Abstract
Alcohol use (i.e., quantity, frequency) and alcohol use disorder (AUD) are common, associated with adverse outcomes, and genetically-influenced. Genome-wide association studies (GWAS) identified genetic loci associated with both. AUD is positively genetically associated with psychopathology, while alcohol use (e.g., drinks per week) is negatively associated or NS related to psychopathology. We wanted to test if these genetic associations extended to life satisfaction, as there is an interest in understanding the associations between psychopathology-related traits and constructs that are not just the absence of psychopathology, but positive outcomes (e.g., well-being variables). Thus, we used Genomic Structural Equation Modeling (gSEM) to analyze summary-level genomic data (i.e., effects of genetic variants on constructs of interest) from large-scale GWAS of European ancestry individuals. Results suggest that the best-fitting model is a Bifactor Model, in which unique alcohol use, unique AUD, and common alcohol factors are extracted. The genetic correlation (rg) between life satisfaction-AUD specific factor was near zero, the rg with the alcohol use specific factor was positive and significant, and the rg with the common alcohol factor was negative and significant. Findings indicate that life satisfaction shares genetic etiology with typical alcohol use and life dissatisfaction shares genetic etiology with heavy alcohol use.
Subject terms: Genomics, Genetics, Psychology
Introduction
Alcohol use is common; 71.7% of individuals in the United States aged 15 years and older endorse drinking at least one drink in the past year1. Alcohol use in excess (e.g., binge drinking, i.e., 4 + drinks/occasion for women and 5 + for men, or heavy drinking, 8 + drinks/week for women and 15 + drinks/week for men)2 is associated with a number of deleterious outcomes including the development of alcohol use disorder (AUD; Ref.3). It is estimated that, in the United States, ~ 6% of individuals meet criteria for a past year AUD4, posing significant public health costs5,6. Given the notable prevalence of and consequences associated with excess alcohol use and AUD, there is a need to better understand their etiology in order to improve prevention and intervention efforts.
Behavioral genetic studies on AUD and alcohol use have estimated the twin-based heritability of AUD to be 0.49 for a review see Ref.7; 95% CI 0.43–0.53 and alcohol use (i.e., quantity and frequency of drinking) to be 0.438; 95% CI 0.31–0.56. Recent molecular work on the genetic influences on AUD and alcohol use have also yielded modest SNP-based heritability () estimates ranging from 5.6 to 13.0% for AUD9; h2 = 0.056, S.E. = 0.00410; h2 = 0.094, S.E. = 0.005, alcohol use11; h2 = 0.13, S.E. = 0.01 and problematic alcohol use, including summary statistics for AUD diagnosis, alcohol dependence (AD) diagnosis and scores on a measure of problems experienced related to one’s use (i.e., Alcohol Use Disorders Identification Test- Problems/AUDIT-P)10; h2 = 0.068, S.E. = 0.004. Genome-wide association studies (GWAS) have identified genome-wide significant SNPs associated with AUD and problematic alcohol use i.e., ADH1B and ADH1C genes; Refs.9,10,12–15 and with alcohol use in the general population e.g., Refs.11,16,17. Furthermore, Kranzler, et al.9 found a positive, significant genetic correlation between alcohol use, as assessed using the Alcohol Use Disorders Test- Consumption (AUDIT-C) questions on quantity and frequency of alcohol use, and AUD (rg = 0.52). Mallard, et al.18 found evidence of a correlated two-factor structure (i.e., “consumption” and problems” [rg = 0.80]) similarly using the AUDIT. Taken together, these findings highlight that the genetic influences on AUD and alcohol use are correlated but not at unity. Studies of alcohol use in population samples are often dominated by the majority of individuals who drink in moderation, which might explain part of the difference. In addition, recent work finds that, phenotypically, most of the variance in alcohol-related consequences is not driven by alcohol use19.
Although extant molecular studies provide a foundation for examining the etiologic underpinnings of alcohol use and AUD, both phenotypes are highly polygenic, indicating a genetic architecture comprised of thousands of causal variants9,13. High polygenicity then necessitates statistical genetic techniques that can be used to summarize the relationships among aggregate genetic risk for alcohol use, AUD, and relevant clinical correlates. Indeed, using linkage disequilibrium score regression LDSR; Ref.20, several groups have found different patterns of associations of alcohol use and AUD with other traits9,10,21.
Further, the genetic relationship between mental health phenotypes (i.e., PTSD, anxiety, and depression) and phenotypes indexing problematic alcohol use/symptoms of AUD versus non-pathological use differs. Our group found significant, positive genetic correlations between posttraumatic stress disorder (PTSD) and AUD-related phenotypes (e.g., AUDIT-P scores, maximum alcohol intake, AUD, and AD), but negative (significant and non-significant) associations between PTSD and alcohol use-related phenotypes (e.g., drinks per week, AUDIT-C; Ref.22. Another group found a near zero genetic correlation between PTSD and the AUDIT-C subscale score (which measures quantity and frequency of use, rather than problems related to drinking)18. LDSC analyses also demonstrated that problematic use (i.e., an as assessment of problems experienced due to alcohol use) and AD are positively correlated with both anxiety23 and depression symptoms24, but that non-pathological alcohol use phenotypes (e.g., quantity, frequency) are not correlated with anxiety23 and negatively correlated depression symptoms24.
While these studies have been useful in giving us a preliminary sense of varying genetic architecture underlying alcohol use and AUD via pairwise genetic associations among constructs, the techniques used do not allow researchers to examine associations while accounting for other factors/constructs. Since the genetic architectures of alcohol use and AUD are correlated but distinct, approaches estimating variance that is common to and unique from these two phenotypes may be useful. Genomic Structural Equation Modeling (gSEM; Ref.25) is a novel statistical genetic technique that builds upon LDSC to fit multivariate models of genetic associations, allowing researchers to identify the latent genetic factor structure of multiple phenotypes. This approach makes it possible to index genetic overlap among phenotypes, as well as variance that is unique to each trait (e.g., alcohol use quantity and frequency vs. AUD). Indeed, work by our group using gSEM found that the best fitting model was one that differentiated genetic factors for alcohol use and AUD relative to models with all alcohol-related indicators loading onto a single factor26. Further, this work demonstrated that the genetic correlation with PTSD for a common alcohol factor indexing shared genetic variance across alcohol indicates was null. This was in comparison to positive and negative associations between the PTSD-AUD factor and the PTSD-alcohol use factor, respectively.
One potential hypothesis for these findings is that those who drink to the point of disorder do so because they are unhappy or unsatisfied with something in their lives. In contrast, individuals who drink at more moderate levels may be consuming alcohol to celebrate happy occasions or because they feel happy or satisfied with their lives. Thus, one might hypothesize that the genetic correlation between life satisfaction and a unique AUD factor might be negative, and the genetic correlation between life satisfaction and a unique alcohol use factor might be positive. Most of the phenotypic work examining associations between alcohol phenotypes and other outcomes has focused on deficits and psychopathology, and this literature has largely neglected protective and/or positive factors that may be associated with alcohol outcomes. Work examining associations between alcohol and positive factors is critical to informing the etiology, prevention and intervention efforts in this area. Examining this question from a genetic perspective allows us to understand the genetic architecture of a range of alcohol phenotypes.
The limited phenotypic work on life satisfaction and alcohol phenotypes suggests that lower levels of satisfaction are associated with more alcohol problems; however, there generally seems to be no association between life satisfaction and alcohol use27,28. Given this discrepant pattern of findings in this rather small literature, and the fact that life satisfaction itself is moderately heritable (h2: 38; Ref.29), it would be useful to know if the genetic correlations, explored in a gSEM framework, between life satisfaction and alcohol phenotypes, follows the pattern outlined above such that on a genetic level, alcohol use and alcohol related problems/AUD show varying results with regard to their association with life satisfaction. If this pattern of findings holds, our work provides additional evidence that the genetic underpinnings of alcohol use vary, at least in part, from those underlying problematic use and/or disorder.
The primary aim of the present paper was to examine the genetic architectures of alcohol use, AUD, and life satisfaction. We sought to examine if the direction of effect between alcohol use and life satisfaction, AUD and life satisfaction, and a combined alcohol factor and life satisfaction were discrepant. We first used gSEM to test whether two or three factor solutions fit better than the one factor model. We then tested the hypothesis that the genetic associations between and alcohol use phenotypes would be significant and positive, and between life satisfaction and AUD would be significant and negative. We also hypothesized that a model separating what is common to all the alcohol items while leaving what is unique to alcohol use and AUD would show even better fit, with the genetic correlations between life satisfaction indices and the common alcohol factor being near zero, life satisfaction and unique alcohol use being even more strongly positive and significant, and life satisfaction and AUD being more strongly negative and significant.
Methods
Summary of cohorts
We obtained summary statistics for alcohol phenotypes and life satisfaction using existing large-scale datasets described below and summarized in Table 1. We only conducted analyses on those of European Ancestry due to the scarcity of large-scale summary statistics available within other ancestral groups.
Table 1.
Descriptive information about phenotypes planned to be included.
| Dataset | Phenotype | Accompanying GWAS | N | N effective |
|---|---|---|---|---|
| PGC-SUD | Alcohol dependence (AD) case/control | Walters et al. 2018 | 45,568 [N = 11,569 cases, N = 34,999 controls] | 34,780 |
| MVP | Alcohol use disorder (AUD) case/control | Kranzler et al. 2019 | 267,391 [N = 55,584 cases, N = 218,807 controls] | 152,332 |
| MVP | Max alcohol consumption | Gelernter et al. 2019b | 126,936 | 126,936 |
| UKB | AUDIT problems | Sanchez-Roige, Palmer et al. 2019 | 121,604 | 121,604 |
| 23andMe | AUDIT total* | Sanchez-Roige, Fontanillas et al. 2019 | 20,328 | 20,328 |
| UKB | AUDIT consumption | Sanchez-Roige, Palmer et al. 2019 | 121,604 | 121,604 |
| MVP | AUDIT consumption | Kranzler et al. 2019 | 206,254 | 206,254 |
| GSCAN and UKB | Drinks per Week | Liu et al. 2019 | 941,280 | 941,280 |
| UKBiobank | Satisfaction-family | Becker et al. 2021 | 168,313 | 168,313 |
| UKBiobank | Satisfaction-finance | Becker et al. 2021 | 169,051 | 169,051 |
| UKBiobank | Satisfaction-friends | Becker et al. 2021 | 168,001 | 168,001 |
| UKBiobank | Satisfaction-work | Becker et al. 2021 | 115,038 | 115,038 |
PGC-SUD psychiatrics genomics consortium substance use disorder workgroup, MVP million veteran program, UKB United Kingdom biobank, GSCAN GWAS & sequencing consortium of alcohol and nicotine use.
*AUDIT-T from 23andMe not included in final models.
Alcohol-related cohorts and phenotypes
AUD-related phenotypes
AUD case/control status came from the Million Veteran Program (MVP) dataset and was defined according to ICD-9 or ICD-10 codes for dependence or abuse diagnoses from Veteran’s Affairs electronic health records (EHR). Participants with at least one inpatient and/or two outpatient alcohol-related ICD-9/10 codes (from 2000 to 2018) were considered to be AUD cases9. AUD case/control status was available for 267,391 participants in MVP (N = 55,584 cases, N = 218,807 controls). Alcohol dependence case/control data came from a PGC-SUD meta-analysis14, which included over 20 datasets. Cases were defined to be meeting criteria for a DSM-IV (and DSM-III-R for one study) diagnosis of alcohol dependence and all controls were alcohol exposed (N = 46,568; N = 11,569 cases, N = 34,999 controls).
Alcohol use-related phenotypes
Drinks per week (DPW), defined as the average number of drinks a participant reported drinking each week, was examined in a combined approach with GSCAN consortium and UK Biobank (UKB)17 (N = 941,280); see Table 1. In studies that reported binned response ranges (e.g., 1–4 drinks), the midpoint of the range was utilized17. The AUDIT29 was available in multiple forms and studies. First, the AUDIT total score (AUDIT-T), was available in the 23andMe dataset30 for 20,328 participants. Second, data from the AUDIT- C subscale, which consists of three items measuring past-year typical quantity and frequency of drinking and frequency of heavy/binge drinking31, were available in two datasets: EHR data from the annual AUDIT-C assessment in MVP collected on individuals between 2007–2017 N = 206,254; Ref.9 and as part of the full 10-item AUDIT in the UK Biobank N = 121,604; Ref.21. Third, the AUDIT-Problems (P) subscale, consisting of 7 items that focus on the problematic consequences of drinking, was used from the UK Biobank (N = 121,604). Finally, in MVP, a quantitative measure of maximum habitual alcohol use in a typical month MaxAlc; Ref.32 was used to reflect typical/habitual maximum use (N = 126,936).
Life satisfaction phenotypes
Summary statistics for the life satisfaction items were taken from the Social Science Genetic Association Consortium (SSGAC; https://www.thessgac.org/). Four items captured the extent to which individuals felt satisfied with their family, friends, work, and finances (e.g., “In general, how satisfied are you with the work that you do?”) with answer choices being 1–6 and higher scores indicating more satisfaction in each domain. These satisfaction items have been shown to be a good proxy for subjective well-being33. The summary statistics coming from genome-wide association studies for each of these four domains were used as indicators of the life satisfaction factor in the current analyses. Within the SSGAC website, satisfaction with family (n = 168,313), friends (n = 168,001), work (115,038), and finances (n = 169,051) were taken from UK Biobank34.
Genotyping, quality control, and imputation
Summary statistics used in these analyses have undergone quality control pipelines applied by the specific consortia (e.g., PGC quality control pipeline including filtering to remove SNPs with imputation information value < 0.90 and minor allele frequency/MAF < 0; 01; Sullivan, 2010). The analytic pipeline for these analyses incorporates additional filtering keeping approximately 1,200,000 SNPs for each phenotype with the exception of the MVP analyses which keep approximately 625,000; see Ref.25 including removing variants that are not SNPs or are strand ambiguous, and removing SNPs based on a minimum N.
Genomic structural equation modeling
We conducted analyses using the GenomicSEM package in R (version 0.0.3; https://github.com/GenomicSEM/GenomicSEM/wiki). GenomicSEM uses a two-stage SEM approach (Grotzinger et al., 2019). In the first stage, the covariance matrix and sampling covariance matrix are estimated for each dataset (see Supplementary Table 1). In the second stage, a SEM is specified and parameters are estimated by minimizing the discrepancy between the model-implied genetic covariance matrix and the empirical covariance matrix. The fit of the model can then be evaluated using standard metrics, including the standardized root mean square residual (SRMR), model chi square, Akaike Information Criterion (AIC), and the Comparative Fit Index (CFI)35,36.
Precomputed linkage disequilibrium (LD) scores were obtained from the 1000 Genomes Project, specifically the Europeans subsample (https://data.broadinstitute.org/alkesgroup/LDSCORE/eur_w_ld_chr.tar.bz2). For case/control samples, liability scale estimates assumed a population prevalence of 15.9% for alcohol dependence and AUD14.
Data analytic plan
Factor analyses: overview of different factor models to be tested
Some of the text from this section is overlapping with our prior published gSEM manuscript26. The first thing we did was we asked whether a common factor model in which all alcohol-related and life satisfaction indicators loading on the same factor (Model A) would fit the data well. If Model A fits best, it would mean that these items are part of one underlying latent factor (versus more than one). Next, we tested whether either a two-factor model with all alcohol items loading on one factor and life satisfaction loading on a second factor (Model B) or a correlated three-factor model allowing for separate life satisfaction, alcohol use, and AUD-related factors (Model C) would provide better fit. If these models fit best it would mean that (Model B) the genetic influences on all alcohol indicators and life satisfaction are distinct, or that (Model C) the genetic influences on the alcohol use indicators are distinct from the AUD indicators and the life satisfaction indicators. We then asked whether a more complicated, Bifactor model that allows for factors common and specific to life satisfaction and alcohol phenotypes (Model D) would fit the data well, and whether the inter-factor correlations between life satisfaction and alcohol use and life satisfaction and AUD would differ from one another when estimating a factor common to all alcohol phenotypes. If this Model D fits best, it suggests that there are genetic influences that are common to all alcohol items, but that there are also importance genetic influences that are specific to alcohol use, and AUD, and also that the genetic influences on life satisfaction are distinct from the alcohol items. In Bifactor models, all items are allowed to load on one common factor and on their specific group factors. The group factors are allowed to correlate with one another, but their correlations with the general factor are usually set to zero. Typically, within Bifactor models, each item loads on the common factor and one specific factor. However, here the 23andMe AUDIT-T item was initially allowed to load on both alcohol use and AUD, as it is composed of items related to both use (AUDIT-C) and problems (AUDIT-P). We hypothesized that, within this framework, the genetic correlation between positive items and AUD would be significant and negative, the correlation between life satisfaction and alcohol use would be significant and positive, and the genetic correlation between life satisfaction and the common alcohol factor would be non-significant.
Factor analyses: determining number of factors
To determine which model best fit the data, we examined the substantive interpretability of each model and its loadings, including the genetic associations between the life satisfaction factor and factors common and specific to alcohol use and AUD. We also examined goodness-of-fit indices with the standard cut-offs for good fit, including a CFI: ≥ 0.9 and SRMR ≤ 0.08 and lower AIC values suggesting better fit and parsimony37,38. We used the zero-order genetic correlations between life satisfaction and alcohol phenotypes generated from this same author group39 to inform which alcohol items would load onto the alcohol use-related factor (i.e., drinks per week, AUDIT-C, and AUDIT-T) or the AUD-related factor (i.e., AUDIT-T, Max Alc, AUDIT-P, AUD, and alcohol dependence).
Results
Zero-order genetic correlations among life satisfaction items
In examining the genetic correlations among the four life satisfaction items, all were significantly correlated with one another (p < 0.001). The associations were all moderate to large (absolute value rg: 0.34–0.85). Thus, we proceeded with including all four items in a one factor model. This one factor model showed great fit to the data (χ: 54.78, AIC = 70.78, CFI = 0.97, SRMR = 0.08). All standardized loadings of items on this factor were stronger than + /− 0.4 (absolute value range: 0.50–0.88).
Estimating initial models: a change to included items
Although the plan had been to include all items described above, upon viewing the loadings of the three factor model, the AUDIT-T from 23andMe had a near zero loading (−0.01, NS) on the AUD factor. Since the AUDIT-T is comprised of items capturing alcohol use (AUDIT-C) and alcohol problems (AUDIT-P), we opted to omit this item from the model entirely, rather than allowing it to load at a near zero level on one of those two factors and potentially water down the factor it loads on. Thus, the steps that are described in the next section pertain to the models we are calling “final”, in which all items described earlier—with the exception of AUDIT-T from 23andMe—were included.
Estimating final models of alcohol and life satisfaction
Common factor model/model A
A single common factor model with all items except for AUDIT-T from 23andMe (Model A) did not fit the data well (χ2 = 3062.65, AIC = 3106.66, CFI = 0.60, SRMR = 0.22; Table 2) (Upon viewing the loadings of the three factor model, although all other loadings were stronger than + /− 0.4 (and p < .001), AUDIT-T from 23andMe had a near zero loading (-0.01, NS) on the AUD factor. Since the AUDIT-T is comprised of items capturing alcohol use and problems, we opted to omit this item from the model entirely. Thus, the steps that are described in this section pertain to the models we are calling “final”, in which all items described earlier—with the exception of AUDIT-T from 23andMe—were included). The loadings indicated that this factor was driven by the alcohol-related factors, while the loadings of the life satisfaction items were small and/or not statistically different from zero (Supplemental Table 2).
Table 2.
Fit indices for Models.
| Model | Χ2 value (df) | χ2 p-value | AIC | CFI | SRMR |
|---|---|---|---|---|---|
| A. Common factor | 3062.66 (44) | p < 0.001 | 3106.66 | 0.60 | 0.22 |
| B. Two correlated factors | 882.33 (43) | p < 0.001 | 928.33 | 0.90 | 0.14 |
| C. Three correlated factors | 762.45 (41) | p < 0.001 | 812.45 | 0.90 | 0.11 |
| D. Bifactor | 553.54 (35) | p < 0.001 | 615.54 | 0.93 | 0.09 |
Two factor model/model B
The two-factor model (Model B) fit somewhat better, but not well (χ2 = 882.33, AIC = 928.33, CFI = 0.90, SRMR = 0.14; Table 2). For Model B, all the alcohol and life satisfaction loadings were significant (Supplemental Table 3). The genetic correlation between the life satisfaction factor and the alcohol factor was small and negative but significant (rg: − 0.07, p < 0.05).
Three factor model/model C
The three-factor model (Model C; Supplemental Table 4) fit adequately (χ2 = 762.45, AIC = 812.45, CFI = 0.90, SRMR = 0.11; Table 2). All of the loadings on these factors were significant. There was a small, negative association between AUD and the life satisfaction factor (rg: − 0.17, p < 0.001). The correlation between the alcohol use and life satisfaction was non-significant (rg: 0.00, NS), but the association between AUD and alcohol use was large, positive, and significant (rg: 0.72, p < 0.001).
Bifactor model/model D
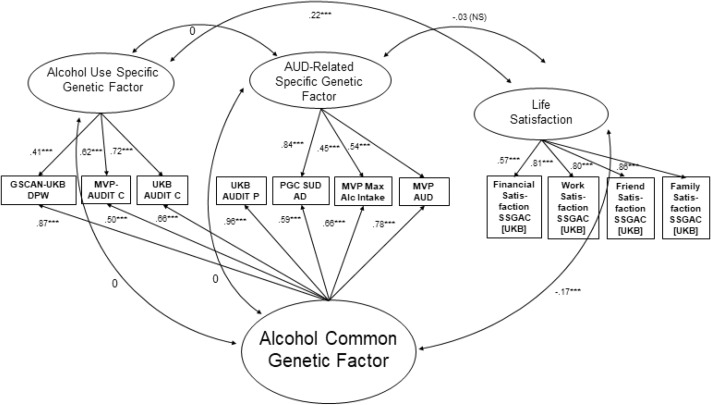
To test whether the associations between the life satisfaction factor, alcohol use, and AUD would shift when accounting for the common variation shared across all alcohol-related items, we fit a Bifactor model (Model D; See Fig. 1 and Supplemental Table 5) in which the alcohol items loaded onto 3 factors: a common factor, a residual alcohol use factor and a residual AUD factor. The correlations across these factors were fixed to zero. Model D also estimated the correlations between the life satisfaction factor and each of the three alcohol factors. Model D fit the data well (χ2 = 553.54, AIC = 615.54, CFI = 0.93, SRMR = 0.09; Table 2) and provided the best fit across the considered models. The genetic correlation between the life satisfaction factor and the common alcohol factor was negative (rg: − 0.17, p < 0.001). The correlation between the life satisfaction factor and the unique AUD factor was not significant (rg − 0.03, NS). Finally, the correlation between the life satisfaction factor and the unique alcohol use factor was positive (rg: 0.22, p < 0.001).
Figure 1.
Depiction of Final, Bifactor Model. Standardized loadings and correlations are depicted; *p < 0.05, **p < 0.01, ***p < 0.001.
Discussion
We employed gSEM to examine multivariate associations between alcohol use, AUD, and life satisfaction. This study builds upon our previous work demonstrating both improved model fit after partitioning genetic variance into separate alcohol use and AUD factors26 and differential genetic associations across these factors and PTSD22. Based on this prior work, we sought to explore potential mechanisms that may contribute to these differences in the genetic architecture between PTSD and alcohol use versus PTSD and more problematic forms of alcohol use, such as AUD. Given the positive genetic correlations between PTSD and AUD compared to the negative or non-significant genetic correlations between PTSD and alcohol use22, we hypothesized that the alcohol use and AUD genetic factors may represent drinking patterns influenced by distinct reasons for drinking. As such, we hypothesized that genetic correlations between unique alcohol use and life satisfaction and unique AUD and life satisfaction would be in opposing directions (i.e., positive and negative, respectively).
Best fitting model and its implications
A Bifactor model partialling out the shared variance across all alcohol-related items was the best fitting model. Therefore, associations between factors in that model are the focus of our interpretation and discussion. Consistent with our hypotheses, we found evidence of a positive genetic correlation between unique alcohol use and life satisfaction. This is consistent with phenotypic research indicating that alcohol use is positively associated with general well-being among low-risk (but not high-risk) drinkers40 and that low-risk drinkers report higher levels of general well-being compared to individuals who abstain from alcohol41,42. Previous research demonstrating that socially-motivated factors may mediate the relationship between happiness and alcohol use43 points to the possibility that individuals who drink alcohol at higher, but not necessarily problematic, levels tend to do so out of a desire to be social and that this may, in turn, be associated with higher general life satisfaction. Previous work demonstrating that social motives for drinking are the most commonly reported reasons for drinking44,45 and have been shown to be positively associated with frequency and quantity of use, but not to heavy drinking or alcohol-related problems46 may lend further support for this theory. Another possible explanation for the positive genetic association between alcohol use and life satisfaction could be the “healthy volunteer” effect, such that large population-based cohorts like those used in the current gSEM analyses tend to be overall healthier and of higher socioeconomic status than the broader population. As such, it is possible that our results are subject to selection bias. This aligns with previous work demonstrating that individuals of higher socioeconomic status tend to endorse more frequent alcohol use in the absence of non-problematic alcohol use18,47.
Revisiting hypotheses about correlations among factors
Our hypothesis that the genetic association between unique AUD and life satisfaction would be negative was not supported, as we did not find evidence for any association between the two genetic factors. Our hypothesis was formulated based on prior work demonstrating positive genetic associations between AUD/AD and PTSD in across both behavioral genetic48 and molecular genetic22,26,49 research designs. We had thought that the AUD factor might represent the propensity to drink for reasons more consistent with negatively valenced intentions, such as to avoid aversive emotional states, specifically in the context of PTSD50. However, prior work suggests that psychopathology and life satisfaction are distinct, albeit related, phenotypes51–53 and, therefore, it is possible our findings reflect a similar genotypic distinction. The null association between life satisfaction and the alcohol factor unique to disordered use suggests that genetic risk for problematic/pathological alcohol use is not associated with genetic risk for life dissatisfaction. This would be consistent with some phenotypic work suggesting that psychopathology and psychological well-being (e.g., life satisfaction, self-realization, social well-being) are not two ends of a single dimension52,53.
We also tested the genetic association between the common factor reflecting shared variance across alcohol use and AUD with life satisfaction. Contrary to our hypothesis that this common factor would show no significant association with life satisfaction as was the case with PTSD in prior work; Ref.26, the common factor was negatively associated with life satisfaction in the present study. One possible explanation for this negative association could be that the genetic signal that is shared across all alcohol use indicators, including measures of alcohol use in the general population, is generally indexing genetic propensities for heavier drinking. This may then indicate patterns of drinking associated with, or motivated by, overall life dissatisfaction. Conversely, it is possible that drinking at high levels leads to greater life dissatisfaction across the domains captured by the indicators of the life satisfaction factor in the present study (e.g., work, family, friends, finances). There is phenotypic support for the bidirectional nature of these two constructs. Specifically, findings from a large (n = 14,083), 15-year longitudinal study of healthy Finnish twins54 showed that life dissatisfaction and adverse alcohol use (including binge drinking, passing out, high use) reciprocally influenced each other over time, and that the magnitude of this relationship increased with heavier alcohol use.
The lack of evidence of a genetic association between AUD and life satisfaction, as well as the negative genetic association between the common alcohol-related factor and life satisfaction were unexpected in light of previous findings presented by our group. Specifically, using gSEM techniques, we reported that AUD is negatively genetically correlated with PTSD and that there was no evidence of a genetic association between PTSD and the common factor representing shared genetic variance across AUD and use26. The discrepancy between these findings and those from the present study highlight a few important points. First, in our prior work, the PTSD factor was capturing what is common between PTSD case/control status and PTSD Re-experiencing symptoms (i.e., the two indicators); that is, the factor is primarily measuring intrusive symptoms such as nightmares, flashbacks, and repetitive, distressing images, typically related to the experienced traumatic event. In contrast, our life satisfaction factor was capturing what is common among indicators of family, friend, work, and financial satisfaction—so some general contentment with all these areas of life. In phenotypic analyses, more intrusive trauma-related (PTSD) symptoms tend to be associated with thought disorders/conditions such as mania psychosis55, while higher satisfaction tends to be associated with more happiness, well-being, and less neuroticism, loneliness, and depressive symptoms33. Second, and relatedly, it seems important to apply gSEM to questions involving positive constructs, such as life satisfaction and other positively valenced constructs associated with psychological well-being, in addition to psychiatric disorders. Doing so not only provides biological support for the notion that life satisfaction and psychopathology are not opposite ends of the same dimension, but also allows for the identification of novel patterns between previously studied phenotypes and other constructs relevant to, but distinct from, psychopathological functioning. As such, incorporation of positively valenced constructs into gSEM provides clinically relevant insight into the potential biological impact of specific treatment intervention and prevention approaches (e.g., interventions to increase life satisfaction).
Limitations
The present findings should be interpreted in light of several limitations. First, the summary statistics that were available for our multiple phenotypes of interest, particularly when collated across various datasets, were limited to individuals of European Ancestry. This is problematic for a number of reasons, including the fact that initial work from our group demonstrated that zero order genetic correlations between PTSD and alcohol-related constructs differed across European Ancestry and admixed populations22. Exploring these associations among more diverse populations will become more feasible as more summary statistics including individuals from other ancestral groups become available. Additionally, while not inclusive of all individuals/populations, particularly outside of European Ancestry, the datasets from which gSEM analyses were conducted in general are large and representative, or are from consortia of numerous studies, adding to the generalizability of the findings. Second, the available summary statistics for alcohol-related phenotypes precluded analyses split by sex, which is problematic given known sex differences in molecular genetic associations between alcohol-related phenotypes and other constructs, such as PTSD49, as well as known sex differences in the prevalence of alcohol-related phenotypes56, motives for drinking46, and relationships between life satisfaction and alcohol use57. Third, due to modeling issues, the 23andMe AUDIT-T item was excluded from each of the models. This choice was made in large part to statistical reasons; however, for substantive reasons as well, it makes sense that this item was not included. It was important for us to examine the genetic architecture of alcohol use and AUD-related phenotypes separately, and the AUDIT-T scale included both consumption and problems items. Thus, it does not make clear sense on which factor(s) that item would load. Additionally, due to other modeling issues, the PGC AD factor was excluded from the unique AUD factor. These modifications resulted in a Bifactor model that differed from that which was run in our earlier paper26. Finally, these findings should be interpreted in the context of the authors not having pre-registered study hypotheses.
Take-home points and implications
Despite these limitations, this study advances the field by applying a novel methodology (i.e., gSEM) to a question relating to a positive psychological construct, which is rare in the field of psychiatric genetics, and is of high importance, given the potential to increase our understanding surrounding the etiology of alcohol phenotypes and prevention of problematic alcohol use. This study also adds to a growing body of work suggesting that the genetic architecture of alcohol use and AUD are distinct and that these distinctions extend to external correlates ranging from psychopathology (e.g., PTSD) and psychological well-being (e.g., life satisfaction). This work also provides some initial evidence for shared etiology across life satisfaction and typical alcohol use, and life dissatisfaction and heavy alcohol use. Although our findings reflect association and not causality, they provide some foundation on which longitudinal, causal models might build. Specifically, work testing whether intervening on life dissatisfaction reduces heavy alcohol use specifically, may be a beneficial next step in this line of research.
Supplementary Information
Acknowledgements
The effort of co-authors was supported by NIAAA (1K01 AA028058 [KB]), NIMH (MH020030-21A1 [DB]; NIMH (R01MH120219 [AG]), and NIA (RF1AG073593 [AG]). The PGC-SUD Working Group receives support from the National Institute on Drug Abuse and the National Institute of Mental Health via MH109532. Statistical analyses for the PGC were carried out on the Genetic Cluster Computer (http://www.geneticcluster.org) hosted by SURFsara and financially supported by the Netherlands Scientific Organization (NWO 480-05-003), along with a supplement from the Dutch Brain Foundation and the VU University Amsterdam. Support for the SSGAC is provided by the U.S. National Science Foundation, the U.S. National Institutes of Health (National Institute on Aging, and the Office for Behavioral and Social Science Research), the Open Philanthropy Project, the Ragnar Söderberg Foundation, the Swedish Research Council, The Jan Wallander and Tom Hedelius Foundation, the European Research Council, and the Pershing Square Fund of the Foundations of Human Behavior. This research has been conducted using data from UK Biobank, a major biomedical database (https://www.ukbiobank.ac.uk/). Research reported herein was supported by the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. We would also like to thank dgGaP (accession phs001672.v7.p1). We also thank Million Veteran Program (MVP) staff, researchers, and volunteers, who have contributed to MVP, and especially participants who previously served their country in the military and now generously agreed to enroll in the study. (See https://www.research.va.gov/mvp/ for more details). The citation for MVP is Gaziano, J.M. et al. Million Veteran Program: A mega-biobank to study genetic influences on health and disease. J Clin Epidemiol 70, 214-23 (2016). This research is based on data from the Million Veteran Program, Office of Research and Development, Veterans Health Administration, and was supported by the Veterans Administration (VA) Cooperative Studies Program (CSP) award #G002.
Author contributions
K.E.B. conducted analyses, wrote Method and Results, and generated the original idea for analyses, S.E.C. wrote Introduction, S.E.H. wrote Discussion, A.G. assisted K.E.B. with analyses, D.B. formatted original data files so they were able to be used within gSEM, R.M.K. completed additional analyses (e.g., computed p-values for zero-order correlations), H.J.E. provided substantial edits, and A.B.A. provided substantial edits and was involved in the original idea formation; all co-authors provided feedback.
Data availability
All data analyzed are summary-level data, most of which are publicly available to qualified investigators (e.g., https://pgc.unc.edu/for-researchers/download-results/). The exception to this is summary-level data coming from the Million Veterans Program (MVP), which is available with a data use agreement through dbGaP.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-40199-1.
References
- 1.Ritchie, H. R., M. Alcohol Consumption., 2018).
- 2.What is Excessive Alcohol Use?https://www.cdc.gov/alcohol/onlinemedia/infographics/excessive-alcohol-use.html (2019).
- 3.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394:781–792. doi: 10.1016/S0140-6736(19)31775-1. [DOI] [PubMed] [Google Scholar]
- 4.Abuse, S. Mental Health Services Administration: Key Substance Use and Mental Health Indicators in the United States: Results from the 2015 National Survey on Drug Use and Health (HHS Publication No. SMA 16–4984, NSDUH Series H-51), 2016. Substance Abuse and Mental Health Services Administration (2017).
- 5.Rehm J, et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction (Abingdon, England) 2017;112:968–1001. doi: 10.1111/add.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the US, 2006. Am. J. Prev. Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 7.Mbarek H, et al. The genetics of alcohol dependence: Twin and SNP-based heritability, and genome-wide association study based on AUDIT scores. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2015;168:739–748. doi: 10.1002/ajmg.b.32379. [DOI] [PubMed] [Google Scholar]
- 8.Vrieze SI, McGue M, Miller MB, Hicks BM, Iacono WG. Three mutually informative ways to understand the genetic relationships among behavioral disinhibition, alcohol use, drug use, nicotine use/dependence, and their co-occurrence: Twin biometry, GCTA, and genome-wide scoring. Behav. Genet. 2013;43:97–107. doi: 10.1007/s10519-013-9584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kranzler HR, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat. Commun. 2019;10:1499. doi: 10.1038/s41467-019-09480-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat. Neurosci. 2020;23:809–818. doi: 10.1038/s41593-020-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clarke TK, et al. Genome-wide association study of alcohol consumption and genetic overlap with other health-related traits in UK Biobank (N=112 117) Mol. Psychiatry. 2017;22:1376–1384. doi: 10.1038/mp.2017.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank J, et al. Genome-wide significant association between alcohol dependence and a variant in the ADH gene cluster. Addict. Biol. 2012;17:171–180. doi: 10.1111/j.1369-1600.2011.00395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelernter J, et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol. Psychiatry. 2014;19:41–49. doi: 10.1038/mp.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walters RK, et al. Transancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat. Neurosci. 2018;21:1656–1669. doi: 10.1038/s41593-018-0275-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edenberg HJ, McClintick JN. Alcohol dehydrogenases, aldehyde dehydrogenases, and alcohol use disorders: A critical review. Alcohol. Clin. Exp. Res. 2018;42:2281–2297. doi: 10.1111/acer.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson EC, et al. The genetic relationship between alcohol consumption and aspects of problem drinking in an ascertained sample. Alcohol. Clin. Experim. Res. 2019;43:1113–1125. doi: 10.1111/acer.14064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu M, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019;51:237–244. doi: 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallard, T. T. et al. Item-Level Genome-Wide Association Study of the Alcohol Use Disorders Identification Test in Three Population-Based Cohorts. American Journal of Psychiatry, appi. ajp. 2020.20091390 (2021). [DOI] [PMC free article] [PubMed]
- 19.Prince MA, Pearson MR, Bravo AJ, Montes KS. A quantification of the alcohol use-consequences association in college student and clinical populations: A large, multi-sample study. Am. J. Addict. 2018;27:116–123. doi: 10.1111/ajad.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Roige S, et al. Genome-wide association study meta-analysis of the alcohol use disorders identification test (AUDIT) in two population-based cohorts. Am. J. Psychiatry. 2019;176:107–118. doi: 10.1176/appi.ajp.2018.18040369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bountress KE, et al. Differences in genetic correlations between posttraumatic stress disorder and alcohol-related problems phenotypes compared to alcohol consumption-related phenotypes. Psychol. Med. 2022 doi: 10.1017/S0033291722002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Colbert SM, et al. Novel characterization of the multivariate genetic architecture of internalizing psychopathology and alcohol use. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2021;186:353–366. doi: 10.1002/ajmg.b.32874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Polimanti R, et al. Evidence of causal effect of major depression on alcohol dependence: Findings from the psychiatric genomics consortium. Psychol. Med. 2019;49:1218–1226. doi: 10.1017/s0033291719000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grotzinger AD, et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat. Hum. Behav. 2019;3:513–525. doi: 10.1038/s41562-019-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bountress KE, et al. Alcohol use and alcohol use disorder differ in their genetic relationships with PTSD: A genomic structural equation modelling approach. Drug Alcohol Depend. 2022;234:109430. doi: 10.1016/j.drugalcdep.2022.109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mies GW, et al. Polygenic risk for alcohol consumption and its association with alcohol-related phenotypes: Do stress and life satisfaction moderate these relationships? Drug Alcohol Depend. 2018;183:7–12. doi: 10.1016/j.drugalcdep.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 28.Murphy JG, McDevitt-Murphy ME, Barnett NP. Drink and be merry? Gender, life satisfaction, and alcohol consumption among college students. Psychol. Addict. Behav. 2005;19:184. doi: 10.1037/0893-164X.19.2.184. [DOI] [PubMed] [Google Scholar]
- 29.Saunders JB, Aasland OG, Babor TF, De la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 30.Sanchez-Roige S, et al. Genome-wide association study of alcohol use disorder identification test (AUDIT) scores in 20 328 research participants of European ancestry. Addict. Biol. 2019;24:121–131. doi: 10.1111/adb.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): An effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch. Intern. Med. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 32.Gelernter J, et al. Genome-wide Association Study of Maximum Habitual Alcohol Intake in >140,000 U.S. European and African American Veterans Yields Novel Risk Loci. Biol. Psychiatry. 2019;86:365–376. doi: 10.1016/j.biopsych.2019.03.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamshidi J, Schofield PR, Gatt JM, Fullerton JM. Phenotypic and genetic analysis of a wellbeing factor score in the UK Biobank and the impact of childhood maltreatment and psychiatric illness. Transl. Psychiatry. 2022;12:113. doi: 10.1038/s41398-022-01874-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker J, et al. Resource profile and user guide of the Polygenic Index Repository. Nat. Hum. Behav. 2021;5:1744–1758. doi: 10.1038/s41562-021-01119-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Browne MW. Asymptotically distribution-free methods for the analysis of covariance structures. Br. J. Math. Stat. Psychol. 1984;37:62–83. doi: 10.1111/j.2044-8317.1984.tb00789.x. [DOI] [PubMed] [Google Scholar]
- 36.Savalei V, Bentler PM. A two-stage approach to missing data: Theory and application to auxiliary variables. Struct. Equ. Modeling. 2009;16:477–497. doi: 10.1080/10705510903008238. [DOI] [Google Scholar]
- 37.Hu LT, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: Conventional criteria versus new alternatives. Struct. Equ. Model. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 38.Kenny, D. A. Measuring Model Fit.http://davidakenny.net/cm/fit.htm (2015).
- 39.Bountress, K. et al. Differences in Genetic Correlations between Posttraumatic Stress Disorder and Alcohol Use Disorder-Related Phenotypes Compared to Alcohol Consumption-Related Phenotypes. medRxiv, 2022.2003.2002.22271415 (2022). 10.1101/2022.03.02.22271415 [DOI] [PMC free article] [PubMed]
- 40.Parackal M, Parackal S. Implication of alcohol consumption on aggregate wellbeing. Perspect. Public Health. 2017;137:220–226. doi: 10.1177/1757913916669538. [DOI] [PubMed] [Google Scholar]
- 41.Appleton A, James R, Larsen J. The association between mental wellbeing, levels of harmful drinking, and drinking motivations: A cross-sectional study of the UK adult population. Int. J. Env. Res. Public Health. 2018;15:1333. doi: 10.3390/ijerph15071333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lucas N, Windsor TD, Caldwell TM, Rodgers B. Psychological distress in non-drinkers: Associations with previous heavy drinking and current social relationships. Alcohol Alcohol. 2010;45:95–102. doi: 10.1093/alcalc/agp080. [DOI] [PubMed] [Google Scholar]
- 43.Zhou J, O’Brien KS, Heim D. Alcohol consumption in sportspeople: The role of social cohesion, identity and happiness. Int. Rev. Sociol. Sport. 2014;49:278–293. doi: 10.1177/1012690213493105. [DOI] [Google Scholar]
- 44.Kuntsche E, et al. Drinking motives and links to alcohol use in 13 European countries. J. Stud. Alcohol Drugs. 2014;75:428–437. doi: 10.15288/jsad.2014.75.428. [DOI] [PubMed] [Google Scholar]
- 45.Wicki M, et al. Different drinking motives, different adverse consequences? Evidence among adolescents from 10 European countries. Drug Alcohol Rev. 2017;36:731–741. doi: 10.1111/dar.12572. [DOI] [PubMed] [Google Scholar]
- 46.Cooper ML. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol. Assess. 1994;6:117. doi: 10.1037/1040-3590.6.2.117. [DOI] [Google Scholar]
- 47.Marees AT, et al. Potential influence of socioeconomic status on genetic correlations between alcohol consumption measures and mental health. Psychol. Med. 2020;50:484–498. doi: 10.1017/S0033291719000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sartor CE, et al. Common genetic and environmental contributions to posttraumatic stress disorder and alcohol dependence in young women. Psychol. Med. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheerin CM, et al. Shared molecular genetic risk of alcohol dependence and posttraumatic stress disorder (PTSD) Psychol. Addict. Behav. 2020 doi: 10.1037/adb0000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hawn SE, Cusack SE, Amstadter AB. A systematic review of the self-medication hypothesis in the context of posttraumatic stress disorder and comorbid problematic alcohol use. J. Trauma. Stress. 2020;33:699–708. doi: 10.1002/jts.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaffer-Hudkins E, Suldo S, Loker T, March A. How adolescents’ mental health predicts their physical health: Unique contributions of indicators of subjective well-being and psychopathology. Appl. Res. Qual. Life. 2010;5:203–217. doi: 10.1007/s11482-010-9105-7. [DOI] [Google Scholar]
- 52.Gigantesco A, et al. The relationship between satisfaction with life and depression symptoms by gender. Front. Psych. 2019;10:419. doi: 10.3389/fpsyt.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Westerhof GJ, Keyes CL. Mental illness and mental health: The two continua model across the lifespan. J. Adult Dev. 2010;17:110–119. doi: 10.1007/s10804-009-9082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koivumaa-Honkanen H, et al. Self-reported life satisfaction and alcohol use: A 15-year follow-up of healthy adult twins. Alcohol Alcohol. 2012;47:160–168. doi: 10.1093/alcalc/agr151. [DOI] [PubMed] [Google Scholar]
- 55.Watson D, Gamez W, Simms LJ. Basic dimensions of temperament and their relation to anxiety and depression: A symptom-based perspective. J. Res. Pers. 2005;39:46–66. doi: 10.1016/j.jrp.2004.09.006. [DOI] [Google Scholar]
- 56.Grant BF, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiat. 2015;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tartaglia S, Gattino S, Fedi A. Life satisfaction and alcohol consumption among young adults at social gatherings. J. Happiness Stud. 2018;19:2023–2034. doi: 10.1007/s10902-017-9907-5. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed are summary-level data, most of which are publicly available to qualified investigators (e.g., https://pgc.unc.edu/for-researchers/download-results/). The exception to this is summary-level data coming from the Million Veterans Program (MVP), which is available with a data use agreement through dbGaP.