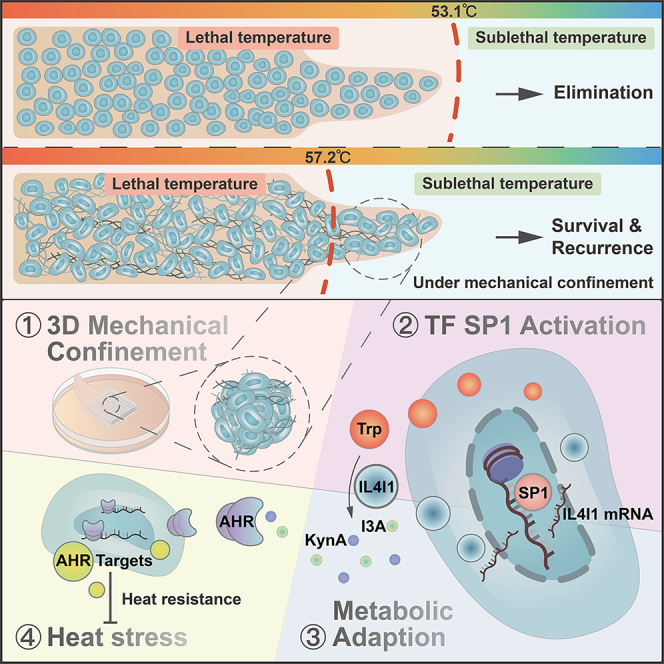
Summary
Mechanical stress can modulate the fate of cells in both physiological and extreme conditions. Recurrence of tumors after thermal ablation, a radical therapy for many cancers, indicates that some tumor cells can endure temperatures far beyond physiological ones. This unusual heat resistance with unknown mechanisms remains a key obstacle to fully realizing the clinical potential of thermal ablation. By developing a 3D bioprinting-based thermal ablation system, we demonstrate that hepatocellular carcinoma (HCC) cells in this 3D model exhibit enhanced heat resistance as compared with cells on plates. Mechanistically, the activation of transcription factor SP1 under mechanical confinement enhances the transcription of Interleukin-4-Induced-1, which catalyzes tryptophan metabolites to activate the aryl hydrocarbon receptor (AHR), leading to heat resistance. Encouragingly, the AHR inhibitor prevents HCC recurrence after thermal ablation. These findings reveal a previously unknown role of mechanical confinement in heat resistance and provide a rationale for AHR inhibitors as neoadjuvant therapy.
Keywords: mechanical confinement, nucleus deformations, tryptophan metabolism, heat resistance, hepatocellular carcinoma
Graphical abstract
Highlights
-
•
HCC cells endure high temperatures under 3D mechanical confinement
-
•
Mechanical confinement renders nucleus deformations of HCC
-
•
Nucleus deformation-mediated SP1 activation increases IL4I1 expression
-
•
IL4I1-driven tryptophan metabolites activate AHR and contribute to heat resistance
Zhang et al. develop a 3D bioprinting-based thermal ablation system and reveal that tryptophan metabolites induced by mechanical confinement play a previously unknown role in the heat resistance of tumor cells. Inhibition of the AHR pathway abrogates the heat resistance and prevents the recurrence of HCC after thermal ablation.
Introduction
Proliferating or migrating cells are subject to mechanical stresses in the confined extracellular space, resulting in deformations of the plasma membrane, cytoskeleton, and nucleus.1,2,3 These deformations adapt cellular behaviors to survive the unfavorable stress through multiple biological processes, such as changes in chromatin accessibility,4 nuclear softening,5 glycolytic regulation,6 or degradation of extracellular collagen.1 Hepatocellular carcinoma (HCC), ranking as the sixth most common cancer and the third leading cause of cancer-related deaths globally,7 mostly arose in fibrotic and cirrhotic livers with dense extracellular matrix (ECM).8,9 Mechanical cues have been demonstrated to play important roles in HCC progression through mechano-sensing pathways such as TGF-β/RAS/MAPK10 and YAP/TAZ,11,12 indicating the key factors provided exclusively by 3D environment should be considered to fully mimic physiological conditions and clinical scenarios.
Thermal ablation is widely accepted for solid neoplasms therapy, including liver, lung, breast, and bone cancers.13,14,15 However, a high recurrence rate after thermal ablation remains the major clinical problem,16,17 due to the induction of protective factors or intrinsic heat resistance during hyperthermia treatment. The conserved heat shock proteins are instantly induced by heat stress to protect cells.18,19 Additionally, we and others have reported that the heat stress-induced alterations of molecular modifications can enhance cell viability and metastasis, leading to postoperative HCC progression.20,21 These findings largely rely on the cell properties in 2D culture, whether some essential properties endowed by the 3D environment contribute to heat resistance remains less known.
There still lacks an appropriate preclinical model for thermal ablation research that can recapitulate the mechanics on proliferating cells with the ECM in vivo. Another requirement for this model is to administer the precise temperature gradients of focal ablation in a 3D scenario. To date, the most common models for thermal ablation are 2D cell culture and animal models.19,20,21,22,23 Obviously, the 2D cell culture does not contain extracellular components to mimic mechanical confinement. Although animal models with the mechanical environment are widely applied to investigate tumor occurrence and progression,24 there are still some problems to address. It is difficult to decouple the factors of mechanics, components, and accurate temperatures to investigate their respective effects on heat resistance in animal models. Besides, tumor variances among individual animals make it difficult to achieve unified energy exposure. In recent years, 3D bioprinting has become a cutting-edge technology in the field of biomedical engineering.25,26 It can combine multiple cells and ECM components to recapitulate pathological structure and microenvironment with adjustable stiffness and growth factors, providing an approach for cancer research.27,28,29 Accordingly, the 3D bioprinted model has advantages to be applied for thermal ablation study.
In this study, we constructed a 3D bioprinting-based thermal ablation system, which possessed thermal properties of the tissue structure and ECM components with high fidelity to the animal model. Using this 3D model, it was feasible to observe the phenotypic and subcellular events with improved resolution of temperature gradients as compared with tumor xenografts, allowing us to interrogate the disparity of heat resistance under 2D and 3D conditions. We leveraged diverse thermal ablation models and clinical data to reveal that the mechanical confinement yielded nucleus deformations and subsequent metabolic adaptation to elicit heat resistance, shedding light on potential therapy regimens post thermal ablation.
Results
3D bioprinted GCL construct
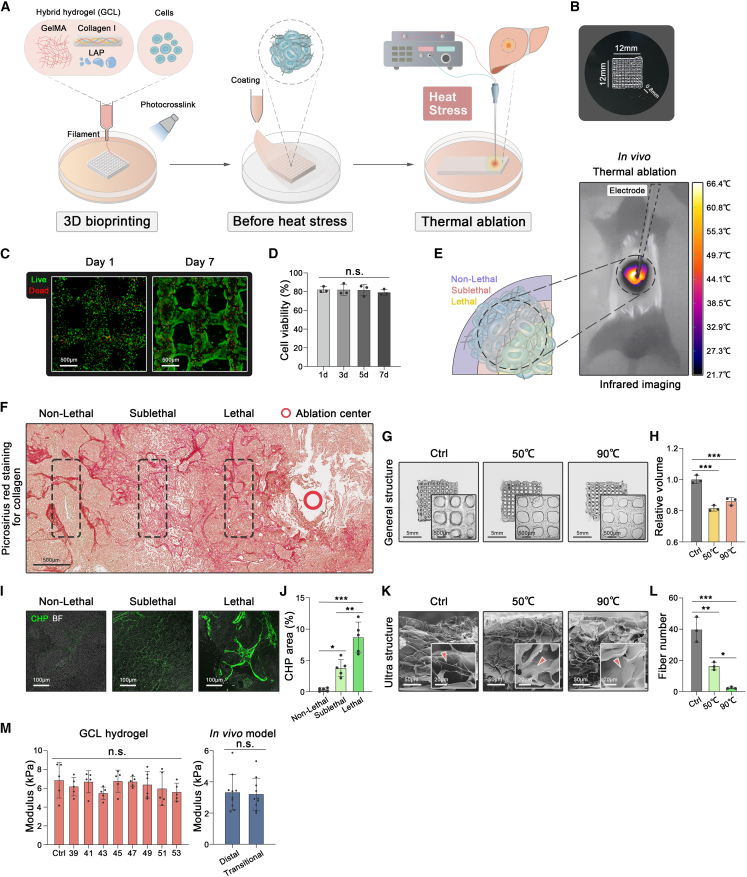
Gelatin methacryloyl (GelMA) has proven optimal biocompatibility and shear-thinning properties for 3D bioprinting and is widely applied in modeling tumor tissues.30,31 To mimic the ECM deposition in the fibrotic microenvironment of HCC, GelMA was combined with collagen I and the auxiliary photoinitiator LAP to generate a hybrid hydrogel GCL (GelMA+Collagen I + LAP) for 3D bioprinting and further thermal ablation (Figure 1A). To ensure the printability and structural-retaining capability during the 3D bioprinting process, the rheological properties of the GCL were characterized. The results showed that a reversible solution-gel temperature of 17.98°C (the point where G’ = G″) warranted the printability of GCL hydrogel (Figure S1A). The mixture of GCL and HCC cell lines was further printed into a 3D grid-like structure, which was structurally stable after photocrosslinked by the UV light (Figure 1B). Human HCC cell lines (MHCC-97H or SNU-449) maintained around 80% viability in the 3D model for 7 days (Figures 1C and 1D, S1B, and S1C), suggesting favorable biocompatibility in line with other studies.32,33
Figure 1.
3D bioprinted GCL construct
(A) The schematic representation of the 3D thermal ablation model using the mixture of GCL hydrogel and HCC cell lines.
(B) A photograph of a 3D bioprinted model with a grid-like structure.
(C and D) Live/dead staining of MHCC-97H and quantifications in 3D model for days 1 and 7 (three biological replicates).
(E) Schematic diagram and infrared imaging of orthotopic HCC tumor during thermal ablation for 30 s in BALB/C nude mice.
(F) Picrosirius red staining of fibrillar collagen in the different regions of the tumor tissue after thermal ablation for 30 s.
(G and H) The images and volume of GCL structure after different heat treatments for 15 min (three biological replicates).
(I and J) Collagen hybridizing peptide staining of fibrillar collagen in the different regions of tumor tissue after thermal ablation for 30 s (five biological replicates).
(K and L) SEM images of fibrillar collagen (red arrows) and quantifications in GCL structure after different heat treatments for 15 min (three biological replicates).
(M) The stiffness of 3D (five biological replicates) and animal (10 biological replicates) models detected by nano-indentation under different temperatures. Mean ± standard deviation. Statistical analysis, one-way ANOVA for (D), (H), (J), (L), and (M, left), two-tailed Student’s t test for (M, right). n.s., not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
We next wondered whether the hydrogel GCL can mimic thermal properties in vivo. On the basis of our previous study,23 an animal model of thermal ablation was established as a reference (Figure 1E). Orthotopic HCC remained an integrated structure in the thermal ablation center (over 90°C), transitional region (around 50°C20), and distal region (normal temperature) (Figure 1F). Likewise, the GCL presented structural stability with reduced volume after subjected to corresponding temperatures (Figures 1G and 1H). Collagen hybridizing peptide (CHP) staining, known to specifically bind unfolded collagen chains to detect collagen damage,34 indicated that the microstructure of collagen fibers in orthotopic tumors began to degrade as the temperature rose (Figures 1I and 1J). Consistently, the scanning electron microscopy (SEM) images showed that collagen fibers of GCL constructs were damaged when treated with 50°C or 90°C (Figures 1K and 1L).
Given that tissue stiffness can affect the migration and invasion of tumor cells,32,35 we measured the stiffness of GCL constructs treated with temperature gradients (37–53°C). Nano-indentation measurement showed no significant alterations in effective Young’s modulus among heat-treated GCL constructs (Figure 1M). In line with the GCL, the animal model showed no significant difference in tissue stiffness between the distal and transitional regions of focal ablation (Figure 1M). Taken together, this 3D model recapitulated the thermal properties of ECM components and mechanical stiffness in the animal model, which was applicable to heating-relevant studies.
3D thermal ablation model
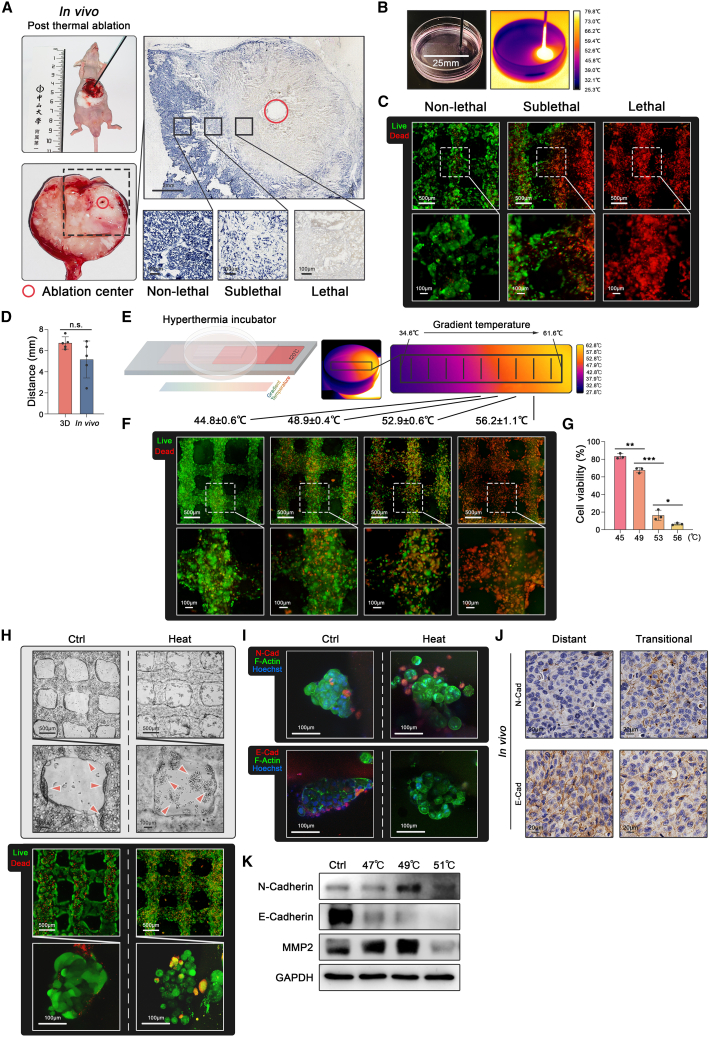
An animal model of thermal ablation requires the temperature gradients to divide the tumor tissue into three regions: lethal, sublethal, and non-lethal temperature regions.13,20,23 NADH staining was applied to validate that HCC cells were dead in the lethal region, partially dead in the sublethal region, and alive in the non-lethal region (Figure 2A). Accordingly, a 3D thermal ablation model was fabricated into a long cuboid construct (25∗10∗0.8mm3) to ensure these three regions (Figure 2B). An electrode of thermal ablation equipment was eccentrically inserted into the 3D model to generate the temperature gradients, which were monitored by infrared imaging (Figure 2B). Live/dead staining in the 3D model showed that HCC cells were dead in the lethal region, partially dead in the sublethal region, and alive in the non-lethal region, replicating the three regions in the animal model (Figure 2C). Additionally, the distance from the lethal to the non-lethal region in the 3D model was analogous to the distance in the animal model (Figure 2D).
Figure 2.
3D thermal ablation model
(A) Orthotopic HCC tumor after thermal ablation for 30 s in BALB/C nude mice. Cell viability determined by NADH staining in the non-lethal, sublethal, and lethal regions of tumor tissue after thermal ablation for 30 s.
(B) A long cuboid construct (25∗10∗0.8 mm3) for thermal ablation (left) monitored by infrared imaging (right).
(C) Live/dead staining of MHCC-97H in the different regions of the 3D model after thermal ablation.
(D) The distance from the lethal to the non-lethal region in 3D and animal models (five biological replicates).
(E) Schematic diagram of a hyperthermal device (left) and its infrared imaging during operation for 15 min (right).
(F and G) Live/dead staining of MHCC-97H and quantifications in the different regions under temperature gradients generated by the homemade hyperthermal device for 15 min (three biological replicates).
(H) The bright field images and live/dead staining of spheroids (red arrows) in the 3D model with or without 49°C treatment for 15 min.
(I) Immunofluorescence on the 3D model with or without 49°C treatment for 15 min and the animal thermal ablation model for N-cadherin (red), E-cadherin (red), F-Actin (green), and Hoechst (blue).
(J) Immunohistochemistry staining of N-cadherin and E-cadherin from the animal thermal ablation model.
(K) E-cadherin, N-cadherin, and MMP2 protein expression detected by western blot assay in the 3D model after different heat treatments. Mean ± standard deviation. Statistical analysis, two-tailed Student’s t test for (D), one-way ANOVA with post Tukey’s comparisons test for (G). n.s., not significant, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
To improve the resolution of temperature detection and identify the accurate temperature in different regions, we applied a hyperthermal device to treat the 3D model with temperature gradients ranging from 37°C to 60°C (Figure 2E). In this 3D thermal ablation model, HCC cells were largely alive in the region below 45°C, more than half the HCC cells were alive in the sublethal temperature region around 49°C, and HCC cells underwent increased death as the temperature reached 53°C and complete death in the region above 56°C (Figures 2F and 2G).
Sublethal heat stress from insufficient thermal ablation has been proven to enhance migratory ability through the alterations of key markers related to metastasis.20,23,36 This 3D thermal ablation model was subsequently used to detect migratory behavior and metastatic markers after heat stress. Interestingly, HCC spheroids detached from the grid-like construct after heat treatment and exhibited loose intercellular junctions with relatively high viability (Figure 2H), indicating the enhanced migratory ability of HCC cells after heat stress. HCC cells in the 3D model upregulated N-cadherin expression and downregulated E-cadherin expression after heat treatment (Figure 2I), which was consistent with the phenomenon in vivo (Figure 2J). These imaging results were confirmed by western blot that MMP2 was additionally upregulated after heat treatment (Figure 2K). Accordingly, we considered that this 3D thermal ablation model supported the heat treatment with precise temperatures as compared with the animal model and provided a platform to explore cell behaviors and molecular mechanisms.
HCC endured high temperatures in the 3D model
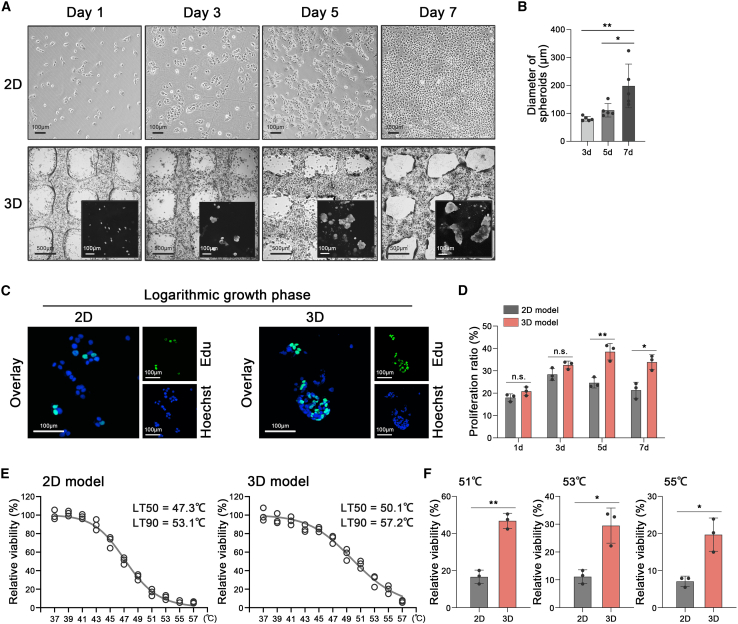
Proliferating cells in 3D models behaved in a spheroid-like structure, mimicking the physiological progress in vivo.27,37,38 In our study, spheroids of MHCC-97H were observed on day 3 after 3D bioprinting. The average diameters of the spheroids on days 3, 5, and 7 were 80.8 μm (±8.4), 115.6 μm (±26.1), and 186.0 μm (±76.4), respectively (Figures 3A and 3B). Similarly, spheroids of SNU-449 had average diameters of 45.3 μm (±6.5), 107.0 μm (±24.4), and 147.6 μm (±41.0) on days 3, 5, and 7 after bioprinting (Figures S2A and S2B). In contrast, MHCC-97H and SNU-449 in the 2D model showed monolayer growth for up to 7 days (Figures 3A and S2A).
Figure 3.
HCC endured high temperatures in the 3D model
(A) Images of the monolayer growth of MHCC-97H in the 2D model (upper) and the spheroids generation of MHCC-97H in the 3D model (lower) on days 1, 3, 5, and 7 (three biological replicates).
(B) Quantification of the diameters of spheroids in the 3D model on days 3, 5, and 7 (n = 5) (three biological replicates).
(C) Immunofluorescence on MHCC-97H in 2D or 3D models during the logarithmic growth phase, stained for EdU (green) and Hoechst (blue) (three biological replicates).
(D) Quantification of proliferative rates of MHCC-97H in 2D or 3D models on days 1, 3, 5, and 7 (n = 3) (three biological replicates).
(E) Cell viability and LT50/LT90 fitted curves of MHCC-97H in the 2D model (left) and 3D model (right), respectively, detected by trypan blue staining and live/dead staining after different heat treatments for 15 min (37–57°C) (three biological replicates).
(F) Cell viability of MHCC-97H in the 2D and 3D models after heat treatment of 51, 53, or 55°C. Mean ± standard deviation (three biological replicates). Statistical analysis, one-way ANOVA with post Tukey’s comparisons test for (B), two-tailed Student’s t test for (D), two-tailed Student’s t test with correction for multiple comparisons for (F). n.s., not significant, ∗p < 0.05, ∗∗p < 0.01.
EdU incorporation assay was performed to detect the proliferative ability of cells in 2D or 3D models. Proliferative rates of MHCC-97H in the 3D model were 20.8%, 32.6%, 38.5%, and 33.9%, respectively, on days 1, 3, 5, and 7 (Figures 3C and 3D). Likewise, the proliferative rates of SNU-449 were 20.6%, 27.4%, 35.9%, and 23.5%, respectively, on days 1, 3, 5, and 7 (Figures S2C and S2D). The logarithmic growth of MHCC-97H and SNU-449 was faster in the 3D model than in the 2D model (Figures 3C and 3D, S2C, and S2D).
The above results prompted us to interrogate the heat resistance of HCC cells under 2D and 3D conditions. The viabilities of HCC cells in 2D or 3D models were detected after heat treatment (37–53°C) for 15 min. Strikingly, we found that the 50% lethal temperature (LT50 = 50.1°C) and 90% lethal temperature (LT90 = 57.2°C) of HCC cells in the 3D model were remarkably higher than the LT50 (47.3°C) and LT90 (53.1°C) in the 2D model as well as the LT50 (47.5°C) and LT90 (53.9°C) in the conventional spheroids culture (Figures 3E and S2E). As shown in Figure 3F, the viability of cells in the 3D model was significantly higher than that in the 2D model after heat treatment of 51, 53, or 55°C. It implied that HCC cells under 3D conditions endured higher temperatures as compared with conventional 2D culture.
IL4I1 promoted HCC survival after heat stress
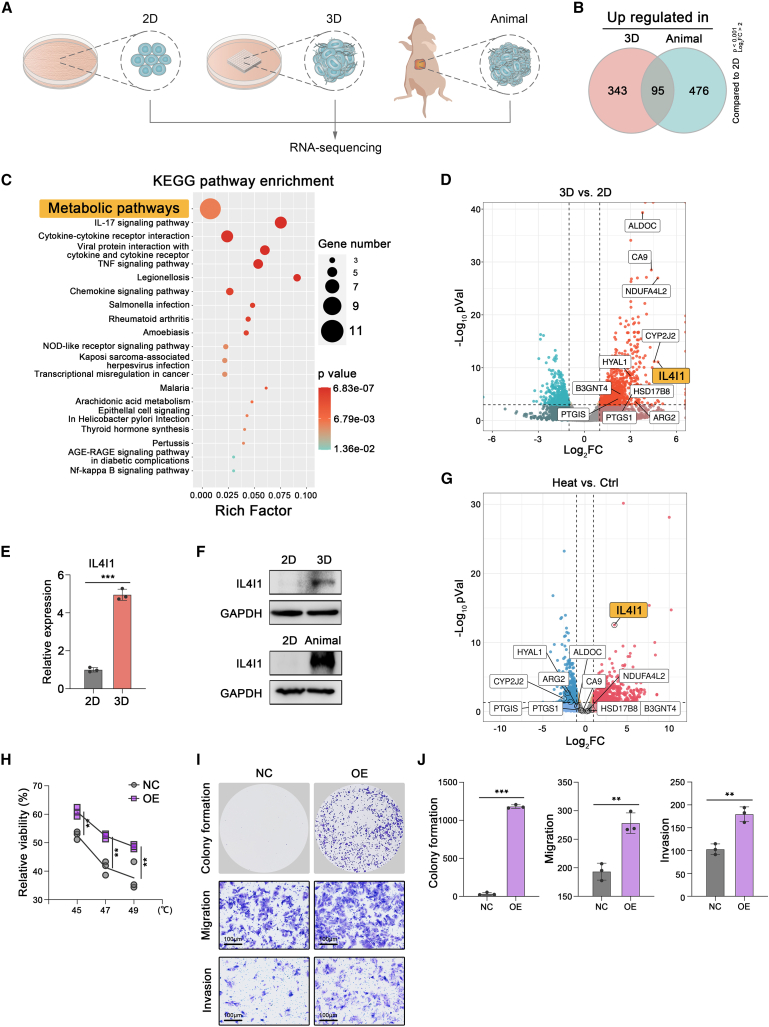
To investigate the specific factors endowed by a bona fide 3D environment and reveal the mechanism of heat resistance in the 3D model, we performed RNA sequencing (RNA-seq) to delineate the transcriptional states of HCC cells in the 2D, 3D, and animal models (Figure 4A). As compared with the 2D model, 95 differentially expressed genes (DEGs) were identified to upregulate in both the 3D model and animal model, suggesting that these 95 genes were specifically regulated by the 3D environment (Figure 4B; Table S1). Further Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis indicated that most of these genes were enriched in “Metabolic pathways” (Figure 4C; Table S2). The volcano plot showed that interleukin-4-Induced-1 (IL4I1) had the highest fold change among the genes in “Metabolic pathways” (Figure 4D). It was further confirmed that IL4I1 expression of HCC cell lines in the 3D and animal models was higher than it in the counterpart 2D model (Figures 4E and 4F, S3A, and S3B).
Figure 4.
IL4I1 promoted HCC survival after heat stress
(A) RNA sequencing for MHCC-97H in 2D, 3D, and animal models (three biological replicates).
(B) Upregulated genes in 3D and animal models, respectively, compared with the 2D model.
(C) The KEGG enrichment of 95 overlapped genes.
(D) Volcano plot of DEGs among “Metabolic pathways” in the 3D model versus 2D model. The names of 11 3D-specific metabolic genes are indicated in the plot.
(E and F) qRT-PCR and western blot assay determining the IL4I1 expression of MHCC-97H in 2D and 3D models (three biological replicates).
(G) Volcano plot of DEGs in HCC cells after heat stress compared with untreated control in the 3D model (three biological replicates). The names of 11 3D-specific metabolic genes are indicated in the plot.
(H) Cell viability of MHCC-97H detected by trypan blue staining in different groups after heat treatments for 15 min (45°C, 47°C, and 49°C) (three biological replicates).
(I and J) Representative images and quantification of the colony formation (4 × 103 cells/well), migration assay (1 × 105 cells/well), and invasion assay (1 × 105 cells/well) in IL4I1-overexpressed and control groups after 47°C treatment for 15 min (three biological replicates). Mean ± standard deviation. Statistical analysis, two-tailed Student’s t test for (E), (H), and (J). ∗∗p < 0.01, ∗∗∗p < 0.001.
IL4I1 has recently been reported to play a pivotal role in tryptophan (Trp) catabolism and promote tumor cell motility and was associated with dismal survival in glioblastoma.39 Besides, IL4I1 can also promote the proliferation, migration, and invasion of ovarian cancer.40 Likewise, high expression of IL4I1 predicted poor overall survival and advanced stage in The Cancer Genome Atlas (TCGA) HCC cohort (Figures S3C and S3D).
To interrogate the key factor among 11 metabolic genes that altered tumor metabolism to induce heat resistance, we performed additional RNA-seq to explore the transcriptional state of HCC cells in the 3D model after heat stress compared with untreated control. Intriguingly, the analysis revealed that among the 11 3D-specific metabolic genes, only IL4I1 was expressed higher in HCC cells after sublethal heat stress than in untreated control (Figure 4G), suggesting IL4I1 was the potential candidate involved in heat resistance. To assess the function of endogenous IL4I1 in 3D conditions under heat stress, we generated stable HCC cell lines with ectopic expression of IL4I1 to simulate the scenario of 3D condition (Figures S3E and S3F). Ectopic IL4I1 had indeed promoted viability of MHCC-97H, Huh7, and PLC/PRF/5 cell lines after heat treatment (45°C, 47°C, 49°C) (Figures 4H, S3G, and S3H). Colony formation revealed that IL4I1 overexpression significantly promoted proliferation in three HCC cell lines treated with 47°C (Figures 4I and 4J, S3G, and S3H). Transwell assay demonstrated that IL4I1 overexpression greatly decreased the migratory and invasive abilities of three HCC cell lines treated with 47°C (Figures 4I and 4J, S3G, and S3H).
SP1 activation increases IL4I1 expression under mechanical confinement
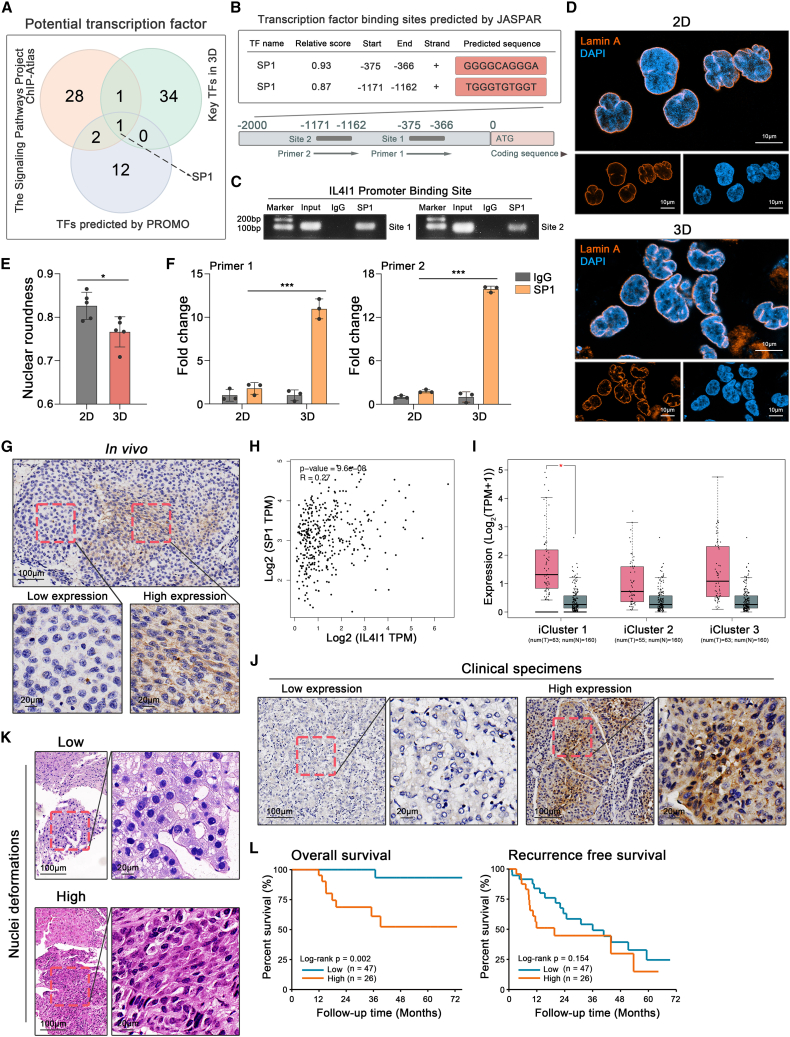
To gain an insight into the regulation of IL4I1, we predicted 15 transcription factors (TFs) of IL4I1 promotor binding sites by PROMO41 (Figure 5A; Table S3). According to The Signaling Pathways Project (https://www.signalingpathways.org/index.jsf) database, 32 TFs were sequenced to bind IL4I1’s promotor based on chromatin immunosuppression sequencing (ChIP-seq) in the liver organ (Figure 5A; Table S3). From the perspective of our RNA-seq, the transcriptional regulatory networks analysis by TTRUST42 (https://www.grnpedia.org/trrust/) depicted that the elevation of DEGs in 3D versus 2D was potentially regulated by 36 key TFs (Figure 5A; Table S3). Overlap among three datasets showed that the transcription factor SP1 was a promising candidate to regulate IL4I1 (Figure 5A).
Figure 5.
SP1 activation increases IL4I1 expression under mechanical confinement
(A) IL4I1 potential transcription factors in PROMO (purple circle), The Signaling Pathways Project (orange circle), and our data using TTRUST (green circle).
(B) SP1 binding sites on IL4I1 promotor predicted by JASPAR database.
(C) Validation of SP1 on IL4I1 promotor by ChIP-PCR (three biological replicates).
(D) The immunofluorescence of nuclear morphology in 2D and 3D models, stained for Lamin A (orange) and DAPI (blue) (three biological replicates).
(E) Quantification of nuclear roundness in 2D and 3D models (three biological replicates).
(F) Putative SP1 binding sites on IL4I1 promotor detected by ChIP-qPCR assay in 2D and 3D models (three biological replicates).
(G) IL4I1 expression in the orthotopic tumor of animal model detected by immunohistochemistry (IHC).
(H) Correlation between IL4I1 and SP1 expression in TCGA HCC dataset.
(I) IL4I1 expression of tumor tissues (red) and normal liver tissues (gray) in TCGA HCC cohort separated by iCluster 1, 2, and 3.
(J) IL4I1 expression in human HCC specimens detected by IHC.
(K) Low or high nucleus deformations showed by hematoxylin-eosin staining.
(L) The Kaplan-Meier curve of overall survival and recurrence-free survival between low and high groups of nuclei deformations. Mean ± standard deviation. Statistical analysis, two-tailed Student’s t test for (E) and (F). ∗p < 0.05, ∗∗∗p < 0.001.
To investigate whether SP1 enhanced IL4I1 transcription in the 3D model, the specific binding sites between SP1 and IL4I1’s promotor were predicted using the JASPAR database (https://jaspar.genereg.net/). The result showed that SP1 had two putative binding sites in IL4I1’s promotor regions (1,500 base pairs upstream from the transcription start site) with relatively high scores (Figure 5B), which was validated in the 3D model by ChIP-PCR (Figure 5C). Stowers et al. have demonstrated that the mechanical confinement in 3D culture promoted SP1 phosphorylation and yielded nucleus deformations that increased chromatin accessibility where SP1 bonded more frequently.4 Consistently, we validated that SP1 phosphorylation at Thr 453 was higher in the 3D model than the 2D model (Figure S4A). The deformed nuclei indeed presented a higher proportion in the 3D model than the 2D model (Figures 5D and 5E), and SP1 was prone to bind IL4I1 promotor particularly in the 3D model (Figure 5F). Additionally, SP1 overexpression upregulated IL4I1 expression in 3D but not 2D (Figure S4B), and SP1 knockdown decreased IL4I1 expression particularly in the 3D model (Figure S4C).
To validate this phenomenon in the animal model, we found that IL4I1 and phosphorylated SP1 were expressed at higher levels along with the increased nuclei density and deformations (Figures 5G and S4D). We further verified that the IL4I1 expression was correlated with SP1 expression in the TCGA HCC dataset (Figure 5H) and higher in the iCluster 1 and 3, designated as the “Proliferation class,” than the iCluster 2, as the “Non-proliferation class.”43 This result implied that the proliferation of HCC cells provided limited space in which the mechanical stress triggered IL4I1 expression (Figure 5I). Clinical specimens in our validation cohort were further employed to analyze the correlation among nuclei density, deformations, and IL4I1 expression. IL4I1 was upregulated with increased nuclei density and deformations, such as in the macrotrabecular subtype, and the heterogeneous and scirrhous phenotype of HCC (Figures 5J, S4E, and S4F). The biopsy samples of HCC patients before thermal ablation in our other validation cohort were employed to calculate nuclei roundness and stratified into low and high groups by the extent of nuclei deformations (Figures 5K and S4G). Higher nuclei deformations were associated with dismal overall survival after thermal ablation, despite the fact that recurrence-free survival did not reach statistically significant differences between the two groups (Figure 5L), which might result from the detrimental effect of mechanical confinement on early recurrence.
The above validation implied that the mechanical confinement upregulated IL4I1 expression through SP1 activation and predicted poor survival of HCC patients after thermal ablation.
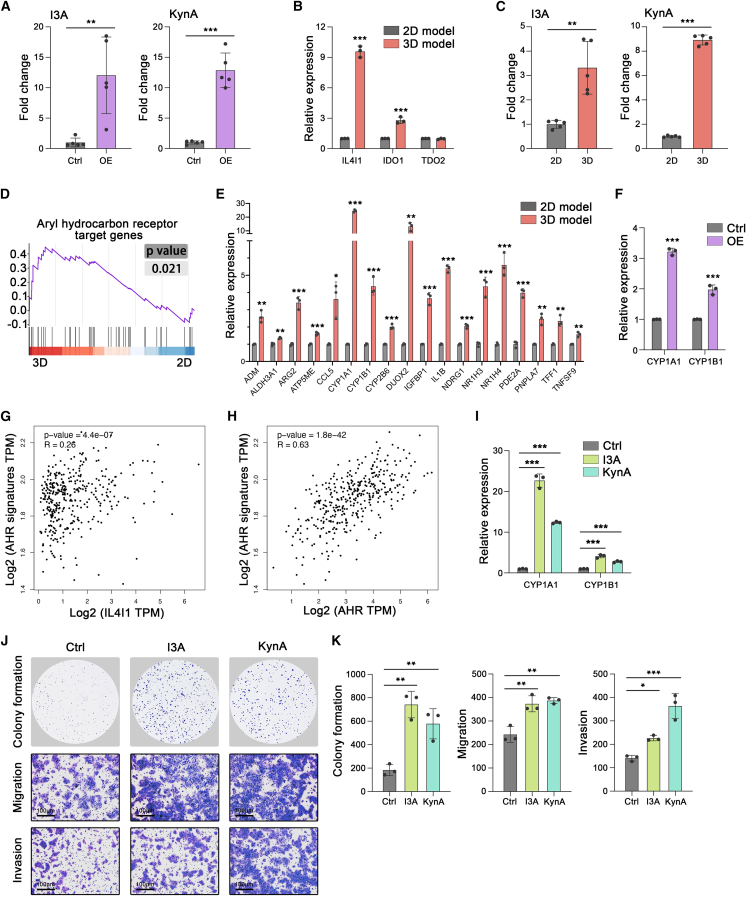
IL4I1-induced AHR activation promotes HCC survival after heat stress
To date, since IL4I1 inhibitor is not available for cancer therapy, we focused on the downstream of IL4I1 for potential targets to improve the efficacy of thermal ablation. IL4I1 can convert tryptophan (Trp) into indole-3-pyruvic acid (I3P), subsequently generating metabolites indole-3-aldehyde (I3A) and Kynurenic acid (KynA).39 Consistently, HCC cells with IL4I1 overexpression indeed elevated the levels of I3A and KynA (Figure 6A). In the 3D condition, we found IL4I1 elevation was higher than indole-amine-2,3-dioxygenase 1 (IDO1) and tryptophan-2,3-dioxygenase (TDO2), the other two Trp-catabolic enzymes (Figure 6B). Moreover, HCC cells in the 3D model generated higher levels of I3A and KynA than that in the 2D model (Figure 6C).
Figure 6.
IL4I1-induced AHR activation promotes HCC survival after heat stress
(A) Relative abundance of I3A and KynA in supernatants of Ctrl and IL4I1 MHCC-97H cells (4 days) (five biological replicates).
(B) mRNA expression of IL4I1, IDO, and TDO2 in 2D and 3D models (three biological replicates).
(C) Relative abundance of I3A and KynA in supernatants of MHCC-97H cells in the 2D and 3D models (4 days) (five biological replicates).
(D) GSEA analysis of AHR pathway in the 3D model versus 2D model.
(E) mRNA expression of AHR signature targets in 2D and 3D models (three biological replicates).
(F) mRNA expression of CYP1A1 and CYP1B1 in IL4I1-overexpressed and control groups of MHCC-97H (three biological replicates).
(G) Correlation between the expression of IL4I1 and AHR-targeted genes in TCGA HCC dataset.
(H) Correlation between the expression of AHR and AHR-targeted genes in TCGA HCC dataset.
(I) mRNA expression of CYP1A1 and CYP1B1 in MHCC-97H treated with I3A (50 μM) or KynA (250 μM) for 8 h (three biological replicates).
(J and K) Representative images and quantification of the colony formation (4 × 103 cells/well), migration assay (1 × 105 cells/well), and invasion assay (1 × 105 cells/well) in MHCC-97H with or without I3A (50 μM) or KynA (250 μM) treatment for 8 h, following 47°C treatment for 15 min (three biological replicates). Mean ± standard deviation. Statistical analysis, two-tailed Student’s t test for (A), (B), (C), (E), and (F), one-way ANOVA with post Tukey’s comparisons test for (I) and (K). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Various tryptophan metabolites can activate the aryl hydrocarbon receptor (AHR) to promote tumor progression.39,44,45,46 Gene set enrichment analysis (GSEA) revealed the enrichment of the AHR pathway in the 3D model (Figure 6D). qRT-PCR was subsequently conducted to confirm that AHR signature targets were notably upregulated in the 3D model as compared with the 2D model (Figures 6E and S5A). To demonstrate that AHR activation in the 3D model depended on IL4I1, we examined canonical AHR targets in HCC cell lines by overexpressing IL4I1. It showed that ectopic IL4I1 induced higher expression of CYP1A1 and CYP1B1 (Figures 6F and S5B). TCGA database also proved the positive correlation between IL4I1 and AHR-targeted genes in clinical HCC specimens (Figure 6G). We concluded that IL4I1 generated tryptophan metabolites (I3A and KynA) that activated the AHR pathway in HCC under the 3D condition.
It was reported that the AHR was expressed higher in HCC than in adjacent normal liver and promoted HCC progression.47,48 We further demonstrated the AHR expression was positively correlated with AHR-targeted genes in TCGA HCC samples, implying that the AHR pathway was activated in HCC (Figure 6H). However, the role of AHR activation in response to heat stress remained less known. We then used I3A or KynA to activate the AHR pathway. It showed that HCC cell lines indeed upregulated CYP1A1 and CYP1B1 expression when subjected to I3A or KynA (Figures 6I and S5C). Further, I3A or KynA significantly enhanced the ability of colony formation, migration, and invasion after heat stress in MHCC-97H, Huh7, and PLC/PRF/5 cell lines (Figures 6J and 6K, and S5D–S5G). Taken together, AHR promoted HCC cell viability and motility after heat stress, hence considered as a potential target to enhance the efficacy of thermal ablation.
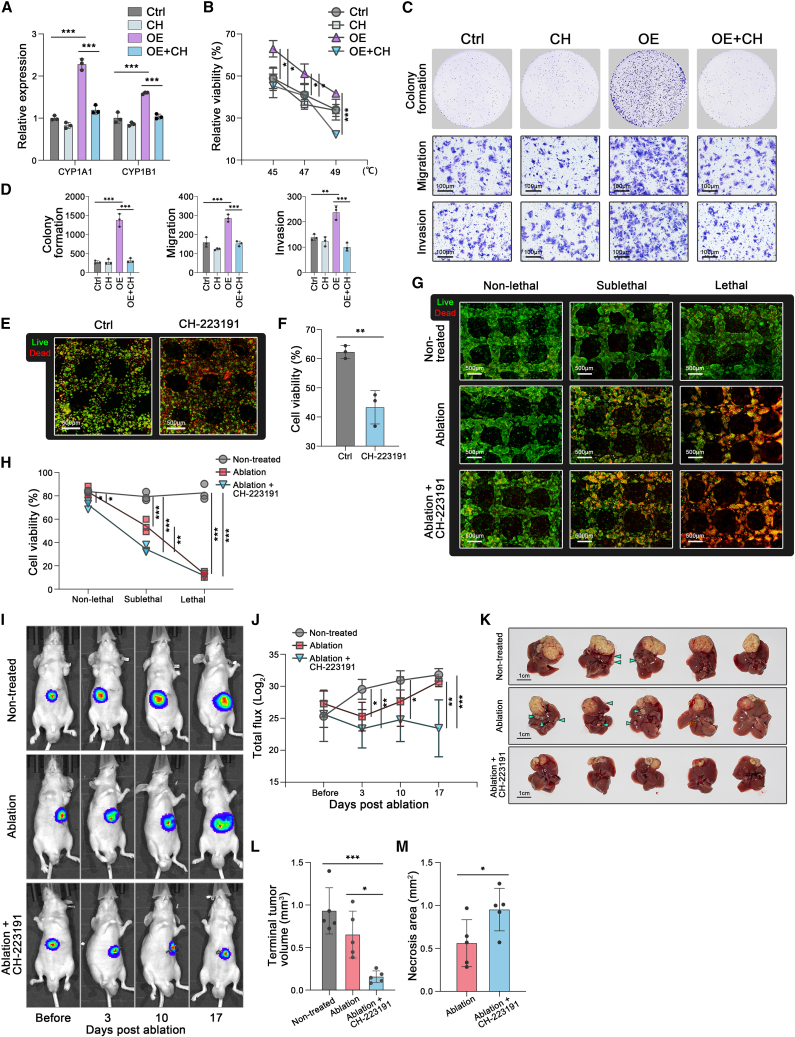
AHR inhibition attenuated IL4I1-driven survival after thermal ablation for HCC
In the 2D model, we previously generated HCC cell lines with stable overexpression of IL4I1 (Figures S3E and S3F) and testified that IL4I1 overexpression facilitated cell viability, colony formation, migration, and invasion after heat stress (Figures 4H–4J, S3G, and S3H). To explore whether AHR inhibition can be a competent combinational regimen with thermal ablation, CH-223191, a widely used AHR inhibitor, was employed to abrogate IL4I1-driven malignancy. CH-223191 can effectively attenuate the IL4I1-driven upregulation but minimally suppress the baseline of AHR targets in HCC cell lines (Figures 7A and S6A). Impressively, the IL4I1-enhanced survival and motility of HCC cells after heat stress were remarkably abrogated by CH-223191. However, CH-223191 had minimal impact on cell survival and motility of HCC cells without IL4I1 overexpression after heat stress (Figures 7B–7D and S6B–S6D).
Figure 7.
AHR inhibition attenuated IL4I1-driven survival after thermal ablation for HCC
(A) mRNA expression of CYP1A1 and CYP1B1 in control, CH-232191 (20 μM), IL4I1-overexpressed and IL4I1-overexpressed+CH-232191 (20 μM) groups of MHCC-97H (three biological replicates).
(B) Cell viability of MHCC-97H detected by trypan blue staining in control, CH-232191 (20 μM), IL4I1-overexpressed, and IL4I1-overexpressed+CH-232191 (20 μM) groups after 47°C treatments for 15 min (45°C, 47°C, and 49°C) (three biological replicates).
(C and D) Representative images and quantification of the colony formation (4 × 103 cells/well), migration assay (1 × 105 cells/well), and invasion assay (1 × 105 cells/well) in control, CH-232191 (20 μM), IL4I1-overexpressed, and IL4I1-overexpressed+CH-232191 (20 μM) groups of MHCC-97H after 47°C treatment for 15 min (three biological replicates).
(E and F) Live/dead staining and quantification of the 3D model in control and CH-232191 (100 μM) groups after thermal ablation (three biological replicates).
(G and H) Live/dead staining and quantification in the non-treatment, ablation monotherapy, and combined ablation and CH-223191 (100 μM) groups in the different regions treated with the hyperthermal device for 15 min (three biological replicates).
(I and J) Tumor burden longitudinally monitored by IVIS imaging in the non-treatment, ablation monotherapy, and combined ablation and CH-223191 (10 mg/kg/d) groups (n = 5 mice/group).
(K and M) Representative images, tumor volume, and intra-tumor necrosis area of dissected orthotopic HCC at the endpoint. Mean ± standard deviation. Statistical analysis, one-way ANOVA with post Tukey’s comparisons test for (A), (B), (D), (H), (J), and (L), two-tailed Student’s t test for (F) and (M). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
In the 3D thermal ablation model with higher IL4I1 expression, we further proved that CH-223191 potently inhibited the AHR pathway (Figure S6E). After heat treatment, HCC cells in the 3D model decreased viability when subjected to CH-223191 (Figures 7E and 7F). Then, we applied the hyperthermal device to observe cell viability in non-lethal, sublethal, and lethal temperature regions (Figures 7G and 7H). The results showed that combined ablation and CH-223191 (“Ablation+CH-223191”) slightly decreased cell viability in the non-lethal region, compared with non-treatment (“Non-treated”) and ablation monotherapy (“Ablation”) (Figures 7G and 7H). Thermal ablation significantly decreased cell viability in the sublethal region, and combination therapy augmented the efficacy of ablation monotherapy to further reduce the viability in the sublethal region (Figures 7G and 7H).
Our next endeavor was to compare the efficacy of thermal ablation monotherapy and combination therapy with CH-223191 in the animal model. To longitudinally visualize the tumor burden before and after thermal ablation treatment by the IVIS imaging system, the luciferase-expressing gene was introduced into MHCC-97H (97H-luc); 97H-luc cells were injected into livers to form orthotopic HCC tumors. Consistent with the clinical scenario, both ablation monotherapy and combination therapy with CH-223191 can significantly reduce tumor burden as compared with non-treatment (Figures 7I and 7J). In line with previous in vitro evidence, combined ablation and CH-223191 significantly shrank the average volume of HCC tumors by 76.0% and increased tumor necrotic region compared with ablation monotherapy on day 17 post ablation (Figures 7K–7M).
To sum up, as depicted in the graphical abstract, mechanical confinement under a 3D environment yielded nucleus deformations that activated SP1 and subsequently induced IL4I1 expression. AHR inhibition was potent to abrogate IL4I1-driven heat resistance of HCC and improve the efficacy of thermal ablation as neoadjuvant therapy.
Discussion
This work demonstrated that the mechanical stress from spatial constraints drove metabolic adaptations that determined the fate of cells under high temperatures. Impressively, tumor cells faced the confined situation and exhibited boosted heat resistance in our 3D thermal ablation model with the recapitulation of ECM and stiffness in vivo. The mechanical confinement from the 3D environment mediated a high proportion of nuclei deformations with activated SP1, triggering the elevation of IL4I1. Moreover, IL4I1-driven tryptophan metabolites activated the AHR pathway and contributed to heat resistance. The involved mechanism inspired a combined regimen of AHR inhibition and thermal ablation, which effectively abrogated the heat resistance and prevented the recurrence of HCC after thermal ablation.
In the animal model of thermal ablation, a narrow region of the tumor tissue spanned a wide range of temperatures when exposed to thermal energy, limiting the investigation of phenotypic and subcellular events under an accurate temperature. The combination of 3D bioprinting and the hyperthermal device was competent to extend the heat distribution with a tailorable configuration of the model size (Figure 2E). The conventional spheroids culture, as the most versatile and commonly used scaffold-free model,49 has been reported to be applied in heat-related studies.50 Cell viability in the conventional spheroids culture was similar to that in the 2D model under different heat treatments. Moreover, the conventional spheroids culture is limited in studying the interaction with extracellular matrix and high-throughput screening for clinical translation.37,51 Despite a plethora of hydrogels to provide an appropriate microenvironment for a 3D in vitro model,52 the collagen-based hydrogel typically used for 3D spheroids culture,53 was prone to disassemble under heat treatments, let alone to achieve tailorable scale (Figure S1C). The hydrogels for 3D bioprinting included the GelMA with LAP crosslinked by UV light,30,31 and the mixture of gelatin and alginate crosslinked by calcium chloride,54 whereas the latter hydrogel was too fragile to operate during thermal ablation. Hence the GCL in our study is more suitable for thermal ablation research, owing to its structural stability during heat treatments. Based on it, a 3D thermal ablation model was developed to replicate the temperature gradients with high resolution and visualize the behaviors of cell survival and motility under precise temperatures. The phenomenon that spheroids detached from the grid-like construct after heat treatment mimicked the first step of metastatic cascade55 (Figure 2H), which was possibly due to the spheroids’ disintegration and MMP2 upregulation (Figures 2J and 2K). In contrast to the 2D model, this 3D model with consecutive heat distribution can screen cell behaviors in situ without the batch variances. To some extent, the 3D model provided a high-throughput potential for personalized medicine.
The 3D bioprinted model can well retain the genomic and transcriptomic states in vivo.56,57,58 In our study, the RNA-seq indicated that 11 metabolic genes were notably expressed higher in both 3D and animal models than in the 2D model (Figure 4D). These genes suggested the metabolic adaptation in the 3D microenvironment and may account for the 2.8°C elevations of LT50 and the 4.1°C elevations of LT90 in the 3D model versus 2D model. We further validated that IL4I1, as the top hit in “Metabolic pathways,” endowed HCC cells with enhanced viability and motility after heat stress. The emerging evidence implies that the other metabolic genes can enhance malignant phenotypes without heat stress. For instance, CYP2J2 promoted the metastasis of colorectal cancer via the epithelial-mesenchymal transition,59 the hypoxia-induced NDUFA4L2 promoted viability and proliferation of HCC,60 and CA9 expression was associated with poor prognosis in several cancers.61,62,63 However, the role of these genes in heat resistance and their regulatory mechanism in 3D condition require further investigation.
Tryptophan metabolism was essential for the generation of an immunosuppressive microenvironment and served as a promising therapeutic target for cancers.64,65 Intriguingly, a recent pan-cancer study manifested that IL4l1 was associated more strongly with AHR activation than any other Trp-catabolic enzymes,39 explaining why the phase III clinical trial of IDO1 inhibitor “Epacadostat” failed.66 Accordingly, IL4I1 may play an important role in the immunosuppressive modulation of HCC under mechanical confinement. Three-dimensional bioprinting enables the construction of complex ecosystems with immune cells, and our 3D thermal ablation model is considered as a suitable candidate for the investigation of the crosstalk between HCC-derived IL4I1 and the immune microenvironment. AHR activation was the consequence of tryptophan degradation. In breast cancer, TDO2-AHR signaling can strengthen metastasis.67 Similarly, our study validated that SP1/IL4I1/AHR axis contributed to enhanced cell mobility of HCC after heat stress, which was significantly abrogated by AHR-targeted inhibition (Figures 7C and 7D). IL4I1 inhibitors are currently in development (see Calithera posters, https://www.calithera.com/preclinical-programs/) and recent patents from MSD (WO2022232333A1), and two AHR inhibitors are in phase I clinical trials (Bayer (NCT04999202) and Ikena Oncology (NCT04200963)). Hence, it is encouraging to apply the IL4I1 inhibitors or AHR inhibitors to clinical trials as a combined regimen with thermal ablation.
In conclusion, this study identified IL4I1 as the key factor that conferred HCC cells with heat resistance under spatial constraints. We highlighted the detrimental effect of mechanical confinement on the recurrence of HCC post thermal ablation. The underlying mechanism provided a strong rationale for AHR inhibitors as neoadjuvant therapy with thermal ablation.
Limitations of the study
Here, we develop a 3D bioprinting-based thermal ablation system to investigate the heat resistance of tumor cells under mechanical confinement. The nude mice are used to establish the animal thermal ablation model as a reference, which helps us get insight into HCC cells per se. IL4I1, as a secreted enzyme, is currently considered a crucial factor modulating the immunosuppressive microenvironment, thus the mechanical confinement is expected to contribute to the HCC recurrence through IL4I1-induced immune suppression. Further development of a 3D bioprinted model can provide a multicellular culture system to interrogate the crosstalk between tumor and immune cells under a 3D environment with high fidelity and controllability.
AHR inhibitor is not effective in suppressing the heat resistance of HCC cells in the 2D model, highlighting that the mechanical confinement-mediated activation of AHR pathway is required for heat resistance. The downstream targets of the AHR pathway included a multitude of known and unknown genes. We noted that at least 20 known AHR downstream targets were upregulated in the 3D model. Additionally, AHR formed a complex with molecular chaperone HSP90, which conservatively protects cells from heat stress.19 Upon ligand binding, AHR dissociates from HSP90 and translocates into the nucleus.68 The interaction between AHR and HSP90 is possibly involved in heat resistance. However, the specific molecular mechanisms by which AHR activation promotes HCC recurrence after thermal ablation warrant further in-depth investigation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-human IL-4I1 | Abcam | Cat#ab222102; RRID: AB_2940764 |
| Rabbit anti-human SP1 | Cell Signaling Technology | Cat#9389S; RRID: AB_2940765 |
| Rabbit anti-human Phospho-SP1 (Thr453) | Affinity | Cat#AF3121; RRID: AB_2834556 |
| Rabbit anti-human GAPDH | Cell Signaling Technology | Cat#5174S; RRID: AB_2940766 |
| Mouse anti-human MMP2 | Proteintech | Cat#66366-1-Ig; RRID: AB_2881746 |
| Mouse anti-human N-Cadherin | Proteintech | Cat#66219-1-Ig; RRID: AB_2881610 |
| Mouse anti-human E-Cadherin | Proteintech | Cat#60335-1-Ig; RRID: AB_2881444 |
| Lamin A | Abcam | Cat#ab26300; RRID: AB_775965 |
| Alexa Fluor 546 goat anti-rabbit IgG (H + L) | Invitrogen | Cat#A-11010; RRID: AB_2534077 |
| Alexa FluorTM 546 Goat anti-Mouse IgG (H + L) Cross-Adsorbed Secondary Antibody | Invitrogen | Cat#A11003; RRID: AB_2534071 |
| DyLight 488 | Invitrogen | Cat#L32470; RRID: AB_2940767 |
| Bacterial and virus strains | ||
| CMV-IL4I1-SV40-EGFP-IRES-Puro | Genecopoeia | Cat#EX-T4715-Lv201 |
| CMV-SP1-SV40-EGFP-IRES-Puro | Genecopoeia | Cat#EX-T2188-Lv201 |
| U6-SP1-SV40-EGFP-IRES-Puro | Genecopoeia | Cat#HSH017640-LVRU6GP-a |
| Biological samples | ||
| HCC patient tissue specimens | the First Affiliated Hospital of Sun Yet-Sen University | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| GelMA Gel | SunP | Cat#SP-BI-G01-2 |
| Lithium phenyl-2,4,6-trimethylbenzoylphosphinate | SunP | Cat#SP-BI-C02-1 |
| 10x DMEM | Sigma-Aldrich | Cat#D2902-10X1L |
| Type I Collagen | IBIDI | Cat#50203 |
| CH-223191 | Selleck | Cat#S7711 |
| Kynurenic acid | Sigma-Aldrich | Cat#K3375 |
| I3A | Sigma-Aldrich | Cat#129445 |
| The Collagen Hybridizing Peptide-5-FAM Conjugate | 3Helix Inc | https://www.3helix.com/product/flu300/ |
| PEG300 | Selleck | Cat#S6704 |
| Triton X-100 | Biosharp | Cat#BS084-100mL |
| Tween 80 | Selleck | Cat#S6702 |
| 3% H2O2 | Sigma-Aldrich | Cat#s9638-1Kg |
| Normal goat serum | Beyotime | Cat#C0265 |
| Primescript™ RT Master Mix (Perfect Real Time) | Takara Bio | Cat#RRO36B |
| SYBR® Premix Ex Taq™ II (Tli RNaseH Plus) | Takara Bio | Cat#RR420A |
| ROX Reference Dye II | Takara Bio | Cat#RR420A |
| Opti-MEM™ I Reduced Serum Medium | Thermo Scientific | Cat#31985070 |
| Puromycin | Solarbio | Cat#IP1280 |
| DMEM | Gibco | Cat#C11995500BT |
| RPMI-1640 | Gibco | Cat#C11875500BT |
| Fetal Bovine Serum (FBS) | Gibco | Cat#A3160801 |
| Penicillin-Streptomycin | Gibco | Cat#10378016 |
| Phosphate Buffered Saline (PBS) | Gibco | Cat#10010001 |
| Dimethyl sulfoxide, DMSO | Solarbio | Cat#D8371 |
| DAPI | Hengsheng | Cat#C0060-DAPI |
| Phalloidin | Sigma-Aldrich | Cat#P5282-.1MG |
| EDTA Antigen Retrieval Solution | Beyotime | Cat#P0085 |
| BCA Protein Assay Kit | Epizyme Biomedical Technology Co., Ltd | Cat#ZJ101 |
| RIPA | Epizyme Biomedical Technology Co., Ltd | Cat#PC101 |
| PhosSTOP™ | Roche | Cat#4906837001 |
| cOmplete™ ULTRA | Roche | Cat#05892970001 |
| 4% paraformaldehyde | Biosharp | Cat#BL539A |
| Critical commercial assays | ||
| Live/dead™ cell imaging kit | Thermo Fisher Scientific | Cat#R37601 |
| NADH-diaphorase staining kit | Genmed | Cat#GMS80039.2 |
| BeyoClick™ EdU Cell Proliferation Kit | Beyotime Biotechnology | Cat#C0071S |
| Picrosirius Red Staining Kit | Abcam | Cat#ab150681 |
| Lipofectamine 3000 transfection kit | Invitrogen | Cat#L3000015 |
| Rneasy Mini Kit | Qiagen | Cat#74104 |
| NovoNGS® CUT&Tag 2.0 High-Sensitivity Kit | NovoProtein | Cat#N259-YH01 |
| Deposited data | ||
| Data files for RNA-sequencing | This paper | GEO: GSE235351 |
| Experimental models: Cell lines | ||
| MHCC97-H cell line | Cell Bank, Chinese Academy of Sciences | RRID: CVCL_4972 |
| SNU-449 cell line | Cell Bank, Chinese Academy of Sciences | RRID: CVCL_0454 |
| Huh7 cell line | Cell Bank, Chinese Academy of Sciences | RRID: CVCL_B7TI |
| PLC/PRF/5 cell line | Cell Bank, Chinese Academy of Sciences | RRID: CVCL_0485 |
| Experimental models: Organisms/strains | ||
| BALB/cNj-Foxn1nu/Gpt | GemPharmatech | Strain NO. D000521 |
| Oligonucleotides | ||
| Primers for PCR, see Table S4 | This paper | N/A |
| Software and algorithms | ||
| ImageJ | Open source | https://imagej.net/ij/index.html |
| GraphPad Software 9.0 | GraphPad Software | https://www.graphpad.com/ |
| Qupath v0.3.0 | Open source | https://qupath.github.io/ |
| KOBAS-i | Bu et al.69 | http://kobas.cbi.pku.edu.cn/ |
| OmicStudio tools | Lianchuan Biotechnology Co., Ltd | https://www.omicstudio.cn/tool |
| Home-for-Researchers | N/A | https://www.aclbi.com/static/index.html#/batch |
| GEPIA2 | Tang et al.70 | http://gepia2.cancer-pku.cn/#index |
| PROMO | Messeguer et al.41 | https://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3 |
| The Signaling Pathways Project database | N/A | https://www.signalingpathways.org/index.jsf |
| TTRUST | Han et al.42 | https://www.grnpedia.org/trrust/ |
| JASPAR database | N/A | https://jaspar.genereg.net/ |
| Other | ||
| 3D bioprinter | SunP, China | N/A |
| Infrared camera | Magnity Electronics Co. Ltd., China | MAG32 |
| Ultra-low attachment 96-well plate | Corning, USA | Cat#CLS3474-24EA |
| MWA machine | Surblate, Vison Medical, USA | N/A |
| IVIS Spectrum | Perkin Elmer | Cat#124262 |
| Discovery Hybrid Rheometer | TA Instruments, USA | N/A |
| Piuma Nanoindenter Instruments | Optics11, Netherlands | N/A |
| Scanning electron microscope | TESCAN MIRA, Czech Republic | TESCAN CLARA |
| Automatic inverted fluorescence microscope | OLYMPUS, Japan | IX83 |
| KF-Pro-020 pathological section scanner | KFBIO, China | N/A |
| Confocal microscope | Olympus, Japan | FV3000 |
| Ultra-performance liquid chromatography | Shimadzu Nexera Series, Japan | N/A |
| Mass Spectrometer | SCIEX Triple Quad 7500 mass spectrometer, USA | N/A |
Resource availability
Lead contact
Requests for resources, reagents, and further information should be directed to and will be addressed by the lead contact, Ming Kuang (kuangm@mail.sysu.edu.cn).
Materials availability
All materials generated in this study will be provided upon request to the lead contact.
Experimental model and subject details
Cell lines
The human HCC cell lines of MHCC-97H, SNU-449, Huh7, and PLC/PRF/5 were acquired from Cell Bank, Chinese Academy of Sciences (Shanghai, China). All cell lines were proved free from mycoplasma contamination and identified by short tandem repeat (STR) analyses. MHCC-97H, Huh7, and PLC/PRF/5 were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (C11995500BT, Gibco, USA) containing 10% fetal bovine serum (A3160801, Gibco, USA), 1% streptomycin and penicillin (10378016, Gibco, USA). SNU-449 were cultured in RPMI-1640 (C11875500BT, Gibco, USA) supplemented with 10% fetal bovine serum (Gibco, USA), 1% streptomycin, and penicillin. All cell lines were cultured in a humidified atmosphere with 5% CO2 at 37°C.
Mouse studies
Animal experiments were approved by the Institutional Care and Animal Use Committee of the First Affiliated Hospital, Sun Yat-sen University, following the Reporting of In Vivo Experiments (ARRIVE) guidelines (Approval No. [2021]034). The animal model was based on the protocol reported in our previous research.23 3-week-old BALB/C nude male mice were purchased from GemPharmatech (Jiangsu, China) and housed in a specific pathogen-free facility. 2 × 106/mL MHCC-97H in 20ul PBS were injected into the orthotopic livers. All the mice with orthotopic tumors were randomly divided into the non-treatment (Non-treated), ablation monotherapy (Ablation), and combined ablation and CH-223191 (10 mg/kg/d) (Ablation+CH-223191) groups. After 3-week growth of orthotopic tumors, the electrode of the MWA machine (Surblate, Vison Medical, USA) was eccentrically inserted into the tumors and generated heat with the power of 15 W for 30 s. An infrared camera was used to monitor the temperature. All the drugs were administered by oral gavage with an oral feeding needle 8 h before the ablation up to the following 3 weeks. Bioluminescence imaging was performed to monitor tumor burden before and after thermal ablation once a week by IVIS Spectrum (Perkin Elmer). After sacrifice, livers and tumors were removed and imaged. The tumor volumes were calculated by using the formula: volume (mm3) = π/6 × length × width.2
Human specimens
Formalin-fixed paraffin-embedded (FFPE) tissue specimens were obtained from HCC patients who underwent radical resection or biopsies before thermal ablation. All patients provided written informed consent. All studies involving human beings were approved by the Institutional Ethical Board of the First Affiliated Hospital of Sun Yet-Sen University (Approval No. [2021]245).
Method details
IL4I1 overexpression
A human IL4I1 cDNA clone was recombined into the lentivirus vector pReceiver-LV201 (fulengen, Inc). HEK-293 T cells were co-transfected with lentiviral vector, Δ 8.9, and VSVG using Lipofectamine 3000 transfection kit (Invitrogen) to produce lentiviral particles. Stable cell lines were generated by infecting MHCC-97H, Huh7, and PLC/PRF/5 with lentivirus containing IL4I1 or nonspecific control. Puromycin was applied to select stable cell clones.
3D bioprinting and culture
GelMA (SP-BI-G01-2, SunP, China) was dissolved in culture medium at 60°C in the water bath, generating the final concentration of 12.5% w/v. 10x DMEM was dissolved in sterilized water with excess NaHCO3. GelMA, lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) (SP-BI-C02-1, SunP, China), 10x DMEM (D2902-10X1L, Sigma, USA), Type I Collagen (50203, IBIDI, Germany), and cell suspensions were mixed in the proportion of 8:1:0.9:9:1 to obtain the bioink containing 5% w/v GelMA, 2.5 mg/ml Collagen I, and 1 × 107/mL cell lines. The bioink was loaded into a sterilized syringe with a 25-gauge needle. The nozzle temperature of a 3D printer (SunP, China) was set at 18°C. The 3D models were printed into a grid-like structure with an extrusion speed of 0.5 mm3/s, which were subsequently photocrosslinked by UV light for 30s to stabilize the structure. The respective medium was immediately added after 3D bioprinting for cell culture and replaced every 2 days.
Heat treatment
When HCC spheroids were generated in 3D bioprinted models, the printed 3D constructs were encapsulated with GCL hydrogel before heat treatment to prevent the spheroids' detachment from the constructs. The water bath, the electrode of microwave ablation (MWA) equipment, and the homemade hyperthermal device were employed to treat the 3D model with heat for 15 min, respectively. An infrared camera (MAG32, Magnity Electronics Co. Ltd., China) was used to monitor temperature gradients. For the 2D model and conventional spheroids formation, cells in a 6cm Petri dish and spheroids in an ultra-low attachment 96-well plate (7007, Corning, USA) were exposed to different temperatures in the water bath for 15 min. For the functional experiments, the medium was replaced with respective reagents 8 h before heat treatment.
Rheological measurement
According to the manufacturer’s instructions, the rheological characteristics of the GCL hydrogel were measured by Discovery Hybrid Rheometer (TA Instruments, USA). Briefly, the GCL hydrogel was prepared before the above procedure of 3D bioprinting and deposited onto the rheometer Peltier plate. Oscillation measurements were performed to acquire the storage modulus (G′) and loss modulus (G”) under different temperatures at 1 Hz frequency of and 0.1% strain amplitude.
Mechanical property detection
After the heat treatment in the 3D model and the conduction of thermal ablation in the animal model, Piuma Nanoindenter Instruments (Optics11, Netherlands) was employed to measure the stiffness of the 3D constructs and tumor tissues, using a silicon nitride cantilever probe with an elastic constant of 0.48N/m and a borosilicate glass ball with 254um radius. The effective Young’s modulus was calculated by the built-in Hertz model. At least five different positions of the same region were measured for each sample.
Microstructure detection
To examine the microstructure of 3D models under different temperatures, samples were gradually dehydrated in ethanol and followed by vacuum freeze-drying overnight. The dried samples were then coated with gold and imaged by a scanning electron microscope (TESCAN MIRA, Czech Republic).
Live and dead analyses
Live and dead analyses in 2D and 3D models were determined one day after heat treatment. Cells in the 2D model were trypsinized, stained with trypan blue, and counted by Countstar Rigel (ALIT Life Sciences, Shanghai, China). Cell viability in the 3D model was determined by the Live/dead cell imaging kit (R37601, Thermo Fisher Scientific, America) according to the manufacturer’s instructions. Briefly, samples were incubated with dye reagents at room temperature for 15 min in the dark. Images were captured immediately under an automatic inverted fluorescence microscope (IX83, OLYMPUS, Japan) and analyzed by ImageJ software. LT50 was calculated by GraphPad Prism 9.0.
Cell proliferation assay
BeyoClick EdU Cell Proliferation Kit (C0071S, Beyotime Biotechnology, China) was applied to detect the proliferation of HCC cell lines in both 2D and 3D models following the manufacturer’s instructions. The samples were labeled with EdU for 2 h and fixed with 4% paraformaldehyde. After incubation with the dying solution, samples were counter-stained with Hoechst 33342. Images were captured immediately under an automatic inverted fluorescence microscope (IX83, OLYMPUS, Japan). Quantitative analyses were performed by ImageJ software.
Picrosirius red staining
A Picrosirius Red Staining Kit (ab150681, Abcam) was employed to reveal the fibrillar collagen of paraffinized tumor sections in the animal model of thermal ablation. The sections were scanned by KF-Pro-020 pathological section scanner (KFBIO, China).
Nicotinamide adenine dinucleotide diaphorase (NADH-diaphorase) staining
To assess the viability of tumor tissues in the animal model post thermal ablation, frozen sections were obtained and stained by an NADH-diaphorase staining kit (GMS80039.2, Genmed, China). The vital cells in situ were stained purple. The sections were scanned by KF-Pro-020 pathological section scanner (KFBIO, China).
Immunohistochemistry
The sections of formalin-fixed and paraffin-embedded tumor tissues were dewaxed and subjected to heat-mediate antigen retrieval using EDTA Antigen Retrieval Solution (Beyotime, China). After incubation of 3% H2O2 to inactivate endogenous peroxidase for 15 min and 10% normal goat serum to block nonspecific sites for 30 min, sections were incubated with antibodies against IL4I1 (1:100, ab222102, Abcam), SP1 (1:100, 9389S, CST) at 4°C overnight. Sections were then stained with K5007Dako REAL EnVision detection kit for 45 min and visualized using 3,3′-diaminobenzidine (DAB, K3468, DAKO, Denmark). The images were obtained by KF-Pro-020 pathological section scanner (KFBIO, China).
Collagen hybridizing peptide staining
The Collagen Hybridizing Peptide-5-FAM Conjugate (CHP, 3Helix Inc, USA) was diluted to 20 μM in PBS, which was treated with 80°C in the water bath for 5 min and quickly quenched to room temperature. Sections were then incubated with CHP solution at 4°C overnight. After washed with PBS, CHP was detected by a confocal microscope (FV3000, Olympus, Japan). The gain and offset values of all images were obtained under standard conditions.
Immunofluorescence staining
3D constructs were fixed with 4% paraformaldehyde for 1 h at room temperature followed by permeabilization with 0.1% Triton X-100 for 10 min. After incubation with the blocking solution of normal goat serum (C-0005, Bioss, China) for 2 h at room temperature, the primary antibodies against human N-cadherin (ab18203, Abcam, England), E-cadherin (60335-1-Ig, Proteintech, USA), lamin A (Abcam, ab26300) were added and incubated at 4°C overnight. After washed with PBS, the constructs were incubated with corresponding secondary antibodies: Alexa Fluor 546 goat anti-rabbit IgG (H + L) (2086712, Invitrogen, USA), Alexa Fluor 546 goat anti-mouse IgG (H + L) (A11003, Invitrogen, USA) or DyLight 488 (Invitrogen, SA5-10110) at room temperature for 3 h and followed by three washes in PBS. The cytoskeleton and nuclei were stained with phalloidin (P5282-.1MG, Sigma, USA) and DAPI (Hengsheng, C0060-DAPI). Immunofluorescence images were captured by a confocal microscope (FV3000, Olympus, Japan). The nuclear roundness was calculated by ImageJ software.
RNA sequencing and bioinformatic analysis
RNA of the 2D, 3D, and animal models were extracted using Rneasy Mini Kit (74104, Qiagen, USA). The RNA samples were transported to Lianchuan Biotechnology Co., Ltd for libraries’ generation and sequencing. Analysis of differentially expressed genes was conducted by the DESeq2 package in the R programming language (R version 4.1.0). The KEGG pathway enrichment was conducted by the website tool KOBAS-i69 (http://kobas.cbi.pku.edu.cn/). GSEA enrichment was conducted and plotted by the OmicStudio tools (https://www.omicstudio.cn/tool). The survival analysis was performed by the website tool Home-for-Researchers (https://www.aclbi.com/static/index.html#/batch). Gene (set) correlation was conducted by the bioinformatic tool GEPIA270 (http://gepia2.cancer-pku.cn/#index).
Chromatin immunoprecipitation (ChIP)
The chromatin immunoprecipitation assay was performed using the NovoNGS CUT&Tag 2.0 High-Sensitivity Kit (NovoProtein, N259-YH01). In brief, cells were trypsinized and enriched by ConA-magnetic beads. Primary antibody against human SP1 (1:50, 9389S, CST) was added in cell suspension and incubated overnight at 4°C. Beads were washed and incubated with secondary antibody for 1 h at a concentration of 10ug/ml. After washed in Dig-Hisalt Buffer, the beads were incubated with proteinA-Tn5 transposome in ChiTag buffer for 1 h at room temperature. Next, cells were mixed in Tagmentation buffer for 1 h at 37°C. The tagmentation reaction was stopped by adding 10% SDS at 55°C for 10 min. Tagment DNA was extracted by Extract Beads and subsequently used to conduct PCR or qRT-PCR.
Quantitative real-time PCR (qRT-PCR)
The RNA or DNA was acquired as previously described. For RNA, reverse transcription was performed by M-MLV reverse transcriptase (Life Technologies, Grand Island, NY). Our primer sequences were shown in Table S4. Real-time PCR was performed with SYBR Green PCR Master Mix (Shanghai, China) on the Thermo Fisher QuantStudio 5 QS instrument. GAPDH was adopted as a reference gene. The relative expression was calculated by the established 2-ΔΔCt or 2-ΔCt method.
Western blot assay
3D constructs were fully homogenized in a tissue grinder with lysis buffer to obtain protein. Protein samples were separated in SDS-PAGE gels and then transferred onto the methanol-activated PVDF membrane (IPVH00010, Millipore, USA). After blocked with 5% non-fat milk in TBS containing 0.1% Tween 20 (TBST), the membrane was incubated with corresponding primary antibodies (see key resources table) at 4°C overnight. After three washes in TBST, the membrane was incubated with the secondary antibody at room temperature for 2 h and detected by Omni-ECL ultra-sensitive chemiluminescence detection kit (SQ201, EpiZyme, USA).
Transwell assay and colony formation
Transwell assay was performed as previously described.36 In short, the MHCC-97H cells, Huh7 cells, and PLC/PRF/5 were heated for 15 min in a water bath at 45°C one day before the transwell assay. DMEM medium with 20% fetal bovine serum (FBS, A3160801, Gibco, USA) was added to the lower chamber. Cell suspension (1×105 cells/well) with 300ul DMEM medium with 1% FBS was placed into the upper chamber. Kynurenic acid (KynA, 250μM, K3375, Sigma-Aldrich, USA), indole-3-aldehyde (I3A, 50μM, 129445, Sigma-Aldrich, USA) or CH223191 (20μM for 2D model and 100μM for 3D model, S7711, Selleck, USA) were added to both lower and upper chamber. MHCC-97H, Huh7, and PLC/PRF/5 were cultured at 37°C for 72 h, 12h, and 24h respectively. Images were captured under a microscope (IX83, OLYMPUS, Japan). The same procedures were applied for the invasion assay, except that the chamber was precoated with Matrigel (Corning, USA). For colony formation assay, cells were seeded at a density of 4×103 cells/well and pretreated with corresponding reagents for 8 h. After heat treatment, cells were cultured in the complete medium with corresponding reagents for 14 days.
LC-MS/MS analysis
The selected tryptophan metabolites I3A and KynA were identified by Liquid Chromatograph-Mass Spectrometer (LC-MS) system consisting of an UPLC (Shimadzu Nexera Series, Japan) connected to an MS (SCIEX Triple Quad 7500 mass spectrometer, USA). 3 × 105cells per 6cm dish were seeded into 5mL complete DMEM and incubated for 4 days, and the conditional media were collected and stored at −80°C. 50mM stock solutions were prepared by adding DMSO to the required amount of I3A and KynA compounds. The stock solutions were diluted with methanol (for LC-MS) to gain the standard sample stocks covering a concentration range from 3 to 150μM. For the quantification of I3A and KynA, 1μL standard sample stocks were added into 300μL cell culture media to gain the standard samples. Subsequently, 300μL acetonitrile was added into 300μL conditional media and the standard samples to precipitate the components from the media, followed by 8000g for 5 min. 150μL supernatants were transferred into 1.5mL glass vials with 150μL inserts. 2μL of the sample was injected for the analysis. The mobile phase for LC analysis consisted of two solutions: A (0.1% formic acid in water) and B (acetonitrile). The chromatography was performed on a Synergi 4μm Fusion-RP column (50 mm × 2 mm×4μm, Synergi 4U Fusion-RP 80A, USA) with a flow rate of 0.3 mL/min at 35°C. The mobile phase gradient program started at 5% of B, 75% of B at 4 min–6 min, then 2% of B at 6.1 min and held for 8min. The MS instrument was operated in the negative ESI mode with a capillary voltage of 1500V. The source temperature was set at 450°C, Ion source gas 1-40psi, Ion source gas 2-50psi, Curtain gas-40 psi, CAD gas-8. The ions were monitored in Multiple Reaction Monitoring (MRM) mode. The SCIEX OS 2.2.0 Analytics was used for data acquisition and analyses.
Quantification and statistical analysis
Nuclear roundness calculation and survival analysis
The nuclear roundness was calculated to quantify and categorize deformation by a semi-automated image process in ImageJ software. For immunofluorescence in the 2D and 3D models, the DAPI signal intensity was applied to segment and outline the nuclei from the background. For clinical specimens, hematoxylin staining was applied to outline the nuclei. The Particle analysis feature was used to obtain the area and perimeter of nuclei. The nuclear roundness was calculated using the formula: roundness = 4π × Area/Perimeter.2 For survival analysis of clinical specimens, the roundness of 0.75 was defined as a cutoff to identify high or low nuclear deformation under the supervision of a qualified pathologist. Survival curves of groups with the low and high nucleus deformations were plotted using the Kaplan-Meier method.
Statistical analysis
The data were analyzed by GraphPad Prism 9.0 and presented with median ±standard deviation (SD). Unless stated otherwise, p values were calculated using a two-tailed Student’s t test between two groups, one-way ANOVA with post Tukey’s comparisons test among multiple groups. p < 0.05 were considered statistically significant. Specific sample size (n) and p value were noted in the text of figure legends.
Acknowledgments
This work is supported by the Joint Funds of the National Natural Science Foundation of China (no. U20A20370), the State Key Program of National Natural Science of China (no. 82130083), the National Science Fund for Distinguished Young Scholars (no. 81825013), the Key Science and Technology Program of Guangzhou (nos. 202206080016 and 202103000076), the National Natural Science Foundation of China (nos. 32101062 and 31970870), and the Guangdong Basic and Applied Basic Research Foundation (nos. 2019A1515110005 and 2022A1515012607).
Author contributions
M.K., C.L., J.W., and G.P.Z. conceived the study. G.P.Z., Z.L.X., and J.J. performed most of the experiments and analyzed the results. Y.T.Z., K.L., and L.Z.L. contributed to 3D model construction. S.L.C., T.H.S., and L.T. collected and analyzed clinical data. S.P. made technical and intellectual contributions. G.P.Z., Y.T.Z., and J.J. wrote the original draft. J.W., C.L., and M.K. supervised the study and reviewed the manuscript. All authors read and approved the manuscript.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: July 20, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2023.101128.
Contributor Information
Ji Wang, Email: wangj683@mail.sysu.edu.cn.
Chun Liu, Email: liuch393@mail.sysu.edu.cn.
Ming Kuang, Email: kuangm@mail.sysu.edu.cn.
Supplemental information
Data and code availability
The RNA sequencing data of HCC cells in the 2D, 3D, and animal models are publicly deposited at Gene Expression Omnibus (GEO accession number: GSE235351). This study does not report custom computer code. Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.
References
- 1.Nader G.P.d.F., Agüera-Gonzalez S., Routet F., Gratia M., Maurin M., Cancila V., Cadart C., Palamidessi A., Ramos R.N., San Roman M., et al. Compromised nuclear envelope integrity drives TREX1-dependent DNA damage and tumor cell invasion. Cell. 2021;184:5230–5246.e22. doi: 10.1016/j.cell.2021.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Lomakin A.J., Cattin C.J., Cuvelier D., Alraies Z., Molina M., Nader G.P.F., Srivastava N., Sáez P.J., Garcia-Arcos J.M., Zhitnyak I.Y., et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science. 2020;370 doi: 10.1126/science.aba2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denais C.M., Gilbert R.M., Isermann P., McGregor A.L., te Lindert M., Weigelin B., Davidson P.M., Friedl P., Wolf K., Lammerding J. Nuclear envelope rupture and repair during cancer cell migration. Science. 2016;352:353–358. doi: 10.1126/science.aad7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stowers R.S., Shcherbina A., Israeli J., Gruber J.J., Chang J., Nam S., Rabiee A., Teruel M.N., Snyder M.P., Kundaje A., Chaudhuri O. Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat. Biomed. Eng. 2019;3:1009–1019. doi: 10.1038/s41551-019-0420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nava M.M., Miroshnikova Y.A., Biggs L.C., Whitefield D.B., Metge F., Boucas J., Vihinen H., Jokitalo E., Li X., García Arcos J.M., et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell. 2020;181:800–817.e22. doi: 10.1016/j.cell.2020.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park J.S., Burckhardt C.J., Lazcano R., Solis L.M., Isogai T., Li L., Chen C.S., Gao B., Minna J.D., Bachoo R., et al. Mechanical regulation of glycolysis via cytoskeleton architecture. Nature. 2020;578:621–626. doi: 10.1038/s41586-020-1998-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.Zhang D.Y., Friedman S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balog S., Li Y., Ogawa T., Miki T., Saito T., French S.W., Asahina K. Development of Capsular Fibrosis Beneath the Liver Surface in Humans and Mice. Hepatology. 2020;71:291–305. doi: 10.1002/hep.30809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su J., Morgani S.M., David C.J., Wang Q., Er E.E., Huang Y.H., Basnet H., Zou Y., Shu W., Soni R.K., et al. TGF-β orchestrates fibrogenic and developmental EMTs via the RAS effector RREB1. Nature. 2020;577:566–571. doi: 10.1038/s41586-019-1897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisso A., Filipuzzi M., Gamarra Figueroa G.P., Brumana G., Biagioni F., Doni M., Ceccotti G., Tanaskovic N., Morelli M.J., Pendino V., et al. Cooperation Between MYC and β-Catenin in Liver Tumorigenesis Requires Yap/Taz. Hepatology. 2020;72:1430–1443. doi: 10.1002/hep.31120. [DOI] [PubMed] [Google Scholar]
- 12.Yuan W.C., Pepe-Mooney B., Galli G.G., Dill M.T., Huang H.T., Hao M., Wang Y., Liang H., Calogero R.A., Camargo F.D. NUAK2 is a critical YAP target in liver cancer. Nat. Commun. 2018;9:4834. doi: 10.1038/s41467-018-07394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu K.F., Dupuy D.E. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat. Rev. Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 14.Lau W.Y., Lai E.C.H. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann. Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu. European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Cucchetti A., Piscaglia F., Cescon M., Colecchia A., Ercolani G., Bolondi L., Pinna A.D. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J. Hepatol. 2013;59:300–307. doi: 10.1016/j.jhep.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Lee D.H., Lee J.M., Lee J.Y., Kim S.H., Yoon J.H., Kim Y.J., Han J.K., Choi B.I. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Pastor R., Burchfiel E.T., Thiele D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19:4–19. doi: 10.1038/nrm.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo M., Meng Z., Moroishi T., Lin K.C., Shen G., Mo F., Shao B., Wei X., Zhang P., Wei Y., Guan K.L. Heat stress activates YAP/TAZ to induce the heat shock transcriptome. Nat. Cell Biol. 2020;22:1447–1459. doi: 10.1038/s41556-020-00602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Su T., Huang M., Liao J., Lin S., Yu P., Yang J., Cai Y., Zhu S., Xu L., Peng Z., Peng S., Chen S., Kuang M. Insufficient Radiofrequency Ablation Promotes Hepatocellular Carcinoma Metastasis Through N6-Methyladenosine mRNA Methylation-Dependent Mechanism. Hepatology. 2021;74:1339–1356. doi: 10.1002/hep.31766. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Bei J., Liu M., Huang J., Xie L., Huang W., Cai M., Guo Y., Lin L., Zhu K. Sublethal heat stress-induced O-GlcNAcylation coordinates the Warburg effect to promote hepatocellular carcinoma recurrence and metastasis after thermal ablation. Cancer Lett. 2021;518:23–34. doi: 10.1016/j.canlet.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Zeng X., Liao G., Li S., Liu H., Zhao X., Li S., Lei K., Zhu S., Chen Z., Zhao Y., et al. Eliminating METTL1-mediated accumulation of PMN-MDSCs prevents HCC recurrence after radiofrequency ablation. Hepatology. 2023;77:1122–1138. doi: 10.1002/hep.32585. [DOI] [PubMed] [Google Scholar]
- 23.Tan L., Chen S., Wei G., Li Y., Liao J., Jin H., Zou Y., Huang M., Peng Z., Guo Y., Peng S., Xu L., Kuang M. Sublethal heat treatment of hepatocellular carcinoma promotes intrahepatic metastasis and stemness in a VEGFR1-dependent manner. Cancer Lett. 2019;460:29–40. doi: 10.1016/j.canlet.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 24.Frese K.K., Tuveson D.A. Maximizing mouse cancer models. Nat. Rev. Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 25.Daly A.C., Prendergast M.E., Hughes A.J., Burdick J.A. Bioprinting for the Biologist. Cell. 2021;184:18–32. doi: 10.1016/j.cell.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedell M.L., Navara A.M., Du Y., Zhang S., Mikos A.G. Polymeric Systems for Bioprinting. Chem. Rev. 2020;120:10744–10792. doi: 10.1021/acs.chemrev.9b00834. [DOI] [PubMed] [Google Scholar]
- 27.Meng F., Meyer C.M., Joung D., Vallera D.A., McAlpine M.C., Panoskaltsis-Mortari A. 3D Bioprinted In Vitro Metastatic Models via Reconstruction of Tumor Microenvironments. Adv. Mater. 2019;31 doi: 10.1002/adma.201806899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolesky D.B., Truby R.L., Gladman A.S., Busbee T.A., Homan K.A., Lewis J.A. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv. Mater. 2014;26:3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 29.Pi Q., Maharjan S., Yan X., Liu X., Singh B., van Genderen A.M., Robledo-Padilla F., Parra-Saldivar R., Hu N., Jia W., et al. Digitally Tunable Microfluidic Bioprinting of Multilayered Cannular Tissues. Adv. Mater. 2018;30 doi: 10.1002/adma.201706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W., Zhang Y.S., Heinrich M.A., De Ferrari F., Jang H.L., Bakht S.M., Alvarez M.M., Yang J., Li Y.C., Trujillo-de Santiago G., et al. Rapid Continuous Multimaterial Extrusion Bioprinting. Adv. Mater. 2017;29:1604630. doi: 10.1002/adma.201604630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heinrich M.A., Bansal R., Lammers T., Zhang Y.S., Michel Schiffelers R., Prakash J. 3D-Bioprinted Mini-Brain: A Glioblastoma Model to Study Cellular Interactions and Therapeutics. Adv. Mater. 2019;31 doi: 10.1002/adma.201806590. [DOI] [PubMed] [Google Scholar]
- 32.Ma X., Yu C., Wang P., Xu W., Wan X., Lai C.S.E., Liu J., Koroleva-Maharajh A., Chen S. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials. 2018;185:310–321. doi: 10.1016/j.biomaterials.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao S., He J., Zhao Y., Liu T., Xie F., Yang H., Mao Y., Pang Y., Sun W. Bioprinting of patient-derived in vitro intrahepatic cholangiocarcinoma tumor model: establishment, evaluation and anti-cancer drug testing. Biofabrication. 2020;12 doi: 10.1088/1758-5090/aba0c3. [DOI] [PubMed] [Google Scholar]
- 34.Hwang J., Huang Y., Burwell T.J., Peterson N.C., Connor J., Weiss S.J., Yu S.M., Li Y. In Situ Imaging of Tissue Remodeling with Collagen Hybridizing Peptides. ACS Nano. 2017;11:9825–9835. doi: 10.1021/acsnano.7b03150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger A.J., Linsmeier K.M., Kreeger P.K., Masters K.S. Decoupling the effects of stiffness and fiber density on cellular behaviors via an interpenetrating network of gelatin-methacrylate and collagen. Biomaterials. 2017;141:125–135. doi: 10.1016/j.biomaterials.2017.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su T., Liao J., Dai Z., Xu L., Chen S., Wang Y., Peng Z., Zhang Q., Peng S., Kuang M. Stress-induced phosphoprotein 1 mediates hepatocellular carcinoma metastasis after insufficient radiofrequency ablation. Oncogene. 2018;37:3514–3527. doi: 10.1038/s41388-018-0169-4. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues T., Kundu B., Silva-Correia J., Kundu S.C., Oliveira J.M., Reis R.L., Correlo V.M. Emerging tumor spheroids technologies for 3D in vitro cancer modeling. Pharmacol. Ther. 2018;184:201–211. doi: 10.1016/j.pharmthera.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Swaminathan S., Hamid Q., Sun W., Clyne A.M. Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication. 2019;11 doi: 10.1088/1758-5090/aafc49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sadik A., Somarribas Patterson L.F., Öztürk S., Mohapatra S.R., Panitz V., Secker P.F., Pfänder P., Loth S., Salem H., Prentzell M.T., et al. IL4I1 Is a Metabolic Immune Checkpoint that Activates the AHR and Promotes Tumor Progression. Cell. 2020;182:1252–1270.e34. doi: 10.1016/j.cell.2020.07.038. [DOI] [PubMed] [Google Scholar]
- 40.Zhao H., Teng Y., Hao W., Li J., Li Z., Chen Q., Yin C., Yue W. Single-cell analysis revealed that IL4I1 promoted ovarian cancer progression. J. Transl. Med. 2021;19:454. doi: 10.1186/s12967-021-03123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Messeguer X., Escudero R., Farré D., Núñez O., Martínez J., Albà M.M. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–334. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 42.Han H., Cho J.W., Lee S., Yun A., Kim H., Bae D., Yang S., Kim C.Y., Lee M., Kim E., et al. TRRUST v2: an expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018;46 doi: 10.1093/nar/gkx1013. D380-d386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Llovet J.M., Montal R., Sia D., Finn R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018;15:599–616. doi: 10.1038/s41571-018-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dean J.W., Zhou L. Cell-intrinsic view of the aryl hydrocarbon receptor in tumor immunity. Trends Immunol. 2022;43:245–258. doi: 10.1016/j.it.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye L.Y., Chen W., Bai X.L., Xu X.Y., Zhang Q., Xia X.F., Sun X., Li G.G., Hu Q.D., Fu Q.H., Liang T.B. Hypoxia-Induced Epithelial-to-Mesenchymal Transition in Hepatocellular Carcinoma Induces an Immunosuppressive Tumor Microenvironment to Promote Metastasis. Cancer Res. 2016;76:818–830. doi: 10.1158/0008-5472.CAN-15-0977. [DOI] [PubMed] [Google Scholar]
- 46.Han Y., Chen Z., Yang Y., Jiang Z., Gu Y., Liu Y., Lin C., Pan Z., Yu Y., Jiang M., Zhou W., Cao X. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology. 2014;59:567–579. doi: 10.1002/hep.26694. [DOI] [PubMed] [Google Scholar]
- 47.Li W., Liang Y., Yang B., Sun H., Wu W. Downregulation of ARNT2 promotes tumor growth and predicts poor prognosis in human hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2015;30:1085–1093. doi: 10.1111/jgh.12905. [DOI] [PubMed] [Google Scholar]
- 48.Hsu S.H., Wang L.T., Chai C.Y., Wu C.C., Hsi E., Chiou S.S., Wang S.N. Aryl hydrocarbon receptor promotes hepatocellular carcinoma tumorigenesis by targeting intestine-specific homeobox expression. Mol. Carcinog. 2017;56:2167–2177. doi: 10.1002/mc.22658. [DOI] [PubMed] [Google Scholar]
- 49.Zanoni M., Cortesi M., Zamagni A., Arienti C., Pignatta S., Tesei A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020;13:97. doi: 10.1186/s13045-020-00931-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brüningk S.C., Rivens I., Box C., Oelfke U., Ter Haar G. 3D tumour spheroids for the prediction of the effects of radiation and hyperthermia treatments. Sci. Rep. 2020;10:1653. doi: 10.1038/s41598-020-58569-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knowlton S., Onal S., Yu C.H., Zhao J.J., Tasoglu S. Bioprinting for cancer research. Trends Biotechnol. 2015;33:504–513. doi: 10.1016/j.tibtech.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 52.Rodrigues J., Heinrich M.A., Teixeira L.M., Prakash J. 3D In Vitro Model (R)evolution: Unveiling Tumor-Stroma Interactions. Trends Cancer. 2021;7:249–264. doi: 10.1016/j.trecan.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Liu C., Lewin Mejia D., Chiang B., Luker K.E., Luker G.D. Hybrid collagen alginate hydrogel as a platform for 3D tumor spheroid invasion. Acta Biomater. 2018;75:213–225. doi: 10.1016/j.actbio.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H., Sun L., Pang Y., Hu D., Xu H., Mao S., Peng W., Wang Y., Xu Y., Zheng Y.C., et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut. 2021;70:567–574. doi: 10.1136/gutjnl-2019-319960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang H.F., Hughes C.S., Li W., He J.Z., Surdez D., El-Naggar A.M., Cheng H., Prudova A., Delaidelli A., Negri G.L., et al. Proteomic Screens for Suppressors of Anoikis Identify IL1RAP as a Promising Surface Target in Ewing Sarcoma. Cancer Discov. 2021;11:2884–2903. doi: 10.1158/2159-8290.Cd-20-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xie F., Sun L., Pang Y., Xu G., Jin B., Xu H., Lu X., Xu Y., Du S., Wang Y., et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials. 2021;265 doi: 10.1016/j.biomaterials.2020.120416. [DOI] [PubMed] [Google Scholar]
- 57.Tang M., Xie Q., Gimple R.C., Zhong Z., Tam T., Tian J., Kidwell R.L., Wu Q., Prager B.C., Qiu Z., et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 2020;30:833–853. doi: 10.1038/s41422-020-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diao J., Zhang C., Zhang D., Wang X., Zhang J., Ma C., Deng K., Jiang T., Jia W., Xu T. Role and mechanisms of a three-dimensional bioprinted microtissue model in promoting proliferation and invasion of growth-hormone-secreting pituitary adenoma cells. Biofabrication. 2019;11 doi: 10.1088/1758-5090/aaf7ea. [DOI] [PubMed] [Google Scholar]
- 59.Kong C., Yan X., Zhu Y., Zhu H., Luo Y., Liu P., Ferrandon S., Kalady M.F., Gao R., He J., et al. Fusobacterium Nucleatum Promotes the Development of Colorectal Cancer by Activating a Cytochrome P450/Epoxyoctadecenoic Acid Axis via TLR4/Keap1/NRF2 Signaling. Cancer Res. 2021;81:4485–4498. doi: 10.1158/0008-5472.Can-21-0453. [DOI] [PubMed] [Google Scholar]
- 60.Lai R.K.H., Xu I.M.J., Chiu D.K.C., Tse A.P.W., Wei L.L., Law C.T., Lee D., Wong C.M., Wong M.P., Ng I.O.L., Wong C.C.L. NDUFA4L2 Fine-tunes Oxidative Stress in Hepatocellular Carcinoma. Clin. Cancer Res. 2016;22:3105–3117. doi: 10.1158/1078-0432.CCR-15-1987. [DOI] [PubMed] [Google Scholar]
- 61.Hyuga S., Wada H., Eguchi H., Otsuru T., Iwgami Y., Yamada D., Noda T., Asaoka T., Kawamoto K., Gotoh K., et al. Expression of carbonic anhydrase IX is associated with poor prognosis through regulation of the epithelialmesenchymal transition in hepatocellular carcinoma. Int. J. Oncol. 2017;51:1179–1190. doi: 10.3892/ijo.2017.4098. [DOI] [PubMed] [Google Scholar]
- 62.Song X., Zhu S., Xie Y., Liu J., Sun L., Zeng D., Wang P., Ma X., Kroemer G., Bartlett D.L., et al. JTC801 Induces pH-dependent Death Specifically in Cancer Cells and Slows Growth of Tumors in Mice. Gastroenterology. 2018;154:1480–1493. doi: 10.1053/j.gastro.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McDonald P.C., Chafe S.C., Brown W.S., Saberi S., Swayampakula M., Venkateswaran G., Nemirovsky O., Gillespie J.A., Karasinska J.M., Kalloger S.E., et al. Regulation of pH by Carbonic Anhydrase 9 Mediates Survival of Pancreatic Cancer Cells With Activated KRAS in Response to Hypoxia. Gastroenterology. 2019;157:823–837. doi: 10.1053/j.gastro.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Platten M., Nollen E.A.A., Röhrig U.F., Fallarino F., Opitz C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019;18:379–401. doi: 10.1038/s41573-019-0016-5. [DOI] [PubMed] [Google Scholar]
- 65.Lemos H., Huang L., Prendergast G.C., Mellor A.L. Immune control by amino acid catabolism during tumorigenesis and therapy. Nat. Rev. Cancer. 2019;19:162–175. doi: 10.1038/s41568-019-0106-z. [DOI] [PubMed] [Google Scholar]
- 66.Long G.V., Dummer R., Hamid O., Gajewski T.F., Caglevic C., Dalle S., Arance A., Carlino M.S., Grob J.J., Kim T.M., et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 2019;20:1083–1097. doi: 10.1016/s1470-2045(19)30274-8. [DOI] [PubMed] [Google Scholar]
- 67.D'Amato N.C., Rogers T.J., Gordon M.A., Greene L.I., Cochrane D.R., Spoelstra N.S., Nemkov T.G., D'Alessandro A., Hansen K.C., Richer J.K. A TDO2-AhR signaling axis facilitates anoikis resistance and metastasis in triple-negative breast cancer. Cancer Res. 2015;75:4651–4664. doi: 10.1158/0008-5472.CAN-15-2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stockinger B., Shah K., Wincent E. AHR in the intestinal microenvironment: safeguarding barrier function. Nat. Rev. Gastroenterol. Hepatol. 2021;18:559–570. doi: 10.1038/s41575-021-00430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bu D., Luo H., Huo P., Wang Z., Zhang S., He Z., Wu Y., Zhao L., Liu J., Guo J., et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021;49 doi: 10.1093/nar/gkab447. W317-w325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tang Z., Kang B., Li C., Chen T., Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47 doi: 10.1093/nar/gkz430. W556-w560. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data of HCC cells in the 2D, 3D, and animal models are publicly deposited at Gene Expression Omnibus (GEO accession number: GSE235351). This study does not report custom computer code. Any additional information required to reanalyze the data reported in this work paper is available from the lead contact upon request.