values were below their negative control used in Chisholm et al. [28].
Thromboembolic events caused by IVIGs are at least in part attributed to remaining coagulation factors [11, 18]. We therefore tested our preparation for the presence of remaining coagulation activity using functional in vitro assays. All assays for specific coagulation factors resulted in signals below the respective detection limit (Table 3 and online resource Fig. S1). Non-activated partial thromboplastin time (NaPTT) is a method to assess activated coagulation factors in comparison to a reference buffer and is required according to the European Pharmacopoeia (Ph. Eur.; 2.6.22), as clotting times below 200 s or less than 80% of the reference buffer without coagulation factors are correlated with adverse reactions [29]. Using this assay, we observed clotting times > 250 s, which were similar to the reference buffer (Table 3). These observations are further confirmed using the TGA method, which resulted in FXIa levels below the detection limit of 1.5 mIU/ml. These functional assays demonstrate the absence of procoagulant activity in Yimmugo®. This is important because in the past, FXIa and prekallikrein/kallikrein were the main biochemical causes for thromboembolic adverse reactions caused by IVIGs [30, 31]. During manufacturing, thrombogenic factors are removed during caprylic acid and low pH treatment (online resource Table S1). We confirmed the absence of representative thrombogenic factors in a process intermediate directly after low pH treatment, demonstrating the efficiency of these process steps in reducing risks related to thromboembolic events (online resource Table S3).
Additionally we analyzed the presence of anti-D and anti-A/B antibodies, which can lead to hemolytic events in patients depending on their blood group [20, 21, 32, 33]. Anti-D antibodies are screened for at the level of donation and are therefore essentially absent in IVIGs, which we confirmed here for Yimmugo® (Table 3). Anti-A/B titers had median values of 1:16 for anti-A and 1:8 for anti-B and were comparable to other comparator IVIGs (Table 3 and online resource Fig. S2), that ranged from 1:8 to 1:32 for anti-A and 1:4 to 1:16 for anti-B in direct agglutination assays.
In summary, high purity is shown for Yimmugo®. We performed a profile of accompanying proteins and activities and demonstrated low levels for all factors that are known/suspected to be related to adverse reactions.
Molecular size distribution
Intact IgG monomers formed by two heavy chains and two light chains connected by disulfide bridges are the predominant state of antibodies in IVIGs. However, IVIGs contain various degrees of other molecular species [34]. These are aggregates, IgG fragments, and IgG dimers. Aggregates (synonym: polymers) and fragments are not functional and can exhibit immunogenicity and are therefore unwanted states of IgG antibodies in IVIGs [35]. Aggregate formation depends on physical parameters during purification steps (shear stress, temperature) and buffer conditions [15, 34]. We therefore used gentle vibromixing [36, 37] during caprylic acid treatment to reduce shear stress during this process step, where proteins are susceptible to aggregation [38]. Indeed, levels of aggregates were very low and fragments were undetectable (Table 4 and online resource Fig. S3). This is likely attributed to the use of vibromixing; although, no systematic comparison between conventional mixing techniques and vibromixing was conducted for this study. These data underline the high degree of structural integrity of IgG molecules in Yimmugo®. In addition, IgG molecules can form dimers. The degree of dimer formation mainly depends on the complexity of the donor pool, protein concentration of the IVIG solution, and on buffer conditions [39, 40]. On a functional level, the significance of dimers in IVIGs is unclear [34], but some evidence suggests that lower dimer levels are favorable [40]. We measured the dimer content in Yimmugo® and four comparator IVIGs. Yimmugo® had a dimer content of 7.1% (± 1.2%), which was comparable to other IVIGs that had an average dimer content of 8.8% (± 2.0%) and a median of 9.3% (Table 4 and online resource Fig. S3). Taken together, Yimmugo® contains very low levels of unwanted oligomeric species, indicative of a high degree of intact and functional antibodies.
Table 4.
Molecular size distribution
| Species | Unit | Mean (median) | SD | N |
|---|---|---|---|---|
| Monomers | Area (%) | 92.9 (93.0) | 1.2 | 19 |
| Dimers | Area (%) | 7.1 (7.0) | 1.2 | 19 |
| Aggregates | Area (%) | 0.03 (0.00) | 0.05 | 19 |
| Fragments | Area (%) | 0.0 (0.0) | 0.0 | 19 |
Properdin removal lowers anticomplementary activity
After the initial process steps of resolubilization, caprylic acid treatment, and anion exchange chromatography, elevated levels of anticomplimentary activity (ACA) in the range of the specification limit of 1 CH50/mg protein [41] are still present in the IgG intermediate. Surprisingly, high ACA levels were caused by properdin, an activator of the antibody independent alternative pathway of the complement system [42]. To deplete properdin from the protein solution, we implemented an additional cation exchange chromatography step in our manufacturing process that was able to efficiently remove properdin (Fig. 3a). To demonstrate functional significance of properdin removal, we performed ACA assays with either purified properdin or process intermediates before and after the cation exchange chromatography step and found that properdin increased anticomplementary activity (Fig. 3b, c). The successful implementation of this process step yielded in an IVIG with very low properdin levels (0.8 ± 0.14 ng/mg protein) and ACA levels (0.3 ± 0.2 CH50/mg protein) (Table 3 and online resource Fig. S1). This predicts excellent tolerability of Yimmugo® in patients as unwanted activation of the complement system is minimized.
Fig. 3.

Properdin binds to complement system components and is depleted by cation exchange chromatography. a Properdin contents were quantified by ELISA before (left) and after (right) samples passed through the cation exchange chromatography column. N = 3. b Yimmugo® drug substance solutions were spiked with the indicated amounts of purified properdin protein and subjected to ACA measurements. A linear fit was applied to the data points. c ACA measurement before (left) and after (right) samples passed through the cation exchange chromatography column using a laboratory downscale model. N = 35. Displayed box plots encompass the 25th–75th percentiles, the middle line indicates the median, the small box indicates the mean, the whiskers extend to the top 5%/top 95% of the distribution of numbers, and diagonal crosses represent the minimum and maximum of the sample set
Potency and functionality
The mode of action of IVIGs in preventing infections is to supply the recipient with pathogen specific antibodies [1]. Titers in Yimmugo® against several high prevalence pathogens have been measured and were found to be present at protective levels for all tested pathogens [43]. We extended this characterization and additionally measured titers against hepatitis, polio, measles, streptolysin O toxin, diphtheria toxin, and Pseudomonas aeruginosa. Again, all titers were present at protective levels (Table 5). In addition to that, we measured the integrity and functionality of antibodies by several in vitro assays (Table 5). Fc part activity of nearly 100% demonstrated a high degree of intact antibodies that were functionally active in phagocytosis assays and antibody dependent cell-mediated cytotoxicity (ADCC) assays (Table 5). These data demonstrate the high degree of structural integrity and functionality and are in line with the excellent efficacy of Yimmugo® in phase III clinical trials [22, 23].
Table 5.
Biological characterization
| Titer assays | ||||
|---|---|---|---|---|
| AB specificity | Unit | Mean (median) | SD | N |
| Hepatitis A virus | U/ml | 1494 (1079) | 861.1 | 3 |
| Hepatitis B virus surface antigen | IU/g protein | 95.9 (80.4) | 53.5 | 19 |
| Anti-polio antibodies type 1 | Relative to standard lot NIH176 | 1.30 (1.03) | 0.7 | 13 |
| Measles virus | Relative to standard lot NIH176 | 1.40 (1.33) | 0.55 | 13 |
| Poliomyelitis antigen | Relative to standard lot NIH176 | 1.66 (1.66) | 1.2 | 2 |
| Streptolysin O toxin | IU/ml | 1611 (1610) | 141.4 | 19 |
| Diphtheria toxin | U/ml | 10.2 (10.9) | 2.1 | 13 |
| Pseudomonas aeruginosa | 1:titre | 40 (40) | N/A | 3 |
| Functional in vitro assays | ||||
|---|---|---|---|---|
| Parameter | Unit | Mean (median) | SD | N |
| Fc part activity | % IgG BRP | 98.0 (95.0) | 7.9 | 19 |
| Phagocytosis (granulocytes) | % | 26 (25) | 1.7 | 3 |
| Phagocytosis (monocytes) | % | 23 (25) | 5.3 | 3 |
| Antibody dependent cell-mediated cytotoxicity (ADCC) | % | 75 (67) | 18.4 | 3 |
Stability and Formulation
Yimmugo® is formulated in a glycine solution at pH 4.8 with low levels of polysorbate 80 as an excipient. These conditions were chosen on the basis of a parameter screen (pH, concentrations of glycin and Polysorbate 80) to find conditions with maximized monomer content (online resource Fig. S4). To demonstrate stability under these conditions, product integrity was monitored over a period of 36 months at 5 °C. As critical surrogates for antibody integrity, we measured Fc part activity (Fig. 4a), dimer content (Fig. 4b), and antibody titers against two representative pathogens (Fig. 4c, d), as well as the aggregate content (Fig. 4e). All measured parameters remained within predefined internal and external (Pharmacopeia) specifications. These data indicate that the functionality is preserved over the entire shelf life period as Fc part activity and pathogen-specific titers remain high. The aggregate formation is very low. Minor increases in aggregate levels is common in IVIGs and has also been observed in other marketed IVIGs (e.g., [44]). Taken together, these data demonstrate excellent stability of Yimmugo®.
Fig. 4.
Stability of Yimmugo®. Drug products were stored at 5 °C and the representative quality attributes Fc part activity (a), dimer content (b), titers against Diphtheria toxin (c) and Streptolysin O toxin (d), as well as aggregate levels (e) were analyzed over the course of 36 months. Measurements were performed in triplicate or more, and error bars represent the sample standard deviation
Discussion
Yimmugo® is a high quality IgG preparation, combining several features of a well-tolerated IVIG. It is obtained from a modern state-of-the-art production plant with an innovative manufacturing process in which human plasma is used to produce the IgG product Yimmugo®, as well as the IgM/IgA/IgG immunoglobulin product trimodulin. Thereby, all available immunoglobulins IgG, IgM, and IgA are purified within one integrated process. This, in our opinion, is a more sustainable approach to produce products with maximum yield from the valuable raw material human plasma, which is a limited resource for which demand is expected to increase even further [7]. Importantly, the use of a combined manufacturing route does not compromise on the quality of Yimmugo®. In our study, we conducted a head-to-head comparison between Yimmugo® and four other currently marketed IVIGs in terms of relevant quality attributes and found that Yimmugo® performed very well across all tested categories. In compendial assays, Yimmugo® exhibited very low ACA, aggregate content, impurities, and no measurable thrombogenic activity. Properdin, which increased ACA activity, was essentially absent in Yimmugo®, because it is retained on the newly implemented cation exchange chromatography column. This resulted in a ⁓ 50% reduction of ACA levels, which shows that properdin contributes to ACA in IVIGs, in addition to other known factors (studied in [15]). Yimmugo® is predominantly composed of monomers, which are the desired antibody states in IVIGs [40]. Most tested impurities were below the respective detection limit. For IgA and IgM, residual amounts of 0.1% were detected. This does not constitute a risk for most patients that naturally have much higher amounts of IgA antibodies [3, 45]. However, patients with certain predispositions, such as common variable immunodeficiency or selective IgA deficiency may—in rare cases—respond with adverse reactions to remaining IgA antibodies. Since these events are very rare, not much data is available and it is unclear whether any dose-dependence exists with regard to residual IgA antibodies [46]. Special guidelines and tests for treating these risk groups exist to minimize their risk of adverse reactions when IVIG treatments are prescribed [47].
Its glycine formulation leads to excellent stability and preserves the monomeric state of antibodies in Yimmugo®. Using titer measurements against common pathogens and functional cell-based in vitro assays, we demonstrated full functionality for the antibodies in Yimmugo®, from epitope binding to phagocytosis. These data in combination with excellent efficacy data from phase III clinical trials [22–24] suggest that Yimmugo® provides functional antibodies at protective levels to patients. Thus, Yimmugo® is a fully functional and well-tolerated new IVIG, that is obtained from a resource-saving manufacturing process.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the technical stuff at the departments of Development, Quality Control, Bioanalysis, and Investigational and Applied Bioscience at Biotest AG for conducting measurements. We thank M. Germer, S. Henrichsen, E. D’Aiuto, and W. Möller for helpful comments on the manuscript.
Funding
This study was funded by Biotest A.G.
Conflict of Interest
O.M. and A.H. are authors of the patent US11325964B2/EP3491018A1 (“Process for preparing immunoglobulin compositions;” assignee: Biotest A.G.). All authors are employees of Biotest AG.
Ethics approval
Not applicable, no clinical trial data are included.
Consent to participate
Not applicable, no clinical trial data are included.
Consent for publication
Not applicable.
Data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Author contributions
C.D., A.H., and O.M. conceived research. C.D., A.H., S.K., and O.M. analyzed data and revised the manuscript. C.D. wrote the manuscript.
Code availability
Not applicable.
Footnotes
Yimmugo® is a registered trademark in the European Union and certain other countries.
Contributor Information
Christian Duellberg, Email: christian.duellberg@biotest.com.
Oliver Maneg, Email: oliver.maneg@biotest.com.
References
- 1.Galeotti C, Kaveri S, Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int Immunol. 2017;29:491–498. doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- 2.Vitiello G, Emmi G, Silvestri E, di Scala G, Palterer B, Parronchi P. Intravenous immunoglobulin therapy: a snapshot for the internist. Intern Emerg Med. Springer-Verlag Italia s.r.l.; 2019. p. 1041–9. [DOI] [PubMed]
- 3.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, et al. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nierhaus A, Berlot G, Kindgen-Milles D, Müller E, Girardis M. Best-practice IgM- and IgA-enriched immunoglobulin use in patients with sepsis. Ann Intensive Care. 2020;10:132. doi: 10.1186/s13613-020-00740-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui J, Wei X, Lv H, Li Y, Li P, Chen Z, et al. The clinical efficacy of intravenous IgM-enriched immunoglobulin (pentaglobin) in sepsis or septic shock: a meta-analysis with trial sequential analysis. Ann Intensive Care. 2019;9:2. doi: 10.1186/s13613-019-0501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giorgia C, Lucio T, Alessandra M, Davide Z, Egidio B, Natalia M. Pentaglobin® efficacy in reducing the incidence of sepsis and transplant-related mortality in pediatric patients undergoing hematopoietic stem cell transplantation: a retrospective study. J Clin Med. 2020;9:1592. doi: 10.3390/jcm9051592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prevot J, Jolles S. Global immunoglobulin supply: steaming towards the iceberg? Curr Opin Allergy Clin Immunol. 2020;20(6):557–564. doi: 10.1097/ACI.0000000000000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Organization WH. Second WHO model list of essential in vitro diagnostics. World Health Organ Tech Rep Ser. 2019.
- 9.Sorensen RU, Hurtado R. Primary immunodeficiencies (PID)—driving diagnosis for optimal care globally. J Allergy Clin Immunol. 2011;127:2. doi: 10.1016/j.jaci.2010.12.475. [DOI] [Google Scholar]
- 10.Hooper JA. The history and evolution of immunoglobulin products and their clinical indications. LymphoSign J. 2015;2:25. doi: 10.14785/lpsn-2014-0025. [DOI] [Google Scholar]
- 11.Guo Y, Tian X, Wang X, Xiao Z. Adverse effects of immunoglobulin therapy. Front Immunol. 2018;63:76. doi: 10.3389/fimmu.2018.01299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Svehag SE. Adverse effects of clinical intervention on the complement system. Complement Inflamm. 1991;56:89. doi: 10.1159/000463207. [DOI] [PubMed] [Google Scholar]
- 13.Tawara T, Hasegawa K, Sugiura Y, Harada K, Miura T, Hayashi S, et al. Complement activation plays a key role in antibody-induced infusion toxicity in monkeys and rats. J Immunol. 2008;1:80. doi: 10.4049/jimmunol.180.4.2294. [DOI] [PubMed] [Google Scholar]
- 14.van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MHJ. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001;11:5. doi: 10.1046/j.1365-2141.2001.03166.x. [DOI] [PubMed] [Google Scholar]
- 15.Buchacher A, Schluga P, Müllner J, Schreiner M, Kannicht C, Weinberger J. Anticomplementary activity of IVIG concentrates—important assay parameters and impact of IgG polymers. Vox Sang. 2010;9:8. doi: 10.1111/j.1423-0410.2009.01271.x. [DOI] [PubMed] [Google Scholar]
- 16.Zang XX, Sun Y, Shen F, Wang D, Zhou L, Wang J, et al. Molecular structure alteration of IgG increased anticomplementary activity of intravenous immunoglobulin. Acta Pharmacol Sin. 1995;1:6. [PubMed] [Google Scholar]
- 17.Ramasamy I, Tran E, Farrugia A. Measurement of anticomplementary activity in therapeutic intravenous immunoglobulin preparations. Biologicals. 1997;2:5. doi: 10.1006/biol.1996.0063. [DOI] [PubMed] [Google Scholar]
- 18.Daniel GW, Menis M, Sridhar G, Scott D, Wallace AE, Ovanesov M, et al. Immune globulins and thrombotic adverse events as recorded in a large administrative database in 2008 through 2010. Transfusion (Paris) 2012;52:86. doi: 10.1111/j.1537-2995.2012.03589.x. [DOI] [PubMed] [Google Scholar]
- 19.Germishuizen WA, Gyure DC, Stubbings D, Burnouf T. Quantifying the thrombogenic potential of human plasma-derived immunoglobulin products. Biologicals. 2014;25:22. doi: 10.1016/j.biologicals.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Bellac CL, Hottiger T, Jutzi MP, Bogli-Stuber K, Sanger M, Hanschmann KM, et al. The role of isoagglutinins in intravenous immunoglobulin–related hemolysis. Transfusion (Paris) 2015;5:5. doi: 10.1111/trf.13113. [DOI] [PubMed] [Google Scholar]
- 21.Cuesta H, el Menyawi I, Hubsch A, Hoefferer L, Mielke O, Gabriel S, et al. Incidence and risk factors for intravenous immunoglobulin-related hemolysis: A systematic review of clinical trial and real-world populations. Transfusion (Paris). John Wiley and Sons Inc; 2022. p. 1894–907. [DOI] [PMC free article] [PubMed]
- 22.Kriván G, Borte M, Harris JB, Lumry WR, Aigner S, Lentze S, et al. Efficacy, safety and pharmacokinetics of a new 10% normal human immunoglobulin for intravenous infusion, BT595, in children and adults with primary immunodeficiency disease. Vox Sang. 2022;87:65. doi: 10.1111/vox.13337. [DOI] [PubMed] [Google Scholar]
- 23.Kriván G, Borte M, Soler-Palacin P, Church JA, Csurke I, Harris JB, et al. BT595, a 10% human normal immunoglobulin, for replacement therapy of primary immunodeficiency disease: results of a subcohort analysis in children. J Clin Immunol. 2022;58:25. doi: 10.1007/s10875-022-01397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Demeter J, Hamed A, László S, Suvajdzic N, Aigner S, Börner B, et al. Efficacy and safety of BT595 (10% human intravenous immunoglobulin) in adult patients with chronic immune thrombocytopenia. Transfus Med. 2022;25:87. doi: 10.1111/tme.12943. [DOI] [PubMed] [Google Scholar]
- 25.Aucouturier P, Mournir S, Preudhomme JL. Distribution of IgG subclass levels in normal adult sera as determined by a competitive enzyme immunoassay using monoclonal antibodies. Diagn Immunol. 1985;3:563. [PubMed] [Google Scholar]
- 26.Tang Y, Cain P, Anguiano V, Shih JJ, Chai Q, Feng Y. Impact of IgG subclass on molecular properties of monoclonal antibodies. MAbs. 2021;1:3. doi: 10.1080/19420862.2021.1993768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bajic G, Degn SE, Thiel S, Andersen GR. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34:77. doi: 10.15252/embj.201591881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chisholm CF, Behnke W, Pokhilchuk Y, Frazer-Abel AA, Randolph TW. Subvisible particles in IVIg formulations activate complement in human serum. J Pharm Sci. 2020;109:996. doi: 10.1016/j.xphs.2019.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funk MB, Gross N, Gross S, Hunfeld A, Lohmann A, Guenay S, et al. Thromboembolic events associated with immunoglobulin treatment. Vox Sang. 2013;105:88. doi: 10.1111/vox.12025. [DOI] [PubMed] [Google Scholar]
- 30.Etscheid M, Breitner-Ruddock S, Gross S, Hunfeld A, Seitz R, Dodt J. Identification of Kallikrein and FXIa as impurities in therapeutic immunoglobulins: Implications for the safety and control of intravenous blood products. Vox Sang. 2012;102:55. doi: 10.1111/j.1423-0410.2011.01502.x. [DOI] [PubMed] [Google Scholar]
- 31.Roemisch J, Kaar W, Zoechling A, Kannicht C, Putz M, Kohla G, et al. Identification of activated FXI as the major biochemical root cause in IVIG Batches associated with thromboembolic events. Analytical and experimental approaches resulting in corrective and preventive measures implemented into the octagam manufacturing process. WebmedCentral Immunotherapy. 2011;2.
- 32.Frazier SK, Higgins J, Bugajski A, Jones AR, Brown MR. Adverse reactions to transfusion of blood products and best practices for prevention. Crit Care Nurs Clin North Am. 2017;75:66. doi: 10.1016/j.cnc.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Basu S, Kaur R, Kaur G. Hemolytic disease of the fetus and newborn: current trends and perspectives. Asian J Transfus Sci. 2011;25:83. doi: 10.4103/0973-6247.75963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kustiawan I, Derksen NIL, Guhr T, Kruithof S, Jiskoot W, Vidarsson G, et al. Dimeric IgG complexes from IVIg are incapable of inducing in vitro neutrophil degranulation or complement activation. PLoS ONE. 2018;13:25. doi: 10.1371/journal.pone.0195729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratanji KD, Derrick JP, Dearman RJ, Kimber I. Immunogenicity of therapeutic proteins: influence of aggregation. J Immunotoxicol. 2014;2:2. doi: 10.3109/1547691X.2013.821564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Orlewski PM, Wang Y, Hosseinalipour MS, Kryscio D, Iggland M, Mazzotti M. Characterization of a vibromixer: experimental and modelling study of mixing in a batch reactor. Chem Eng Res Des. 2018;137:253. doi: 10.1016/j.cherd.2018.08.003. [DOI] [Google Scholar]
- 37.Wójtowicz R. Flow pattern and power consumption in a vibromixer. Chem Eng Sci. 2017;172:364. doi: 10.1016/j.ces.2017.07.010. [DOI] [Google Scholar]
- 38.Trapp A, Faude A, Hörold N, Schubert S, Faust S, Grob T, et al. Multiple functions of caprylic acid-induced impurity precipitation for process intensification in monoclonal antibody purification. J Biotechnol. 2018;279:85. doi: 10.1016/j.jbiotec.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Tankersley DL, Preston MS, Finlayson JS. Immunoglobulin G dimer: an idiotype-anti-idiotype complex. Mol Immunol. 1988;25:489. doi: 10.1016/0161-5890(88)90088-0. [DOI] [PubMed] [Google Scholar]
- 40.Bolli R, Woodtli K, Bärtschi M, Höfferer L, Lerch P. l-Proline reduces IgG dimer content and enhances the stability of intravenous immunoglobulin (IVIG) solutions. Biologicals. 2010;38:225. doi: 10.1016/j.biologicals.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Buchacher A, Curling JM. Current manufacturing of human plasma immunoglobulin G. Biopharmaceutical processing: development, design, and implementation of manufacturing processes. 2018.
- 42.Chen JY, Cortes C, Ferreira VP. Properdin: a multifaceted molecule involved in inflammation and diseases. Mol Immunol. 2018;102:587. doi: 10.1016/j.molimm.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hein C, Marschall V, Braun V, Schüttrumpf J, Germer M. Rising anti-SARS-CoV-2 titer in a human immunoglobulin preparation. Int J Blood Transf Immunohematol [Internet]. 2023;13:1–8. Available from: https://www.ijbti.com/archive/article-full-text/100076Z02CH2023
- 44.Mersich C, Ahrer K, Buchacher A, Ernegger T, Kohla G, Kannicht C, et al. Biochemical characterization and stability of immune globulin intravenous 10% liquid (Panzyga®) Biologicals. 2017;45:33–38. doi: 10.1016/j.biologicals.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Mathew SK, Anjum F. Transfusion selective IgA deficiency. StatPearls. 2021. [PubMed]
- 46.Martinez C, Wallenhorst C, van Nunen S. Intravenous immunoglobulin and the current risk of moderate and severe anaphylactic events, a cohort study. Clin Exp Immunol. 2021;206:384–394. doi: 10.1111/cei.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2014;136:1186–1205. doi: 10.1016/j.jaci.2015.04.049. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.