Abstract
The maidenhairtree polysaccharides (MTPs) have important application prospects. So, the extraction, purification, structure, derivatization and biological activities of polysaccharides from leaves, fruits, and testae of maidenhairtree were disscussed. Polysaccharides were extracted by collaborative extraction methods such as ultrasound-assisted extraction and microwave-assisted extraction. The ultrasound-assisted extraction had higher content and higher efficiency. The structural characteristics and structure-activity relationship of maidenhairtree polysaccharides were studied in order to provide theoretical basis and technical support for the further development and utilization of maidenhairtree polysaccharides.
Keywords: Maidenhairtree polysaccharides, Ultrasound-assisted extraction, Purification, Analysis
1. Introduction
Maidenhairtree is with homology of medicine and food. It can nourish lung, relieve cough and diarrhea, kill insects and kill bacteria. Maidenhairtree not only has good ornamental value, but also is widely planted in the world, which adds a force to the evolution of air and environmental beautification. Maidenhairtree is a precious fast-growing plant. The leaves, seeds and seed coats of maidenhairtree are valuable materials [1]. Its white fruit tastes delicious, tastes delicate and has high nutritional value. However, there is a widespread misconception that “white fruit is harmful,” which limits how white fruit is used and processed and presents significant development challenges. Studying and exploring its sophisticated deep processing are critical tasks. It was displayed in Fig. 1.
Fig. 1.
Leaf, seed and seed coat of maidenhairtree.
In recent years, plant polysaccharides have been widely used in health food, cosmetics, medicine, basic research and other fields. Maidenhairtree polysaccharides (MTPs) are isolated from maidenhairtree leaves, fruits and testae. The polysaccharide content in maidenhairtree testae is relatively rich, followed by fruits, and the polysaccharide content in leaves is relatively small. Maidenhairtree polysaccharides have good effects on resisting tumors [2], inflammation [3], diabetes [4], and also have natural biological activities such as immune regulation [5] and anti-aging [6]. It has small side effects, high safety and wide sources. Maidenhairtree polysaccharide (MTP) affects the turn of events and capability of bone marrow-inferred dendritic cells. MTP can be utilized for the therapy of specific growths (like gastric disease, melanoma) and other irresistible circumstances. MTP can likewise be utilized as an adjuvant for DC immunization readiness [7]. A water-soluble polysaccharide with anti-inflammatory activity was isolated and purified from maidenhairtree. Its main function is to inhibit NF-κB and MAPK signaling pathways, so as to achieve the purpose of anti-inflammatory. It shows that the maidenhairtree polysaccharide can be used to develop anti-inflammatory agents or nutritional health products [8]. Maidenhairtree polysaccharide has extraordinary application likely in medication and useful food, and it is of incredible pragmatic importance to complete extensive turn of events. Notwithstanding, the construction, movement and instrument of activity of different polysaccharides extricated from maidenhairtree leaves, seeds, products of the soil should be additionally summed up and gotten to the next level. Hence, in light of the important writing on maidenhairtree polysaccharides at home and abroad in the previous 10 years, this paper further thinks about the readiness strategies for maidenhairtree polysaccharides, and thoroughly presents its construction, its relationship with organic action and application fields, which gives a helpful reference values to scientists.
2. Preparation of maidenhairtree polysaccharides
Lately, the turn of events and usage of restorative plant assets at home and abroad stand out, and its turn of events and clinical application have become increasingly broad. Among them, plant extraction with polysaccharides as the super natural substance is the essential stage [9]. With the advancement of science and innovation and the ceaseless endeavors of specialists, all the more new instruments and specialized means will be utilized to extricate important parts from regular plants, consequently speeding up the turn of events and use of medications.
In the extraction process of maidenhairtree polysaccharide, the ratio of material to liquid, the temperature of polysaccharide extraction and the extraction time have certain influence on the extraction effect [10], [11]. The leaching rate of maidenhairtree polysaccharide is related to the ratio of solid to liquid. Firstly, it increases with the decrease of the ratio of solid to liquid (that is, the amount of extraction solvent increases), when the ratio of material to liquid reached a certain ratio, the extraction rate of maidenhairtree polysaccharide was the highest, and then there would be a downward trend. This may be because when the extraction solvent was less, there was a part of the polysaccharide that could not be dissolved in time, so that the maidenhairtree polysaccharide was not completely dissolved. When the extraction solvent continued to increase and the total amount of polysaccharide was certain, the extraction rate of maidenhairtree polysaccharide decreased. It has been proved that the extraction rate of maidenhairtree polysaccharide was the best when the ratio of material to liquid was about 1:25. With an increase in extraction temperature, the rate of maidenhairtree polysaccharide extraction gradually increased. The increase of temperature would increase the dissolution rate of polysaccharides. If the temperature continued to rise, the polysaccharides would be easily burnt, but the extracted polysaccharides would be reduced. Therefore, the extraction temperature was controlled at about 75 ℃ [12]. The leaching rate of maidenhairtree polysaccharide increased with the passage of time. When the extraction time was about 3 h, the leaching rate of polysaccharide was the highest. If the time was prolonged, the leaching rate would be reduced, which was time-consuming and could not obtain high yield polysaccharide. Therefore, the best extraction time should be selected to obtain more polysaccharide yield. The main reason may be that maidenhairtree polysaccharides are dissolved in aqueous solution and are already saturated. If the extraction time is too long, maidenhairtree polysaccharides will be lost. The extraction times may also affect the extraction rate of polysaccharides. Choosing the appropriate extraction times can not only maximize the saving of experimental time, avoid wasting resources, but also obtain polysaccharides with higher extraction rate [13].
On this basis, the single factor method can be used to test the factors that have the greatest influence on the extraction rate of polysaccharides, and then the orthogonal test method is used to test the three factors with the greatest influence in the early stage, and the extraction process conditions with the best yield of maidenhairtree polysaccharides are determined [14], which provides a theoretical reference for further research and development of maidenhairtree polysaccharides in the future.
2.1. Comparison of extraction methods
-
a)
Single extraction method: hot water extraction, water extraction and alcohol precipitation, enzyme-assisted extraction, microwave-assisted extraction, and ultrasonic-assisted extraction [15].
-
b)
Collaborative extraction methods: ultrasound-water extraction, microwave-water extraction, and ultrasound-enzyme extraction.
-
c)
New technology extraction applications: ultra-high pressure technology, ultrafiltration membrane technology, semi-bionic technology, multiphase system, supercritical fluid extraction technology [16].
2.1.1. Single extraction method
The principle of single extraction method was as follows. The advantages and disadvantages were compared in Table 1a.
-
(1)
Hot water extraction(HWE)
Table 1a.
Comparison of advantages and disadvantages of single extraction method.
| method | advantage | shortcomings |
|---|---|---|
| Hot water extraction(HWE) | Compared with other extraction processes, it has the advantages of simple and convenient, no need for other auxiliary equipment, easy to take materials and so on. | ① Easy to be affected by extraction time, temperature and the ratio of water to raw materials; ② The extraction time is long and takes many times, and the precipitation of crude polysaccharide requires a large amount of ethanol (4–10 times); ③ Can only extract extracellular polysaccharides, cannot destroy the plant cell wall and plasma membrane; ④ Low extraction rate: At high temperature, the polysaccharide is easily degraded, resulting in poor extraction efficiency. |
| Enzyme assisted extraction method(EAM) | ① Enzymes are characterized by specificity and selectivity; ② High extraction efficiency, simple separation and purification, increased polysaccharide activity, high reaction compatibility, and mild reaction conditions; ③ Easy to operate, green environmental protection, reduce the use of chemical reagents, conducive to the utilization of resources and environmental improvement, low investment cost and energy consumption. |
① The high price of enzyme limits its large-scale application; ② Several factors such as enzyme concentration, extraction temperature and pH value also affect the biological function of polysaccharides. |
| Microwave-assisted extraction(MAE) | ① It has the characteristics of high permeability and selectivity, less time, small solvent demand and low energy consumption; ② Economical and feasible, save time, environmental protection. |
The energy consumption is large, the microwave power is slightly higher, and it is easy to produce charring and destroy the structure of polysaccharides. |
| Ultrasonic-assisted extraction(UAE) | ① Improve permeability and capillary effect; ② The extraction time of polysaccharides can be significantly shortened with less impurities, which simplifies the post-processing steps; ③ Heat effect will be produced in the process of ultrasound, increasing the solubility of active ingredients. |
The structure and molecular weight of polysaccharides were affected, which led to the change of biological activity. |
The hot water extraction method is a traditional polysaccharide extraction method and the earliest leaching method. The extraction temperature affects the rate and yield of HWE leaching polysaccharide, followed by the ratio of material to liquid and extraction time [17]. The hot water cooking extraction device has the advantages of simple process, no complex equipment, single extraction solvent, no other solvent pollution, environmental protection and low cost [18]. However, the traditional extraction method has a large number of water-soluble impurities, and it is difficult to separate polysaccharides from cells, resulting in low extraction efficiency of target components and difficulty in controlling temperature. Compared with physical methods such as microwave and ultrasonic extraction, the activity of plant polysaccharides extracted by hot water is not high and the yield is low.
-
(2)
Enzyme-assisted extraction method(EAE)
Enzymes play an important role in the body and are very important catalysts. Enzymatic hydrolysis is not only an auxiliary process of water extraction, but also has the function of impurity removal and purification. Enzyme-assisted extraction can increase the activity of polysaccharides. Under suitable conditions, adding a certain amount of enzymes (such as protease, cellulase, pectinase) [15], stirring constantly during the extraction process and adjusting the pH, this step is crucial, because the pH value of the solution is one of the most important factors affecting the yield of polysaccharides [19].
Enzymatic hydrolysis is an effective extraction method for accelerating the dissolution of polysaccharides by enzymatic hydrolysis or destroying the structure of cell wall to release active polysaccharides from plant cells. The maidenhairtree leaf polysaccharide obtained by cellulase hydrolysis after a series of extraction processes is a light gray powder with good water solubility and insoluble in organic reagents such as ethanol. The polysaccharides isolated from maidenhairtree leaves were yellow-white powder by the combination of ultrasonic and enzymatic methods. The polysaccharides were mainly composed of Gal, Ara, Man and Rha monosaccharides.
-
(3)
Ultrasonic-assisted extraction(UAE)
There are many kinds of single extraction methods for polysaccharides. Among them, ultrasonic method has been widely concerned because of its low energy consumption, short time, high efficiency, and the advantages of mild heating temperature and no destruction of active ingredients during ultrasonic extraction [20]. The basic principle: Using the resonance and cavitation caused by ultrasound, it can break through the cell wall of plants and promote the penetration of solvents into cells, so as to maximize the dissolution of polysaccharides and increase the extraction efficiency [21]. First, ultrasound can destroy the cell wall of plants in a matter of seconds, even the whole plant. Secondly, vibration can promote the uniform release of polysaccharides from cells and disperse them in solvents for better dissolution and extraction [22]. This method combined with solvent extraction is a potential method for rapid extraction of high-yield polysaccharides. At the same time, the ultrasonic extraction process can prevent the influence of high temperature on the active substances in the extraction process. The following is the basic flow chart of ultrasonic-assisted extraction of maidenhairtree polysaccharides (Fig. 2).
Fig. 2.
Flow chart of ultrasonic-assisted extraction of maidenhairtree polysaccharides [23].
The yield and purity of polysaccharides were affected by ultrasonic frequency and power [24]. Increasing the ultrasonic power can accelerate the circulation rate of water, enhance the mass transfer ability, improve the solubility of polysaccharides, and the extraction rate and purity of maidenhairtree polysaccharides will increase. When the ultrasonic power is increased to a certain value, the extraction rate of maidenhairtree polysaccharides is the highest. However, when the ultrasonic power is greater than this limit, the extraction rate of maidenhairtree polysaccharides will decrease, so that the extraction rate and purity of polysaccharides will decrease [25]. This is because the dissolution of polysaccharides will reach saturation and will not increase indefinitely with the increase of ultrasonic power. Ultrasonic power is generally selected at about 350–550 W for the best. When the intensity of ultrasonic wave was constant, the purity and yield of maidenhairtree polysaccharide increased with the increase of ultrasonic times. The number of ultrasounds continued to increase and its growth tended to be gentle, and then there was no significant effect; after more than 500 times, its yield gradually decreased (Table 1b). It can be seen that the longer the ultrasonic treatment time, the lower the yield. The reason for this phenomenon may be that the mechanical effect of ultrasound is relatively large. Long-term treatment will change the structure of high-molecular-weight polysaccharides, and the loss of polysaccharides will increase during the later processing. Therefore, the yield and purity were high in the range of 350–450 times. The main factors affecting the extraction rate of polysaccharide were solid-liquid ratio, time and ultrasonic power [26].
Table 1b.
Ultrasonic-assisted extraction of maidenhairtree polysaccharide optimum conditions and extraction rate analysis.
| Liquid-material ratio(g:mL) | Ultrasonic power | Ultrasonic time | Temperature | Extraction rate | |
|---|---|---|---|---|---|
| Polysaccharides of maidenhairtree leaf | 1:20 | 300 W | 40 min | 50 ℃ | 4.60 % |
| – | 500 W | 50 min | 60 ℃ | 4.33 % | |
| 1:40 | 600 W | 50 min | 84 ℃ | 6.61 % | |
| Maidenhairtree seed polysaccharid | – | 373 W | 41 min | 52 ℃ | 5.91 % |
| – | 400 W | 40 min | 50 ℃ | 5.89 % | |
| Maidenhairtree exocarp polysaccharid | 1:30 | 162 W | 120 min | 100 ℃ | 16.529 % |
It can be seen from Table 1b that under the optimal extraction process of ultrasound-assisted extraction of maidenhairtree polysaccharides, maidenhairtree exocarp contains a large amount of polysaccharides, accounting for about 12 %, which is a widely used polysaccharide substance. Maidenhairtree exocarp polysaccharide is a kind of yellowish-brown powder, without any smell, slight sweetness, no toxicity and side effects on human body. When maidenhairtree reaches maturity, the outer seed coat will begin to smell. The enormous waste of maidenhairtree resources can be improved, and environmental contamination can be decreased, by recycling the outer seed coat of the plant.
-
(4)
Microwave-assisted extraction(MAE)
Microwave extraction technology uses different frequencies of electromagnetic waves to form molecular vibrations in water, accumulate heat, and continue to heat, causing cell contraction to cause cell rupture [27].The extraction solvent satisfies a transparent or translucent state and is selective. This characteristic of the solvent sometimes limits the application of microwave-assisted extraction of polysaccharides. Using maidenhairtree fruit as raw material, after microwave extraction, the polysaccharide was milky white, without phenolic hydroxyl and reducing sugar. The crude polysaccharide contained more proteins and polypeptides, which could be removed by Sevag method. The existing experiments showed that the microwave extraction method had better extraction effect on maidenhairtree polysaccharide. When the ratio of material to liquid was 1:40 g/mL, the microwave power was about 700 W, the temperature was set at 50 °C, and the processing time was 5 min, the yield of maidenhairtree polysaccharide was 4.87 %. With 480 W microwave power, 1:30 g/mL solid–liquid ratio, extraction 2 times, 8 min each time, the extraction rate of crude polysaccharide from maidenhairtree leaves was 14.70 %. Maidenhairtree exopleura was used as raw material. The ratio of material to liquid was 1: 29, the power was 700 W, the microwave heating time was 13 min, and then soaked in boiling water bath for about 160 min. The yield was up to 10 %. The main factors affecting the yield of maidenhairtree polysaccharide are: time < solid-liquid ratio < microwave power.
The extraction rate of maidenhairtree polysaccharides by four single extraction methods, ranked from small to large, is HWE, UAM, EAM, and MAE, according to experimental research. The ability of the physical and mechanical destruction method to biodegrade the plant cell wall and significantly increase the solubility of polysaccharides may be the cause of this condition. Under the ideal process conditions, maidenhairtree polysaccharides were extracted using a variety of single extraction techniques. The best method was microwave extraction, with extraction rate as the reference value. The yield of polysaccharides extracted from maidenhairtree fruit was consistently higher than that from maidenhairtree leaves, showing that the polysaccharide content in maidenhairtree fruit was relatively high.
2.1.2. New technology extraction applications
-
(1)
Ultrafiltration membrane technology
Ultrafiltration is a membrane separation technology (MST). The commonly used membrane separation methods include microfiltration, nano-filtration, reverse osmosis, etc. Ultrafiltration and microfiltration are the most commonly used extraction technologies for polysaccharide extraction. Ultrafiltration membrane is a new and efficient separation technology, which uses a selective permeable membrane as a medium and applies different potentials and pressures on both sides of the membrane to form a driving force. According to the principle of screening, the polysaccharide was selectively passed through the membrane and concentrated and purified again [28]. The pore size of ultrafiltration membrane was 1–100 nm, the molecular weight of interception was 1×103 – 1×106 Da, and the material with molecular weight greater than 1×103 Da was intercepted, which could fundamentally realize the separation and purification of plant polysaccharides. This technology has the advantages of high efficiency, energy saving and no pollution [29]: ① It is a physical method without introducing any impurities. ② The conditions were mild and had no effect on the biological activity of polysaccharides at room temperature. ③ It can realize gradient extraction, extraction and separation at the same time, and can improve the purity [30].
-
(2)
Semi-bionic extraction(SBE)
The semi-bionic extraction technique is an extraction technology built on the “gray thinking mode” of cybernetics. Also need to use a certain pH acid and lye extraction in turn in turn can increase the medication bioavailability by imitating the process of oral drug distribution through the digestive system. Polysaccharide macromolecules with a high concentration of active components have been synthesized using a new extraction technique developed from a biopharmaceutical perspective [31]. SBE has the following advantages: (1) The extraction of polysaccharides does not use organic solvents, so there is no residual organic solvent, which makes the operation of separating and purifying polysaccharides more convenient, not only protecting the environment, but also saving costs; (2) The extraction temperature is relatively low, reducing the effect of temperature on the active components of polysaccharides. However, SBE also has some shortcomings, such as the high cost of artificial gastrointestinal fluid and the waste of time due to the emptying and rapid propulsion of the digestive tract.
-
(3)
Supercritical fluid extraction technology(SFE)
Supercritical fluid extraction (SFE) is a new physical extraction technology that emerged in the early 1980s. It is a fluid between gas and liquid, above the critical temperature (Tc) and critical pressure (Pc) as an extractant [32], selectively separating and purifying substances with different relative molecular mass, boiling point and polarity. Commonly used supercritical fluids with stable chemical properties are: CH2 = CH2, CO2, CH3OH, H2O, C2H6, etc. CO2 is the most commonly used solvent in SFE because of its relatively easy to achieve operating temperature and pressure, and it is easy to reach supercritical state (critical temperature is 31.1℃, critical pressure is 7.38 MPa) [33]. FE-CO2 technology can provide extraction conditions that cannot be obtained by ordinary solvents, greatly improving the extraction rate, and has the characteristics of safety, non-toxicity, low extraction temperature, non-flammability, no residue, and high efficiency [34]. It has been widely used in food, spices, and plant polysaccharides.
2.1.3. Collaborative extraction methods
In addition to a single method to extract polysaccharides, many studies have shown that synergistic extraction has the advantages of a variety of single extraction methods, overcomes the shortcomings of single extraction, changes the traditional single method preparation mode, improves the extraction and purification of polysaccharides, and enhances their biological activities [35]. The benefits of combining extraction techniques with various technologies are shown in Table 1c. According to studies, the order of water extraction methods using synergic technology from highest to lowest yield was water extraction with ultrasound, water extraction with enzymes, water extraction with microwaves, and water extraction with alcohol precipitation. Additionally, there are many synergistic extraction methods such as ultrasonic-assisted semi-bionic extraction, semi-bionic-enzyme extraction, supercritical extraction-ultrasonic extraction, etc., to extract polysaccharides to the greatest extent to improve the efficiency of biological utilization.
Table 1c.
Advantages of collaborative extraction methods.
| Method | Advantage |
|---|---|
| Ultrasonic-assisted water extraction | It has the characteristics of short time, low energy consumption and high product extraction rate, which can meet the needs of large-scale production; |
| Microwave-enzymatic extraction method | It has the advantages of high efficiency, simple operation, high yield, easy separation and purification of products; |
| Ultrasonic – microwave extraction | Safer, detection of large sample size, less affected by solvent, reduce extraction time and reduce energy consumption; |
| Ultrasonic assisted – enzyme extraction | High extraction efficiency, mild extraction conditions and less impurities in the product. |
The traditional polysaccharide extraction technology cannot be ignored in large-scale production. Traditional technology and modern technology complement each other. In practice, in order to achieve the purpose of short time and high purity, the use of advanced technical means for organic integration is the future preparation, separation and purification of active polysaccharides. For example, experiments have shown that the cellulase-microwave-assisted extraction method is used to extract the polysaccharide of maidenhairtree testae, and the enzymolysis is carried out at 50℃ for 1 h. The optimal extraction process is: extraction temperature 100℃, extraction 3 times, each time takes 6 min. The content of maidenhairtree exopleura polysaccharide was about 9.80 % by phenol sulfuric acid method, and the weight average molecular weight was about 16,320 by HPGFC. Compared with the single extraction method, the extraction method has the advantages of environmental friendliness, economic efficiency and high polysaccharide extraction rate.
2.2. Extraction status analysis
Maidenhairtree polysaccharide offers a wide range of physiological effects, including the anti-aging, lipid-lowering, liver protection, anti-cancer, antioxidant activity, and so on [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]. It also has potential medicinal benefit and future medical advancement chances. In order to extract the polysaccharide from maidenhairtree, Zhang, et al. [60] developed an environmentally friendly polyethylene glycol (PEG) solvent and combined it with ultrasonic-assisted enzymatic method. Through experiments, better extraction conditions were obtained, and polysaccharides were extracted from maidenhairtree leaves. Under the optimum conditions, the polysaccharide yield can reach 7.29±0.21 %. An Arabic polysaccharide that might successfully prevent cerebral ischemia/reperfusion injury and had a good protective effect was isolated by Yang et al. [61] from maidenhairtree leaves. Cui et al. [62] maidenhairtree polysaccharides (GBLP as a reducing agent and stabilizer) with good stability and biocompatibility have prepared palladium nanoparticles (Pdn-GBLPNPs) with good accuracy, high selectivity, wide linear range and low detection limit by a green and environmentally friendly process, which has good applications in biomedical technology.
2.3. Separation and purification
Typically, the polysaccharides found in maidenhairtree are unrefined. The two basic processes for removing additional contaminants are refining and fractionation. To create homogeneous polysaccharides with a comparatively high purity, non-polysaccharide components such proteins, lipids, phenols, flavonoids, pigments, nucleic acids, and other contaminants, such as organic and inorganic chemicals coupled with plant polysaccharides, need first be eliminated. Getting the necessary uniform dispersion of polymer is the ultimate goal of polysaccharide purification. In general, there are two steps in the purification of polysaccharides: first, contaminants from raw polysaccharides are removed, and then different separation techniques are used to separate polysaccharides to produce the desired target product [63]. The neutral polysaccharide was separated by salt gradient using ion exchange chromatography in order to purify maidenhairtree polysaccharide. Polysaccharides of different molecular weights were separated using particle size exclusion chromatography.
The traditional processes for refining crude polysaccharides include:
-
(a)
Decolorization, which uses trichloroacetic acid (good effect but easy to destroy), activated carbon adsorption (stronger affinity but greater loss), hydrogen peroxide (pigments containing unsaturated double bonds, hydroxyl groups, and aromatic rings), protease (mild and efficient), ion exchange (high decolorization rate and retention rate), and other techniques [64];
-
(b)
The Sevage method, trichloroacetic acid (TCA), sodium chloride, calcium chloride, and other processes are the most popular and efficient ways to remove proteins [65];
-
(c)
Fractionation purification: water used in dialysis, ethanol treatment (precipitation), and ultrafiltration (ultrapure, distillation). According to their size, charge, and chemical effects, polysaccharides were separated using resin columns, gel permeation chromatography, ion exchange chromatography, and affinity chromatography.
2.3.1. Step alcohol sink
According to relevant data, the concentration of ethanol has a certain effect on the yield of polysaccharides. There are usually the following rules: when the concentration is low, polysaccharides, proteins, cellulose, starch and other substances with relatively large molecular weight are deposited. The medium molecular weight polysaccharide was mainly precipitated in 50%-60 % ethanol [66], and the impurity protein content was low. Higher concentrations (not less than 65%) deposited mostly oligo- and polysaccharides, with lower molecular weights for the polysaccharides produced at these concentrations. A series of experiments showed that when the concentration of ethanol was less than 60 %, the desired polysaccharide product could not be obtained and the polysaccharide could not be extracted. The results are shown in Table 1d.
Table 1d.
Effect of ethanol concentration on yield and purity of maidenhairtree polysaccharides.
| Ethanol concentration % | purity % | yield % | Precipitation phenomenon |
|---|---|---|---|
| 30 | — | — | No precipitation phenomenon |
| 40 | — | — | No precipitation phenomenon |
| 50 | 37.92 | 0.25 | Precipitation begins to precipitate |
| 60 | 58.20 | 1.62 | There is a large amount of precipitation. |
| 70 | 70.40 | 0.55 | Sedimentation continues to occur |
| 80 | 73.20 | 0.77 | Precipitation phenomenon is not obvious |
2.3.2. Column chromatography
Anion exchange chromatography (AEC) and gel filtration chromatography (GFC) are the two most common and straightforward polysaccharide fractionation techniques used today (Table 1e). Diethylamine ethyl (DEAE)-cellulose anion exchange column chromatography is usually used in combination with gel column chromatography [67]. It has been frequently employed by researchers due to its great properties in plant polysaccharide distillation. In column chromatography, cellulose is a regularly employed stationary phase. In order to prevent the non-specific adsorption of cellulose molecules on polysaccharides, it is balanced with alcohol. With the most recent eluents, the polysaccharide fractions can be eluted (such as water, buffer, and some organic solvents). There will be more theoretical plates because of the smaller cellulose particles and bigger surface area. The outcomes demonstrated that polysaccharides' classification effect improved. However, column chromatography is not widely used because of its low efficiency, sluggish flow rate, and lengthy time commitment, limited to the laboratory use more.
Table 1e.
Separation and purification of by AEC and GFC.
| Anion exchange chromatography(AEC) | Gel filtration chromatography(GFC) | |
|---|---|---|
| Principle | Polysaccharides are separated according to charge and polarity indices | Fractional distillation according to polysaccharide size and shape |
| Elution order | ① Acidic polysaccharides with high uronic acid content could strongly bind to anion resin, while neutral polysaccharides had no interaction with anion resin, so they were eluted first. ② Fractionation is performed by using a combination of gradient elution practices and different ionic eluents |
① Macromolecules and high molecular weight polysaccharides do not enter the pores of the gel and are first eluted; ② Polysaccharides with small and low molecular weights block the pores of the gel and are finally eluted. |
| Chromatographic column | Diethylaminoethyl(DEAE) | Agarose, agar propylene, Superdex, biogel and agarose |
| Anion exchanger/Gel selection | Depends on small-scale experiments and the concentration of uronic acid in the polysaccharide | Much depends on the nature and source of the polysaccharide to be fractionated |
| limitation | Long time consuming; According to different uses, different resins need to be selected, and the general scope of application is small. | Instruments are expensive, inefficient, lack automation and difficult to scale up. |
Polysaccharides were extracted from maidenhairtree leaves, fruits and testae by different extraction methods. A variety of different homogeneous components were obtained by ion exchange-gel column chromatography and molecular sieve-gel column chromatography. Their total sugar content, polysaccharide molecular weight and monosaccharide composition were different. The Congo red experiment was used to study the red shift of the maximum absorption wavelength of the homogeneous polysaccharide isolated and purified, revealing that its structure has a triple helix shape. The above components were analyzed by FT-IR and HPLC. The results showed that they all contained the characteristic absorption peaks of polysaccharides, including β-glycosidic bonds and pyranose rings. They were composed of different monosaccharides, and the molar ratio of monosaccharides was also significantly different.
2.3.3. Other separation methods
Liquid selective adsorption chromatography, sometimes referred to as affinity chromatography (AC), is another analytical technique used in the fractionation of polysaccharides. It divides polysaccharides based on their ability to bind to stationary phases. Stationary phases in affinity chromatography are immobilized ligands [68]. Many immobilized lectins that are sold commercially can be employed as AC ligands. Concanavalin A, wheat germ agglutinin, and agarose have all been used to separate glycoproteins as of late. Although affinity chromatography has the potential to separate materials on both a small and large scale, the conventional screening of polysaccharide coordination takes a long time. Future research will need to focus on examining the fundamentals of polysaccharide classification and separation.
2.4. Basic extraction step design
Basic steps: raw material picking and cleaning → drying → grinding → sifting → petroleum ether degreasing → filtration and drying → Adding water → constant temperature leaching for a certain time → filtration → centrifugation → concentration in constant temperature water bath → protein removal by Sevage → centrifugation → ethanol (not less than 65%) precipitation → centrifugation after a period of time → drying.
Main points of operation:
-
(a)
The water extraction and alcohol precipitation method is to use the physical and chemical properties of polysaccharides-dissolved in water, insoluble in organic solvents such as alcohol and ether to precipitate polysaccharides. Generally, water extraction can extract most of the polar substances, but in ethanol, the solubility of this polar molecule is very low or insoluble, so when the concentration of ethanol reaches a certain level, a large amount of polysaccharide precipitate will be produced. Using this principle, impurities can be selectively removed from the extract according to the different concentrations of ethanol. Generally, the ethanol content is more than 60 %, and starch and protein can be effectively removed.
-
(b)
Sevage method to remove protein, protein will be affected by chemical factors, resulting in its function, activity, physical and chemical properties completely lost, so the extract and Savage reagent (n-butanol: chloroform = 1: 5) mixed can effectively remove protein components.
3. Structure analysis of maidenhairtree polysaccharides
Polysaccharide has a complicated structural makeup, making quality control challenging [69]. Chemical make-up, molecular weight, primary chain make-up, degree of branching, degree of polymerization, glycosidic bond, and three-dimensional conformation are examples of structural characteristics [70]. Plant polysaccharides form long chains by α-or β-glycosidic bonds as bridges to connect monosaccharides of the same or different species. Taking plant polysaccharide rhamnuronic acid-II with complex structure as an example, 21 different glycosidic bonds were connected to 13 different monosaccharide compositions [71]. Galactomannan can be dissolved in water, with β- (1 → 4) -linked D-mannan and a single α- (1 → 6) -linked D-gap residue. The mannose unit in galactomannan is more than the galactose unit. Some polysaccharides are unique to specific sources.
Different extraction methods and processes will lead to some differences in MTP monosaccharide composition. The molecular structure of MTP determines its biological activity, so studying its structural characteristics is a key step in exploring its biological activity. The primary techniques used to investigate the composition and structure of MTP include high performance gel permeation chromatography (HPGPC), Fourier transform infrared spectroscopy (FT-IR), gas chromatography-mass spectrometry (GC–MS), high performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), ultraviolet spectrophotometry (UV), and capillary zone electrophoresis-amperometry (CZE-AD) [72]. An analytical technique frequently employed to ascertain the structural characteristics of MTP was shown in Table 1f.
Table 1f.
Analysis methods for determination of primary structure of MTP chain.
| Projects measured | Common method |
|---|---|
| Determination of relative molecular mass | Gel chromatography, vapor pressure osmometer method, end base method, light scattering method, ultrafiltration method, etc. |
| Monosaccharide composition and molecular proportion | Acid hydrolysis, gas chromatography – high performance liquid chromatography, paper chromatography. |
| Pyran ring or furan ring form | Infrared spectrometry(IR). |
| Connection order | Smith degradation, Nuclear magnetic resonance(NMR). |
| α-or β-isomers | Enzymatic hydrolysis, Nuclear magnetic resonance. |
| Hydroxyl substitution | Mass spectrum(MS), NMR, Periodate oxidation. |
IR analysis of maidenhairtree polysaccharide extracted by cellulase hydrolysis showed that the structure of maidenhairtree polysaccharide contained α-D-glucopyranose. Maidenhairtree polysaccharides obtained by three different extraction processes (Hot water extraction, Microwave extraction, and Shaker extraction) were named MTP-I, MTP-II, and MTP-III, respectively. The results of infrared spectrum analysis showed that the maidenhairtree polysaccharides obtained by the above three extraction methods contained α-type glycosidic bonds. MTP-I and MTP-III had an absorption peak at 930 cm−1, indicating that they had β-type glycosidic bonds. The absorption peak at 530 cm−1 indicated that there was a carbonyl group. MTP-II has neither β-glycosidic bond nor carbonyl group, which is because microwave may destroy β-glycosidic bond and carbonyl group, thus changing its molecular structure. It also further illustrates that different extraction methods will affect the structure of polysaccharides.
3.1. Monosaccharide composition
Maidenhairtree polysaccharide can be extracted from its leaves, outer seed coat and fruits, including maidenhairtree polysaccharide, maidenhairtree polysaccharide and maidenhairtree maidenhairtree polysaccharide. Most studies show that MTP is a heteropoly sugar. It consists of mannose (Man), rhamnose (Rha), glucuronic acid (GlcUA), galacturonic acid (GalA), glucose (Glc), galactose (Gal) and arabinose (Ara) [73], The molecular weight ranges from 12 to 3500 kDa.
Analysis of monosaccharide compositions:
By using an ultrasonic-enzymatic water-soluble alcohol precipitation method, Wang et al. [74] isolated polysaccharides from maidenhairtree leaves and examined their structure and chemical makeup. The acid polysaccharide in maidenhairtree that had been isolated had a molecular weight of 12.08 kDa. Rhamnose, arabinose, xylose, mannose, glucose, and galactose were among the monosaccharides, and their molar ratios were 5.56: 3.84: 2.34: 7.54: 8.34: 1. The basic chain structure was thought to beβ-D-Rhap- (1→, β-L-Xylp- (1 → 4) -β-D-Manp- (1 → 4) -β-D-Glcp- (1 → 6) -β-D-Manp- (1 → 3) -β-L-Araf- (1 → 4) -β-D-Galp- (1 → 3,1 → 4) -β-D-Glcp- (1→.
Mao et al. [75] used the HPLC method of pre-column derivatives of 1-phenyl-3-methyl-5-pyrazolone (PMP) to simultaneously detect six different monosaccharides. The findings demonstrated that the exoderm polysaccharides of maidenhairtree were acidic heteropolysaccharides, with mannose, rhamnose, D-galacturonic acid, glucose, galactose, and arabinose serving as their primary constituents.
3.2. Main chain structure and branching degree
Heteropolysaccharides are also called heteropolysaccharides. After hydrolysis, they not only produce various types of monosaccharides, but also some monosaccharide derivatives (such as amino sugars and uronic acids), and even some acidic substances. The spatial structure and unit composition of proteins and nucleic acids are familiar, and polysaccharides also have primary structure and spatial conformation [76]. The primary structure of polysaccharides is divided into main chain type and branch chain type. Main chain characteristics: connection mode, order and glycosyl composition; the branched characteristics mainly include the length of the branch of the polysaccharide, the position and type of the main chain [77]. Therefore, the primary structure of polysaccharides is very complex because of its diverse components and various permutations and combinations, such as upright, curved, and some contain more branched chains. The carbohydrate structure above the second order is composed of the special conformation of polysaccharides. The active polysaccharide structure can be roughly divided into four categories: stretchable strip, wrinkled strip, coiled spiral, and irregularly curved.
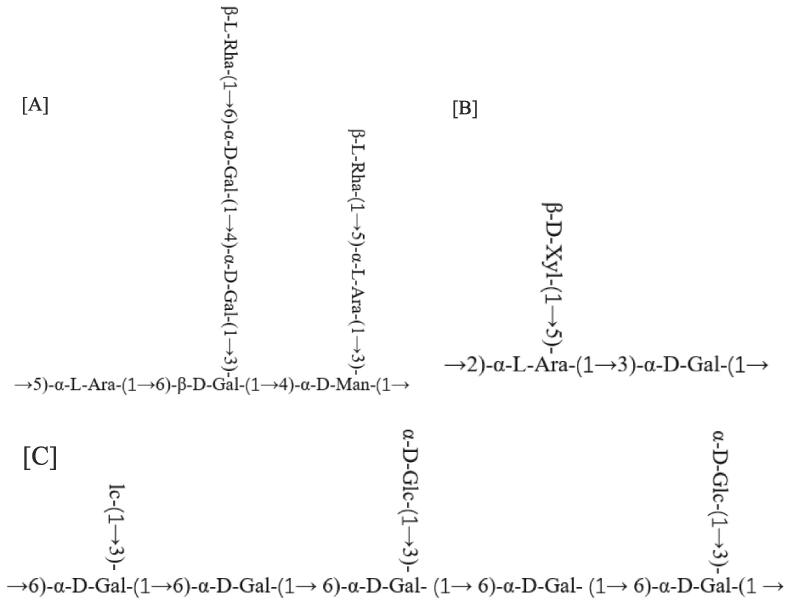
The structure of maidenhairtree polysaccharide was analyzed by means of complete and partial acid hydrolysis, Smith degradation, methylation analysis, infrared absorption spectroscopy and nuclear magnetic resonance. The results showed that maidenhairtree polysaccharide was an acidic heteropolysaccharide, mainly including rhamnose, arabinose, galactose and mannose. The branched chain of polysaccharides was composed of (1 → 5) -α-L-Ara, (1 → 4) -α-D-Gal and (1 → 6) -α-D-Gal, with β-L-Rha as the end, and the main chain was composed of (1 → 5) -α-L-Ara, (1 → 3, 1 → 6) -β-D-Gal and (1 → 3, 1 → 4) -α-D-Man, as shown in Fig. 3[A]. In Fig. 3[B], maidenhairtree polysaccharide is mainly composed of xylose, arabinose and glucose. The branch chain of the polysaccharide is composed of β-D-Xyl, and the main chain is composed of (1 → 2, 1 → 5) -α-l-arabinose and (1 → 3) -α-D-glucose. The main structural unit of maidenhairtree polysaccharide in Fig. 3 [C] isα-D- (1 → 6), with an average of one branch chain every other Gal. The main chain is mainly composed of galactose and (1 → 6), (1 → 3, 1 → 6) glycosidic bonds alternately. α-D-Glc is distributed on the branches closely connected to the main chain, and indirectly consists of a small amount of Glc- (1 → 3). Different possible structural units of maidenhairtree polysaccharides were shown in Fig. 3.
Fig. 3.
Primary structural units of different maidenhairtree polysaccharides.
3.3. Molecular morphology
The biological activity of polysaccharides is closely related to factors such as molecular weight, type and number of substituents, type of glycosidic bonds, number of branched chains, and spatial arrangement. The in-depth study of the structure of polysaccharides can provide a basis for its functional characteristics and mechanisms. SEM observation showed that GBLP had certain pores and irregular distribution, which was related to the intermolecular force effect of polysaccharides. The structure had an important influence on its properties, biological activity and structure-activity relationship. Different extraction methods of maidenhairtree polysaccharide will lead to certain differences in the structure of maidenhairtree polysaccharide. The SEM analysis findings of maidenhairtree seed polysaccharides treated by HWE, UTE, ETE, and UETE were displayed in Fig. 4 [78]. As seen in Fig. 4 A, HWE-polysaccharide exhibited a significant number of tiny, compact spherical forms. According to Fig. 4 B, UET-polysaccharide had pores, an uneven surface, and an unusual geometric shape. Fig. 4 C and 4 D demonstrated the spongy surface shape of ETE – polysaccharide and UETE – polysaccharide. Gong et al. [79] observed the structure of maidenhairtree polysaccharides by scanning electron microscopy. As demonstrated in Fig. 4(E and F), the structure was thin, wide, flaky, smooth on the surface, and accompanied by certain strip structures with polysaccharide structure.
Fig. 4.
A, B, C and D were SEM images of maidenhairtree seed polysaccharide. (E) and (F) were SEM images of maidenhairtree leaves.
4. Activities of maidenhairtree polysaccharides
Because of its rich composition and varied applications, plant polysaccharide research has recently advanced quickly. Plant polysaccharide is a kind of substance with more than one important biological activity, which is closely related to the physiological function of the body. Through the study of pharmacology and pharmacodynamics, it shows that it can activate and enhance the immune function of the body quickly and efficiently, prevent oxidation, treat inflammation, virus and tumor, regulate blood sugar and repair autoimmune diseases.
We have reason to believe that in the 21st century, just as the era of genetic protein has made a great contribution to human health, active polysaccharides will also be our main health guardians.
4.1. Effect of structure on activity
4.1.1. Antioxidant activity, free radical scavenging and anti-aging function
The aging of the human body is essentially the continuous generation and disappearance of free radicals in the body. The forms of free radicals in the body are also diverse. They can be directly produced by DPPH free radicals, hydroxyl radicals, superoxide anion radicals, etc., and can also be released as intermediates and final products of the reaction. If a large number of free radicals in the body are generated or cleared too slowly, they will cause damage to human cells, organs and other biological macromolecules, thus accelerating the aging process of the body and causing some chronic diseases. Studies have shown that Lentinula edodes, corn silk, onion, Maidenhairtree leaves, and Lycium barbarum polysaccharides can scavenge free radicals, inhibit lipid peroxidation, and reduce the production of malondialdehyde; in addition, it can also enhance the activity of antioxidant enzymes in the human body and inhibit the lipid peroxidation damage caused by free radicals. The polysaccharides from leaves, seeds and peels of maidenhairtree could effectively inhibit and scavenge free radicals as well as exert the antioxidant activity [80]. The amount of polysaccharides favorably associated with the antioxidant activity. Influencing variables: The polysaccharide molecular weight and monosaccharide content may be impacted by the extraction technique. The characteristics of monosaccharides will vary depending on their various components. For instance, a high content of rhamnose and low molecular weight monosaccharides will result in polysaccharides with strong antioxidant activity, which will alter the polysaccharides found in maidenhairtree.
4.1.2. Antitumor activity
In cell models, animal disease models, and clinical situations, plant polysaccharides show a great deal of promise in the treatment of oxidative stress and cancer-causing disorders. These polysaccharides commonly use ros-centric mechanisms, including mitochondrial autophagy, MAPK, and JNK, as well as transcription factor-related pathways, including NF-KB and HIF, whether or not additional inflammatory and death receptor pathways are also implicated, certain polysaccharides may also have an impact on tumorigenic pathways, such as Wnt (a secreted glycoprotein) and p53 (a tumor-suppressor protein and transcription factor). It was shown in Fig. 5.
Fig. 5.
Summarized the signaling pathways involved in oxidative stress-induced pathological events and cancer development [2].
One of the mechanisms of tumor inhibition is to induce cell apoptosis and differentiation [81]. At present, existing experiments have shown that polysaccharides can indirectly improve the immune function of the body by directly acting on the apoptosis of tumor cells, and have β-1,6 glycosidic bonds in its main chain, which has anticancer effects. Polysaccharide is an effective anticancer agent. It is non-toxic to human body and has no side effects. It is an important progress in the field of medicine and is related to human life and health [82]. The results showed that maidenhairtree exopleura polysaccharide had obvious effect on inducing apoptosis and differentiation of tumor cells, which could reduce the pain of gastric cancer patients, enhance appetite and reduce tumor volume, so as to achieve the purpose of treating gastric cancer. However, and there are significant limits in both clinical and scientific research as a result of the complicated chemical composition of polysaccharides, varying quality standards, and some flaws. As The Times continues to advance, the investigation of polysaccharides is being deepened via the application of novel separation and analytical techniques that have helped to clarify their structure and the important connection between their structural functions [83].
4.1.3. Immune regulation
A large number of pharmacological and clinical trials have found that fungal polysaccharides, animal polysaccharides, and plant polysaccharides have certain immune enhancement effects. Acting as a host immune enhancer (HDP) is the most important and basic function of this kind of polysaccharide. Its mechanism of action is similar to that of traditional Chinese medicine. It shows the regulation of host immunity in pharmacology, promotes the maturation, differentiation and proliferation of immune cells, improves the balance of host cells, and enhances the response of host cells to factors such as lymph factors and hormones [84]. Too many cytokines and inflammatory mediators may lead to systemic inflammatory response syndrome (SIRS), even tissue damage and endotoxic shock [4]. According to studies, PGBL has a strong activating and non-specific immune regulation effect since it might considerably reduce the activities of NK cells, T cells, and lymphocytes in mice and increase the phagocytic index of peritoneal phagocytic cells on chicken erythrocytes [85].
Jiang et al. [86] isolated GBSPII-1, a new acidic heteroposaccharide with a molecular weight of 34 kDa, from the fresh peel of maidenhairtree. By suppressing gene expression, GBSII-1 regulated the development of Staphylococcus aureus biofilm and lowers the release of polysaccharide intercellular adhesion (PIA). It possessed antibacterial effect on Staphylococcus aureus and was a potential biocontrol agent for developing new treatment plans for illnesses caused by Staphylococcus aureus that were linked to biofilms. It caused Staphylococcus aureus to fail to form biofilms at a dose of 100 g/L of GBSPII-1.
It was evaluated the antiviral activity and action mode of polysaccharides extracted from maidenhairtree exocarp against porcine Epidemic diarrhea virus (PEDV). The results showed that maidenhairtree exocarp polysaccharides may be involved in the interaction of PEDV-Vero cells, because the attachment and entry of the virus into Vero cells were hindered by polysaccharides. Therefore, it had an effective inhibitory effect on the virus attachment and entry steps of the PEDV life cycle and is a new candidate agent for PEDV treatment.
The antiviral efficacy and mechanism of action of polysaccharides derived from maidenhairtree exocarp against swine epidemic diarrhea virus (PEDV) were assessed by [87]. The results showed that the interaction between maidenhairtree exocarp polysaccharides and PEDV-Vero cells was related. As a result, this technique efficiently prevents PEDV from attaching to and invading host cells, offering a fresh approach to treating PEDV.
Li et al. [88] purified RG-I polysaccharide (MTP-A2b) having a molecular weight of 44 kDa and extracted GBLP-A2 acidic polysaccharide from maidenhairtree leaves. The findings demonstrated that GBLP-A2 might dramatically raise VEGF and HGF levels in alopecia areata mouse skin tissue while lowering serum inflammatory factor levels.
4.1.4. Other biological activity
In rats with non-alcoholic fatty liver disease (NAFLD) brought on by a high-fat diet (HFD), water-soluble maidenhairtree polysaccharide (GBLP) was isolated and its preventive effects were assessed [89]. Oral administration of GBLP could dramatically improve lipid alterations, improve insulin resistance, retain liver function, and boost antioxidant capacity in NAFLD rats. The findings indicated that GBLP seemed to have some protective effects in NAFLD, and these effects might be connected to IR attenuation, liver function preservation, enhancement of the antioxidant defense system, and reduction of lipid peroxidation.
A water-soluble polysaccharide found in maidenhairtree leaves has been demonstrated to lessen depression brought on by stress [90]. According to the findings, probiotics, notably Lactobacillus reuteri, help GBLP control the gut-brain axis and treat depression-related intestinal diseases. The richness of lactobacillus species, which has been demonstrated to be a route for depression, was increased by GBLP similarly to the antidepressant Paxil. The polysaccharides found in maidenhairtree leaves have been proposed as a potential pharmacological target and therapeutic approach for depression.
Zhu and Xie [76] used aerobic exercise combined with maidenhairtree polysaccharide to treat diabetic rats. The study found that after the diabetic rats were injected with maidenhairtree polysaccharide through aerobic exercise, the secretion of insulin increased, and the body weight and blood glucose of the rats could be controlled. Therefore, it is suggested that the mechanism of maidenhairtree polysaccharide may promote the repair and regeneration of islet cells in vivo, thereby improving the insulin pathology of diabetic rats.
Oh et al. [91] used ethanol precipitation, hot water extraction, and column chromatography to extract polysaccharides from the maidenhairtree outer seed coat. It was discovered that maidenhairtree's outer seed coat polysaccharides prevented rice sheath blight in all treatment doses. According to the findings, maidenhairtree was a type of natural substance, and the polysaccharide derived from maidenhairtree exhibits antifungal activity against rice fusarium wilt.
The MTP's chemical make-up was displayed in Table 1g. It showed that the molecular weight, content, molar ratio, and sugar chain structure of MTP obtained by various extraction and purification techniques are variable, by analyzing the maidenhairtree polysaccharide's molecular weight using high pressure gel filtration chromatography, we can draw the polysaccharide from exopleura of maidenhairtree as low molecular weight polysaccharides, maidenhairtree leaf and maidenhairtree nut polysaccharide of macromolecular weight polysaccharide. At present, we cannot uniformly define the structural characteristics of MTP.
Table 1g.
Chemical composition and biological activities of MTPs.
| Extraction method | Polysaccharide monomer | Purification method | Molecular weight | Monosaccharide composition | Molar ratio | Main chain structure/glycosidic bond | Bioactivity | References | |
|---|---|---|---|---|---|---|---|---|---|
| Polysaccharides of maidenhairtree leaf | Ultrasonic assisted – enzyme extraction (The mass ratio of cellulase to pectinase was 1:2) | GBLP11 GBLP22 GBLP33 |
DEAE-52 cellulose, Sephadex G100 column, neutral protease and TCA | 194.43 KDa 5167.3 KDa 45.98 KDa |
Glc, Gal, Ara, Rha | 62.2:1.98:3.84:1.24 3.57:60.8:4.67:0.36 5.34:48.8:1.70:8.97 |
— | Antioxidant activity | [92] |
| Supercritical CO2 fluid extraction (SFE-CO2) | GBLP-1 GBLP-2 GBLP-3 |
— | 218.5 kDa 78.83 kDa 44.66 kDa |
Man, Rha, GlcA, GalA, Glc, Gal, Ara | 3.64:4.25:8.48:9.10:4.39: 50.30:19.84 |
— | Hypoglycemic and antioxidant properties | [79] | |
| Maidenhairtree exocarp polysaccharide | Hot water extraction | GBEP-NN (Neutral polysaccharide) | DEAE-Sepharose Fast Flow column and Superdex 200 column | 4.57 kDa | Ara, Man, Glc, Gal | 1.88:2.41:1.53:1:2 | Glucopyranoside bond | Antioxidant activity | [6] |
| GBEP-AA (Acidic polysaccharide) | DEAE-Sepharose Fast Flow column and Sevag method | 5.68 kDa | Man, Rha, GlcUA, GalA, GalN, Glc, Gal, Xyl, Ara | 11.85:15.98:1:5.05:15.98: 193.52:3.34:14.56:2.67 |
Polysaccharides containing uronic acid | ||||
| Maidenhairtree seed polysaccharide | Ultrasonic extraction processat 350 w for 30 min | UTE- polysaccharide | Sevag method | 1340 kDa | Man, Rha, Glc | 8.25:1.00:1.53 | — | Antioxidant activity | [78] |
| — | — | — | 1600 kDa | Rha, Ara, Gal, Glc and Man | 3.50:8.50:3.40:1.80:1.00 | The molecule structure is formed through 1 → 4 or 1 → 6 binding | Antitumor activity | [93] | |
4.2. Effect of structure on activity after derivatization
The bioactivity of polysaccharides is tightly correlated with their structural characteristics, and the characteristics of their derivatives also heavily depend on modification techniques and processing settings. This is because different modification techniques and processing conditions will have different effects on the level of substitution, molecular weight, conformation, and solubility of derivatives of bioactive polysaccharides. The following methods of chemical modification are frequently used: sulfation, phosphorylation, selenization, benzylation, carboxymethylation, etc. Many structural analytical techniques can be used to demonstrate that the native polysaccharide has already been successfully changed. Examples are Fourier Modified polysaccharide FTIR spectra frequently exhibit the emergence of new peaks (s) or a rise in the intensity of pre-existing peaks. Nuclear magnetic resonance studies of its polysaccharide derivatives (physicochemical characteristics and IH-NMR) revealed extra peaks or shifted peaks. A large number of studies have shown that chemical modification of polysaccharides can significantly improve their biological activity, in particular, they can show new activity, and have good physiological characteristics in maintaining health and preventing diseases. Currently, polysaccharide derivatives have made significant progress in antiviral, anti-inflammatory, antibacterial activities [94], anti-tumor, anti-oxidation, and other investigations, opening up a wide range of potential therapeutic options for viral disorders.
4.2.1. Sulfate polysaccharide
Sulfated polysaccharides are polysaccharides with sulfate groups on the sugar group, which can be obtained by natural or sulfate structural modification. It is one of the most studied antiviral polysaccharides. For example, natural sulfate polysaccharides such as chondroitin sulfate and heparin have biological functions such as sterilization and resistance to viruses. The sulfated polysaccharide activity of homogeneous components is higher than that of hybrid sulfated polysaccharides. If the polysaccharide is mainly composed of β- (1 → 3)-D-glucan, its biological activity is higher than that of β-(1 → 6)-D-glucan, so that the research of sulfate polysaccharides has attracted much attention. At present, the commonly used sulfonation modification processes are: chlorosulfonic acid-pyridine method, concentrated sulfuric acid method, sulfur trioxide-pyridine method [95]. The result of modification is to add sulfate groups to the polysaccharide free radicals to increase their biological activity.
4.2.2. Acetylated polysaccharide
Acetylation of polysaccharides is one of the commonly used chemical modification methods, which involves the substitution of hydroxyl oxygen or amino nitrogen of polysaccharides by acetyl, and its main purpose is to modify the branched chain of polysaccharides. After acetylation, more polysaccharides can be dissolved in water to enhance their biological activity, and lessen their toxicity and negative consequences. Although there are differences in the methods of acetylation of polysaccharides, acetic anhydride is usually used as a derivative [96]. Generally, the acetic anhydride-pyridine method is used to introduce acetyl groups. The polysaccharide is dissolved in an organic solvent, and then pyridine is added as a solubilizer, which can accelerate the reaction speed, increase the substitution of acetylation, and react with acetic anhydride under continuous stirring (Fig. 6). The polysaccharide product was determined by infrared spectroscopy. It was observed that the absorption peak increased with the increase of acetyl substitution degree in the wavelength range of 1735–1729 cm−1, indicating that the modification was successful. If the stretching vibration intensity of hydroxyl group is much higher than that of carbonyl group, the substitution rate of acetyl group is very low. Therefore, 13C NMR can be used to further determine the modified site of acetyl group. The molecular weight, monosaccharide composition and spatial structure of polysaccharides will be changed by acetyl groups with different contents and substitution sites, and there are differences in water solubility, emulsification degree and crystallization degree [97]. The combination of polysaccharides and acetyl groups can improve the effects of antioxidant, hypoglycemic and anticancer.
Fig. 6.
Reaction mechanism of acetylated polysaccharide.
Four water-soluble polysaccharides GBEPP-11, GBEPP-22, GBEPP-33 and GBEPP-44 were isolated and purified by cellulose DEAE-52 and Sephadex G-100 column chromatography [98]. The anti-tumor effect of GBEPP-11, GBEPP-22, GBEPP-33 and GBEPP-44 in vitro was detected by MTT method. The experiment proved that its inhibitory effect on tumor cells was dose-dependent. Leukemia cells (U937) were the tumor cells that were most effectively inhibited by polysaccharides, and the rate of inhibition rose as MW decreased. Acetylated GBEPP-11 had stronger activity than unacetylated GBEPP-11, and at a concentration of 640 g/mL, the inhibition rate was 78.97%.
4.2.3. Phosphorylated polysaccharide
Phosphorylated polysaccharides are a class of substances commonly found in nature, with diverse forms and complex structures. According to the morphology of phosphate ester, natural phosphorylated polysaccharides can be divided into polysaccharide phosphate monoester and polysaccharide phosphate diester [99]. Because there are few kinds of polysaccharides extracted from nature, people often use chemical modification to synthesize sulfated polysaccharides. Phosphoric acid and its anhydride, phosphorus oxychloride, phosphoric acid are commonly used for phosphorylation of polysaccharides [57].
4.2.4. Seleno-polysaccharide
The bioavailability of selenium can be increased and the body can more readily absorb selenium when combined with polysaccharide. Polysaccharides that contain selenium are a valuable source of dietary supplements. Selenium deficiency can cause a decline in body function, thereby increasing the risk of highly pathogenic diseases. There are numerous methods for selenizing polysaccharides, such as the nitric acid-sodium selenite method and the selenium oxide pyridine approach. Selenium can partially prevent the spread of the HIV and influenza viruses. At present, studies have shown that selenium polysaccharides can inhibit the adsorption of viruses on human cells, thereby effectively controlling the spread of bacteria.
For the first time, it was revealed by Chen et al. [100] that Se-GBLP isolated from maidenhairtree leaves can somewhat suppress the development of human bladder tumor T24 cells, leading to a reduction in cell activity as a result of apoptotic cell death. Se-GBLP is a potential medicine for the detection or treatment of human bladder cancer, and it merits further research. Additionally, research has demonstrated that selenium polysaccharides derived from therapeutic plants have potent anticancer action against a range of human cancer cells, including breast cancer.
5. Application of maidenhairtree polysaccharides
Vegetables, algae, fruits, beans, fungi and other natural foods contain a variety of chemical components of polysaccharides, which can be easily extracted from these substances for special purposes. Because of its rich polysaccharide content, it has been widely developed in the world, and is used in the development of biological materials such as food, pharmaceutical, textile, papermaking, and the preparation of biodegradable packaging materials [101].
5.1. Food field
Because of its few adverse effects, polysaccharides are a significant class of biological macromolecules and are regarded as the best raw dietary supplements for health. The physicochemical properties of polysaccharides are greatly influenced by their internal molecular characteristics and external environmental variables. Food polysaccharides' functions and uses in many types of foods are influenced by their physicochemical properties, such as hydration, gelation, emulsification, and thickening [102]. For instance, high hydrophilic and gelling polysaccharides are desirable in frozen foods, while surfactant polysaccharides have considerable application potential in the manufacture of emulsion foods. Polysaccharides have been shown to be extremely useful in food applications as stabilizers, thickeners, emulsifiers, and humectants [103]. They can also be employed as additives to affect the physical, chemical, and textural aspects of food in the food industry for allied food businesses. Polysaccharide, which serves as an oxygen barrier to lengthen food shelf life, has received greater attention in recent years as a promising food packaging material. Overall, whether constructing or enhancing food texture, a rational polysaccharide choice requires a basic understanding of its characteristics.
Maidenhairtree is a natural plant dietary supplement rich in protein, minerals, vitamins and other nutrients. Maidenhairtree seeds have been used for thousands of years, and the polysaccharides extracted from them have been found in the food field to be used in the production of energy drinks, candy, canned food and biopreservative, among others (Table 1h) [104].
Table 1h.
Application of derived polysaccharides in food field.
| Derivatized polysaccharides | Application in food | References |
|---|---|---|
| Carboxymethylated polysaccharide | Food additives, food packaging, food bioactive ingredient carrier, food analysis sensor, functional food, etc.; | [105] |
| Acetylated polysaccharide | Green food materials, bioactive ingredient carriers and functional food, etc.; | [97] |
| Sulfate polysaccharide | Nutraceuticals, food packaging, additives that can also be used to maintain, stabilize and construct processed foods, widely used in food preparation, Such as seasonings, jams, jelly, other dairy products as additives, with stable, gelation, emulsification and thickening properties. | [106] |
5.2. Medical field
Polysaccharides are a novel class of medication that have excellent efficacy and low toxicity. It has recently been the subject of research recently. In many situations, the vaccine merely produces a mild immune response that is insufficient to stop infection. Therefore, vaccine adjuvants are needed to enhance the immune response when preparing vaccines. Polysaccharides belong to a class of natural polymers composed of sugar-linked carbohydrate monomers. Polysaccharides can activate giant cells, T cells, B cells and other cells, and promote the production of cytokines, antibodies, complement molecules and other immune-related molecules. Polysaccharide can be used as a vaccine adjuvant, not only can enhance the specific antigen immune system, but also can improve their immune ability [7]. Direct evidence and justification are provided by GSPS for their prospective application in DC vaccine development as powerful adjuvants and immune-enhancers. The types of polysaccharides added to the vaccine mainly include dextran, mannan and derivatives.
Xinkang capsules are one example of a preparation that contains maidenhairtree polysaccharides as a therapeutic ingredient. These preparations have shown promise in fighting cancer, slowing the aging process, and reducing the formation of bacteria. Traditional Chinese medicine has employed maidenhairtree formulations to treat a variety of ailments, including tinnitus, anxiety, dementia, allergies, liver fibrosis, hepatocellular carcinoma, and cardiovascular health. These herbal treatments have been proven to be both safe and effective [107].
6. Conclusion and prospective
Polysaccharide is a kind of widespread natural product, which has broad application prospects in medicine, food, skin care products, health care products and other fields. It not only opens up a broad market for the development and utilization of maidenhairtree polysaccharides, but also provides a good opportunity for the processing of maidenhairtree leaves, seeds, exocarps and other resources in China. maidenhairtree polysaccharide is an acidic heteropolysaccharide, which is mainly composed of galactose, arabinose, rhamnose, mannose, glucose and galacturonic acid. It is composed of α-D-and β-D-glycosidic bonds. It has anti-cancer, anti-hyperlipidemia, anti-diabetes, anti-oxidation, anti-depression and other activities. Among them, the content of polysaccharides in maidenhairtree exopleura is the most abundant, followed by maidenhairtree leaves and Maidenhairtree fruits. Therefore, the extraction of polysaccharides from maidenhairtree exopleura can be further improved. At present, the study of polysaccharides in natural products in China is still a hot topic. Different extraction methods will affect the properties of maidenhairtree polysaccharides. To achieve more effective separation and purification of polysaccharides is the premise and guarantee for the effective utilization of maidenhairtree resources, and also the only way to realize the industrialization and application of maidenhairtree polysaccharides.
In recent years, domestic and foreign scholars have done a lot of research on the extraction and purification of maidenhairtree polysaccharide. However, due to the lack of in-depth research and systematicness, it is still in the preliminary exploration period of the laboratory. Polysaccharide is a macromolecular substance, its structure is very complex, and the mechanism of action of most polysaccharides is not clear. Some modern extraction techniques with high extraction rate and green environmental protection have not been widely used. Therefore, in the future, we should strengthen the research on the extraction and purification of maidenhairtree polysaccharides, spend a lot of time to discover the specific mechanism of polysaccharides, and learn from the mature experience of other natural polysaccharide extraction and purification technologies (such as Lycium barbarum polysaccharides, Ganoderma lucidum polysaccharides, ginseng polysaccharides, etc.). The extraction and purification technology of maidenhairtree polysaccharides was systematically and deeply studied and innovated without affecting its functional activity, so as to achieve the goal of industrial application.
In-depth study of its structural characteristics and the relationship between its structure and the biological activity of maidenhairtree polysaccharide provide theoretical basis and technical support for the further development and utilization of maidenhairtree polysaccharide. At the same time, it can also supplement the information database of the extraction and purification, structure and activity of maidenhairtree polysaccharide, and the efficient extraction and purification of maidenhairtree polysaccharide can be better applied to clinical, food and other fields. In order to maximize the use of maidenhairtree polysaccharide products in people's daily life and improve people's physique and health, it is necessary to conduct in-depth research on its derivatives and develop more superior and more biologically active polysaccharides to realize the important utilization value of maidenhairtree polysaccharides.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Liu, Y. X., H. W. Xin, Y. C. Zhang, F. Y. Che, N. Shen and Y. L. Cui (2022). Leaves, seeds and exocarp of Maidenhairtree L. (Maidenhairtreeaceae): A Comprehensive Review of Traditional Uses, phytochemistry, pharmacology, resource utilization and toxicity. JOURNAL OF ETHNOPHARMACOLOGY 298. [DOI] [PubMed]
- 2.Jiao R., Liu Y., Gao H., Xiao J., So K.F. The Anti-Oxidant and Antitumor Properties of Plant Polysaccharides. Am J Chin Med. 2016;44(3):463–488. doi: 10.1142/S0192415X16500269. [DOI] [PubMed] [Google Scholar]
- 3.Cheng J.T., Guo C., Cui W.J., Zhang Q., Wang S.H., Zhao Q.H., Liu D.W., Zhang J., Chen S., Chen C., Liu Y., Pan Z.H., Liu A. Isolation of two rare N-glycosides from Maidenhairtree and their anti-inflammatory activities. Sci Rep. 2020;10(1):5994. doi: 10.1038/s41598-020-62884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noor, E. T., R. Das, M. S. Lami, A. J. Chakraborty, S. Mitra, T. E. Tallei, R. Idroes, A. A. R. Mohamed, M. J. Hossain, K. Dhama, G. Mostafa-Hedeab and T. B. Emran (2022). Maidenhairtree: A Treasure of Functional Phytochemicals with Multimedicinal Applications. EVIDENCE-BASED COMPLEMENTARY AND ALTERNATIVE MEDICINE 2022. [DOI] [PMC free article] [PubMed]
- 5.Yin M., Zhang Y., Li H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front Immunol. 2019;10:145. doi: 10.3389/fimmu.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J., Zhang T., Jiang B., Mu W., Miao M. Characterization and antioxidant activity of Maidenhairtree exocarp polysaccharides. Carbohydr Polym. 2012;87(1):40–45. doi: 10.1016/j.carbpol.2011.06.083. [DOI] [PubMed] [Google Scholar]
- 7.Sun B., Yu S., Zhao D., Guo S., Wang X., Zhao K. Polysaccharides as vaccine adjuvants. Vaccine. 2018;36(35):5226–5234. doi: 10.1016/j.vaccine.2018.07.040. [DOI] [PubMed] [Google Scholar]
- 8.Ye J., Ye C., Huang Y., Zhang N., Zhang X., Xiao M. Maidenhairtree sarcotesta polysaccharide inhibits inflammatory responses through suppressing both NF-kappaB and MAPK signaling pathway. J Sci Food Agric. 2019;99(5):2329–2339. doi: 10.1002/jsfa.9431. [DOI] [PubMed] [Google Scholar]
- 9.Xie J.H., Jin M.L., Morris G.A., Zha X.Q., Chen H.Q., Yi Y., Li J.E., Wang Z.J., Gao J., Nie S.P., Shang P., Xie M.Y. Advances on Bioactive Polysaccharides from Medicinal Plants. Crit Rev Food Sci Nutr. 2016;56(Suppl 1):S60–S84. doi: 10.1080/10408398.2015.1069255. [DOI] [PubMed] [Google Scholar]
- 10.Xu Q., Shen Y.Y., Wang H.F., Zhang N.P., Xu S., Zhang L. Application of response surface methodology to optimise extraction of flavonoids from fructus sophorae. FOOD CHEMISTRY. 2013;138(4):2122–2129. doi: 10.1016/j.foodchem.2012.11.099. [DOI] [PubMed] [Google Scholar]
- 11.Wang, G. X., H. Y. Wang, Y. Z. Yang, C. G. Li, Q. Luo and D. Song (2019). EXTRACTION OF POLYSACCHARIDES FROM THE MESOSPERM OF MAIDENHAIRTREE L. BY AQUEOUS ENZYMATIC EXTRACTION. BANGLADESH JOURNAL OF BOTANY 48(3): 711-717.
- 12.Jiang B., Zhang H., Liu C., Wang Y., Fan S. Extraction of water-soluble polysaccharide and the antioxidant activity from Maidenhairtree leaves. Medicinal Chemistry Research. 2009;19(3):262–270. [Google Scholar]
- 13.Han H.P., Xie H.C. A study on the extraction and purification process of lily polysaccharide and its anti-tumor effect. Afr J Tradit Complement Altern Med. 2013;10(6):485–489. doi: 10.4314/ajtcam.v10i6.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Wang F., Jing Y., Wang Y., Lin P., Yang L. Application of Orthogonal Design to Optimize Extraction of Polysaccharide from Cynomorium songaricum Rupr (Cynomoriaceae) Tropical Journal of Pharmaceutical Research. 2015;14(7) [Google Scholar]
- 15.Huang G., Chen F., Yang W., Huang H. Preparation, deproteinization and comparison of bioactive polysaccharides. Trends in Food Science & Technology. 2021;109:564–568. [Google Scholar]
- 16.Li R., Xia Z., Li B., Tian Y., Zhang G., Li M., Dong J. Advances in Supercritical Carbon Dioxide Extraction of Bioactive Substances from Different Parts of Maidenhairtree L. Molecules. 2021;26(13) doi: 10.3390/molecules26134011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang S.J., Liu S.S., Qin M.S. Effects of Extraction Conditions on Crude Polysaccharides and Antioxidant Activities of the Lion's Mane Medicinal Mushroom, Hericium erinaceus (Agaricomycetes) INTERNATIONAL JOURNAL OF MEDICINAL MUSHROOMS. 2019;21(10):1007–1018. doi: 10.1615/IntJMedMushrooms.2019032566. [DOI] [PubMed] [Google Scholar]
- 18.Cheng S., He F., Fu L., Zhang Y. Polysaccharide from rubescens: extraction, optimization, characterization and antioxidant activities. RSC Adv. 2021;11(31):18974–18983. doi: 10.1039/d1ra01365c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.You Q., Yin X., Zhao Y. Enzyme assisted extraction of polysaccharides from the fruit of Cornus officinalis. Carbohydrate Polymers. 2013;98(1):607–610. doi: 10.1016/j.carbpol.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Nai J., Zhang C., Shao H., Li B., Li H., Gao L., Dai M., Zhu L., Sheng H. Extraction, structure, pharmacological activities and drug carrier applications of Angelica sinensis polysaccharide. Int J Biol Macromol. 2021;183:2337–2353. doi: 10.1016/j.ijbiomac.2021.05.213. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D.Y., Wan Y., Xu J.Y., Wu G.H., Li L., Yao X.H. Ultrasound extraction of polysaccharides from mulberry leaves and their effect on enhancing antioxidant activity. Carbohydr Polym. 2016;137:473–479. doi: 10.1016/j.carbpol.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad M.M., Chatha S.A.S., Iqbal Y., Hussain A.I., Khan I., Xie F. Recent trends in extraction, purification, and antioxidant activity evaluation of plant leaf-extract polysaccharides. Biofuels, Bioproducts and Biorefining. 2022;16(6):1820–1848. [Google Scholar]
- 23.Zhu W., Xue X., Zhang Z. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int J Biol Macromol. 2016;91:132–142. doi: 10.1016/j.ijbiomac.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 24.Hariharasubramanian J., Baik O.D., Singhal R.S. ULTRASOUND-ASSISTED EXTRACTION (UAE) OF POLYSACCHARIDE FROM DRIED BIOMASS OF SARGASSUM MUTICUM.“. TRANSACTIONS OF THE ASABE. 2015;58(5):1363–1370. [Google Scholar]
- 25.Chen R., Li Y., Dong H., Liu Z., Li S., Yang S., Li X. Optimization of ultrasonic extraction process of polysaccharides from Ornithogalum Caudatum Ait and evaluation of its biological activities. Ultrason Sonochem. 2012;19(6):1160–1168. doi: 10.1016/j.ultsonch.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Zhao Q., Kennedy J.F., Wang X., Yuan X., Zhao B., Peng Y., Huang Y. Optimization of ultrasonic circulating extraction of polysaccharides from Asparagus officinalis using response surface methodology. Int J Biol Macromol. 2011;49(2):181–187. doi: 10.1016/j.ijbiomac.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Huang H., Huang G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem Biol Drug Des. 2020;96(5):1209–1222. doi: 10.1111/cbdd.13794. [DOI] [PubMed] [Google Scholar]
- 28.Marcati A., Ursu A.V., Laroche C., Soanen N., Marchal L., Jubeau S., Djelveh G., Michaud P. Extraction and fractionation of polysaccharides and B-phycoerythrin from the microalga Porphyridium cruentum by membrane technology. Algal Research. 2014;5:258–263. [Google Scholar]
- 29.Xie J.-H., Shen M.-Y., Nie S.-P., Zhao Q., Li C., Xie M.-Y. Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydrate Polymers. 2014;101:479–483. doi: 10.1016/j.carbpol.2013.09.075. [DOI] [PubMed] [Google Scholar]
- 30.Tang H.L., Chen C., Wang S.K., Sun G.J. Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int J Biol Macromol. 2015;77:235–242. doi: 10.1016/j.ijbiomac.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 31.Li P., Liang Z.H., Jiang Z., Qiu Z., Du B., Liu Y.B., Li W.Z., Tan L.H. Supercritical fluid extraction effectively removes phthalate plasticizers in spores of Ganoderma lucidum. Food Sci Biotechnol. 2018;27(6):1857–1864. doi: 10.1007/s10068-018-0404-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dias A.L.B., de Aguiar A.C., Rostagno M.A. Extraction of natural products using supercritical fluids and pressurized liquids assisted by ultrasound: Current status and trends. Ultrasonics Sonochemistry. 2021;74 doi: 10.1016/j.ultsonch.2021.105584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R., Wu G., Du L., Shao J., Liu F., Yang Z., Liu D., Wei Y. Semi-bionic extraction of compound turmeric protects against dextran sulfate sodium-induced acute enteritis in rats. J Ethnopharmacol. 2016;190:288–300. doi: 10.1016/j.jep.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 34.Zou X., Liu Y., Tao C., Liu Y., Liu M., Wu J., Lv Z. CO2 supercritical fluid extraction and characterization of polysaccharide from bamboo (Phyllostachys heterocycla) leaves. Journal of Food Measurement and Characterization. 2017;12(1):35–44. [Google Scholar]
- 35.He L., Yan X., Liang J., Li S., He H., Xiong Q., Lai X., Hou S., Huang S. Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohydr Polym. 2018;198:101–108. doi: 10.1016/j.carbpol.2018.06.073. [DOI] [PubMed] [Google Scholar]
- 36.Chen F., Huang G., Huang H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydr. Polym. 2021;252 doi: 10.1016/j.carbpol.2020.117179. [DOI] [PubMed] [Google Scholar]
- 37.Zhou S., Huang G. Extraction, derivatization, and antioxidant activity of Morinda citrifolia polysaccharide. Chem. Bio. Drug Des. 2022;99:603–608. doi: 10.1111/cbdd.14023. [DOI] [PubMed] [Google Scholar]
- 38.Lin B., Huang G. An important polysaccharide from fermentum. Food Chemistry: X. 2022;15 doi: 10.1016/j.fochx.2022.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li B., Huang G. Preparation, structure-function relationship and application of Grifola umbellate polysaccharides. Industrial Crops and Products. 2022;186 [Google Scholar]
- 40.Zhou S., Huang G., Huang H. Extraction, derivatization and antioxidant activities of onion polysaccharide. Food Chemistry. 2022;388 doi: 10.1016/j.foodchem.2022.133000. [DOI] [PubMed] [Google Scholar]
- 41.Tang Z., Huang G. Extraction, structure, and activity of polysaccharide from Radix astragali. Biomedicine and Pharmacotherapy. 2022;150 doi: 10.1016/j.biopha.2022.113015. [DOI] [PubMed] [Google Scholar]
- 42.Lin B., Huang G. Extraction, isolation, purification, derivatization, bioactivity, structure-activity relationship, and application of polysaccharides from White jellyfungus. Biotechnology and Bioengineering. 2022;119(6):1359–1379. doi: 10.1002/bit.28064. [DOI] [PubMed] [Google Scholar]
- 43.Tang Q., Huang G. Improving method, properties and application of polysaccharide as emulsifier. Food Chemistry. 2022;376 doi: 10.1016/j.foodchem.2021.131937. [DOI] [PubMed] [Google Scholar]
- 44.Li J., Fan Y., Huang G., Huang H. Extraction, structural characteristics and activities of Zizylphus vulgaris polysaccharides. Industrial Crops and Products. 2022;178 [Google Scholar]
- 45.Zhou S., Huang G. Extraction, derivatization, and antioxidant activity of Morinda citrifolia polysaccharide. Chemical Biology and Drug Design. 2022;99(4):603–608. doi: 10.1111/cbdd.14023. [DOI] [PubMed] [Google Scholar]
- 46.Yang W., Yang Z., Zou Y., Sun X., Huang G. Extraction and deproteinization process of polysaccharide from purple sweet potato. Chemical Biology and Drug Design. 2022;99(1):111–117. doi: 10.1111/cbdd.13935. [DOI] [PubMed] [Google Scholar]
- 47.Fan Y., Zhou X., Huang G. Preparation, structure, and properties of tea polysaccharide. Chemical Biology and Drug Design. 2022;99(1):75–82. doi: 10.1111/cbdd.13924. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W., Huang G. Preparation, structural characteristics, and application of taro polysaccharides in food. J. Sci. Food Agric. 2022;102:6193–6201. doi: 10.1002/jsfa.12058. [DOI] [PubMed] [Google Scholar]
- 49.Zhou S., Huang G. Extraction, structural analysis and antioxidant activity of aloe polysaccharide. Journal of Molecular Structure. 2023;1273 [Google Scholar]
- 50.Mei X., Tang Q., Huang G., Long R., Huang H. Preparation, structural analysis and antioxidant activities of phosphorylated (1→3)-â-d-glucan. Food Chem. 2020;309 doi: 10.1016/j.foodchem.2019.125791. [DOI] [PubMed] [Google Scholar]
- 51.Benchamas G., Huang G., Huang S., Huang H. Preparation and biological activities of chitosan oligosaccharides. Trends in Food Science and Technology. 2021;107:38–44. [Google Scholar]
- 52.Liu Y., Huang G. Extraction and derivatisation of active polysaccharides. Journal of Enzyme Inhibition and Medicinal Chemistry. 2019;34(1):1690–1696. doi: 10.1080/14756366.2019.1660654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang W., Huang G. Extraction methods and activities of natural glucans. Trends in Food Science and Technology. 2021;112:50–57. [Google Scholar]
- 54.Yang W., Huang G., Chen F., Huang H. Extraction/synthesis and biological activities of selenopolysaccharide. Trends Food Sci. Technol. 2021;109:211–218. [Google Scholar]
- 55.Zhou S., Huang G. Preparation, structure and activity of polysaccharide phosphate esters. Biomedicine & Pharmacotherapy. 2021;144 doi: 10.1016/j.biopha.2021.112332. [DOI] [PubMed] [Google Scholar]
- 56.Zhou S., Huang G., Chen G. Extraction, structural analysis, derivatization and antioxidant activity of polysaccharide from Chinese yam. Food Chemistry. 2021;361 doi: 10.1016/j.foodchem.2021.130089. [DOI] [PubMed] [Google Scholar]
- 57.Li J., Shi H., Yu J., Lei Y., Huang G., Huang H. Extraction and properties of Ginkgo biloba leaf polysaccharide and its phosphorylated derivative. Industrial Crops & Products. 2022;189 [Google Scholar]
- 58.Li J., Chen Z., Shi H., Yu J., Huang G., Huang H. Ultrasound-assisted extraction and properties of polysaccharide from Ginkgo biloba leaves. Ultrasonics Sonochemistry. 2023;93 doi: 10.1016/j.ultsonch.2023.106295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou S., Huang G. Extraction, structure characterization and biological activity of polysaccharide from coconut peel. Chemical and Biological Technologies in Agriculture. 2023;10(1):15. [Google Scholar]
- 60.Zhang L., Guo S., Wang M., He L. PEG-based ultrasound-assisted enzymatic extraction of polysaccharides from Maidenhairtree leaves. Int J Biol Macromol. 2015;80:644–650. doi: 10.1016/j.ijbiomac.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 61.Yang Y., Liu P., Chen L., Liu Z., Zhang H., Wang J., Sun X., Zhong W., Wang N., Tian K., Zhao J. Therapeutic effect of Maidenhairtree polysaccharide in rats with focal cerebral ischemia/reperfusion (I/R) injury. Carbohydr Polym. 2013;98(2):1383–1388. doi: 10.1016/j.carbpol.2013.07.045. [DOI] [PubMed] [Google Scholar]
- 62.Cui Y., Lai X., Liu K., Liang B., Ma G., Wang L. Maidenhairtree leaf polysaccharide stabilized palladium nanoparticles with enhanced peroxidase-like property for the colorimetric detection of glucose. RSC Adv. 2020;10(12):7012–7018. doi: 10.1039/d0ra00680g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bilal M., Gul I., Basharat A., Qamar S.A. Polysaccharides-based bio-nanostructures and their potential food applications. Int J Biol Macromol. 2021;176:540–557. doi: 10.1016/j.ijbiomac.2021.02.107. [DOI] [PubMed] [Google Scholar]
- 64.Zeng P., Li J., Chen Y., Zhang L. The structures and biological functions of polysaccharides from traditional Chinese herbs. Prog Mol Biol Transl Sci. 2019;163:423–444. doi: 10.1016/bs.pmbts.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheng H., Huang G. Extraction, characterisation and antioxidant activity of Allium sativum polysaccharide. Int J Biol Macromol. 2018;114:415–419. doi: 10.1016/j.ijbiomac.2018.03.156. [DOI] [PubMed] [Google Scholar]
- 66.Shi L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int J Biol Macromol. 2016;92:37–48. doi: 10.1016/j.ijbiomac.2016.06.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ren Y., Bai Y., Zhang Z., Cai W., Del Rio Flores A. The Preparation and Structure Analysis Methods of Natural Polysaccharides of Plants and Fungi: A Review of Recent Development. Molecules. 2019;24(17) doi: 10.3390/molecules24173122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Vaquero M., Rajauria G., O'Doherty J.V., Sweeney T. Polysaccharides from macroalgae: Recent advances, innovative technologies and challenges in extraction and purification. Food Res Int. 2017;99(Pt 3):1011–1020. doi: 10.1016/j.foodres.2016.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Tian X., Liang T., Liu Y., Ding G., Zhang F., Ma Z. Extraction, Structural Characterization, and Biological Functions of Lycium Barbarum Polysaccharides: A Review. Biomolecules. 2019;9(9) doi: 10.3390/biom9090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fan W., Yan W., Xu Z., Ni H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf B Biointerfaces. 2012;90:21–27. doi: 10.1016/j.colsurfb.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 71.Ndeh D., Rogowski A., Cartmell A., Luis A.S., Basle A., Gray J., Venditto I., Briggs J., Zhang X., Labourel A., Terrapon N., Buffetto F., Nepogodiev S., Xiao Y., Field R.A., Zhu Y., O'Neil M.A., Urbanowicz B.R., York W.S., Davies G.J., Abbott D.W., Ralet M.C., Martens E.C., Henrissat B., Gilbert H.J. Complex pectin metabolism by gut bacteria reveals novel catalytic functions. Nature. 2017;544(7648):65–70. doi: 10.1038/nature21725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferreira I.C., Heleno S.A., Reis F.S., Stojkovic D., Queiroz M.J., Vasconcelos M.H., Sokovic M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry. 2015;114:38–55. doi: 10.1016/j.phytochem.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 73.Ren Q., Chen J., Ding Y., Cheng J., Yang S., Ding Z., Dai Q., Ding Z. In vitro antioxidant and immunostimulating activities of polysaccharides from Maidenhairtree leaves. Int J Biol Macromol. 2019;124:972–980. doi: 10.1016/j.ijbiomac.2018.11.276. [DOI] [PubMed] [Google Scholar]
- 74.Wang, F. F., S. H. Ye, Y. Ding, Z. Y. Ma, Q. K. Zhao, M. Zang and Y. Li (2022). Research on structure and antioxidant activity of polysaccharides from Maidenhairtree leaves. JOURNAL OF MOLECULAR STRUCTURE 1252.
- 75.Mao Ll Fau - Chen, Y., B.-Y. Chen Y Fau - Hu, A.-H. Hu By Fau - Xu and A. H. Xu (2014). Analysis of monosaccharide compositions in polysaccharides from exopleura of Maidenhairtree. (1001-5302 (Print)). [PubMed]
- 76.Zhu R.K., Xie J. Effect of aerobic exercise combined with maidenhairtree polysaccharide on weight, blood glucose and glycosylated serum protein in diabetic rats. PAKISTAN JOURNAL OF PHARMACEUTICAL SCIENCES. 2018;31(3):1045–1050. [PubMed] [Google Scholar]
- 77.Luan F., Ji Y., Peng L., Liu Q., Cao H., Yang Y., He X., Zeng N. Extraction, purification, structural characteristics and biological properties of the polysaccharides from Codonopsis pilosula: A review. Carbohydr Polym. 2021;261 doi: 10.1016/j.carbpol.2021.117863. [DOI] [PubMed] [Google Scholar]
- 78.Hu J., Liu Y., Cheng L., Shi R., Qayum A., Bilawal A., Gantumur M.A., Hussain M.A., Jiang Z., Tian B. Comparison in bioactivity and characteristics of Maidenhairtree seed polysaccharides from four extract pathways. Int J Biol Macromol. 2020;159:1156–1164. doi: 10.1016/j.ijbiomac.2020.05.129. [DOI] [PubMed] [Google Scholar]
- 79.Gong T., Liu S., Wang H., Zhang M. Supercritical CO2 fluid extraction, physicochemical properties, antioxidant activities and hypoglycemic activity of polysaccharides derived from fallen Maidenhairtree leaves. Food. Bioscience. 2021;42 [Google Scholar]
- 80.Cui N., Zhang L., Quan M., Xu J. Profile of the main bioactive compounds and in vitro biological activity of different solvent extracts from Maidenhairtree exocarp. RSC Adv. 2020;10(73):45105–45111. doi: 10.1039/d0ra09490k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gharibzahedi S.M.T., Marti-Quijal F.J., Barba F.J., Altintas Z. Current emerging trends in antitumor activities of polysaccharides extracted by microwave- and ultrasound-assisted methods. International Journal of Biological Macromolecules. 2022;202:494–507. doi: 10.1016/j.ijbiomac.2022.01.088. [DOI] [PubMed] [Google Scholar]
- 82.Lin J., Li P., Chen K.-S. Advance in studies on anti-tumor activity of polysaccharides in latest five years. China journal of Chinese materia medica. 2013;38(8):1116–1125. [PubMed] [Google Scholar]
- 83.Huang G., Huang H. The derivatization and antitumor mechanisms of polysaccharides. Future Med Chem. 2017;9(16):1931–1938. doi: 10.4155/fmc-2017-0132. [DOI] [PubMed] [Google Scholar]
- 84.Xia G.-X., Wu Y.-M., Bi Y.-F., Chen K., Zhang W.-W., Liu S.-Q., Zhang W.-J., Liu R.-H. Antimicrobial Properties and Application of Polysaccharides and Their Derivatives. Chinese Journal of Polymer Science. 2020;39(2):133–146. [Google Scholar]
- 85.Zhang C., Lin L., Li G., Ma J., Han X., Fei R. PGBL inhibits the RAW 264.7 cells to express inflammatory factor. Biomed Mater Eng. 2015;26 Suppl 1:S2069. doi: 10.3233/BME-151512. S2075. [DOI] [PubMed] [Google Scholar]
- 86.Jiang H., Luan Z., Fan Z., Wu X., Xu Z., Zhou T., Wang H. Antibacterial, Antibiofilm, and Antioxidant Activity of Polysaccharides Obtained from Fresh Sarcotesta of Maidenhairtree: Bioactive Polysaccharide that Can Be Exploited as a Novel Biocontrol Agent. Evid Based Complement Alternat Med. 2021;2021:5518403. doi: 10.1155/2021/5518403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J.H., Park J.S., Lee S.W., Hwang S.Y., Young B.E., Choi H.J. Porcine epidemic diarrhea virus infection: inhibition by polysaccharide from maidenhairtree exocarp and mode of its action. Virus Res. 2015;195:148–152. doi: 10.1016/j.virusres.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 88.Li, Y. N., Y. Sheng, J. Y. Liu, G. Y. Xu, W. W. Yu, Q. W. Cui, X. C. Lu, P. G. Du and L. P. An (2022). “Hair-growth promoting effect and anti-inflammatory mechanism of Maidenhairtree polysaccharides.” CARBOHYDRATE POLYMERS 278. [DOI] [PubMed]
- 89.Yan Z., Fan R., Yin S., Zhao X., Liu J., Li L., Zhang W., Ge L. Protective effects of Maidenhairtree leaf polysaccharide on nonalcoholic fatty liver disease and its mechanisms. Int J Biol Macromol. 2015;80:573–580. doi: 10.1016/j.ijbiomac.2015.05.054. [DOI] [PubMed] [Google Scholar]
- 90.Chen P., Hei M., Kong L., Liu Y., Yang Y., Mu H., Zhang X., Zhao S., Duan J. One water-soluble polysaccharide from Maidenhairtree leaves with antidepressant activities via modulation of the gut microbiome. Food Funct. 2019;10(12):8161–8171. doi: 10.1039/c9fo01178a. [DOI] [PubMed] [Google Scholar]
- 91.Oh T.S., Koo H.M., Yoon H.R., Jeong N.S., Kim Y.J., Kim C.H. Antifungal Action of Maidenhairtree Outer Seedcoat on Rice Sheath blight. PLANT PATHOLOGY JOURNAL. 2015;31(1):61–66. doi: 10.5423/PPJ.NT.03.2014.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C.-W., Wang C.-Z., Tao R. Characterization and antioxidant activities of polysaccharides extracted from enzymatic hydrolysate of Maidenhairtree leaves. Journal of Food Biochemistry. 2017;41(3) [Google Scholar]
- 93.Chen Y., Meng Y., Cao Y., Wen H., Luo H., Gao X., Shan F. Novel analysis of maturation of murine bone-marrow-derived dendritic cells induced by Maidenhairtree Seed Polysaccharides. Hum Vaccin Immunother. 2015;11(6):1387–1393. doi: 10.1080/21645515.2015.1023971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abd El-Hack M.E., El-Saadony M.T., Shafi M.E., Zabermawi N.M., Arif M., Batiha G.E., Khafaga A.F., Abd El-Hakim Y.M., Al-Sagheer A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int J Biol Macromol. 2020;164:2726–2744. doi: 10.1016/j.ijbiomac.2020.08.153. [DOI] [PubMed] [Google Scholar]
- 95.Cai Y., Chen P., Wu C., Duan J., Bai H. Sulfated modification and biological activities of polysaccharides derived from Zizyphus jujuba cv. Jinchangzao. Int J Biol Macromol. 2018;120(Pt A):1149–1155. doi: 10.1016/j.ijbiomac.2018.08.141. [DOI] [PubMed] [Google Scholar]
- 96.Simsek M., Asiyanbi-Hammed T.T., Rasaq N., Hammed A.M. Progress in Bioactive Polysaccharide-Derivatives: A Review. Food Reviews International. 2021:1–16. [Google Scholar]
- 97.Wang X., Wang Z., Shen M., et al. Acetylated polysaccharides: Synthesis, physicochemical properties, bioactivities, and food applications. Critical Reviews in Food Science and Nutrition. 2022 doi: 10.1080/10408398.2022.2146046. [DOI] [PubMed] [Google Scholar]
- 98.Wu X., Mao G., Zhao T., Zhao J., Li F., Liang L., Yang L. Isolation, purification and in vitro anti-tumor activity of polysaccharide from Maidenhairtree sarcotesta. Carbohydrate Polymers. 2011;86(2):1073–1076. [Google Scholar]
- 99.Chen L., Huang G. The antiviral activity of polysaccharides and their derivatives. Int J Biol Macromol. 2018;115:77–82. doi: 10.1016/j.ijbiomac.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 100.Chen D., Sun S., Cai D., Kong G. Induction of mitochondrial-dependent apoptosis in T24 cells by a selenium (Se)-containing polysaccharide from Maidenhairtree L. leaves. Int J Biol Macromol. 2017;101:126–130. doi: 10.1016/j.ijbiomac.2017.03.033. [DOI] [PubMed] [Google Scholar]
- 101.Lovegrove A., Edwards C.H., De Noni I., Patel H., El S.N., Grassby T., Zielke C., Ulmius M., Nilsson L., Butterworth P.J., Ellis P.R., Shewry P.R. Role of polysaccharides in food, digestion, and health. Crit Rev Food Sci Nutr. 2017;57(2):237–253. doi: 10.1080/10408398.2014.939263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang X., Li A., Li X., Sun L., Guo Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends in Food Science & Technology. 2020;102:1–15. [Google Scholar]
- 103.Martínez-Abad A., Ruthes A.C., Vilaplana F. Enzymatic-assisted extraction and modification of lignocellulosic plant polysaccharides for packaging applications. Journal of Applied Polymer Science. 2016;133(2) [Google Scholar]
- 104.Boateng I.D., Yang X.M. Maidenhairtree L. seed; A comprehensive review of bioactives, toxicants, and processing effects. INDUSTRIAL CROPS AND PRODUCTS. 2022;176 [Google Scholar]
- 105.Xie L.M., Shen M.Y., Wang Z.J., Xie J.H. Structure, function and food applications of carboxymethylated polysaccharides: A comprehensive review. TRENDS IN FOOD SCIENCE & TECHNOLOGY. 2021;118:539–557. [Google Scholar]
- 106.Muthukumar J., Chidambaram R., Sukumaran S. Sulfated polysaccharides and its commercial applications in food industries-A review. J Food Sci Technol. 2021;58(7):2453–2466. doi: 10.1007/s13197-020-04837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yip K.C.E., Lin C.H., Wu J.S., Lin Y.H., Hsieh P.C., Chu V., Tsai F.M., Chen M.L., Tzeng I.S., Lin J.W., Kuo C.Y. The Role of Maidenhairtree in Human Diseases. CURRENT TOPICS IN NUTRACEUTICAL RESEARCH. 2021;19(4):405–408. [Google Scholar]